Gliogenesis and Glial Pathology in Depression (original) (raw)
. Author manuscript; available in PMC: 2010 Aug 10.
Published in final edited form as: CNS Neurol Disord Drug Targets. 2007 Jun;6(3):219–233. doi: 10.2174/187152707780619326
Abstract
Recent research has changed the perception of glia from being no more than silent supportive cells of neurons to being dynamic partners participating in brain metabolism and communication between neurons. This discovery of new glial functions coincides with growing evidence of the involvement of glia in the neuropathology of mood disorders. Unanticipated reductions in the density and number of glial cells are reported in fronto-limbic brain regions in major depression and bipolar illness. Moreover, age-dependent decreases in the density of glial fibrillary acidic protein (GFAP) - immunoreactive astrocytes and levels of GFAP protein are observed in the prefrontal cortex of younger depressed subjects. Since astrocytes participate in the uptake, metabolism and recycling of glutamate, we hypothesize that an astrocytic deficit may account for the alterations in glutamate/GABA neurotransmission in depression. Reductions in the density and ultrastructure of oligodendrocytes are also detected in the prefrontal cortex and amygdala in depression. Pathological changes in oligodendrocytes may be relevant to the disruption of white matter tracts in mood disorders reported by diffusion tensor imaging. Factors such as stress, excess of glucocorticoids, altered gene expression of neurotrophic factors and glial transporters, and changes in extracellular levels of neurotransmitters released by neurons may modify glial cell number and affect the neurophysiology of depression. Therefore, we will explore the role of these events in the possible alteration of glial number and activity, and the capacity of glia as a promising new target for therapeutic medications. Finally, we will consider the temporal relationship between glial and neuronal cell pathology in depression.
Glial cells were first identified as non-neuronal elements in the nineteenth century by the anatomist R. Virchow (after [1]). At that time glia were thought to be no more than silent supportive “glue” for neurons and that glia were unable to participate in information processing. In the past two decades, research has changed this perception and provided evidence for glia being important dynamic partners of neuronal cells actively participating in brain metabolism, synaptic neurotransmission and communication between neurons [2]. The discovery of new glial functions coincides with growing evidence for the involvement of glia in the neuropathology of neurological [3] and more recently, psychiatric disorders, such as major depression and manic-depressive (bipolar) illness (reviewed in [4])
TYPES OF GLIAL CELLS
Glia are the most numerous cells in the human brain, outnumbering neurons by a ratio of ten to one [5] (Fig. 1). This ratio drops to one-to-one in rodents [5, 6] implying that increases in glia over neurons are associated with the progressive development of higher brain functions [7]. Although anecdotal, it is interesting that in the brain of Albert Einstein, the neuron/glia ratio in the associational parieto-temporal cortical area 39 was found to be smaller (suggesting increases in glial numbers) [3]. An enlargement of astroglial intralaminar processes was also observed in few areas of parietal cortex the brain of the genius as compared to several age-matched control brains [9].
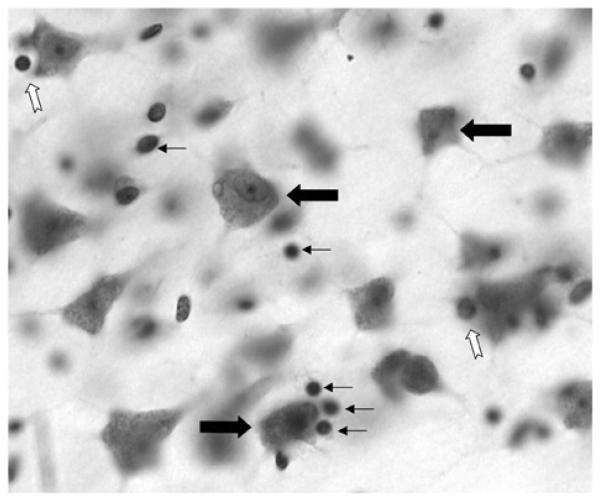
Fig. (1).
Photomicrograph showing a Nissl stained 40 μm thick section from the dorsolateral prefrontal cortex of human postmortem brain. Large black arrows indicate neuronal cell bodies. Small black arrows show the location of glial cells and the white arrows display examples of perineuronal oligodendrocytes. The image was taken using the 40x immersion oil objective of a Nikon Eclipse E600 microscope.
Glia of the Central Nervous System (CNS), collectively called neuroglia, can be divided into three main types: astrocytes and oligodendrocytes (both of ectodermal origin and together called macroglia) and microglia (originating from monocyte-macrophage lineage) [10]. Each type of glia is characterized by specific morphology and functions and they can be distinguished from each other by immunohistochemistry, metallic impregnation methods or electron microscopy.
The three types of CNS glial cells play a crucial role in the proper functioning of neurons. Some glial functions are more specialized and restricted to one morphological type of glia (e.g., oligodendrocytes produce myelin to insulate axons), while other functions are shared by two or three types of glia (e.g. both astrocytes and microglia participate in the response to neuronal injury and secrete neurotrophic factors). The functions of astroglia are the most complex among glial cells, and therefore most of this review will be devoted to astrocytic functions, and particularly those that may be relevant to the pathophysiology of depression.
ASTROCYTES
Astrocytes or astroglia are the most numerous and versatile type of glial cell. In the cerebral cortex of the rat, about 90% of cortical tissue volume is made up by astrocytes, whereas the remaining 10% consists of neuronal cell bodies and blood vessels [11]. Two different forms of astrocytes which differ morphologically and biochemically are found in both gray and white matter of the cerebral cortex [12]. Protoplasmic astrocytes predominate in gray matter, whereas fibrous astrocytes are abundant in white matter. Protoplasmic astrocytes have large spherical nuclei (exceeding the size of oligodendrocyte and microglia nuclei) and multiple thick processes radiating from the cell body [13] (Fig. 2A). In contrast to protoplasmic astrocytes, fibrous astrocytes have ovoid nuclei and fewer, longer and relatively thin processes with very few branches, as compared to the protoplasmic ones (Fig. 2B). The processes of both astrocytic types are often attached to the blood vessels and form expansions called end-feet, which envelop blood vessels and form a limiting membrane [10]. Astrocytes thereby play a central role in building and maintaining the blood-brain barrier (which is formed by tight junctions between the endothelial cells of capillaries) to restrict the passage of soluble molecules and toxic substances from the blood to the brain [14].
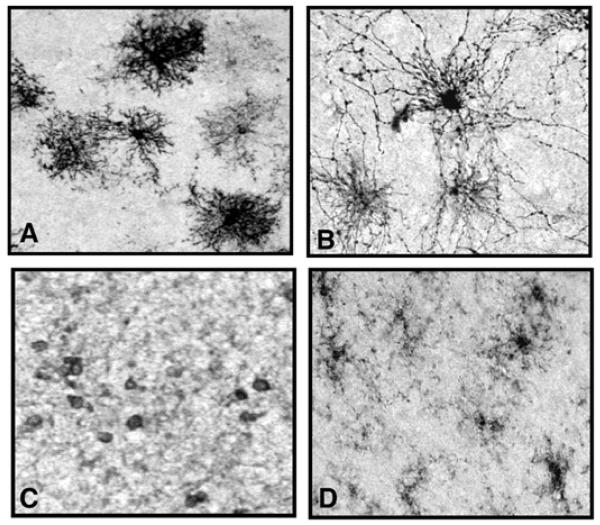
Fig. (2).
Photomicrographs displaying the different types of immunopositive neuroglia in the prefrontal cortex of a psychiatrically normal human subject. Images A and B show two types of astroglia both immunostained with an anti-GFAP (glial fibrillary acid protein) antibody which is a marker for reactive astroglia. Protoplasmic astrocytes (A) are found mainly in cortical gray matter, and fibrous astrocytes (B) are found mainly in cortical white matter. Image C displays oligodendrocytes in cortical white matter which are immunostained with anti CNPase antibody, a marker for mature oligodendrocytes. Image D illustrates microglia in white matter immunostained for anti LFA-1 antibody, a marker of active microglia. All photomicrographs were taken with the 20x objective of a Nikon Eclipse E600 microscope.
Glucose metabolism
Astrocytic end-feet are enriched with glucose transporters and envelope virtually all capillary walls in the brain to permit astrocytes to take up glucose, the main energy substrate for neurons and glia. In astrocytes, glucose undergoes the process of glycolysis and oxidative phosphorylation, processes that are believed to provide the observed signal in functional magnetic resonance imaging and positron emission tomography [15-17]. Astroglia are also able to control blood flow requirements by inducing local vasoconstriction or vasodilation responses [18, 19]. Thus, defective or missing astrocytes may compromise brain function and structure as reported by in vivo neuroimaging studies in depressed patients.
Neurotransmitter modulation
Cell culture and in vivo studies reveal that astrocytes express virtually all of the receptor systems, and ion channels found in neurons [20-30]. Although alterations in the density of monoaminergic receptors (reviewed in [31]) and subunits of the NMDA receptor [32, 33] are reported in depression, no studies to date have investigated whether neuronal versus glial receptors are involved in these changes in receptor binding. Astrocytes modulate extraneuronal levels of neurotransmitters via specialized transporters located on astrocytic processes. These transporter systems regulate both the availability and reuptake of neurotransmitters at the synapse [34]. Studies in primary astrocyte cultures and in vivo studies demonstrate that these glia are able to take up glutamate [35-38], GABA [39-42], serotonin [43, 44], dopamine [45], norepinephrine [45, 46] and histamine [47] and they contain the enzymes (i.e., monoamine oxidase (MAO), or catechol-O- methyl transferase (COMT) [48-50], required for further regulation of the extracellular concentrations of these neurotransmitters. Moreover, the ability of cultured astrocytes to take up serotonin can be blocked by fluoxetine or paroxetine [43, 51], suggesting that glia along with neurons may participate in the therapeutic effects of these compounds in depression. Astrocytes thereby constitute a crucial part of the tripartite synapse - presynaptic neuron (axonal terminal), astrocytic processes, postsynaptic neuron (dendritic spine) - and promote efficient signaling between neurons [52].
Glutamate neurotransmission
Astroglia are actively involved in the uptake, metabolism and recycling of glutamate. Through glutamate uptake, astrocytes are able to regulate the level of extracellular glutamate through a specialized transporter, the glial glutamate transporter (GLT-1) in rodents. The rodent GLT-1 is homologous to the excitatory amino acid transporter-2 (EAAT-2) in the human brain [36, 37]. The glial glutamate transporter is located only on astrocytes and is highly expressed in the gray matter of the cerebral cortex [35, 36, 38]. Strikingly, 90% of total glutamate uptake involves GLT-1 [53-56]. Since an abnormally elevated concentration of extracellular glutamate leads to neuronal death, astrocytes appear to play a key role in neuroprotection by decreasing extracellular levels of glutamate [3, 57]. Indeed, decreased levels of extracellular glutamate protect neurons from cell death in mixed cell cultures of astrocytes and neurons [58]. Further evidence of a protective role for astrocytes in the uptake of glutamate is revealed in studies where a deletion of the glial glutamate transporter GLT-I gene in mice results in excitotoxic damage [21], and an antisense knockdown of the glial (but not neuronal) glutamate transporter in rat brain exacerbates neuronal damage [58, 59]. In addition, loss of astrocytic GFAP was shown to decrease both, glial and neuronal glutamate transporters indicating the role of GFAP in regulating the level of glutamate [60].
The metabolism and recycling of glutamate is a further contribution of astrocytes to excitatory amino acid neurotransmission. After its uptake by astrocytes, glutamate is converted to glutamine by the astrocytic specific enzyme, glutamine synthetase [61]. Glutamine is then released by the astrocytes, and taken up by the neuronal terminals where it is reconverted to glutamate, and to GABA for the replenishment of pools of these neurotransmitters [62]. The glutamate-glutamine cycle between neurons and glia is a major metabolic flux reflecting the synaptic release of glutamate [63]. Evidence for potential dysfunction of this cycle in depression comes form recent neuroimaging and postmortem human studies. The use of magnetic resonance spectroscopy reveals altered levels of cortical glutamate and GABA in patients with major depression [64, 65]. This in vivo evidence of altered amino acid neurotransmitters in cerebral cortex in depression may be related to observations of reduced density of astrocytes, pyramidal glutamatergic neurons and GABAergic interneurons detected in sections from postmortem prefrontal cortex of subjects with major depression [66-69]. Finally, decreases in the expression of mRNA for the astrocytic glutamate markers, glial glutamate transporter and glutamine synthetase were observed in postmortem brain tissue from subjects with major depression [70]. Hence, in depression astrocytic glutamate pathology appears to be linked to reductions in the density of astroglial cells and GFAP (see glia/glutamate model of depression below).
