miR-19 is a key oncogenic component of mir-17-92 (original) (raw)
Abstract
Recent studies have revealed the importance of multiple microRNAs (miRNAs) in promoting tumorigenesis, among which mir-17-92/Oncomir-1 exhibits potent oncogenic activity. Genomic amplification and elevated expression of mir-17-92 occur in several human B-cell lymphomas, and enforced mir-17-92 expression in mice cooperates with c-myc to promote the formation of B-cell lymphomas. Unlike classic protein-coding oncogenes, mir-17-92 has an unconventional gene structure, where one primary transcript yields six individual miRNAs. Here, we functionally dissected the individual components of mir-17-92 by assaying their tumorigenic potential in vivo. Using the Eμ-myc model of mouse B-cell lymphoma, we identified miR-19 as the key oncogenic component of mir-17-92, both necessary and sufficient for promoting _c-myc_-induced lymphomagenesis by repressing apoptosis. The oncogenic activity of miR-19 is at least in part due to its repression of the tumor suppressor Pten. Consistently, miR-19 activates the Akt–mTOR (mammalian target of rapamycin) pathway, thereby functionally antagonizing Pten to promote cell survival. Our findings reveal the essential role of miR-19 in mediating the oncogenic activity of mir-17-92, and implicate the functional diversity of mir-17-92 components as the molecular basis for its pleiotropic effects during tumorigenesis.
Keywords: Cancer, apoptosis, c-myc, microRNAs, mir-17-92, mir-19
MicroRNAs (miRNAs) encode small, regulatory RNAs that control gene expression predominantly through post-transcriptional repression (Ambros 2004; Zamore and Haley 2005; Bartel 2009). Nascent transcripts from miRNA genes (pri-miRNAs) contain one or multiple unique stem–loop structures. Mature miRNAs, ranging from 18 to 24 nucleotides (nt) in length, are initially embedded within one arm of the hairpin stems. These pri-miRNAs are processed sequentially by the ribonuclease III enzymes Drosha and Dicer to yield the mature miRNA duplexes (Kim et al. 2009). As the mature duplex is formed, the miRNA strand is then incorporated into the RNA-induced silencing complex (RISC) to mediate post-transcriptional regulation of specific mRNAs, primarily through mRNA degradation and/or translational repression (Filipowicz et al. 2008). The target recognition by miRNAs is achieved through imperfect complementarity. The seed region of the mature miRNA (nucleotides 2–8) is often complementary to sites within the target mRNAs, forming a “seed match” in an otherwise imperfect base-pairing (Bartel 2009). The small size of miRNAs, combined with imperfect target recognition, provide miRNAs with the enormous capacity and versatility to act as global gene regulators in diverse developmental and physiological processes. Recent bioinformatic predictions and several experimental validations also suggest that each miRNA is likely to regulate hundreds of mRNA targets and fine-tune their expression in a cell type-dependent and context-dependent manner (Baek et al. 2008; Selbach et al. 2008; Chi et al. 2009).
The connection between miRNAs and cancer was first implicated by their frequent genomic alteration and dysregulated expression in various human tumors (Calin et al. 2004; Lu et al. 2005; He et al. 2007b). Multiple miRNAs were subsequently identified to promote or suppress oncogenesis, presumably by modulating gene expression in the oncogenic and tumor suppressor networks (He et al. 2005, 2007a; Johnson et al. 2005; Kumar et al. 2008; Kota et al. 2009). One of the first oncogenic miRNAs identified was mir-17-92 (also known as oncomir-1), a miRNA polycistron with pleiotropic functions in cell survival, proliferation, differentiation, and angiogenesis (Hayashita et al. 2005; He et al. 2005; Lu et al. 2005; O'Donnell et al. 2005; Dews et al. 2006; Ventura et al. 2008). mir-17-92 is the primary target of the genomic amplification 13q31 that occurs in Burkitt's lymphoma, diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma, follicular lymphoma, and several other solid tumor types (Ota et al. 2004; He et al. 2005; Tagawa and Seto 2005; Tagawa et al. 2007; Inomata et al. 2009; Navarro et al. 2009). Additionally, mir-17-92 is highly expressed in a range of hematopoietic malignancies, particularly in B-cell lymphomas (Lu et al. 2005; Tagawa and Seto 2005; Navarro et al. 2009). Although transgenic mice with moderate mir-17-92 overexpression only develop lymphoproliferative phenotypes (Xiao et al. 2008), enforced high-level expression of mir-17-92, in conjunction with c-myc, has potent transformation potential in mouse B cells in vivo, largely due to its ability to repress apoptosis (He et al. 2005) This observation is consistent with the recurring mir-17-92 amplifications in _MYC_-rearranged Burkitt's lymphomas and DLBCLs in humans (Tagawa et al. 2007). The survival effect of mir-17-92 is also evident in the normal development of the B-cell compartment, as mir-17-92 deficiency leads to premature cell death during pro-B-to-pre-B transition (Ventura et al. 2008).
Unlike classic protein-coding oncogenes, where one transcript generally gives rise to one protein product, the mir-17-92 miRNA cluster produces a single polycistronic primary transcript that yields six individual mature miRNAs. The distinct mature miRNA sequence of these mir-17-92 components dictates the specificity of their target regulation, and ultimately can determine the functional specificity. Here we report the functional dissection of mir-17-92 in the context of B-cell transformation in vivo, and reveal the essential role of miR-19 in mediating the oncogenic activity of mir-17-92. In the Eμ-myc model of Burkitt's lymphoma, miR-19 is both necessary and sufficient for mir-17-92 to promote _c-myc_-induced B lymphomagenesis. The oncogenic activity of miR-19 is at least in part mediated by the PI3K (phosphatidylinositol 3-kinase)–Akt–mTOR (mammalian target of rapamycin) pathway, as enforced miR-19 expression dampens the expression of the tumor suppressor Pten, thus activating the Akt–mTOR signaling to promote cell survival. Our findings demonstrate the functional diversity of mir-17-92 components, and characterize the key molecular mechanism through which the mir-17-92 polycistron promotes malignant transformation in the B-cell compartment.
Results
mir-17-92 encodes a miRNA polycistron that yields six individual components
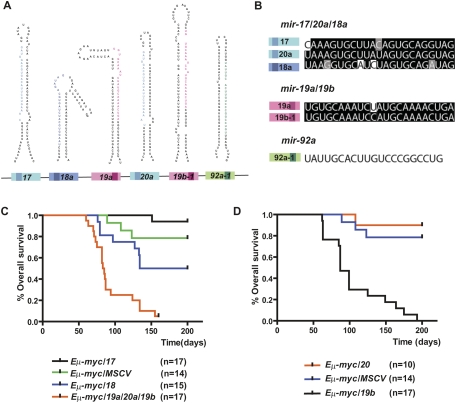
The mir-17-92 miRNA cluster produces a single polycistronic primary transcript that yields six mature miRNAs: miR-17, miR-18a, miR-19a, miR-20a, miR-19b, and miR-92a (Fig. 1A). This unique structural feature of mir-17-92, shared by a large number of miRNA genes in mammalian genomes, may constitute the molecular basis for its pleiotropic functions in a cell type-dependent and context-dependent manner. Based on sequence homology, the six mir-17-92 components can be categorized into three miRNA families: miR-17/20a/18a, miR-19a/19b, and miR-92a (Fig. 1B). miR-17 and miR-20a miRNAs are closely related homologs, differing by 2 nt outside the seed sequence (Fig. 1B). A related miRNA miR-18a has a similar yet not identical seed region, but shares significant sequence identity with mir-17/20a overall (Fig. 1B). The second miRNA family contains miR-19a and miR-19b, which differ only by a single nucleotide at position 11, a region minimally important for target recognition (Fig. 1B; Lewis et al. 2003, 2005; Farh et al. 2005; Grimson et al. 2007). Finally, the unique seed region of miR-92a distinguishes it from all of the other mir-17-92 components (Fig. 1B). All six mir-17-92 miRNAs can repress many target mRNAs either cooperatively or individually; both mechanisms could lead to cell type-dependent and context-dependent functional readout.
