Imprinting Disorders and Assisted Reproductive Technology (original) (raw)
. Author manuscript; available in PMC: 2009 Dec 1.
Published in final edited form as: Semin Reprod Med. 2009 Aug 26;27(5):417–428. doi: 10.1055/s-0029-1237430
Abstract
Worldwide use of assisted reproductive technology (ART) accounts for an estimated 1 to 3% of births. Since 2002, a series of reports have suggested an increased risk of imprinting disorders (Beckwith-Wiedemann syndrome and Angelman syndrome) in children conceived by ART. Definitive conclusions are difficult to substantiate due to the rarity of imprinting disorders and the variability in ART protocols. Despite these limitations, there is biological plausibility for alteration in nongenomic inheritance caused by ART. Animal studies have shown that ART procedures can alter normal imprinting, specifically DNA methylation patterns. Collectively, studies suggest an association between ART and loss of maternal methylation. More recent reports examined a possible association between ART and global hypomethylation of DNA. Three other imprinting disorders (Silver-Russell syndrome, maternal hypomethylation syndrome, and retinoblastoma) have also been implicated, but there is insufficient evidence to establish an association of these syndromes with ART. Based on current evidence, the absolute risk of imprinting disorders after ART remains small and does not warrant routine screening. Large prospective studies are needed to better understand the risks associated with imprinting disorders, imprinting defects, and ART.
Keywords: Assisted reproductive technology, ART, imprinting disorders, nongenomic inheritance, loss of maternal methylation, DNA methylation, BWS, AS
The use of assisted reproductive technologies (ART) worldwide has increased exponentially since the first report in 1978. It is estimated that slightly > 1% of total births in the United States1 and 3% of total births in developed countries result from the use of ART. In the United States between 1996 and 2005, the total number of live-birth deliveries increased more than 2.5 times from 14,507 in 1996 to 38,910 in 2005.2 As the use of ART increases worldwide, it is important to consider the possible risks involved as well as the unknown potential long-term consequences.
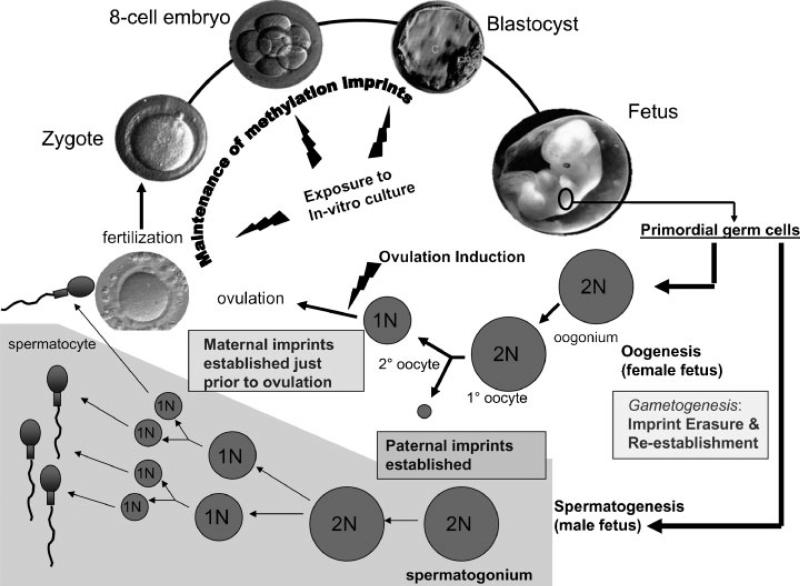
Starting in 2002, a series of reports raised concern that there might be an increased incidence of imprinting disorders in children conceived by ART. Animal studies have demonstrated biological plausibility for an association between ART and alteration in nongenomic inheritance, lending credence to the possibility that ART might alter nongenomic inheritance in humans. Genomic imprinting is an epigenetic process that allows for some genes to be expressed from only one parental allele while silencing the other parental allele. Two important regulators of imprinted gene expression are DNA methylation and histone modification. Most studies have focused on the effect of ART on DNA methylation because it is one of the best studied DNA modifications, and there is little current evidence to suggest an effect of ART on histone modification.3 Many imprinted genes are clustered together with common regulatory mechanisms such as differentially methylated regions (DMRs) where methylation occurs at CpG-rich regions of DNA, typically on the nonexpressed allele of the imprinted gene. Epigenetic reprogramming of DNA methylation patterns critical for establishing and maintaining imprinting occurs during germ cell development and preimplantation development,4 which are two developmental stages potentially vulnerable to ART5 (Fig. 1).
Figure 1.
Assisted reproductive technology (ART) procedures occur during developmental periods when genomic imprints have been shown to be vulnerable in animal studies. Gametogenesis in both males and females represents a critical stage because genomic imprints are erased and reestablished in developing gametes. The paternal allele (male gamete) imprints are established much earlier during gametogenesis than the maternal allele (female gamete) imprints, making it more likely that ART (especially ovulation induction) would affect maternal allele imprints. Postfertilization, there is a second critical time during which there is global demethylation of nonimprinted sequences with maintenance of imprinted sequences. ART procedures expose the developing embryo to in vitro culture and manipulation during this time of methylation maintenance.
Most evidence related to ART suggests altered methylation status of the maternal allele. Regarding germ cell development, ART is unlikely to cause defects of the paternal allele (male gamete) imprints because they are established earlier during gametogenesis than the maternal allele (female gamete) imprints (for review, see Manipalviratn et al, 20096). Specifically, studies have demonstrated completed establishment of paternal imprints as early as the diploid spermatogonium,7 and at least one study has demonstrated normal imprinting in spermatogonia selected from seminiferous tubules of infertile men.8 In contrast, animals studies of epigenetic reprogramming in the oocyte suggest that imprints are established in growing oocytes during the primordial to antral follicle transition9,10 and are not completed for some genes until just prior to ovulation. For this reason, maternal imprinting mechanisms could be vulnerable to ovarian stimulation (Fig. 1). In addition, at least one report11 has suggested that the maternal genome may be more vulnerable to imprinting and methylation defects than the paternal genome during preimplantation development (postfertilization) when embryos are exposed to in vitro culture media.
Because of these concerns, it is important to scrutinize the strength of current evidence regarding the association between ART and imprinting. In general, rigorous analysis of the issue is complicated by the variability in ART protocols and the rarity of imprinting disorders. Some of the factors that deserve scrutiny are the lack of appropriate control groups, potential for ascertainment and recall bias, inadequate control of confounding factors, and low statistical power resulting from inappropriate sample size.12 In addition, many reports are retrospective observational studies that could be subject to bias and imprecision of timing of correlated exposures but are notably more efficient for rare outcomes.13 In this article, we summarize the current evidence concerning an association between ART and imprinting disorders including Beckwith-Wiedemann syndrome (BWS), Angelman syndrome (AS), Silver-Russell syndrome (SRS), maternal hypomethylation syndrome, and retinoblastoma.
