Seven Transmembrane Receptors as Shapeshifting Proteins: The Impact of Allosteric Modulation and Functional Selectivity on New Drug Discovery (original) (raw)
Abstract
It is useful to consider seven transmembrane receptors (7TMRs) as disordered proteins able to allosterically respond to a number of binding partners. Considering 7TMRs as allosteric systems, affinity and efficacy can be thought of in terms of energy flow between a modulator, conduit (the receptor protein), and a number of guests. These guests can be other molecules, receptors, membrane-bound proteins, or signaling proteins in the cytosol. These vectorial flows of energy can yield standard canonical guest allostery (allosteric modification of drug effect), effects along the plane of the cell membrane (receptor oligomerization), or effects directed into the cytosol (differential signaling as functional selectivity). This review discusses these apparently diverse pharmacological effects in terms of molecular dynamics and protein ensemble theory, which tends to unify 7TMR behavior toward cells. Special consideration will be given to functional selectivity (biased agonism and biased antagonism) in terms of mechanism of action and potential therapeutic application. The explosion of technology that has enabled observation of diverse 7TMR behavior has also shown how drugs can have multiple (pluridimensional) efficacies and how this can cause paradoxical drug classification and nomenclatures.
I. Receptors as Allosteric Proteins
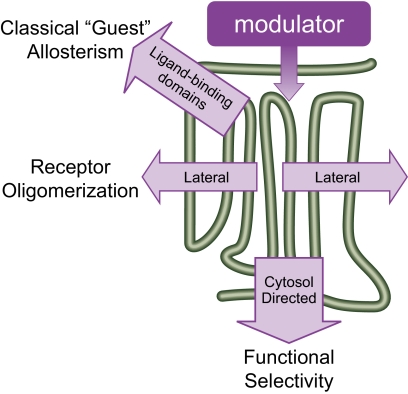
Seven transmembrane receptors (7TMRs1) are molecules, situated as intrinsic plasma membrane proteins, that bind to natural ligands approaching from one milieu (extracellular) and respond by activating signaling cascades emanating from molecular interactions in a distinct (cytosolic) milieu. Their fundamental nature requires extracellular ligand binding to result in a dynamic change in receptor conformation that is reflected in exposure of a signaling domain at the cytosolic surface, which interacts with the classic proximal effecter partner, a heterotrimeric G protein. However, not only are these regions of classic function important, but they also provide their respective regions for the binding of allosteric ligands from the extracellular space and the cytosol. In addition, the intramembranous surfaces of 7TMRs within the plane of the membrane provide still more sites for possible allosteric action. These three allosteric vectors, directed toward 1) the ectodomain, 2) the cytosolic face, and 3) the intramembranous faces of 7TMRs (Fig. 1), provide numerous opportunities for functional selectivity of the action of drugs (see section V.C.2.c). Such functional selectivity can even manifest itself differently at the same receptor expressed in distinct cellular environments that are present not only in different cells in different organs but even in the same type of cell in a single organ that might be affected differentially by its local environment.
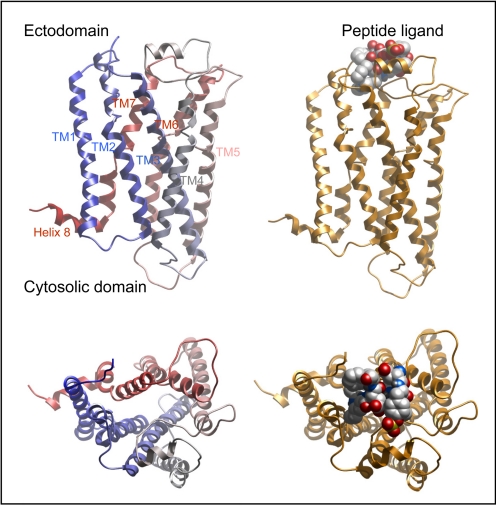
Fig. 1.
Typical topology of 7TMRs. Shown is a representative structure of a family A 7TMR with a peptide ligand. The extracellular ectodomain is shown in the top of the lateral views, whereas the top views provide appreciation for the helical bundle domain. The cytosolic region is shown at the bottom of the lateral views. The transmembrane segments are colored blue to red from amino terminus toward carboxyl terminus of the receptor. Shown at the carboxyl-terminal end of TM7 is the extension into another helix 8 that lies adjacent to the cytosolic face of the lipid bilayer.
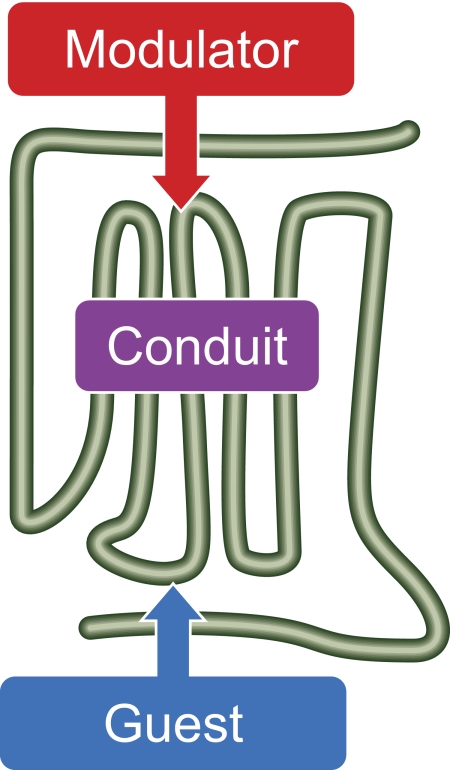
This review considers 7TMRs as a means of information transfer from the extracellular space to the cytosol. As will be seen, there is two-way transfer of information that communicates the state and needs of the cell to the extracellular space. They do this through a change in shape, specifically referred to as a change in conformation. The mechanism by which this occurs is allosterism. The term allosteric is derived from the Greek word allos, meaning “else” or “different.” Early ideas about allosterism related to the explanation of how products of enzyme pathways, structurally unrelated to the substrates, could influence the activity of some bacterial enzymes (Umbarger, 1956). Ideas then progressed toward the postulate that these effects occurred not through mutual exclusion and steric hindrance of substrate from the active site but rather through the interaction of molecules at topographical and stereochemically distinct sites (Changeux, 1961; Monod and Jacob, 1961). The remoteness of these sites of interaction was suggested by early discussions of these interactions being “teleonomic” (Monod and Jacob, 1961). In biochemical terms, allosterism relates to a change in shape and activity of a protein that results from combination with another substance at a point other than the chemically active site. It is important to note that allosterism must be defined in terms of the bodies involved and can change, both in quality and quantity of effect, for the same protein with different interactants. It is useful to define allosterism in terms of three interacting species: the modulator, a ligand or protein that binds to a conduit (usually a protein) that transduces the thermodynamic allosteric energy to a guest, which receives the influence of the modulator through the conduit (Fig. 2). It should also be noted that allosteric effects are reciprocal in that guests impart the same allosteric energy through the conduit back to the modulator (Tränkle et al., 1999). In this sense, the roles of modulator and guest become interchangeable in that the effect of the modulator on the guest is identical to the effect of the guest on the modulator. Allosterism is an extremely important biochemical mechanism (even having been referred to as the “second secret of life,” second only to the genome) (Fenton, 2008) because it allows proteins to sense their environment and react to it. The power of the mechanism emanates from the ability of the protein to sense from sites other than the active site or the site being modulated. Therefore, the active site is free to function until changes in the environment dictate that a change should occur. As a preface to discussion of 7TMR function, it is useful to consider their structure.
Fig. 2.
Allosteric systems. Energy is transmitted between loci of binding for the modulator and that of the guest. Energy transfer is reciprocal (Tränkle et al., 1999); therefore, the role of modulator and guest are operationally interchangeable. For an extracellular modulator binding site, the guest binding site can be extracellular, within the plane of the membrane, or facing the cytosol.
II. The Structural Organization of Seven Transmembrane Receptors
Members of the superfamily of 7TMRs are believed to have evolved from a common precursor, retaining their heptahelical architecture and their physical and functional coupling to heterotrimeric G proteins yet being distributed into families and subfamilies that have distinctive structural elements and distinctive themes for ligand binding, receptor activation, and receptor regulation (Kolakowski, 1994; Fredriksson et al., 2003; Römpler et al., 2007). Although primary structural information has been used to divide this superfamily into major families, tertiary structural information is available for intact receptors in only one of these families.
There are finally high-resolution three-dimensional structural data for a few members of the largest group of 7TMRs, representing family A (the rhodopsin/β-adrenergic receptor family) (Cherezov et al., 2007; Rasmussen et al., 2007; Rosenbaum et al., 2007; Hanson et al., 2008; Jaakola et al., 2008; Warne et al., 2008). These have confirmed many of the de novo structural predictions and interpretations of lower resolution structures that had been made previously and have finally provided a level of molecular detail adequate to explain many pharmacological observations and many of the general concepts of 7TMR allosterism. This family includes many of the receptors for biogenic amines and small peptides. The transmembrane segments of family A 7TMRs contain most of the signature sequences defining this family, making it no surprise that the structures of the helical bundle regions of these 7TMRs seem to be highly conserved.
7TMR family B has substantially fewer members yet contains several strong candidates for the development of potentially important drugs (Harmar, 2001; Hoare, 2005). The natural ligands for these receptors are all moderately large peptides, such as calcitonin, parathyroid hormone, and glucagon, that have diffuse pharmacophoric domains. This family of receptors has a characteristic long amino-terminal tail containing six conserved cysteine residues that contribute to three conserved disulfide bonds that provide structural stability and a conserved cleft for the docking of the often helical carboxyl-terminal region of the peptide ligands (Grace et al., 2004). This helps to orient the amino terminus of these peptides, which is critical for biological activity toward the receptor helical bundle region (Tan et al., 2006). It is noteworthy that, based on the absence of the signature sequences from family A and on the conservation of a distinct set of residues and the polarity of these residues in family B, the helical bundle of family B 7TMRs is predicted to be structurally distinct from that of family A 7TMRs (Donnelly, 1997; Tams et al., 1998). No direct experimental data yet exist to provide insights into the structure of this important domain of these receptors.
Family C 7TMRs also have a very large amino-terminal domain, often with a Venus flytrap-like structure (not found in the orphans in this family), that seems to play important roles in natural ligand binding (de Vos et al., 1992; White et al., 1998; Kunishima et al., 2000). The ligands for these receptors are actually quite diverse, including large glycoprotein hormones and very small molecules such as glutamate, calcium, GABA, and some taste molecules and l-α-amino acids. The 22 subtypes of receptors that have been described in this family are divided into four groups, including 1) the metabotropic glutamate receptors; 2) the calcium-sensing receptor, taste receptors, and GPRC6A; 3) the type B GABA receptors; and 4) orphan receptors. Dimerization is a fundamental theme for these receptors, with important functional impact of homo- versus heterodimerization. This theme will be discussed in depth below.
Themes for molecular interactions with 7TMRs are conserved throughout the superfamily, although the details of some of these themes diverge in individual families. These themes include 1) interactions with the extracellular face of the receptors, including ligands, both natural and pharmaceutical (guest allostery); 2) interactions with the cytosolic face of the receptors, including heterotrimeric G proteins, as well as other key cytosolic proteins (cytosolic allostery: biased agonism and antagonism); and 3) interactions in the plane of the lipid bilayer, including interactions with other 7TMRs and other membrane proteins (lateral allostery: oligomerization).
A. Structure and Interaction of Ligands with Seven Transmembrane Receptors
1. Interaction of Seven Transmembrane Receptors with Natural Ligands.
The mode of natural ligand binding and the structures of the ligands correlate with positions within the proposed minimally plausible phylogenetic tree of 7TMR evolution (Kolakowski, 1994). In fact, the position within this tree has often been the best clue to the identity of the natural ligand that recognizes a particular orphan receptor. Family A contains rhodopsin and many of the biogenic amine receptors in which the natural ligands typically dock within the helical bundle at the level of the lipid bilayer (Ji et al., 1998). This family also includes a large number of receptors that bind peptide ligands, particularly those having a focused pharmacophoric region at the carboxyl terminus of the ligand. Such ligands typically have binding determinants closer to the outside surface of the plasma membrane, often with contributions of external loop and tail regions of their receptors, although some have been postulated to dip into the helical bundle. The greatest degree of similarity among family A 7TMRs resides within their transmembrane segments. Indeed, three regions of these receptors that have been postulated to play critical roles in the conformational changes in these receptors, are associated with their activation, and have been identified as “microswitches” are within these segments (Nygaard et al., 2009). These include Trp6.50 (the W in the CWxP motif in TM6) at the bottom of the major intrahelical ligand-binding pocket, Tyr7.53 (the Y in the NPxxY motif in TM7) that is believed to connect the bottom of TM7 and helix 8 at the cytosolic surface of the lipid bilayer, and Arg3.50 (the R in the D/ERY motif at the bottom of TM3) at the cytosolic face of TM3, where it probably interacts with the G protein. These are postulated to contribute to an extended set of toggle switch movements that link ligand binding to a region accessible from the extracellular milieu to conformational changes that facilitate protein-protein interactions at the cytosolic interface and result in intracellular signaling events.
An allosteric molecule such as a 7TMR is capable of assuming multiple conformations, some of which may be “active” (i.e., facilitating the coupling or association with a molecule that initiates a signaling cascade) (Kobilka and Deupi, 2007). Some of the conformations are clearly inactive and may be pharmacologically silent. It is possible that only a subset of conformations will interact with naturally occurring regulatory molecules. There is an equilibrium between the dynamic conformations of 7TMRs, with many “locked” into inactive, nonsignaling, low-energy states, whereas some are capable of constitutive activity, reflecting assumption of an active conformation for at least a portion of the time (Kobilka and Deupi, 2007). The “microswitches,” we are beginning to understand, are likely to play important roles in protecting the receptor from the classic active conformation when they are in the “off” position and in stabilizing this conformation when they are in the “on” position (Schwartz et al., 2006). As we understand more about allosteric ligands, it is likely that they, too, will be discovered to stabilize a variety of conformations, ranging from the “full on” conformation (which may be typical of a full allosteric agonist), to “variant partially on” conformations (which may be typical of biased agonists or allosteric modulators), and to “off” conformations (which may be typical of allosteric inhibitors). It is particularly interesting that there are examples where even natural ligands might express differential functional profiles at a naturally occurring receptor (Picchio et al., 2008; Moxley et al., 2009) (see section V.C.2.c), as was first described for CCL19/CCL21 at the CCR7 receptor (Kohout et al., 2004). Although the specific structural basis for this is not yet clear, it is consistent with the notion that these ligands stabilize distinct active conformations that facilitate different patterns of proximal effector interactions.
For many years, the prototypical 7TMR structure was based on rhodopsin, for which there was biochemical and biophysical data, including the only high-resolution crystal structure (Palczewski et al., 2000). Since 2000, a number of inactive, dark-state crystal structures of various states of bovine rhodopsin and thermostable mutants have been reported (Okada et al., 2004; Standfuss et al., 2007). Since 2007, two different structures of the β2-adrenergic receptor, the β1-adrenergic receptor, squid rhodopsin, and the adenosine A2a receptor bound to antagonists were reported (Cherezov et al., 2007; Rasmussen et al., 2007; Rosenbaum et al., 2007; Hanson et al., 2008; Jaakola et al., 2008; Warne et al., 2008). All of these have highly similar helical bundles, whereas the loop and tail regions were quite divergent. These structures also highlight a major funnel-shaped intrahelical ligand-binding pocket, and extracellular loop 2b sits above the entry, perhaps playing a gating role (Nygaard et al., 2009).
It was only in 2008, when a crystal structure was solved for opsin associated with a carboxyl-terminal peptide fragment of its Gα subunit, transducin, that the first minimally active conformation of a family A 7TMR could be directly appreciated (Scheerer et al., 2008). This structure suggested the presence of a substantial change in the arrangement of the helical bundle, with prominent movement of TM6. Clearly, it will be critical to elucidate additional active structures of these receptors in the future.
Indeed, analogous insights have come from an in silico molecular modeling analysis of inactive and predicted active structures of the β2-adrenergic receptor (Katritch et al., 2009). In this work, the authors used a powerful energy-based computational approach directed by ligand structures and complemented by mutagenesis data to gain insights into the activation mechanism for this family A 7TMR. In this report, the authors predict an activation mechanism that is accompanied by tilting of the extracellular portion of TM5 toward the receptor axis. This movement is shown to facilitate interaction of the full and partial agonist ligands with this receptor, with the ligand tail interacting with side chains of Asp113 and Asn312 and the ligand head interacting with side chains of Ser203, Ser204, and Ser207. The kinetics of achieving the active conformation are supported by two fast steps, representing agonist fit into a loose binding pocket and conformational changes facilitating full engagement in the binding pocket, and a slow step representing more substantial movements and deformations of the transmembrane helices.
The natural ligands for family B 7TMRs are all moderately large peptides with diffuse pharmacophoric regions, the amino terminus of the peptides playing important roles in receptor activation (Ulrich et al., 1998). This is quite distinct from peptide ligands for family A 7TMRs. Sequence and conservation analysis of the predicted transmembrane segments of family B 7TMRs has suggested a very different structure than that present in family A 7TMRs (Donnelly, 1997; Tams et al., 1998). The longer amino-terminal tail region of family B 7TMRs is quite conserved, including the presence of six cysteines and three disulfide bonds, and structure-activity, mutagenesis, and photoaffinity labeling studies have all supported an important role of this region in natural ligand binding. Indeed, recent solution of NMR and crystal structures of amino-terminal domains of several family B 7TMRs, including ligand-associated states, has confirmed this and has provided some insights into the mode of ligand binding (Grace et al., 2004, 2007; Parthier et al., 2007; Sun et al., 2007a; Pioszak and Xu, 2008; Pioszak et al., 2008; Runge et al., 2008).
The characteristic structure of the amino-terminal region of family B 7TMRs includes two antiparallel β-sheets, three disulfide bonds, an amino-terminal α-helix, and multiple loop regions. The carboxyl-terminal regions of the natural peptide ligands for these receptors that are often in helical structures are believed to occupy a hydrophobic binding cleft between the amino-terminal α-helix and loop structures. Although there are minor differences in the docking of these ligands in the NMR and crystal structures relative to predictions coming from other types of experimental constraints, such as photoaffinity labeling, the theme of docking in this region has been quite consistent. Unfortunately, the orientation of the receptor amino terminus relative to the helical bundle of the family B 7TMRs is unknown, and there are not yet adequate constraints to guide this process. Here, unlike the relative consistency of predictions for site of peptide docking, the predictions for domain orientation are radically different and diverse—even the face of the amino-terminal domain that is directed toward the helical bundle varies from model to model. Additional experimental constraints (or an intact receptor structure) clearly will be necessary to advance this field.
Family C 7TMRs also have large amino-terminal domains that are quite important in natural ligand binding. Here, crystal structures of this region have nicely defined structures and mode of ligand binding (de Vos et al., 1992). The Venus flytrap domain contains two globular domains arranged as a central β-sheet flanked on both sides by α-helices, connected by a hinge region with a central cleft. This domain is often situated above a cysteine-rich domain of unknown function that connects it to the transmembrane helical bundle domain. The cysteine-rich domain contains nine conserved cysteines that form four intradomain disulfide bonds and one disulfide bond directed to the Venus flytrap domain. The disulfides seem to be critical for function and are believed to add a degree of rigidity to the structure. It is noteworthy that constitutive dimerization of the family C 7TMRs has been the theme. The type B GABA receptor and the taste1 receptors in this family exist as heterodimers, composed of two distinct structures, whereas the calcium-sensing receptor and the metabotropic glutamate receptors form homodimers. The latter are quite interesting in that the homodimeric structures are established by both covalent and noncovalent interactions. The inter-receptor disulfide bond is located in a loop of the Venus flytrap domain. One large protein ligand can span both protomers of this dimeric amino-terminal structure. Here in family C 7TMRs, as in family B 7TMRs, we have little information about the structure of the helical bundle region. However, there has at least been consistency in the proposed orientation of the amino-terminal and helical bundle domains for this family.
In summary, essentially all 7TMRs follow the structural theme of possessing seven transmembrane helical segments that arrange in a bundle in the lipid bilayer. Most of these couple with heterotrimeric G proteins as the dominant physiological effector pathway. Three major families within this superfamily follow distinct structural themes that even include predicted differences in the structures of the seven-helix bundle. Each family follows its unique themes for types of natural ligands, mechanisms of activation, and importance of interaction with other endogenous proteins within the cell.
2. Interaction of Seven Transmembrane Receptors with Drugs.
Interactions with pharmaceutical ligands (drugs) follow few rules, requiring only the presence of “druggable pockets” within the receptors to provide adequate binding energy to stabilize the drug-receptor complex. Here, the diversity of the 7TMR families and their structures and themes for natural ligand binding provide many options for drug action. All the families are likely to provide the opportunity for small-molecule ligand binding within the helical bundles. For many family A 7TMRs, that also represents the orthosteric natural ligand binding pocket, whereas for others, this is an allosteric binding pocket. There are examples of use of this docking site for agonists, partial agonists, and antagonists. The theoretical advantages of allosteric ligands have been extensively reviewed recently (Kenakin, 2004, 2007; Leach et al., 2007; Schwartz and Holst, 2007). It is interesting that all the concepts of allosteric modulation are also likely to be relevant to regions of the receptors where there are no distinct “pockets.” Examples of this are the effects that have been attributed to lipid microenvironments of the receptor. Although this has not been studied in great detail, many observations support variations in signaling based on such interactions with the intramembranous faces of 7TMRs (Harikumar et al., 2007, 2008; Gao et al., 2009).
For family A 7TMRs, there are now many examples of small-molecule drugs with a broad spectrum of activities. Detailed insights into molecular mechanisms of binding exist for a limited number of these drugs, particularly those docking within the structurally constrained helical bundle. There is clear evidence for some of these small molecules to dock closer to or even at the surface of these receptors (Ji et al., 1998). In general, as the site of docking of such ligands moves toward the surface to include external loop and tail regions of the receptors, our understanding of the molecular basis of this process becomes less well defined. This probably reflects the great diversity of structures of these less-well defined regions and their greater degrees of structural freedom. Essentially all such ligands for family A 7TMRs that we currently understand bind within a funnel-shaped region with its apex within the helical bundle. There are two relatively well defined pockets in these receptors that are established by the confluence of TM3 with TM2, TM6, and TM7 (shallow pocket), and that of TM3 with TM4, TM5, and TM6 (deeper pocket) (Schwartz, 1994).
The opportunities for distinct “druggable” pockets in family B and family C 7TMRs seem to be greater, reflecting the larger size and conserved structural complexity of the amino-terminal domains of those receptors. Although the amino-terminal regions of these receptors are larger than thpse of family A receptors, providing more opportunities for sites of binding, it may also be more challenging to elicit and stabilize a relevant conformational change by binding a small molecule to such regions. Antagonists targeting such regions may be easier to develop than agonists. Given what we believe we understand about the structure-activity considerations and docking of natural peptide ligands to family B 7TMRs (Hoare, 2005), the binding cleft in the amino terminus of the receptor that interacts with the carboxyl-terminal region of the peptides is probably a prime target for small-molecule antagonist docking (Grace et al., 2007). Likewise, once we understand the region of these receptors that interacts with the amino-terminal end of the natural peptide ligands (postulated to be somewhere within the second or third extracellular loop regions), this should provide a fertile site for the action of a broad range of ligands. There is theoretical reason to believe that these can range from blocking antagonists to mimicking full or partial agonists. This still leaves the helical bundle region for allosteric drug action at these receptors. In addition, recent data suggest that the “hinge” region linking the amino-terminal domain and the helical bundle domain of these receptors may also contain a “druggable” pocket that can have agonist activity (Dong et al., 2009). The structural details of this region are still poorly defined.
In summary, each family of 7TMRs has its own unique mode of natural ligand binding that reflects its unique structure. Although this represents the binding of orthosteric ligands, there are many opportunities for the binding of allosterically active drugs. Each 7TMR family provides several opportunities for the development of allosteric modulators.
III. Receptor Conformation as Protein Ensembles
Theoretical modeling (Crozier et al., 2003; Spijker et al., 2006) and experimental data [Gether, 2000 (i.e., site-directed spin labeling, site-directed fluorescence quenching, sulfhydryl accessibility, disulfide cross-linking); see methods reviewed in Meng and Bourne, 2001; Hubbell et al., 2003; Kobilka, 2002; Park et al., 2008] show that receptor proteins exist as collections (termed “ensembles”) of tertiary conformations. The differences in these conformations need not be large. For example, changes of as little as 1 Å can lead to profound effects on the activity of enzymes and receptors (Koshland, 1998). Receptors sample these conformations (i.e., roll on a funnel-shaped “energy landscape”) (Frauenfelder et al., 1988, 1991; Woodward, 1993; Dill and Chan, 1997; Hilser and Freire, 1997; Miller and Dill, 1997; Hilser et al., 1998, 2006; Freire, 1998; Ma et al., 2002) according to changes in the thermal energy in the system. Thus, proteins are dynamically fluctuating macromolecules constantly changing conformations (described as “breathing” by Englander and Kallenbach, 1983) and taking conformational excursions away from a canonical native structure (Liu et al., 2006a,b). This dynamic view of proteins is supported by computer simulations and NMR data (Volkman et al., 2001; Ikeguchi et al., 2005; Bahar et al., 2007; Henzler-Wildman et al., 2007) or electron paramagnetic resonance (Blackburn et al., 2009). In terms of the number of states sampled by a protein, early models of two discrete protein states (Monod et al., 1965) have been extended to models describing thermal fluctuations over a continuum of states (Kotani, 1968). In fact, there are probably a vast number of conformations associated with energy wells in the landscape and thus are frequented more often than random chance in the normal course of conformational sampling. Moreover, the bottom of the energy wells are probably “rugged,” allowing for a range of nearly isoenergetic conformers; the more flexible is the protein, the larger the ensemble of conformers (Kumar et al., 2000). The energy wells relate to the population times spent in each conformation; a high population time would correspond to a conformation that the protein commonly adopts, whereas a low population time would correspond to a relatively rare conformation. Because allostery involves changes in protein conformation, the ability of a protein (receptor) to take on new conformations is related to the ability of the protein to be allosterically modulated. Therefore, a protein with an already rigid structure is less inclined to be allosterically modulated than a protein with a high degree of intrinsic disorder. Thus, there must be a balance between thermodynamic stability to support specificity (Janin and Wodak, 1983; Frauenfelder, 1989; Gerstein et al., 1994) and flexibility to mediate conformational change to catalyze biochemical reaction pathways (Livesay et al., 2004). Molecular dynamics has been used to determine that signaling proteins have an unusual amount of intrinsic disorder, making them ideal candidates to be allosterically modulated (Liu et al., 2006b; Hilser and Thompson, 2007). As stated by Mittag and Forman-Kay (2007), “‖ all states that are accessible to proteins, whether they contain stably folded globular structure, stretches of transiently populated structural motifs or little structure, are likely to be exploited by living cells for some function.” If a conformation leads to a defined cytosolic outcome (i.e., second messenger production, internalization, phosphorylation, binding of cytosolic protein) then it may be operationally defined as an “active state.” The biological activities controlled by a given receptor will be mediated by the energy-weighted contributions of the component microstates of the ensemble (Hilser et al., 2006). Thus, there could be ensembles for a number of cellular functions modulated by the receptor (Kenakin, 2002b). A reasonable model for ligand interaction with such a system describes differential binding to the various states; the greater the affinity a ligand has for a particular state, the greater the binding.
In summary, a collection of receptor proteins should be thought of as a dynamic system of interchanging conformations, not a single static tertiary conformation. The ability of a given protein to change conformation may be linked to its ability to be allosterically modulated and also to induce a cellular response through binding to cytosolic signaling proteins.
IV. Allosteric Transitions within Receptor Ensembles
To understand the interaction of molecules with various receptor conformations, it is useful to discuss mechanisms of interaction of molecules with protein ensembles. Two extreme views are embodied in the concepts of “conformational induction” and “conformational selection.” Induction is a product of the 50-year-old concept proposed by Koshland (1958), wherein the molecule contributes energy to cause a change in the conformation of the receptor. Conformational selection is rooted in the “population shift” model emanating from the Monod-Wyman-Changeux model of allostery (Monod et al., 1965) whereby molecules selectively bind to pre-existing conformations to stabilize them and thus bias the system toward a predominance of those conformations (Bosshard, 2001; James and Tawfik, 2003). Conformational induction and selection can be viewed in terms of jumps on dynamic energy landscapes for receptors. Thus, a receptor may have a canonical low-energy conformation and somewhat higher energy “active state” conformation. For both mechanisms, binding of the ligand causes the active state to become the preferred low-energy state. In terms of the conformational selection hypothesis, there is a small probability that the receptor pre-exists in the active state without ligand bound. The ligand binds to this conformation, thus stabilizing it and driving the equilibrium toward a more stabilized active state. Within the conformational induction scenario, the ligand binds to the low-energy inactive state of the receptor to cause a conformational change in the protein to the active state. Molecular simulations favor conformational shift models for the binding of small molecules to proteins (in pharmacological terms, drug-like molecules to receptors) (Okazaki and Takada, 2008). It should be noted that a selection of a relatively rare pre-existing conformation would thermodynamically resemble induction (Kenakin, 1996). For example, kinetic experiments for glutathione transferase (Stella et al., 1998; Nieslanik et al., 1999) and ester hydrolyzing antibodies (Geyer et al., 1996; Lindner et al., 1999) show that what was considered conformational induction was equally consistent with pre-existing equilibria between high- and low-affinity conformations.
Conformational selection was proposed by Burgen (1981) to account for pharmacological efficacy of molecules acting on drug receptors. Specifically, biologically active molecules bind selectively to certain receptor conformations that mediate physiological activity (so called active conformations) and thus enrich their presence in the ensemble (Burgen, 1981). This process, articulated as Le Chatelier's principle, leads to enrichment of the preferred conformations at the expense of others (i.e., if a dynamic equilibrium is disturbed by changing the conditions, the position of the equilibrium moves to counteract the change). The various members of the stabilized ensembles may or may not have distinct biological activity (Onaran and Costa, 1997; Onaran et al., 2000). If they interact with cytosolic signaling proteins, then a unique direct signal may be obtained from ligand binding; this will be discussed specifically in section V.C. The resulting stabilized conformation may have no interaction with cellular proteins but may change the behavior of the receptor toward another ligand; this falls under the classification of classic guest allostery (see section V.A).
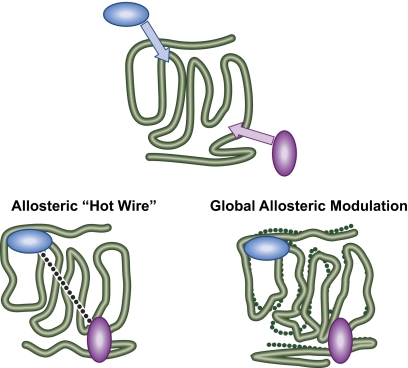
A traditional view of allosteric linkage has proposed specific pathways that link the allosteric modulation site with the guest (substrate, endogenous ligand) binding site, in effect an energetic “hot wire” joining the sites (Lockless and Ranganathan, 1999; Datta et al, 2008) (Fig. 3, left). This view is now largely supplanted by a more general view involving population dynamics relating allosteric effect to order/disorder transitions to mediate long range allosteric communication (Liu et al., 2006a). Within this latter idea, the coupling between sites depends upon the intrinsic stabilities of the domains and the interactions between them, which, in turn, depend upon probability distributions resulting from the conformational energies within the receptor protein. Thus, the energy balance within the protein (i.e., which receptor states are most stable and which states bind ligand) and not a mechanical link between sites mediates the energetics of allosterism (Hilser and Thompson, 2007). Although stabilization of conformations usually is the cause of the existence of allosterically distinct states, even thermal energy can cause allosteric modulation through changes in domain stability (Cooper and Dryden, 1984; Popovych et al., 2006). There is evidence to suggest that the binding of ligands to protein ensembles reduces motion (Park et al., 2008). For example, there is a significant reduction in conformational heterogeneity of the protein Sem-5 upon binding of its peptide ligand (Ferreon and Hilser, 2003). This is consistent with the notion that exposure of certain loci on 7TMRs to G proteins initiates interaction and that the inactive state of the receptor is a closed conformation shielding these sites from the cytosol. Consistent with this notion is the fact that an eleven-amino acid peptide sequence from the C-terminal region of the third intracellular loop of the β-adrenoceptor (Thr284–Thr291) has the ability to initiate Gs-mediated adenylate cyclase activation in turkey erythrocytes (Münch et al., 1991). In general this suggests that the special conformations (i.e., those that shield active sequences of the 7TMR from cytosolic protein binding) are the inactive (closed) conformations. Circumstantial evidence for this comes from mutation studies. For example, point mutation at position 293 of α1B-adrenoceptor with 22 different amino acids yields 22 mutants, all of which demonstrate constitutive activity for inositol phosphate production (i.e., they are active states) (Kjelsberg et al., 1992). This suggests that there are a number of active states for 7TMRs that are associated with disorder and relatively fewer inactive states.
Fig. 3.
Two proposed modes of allostery. The allosteric “hot wire” proposes a preferred energy link between an allosteric binding site and the guest site; in the past, this has been an assumed mechanism. Global allosteric modulation predicts that changes at the guest allosteric site are part of global conformational variations within an ensemble of conformations.
A useful idea to describe the new behaviors assigned to an allosterically modulated receptor is to consider that ligand binding essentially moves the receptor onto another energy landscape (Peleg et al., 2001). Within this idea, the native and allosteric ensembles have globally distinct conformations (Fig. 3, right) and many other regions of the receptor proteins may differ in addition to the binding sites for the modulator and guest. This idea is supported by the ability of allosteric modulators to cause disruption of the interactions of huge proteins that bind to each other at numerous loci. For example, mutation and structural data suggest that the CCR5 chemokine receptor and HIV-1 viral coat binding protein gp120 interact at numerous points in the fusion and subsequent viral infection process (Atchison et al., 1996; Doms and Peiper, 1997; Doranz et al., 1997; Picard et al., 1997; Rucker et al., 1997). This situation is not readily amenable to blockade through steric interaction of small drug-like molecules. The low molecular weight drug-like inhibitors that block HIV-1 entry have been shown to do so through an allosteric mechanism (Watson et al., 2005; Muniz-Medina et al., 2009); this, in turn, is consistent with a global change in CCR5 conformation to interfere with numerous regions of interaction between CCR5 and gp120.
