Evidence for a Frontoparietal Control System Revealed by Intrinsic Functional Connectivity (original) (raw)
Abstract
Two functionally distinct, and potentially competing, brain networks have been recently identified that can be broadly distinguished by their contrasting roles in attention to the external world versus internally directed mentation involving long-term memory. At the core of these two networks are the dorsal attention system and the hippocampal-cortical memory system, a component of the brain's default network. Here spontaneous blood-oxygenation-level-dependent (BOLD) signal correlations were used in three separate functional magnetic resonance imaging data sets (n = 105) to define a third system, the frontoparietal control system, which is spatially interposed between these two previously defined systems. The frontoparietal control system includes many regions identified as supporting cognitive control and decision-making processes including lateral prefrontal cortex, anterior cingulate cortex, and inferior parietal lobule. Detailed analysis of frontal and parietal cortex, including use of high-resolution data, revealed clear evidence for contiguous but distinct regions: in general, the regions associated with the frontoparietal control system are situated between components of the dorsal attention and hippocampal-cortical memory systems. The frontoparietal control system is therefore anatomically positioned to integrate information from these two opposing brain systems.
INTRODUCTION
The brain is organized into multiple systems that have distinct and potentially competing functional roles. Two such systems have been extensively studied. The first is the dorsal attention system that is associated with externally directed cognition including covert and overt shifts of spatial attention, eye movements, and hand-eye coordination (Corbetta and Shulman 2002). This brain system includes regions in the frontal eye fields, ventral premotor cortex, superior parietal lobule, intraparietal sulcus, and motion-sensitive middle temporal area (MT+). Recent analysis of the dorsal attention system based on functional magentic resonance imaging (fMRI) measures of spontaneous activity reveals that the regions within the system are strongly positively correlated (Fox et al. 2005, 2006; Laufs et al. 2003; Vincent et al. 2006). This system tracks performance on tasks involving search and detection. Specifically, activity in the dorsal attention system is increased at the onset of search displays, maintains activity while awaiting a target, and further increases activity when targets are detected (Corbetta and Shulman 2002; Shulman et al. 2003). These properties are consistent with the system supporting externally directed cognition.
The second system—the hippocampal-cortical memory system—is part of a network of regions that are active during passive mental states linked to internally directed cognition including recollection of the past and thinking about the future (often labeled the default network) (Buckner et al. 2008; Raichle et al. 2001).1 This brain system includes regions in ventral medial prefrontal cortex, posterior inferior parietal lobule, retrosplenial cortex, posterior cingulate, and the lateral temporal lobe. Analysis of the hippocampal-cortical memory system using fMRI measures of spontaneous activity reveals a tightly coupled set of regions (Buckner et al. 2008; Greicius et al. 2004; Kahn et al. 2008; Vincent et al. 2006) that overlap regions active during episodic memory retrieval (Maguire 2001; Svoboda et al. 2006; Vilberg and Rugg 2008; Vincent et al. 2006; Wagner et al. 2005).
Of particular interest, several recent studies show that spontaneous activity within the dorsal attention system and the hippocampal-cortical memory system is negatively correlated—leading to their being described as anticorrelated brain systems (Fox et al. 2005; Fransson 2005). While there are challenges for interpreting the physiological meaning of negative correlations in BOLD data preprocessed to eliminate the global mean signal (Buckner et al. 2008), the negative correlation between these systems suggests segregation of competing processes. In the instance of the dorsal attention and hippocampal-cortical memory systems, this segregation may arise from their divergent roles in processing information from the external world versus internal mentation. This intriguing observation raises the question of how these two systems interact and whether there are control systems that either integrate information from the two systems or regulate their activity.
Here we used analysis of BOLD-measured spontaneous activity fluctuations to map a third functional system—which we label the _frontoparietal control system_—in relation to the dorsal attention and hippocampal-cortical memory systems. Our motivation to undertake this exploration is the extensive literature that suggests executive control systems guide decision-making by integrating information from the external environment with stored internal representations (Miller 2000). Tasks that require simultaneous consideration of multiple interdependent contingencies (Kroger et al. 2002) or conflicting stimulus-response mappings (Crone et al. 2006), as well as the process of integrating working memory with attentional resource allocation (Koechlin et al. 1999), all engage a similar system that includes anterior cingulate, lateral parietal, and prefrontal cortex.
The anterior prefrontal cortex (aPFC), in particular, is most responsive during tasks that demand integrating the outcomes of multiple cognitive operations in the pursuit of a higher behavioral goal (Ramnani and Owen 2004). Koechlin and Hyafil (2007) have recently noted that “lateral prefrontal regions select and maintain the task set governing ongoing action, whereas the frontopolar cortex [aPFC] enables previously selected task sets to be maintained in a pending state for subsequent automatic retrieval and execution on completion of the ongoing one,” which suggests that the aPFC is at the apex of the cognitive control hierarchy (see also Badre and D'Esposito 2007; Buckner 2003; Christoff and Gabrieli 2000). Further, there is reason to believe that this area of the brain is greatly expanded in humans relative to macaques and apes (Öngür et al. 2003; Semendeferi et al. 2001). For these reasons, the aPFC was selected for the initial functional connectivity analyses of a potential cognitive control system. We specifically hypothesized the existence of a brain system associated with aPFC that is dissociable from these other two systems, which are involved in visuospatial attention (linked to MT+) and the reconstruction of long-term memories (linked to the hippocampal formation; HF). Given the potential close juxtaposition of regions constituting these distinct systems, we used high-resolution data and topographical analysis to identify and explore the organization of the frontoparietal control system in relation to the dorsal attention and hippocampal-cortical memory systems.
METHODS
Overview
The main goal of the present study is to describe the functional anatomy of the frontoparietal control system relative to adjacent brain systems. We began by examining correlations between spontaneous BOLD fluctuations in a seed region of interest (ROI) and spontaneous fluctuations in each voxel of the brain. Using procedures similar to previous studies (e.g., Fox et al. 2005; Vincent et al. 2006), functional correlation maps were computed to differentiate the dorsal attention system (MT+ correlated regions) and the hippocampal-cortical memory system (HF correlated regions) from the frontoparietal control system (aPFC-correlated regions). A qualitative dissociation between MT+, aPFC, and the HF functional correlation maps emerged, which was formally tested in an additional data set (dataset 3) using ROIs defined in data set 2. In addition, we used high-resolution fMRI (dataset 2; 50 subjects; dataset 3; 45 subjects) to illustrate the precise anatomical organization of the frontoparietal control system relative to adjacent systems.
Participants, task, and data acquisition
In total, 105 normal right-handed young adults participated in the present study (Table 1). All fMRI data were collected during a continuous passive fixation task in which participants fixated a cross-hair with no task instructions other than to stay awake and remain still. Ten (dataset 1), 50 (dataset 2), and 45 (dataset 3) participants completed two to four fMRI runs of fixation. Imaging data were collected either on a 3T Allegra system at Washington University School of Medicine (dataset 1) or a 3T TimTrio system at Massachusetts General Hospital (datasets 2 and 3; Siemens, Erlangen, Germany). Structural data (for atlas transformation) included a sagittal T1-weighted MP-RAGE structural image and an additional T2-weighted fast spin echo scan in dataset 1. Dataset 1 was collected using a gradient echo, echo-planar sequence sensitive to BOLD contrast (for parameters see Table 1). Simultaneous electroencephalography (EEG) data were acquired during functional imaging in dataset 1 but were not used in the present analysis. In dataset 1, the volume TR included a 1-s pause between frames. Dataset 1 has been used in previous analyses of spontaneous BOLD correlations (Fox et al. 2005, 2006; Vincent et al. 2006, 2007) and can be freely obtained as part of the open BrainScape data archive (www.brainscape.org). Datasets 2 and 3 were acquired using a gradient-echo echo-planar pulse sequence using a 12-channel phased-array whole-head coil (for parameters, see Table 1). The relatively long TR (5 s) enabled whole brain coverage with high spatial resolution (2 mm3). Sparse temporal sampling is sufficient for the present purposes because the signals of interest are of low frequency (<0.1 Hz). Maps of the spatial distribution of signal-to-noise ratio for datasets 2 and 3 are provided in a previous publication (Kahn et al. 2008).
TABLE 1.
Participants and MR scanning parameters
| Dataset 1 | Dataset 2 | Dataset 3 | |
|---|---|---|---|
| n | 10 (7 male) | 50 (23 male) | 45 (18 male) |
| Mean age, yr | 23.2 ± 2.6 | 23.6 ± 5.3 | 22.3 ± 2.9 |
| Field strength, T | 3 | 3 | 3 |
| BOLD TR, s | 3.013 | 5.000 | 5.000 |
| BOLD TE, ms | 25 | 30 | 30 |
| BOLD flip angle,° | 90 | 90 | 90 |
| Voxel size, mm | 4 × 4 × 4 | 2 × 2 × 2 | 2 × 2 × 2 |
| Time points per run | 110 | 66 | 76 |
| No. runs | 3 | 2 | 4 |
Processing of functional data
fMRI preprocessing steps included, first, compensation of systematic, slice-dependent time shifts; second, elimination of systematic odd-even slice intensity differences due to interleaved acquisition; and, third, rigid body correction for interframe head motion within and across runs. Step three provided a record of head position within and across all fMRI runs. Each fMRI run was intensity scaled (1 multiplicative constant over all voxels and volumes) to yield a whole brain mode value of 1,000 (Ojemann et al. 1997). Atlas registration was achieved by computing affine transforms connecting the fMRI run first volume (averaged over all runs after cross-run realignment) with the T2- and T1-weighted structural images within each participant (Ojemann et al. 1997). The transformation between the participant's T1-weighted structural image and an atlas representative template was then computed. Our atlas representative template includes MP-RAGE data from 12 normal young adults and was made to conform to the MNI152 (ICBM-152) atlas using procedures as outlined in Buckner et al. (2004). To prepare the fMRI data for the present main analyses, each fMRI run was transformed to atlas space and resampled to 2 mm cubic voxels. The first four volumes of each run were not processed for fMRI analysis beyond their use in registration. fMRI data are shown either on slices of our custom atlas template or on the inflated population average landmark surface (PALS) using CARET software (Van Essen 2005).