Another aspect of astrocytic involvement in glutamate neurotransmission is participation in NMDA receptor activity. Astrocytic foot processes are the exclusive source of Dserine, which is an important endogenous ligand for the co-activation of the NMDA receptor [71, 72]. D-serine is an agonist of the glycine site of NMDA receptors and it is considered to be greater than three times more potent as glycine at that site [73]. Thus, astrocytes may regulate NMDA receptor activity through regulating the amount of D-serine that is available [74]. NMDA receptor subunits are also located on distal astrocytic processes and occasionally on astrocytic cell bodies in cerebral cortex in rodents and humans [75-77]. Interestingly, recent studies in postmortem brain tissue from subjects with major depression report alterations in specific subunits of NMDA receptors [32], however, it is not known whether these changes involve the neuronal or glial NMDA receptor. The potential clinical relevance of these basic research findings comes from a new study reporting that ketamine, an NMDA-receptor antagonist, may be a promising medication for treatment-resistant depression [78].
Brain signaling and Synaptic Plasticity
Although there is no direct evidence that glia are involved in electrical signaling, compelling studies over the past 15 years suggest that astrocytes are 'excitable', in the sense that, when activated by internal or external signals, they deliver specific messages to neighbouring neurons, glia and blood vessels [79, 80]. For example, under high frequency stimulation, glutamate can activate astrocytic glutamatergic receptors leading to a rise in the intracellular concentration of astrocytic Ca++. Increased astrocytic Ca++ levels can promote Ca++ waves in adjacent astrocytes connected through gap junctions. Such connections permit the spread of modulatory signals to neighboring synapses and may provide the basis for synaptic plasticity [81].
Astrocytes also play an apparently crucial role in promoting the in vitro development and modeling of synapses [62] (for further references see [82]). The addition of astroglia-conditioned medium to cultures of CNS neurons from rats increases the number of functionally mature synapses by sevenfold [83], whereas the removal of astrocytic media from neurons yields a decrease of synaptic contacts [84].
Immune Response, Neurodegeneration and Neurotrophic Factors
Astrocytes appear to play a role in inflammatory and neurodegenerative processes. Reactive astrocytes along with microglia and oligodendrocytes are sources and targets of various inflammatory cytokines (e.g., interleukins, tumor necrosis factor), which are expressed under pathological conditions and involved in the regulation of neuroinflammation and immune and tissue repair processes [85].
Astrocytes and microglia actively respond to neuronal injury, and thereby participate in the neurodegeneration process. In response to injury astrocytes become activated and increase their number and size and the expression of their cytoskeletal protein, glial fibrilary acidic protein (GFAP) [86, 87]. Activated astrocytes also synthesize and release neurotrophic factors, such as nerve growth factor (NGF), brain derived neurotrophic factor (BDNF), glial derived neurotrophic factor (GDNF), fibroblast growth factor 2 (FGF2) and neurotrophins 3 and 4/5 [88-92] (for further review see [93]). These growth factors are important for neuronal survival, growth and differentiation, and the enhancement of synaptic plasticity and synaptic efficiency. Moreover, reactive astrocytes are a source of factors involved in glia-mediated angiogenesis, neurogenesis and migration of neuronal precursors (e.g., endothelins, vascular endothelial growth factor (VEGF) and angiopeietin 1and 2 [94]. The withdrawal of neurotrophic factors, potentially from glial sources, is associated with vulnerability to cell death or damage [88, 93]. Preclinical and human postmortem studies strongly suggest that deficienciens in neurotrophic factors (e.g. BDNF, FGF2) and impairments of neuroplasticity may be crucial in the pathophysiology of depression [92, 95-98]. Glial cells are suggested as potential new targets for the action of antidepressant treatments since repeated administration of antidepressant medications and electroconvulsive seizure increases the expression of BDNF and other neurotrophic and angiogenic factors synthesized by glia (in addition to neurons) [92, 96, 97, 99-102].
Estrogen has been proposed as another neuroprotective factor in the CNS [103-105]. Neuroprotection by estrogen can be mediated directly by neurons or indirectly by glial cells [105-107]. Estrogen receptors have been detected in vivo in astrocytes [108, 109] and oligodendrocytes [110] and in primary cultures of microglia [111]. A recent cell culture study reveals that estrogen can increase the expression of astrocytic glutamate transporters (via nuclear estrogen receptors), thereby decreasing extracellular levels of glutamate and potentially preventing neuronal death related to high levels of glutamate [112]. Moreover, up-regulation of mRNA and protein level of glutamine synthetase (an astrocyte-specific enzyme which converts glutamate to glutamine) by estradiol (one of the estrogens) was observed in the hypothalamus and hippocampus of ovariectomised mice suggesting the important role of astroglia in hormonal modulation of glutamatergic neurotransmission. Thus, the deficit in astrocytes reported by postmortem cell counting studies in depression could contribute to impaired estrogen-mediated neuroprotection. Other sex hormones may also be involved in this impairement as astrocytes express receptors for androgens (e.g., testosterone) [113-115].
OLIGODENDROCYTES
Oligodendrocytes or oligodendroglia are smaller than astrocytes and have fewer, less branched processes [13]. Oligodendrocytes are found in both gray and white matter of the CNS. There are two main types of cortical oligodendrocytes: 1) perineuronal oligodendrocytes which are found in gray matter in close proximity to neuronal cell bodies and sometimes appear as if embedded in the neuronal cytoplasm when observed in Nissl-stained material, (see white arrows in Fig. 1 and 2) interfascicular oligodendrocytes found in the white matter (Fig. 2C). Interfascicular oligodendrocytes ensheath unmyelinated fibers with myelin forming rows between bundles of axons and isolating different neuronal pathways. It is noteworthy to mention that a new type of non-myelinating oligodendrocytes (so-called NG-2 glia or synantocytes) has been found in the gray and white matter of adult CNS. During brain development precursor cells for mature oligodendrocytes are NG-2 positive thus, suggesting that NG-2 glia may encompass a population of oligodendrocyte precursor cells. The NG-2 glia participate in degenerative and demyelination processes of the CNS (reviewed in [116]). Moreover, oligodendrocyte precursor cells in the hippocampus receive glutamatergic synapses from neighbouring pyramidal neurons thus, likely participating in fast excitatory neuro-transmission [116, 117]. Other functions of NG-2 glia are yet to be established.
Mature oligodendrocytes promote myelination of axons in the CNS and express myelin related genes. A new mechanism has been suggested for myelination: neuronal activity promotes myelination of axons by mature oligodendrocytes at a late stage of development and possibly into adult life [118]. This activity-dependent mechanism of myelination in adults might constitute an unforeseen form of CNS plasticity. Both perineuronal and interfascicular oligodendrocytes, as with astrocytes, produce neurotrophic factors and cytokines involved in anti-inflammatory and axonal repair processes [85, 93].
Oligodendrocytes express various subtypes of glutamate receptors [20], among them the NMDA receptors [28], which are expressed in oligodendrocytic myelinating processes and are activated in certain pathological conditions [119]. In ischemia, interestingly, antagonists at NMDA receptors slow the loss of action potentials in white matter and reduce damage to white matter [120, 121]. Susceptible populations of oligodendrocyte progenitors have been associated with predisposition to, and with the extent of, white matter damage [122]. Moreover, developmental alteration of immature oligodendrocytes may play a key role in the proper maturation of cerebral white matter tracts [123]. In elderly subjects with depression, the detection of increased density and size of so-called hyperintensities in frontal lobe white matter (reviewed in [124, 125]) suggests that the pathology of oligodendrocytes observed in postmortem tissue in depression may be related to disruptions of white matter tracts reported by recent diffusion tensor imaging studies in depressed subjects [126].
MICROGLIA
Microglial cells are distinguished from other types of glial cells by the presence of small nuclei surrounded by scant cytoplasm and short, tortuous processes [13] (Fig. 2D). Unlike other cell types in the CNS, microglia are ontogenetically related to the mononuclear phagocyte lineage and are uniquely capable of rapid activation in response to any pathological changes in the CNS [10]. Microglia are the macrophages of the brain and they respond quickly to any changes in the structural integrity of the brain (e.g. head injury, infection) (after [127]). Activated microglia proliferate and migrate to the site of brain injury where they became highly phagocytic toward damaged neurons or myelin. In addition to their phagocytic function, microglia respond to any imbalances in ion homeostasis and also participate in the immune response. It is noteworthy that stress and glucocorticoids induce a pro-inflammatory response in microglia [128] – in light of evidence that the hypothalamic-pituitary-adrenal axis is activated in many with depression, cortisol may modulate microglia in stress-related brain disorders. Microglia express cytokines and neurotrophic factors, as do astrocytes, and microglia apprear to participate in neuroprotection [85]. On the other hand, microglia produce a variety of toxic molecules (e.g. superoxide anions, hydroxyl radicals, hydrogen peroxidase, nitric oxide) that may damage neurons or other glial cells [129, 130]. In sum, the proper number and activity of microglial cells is also crucial for integrity of the CNS.
GLIAL CELL PATHOLOGY IN DEPRESSION
As outlined above, an impairment of several glial functions (glutamate metabolism, deficiency in neurotrophic and angiogenic factors, disturbed myelination) are likely to contribute to the pathophysiology of depression. Altered number and morphology of glial cells described below, may be the cause or consequence of glial dysfuction in mood disorders.
Glial cell number
Several cell counting studies in postmortem brain tissue of subjects diagnosed with major depressive disorders (MDD) and/or manic-depressive (bipolar) disease (BPD) consistently report prominent decreases in the packing density and number of the general population of Nissl-stained glial cells. Moreover, alterations in the size and ultrastructure of glial nuclei are also detected in depressed subjects as compared to age-matched non-psychiatric controls. This glial pathology is suggestive of a loss of glial cells and it is observed in specific fronto-limbic brain regions such as, the subgenual region of the anterior cingulate cortex (ventral part of Brodmann's area 24) [131], dorsolateral prefrontal cortex (Brodmann's area 9) [132, 133] supragenual anterior cingulate cortex, dorsal part of area 24 [134] and orbitofrontal cortex (area 47) [133]. The opposite pattern, i.e., an increase in glial cell density has been reported in hippocampal CA subfields and in the granule cell layer of the dentate gyrus in subjects with MDD [135]. Increases in glial cell packing density detected postmortem in MDD implicate a reduction in glial processes rather than loss of glial cells and they may be related to decreases in hippocampal volume noted by neuroimaging studies in MDD. Glial cell pathology in mood disorders may not be universally noted throughout the cerebral cortex. Changes in glial cell density or number were not found in the sensorimotor cortex in either MDD or BPD populations [131]. Recent reports suggest a lack of marked glial pathology in the supragenual part of the anterior cingulate cortex [136] and the entorhinal cortex in BPD and MDD [137], as well as in the most rostral part of the orbitofrontal cortex corresponding to the transitional cortex between Broadmann's areas 10 and 47 in MDD subjects [133]. Glial pathology in mood disorders has not been systematically studied in subcortical structures to date. Only one study reports a significant reduction in glial number in the amygdala in MDD and unmedicated BPD subjects [137].
Glial cell size
Glial cell size and shape, in addition to density, appear to be affected in mood disorders. Size of glial cell bodies (corresponding to glial cell nuclei in Nissl stained material) was to date, estimated in six different studies. In three of these investigations glial size was found to be increased [133, 136, 138], whereas three other studies found glial size to be unchanged in MDD or BPD [132, 135, 139]. Significant increases in glial size were observed in the dorsolateral prefrontal cortex in BPD [138] and to a smaller degree in MDD subjects [133] and in the anterior cingulate cortex in MDD subjects [136] as compared to non-psychiatric controls. Furthermore, in the dorsolateral prefrontal cortex in BPD there were also changes in the shape of glial nuclei to a less rounded conformation [138]. Reductions in glial density paralleled by an increase in the size of glial nuclei suggest that some compensatory mechanisms might be taking place. It could be speculated that decreased density of glial cells is indicative of a decrease in the number of normally functioning glial cells. At the same time glial cells that survive and are not damaged might be forced to work harder to support the metabolic needs of the surrounding neurons. As a consequence, the nuclei of these glial cells are enlarged in size and changed in shape. Glutamate-induced swelling of astroglia reported in animal cell cultures [48] could also account for enlarged glial cells in depression. Interestingly, enlargement in sizes of glial nuclei seem to be specific to depressive disorders as no change in glial sizes are reported in schizophrenia [140, 141] and smaller glial sizes are observed in postmortem brain tissue from alcoholics with and without depression [142].
