Phosphorylation by Akt1 Promotes Skp2 Cytoplasmic Localization and Impairs APC/Cdh1-mediated Skp2 Destruction (original) (raw)
. Author manuscript; available in PMC: 2010 Jul 27.
Published in final edited form as: Nat Cell Biol. 2009 Mar 8;11(4):397–408. doi: 10.1038/ncb1847
Abstract
Deregulated Skp2 function promotes cell transformation, and this is consistent with observations of Skp2 over-expression in many human cancers. However, the mechanisms underlying elevated Skp2 expression remain elusive. Here we report that the serine/threonine protein kinase Akt1, but not Akt2, directly controls Skp2 stability by a mechanism that involves degradation by the APC/Cdh1 ubiquitin ligase complex. We further show that Akt1 phosphorylates Skp2 at Ser72, which is required to disrupt the interaction between Cdh1 and Skp2. In addition, we show that Ser72 is localized within a putative Nuclear Localization Sequence (NLS) and that phosphorylation of Ser72 by Akt leads to Skp2 cytoplasmic translocation. This finding expands our knowledge of how specific signaling kinase cascades influence proteolysis governed by APC/Cdh1 complexes, and provides evidence that elevated Akt activity and cytoplasmic Skp2 expression may be causative for cancer progression.
The SCF/Skp2 E3 ubiquitin ligase complex regulates the destruction of numerous cell cycle regulators including p27, FOXO1 and p1301. Elevated Skp2 expression is frequently observed in many tumors including breast and prostate carcinomas2,3. However, the molecular mechanisms underlying elevated Skp2 expression have not been fully explored. We and others have identified Cdh1 as the E3 ligase that promotes Skp2 destruction4,5. In contrast to the frequency of Skp2 overexpression, loss of Cdh1 is not a frequent event in human cancer. On the other hand, hyperactivation of the Akt pathway is considered a hallmark of many cancers and it has been reported that activation of the PI 3-K (phosphoinositide 3-kinase)/Akt pathway enhances p27 destruction6. This suggests that sustained Akt activity can influence Skp2 activity7,8.
The Akt family of kinases includes three closely related family members designated Akt1, Akt2 and Akt39. Since most of the upstream regulators and downstream mediators of the Akt pathway are either oncogenes or tumor suppressors, it is not surprising to find that Akt activity is abnormally elevated in most human cancers10. Enhanced Akt signaling in tumor cells can suppress apoptosis by promoting the phosphorylation and subsequent cytoplasmic localization of many downstream pro-apoptotic proteins targets such as Bad11, FOXO112 and FOXO3a13. Akt upregulation can also promote cell growth by inactivating the negative cell cycle regulators p2114 and p2715–17. Most studies exploring a role for the Akt pathway in cell cycle progression, survival and cancer progression have generally assumed that all three isoforms function in overlapping, redundant roles. However, recent studies have begun to suggest isoform-specific functions for Akt18,19,20.
Here we evaluated the mechanism by which Akt controls Skp2 stability as well as Skp2 subcellular localization. Our findings provide a mechanistic explanation for elevated Skp2 expression as well as Skp2 cytoplasmic staining in tissues derived from advanced breast and prostate cancers21,22.
Results
Skp2 expression is regulated by the PI 3-K/Akt pathway
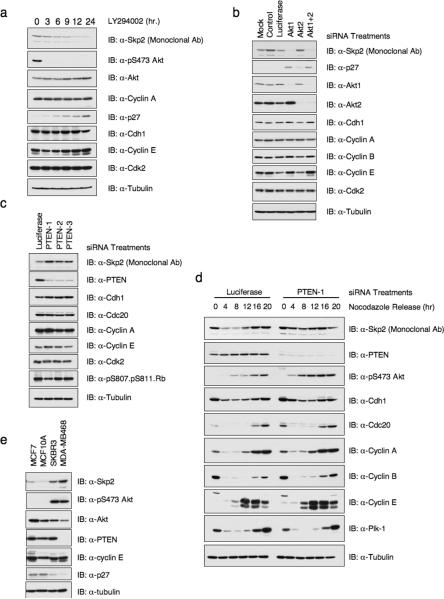
Recent reports suggested that the PI 3-K/Akt pathway regulates Skp2 expression levels by unknown mechanism(s)6,23. To investigate the contribution of Akt signaling in Skp2 expression, we treated HeLa and PC3 cells with the PI 3-K inhibitor LY294002, and observed a time-dependent decrease in Skp2 protein levels concomitant with a robust inhibition of PI 3-K activity as revealed by the loss of phospho-Akt (pS473). However, the expression of Cdh1, the known E3 ligase of Skp2, was not affected by LY294002. The expression of other Cdh1 substrates such as Cyclin A did not respond to inhibition of PI 3-K activity either (Fig. 1a, Supplementary information Fig. S1a–c). Secondly, we used IGF-1, which potently activates PI 3-K in all cell types, and observed increased Skp2 protein levels also concomitant with enhanced Akt phosphorylation (Supplementary information Fig. S1d and S1e). Fig. 1b shows that specific depletion of Akt1, but not Akt2, markedly decreases Skp2 protein levels in HeLa cells, and induces its downstream target p27. However, depletion of Akt1 did not change the expression of Cdh1 and other Cdh1 substrates we examined (Fig. 1b). Similar results were also obtained in U2OS cells and SKBR3 cells (Supplementary information Fig. S1f and S1g). Conversely, inactivation of PTEN, which results in elevated Akt activity, leads to upregulation of Skp2 in both asynchronized and synchronized HeLa cells (Fig. 1c and 1d). This finding is further supported by the positive correlation between Skp2 expression and Akt activity in a panel of breast cancer cell lines (Fig. 1e). Furthermore, the suppression of Akt activity by LY294002 in both MDA-MB468 and SKBR3 cells leads to downregulation of Skp2 expression, providing further evidence that elevated Akt activity is one major cause for the observed upregulation of Skp2 in these two cell lines (Supplementary information Fig. S1b and S1c). Thus, in agreement with previous reports6,8, the PI 3-K pathway regulates Skp2 expression, and moreover, this occurs selectively through Akt1 signaling.
Figure 1. Human Skp2 protein levels are regulated by the PTEN/PI 3-K/Akt pathway.
(a) Immunoblot analysis of HeLa cells treated with 20μM of the PI 3-K inhibitor LY294002 for the indicated duration of time.
(b–c) Immunoblot analysis of HeLa cells transfected with the indicated siRNA oligonucleotides. The control lane is scrambled E2F-1 siRNA; Luciferase, siRNA against firefly luciferase; PTEN1–3; three independent PTEN siRNA oligos; siRNA, short interfering RNA.
(d) Immunoblot analysis of HeLa cells transfected with the indicated siRNA oligos, after synchronization with nocodazole and release.
(e) Immunoblot analysis of indicated cell lines cultured in serum-free medium. Whole cell lysates were isolated in the presence of phosphatase inhibitors.
Full-length blots are provided in the Supplementary Information, Fig. S11.
Consistent with previous report, we found that inactivation of PTEN in mouse embryonic fibroblasts (MEFs) led to mild upregulation (1.5–1.7 fold) of mouse Skp2 levels (Supplementary information Fig. S1h)8. However, this is not likely to operate through the Akt pathway since downregulation of either Akt1 or Akt2 by shRNA in MEFs did not affect mouse Skp2 protein levels (Supplementary information Fig. S1i). These results indicate that Akt signaling differs in mouse versus human cells with respect to regulation of Skp2 expression.
PTEN/PI 3-K/Akt pathway regulates both Skp2 transcription and Skp2 stability
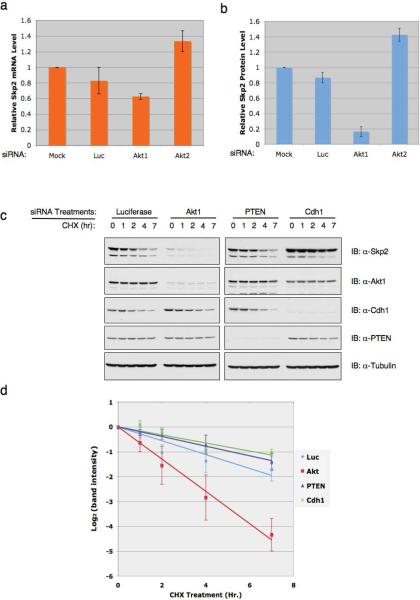
Next, we asked how Akt mechanistically regulates Skp2 expression. We found that in agreement with previous reports24,25, inactivation of Akt1, but not Akt2 leads to 40% reduction of Skp2 mRNA levels (Fig. 2a). This is possibly through either E2F1 or NF-κB pathways that are subjective to Akt1 regulation24,25. However, we observed a much greater (about 8 fold) reduction of Skp2 protein abundance (Fig. 2b), arguing that Akt1 could also regulate Skp2 expression post-translationally. To further address this possibility, we assessed alterations of endogenous Skp2 half-life after modulating the PTEN/Akt pathway. We found that inactivation of Akt1 by siRNA shortened endogenous Skp2 half-life, while depletion of PTEN, in a similar fashion to depletion of Cdh1, stabilized Skp2 (Fig. 2c, 2d and Supplementary information Fig. S2).
