Lymphatic Tissue Fibrosis Is Associated with Reduced Numbers of Naïve CD4+ T Cells in Human Immunodeficiency Virus Type 1 Infection (original) (raw)
Abstract
The organized structure of lymphatic tissues (LTs) constitutes a microenvironment referred to as a niche that plays a critical role in immune system homeostasis by promoting cellular interactions and providing access to cytokines and growth factors on which cells are dependent for survival, proliferation, and differentiation. In chronic human immunodeficiency virus type 1 (HIV-1) infection, immune activation and inflammation result in collagen deposition and disruption of this LT niche. We have previously shown that these fibrotic changes correlate with a reduction in the size of the total population of CD4+ T cells. We now show that this reduction is most substantial within the naïve CD4+ T-cell population and is in proportion to the extent of LT collagen deposition in HIV-1 infection. Thus, the previously documented depletion of naïve CD4+ T cells in LTs in HIV-1 infection may be a consequence not only of a decreased supply of thymic emigrants or chronic immune activation but also of the decreased ability of those cells to survive in a scarred LT niche. We speculate that LT collagen deposition might therefore limit repopulation of naïve CD4+ T cells with highly active antiretroviral therapy, and thus, additional treatments directed to limiting or reversing inflammatory damage to the LT niche could potentially improve immune reconstitution.
Human immunodeficiency virus (HIV)/AIDS is associated with profound depletion of CD4+ T cells in peripheral blood and throughout the secondary peripheral (22, 23, 26) and gut-associated lymphoid tissues (3, 15, 21, 25), where most of these cells reside. Multiple mechanisms have been proposed to explain this depletion, including decreased thymic output (7, 8), direct viral cytopathicity (1, 4, 5, 13, 19, 24), T-cell-mediated cytolysis of infected cells (20), chronic immune activation leading to increased rates of apoptosis and attrition of CD4+ T-cell naïve and memory pools, and most recently, disruption of homeostatic mechanisms that maintain normal-sized T-cell populations by collagen deposition of the T-cell zone (TZ) niche on which T cells are dependent for survival and growth (22).
The last mechanism was suggested by the observation of an inverse correlation between the extent of collagen deposition and the size of the population of CD4+ T cells. Of importance, it has been shown in studies of the mouse immune system that naïve CD4+ T cells are particularly dependent on an organized lymphatic tissue (LT) structure to facilitate both movement and contact with self-peptide major histocompatibility complexes and access to cytokines and growth factors necessary for survival (6, 11, 14, 17); we therefore reasoned that disruption of the normal TZ architecture by fibrosis might be playing an important role in the well-documented and substantial depletion of naïve CD4+ T cells in peripheral blood and LT in late HIV-1 infection.
Under this hypothesis, the depletion before treatment and slow repletion of the naïve CD4+ T-cell pool with antiretroviral therapy (ART) are due not only to the limited ability of the thymus to generate new naïve CD4+ T cells in the face of the homeostatic strain imposed by chronic immune activation of the T-cell pools (9, 10) but also to the detrimental effects of collagen deposition and disruption of the niche on the survival of naïve T cells. The major predictions of this hypothesis are that LT collagen should be correlated with the reduced size of the naïve CD4+ T-cell population before treatment and subsequent recovery of the CD4+ T-cell population with ART. In this report, we show that LT fibrosis in fact negatively impacts naïve T-cell populations during infection and therefore could limit recovery with ART.
MATERIALS AND METHODS
Patients were recruited into the University of Minnesota Institutional Review Board-approved protocol and were seen in the University of Minnesota NIH-funded General Clinical Research Center, using entry criteria that have been previously described (22). All study-related procedures were carried out in the University of Minnesota General Clinical Research Center except for the excisional inguinal lymph node biopsy, which was completed in a research clinic at the University of Minnesota with facilities for biopsy under local anesthetic.
At the time of biopsy, peripheral blood was obtained by venipuncture for T-cell subset analysis. The inguinal lymph node was obtained and processed as described previously (22).
