Transcription Activator Interactions with Multiple SWI/SNF Subunits (original) (raw)
Abstract
We have previously shown that the yeast SWI/SNF complex stimulates in vitro transcription from chromatin templates in an ATP-dependent manner. SWI/SNF function in this regard requires the presence of an activator with which it can interact directly, linking activator recruitment of SWI/SNF to transcriptional stimulation. In this study, we determine the SWI/SNF subunits that mediate its interaction with activators. Using a photo-cross-linking label transfer strategy, we show that the Snf5, Swi1, and Swi2/Snf2 subunits are contacted by the yeast acidic activators, Gcn4 and Hap4, in the context of the intact native SWI/SNF complex. In addition, we show that the same three subunits can interact individually with acidic activation domains, indicating that each subunit contributes to binding activators. Furthermore, mutations that reduce the activation potential of these activators also diminish its interaction with each of these SWI/SNF subunits. Thus, three distinct subunits of the SWI/SNF complex contribute to its interactions with activation domains.
The yeast SWI/SNF complex alters nucleosome structure in an ATP-dependent reaction, and SWI/SNF activity can lead to the relief of chromatin-mediated repression of transcription (for reviews, see references 30, 50, 64, and 70). SWI/SNF is part of a large family of ATP-dependent chromatin remodeling enzymes, which have been purified and characterized from yeast, Drosophila, Xenopus, and human sources. Yeast SWI/SNF consists of 11 identified subunits, including a highly conserved ATPase, Swi2/Snf2. A major consequence of SWI/SNF action is the increased accessibility of transcription factors to nucleosomal DNA (10). The complex is able to catalyze the movement of histones along DNA in cis, as well as the displacement of histones from DNA in trans (47, 51, 68). Recently, it was shown that SWI/SNF generates superhelical torsion within linear DNA fragments and changes in DNA twist, providing a means for the repositioning of nucleosomes along DNA (17, 24).
Yeast SWI/SNF is able to bind to both DNA and nucleosomes; however, it does so without sequence specificity (9, 52). DNA expression microarray analysis has shown that levels of only a subset (ca. 5%) of yeast genes are affected by the loss of SWI/SNF ATPase activity, including the acid phosphatase and mating-type specific genes (25, 56). Many recent studies have indicated that yeast SWI/SNF and its human counterparts are able to interact with sequence-specific transcription factors, which may recruit the complex to specific genes. A human SWI/SNF complex, E-RC1, functionally and physically interacts with erythroid Krüppel-like factor (EKLF) to increase transcription of the β-globin gene (2, 27, 36), and C/EBPβ interacts with hSWI/SNF to activate myeloid genes (33). Recently, BRG1-containing hSWI/SNF was shown to associate with human heat shock factor 1 (hHSF1) (57). Interactions between SWI/SNF and the glucocorticoid receptor have also been reported (16, 45, 65, 73).
Work from our lab and others has shown that purified yeast SWI/SNF directly interacts with the acidic activation domains of the herpesvirus VP16 protein, yeast Gcn4, and yeast Hap4 (43, 44, 74). Yeast SWI/SNF does not interact with glutamine-rich or proline-rich activators (44, 74). Additionally, the yeast activator Swi5 has been shown to interact directly with SWI/SNF in vitro and to recruit the complex to the HO endonuclease gene in vivo (8, 35, 44). The yeast SWI/SNF complex is able to activate transcription from chromatin templates in vitro. However, in the presence of an excess of nonspecific competitor chromatin, transcription stimulation by the complex requires an activator that can target it to the promoter (44).
SWI/SNF has also been shown to play a role in the repression of some genes (41, 60). Genome wide microarray analysis showed that a subset of yeast genes was upregulated by inactivation of SWI/SNF activity (25, 56). Indeed, it was reported that yeast SWI/SNF can interact with gene-specific repressors (12), suggesting that promoter-specific recruitment is a common mechanism for activation and repression of SWI/SNF-regulated genes.
It is becoming apparent that many genes require the function of both ATP-dependent remodeling complexes and histone acetyltransferase (HAT) complexes for their full expression (reference 15 and references therein). Yeast HAT complexes have been shown to interact with a similar set of acidic activators as SWI/SNF (5, 61). By itself, yeast SWI/SNF requires the continuous binding of the activator to remain associated with the promoter in vitro (23). Importantly, histone acetylation by these HAT complexes is able to retain SWI/SNF at the promoter once the activator dissociates (23), linking the functions of HAT complexes and SWI/SNF and indicating an importance for their sequential recruitment to the same promoter.
In this study, we determined the subunits of the yeast SWI/SNF complex that are able to physically interact with activators. We show that three different evolutionarily conserved subunits—Snf5, Swi1, and Swi2/Snf2—can directly contact acidic activators individually, as well as when part of the intact complex.
MATERIALS AND METHODS
Purification of GST fusion proteins.
Glutathione _S_-transferase (GST) fusion proteins were expressed in bacteria and purified essentially as described in the manufacturer's protocol (Amersham Pharmacia Biotech, Piscataway, N.J.). In brief, cells were grown to an optical density at 600 nm (OD600) of ca. 1.0. GST, GST-VP16, GST-VP16Δ456, GST-Hap4, GST-Pho4FL, GST-Pho4AD, and GST-Swi5FL were induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 3 h at 37°C. GST-Gcn4 and GST-Gcn4[3,5,6,7] were induced with 0.25 mM IPTG for 4 to 6 h at 30°C. Cells were harvested and resuspended in 1× phosphate-buffered saline (PBS) containing 1 mM phenylmethylsulfonyl fluoride (PMSF), 2 μg of leupeptin/ml, 2 μg of pepstatin A/ml, and 0.5 mM dithiothreitol (DTT). The fusion proteins were cross-linked to glutathione-Sepharose 4B with dimethylpimelimidate as described previously for coupling antibodies (22). Protein purity and cross-linking efficiency were checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie blue staining. For GST pull-down experiments, protein amounts were normalized by Coomassie blue staining and compared to a titration of bovine serum albumin (BSA). For far-Western analyses, GST fusion proteins were eluted from the resin with glutathione, and protein amounts were determined by a Bradford protein assay (Bio-Rad, Hercules, Calif.). For photo-cross-linking experiments, the GST tag was cleaved from Gcn4 by using biotin-tagged Factor Xa protease (Roche Molecular Biochemicals, Geneva, Switzerland) that was subsequently removed from the Gcn4 by using streptavidin resin. The GST was separated from the Gcn4 after cleavage by using glutathione Sepharose 4B.
Affinity purification of the yeast SWI/SNF complex.
Yeast SWI/SNF complex was purified from strain YJW426 (this study), which contains a COOH-terminal FLAG tag integrated at the genomic SNF6 gene in strain CY396 (10). The Snf6 open reading frame (ORF) and ca. 1 kb of upstream sequence were amplified by PCR and cloned in frame with a COOH-terminal FLAG tag, followed by 250 bp of CYC1 downstream sequence into YIplac128. The FLAG tag was integrated by homologous recombination by using a PCR product that contained the COOH terminus of the Snf6 ORF, the FLAG tag and CYC1 terminator sequence, the LEU2 gene sequence, and the Snf6 downstream sequence. The integration of the FLAG tag at the SNF6 locus was verified by PCR. The yeast cells were grown in synthetic complete (lacking −leucine) media to an OD600 of ca. 1.0. The preparation of the whole-cell extract and purification over Ni2+-nitrilotriacetic acid (NTA) agarose was carried out as described previously (19), except with buffers described elsewhere (38). The 500 mM imidazole elution was directly loaded onto a 1-ml MonoQ ion-exchange column, and bound proteins were eluted with a 25-ml gradient of 100 to 500 mM NaCl MonoQ buffer (19). Fractions were monitored for the presence of SWI/SNF complex by immunoblotting with SWI/SNF-specific antibodies, and fractions containing SWI/SNF were pooled and incubated with 100 μl of FLAG M2 agarose affinity gel (Sigma, St. Louis, Mo.). SWI/SNF was eluted in 100 μl of MonoQ buffer (250 mM NaCl) containing 0.5 mg of FLAG peptide/ml. The FLAG resin was incubated with the FLAG peptide for ca. 45 min, while being mixed on a wheel at 4°C, and the elution was repeated three more times. The purity of the complex was examined by SDS-PAGE and silver staining. The approximate concentration of SWI/SNF complex in each fraction was determined by using quantitative Western blotting and comparing it to a standard curve of recombinant GST-TafII30.
Photo-cross-linking label transfer assays.