ALTERATIONS IN ASTROCYTES AND OLIGODENDROCYTES
All of the above mentioned studies on glial deficit in depression were conducted on a general population of Nissl-stained glial cells which does not permit a confident distinction between different morphological glial types. Examination of different distinctive CNS glial cell types with specific immunohistochemistry demonstrates the presence of pathology in astrocytes and oligodendrocytes in major depression and bipolar disorder. Microglia have not yet been studied systematically in mood disorders. Our recent preliminary study of immunoreactive microglia stained with LFA-1 antibody (lymphocyte function associated antigen 1) in the white matter of the orbitofrontal cortex suggests no difference in the area fraction covered by immunopositive microglia in elderly depressed subjects as compared to age-matched non-depressed controls [143]. Whether the same is true for prefrontal gray matter of orbitofrontal cortex and younger depressed subjects remains to be determined.
Oligodendrocytes
The involvement of oligodendrocytes in general glial pathology is suggested by a number of human postmortem studies. Reductions in the level of immunoreactive myelin basic protein (MBP), a marker for mature oligodendrocytes, were found in homogenates from the anterior frontal cortex of depressesd subjects [144]. In addition, ultrastructural changes in oligodendrocytes were observed in the dorsolateral prefrontal and anterior frontal cortex in MDD and BPD subjects [145, 146]. Moreover, a reduction in the expression of key oligodendrocyte-related and myelin-related genes was recently reported in the temporal cortex in MDD [147] and in the dorsolateral prefrontal cortex in BPD [148]. Reduced density of oligodendrocytes has been suggested in the amygdala of MDD, and unmedicated BPD patients, however in this study, the distinction between oligodendroglia and other glial cell types was made solely based on general Nissl-staining rather than specific immunohistochemistry [137]. Thus, it is reasonable to propose that reductions in the volume of frontal white matter, enlargement of vascular lesions in frontal and periventricular white matter and changes in diffusion anisotropy of frontal white matter tracts reported in elderly subjects with MDD and BPD may be related to oligodendrocyte deficit.
Astrocytes
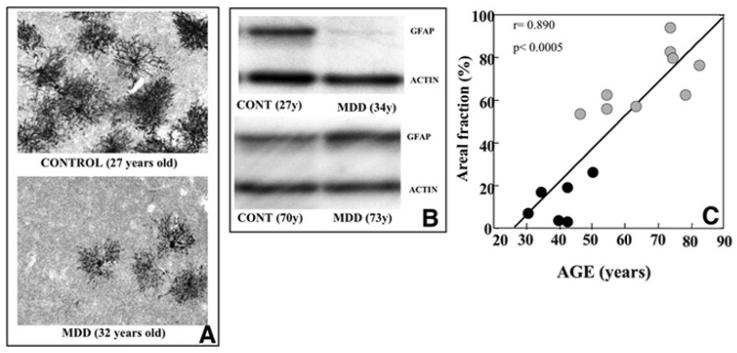
Several postmortem studies report alterations in the packing density and protein expression of astroglia in mood disorders. Immunohistochemical examination of glial fibrillary acidic protein (GFAP), a cytoskeletal marker for reactive astrocytes, in the dorsolateral prefrontal cortex indicates the involvement of astrocytes in the overall glial pathology reported in MDD [68, 69] (Fig. 3A). Comparison of the area fraction covered by immunoreactive astrocytes revealed a dramatic 75% reduction in young subjects (30-45 years old) with MDD as compared to age-matched non-depressed controls and older subjects (46-86 years old) with MDD [68]. A strong positive correlation between the packing density of GFAP-immunoreactive astrocytes and age was detected in this study only in the MDD and not in the control group further supporting the specificity of the astrocytic deficit in younger depressed (Fig. 3C). The subgroup of younger adults with MDD also had a shorter duration of depression and most of these subjects were suicide victims. A subsequent estimation of GFAP protein level by Western immunoblot analysis in the same young and older subjects with MDD confirmed that the relative level of GFAP protein was significantly decreased by 7 fold in the younger subjects (<60) with MDD as compared to the older subjects (>60) with MDD and older controls [69] (Fig. 3B). Preliminary results from our ongoing study in another prefrontal region, orbitofrontal cortex, indicate that GFAP level is again reduced in younger but not older depressed subjects. This is in agreement with the results of another cell counting study in which density of the general (Nissl-stained) population of glial cells was estimated in the orbitofrontal cortex in elderly depressed (>60) and age-matched control subjects [66]. No significant differences in either the overall (all six layers combined) or laminar density of glial cells were found between the two groups of elderly subjects.
Fig. (3).
Photomicrographs (A) of the distribution of glial fibrillary acidic protein (GFAP) immunoreactivity in the prefrontal cortex of a pair of young subjects. The top image displays a control subject (27 years old,) with the bottom image illustrating a subject diagnosed with major depressive disorder (MDD) (32 years old,). Note a much reduced GFAP immunolabeling in the MDD subject. Blot immunolabeling of GFAP in depicted in part B of the figure. Two pairs of subjects, control and MDD, display two bands identified as GFAP (50kDa) and actin (42kDa). For each pair the ages of the subjects are indicated in parentheses. Note that GFAP levels from the younger depressed subject are markedly lower than those of the young control subject, whereas, the older depressed subject has more GFAP than its matched control. Scatter plot (C) illustrates the significant positive correlation between areal fraction of GFAP-immunoreactive astrocytes and age in the prefrontal cortex of subjects with MDD. Note that lower values for GFAP areal fraction were found in younger depressed subjects as opposed to older subjects with depression.
In agreement with the above, several other studies, carried out on a mixture of mostly young and few older subjects, demonstrate alterations in GFAP and other astrocytic markers in mood disorders. Changes in different forms of GFAP protein were reported by a proteomic study in frontal cortex homogenates from mood disorder subjects [149]. Reduced GFAP staining was also detected in the hippocampus of steroid-treated and depressed patients [150] and in stressed animals [151]. As mentioned above, decreases in the expression of genes for astrocytic specific proteins, glia glutamate transporter and enzyme glutamine synthetase were observed in frontal cortex tissue from depressed patients [70]. Elevated levels of another astrocytic marker, calcium binding protein s100ß, were detected in subjects with MDD or unmedicated BPD and antidepressants were able to reduce these levels [152-154]. In contrast to other astrocytic proteins an excess of s100ß has a toxic effect on neurons [155].
In summary, all of the above mentioned findings indicate that lower density of astrocytes and decreased GFAP expression are associated with younger depressed subjects who had early onset of depression and that an increase in GFAP expression is not simply related to biological aging, it may also be associated with the progression of cellular changes of depressive illness [66, 156]. This last observation implies that the involvement of GFAP expression is different in early versus late life depression. Increasing clinical evidence confirms that late onset depression (first depressive episode when older than 50 years) differs from early-onset depression by its etiology, phenomenology and cerebrovascular pathology [157-159].
NEURONAL PATHOLOGY IN DEPRESSION
The pathology of neurons in depression is less obvious than the pathology of glial cells as it is not found in all of the brain regions where glial pathology has been observed [131, 132, 134, 137]. Moreover, if changes in cell packing density and/or size of neurons are detected, the magnitude of these changes are smaller than that of glial cells. Brain regions in which some neuronal pathology has been detected include dorsolateral prefrontal cortex [132, 133, 138], orbitofrontal cortex [66, 133, 139], anterior cingulate cortex [134, 136, 160-162] and hippocampus [135]. Subjects in these studies were a mixture of mostly young and few older individuals with major depression or bipolar disorder. For example, in the same postmortem study on younger subjects with MDD (average age 53), in which we found prominent reductions in glial cell density, we observed only subtle changes in the mean size and density of specific size classes of neurons. These alterations were observed in specific layers of the prefrontal cortex and not in the overall population of prefrontal neurons [133]. Moreover, there was a trend for a negative correlation between the duration of depression and sizes of neuronal cell bodies. The longer the duration of illness, the smaller the neurons were, suggesting the progression of neuronal damage with the progression of the disease. The observation on smaller neuronal sizes in depressed subjects has been confirmed by three independent studies [132, 134, 135]. The above results indicate that neuronal pathology at a younger age in MDD is less severe than the pathology of glial cells. In contrast, a comparison of neuronal density between elderly (>60 years old) and younger (<50 years old) depressed subjects revealed that neuronal pathology in the orbitofrontal cortex is much more severe in elderly than in younger subjects with MDD [66].
Morphometric analysis of neurons and glia in elderly subjects with MDD (>60 years old) and age-matched non-depressed controls revealed that the density of pyramidal neurons was significantly (20-60%) reduced below the control values in all cortical layers in the MDD group [66]. These reductions were most severe in cortical layers III and V which contain pyramidal glutamatergic neurons giving rise to prefronto-striatal, prefronto-amygdalar and prefronto-cortical (cingulate, hippocampal, temporal and parietal) projections and that receive thalamo-cortical fibers. This observation in postmortem tissue is in agreement with the in vivo neuroimaging studies revealing functional and volumetric abnormalities in the above mentioned regions and increased density of white matter hyperintensities (corresponding to vascular lesions) in the ORB cortex and striatum (reviewed in [124]). Moreover, our preliminary observations of an inverse correlation between neuronal density and the density of blood vessel profiles from the same subjects [163] further suggests a link between neuronal and vascular pathology in elderly depressed (see Fig. 4).
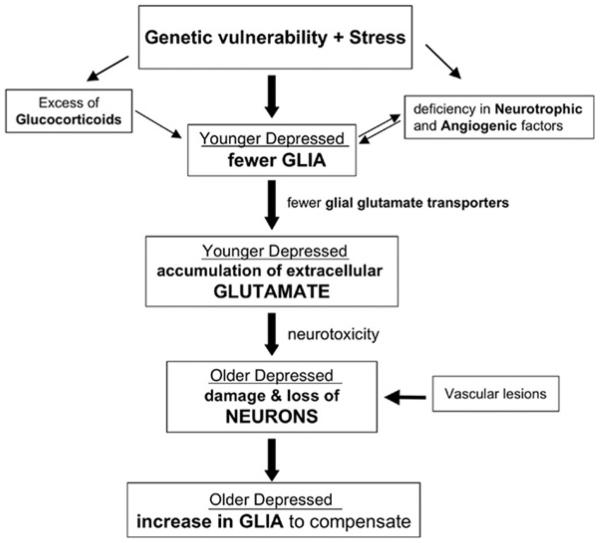
Fig. (4).
Hypothetical scheme of the progression of cell pathology in depression from young adulthood to old age This scheme incorporates the relationships between cellular, glutamate, glucocorticoid, and neurotrophic factor alterations in depression. It is proposed that a combination of genetic and environmental (e.g. stress) factors at the early stages of depressive illness could lead initially to the pathology of glial cells, and consequently to the pathology of neurons later in life as depressive illness progresses. Among the factors contributing to early glial pathology might be stress-related elevations in glucocorticoids and/or a deficiency in neurotrophic and angiogenic factors. It is further proposed that the loss of glial cells at early stages of depression may lead to decreased expression of glial glutamate transporters and a concomitant reduction in the uptake of glutamate from the synaptic cleft. As a consequence, an excess of extracellular glutamate may contribute to neuronal damage or neuronal death as depressed subjects get older. In light of the ability of glia to proliferate in response to neuronal injury, it could be hypothesized that neuronal injury in depression could result in glial proliferation in elderly subjects with depression. However, other factors (vascular lesions, age-related atrophy of neurons and fiber tracts, and medical comorbidity) in addition to glial deficits could contribute to neuronal pathology in older depressed subjects, particularly in those with the first onset of depression in late-life. Thus, the box “Vascular Lesions” mostly represents a new cohort of patients with late onset depression who might not have had glial pathology earlier in life (for further details please refer to the section: “Model of Glial/Neuronal Cell Pathology in Depression”).
In summary, older subjects with MDD show a prominent reduction in pyramidal (presumably glutamatergic) neurons whereas, younger subjects with MDD are characterized by mild neuronal and prominent astroglial pathology (manifested by the reduced expression of GFAP protein) [66, 156].
