Understanding and targeting resistance to anti-angiogenic therapies (original) (raw)
Abstract
Therapies targeting tumor angiogenesis are used in a variety of malignancies, however not all patients benefit from treatment and impact on tumor control may be transient and modest. Mechanisms of resistance to anti-angiogenic therapies can be broadly categorized into VEGF-axis dependent alterations, non-VEGF pathways, and stromal cell interactions. Complimentary combinations of agents that inhibit alternative mechanisms of blood vessel formation may optimize inhibition of angiogenesis and improve clinical benefit for patients. The purpose of this review is to detail the preclinical evidence for mechanisms of angiogenic resistance and provide an overview of novel therapeutic approaches exploiting these pathways.
Key Words: Cancer, angiogenesis, resistance, VEGF, vascular endothelial growth factor, colorectal cancer
Introduction
Therapies targeting angiogenesis are an integral modality of modern anti-tumor treatment for a number of malignancies, in particular metastatic colorectal cancer (CRC). Broadly, tumor angiogenesis relies on a highly complex program of endothelial cell migration and proliferation, growth factor signaling, extracellular matrix remodeling, and stromal cell interaction. The primary proangiogenic driver of this process is VEGF, also known as VEGF-A. The VEGF family includes 5 ligands, VEGFA, VEGFB, VEGFC, VEGFD, and placental growth factor (PlGF), three receptors, VEGFR1 (fms-like tyrosine kinase 1/Flt-1), VEGFR2 (Flk-1/KDR), and VEGFR3 (Flt-4), and 2 co-receptors neuropillin 1 and 2 (NRP1/2). All of these receptors and co-receptors are expressed on endothelial cell, although they may also be present on other cells. VEFGR1 binds to VEGFA, VEGFB, and PlGF, while ligands for VEGFR2 include VEGFA as well as VEGFC and VEGD. VEGFR2 is widely considered the primary receptor mediating angiogenesis; and VEGFR1 and VEGFR3 are classically involved in monocyte chemotaxis, hematopoietic stem cell survival, and lymphangiogenesis, respectively (1). Currently, the most common approaches to inhibition of the VEGF axis include: binding of VEGF ligands (i.e., using a monoclonal antibody or soluble receptor), small molecular inhibition of receptor tyrosine kinase (RTK) and downstream targets, and steric blockade of the VEGFRs (using a monoclonal antibody). FDA approved agents with anti-VEGF properties include bevacizumab, ziv-aflibercept, and multiple small molecule RTK inhibitors (i.e., sorafenib, sunitinib, pazopanib, axitinib, cabozantinib, and regorafenib). Bevacizumab, ziv-aflibercept, and regorafenib are all approved for use in metastatic CRC.
Over the past three decades, a number of additional complementary angiogenic pathways have been described (2,3). These pathways rely on key proteins such as hypoxia inducible factor (HIF), platelet derived growth factor (PDGF), fibroblast growth factor (FGF), angiopoietin (Ang), and Notch, along with various inflammatory mediators of angiogenesis. Attention has shifted in recent years to non-VEGF mechanisms of blood vessel formation in the context of understanding resistance to anti-angiogenic therapies. For example in the setting of bevacizumab, not all patients derive clinical benefit from treatment, and duration of response can be highly varied. Furthermore, clinical gains in overall survival have been quite modest in several different malignancies including breast and non-small cell lung cancer (NSCLC). Alterations in critical angiogenic pathways likely provide an explanation for the heterogeneity in clinical outcomes with VEGF-axis directed therapies. Angiogenic resistance mechanisms can be generally categorized into VEGF-axis dependent alterations, non-VEGF pathways, and stromal cell interactions (Figure 1). These broad categories are not mutually exclusive, and given the coordination of both physiological and pathological angiogenesis, multiple factors and pathways are likely to be relevant in any given patient. The purpose of this review is to detail the preclinical evidence for mechanisms of angiogenic resistance and provide an overview of novel therapeutic approaches exploiting these pathways.
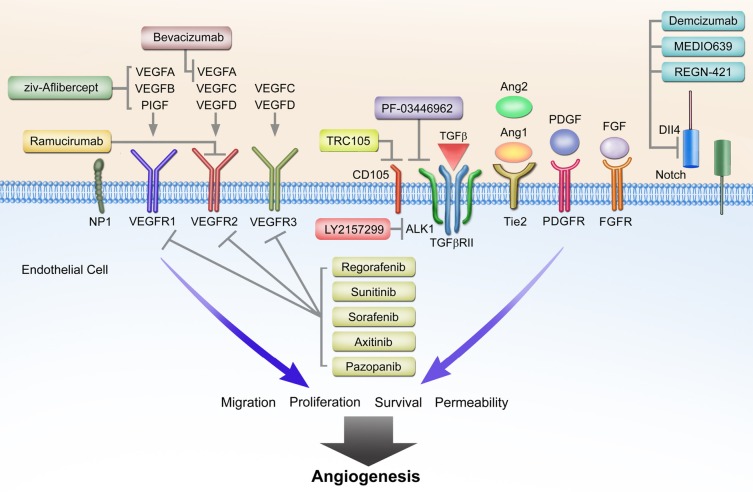
Figure 1.
VEGF-axis dependent and non-VEGF mediated mechanisms of resistance to anti-angiogenic therapies. Non-VEGF axis receptors include TGF-β receptor, Tie2, PDGFR, FGFR, and Dll4-Notch. General mechanisms of action for ziv-Aflibercept, bevacizumab, ramucirumab, and VEGFR activity of RTK inhibitors are depicted. Novel targeted therapies include TRC105, PF-03446962, LY2157299, demcizumab, MEDI0639, and RGN-421 inhibiting TGF-β and Dll4-Notch signaling
VEGF-axis dependent resistance mechanisms
VEGFA is upregulated in most malignancies and in response to the administration of anti-angiogenic therapies. In the setting of VEGFA inhibition by bevacizumab; however, PlGF, VEGB, VEGFC, and VEGFD may contribute to VEGFR signaling and ultimately tumor angiogenesis. In patients with mCRC receiving FOLFIRI with bevacizumab, plasma levels of PlGF, VEGFC and VEGFD were elevated prior to or at time of disease progression (4,5). The role of PlGF in tumor blood vessel formation remains controversial with much evidence published showing both pro- and anti-angiogenic effects in preclinical studies (6,7). Likewise, while VEGFB is upregulated in multiple malignancies including colorectal cancer, the precise function of VEGFB in tumor angiogenesis remains undefined, but may involve promoting tumor cell migration (8). Ziv-aflibercept (Zaltrap, VEGF-Trap) is composed of the extracellular domains of VEGFR1 and VEGFR2 linked to an IgG1 backbone. Ziv-aflibercept binds not only VEGFA but also VEGFB and PlGF. At this point, it is not known if binding VEGFB and PlGF are important to the efficacy or toxicity of ziv-aflibercept. In patients with refractory mCRC with prior oxaliplatin-based therapy, ziv-aflibercept with FOLFIRI resulted in improved overall survival (HR 0.82; 13.5 vs. 12.1 months) compared to chemotherapy alone (9). However, a recent study of ziv-aflibercept with docetaxel in patients with metastatic non-small cell lung cancer failed to meet its primary end point with overall survival (10,11).
