T Cell Responses during Influenza Infection: Getting and Keeping Control (original) (raw)
. Author manuscript; available in PMC: 2012 May 1.
Published in final edited form as: Trends Immunol. 2011 Mar 23;32(5):225–231. doi: 10.1016/j.it.2011.02.006
Abstract
The 2009 influenza pandemic highlights the threat that type A influenza poses to human health. Thus there is an urgency to understand the pathobiology of influenza infection and the contribution of the host immune response to both virus elimination and the development of lung injury. This review focuses on the T cell arm of the adaptive host immune response to influenza and assesses recent developments in our understanding of the induction of primary influenza virus-specific T cell responses by antigen-presenting cells, the interaction of activated effector T cells with antigen-bearing cells in the infected lungs, and the contribution of influenza-specific effector T cells to the development and control of lung injury and inflammation during infection.
The continuing threat of influenza infection
In 1918 – 1919, an outbreak of severe respiratory infection, the so-called “Spanish flu”, occurred which rapidly spread through the human population and resulted in an estimated 20 - 50 million deaths worldwide. The agent causing this catastrophe was subsequently revealed to be a type A influenza (IAV) virus strain. Unique features of IAV structure, replication, transmissibility among certain species and the existence of zoonotic reservoirs make this viral pathogen ideally suited to escape immune recognition and to produce both epidemic (seasonal) disease outbreaks, as well as sporadic pandemic infections. Since the identification of the cause of influenza as a “filterable virus” increasing emphasis has been placed on the analysis of the host response to this virus, not only to improve vaccination strategies, but also to understand the role of the innate and adaptive immune system in controlling/clearing infection and in the development of lung inflammation and injury.
As recently reviewed [1-3], evidence is mounting from analysis of sporadic cases of human infection with avian high pathogenic influenza strains, the more recent outbreak of pandemic A/2009 swine origin IAV infection in humans, as well as the results of experimental IAV infection models, to support the concept that the innate and adaptive immune response to IAV, while critical for virus control and recovery, is also a major contributor to the injury produced by infection. In this review we limit our focus to the adaptive immune T cell response to influenza.
We describe recent findings on the induction of primary CD8+ and CD4+ T cell responses to influenza, the interaction of these antiviral effector T cells with different cell types within the infected lungs and the dual role of anti-viral T cells as pro-inflammatory effectors and as regulators of inflammation in the influenza-infected lungs.
Control of T cell activation and differentiation by respiratory dendritic cells
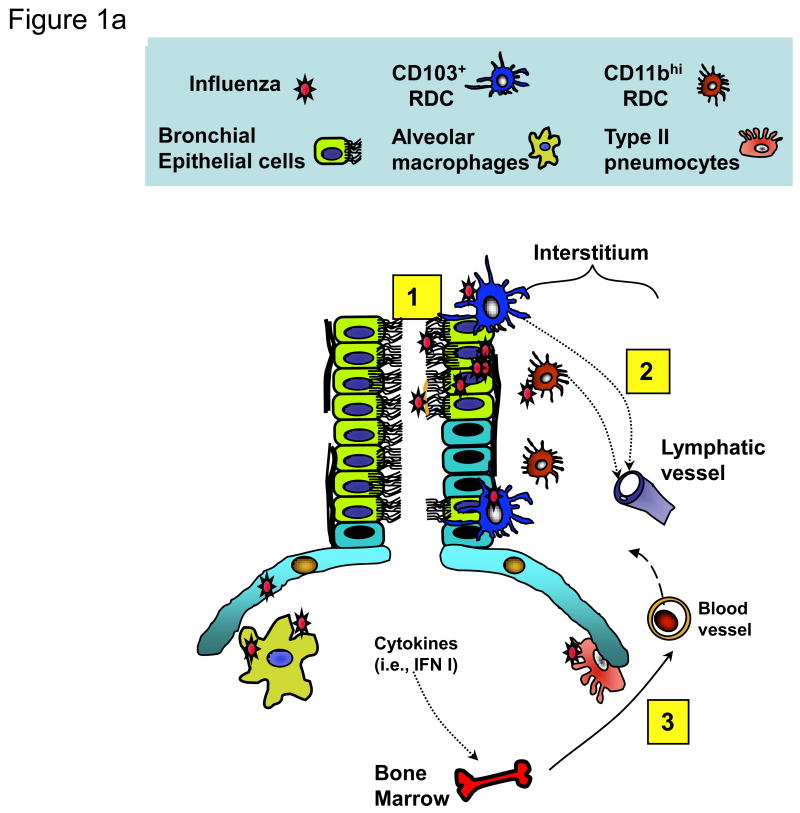
The outcome of viral infections is determined by a series of intricate interactions between the pathogen and host. At mucosal surfaces like the respiratory tract (RT), epithelial cells lining the RT are not only the primary cell types that support the productive replication of IAV, but also serve as the first line of host defense against infection (reviewed in [4, 5]). The respiratory epithelial layer is a heterogeneous array of CD45- cells, including ciliated and non-ciliated airway lining cells and alveolar type I and II epithelial cells (Figure 1a). Influenza infection of epithelial cells activates these resident cells by one or more distinct signaling mechanisms to produce type 1 interferons (IFNs) and a spectrum of inflammatory cytokines and chemokines. This, in turn, orchestrates the recruitment of innate and adaptive immune cells into the infected lungs, and modulates the function of these immune cells (recently reviewed in [4-7]). The infected RT is the site of initiation of adaptive immune T cell responses via the capture of viral (or inhaled) antigen by resident respiratory dendritic cells (RDCs) and concomitant RDC activation, leading to their migration to the draining lymph node (DLN) [8, 9]. The DC network in the murine RT is composed of several distinct RDC subsets which differ in phenotype, anatomic distribution, and function [10, 11]. Major RDC subsets in the normal (non-inflamed) murine lung include conventional RDC (cRDC) and plasmacytoid DC (pDC). cRDC can be further categorized into CD103+CD11b+/- (referred to here as CD103+ RDC), CD103-CD11bhi (CD11bhi RDC), and monocytic RDC (Figure 1a). Their likely counterparts have been detected in human lungs [12, 13].