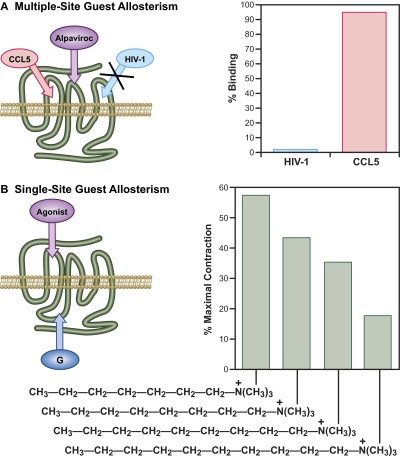
In terms of the relative geography of binding of modulators and guests, there is a wide range of distances between these in various proteins. For example, the binding site for 5-chloro-1_H_-indole-2-carboxylic acid (1-(4-fluorobenzyl)-2-(4-hydroxypiperidin-1-yl)-2-oxoethyl)amide (CP320626) for glycogen phosphorylase b is 33 Å from the catalytic site and 15 Å from the site for cyclic AMP (Oikonomakos et al., 2000). If it is accepted that a direct connection (i.e., “hot wire”) between the sites need not exist, then there is no limitation as to the distance between energetically linked allosteric and active sites on receptors. It is intuitively simple to understand how an allosteric modulator could have different effects on different guests (probe dependence) if those guests bind in different regions of the protein; this will be referred to as “multiple-site guest allostery” (Fig. 4A). For example, experiments with chimeric CCR-5 chemokine receptor and HIV-1 entry inhibitors have shown that portions of CCR5 that interact with the endogenous chemokine agonist macrophage inflammatory protein type 1α differ from those that interact with HIV-1 gp120 (Blanpain et al., 1999a,b; Howard et al., 1999). Likewise, there is evidence to suggest that the peptide chemokine CCL5 binds to regions of the receptor different from those that bind one of these, namely the HIV entry-inhibitor Sch-C (Wu et al., 1997; Blanpain et al., 2003; Tsamis et al., 2003). Thus, binding at an allosteric site presumably stabilizes an ensemble of conformations, the members of which may have regions of the protein considerably different from the native ensemble. In fact, it has been shown that mutation in regions of receptor proteins can cause dramatic changes in the overall conformation of the protein (Gekko et al., 2004; Lu et al., 2005). From this standpoint, it might be expected that a conformational change in one region of the receptor would rarely if ever be linked to the same conformational change in another portion of the receptor.
Fig. 4.
Classification of allostery on the basis of number of sites. A, multiple site allostery involves the interaction of more than one guest with the modulator. Probe dependence results in different effects on each guest for a given modulator. The example shown shows the effect of the CCR5 modulator aplaviroc on CCL5 (no effect on binding) and HIV-1 (complete inhibition of binding) (Watson et al., 2005). B, probe dependence can also result from the interaction of different modulators at the same modulator binding site for the same guest. The different abilities of alkyltrimethylammonium compounds to initiate contraction of guinea pig ileum as mediated by G protein binding is a classic example (Stephenson, 1956). Both the modulator(s) and G proteins bind at a single site.
In addition to multiple guest allostery, it also is common to observe different allosteric effects emanating from interactions at a common modulator binding site (Fig. 4B). The classic example of this is the effects of muscarinic receptor alkyltrimethylammonium agonists in guinea pig ileum, which provided the basis for the concept of agonist efficacy. Specifically, R. P. Stephenson (1956) showed that a very similar series of molecules, which presumably bound to the same loci on the muscarinic receptor, nevertheless had very different abilities to produce muscle contraction (Fig. 4B). On the basis of these data, the concept of agonist efficacy was proposed; i.e., though the molecules presumably bound to the same site on the receptor, the way they bound produced different effects on the receptor. Nuances in agonist binding to a common site have been observed with computational, X-ray and binding methods (Hogner et al., 2002; Bhattacharya et al., 2008). The activation of 7TMRs by agonists is an allosteric system consisting of a modulator (agonist) interacting with a conduit (receptor) to affect a guest (G protein). Conventional guest allostery (modulator affecting the binding of another ligand or protein as a guest) is identical only what is referred to as efficacy for cellular response is generally referred to as cooperativity in guest allosteric systems. However, the fact that several different allosteric modulators can bind to the same allosteric site but produce very different effects is still true. For example, structurally very diverse allosteric modulators of CCR5 [aplaviroc, maraviroc, vicriviroc, N,_N_-dimethyl-_N_-(4-(((2-(4-methylphenyl)-6,7-dihydro-5_H_-benzocyclohepten-8-yl)carbonyl)amino)benzyl)tetrahydro-2_H_-pyran-4-aminium chloride (TAK-779), and 1-acetyl-piperidine-4-carboxylic acid {3-[4-(4-carbamoyl-benzyl)-piperidin-1-yl]-propyl}-(3-chloro-4-methyl-phenyl)-amide (TAK-220)] have all been shown to bind to a single allosteric site on the CCR5 receptor (Maeda et al., 2006; Kondru et al., 2008). However, mutagenesis studies showed that each of the modulators displayed a unique interaction profile with the amino acids in the binding pocket. Similar data have been shown for the CCR5 modulators TAK-779, 4-[1-(2,4-dimethyl-3-pyridinylcarbonyl)-4-methyl-4-piperidinyl]-2(S)-methyl-1-[1(S)-[4-(trifluoromethyl)phenyl]ethyl]piperazine (AD101), and (Z)-(4-bromophenyl){1′-[(2,4-dimethyl-1-oxido-3-pyridinyl)carbonyl]-4′-methyl-1,4′-bipiperidin-4-yl}methanone _O_-ethyloxime (SCH-C) (Seibert et al., 2006).
In summary, a ligand can be thought to enter a collection of interchanging protein conformations (a “conformational cafeteria”) and through a process of conformational selection, stabilize select conformations at the expense of others. These stabilized conformations may have pharmacological function, thereby linking the ligand-receptor binding process to a pharmacologic response (efficacy). Ligands may stabilize global conformations through binding at sites distal from the sites binding signaling proteins and endogenous hormones and neurotransmitters. Finally, binding of different ligands in different ways to a common site can lead to different allosteric consequences for the receptor (differing ligand efficacies).
V. The Vectorial Nature of Allostery
In a protein ensemble world, the ramifications of modulator binding to receptor protein have no boundary and allosteric effect can be exerted throughout the protein. It is useful to consider these effects as a vector of energy, because this allows both the classification and unification of allostery for documented effects of ligands on 7TMRs. Therefore, considering a molecule as the modulator acting on the 7TMR conduit protein, the vector can be described in terms of the location of the guest. Thus, if the guest is another ligand cobinding with the modulator, this will be referred to as classic guest allostery (Fig. 5). If the guest interacts with the conduit along the plane of the membrane [either another receptor as receptor oligomerization or auxiliary protein, such as a receptor activity-modifying membrane protein (RAMP)], this will be referred to as laterally directed allostery (Fig. 5). Finally, if the guest resides in the cytosol, then modulators can cause alteration of cellular function; i.e., all 7TMR agonists are allosteric modulators. There are new data to show that these effects are considerably more complex than described previously, leading to a phenomenon referred to as functional selectivity or biased agonism or antagonism. This will be discussed under the heading of cytosolic allostery (Fig. 5) (see section V.C.2).
Fig. 5.
The vectorial nature of 7TMR allostery. Modulators can affect binding and function of other ligands cobinding to the receptor (classic guest allostery); of the cobinding of other proteins along the plane of the membrane (other receptors or membrane-binding proteins such as RAMPs); or of cytosolic signaling proteins, such as G proteins or β-arrestin. These effects can be simultaneous and all emanate from the same mechanism(s) of allosteric change within the conduit 7TMR protein.
A. Classic Guest Allosterism
The awareness of allosteric ligands has increased sharply over the past decade. This is consistent with the change in screening strategies in industry for new molecules. Specifically, to detect biological activity in large libraries of molecules, a robust, rapid, robotically friendly screening format must be used; until recently, this has been radioligand binding, which is biased toward detecting orthosteric effects. Technology now is providing rapid robust screening methods in formats that detect biological function, and with this change in screening has come an increase in the number of allosteric molecules discovered (Rees et al., 2002). Allosterism is clearly powerful and is an obvious mechanism for natural control of physiological systems. Although several examples of feedback inhibition of enzymes are known (Roberts et al., 1955; Pardee and Yates, 1956; Umbarger, 1956), it is still surprising that there are so few known natural allosteric modulators in the body. However, on the other hand, because allosteric modulators are usually structurally dissimilar to endogenous ligands, it would be difficult to identify natural modulators within the myriad of natural peptides present in the body (Lindsley and Rutter, 2006). At present, only a few natural allosteric modulators are known. For example, an unnatural d-amino acid, d-serine formed in the brain by serine racemase is a potent allosteric modulator of the _N_-methyl-d-aspartate (NMDA) receptor (Tsai and Coyle, 2002). Likewise, the calcium receptor is activated allosterically (enhanced sensitivity to Ca2+) by several classes of l-amino acids including l-phenylalanine and l-tryptophan (Lee et al., 2007). Another natural allosteric modulator initially purifed from rat brain is the tetrapeptide Leu-Ser-Ala-Leu (LSAL; later named 5-HT moduline), which selectively reduces the binding of 5-HT to the 5-HT1B receptor (Massot et al., 1996; Rousselle et al., 1996).
1. Unique Properties of Allosteric Modulators.
Feedback studies on the effects of enzyme products on the rate of enzyme reactions were among the earliest explorations of allosteric mechanisms. Within this scenario, the binding of a molecule (modulator) to an allosteric site on the protein leads to a change in behavior of the protein toward the binding of another molecule (guest) at a different site. This change in behavior can be an inhibition or potentiation of effect of the guest. If the guest is a pharmacologically active agonist (with affinity and efficacy), then the affinity and efficacy of that molecule can be affected in different ways. Allosterism is a powerful mechanism that can induce powerful changes in receptor behavior; several reviews describing allosterism in receptors and allosteric molecules have been written (Christopoulos, 2002; Christopoulos and Kenakin, 2002; Goudet et al., 2004; Noeske et al., 2006; Kenakin, 2007a; Leach et al., 2007; May et al., 2007; Conn et al., 2009a). It is useful to consider some general properties of guest allostery as they pertain to the therapeutic profiles of drugs.
Allosteric modulators, by virtue of the fact that they may stabilize different global conformations of the receptor, have the potential to disrupt protein-protein interactions of very large proteins. As discussed previously, the multiple contacts between the chemokine receptor CCR5 and HIV-1 viral coat protein gp120 can be successfully blocked by small-molecule allosteric modulators, leading to an effective prevention of HIV-1 entry and subsequent progression to AIDS. In theory, orthosteric antagonist ligands have the potential to do the same for guest molecules removed from the orthosteric binding site (i.e., inverse agonists for the binding site for G proteins). However, this is also an allosteric effect in that the “orthosteric” antagonist actually becomes an allosteric modulator for the guest molecule G protein as the receptor changes shape according to energy constraints put on the molecule through binding of the orthosteric antagonist. A distinction must be made between orthosteric effects where the antagonist sterically hinders the access of the agonist to its binding site and any other change in conformation that results from the binding of the orthosteric antagonist to the receptor, such as stabilization of the inactive receptor state for inverse agonists.
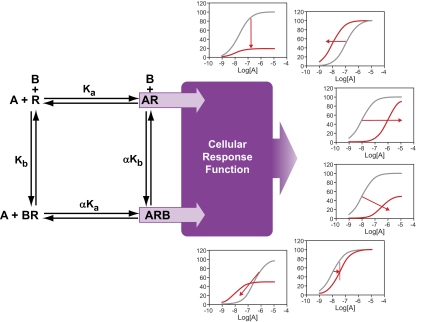
A unique feature of allosteric modulators, with respect to guest ligand binding, is the fact that both the modulator and guest can bind simultaneously to the receptor protein. In fact, the interaction between these two ligands occurs through a change in conformation of the conduit protein (receptor). This gives allosteric modulators their three characteristic and unique properties: saturability of effect, probe dependence, and differential modulation of ligand affinity and efficacy. Thus, allosteric modulators are permissive with respect to the behavior of the receptor (Kenakin, 2005); this is in contrast to orthosteric ligands, which have a pre-emptive behavior. In this latter case, once the antagonist occupies the receptor, the agonist cannot. The permissive nature of allosteric interaction is depicted in early models of receptor allosterism (Stockton et al., 1983; Ehlert, 1988) (Fig. 6). The receptor may cobind a probe ligand (in functional studies, this is an agonist; in binding studies, a radioligand) denoted [A] in the figure and a modulator (denoted [B]). Both the probe-receptor complex ([AR]) and the ternary complex ([ABR]) can have pharmacologically relevant behaviors (i.e., may produce response). The ABR complex has three potential behaviors, relative to the AR complex made naturally when the modulator is not present. These behaviors are antagonism [allosteric antagonists are also referred to as negative allosteric modulators (NAMs); potentiation of response, positive allosteric modulators (PAMs)] and direct allosteric agonism.
Fig. 6.
Basic model for allosteric interaction of a modulator (A or B) interacting with a receptor (R) to affect the interaction of a guest (counterpart A or B). If A is an agonist, then agonism can emanate from the species AR and/or the allosterically modified species ARB. The modulator would then be B, which can affect the affinity of the agonist for the receptor (through α) or the efficacy of the receptor (relative efficacy of the species ARB/AR described as β). This can result in a range of new responses to ligand A from potentiation to antagonism. The binding species are from the model given by Ehlert (1988); the response components can be added through melding of that model with the operational model for receptor function (Black and Leff, 1983) to yield a general functional model of allosteric 7TMR function (Ehlert, 2005; Kenakin, 2005; Price et al., 2005).
In preparation for discussion of properties of allosteric systems, it is useful to define two general cooperative effects of modulators on 7TMRs. Considering agonism, the agonist will have an affinity for the receptor and also a value for intrinsic efficacy defining the power of that agonist to induce a defined response. These affinities and efficacies themselves are products of the allosteric effect of agonists on 7TMRs; this will be discussed further in the section on functional selectivity (see section V.C.2). However, for the purposes of discussing classic guest allostery for cobinding ligands, agonists will be considered as guest probes of receptor behavior that can be modified by the modulating ligand. Therefore, a modulator can have varying effects (denoted as cooperativity values) on the activity of probe molecules such as agonists and radioligands. Two values that will be used to describe modulatory effects on agonism will be α, the change in the affinity of receptor for an agonist observed after modulator binding, and β, the effect on agonist efficacy. There are no constraints as to the vectorial effect of each of these on a given 7TMR; i.e., a modulator may increase affinity and reduce efficacy, increase both, decrease both, or decrease affinity and increase efficacy. These cooperativity values are described in terms of their maximum value upon saturation of the allosteric site. Thus, an α value of 10 denotes a 10-fold increase in the affinity of the receptor for the probe upon complete saturation of binding of the modulator to the allosteric site.
Saturability of effect simply refers to the fact that, whatever the allosteric effect, it will reach an asymptote maximum when there is complete occupancy of the allosteric site. Competitive orthosteric ligands can produce theoretically infinite competitive effects as long as the concentrations of the competing ligands are manipulated in the correct manner. Therefore, a competitive antagonist can produce a dextral displacement of an agonist concentration response curve that is limited only by the experimental or viability constraints of the system. In addition, if given in sufficiently high concentration, an orthosteric antagonist will render the receptor protein completely unresponsive to guest effect through mass action. In contrast, an allosteric modulator that produces a 10-fold reduction in the affinity of a guest ligand (α = 0.1) will produce up to a 10-fold shift in the binding curve to that guest ligand and no more. Thus, for low levels of allosteric modulation, the protein may still be responsive to the guest ligand; i.e., effects can be modulated, not obliterated. For example, 101.10 (Arg-Tyr-Thr-Val-Glu-Leu-Ala), a peptide allosteric antagonist of IL-1 receptors produces a maximum 18-fold dextral displacement of an 125I-IL-1β radioligand binding curve, and no increase in the concentration of 101.10 will reduce the affinity for 125I-IL-1β beyond that level (Quiniou et al., 2008). These effects often are made manifest as curves that show that the radioligand cannot be completely displaced by the allosteric molecule (for example, see Rominger et al., 2009). These patterns have on occasion been incorrectly termed “partial” antagonism.
Because an allosterically modulated receptor has new properties toward guest ligands, the affinity and efficacy can be modulated in different ways; it is worth considering the possible effects than can lead to antagonism. For example, allosteric antagonists may only reduce agonist affinity and not affect agonist efficacy (in which case it will resemble a limited effect competitive antagonist), or they can reduce both affinity and efficacy (to produce either mixed competitive/noncompetitive effects). It is noteworthy that they also can reduce efficacy without altering affinity. For example, the CCR5 allosteric modulator aplaviroc minimally affects the binding of the chemokine CCL5 to the receptor but completely blocks CCL5-mediated agonism (Maeda et al., 2004; Watson et al., 2005). Likewise, the noncompetitive metabotropic glutamate receptor 1 antagonist 7-hydroxyiminocyclopropan[_b_]chromen-1a-carboxylic acid ethyl ester inhibits receptor signaling without interfering with glutamate binding (Litschig et al., 1999). An even more interesting effect is seen with the NMDA receptor allosteric antagonist ifenprodil. This antagonist actually increases the affinity of the agonist NMDA but blocks its efficacy (Kew et al., 1996). Because allosteric effects are reciprocal, this means that NMDA also increases the affinity of ifenprodil as a blocking agent. This adds the intriguing antagonist property of increased potency in systems where agonist tone is high; i.e., the blocker gets better the more the system is driven. A similar effect is observed for the cannabinoid CB1 receptor allosteric modulator 5-chloro-3-ethyl-1_H_-indole-2-carboxylic acid [2-(4-piperidin-1-ylphenyl)ethyl]amide (Org27569) (Price et al., 2005).
The previous discussion has been confined to classic guest allostery whereby a modulator alters the interaction of a guest molecule (usually binding from the extracellular space) with the receptor. These effects can result in a diminution of guest agonism (allosteric antagonism) or potentiation of guest agonism (potentiation with molecules referred to as PAMs). The potentiation of agonist effect can occur through an increase in endogenous agonist affinity [i.e., brucine for acetylcholine agonists, (Jakubík et al., 1997)] or efficacy [i.e., hexapeptide growth hormone secretagogue for Ghrelin receptors, Holst et al., 2009)].
Potentiation of endogenous response can only occur in a permissive system (Kenakin, 2005) that allows cobinding of the modulator and the endogenous agonist, so this mechanism is accessible only through allosteric molecules. One of the main unique features of positive allosteric modulators (PAMs) is the retention of complex patterns of excitation. This occurs because the PAM effect is proportional to the endogenous physiologic tone already present in the system. In this way, geographical patterns of innervation, such as those found in the CNS, can be potentiated in correct proportion to the function of the healthy system.
As discussed in the section(s) on functional selectivity and biased agonists (section V.C.2), there is no simple relationship between receptor binding of an agonist and the activation of the spectrum of signaling pathways that are mediated by that receptor; i.e., there is heterogeneity in the pathways activated by different agonists. This is a result of the change in energy landscape produced by the binding of an allosteric modulator potentially to cause a completely different array of behaviors of the receptor toward binding of any guest ligand. This extends to PAMs as well in that the signaling pattern of a given agonist acting on a receptor may change in the presence of an allosteric antagonist (see section V.C.2.d) or a PAM. For instance, allosteric potentiators of mGluR receptors have been shown to differentially potentiate mGluR5-mediated calcium transients and ERK1/2 phosphorylation (Zhang et al., 2005). In general, a number of PAMs are currently being investigated for therapeutic activity (see Bridges and Lindsley, 2008).
The other major effect of allosteric modulators emanating from the property of permissiveness of binding of coligands is probe dependence. Thus, the actual allosteric effect that a given modulator has on a series of guest molecules may be quite different for different guests (Hejnová et al., 1995; Maass and Mohr, 1996; Jakubík et al., 1997). For example, the CCR5 receptor modulator aplaviroc blocks the binding of the chemokine CCL3 to the receptor but not the binding of CCL5 (Watson et al., 2005). This unique property can be exploited therapeutically to enhance therapeutic potential, reduce secondary effects, and prosecute targets the natural function of which cannot be compromised (see V.C.3).
Another feature of allosteric modulators is extraordinary selectivity for receptor types (Melchiorre et al., 1989; Ellis et al., 1991; Jakubík et al., 1995; Liang et al., 1996; Gnagey et al., 1999; Johnson et al., 2004). This may result from binding to sites on the protein that are unique for a particular protein, as opposed to sites common to a set of proteins (i.e., binding site for common neurotransmitter or hormone agonist, ATP substrate binding, etc.). For example, although it is difficult to demonstrate selectivity for kinases at ATP binding sites (because they are common to all kinases), an allosteric antagonist that binds to another site and modulates the ATP site may be selective. Imatinib blocks the Bcr-abl fusion protein with kinase activity in myelogenous leukemia through an allosteric mechanism but does not alter ATP interaction with the catalytic domain of other kinases (Adrián et al., 2006). Yet another advantage of allosteric molecules is that they simply act on the physiological tone that is already present; this preserves complex patterns of activation (such as those due to innervation networks in the brain) for better physiological effect (Jakubík et al., 1997; Dolezal and Tucek, 1998; Lazareno et al., 1998; Möhler et al., 2002).
In keeping with the mechanism of creating new energy landscapes for receptors, allosteric modulators basically create new ensembles. The way these new ensembles interact with different guests defines the pharmacology of the modulator. This has ramifications for disease-related alterations in guest molecules. For example, HIV-1 infection is mediated by the interaction of the viral coat protein gp120 with the CCR5 receptor, and the virus is known to continually mutate and alter the composition and conformation of gp120 in its routine realm of existence (Wyatt and Sodroski, 1998; Poignard et al., 2001). The therapeutic relevance of this activity is that the virus will probably learn to use whatever form various HIV-1 entry inhibitors impose on the CCR5 receptor; i.e., viral resistance is inevitable (Trkola et al., 2002; Kuhmann et al., 2004). For an orthosteric mechanism, the “blocked” CCR5 receptor would be identical for all blockers; therefore, once viral resistance was attained, all orthosteric CCR5 entry inhibitors would show resistance. However, because allosteric ligands create new ensembles, there is no rule a priori to suggest that the various ensembles stabilized by different allosteric molecules will be identical; in fact, antibody binding evidence suggests that the opposite is true. It has been shown that the antibody binding profiles of Ab45531 and Ab45523 differ for CCR5 in the presence of the allosteric HIV-1 entry inhibitors TAK779, SCH-C, and aplaviroc (Kenakin, 2007b), further suggesting that the conformations stabilized by these allosteric modulators differ from each other. This suggests that different allosteric modulators may produce different conformations that do not resemble each other except for the fact that none of them support HIV-1 entry. Under these circumstances, it would be predicted that viral resistance to one allosteric entry inhibitor could be overcome by use of another, because a different conformation of the receptor (one that the virus has not encountered) would be formed.
In summary, allosteric ligands, by virtue of the fact that they provide a new energy landscape for the receptor, can essentially create a new receptor. If the allosterically modified receptor prefers new global conformations, the interaction with large proteins (involving multiple binding loci) may be altered. In addition, allosteric ligands have unique properties in that they produce saturable and probe-dependent effects; these can lead to correspondingly special therapeutic properties. Finally, the adoption of new conformations leads to potentially dissociable effects on cobinding ligand efficacy and affinity, and the combination of these two properties can also produce unique allosteric modulator behavior. It should be stressed that there are no theoretical limitations on the effects of an allosteric modulator on the function of other ligands on the receptor; i.e., they may be antagonized (NAMs), potentiated (PAMs), or unaffected.
2. Allosteric Agonists.
Because allosteric modulation can result in a globally different receptor, there also are no limits to the potential direct effects a modulator can have (Kenakin, 2007). Given this, the interaction of the receptor with other guests can simultaneously be altered. When this occurs for cytosolic guests that mediate signaling, then a direct agonist effect of the modulator on cell function may be observed. Allosteric agonists produce direct activation of receptors through binding at a site other than the binding site for endogenous agonists. PAMs often can be shown to produce direct agonism in highly sensitive systems, a finding that perhaps should not be surprising, because PAMs stabilize agonist-activated conformations of receptors to produce potentiation of effect (Schwartz and Holst, 2007). Allosteric agonists may pose unique problems in validations because the resulting allosteric agonism may not be sensitive to conventional orthosteric antagonists. For example, the muscarinic receptor agonism produced by alcuronium is insensitive to the blocking effects of the orthosteric antagonist quinuclidinyl benzilate (Jakubík et al., 1996). Likewise, the effects of the peptide agonists for CXCR4 receptors RSVM and ASLW are insensitive to the antagonist AMD3100 (Sachpatzidis et al., 2003). In addition, as discussed in terms of allosteric modulators in general, allosteric agonists have the potential for great selectivity because they target unique loci on receptor subtype proteins (Jones et al., 2008). Allosteric agonists also have been shown to be functionally selective and bias their signaling toward different pathways mediated by the receptor (Thomas et al., 2008); these ideas will be developed more fully in the following sections on functional selectivity and agonist bias (section V.C.2.c). Similar to positive signaling effect through allosteric modulation, allosteric antagonists (NAMs) also have the potential to induce a negative direct effect on the receptor (allosteric inverse agonism). In this case, the direct allosterically stabilized conformation of the receptor would not interact with signaling proteins to produce visible positive response. In addition, as with orthosteric inverse agonists, an allosteric inverse agonist would reverse constitutive activity to produce inverse agonism in constitutively active receptor systems. Finally, a new unique class of ligand has emerged, specifically for muscarinic receptors. Termed “bitopic,” these molecules interact with both the orthosteric and allosteric site on the receptor to self-modulate their own activity (Valant et al., 2008, 2009).
3. Seven Transmembrane Receptor Allostery and New Drug Discovery.
In the quest for new allosteric ligands, the fact that the species of interest is a ternary complex of modulator/conduit/guest has ramifications for the screening of modulators. A new mode of screening must be adopted in which new molecules are tested in assays containing a low level of cobinding ligand already present. The resulting data describes ligands in terms of the concentration at which an effect is observed versus the effect on the response (or binding) of the reference ligand. Therefore, two parameters are needed to fully describe an allosteric modulator: the affinity of the modulator for the 7TMR and the cooperativity observed with the coligand (i.e., a value for change in affinity or potency, etc). In this sense, reporting data for modulators is the same as for agonists in which a potency (EC50) and maximal response is needed to describe the profile of activity. For a modulator, an added complication is the fact that the identity of the cobinding ligand is also relevant because probe dependence can make modulator effects differ for different guest molecules. In this regard, probe dependence requires that, wherever possible, the natural endogenous ligand interacting with the receptor be present in the screening milieu to detect physiologically relevant interactions. For example, when screening for cholinergic PAMs for use in Alzheimer's disease, it would be chemically preferable to use a stable cholinergic ligand in the place of acetylcholine, an unstable molecule that is difficult to control under screening conditions. However, many PAMs potentiate surrogate ligands (such as arecoline and carbachol) but actually inhibit acetylcholine (Jakubík et al., 1997) and thus are not therapeutically useful. This type of probe dependence could lead screening efforts astray in that nonphysiologically relevant potentiators of agonism would be discovered.
Another possible consideration is kinetics, because many allosteric modulators have an unusually long requirement for attainment of kinetic equilibrium (long times of onset on and offset off of the receptor). For example, the allosteric CCR5 modulator aplaviroc has a requirement of 2 to 3 h for onset and has a _t_1/2 for dissociation on the order of hundreds of hours (Watson et al., 2005). Likewise, persistent kinetics have been reported for muscarinic allosteric modulators (Jakubík et al., 2002; Machová et al., 2007) and p38 kinase inhibitors (Pargellis et al., 2002). In keeping with the notion that an allosteric site may be distant from the endogenous agonist binding site and may in fact be part of a relatively rare ensemble present only for a fraction of the lifetime of energy landscape, it has been proposed that this is the reason some allosteric modulators are slow to bind to the protein. In practical terms, this suggests that exceedingly long incubation times may be required to detect weak allosteric modulators in a screening environment.
To fully characterize an allosteric effect, the modulator/conduit/guest ensemble must be specified and the effects of the modulator on the guest characterized quantitatively. This can be done by comparing data with output from quantitative models (Ehlert, 2005; Kenakin, 2005; Price et al., 2005), where the effect on affinity (α) and efficacy (β) can be calculated. For functional studies, a powerful tool that also can be used is the measurement of allosteric modulation of agonist effect through calculation of changes in agonist “activity ratios” (the ratio of the maximal response to the agonist and the EC50 molar concentration that produces half-maximal response) (Ehlert, 2005).
In summary, drug discovery for allosteric modulators requires certain special considerations such as the need to quantify both potency and cooperativity, the acknowledgment of probe dependence (the natural agonist must be used), and the accommodation of possibly slow kinetics. A simple combination of the Ehlert (1988) and Black/Leff (Black and Leff, 1983) models for allosterism and operational agonism, respectively, can be used to assign quantitative parameters to allosteric modulators.
4. Therapeutic Application of Allosteric Modulators.
In light of the ability of PAMs to preserve complex patterns of agonism, central nervous system targets in which the endogenous system is failing are obvious areas where these molecules can be used therapeutically in diseases such as Alzheimer's disease and in other cases of failing cholinergic innervation (Bartus et al., 1982). One clinical approach has been to potentiate cholinergic effect through cholinesterase inhibition (Flicker, 1999), but this strategy seems to have limited practical value (Nordberg and Svensson, 1998; Rogers et al., 1998; Maelicke et al., 2000). Coupled with the obvious potential liability to be encountered with cholinesterase inhibition, it may be that cholinesterase inhibitors produce relatively nonselective increases in cholinergic function through both nicotinic and muscarinic receptors (Fisher, 1999; Maelicke et al., 2000), whereas selective potentiation of nicotinic responses is required (Rogers et al., 1998). Under these circumstances, a preferable approach would be to selectively potentiate nicotinic receptor function through PAMs (Maelicke and Albuquerque, 1996; Krause et al., 1998). Likewise, potentiation of the effects of adenosine has been proposed to be beneficial in localized areas of oxygen deficit such as angina, myocardial infarction, and stroke (Fredholm, 2007; Romagnoli et al., 2008). In addition to cardiovascular disease, PAMs have also been postulated to be of value in the treatment of psychoses and cognitive disorders via potentiation of mGluR5-mediated responses (O'Brien et al., 2003). With regard to schizophrenia, it has been proposed that positive modulation of mGluR2 and mGluR3 receptors could be beneficial in treating positive symptoms, whereas positive modulation of mGluR5 could be useful for treating all major symptoms [positive, negative and cognitive (Conn et al., 2009b)]. Likewise, selective potentiation of muscarinic m4 receptor responses to regulate brain levels in psychosis with the PAM 3-amino-5-chloro-6-methoxy-4-methyl-thieno[2,3-_b_]pyridine-2-carboxylic acid cyclopropylamide (LY2033298) has been proposed as a novel antipsychotic drug (Chan et al., 2008). A comprehensive description of the application of allosteric molecules to a range of psychiatric disorders is given in Conn et al. (2009a).
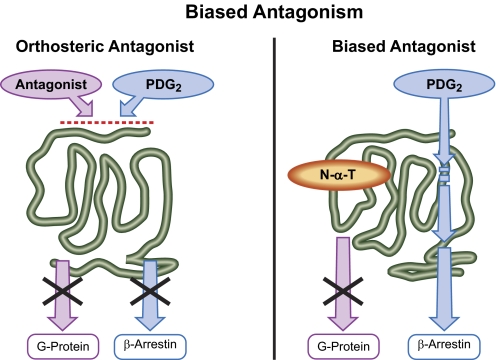
In terms of receptor antagonism, allosteric modulation has certain distinct advantages, especially in terms of probe dependence, where there is the possibility of blocking some interactants for a receptor but not others. For example, blockade of the CCR5 receptor is a mechanism to prevent HIV-1 infection. However, high levels of chemokines have been associated with delay of AIDS progression (Lori et al., 1997; Ullum et al., 1998; Garzino-Demo et al., 1999; Shieh et al., 2001; Heredia et al., 2003; Rogez et al., 2003; Xiang et al., 2004), suggesting it may be advantageous to preserve CCR5-chemokine interaction in AIDS. In fact, a study of 1064 patients infected with HIV in 57 populations around the world showed a strong inverse correlation between the gene copy number for the chemokine CCL3L1 (this is variable in humans) and progression to AIDS. Specifically, patients with high CCL3L1 gene copy number showed a highly statistically significantly greater survival rate compared with patients with low CCL3L1 copy number (Gonzalez et al., 2005). The mechanism for this effect is thought to be removal of CCR5 cell receptors through internalization into the cytosol (Alkhatib et al., 1997; Amara et al., 1997; Mack et al., 1998), and an inverse link between CCL3L1 and CCR5 receptor levels has been reported (Ketas et al., 2007). Therefore, a functionally selective CCR5 modulator that blocked the binding of HIV-1 gp120 protein but otherwise preserved CCL3L1 function through the receptor (to enable the chemokine to internalize the receptor) would theoretically offer a more efficacious profile (Muniz-Medina et al., 2009).
B. Lateral Allostery (Dimerization, Complexation)
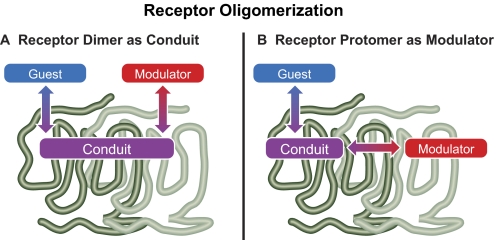
1. Receptor Oligomerization and Structure.
The concept of 7TMRs associating with each other within the plane of the plasma membrane has been the focus of extensive debate (Bouvier, 2001; Angers et al., 2002; James et al., 2006; Milligan et al., 2006). Having seven transmembrane segments gives these molecules every opportunity for nonspecific hydrophobic interaction and aggregation with other membrane proteins. Anyone who works with these molecules in the laboratory has had the chance to see this behavior firsthand. It is because of this behavior that many groups have been skeptical about 7TMR oligomerization. There are now a series of experimental manipulations, including saturation experiments and use of very low levels of expression in recombinant systems that are analogous to those that exist in nature, that are required as part of the evaluation of true, biologically relevant 7TMR oligomerization. Even with these manipulations yielding positive results, skeptics remain. However, there are compelling data to support the existence of structurally specific oligomerization of some 7TMRs, many of these interactions having substantial functional implications.
Although the interactions between single transmembrane tyrosine kinase receptors have long been recognized as providing the fundamental mechanism for their cross-phosphorylation, no such clear function has been identified for 7TMR association. Nevertheless, there are examples of 7TMR association (oligomerization) affecting the affinity and specificity of ligand binding, the pattern of signaling, and internalization. For 7TMRs in family A, the full spectrum of observations have been recorded, from having no effect on function to each of the described functional changes, and from ligand binding leading to dissociation of the oligomeric complex to having no effect. There are clearly no rules yet for which subset of such receptors oligomerizes or which has a particular functional effect.
It is interesting that family C 7TMRs have a very compelling structural and functional story for their association. Many family C 7TMRs seem to require association as dimers, some of these even acquiring covalent stabilization with disulfide bonds. There are beautiful crystal structures of dimeric complexes of amino-terminal domains of family C 7TMRs, with a single natural ligand spanning the protomers (de Vos et al., 1992). Homodimeric calcium-sensing receptors and metabotropic glutamate receptors can have disulfide bonds linking their Venus flytrap domains, as well as possessing a number of other noncovalent stabilizing interactions.
Family B 7TMRs have also recently been shown to associate as stable homo-dimers. The structural basis of this, at least for the prototypic secretin receptor, is the lipid-exposed face of TM4 (Harikumar et al., 2007; Harikumar et al., 2008; Gao et al., 2009). This complex has been postulated as being important for the structural stabilization of the high affinity complex with G protein. It will be interesting to determine whether this pattern is reproduced for other members of family B.
a. Interactions of seven transmembrane receptors with other membrane proteins.
The most dramatic and interesting association with 7TMRs was identified in an attempt to clone the receptor for calcitonin gene-related peptide. In that effort, using expression cloning, a single transmembrane protein was identified that ultimately was named RAMP (Foord and Marshall, 1999). Subsequently, two homologous RAMPs were cloned (RAMP1, RAMP2, and RAMP3). The story for RAMP association with 7TMRs and how they can modify the function of those receptors is indeed fascinating (Hay et al., 2006).
