Cellular senescence is an important mechanism of tumor regression upon c-Myc inactivation (original) (raw)
Abstract
Oncogene-induced senescence is an important mechanism by which normal cells are restrained from malignant transformation. Here we report that the suppression of the c-Myc (MYC) oncogene induces cellular senescence in diverse tumor types including lymphoma, osteosarcoma, and hepatocellular carcinoma. MYC inactivation was associated with prototypical markers of senescence, including acidic β-gal staining, induction of p16INK4a, and p15INK4b expression. Moreover, MYC inactivation induced global changes in chromatin structure associated with the marked reduction of histone H4 acetylation and increased histone H3 K9 methylation. Osteosarcomas engineered to be deficient in p16INK4a or Rb exhibited impaired senescence and failed to exhibit sustained tumor regression upon MYC inactivation. Similarly, only after lymphomas were repaired for p53 expression did MYC inactivation induce robust senescence and sustained tumor regression. The pharmacologic inhibition of signaling pathways implicated in oncogene-induced senescence including ATM/ATR and MAPK did not prevent senescence associated with MYC inactivation. Our results suggest that cellular senescence programs remain latently functional, even in established tumors, and can become reactivated, serving as a critical mechanism of oncogene addiction associated with MYC inactivation.
Keywords: oncogene addiction, tumorigenesis, tumor maintenance
The c-myc proto-oncogene encodes a transcription factor that is known to play a crucial role in various biological processes such as cellular proliferation, growth, apoptosis, metabolism, adhesion, protein synthesis, DNA replication, and angiogenesis (1–3). The overexpression of c-Myc (MYC) is associated with tumorigenesis in a wide range of human cancers by causing inappropriate gene expression, resulting in autonomous cellular proliferation, while blocking cellular differentiation (4, 5). Myc is likely to contribute to tumorigenesis through an exaggeration of its physiologic functions.
c-Myc forms heterodimeric complexes with Max to bind to a DNA-motif called the E-box, thereby activating gene expression. In addition, Max heterodimerizes with Mad family members to antagonize Myc's ability to induce transcription (6). Both Myc:Max and Mad:Max complexes modulate gene expression by several mechanisms, including recruitment of coregulatory complexes that remodel the chromatin structure. Myc is known to associate with histone acetyl-transferase complexes such as TRRAP to induce the acetylation of nucleosomal histones, which results in transcriptional activation (7). However, Mad recruits histone deacetylases like Sin3, subsequently repressing transcription of its target genes (8–10). The Myc:Max network has been implicated in the regulation of hundreds of genes (2).
To understand how MYC initiates and maintains tumorigenesis, we and other groups have generated transgenic mice that conditionally overexpress MYC under the regulation of tissue specific promoters (11). Using the tetracycline regulatory system (Tet-off), we overexpressed MYC in different cellular lineages resulting in the formation of lymphomas, osteosarcomas, and hepatocellular carcinomas. The inactivation of MYC stereotypically causes sustained tumor regression, although the specific consequences depend upon the cell type (11–14). Upon MYC inactivation, lymphomas undergo proliferative arrest, differentiation, and apoptosis associated with sustained regression in > 70% of the transgenic mice. MYC inactivation in osteosarcoma results in the differentiation into mature osteocytes and the formation of bone but is not associated with significant apoptosis. In hepatocellular carcinoma, MYC inactivation also results in differentiation of tumor cells, in this case, into normal hepatocytes and biliary cells; however, a subpopulation of the cells retains its neoplastic properties upon MYC inactivation, hence exhibiting tumor dormancy. The inactivation of oncogenes such as MYC can reverse the process of transformation even in tumors with genomic complexity (15). However, the molecular mechanism by which the inactivation of MYC or other oncogenes induces tumor regression is still unclear.
Oncogene-induced senescence (OIS) is defined as irreversible cell cycle arrest of normal cells upon overexpression of oncogenes such as Ras and Raf (16, 17). OIS is believed to act as a barrier for tumorigenesis both in mouse and human tissues (17–21). Even though its molecular mechanism is not well understood, cellular senescence is accompanied by hallmark features including increased acidic β-gal activity (22) and up-regulation of cell cycle inhibitors like p15INK4b, p16INK4a, and p21CIP (18, 20, 22–24). In addition, OIS is associated with global changes in chromatin structure, in particular acetylation and methylation of histones (21, 25), leading to heterochromatin formation (18). Signaling pathways such as the DNA damage response (26, 27), mitogen-activated protein kinase (MAPK) (16, 28, 29), and the phosphoinositide-3 kinase (30, 31) are implicated in OIS. Interestingly, tumor cells can reactivate senescence pathways after treatment with chemotherapeutic agents, strongly suggesting that this process is retained in tumor cells (32).
