Early events governing memory CD8+ T-cell differentiation (original) (raw)
Abstract
Understanding the regulation of the CD8+ T-cell response and how protective memory cells are generated has been intensely studied. It is now appreciated that a naive CD8+ T cell requires at least three signals to mount an effective immune response: (i) TCR triggering, (ii) co-stimulation and (iii) inflammatory cytokines. Only recently have we begun to understand the molecular integration of those signals and how early events regulate the fate decisions of the responding CD8+ T cells. This review will discuss the recent findings about both the extracellular and intracellular factors that regulate the destiny of responding CD8+ T cells.
Keywords: CD8 T cell, CTL, differentiation, memory
Introduction
The immune system exists in a delicate state that enables the host to swiftly and effectively battle pathogenic insults, yet remain tolerant of self-antigens. To this end, T-cell activation is tightly regulated and was originally proposed to be mediated by two signals: (i) TCR triggering by antigen and (ii) a co-stimulatory signal from the accessory cell presenting antigen (1–4). Steinman and Witmer (5) later demonstrated that the accessory cell responsible for T-cell activation was primarily a dendritic cell.
The theory of T-cell activation was further extended when Janeway (6) and Matzinger (7) proposed that microbial products or cellular danger signals, respectively, may regulate the co-stimulatory signals delivered by the antigen-presenting cell (APC), thus regulating the outcome of the T-cell response (tolerance versus activation). Furthermore, a third signal mediated by cytokines, which can also be regulated by host-extrinsic and -intrinsic signals, is crucial in regulating T-cell activation versus tolerance (8, 9). How these three signals regulate effector and memory T-cell differentiation continues to be the subject of considerable research efforts.
Our knowledge of effector and memory CD8+ T-cell differentiation pathways has grown significantly due in large part to technical advances, such as multiparameter flow cytometry, knockout and transgenic mice, gene arrays, peptide–MHC (p–MHC) tetrameric reagents and the application of laser-based confocal and two-photon microscopy. All together, these techniques have enabled us to isolate, quantify, characterize and localize antigen-specific T cells throughout the entire immune response. From these studies, CD8+ T-cell responses can be grossly divided into four phases: (i) activation/initiation, (ii) clonal expansion, (iii) contraction and (iv) memory. This dynamic process enables the host to maintain a broad repertoire of antigen-specific T cells under homeostatic conditions while specifically selecting useful clones during pathogenic insult to fight off infection and subsequently maintaining those clones at higher frequencies compared with non-selected clones to protect the host against future encounters with the same pathogen.
We hypothesize that the activation/initiation and early expansion stages are crucial in determining the fate of the responding CD8+ T cells. From our studies using confocal microscopy and in situ tetramer analysis (10, 11), plus the implementation of two-photon microscopy by others (12–16), it is clear that activated CD8+ T cells are in intimate contact with APCs for extended periods of time. The activation and early expansion phases therefore create an opportunity for responding CD8+ T cells to integrate the signals necessary for mounting an effective immune response.
This review will discuss the heterogeneity observed during the CD8+ T-cell response and the roles antigenic stimulation, co-stimulation and inflammatory cytokines play in regulating the activation, expansion and differentiation of effector and memory CD8+ T cells; furthermore, we will highlight recent work that sheds light on the intracellular signaling networks that are critical for the integration of these signals.
Phenotypic heterogeneity exists throughout the CD8+ T-cell response
The origins of a memory CD8+ T cell have been intensely studied because knowledge about their origins could significantly enhance our ability to develop rational vaccines. Studies aiming to identify the origins of the memory CD8+ T-cell population have demonstrated that substantial heterogeneity exists throughout the CD8+ T-cell response.
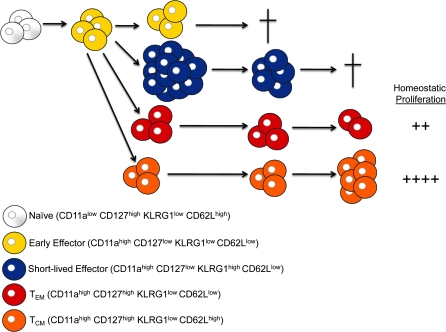
Originally, it was observed that a small proportion of effector CD8+ T cells retained or reexpressed IL-7Rα at the peak of the immune response and that those cells subsequently survived to form the memory population, thus identifying a memory precursor cell for the first time (17, 18). More recently, the phenotype of the memory precursor CD8+ T cell has been refined using the expression patterns of IL-7Rα, killer-cell lectin-like receptor G1 (KLRG1) and CD27 (19–23). Using this panel of markers, memory precursor CD8+ T cells are identified as IL-7Rαhigh KLRG1low CD27high (memory precursor effector cell, MPEC), whereas short-lived effector CD8+ T cells are IL-7Rαlow KLRG1high CD27low (short-lived effector cell, SLEC) (Fig. 1).