MODEL OF GLIAL/NEURONAL CELL PATHOLOGY IN DEPRESSION
Based on all the findings on glial and neuronal pathology outlined above, we have proposed a hypothetical scheme of the sequence of events in the progression of cell pathology in depression (Fig. 4). This scheme seeks to clarify the relationship between cellular changes, glutamate, glucocorticoids, and neurotrophic factors in depression. It is proposed that a combination of genetic and environmental (e.g. stress) factors at the early stages of depressive illness could lead initially to the pathology of glial cells, and consequently to the pathology of neurons later in life as depressive illness progresses. Among the factors contributing to early glial pathology might be stress-related elevations in glucocorticoids and/or a deficiency in neurotrophic and angiogenic factors. Psychosocial stress and an excess of glucocorticoids inhibits the proliferation of astrocytes and oligodendrocytes in animal studies (see section on Gliogenesis below), and glucocorticoids alter the expression of GFAP in depressed patients [150]. Moreover, reductions in neurotrophic and angiogenic factors are observed in animal stress models related to depression (reviewed in [96]) in human postmortem brain tissue from depressed suicide victims [98] and in plasma of depressed patients attempting suicide [164]. The chronic administration of antidepressant drugs or electroconvulsive seizures can reverse this stress-induced deficit by increasing the expression of neurotrophic and glia-derived factors, as well as other glial markers [96, 99, 100, 102, 165-167] and suggests that glia may serve as new targets for the therapeutic action of antidepressant treatment. It is further proposed that the loss of glial cells at early stages of depression may lead to decreased expression of glial glutamate transporters and a concomitant reduction in the uptake of glutamate from the synaptic cleft [65]. As a consequence, an excess of extracellular glutamate may contribute to neuronal damage or neuronal death as depressed subjects get older. In light of the ability of glia to proliferate in response to neuronal injury, it could be hypothesized that neuronal injury in depression could result in glial proliferation in elderly subjects with depression. In fact, glial proliferation and decrements in neuronal density have been observed in the orbitofrontal cortex of elderly depressed, as compared to younger depressed subjects [66]. Such a model is supported by the observation in postmortem cell counting studies of more prominent glial pathology in younger depressed subjects and more prominent neuronal pathology in older depressed subjects. However, other factors in addition to glial deficits could contribute to neuronal pathology in older depressed subjects particularly, but not exclusively, in those with the first onset of depression in late-life (e.g., after age of 50 or 60). Among these factors are vascular lesions, age-related atrophy of neurons and fiber tracts, and medical comorbidity [168-171]. Thus, it could be hypothesized that a cohort of patients with the first episode of depression in late-life might not have had glial pathology earlier in life (see Fig. 4).
The concept of lower glial number in younger depressed subjects leading to a diminished level of glial glutamate transporters and increased level of extracellular glutamate is supported by neuroimaging and postmortem studies. Elevated levels of glutamate are reported in depressed patients, as assessed by magnetic resonance spectroscopy [65, 172] and decreases in gene expression for glial markers of glutamate neurotransmission are observed in homogenates from the postmortem frontal cortex of depressed subjects [70]. Recent studies indicate that enhanced uptake of glutamate might have an antidepressant-like effect in murine behavioral model related to depression [173] and that ketamine, an antagonist at the NMDA-receptor, may be a promising medication for treatment-resistant depression [78]. The involvement of glial cells in these potentially novel therapeutic mechanisms is yet to be determined.
GLIOGENESIS
Reduced neurogenesis in the hippocampus has been proposed as a mechanism contributing to the pathophysiology of depression while increased neurogenesis may mediate the effects of antidepressants [164, 174, 175] (see also article on Neurogenesis in this issue). As outlined above alterations in glial and neuronal numbers are very prominent in brain areas (e.g. the prefrontal cortex) other than the hippocampus, and in these areas very little or no neurogenesis occurs in the adult [176-179]. Glial cells, unlike neurons, retain their ability to proliferate in most brain areas of postnatal and adult subjects [177, 180-182]. For example, in the neocortex and underlying white matter of adult mice production of oligodendrocytes and astrocytes is quite active with the proportions of these two cell types varying according to the brain area [181, 182]. The generation of astrocytes is also detectable in the neocortex and the hippocampus of adult human brain [176, 183]. Although the majority of newly generated cells in the adult rat hippocampal dentate gyrus are neurons (about 75%), there is still 15% of new cells that are positive for the astrocytic marker GFAP and might be astrocytes [164, 174, 184]. This neuron to glia ratio does not change with antidepressant treatments [164, 174], indicating that these treatments increase the number of newly generated glial cells in the adult brain. As mentioned above, in the hippocampus of MDD subjects although increases in neuronal and glial cell packing densities are observed, there is still a tendency for a reduction in the number of glial cells per neuron [185]. Thus, the question remains whether or not the generation of new glial cells in the hippocampus, neocortex and the amygdala is involved in the pathophysiology of depression and the effects of antidepressant treatments.
DEVELOPMENTAL GLIOGENETIC FACTORS
The regulation of gliogenesis in the mature brain is a legitimate target for study. However, as described above glial deficit is detected in relatively young subjects with depression. Thus, the possibility exists that there are developmental factors influencing gliogenesis that contribute to the etiopathology of or the vulnerability to depression. During development several molecular factors acting in different combinations determine the fate of multipotent precursors cells that eventually will produce either neurons or glial cells. For example, basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF) have proven to be crucial to the proliferation and survival of multipotent neural precursors [186]. Moreover, later in development, EGF is a required factor for proliferation of neuronal precursors, but not for glial cells. It is now well established that radial glial cells in the developing CNS are multipotent cells that generate separate precursors for neurons and mature glial cells [187, 188]. Several progenitors for glial cells have been described and are distinguished according to specific combinations of marker proteins [189]. In vitro, precursors for glial cells contain the A2B5 marker and generate oligodendrocytes and astrocytes of different developmental types (type I and type II). Since A2B5 precursors do not produce neurons they have been named glial restricted precursors (GRP). Other progenitors denoted as O-2A are capable of producing oligodendrocytes and type II astrocytes, although when transplanted to the CNS they only produce oligodendrocytes. Additional precursors for oligodendrocytes and astrocytes have been described, although the possibility that they are intermediate forms of the ones mentioned above cannot be ruled out [189]. Interestingly both O-2A and A2B5 cells are also found in various regions of the adult CNS and have potential to generate oligodendrocytes, astrocytes or both [190].
Glial precursors differentiate into astrocytes or oligodendrocytes driven by specific factors in the cell's microenvironment. For example, glial precursors exposed to platelet derived growth factor (PDGF) differentiate into oligodendrocytes whereas, when exposed to ciliary neurotrophic factor (CNTF), bone morphogenetic protein (BMP), interleukin 6, or leukemia inhibitory factor (LIF), they preferentially produce astrocytes [191, 192]. Basic FGF (bFGF) is not only important in early neural development for the maintenance of multipotent precursors, but also postnatally when it is synthesized by astrocytes and some neurons and induces the oligodendrocyte lineage in glial precursors [193]. Also there is recent, strong evidence that transforming growth factor alpha promotes survival and proliferation of human astrocytes [194].
In the proliferative zones of the adult brain, some of the factors mentioned above exert effects on the production of glial cells similar to those during development [195]. Accordingly, alterations in gliogenetic factors during development or in the adult brain have the potential to participate in the pathophysiology of depression or be manipulated to achieve antidepressant effects. In this regard, bFGF may be paradigmatic. Basic FGF is a well-known stimulator of astrocyte and oligodendrocyte proliferation [196, 197]. The mRNA level of bFGF has been shown to be reduced in the dorsolateral prefrontal cortex of patients with major depression [92, 198]. This factor may be also involved in the action of antidepressants because treatment of rats with antidepressants increases bFGF expression in the hippocampus and neocortex [199, 200]. It is then possible that the actions of bFGF on astrocytes and oligodendrocytes are part of the mechanisms contributing to depression or to the actions of antidepressant treatments [93].
OTHER GLIOGENETIC FACTORS
Besides the ontogenetic factors present in the nervous tissue that guide the proliferation and differentiation of glial cells, the interaction of astrocytes with blood vessels is of special interest. Astrocytes are functionally and structurally associated to blood vessels and are able to control brain blood flow to provide for the energetic and nutritional demands of neuronal activity. Thus, it is not surprising that during development and adulthood factors released by endothelial cells of blood vessels regulate the proliferation or differentiation of astrocytes. Mi et al. [201] demonstrated that endothelial cells directly induce differentiation in optic nerve astrocytes and that this is mediated by LIF. Other molecules that are released by endothelial cells or astrocytes which participate in the genesis of blood vessels, such as endothelin-1 and VEGF, enhance astrocyte proliferation [202, 203]. Conversely, signals produced by astrocytes act directly on endothelial cells to enhance vascularization [204]. Recent studies have shown that ECT, a treatment with antidepressant properties, results in enhanced expression of angiogenic and gliogenic factors [101]. Since recent research in depression has detected alterations in blood flow and vascular morphology [163, 170, 171, 205], the possibility exists that deficits in astrogliogenesis follow and/or cause vascular disturbances in depression.
Although this review does not focus on the regulation of glial proliferation after brain injury, it is worth a mention that excess glutamate released to the extracellular space, as a consequence of brain trauma or epileptic seizures, is capable of altering the proliferation of astrocytes and oligodendrocytes [206]. Glutamate acting on AMPA receptors reduces the proliferation of oligodendroglial precursors [207]. In contrast, activation of glutamate metabotropic receptors in astrocytes results in increased astrocyte proliferation [208]. Proliferation of astrocytes and oligodendrocytes is also regulated by beta-adrenergic receptors, where activation of these receptors inhibits proliferation of cortical astrocytes [209, 210] but increases astrogliogenesis in the optic nerve [210, 211]. This suggests that effects of neurotransmitters on astrocyte proliferation depend on the localization or the final stage of differentiation of astrocytes. As there is evidence for the involvement of both the glutamatergic and noradrenergic systems in depression [31, 64], an effect on the proliferative properties of glial cells might occur in the brain of depressed subjects. It is unknown, however, how the balance of glutamatergic and noradrenergic activities affects the survival and/or proliferative responses of astrocytes, oligodendrocytes and their precursors, and clearly more research is needed on this important issue.
Due to the high co-morbidity of depression or depressive symptoms with alcoholism it is noteworthy that ethanol have been shown to significantly affect the proliferation of glial cells. Ethanol has been reported to inhibit proliferation of astrocytes and astrocyte precursors in vivo and in vitro [212-215]. However, neuronal damage following alcohol abuse can result in activation of astrocyte proliferation and astrogliosis [216, 217]. Recent postmortem cell counting studies in the prefrontal cortex of neurologically “uncomplicated” alcoholics with and without depression have revealed marked reductions in the density of glial cells in the dorsolateral prefrontal and orbitofrontal cortices [142, 218]. These reductions were most prominent in alcoholics with comorbid depression [142].
GLIOGENESIS IN ANIMAL MODELS OF DEPRESSION
Critical to establishing the mechanisms by which gliogenetic alterations may contribute to the pathology or the therapy of depression is the examination of gliogenesis in animal models of physiological or behavioral aspects of depression. In recent years, models of psychosocial stress, stress-related endocrine alterations and olfactory bulb removal have been approached from the perspective of glial cell proliferation. These models have shown that gliogenesis may contribute to the cause or consequence of depression-like symptoms.
In a tree shrew model for aspects of depression related to psychosocial stress Lucassen and colleagues [219] have found that stress results in decreased cytogenesis and increased cell death in the hippocampus. Interestingly, increased labeling for apoptosis after stress appears to occur mainly in glial cells, suggesting that these cells might be the target of early neuropathological changes. More recently Czeh et al. 2006 [151] reported that psychosocial stress actually resulted in a remarkable 25% decrease in the number of GFAP immunoreactive astroglia in the hippocampus, and this number was highly correlated with a reduction in hippocampal volume. In the same model there is rather a decrease in apoptotic markers after chronic stress suggesting adaptive mechanisms in the hippocampus [220]. Thus it could be proposed that prolonged exposure to stress may contribute to the absence of detectable cell loss in the hippocampus of depressed subjects [185]. This is in contrast with findings of decreased numbers of glial and neuronal cells in prefrontal cortical areas in depression (see above). Regarding the differences in cell proliferation between the hippocampus and other cortical areas, it is interesting to note that ischemia and stroke in animal models result in increased cell genesis in the hippocampus and neocortex [221, 222]. However, in the neocortex only gliogenesis but not neurogenesis is significantly increased, while in the hippocampus both neurogenesis and gliogenesis are affected. This opposite pattern of cytogenesis between neocortex and hippocampus might be related to differences in the cell density observed by postmortem studies in depression. Increases in neuronal and glia cell density were detected in the hippocampus, whereas reduced cell density was found in the prefrontal cortex (see section above on pathology of glia and neurons in depression).
Nevertheless, further investigation is necessary into the possibility that precursors for the genesis of astrocytes and oligodendrocytes are affected by stress, and hormonal or neurotransmitter disturbances associated with depression. It is noteworthy, that while stress and an excess of corticosteroids appear to result in impaired proliferation of oligodendrocytes and astrocytes, proliferation of microglial cells is, in contrast stimulated by stress-related elevation of glucocorticoids [129].