To obtain LT CD4+ T cells for flow cytometry, a portion of the LT was placed on ice immediately after biopsy, and within 24 h, CD4+ T cells were isolated by gently separating cells from surrounding tissue using a mesh screen. Peripheral blood mononuclear cells were isolated by density centrifugation of venous blood with Ficoll (Upsula, Sweden). One million cells were washed once in a fluorescence-activated cell sorter (FACS) wash (phosphate-buffered saline supplemented with 0.1% sodium azide and 2% bovine serum albumin [Sigma, St. Louis, MO]). After aspiration of the supernatant, cells were stained with CD4 PerCp, CD8 allophycocyanin, CD27 phycoerythrin, and CD45RO fluorescein isothiocyanate (all obtained from BD Pharmingen, San Diego, CA). Antibodies were incubated with the cells for 30 min at 4°C, followed by another FACS wash. Cells were then fixed with 1% paraformaldahyde (Electron Microscopy Sciences, Ft. Washington, PA) and analyzed on a FACSCalibur (BD Pharmingen). Lymphocytes were gated based upon characteristic forward and side scatter properties, followed by separation into CD4+ T cells and CD8+ T cells based upon expression of CD4 and CD8. Naïve T cells were classified by bright expression of CD27 without expression of CD45RO as previously described (2). Central memory T cells were classified by coexpression of CD27 and CD45RO, and effector memory T cells were classified by lack of CD27 expression.
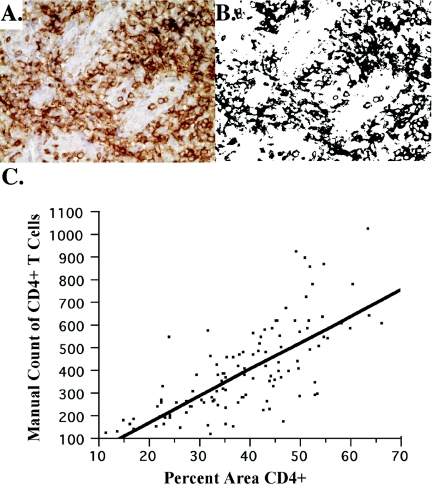
We used immunohistochemistry to quantify the size of the overall and naïve CD4+ T-cell population. Briefly, 4-μm sections of LT were reacted with antibodies to CD4 (clone If 6°; Novocastra, Newcastle upon Tyne, United Kingdom), and approximately 18 images of the T-cell zone were captured by standard light microscopy and transported into Photoshop CS (Adobe, San Jose, CA). The area that stained positive for CD4 was quantified using tools from Reindeer Graphics (Asheville, N.C.) and expressed as the area occupied by CD4+ T cells/μm2. Previously, we used image analysis to calculate the number of cells/μm2 (22) as opposed to reporting the area of TZ staining positive for CD4; however, using the Reindeer Graphics tools and reporting the area positive for CD4 is a more efficient way of image analysis for a large volume of images. Analysis of 100 images from a total of 20 patients in which CD4 cells in each image were hand counted demonstrated good correlation with the automated quantification of the area occupied by CD4+ T cells (Fig. 1). The numbers of naïve, effector, and central memory CD4+ T cells in LT were determined as the fraction of each population (from flow cytometry analysis) of the total area staining positive for CD4.
FIG. 1.
Comparison of CD4+ area to actual count. The percent area that stains with diaminobenzidine (i.e., CD4+ T cells) is compared to the number of cells manually counted in the image. A total of 117 images (the 1st, 10th, and 18th images from each subject in the analysis) were hand counted using MetaMorph (Trenton, NJ) analysis, where each cell is identified by placing a small hash mark over it with the mouse and the computer tallies the number of hash marks. In panel A, 156 cells were counted. Using Photoshop and the image analysis tools, the percent area CD4+ was found to be 31% (B). Panel C shows the correlation between all images from the 32 patients (P < 0.0001, r = 0.73) and can be described by the following equation: number of CD4+ T cells = (−61.06389 + 11.76418) × % CD4+ area.