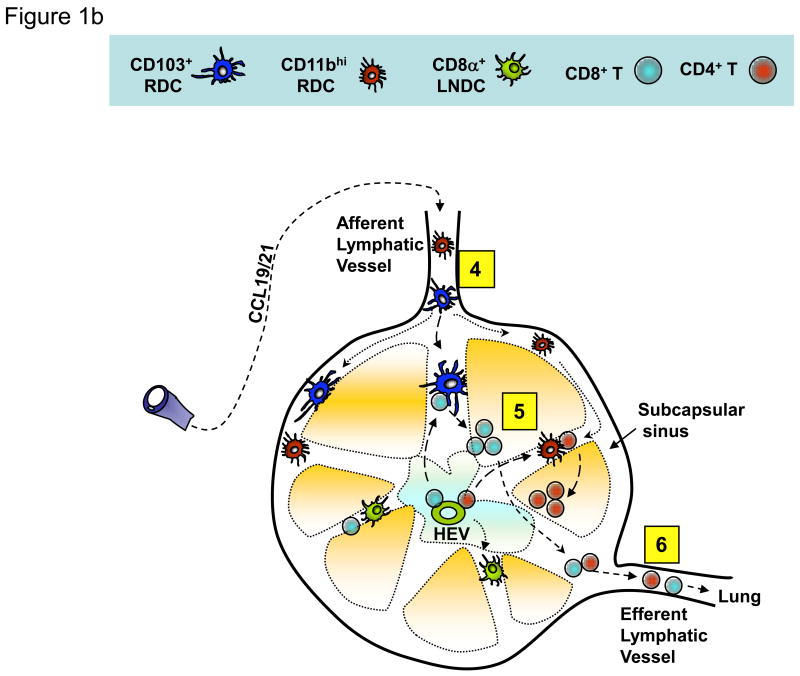
Figure 1. Migrant respiratory dendritic cell (RDC) antigen uptake, migration and T cell activation in response to influenza virus infection.
(a) Influenza virus enters the respiratory tract and replicates (undergoes “productive” infection) in the respiratory epithelial cells lining the airways and, in more severe infections, the alveolar lining type I and II pneumocytes (1). The viral antigens generated are sampled by distinct subsets of resident RDCs that are located within or at the margins of the epithelial mucosal lining (i.e., CD103+ RDCs) or within the pulmonary interstitium (i.e., CD11bhi RDCs). Antigen acquisition by RDCs, along with virus-induced local inflammatory stimuli, triggers upregulation of CC-chemokine receptor 7 on the responding RDCs, which enables the chemokine-dependent migration of the activated RDC from the infected lung through the afferent lymphatics to the lung-draining lymph nodes (DLN) (2). IAV infection of epithelial cells along with virus-induced activation of lung resident innate immune cells orchestrates the recruitment of CD45+ innate immune/inflammatory cells from the circulation (and ultimately the bone marrow) to the site of infection (3). (b) Antigen-bearing migrant CD103+ and CD11bhi RDCs within the DLN (4) present processed IAV antigenic peptides to naive virus-specific T cells (5). Activated T cells undergo clonal expansion and differentiate into effectors capable of migrating through the efferent lymphatics and into the blood via the thoracic duct (6) and ultimately home to the infected lungs where they exert their anti-viral effector activity.
The two major cRDC subsets, CD103+ and CD11bhi RDC, exhibit several features characteristic of DC found in extralymphoid mucosal sites, e.g., the RT and gut. In the normal lungs, these cRDC are localized at distinct anatomical sites (e.g., intraepithelial localization for CD103+ RDC and submucosal/interstitial distribution for CD11bhi RDC), where they monitor the RT and “sample” the local environment (see Figure 1a) [10, 11]. Following an inflammatory stimulus like virus infection, the viral antigen-bearing activated RDC migrate to the DLN along a chemokine gradient dependent on expression of the chemokine receptor CCR7 (Figure 1b) [10, 14]. Perhaps the most important function of RDC in the infected RT is the capture of antigen derived from IAV for delivery to DLN. In the case of IAV, the acquisition of viral antigen in the RT by cRDC is most likely achieved by direct infection of migratory RDC [15, 16] and presumably phagocytic engulfment of cell-free virions or dying/dead infected cells containing viral antigen (e.g., respiratory epithelia), although this latter mechanism while inferred [10, 27] has not been formally demonstrated. Viral antigen transfer from migrant antigen-bearing RDC to LN-resident CD8α+ DC for presentation to naïve T cells has also been reported [17]. The relative contribution of these various mechanisms of antigen uptake and their impact on antigen presentation efficiency and the subsequent anti-viral immune response is at present poorly understood.