Given the close association of stress-related behavioral and endocrine changes to depressive symptoms, the work of Alonso [223] on glial cell proliferation in the adult rat hippocampus is of special interest. The author has found that prolonged treatment with corticosterone significantly reduced the generation of glial cells in the hippocampus. In addition, using a similar model Wennstromm et al. [224] recently demonstrated that ECT prevents reductions in hippocampal gliogenesis caused by corticosterone. These data on the hippocampus are consistent with a recent suggestion that gliogenesis might be involved in the antidepressant effects of ECT [102]. The type of glial cell sensitive to corticosterone and ECT treatments in the above studies is the NG2 cell [223, 224], an abundant glial cell type of uncertain function, that includes precursors of the oligodendrocyte lineage [225-227]. Since the numbers of glial cells are reduced in some brain regions (prefrontal cortex, hippocampus, amygdala) in depression, the question remains whether the NG2 glia in these regions are reduced, or if the genesis of other glial cell types is also diminished. In culture, other research has shown that glucocorticoids inhibit the proliferation of astrocytes and appear to mediate the antiproliferative effect of the neural cell adhesion molecule (NCAM) on these cells [228-230]. Surprisingly, ECT increases the genesis of glial cells in the amygdala [224], which suggests that alterations in gliogenesis have to be observed with consideration to specific anatomical location of the circuits that regulate the emotional aspects of behavior.
Another well-established model of behavioral, biochemical and hormonal alterations associated with depression is olfactory bulb removal (bulbectomy) in the rat [231, 232]. Keilhoff et al. [233] have recently demonstrated that bulbectomy results in decreased cell proliferation in the hippocampus and the subventricular zone (SVZ, which generates precursors for neurons and glial cells in the olfactory bulb). By contrast, in the basolateral amygdala those researchers detected an increase in cell genesis. Treatment with the antidepressant imipramine prevented a reduction in cell genesis in the hippocampus, but not the increase in the amygdala. In addition, bulbectomy increased cell death by apoptosis in the SVZ but not in the hippocampus or amygdala. These experiments add evidence to the notion that altered cell genesis may play a role in the pathophysiology of depression. The results show that the direction of changes is dependent on the brain area and that both cell genesis and cell death (that is cell turnover) have to be taken into account when explaining alterations of cell numbers associated with the pathophysiology of depression.
Although stress and related hormonal changes are generally associated with the suppression of gliogenesis, other behavioral interventions in animal models like environmental enrichment or increased physical exercise have been shown to increase gliogenesis and neurogenesis in the hippocampus and neocortex of normal animals [234-238]. Increases in gliogenesis in animal models of environmental enrichment does not evenly affect the three main classes of glial cells. Ehninger et al. [236] have shown, in the mouse, that enrichment and exercise stimulated the proliferation of astrocytes particularly in layer 1 of the cingulate and motor cortices, but very few oligodendrocytes were newly generated. A significant increase in proliferation was also traced to microglial cells [238].
It is also possible that stimulation of glial cell proliferation and differentiation by behavioral or cognitive treatments may be more significant in diseased than in normal brain. For example, in stroke-lesioned rats environmental enrichment leads to an increase in the generation of neurons and glial cells originated from the subventricular zone (SVZ). Neuronal precursors produced in the SVZ migrate towards the stroke site in the neocortex, but they do not differentiate into neurons, even if the enriched environment reduces the functional impairments caused by focal cortical ischemia [221, 222]. In contrast, the enriched environment enhances the generation of astroglia and NG2 cells, not only near the ischemic area, but all over the neocortex [222]. In a rat model of Parkinson's disease this compound produces a specific reduction in the production of newborn astrocytes in the substantia nigra. Interestingly, exposure to an enriched environment during the postsurgical period produced elevated numbers of NG2 s and GFAP-immunoreactive cells in the substantia nigra and improved motor behavior [239]. Since enriched environments and exercise have been shown to have antidepressant effects, the results in animal models of neurological disorders point to the possibility that targeted and controlled enhancement of glial proliferation or survival may add to the arsenal of therapeutic tools for the treatment of depression. Understanding the molecular and cellular mechanisms mediated by glial cells which ultimately result in improved emotional outcomes, may represent a new avenue for therapeutic approaches to depression.
CONCLUSIONS
Glial cells perform complex functions in the developing as well as mature brain. These functions include the generation of neural cell precursors, support of neuronal metabolism, control of neurotransmitter turnover and release, and regulation of angiogenesis and cerebral blood flow. Disturbance of these functions or alterations in the number and/or morphology of glial cells in brain regions involved in emotional aspects of behavior may be an etiological factor in the onset or progression of depressive disorders. Although no obvious neuropathology or neurodegeneration is found in the brain of depressed subjects (with the possible exception of elderly depressed), mounting evidence indicates that frontal cortical areas of subjects with major depression or bipolar disorder have lower numbers of glial cells than non-psychiatric controls. These glial deficits may be the result of altered gliogenesis as various factors that impair glial cell proliferation are likely to be altered in the cortex in depression. Among the known factors that regulate proliferation of glia with the potential to affect the neurophysiology of depression are growth and differentiation proteins in the developing and adult brain, stress-related hormones and related molecules, neurotransmitters like glutamate or noradrenaline, ethanol, and blood-vessel derived factors. Further studies are needed to address the actions of these and other gliogenic factors during brain development, at the onset and during the progression of depression. Such studies may determine whether therapies based on gliogenic factors will alleviate depression. Furthermore, it remains to be established whether changes in glial physiology and number are so-called state markers present only during episodes of depression or are trait markers present also during periods of remission.
ACKNOWLEDGEMENTS
Research presented in this review was supported in part by grants: MH60451, MH61578, MH67996, MH63187, The Alcoholic Beverage Medical Research Foundation and RR17701. The authors thank Dr. C. Stockmeier for critical reading of this manuscript and Dr. D. Maciag and G. O'Dwyer for editorial assistance.
ABBREVIATIONS
AMPA
Alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid
A2B5
Marker for a glial progenitor cell that differentiates into oligodendrocytes or type 1 or type 2 astrocytes
BDNF
Brain derived neurotrophic factor
BMP
Bone morphogenetic protein
bFGF
Basic fibroblast growth factor
BPD
Bipolar disorder
Ca++
Calcium
CNS
Central nervous system
CNTF
Ciliary neurotrophic factor
COMT
Catechol-O-methyl transferase
EAAT-2
Excitatory amino acid transporter 2
ECT
Electroconvulsive therapy
EGF
Epidermal growth factor
FGF2
Fibroblast growth factor-2
GABA
Gamma-aminobutyric acid
GDN
Glial derived neurotrophic factor
GFAP
Glial fibrillary acidic protein
GLT-1
Glutamate transporter-1
GRP
Glial restricted precursors
LFA-1
Lymphocyte function associated antigen-1
LIF
Leukemia inhibitory factor
MAO
Monoamine oxidase
MBP
Myelin basic protein
MDD
Major depressive disorder
mRNA
Messenger ribonucleic acid
NCAM
Neural cell adhesion molecule
NGF
Nerve growth factor
NG2
Specific type of glia identified by their expression of chondroitin sulphate proteoglycan
NMDA
N-methyl-d-aspartic acid
O-2A
Progenitor cells which are capable of differentiating into oligodendrocytes or type 2 astrocytes
PDGF
Platelet derived growth factor
SVZ
Subventricular zone
S100ß
Calcium binding protein, a marker for astrocytes
VEGF
Vascular endothelial growth factor
REFERENCES
- 1.Kettenmann H, Ransom BR. In: Neuroglia. 2nd ed. Kettenmann H, Ransom BR, editors. Oxford University Press; New York: 2005. p. 1. [Google Scholar]
- 2.Volterra A, Meldolesi J. Nat. Rev. Neurosci. 2005;6:626. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- 3.Seifert G, Schilling K, Steinhauser C. Nat. Rev. Neurosci. 2006;7:194. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- 4.Rajkowska G. The Neuroscientist. 2003;9:273. doi: 10.1177/1073858403252773. [DOI] [PubMed] [Google Scholar]
- 5.Kandel ER. In: Principles of neuronal science. 4th ed. Kandel ER, Schwartz JH, Jessell TM, editors. McGraw-Hill; 2000. [Google Scholar]
- 6.Sulston JE, Schierenberg E, White JG, Thomson JN. Dev. Biol. 1983;100:64. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 7.Nedergaard M. Science. 1994;263:1768. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- 8.Diamond MC, Scheibel AB, Murphy GM, Jr., Harvey T. Exp. Neurol. 1985;88:198. doi: 10.1016/0014-4886(85)90123-2. [DOI] [PubMed] [Google Scholar]
- 9.Colombo JA, Reisin HD, Miguel-Hidalgo JJ, Rajkowska G. Brain Res. Brain Res. Rev. 2006;52:257. doi: 10.1016/j.brainresrev.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naftel JP, Ard MD, Fratkin JD, Hutchins JB. In: Fundamental Neuroscience for basic and clinical applications. 3rd ed. Haines DE, editor. Elsevier Inc.; Philadelphia: 2006. p. 25. [Google Scholar]
- 11.Chao TI, Rickmann M, Wolff JR. In: The tripartite synapse. Glia in synaptic transmission. Volterra A, Magistretti PJ, Haydon PG, editors. Oxford University Press; New York: 2002. p. 3. [Google Scholar]
- 12.Miller RH, Raff MC. J. Neurosci. 1984;4:585. doi: 10.1523/JNEUROSCI.04-02-00585.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fawcett D. Bloom and Fawcett. A textbook of histology. Chapman & Hall; New York, London: 1994. p. 355. [Google Scholar]
- 14.Kettenmann H, Ransom BR. Neuroglia. 2nd ed. Oxford University Press; New York: 2005. [Google Scholar]
- 15.Magistretti PJ, Pellerin L. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1999;354:1155. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raichle ME. Nature. 2001;412:128. doi: 10.1038/35084300. [DOI] [PubMed] [Google Scholar]
