Phase II Trial of Infusional Fluorouracil, Irinotecan, and Bevacizumab for Metastatic Colorectal Cancer: Efficacy and Circulating Angiogenic Biomarkers Associated With Therapeutic Resistance (original) (raw)
Abstract
Purpose
We investigated the efficacy of fluorouracil (FU), leucovorin, irinotecan, and bevacizumab (FOLFIRI + B) in a phase II trial in patients previously untreated for metastatic colorectal cancer (mCRC), and changes during treatment in plasma cytokines and angiogenic factors (CAFs) as potential markers of treatment response and therapeutic resistance.
Patients and Methods
We conducted a phase II, two-institution trial of FOLFIRI + B. Each 14-day cycle consisted of bevacizumab (5 mg/kg), irinotecan (180 mg/m2), bolus FU (400 mg/m2), and leucovorin (400 mg/m2) followed by a 46-hour infusion of FU (2,400 mg/m2). Levels of 37 CAFs were assessed using multiplex-bead assays and enzyme-linked immunosorbent assay at baseline, during treatment, and at the time of progressive disease (PD).
Results
Forty-three patients were enrolled. Median progression-free survival (PFS), the primary end point of the study, was 12.8 months. Median overall survival was 31.3 months, with a response rate of 65%. Elevated interleukin-8 at baseline was associated with a shorter PFS (11 v 15.1 months, P = .03). Before the radiographic development of PD, several CAFs associated with angiogenesis and myeloid recruitment increased compared to baseline, including basic fibroblast growth factor (P = .046), hepatocyte growth factor (P = .046), placental growth factor (P < .001), stromal-derived factor-1 (P = .04), and macrophage chemoattractant protein-3 (P < .001).
Conclusion
Efficacy and tolerability of FOLFIRI + B eared favorable to historical controls in this single arm study. Before radiographic progression, there was a shift in balance of CAFs, with a rise in alternate pro-angiogenic cytokines and myeloid recruitment factors in subsets of patients that may represent mechanisms of resistance.
INTRODUCTION
Therapies incorporating the monoclonal antibody bevacaziumab, an inhibitor of vascular endothelial growth factor (VEGF), have demonstrated efficacy in metastatic colorectal cancer.1–3 The majority of previously untreated metastatic colorectal cancer patients are treated with bevacizumab (B) in combination with oxaliplatin and fluorouracil (FOLFOX).4 Although the literature suggests an equivalent efficacy for FOLFIRI and FOLFOX without a monoclonal antibody, there are few reports on the FOLFIRI + B regimen in previously untreated patients.5,6
Despite the benefit provided by bevacizumab-based regimens for patients with metastatic colorectal cancer, clinical resistance usually develops. Extensive preclinical work has suggested that alternate proangiogenic factors may modulate sensitivity to anti-VEGF therapy and allow regrowth of tumor-associated vasculature.7 Additional studies have implicated infiltrating monocytic cells in the angiogenic switch, recruited by cytokines derived from tumor or tumor-associated stroma.8 However, clinical studies incorporating analysis of these potential cytokines are limited by the number and time points of collected samples.
This phase II study we report here was designed to determine the efficacy of the FOLFIRI + B regimen and to explore both predictors of sensitivity and potential mechanisms of resistance to FOLFIRI + B. We report the clinical efficacy of this regimen and identified elevation of several proangiogenic cytokines before, and at the time of, progression on this regimen.
PATIENTS AND METHODS
Patients and Eligibility Criteria
Patients were enrolled from M. D. Anderson Cancer Center and Lyndon B. Johnson General Hospital, a county hospital affiliated with The University of Texas, Houston, TX. Enrollment began in January 2005 and completed in January 2007.
Eligible patients were ≥ 18 years old, and were required to have histologically confirmed colorectal cancer with measurable metastatic disease per RECIST (Response Evaluation Criteria in Solid Tumors), with no prior chemotherapy for metastatic disease and with at least 6 months elapsed from completion of any adjuvant therapy. All patients had adequate hepatic, renal, and marrow functions, and Eastern Cooperative Oncology Group performance status of ≤ 2. Written informed consent was obtained from each patient after roval of the clinical study from both institutional review boards.
Treatment
The FOLFIRI + B regimen consisted of bevacizumab (5 mg/kg), irinotecan (180 mg/m2), bolus fluorouracil (400 mg/m2), and leucovorin (400 mg/m2), followed by a 46-hour infusion of fluorouracil (2,400 mg/m2). Patients were treated with bevacizumab alone on day 1, starting FOLFIRI + B on day 15. This single dose of bevacizumab was administered to allow correlative studies to be completed before and after bevacizumab alone.
Dose reductions were required for all grade 3 or 4 toxicities attributed to study medications. Bevacizumab was not dose reduced. Treatment was continued until disease progression, unacceptable toxicities, or withdrawal of consent. Adverse event grading was performed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 3.0.
Statistical Analysis
The primary objective of the trial was PFS, with a null hypothesis of an 8 months PFS for FOLFIRI alone.9–11 The target enrollment of 43 patients provided an 80% power to detect a 4-month improvement in PFS, with an α of .05.2 The secondary clinical objectives included evaluation of response rate, overall survival, and toxicity. PFS was defined as time from study enrollment to progression of disease or death, without censoring for treatment discontinuation. Overall survival was defined as the time from study enrollment to death from any cause.
Plasma Sample Collection and Analysis
Venous blood was drawn and immediately processed for plasma at baseline, immediately before each cycle of chemotherapy (including a sample 2 weeks after single-agent bevacizumab in the first cycle), and, when available, at the time of progression. On retrospective review of computed tomography imaging, we identified the plasma sample associated with the best radiographic response for all patients. This sample (henceforth denoted as “before progression”) represented a point before the development of progressive disease by RECIST, which occurred a median of 8 weeks later. Plasma was stored at −80°C until analysis.