The interaction of naïve T cells with professional antigen-presenting cells in the secondary lymphoid organs sculpt the activation profile and transcriptional signature of the responding T cells, which in turn shapes the activated T cell differentiation program (i.e., expansion, lineage commitment, effector phenotype, egress from the DLN through efferent lymphatics, and survival into memory) (Figure 1b) [18-20]. Among the DC populations present in the DLN of IAV infected mice (i.e., migrant CD103+, CD11bhi, and monocytic cRDC, pDC, CD8α+/- LNDC), current evidence suggests that the migrant CD103+ and CD11bhi RDCs serve a prominent (primary) role as APCs for naïve IAV-specific T cells. But these populations also appear to differ in their capacity to activate IAV-specific CD8+ T cells, with CD103+ migrant RDCs serving as the most potent APC following IAV infection [10]. The CD103+ RDCs (but not the CD11bhi RDCs) also have the capacity to capture non-infectious virus preparations delivered into the RT, and then process and present IAV antigens to CD8+ T cells [10]. In keeping with the prominent role of CD103+ RDC in priming anti-viral CD8+ T cells, selective depletion of langerin-expressing CD103+ RDCs prior to IAV infection results in a diminished CD8+ CTL response and impaired virus clearance from the lungs with subsequent infection [21]. Recently, mice constitutively lacking CD103+ RDCs (Batf3-deficient mice) have been described [22] but studies on the CD8+ T cell responses to IAV in these mice have not as yet been reported.
After the initial wave of antigen presentation to CD8+ T cells, which is dominated by CD103+ RDCs (1- 4 dpi), IAV antigen is continuously replenished in the DLN in large by migrant CD11bhi RDCs [10] during the peak of virus replication (perhaps amplifying the pool of the effector CD8 T cells in the DLN [23]) and this antigen “pool” remains detectable after resolution of the acute infection [24]. Temporal regulation of antigen presentation by distinct DC subsets over time, linked with the extent of recruitment of naïve precursors, might also influence the amplitude and immunodominance hierarchy of the responding T cells [25]. In parallel with cRDCs, pDCs, a major DC subset specialized in producing type I IFNs, have been reported to capture virally-derived Ag in the lung. However, antigen-positive pDCs in the DLN fail to induce CD8+ T cell responses during sublethal IAV infection [21, 26]. Interestingly, pDCs can play a regulatory role in enhancing mortality during lethal infection by eliminating virus-specific CD8+ T cells via Fas/FasL interaction [27]. Similar to the stimulation of CD8+ T cells, migrant cRDCs exhibit a potent capability to trigger naive CD4+ T cell activation, although other DC subsets can also trigger activation, albeit with lower efficiency [10]. The significance of this hierarchy of APC function and the distribution of various activities among DC subsets for IAV pathogenesis and the host immune response remains to be determined.
T cell responses within the influenza infected lungs
Along with their roles as initiators and regulators of innate and adaptive immune responses to influenza, respiratory epithelial cells are the primary target of the immune response because they are the major cell types capable of supporting “productive” infection (release of fully infectious virions) by most influenza strains [4, 6]. Thus infected respiratory epithelial cells not only regulate the immune response, but also represent an essential target of immune recognition whose elimination ultimately results in virus clearance from the lungs.
A second more recently appreciated regulator of effector T (Te) cell function in the IAV-infected lungs are the CD45+ myeloid lineage-derived inflammatory granulocytes and mononuclear cells that infiltrate the lung interstitium (and airways) in response to infection [28, 29]. These myeloid lineage cell types are capable of being infected by IAV in vitro and in vivo [16] but their capacity to support productive virus replication in the infected lungs is uncertain. In both severe human influenza infections, and in experimental models, neutrophils and tissue monocyte/macrophage lineage cells, as well as several types of activated APCs including pDC, CD11c+/hi, MHC II+ inflammatory mononuclear cells (also classified as TNF-α and iNOS-producing TipDC or CCR2+ inflammatory monocytes), and the newly identified late-activator APC (LAPC), are (together with lymphocytes) prominent cell types that make up the inflammatory infiltrates in the influenza-infected lung interstitium and airways [28, 30-33].
The contribution of these inflammatory cell types to virus clearance and lung injury is currently unclear and controversial (see Table 1). Although a macrophage lineage cell type that is recruited into the infected lungs has been implicated as a cytolytic effector cell that is capable of destroying influenza-infected respiratory epithelial cells, destruction of epithelial cells also results in enhanced inflammation associated with the recruitment of inflammatory CCR2+ mononuclear cells ([28, 31, 32] and see Table 1). However, germane to this discussion, emerging evidence from our laboratory [34] and others [29, 32] suggests that inflammatory mononuclear cells might play a role in promoting growth and survival of virus-specific CD8+ Te and in dictating the effector cytokine production by the responding CD8+ T cells in the infected lungs.
Table 1. Cell Types Regulating Pulmonary Injury During Influenza Infection.