In the phase III MAX trial of patients with metastatic colorectal cancer receiving capecitabine and mitomycin with or without bevacizumab, levels of VEGF- D measured by IHC in formalin fixed paraffin embedded tumor samples predicted for benefit from bevacizumab (12). Parent VEGF-D binds to VEGFR-3, however proteolytically processed VEGF-D binds with high affinity to VEGFR2 promoting angiogenesis, and potentially bypassing VEGF-A inhibition (13). VEGFR2 blockade by ramucirumab, for example, would theoretically be sufficient to block processed VEGFD activity on VEGFR2, but not activation of VEGFR3 signaling. Likewise, VEGFC is strongly implicated in angiogenesis and tumor progression in preclinical models, and has also been implicated in resistance to anti-VEGFA directed therapies (14). Importantly, while VEGFR3 is classically described as contributing to lymphangiogenesis, it is expressed on tumor endothelial cells and is involved in angiogenic sprouting and endothelial cell proliferation. In vivo stimulation of VEGFR3 is capable of sustaining angiogenesis in the setting of VEGFR2 inhibition; and combined VEGFR2 and VEGFR3 blockade demonstrates additive inhibitory effects on tumor growth (15). Taken together, approaches utilizing novel combinations to account for the proangiogenic effects of VEGFC and VEGFD on both VEGFR2 and VEGFR3 should be considered with future anti-angiogenic regimens.
Non-VEGF modulators of angiogenesis
The FGF family of growth factors is an important and potent mediator of tumor angiogenesis (16). In some model systems, FGF2 or bFGF has even greater proangiogenic effect than VEGFA, and acts synergistically with VEGFA to induce angiogenesis via endothelial cell proliferation, survival, and migration (17). Importantly, combinations of anti-VEGF and anti-FGF agents also act synergistically to inhibit angiogenesis and tumor growth (18). The interplay between FGF and VEGF signaling is likely mediated through multiple mechanisms including upregulation of NRP1 and hypoxia-inducible factor 1 (HIF1) resulting in increased VEGF signaling (19,20). Preclinical models demonstrate that FGF2 levels increase with VEGF-axis inhibition, and FGF blockade reduces tumor growth in anti-VEGF resistant in vivo models (21,22). Kopetz et al. showed that plasma FGF levels, along with PDGF, increased prior to disease progression in patients with metastatic colorectal cancer receiving FOLFIRI with bevacizumab (4). Similar temporal changes in circulating FGF2 levels in response to VEGF axis inhibition and disease progression have been documented in glioblastoma patients as well (23). Based on the results by Kopetz et al. and others, PDGF may also contribute along with FGF to the proangiogenic mileu implicated in VEGF resistance. PDGF is known to be involved in pericyte recruitment and tumor vessel coverage, as well as endothelial cell function (24). Additionally, VEGFA and FGF2 signaling results in upregulation of PDGF and PDGFR expression on endothelial cells (25), while combined VEGFR2 and PDGF inhibition is sufficient to overcome anti-VEGF resistance in vivo using murine tumor xenografts (26).
PDGFR activity is common in most currently approved RTK inhibitors, however a growing number of novel agents in early phase trials demonstrate activity against FGFR in addition to VEGFR. Brivanib (BMS-582664) has been evaluated in combination with cetuximab in patients with metastatic colorectal cancer; despite improvement in PFS, however OS was unchanged compared to cetuximab alone (27). Dovitinib as well is undergoing phase III evaluation in metastatic renal cancer, and several phase II studies in colorectal cancer and other malignancies are actively recruiting patients (NCT01676714). Several other RTK inhibitors with FGFR activity are also being evaluated including AZD4547 and Nintedanib in phase I and II trials, however no results in colorectal cancer patient populations have been reported. Combined VEGFR and PDGFR blockade using sunitinib has been evaluated recently in metastatic CRC patients. Unfortunately, combination sunitinib with or without FOLFIRI failed to improve PFS (28).
Delta-like ligand 4 (Dll4), a ligand for Notch, is expressed on arterial endothelial cells surfaces and upregulated in multiple malignancies. Together, Dll4 and Notch have been implicated in anti-angiogenic resistance, specifically with VEGFA targeted therapies (29,30). Dll4 and Notch are upregulated by VEGFA, and under physiologic conditions act as a negative feedback mechanism for vessel sprouting and angiogenesis (30). Paradoxically, inhibition of Dll4 in tumor models results hypervascularity with abnormal vessels, reduced perfusion and improved tumor growth inhibition (31,32). Interestingly, upregulation of Dll4 induced bevacizumab resistance, and was in turn overcome by Notch inhibition with dibenzazepine, a γ-secretase inhibitor (33) (which in inhibits Notch singaling). In vivo inhibition of Dll4 in pancreatic and ovarian tumor xenografts results in potent growth inhibition (34,35). Hu et al. also demonstrated that tissue Dll4 levels were predictive of clinical outcomes and response to anti-VEGF treatment in patients with ovarian cancer. Furthermore, Dll4 downregulation with siRNA in combination with anti-VEGF therapy resulted in greater tumor growth inhibition than with each agent alone (35).