Figure 1.
miR-19b phenocopies the oncogenic effects of mir-17-92 in the Eμ-myc model. (A) Gene structure of the mir-17-92 polycistronic cluster. (Light-colored boxes) Pre-miRNAs; (dark-colored boxes) mature miRNAs. Homologous miRNA components are indicated by the same or similar colors. (B) mir-17-92 components belong to three miRNA families: miR-17/20a/18 (blue), miR-19 (red), and miR-92a (green). Mature miRNA sequence alignments are shown for each family. Based on sequence identity, miR-17 and miR-20a are closely related homologs, sharing significant sequence identity with miR-18a, but containing a slightly different seed (74% identity among all three). miR-19a and miR-19b differ by a single nucleotide at position 11, and are likely to regulate the same mRNA targets (96% identity). miR-92a has a unique seed sequence that distinguishes it from other components. (C) The mir-19a/20/19b subcluster accelerates _c-myc_-induced lymphomagenesis. Irradiated mice reconstituted with the Eμ-myc/+ HSPCs overexpressing miR-17, miR-18a, mir-19a/20a/19b, or a MSCV control vector were monitored weekly beginning 4 wk post-transplantation. The Kaplan-Meier curves represent percentage of overall survival. (D) miR-19b accelerates _c-myc_-induced lymphomagenesis. The mir-19a/20/19b subcluster was further divided into miR-19b and miR-20a, each overexpressed in the Eμ-myc/+ HSPCs before transplantation into lethally irradiated recipient animals. Reconstituted mice were monitored weekly starting 4 wk post-transplantation. The Kaplan-Meier curve indicates miR-19b has a strong oncogenic effect.
miR-19 exhibits potent oncogenic activity in the Eμ-myc model
Previously, we chose the Eμ-myc model of Burkitt's lymphoma to evaluate the oncogenic activity of mir-17-92 in vivo because genomic amplification and up-regulated expression of mir-17-92 were both observed in human Burkitt's lymphomas (Tagawa et al. 2007). Recent studies also indicated the association between recurring mir-17-92 genomic amplification and MYC rearrangement in Burkitt's lymphomas and DLBCLs, further implicating a functional cooperation between these two lesions (Tagawa et al. 2007). The Eμ-myc transgenic mice carry a c-myc oncogene driven by the immunoglobulin heavy-chain enhancer (Eμ), which is a powerful system in Burkitt's lymphoma. These mice exhibit c-myc overexpression specifically in the B-cell lineage, and ultimately acquire late-onset B-cell lymphomas with a latency of ∼6 mo (Adams et al. 1985). To examine the oncogenic potentials of any candidate miRNA, the Eμ-myc/+ hematopoietic stem and progenitor cells (HSPCs) can be infected with murine stem cell viruses (MSCVs) overexpressing the miRNA, and then transplanted into lethally irradiated recipient animals (Supplemental Fig. S1B; Schmitt et al. 2000). The oncogenic potential can be evaluated by the acceleration of _c-myc_-induced lymphomagenesis in this adoptive transfer model (Supplemental Fig. S1B).
The first evidence that individual mir-17-92 components may contribute differently to its oncogenic potential comes from our previous observation, in which mir-17-19b, a truncated mir-17-92 cluster lacking miR-92a (Supplemental Fig. S1A), can greatly accelerate _c-myc_-induced tumorigenesis in the Eμ-myc model (He et al. 2005). The fact that miR-92a is dispensable for the oncogenic activity prompted us to further divide the mir-17-19b cluster to functionally dissect the oncogenic potentials of each individual miRNA component (Supplemental Fig. S1A).
We initially divided the oncogenic mir-17-19b miRNA polycistron into three subclusters: miR-17, miR-18a, and mir-19a/20a/19b (Supplemental Fig. S1A). Overexpression of the control vector (MSCV) or miR-17 in this adoptive transfer model had minimal oncogenic effects, causing late-onset B lymphomas with incomplete penetrance (Fig. 1C). In contrast, animals coexpressing mir-19a/20a/19b and c-myc developed highly malignant, early-onset B lymphomas, with a median survival at 84 d post-transplantation (P < 0.0001, log rank test) (Fig. 1C). Interestingly, miR-18a overexpression caused a moderate acceleration of lymphomagenesis in the Eμ-myc model (Fig. 1C). Although it is unlikely that miR-18a is the major oncogenic component of mir-17-19b, it may play accessory roles to facilitate miR-19a/20a/19b to promote malignant transformation in the Eμ-myc model. Retroviruses driving mir-17-19b and each of the subclusters had similar levels of overexpression, indicating that the observed difference in oncogenic effects reflected true functional differences among mir-17-92 components, rather than differences in expression level (data not shown).
The three miRNAs encoded by mir-19a/20a/19b belong to two distinct families: miR-19 (including miR-19a and miR-19b) and miR-20a. While miR-20a exhibited minimal oncogenic collaboration with c-myc, miR-19b and c-myc resulted in highly malignant B lymphomas with nearly complete penetrance (median survival 87 d; P < 0.0001, log rank test) (Fig. 1D). Given the sequence homology between miR-19a and miR-19b and their 1-nt divergence at a region nonessential for miRNA target recognition, it is possible that miR-19a can also accelerate _c-myc_-induced lymphomagenesis. Similar to mir-19a/20a/19b, miR-19b phenocopied the oncogenic effects of mir-17-19b. Interestingly, mice overexpressing miR-19b exhibited a slightly longer life span after reconstitution (Fig. 3C, below) when compared with those overexpressing miR-17-19b, a difference likely due to functional cooperation among miR-19b, miR-19a, and possibly miR-18a.