| Cell types | Potential mechanisms | References |
|---|---|---|
| Injury promoting cells | ||
| Neutrophils | ROS, pro-inflammatory | [65, 66] |
| cytokines | ||
| Inflammatory monocytes/Tip-DCs | ROS, TRAIL, iNOS, proinflammatory cytokines | [28, 31, 32] |
| Effector T cells | Proinflammatory cytokines,cytolytic granules | [36] |
| Injury controlling cells | ||
| Neutrophils | Unknown | [30] |
| Regulatory T cells | IL-10, TGF-β | [55, 64] |
| Effector T cells | IL-10, CD200, NKG2A, TGF-β, | [3, 37, 54, 58] |
| PD-1/Tim-3/Lag-3 | ||
| Epithelial cells | CD200 | [58, 59] |
A large body of evidence that has accumulated over a number of years from experimental models of influenza infection supports the view that CD4+ and CD8+ T cells, as well as B cells (antibody), each make an important contribution to the control of influenza virus replication and virus clearance in the infected lungs during primary infection [1]. CD8+ Te (so called Tc1 or conventional CTL) destroy IAV-infected cells by perforin/granzyme-dependent granule exocytosis and by FasL/Fas-mediated apoptosis following TCR engagement in the infected lungs [35]. Under these conditions, CD8+ Te also produce pro-inflammatory cytokines (e.g., IFN-γ and TNF-α) that can contribute to the recruitment and activation of innate inflammatory cells, in particular, inflammatory CD11chi DC and pDC [36], as well as regulatory cytokines such as IL-10 ([37]). Along with the necessary encounter of naïve CD8+ T cells with migrant RDCs for initial activation in the DLN, recent evidence suggests that within the infected lungs, recruited CD8+ Te might interact with inflammatory DCs for optimum in vivo antiviral effector activity, i.e., virus clearance and prolonged CD8+ Te survival [29]. IL-15 produced in the infected lungs has been implicated in providing such a survival stimulus [38, 39]. It is also noteworthy that the engagement of costimulatory receptors GITR and 4-1BB by their respective ligands during IAV infection likewise results in the prolonged CD8+ Te survival and enhanced virus clearance in the infected lungs (Figure 2). Precisely where in the process of CD8+ T cell activation and differentiation these ligand/receptor interactions occur, and the cell type(s) expressing the ligands, has not been firmly established [40, 41].
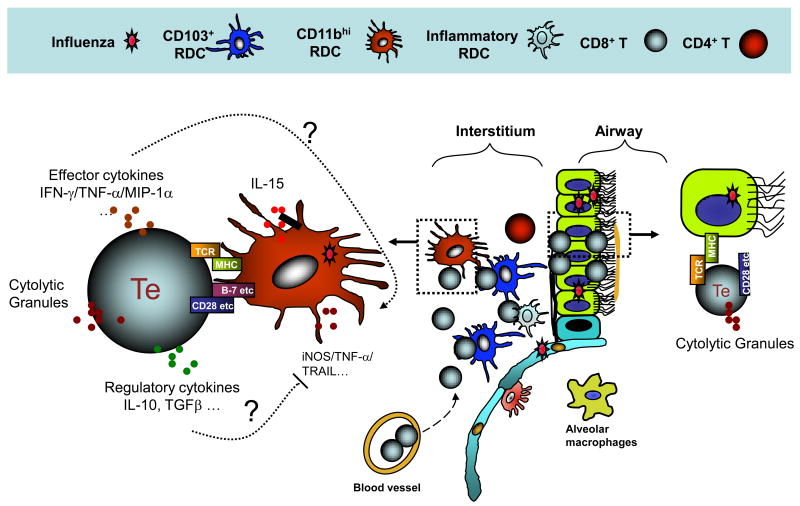
Figure 2. Interactions between antigen-presenting cells (APCs) and antiviral effector T cells (Te) in the influenza-infected lungs.
CD45+ inflammatory/innate immune cells recruited to the IAV-infected lungs can participate in the host response to infection as mediators and regulators of pulmonary injury, through the production of pro-inflammatory and regulatory molecules (see Table 1), as well as APCs capable of enhancing Te function and proliferation through antigen-dependent APC-Te interactions. This antigen presenting activity appears to be mainly restricted to recruited inflammatory DCs and possibly monocytes/macrophages. APCs produce IL-15 and other molecules, which also facilitate Te cell recruitment to and survival in the infected lungs. The outcome of the encounter between the Te and the APC in the infected lung interstitium (e.g., production of pro-inflammatory cytokines by the Te cells) is controlled by TCR engagement, recognition of peptide/MHC complexes, and by co-stimulatory (and possibly co-inhibitory) receptor/ligand interactions between the Te and the APC within the infected lung interstitium and airways. The proinflammatory cytokines produced by Te cells contribute to lung inflammation while the regulatory cytokines, such as IL-10, released by Te cells help to restrain the excessive pulmonary inflammation and injury associated with more severe IAV infection. Recent evidence raises the possibility that the interaction of CD8+ Te with IAV-infected respiratory epithelium might result in the expression of only a subset of Te effector activities (e.g., cytolysis).
It has recently been shown that interaction of CD8+ Te with antigen-bearing CD45+ inflammatory monocytic cells (e.g., DCs) in the lung interstitium results in the expression of the full spectrum of CTL effector activity, including cytolysis and the release of pro-inflammatory cytokines. In contrast, interaction of the CTL with infected CD45- respiratory epithelial cells only results in infected target cell lysis [34]. Furthermore, the release of pro-inflammatory cytokines after CD8+ Te interaction with the infiltrating inflammatory DCs is in part controlled by costimulatory receptor/ligand interactions, notably CD28 on CD8+ Te and its ligands, CD80 and CD86, displayed by infiltrating inflammatory CD11chi inflammatory DCs [34] (Figure 2).
Perhaps the most important contribution of IAV-specific CD4+ T cells is their ability to “help” anti-viral B cell responses, resulting in antibody class switch, affinity maturation and long lived plasma cell generation. We can now relegate this CD4+ T cell effector activity to a distinct CD4+ T cell subset, that is the T follicular helper cell (Tfh) [42]. Rather than acting in the infected lung, the Tfh is believed to carry out its function in secondary lymphoid tissues. However, the formation of iBALT after recovery from virus infection [43, 44] potentially allows Tfh cells to function in these lung-associated lymphoid aggregates during recall responses. At present there is a paucity of information on the regulation of Tfh cell responses in experimental influenza infection, but this is an area of intense interest because of its implications for vaccination and immunological memory. However, it should be noted that there is also evidence in the experimental murine model for a role of T cell-independent neutralizing IgM antibody production by B-1 B cells following primary influenza infection [45].
