Increased mitophagy in the skeletal muscle of spinal and bulbar muscular atrophy patients - PubMed (original) (raw)
. 2017 Mar 15;26(6):1087-1103.
doi: 10.1093/hmg/ddx019.
Adriana Malena 1, Marco Spinazzi 2, Maria Andrea Desbats 3, Leonardo Salviati 3, Aaron P Russell 4, Giovanni Miotto 5 6, Laura Tosatto 7, Elena Pegoraro 1, Gianni Sorarù 1, Maria Pennuto 7, Lodovica Vergani 1
Affiliations
- PMID: 28087734
- PMCID: PMC5409076
- DOI: 10.1093/hmg/ddx019
Increased mitophagy in the skeletal muscle of spinal and bulbar muscular atrophy patients
Doriana Borgia et al. Hum Mol Genet. 2017.
Abstract
Spinal and bulbar muscular atrophy (SBMA) is a neuromuscular disorder caused by polyglutamine expansion in the androgen receptor (AR) and characterized by the loss of lower motor neurons. Here we investigated pathological processes occurring in muscle biopsy specimens derived from SBMA patients and, as controls, age-matched healthy subjects and patients suffering from amyotrophic lateral sclerosis (ALS) and neurogenic atrophy. We detected atrophic fibers in the muscle of SBMA, ALS and neurogenic atrophy patients. In addition, SBMA muscle was characterized by the presence of a large number of hypertrophic fibers, with oxidative fibers having a larger size compared with glycolytic fibers. Polyglutamine-expanded AR expression was decreased in whole muscle, yet enriched in the nucleus, and localized to mitochondria. Ultrastructural analysis revealed myofibrillar disorganization and streaming in zones lacking mitochondria and degenerating mitochondria. Using molecular (mtDNA copy number), biochemical (citrate synthase and respiratory chain enzymes) and morphological (dark blue area in nicotinamide adenine dinucleotide-stained muscle cross-sections) analyses, we found a depletion of the mitochondria associated with enhanced mitophagy. Mass spectrometry analysis revealed an increase of phosphatidylethanolamines and phosphatidylserines in mitochondria isolated from SBMA muscles, as well as a 50% depletion of cardiolipin associated with decreased expression of the cardiolipin synthase gene. These observations suggest a causative link between nuclear polyglutamine-expanded AR accumulation, depletion of mitochondrial mass, increased mitophagy and altered mitochondrial membrane composition in SBMA muscle patients. Given the central role of mitochondria in cell bioenergetics, therapeutic approaches toward improving the mitochondrial network are worth considering to support SBMA patients.
© The Author 2017. Published by Oxford University Press.
Figures
Figure 1
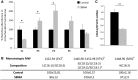
Atrophic and hypertrophic fibers in the skeletal muscle of SBMA patients. (A) Representative images of H&E-stained cryosections of control and SBMA quadriceps muscles. Scale bar, 40 µm. (B) Table showing the mean values of the atrophy and hypertrophy indexes in SBMA, ALS, and neurogenic patients and control subjects. The values, quantified as described in Materials and Methods, are expressed as mean ± SEM. Significance by ANOVA + LDS Fisher post doc: atrophy index: P < 0.001 SBMA, ALS and neurogenic atrophy patients versus controls; hypertrophy index: P < 0.001 SBMA versus controls, P < 0.001 SBMA versus ALS patients, P < 0.001 SBMA versus neurogenic patients. (C) Representative NADH-stained quadriceps muscle sections of control and SBMA subjects. Scale bar, 80 μm. (D) Analysis of the hypertrophy index in oxidative and glycolytic fibers. Graph, mean ± SEM, n = 4 SBMA patients and 5 control subjects. Significance by Student’s t test: **P < 0.01, ***P < 0.001.
Figure 2
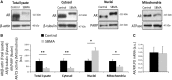
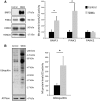
Aberrant subcellular localization of polyglutamine-expanded AR in the muscle of SBMA patients. (A) Western blotting analysis of AR levels in total lysates, cytosolic, nuclear and mitochondrial fractions from quadriceps muscles of SBMA patients and control subjects. β-actin, β-tubulin, PARP and ATPase were used as loading controls. (B) Quantification of AR in total lysates, and nuclear, cytosolic and mitochondrial fractions. Graph, mean ± SEM, n = 7 SBMA patients and 7 control subjects. Poly(ADP-ribose) polymerase (PARP), citrate synthase (CS). Significance by Student’s t test: *P < 0.05, **P < 0.01. (C) Real-time PCR analysis of the AR mRNA transcript levels normalized to large ribosomal protein (RPLPO) mRNA in the muscle of SBMA patients and control individuals. Graph, mean ± SEM, n = 6 SBMA patients and 3 control subjects. Significance by Student’s t test: *P < 0.05, **P < 0.01.