Figure 3.
miR-19 miRNAs are essential for the oncogenic activity of mir-17-19b. (A) A schematic representation of the gene structural organization of mir-17-19b, mir-17-19b-Mut19, and miR-19b. 19a* and 19b* indicate miR-19 mutations. (B) miR-19 mutations specifically affected miR-19 expression in mir-17-19b. 3T3 cells were infected with MSCV-mir-17-19b, MSCV-mir-17-19b-Mut19, or control MSCV vectors. Expression levels of miR-17, miR-18a, miR-19a, miR-20a, and miR-19b were determined using TaqMan miRNA assays. miR-19 mutations specifically affected the expression of miR-19a and miR-19b, but not that of the adjacent mir-17-19b components. Error bars indicate SD (n = 3). (C) miR-19 is both necessary and sufficient for the oncogenic effect of mir-17-19b. Overexpression of miR-19b and mir-17-19b accelerated _c-myc_-induced lymphomagenesis to a similar degree, shown in Kaplan-Meier curves as the percentage of overall survival. Mutations in miR-19 greatly decreased the oncogenic activity of mir-17-19b in the Eμ-myc model. We compared the oncogenic effects of mir-17-19b, miR-19b, and mir-17-19b-Mut19 in the same adoptive transfer experiment. (D) miR-19 is both necessary and sufficient for the cell survival effect of mir-17-19b in vivo. Representative lymphomas from Eμ-myc, Eμ-myc/19b, and Eμ-myc/17-19b-Mut19 were stained for H&E and caspase-3, which indicated that the miR-19 mutations significantly compromised cell survival effects of mir-17-19b, while miR-19b overexrepssion suppresses apoptosis. The Eμ-myc/19b and Eμ-myc/17-19b-Mut19 tumors shown here were both IgM-negative B lymphomas. (Arrow) “Starry sky” feature of apoptotic tumor cells; (arrowhead) apoptotic cells with positive caspase-3 staining. Bar, 50 μm. (E) Quantification of caspase-3 staining of representative tumors from D as percentage of positive cells. For Eμ-myc/19b and Eμ-myc/17-19b-Mut19 tumors, only IgM-negative tumors were selected for this comparison (n = 3; error bars represent SEM).
Histopathological and immunophenotyping analyses confirmed the phenotypic similarities among the Eμ-myc/miR-19b (Eμ-myc/19b), Eμ-myc/mir-19a/20a/19b (Eμ-myc/19a/20a/19b), and Eμ-myc/mir-17-19b (Eμ-myc/17-19b) lymphomas. These mice developed advanced lymphomas, with massive enlargement of lymph nodes, splenic hyperplasia, and leukemia. In all cases, lymphoma cells invaded the thymus and bone marrow, as well as visceral organs outside the lymphoid compartment, including the liver, lung, and, occasionally, kidney (Fig. 2A; He et al. 2005). While control Eμ-myc lymphomas exhibited a high mitotic index accompanied by extensive cell death as shown by TUNEL staining (Fig. 2B; Supplemental Fig. S3), the Eμ-myc/19a/20a/19b and Eμ-myc/19b lymphomas, similar to Eμ-myc/17-19b lymphomas, had greatly reduced apoptosis without affecting cell proliferation (Fig. 2B; Supplemental Fig. S3). Although mir-17-92 has been implicated to promote cell proliferation in other cellular contexts (Ventura et al. 2008), mir-17-19b, mir-19a/20a/19b, and miR-19b overexpression did not enhance cell proliferation in the Eμ-myc model. The lack of proliferative effects in the B-lymphoma cells is likely due to the high level of basal proliferation resulting from c-myc overexpression. Additionally, control Eμ-myc mice frequently develop more mature B-cell lymphomas, but the Eμ-myc/19b, Eμ-myc/19a/20a/19b, and Eμ-myc/17-19b tumors were derived primarily from progenitor B cells (Fig. 2C; Supplemental Table S1; Supplemental Fig. S2), suggesting a functional preference for these miRNAs to transform B-cell progenitors under our experimental conditions. These observations were consistent with the B-cell phenotypes in the mir-17-92 knockout mice, in which the pro-B-to-pre-B transition was marked by extensive apoptosis (Ventura et al. 2008).
Figure 2.
Eμ-myc/17-19 and Eμ-myc/19b lymphomas have similar pathological and immunological features. (A) Eμ-myc/19b lymphomas are highly invasive. H&E staining of the liver, lung, and kidney showed aggressive invasion by Eμ-myc/19b tumor cells, which was highly analogous to that of the Eμ-myc/19a/20a/19b lymphomas. In particular, both perivascular and parenchymal invasion of the liver were observed. (B) Overexpression of miR-19b represses _c-myc_-induced apoptosis. The Eμ-myc/19b and Eμ-myc/19a/20a/19b lymphomas had similar proliferation rates to those of Eμ-myc/MSCV controls, demonstrated by Ki67 staining. However, exogenous expression of miR-19b or mir-19a/20/19b greatly decreased apoptosis in the Eμ-myc tumors, confirmed by TUNEL and H&E staining of Eμ-myc/17-19 lymph node tumors. The “starry sky” morphology of cell clusters undergoing apoptosis (black arrows), a hallmark of Eμ-myc/MSCV lymphomas, was absent in Eμ-myc/19b and Eμ-myc/19a/20a/19b tumors. (C) miR-19b and mir-17-19b preferably transform progenitor B cells. Flow cytometric immunophenotyping of representative Eμ-myc, Eμ-myc/17-19, and Eμ-myc/19 lymphomas. While the majority of Eμ-myc tumors consisted of CD19-positive and IgM-positive B cells, the Eμ-myc/19b and Eμ-myc/17-19 tumor cells bore cellular characteristics of progenitor B cells, positive for CD19 but not for surface IgM.
miR-19 components are required for the oncogenic activity of mir-17-92
Among all of the mir-17-92 components, miR-19 is sufficient for accelerating _c-myc_-induced lymphomagenesis. To determine whether miR-19 miRNAs are also necessary for the oncogenic effects of mir-17-19b, we introduced mutations into miR-19a and miR-19b within the mir-17-19b construct (designated as mir-17-19b-Mut19). In so doing, we disrupted the hairpin stem of both miR-19a and miR-19b to abolish the biogenesis of miR-19 miRNAs while preventing generation of cryptic miRNAs. These mutations specifically disabled miR-19 biogenesis, without affecting that of miR-17, miR-18a, and miR-20a within the same construct (Fig. 3A,B). Consistent with miR-19 being the key oncogenic component, the miR-19 mutations greatly compromised the oncogenic capacity of mir-17-19b, causing delayed tumor onset, incomplete penetrance, and extended life span (median survival 141 d; P < 0.001, log rank test) (Fig. 3C). In Eμ-myc/mir-17-19b-Mut19 (Eμ-myc/17-19b-Mut19) animals that ultimately developed late-onset lymphomas, the miR-19 mutations significantly compromised cell survival without affecting cell proliferation (Fig. 3D,E; Supplemental Fig. S3). In comparison, tumors derived from oncogenic collaboration between c-myc and mir-17-19b or miR-19b alone exhibited greatly reduced apoptosis (Figs. 2B, 3D,E). The miR-19 mutations not only disrupted cell survival, but also compromised the ability of mir-17-19b to preferentially transform progenitor B cells. This was suggested by the immunophenotyping analyses on Eμ-myc/17-19b-Mut19 B-cell tumors, which exhibited more cell type heterogeneity compared with Eμ-myc/17-19b tumors (Supplemental Table S1). Taken together, these findings establish miR-19 miRNAs as the key oncogenic component within mir-17-92, promoting tumorigenesis seemingly through the repression of _c-myc_-induced apoptosis.
miR-19 specifically dampens the expression level of the tumor suppressor Pten
miR-19 is the key oncogenic component of mir-17-92, and therefore _miR-19_-specific targets are likely to mediate the oncogenic effects of mir-17-92. Among hundreds of miR-19 targets predicted by TargetScan and RNA22 (Lewis et al. 2005; Miranda et al. 2006; Grimson et al. 2007), the tumor suppressor Pten is a prominent candidate, due to its important functions in promoting apoptosis and tumor suppression. Pten is a negative regulator of the PI3K signaling. In response to a variety of extracellular signals, the PI3K pathway elicits diverse cellular responses to promote cell survival, rapid proliferation, and cell growth. Pten has been implicated as a target of mir-17-92 by luciferase reporter assays and Western analyses in cell culture-based experiments (Takakura et al. 2008; Xiao et al. 2008). However, it is not clear how individual mir-17-92 components contribute to the repression of Pten and, more importantly, whether the down-regulation of Pten by mir-17-92 leads to any functional impact on cell survival.