Multiple phase I and II studies are ongoing evaluating novel Dll4 inhibitors. Demcizumab (OMP-21M18), a monoclonal antibody targeting Dll4, is now being evaluated in phase II clinical trials. The phase I results have not yet been reported, but phase II studies in combination with chemotherapies are currently enrolling for pancreatic cancer, metastatic colorectal cancer, and NSCLC patients (NCT01189942, NCT01189929, NCT01189968). Promising preclinical results showing promotion of hypervascularity with mural cell coverage have been demonstrated for MEDI0639, consistent with Dll4-Notch disruption (36). Phase I studies in patients with advanced solid malignancies are ongoing as well for MEDI0639 and REGN-421. The efficacy of γ-secretase inhibition is also being tested, given promising phase I results with R04929097 and MK-0752 (37,38).
The Angiopoietin (Ang)-Tie axis plays an integral role in tumor blood vessel development as well. Both Ang1 and Ang2 are upregulated in numerous malignancies including non-small cell lung, gastric, and colorectal carcinomas (39). However, each ligand has differential effects on the Tie2 signaling, which is typically localized to activated tumor endothelium. Ang1 binds Tie2 resulting in decreased vascular permeability and promotion of vessel maturation and stabilization. Ang2, on the other hand, antagonizes Ang1 and induces neovascularization by destabilizing endothelial cell-pericyte junctions and promotes endothelial cell survival, migration, and proliferation (40). Accordingly, it is well established that higher ratios of Ang2 to Ang1 levels predict worse clinical outcomes (41-43). While some controversy exists regarding the effect of Ang-Tie on tumor angiogenesis in specific models, the effect of, at least, Ang2 signaling appears to be highly dependent on other progangiogenic cytokines being present, such as VEGFA (39,40,44). Indeed, ectopic Ang2 expression interferes with VEGFR2 blockade, and combined inhibition of Ang2 and VEGFA produces greater reduction in angiogenesis in preclinical models (45-47).
A number of novel agents targeting the Ang-Tie axis are currently in clinical development (48). Most notably, Regorafenib, a multi-target RTK inhibitor with VEGFR1-3 and Tie2 activity, demonstrated efficacy in the 3rd line setting for both metastatic colorectal cancer and gastrointestinal stromal tumor (49,50). Trebananib (AMG386) is a peptide-Fc fusion protein that inhibits the interaction between Ang1/2 and Tie2, and has demonstrated tolerability but mixed efficacy in phase II trials (51-54). Several phase III studies ongoing are evaluating combination trebananib with paclitaxel, carboplatin, or pegylated liposomal doxorubicin. CovX-060 (PF04856884) is an Ang-2 specific peptide linked to IgG, which demonstrated safety in phase I trials and is being evaluated in patients with metastatic renal cell carcinoma (NCT00982657) (55,56). REGN-910 and MEDI-3617 are both Ang2 specific monoclonal antibodies, which are currently in phase I development (NCT01248949, NCT01688960, NCT01271972).
As cell proliferation and tumor growth outstrips blood vessel supply of oxygen, tumor cell hypoxia becomes a key driver of angiogenesis. Upregulation of the transcription factor, HIF-1, is central in the cellular response to reduced oxygen tension, and has multiple downstream effects including promotion of VEGFA, VEGFRs, PlGF, Ang1/2, and PDGF (57). Furthermore, HIF-1 activation is intimately involved in promoting cell survival, endothelial cell migration, anaerobic metabolism, and metastasis; and elevated tissue levels correlate with worse prognosis in a number of malignancies (58,59). Indeed, in colorectal cancer patients, HIF-1 levels are an independent predictor of poor survival (60). Extensive preclinical evidence for both direct and indirect strategies to inhibit of HIF-1 activation has been published (61). Approaches to indirect inhibition of HIF-1 have focused on blockade of factors mediating response to hypoxia including PI3-kinase, insulin-like growth factor, and mTOR (57). EZN-2968, an antisense oligonucleotide, which blocks HIF-1 alpha mRNA and is currently in a phase I development (NCT01120288). Combinations of VEGF and mTOR inhibitors have to date been unsuccessful, including in colorectal cancer (62-65).
TGF-β is another regulator of endothelial cell function and angiogenesis. TGF-β is a ligand for type II TGF-β receptors and CD105 (endoglin), which form heterotetrameric complexes with type I receptors, resulting in an intracellular signaling cascade via phosphorylation Smad proteins 1/5/8 (66). Activin receptor-like kinase 1 (ALK-1) is a type I TGF-β transmembrane receptor with restricted expression to proliferating endothelial cells and is critical to TGF-β mediated angiogenesis (66). ALK-1 is present on the vascular endothelium and circulating endothelial cells in numerous malignancies including, breast, prostate, renal cell, and colorectal carcinomas (67). Following phosphorylation of Smads 1/5/8, upregulation occurs of multiple genes including Id1, stimulating endothelial cell proliferation, migration, and tubule formation and culminating in the activation phase of angiogenesis (68). CD105 is also expressed on proliferating endothelial cell surfaces and modulates angiogenesis through TGF-β signaling via ALK-1 and SMAD proteins (69). In addition to being heavily overexpressed on tumor vessel endothelium (70), increased CD105 expression correlates inversely with clinical outcome in a variety of malignancies, including colorectal cancer (71). Intriguingly, VEGF blockade increases CD105 expression in multiple preclinical models, suggesting a role for CD105 in circumventing anti-VEGF therapy (72). The intimate role TGF-β with ALK1 and CD105 in endothelial cell function, highlights a promising avenue of exploitation for targeted inhibition of tumor angiogenesis.
Several TGF-β inhibitors are currently being developed in clinical trials. PF-03446962 is a IgG2 monoclonal antibody with potent specificity against human ALK-1 (67). Interestingly, melanoma xenografts with acquired overexpression of human VEGF-A and RTK-inhibitor resistance, demonstrate increased tumor growth inhibition when bevacizumab is used in conjunction with PF-03446962, thus suggesting ALK-1 signaling is involved in bevacizumab resistance. Phase I trials are currently ongoing evaluating the safety profile of PF-03446962 in patients with all type of cancers (NCT00557856, NCT01337050). TRC105 is a novel IgG monoclonal antibody directed at CD105, and has been well tolerated in the initial phase I study (73). Several phase I/II studies are ongoing using TRC105 in combination with RTK inhibitors and bevacizumab (NCT01332721, NCT01306058). Finally, LY2157299 is a small molecular inhibitor with a pyrazole structure and specificity for TGF-β type I receptors (74). LY2157299 safety has been reported in two clinical trials in the past several years (75,76). Multiple phase II studies are ongoing in patients with advanced pancreatic cancer, recurrent glioma, and hepatocellular carcinoma (Table 1).
