Toll-like Receptor 9–Dependent and –Independent Dendritic Cell Activation by Chromatin–Immunoglobulin G Complexes (original) (raw)
Abstract
Dendritic cell (DC) activation by nucleic acid–containing immunoglobulin (Ig)G complexes has been implicated in systemic lupus erythematosus (SLE) pathogenesis. However, the mechanisms responsible for activation and subsequent disease induction are not completely understood. Here we show that murine DCs are much more effectively activated by immune complexes that contain IgG bound to chromatin than by immune complexes that contain foreign protein. Activation by these chromatin immune complexes occurs by two distinct pathways. One pathway involves dual engagement of the Fc receptor FcγRIII and Toll-like receptor (TLR)9, whereas the other is TLR9 independent. Furthermore, there is a characteristic cytokine profile elicited by the chromatin immune complexes that distinguishes this response from that of conventional TLR ligands, notably the induction of BAFF and the lack of induction of interleukin 12. The data establish a critical role for self-antigen in DC activation and explain how the innate immune system might drive the adaptive immune response in SLE.
Keywords: systemic lupus erythematosus, innate immunity, Fc receptor, BAFF, tumor necrosis factor
Introduction
SLE is characterized by the loss of tolerance to self-antigens and the consequent production of autoantibodies (1). The predominant autoantigenic targets are nucleic acid–containing macromolecules such as chromatin or ribonucleoprotein particles (1). These autoantigens are continuously being released or exposed to the extracellular milieu as a result of apoptosis, but multiple mechanisms normally exist to ensure that they are rapidly cleared and degraded. A number of murine models have been developed that have in common the impaired clearance of apoptotic material. These include mice deficient in C1q, DNaseI, serum amyloid P component, or the membrane tyrosine kinase c-mer (2–6). Remarkably, lupus-like autoimmunity is seen in all these different models, suggesting that the autoantigens themselves might be driving the autoimmune response in SLE. The presence of large amounts of circulating apoptotic cells and nucleosomes in SLE patients (7, 8), and the identification of C1q or DNase I genetic deficiencies in certain individuals (9, 10), are consistent with a role for autoantigen in the human disease. However, a critical factor in the development of autoimmune disease is not only the presence of autoantigen, but also the manner in which the autoantigen is presented to the immune system.
DCs can induce either T cell tolerance or strong innate and adaptive immunity to specific antigen (11). In general, tolerance is initiated when DCs are immature (unactivated), whereas the initiation of immunity requires an effective DC maturation (“danger”) signal (12). In the context of autoimmune disease, DC uptake of apoptotic cells in the absence of a maturation signal induces tolerance (13). However, this benign presentation of antigen can be reversed by the coordinate engagement of Toll-like receptors (TLRs), CD40, or activating Fcγ receptors that provide this maturation signal in vivo (11, 14, 15).
DCs also play a key role in B cell survival and adaptive antibody responses. The production of the TNF family ligands BAFF and APRIL are particularly important in this respect (16, 17). Overexpression of BAFF in transgenic animals leads to lupus-like disease (18, 19), and elevated serum levels of BAFF are found in patients with SLE and other systemic autoimmune disorders (17, 20). However, the stimuli that lead to BAFF production in autoimmune disease are not well understood. Therefore, it is important to identify ligands that induce BAFF production, particularly in the context of autoimmune disease.
Immune complexes consisting of DNA and anti–double stranded DNA antibodies isolated from the sera of patients with SLE can induce plasmacytoid DCs to produce high levels of IFN-α (21), a cytokine thought to be involved in SLE pathogenesis (22–24). To eventually limit the consequences of DC activation by these immune complexes, it will be necessary to understand the mechanisms whereby this activation occurs. In this regard, we have recently found that similar chromatin-containing immune complexes can activate IgG2a-specific rheumatoid factor B cells by a dual receptor mechanism. The IgG autoantibody is bound and internalized by the B cell receptor and the chromatin is then able to engage a TLR, most likely TLR9, in a cytoplasmic compartment (25). Therefore, we hypothesized that a similar dual receptor mechanism might be operating in DCs, with an Fcγ receptor (instead of the B cell receptor) serving to internalize the chromatin and deliver it to TLR9. To test this premise, we evaluated the relative efficiency of chromatin-containing immune complexes on the activation of DCs derived from wild-type, Fcγ receptor–deficient, MyD88-deficient, and TLR9-deficient mice. These studies have demonstrated both a TLR9-dependent and -independent pathway in this activation process and have further revealed distinctive functional properties of DCs activated via these mechanisms.
Materials and Methods
Mice.
BALB/cJ and FcγRIII-deficient mice (B6.129P2-Fcgr3 tm1Sjv; backcrossed six generations to C57BL/6) were obtained from The Jackson Laboratory. C57BL/6, Fc receptor common γ chain–deficient (B6.129P2-Fcerg1 tm1; backcrossed 12 generations to C57BL/6), and FcγRII-deficient mice (B6.129-Fcgr2 tm1; backcrossed 12 generations to C57BL/6), were obtained from Taconic. MyD88-deficient mice (backcrossed 12 generations to C57BL/6; reference 26) and TLR9-deficient mice (backcrossed three generations to BALB/c; reference 27) were provided by D. Golenbock (University of Massachusetts, Worcester, MA) and A. Krieg and P. Payette (Coley Pharmaceuticals, Ottawa, Canada), respectively. All mice were maintained at the Boston University School of Medicine Laboratory Animal Sciences Center in accordance with the regulations of the American Association for the Accreditation of Laboratory Animal Care.
Preparation of Nucleosomes.
We used nucleosomes purified from bovine thymus as described previously (28) according to the method of Burlingame and Rubin (29). In brief, nuclei isolated from bovine thymus (Pel-Freez Biologicals) were physically disrupted and then digested with micrococcal nuclease (Worthington Biochemical). The digested nucleosome/chromatin fragments were then separated on a 5–30% sucrose gradient to obtain mononucleosomes, di-nucleosomes, tri-nucleosomes, and higher oligomers. Nucleosome fraction 4 contained mainly mononucleosomes, whereas nucleosome fraction 7 contained mainly di-nucleosomes and tri-nucleosomes (28). The nucleosome fractions were extensively dialyzed against PBS, aliquoted, and frozen at −80°C until used.
mAbs and Immune Complexes.
The nucleosome-specific mAbs PL2-3 (IgG2a) and PL2-8 (IgG2b) were provided by M. Monestier (Temple University, Philadelphia, PA), and the anti-TNP mAb Hy1.2 (IgG2a) was provided by M. Shlomchik (Yale University, New Haven, CT). To prepare chromatin-enriched supernatant, MRL+/+ spleen cells at 5 × 106/ml were cultured in RPMI 1640 with 10% FCS for 48 h. The supernatant was then collected, 0.2 μM filtered, and stored at −80°C until needed. Chromatin is spontaneously released from spleen cells in short-term in vitro culture (30), and we have demonstrated previously the ability of PL2-3 to form immune complexes with this released chromatin (25, 31). Immune complexes comprised of antinucleosome mAb and chromatin (chromatin immune complex [chromatin IC]) for use in the binding studies shown in Fig. 1, a and c, were made by premixing PL2-3 (50 μg/ml final concentration) with chromatin-enriched supernatant (12.5% of final volume) at 37°C for 30 min before addition to the assay. Chromatin IC for use in the DC activation studies was made by adding the PL2-3 and PL2-8 (50 μg/ml final concentration) and chromatin-enriched supernatant (12.5% of total well volume) directly to the culture wells. Nucleosome/antinucleosome immune complexes for use in the DC activation studies were made by adding the PL2-3 (50 μg/ml final concentration) and the various bovine thymus nucleosome fractions (4 μg/ml final concentration) directly to the culture wells. Immune complexes comprised of anti-TNP mAb and TNP-BSA (protein immune complex [protein IC]) for use in both the binding and activation studies were made by premixing 50 μg/ml Hy1.2 (50 μg/ml final concentration) with TNP-BSA (12.5 μg/ml final concentration) at 37°C for 30 min before addition to the assay.