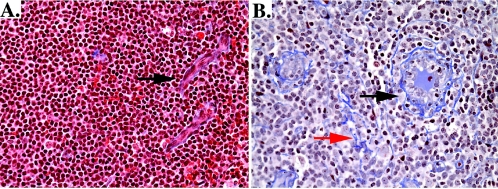
To quantify collagen in the TZ, we used methods similar to those previously published (22). Sections (4 μm) were stained using a modified trichrome stain to identify collagen fibers, and approximately 18 images from the TZ were captured and transported into Photoshop CS (Adobe, San Jose, CA). New image analysis tools from Reindeer Graphics (Asheville, NC) provided better sensitivity for isolating collagen fibers to quantify the percent area occupied by collagen. A sample of an HIV-infected and uninfected lymph node stained with trichrome to identify collagen fibers is presented in Fig. 2.
FIG. 2.
Example of collagen deposition in inguinal lymph node tissue from two patients, one without HIV infection (A) and one with HIV infection and AIDS (B). In panel A, there is minimal deposition of collagen that is found mostly around high endothelial venules (black arrow), as expected. In panel B, there is significant deposition of collagen that is increased around the high endothelial venules (black arrow) and is also found throughout the T-cell zone (red arrow).
RESULTS
We studied a total of 33 patients: 9 HIV-uninfected persons considered to be at risk for HIV and 24 ART naïve HIV-positive individuals at all stages of infection. Demographic characteristics and peripheral blood CD4+ T-cell counts are presented in Table 1.
TABLE 1.
Patient population
| Characteristic | HIV-negative patients (n = 9) | Presymptomatic patients (n = 18) | AIDS patients (n = 6) |
|---|---|---|---|
| Ethnicity | |||
| Black (no. of patients) | 1 | 3 | 2 |
| White (no. of patients) | 8 | 14 | 4 |
| Latino (no. of patients) | 0 | 1 | 0 |
| Male (no. of patients) | 8 | 17 | 6 |
| Female (no. of patients) | 1 | 1 | 0 |
| Median (range) age | 39 (22-52) | 37 (27-63) | 44 (36-50) |
| Median (range) CD4+ T-cell count | 809 (392-1,487) | 485 (221-1,058) | 93 (3-188) |
We compared the sizes of the LT naïve and memory CD4+ T-cell populations in HIV-1-uninfected and -infected individuals in the presymptomatic stage of infection to AIDS and found that the size of the naïve pool was smaller in infected individuals and that the greatest decreases were in patients with AIDS (Table 2). By contrast, neither the total nor central memory pools of CD4+ T cells in LT were altered in HIV-infected persons, but the effector memory population was significantly increased (Table 2), consistent with previous reports (2, 18).
TABLE 2.
Mean size of LT CD4+ naïve and memory populations
| CD4 population | Mean area (104 μm2) of cell population ina: | P valueb | ||
|---|---|---|---|---|
| HIV-negative patients | Presymptomatic patients | AIDS patients | ||
| Total | 5.96 | 4.38 | 3.34 | 0.00001 |
| Naïve | 3.59 | 2.13 | 0.65 | 0.0001 |
| Total memory | 2.40 | 2.25 | 2.70 | 0.5 |
| Effector memory | 0.11 | 0.13 | 0.51 | 0.0049 |
| Central memory | 2.30 | 2.12 | 2.12 | 0.9 |
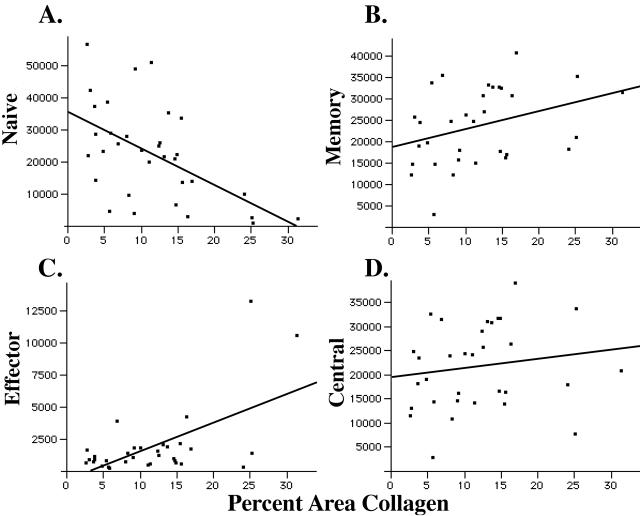
We next compared the sizes of the naïve and memory CD4 populations in LT to the amount of fibrosis measured in the TZ and found that the extent of fibrosis correlated most strongly with reductions in the size of the naïve pool of T cells (_r_2 = 0.55, P = 0.0008) (Fig. 3A). TZ fibrosis was also stage dependent (Table 3). The median percent area of the TZ occupied by collagen increased from 5.3% in the HIV-uninfected patients to 9.5% in presymptomatic patients to 24.5% in patients with AIDS. Naïve T-cell numbers were most severely affected by LT fibrosis in the later stages of infection. Of interest, in the nine HIV-uninfected subjects, the range for quantity of collagen in the TZ was 2.6% to 15.6%, and the relationship with the size of the naïve T-cell population remained significant (_r_2 = 0.44, P = 0.05).