Table 1. Selected ongoing trials involving non-VEGF mediated pathways of angiogenesis.
Stromal dependent mechanisms of resistance
The contribution of various cells in the tumor stroma to angiogenesis is well established (77). Bone marrow derived cells (BMDCs) are comprised of both endothelial and pericyte progenitors, as well as proangiogenic, tumor infiltrating immune cells (78). Circulating, Flk1+ (VEGFR2+) endothelial progenitors in particular deposit into the vessel lumen at sites of active angiogenesis in vivo and contribute to vessel formation (79,80). Disruption of endothelial cell progenitor function results in decreased angiogenesis of tumor xenografts and increased animal survival (81). Furthermore, anti-VEGR2 monoclonal antibodies reduce endothelial progenitor cells in murine models and inhibit tumor growth (82). Additionally, resistance to tumor vascular disrupting agents is mediated by endothelial progenitors and can be overcome by VEGFR and PDGFR inhibition (83). Increased levels of circulating VEGFR2+ BMDC progenitors are associated with worse overall survival compared to low levels in patients with advanced cancer (84). Accordingly, suppression of endothelial cell progenitors may be one of the underlying mechanisms of anti-VEGF therapies.
A number of distinct immune cells are also recruited to the tumor microenvironment by secreted cytokines (including G-CSF, PlGF, stromal derived factor 1α) and release proangiogenic factors which influence resistance to anti-VEGF therapies (77,85,86). For example, tumor infiltration by CD11b+Gr1+ myeloid cells is associated with anti-VEGF resistance; recruitment of these cells confers resistance by release of the proangiogenic factor Bv8 (87,88). Tie2 expressing monocytes or macrophages are physically associated with tumor vessels as well in a number of malignancies, and promote angiogenesis via paracrine release of factors including VEGFA (89,90). Ang2 secreted by tumor cells alters the genetic phenotype of Tie2+ monocytes/macrophages and increases expression of multiple proangiogenic genes (91). Indeed, inhibition of Ang2 signaling is effective at decreasing tumor growth and angiogenesis by impeding upregulation of Tie2 and association of Tie2+ cells with blood vessels (92). Future strategies aimed at mediating the inflammatory cytokines driving recruitment of various BMDCs and blunting proangiogenic signals are a promising avenue of research.
Biomarkers
Given the prominent use of anti-angiogenic agents in colorectal cancer and other malignancies today, predictive biomarkers are urgently needed in order to maximize clinical benefit, decreased unnecessary drug toxicity, and improve costs of cancer care. Biomarkers to predict benefit and guide use of therapies targeting angiogenesis can be measured at baseline (pretreatment) or by relative change during treatment. While baseline measurement may reflect preexisting, intrinsic mechanisms of resistance, angiome changes during treatment may offer insight into acquired or upregulated pathways of angiogenesis. Thus far, biomarkers for both have remained elusive for a multitude of reasons (93). Aside from the complexity of tumor angiogenesis, a major consideration is that robust blood and tissue based biomarker programs were not often embedded into large randomized trials, where such work is best done. In addition, many targets are of low abundance and are highly processed, and reagents for many targets are often limited.
Plasma VEGFA levels in a variety of malignancies, including colorectal cancer, have well-established prognostic value. However plasma VEGF levels have generally not been predictive of benefit with anti-angiogenic therapy (94). Recently, a VEGF assay that is preferential for small VEGF isoforms was reported to predict for benefit from bevacizumab in metastatic breast, pancreatic, and gastric cancers. However these results were of only borderline statistical significance and this assay was not predictive of benefit in colorectal, non-small cell lung, or renal cell cancers. The reason for these differences is not yet known, but may relate to differences in biology or in differences in sample handling across these trials (95). Biomarker analyses from other phase III trial with bevacizumab have plasma VEGF-D, SDF1, and Ang2 in pancreatic cancer and tumor tissue VEGFD measured by IHC in colorectal cancer (12,96). Phase III trials with pazopanib and bevacizumab have implicated high IL6 levels as a predictor of benefit from these agents in renal cell cancer (97).
Intriguingly, anti-angiogenic VEGFA isoforms have been described, although this field remains controversial (98). VEGFxxxb isoforms have anti-angiogenic properties and bind to bevacizumab; bevacizumab binding to these isoforms would theoretically deplete both bevacizumab and the anti-angiogenic VEGFxxxb ligands. Conversely low levels of VEGFxxxb would be predicted to describe a more VEGFA dependent and bevacizumab sensitive state. Interestingly, in an analysis of a subset of patients with tumor available from the 2nd line E3200 study (FOLFOX +/- bevacizumab) low ratios of VEGF165b:VEGFtotal measured in tissue samples of mCRC patients correlated with clinical benefit from addition of bevacizumab compared to chemotherapy alone; and no benefit from bevacizumab was seen when the VEGF165b:VEGFtotal ratio was elevated (99).
Genetic polymorphisms in VEGFA and VEGFR1 may influence angiogenic potential involving the tumor vasculature and response to anti-angiogenic therapies. Accordingly, multiple studies have investigated VEGF genotypes and clinical outcomes. Schneider et al. reported that the VEGF-2578 AA genotype was associated with both an overall survival benefit and less toxicity in the phase III E2100 trial of paclitaxel with or without bevacizumab in metastatic breast cancer (100). Furthermore, certain single nucleotide polymorphisms (SNPs) correlated with clinical outcomes in the AViTA and AVOREN trails, using bevacizumab in patients with metastatic pancreatic cancer and metastatic RCC, respectively. VEGFR1 SNP rs9582036 was associated with progression free survival in both trials, and overall survival for patients AC and CC genotypes. No genetic interaction was seen in placebo groups (93). Circulating endothelial cells and tumor vessel imaging with dynamic contrast-enhanced magnetic resonance imaging are also other emerging areas of biomarker research (93,101,102). While exploratory angiogenic biomarker analysis should continue in early phase studies, future phase III trials should have prospective angiogenic marker analysis incorporated into the study design to aid and expedite validation and clinical implementation. For example, the ongoing phase III MERiDIAN trial is evaluating paclitaxel with or without bevacizumab in patients with metastatic breast cancer, stratified by pretreatment plasma VEGF level (101).
