Small interfering RNA-induced TLR3 activation inhibits blood and lymphatic vessel growth (original) (raw)
Abstract
Neovascularization in response to tissue injury consists of the dual invasion of blood (hemangiogenesis) and lymphatic (lymphangiogenesis) vessels. We reported recently that 21-nt or longer small interfering RNAs (siRNAs) can suppress hemangiogenesis in mouse models of choroidal neovascularization and dermal wound healing independently of RNA interference by directly activating Toll-like receptor 3 (TLR3), a double-stranded RNA immune receptor, on the cell surface of blood endothelial cells. Here, we show that a 21-nt nontargeted siRNA suppresses both hemangiogenesis and lymphangiogenesis in mouse models of neovascularization induced by corneal sutures or hindlimb ischemia as efficiently as a 21-nt siRNA targeting vascular endothelial growth factor-A. In contrast, a 7-nt nontargeted siRNA, which is too short to activate TLR3, does not block hemangiogenesis or lymphangiogenesis in these models. Exposure to 21-nt siRNA, which we demonstrate is not internalized unless cell-permeating moieties are used, triggers phosphorylation of cell surface TLR3 on lymphatic endothelial cells and induces apoptosis. These findings introduce TLR3 activation as a method of jointly suppressing blood and lymphatic neovascularization and simultaneously raise new concerns about the undesirable effects of siRNAs on both circulatory systems.
Keywords: angiogenesis, innate immunity, lymphangiogenesis, ischemia, wound healing
Blood and lymphatic vessels both participate in the wound healing response, restoration of circulation, and systemic immune surveillance and activation. Pathological responses of these parallel circulatory systems can lead to diseases as varied as age-related macular degeneration, atherosclerosis, cancer, lymphedema, and rheumatoid arthritis (1). Collectively, exuberant or inadequate responses of angiogenesis are estimated to affect nearly 2 billion people (2, 3). The prevalence of such diseases has led to the development of myriad antiangiogenic strategies, such as antibodies, soluble receptors, and receptor antagonists, targeting the various growth factors and cytokines that are involved in aberrant neovascularization.
Among the various proposed strategies, small interfering RNAs (siRNAs) have attracted much attention as a new therapeutic platform for achieving target-specific gene silencing via double-stranded RNA (dsRNA)-mediated RNA interference (RNAi). However, the combined challenges of intracellular delivery and unintended “off-target” effects remain formidable (4). The polyanionic nature and molecular size of siRNA are impediments to their entry into mammalian cells (5–7). In addition, siRNAs have both sequence-specific (8–10) and sequence-independent (11–13) off-target effects, some of which are better understood than others.
We reported recently the surprising finding that 21-nt or longer siRNAs suppress hemangiogenesis in mouse models of choroidal and dermal neovascularization not via RNAi but by activating cell surface Toll-like receptor 3 (TLR3) on blood endothelial cells (BECs) in a sequence- and target-independent manner (12). TLRs comprise a family of innate immune receptors that recognize various pathogen-associated molecular patterns. TLR3 is a sensor of dsRNA (14), such as those found in viral genomes or replication intermediates, that undergoes dimerization (15–18) and phosphorylation (19) to initiate a signaling cascade that can ultimately result in apoptotic cell death (20–23). In this study, we sought to further explore this newly defined intersection between angiogenesis and innate immunity. We determined whether the generic antihemangiogenic effects of siRNAs extended to other well-established and clinically relevant mouse models of neovascularization in response to corneal suture injury or hindlimb ischemia, and whether siRNA-mediated TLR3 activation also suppressed lymphangiogenesis. We used a 21-nt siRNA targeting mouse vascular endothelial growth factor-A (21nt-siRNA-m_Vegfa_), a potent angiogenic growth factor (24); a 21-nt siRNA targeting the nonmammalian gene firefly luciferase (21nt-siRNA-Luc); and a 7-nt siRNA targeting Luc, which we have shown does not activate TLR3 (12). Because 21-nt siRNAs in phase 2/3 clinical trials in patients with ocular diseases are unformulated, we also studied whether unformulated 21-nt siRNA is internalized by lymphatic endothelial cells (LECs) and activates TLR3.
Results
21-Nucleotide siRNAs Suppress Corneal Neovascularization Regardless of Target.
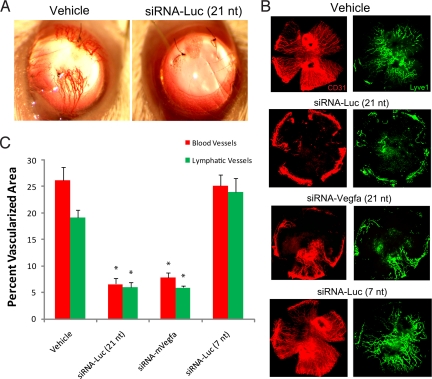
Corneal suture injury induced a robust neovascular response that was evident at 14 days after injury (Fig. 1A). The densities of CD31+ LYVE-1− blood vessels and CD31+ LYVE-1+ lymphatic vessels were quantified by morphometric analysis of immunolabeled flat mounts (Fig. 1B). A single intracorneal injection of 21nt-siRNA-m_Vegfa_ at the time of suture placement dramatically suppressed both hemangiogenesis (70% ± 4%) and lymphangiogenesis (69% ± 2%) compared with vehicle injection (Fig. 1 B and C). Surprisingly, 21nt-siRNA-Luc suppressed hemangiogenesis (77% ± 13%) and lymphangiogenesis (65% ± 6%) as effectively as 21nt-siRNA-m_Vegfa_ (Fig. 1). In contrast, 7-nt siRNA-Luc, which does not activate TLR3, did not suppress either blood (96% ± 9% of vehicle treatment) or lymphatic (124% ± 16% of vehicle treatment) vessel growth into the cornea (Fig. 1 B and C). These data demonstrate a sequence-independent ability of siRNA to suppress corneal neovascularization that correlates with its ability to activate TLR3.