BECKWITH-WIEDEMANN SYNDROME
BWS (MIM 130650) is a heterogeneous congenital overgrowth syndrome with an estimated incidence of 1 in 13,700 live births.14 Some of the more frequently occurring clinical features include macroglossia, abdominal wall defects, macrosomia, neonatal hypoglycemia, nephromegaly, and other developmental anomalies.15 In addition, patients with BWS have an increased risk of developing embryonal tumors (7.5%) including Wilms tumor, adrenocortical carcinoma, hepatoblastoma, and rhabdomyosarcoma.16,17
BWS is a multigenic disorder resulting from genetic or epigenetic alterations within two imprinted domains of growth regulatory genes on chromosome 11p15.18 The majority of BWS cases (85%) occur sporadically, and there is evidence to support the possibility of regulatory interactions between the two imprinted domains.18 Domain 1 includes the imprinting center DMR1, which regulates two genes insulinlike growth factor 2 (IGF2) and H19.19 Normally, there is maternal expression of H19 and paternal expression of IGF2.Accounting for a small percentage of BWS cases is the so-called _H19_-dependent IGF2 biallelic expression where gain of methylation at the maternal H19 and DMR1 will lead to loss of H19 expression and biallelic IGF2 expression.19 Domain 2 contains the imprinting center KvDMR1 (also called KvDMR or DMR2) and certain genes relevant to BWS etiology including KCNQ1OT1 (also known as LIT1), KCNQ1, and CDKN1C. Maternal methylation of KvDMR1 normally silences KCNQ1OT1 on the maternal allele so that the gene is only expressed from the paternal allele.19 The most common molecular subgroup (50 to 60% of sporadic BWS cases) exhibits loss of maternal methylation of KvDMR1.19 Loss of methylation of KvDMR1 is associated with biallelic expression of KCNQ1OT119 and reduced expression of CDKN1C,a tumorsuppressorand apotential negative regulatorof fetal growth.19 Early studies examining the increased incidence of female monozygotic twins among patients with BWS revealed that most cases of monozygotic twins were discordant for BWS and possessed an imprinting defect at KCNQ1OT1 on chromosome 11p15.20,21 Because monozygotic twinning is known to occur at gestational days 8 to 10, these findings led to the hypothesis that KCNQ1OT1 was especially vulnerable to loss of imprinting arising from deficient maintenance of DNA methylation during a critical stage of preimplantation development.21
In 2003, three case series from BWS registries in the United States and Europe reported an increased proportion of individuals with BWS after ART compared with the accepted frequency of ART use in the general population. DeBaun et al22 published a case report of seven children with sporadic BWS born after ART (two in vitro fertilization [IVF] and five intracytoplasmic injection [ICSI]). Four BWS cases after ART were identified from the National Cancer Institute (NCI) BWS Registry, but this older questionnaire had not specifically asked about ART procedures. The other three BWS cases after ART were identified from the more recently established Washington University BWS Registry, which started in 2001 and systematically inquired about ART procedures on an updated version of the NCI BWS registry questionnaire.22 Using only the three BWS cases after ART that were part of the systematic assessment for ART procedures, the authors estimated the prevalence of ART in the BWS registry to be 4.6% (3 of 65).22 The authors compared this prevalence to the most recent data that were available from the Centers for Disease Control and Prevention (CDC) in 1999 of 0.76% total live births after ART in the general population, and thus estimated a sixfold increase in BWS in children born after ART.22 Samples from six of the seven affected children were tested for the molecular basis of their BWS. Five of the six patients tested had imprinting defects (Table 1), specifically hypomethylation of the LIT1 differentially methylated region (also known as KvDMR1).22 In addition to this defect, one of the six patients also had hypermethylation of the H19 DMR (also known as DMR1).22 Because CDC data for ART usage for 2000 to 2002 was subsequently reported to range from 0.9% to > 1%,23–25 the authors’ estimate of the increase in BWS in children born after ART might be slightly high.
Table 1.
Molecular Mechanism for Beckwith-Wiedemann Syndrome after Assisted Reproductive Technology
| Citation | No. of BWS Pts Post-ART | No. of BWS Pts Post-ART Tested | No. of BWS Pts Post-ART with LOM* | Percentage of BWS Pts Post-ART Tested with LOM | Percentage of Total BWS Pts Post-ART with LOM |
|---|---|---|---|---|---|
| DeBaun et al22 | 7 | 6 | 5 | 83.3 | 71.4 |
| Maher et al26 | 6 | 2 | 2 | 100.0 | 33.3 |
| Gicquel et al27 | 6 | 6 | 6 | 100.0 | 100.0 |
| Halliday et al28 | 4 | 3 | 3 | 100.0 | 75.0 |
| Sutcliffe et al32 | 11 | 8 | 8 | 100.0 | 72.7 |
| Bowdin et al33 | 1 | 1 | 1 | 100.0 | 100.0 |
| Lim et al36 | 25 | 25 | 24 | 96.0 | 96.0 |
| Average percentages | 97.0 | 78.3 | |||
| Estimated percentage of BWS cases caused by LOM in the general BWS population | 50–60 |
A case report from the United Kingdom by Maher et al26 identified six cases of BWS after ART (three IVF and three ICSI) in 149 BWS patients who had been referred for uniparental disomy analysis at the BWS Research Group at the Birmingham University Section of Medical Genetics and/or West Midlands Molecular Genetics Service. Assuming an overall frequency of 0.997% of births after IVF or ICSI in the general UK population from 1995 to 2000, the authors concluded that identification of 6 BWS patients after IVF or ICSI of the 149 was significantly greater than the expected 1.73 patients.26 The authors measured the excess risk of a child being affected by BWS following IVF or ICSI to fall within a 95% confidence interval (CI) of 1.5 to 8.8.26 Four of the six BWS patients were tested and found negative for uniparental disomy. Two of the six cases (one IVF and one ICSI) were tested, and both were found positive for loss of maternal methylation of KvDMR126 (Table 1). One of the IVF cases, for whom the genetic results were not available, occurred in twin 2 of a pair of female monozygotic twins discordant for BWS.26 Female monozygotic twinning with discordance for BWS could be considered a confounding factor in this case because it is known that BWS occurs with greater frequency in monozygotic twins independent of ART.
The third case-report,27 published in 2003, was from a reference center in France for the molecular diagnosis of BWS and reported 6 cases of BWS following ART (4 IVF, 2 ICSI, 1 frozen embryo transfer, and embryo transfer days varying from 2 to 5 days) of 149 BWS patients. All six cases of BWS after ART showed isolated loss of methylation of KvDMR1 with a demethylation index varying from 72 to 100%.27 The investigators estimated the prevalence of ART in their BWS population (4%) to be three times higher than the prevalence of ART in the general French population (1.3%).27 Based on these data, the investigators estimated an odds ratio (OR) of 3.2 (95% CI, 1.4 to 7.3) for exposure to ART and risk of BWS.27 They found no association between specific ART procedures and the risk of BWS.