Several processing steps were used to condition the fMRI data for analysis of voxel-based correlations (described in Fox et al. 2005; Vincent et al. 2006). Data were spatially smoothed with either a 6-mm (dataset 1) or 4-mm (datasets 2 and 3) full width at half-maximum (FWHM) Gaussian kernel. Datasets 2 and 3 were smoothed with a smaller kernel to maintain resolution. Temporal band-pass filtering removed frequencies >0.08 Hz. Several sources of spurious variance along with their temporal derivatives were then removed from the data through linear regression: 1) six parameters obtained by rigid body correction of head motion, 2) the whole-brain signal averaged over a fixed region in atlas space, and 3) the signal from a ventricular region of interest and a region centered in the white matter. In this manner, variance unlikely to be involved in spatially specific regional correlations was removed from the data. The global (whole brain) signal may correlate with respiration-induced fMRI signal fluctuations (Birn et al. 2006; Wise et al. 2004) or result from global fluctuations in neuronal activity. Removing signals correlated with ventricles and white matter is an additional means of reducing nonneuronal contributions to BOLD correlations (Bartels and Zeki 2005; Fox et al. 2005).
Construction of ROIs
The atlas coordinates of all regions of interest used in this study are given in Table 2. Left aPFC was defined from a previous study of cognitive control (Koechlin et al. 1999). For Fig. 1, the right hemisphere aPFC seed was generated as the x-flipped version of the left aPFC region. Left MT+ and HF were transformed into MNI space from a previous study conducted in a different atlas space (Vincent et al. 2006). The HF region includes the posterior extent of the hippocampal formation that is most strongly correlated with regions in lateral parietal cortex (Kahn et al. 2008). Right hemisphere seed regions (right aPFC, MT+, and HF) were generated from the left aPFC, MT+, and HF functional correlation maps generated in dataset 2. All seed regions were ∼2 cm3. These regions were then used as seed regions to create functional correlation maps in datasets 1–3.
TABLE 2.
Regions of interest
| x | y | z | Volume, cm3 | |
|---|---|---|---|---|
| Seed regions of interest | ||||
| Left middle temporal area (lMT+) | −45 | −69 | −2 | 1.99 |
| Right middle temporal area (rMT+) | 50 | −69 | −3 | 2.16 |
| Left anterior prefrontal cortex (laPFC) | −36 | 57 | 9 | 2.14 |
| Right anterior prefrontal cortex (raPFC) | 34 | 52 | 10 | 1.97 |
| Left hippocampal formation (lHF) | −21 | −25 | −14 | 2.00 |
| Right hippocampal formation (rHF) | 24 | −19 | −21 | 2.22 |
| MT correlated regions of interest | ||||
| Left frontal eye fields (lFEF) | −25 | −8 | 50 | 2.14 |
| Right frontal eye fields (rFEF) | 27 | −8 | 50 | 2.17 |
| Left superior parietal lobule (lSPL) | −27 | −52 | 57 | 2.10 |
| Right superior parietal lobule (rSPL) | 24 | −56 | 55 | 2.13 |
| aPFC correlated regions of interest | ||||
| Anterior cingulate cortex (aCC) | 3 | 31 | 27 | 2.14 |
| Left dorsolateral prefrontal cortex (ldlPFC) | −50 | 20 | 34 | 2.13 |
| Right dorsolateral prefrontal cortex (rdlPFC) | 46 | 14 | 43 | 2.17 |
| Left anterior insula (laINS) | −31 | 21 | −1 | 2.15 |
| Right anterior insula (raINS) | 31 | 22 | −2 | 2.13 |
| Left anterior inferior parietal lobule (laIPL) | −52 | −49 | 47 | 2.22 |
| Right anterior inferior parietal lobule (raIPL) | 52 | −46 | 46 | 2.13 |
| HF correlated regions of interest | ||||
| Ventromedial prefrontal cortex (vmPFC) | 0 | 51 | −7 | 2.16 |
| Posterior cingulate cortex (pCC) | 1 | −55 | 17 | 2.13 |
| Left posterior inferior parietal lobule (lpIPL) | −47 | −71 | 29 | 2.15 |
| Right posterior inferior parietal lobule (rpIPL) | 50 | −64 | 27 | 2.17 |
FIG. 1.
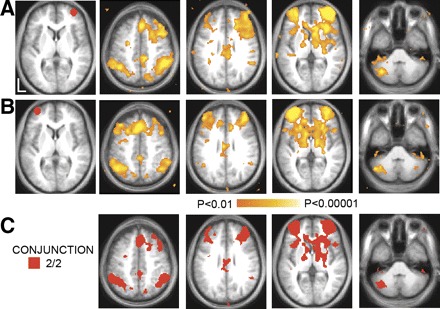
Significant voxel-wise correlations in 10 humans associated with regions of interest in the right (A) and left (B) anterior prefrontal cortex (aPFC). Regions are shown in red to the left of the statistical maps. C: conjunction map showing regions consistently correlated with both aPFC regions.
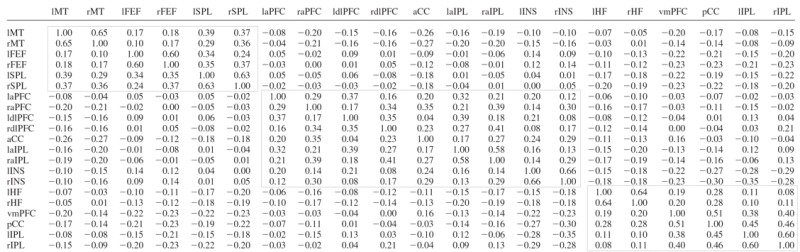
A group-averaged, Fisher's _z_-transformed correlation map was generated for left HF, right HF, left aPFC, right aPFC, left MT+, and right MT+ in dataset 2. These maps were collapsed over hemisphere by averaging to obtain three maps, derived from intrinsic correlations with MT+, aPFC, and HF. Peak search of the MT+, aPFC, and HF correlation maps identified several local maxima across the brain. Several ROIs were defined around these peaks to include volumes of ∼2 cm3. These regions then were carried forward as a priori ROI for hypothesis-driven tests on dataset 3. The full correlation matrix is shown in Table 3. Functional correlation matrix of regions listed in Table 2.
TABLE 3.
A central goal of the analysis was to identify parietal regions correlated with the HF, aPFC, or MT+. Regions were defined from the dataset 2 correlation maps and then used for regional statistical analyses in an independent data set (dataset 3). This strategy represents a conservative approach to the problem of multiple comparisons and allows for unbiased measurement of correlation strength (Vincent et al. 2006).
Regional and voxel-wise computation of functional correlations
The correlation coefficient between region pairs were computed as
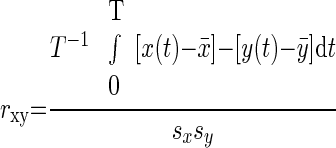
where x(t) and y(t) are the regional time courses with means x̄ and ȳ and SDs s x and s y. The period of integration, T, corresponds to multiple, concatenated BOLD runs, excluding the first four (premagnetization steady state) functional volumes of each run. The results of pair-wise correlation analyses are shown in Table 3.
Correlation maps were computed using the previously described modification (Fox et al. 2005; Vincent et al. 2006) of the method originally described by Biswal and colleagues (1995). Thus for a selected regional time course, x(t), the correlation map was computed as
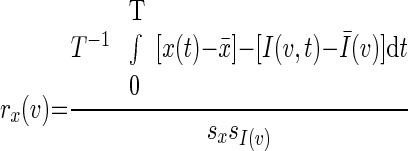
where I (v,t) is image intensity at locus v and time t; Ī(v,t) and s I(v)are the voxel-wise mean and SD. At each voxel, v, r x(v) is the correlation with the regional time course, x(t). Voxel-wise application of Fisher's z transform yielded maps, z x(v), with values at each voxel that theoretically are nearly normally distributed over the population.
Evaluation of significance
To assess voxel-wise statistical significance of functional correlations at the group level, _t_-test versus the null hypothesis of no correlation were performed on the z-transformed correlation coefficient maps, z x(v). A Student's _t_-statistic map (random effects analysis; uncorrected) was computed for each data set and thresholded at a significance level of P < 0.01 (Figs. 1–5 and 7). Figure 6 shows a convergence map of all three datasets after multiple comparison correction with a threshold of P < 0.05 and a voxel extent of 5 using the FDR function of SPM2 (SPM2, Wellcome Department of Cognitive Neurology, London, UK). The convergence map was computed by adding the number of data sets that reached significance. In addition to the convergence map, a conjunction map (Fig. 7) was created for each system in dataset 3. The conjunction map displays all voxels significantly correlated (P < 0.01) with two seeds in each network (MT+ and SPL for the dorsal attention system; aPFC and aIPL for the frontoparietal control system; HF and pIPL for the hippocampal-cortical memory system). The conjunction map shows voxels consistently correlated within a system as well as the overlap between systems. All maps were shown on slices in MNI atlas space (Evans et al. 1993) or projected on the left and right cerebral hemispheres of the inflated PALS surface using Caret software (Van Essen 2005).