Fig. 1.
The 21-nt targeted and nontargeted siRNA inhibited suture-induced corneal neovascularization. (A) Fourteen days after surgery, representative photographs of vehicle-treated corneas (Left) demonstrate marked corneal neovascularization, whereas a single intracorneal injection of 21nt-siRNA-Luc (Right) abolished this suture-induced response. (B) Representative corneal flat mounts immunolabeled with anti-CD31 (red) and anti-LYVE-1 (green) antibodies show that 21nt-siRNA-Luc (nontargeted) and 21nt-siRNA-m_Vegfa_ (targeted) reduced corneal hemangiogenesis and lymphangiogenesis compared with vehicle treatment, whereas 7-nt siRNA-Luc did not. (C) Quantification (mean ± SEM) shows significant reduction in the area of the cornea occupied by blood (CD31+ LYVE-1−) and lymphatic (CD31+ LYVE-1+) vessels in eyes treated with 21nt-siRNA-Luc or 21nt-siRNA-m_Vegfa_ compared with the vehicle control group. *, P = 0.001, n = 8 mice per group. Mann–Whitney U test. There was no difference in angiogenesis between 7-nt siRNA-Luc and vehicle control groups.
21-Nucleotide siRNAs Suppress Hindlimb Neovascularization Regardless of Target.
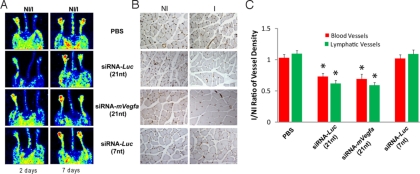
Hindlimb ischemia was induced by femoral artery ligation, and siRNAs were administered intramuscularly at the time of surgery and 2 days thereafter. Color laser doppler imaging 2 days after ligation revealed significant reduction in blood flow in the injured limbs of all experimental groups. However, by day 7, only the vehicle control and 7-nt siRNA-Luc_-injected limbs exhibited vascular rescue that was comparable to the contralateral untreated, nonischemic limb (Fig. 2A). Limbs injected with 21nt-siRNA-Luc or 21nt-siRNA-m_Vegfa exhibited suppressed revascularization and diminished perfusion. There was a corresponding reduction in CD31+ capillary density in the 21nt-siRNA-_Luc_-treated and 21nt-siRNA-m_Vegfa_-treated limbs compared with the vehicle-treated or 7-nt siRNA-_Luc_-treated groups (Fig. 2B). Quantification of hindlimb muscle blood and lymphatic vessels with CD31/LYVE-1 immunohistochemistry, represented as ischemic to nonischemic ratio, demonstrated a 29–33% reduction in hemangiogenesis and a 43–46% reduction in lymphangiogenesis with intramuscular injection of 21-nt siRNA independently of sequence or target (Fig. 2C). In contrast, 7-nt siRNA-Luc did not suppress either hemangiogenesis (99% ± 6% of vehicle treatment) or lymphangiogenesis (99% ± 6% of vehicle treatment).
Fig. 2.
The 21-nt targeted and nontargeted siRNA inhibited hindlimb ischemia-induced neovascularization. (A) Color laser doppler studies were performed at 2 and 7 days after femoral artery ligation (NI, nonischemic; I, ischemic). The blue areas denote low flow/ischemic regions, whereas red denotes normal perfusion. Representative images show that whereas animals treated with intramuscular PBS demonstrate substantial reperfusion of the limb at 7 days, those treated with 21nt-siRNA-Luc or 21nt-siRNA-m_Vegfa_ injections did not. Injection of 7-nt siRNA-Luc did not inhibit hindlimb vascular perfusion and was comparable to PBS control. (B) CD31 immunostaining of capillaries in nonischemic (NI) and ischemic (I) muscles of lower limbs revealed a robust neovascular response at 7 days in the PBS-treated ischemic hindlimb. Treatment with siRNA-Luc or 21nt-siRNA-m_Vegfa_ resulted in significantly reduced CD31 capillary staining in ischemic hindlimb muscles. The 7-nt siRNA-Luc did not exhibit this angioinhibitory effect. (C) Quantification (mean ± SEM) of blood and lymphatic capillary density, expressed as a ratio of ischemic to nonischemic hindlimb, shows that 21nt-siRNA-Luc or 21nt-siRNA-m_Vegfa_ reduced both hemangiogenesis (∗, P = 0.016, Mann–Whitney U test) and lymphangiogenesis (∗, P = 0.008) compared with vehicle treatment. n = 5 mice per group. There was no difference in angiogenesis between 7-nt siRNA-Luc and vehicle control groups.