Photo-cross-linking experiments were done as described previously (1), with the following variations. Recombinant GST-Hap4, Gcn4, and GST were incubated with 30 eq of Sulfo-SBED {sulfosuccinimidyl [2-6-(biotinamido)-2-(_p_-azidobenzamido)-hexanoamido] ethyl-1,3′-dithiopropionate; Pierce, Rockford, Ill.} in dimethyl fluoride to conjugate the cross-linking reagent to the activator. The excess cross-linking reagent was removed from the GST-Hap4 and Gcn4 by MonoQ ion-exchange chromatography. GST-Hap4 and Gcn4 bound the column and were eluted with a salt step gradient. Fractions containing peak amounts of activator were determined by immunoblotting with streptavidin-horseradish peroxidase (HRP), which recognizes the biotin tag on the cross-linking reagent. GST that was conjugated with Sulfo-SBED was separated from excess cross-linking reagent by running through a G25 Sepharose spin column. The conjugated activator (ca. 40 nM of GST-Hap4 and 60 nM of GST as the negative control) and a 300 nM concentration of Gcn4 was mixed in a 1.5-ml microfuge tube with affinity-purified SWI/SNF complex (30 to 100 nM) in a pull-down buffer (50 mM HEPES, pH 7.5; 150 mM NaCl; 10% glycerol; 0.1% Tween 20) in a 20-μl reaction mixture. The reaction mixture was exposed to UV light (XX-15B lamp; Spectroline, Westbury, N.Y.) at a distance of 6 cm for 8 min at room temperature. A quantity of 4× SDS sample buffer (lacking DTT and β-mercaptoethanol) containing 8 mM _N_-ethylmaleimide (in dimethyl fluoride) was added, and the reaction mixtures were incubated for 10 min at room temperature. DTT was added to a final concentration of 100 mM, and 18 μl of each reaction mixture was loaded on a 4 to 15% (or 5%, Fig. 3B) polyacrylamide gel. The proteins were transferred to polyvinylidene difluoride (PVDF) membrane, blocked for 30 min with 3% BSA (in PBS-Tween 20 [PBS-T]), and probed with a 1:3,000 dilution of streptavidin-HRP (Gibco-BRL, Rockville, Md.) for 1 h. After the proteins were rinsed with PBS-T, the blot was incubated with 2% milk for 30 min, which helps to reduce the background. Finally, the blots were rinsed again in PBS-T and developed by enhanced chemiluminescence (Amersham Pharmacia Biotech). The blots were stripped prior to probing with SWI/SNF-specific antisera by incubating the blots in 62.5 mM Tris (pH 6.8), 2% SDS, and 100 mM β-mercaptoethanol for 10 min at 50°C, while rotating the blots in a hybridization oven. It seems that a percentage of activator bound subunit is resistant to DTT cleavage, since there is a reduction in the SWI/SNF subunit signal after exposure to UV light. This is most notable for the Swi2/Snf2 subunit, which actually runs as a doublet after exposure to UV light (Fig. 2C, lanes 4 and 12). The slower-migrating product most likely represents Swi2/Snf2 that remains covalently attached to the activator.
FIG. 3.
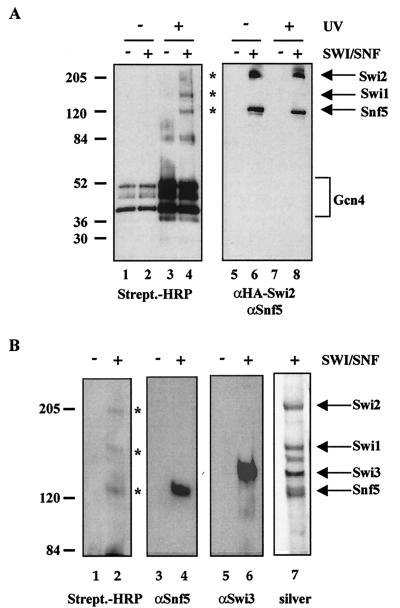
Interaction of Snf5, Swi1, and Swi2/Snf2 in the context of intact SWI/SNF with Gcn4. (A) Photo-cross-linking reactions were carried out as in Fig. 2C, except that full-length Gcn4 (the GST tag was cleaved and removed) was conjugated with the cross-linking reagent and incubated with purified SWI/SNF. The asterisks indicate photo-cross-linked SWI/SNF subunits. (B) Reaction mixtures identical to those shown in panel A were separated on an SDS-5% polyacrylamide gel, instead of an SDS-4 to 15% gradient polyacrylamide gel. The asterisks indicate photo-cross-linked products.
FIG. 2.
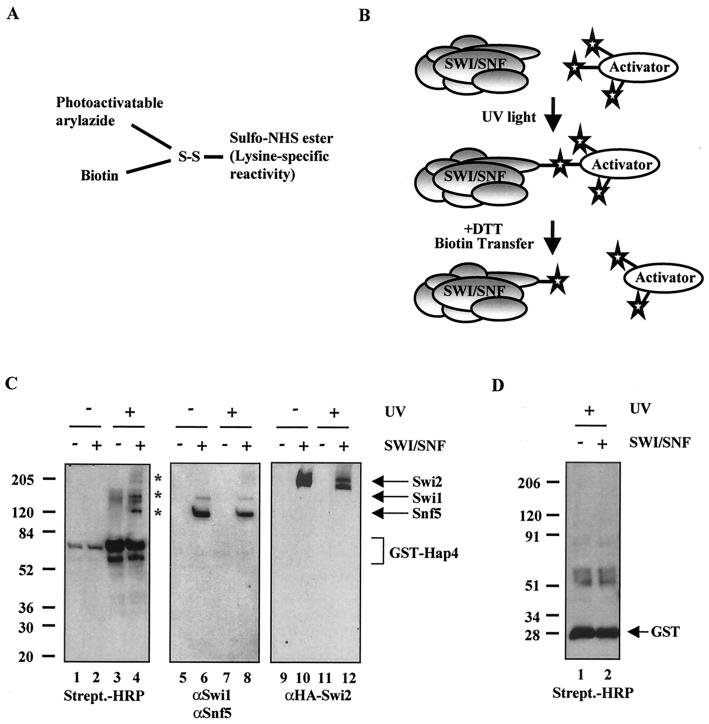
Interaction of Snf5, Swi1, and Swi2/Snf2 in the context of intact SWI/SNF with Hap4. (A) Diagram of the photo-cross-linking reagent Sulfo-SBED. (B) Schematic of the photo-cross-linking experiments. Photo-cross-linking experiments were performed with purified SWI/SNF complex and an activator that was previously conjugated with Sulfo-SBED. Where indicated, the reaction mixtures were exposed to UV light (312 nm) at 6 cm for 8 min at room temperature. The activator was separated from interacting SWI/SNF subunits upon the addition of DTT, which reduces the disulfide bond. (C) Snf5, Swi1, and Swi2/Snf2 are cross-linked by GST-Hap4. The photo-cross-linking reaction mixtures were run on an SDS-4 to 15% gradient polyacrylamide gel. The proteins were transferred to PVDF membrane and immunoblotted with streptavidin-HRP or the indicated SWI/SNF-specific antibodies. The asterisks indicate photo-cross-linked SWI/SNF subunits. (D) SWI/SNF subunits are not cross-linked by GST alone. The photo-cross-linking experiment was performed as described for panels B and C.
Identification of photo-cross-linked products was confirmed by streptavidin-agarose pull-down analyses under denaturing conditions. Photo-cross-linking reactions were carried out as described above, except following UV exposure, a 4× SDS sample buffer (lacking bromophenol blue dye, DTT, and β-mercaptoethanol) containing 8 mM _N_-ethylmaleimide was added, in which the final SDS concentration was ca. 2%. DTT was then added as described above to a final concentration of 100 mM. The samples were then heated to 95°C for 5 min and cooled slowly to room temperature. Twenty volumes of radioimmunoprecipitation assay (RIPA) buffer (lacking SDS) (150 mM NaCl, 1% NP-40, 0.5% deoxycholic acid, 50 mM Tris [pH 8.0]), including 1 mM PMSF, 2 μg of pepstatin A/ml, and 2 μg of leupeptin/ml, were added to each reaction. The final concentration of SDS was then brought up to ca. 0.5%. The reaction mixtures were then incubated with streptavidin-agarose beads (ImmunoPure Immobilized Streptavidin; Pierce) that were previously washed in RIPA buffer and mixed gently overnight on a wheel at 4°C. The beads were washed three times in RIPA buffer, and the protein associated with the beads was loaded on a 4 to 15% polyacrylamide gel and transferred to a PVDF membrane, and the presence of SWI/SNF subunits was detected by using SWI/SNF-specific antisera.
SWI/SNF subunit constructs and in vitro transcription and/or translation.
Each SWI/SNF gene was amplified by PCR from yeast genomic DNA. The ORF sequences were cloned in frame into the vector pRSETA (Invitrogen, Carlsbad, Calif.), which contains a T7 promoter and an amino-terminal His6 tag. The proteins were expressed with T7 polymerase by using a TNT-coupled reticulocyte lysate system (Promega, Madison, Wis.) that generated [35S]methionine-labeled product. The reactions were carried out as described in the manufacturer's protocol, with 1 μg of DNA in each reaction. The translated proteins were analyzed by SDS-PAGE and fluorography (En3Hance was from NEN Life Science Products, Boston, Mass.).
GST pull-down assays.
