CD28-dependent Activation of Protein Kinase B/Akt Blocks Fas-mediated Apoptosis by Preventing Death-inducing Signaling Complex Assembly (original) (raw)
Abstract
The T cell costimulatory molecule CD28 is important for T cell survival, yet both the signaling pathways downstream of CD28 and the apoptotic pathways they antagonize remain poorly understood. Here we demonstrate that CD4+ T cells from CD28-deficient mice show increased susceptibility to Fas-mediated apoptosis via a phosphatidylinositol 3-kinase (PI3K)-dependent pathway. Protein kinase B (PKBα/Akt1) is an important serine/threonine kinase that promotes survival downstream of PI3K signals. To understand how PI3K-mediated signals downstream of CD28 contribute to T cell survival, we examined Fas-mediated apoptosis in T cells expressing an active form of PKBα. Our data demonstrate that T cells expressing active PKB are resistant to Fas-mediated apoptosis in vivo and in vitro. PKB transgenic T cells show reduced activation of caspase-8, BID, and caspase-3 due to impaired recruitment of procaspase-8 to the death-inducing signaling complex (DISC). Similar alterations are seen in T cells from mice which are haploinsufficient for PTEN, a lipid phosphatase that regulates phosphatidylinositol-3,4,5-trisphosphate (PIP3) and influences PKBα activity. These findings provide a novel link between CD28 and an important apoptosis pathway in vivo, and demonstrate that PI3K/PKB signaling prevents apoptosis by inhibiting DISC assembly.
Keywords: CD28, PKB/Akt, apoptosis, Fas, caspase-8
Introduction
T cell costimulatory molecules play an important role in T cell activation and survival. The best characterized of these molecules is CD28, which plays a multifaceted role in regulating T cell function after interaction with its ligands, CD80 (B7.1) and CD86 (B7.2). Studies have shown that CD28 plays an important role in early events during T cell activation, survival, tolerance, and the development of Th2 responses (1, 2). Costimulation by CD28 has been shown to promote T cell viability via the upregulation of the antiapoptotic molecule Bcl-XL (3–7); however, the apoptotic pathways that are altered by CD28 costimulation remain unknown. In addition, the signaling pathways that relay CD28 survival signals are largely undefined in vivo.
One molecule involved in influencing T cell survival is the serine/threonine kinase protein kinase B (PKB/Akt)* (8–11). PKB is readily activated in response to phosphatidylinositol-3,4,5-trisphosphate (PIP3), a lipid second messenger generated by phosphatidylinositol 3-kinase (PI3K) (12, 13). PI3K-mediated generation of PIP3 functions to recruit PKB and the protein kinase PDK1 to the plasma membrane, allowing for direct phosphorylation and activation of PKB. Phosphorylation on Thr-308 and Ser-473 is required for the full activation of PKBα, which then dissociates from the plasma membrane and phosphorylates a variety of substrates (13). The phosphatase PTEN antagonizes this process by selectively cleaving the D3 phosphate from PIP3 (14, 15). PKB can be activated in a PI3K-dependent manner downstream of CD28 (16, 17), indicating that PKB may be a key player in mediating antiapoptotic signals via CD28.
The balance between survival and death is of critical physiological importance, as apoptotic death is essential in many phases of lymphocyte development and function (18, 19). The Fas/FasL signaling pathway highlights the importance of apoptosis for the normal function and maintenance of the immune system (20). Lpr and gld mice harboring natural mutations in fas or fas ligand genes respectively develop a systemic autoimmune disorder marked by an accumulation of activated T and B cells, autoantibody production, and other features of autoimmunity (21). Mutations in Fas or FasL genes or other genes influencing the Fas signaling pathway in humans can lead to an _lpr_-like disease with characteristic nonmalignant lymphadenopathy known as autoimmune lymphoproliferative syndrome (ALPS) or Canale-Smith Syndrome (22–25). Given the importance of normal apoptotic signaling to proper T cell function, identifying the signaling networks which influence death receptor–induced apoptosis in lymphocytes is of great importance.
This study examines the influence of CD28-mediated costimulatory signals on Fas-mediated apoptosis in T cells. Using a variety of transgenic and gene-deficient mice, we have examined the PI3K/PKB signaling pathway downstream of CD28 and have identified a role for CD28, PI3K, and PKB in preventing Fas-mediated death in vivo. Here we demonstrate that the CD28-PI3K-PTEN-PKB signaling axis is involved in preventing Fas-mediated death by regulating the assembly of the Fas death-inducing signaling complex (DISC).
Materials and Methods
Mice.
hCD2-gag-PKB (B6/PKB, backcrossed eight generations to C57BL/6J) (8), PTEN+/− (14), and B6/lpr/lpr (21) mice have been described previously. MRL/MpJ (MRL), MRL/MpJ/lpr/lpr (MRL/lpr), and C57BL/6J (B6) mice were obtained from the The Jackson Laboratory. TCR-transgenic mice were previously generated using α and β chains isolated from CTL clone P14, which recognizes the LCMV glycoprotein (peptide p33–41) in the context of H-2Db (26). Mice homozygous for the P14 TCR transgene (327 line) were bred with heterozygous gag-PKB mice to generate P14/PKB offspring. B6/PKB mice were backcrossed seven generations to MRL/MpJ mice to generate MRL/PKB mice. All mice were maintained in a specific pathogen-free environment at the Ontario Cancer Institute according to institutional guidelines.
Reagents and Antibodies.
Recombinant hCD8–mFasL fusion protein was generously supplied by Mark Bray. Purified anti-CD3 (2C11), anti-CD28 (37.51), and staphylococcal enterotoxin B (SEB) was purchased from BD Biosciences. The LCMV glycoprotein peptide p33 (KAVYNFATM) was synthesized and purified as described previously (27). The following monoclonal antibodies were used for flow cytometry (either FITC-, PE-, or biotin-conjugated): anti-CD4 (FITC or PE), anti-CD8–FITC, anti-CD3 (FITC or biotin), anti-B220–PE, anti-CD19–PE, anti-CD69–biotin, anti-CD23–FITC, anti-Vα2–biotin, anti-Vβ8–biotin, and anti-Fas–biotin (BD Biosciences). Biotinylated antibodies were detected with streptavidin-red 670 (GIBCO BRL).
Flow Cytometry.
Thymus, spleen, Peyer's patches, and lymph nodes from 12-wk-old mice (MRL strains) were removed and placed in sterile HBSS. Single cell suspensions of lymphoid organs were generated by gently pressing organs through sterile wire mesh. Cells were resuspended in IMDM, supplemented with 10% heat-inactivated FCS (GIBCO BRL), 50 μM β-mercaptoethanol, 2 mM glutamine, and 0.1% penicillin/streptomycin, and counted. For flow cytometric analysis, 0.2–1 × 106 cells were stained with combinations of monoclonal antibodies at 4°C in PBS containing 2% FCS and 2% NaN3. All flow cytometric analysis was performed on a FACScan™ instrument (Becton Dickinson). Samples were gated for live cells based upon forward and side scatter parameters (10,000 events/sample) and analyzed using CELLQuest™ software.
Fas Apoptosis Assays.
Splenocytes from 10–12-wk-old mice were isolated and cultured with plate-bound anti-CD3 (2C11; 10 μg/ml) and anti-CD28 (37.51; 5 μg/ml; BD Biosciences) for 24 h, followed by culture in media containing murine recombinant IL-2 (50 U/ml; PeproTech) for 4 d. Viable, activated lymphocytes were isolated using Lympholyte-M (Cedarlane), cultured in the presence of IL-2, and treated with recombinant hCD8-mFasL for various time points. Fas-specific apoptosis was determined by flow cytometry using Annexin V and PI or 7AAD staining (R&D Systems). Mitochondrial permeability was assessed via flow cytometry using DiOC6 (gift from Josef Penninger, Amgen Institute, Toronto, Canada) according to established protocols.
In Vivo SEB- and Peptide-mediated Deletion.
For SEB-mediated deletion experiments, SEB (80 μg/mouse) was injected intravenously into 8–12-wk-old mice. The percentage of Vβ8+CD4+ and Vβ6+CD4+ T cells in peripheral blood was determined by flow cytometry every 3–7 d for 1 mo. Peptide-specific deletion of P14 TCR transgenic T cells was determined by measuring the percentage of Vα2+CD8+ T cells in peripheral blood of P14 TCR transgenic animals (P14 or P14/PKB) after intravenous injection with p33 peptide (5 μg/mouse, injected three times over 6 d).
Western Blot Analysis.
Single-cell suspensions were lysed by incubation on ice in Gentle Soft Buffer (10 mM NaCl, 20 mM PIPES, pH 7.4, 0.5% NP-40, 5 mM EDTA, 5 μg/ml leupeptin, 1 mM benzamidine, 0.5 mM NaF, and 100 μM Na3VO4) for 20 min. Lysates were cleared by centrifugation and supernatants were normalized for total protein (Bio-Rad Laboratories). Proteins were resolved by 10–12% SDS-PAGE, electroblotted to PVDF membrane (Costar), blocked in 5% TBS, 0.05% Tween-20, and probed with primary antibodies. Anti-PKB, anti-phospho-S473 PKB, and anti-PARP antibodies were purchased from Cell Signaling Technology. Anti–caspase-3, anti-Fas-associated molecule with a death domain (FADD), and anti-Fas are from BD Transduction Laboratories. Anti–GSK-3 and anti-FLICE-inhibitory protein (FLIP) antibodies were purchased from Upstate Biotechnology. Anti-Bid, anti–cIAP-1, and anti–cIAP-2 were purchased from R&D Systems. Rabbit polyclonal anti–caspase-8 was a gift from Dr. Razqallah Hakem. After incubation with horseradish peroxidase–conjugated goat anti–rabbit, goat anti–mouse, or mouse anti–goat antibody (Santa Cruz Biotechnology, Inc.), bound immunoglobulins were detected using enhanced chemiluminescence (ECL; Amersham Biotech) according to manufacturer's directions.
Caspase Activity Assays.
Fas-specific apoptosis was induced in activated T cells as described. Caspase activity was determined using caspase-3- or caspase-8-specific colorimetric protease assay kits as per manufacturer's instructions (Chemicon). In brief, FasL-stimulated (10 μg/ml) or unstimulated T cells were lysed and protein levels normalized to 2 mg/ml. Samples were incubated in the presence of reaction buffer and colorimetric caspase substrate for 90 min at 37°C (caspase-3 substrate: DEVD-_p_NA, caspase-8 substrate: IETD-_p_NA). OD values at 405 nm were determined for each sample and corrected for spontaneous release of _p_NA. Fold increase in caspase activity is expressed as the ratio of activity in FasL-treated samples relative to untreated controls. All time points were measured in triplicate, and results expressed as the mean ± SEM.
DISC Analysis.