Fig. 1.
Early cell fate determination model of effector and memory CD8+ T-cell differentiation. In this model, naive CD8+ T cells become activated and form an EEC population, which is CD127low KLRG1low CD27high. Next, three populations of effector cells can be identified by the peak of the CD8+ T-cell response—SLECs that are CD127low KLRG1high CD27low CD62Llow, TEM MPECs that are CD127high KLRG1low CD27high CD62Llow and TCM MPECs that are CD127high KLRG1low CD27high CD62Lhigh. Over time, the EEC and SLEC populations are lost through apoptosis, whereas both the MPEC populations remain long term in the host forming the memory CD8+ T-cell population. Additionally, with time, the memory population transitions from being predominately TEM in phenotype to TCM in nature as a result of the increased homeostatic proliferation rate of the TCM population.
We have recently identified a population of activated CD8+ T cells with a phenotype of IL-7Rαlow KLRG1low CD27high that appears to be the first effector CD8+ T-cell population generated and gives rise to all other subsets [(23) and our unpublished observation] (Fig. 1). Additionally, all three of the previously mentioned effector CD8+ T-cell populations have been shown to express effector molecules, such as IFNγ or granzyme B (19, 20, 24), which fits with Cre-recombinase-mediated genetic labeling experiments (25).
Furthermore, the memory T-cell population contains additional heterogeneity, which is defined by CD62 ligand (CD62L) and CCR7. Originally observed by Hamann et al. (26) and Sallusto et al. (27), two broad phenotypes of memory T cells have been described, CD62Lhigh CCR7high central memory T cells (TCM) and CD62Llow CCR7low effector memory T cells (TEM). Later, it was shown that TCM cells preferentially localized to lymph nodes, whereas TEM cells preferentially were found in peripheral tissues, such as the lungs, liver and intestines (28, 29). Interestingly, our laboratory has recently found that the IL-7Rαhigh KLRG1low CD27high memory precursor population can be further split into CD62Lhigh and CD62Llow populations, whereas early effector cell (EEC) and SLEC are largely CD62Llow, suggesting that this dichotomy in memory subsets is set early during CD8+ T-cell differentiation (30).
Elegant work from Busch and colleagues demonstrated that a single naive CD8+ T cell is capable of generating the same diversity and heterogeneity within both the effector and the memory cell populations as a polyclonal naive population (31). Naive CD8+ T cells are therefore extremely pliable and must integrate numerous signals that determine their fate. Detailed knowledge about what drives memory subset differentiation will be crucial in the design of novel adjuvants and vaccines.
Extracellular signals regulating effector and memory CD8+ T-cell heterogeneity
To account for the heterogeneity within the effector CD8+ T-cell population, we recently proposed an early fate determination model (32). In this model, CD8+ T-cell activation results in the formation of an EEC population that is IL-7Rαhigh KLRG1low CD27high granzyme Bhigh. This early effector CD8+ T-cell population then differentiates into SLEC (IL-7Rαlow KLRG1high CD27low), TCM precursors (IL-7Rαhigh KLRG1low CD27high CD62Lhigh) and TEM precursors (IL-7Rαhigh KLRG1low CD27high CD62Llow). We envision that signals originating during T-cell activation and the early expansion stage will be crucial in directing the differentiation of these three effector CD8+ T-cell populations. As proposed in the three-signal model of T-cell activation (33), the expansion and differentiation of effector and memory CD8+ T cells could be directed by TCR-mediated signals, co-stimulation and/or inflammatory cytokines.
Initiation of T-cell responses is a highly orchestrated process whereby antigen is enriched in the T-cell zones of secondary lymphoid organs by migration of dendritic cells from the periphery to these zones (34). Since the probability of any given APC bearing a particular antigen is rare, this process of enrichment serves to enhance the probability of equally rare naive T cells that are specific for that antigen being able to interact with that APC; moreover, activated dendritic cells that are presenting antigens from pathogens are ‘licensed’ to produce chemokines that aid in the recruitment of naive CD8+ T cells to antigen-presenting dendritic cells (35, 36).