Figure 2. The PTEN/PI 3-K/Akt pathway regulates both Skp2 transcription and Skp2 stability.
(a) Real-time RT-PCR analysis to examine the relative Skp2 mRNA expression levels in HeLa cells transfected with the indicated siRNA oligonucleotides. Three independent sets of experiments were performed to generate the error bar. The error bars represent s.d.
(b) Immunoblot analysis to examine the relative Skp2 protein levels in HeLa cells transfected with the indicated siRNA oligonucleotides. Three independent sets of experiments were performed to generate the error bar. The error bars represent s.d.
(c) HeLa cells were transfected with indicated siRNA oligos. 40 hr post-transfection, cells were treated with 20 μg/ml cycloheximide. At the indicated time points, whole-cell lysates were prepared and immunoblots were probed with indicated antibodies.
(d) Quantification of the band intensities in c. Skp2 band intensity was normalized to Tubulin, then normalized to the t=0 controls. Three independent sets of experiments were performed to generate the error bar. The error bars represent s.d.
Full-length blots are provided in the Supplementary Information, Fig. S11.
Akt1 interacts with and phosphorylates Skp2 at Ser72
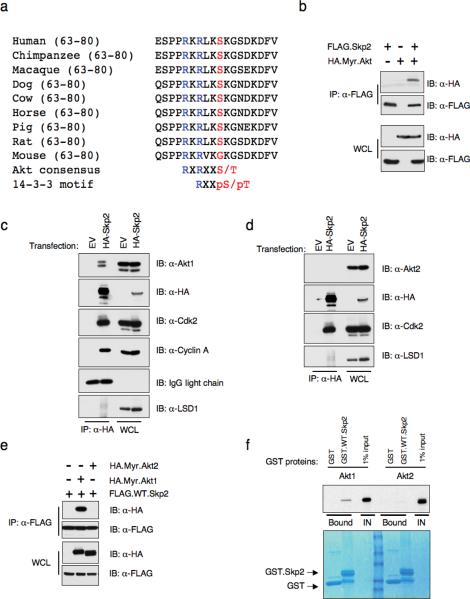
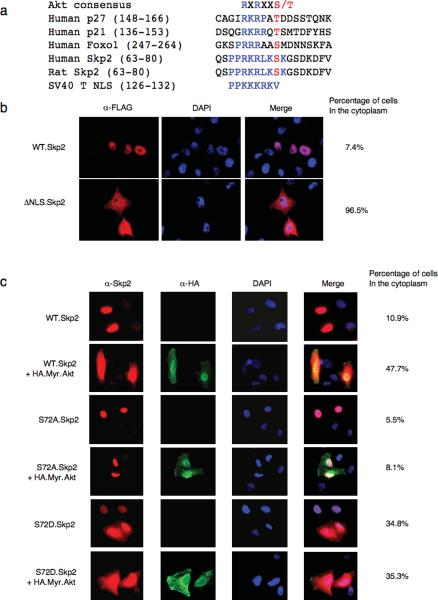
Sequence analysis revealed that human Skp2 contains an Akt consensus phosphorylation site at Ser72, which conforms to the optimal Akt motif RxRxxS/T. The motif surrounding Ser72 is also conserved in Skp2 orthologues in all mammals, except mouse (Fig 3a). We therefore reasoned that Skp2 is an Akt substrate whereby phosphorylation may influence Skp2 stability. However, since mouse Skp2 lacks the Ser72 site, it is likely that mouse Skp2 is not an optimal Akt substrate. Thus, it explains why loss of Akt1 would not affect Skp2 expression in MEFs (Supplementary information Fig. S1i). On the other hand, the absence of Ser72 in xenopus and zebrafish suggests that the Akt/Skp2 regulatory pathway might be a relatively late event acquired during evolution.
Figure 3. The Skp2 protein contains a canonical Akt phosphorylation site at S72 and interacts with Akt1, but not Akt2 in vivo.
(a) Sequence alignment of the putative Akt phosphorylation site at S72 in Skp2 from different species.
(b) Immunoblot (IB) analysis of whole cell lysates (WCL) and immunoprecipitates (IP) derived from 293T cells transfected with HA.Myr.Akt and FLAG.Skp2 constructs.
(c–d) Immunoblot (IB) analysis of whole cell lysates (WCL) and immunoprecipitates (IP) derived from 293T cells transfected with HA.Skp2 or empty vector (EV). Cdk2 and Cylin A antibodies were used as positive controls to detect the interaction with Skp2, while the LSD1 antibody was used as a negative control.
(e) Immunoblot (IB) analysis of whole cell lysates (WCL) and immunoprecipitates (IP) derived from 293T cells transfected with indicated HA.Myr.Akt1 or HA-Myr.Akt2 and FLAG.Skp2 constructs.
(f) Autoradiography of 35S-labelled Akt1 or Akt2 bound to the indicated GST-fusion proteins.
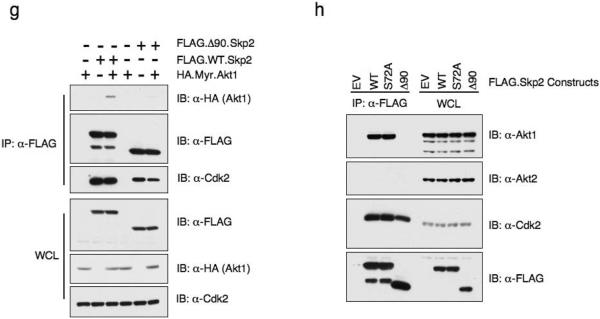
(g) Immunoblot (IB) analysis of whole cell lysates (WCL) and immunoprecipitates (IP) derived from 293T cells transfected with indicated HA.Myr.Akt1 and FLAG.Skp2 constructs.
(h) Immunoblot (IB) analysis of whole cell lysates (WCL) and immunoprecipitates (IP) derived from 293T cells transfected with indicated FLAG.Skp2 construct or empty vector (EV). Cdk2 antibody was used as a positive control to detect the interaction with Skp2.
Full-length blots are provided in the Supplementary Information, Fig. S11.
Consistent with the hypothesis that Skp2 is a putative Akt substrate, an activated allele of Akt1 (Myr.Akt1) interacts with Skp2 as detected by co-immunoprecipitation (Fig. 3b). Furthermore, when overexpressed in 293T cells, ectopically expressed Skp2 specifically interacts with endogenous Akt1, but not Akt2 (Fig. 3c and 3d). In support of this finding, using both in vivo co-immunoprecipitation (Fig. 3e) and in vitro GST-pull down (Fig. 3f) assays, we were able to show that Skp2 specifically interacts with Akt1, but not Akt2. Moreover, we demonstrated that the Skp2 constructs lacking the first 90 amino acids failed to interact with both ectopically overexpressed Akt1 (Fig. 3g) and endogenous Akt1 (Fig. 3h). These results provide further evidence for the molecular mechanism for the Akt1 isoform-specific regulation of Skp2.