Following these three case series in 2003,22,26,27 a case-control study28 examined the rate of IVF in BWS cases and in the non-BWS controls in the population of the state of Victoria in Australia. All cases of BWS diagnosed by a clinical geneticist from 1983 to 2003 in Victoria were identified. For each BWS case, four liveborn controls were randomly selected from singletons born within 1 month of the index case and in which the maternal age was within 12 months of the index case.28 ART was identified in 4 of 37 cases of BWS (3 IVF, 1 ICSI) (10.81%) and in 1 of 148 matched controls (0.67%) leading to a measured OR of 17.8 (95% CI, 1.8 to 432.9) and a p value equal to 0.006.28 The average maternal age for BWS cases was 27.0 years.28 Three of the four patients with BWS after IVF were tested, and all three were found to have isolated loss of maternal methylation (Table 1) at the KVDMR1/LIT1 locus of 11p15.5.28 In contrast, this molecular mechanism was observed in 46% of the Victorian BWS population overall.28 Additionally, the authors estimated the absolute risk of BWS in the IVF population to be 1 in 4000 live births, which was nine times greater than the risk of BWS in the general population.28
In 2005, Chang et al29 reexamined the charts of the BWS patients in the combined NCI BWS Registry and the Washington University BWS Registry as DeBaun et al22 had done in 2003. In this study,29 questionnaires completed by parents from the original NCI BWS registry (n = 262) from 1994 to 2001 were reviewed. Notably, this older questionnaire did not include specific questions about ART. Based on positive responses to ART questions on the newer BWS questionnaire (used starting in 2001), investigators secured reproductive endocrine records for all women who had possibly undergone ovarian stimulation. The authors of this study broadly defined ART to include artificial insemination and ovulation induction. (Although the CDC definition limits ART to procedures that involve the manipulation of both oocytes and sperm in the laboratory, there is biological plausibility forthe inclusionofatleastovulation induction because, as suggested earlier, this procedure could potentially interfere with the establishment of imprinting in the oocyte during its primordial to antral follicle transition,9,10 and even up the time of ovulation.) Using this broad definition of ART, Chang et al identified 19 BWS patients born after ART from the BWS registry (n = 341).29 There were five twins of unknown zygosity in the group of 19 BWS patients born after ART. The authors29 successfully obtained records for 12 of the 19 patients identified with BWS after ART showing that 5 were associated with IVF, 5 with ICSI, and 2 with ovarian stimulation alone (one gonadotropin followed by intrauterine insemination and the other clomiphene citrate followed by artificial insemination). The average age of mothers of children with BWS born after ART in their studygroup was 35.2 years compared with 29.4 years for mothers of children with BWS conceived naturally.29 Instead of estimating a fold increase in the risk of BWS after ART, the authors sought to identify positive associations between infertility/ART procedures and the risk of BWS. However, the authors were not able to identify any association between BWS and the cause for infertility, the number or timing of embryos transferred, the type of culture media used, or the type of ART used.29 The only common feature among the identified cases was the use of ovarian stimulation medication (1 clomiphene citrate and 11 gonadotropin).29 Although the authors could not exclude the possibility of an association between imprinting disorders and infertility, they interpreted these data to indicate that manipulation of oocyte maturation or folliculogenesis might result in altered timing of imprinted genes.29
A Danish National Cohort study,30 designed to compare the incidence of imprinting diseases in singletons born after IVF to the incidence in naturally conceived children, tracked all singletons that were born in Denmark from January 1, 1995, to December 31, 2001, using the Birth Registry. The investigators linked these data to the IVF Registry to identify the number of singletons born after IVF. The authors were able to identify 442,349 naturally conceived children with a mean follow-up of 4.5 years and 6052 children born after IVF (of these 1680 after ICSI) for whom the mean follow-up was 4.1 years.30 Using the patient's personal identification numbers in the National Register of Patients and the National Register of Psychiatric Diseases, the authors searched for specific diagnostic codes for known imprinting diseases (with the notable exception of AS) as well as codes for diseases with clinical symptoms that might be associated with imprinting disorders in children who had not been specifically diagnosed with an imprinting disorder. Because 54 children in the naturally conceived cohort were identified with imprinting disorders (0 BWS, 44 kidney cancer, 5 retinoblastoma, 3 Prader-Willi syndrome, and 2 SRS), and assuming the same frequency in IVF children, the authors expected to find 0.74 children born after IVF with imprinting disorders.30 However, the authors identified no children with imprinting disorders in the IVF cohort,30 and they concluded there was therefore no increased risk of imprinting disorders in children born after IVF.
Another large population-based study31 was designed to assess the risk of congenital malformations after different IVF procedures and included all children born after IVF from 1982 to 2001 based on the records of all IVF clinics in Sweden. To ascertain the presence of congenital malformations in these children, diagnostic codes were linked from the Swedish Medical Birth Register, the Swedish Registry of Congenital Malformations, and the Swedish Hospital Discharge Register.31 The authors identified 16,280 children born after IVF, of whom 811 had a diagnosis of congenital malformation, but there were no identified cases of BWS after IVF.31
In a British survey published in 2006,32 conception history-specific questionnaires were sent to the families of 213 children known to have BWS. The investigators received 83 replies (39% response rate) with 79 being identified as sporadic cases, and of those, 11 patients with BWS (14%) were conceived after ART (1 IVF, 5 ICSI, and 5 fertility drugs only).32 To adjust for a possible biased response rate, the authors assumed that all nonresponders (n = 209 including nonresponders from BWS, AS, and Prader-Willi syndrome groups) were natural conceptions giving a minimum of 5% of patients with BWS who were conceived by ART procedures.32 More specifically, the authors estimated that 2.9% of BWS patients (6 of 209) were conceived by IVF/ ICSI (95% CI, 1.4 to 6.3%) and compared this value to the estimated incidence of IVF/ICSI in the general UK population of 0.8%, thus reporting a significantly higher frequency of IVF/ICSI than in the general population.32 Molecular analysis was performed on eight of the BWS cases after ART, and 100% of children tested were found to have loss of maternal methylation at the KvDMR1 (also known as DMR2) imprinting control region,32 which is the greater than the expected 50 to 60% of sporadic BWS patients with this epigenetic change.19 Given the results of the survey, the authors32 concluded there was an association between ART and BWS, but they noted that the absolute frequency of BWS after ART was < 1%.
A questionnaire-based cohort study33 included the families of 2492 children born after IVF in the Republic of Ireland (from 1989 to 2002) and central England (from 1997 to 2003) who were sent screening questionnaires designed to detect cases of BWS both clinically diagnosed and previously unrecognized. With a response rate of 61%, data for 1524 children were reviewed,33 and 47 of 70 children (67%) accepted and were given a detailed clinical assessment.33 One case of BWS was identified in this cohort, and molecular analysis confirmed loss of methylation at KvDMR1.33 The data from this survey suggested the prevalence of BWS after ART to be 1 in 152433 compared with a prevalence of 1 in 14,500 for BWS in the general population, suggesting an increased relative risk of BWS in children born after ART.
A nationwide survey34 performed in the Netherlands queried families of children with BWS born between January 1, 1983, and December 31, 2003. Families were identified from BWS support groups and given questionnaires regarding infertility and etiology of BWS (confirmed with records from the Dutch Diagnostic Molecular Genetics Laboratories). In this study, the authors34 broadly defined ART to include the use of fertility drugs to induce ovarian stimulation and ovulation, intrauterine insemination, and donor insemination. There was a response rate of 54.3% (n = 75), but only 71 cases of BWS were eligible for the study. The authors identified six cases of BWS after ART of any kind (four IVF/ICSI, one IUI with donor sperm, and one ovarian stimulation).34 With four cases of BWS after IVF, the authors estimated a prevalence of 5.6% for IVF in BWS cases compared with 0.92% in the Dutch population giving a relative risk of 6.1.34 All six cases of BWS after ART demonstrated hypomethylation of KCNQ1OT1 as the underlying molecular mechanism of disease.34 There were 15 cases of BWS after fertility problems of any kind (11.1% versus 5.9% in Dutch population; relative risk (RR) 2.0).34 The average maternal age was 30.59 years (p = 0.03) versus the Dutch population average maternal age of 29.68 years.34 Because the RR (3.0) of imprinting disorders (BWS, AS, and Prader-Willi syndrome) after infertility resembled that in the general population, the authors concluded that after correction for impaired infertility there was no increased incidence of BWS, AS, or Prader-Willi syndrome attributable to ART.