GST fusion proteins (3 to 6 μg) cross-linked to glutathione-Sepharose 4B resin were incubated with 5 μl of the reticulocyte lysate reaction mixture containing a labeled SWI/SNF subunit in a pull-down buffer (50 mM HEPES, pH 7.5; 1 mM EDTA; 150 mM NaCl; 10% glycerol; 0.1% Tween 20; 0.5 mM DTT; 1 mM PMSF; 2 μg of leupeptin/ml; 2 μg of pepstatin A/ml) for at least 2 h at 4°C, while being mixed on a rotating wheel. The beads were washed three times with pull-down buffer and resuspended in 1× SDS loading dye. A total of 50% of the input and supernatants and 100% of the proteins associated with the beads were subjected to SDS-PAGE and fluorography, and radiolabeled SWI/SNF subunits were visualized by autoradiography. GST pull-down assays from yeast whole-cell extracts were done similarly, except that 500 μg of total yeast protein was incubated with the GST fusion proteins and the presence of SWI/SNF in the supernatant or associated with the beads was monitored by immunoblotting. In the experiment shown in Fig. 7A, affinity-purified SWI/SNF complex was added with the GST fusion proteins, and the presence of SWI/SNF in the supernatants or associated with the beads was detected by immunoblotting with SWI/SNF-specific antisera.
FIG. 7.
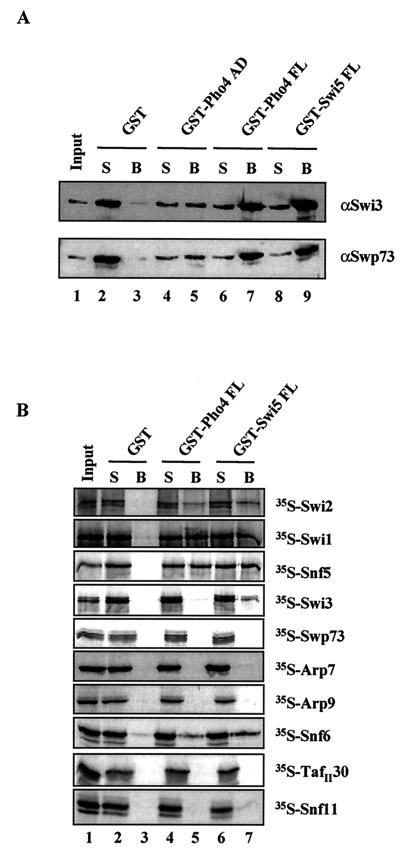
Interaction of SWI/SNF subunits with the yeast activators Pho4 and Swi5. (A) Purified SWI/SNF interacts with the acidic activator Pho4. GST pull-down assays were done with the GST fusion proteins indicated and affinity-purified SWI/SNF complex. The presence of SWI/SNF in the supernatant or associated with the beads was detected by immunoblotting with antibodies against the SWI/SNF subunits Swi3 and Swp73. (B) Individual SWI/SNF subunit interactions with Pho4 and Swi5. GST pull-down assays were performed as for Fig. 5 with the GST fusion proteins and 35S-labeled SWI/SNF subunits indicated. Radiolabeled SWI/SNF subunits were visualized by autoradiography.
Far-Western assays.
Far-Western assays were done primarily as described previously (37). Approximately 6 μg of each GST fusion protein was subjected to SDS-PAGE and transferred to a PVDF membrane. The protein blot was denatured, renatured, and blocked with BSA prior to incubation in a 20-ml volume of 2% milk supplemented with a 50-μl reticulocyte lysate transcription and translation reaction mixture containing a 35S-labeled SWI/SNF subunit. After multiple washes, the blot was dried, and protein-protein interactions were visualized by autoradiography. The signal was enhanced by using a low-energy-intensifying screen (Kodak).
RESULTS
Targets for acidic activators within intact SWI/SNF complex.
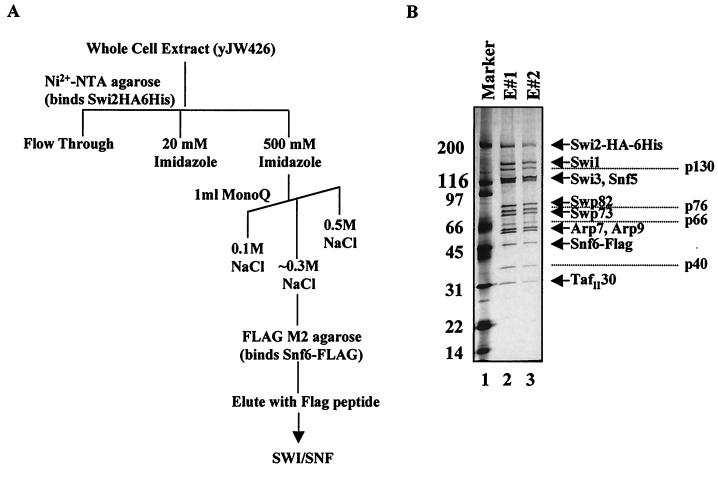
To determine the subunits of the SWI/SNF complex mediating its interaction with acidic activation domains, we utilized a photo-cross-linking affinity label transfer method. This method was recently used to determine that Tra1 was a target of activators within the SAGA and NuA4 yeast HAT complexes (1, 5). A similar strategy was previously used to establish Srb4 as an activator target within RNA polymerase II holoenzyme (32). For these experiments, we used highly purified yeast SWI/SNF complex prepared by using a double affinity purification method (Fig. 1A). A COOH-terminal FLAG tag was integrated at the SNF6 locus in strain CY396, which contains a COOH-terminal HA and histidine tag on the Swi2/Snf2 subunit (10). The whole-cell extract was purified over Ni2+-NTA agarose, followed by ion-exchange chromatography. Peak fractions that contained SWI/SNF were pooled and incubated with FLAG M2 agarose affinity gel and subsequently eluted with FLAG peptide. The SWI/SNF from this purification is largely free of contaminating proteins as determined by SDS-PAGE and silver staining (Fig. 1B, lanes 2 and 3).
FIG. 1.
Affinity purification of the yeast SWI/SNF complex. (A) Diagram of the SWI/SNF purification scheme. Double affinity-purified yeast SWI/SNF complex was used in photo-cross-linking experiments. (B) The purified complex was separated on an SDS-4 to 15% gradient polyacrylamide gel and found to be largely free of contaminating proteins as visualized by silver staining. Known SWI/SNF subunits are indicated to the right of the panel, and unknown proteins are labeled according to their approximate molecular weight. E#1 and E#2 indicate two subsequent Flag peptide elutions from the Flag M2 agarose affinity gel.
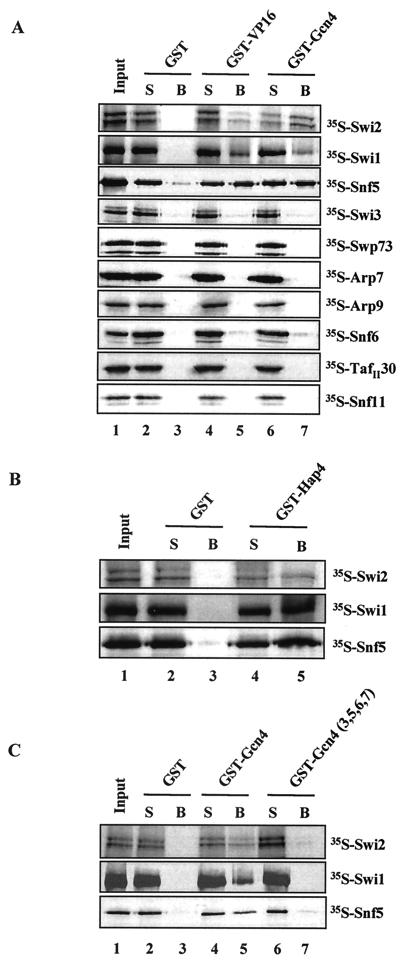
For the photo-cross-linking experiments, we used the cross-linking reagent Sulfo-SBED, which is composed of a sulfo-NHS ester that specifically reacts with lysines, a photoactivatable arylazide, a disulfide bond, and a biotin label (Fig. 2A) (Pierce). A schematic of the photo-cross-linking assay is shown in Fig. 2B. First, the photo-cross-linking reagent was conjugated to lysines within the acidic activator. The purified conjugated activator was then incubated with affinity-purified SWI/SNF complex (Fig. 1) and exposed to UV light (312 nm). Upon UV exposure, the arylazide forms a covalent linkage with SWI/SNF subunits that are located within ca. 20 Å of the site of interaction. The disulfide bond is then reduced with DTT, separating the activator from the biotin arylazide on any SWI/SNF subunits that it interacted with (Fig. 2B). The transferred biotin label is detected by immunoblotting with HRP-conjugated streptavidin. We found that GST-Hap4 cross-linked to Snf5, Swi1, and Swi2/Snf2 (Fig. 2C, lane 4).