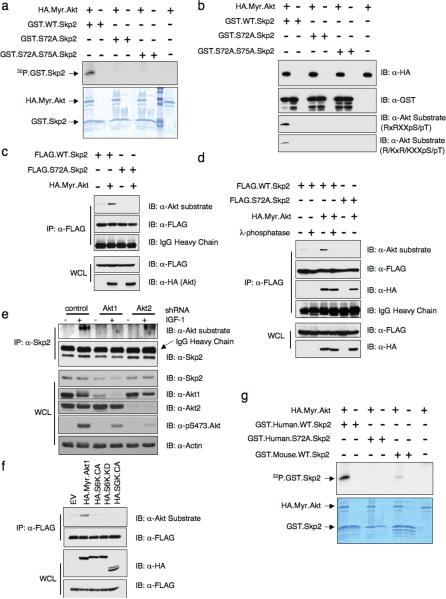
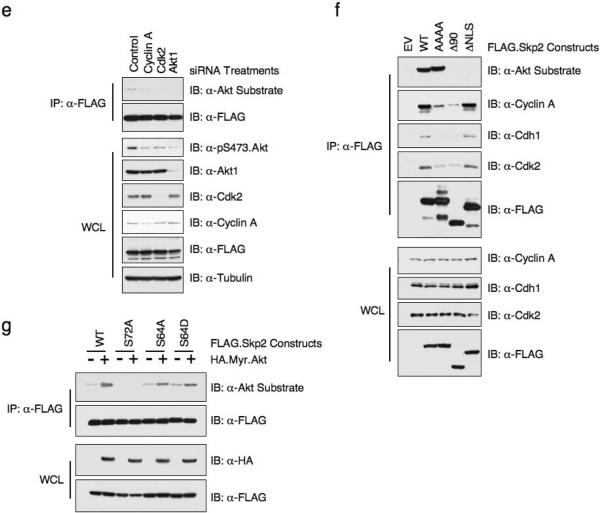
To test if Skp2 is an Akt substrate, we first performed in vitro kinase assays. Fig. 4a shows that Akt1 phosphorylates wild-type Skp2, but not the S72A mutant. Furthermore, we demonstrate that Akt could phosphorylate Skp2 as efficiently as it does to another known Akt substrate, Mdm226 (Supplementary information Fig. S3a). We also examined Skp2 phosphorylation using a substrate-directed phospho-specific antibody that recognizes the optimal Akt consensus phosphorylation motif27 (Fig. 4b). Since the ScanSite program showed that there are additional suboptimal putative Akt phosphorylation sites present in human Skp2, including Thr21, Ser75 and Ser157, to pinpoint the exact Akt phosphorylation site, we performed mass spectrometric analysis of GST.WT.Skp2 subsequent to incubation with Myr.Akt. This analysis revealed that Ser72 is the only phosphorylation event identified under these experimental conditions (Supplementary information Fig. S3b). Next, we found that expression of activated Akt significantly enhanced the phosphorylation of wild-type Skp2, whereas Skp2.S72A phosphorylation was not detected (Fig. 4c). The reactivity of Skp2 with the Akt substrate antibody was reversed when the cell lysates were incubated with lambda phosphatase (Fig. 4d). Additionally, Skp2 phosphorylation was detected by the Akt substrate antibody in IGF-1-stimulated cells, and moreover, phosphorylation was reduced in cells transduced with Skp2 siRNA or Akt1, but not Akt2, shRNA (Fig. 4e, Supplementary information Fig. S3f). Although both Akt1 and Akt2 phosphorylated Skp2 at relatively similar efficiency in vitro (Supplementary information Fig. S3c), when ectopically overexpressed in HeLa cells, Akt1 is more potent than Akt2 in phosphorylating the Skp2 protein (Supplementary information Fig. S3d). These results are thus consistent with the notion that endogenous Akt1, but not Akt2, directly phosphorylates Skp2 in cells, and suggests that this may be causally linked to decreased Skp2 expression after Akt1 depletion (Fig. 1b). It was recently demonstrated that besides Akt, other AGC family kinase such as SGK could also phosphorylate p27, a well-known Akt substrate28. We found that Akt is the only kinase capable of phosphorylating Skp2 in vivo while both S6K and SGK failed to phosphorylate Skp2 (Fig. 4f).
Figure 4. Akt phosphorylates the human Skp2 protein at the Ser72 site.
(a) Akt phosphorylates Skp2 in vitro at S72. HA.Myr.Akt was transfected into 293T cells, recovered by anti-HA immunoprecipitation and incubated with 5 μg of indicated GST.Skp2 in the presence of γ-32P-ATP. The kinase reaction products were resolved by SDS-PAGE and phosphorylation was detected by autoradiography.
(b) Akt phosphorylates Skp2 in vitro at S72. HA.Myr.Akt was transfected into 293T cells, recovered by anti-HA immunoprecipitation and incubated with 5 μg of indicated GST.Skp2 in the presence of cold ATP. The kinase reaction products were resolved by SDS-PAGE and phosphorylation was detected by the phospho-Akt-substrate antibody that recognizes either the RXRXXpS/pT or the R/KXR/KXXpS/pT motif.
(c) Immunoblot (IB) analysis of whole-cell lysates (WCL) and immunoprecipitates (IP) derived from HeLa cells transfected with HA.Myr.Akt and FLAG.Skp2. WT, wild-type Skp2; S72A, mutant Skp2 at the Akt site.
(d) Immunoblot (IB) analysis of whole-cell lysates (WCL) and immunoprecipitates (IP) derived from 293T cells transfected with HA.Myr.Akt and indicated FLAG.Skp2 plasmids. Where indicated, the whole cell lysates were treated with λ-phosphatase prior to immunoprecipitation.
(e) Immunoblot analysis of whole cell lysates (WCL) and immunoprecipitates (IP) derived from HeLa cells infected with Akt1 and Akt2 lentiviral shRNA. Endogenous Skp2 was immunoprecipitated with anti-Skp2 and immunoblotted with the Akt substrate directed phospho-antibody.
(f) Immunoblot analysis of whole cell lysates (WCL) and immunoprecipitates (IP) derived from HeLa cells transfected with the indicated kinases and FLAG.Skp2 construct.
(g) Mouse Skp2 protein is a poor Akt substrate. HA.Myr.Akt was transfected into 293T cells, recovered by anti-HA immunoprecipitation and incubated with 5 μg of indicated GST.Skp2 in the presence of γ-32P-ATP. The kinase reaction products were resolved by SDS-PAGE and phosphorylation was detected by autoradiography.
Full-length blots are provided in the Supplementary Information, Fig. S11.
High stringency Akt sites are not found in mouse Skp2, although there are several suboptimal sites, including Thr21, Ser75 and Ser133. This low stringency is consistent with the failure of the phospho-Akt substrate antibody to recognize phosphorylation at any of these sites in mouse Skp2 (Supplementary information Fig. S3e). Furthermore, we only observed a very weak incorporation of γ-32P into a mouse GST.Skp2 fusion protein after incubation with Myr.Akt in vitro (Fig. 4g).
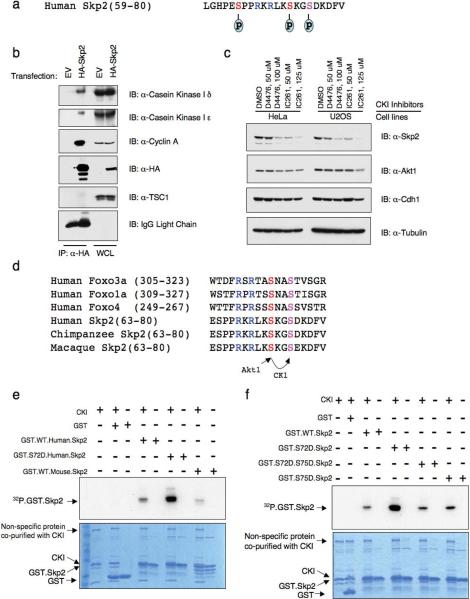
Phosphorylation of Skp2 Ser72 site by Akt1 triggers the subsequent phosphorylation of the Ser75 site by Casein Kinase I
To better understand the phosphorylation events of human Skp2 protein in vivo, we immunoprecipitated the ectopically expressed HA-Skp2 protein and analyzed its phosphorylation status by mass spectrum methods. As illustrated in Fig. 5a, we identified phosphorylation of the Ser72 Akt site, the Ser75 site, and a previously reported Ser64 site29 (Supplementary information Fig. S4a–2b). Sequence analysis revealed that the Ser75 site is a putative Casein Kinase I site. Consistent with this, the Casein Kinase I ε isoform was found to specifically associate with Skp2 protein by mass spectrum analysis (data not shown). This finding was further validated by immunoblot analysis of Skp2 immunoprecipitates (Fig. 5b). In addition, inhibition of the CKI kinase activity with specific CKI kinase inhibitors resulted in a decrease of Skp2 expression level in both HeLa and U2OS cell lines (Fig. 5c), indicating that CKI is involved in regulating Skp2 stability by directly phosphorylating the Skp2 protein.
Figure 5. Phosphorylation of Skp2 at Ser72 triggers the subsequent phosphorylation of the Ser75 site by Casein Kinase I.
(a) In vivo Skp2 phosphorylation sites detected by mass spectrum analysis.