RAMP1/calcitonin-like receptor behaves phenotypically as a calcitonin gene-related peptide receptor, whereas RAMP2 and RAMP3 association with the calcitonin-like receptor exhibits adrenomedullin phenotypes. RAMP association with the calcitonin receptor assumes an amylin receptor phenotype. Other family B 7TMRs have also been shown to associate with RAMPs and to elicit no particular pharmacological profile. These include parathyroid hormone (PTH) 1, PTH2, VPAC1, and glucagon receptors; the VPAC1 receptor is able to associate with all three RAMPs, PTH1, and glucagon receptors able to associate with only RAMP2, and the PTH2 receptor is able to associate only with RAMP3. In contrast, other family B 7TMRs, including VPAC2, growth hormone-releasing hormone, GLP-1, and GLP-2 receptors seem to not associate with RAMPs. The structural basis for RAMP association with these receptors has been postulated to be affected by amino-terminal regions and by transmembrane segments, but no clear theme has yet emerged.
It is likely that other transmembrane proteins will also follow this theme and associate with 7TMRs within the lipid bilayer. It is not yet clear what such interactions might exist, their stability, or their functional importance. Such interactions could well be contributing to some of the observed differences in function and regulation of specific receptors expressed in different natural cellular environments, or even in the differences in receptor function and regulation in pathological environments, such as in tumor cells.
b. Influence of the lipid microenvironment.
Lipids are known to play important roles in 7TMR signaling (Escribá et al., 2007; Paila and Chattopadhyay, 2009). Many 7TMRs are themselves reversibly palmitoylated, typically within their carboxyl-terminal tail, where this modification helps to form a fourth intracellular loop region that has been shown to have regulatory properties. In addition, fatty acid acylation of Gα subunits and isoprenylation of Gγ subunits helps to direct these to the plasma membrane for their interaction with the receptors. Recent crystal structures of rhodopsin have also clearly demonstrated preferential sites of interaction with cholesterol. Many observations over the years have suggested that the lipid microenvironment of some particularly sensitive 7TMRs could affect their function, typically by affecting G protein coupling. Although the lipid-exposed faces of the 7TMR helical bundles can have allosteric effects on these membrane proteins, this may be difficult to take advantage of in a direct way by interacting drugs. It is hard to achieve specificity of interaction in the absence of a specific pocket to target. However, indirect effects on sensitive receptors may well be possible.
2. Receptor Oligomerization and Function.
There is considerable evidence to indicate that 7TMRs can form and function as homodimers and heterodimers (Milligan and Smith, 2007; Gurevich and Gurevich, 2008; Milligan, 2008; for reviews, see Franco et al., 2008a) and that these dimers may have therapeutic relevance (Bouvier, 2001; Breitwieser, 2004; Franco et al., 2005, 2008b). There also are mathematical models to detect, characterize, and quantify receptor dimerization on the level of binding (Durroux, 2005; Albizu et al., 2006; Franco et al., 2006; Casadó et al., 2007; Giraldo, 2008) and function (Franco et al., 2006). Association of receptors within cell membranes constitutes the lateral transduction of information through allosterism; it is worth considering some types of interactions that have been reported.
There are several ways to examine lateral allosterism with ligands and proteins changing roles of modulator, conduit, and guest. Among the most commonly reported data are studies of ligands interacting with each other through a receptor dimer. Phenotypes observed for receptor dimers are mediated by laterally induced allosteric effects that change the conformation(s) of the receptors involved. In fact, such effects have directly been observed in a leukotriene B4 dimer through a fluorescently labeled 5-hydroxy-tryptophan labeled protomer (Damian et al., 2006). This can lead to production of a unique therapeutic effect because the activity of ligands is conferred by systems only producing receptor dimers. In these types of systems, the conduit is the dimeric receptor complex with the ligands taking on the role of modulator and guest; see Fig. 7A. The pharmacologic effect is transferred through one receptor and made manifest in the dimeric receptor complex, an effect referred to as “off-target” allosterism (Ballesteros and Ransom, 2006). These effects can be seen on the level of receptor binding. For example, heterodimerization of somatostatin SSTR5 and the dopamine D2 receptors allows crossed activity of dopamine and somatostatin ligands. Specifically, the potency of the somatostatin ligand SST-14 (in competition binding with the radioligand 125I-Leu8-d-Trp22-Tyr25-SST-28) is increased 30-fold by the dopamine agonist quinpirole and reduced 80% by the dopamine antagonist sulpiride in cells containing SSTR5/D2 heterodimers (Fig. 7A) (Rocheville et al., 2000). Likewise, heterodimerization of CCR2b and CCR5 receptors causes the CCR5 receptor, normally insensitive to MCP-1 (monocyte chemoattractant protein-1) binding, to bind MCP-1; likewise, the CCR2b receptor, normally insensitive to the CCR5 chemokine ligand CCL4 binds CCL4 when dimerized with CCR5 (El-Asmar et al., 2005). Studies with a dopamine D1-dopamine/D3-dopamine receptor heterodimer (D1-D3 dimer) show that binding of agonists to the D3 protomer of the dimer complex increases the affinity of the D1 receptor for agonists within the dimer (Marcellino et al., 2008). Other examples include the reduction in the affinity of adenosine A1 protomers for agonists upon binding of adenosine A2A receptor agonists to A2A receptors in an A1-A2A receptor heterodimer (Ciruela et al., 2006) and reduction in the affinity of dopamine D2 receptors for dopamine through stimulation of adenosine A2A receptors in a A2A-D2 heterodimer (Ferre et al., 1991). These effects can be seen in functional studies as well. Thus, the agonist effects of orexin-A can be reversed by the cannabinoid receptor CB1 inverse agonist SR141716A through formation of an orexin-1/CB1 receptor heterodimer (Hilairet et al., 2003). Conformational changes in dimers open the possibility of changes in receptor-mediated signaling in cells. Under these circumstances, the receptor dimer is considered a single species (Levoye et al., 2006) to affect guest cytosolic signaling proteins. Changes in receptor sensitivity to agonists upon dimerization have been well characterized, notably for opioid receptor heterodimers. For instance, reduced potency and changes in the agonist rank order of potencies to synthetic opioid agonists for μ- and δ-opioid receptors have been shown in systems in which μ- and δ-opioid receptors are coexpressed. In contrast to the reduced synthetic ligand potencies, enhanced affinities for the peptide agonists endomorphin-1 and Leu-enkephalin were seen in these systems (George et al., 2000). Striking alterations have been observed with dimerization involving actual changes in the nature of receptor signals upon receptor dimerization (Ferré et al., 2007). Thus, dopamine D2 receptors, normally coupled to Gi-o proteins as monomers, switch their coupling to Gq/11 proteins upon dimerization with dopamine D1 receptors when the D1 receptor is coactivated with agonist (Rashid et al., 2007). In addition, although the ligand 6-chloro-2,3,4,5-tetrahydro-3-methyl-1-(3-methylphenyl)-1_H_-3-benzazepine-7,8-diol (SKF83959) does not activate adenylate cyclase or Gq through either D1 or D2 dopamine receptor monomers, it does activate Gq/11 via the D1-D2 heterodimer (Rashid et al., 2007). Likewise, the potency of orexin-A, in producing phosphorylation of ERK1/2 is increased 100-fold upon heterodimerization of the orexin-1 receptor with cannabinoid CB1 receptors, but the effect is pathway-dependent. Specifically, no increased potency for orexin-A inositol phosphate production is observed upon heterodimerization (Hilairet et al., 2003).
Fig. 7.
Two types of allosteric system derived from receptor oligomers. A, the receptor dimer becomes a completely new conduit and ligands interact through this new conduit with revised interactive behaviors. For example, the dopamine antagonists quinpirole and sulpiride bind to a somatostatin SSTR5-dopamine receptor D2 heterodimer to block the binding of somatostatin (Rocheville et al., 2000). B, another receptor (or membrane bound protein such as RAMP) becomes the modulator that confers new reactivity toward a guest through a receptor. For example, the bradykinin-2 receptor can act as a modulator and bind to the angiotensin1 receptor to change the binding characteristics of angiotensin-1 (AbdAlla et al., 2000).
In addition to receptors, allosteric information can be transducer laterally to other membrane proteins such as RAMPs to yield unique functional receptor phenotypes (Hay et al., 2004, 2006; Udawela et al., 2006). For example, human calcitonin is 20-fold more potent than rat amylin in melanophores transfected to express human calcitonin receptors (Gq protein activation); this relative potency is reversed by the cotransfection of RAMP3 (amylin becomes 3-fold more potent than human calcitonin) (Armour et al., 1999). Thus human calcitonin and rat amylin become modulators for the conduit human calcitonin receptor-RAMP complex with Gq protein as the guest. It can be seen that other receptors or membrane proteins cobinding to the receptor can confer functional selectivity upon systems in a manner similar to external allosteric modulators.
Although it is customary to consider small molecules or peptides as allosteric modulators, it should be noted that large cytosolic proteins can be considered allosteric modulators as well. Although this has been well established in the case of G proteins (Kostenis, 2006; Kostenis et al., 2005), it also is true of membrane-bound proteins. Under these circumstances, the receptor is the conduit protein acted on by a second dimerizing receptor, which becomes the allosteric modulator sensed by guest ligands (Fig. 7B). Thus, the efficacy of angiotensin I in HEK293 cells increases upon heterodimerization of the angiotensin receptor with bradykinin-2 receptors (Fig. 6B) (AbdAlla et al., 2000). These types of effects can be seen with antagonists as well. Thus, the affinity of caffeine for the adenosine A2A receptor falls by a factor of 10 when the receptor heterodimerizes with the adenosine A1 receptor (Ciruela et al., 2006). The cannabinoid CB1 receptor becomes a modulator of the orexin-1 receptor, causing it to be actively internalized (in this case, the guest is the cytosolic component that interacts with the receptor during internalization, presumably β-arrestin) (Ellis et al., 2006). In addition to receptors acting as active modulators, other membrane proteins can fill this role as well. For example, RAMP3 actively modulates human calcitonin receptors to cause a 7-fold reduction in the affinity of the amylin antagonist AC66 (salmon calcitonin [8–32], p_K_B for blockade of amylin response 9.7 without RAMP3; coexpression of RAMP3, p_K_B = 8.85) (Armour et al., 1999).
In summary, there are two general mechanisms by which lateral allosterism can affect drug effect with dimerization. In one, the dimerizing protein (other receptor, RAMP) binds to the receptor to form a new conduit with allosterically altered characteristics toward both modulators and guest (Fig. 7A). The other involves the cobinding protomer (other receptor, RAMP) as the modulator in a multisite allosteric system in which the protomer alters the signaling effects of the agonist-bound receptor as it interacts with cytosolic molecules (Fig. 7B). This is an increasingly described phenomenon in the literature with possible therapeutic application in new drug discovery.
3. Therapeutic Application(s) of Receptor Oligomers.
For some receptors (i.e., tyrosine-kinase receptors), dimerization is a known mechanism of action (Heldin, 1995). The increasing importance of dimerization for 7TMRs naturally suggests its possible relevance to drug discovery (George et al., 2002). Thus, if new drug-sensitive phenotypes for existing receptors are formed upon dimerization, then selective drug effect could result. There are provocative links between therapeutic profiles of some drugs and dimers. For example, functional selectivity in antagonism for typical and atypical antipsychotic interaction with 5-HT2A receptors has been linked with receptor dimerization (Brea et al., 2009). Oligomerization seems to be especially prevalent among opioid receptors; this has been shown to yield many opioid receptor phenotypes in tissues (defined as μ1, μ2, δ1, δ2, κ1, κ2, κ3). In terms of acquisition of new drug sensitivity through oligomerization, the agonist 6′-guanidinoaltrindole (6′-GNTI) produces no agonist response at δ-opioid receptors and very little at κ-opioid receptors. However, this agonist produces powerful responses on the heterodimer of δ- and κ-opioid receptors (Waldhoer et al., 2005). These responses to 6′-GNTI are blocked by antagonists of both δ- or κ-opioid receptors. Of possible therapeutic relevance is the fact that 6′-GNTI produces analgesia only when administered into the spinal cord, suggesting that the dimerization is organ-specific. This could lead to reduction in side effects with spinal analgesia as a projected drug phenotype. Likewise, complexes of glutamate receptors mGluR2 and serotonin receptor type 5-HT2A have been specifically associated with hallucinogenic responses in schizophrenia (González-Maeso et al., 2008).
An intuitively obvious advantage of targeting receptor dimers therapeutically is the possibility of reducing global activity to one of tissue-specific relevance. However, an opposite effect may be useful as well. Chemokine signaling is known to be particularly pleiotropic with chemokines showing cross-reactivity to a number of chemokine receptor types (Mantovani, 1999; Wells et al., 2006). In cases in which more than one chemokine receptor is targeted in inflammatory disease, the production of receptor dimers can confer sensitivity of multiple receptor types for a single antagonist. Such effects have been noted for CCR2/CXCR4 receptor heterodimers (Sohy et al., 2007).
C. Cytosolic Allostery: Functional Selectivity and Biased Ligands
1. Functional Selectivity and Structure.
a. Interactions with G proteins.
Despite the evolved diversity in themes for natural ligand binding and even differences in the structures of the helical bundle regions, all the members of this superfamily associate with a small number of heterotrimeric G proteins (Bourne, 2006; Johnston and Siderovski, 2007; Oldham and Hamm, 2008). As noted above, only in family A 7TMRs do we have a clear picture of the types of molecular motion associated with the exposure of the G protein-binding region upon agonist binding. This region is exposed on the cytosolic surface of the helical bundle and tends to have contributions from the bottom of key helical segments, particularly TM6. Residues within each of the cytosolic loop regions and the proximal carboxyl-terminal tail region have been reported to influence G protein coupling, based largely on loss-of-function site-directed mutagenesis studies. It is particularly interesting that mutations in these regions have been reported to differentially affect coupling with one particular G protein, with coupling to other G proteins being undisturbed (Wu et al., 1999). Therefore, although there may be overlap in the coupling interface for different G proteins (something we would expect, based on the large relative size of the heterotrimeric G protein complex), the details of the interactions with each specific G protein are distinct and can be differentially modified. An extension of this is the prediction that different drugs might stabilize the coupling with distinct G proteins in a differential manner. Indeed, such observations have already been made. Our understanding of the molecular basis of G protein coupling has recently improved substantially, based on the crystal structure of opsin coupled with the peptide representing the carboxyl terminus of transducin (Scheerer et al., 2008).
The assumption is that family B and family C 7TMRs couple to G proteins in a similar manner, but there are minimal data to support this at the current time. Site-directed mutagenesis of residues in similar cytosolic face regions of these receptors has also produced loss-of-function phenotypes, but the details of the molecular basis for exposure of these regions for G protein association is currently unclear (Chan et al., 2001; Tams et al., 2001).
b. Interactions of seven transmembrane receptors with other cytosolic proteins.
Arrestin represented one of the first proteins, other than heterotrimeric G proteins, shown to directly interact with 7TMRs. Visual arrestin was shown to bind to phosphorylated rhodopsin in 1985 (Kühn et al., 1984; Pfister et al., 1985). Later, it became clear that there was a family of arrestins, with a second visual arrestin identified in cones and with two nonvisual arrestins identified (arrestins 2 and 3, or β-arrestin 1 and 2) (Prossnitz and Sklar, 2006; Gurevich et al., 2008). There has been a consistent theme of these cytosolic proteins interacting with phosphorylated regions of 7TMRs, particularly those representing the sites of action of G protein-coupled receptor kinase action. It is also noteworthy that nonphosphorylated intracellular loop and tail regions of 7TMRs have also been shown to contribute to association with arrestins. This binding has been described as sequential and multisite, with both activation sensor that seems to bind to receptor determinants that are exposed upon conformational change (activation) and a phosphate sensor that binds to the sites of receptor phosphorylation. Having these two binding determinants provides the possibility of modifying affinity of binding, further conformational changes in the arrestin that can expose sites for binding of other associated proteins, and that can regulate release and recycling of the arrestin in the regulatory cycle.
7TMR-arrestin association has typically been described as contributing to receptor desensitization via uncoupling the receptor from its heterotrimeric G protein and via internalization by moving the receptor off the cell surface where ligands would interact with it. The latter seems to be mediated by the direct binding of arrestins 2 and 3 to clathrin, a major structural element of the endocytic machinery. Arrestin also associates with other proteins involved in trafficking and endocytosis, including phosphoinositides, activator protein 2, _N_-ethylmaleimide-sensitive factor, ADP-ribosylation factor 6, and ADP-ribosylation factor nucleotide-binding-site opener (i.e., ARNO) (Shenoy and Lefkowitz, 2005; DeWire et al., 2007). Arrestins also act as more general scaffolds, binding several MAPK family members and nonreceptor tyrosine kinases that may be critical for many intracellular signaling events. They also can direct endocytosed proteins for ubiquitination, by associating with E3 ubiquitin ligase Mdm2. The structural basis for the interaction of arrestin molecules with both 7TMRs and other signaling and regulatory molecules has been thoroughly explored, with distinct determinants for many of these processes (Gurevich and Gurevich, 2006). These can even be dissociated by means of use of various arrestin mutants. It is particularly interesting that receptor-arrestin signalsomes do not require heterotrimeric G proteins for their signaling. Indeed, this is the molecular basis first identified as mediating biased agonism at 7TMRs.
Another group of important 7TMR-interacting proteins are PDZ domain proteins (Bockaert et al., 2004). Many 7TMRs express an endogenous PDZ ligand at their distal carboxyl termini (Bockaert et al., 2003). This typically involves the terminal three or four residues, representing the minimal sequence that binds to PDZ domains. Three classes of PDZ ligands have been described: class I (-E-S/T-xV/I), class II (-Φ-x-Φ-), and class III (-ψ-x-Φ-), in which ψ represents an acidic residue and Φ represents a hydrophobic residue (Sheng and Sala, 2001). The most studied PDZ-containing partner of 7TMRs is the Na+/H+ exchange regulator factor 1. Many other similar partners have been described previously (C kinase 1 protein, Golgi reassembly stacking protein, glutamate receptor interacting protein, and nebulin molecules). A PDZ domain typically consists of 80 to 100 residues forming six β-strands and two α-helices. The carboxyl-terminal tail of the 7TMR is believed to interact with an elongated surface groove that is situated between the second β-strand and the second α-helix in an antiparallel manner. The functional implications of such interactions are as diverse as the PDZ domain-containing proteins. Functions related to intracellular trafficking, transcriptional regulation, and cell growth have been postulated.
2. Operational Mechanisms of Functional Selectivity.
a. Historical perspective.
Perhaps the most important single concept in pharmacology is that of the receptor and its role as the primary recognition unit for neurotransmitters and hormones (Rang, 2006). This idea put order in otherwise diverse physiological responses to chemicals; by implication, the receptor was thought to be the minimal unit of interaction transmitting chemical signals to cells. The necessary corollary to this idea is that the relative potency of activators (agonists) of this minimal unit should be constant if measured in the same tissue under the same conditions. This is because the relative potency of agonists would then be a complex function of parameters unique only to the chemical structures of the agonists, namely affinity and efficacy. This idea formed the basis of receptor classification before biochemical characterization of receptors was possible, and it was enormously valuable in the classification of receptor types and subtypes.
Over the past 20 years, instances in which the relative activity of agonists did not adhere to the predictions of simple receptor theory were noted with some agonists for some receptors. (Roth and Chuang, 1987; Mottola et al., 1991; Roerig et al., 1992 Fisher et al., 1993; Gurwitz et al., 1994; Lawler et al., 1994; Ward et al., 1995; Heldman et al., 1996; Mailman et al., 1998; Lawler et al., 1999). To consider these data further, it is important to note the reliance of the predictions of constant agonist potency ratios on the definition of efficacy. Until the 1980s, the scale for efficacy was system response either in the form of a complex whole-tissue response (e.g., contraction, secretion) or defined single biochemical measure (e.g., cyclic AMP). Although it was known that 7TM receptors are pleiotropic with respect to the number of cytosolic elements with which they can interact (e.g., multiple G proteins) (Offermanns et al., 1994; Prather et al., 1994, 2000; Gudermann et al., 1996; Wise et al., 1999; Burford et al., 2000; Albert and Robillard, 2002; reviewed in Hermans, 2003), measures of response and efficacy at that time were single amalgamated measures of cell activation. Early formulations of receptor activation of 7TM receptors considered the receptors mainly as rheostats controlling a single uniform signal varying only in intensity. In terms of this concept, differential signaling could still occur but only in terms of a strength-of-signal scale; i.e., the most sensitive signal would be activated first followed by less efficiently coupled processes. Such effects could be mediated by agonist efficacy and/or the receptor density on the cell membrane. For example, the opioid receptor agonist [d-Ala2-d-Leu5]enkephalin inhibits basal cyclic AMP in NG 108-115 cells (Costa et al., 1988) and also stimulates high-affinity GTPase. Reduction of the receptor density through alkylation produces a preparation less sensitive to opioid receptor stimulation; under these conditions, the less sensitive response (GTPase stimulation) is eliminated and only the most sensitive response (inhibition of adenylate cyclase) remains. A similar effect has been observed for muscarinic receptor contraction of guinea pig ileum. In this case, oxotremorine is 2-fold more potent than carbachol as an agonist. However, upon reduction of the receptor density through alkylation with phenoxybenzamine, the responses to oxotremorine are completely eliminated, whereas the response to carbachol is diminished but still present. The reason for this disparity in agonism is the fact that oxotremorine is a high-affinity but low-efficacy agonist (more sensitive to decreases in receptor number) and carbachol is a low-affinity but high-efficacy agonist (more resistant to diminution of tissue sensitivity). These data indicate how the strength of a pharmacological signal can appear as functional selectivity. However, this mechanism yields data that does not require the postulate of separate agonist-induced receptor active states (Kenakin, 1995a). Such strength-of-signal effects produce cell-based functional selectivity that is not associated with selective stabilization of receptor conformation by agonists (Kenakin, 2007c). The fact that this effect is cell-dependent makes it difficult to harness under clinical conditions and thus of limited therapeutic interest and relevance. A typical pattern of functional selectivity based on stimulus strength would show a low- and high-efficacy agonist both capable of producing activation of one signaling pathway and the agonist of higher efficacy showing activation of the second pathway, whereas the lower efficacy does not.
Once multiple measures of efficacy could be determined from a single receptor it became clear that the idea of the receptor itself being the minimal trafficking unit for chemical information to the cell was obsolete. One of the earliest and most striking examples of lack of adherence to the idea that the receptor is the minimal unit of signal transduction for the cell was found with PACAP. In LLC-PK1 cells transfected with PACAP receptor, the relative potencies of the PACAP analogs PACAP1–27 and PACAP1–38 for increasing cellular cyclic AMP and inositol phosphate were measured. In complete contradiction to predictions of simple receptor theory, the two agonists reversed their relative order of potency for the two signaling pathways mediated by the same receptor. Thus, whereas the relative potency for cyclic AMP was PACAP1–27 > PACAP1–38, this order was reversed for inositol phosphate production (PACAP1–27 < PACAP1–38) (Spengler et al., 1993). These data definitively demonstrated that the two agonists were not activating the receptor in the same manner but rather that there is a uniqueness to the activation related to each agonist that expressed itself as a different bias in signaling.
The first formal model to account for these digressions postulated that it was the ligand-receptor complex, not the receptor, that constituted the minimal recognition unit for cytosolic elements interacting with the receptor, in essence what was called the “receptor active state” made by the ligand (Kenakin, 1995a). This model was adapted from an earlier one that describes the interaction of a single receptor with two G proteins (Kenakin and Morgan, 1989). Thus, agonist-selective active states, formed by different agonists, could bias the activation of cellular signaling pathways and be functionally selective. Although originally defined for G protein-receptor interaction, functional selectivity extends beyond processes mediated by G proteins. Another early indication that ligands could stabilize receptor conformations that mediated effects beyond G protein activation was the observation that antagonists, devoid of agonist activating activity, nevertheless produced active internalization of receptors (Roettger et al., 1997; Gray and Roth, 2001). This disproved the notion that antagonists were just inert ligands that occluded access to the binding site for endogenous agonists on receptors; this idea will be developed in later discussion of the classification and nomenclature of antagonists (section V.C.2.f).
b. Ligand-specific seven transmembrane receptor conformations.
The observation of differential signaling has supported the notion that molecules produce ligand-specific conformations in the cell membrane (Palanche et al., 2001; reviewed in Kenakin, 2001, 2002a,b, 2003). In fact, the ability of ligands to stabilize different conformations of 7TMRs has been confirmed directly with a number of technological approaches, including fluorescent probes on receptors (Gether et al., 1995; Ghanouni et al., 2001; Kobilka and Gether, 2002), plasmon-waveguide resonance spectroscopy (Hruby and Tollin, 2007; Georgieva et al., 2008), fluorescence resonance energy transfer studies (Vilardaga et al., 2003, 2005; Swaminath et al., 2004, 2005; Granier et al., 2007; Lohse et al., 2008; Zürn et al., 2009), bioluminescence resonance energy transfer studies (Galandrin et al., 2008; Lohse et al., 2008), circular dichroism (Banères et al., 2005), X-ray crystallography (Okada and Palczewski, 2001), antibody binding (Tutor et al., 2007), site-directed mutagenesis and molecule modeling (Pellissier et al., 2009), and kinetic studies (Swaminath et al., 2004). As mentioned previously, indirect cytosolic signaling probes have been used for a number of years to detect ligand-specific receptor conformations. The general idea is that if ligands stabilize different conformations, then signaling molecules should detect these effects by differential engagement of signaling proteins. For example, experiments such as the transfection of the human calcitonin receptor into wild-type HEK cells and HEK cells cotransduced with Gαs protein showed that agonists such as eel and porcine calcitonin actually reversed their relative potency in cells enriched with Gαs protein (Watson et al., 2000). These data were best explained by the postulate that eel and porcine calcitonin stabilize different active state conformations of the calcitonin receptor and that these had higher affinities for Gαs protein.
In general, if it is accepted that ligands stabilize different global ensembles of conformations and that different cytosolic guests (e.g., signaling proteins) interact with different parts of 7TMRs (Ikezu et al., 1992; Jones and Hinkle, 2008), then it would be expected that multiple probe allostery would show probe dependence. For example, different structural elements of β-arrestin, which itself has been shown to undergo changes in conformation with receptor binding (Xiao et al., 2004), have been shown to interact with different forms of the receptor (Hanson and Gurevich, 2006). Therefore, on a theoretical level, agonist-selective stabilization of different receptor conformations would be predicted to result in bias in coupling to different cytosolic proteins. In practice, there has been a great deal of evidence to show biased agonist effects that are consistent with the stabilization of ligand-specific receptor active states over the past 15 years (see section V.C.2.b). Only recently has biased signaling of the MAPK pathway through G protein and G protein-independent pathways been directly correlated with unique receptor conformations as measured by bioluminescence resonance energy transfer (Galandrin et al., 2008).
One of the more direct methods of demonstrating that receptors can form more than one active functional state is the observance of protean agonism. Protean agonists are ligands that produce a receptor active state that is capable of initiating signal where there is none by stabilizing a receptor active state that is less efficacious than the naturally occurring, spontaneously formed constitutive active state (Kenakin, 1995b, 2001). Thus, protean agonists produce positive agonism in quiescent nonconstitutive systems and inverse agonism in constitutively active systems. These molecules were named after the Greek sea-god Proteus (son of Poseidon), who could change shape at will depending on his environment and needs (Kenakin, 1995b). Experimental examples of a protean agonist are dichloroisoproterenol for β-adrenoceptors (Chidiac et al., 1996) and levomedetomidine for α2A-adrenoceptors (Jansson et al., 1998).
The selective stimulation of cellular pathways by different agonists acting through a single receptor been referred to by many names: stimulus trafficking (Kenakin, 1995a), functional dissociation (Whistler et al., 1999), biased agonism (Jarpe et al., 1998), biased inhibition (Kudlacek et al., 2002), differential engagement (Manning, 2002), discrete activation of transduction (Gurwitz et al., 1994), and functional selectivity (Lawler et al., 1999; Kilts et al., 2002; Shapiro et al., 2003). The term functional selectivity, coined by the Mailman group as early as 1994 (Lawler et al., 1994), is a commonly used term for this effect. Proposed as a universal thermodynamic mechanism for all 7TMRs (Kenakin, 1995a), it has been described for many receptors and discussed as a general receptor mechanism (Gurwitz and Haring, 2003; Hermans, 2003). The list of 7TMRs for which functionally selective signaling has reported is extensive; for a partial listing, see Table 1. Cytosolic allostery resulting in differential trafficking of ligand information can involve different modulator/conduit/guest arrays, and it is useful to differentiate these for discussion.
TABLE 1.
Examples of receptors that demonstrate biased agonism
In summary, when the conformation of 7TM receptors is examined, either directly with biochemical or biophysical methods or indirectly through the response of guest molecules, such as signaling proteins in cells, it is clear that a wide range of conformational ensembles can be stabilized by different modulators. Under these circumstances, it is not guaranteed that a single assay format will detect all known effects of a ligand, and it cannot be assumed that two ligands will produce the same repertoire of receptor behaviors that would correspondingly produce the same array of signals. This latter effect will now be considered as a system whereby a modulator is an agonist acting through a receptor to signaling proteins (G proteins, β-arrestin etc.) as guests; this can result in biased agonism.
c. Biased agonism.
As preferred ensembles of conformations interact with cytosolic signaling proteins, biased activation of signaling cascades ensues. The fact that collections of cytosolic proteins interact with most 7TMRs combined with the notion that regions of proteins change with respect to each other in nonconcerted ways (i.e., different regions move at different rates; therefore, an ensemble will never contain identical tertiary conformations) necessarily leads to the conclusion that different ensembles will have differential sensitivity for any collection of signaling proteins in a cell (see multiprobe allostery). This is the thermodynamic mechanism of biased signaling (for reviews, see Kenakin, 2002a,b, 2006, 2007b; Hermans, 2003; Kukkonen, 2004; Perez and Karnik, 2005). It has been known for some time that the interaction of β-arrestin with 7TMRs is very important for the cessation of G protein signaling (Ferguson, 2001; Pierce and Lefkowitz, 2001; Luttrell and Lefkowitz, 2002) and the internalization of 7TMRs (by linking receptors to endocytic machinery such as clathrin and clathrin adaptor activator protein 2) (Goodman et al., 1996; Laporte et al., 2000, 2002; Kim and Benovic, 2002; Edeling et al., 2006). However, it has also become apparent that β-arrestin mediates signaling in its own right (Luttrell et al., 1999; Luttrell and Lefkowitz, 2002; Terrillon and Bouvier, 2004; Lefkowitz and Shenoy, 2005; Luttrell, 2005; Lefkowitz et al., 2006; Smith and Luttrell, 2006; DeWire et al., 2007). This latter idea describes a complex between the receptor, β-arrestin, and various cytosolic MAP kinases to form a “signalsome” that can produce a low-level, long-lasting cellular signal through activation of ERK1/2. p38 MAPK and c-Jun NH2-terminal kinase also function as scaffolds to connect 7TMR complexes to tyrosine kinase c-Src, phosphatidylinositol 3-kinase/AKT, and nuclear factor-κB pathways (van Biesen et al., 1996; Claing et al., 2002; Gáborik and Hunyady, 2004; Lefkowitz and Whalen, 2004; Lefkowitz and Shenoy, 2005; Vroon et al., 2006).
Biased signaling can roughly be divided into the activation, by an agonist-bound 7TMR, of processes that produce rapid transient G protein-mediated responses, low-level, long-lasting β-arrestin-mediated responses, desensitization of response (usually G protein response largely through β-arrestin binding to the receptor) (Kelly et al., 2008), and removal of the receptor from the cell surface through the process of internalization. Agonist-mediated internalization of receptors can further be differentiated by the long-term fate of the internalized receptors. Although some internalization effects result in rapid recycling of the receptor back to the surface of the cell, other ligands induce much longer term removal and destruction of the receptor from the cell surface (i.e., Mack et al., 1998; Ryman-Rasmussen et al., 2007). Thus, biased agonists produce one or more of these activities to varying degrees.
Almost all agonists for 7TMRs have been characterized through measurement of G protein signals mediating cell second messengers or calcium. However, because the measurement of β-arrestin signaling effects are becoming routine, agonists are now being classified as actually being biased toward the β-arrestin system. For example, PTH activates extracellular signal-related kinase through separate G protein-related and G protein-independent pathways. However, analogs of PTH can separate stimulus through the PTH receptor to these pathways. For example, ERK1/2 is stimulated primarily via the G protein pathway by [Trp1]PTHrp-(1–36) and mainly through the β-arrestin-dependent (and G protein-independent) pathway by PTH-1A [[d-Trp12,Tyr34]PTH-(7–34) (Gesty-Palmer et al., 2006)]. Likewise, the Substance P analog [d-Arg1,d-Phe5,d-Trp7,9,Leu11]Substance P and bombesin both activate ERK1/2 through the bombesin/gastrin-releasing peptide receptor. However, the effects of [d-Arg1,d-Phe5,d-Trp7,9,Leu11]Substance P are pertussis-sensitive (indicating a Gi protein dependence of the effect), whereas the responses to bombesin are not. In fact, bombesin stimulates the ERK1/2 pathway through a G protein-independent β-arrestin effect (MacKinnon et al., 2001). Likewise, angiotensin produces activation of G proteins and β-arrestin through binding to angiotensin type 1A receptors. However, the angiotensin agonist analog SII (Sar1,Ile4,Ile8-AngII) (Holloway et al., 2002) is “perfectly biased” toward producing activation of only the β-arrestin pathway through this receptor (Ahn et al., 2003; Wei et al., 2003). Other examples of perfect bias for β-arrestin activation can be found in standard β-blockers. Thus propranolol, a known inverse agonist for Gαs protein interaction with β2-adrenoceptors (Baker et al., 2003) produces activation of β-arrestin (Azzi et al., 2003; Galandrin et al., 2008). Similar profiles are observed for carvedilol (Wisler et al., 2007) and bucindolol (Galandrin et al., 2008). In addition, just as there is heterogeneity in the G protein array available for 7TMR interaction, there are data to suggest that β-arrestin is functionally diverse as well (Shukla et al., 2008). Finally, it should be noted that 7TMR signaling and behavior can also be mediated by other cytosolic proteins in addition to G proteins and β-arrestin (Bockaert and Pin, 1999; Heuss and Gerber, 2000; Brady and Limbird, 2002; Bockaert et al., 2004; Lanier, 2004; Gavarini et al., 2006; Sun et al., 2007b). Current data for agonist bias most often report effects seen with synthetic agonists, but there is at least one 7TMR in which it is operative for natural ligands. CCL19 and CCL21, two natural agonists for the CCR7 chemokine receptor, both produce G protein activation but differ in that only CCL19 (not CCL21) causes receptor agonist-dependent phosphorylation and recruitment of β-arrestin to terminate the G protein stimulus (Kohout et al., 2004).