FIG. 3.
T-cell zone fibrosis is correlated with the size of the LT naïve and effector memory population. The sizes of the naïve (A), overall memory (B), effector memory (C), and central memory (D) CD4+ T-cell populations are compared to the amount of TZ fibrosis. There is a negative and significant correlation between the size of the naïve CD4+ T-cell population and TZ fibrosis (_r_2 = −0.55, P = 0.0008); however, there is no correlation between the size of the memory CD4+ T cells and TZ fibrosis (_r_2 = 0.35). However, when we compared the sizes of the effector and central memory subpopulations, we found a positive and significant correlation between the effector memory cells and TZ fibrosis (_r_2 = 0.59, P = 0.0003) and no correlation with central memory cells (_r_2 = 0.16).
TABLE 3.
Median area of TZ occupied by collagen
| Characteristic | HIV-negative patients | Presymptomatic patients | AIDS patients |
|---|---|---|---|
| Median area of fibrosis (%)a | 5.34 | 9.52 | 24.5 |
| Range of collagen quantity (%) | 2.6-15.6 | 2.8-17.0 | 11.0-31.3 |
We found no relationship between the size of the overall memory population of CD4+ T cells and TZ fibrosis, but there was a significant positive correlation between the increased size of the effector subset of memory cells and TZ fibrosis (_r_2 = 0.59, P = 0.0003) (Fig. 3D). Collectively, these findings are consistent with the hypothesis that TZ fibrosis significantly impairs the capacity of the LT niche to maintain normal populations of naïve CD4+ T cells and the hypothesis that immune activation accompanying infection drives both the fibrosis and the relative increases in memory cells with an effector phenotype.
DISCUSSION
The association documented here of higher levels of TZ fibrosis and reduced populations of CD4+ naïve T cells compared to HIV-1-uninfected controls before treatment points to an important role for the LT milieu in the pathogenesis of CD4+ T-cell depletion and reconstitution. Thus, in addition to other established mechanisms of depletion (e.g., altered thymopoiesis, increased activation, and virus-induced cytopathicity) (7, 8), we believe that fibrosis in the LT niche alters survival, growth, and trafficking of naïve T cells through physical constraints, cell-cell interactions, and access to the cytokines required to maintain normal-sized populations of cells as well as restore the population after depletion. These same restraints on homeostasis and proliferation may apply to the peripheral memory pool as well and may account for the surprising finding that following highly active antiretroviral therapy, the depleted niche is not refilled by the expected robust proliferation of memory cells (12, 16).
An important observation from this is that the amount of fibrosis in the TZ is an important factor in the size of the resident T-cell population for all subjects and not just those with HIV infection. A few of the HIV-negative subjects had a surprisingly high amount of TZ fibrosis, suggesting that any preexisting damage to the LT niche that supports the naïve T-cell population might be a critical factor in determining both the course of HIV disease and immunologic recovery from other conditions (e.g., bone marrow transplant, chemotherapy, etc.) Further studies will need to be done to determine this.
We believe that the underlying causes for fibrosis in the HIV-infected subjects are likely to be many but are all related to chronic immune activation and inflammation. For example, we found a significant relationship between the size of the effector memory CD4 T-cell population and the amount of fibrosis in the TZ. Effector memory cells mediate an inflammatory response and could contribute to the ongoing deposition of collagen in the TZ. It will be of interest to see how results of similar studies in gut and other tissues parallel the interesting results we present here. Whatever the mechanisms, we believe our findings suggest that measurement of LT fibrosis may have predictive value with respect to the potential for overall immune reconstitution and point to a potential role for anti-inflammatory agents in both slowing depletion without ART and improving immune reconstitution with ART.