Figure 1.
Chromatin IC and protein IC bind DCs comparably. (a) Protein IC (anti-TNP IgG2a mAb plus TNP-BSA) and chromatin IC (antinucleosome IgG2a mAb plus spent culture fluid) were tested for immune complex formation in a C1q binding assay and compared with monomeric or heat-aggregated (HA) mouse IgG. (b) FACS® analysis of bone marrow–derived DCs from wild-type C57BL/6 mice purified with anti-CD11c magnetic beads. (c) The ability of the monomeric anti-TNP mAb (α-TNP mAb) and the immune complexes described above to bind to bone marrow–derived DCs from wild-type C57BL/6 mice was compared by measuring the amount of IgG2a bound to the cell surface using flow cytometry.
DC Preparation.
Bone marrow cells were extracted from the mice and layered onto Lympholyte-M (Cedarlane) density separation medium. Cells at the interface were cultured in complete medium (RPMI 1640 with 10% heat-inactivated FCS, 100 U/ml penicillin G, 100 μg/ml streptomycin, and 290 μg/ml l-glutamine) together with 6.7 ng/ml recombinant mouse GM-CSF (BD Biosciences) and 400 pg/ml recombinant mouse IL-4 (R&D Systems). On day 6, the cells were collected and the CD11c+ cells were isolated using magnetic bead positive selection with anti-CD11c beads (Miltenyi Biotec). Purity was assessed by flow cytometry after staining with anti–CD11c-FITC, anti–CD11b-PE and anti–CD8α-PE mAbs (BD Biosciences). Consistently, >90% of the cells were CD11c+ CD11b+ CD8α−, in keeping with a myeloid DC phenotype.
Immune Complex Binding to DCs.
The immune complexes (prepared as described above) or the monomeric anti-TNP mAb Hy1.2, both at 50 μg/ml final antibody concentration, were added to 106 DCs from wild-type C57BL/6 mice in 100 μl Hanks' balanced salt solution with 5% FCS on ice for 1 h. Cells were then washed twice and incubated with 500 ng/ml biotin-conjugated goat F(ab′)2 anti–mouse IgG2a (Southern Biotechnology Associates, Inc.) on ice for 1 h. Cells were again washed twice and incubated with 20 μg/ml streptavidin-PE (Biomeda Corp.) on ice for 1 h. After two final washes, the cells were analyzed by flow cytometry to detect the relative amount of bound IgG2a.
DC Activation for Cytokine Measurement.
3 × 105 DCs were seeded in 48-well tissue culture plates and cultured in complete medium (as above) with the appropriate ligands in a total well volume of 600 μl, together with 6.7 ng/ml GM-CSF and 400 pg/ml IL-4. Ligands included the nucleosome-specific mAbs PL2-3 (IgG2a) and PL2-8 (IgG2b; both at 50 μg/ml), 10 μg/ml LPS (Sigma-Aldrich), 100 μg/ml poly(I:C) (Sigma-Aldrich), 6 μg/ml stimulatory CpG phosphorothioate oligodeoxynucleotide (sODN) 1826 (32), 5′-TCCATGACGTTCCTGACGTT (Oligo's Etc), 1 μg/ml of the TLR7 agonist R848 (InvivoGen; reference 33), and 50 μg/ml protein IC (prepared as described above). In most experiments, the cells were preactivated before addition of the ligands with a CD40L–CD8 fusion protein and supernatant from the anti-CD8 B cell hybridoma 53-6.72 (American Type Culture Collection) as described previously (34) to amplify the cytokine response (21, 35–37). Chromatin-enriched supernatants (12.5% of total well volume, prepared as described above) were added to all wells in the cultures. In certain experiments, 4 μg/ml of the purified nucleosome fractions 4 and 7 (prepared as described above) were added instead of the chromatin-enriched supernatants. In these experiments, the DCs were not preactivated with the CD40L–CD8 fusion protein and anti-CD8 B cell hybridoma supernatant. In some experiments, the inhibitory CpG sODN 2088 (38), 5′-TCCTGGCGGGGAAGT-3′ (Oligo's Etc), the control sODN 2138, 5′-TCCATGAGCTTCCTGAGCTT-3′ (Coley Pharmaceutical Group), the control sODN 1982 (39), 5′-TCCAGGACTTCTCTCAGGTT-3′ (Coley Pharmaceutical Group), or chloroquine (Sigma-Aldrich) were added to the cultures 30 min before the addition of the ligands. After 48 h, supernatants were collected and stored at −20°C until cytokine measurements were performed. All antibodies and protein antigens used in the assays had an endotoxin level <0.06 EU/ml as measured by Limulus Amebocyte Lysate ELISA (Bio-Whittaker).
DC Costimulatory Molecule Expression.
Day 6 DC cultures derived from bone marrows of wild-type BALB/cJ and TLR9-deficient mice were incubated together with 6.7 ng/ml GM-CSF and 400 pg/ml IL-4 for 24 h together with the following stimuli: 4 μg/ml nucleosome fraction 7 alone, nucleosome/antinucleosome immune complexes (50 μg/ml of the antinucleosome mAb PL2-3 and 4 μg/ml nucleosome fraction 7), protein IC (50 μg/ml of the anti-TNP mAb Hy1.2 and 12.5 μg/ml TNP-BSA), 10 μg/ml LPS, or 6 μg/ml stimulatory CpG sODN 1826. After 24 h of incubation, DCs were double stained with anti–CD11c-PE (HL3; BD Biosciences) and anti–CD86-FITC (GL1; BD Biosciences) and analyzed by flow cytometry. The DCs used in these studies were not first purified with anti-CD11c magnetic beads because use of the beads was found to induce CD86 expression.
ELISAs.
TNF-α and IL-12 p70 ELISAs were performed according to the manufacturer's instructions (BD Biosciences). The C1q ELISA was performed as described previously (28). The BAFF ELISA was performed using monoclonal rat anti–mouse BAFF antibodies and recombinant murine BAFF, which is now available from Apotech. The level of sensitivity of the assay is 150 pg/ml.
Results
Chromatin IC, But Not Protein IC, Induces TNF-α.
We began by comparing the stimulatory ability of immune complexes comprised of chromatin and antinucleosome mAb (chromatin IC) to isotype-matched immune complexes comprised of TNP-conjugated BSA (TNP-BSA) and anti-TNP mAb (protein IC). To compare the extent of immune complex formation in our standard protein IC and chromatin IC preparations, we determined their relative ability to bind to C1q, as immune complexes bind C1q more avidly than monomeric IgG does (40). Both immune complex preparations bound more strongly to C1q than uncomplexed IgG did, with the protein IC binding a little better than the chromatin IC (Fig. 1 a), and thus potentially consisting of slightly larger complexes.