The expression of direct effector activity, i.e. cytolytic potential, by activated IAV-specific CD4+ Te was first suggested by early work in the murine model demonstrating the ability of clones of MHC II-restricted CD4+ T cells to lyse influenza infected target cells and subsequently to mediate virus clearance, albeit inefficiently, following adoptive transfer into infected B cell-deficient recipients [46]. More recently, the ability of cytolytic CD4+ Te to promote influenza virus clearance in vivo has been reported to be dependent on perforin expression [47]. An as yet unresolved issue concerning the in vivo cytolytic potential of CD4+ Te is the nature of the MHC II+ productively infected target cell type(s) recognized in the infected lungs because only CD45- alveolar type II pneumocytes are constitutively MHC II+ (in both humans and rodents [48]) and upregulate MHC II upon infection. Perhaps this MHC II-restricted cytolytic activity is uniquely geared to eliminate infected type II alveolar cells, or MHC II expression on this cell type might be linked to regulatory T cell (Treg) function in the influenza-infected lungs [49].
The Ying and Yang of injury control by effector T cell immune responses in the infected lung
Along with the ability to clear IAV infection, anti-viral T cells can be a significant contribution to pulmonary inflammation and injury. The mechanisms of T cell-induced injury might be both direct and indirect. Anti-viral CD8+ and CD4+ T cells are able to lyse antigen-bearing lung epithelial cells directly and can contribute to airway exudates during the process of viral clearance. Anti-viral T cells are also the major sources of pro-inflammatory cytokines/chemokines (e.g., IFN-γ, TNF-α, IL-17, and MIP-1α) that contribute to virus-induced pulmonary inflammation and damage during influenza infection [36, 50]. Furthermore, infiltrating anti-viral T cells might also contribute to lung damage through indirect mechanisms by activating and recruiting other injury promoting cells, such as the Tip-DC [28, 31, 32]. Selective dampening of the proinflammatory activity of anti-viral T cells, therefore, might be beneficial during influenza infection.
Compared to the directional release of cytotoxic granules during infected target cell lysis, proinflammatory cytokines released by T cells can indiscriminately target many cells and non-specifically damage the infected RT. As noted above, the strength of signal delivered to the Te by the infected target cells that is required to trigger the release of cytotoxic granules is apparently less than that for the production of proinflammatory cytokines. This is because the release of cytokines (but not cytotoxic granules) by the Te requires co-stimulatory signaling by CD28 [34]. Therefore, therapeutic strategies to disrupt the interaction between CD28 (on T cells) and CD80 and CD86 (on CD45+ CD11c+ inflammatory DC in the infected RT), might ameliorate excessive pulmonary inflammation/injury without affecting virus clearance. In a similar context, OX-40 is a co-stimulatory receptor, which promotes T cell proliferation and survival. In experimental IAV infection in vivo, blockade of OX-40 co-stimulation reduces T cell infiltration, proliferation and cytokine production in the RT, and associated weight loss and pulmonary injury, without affecting viral clearance [51, 52]. On the other hand, blockade of the ICOS co-stimulatory receptor on T cells during IAV infection likewise reduces T cell proliferation, accumulation and cytokine production in the RT, but does not diminish pulmonary inflammation [51]. This dichotomy is likely due to the differential effects of ICOS and OX-40 blockade on viral clearance. ICOS blockade, but not OX-40 blockade, significantly enhances viral persistence in the IAV-infected lungs and so the beneficial effect of ICOS blockade on T cell-mediated inflammation is likely compensated for by enhanced injury due to virus infection [51, 53]. In addition to cell surface costimulatory receptor/ligand interaction, soluble mediators including inflammatory cytokines could serve a similar function to increase the strength of signaling to the Te.
Pulmonary Te cells express not only co-stimulatory molecules, but also co-inhibitory molecules. In contrast to the blockade of co-stimulatory molecules, the prevention of the signaling mediated by the co-inhibitory molecules can lead to excessive cellular proliferation and cytokine production by Te cells. NKG2A is an inhibitory receptor expressed on CD8+ Te cells that can engage the nonclassical MHC class Ib molecule, Qa-1b. Lung infiltrating anti-viral T cells selectively express high levels of NKG2A and blocking the action of NKG2A on CD8+ T cells during IAV infection enhances TNF-α dependent inflammation [54]. The expression of additional known inhibitory molecules, such as Lag-3, PD-1 and Tim-3 (reviewed in [3]), by pulmonary CD8+ Te cells has also been reported. The impact of the latter molecules on RT inflammation during influenza infection is currently not known, but might play an important role in preventing excess inflammation in the IAV-infected RT.