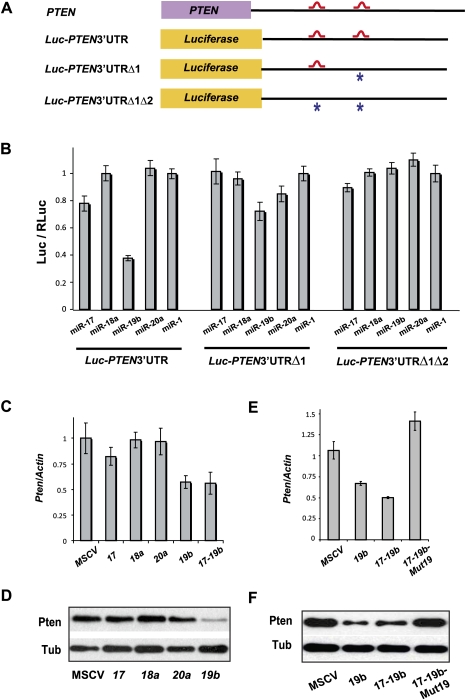
Overexpression of miR-19b and _mir-17-19b_—but not miR-17, miR-18a, miR-20a, or MSCV control—significantly down-regulated the endogenous level of Pten mRNA and protein in NIH-3T3 cells (Fig. 4C,D). Similarly, Pten down-regulation by miR-19b and mir-17-19b was observed in mouse primary B-cell culture (Supplemental Fig. S4B). The ability of mir-17-19b to down-regulate Pten in both cell types depended on the intact miR-19 components within this cluster, since mir-17-19b/Mut19 failed to dampen Pten expression in either 3T3 cells or primary B-cell culture (Fig. 4E,F; Supplemental Fig. S4A,B). These findings suggest that miR-19 is the mir-17-92 component that is both necessary and sufficient to mediate Pten repression. Interestingly, the level of Pten repression by miR-19 was also dependent on cell types and physiological contexts, possibly due to differences in basal levels of miR-19 and Pten expression (Hwang et al. 2009; data not shown).
Figure 4.
Pten is a mir-17-19b target specifically regulated by miR-19. (A) Schematic representation of the PTEN 3′UTR and its _miR-19_-binding sites. Two _miR-19_-binding sites (shown in red) can be found in the human PTEN 3′UTR. The PTEN 3′UTR with mutations (designated with asterisks) at one (_PTEN_3′UTRΔ1) or both (_PTEN_3′UTRΔ1Δ2) miR-19 sites, as well as the wild-type counterpart (_PTEN_3′UTR), were each cloned downstream from a luciferase reporter (Luc). (B) Specific repression of _Luc-PTEN_3′UTR reporter by miR-19. _Luc-PTEN_3′UTR was cotransfected with mimics of miR-17, miR-18a, miR-19b, miR-20a, and control miR-1. Only miR-19b significantly repressed the reporter expression. Cotransfection with a luciferase construct carrying one mutated miR-19b site in the PTEN 3′UTR (Luc-_PTEN_3′UTRΔ1) partially derepressed the Luc reporter, and cotransfection of a construct with mutations in both miR-19 sites (_Luc-PTEN_3′UTRΔ1Δ2) completely derepressed the Luc reporter. (C,D) miR-19b specifically represses endogenous Pten expression level. Using real-time PCR analysis (C) and Western analysis (D), down-regulation of endogenous Pten mRNA and protein can be detected in serum-starved NIH-3T3 cells infected with miR-19b and mir-17-19b. In comparison, overexpression of miR-17, miR-18a, miR-20a, and control vector (MSCV) in these cells has minimal effects on the endogenous Pten level. (E,F) miR-19 is both necessary and sufficient to mediate the Pten repression by mir-17-19b. Repression of endogenous Pten mRNA (E) and protein (F) can be detected in serum-starved NIH-3T3 cells infected with miR-19b and mir-17-19b. In comparison, mir-17-19b-Mut19 failed to repress the endogenous Pten level.
The human PTEN 3′ untranslated region (UTR) contains two _miR-19_-binding sites (Fig. 4A; Miranda et al. 2006). A luciferase reporter, when fused with the wild-type human PTEN 3′UTR, was significantly repressed by overexpression of miR-19b, but not by miR-17, miR-18a, miR-20a, or miR-1 control (Fig. 4B). Mutations of one or both _miR-19_-binding sites in the luc-PTEN 3′UTR construct either partially or completely derepressed the luciferase reporter when coexpressed with miR-19b (Fig. 4A,B). These findings suggest that direct binding between miR-19 and PTEN 3′UTR is required for PTEN repression by miR-19.
miR-19 functionally antagonizes Pten to promote cell survival
miR-19 is evolutionarily conserved across many vertebrate species (Griffiths-Jones 2006). Between Xenopus and mammals, the sequences of miR-19a and miR-19b are identical, and two putative _miR-19_-binding sites are also present in the Xenopus pten mRNA. These observations implicate a selective pressure to preserve the pten regulation by miR-19 during ∼350 million years of evolution. To determine whether Pten down-regulation by miR-19 had any functional impact, we examined their functional interaction using an in vivo apoptosis assay in Xenopus embryos.