FIG. 5.
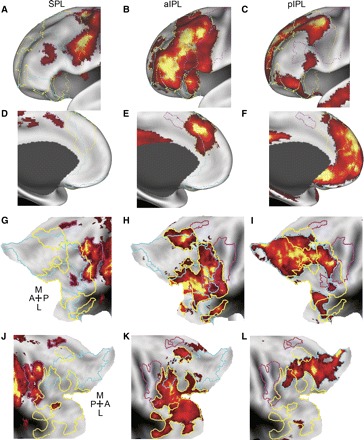
The aIPL is functionally correlated with a many regions in frontal cortex that are roughly spatially interposed between functional correlations associated with the SPL and the pIPL. Significant spontaneous BOLD correlations associated with seed regions in left SPL (A, D, G, and J), aIPL (B, E, H, and K), and the pIPL (C, F, I, and L) in dataset 3 are displayed on the anterior-lateral inflated surface of the left hemisphere (A_–_C), the anterior-medial surface of the left hemisphere (D_–_F), and the flattened representation of the left (G_–_I) and right (J_–_L) hemispheres. Borders are drawn around the frontal and insular correlations associated with each seed region. The colors magenta, yellow, and cyan correspond to SPL, aIPL, and pIPL parietal correlations, respectively. A directional compass is provided for reference.
FIG. 7.
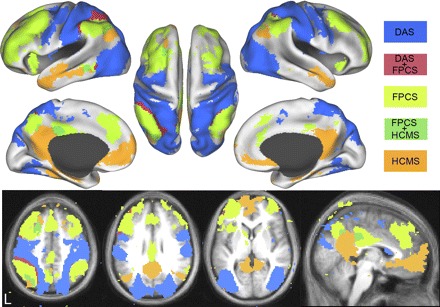
Intrinsically defined dorsal attention (DAS), frontoparietal control (FPCS), and hippocampal-cortical memory (HCMS) systems and the overlap between them from dataset 3. Voxels in the DAS include regions correlated with MT+ and SPL and are shown in blue. Voxels in the FPCS include regions correlated with aPFC and aIPL and are shown in light green. Voxels in the HCMS include regions correlated with HF and pIPL and are shown in orange. Voxels significantly correlated with the DAS and FPCS are shown in red. Voxels significantly correlated with the HCMS and FPCS are shown in dark green. Data are displayed on the lateral, medial, and dorsal surfaces of the left and right hemispheres as well as MNI atlas space axial and sagittal slices.
FIG. 6.
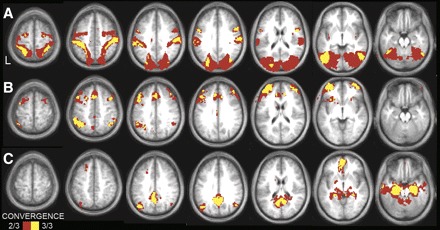
Convergence analyses illustrate the cortical topography of regions that correlate with left MT+ complex (A), aPFC (B), and the left HF (C) across the three independent data sets. Convergent correlations are overlaid on axial slices of an average MNI atlas space template. The correlation with seed regions were identified at a threshold of P < 0.05 (multiple comparison corrected) in each of the 3 independent data sets. Voxels over threshold in 2 of 3 data sets are shown in red; 3 of 3 data sets are shown in yellow. MT+ convergence is observed in premotor cortex and MT+ extending along the intraparietal and superior parietal cortex to postcentral gyrus. aPFC convergence is observed in the anterior and dorsolateral prefrontal cortex, anterior inferior parietal lobule, anterior cingulate, and anterior insula. HF convergence is observed in the posterior inferior parietal lobule, ventral medial prefrontal, lateral temporal, as well as along the medial surface extending from the retrosplenial cortex into posterior cingulate.
RESULTS
To identify the spatial distribution of the frontoparietal control system, we correlated the time course of BOLD fluctuations averaged within a priori defined aPFC seed regions with the time course of each voxel across the brain. Voxels significantly correlated with either left or right aPFC in dataset 1 are shown in Fig. 1, A and B. A conjunction analysis was performed to visualize voxels that were significantly correlated with both the left and right seed regions (Fig. 1_C_). Regions correlated with fluctuations in the aPFC included dorsomedial frontal cortex/anterior cingulate, anterior insula, the head of the caudate nucleus, lateral cerebellum, anterior inferior parietal lobule (aIPL), and an extensive region within the dorsolateral prefrontal cortex extending to the frontal pole.2
For comparison to the map of regions correlated with activity in aPFC, we have also plotted the functional correlations associated with visual area MT+ and the HF (Fig. 2), which identify the dorsal attention and hippocampal-cortical memory systems, respectively (Vincent et al. 2006). Group-averaged statistical maps (dataset 1, n = 10) display the distribution of voxels temporally correlated with spontaneous fluctuations in each ROI. Regions correlated with fluctuations in area MT+ included the frontal eye fields, intraparietal sulcus, and superior parietal lobule (SPL). Regions correlated with the HF included ventromedial prefrontal cortex, dorsal frontal cortex, lateral temporal cortex, posterior cingulate cortex, and ventral posterior inferior parietal lobule (pIPL). Of importance, the regions correlated with aPFC appeared to be spatially interposed between regions within the dorsal attention and hippocampal-cortical memory systems, especially within parietal cortex.
FIG. 2.
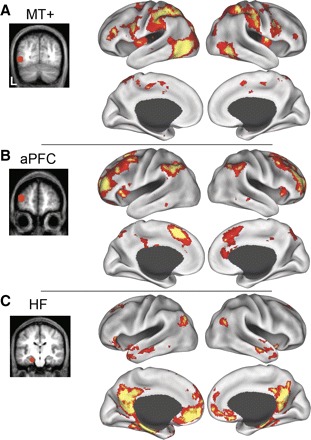
Significant spontaneous blood-oxygen-level-dependent (BOLD) correlations associated with seed regions in left middle temporal motion complex (MT+, A), aPFC (B), and the hippocampal formation (HF; C) in dataset 1 are displayed on the lateral and medial inflated surfaces of the left and right hemisphere. The seed regions used to compute the correlation maps are shown on coronal slices to the left of their associated functional correlation maps.
To further characterize the spatial relation among the dorsal attention, frontoparietal control, and hippocampal-cortical memory systems within parietal cortex, we acquired high-resolution (2 mm3) resting functional MRI in 50 subjects (dataset 2). Figure 3 shows parietal correlations associated with the MT+, aPFC, and HF seed regions in dataset 2. The correlations associated with MT+ encircled parietal cortex extending from extrastriate cortex into primary and secondary visual cortices, across the medial intraparietal sulcus and superior parietal lobule into the postcentral gyrus. The MT+ correlations covered most of posterior parietal cortex but omitted a large region on the inferior parietal lobule and lateral intraparietal sulcus. Two nearly nonoverlapping regions that were correlated with either aPFC or HF occupied the remaining portion of this lateral parietal region. The aPFC region was correlated with the aIPL extending into lateral intraparietal sulcus. The HF region correlated with the pIPL. Correlation patterns were bilateral and similar across both the left and right hemispheres (Fig. 3, D_–_F and G_–_I).
FIG. 3.

The anterior prefrontal cortex is functionally correlated with a lateral parietal region that is spatially interposed between functional correlations associated with the MT+ and the HF. Significant spontaneous BOLD correlations associated with seed regions in left MT+ (A, D, and G), aPFC (B, E, and H), and the HF (C, F, and I) in dataset 2 are displayed on the posterior-lateral inflated surface of the left hemisphere (A_–_C) and the flattened representation of the left (D_–_F) and right (G_–_I) hemispheres. Borders are drawn around the parietal correlations associated with each seed region. The colors magenta, yellow, and cyan correspond to MT+, aPFC, and HF parietal correlations, respectively. A directional compass and sulcal labels are provided for reference. G: medial (M), lateral (L), anterior (A), posterior (P), intraparietal sulcus (IPS), central sulcus (CS), sylvian fissure (SF).
Next, we asked if the parietal ROIs (SPL, aIPL, and pIPL) produced similar maps to the seed ROIs (MT+, aPFC, and HF) from which they were derived. We correlated the time course of BOLD fluctuations averaged within a priori defined ROIs within SPL, aIPL, and pIPL (defined in dataset 2). Group-averaged statistical maps (dataset 3, n = 45) showing the distribution of voxels temporally correlated with spontaneous fluctuations in each parietal ROI are displayed in Fig. 4. Comparison of Figs. 2 and 4 suggests that the systems correlated with the seed ROI (MT+, aPFC, and HF) and each parietal ROI (SPL, aIPL, and pIPL) are highly similar.
FIG. 4.
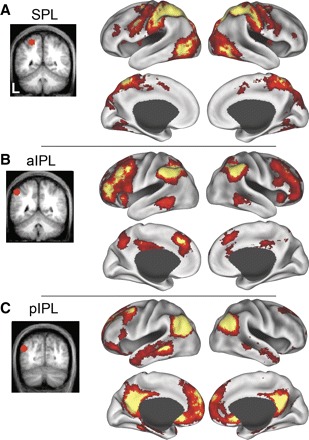
Significant spontaneous BOLD correlations associated with seed regions in left superior parietal lobule (SPL, A), anterior inferior parietal lobule (aIPL, B), and posterior inferior parietal lobule (pIPL, C) in dataset 3 are displayed on the lateral and medial inflated surfaces of the left and right hemispheres. The seed regions used to compute the correlation maps are shown on coronal slices to the left of their associated functional correlation maps.