Noting that most epimutations implicated in the various imprinting disorders following ART involved loss of methylation at maternal alleles, Rossignol et al35 hypothesized there should be imprinting defects at other loci in children after ART. The investigators analyzed the methylation patterns of four imprinted genes in 40 patients with BWS who had loss of methylation of the maternal allele at KvDMR1. Eleven of the 40 were post-ART (8 IVF, 3 ICSI, 1 cryopreserved embryo, varying numbers of days before embryo transfer), and 29 of the 40 were naturally conceived (4 monozygotic twins with no mention of discordance).35 Hypomethylation of at least one locus other than KvDMR1 was demonstrated in 3 of 11 (27%) post-ART BWS cases, and 7 of 29 (24%) naturally conceived BWS cases.35 Of note, the authors reported no significant phenotypic difference between BWS cases with and without additional hypomethylation. Given the similar rates of hypomethylation in the two groups, the authors concluded that ART was not specifically associated with loss of methylation at various imprinted loci.35
A recently published report by Lim et al36 compared the clinical phenotype and molecular features of 25 post-ART (12 IVF and 13 ICSI) children with BWS to 87 non-ART children with BWS who had KvDMR1 loss of methylation. Twenty-four of the 25 post-ART children with BWS had KvDMR1 loss of methylation, and the remaining case had no identifiable molecular cause including methylation error.36 Because KvDMR1 loss of methylation is estimated to account for 50 to 60% of sporadic BWS cases,19 the authors concluded that 24 of 25 (96%) was a significantly increased proportion.36 There was no statistical difference (p = 0.6) for mean methylation index in the two groups, which was 4.6% for post-ART BWS and 7.6% for non-ART BWS cases.36 Analysis of the methylation status of three additional DMRs revealed an additional loss of methylation of 37.5% (3 of 8) in the post-ART group compared with 6.4% (3 of 47) in the non-ART group (p = 0.034).36 For comparison, all 4 DMRs were analyzed in 20 normal controls in whom no methylation abnormalities were detected.36 The numbers were small, but the authors detected no significant differences in phenotype between non-ART BWS patients with additional DMR hypomethylation (3 of 47) and those without (44 of 47).36 The authors of this study36 concluded there was an increased loss of methylation in children with BWS born after ART. Despite not observing a phenotypic difference with the additional loss of methylation in the larger non-ART group, the authors postulated there might be variable combinations of epigenetic alterations at imprinted DMRs leading to some cases of abnormal growth and development in children after ART.36
As noted earlier, the estimated prevalence of ART in BWS populations ranged from 2.9 to 5.6% as reported from four case series reports22,26,27,29 and two survey studies32,34 where the families of BWS children were questioned about ART. The only case-control study to date28 showed the estimated prevalence of ART in BWS populations to be as high as 10.81%. Despite limitations of study design in some of these reports, these reports have suggested an association between ART and BWS. In contrast, two national cohort studies30,31 failed to show association between ART and BWS after no cases of BWS after ART were identified. Regarding the absolute risk of BWS after ART, two of the studies28,33 have reported an absolute risk of < 1% of BWS after ART, suggesting that routine screening of BWS in children born after ART is not warranted. Regarding the molecular etiology of BWS, several reports have substantiated an altered maternal imprint in children with BWS who were born after ART. Maternal loss of methylation at KvDMR1 is estimated to account for 50 to 60% of sporadic BWS cases.19 However, the studies reviewed here reported that between 83.3% and 100% (Table 1) of patients with BWS after ART tested for molecular etiology were found to have maternal loss of methylation at KvDMR1. Although there seems to be an increased rate of loss of methylation in BWS children born after ART, the only two available studies35,36 provide conflicting information about the possible association between ART and a more global demethylation pattern. Although there have been limitations in the studies; taken together, current evidence suggests an association between ART and BWS.
ANGELMAN SYNDROME
AS (MIM 105830) is a rare neurogenetic syndrome with an estimated prevalence among children and young adults of between 1 in 10,000 and 1 in 20,000.37 Classic features of the syndrome include severe developmental delay, frequent laughter, unsteady gait, and a seizure disorder.38 AS is a classic example of genetic imprinting because the syndrome results from loss of maternal expression of the gene UBE3A located at chromosome 15q11–1339,40 known to be imprinted in the brain.41 The four genetic mechanisms known to cause AS are deletion of maternal UBE3A (68% of cases), intragenic UBE3A mutation on maternal allele (13% of cases), uniparental disomy (3% of cases), and imprinting defect turning off the maternal allele (6% of cases).37
In 2002, Cox et al42 published a report suggesting that ICSI may increase the risk of imprinting defects. The authors reported two cases of AS post-ICSI secondary to male infertility. Molecular analysis by methylation-specific PCR and Southern blotting with the SNRPN probe demonstrated that both cases showed hypomethylation of the SNRPN locus,42 a sporadic imprinting defect on the maternal chromosome. Additionally, the investigators ruled out mutation at the AS imprinting center in both cases. Given the low proportion of AS cases in the general population attributed to imprinting defects (estimates range from ~4 to 6%), the incidence of AS with an imprinting defect as the molecular etiology was approximated by the authors to be ~1 in 300,000.42 Thus identification of two of these cases raised concerns for an association between the use of ISCI and AS caused by imprinting defects.
In 2003, Østavik et al43 reported a third case of a girl with AS following conception by ICSI. In contrast to the previous two case reports,42 the biological father had normal sperm analyses on multiple occasions.43 However, both the mother and the maternal grandmother suffered from reproductive difficulties leading to spontaneous abortions and a stillbirth, suggesting a defect of oogenesis was possible in this case.43 Again, molecular analysis was performed using methylation-specific PCR and Southern blotting with the SNRPN probe that demonstrated absence of the normally methylated maternal band.43 A mutation at the AS imprinting center was unlikely based on molecular analysis and both parents showed normal chromosomes and normal methylation patterns,43 making this the third case of AS post-ICSI attributable to a sporadic imprinting defect.