(b) Immunoblot (IB) analysis of whole cell lysates (WCL) and immunoprecipitates (IP) derived from 293T cells transfected with HA.Skp2. The Cylin A antibody was used as a positive control to detect the interaction with Skp2, while the TSC1 antibody was used as a negative control.
(c) Immunoblot analysis of HeLa and U2OS cells treated with the Casein Kinase I inhibitors D4476 and IC261 at the indicated concentrations for 12 hours.
(d) Protein sequence illustration of the phosphorylation of the Ser75 site by Casein Kinase I (CKI), which is triggered by the phosphorylation of the Ser72 site by Akt1. The same sequential phosphorylation cascade has been described in the Foxo family of transcription factors.
(e–f) CKI phosphorylates Skp2 in vitro at S75. Purified CKI protein (from New England Biolabs) was incubated with 5 μg of indicated GST.Skp2 in the presence of γ-32P-ATP. The kinase reaction products were resolved by SDS-PAGE and phosphorylation was detected by autoradiography.
Full-length blots are provided in the Supplementary Information, Fig. S11.
We reasoned that phosphorylation of Ser72 might create a priming site that facilitates phosphorylation of Ser75 by Casein Kinase I, a mechanism that has been reported in the phosphorylation of the Foxo family of transcription factors30 (Fig. 5d). Indeed, we found that substitution of Ser72 to Asp to mimic Akt phosphorylation enhanced phosphorylation of this S72D.Skp2 mutant by CKI (Fig. 5e and Supplementary information Fig. S4c). On the other hand, mutation of the Ser75 site reduced phosphorylation indicating that CKI-mediated phosphorylation of Skp2 occurs at the Ser75 site (Fig. 5f). These data suggest that CKI may function as the Ser75 kinase after a priming phosphorylation of Ser72 by Akt1.
Overexpression of Akt protects Skp2 from Cdh1-mediated destruction
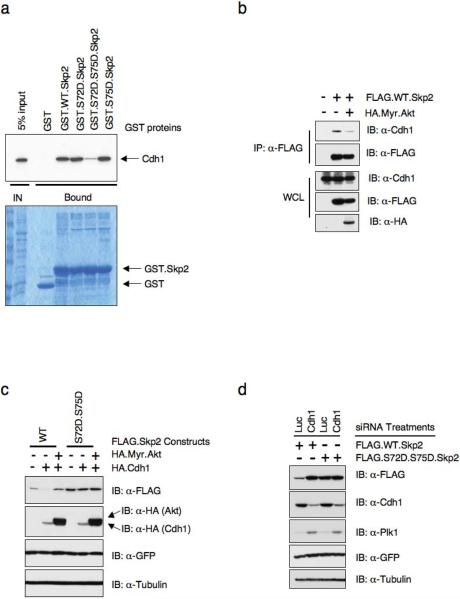
Our previous studies suggested that a region of Skp2 between amino acids 46 and 904,5, which contains both the Akt and CKI phosphorylation sites, is both sufficient and required for interaction with Cdh1. Thus, we further examined how Akt and/or CKI phosphorylation affects the interaction between Cdh1 and Skp2. We find that substitution of both Ser72 and Ser75 with the phospho-mimetic amino acids disrupted the interaction between Skp2 and Cdh1 as detected by both in vitro GST-pull down assays (Fig. 6a and Supplementary information Fig. S4d) and in vivo Co-immunoprecipitation analysis (Supplementary information Fig. S4e). In support of this finding, we further demonstrated that overexpression of activated Akt resulted in reduced interaction between Skp2 and Cdh1 in vivo (Fig. 6b). Next, we asked whether this leads to the stabilization of Skp2. In keeping with previous reports4,5, we found that expression of Cdh1 downregulated wild-type Skp2, while overexpression of activated Akt abolished Cdh1-mediated Skp2 destruction. Importantly, phospho-mimetic mutants of S72 and S75 (S72D and S75D) were resistant to Cdh1-mediated destruction (Fig. 6c). Conversely, depletion of endogenous Cdh1 with siRNA upregulated wild-type Skp2, but not the Skp2 S72D/S75D phospho-mimetic mutant, further supporting the idea that the S72D/S75D mutant is resistant to Cdh1-mediated destruction (Fig. 6d).
Figure 6. Phosphorylation of Skp2 by Akt1 protects Skp2 from Cdh1-mediated destruction.
(a) Autoradiography of 35S-labelled Cdh1 bound to the indicated GST-fusion proteins.
(b) Immunoblot analysis of whole cell lysates (WCL) and immunoprecipitates (IP) derived from 293T cells transfected with the FLAG.Skp2 construct in the presence or absence of HA.Myr.Akt.
(c) Immunobot analysis of HeLa cells transfected with the indicated FLAG.Skp2 and HA.Cdh1 plasmids in the presence or absence of HA.Myr.Akt. A plasmid encoding GFP was used as a negative control for transfection efficiency.
(d) Immunoblot analysis of HeLa cells transfected with the indicated FLAG.Skp2 plasmids and siRNA oligos. A plasmid encoding GFP was included as negative control for transfection efficiency.
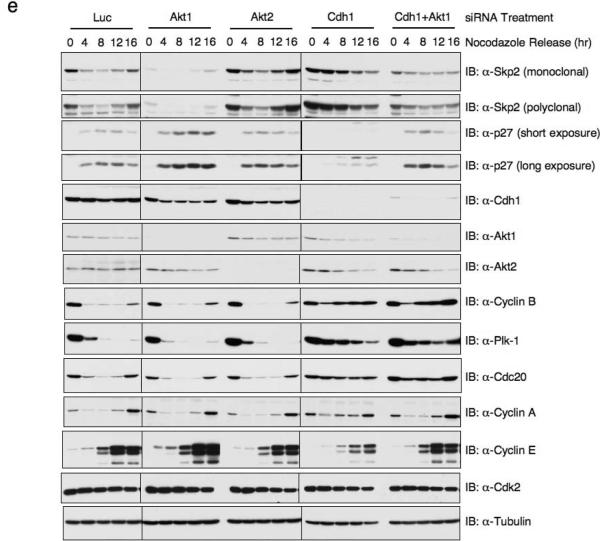
(e) Immunoblot analysis of HeLa cells transfected with the indicated siRNA oligos, synchronized by growth in nocodazole, and then released for the indicated periods of time.
Cdh1 activity is required for Akt1 in regulating Skp2 expression levels
Although to date Cdh1 is the only identified E3 ligase that targets Skp2, Akt1-mediated Skp2 regulation could occur through either Cdh1-dependent or Cdh1-independent mechanisms. To further test the contribution of Cdh1 in Akt-dependent regulation of Skp2, we used siRNA. Fig. 6e shows that depletion of Akt1, but not Akt2, leads to a reduction of Skp2 protein levels and subsequent accumulation of p27. Conversely, depletion of Cdh1 enhanced upregulation of Skp2 and downregulation of p27. Interestingly, Skp2 levels were restored to normal control levels when both Cdh1 and Akt1 were concomitantly depleted. This result suggests that Cdh1 is required for the ability of Akt1 to regulate Skp2. Thus, in normal cycling cells, the ability of Akt1 to phosphorylate Skp2 at S72 protects Skp2 from Cdh1-mediated degradation, such that loss of Akt1 leads to enhanced Skp2 degradation primarily through the Cdh1-dependent destruction pathway. Consistent with this, we also found that in T98G cells released from serum starvation, Skp2 expression showed up earlier than most APC/Cdh1 substrates (Data not shown)31,32.
Phosphorylation of Skp2 at Ser72 by Akt affects Skp2 protein stability
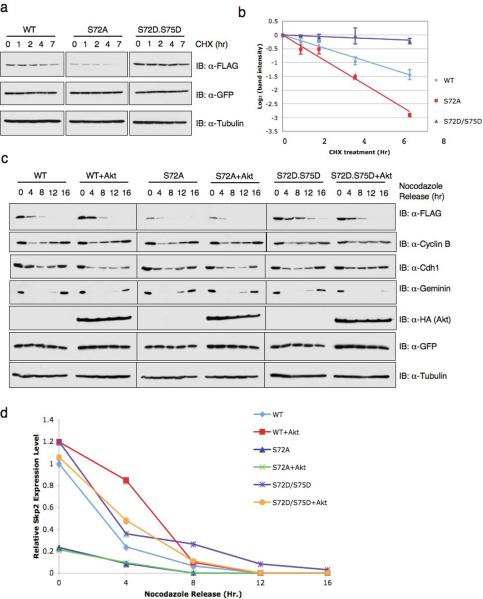
Time course experiments in cells expressing Skp2 mutants and treated with the protein synthesis inhibitor cycloheximide revealed that the effects of Akt on Skp2 protein levels are due to alterations in the half-life of the Skp2 protein (Fig. 7a and 7b). To further investigate the effects of Akt1 activity on Skp2 protein levels, various Skp2 constructs were transfected with or without activated Myr.Akt and their expression levels were monitored during the cell cycle progression. As shown in Figs. 7c and 7d, wild-type Skp2 protein levels rapidly decline in early G1 when APC/Cdh1 is active, and expression of activated Akt delayed the rate of degradation. The S72A mutant was degraded significantly more rapidly than wild-type Skp2. Conversely, the S72D/S75D mutant was degraded with slower kinetics than wild-type Skp2. Collectively, these data demonstrate that Akt phosphorylation influences the destruction of Skp2 governed by APC/Cdh1.