The three parietal correlation maps (SPL, aIPL, and pIPL) appeared to be roughly spatially interposed within medial and lateral prefrontal cortex. Figure 5 shows the medial and lateral frontal correlations associated with the SPL, aIPL, and pIPL seed regions in dataset 3. The SPL correlations extended from the dorsal premotor cortex (in or around the frontal eye fields), the ventral frontal cortex, ventral dorsolateral prefrontal cortex, and the middle insula. Along the medial surface, MT+ was correlated with pre-supplementary motor area. Correlations with aPFC extended from aPFC along the middle frontal gyrus into dorsolateral prefrontal cortex as well as the anterior insula. Medially, aPFC correlated with dorsal frontal cortex/anterior cingulate cortex. Correlations with HF were sparse in lateral frontal cortex but were observed within ventral frontal cortex (just anterior to the frontal operculum) and in superior frontal cortex. Medially, HF correlations extended from ventral frontal cortex dorsally over the genu of the anterior cingulate as well as the dorsomedial frontal pole. Correlation patterns were bilateral and similar across both the left and right hemispheres (Fig. 5, G_–_I and J_–_L).
To assess the reliability of our correlation maps, we created a convergence map showing the number of data sets correlated with each seed region (left MT+, aPFC, and HF). Figure 6 shows voxels that are consistently correlated with the seed regions across datasets 1, 2, and 3. The correlation maps were highly consistent.
Figure 7 shows a conjunction analysis of two regions within each putative functional system. The dorsal attention system was defined as voxels correlated with both MT+ and SPL. The frontoparietal control system was defined as voxels correlated with aPFC and aIPL. The hippocampal-cortical memory system was defined as voxels correlated with HF and pIPL. Overlap between the hippocampal-cortical and the frontoparietal control system was observed in posterior cingulate, lateral parietal, dorsal frontal, and lateral temporal cortex. Overlap between the dorsal attention and the frontoparietal control system was observed in intraparietal sulcus, lateral temporal cortex, and lateral prefrontal cortex. Overall, there was very little overlap between the three systems.
To assess the strength of the cross-correlations within each system, we created a full correlation matrix of the regions within each of the three systems in dataset 3 (regions defined in dataset 2). The cross-correlation matrix is shown in Table 3 with black boxes around each functional system.
To assess the reliability of the correlation between each of the seed regions and parietal cortex, we defined ROIs within multiple regions around the coordinate of peak correlation in each of the seed region correlation maps from dataset 2 (Table 2) and computed the inter-regional cross-correlations between the seed regions (MT+, aPFC, and HF) and a priori parietal ROIs (SPL, aIPL, and pIPL) in the independent dataset 3. Table 3 displays quantitative results. As expected from datasets 1 and 2, the left and right MT+ were significantly positively correlated with both left and right SPL (all, P < 0.001), the left and right aPFC were significantly positively correlated with both left and right aIPL (all, _P_ ≤ 0.01), and the left and right HF were significantly positively correlated with both left and right pIPL (all, _P_ < 0.005). None of the seed regions were positively correlated with any of the parietal regions hypothesized to be outside of the seed region's network (all, _P_ > 0.05). Thus the pattern of correlation in dataset 3 is highly consistent with the results from dataset 2. This result is not surprising given the robustness of the effects but is not mandated as the regional definitions were constructed independently (dataset 2) from the test data (dataset 3). These results formally demonstrate that the three adjacent parietal ROIs dissociate based on their differential correlation with MT+, aPFC, and HF.
Previously, we reported no significant lateralization in the cross-correlations (effect of hemisphere) for parietal regions within the dorsal attention and hippocampal-cortical memory systems (Vincent et al. 2006). We re-examined the lateralization of correlation in dataset 3 of the present study, which has considerably more power than the previous study (45 vs. 25 participants), and found a significant effect of hemisphere for laPFC to aIPL, raPFC to aIPL, and rMT to SPL (all P < 0.001). For each region pair, the seed region was most correlated with the parietal region in the ipsilateral hemisphere of the seed. These results suggest that the frontoparietal control system is lateralized.
DISCUSSION
The brain is organized into multiple distributed and parallel large-scale systems. Two major systems—the dorsal attention system (Fox et al. 2006) and hippocampal-cortical memory system (Vincent et al. 2006)—have been found to be negatively correlated in a manner consistent with their roles in opposed modes of behavior (Fox et al. 2005; Fransson 2005). The dorsal attention system selects information from the sensorium under the direction of top-down influences to enable the performance of externally oriented tasks, e.g., eye-hand coordination; the hippocampal-cortical memory system participates in declarative memory, particularly in the context of episodic memory (e.g., autobiographical memory). Here we report a detailed spatial characterization of a third system—the frontoparietal control system—that is anatomically interposed between the other two. Thus the frontoparietal control system may be uniquely positioned to integrate information coming from the other two systems and to adjudicate between potentially competing inner- versus outer-directed processes.
Frontoparietal control system
Each region in the brain has a unique spatial distribution of functional connectivity. Some regions have functional connectivity that is highly similar. We have referred to those regions as a “system” or “network.” A system in the presently used sense means a set of widely distributed brain regions that exhibit consistently correlated spontaneous activity fluctuations and characteristically respond in concert during conventional, task-related fMRI experiments. We presume the systems are supported by mono- and polysynaptic anatomic connectivity (Buckner et al. 2008; Vincent et al. 2007). We have previously detailed similarities between resting state correlation maps and fMRI responses in descriptions of the dorsal attention (Fox et al. 2006) and hippocampal-cortical memory systems (Vincent et al. 2006), sometimes using alternative nomenclature e.g., the “task positive” and “task negative” systems (Fox et al. 2005).
Here we report that the frontoparietal control system includes the anterior prefrontal, dorsolateral prefrontal, dorsomedial superior frontal/anterior cingulate, anterior inferior parietal lobule, and anterior insular cortex. The frontoparietal control system is anatomically interposed between the systems functionally correlated with the HF and MT+ in multiple lobes (prefrontal, lateral temporal, parietal; Figs. 3 and 5). The interposition relation is especially clear in parietal cortex. The distinction between the three systems as well as their close anatomic proximity can be appreciated most clearly in Fig. 7. Table 3 provides a formal, quantitative dissociation between parietal regions linked to the three distinct systems.
Relation of the present results to previous functional connectivity studies
Previous reports have examined the pattern of correlations associated with the HF (Buckner et al. 2008; Greicius et al. 2004; Kahn et al. 2008; Vincent et al. 2006; Wang et al. 2006) and area MT+ (Fox et al. 2005; Vincent et al. 2006). Concordant with previous results, we found that the HF is functionally correlated with the ventral medial prefrontal cortex, lateral temporal cortex, the posterior inferior parietal lobule, and the retrosplenial cortex extending into the posterior cingulate. Further, as previously described, MT+ is functionally correlated with medial intraparietal sulcus, superior parietal cortex, postcentral gyrus, premotor cortex, frontal eye fields, insula, dorsolateral prefrontal cortex, and extrastriate cortex.
Using independent component analysis, Damoiseaux and colleagues (2006) identified a system that resembles the present aPFC-correlated system (their Fig. 1, C and D). Their results differ from the present results in splitting the system into right and left hemisphere components, a difference that may be attributable to computational technique. [However, we note a significant effect of lateralization in the frontoparietal control system (see results).] Also using ICA, Seeley and colleagues (2007) reported a bilateral system similar to the present frontoparietal control system. However, Seeley and colleagues split the system into two parts, one associated with “salience” (including dorsal anterior cingulate and orbital fronto-insular cortex), the other associated with “executive control” (including dorsolateral frontal and parietal cortex). Seeley and colleagues reported a significant negative correlation between the functional connectivity within their “executive control” system and the time required to complete the Trail Making Test, which measures the ability to switch between competing mental sets while performing visuomotor search task. Dosenbach et al. (2007) have also examined functional correlations within a region in or near the presently described frontoparietal system and distinguished between cinguloopercular and frontoparietal networks as well. Here we report statistically significant correlations between both frontal and parietal regions and voxels in or near the cinguloopercular system (Figs. 1, 2, and 4–7; see also Table 3).
We note that the functional significance of the present correlation results is limited because it remains unclear how correlations in BOLD signals relate to correlations in underlying neuronal activity (but see Nir et al. 2008; Shmuel and Leopold 2008). Moreover, it is unclear whether the observed correlations between two regions indicate direct interactions or signals shared in common from some other source. Future work should explore alternative methods of examining functional interactions within and between the systems described in the present work. For example, the present correlation results might be refined by further examining inter-regional correlations after deconvolution of the hemodynamic signal, which may more accurately reflect underlying neuronal activity. (e.g., Gilteman et al. 2003). In addition, methods such as structural equation modeling (Gonçalves and Hall 2003) and Granger causality (Goebel et al. 2003) may shed light on the direction of causality as well as whether the observed correlations are direct or indirect.