Next, we evaluated binding of the immune complex preparations to our DC population. DCs in these studies consisted of a highly enriched population bearing the characteristic CD11c+ CD11b+ CD8α− phenotype of myeloid DCs (Fig. 1 b). Murine bone marrow–derived myeloid DCs express TLR9 and respond well to TLR9 ligands such as CpG sODN (27). Because these DCs also express FcγRI, FcγRII, and FcγRIII (15, 41), and have been reported to undergo maturation on incubation with protein ICs (42), they were an appropriate cell type for our studies. We found that the protein and chromatin IC bound similarly to the DCs and both bound better than uncomplexed antibody (Fig. 1 c). Thus, it is unlikely that any difference in stimulatory capacity between the two types of immune complexes could be attributable to binding efficiency.
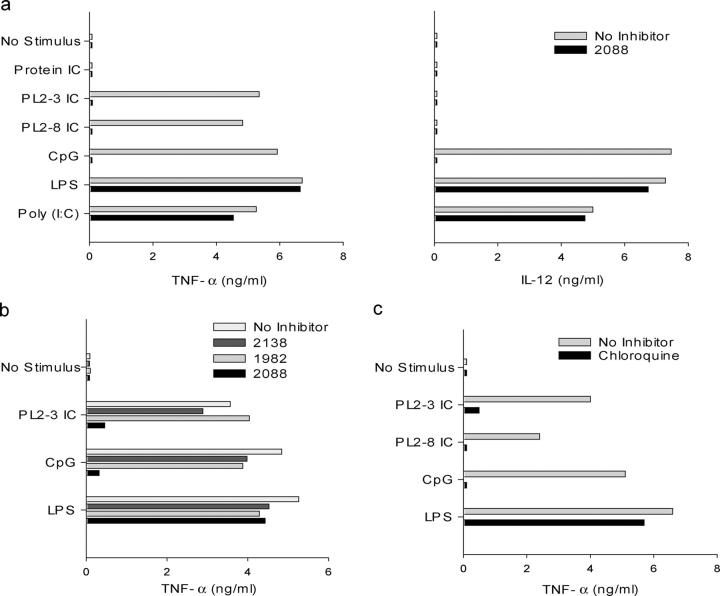
We then used TNF-α and IL-12 production as a readout to compare the functional activities of protein and chromatin IC. DC production of these proinflammatory cytokines requires a strong activation/maturation signal such as that mediated by TLR engagement (43). We found that stimulation of the DCs with the conventional TLR3, TLR4, and TLR9 ligands, poly(I:C), LPS, and hypomethylated CpG sODN, respectively, resulted in the production of TNF-α and IL-12 p70 as has been reported by others (Fig. 2 a; references 44 and 45). Protein IC elicited no cytokine production. Strikingly, however, chromatin IC elicited levels of TNF-α comparable to conventional TLR ligands (Fig. 2 a). This was seen with both IgG2a and IgG2b chromatin IC (PL2-3 IC and PL2-8 IC, respectively). Also notable was the complete absence of IL-12 production in the chromatin IC cultures (Fig. 2 a). This split cytokine profile, with selective inhibition of IL-12 but intact TNF-α production, is similar to that reported for macrophage activation by a combination of LPS and Fcγ receptor cross-linking reagents (46). Therefore, similar to our observations with autoreactive rheumatoid factor B cells (25), chromatin IC can activate DCs under conditions in which protein IC cannot, and this activation process leads to a cytokine profile distinct from that elicited by conventional TLR ligands.
Figure 2.
Chromatin IC, but not protein IC, induce TNF-α production. Bone marrow–derived DCs from wild-type C57BL/6 mice were preincubated for 30 min with or without (a) 12 μg/ml inhibitory CpG sODN 2088, (b) sODN 2138, sODN 1982, sODN 2088 (all at 1 μg/ml), and (c) 20 μg/ml chloroquine before the addition of 50 μg/ml protein IC, 50 μg/ml chromatin IC (PL2-3, IgG2a; PL2-8, IgG2b), 6 μg/ml of the stimulatory CpG sODN 1826, 10 μg/ml LPS, and 100 μg/ml poly(I:C). TNF-α and IL-12 p70 concentrations in supernatants collected after 48 h were determined by ELISA. The data are representative of nine (a), three (b), and three experiments (c).
TLR9 Is Involved in Activation by Chromatin IC.
To determine whether DC activation by chromatin IC involved TLR9, we attempted to block the response with known inhibitors of the TLR9-dependent CpG sODN-driven signaling pathway. The nuclease-resistant sODN 2088 has been found to effectively block activation by stimulatory CpG sODN such as 1826, which is TLR9 dependent, but does not inhibit activation through either TLR2 or TLR4 (25, 38, 47). The addition of sODN 2088 to the cultures blocked the chromatin IC induction of TNF-α and also CpG-mediated cytokine production, but had no effect on cytokine production induced by the TLR3 ligand poly- (I:C) or the TLR4 ligand LPS (Fig. 2 a). The inhibitory effect of sODN 2088 was specific as no inhibition was seen with the control sODNs 2138 and 1982 (Fig. 2 b). We observed similar results with chloroquine (Fig. 2 c), an agent that also inhibits CpG-mediated activation but has no effect on activation through TLR2 or TLR4 (25, 32, 48). Thus, based on the inhibitor studies, it appeared that TNF-α production induced by the chromatin IC involved TLR9 activation.
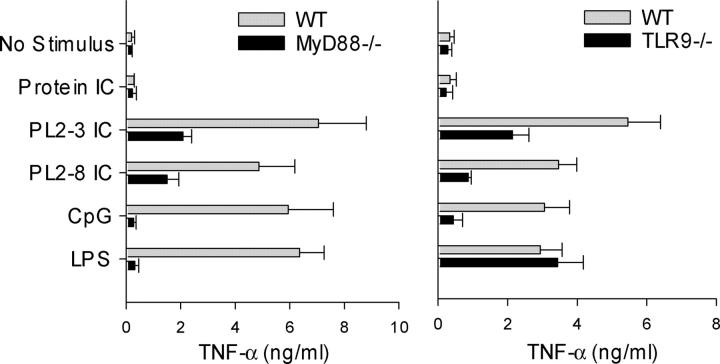
To confirm these findings, studies were performed with DCs from both MyD88-deficient mice, which are unresponsive to almost all TLR ligands in terms of cytokine production, and TLR9-deficient mice (Fig. 3). TNF-α production induced by the chromatin IC was substantially, but not completely, inhibited in DCs from these mice compared with wild-type controls. These results not only demonstrate an important role for TLR9 in DC activation by chromatin IC, but also indicate the existence of an as yet unidentified TLR9 and MyD88-independent pathway.
Figure 3.
Chromatin IC–induced TNF-α production involves MyD88 and TLR9. Bone marrow–derived DCs from wild-type C57BL/6 and MyD88-deficient mice (left), or wild-type BALB/c and TLR9-deficient mice (right), were cultured with protein IC, chromatin IC (PL2-3, IgG2a; PL2-8, IgG2b), the stimulatory CpG sODN 1826, and LPS. TNF-α concentrations in supernatants collected after 48 h were determined by ELISA. Data represent mean + SEM of three (left) and five experiments (right).
FcγRIII Is Required for Chromatin IC–mediated Activation.