Figure 3
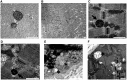
Mitochondrial abnormalities in the muscle of SBMA patients. (A) Representative image of TEM analysis of control muscle specimens. Representative images of TEM analysis of SBMA muscle specimens. (B) TEM analysis revealed myofibrillar disorganization and Z-line streaming. (C, D) Degenerated mitochondria with dense matrix (arrows). (E, F) Mitochondria with dilated-hypodense matrix and swelling (asterisks). Scale bar, 1 μm.
Figure 4
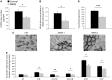
Reduction of mitochondria in the muscle of SBMA patients. (A) Real-time PCR analysis of mtDNA copy number measured as the ratio between cytochrome c oxidase II (COII) and nuclear amyloid precursor protein (APP) genes. Graph, mean ± SEM, n = 13 SBMA patients and 14 control subjects. (B) Mitochondrial mass expressed as the percentage of dark blue area in NADH-stained muscle cross-sections. Graph, mean ± SEM, n = 6 SBMA patients and 5 control subjects. (C) NADH analysis of quadriceps muscle of control and SBMA subjects. Scale bar, 80 μm. (D) Citrate synthase (CS) activity, expressed as nmol min−1 mg−1 of protein. Graph, mean ± SEM, n = 6 SBMA patients and 4 control subjects. (E) Activity of the respiratory chain complexes I–IV, expressed as nmol min−1 mg−1 of protein. Graph, mean ± SEM, n = 6 SBMA patients and 4 control subjects. Significance by Student’s t test: *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 5
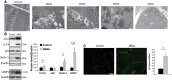
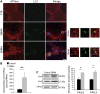
Increased autophagy in the muscle of SBMA patients. (A) TEM analysis of quadriceps muscle biopsies derived from two SBMA patients revealed the presence of autophagic vacuoles (AV). Scale bar, 1 μm. (B) Western blotting analysis of LC3-II, p62, Beclin-1 and LAMP1 levels. β-actin was used as loading control. Graph, mean ± SEM, n = 10 SBMA patients and 5 control subjects (top panels), and 9 SBMA patients and 9 control subjects (bottom panel). (C) Immunofluorescence analysis of LC3 in control and SBMA muscle tissues. The number of autophagosomes/fiber was measured as the number of green object-for-fiber (puncta/fiber). Graph, mean ± SEM, n = 50 fibers from each muscle sample derived from 11 SBMA patients and 10 control subjects. LC3 was detected using a specific antibody. Scale bar, 20 μm. Significance by Student’s t test: *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 6
Mitophagy is induced in the muscle of SBMA patients. (A, B) Western blotting analysis of BNIP3, PINK1, PARK2 and ubiquitin levels in mitochondria isolated from the quadriceps muscle of SBMA patients and controls subjects normalized to CS activity. TOM20 and ATPase were used as loading controls. Graph, mean ± SEM, n = 5 SBMA patients and 5 control subjects. Significance by Student’s t test: *P < 0.05.
Figure 7
Increased mitophagy and mitochondrial fission proteins in the muscle of SBMA patients. (A) Representative images of anti-LC3 (green) and anti-ATPase (red) immunostaining of control and SBMA muscle tissues. Zoom: magnification of the marked area (circle). Scale bar, 20 μm. (B) Number of colocalized autophagosome-mitochondria (yellow puncta), measured as number of yellow puncta/fiber. Graph, mean ± SEM, n = 11 SBMA patients and 10 control subjects, n of fibers: 50 for each sample. (C) Western blotting analysis of Drp1 and hFis1 levels normalized to CS activity in mitochondria isolated from the quadriceps muscle of SBMA patients and control subjects. ATPase was used as loading control. Graph, mean ± SEM, n = 4 SBMA patients and 4 control subjects. Significance by Student’s t test: *P < 0.05, **P < 0.01.