Relation between the frontoparietal control system and conventional task-based fMRI studies
The frontoparietal control system identified by the present correlation analysis includes lateral frontopolar cortex, anterior prefrontal cortex, dorsolateral prefrontal cortex, anterior cingulate/medial frontal cortex, lateral cerebellum, anterior insula, caudate, and the anterior inferior parietal lobule. This constellation of regions is commonly engaged by tasks that require controlled processing of information (for reviews, see Botvinick et al. 2004; Dosenbach et al. 2007; Gruber and Goshke 2004; Ramnani and Owen 2004). Task paradigms eliciting controlled processing related to the simultaneous consideration of multiple interdependent contingencies (Kroger et al. 2002), conflicting stimulus-response mappings (Crone et al. 2006), and integrating working memory with attentional resource allocation (Koechlin et al. 1999) commonly engage the frontoparietal control system. In addition, many of the regions in the frontoparietal control system show sustained activity over the duration of a task block (Dosenbach et al. 2006; Velanova et al. 2003; Yarkoni et al. 2005), which may be related to a requirement for integration of information throughout the block or the sustained maintenance of the task set.
Previous research has suggested roles for regions within the frontoparietal control system. Anterior cingulate cortex commonly increases activity after the commission of errors (Carter et al. 1998; Dosenbach et al. 2006), which is consistent with a role for this region in conflict monitoring during task performance (Botvinick et al. 2004). The anterior cingulate cortex has also been implicated in the instantiation of action sets as it transiently responds at the start of task blocks (Dosenbach et al. 2006). This result is consistent with observation that lesions in or around anterior cingulate cortex lead to impaired initiation of voluntary actions (Cohen et al. 1999). The aIPL often increases activity during tasks involving working memory and may serve as a temporary storage buffer (Gruber 2001). The aPFC has been associated with the coordination of information processing and information transfer between multiple brain regions during the performance of concurrent tasks (Koechlin et al. 1999; Ramnani and Owen 2004). One possibility is that information represented in the anterior cingulate cortex and aIPL is integrated in aPFC during decision-making. In addition, the set of prefrontal regions that we found to be functionally correlated with both the aPFC and the aIPL may correspond to the cluster of prefrontal regions thought to reflect a hierarchical organization of control (Badre and D'Esposito 2007; Buckner 2003; Christoff and Gabrieli 2001; Koechlin and Hyafil 2007; Koechlin et al. 2003).
In the present article, we have described the frontoparietal system correlated with the aPFC as a “frontoparietal control system” because many of the regions within this system are activated during tasks requiring cognitive control or executive function. However, the regions in this system may be involved in many processes and functions that must be examined with forward hypotheses and “task-based” cognitive neuroscience methods. Therefore our description of this system as a “control system” is tentative.
Relation between the hippocampal-cortical memory and frontoparietal control systems
Both the anterior and posterior portions of the inferior parietal lobule, which are correlated with the aPFC and HF, respectively, commonly increase and decrease activity concurrently during task performance. For example, Shulman and colleagues (1997) reported two distinct foci in the inferior parietal lobule (1 anterior and the other posterior) that show activity decreases during cognitively demanding tasks. In addition, a recent meta-analysis of the default network reported that activity decreases in the inferior parietal lobule include the posterior region correlated with the HF but also extend anteriorly into a region that closely resembles the aIPL region that is correlated with the aPFC (Buckner et al. 2008). Thus in many instances, the presence of distinct regions within the inferior parietal lobule is missed because the regions track each other in some task contexts. Our results demonstrate a clear dissociation that clarifies several prior observations.
The dissociation between parietal regions differentially associated with aPFC and HF that we present here informs the dissociation between brain regions that respond to familiarity and recollection based on long-term memory decisions (Henson et al. 1999; Wheeler and Buckner 2004; Yonelinas et al. 2005; see Cabeza et al. 2008; Vilberg and Rugg 2008; Wagner et al. 2005 for reviews). These earlier studies and reviews have noted discordant properties of distinct parietal regions during memory retrieval tasks. The present observation that this region contains distinct (but closely juxtaposed) areas associated with the frontoparietal control system and the hippocampal-cortical memory system provides clarification. Specifically, a recent study by Yonelinas and colleagues (2005) asked participants to either judge their confidence that a presented item was previously seen during a study session (familiarity-based recognition) or to indicate that they recollected a specific detail about the previous presentation of the item (recollection-based recognition). They reported that activity in a system of brain regions that included anterior lateral prefrontal cortex, superior anterior inferior parietal lobule, and the precuneus positively correlated with confidence ratings. In contrast, a distinct brain system including medial prefrontal cortex, posterior cingulate, and the ventral-posterior inferior parietal lobule was more active during recollection judgments than high confidence recognition judgments. Here we have shown that the systems segregated by recollection/familiarity long-term memory paradigms are intrinsically represented in the correlation structure of spontaneous activity recorded in the absence of task performance.
What is the function of the aPFC and HF correlated systems in long-term memory judgments? The hippocampal-cortical memory system has been associated with recollection memory (Vincent et al. 2006). Numerous studies have indicated that the hippocampal formation, posterior inferior parietal lobule, and posterior cingulate/retrosplenial cortex show recollective-sensitive activity increases when retrieval trials are sorted by remember/know, source recollection, and study-depth status (e.g., Eldridge et al. 2000; Henson et al. 1999; Wheeler and Buckner 2004; for reviews, see Cabeza et al. 2008; Vilberg and Rugg 2008; Wagner et al. 2005). Autobiographical memory tasks, which include both memory demands and self-referential components, increase activity in all components of the hippocampal-cortical memory system including the parietal and posterior cingulate regions as well as the medial prefrontal cortex (Andreasen et al. 1995; Cabeza et al. 2004; Maddock et al. 2003; Maguire et al. 2001; Steinvorth et al. 2006). Thus this system is likely involved in the reconstruction of long-term memories during retrieval tasks and other tasks that utilize past experiences to make prospectively oriented decisions (Schacter et al. 2007).
By contrast, the frontoparietal control system has been associated with familiarity-based memory retrieval decisions. However, the frontoparietal control system is not likely to support functions specific to long-term memory. First, this system commonly increases activity during many working memory and decision-making tasks (Crone et al. 2006; Koechlin et al. 1999; Kroger et al. 2002) in addition to memory-retrieval tasks. Second, this system appears to show robust retrieval success effects when old items are relatively infrequent (25:75 old to new item ratio), but these effects are either nonsignificant or reversed when the old to new item ratio is reversed (75:25) (Herron et al. 2004). Therefore these regions appear to be sensitive to target probabilities. These data might suggest that activity increases in these regions could be related to the task-relevance of the probe. Alternatively, during memory retrieval, the frontoparietal control system may be recruited when judgments are uncertain and further analysis of the stimulus in relation to mnemonic representations is required (see for example Atkinson and Juola 1974; Balota and Chumbley 1984). Specifically, the control system may be engaged when decisions are made in situations where the stimulus strength falls between the low and the high familiarity decision criterion during retrieval.
Relation between the dorsal attention and frontoparietal control systems
Both the anterior inferior parietal lobule and the superior parietal lobule, which are correlated with the aPFC and MT+, respectively, commonly increase activity concurrently during task performance. For example, Crone and colleagues (2006) reported activity increases in both superior and inferior parietal lobule when participants switched from univalent rules to bivalent rules during a stimulus-response association task. Further, Sapir et al. (2005) demonstrated that while regions within the dorsal attention system were modulated during the processing of behaviorally relevant visual cues, a region in the aIPL of the frontoparietal control system was highly predictive of participants' accuracy and potentially the degree of utilization of the cued information in the control of spatial attention. These data suggest that the parietal region of both dorsal attention and frontoparietal control systems may transfer behaviorally relevant information during tasks requiring attention and discrimination.
One of the most striking and unpredicted results of our study was the dissociation between the frontoparietal control and dorsal attention systems. The dorsal attention system (also known as the oculomotor system) has been implicated in eye movements, overt and covert spatial attention, and the generation of motor plans via transformations of sensory inputs from multiple modalities (Anderson and Buneo 2002; Corbetta and Shulman 2002). The frontoparietal control system has been implicated in monitoring of conflict, the updating and implementation of goal-directed behavior, and the integration of sensory information with internal representations of intentions to coordinate behavior (Miller and Cohen 2001; Ramnani and Owen 2004). Both of these systems have been implicated in working memory performance (Gruber and Goschke 2004). As a result of the highly similar topography of the two systems, the regions within these systems may have been confused in the past. Our results suggest that further progress in understanding the anatomical correlates of externally directed attention and cognitive control will require careful disambiguation of these two systems.
Distinct functional properties of the frontoparietal control system and the dorsal attention system have also been foreshadowed by results from carefully designed working memory paradigms. For example, Gruber (2001) instructed participants to perform a verbal working memory task under single-task conditions, dual-task performance that allowed rehearsal (finger tapping), and dual-task performance involving articulatory suppression (counting). During the single-task and the dual-task without articulatory suppression (finger tapping) conditions, parts of the dorsal attention system including ventral premotor cortex and the superior parietal lobule/intraparietal sulcus showed increased activity. However, in the dual-task with articulatory suppression (counting) condition, many regions in the frontoparietal control system increased activity. The authors suggested that this system increases activity when the subjects are forced to refrain from active rehearsal (via articulatory suppression) during the verbal working memory. Alternatively, the frontoparietal control system may be recruited during articulatory suppression because articulatory suppression leads to less certain judgments and further analysis of the stimulus in relation to memory of the stimulus set is required (see for example Atkinson and Juola 1974; Balota and Chumbley 1984).