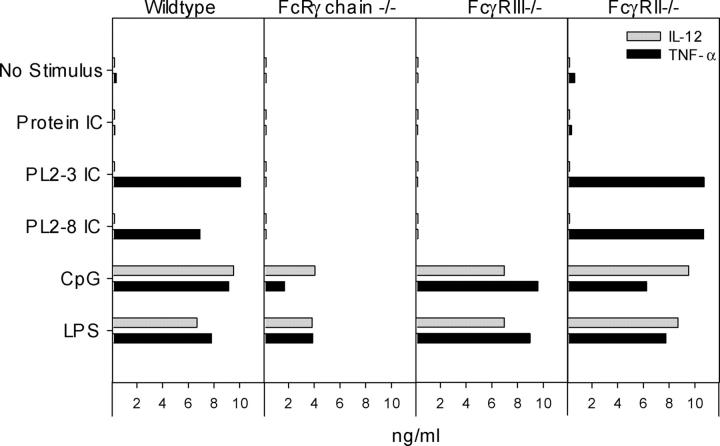
Our original hypothesis was that Fcγ receptors would be required for the uptake of chromatin IC and their delivery to TLR9 in an intracellular compartment. To identify the relevant FcγR, we used DCs from various FcγR-deficient mice. The high affinity FcγRI, which binds monomeric IgG, and the low affinity FcγRIII, which binds immune complexes, are stimulatory Fc receptors and both use the Fc receptor common γ chain (49). In the absence of the Fc receptor common γ chain or of FcγRIII alone, chromatin IC did not induce TNF-α production (Fig. 4). However, TNF-α production was not inhibited in DCs from mice deficient in the inhibitory low affinity receptor FcγRII. Thus, FcγRIII is required for DC activation by chromatin IC.
Figure 4.
FcγRIII is required for chromatin IC–induced TNF-α production. Bone marrow–derived DCs from wild-type C57BL/6, Fc receptor common γ chain–deficient (FcRγ chain −/−), FcγRIII-deficient (FcγRIII−/−), and FcγRII-deficient (FcγRII−/−) mice were cultured with protein IC, chromatin IC (PL2-3, IgG2a; PL2-8, IgG2b), the stimulatory CpG sODN 1826, and LPS. TNF-α and IL-12 p70 concentrations in supernatants collected after 48 h were determined by ELISA. The data are representative of three experiments.
Chromatin IC–induced BAFF Production Is TLR9 Independent.
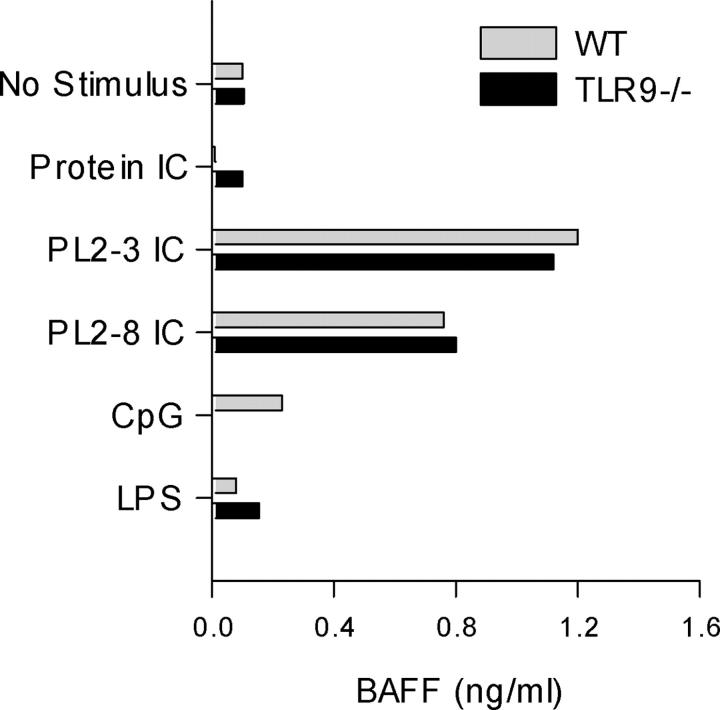
BAFF is a fundamental survival factor for B cells made by DCs and other antigen-presenting cells that is implicated in the pathogenesis of SLE and other systemic autoimmune disorders (16, 17). Therefore, we tested the capacity of chromatin IC to induce BAFF production. Remarkably, it was found that the chromatin IC did in fact elicit BAFF production, whereas little if any BAFF production was elicited by the conventional TLR ligands (Fig. 5). Protein IC also failed to elicit BAFF production. Even though DCs derived from the TLR9-deficient mice produced markedly reduced levels of TNF-α in response to chromatin IC, they made levels of BAFF that were comparable to wild-type cells. Thus, consistent with the limited ability of the TLR ligands to elicit BAFF, it was found that BAFF production induced by the chromatin IC was TLR9 independent and must therefore involve a separate signaling cascade.
Figure 5.
BAFF induction by chromatin IC is TLR9 independent. Bone marrow–derived DCs from wild-type BALB/c and TLR9-deficient mice were cultured with protein IC, chromatin IC (PL2-3, IgG2a; PL2-8, IgG2b), the stimulatory CpG sODN 1826, and LPS. BAFF concentration in supernatants collected after 48 h was determined by ELISA. The data are representative of three experiments.
Nucleosomes Complexed with Antinucleosome mAb Activate DCs.
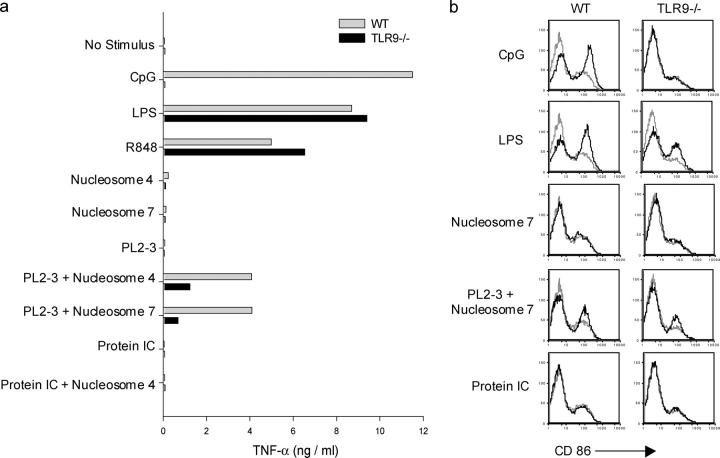
The studies above showing chromatin IC–induced DC activation were performed by allowing the antinucleosome mAbs to spontaneously form immune complexes in cultures containing both spleen cell culture supernatants (as a source of enriched chromatin), as well as reagents to cross-link CD40 on the DCs (to amplify the response). All experimental wells, not just those with antinucleosome mAb, contained identical amounts of spleen cell culture supernatant and CD40 cross-linking reagents, making it unlikely that nonchromatin components in the cultures were responsible for the chromatin IC–induced activation observed. Nevertheless, to more directly demonstrate the requirement for chromatin in DC activation, we performed experiments using purified calf thymus nucleosomes as a source of chromatin. We used micrococcal nuclease-digested nucleosome/chromatin fragments that we further purified on sucrose gradients to give fractions comprising different multimers of nucleosome ranging from mainly mononucleosomes (fraction 4) to mainly di-nucleosomes and tri-nucleosomes (fraction 7; reference 28). We had shown that these nucleosome fractions bound specifically to antinucleosome mAbs, including PL2-3, but not to isotype-matched anti-hapten mAbs (28). We added PL2-3 and nucleosome fractions 4 and 7 to the DC cultures in the absence of spleen cell culture supernatants and in the absence of CD40 cross-linking reagents, and found that DC TNF-α production was induced when both PL2-3 and nucleosomes were present together in the cultures, but was not induced when these immune complex components were added individually (Fig. 6 a). TNF-α production was not enhanced by the addition of nucleosomes to cultures containing protein IC. Remarkably, TNF-α production induced by the nucleosome–PL2-3 complexes was substantially reduced in DCs from TLR9-deficient mice as compared with wild-type mice (Fig. 6 a).