Hence, we hypothesized that one possible mechanism by which suppression of oncogenes such as MYC induces tumor regression is by restoring cellular senescence programs. To interrogate this possibility, we examined the consequences of MYC inactivation in murine transgenic lymphoma, hepatocellular carcinoma, and osteosarcoma.
Results
Tumor Regression upon MYC Inactivation Is Associated with Increased Cellular Senescence Markers.
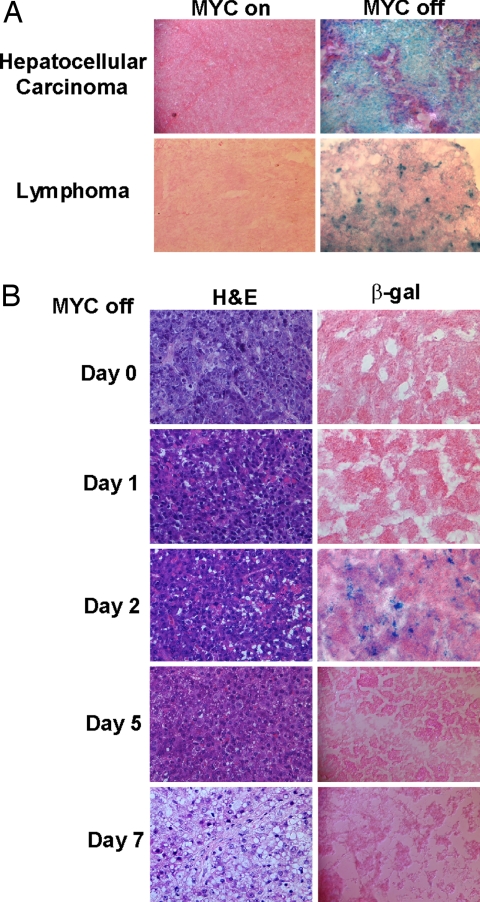
The suppression of MYC in primary MYC-induced lymphoma and hepatocellular carcinoma resulted in senescence-associated acidic β-gal (SA-β-Gal) staining (Fig. 1A and data not shown). Moreover, MYC inactivation in transplanted liver tumors also was associated with induced SA-β-Gal activity (Fig. 1B). Notably, after 5 days of MYC inactivation, liver tumors no longer exhibited SA-β-Gal activity. As previously reported, at this time point, the remaining tumor cells have differentiated into normal liver cells (14). Therefore, tumor regression in vivo appeared to be associated with cellular senescence in primary lymphoma and liver tumors as well as in transplanted liver tumors.
Fig. 1.
MYC inactivation induces cellular senescence in primary tumors in vivo. (A) Acidic β-gal (β-gal) staining was conducted for primary hepatocelullar carcinoma and lymphoma 2 days and 4 days after MYC inactivation. Primary MYC-induced hepatocellular carcinoma was transplanted to SCID mice s.c (14). (B) Tumor tissues were stained with acidic β-gal (22) or hematoxylin and eosin at different time points upon MYC inactivation.
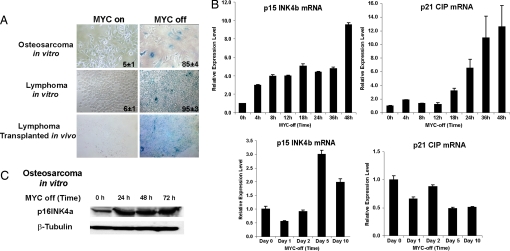
One possible explanation for our results is that cellular senescence is occurring secondarily to a host-dependent mechanism such as hypoxia associated with tumor regression (33, 34). To evaluate whether cellular senescence occurs through a cell-autonomous versus a host-dependent mechanism, the consequences of MYC inactivation were examined in lymphoma and osteosarcoma-derived cell lines in vitro (12, 13). Multiple independent tumors exhibited induction of SA-β-Gal activity upon MYC inactivation [Fig. 2A and supporting information (SI) Fig. 5]. In addition, transplanted lymphoma cell lines also exhibited SA-β-Gal activity upon MYC inactivation in vivo (Fig. 2A). Hence, MYC inactivation results in cellular senescence through a cell-autonomous mechanism.
Fig. 2.
MYC inactivation induces expression of cellular senescence markers in vitro and in vivo. (A) (Top and Middle) Acidic β-gal staining was conducted for osteosarcoma and lymphoma cell lines before and after MYC inactivation for 48 and 24 h, respectively. Means and standard deviations of the percentages of SA-β-gal-positive cells are indicated. (Bottom) s.c.-injected MYC-induced lymphoma cell line 6780 with MYC on or 3 days after MYC inactivation stained with eosin and β-gal activities. (B) Real-time PCR for p15INK4b and p21CIP were done in an osteosaroma cell line in vitro (Upper) and hepatocellular carcinoma in vivo (Lower) normalized by GAPDH. (C) Western blots with osteosarcoma cells with MYC on or MYC off conditions at various time points for p16INK4a expression.