Figure 7. Phosphorylation of Skp2 at Ser72 by Akt affects Skp2 protein stability.
(a) HeLa cells were transfected with the indicated FLAG.Skp2 plasmids. 20 hr post-transfection, cells were split into 60 mm dishes, and after another 20 hr, treated with 20 μg/ml cycloheximide. At the indicated time points, whole-cell lysates were prepared and immunoblots were probed with the indicated antibodies.
(b) Quantification of the band intensities in a. Skp2 band intensity was normalized to GFP, then normalized to the t=0 controls. Three independent sets of experiments were performed to generate the error bar. The error bars represent s.d.
(c) Immunoblot analysis of HeLa cells transfected with limiting amounts of FLAG.Skp2 (wild-type; S72A; S72D/S75D) plasmids with or without HA.Myr.Akt, along with a green fluorescent protein (GFP) as transfection control. HeLa cells were synchronized in M phase with nocodazole, and then released to the G1 phase for the indicated time periods.
(d) Quantitation of the band intensities in c. Skp2 band intensity was normalized to Tubulin, then normalized to the t=0 control of wild-type Skp2.
(e) Immunoblot analysis of whole cell lysates (WCL) and immunoprecipitates (IP) derived from HeLa cells transfected with the FLAG.Skp2 construct together with the indicated siRNA oligos.
(f) Immunoblot analysis of whole cell lysates (WCL) and immunoprecipitates (IP) derived from 293T cells transfected with the indicated FLAG.Skp2 constructs.
(g) Immunoblot analysis of whole cell lysates (WCL) and immunoprecipitates (IP) derived from HeLa cells transfected with the indicated FLAG.Skp2 constructs in the presence or absence of HA.Myr.Akt.
Full-length blots are provided in the Supplementary Information, Fig. S11.
As mentioned previously, using mass spectrometry we found that in addition to Ser72 and Ser75 phosphorylation, the Ser64 site of Skp2 is also phosphorylated in vivo (Fig. 5a), and cyclin A/Cdk2 complex was indicated to be the responsible kinase29. In agreement with a recent report32, we found that phosphoryation of Skp2 by cyclin A/Cdk2 at Ser64 also stabilizes Skp2 (Supplementary information Fig. S5a–S5b). However, phosphorylation of Ser64 did not affect the interaction between Skp2 and Cdh1 in vitro (Supplementary information Fig. S5c) or in vivo (Supplementary information Fig. S4e), indicating that CyclinA/Cdk2 affects Skp2 protein stability through a different mechanism than Akt.
Because both Cyclin A/Cdk2 and Akt affect Skp2 stability, we next sought to investigate the potential connection between these two kinases. We found that neither overexpression of Myr.HA.Akt nor depletion of Akt significantly affects Cyclin A or Cdk2 expression levels or their kinase activity (Supplementary information Fig. S6a, S6b). On the other hand, inactivation of either Cyclin A and Cdk2 leads to a significant decrease of Akt activity, as illustrated by the decrease in pS473 Akt signals (Supplementary information Fig. S6c, S6d) and decreased efficiency in phosphorylating Skp2 (Fig. 7e). This indicates that Cyclin A/Cdk2 could execute their function partially through activation of the Akt kinase. However, Akt can efficiently phosphorylate a Skp2 mutant (AAAA.Skp2) which fails to interact with Cyclin A/Cdk2 complex33 (Fig. 7f) as well as Skp2 mutants where the Ser64 site is substituted with either Ala or Asp (S64A.Skp2 and S64D.Skp2) (Fig. 7g). This indicates that phosphorylation of Skp2 by Akt is independent of the phosphorylation event occurring at Skp2 Ser64.
Akt phosphorylation of Skp2 promotes its cytoplasmic translocation
Akt has been reported to play a major role in the cellular localization of many of its substrates, including p21, p27 and FOXO114–17,34. Amino acid alignment of human Skp2 with known Akt substrates revealed that Skp2 also contains a putative Nuclear Localization Sequence (NLS), and that Ser72 is located within this NLS (Fig. 8a). Immunofluorescence experiments revealed that the bulk of cellular Skp2 is localized in the nucleus in normally dividing cells. However, a mutant Skp2 deleted in the putative NLS (ΔNLS.Skp2) localized predominantly in the cytoplasm (Fig. 8b, and Supplementary information Fig. S8a–S8b). Previous studies have also indicated that, at least in the case of p21, p27 and FOXO, phosphorylation of serine and/or threonine residues adjacent to the NLS leads to a masking of the NLS mediated by the binding of 14-3-3. Similarly, we and the Pandolfi group found that the interaction between 14-3-3 and Skp2 was enhanced by activated Myr.Akt (Supplementary information Fig. S7a, S7b). Furthermore, we found that the ability to interact with 14-3-3 is largely decreased in the S72A and ΔNLS Skp2 and that the Skp2 mutant lacking the first 90 amino acids failed to interact with 14-3-3 at all (Supplementary information Fig. S7c, S7d). It has been reported previously that phosphorylation of BAD at Ser136 by Akt promotes its interaction with 14-3-3, disrupting its interaction with Bcl-XL35. However, we found that blockage of Akt-induced 14-3-3 interaction with Skp2 by the R18 peptide36 does not affect the interaction between Skp2 and Cdh1 (Supplementary information Fig. S7e, S7f).
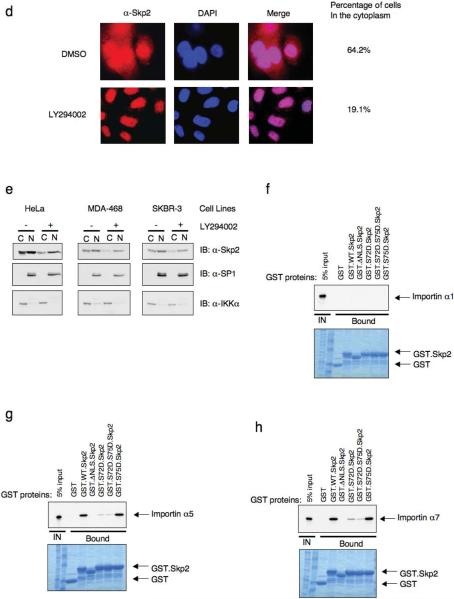
Figure 8. Akt phosphorylation of Skp2 promotes its cytoplasmic translocation.
(a) Sequence alignment of Skp2 with p27, p21 and FOXO1 Nuclear Localization Sequence.
(b) Immunofluorescence and DAPI staining of HeLa cells transfected with FLAG-wild-type or ΔNLS Skp2 plasmids. Scale bars are 20 μm.
(c) Immunofluorescence and DAPI staining of HeLa cells transfected with indicated FLAG.Skp2 in the presence or absence of HA.Myr.Akt constructs. Scale bars are 20 μm.
(d) Immunofluorescence and DAPI staining of SKBR3 cells treated with LY294002 (or DMSO as negative control) for 12 hours. Scale bars are 20 μm.
(e) Immunoblot analysis of Nuclear (N) and Cytoplasmic (C) fraction of HeLa, MDA-MB468 and SKBR3 cells treated with LY294002 (or DMSO as a negative control) for 12 hours.
(f–h) Autoradiography of 35S-labelled importin α1 (f), importin α5 (g) and importin α7 (h) bound to the indicated GST-fusion proteins.