Figure 8
Reduced cardiolipin levels and biosynthesis in the muscle of SBMA patients. (A) Mass spectrometry lipidomic analysis of mitochondrial membranes isolated from SBMA patients and control subjects. Cardiolipin (CL), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylcholine (PC) and phosphatidylinositol (PI) amounts were normalized to CS activity. Graph, mean ± SEM, n = 4 SBMA patients and 4 control subjects. (B) Composition of CL molecular species in control and SBMA muscle samples. The values are expressed as mean ± SEM of two independent experiments. #Percentage of the molecular species/total CL. *Range of the major combinations among the shown acyl moieties. (C) Real-time PCR analysis of cardiolipin synthase (CRLS1) normalized to RPLPO in the muscle of SBMA patients and control subjects. Graph, mean ± SEM, n = 5 SBMA patients and 6 control subjects. Significance by Student’s t test: *P < 0.05, **P < 0.01.
Figure 9
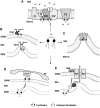
Cartoon representing cardiolipin functions in mitochondria. (A) Cardiolipin (CL) is essential for normal electron transport and proton translocation activity of complex I, III and IV and, with its negative charged head group, CL attracts and provides a local pool of protons necessary for complex V function. CL also promotes the assembling of OXPHOS complexes into supercomplexes, which implies an improvement of electron transfer and a reduction in ROS production. (B) CL is involved in mitochondrial dynamics. It is critical for the fusion of the IMMs via its interaction with optic atrophy (Opa1), promoting its dimerization and enhancing its GTPase activity. CL also has a role in the fission pathway. After its transfer from the IMM to the OMM, it mediates Drp1 recruitment to mitochondrial membrane surface and enhances Drp1 GTPase activity. (C) In concert with complex V, CL promotes cristae formation. CL structure composed of two phosphatidyl moieties with a single glycerol group promotes negative curvature of membrane. (D) CL externalization from IMM to OMM acts as an ‘eat-me-signal’ for the autophagic machinery, promoting mitophagy. The negative charged head group of externalized CL interacts with the basic CL binding sites of LC3, which mediates both autophagosome formation and cargo recognition. LC3 recognizes CL more effectively than its metabolites and oxidized CL, suggesting that CL oxidation is not a prerequisite for mitochondria elimination through mitophagy. (E) CL plays a role in apoptosis. During apoptosis initiation, CL undergoes peroxidation catalyzed by cytochrome c. Mitochondrial injuries generate ROS, which cause a significant amount of CL to flip to the outer leaflet of IMM, where it binds to cytochrome c. After peroxidation and externalization, CL binds a set of apoptotic proteins such as caspase-8, which are recruited to the mitochondrial surface. Caspase-8 cleaves Bid to a truncated form (tBid), which induces Bax/Bak oligometization thereby permeabilizing the membrane and releasing cytochrome c. AV, autophagic vacuole; cyt c, cytochrome c; IMS, intermembrane space; IMM, inner mitochondrial membrane; OMM, outer mitochondrial membrane.
Similar articles
- Muscle expression of mutant androgen receptor accounts for systemic and motor neuron disease phenotypes in spinal and bulbar muscular atrophy.
Cortes CJ, Ling SC, Guo LT, Hung G, Tsunemi T, Ly L, Tokunaga S, Lopez E, Sopher BL, Bennett CF, Shelton GD, Cleveland DW, La Spada AR. Cortes CJ, et al. Neuron. 2014 Apr 16;82(2):295-307. doi: 10.1016/j.neuron.2014.03.001. Neuron. 2014. PMID: 24742458 Free PMC article. - Distinct Etiological Roles for Myocytes and Motor Neurons in a Mouse Model of Kennedy's Disease/Spinobulbar Muscular Atrophy.
Ramzan F, McPhail M, Rao P, Mo K, Halievski K, Swift-Gallant A, Mendoza-Viveros L, Cheng HY, Monks DA. Ramzan F, et al. J Neurosci. 2015 Apr 22;35(16):6444-51. doi: 10.1523/JNEUROSCI.3599-14.2015. J Neurosci. 2015. PMID: 25904795 Free PMC article. - Glycolytic-to-oxidative fiber-type switch and mTOR signaling activation are early-onset features of SBMA muscle modified by high-fat diet.