T-cell activation is fundamentally dependent on the interaction of the TCR with cognate antigen presented by the appropriate MHC molecule. Previous reports have demonstrated that the overall strength of TCR stimulation, which is the sum of the magnitude and duration of TCR–p–MHC interaction, can direct the magnitude of the T-cell response (37–40), but this does not seem to drastically alter effector CD8+ differentiation (19). Co-stimulatory molecules from both the CD28 family and the tumor necrosis factor receptor family have been shown to be important in enhancing proliferation and survival of activated CD8+ T cells (41, 42), but little is known about the role of co-stimulatory molecules in regulating effector and memory CD8+ T-cell differentiation. Interestingly, both the CD28 and CD27 pathways appear to regulate IL-2 production (43, 44), which we and others have recently demonstrated to be important in regulating the differentiation of SLECs (23, 45, 46).
CD4+ T cells are important for the up-regulation of CD25 (IL-2Rα) on responding CD8+ T cells (23). CD4+ T-cell ‘help’ to responding CD8+ T cells has been a confounding area of research: Certain CD8+ T-cell responses are dependent on CD4+ T-cell help for expansion (23, 47, 48); in other responses, expansion and memory development are normal in the absence of CD4+ T-cell help (23, 48); and in still other responses where CD4+ T cells are absent, expansion is normal, but memory generation is abnormal (49–54). Intriguingly, the route of infection with a given pathogen can alter the CD4+ T-cell dependence of the response. For example, intra-nasal infection with vaccinia virus is CD4+ T cell independent for CD8+ T-cell expansion (55), but the CD8+ T-cell response to intra-peritoneal infection is CD4+ T cell dependent (23). Likely, the inflammatory milieu induced by infection through these different routes as well as the type of APC that is involved may differ. To this point, both IL-12 and IFNα/β are differentially expressed during different infections and both enhance CD8+ T-cell proliferation and/or survival (9, 56, 57); furthermore, inflammation induced by unmethylated CpG DNA or infection induces SLEC differentiation in an IL-12-dependent mechanism (19, 58, 59). In contrast, priming environments lacking high levels of inflammation tend to favor MPEC development (19, 59–61). The factors required for SLEC and MPEC survival also differ. SLEC survival is promoted by IL-15 (19, 21), which counteracts the apoptotic actions of transforming growth factor β on the SLEC population (22). MPEC survival is enhanced by complexes comprising anti-IL-7 plus IL-7 (21) and their long-term maintenance and proliferation are dependent on IL-15 (19, 21). The SLEC/MPEC populations are thus dynamically regulated by multiple factors, which we are only beginning to understand.
In terms of regulating the fate decision of memory precursors into either TCM or TEM populations, our current data indicate that limiting antigen availability early during infection enhances the proportion of memory precursor cells that are CD62Lhigh (30), which fits with previous observations that naive CD8+ T-cell precursor frequency regulates TCM/TEM differentiation (62–64); however, we postulate that dampening TCR signaling will alter the pattern of co-stimulatory and cytokine signals the responding CD8+ T cells can receive downstream. Indeed, we have observed that limiting antigen availability reduces the expression of both CD25 and programmed death-1 (PD-1) on the responding CD8+ T cells (30). Interestingly, both in vitro and in vivo IL-2 signaling is associated with TEM development, whereas IL-15 signaling enhances TCM differentiation (30, 46, 65–67).
Additionally, IL-21 stimulation of in vitro activated CD8+ T cells leads to a high frequency of TCM-like cells (68), which could be due to the ability of IL-21 to limit CD25 expression (69). The absence of CD4+ T-cell help is also associated with an increased TCM population (51). We hypothesize that the ‘helpless’ phenotype is also the result of altered IL-2 signaling because CD4+ T cells can serve as a major source of IL-2 for responding CD8+ T cells (70) and CD4+ T cells can regulate CD25 expression levels on responding CD8+ T cells (23). Thus, we propose that the IL-2/IL-15 signaling pathways are crucial in the fate determination of TCM and TEM early in the CD8+ T-cell response (Fig. 2).
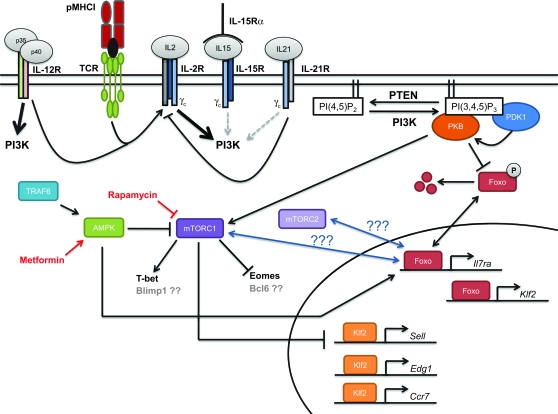
Fig. 2.