We initially chose to conjugate GST-Hap4 with the cross-linking reagent, due to the availability of lysines within its acidic activation domain. The region of Hap4 used in this study was amino acids 330 through 554, which contains 14 lysines. Of additional note is that the GST portion of this fusion protein contains 21 lysines. The detection of the biotin-labeled (i.e., cross-linked) subunits was dependent on SWI/SNF (Fig. 2C, compare lanes 3 and 4), UV exposure (Fig. 2C, compare lanes 2 and 4), and the addition of DTT (data not shown). The membranes were stripped and reprobed, or the remaining portion of the reaction mixture was analyzed with Snf5, Swi1, and Swi2/Snf2 (HA)-specific antibodies to indicate the mobility of these subunits by SDS-PAGE (Fig. 2C, lanes 5 through 12). To rule out that the photo-cross-linking reagent conjugated to lysines within the GST portion of the fusion protein was leading to the biotin-labeled products, we performed the experiment with GST alone and found that it does not significantly cross-link to any SWI/SNF subunits (Fig. 2D). The GST-Hap4 is biotin labeled (doublet at ca. 80 kDa) upon irradiation with UV light due to the dimerization of the GST portion of this fusion protein (Fig. 2C, compare lanes 1 and 2 to lanes 3 and 4). This background precluded the detection of possible activator targets in this region of the gel.
To determine whether a different yeast acidic activator contacts the same subunits within SWI/SNF or has unique subunit targets, we conjugated full-length Gcn4 with the lysine-specific cross-linking reagent. Gcn4 contains nine lysines within its acidic activation domain. We found that Snf5, Swi1, and Swi2/Snf2 were biotin labeled when UV cross-linked with conjugated Gcn4 (Fig. 3A, lane 4), which is the same result that we found with GST-Hap4. Again, the photo-cross-linked products were dependent on UV exposure (Fig. 3A, compare lanes 2 and 4) and the presence of the SWI/SNF complex (Fig. 3A, compare lanes 3 and 4). Gcn4 contains a leucine zipper domain that allows dimerization, leading to the background biotin labeling of Gcn4 itself. The membranes were stripped and immunoblotted with Snf5- and Swi2-specific antibodies to indicate their electrophoretic mobility (Fig. 3A, lanes 5 through 8). These results demonstrate that two yeast activators, Hap4 and Gcn4, contact the same three subunits within the native SWI/SNF complex.
The Snf5 and Swi3 subunits of yeast SWI/SNF run very close together on the 4 to 15% polyacrylamide gradient gels used in the above photo-cross-linking experiments (see Fig. 1, lanes 2 and 3). To examine whether Swi3 is an additional target for acidic activators within yeast SWI/SNF, the photo-cross-linking experiment above was repeated, followed by separation of the proteins on a 5% polyacrylamide gel. Snf5 and Swi3 were clearly separated as indicated by silver staining of the purified SWI/SNF complex used in the reaction (Fig. 3B, lane 7). We found that Snf5, along with Swi1 and Swi2/Snf2, were cross-linked by conjugated Gcn4, whereas Swi3 was not significantly biotin labeled (Fig. 3B, lane 2). The membrane was subsequently immunoblotted with Snf5- and Swi3-specific antisera to indicate the mobility of these two SWI/SNF subunits (Fig. 3B, lanes 4 and 6).
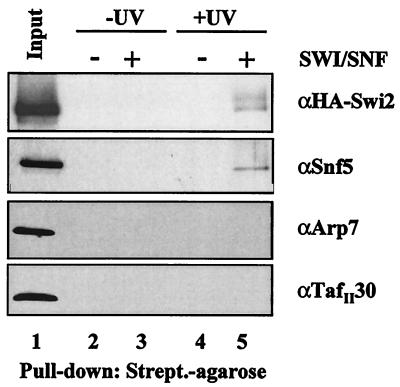
As an additional approach to confirm the identity of the subunits that are biotin labeled during the photo-cross-linking experiments, we used a denaturing pull-down assay. The photo-cross-linking reaction was carried out as in Fig. 3A and B with the Gcn4 activator. After exposure to UV light, the reaction mixtures were heat denatured and incubated with streptavidin-agarose beads. Subunits that were biotin labeled during the photo-cross-linking reaction were able to associate with the streptavidin resin. The proteins associated with the bead fraction were then separated on a 4 to 15% polyacrylamide gel and transferred to a PVDF membrane, followed by immunoblotting with SWI/SNF-specific antisera. We found that both the Snf5 and Swi2/Snf2 subunits are pulled down with the streptavidin-agarose, whereas other SWI/SNF subunits, Arp7 and TafII30, are not (Fig. 4). The presence of Snf5 and Swi2/Snf2 associated with the beads is dependent on both UV exposure (Fig. 4, compare lanes 3 and 5) and the presence of SWI/SNF complex (Fig. 4, compare lanes 4 and 5). Double affinity-purified SWI/SNF complex is used as a control for the Western analysis in this experiment and represents the approximate amount of complex that was put into the initial photo-cross-linking reactions (Fig. 4, lane 1). The Swi1 antibody was barely able to detect the Swi1 present in the input lane, so Swi1 was not picked up in the experimental lanes (data not shown). The strength of the antisera is a critical variable in this assay. We conclude that this approach strengthens our finding that Snf5 and Swi2/Snf2 are targets for acidic transcription activators.
FIG. 4.
Identification of biotinylated SWI/SNF subunits after photo-cross-linking. Reaction mixtures identical to those shown in Fig. 3 were heat denatured in the presence of SDS, followed by incubation with streptavidin-agarose beads. The precipitated material was separated on an SDS-4 to 15% gradient polyacrylamide gel, transferred to PVDF, and immunoblotted with SWI/SNF-specific antisera.
Individual interaction of Snf5, Swi1, and Swi2/Snf2 with acidic activators.
The cross-linking data presented above indicate that Snf5, Swi1, and Swi2/Snf2 are all contacted or are in close proximity to activators bound to the SWI/SNF complex. To determine which of these subunits actually contributes directly to activator binding, we tested recombinant forms of these and other SWI/SNF subunits individually for activator interaction. The ORF of each subunit was amplified from yeast genomic DNA by PCR, cloned under the control of the T7 promoter, and expressed by using an in vitro transcription and translation system. Radiolabeled methionine was incorporated into the proteins during translation with a rabbit reticulocyte lysate. To test the ability of each subunit to interact with acidic activators, we used GST pull-down assays. GST activation domain fusion proteins were incubated with radiolabeled SWI/SNF subunits, and the presence of the SWI/SNF subunit in the supernatant or associated with the GST activator-bound beads was detected by fluorography.
Snf5, Swi1, and Swi2/Snf2 interacted with the activation domain of VP16 (amino acids 413 to 490), as well as the full-length yeast activator, Gcn4, as indicated by the presence of each radiolabeled subunit in the bound fraction (Fig. 5A, lanes 5 and 7). The same three subunits were also able to interact with the acidic activation domain of yeast Hap4 (amino acids 330 to 554) (Fig. 5B, lane 5). The Snf6 subunit interacted weakly with acidic activators by using this assay. We were unable to clone the Swp82 subunit, because the sequence is unpublished (B. Cairns, unpublished data). As an alternative, the SWI/SNF complex present in a _swp82_Δ strain was analyzed for its ability to interact with activators. Whole-cell extracts were prepared from both a wild-type and _swp82_Δ strain and GST pull-down assays were performed. GST-acidic activator fusion proteins were able to pull down SWI/SNF subunits from both extracts (data not shown), suggesting that Swp82 was not critical for the interaction of SWI/SNF and the activators tested. However, due to the possible redundancy among subunits, we cannot rule out that Swp82 contributes to activator interaction.
FIG. 5.
Interaction of Snf5, Swi1, and Swi2/Snf2 with acidic activators but not with activation domain mutants by GST pull-down analysis. (A) GST pull-down assays were performed with the GST fusion proteins indicated bound to glutathione-Sepharose beads and individually expressed 35S-labeled SWI/SNF subunits. A total of 50% of each input and supernatant (S) and 100% of the proteins associated with the beads (B) were loaded on SDS-8, 10, or 12% polyacrylamide gels, depending on the size of the labeled subunit. The gels were fixed, enhanced, and dried, and radiolabeled proteins were visualized by autoradiography. (B) GST pull-down assays were performed as for panel A, with GST or GST-Hap4 (amino acids 330 through 554) and the 35S-labeled SWI/SNF subunits indicated. (C) GST pull-down as-says were performed as for panels A and B, with the GST fusion proteins and 35S-labeled SWI/SNF subunits indicated. Gcn4[3,5,6,7] contains mutations in hydrophobic residues within its activation domain that reduce its activation potential.