Each sample was analyzed in duplicate and as previously described with no more than one prior freeze-thaw cycle.12 Thirty-seven factors with angiogenic and/or monocyte recruitment activity and present on commercially available multiplex bead suspension bead arrays (Biosource, Camarillo, CA; LINCO Research, St Charles, MO) and enzyme-linked immunosorbent assays (vascular endothelial growth factor [VEGF], soluble VEGFR2 [sVEGFR2], placental growth factor [PlGF]; R&D Systems, Minneapolis, MN) were analyzed per the manufacturers' directions (factors listed in Appendix Table A1, online only, and grouped by class). Samples were allocated onto the arrays using a randomized block design. The relationship between continuous variables was assessed by Spearman correlation. Survival and progression-free survival distributions were summarized by Kaplan-Meier methods and compared using log-rank tests. All P values are two sided.
Using Wilcoxon tests, we assessed which factors differed significantly between different time points, specifically from baseline (pretreatment) until after (A) bevacizumab, (B) after bevacizumab + FOLFIRI, (C) before progression, and (D) after progression, plus comparisons from(E) bevicizumab + FOLFIRI to best before progression, and from (F) before progression until after progression, and from (G) bevicizumab + FOLFIRI to progression. Given the large number of comparisons, we adjusted for multiple testing using the false discovery rate methods, which is a standard multiple test adjustment procedure.13 Specifically, we apply the fdrtool method to map each P value to a q value, which can be interpreted as the probability that the given factor is a false discovery.14,15 We flagged as significant any factor with q value less than 0.05, meaning that we are at least 95% certain it is a true discovery.
RESULTS
Baseline Characteristics
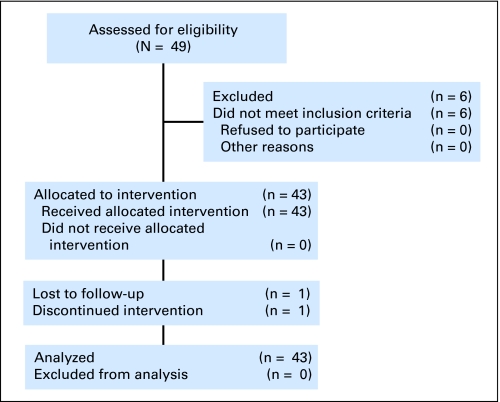
Forty-three patients were enrolled, as shown in Figure 1. The median age of the patients was 57 years (range, 26 to 78), and 41% were female. Fourteen percent of patients self-reported to be of Hispanic ethnicity, 10% African American, and 74% white. Eastern Cooperative Oncology Group performance status scores were 0, 1, and 2 in 42%, 56%, and 2% of the patients, respectively. Primary tumors were graded as poorly differentiated in 14% and moderately differentiated in the remainder. Paired plasma samples were obtained for 40 patients at baseline, after single-agent bevacizumab, after FOLFIRI + B, and before progression. Nineteen samples were available at the time of radiographic progression. The number of samples varied due to logistic failure to obtain all samples or patient withdrawal for reasons besides progression.
Fig 1.
CONSORT diagram.
Efficacy
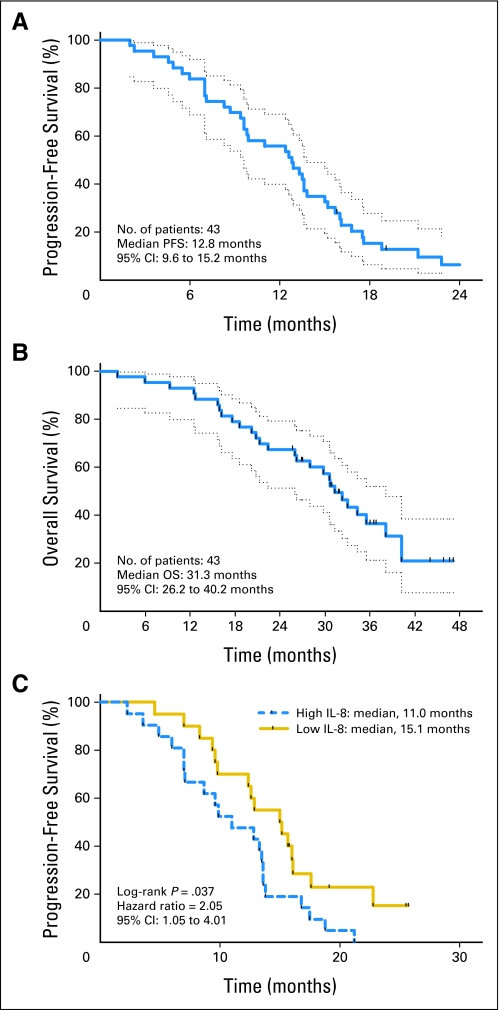
The median PFS, the primary study end point, was 12.8 months, as shown in Figure 2A. Median overall survival was 31.3 months (Fig 2B). On-treatment PFS, in which patients whose initial treatment ceased without progression were censored at the date of last computed tomography scan, was 10.2 months.
Fig 2.
Kaplan-Meier plots. (A) Progression-free survival and (B) overall survival for patients treated with FOLFIRI + B. The 1-year and 2-year survivals were 93% (95% CI, 98% to 80%) and 65% (95% CI, 78% to 48%), respectively. (C) Progression-free survival for patients with interleukin-8 levels above and below the median value of 3.7 pg/mL.
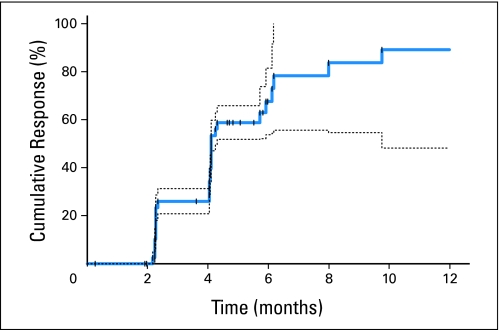
Twenty-eight patients had confirmed partial responses, for an overall response rate of 65% (95% CI, 49% to 79%). One additional patient had an unconfirmed partial response. Responses were observed at a median of 4.1 months, with 23%, 28%, 9%, and 5% of patients obtaining a response on the first, second, third, and fourth or later restaging studies, respectively (Appendix Fig A1, online only). Four patients were subsequently able to undergo resection of metastatic disease.
Toxicities
Fifty-six percent of patients required dose reductions for toxicity during the course of treatment. The rate of grade 3 or 4 toxicities was low, as presented in Table 1. Eight patients stopped treatment for toxicities. Neutropenia was the most common hematologic adverse event, with rare febrile neutropenia. Six patients had grade 2 diarrhea. Adverse events related to bevacizumab included bleeding, proteinuria, and hypertension. In addition, there was one possible localized bowel perforation managed conservatively. All patients had their blood pressure controlled, requiring a median of two blood pressure medications. Nasal epistaxis was seen in 45% patients, associated with mild nasal mucosal ulceration in most cases. Five patients stopped protocol therapy for surgical procedures. No wound healing complications were noted.
Table 1.
Adverse Events
| Adverse Event | Grade 3 or 4 | All Grades | ||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Neutropenia | 17 (3 grade 4) | 40 | 27 | 64 |
| Hypertension | 8 | 19 | 15 | 36 |
| Venous thromboembolism | 8 (5 grade 4) | 19 | 12 | 29 |
| Fatigue | 6 | 14 | 38 | 90 |
| Diarrhea | 1 | 2 | 30 | 71 |
| Vomiting | 1 | 2 | 25 | 60 |
| Hemoglobin | 1 | 2 | 22 | 52 |
| Neutropenic fever | 1 | 2 | 1 | 2 |
| Alopecia | — | — | 30* | 71* |
| Nose bleed/nasal septal ulceration | 0 | 0 | 19 | 45 |
| Mucositis | 0 | 0 | 19 | 45 |
| Proteinuria | 0 | 0 | 13 | 31 |
| Constipation | 0 | 0 | 9 | 21 |
| Anorexia | 0 | 0 | 5 | 12 |
Cytokines and Angiogenic Factors at Baseline and After Initial Treatment
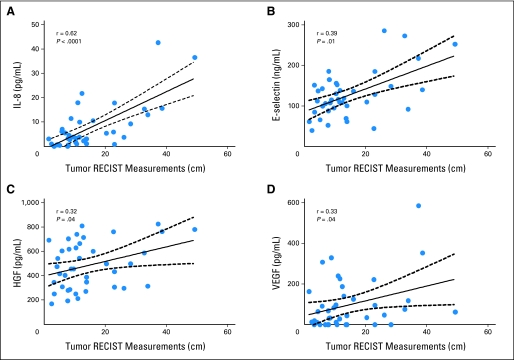
In the 40 evaluable patients, neither the baseline levels of VEGF nor those of VEGFR2 were associated with differences in PFS or overall survival. However, when dichotomized at the median value (3.7 pg/mL), high interleukin (IL)-8 levels were associated with a shorter PFS (11 v 15.1 months; P = .03), and were correlated with increased tumor volume (Spearman r = .62; P < .001; Fig 2C). A similar correlation was found between tumor volume and baseline VEGF, hepatocyte growth factor (HGF), and E-selectin, although none of these were correlated with subsequent outcomes after treatment (Appendix Fig A2, online only).
The modulation of each marker after treatment with single-agent bevacizumab and after one cycle FOLFIRI + B was evaluated. When compared with baseline levels, treatment with single-agent bevacizumab revealed an increase in PlGF (P = .01), eotaxin (P = .01), and sVEGFR2 (P = .03), and a decrease in E-selectin (P < .001). After one cycle of FOLFIRI + B, these same changes in PlGF, eotaxin, sVEGFR2, and E-selectin continued; in addition, matrix metallopeptidase-9 and IL-8 levels were reduced (P < .001 and P = .01, respectively; Appendix Table A2).
Cytokine and Angiogenic Factor Modulation Before and at Progression
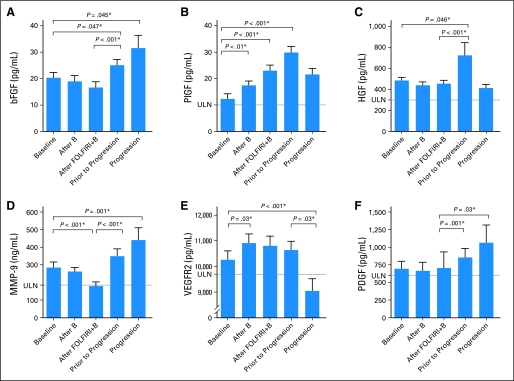
In order to understand the dynamic changes in circulating factors during the emergence of therapeutic resistance, we evaluated profiles of cytokines and angiogenic factors before progression, when we hypothesized that angiogenic activity may have been restarting, and at the time of radiographic progression. Several proangiogenic cytokines were elevated before progression, notably basic fibroblast growth factor (bFGF), PlGF, and HGF (Fig 3) At the time of radiographic progression, bFGF, and MMP-9 were increased compared with the levels after a single dose of FOLFIRI + B (P < .001 for both), although HGF was no longer elevated. At the time of radiographic progression, sVEGFR2 decreased to below baseline levels (P = .005). Compared with levels after FOLFIRI + B, PDGF levels were increased before progression (P = 0.001). In a sensitivity analysis, these same trends persisted when analysis was limited to patients with samples available for all five time points (data not shown).
Fig 3.
Cytokine values at baseline, after a single dose of bevacizumab, after a single dose of fluorouracil, leucovorin, irinotecan, and bevacizumab (FOLFIRI + B), before progression, and at the time of progression. (*) Significantly different after multiple comparison correction, with significance defined by local false discovery rate q less than 0.05. ULN, upper limit of normal.
Changes in some of the cytokines were correlated with each other (eg, bFGF, IL-8, E-selectin, and PDGF), although there was a wide spectrum of modulation between patients (Appendix Fig A3, online only). Despite this large interpatient variability, a subset of patients had no discernable increase in alternate/redundant proangiogenic factors before progression or at the time of progression.