Model of the intracellular signaling network regulating T-cell trafficking and differentiation. Antigen-specific CD8+ T cells are activated following engagement of the TCR with cognate peptide antigen presented in the context of MHC-I (p–MHCI). TCR triggering together with inflammatory cytokines of the γc and IL-12 families can regulate T-cell trafficking and differentiation largely through the PI3K pathway. IL-2 signaling appears to be very central in this process as both TCR engagement and IL-12-mediated signaling enhance CD25 expression, whereas IL-21-mediated signaling inhibits CD25 expression. In terms of PI3K activation, both IL-2 and IL-12 strongly activate PI3K, whereas IL-15 and IL-21 weakly activate the PI3K pathway. Strong activation of PI3K results in conversion of PI(4,5)P2 into PI(3,4,5)P3. Accumulation of PI(3,4,5)P3 results in the recruitment of both PKB/Akt and PDK1 to the cell membrane. PDK1 can then phosphorylate PKB/Akt, resulting in its activation. PKB can then phosphorylate Foxo proteins, which results in their exclusion from the nucleus and degradation, which ultimately results in cells being unable to express Foxo target genes, such as Il7ra and Klf2. Inhibition of Foxo-dependent expression of Klf2 will also alter expression of Klf2 target genes, such as Sell (L-selectin/CD62L), Edg1 (S1P1) and Ccr7. PKB can also activate the mTORC1 complex. Activation of mTOR is known to enhance T-bet expression while repressing Eomes expression, which can regulate the fate of the responding CD8+ T cell, but whether Blimp1 or Bcl6 expression is altered by mTOR remains unresolved; furthermore, mTORC1 activity directly regulates Klf2 function. The mTORC1 complex can also be regulated by AMP-activated kinase (AMPK) activity, which inhibits mTORC1 activity. AMPK is a nutrient-sensing pathway that is activated by TNF receptor associated factor 6. Interestingly, rapamycin and metformin can have adjuvant-like functions by targeting the mTORC1 complex and AMPK, respectively.
Processing the extracellular cues: understanding the molecular networks responsible for generating effector and memory heterogeneity
Recent work has begun to elucidate how signals mediated through the TCR, co-stimulatory molecules and cytokine receptors are integrated by to determine the fate of the responding CD8+ T cells. Current data demonstrate that the phosphatidylinositol 3-kinase (PI3K) and mammalian target of rapamycin (mTOR) signaling networks play central roles in regulating T-cell migration (66, 71) and effector/memory T-cell differentiation (72–74). These pathways appear to be central in integrating signals from TCR engagement and inflammatory cytokines, such as IL-2, IL-12, IL-15 and IL-21 (45, 66, 74) (Fig. 2).
PI3K converts phophoinositide (4,5) bisphosphate [PI(4,5)P2] into phophoinositide (3,4,5) trisphosphate [PI(3,4,5)P3], which can recruit pleckstrin-homology-domain-containing proteins to the intracellular leaflet of the cell membrane. Two of these proteins are phosphoinositide-dependent kinase 1 (PDK1) and protein kinase B (PKB)/Akt. Recruitment of PKB/Akt alone to PI(3,4,5)P3 results in cellular proliferation but does not change migratory properties (75); however, co-recruitment of PDK1 to PI(3,4,5)P3 results in further phosphorylation of PKB/Akt, enhancing PKB/Akt activity (75). This highly phosphorylated PKB/Akt can then phosphorylate forkhead box (Foxo) proteins, as well as activate mTOR. The activation of mTOR will inhibit Kruppel-like factor 2 (Klf2) function, thus altering expression of CD62L, sphingosine-1-phosphate receptor 1 (S1P1), and CCR7 (66), all of which are known targets of Klf2 (76).
Phosphorylation of Foxo proteins will exclude them from the nucleus (77), thus limiting Klf2 and Il7ra transcription (71, 78, 79). Currently, the interplay between mTOR and Foxo1 in regulating Klf2 expression and/or function remains unknown; furthermore, phosphatase and tensin homologue deleted on chromosome 10 (PTEN) is known to oppose PI3K activity by converting PI(3,4,5)P3 back into PI(4,5)P2 (80). Intriguingly, PTEN-null T cells, which accumulate high levels of PI(3,4,5)P3, do not express CD62L or CCR7 and deletion of PDK1 from PTEN-null thymocytes rescues CD62L and CCR7 expression (81).