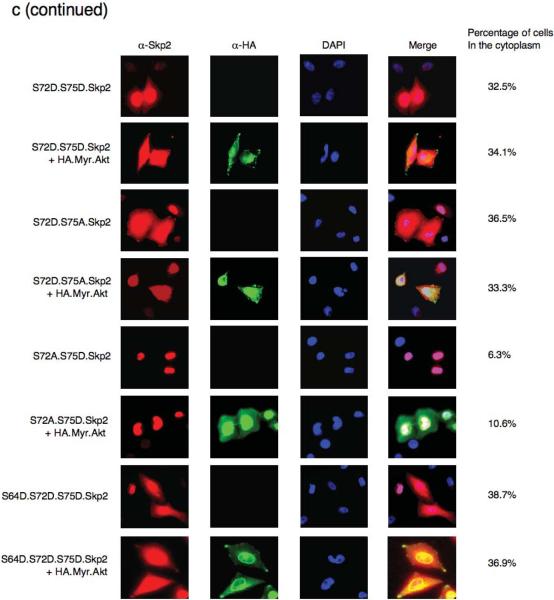
There are also documented examples where phosphorylation of serine or threonine residues near or within the NLS reduces the interaction between the NLS and the importin protein complex, thus affecting nuclear import37,38. Using both immunofluorescence microscopy and cellular fractionation, we found that expression of activated Myr.Akt promotes the cytoplasmic localization of wild-type Skp2 (Fig. 8c and Supplementary information Fig. S7g). In contrast, the non-phosphorylatable Skp2.S72A mutant is restricted to the nuclear compartment in the presence of activated Akt. Conversely, a significant fraction of the phospho-mimetic Skp2.S72D, Skp2.S72D.S75A and Skp2.S72D.S75D is located in the cytoplasm and its localization is unaffected by expression of activated Akt (Fig. 8c and Supplementary information Fig. S9). Furthermore, we found that there is a significant pool of cytoplasmic Skp2 distribution in SKBR3 cells, which harbor elevated Akt activity (Fig. 1e), and that inhibition of Akt activity by LY294002 results in translocation of Skp2 into the nucleus (Fig. 8d and 8e). We found that the Skp2 protein specifically interacts with importin α5 and α7 but not α1, and that phosphorylation of the Ser72 site by Akt is sufficient to disrupt the interaction between Skp2 and importin α5 and α7 (Fig. 8f–8h). Because the importin complex plays a critical role in transporting proteins into the nucleus, we reasoned that the dissociation of Skp2 with the importin complex would retain Skp2 in the cytoplasm. Since the Ser64 site is very close to the putative NLS, we also investigated the potential effects of Ser64 phosphorylation on Skp2 cellular localization. In agreement with previous report32, we found that the phosphorylation status of Ser64 did not affect the cellular localization of Skp2 (Supplementary information Fig. S8c). We further demonstrated that while phosphorylation of Skp2 by Akt at Ser72 abolished the interaction between Skp2 and the importin complex, phosphorylation of Ser64 was not sufficient to disrupt the interaction between Skp2 and the importin complex (Supplementary information Fig. S10a–S10c).
Discussion
The data presented above provide evidence for a novel mechanism by which Akt1-mediated phosphorylation of Skp2 at Ser72 protects Skp2 from Cdh1-mediated destruction through disruption of the interaction between Skp2 and its E3 ligase Cdh1, as well as inducing Skp2 cytoplasmic translocation. The Ser72 phosphorylation site on human Skp2 is not present in the mouse sequence. Similar inter-species differences have been reported for other Akt substrates including p2717 and caspase-939. However, the Ser72 site is conserved in most large mammals (Fig. 3a). It is plausible that for larger animals with a longer life span than mice, cell cycle control is more stringent, illustrated by the additional layer of Akt regulation on Skp2 stability.
For most SCF/F-box complexes, the regulation of substrate recognition occurs at the level of the substrate, while the interaction of Cdh1 and Cdc20 with their substrates usually does not require any post-translational modifications40. Our finding provides another unique mechanism for the selective degradation of Cdh1 downstream targets. This protective mechanism mediated by the Akt pathway is very similar to the Cdk2/cyclin E complex, which protects Cdc6 from Cdh1-mediated destruction41.
The Akt pathway functions to promote both cell survival and cell growth by inactivating many of its downstream substrates9. In the case of p27, p21 and FOXO proteins, Akt phosphorylation triggers the recruitment of 14-3-3 which results in the masking of the NLS and subsequent cytoplasmic translocation37. We also observed an enhanced interaction of 14-3-3 with Skp2 in cells expressing activated Akt. Moreover, phosphorylation of Skp2 by Akt at Ser72 greatly reduces the interaction between Skp2 and importin. It is possible that both of these mechanisms contribute to the cytoplasmic translocation of Skp2 subsequent to Akt phosphorylation42,43. Thus our results offer a molecular mechanism for Skp2 cytoplasmic localization, which has been observed in many clinical tumor samples and is correlated with aggressive malignancy and poor diagnosis3,21,22,44.
Interestingly, our data point to Akt isoform specificity in the regulation of Skp2 protein stability (Fig. 4). Furthermore, we demonstrated that when overexpressed in 293T cells, human Skp2 specifically interacts with endogenous Akt1, but not Akt2 (Fig. 3c and 3d), although the precise mechanism by which Akt1 can, whereas Akt2 cannot, signal to Skp2 has yet to be defined.
Collectively, our results provide novel insight into how Akt activity could influence the Skp2/p27 pathway, which is a known hotspot for mutations in human cancer. On one level, our finding provides a mechanism whereby Akt influences cell cycle progression. On another level, we offer a novel mechanism by which Akt affects the degradation order of specific APC/Cdh1 substrates. Ultimately, these data may provide the rationale to develop specific Akt1 inhibitors as efficient anti-cancer drugs.
Experimental Procedures
Plasmids
FLAG.Skp2, HA.Skp2 and HA.Myr.Akt1 plasmids were described previously5,45. HA.Myr.Akt2 plasmid was purchased from Addgene. The first 90 amino acids of human Skp2 protein was fused in frame with the GST protein to create the pGEX.WT.human.Skp2 construct. Mouse Skp2 cDNA was amplified from a mouse cDNA library (kind gift from Dr. Ronald Depinho) using the Pfu polymerase (Stratagene). Full-length mouse Skp2 cDNA was subcloned into the pCMV-FLAG vector (Sigma) to create the FLAG.mouse.Skp2 construct and the first 90 amino acids of mouse Skp2 was fused in frame with the GST protein to create the pGEX.WT.mouse.Skp2 construct. Skp2 mutants were generated using the QuikChange XL Site-Directed Mutagenesis Kit (Stratagene). HA.Cdh1 construct was obtained from Dr. Peter Jackson. HA.S6K.CA and HA.S6K.KD constructs were obtained from Dr. John Blennis. HA.SGK.CA construct was a kind gift from Dr. Sussanne Conzen. The importin α1, importin α5 and importin α7 plasmids were obtained from the DF/HCC DNA Resource Core.
Antibodies and Reagents
Anti-Akt antibody (9272), anti-Akt2 antibody (5B5), anti-phospho-Akt antibody (4051), and anti-phospho-Akt substrate (9614) were purchased from Cell Signaling. Anti-p27 antibody (SC-528), polyclonal anti-HA antibody (SC-805), polyclonal anti-Skp2 antibody (SC-7164), anti-cyclin A antibody (SC-751), anti-cyclin B antibody (SC-245), anti-Cdc20 antibody (SC-8358), anti-Casein Kinase I δ antibody (SC-6473), anti-14-3-3 β antibody (SC-629), anti-Geminin antibody (SC-13015), anti-Plk1 antibody (SC-17783), anti-cyclin E antibody (SC-247), anti-SP1 antibody (SC-59), anti-IKKalpha antibody (SC-7184), anti-TSC1 antibody (SC-13013) were purchased from Santa Cruz. Anti-tubulin antibody (T-5168), polyclonal anti-FLAG antibody (F2425), monoclonal anti-FLAG antibody (F-3165), peroxidase-conjugated anti-mouse secondary antibody (A4416) and peroxidase-conjugated anti-rabbit secondary antibody (A4914) were purchased from Sigma. Monoclonal anti-HA antibody (MMS-101P) was purchased from Convace. Anti-GFP antibody (632380), monoclonal anti-Skp2 antibody (32–3400) and polyclonal anti-Cdh1 antibody (34–2000) were purchased from Invitrogen. Monoclonal anti-Cdh1 (CC43) was purchased from Oncogene. Anti-Casein Kinase I ε antibody (AP7403a) was purchased from Abgent.