In 2005, a cohort study44 in Germany investigated the association between infertility treatment and imprinting defects leading to AS. The investigators44 sent questionnaires pertaining to method of conception and time to pregnancy (TTP) to all 270 members of the German Angelman Syndrome Support group and received 79 valid questionnaires (30% response rate). If infertility/subfertility (infertility treatment of any kind or TTP > 2 years) was reported by the families, the authors requested a blood sample or buccal smear from the AS patient as well as the parents. Sixteen children with AS were identified as having been born to couples with subfertility (20%).44 Four of these 16 children were identified as having a sporadic imprinting defect as the molecular cause of AS (1 ICSI after TTP > 2 years, 1 hormone therapy after TTP > 2 years, 2 TTP > 2 years without treatment).44 Assuming that roughly 4% of AS cases are caused by imprinting defects, the investigators found that the relative risk of having an AS child with an imprinting defect increased by the same factor of 6.25 (95% CI, 0.70 to 22.57) for untreated couples with TTP > 2 years and also for couples who were treated with either ICSI or hormone therapy alone,44 although the increase was not statistically significant. Couples with TTP > 2 years who received therapy were estimated to have a significantly increased relative risk of 12.50 (95% CI, 1.40 to 45.13).44 Of note, molecular analysis showed that one of the three AS children post-ICSI had an imprinting defect as the underlying molecular mechanism, and the other two AS children post-ICSI had the most common molecular mechanism of AS, namely deletion of the UBE3A gene.44 The authors concluded that although the absolute risk remains small, subfertile couples have an increased relative risk of conceiving a child with AS with an imprinting defect, and that the addition of therapy (hormone therapy, ICSI, or both) further increased the risk.44 These results led the authors to postulate there may be a common cause for subfertility and imprinting defects and also that superovulation, instead of ICSI, might increase the risk of imprinting defects.44
In the previously mentioned British survey published by Sutcliffe et al,32 conception history specific questionnaires were sent to the families of 384 children known to have AS. The investigators identified 75 sporadic cases of AS of 81 replies (21% response rate). Of the 75 sporadic cases, 3 cases of AS (4%) were conceived after ART (1 artificial insemination by donor, 1 intrauterine insemination by donor, 1 mother had previously used IVF).32 Molecular analysis was performed on all three of the AS cases conceived by ART. One AS child who was conceived by intrauterine insemination by donor and whose parents had previously used IVF demonstrated loss of maternal methylation at the SNRPN imprinting control region.32 If the mother had previously used IVF, then presumably her ovaries had been stimulated, which raises the question of an association between ovulation induction and imprinting defects leading to AS. The two remaining cases of AS post-ART (it was not clear which ART procedures were used) exhibited deletion of the maternal UBE3A gene,32 the most common molecular mechanism leading to AS. The authors32 concluded that given their results and the results of previous studies, there appeared to be an association between ART conception and AS cases caused by loss of maternal allele methylation.
The previously referenced nationwide survey in the Netherlands by Doornbos et al34 included 135 families of AS children who had been born between January 1, 1983, and December 31, 2003. These children, identified from AS support groups, were sent questionnaires relating to conception and AS molecular etiology.34 The authors identified four cases of AS after ART (6.3% versus 2.1% in the Dutch population; RR = 3.0) of any kind. The investigators identified no cases of AS after IVF/ICSI, one case after intrauterine insemination, and three cases of AS after ovulation induction alone (4.8% versus 0.39% in the Dutch population; RR 12.3).34 Thus the authors noted a significant association between risk of AS and ovulation induction,34 a finding that supported the earlier report by Ludwig et al.44 In addition, there were 12 cases of AS born to families with fertility problems of any kind (19% versus 5.9% in the Dutch population; RR = 3.4).34 These results again supported the finding by Ludwig et al,44 who reported a 20% incidence of subfertility in the AS study group. Additionally, the average maternal age of the AS study group was significantly increased at 30.64 years versus the Dutch population average maternal age of 29.68 years.34 Two of the four AS cases after ART exhibited deletion of the maternal UBE3A gene, one case had the etiology confirmed but was not specified, and the molecular analysis of the fourth case was not provided. Thus no conclusion based on molecular analysis could be drawn. The authors34 concluded that after correction for subfertility, there was no increased relative risk of imprinting disorders (including AS) after ART.
In the previously mentioned questionnaire-based cohort study by Bowdin et al,33 the families of 2492 children born after IVF in the Republic of Ireland (from 1989 to 2002) and central England (from 1997 to 2003) were sent screening questionnaires designed to detect cases of AS both clinically diagnosed and previously unrecognized. In a cohort of 1524 children, no cases of AS were detected.33 Considering the relatively small cohort size and the exceptional rarity of AS arising from an imprinting defect estimated by Bowdin et al33 to be 1 in 750,000 (previously estimated by Cox et al42 to be 1 in 300,000), the authors concluded there was an 85% chance that the study would not find a 50-fold increased risk of AS after ART.33
A study by Manning et al45 prospectively analyzed blood samples from 92 children born after ICSI. The investigators focused on DNA methylation defects in the chromosome 15q11–13 region, which is known to be associated with AS and Prader-Willi syndrome. Using sodium bisulphate treatment followed by methylationspecific polymerase chain reaction, the investigators found that in all 92 children the methylation patterns at the 15q11–13 region were identical to normal controls.45 In 2008, a retrospective study46 examined various outcomes from ICSI and analyzed the methylation patterns by multiplex PCR of 53 samples from children post-ICSI. The authors found no abnormal patterns at the 15q11–13 region.46 Although neither study reported an increased risk of methylation defect associated with ICSI, the sample size of both of these studies may have been insufficient to detect an increased prevalence of imprinting defects following ICSI given the relative rarity of imprinting abnormalities causing AS.
Combining the incidence of AS caused by an imprinting defect (1 in 300,000)42 with the relative infrequency of ART use in the general population (1 to 3%), the finding of five reported cases of AS caused by imprinting defects in children born after ART42–44 is suggestive but not conclusive for an association between ART and AS due to an imprinting defect. Furthermore, an association with ICSI has been proposed because four of these five AS cases with imprinting defect were conceived by ICSI.42–44 However, it is possible that superovulation rather than ICSI may increase the risk of imprinting defects44 because all ICSI patients presumably receive ovulation induction, and there have been cases of AS caused by imprinting defects occurring after ovulation induction alone. Two studies have also suggested a tentative association between subfertility and AS with the identification of two cases of AS caused by imprinting defects in patients whose parents had taken > 2 years to become pregnant44 and one case of AS caused by an imprinting defect in a child who was conceived by parents who used intrauterine insemination and who had previously used IVF.32 In the Dutch survey,34 there were four cases of AS following TTP > 1 year without the use of infertility therapy, but the molecular cause of AS was not documented in any of these cases. Taken together, the strength of current evidence for an association of AS caused by imprinting defect with ART is more tenuous than that for BWS.
OTHER IMPRINTING DISORDERS WITH A POSSIBLE ASSOCIATION TO ART
Silver-Russell Syndrome
SRS (MIM 180860) is a rare disorder with an estimated incidence of 1 in 100,000 live births.47 SRS is a clinically heterogeneous disorder associated with intrauterine growth retardation, failure to thrive, characteristic triangular facies, clinodactyly, and genitourinary malformations. SRS has a complex genetic etiology usually involving chromosome 7 or 11 with multiple candidate imprinted genes on chromosome 7 and one elucidated molecular mechanism being methylation defects of H19 imprinted domains on chromosome 11.48 Two Swedish reports31,49 published in 2005 identified two cases of SRS after ICSI, which given the rarity of the syndrome combined with the relative rarity of ICSI was suggestive of an increased risk of SRS following ICSI. Unfortunately, no molecular analysis was available on either patient. In 2006, Bliek et al50 reported the third case of SRS associated with ICSI. Molecular analysis was performed of this third case and showed hypomethylation of the H19 promoter. A fourth case has been described more recently51 of a twin girl with SRS conceived by IVF demonstrating partial hypermethylation at the examined DMR of PEG1/MEST and mild hypermethylation in her father. Of note, the authors stated it was unknown if the partial hypermethylation was related to the SRS phenotype in this patient.51 Given the rarity of this syndrome and the one report of hypomethylation at H19, these reports taken together raised suspicion but are inconclusive regarding an association between ART (specifically ICSI) and an increased incidence of SRS.