To confirm further that MYC inactivation was resulting in cellular senescence, we examined the expression of additional molecular markers (17, 18, 20, 23, 32). Among them, p15INK4b and p21CIP have been shown to be MYC targets (35, 36). Upon MYC inactivation in vitro (SI Fig. 6), osteosarcoma cell lines exhibited the induction of p15INK4b and p21CIP mRNA expression by quantitative RT-PCR (Fig. 2B) and p16INK4a protein expression as shown by Western blot analysis (Fig. 2C). Similarly, upon MYC inactivation in vivo (SI Fig. 6), hepatocellular carcinomas exhibited induction of p15INK4b but did not exhibit induction of p21CIP mRNA expression (Fig. 2B). Thus, MYC inactivation resulted in the induction of p16INK4a and p15INK4b and variably p21CIP, which has been associated with cellular senescence.
MYC Inactivation Induces Global Changes in Chromatin Structure.
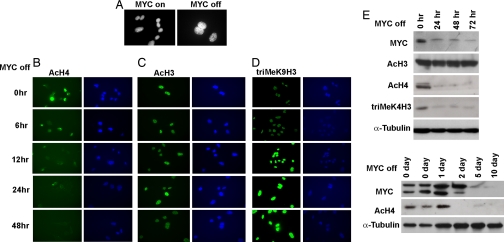
Cellular senescence is known to be associated with changes in chromatin structure (18, 25). Moreover, MYC recently has been shown to globally influence the acetylation and methylation of histones (10). Therefore, we examined global chromatin modifications associated with cellular senescence in osteosarcoma cell lines in vitro and hepatocellular carcinoma in vivo. First, we found heterochromatin formation upon MYC inactivation in osteosarcoma (Fig. 3A). After 12 h of MYC inactivation, there was marked global reduction of total acetylation of histone H4 detected by immunofluorescence and Western blot analysis (Fig. 3 B and E), reduction of trimethyl K4 histone H3 (Fig. 3E), and an increase of trimethyl K9 histone H3 (Fig. 3D). In contrast, there was no change in total acetylation of histone H3 (Fig. 3 C and E). Also, there was a global reduction in total histone H4 acetylation in transplanted hepatocellular carcinomas after 2 days of MYC inactivation (Fig. 3E). Hence, MYC inactivation results in global changes of histone modifications that have been associated with cellular senescence.
Fig. 3.
MYC inactivation induces global changes in chromatin structure. Bone tumor cells generated from mice were cultured in vitro and treated with 20 ng/ml doxycycline to inactivate MYC expression. (A) Heterochromatin formation was shown by DAPI staining at 48 h after MYC inactivation. (B–D) Immunofluorescence staining with anti-acetyl histone H4 (B), anti-acetyl histone H3 antibody (C), and anti-trimethyl K9 histone H3 antibody (D) upon MYC inactivation for 0, 6, 12, 24, and 48 h. There was a great reduction of MYC expression after 4 h of treatment of doxycycline (SI Fig. 7). (E) Western blots for MYC on and MYC off bone tumor cells in vitro and transplanted primary hepatocullar carcinoma in vivo probed with antibodies against MYC, acetyl histone H4, acetyl histone H3, trimethyl K4 histone H3, and α-tubulin.
Cellular Senescence Is Required for Tumor Regression upon MYC Inactivation.
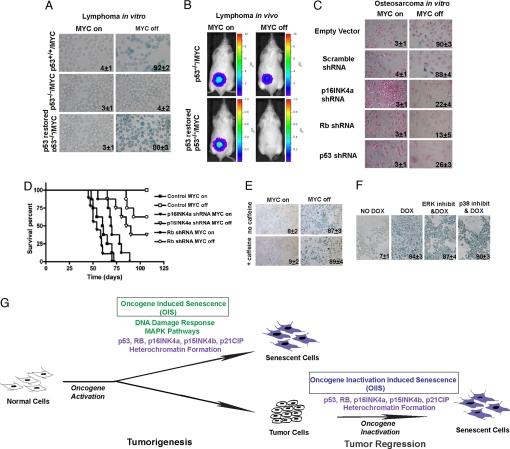
Cellular senescence requires the function of multiple tumor suppressors including p53, Rb, and p16INK4a (17, 18, 24, 32). We evaluated the effects of the loss of expression of these genes on the ability of MYC inactivation to induce senescence. Recently, we have described the generation of lymphoma cell lines that express p53, are engineered to be negative for p53, or have been subsequently restored for p53 expression by using a retroviral vector (33). We found that only lymphoma cell lines that expressed endogenous or restored p53, but not p53 deficient tumors, exhibited SA-β-Gal activity upon MYC inactivation (Fig. 4A). In addition, p53 expression was required for complete tumor regression upon MYC inactivation when transplanted into syngeneic hosts as quantified by bioluminescence imaging (Fig. 4B). Thus, the loss of p53 prevents both cellular senescence and complete tumor regression upon MYC inactivation.