G protein-dependent and -independent (β-arrestin-mediated) signals converge to produce ERK phosphorylation (Kim et al., 2009), the source of which often can be differentiated kinetically (Ahn et al., 2004 Gesty-Palmer et al., 2006; DeWire et al., 2007). Specifically, the G protein response is rapid and transient, whereas the β-arrestin response is sustained, and these two signals can have different consequences for the cell (see section V.C.3). Biochemical information about the origin and control of these two signals Leads to the study of factors that emphasize different signaling pathways in cells, hopefully for therapeutic advantage. In general, interactions of receptors with multiple signaling proteins create the ideal conditions for biased agonism if selective receptor conformations are stabilized by different agonists. On a theoretical level, biased effects should be anticipated and signaling identical to endogenous natural agonists should not be expected.
In addition to acute cellular events, there are other long-term consequences to 7TMR-mediated signaling. For example, the generation of βγ subunits from G protein activation also can produce cross-activation of pathways and ligand-dependent nuances in cellular response (Rives et al., 2009). Yet an additional consequence of signaling through agonists can be observed upon chronic activation, which involves the restructuring of the agonist signaling system. For example, regulation of G proteins can result from agonist activation as in the up-regulation of Gα12 and down-regulation of Gαi3 proteins by μ-opioid receptors in Chinese hamster ovary cells (Xu et al., 2008). Likewise, the ligand-specific expression of β-arrestin2 and G protein receptor kinase (GRK) 2 is modulated through receptor by activation by etorphine but not morphine (Narita et al., 2006; Zheng et al., 2008).
One of the major mechanisms of coding for functional selectivity of 7TMRs is the phosphorylation of the ligand-stabilized receptor by GRKs (Pitcher et al., 1998) at the C-terminal (Mendez et al., 2000; Seibold et al., 2000; Kara et al., 2006; Liu et al., 2008) and/or second intracellular loops (Nakamura et al., 1998; Kim et al., 2001) of 7TMRs. This “barcoding” (Zidar et al., 2009) of the receptor by GRK phosphorylation forms the link between the agonist (as it stabilizes unique 7TMR conformations) and the rest of the cytosolic machinery.
In summary, the fact that a number of signaling proteins interact with the receptor at the cytosolic face of the cell membrane at different binding loci opens the possibility of differential interaction of the receptor with these signaling proteins because it forms different conformations. Because different ligands can stabilize different receptor conformations, this naturally leads to the conclusion that these same ligands differentially activate cell signaling; i.e., they can bias the activation to some pathways over others. This type of differential signaling can involve different G proteins, direct activation of β-arrestin as a signaling molecule, or other cellular components such as GRK.
d. Biased antagonism.
As discussed in the section on guest allostery (section V.A.1), modulators produce permissive effects by virtue of the fact that they bind to their own site on the receptor and allow other ligands (i.e., agonists) to bind to the receptor as well. This can produce direct effect such as antagonism or potentiation, but it can also alter the direct effects of the cobinding guest ligand. Thus, the agonist signaling profiles of agonists can be altered by allosteric modulators; this, in turn, can produce a bias in the activation pattern of the agonist. Such effects are referred to as biased antagonism. It should be noted that this term specifically describes the imposition of biased agonism upon an agonist interacting with the receptor after antagonist binding and does not refer to any direct effects (such as β-arrestin activation or internalization) that the antagonist may possess as well.
In terms of imposing biased agonism on natural agonists, _N_α-tosyltryptophan, the allosteric modulator for CRTH2 receptors, causes the natural agonist prostaglandin D2 to change its signaling pattern from activation of Gi and β-arrestin to activation of Gi alone, with no concomitant β-arrestin interaction (Fig. 8) (Mathiesen et al., 2005). Likewise, the natural neurokinin 2 receptor agonist neurokinin A activates Gs and Gq proteins, but the allosteric modulator N,_N_-(2-methylnaphthyl-benzyl)-2-aminoacetonitrile (LP1805) changes this pattern to one of enhanced Gq and blockade of Gs activation (Maillet et al., 2007). The peptide antagonist of the IL-1 receptor 101.10 blocks certain IL-1 signaling pathways but not others (Quiniou et al., 2008). Likewise, a striking disparity in the efficacies of a range of antipsychotic dopamine D2 antagonists in blocking G protein-mediated effects and β-arrestin has been described previously (Masri et al., 2008). In addition, the antagonist _N_-(2-adamantyloxy)carbonyl-α-Me-d-Trp-d-_cis_-Hyp-(_o,p_-dichlorophenol) (RB213) has been reported to show pathways for selective antagonism for type 2 cholecystokinin receptor-mediated inositol phosphate formation and arachidonic acid release (Pommier et al., 1999). In general, any therapeutic situation in which biased agonism may be beneficial qualifies also for intervention with a biased antagonist and this opens new vistas for therapy (see section V.C.3).
Fig. 8.
Two contrasting mechanisms of receptor antagonism. A, orthosteric antagonists block agonist activation of the receptor and all functions mediated by that activation are uniformly inhibited. For example, prostaglandin D2 is the endogenous agonist for CRTH2 receptors causing activation of Gαs protein and β-arrestin. An orthosteric antagonist blocking PDG2 binding to the receptor would block both pathways. B, the allosteric modulator _N_-α-tosyltryptophan (N-α-T) blocks the Gαs protein activation of CRTH2 through PDG2 but still allows the agonist to active β-arrestin (Mathiesen et al., 2005).
e. Functional selectivity and new drug discovery.
The discovery that ligands can stabilize unique receptor conformations with pharmacologically relevant properties theoretically increases the scope for therapeutically targeted selective drug effect (see section V.C.3). Progress in the characterization of functional selectivity has largely been the result of being able to monitor multiple signaling pathways in cells and observing differential activation of those pathways by various ligands. The ultimate result of biased agonism and/or biased antagonism is a phenotypic response that can be unique to a given cell. The first step in the prosecution of this mechanism for new drug discovery is to have the ability to detect the effect. To this end, there has been vast progress in technology in pharmacological assay systems. One of the most facile approaches has been the return to whole-system real-time response reading of pharmacologic response not from isolated tissues but rather from human cells in culture.
Recent technological advances have expanded possible modes of detection of receptor function. The importance of 7TMR and β-arrestin association for functionally selective effects is obvious, and with this realization has come an increase in the technology available to directly measure these effects. A great deal of information is obtained from high-content assays based on imaging techniques that use fluorescent signals to yield information about receptor interaction with β-arrestin and subsequent movement of the receptor/β-arrestin complex within the cytoplasm (Milligan, 2003; Lefkowitz and Whalen, 2004; Fredriksson and Schiöth, 2005). These responses can be monitored directly through observation of receptor/β-arrestin green fluorescent protein complexes (Oakley et al., 2002; Ghosh et al., 2005; Ross et al., 2008; Hanson et al., 2009; van der Lee et al., 2009), with bioluminescence resonance energy transfer (Milligan, 2004; Hamdan et al., 2005), with enzyme fragment complementation (Olson and Eglen, 2007; Zhao et al., 2008; van der Lee et al., 2009), or with protease-activated transcriptional reporter genes (Barnea et al., 2008; Verkaar et al., 2008). Green fluorescent protein and immunofluorescence-based technologies can also be multiplexed to gain multiple readouts from the same cell to compare signaling pathways (Henriksen et al., 2008).
There also have been tremendous advances in technology for the observation of integrated whole-cell pharmacologic responses. Resonant waveguide grating technology has led to the use of optical biosensors that can measure dynamic mass redistribution signals from whole cells (Fang et al., 2006). This technology can detect interactions of 7TMRs with many cytosolic signaling molecules, such as G proteins and β-arrestin, at a depth of 150 to 200 nm within the cell and can also detect receptor internalization. The resulting dynamic mass redistribution signal is a noninvasive cell-based technology that can measure virtually any receptor activation in any cell type in systems such as the Epic (Fang et al., 2005a,b). The responses are obtained in real time and have characteristic kinetic patterns that can be used to identify specific signaling pathways. This technique has been applied to the detection and quantification of functional selectivity in live whole-cell assays (Cunningham et al., 2004; Fang, 2006; Yu et al., 2006; Fang and Ferrie, 2008). Another type of technology that can be used for the same purpose employs alterations in the electrical impedance of layers of cells in culture caused by receptor-mediated changes in cell mass redistribution (Verdonk et al., 2006; McGuinness, 2007; Peters et al., 2007; Shiau et al., 2008; Peters and Scott, 2009). In general, whole-cell responses are the result of the integration of numerous pathway activations and as such are ideal for detecting bias in signaling. These formats also are preferable for detecting agonist phenotypes that will be relevant in the therapeutic situation. The use of these techniques in primary cells adds a further advantage to this approach.
There are two points of control in functionally selective system; the receptor conformation and the cell. Both of these furnish the control units for allostery, namely the ligand structure (as the modulator) and the signaling molecules [as the guest(s)]. The ligand control by chemical structure is ideal for drug discovery because it theoretically enables medicinal chemists to control the receptor conformation stabilized and subsequently the signaling pathway selectively activated. The second factor, however, is deleterious to orderly drug discovery because it imposes a cell type dependence on functionally selective effects that is difficult to control in discovery programs. This is because new drug candidates usually are tested and optimized in cell systems different from the therapeutic ones.
There are a number of phenotypic drug responses associated with cell types that are not specifically ascribed to mechanisms but are nevertheless noted in the literature (i.e., levomedetomidine for α2A-adrenoceptors (Jansson et al., 1998; Kukkonen et al., 2001), isoprostane 8-iso-prostaglandin F for thromboxane A2 receptors (Weber and Markillie, 2003), and quinpirole for dopamine D3 and D2 receptors (Zaworski et al., 1999; Alberts et al., 2000). In addition, cells can adjust their signaling capability according to their needs through control of reagents required for receptor phosphorylation [i.e., through GRKs (Lohse, 1993; Zamah et al., 2002; Ribas et al., 2007; Tobin et al., 2008) or protein kinase A (Daaka et al., 1997; Tobin, 2008)] and internalization. For instance, immune cells dynamically regulate GRK and arrestin levels according to levels of inflammation (Chuang et al., 1992; Vroon et al., 2006). This can lead to differences in receptor phosphorylation “barcoding” that can go on to target receptors for different signaling pathways. Cell-type dependence is observed for phosphorylation of different residues in somatostatin type 2A receptors when studied in Chinese hamster ovary, pituitary, and GH4C1 cells (Liu et al., 2009). Likewise, different cell type-dependent phosphorylation of muscarinic m3 receptors has been observed (Torrecilla et al., 2007). Cell variability with functionally selective effects has also been described in terms of differences in receptor-G protein relative stoichiometry (Kenakin, 1997a; González-Maeso et al., 2002; Ernst et al., 2007; Philip et al., 2007). For example, the β3-adrenoceptor ligands (R,R)-5-[2-[[2-(3-chlorophenyl)-2-hydroxyethyl]-amino]-propyl]1,3-benzodioxole-2,2-decarboxylate (CL316243) and 3-(2-ethylphenoxy)-1-[(1,S)-1,2,3,4-tetrahydronapth-1-ylamino]-2_S_-2-propanol oxalate (SR59230A) both elevate cyclic AMP and activate ERK1/2 and p38 MAPK phosphorylation but with reverse potencies for these pathways (they are functionally selective) (Sato et al., 2007). However, the dominant pathways activated by each agonist change with expression level of β3-adrenoceptors, with a resulting difference in the overall potency of these ligands in producing cellular activation (Michel and Alewijnse, 2007; Sato et al., 2007).
Levels of GRKs are high and dynamically regulated in immune cells (Chuang et al., 1992; Mak et al., 2002). In addition to intrinsic differences in GRK and β-arrestin levels in different cell types, levels of these proteins also have been shown to vary in disease. For example, down-regulation of GRK2 and GRK6 has been observed in patients with rheumatoid arthritis and multiple sclerosis (Lombardi et al., 1999; Giorelli et al., 2004; Vroon et al., 2005) and in rat immune cells from rats with adjuvant arthritis (Lombardi et al., 2001; Vroon et al., 2003). Overexpression of GRK2 in vascular smooth muscle has been shown to lead to hypertension and cardiac hypertrophy (Eckhart et al., 2002). Because receptor desensitization, phosphorylation, and internalization are known to be modulated by expression levels of GRKs and β-arrestin (Ménard et al., 1997; Schlador and Nathanson, 1997), these effects can lead to cell-type dependence for biased ligands.
One behavior of 7TMRs that has been shown to be particularly cell-type dependent is receptor internalization. For example, CB1 cannabinoid receptors have been shown to have varying patterns of phosphorylation leading to different internalizing behavior in HEK293 and AtT20 cells (Daigle et al., 2008). Receptor internalization has been extensively studied for opioid receptors, where it has been shown that opioid receptor internalization can be cell-type dependent (Zhang et al., 1998; Whistler and von Zastrow, 1999; Bailey et al., 2003; Bohn et al., 2004; Koch et al., 2005). This poses a practical problem for prediction of the propensity for new opioid ligands to internalize receptors. It is noteworthy that Groer et al. (2007) have shown that opioid receptors in HEK293 cells do not internalize with morphine or herkinorin activation. However, after transfection of HEK293 cells with GRK2 and β-arrestin2, morphine does internalize opioid receptors, but herkinorin still does not. This may suggest a characteristic property of the morphine and herkinorin scaffolds, namely that morphine can lead to receptor internalization in cells with sufficient reagents to support the process (GRK2 and β-arrestin) whereas herkinorin will not internalize receptors in any cell type irrespective of the levels of GRK and β-arrestin. Thus, the testing of opioid agonists in GRK2-transfected cells could function as characteristic assay for the detection of opioid receptor internalizing activity in ligands.
The data with morphine and herkinorin suggest an approach to control for, or at least predict, cell-type dependence would be through the use of transfection for purpose assays. In theory, these could be used to identify characteristic properties of functionally selective ligands as predictors of activity in different cell types (Kenakin, 2009b). The association of GRKs with general effects on 7TMRs (Kim et al., 2005; Ren et al., 2005) [e.g., GRK2,3 for desensitization and GRK5,6 for signalsome response (Reiter and Lefkowitz, 2006)] opens the possibility of designing biased assays to detect selectivity for desensitization and non G protein mediated β-arrestin-based signaling. The actual association of the type of GRK with biochemical effects on the receptor may be receptor-type-dependent, because the rule of GRK2 for desensitization and GRK5/6 for β-arrestin signaling does not hold true for all receptor and cell types. For example, overexpression of GRKs 2, 5, and especially 6 has been shown to cause leukotriene B4 receptor desensitization in Cos-2 cells (Gaudreau et al., 2002).
For functional selectivity to be therapeutically applied, structure-activity relationships for biased signaling must be understood. A prerequisite for this is a scale for quantifying bias. A useful starting point is to describe agonism in terms of the Black/Leff operational model, in which affinity (denoted as _K_A, the equilibrium dissociation constant of the agonist-receptor complex) and efficacy (denoted as τ, a parameter encompassing both the efficacy of the agonist and the sensitivity of the system) (Black and Leff, 1983; Black et al., 1985). Agonist bias must be described both in terms of affinity and efficacy because both can change with allosteric modulation within descriptions of agonism with the operational model (Kenakin, 2009a). Figure 9 shows the calcium transient response to a range of agonists for the calcitonin receptor in wild-type HEK293 cells and cells stably transfected to increase levels of Gαs protein. It can be seen from Fig. 9, A and B, that the potency ratios of the agonists differ in the different cellular background(s). For each cell background, values of (τ/_K_A), relative to a chosen standard for the system, which in this case is amylin, are calculated to quantify the relative ability of each agonist to produce response (τ/_K_A is referred to as the transduction ratio). For wild-type HEK cells this is 19.05:5.5:1.91:1 for eel calcitonin > porcine calcitonin > rat calcitonin > amylin. It is noteworthy that in Gαs-transfected cells, this relative order changes to 100:26:21.9:1 for porcine calcitonin > rat calcitonin > eel calcitonin > amylin. Thus, the change in only cell background produces a fundamental change in relative agonist potency ratios. A comparison of relative τ/_K_A values can now be used to compare across cell type (Fig. 9, columns labeled “bias” in table). It can be seen that whereas the cotransfection of Gαs into the HEK cell have minimal effect on the transduction ratio for eel calcitonin (relative to amylin) but considerable effect on the agonism produced by porcine calcitonin and rat calcitonin. Because relative τ/_K_A scales theoretically cancel system effects, calculated bias could be used by medicinal chemists to systematically explore agonist bias in a chemical series. The bias observed for these agonists has been proposed to be due to the stabilization of different active states of the calcitonin receptor with differing relative affinity for Gαs protein (Watson et al., 2000). In terms of this hypothesis, these data indicate that rat and porcine calcitonin have a greater bias for activating Gαs protein (over other G proteins) than do amylin and eel calcitonin (bias in order of increasing relative interaction with Gαs protein as porcine calcitonin > rat calcitonin > rat amylin = eel calcitonin).
Fig. 9.
Differences in relative agonist activity with changes in cellular background. A, calcium transient response for activation of human calcitonin receptors transfected into HEK293 cells by eel (●), porcine (○), and rat (▴) calcitonin and rat amylin (▵). B, responses to the same agonists in HEK293 cells transfected with Gαs protein. Note that the relative potencies of the agonists change. The table shows the activity of the agonists in terms of their log transduction ratios [Log (τ/_K_A)] values in the two cell hosts and their relative activity in terms of the reference agonist amylin [ΔLog(τ/_K_A) values]. A measure of the relative effect of Gαs-transfection on each agonist is given as the bias. Data from Watson et al. (2000).
Another useful measurement for describing agonist effect has been given by Ehlert (2005) and Tran et al. (2009). Specifically, when dose-response relationships can be described by a rectangular hyperbola, the maximum response (denoted Max) divided by the EC50 (the molar concentration producing half-maximal effect) describes the ratio of the affinity and the efficacy of the agonist [referred to as the activity ratio (Max/EC50)]. It can be shown that even when a rectangular hyperbolic relationship between concentration and response is not the case, Max/EC50 values are still a useful measure of agonism. Of note is the fact that Max/EC50 values are unique for the particular response pathway being measured; therefore, they serve to characterize the ability of a given agonist to produce activation of a given signaling pathway. Therefore, if two pathways are measured, the relative activation of one (as a function of the activation of the other) can be used to determine signaling preference to yield a measure of bias.
It is interesting to note the subtle structure-activity relationships that have been reported for biased ligands. For example, a single substitution of an oxygen for a nitrogen in the herkinorin chemical scaffold converts an opioid receptor agonist that activates G proteins and causes the μ opioid receptor to associate with β-arrestin and become internalized to the cytoplasm to an agonist that activates only G protein but does not cause interactions with β-arrestin or receptor internalization (Tidgewell et al., 2008). Within a series of phenylethylamines, a considerable bias for association of β2-adrenoceptors with β-arrestin was observed for selective compounds containing an ethyl substitution of the α-carbon (Drake et al., 2008). Stereoisomers of fenoterol differentially activate Gs and Gi proteins in rat cardiomyocytes (Woo et al., 2009). Likewise, 5-HT2C (Miller et al., 2000) and dopamine D2 (Gay et al., 2004) ligands demonstrate more than 100-fold differences in bias with relatively small changes in chemical structure, and no change in overall affinity. In general, it should be noted that bias within agonists does not require large differences in chemical structure.
f. Functional selectivity and drug nomenclature.
The ability to see multiple signaling pathways linked to receptors has revealed the lack of concordance of receptor behaviors when bound to ligands. When Stephenson (1956) defined “efficacy,” the only indication of receptor activation available in his experiments was contraction of guinea pig ileum. Now we are able to visualize separate components of whole-cell respons, and the data show previously unknown diversity. Thus, for functionally selective biased agonists, it can be seen that some cellular pathways are activated and some are not (e.g., G protein versus β-arrestin). This is posing interesting problems for the nomenclature of drugs (Kenakin, 2008). The canonical definitions of agonist and antagonist are ligands that produce activation of cells and inhibition of ligand-mediated activation, respectively. However, it is now known that ligands can be antagonists for some pathways and agonists for others (Azzi et al., 2003; Wisler et al., 2007, Galandrin et al., 2008); i.e., propranolol, carvedilol, and bucindolol block β2-adrenoceptor-mediated elevations in cyclic AMP but activate ERK through β-arrestin. Likewise, a PTH inverse agonist for Gαs protein activation [d-Trp12,Tyr34PTH(7–34)] (Gardella et al., 1996) also produces a positive signal via β-arrestin in the absence of Gαs or Gαq stimulation (Gesty-Palmer et al., 2006). Even within the realms of activation of G-protein pathways, conflicting effects are observed. For example, the cannabinoid CB1 receptor agonist desacetyl levonantradol is a positive agonist for Gi1 and Gi2 but an inverse agonist for Gi3; similarly, the CB1 ligand methanandamide is an inverse agonist for Gi1 and Gi2 and a positive agonist for Gi3 (Mukhopadhyay and Howlett. 2005). In addition, the 5-HT2C ligand 6-chloro-5-methyl-1-[6-(2-methylpyridin-3-yloxy)pyridin-3-ylcarbamoyl]indoline (SB242084) is an inverse agonist for Gαi protein-mediated phospholipase-2 arachidonic acid release and a positive agonist for phospholipase C-mediated inositol phosphate production (De Deurwaerdère et al., 2004). In addition, many antagonists have been shown to actively internalize receptors. Such divergences in efficacy have been used to propose that efficacy be considered to be “pluridimensional”; i.e., ligands may have a range of different efficacies to cause a range of receptor behaviors (Galandrin and Bouvier, 2006).
The classification of drugs through their behaviors in cellular systems (e.g., full agonist, partial agonist, etc.) has long been known to be a poor practice simply because of the ability of cellular systems to change these behaviors with factors such as receptor density or signaling components. This has now been made clearly apparent by the additional behaviors that ligands can be seen to have in addition to simple cellular agonism. These behaviors can serve to modify primary behaviors and, in some cases, offer directly opposing behaviors (positive versus inverse agonism). These factors necessarily suggest drug classifications and nomenclatures more closely associated with pharmacologic processes. It also opens the possibility of complex pharmacological patterns of effect that could lead to cell phenotypic behavior.
3. Therapeutic Application of Functional Selectivity.
Many 7TMRs mediate pleiotropic signaling, in many cases through multiple G proteins (see below). Selection of some of these pathways over others at the G protein level has been postulated as a means of obtaining therapeutically favorable signaling bias in agonists. For example, activation of thyrotropin (thyrotropin-stimulating hormone) receptors leads to thyroid growth and differentiation through activation of Gs protein, whereas activation of Gq protein is required for stimulation of thyroid synthesis (Vassart and Dumont, 1992). Under these circumstances, selective stimulation of thyroid hormone could be achieved through selective Gq protein activation. Likewise, β-Adrenoceptor agonists are known to couple to Gs protein to elevate and Gi proteins to reduce cardiac cell cyclic AMP. Gi-mediated decreases in cyclic AMP production are accentuated in congestive heart failure (Xiao et al., 2003), and the balance of cell death and survival in this disease is related to the relative β-adrenoceptor agonist-induced activation of Gs and Gi protein (Shizukuda and Buttrick, 2002). The fact that different β-adrenoceptor agonists have differential bias toward the Gs and Gi signaling pathways (Pönicke et al., 2006) suggests that control of selective signaling for this receptor may be useful in the treatment of congestive heart failure. Likewise, activation of histamine H1 receptors can lead to elevation of cyclic AMP and inositol phosphate and activation of phospholipase C (Hill et al., 1997). Studies with the biased histamine receptor agonist (1_R_,3_S_)-(−)-_trans_-1-phenyl-3-dimethylamino-1,2,3,4-tetrahydronaphthalene (Moniri et al., 2004) suggest that a selective activation of adenylate cyclase in the brain (of possible value in the treatment of neuropsychiatric and neurodegenerative disorders involving catecholamine transmission) could be produced without the debilitating histamine-mediated allergic responses and hyperalgesia (Galeotti et al., 2004).
In addition to multiple G protein coupling, 7TMRs can couple to other signaling proteins, a common motif being activation of G proteins to produce rapid transient signaling and activation of β-arrestin, which then can serve as a scaffold for MAP kinases to produce long-term, low-level signaling. In most cases, natural hormones and transmitters cause activation of all of these pathways, but there are examples in which selection of signaling in disease can lead to improved therapeutic effect. For example, nicotinic acid activation of the GPR109 receptor is effective in lowering triglycerides and elevating high-density lipoprotein (Canner et al., 1986; Pike, 2005). However, serious cutaneous flushing limits the use of nicotinic acid as an antilipolytic (Pike, 2005). Studies in GPR109 containing human cells with nicotinic acid have identified a cyclic AMP lowering effect as a result of activation of Gi protein and an activation of cytosolic phospholipase A2 as a result of β-arrestin 1 binding to the receptor (Walters et al., 2009). This latter effect is related to arachidonate release, a precursor to prostaglandin D2, which is a potent cause of cutaneous flushing. Studies in β-arrestin-null mice show beneficial effects of nicotinic acid on serum-free fatty acid levels but reduced flushing indicating that an agonist of GPR109, which was devoid of effects on β-arrestin (biased toward Gi protein activation), could be a superior treatment for dyslipidemia (Walters et al., 2009). It is noteworthy that a series of pyrazole GPR109 receptor agonists devoid of the ability to internalize GPR109 and activate ERK fail to induce flushing (Richman et al., 2007).
The activation of α2-adrenoceptors results in a beneficial hypotensive response in hypertension but also an unwanted sedation (Kukkonen, 2004). This latter effect can be assessed through observation of reduced coordination and balance in the rotorod test in mice. The resistance to impairment of rotorod coordination to the α2-adrenoceptor agonist 5-bromo-_N_-(4,5-dihydro-1_H_-imidazol-2-yl)-6-quinoxalinamine (UK-14,304) in β-arrestin 2 knockout mice suggests that sedation may be related to the β-arrestin signaling pathway (Wang et al., 2004). These data further suggest that an α2-adrenoceptor biased agonist devoid of β-arrestin 2 stimulation properties may be a therapeutic improvement (Schmid and Bohn, 2009).
Another case in which a reduced spectrum of agonism has been proposed to be therapeutically advantageous is in the treatment of schizophrenia with the antipsychotic aripiprazole. Specifically, the selectively reduced ability of this partial agonist to produce MAPK phosphorylation (versus inhibition of cyclic AMP accumulation and arachidonate release) coupled with its lack of ability to cause internalization of receptors has been proposed as a biased agonist profile responsible for the therapeutic superiority of aripiprazole in the clinic (Grady et al., 2003; Urban et al., 2007).
Selective β-Arrestin signaling also has been associated with possible beneficial therapeutic effect. For example, PTH regulates calcium homeostasis and bone metabolism, and agonism at the level of the PTH receptor theoretically should produce useful effects in osteoporosis. Specifically, intermittent administration of PTH can increase bone mass by stimulating osteoblasts. However, PTH also can cause stimulation of bone resorption (a negative effect in osteoporosis) through the coupling of osteoblasts to osteoclasts. In mice devoid of β-arrestin 2, PTH does not stimulate bone formation or increase the number of osteoclast cells (Ferrari et al., 2005). These data suggest that an improved beneficial effect of PTH stimulation could be obtained in osteoporosis through selective activation of β-arrestin 2 (Schmid and Bohn, 2009). Data showing that analogs of PTH can produce selective stimulation of G protein and β-arrestin pathways (Bisello et al., 2002; Gesty-Palmer et al., 2006) offers the promise of possibly improved therapy of osteoporosis through biased agonism (Gesty-Palmer et al., 2009).
There is considerable evidence to show that ligand-induced receptor internalization may limit therapeutically relevant chronic agonism. For example, opioids demonstrate diversity in their ability to internalize opioid receptors. Enkephalins avidly internalize receptors, whereas morphine produces cell-type-dependent and variable internalization (Keith et al., 1998). This effect is attributed to the inability of morphine to induce receptor internalization in some cell types (Zhang et al., 1998). Opioid ligands such as herkinorin, which is biased toward producing even less β-arrestin receptor interaction than morphine (Groer et al., 2007; Xu et al., 2007), may provide the key to opioid receptor-mediated analgesia with less concomitant desensitizing effect (Varga et al., 2004). It is noteworthy that it has been shown that the beneficial analgesic effects of morphine are enhanced and prolonged in β-arrestin 2 knockout mice (Bohn et al., 1999; Raehal et al., 2005). These data are consistent with the notion that β-arrestin association of opioid receptors leads to desensitization and receptor internalization. In addition, the negative effects of morphine (respiratory depression, constipation) are dramatically decreased in knockout mice, suggesting that β-arrestin 2 activation by morphine is associated with these negative effects as well. Another therapeutic area in which receptor internalization may be important in is the therapy of Parkinsonism. Specifically, the dopamine agonist (1_R_,3_S_)-3-(1′-adamantyl)-1-aminomethyl-3,4-dihydro-5,6-dihydroxy-1_H_-2-benzopyran (A-77636) has been shown to be limited as a therapy for Parkinson's disease (Lin et al., 1996), an effect hypothesized to be related to its powerful and prolonged (>48 h) internalization of dopamine D1 receptors (Ryman-Rasmussen et al., 2007).
On the other hand, association of receptors to β-arrestin not only mediates receptor desensitization and internalization but also can produce G protein-independent activation of extracellular signal-regulated kinases, which may, in turn, be therapeutically beneficial. One of the most striking examples of possible delineation of beneficial and deleterious effects of these pathways is seen for the angiotensin receptor (Fig. 10). Angiotensin has been shown to produce a range of effects on the cardiovascular system relating to its ability to induce Gq protein activation of phospholipase C, AT1 receptor phosphorylation by GRKs, and recruitment of β-arrestin to both internalize the receptor and produce G protein-independent signaling (Anborgh et al., 2000; Ahn et al., 2003; Violin et al., 2006). Although the Gq protein-mediated effects of angiotensin II can lead to hypertension and other deleterious cardiovascular effects, selective activation of β-arrestin may be beneficial both through a modulatory effect on G protein signaling by angiotensin II. An interesting effect is observed with SII, a β-arrestin biased agonist of the AT1 receptor. Specifically, this agonist causes AT1-receptor association with β-arrestin with no concomitant Gq protein effect (Holloway et al., 2002; Wei et al., 2003; Ahn et al., 2004). In contrast, SII blocks Gq activation by angiotensin II and produces a separate β-arrestin-mediated antiapoptotic effect (Revankar et al., 2004; Rajagopal et al., 2005; Violin and Lefkowitz, 2007).
Fig. 10.
Biased agonism at the angiotensin II receptor. The natural ligand angiotensin II produces activation of G proteins, which can produce a deleterious pressor response in hypertension and heart failure and also stimulates β-arrestin to initiate ERK, Akt, and phosphatidylinositol 3-kinase signals, which could be cytoprotective. The biased agonist SII produces only activation of β-arrestin to induce cytoprotection but not pressor response. Moreover, receptor occupancy of the receptor by SII prevents activation by endogenous angiotensin (the receptor is blocked), thereby further protecting against endogenous pressor response. Data from Violin and Lefkowitz (2007).
Another realm in which limitation of signaling has been proposed for therapeutic advantage is in the use of biased antagonists. For example, the blockade of the angiotensin AT1 receptor is an extensive strategy in the treatment of hypertension; cardiac hypertrophy, failure, and arrhythmia; and diabetic nephropathy. However, most of these effects are related to blockade of the AT1-mediated activation of G proteins (i.e., Gq protein-dependent vasoconstriction and hypertrophic growth), whereas angiotensin-mediated activation β-arrestin 2 has been associated with cardioprotective responses (Hunton et al., 2005; Zhai et al., 2005; Rajagopal et al., 2006). Therefore, a functionally selective AT1 receptor antagonist that blocked Gq protein activation but allowed β-arrestin 2 activation could be a superior therapy (Aplin et al., 2009). Another variation of AT1 receptor biased antagonism relates to activation of β-arrestin 1. In this case, angiotensin activation of β-arrestin 1 has been associated with harmful up-regulation of the aldosterone system, leading to promotion of postmyocardial infarction, adverse cardiac remodeling, and progression of heart failure (Weber, 2001; Connell and Davies, 2005; Marney and Brown, 2007). Therefore, antagonism of AT1 activation of β-arrestin 1 may have selectively beneficial effects in cardiovascular therapy (Lymperopoulos et al., 2009).
The antagonism of serotonin receptors is a very important aspect of therapy for depression and schizophrenia. Serotonin pleiotropically activates signaling pathways, some of which (for example, the β-arrestin system) may be selectively associated with visual and auditory hallucinations (Cussac et al., 2008). It has been postulated that biased antagonism of the serotonin system in the central nervous system may produce advantageous therapeutic effect (selective effects on hallucinations); this is currently under investigation (Schmid et al., 2008). Likewise, the association of serotonin receptors with the cytosolic phosphatase with tensin homology has been linked with propensity for addiction (Ji et al., 2006). Therefore, a ligand that selectively interferes with this interaction may be beneficial in the treatment of addiction.
There is no reason a priori to believe that improved therapeutic profiles should result only from reduction in signaling capability. Analogs of Substance-P such as SP-D ([d-Arg1,d-Phe5,d-Trp7,8,Leu11]substance P) and SP-G ([Arg6,d-Trp7,9,_N_-Me-Phe8]substance P(6–11)) gain valuable antitumor activity through addition of a biased activation of Gi proteins in small-cell lung cancer (MacKinnon et al., 2005), which is a particularly aggressive cancer characterized by aberrant tumor expression of receptors for gastrin-releasing peptide and arginine vasopressin (Carroll et al., 1999; Jensen et al., 2001); blockade of these receptors inhibits the mitogenic and morphogenic effects of these neuropeptides. However, it has been proposed that the biased stimulating effects of SP-D and SP-G (Jarpe et al., 1998) [i.e., activation of ERK signaling (MacKinnon et al., 2001; Djanani et al., 2003)] in addition to blockade of gastrin-releasing peptide and arginine vasopressin produces the unique antiproliferative properties to SP-D and SP-G (MacKinnon et al., 2005).
It has been shown that, in addition to blockade of β-adrenoceptor G protein activation, some β-blockers produce activation of ERK through β-arrestin (Azzi et al., 2003). In view of the fact that a number of β-blockers have been tested in clinical trial for therapy of congestive heart failure and only a few have shown beneficial effects (Metra et al., 2004), this ERK-stimulating effect may be relevant. In particular, carvedilol has been identified as producing beneficial effects in congestive heart failure, and it also has been shown to produce β-arrestin-mediated activation of ERK (Wisler et al., 2007).
Another added property of biased ligands, in this case antagonists, is the ability to actively internalize receptors. In this way, a long-lasting blockade of signaling to a given system can be produced by the removal of the receptor from the cell surface. As discussed previously (section V.A.4), this has obvious advantages for AIDS therapy and prevention by removing the CCR5 receptor, the site of HIV-1 entry. In addition, many tumors overexpress 7TMRs, leading to postulates that activation of these new receptor populations support tumor progression, invasion, and metastasis (Li et al., 2005). In addition, these enormously elevated receptor populations would be predicted to be constitutively active as well, thereby elevating cellular second messenger levels that would, in turn, promote tumor growth (Kenakin, 2001). The ability of antagonists for these 7TMRs to internalize (and thus remove from the cell surface) these receptors without activating them would be postulated to be a useful strategy in cancer (Bosier and Hermans, 2007). Likewise, the ability to internalize 5-HT2A receptors has been proposed to contribute to the unique therapeutic profile of the antipsychotic drug clozapine (Willins et al., 1998, 1999). In addition, reduction of 5-HT agonism in the treatment of major depression, which may be augmented by 5-HT antagonists that cause receptor internalization (Gray and Roth, 2001), has been associated with alleviation of severely pessimistic and dysfunctional attitudes in depressed patients (Meyer et al., 2003).