T cells (15–20 × 106) were incubated with FasL (10 μg/ml) or PBS for 1 h at 37°C, then lysed in IP/lysis buffer (150 nM NaCl, 50 mM Tris, pH 7.5, 1 mM EDTA, 1 mM DTT, 1% NP-40, 10 mM β-phosphoglycerate, 0.1 mM NaF, 1 mM NaVO4, and small peptide inhibitors). Lysates were precleared by incubation with 30 μl protein G-agarose beads (50% slurry in IP/lysis buffer). The DISC was immunoprecipitated for 3 h at 4°C using anti-Fas antibody (Jo2 clone; BD Biosciences) and protein-G-sepharose (Amersham Biotech). After immunoprecipitation, the beads were washed five times with IP/lysis buffer, resuspended in SDS-PAGE sample buffer, and boiled. Immunoprecipitated proteins and precleared lysates were resolved via 10–12% SDS-PAGE and transferred to PVDF membrane. Membranes were probed with antibodies specific for Fas, FADD, and procaspase-8, and resolved by ECL.
Results
CD28 Costimulation Inhibits Fas-mediated Apoptosis.
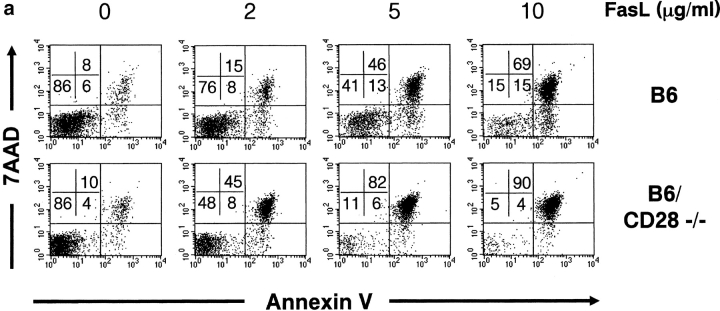
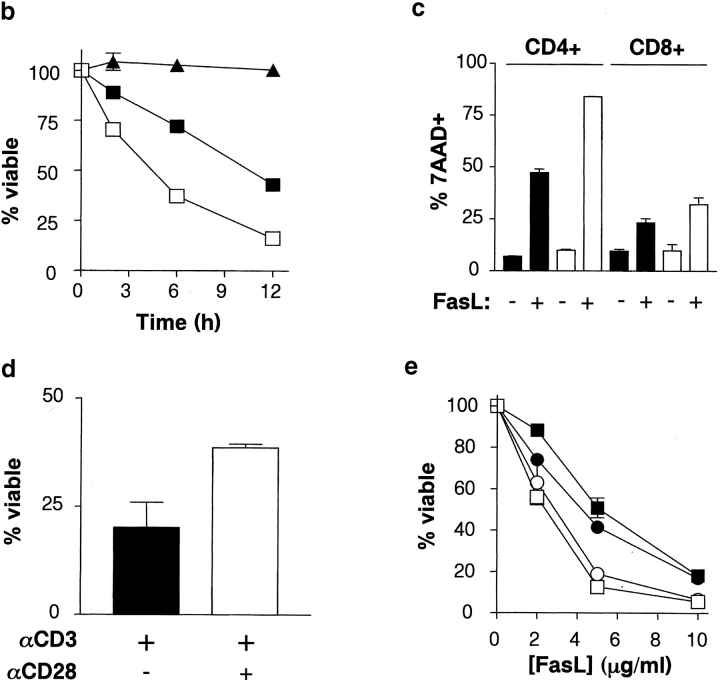
To determine whether CD28 plays a role in Fas-mediated apoptosis, T cells from C57Bl/6 and CD28−/− (C57Bl/6 background) mice were activated with plate-bound anti-CD3 and anti-CD28 antibodies, followed by culture in the presence of IL-2. Fas-dependent apoptosis of viable T cells was induced after 4 d of culture through the addition of FasL. As shown in Fig. 1 a, CD4+ T cells lacking CD28 displayed dramatic sensitivity to Fas-mediated cell death relative to wild-type controls at various concentrations of FasL. This susceptibility to Fas-mediated cell death was characterized by both a decreased number of viable cells (AnnexinV-7AAD−, bottom left quadrant) and an increased proportion of apoptotic cells (AnnexinV+ 7AAD+, top right quadrant) when compared with controls, particularly at low concentrations of FasL which have little impact on wild-type cells. The kinetics of cell death over time revealed that CD28−/−CD4+ T cells underwent apoptosis with faster kinetics than wild-type cells, whereas T cells from lpr mice which have defective Fas signaling displayed no detectable increase in apoptosis after the addition of FasL (Fig. 1 b). Further assessment revealed that the lack of CD28 resulted in increased Fas-mediated death in both T cell subsets, but CD4+ T cells were clearly more sensitive to FasL treatment than CD8+ cells (Fig. 1 c). T cells from CD28−/− mice displayed normal surface expression of Fas (unpublished data), suggesting that the survival defect was not due to enhanced expression of Fas. Thus, our results indicate that Fas-mediated apoptosis of peripheral T cells is enhanced in the absence of CD28 expression, indicating that CD28 is an important guardian against Fas-mediated apoptosis in T cells.
Figure 1.
CD28-associated PI3K activity confers protection against Fas-mediated apoptosis. (a) Increased sensitivity of CD4+CD28−/− T cells to Fas-mediated apoptosis. Splenocytes were cultured with anti-CD3 and anti-CD28 antibodies and IL-2 for 4 d, and apoptosis induced by FasL. CD4+ cell death was measured 6 h after FasL treatment by Annexin V-FITC, CD4-PE, and 7AAD staining. The proportion of cells in each quadrant is indicated. Results are representative of four independent experiments. (b) Time course of Fas-mediated death for CD28−/− T cells. Activated, viable T cells were treated with 5 μg/ml hCD8-mFasL, and apoptosis measured as in panel a. C57BL/6 (B6), filled squares; CD28−/− (B6/CD28−/−), open squares; lpr (B6/lpr/lpr), filled triangles. (c) FasL preferentially kills CD4+ T cells. Activated, viable T cells were left untreated (−FasL) or treated with 5 μg/ml FasL (+FasL), and percent dead CD4+ and CD8+ cells were determined by staining with 7AAD after 6 h. Wild-type, black bars; CD28−/−, white bars. (d) CD28 ligation enhances T cell survival. Wild-type sorted T cells were cultured with anti-CD3 (black bars) or anti-CD3 and CD28 (white bars) antibodies and IL-2, and apoptosis induced by FasL. Cell death was measured 12 h after FasL treatment. (e) Enhanced Fas-mediated apoptosis in T cells with defective CD28-dependent P13K signals. Dose response of Fas-mediated death of activated, viable CD4+ T cells 6 h after the addition of FasL. Apoptosis was measured as in panel a. Wild-type, filled squares; CD28−/−, open squares; WT CD28 Tg, filled circles; Y170F Tg-1 (impaired PI3K binding), open circles.
Fas-mediated Apoptosis Is Blocked by the Activation of PI3K Downstream of CD28.
The interaction between CD28 and B7 results in tyrosine phosphorylation of the cytoplasmic tail of CD28, promoting the recruitment of SH2-domain–containing adaptor and signaling proteins to CD28. Ligation of CD28 can enhance T cell survival, as CD4+ T cells stimulated with anti-CD3 and anti-CD28 antibodies demonstrated greater resistance to Fas-mediated apoptosis than T cells activated with anti-CD3 alone (Fig. 1 d). One important signaling molecule recruited to CD28 is the lipid kinase PI3K, which binds the phosphorylated tyrosine residue 170 (Y170) within the intracellular domain of CD28. CD28 has been shown to activate PI3K and increase PIP3 levels independent from signals generated by the TCR (28). CD28-deficient mice expressing a mutant form of CD28 unable to bind PI3K are particularly sensitive to radiation-induced apoptotic cell death, suggesting that PI3K signaling downstream of Y170 may be important for survival (7). To examine whether PI3K activation downstream of CD28 was required for protection from Fas-induced apoptosis, activated T cells from CD28-deficient animals expressing either a wild-type CD28 transgene (WT Tg) or containing an inactivating mutation at tyrosine 170 (Y170F Tg-1) were treated with FasL, and Fas-specific cell death was measured. Whereas CD4+ T cells from wild-type animals and CD28−/− animals expressing wild-type CD28 underwent apoptosis at similar rates, both CD28−/− and Y170F transgenic T cells displayed an accelerated rate of death in response to FasL treatment (Fig. 1 e). Therefore, the antagonistic effects of CD28 against Fas signaling are dependent upon CD28-mediated induction of the PI3K pathway.
Fas-mediated Apoptosis Is Impaired in T Cells Expressing Active PKB.
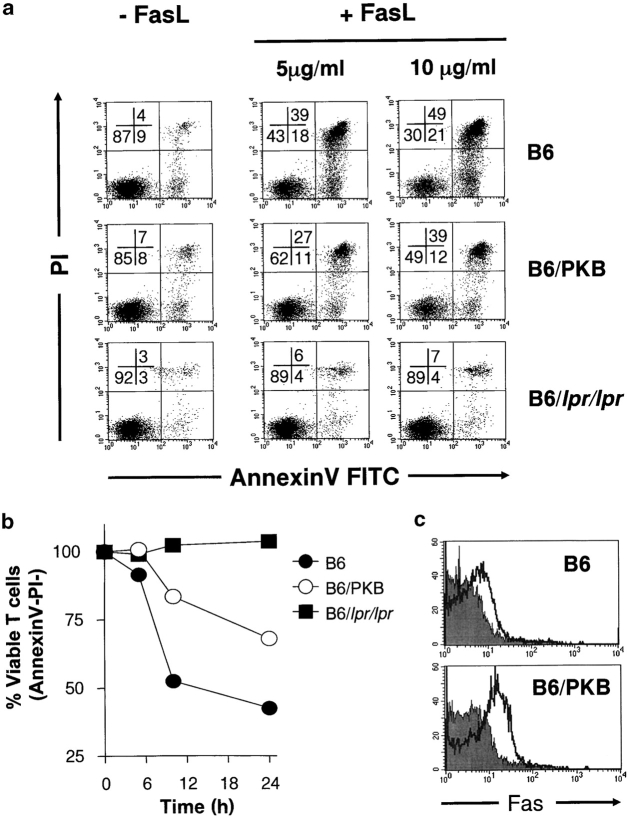
PI3K-mediated generation of the lipid second messenger PIP3 leads to the phosphorylation and activation of PKB/Akt, a survival-promoting kinase in T cells (8, 9). Studies have shown that PKB is activated in a PI3K-dependent manner downstream of CD28 (16, 17). In addition, analysis of T cells from the altered CD28-Y170F–transgenic mice have demonstrated that CD28-specific phosphorylation of PKB was decreased (7), confirming the link between CD28 and PKB activation. Thus, to further evaluate the mechanism of how the CD28-PI3K-PKB pathway influences apoptosis, we analyzed Fas-mediated T cell death in transgenic mice which express active PKBα (gag-PKB) in the T cell lineage (8). Splenocytes from control and gag-PKB transgenic animals were activated and Fas-mediated apoptosis was induced as indicated previously. As shown in Fig. 2 a, T cells expressing the gag-PKB transgene (B6/PKB) displayed a marked decrease in Fas-mediated cell death relative to wild-type controls (B6) at various concentrations of FasL. Observing the nonapoptotic (AnnexinV−PI−) T cell populations over time revealed that PKB transgenic T cells underwent apoptosis with slower kinetics than wild-type cells, suggesting an impaired response to FasL rather than a complete block in Fas-mediated apoptosis (Fig. 2 b). T cells deficient in Fas signaling (B6/lpr/lpr) displayed no detectable increase in apoptosis after the addition of FasL (Fig. 2, a and b). The resistance to Fas-mediated apoptosis in PKB transgenic T cells could not be attributed to decreased Fas expression as T cells bearing the gag-PKB transgene surprisingly displayed both elevated levels of Fas mRNA and 2–3-fold higher Fas surface expression over wild-type controls (Fig. 2 c, and unpublished data). Thus, despite evidence of increased Fas expression, our data indicates that Fas-mediated apoptosis is impaired in PKB transgenic T cells.