Figure 6.
Nucleosome/antinucleosome complexes activate DCs. (a) Bone marrow–derived DCs from wild-type BALB/c and TLR9-deficient mice were cultured with the stimulatory CpG sODN 1826, LPS, R848, nucleosome fraction 4 (nucleosome 4; mainly mononucleosomes), nucleosome fraction 7 (nucleosome 7; mainly di-nucleosomes and tri-nucleosomes), the antinucleosome IgG2a mAb PL2-3, PL2-3 and nucleosome 4, PL2-3 and nucleosome 7, protein IC (anti-TNP IgG2a mAb plus TNP-BSA), and protein IC and nucleosome 4. TNF-α concentrations in supernatants collected after 24 h were determined by ELISA. The data are representative of three experiments. (b) Day 6 bone marrow–derived DC cultures (not CD11c purified) from wild-type BALB/c and TLR9-deficient mice were cultured with stimuli as shown. After 24 h of incubation, DCs were double stained with anti–CD11c-PE and anti–CD86-FITC. CD86 expression of the CD11c+ population gate is shown with stimulus-induced staining intensity (black) compared with staining intensity of the nonstimulated cultures (gray). The data are representative of three experiments.
We also determined the ability of the nucleosome–PL2-3 complexes to increase the expression on DCs of the costimulatory molecule CD86. The nucleosome–PL2-3 complexes, but not the protein IC, induced CD86 up-regulation, although the extent of up-regulation was less than that seen with either CpG or LPS (Fig. 6 b). The addition of identical amounts of nucleosome to the protein IC cultures failed to induce CD86 up-regulation (not depicted). CD86 up-regulation induced by the nucleosome–PL2-3 complexes was observed in DCs from both wild-type and TLR9-deficient mice, indicating that a TLR9-independent pathway is sufficient for this effect.
Discussion
Dysregulated DC activation is implicated in the pathogenesis of SLE (22). Here we show that immune complexes containing self-antigen (chromatin), in contrast to immune complexes containing conventional protein antigen, activate myeloid DCs through both TLR9-dependent and -independent pathways.
The stimulatory capacity of the chromatin IC requires interaction with the low affinity Fc receptor, FcγRIII. Other investigators have identified FcγRIII as the critical IgG receptor mediating disease induction by autoantigen-containing immune complexes in a mouse rheumatoid arthritis model (50). In general, engagement of FcγRIII by immune complexes has two major consequences (51). The first is to initiate signaling cascades via the cytoplasmic ITAM motif leading to proinflammatory cellular phenotypes such as degranulation and the transcription of cytokine genes. The second is to mediate the uptake of the immune complexes and their delivery to distinct intracellular compartments, leading to efficient antigen presentation in both the MHC class I and II pathways (52). In this study, FcγRIII engagement alone was insufficient to induce DC activation as protein IC failed to elicit either cytokine production or up-regulation of CD86. Previous studies have shown variability in the ability of immune complexes to up-regulate costimulatory molecule expression on DCs (15, 42, 53, 54). The ability of the chromatin IC and the nucleosome/antinucleosome complexes, but not the protein IC, to induce both cytokine production and CD86 up-regulation, indicated that there must be additional activation pathways dependent on the autoantigen itself. In the case of the chromatin IC, FcγRIII functions to transport the complex to an internal compartment where the chromatin autoantigen is then able to induce DC activation.
Vertebrate DNA is generally nonstimulatory (55, 56), unless it is transfected into cells (57–60), supporting the concept that internalization of chromatin is important for cell activation. Furthermore, TLR9 is located in an intracellular compartment and therefore it is likely that delivery of ligand to this intracellular compartment is required for TLR9 stimulation (61, 62). We investigated the role of TLR9 in DC activation by chromatin IC because previous work demonstrated that endogenous chromatin activates autoreactive B cells if the chromatin is internalized via the B cell receptor, and that a TLR, most likely TLR9, is required for this activation (25). This study demonstrates an important role for TLR9 in DC activation by chromatin IC and, in addition, points to a TLR9-independent pathway that contributes to TNF-α production and is required for BAFF production.
TLR-independent DNA-reactive signaling pathways have been identified. Drosophila deficient in caspase-activated DNase and DNase II-like acid DNase accumulate endogenous intracellular DNA that leads to activation of innate immunity via the Toll-independent immune deficiency pathway (63). It remains to be established whether an analogous pathway exists in man, but mice deficient in caspase-activated DNase and DNase II accumulate DNA intracellularly and demonstrate innate immune system activation (64). Our findings are directly relevant to human SLE because DNA-containing immune complexes purified from the sera of patients with SLE induce plasmacytoid DCs to produce IFN-α (21), a cytokine implicated in SLE pathogenesis (22–24). The effect of DNA-containing immune complexes on other human DC subsets remains to be determined. However, it is likely that the relative importance of the TLR9-dependent and -independent pathways will differ in various DC subsets depending on the level of TLR9 expression, with TLR9 in humans being highly expressed in plasmacytoid DCs and less or no expression found in other subsets (65, 66).
The involvement of TLR9 in DC activation by chromatin IC raises the question of the nature of the mammalian chromatin able to engage TLR9, as the immune stimulatory effects of bacterial DNA mediated through TLR9 are dependent upon the presence of unmethylated CpG motifs (55). Bacterial DNA contains many more unmethylated CpG motifs than vertebrate DNA (67). However, despite this CpG suppression and methylation compared with bacterial DNA, vertebrate DNA nevertheless contains many unmethylated CpG motifs that could potentially serve as stimulatory ligands for TLR9 (68). Examples include the 37,000 mouse/45,000 human CpG islands that incorporate clusters of unmethylated CpG di-nucleotides (69). In addition to stimulatory CpG motifs, both vertebrate and nonvertebrate DNA also contain inhibitory motifs capable of blocking the effect of stimulatory CpG motifs (70). The inhibitory activity is critically dependent on base sequence. Two major nucleotide blocks within the inhibitory motif are essential for activity, a 5′ pyrimidine-rich block and a 3′ poly G block (either a G tetrad or G triplet), with the length of the intervening sequence between the 5′ and 3′ blocks also contributing to inhibitory activity (39). Consistent with this, we observed inhibition of chromatin IC–induced DC activation with sODN 2088, but not with the two control sODNs which both lacked a 3′ poly G sequence (Fig. 2 b). Although the precise mechanism of action of the inhibitory motifs has not been defined, sODN 2088 appears to specifically block stimulatory CpG-mediated activation and does not block activation mediated through TLR2, TLR4, CD40, or the B cell receptor (25, 38, 47). Thus, the ability of sODN 2088 to block chromatin IC–induced DC TNF-α production in our studies is consistent with a role for TLR9 in this process.