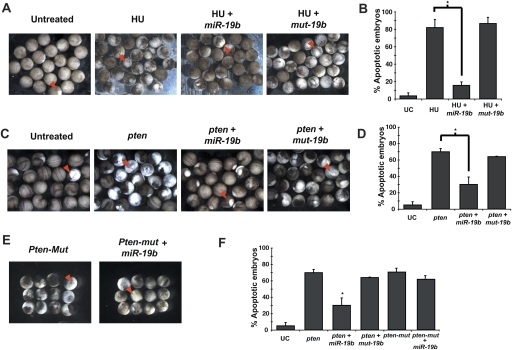
Not only is miR-19 conserved at the sequence level in Xenopus, its anti-apoptotic function is also preserved, evident by its ability to repress hydroxyurea-induced cell death. When subjected to hydroxyurea treatment, Xenopus embryos undergo apoptosis, often characterized by a whitish color, cell blebbing, and disruption of cell adhesion after the mid-blastula transition (Walker and Harland 2009). Injection of miR-19b considerably rescued hydroxyurea-induced apoptosis (Fig. 5A,B). Consistent with Pten being a key target of miR-19, injection of miR-19 and pten individually gave rise to opposite phenotypes, with miR-19 promoting cell survival (Fig. 5A,B) and pten inducing apoptosis (Fig. 5C,D). Coinjection of miR-19b and pten, however, significantly rescued _pten_-induced apoptosis, but a mutated miR-19b (mut-19b) with an altered seed sequence failed to impact the pten function (Fig. 5C,D). When we introduced mutations in the two _miR-19_-binding sites in the pten cDNA without affecting pten protein coding, the mutated pten mRNA (pten-mut) retained the ability to potently induce cell death. In this case, miR-19 injection failed to rescue the apoptotic effects of pten-Mut (Fig. 5E,F). Altogether, our findings indicate that the repression of Pten by miR-19 occurs not only at the expression level, but also at the functional level, both of which are dependent on the intact _miR-19_-binding sites within the Pten mRNA.
Figure 5.
miR-19b functionally antagonizes _pten_-induced apoptosis in Xenopus embryos. (A,B) miR-19b rescues hydroxyurea (HU)-induced apoptosis in X. laevis embryos. Injection of miR-19b mimics into X. laevis embryos rescued apoptosis caused by hydroxyurea treatment. The mutated miR-19b with an altered seed region (mut-19b) failed to rescue hydroxyurea-induced apoptosis. Embryos undergoing apoptosis were marked by cell blebbing, disruption of cell adhesion, and a characteristic white color (red arrowhead). Hydroxyurea-treated embryos appeared more pigmented than control embryos, largely due to developmental arrest. (B) Apoptosis was quantified for untreated and hydroxyurea-treated embryos, as well as the hydroxyurea-treated embryos coinjected with either miR-19b mimics or mut-19b mimics (n = 3 experiments with >25 embryos in each group; [*] P < 0.05). (_C_,_D_) Injection of _miR-19b_ rescued _pten_-induced apoptosis in _Xenopus_ embryos. Injection of full-length _pten_ mRNA into _Xenopus_ embryos led to widespread apoptosis. Injection of _miR-19b_, but not _mut-19b_, significantly rescued the proapoptotic effect of _pten_. (_E_,_F_) Disruption of base-pairing between _miR-19_ and _pten_ mRNA abolished their functional antagonism. Mutations in the _miR-19_-binding sites within the _pten_ mRNA (_pten-mut_) did not abrogate the proapoptotic effects of _pten_, but did eliminate the ability of _miR-19b_ to repress the apoptosis (_n_ = 3 experiments with >25 embryos in each group; [*] P < 0.05). All error bars represent SEM.
miR-19 activates the Akt–mTOR pathway both in vitro and in vivo
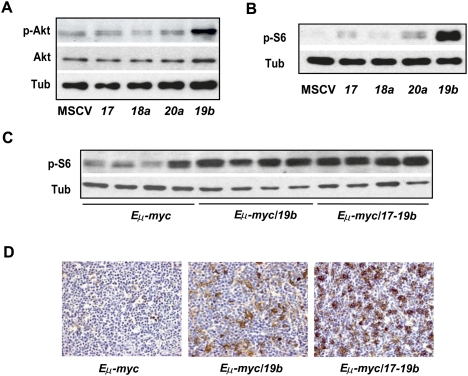
PTEN is a tumor suppressor that is mutated or deleted in multiple tumor types with high frequency (Di Cristofano and Pandolfi 2000; Knobbe et al. 2008). It acts to repress the intracellular level of phosphatidylinositol-3,4,5-trisphosphate in cells, thus negatively regulating the PI3K pathway and its downstream effectors, Akt and mTOR (Comer and Parent 2002; Rossi and Weissman 2006). Consistent with the ability of miR-19 to repress Pten expression and function, miR-19b overexpression in NIH-3T3 cells specifically activated the PI3K pathway, leading to increased phosphorylation of Akt without affecting the overall level of Akt (Fig. 6A). miR-19b or mir-17-19b overexpression also caused a significant increase in phosphorylation of S6 ribosomal protein due to the activated mTOR pathway (Supplemental Fig. S4D). Surprisingly, miR-17 and miR-20a overexpression slightly increased the phospho-S6 level compared with the vector control, but this increase might be achieved through a mechanism independent of Pten or Akt (Fig. 6A,B). Using Western analyses and immunohistochemistry assays, a high level of phospho-S6 protein was observed in a cohort of Eμ-myc/19b and Eμ-myc/17-19b tumors, suggesting that miR-19 and mir-17-19b were able to activate the mTOR pathway in vivo (Fig. 6C,D), at least in part through the repression on Pten. In comparison, the control Eμ-myc tumors generally exhibited a low level of phospho-S6 protein, although a certain degree of heterogeneity existed among different tumors. Although mir-17-19b and miR-19b were equally potent in inducing phospho-S6 in vitro (Supplemental Fig. S4D), the effect of mir-17-19b in vivo was more potent (Fig. 6C,D), possibly due to a degree of cooperative effects from the other miRNA components. The late-onset Eμ-myc/17-19b-Mut19 lymphomas had varying levels of phospho-S6, yet this cohort of lymphomas as a whole exhibited a decrease in the phospho-S6 level compared with Eμ-myc/17-19b tumors (Supplemental Fig. S4E).
Figure 6.
miR-19 and mir-17-19b activates the Akt–mTOR pathway. (A) miR-19 is a key mir-17-19b component to activate the Akt–mTOR pathway. Using Western analysis, increased phospho-Akt level was detected in serum-starved 3T3 cells infected with miR-19b, but not miR-17, miR-18a, miR-20a, and the control vector (MSCV). In comparison, the overall Akt level was not affected by miR-19b. (B) miR-19 induces an increase in phosphorylation of S6 ribosomal protein. Enforced expression of miR-19b strongly promoted the S6 phosphorylation as compared with the rest of mir-17-19b components. (C,D) Enforced miR-19b or mir-17-19b expression in the Eμ-myc model led to an increased level of phospho-S6 in lymphomas. Cells derived from the Eμ-myc, Eμ-myc/19b, and Eμ-myc/17-19b lymphomas were analyzed by Western (C) and immunohistochemistry (D). Both Eμ-myc/19b and Eμ-myc/17-19b lymphomas exhibited a high level of phospho-S6, although mir-17-19b seemed to have a stronger effect. In comparison, Eμ-myc tumors exhibited a low level of phospho-S6 and more variation among different samples, possibly reflecting the differences in the secondary oncogenic lesions. In all Western analyses, tubulin (Tub) was used as a normalization control.