Figure 2.
Impaired Fas-mediated apoptosis in PKB-transgenic mice. Splenocytes from C57BL/6 (B6), PKB-transgenic (B6/PKB), or lpr (B6/lpr/lpr) mice were activated with anti-CD3 and anti-CD28 antibodies and IL-2 for 4 d, followed by induction of Fas-specific apoptosis by addition of FasL. (a) Reduced FasL-induced apoptosis of activated PKB-transgenic T cells. Activated, viable T cells were left untreated (−FasL) or treated (+FasL) with various concentrations of FasL and cell death measured at 10 h by AnnexinV-FITC and PI staining. The proportion of cells in each quadrant is indicated. Results are representative of six independent experiments. (b) Time course of Fas-mediated death. Activated, viable T cells were treated with 5 μg/ml FasL, and apoptosis measured as in panel a. C57BL/6 (B6), closed circles; PKB transgenic (B6/PKB), open circles; lpr (B6/lpr/lpr), closed squares. (c) Elevated Fas surface expression on PKB transgenic T cells. Fas surface expression (open histogram) on activated T cells from C57BL/6 (B6) or PKB transgenic (B6/PKB) animals. IgG2a isotype control antibody staining is shown (shaded histogram).
Active PKB Inhibits Fas-mediated Death, but Not Antigen-specific Death, In Vivo.
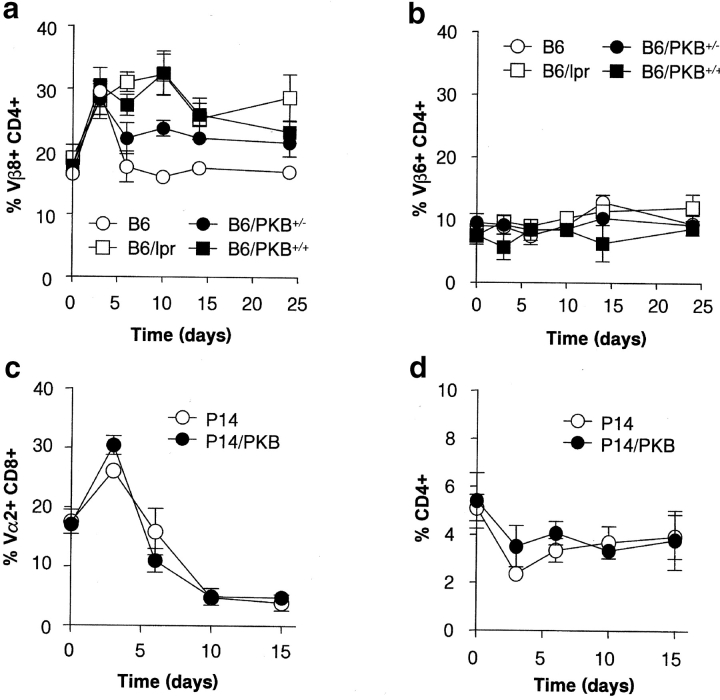
To further evaluate the role of active PKB on Fas-induced apoptosis, various in vivo analyses were performed. As Fas-FasL signaling has been implicated in the control of peripheral T cell deletion under certain conditions (29, 30), we examined whether in vivo peripheral T cell deletion was impaired in PKB transgenic mice. Wild-type mice or mice expressing one or two alleles of the gag-PKB transgene were challenged with SEB, a superantigen which induces expansion and Fas/FasL-dependent deletion of Vβ8+ T cells (31, 32). As shown in Fig. 3 a, we observed comparable expansion of Vβ8+CD4+ T cells in both wild-type and PKB transgenic animals 4 d after injection of SEB. However, deletion was significantly impaired in PKB transgenic animals, and the deletion of Vβ8+CD4+ T cells never reached that of wild-type levels up to 3 wk after SEB injection. Moreover, mice homozygous for the gag-PKB allele (B6/PKB+/+) displayed dramatic resistance of deletion in response to SEB, indicating that this process could be influenced by the amount of active PKB expressed in T cells. Impaired deletion of Vβ8+CD4+ T cells was observed in lpr mice, indicating that the mechanism of deletion is dependent upon functional Fas signals. The percentage of Vβ6+CD4+ T cells from each set of animals did not vary significantly over time, confirming the response to SEB was specific to Vβ8+ T cells (Fig. 3 b). These observations suggest that PKB can inhibit Fas/FasL-dependent T cell deletion in vivo. In contrast, peptide-induced deletion of T cell receptor (TCR) transgenic T cells (P14 TCR transgene, MHC class I restricted), which employs a Fas-independent mechanism for peripheral tolerance (33, 34), was unaffected by transgenic PKB expression (Fig. 3 c). Collectively, our results indicate that PKB activity can influence peripheral deletion under certain conditions; while it displays no effect on Fas-independent deletion mechanisms induced by peptide, PKB can antagonize Fas signaling–dependent T cell deletion by SEB in vivo.
Figure 3.
Impaired Fas-dependent T cell deletion in PKB transgenic mice. (a and b) Impaired SEB-mediated deletion of PKB-transgenic T cells. Control C57BL/6 mice (B6, open circles), Fas-deficient lpr mice (B6/lpr, open squares), or animals expressing one or two gag-PKB alleles (B6/PKB+/−, filled circles, and B6/PKB+/+, filled squares, respectively) were injected with SEB (80 μg) and the proportion of Vβ8+CD4+ T cells from peripheral blood was measured by flow cytometry (a). The percentage of Vβ6+CD4+ T cells was measured as a negative control (b). (c) Normal peptide-mediated deletion of PKB-transgenic T cells. P14 TCR (open circles) or P14 TCR/PKB (filled circles) transgenic mice were injected with p33 peptide (three times with 5 μg over 6 d) and the proportion of Vα2+CD8+ T cells from peripheral blood was measured by flow cytometry. The percentage of CD4+ T cells was measured as a negative control (d).
As PKB can promote survival in response to a variety of apoptotic stimuli (8), we wanted to further examine the importance of PKB in regulating Fas-mediated death in vivo. Previous studies have demonstrated that the absence of Fas signaling in lpr mice leads to the development of a lymphoproliferative disorder and autoimmune disease. Mice harboring lpr and gld mutations in the MRL genetic background display a severe lymphoproliferative disorder accompanied by autoantibody and rheumatoid factor production, glomerulonephritis, arthritis, and early mortality. In contrast, C57BL6/lpr mice develop lymphadenopathy and splenomegaly with lesser severity and slower kinetics than MRL mice (35). Therefore, to further strengthen the link between PKB and Fas in vivo, we examined whether the genetic susceptibility loci in the MRL background was sufficient to promote a lymphoproliferative disorder in the context of the gag-PKB transgene. Our previous studies have shown that heterozygous PKB transgenic mice have normal lymphocyte subsets at 12 wk of age (8, 36). However, at 12 wk of age, significant expansion of both T and B cells were observed in MRL/PKB mice relative to MRL controls within a subset of lymphoid compartments (Table I). This increase was primarily observed in the spleen, mesenteric and inguinal lymph nodes, and Peyer's patches. The most pronounced increase was observed in the Peyer's patches, where the total number of B cells, and CD4+ and CD8+ T cells exceeded that of MRL/lpr mice of the same age (Table I). Interestingly, the massive expansion of B220+CD3+CD4−CD8− (double negative [DN]) T cells observed in MRL/lpr mice was notably absent in MRL/PKB mice.
Table I.
Enlarged Secondary Lymphoid Organs in MRL/PKB Transgenic Mice
| Cell populations | MRL | MRL/PKB | MRL/lpr |
|---|---|---|---|
| Spleen | |||
| Total cells (×107) | 4.14 ± 1.22 | 10.6 ± 1.1 | 13.3 ± 1.3 |
| B cells (×107) | 1.05 ± 0.97 | 3.90 ± 0.66 | 3.58 ± 0.21 |
| T cells (×107) | 1.4 ± 0.7 | 3.9 ± 0.5 | 5.2 ± 0.3 |
| CD4+ (×106) | 7.7 ± 0.6 | 22.9 ± 2.6 | 23.2 ± 1.5 |
| CD8+ (×106) | 4.4 ± 0.3 | 11.0 ± 1.0 | 10.8 ± 0.7 |
| DN cells (×106) | 3.1 ± 0.8 | 5.7 ± 1.3 | 25.7 ± 1.6 |
| Peyer's patches | |||
| Total cells (×104) | 5.31 ± 3.46 | 103.3 ± 16.8 | 95.7 ± 14.2 |
| B cells (×104) | 2.06 ± 1.37 | 51.9 ± 10.2 | 20.2 ± 3.5 |
| T cells (×104) | 2.10 ± 1.37 | 37.4 ± 7.0 | 53.0 ± 8.0 |
| CD4+ (×104) | 1.39 ± 0.93 | 29.0 ± 6.1 | 18.7 ± 3.1 |
| CD8+ (×103) | 4.7 ± 2.7 | 71.0 ± 11.6 | 66.9 ± 9.8 |
| DN cells (×103) | 3.7 ± 1.2 | 26.8 ± 7.6 | 150.8 ± 49.4 |
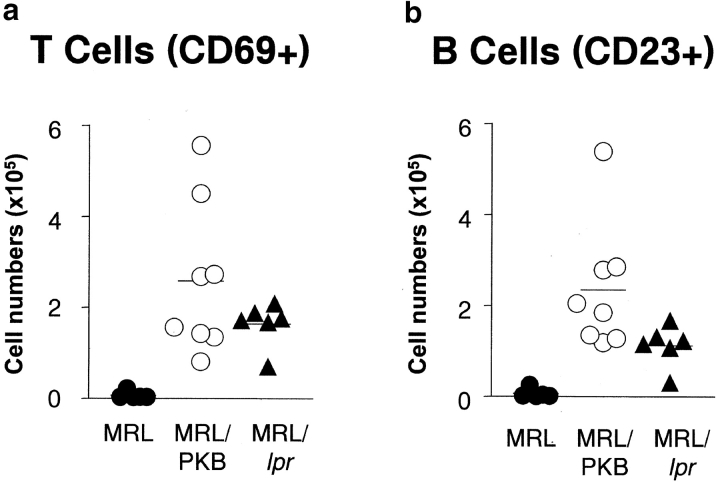
Analysis of activation markers revealed a striking increase in the number of activated lymphocytes in MRL/PKB mice. Both the percentage and total number of CD69+ and CD44+ T cells (Fig. 4 a, and unpublished data) as well as CD23+ B cells (Fig. 4 b) were notably increased in the Peyer's patches of MRL/PKB mice and comparable to that observed in MRL/lpr mice. These data demonstrate that gag-PKB expression, similar to defective Fas expression, can promote lymphoproliferation in the context of MRL background genes. Moreover, this phenotype must be driven by T cells, as expression of gag-PKB is restricted to the T cell lineage (36). Together these studies demonstrate that PKB is involved in regulating Fas-mediated death at the functional and genetic level.