T cells (both effector and Treg) not only serve as potential contributors to lung inflammation and injury, but they might also act to control/inhibit excessive inflammation and injury during IAV infection. Foxp3+ CD25+ regulatory T cells (Tregs) are a specialized CD4+ cell type capable of limiting excessive immune responses induced by self-antigen and infection [55]. Depletion of Tregs during IAV infection results in enhanced CD8+ T cell responses and transfer of exogenous Tregs suppressed innate immune cell-mediated pathology in immune-deficient mice during influenza infection [56, 57]. Thus, Tregs are able to dampen both exaggerated innate and adaptive immune responses against IAV. Along with Tregs, lung-infiltrating Te cells also exhibit potential regulatory function to control excessive immune-mediated inflammation during IAV infection. For example, like respiratory epithelial cells, lung-infiltrating Te cells also express CD200 (albeit at a lower level than epithelial cells) in the IAV-infected RT [58]. Engagement of the CD200R on lung macrophages by CD200-expressing epithelial cells suppresses macrophage injury potential and thereby maintains lung immune homeostasis [58, 59].
IL-10 is a major regulatory cytokine that has powerful inhibitory effects on both innate and adaptive immune cells [60]. Interestingly, Te cells, in particular CD8+ Te cells, are the major cellular source of IL-10 at the IAV-infected murine RT [37]. The IL-10 produced by Te cells plays a crucial role in inhibiting excessive pulmonary inflammation during influenza infection as blockade of the action of IL-10 at the time of Te cell infiltration to the lung results in lethal pulmonary inflammation and injury [37]. IL-10 production by Te cells is restricted primarily to the infected RT, suggesting that Te cells are able to sense an inflamed environment and in response trigger production of this anti-inflammatory cytokine [61]. Interestingly, although the blockade of IL-10 receptor at the time of T cell infiltration results in excessive pulmonary inflammation, mice constitutively deficient in IL-10 are more resistant to lethal IAV infection, perhaps due to enhanced viral clearance associated with elevated Th17 and antibody responses [37, 62, 63]. Thus, IL-10 can be either beneficial in controlling lung inflammation or detrimental by inhibiting the initiation of effective anti-viral immunity. In sum, the exact in vivo function of IL-10 might vary depending upon the timing and anatomic site of production and the nature of the cellular targets of IL-10 during infection. TGF-β is another major anti-inflammatory cytokine and the blockade of TGF-β activity results in enhanced host lethality during IAV infection [64]. Although the cellular sources of TGF-β in IAV-infected lungs are currently unknown, T cells, especially Tregs, are an important source of TGF-β [55]. Characterizing the cellular sources, target cell types, and mechanism of TGF-β produced in the IAV-infected lung will further understanding of how local immune responses are regulated in the lung, the target organ of respiratory virus replication.
Concluding remarks
Over the past several years, we have gained new insight into the induction of anti-viral T cell responses in the DLN, and the expression of effector and regulatory activity by these T cells in the infected lungs during IAV infection. The identification of crucial roles for migrant RDCs in the priming of IAV-specific T cells, and for inflammatory DCs in further maintaining and controlling local T cell responses in the lung, have established DC as central regulators of the magnitude and quality of anti-viral T cell responses in both the DLN and the infected lungs. Furthermore, the realization that engagement of various co-stimulatory/inhibitory receptors and production of key regulatory cytokines by Te cells might “fine-tune” the degree of pulmonary inflammation and the tempo of viral clearance opens up potential new avenues for therapeutic intervention in IAV infection. However, it is clear that the function of the Te cells is tightly regulated both temporally (days post-infection) and spatially (i.e., their activity within different lung compartments, such as the airway versus the interstitium). Consequently, strategies to control excess pulmonary inflammation during infection could also impede virus clearance. To date, the published data suggest that interventions to interfere with the release of pro-inflammatory mediators (i.e., IFNγ or TNFα) or to enhance the production of anti-inflammatory mediators (i.e., IL-10 or TGFβ) by Te cells might be most effective when IAV replication is simultaneously controlled by anti-viral agents.
Acknowledgments
We thank members of the Braciale laboratory for helpful discussions and colleagues who provided recent publications. As a result of space limitations we apologize for being unable to cite many primary references relevant to the topic of this review. This study was supported by grants from the National Institutes of Health (RO1 AI-15608, RO1 AI-37293, RO1 HL-33391, and U-19 AI-83024) to T.J. Braciale.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maines TR, et al. Pathogenesis of emerging avian influenza viruses in mammals and the host innate immune response. Immunol Rev. 2008;225:68–84. doi: 10.1111/j.1600-065X.2008.00690.x. [DOI] [PubMed] [Google Scholar]
- 2.Peiris JS, et al. Host response to influenza virus: protection versus immunopathology. Curr Opin Immunol. 2010;22:475–481. doi: 10.1016/j.coi.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rouse BT, Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nat Rev Immunol. 2010;10:514–526. doi: 10.1038/nri2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanders CJ, et al. Respiratory epithelial cells in innate immunity to influenza virus infection. Cell Tissue Res. 2010 doi: 10.1007/s00441-010-1043-z. [DOI] [PubMed] [Google Scholar]
- 5.Holt PG, et al. Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol. 2008;8:142–152. doi: 10.1038/nri2236. [DOI] [PubMed] [Google Scholar]
- 6.Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008;8:193–204. doi: 10.1038/nri2275. [DOI] [PubMed] [Google Scholar]