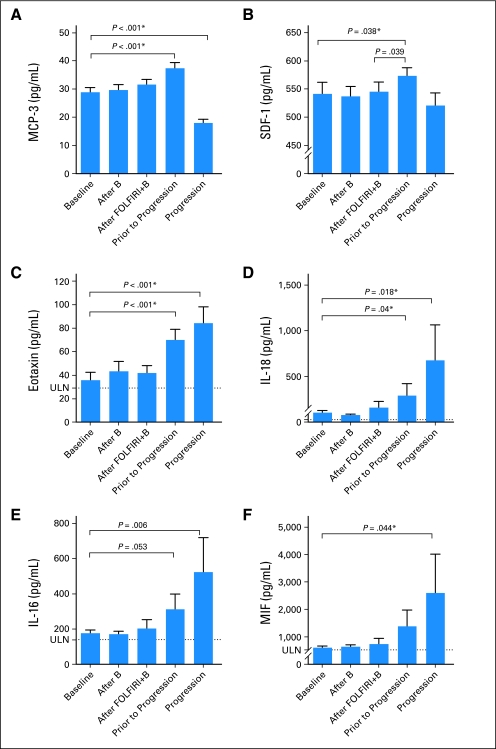
Because myeloid cell recruitment has been hypothesized to play a role in resistance to antiangiogenic therapy, we analyzed cytokines with known roles in myeloid cell differentiation and recruitment. Before progression, there was an increase in SDF-1 (P = .038) and MCP-3 (P < .001), two factors known to be potent chemoattractants for myeloid cells and hematopoietic progenitor cells. Intriguingly, several factors associated with eosinophil recruitment and differentiation were also elevated before progression and at the time of progression. Eotaxin, the level of which was increased by single-agent bevacizumab, was further increased before progression and at the time of progression (P < .001, for both). Similarly, IL-16, IL-18, and macrophage inhibitory factor were also higher at the time of progression than at baseline (Appendix Fig A4, online only).
DISCUSSION
The progression-free survival of 12.8 months and response rate of 65% for FOLFIRI + B compares favorably with historical controls, with low rates of toxicity. A previously published study has demonstrated the efficacy of FOLFIRI when combined with bevacizumab in 57 patients, although there are no published results of larger prospective studies of this regimen despite its widespread use.1,16 The overall survival of 31.3 months compares favorably with 20.3 months reported with bolus fluorouracil, leucovorin, irinotecan, and bevacizumab and 16 to 23 months reported with FOLFIRI alone.1,2,17 Unlike the older irinotecan and bolus fluorouracil regimens, which resulted in severe diarrhea and neutropenia, FOLFIRI + B is well tolerated.18
The cytokine analyses demonstrated that alternate proangiogenic cytokines are modulated by bevacizumab and FOLFIRI + B, and that they are increased before disease progression in a subset of patients receiving this bevacizumab-containing regimen. Single-agent bevacizumab and FOLFIRI + B resulted in lower average levels of bFGF, MMP-9, IL-8, and E-selectin. Before progression, mean bFGF, PlGF, MMP-9, PDGF, and HGF increased, among others. In addition, a subset of factors associated with monocyte recruitment was elevated at disease progression.
The mean bFGF level decreased after one cycle of FOLFIRI + B but was elevated before and at the time of progression. In preclinical studies, bFGF has been shown to be a compensatory angiogenic factor after VEGF inhibition, resulting in renewed angiogenesis independent of VEGF.19,20 Previously, bFGF has been shown to increase at the time of progression in patients with glioblastoma treated with the VEGF receptor tyrosine kinase inhibitor cediranib.21 However, a study of bevacizumab with or without interferon-α2b in melanoma patients did not demonstrate any changes in bFGF levels during treatment.22 Similarly, modulation of bFGF was not seen after a limited course of single-agent bevacizumab in rectal cancer.23
PlGF, a ligand for VEGFR1 that is involved with angiogenesis but not bound by bevacizumab, was increased in a subset of patients after administration of bevacizumab and FOLFIRI + B. A similar increase has been shown within weeks after initiation of a VEGF receptor tyrosine kinase inhibitor, and as soon as 2 days after administration of bevacizumab.21,23-26 In our study, average PlGF was highest at the point before progression and subsequently decreased with the development of radiographically evident progressive disease. This is concordant with a prior report that PlGF is decreased at the time of progression, as this study did not incorporate time points before progression.21 It remains unclear whether PlGF is sufficient to restore angiogenesis in the presence of VEGF inhibition, although this is suggested in preclinical models.27
A similar pattern of peak average levels before the development of radiographically evident progressive disease was seen with HGF and, to a lesser extent with stem cell factor (SCF)/c-kit ligand, with average levels returning to normal at the time of radiographic progressive disease. HGF, via activation of the Met receptor, is a potent angiogenic factor that stimulates VEGF and IL-8 expression while inhibiting negative regulators of angiogenesis.28–31 The finding of high levels of PlGF, HGF, and SCF levels in a subset of patients before progression suggests that these factors, known to be regulated by HIF-1α, may reflect a hypoxia response. As establishment of functional vasculature is a prerequisite for disease growth and predates disease progression, the hypoxic response as measured by cytokines in circulation may be resolving at the time of radiographically evident progressive disease.
Soluble VEGFR2 increased after a single dose of bevacizumab and remained elevated with continued treatment with FOLFIRI + B until decreasing below baseline on the development of progressive disease. This is in contrast to the consistent data demonstrating an initial decrease in sVEGFR2 after treatment with VEGFR TKIs with or without cytotoxic chemotherapy, with rising sVEGFR2 at the time of progression, and likely represents a distinction between the modulation profiles of bevacizumab and VEGF TKIs.26,32,33 In support of this, treatment with a single dose of bevacizumab resulted in an initial increase in sVEGFR2 in a neoadjuvant rectal cancer study.23 In our study, changes in VEGFR2 were not correlated with outcomes with this regimen. MMP-9 has previously been shown to decrease after platinum-based chemotherapy with and without vandetanib in non–small-cell lung cancer patients, and rising levels on treatment were associated with tumor progression, although the latter was not seen in this study.12,34
Myeloid cell recruitment to the tumor bed via several cytokines including PlGF and SDF-1 has been described previously and increased levels of these factors were seen in this study before progression.35–38 Infiltrating myeloid-lineage cells release additional proangiogenic factors and may modulate response to antiangiogenic therapies.39 Interestingly, eotaxin and IL-16, both potent chemoattractants for eosinophils, were elevated in many patients at the time of progression.40 Eosinophil granules contain multiple proangiogenic factors including VEGF and bFGF, but have not been previously associated with resistance to VEGF inhibition in the clinic.39 IL-18, the expression of which is tightly correlated to IL-16 and also rises at progression, is a pro-inflammatory cytokine expressed by macrophages and monocytes and has been shown to stimulate migration of endothelial cells and induce angiogenesis in vivo.41,42
It should be noted that this study is exploratory in nature and has several limitations. Multiple cytokines were evaluated in a modest sample size, leading to the possibility of false positive results, although multiple-comparison testing methods were utilized. Circulating cytokine levels may not reflect the local concentrations of these factors in the tumor microenvironment, and may also reflect a response of non-neoplastic, host tissue to the treatment. Additional studies will clearly be needed to determine whether specific factors associated with resistance in this study are consistent and causative of resistance to bevacizumab-containing regimens. The single-arm study design, likewise, does not allow us to distinguish between cytokine changes induced by bevacizumab and those induced by the cytotoxic chemotherapy component of the regimen. Nevertheless, the associations between specific circulating pro-angiogenic and myeloid recruitment factors and the emergence of therapeutic resistance suggest several potential mechanisms that merit further investigation.