BAFF overexpression in transgenic animals leads to lupus-like autoimmunity, and BAFF serum levels are elevated in patients with SLE and other systemic autoimmune disorders (18, 19). IFN-α, IFN-γ, CD40L, and IL-10 induce BAFF production by human DCs (16, 71), although less is known about stimuli that elicit BAFF in murine DCs. In addition to DCs, BAFF is also made by monocytes, macrophages, and neutrophils (17, 72), but the stimuli that induce BAFF production in autoimmunity are not well defined. The demonstration that chromatin IC can induce BAFF production by myeloid DCs identifies a potentially pivotal mechanism whereby BAFF production could be chronically elicited in SLE patients with circulating immune complexes.
It is possible that the dual receptor paradigm shown here for complexed chromatin autoantigens might also apply to other lupus autoantigens and other cell types. For example, small nuclear ribonucleoproteins (RNA/protein particles) are a common autoantigen specificity in SLE (1) and could potentially engage either TLR3 or protein kinase R, both of which can mediate double stranded RNA–induced DC activation (37, 44). The demonstration that mRNA is an endogenous ligand for TLR3 supports this possibility (73). In addition, RNA/protein autoantigens could also engage TLR7, which has recently been shown to recognize single stranded RNA and thereby mediate DC activation (74–76). Intriguingly, it was reported that this recognition by TLR7 of single stranded RNA is not species specific and that both self- and viral RNA induce DC activation (74, 75). The predominant intracellular location of TLR3 and protein kinase R in DCs (37, 77), and the requirement for normal endosomal acidification in single stranded RNA–mediated DC activation (75, 76), suggests that internalization of the RNA would be required for any such RNA-induced DC activation in SLE. Although it is established that immune complexes consisting of DNA and anti–double stranded DNA antibodies can induce IFN-α production by human plasmacytoid DCs, less is known about the effect of these complexes on other human cell types. Potential effects on human DCs of the DC1 type or on neutrophils should be considered, as both express activating Fcγ receptors (49, 78) and can produce BAFF (16, 72). These possibilities need to be addressed in future studies.
Acknowledgments
We thank M. Monestier for providing the antinucleosome mAbs, M. Shlomchik and A. Krieg for helpful discussions and critical reading of the manuscript, and T. Ling for technical assistance.
This work was supported by grants from the National Institutes of Health, the National Kidney Foundation, and the Lupus Research Institute to I.R. Rifkin.
Abbreviations used in this paper: chromatin IC, chromatin immune complex; protein IC, protein immune complex; sODN, phosphorothioate oligodeoxynucleotide; TLR, Toll-like receptor.
References
- 1.Kotzin, B.L. 1996. Systemic lupus erythematosus. Cell. 85:303–306. [DOI] [PubMed] [Google Scholar]
- 2.Botto, M., C. Dell'Agnola, A.E. Bygrave, E.M. Thompson, H.T. Cook, F. Petry, M. Loos, P.P. Pandolfi, and M.J. Walport. 1998. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 19:56–59. [DOI] [PubMed] [Google Scholar]
- 3.Napirei, M., H. Karsunky, B. Zevnik, H. Stephan, H.G. Mannherz, and T. Moroy. 2000. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat. Genet. 25:177–181. [DOI] [PubMed] [Google Scholar]
- 4.Bickerstaff, M.C.M., M. Botto, W.L. Hutchinson, J. Herbert, G.A. Tennent, A. Bybee, D.A. Mitchell, H.T. Cook, P.J.G. Butler, M.J. Walport, et al. 1999. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nat. Med. 5:694–697. [DOI] [PubMed] [Google Scholar]
- 5.Cohen, P.L., R. Caricchio, V. Abraham, T.D. Camenisch, J.C. Jennette, R.A. Roubey, H.S. Earp, G. Matsushima, and E.A. Reap. 2002. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J. Exp. Med. 196:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott, R.S., E.J. McMahon, S.M. Pop, E.A. Reap, R. Caricchio, P.L. Cohen, H.S. Earp, and G.K. Matsushima. 2001. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 411:207–211. [DOI] [PubMed] [Google Scholar]
- 7.Rumore, P.M., and C.R. Steinman. 1990. Endogenous circulating DNA in systemic lupus erythematosus. Occurrence as multimeric complexes bound to histone. J. Clin. Invest. 86:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams, R.C., Jr., C.C. Malone, C. Meyers, P. Decker, and S. Muller. 2001. Detection of nucleosome particles in serum and plasma from patients with systemic lupus erythematosus using monoclonal antibody 4H7. J. Rheumatol. 28:81–94. [PubMed] [Google Scholar]
- 9.Morgan, B.P., and M.J. Walport. 1991. Complement deficiency and disease. Immunol. Today. 12:301–306. [DOI] [PubMed] [Google Scholar]
- 10.Yasutomo, K., T. Horiuchi, S. Kagami, H. Tsukamoto, C. Hashimura, M. Urushihara, and Y. Kuroda. 2001. Mutation of DNASE1 in people with systemic lupus erythematosus. Nat. Genet. 28:313–314. [DOI] [PubMed] [Google Scholar]
- 11.Steinman, R.M., D. Hawiger, and M.C. Nussenzweig. 2003. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21:685–711. [DOI] [PubMed] [Google Scholar]
- 12.Savill, J., I. Dransfield, C. Gregory, and C. Haslett. 2002. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2:965–975. [DOI] [PubMed] [Google Scholar]
- 13.Steinman, R.M., S. Turley, I. Mellman, and K. Inaba. 2000. The induction of tolerance by dendritic cells that have captured apoptotic cells. J. Exp. Med. 191:411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sparwasser, T., R.M. Vabulas, B. Villmow, G.B. Lipford, and H. Wagner. 2000. Bacterial CpG-DNA activates dendritic cells in vivo: T helper cell-independent cytotoxic T cell responses to soluble proteins. Eur. J. Immunol. 30:3591–3597. [DOI] [PubMed] [Google Scholar]
- 15.Kalergis, A.M., and J.V. Ravetch. 2002. Inducing tumor immunity through the selective engagement of activating Fcγ receptors on dendritic cells. J. Exp. Med. 195:1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Litinskiy, M.B., B. Nardelli, D.M. Hilbert, B. He, A. Schaffer, P. Casali, and A. Cerutti. 2002. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 3:822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackay, F., P. Schneider, P. Rennert, and J. Browning. 2003. BAFF and APRIL: a tutorial on B cell survival. Annu. Rev. Immunol. 21:231–264. [DOI] [PubMed] [Google Scholar]