VI. Conclusions
It is useful to consider 7TMRs as disordered allosteric proteins that exhibit modulator/conduit/guest behavior with a number of guests in the extracellular space and cytosol. The intrinsic disorder in various regions of 7TMRs allows them to adopt numerous conformations, a behavior pattern that can be described as the protein rolling on an energy landscape. The binding of an allosteric ligand changes the thermodynamics of the protein such that it basically moves onto a new energy landscape. This new landscape dictates new behaviors that may manifest themselves as increased or decreased reactivity to other ligands, membrane-bound proteins, or cytosolic signaling proteins. The ability to separately observe these behaviors shows that ligands break the bounds of such classifications as agonist and antagonist and can show a wide variety of mixed effects. The challenge will be to identify these phenotypes and understand how they can be uniquely applied to therapeutics. The first steps in the process of integrating appreciation of general 7TMR allosteric behavior into new drug discovery is to detect the effects through targeted pharmacologic assays, quantifying biased responses and controlling their expression in chemical scaffolds to enhance biased effects. It is hoped that with a better understanding of the complex capabilities of 7TMRs and the access to assays that enable us to see how molecules manipulate 7TMR ensemble behavior, more selective and efficacious drugs will emerge for testing in the clinic.
Acknowledgments.
This work was supported in part by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grants DK32878, DK46577].
1
Abbreviations:
101.10
peptide sequence RYTVELA
5-HT
5-hydroxytryptamine
6′-GNTI
6′-guanidinoaltrindole
7TMR
seven transmembrane receptor
A-77636
(1_R_,3_S_)-3-(1′-adamantyl)-1-aminomethyl-3,4-dihydro-5,6-dihydroxy-1_H_-2-benzopyran
AD101
4-[1-(2,4-dimethyl-3-pyridinylcarbonyl)-4-methyl-4-piperidinyl]-2(S)-methyl-1-[1(S)-[4-(trifluoromethyl)phenyl]ethyl]piperazine
AMD3100
1,1′-[1,4-phenylenebis(methylene)]bis-1,4,8,11 tetraazacyclotetradecane octahydrochloride
AT
angiotensin
CB
cannabinoid
CCL
chemokine
CCR
chemokine
(R,R)-5-[2-[[2-(3-chlorophenyl)-2-hydroxyethyl]-amino]-propyl]1,3-benzodioxole-2,2-decarboxylate
CP320626
5-chloro-1_H_-indole-2-carboxylic acid (1-(4-fluorobenzyl)-2-(4-hydroxypiperidin-1-yl)-2-oxoethyl)amide
CRTH
chemoattractant receptor-homologous molecule expressed on TH2 cells
CXCR
chemokine receptor
ERK
extracellular signal-regulated kinase
GLP
glucagon-like receptor
GRK
G protein receptor kinase
HEK
human embryonic kidney
IL
interleukin
LP1805
N,_N_-(2-methylnaphthyl-benzyl)-2-aminoacetonitrile
LY2033298
3-amino-5-chloro-6-methoxy-4-methyl-thieno[2,3-_b_]pyridine-2-carboxylic acid cyclopropylamide
MAP
mitogen-activated protein
MAPK
mitogen-activated protein kinase
MCP-1
monocyte chemoattractant protein-1
mGluR
metabotropic glutamate receptor
NAM
negative allosteric modulator
NMDA
_N_-methyl-d-aspartate
Org27569
(5-chloro-3-ethyl-1_H_-indole-2-carboxylic acid [2-(4-piperidin-1-ylphenyl)ethyl]amide)
PACAP
pituitary adenylate cyclase-activating polypeptide
PAM
positive allosteric modulator
PDZ
postsynaptic density 95/disc-large/zona occludens
PTH
parathyroid hormone
RAMP
receptor activity-modifying membrane protein
RB213
_N_-(2-adamantyloxy)carbonyl-α-Me-d-Trp-d-_cis_-Hyp-(_o,p_-dichlorophenol)
SB242,084
6-chloro-5-methyl-1-[6-(2-methylpyridin-3-yloxy)pyridin-3-ylcarbamoyl]indoline
SCH-C
(Z)-(4-bromophenyl){1′-[(2,4-dimethyl-1-oxido-3-pyridinyl)carbonyl]-4′-methyl-1,4′-bipiperidin-4-yl}methanone _O_-ethyloxime
SII
Sar1,Ile4,Ile8-AngII
6-chloro-2,3,4,5-tetrahydro-3-methyl-1-(3-methylphenyl)-1_H_-3-benzazepine-7,8-diol
SP-D
[d-Arg1,d-Phe5,d-Trp7,8,Leu11]substance P
SP-G
[Arg6,d-Trp7,9,_N_-Me-Phe8]substance P(6–11)
SR141716
_N_-(piperidino-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-pyrazole-3-carboxamide
SR59230A
3-(2-ethylphenoxy)-1-[(1,S)-1,2,3,4-tetrahydronapth-1-ylamino]-2_S_-2-propanol oxalate
SSTR
somatostatin receptor subtype 5
TAK-220
1-acetyl-piperidine-4-carboxylic acid {3-[4-(4-carbamoyl-benzyl)-piperidin-1-yl]-propyl}-(3-chloro-4-methyl-phenyl)-amide
TAK-779
]N,_N_-dimethyl-_N_-(4-(((2-(4-methylphenyl)-6,7-dihydro-5_H_-benzocyclohepten-8-yl)carbonyl)amino)benzyl)tetrahydro-2_H_-pyran-4-aminium chloride
TM
transmembrane
UK-14,304
5-bromo-_N_-(4,5-dihydro-1_H_-imidazol-2-yl)-6-quinoxalinamine
VPAC
vasoactive intestinal polypeptide-pituitary adenylate cyclase-activating polypeptide.
References
- AbdAlla S, Lother H, Quitterer U. (2000) AT1-receptor heterodimers show enhanced G-protein activation and altered receptor sequestration. Nature 407:94–98 [DOI] [PubMed] [Google Scholar]
- Adrián FJ, Ding Q, Sim T, Velentza A, Sloan C, Liu Y, Zhang G, Hur W, Ding S, Manley P, et al. (2006) Allosteric inhibitors of Bcr-abl-dependent cell proliferation. Nat Chem Biol 2:95–102 [DOI] [PubMed] [Google Scholar]
- Ahn S, Nelson CD, Garrison TR, Miller WE, Lefkowitz RJ. (2003) Desensitization, internalization, and signaling functions of beta-arrestins demonstrated by RNA interference. Proc Natl Acad Sci USA 100:1740–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. (2004) Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem 279:35518–35525 [DOI] [PubMed] [Google Scholar]
- Akam EC, Challiss RA, Nahorski SR. (2001) Gq/11 and Gi/1o activation profiles in CHO cells expressing human muscarinic acetylcholine receptors: dependence on agonist as well as receptor-subtype. Br J Pharmacol 132:950–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert PR, Robillard L. (2002) G protein specificity: traffic direction required. Cell Signal 14:407–418 [DOI] [PubMed] [Google Scholar]
- Alberts GL, Pregenzer JF, Im WB. (2000) Advantages of heterologous expression of human D2long dopamine receptors in human neuroblastoma SH-SY5Y over human embryonic kidney 293 cells. Br J Pharmacol 131:514–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albizu L, Balestre MN, Breton C, Pin JP, Manning M, Mouillac B, Barberis C, Durroux T. (2006) Probing the existence of G protein-coupled receptor dimers by positive and negative ligand-dependent cooperative binding. Mol Pharmacol 70:1783–1791 [DOI] [PubMed] [Google Scholar]
- Alkhatib G, Locati M, Kennedy PE, Murphy PM, Berger EA. (1997) HIV-1 coreceptor activity of CCR5 and its inhibition by chemokines: independence from G protein signaling and importance of coreceptor downmodulation. Virology 234:340–348 [DOI] [PubMed] [Google Scholar]
- Allouche S, Polastron J, Hasbi A, Homburger V, Jauzac P. (1999) Differential G-protein activation by alkaloid and peptide opioid agonists in the human neuroblastoma cell line SK-N-BE. Biochem J 342:71–78 [PMC free article] [PubMed] [Google Scholar]
- Amara A, Gall SL, Schwartz O, Salamero J, Montes M, Loetscher P, Baggiolini M, Virelizier JL, Arenzana-Seisdedos F. (1997) HIV coreceptor downregulation as antiviral principle: SDF-1α-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med 186:139–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anborgh PH, Seachrist JL, Dale LB, Ferguson SS. (2000) Receptors/beta arrestin complex formation and the differential trafficking and resensitization of beta2-adrenergic and angiotensin II type 1A receptors. Mol Endocrinol 14:2040–2053 [DOI] [PubMed] [Google Scholar]
- Angers S, Salahpour A, Bouvier M. (2002) Dimerization: an emerging concept for G protein-coupled receptor ontogeny and function. Annu Rev Pharmacol Toxicol 42:409–435 [DOI] [PubMed] [Google Scholar]
- Aplin M, Bonde MM, Hansen JL. (2009) Molecular determinants of angiotensin II type 1 receptor functional selectivity. J Mol Cell Cardiol 46:15–24 [DOI] [PubMed] [Google Scholar]
- Armour SL, Foord S, Kenakin T, Chen WJ. (1999) Pharmacological characterization of receptor-activity-modifying proteins (RAMPs) and the human calcitonin receptor. J Pharmacol Toxicol Methods 42:217–224 [DOI] [PubMed] [Google Scholar]
- Arttamangkul S, Quillinan N, Low MJ, von Zastrow M, Pintar J, Williams JT. (2008) Differential activation and trafficking of micro-opioid receptors in brain slices. Mol Pharmacol 74:972–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison RE, Gosling J, Monteclaro FS, Franci C, Digilio L, Charo IF, Goldsmith MA. (1996) Multiple extracellular elements of CCR5 and HIV-1 entry: dissociation from response to chemokines. Science 274:1924–1926 [DOI] [PubMed] [Google Scholar]
- Azzi M, Charest PG, Angers S, Rousseau G, Kohout T, Bouvier M, Piñeyro G. (2003) β-Arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc Natl Acad Sci USA 100:11406–11411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar I, Chennubhotla C, Tobi D. (2007) Intrinsic dynamics of enzymes in the unbound state and relation to allosteric regulation. Curr Opin Struct Biol 17:633–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CP, Couch D, Johnson E, Griffiths K, Kelly E, Henderson G. (2003) μ-Opioid receptor desensitization in mature rat neurons: lack of interaction between DAMGO and morphine. J Neurosci 23:10515–10520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JG, Hall IP, Hill SJ. (2003) Agonist and inverse agonist actions of β-blockers at the human β2-adrenoceptor provide evidence for agonist-directed signaling. Mol Pharmacol 64:1357–1369 [DOI] [PubMed] [Google Scholar]
- Ballesteros J, Ransom J. (2006) In-target versus off-target allosteric modulators of GPCRs. Drug Discov Today Ther Strateg 3:445–450 [Google Scholar]
- Banères JL, Mesnier D, Martin A, Joubert L, Dumuis A, Bockaert J. (2005) Molecular characterization of a purified 5-HT4 receptor: a structural basis for drug efficacy. J Biol Chem 280:20253–20260 [DOI] [PubMed] [Google Scholar]
- Barnea G, Strapps W, Herrada G, Berman Y, Ong J, Kloss B, Axel R, Lee KJ. (2008) The genetic design of signaling cascades to record receptor activation. Proc Natl Acad Sci USA 105:64–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. (1982) The cholinergic hypothesis of geriatric memory dysfunction. Science 217:408–414 [DOI] [PubMed] [Google Scholar]
- Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. (1998) Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol 54:94–104 [PubMed] [Google Scholar]
- Beyermann M, Heinrich N, Fechner K, Furkert J, Zhang W, Kraetke O, Bienert M, Berger H. (2007) Achieving signalling selectivity of ligands for the corticotropin-releasing factor type 1 receptor by modifying the agonist's signalling domain. Br J Pharmacol 151:851–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Hall SE, Li H, Vaidehi N. (2008) Ligand-stabilized conformational states of human beta(2) adrenergic receptor: insight into G-protein-coupled receptor activation. Biophys J 94:2027–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisello A, Chorev M, Rosenblatt M, Monticelli L, Mierke DF, Ferrari SL. (2002) Selective ligand-induced stabilization of active and desensitized parathyroid hormone type 1 receptor conformations. J Biol Chem 277:38524–38530 [DOI] [PubMed] [Google Scholar]
- Black JW, Leff P. (1983) Operational models of pharmacological agonism. Proc R Soc Lond B Biol Sci 220:141–162 [DOI] [PubMed] [Google Scholar]
- Black JW, Leff P, Shankley NP, Wood J. (1985) An operational model of pharmacological agonism: the effect of E/[A] curve shape on agonist dissociation constant estimation. Br J Pharmacol 84:561–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn ME, Veloro AM, Fanucci GE. (2009) Monitoring inhibitor-induced conformational population shifts in HIV-1 protease by pulsed EPR spectroscopy. Biochemistry 48:8765–8767 [DOI] [PubMed] [Google Scholar]
- Blanpain C, Doranz BJ, Vakili J, Rucker J, Govaerts C, Baik SS, Lorthioir O, Migeotte I, Libert F, Baleux F, et al. (1999a) Multiple charged and aromatic residues in CCR5 amino-terminal domain are involved in high affinity binding of both chemokines and HIV-1 Env protein. J Biol Chem 274:34719–34727 [DOI] [PubMed] [Google Scholar]
- Blanpain C, Doranz BJ, Bondue A, Govaerts C, De Leener A, Vassart G, Doms RW, Proudfoot A, Parmentier M. (2003) The core domain of chemokines binds CCR5 extracellular domains while their amino terminus interacts with the transmembrane helix bundle. J Biol Chem 278:5179–5187 [DOI] [PubMed] [Google Scholar]
- Blanpain C, Lee B, Vakili J, Doranz BJ, Govaerts C, Migeotte I, Sharron M, Dupriez V, Vassart G, Doms RW, et al. (1999b) Extracellular cysteines of CCR5 are required for chemokine binding, but dispensable for HIV-1 coreceptor activity. J Biol Chem 274:18902–18908 [DOI] [PubMed] [Google Scholar]
- Bockaert J, Fagni L, Dumuis A, Marin P. (2004) GPCR interacting proteins (GIP). Pharmacol Ther 103:203–221 [DOI] [PubMed] [Google Scholar]
- Bockaert J, Marin P, Dumuis A, Fagni L. (2003) The ‘magic tail’ of G protein-coupled receptors: an anchorage for functional protein networks. FEBS Lett 546:65–72 [DOI] [PubMed] [Google Scholar]
- Bockaert J, Pin JP. (1999) Molecular tinkering of G protein-coupled receptors: an evolutionary success.EMBO J 18:1723–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. (1999) Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 286:2495–2498 [DOI] [PubMed] [Google Scholar]
- Bohn LM, Dykstra LA, Lefkowitz RJ, Caron MG, Barak LS. (2004) Relative opioid efficacy is determined by the complements of the G protein-coupled receptor desensitization machinery. Mol Pharmacol 66:106–112 [DOI] [PubMed] [Google Scholar]
- Bonhaus DW, Chang LK, Kwan J, Martin GR. (1998) Dual activation and inhibition of adenylyl cyclase by cannabinoid receptor agonists: evidence for agonist-specific trafficking of intracellular responses. J Pharmacol Exp Ther 287:884–888 [PubMed] [Google Scholar]
- Bosier B, Hermans E. (2007) Versatility of GPCR recognition by drugs: from biological implications to therapeutic relevance. Trends Pharmacol Sci 28:438–446 [DOI] [PubMed] [Google Scholar]
- Bosier B, Hermans E, Lambert D. (2008) Differential modulation of AP-1- and CRE-driven transcription by cannabinoid agonists emphasizes functional selectivity at the CB1 receptor. Br J Pharmacol 155:24–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosshard HR. (2001) Molecular recognition by induced fit: how fit is the concept? News Physiol Sci 16:171–173 [DOI] [PubMed] [Google Scholar]
- Bourne HR. (2006) G-proteins and GPCRs: from the beginning, in GPCRs: From Deorphanization to Lead Structure Identification (Ernst Schering Foundation Symposium Proceedings) (Bourne H, Horuk R, Kuhnke J, Michel H. eds) pp 1–21, Springer, Berlin: [DOI] [PubMed] [Google Scholar]
- Bouvier M. (2001) Oligomerization of G-protein-coupled transmitter receptors. Nat Rev Neurosci 2:274–286 [DOI] [PubMed] [Google Scholar]
- Brady AE, Limbird LE. (2002) G protein-coupled receptor interacting proteins: emerging roles in localization and signal transduction. Cell Signal 14:297–309 [DOI] [PubMed] [Google Scholar]
- Brea J, Castro M, Giraldo J, López-Giménez JF, Padín JF, Quintián F, Cadavid MI, Vilaró MT, Mengod G, Berg KA, et al. (2009) Evidence for distinct antagonist-revealed functional states of 5-hydroxytryptamine2A receptor homodimers. Mol Pharmacol 75:1380–1391 [DOI] [PubMed] [Google Scholar]
- Breitwieser GE. (2004) G protein-coupled receptor oligomerization: implications for G protein activation and cell signaling. Circ Res 94:17–27 [DOI] [PubMed] [Google Scholar]
- Bridges TM, Lindsley CW. (2008) G-protein-coupled receptors: from classical modes of modulation to allosteric mechanisms. ACS Chem Biol 3:530–541 [DOI] [PubMed] [Google Scholar]
- Burford NT, Wang D, Sadée W. (2000) G-protein coupling of μ-opioid receptors (OP3): elevated basal signalling activity. Biochem J 348:531–537 [PMC free article] [PubMed] [Google Scholar]
- Burgen AS. (1981) Conformational changes and drug action. Fed Proc 40:2723–2728 [PubMed] [Google Scholar]
- Carroll RE, Matkowskyj KA, Chakrabarti S, McDonald TJ, Benya RV. (1999) Aberrant expression of gastrin-releasing peptide and its receptor by well-differentiated colon cancers in humans. Am J Physiol 276:G655–G665 [DOI] [PubMed] [Google Scholar]
- Canner PL, Berge KG, Wenger NK, Stamler J, Friedman L, Prineas RJ, Friedewald W. (1986) Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 8:1245–1255 [DOI] [PubMed] [Google Scholar]
- Casadó V, Cortés A, Ciruela F, Mallol J, Ferré S, Lluis C, Canela EI, Franco R. (2007) Old and new ways to calculate the affinity of agonists and antagonists interacting with G-protein-coupled monomeric and dimeric receptors: the receptor-dimer cooperativity index. Pharmacol Ther 116:343–354 [DOI] [PubMed] [Google Scholar]
- Cescato R, Schulz S, Waser B, Eltschinger V, Rivier JE, Wester HJ, Culler M, Ginj M, Liu Q, Schonbrunn A, et al. (2006) Internalization of sst2, sst3, and sst5 receptors: effects of somatostatin agonists and antagonists. J Nucl Med 47:502–511 [PubMed] [Google Scholar]
- Chan KY, Pang RT, Chow BK. (2001) Functional segregation of the highly conserved basic motifs within the third endoloop of the human secretin receptor. Endocrinology 142:3926–3934 [DOI] [PubMed] [Google Scholar]
- Chan WY, McKinzie DL, Bose S, Mitchell SN, Witkin JM, Thompson RC, Christopoulos A, Lazareno S, Birdsall NJ, Bymaster FP, et al. (2008) Allosteric modulation of the muscarinic M4 receptor as an approach to treating schizophrenia. Proc Natl Acad Sci USA 105:10978–10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux JP. (1961) The feedback control mechanisms of biosynthetic l-threonine deaminase by l-isoleucine. Cold Spring Harbor Symp Quant Biol 26:313–318 [DOI] [PubMed] [Google Scholar]
- Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, et al. (2007) High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science 318:1258–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidiac P, Nouet S, Bouvier M. (1996) Agonist-induced modulation of inverse agonist efficacy at the beta 2-adrenergic receptor. Mol Pharmacol 50:662–669 [PubMed] [Google Scholar]
- Christopoulos A. (2002) Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nat Rev Drug Discov 1:198–210 [DOI] [PubMed] [Google Scholar]
- Christopoulos A, Kenakin T. (2002) G protein-coupled receptor allosterism and complexing. Pharmacol Rev 54:323–374 [DOI] [PubMed] [Google Scholar]
- Chuang TT, Sallese M, Ambrosini G, Parruti G, De Blasi A. (1992) High expression of beta-adrenergic receptor kinase in human peripheral blood leukocytes. Isoproterenol and platelet activating factor can induce kinase translocation. J Biol Chem 267:6886–6892 [PubMed] [Google Scholar]
- Ciruela F, Casadó V, Rodrigues RJ, Luján R, Burgueño J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, et al. (2006) Presynaptic control of striatal glutamergic neurotransmission by adenosine A1–A2A receptor heteromers. J Neurosci 26:2080–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claing A, Laporte SA, Caron MG, Lefkowitz RJ. (2002) Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog Neurobiol 66:61–79 [DOI] [PubMed] [Google Scholar]
- Conn PJ, Christopoulos A, Lindsley CW. (2009a) Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov 8:41–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Lindsley CW, Jones CK. (2009b) Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol Sci 30:25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell JM, Davies E. (2005) The new biology of aldosterone. J Endocrinol 186:1–20 [DOI] [PubMed] [Google Scholar]
- Cooper A, Dryden DT. (1984) Allostery without conformational change. A plausible model. Eur Biophys J 11:103–109 [DOI] [PubMed] [Google Scholar]
- Cordeaux Y, Briddon SJ, Megson AE, McDonnell J, Dickenson JM, Hill SJ. (2000) Influence of receptor number on functional responses elicited by agonists acting at the human adenosine A1 receptor: evidence for signaling pathway-dependent changes in agonist potency and relative intrinsic activity. Mol Pharmacol 58:1075–1084 [DOI] [PubMed] [Google Scholar]
- Cordeaux Y, Nickolls SA, Flood LA, Graber SG, Strange PG. (2001) Agonist regulation of D2 dopamine receptor/G protein interaction. Evidence for agonist selection of G protein subtype. J Biol Chem 276:28667–28675 [DOI] [PubMed] [Google Scholar]
- Costa T, Klinz FJ, Vachon L, Herz A. (1988) Opioid receptors are coupled tightly to G proteins but loosely to adenylate cyclase in NG108–15 cell membranes. Mol Pharmacol 34:744–754 [PubMed] [Google Scholar]
- Crozier PS, Stevens MJ, Forrest LR, Woolf TB. (2003) Molecular dynamics simulation of dark-adapted rhodopsin in an explicit membrane bilayer: coupling between local retinal and larger scale conformational change. J Mol Biol 333:493–514 [DOI] [PubMed] [Google Scholar]
- Cunningham BT, Li P, Schulz S, Lin B, Baird C, Gerstenmaier J, Genick C, Wang F, Fine E, Laing L. (2004) Label-free assays on the BIND system. J Biomol Screen 9:481–490 [DOI] [PubMed] [Google Scholar]
- Cussac D, Boutet-Robinet E, Ailhaud MC, Newman-Tancredi A, Martel JC, Danty N, Rauly-Lestienne I. (2008) Agonist-directed trafficking of signalling at serotonin 5-HT2A, 5-HT2B and 5-HT2C-VSV receptors mediated Gq/11 activation and calcium mobilisation in CHO cells. Eur J Pharmacol 594:32–38 [DOI] [PubMed] [Google Scholar]
- Daigle TL, Kwok ML, Mackie K. (2008) Regulation of CB1 cannabinoid receptor internalization by a promiscuous phosphorylation-dependent mechanism. J Neurochem 106:70–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daaka Y, Luttrell LM, Lefkowitz RJ. (1997) Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature 390:88–91 [DOI] [PubMed] [Google Scholar]
- Damian M, Martin A, Mesnier D, Pin JP, Banères JL. (2006) Asymmetric conformational changes in a GPCR dimer controlled by G-proteins. EMBO J 25:5693–5702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta D, Scheer JM, Romanowski MJ, Wells JA. (2008) An allosteric circuit in caspase-1. J Mol Biol 381:1157–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Deurwaerdère P, Navailles S, Berg KA, Clarke WP, Spampinato U. (2004) Constitutive activity of the serotonin2C receptor inhibits in vivo dopamine release in the rat striatum and nucleus accumbens. J Neurosci 24:3235–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos AM, Ultsch M, Kossiakoff AA. (1992) Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science 255:306–312 [DOI] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. (2007) Beta-arrestins and cell signaling. Annu Rev Physiol 69:483–510 [DOI] [PubMed] [Google Scholar]
- Dill KA, Chan HS. (1997) From Levinthal to pathways to funnels. Nat Struct Biol 4:10–19 [DOI] [PubMed] [Google Scholar]
- Djanani A, Kaneider NC, Sturn D, Wiedermann CJ. (2003) Agonist function of the neurokinin receptor antagonist, [d-Arg1,d-Phe5,d-Trp7,9,Leu11] substance P, in monocytes. Regul Pept 115:123–129 [DOI] [PubMed] [Google Scholar]
- Dolezal V, Tucek S. (1998) The effects of brucine and alcuronium on the inhibition of [3H] acetylcholine release from rat striatum by muscarinic receptor agonists. Br J Pharmacol 124:1213–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms RW, Peiper SC. (1997) Unwelcomed guests with master keys: how HIV uses chemokine receptors for cellular entry. Virology 235:179–190 [DOI] [PubMed] [Google Scholar]
- Dong M, Cox RF, Miller LJ. (2009) Juxtamembranous region of the amino terminus of the family B G protein-coupled calcitonin receptor plays a critical role in small-molecule agonist action. J Biol Chem 284:21839–21847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly D. (1997) The arrangement of the transmembrane helices in the secretin receptor family of G-protein-coupled receptors. FEBS Lett 409:431–436 [DOI] [PubMed] [Google Scholar]
- Doranz BJ, Lu ZH, Rucker J, Zhang TY, Sharron M, Cen YH, Wang ZX, Guo HH, Du JG, Accavitti MA, et al. (1997) Two distinct CCR5 domains can mediate coreceptor usage by human immunodeficiency virus type 1.J Virol 71:6305–6314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake MT, Violin JD, Whalen EJ, Wisler JW, Shenoy SK, Lefkowitz RJ. (2008) β-arrestin-biased agonism at the β2-adrenergic receptor. J Biol Chem 283:5669–5676 [DOI] [PubMed] [Google Scholar]
- Durroux T. (2005) Principles: a model for the allosteric interactions between ligand binding sites within a dimeric GPCR. Trends Pharmacol Sci 26:376–384 [DOI] [PubMed] [Google Scholar]
- Eckhart AD, Ozaki T, Tevaearai H, Rockman HA, Koch WJ. (2002) Vascular-targeted overexpression of G protein-coupled receptor kinase-2 in transgenic mice attenuates β-adrenergic receptor signaling and increases resting blood pressure. Mol Pharmacol 61:749–758 [DOI] [PubMed] [Google Scholar]
- Edeling MA, Mishra SK, Keyel PA, Steinhauser AL, Collins BM, Roth R, Heuser JE, Owen DJ, Traub LM. (2006) Molecular switches involving the AP-2 β2 appendage regulate endocytic cargo selection and clathrin coat assembly. Dev Cell 10:329–342 [DOI] [PubMed] [Google Scholar]
- Ehlert FJ. (1988) Estimation of the affinities of allosteric ligands using radioligand binding and pharmacological null methods. Mol Pharmacol 33:187–194 [PubMed] [Google Scholar]
- Ehlert FJ. (2005) Analysis of allosterism in functional assays. J Pharmacol Exp Ther 315:740–754 [DOI] [PubMed] [Google Scholar]
- El-Asmar L, Springael JY, Ballet S, Andrieu EU, Vassart G, Parmentier M. (2005) Evidence for negative binding cooperativity with CCR5-CCR2b heterodimers. Mol Pharmacol 67:460–469 [DOI] [PubMed] [Google Scholar]
- Ellis J, Huyler J, Brann MR. (1991) Allosteric regulation of cloned M1–M5 muscarinic receptor subtypes. Biochem Pharmacol 42:1927–1932 [DOI] [PubMed] [Google Scholar]
- Ellis J, Pediani JD, Canals M, Milasta S, Milligan G. (2006) Orexin-1 receptor-cannabinoid CB1 receptor heterodimerization results in both ligand-dependent and -independent coordinated alterations of receptor localization and function. J Biol Chem 281:38812–38824 [DOI] [PubMed] [Google Scholar]
- Englander SW, Kallenbach NR. (1983) Hydrogen exchange and structural dynamics of proteins and nucleic acids. Q Rev Biophys 16:521–655 [DOI] [PubMed] [Google Scholar]
- Engström M, Savola JM, Wurster S. (2006) Differential efficacies of somatostatin receptor agonists for G-protein activation and desensitization of somatostatin receptor subtype 4-mediated responses. J Pharmacol Exp Ther 316:1262–1268 [DOI] [PubMed] [Google Scholar]
- Ernst OP, Gramse V, Kolbe M, Hofmann KP, Heck M. (2007) Monomeric G-protein coupled receptor rhodopsin in solution activates its G protein transducin at the diffusion limit. Proc Natl Acad Sci USA 104:10859–10864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escribá PV, Wedegaertner PB, Goñi FM, Vögler O. (2007) Lipid-protein interactions in GPCR-associated signaling. Biochim Biophys Acta 1768:836–852 [DOI] [PubMed] [Google Scholar]
- Fang Y, Ferrie AM, Fontaine NH, Yuen PK. (2005a) Characteristics of dynamic mass redistribution of epidermal growth factor receptor signaling in living cells measured with label-free optical biosensors. Anal Chem 77:5720–5725 [DOI] [PubMed] [Google Scholar]
- Fang Y, Li GG, Peng J. (2005b) Optical biosensor provides insights for bradykinin B2 receptor signaling in A431 cells. FEBS Lett 579:6365–6374 [DOI] [PubMed] [Google Scholar]
- Fang Y. (2006) Label-free cell-based assays with optical biosensors in drug discovery. Assay Drug Dev Technol 4:583–595 [DOI] [PubMed] [Google Scholar]
- Fang Y, Ferrie AM, Fontaine NH, Mauro J, Balakrishnan J. (2006) Resonant waveguide grating biosensor for living cell sensing. Biophys J 91:1925–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Ferrie AM. (2008) Label-free optical biosensor for ligand-directed functional selectivity acting on β2-adrenoceptor in living cells. FEBS Lett 582:558–564 [DOI] [PubMed] [Google Scholar]
- Fenton AW. (2008) Allostery: an illustrated definition for the ‘second secret of life’. Trends Biochem Sci 33:420–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SS. (2001) Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev 53:1–24 [PubMed] [Google Scholar]
- Ferrari SL, Pierroz DD, Glatt V, Goddard DS, Bianchi EN, Lin FT, Manen D, Bouxsein ML. (2005) Bone response to intermittent parathyroid hormone is altered in mice null for β-arrestin2. Endocrinology 146:1854–1862 [DOI] [PubMed] [Google Scholar]
- Ferré S, Ciruela F, Woods AS, Lluis C, Franco R. (2007) Functional relevance of neurotransmitter receptor heteromers in the central nervous system. Trends Neurosci 30:440–446 [DOI] [PubMed] [Google Scholar]
- Ferre S, von Euler G, Johansson B, Fredholm BB, Fuxe K. (1991) Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes. Proc Natl Acad Sci USA 88:7238–7241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreon JC, Hilser VJ. (2003) Ligand-induced changes in dynamics in the RT loop of the C-terminal SH3 domain of Sem-5 indicate cooperative conformational coupling. Protein Sci 12:982–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A, Heldman E, Gurwitz D, Haring R, Barak D, Meshulam H, Marciano D, Brandeis R, Pittel Z, Segal M. (1993) Selective signaling via unique M1 muscarinic agonists. Ann NY Acad Sci 695:300–303 [DOI] [PubMed] [Google Scholar]
- Fisher A. (1999) Muscarinic receptor agonists in Alzheimer's disease: more than just a symptomatic treatment? CNS Drugs 12:197–214 [Google Scholar]
- Flicker L. (1999) Acetylcholinesterase inhibitors for Alzheimer's disease. BMJ 318:615–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foord SM, Marshall FH. (1999) RAMPs: accessory proteins for seven transmembrane domain receptors. Trends Pharmacol Sci 20:184–187 [DOI] [PubMed] [Google Scholar]
- Franco R, Casadó V, Cortés A, Mallol J, Ciruela F, Ferré S, Lluis C, Canela EI. (2008a) G-protein-coupled receptor heteromers: function and ligand pharmacology. Br J Pharmacol 153:S90–S98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco R, Casadó V, Cortés A, Pérez-Capote K, Mallol J, Canela E, Ferré S, Lluis C. (2008b) Novel pharmacological targets based on receptor heteromers. Brain Res Rev 58:475–482 [DOI] [PubMed] [Google Scholar]
- Franco R, Casadó V, Mallol J, Ferrada C, Ferré S, Fuxe K, Cortés A, Ciruela F, Lluis C, Canela EI. (2006) The two-state dimer receptor model: a general model for receptor dimers. Mol Pharmacol 69:1905–1912 [DOI] [PubMed] [Google Scholar]
- Franco R, Casadó V, Mallol J, Ferré S, Fuxe K, Cortés A, Ciruela F, Lluis C, Canela EI. (2005) Dimer-based model for heptaspanning membrane receptors. Trends Biochem Sci 30:360–366 [DOI] [PubMed] [Google Scholar]
- Frauenfelder H. (1989) New looks at protein motions. Nature 338:623–624 [Google Scholar]
- Frauenfelder H, Parak F, Young RD. (1988) Conformational substates in proteins. Annu Rev Biophys Biophys Chem 17:451–479 [DOI] [PubMed] [Google Scholar]
- Frauenfelder H, Sligar SG, Wolynes PG. (1991) The energy landscapes and motions of proteins. Science 254:1598–1603 [DOI] [PubMed] [Google Scholar]
- Fredholm BB. (2007) Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ 14:1315–1323 [DOI] [PubMed] [Google Scholar]
- Fredriksson R, Lagerström MC, Lundin LG, Schiöth HB. (2003) The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol 63:1256–1272 [DOI] [PubMed] [Google Scholar]
- Fredriksson R, Schiöth HB. (2005) The repertoire of G-protein-coupled receptors in fully sequenced genomes. Mol Pharmacol 67:1414–1425 [DOI] [PubMed] [Google Scholar]
- Freire E. (1998) Statistical thermodynamic linkage between conformational and binding equilibria. Adv Protein Chem 51:255–279 [DOI] [PubMed] [Google Scholar]
- Gáborik Z, Hunyady L. (2004) Intracellular trafficking of hormone receptors. Trends Endocrinol Metab 15:286–293 [DOI] [PubMed] [Google Scholar]
- Galandrin S, Bouvier M. (2006) Distinct signaling profiles of beta1 and beta2 adrenergic receptor ligands toward adenylyl cyclase and mitogen-activated protein kinase reveals the pluridimensionality of efficacy. Mol Pharmacol 70:1575–1584 [DOI] [PubMed] [Google Scholar]
- Galandrin S, Oligny-Longpré G, Bonin H, Ogawa K, Galés C, Bouvier M. (2008) Conformational rearrangements and signaling cascades involved in ligand-biased mitogen-activated protein kinase signaling through the β1-adrenergic receptor. Mol Pharmacol 74:162–172 [DOI] [PubMed] [Google Scholar]