21-Nucleotide siRNA Is Not Internalized by LECs.
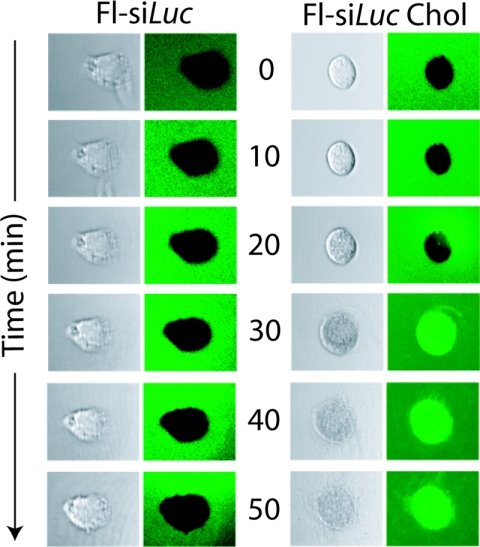
We demonstrated previously that naked, unmodified siRNA does not enter mouse or human BECs (12). Several other reports also have stressed the hurdles of cellular delivery of unmodified siRNA in vivo, given that in its naked form it does not enter mammalian cells because of its size and polyanionic structure (5–7, 25, 26). To determine whether LECs internalize naked 21-nt siRNA, we treated mouse LEC (mLEC) cultures with fluorescein-tagged siRNA-Luc that was either unmodified (Fl-siRNA-Luc) or conjugated to cholesterol (Fl-siRNA-_Luc_-chol), a previously described cell-permeating modification (25, 27). By using time-lapse confocal microscopy, we visualized the internalization of Fl-siRNA-_Luc_-chol at 20–30 min after exposure, but at no time point did we observe the cellular uptake of unmodified, naked Fl-siRNA-Luc (Fig. 3). These data imply that 21-nt siRNA executes its antilymphangiogenic activity without entering LECs.
Fig. 3.
The 21-nt siRNA requires a cell-permeating entity for internalization by LECs. mLEC cultures were exposed to fluorescein-conjugated 21nt-siRNA-Luc (Fl-si_Luc_), followed by time-lapse confocal microscopy (left column, Nomarski; right column, green channel). When Fl-si_Luc_ was conjugated with cholesterol (Fl-si_Luc_ Chol), a cell-permeant entity, there was robust internalization by mLECs. Images representative of 3 independent experiments are shown.
TLR3 Is Expressed on BECs and LECs.
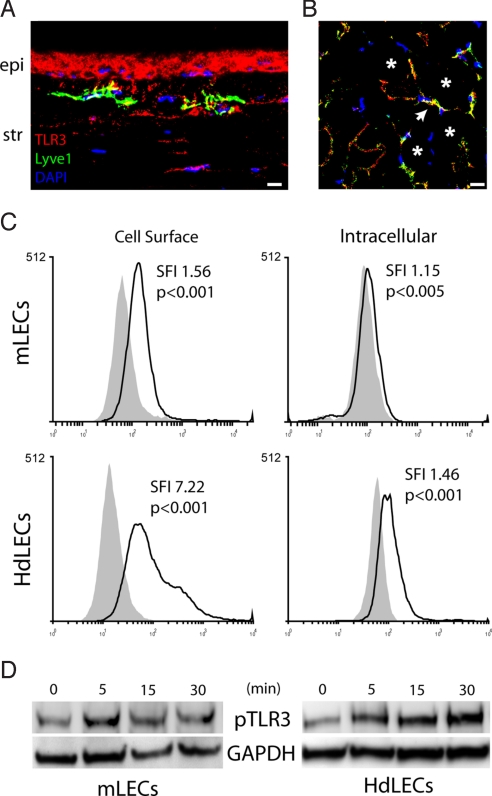
We performed immunofluorescence studies in vascularized mouse corneas and hindlimb muscles to determine whether TLR3 is expressed on LECs and BECs. LYVE-1+ lymphatic vessels in both the cornea and muscle were strongly immunoreactive for TLR3 (Fig. 4 A and B), providing in vivo confirmation of the recent finding that LECs in culture express TLR3 (28). Blood vessels in vascularized corneas and hindlimb muscle also were immunolabeled by anti-TLR3 antibodies (Fig. S1). We found by using flow cytometry that TLR3 expression was significantly more abundant on the cell surface of mLECs and human dermal LECs (HdLECs) than in the intracellular compartment (Fig. 4C), suggesting that LEC-expressed TLR3 is able to interact with 21-nt siRNA in vivo.
Fig. 4.
TLR3 expression on LECs and phosphorylation by 21-nt siRNA. (A and B) TLR3 (red) expression was visualized on LYVE-1+ (green) LECs by immunofluorescence within the stroma (str) of sutured corneas (A) and in the capillary vessels (arrow) amidst the skeletal muscle fibers (asterisk) in hindlimb muscles (B) in mice. TLR3 expression is also visible in the corneal epithelium (epi; A). Cell nuclei stain blue with DAPI. Images are representative of 5 mice. (C) Flow cytometry revealed that mLECs and HdLECs expressed significantly greater amounts of TLR3 on the cell surface than in the intracellular compartment. SFI, standardized fluorescence index (ratio of geometric means of cell populations). P values reflect statistical comparison between curves based on Kolmogorov–Smirnov tests. (D) The 21nt-siRNA-_Luc_-treated mLECs and HdLECs demonstrated a rapid increase in pTLR3 levels within 5 min of stimulation. Images representative of 3 independent experiments are shown (A–D). [Scale bar (A and B): 10 μm].