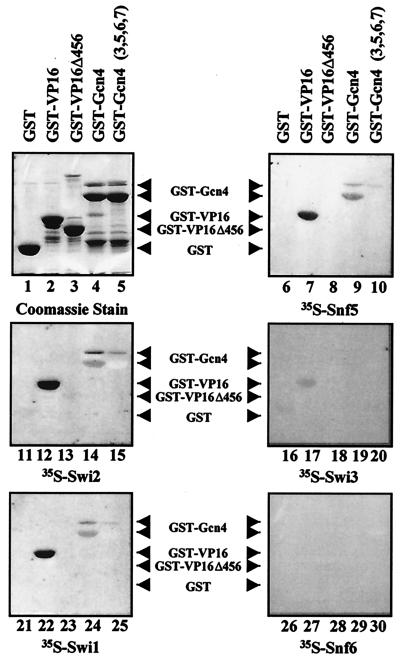
To confirm the results of the GST pull-down experiments, we also examined the ability of SWI/SNF subunits to interact with acidic activators by far-Western analysis. GST-activator fusion proteins were separated by SDS-PAGE and transferred to a PVDF membrane. The proteins were subjected to a series of guanidine-HCl incubations for renaturation, and then the membranes were incubated with a rabbit reticulocyte lysate reaction mixture containing a radiolabeled SWI/SNF subunit. Snf5, Swi1, and Swi2/Snf2 were able to interact with both the activation domain of VP16 and full-length Gcn4, which is consistent with our pull-down results (Fig. 6, lanes 7 and 9 for Snf5, lanes 12 and 14 for Swi2/Snf2, and lanes 22 and 24 for Swi1). Preparations of GST-Gcn4 contain two major products (Fig. 6, lanes 4 and 5), and both have been reported to contain the activation domain (43). Snf5, Swi1, and Swi2/Snf2 all appear to interact more strongly with the full-length activator. Swi3 and Snf6 did not significantly interact with either VP16 or Gcn4 (Fig. 6, lanes 17 and 19 for Swi3 and lanes 27 and 29 for Snf6). The Coomassie blue-stained gel indicates the mobility of each fusion protein on SDS-PAGE (Fig. 6, lanes 1 to 5).
FIG. 6.
Interaction of Snf5, Swi1, and Swi2/Snf2 with acidic activators but not with activation domain mutants by far-Western analysis. Far-Western analysis was used to confirm the interactions of Snf5, Swi1, and Swi2/Snf2 with GST-VP16 and GST-Gcn4. GST fusion proteins were run on a SDS-10% polyacrylamide gel and transferred to PVDF membrane. After denaturation and renaturation steps, the blots were incubated with in vitro transcription and translation rabbit reticulocyte lysate reactions containing the 35S-labeled SWI/SNF subunit indicated below each panel. A COOH-terminally truncated VP16 activation domain (GST-VP16Δ456) and full-length Gcn4 that contained hydrophobic cluster mutations in its activation domain (GST-Gcn4 [3,5,6,7]) were also tested for interaction with the 35S-labeled subunits. The interaction of the radiolabeled subunits with the GST fusions on the blot was visualized by autoradiography.
Effect of activation domain mutations on the interaction of Snf5, Swi1, and Swi2/Snf2 with acidic activators.
It has previously been shown that mutations in the activation domains of transcription activators reduced or eliminated interaction with the SWI/SNF complex (43, 44). In this study, we demonstrate that the interaction of individual SWI/SNF subunits with activators was also affected by the same mutations, indicating that these interactions are specific. A COOH-terminal truncated form of the VP16 activation domain (VP16Δ456), known to reduce the transcriptional activity of Gal4-VP16 to 30 to 50% of that of the wild type (3, 59), was unable to interact with Snf5, Swi1, and Swi2/Snf2 as determined by far-Western analysis (Fig. 6, compare lanes 7 and 8 for Snf5, lanes 12 and 13 for Swi2/Snf2, and lanes 22 and 23 for Swi1). Additionally, GST-VP16Δ456 did not interact with these subunits as determined by pull-down assays (data not shown). Hydrophobic cluster mutations within the Gcn4 activation domain have been shown to affect its transcriptional activity (13, 26). Gcn4[3,5,6,7], a mutant that has 10 hydrophobic residues within the activation domain changed to alanines, was previously shown to reduce the interaction of Gcn4 with purified SWI/SNF and with SWI/SNF complex in yeast whole-cell extracts (43). We found that these mutations also diminished the interaction of Gcn4 with the individual subunits Snf5, Swi1, and Swi2/Snf2 in both pull-down and far-Western experiments (Fig. 5C, compare lanes 5 and 7; Fig. 6, compare lanes 9 and 10 for Snf5, lanes 14 and 15 for Swi2/Snf2, and lanes 24 and 25 for Swi1). The finding that the same activator mutations affect interactions with three different subunits in a similar manner suggests that there might be a common mechanism of recruitment.
Interaction of SWI/SNF subunits with the yeast Pho4 and Swi5 activators.
We extended our study by investigating the interaction of SWI/SNF with the yeast activators, Pho4 and Swi5, which associate with SWI/SNF-dependent genes (for examples, see references 8, 20, and 34). Genome-wide microarray gene expression analysis has found that many genes in the acid phosphatase family are downregulated in yeast strains deficient in SWI/SNF activity (25, 56). For example, the expression of PHO84, which encodes a high-affinity phosphate transporter, was found to be reduced 10- or 30-fold in rich media in two independent studies (25, 56). PHO84 is regulated by the Pho4 activator and, in addition, the expression of two other Pho4-dependent genes encoding secreted acid phosphatases, PHO11 and PHO12, is also reduced in a Swi2/Snf2 mutant (25, 56). The PHO8 gene is another example of a Pho4-dependent gene that also requires SWI/SNF to alter the chromatin structure of its promoter (20). The cell cycle-regulated yeast Swi5 activator has been shown to bind the upstream region of the HO endonuclease gene, and there is strong evidence that this activator is responsible for recruiting the SWI/SNF complex to the HO promoter (8, 44).
We set out to determine whether similar SWI/SNF subunits that contacted Gcn4, Hap4, and VP16 were also targets for Pho4 and Swi5. First, to demonstrate that the acidic Pho4 activator was able to directly interact with purified SWI/SNF complex, we used GST pull-down assays. We tested both full-length Pho4 (amino acids 1 through 312) and a region consisting of amino acids 1 through 109, which contains an acidic activation domain (39, 46). We found that affinity-purified SWI/SNF complex was able to interact with full-length Pho4 and, albeit slightly more weakly, with only the activation domain of Pho4. This is indicated by the presence of the Swi3 and Swp73 subunits of SWI/SNF associated with the GST-Pho4 beads (Fig. 7A, lanes 4 through 7). The SWI/SNF complex did not interact with GST alone (Fig. 7A, lanes 2 and 3) and, as a positive control, the interaction between SWI/SNF and the full-length Swi5 activator is shown (Fig. 7A, lanes 8 and 9 [see also reference 44]).
Next, we used GST pull-down analysis to determine which subunits of SWI/SNF facilitate its interaction with Pho4. We found that Swi2/Snf2, Swi1, and Snf5 were each able to individually interact with full-length Pho4 (Fig. 7B, lane 5). These results were consistent with what we observed with VP16, Gcn4, and Hap4 (see Fig. 5). Additionally, we found that Snf6, a subunit that interacted very weakly with VP16 and Gcn4, interacted with Pho4 (Fig. 7B, lane 5). We also looked at the interaction of individual SWI/SNF subunits with the Swi5 activator. Again, we found that Swi2/Snf2, Swi1, Snf5, and Snf6 interacted with Swi5, in addition to a weak interaction with Swi3 (Fig. 7B, lane 7). Thus, we conclude that the Swi2/Snf2, Swi1, and Snf5 subunits of SWI/SNF are able to interact with the Pho4 and Swi5 yeast activators, confirming the results described above for VP16, Gcn4, and Hap4. Potential interactions of Pho4 with Snf6 and Swi5 with Snf6 and Swi3 would have to be confirmed with intact SWI/SNF complex before we would consider them bona fide activator interaction subunits. These additional experiments with two activators that regulate SWI/SNF-dependent genes support the idea that there is a common mechanism of SWI/SNF recruitment by different activators and that this recruitment is mediated by a common set of SWI/SNF subunits.
DISCUSSION
Multiple activator targets within one complex.