- Galeotti N, Malmberg-Aiello P, Bartolini A, Schunack W, Ghelardini C. (2004) H1-receptor stimulation induces hyperalgesia through activation of the phospholipase C-PKC pathway. Neuropharmacology 47:295–303 [DOI] [PubMed] [Google Scholar]
- Gao F, Harikumar KG, Dong M, Lam PC, Sexton PM, Christopoulos A, Bordner A, Abagyan R, Miller LJ. (2009) Functional importance of a structurally distinct homodimeric complex of the family B G protein-coupled secretin receptor. Mol Pharmacol 76:264–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Jacobson KA. (2008) Translocation of arrestin induced by human A3 adenosine receptor ligands in an engineered cell line: comparison with G protein-dependent pathways. Pharmacol Res 57:303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardella TJ, Luck MD, Jensen GS, Schipani E, Potts JT, Jr, Jüppner H. (1996) Inverse agonism of amino-terminally truncated parathyroid hormone (PTH) and PTH-related peptide (PTHrP) analogs revealed with constitutively active mutant PTH/PTHrP receptors. Endocrinology 137:3936–3941 [DOI] [PubMed] [Google Scholar]
- Garzino-Demo A, Moss RB, Margolick JB, Cleghorn F, Sill A, Blattner WA, Cocchi F, Carlo DJ, DeVico AL, Gallo RC. (1999) Spontaneous and antigen-induced production of HIV-inhibitory β-chemokines are associated with AIDS-free status. Proc Natl Acad Sci USA 96:11986–11991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudreau R, Le Gouill C, Venne MH, Stankova J, Rola-Pleszczynski M. (2002) Threonine 308 within a putative casein kinase 2 site of the cytoplasmic tail of leukotriene B4 receptor (BLT1) is crucial for ligand-induced, G-protein-coupled receptor-specific kinase 6-mediated desensitization. J Biol Chem 277:31567–31576 [DOI] [PubMed] [Google Scholar]
- Gavarini S, Bécamel C, Altier C, Lory P, Poncet J, Wijnholds J, Bockaert J, Marin P. (2006) Opposite effects of PSD-95 and MPP3 PDZ proteins on serotonin 5-hydroxytryptamine2C receptor desensitization and membrane stability. Mol Biol Cell 17:4619–4631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay EA, Urban JD, Nichols DE, Oxford GS, Mailman RB. (2004) Functional selectivity of D2 receptor ligands in a Chinese hamster ovary hD2L cell line: evidence for induction of ligand-specific receptor states. Mol Pharmacol 66:97–105 [DOI] [PubMed] [Google Scholar]
- Gekko K, Obu N, Li J, Lee JC. (2004) A linear correlation between the energetics of allosteric communication and protein flexibility in the Escherichia coli cyclic AMP receptor protein revealed by mutation-induced changes in compressibility and amide hydrogen-deuterium exchange. Biochemistry 43:3844–3852 [DOI] [PubMed] [Google Scholar]
- George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, O'Dowd BF. (2000) Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. J Biol Chem 275:26128–26135 [DOI] [PubMed] [Google Scholar]
- George SR, O'Dowd BF, Lee SP. (2002) G-protein-coupled receptor oligomerization and its potential for drug discovery. Nat Rev Drug Discov 1:808–820 [DOI] [PubMed] [Google Scholar]
- Georgieva T, Devanathan S, Stropova D, Park CK, Salamon Z, Tollin G, Hruby VJ, Roeske WR, Yamamura HI, Varga E. (2008) Unique agonist-bound cannabinoid CB1 receptor conformations indicate agonist specificity in signaling. Eur J Pharmacol 581:19–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein M, Lesk AM, Chothia C. (1994) Structural mechanisms for domain movements in proteins. Biochemistry 33:6739–6749 [DOI] [PubMed] [Google Scholar]
- Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, Eckhardt AE, Cowan CL, Spurney RF, Luttrell LM, et al. (2006) Distinct β-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J Biol Chem 281:10856–10864 [DOI] [PubMed] [Google Scholar]
- Gesty-Palmer D, Flanner P, Yuan L, Corsino L, Spurney R, Lefkowitz RJ, Luttrell LM. (2009) A β-Arrestin–biased agonist of the parathyroid hormone receptor (PTH1R) promotes bone formation independent of G protein activation. Sci Transl Med 1:1ra1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gether U, Lin S, Kobilka BK. (1995) Fluorescent labeling of purified beta 2 adrenergic receptor. Evidence for ligand-specific conformational changes. J Biol Chem 270:28268–28275 [DOI] [PubMed] [Google Scholar]
- Gether U. (2000) Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr Rev 21:90–113 [DOI] [PubMed] [Google Scholar]
- Geyer M, Schweins T, Herrmann C, Prisner T, Wittinghofer A, Kalbitzer HR. (1996) Conformational transitions in p21_ras_ and its complexes with the effector protein Raf-RBD and GTPase activating protein GAP. Biochemistry 35:10308–10320 [DOI] [PubMed] [Google Scholar]
- Ghanouni P, Gryczynski Z, Steenhuis JJ, Lee TW, Farrens DL, Lakowicz JR, Kobilka BK. (2001) Functionally different agonists induce distinct conformations in the G protein coupling domain of the beta 2 adrenergic receptor. J Biol Chem 276:24433–24436 [DOI] [PubMed] [Google Scholar]
- Ghosh RN, DeBiasio R, Hudson CC, Ramer ER, Cowan CL, Oakley RH. (2005) Quantitative cell-based high-content screening for vasopressin receptor agonists using transfluor technology. J Biomol Screen 10:476–484 [DOI] [PubMed] [Google Scholar]
- Giorelli M, Livrea P, Trojano M. (2004) Post-receptorial mechanisms underlie functional disregulation of β2-adrenergic receptors in lymphocytes from multiple sclerosis patients. J Neuroimmunol 155:143–149 [DOI] [PubMed] [Google Scholar]
- Giraldo J. (2008) On the fitting of binding data when receptor dimerization is suspected. Br J Pharmacol 155:17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnagey AL, Seidenberg M, Ellis J. (1999) Site-directed mutagenesis reveals two epitopes involved in the subtype selectivity of the allosteric interactions of gallamine at muscarinic acetylcholine receptors. Mol Pharmacol 56:1245–1253 [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Kulkarni H, Bolivar H, Mangano A, Sanchez R, Catano G, Nibbs RJ, Freedman BI, Quinones MP, Bamshad MJ, et al. (2005) The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility. Science 307:1434–1440 [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, López-Giménez JF, Zhou M, Okawa Y, Callado LF, Milligan G, et al. (2008) Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452:93–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maeso J, Rodríguez-Puertas R, Meana JJ. (2002) Quantitative stoichiometry of G-proteins activated by mu-opioid receptors in postmortem human brain. Eur J Pharmacol 452:21–33 [DOI] [PubMed] [Google Scholar]
- Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. (1996) β-Arrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor. Nature 383:447–450 [DOI] [PubMed] [Google Scholar]
- Goudet C, Binet V, Preseau L, Pini JP. (2004) Allosteric modulators of class-C G-protein-coupled receptors open new possibilities for therapeutic application. Drug Discov Today Ther Strateg 1:1250–1330 [Google Scholar]
- Grace CR, Perrin MH, DiGruccio MR, Miller CL, Rivier JE, Vale WW, Riek R. (2004) NMR structure and peptide hormone binding site of the first extracellular domain of a type B1 G protein-coupled receptor. Proc Natl Acad Sci USA 101:12836–12841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace CR, Perrin MH, Gulyas J, Digruccio MR, Cantle JP, Rivier JE, Vale WW, Riek R. (2007) Structure of the N-terminal domain of a type B1 G protein-coupled receptor in complex with a peptide ligand. Proc Natl Acad Sci USA 104:4858–4863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady MA, Gasperoni TL, Kirkpatrick P. (2003) Aripiprazole. Nat Rev Drug Discov 2:427–428 [DOI] [PubMed] [Google Scholar]
- Granier S, Kim S, Shafer AM, Ratnala VR, Fung JJ, Zare RN, Kobilka B. (2007) Structure and conformational changes in the C-terminal domain of the beta2-adrenoceptor: insights from fluorescence resonance energy transfer studies. J Biol Chem 282:13895–13905 [DOI] [PubMed] [Google Scholar]
- Gray JA, Roth BL. (2001) Paradoxical trafficking and regulation of 5-HT(2A) receptors by agonists and antagonists. Brain Res Bull 56:441–451 [DOI] [PubMed] [Google Scholar]
- Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano TE, Bohn LM. (2007) An opioid agonist that does not induce mu-opioid receptor–arrestin interactions or receptor internalization. Mol Pharmacol 71:549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudermann T, Kalkbrenner F, Schultz G. (1996) Diversity and selectivity of receptor-G protein interaction. Annu Rev Pharmacol Toxicol 36:429–459 [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV. (2006) The structural basis of arrestin-mediated regulation of G-protein-coupled receptors. Pharmacol Ther 110:465–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV. (2008) How and why do GPCRs dimerize? Trends Pharmacol Sci 29:234–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV, Cleghorn WM. (2008) Arrestins as multi-functional signaling adaptors. Handb Exp Pharmacol (186):15–37 [DOI] [PubMed] [Google Scholar]
- Gurwitz D, Haring R. (2003) Ligand-selective signaling and high-content screening for GPCR drugs. Drug Discov Today 8:1108–1109 [DOI] [PubMed] [Google Scholar]
- Gurwitz D, Haring R, Heldman E, Fraser CM, Manor D, Fisher A. (1994) Discrete activation of transduction pathways associated with acetylcholine m1 receptor by several muscarinic ligands. Eur J Pharmacol 267:21–31 [DOI] [PubMed] [Google Scholar]
- Hall DA, Beresford IJ, Browning C, Giles H. (1999) Signalling by CXC-chemokine receptors 1 and 2 expressed in CHO cells: a comparison of calcium mobilization, inhibition of adenylyl cyclase and stimulation of GTPγS binding induced by IL-8 and GROα. Br J Pharmacol 126:810–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdan FF, Audet M, Garneau P, Pelletier J, Bouvier M. (2005) High-throughput screening of G protein-coupled receptor antagonists using a bioluminescence resonance energy transfer 1-based β-arrestin2 recruitment assay. J Biomol Screen 10:463–475 [DOI] [PubMed] [Google Scholar]
- Hanson BJ, Wetter J, Bercher MR, Kopp L, Fuerstenau-Sharp M, Vedvik KL, Zielinski T, Doucette C, Whitney PJ, Revankar C. (2009) A homogeneous fluorescent live-cell assay for measuring 7-transmembrane receptor activity and agonist functional selectivity through beta-arrestin recruitment. J Biomol Screen 14:798–810 [DOI] [PubMed] [Google Scholar]
- Hanson MA, Cherezov V, Griffith MT, Roth CB, Jaakola VP, Chien EY, Velasquez J, Kuhn P, Stevens RC. (2008) A specific cholesterol binding site is established by the 2.8 A structure of the human beta2-adrenergic receptor. Structure 16:897–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson SM, Gurevich VV. (2006) The differential engagement of arrestin surface charges by the various functional forms of the receptor. J Biol Chem 281:3458–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harikumar KG, Happs RM, Miller LJ. (2008) Dimerization in the absence of higher-order oligomerization of the G protein-coupled secretin receptor. Biochim Biophys Acta 1778:2555–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harikumar KG, Pinon DI, Miller LJ. (2007) Transmembrane segment IV contributes a functionally important interface for oligomerization of the class II G protein-coupled secretin receptor J Biol Chem 282:30363–30372 [DOI] [PubMed] [Google Scholar]
- Harmar AJ. (2001) Family-B G-protein-coupled receptors. Genome Biol 2:REVIEWS3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay DL, Christopoulos G, Christopoulos A, Sexton PM. (2004) Amylin receptors: molecular composition and pharmacology. Biochem Soc Trans 32:865–867 [DOI] [PubMed] [Google Scholar]
- Hay DL, Poyner DR, Sexton PM. (2006) GPCR modulation by RAMPs. Pharmacol Ther 109:173–197 [DOI] [PubMed] [Google Scholar]
- Heldin CH. (1995) Dimerization of cell surface receptors in signal transduction. Cell 80:213–223 [DOI] [PubMed] [Google Scholar]
- Hejnová L, Tucek S, el-Fakahany EE. (1995) Positive and negative allosteric interactions on muscarinic receptors. Eur J Pharmacol 291:427–430 [DOI] [PubMed] [Google Scholar]
- Heldman E, Barg J, Fisher A, Levy R, Pittel Z, Zimlichman R, Kushnir M, Vogel Z. (1996) Pharmacological basis for functional selectivity of partial muscarinic receptor agonists. Eur J Pharmacol 297:283–291 [DOI] [PubMed] [Google Scholar]
- Henriksen U, Fog J, Loechel F, Praestegaard M. (2008) Profiling of multiple signal pathway activities by multiplexing antibody and GFP-based translocation assays. Comb Chem High Throughput Screen 11:537–544 [DOI] [PubMed] [Google Scholar]
- Henzler-Wildman KA, Thai V, Lei M, Ott M, Wolf-Watz M, Fenn T, Pozharski E, Wilson MA, Petsko GA, Karplus M, et al. (2007) Intrinsic motions along an enzymatic reaction trajectory. Nature 450:838–844 [DOI] [PubMed] [Google Scholar]
- Heredia A, Davis C, Amoroso A, Dominique JK, Le N, Klingebiel E, Reardon E, Zella D, Redfield RR. (2003) Induction of G1 cycle arrest in T lymphocytes results in increased extracellular levels of β-chemokines: a strategy to inhibit R5 HIV-1. Proc Natl Acad Sci USA 100:4179–4184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans E. (2003) Biochemical and pharmacological control of the multiplicity of coupling at G-protein-coupled receptors. Pharmacol Ther 99:25–44 [DOI] [PubMed] [Google Scholar]
- Heuss C, Gerber U. (2000) G-protein-independent signaling by G-protein-coupled receptors. Trends Neurosci 23:469–475 [DOI] [PubMed] [Google Scholar]
- Hilairet S, Bouaboula M, Carrière D, Le Fur G, Casellas P. (2003) Hypersensitization of the Orexin 1 receptor by the CB1 receptor: evidence for cross-talk blocked by the specific CB1 antagonist, SR141716. J Biol Chem 278:23731–23737 [DOI] [PubMed] [Google Scholar]
- Hill SJ, Ganellin CR, Timmerman H, Schwartz JC, Shankley NP, Young JM, Schunack W, Levi R, Haas HL. (1997) International Union of Pharmacology. XIII. Classification of histamine receptors. Pharmacol Rev 49:253–278 [PubMed] [Google Scholar]
- Hilser J, Freire E. (1997) Predicting the equilibrium protein folding pathway: structure-based analysis of staphylococcal nuclease. Protein Struct Funct Genet 27:171–183 [DOI] [PubMed] [Google Scholar]
- Hilser VJ, Dowdy D, Oas TG, Freire E. (1998) The structural distribution of cooperative interactions in proteins: analysis of the native state ensemble. Proc Natl Acad Sci USA 95:9903–9908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilser VJ, García-Moreno EB, Oas TG, Kapp G, Whitten ST. (2006) A statistical thermodynamic model of the protein ensemble. Chem Rev 106:1545–1558 [DOI] [PubMed] [Google Scholar]
- Hilser VJ, Thompson EB. (2007) Intrinsic disorder as a mechanism to optimize allosteric coupling in proteins. Proc Natl Acad Sci USA 104:8311–8315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare SR. (2005) Mechanisms of peptide and nonpeptide ligand binding to class B G-protein-coupled receptors. Drug Discovery Today 10:417–427 [DOI] [PubMed] [Google Scholar]
- Hogner A, Kastrup JS, Jin R, Liljefors T, Mayer ML, Egebjerg J, Larsen IK, Gouaux E. (2002) Structural basis for AMPA receptor activation and ligand selectivity: crystal structures of five agonist complexes with the GluR2 binding core. J Mol Biol 322:93–109 [DOI] [PubMed] [Google Scholar]
- Holloway AC, Qian H, Pipolo L, Ziogas J, Miura S, Karnik S, Southwell BR, Lew MJ, Thomas WG. (2002) Side-chain substitutions within angiotensin II reveal different requirements for signaling, internalization, and phosphorylation of type 1A angiotensin receptors. Mol Pharmacol 61:768–777 [DOI] [PubMed] [Google Scholar]
- Holst B, Frimurer TM, Mokrosinski J, Halkjaer T, Cullberg KB, Underwood CR, Schwartz TW. (2009) Overlapping binding site for the endogenous agonist, small-molecule agonists, and ago-allosteric modulators on the ghrelin receptor. Mol Pharmacol 75:44–59 [DOI] [PubMed] [Google Scholar]
- Howard OM, Shirakawa AK, Turpin JA, Maynard A, Tobin GJ, Carrington M, Oppenheim JJ, Dean M. (1999) Naturally occurring CCR5 extracellular and transmembrane domain variants affect HIV-1 co-receptor and ligand binding function. J Biol Chem 274:16228–16234 [DOI] [PubMed] [Google Scholar]
- Hruby VJ, Tollin G. (2007) Plasmon-waveguide resonance (PWR) spectroscopy for directly viewing rates of GPCR/G-protein interactions and quantifying affinities. Curr Opin Pharmacol 7:507–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell WL, Altenbach C, Hubbell CM, Khorana HG. (2003) Rhodopsin structure, dynamics, and activation: a perspective from crystallography, site-directed spin labeling, sulfhydryl reactivity, and disulfide cross-linking. Adv Protein Chem 63:243–290 [DOI] [PubMed] [Google Scholar]
- Hunton DL, Barnes WG, Kim J, Ren XR, Violin JD, Reiter E, Milligan G, Patel DD, Lefkowitz RJ. (2005) Beta-arrestin 2-dependent angiotensin II type 1A receptor-mediated pathway of chemotaxis. Mol Pharmacol 67:1229–1236 [DOI] [PubMed] [Google Scholar]
- Ikeguchi M, Ueno J, Sato M, Kidera A. (2005) A protein structural change upon ligand binding: linear response theory. Phys Rev Lett 94:078102 [DOI] [PubMed] [Google Scholar]
- Ikezu T, Okamoto T, Ogata E, Nishimoto I. (1992) Amino acids 356–372 constitute a Gi-activator sequence of the alpha 2-adrenergic receptor and have a Phe substitute in the G protein-activator sequence motif. FEBS Lett 311:29–32 [DOI] [PubMed] [Google Scholar]
- Jaakola VP, Griffith MT, Hanson MA, Cherezov V, Chien EY, Lane JR, Ijzerman AP, Stevens RC. (2008) The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science 322:1211–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubík J, Bacáková L, el-Fakahany EE, Tucek S. (1995) Subtype selectivity of the positive allosteric action of alcuronium at cloned M1–M5 muscarinic acetylcholine receptors. J Pharmacol Exp Ther 274:1077–1083 [PubMed] [Google Scholar]
- Jakubík J, Bacáková L, El-Fakahany EE, Tucek S. (1997) Positive cooperativity of acetylcholine and other agonists with allosteric ligands on muscarinic acetylcholine receptors. Mol Pharmacol 52:172–179 [DOI] [PubMed] [Google Scholar]
- Jakubík J, Bacáková L, Lisá V, el-Fakahany EE, Tucek S. (1996) Activation of muscarinic acetylcholine receptors via their allosteric binding sites. Proc Natl Acad Sci USA 93:8705–8709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubík J, Tucek S, El-Fakahany EE. (2002) Allosteric modulation by persistent binding of xanomeline of the interaction of competitive ligands with the M1 muscarinic acetylcholine receptor. J Pharmacol Exp Ther 301:1033–1041 [DOI] [PubMed] [Google Scholar]
- James JR, Oliveira MI, Carmo AM, Iaboni A, Davis SJ. (2006) A rigorous experimental framework for detecting protein oligomerization using bioluminescence resonance energy transfer. Nature Methods 3:1001–1006 [DOI] [PubMed] [Google Scholar]
- James LC, Tawfik DS. (2003) Conformational diversity and protein evolution—a 60-year-old hypothesis revisited. Trends Biochem Sci 28:361–368 [DOI] [PubMed] [Google Scholar]
- Janin J, Wodak SJ. (1983) Structural domains in proteins and their role in the dynamics of protein function. Prog Biophys Mol Biol 42:21–78 [DOI] [PubMed] [Google Scholar]
- Jansson CC, Kukkonen JP, Näsman J, Huifang G, Wurster S, Virtanen R, Savola JM, Cockcroft V, Akerman KE. (1998) Protean agonism at α2A-adrenoceptors. Mol Pharmacol 53:963–968 [PubMed] [Google Scholar]
- Jarpe MB, Knall C, Mitchell FM, Buhl AM, Duzic E, Johnson GL. (1998) [D-Arg1,D-Phe5,D-Trp7,9,Leu11]Substance P acts as a biased agonist toward neuropeptide and chemokine receptors. J Biol Chem 273:3097–3104 [DOI] [PubMed] [Google Scholar]
- Jensen JA, Carroll RE, Benya RV. (2001) The case for gastrin-releasing peptide acting as a morphogen when it and its receptor are aberrantly expressed in cancer. Peptides 22:689–699 [DOI] [PubMed] [Google Scholar]
- Ji SP, Zhang Y, Van Cleemput J, Jiang W, Liao M, Li L, Wan Q, Backstrom JR, Zhang X. (2006) Disruption of PTEN coupling with 5-HT2C receptors suppresses behavioral responses induced by drugs of abuse. Nat Med 12:324–329 [DOI] [PubMed] [Google Scholar]
- Ji TH, Grossmann M, Ji I. (1998) G protein-coupled receptors. I. Diversity of receptor-ligand interactions. J Biol Chem 273:17299–17302 [DOI] [PubMed] [Google Scholar]
- Johnson MP, Nisenbaum ES, Large TH, Emkey R, Baez M, Kingston AE. (2004) Allosteric modulators of metabotropic glutamate receptors: lessons learnt from mGlu1, mGlu2 and mGlu5 potentiators and antagonists. Biochem Soc Trans 32:881–887 [DOI] [PubMed] [Google Scholar]
- Johnston CA, Siderovski DP. (2007) Receptor-mediated activation of heterotrimeric G-proteins: current structural insights. Mol Pharmacol 72:219–230 [DOI] [PubMed] [Google Scholar]
- Jones BW, Hinkle PM. (2008) Arrestin binds to different phosphorylated regions of the thyrotropin-releasing hormone receptor with distinct functional consequences. Mol Pharmacol 74:195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CK, Brady AE, Davis AA, Xiang Z, Bubser M, Tantawy MN, Kane AS, Bridges TM, Kennedy JP, Bradley SR, et al. (2008) Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor regulates amyloid processing and produces antipsychotic-like activity in rats. J Neurosci 28:10422–10433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen R, Kubale V, Vrecl M, Schwartz TW, Elling CE. (2007) Oxyntomodulin differentially affects glucagons-like peptide-1 receptor β-arrestin recruitment and signaling through Gαs. J Pharmacol Exp Ther 322:148–154 [DOI] [PubMed] [Google Scholar]
- Kara E, Crépieux P, Gauthier C, Martinat N, Piketty V, Guillou F, Reiter E. (2006) A phosphorylation cluster of five serine and threonine residues in the C-terminus of the follicle-stimulating hormone receptor is important for desensitization but not for β-arrestin-mediated ERK activation. Mol Endocrinol 20:3014–3026 [DOI] [PubMed] [Google Scholar]
- Katritch V, Reynolds KA, Cherezov V, Hanson MA, Roth CB, Yeager M, Abagyan R. (2009) Analysis of full and partial agonists binding to beta2-adrenergic receptor suggests a role of transmembrane helix V in agonist-specific conformational changes. J Mol Recognit 22:307–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith DE, Anton B, Murray SR, Zaki PA, Chu PC, Lissin DV, Monteillet-Agius G, Stewart PL, Evans CJ, von Zastrow M. (1998) mu-Opioid receptor internalization: opiate drugs have differential effects on a conserved endocytic mechanism in vitro and in the mammalian brain. Mol Pharmacol 53:377–384 [PubMed] [Google Scholar]
- Kelly E, Bailey CP, Henderson G. (2008) Agonist-selective mechanisms of GPCR desensitization. Br J Pharmacol 153:S379–S388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. (1995a) Agonist-receptor efficacy. II. Agonist-trafficking of receptor signals. Trends Pharmacol Sci 16:232–238 [DOI] [PubMed] [Google Scholar]
- Kenakin T. (1997a) Differences between natural and recombinant G protein-coupled receptor systems with varying receptor/G-protein stoichiometry. Trends Pharmacol Sci 18:456–464 [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2001) Inverse, protean, and ligand-selective agonism: matters of receptor conformation. FASEB J 15:598–611 [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2002a) Drug efficacy at G protein-coupled receptors. Annu Rev Pharmacol Toxicol 42:349–379 [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2002b) Efficacy at G-protein-coupled receptors. Nat Rev Drug Discov 1:103–110 [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2003) Ligand-selective receptor conformations revisited: the promise and the problem. Trends Pharmacol Sci 24:346–354 [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2004) Allosteric modulators: the new generation of receptor antagonist. Mol Interv 4:222–229 [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2005) New concepts in drug discovery: collateral efficacy and permissive antagonism. Nat Rev Drug Discov 4:919–927 [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2007a)Allosteric agonist modulators. J Recept Signal Transduct Res 27:247–259 [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2007b)Collateral efficacy in drug discovery: taking advantage of the good (allosteric) nature of 7TM receptors. Trends Pharmacol Sci 28:407–415 [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2007c)Functional selectivity through protean and biased agonism: who steers the ship? Mol Pharmacol 72:1393–1401 [DOI] [PubMed] [Google Scholar]
- Kenakin TP. (1995b) Pharmacological proteus. Trends Pharmacol Sci 16:256–258 [DOI] [PubMed] [Google Scholar]
- Kenakin TP. (1996) Receptor conformational induction versus selection: all part of the same energy landscape. Trends Pharmacol Sci 17:190–191 [Google Scholar]
- Kenakin TP. (2006) Collateral efficacy as pharmacological problem applied to new drug discovery. Expert Opin Drug Discov 1:635–652 [DOI] [PubMed] [Google Scholar]
- Kenakin TP. (2008) Pharmacological onomastics: what's in a name? Br J Pharmacol 153:432–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin TP. (2009a) ′7TM receptor allostery: putting numbers to shapeshifting proteins. Trends Pharmacol Sci 30:460–469 [DOI] [PubMed] [Google Scholar]
- Kenakin TP. (2009b) Cellular assays as portals to seven-transmembrane receptor-based drug discovery. Nat Rev Drug Discov 8:617–626 [DOI] [PubMed] [Google Scholar]
- Kenakin TP, Morgan PH. (1989) Theoretical effects of single and multiple transducer receptor coupling proteins on estimates of the relative potency of agonists. Mol Pharmacol 35:214–222 [PubMed] [Google Scholar]
- Ketas TJ, Kuhmann SE, Palmer A, Zurita J, He W, Ahuja SK, Klasse PJ, Moore JP. (2007) Cell surface expression of CCR5 and other host factors influence the inhibition of HIV-1 infection of human lymphocytes by CCR5 ligands. Virology 364:281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew JNC, Trube G, Kemp JA. (1996) A novel mechanism of activity-dependent NMDA receptor antagonism describes the effect of ifenprodil in rat cultured cortical neurons. J Physiol 497.3: 761–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts JD, Connery HS, Arrington EG, Lewis MM, Lawler CP, Oxford GS, O'Malley KL, Todd RD, Blake BL, Nichols DE, et al. (2002) Functional selectivity of dopamine receptor agonists. II. Actions of dihydrexidine in D2L receptor-transfected MN9D cells and pituitary lactotrophs. J Pharmacol Exp Ther 301:1179–1189 [DOI] [PubMed] [Google Scholar]
- Kim J, Ahn S, Rajagopal K, Lefkowitz RJ. (2009) Independent beta-arrestin2 and Gq/protein kinase Czeta pathways for ERK stimulated by angiotensin type 1A receptors in vascular smooth muscle cells converge on transactivation of the epidermal growth factor receptor. J Biol Chem 284:11953–11962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Ahn S, Ren XR, Whalen EJ, Reiter E, Wei H, Lefkowitz RJ. (2005) Functional antagonism of different G protein-coupled receptor kinases for β-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci USA 102:1442–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KM, Valenzano KJ, Robinson SR, Yao WD, Barak LS, Caron MG. (2001) Differential regulation of the dopamine D2 and D3 receptors by G protein-coupled receptor kinases and β-arrestins. J Biol Chem 276:37409–37414 [DOI] [PubMed] [Google Scholar]
- Kim YM, Benovic JL. (2002) Differential roles of arrestin-2 interaction with clathrin and adaptor protein 2 in G protein-coupled receptor trafficking. J Biol Chem 277:30760–30768 [DOI] [PubMed] [Google Scholar]
- Kjelsberg MA, Cotecchia S, Ostrowski J, Caron MG, Lefkowitz RJ. (1992) Constitutive activation of the α1B-adrenergic receptor by all amino acid substitutions at a single site. Evidence for a region which constrains receptor activation. J Biol Chem 267:1430–1433 [PubMed] [Google Scholar]
- Kobilka BK. (2002) Agonist-induced conformational changes in the β2 adrenergic receptor. J Pept Res 60:317–321 [DOI] [PubMed] [Google Scholar]
- Kobilka BK, Deupi X. (2007) Conformational complexity of G-protein-coupled receptors. Trends Pharmacol Sci 28:397–406 [DOI] [PubMed] [Google Scholar]
- Kobilka BK, Gether U. (2002) Use of fluorescence spectroscopy to study conformational changes in the beta 2-adrenoceptor. Methods Enzymol 343:170–182 [DOI] [PubMed] [Google Scholar]
- Koch T, Widera A, Bartzsch K, Schulz S, Brandenburg LO, Wundrack N, Beyer A, Grecksch G, Höllt V. (2005) Receptor endocytosis counteracts the development of opioid tolerance. Mol Pharmacol 67:280–287 [DOI] [PubMed] [Google Scholar]
- Kohout TA, Nicholas SL, Perry SJ, Reinhart G, Junger S, Struthers RS. (2004) Differential desensitization, receptor phosphorylation, beta-arrestin recruitment, and ERK1/2 activation by the two endogenous ligands for the CC chemokine receptor 7. J Biol Chem 279:23214–23222 [DOI] [PubMed] [Google Scholar]
- Kolakowski LF. (1994) GCRDb: a G-protein-coupled receptor database, pp 1–7 [PubMed] [Google Scholar]
- Kondru R, Zhang J, Ji C, Mirzadegan T, Rotstein D, Sankuratri S, Dioszegi M. (2008) Molecular interactions of CCR5 with major classes of small-molecule anti-HIV CCR5 antagonists. Mol Pharmacol 73:789–800 [DOI] [PubMed] [Google Scholar]
- Koshland DE. (1958) Application of a theory of enzyme specificity to protein synthesis. Proc Natl Acad Sci USA 44:98–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland DE., Jr (1998) Conformational changes: how small is big enough? Nat Med 4:1112–1114 [DOI] [PubMed] [Google Scholar]
- Kostenis E. (2006) G proteins in drug screening: from analysis of receptor-G protein specificity to manipulation of GPCR-mediated signalling pathways. Curr Pharm Des 12:1703–1715 [DOI] [PubMed] [Google Scholar]
- Kostenis E, Waelbroeck M, Milligan G. (2005) Techniques: promiscuous Galpha proteins in basic research and drug discovery. Trends Pharmacol Sci 26:595–602 [DOI] [PubMed] [Google Scholar]
- Kotani M. (1968) Fluctuation in quaternary structure of proteins and cooperative ligand binding. I. Generalizations of Monod-Wyman-Changeux model of allosteric enzymes. Prog Theor Phys SupplExtra Number 233–241 [Google Scholar]
- Krause RM, Buisson B, Bertrand S, Corringer PJ, Galzi JL, Changeux JP, Bertrand D. (1998) Ivermectin: a positive allosteric effector of the alpha7 neuronal nicotinic acetylcholine receptor. Mol Pharmacol 53:283–294 [DOI] [PubMed] [Google Scholar]
- Krueger KM, Witte DG, Ireland-Denny L, Miller TR, Baranowski JL, Buckner S, Milicic I, Esbenshade TA, Hancock AA. (2005) G protein-dependent pharmacology of histamine H3 receptor ligands: evidence for heterogeneous active state receptor conformations. J Pharmacol Exp Ther 314:271–281 [DOI] [PubMed] [Google Scholar]
- Kudlacek O, Waldhoer M, Kassack MU, Nickel P, Salmi JI, Freissmuth M, Nanoff C. (2002) Biased inhibition by a suramin analogue of A1-adenosine receptor/G protein coupling in fused receptor/G protein tandems: the A1 adenosine receptor is predominantly coupled to Goalpha in human brain. Naunyn Schmiedebergs Arch Pharamcol 365:8–16 [DOI] [PubMed] [Google Scholar]
- Kuhmann SE, Pugach P, Kunstman KJ, Taylor J, Stanfield RL, Snyder A, Strizki JM, Riley J, Baroudy BM, Wilson IA, et al. (2004) Genetic and phenotypic analyses of human immunodeficiency virus type 1 escape from a small-molecule CCR5 inhibitor. J Virol 78:2790–2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn H, Hall SW, Wilden U. (1984) Light-induced binding of 48-kDa protein to photoreceptor membranes is highly enhanced by phosphorylation of rhodopsin. FEBS Lett 176:473–478 [DOI] [PubMed] [Google Scholar]
- Kukkonen JP. (2004) Regulation of receptor-coupling to (multiple) G proteins. A challenge for basic research and drug discovery. Receptors and Channels 10:167–183 [DOI] [PubMed] [Google Scholar]
- Kukkonen JP, Jansson CC, Akerman KE. (2001) Agonist trafficking of G(i/o)-mediated alpha(2A)-adrenoceptor responses in HEL 92.1.7 cells. Br J Pharmacol 132:1477–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Ma B, Tsai CJ, Sinha N, Nussinov R. (2000) Folding and binding cascades: dynamic landscapes and population shifts. Protein Sci 9:10–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, Nakanishi S, Jingami H, Morikawa K. (2000) Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature 407:971–977 [DOI] [PubMed] [Google Scholar]
- Kurrasch-Orbaugh DM, Watts VJ, Barker EL, Nichols DE. (2003) Serotonin 5-hydroxytryptamine 2A receptor-coupled phospholipase C and phospholipase A2 signaling pathways have different receptor reserves. J Pharmacol Exp Ther 304:229–237 [DOI] [PubMed] [Google Scholar]