Future directions
A wealth of evidence has been published in the past decade collectively affirming that VEGF-axis directed therapies confer clinical benefit along the continuum of care for patients with metastatic CRC (11,50,103). Within the past year, novel approaches to targeting agiogenesis have also yielded benefit in phase III trials with regorafenib and ziv-aflibercept. While the clinical effect of anti-VEGF targeted therapies may be well established in this population, not all patients experience benefit. Furthermore, patients inevitably progress while on anti-angiogenic treatment, and the ultimate improvement in overall survival can be modest. There are numerous complementary angiogenic pathways, which may be deregulated or circumvent the mechanism of action for current targeted agents. Alternative mechanisms of tumor vessel formation may explain the various clinical phenotypes of initial treatment nonresponse or inducible resistance to anti-angiogenesis therapies.
Rational combinations of anti-angiogenic agents are needed to overcome resistance mechanisms and exploit alternative pathways of tumor blood vessel formation. Both “vertical” (targeting multiple levels of the same pathway) and “horizontal” strategies (covering multiple different angiogenic pathways) have been attempted in several different tumor types and reviewed recently (104). Although several of these combinations have demonstrated encouraging anti-tumor activity, the unfavorable side effect profiles have proven to be difficult to overcome. Future strategies involving non-overlapping toxicity profiles of anti-angiogenic agents and dosing adjustments based on pharmacokinetic/pharmacodynamics data should be employed to optimize tolerability and balance anti-tumor effect. Lastly, routine incorporation of predictive biomarkers is imperative to tailor patient selection and increase therapeutic efficacy of novel drug combinations.
Conclusions
Mechanisms of resistance to anti-angiogenic therapies can broadly be categorized by involvement of the VEGF-axis, stromal cell interaction, and non-VEGF pathways. These mechanisms rely on a number of distinct but interrelated paracrine signaling factors and intracellular cascades. Clinical approaches targeting multiple pathways involving VEGFC, VEGFD, Tie2-Ang2, Dll4-Notch, and TGF-β may have greater benefit than monotherapies blocking VEGFA or VEGFR2 signaling alone, for example. Numerous clinical trials are ongoing to evaluate targeted therapies with specificity for these resistance mechanisms. Incorporation of biomarkers in future clinical trials will be critical to the development of next generation anti-angiogenic regimens.
Acknowledgements
Disclosure: Clarke—None; Hurwitz—None; Stock—None; Employment—None; Honoraria—Roche; Advisory—Genentech/Roche, Lilly, Sanofi, Regeneron, GSK, Pfizer, BMS, Tracon; Research Support—Genentech/Roche, GSK, BMS, Tracon.
References
- 1.Ferrara N.Vascular endothelial growth factor as a target for anticancer therapy. Oncologist 2004;9Suppl 1:2-10 [DOI] [PubMed] [Google Scholar]
- 2.Potente M, Gerhardt H, Carmeliet P.Basic and therapeutic aspects of angiogenesis. Cell 2011;146:873-87 [DOI] [PubMed] [Google Scholar]
- 3.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med 2011;17:1359-70 [DOI] [PubMed] [Google Scholar]
- 4.Kopetz S, Hoff PM, Morris JS, et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol 2010;28:453-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lieu CH, Tran HT, Jiang Z, et al. The association of alternate VEGF ligands with resistance to anti-VEGF therapy in metastatic colorectal cancer. ASCO Meeting 2011;29:abstr 3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hedlund EM, Yang X, Zhang Y, et al. Tumor cell-derived placental growth factor sensitizes antiangiogenic and antitumor effects of anti-VEGF drugs. Proc Natl Acad Sci U S A 2013;110:654-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carmeliet P, Moons L, Luttun A, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med 2001;7:575-83 [DOI] [PubMed] [Google Scholar]
- 8.Fischer C, Mazzone M, Jonckx B, et al. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer 2008;8:942-56 [DOI] [PubMed] [Google Scholar]
- 9.Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 2012;30:3499-506 [DOI] [PubMed] [Google Scholar]
- 10.Ramlau R, Gorbunova V, Ciuleanu TE, et al. Aflibercept and Docetaxel versus Docetaxel alone after platinum failure in patients with advanced or metastatic non-small-cell lung cancer: a randomized, controlled phase III trial. J Clin Oncol 2012;30:3640-7 [DOI] [PubMed] [Google Scholar]
- 11.Arnold D, Andre T, Bennouna J, et al. Bevacizumab (BEV) plus chemotherapy (CT) continued beyond first progression in patients with metastatic colorectal cancer (mCRC) previously treated with BEV plus CT: Results of a randomized phase III intergroup study (TML study). ASCO Meeting 2012;30:CRA3503. [Google Scholar]
- 12.Weickhardt AJ, Williams D, Lee C, et al. Vascular endothelial growth factors (VEGF) and VEGF receptor expression as predictive biomarkers for benefit with bevacizumab in metastatic colorectal cancer (mCRC): Analysis of the phase III MAX study. J Clin Oncol 2011;29:abstr 3531.