Importantly, these results suggest that plasma cytokine levels at the time of progression are different from earlier changes before progression, when compensatory angiogenic factors may be stimulating new vessel growth in preparation for clinically evident progression. Given the lack of single-agent activity of bevacizumab, resistance that develops to the cytotoxic components may manifest in progressing disease without an “angiogenic switch.” Indeed, approximately a third of patients did not have an identifiable increase in alternate angiogenic factors before progression. It remains an intriguing hypothesis that similar analyses might identify a subset of patients progressing on a bevacizumab regimen that may continue to benefit from further bevacizumab therapy.43,44 Conversely, one could speculate that those patients with significant elevations in PlGF, bFGF, or HGF at progression might not benefit from continuation of bevacizumab in second-line therapy, but may benefit from further antiangiogenic therapy using VEGFR TKIs, bFGF inhibitors, or MET inhibitors, respectively. Further studies are planned to explore this speculation, validate these findings, and to better quantify the apparent interpatient heterogeneity of cytokine changes.
In conclusion, this study demonstrates the long progression-free and overall survival of patients who received the FOLFIRI + B regimen. Elevations of pro-angiogenic cytokines, notably bFGF, PlGF, and HGF, were observed in subsets of patients before radiographic evidence of disease progression. Prospective measurements of these or other pro-angiogenic cytokines may provide a mechanism to individualize continued antiangiogenic therapy after progression on a bevacizumab-containing regimen.
Acknowledgment
We thank Kathyrn McKee for technical assistance, B.J. Clayton, Yvonne Lassere, Gail Bland, and Editha Baer for research support, and Michael Worley for manuscript review.
Appendix
Fig A1.
Cumulative time to radiographic response by Response Evaluation Criteria in Solid Tumors, demonstrating continued accumulation of response after extended treatment. Dashed lines represent 95% CIs for cumulative response rate.
Fig A2.
Correlation of baseline cytokine and angiogenic factor levels and tumor volume by sum of tumor unidimensional measurement according to Response Evaluation Criteria in Solid Tumors (RECIST) methodology.
Fig A3.
Unsupervised clustering analysis of cytokines and angiogenic factors before progression (log base 2 change from baseline without normalization or centering).
Fig A4.
Cytokine values at baseline, after single dose of bevacizumab, after a single dose of fluorouracil, leucovorin, irinotecan, and bevacizumab (FOLFIRI + B), before progression, and at the time of progression for factors associated with myeloid cell recruitment. (*) Significantly different after multiple comparison correction, with significance defined by local false discovery rate q less than 0.05. ULN, upper limit of normal.
Table A1.
Cytokines and Angiogenic Factors Analyzed
| Proangiogenic factor |
|---|
| FGF, basic |
| IL-6 |
| IL-8 |
| GRO-α |
| HGF |
| MMP-9 |
| PDGF |
| PlGF |
| TNF-α |
| Anti-angiogenic factors |
| IFN-α2 |
| IFN-γ |
| IP-10 |
| MIG |
| TRAIL |
| Markers of endothelial cell function or damage |
| E-selectin |
| sVEGFR-2 |
| Hematopoietic growth factors |
| G-CSF |
| GM-CSF |
| M-CSF |
| SCF-1 |
| SCGF-β |
| Other interleukins |
| IL-1RA |
| sIL-2R |
| IL-3 |
| IL-4 |
| IL-9 |
| IL-12 |
| IL-13 |
| IL-18 |
| Monocyte chemotaxis |
| CTACK |
| Eotaxin |
| IL-16 |
| MCP-3 |
| MIF |
| MIP-1α |
| MIP-1β |
| SDF-1α |
Table A2.