Polyclonal anit-Akt1 isoform-specific antibody was produced in house by immunizing rabbits with a synthetic peptide (VDSERRPHFPQFSYSASGTA). Oligofectamine, Lipofectamine and Plus reagent were purchased from Invitrogen. Recombinant human IGF-1 was purchased from R&D systems.
siRNAs
Human Akt1 siRNA oligo (sense, 5'-GAGUUUGAGUACCUGAAGCUGUU-3') and human Akt2 siRNA oligo (sense, 5'-GCGUGGUGAAUACAUCAAGACUU-3') have been validated previously20 and were purchased from Dharmacon, or sequences cloned into the pLKO lentiviral expression system and virus generated in 293T cells for infection, as described20. Mouse Akt1 and mouse Akt2 siRNA oligos have been validated by Laurie Benjamin's laboratory (data not shown), and sequences were cloned into the pLKO lentiviral expression system. PTEN-1 (sense, 5'-AGGCACAAGAGGCCCUAGA-3'), PTEN-2 (sense, 5'-AAGAGGAUGGAUUCGACUUAG-3'), PTEN-3 (sense, 5'-AUCGUUAGCAGAAACAAAAGG-3') have been validated previously6,46 and purchased from Dharmacon. Luciferase GL2 siRNA oligo was purchased from Dharmacon, and the Cdh1 siRNA oligo has been described before5. Cdk2, cyclin E and Cylin A siRNA oligos have been described previously47. As described previously, siRNA oligos were transfected into subconfluent cells using Oligofectamine or Lipofectamine 2000 (Invitrogen) according to the manufacturer's instruction5,48.
Cell culture and Cell Synchronization
Cell culture including synchronization and transfection have been described5. Where indicated, the PI 3-K inhibitor LY294002 (Sigma) or cycloheximide (Sigma) were added to the cell culture media. INK4a−/− mouse embryonic fibroblasts (MEFs) and INK4a−/−.PTENloxp/loxp MEFs were a kind gift from Dr. Ronald Depinho.
Immunoblots and Immunoprecipitation
Cells were lysed in EBC (50 mM Tris pH 8.0, 120 mM NaCl, 0.5% NP-40) buffer supplemented with protease inhibitors (Complete Mini, Roche) and phosphatase inhibitors (phosphatase inhibitor cocktail set I and II, Calbiochem). The protein concentrations of the lysates were measured using the Bio-Rad protein assay reagent on a Beckman Coulter DU-800 machine. The lysates were then resolved by SDS PAGE and immunoblotted with indicated antibodies. For immunoprecipitation, 800 μg lysates were incubated with the appropriate antibody (1–2 μg) for 3–4 hours at 4 °C followed by an one hour-incubation with Protein A sepharose beads (GE Healthcare). Immuno complexes were washed five times with NETN buffer (20 mM Tris, pH 8.0, 100 mM NaCl, 1 mM EDTA and 0.5% NP-40) before being resolved by SDS PAGE and immunoblotted with indicated antibodies. Quantification of the immunoblot band intensity was performed with ImageJ software.
Skp2 Binding Assays
Binding to immobilized GST proteins was performed as described before5.
Cellular Fractionation
The NE-PER kit (Pierce) was used to perform cellular fractionation according to the manufacturer's instruction. Buffers were supplemented with both protease inhibitor (Roche) and phosphatase inhibitors (Calbiochem).
Real-time RT-PCR Analysis
RNA was extracted using Qiagen RNeasy mini kit, and reverse transcription reaction was performed using the ABI Taqman Reverse Transcriptional Reagents (N808-0234). After mixing the resulting template with Skp2 (Hs00180634-m1) or GAPDH (Hs99999905-m1) primers and ABI Taqman Fast Universal PCR Master Mix (4352042), the real-time RT-PCR reaction was performed with the ABI-7500 Fast Real-time PCR system.
Indirect Immunofluorescence Microscopy
Cells grown on cover-slips were fixed in 4% paraformaldehyde and permeabilized with 0.2% Triton-X100. The cells were stained with polyclonal anti-HA antibody (Santa Cruz) and monoclonal anti-Skp2 antibody (Invitrogen) in blocking buffer (3% BSA/PBS) for 30 min, and then rinsed and incubated with secondary Alexa Fluor 594-conjugated anti-mouse antibody and Alexa Fluor 488-conjugated anti-Rabbit antibody (Invitrogen) for 1 hour. Cells were then rinsed with PBS, stained with DAPI and mounted. The slides were examined using a fluorescence microscope (Eclipse TE300, Nikon) and digital image analysis software (IPLab, Scanalytics).
Protein Degradation Analysis
Cells were transfected with a plasmid encoding a FLAG-tagged version of the protein of interest along with a plasmid encoding GFP as a negative control. For half-life studies, cycloheximide (20 μg/ml, Sigma) was added to the media 40 hours post-transfection. At various time points thereafter, cells were lysed and protein abundances were measured by immunoblot analysis. Where indicated, HA.Cdh1 and/or HA.Myr.Akt constructs were cotransfected into the cells to examine their effects on the abundance of the protein of interest.
In vitro Kinase Assay
293T cells were transfected with HA.Myr.Akt. 48 hours later, Akt was immunoprecipitated using HA-matrix (Roche). Afterwards, it was incubated with 5 μg of GST.Skp2 proteins (wild type or S72A mutant) in the presence of 5 μCi [γ-32P] ATP and 20 μM cold ATP in the Akt kinase reaction buffer for 15–30 minutes. The reaction was stopped by the addition of SDS-containing lysis buffer, resolved on SDS PAGE, and detected by autoradiography. Casein Kinase I was purchased from New England Biolab. The Casein Kinase I in vitro kinase assays were performed according to the manufacturer's instructions. Cdk2 kinase assay was performed as described previously47.
Supplementary Material
Supplemental Figure Legends
Supplemental Figures
Acknowledgement
We thank William Kaelin, Jr., Lewis Cantley, Roya Khosravi-Far and Susan Glueck for critical reading of the manuscript, James DeCaprio, Christoph Geisen, Ronald Depinho, Laurie Benjamin, Sussanne Conzen, John Blennis and Peter Jackson for providing reagents; Ross Tomaino for his kind assistance on the mass spectrum analysis, Isaac Robinovitz for his technical support on the fluorescence microscopy, Pier Paolo Pandolfi for sharing unpublished data, and members of the Wei and Toker labs for useful discussions. Wenyi Wei is a Leukemia and Lymphoma Society Special Fellow, Kimmel Scholar and V Scholar. This work was supported in part by the Harvard Medical School Milton Fund (W.W.) and the Emerald Foundation, and by grants from the National Institutes of Health (W.G.K., CA076120; A.T., CA122099) and the Susan G. Komen Breast Cancer Foundation (R.C. 0706963).