- Lanier SM. (2004) AGS proteins, GPR motifs and the signals processed by heterotrimeric G proteins. Biol Cell 96:369–372 [DOI] [PubMed] [Google Scholar]
- Laporte SA, Oakley RH, Holt JA, Barak LS, Caron MG. (2000) The interaction of beta-arrestin with the AP-2 adaptor is required for the clustering of beta 2-adrenergic receptor into clathrin-coated pits. J Biol Chem 275:23120–23126 [DOI] [PubMed] [Google Scholar]
- Laporte SA, Miller WE, Kim KM, Caron MG. (2002) Beta-arrestin/AP-2 interaction in G protein-coupled receptor internalization: identification of a beta-arrestin binging site in beta 2-adaptin. J Biol Chem 277:9247–9254 [DOI] [PubMed] [Google Scholar]
- Lawler CO, Watts VJ, Booth RG, Southerland SB, Mailman RB. (1994) Discrete functional selectivity of drugs: OPC-14597, a selective antagonist for post-synaptic dopamine D2 receptors (Abstract). Soc Neurosci Abstr 20:525 [Google Scholar]
- Lawler CP, Prioleau C, Lewis MM, Mak C, Jiang D, Schetz JA, Gonzalez AM, Sibley DR, Mailman RB. (1999) Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology 20:612–627 [DOI] [PubMed] [Google Scholar]
- Lazareno S, Gharagozloo P, Kuonen D, Popham A, Birdsall NJ. (1998) Subtype selective positive cooperative interactions between brucine analogues and acetylcholine at muscarinic receptors: radioligand binding studies. Mol Pharmacol 53:573–589 [DOI] [PubMed] [Google Scholar]
- Leach K, Sexton PM, Christopoulos A. (2007) Allosteric GPCR modulators: taking advantage of permissive receptor pharmacology. Trends Pharmacol Sci 28:382–389 [DOI] [PubMed] [Google Scholar]
- Lee HJ, Mun HC, Lewis NC, Crouch MF, Culverston EL, Mason RS, Conigrave AD. (2007) Allosteric activation of the extracellular Ca2+-sensing receptor by L-amino acids enhances ERK1/2 phosphorylation. Biochem J 404:141–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK. (2005) Transduction of receptor signals by beta-arrestins. Science 308:512–517 [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Whalen EJ. (2004) β-Arrestins: traffic cops of cell signaling. Curr Opin Cell Biol 16:162–168 [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Rajagopal K, Whalen EJ. (2006) New roles for beta-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol Cell 24:643–652 [DOI] [PubMed] [Google Scholar]
- Levoye A, Dam J, Ayoub MA, Guillaume JL, Couturier C, Delagrange P, Jockers R. (2006) The orphan GPR50 receptor specifically inhibits MT1 melatonin receptor function through heterodimerization. EMBO J 25:3012–3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Huang S, Peng SB. (2005) Overexpression of G protein-coupled receptors in cancer cells: involvement in tumor progression. Int J Oncol 27:1329–1339 [PubMed] [Google Scholar]
- Liang JS, Carsi-Gabrenas J, Krajewski JL, McCafferty JM, Purkerson SL, Santiago MP, Strauss WL, Valentine HH, Potter LT. (1996) Anti-muscarinic toxins from Dendroaspis angusticeps. Toxicon 34:1257–1267 [DOI] [PubMed] [Google Scholar]
- Lin CW, Bianchi BR, Miller TR, Stashko MA, Wang SS, Curzon P, Bednarz L, Asin KE, Britton DR. (1996) Persistent activation of the dopamine D1 receptor contributes to prolonged receptor desensitization: studies with A-77636. J Pharmacol Exp Ther 276:1022–1029 [PubMed] [Google Scholar]
- Lindner AB, Eshhar Z, Tawfik DS. (1999) Conformational changes affect binding and catalysis by ester-hydrolysing antibodies. J Mol Biol 285:421–430 [DOI] [PubMed] [Google Scholar]
- Lindsley JE, Rutter J. (2006) Whence cometh the allosterome? Proc Natl Acad Sci USA 103:10533–10535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litschig S, Gasparini F, Rueegg D, Stoehr N, Flor PJ, Vranesic I, Prézeau L, Pin JP, Thomsen C, Kuhn R. (1999) CPCCOEt, a noncompetitive metabotropic glutamate receptor 1 antagonist, inhibits receptor signaling without affecting glutamate binding. Mol Pharmacol 55:453–461 [PubMed] [Google Scholar]
- Liu J, Perumal NB, Oldfield CJ, Su EW, Uversky VN, Dunker AK. (2006a) Intrinsic disorder in transcription factors. Biochemistry 45:6873–6888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Bee MS, Schonbrunn A. (2009) Site specificity of agonist and second messenger-activated kinases for somatostatin receptor subtype 2A (Sst2A) phosphorylation. Mol Pharmacol 76:68–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Cescato R, Dewi DA, Rivier J, Reubi JC, Schonbrunn A. (2005) Receptor signaling and endocytosis are differentially regulated by somatostatin analogs. Mol Pharmacol 68:90–101 [DOI] [PubMed] [Google Scholar]
- Liu Q, Dewi DA, Liu W, Bee MS, Schonbrunn A. (2008) Distinct phosphorylation sites in the SST2A somatostatin receptor control internalization, desensitization, and arrestin binding. Mol Pharmacol 73:292–304 [DOI] [PubMed] [Google Scholar]
- Liu T, Whitten ST, Hilser VJ. (2006b) Ensemble-based signatures of energy propagation in proteins: a new view of an old phenomenon. Proteins 62:728–738 [DOI] [PubMed] [Google Scholar]
- Livesay DR, Dallakyan S, Wood GG, Jacobs DJ. (2004) A flexible approach for understanding protein stability. FEBS Lett 576:468–476 [DOI] [PubMed] [Google Scholar]
- Lockless SW, Ranganathan R. (1999) Evolutionary conserved pathways of energetic connectivity in protein families. Science 286:295–299 [DOI] [PubMed] [Google Scholar]
- Lohse MJ. (1993) Molecular mechanisms of membrane receptor desensitization. Biochim Biophys Acta 1179:171–188 [DOI] [PubMed] [Google Scholar]
- Lohse MJ, Hein P, Hoffmann C, Nikolaev VO, Vilardaga JP, Bünemann M. (2008) Kinetics of G-protein-coupled receptor signals in intact cells. Br J Pharmacol 153:S125–S132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi MS, Kavelaars A, Cobelens PM, Schmidt RE, Schedlowski M, Heijnen CJ. (2001) Adjuvant arthritis induces down-regulation of G protein-coupled receptor kinases in the immune system. J Immunol 166:1635–1640 [DOI] [PubMed] [Google Scholar]
- Lombardi MS, Kavelaars A, Schedlowski M, Bijlsma JW, Okihara KL, Van de Pol M, Ochsmann S, Pawlak C, Schmidt RE, Heijnen CJ. (1999) Decreased expression and activity of G-protein-coupled receptor kinases in peripheral blood mononuclear cells of patients with rheumatoid arthritis. FASEB J 13:715–725 [DOI] [PubMed] [Google Scholar]
- Lori F, Jessen H, Foli A, Lisziewicz J, Matteo PS. (1997) Long-term suppression of HIV-1 by hydroxyurea and didanosine. JAMA 277:1437–1438 [PubMed] [Google Scholar]
- Lu ZL, Gallagher R, Sellar R, Coetsee M, Millar RP. (2005) Mutations remote from the human gonadotropin-releasing hormone (GnRH) receptor-binding sites specifically increase binding affinity for GnRH II but not GnRH I: evidence for ligand-selective, receptor-active conformations. J Biol Chem 280:29796–29803 [DOI] [PubMed] [Google Scholar]
- Lu ZL, Coetsee M, White CD, Millar RP. (2007) Structural determinants for ligand-receptor conformational selection in a peptide G protein-coupled receptor. J Biol Chem 282:17921–17929 [DOI] [PubMed] [Google Scholar]
- Luttrell LM. (2005) Composition and function of G protein-coupled receptor signalsomes controlling mitogen-activated protein kinase activity. J Mol Neurosci 26:253–264 [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, et al. (1999) β-Arrestin-dependent formation of β2-adrenergic receptor-Src protein kinase complexes. Science 283:655–661 [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Lefkowitz RJ. (2002) The role of β-arrestins in the termination and transduction of G-protein-coupled receptors. J Cell Sci 115:455–465 [DOI] [PubMed] [Google Scholar]
- Lymperopoulos A, Rengo G, Zincarelli C, Kim J, Soltys S, Koch WJ. (2009) An adrenal β-arrestin-1 mediated signaling pathway underlies angiotensin II-induced aldosterone production in vitro and in vivo. Proc Natl Acad Sci USA 106:5825–5830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Shatsky M, Wolfson HJ, Nussinov R. (2002) Multiple diverse ligands binding at a single protein site: a matter of pre-existing conformations. Protein Sci 11:184–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass A, Mohr K. (1996) Opposite effects of alcuronium on agonist and antagonist binding to muscarinic receptors. Eur J Pharmacol 305:231–234 [DOI] [PubMed] [Google Scholar]
- Machová E, Jakubík J, El-Fakahany EE, Dolezal V. (2007) Wash-resistantly bound xanomeline inhibits acetylcholine release by persistent activation of presynaptic M(2) and M(4) muscarinic receptors in rat brain. J Pharmacol Exp Ther 322:316–323 [DOI] [PubMed] [Google Scholar]
- Mack M, Luckow B, Nelson PJ, Cihak J, Simmons G, Clapham PR, Signoret N, Marsh M, Stangassinger M, Borlat F, et al. (1998) Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of HIV infectivity. J Exp Med 187:1215–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon AC, Tufail-Hanif U, Lucas CD, Jodrell D, Haslett C, Sethi T. (2005) Expression of V1A and GRP receptors leads to cellular transformation and increased sensitivity to substance-P analogue-induced growth inhibition. Br J Cancer 92:522–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon AC, Waters C, Jodrell D, Haslett C, Sethi T. (2001) Bombesin and substance P analogues differentially regulate G-protein coupling to the bombesin receptor. Direct evidence for biased agonism. J Biol Chem 276:28083–28091 [DOI] [PubMed] [Google Scholar]
- Maeda K, Das D, Ogata-Aoki H, Nakata H, Miyakawa T, Tojo Y, Norman R, Takaoka Y, Ding J, Arnold GF, et al. (2006) Structural and molecular interactions of CCR5 inhibitors with CCR5. J Biol Chem 281:12688–12698 [DOI] [PubMed] [Google Scholar]
- Maeda K, Nakata H, Koh Y, Miyakawa T, Ogata H, Takaoka Y, Shibayama S, Sagawa K, Fukushima D, Moravek J, et al. (2004) Spirodiketopiperazine-based CCR5 inhibitor which preserves CC-chemokine/CCR5 interactions and exerts potent activity against R5 human immunodeficiency virus type 1 in vitro. J Virol 78:8654–8662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maelicke A, Albuquerque EX. (1996) New approach to drug therapy in Alzheimer's dementia. Drug Discov Today 1:53–59 [Google Scholar]
- Maelicke A, Schrattenholz A, Samochocki M, Radina M, Albuquerque EX. (2000) Allosterically potentiating ligands of nicotinic receptors as a treatment strategy for Alzheimer's disease. Behav Brain Res 113:199–206 [DOI] [PubMed] [Google Scholar]
- Maillet EL, Pellegrini N, Valant C, Bucher B, Hibert M, Bourguignon JJ, Galzi JL. (2007) A novel, conformation-specific allosteric inhibitor of the tachykinin NK2 receptor (NK2R) with functionally selective properties. FASEB J 21:2124–2134 [DOI] [PubMed] [Google Scholar]
- Mailman RB, Lawler CP, Lewis MM, Blake B. (1998) Functional effects of novel dopamine ligands: dihydrexidine and Parkinson's disease as a first step, in Dopamine Receptor Subtypes: From Basic Science to Clinical Application (Jenner P, Demirdamar R. eds) pp 64–83, IOS Press, Amsterdam: [Google Scholar]
- Mak JC, Hisada T, Salmon M, Barnes PJ, Chung KF. (2002) Glucocorticoids reverse IL-1β-induced impairment of β-adrenoceptor-mediated relaxation and up-regulation of G-protein-coupled receptor kinases. Br J Pharmacol 135:987–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning DR. (2002) Measures of efficacy using G proteins as endpoints: differential engagement of G proteins through single receptors. Mol Pharmacol 62:451–452 [DOI] [PubMed] [Google Scholar]
- Mantovani A. (1999) The chemokine system: redundancy for robust outputs. Immunol Today 20:254–257 [DOI] [PubMed] [Google Scholar]
- Marcellino D, Ferré S, Casadó V, Cortés A, Le Foll B, Mazzola C, Drago F, Saur O, Stark H, Soriano A, et al. (2008) Identification of dopamine D1–D3 receptor heteromers. Indications for a role of synergistic D1–D3 receptor interactions in the striatum. J Biol Chem 283:26016–26025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marney AM, Brown NJ. (2007) Aldosterone and end-organ damage. Clin Sci 113:267–278 [DOI] [PubMed] [Google Scholar]
- Masri B, Salahpour A, Didriksen M, Ghisi V, Beaulieu JM, Gainetdinov RR, Caron MG. (2008) Antagonism of dopamine D2 receptor/β-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc Natl Acad Sci USA 105:13656–13661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massot O, Rousselle JC, Fillion MP, Grimaldi B, Cloëz-Tayarani I, Fugelli A, Prudhomme N, Seguin L, Rousseau B, Plantefol M, et al. (1996) 5-Hydroxytryptamine-moduline, a new endogenous cerebral peptide, controls the serotonergic activity via its specific interaction with 5-hydroxytryptamine1B/1D receptors. Mol Pharmacol 50:752–762 [PubMed] [Google Scholar]
- Mathiesen JM, Ulven T, Martini L, Gerlach LO, Heinemann A, Kostenis E. (2005) Identification of indole derivatives exclusively interfering with a G protein-independent signaling pathway of the prostaglandin D2 receptor CRTH2. Mol Pharmacol 68:393–402 [DOI] [PubMed] [Google Scholar]
- May LT, Leach K, Sexton PM, Christopoulos A. (2007) Allosteric modulation of G protein-coupled receptors. Annu Rev Pharmacol Toxicol 47:1–51 [DOI] [PubMed] [Google Scholar]
- McGuinness R. (2007) Impedance-based cellular assay technologies: recent advances, future promise. Curr Opin Pharmacol 7:535–540 [DOI] [PubMed] [Google Scholar]
- McLaughlin JN, Shen L, Holinstat M, Brooks JD, Dibenedetto E, Hamm HE. (2005) Functional selectivity of G protein signaling by agonist peptides and thrombin for the protease-activated receptor-1. J Biol Chem 280:25048–25059 [DOI] [PubMed] [Google Scholar]
- Melchiorre C, Minarini A, Angeli P, Giardina D, Gulini U, Quaglia W. (1989) Polymethylene tetraamines as muscarinic receptor probes. Trends Pharmacol Sci 10Suppl:55–59 [PubMed] [Google Scholar]
- Meller E, Puza T, Diamond J, Lieu HD, Bohmaker K. (1992) Comparative effects of receptor inactivation, 17 beta-estradiol and pertussis toxin on dopaminergic inhibition of prolactin secretion in vitro. J Pharmacol Exp Ther 263:462–469 [PubMed] [Google Scholar]
- Ménard L, Ferguson SS, Zhang J, Lin FT, Lefkowitz RJ, Caron MG, Barak LS. (1997) Synergistic regulation of β2-adrenergic receptor sequestration: intracellular complement of β-adrenergic receptor kinase and β-arrestin determine kinetics of internalization. Mol Pharmacol 51:800–808 [PubMed] [Google Scholar]
- Mendez A, Burns ME, Roca A, Lem J, Wu LW, Simon MI, Baylor DA, Chen J. (2000) Rapid and reproducible deactivation of rhodopsin requires multiple phosphorylation sites. Neuron 28:153–164 [DOI] [PubMed] [Google Scholar]
- Meng EC, Bourne HR. (2001) Receptor activation: what does the rhodopsin structure tell us? Trends Pharmacol Sci 22:587–593 [DOI] [PubMed] [Google Scholar]
- Metra M, Cas LD, di Lenarda A, Poole-Wilson P. (2004) Beta-blockers in heart failure: are pharmacological differences clinically important? Heart Fail Rev 9:123–130 [DOI] [PubMed] [Google Scholar]
- Meyer JH, McMain S, Kennedy SH, Korman L, Brown GM, DaSilva JN, Wilson AA, Blak T, Eynan-Harvey R, Goulding VS, et al. (2003) Dysfunctional attitudes and 5-HT2 receptors during depression and self-harm. Am J Psychiat 160:90–99 [DOI] [PubMed] [Google Scholar]
- Michel MC, Alewijnse AE. (2007) Ligand-directed signaling: 50 ways to find a lover. Mol Pharmacol 72:1097–1099 [DOI] [PubMed] [Google Scholar]
- Miller DW, Dill KA. (1997) Ligand binding to proteins: the binding landscape model. Protein Sci 6:2166–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, Robichaud AJ, Largent B. (2000) 5-HT2C receptor-mediated activation of IP3 and cGMP in HEK293 cells: evidence for agonist directed trafficking (Abstract). Soc Neurosci Abstr 26:46.6 [Google Scholar]
- Milligan G. (2003) High-content assays for ligand regulation of G-protein-coupled receptors. Drug Discov Today 8:579–585 [DOI] [PubMed] [Google Scholar]
- Milligan G. (2004) Applications of bioluminescence- and fluorescence resonance energy transfer to drug discovery at G protein-coupled receptors. Eur J Pharm Sci 21:397–405 [DOI] [PubMed] [Google Scholar]
- Milligan G. (2008) A day in the life of a G protein-coupled receptor: the contribution to function of G protein-coupled receptor dimerization. Br J Pharmacol 153:S216–S229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G, Canals M, Pediani JD, Ellis J, Lopez-Gimenez JF. (2006) The role of GPCR dimerisation/oligomerisation in receptor signalling, in GPCRs: From Deorphanization to Lead Structure Identification (Ernst Schering Foundation Symposium Proceedings) (Bourne H, Horuk R, Kuhnke J, Michel H. eds) pp 145–161, Springer, Berlin: [DOI] [PubMed] [Google Scholar]
- Milligan G, Smith NJ. (2007) Allosteric modulation of heterotrimeric G-protein-coupled receptors. Trends Pharmacol Sci 28:615–620 [DOI] [PubMed] [Google Scholar]
- Mittag T, Forman-Kay JD. (2007) Atomic-level characterization of disordered protein ensembles. Curr Opin Struct Biol 17:3–14 [DOI] [PubMed] [Google Scholar]
- Möhler H, Fritschy JM, Rudolph U. (2002) A new benzodiazepine pharmacology. J Pharmacol Exp Ther 300:2–8 [DOI] [PubMed] [Google Scholar]
- Moniri NH, Covington-Strachan D, Booth RG. (2004) Ligand-directed functional heterogeneity of histamine H1 receptors: novel dual-function ligands selectively activate and block H1-mediated phospholipase C and adenylyl cyclase signaling. J Pharmacol Exp Ther 311:274–281 [DOI] [PubMed] [Google Scholar]
- Monod J, Jacob F. (1961) Teleonomic mechanisms in cellular metabolism, growth, and differentiation. Cold Spring Harbor Symp Quant Biol 26:389–401 [DOI] [PubMed] [Google Scholar]
- Monod J, Wyman J, Changeux JP. (1965) On the nature of allosteric transitions: a plausible model. J Mol Biol 12:88–118 [DOI] [PubMed] [Google Scholar]
- Mottola DM, Cook LL, Jones SR, Booth RG, Nichols DE, Mailman RB. (1991) Dihydrexidine, a selective dopamine receptor agonist that may discriminate postsynaptic D2 receptors (Abstract). Soc Neurosci Abstr 17:818 [Google Scholar]
- Mottola DM, Kilts JD, Lewis MM, Connery HS, Walker QD, Jones SR, Booth RG, Hyslop DK, Piercey M, Wightman RM, Lawler CP, Nichols DE, Mailman RB. (2002) Functional selectivity of dopamine receptor agonists. I. Selective activation of postsynaptic dopamine D2 receptors linked to adenylate cyclase. J Pharmacol Exp Ther 301:1166–1178 [DOI] [PubMed] [Google Scholar]
- Moxley R, Day E, Brown K, Mahnke M, Zurini M, Schmitz R, Jones CE, Jarai G. (2009) Cloning and pharmacological characterization of CCR7, CCL21 and CCL19 from Macaca fascicularis. Eur J Pharm Sci 37:264–271 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Howlett AC. (2005) Chemically distinct ligands promote differential CB1 cannabinoid receptor-Gi protein interactions. Mol Pharmacol 67:2016–2024 [DOI] [PubMed] [Google Scholar]
- Münch G, Dees C, Hekman M, Palm D. (1991) Multisite contacts involved in coupling of the beta-adrenergic receptor with the stimulatory guanine-nucleotide-binding regulatory protein. Structural and functional studies by beta-receptor-site-specific synthetic peptides. Eur J Biochem 198:357–364 [DOI] [PubMed] [Google Scholar]
- Muniz-Medina VM, Jones S, Maglich JM, Galardi C, Hollingsworth RE, Kazmierski WM, Ferris RG, Edelstein MP, Chiswell KE, Kenakin TP. (2009) The relative activity of “function sparing” HIV-1 entry inhibitors on viral entry and CCR5 internalization: is allosteric functional selectivity a valuable therapeutic property? Mol Pharmacol 75:490–501 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Hipkin RW, Ascoli M. (1998) The agonist-induced phosphorylation of the rat follitropin receptor maps to the first and third intracellular loops. Mol Endocrinol 12:580–591 [DOI] [PubMed] [Google Scholar]
- Narita M, Suzuki M, Narita M, Niikura K, Nakamura A, Miyatake M, Yajima Y, Suzuki T. (2006) Mu-opioid receptor internalization-dependent and -independent mechanisms of the development of tolerance to mu-opioid receptor agonists: comparison between etorphine and morphine. Neuroscience 138:609–619 [DOI] [PubMed] [Google Scholar]
- Nickolls SA, Fleck B, Hoare SR, Maki RA. (2005) Functional selectivity of melanocortin 4 receptor peptide and nonpeptide agonists: evidence for ligand-specific conformational states. J Pharmacol Exp Ther 313:1281–1288 [DOI] [PubMed] [Google Scholar]
- Nieslanik BS, Dabrowski MJ, Lyon RP, Atkins WM. (1999) Stopped-flow kinetic analysis of the ligand-induced coil-helix transition in glutathione _S_-transferase A1-1: evidence for a persistent denatured state. Biochemistry 38:6971–6980 [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Hoffmann C, Bünemann M, Lohse MJ, Vilardaga JP. (2006) Molecular basis of partial agonoism at the neurotransmitter α2A-adrenoceptor and Gi-protein heterotrimer. J Biol Chem 281:24506–24511 [DOI] [PubMed] [Google Scholar]
- Noeske T, Gutcaits A, Parsons CG, Weil T. (2006) Allosteric modulation of family 3 GPCRs. QSAR Comb Sci 25:134–146 [Google Scholar]
- Nordberg A, Svensson AL. (1998) Cholinesterase inhibitors in the treatment of Alzheimer's disease: a comparison of tolerability and pharmacology. Drug Saf 19:465–480 [DOI] [PubMed] [Google Scholar]
- Nygaard R, Frimurer TM, Holst B, Rosenkilde MM, Schwartz TW. (2009) Ligand binding and micro-switches in 7TM receptor structures. Trends Pharmacol Sci 30:249–259 [DOI] [PubMed] [Google Scholar]
- Oakley RH, Hudson CC, Cruickshank RD, Meyers DM, Payne RE, Jr., Rhem SM, Loomis CR. (2002) The cellular distribution of fluorescently labeled arrestins provides a robust, sensitive, and universal assay for screening G protein-coupled receptors. Assay Drug Dev Technol 1:21–30 [DOI] [PubMed] [Google Scholar]
- O'Brien JA, Lemaire W, Chen TB, Chang RS, Jacobson MA, Ha SN, Lindsley CW, Schaffhauser HJ, Sur C, Pettibone DJ, et al. (2003) A family of highly selective allosteric modulators of the metabotropic glutamate receptor subtype 5. Mol Pharmacol 64:731–740 [DOI] [PubMed] [Google Scholar]
- Offermanns S, Wieland T, Homann D, Sandmann J, Bombien E, Spicher K, Schultz G, Jakobs KH. (1994) Transfected muscarinic acetylcholine receptors selectively couple to Gi-type proteins and Gq/11. Mol Pharmacol 45:890–898 [PubMed] [Google Scholar]
- Oikonomakos NG, Skamnaki VT, Tsitsanou KE, Gavalas NG, Johnson LN. (2000) A new allosteric site in glycogen phosphorylase b as a target for drug interactions. Structure 8:575–584 [DOI] [PubMed] [Google Scholar]
- Okada T, Palczewski K. (2001) Crystal structure of rhodopsin: implications for vision and beyond. Curr Opin Struct Biol 11:420–426 [DOI] [PubMed] [Google Scholar]
- Okada T, Sugihara M, Bondar AN, Elstner M, Entel P, Buss V. (2004) The retinal conformation and its environment in rhodopsin in light of a new 2.2 A crystal structure. J Mol Biol 342:571–583 [DOI] [PubMed] [Google Scholar]
- Okazaki K, Takada S. (2008) Dynamic energy landscape view of coupled binding and protein conformational change: induced-fit versus population-shift models. Proc Natl Acad Sci USA 105:11182–11187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham WM, Hamm HE. (2008) Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol 9:60–71 [DOI] [PubMed] [Google Scholar]
- Olson KR, Eglen RM. (2007) Beta galactosidase complementation: a cell-based luminescent assay platform for drug discovery. Assay Drug Dev Technol 5:137–144 [DOI] [PubMed] [Google Scholar]
- Onaran HO, Costa T. (1997) Agonist efficacy and allosteric models of receptor action. Ann N Y Acad Sci 812:98–115 [DOI] [PubMed] [Google Scholar]
- Onaran HO, Scheer A, Cotecchia S, Costa T. (2000) A look at receptor efficacy. From the signaling network of the cell to the intramolecular motion of the receptor, in The Pharmacology of Functional, Biochemical, and Recombinant Systems (Handbook of Experimental Pharmacology) (Kenakin TP, Angus JA. eds) vol. 148, pp 217–280, Springer, Heidelberg, Germany: [Google Scholar]
- Oppermann M, Mack M, Proudfoot AE, Olbrich H. (1999) Differential effects of CC chemokines on CC chemokine receptor 5 (CCR5) phosphorylation and identification of phosphorylation sites on the CCR5 carboxyl terminus. J Biol Chem 274:8875–8885 [DOI] [PubMed] [Google Scholar]
- Paila YD, Chattopadhyay A. (2009) The function of G-protein coupled receptors and membrane cholesterol: specific or general interaction? Glycoconjugate Journal 26:711–720 [DOI] [PubMed] [Google Scholar]
- Palanche T, Ilien B, Zoffmann S, Reck MP, Bucher B, Edelstein SJ, Galzi JL. (2001) The neurokinin A receptor activates calcium and cAMP responses through distinct conformational states. J Biol Chem 276:34853–34861 [DOI] [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, et al. (2000) Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289:739–745 [DOI] [PubMed] [Google Scholar]
- Pardee AB, Yates RA. (1956) Control of pyrimidine biosynthesis in Escherichia coli by a feed-back mechanism. J Biol Chem 221:757–770 [PubMed] [Google Scholar]
- Pargellis C, Tong L, Churchill L, Cirillo PF, Gilmore T, Graham AG, Grob PM, Hickey ER, Moss N, Pav S, et al. (2002) Inhibition of p38 MAP kinase by utilizing a novel allosteric binding site. Nat Struct Biol 9:268–272 [DOI] [PubMed] [Google Scholar]
- Park PS, Lodowski DT, Palczewski K. (2008) Activation of G protein-coupled receptors: beyond two-state models and tertiary conformational changes. Annu Rev Pharmacol Toxicol 48:107–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthier C, Kleinschmidt M, Neumann P, Rudolph R, Manhart S, Schlenzig D, Fanghänel J, Rahfeld JU, Demuth HU, Stubbs MT. (2007) Crystal structure of the incretin-bound extracellular domain of a G protein-coupled receptor. Proc Natl Acad Sci USA 104:13942–13947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg G, Ghanouni P, Kobilka BK, Zare RN. (2001) Single-molecule spectroscopy of the beta(2) adrenergic receptor: observation of conformational substates in a membrane protein. Proc Natl Acad Sci USA 98:8469–8474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellissier LP, Sallander J, Campillo M, Gaven F, Queffeulou E, Pillot M, Dumuis A, Claeysen S, Bockaert J, Pardo L. (2009) Conformational toggle switches implicated in basal constitutive and agonist-induced activated states of 5-hydroxytryptamine-4 receptors. Mol Pharmacol 75:982–990 [DOI] [PubMed] [Google Scholar]
- Perez DM, Karnik SS. (2005) Multiple signaling states of G-protein-coupled receptors. Pharmacol Rev 57:147–161 [DOI] [PubMed] [Google Scholar]
- Peters MF, Knappenberger KS, Wilkins D, Sygowski LA, Lazor LA, Liu J, Scott CW. (2007) Evaluation of cellular dielectric spectroscopy, a whole-cell, label-free technology for drug discovery on Gi-coupled GPCRs. J Biomol Screen 12:312–319 [DOI] [PubMed] [Google Scholar]
- Peters MF, Scott CW. (2009) Evaluating cellular impedance assays for the detection of GPCR pleiotropic signaling and functional selectivity. J Biomol Screen 14:246–255 [DOI] [PubMed] [Google Scholar]
- Pfister C, Chabre M, Plouet J, Tuyen VV, De Kozak Y, Faure JP, Kühn H. (1985) Retinal S antigen identified as the 48K protein regulating light-dependent phosphodiesterase in rods. Science 228:891–893 [DOI] [PubMed] [Google Scholar]
- Philip F, Sengupta P, Scarlata S. (2007) Signaling through a G-protein coupled receptor and its corresponding G protein follows a stoichiometrically limited model. J Biol Chem 282:19203–19216 [DOI] [PubMed] [Google Scholar]
- Picard L, Simmons G, Power CA, Meyer A, Weiss RA, Clapham PR. (1997) Multiple extracellular domains of CCR-5 contribute to human immunodeficiency virus type 1 entry and fusion. J Virol 71:5003–5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picchio MC, Scala E, Pomponi D, Caprini E, Frontani M, Angelucci I, Mangoni A, Lazzeri C, Perez M, Remotti D, et al. (2008) CXCL13 is highly produced by Sézary cells and enhances their migratory ability via a synergistic mechanism involving CCL19 and CCL21 chemokines. Cancer Res 68:7137–7146 [DOI] [PubMed] [Google Scholar]
- Pierce KL, Lefkowitz RJ. (2001) Classical and new roles of beta-arrestins in the regulation of G-protein-coupled receptors. Nat Rev Neurosci 2:727–733 [DOI] [PubMed] [Google Scholar]
- Pike NB. (2005) Flushing out the role of GPR109A (HM74A) in the clinical efficacy of nicotinic acid. J Clin Invest 115:3400–3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pioszak AA, Parker NR, Suino-Powell K, Xu HE. (2008) Molecular recognition of corticotropin-releasing factor by its G-protein-coupled receptor CRFR1. J Biol Chem 283:32900–32912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pioszak AA, Xu HE. (2008) Molecular recognition of parathyroid hormone by its G protein-coupled receptor. Proc Natl Acad Sci USA 105:5034–5039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher JA, Freedman NJ, Lefkowitz RJ. (1998) G protein-coupled receptor kinases. Annu Rev Biochem 67:653–692 [DOI] [PubMed] [Google Scholar]
- Poignard P, Saphire EO, Parren PW, Burton DR. (2001) gp120: biologic aspects of structural features. Annu Rev Immunol 19:253–274 [DOI] [PubMed] [Google Scholar]
- Pommier B, Da Nascimento S, Dumont S, Bellier B, Million E, Garbay C, Roques BP, Noble F. (1999) The cholecystokininB receptor is coupled to two effector pathways through pertussis toxin-sensitive and -insensitive G proteins. J Neurochem 73:281–288 [DOI] [PubMed] [Google Scholar]
- Pönicke K, Gröner F, Heinroth-Hoffmann I, Brodde OE. (2006) Agonist-specific activation of the beta2-adrenoceptor/Gs-protein and beta2-adrenoceptor/Gi-protein pathway in adult rat ventricular cardiomyocytes. Br J Pharmacol 147:714–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovych N, Sun S, Ebright RH, Kalodimos CG. (2006) Dynamically driven protein allostery. Nat Struct Mol Biol 13:831–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather PL, Loh HH, Law PY. (1994) Interaction of delta-opioid receptors with multiple G proteins: a non-relationship between agonist potency to inhibit adenylate cyclase and to activate G proteins. Mol Pharmacol 45:997–1003 [PubMed] [Google Scholar]
- Prather PL, Martin NA, Breivogel CS, Childers SR. (2000) Activation of cannabinoid receptors in rat brain by WIN 55212-2 produces coupling to multiple G protein α-subunits with different potencies. Mol Pharmacol 57:1000–1010 [PubMed] [Google Scholar]
- Price MR, Baillie GL, Thomas A, Stevenson LA, Easson M, Goodwin R, McLean A, McIntosh L, Goodwin G, Walker G, et al. (2005) Allosteric modulation of the cannabinoid CB1 receptor. Mol Pharmacol 68:1484–1495 [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Sklar LA. (2006) Modulation of GPCR conformations by ligands, g-proteins, and arrestins, in GPCRs: From Deorphanization to Lead Structure Identification (Ernst Schering Foundation Symposium Proceedings) (Bourne H, Horuk R, Kuhnke J, Michel H. eds) pp 211–228, Springer, Berlin: [PubMed] [Google Scholar]
- Proudfoot AE, Buser R, Borlat F, Alouani S, Soler D, Offord RE, Schröder JM, Power CA, Wells TN.-M, Power CA, Wells TNC. (1999) Amino-terminally modified RANTES analogues demonstrate differential effects on RANTES receptors. J Biol Chem 274:32478–32485 [DOI] [PubMed] [Google Scholar]
- Quiniou C, Sapieha P, Lahaie I, Hou X, Brault S, Beauchamp M, Leduc M, Rihakova L, Joyal JS, Nadeau S, et al. (2008) Development of a novel noncompetitive antagonist of IL-1 receptor. J Immunol 180:6977–6987 [DOI] [PubMed] [Google Scholar]
- Raehal KM, Walker JK, Bohn LM. (2005) Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther 314:1195–1201 [DOI] [PubMed] [Google Scholar]
- Rajagopal K, Lefkowitz RJ, Rockman HA. (2005) When 7 transmembrane recepors are not G protein-coupled receptors. J Clin Invest 115:2971–2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal K, Whalen EJ, Violin JD, Stiber JA, Rosenberg PB, Premont RT, Coffman TM, Rockman HA, Lefkowitz RJ. (2006) β-arrestin-2 mediated inotropic effects of the angiotensin II type 1A receptor in isolated cardiac myocytes. Proc Natl Acad Sci USA 103:16284–16289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang HP. (2006) The receptor concept: pharmacology's big idea. Br J Pharmacol 147:S9–S16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, O'Dowd BF, George SR. (2007) D1–D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci USA 104:654–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, et al. (2007) Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature 450:383–387 [DOI] [PubMed] [Google Scholar]