Figure 4.
Increased frequency of activated lymphocytes in MRL/PKB transgenic mice. Total numbers of activated T cells (a) and B cells (b) in Peyer's patches of 12-wk-old MRL mice (filled circles, n = 5), MRL/PKB transgenic mice (open circles, n = 8), and MRL/lpr (filled triangles, n = 5) mutant mice. Accumulation of activated cells in MRL/PKB mice relative to MRL/lpr mice is shown. Mean scores for each genotype are indicated.
Active PKB Leads to Reduced Activation of the Fas Apoptotic Pathway.
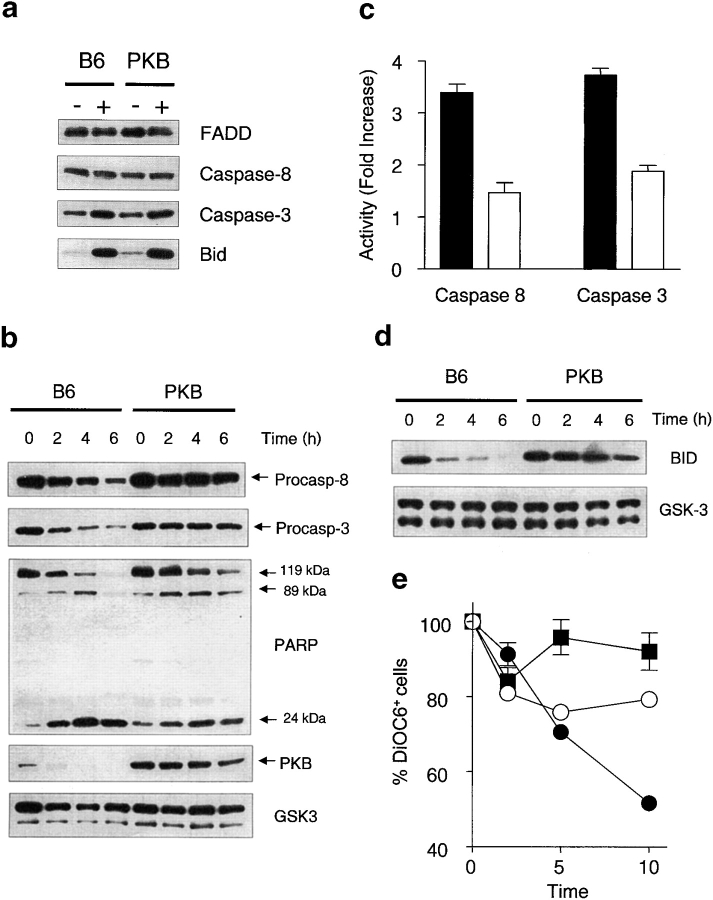
To understand how PKB influences Fas death, we examined various molecules in the apoptotic cascade triggered by Fas. Several components are involved in transducing the apoptotic signal downstream of Fas, including the adaptor molecule FADD and the initiating caspase in the pathway, caspase-8 (37). Procaspase-8 is recruited to Fas by FADD, where it becomes activated by proteolysis and initiates cell death by activating other death-inducing molecules including procaspase-3 and Bid. No difference in mRNA or protein expression levels for FADD, procaspase-8, procaspase-3, and Bid was observed in activated T cells between wild-type and PKB transgenic T cells (Fig. 5 a, and unpublished data), suggesting that PKB activity did not influence the expression of these components. An alternate possibility is that CD28-dependent PKB activity regulates the activation of these components, leading us to examine the activation of caspases downstream of Fas. Surprisingly, we found that both the processing of procaspase-8 and procaspase-3 was significantly impaired in PKB transgenic T cells (Fig. 5 b). Examination of caspase activity in lysates from these T cells also revealed a 2–3-fold decrease in the functional activity of caspase-8 and caspase-3 in PKB transgenic animals relative to controls (Fig. 5 c). The degradation of PARP, a cellular target of caspase-3, was also impaired in PKB-transgenic T cells, indicating decreased Fas-dependent caspase activity in vivo (Fig. 5 b). Interestingly, we also observed rapid loss of PKB in wild-type cells but not in PKB transgenic T cells (Fig. 5 b), consistent with recent observations that PKB is cleaved by caspases during apoptosis (38, 39). Given the effect of PKB on caspase activation in T cells, it is understandable why PKB is a target of caspases during the apoptotic process; degradation of PKB after death receptor activation may act as a positive regulatory loop, ensuring that the cell becomes sensitive to Fas signals. Collectively, these data indicate that PKB activity is able to inhibit the activation of caspases downstream of Fas. Moreover, PKB appears to be acting at the apical point of Fas-dependent caspase activation, the processing of procaspase-8.
Figure 5.
PKB inhibits the activation of caspase-8 and multiple downstream targets of Fas. (a) Normal expression of components of the Fas death pathway. FADD, procaspase-8, procaspase-3, and Bid protein levels were determined in naive (−) or activated (+) T cells from wild-type (B6) or PKB transgenic (PKB) mice via Western blotting. (b) PKB prevents processing of procaspases-8 and -3, and degradation of PARP. Activated CD4+ T cells from C57BL/6 (B6) or PKB transgenic (PKB) animals cultured in the presence of IL-2 were left untreated (−FasL) or treated (+FasL) with 10 μg/ml FasL for various time points. Cell lysates were immunodetected for the presence of procaspase-8, procaspase-3, PARP, and PKB. Antibodies directed against GSK-3 were used to assess protein loading. (c) Decreased caspase activity in PKB transgenic T cells. Caspase-8- or caspase-3–specific activity was determined in cell lysates 4 h after addition of FasL. Fold increase in activity determined as ratio of activity relative to untreated cells. Mean values ± SEM are shown for one representative experiment performed in triplicate. (d) Active PKB prevents processing of Bid. Bid protein levels were detected in CD4+ T cell lysates after addition of FasL using antibodies directed against Bid. (e) Decreased mitochondrial permeability in PKB transgenic T cells. Specific loss of DiOC6+ staining was measured in T cells from C57BL/6 (closed circles), PKB transgenic (open circles), or lpr (filled squares) mice via flow cytometry after addition of FasL. Mean values ± SEM for samples performed in triplicate are shown.
One of the accompanying effects of Fas-mediated caspase-8 activation is the activation of a pro-apoptotic amplification loop involving the mitochondria, a process that involves the processing of the Bcl-2 family member Bid by caspase-8 (40, 41). We therefore analyzed the level of Bid processing (as measured by loss of full-length Bid) in PKB-transgenic T cells after Fas treatment and found that its degradation was impaired (Fig. 5 d). In addition, we observed a slower loss of mitochondrial membrane integrity in PKB-transgenic T cells after FasL treatment (Fig. 5 e). Together these data indicate that the mitochondrial-dependent apoptotic events driven by caspase-8 after Fas ligation can be abrogated by gag-PKB activity in T cells.
PTEN+/− T Cells Show Impaired Fas Death and Caspase-8 Activation.
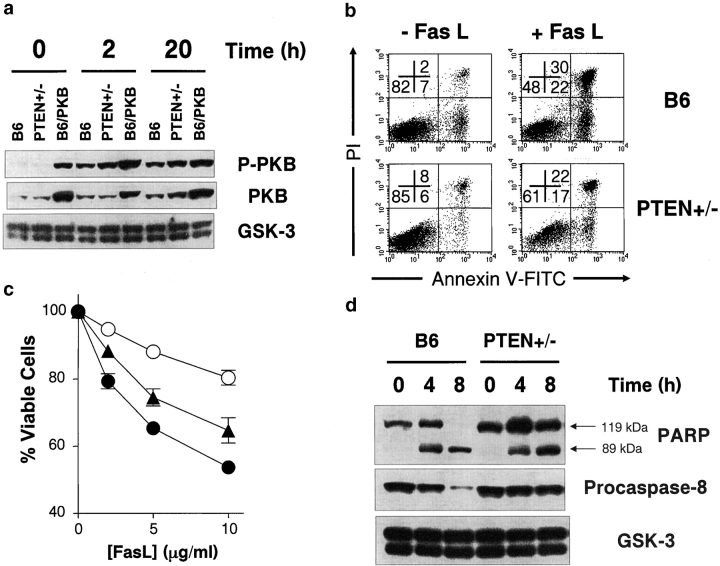
PI3K activity leads to an increase in the intracellular concentration of the phospholipid PIP3, a process which, in turn, is negatively regulated by the lipid phosphatase PTEN. PTEN acts by selectively cleaving the D3 phosphate from PIP3 (14, 15). A reduction in PTEN activity leads to hyperphosphorylation of PKB and resistance to apoptosis induced by Fas-antagonistic antibodies (42). Therefore, to further confirm the association between CD28-PI3K-PKB signaling and Fas, we examined PKB activity and the induction of Fas apoptosis in PTEN+/− T cells. After stimulation with anti-CD3 and anti-CD28 antibodies, we observed elevated levels of phosphorylated PKB in PTEN+/− T cells relative to control cells, although the amount of phosphorylated PKB was considerably less than that observed in PKB transgenic T cells (Fig. 6 a). As seen in Fig. 6 b, Fas-dependent apoptosis was impaired in activated PTEN+/− splenocytes as noted by a slower loss of the viable T cell population after treatment with FasL. Resistance to Fas-mediated cell death in these T cells correlated with the level of PKB phosphorylation; 5 h after FasL treatment, PTEN+/− T cells showed greater survival relative to wild-type cells, but died at a faster rate relative to PKB transgenic T cells (Fig. 6 c). PARP cleavage was clearly decreased in PTEN+/− T cells, suggesting that caspase-dependent cleavage of cellular proteins downstream of Fas was impaired due to the loss of one PTEN allele (Fig. 6 d, top panel). Importantly, FasL-dependent processing of procaspase-8 was also impaired in PTEN+/− T cells relative to controls (Fig. 6 d, middle panel). Collectively, these data indicate that resistance to Fas-mediated apoptosis in PKB transgenic and PTEN+/− mice is mediated by a similar mechanism, impaired caspase-8 activation downstream of Fas, and reiterates the inhibitory effect of PKB activity on procaspase-8 processing.
Figure 6.
PTEN+/− T cells show decreased Fas-induced apoptosis and caspase-8 activation. (a) Enhanced PKB activation in PTEN+/− T cells. T cells from C57BL/6 (B6), PTEN+/−, or PKB transgenic (B6/PKB) mice were stimulated with soluble anti-CD3 and anti-CD28 antibodies for the indicated times and the amount of phosphorylated PKB (P-PKB) and total PKB were analyzed via Western blot. Anti-GSK-3 antibodies were used to assess protein loading. (b) Activated PTEN+/− T cells show decreased susceptibility to Fas-mediated apoptosis. Activated, viable T cells were treated with FasL and cell death measured at 5 h by AnnexinV-FITC and PI staining. The proportion of cells in each quadrant is indicated. (c) Dose-dependent Fas-resistance in PTEN+/− T cells. Activated, viable T cells from C57BL/6 (B6, filled circles), gag-PKB transgenic (B6/PKB, open circles), and PTEN+/− (filled triangles) mice were treated with various concentrations of FasL and cell death measured at 8 h. Viability was determined as percent AnnexinV-PI- relative to untreated controls and performed in triplicate. Results are representative of two independent experiments. (d) Decreased PARP cleavage and procaspase-8 processing in PTEN+/− T cells. Cell lysates were analyzed for the presence of PARP (top panel) and procaspase-8 (middle panel) by Western blot. Antibodies directed against GSK-3 were used to assess protein loading (bottom panel).