- 18.Mackay, F., S.A. Woodcock, P. Lawton, C. Ambrose, M. Baetscher, P. Schneider, J. Tschopp, and J.L. Browning. 1999. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med. 190:1697–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gross, J.A., J. Johnston, S. Mudri, R. Enselman, S.R. Dillon, K. Madden, W. Xu, J. Parrish-Novak, D. Foster, C. Lofton-Day, et al. 2000. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 404:995–999. [DOI] [PubMed] [Google Scholar]
- 20.Zhang, J., V. Roschke, K.P. Baker, Z. Wang, G.S. Alarcon, B.J. Fessler, H. Bastian, R.P. Kimberly, and T. Zhou. 2001. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J. Immunol. 166:6–10. [DOI] [PubMed] [Google Scholar]
- 21.Vallin, H., A. Perers, G.V. Alm, and L. Ronnblom. 1999. Anti-double-stranded DNA antibodies and immunostimulatory plasmid DNA in combination mimic the endogenous IFN-alpha inducer in systemic lupus erythematosus. J. Immunol. 163:6306–6313. [PubMed] [Google Scholar]
- 22.Blanco, P., A.K. Palucka, M. Gill, V. Pascual, and J. Banchereau. 2001. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 294:1540–1543. [DOI] [PubMed] [Google Scholar]
- 23.Baechler, E.C., F.M. Batliwalla, G. Karypis, P.M. Gaffney, W.A. Ortmann, K.J. Espe, K.B. Shark, W.J. Grande, K.M. Hughes, V. Kapur, et al. 2003. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. USA. 100:2610–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rozzo, S.J., J.D. Allard, D. Choubey, T.J. Vyse, S. Izui, G. Peltz, and B.L. Kotzin. 2001. Evidence for an interferon-inducible gene, Ifi202, in the susceptibility to systemic lupus. Immunity. 15:435–443. [DOI] [PubMed] [Google Scholar]
- 25.Leadbetter, E.A., I.R. Rifkin, A.M. Hohlbaum, B.C. Beaudette, M.J. Shlomchik, and A. Marshak-Rothstein. 2002. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 416:603–607. [DOI] [PubMed] [Google Scholar]
- 26.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 9:143–150. [DOI] [PubMed] [Google Scholar]
- 27.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, et al. 2000. A Toll-like receptor recognizes bacterial DNA. Nature. 408:740–745. [DOI] [PubMed] [Google Scholar]
- 28.Rifkin, I.R., E.A. Leadbetter, B.C. Beaudette, C. Kiani, M. Monestier, M.J. Shlomchik, and A. Marshak-Rothstein. 2000. Immune complexes present in the sera of autoimmune mice activate rheumatoid factor B cells. J. Immunol. 165:1626–1633. [DOI] [PubMed] [Google Scholar]
- 29.Burlingame, R.W., and R.L. Rubin. 1990. Subnucleosome structures as substrates in enzyme-linked immunosorbent assays. J. Immunol. Methods. 134:187–199. [DOI] [PubMed] [Google Scholar]
- 30.Bell, D.A., B. Morrison, and P. VandenBygaart. 1990. Immunogenic DNA-related factors. Nucleosomes spontaneously released from normal murine lymphoid cells stimulate proliferation and immunoglobulin synthesis of normal mouse lymphocytes. J. Clin. Invest. 85:1487–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viglianti, G.A., C.M. Lau, T.M. Hanley, B.A. Miko, M.J. Shlomchik, and A. Marshak-Rothstein. 2003. Activation of autoreactive B cells by CpG dsDNA. Immunity. 19:837–847. [DOI] [PubMed] [Google Scholar]
- 32.Yi, A.K., R. Tuetken, T. Redford, M. Waldschmidt, J. Kirsch, and A.M. Krieg. 1998. CpG motifs in bacterial DNA activate leukocytes through the pH-dependent generation of reactive oxygen species. J. Immunol. 160:4755–4761. [PubMed] [Google Scholar]
- 33.Hemmi, H., T. Kaisho, O. Takeuchi, S. Sato, H. Sanjo, K. Hoshino, T. Horiuchi, H. Tomizawa, K. Takeda, and S. Akira. 2002. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3:196–200. [DOI] [PubMed] [Google Scholar]
- 34.Rothstein, T.L., J.K. Wang, D.J. Panka, L.C. Foote, Z. Wang, B. Stanger, H. Cui, S.T. Ju, and A. Marshak-Rothstein. 1995. Protection against Fas-dependent Th1-mediated apoptosis by antigen receptor engagement in B cells. Nature. 374:163–165. [DOI] [PubMed] [Google Scholar]
- 35.Krug, A., A. Towarowski, S. Britsch, S. Rothenfusser, V. Hornung, B. Bals, T. Giese, H. Engelmann, S. Endres, A.M. Krieg, et al. 2001. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur. J. Immunol. 31:3026–3037. [DOI] [PubMed] [Google Scholar]
- 36.Edwards, A.D., S.P. Manickasingham, R. Sporri, S.S. Diebold, O. Schulz, A. Sher, T. Kaisho, S. Akira, and C. Reis e Sousa. 2002. Microbial recognition via Toll-like receptor-dependent and -independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. J. Immunol. 169:3652–3660. [DOI] [PubMed] [Google Scholar]
- 37.Diebold, S.S., M. Montoya, H. Unger, L. Alexopoulou, P. Roy, L.E. Haswell, A. Al-Shamkhani, R. Flavell, P. Borrow, and C. Reis e Sousa. 2003. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature. 424:324–328. [DOI] [PubMed] [Google Scholar]
- 38.Lenert, P., L. Stunz, A.K. Yi, A.M. Krieg, and R.F. Ashman. 2001. CpG stimulation of primary mouse B cells is blocked by inhibitory oligodeoxyribonucleotides at a site proximal to NF-kappaB activation. Antisense Nucleic Acid Drug Dev. 11:247–256. [DOI] [PubMed] [Google Scholar]
- 39.Lenert, P., W. Rasmussen, R.F. Ashman, and Z.K. Ballas. 2003. Structural characterization of the inhibitory DNA motif for the type A (D)-CpG-induced cytokine secretion and NK-cell lytic activity in mouse spleen cells. DNA Cell Biol. 22:621–631. [DOI] [PubMed] [Google Scholar]
- 40.Uwatoko, S., M. Mannik, I.R. Oppliger, M. Okawa-Takatsuji, S. Aotsuka, R. Yokohari, G. Seki, S. Taniguchi, K. Suzuki, and K. Kurokawa. 1995. C1q-binding immunoglobulin G in MRL/l mice consists of immune complexes containing antibodies to DNA. Clin. Immunol. Immunopathol. 75:140–146. [DOI] [PubMed] [Google Scholar]
- 41.Tan, P.S., A.L. Gavin, N. Barnes, D.W. Sears, D. Vremec, K. Shortman, S. Amigorena, P.L. Mottram, and P.M. Hogarth. 2003. Unique monoclonal antibodies define expression of Fc gamma RI on macrophages and mast cell lines and demonstrate heterogeneity among subcutaneous and other dendritic cells. J. Immunol. 170:2549–2556. [DOI] [PubMed] [Google Scholar]
- 42.Regnault, A., D. Lankar, V. Lacabanne, A. Rodriguez, C. Thery, M. Rescigno, T. Saito, S. Verbeek, C. Bonnerot, P. Ricciardi-Castagnoli, et al. 1999. Fcγ receptor–mediated induction of dendritic cell maturation and major histocompatibility complex class I–restricted antigen presentation after immune complex internalization. J. Exp. Med. 189:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675–680. [DOI] [PubMed] [Google Scholar]
- 44.Alexopoulou, L., A.C. Holt, R. Medzhitov, and R.A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 413:732–738. [DOI] [PubMed] [Google Scholar]
- 45.Kaisho, T., and S. Akira. 2001. Dendritic-cell function in Toll-like receptor- and MyD88-knockout mice. Trends Immunol. 22:78–83. [DOI] [PubMed] [Google Scholar]