Fig. 4.
Loss of p53, Rb, or p16INK4a affects cellular senescence and tumor regression upon MYC inactivation. (A) Acidic β-gal staining for MYC, p53−/−/MYC, and p53 restored lymphomas in MYC on and MYC off after 24 h. Means and standard deviations of the percentages of SA-β-gal-positive cells are indicated. Bioluminescence imaging of tumor cell elimination of p53-negative versus p53-restored lymphomas before and after MYC inactivation. (B) Luciferase-labeled lymphoma cells (107) were inoculated into syngeneic mice, then imaged by bioluminescence imaging on the day of MYC inactivation (MYC On) and 9 days after MYC inactivation (MYC Off). (C) Acidic β-gal staining for osteosarcoma cells infected with scramble shRNA, Rb shRNA, p16INK4a shRNA, or p53 shRNA in MYC on and MYC off for 48 h. The cells were counterstained with eosin. (D) Tumor-free survival of mice that had been injected with 105 bone tumor cells infected with vector control or RB or p16INK4a shRNA vectors. (E) MYC-induced lymphoma cells were treated with or without 5 mM caffeine 1 h before turning MYC off. SA-β-Gal staining was performed 24 h later after MYC inactivation. Means and standard deviations of the percentages of SA-β-gal-positive cells are indicated. (F) MYC-induced lymphomas were treated with the ERK1/2 pathway inhibitor (U0126, 10 μM) or p38-MAPK pathway inhibitor (SB203580, 10 μM) for 48 h with MYC off conditions. (G) Cells were collected for SA-β-gal staining. A model for OIS and oncogene inactivation-induced senescence is illustrated here.
Next, we suppressed Rb, p16INK4a, or p53 expression in osteosarcoma cell lines by retrovirally delivered short hairpin RNA (shRNA) (SI Fig. 8). Cells in which Rb, p16INK4a, or p53 was suppressed, but not in the scramble control, exhibited loss of SA-β-Gal staining upon MYC inactivation (Fig. 4C). Upon MYC inactivation, tumor cells with suppressed p16INK4a, p53, or Rb exhibited continued cellular proliferation (18.09%, 12.10%, or 13.54% versus 0.37%, respectively; SI Fig. 9_A_). These shRNAs also impeded the block of cell growth induced by MYC inactivation assayed by colony formation (SI Fig. 9_B_). As a negative control, tumor cells expressing a scrambled shRNA control did not exhibit changes in cellular proliferation or growth upon MYC inactivation. Thus, the knockdown of p16INK4a, Rb, or p53 expression suppressed both cellular senescence and cell cycle arrest upon MYC inactivation.
We examined whether the perturbation of cellular senescence programs influenced the ability of MYC inactivation to induce sustained tumor regression in osteosarcoma. Upon MYC inactivation, tumors expressing shRNA against p16INK4a or Rb exhibited sustained regression in only 40% (P = 0.0085) or 60% (P = 0.0628) of mice, respectively. In marked contrast, control tumors exhibited 100% sustained tumor regression (Fig. 4D). Thus, inhibition of p16INK4a or Rb prevents MYC inactivation from inducing sustained tumor regression of osteosarcoma in vivo.
DNA Damage Response and MAPK Pathways Are Not Required for Senescence Induced by MYC Inactivation.
Oncogene activation induced senescence in normal cells recently has been shown to be mediated by specific signaling pathways including ATM/ATR (26, 27) and MAPK (16, 28, 29). To test whether senescence upon MYC-inactivation in tumor cells similarly depends on DNA-repair pathways, lymphoma cells were treated with the ATM/ATR inhibitor caffeine (27, 37). However, we did not observe any decrease in SA-β-Gal-positive cells (Fig. 4E and SI Fig. 10). OIS also appears to require ERK1/2 and p38-MAPK signaling (16, 28, 29). Pharmacologic inhibition of ERK1/2 or p38-MAPK using U0126 or SB203580, respectively, at 10 μM for 48 h did not prevent MYC inactivation-induced senescence as monitored by SA-β-Gal staining, p15INK4b, and p21CIP expression (Fig. 4F and SI Fig. 11).