- 17.Rossi D. J. Nat. Neurosci. 2006;9:159. doi: 10.1038/nn0206-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Nat. Neurosci. 2003;6:43. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 19.Parri R, Crunelli V. Nat. Neurosci. 2003;6:5. doi: 10.1038/nn0103-5. [DOI] [PubMed] [Google Scholar]
- 20.Kettenmann H, Steinhauser C. In: Neuroglia. 2nd ed. Kettenmann H, Ransom BR, editors. Oxford University Press; New York: 2005. p. 131. [Google Scholar]
- 21.Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Science. 1997;276:1699. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- 22.Khan ZU, Koulen P, Rubinstein M, Grandy DK, Goldman-Rakic PS. Proc. Natl. Acad. Sci. USA. 2001;98:1964. doi: 10.1073/pnas.98.4.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azmitia EC, Gannon PJ, Kheck NM, Whitaker_Azmitia PM. Neuropsychopharmacology. 1996;14:35. doi: 10.1016/S0893-133X(96)80057-1. [DOI] [PubMed] [Google Scholar]
- 24.Azmitia EC. Brain Res. Bull. 2001;56:413. doi: 10.1016/s0361-9230(01)00614-1. [DOI] [PubMed] [Google Scholar]
- 25.Wu C, Singh SK, Dias P, Kumar S, Mann DM. Exp. Neurol. 1999;158:529. doi: 10.1006/exnr.1999.7105. [DOI] [PubMed] [Google Scholar]
- 26.Hirst WD, Cheung NY, Rattray M, Price GW, Wilkin GP. Brain Res. Mol. Brain Res. 1998;61:90. doi: 10.1016/s0169-328x(98)00206-x. [DOI] [PubMed] [Google Scholar]
- 27.Carson MJ, Thomas EA, Danielson PE, Sutcliffe JG. Glia. 1996;17:317. doi: 10.1002/(SICI)1098-1136(199608)17:4<317::AID-GLIA6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 28.Steinhauser C, Gallo V. Trends Neurosci. 1996;19:339. doi: 10.1016/0166-2236(96)10043-6. [DOI] [PubMed] [Google Scholar]
- 29.Verkhratsky A, Steinhauser C. Brain Res. Brain Res. Rev. 2000;32:380. doi: 10.1016/s0165-0173(99)00093-4. [DOI] [PubMed] [Google Scholar]
- 30.Steinhauser C, Jabs R, Kettenmann H. Hippocampus. 1994;4:19. doi: 10.1002/hipo.450040105. [DOI] [PubMed] [Google Scholar]
- 31.Stockmeier CA, Jurjus G. In: The postmortem brain in psychiatric research. Agam G, Everall IP, Belmaker RH, editors. Kluwer Academic Publishers; Boston, Dordrecht, London: 2002. p. 363. [Google Scholar]
- 32.Karolewicz B, Stockmeier CA, Ordway GA. Neuropsychopharmacology. 2005;30:1557. doi: 10.1038/sj.npp.1300781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nowak G, Ordway GA, Paul IA. Brain Res. 1995;675:157. doi: 10.1016/0006-8993(95)00057-w. [DOI] [PubMed] [Google Scholar]
- 34.Verkhratsky A, Orkand RK, Kettenmann H. Physiol. Rev. 1998;78:99. doi: 10.1152/physrev.1998.78.1.99. [DOI] [PubMed] [Google Scholar]
- 35.Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Neuron. 1994;13:713. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 36.Furuta A, Takashima S, Yokoo H, Rothstein JD, Wada K, Iwaki T. Brain Res. Dev. Brain Res. 2005;155:155. doi: 10.1016/j.devbrainres.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E, Volterra A. Nat. Neurosci. 2004;7:613. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- 38.Huang YH, Sinha SR, Tanaka K, Rothstein JD, Bergles DE. J. Neurosci. 2004;24:4551. doi: 10.1523/JNEUROSCI.5217-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minelli A, DeBiasi S, Brecha NC, Zuccarello LV, Conti F. J. Neurosci. 1996;16:6255. doi: 10.1523/JNEUROSCI.16-19-06255.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hertz L, Wu PH, Schousboe A. Neurochem. Res. 1978;3:313. doi: 10.1007/BF00965577. [DOI] [PubMed] [Google Scholar]
- 41.Lopez-Corcuera B, Liu QR, Mandiyan S, Nelson H, Nelson N. J. Biol. Chem. 1992;267:17491. [PubMed] [Google Scholar]
- 42.Wu Q, Wada M, Shimada A, Yamamoto A, Fujita T. Brain Res. 2006;1075:100. doi: 10.1016/j.brainres.2005.12.109. [DOI] [PubMed] [Google Scholar]
- 43.Bal N, Figueras G, Vilaro MT, Sunol C, Artigas F. Eur. J. Neurosci. 1997;9:1728. doi: 10.1111/j.1460-9568.1997.tb01530.x. [DOI] [PubMed] [Google Scholar]
- 44.Hirst WD, Price GW, Rattray M, Wilkin GP. Neurochem. Int. 1998;33:11. doi: 10.1016/s0197-0186(05)80003-8. [DOI] [PubMed] [Google Scholar]
- 45.Takeda H, Inazu M, Matsumiya T. Naunyn Schmiedebergs Arch. Pharmacol. 2002;366:620. doi: 10.1007/s00210-002-0640-0. [DOI] [PubMed] [Google Scholar]
- 46.Inazu M, Takeda H, Matsumiya T. J. Neurochem. 2003;84:136. doi: 10.1046/j.1471-4159.2003.01514.x. [DOI] [PubMed] [Google Scholar]
- 47.Inazu M, Takeda H, Matsumiya T. J. Neurochem. 2003;84:43. doi: 10.1046/j.1471-4159.2003.01566.x. [DOI] [PubMed] [Google Scholar]
- 48.Hansson E, Muyderman H, Leonova J, Allansson L, Sinclair J, Blomstrand F, Thorlin T, Nilsson M, Ronnback L. Neurochem. Int. 2000;37:317. doi: 10.1016/s0197-0186(00)00033-4. [DOI] [PubMed] [Google Scholar]
- 49.Pelton EW, 2nd, Kimelberg HK, Shipherd SV, Bourke RS. Life Sci. 1981;28:1655. doi: 10.1016/0024-3205(81)90322-2. [DOI] [PubMed] [Google Scholar]
- 50.Fitzgerald LW, Kaplinsky L, Kimelberg HK. J. Neurochem. 1990;55:2008. doi: 10.1111/j.1471-4159.1990.tb05789.x. [DOI] [PubMed] [Google Scholar]
- 51.Dave V, Kimelberg HK. J. Neurosci. 1994;14:4972. doi: 10.1523/JNEUROSCI.14-08-04972.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Volterra A, Magistretti PJ, Haydon PG. The tripartite synapse. Glia in synaptic transmission. Oxford University Press; New York: 2002. [Google Scholar]
- 53.Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. J. Neurosci. 1995;15:1835. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lehre KP, Danbolt NC. J. Neurosci. 1998;18:8751. doi: 10.1523/JNEUROSCI.18-21-08751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chaudhry FA, Lehre KP, van Lookeren Campagne M, Ottersen OP, Danbolt NC, Storm-Mathisen J. Neuron. 1995;15:711. doi: 10.1016/0896-6273(95)90158-2. [DOI] [PubMed] [Google Scholar]
- 56.Haugeto O, Ullensvang K, Levy LM, Chaudhry FA, Honore T, Nielsen M, Lehre KP, Danbolt NC. J. Biol. Chem. 1996;271:2771. doi: 10.1074/jbc.271.44.27715. [DOI] [PubMed] [Google Scholar]
- 57.Rothstein JD, Jin L, Dykes-Hoberg M, Kuncl RW. Proc. Natl. Acad. Sci. USA. 1993;90:6591. doi: 10.1073/pnas.90.14.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Neuron. 1996;16:675. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 59.Rao VL, Dogan A, Todd KG, Bowen KK, Kim BT, Rothstein JD, Dempsey RJ. J. Neurosci. 2001;21:1876. doi: 10.1523/JNEUROSCI.21-06-01876.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hughes EG, Maguire JL, McMinn MT, Scholz RE, Sutherland ML. Brain Res. Mol. Brain Res. 2004;124:114. doi: 10.1016/j.molbrainres.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 61.Rothman D, Lebon V, Shulman RG. In: The tripartite synapse. Glia in synaptic transmission. Volterra A, Magistretti PJ, Haydon PG, editors. Oxford University Press; New York: 2002. p. 85. [Google Scholar]
- 62.Pfrieger FW, Barres BA. Science. 1997;277:1684. doi: 10.1126/science.277.5332.1684. [DOI] [PubMed] [Google Scholar]
- 63.Shen J. Biol. Psychiatry. 2006;59:883. doi: 10.1016/j.biopsych.2005.07.042. [DOI] [PubMed] [Google Scholar]
- 64.Yildiz-Yesiloglu A, Ankerst DP. Psychiatry Res. 2006;147:1. doi: 10.1016/j.pscychresns.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 65.Kugaya A, Sanacora G. CNS Spectr. 2005;10:808. doi: 10.1017/s1092852900010403. [DOI] [PubMed] [Google Scholar]
- 66.Rajkowska G, Miguel-Hidalgo JJ, Dubey P, Stockmeier CA, Krishnan KR. Biol. Psychiatry. 2005;58:297. doi: 10.1016/j.biopsych.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rajkowska G, O'Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. Neuropsychopharmacology. 2007;32:471. doi: 10.1038/sj.npp.1301234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miguel-Hidalgo JJ, Baucom C, Dilley G, Overholser JC, Meltzer HY, Stockmeier CA, Rajkowska G. Biol. Psychiatry. 2000;48:861. doi: 10.1016/s0006-3223(00)00999-9. [DOI] [PubMed] [Google Scholar]
- 69.Si X, Miguel-Hidalgo JJ, O'Dwyer G, Stockmeier CA, Rajkowska G. Neuropsychopharmacology. 2004;29:2088. doi: 10.1038/sj.npp.1300525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP, Myers RM, Bunney WE, Jr., Akil H, Watson SJ, Jones EG. Proc. Natl. Acad. Sci, USA. 2005;102:15653. doi: 10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnson JW, Ascher P. Nature. 1987;325:529. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- 72.Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. Cell. 2006;125:775. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 73.Miyazaki J, Nakanishi S, Jingami H. Biochem. J. 1999;340(Pt 3):687. [PMC free article] [PubMed] [Google Scholar]
- 74.Wolosker H, Blackshaw S, Snyder SH. Proc. Natl. Acad. Sci, USA. 1999;96:1340. doi: 10.1073/pnas.96.23.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Conti F, Barbaresi P, Melone M, Ducati A. Cereb. Cortex. 1999;9:110. doi: 10.1093/cercor/9.2.110. [DOI] [PubMed] [Google Scholar]
- 76.Conti F, DeBiasi S, Minelli A, Melone M. Glia. 1996;17:254. doi: 10.1002/(SICI)1098-1136(199607)17:3<254::AID-GLIA7>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 77.Lalo U, Pankratov Y, Kirchhoff F, North RA, Verkhratsky A. J. Neurosci. 2006;26:2673. doi: 10.1523/JNEUROSCI.4689-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zarate CA, Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. Arch. Gen. Psychiatry. 2006;63:856. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 79.Verkhratsky A, Kettenmann H. Trends Neurosci. 1996;19:346. doi: 10.1016/0166-2236(96)10048-5. [DOI] [PubMed] [Google Scholar]
- 80.Charles A, Giaume C. In: The tripartite synapse. Glia in synaptic transmission. Volterra A, Magistretti PJ, Haydon PG, editors. Oxford University Press; New York: 2002. p. 110. [Google Scholar]
- 81.Hassinger TD, Atkinson PB, Strecker GJ, Whalen LR, Dudek FE, Kossel AH, Kater SB. J. Neurobiol. 1995;28:159. doi: 10.1002/neu.480280204. [DOI] [PubMed] [Google Scholar]
- 82.Pfrieger FW. In: The tripartite synapse. Glia in synaptic transmission. Volterra A, Magistretti PJ, Haydon PG, editors. Oxford University Press; New York: 2002. p. 24. [Google Scholar]
- 83.Nagler K, Mauch DH, Pfrieger FW. J. Physiol. 2001;533:665. doi: 10.1111/j.1469-7793.2001.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Science. 2001;291:657. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- 85.Aloisi F. In: Neuroglia. 2nd ed. Kettenmann H, Ransom BR, editors. Oxford University Press; New York: 2005. p. 285. [Google Scholar]
- 86.Laping NJ, Teter B, Nichols NR, Rozovsky I, Finch CE. Brain Pathol. 1994;4:259. doi: 10.1111/j.1750-3639.1994.tb00841.x. [DOI] [PubMed] [Google Scholar]
- 87.Mongin AA, Kimelberg HK. In: Neuroglia. 2nd ed. Kettenmann H, Ransom BR, editors. Oxford University Press; New York: 2005. p. 550. [Google Scholar]
- 88.Connor B, Dragunow M. Brain Res. Brain. Res. Rev. 1998;27:1. doi: 10.1016/s0165-0173(98)00004-6. [DOI] [PubMed] [Google Scholar]
- 89.Lindahl P, Johansson BR, Leväen P, Betsholtz C. Science. 1997;277:242. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 90.Malin SA, Molliver DC, Koerber HR, Cornuet P, Frye R, Albers KM, Davis BM. J. Neurosci. 2006;26:8588. doi: 10.1523/JNEUROSCI.1726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Erickson JT, Brosenitsch TA, Katz DM. J. Neurosci. 2001;21:581. doi: 10.1523/JNEUROSCI.21-02-00581.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Turner CA, Akil H, Watson SJ, Evans S. J. Biol. Psychiatry. 2006;59:1128. doi: 10.1016/j.biopsych.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 93.Reuss B, Unsicker K. In: Neuroglia. 2nd ed. Kettenmann H, Ransom BR, editors. Oxford University Press; New York: 2005. p. 377. [Google Scholar]