DISC Assembly Is Impaired in the Presence of Active PKB.
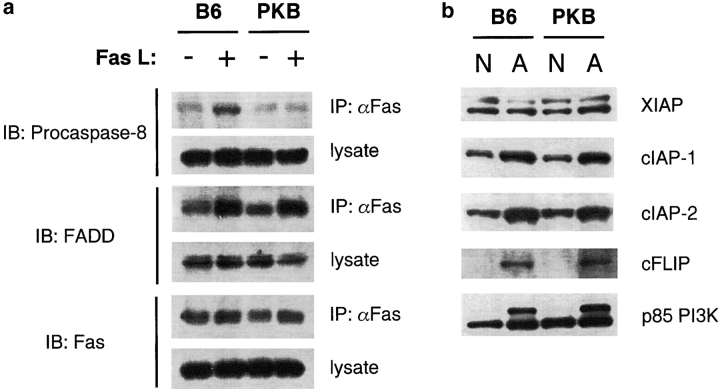
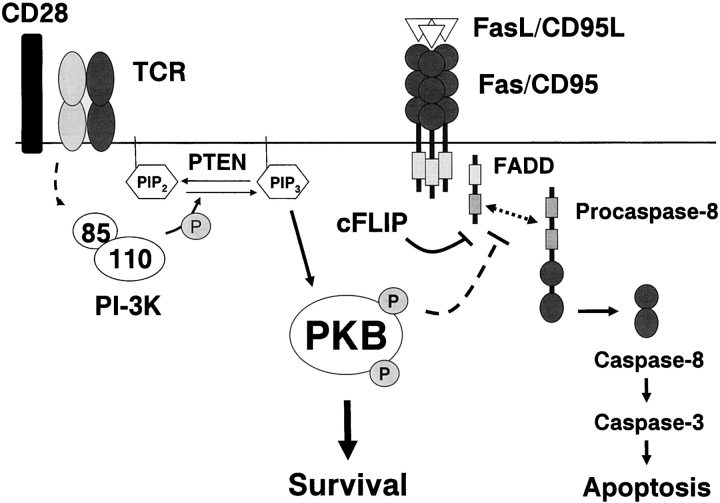
How does PKB block caspase-8 activation? Fas activation results in the recruitment of the death effector molecule FADD to the cytoplasmic tail of Fas (43), which in turn binds procaspase-8 (44), leading to the formation of a “death-inducing signaling complex,” or DISC. DISC formation ultimately leads to caspase-8 activation and the full execution of apoptosis (45). The deficiency in procaspase-8 processing in PKB transgenic T cells implied that DISC formation may be affected in the presence of gag-PKB, leading us to analyze components of the DISC in wild-type and gag-PKB transgenic T cells after treatment with FasL. FADD recruitment to the DISC was comparable in both wild-type and PKB transgenic T cells (Fig. 7 , middle panel), suggesting that the initial events important to DISC assembly were unaltered by transgenic gag-PKB expression. However, analysis of procaspase-8 levels in the DISC revealed that procaspase-8 recruitment to the DISC was markedly impaired in T cells expressing gag-PKB (Fig. 7, top panel). These data provide the first insights into the mechanism of how PI3K-PKB signaling promotes survival from death receptor-induced apoptosis.
Figure 7.
(a) PKB inhibits the recruitment of procaspase-8 to the DISC. T cells from C57BL/6 (B6) and PKB transgenic (PKB) mice were activated with anti-CD3 and anti-CD28 antibodies for 24 h, followed by culture with IL-2 for 4 d. Fas was immunoprecipitated from activated T cells either left untreated (−) or treated (+) with FasL (10 μg/ml) for 1 h. T cells were maintained in IL-2 during stimulation with FasL. Immunoprecipitates were blotted with antibodies specific for procaspase-8, FADD, or Fas. Relative protein expression of DISC components in precleared lysates is shown. (b) Expression of IAPs and FLIP in PKB transgenic T cells. Protein levels of XIAP, cIAP-1, cIAP-2, and cFLIP in naive (N, no stimulation for 24 h) or activated (A, anti-CD3 and anti-CD28 treatment for 24 h) sorted CD4+ T cells from wild-type (B6) and PKB transgenic (PKB) mice were assessed by Western blot. Levels of p85 PI3K were measured to assess protein loading.
Several effector proteins may be involved in regulating DISC formation and caspase activation downstream of Fas. The cellular inhibitors of apoptosis (IAPs) are potent inhibitors of caspase activation and can inhibit apoptosis induced by both death receptors and mitochondrial-dependent pathways. The up-regulation of cIAP-1 and cIAP-2 has been shown to suppress TNF-α–induced apoptosis through recruitment to the TNFR1 signaling complex, suggesting that induction of these IAP molecules may act to inhibit caspase-8 activation during DISC formation (46). These results led us to speculate that cIAP-1 and cIAP-2 protein levels may be deregulated in activated PKB transgenic T cells. However, whereas both proteins were induced upon activation, we did not detect any appreciable difference in cIAP-1 or cIAP-2 protein levels between wild-type and PKB transgenic CD4+ T cells (Fig. 7 b). XIAP (X chromosome-linked IAP) is an IAP isoform that is highly expressed in T cells and potently antiapoptotic (47, 48), but we did not detect differences in XIAP protein levels between wild-type and PKB CD4+ T cells. Recent reports have suggested that PKB can regulate expression of c-FLIP (49, 50), a cellular inhibitor of caspase-8. FLIP, FADD, and procaspase-8 contain death effector domains (DEDs), protein modules that facilitate binding via homophilic protein interactions. FLIP can heterodimerize with FADD or procaspase-8 through binding of their DEDs, thus acting as a molecular inhibitor of DISC formation by blocking procaspase-8 recruitment to FADD (51–53). However, we were unable to detect differences in c-FLIP protein or mRNA between wild-type and PKB transgenic animals following CD4+ T cell activation (Fig. 7 b, and unpublished data). These results suggest that PKB is preventing association of procaspase-8 with the DISC, but through a mechanism independent of c-FLIP or IAP protein expression.
Discussion
CD28 Engagement Promotes Survival by Antagonizing Fas-mediated Apoptosis.
In this study, we demonstrate that CD28 is required to transmit signals that protect T cells from Fas-mediated death, a process that requires activation of the PI3K-PKB signaling pathway. This connection between CD28-dependent PKB activity and the Fas apoptotic pathway is significant, as the physiological role of CD28 in regulating T cell apoptosis has remained unclear. Although many of the interactions and associations between segments of this pathway have been analyzed using in vitro models, the literature has remained controversial (for a review, see reference 2). In some studies, costimulation by CD28 has been shown to promote T cell survival (3–5), while other groups have found that T cell apoptosis is unaffected by CD28 engagement (54, 55). CD28 engagement promotes the expression of Bcl-XL, a molecule that prevents cell death by blocking apoptotic events at the mitochondria. Certainly, PI3K signals may play a role in preventing spontaneous apoptosis by regulating Bcl-XL levels. Studies using pharmacological inhibitors of PI3K (56) or CD28 mutants (7, 57) indicate that PI3K activity can regulate Bcl-XL expression. However, the role of Bcl-XL in modulating Fas-induced apoptosis remains controversial. Although previous experiments have correlated the expression of Bcl-XL with impaired Fas death (3–5), conflicting reports from other groups suggest that Bcl-XL expression is not sufficient to protect from Fas-induced apoptosis (58). Thus, PI3K signaling downstream of CD28 may serve a dual role: preventing spontaneous apoptosis via upregulation of Bcl-XL and antagonizing death receptor–mediated apoptosis through the activation of PKB.
Although several studies have examined the role of CD28-mediated costimulation in Fas-mediated apoptosis, the influence of CD28 upon Fas death signaling has remained unclear. Vallejo et al. have isolated human T cell lines from patients with chronic inflammatory diseases which lack CD28 expression; however, contrary to our work, these CD28null T cells are resistant to Fas-mediated death (59). Using a different approach, Kirchoff et al. demonstrated that triggering CD28 on Fas-susceptible T cells can render those cells resistant to Fas-induced apoptosis (60). This contrasts with previous work showing that ligation of CD28 has no influence upon activation-induced cell death (55). Therefore, the literature is inconclusive, with different studies using very different models. Our experiments are the first to demonstrate from a genetic standpoint that primary T cells lacking CD28 are more susceptible to Fas-induced apoptosis.
Previous studies using transient transfection experiments in cell lines have suggested that PI3K-PKB signaling can influence Fas-mediated apoptosis (61, 62). However, our loss- and gain-of-function approach provides the first in vivo evidence that PKB is responsible for inhibiting Fas-mediated apoptosis downstream of CD28. Our findings suggest that CD28-mediated costimulation is critical for maintaining an apoptosis-insensitive state in T cells after activation in response to antigen. The susceptibility of T cells to Fas-induced death changes over the course of an immune response (63). Naive T lymphocytes are resistant to Fas-induced apoptosis and remain refractive to death receptor signaling upon activation. DISC formation is incomplete in Fas-resistant activated T cells, marked by an inability to recruit and process procaspase-8 despite the normal recruitment of FADD (63). In addition, amplification of Fas signals via the mitochondrial-dependent apoptotic pathway is limited in these cells through increased expression the antiapoptotic molecule Bcl-XL (3). In contrast, activated T cells become sensitive to apoptosis after extended activation in the presence of IL-2 (64). These Fas-susceptible T cells are able to form a functional DISC, leading to extensive caspase-8 activation (63). In our studies PKB also prevents Fas-mediated apoptosis at the level of DISC formation. Therefore, it is possible that PKB signals downstream of CD28 are critical during the early stage after activation in order to ensure that T cells are refractive to Fas-induced apoptosis. Our studies demonstrate that CD28 is selectively required to protect T cells from death signals propagated by Fas, and that signals provided by PKB mediate this process by interfering with the assembly of the Fas apoptotic machinery (Fig. 8) . However, our studies cannot exclude the contribution of other receptors expressed on the surface of T cells that may also influence Fas death during T cell activation.
Figure 8.
Activation of PKB by TCR/CD28 inhibits Fas-mediated death. In T cells, PKB is activated downstream of the TCR and CD28 by PI3K, a process antagonized by the lipid phosphatase PTEN. Triggering of Fas by FasL leads to recruitment of procaspase-8 to the DISC via binding to a FADD-Fas complex. Processing and activation of procaspase-8 leads to activation of downstream effectors (caspase-3, Bid) and subsequently to apoptosis. FLIP antagonizes this process through direct binding and inhibition of procaspase-8. PKB inhibits processing of procaspase-8 by preventing its translocation to the DISC, independent of FLIP expression.