Maternal Hypomethylation Syndrome
In 2006, Mackay et al52 proposed a novel syndrome called “maternal hypomethylation syndrome” in a cohort of transient neonatal diabetes patients whose underlying molecular etiology of disease was loss of methylation. Although the use of ART was not specifically mentioned in the study, the authors did report that two mothers were known to have experienced a period of infertility and that the maternal age was > 35 years in three cases.52 Two studies35,53 have examined a maternal hypomethylation syndrome in association with ART. Neither has confirmed an association between ART and the finding of hypomethylation at multiple imprinted loci. As described earlier, Rossignol et al35 found similar percentages of BWS patients both post-ART (27%) and naturally conceived (24%) who had hypomethylation at multiple loci. The investigators were not able to show an association between hypomethylation and any specific ART procedure.35 These results indicated that ART was not specifically involved in loss of methylation at multiple loci. In a cohort of 149 BWS patients of whom 81 had loss of maternal methylation at KCNQ1OT1, Bliek et al53 found 17 patients with hypomethylation at multiple maternally methylated loci. Of the 17, 1 patient was born after IVF and a second patient, who was a twin, was conceived after the use of unspecified hormone treatment for infertility.53 Including only the IVF patient in their analysis of RR of maternal hypomethylation syndrome after ART, the authors calculated an insignificant p value when comparing the incidence of IVF associated with BWS cases of multiple loss of methylation (0.06) to the incidence of IVF in BWS cases with isolated loss of KCNQ1OT1 methylation (0.09).53 If the authors had broadly defined ART to include the patient conceived after hormone therapy, it is uncertain how this might have impacted the RR of maternal hypomethylation syndrome following ART. The authors concluded that ART can be excluded as a cause of loss of methylation at various maternal loci because neither Rossignol et al35 nor their own study53 demonstrated an increased frequency of ART-associated cases. Although the current evidence is limited, there is no suggestion of an association between ART and a maternal hypomethylation syndrome.
Retinoblastoma
Retinoblastoma is a rare childhood tumor of the retina occurring in both a familial (40%) and sporadic form (60%) with an average incidence of 1 per 17,000 live births.54 Tumorigenesis results from inactivation of the tumor suppressor gene RB1 on both alleles on chromosome 13 usually by mutation or deletion. Studies into epigenetic causes of retinoblastoma have demonstrated that hypermethylation of the RB1 gene may play a role in tumor development by reducing the activity of RB1 especially in sporadic cases.55–57 In 2003, Moll et al58 published a report of five children with retinoblastoma born after IVF in the Netherlands between November 2000 and February 2002. Estimating the proportion of children born after IVF in the Netherlands during that period to be between 1% and 1.5%, the authors58 estimated an expected 0.69 cases of retinoblastoma in children post-IVF. The occurrence of five cases post-IVF showed a significantly increased relative risk of approximately five to sevenfold.58 A British study59 identified 24 cases of retinoblastoma in 358,270 live births between January 1, 1989, and December 31, 2001. During that period, there were 176 births after IVF with no cases of retinoblastoma identified.59 In a Danish national cohort study30 that included all singletons born in Denmark from 1995 to 2001, retinoblastoma was identified in none of the 6052 singletons post-IVF who were followed for a mean of 4.1 years. Despite the significant association shown by Moll et al,58 no other studies have been able to confirm the association between ART and retinoblastoma, although arguably, the sample size in these studies is relatively small for the rarity of retinoblastoma.
CONCLUSION
Several studies have suggested a possible link between ART and various imprinting disorders including BWS, AS, SRS, maternal hypomethylation syndrome, and retinoblastoma. Concern seems warranted because ART procedures including ovarian stimulation and the manipulation of preimplantation embryos occur during critical developmental periods (Fig. 1) when genomic imprints have been shown to be vulnerable in animal studies. Although evidence is limited, the strongest data in humans suggest that there may be an increased risk of BWS in children conceived by ART. Independent studies have reported an association between ART and BWS. Furthermore, the finding of a greater percentage of BWS cases caused by loss of maternal methylation after ART than in the general BWS population (Table 1) supports the association and is consistent with altered imprinting due to ART. Similarly, an association between ART and AS was suggested by the reports of five cases of AS caused by imprinting defects (e.g., loss of methylation at the SNRPN imprinting control region) on the maternal allele in children conceived by ART. The frequent finding of hypomethylation of maternal alleles leading to imprinting disorders in children conceived by ART lends credence to a link between ART and sporadic global demethylation at multiple loci. This question has not yet been settled, but it highlights the need for careful follow-up of ART-conceived children into adulthood because small epigenetic changes could have consequences, affect long-term morbidity, or have transgenerational effects.
Due to the variation in ART protocols worldwide, limitations in sample size, and the inability to control for all the variables and confounders involved, it has been difficult to rigorously relate an association between imprinting disorders and any one specific ART procedure (e.g., IVF, ICSI, embryo culture). This has led some investigators to conclude that the increased risk of imprinting disorders is due to underlying subfertility or ovarian stimulation without subsequent in vitro procedures. However, that association remains unproven. Prospective studies are needed to examine these associations and test for potential long-term effects of ART. In the meantime, routine screening for imprinting disorders is not justified in offspring conceived by ART because the absolute risk of imprinting disorders is small. Nevertheless, parents seeking ART should be counseled about the possible increased risk of imprinting disorders.
ACKNOWLEDGMENTS
Supported in part by the Intramural Research Program of the National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland. Carter Owen was supported by the Clinical Research Training Program, a public-private partnership supported jointly by the NIH and Pfizer, Inc. via a grant to the Foundation for NIH from Pfizer, Inc.
Footnotes
Epigenetics in Reproduction; Guest Editors, James H. Segars, Jr., M.D., and Kjersti M. Aagaard-Tillery, M.D., Ph.D.