To identify factors that can regulate the mTOR and PI3K pathways, Cantrell and colleagues have utilized a simple in vitro CD8+ T-cell activation assay. Using this system, they demonstrate that IL-2 strongly activates the mTOR and PI3K pathways, resulting in repression of CD62L, CCR7 and S1P1 expression, whereas IL-15 only weakly activates the mTOR and PI3K pathways and CD62L, CCR7 and S1P1 expression is maintained (66, 82). Others have used a similar system to show that IL-2 represses Il7ra expression in a PI3K- and PKB/Akt-dependent manner (83). Our in vivo studies using CD25−/− CD8+ T cells and IL-15−/− mice support those findings, with CD25−/− CD8+ T cells tending to be more TCM like after infection, whereas CD8+ T cells from IL-15−/− mice were more TEM like (30).
Additionally, a recent report from Ahmed and colleagues similarly found that CD25low effector CD8+ T cells preferentially became TCM cells (46); furthermore, CD25−/− or CD25low CD8+ T cells preferentially form the MPEC population (23, 46). High levels of IL-2 are known to enhance Blimp1 expression, while repressing B-cell CLL/lymphoma 6 (Bcl6) expression (45), fitting with the fact that PR-domain-containing (_Prdm1_−/−) memory CD8+ T cells transition to a CD62Lhigh phenotype more rapidly (84), whereas _Bcl6_−/− CD8+ T cells have a decreased frequency of TCM cells (85); furthermore, CD4+ T-cell help is also known to result in decreased CD62L expression on antigen-specific CD8+ T cells (51), which is likely due to the fact that CD4+ T cells provide help to the responding CD8+ T cells through up-regulation of CD25 and production of IL-2 (23, 70).
As discussed above, unmethylated CpG and IL-12 have been shown to drive SLEC differentiation (19, 20, 58, 59). More recently, IL-12 was shown to enhance mTOR activity through a PI3K- and signal transducer and activator of transcription 4 (STAT4)-dependent pathway, resulting in enhanced expression of T-bet and repression of Eomes expression (74); furthermore, it was recently reported that modulating mTOR activity by the administration of low doses of rapamycin, an inhibitor of mTORC1, resulted in an enlarged memory population (72, 73). Additionally, enhancement of AMP-activated kinase activity through the administration of metformin also enhanced memory CD8+ T-cell formation (73), which has previously been shown to inhibit mTOR activity (86). Dampening mTOR activity by administering rapamycin appears to operate by shifting the Eomes:T-bet ratio toward Eomes dominance (74), which again favors memory differentiation (87). Skewing the Eomes:T-bet ratio toward Eomes would also enhance TCM emergence because _Tbx21_−/− CD8+ T cells have been shown to become CD62Lhigh more rapidly (88), which fits with the observation that rapamycin-treated mice have a shift of their memory CD8+ T-cell population toward TCM phenotype (72). How inhibition of mTORC1 regulates the emergence of memory CD8+ T cells is, however, ill defined. We have thus begun to understand the molecular regulation of effector and memory CD8+ T-cell differentiation, but much still remains unresolved.
Conclusions and future prospects
As discussed here, we have learned an extraordinary amount about effector and memory CD8+ T-cell development over the past few decades, but many questions still remain unsolved. The signals necessary for T-cell activation have been well characterized, but an understanding of the complexity surrounding each of these factors is lacking. For example, why are so many co-stimulators necessary to mount effective CD8 T-cell responses? Also, how do the many inflammatory cytokines produced during a response by multiple cell types cooperate to regulate CD8+ T-cell expansion and differentiation? Another important aspect is the temporal regulation of these events. As such, is heterogeneity already established in the CD127low KLRG1low CD27low early effector ‘stem cell’ population?
Intriguingly, recent molecular studies have demonstrated that nutrient-sensing and metabolic regulation appear to be crucial in regulating T-cell trafficking (78) and memory development (73), which extends early studies that demonstrated changes in the metabolic state of responding T cells (89). Vitamin D signaling has recently been shown to be necessary for optimal TCR signaling (90). Additionally, retinoic acid, a vitamin A-derived product, has been demonstrated to be important in ‘imprinting’ a gut-homing phenotype (91). Therefore, the range of factors that can regulate T-cell expansion, migration and differentiation now extend well beyond the world of cytokines. Thus, although many of the secrets surrounding effector and memory T-cell development have been unlocked, many more remain to be revealed that could be exploited in the development of adjuvants and vaccines.
Funding
Work in the Lefrançois laboratory has been supported by National Institutes of Health grants (AI051583, AI076457, AI041576, AI078289, and P01 AI056172 to L.L. and F32 AI074277 to J.J.O.).
Acknowledgments
The authors thank all the members of the Lefrançois laboratory and Dr Edward Usherwood and Michael Molloy for insightful discussions shaping this paper.