Type I and II Pathways: Association with PKB Activity.
Scaffidi et al. have identified two alternate biochemical pathways that are associated with Fas-induced apoptosis (58). Type I cells primarily employ a caspase cascade to induce death; ligation of Fas in these cells leads to the recruitment and activation of large amounts of procaspase-8 at the DISC and subsequent activation of caspase-3. In type II cells, very little procaspase-8 is recruited to the DISC and is cleaved with slower kinetics. As a result, amplification of death signals via caspase-8–mediated cleavage of Bid and subsequent triggering of the mitochondria-dependent apoptotic pathway is required to induce apoptosis. Using the Jurkat T cell line as a model, Scaffidi suggests that Fas-resistant lymphocytes act as Type II cells. It is suggested that T cells acquire Fas sensitivity via the down-regulation of the Fas-signaling inhibitor c-FLIP (65), which would allow for normal formation of the DISC. However, Jurkat T cells have been reported to have defects in PTEN expression, leading to elevated PIP3 levels and constitutive PKB phosphorylation (66). Our results demonstrate that PKB transgenic T cells resemble type II cells: binding of FADD to the DISC is normal, but association of procaspase-8 is impaired. This suggests that the distinction between type I and type II cells may be influenced by elevated levels of PKB activity. Thus, through several lines of evidence, PKB is emerging as a critical gatekeeper for survival, restricting the progression of T cells from an apoptosis-resistant to apoptosis-susceptible state by preventing the association of procaspase-8 with the DISC. PKB activation in naive lymphocytes may be a key element for preventing Fas-induced apoptosis during the initial phase of a T cell response.
Susceptibility to Fas-induced Death, Homeostasis, and Autoimmunity.
Several groups (36, 42, 67) have implicated the PI3K/PKB signaling axis as a key regulator of lymphocyte homeostasis and autoimmunity, although the molecular mechanisms underlying this process in vivo have not been elucidated. The fact that hyperactivation of the PI3K/PKB pathway, either through activation of PI3K or PKB or loss of PTEN expression, leads to the development of autoimmune disorders bearing similarities with lpr and gld mice, is consistent with the impaired Fas/FasL-induced apoptosis observed in lymphocytes from these mice. Our findings indicate that active PKB can block Fas-mediated caspase-8 activation by preventing DISC formation. This observation provides a molecular mechanism to explain why gag-PKB transgenic and PTEN+/− heterozygous mice develop lymphoproliferative disorders similar to that observed in lpr and gld mice (19, 21, 36, 42). In addition, the accelerated disease observed in the PKB transgenic mice on an MRL background compared with the C57Bl/6 background (8, 36), provides further in vivo support for the role of PKB in promoting a defect in Fas death. This demonstrates that the genetic factors that contribute to the phenotype associated with the absence of proper Fas signaling also contribute to accelerating the PKB-associated disease.
Although it is well established that Fas is a potent inducer of apoptosis, recent evidence indicates that the physiological significance of Fas in regulating cell death varies depending on tissue and activation state. For example, naive T cells are refractive to Fas death signals whereas activated T cells become susceptible to Fas-mediated death (37). However, susceptibility to Fas-mediated death also varies within the T cell subset itself. Our results demonstrate that CD4+ T cells are ∼2 times more sensitive to apoptosis upon to exposure to FasL than are CD8+ T cells, regardless of PKB expression. Moreover, whereas SEB-mediated deletion of CD4+ T cells is impaired in mice that have impaired Fas signaling (both lpr and gag-PKB mice), peptide-specific deletion of CD8+ T cells occurs normally in the absence of normal Fas signaling (our results, and references 33 and 34). There also appears to be differential susceptibility to Fas-mediated apoptosis within the CD4+ subset. TH2 cells have been shown to display greater resistance to Fas-mediated death than TH1 cells, due in part to incomplete processing of procaspase-8 at the Fas-DISC (68).
Potential Ways that PKB Can Inhibit Fas-mediated Death.
The mechanism by which CD28-dependent PKB signaling inhibits procaspase-8 recruitment to the DISC remains unknown. PKB may act directly through phosphorylation of downstream effectors or indirectly via regulation of transcription factors such as Forkhead family members (69), c-myb (70), and nuclear factor (NF)-κB (8, 71–73). PKB has been shown to inhibit the apoptotic processes at the level of the mitochondria by phosphorylating the Bcl-2 family member BAD (74, 75) or Caspase-9 (76). BAD is proapoptotic when overexpressed in cytokine-dependent cell lines and transgenic overexpression of BAD in thymocytes renders them hypersensitive toward several types of apoptotic death including treatment with anti-Fas antibodies (77). However, previous evidence suggests that BAD is not a relevant target for PKB in T cells. Both our group and Mok et al. demonstrate that BAD protein expression in primary T lymphocytes is extremely low, and, as demonstrated by the latter, induced only in response to apoptotic stimuli such as treatment with γ-irradiation or dexamethasone. In addition, our previous studies have shown that BAD is not phosphorylated in gag-PKB transgenic T cells (8). Evidence also suggests that caspase-9 is not a natural target for PKB in mouse T cells. Although it does lie downstream of the mitochondria, mouse caspase-9 lacks the PKB phosphorylation sites found in human caspase-9 and, as such, cannot be phosphorylated by active PKB in vitro (78). It is unlikely that PKB affects the association of procaspase-8 with FADD via posttranslational modification, as these molecules both lack PKB phosphorylation consensus sites (79), although this does not rule out the possibility that PKB is acting on other molecules that inhibit this process. Alternatively, PKB may affect the transcriptional upregulation of molecules that can inhibit FADD or procaspase-8, possibly through the binding of their death effector domains.
Recent reports suggest that PKB may influence Fas-induced apoptosis through regulation the expression of c-FLIP (49, 50), although this remains controversial, as PKB does not influence FLIP expression in other tissues (80). These data, combined with our observation that FLIP levels are not altered due to gag-PKB expression, indicate that PKB-mediated control of FLIP expression may not be universal to all tissues. Collectively this suggests that PKB prevents association of procaspase-8 with the DISC, but through a mechanism independent of c-FLIP expression. Interestingly, mice overexpressing c-FLIP also develop a mild polyclonal lymphoproliferative disease, although with differences to that observed in lpr and gag-PKB mouse strains (65). Mice overexpressing FLIP show a large accumulation of activated B cells accompanied by autoantibody production and immune complex–mediated glomerulonephritis, but no significant increase in activated T cells. Finally, it is important to note that PKB can act upon many apoptotic pathways, many of which are not influenced by c-FLIP. Although c-FLIP can prevent apoptosis induced by death receptors, it cannot block death induced by other agents such as γ-irradiation, processes which are inhibited by PKB activity (8, 9, 81). Collectively, these data suggest that FLIP and PKB antagonize Fas-mediated apoptosis through different mechanisms in T cells. Further research is required to identify transcriptional targets of PKB that may play a role in this process.
Concluding Remarks.
Our results add a new perspective to the role of costimulation in T lymphocytes. Although previous studies have linked CD28 with survival, a direct role for CD28 in preventing Fas-mediated apoptosis has not been clearly demonstrated. It is becoming apparent that several receptor-ligand pairs play a dual role in the regulation of costimulation/proliferation and apoptosis/survival. Fas has been reported to influence T cell proliferation using the downstream effectors FADD and FLIP independent from its ability to induce apoptosis (82). Similarly, our results have now implicated CD28 in the regulation of Fas-induced apoptosis in addition to its well-defined role as a regulator of activation and proliferation in T lymphocytes. This indicates that key modulators of T lymphocyte function possess the capacity to control both proliferation and apoptosis.
The cross-talk between CD28-dependent PKB signaling and the Fas apoptotic pathway provides a mechanistic explanation for the success of costimulatory blockade treatment during organ transplantation. Systemic administration of chimeric CTLA4–Ig fusion protein, which blocks the CD28–B7 interaction in vivo, suppresses allograft rejection and promotes transplant tolerance (83), a process that requires functional FasL expression by the graft (84). Recent reports indicate that this costimulation blockade causes extensive apoptosis of graft-infiltrating CD4+ and CD8+ T cells (85), mirroring the enhanced death we observe in CD28-deficient T cells treated with FasL. Our findings identify the mechanism by which CD28-mediated PKB activation promotes survival in T cells, and has profound implications for the role of costimulation in the regulation of lymphocyte homeostasis, response to pathogens, and transplantation biology.
Acknowledgments
We thank Dr. Astar Winoto for advice, Dr. Mark Bray and Dr. Sudha Arya at the Amgen Research Institute for supplying recombinant hCD8–mFasL fusion protein, Megan Cully for providing PTEN+/− mice, Rosa Pileggi for administrative assistance, and the Toronto Maple Leafs.
This work was supported by the Canadian Institutes for Health Research (CIHR; to J.R. Woodgett), the Terry Fox Program Project Grant, and the National Cancer Institute of Canada, with funds from the Canadian Cancer Society (to P.S. Ohashi). R.G. Jones is supported by the CIHR (K.M. Hunter Award). J.R. Woodgett is a CIHR Senior Scientist and an International Scholar of the Howard Hughes Medical Institute (HHMI). P.S. Ohashi holds a Canada Research Chair in Infection and Immunity.
Footnotes
*
Abbreviations used in this paper: DISC, death-inducing signaling complex; FADD, Fas-associated molecule with a death domain; FLIP, FLICE-inhibitory protein; PIP3, phosphatidylinositol-3,4,5-trisphosphate; PI3K, phosphatidylinositol 3′-kinase; PKB, protein kinase B; SEB, staphylococcal enterotoxin B.