- Rees S, Morrow D, Kenakin T. (2002) GPCR drug discovery through the exploitation of allosteric drug binding sites. Receptors and Channels 8:261–268 [PubMed] [Google Scholar]
- Reiter E, Lefkowitz RJ. (2006) GRKs and beta-arrestins: roles in receptor silencing, trafficking, and signaling. Trends Endocrinol Metab 17:159–165 [DOI] [PubMed] [Google Scholar]
- Ren XR, Reiter E, Ahn S, Kim J, Chen W, Lefkowitz RJ. (2005) Different G protein-coupled receptor kinases govern G protein and β-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci USA 102:1448–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revankar CM, Vines CM, Cimino DF, Prossnitz ER. (2004) Arrestins block G protein-coupled receptor-mediated apoptosis. J Biol Chem 279:24578–24584 [DOI] [PubMed] [Google Scholar]
- Ribas C, Penela P, Murga C, Salcedo A, García-Hoz C, Jurado-Pueyo M, Aymerich I, Mayor F., Jr (2007) The G protein-coupled receptor kinase (GRK) ineractome: role of GRKs in GPCR regulation and signaling. Biochim Biophys Acta 1768:913–922 [DOI] [PubMed] [Google Scholar]
- Richman JG, Kanemitsu-Parks M, Gaidarov I, Cameron JS, Griffin P, Zheng H, Guerra NC, Cham L, Maciejewski-Lenoir D, Behan DP, et al. (2007) Nicotinic acid receptor agonists differentially activate downstream effectors. J Biol Chem 282:18028–18036 [DOI] [PubMed] [Google Scholar]
- Rives ML, Vol C, Fukazawa Y, Tinel N, Trinquet E, Ayoub MA, Shigemoto R, Pin JP, Prézeau L. (2009) Crosstalk between GABAB and mGlu1a receptors reveals new insight into GPCR signal integration. EMBO J 28:2195–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R, Abelson P, Cowie D, Bolton E, Britten R. (1955) Studies of Biosynthesis in Escherichia coli, p 607, Carnegie Institution of Washington, Washington, DC: [Google Scholar]
- Rocheville M, Lange DC, Kumar U, Patel SC, Patel RC, Patel YC. (2000) Receptors for dopamine and somatostatin: formation of hetero-oligomers with enhanced functional activity. Science 288:154–157 [DOI] [PubMed] [Google Scholar]
- Roerig SC, Loh HH, Law PY. (1992) Identification of three separate guanine nucleotide-binding proteins that interact with the δ-opioid receptor in NG108-15 neuroblastoma x glioma hybrid cells. Mol Pharmacol 41:822–831 [PubMed] [Google Scholar]
- Roettger BF, Ghanekar D, Rao R, Toledo C, Yingling J, Pinon D, Miller LJ. (1997) Antagonist-stimulated internalization of the G protein-coupled cholecystokinin receptor. Mol Pharmacol 51:357–362 [PubMed] [Google Scholar]
- Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. (1998) A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer's disease. Donepezil Study Group. Neurology 50:136–145 [DOI] [PubMed] [Google Scholar]
- Rogez C, Martin M, Dereuddre-Bosquet N, Martal J, Dormont D, Clayette P. (2003) Anti-human immunodeficiency virus activity of tau interferon in human macrophages: involvement of cellular factors and β-chemokines. J Virol 77:12914–12920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnoli R, Baraldi PG, Carrion MD, Cara CL, Cruz-Lopez O, Iaconinoto MA, Preti D, Shryock JC, Moorman AR, Vincenzi F, et al. (2008) Synthesis and biological evaluation of 2-amino-3-(4-chlorobenzoyl)-4-[N-(substituted)piperazin-1-yl]thiophenes as potent allosteric enhancers of the A1 adenosine receptor. J Med Chem 51:5875–5879 [DOI] [PubMed] [Google Scholar]
- Rominger CM, Bee WL, Copeland RA, Davenport EA, Gilmartin A, Gontarek R, Hornberger KR, Kallal LA, Lai Z, Lawrie K, et al. (2009) Evidence for allosteric interactions of antagonist binding to the smoothened receptor. J Pharmacol Exp Ther 329:995–1005 [DOI] [PubMed] [Google Scholar]
- Römpler H, Stäubert C, Thor D, Schulz A, Hofreiter M, Schöneberg T. (2007) G protein-coupled time travel: evolutionary aspects of GPCR research. Mol Interv 7:17–25 [DOI] [PubMed] [Google Scholar]
- Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Yao XJ, Weis WI, Stevens RC, et al. (2007) GPCR engineering yields high-resolution structural insights into beta2-adrenergic receptor function. Science 318:1266–1273 [DOI] [PubMed] [Google Scholar]
- Ross DA, Lee S, Reiser V, Xue J, Alves K, Vaidya S, Kreamer A, Mull R, Hudak E, Hare T, et al. (2008) Mulitplexed assays by high-content imaging for assessment of GPCR activity. J Biomol Screen 13:449–455 [DOI] [PubMed] [Google Scholar]
- Roth BL, Chuang DM. (1987) Multiple mechanisms of serotonergic signal transduction. Life Sci 41:1051–1064 [DOI] [PubMed] [Google Scholar]
- Rousselle JC, Massot O, Delepierre M, Zifa E, Rousseau B, Fillion G. (1996) Isolation and characterization of an endogenous peptide from rat brain interacting specifically with the serotonergic 1B receptor subtypes. J Biol Chem 271:726–735 [DOI] [PubMed] [Google Scholar]
- Rucker J, Edinger AL, Sharron M, Samson M, Lee B, Berson JF, Yi Y, Margulies B, Collman RG, Doranz BJ, et al. (1997) Utilization of chemokine receptors, orphan receptors, and herpesvirus-encoded receptors by diverse human and simian immunodeficiency viruses. J Virol 71:8999–9007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runge S, Thøgersen H, Madsen K, Lau J, Rudolph R. (2008) Crystal structure of the ligand-bound glucagon-like peptide-1 receptor extracellular domain. J Biol Chem 283:11340–11347 [DOI] [PubMed] [Google Scholar]
- Ryman-Rasmussen JP, Griffith A, Oloff S, Vaidehi N, Brown JT, Goddard WA, 3rd, Mailman RB. (2007) Functional selectivity of dopamine D1 receptor agonists in regulating the fate of internalized receptors. Neuropharmacology 52:562–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryman-Rasmussen JP, Nichols DE, Mailman RB. (2005) Differential activation of adenylate cyclase and receptor internalization by novel dopamine D1 receptor agonists. Mol Pharmacol 68:1039–1048 [DOI] [PubMed] [Google Scholar]
- Sachpatzidis A, Benton BK, Manfredi JP, Wang H, Hamilton A, Dohlman HG, Lolis E. (2003) Identification of allosteric peptide agonists of CXCR4. J Biol Chem 278:896–907 [DOI] [PubMed] [Google Scholar]
- Sagan S, Karoyan P, Chassaing G, Lavielle S. (1999) Further delineation of the two binding sites (R*(n)) associated with tachykinin neurokinin-1 receptors using [3-prolinomethionine11]SP analogues. J Biol Chem 274:23770–23776 [DOI] [PubMed] [Google Scholar]
- Sato M, Horinouchi T, Hutchinson DS, Evans BA, Summers RJ. (2007) Ligand-directed signaling at the β-adrenoceptor produced by 3-(2-Ethylphenoxy)-1-[(1,S)-1,2,3,4-tetrahydronapth-1-ylamino]-2_S_-2-propanol oxalate (SR59230A) relative to receptor agonists. Mol Pharmacol 72:1359–1368 [DOI] [PubMed] [Google Scholar]
- Schlador ML, Nathanson NM. (1997) Synergistic regulation of m2 muscarinic acetylcholine receptor desensitization and sequestration by G protein-coupled receptor kinase-2 and beta-arrestin-1. J Biol Chem 272:18882–18890 [DOI] [PubMed] [Google Scholar]
- Scheerer P, Park JH, Hildebrand PW, Kim YJ, Krauss N, Choe HW, Hofmann KP, Ernst OP. (2008) Crystal structure of opsin in its G-protein-interacting conformation. Nature 455:497–502 [DOI] [PubMed] [Google Scholar]
- Schmid CL, Bohn LM. (2009) Physiological and pharmacological implications of beta-arrestin regulation. Pharmacol Ther 121:285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Raehal KM, Bohn LM. (2008) Agonist-directed signaling of the serotonin 2A receptor depends on β-arrestin-2 interactions in vivo. Proc Natl Acad Sci USA 105:1079–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz TW. (1994) Locating ligand-binding sites in 7TM receptors by protein engineering. Curr Opin Biotech 5:434–444 [DOI] [PubMed] [Google Scholar]
- Schwartz TW, Frimurer TM, Holst B, Rosenkilde MM, Elling CE. (2006) Molecular mechanism of 7TM receptor activation—a global toggle switch model. Annu Rev Pharmacol Toxicol 46:481–519 [DOI] [PubMed] [Google Scholar]
- Schwartz TW, Holst B. (2007) Allosteric enhancers, allosteric agonists and ago-allosteric modulators: where do they bind and how do they act? Trends Pharmacol Sci 28:366–373 [DOI] [PubMed] [Google Scholar]
- Seibert C, Ying W, Gavrilov S, Tsamis F, Kuhmann SE, Palani A, Tagat JR, Clader JW, McCombie SW, Baroudy BM, et al. (2006) Interaction of small molecule inhibitors of HIV-1 entry with CCR5. Virology 349:41–54 [DOI] [PubMed] [Google Scholar]
- Seifert R, Gether U, Wenzel-Seifert K, Kobilka BK. (1999) Effects of guanine, inosine, and xanthine nucleotides on β2-adrenergic receptor/Gs interactions: evidence for multiple receptor conformations. Mol Pharmacol 56:348–358 [DOI] [PubMed] [Google Scholar]
- Seibold A, Williams B, Huang ZF, Friedman J, Moore RH, Knoll BJ, Clark RB. (2000) Localization of the sites mediating desensitization of the β2-adrenergic receptor by the GRK pathway. Mol Pharmacol 58:1162–1173 [DOI] [PubMed] [Google Scholar]
- Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR, Roth BL, Mailman R. (2003) Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology 28:1400–1411 [DOI] [PubMed] [Google Scholar]
- Sheng M, Sala C. (2001) PDZ domains and the organization of supramolecular complexes. Annu Rev Neurosci 24:1–29 [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. (2005) Seven-transmembrane receptor signaling through beta-arrestin. Sci STKE (308):cm10 [DOI] [PubMed] [Google Scholar]
- Shiau AK, Massari ME, Ozbal CC. (2008) Back to basics: label-free technologies for small molecule screening. Comb Chem High Throughput Screen 11:231–237 [DOI] [PubMed] [Google Scholar]
- Shieh B, Yan YP, Ko NY, Liau YE, Liu YC, Lin HH, Chen PP, Li C. (2001) Detection of elevated serum beta-chemokine levels in seronegative Chinese individuals exposed to human immunodeficiency virus type 1. Clin Infect Dis 33:273–279 [DOI] [PubMed] [Google Scholar]
- Shizukuda Y, Buttrick PM. (2002) Subtype specific roles of β-adrenergic receptors in apoptosis of adult rat ventricular myocytes. J Mol Cell Cardiol 34:823–831 [DOI] [PubMed] [Google Scholar]
- Shoemaker JL, Ruckle MB, Mayeux PR, Prather PL. (2005) Agonist-directed trafficking of response by endocannabinoids acting at CB2 receptors. J Pharmacol Exp Ther 315:828–838 [DOI] [PubMed] [Google Scholar]
- Shukla AK, Violin JD, Whalen EJ, Gesty-Palmer D, Shenoy SK, Lefkowitz RJ. (2008) Distinct conformational changes in β-arrestin report biased agonism at seven transmembrane receptors. Proc Nat Acad Sci USA 105:9988–9993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NJ, Luttrell LM. (2006) Signal switching, crosstalk, and arrestin scaffolds: novel G protein-coupled receptor signaling in cardiovascular disease. Hypertension 48:173–179 [DOI] [PubMed] [Google Scholar]
- Sohy D, Parmentier M, Springael JY. (2007) Allosteric transinhibition by specific antagonists in CCR2/CXCR4 heterodimers. J Biol Chem 282:30062–30069 [DOI] [PubMed] [Google Scholar]
- Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, Journot L. (1993) Differential signal transduction by five splice variants of the PACAP receptor. Nature 365:170–175 [DOI] [PubMed] [Google Scholar]
- Spijker P, Vaidehi N, Freddolino PL, Hilbers PA, Goddard WA., 3rd (2006) Dynamic behavior of fully solvated beta2-adrenergic receptor, embedded in the membrane with bound agonist or antagonist. Proc Natl Acad Sci USA 103:4882–4887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standfuss J, Xie G, Edwards PC, Burghammer M, Oprian DD, Schertler GF. (2007) Crystal structure of a thermally stable rhodopsin mutant. J Mol Biol 372:1179–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella L, Caccuri AM, Rosato N, Nicotra M, Lo Bello M, De Matteis F, Mazzetti AP, Federici G, Ricci G. (1998) Flexibility of helix 2 in the human glutathione transferase P1–1. Time-resolved fluorescence spectroscopy. J Biol Chem 273:23267–23273 [DOI] [PubMed] [Google Scholar]
- Stephenson RP. (1956) A modification of receptor theory. Br J Pharmacol Chemother 11:379–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockton JM, Birdsall NJ, Burgen AS, Hulme EC. (1983) Modification of the binding properties of muscarinic receptors by gallamine. Pharmacol 23:551–557 [PubMed] [Google Scholar]
- Sun C, Song D, Davis-Taber RA, Barrett LW, Scott VE, Richardson PL, Pereda-Lopez A, Uchic ME, Solomon LR, Lake MR, et al. (2007a) Solution structure and mutational analysis of pituitary adenylate cyclase-activating polypeptide binding to the extracellular domain of PAC1-RS. Proc Natl Acad Sci USA 104:7875–7880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, McGarrigle D, Huang XY. (2007b) When a G protein-coupled receptor does not couple to a G protein. Mol BioSyst 3:849–854 [DOI] [PubMed] [Google Scholar]
- Swaminath G, Deupi X, Lee TW, Zhu W, Thian FS, Kobilka TS, Kobilka B. (2005) Probing the beta2 adrenoceptor binding site with catechol reveals differences in binding and activation by agonists and partial agonists. J Biol Chem 280:22165–22171 [DOI] [PubMed] [Google Scholar]
- Swaminath G, Xiang Y, Lee TW, Steenhuis J, Parnot C, Kobilka BK. (2004) Sequential binding of agonists to the beta2 adrenoceptor. Kinetic evidence for intermediate conformational states. J Biol Chem 279:686–691 [DOI] [PubMed] [Google Scholar]
- Takasu H, Gardella TJ, Luck MD, Potts JT, Jr, Bringhurst FR. (1999) Amino-terminal modifications of human parathyroid hormone (PTH) selectively alter phospholipase C signaling via type 1 PTH receptor: implications for design of signal-specific PTH ligands. Biochemistry 38:13453–13460 [DOI] [PubMed] [Google Scholar]
- Tams JW, Knudsen SM, Fahrenkrug J. (1998) Proposed arrangement of the seven transmembrane helices in the secretin receptor family. Recept Channels 5:79–90 [PubMed] [Google Scholar]
- Tams JW, Knudsen SM, Fahrenkrug J. (2001) Characterization of a G protein coupling “YL” motif of the human VPAC1 receptor, equivalent to the first two amino acids in the “DRY” motif of the rhodopsin family. J Mol Neurosci 17:325–330 [DOI] [PubMed] [Google Scholar]
- Tan YV, Couvineau A, Murail S, Ceraudo E, Neumann JM, Lacapère JJ, Laburthe M. (2006) Peptide agonist docking in the N-terminal ectodomain of a class II G protein-coupled receptor, the VPAC1 receptor. Photoaffinity, NMR, and molecular modeling. J Biol Chem 281:12792–12798 [DOI] [PubMed] [Google Scholar]
- Tateyama M, Kubo Y. (2006) Dual signaling is differentially activated by different active states of the metabotropic glutamate receptor 1α. Proc Natl Acad Sci USA 103:1124–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrillon S, Bouvier M. (2004) Receptor activity-independent recruitment of β-arrestin2 reveals specific signalling modes. EMBO J 23:3950–3961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RL, Mistry R, Langmead CJ, Wood MD, Challiss RA. (2008) G protein coupling and signaling pathway activation by m1 muscarinic acetylcholine receptor orthosteric and allosteric agonists. J Pharmacol Exp Ther 327:365–374 [DOI] [PubMed] [Google Scholar]
- Tidgewell K, Groer CE, Harding WW, Lozama A, Schmidt M, Marquam A, Hiemstra J, Partilla JS, Dersch CM, Rothman RB, et al. (2008) Herkinorin analogues with differential β-arrestin-2 interactions. J Med Chem 51:2421–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin AB. (2008) G-protein-coupled receptor phosphorylation: where, when and by whom. Br J Pharmacol 153:S167–S176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin AB, Butcher AJ, Kong KC. (2008) Location, location, location… site-specific GPCR phosphorylation offers a mechanism for cell-type-specific signalling. Trends Pharmacol Sci 29:413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrecilla I, Spragg EJ, Poulin B, McWilliams PJ, Mistry SC, Blaukat A, Tobin AB. (2007) Phosphorylation and regulation of a G protein-coupled receptor by protein kinase CK2. J Cell Biol 177:127–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran JA, Chang A, Matsui M, Ehlert FJ. (2009) Estimation of relative microscopic affinity constants of agonists for the active state of the receptor in functional studies on M2 and M3 muscarinic receptors. Mol Pharmacol 75:381–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tränkle C, Weyand O, Schröter A, Mohr K. (1999) Using a radioalloster to test predictions of the cooperativity model for gallamine binding to the allosteric site of muscarinic acetylcholine M(2) receptors. Mol Pharmacol 56:962–965 [DOI] [PubMed] [Google Scholar]
- Trkola A, Kuhmann SE, Strizki JM, Maxwell E, Ketas T, Morgan T, Pugach P, Xu S, Wojcik L, Tagat J, et al. (2002) HIV-1 escape from a small molecule, CCR5-specific entry inhibitor does not involve CXCR4 use. Proc Natl Acad Sci USA 99:395–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. (2002) Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol 42:165–179 [DOI] [PubMed] [Google Scholar]
- Tsamis F, Gavrilov S, Kajumo F, Seibert C, Kuhmann S, Ketas T, Trkola A, Palani A, Clader JW, Tagat JR, et al. (2003) Analysis of the mechanism by which the small molecule CCR5 antagonists SCH-351125 and SCH-350581 inhibit human immunodeficiency virus type 1 entry. J Virol 77:5201–5208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutor AS, Penela P, Mayor F., Jr (2007) Anti-β1-adrenergic receptor autoantibodies are potent stimulators of the ERK1/2 pathway in cardiac cells. Cardiovasc Res 76:51–60 [DOI] [PubMed] [Google Scholar]
- Udawela M, Christopoulos G, Tilakaratne N, Christopoulos A, Albiston A, Sexton PM. (2006) Distinct receptor activity-modifying protein domains differentially modulate interaction with calcitonin receptors. Mol Pharmacol 69:1984–1989 [DOI] [PubMed] [Google Scholar]
- Ullum H, Cozzi Lepri A, Victor J, Aladdin H, Phillips AN, Gerstoft J, Skinhøj P, Pedersen BK. (1998) Production of β-chemokines in human immunodeficiency virus (HIV) infection: evidence that high levels of macrophage inflammatory protein 1β are associated with a decreased risk of HIV disease progression. J Infect Dis 177:331–336 [DOI] [PubMed] [Google Scholar]
- Ulrich CD, 2nd, Holtmann M, Miller LJ. (1998) Secretin and vasoactive intestinal peptide receptors: members of a unique family of G protein-coupled receptors. Gastroenterology 114:382–397 [DOI] [PubMed] [Google Scholar]
- Umbarger HE. (1956) Evidence for a negative feedback mechanism in the biosynthesis of isoleucine. Science 123:848 [DOI] [PubMed] [Google Scholar]
- Urban JD, Vargas GA, von Zastrow M, Mailman RB. (2007) Aripiprazole has functionally selective action at dopamine D2 receptor-mediated signaling pathways. Neuropsychopharmacology 32:67–77 [DOI] [PubMed] [Google Scholar]
- Valant C, Gregory KJ, Hall NE, Scammells PJ, Lew MJ, Sexton PM, Christopoulos A. (2008) A novel mechanism of G protein-coupled receptor functional selectivity. Muscarinic partial agonist McN-A-343 as a bitopic orthosteric/allosteric ligand. J Biol Chem 283:29312–29321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valant C, Sexton PM, Christopoulos A. (2009) Orthosteric/allosteric bitopic ligands: going hybrid at GPCRs. Mol Interv 9:125–135 [DOI] [PubMed] [Google Scholar]
- van Biesen T, Luttrell LM, Hawes BE, Lefkowitz RJ. (1996) Mitogenic signaling via G protein-coupled receptors. Endocr Rev 17:698–714 [DOI] [PubMed] [Google Scholar]
- van der Lee MM, Blomenröhr M, van der Doelen AA, Wat JW, Smits N, Hanson BJ, van Koppen CJ, Zaman GJ. (2009) Pharmacological characterization of receptor redistribution and β-arrestin recruitment assays for can cannabinoid receptor 1. J Biomol Screen 14:811–823 [DOI] [PubMed] [Google Scholar]
- Van Rampelbergh J, Gourlet P, De Neef P, Robberecht P, Waelbroeck M. (1996) Properties of the pituitary adenylate cyclase-activating polypeptide I and II receptors, vasoactive intestinal peptide1, and chimeric amino-terminal pituitary adenylate cyclase-activating polypeptide/vasoactive intestinal peptide1 receptors: evidence for multiple receptor states. Mol Pharmacol 50:1596–1604 [PubMed] [Google Scholar]
- Varga EV, Navratilova E, Stropova D, Jambrosic J, Roeske WR, Yamamura HI. (2004) Agonist-specific regulation of the δ-opioid receptor. Life Sci 76:599–612 [DOI] [PubMed] [Google Scholar]
- Vassart G, Dumont JE. (1992) The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev 13:596–611 [DOI] [PubMed] [Google Scholar]
- Verdonk E, Johnson K, McGuinness R, Leung G, Chen YW, Tang HR, Michelotti JM, Liu VF. (2006) Cellular dielectric spectroscopy: a label-free comprehensive platform for functional evaluation of endogenous receptors. Assay Drug Dev Technol 4:609–619 [DOI] [PubMed] [Google Scholar]
- Verkaar F, van Rosmalen JW, Blomenröhr M, van Koppen CJ, Blankesteijn WM, Smits JF, Zaman GJ. (2008) G protein-independent cell-based assays for drug discovery on seven-transmembrane receptors. Biotechnol Annu Rev 14:253–274 [DOI] [PubMed] [Google Scholar]
- Vilardaga JP, Bünemann M, Krasel C, Castro M, Lohse MJ. (2003) Measurement of the millisecond activation switch of G protein-coupled receptors in living cells. Nat Biotechnol 21:807–812 [DOI] [PubMed] [Google Scholar]
- Vilardaga JP, Steinmeyer R, Harms GS, Lohse MJ. (2005) Molecular basis of inverse agonism in a G-protein coupled receptor. Nat Chem Biol 1:25–28 [DOI] [PubMed] [Google Scholar]
- Violin JD, Dewire SM, Barnes WG, Lefkowitz RJ. (2006) G protein-coupled receptor kinase and beta-arrestin-mediated desensitization of the angiotensin II type 1A receptor elucidated by diacylglycerol dynamics. J Biol Chem 281:36411–36419 [DOI] [PubMed] [Google Scholar]
- Violin JD, Lefkowitz RJ. (2007) β-Arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci 28:416–422 [DOI] [PubMed] [Google Scholar]
- Volkman BF, Lipson D, Wemmer DE, Kern D. (2001) Two-state allosteric behavior in a single-domain signaling protein. Science 291:2429–2433 [DOI] [PubMed] [Google Scholar]
- Vroon A, Lombardi MS, Kavelaars A, Heijnen CJ. (2003) Changes in the G-protein-coupled receptor desensitization machinery during relapsing-progressive experimental allergic encephalomyelitis. J Neuroimmunol 137:79–86 [DOI] [PubMed] [Google Scholar]
- Vroon A, Heijnen CJ, Kavelaars A. (2006) GRKs and arrestins: regulators of migration and inflammation. J Leukocyt Biol 80:1214–1221 [DOI] [PubMed] [Google Scholar]
- Vroon A, Kavelaars A, Limmroth V, Lombardi MS, Goebel MU, Van Dam AM, Caron MG, Schedlowski M, Heijnen CJ. (2005) G protein-coupled receptor kinase 2 in multiple sclerosis and experimental autoimmune encephalomyelitis. J Immunol 174:4400–4406 [DOI] [PubMed] [Google Scholar]
- Waldhoer M, Fong J, Jones RM, Lunzer MM, Sharma SK, Kostenis E, Portoghese PS, Whistler JL. (2005) A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. Proc Natl Acad Sci USA 102:9050–9055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters RW, Shukla AK, Kovacs JJ, Violin JD, DeWire SM, Lam CM, Chen JR, Muehlbauer MJ, Whalen EJ, Lefkowitz RJ. (2009) β-Arrestin 1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice. J Clin Invest 119:1312–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhao J, Brady AE, Feng J, Allen PB, Lefkowitz RJ, Greengard P, Limbird LE. (2004) Spinophilin blocks arrestin actions in vitro and in vivo at G protein-coupled receptors. Science 304:1940–1944 [DOI] [PubMed] [Google Scholar]
- Ward JS, Merritt L, Calligaro DO, Bymaster FP, Shannon HE, Sawyer BD, Mitch CH, Deeter JB, Peters SC, Sheardown MJ. (1995) Functionally selective M1 muscarinic agonists. 3. Side chains and azacycles contributing to functional muscarinic selectivity among pyrazinylazacycles. J Med Chem 38:3469–3481 [DOI] [PubMed] [Google Scholar]
- Warne T, Serrano-Vega MJ, Baker JG, Moukhametzianov R, Edwards PC, Henderson R, Leslie AG, Tate CG, Schertler GF. (2008) Structure of a beta1-adrenergic G-protein-coupled receptor. Nature 454:486–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C, Chen G, Irving P, Way J, Chen WJ, Kenakin T. (2000) The use of stimulus-biased assay systems to detect agonist-specific receptor active states: implications for the trafficking of receptor stimulus by agonists. Mol Pharmacol 58:1230–1238 [DOI] [PubMed] [Google Scholar]
- Watson C, Jenkinson S, Kazmierski W, Kenakin T. (2005) The CCR5 receptor-based mechanism of action of 873140, a potent allosteric noncompetitive HIV entry inhibitor. Mol Pharmacol 67:1268–1282 [DOI] [PubMed] [Google Scholar]
- Weber KT. (2001) Aldosterone in congestive heart failure. N Engl J Med 345:1689–1697 [DOI] [PubMed] [Google Scholar]
- Weber TJ, Markillie LM. (2003) Regulation of activator protein-1 by 8-iso-prostaglandin E2 in a thromboxane A2 receptor-dependent and -independent manner. Mol Pharmacol 63:1075–1081 [DOI] [PubMed] [Google Scholar]
- Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM, Lefkowitz RJ. (2003) Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci USA 100:10782–10787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells TN, Power CA, Shaw JP, Proudfoot AE. (2006) Chemokine blockers—therapeutics in the making? Trends Pharmacol Sci 27:41–47 [DOI] [PubMed] [Google Scholar]
- Wenzel-Seifert K, Seifert R. (2000) Molecular analysis of β2-adrenoceptor coupling to Gs-, Gi-, and Gq-proteins. Mol Pharmacol 58:954–966 [DOI] [PubMed] [Google Scholar]
- Werry TD, Gregory KJ, Sexton PM, Christopoulos A. (2005) Characterization of serotonin 5-HT2C receptor signaling to extracellular signal-regulated kinases 1 and 2. J Neurochem 93:1603–1615 [DOI] [PubMed] [Google Scholar]
- Whistler JL, Chuang HH, Chu P, Jan LY, von Zastrow M. (1999) Functional dissociation of mu opioid receptor signaling and endocytosis: implications for the biology of opiate tolerance and addiction. Neuron 23:737–746 [DOI] [PubMed] [Google Scholar]
- Whistler JL, von Zastrow M. (1999) Dissociation of functional roles of dynamin in receptor-mediated endocytosis and mitogenic signal transduction. J Biol Chem 274:24575–24578 [DOI] [PubMed] [Google Scholar]
- White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH, Barnes AA, Emson P, Foord SM, Marshall FH. (1998) Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature 396:679–682 [DOI] [PubMed] [Google Scholar]
- Willins DL, Alsayegh L, Berry SA, Backstrom JR, Sanders-Bush E, Friedman L, Khan N, Roth BL. (1998) Serotonergic antagonist effects on trafficking of serotonin 5-HT2A receptors in vitro and in vivo. Ann NY Acad Sci 861:121–127 [DOI] [PubMed] [Google Scholar]
- Willins DL, Berry SA, Alsayegh L, Backstrom JR, Sanders-Bush E, Friedman L, Roth BL. (1999) Clozapine and other 5-hydroxytryptamine-2A receptor antagonists alter the subcellular distribution of 5-hydroxytryptamine-2A receptors in vitro and in vivo. Neuroscience 91:599–606 [DOI] [PubMed] [Google Scholar]
- Wise A, Sheehan M, Rees S, Lee M, Milligan G. (1999) Comparative analysis of the efficacy of A1 adenosine receptor activation of Gi/0α G proteins following coexpression of receptor and G protein and expression of A1 adenosine receptor Gi/oα fusion proteins. Biochemistry 38:2272–2278 [DOI] [PubMed] [Google Scholar]
- Wisler JW, DeWire SM, Whalen EJ, Violin JD, Drake MT, Ahn S, Shenoy SK, Lefkowitz RJ. (2007) A unique mechanism of β-blocker action: carvedilol stimulates β-arrestin signaling. Proc Natl Acad Sci USA 104:16657–16662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo AY, Wang TB, Zeng X, Zhu W, Abernethy DR, Wainer IW, Xiao RP. (2009) Stereochemistry of an agonist determines coupling preference of beta2-adrenoceptor to different G proteins in cardiomyocytes. Mol Pharmacol 75:158–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward C. (1993) Is the slow exchange core the protein folding core? Trends Biochem Sci 18:359–360 [DOI] [PubMed] [Google Scholar]
- Wu L, LaRosa G, Kassam N, Gordon CJ, Heath H, Ruffing N, Chen H, Humblias J, Samson M, Parmentier M, et al. (1997) Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J Exp Med 186:1373–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SV, Yang M, Avedian D, Birnbaumer M, Walsh JH. (1999) Single amino acid substitution of serine82 to asparagine in first intracellular loop of human cholecystokinin (CCK)-B receptor confers full cyclic AMP responses to CCK and gastrin. Mol Pharmacol 55:795–803 [PubMed] [Google Scholar]
- Wyatt R, Sodroski J. (1998) The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280:1884–1888 [DOI] [PubMed] [Google Scholar]
- Xiang J, George SL, Wünschmann S, Chang Q, Klinzman D, Stapleton JT. (2004) Inhibition of HIV-1 replication by GB virus C infection through increases in RANTES, MIP-lα, MIP-1β, and SDF-1. Lancet 363:2040–2046 [DOI] [PubMed] [Google Scholar]
- Xiao K, Shenoy SK, Nobles K, Lefkowitz RJ. (2004) Activation-dependent conformational changes in β-arrestin 2. J Biol Chem 279:55744–55753 [DOI] [PubMed] [Google Scholar]
- Xiao RP, Zhang SJ, Chakir K, Avdonin P, Zhu W, Bond RA, Balke CW, Lakatta EG, Cheng H. (2003) Enhanced Gi signaling selectively negates β2-adrenergic receptor (AR)–but not β1-AR-mediated positive inotropic effect in myocytes from failing rat hearts. Circulation 108:1633–1639 [DOI] [PubMed] [Google Scholar]
- Xu H, Partilla JS, Wang X, Rutherford JM, Tidgewell K, Prisinzano TE, Bohn LM, Rothman RB. (2007) A comparison of noninternalizing (herkinorin) and internalizing (DAMGO) μ-opioid agonists on cellular markers related to opioid tolerance and dependence. Synapse 61:166–175 [DOI] [PubMed] [Google Scholar]
- Xu H, Wang X, Partilla JS, Bishop-Mathis K, Benaderet TS, Dersch CM, Simpson DS, Prisinzano TE, Rothman RB. (2008) Differential effects of opioid agonists on G-protein expression in CHO cells expressing cloned human opioid receptors. Brain Res Bull 77:49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu N, Atienza JM, Bernard J, Blanc S, Zhu J, Wang X, Xu X, Abassi YA. (2006) Real-time monitoring of morphological changes in living cells by electronic cell sensor assays: an approach to study G protein-coupled receptors. Anal Chem 78:35–43 [DOI] [PubMed] [Google Scholar]
- Zamah AM, Delahunty M, Luttrell LM, Lefkowitz RJ. (2002) Protein kinase A-mediated phosphorylation of the beta 2-adrenergic receptor regulates its coupling to Gs and Gi. Demonstration in a reconstituted system.. J Biol Chem 277:31249–31256 [DOI] [PubMed] [Google Scholar]
- Zaworski PG, Alberts GL, Pregenzer JF, Im WB, Slightom JL, Gill GS. (1999) Efficient functional coupling of the human D3 dopamine receptor to G(o) subtype of G proteins in SH-SY5Y cells. Br J Pharmacol 128:1181–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai P, Yamamoto M, Galeotti J, Liu J, Masurekar M, Thaisz J, Irie K, Holle E, Yu X, Kupershmidt S, Roden DM, Wagner T, Yatani A, Vatner DE, Vatner SF, Sadoshima J., et al. (2005) Cardiac-specific overexpression of AT1 receptor mutant lacking G alpha q/G alpha i coupling causes hypertrophy and bradycardia in transgenic mice. J Clin Invest 115:3045–3056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, Caron MG. (1998) Role for G protein-coupled receptor kinase in agonist-specific regulation of μ-opioid receptor responsiveness. Proc Natl Acad Sci USA 95:7157–7162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Rodriguez AL, Conn PJ. (2005) Allosteric potentiators of metabotropic glutamate receptor subtype 5 have differential effects on different signaling pathways in cortical astrocytes. J Pharmacol Exp Ther 315:1212–1219 [DOI] [PubMed] [Google Scholar]
- Zhao X, Jones A, Olson KR, Peng K, Wehrman T, Park A, Mallari R, Nebalasca D, Young SW, Xiao SH. (2008) A homogeneous enzyme fragment complementation-based β-arrestin translocation assay for high-throughput screening of G-protein-coupled receptors. J Biomol Screen 13:737–747 [DOI] [PubMed] [Google Scholar]
- Zheng H, Loh HH, Law PY. (2008) β-Arrestin-dependent μ-opioid receptor activated receptor-activated extracellular signal-regulated kinases (ERKs) translocate to nucleus in contrast to G protein-dependent ERK activation. Mol Pharmacol 73:178–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidar DA, Violin JD, Whalen EJ, Lefkowitz RJ. (2009) Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc Natl Acad Sci USA 106:9649–9654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zürn A, Zabel U, Vilardaga JP, Schindelin H, Lohse MJ, Hoffmann C., Schindlein H, Lohse MJ, Hoffmann C. (2009) Fluorescence resonance energy transfer analysis of α2a-adrenergic receptor activation reveals distinct agonist-specific conformational changes. Mol Pharmacol 75:534–541 [DOI] [PubMed] [Google Scholar]