REFERENCES
- 1.Centers for Disease Control and Prevention . 2005 assisted reproductive technology success rates: national summary and fertility clinic reports. Centers for Disease Control and Prevention; Atlanta, GA: 2007. American Society for Reproductive Medicine SfART. [Google Scholar]
- 2.Wright VC, Schieve LA, Reynolds MA, Jeng G. Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (CDC). Assisted reproductive technology surveillance—United States, 2002. MMWR Surveill Summ. 2005;54(2):1–24. [PubMed] [Google Scholar]
- 3.Huang JC, Lei ZL, Shi LH, et al. Comparison of histone modifications in in vivo and in vitro fertilization mouse embryos. Biochem Biophys Res Commun. 2007;354(1):77–83. doi: 10.1016/j.bbrc.2006.12.163. [DOI] [PubMed] [Google Scholar]
- 4.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293(5532):1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 5.Wilkins-Haug L. Assisted reproductive technology, congenital malformations, and epigenetic disease. Clin Obstet Gynecol. 2008;51(1):96–105. doi: 10.1097/GRF.0b013e318161d25a. [DOI] [PubMed] [Google Scholar]
- 6.Manipalviratn S, DeCherney A, Segars J. Imprinting disorders and assisted reproductive technology. Fertil Steril. 2009;91(2):305–315. doi: 10.1016/j.fertnstert.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allegrucci C, Thurston A, Lucas E, Young L. Epigenetics and the germline. Reproduction. 2005;129(2):137–149. doi: 10.1530/rep.1.00360. [DOI] [PubMed] [Google Scholar]
- 8.Hartmann S, Bergmann M, Bohle RM, Weidner W, Steger K. Genetic imprinting during impaired spermatogenesis. Mol Hum Reprod. 2006;12(6):407–411. doi: 10.1093/molehr/gal040. [DOI] [PubMed] [Google Scholar]
- 9.Hajkova P, Erhardt S, Lane N, et al. Epigenetic reprogramming in mouse primordial germ cells. Mech Dev. 2002;117(1–2):15–23. doi: 10.1016/s0925-4773(02)00181-8. [DOI] [PubMed] [Google Scholar]
- 10.Obata Y, Kaneko-Ishino T, Koide T, et al. Disruption of primary imprinting during oocyte growth leads to the modified expression of imprinted genes during embryo-genesis. Development. 1998;125(8):1553–1560. doi: 10.1242/dev.125.8.1553. [DOI] [PubMed] [Google Scholar]
- 11.Jacob S, Moley KH. Gametes and embryo epigenetic reprogramming affect developmental outcome: implication for assisted reproductive technologies. Pediatr Res. 2005;58(3):437–446. doi: 10.1203/01.PDR.0000179401.17161.D3. [DOI] [PubMed] [Google Scholar]
- 12.Schieve LA, Rasmussen SA, Reefhuis J. Risk of birth defects among children conceived with assisted reproductive technology: providing an epidemiologic context to the data. Fertil Steril. 2005;84(5):1320–1324. doi: 10.1016/j.fertnstert.2005.04.066. discussion 1327. [DOI] [PubMed] [Google Scholar]
- 13.Buck Louis GM, Schisterman EF, Dukic VM, Schieve LA. Research hurdles complicating the analysis of infertility treatment and child health. Hum Reprod. 2005;20(1):12–18. doi: 10.1093/humrep/deh542. [DOI] [PubMed] [Google Scholar]
- 14.Thorburn MJ, Wright ES, Miller CG, Smith-Read EH. Exomphalos-macroglossia-gigantism syndrome in Jamaican infants. Am J Dis Child. 1970;119(4):316–321. doi: 10.1001/archpedi.1970.02100050318006. [DOI] [PubMed] [Google Scholar]
- 15.Elliott M, Bayly R, Cole T, Temple IK, Maher ER. Clinical features and natural history of Beckwith-Wiedemann syndrome: presentation of 74 new cases. Clin Genet. 1994;46(2):168–174. doi: 10.1111/j.1399-0004.1994.tb04219.x. [DOI] [PubMed] [Google Scholar]
- 16.Wiedemann H. Tumours and hemihypertrophy associated with Wiedemann-Beckwith syndrome. Eur J Pediatr. 1983;141:129. [Google Scholar]
- 17.Junien C. Beckwith-Wiedemann syndrome, tumourigenesis and imprinting. Curr Opin Genet Dev. 1992;2(3):431–438. doi: 10.1016/s0959-437x(05)80154-6. [DOI] [PubMed] [Google Scholar]
- 18.Weksberg R, Smith AC, Squire J, Sadowski P. Beckwith-Wiedemann syndrome demonstrates a role for epigenetic control of normal development. Hum Mol Genet. 2003;12(Spec No 1):R61–68. doi: 10.1093/hmg/ddg067. [DOI] [PubMed] [Google Scholar]
- 19.Weksberg R, Shuman C, Smith AC. Beckwith-Wiedemann syndrome. Am J Med Genet C Semin Med Genet. 2005;137C(1):12–23. doi: 10.1002/ajmg.c.30058. [DOI] [PubMed] [Google Scholar]
- 20.Clayton-Smith J, Read AP, Donnai D. Monozygotic twinning and Wiedemann-Beckwith syndrome. Am J Med Genet. 1992;42(4):633–637. doi: 10.1002/ajmg.1320420440. [DOI] [PubMed] [Google Scholar]
- 21.Weksberg R, Shuman C, Caluseriu O, et al. Discordant KCNQ1OT1 imprinting in sets of monozygotic twins discordant for Beckwith-Wiedemann syndrome. Hum Mol Genet. 2002;11(11):1317–1325. doi: 10.1093/hmg/11.11.1317. [DOI] [PubMed] [Google Scholar]
- 22.DeBaun MR, Niemitz EL, Feinberg AP. Association of in vitro fertilization with Beckwith-Wiedemann syndrome and epigenetic alterations of LIT1 and H19. Am J Hum Genet. 2003;72(1):156–160. doi: 10.1086/346031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention . 2000 Assisted Reproductive Technology Success Rates: National Summary and Fertility Clinic Reports. Centers for Disease Control and Prevention; Atlanta, GA: 2002. American Society for Reproductive Medicine SART. [Google Scholar]
- 24.Centers for Disease Control and Prevention . 2001 Assisted Reproductive Technology Success Rates: National Summary and Fertility Clinic Reports. Centers for Disease Control and Prevention; Atlanta, GA: 2003. American Society for Reproductive Medicine SART. [Google Scholar]
- 25.Centers for Disease Control and Prevention . 2002 Assisted Reproductive Technology Success Rates: National Summary and Fertility Clinic Reports. Centers for Disease Control and Prevention; Atlanta, GA: 2004. American Society for Reproductive Medicine SART. [Google Scholar]
- 26.Maher ER, Brueton LA, Bowdin SC, et al. Beckwith-Wiedemann syndrome and assisted reproduction technology (ART). J Med Genet. 2003;40(1):62–64. doi: 10.1136/jmg.40.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gicquel C, Gaston V, Mandelbaum J, Siffroi JP, Flahault A, Le Bouc Y. In vitro fertilization may increase the risk of Beckwith-Wiedemann syndrome related to the abnormal imprinting of the KCN1OT gene. Am J Hum Genet. 2003;72(5):1338–1341. doi: 10.1086/374824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halliday J, Oke K, Breheny S, Algar E, J Amor D. Beckwith-Wiedemann syndrome and IVF: a case-control study. Am J Hum Genet. 2004;75(3):526–528. doi: 10.1086/423902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang AS, Moley KH, Wangler M, Feinberg AP, DeBaun MR. Association between Beckwith-Wiedemann syndrome and assisted reproductive technology: a case series of 19 patients. Fertil Steril. 2005;83(2):349–354. doi: 10.1016/j.fertnstert.2004.07.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lidegaard O, Pinborg A, Andersen AN. Imprinting diseases and IVF: Danish National IVF cohort study. Hum Reprod. 2005;20(4):950–954. doi: 10.1093/humrep/deh714. [DOI] [PubMed] [Google Scholar]