References
- 1.Lafferty KJ, Cunningham AJ. A new analysis of allogeneic interactions. Aust. J. Exp. Biol. Med. Sci. 1975;53:27. doi: 10.1038/icb.1975.3. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham AJ, Lafferty KJ. A simple, conservative explanation of the H-2 restriction of interactions between lymphocytes. Scand. J. Immunol. 1977;6:1. doi: 10.1111/j.1365-3083.1977.tb00317.x. [DOI] [PubMed] [Google Scholar]
- 3.Weiss A, Wiskocil RL, Stobo JD. The role of T3 surface molecules in the activation of human T cells: a two-stimulus requirement for IL 2 production reflects events occurring at a pre-translational level. J. Immunol. 1984;133:123. [PubMed] [Google Scholar]
- 4.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J. Exp. Med. 1987;165:302. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinman RM, Witmer MD. Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc. Natl Acad. Sci. USA. 1978;75:5132. doi: 10.1073/pnas.75.10.5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janeway CA., Jr. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol. Today. 1992;13:11. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 7.Matzinger P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994;12:991. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 8.Curtsinger JM, Schmidt CS, Mondino A, et al. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J. Immunol. 1999;162:3256. [PubMed] [Google Scholar]
- 9.Curtsinger JM, Johnson CM, Mescher MF. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J. Immunol. 2003;171:5165. doi: 10.4049/jimmunol.171.10.5165. [DOI] [PubMed] [Google Scholar]
- 10.Khanna KM, McNamara JT, Lefrancois L. In situ imaging of the endogenous CD8 T cell response to infection. Science. 2007;318:116. doi: 10.1126/science.1146291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khanna KM, Aguila CC, Redman JM, Suarez-Ramirez JE, Lefrancois L, Cauley LS. In situ imaging reveals different responses by naive and memory CD8 T cells to late antigen presentation by lymph node DC after influenza virus infection. Eur. J. Immunol. 2008;38:3304. doi: 10.1002/eji.200838602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- 13.Bousso P, Robey E. Dynamics of CD8(+) T cell priming by dendritic cells in intact lymph nodes. Nat. Immunol. 2003;4:579. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- 14.Mempel TR, Henrickson SE, von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 15.Celli S, Garcia Z, Bousso P. CD4 T cells integrate signals delivered during successive DC encounters in vivo. J. Exp. Med. 2005;202:1271. doi: 10.1084/jem.20051018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henrickson SE, Mempel TR, Mazo IB, et al. T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nat. Immunol. 2008;9:282. doi: 10.1038/ni1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schluns KS, Kieper WC, Jameson SC, Lefrançois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 2000;1:426. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 18.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003;4:1191. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 19.Joshi NS, Cui W, Chandele A, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J. Exp. Med. 2008;205:625. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubinstein MP, Lind NA, Purton JF, et al. IL-7 and IL-15 differentially regulate CD8+ T-cell subsets during contraction of the immune response. Blood. 2008;112:3704. doi: 10.1182/blood-2008-06-160945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanjabi S, Mosaheb MM, Flavell RA. Opposing effects of TGF-beta and IL-15 cytokines control the number of short-lived effector CD8+ T cells. Immunity. 2009;31:131. doi: 10.1016/j.immuni.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obar JJ, Molloy MJ, Jellison ER, et al. CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. Proc. Natl Acad. Sci. USA. 2010;107:193. doi: 10.1073/pnas.0909945107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lefrancois L, Obar JJ. Once a killer, always a killer: from cytotoxic T cell to memory cell. Immunol. Rev. 2010;235:206. doi: 10.1111/j.0105-2896.2010.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bannard O, Kraman M, Fearon DT. Secondary replicative function of CD8+ T cells that had developed an effector phenotype. Science. 2009;323:505. doi: 10.1126/science.1166831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamann D, Baars PA, Rep MH, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 1997;186:1407. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 28.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 29.Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 30.Obar JJ, Lefrancois L. Early signals during CD8+ T cell priming regulate the generation of central memory cells. J. Immunol. 2010 doi: 10.4049/jimmunol.1000492. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stemberger C, Huster KM, Koffler M, et al. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity. 2007;27:985. doi: 10.1016/j.immuni.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 32.Obar JJ, Lefrancois L. Memory CD8+ T cell differentiation. Ann. N. Y. Acad. Sci. 2010;1183:251. doi: 10.1111/j.1749-6632.2009.05126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mescher MF, Agarwal P, Casey KA, Hammerbeck CD, Xiao Z, Curtsinger JM. Molecular basis for checkpoints in the CD8 T cell response: tolerance versus activation. Semin. Immunol. 2007;19:153. doi: 10.1016/j.smim.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 2000;343:1020. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 35.Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 36.Semmling V, Lukacs-Kornek V, Thaiss CA, et al. Alternative cross-priming through CCL17-CCR4-mediated attraction of CTLs toward NKT cell-licensed DCs. Nat. Immunol. 2010;11:313. doi: 10.1038/ni.1848. [DOI] [PubMed] [Google Scholar]
- 37.Wherry EJ, Puorro KA, Porgador A, Eisenlohr LC. The induction of virus-specific CTL as a function of increasing epitope expression: responses rise steadily until excessively high levels of epitope are attained. J. Immunol. 1999;163:3735. [PubMed] [Google Scholar]
- 38.Wherry EJ, McElhaugh MJ, Eisenlohr LC. Generation of CD8(+) T cell memory in response to low, high, and excessive levels of epitope. J. Immunol. 2002;168:4455. doi: 10.4049/jimmunol.168.9.4455. [DOI] [PubMed] [Google Scholar]
- 39.Bullock TN, Mullins DW, Engelhard VH. Antigen density presented by dendritic cells in vivo differentially affects the number and avidity of primary, memory, and recall CD8+ T cells. J. Immunol. 2003;170:1822. doi: 10.4049/jimmunol.170.4.1822. [DOI] [PubMed] [Google Scholar]
- 40.Prlic M, Hernandez-Hoyos G, Bevan MJ. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J. Exp. Med. 2006;203:2135. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu. Rev. Immunol. 2005;23:515. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 42.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 2005;23:23. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 43.Shahinian A, Pfeffer K, Lee KP, et al. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 44.Peperzak V, Xiao Y, Veraar EA, Borst J. CD27 sustains survival of CTLs in virus-infected nonlymphoid tissue in mice by inducing autocrine IL-2 production. J. Clin. Invest. 2010;120:168. doi: 10.1172/JCI40178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 47.Shedlock DJ, Whitmire JK, Tan J, MacDonald AS, Ahmed R, Shen H. Role of CD4 T cell help and costimulation in CD8 T cell responses during Listeria monocytogenes infection. J. Immunol. 2003;170:2053. doi: 10.4049/jimmunol.170.4.2053. [DOI] [PubMed] [Google Scholar]
- 48.Marzo AL, Vezys V, Klonowski KD, et al. Fully functional memory CD8 T cells in the absence of CD4 T cells. J. Immunol. 2004;173:969. doi: 10.4049/jimmunol.173.2.969. [DOI] [PubMed] [Google Scholar]
- 49.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 50.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 51.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat. Immunol. 2004;5:927. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bachmann MF, Schwarz K, Wolint P, et al. Cutting edge: distinct roles for T help and CD40/CD40 ligand in regulating differentiation of proliferation-competent memory CD8+ T cells. J. Immunol. 2004;173:2217. doi: 10.4049/jimmunol.173.4.2217. [DOI] [PubMed] [Google Scholar]
- 54.Khanolkar A, Fuller MJ, Zajac AJ. CD4 T cell-dependent CD8 T cell maturation. J. Immunol. 2004;172:2834. doi: 10.4049/jimmunol.172.5.2834. [DOI] [PubMed] [Google Scholar]
- 55.Fuse S, Tsai CY, Molloy MJ, et al. Recall responses by helpless memory CD8+ T cells are restricted by the up-regulation of PD-1. J. Immunol. 2009;182:4244. doi: 10.4049/jimmunol.0802041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thompson LJ, Kolumam GA, Thomas S, Murali-Krishna K. Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation. J. Immunol. 2006;177:1746. doi: 10.4049/jimmunol.177.3.1746. [DOI] [PubMed] [Google Scholar]
- 57.Keppler SJ, Theil K, Vucikuja S, Aichele P. Effector T-cell differentiation during viral and bacterial infections: role of direct IL-12 signals for cell fate decision of CD8(+) T cells. Eur. J. Immunol. 2009;39:1774. doi: 10.1002/eji.200839093. [DOI] [PubMed] [Google Scholar]