Although Pten deficiency in mice frequently gives rise to T-cell malignancy, Pten heterozygosity appears to partially phenocopy mir-17-19b overexpression, and cooperates with c-myc in the Eμ-myc model to moderately accelerate B lymphomagenesis (Wendel et al. 2006). However, the oncogenic effect of miR-19b or mir-17-19b is more potent than Pten heterozygosity in the Eμ-myc lymphoma model. Pten is a haploinsufficient tumor suppressor whose dosage proportionally impacts its tumor suppressor activity and inversely correlates with tumor progression in a cell type-dependent manner (Trotman et al. 2003). It is likely that miR-19b or mir-17-19b overexpression, when compared with Pten heterozygosity, causes greater Pten repression (Supplemental Fig. S4C), and thus a stronger B-cell transformation in the Eμ-myc model. Interestingly, in non-Hodgkin's B-cell lymphomas (NHLs) including Burkitt's lymphoma and DLBCL, recurring deletions and rearrangements of 10q23, which harbors PTEN, are observed in 5%–10% of patients (Butler et al. 1999). In addition, the reduction or loss of PTEN expression is often associated with inferior survival (Abubaker et al. 2007; Robledo et al. 2009). These findings implicate the importance of the PTEN pathway in the progression and prognosis of specific human B-cell malignancies. Besides PTEN deletion and mutations, _mir-17-92_-mediated PTEN repression may be an additional mechanism to disrupt the PTEN function and to promote malignant transformation in human B cells. In line with this hypothesis, a recent study described a subset of DLBCLs in which the amplification of mir-17-92 and the deletion of PTEN are mutually exclusive (Lenz et al. 2008).
Discussion
The small size of miRNAs, combined with their imperfect base-pairing for target recognition, allows miRNAs to regulate many mRNA targets. The strong cooperation between miR-19 and c-myc can result from the collective impacts on many miR-19 targets, of which Pten is a key target, but it may not be the only one. Given the robust induction of phospho-S6 by miR-19, it is possible that other negative regulators of the PI3K–AKT–mTOR pathway, in addition to Pten, are coordinately regulated. It is also conceivable that additional pathways regulated by miR-19 could synergize with down-regulated Pten to promote cell survival and malignant transformation. Increasing evidence suggests that the dosage of tumor suppressor genes has a significant impact on their functional readout. A number of tumor suppressors are haploinsufficient, and hypomorphic mutations in tumor suppressors are frequent in cancer (Payne and Kemp 2005). Given the importance of miRNAs in human cancer and their unique mechanism of action, partial repression of tumor suppressors is likely a novel mechanism through which miRNA oncogenes promote malignant transformation.
Polycistronic gene structure is frequently observed in miRNA loci (Ambros 2004; He and Hannon 2004; Zamore and Haley 2005). Since each miRNA has the potential to regulate hundreds of target mRNAs, a miRNA polycistron containing multiple components may possess a greater capacity for gene regulation, thus yielding pleiotropic biological effects through complex mechanisms of coordination. In the context of B-cell transformation in the _Eμ-m_yc model, miR-19 miRNAs are identified as the key oncogenic components of mir-17-92 to cooperate with c-myc. The other mir-17-92 components either are dispensable for oncogenesis, or, as in the case of miR-18a, may play an accessory role to enhance oncogenic potential. As a strong oncogene, mir-17-92 can regulate multiple cellular processes to favor malignant transformation, causing decreased cell death, rapid cell proliferation, and increased angiogenesis (He et al. 2005; O'Donnell et al. 2005; Dews et al. 2006; Ventura et al. 2008). However, it remains unclear how this polycistronic miRNA regulates coordinated biological processes to achieve malignant transformation. It is conceivable that individual mir-17-92 components function cooperatively to impact multiple cellular processes, although more complex mechanisms of coordination may also exist. A recent study indicates that miR-17 overexpression in mice decreases cell proliferation, adhesion, and migration, raising a possibility that components of mir-17-92 can both positively and negatively regulate the same cellular process to achieve homeostasis (Shan et al. 2009). It is possible that cell types and biological contexts may also determine the exact mechanisms of coordination among polycistronic miRNA components. We are just beginning to understand the functional complexity of polycistronic miRNAs, which have an enormous capacity for gene regulation and complex coordination among different components. The unique gene structure of these miRNA polycistrons can ultimately underlie the molecular basis for their pleiotropic functions in the oncogenic and tumor suppressor network.
Materials and methods
Molecular construction of mir-17-92 subclusters
The subclusters of mir-17-92 were amplified by PCR and subsequently cloned into the XhoI and EcoRI sites of the MSCV retrovirus vectors. In these vectors, miRNAs were placed downstream from the LTR promoter, which is followed by either a SV40-GFP cassette (for all in vivo experiments) or a PGK-Puro-Ires-GFP cassette (for all in vitro experiments) (Hemann et al. 2005). To construct the MSCV_-17-19b/Mut19_ vector, a 12-nt mutation was introduced into the hairpin stem of pre-mir-19a and pre_-mir-19b_ using the QuickChangeXL mutagenesis kit (Strategene). For the MSCV_-17-19b/Mut19_ vector, the loss of miR-19 expression and the normal expression of the other mir-17-19b components were validated using the TaqMan miRNA assays (Applied Biosystems).
Adoptive transfer of Eμ-myc HSPCs for lymphomagenesis
Fetal liver-derived HSPCs were isolated from embryonic day 13.5–15.5 (E13.5–E15.5) Eμ-myc/+ embryos, and were transduced with MSCV alone or MSCV expressing various mir-17-92 subclusters. The MSCV retroviral vector used in our studies contains a SV40-GFP cassette that allows us to monitor transduced HSPCs both in vitro and in vivo. Infected HSPCs were subsequently transplanted into 8- to 9-wk-old, lethally irradiated C57BL/6 recipient mice. Tumor onset was subsequently monitored by weekly palpation, and tumor samples were either collected into formalin for histopathological studies, or prepared as single-cell suspension for FACS analysis.
Luciferase assays
Human PTEN 3′UTR were amplified from the genomic DNA (forward primer, 5′-CACCAAGATGGCACTTTCCCGTTT-3′; reverse primer, 5′-TGGCAAACATGTTCAAGAGGAGCT-3′), which contains two _miR-19_-binding sites that are also conserved in mice. Mutagenesis of these two _miR-19_-binding sites was carried out using the QuickChangeXL mutagenesis kit (Strategene). The wild-type PTEN 3′UTR, as well as the mutated PTEN 3′UTR fragments, were each cloned downstream from a firefly luciferase reporter. These firefly luciferase constructs that contain either the wild-type or the mutated PTEN 3′UTR were each transfected into the Dicer-deficient Hct116 cells (Cummins et al. 2006), together with a Renilla luciferase construct for normalization control, and 50 nM miRNA mimics for mir-17, mir-18, miR-19b, miR-20a, and mir-1, respectively. These miRNA mimics were generated by annealing two complementary RNA oligos (IDT). Luciferase activity of each construct was determined by dual luciferase assay (Promega) 48 h post-transfection.
Cell culture
NIH-3T3 cells were cultured in DMEM with 10% bovine serum, and were kept 30%–40% confluent throughout the entire cell culture experiment. Primary B cells were prepared from mouse bone marrows, and were cultured in RPMI medium with 10% fetal bovine serum (FBS), 50 μM β-mercaptoethanol, and 2 ng/mL Il-7. NIH-3T3 cells and mouse primary B-cell cultures were infected by MSCV retroviruses expressing mir-17-92 subclusters, and were selected by puromycin for 2 d. Serum-starved NIH-3T3 cells were prepared by incubating the cells with DMEM without serum for 12 h before harvesting the cell lysate. MSCV-infected primary B cells were sorted based on GFP, and were then subjected to Western analyses or real-time PCR analyses as described below.