21-Nucleotide siRNA Induces TLR3 Phosphorylation and Apoptosis.
To assess whether 21-nt siRNA activated TLR3 directly, we performed Western blotting using a phospho-specific anti-TLR3 antibody. Exposure to 21nt-siRNA-Luc triggered TLR3 phosphorylation in mLEC and HdLEC cultures within 5 min after stimulation (Fig. 4D). This rapid time course of receptor phosphorylation is consistent with cell surface receptor activation, especially because we have shown that 21-nt siRNA is not internalized by these cells. Moreover, long dsRNAs, such as poly(I:C), which is internalized via scavenger receptors (7, 29), effect much slower kinetics (≈15–60 min) of phosphorylation (19, 30). The baseline levels of TLR3 phosphorylation may be due to the presence of dsRNA from cell culture senescence or low levels of constitutive TLR3 dimerization. We also found at 24 h after exposure to 21nt-siRNA-Luc a 64% ± 16% (n = 3 per group; P < 0.05 by Mann–Whitney U test) increase in apoptosis in HdLECs, as monitored by flow cytometric detection of annexin-V+/PI− cells, compared with 7-nt siRNA-Luc, which does not activate TLR3. The 21nt-siRNA-Luc treatment after corneal suture injury led to marked increases in the protein levels of CXCL10 and IL-12, proapoptotic molecules known to be induced by TLR3 activation (Fig. S2).
Discussion
The results presented here establish 21-nt siRNAs as sequence- and target-independent inhibitors of both blood and lymphatic vessels in multiple systems. Coupled with our earlier demonstration that 21-nt siRNAs inhibit hemangiogenesis in mouse models of choroidal and dermal neovascularization, as well the widespread expression of TLR3 on both vascular endothelial cells from various tissue beds (12, 28, 31, 32), it is likely that these RNA duplexes are generic inhibitors of both components of neovascularization. Such target-independent antiangiogenic activities can be serendipitous, as in the case of corneal neovascularization, which is responsible for blindness in nearly 10 million people (33) and promotes rejection of corneal allografts, the most commonly transplanted solid tissue. Conversely, 21-nt siRNAs may induce undesirable effects in the context of ischemic vascular disease or physiological cyclic angiogenesis. These concerns are particularly salient, given ongoing clinical trials of systemically delivered siRNAs.
Although it is well accepted that long dsRNAs, such as poly(I:C) or those of viral origin, bind TLR3 (14), the precise minimum length of dsRNA required to interact with and activate TLR3 has been the focus of intense investigation. More than 30 years ago, it was postulated that 20 nt of an RNA duplex comprises the minimum binding region to an unknown dsRNA receptor that drives IFN production (34). Gel filtration studies (35) and cell culture experiments (36) have shown that 21-nt dsRNAs can bind and initiate TLR3 signaling, respectively. We showed previously by using flow cytometry that binding of 21-nt siRNAs to mouse retinal and choroidal cells is eliminated by deletion of Tlr3 or competition with soluble TLR3 or poly(I:C) (12). We also showed that human choroidal BECs expressing a hypomorphic variant of TLR3 are resistant to the cytotoxic effects of 21-nt siRNAs. Further, we showed that the antihemangiogenic effects of multiple 21-nt siRNAs in mice are abolished by deletion of Tlr3 or several of its downstream mediators, or by cell surface receptor-neutralizing antibodies. In addition, our molecular modeling studies revealed a topological basis for the interaction of 21-nt siRNAs with the active TLR3 dimer. In contrast, some structural studies have reported that efficient binding of dsRNA to TLR3 requires a minimum of 40–50 nt (16, 37). The recent identification of a second binding site for dsRNA in TLR3 confirms the ability of 21-nt siRNA to bind this immune receptor (38) and helps to resolve some of the conflicting biological and structural data. Our data that 21-nt siRNA phosphorylates TLR3 demonstrate that dsRNAs of this minimum length directly activate this immune sensor.
The inability of 21-nt siRNAs to enter mammalian cells without cell-permeating moieties has been well documented (5–7, 12, 25, 26). Here, we showed by using time-lapse imaging that 21-nt siRNAs are not internalized by LECs and yet can induce rapid TLR3 phosphorylation, demonstrating that such short RNA duplexes have extracellular biological activity. We also showed that cholesterol conjugation enables 21-nt siRNAs to penetrate LECs, confirming earlier reports (25, 27) and extending our earlier findings that such modified siRNAs enter BECs (12). Although these data further affirm the viability of this cell permeation approach for potentially enabling bona fide intracellular RNAi, it should be noted that we have demonstrated that cholesterol-conjugated siRNA cannot only execute RNAi but also activate intracellular TLR3 (12). Collectively, our findings reveal a new facet of the unintended effects of siRNA that could be carefully harnessed for therapeutic advantage in angiogenesis-driven diseases that affect 30% of the world's population and that should be vigilantly monitored in ongoing clinical trials of these drugs in nonangiogenic diseases.