- 13.Leppänen VM, Jeltsch M, Anisimov A, et al. Structural determinants of vascular endothelial growth factor-D receptor binding and specificity. Blood 2011;117:1507-15 [DOI] [PubMed] [Google Scholar]
- 14.Ye J, Wu X, Wu D, et al. miRNA-27b Targets Vascular Endothelial Growth Factor C to Inhibit Tumor Progression and Angiogenesis in Colorectal Cancer. PLoS One 2013;8:e60687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tammela T, Zarkada G, Wallgard E, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature 2008;454:656-60 [DOI] [PubMed] [Google Scholar]
- 16.Lieu C, Heymach J, Overman M, et al. Beyond VEGF: inhibition of the fibroblast growth factor pathway and antiangiogenesis. Clin Cancer Res 2011;17:6130-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pepper MS, Ferrara N, Orci L, et al. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem Biophys Res Commun 1992;189:824-31 [DOI] [PubMed] [Google Scholar]
- 18.Compagni A, Wilgenbus P, Impagnatiello MA, et al. Fibroblast growth factors are required for efficient tumor angiogenesis. Cancer Res 2000;60:7163-9 [PubMed] [Google Scholar]
- 19.Liu W, Parikh AA, Stoeltzing O, et al. Upregulation of neuropilin-1 by basic fibroblast growth factor enhances vascular smooth muscle cell migration in response to VEGF. Cytokine 2005;32:206-12 [DOI] [PubMed] [Google Scholar]
- 20.Shi YH, Bingle L, Gong LH, et al. Basic FGF augments hypoxia induced HIF-1-alpha expression and VEGF release in T47D breast cancer cells. Pathology 2007;39:396-400 [DOI] [PubMed] [Google Scholar]
- 21.Casanovas O, Hicklin DJ, Bergers G, et al. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 2005;8:299-309 [DOI] [PubMed] [Google Scholar]
- 22.Allen E, Walters IB, Hanahan D. Brivanib, a dual FGF/VEGF inhibitor, is active both first and second line against mouse pancreatic neuroendocrine tumors developing adaptive/evasive resistance to VEGF inhibition. Clin Cancer Res 2011;17:5299-310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 2007;11:83-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostman A.PDGF receptors-mediators of autocrine tumor growth and regulators of tumor vasculature and stroma. Cytokine Growth Factor Rev 2004;15:275-86 [DOI] [PubMed] [Google Scholar]
- 25.Kano MR, Morishita Y, Iwata C, et al. VEGF-A and FGF-2 synergistically promote neoangiogenesis through enhancement of endogenous PDGF-B-PDGFRbeta signaling. J Cell Sci 2005;118:3759-68 [DOI] [PubMed] [Google Scholar]
- 26.Erber R, Thurnher A, Katsen AD, et al. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J 2004;18:338-40 [DOI] [PubMed] [Google Scholar]
- 27.Siu LL, Shapiro JD, Jonker DJ, et al. Final analysis of the phase III randomized trial of cetuximab (CET) plus either brivanib alaninate (BRIV) or placebo in patients (pts) with chemotherapy refractory, K-RAS wild-type (WT), metastatic colorectal carcinoma (mCRC): The NCIC Clinical Trials Group and AGITG CO.20 trial. ASCO Meeting 2012;30:abstr 3504. [DOI] [PubMed] [Google Scholar]
- 28.Carrato A, Swieboda-Sadlej A, Staszewska-Skurczynska M, et al. Fluorouracil, leucovorin, and irinotecan plus either sunitinib or placebo in metastatic colorectal cancer: a randomized, phase III trial. J Clin Oncol 2013;31:1341-7 [DOI] [PubMed] [Google Scholar]
- 29.Jubb AM, Turley H, Moeller HC, et al. Expression of delta-like ligand 4 (Dll4) and markers of hypoxia in colon cancer. Br J Cancer 2009;101:1749-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thurston G, Noguera-Troise I, Yancopoulos GD. The Delta paradox: DLL4 blockade leads to more tumour vessels but less tumour growth. Nat Rev Cancer 2007;7:327-31 [DOI] [PubMed] [Google Scholar]
- 31.Noguera-Troise I, Daly C, Papadopoulos NJ, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature 2006;444:1032-7 [DOI] [PubMed] [Google Scholar]
- 32.Ridgway J, Zhang G, Wu Y, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature 2006;444:1083-7 [DOI] [PubMed] [Google Scholar]
- 33.Li JL, Sainson RC, Oon CE, et al. DLL4-Notch signaling mediates tumor resistance to anti-VEGF therapy in vivo. Cancer Res 2011;71:6073-83 [DOI] [PubMed] [Google Scholar]
- 34.Yen WC, Fischer MM, Hynes M, et al. Anti-DLL4 has broad spectrum activity in pancreatic cancer dependent on targeting DLL4-Notch signaling in both tumor and vasculature cells. Clin Cancer Res 2012;18:5374-86 [DOI] [PubMed] [Google Scholar]
- 35.Hu W, Lu C, Dong HH, et al. Biological roles of the Delta family Notch ligand Dll4 in tumor and endothelial cells in ovarian cancer. Cancer Res 2011;71:6030-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jenkins DW, Ross S, Veldman-Jones M, et al. MEDI0639: a novel therapeutic antibody targeting Dll4 modulates endothelial cell function and angiogenesis in vivo. Mol Cancer Ther 2012;11:1650-60 [DOI] [PubMed] [Google Scholar]
- 37.Tolcher AW, Messersmith WA, Mikulski SM, et al. Phase I study of RO4929097, a gamma secretase inhibitor of Notch signaling, in patients with refractory metastatic or locally advanced solid tumors. J Clin Oncol 2012;30:2348-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krop I, Demuth T, Guthrie T, et al. Phase I pharmacologic and pharmacodynamic study of the gamma secretase (Notch) inhibitor MK-0752 in adult patients with advanced solid tumors. J Clin Oncol 2012;30:2307-13 [DOI] [PubMed] [Google Scholar]
- 39.Shim WS, Ho IA, Wong PE. Angiopoietin: a TIE(d) balance in tumor angiogenesis. Mol Cancer Res 2007;5:655-65 [DOI] [PubMed] [Google Scholar]
- 40.Cascone T, Heymach JV. Targeting the angiopoietin/Tie2 pathway: cutting tumor vessels with a double-edged sword? J Clin Oncol 2012;30:441-4 [DOI] [PubMed] [Google Scholar]
- 41.Ahmad SA, Liu W, Jung YD, et al. The effects of angiopoietin-1 and -2 on tumor growth and angiogenesis in human colon cancer. Cancer Res 2001;61:1255-9 [PubMed] [Google Scholar]
- 42.Mitsuhashi N, Shimizu H, Ohtsuka M, et al. Angiopoietins and Tie-2 expression in angiogenesis and proliferation of human hepatocellular carcinoma. Hepatology 2003;37:1105-13 [DOI] [PubMed] [Google Scholar]