- 94.Acker T, Beck H, Plate KH. Mech. Dev. 2001;108:45. doi: 10.1016/s0925-4773(01)00471-3. [DOI] [PubMed] [Google Scholar]
- 95.Fuchs E, Czeh B, Kole MH, Michaelis T, Lucassen PJ. Eur. Neuropsychopharmacol. 2004;14(Suppl. 5):S481. doi: 10.1016/j.euroneuro.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 96.Duman RS, Monteggia LM. Biol. Psychiatry. 2006;59:1116. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 97.Manji HK, Quiroz JA, Sporn J, Payne JL, Denicoff K, N AG, Zarate CA, Jr., Charney DS. Biol. Psychiatry. 2003;53:707. doi: 10.1016/s0006-3223(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 98.Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Arch. Gen. Psychiatry. 2003;60:804. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- 99.Newton SS, Girgenti MJ, Collier EF, Duman RS. Eur. J. Neurosci. 2006;24:819. doi: 10.1111/j.1460-9568.2006.04958.x. [DOI] [PubMed] [Google Scholar]
- 100.Newton SS, Collier EF, Hunsberger J, Adams D, Terwilliger R, Selvanayagam E, Duman RS. J. Neurosci. 2003;23:10841. doi: 10.1523/JNEUROSCI.23-34-10841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ongur D, Heckers S. Harv. Rev. Psychiatry. 2004;12:253. doi: 10.1080/10673220490886185. [DOI] [PubMed] [Google Scholar]
- 102.Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Biol. Psychiatry. 2001;50:260. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- 103.Behl C, Davis JB, Klier FG, Schubert D. Brain Res. 1994;645:253. doi: 10.1016/0006-8993(94)91659-4. [DOI] [PubMed] [Google Scholar]
- 104.Wise PM, Dubal DB, Wilson ME, Rau SW. J. Neurocytol. 2000;29:401. doi: 10.1023/a:1007169408561. [DOI] [PubMed] [Google Scholar]
- 105.Mhyre AJ, Dorsa DM. Neuroscience. 2006;138:851. doi: 10.1016/j.neuroscience.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 106.Garcia-Segura LM, Naftolin F, Hutchison JB, Azcoitia I, Chowen JA. J. Neurobiol. 1999;40:574. [PubMed] [Google Scholar]
- 107.Santagati S, Melcangi RC, Celotti F, Martini L, Maggi A. J. Neurochem. 1994;63:2058. doi: 10.1046/j.1471-4159.1994.63062058.x. [DOI] [PubMed] [Google Scholar]
- 108.Hosli E, Jurasin K, Ruhl W, Luthy R, Hosli L. Int. J. Dev. Neurosci. 2001;19:11. doi: 10.1016/s0736-5748(00)00082-4. [DOI] [PubMed] [Google Scholar]
- 109.Pawlak J, Karolczak M, Krust A, Chambon P, Beyer C. Glia. 2005;50:270. doi: 10.1002/glia.20162. [DOI] [PubMed] [Google Scholar]
- 110.Platania P, Laureanti F, Bellomo M, Giuffrida R, Giuffrida-Stella AM, Catania MV, Sortino MA. Neuroendocrinology. 2003;77:334. doi: 10.1159/000070899. [DOI] [PubMed] [Google Scholar]
- 111.Bruce-Keller AJ, Keeling JL, Keller JN, Huang FF, Camondola S, Mattson MP. Endocrinology. 2000;141:3646. doi: 10.1210/endo.141.10.7693. [DOI] [PubMed] [Google Scholar]
- 112.Pawlak J, Brito V, Kuppers E, Beyer C. Brain Res. Mol. Brain Res. 2005;138:1. doi: 10.1016/j.molbrainres.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 113.Garcia-Segura LM, McCarthy MM. Endocrinology. 2004;145:1082. doi: 10.1210/en.2003-1383. [DOI] [PubMed] [Google Scholar]
- 114.Garcia-Ovejero D, Veiga S, Garcia-Segura LM, Doncarlos LL. J. Comp. Neurol. 2002;450:256. doi: 10.1002/cne.10325. [DOI] [PubMed] [Google Scholar]
- 115.Finley SK, Kritzer MF. J. Neurobiol. 1999;40:446. [PubMed] [Google Scholar]
- 116.Butt A. In: Neuroglia. Kettenmann H, Ransom BR, editors. Oxford University Press; New York: 2005. p. 36. [Google Scholar]
- 117.Bergles DE, Roberts JD, Somogyi P, Jahr CE. Nature. 2000;405:187. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- 118.Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, Fields RD. Neuron. 2006;49:823. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Karadottir R, Cavelier P, Bergersen LH, Attwell D. Nature. 2005;438:1162. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tekkok SB, Goldberg MP. J. Neurosci. 2001;21:4237. doi: 10.1523/JNEUROSCI.21-12-04237.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schabitz WR, Li F, Fisher M. Stroke. 2000;31:1709. doi: 10.1161/01.str.31.7.1709. [DOI] [PubMed] [Google Scholar]
- 122.Riddle A, Luo NL, Manese M, Beardsley DJ, Green L, Rorvik DA, Kelly KA, Barlow CH, Kelly JJ, Hohimer AR, Back SA. J. Neurosci. 2006;26:3045. doi: 10.1523/JNEUROSCI.5200-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Drobyshevsky A, Song SK, Gamkrelidze G, Wyrwicz AM, Derrick M, Meng F, Li L, Ji X, Trommer B, Beardsley DJ, Luo NL, Back SA, Tan S. J. Neurosci. 2005;25:5988. doi: 10.1523/JNEUROSCI.4983-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Taylor WD, Krishnan KR. In: Brain imaging in affective disorders. Soares JC, editor. Marcel Dekker, Inc; New York, NY: 2003. p. 53. [Google Scholar]
- 125.Taylor WD, MacFall JR, Steffens DC, Payne ME, Provenzale JM, Krishnan KR. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27:539. doi: 10.1016/S0278-5846(02)00358-5. [DOI] [PubMed] [Google Scholar]
- 126.Taylor WD, MacFall JR, Payne ME, McQuoid DR, Provenzale JM, Steffens DC, Krishnan KR. Am. J. Psychiatry. 2004;161:1293. doi: 10.1176/appi.ajp.161.7.1293. [DOI] [PubMed] [Google Scholar]
- 127.Kreutzberg GW. Trends Neurosci. 1996;19:312. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 128.Nair A, Bonneau RH. J. Neuroimmunol. 2006;171:72. doi: 10.1016/j.jneuroim.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 129.Boje KM, Arora PK. Brain Res. 1992;587:250. doi: 10.1016/0006-8993(92)91004-x. [DOI] [PubMed] [Google Scholar]
- 130.Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK. J. Immunol. 1992;149:2736. [PubMed] [Google Scholar]
- 131.Ongur D, Drevets WC, Price JL. Proc. Natl. Acad. Sci. USA. 1998;95:13290. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Cereb. Cortex. 2002;12:386. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- 133.Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Biol. Psychiatry. 1999;45:1085. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 134.Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Arch. Gen. Psychiatry. 2001;58:545. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- 135.Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Uylings HB, Friedman L, Rajkowska G. Biol. Psychiatry. 2004;56:640. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chana G, Landau S, Beasley C, Everall IP, Cotter D. Biol. Psychiatry. 2003;53:1086. doi: 10.1016/s0006-3223(03)00114-8. [DOI] [PubMed] [Google Scholar]
- 137.Bowley MP, Drevets WC, Ongur D, Price JL. Biol. Psychiatry. 2002;52:404. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- 138.Rajkowska G, Halaris A, Selemon LD. Biol. Psychiatry. 2001;49:741. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- 139.Cotter D, Hudson L, Landau S. Bipolar Disord. 2005;7:358. doi: 10.1111/j.1399-5618.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 140.Rajkowska G, Selemon LD, Goldman-Rakic PS. Arch. Gen. Psychiatry. 1998;55:215. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- 141.Selemon LD, Rajkowska G, Goldman-Rakic PS. J. Comp. Neurol. 1998;392:402. [PubMed] [Google Scholar]
- 142.Miguel-Hidalgo JJ, Wei J, Overholser JC, Jurjus G, Stockmeier CA, Rajkowska G. Biol. Psychiatry. 2002;52:1121. doi: 10.1016/s0006-3223(02)01439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Van Otterloo ES, Miguel-Hidalgo JJ, Stockmeier CA, Rajkowska G. Program No. 915.2. 2005 Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: 2005. Online. 2005. [Google Scholar]
- 144.Honer WG, Falkai P, Chen C, Arango V, Mann JJ, Dwork AJ. Neuroscience. 1999;91:1247. doi: 10.1016/s0306-4522(98)00679-4. [DOI] [PubMed] [Google Scholar]
- 145.Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Schizophr. Res. 2004;67:269. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- 146.Uranova N, Orlovskaya D, Vikhreva O, Zimina I, Kolomeets N, Vostrikov V, Rachmanova V. Brain Res. Bull. 2001;55:597. doi: 10.1016/s0361-9230(01)00528-7. [DOI] [PubMed] [Google Scholar]
- 147.Aston C, Jiang L, Sokolov BP. J. Neurosci. Res. 2004;77:858. doi: 10.1002/jnr.20208. [DOI] [PubMed] [Google Scholar]
- 148.Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, Starkey M, Webster MJ, Yolken RH, Bahn S. Lancet. 2003;362:798. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- 149.Johnston-Wilson NL, Sims CD, Hofmann JP, Anderson L, Shore AD, Torrey EF, Yolken RH. Mol. Psychiatry. 2000;5:142. doi: 10.1038/sj.mp.4000696. [DOI] [PubMed] [Google Scholar]
- 150.Muller MB, Lucassen PJ, Yassouridis A, Hoogendijk WJ, Holsboer F, Swaab DF. Eur. J. Neurosci. 2001;14:1603. doi: 10.1046/j.0953-816x.2001.01784.x. [DOI] [PubMed] [Google Scholar]
- 151.Czeh B, Simon M, Schmelting B, Hiemke C, Fuchs E. Neuropsychopharmacology. 2006;31:1616. doi: 10.1038/sj.npp.1300982. [DOI] [PubMed] [Google Scholar]
- 152.Aroor AR, Baker RC. Journal Of Neuroscience Research. 1997;50:1010. doi: 10.1002/(SICI)1097-4547(19971215)50:6<1010::AID-JNR11>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 153.Schroeter ML, Abdul-Khaliq H, Diefenbacher A, Blasig IE. Neuroreport. 2002;13:1675. doi: 10.1097/00001756-200209160-00021. [DOI] [PubMed] [Google Scholar]
- 154.Machado-Vieira R, Lara DR, Portela LV, Goncalves CA, Soares JC, Kapczinski F, Souza DO. Eur. Neuropsychopharmacol. 2002;12:269. doi: 10.1016/s0924-977x(02)00029-9. [DOI] [PubMed] [Google Scholar]
- 155.Barger SW, Van Eldik LJ. J. Biol. Chem. 1992;267:9689. [PubMed] [Google Scholar]
- 156.Rajkowska G. Biol. Psychiatry. 2000;48:766. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- 157.Heun R, Kockler M, Papassotiropoulos A. Int. J. Geriat. Psychiatry. 2000;15:1138. doi: 10.1002/1099-1166(200012)15:12<1138::aid-gps266>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 158.Krishnan K, Hays J, Tupler L, George L, Blazer D. Am. J. Psychiatry. 1995;152:785. doi: 10.1176/ajp.152.5.785. [DOI] [PubMed] [Google Scholar]
- 159.Lavretsky H, Lesser IM, Wohl M, Miller BL. Am. J. Geriatr. Psychiatry. 1998;6:248. [PubMed] [Google Scholar]
- 160.Cotter D, Landau S, Beasley C, Stevenson R, Chana G, MacMillan L, Everall I. Biol. Psychiatry. 2002;51:377. doi: 10.1016/s0006-3223(01)01243-4. [DOI] [PubMed] [Google Scholar]
- 161.Bouras C, Kovari E, Hof PR, Riederer BM, Giannakopoulos P. Acta. Neuropathol. (Berl) 2001;102:373. doi: 10.1007/s004010100392. [DOI] [PubMed] [Google Scholar]
- 162.Benes FM, Vincent SL, Todtenkopf M. Biol. Psychiatry. 2001;50:395. doi: 10.1016/s0006-3223(01)01084-8. [DOI] [PubMed] [Google Scholar]
- 163.Rajkowska G. Biol. Psychiatry. 2005;57:210S. [Google Scholar]