Materials and Methods
Animals and Reagents.
Balb/C and C57BL/6J mice were purchased from The Jackson Laboratory. For all procedures, anesthesia was achieved by i.p. injection of 50 mg/kg ketamine hydrochloride (Fort Dodge Animal Health, Wyeth) and 10 mg/kg xylazine (Phoenix Scientific). Experiments were approved by the University of Kentucky Institutional Animal Care and Use Committee board, IGB veterinarian, and conformed to European Directives no. 86/609, Italian D.L. 116 (January 27, 1992), and the Association for Research in Vision and Ophthalmology Statement on Animal Research. Nontargeted siRNAs were 21nt-siRNA-Luc (firefly luciferase) or 7-nt siRNA-Luc. Targeted 21nt-siRNA-m_Vegfa_ or vehicle buffer (PBS) was used for comparison. These sequences (5′→3′) of the sense strand of the siRNAs (Dharmacon) are as follows: 21nt-siRNA-m_Vegfa_: CGAUGAAGCCCUGGAGUGCdTdT; 21nt-siRNA-Luc: UAAGGCUAUGAAGAGAUACdTdT; 7-nt siRNA-Luc: UAAGGdTdT.
Suture-Induced Corneal Neovascularization.
siRNA (300 pmol) or vehicle buffer (PBS) was injected (33-gauge needle; Ito) at the center of the cornea at varying angles (4 injections of 2 μL each into 4 quadrants) into the corneal stroma. Delivery to the entire cornea was ascertained by verifying the spread of temporary stromal swelling throughout the entire cornea. Two interrupted 11-0 nylon sutures (Mani) were placed in Balb/C mice midway between the central corneal apex and the limbus (≈1.25 mm from the limbus). Fourteen days after suture, corneas were excised, and lymphatic and blood vessel growth was evaluated.
Corneal Flat Mounts.
After euthanasia, the corneas were isolated, washed in PBS, and fixed in acetone for 20 min at RT. They then were washed in 0.1% Tween-20 in PBS and blocked on 3% BSA in PBS for 48 h. Incubation with rabbit anti-mouse LYVE-1 antibody (1:333; Abcam) and rat anti-mouse CD31 antibody (1:50; BD Biosciences) was performed for 48 h at 4 °C. The corneas were again washed in 0.1% Tween-20 in PBS and incubated with Alexa Fluor 488-conjugated goat anti-rabbit (1:200) and Alexa Fluor 594-conjugated goat anti-rat (1:200; both from Invitrogen) for 2 h. Corneal flat mounts were visualized under fluorescent microscopy (Nikon Eclipse TE2000-E) and analyzed with ImageJ (National Institutes of Health).
Hindlimb Ischemia.
C57BL/6J mice underwent unilateral proximal right femoral artery ligation and excision distal to the deep femoral artery as described previously (39). The nonischemic left limb underwent a sham surgery without arterial ligation. Immediately after surgery and after 48 h, siRNA (1.5 nmol) or PBS was intramuscularly administered to each hindlimb. siRNAs were delivered in a volume of 35 μL (5 μL in the middle area of the tibialis anterior muscle and 2 injections of 15 μL in the middle area of the left and right lobes of the gastrocnemius muscle). Seven days later, both anterior and posterior muscles from ischemic and nonischemic hindlimbs were harvested and processed for immunohistochemical analysis. Once explanted, muscles were divided into 2 parts by a central transversal cut, and sections were prepared starting from the exposed central area versus distal and proximal sides of the muscles. Five nonserial sections of each muscle were analyzed for each animal. Capillaries were stained with anti-CD31 (BD Biosciences) or anti-LYVE-1 antibodies (Abcam) and then with biotin-labeled goat anti-rat secondary antibodies (DAKO). Capillary number was normalized to myocyte number.
Color Laser Doppler Analysis.
Color laser doppler analyses were performed 2 and 7 days after femoral artery ligation by using a dedicated Laser Doppler Perfusion Imaging System (Petimed AB) with high resolution in single mode. Hindlimbs were depilated, and mice were placed on a heating plate at 37 °C. The distance between the scanner head and tissue surface was 8 cm. An area of 5 × 5 cm was sequentially scanned, and blood flow 1 mm under the surface was measured. Color-coded images were recorded, and analyses were performed by calculating the average perfusion of the right and left distal limb. Dark blue color implied low or absent perfusion, whereas red implied maximal perfusion.
Cellular Internalization of siRNAs.
To analyze cellular internalization of siRNAs, 1,000 cells per well of mLECs (40) were seeded onto an 8-well Chamber slide (Lab-Tek II) and cultured in DMEM media containing 10% FBS for 24 h. Cells were serum-starved overnight and placed in confocal microscope incubator stage (Leica SP5), and 50 μg of either Fl-siRNA-Luc or Fl-siRNA-_Luc_-chol was added to each well. Time-lapse live images of brightfield and FITC channels were acquired simultaneously at 5-min intervals for 50 min.
TLR3 Phosphorylation Assay.