- 31.Källén B, Finnström O, Nygren KG, Olausson PO. In vitro fertilization (IVF) in Sweden: infant outcome after different IVF fertilization methods. Fertil Steril. 2005;84(3):611–617. doi: 10.1016/j.fertnstert.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 32.Sutcliffe AG, Peters CJ, Bowdin S, et al. Assisted reproductive therapies and imprinting disorders—a preliminary British survey. Hum Reprod. 2006;21(4):1009–1011. doi: 10.1093/humrep/dei405. [DOI] [PubMed] [Google Scholar]
- 33.Bowdin S, Allen C, Kirby G, et al. A survey of assisted reproductive technology births and imprinting disorders. Hum Reprod. 2007;22(12):3237–3240. doi: 10.1093/humrep/dem268. [DOI] [PubMed] [Google Scholar]
- 34.Doornbos ME, Maas SM, McDonnell J, Vermeiden JP, Hennekam RC. Infertility, assisted reproduction technologies and imprinting disturbances: a Dutch study. Hum Reprod. 2007;22(9):2476–2480. doi: 10.1093/humrep/dem172. [DOI] [PubMed] [Google Scholar]
- 35.Rossignol S, Steunou V, Chalas C, et al. The epigenetic imprinting defect of patients with Beckwith-Wiedemann syndrome born after assisted reproductive technology is not restricted to the 11p15 region. J Med Genet. 2006;43(12):902–907. doi: 10.1136/jmg.2006.042135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim D, Bowdin SC, Tee L, et al. Clinical and molecular genetic features of Beckwith-Wiedemann syndrome associated with assisted reproductive technologies. Hum Reprod. 2009;24(3):741–747. doi: 10.1093/humrep/den406. [DOI] [PubMed] [Google Scholar]
- 37.Williams CA. Neurological aspects of the Angelman syndrome. Brain Dev. 2005;27(2):88–94. doi: 10.1016/j.braindev.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 38.Williams CA, Zori RT, Hendrickson J, et al. Angelman syndrome. Curr Probl Pediatr. 1995;25(7):216–231. doi: 10.1016/s0045-9380(06)80036-8. [DOI] [PubMed] [Google Scholar]
- 39.Kishino T, Lalande M, Wagstaff J. UBE3A/E6-AP mutations cause Angelman syndrome. Nat Genet. 1997;15(1):70–73. doi: 10.1038/ng0197-70. [DOI] [PubMed] [Google Scholar]
- 40.Matsuura T, Sutcliffe JS, Fang P, et al. De novo truncating mutations in E6-AP ubiquitin-protein ligase gene (UBE3A) in Angelman syndrome. Nat Genet. 1997;15(1):74–77. doi: 10.1038/ng0197-74. [DOI] [PubMed] [Google Scholar]
- 41.Rougeulle C, Glatt H, Lalande M. The Angelman syndrome candidate gene, UBE3A/E6-AP, is imprinted in brain. Nat Genet. 1997;17(1):14–15. doi: 10.1038/ng0997-14. [DOI] [PubMed] [Google Scholar]
- 42.Cox GF, Bürger J, Lip V, et al. Intracytoplasmic sperm injection may increase the risk of imprinting defects. Am J Hum Genet. 2002;71(1):162–164. doi: 10.1086/341096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ørstavik KH, Eiklid K, van der Hagen CB, et al. Another case of imprinting defect in a girl with Angelman syndrome who was conceived by intracytoplasmic semen injection. Am J Hum Genet. 2003;72(1):218–219. doi: 10.1086/346030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ludwig M, Katalinic A, Gross S, Sutcliffe A, Varon R, Horsthemke B. Increased prevalence of imprinting defects in patients with Angelman syndrome born to subfertile couples. J Med Genet. 2005;42(4):289–291. doi: 10.1136/jmg.2004.026930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manning M, Lissens W, Bonduelle M, et al. Study of DNA-methylation patterns at chromosome 15q11-q13 in children born after ICSI reveals no imprinting defects. Mol Hum Reprod. 2000;6(11):1049–1053. doi: 10.1093/molehr/6.11.1049. [DOI] [PubMed] [Google Scholar]
- 46.Neri QV, Takeuchi T, Palermo GD. An update of assisted reproductive technologies results in the United States. Ann N Y Acad Sci. 2008;1127:41–48. doi: 10.1196/annals.1434.017. [DOI] [PubMed] [Google Scholar]
- 47.Perkins RM, Hoang-Xuan MT. The Russell-Silver syndrome: a case report and brief review of the literature. Pediatr Dermatol. 2002;19(6):546–549. doi: 10.1046/j.1525-1470.2002.00230.x. [DOI] [PubMed] [Google Scholar]
- 48.Abu-Amero S, Monk D, Frost J, Preece M, Stanier P, Moore GE. The genetic aetiology of Silver-Russell syndrome. J Med Genet. 2008;45(4):193–199. doi: 10.1136/jmg.2007.053017. [DOI] [PubMed] [Google Scholar]
- 49.Svensson J, Björnståhl A, Ivarsson SA. Increased risk of Silver-Russell syndrome after in vitro fertilization? Acta Paediatr. 2005;94(8):1163–1165. doi: 10.1111/j.1651-2227.2005.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 50.Bliek J, Terhal P, van den Bogaard MJ, et al. Hypomethylation of the H19 gene causes not only Silver-Russell syndrome (SRS) but also isolated asymmetry or an SRS-like phenotype. Am J Hum Genet. 2006;78(4):604–614. doi: 10.1086/502981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kagami M, Nagai T, Fukami M, Yamazawa K, Ogata T. Silver-Russell syndrome in a girl born after in vitro fertilization: partial hypermethylation at the differentially methylated region of PEG1/MEST. J Assist Reprod Genet. 2007;24(4):131–136. doi: 10.1007/s10815-006-9096-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mackay DJ, Boonen SE, Clayton-Smith J, et al. A maternal hypomethylation syndrome presenting as transient neonatal diabetes mellitus. Hum Genet. 2006;120(2):262–269. doi: 10.1007/s00439-006-0205-2. [DOI] [PubMed] [Google Scholar]
- 53.Bliek J, Verde G, Callaway J, et al. Hypomethylation at multiple maternally methylated imprinted regions including PLAGL1 and GNAS loci in Beckwith-Wiedemann syndrome. Eur J Hum Genet. 2009;17(5):611–619. doi: 10.1038/ejhg.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moll AC, Kuik DJ, Bouter LM, et al. Incidence and survival of retinoblastoma in The Netherlands: a register based study 1862–1995. Br J Ophthalmol. 1997;81(7):559–562. doi: 10.1136/bjo.81.7.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greger V, Passarge E, Höpping W, Messmer E, Horsthemke B. Epigenetic changes may contribute to the formation and spontaneous regression of retinoblastoma. Hum Genet. 1989;83(2):155–158. doi: 10.1007/BF00286709. [DOI] [PubMed] [Google Scholar]
- 56.Greger V, Debus N, Lohmann D, Höpping W, Passarge E, Horsthemke B. Frequency and parental origin of hyper-methylated RB1 alleles in retinoblastoma. Hum Genet. 1994;94(5):491–496. doi: 10.1007/BF00211013. [DOI] [PubMed] [Google Scholar]
- 57.Ohtani-Fujita N, Dryja TP, Rapaport JM, et al. Hyper-methylation in the retinoblastoma gene is associated with unilateral, sporadic retinoblastoma. Cancer Genet Cytogenet. 1997;98(1):43–49. doi: 10.1016/s0165-4608(96)00395-0. [DOI] [PubMed] [Google Scholar]
- 58.Moll AC, Imhof SM, Cruysberg JR, Schouten-van Meeteren AY, Boers M, van Leeuwen FE. Incidence of retinoblastoma in children born after in-vitro fertilisation. Lancet. 2003;361(9354):309–310. doi: 10.1016/S0140-6736(03)12332-X. [DOI] [PubMed] [Google Scholar]
- 59.Bradbury BD, Jick H. In vitro fertilization and childhood retinoblastoma. Br J Clin Pharmacol. 2004;58(2):209–211. doi: 10.1111/j.1365-2125.2004.02109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]