Rocchi A, Milioto C, Parodi S, Armirotti A, Borgia D, Pellegrini M, Urciuolo A, Molon S, Morbidoni V, Marabita M, Romanello V, Gatto P, Blaauw B, Bonaldo P, Sambataro F, Robins DM, Lieberman AP, Sorarù G, Vergani L, Sandri M, Pennuto M. Rocchi A, et al. Acta Neuropathol. 2016 Jul;132(1):127-44. doi: 10.1007/s00401-016-1550-4. Epub 2016 Mar 12. Acta Neuropathol. 2016. PMID: 26971100 Free PMC article. - X-Linked Spinal and Bulbar Muscular Atrophy: From Clinical Genetic Features and Molecular Pathology to Mechanisms Underlying Disease Toxicity.
Cortes CJ, La Spada AR. Cortes CJ, et al. Adv Exp Med Biol. 2018;1049:103-133. doi: 10.1007/978-3-319-71779-1_5. Adv Exp Med Biol. 2018. PMID: 29427100 Review. - Pathogenesis and therapy of spinal and bulbar muscular atrophy (SBMA).
Katsuno M, Tanaka F, Adachi H, Banno H, Suzuki K, Watanabe H, Sobue G. Katsuno M, et al. Prog Neurobiol. 2012 Dec;99(3):246-56. doi: 10.1016/j.pneurobio.2012.05.007. Epub 2012 May 15. Prog Neurobiol. 2012. PMID: 22609045 Review.
Cited by
- Bone metastases induce metabolic changes and mitophagy in mice.
Wilcox-Hagerty J, Xu H, Hain BA, Arnold AC, Waning DL. Wilcox-Hagerty J, et al. Exp Physiol. 2021 Feb;106(2):506-518. doi: 10.1113/EP089130. Epub 2021 Jan 6. Exp Physiol. 2021. PMID: 33369797 Free PMC article. - LSD1/PRMT6-targeting gene therapy to attenuate androgen receptor toxic gain-of-function ameliorates spinobulbar muscular atrophy phenotypes in flies and mice.
Prakasam R, Bonadiman A, Andreotti R, Zuccaro E, Dalfovo D, Marchioretti C, Tripathy D, Petris G, Anderson EN, Migazzi A, Tosatto L, Cereseto A, Battaglioli E, Sorarù G, Lim WF, Rinaldi C, Sambataro F, Pourshafie N, Grunseich C, Romanel A, Pandey UB, Contestabile A, Ronzitti G, Basso M, Pennuto M. Prakasam R, et al. Nat Commun. 2023 Feb 6;14(1):603. doi: 10.1038/s41467-023-36186-9. Nat Commun. 2023. PMID: 36746939 Free PMC article. - Fusion or Fission: The Destiny of Mitochondria In Traumatic Brain Injury of Different Severities.
Di Pietro V, Lazzarino G, Amorini AM, Signoretti S, Hill LJ, Porto E, Tavazzi B, Lazzarino G, Belli A. Di Pietro V, et al. Sci Rep. 2017 Aug 23;7(1):9189. doi: 10.1038/s41598-017-09587-2. Sci Rep. 2017. PMID: 28835707 Free PMC article. - Targeting Mitochondrial Network Disorganization is Protective in C. elegans Models of Huntington's Disease.
Machiela E, Rudich PD, Traa A, Anglas U, Soo SK, Senchuk MM, Van Raamsdonk JM. Machiela E, et al. Aging Dis. 2021 Oct 1;12(7):1753-1772. doi: 10.14336/AD.2021.0404. eCollection 2021 Oct. Aging Dis. 2021. PMID: 34631219 Free PMC article. - Chronic Intermittent Hypoxia-Induced Diaphragm Muscle Weakness Is NADPH Oxidase-2 Dependent.
Drummond SE, Burns DP, El Maghrani S, Ziegler O, Healy V, O'Halloran KD. Drummond SE, et al. Cells. 2023 Jul 12;12(14):1834. doi: 10.3390/cells12141834. Cells. 2023. PMID: 37508499 Free PMC article.
References
- Kennedy W.R., Alter M., Sung J.H. (1968) Progressive proximal spinal and bulbar muscular atrophy of late onset. A sex-linked recessive trait. Neurology, 18, 671–680. - PubMed
- La Spada A.R., Wilson E.M., Lubahn D.B., Harding A.E., Fischbeck K.H. (1991) Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature, 352, 77–79. - PubMed
- Orr H.T., Zoghbi H.Y. (2007) Trinucleotide repeat disorders. Annu.Rev. Neurosci., 30, 575–621. - PubMed
- Katsuno M., Adachi H., Kume A., Li M., Nakagomi Y., Niwa H., Sang C., Kobayashi Y., Doyu M., Sobue G. (2002) Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Neuron, 35, 843–854. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous