Loss of Apc allows phenotypic manifestation of the transforming properties of an endogenous K-ras oncogene in vivo (original) (raw)
Abstract
Oncogenic mutations in the K-ras gene occur in ≈50% of human colorectal cancers. However, the precise role that K-ras oncogenes play in tumor formation is still unclear. To address this issue, we have conditionally expressed an oncogenic K-_ras_V12 allele in the small intestine of adult mice either alone or in the context of Apc deficiency. We found that expression of K-_ras_V12 does not affect normal intestinal homeostasis or the immediate phenotypes associated with Apc deficiency. Mechanistically we failed to find activation of the Raf/MEK/ERK pathway, which may be a consequence of the up-regulation of a number of negative feedback loops. However, K-_ras_V12 expression accelerates intestinal tumorigenesis and confers invasive properties after Apc loss over the long term. In renal epithelium, expression of the oncogenic K-_ras_V12 allele in the absence of Apc induces the rapid development of renal carcinoma. These tumors, unlike those of intestinal origin, display activation of the Raf/MEK/ERK and Akt signaling pathways. Taken together, these data indicate that normal intestinal and kidney epithelium are resistant to malignant transformation by an endogenous K-ras oncogene. However, activation of K-_ras_V12 after Apc loss results in increased tumorigenesis with distinct kinetics. Whereas the effect of K-ras oncogenes in the intestine can been observed only after long latencies, they result in rapid carcinogenesis in the kidney epithelium. These data imply a window of opportunity for anti-K-ras therapies after tumor initiation in preventing tumor growth and invasion.
Keywords: colorectal cancer, renal carcinoma, Wnt signaling
Although the molecular genetic events that occur in colorectal cancer have been known for many years, the selective advantage conferred by these mutations has remained unclear (1, 2). Of these mutations, inactivation of Apc is the most frequent and appears to be the key early event initiating colorectal cancer (3). Recent in vivo studies using inducible gene deletion have allowed the precise function of Apc in the adult intestine to be defined (4). These studies have implicated two major functions of Apc in facilitating tumor suppression, namely controlling differentiation and migration.
K-ras is mutated in ≈40–50% of colorectal tumors at a relatively early stage of the carcinogenic process and, despite extensive research, the primary reason for this high frequency remains unclear (5). Ras proteins are prototypic small GTPases that act as molecular switches, transducing signals from the cell surface (from receptor and nonreceptor tyrosine kinases and G protein-coupled receptors) to the nucleus (5). The most studied downstream effector pathways are the mitogenic Raf-MEK-ERK pathway and the survival phosphatidylinositol 3-kinase pathway. In vitro studies have shown that oncogenic K-ras is required for proliferation, cytoskeleton organisation, adhesion, motility, and invasion of colorectal cancer cells. In these studies, activation of the downstream Raf-MEK-ERK pathways has been demonstrated, and inhibitors to this pathway have been shown to suppress the phenotypes induced by oncogenic K-ras (6).
To address the role of oncogenic K-ras in vivo, a number of strategies have been used. Most have relied on transgenic overexpression of oncogenic K-ras mutants (7). A key difficulty in interpreting these studies is that K-ras is normally mutated but not overexpressed in tumors. Thus, overexpression of oncogenic K-ras may yield very different phenotypes to simple mutation. When overexpressed from the villin promoter, mice developed tumors from 8 months of age, although no effects were seen on normal epithelium (7). As with in vitro studies, activation of Raf-MEK-ERK was observed in both normal and adenomatous tissue. In contrast, expression of oncogenic K-ras from the fatty acid-binding protein promoter only resulted in a proapoptotic phenotype in the context of a simian virus 40 T antigen transgene. In this study, the lack of phenotype mirrored an inability to see up-regulation of Raf-MEK-ERK signaling (8).
More sophisticated mouse models have been designed in which mutant alleles have been knocked in at the endogenous locus. Moreover, these mutant alleles have been designed so their expression can be controlled in a spatial and temporal manner using tissue-specific Cre and inducible Cre transgenic strains. One of these models involves the expression of an oncogenic K_-ras_ allele under the control of a floxed STOP transcriptional cassette (9, 10). This oncogenic k-ras allele remains transcriptionally silent until the STOP is removed by a Cre recombinase. These models not only offer the advantage of the controlled expression of the desired knocked-in mutation, but the fact that conditional knockin allele is expressed from its endogenous promoter (9, 10).
These models have been used to demonstrate that endogenous K-ras oncogenes, activated in different tissues and at different times during embryonic or postnatal development, can induce myeloproliferative disease and carcinomas in various tissues including lung, pancreas, and ovaries (9–12). So far, there are no reports for a role of endogenous K-ras oncogenes in intestinal or colon tumors. Apc_min_ mice carrying an oncogenic K-ras allele whose expression required spontaneous recombination failed to display any phenotype significantly distinct of the Apc_min_ strain (9). Whether these observations were caused by a lack of synergy between the Apc and K-ras mutations or the low percentage of cells expressing the oncogenic K-ras allele could not be determined.
Here, we define the role of an endogenous K-ras oncogene in the adult intestine by using an inducible cre-lox approach. We show that although expression of the oncogenic K-ras allele has little or no effect on intestinal homeostasis or the immediate phenotype after Apc loss the K-ras oncogene does accelerate tumor formation and invasion. Importantly, we also show that expression of an endogenous K-ras oncogene in kidney cells accelerates the formation of renal carcinomas (RCs) after loss of Apc in the renal epithelium.
Results and Discussion
Activation of K-ras Does Not Alter Intestinal Homeostasis.
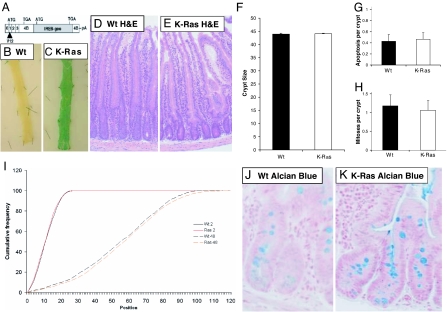
We investigated whether expression of an oncogenic K-_ras_V12 allele would alter intestinal homeostasis. To this end, we intercrossed K-ras(+/LSLV12) mice bearing a conditional knocked-in K-_ras_V12 allele whose expression requires Cre-mediated elimination of a STOP transcriptional cassette (10) (Fig. 1A) to mice bearing the cytochrome p450 inducible AhCre transgene (13). After induction with β-naphthoflavone, the AhCre transgene delivers very high penetrance Cre-mediated recombination in both the intestine and liver, with subsequent expression of the oncogenic K-_ras_V12 allele in these tissues. This targeted allele also carries an IRES-betageo sequence that allows monitoring of successful Cre-mediated recombination through LacZ activity. Therefore, to confirm excision of the STOP cassette, we performed β-gal staining on intestinal whole mounts (Fig. 1 B and C). Nearly all intestinal cells stained positive for LacZ, indicating successful recombination of the knockin K-_ras_V12 allele throughout the intestine.
Fig. 1.
Expression of oncogenic K-_ras_V12 has no impact on intestinal homeostasis. (A) Schematic diagram of the transcript derived from the conditional K-_ras_V12 allele. (B and C) Intestinal whole mounts showing β-gal expression in AhCre+/T;K-ras+/+ (WT) (B) and AhCre+/T;K-ras+/LSLV12 (K-Ras) (C). Note that the intestinal whole mount from the AhCre+/T;K-ras+/LSLV12 mouse is almost exclusively composed of LacZ-positive cells indicative of Cre-mediated K-_ras_V12 expression. (D and E) H&E-stained section from AhCre+/T;K-ras+/+ (WT) (D) and AhCre+/T;K-ras+/LSLV12 (K-Ras) (E) intestines. No gross changes were observed between these samples. (F_–_H) Graphs showing that crypt size, mitoses, and apoptotic indices are not altered between AhCre+/T;K-ras+/+ (WT) (filled bars) and AhCre+/T;K-ras+/LSLV12 (K-Ras) (empty bars) intestines. All of the above studies were performed 3 days after Cre induction. (I) Expression of oncogenic K-_ras_V12 does not affect migration or S-phase labeling. Mice were treated with BrdU and then culled either 2 or 48 h later at days 3 and 5 after recombination, respectively. The position of labeled cells was then scored relative to the base of the crypt and cumulative distribution plots generated. AhCre+/T;K-ras+/+ (WT) mice are indicated by black lines, and AhCre+/T; K-ras+/LSLV12 (K-Ras) mice are indicated by red lines. Solid lines, 2 h. Broken lines, 48 h. No differences were observed between the two genotypes at either time points (P = 0.5, Kolmorov–Smirnov). However, significant intestinal enterocyte migration was observed in both genotypes over the 48-h period of the experiment (P > 0.01 Kolmorov–Smirnov). (J and K) Alcian blue staining for goblet cells performed on AhCre+/T;K-ras+/+ (WT) (J) and AhCre+/T;K-ras+/LSLV12 (K-Ras) (K) intestines. No significant difference was found between genotypes (P = 0.4 Mann–Whitney). (Magnifications: ×40.)
We next characterized the consequences of expressing the oncogenic K-_ras_V12 allele in the small intestine. Histological analysis revealed no major alterations in the architecture of the crypt-villus axis at 3 days after Cre induction. Crypt size, mitotic index, and levels of apoptosis were scored from these sections and found not to vary significantly between experimental and control tissue at day 3 postinduction (PI). For all comparisons n ≥ 3 and Mann–Whitney U P values were ≥0.40 (Fig. 1 F_–_H). Levels of apoptosis were also confirmed independently with immunohistochemistry (IHC) staining for active caspase 3 (14) (data not shown). This failure to observe phenotypic change was consistently observed in mice up to 180 days after Cre induction.
Given previous data suggesting that oncogenic activation of K-ras may alter migration of cells, we determined whether cells expressing the oncogenic K-_ras_V12 allele were able to migrate normally along the crypt-villus axis. This “migration” refers to the upward displacement of intestinal epithelial cells and thus differs from classical cell migration. Mice at day 3 PI were injected with BrdU and killed after 2 or 48 h. Comparison of the distribution of BrdU-positive cells along the axis allows an assessment of the degree of movement along the crypt-villus axis. As shown in Fig. 1I, cells expressing K-_ras_V12 showed a normal migration pattern between 2 and 48 h postlabeling compared with controls (Kolmogorov-Smirnov test, P = 0.5). The 2-h BrdU labeling experiment described above demonstrated that cells expressing oncogenic K-_ras_V12 proliferated normally, and that the location and size of the proliferative compartment within the crypt did not differ from controls (P = 0.4, Kolmogorov-Smirnov).
The presence and correct localization of all differentiated intestinal cell lineages was confirmed by use of specific stains or IHC against markers of the differentiated cell types. Alcian blue staining for goblet cells showed that there were similar numbers of goblet cells (Fig. 1 H_–_J); Grimelius staining showed that there was no significant change in the number of enteroendocrine cells (for all comparisons n ≥ 3 and Mann–Whitney U P values were ≥0.39). Antilysozyme staining for paneth cells and antivillin staining for villus enterocytes also revealed no gross change in the number or localization of these cell types (data not shown).
Activation of K-ras Does Not Alter the Levels of Proliferation or Apoptosis After Apc Loss.
In colorectal cancer, K_-ras_ mutations occur after Apc mutations, so we next examined whether Cre-mediated expression of oncogenic K-_ras_V12 affected the immediate phenotypes of Apc deficiency. This was achieved by intercrossing AhCre+/T, K-ras+/LSLV12 animals with Apcfl/fl mice bearing a loxP flanked Apc 580 allele (15). The resulting AhCre+/T, K-ras+/LSLV12, Apcfl/fl mice were then induced with β-naphthoflavone.
Histological analysis revealed that K-_ras_V12 expression had little impact on the previously reported phenotypes of Apc deficiency (4) (Fig. 4 B and C, which is published as supporting information on the PNAS web site). Crypt size, mitotic index, and levels of apoptosis all were increased compared with WT mice but were indistinguishable to that of _Apc_-deficient mice (for all comparisons n ≥ 3 and Mann–Whitney U P values were ≥0.40) (Fig. 4 D_–_F). IHC for BrdU and Ki67 showed K-_ras_V12 expression did not increase levels of cell turnover, with proliferation independent of position in both genotypes. Likewise, K-_ras_V12 expression did not significantly alter the number of enteroendocrine cells, the mislocalization of paneth cells, or the reduction in villin expression after Apc loss (data not shown). However, there was significant rescue in the number of goblet cells after Apc loss [34 ± 14 (Apcfl/fl) vs. 113 ± 10 (K-ras(+/LSLV12), Apcfl/fl), P = 0.04 Mann–Whitney, n = 3] (Fig. 4 I_–_J). Taken together, these data suggest that Apc loss alone is sufficient to induce the vast majority of changes that occur at the earliest stages of neoplasia and that K-_ras_V12 expression at these stages confers little, if any, additional phenotype.
Raf-MEK-ERK and Wnt Signaling Pathways Are Not Dysregulated After Expression of Oncogenic K-_ras_V12.
Given our failure to observe any phenotypic change after expression of the oncogenic K-_ras_V12 allele, we next investigated whether the Raf-MEK-ERK pathway was activated. IHC for P-MEK and P-ERK indicated that these phospho-proteins are normally present in the cytoplasm of intestinal enterocytes and that expression of oncogenic K-ras_V12, either alone or in the context of Apc deficiency, did not affect either the levels or the localization of these proteins (Fig. 5_a A_–_I, which is published as supporting information on the PNAS web site). These observations provide a molecular explanation for our failure to see phenotypic changes after activation of K-ras_V12 expression. These data also argue against the notion that Apc inhibits activation of the Raf-MEK-ERK pathway in vivo, as recently suggested (16). Interestingly, nuclear P-MEK and P-ERK were observed in goblet cell nuclei, suggesting that this pathway may be relevant to goblet cell differentiation (Fig. 5_aF). This may also explain the partial rescue of goblet cell loss, which we report in _Apc_-deficient cells that express the oncogenic K-_ras_V12 allele.
We next investigated expression of cell-cycle proteins cyclin D1 and p21, which have also been reported to become up-regulated after expression of K-ras oncogenes (12). However, IHC showed no changes of localization or expression of either p21 or cyclin D1 after K-ras activation (data not shown). Previous studies have suggested that oncogenic K-ras may also activate Wnt signaling (17). Within the intestinal crypt, levels of Wnt signaling are crucial for the establishment of cell positioning, differentiation, and proliferation (4). We therefore examined expression of β-catenin after Cre-mediated expression of the oncogenic K-_ras_V12 allele in otherwise normal epithelium. No changes were observed in either the level or location of β-catenin in cells expressing oncogenic K-ras_V12 as determined by IHC (Fig. 5_a K_–_L). These data, in conjunction with the failure to see changes in crypt kinetics or up-regulation of the Wnt targets CD44 and C-Myc (WT vs. K-ras(+/LSLV12), 1.31- and 0.83-fold by quantitative RT-PCR (qRT-PCR), respectively, Mann–Whitney P > 0.4), argue that Wnt signaling was unaltered despite oncogenic K-_ras_V12 expression.
We also determined whether expression of the K-_ras_V12 allele altered Wnt signaling after Apc gene deletion. Irrespective of K-ras_V12 expression status, β-catenin translocated to the nucleus (Fig. 5_a M and N), causing transcriptional activation of the canonical Wnt target genes CD44 and C-Myc (WT vs. Apcfl/fl, 6.72-fold, 1.88-fold, WT vs. K-ras(+/LSLV12), Apcfl/fl 5.33-fold, 2.72-fold changes, respectively by qRT-PCR). Wnt signaling was not accentuated after K-_ras_V12 expression in Apc-deficient cells with the levels of induction of CD44 and C-Myc unchanged (0.79- and 1.46-fold change, respectively by qRT-PCR, Mann–Whitney P > 0.2).
Up-Regulation of Negative Regulators of Raf-MEK-ERK Signaling.
Previously we have shown that numerous negative feedback loops are activated after Apc loss in vivo (4). Thus, we observe up-regulation of Axin-2, Wisp1, Wif-1, and Frizzled 6 after Apc loss. However, in the absence of Apc, these proteins are unable to prevent dysregulated Wnt signaling (2) (Fig. 5_aM_). A different scenario exists after expression of the oncogenic K-_ras_V12 allele, as a number of studies have indicated that activation of the Ras signaling pathway can be attenuated through reducing the phosphorylation status of the downstream proteins Raf-MEK-ERK (18). Importantly, this has been the rationale behind developing kinase inhibitors to Raf and a number of other downstream effectors of Ras signaling.
To further explore the molecular consequences of oncogenic K-_ras_V12 expression, we performed microarray experiments using MOE430_2 chips comparing control and AhCre+, K-ras(+/LSLV12) intestines. Using significance analysis of microarray analysis and a false discovery rate of 5% we identified a small number of changes specific to K-_ras_V12 expression. Of these, a number have previously been associated with Ras or G protein receptor signaling (Table 1, which is published as supporting information on the PNAS web site). Regulator of G protein signaling-5 (RGS5), a potential negative regulator of Ras signaling, was up-regulated in the array and confirmed by qRT-PCR to be 3-fold elevated after Cre-mediated induction of K-_ras_V12 expression. This protein was originally identified in a screen for regulators of G protein signaling and has been shown to attenuate ERK activation in vivo (19, 20).
The most studied phosphatases of the Raf-MEK-ERK pathway are the Dusp/MKP phosphatases. Our previous microarray analysis of Apc-deficient intestines had suggested that Dusp4/Mkp2 and Dusp9/Mkp4 were both up-regulated after Apc loss. To investigate this we performed IHC against Dusp4 after Apc loss and confirmed Dusp4 to be up-regulated immediately after Apc loss and to be independent of K-ras status (Fig. 6, which is published as supporting information on the PNAS web site). We also used qRT-PCR to confirm 5-fold up-regulation of Dusp9 after Apc loss, which again was independent of K-_ras_V12 expression. Previous studies have shown that K-_ras_-mediated transformation of pancreatic cells in vitro can be suppressed by cotransfection with vectors containing Dusp4 (21). Deregulation of Dusp4 and Dusp9 therefore provides a ready mechanism to explain the lack of additional phenotype we see after K-_ras_V12 expression.
Expression of K-_ras_V12 Accelerates Tumorigenesis and Causes Invasion After Apc Loss.
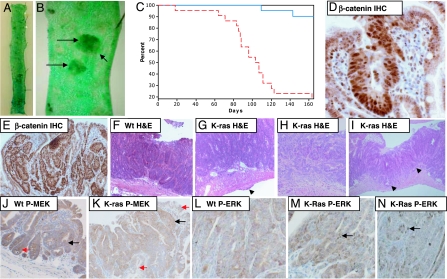
After establishing that K-_ras_V12 expression has no detectable impact on the immediate consequences of Apc loss, we next investigated whether expression of this oncogenic allele could accelerate the appearance of adenomas. Cohorts of (AhCre+/T, K-ras+/+, Apc+/+), (AhCre+/T, K-ras+/LSLV12, Apc+/+), (AhCre+/T, K-ras+/+, Apc+/fl), and (AhCre+/T, K-ras+/LSLV12, Apc+/fl) mice were induced with β-naphthoflavone at weaning (3 × 80 mg/kg β-naphthoflavone i.p.). This protocol effectively renders the animals either WT or constitutively heterozygous for Apc from weaning, and additionally either WT or activated for K-ras. Cohorts were aged until they developed symptoms of intestinal neoplasia (anaemia, pale feet, hunching, and swollen abdomen). Fig. 2A and B shows an intestinal whole mount of an AhCre+/T, K-ras+/LSLV12, Apc+/fl mouse at 150 days after induction of Cre expression stained for β-gal activity. LacZ staining was observed in both normal and adenomatous tissue throughout the intestine, consistent with high levels of Cre-induced expression of the conditional K-_ras_V12 allele. All adenomas were characterized by nuclear β-catenin, strongly implicating loss of the remaining Apc allele (Fig. 2 D and E).
Fig. 2.
Expression of endogenous K-_ras_V12 accelerates tumor growth and invasion within the intestine. (A) Intestinal whole mounts showing β-gal expression in AhCre+/T, K-ras+/LSLV12, Apc+/fl 150 days after Cre induction. (B) Close-up showing LacZ-positive blue intestinal adenomas. (C) Kaplan–Meier curve showing survival of AhCre+/T, K-ras+/LSLV12, Apc+/fl (red dashed line) and AhCre+/T, K-ras+/+, Apc+/fl (blue line) after Cre induction. Expression of oncogenic K-_ras_V12 rapidly accelerates tumorigenesis. (D and E) β-catenin relocalizes to the nuclei of all adenomatous cells in AhCre+/T, K-ras+/LSLV12, Apc+/fl tumors, indicating deregulation of the Wnt pathway. (F) H&E-stained section of an adenoma from AhCre+/T, K-ras+/+, Apc+/fl mouse. Note the lack of any signs of invasion. (G) Adenoma from AhCre+/T, K-ras+/LSLV12, Apc+/fl showing scarring on the outer surface of the intestine (denoted by arrows), which occurred frequently in AhCre+/T, K-ras+/LSLV12, Apc+/fl mice but was not seen in control AhCre+/T, K-ras+/+, Apc+/fl mice. In humans such external (serosal) thickening below a tumor is generally associated with adenocarcinomas rather than adenomas. (H and I) Adenocarcinoma from AhCre+/T, K-ras+/LSLV12, Apc+/fl showing invasion into the muscle wall (shown by arrows in I). (J and K) P-Mek staining of adenomas arising in a AhCre+/T, K-ras+/+, Apc+/fl mouse (J) and a AhCre+/T, K-ras+/LSLV12, Apc+/fl mouse (K). Note cytoplasmic P-Mek staining is more intense in the adenoma from the AhCre+/T, K-ras+/LSLV12, Apc+/fl mouse (black arrows) compared with surrounding normal mucosa (red arrows). This is not the case in the AhCre+/T, K-ras+/+, Apc+/fl adenomas (J). (L_–_N) P-ERK 1/2 staining of adenomas from AhCre+/T, K-ras+/+, Apc+/fl (L) and AhCre+/T, K-ras+/LSLV12, Apc+/fl (M and N). Note that nuclear P-ERK 1/2 was not observed in adenomas from the AhCre+/T, K-ras+/+, Apc+/fl mouse even in large adenomas (L). However, nuclear staining was evident in a number of adenomas from AhCre+/T, K-ras+/LSLV12, Apc+/fl mice (M and N). Black arrows shows nuclear staining in adenomas. Wt represents AhCre+/T, K-ras+/+, Apc+/fl and K-Ras represents AhCre+/T, K-ras+/LSLV12, Apc+/fl. (Magnifications: D_–_N, ×400.)
Fig. 2C shows that expression of K-_ras_V12 rapidly accelerated intestinal tumorigenesis after Apc loss. By 165 days, 17 of 21 (81%) AhCre+/T, K-ras+/LSLV12, Apc+/fl animals had become symptomatic of disease and were culled because of the development of multiple intestinal tumors. Instead, only 2 of 20 (10%) AhCre+/T, K-ras+/+, Apc+/fl mice had a similar phenotype (χ2 = 20.739, df = 1, P = 0.0001). At death, there was no significant difference in tumor number or size (Mann–Whitney P >0.4, n = 18 and n = 6), indicating that mice were being culled when they had a similar tumor burden.
As K-ras oncogenes had previously been associated with increased mobility of colorectal cancer cells in vitro (6), we investigated whether the invasive status of the tumors was altered in AhCre+/T, K-ras+/LSLV12, Apc+/fl mice. No adenocarcinomas were found in 100 tumors analyzed in AhCre+/T, K-ras+/+, Apc+/fl mice. These data consolidate data in the Apc+/Min mouse, which rarely, if ever, show progression to adenocarcinoma and do not show K-ras mutations. However 17 of 100 tumors arising in AhCre+/T, K-ras+/LSLV12, Apc+/fl double mutant mice showed morphological evidence of invasion into the smooth muscle and were classified as adenocarcinomas (Fig. 2 F_–_I). This significant increase in invasion (χ2 = 18.579, df = 1, P = 0.001) was observed despite the early onset of tumor formation in this cohort.
We next investigated whether this increased tumorigenesis was associated with activation of the Raf-MEK-ERK pathway. Previous studies in vivo in both the colon and lung had failed to see dysregulation of these pathways after K-ras oncogene expression, even within tumors (9, 12). Although we did not see gross activation of Raf-MEK-ERK signaling in all intestinal adenomas, we did observe higher levels of P-MEK and occasional nuclei positive for P-ERK in a proportion of the small intestinal adenomas (≈10%) arising in AhCre+/T, K-ras+/LSLV12, Apc+/fl mice. Similar changes were not observed in the AhCre+/T, K-ras+/+, Apc+/fl cohort. There was also no gross activation of the Akt signaling pathways with similar patterns and levels of expression of P-AktSer-473 and its downstream effectors mTorSer-2448 and S6 kinaseThr-421,Ser-424 (data not shown). These data suggest either that relatively low-level activation of the Raf-MEK-ERK pathway is sufficient to mediate the effects of the K-_ras_V12 oncogene or that alternate pathways are responsible.
The K-_ras_V12 Oncogene Accelerates RC with Concomitant Activation of Raf-MEK-ERK and Akt Signaling Pathways.
The AhCre transgene also drives constitutive expression of Cre recombinase in the renal epithelium without causing Apc loss in the intestine. This property has been used to drive homozygous deletion of Apc in AhCre+, Apc fl/fl animals. Indeed, these mice spontaneously develop RC with an incidence of 30% at 12 months at age. These tumors invariably display loss of both Apc alleles. The relative low frequency of tumors, despite high levels of Cre-mediated recombination, appears to reflect spontaneous deletion of the majority of Apc-deficient cells in a p53-dependent manner (22).
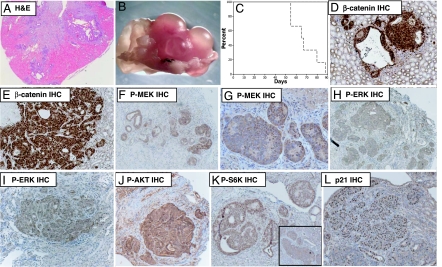
To investigate whether expression of the K-_ras_V12 oncogene accelerates RC in these mice, we first examined kidneys from (AhCre+/T, K-ras+/+, Apc+/+), (AhCre+/T, K-ras+/LSLV12, Apc+/+), (AhCre+/T, K-ras+/+, Apcfl/fl), and (AhCre+/T, K-ras+/LSLV12, Apc fl/fl) mice at 2 months of age (n = 12). At this time point no lesions were found in (AhCre+/T, K-ras+/+, Apc+/+), (AhCre+/T, K-ras+/LSLV12, Apc+/+), or (AhCre+/T, K-ras+/+, Apcfl/fl) animals. However, all floxed Apc mice expressing the K-_ras_V12 allele (AhCre+/T, K-ras+/LSLV12, Apcfl/fl mice) were characterized by RC in addition to many smaller lesions (Fig. 3A). To further analyze the onset of RC we then aged cohorts of (AhCre+/T, K-ras+/+, Apcfl/fl) and (AhCre+/T, K-ras+/LSLV12, Apcfl/fl) mice (n = 10) until they became symptomatic of disease (swollen, blood in urine, anemic). By 4 months of age all double mutant AhCre+/T, K-ras+/LSLV12, Apcfl/fl animals became ill and were confirmed to have RC (Fig. 3 B and C). At this time point, no RC was observed in the AhCre+/T, K-ras+/+, Apcfl/fl cohort. Molecular analysis revealed nuclear β-catenin within every kidney lesion, implying loss of the WT Apc alleles (Fig. 3 D and E). P21 and cyclin D1 was also up-regulated within most lesions (Fig. 3L and data not shown). Critically, we observed up-regulation of P-MEK and nuclear P-ERK, indicating that the Raf-MEK-ERK pathway was activated within these tumors (Fig. 3 F_–_I). We also observed up-regulation of P-AktSer-473 and the downstream effectors mTOR2448 and S6 kinaseSer-421/Thr-424 (Fig. 3 J and K). Up-regulation of Akt signaling and particularly mTOR has previously be associated in RC because of loss of the tuberous sclerosis tumor suppressor genes (TSC1 or TSC2) (23).
Fig. 3.
Oncogenic K-_ras_V12 rapidly accelerates renal cell carcinoma caused by Apc deficiency. (A) H&E-stained section of kidney from AhCre+/T, K-ras+/LSLV12, Apcfl/fl at 2 months of age. Note the presence of multiple kidney lesions. (B) Macroscopic image showing grossly altered kidney morphology of AhCre+/T, K-ras+/LSLV12, Apcfl/fl at 3 months of age. (C) Kaplan–Meier curve showing survival of AhCre+/T, K-ras+/LSLV12, Apcfl/fl (dashed line) and AhCre+/T, K-ras+/+, Apcfl/fl (solid line) after Cre induction (log rank P = 0.001, n = 10). All AhCre+/T, K-ras+/LSLV12, Apcfl/fl had to be culled by 4 months of age because of kidney tumor burden. At this stage no AhCre+/T, K-ras+/+, Apcfl/fl mice showed any overt signs of illness or had developed kidney tumors. (D and E) β-catenin is up-regulated and relocalized to the nuclei of all kidney lesions of AhCre+/T, K-ras+/LSLV12, Apcfl/fl mice. (F and G) Up-regulation of cytoplasmic P-MEK, which was observed in all kidney lesions. (H and I) Up-regulation of P-ERK 1/2, again observed in all kidney lesions of AhCre+/T, K-ras+/LSLV12, Apcfl/fl mice. (J) Up-regulation of P-Akt in kidney lesions arising in AhCre+/T, K-ras+/LSLV12, Apcfl/fl mice. (K) Nuclear P-S6 kinase in kidney lesions arising in AhCre+/T, K-ras+/LSLV12, Apc fl/fl. (Inset) Up-regulation of P-mTOR in the kidney lesions. (L) Up-regulation of p21 in kidney lesions arising in AhCre+/T, K-ras+/LSLV12, Apcfl/fl mice. (Magnifications: A, ×40; B_–_L, ×400.)
The relative lack of lesions in the AhCre+/T, K-ras+/+, Apcfl/fl mice at these stages suggests that expression of the oncogenic K-_ras_V12 allele allows the survival of Apc-deficient cells during the early stages of tumorigenesis. This parallels our previous study where we found p53 deficiency also accelerated tumorigenesis, although this effect was less marked than that observed here. K-_ras_V12 expression is also likely to drive increased lesion growth, as Apc-deficient kidneys are characterized by β-catenin-positive foci at 3 months but these do not progress to RC until many months later (22).