- 7.McGill J, et al. Innate immune control and regulation of influenza virus infections. J Leukoc Biol. 2009;86:803–812. doi: 10.1189/jlb.0509368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plantinga M, et al. Origin and functional specializations of DC subsets in the lung. Eur J Immunol. 2010;40:2112–2118. doi: 10.1002/eji.201040562. [DOI] [PubMed] [Google Scholar]
- 9.Strickland DH, et al. Epithelial-dendritic cell interactions in allergic disorders. Curr Opin Immunol. 2010;22:789–794. doi: 10.1016/j.coi.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Kim TS, Braciale TJ. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS One. 2009;4:e4204. doi: 10.1371/journal.pone.0004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sung SS, et al. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol. 2006;176:2161–2172. doi: 10.4049/jimmunol.176.4.2161. [DOI] [PubMed] [Google Scholar]
- 12.Masten BJ, et al. Characterization of myeloid and plasmacytoid dendritic cells in human lung. J Immunol. 2006;177:7784–7793. doi: 10.4049/jimmunol.177.11.7784. [DOI] [PubMed] [Google Scholar]
- 13.Demedts IK, et al. Identification and characterization of human pulmonary dendritic cells. Am J Respir Cell Mol Biol. 2005;32:177–184. doi: 10.1165/rcmb.2004-0279OC. [DOI] [PubMed] [Google Scholar]
- 14.del Rio ML, et al. CD103- and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. J Immunol. 2007;178:6861–6866. doi: 10.4049/jimmunol.178.11.6861. [DOI] [PubMed] [Google Scholar]
- 15.Hao X, et al. Differential response of respiratory dendritic cell subsets to influenza virus infection. J Virol. 2008;82:4908–4919. doi: 10.1128/JVI.02367-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manicassamy B, et al. Analysis of in vivo dynamics of influenza virus infection in mice using a GFP reporter virus. Proc Natl Acad Sci U S A. 2010;107:11531–11536. doi: 10.1073/pnas.0914994107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belz GT, et al. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc Natl Acad Sci U S A. 2004;101:8670–8675. doi: 10.1073/pnas.0402644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masson F, et al. Dendritic cells: driving the differentiation programme of T cells in viral infections. Immunol Cell Biol. 2008;86:333–342. doi: 10.1038/icb.2008.15. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence CW, Braciale TJ. Activation, differentiation, and migration of naive virus-specific CD8+ T cells during pulmonary influenza virus infection. J Immunol. 2004;173:1209–1218. doi: 10.4049/jimmunol.173.2.1209. [DOI] [PubMed] [Google Scholar]
- 20.Bedoui S, Gebhardt T. Interaction between dendritic cells and T cells during peripheral virus infections: a role for antigen presentation beyond lymphoid organs. Curr Opin Immunol. 2010 doi: 10.1016/j.coi.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 21.GeurtsvanKessel CH, et al. Clearance of influenza virus from the lung depends on migratory langerin+CD11b- but not plasmacytoid dendritic cells. J Exp Med. 2008;205:1621–1634. doi: 10.1084/jem.20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edelson BT, et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med. 2010;207:823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ballesteros-Tato A, et al. Temporal changes in dendritic cell subsets, cross-priming and costimulation via CD70 control CD8(+) T cell responses to influenza. Nat Immunol. 2010;11:216–224. doi: 10.1038/ni.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim TS, et al. Antigen persistence and the control of local T cell memory by migrant respiratory dendritic cells after acute virus infection. J Exp Med. 2010;207:1161–1172. doi: 10.1084/jem.20092017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.La Gruta NL, et al. Primary CTL response magnitude in mice is determined by the extent of naive T cell recruitment and subsequent clonal expansion. J Clin Invest. 2010;120:1885–1894. doi: 10.1172/JCI41538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf AI, et al. Plasmacytoid dendritic cells are dispensable during primary influenza virus infection. J Immunol. 2009;182:871–879. doi: 10.4049/jimmunol.182.2.871. [DOI] [PubMed] [Google Scholar]
- 27.Langlois RA, Legge KL. Plasmacytoid dendritic cells enhance mortality during lethal influenza infections by eliminating virus-specific CD8 T cells. J Immunol. 2010;184:4440–4446. doi: 10.4049/jimmunol.0902984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin KL, et al. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J Immunol. 2008;180:2562–2572. doi: 10.4049/jimmunol.180.4.2562. [DOI] [PubMed] [Google Scholar]
- 29.McGill J, et al. Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. J Exp Med. 2008;205:1635–1646. doi: 10.1084/jem.20080314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tate MD, et al. Neutrophils ameliorate lung injury and the development of severe disease during influenza infection. J Immunol. 2009;183:7441–7450. doi: 10.4049/jimmunol.0902497. [DOI] [PubMed] [Google Scholar]
- 31.Herold S, et al. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J Exp Med. 2008;205:3065–3077. doi: 10.1084/jem.20080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aldridge JR, Jr, et al. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc Natl Acad Sci U S A. 2009;106:5306–5311. doi: 10.1073/pnas.0900655106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoo JK, et al. Identification of a novel antigen-presenting cell population modulating antiinfluenza type 2 immunity. J Exp Med. 2010;207:1435–1451. doi: 10.1084/jem.20091373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hufford MM, et al. Antiviral CD8+ T cell effector activities in situ are regulated by target cell type. J Exp Med. 2011;208:167–180. doi: 10.1084/jem.20101850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Topham DJ, et al. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- 36.La Gruta NL, et al. A question of self-preservation: immunopathology in influenza virus infection. Immunol Cell Biol. 2007;85:85–92. doi: 10.1038/sj.icb.7100026. [DOI] [PubMed] [Google Scholar]