We have carried out an unbiased systematic study to determine the subunits of the yeast SWI/SNF complex that interact with transcription activators. We have demonstrated that three yeast SWI/SNF subunits, Snf5, Swi1, and Swi2/Snf2, interact directly with acidic activation domains. Importantly, each of these subunits is able to contact acidic activators while part of the intact SWI/SNF complex. Multiple targets within the same coactivator complex for activator interaction and promoter selectivity have also been suggested for TFIID complexes (6, 62), the Drosophila Brahma complex (28), and the yeast mediator complex (42). It is possible that the multiple activator targets within SWI/SNF allow for promoter selectivity, where one subunit facilitates interaction with an activator at one promoter and one of the other subunits brings SWI/SNF in contact with an activator at another promoter. Also, the subunits may mediate interaction at promoters with different affinities, and a combinatorial effect allows for a stronger interaction when needed. Other possibilities are that the three subunits form an interface that is necessary for activator interaction or that one of the subunits is more important for initiating the interaction with activators in vivo and that the others work to stabilize the association of SWI/SNF with the promoter. Alternatively, these subunits may have redundant roles in SWI/SNF recruitment. It will be critical to construct activator interaction mutants of Snf5, Swi1, and Swi2/Snf2 and to make combinations of these mutations and then use them to distinguish between these different possibilities. The generation of these mutants may prove to be difficult, considering the possible redundancy among subunits. Furthermore, the deletion of these subunits is very detrimental to the integrity and function of the complex, and many subunits seem to be dependent on others for their own stability (48, 49; K. E. Neely, unpublished observations). If the activator interaction domain of a subunit overlaps the region necessary for complex integrity or catalytic activity (in the case of Swi2/Snf2), it will be hard to determine the specific effects of a block in SWI/SNF recruitment. These studies are currently under way in our laboratory, and creating an activator interaction-deficient SWI/SNF complex will allow us to test the effects of lack of recruitment on specific gene expression.
Mechanisms of SWI/SNF recruitment in yeast and humans.
The role that individual SWI/SNF subunits play in the function of the complex is not clearly understood. We have begun a functional dissection of the yeast complex by determining the subunits that are involved in promoter recruitment. Snf5, Swi1, and Swi2/Snf2 are all conserved in humans, which may indicate a common mode for the recruitment of chromatin remodeling activity among species. Several human SWI/SNF subunits have been implicated in interactions with different domains of transcription factors. The BAF155 and BAF170 subunits (two yeast Swi3 homologs), along with the BRG1 subunit (a yeast Swi2/Snf2 homolog), directly interact with the zinc finger DNA-binding domain of EKLF (27, 36). A second yeast Swi2/Snf2 homolog, hBrm, directly interacts with the transactivation domain of C/EBPβ (33). BRG1 and hBrm are mutually exclusive within mammalian SWI/SNF complexes; however, a number of proteins are common to both hBrm- and BRG1-associated complexes (66, 67). A recent report demonstrated that BRG1-containing SWI/SNF functionally and physically interacts with hHSF1, and acidic and hydrophobic residues in two C-terminal activation domains of hHSF1 are important for this interaction (57). However, it still remains to be seen if hHSF1 directly interacts with BRG1 or associates with the complex via another hSWI/SNF subunit. The importance of hydrophobic residues in the interaction of hSWI/SNF with hHSF1 is consistent with the finding that hydrophobic patches in the Gcn4 activation domain are important for both interaction with yeast SWI/SNF and its transcriptional potency (13, 26, 43).
Another subunit of hSWI/SNF, hSnf5/Ini1, has recently been shown to interact with the proto-oncogene c-Myc and the viral transcriptional activator, EBNA2, via domains that are distinct from their transactivation domains (7, 71, 72). This subunit was originally identified via a yeast two-hybrid assay as a protein that binds to the human immunodeficiency virus type 1 integrase, hence the name Ini1 (for integrase interactor 1) (29), and by another lab due to its sequence homology to yeast Snf5 (40).
BAF250, a yeast Swi1 homolog and component of the human SWI/SNF-A complex, directly interacts with the glucocorticoid receptor (45). Our systematic study supports a conserved function (from yeast to humans) for the Snf5, Swi1, and Swi2/Snf2 subunits in SWI/SNF recruitment by transcription activators. Our finding of direct interactions of these yeast SWI/SNF subunits with activators as part of native complexes demonstrates that the interacting surfaces of these subunits are indeed available for activator interactions in their natural context, which has not yet been demonstrated for their human counterparts.
Data are emerging that link two subunits of human SWI/SNF complexes, hSnf5/Ini1 and BRG1, to cancer. Interestingly, these subunits were found in our current study, as well as in other recent studies, as targets for activators in the recruitment of SWI/SNF. This opens the possibility that improper SWI/SNF recruitment to its target genes results in the onset of cancer. Studies have found that the hSnf5/Ini1 gene is mutated in many pediatric cancers (11, 18, 54, 58, 63), indicating that it is a tumor suppressor gene. Furthermore, heterozygous Snf5/Ini1 knockout mice develop nervous system and soft tissue sarcomas, whereas homozygous mutations result in embryonic lethality (21, 31, 53). A second component of human SWI/SNF complexes, BRG1, has also been implicated as a tumor suppressor and as a target for mutation in human cancer (69). BRG1 is also an important regulator of cell growth through its interactions with the tumor suppressor pRb (14, 55, 75). A BRG1-containing SWI/SNF complex was recently purified via association with the tumor suppressor and transcriptional regulator BRCA1 through a direct interaction between BRG1 and BRCA1 (4). Our experiments have shown that the yeast homologs of hSnf5/Ini1 and BRG1 can facilitate interactions of the SWI/SNF chromatin remodeling complex with DNA-binding transcription activators. Other studies have demonstrated the interaction of these human SWI/SNF subunits with DNA-binding factors (see above). These studies suggest that hSnf5 and BRG1 are essential for the proper regulation of genes encoding other tumor suppressors by facilitating the recruitment of SWI/SNF. In addition, these SWI/SNF subunits might link chromatin-remodeling activity to target genes of some tumor suppressor proteins, namely, pRb and BRCA1, via direct interactions with these proteins.
Acknowledgments
We thank M. Green, A. Hinnebusch, D. Stillman, and S. Berger for expression plasmids; J. Reese for αTafII30 antisera; E. T. Young for αSwi1/Adr6 antisera; B. Cairns and B. Laurent for antisera to other SWI/SNF subunits; and B. Cairns for the _swp82_Δ strain (YBC381). We thank M. Carrozza for assistance with chromatography and S. Alley, F. Ishmael, and S. Benkovic at Penn State for the use of equipment and for advice on the photo-cross-linking studies. Finally, we thank the members of the Workman, Simpson, and Reese labs at Penn State for many valuable discussions.
This work was supported by NIGMS grant R37 GM47867 to J.L.W. L.H. is supported by a postdoctoral fellowship from the Canadian Institute of Health Research, C.E.B. is supported by a Leukemia and Lymphoma Society fellowship, and J.L.W. is an Associate Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Alley, S. C., A. D. Jones, P. Soumillion, and S. J. Benkovic. 1999. The carboxyl terminus of the bacteriophage T4 DNA polymerase contacts its sliding clamp at the subunit interface. J. Biol. Chem. 274**:**24485-24489. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong, J. A., J. J. Bieker, and B. M. Emerson. 1998. A SWI/SNF-related chromatin remodeling complex, E-RC1, is required for tissue-specific transcriptional regulation by EKLF in vitro. Cell 95**:**93-104. [DOI] [PubMed] [Google Scholar]
- 3.Berger, S. L., W. D. Cress, A. Cress, S. J. Triezenberg, and L. Guarente. 1990. Selective inhibition of activated but not basal transcription by the acidic activtation domain of VP16: evidence for transcriptional adaptors. Cell 61**:**1199-1208. [DOI] [PubMed] [Google Scholar]