Modulation of Cytokines and Angiogenic Factors by Treatment
| Parameter | Baseline | After Bevacizumab | After Bevacizumab + FOLFIRI | Prior to Progression | Progression | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (pg/mL)* | Median (pg/mL)* | Mean (pg/mL) | Median (pg/mL) | % Baseline† | Mean (pg/mL) | Median (pg/mL) | % Baseline† | Mean (pg/mL) | Median (pg/mL) | % Baseline† | Mean (pg/mL) | Median (pg/mL) | % Baseline† | |
| CTACK | 832.5 | 822.6 | 869.6 | 862.8 | 109% | 927.9 | 917.4 | 111%ठ| 893.9 | 775.9 | 107% | 784.9 | 772.3 | 101% |
| Eotaxin | 35.9 | 28.3 | 43.4 | 29.4 | 125%‡ | 42.0 | 30.5 | 135%‡§‖ | 70.1 | 52.0 | 213%‡ | 84.5 | 66.1 | 285%‡ |
| E-Selectin, ng/mL | 127.2 | 115.9 | 108.3 | 100.4 | 84%‡ | 90.2 | 82.5 | 72%‡‖ | 105.1 | 101.2 | 93%‡ | 95.1 | 95.7 | 72%‡ |
| FGF, basic | 20.4 | 19.8 | 19.0 | 15.6 | 94% | 16.7 | 13.9 | 83%§‖ | 25.2 | 21.6 | 114%‡ | 31.6 | 25.4 | 139%‡ |
| G-CSF¶ | 20.4 | 18.2 | 19.2 | 18.6 | 96% | 17.7 | 15.9 | 86%§‖ | 22.2 | 22.3 | 114%§ | 14.3 | 13.0 | 65%‡ |
| GM-CSF | 52.6 | 20.2 | 64.8 | 20.7 | 97% | 40.8 | 19.6 | 96%§ | 39.9 | 25.4 | 108% | 37.9 | 32.1 | 179%‡ |
| GRO-α | 113.3 | 113.7 | 108.8 | 107.8 | 102% | 111.1 | 109.7 | 98%§‖ | 136.3 | 133.7 | 121%‡§ | 47.0 | 16.3 | 26%‡ |
| HGF | 486.4 | 465.8 | 443.2 | 417.6 | 90% | 457.1 | 432.8 | 98%§‖ | 726.5 | 533.7 | 113%‡ | 414.6 | 386.3 | 80% |
| IFN-α2 | 140.8 | 139.9 | 137.0 | 132.3 | 98% | 141.8 | 133.3 | 100%§‖ | 153.9 | 150.8 | 106%‡ | 134.3 | 121.9 | 95% |
| IFN-γ | 161.1 | 129.1 | 166.2 | 127.7 | 99% | 142.7 | 122.0 | 90%§‖ | 174.8 | 162.3 | 116%‡ | 149.2 | 153.6 | 115% |
| IL-1ra | 227.6 | 139.9 | 226.0 | 131.7 | 93% | 183.8 | 120.6 | 82%‡§‖ | 277.8 | 166.1 | 110% | 546.9 | 212.3 | 161%‡ |
| IL2R-α | 199.3 | 197.2 | 207.8 | 184.0 | 101% | 208.1 | 194.0 | 102% | 217.0 | 202.5 | 110% | 215.6 | 182.6 | 119% |
| IL-3 | 104.2 | 90.0 | 102.6 | 99.8 | 99% | 101.3 | 90.6 | 100%§ | 114.9 | 109.1 | 105%§ | 76.1 | 67.3 | 81% |
| IL-4 | 1.6 | 1.4 | 1.6 | 1.5 | 103% | 1.5 | 1.4 | 90%§‖ | 1.9 | 1.8 | 121%‡ | 1.9 | 1.8 | 126% |
| IL-6 | 15.3 | 9.4 | 13.9 | 8.6 | 102% | 10.2 | 7.9 | 93%§‖ | 11.5 | 9.4 | 110% | 10.9 | 10.7 | 102% |
| IL-8 | 7.4 | 3.7 | 6.5 | 3.8 | 90% | 7.8 | 2.4 | 57%‡ | 5.9 | 3.6 | 96% | 7.6 | 4.0 | 100% |
| IL-9 | 51.0 | 37.8 | 47.1 | 31.6 | 103% | 36.4 | 25.5 | 67%‡§‖ | 56.2 | 39.6 | 109% | 47.9 | 35.9 | 68% |
| IL-12 | 443.1 | 402.2 | 465.5 | 437.5 | 101% | 443.3 | 394.0 | 102%§‖ | 449.8 | 411.5 | 94% | 522.5 | 407.2 | 107% |
| IL-13 | 11.1 | 8.6 | 10.7 | 6.1 | 87% | 7.9 | 3.6 | 63%‡‖ | 10.4 | 6.5 | 74% | 10.8 | 8.8 | 76% |
| IL-16 | 177.7 | 152.3 | 172.6 | 145.3 | 102% | 203.8 | 140.3 | 97%§‖ | 313.0 | 164.1 | 109% | 523.7 | 194.0 | 121%‡ |
| IL-18 | 105.1 | 70.3 | 80.9 | 68.8 | 100% | 159.4 | 76.4 | 112%§ | 290.3 | 91.1 | 120%‡ | 676.0 | 74.9 | 209%‡ |
| IP-10 | 495.1 | 405.2 | 503.9 | 451.7 | 104% | 464.3 | 391.7 | 104% | 555.9 | 453.1 | 116% | 351.5 | 269.4 | 79% |
| MCP-3 | 29.0 | 29.3 | 29.7 | 28.0 | 99% | 31.7 | 31.5 | 108%§‖ | 37.5 | 33.7 | 128%‡§ | 18.1 | 19.1 | 57%‡ |
| M-CSF | 27.3 | 24.1 | 26.3 | 24.5 | 97% | 28.8 | 26.2 | 99%§‖ | 33.5 | 30.4 | 114%§ | 19.0 | 11.4 | 68%‡ |
| MIF | 606.4 | 506.5 | 638.7 | 516.6 | 97% | 735.9 | 432.0 | 85%‖ | 1385.1 | 540.6 | 104% | 2596.0 | 793.0 | 107%‡ |
| MIG | 480.0 | 364.7 | 495.6 | 393.6 | 106% | 451.8 | 405.3 | 104% | 605.0 | 424.1 | 100%§ | 419.4 | 206.7 | 71% |
| MIP-1α | 7.0 | 6.3 | 7.1 | 6.6 | 98% | 6.6 | 6.1 | 93%‖ | 7.6 | 7.2 | 108%‡§ | 6.7 | 6.7 | 102% |
| MIP-1β | 40.3 | 36.5 | 41.6 | 38.4 | 97% | 38.2 | 33.6 | 83%‡ | 40.7 | 37.6 | 95% | 41.7 | 34.5 | 108% |
| MMP-9, ng/mL | 285.3 | 234.3 | 262.2 | 227.5 | 94% | 179.9 | 162.9 | 59%‡§‖ | 349.8 | 308.9 | 115%§ | 441.2 | 322.6 | 178%‡ |
| PDGF | 695.5 | 515.7 | 668.6 | 490.8 | 104% | 707.0 | 481.1 | 80%§‖ | 854.0 | 639.0 | 107% | 1066.2 | 684.2 | 178% |
| PlGF | 12.6 | 12.4 | 17.4 | 19.7 | 174%‡ | 23.0 | 23.2 | 229%‡‖ | 29.9 | 29.0 | 319%‡§ | 21.6 | 20.4 | 123% |
| SCF | 63.1 | 61.4 | 63.3 | 65.0 | 104% | 64.4 | 61.4 | 106%§ | 75.5 | 73.7 | 120%‡§ | 59.7 | 57.8 | 101% |
| SCGF-β, ng/mL | 27.5 | 25.2 | 26.2 | 24.6 | 102% | 27.0 | 25.3 | 110%‖ | 31.8 | 30.7 | 125% | 27.1 | 22.0 | 125% |
| SDF-1 | 541.8 | 528.4 | 537.3 | 533.9 | 101% | 545.4 | 514.8 | 101%‖ | 573.5 | 562.6 | 107%‡ | 521.1 | 490.9 | 94% |
| TNF-α | 37.6 | 25.3 | 46.5 | 28.6 | 110% | 35.6 | 22.9 | 100% | 37.0 | 31.2 | 102%§ | 42.1 | 38.6 | 135%‡ |
| TRAIL | 227.9 | 222.1 | 222.7 | 214.8 | 103% | 231.5 | 216.8 | 100%‖ | 257.2 | 248.8 | 111%‡ | 259.8 | 231.4 | 106%‡ |
| VEGFR2, ng/mL | 10.3 | 10.4 | 10.9 | 10.7 | 103%‡ | 10.8 | 10.5 | 107%‡§ | 10.6 | 10.8 | 104%§ | 9.1 | 8.7 | 91%‡ |
Footnotes
Supported by Genentech, and Grants No. NIH CA090810 (J.A., S.K., P.H.) and CA136980 (S.K.). J.V.H. is a Damon Runyan–Lilly Clinical Investigator supported in part by the Damon Runyan Cancer Research Foundation (CI 24-04) and the Physician Scientist Program at M.D. Anderson Cancer Center.