- 37.Sun J, et al. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15:277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGill J, et al. IL-15 trans-presentation by pulmonary dendritic cells promotes effector CD8 T cell survival during influenza virus infection. J Exp Med. 2010;207:521–534. doi: 10.1084/jem.20091711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verbist KC, et al. A Role for IL-15 in the Migration of Effector CD8 T Cells to the Lung Airways following Influenza Infection. J Immunol. 2011;186:174–182. doi: 10.4049/jimmunol.1002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snell LM, et al. CD8 T cell-intrinsic GITR is required for T cell clonal expansion and mouse survival following severe influenza infection. J Immunol. 2010;185:7223–7234. doi: 10.4049/jimmunol.1001912. [DOI] [PubMed] [Google Scholar]
- 41.Lin GH, et al. Endogenous 4-1BB ligand plays a critical role in protection from influenza-induced disease. J Immunol. 2009;182:934–947. doi: 10.4049/jimmunol.182.2.934. [DOI] [PubMed] [Google Scholar]
- 42.Yu D, Vinuesa CG. The elusive identity of T follicular helper cells. Trends Immunol. 2010;31:377–383. doi: 10.1016/j.it.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 43.GeurtsvanKessel CH, et al. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J Exp Med. 2009;206:2339–2349. doi: 10.1084/jem.20090410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moyron-Quiroz JE, et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10:927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 45.Choi YS, Baumgarth N. Dual role for B-1a cells in immunity to influenza virus infection. J Exp Med. 2008;205:3053–3064. doi: 10.1084/jem.20080979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Graham MB, et al. Influenza virus-specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J Exp Med. 1994;180:1273–1282. doi: 10.1084/jem.180.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown DM, et al. CD4 T cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J Immunol. 2006;177:2888–2898. doi: 10.4049/jimmunol.177.5.2888. [DOI] [PubMed] [Google Scholar]
- 48.Debbabi H, et al. Primary type II alveolar epithelial cells present microbial antigens to antigen-specific CD4+ T cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L274–279. doi: 10.1152/ajplung.00004.2005. [DOI] [PubMed] [Google Scholar]
- 49.Gereke M, et al. Alveolar type II epithelial cells present antigen to CD4(+) T cells and induce Foxp3(+) regulatory T cells. Am J Respir Crit Care Med. 2009;179:344–355. doi: 10.1164/rccm.200804-592OC. [DOI] [PubMed] [Google Scholar]
- 50.Crowe CR, et al. Critical role of IL-17RA in immunopathology of influenza infection. J Immunol. 2009;183:5301–5310. doi: 10.4049/jimmunol.0900995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Humphreys IR, et al. A critical role for OX40 in T cell-mediated immunopathology during lung viral infection. J Exp Med. 2003;198:1237–1242. doi: 10.1084/jem.20030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hussell T, et al. Co-stimulation: novel methods for preventing viral-induced lung inflammation. Trends Mol Med. 2004;10:379–386. doi: 10.1016/j.molmed.2004.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Humphreys IR, Edwards L, Snelgrove RJ, Rae AJ, Coyle AJ, Hussell T. A critical role for ICOS co-stimulation in immune containment of pulmonary influenza virus infection. Eur J Immunol. 2006;36:2928–2938. doi: 10.1002/eji.200636155. [DOI] [PubMed] [Google Scholar]
- 54.Zhou J, et al. Cutting edge: engagement of NKG2A on CD8+ effector T cells limits immunopathology in influenza pneumonia. J Immunol. 2008;180:25–29. doi: 10.4049/jimmunol.180.1.25. [DOI] [PubMed] [Google Scholar]
- 55.Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions (*) Annu Rev Immunol. 2009;27:551–589. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- 56.Haeryfar SM, et al. Regulatory T cells suppress CD8+ T cell responses induced by direct priming and cross-priming and moderate immunodominance disparities. J Immunol. 2005;174:3344–3351. doi: 10.4049/jimmunol.174.6.3344. [DOI] [PubMed] [Google Scholar]
- 57.Antunes I, Kassiotis G. Suppression of innate immune pathology by regulatory T cells during Influenza A virus infection of immunodeficient mice. J Virol. 2010;84:12564–12575. doi: 10.1128/JVI.01559-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Snelgrove RJ, et al. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat Immunol. 2008;9:1074–1083. doi: 10.1038/ni.1637. [DOI] [PubMed] [Google Scholar]
- 59.Rygiel TP, et al. Lack of CD200 enhances pathological T cell responses during influenza infection. J Immunol. 2009;183:1990–1996. doi: 10.4049/jimmunol.0900252. [DOI] [PubMed] [Google Scholar]
- 60.Couper KN, et al. IL-10: The Master Regulator of Immunity to Infection. J Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 61.Palmer EM, et al. IFNgamma-producing, virus-specific CD8+ effector cells acquire the ability to produce IL-10 as a result of entry into the infected lung environment. Virology. 2010;404:225–230. doi: 10.1016/j.virol.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McKinstry KK, et al. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 2009;182:7353–7363. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun K, et al. A detrimental effect of interleukin-10 on protective pulmonary humoral immunity during primary influenza A virus infection. J Virol. 2010;84:5007–5014. doi: 10.1128/JVI.02408-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carlson CM, et al. Transforming growth factor-beta: activation by neuraminidase and role in highly pathogenic H5N1 influenza pathogenesis. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.White MR, et al. Respiratory innate immune proteins differentially modulate the neutrophil respiratory burst response to influenza A virus. Am J Physiol Lung Cell Mol Physiol. 2005;289:L606–616. doi: 10.1152/ajplung.00130.2005. [DOI] [PubMed] [Google Scholar]
- 66.Snelgrove RJ, et al. An absence of reactive oxygen species improves the resolution of lung influenza infection. Eur J Immunol. 2006;36:1364–1373. doi: 10.1002/eji.200635977. [DOI] [PubMed] [Google Scholar]