- 43.Sfiligoi C, de Luca A, Cascone I, et al. Angiopoietin-2 expression in breast cancer correlates with lymph node invasion and short survival. Int J Cancer 2003;103:466-74 [DOI] [PubMed] [Google Scholar]
- 44.Huang H, Bhat A, Woodnutt G, et al. Targeting the ANGPT-TIE2 pathway in malignancy. Nat Rev Cancer 2010;10:575-85 [DOI] [PubMed] [Google Scholar]
- 45.Chae SS, Kamoun WS, Farrar CT, et al. Angiopoietin-2 interferes with anti-VEGFR2-induced vessel normalization and survival benefit in mice bearing gliomas. Clin Cancer Res 2010;16:3618-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hashizume H, Falcón BL, Kuroda T, et al. Complementary actions of inhibitors of angiopoietin-2 and VEGF on tumor angiogenesis and growth. Cancer Res 2010;70:2213-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daly C, Eichten A, Castanaro C, et al. Angiopoietin-2 functions as a Tie2 agonist in tumor models, where it limits the effects of VEGF inhibition. Cancer Res 2013;73:108-18 [DOI] [PubMed] [Google Scholar]
- 48.Gerald D, Chintharlapalli S, Augustin HG, et al. Angiopoietin-2: an attractive target for improved antiangiogenic tumor therapy. Cancer Res 2013;73:1649-57 [DOI] [PubMed] [Google Scholar]
- 49.Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:295-302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12 [DOI] [PubMed] [Google Scholar]
- 51.Eatock MM, Tebbutt NC, Bampton CL, et al. Phase II randomized, double-blind, placebo-controlled study of AMG 386 (trebananib) in combination with cisplatin and capecitabine in patients with metastatic gastro-oesophageal cancer. Ann Oncol 2013;24:710-8 [DOI] [PubMed] [Google Scholar]
- 52.Peeters M, Strickland AH, Lichinitser M, et al. A randomised, double-blind, placebo-controlled phase 2 study of trebananib (AMG 386) in combination with FOLFIRI in patients with previously treated metastatic colorectal carcinoma. Br J Cancer 2013;108:503-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karlan BY, Oza AM, Richardson GE, et al. Randomized, double-blind, placebo-controlled phase II study of AMG 386 combined with weekly paclitaxel in patients with recurrent ovarian cancer. J Clin Oncol 2012;30:362-71 [DOI] [PubMed] [Google Scholar]
- 54.Rini B, Szczylik C, Tannir NM, et al. AMG 386 in combination with sorafenib in patients with metastatic clear cell carcinoma of the kidney: a randomized, double-blind, placebo-controlled, phase 2 study. Cancer 2012;118:6152-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mendelson DS, Rosen LS, Gordon MS, et al. First-in-human dose-escalation safety and PK trial of a novel humanized monoclonal CovX body dual inhibitor of angiopoietin 2 and vascular endothelial growth factor. ASCO Meeting 2011;29:abstr 3055. [Google Scholar]
- 56.Rosen LS, Mendelson DS, Gordon MS, et al. Phase Ib safety trial of CVX-060, an intravenous humanized monoclonal CovX body inhibiting angiopoietin 2 (Ang-2), with sunitinib. ASCO Meeting 2012;30:abstr 3032. [Google Scholar]
- 57.Yang Y, Sun M, Wang L, et al. HIFs, angiogenesis, and cancer. J Cell Biochem 2013;114:967-74 [DOI] [PubMed] [Google Scholar]
- 58.Koh MY, Lemos R, Jr, Liu X, et al. The hypoxia-associated factor switches cells from HIF-1α- to HIF-2α-dependent signaling promoting stem cell characteristics, aggressive tumor growth and invasion. Cancer Res 2011;71:4015-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer 2011;11:393-410 [DOI] [PubMed] [Google Scholar]
- 60.Kwon HC, Kim SH, Oh SY, et al. Clinicopathological significance of p53, hypoxia-inducible factor 1alpha, and vascular endothelial growth factor expression in colorectal cancer. Anticancer Res 2010;30:4163-8 [PubMed] [Google Scholar]
- 61.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer 2003;3:721-32 [DOI] [PubMed] [Google Scholar]
- 62.Strickler JH, Starodub AN, Jia J, et al. Phase I study of bevacizumab, everolimus, and panobinostat (LBH-589) in advanced solid tumors. Cancer Chemother Pharmacol 2012;70:251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Négrier S, Gravis G, Pérol D, et al. Temsirolimus and bevacizumab, or sunitinib, or interferon alfa and bevacizumab for patients with advanced renal cell carcinoma (TORAVA): a randomised phase 2 trial. Lancet Oncol 2011;12:673-80 [DOI] [PubMed] [Google Scholar]
- 64.Harshman LC, Barbeau S, McMillian A, et al. A Phase II Study of Bevacizumab and Everolimus as Treatment for Refractory Metastatic Renal Cell Carcinoma. Clin Genitourin Cancer 2013;11:100-6 [DOI] [PubMed] [Google Scholar]
- 65.Altomare I, Bendell JC, Bullock KE, et al. A phase II trial of bevacizumab plus everolimus for patients with refractory metastatic colorectal cancer. Oncologist 2011;16:1131-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cunha SI, Pietras K. ALK1 as an emerging target for antiangiogenic therapy of cancer. Blood 2011;117:6999-7006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu-Lowe DD, Chen E, Zhang L, et al. Targeting activin receptor-like kinase 1 inhibits angiogenesis and tumorigenesis through a mechanism of action complementary to anti-VEGF therapies. Cancer Res 2011;71:1362-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tian M, Neil JR, Schiemann WP. Transforming growth factor-β and the hallmarks of cancer. Cell Signal 2011;23:951-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lebrin F, Goumans MJ, Jonker L, et al. Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. EMBO J 2004;23:4018-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang LY, Lu WQ, Huang GW, et al. Correlation between CD105 expression and postoperative recurrence and metastasis of hepatocellular carcinoma. BMC Cancer 2006;6:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li C, Gardy R, Seon BK, et al. Both high intratumoral microvessel density determined using CD105 antibody and elevated plasma levels of CD105 in colorectal cancer patients correlate with poor prognosis. Br J Cancer 2003;88:1424-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bockhorn M, Tsuzuki Y, Xu L, et al. Differential vascular and transcriptional responses to anti-vascular endothelial growth factor antibody in orthotopic human pancreatic cancer xenografts. Clin Cancer Res 2003;9:4221-6 [PubMed] [Google Scholar]