- 46.Sutterwala, F.S., G.J. Noel, R. Clynes, and D.M. Mosser. 1997. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J. Exp. Med. 185:1977–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lenert, P., A.K. Yi, A.M. Krieg, L.L. Stunz, and R.F. Ashman. 2003. Inhibitory oligonucleotides block the induction of AP-1 transcription factor by stimulatory CpG oligonucleotides in B cells. Antisense Nucleic Acid Drug Dev. 13:143–150. [DOI] [PubMed] [Google Scholar]
- 48.Macfarlane, D.E., and L. Manzel. 1998. Antagonism of immunostimulatory CpG-oligodeoxynucleotides by quinacrine, chloroquine, and structurally related compounds. J. Immunol. 160:1122–1131. [PubMed] [Google Scholar]
- 49.Ravetch, J.V., and S. Bolland. 2001. IgG Fc receptors. Annu. Rev. Immunol. 19:275–290. [DOI] [PubMed] [Google Scholar]
- 50.Ji, H., K. Ohmura, U. Mahmood, D.M. Lee, F.M. Hofhuis, S.A. Boackle, K. Takahashi, V.M. Holers, M. Walport, C. Gerard, et al. 2002. Arthritis critically dependent on innate immune system players. Immunity. 16:157–168. [DOI] [PubMed] [Google Scholar]
- 51.Takai, T. 2002. Roles of Fc receptors in autoimmunity. Nat. Rev. Immunol. 2:580–592. [DOI] [PubMed] [Google Scholar]
- 52.Amigorena, S., and C. Bonnerot. 1999. Fc receptor signaling and trafficking: a connection for antigen processing. Immunol. Rev. 172:279–284. [DOI] [PubMed] [Google Scholar]
- 53.Dhodapkar, K.M., J. Krasovsky, B. Williamson, and M.V. Dhodapkar. 2002. Antitumor monoclonal antibodies enhance cross-presentation of cellular antigens and the generation of myeloma-specific killer T cells by dendritic cells. J. Exp. Med. 195:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amigorena, S. 2002. Fcγ receptors and cross-presentation in dendritic cells. J. Exp. Med. 195:F1–F3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krieg, A.M., A.K. Yi, S. Matson, T.J. Waldschmidt, G.A. Bishop, R. Teasdale, G.A. Koretzky, and D.M. Klinman. 1995. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 374:546–549. [DOI] [PubMed] [Google Scholar]
- 56.Messina, J.P., G.S. Gilkeson, and D.S. Pisetsky. 1991. Stimulation of in vitro murine lymphocyte proliferation by bacterial DNA. J. Immunol. 147:1759–1764. [PubMed] [Google Scholar]
- 57.Suzuki, K., A. Mori, K.J. Ishii, J. Saito, D.S. Singer, D.M. Klinman, P.R. Krause, and L.D. Kohn. 1999. Activation of target-tissue immune-recognition molecules by double-stranded polynucleotides. Proc. Natl. Acad. Sci. USA. 96:2285–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ishii, K.J., K. Suzuki, C. Coban, F. Takeshita, Y. Itoh, H. Matoba, L.D. Kohn, and D.M. Klinman. 2001. Genomic DNA released by dying cells induces the maturation of APCs. J. Immunol. 167:2602–2607. [DOI] [PubMed] [Google Scholar]
- 59.Magnusson, M., S. Magnusson, H. Vallin, L. Ronnblom, and G.V. Alm. 2001. Importance of CpG dinucleotides in activation of natural IFN-α-producing cells by a lupus-related oligodeoxynucleotide. Scand. J. Immunol. 54:543–550. [DOI] [PubMed] [Google Scholar]
- 60.Zhu, F.G., C.F. Reich, and D.S. Pisetsky. 2003. Effect of cytofectins on the immune response of murine macrophages to mammalian DNA. Immunology. 109:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahmad-Nejad, P., H. Hacker, M. Rutz, S. Bauer, R.M. Vabulas, and H. Wagner. 2002. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur. J. Immunol. 32:1958–1968. [DOI] [PubMed] [Google Scholar]
- 62.Latz, E., A. Schoenemeyer, A. Visintin, K.A. Fitzgerald, B.G. Monks, C.F. Knetter, E. Lien, N.J. Nilsen, T. Espevik, and D.T. Golenbock. 2004. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 5:190–198. [DOI] [PubMed] [Google Scholar]
- 63.Mukae, N., H. Yokoyama, T. Yokokura, Y. Sakoyama, and S. Nagata. 2002. Activation of the innate immunity in Drosophila by endogenous chromosomal DNA that escaped apoptotic degradation. Genes Dev. 16:2662–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kawane, K., H. Fukuyama, H. Yoshida, H. Nagase, Y. Ohsawa, Y. Uchiyama, K. Okada, T. Iida, and S. Nagata. 2003. Impaired thymic development in mouse embryos deficient in apoptotic DNA degradation. Nat. Immunol. 4:138–144. [DOI] [PubMed] [Google Scholar]
- 65.Hornung, V., S. Rothenfusser, S. Britsch, A. Krug, B. Jahrsdorfer, T. Giese, S. Endres, and G. Hartmann. 2002. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168:4531–4537. [DOI] [PubMed] [Google Scholar]
- 66.Kadowaki, N., S. Ho, S. Antonenko, R.W. Malefyt, R.A. Kastelein, F. Bazan, and Y.J. Liu. 2001. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bird, A.P. 1987. CpG islands as gene markers in the vertebrate nucleus. Trends Genet. 3:342–347. [Google Scholar]
- 68.Krieg, A.M. 2002. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20:709–760. [DOI] [PubMed] [Google Scholar]
- 69.Cross, S.H., M. Lee, V.H. Clark, J.M. Craig, A.P. Bird, and W.A. Bickmore. 1997. The chromosomal distribution of CpG islands in the mouse: evidence for genome scrambling in the rodent lineage. Genomics. 40:454–461. [DOI] [PubMed] [Google Scholar]
- 70.Krieg, A.M., T. Wu, R. Weeratna, S.M. Efler, L. Love-Homan, L. Yang, A.-K. Yi, D. Short, and H.L. Davis. 1998. Sequence motifs in adenoviral DNA block immune activation by stimulatory CpG motifs. Proc. Natl. Acad. Sci. USA. 95:12631–12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nardelli, B., O. Belvedere, V. Roschke, P.A. Moore, H.S. Olsen, T.S. Migone, S. Sosnovtseva, J.A. Carrell, P. Feng, J.G. Giri, et al. 2001. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood. 97:198–204. [DOI] [PubMed] [Google Scholar]
- 72.Scapini, P., B. Nardelli, G. Nadali, F. Calzetti, G. Pizzolo, C. Montecucco, and M.A. Cassatella. 2003. G-CSF–stimulated neutrophils are a prominent source of functional BLyS. J. Exp. Med. 197:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kariko, K., H. Ni, J. Capodici, M. Lamphier, and D. Weissman. 2004. mRNA is an endogenous ligand for Toll-like receptor 3. J. Biol. Chem. 279:12542–12550. [DOI] [PubMed] [Google Scholar]
- 74.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 303:1526–1529. [DOI] [PubMed] [Google Scholar]
- 75.Diebold, S.S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 303:1529–1531. [DOI] [PubMed] [Google Scholar]
- 76.Lund, J.M., L. Alexopoulou, A. Sato, M. Karow, N.C. Adams, N.W. Gale, A. Iwasaki, and R.A. Flavell. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA. 101:5598–5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matsumoto, M., K. Funami, M. Tanabe, H. Oshiumi, M. Shingai, Y. Seto, A. Yamamoto, and T. Seya. 2003. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J. Immunol. 171:3154–3162. [DOI] [PubMed] [Google Scholar]
- 78.Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. [DOI] [PubMed] [Google Scholar]