Interposition of control system among dorsal attention and hippocampal-cortical memory systems
An intriguing result of our analyses is the observation that the frontoparietal control system is largely spatially interposed between the dorsal attention and hippocampal-cortical memory systems. As shown in Fig. 3, the MT+, aPFC, and HF functional correlations to lateral parietal cortex are largely nonoverlapping. The anterior inferior parietal lobule linked to aPFC fits between the posterior parietal region associated with the HF and the superior parietal region associated with area MT+. As discussed in the preceding text, several studies report concurrent activity increases or decreases of either the aIPL and pIPL (Buckner et al. 2008; Shulman et al. 1997) or the aIPL and SPL (Crone et al. 2006; Sapir et al. 2005). In addition, a sustained increase in activity within the anterior parietal lobule during task performance is commonly reported (Dosenbach et al. 2006; Velanova et al. 2003; Yarkoni et al. 2005), which suggests this region is involved in a wide variety of tasks. These results suggest that the close interposition of the aIPL among pIPL and SPL regions may function to facilitate the transfer of information between either the dorsal attention or hippocampal-cortical memory systems and the frontoparietal control system during task performance. The aIPL component of the control system may be recruited during tasks that demand a temporary buffer. For example, during articulatory suppression, the aIPL may hold information on-line in working memory (Gruber 2001), and during long-term memory retrieval, the aIPL may represent retrieved information in a form accessible to decision-making processes (Wagner et al. 2005). Alternatively, increases in activity within aIPL may reflect processes recruited to maintain an attentional set during demanding tasks.
A common theory is that lateral prefrontal cortex has been associated with the abstract representation of the stimulus-related information and stimulus response mappings that are used to plan behavioral decisions (Miller and Cohen 2001). The anterior cingulate cortex is thought to monitor conflict on trials with multiple competing responses and signal the need for greater cognitive control on subsequent trials (Carter et al. 1998; Kerns et al. 2004; Liston et al. 2006; MacDonald et al. 2000). Recently it has been proposed that a region in parietal cortex processes stimulus-related or perceptual conflict because it shows greater activity on trials where the irrelevant dimension of a stimulus is highly salient (Liston et al. 2006). Further, this increase in stimulus conflict related activity in parietal cortex is associated with greater activity in lateral prefrontal cortex on subsequent trials (Liston et al. 2006). A conflict hypothesis has the potential to account for many incidences of aIPL activation across tasks as diverse as long-term memory retrieval, cognitive control, and spatial attention. In addition, the interposition of the aIPL among externally directed SPL and internally directed pIPL may make the aIPL an ideal location to integrate perceptual information and detect conflict emerging from the results of processes occurring in posterior associative cortices.
For example, using a recognition memory task, Herron and colleagues (2004) described a parietal region in or near the aIPL with activity that increased as the probability of an old item on the retrieval test went from high to low. One potential explanation is that the participants experienced greater conflict when making judgments on old items when old items were rare. In another recognition experiment, Kim and Cabeza (2007) reported two parietal regions that were activated during retrieval. In contrast to the more posterior-ventral region (in or near our pIPL), the dorsal-anterior region (in or near our aIPL) was more active during low confidence versus high confidence judgments; this may reflect greater conflict on low confidence recognition trials. In a recognition memory paradigm, Velanova et al. (2003), manipulated the number of times the participants viewed study words at encoding (1 vs. 20 times) and found that a region in or near the aIPL had greater activity on recognition trials that followed a single encoding trial versus 20 encoding trials, which may have resulted from greater conflict on the words that were minimally rehearsed. In a working memory task, Gruber (2001) found that a region in or near the aIPL was most active for trials with articulatory suppression versus no articulatory suppression. The process of articulatory suppression may have increased conflict at the time of decision and resulted in the enhancement of activity in the aIPL. In a parametric study of relational complexity using a task similar to Raven's Progressive Matrices (Ravin 1941), Kroger and colleagues (2002) found that as the complexity of the matrix problems increased, the frontoparietal control system including bilateral aIPL increased activity. Once again, this increase in parietal cortex may be attributable to stimulus-related conflict that would be highest in the most complex matrices. Future task-related studies will be required to elucidate the function of the aIPL component of the frontoparietal control system and determine whether activity in this region is best accounted for by conflicting processes generated by the stimulus or context of the task.
Spontaneous BOLD fluctuations, default activity, and spontaneous cognition
In the last few years, activity during the resting state has become a major topic of research in functional neuroimaging (for reviews and commentary, see Buckner and Vincent 2007; Fox and Raichle 2007; Haughton and Biswal 1998; Raichle and Mintun 2006). Resting state studies may be central to information processing and provide insight into anatomy, disease, and spontaneous cognition (Buckner and Vincent 2007). However, the two types of resting state activity that have received the majority of research must be considered separately.
First, both blocked and event-related analyses based on positron emission tomography (PET) and fMRI have revealed a highly stereotypic pattern of brain regions that manifest greater activity during passive task states as compared with many forms of active task states (Binder et al. 1999; Buckner et al. 2008; Mazoyer et al. 2001; McKiernan et al. 2003; Raichle et al. 2001; Shulman et al. 1997). This network includes medial prefrontal cortex, the medial temporal lobes, lateral parietal cortex, and posterior cingulate extending into retrosplenial cortex. The consistency of this activity pattern in undirected task states as well as its metabolic properties led to the designation of the activity observed in rest states as reflecting a “default mode” of brain function (Raichle et al. 2001). This type of spontaneous activity is thought to relate to mind-wandering and spontaneous use of recollection and future-oriented thought (Buckner et al. 2008; Mason et al. 2007).
Second, analyses of the temporal dynamics of fMRI measured activity during rest have further revealed that networks of regions spontaneously increase and decrease activity together in a correlated manner, including within the default network (De Luca et al. 2006; Greicius et al. 2003, 2004; Laufs et al. 2003). Correlations in spontaneous BOLD fluctuations, however, can be found in systems outside of the default network. Complex, structured spontaneous activity patterns emerge in many brain systems including sensory-motor, attention, cognitive control, long-term memory, and language (e.g., Biswal et al. 1995; Fox et al. 2006; Hampson et al. 2002; Lowe et al. 1998; Vincent et al. 2006). These results demonstrate that the brain contains highly organized, spontaneous patterns of functional activity at rest. However, similar patterns of spontaneous correlations are observed during sleep and anesthesia (Greicius et al. 2008; Horovitz et al. 2008; Vincent et al. 2007) and during task states (Fransson 2006). Therefore coherent spontaneous BOLD fluctuations cannot exclusively be a reflection of conscious mental activity but may reflect a more fundamental or intrinsic property of functional brain organization.
These two types of spontaneous activity constitute a significant amount of the brain's metabolic budget, but the precise functions of this activity remain poorly understood (Raichle and Mintun 2006). Relevant to the present paper, we use the presence of spontaneous, intrinsic activity to define the frontoparietal control system but our observations do not shed insight into the function of spontaneous BOLD fluctuations themselves.
Relation to nonhuman primate work
Finally, we wish to speculate about the evolutionary history of the frontoparietal system. The seed region used to generate the frontoparietal control system is in or around Brodmann area 10. Previous cytoarchitectonic and histological studies have shown that area 10 is greatly expanded in both absolute size and percentage of whole brain volume in humans relative to macaques and apes (Öngür et al. 2003; Semendeferi et al. 2001). More recently, Van Essen and Dierker (2007) used anatomical MRI to generate a map of estimated cortical expansion between macaque and human (based on 23 landmark constraints). Two of the regions that showed the greatest expansion included anterior lateral prefrontal cortex and anterior inferior parietal lobule—two of the prominent components of the frontoparietal control system. Collectively, these studies suggest that the human frontoparietal control system may be especially important in the evolution of the human brain.
GRANTS
This work was supported by National Institutes of Health Grants AG-021910 and NS-06833, the Mallinckrodt Institute of Radiology, AAMCBR, the James S McDonnell Foundation, and the Howard Hughes Medical Institute.
Acknowledgments
We thank L. J. Larson-Prior, J. Zempel, and J. R. Andrews-Hanna for providing data. D. C. Van Essen generously provided use of Caret software. We also thank O. Gruber and M. D. Fox for informative discussions.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “_advertisement_” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
1
The default network (Raichle et al. 2001) has been demonstrated to be a set of interacting subsystems that are utilized together during passive task states, connected through hubs including the posterior cingulate (Buckner et al. 2008; Hagmann et al. 2008; Fransson and Marrelec 2008). The hippocampal-cortical memory system described here is a component of the default network but does not include the dorsal extent of medial prefrontal cortex or the anterior components of inferior parietal cortex that are prominent in the default network (Buckner et al. 2008). As the analysis will reveal, it is likely that the default network encompasses both the hippocampal-cortical memory system and parts the frontoparietal control system we describe here (see discussion).
2
In Fig. 1, we found correlations in the white matter near the anterior prefrontal cortex seed region, which may be due to the correlated noise near the seed region and potentially susceptibility-related distortion. The higher resolution data sets had less white matter correlations (see Fig. 6).