Presented in part in oral format at American Society of Clinical Oncology (ASCO) Gastrointestinal Cancer Symposium January 15-17, 2009, San Francisco, CA, and presented at 43rd Annual Meeting of the ASCO June 1-5, 2007, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00354978.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Scott Kopetz, Roche (C); Paulo M. Hoff, Roche (C); Lee M. Ellis, Genentech (C), Amgen (C), Schering-Plough (C), Roche (C), AstraZeneca (C); John V. Heymach, AstraZeneca (C), Genentech (C), GlaxoSmithKline (C) Stock Ownership: None Honoraria: Scott Kopetz, Pfizer; Paulo M. Hoff, Roche; John V. Heymach, AstraZeneca, Genentech, GlaxoSmithKline Research Funding: Robert A. Wolff, Eli Lilly; Michael J. Overman, sanofi-aventis; Vicente Valero, Genentech; Lee M. Ellis, sanofi-aventis, ImClone Systems; John V. Heymach, AstraZeneca, GlaxoSmithKline Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Scott Kopetz, Paulo M. Hoff, James L. Abbruzzese, John V. Heymach
Financial support: James L. Abbruzzese, John V. Heymach
Administrative support: Scott Kopetz, Christopher Lieu, James L. Abbruzzese
Provision of study materials or patients: Scott Kopetz, Robert A. Wolff, Cathy Eng, Katrina Y. Glover, Vicente Valero
Collection and assembly of data: Scott Kopetz, Rosie Adinin, Vicente Valero, Shaoyu Yan, Hai T. Tran, John V. Heymach
Data analysis and interpretation: Scott Kopetz, Jeffrey S. Morris, Katrina Y. Glover, Michael J. Overman, Sijin Wen, Christopher Lieu, Shaoyu Yan, Hai T. Tran, Lee M. Ellis, John V. Heymach
Manuscript writing: Scott Kopetz, Paulo M. Hoff, Michael J. Overman, Lee M. Ellis, John V. Heymach
Final approval of manuscript: Scott Kopetz, Paulo M. Hoff, Jeffrey S. Morris, Robert A. Wolff, Cathy Eng, Katrina Y. Glover, Rosie Adinin, Michael J. Overman, Vicente Valero, Shaoyu Yan, Hai T. Tran, Lee M. Ellis, James L. Abbruzzese, John V. Heymach
REFERENCES
- 1.Fuchs CS, Marshall J, Mitchell E, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: Results from the BICC-C study. J Clin Oncol. 2007;25:4779–4786. doi: 10.1200/JCO.2007.11.3357. [DOI] [PubMed] [Google Scholar]
- 2.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 3.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 4.Politano S, Overman M, Pathak P, et al. Second-line chemotherapy use in metastatic colon cancer varies by disease responsiveness. Clin Colorectal Cancer. 2008;7:55–59. doi: 10.3816/CCC.2008.n.008. [DOI] [PubMed] [Google Scholar]
- 5.Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 6.Colucci G, Gebbia V, Paoletti G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: A multicenter study of the Gruppo Oncologico Dell'Italia Meridionale. J Clin Oncol. 2005;23:4866–4875. doi: 10.1200/JCO.2005.07.113. [DOI] [PubMed] [Google Scholar]
- 7.Ellis LM, Hicklin DJ. Pathways mediating resistance to vascular endothelial growth factor-targeted therapy. Clin Cancer Res. 2008;14:6371–6375. doi: 10.1158/1078-0432.CCR-07-5287. [DOI] [PubMed] [Google Scholar]
- 8.Shojaei F, Ferrara N. Role of the microenvironment in tumor growth and in refractoriness/resistance to anti-angiogenic therapies. Drug Resist Updat. 2008;11:219–230. doi: 10.1016/j.drup.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Bouzid K, Khalfallah S, Tujakowski J, et al. A randomized phase II trial of irinotecan in combination with infusional or two different bolus 5-fluorouracil and folinic acid regimens as first-line therapy for advanced colorectal cancer. Ann Oncol. 2003;14:1106–1114. doi: 10.1093/annonc/mdg288. [DOI] [PubMed] [Google Scholar]
- 10.Leonard P, Seymour MT, James R, et al. Phase II study of irinotecan with bolus and high dose infusional 5-FU and folinic acid (modified de Gramont) for first or second line treatment of advanced or metastatic colorectal cancer. Br J Cancer. 87:1216–1220. doi: 10.1038/sj.bjc.6600641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maiello E, Giuliani F, Gebbia V, et al. FOLFIRI with or without celecoxib in advanced colorectal cancer: A randomized phase II study of the Gruppo Oncologico dell'Italia Meridionale (GOIM) Ann Oncol. 2006;17:vii55–59. doi: 10.1093/annonc/mdl952. [DOI] [PubMed] [Google Scholar]
- 12.Hanrahan E, Lin HY, Kim ES, et al. Distinct patterns of cytokine and angiogenic factor modulation and markers of benefit for vandetanib and/or chemotherapy in non-small cell lung cancer patients. J Clin Oncol. doi: 10.1200/JCO.2009.22.4279. doi: 10.1200/JCO.2009.22.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. JRSS-B. 1995;57:289–300. [Google Scholar]
- 14.Storey J. The positive false discovery rate: A Bayesian interpretation and the q-values. Ann Stat. 2003;31:2013–2035. [Google Scholar]
- 15.Strimmer K. fdrtool: A versatile R package for estimating local and tail area-based false discovery rates. Bioinformatics. 2008;24:1461–1462. doi: 10.1093/bioinformatics/btn209. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs CS, Marshall J, Barrueco J. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: Updated results from the BICC-C study. J Clin Oncol. 2008;26:689–690. doi: 10.1200/JCO.2007.15.5390. [DOI] [PubMed] [Google Scholar]