- 58.Cui W, Joshi NS, Jiang A, Kaech SM. Effects of Signal 3 during CD8 T cell priming: bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine. 2009;27:2177. doi: 10.1016/j.vaccine.2009.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pham NL, Badovinac VP, Harty JT. A default pathway of memory CD8 T cell differentiation after dendritic cell immunization is deflected by encounter with inflammatory cytokines during antigen-driven proliferation. J. Immunol. 2009;183:2337. doi: 10.4049/jimmunol.0901203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nat. Immunol. 2004;5:809. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- 61.Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat. Med. 2005;11:748. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- 62.Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat. Immunol. 2005;6:793. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wherry EJ, Teichgraber V, Becker TC, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003;4:225. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 65.Manjunath N, Shankar P, Wan J, et al. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J. Clin. Invest. 2001;108:871. doi: 10.1172/JCI13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sinclair LV, Finlay D, Feijoo C, et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat. Immunol. 2008;9:513. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Casey KA, Mescher MF. IL-21 promotes differentiation of naive CD8 T cells to a unique effector phenotype. J. Immunol. 2007;178:7640. doi: 10.4049/jimmunol.178.12.7640. [DOI] [PubMed] [Google Scholar]
- 69.Hinrichs CS, Spolski R, Paulos CM, et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilson EB, Livingstone AM. Cutting edge: cD4+ T cell-derived IL-2 is essential for help-dependent primary CD8+ T cell responses. J. Immunol. 2008;181:7445. doi: 10.4049/jimmunol.181.11.7445. [DOI] [PubMed] [Google Scholar]
- 71.Fabre S, Carrette F, Chen J, et al. FOXO1 regulates L-Selectin and a network of human T cell homing molecules downstream of phosphatidylinositol 3-kinase. J. Immunol. 2008;181:2980. doi: 10.4049/jimmunol.181.5.2980. [DOI] [PubMed] [Google Scholar]
- 72.Araki K, Turner AP, Shaffer VO, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pearce EL, Walsh MC, Cejas PJ, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Waugh C, Sinclair L, Finlay D, Bayascas JR, Cantrell D. Phosphoinositide (3,4,5)-triphosphate binding to phosphoinositide-dependent kinase 1 regulates a protein kinase B/Akt signaling threshold that dictates T-cell migration, not proliferation. Mol. Cell Biol. 2009;29:5952. doi: 10.1128/MCB.00585-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carlson CM, Endrizzi BT, Wu J, et al. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- 77.Hedrick SM. The cunning little vixen: foxo and the cycle of life and death. Nat. Immunol. 2009;10:1057. doi: 10.1038/ni.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kerdiles YM, Beisner DR, Tinoco R, et al. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat. Immunol. 2009;10:176. doi: 10.1038/ni.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ouyang W, Beckett O, Flavell RA, Li MO. An essential role of the Forkhead-box transcription factor Foxo1 in control of T cell homeostasis and tolerance. Immunity. 2009;30:358. doi: 10.1016/j.immuni.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harris SJ, Parry RV, Westwick J, Ward SG. Phosphoinositide lipid phosphatases: natural regulators of phosphoinositide 3-kinase signaling in T lymphocytes. J. Biol. Chem. 2008;283:2465. doi: 10.1074/jbc.R700044200. [DOI] [PubMed] [Google Scholar]
- 81.Finlay DK, Sinclair LV, Feijoo C, et al. Phosphoinositide-dependent kinase 1 controls migration and malignant transformation but not cell growth and proliferation in PTEN-null lymphocytes. J. Exp. Med. 2009;206:2441. doi: 10.1084/jem.20090219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cornish GH, Sinclair LV, Cantrell DA. Differential regulation of T-cell growth by IL-2 and IL-15. Blood. 2006;108:600. doi: 10.1182/blood-2005-12-4827. [DOI] [PubMed] [Google Scholar]
- 83.Xue HH, Kovanen PE, Pise-Masison CA, et al. IL-2 negatively regulates IL-7 receptor alpha chain expression in activated T lymphocytes. Proc. Natl Acad. Sci. USA. 2002;99:13759. doi: 10.1073/pnas.212214999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rutishauser RL, Martins GA, Kalachikov S, et al. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ichii H, Sakamoto A, Kuroda Y, Tokuhisa T. Bcl6 acts as an amplifier for the generation and proliferative capacity of central memory CD8+ T cells. J. Immunol. 2004;173:883. doi: 10.4049/jimmunol.173.2.883. [DOI] [PubMed] [Google Scholar]
- 86.Gwinn DM, Shackelford DB, Egan DF, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell. 2008;30:214. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Intlekofer AM, Takemoto N, Wherry EJ, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 2005;6:1236. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 88.Intlekofer AM, Takemoto N, Kao C, et al. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J. Exp. Med. 2007;204:2015. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jones RG, Thompson CB. Revving the engine: signal transduction fuels T cell activation. Immunity. 2007;27:173. doi: 10.1016/j.immuni.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 90.von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat. Immunol. 2010;11:344. doi: 10.1038/ni.1851. [DOI] [PubMed] [Google Scholar]
- 91.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]