Two previous studies have shown activation of the Raf-MEK-ERK pathways in tumors in vivo after endogenous K-ras oncogene activation. Notably, one of these studies required coincident loss of PTEN in endometrioid ovarian adenocarcinoma (24). The second study was performed in pancreatic adenocarcinomas after combined expression of an oncogenic K-ras allele and loss of p53 (25). Together with the data presented here, these studies argue that both cellular and genetic contexts are critical determinants of the consequences of K-ras mutation. The observed differences in renal, pancreatic, and intestinal epithelium may reflect the relatively high levels of P-MEK and P-ERK staining in normal intestinal enterocytes compared with normal renal or pancreatic epithelium. Thus, the effects of K-ras mutation may be less aggressive in tissues (such as the intestinal epithelium) in which these pathways are normally activated and where the appropriate feedback loops are expressed. In this light it is of interest to note that within Madin Darby canine kidney cells loss of active ERK/MEK signaling restores cells to an epithelial state (26).
In the intestine, oncogenic K-ras expression leads to both accelerated adenoma development and increased invasiveness, but only after Apc deficiency. The latter effect is of particular note as one of the immediate phenotypes of Apc deficiency is a complete loss of cell migration and coincident up-regulation of proteins such as TIAM1 (27) and the EphB receptors (28). The later stages of colorectal cancer are marked by a progressive deregulation of proteins, including E-Cadherin, and the EphB receptors, which may reverse this consequence of Apc loss and so permit invasion. Our data suggest that expression of oncogenic K-ras provides a further mechanism for this reversal (28).
In summary, we have elucidated the consequences of expressing an endogenous K-_ras_V12 oncogene in intestinal and renal epithelium. Expression of the K-_ras_V12 oncogene in these tissues by itself had no phenotypic consequences. However, Apc deficiency makes these cells permissive for the tumorigenic properties of K-ras oncogenes. These tissues, however, differ markedly in the specific contributions of K-_ras_V12 expression. Whereas the renal epithelium is exquisitely sensitive to the consequences of K-_ras_V12 expression in the absence of Apc, expression of oncogenic K-_ras_V12 in the intestinal epithelium has little or no impact on physiology and does not accentuate the immediate effects of Apc deficiency. However, over the long term, oncogenic K-_ras_V12 expression accelerates both intestinal adenoma formation and invasion. These data demonstrate distinct tissue-specific responses to K-ras oncogenes, differences that may reflect the activation state of the Raf-MEK-ERK pathway in these tissues.
Materials and Methods
Experimental Animals.
All procedures were conducted according to U.K. Home Office regulations. Mice were maintained on an outbred background, and all mice were genotyped as described for the targeted Apc580s allele (15), K-_ras_V12 allele (10), and the AhCre transgene (13). Cre activity was induced in control and experimental mice by 3 i.p. injections of 80 mg/kg β-naphthoflavone within 24 h (4, 13).
Assaying Crypt Size, Apoptosis, Mitosis, and S-Phase Labeling in Vivo.
Apoptosis, crypt size, and mitotic index were scored from H&E-stained sections as described (4). For each analysis, 25 full crypts were scored from at least three mice of each genotype. For BrdU labeling, mice were injected either 2 h or 48 h before culling with 0.25 ml of BrdU (Amersham, Piscataway, NJ), and staining was performed using an anti-BrdU antibody (BD, Franklin Lakes, NJ) at 1 part in 100. At least three mice were used for each genotype and time point
β-Gal Analysis.
Intestinal whole mounts were prepared, fixed, and exposed to X-Gal substrate by using a method as reported (4).
Histology and IHC.
Intestinal tissue was fixed in ice-cold 10% neutral buffered formalin for no longer than 24 h before being processed into paraffin blocks according to standard procedures. Tissue sections (5 μm) were either stained with H&E for histological analysis or used for IHC. The details of the antibodies used are given in Supporting Text, which is published as supporting information on the PNAS web site.
RNA Extraction and Affymetrix Microarray Analysis.
Eight-week-old male mice were used for the array analysis. Three centimeters of the small intestine located 7 cm from the stomach was placed in RNA_later_ (Ambion, Austin, TX) (after removing any mesentery and ensuring that no Peyer’s patches were present). The tissue was then homogenized in Trizol reagent, and RNA was extracted by using standard phenol-chloroform methodologies. Biotinylated target cRNA was generated as described (4). Affymetrix (Santa Clara, CA) gene arrays were run at the Cancer Research U.K. facility at the Paterson Institute for Cancer Research. Samples were hybridized to MOE430_2 chips. In total, six chips were used: three for WT intestine and three for K-_ras_v12 intestine (each chip represented RNA made from an individual mouse intestine). Raw signal intensities were initially screened by using the MaxD/View program to remove false-positive and false-negative signals (using a cutoff of at least one P value for each transcript < 0.2). The data were then normalized by global normalization, using the MaxD/View-Program (4). qRT-PCR was performed as described in Supporting Text.
Supplementary Material
Supporting Information
Acknowledgments
This work was supported by Cancer Research U.K.