Acknowledgments
We thank Frank Rhame, Leslie Bakken, Margaret Simpson, and Alan Lifson for their continued support of these studies. In addition, we thank Patricia Johnson of the Department of Medicine, Sally McCasland and Mindie Orey of the Surgical Pathology Department, Ann Maruska, Debbie Spade, and Karen Scherping of the Immunohistology Laboratory of the Fairview-University Medical Center for their help with preparation and staining of the lymphatic tissues, Joe Schnide for his assistance with information technology, and Tim Leonard for his assistance in the preparation of the figures.
This study was supported by grants P130-CA79458-01, 1RO1DE12934-01, MO1 RR00400, 2UO1 AI041535, RO1 AI54232-01A2, and R37 AI 28246.
REFERENCES
- 1.Ahsan, N., and E. Langhoff. 1998. Immunopathogenesis of human immunodeficiency virus. Semin. Nephrol. 18**:**422-435. [PubMed] [Google Scholar]
- 2.Brenchley, J. M., N. J. Karandikar, M. R. Betts, D. R. Ambrozak, B. J. Hill, L. E. Crotty, J. P. Casazza, J. Kuruppu, S. A. Migueles, M. Connors, M. Roederer, D. C. Douek, and R. A. Koup. 2003. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 101**:**2711-2720. [DOI] [PubMed] [Google Scholar]
- 3.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200**:**749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao, J., I. W. Park, A. Cooper, and J. Sodroski. 1996. Molecular determinants of acute single-cell lysis by human immunodeficiency virus type 1. J. Virol. 70**:**1340-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casella, C. R., and T. H. Finkel. 1997. Mechanisms of lymphocyte killing by HIV. Curr. Opin. Hematol. 4**:**24-31. [DOI] [PubMed] [Google Scholar]
- 6.Dai, Z., and F. G. Lakkis. 2001. Cutting edge: secondary lymphoid organs are essential for maintaining the CD4, but not CD8, naive T cell pool. J. Immunol. 167**:**6711-6715. [DOI] [PubMed] [Google Scholar]
- 7.Dion, M. L., J. F. Poulin, R. Bordi, M. Sylvestre, R. Corsini, N. Kettaf, A. Dalloul, M. R. Boulassel, P. Debre, J. P. Routy, Z. Grossman, R. P. Sekaly, and R. Cheynier. 2004. HIV infection rapidly induces and maintains a substantial suppression of thymocyte proliferation. Immunity 21**:**757-768. [DOI] [PubMed] [Google Scholar]
- 8.Douek, D. C., M. R. Betts, B. J. Hill, S. J. Little, R. Lempicki, J. A. Metcalf, J. Casazza, C. Yoder, J. W. Adelsberger, R. A. Stevens, M. W. Baseler, P. Keiser, D. D. Richman, R. T. Davey, and R. A. Koup. 2001. Evidence for increased T cell turnover and decreased thymic output in HIV infection. J. Immunol. 167**:**6663-6668. [DOI] [PubMed] [Google Scholar]
- 9.Douek, D. C., R. D. McFarland, P. H. Keiser, E. A. Gage, J. M. Massey, B. F. Haynes, M. A. Polis, A. T. Haase, M. B. Feinberg, J. L. Sullivan, B. D. Jamieson, J. A. Zack, L. J. Picker, and R. A. Koup. 1998. Changes in thymic function with age and during the treatment of HIV infection. Nature 396**:**690-695. [DOI] [PubMed] [Google Scholar]
- 10.Douek, D. C., L. J. Picker, and R. A. Koup. 2003. T cell dynamics in HIV-1 infection. Annu. Rev. Immunol. 21**:**265-304. [DOI] [PubMed] [Google Scholar]
- 11.Dummer, W., B. Ernst, E. LeRoy, D. Lee, and C. Surh. 2001. Autologous regulation of naive T cell homeostasis within the T cell compartment. J. Immunol. 166**:**2460-2468. [DOI] [PubMed] [Google Scholar]
- 12.Freitas, A. A., and B. Rocha. 2000. Population biology of lymphocytes: the flight for survival. Annu. Rev. Immunol. 18**:**83-111. [DOI] [PubMed] [Google Scholar]