Real-time PCR analysis and Western analysis
TaqMan miRNA assays (Applied Biosystems) were used to measure the level of mature miRNAs. The mRNA level for Pten (forward primer, 5′-CACAATTCCCAGTCAGAGGCGC-3′; reverse primer, 5′-GCTGGCAGACCACAAACTGAGGA-3′) was determined using real-time PCR analysis with SYBR (Applied Biosystems). Actin was used as a normalization control in all our RT-QPCR experiments (forward primer, 5′-GATCTGGCACCACACCTTCT-3′; reverse primer, 5′-GGGGTGTTGAAGGTCTCAAA-3′). For Western analyses, Pten (Cell Signaling), Akt (Cell Signaling), phospho-Akt (Ser473; Cell Signaling), and phospho-S6 (Cell Signaling) antibodies were used at 1:1000, and tubulin antibody (Sigma) was used at 1:4000.
Histopathology
Tissue samples were fixed in formalin, embedded in paraffin, sectioned into 5-μm sections, and stained with haematoxylin and eosin (H&E). For Ki-67 (rabbit anti-Ki67; NovoCastra), caspase-3 (AF835; R&D Systems), and PCNA (MS-106P; Lab Vision Corp.) detection, representative sections were deparaffinized and rehydrated in graded alcohols before being subjected to antigen retrieval treatment in a vegetable steamer. Detection of antibody staining was carried out following standard procedures from the avidin-biotin immunoperoxidase methods. Diaminobenzidine (Invitrogen) was used as the chromogen and haematoxylin was used as the nuclear counterstain. Analysis of the apoptotic rate by TUNEL assay was performed according to a published protocol (Di Cristofano et al. 1998).
Flow cytometry
Lymphoma cells harvested from the animals were resuspended in 10% FBS/phosphate-buffered saline (PBS) to reach a concentration of 107 cells per milliliter. Twenty microliters of the cell suspension were stained with various antibodies diluted in 10% FBS/PBS for 1 h. Subsequently, cells were washed with 2% FBS/PBS and resuspended in 10% FBS/PBS for flow cytometry analysis. Antibodies used for our FACS analyses include PE anti-mouse IgM (eBioscience, 12-5790), APC-Cy7 B220 (BD Pharmingen, 552094), CD4 APC-Cy7 (BD Pharmingen, 552051), PE-CD8 (BD Pharmingen, 553032), PE-CD25 (BD Pharmingen, 553866), and APC-CD19 (Biolegend, 115511).
Apoptotic assays
Xenopus laevis eggs were collected and fertilized, and embryos were cultured by standard procedures. The miR-19b mimics were produced from the annealing products of 5′-UGUGCAAAUCCAUGCAAAACUGA-3′ and 5′-AGUUUUGCAGGUUUGCAUCCAUU-3′ (IDT). Two complimentary RNA oligos were combined and diluted to a stock concentration of 1 μg/μL, heated for 1 min to 80°C, and then allowed to cool to room temperature to form duplexes. The same was done to generate the mutated miR-19 mimics, mut-19b, using 5′-UCAGGUAAUCCAUGCAAAACUGA-3′ and 5′-AGUUUUGCAGGUUACCUUCGAUU-3′. The Pten-mut construct was made by two consecutive QuickChange reactions of accession plasmid BC161129 with primers 5′-ACAAATTTAGCTGCAGAGTAG-3′ and 5′-GGTCTTCAAACGGATACTGAG-3′, and 5′-GCAGAGTGAGGGGAGCGGGT-3′ and 5′-CGGGGAAAGGTTGGCACCCG-3′. Xenopus tropicalis pten and pten-mut RNAs were made using the mMessage SP6 kit (Ambion) on Not1-cut plasmid. Embryos were injected into both cells at the two-cell stage with 2 ng of each RNA. Hydroxyurea (Sigma) was diluted to a final concentration of 15 mM and embryos were treated from 2 h post-fertilization until stage 10.5. All embryos undergoing apoptosis of any cells were scored as positive.
Acknowledgments
We thank B. Zude, A. Basila, Y. Choi, and K. Lehet for technical assistance, and A. Winoto, D. Raulet, G. Barton, L. Coscoy, J. Liu, and R. Vance for stimulating discussions and helpful input. We also thank M. Schlissel and G.S. Martin for careful reading of our manuscript, and J.M. Silva, A. Economides, M. Schlissel, C. Miething, A. Thomas-Tikhonenko, D. Schulz, P. Garcia, M. Sohaskey, D. Stafford, M. Tokuyama, K. Chow, E. Cadera, W. Cousin, L.C. Trotman, and E. Hernando for sharing reagents and helpful discussions. We are particularly grateful to M. Schriok and B. Colpo for unconditional support. L.H. is a Searle Scholar, and is supported by the pathway to independence grant, an RO1 grant from the NCI, and the new faculty award from CIRM. S.W.L. and G.J.H. are both HHMI investigators, and are supported by a program project grant from the NCI.