Primary HdLECs (Lonza) were cultured in EGM-2MV (Lonza) containing 5% FBS until 80–90% confluence, and they were serum-starved with basal media (MCDB131) overnight before stimulation with siRNA-Luc (10 μg/mL). mLECs were cultured in 10% FBS DMEM (Gibco) media, serum-starved with serum-free DMEM media overnight, and stimulated as above. At the indicated time points, cells were washed with ice-cold TBS containing 1 mM sodium orthovanadate and 5 mM sodium fluoride and were scraped in 100 μL of Nonidet P-40 lysis buffer (50 mM Tris, 150 mM NaCl, and 1% Nonidet P-40) supplemented with additional protease inhibitors (Roche). Equal quantities of total protein were separated by SDS/PAGE, followed by Western blotting with anti-phospho-TLR3 antibody (pY759; Imgenex). GAPDH served as control for equal loading.
TLR3 Immunofluorescence on Cornea and Muscle Lymphatic and Blood Vessels.
Mouse eyes treated with corneal suture placement for 10–14 days and uninjured hindlimb muscle (n = 3) were harvested, embedded in OCT, and snap-frozen in liquid nitrogen. Sections (10 μm) were fixed in ice-cold acetone for 10 min at 4 °C, washed in PBS, and blocked with PBS-5% normal goat serum-5% normal donkey serum for 1 h at RT. Slides were incubated with rabbit anti-mouse TLR3 (1:50; Imgenex) overnight at 4 °C, followed by washes and detection with goat anti-rabbit IgG conjugated to Alexa Fluor 594 (1:200; Molecular Probes). For labeling of lymphatic and blood endothelial cells, sections were incubated with either goat anti-mouse LYVE-1 (2 μg/mL; R&D Systems) or rat anti-mouse CD31 (1:50; clone MEC13.3; BD Biosciences) for 1 h at RT, followed by detection with donkey anti-goat IgG or goat anti-rat IgG conjugated to Alexa Fluor 488 (1:200; Molecular Probes). Slides were treated with DAPI for nuclear counterstain, coverslipped in Vectashield (Vector Laboratories), and visualized by confocal microscopy (Leica SP-5).
FACS Analysis of TLR3 on HdLECs.
Briefly, HdLECs were cultivated in EGM-2MV (omitting gentamicin, hydrocortisone; Lonza) with 10% FBS (Gibco) at 37 °C and 5% CO2. Cells (106) were harvested at 70–80% confluency with 0.04% EDTA, followed by blocking in PBS-10% mouse serum containing 0.1 mg/mL normal human IgG and 0.1% NaN3. For surface TLR3 staining, cells were incubated with phycoerythrin-conjugated anti-human TLR3 (10 μg/mL; clone TLR3.7; eBioscience) for 1 h at 4 °C. For intracellular TLR3 staining, cells were fixed and permeabilized with Leucoperm (Serotec) according to the manufacturer's instructions. Cells then were stained with conjugated primary antibody for TLR3 (eBioscience) in the presence of 10% mouse serum for 30 min at RT. Surface and intracellular controls were stained with phycoerythrin-conjugated mouse IgGκ1 isotype (BD Biosciences) at the same concentrations. Samples were resuspended in 1% PFA and analyzed on an LSRII flow cytometer (Becton Dickinson) with Flowjo (Treestar), with a minimum of 20,000 events. Kolmogorov–Smirnov statistics were used to compare differences between groups.
FACS Analysis of TLR3 on mLECs.
mLECs were cultivated in DMEM with 10% FBS at 37 °C and 5% CO2. Cells (106) were harvested at 70–80% confluency with 0.04% EDTA and were resuspended in 100 μL of PBS with 0.1% BSA and 0.01% NaN3. For surface TLR3 staining, cells were pretreated with Fc Block (10 μg/mL; BD Biosciences) for 15 min on ice and then incubated with anti-mouse TLR3 (2.5 μg/mL; clone 313129; R&D Systems) for 1 h. For intracellular TLR3 staining, cells were fixed and permeabilized with Leucoperm (Serotec) according to the manufacturer's instructions. Cells then were stained with primary antibody in the presence of 10% normal goat serum for 1 h at RT. Surface and intracellular controls were stained with rat IgG2a isotype (R&D Systems) at the same concentrations. All samples were washed 2 times in PBS and then stained with Alexa Fluor 488 goat anti-rat IgG (1:200; Invitrogen) for 1 h [on ice for surface samples and room temperature (RT) for intracellular samples]. After washing in PBS 3 times, cells were resuspended in 1% PFA and analyzed on an LSRII flow cytometer (Becton Dickinson) with Flowjo (Treestar), with a minimum of 20,000 events. Kolmogorov–Smirnov statistics were used to compare differences between groups.
In Vitro HdLEC Apoptosis Assay.
HdLECs were cultivated in EGM-2MV with 5% FBS until 70% confluence, followed by sensitization with IFN-α/β (1,000 U/mL each) for 24 h. Cells were treated either with PBS, 21nt-siRNA-Luc (10 μg/mL), or a molar equivalent of 7-nt siRNA-Luc (3.33 μg/mL) in EGM-2MV (Lonza) with 2% FBS (Gibco). After 24 h, cells were harvested with 0.05% trypsin-EDTA (Gibco), washed in PBS, and resuspended in annexin-V staining buffer (BD Biosciences) at 106 cells per milliliter. Aliquots of 100 μL then were incubated with 5 μL of FITC-conjugated annexin-V (BD Biosciences) and 5 μL of propidium iodide (PI; BD Biosciences) for 15 min at 25 °C. Cells were immediately analyzed, and annexin V+/PI− cells were calculated by using Cellquest Pro (BD Biosciences).