Therefore, we conclude that the inhibition of DNA-damage response, ERK1/2, and p38-MAPK signaling pathways are not sufficient to prevent cellular senescence upon MYC inactivation.
Discussion
Our results illustrate that cellular senescence is an important mechanism of sustained tumor regression upon MYC oncogene inactivation in hematopoietic tumors, osteosarcomas, and hepatocellular carcinomas. MYC inactivation was associated with several molecular features of cellular senescence including elevated acidic-β-gal activity, increased expression of the cell cycle inhibitors p15INK4b and p16INK4a, the formation of heterochromatin, and characteristic changes in chromatin structure like histone H3 K9 methylation. Importantly, we observed that MYC inactivation either in vitro or in vivo results in cellular senescence, strongly suggesting that senescence programs are activated through cell autonomous programs, as opposed to indirect consequences of tumor involution and local hypoxia.
Moreover, both cellular senescence and sustained tumor regression upon MYC inactivation were shown to require the expression of p16INK4a, Rb, and p53, and the reconstitution of p53 expression correspondingly restored the ability of MYC inactivation to induce senescence. Hence, the suppression of MYC oncogene appears to induce senescence through at least some pathways overlapping with oncogene activation-induced senescence (19–21, 24). Furthermore, MYC may be able to block the function of tumor-suppressor genes including p16INK4a, Rb, and p53 (38). Hence, inactivating MYC now may restore their functions. Even though the mechanism by which MYC inactivation inducing cellular senescence is not clear and needs further examination, our results suggest that unlike OIS in normal cells, the inhibition of ATM/ATR or MAPK pathways does not impede oncogene inactivation induced senescence in tumor cells. Because MYC is downstream of MAPK pathways (39), our results suggest that inactivating MYC is capable of executing senescence programs independently of upstream regulatory pathways.
Previously, we have shown that the suppression of the MYC oncogene results in distinct specific consequences in different types of tumors. Here, we illustrate that in many types of cancers, MYC inactivation induces tumor regression ultimately through a common convergent pathway associated with cellular senescence. Hence, in hematopoietic tumors, MYC inactivation resulted in an initial proliferative arrest, differentiation, and senescence, subsequently followed by complete elimination through apoptosis (12). In osteosarcoma, MYC inactivation resulted in proliferative arrest and differentiation of tumor cells into mature osteocytes associated with senescence, but no significant apoptosis (13). Finally, in hepatocellular carcinoma, tumors underwent senescence, differentiation, and apoptosis (14). We noted that the remaining differentiated hepatocytes did not exhibit senescence, which possibly could explain why MYC reactivation restored their neoplastic properties (14). Thus, cellular senescence pathways appear to be an important component of oncogene addiction associated with MYC overexpression.
Notably, recent reports have variously suggested or refuted that knockout of Myc results in senescence. One report demonstrated that the knockout of one allele of c-myc in human fibroblasts results in senescence through p16INK4a and Bmi-1 (40). However, other reports showed that the complete knockout of either n-myc or c-myc, respectively, does not induce cellular senescence in normal cells (10, 41). Our results suggest that MYC inactivation in tumor cells more generally results in senescence.
An important implication of our results is that cellular senescence pathways must remain intact in tumors as has been suggested (32). Inactivation of MYC appeared to reactivate cellular senescence pathways that then must be silenced in tumor cells. This shift in balance between tumor-promoting and tumor-suppressing signals has been suggested to be integral to the phenomena of oncogene addiction (42). Furthermore, recently it was suggested that oncogene inactivation may induce apoptosis in tumors by flipping the balance of pro- and antiapoptotic pathways (43). Indeed, our observation supports this conclusion and illustrates that one possible overriding mechanism is that upon oncogene inactivation tumors undergo cellular senescence. The subsequent decision of whether tumor cells undergo senescence later accompanied by their complete elimination through apoptosis depends on other factors specific to a given type of cancer.
Our studies suggest that cellular senescence may be an important general mechanism by which targeted therapeutics induces tumor regression. It long has been postulated that MYC plays a role in the immortalization of tumor cells (44), so it is perhaps less surprising that the inactivation of MYC could result in senescence. However, it remains to be seen whether suppressing other oncogenes in murine and even more importantly human tumors will result in senescence. In this regard, we have preliminary results indicating that inactivation of K-Ras in lymphoma also induced senescence (unpublished results), suggesting that this oncogene inactivation induced senescence (OIIS) may be a general mechanism of tumor regression upon oncogene inactivation (Fig. 4G). Previous reports (45, 46) suggested that activating apoptosis or cellular senescence could serve as an intrinsic mechanism for tumor regression. Recent observations support the idea that the restoration of p53 function may contribute to tumor regression by inducing senescence (47, 48).