- 73.Rosen LS, Hurwitz HI, Wong MK, et al. A phase I first-in-human study of TRC105 (Anti-Endoglin Antibody) in patients with advanced cancer. Clin Cancer Res 2012;18:4820-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bueno L, de Alwis DP, Pitou C, et al. Semi-mechanistic modelling of the tumour growth inhibitory effects of LY2157299, a new type I receptor TGF-beta kinase antagonist, in mice. Eur J Cancer 2008;44:142-50 [DOI] [PubMed] [Google Scholar]
- 75.Calvo-Aller E, Baselga J, Glatt S, et al. First human dose escalation study in patients with metastatic malignancies to determine safety and pharmacokinetics of LY2157299, a small molecule inhibitor of the transforming growth factor-beta receptor I kinase. ASCO Meeting 2008;26:abstr 14554. [Google Scholar]
- 76.Azaro A, Baselga J, Sepulveda JM, et al. The oral transforming growth factor-beta (TGF-ss) receptor I kinase inhibitor LY2157299 plus lomustine in patients with treatment-refractory malignant glioma: The first human dose study. ASCO Meeting 2012;30:abstr 2042. [Google Scholar]
- 77.Ferrara N.Pathways mediating VEGF-independent tumor angiogenesis. Cytokine Growth Factor Rev 2010;21:21-6 [DOI] [PubMed] [Google Scholar]
- 78.Bergers G, Hanahan D.Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer 2008;8:592-603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964-7 [DOI] [PubMed] [Google Scholar]
- 80.Sun XT, Yuan XW, Zhu HT, et al. Endothelial precursor cells promote angiogenesis in hepatocellular carcinoma. World J Gastroenterol 2012;18:4925-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gao D, Nolan DJ, Mellick AS, et al. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science 2008;319:195-8 [DOI] [PubMed] [Google Scholar]
- 82.Shaked Y, Bertolini F, Man S, et al. Genetic heterogeneity of the vasculogenic phenotype parallels angiogenesis; Implications for cellular surrogate marker analysis of antiangiogenesis. Cancer Cell 2005;7:101-11 [DOI] [PubMed] [Google Scholar]
- 83.Taylor M, Billiot F, Marty V, et al. Reversing resistance to vascular-disrupting agents by blocking late mobilization of circulating endothelial progenitor cells. Cancer Discov 2012;2:434-49 [DOI] [PubMed] [Google Scholar]
- 84.Massard C, Borget I, Le Deley MC, et al. Prognostic value of circulating VEGFR2+ bone marrow-derived progenitor cells in patients with advanced cancer. Eur J Cancer 2012;48:1354-62 [DOI] [PubMed] [Google Scholar]
- 85.Sennino B, McDonald DM. Controlling escape from angiogenesis inhibitors. Nat Rev Cancer 2012;12:699-709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shojaei F, Wu X, Qu X, et al. G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc Natl Acad Sci U S A 2009;106:6742-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shojaei F, Wu X, Malik AK, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol 2007;25:911-20 [DOI] [PubMed] [Google Scholar]
- 88.Shojaei F, Wu X, Zhong C, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature 2007;450:825-31 [DOI] [PubMed] [Google Scholar]
- 89.De Palma M, Venneri MA, Galli R, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 2005;8:211-26 [DOI] [PubMed] [Google Scholar]
- 90.De Palma M, Naldini L.Angiopoietin-2 TIEs up macrophages in tumor angiogenesis. Clin Cancer Res 2011;17:5226-32 [DOI] [PubMed] [Google Scholar]
- 91.Coffelt SB, Tal AO, Scholz A, et al. Angiopoietin-2 regulates gene expression in TIE2-expressing monocytes and augments their inherent proangiogenic functions. Cancer Res 2010;70:5270-80 [DOI] [PubMed] [Google Scholar]
- 92.Mazzieri R, Pucci F, Moi D, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell 2011;19:512-26 [DOI] [PubMed] [Google Scholar]
- 93.Lambrechts D, Lenz HJ, de Haas S, et al. Markers of response for the antiangiogenic agent bevacizumab. J Clin Oncol 2013;31:1219-30 [DOI] [PubMed] [Google Scholar]
- 94.Hegde PS, Jubb AM, Chen D, et al. Predictive impact of circulating vascular endothelial growth factor in four phase III trials evaluating bevacizumab. Clin Cancer Res 2013;19:929-37 [DOI] [PubMed] [Google Scholar]
- 95.Jayson GC, de Haas S, Delmar P, et al. Evaluation of plasma VEGFA as a potential predictive pan-tumour biomarker for bevacizumab. 2011 European Multidisciplinary Cancer Congress 2011;47:S96. [Google Scholar]
- 96.Nixon AB, Pang H, Starr M, et al. Prognostic and predictive blood-based biomarkers in patients with advanced pancreatic cancer: Results from CALGB 80303. ASCO Meeting 2011;29:abstr 10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu Y, Tran HT, Lin Y, et al. Circulating baseline plasma cytokines and angiogenic factors (CAF) as markers of tumor burden and therapeutic response in a phase III study of pazopanib for metastatic renal cell carcinoma (mRCC). ASCO Meeting 2011;29:abstr 4553. [Google Scholar]
- 98.Harris S, Craze M, Newton J, et al. Do anti-angiogenic VEGF (VEGFxxxb) isoforms exist? A cautionary tale. PLoS One 2012;7:e35231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bates DO, Catalano PJ, Symonds KE, et al. Association between VEGF splice isoforms and progression-free survival in metastatic colorectal cancer patients treated with bevacizumab. Clin Cancer Res 2012;18:6384-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schneider BP, Wang M, Radovich M, et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol 2008;26:4672-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schneider BP, Shen F, Miller KD. Pharmacogenetic biomarkers for the prediction of response to antiangiogenic treatment. Lancet Oncol 2012;13:e427-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.O’Connor JP, Jackson A, Parker GJ, et al. Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies. Nat Rev Clin Oncol 2012;9:167-77 [DOI] [PubMed] [Google Scholar]
- 103.Wagner AD, Arnold D, Grothey AA, et al. Anti-angiogenic therapies for metastatic colorectal cancer. Cochrane Database Syst Rev 2009;(3):CD005392. [DOI] [PubMed] [Google Scholar]
- 104.Moreno Garcia V, Basu B, Molife LR, et al. Combining antiangiogenics to overcome resistance: rationale and clinical experience. Clin Cancer Res 2012;18:3750-61 [DOI] [PubMed] [Google Scholar]