References
- 1.Cardozo T, Pagano M. The SCF ubiquitin ligase: insights into a molecular machine. Nat Rev Mol Cell Biol. 2004;5:739–51. doi: 10.1038/nrm1471. [DOI] [PubMed] [Google Scholar]
- 2.Gstaiger M, et al. Skp2 is oncogenic and overexpressed in human cancers. Proc Natl Acad Sci U S A. 2001;98:5043–8. doi: 10.1073/pnas.081474898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Signoretti S, et al. Oncogenic role of the ubiquitin ligase subunit Skp2 in human breast cancer. J Clin Invest. 2002;110:633–41. doi: 10.1172/JCI15795. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Bashir T, Dorrello NV, Amador V, Guardavaccaro D, Pagano M. Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature. 2004;428:190–3. doi: 10.1038/nature02330. [DOI] [PubMed] [Google Scholar]
- 5.Wei W, et al. Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature. 2004;428:194–8. doi: 10.1038/nature02381. [DOI] [PubMed] [Google Scholar]
- 6.van Duijn PW, Trapman J. PI3K/Akt signaling regulates p27(kip1) expression via Skp2 in PC3 and DU145 prostate cancer cells, but is not a major factor in p27(kip1) regulation in LNCaP and PC346 cells. Prostate. 2006;66:749–60. doi: 10.1002/pros.20398. [DOI] [PubMed] [Google Scholar]
- 7.Andreu EJ, et al. BCR-ABL induces the expression of Skp2 through the PI3K pathway to promote p27Kip1 degradation and proliferation of chronic myelogenous leukemia cells. Cancer Res. 2005;65:3264–72. doi: 10.1158/0008-5472.CAN-04-1357. [DOI] [PubMed] [Google Scholar]
- 8.Mamillapalli R, et al. PTEN regulates the ubiquitin-dependent degradation of the CDK inhibitor p27(KIP1) through the ubiquitin E3 ligase SCF(SKP2) Curr Biol. 2001;11:263–7. doi: 10.1016/s0960-9822(01)00065-3. [DOI] [PubMed] [Google Scholar]
- 9.Woodgett JR. Recent advances in the protein kinase B signaling pathway. Curr Opin Cell Biol. 2005;17:150–7. doi: 10.1016/j.ceb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Parsons R. Human cancer, PTEN and the PI-3 kinase pathway. Semin Cell Dev Biol. 2004;15:171–6. doi: 10.1016/j.semcdb.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Datta SR, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 12.Zhao X, et al. Multiple elements regulate nuclear/cytoplasmic shuttling of FOXO1: characterization of phosphorylation- and 14-3-3-dependent and -independent mechanisms. Biochem J. 2004;378:839–49. doi: 10.1042/BJ20031450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunet A, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 14.Zhou BP, et al. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol. 2001;3:245–52. doi: 10.1038/35060032. [DOI] [PubMed] [Google Scholar]
- 15.Viglietto G, et al. Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27(Kip1) by PKB/Akt-mediated phosphorylation in breast cancer. Nat Med. 2002;8:1136–44. doi: 10.1038/nm762. [DOI] [PubMed] [Google Scholar]
- 16.Liang J, et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med. 2002;8:1153–60. doi: 10.1038/nm761. [DOI] [PubMed] [Google Scholar]
- 17.Shin I, et al. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med. 2002;8:1145–52. doi: 10.1038/nm759. [DOI] [PubMed] [Google Scholar]
- 18.Whiteman EL, Cho H, Birnbaum MJ. Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab. 2002;13:444–51. doi: 10.1016/s1043-2760(02)00662-8. [DOI] [PubMed] [Google Scholar]
- 19.Heron-Milhavet L, et al. Only Akt1 is required for proliferation, while Akt2 promotes cell cycle exit through p21 binding. Mol Cell Biol. 2006;26:8267–80. doi: 10.1128/MCB.00201-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irie HY, et al. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J Cell Biol. 2005;171:1023–34. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drobnjak M, et al. Altered expression of p27 and Skp2 proteins in prostate cancer of African-American patients. Clin Cancer Res. 2003;9:2613–9. [PubMed] [Google Scholar]
- 22.Radke S, Pirkmaier A, Germain D. Differential expression of the F-box proteins Skp2 and Skp2B in breast cancer. Oncogene. 2005;24:3448–58. doi: 10.1038/sj.onc.1208328. [DOI] [PubMed] [Google Scholar]
- 23.Shapira M, Kakiashvili E, Rosenberg T, Hershko DD. The mTOR inhibitor rapamycin down-regulates the expression of the ubiquitin ligase subunit Skp2 in breast cancer cells. Breast Cancer Res. 2006;8:R46. doi: 10.1186/bcr1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reichert M, Saur D, Hamacher R, Schmid RM, Schneider G. Phosphoinositide-3-kinase signaling controls S-phase kinase-associated protein 2 transcription via E2F1 in pancreatic ductal adenocarcinoma cells. Cancer Res. 2007;67:4149–56. doi: 10.1158/0008-5472.CAN-06-4484. [DOI] [PubMed] [Google Scholar]
- 25.Barre B, Perkins ND. A cell cycle regulatory network controlling NF-kappaB subunit activity and function. Embo J. 2007;26:4841–55. doi: 10.1038/sj.emboj.7601899. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Zhou BP, et al. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol. 2001;3:973–82. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, et al. Phosphoprotein analysis using antibodies broadly reactive against phosphorylated motifs. J Biol Chem. 2002;277:39379–87. doi: 10.1074/jbc.M206399200. [DOI] [PubMed] [Google Scholar]
- 28.Hong F, et al. mTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol Cell. 2008;30:701–11. doi: 10.1016/j.molcel.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 29.Yam CH, Ng RW, Siu WY, Lau AW, Poon RY. Regulation of cyclin A-Cdk2 by SCF component Skp1 and F-box protein Skp2. Mol Cell Biol. 1999;19:635–45. doi: 10.1128/mcb.19.1.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran H, Brunet A, Griffith EC, Greenberg ME. The many forks in FOXO's road. Sci STKE. 2003;2003:RE5. doi: 10.1126/stke.2003.172.re5. [DOI] [PubMed] [Google Scholar]
- 31.Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999;1:193–9. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 32.Rodier G, Coulombe P, Tanguay PL, Boutonnet C, Meloche S. Phosphorylation of Skp2 regulated by CDK2 and Cdc14B protects it from degradation by APC(Cdh1) in G1 phase. Embo J. 2008;27:679–91. doi: 10.1038/emboj.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji P, et al. Skp2 contains a novel cyclin A binding domain that directly protects cyclin A from inhibition by p27Kip1. J Biol Chem. 2006;281:24058–69. doi: 10.1074/jbc.M603105200. [DOI] [PubMed] [Google Scholar]
- 34.Vogt PK, Jiang H, Aoki M. Triple layer control: phosphorylation, acetylation and ubiquitination of FOXO proteins. Cell Cycle. 2005;4:908–13. doi: 10.4161/cc.4.7.1796. [DOI] [PubMed] [Google Scholar]
- 35.Chiang CW, et al. Protein phosphatase 2A dephosphorylation of phosphoserine 112 plays the gatekeeper role for BAD-mediated apoptosis. Mol Cell Biol. 2003;23:6350–62. doi: 10.1128/MCB.23.18.6350-6362.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang B, et al. Isolation of high-affinity peptide antagonists of 14-3-3 proteins by phage display. Biochemistry. 1999;38:12499–504. doi: 10.1021/bi991353h. [DOI] [PubMed] [Google Scholar]
- 37.Poon IK, Jans DA. Regulation of nuclear transport: central role in development and transformation? Traffic. 2005;6:173–86. doi: 10.1111/j.1600-0854.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- 38.Fabbro M, Henderson BR. Regulation of tumor suppressors by nuclear-cytoplasmic shuttling. Exp Cell Res. 2003;282:59–69. doi: 10.1016/s0014-4827(02)00019-8. [DOI] [PubMed] [Google Scholar]
- 39.Fujita E, et al. Akt phosphorylation site found in human caspase-9 is absent in mouse caspase-9. Biochem Biophys Res Commun. 1999;264:550–5. doi: 10.1006/bbrc.1999.1387. [DOI] [PubMed] [Google Scholar]
- 40.Harper JW, Burton JL, Solomon MJ. The anaphase-promoting complex: it's not just for mitosis any more. Genes Dev. 2002;16:2179–206. doi: 10.1101/gad.1013102. [DOI] [PubMed] [Google Scholar]
- 41.Mailand N, Diffley JF. CDKs promote DNA replication origin licensing in human cells by protecting Cdc6 from APC/C-dependent proteolysis. Cell. 2005;122:915–26. doi: 10.1016/j.cell.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 42.Hennekes H, Peter M, Weber K, Nigg EA. Phosphorylation on protein kinase C sites inhibits nuclear import of lamin B2. J Cell Biol. 1993;120:1293–304. doi: 10.1083/jcb.120.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang F, White RL, Neufeld KL. Cell density and phosphorylation control the subcellular localization of adenomatous polyposis coli protein. Mol Cell Biol. 2001;21:8143–56. doi: 10.1128/MCB.21.23.8143-8156.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dowen SE, Scott A, Mukherjee G, Stanley MA. Overexpression of Skp2 in carcinoma of the cervix does not correlate inversely with p27 expression. Int J Cancer. 2003;105:326–30. doi: 10.1002/ijc.11066. [DOI] [PubMed] [Google Scholar]
- 45.Yoeli-Lerner M, et al. Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol Cell. 2005;20:539–50. doi: 10.1016/j.molcel.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 46.Hamada K, et al. The PTEN/PI3K pathway governs normal vascular development and tumor angiogenesis. Genes Dev. 2005;19:2054–65. doi: 10.1101/gad.1308805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang GJ, et al. Bioluminescent imaging of Cdk2 inhibition in vivo. Nat Med. 2004;10:643–8. doi: 10.1038/nm1047. [DOI] [PubMed] [Google Scholar]
- 48.Wei W, Jin J, Schlisio S, Harper JW, Kaelin WG., Jr. The v-Jun point mutation allows c-Jun to escape GSK3-dependent recognition and destruction by the Fbw7 ubiquitin ligase. Cancer Cell. 2005;8:25–33. doi: 10.1016/j.ccr.2005.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure Legends
Supplemental Figures