Measurement of Cytokines.
Lysates from sutured mouse corneas treated with either PBS or 21nt-siRNA-Luc (300 pmol) were used to quantify CXCL10 and IL-12 protein levels by fluorescent bead assay technology (Beadlyte; Millipore) per the manufacturer's instructions. Values were normalized to total protein concentrations (Bradford Assay; Bio-Rad).
Statistics.
Results are expressed as mean ± SEM, with P < 0.05 considered statistically significant. Kolmogorov–Smirnov statistics were used to compare differences between groups in flow cytometry assays. Differences between groups in other experiments were compared by using Mann–Whitney U test, and 2-tailed P values are reported.
Supplementary Material
Supporting Information
Acknowledgments.
We thank R. King, L. Xu, K. Emerson, M. McConnell, J. Ebersole, J. Stevens, V. Mercadante, and the IGB animal house staff for technical assistance, and R. Mohan, A. M. Rao, G. S. Rao, and K. Ambati for discussions. J.A. was supported by the National Eye Institute/National Institutes of Health (NEI/NIH), a Doris Duke Distinguished Clinical Scientist Award, a Burroughs Wellcome Fund Clinical Scientist Award in Translational Research, the Macula Vision Research Foundation, the E. Matilda Ziegler Foundation for the Blind, the Dr. E. Vernon Smith and Eloise C. Smith Macular Degeneration Endowed Chair, Lew R. Wassermann Merit & Physician Scientist Awards [Research to Prevent Blindness (RPB)], the American Health Assistance Foundation, and a departmental challenge grant from RPB; R.J.C.A. by RPB and Fight for Sight; M.E.K by the International Retinal Research Foundation Dr. Charles Kelman Postdoctoral Scholar Award; B.K.A. by the NEI/NIH, a Veterans' Affairs Merit Award, and the Department of Defense; and S.D.F. by Associazione Italiana Ricerca sul Cancro Grant 4840 and Telethon Italy Grant GGP08062.
Footnotes
Conflict of interest statement: J.A. is named as inventor on a patent application filed by the University of Kentucky on TLR3.
This article is a PNAS Direct Submission.
References
- 1.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 3.Fenwick A. Waterborne infectious diseases–could they be consigned to history? Science. 2006;313:1077–1081. doi: 10.1126/science.1127184. [DOI] [PubMed] [Google Scholar]
- 4.de Fougerolles A, Vornlocher HP, Maraganore J, Lieberman J. Interfering with disease: A progress report on siRNA-based therapeutics. Nat Rev Drug Discov. 2007;6:443–453. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song E, et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat Biotechnol. 2005;23:709–717. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- 6.Chiu YL, Ali A, Chu CY, Cao H, Rana TM. Visualizing a correlation between siRNA localization, cellular uptake, and RNAi in living cells. Chem Biol. 2004;11:1165–1175. doi: 10.1016/j.chembiol.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Saleh MC, et al. The endocytic pathway mediates cell entry of dsRNA to induce RNAi silencing. Nat Cell Biol. 2006;8:793–802. doi: 10.1038/ncbl439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson AL, et al. Expression profiling reveals off-target gene regulation by RNAi. Nat Biotechnol. 2003;21:635–637. doi: 10.1038/nbt831. [DOI] [PubMed] [Google Scholar]
- 9.Hornung V, et al. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med. 2005;11:263–270. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- 10.Judge AD, et al. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol. 2005;23:457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- 11.Sledz CA, Holko M, de Veer MJ, Silverman RH, Williams BR. Activation of the interferon system by short-interfering RNAs. Nat Cell Biol. 2003;5:834–839. doi: 10.1038/ncb1038. [DOI] [PubMed] [Google Scholar]
- 12.Kleinman ME, et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robbins M, et al. Misinterpreting the therapeutic effects of siRNA caused by immune stimulation. Hum Gene Ther. 2008;19:991–999. doi: 10.1089/hum.2008.131. [DOI] [PubMed] [Google Scholar]
- 14.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 15.Choe J, Kelker MS, Wilson IA. Crystal structure of human toll-like receptor 3 (TLR3) ectodomain. Science. 2005;309:581–585. doi: 10.1126/science.1115253. [DOI] [PubMed] [Google Scholar]
- 16.Leonard JN, et al. The TLR3 signaling complex forms by cooperative receptor dimerization. Proc Natl Acad Sci USA. 2008;105:258–263. doi: 10.1073/pnas.0710779105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ranjith-Kumar CT, et al. Biochemical and functional analyses of the human Toll-like receptor 3 ectodomain. J Biol Chem. 2007;282:7668–7678. doi: 10.1074/jbc.M610946200. [DOI] [PubMed] [Google Scholar]