- 4.Bochar, D. A., L. Wang, H. Beniya, A. Kinev, Y. Xue, W. S. Lane, W. Wang, F. Kashanchi, and R. Shiekhattar. 2000. BRCA1 is associated with a human SWI/SNF-related complex: linking chromatin remodeling to breast cancer. Cell 102**:**257-265. [DOI] [PubMed] [Google Scholar]
- 5.Brown, C. E., L. Howe, K. Sousa, S. C. Alley, M. J. Carrozza, S. Tan, and J. L. Workman. 2001. Recruitment of HAT complexes by direct activator interactions with the ATM-related Tra1 subunit. Science 292**:**2333-2337. [DOI] [PubMed] [Google Scholar]
- 6.Chen, J.-L., L. D. Attardi, C. P. Verrijzer, K. Yokomori, and R. Tjian. 1994. Assembly of recombinant TFIID reveals differential coactivator requirements for distinct transcriptional activators. Cell 79**:**93-105. [DOI] [PubMed] [Google Scholar]
- 7.Cheng, S. W., K. P. Davies, E. Yung, R. J. Beltran, J. Yu, and G. V. Kalpana. 1999. c-MYC interacts with INI1/hSNF5 and requires the SWI/SNF complex for transactivation function. Nat. Genet. 22**:**102-105. [DOI] [PubMed] [Google Scholar]
- 8.Cosma, M. P., T. Tanaka, and K. Nasmyth. 1999. Ordered recruitment of transcription and chromatin remodeling factors to a cell cycle-and developmentally regulated promoter. Cell 97**:**299-311. [DOI] [PubMed] [Google Scholar]
- 9.Côté, J., C. L. Peterson, and J. L. Workman. 1998. Perturbation of nucleosome core structure by the SWI/SNF complex persists after its detachment, enhancing subsequent transcription factor binding. Proc. Natl. Acad. Sci. USA 95**:**4947-4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Côté, J., J. Quinn, J. L. Workman, and C. L. Peterson. 1994. Stimulation of GAL4 derivative binding to nucleosomal DNA by the yeast SWI/SNF complex. Science 265**:**53-60. [DOI] [PubMed] [Google Scholar]
- 11.DeCristofaro, M. F., B. L. Betz, W. Wang, and B. E. Weissman. 1999. Alteration of hSNF5/INI1/BAF47 detected in rhabdoid cell lines and primary rhabdomyosarcomas but not Wilms' tumors. Oncogene 18**:**7559-7565. [DOI] [PubMed] [Google Scholar]
- 12.Dimova, D., Z. Nackerdien, S. Furgeson, S. Eguchi, and M. A. Osley. 1999. A role for transcriptional repressors in targeting the yeast Swi/Snf complex. Mol. Cell 4**:**75-83. [DOI] [PubMed] [Google Scholar]
- 13.Drysdale, C. M., E. Dueñas, B. M. Jackson, U. Reusser, G. H. Braus, and A. G. Hinnebusch. 1995. The transcriptional activator GCN4 contains multiple activation domains that are critically dependent on hydrophobic amino acids. Mol. Cell. Biol. 15**:**1220-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunaief, J. L., B. E. Strober, S. Guha, P. A. Khavari, K. Ålin, J. Luban, M. Begemann, G. R. Crabtree, and S. P. Goff. 1994. The retinoblastoma protein and BRG1 form a complex and cooperate to induce cell cycle arrest. Cell 79**:**119-130. [DOI] [PubMed] [Google Scholar]
- 15.Fry, C. J., and C. L. Peterson. 2001. Chromatin remodeling enzymes: who's on first? Curr. Biol. 11**:**R185-R197. [DOI] [PubMed] [Google Scholar]
- 16.Fryer, C. J., and T. K. Archer. 1998. Chromatin remodelling by the glucocorticoid receptor requires the BRG1 complex. Nature 393**:**88-91. [DOI] [PubMed] [Google Scholar]
- 17.Gavin, I., P. J. Horn, and C. L. Peterson. 2001. SWI/SNF chromatin remodeling requires changes in DNA topology. Mol. Cell 7**:**97-104. [DOI] [PubMed] [Google Scholar]
- 18.Grand, F., S. Kulkarni, A. Chase, J. M. Goldman, M. Gordon, and N. C. Cross. 1999. Frequent deletion of hSNF5/INI1, a component of the SWI/SNF complex, in chronic myeloid leukemia. Cancer Res. 59**:**3870-3874. [PubMed] [Google Scholar]
- 19.Grant, P. A., S. L. Berger, and J. L. Workman. 1999. Identification and analysis of native nucleosomal histone acetyltransferase complexes. Methods Mol. Biol. 119**:**311-317. [DOI] [PubMed] [Google Scholar]
- 20.Gregory, P. D., A. Schmid, M. Zavari, M. Münsterkötter, and W. Hörz. 1999. Chromatin remodelling at the PHO8 promoter requires SWI-SNF and SAGA at a step subsequent to activator binding. EMBO J. 18**:**6407-6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guidi, C. J., A. T. Sands, B. P. Zambrowicz, T. K. Turner, D. A. Demers, W. Webster, T. W. Smith, A. N. Imbalzano, and S. N. Jones. 2001. Disruption of Ini1 leads to peri-implantation lethality and tumorigenesis in mice. Mol. Cell. Biol. 21**:**3598-3603.11313485 [Google Scholar]
- 22.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 23.Hassan, A. H., K. E. Neely, and J. L. Workman. 2001. Histone acetyltransferase complexes stabilize SWI/SNF binding to promoter nucleosomes. Cell 104**:**817-827. [DOI] [PubMed] [Google Scholar]
- 24.Havas, K., A. Flaus, M. Phelan, R. Kingston, P. A. Wade, D. M. J. Lilley, and T. Owen-Hughes. 2000. Generation of superhelical torsion by ATP-dependent chromatin remodeling activities. Cell 103**:**1133-1142. [DOI] [PubMed] [Google Scholar]
- 25.Holstege, F. C. P., E. G. Jennings, J. J. Wyrick, T. I. Lee, C. J. Hengartner, M. R. Green, T. R. Golub, E. S. Lander, and R. A. Young. 1998. Dissecting the regulatory circuitry of a eukaryotic genome. Cell 95**:**717-728. [DOI] [PubMed] [Google Scholar]
- 26.Jackson, B. M., C. M. Drysdale, K. Natarajan, and A. G. Hinnebusch. 1996. Identification of seven hydrophobic clusters in GCN4 making redundant contributions to transcriptional activation. Mol. Cell. Biol. 16**:**1557-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kadam, S., G. S. McAlpine, M. L. Phelan, R. E. Kingston, K. A. Jones, and B. M. Emerson. 2000. Functional selectivity of recombinant mammalian SWI/SNF subunits. Genes Dev. 14**:**2441-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kal, A. J., T. Mahmoudi, N. B. Zak, and C. P. Verrijzer. 2000. The Drosophila Brahma complex is an essential coactivator for the trithorax group protein Zeste. Genes Dev. 14**:**1058-1071. [PMC free article] [PubMed] [Google Scholar]
- 29.Kalpana, G. V., S. Marmon, W. Wang, G. R. Crabtree, and S. P. Goff. 1994. Binding and stimulation of HIV-1 integrase by a human homolog of yeast transcription factor SNF5. Science 266**:**2002-2006. [DOI] [PubMed] [Google Scholar]
- 30.Kingston, R. E., and G. J. Narlikar. 1999. ATP-dependent remodeling and acetylation as regulators of chromatin fluidity. Genes Dev. 13**:**2339-2352. [DOI] [PubMed] [Google Scholar]
- 31.Klochendler-Yeivin, A., L. Fiette, J. Barra, C. Muchardt, C. Babinet, and M. Yaniv. 2000. The murine SNF5/INI1 chromatin remodeling factor is essential for embryonic development and tumor suppression. EMBO Rep. 1**:**500-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koh, S. S., A. Z. Ansari, M. Ptashne, and R. A. Young. 1998. An activator target in the RNA polymerase II holoenzyme. Mol. Cell 1**:**895-904. [DOI] [PubMed] [Google Scholar]
- 33.Kowenz-Leutz, E., and A. Leutz. 1999. A C/EBPβ isoform recruits the SWI/SNF complex to activate myeloid genes. Mol. Cell 4**:**735-743. [DOI] [PubMed] [Google Scholar]
- 34.Krebs, J. E., C. J. Fry, M. L. Samuels, and C. L. Peterson. 2000. Global role for chromatin remodeling enzymes in mitotic gene expression. Cell 102**:**587-598. [DOI] [PubMed] [Google Scholar]
- 35.Krebs, J. E., M. H. Kuo, C. D. Allis, and C. L. Peterson. 1999. Cell cycle-regulated histone acetylation required for expression of the yeast HO gene. Genes Dev. 13**:**1412-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee, C.-H., M. R. Murphy, J.-S. Lee, and J. H. Chung. 1999. Targeting a SWI/SNF-related chromatin remodeling complex to the β-globin promoter in erythroid cells. Proc. Natl. Acad. Sci. USA 96**:**12311-12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lieberman, P. M., and A. J. Berk. 1991. The Zta _trans_-activator protein stabilizes TFIID association with promoter DNA by direct protein-protein interaction. Genes Dev. 5**:**2441-2454. [DOI] [PubMed] [Google Scholar]
- 38.Logie, C., and C. L. Peterson. 1999. Purification and biochemical properties of yeast SWI/SNF complex. Methods Enzymol. 304**:**726-741. [DOI] [PubMed] [Google Scholar]