REFERENCES
- Andersen and Buneo 2002.Andersen RA**, Buneo CA.** Intentional maps in posterior parietal cortex. Annu Rev Neurosci 25: 189–220, 2002 [DOI] [PubMed] [Google Scholar]
- Andreasen et al. 1995.Andreasen NC**, O'Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, Ponto LL, Hichwa RD.** Remembering the past: two facets of episodic memory explored with positron emission tomography. Am J Psychiatry 152: 1576–1585, 1995 [DOI] [PubMed] [Google Scholar]
- Atkinson and Juola 1974.Atkinson RC**, Juola JF.** Search and decision processes in recognition memory. In: Contemporary Developments in Mathematical Psychology, edited by Krantz DH, Atkinson RC, Luce RD, Suppes P. San Diego, CA: Freeman, 1974, vol. 1, p. 242–293. [Google Scholar]
- Badre and D'Esposito 2007.Badre D**, D'Esposito M.** Functional magnetic resonance imaging evidence for a hierarchical organization in the prefrontal cortex. J Cogn Neurosci 19: 2082–2099, 2007 [DOI] [PubMed] [Google Scholar]
- Balota and Chumbley 1984.Balota DA**, Chumbley JI.** Are lexical decisions a good measure of lexical access? The role of word frequency in the neglected decision stage. J Exp Psychol Hum Percept Perform. 10: 340–357, 1984 [DOI] [PubMed] [Google Scholar]
- Bartels and Zeki 2005.Bartels A**, Zeki S.** The chronoarchitecture of the cerebral cortex. Philos Trans R Society Lond B Biol Sci 360: 733–750, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder et al. 1999.Binder JR**, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW.** Conceptual processing during the conscious resting state: a functional MRI study. J Cogn Neurosci 11: 80–93, 1999 [DOI] [PubMed] [Google Scholar]
- Birn et al. 2006.Birn RM**, Diamond JB, Smith MA, Bandettini PA.** Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. NeuroImage 31: 1536–1548, 2006 [DOI] [PubMed] [Google Scholar]
- Biswal et al. 1995.Biswal B**, Yetkin FZ, Haughton VM, Hyde JS.** Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34: 537–541, 1995 [DOI] [PubMed] [Google Scholar]
- Botvinick et al. 2004.Botvinick MM**, Cohen JD, Carter CS.** Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci 8: 539–546, 2004 [DOI] [PubMed] [Google Scholar]
- Buckner 2003.Buckner RL**.** Functional-anatomic correlates of control processes in memory. J Neurosci 23: 3999–4004, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner et al. 2008.Buckner RL**, Andrews-Hanna JR, Schacter DL.** The brain's default network: anatomy, function, and relevance to disease. Ann NY Acad Sci 1124: 1–38, 2008 [DOI] [PubMed] [Google Scholar]
- Buckner and Carroll 2007.Buckner RL**, Carroll DC.** Self-projection and the brain. Trends Cogn Sci 11: 49–57, 2007 [DOI] [PubMed] [Google Scholar]
- Buckner et al. 2004.Buckner RL**, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ.** A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. NeuroImage 23: 724–738, 2004 [DOI] [PubMed] [Google Scholar]
- Buckner and Vincent 2007.Buckner RL**, Vincent JL.** Unrest at rest: default activity and spontaneous network correlations. Neuroimage 37: 1091–1096, 2007 [DOI] [PubMed] [Google Scholar]
- Cabeza et al. 2008.Cabeza R**, Ciaramelli E, Olson IR, Moscovitch M.** The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci 9: 613–625, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza et al. 2004.Cabeza R**, Prince SE, Daselaar SM, Greenberg DL, Budde M, Dolcos F, LaBar KS, Rubin DC.** Brain activity during episodic retrieval of autobiographical and laboratory events: An fMRI study using a novel photo paradigm. J Cogn Neurosci 16: 1583–1594, 2004 [DOI] [PubMed] [Google Scholar]
- Carter et al. 1998.Carter CS**, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD.** Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280: 747–749, 1998 [DOI] [PubMed] [Google Scholar]
- Cohen et al. 1999.Cohen RA**, Kaplan RF, Zuffante P, Moser DJ, Jenkins MA, Salloway S, Wilkinson H.** Alteration of intention and self-initiated action associated with bilateral anterior cingulotomy. J Neuropsychiatry Clin Neurosci 11: 444–453, 1999 [DOI] [PubMed] [Google Scholar]
- Corbetta and Shulman 2002.Corbetta M**, Shulman GL.** Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3: 201–215, 2002 [DOI] [PubMed] [Google Scholar]
- Christoff and Gabrieli 2000.Christoff K**, Gabrieli JDE.** The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology 28: 168–186, 2000 [Google Scholar]
- Crone et al. 2006.Crone EA**, Wendelken C, Donohue SE, Bunge SA.** Neural evidence for dissociable components of task-switching. Cereb Cortex 16: 475–486, 2006 [DOI] [PubMed] [Google Scholar]
- Damoiseaux et al. 2006.Damoiseaux JS**, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF.** Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA 103: 13848–13853, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca et al. 2006.De Luca M**, Beckmann CF, De Stefano N, Matthews PM, Smith SM.** fMRI resting state networks define distinct modes of long-distance interactions in the human brain. NeuroImage 29: 1359–1367, 2006 [DOI] [PubMed] [Google Scholar]
- Dosenbach et al. 2007.Dosenbach NU**, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE.** Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA 104: 11073–11078, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach et al. 2006.Dosenbach NU**, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE.** A core system for the implementation of task sets. Neuron 50: 799–812, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge et al. 2000.Eldridge LL**, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA.** Remembering episodes: a selective role for the hippocampus during retrieval. Nat Neurosci 3: 1149–1152, 2000 [DOI] [PubMed] [Google Scholar]
- Evans et al. 1993.Evans AC**, Collins DL, Mills SR, Brown ED, Kelly RL, Peters TM.** 3D statistical neuroanatomical models from 305 MRI volumes. In: Nuclear Science Symposium & Medical Imaging Conference: 1993 IEEE conference record, New York: IEEE.
- Fox et al. 2006.Fox MD**, Corbetta M, Snyder AZ, Vincent JL, Raichle ME.** Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA 103: 100046–100051, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox and Raichle 2007.Fox MD**, Raichle ME.** Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8: 700–711, 2007 [DOI] [PubMed] [Google Scholar]
- Fox et al. 2005.Fox MD**, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME.** The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102: 9673–9678, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson 2005.Fransson P**.** Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp 26: 15–29, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson 2006.Fransson P**.** How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia 44, 2836–2845, 2006 [DOI] [PubMed] [Google Scholar]
- Fransson and Marrelec 2008.Fransson P**, Marrelec G.** The precuneus/posterior cingulated cortex plays a pivitol role in the default mode network: evidence from a partial correlation network analysis. Neuroimage 42: 1178–1184, 2008 [DOI] [PubMed] [Google Scholar]
- Gitelman et al. 2003.Gitelman DR**, Penny WD, Ashburner J, Friston KJ.** Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. NeuroImage 19: 200–207, 2003 [DOI] [PubMed] [Google Scholar]
- Goebel et al. 2003.Goebel R**, Roebroeck A, Kim DS, Formisano E.** Investigating directed cortical interactions in time-resolved fMRI data using vector autoregressive modeling and Granger causality mapping. Magn Reson Imaging 21: 1251–1261, 2003 [DOI] [PubMed] [Google Scholar]
- Gonçalves and Hall 2003.Gonçalves MS**, Hall DA.** Connectivity analysis with structural equation modeling: an example of the effects of voxel selection. NeuroImage 20: 1455–1467, 2003 [DOI] [PubMed] [Google Scholar]
- Greicius et al. 2003.Greicius MD**, Krasnow B, Reiss AL, Menon V.** Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100: 253–258, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius et al. 2008.Greicius MD**, Kiviniemi V, Tervonen O, Vainionpää V, Alahuhta S, Reiss AL, Menon V.** Persistent default-mode network connectivity during light sedation. Hum Brain Mapp 29: 839–847, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius et al. 2004.Greicius MD**, Srivastava G, Reiss AL, Menon V.** Default-mode network activity distinguishes Alzheimer's disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci USA 101: 4637–4642, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber 2001.Gruber O**.** Effects of domain-specific interference on brain activation associated with verbal working memory task performance. Cereb Cortex 11: 1047–1055, 2001 [DOI] [PubMed] [Google Scholar]
- Gruber and Goschke 2004.Gruber O**, Goschke T.** Executive control emerging from dynamic interactions between brain systems mediating language, working memory and attentional processes. Acta Psychol 115: 105–121, 2004 [DOI] [PubMed] [Google Scholar]
- Hagmann et al. 2008.Hagmann P**, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O.** Mapping the structural core of human cerebral cortex. PLoS Biol 6: 1479–1493, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson et al. 2002.Hampson M**, Peterson BS, Skudlarski P, Gatenby JC, Gore JC.** Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp 15: 247–262, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughton and Biswal 1998.Haughton V**, Biswal, B.** Clinical application of basal regional cerebral blood flow fluctuation measurements by fMRI. Adv Exp Med Biol 454: 583–590, 1998 [DOI] [PubMed] [Google Scholar]
- Henson et al. 1999.Henson RNA**, Rugg MD, Shallice T, Josephs O, Dolan RJ.** Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J Neurosci 19: 3962–3972, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron et al. 2004.Herron JE**, Henson RNA, Rugg MD.** Probability effects on the neural correlates of retrieval success: an fMRI study. NeuroImage 21: 302–310, 2004 [DOI] [PubMed] [Google Scholar]