- 17.Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: The Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670–1676. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 18.Rothenberg ML, Meropol NJ, Poplin EA, et al. Mortality associated with irinotecan plus bolus fluorouracil/leucovorin: Summary findings of an independent panel. J Clin Oncol. 2001;19:3801–3807. doi: 10.1200/JCO.2001.19.18.3801. [DOI] [PubMed] [Google Scholar]
- 19.Yoshiji H, Harris SR, Thorgeirsson UP. Vascular endothelial growth factor is essential for initial but not continued in vivo growth of human breast carcinoma cells. Cancer Res. 1997;57:3924–3928. [PubMed] [Google Scholar]
- 20.Casanovas O, Hicklin DJ, Bergers G, et al. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varker KA, Biber JE, Kefauver C, et al. A randomized phase 2 trial of bevacizumab with or without daily low-dose interferon alfa-2b in metastatic malignant melanoma. Ann Surg Oncol. 2007;14:2367–2376. doi: 10.1245/s10434-007-9389-5. [DOI] [PubMed] [Google Scholar]
- 23.Willett CG, Duda DG, di Tomaso E, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: A multidisciplinary phase II study. J Clin Oncol. 2009;27:3020–3026. doi: 10.1200/JCO.2008.21.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 25.Willett CG, Boucher Y, Duda DG, et al. Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: Continued experience of a phase I trial in rectal cancer patients. J Clin Oncol. 2005;23:8136–8139. doi: 10.1200/JCO.2005.02.5635. [DOI] [PubMed] [Google Scholar]
- 26.Zhu AX, Sahani DV, Duda DG, et al. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: A phase II study. J Clin Oncol. 2009 doi: 10.1200/JCO.2008.20.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer C, Jonckx B, Mazzone M, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 28.Bussolino F, Di Renzo MF, Ziche M, et al. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119:629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang YW, Su YL, Volpert OV, et al. Hepatocyte growth factor/scatter factor mediates angiogenesis through positive VEGF and negative thrombospondin 1 regulation. Proc Natl Acad Sci U S A. 2003;100:12718–12723. doi: 10.1073/pnas.2135113100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xin XH, Yang SY, Ingle G, et al. Hepatocyte growth factor enhances vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. American J Pathol. 2001;158:1111–1120. doi: 10.1016/S0002-9440(10)64058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong G, Chen Z, Li ZY, et al. Hepatocyte growth factor/scatter factor-induced activation of MEK and P13K signal pathways contributes to expression of proangiogenic cytokines interleukin-8 and vascular endothelial growth factor in head and neck squamous cell carcinoma. Cancer Res. 2001;61:5911–5918. [PubMed] [Google Scholar]
- 32.Walshe JM, Denduluri N, Berman AW, et al. Effect of bevacizumab (BV) and chemotherapy (CT) on serum levels of vascular endothelial growth factor receptor-2 (sVEGFR-2) in patients with inflammatory and locally advanced breast cancer. J Clin Oncol (Meeting Abstracts) 2006;24(suppl):602s. abstr 13003. [Google Scholar]
- 33.Denduluri N, Yang SX, Berman AW, et al. Circulating biomarkers of bevacizumab activity in patients with breast cancer. Cancer Biol Ther. 2008;7:15–20. doi: 10.4161/cbt.7.1.5337. [DOI] [PubMed] [Google Scholar]
- 34.Mihaylova Z, Ludovini V, Gregorg V, et al. Serum level changes of matrix metalloproteinases 2 and 9, vascular endothelial growth factor and epidermal growth factor receptor during platinum-based chemotherapy in advanced non-small cell lung cancer patients. J BUON. 2007;12:105–111. [PubMed] [Google Scholar]
- 35.Shojaei F, Wu X, Qu X, et al. G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc Natl Acad Sci U S A. 2009;106:6742–6747. doi: 10.1073/pnas.0902280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rafii S, Lyden D, Benezra R, et al. Vascular and haematopoietic stem cells: Novel targets for anti-angiogenesis therapy? Nat Rev Cancer. 2002;2:826–835. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- 37.Rehman J, Li JL, Orschell CM, et al. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 38.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 39.Murdoch C, Muthana M, Coffelt SB, et al. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 40.Ferland C, Flamand N, Davoine F, et al. IL-16 activates plasminogen-plasmin system and promotes human eosinophil migration into extracellular matrix via CCR3-chemokine-mediated signaling and by modulating CD4 eosinophil expression. J Immunol. 2004;173:4417–4424. doi: 10.4049/jimmunol.173.7.4417. [DOI] [PubMed] [Google Scholar]
- 41.Okamura H, Tsutsi H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 42.Park CC, Morel JC, Amin MA, et al. Evidence of IL-18 as a novel angiogenic mediator. J Immunol. 2001;167:1644–1653. doi: 10.4049/jimmunol.167.3.1644. [DOI] [PubMed] [Google Scholar]
- 43.Ellis LM, Haller DG. Bevacizumab beyond progression: Does this make sense? J Clin Oncol. 2008;26:5313–5315. doi: 10.1200/JCO.2008.17.4540. [DOI] [PubMed] [Google Scholar]
- 44.Grothey A, Sugrue MM, Purdie DM, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: Results from a large observational cohort study (BRiTE) J Clin Oncol. 2008;26:5326–5334. doi: 10.1200/JCO.2008.16.3212. [DOI] [PubMed] [Google Scholar]