References
- 1.Lenschow, D.J., T.L. Walunas, and J.A. Bluestone. 1996. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 14:233–258. [DOI] [PubMed] [Google Scholar]
- 2.Alegre, M.-L., K.A. Frauwirth, and C.B. Thompson. 2001. T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol. 1:220–228. [DOI] [PubMed] [Google Scholar]
- 3.Boise, L.H., A.J. Minn, P.J. Noel, C.H. June, M.A. Accavitti, T. Lindsten, and C.B. Thompson. 1995. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-xL. Immunity. 3:87–98. [DOI] [PubMed] [Google Scholar]
- 4.Radvanyi, L., Y. Shi, H. Vaziri, A. Sharma, R. Dhala, G.B. Mills, and R.G. Miller. 1996. CD28 costimulation inhibits TCR-induced apoptosis during a primary T cell response. J. Immunol. 156:1788–1798. [PubMed] [Google Scholar]
- 5.Sperling, A.I., J.A. Auger, B.D. Ehst, I.C. Rulifson, C.B. Thompson, and J.A. Bluestone. 1996. CD28/B7 interactions deliver a unique signal to naive T cells that regulates cell survival but not early proliferation. J. Immunol. 157:3909–3917. [PubMed] [Google Scholar]
- 6.Dahl, A.M., C. Klein, P.G. Andres, C.A. London, M.P. Lodge, R.C. Mulligan, and A.K. Abbas. 2000. Expression of Bcl-XL restores cell survival, but not proliferation and effector differentiation, in CD28-deficient T lymphocytes. J. Exp. Med. 191:2031–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okkenhaug, K., L. Wu, K.M. Garza, J. La Rose, W. Khoo, B. Odermatt, T.W. Mak, P.S. Ohashi, and R. Rottapel. 2001. A point mutation in CD28 distinguishes proliferative signals from survival signals. Nat. Immunol. 2:325–332. [DOI] [PubMed] [Google Scholar]
- 8.Jones, R.G., M. Parsons, M. Bonnard, V.S.F. Chan, W.C. Yeh, J.R. Woodgett, and P.S. Ohashi. 2000. Protein kinase B regulates T lymphocyte survival, nuclear factor kappaB activation, and Bcl-X(L) levels in vivo. J. Exp. Med. 191:1721–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, W.S., P.-S. Xu, K. Gottlob, M.-L. Chen, K. Sokol, T. Shiyanova, I. Roninson, W. Weng, R. Suzuki, K. Tobe, et al. 2001. Growth retardation and increased apoptosis in mice with homozygous disruption of the akt1 gene. Genes Dev. 15:2203–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Parjis, L., Y. Refaeli, J.D. Lord, B.H. Nelson, A.K. Abbas, and D. Baltimore. 1999. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity. 11:281–288. [DOI] [PubMed] [Google Scholar]
- 11.Kelly, E., A. Won, Y. Refaeli, and L. Van Parjis. 2002. IL-2 and related cytokines can promote T cell survival by activating AKT. J. Immunol. 168:597–603. [DOI] [PubMed] [Google Scholar]
- 12.Scheid, M., and J.R. Woodgett. 2001. PKB/AKT: functional insights from genetic models. Nat. Rev. Mol. Cell Biol. 2:760– 768. [DOI] [PubMed] [Google Scholar]
- 13.Chan, T.O., S.E. Rittenhouse, and P.N. Tsichlis. 1999. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu. Rev. Biochem. 68:965–1014. [DOI] [PubMed] [Google Scholar]
- 14.Stambolic, V., A. Suzuki, J.L. de la Pompa, G.M. Brothers, C. Mirtsos, T. Sasaki, J. Ruland, J.M. Penninger, D.P. Siderovski, and T.W. Mak. 1998. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 95:29–39. [DOI] [PubMed] [Google Scholar]
- 15.Maehama, T., and J.E. Dixon. 1998. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 273:13375–13378. [DOI] [PubMed] [Google Scholar]
- 16.Parry, R.V., K. Reif, G. Smith, D.M. Sanson, B.A. Hemmings, and S.G. Ward. 1997. Ligation of the T cell co-stimulatory receptor CD28 activates the serine-threonine protein kinase protein kinase B. Eur. J. Immunol. 27:2495–2501. [DOI] [PubMed] [Google Scholar]
- 17.Kane, L.P., P.G. Andres, K.C. Howland, A.K. Abbas, and A. Weiss. 2001. Akt provides the CD28 costimulatory signal for up-regulation of IL-2 and IFN-gamma but not TH2 cytokines. Nat. Immunol. 2:37–44. [DOI] [PubMed] [Google Scholar]
- 18.Sebzda, E., S. Mariathasan, T. Ohteki, R. Jones, M.F. Bachmann, and P.S. Ohashi. 1999. Selection of the T cell repertoire. Annu. Rev. Immunol. 17:829–874. [DOI] [PubMed] [Google Scholar]
- 19.Lenardo, M.J., F.K.M. Chan, F. Hornung, H. McFarland, R. Siegel, J. Wang, and L. Zheng. 1999. Mature T lymphocyte apoptosis - immune regulation in a dynamic and unpredictable environment. Annu. Rev. Immunol. 17:221–253. [DOI] [PubMed] [Google Scholar]
- 20.Siegel, R.M., F.K. Chan, H.J. Chun, and M.J. Lenardo. 2000. The multifaceted role of Fas signaling in immune cell homeostasis and autoimmunity. Nat. Immunol. 1:469–474. [DOI] [PubMed] [Google Scholar]
- 21.Nagata, S., and T. Suda. 1995. Fas and Fas ligand: lpr and gld mutations. Immunol. Today. 16:39–43. [DOI] [PubMed] [Google Scholar]
- 22.Rieux-Laucat, F., F. Le Deist, C. Hivroz, I.A.G. Roberts, K.M. Debatin, A. Fischer, and J.P. de Villartay. 1995. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 268:1347–1349. [DOI] [PubMed] [Google Scholar]
- 23.Fisher, G.H., F.J. Rosenberg, S.E. Straus, J.K. Dale, L.A. Middelton, A.Y. Lin, W. Strober, M.J. Lenardo, and J.M. Puck. 1995. Dominant interfering Fas gene mutations. Cell. 81:935–946. [DOI] [PubMed] [Google Scholar]
- 24.Drappa, J., A.K. Vaishnaw, K.E. Sullivan, J.-L. Chu, and K.B. Elkon. 1996. Fas gene mutations in the Canale-Smith syndrome, an inherited lymphoproliferative disorder associated with autoimmunity. N. Engl. J. Med. 335:1643–1649. [DOI] [PubMed] [Google Scholar]
- 25.Wang, J., L. Zheng, A. Lobito, F.K. Chan, J. Dale, M. Sneller, X. Yao, J.M. Puck, S.E. Straus, and M.J. Lenardo. 1999. Inherited human Caspase 10 mutations underlie defective lymphocyte and dendritic cell apoptosis in autoimmune lymphoproliferative syndrome type II. Cell. 98:47–58. [DOI] [PubMed] [Google Scholar]
- 26.Pircher, H., K. Bürki, R. Lang, H. Hengartner, and R. Zinkernagel. 1989. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 342:559–561. [DOI] [PubMed] [Google Scholar]
- 27.Sebzda, E., T.M. Kündig, C.T. Thomson, K. Aoki, S.Y. Mak, J. Mayer, T.M. Zamborelli, S. Nathenson, and P.S. Ohashi. 1996. Mature T cell reactivity altered by a peptide agonist that induces positive selection. J. Exp. Med. 183:1093–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward, S.G., J. Westwisck, N.D. Hall, and D.M. Sansom. 1993. Ligation of CD28 receptor by B7 induces formation of D-3 phosphoinositides in T lymphocytes independently of T cell receptor/CD3 activation. Eur. J. Immunol. 23:2572–2577. [DOI] [PubMed] [Google Scholar]
- 29.Russell, J.H., B. Rush, C. Weaver, and R. Wang. 1993. Mature T cells of autoimmune lpr/lpr mice have a defect in antigen-stimulated suicide. Proc. Natl. Acad. Sci. USA. 90:4409–4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singer, G.G., and A.K. Abbas. 1994. The Fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1:365–371. [DOI] [PubMed] [Google Scholar]
- 31.Kawabe, Y., and A. Ochi. 1991. Programmed cell death and extrathymic reduction of Vβ8+ CD4+ T cells in mice tolerant to Staphylococcus aureus enterotoxin B. Nature. 349:245–248. [DOI] [PubMed] [Google Scholar]
- 32.Bonfoco, E., P.M. Stuart, T. Brunner, T. Lin, T.S. Griffith, Y. Gao, H. Nakajima, P.A. Henkart, T.A. Ferguson, and D.R. Green. 1998. Inducible nonlymphoid expression of Fas ligand is responsible for superantigen-induced peripheral deletion of T cells. Immunity. 9:711–720. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen, L.T., K. McKall-Faienza, A. Zakarian, D.E. Speiser, T.W. Mak, and P.S. Ohashi. 2000. TNF receptor 1 (TNFR1) and CD95 are not required for T cell deletion after virus infection but contribute to peptide-induced deletion under limited conditions. Eur. J. Immunol. 30:683–688. [DOI] [PubMed] [Google Scholar]
- 34.Reich, A., H. Korner, J.D. Sedgwick, and H. Pircher. 2000. Immune down-regulation and peripheral deletion of CD8 T cells does not require TNF receptor-ligand interactions nor CD95. Eur. J. Immunol. 30:678–682. [DOI] [PubMed] [Google Scholar]
- 35.Vidal, S., D.H. Kono, and A.N. Theofilopoulos. 1998. Loci predisposing to autoimmunity in MRL-Fas lpr and C57BL/6-Fas lpr mice. J. Clin. Invest. 101:696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parsons, M.J., R.G. Jones, M.S. Tsao, B. Odermatt, P.S. Ohashi, and J.R. Woodgett. 2001. Expression of active PKB in T cells perturbs both T and B cell homeostasis and promotes inflammation. J. Immunol. 167:42–48. [DOI] [PubMed] [Google Scholar]
- 37.Krammer, P.H. 2000. CD95's deadly mission in the immune system. Nature. 407:789–795. [DOI] [PubMed] [Google Scholar]
- 38.Rokudai, S., N. Fujita, Y. Hashimoto, and T. Tsuruo. 2000. Cleavage and inactivation of antiapoptotic Akt/PKB by caspases during apoptosis. J. Cell. Physiol. 182:290–296. [DOI] [PubMed] [Google Scholar]
- 39.Bachelder, R.E., M.A. Wendt, N. Fujita, T. Tsuruo, and A.M. Mercurio. 2001. The cleavage of Akt/protein kinase B by death receptor signaling is an important event in detachment-induced apoptosis. J. Biol. Chem. 276:34702–34707. [DOI] [PubMed] [Google Scholar]
- 40.Luo, X., I. Budihardjo, H. Zou, C. Slaughter, and X. Wang. 1998. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 94:481–490. [DOI] [PubMed] [Google Scholar]
- 41.Li, H., H. Zhu, C.J. Xu, and J. Yuan. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 94:491–501. [DOI] [PubMed] [Google Scholar]
- 42.Di Cristofano, A., P. Kotsi, Y.F. Peng, C. Cordon-Cardo, K.B. Elkon, and P.P. Pandolfi. 1999. Impaired Fas response and autoimmunity in Pten+/− mice. Science. 285:2122–2125. [DOI] [PubMed] [Google Scholar]
- 43.Chinnaiyan, A.M., K. O'Rourke, M. Tewari, and V.M. Dixit. 1995. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 81:505–512. [DOI] [PubMed] [Google Scholar]
- 44.Boldin, M.P., T.M. Goncharov, Y.V. Goltsev, and D. Wallach. 1996. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1-and TNF receptor-induced cell death. Cell. 85:803–815. [DOI] [PubMed] [Google Scholar]