- Horovitz et al. 2008.Horovitz SG**, Fukunaga M, de Zwart JA, van Gelderen P, Fulton SC, Balkin TJ, Duyn JH.** Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI study. Hum Brain Mapp 29: 671–682, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins and Watts 1968.Jenkins GM**, Watts DG.**Spectral Analysis and its Applications. Boca Raton, FL: Emerson-Adams, 1968
- Kahn et al. 2008.Kahn I**, Andrews-Hanna JR, Vincent JL, Snyder AZ, Buckner RL.** Distinct cortical anatomy linked to subdivisions of the medial temporal lobe revealed by intrinsic functional connectivity, J Neurophysiol 100: 129–139, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenet et al. 2003.Kenet T**, Bibitchkov D, Tsodyks M, Grinvald A, Arieli A.** Spontaneously emerging cortical representations of visual attributes. Nature 425: 954–956, 2003 [DOI] [PubMed] [Google Scholar]
- Kerns et al. 2007.Kerns JG**, Cohen JD, MacDonald AW 3rd, Cho RY, Stenger VA, Carter CS.** Anterior cingulate conflict monitoring and adjustments in control. Science 303: 1023–1026, 2004. [DOI] [PubMed] [Google Scholar]
- Kim and Cabeza 2007.Kim H**, Cabeza R.** Trusting our memories: dissociating the neural correlates of confidence in veridical versus illusory memories. J Neurosci 27: 12190–12197, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin et al. 1999.Koechlin E**, Basso G, Pietrini P, Panzer S, Grafman J.** The role of the anterior prefrontal cortex in human cognition. Nature 399: 148–151, 1999 [DOI] [PubMed] [Google Scholar]
- Koechlin and Hyafil 2007.Koechlin E**, Hyafil A.** Anterior prefrontal function and the limits of human decision making. Science 318: 594–598, 2007 [DOI] [PubMed] [Google Scholar]
- Koechlin et al. 2003.Koechlin E**, Ody C, Kouneiher F.** The architecture of cognitive control in the human prefrontal cortex. Science 302: 1181–1185, 2003 [DOI] [PubMed] [Google Scholar]
- Kroger et al. 2002.Kroger JK**, Sabb FW, Fales CL, Bookheimer SY, Cohen MS, Holyoak KJ.** Recruitment of anterior dorsolateral prefrontal cortex in human reasoning: a parametric study of relational complexity. Cereb Cortex 12: 477–485, 2002 [DOI] [PubMed] [Google Scholar]
- Laufs et al. 2003.Laufs H**, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, Kleinschmidt A.** Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proc Natl Acad Sci USA 100: 11053–11058, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston et al. 2006.Liston C**, Matalon S, Hare TA, Davidson MC, Casey BJ.** Anterior cingulate and posterior parietal cortices are sensitive to dissociable forms of conflict in a task-switching paradigm. Neuron 50: 643–653, 2006 [DOI] [PubMed] [Google Scholar]
- Lowe et al. 1998.Lowe MJ**, Mock BJ, Sorenson JA.** Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. NeuroImage 7: 119–132, 1998 [DOI] [PubMed] [Google Scholar]
- MacDonald et al. 2003.MacDonald AW 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288: 1835–1838, 2004. [DOI] [PubMed] [Google Scholar]
- Maddock et al. 2003.Maddock RJ**, Garrett AS, Buonocore MH.** Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp 18: 30–41, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire 2001.Maguire EA**.** Neuroimaging studies of autobiographical event memory. Philos Trans R Society Lond B Biol Sci 356: 1441–1451, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason et al. 2001.Mason MF**, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN.** Wandering minds: the default network and stimulus-independent thought. Science 315: 393–395, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer et al. 2001.Mazoyer B**, Zago L, Mellet E, Bricogne S, Etard O, Houdé O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N.** Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull 54: 287–298, 2001 [DOI] [PubMed] [Google Scholar]
- McKiernan et al. 2003.McKiernan KA**, Kaufman JN, Kucera-Thompson J, Binder JR.** 2003. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci 15: 394–408, 2003 [DOI] [PubMed] [Google Scholar]
- Mesulam 1990.Mesulam M-M**.** Large-scale neurocognitive networks and distributed processing from attention, language, and memory. Ann Neurol 28: 597–613, 1990 [DOI] [PubMed] [Google Scholar]
- Miller 2000.Miller EK**.** The prefrontal cortex and cognitive control. Nat Rev Neurosci 1: 59–65, 2000 [DOI] [PubMed] [Google Scholar]
- Miller and Cohen 2001.Miller EK**, Cohen JD.** An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202, 2001 [DOI] [PubMed] [Google Scholar]
- Nir et al. 2008.Nir Y**, Mukamel R, Dinstein I, Privman E, Harel M, Fisch L, Gelbard-Sagiv, H, Kipervasser S, Andelman F, Neufeld MY, Kramer U, Arieli A, Fried I, Malach R.** Interhemisphere correlations of slow spontaneous neuronal fluctuations revealed in human sensory cortex. Nat Neurosci 11: 1100–1108, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann et al. 1997.Ojemann JG**, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE.** Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage 6: 156–167, 1997 [DOI] [PubMed] [Google Scholar]
- Öngür et al. 2003.Öngür D**, Ferry AT, Price JL.** Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol 460: 425–449, 2003 [DOI] [PubMed] [Google Scholar]
- Raichle et al. 2001.Raichle ME**, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL.** A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle and Mintun 2006.Raichle ME**, Mintun MA.** Brain work and brain imaging. Annu Rev Neurosci 29: 449–476, 2006 [DOI] [PubMed] [Google Scholar]
- Ramnani and Owen 2004.Ramnani N**, Owen AM.** Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci 5: 184–194, 2004 [DOI] [PubMed] [Google Scholar]
- Ravin 1941.Ravin JC**.** Standardization of progressive matrices, 1938. Br J Med Psychol 19: 137–150, 1941 [Google Scholar]
- Sapir et al. 2005.Sapir A**, d'Avossa G, McAvoy M, Shulman GL, Corbetta M.** Brain signals for spatial attention predict performance in a motion discrimination task. Proc Natl Acad Sci USA 102: 17810–17815, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley et al. 2007.Seeley WW**, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD.** Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27: 2349–2356, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semendeferi et al. 2001.Semendeferi K**, Armstrong E, Schleicher A, Zilles K, Van Hoesen GW.** Prefrontal cortex in humans and apes: a comparative study of area 10. Am J Phys Anthropol 114: 224–241, 2001 [DOI] [PubMed] [Google Scholar]
- Schacter et al. 2007.Schacter DL**, Addis DR, Buckner RL.** Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci 8: 657–661, 2007 [DOI] [PubMed] [Google Scholar]
- Shmuel and Leopold 2008.Shmuel A**, Leopold DA.** Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: implications for functional connectivity at rest. Hum Brain Mapp 29: 751–761, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman et al. 1997.Shulman GL**, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE.** Common blood flow changes across visual tasks. II. Decreases in cerebral cortex. J Cogn Neurosci 9: 648–663, 1997 [DOI] [PubMed] [Google Scholar]
- Shulman et al. 2003.Shulman GL**, McAvoy MP, Cowan MC, Astafiev SV, Tansy AP, d'Avossa G, Corbetta M.** Quantitative analysis of attention and detection signals during visual search. J Neurophys 90: 3384–3397, 2003 [DOI] [PubMed] [Google Scholar]
- Steinvorth et al. 2006.Steinvorth S**, Corkin S, Halgren E.** Ecphory of autobiographical memories: an fMRI study of recent and remote memory retrieval. NeuroImage 30: 285–298, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda et al. 2006.Svoboda E**, McKinnon MC, Levine B.** The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia 44: 2189–2208, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen 2005.Van Essen DC**.** A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. NeuroImage 28: 635–662, 2005 [DOI] [PubMed] [Google Scholar]
- Van Essen and Dierker 2007.Van Essen DC**, Dierker DL.** Surface-based and probabilistic atlases of primate cerebral cortex. Neuron 56: 209–225, 2007 [DOI] [PubMed] [Google Scholar]
- Velanova et al. 2003.Velanova K**, Jacoby LL, Wheeler ME, McAvoy MP, Petersen SE, Buckner RL.** Functional–anatomic correlates of sustained and transient processing components engaged during controlled retrieval. J Neurosci 23: 8460–8470, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg and Rugg 2008.Vilberg KL**, Rugg MD.** Memory retrieval and parietal cortex: a review of evidence from a dual-process perspective. Neuropsychologia 46: 1787–1799, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent et al. 2007.Vincent JL**, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zepnpel JM, Snyder LH, Corbetta M, Raichle ME.** Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447: 83–86, 2007 [DOI] [PubMed] [Google Scholar]
- Vincent et al. 2006.Vincent JL**, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL.** Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophys 96: 3517–3531, 2006 [DOI] [PubMed] [Google Scholar]
- Wagner et al. 2005.Wagner AD**, Shannon BJ, Kahn I, Buckner RL.** Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci 9: 445–453, 2005 [DOI] [PubMed] [Google Scholar]
- Wang et al. 2006.Wang L**, Zang Y, He Y, Liang M, Zhang X, Tian L, Wu T, Jiang T, Li K.** Changes in hippocampal connectivity in the early stages of Alzheimer's disease: evidence from resting state fMRI. NeuroImage 31: 496–504, 2006 [DOI] [PubMed] [Google Scholar]
- Wheeler and Buckner 2004.Wheeler ME**, Buckner RL.** Functional-anatomic correlates of remembering and knowing. Neuroimage 21: 1337–1349, 2004 [DOI] [PubMed] [Google Scholar]
- Wise et al. 2004.Wise RG**, Ide K, Poulin MJ, Traceya I.** Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. NeuroImage 21: 1652–1664, 2004 [DOI] [PubMed] [Google Scholar]
- Yarkoni et al. 2005.Yarkoni T**, Gray JR, Chrastil ER, Barch DM, Green L, Braver TS.** Sustained neural activity associated with cognitive control during temporally extended decision making. Brain Res Cogn Brain Res 23: 71–84, 2005 [DOI] [PubMed] [Google Scholar]
- Yonelinas et al. 2005.Yonelinas AP**, Otten LJ, Shaw KN, Rugg MD.** Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci 25: 3002–3008, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]