- 39.McAndrew, P. C., J. Svaren, S. R. Martin, W. Hörz, and C. R. Goding. 1998. Requirements for chromatin modulation and transcription activation by the Pho4 acidic activation domain. Mol. Cell. Biol. 18**:**5818-5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muchardt, C., C. Sardet, B. Bourachot, C. Onufryk, and M. Yaniv. 1995. A human protein with homology to Saccharomyces cerevisiae SNF5 interacts with the potential helicase hbrm. Nucleic Acids Res. 23**:**1127-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy, D. J., S. Hardy, and D. A. Engel. 1999. Human SWI-SNF component BRG1 represses transcription of the c_-fos_ gene. Mol. Cell. Biol. 19**:**2724-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Myers, L. C., C. M. Gustafsson, K. C. Hayashibara, P. O. Brown, and R. D. Kornberg. 1999. Mediator protein mutations that selectively abolish activated transcription. Proc. Natl. Acad. Sci. USA 96**:**67-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Natarajan, K., B. M. Jackson, H. Zhou, F. Winston, and A. G. Hinnebusch. 1999. Transcriptional activation by Gcn4p involves independent interactions with the SWI/SNF complex and the SRB/Mediator. Mol. Cell 4**:**657-664. [DOI] [PubMed] [Google Scholar]
- 44.Neely, K. E., A. H. Hassan, A. E. Wallberg, D. J. Steger, B. R. Cairns, A. P. H. Wright, and J. L. Workman. 1999. Activation domain-mediated targeting of the SWI/SNF complex to promoters stimulates transcription from nucleosome arrays. Mol. Cell 4**:**649-655. [DOI] [PubMed] [Google Scholar]
- 45.Nie, Z., Y. Xue, D. Yang, S. Zhou, B. J. Deroo, T. K. Archer, and W. Wang. 2000. A specificity and targeting subunit of a human SWI/SNF family-related chromatin-remodeling complex. Mol. Cell. Biol. 20**:**8879-8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogawa, N., and Y. Oshima. 1990. Functional domains of a positive regulatory protein, PHO4, for transcriptional control of the phosphatase regulon in Saccharomyces cerevisiae. Mol. Cell. Biol. 10**:**2224-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Owen-Hughes, T., R. T. Utley, J. Côté, C. L. Peterson, and J. L. Workman. 1996. Persistant site-specific remodeling of a nucleosome array by transient action of the SWI/SNF complex. Science 273**:**513-516. [DOI] [PubMed] [Google Scholar]
- 48.Peterson, C. L., A. Dingwall, and M. P. Scott. 1994. Five SWI/SNF gene products are components of a large multisubunit complex required for transcriptional enhancement. Proc. Natl. Acad. Sci. USA 91**:**2905-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peterson, C. L., and I. Herskowitz. 1992. Characterization of the yeast SWI1, SWI2, and SWI3 genes, which encode a global activator of transcription. Cell 68**:**573-583. [DOI] [PubMed] [Google Scholar]
- 50.Peterson, C. L., and J. L. Workman. 2000. Promoter targeting and chromatin remodeling by the SWI/SNF complex. Curr. Opin. Genet. Dev. 10**:**187-192. [DOI] [PubMed] [Google Scholar]
- 51.Phelan, M. L., G. R. Schnitzler, and R. E. Kingston. 2000. Octamer transfer and creation of stably remodeled nucleosomes by human SWI-SNF and its isolated ATPases. Mol. Cell. Biol. 20**:**6380-6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quinn, J., A. M. Fyrberg, R. W. Ganster, M. C. Schmidt, and C. L. Peterson. 1996. DNA-binding properties of the yeast SWI/SNF complex. Nature 379**:**844-847. [DOI] [PubMed] [Google Scholar]
- 53.Roberts, C. W. M., S. A. Galusha, M. E. McMenamin, C. D. M. Fletcher, and S. H. Orkin. 2000. Haploinsufficiency of Snf5 (integrase interactor 1) predisposes to malignant rhabdoid tumors in mice. Proc. Natl. Acad. Sci. USA 97**:**13796-13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sévenet, N., A. Lellouch-Tubiana, D. Schofield, K. Hoang-Xuan, M. Gessler, D. Birnbaum, C. Jeanpierre, A. Jouvet, and O. Delattre. 1999. Spectrum of hSNF5/INI1 somatic mutations in human cancer and genotype-phenotype correlations. Hum. Mol. Genet. 8**:**2359-2368. [DOI] [PubMed] [Google Scholar]
- 55.Strober, B. E., J. L. Dunaief, S. Guha, and S. P. Goff. 1996. Functional interactions between hBRM/hBRG1 transcriptional activators and the pRB family of proteins. Mol. Cell. Biol. 16**:**1576-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sudarsanam, P., V. R. Iyer, P. O. Brown, and F. Winston. 2000. Whole-genome expression analysis of snf/swi mutants of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 97**:**3364-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sullivan, E. K., C. S. Weirich, J. R. Guyon, S. Sif, and R. E. Kingston. 2001. Transcriptional activation domains of human heat shock factor 1 recruit human SWI/SNF. Mol. Cell. Biol. 21**:**5826-5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor, M. D., N. Gokgoz, I. L. Andrulis, T. G. Mainprize, J. M. Drake, and J. T. Rutka. 2000. Familial posterior fossa brain tumors of infancy secondary to germline mutation of the hSNF5 gene. Am. J. Hum. Genet. 66**:**1403-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Triezenberg, S. J., R. C. Kingsbury, and S. L. McKnight. 1988. Functional dissection of VP16, the transactivator of herpes simplex virus immediate early gene expression. Genes Dev. 2**:**718-729. [DOI] [PubMed] [Google Scholar]
- 60.Trouche, D., C. LeChalony, C. Muchardt, M. Yaniv, and T. Kouzarides. 1997. RB and hbrm cooperate to repress the activation functions of E2F1. Proc. Natl. Acad. Sci. USA 94**:**11268-11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Utley, R. T., K. Ikeda, P. A. Grant, J. Côté, D. J. Steger, A. Eberharter, S. John, and J. L. Workman. 1998. Transcriptional activators direct histone acetyltransferase complexes to nucleosomes. Nature 394**:**498-502. [DOI] [PubMed] [Google Scholar]
- 62.Verrijzer, C. P., and R. Tjian. 1996. TAFs mediate transcriptional activation and promoter selectivity. Trends Biochem. Sci. 21**:**338-342. [PubMed] [Google Scholar]
- 63.Versteege, I., N. Sévenet, J. Lange, M.-F. Rousseau-Merck, P. Ambros, R. Handgretinger, A. Aurias, and O. Delattre. 1998. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature 394**:**203-206. [DOI] [PubMed] [Google Scholar]
- 64.Vignali, M., A. H. Hassan, K. E. Neely, and J. L. Workman. 2000. ATP-dependent chromatin remodeling complexes. Mol. Cell. Biol. 20**:**1899-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wallberg, A. E., K. E. Neely, A. H. Hassan, J.-Å. Gustafsson, J. L. Workman, and A. P. H. Wright. 2000. Recruitment of the SWI-SNF chromatin remodeling complex as a mechanism of gene activation by the glucocorticoid receptor τ1 activation domain. Mol. Cell. Biol. 20**:**2004-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang, W., J. Côté, Y. Xue, S. Zhou, P. A. Khavari, S. R. Biggar, C. Muchardt, G. V. Kalpana, S. P. Goff, M. Yaniv, J. L. Workman, and G. R. Crabtree. 1996. Purification and biochemical heterogeneity of the mammalian SWI-SNF complex. EMBO J. 15**:**5370-5382. [PMC free article] [PubMed] [Google Scholar]
- 67.Wang, W., Y. Xue, S. Zhou, A. Kuo, B. R. Cairns, and G. R. Crabtree. 1996. Diversity and specialization of mammalian SWI/SNF complexes. Genes Dev. 10**:**2117-2130. [DOI] [PubMed] [Google Scholar]
- 68.Whitehouse, I., A. Flaus, B. R. Cairns, M. F. White, J. L. Workman, and T. Owen-Hughes. 1999. Nucleosome mobilization catalysed by the yeast SWI/SNF complex. Nature 400**:**784-787. [DOI] [PubMed] [Google Scholar]
- 69.Wong, A. K., F. Shanahan, Y. Chen, L. Lian, P. Ha, K. Hendricks, S. Ghaffari, D. Iliev, B. Penn, A. M. Woodland, R. Smith, G. Salada, A. Carillo, K. Laity, J. Gupte, B. Swedlund, S. V. Tavtigian, D. H. Teng, and E. Lees. 2000. BRG1, a component of the SWI-SNF complex, is mutated in multiple human tumor cell lines. Cancer Res. 60**:**6171-6177. [PubMed] [Google Scholar]
- 70.Workman, J. L., and R. E. Kingston. 1998. Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu. Rev. Biochem. 67**:**545-579. [DOI] [PubMed] [Google Scholar]
- 71.Wu, D. Y., G. V. Kalpana, S. P. Goff, and W. H. Schubach. 1996. Epstein-Barr virus nuclear protein 2 (EBNA2) binds to a component of the human SNF-SWI complex, hSNF5/Ini1. J. Virol. 70**:**6020-6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu, D. Y., A. Krumm, and W. H. Schubach. 2000. Promoter-specific targeting of human SWI-SNF complex by Epstein-Barr virus nuclear protein 2. J. Virol. 74**:**8893-8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoshinaga, S. K., C. L. Peterson, I. Herskowitz, and K. R. Yamamoto. 1992. Roles of SWI1, SWI2, and SWI3 proteins for transcriptional enhancement by steroid receptors. Science 258**:**1598-1604. [DOI] [PubMed] [Google Scholar]
- 74.Yudkovsky, N., C. Logie, S. Hahn, and C. L. Peterson. 1999. Recruitment of the SWI/SNF chromatin remodeling complex by transcriptional activators. Genes Dev. 13**:**2369-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang, H. S., M. Gavin, A. Dahiya, A. A. Postigo, D. Ma, R. X. Luo, J. W. Harbour, and D. C. Dean. 2000. Exit from G1 and S phase of the cell cycle is regulated by repressor complexes containing HDAC-Rb-hSWI/SNF and Rb-hSWI/SNF. Cell 101**:**79-89. [DOI] [PubMed] [Google Scholar]