- 45.Kischkel, F.C., S. Hellbardt, I. Behrmann, M. Germer, M. Pawlilta, P.H. Krammer, and M.E. Peter. 1995. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 14:5579–5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang, C.-Y., M.W. Mayo, R.G. Korneluk, D.V. Goeddel, and A.S. Baldwin, Jr. 1998. NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-lAP1 and c-lAP2 to suppress caspase-8 activation. Science. 281:1680–1683. [DOI] [PubMed] [Google Scholar]
- 47.Liston, P., N. Roy, K. Tamai, C. Lefebvre, S. Baird, G. Cherton-Horvat, R. Farahani, M. McLean, J.E. Ikeda, A. MacKenzie, and R.G. Korneluk. 1996. Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature. 379:349–353. [DOI] [PubMed] [Google Scholar]
- 48.Tang, G., Y. Minemoto, B. Dibling, R.H. Purcell, Z. Li, M. Karin, and A. Lin. 2001. Inhibition of JNK activation through NF-kappaB target genes. Nature. 414:313–317. [DOI] [PubMed] [Google Scholar]
- 49.Suhara, T., T. Mano, B.E. Oliveira, and K. Walsh. 2001. Phosphatidylinositol 3-kinase/Akt signaling controls endothelial cell sensitivity to Fas-mediated apoptosis via regulation of FLICE-inhibitory protein (FLIP). Circ. Res. 89:13–19. [DOI] [PubMed] [Google Scholar]
- 50.Panka, D.J., T. Mano, T. Suhara, K. Walsh, and J.W. Mier. 2001. Phosphatidylinositol 3-kinase/Akt activity regulates c-FLIP expression in tumor cells. J. Biol. Chem. 276:6893–6896. [DOI] [PubMed] [Google Scholar]
- 51.Thome, M., P. Schneider, K. Hofmann, H. Fickenscher, E. Meini, F. Neipel, C. Mattmann, K. Burns, J.L. Bodmer, M. Schroter, et al. 1997. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 386:517–521. [DOI] [PubMed] [Google Scholar]
- 52.Irmler, M., M. Thome, M. Hahne, P. Schneider, K. Hofmann, V. Steiner, J.-L. Bodmer, M. Schroter, K. Burns, C. Mattmann, et al. 1997. Inhibition of death receptor signals by cellular FLIP. Nature. 388:190–195. [DOI] [PubMed] [Google Scholar]
- 53.Rasper, D.M., J.P. Vaillancourt, S. Hadano, V.M. Hontzager, I. Seiden, S.L. Keen, P. Tawa, S. Xanthoudakis, J. Nasir, D. Martindale, et al. 1998. Cell death attenuation by ‘Usurpin’, a DED-caspase homologue that precludes caspase-8 recruitment and activation by the CD95 (Fas, APO-1) receptor complex. Cell Death Differ. 5:271–288. [DOI] [PubMed] [Google Scholar]
- 54.Boehme, S.A., L. Zheng, and M.J. Lenardo. 1995. Analysis of the CD4 coreceptor and activation-induced costimulatory molecules in antigen-mediated mature T lymphocyte death. J. Immunol. 155:1703–1712. [PubMed] [Google Scholar]
- 55.van Parijs, L., A. Ibraghimov, and A.K. Abbas. 1996. The roles of costimulation and Fas in T cell apoptosis and peripheral tolerance. Immunity. 4:321–328. [DOI] [PubMed] [Google Scholar]
- 56.Collette, Y., D. Razanajaone, M. Ghiotto, and D. Olive. 1997. CD28 can promote T cell survival through a phosphatidylinositol 3-kinase-independent mechanism. Eur. J. Immunol. 27:3283–3289. [DOI] [PubMed] [Google Scholar]
- 57.Burr, J.S., N.D.L. Savage, G.E. Messah, S.L. Kimzey, A.S. Shaw, R.H. Arch, and J.M. Green. 2001. Cutting Edge: Distinct motifs within CD28 regulate T cell proliferation and induction of Bcl-XL. J. Immunol. 166:5331–5335. [DOI] [PubMed] [Google Scholar]
- 58.Scaffidi, C., S. Fulda, A. Srinivasan, C. Friesen, F. Li, K.J. Tomaselli, K.M. Debatin, P.H. Krammer, and M.E. Peter. 1998. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 17:1675–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vallejo, A.N., M. Schirmer, C.M. Weyand, and J.J. Goronzy. 2000. Clonality and longevity of CD4+CD28null T cells are associated with defects in apoptotic pathways. J. Immunol. 165:6301–6307. [DOI] [PubMed] [Google Scholar]
- 60.Kirchhoff, S., W.W. Muller, M. Li-Weber, and P.H. Krammer. 2000. Up-regulation of c-FLIPshort and reduction of activation-induced cell death in CD28-costimulated human T cells. Eur. J. Immunol. 30:2765–2774. [DOI] [PubMed] [Google Scholar]
- 61.Hausler, P., G. Papoff, A. Eramo, K. Reif, D. Cantrell, and G. Ruberti. 1998. Protection of CD95-mediated apoptosis by activation of phosphatidylinositide 3-kinase and protein kinase B. Eur. J. Immunol. 28:57–69. [DOI] [PubMed] [Google Scholar]
- 62.Rohn, J.L., A.-O. Hueber, N.J. McCarthy, D. Lyon, P. Navarro, B.M. Burgering, and G.I. Evan. 1998. The opposing roles of the Akt and c-Myc signalling pathways in survival from CD95-mediated apoptosis. Oncogene. 17:2811–2818. [DOI] [PubMed] [Google Scholar]
- 63.Peter, M.E., F.C. Kischkel, C.G. Scheuerpflug, J.P. Medema, K.-M. Debatin, and P.H. Krammer. 1997. Resistance of cultured peripheral T cells towards activation-induced cell death involves a lack of recruitment of FLICE (MACH/caspase 8) to the CD95 death-inducing signaling complex. Eur. J. Immunol. 27:1207–1212. [DOI] [PubMed] [Google Scholar]
- 64.Lenardo, M.J. 1991. Interleukin-2 programs mouse alpha/beta T lymphocytes for apoptosis. Nature. 353:858–861. [DOI] [PubMed] [Google Scholar]
- 65.van Parijs, L., Y. Refaeli, A.K. Abbas, and D. Baltimore. 1999. Autoimmunity as a consequence of retrovirus-mediated expression of C-FLIP in lymphocytes. Immunity. 11:763–770. [DOI] [PubMed] [Google Scholar]
- 66.Shan, X., M.J. Czar, S.C. Bunnell, P. Liu, Y. Liu, P.L. Schwartzberg, and R.L. Wange. 2000. Deficiency of PTEN in Jurkat T cells causes constitutive localization of Itk to the plasma membrane and hyperresponsiveness to CD3 stimulation. Mol. Cell. Biol. 20:6945–6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Borlado, L.R., C. Redondo, B. Alverez, C. Jimenez, L.M. Criado, J. Flores, M.A. Marcos, C. Martinez-A, D. Balomenos, and A.C. Carrera. 2000. Increased phosphoinositide 3-kinase activity induces a lymphoproliferative disorder and contributes to tumor generation in vivo. FASEB J. 14:895–903. [DOI] [PubMed] [Google Scholar]
- 68.Varadhachary, A.S., M.E. Peter, S.N. Perdow, P.H. Krammer, and P. Salgame. 1999. Selective up-regulation of phosphatidylinositol 3′-kinase activity in Th2 cells inhibits caspase-8 cleavage at the death-inducing complex: a mechanism for Th2 resistance from Fas-mediated apoptosis. J. Immunol. 163:4772–4779. [PubMed] [Google Scholar]
- 69.Kops, G.J., and B.M. Burgering. 1999. Forkhead transcription factors: new insights into protein kinase B (c-akt) signaling. J. Mol. Med. 77:656–665. [DOI] [PubMed] [Google Scholar]
- 70.Lauder, A., A. Castellanos, and K. Weston. 2001. c-myb transcription is activated by protein kinase B (PKB) following interleukin-2 stimulation of T cells and is required for PKB-mediated protection from apoptosis. Mol. Cell. Biol. 21:5797–5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ozes, O.N., L.D. Mayo, J.A. Gustin, S.R. Pfeffer, L.M. Pfeffer, and D.B. Donner. 1999. NF-κB activation by tumor necrosis factor requires the Akt serine-threonine kinase. Nature. 401:82–85. [DOI] [PubMed] [Google Scholar]
- 72.Romashkova, J.A., and S.S. Makarov. 1999. NF-κB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 401:86–90. [DOI] [PubMed] [Google Scholar]
- 73.Kane, L.P., V.S. Shapiro, D. Stokoe, and A. Weiss. 1999. Induction of NF-κB by the Akt/PKB kinase. Curr. Biol. 9:601–604. [DOI] [PubMed] [Google Scholar]
- 74.Datta, S.R., H. Dudek, X. Tao, S. Masters, H. Fu, Y. Gotoh, and M.E. Greenberg. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 91:231–241. [DOI] [PubMed] [Google Scholar]
- 75.del Peso, L., M. Gonzalez-Garcia, C. Page, R. Herrera, and G. Nunez. 1997. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 278:687–689. [DOI] [PubMed] [Google Scholar]
- 76.Cardone, M.H., N. Roy, H.R. Stennicke, G.S. Salvesen, T.F. Franke, E. Stanbridge, S. Frisch, and J.C. Reed. 1998. Regulation of cell death protease caspase-9 by phosphorylation. Science. 282:1318–1321. [DOI] [PubMed] [Google Scholar]
- 77.Mok, C., G. Gil-Gomez, O. Williams, M. Coles, S. Taga, M. Tolaini, T. Norton, D. Kioussis, and H.J.M. Brady. 1999. Bad can act as a key regulator of T cell apoptosis and T cell development. J. Exp. Med. 189:575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fujita, E., A. Jinbo, H. Matuzaki, H. Konishi, U. Kikkawa, and T. Momoi. 1999. Akt phosphorylation site found in human caspase-9 is absent in mouse caspase-9. Biochem. Biophys. Res. Commun. 264:550–555. [DOI] [PubMed] [Google Scholar]
- 79.Alessi, D.R., F.B. Caudwell, M. Andjelkovic, B.A. Hemmings, and P. Cohen. 1996. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 399:333–338. [DOI] [PubMed] [Google Scholar]
- 80.Hatano, E., and D.A. Brenner. 2001. Akt protects mouse hepatocytes from TNF-alpha- and Fas-mediated apoptosis through NF-kappaB activation. Am. J. Physiol. Gastrointest. Liver Physiol. 281:G1357–G1368. [DOI] [PubMed] [Google Scholar]
- 81.Kataoka, T., M. Schroter, M. Hahne, P. Schneider, M. Irmler, M. Thome, C.J. Froelich, and J. Tschopp. 1998. FLIP prevents apoptosis induced by death receptors but not by perforin/granzyme B, chemotherapeutic drugs, and gamma irradiation. J. Immunol. 161:3936–3942. [PubMed] [Google Scholar]
- 82.Thome, M., and J. Tschopp. 2001. Regulation of lymphocyte proliferation and death by FLIP. Nat. Rev. Immunol. 1:50–58. [DOI] [PubMed] [Google Scholar]
- 83.Salomon, B., and J.A. Bluestone. 2001. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu. Rev. Immunol. 19:225–252. [DOI] [PubMed] [Google Scholar]
- 84.Bellgrau, D., D. Gold, H. Selawry, J. Moore, A. Franzusoff, and R.C. Duke. 1995. A role for CD95 ligand in preventing graft rejection. Nature. 377:630–632. [DOI] [PubMed] [Google Scholar]
- 85.Li, W., L. Lu, Z. Wang, L. Wang, J.J. Fung, A.W. Thomson, and S. Qian. 2001. Costimulation blockade promotes the apoptotic death of graft-infiltrating T cells and prolongs survival of hepatic allografts from FLT3L-treated donors. Transplantation. 72:1423–1432. [DOI] [PubMed] [Google Scholar]