We recognize that there are likely to be multiple mechanisms by which oncogene inactivation results in tumor regression, including both cell intrinsic mechanisms and host dependence mechanisms such as the inhibition of angiogenesis (33). Here we uncover that cellular senescence appears to be a common critical, tumor cell-intrinsic mechanism for MYC-associated oncogene addiction.
Materials and Methods
Cell Culture, Western Blots, and RT-PCR.
Cell lines derived from osteogenic sarcomas were cultured in DMEM supplemented with 10% FBS/1% penicillin/streptomycin/1% l-glutamine/1% nonessential amino acids. Lymphoma derived cell lines were grown in RPMI medium 1640 with 10% FBS/1% penicillin/Streptomycin/1% l-glutamine/50 μM 2-mercaptoethanol. To inactivate MYC expression, 20 ng/ml doxycycline was added to the medium. To inhibit ATM and ATR, 1 h before the doxycycline treatment, 5 mM caffeine (Sigma, St. Louis, MO) were added to the tissue culture medium (37). For colony formation assay, 4.25 × 10_e_3 of bone tumor cells were seeded on 10-cm tissue culture plates with no doxycycline or 20 ng/ml doxycyline in the medium. Four days after, cells were fixed with methanol and stained with 0.5% methylene blue in 85% ethanol. Antibodies for Western blots and primers for real-time PCR are listed in SI Methods.
Immunofluorescence.
Cells were fixed with 4% paraformaldehyde in PBS for 15 min at room temperature for anti-acetyl histone H4 (06-598; Upstate Biotechnology) and trimethyl K9 histone H3 (8898; Abcam). For anti-acetyl histone H3 (06-599; Upstate Biotechnology) staining, cells were fixed with 95% ethanol and 5% acetic acid for 1 min at room temperature. Cells were permeabilized with 0.2% Triton X-100 (EM Science, Gibbstown, NJ) in PBS for 10 min at room temperature. FITC-labeled secondary antibody (F-0382; Sigma) were applied at the concentration of 1:500. Images were taken with Nikon E800 scope. Senescence-associated heterochromatin staining was conduced as described (18).
Animal Experiments.
Tet-_o_-MYC transgenic mice have been described (12). All animal experiments were performed by following the guidelines from Administrative Panel on Laboratory Animal Care at Stanford University (protocol 8144). To inactivate MYC expression, drinking water was supplemented with 200 μg/ml doxycycline. β-Gal staining of tumors were conduced as described (32).
In Vivo Bioluminescence Imaging.
p53−/− and p53-restored tumor cells, expressing the luciferase enzyme, were injected i.p. or s.c. into syngeneic mice. Tumors were allowed to develop until reaching approximately the same bioluminescent signal. Tumor regression then was induced by doxycycline treatment (200 μg/ml). Transgenic mice were anesthetized with a combination of inhaled isoflorane/oxygen delivered by the Xenogen XGI-8 5-port Gas Anesthesia System. An aqueous solution of the substrate d-luciferin (150 mg/kg) was injected into the animal's peritoneal cavity 10 min before imaging. Animals then were placed into a light-tight chamber and imaged with an IVIS-100 cooled CCD camera (Xenogen, Alameda, CA).
Retrovirus Constructs, Virus Production, and Tumor Cell Infection.
Vectors containing RNAi sequences against p16INK4a and Rb (MSCV-LMP), MSCV-IRES-GFP. and MSCV-p53-IRES-GFP constructs were all kindly provided by S. Lowe (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY). Scramble shRNA sequences were designed (AGCGCGCTTTGTAGGATTCG) and cloned in to the MSCV-LMP vector as described (Open Biosystems). MSCV-LUC-IRES-GFP and MSCV-Puro-LUC constructs, a modified version of the pDON plasmid vector (Takara Mirus Bio, Madison, WI), was kindly provided by Luis Soares (Stanford University). Tumor cells were incubated with retroviruses containing supernatants for 12 h at 32°C in media containing 4 μg/ml polybrene. Cells then were expanded at 37°C for an additional 48 h, and GFP-expressing cells were purified by flow cytometry on a FACS Vantage (Becton Dickinson). Cells containing MSCV-Puro-LUC were selected with puromycin. The restoration of p53 protein expression was confirmed by Western blot analysis.