- 164.Kim YK, Lee HP, Won SD, Park EY, Lee HY, Lee BH, Lee SW, Yoon D, Han C, Kim DJ, Choi SH. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31:78. doi: 10.1016/j.pnpbp.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 165.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. J. Neurosci. 2000;20:9104. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nibuya M, Morinobu S, Duman RS. J. Neurosci. 1995;15:7539. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Angelucci F, Aloe L, Jimenez-Vasquez P, Mathe AA. J. Ect. 2002;18:138. doi: 10.1097/00124509-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 168.Alexopoulos GS, Kiosses DN, Murphy C, Heo M. Neuropsychopharmacology. 2004;29:2278. doi: 10.1038/sj.npp.1300557. [DOI] [PubMed] [Google Scholar]
- 169.Krishnan KR. Biol. Psychiatry. 2002;52:185. doi: 10.1016/s0006-3223(02)01349-5. [DOI] [PubMed] [Google Scholar]
- 170.Kumar A, Bilker W, Jin Z, Udupa J. Neuropsychopharmacology. 2000;22:264. doi: 10.1016/S0893-133X(99)00124-4. [DOI] [PubMed] [Google Scholar]
- 171.Krishnan KR, Taylor WD, McQuoid DR, MacFall JR, Payne ME, Provenzale JM, Steffens DC. Biol. Psychiatry. 2004;55:390. doi: 10.1016/j.biopsych.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 172.Sanacora G, Rothman DL, Mason G, Krystal JH. Ann. N.Y. Acad. Sci. 2003;1003:292. doi: 10.1196/annals.1300.018. [DOI] [PubMed] [Google Scholar]
- 173.Mineur YS, Picciotto MR, Sanacora G. Biol. Psychiatry. 2007;61:250. doi: 10.1016/j.biopsych.2006.04.037. [DOI] [PubMed] [Google Scholar]
- 174.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Science. 2003;301:805. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 175.Malberg JE, Duman RS. Neuropsychopharmacology. 2003;28:1562. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- 176.Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Bjork-Eriksson T, Nordborg C, Gage FH, Druid H, Eriksson PS, Frisen J. Proc. Natl. Acad. Sci. USA. 2006;103:12564. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kornack DR, Rakic P. Science. 2001;294:2127. doi: 10.1126/science.1065467. [DOI] [PubMed] [Google Scholar]
- 178.Kornack DR, Rakic P. Proc. Natl. Acad. Sci. USA. 1999;96:5768. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Koketsu D, Mikami A, Miyamoto Y, Hisatsune T. J. Neurosci. 2003;23:937. doi: 10.1523/JNEUROSCI.23-03-00937.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gensert JM, Goldman JE. J. Neurobiol. 2001;48:75. [PubMed] [Google Scholar]
- 181.McCarthy GF, Leblond CP. J. Comp. Neurol. 1988;271:589. doi: 10.1002/cne.902710409. [DOI] [PubMed] [Google Scholar]
- 182.Kraus-Ruppert R, Laissue J, Burki H, Odartchenko N. J. Comp. Neurol. 1973;148:211. doi: 10.1002/cne.901480206. [DOI] [PubMed] [Google Scholar]
- 183.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Nat. Med. 1998;4:1313. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 184.Rietze R, Poulin P, Weiss S. J. Comp. Neurol. 2000;424:397. [PubMed] [Google Scholar]
- 185.Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Uylings HB, Friedman L, Rajkowska G. Biol. Psychiatry. 2004;56:640. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Weiss S, Reynolds BA, Vescovi AL, Morshead C, Craig CG, van der Kooy D. Trends Neurosci. 1996;19:387. doi: 10.1016/s0166-2236(96)10035-7. [DOI] [PubMed] [Google Scholar]
- 187.Malatesta P, Hack MA, Hartfuss E, Kettenmann H, Klinkert W, Kirchhoff F, Gotz M. Neuron. 2003;37:751. doi: 10.1016/s0896-6273(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 188.Campbell K, Gotz M. Trends Neurosci. 2002;25:235. doi: 10.1016/s0166-2236(02)02156-2. [DOI] [PubMed] [Google Scholar]
- 189.Lee JC, Mayer-Proschel M, Rao MS. Glia. 2000;30:105. doi: 10.1002/(sici)1098-1136(200004)30:2<105::aid-glia1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 190.Noble M, Wren D, Wolswijk G. Semin. Cell Biol. 1992;3:413. doi: 10.1016/1043-4682(92)90012-k. [DOI] [PubMed] [Google Scholar]
- 191.Bonni A, Sun Y, Nadal-Vicens M, Bhatt A, Frank DA, Rozovsky I, Neil S, Yancopoulos GD, Greenberg ME. Science. 1997;278:477. doi: 10.1126/science.278.5337.477. [DOI] [PubMed] [Google Scholar]
- 192.Rajan P, McKay RD. J. Neurosci. 1998;18:3620. doi: 10.1523/JNEUROSCI.18-10-03620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rogister B, Ben-Hur T, Dubois-Dalcq M. Mol. Cell. Neurosci. 1999;14:287. doi: 10.1006/mcne.1999.0790. [DOI] [PubMed] [Google Scholar]
- 194.Sharif A, Prevot V, Renault-Mihara F, Allet C, Studler JM, Canton B, Chneiweiss H, Junier MP. Oncogene. 2006;25:4076. doi: 10.1038/sj.onc.1209443. [DOI] [PubMed] [Google Scholar]
- 195.Horner PJ, Palmer TD. Trends Neurosci. 2003;26:597. doi: 10.1016/j.tins.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 196.Skaper SD, Varon S. J. Cell. Physiol. 1987;130:453. doi: 10.1002/jcp.1041300320. [DOI] [PubMed] [Google Scholar]
- 197.Hunter KE, Sporn MB, Davies AM. Glia. 1993;7:203. doi: 10.1002/glia.440070303. [DOI] [PubMed] [Google Scholar]
- 198.Evans SJ, Choudary PV, Neal CR, Li JZ, Vawter MP, Tomita H, Lopez JF, Thompson RC, Meng F, Stead JD, Walsh DM, Myers RM, Bunney WE, Watson SJ, Jones EG, Akil H. Proc. Natl. Acad. Sci. USA. 2004;101:15506. doi: 10.1073/pnas.0406788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mallei A, Shi B, Mocchetti I. Mol. Pharmacol. 2002;61:1017. doi: 10.1124/mol.61.5.1017. [DOI] [PubMed] [Google Scholar]
- 200.Maragnoli ME, Fumagalli F, Gennarelli M, Racagni G, Riva MA. Biol. Psychiatry. 2004;55:1095. doi: 10.1016/j.biopsych.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 201.Mi H, Haeberle H, Barres BA. J. Neurosci. 2001;21:1538. doi: 10.1523/JNEUROSCI.21-05-01538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tabernero A, Jimenez C, Velasco A, Giaume C, Medina JM. J. Neurochem. 2001;78:890. doi: 10.1046/j.1471-4159.2001.00476.x. [DOI] [PubMed] [Google Scholar]
- 203.Krum JM, Khaibullina A. Exp. Neurol. 2003;181:241. doi: 10.1016/s0014-4886(03)00039-6. [DOI] [PubMed] [Google Scholar]
- 204.Abbott NJ. J. Anat. 2002;200:629. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Drevets WC. Prog. Brain Res. 2000;126:413. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- 206.Huttmann K, Sadgrove M, Wallraff A, Hinterkeuser S, Kirchhoff F, Steinhauser C, Gray WP. Eur. J. Neurosci. 2003;18:2769. doi: 10.1111/j.1460-9568.2003.03002.x. [DOI] [PubMed] [Google Scholar]
- 207.Gallo V, Zhou JM, McBain CJ, Wright P, Knutson PL, Armstrong RC. J. Neurosci. 1996;16:2659. doi: 10.1523/JNEUROSCI.16-08-02659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Liao SL, Chen C. J. Neuroreport. 2001;12:3519. doi: 10.1097/00001756-200111160-00029. [DOI] [PubMed] [Google Scholar]
- 209.Hodges-Savola C, Rogers SD, Ghilardi JR, Timm DR, Mantyh PW. Glia. 1996;17:52. doi: 10.1002/(SICI)1098-1136(199605)17:1<52::AID-GLIA5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 210.Kurino M, Fukunaga K, Ushio Y, Miyamoto E. J. Neurochem. 1996;67:2246. doi: 10.1046/j.1471-4159.1996.67062246.x. [DOI] [PubMed] [Google Scholar]
- 211.Feinstein DL, Rozelman E. Neurosci. Lett. 1997;223:37. doi: 10.1016/s0304-3940(97)13402-4. [DOI] [PubMed] [Google Scholar]
- 212.Kane CJ, Berry A, Boop FA, Davies DL. Brain Res. 1996;731:39. doi: 10.1016/0006-8993(96)00456-8. [DOI] [PubMed] [Google Scholar]
- 213.Guizzetti M, Costa LG. J. Neurochem. 1996;67:2236. doi: 10.1046/j.1471-4159.1996.67062236.x. [DOI] [PubMed] [Google Scholar]
- 214.Vallés S, Pitarch J, Renau-Piqueras J, Guerri C. J. Neurochem. 1997;69:2484. doi: 10.1046/j.1471-4159.1997.69062484.x. [DOI] [PubMed] [Google Scholar]
- 215.Davies DL, Vernadakis A. Brain Res. 1984;318:27. doi: 10.1016/0165-3806(84)90059-2. [DOI] [PubMed] [Google Scholar]
- 216.Goodlett CR, Peterson SD, Lundahl KR, Pearlman AD. Alcohol. Clin. Exp. Res. 1997;21:1010. [PubMed] [Google Scholar]
- 217.Guerri C, Renau-Piqueras J. Mol. Neurobiol. 1997;15:65. doi: 10.1007/BF02740616. [DOI] [PubMed] [Google Scholar]
- 218.Miguel Hidalgo JJ, Overholser J, Meltzer H, Stockmeier C, Rajkowska G. Alcohol. Clin. Exp. Res. 2006;30:1845. doi: 10.1111/j.1530-0277.2006.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lucassen PJ, Fuchs E, Czeh B. Biol. Psychiatry. 2004;55:789. doi: 10.1016/j.biopsych.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 220.Heine VM, Maslam S, Zareno J, Joels M, Lucassen PJ. Eur. J. Neurosci. 2004;19:131. doi: 10.1046/j.1460-9568.2003.03100.x. [DOI] [PubMed] [Google Scholar]
- 221.Komitova M, Mattsson B, Johansson BB, Eriksson PS. Stroke. 2005;36:1278. doi: 10.1161/01.STR.0000166197.94147.59. [DOI] [PubMed] [Google Scholar]
- 222.Komitova M, Perfilieva E, Mattsson B, Eriksson PS, Johansson BB. Exp. Neurol. 2006;199:113. doi: 10.1016/j.expneurol.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 223.Alonso G. Glia. 2000;31:219. doi: 10.1002/1098-1136(200009)31:3<219::aid-glia30>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 224.Wennstrom M, Hellsten J, Ekstrand J, Lindgren H, Tingstrom A. Biol. Psychiatry. 2006;59:178. doi: 10.1016/j.biopsych.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 225.Nishiyama A, Chang A, Trapp BD. J. Neuropathol. Exp. Neurol. 1999;58:1113. doi: 10.1097/00005072-199911000-00001. [DOI] [PubMed] [Google Scholar]
- 226.Kimelberg HK. Neurochem. Int. 2004;45:191. doi: 10.1016/j.neuint.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 227.Butt AM, Hamilton N, Hubbard P, Pugh M, Ibrahim M. J. Anat. 2005;207:695. doi: 10.1111/j.1469-7580.2005.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Crossin KL, Tai MH, Krushel LA, Mauro VP, Edelman GM. Proc. Natl. Acad. Sci. USA. 1997;94:2687. doi: 10.1073/pnas.94.6.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Krushel LA, Sporns O, Cunningham BA, Crossin KL, Edelman GM. Proc. Natl. Acad. Sci. USA. 1995;92:4323. doi: 10.1073/pnas.92.10.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sporns O, Edelman GM, Crossin KL. Proc. Natl. Acad. Sci. USA. 1995;92:542. doi: 10.1073/pnas.92.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kelly JP, Wrynn AS, Leonard BE. Pharmacol. Ther. 1997;74:299. doi: 10.1016/s0163-7258(97)00004-1. [DOI] [PubMed] [Google Scholar]
- 232.Lumia AR, Teicher MH, Salchli F, Ayers E, Possidente B. Brain Res. 1992;587:181. doi: 10.1016/0006-8993(92)90995-l. [DOI] [PubMed] [Google Scholar]
- 233.Keilhoff G, Becker A, Grecksch G, Bernstein HG, Wolf G. Neuropsychopharmacology. 2006;31:1165. doi: 10.1038/sj.npp.1300924. [DOI] [PubMed] [Google Scholar]
- 234.Walsh RN, Cummins RA. Int. J. Neurosci. 1979;9:209. doi: 10.3109/00207457909147675. [DOI] [PubMed] [Google Scholar]
- 235.Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. J. Neurobiol. 1999;39:569. doi: 10.1002/(sici)1097-4695(19990615)39:4<569::aid-neu10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 236.Ehninger D, Kempermann G. Cereb. Cortex. 2003;13:845. doi: 10.1093/cercor/13.8.845. [DOI] [PubMed] [Google Scholar]
- 237.Kempermann G, Kuhn HG, Gage FH. Nature. 1997;386:493. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 238.Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG. Eur. J. Neurosci. 2003;17:2042. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- 239.Steiner B, Winter C, Hosman K, Siebert E, Kempermann G, Petrus DS, Kupsch A. Exp. Neurol. 2006;199:291. doi: 10.1016/j.expneurol.2005.11.004. [DOI] [PubMed] [Google Scholar]