Abbreviations
PI
postinduction
IHC
immunohistochemistry
qRT-PCR
quantitative RT-PCR
RC
renal carcinoma
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kinzler KW, Vogelstein B. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 2.Bienz M, Clevers H. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 3.Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D, et al. Science. 1991;253:661–663. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 4.Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP, Batlle E, Simon-Assmann P, Clevers H, Nathke IS, et al. Genes Dev. 2004;18:1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malumbres M, Barbacid M. Nat Rev Cancer. 2003;3:459–65. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 6.Pollock CB, Shirasawa S, Sasazuki T, Kolch W, Dhillon AS. Cancer Res. 2005;65:1244–1250. doi: 10.1158/0008-5472.CAN-04-1911. [DOI] [PubMed] [Google Scholar]
- 7.Janssen KP, el-Marjou F, Pinto D, Sastre X, Rouillard D, Fouquet C, Soussi T, Louvard D, Robine S. Gastroenterology. 2002;123:492–504. doi: 10.1053/gast.2002.34786. [DOI] [PubMed] [Google Scholar]
- 8.Coopersmith CM, Chandrasekaran C, McNevin MS, Gordon JI. J Cell Biol. 1997;138:167–179. doi: 10.1083/jcb.138.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, Jacks T. Nature. 2001;410:1111–1116. doi: 10.1038/35074129. [DOI] [PubMed] [Google Scholar]
- 10.Guerra C, Mijimolle N, Dhawahir A, Dubus P, Barradas M, Serrano M, Campuzano V, Barbacid M. Cancer Cell. 2003;4:111–120. doi: 10.1016/s1535-6108(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 11.Chan IT, Kutok JL, Williams IR, Cohen S, Kelly L, Shigematsu H, Johnson L, Akashi K, Tuveson DA, Jacks T, Gilliland DG. J Clin Invest. 2004;113:528–538. doi: 10.1172/JCI20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuveson DA, Shaw AT, Willis NA, Silver DP, Jackson EL, Chang S, Mercer KL, Grochow R, Hock H, Crowley D, et al. Cancer Cell. 2004;5:375–387. doi: 10.1016/s1535-6108(04)00085-6. [DOI] [PubMed] [Google Scholar]
- 13.Ireland H, Kemp R, Houghton C, Howard L, Clarke AR, Sansom OJ, Winton DJ. Gastroenterology. 2004;126:1236–1246. doi: 10.1053/j.gastro.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Marshman E, Ottewell PD, Potten CS, Watson AJ. J Pathol. 2001;195:285–292. doi: 10.1002/path.967. [DOI] [PubMed] [Google Scholar]
- 15.Shibata H, Toyama K, Shioya H, Ito M, Hirota M, Hasegawa S, Matsumoto H, Takano H, Akiyama T, Toyoshima K, et al. Science. 1997;278:120–123. doi: 10.1126/science.278.5335.120. [DOI] [PubMed] [Google Scholar]
- 16.Park KS, Jeon SH, Kim SE, Bahk YY, Holmen SL, Williams BO, Chung KC, Surh YJ, Choi KY. J Cell Sci. 2006;119:819–827. doi: 10.1242/jcs.02779. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Mizukami Y, Zhang X, Jo WS, Chung DC. Gastroenterology. 2005;128:1907–1918. doi: 10.1053/j.gastro.2005.02.067. [DOI] [PubMed] [Google Scholar]
- 18.Kolch W. Nat Rev Mol Cell Biol. 2005;6:827–837. doi: 10.1038/nrm1743. [DOI] [PubMed] [Google Scholar]
- 19.Cho H, Kozasa T, Bondjers C, Betsholtz C, Kehrl JH. FASEB J. 2003;17:440–442. doi: 10.1096/fj.02-0340fje. [DOI] [PubMed] [Google Scholar]
- 20.Cismowski MJ, Takesono A, Ma C, Lizano JS, Xie X, Fuernkranz H, Lanier SM, Duzic E. Nat Biotechnol. 1999;17:878–883. doi: 10.1038/12867. [DOI] [PubMed] [Google Scholar]
- 21.Yip-Schneider MT, Lin A, Marshall MS. Biochem Biophys Res Commun. 2001;280:992–997. doi: 10.1006/bbrc.2001.4243. [DOI] [PubMed] [Google Scholar]
- 22.Sansom OJ, Reed KR, Griffiths D, Winton DJ, Clarke AR. Oncogene. 2005;24:8205–8210. doi: 10.1038/sj.onc.1208956. [DOI] [PubMed] [Google Scholar]
- 23.Hay N. Cancer Cell. 2005;8:179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Dinulescu DM, Ince TA, Quade BJ, Shafer SA, Crowley D, Jacks T. Nat Med. 2005;11:63–70. doi: 10.1038/nm1173. [DOI] [PubMed] [Google Scholar]
- 25.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 26.Schramek H, Feifel E, Marschitz I, Golochtchapova N, Gstraunthaler G, Montesano R. Am J Physiol. 2003;285:C652–C661. doi: 10.1152/ajpcell.00463.2002. [DOI] [PubMed] [Google Scholar]
- 27.Malliri A, Rygiel TP, van der Kammen RA, Song JY, Engers R, Hurlstone AF, Clevers H, Collard JG. J Biol Chem. 2006;281:543–548. doi: 10.1074/jbc.M507582200. [DOI] [PubMed] [Google Scholar]
- 28.Batlle E, Bacani J, Begthel H, Jonkheer S, Gregorieff A, van de Born M, Malats N, Sancho E, Boon E, Pawson T, et al. Nature. 2005;435:1126–1130. doi: 10.1038/nature03626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information