- 18.Takada E, et al. C-terminal LRRs of human Toll-like receptor 3 control receptor dimerization and signal transmission. Mol Immunol. 2007;44:3633–3640. doi: 10.1016/j.molimm.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 19.Sarkar SN, et al. Novel roles of TLR3 tyrosine phosphorylation and PI3 kinase in double-stranded RNA signaling. Nat Struct Mol Biol. 2004;11:1060–1067. doi: 10.1038/nsmb847. [DOI] [PubMed] [Google Scholar]
- 20.Han KJ, et al. Mechanisms of the TRIF-induced interferon-stimulated response element and NF-kappaB activation and apoptosis pathways. J Biol Chem. 2004;279:15652–15661. doi: 10.1074/jbc.M311629200. [DOI] [PubMed] [Google Scholar]
- 21.Kaiser WJ, Offermann MK. Apoptosis induced by the toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. J Immunol. 2005;174:4942–4952. doi: 10.4049/jimmunol.174.8.4942. [DOI] [PubMed] [Google Scholar]
- 22.Salaun B, Coste I, Rissoan MC, Lebecque SJ, Renno T. TLR3 can directly trigger apoptosis in human cancer cells. J Immunol. 2006;176:4894–4901. doi: 10.4049/jimmunol.176.8.4894. [DOI] [PubMed] [Google Scholar]
- 23.Yang Z, et al. Toll-like receptor 3 and geographic atrophy in age-related macular degeneration. N Engl J Med. 2008;359:1456–1463. doi: 10.1056/NEJMoa0802437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrara N, Mass RD, Campa C, Kim R. Targeting VEGF-A to treat cancer and age-related macular degeneration. Annu Rev Med. 2007;58:491–504. doi: 10.1146/annurev.med.58.061705.145635. [DOI] [PubMed] [Google Scholar]
- 25.Soutschek J, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 26.Patil ML, et al. Surface-modified and internally cationic polyamidoamine dendrimers for efficient siRNA delivery. Bioconjug Chem. 2008;19:1396–1403. doi: 10.1021/bc8000722. [DOI] [PubMed] [Google Scholar]
- 27.Wolfrum C, et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol. 2007;25:1149–1157. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- 28.Pegu A, et al. Human lymphatic endothelial cells express multiple functional TLRs. J Immunol. 2008;180:3399–3405. doi: 10.4049/jimmunol.180.5.3399. [DOI] [PubMed] [Google Scholar]
- 29.Limmon GV, et al. Scavenger receptor class-A is a novel cell surface receptor for double-stranded RNA. FASEB J. 2008;22:159–167. doi: 10.1096/fj.07-8348com. [DOI] [PubMed] [Google Scholar]
- 30.Sarkar SN, Smith HL, Rowe TM, Sen GC. Double-stranded RNA signaling by Toll-like receptor 3 requires specific tyrosine residues in its cytoplasmic domain. J Biol Chem. 2003;278:4393–4396. doi: 10.1074/jbc.C200655200. [DOI] [PubMed] [Google Scholar]
- 31.Heidemann J, et al. Expression of IL-12-related molecules in human intestinal microvascular endothelial cells is regulated by TLR3. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1315–G1324. doi: 10.1152/ajpgi.00142.2007. [DOI] [PubMed] [Google Scholar]
- 32.Lundberg AM, et al. Key differences in TLR3/poly I:C signaling and cytokine induction by human primary cells: A phenomenon absent from murine cell systems. Blood. 2007;110:3245–3252. doi: 10.1182/blood-2007-02-072934. [DOI] [PubMed] [Google Scholar]
- 33.Lee P, Wang CC, Adamis AP. Ocular neovascularization: an epidemiologic review. Surv Ophthalmol. 1998;43:245–269. doi: 10.1016/s0039-6257(98)00035-6. [DOI] [PubMed] [Google Scholar]
- 34.Greene JJ, et al. Interferon induction and its dependence on the primary and secondary structure of poly(inosinic acid). poly(cytidylic acid) Biochemistry. 1978;17:4214–4220. doi: 10.1021/bi00613a016. [DOI] [PubMed] [Google Scholar]
- 35.Bell JK, et al. The molecular structure of the Toll-like receptor 3 ligand-binding domain. Proc Natl Acad Sci USA. 2005;102:10976–10980. doi: 10.1073/pnas.0505077102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kariko K, Bhuyan P, Capodici J, Weissman D. Small interfering RNAs mediate sequence-independent gene suppression and induce immune activation by signaling through toll-like receptor 3. J Immunol. 2004;172:6545–6549. doi: 10.4049/jimmunol.172.11.6545. [DOI] [PubMed] [Google Scholar]
- 37.Liu L, et al. Structural basis of toll-like receptor 3 signaling with double-stranded RNA. Science. 2008;320:379–381. doi: 10.1126/science.1155406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pirher N, Ivicak K, Pohar J, Bencina M, Jerala R. A second binding site for double-stranded RNA in TLR3 and consequences for interferon activation. Nat Struct Mol Biol. 2008;15:761–763. doi: 10.1038/nsmb.1453. [DOI] [PubMed] [Google Scholar]
- 39.Gigante B, Morlino G, Gentile MT, Persico MG, De Falco S. Plgf-/-eNos-/- mice show defective angiogenesis associated with increased oxidative stress in response to tissue ischemia. FASEB J. 2006;20:970–972. doi: 10.1096/fj.05-4481fje. [DOI] [PubMed] [Google Scholar]
- 40.Ando T, et al. Isolation and characterization of a novel mouse lymphatic endothelial cell line: SV-LEC. Lymphat Res Biol. 2005;3:105–115. doi: 10.1089/lrb.2005.3.105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information