Footnotes
References
- Abubaker J, Bavi PP, Al-Harbi S, Siraj AK, Al-Dayel F, Uddin S, Al-Kuraya K. PIK3CA mutations are mutually exclusive with PTEN loss in diffuse large B-cell lymphoma. Leukemia. 2007;21:2368–2370. doi: 10.1038/sj.leu.2404873. [DOI] [PubMed] [Google Scholar]
- Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, Palmiter RD, Brinster RL. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318:533–538. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MP, Wang SI, Chaganti RS, Parsons R, Dalla-Favera R. Analysis of PTEN mutations and deletions in B-cell non-Hodgkin's lymphomas. Genes Chromosomes Cancer. 1999;24:322–327. [PubMed] [Google Scholar]
- Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi SW, Zang JB, Mele A, Darnell RB. Argonaute HITS-CLIP decodes microRNA–mRNA interaction maps. Nature. 2009;460:479–486. doi: 10.1038/nature08170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer FI, Parent CA. PI 3-kinases and PTEN: How opposites chemoattract. Cell. 2002;109:541–544. doi: 10.1016/s0092-8674(02)00765-1. [DOI] [PubMed] [Google Scholar]
- Cummins JM, He Y, Leary RJ, Pagliarini R, Diaz LA, Jr, Sjoblom T, Barad O, Bentwich Z, Szafranska AE, Labourier E, et al. The colorectal microRNAome. Proc Natl Acad Sci. 2006;103:3687–3692. doi: 10.1073/pnas.0511155103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, Wentzel E, Furth EE, Lee WM, Enders GH, Mendell JT, et al. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet. 2006;38:1060–1065. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristofano A, Pandolfi PP. The multiple roles of PTEN in tumor suppression. Cell. 2000;100:387–390. doi: 10.1016/s0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- Farh KK, Grimson A, Jan C, Lewis BP, Johnston WK, Lim LP, Burge CB, Bartel DP. The widespread impact of mammalian microRNAs on mRNA repression and evolution. Science. 2005;310:1817–1821. doi: 10.1126/science.1121158. [DOI] [PubMed] [Google Scholar]
- Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- Griffiths-Jones S. miRBase: The microRNA sequence database. Methods Mol Biol. 2006;342:129–138. doi: 10.1385/1-59745-123-1:129. [DOI] [PubMed] [Google Scholar]
- Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: Determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashita Y, Osada H, Tatematsu Y, Yamada H, Yanagisawa K, Tomida S, Yatabe Y, Kawahara K, Sekido Y, Takahashi T. A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65:9628–9632. doi: 10.1158/0008-5472.CAN-05-2352. [DOI] [PubMed] [Google Scholar]
- He L, Hannon GJ. MicroRNAs: Small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007a;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, He X, Lowe SW, Hannon GJ. microRNAs join the p53 network—Another piece in the tumour-suppression puzzle. Nat Rev Cancer. 2007b;7:819–822. doi: 10.1038/nrc2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemann MT, Bric A, Teruya-Feldstein J, Herbst A, Nilsson JA, Cordon-Cardo C, Cleveland JL, Tansey WP, Lowe SW. Evasion of the p53 tumour surveillance network by tumour-derived MYC mutants. Nature. 2005;436:807–811. doi: 10.1038/nature03845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang HW, Wentzel EA, Mendell JT. Cell–cell contact globally activates microRNA biogenesis. Proc Natl Acad Sci. 2009;106:7016–7021. doi: 10.1073/pnas.0811523106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata M, Tagawa H, Guo YM, Kameoka Y, Takahashi N, Sawada K. MicroRNA-17-92 down-regulates expression of distinct targets in different B-cell lymphoma subtypes. Blood. 2009;113:396–402. doi: 10.1182/blood-2008-07-163907. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- Knobbe CB, Lapin V, Suzuki A, Mak TW. The roles of PTEN in development, physiology and tumorigenesis in mouse models: A tissue-by-tissue survey. Oncogene. 2008;27:5398–5415. doi: 10.1038/onc.2008.238. [DOI] [PubMed] [Google Scholar]
- Kota J, Chivukula RR, O'Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M, Clark KR, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar MS, Erkeland SJ, Pester RE, Chen CY, Ebert MS, Sharp PA, Jacks T. Suppression of non-small cell lung tumor development by the let-7 microRNA family. Proc Natl Acad Sci. 2008;105:3903–3908. doi: 10.1073/pnas.0712321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz G, Wright GW, Emre NC, Kohlhammer H, Dave SS, Davis RE, Carty S, Lam LT, Shaffer AL, Xiao W, et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci. 2008;105:13520–13525. doi: 10.1073/pnas.0804295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- Miranda KC, Huynh T, Tay Y, Ang YS, Tam WL, Thomson AM, Lim B, Rigoutsos I. A pattern-based method for the identification of microRNA binding sites and their corresponding heteroduplexes. Cell. 2006;126:1203–1217. doi: 10.1016/j.cell.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Navarro A, Bea S, Fernandez V, Prieto M, Salaverria I, Jares P, Hartmann E, Mozos A, Lopez-Guillermo A, Villamor N, et al. MicroRNA expression, chromosomal alterations, and immunoglobulin variable heavy chain hypermutations in Mantle cell lymphomas. Cancer Res. 2009;69:7071–7078. doi: 10.1158/0008-5472.CAN-09-1095. [DOI] [PubMed] [Google Scholar]
- O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- Ota A, Tagawa H, Karnan S, Tsuzuki S, Karpas A, Kira S, Yoshida Y, Seto M. Identification and characterization of a novel gene, C13orf25, as a target for 13q31-q32 amplification in malignant lymphoma. Cancer Res. 2004;64:3087–3095. doi: 10.1158/0008-5472.can-03-3773. [DOI] [PubMed] [Google Scholar]
- Robledo C, Garcia JL, Caballero D, Conde E, Arranz R, Flores T, Grande C, Rodriguez J, Garcia E, Saez AI, et al. Array comparative genomic hybridization identifies genetic regions associated with outcome in aggressive diffuse large B-cell lymphomas. Cancer. 2009;115:3728–3737. doi: 10.1002/cncr.24430. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Weissman IL. Pten, tumorigenesis, and stem cell self-renewal. Cell. 2006;125:229–231. doi: 10.1016/j.cell.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Schmitt CA, Wallace-Brodeur RR, Rosenthal CT, McCurrach ME, Lowe SW. DNA damage responses and chemosensitivity in the E mu-myc mouse lymphoma model. Cold Spring Harb Symp Quant Biol. 2000;65:499–510. doi: 10.1101/sqb.2000.65.499. [DOI] [PubMed] [Google Scholar]
- Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- Shan SW, Lee DY, Deng Z, Shatseva T, Jeyapalan Z, Du WW, Zhang Y, Xuan JW, Yee SP, Siragam V, et al. MicroRNA MiR-17 retards tissue growth and represses fibronectin expression. Nat Cell Biol. 2009;11:1031–1038. doi: 10.1038/ncb1917. [DOI] [PubMed] [Google Scholar]
- Tagawa H, Seto M. A microRNA cluster as a target of genomic amplification in malignant lymphoma. Leukemia. 2005;19:2013–2016. doi: 10.1038/sj.leu.2403942. [DOI] [PubMed] [Google Scholar]
- Tagawa H, Karube K, Tsuzuki S, Ohshima K, Seto M. Synergistic action of the microRNA-17 polycistron and Myc in aggressive cancer development. Cancer Sci. 2007;98:1482–1490. doi: 10.1111/j.1349-7006.2007.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura S, Mitsutake N, Nakashima M, Namba H, Saenko VA, Rogounovitch TI, Nakazawa Y, Hayashi T, Ohtsuru A, Yamashita S. Oncogenic role of miR-17-92 cluster in anaplastic thyroid cancer cells. Cancer Sci. 2008;99:1147–1154. doi: 10.1111/j.1349-7006.2008.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotman LC, Niki M, Dotan ZA, Koutcher JA, Di Cristofano A, Xiao A, Khoo AS, Roy-Burman P, Greenberg NM, Van Dyke T, et al. Pten dose dictates cancer progression in the prostate. PLoS Biol. 2003;1:E59. doi: 10.1371/journal.pbio.0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, Newman J, Bronson RT, Crowley D, Stone JR, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132:875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JC, Harland RM. microRNA-24a is required to repress apoptosis in the developing neural retina. Genes & Dev. 2009;23:1046–1051. doi: 10.1101/gad.1777709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendel HG, Malina A, Zhao Z, Zender L, Kogan SC, Cordon-Cardo C, Pelletier J, Lowe SW. Determinants of sensitivity and resistance to rapamycin–chemotherapy drug combinations in vivo. Cancer Res. 2006;66:7639–7646. doi: 10.1158/0008-5472.CAN-06-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, Henderson JM, Kutok JL, Rajewsky K. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol. 2008;9:405–414. doi: 10.1038/ni1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamore PD, Haley B. Ribo-gnome: The big world of small RNAs. Science. 2005;309:1519–1524. doi: 10.1126/science.1111444. [DOI] [PubMed] [Google Scholar]