- 13.Gandhi, R. T., B. K. Chen, S. E. Straus, J. K. Dale, M. J. Lenardo, and D. Baltimore. 1998. HIV-1 directly kills CD4+ T cells by a Fas-independent mechanism. J. Exp. Med. 187**:**1113-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gretz, J. E., C. C. Norbury, A. O. Anderson, A. E. Proudfoot, and S. Shaw. 2000. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J. Exp. Med. 192**:**1425-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guadalupe, M., E. Reay, S. Sankaran, T. Prindiville, J. Flamm, A. McNeil, and S. Dandekar. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77**:**11708-11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haase, A. T. 1999. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu. Rev. Immunol. 17**:**625-656. [DOI] [PubMed] [Google Scholar]
- 17.Kaldjian, E. P., J. E. Gretz, A. O. Anderson, Y. Shi, and S. Shaw. 2001. Spatial and molecular organization of lymph node T cell cortex: a labyrinthine cavity bounded by an epithelium-like monolayer of fibroblastic reticular cells anchored to basement membrane-like extracellular matrix. Int. Immunol. 13**:**1243-1253. [DOI] [PubMed] [Google Scholar]
- 18.Legac, E., B. Autran, H. Merle-Beral, C. Katlama, and P. Debre. 1992. CD4+CD7−CD57+ T cells: a new T-lymphocyte subset expanded during human immunodeficiency virus infection. Blood 79**:**1746-1753. [PubMed] [Google Scholar]
- 19.Lenardo, M. J., S. B. Angleman, V. Bounkeua, J. Dimas, M. G. Duvall, M. B. Graubard, F. Hornung, M. C. Selkirk, C. K. Speirs, C. Trageser, J. O. Orenstein, and D. L. Bolton. 2002. Cytopathic killing of peripheral blood CD4+ T lymphocytes by human immunodeficiency virus type 1 appears necrotic rather than apoptotic and does not require env. J. Virol. 76**:**5082-5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMichael, A. J., and S. L. Rowland-Jones. 2001. Cellular immune responses to HIV. Nature 410**:**980-987. [DOI] [PubMed] [Google Scholar]
- 21.Mehandru, S., M. A. Poles, K. Tenner-Racz, A. Horowitz, A. Hurley, C. Hogan, D. Boden, P. Racz, and M. Markowitz. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200**:**761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schacker, T. W., P. L. Nguyen, G. J. Beilman, S. Wolinsky, M. Larson, C. Reilly, and A. T. Haase. 2002. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J. Clin. Investig. 110**:**1133-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schacker, T. W., P. L. Nguyen, E. Martinez, C. Reilly, J. M. Gatell, A. Horban, E. Bakawska, B. Berzins, R. van Leeuwen, S. Wolinsky, A. T. Haase, and R. L. Murphy. 2002. Persistent abnormalities in lymphoid tissues of human immunodeficiency virus-infected persons successfully treated with anti-retroviral therapy. J. Infect. Dis. 186**:**1092-1097. [DOI] [PubMed] [Google Scholar]
- 24.Stewart, S. A., B. Poon, J. B. Jowett, and I. S. Chen. 1997. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J. Virol. 71**:**5579-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280**:**427-431. [DOI] [PubMed] [Google Scholar]
- 26.Zhang, Z. Q., D. W. Notermans, G. Sedgewick, W. Cavert, S. Wietgrefe, M. Zupancic, K. Gebhard, K. Henry, L. Boies, Z. Chen, M. Jenkins, R. Mills, H. McDade, C. Goodwin, C. M. Schuwirth, S. A. Danner, and A. T. Haase. 1998. Kinetics of CD4+ T cell repopulation of lymphoid tissues after treatment of HIV-1 infection. Proc. Natl. Acad. Sci. USA 95**:**1154-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]