Supplementary Material
Supporting Information
Acknowledgments
We dedicate this work in memory of Arthur Lantz. We thank the many members of the D.W.F. laboratory for generously providing their suggestions and Dr. Scott Lowe for generously providing us with the shRNA retroviral vectors for p16INK4a, p53, and RB. This work was supported by National Cancer Institute Grants R01-CA85610, R01-CA105102, 3R01CA089305–03S1, NIH/NCI ICMIC P50, and NIH/NCI 1P20 CA112973; Leukemia and Lymphoma Society; Burroughs Wellcome Fund; and the Damon Runyon Lilly Clinical Investigator Award (to D.W.F.); Leukemia and Lymphoma Society (A.C.F.); Lymphoma Research Foundation (J.v.R.); and Howard Hughes Medical Institute (P.B.).
Abbreviations
MYC
c-Myc
OIS
oncogene-induced senescence
Sa-β-gal
senescence-associated acidic β-gal
shRNA
short hairpin RNA.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Dang CV, O'Donnell KA, Zeller KI, Nguyen T, Osthus RC, Li F. Semin Cancer Biol. 2006;16:253–264. doi: 10.1016/j.semcancer.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Oster SK, Ho CS, Soucie EL, Penn LZ. Adv Cancer Res. 2002;84:81–154. doi: 10.1016/s0065-230x(02)84004-0. [DOI] [PubMed] [Google Scholar]
- 3.Secombe J, Pierce SB, Eisenman RN. Cell. 2004;117:153–156. doi: 10.1016/s0092-8674(04)00336-8. [DOI] [PubMed] [Google Scholar]
- 4.Adhikary S, Eilers M. Nat Rev Mol Cell Biol. 2005;6:635–645. doi: 10.1038/nrm1703. [DOI] [PubMed] [Google Scholar]
- 5.Dang CV. Mol Cell Biol. 1999;19:1–11. doi: 10.1128/mcb.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelengaris S, Khan M, Evan G. Nat Rev Cancer. 2002;2:764–776. doi: 10.1038/nrc904. [DOI] [PubMed] [Google Scholar]
- 7.Bouchard C, Dittrich O, Kiermaier A, Dohmann K, Menkel A, Eilers M, Luscher B. Genes Dev. 2001;15:2042–2047. doi: 10.1101/gad.907901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez PC, Frank SR, Wang L, Schroeder M, Liu S, Greene J, Cocito A, Amati B. Genes Dev. 2003;17:1115–1129. doi: 10.1101/gad.1067003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank SR, Schroeder M, Fernandez P, Taubert S, Amati B. Genes Dev. 2001;15:2069–2082. doi: 10.1101/gad.906601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knoepfler PS, Zhang XY, Cheng PF, Gafken PR, McMahon SB, Eisenman RN. EMBO J. 2006;25:2723–2734. doi: 10.1038/sj.emboj.7601152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felsher DW. Nat Rev Cancer. 2003;3:375–380. doi: 10.1038/nrc1070. [DOI] [PubMed] [Google Scholar]
- 12.Felsher DW, Bishop JM. Mol Cell. 1999;4:199–207. doi: 10.1016/s1097-2765(00)80367-6. [DOI] [PubMed] [Google Scholar]
- 13.Jain M, Arvanitis C, Chu K, Dewey W, Leonhardt E, Trinh M, Sundberg CD, Bishop JM, Felsher DW. Science. 2002;297:102–104. doi: 10.1126/science.1071489. [DOI] [PubMed] [Google Scholar]
- 14.Shachaf CM, Kopelman AM, Arvanitis C, Karlsson A, Beer S, Mandl S, Bachmann MH, Borowsky AD, Ruebner B, Cardiff RD, et al. Nature. 2004;431:1112–1117. doi: 10.1038/nature03043. [DOI] [PubMed] [Google Scholar]
- 15.Karlsson A, Giuriato S, Tang F, Fung-Weier J, Levan G, Felsher DW. Blood. 2003;101:2797–2803. doi: 10.1182/blood-2002-10-3091. [DOI] [PubMed] [Google Scholar]
- 16.Zhu J, Woods D, McMahon M, Bishop JM. Genes Dev. 1998;12:2997–3007. doi: 10.1101/gad.12.19.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 18.Narita M, Nunez S, Heard E, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW. Cell. 2003;113:703–716. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- 19.Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, Majoor DM, Shay JW, Mooi WJ, Peeper DS. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 20.Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, Benguria A, Zaballos A, Flores JM, Barbacid M, et al. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 21.Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, Stein H, Dorken B, Jenuwein T, Schmitt CA. Nature. 2005;436:660–665. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- 22.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan HM, Narita M, Lowe SW, Livingston DM. Genes Dev. 2005;19:196–201. doi: 10.1101/gad.1280205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, et al. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang R, Poustovoitov MV, Ye X, Santos HA, Chen W, Daganzo SM, Erzberger JP, Serebriiskii IG, Canutescu AA, Dunbrack RL, et al. Dev Cell. 2005;8:19–30. doi: 10.1016/j.devcel.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, Schurra C, Garre M, Nuciforo PG, Bensimon A, et al. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- 27.Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou LV, Kolettas E, Niforou K, Zoumpourlis VC, et al. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- 28.Cammarano MS, Nekrasova T, Noel B, Minden A. Mol Cell Biol. 2005;25:9532–9542. doi: 10.1128/MCB.25.21.9532-9542.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorenzini A, Tresini M, Mawal-Dewan M, Frisoni L, Zhang H, Allen RG, Sell C, Cristofalo VJ. Exp Gerontol. 2002;37:1149–1156. doi: 10.1016/s0531-5565(02)00133-x. [DOI] [PubMed] [Google Scholar]
- 30.Collado M, Medema RH, Garcia-Cao I, Dubuisson ML, Barradas M, Glassford J, Rivas C, Burgering BM, Serrano M, Lam EW. J Biol Chem. 2000;275:21960–21968. doi: 10.1074/jbc.M000759200. [DOI] [PubMed] [Google Scholar]
- 31.Courtois-Cox S, Genther Williams SM, Reczek EE, Johnson BW, McGillicuddy LT, Johannessen CM, Hollstein PE, MacCollin M, Cichowski K. Cancer Cell. 2006;10:459–472. doi: 10.1016/j.ccr.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitt CA, Fridman JS, Yang M, Lee S, Baranov E, Hoffman RM, Lowe SW. Cell. 2002;109:335–346. doi: 10.1016/s0092-8674(02)00734-1. [DOI] [PubMed] [Google Scholar]
- 33.Giuriato S, Ryeom S, Fan AC, Bachireddy P, Lynch RC, Rioth MJ, van Riggelen J, Kopelman AM, Passegue E, Tang F, et al. Proc Natl Acad Sci USA. 2006;103:16266–16271. doi: 10.1073/pnas.0608017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welford SM, Bedogni B, Gradin K, Poellinger L, Broome Powell M, Giaccia AJ. Genes Dev. 2006;20:3366–3371. doi: 10.1101/gad.1471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gartel AL, Ye X, Goufman E, Shianov P, Hay N, Najmabadi F, Tyner AL. Proc Natl Acad Sci USA. 2001;98:4510–4515. doi: 10.1073/pnas.081074898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staller P, Peukert K, Kiermaier A, Seoane J, Lukas J, Karsunky H, Moroy T, Bartek J, Massague J, Hanel F, et al. Nat Cell Biol. 2001;3:392–399. doi: 10.1038/35070076. [DOI] [PubMed] [Google Scholar]
- 37.Sarkaria JN, Busby EC, Tibbetts RS, Roos P, Taya Y, Karnitz LM, Abraham RT. Cancer Res. 1999;59:4375–4382. [PubMed] [Google Scholar]
- 38.Felsher DW, Zetterberg A, Zhu J, Tlsty T, Bishop JM. Proc Natl Acad Sci USA. 2000;97:10544–10548. doi: 10.1073/pnas.190327097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sears R, Nuckolls F, Haura E, Taya Y, Tamai K, Nevins JR. Genes Dev. 2000;14:2501–2514. doi: 10.1101/gad.836800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guney I, Wu S, Sedivy JM. Proc Natl Acad Sci USA. 2006;103:3645–3650. doi: 10.1073/pnas.0600069103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oskarsson T, Essers MA, Dubois N, Offner S, Dubey C, Roger C, Metzger D, Chambon P, Hummler E, Beard P, et al. Genes Dev. 2006;20:2024–2029. doi: 10.1101/gad.381206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weinstein IB. Science. 2002;297:63–64. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- 43.Sharma SV, Gajowniczek P, Way IP, Lee DY, Jiang J, Yuza Y, Classon M, Haber DA, Settleman J. Cancer Cell. 2006;10:425–435. doi: 10.1016/j.ccr.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grandori C, Wu KJ, Fernandez P, Ngouenet C, Grim J, Clurman BE, Moser MJ, Oshima J, Russell DW, Swisshelm K, et al. Genes Dev. 2003;17:1569–1574. doi: 10.1101/gad.1100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lowe SW, Cepero E, Evan G. Nature. 2004;432:307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- 46.Sharpless NE, DePinho RA. Nature. 2005;436:636–637. doi: 10.1038/436636a. [DOI] [PubMed] [Google Scholar]
- 47.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, Newman J, Reczek EE, Weissleder R, Jacks T. Nature. 2007;445:661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information