Designing vaccines based on biology of human dendritic cell subsets (original) (raw)
. Author manuscript; available in PMC: 2011 Oct 29.
Abstract
The effective vaccines developed against a variety of infectious agents, including polio, measles and Hepatitis B, represent major achievements in medicine. These vaccines, usually composed of microbial antigens, are often associated with an adjuvant that activates dendritic cells (DCs). Many infectious diseases are still in need of an effective vaccine including HIV, malaria, hepatitis C and tuberculosis. In some cases, the induction of cellular rather than humoral responses may be more important as the goal is to control and eliminate the existing infection rather than to prevent it. Our increased understanding of the mechanisms of antigen presentation, particularly with the description of DC subsets with distinct functions, as well as their plasticity in responding to extrinsic signals, represent opportunities to develop novel vaccines. In addition, we foresee that this increased knowledge will permit us to design vaccines that will reprogram the immune system to intervene therapeutically in cancer, allergy and autoimmunity.
Introduction
Vaccines can be preventive or therapeutic. Preventive vaccines go back as far as 200 B.C. when in ancient China and India, powdered scabs from people infected with smallpox were administered to protect against disease. The word vaccination was first used by Edward Jenner in 1796 to describe the injection of smallpox derived from cows (L. vaccae, cow). Louis Pasteur discovered that animals and people could be protected against disease after exposure to attenuated microbes. Most, if not all, preventive vaccines are designed to initiate protective humoral immune responses. However, many pathogens, for which no efficient vaccines are available, are still affecting mankind with diseases such as human immunodeficiency virus (HIV)–induced acquired immune deficiency syndrome, plasmodium-induced malaria, virus-induced hepatitis C, and _Mycobacterium_-induced tuberculosis. Most of these appear to be chronic diseases for which it is thought that strong cellular immunity, in particular cytotoxic T cells, is necessary to eliminate the cells that are infected with the causative agent. Thus, therapeutic vaccines are needed to eliminate existing disease as much as prophylactic vaccines that might block the initial infection. Vaccines have yet to be developed in noninfectious settings, where they have the potential to prevent and treat cancer, allergy, and chronic inflammation.
A more detailed understanding of the mechanisms leading to strong cellular immunity is necessary to enable rational approaches to vaccine design. Two recent conceptual breakthroughs in this regard have been i) our understanding that dendritic cells (DCs) play a pivotal role in initiating the immune response to foreign antigens (Figure 1) and ii) the realization that adjuvants act primarily because they are DC activators. Preventive vaccines are based on the concept of transitioning from no immunity to immunity by generating new CD4+ or CD8+ T effector cells by “priming” a new immune response. Therapeutic vaccines in chronic infections (or cancer) have two objectives: one is priming whereas the other is the modulation or reprogramming of memory cells, i.e., to transition from one type of immunity to another (e.g., regulatory to cytotoxic). These two types of vaccination might necessitate distinct approaches, facilitated by exploiting the diversity of DCs including their different subsets and functional plasticity. Direct modulation of T cell responses and populations may also contribute to vaccine effectiveness, although in practice this option may only be applicable to the treatment of patients with pre-existing disease by therapeutic vaccination.
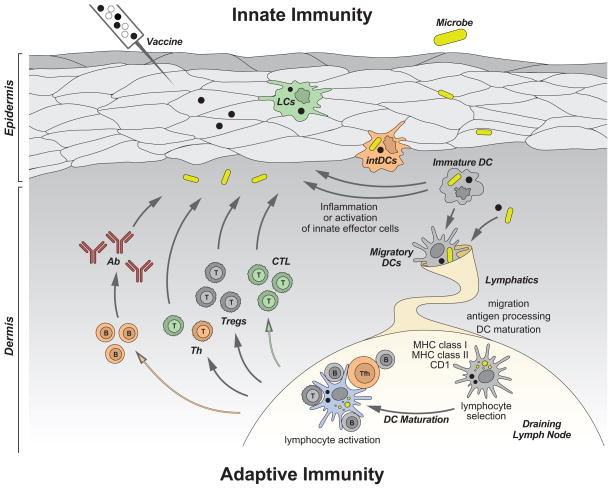
Figure 1. Dendritic cells.
DCs reside in the tissue where they are poised to capture antigens, be it microbes or vaccines. DCs recognize microbes (vaccines), and secrete cytokines (e.g. IFN-α), directly through pattern recognition receptors, or indirectly through stromal cells that sense microbes (vaccines). Cytokines secreted by DCs in turn activate effector cells of innate immunity such as eosinophils, macrophages and NK cells. Activation triggers DCs migration towards secondary lymphoid organs and simultaneous activation (maturation). These migratory DCs display antigens in the context of classical MHC class I and class II or non-classical CD1 molecules, which allow selection of rare antigen-specific T lymphocytes. Activated T cells drive DCs towards their terminal maturation, which induces further expansion and differentiation of lymphocytes. Activated T lymphocytes traverse inflamed epithelia and reach the injured tissue, where they eliminate microbes and/or microbe-infected cells. B cells, activated by DCs and T cells, differentiate into plasma cells that produce antibodies against the initial pathogen. Antigen can also drain into lymph nodes without involvement of peripheral tissue DCs and be captured and presented by lymph node resident DCs. Antigen capture by interstitial DCs (intDCs; orange) will preferentially lead to generation of humoral immunity whereas antigen capture by Langerhans cells (LCs; green) will preferentially lead to generation of cellular immunity.
The challenge of eliciting the right immune response
The protective and/or therapeutic efficacy of vaccination is directly linked to the type and the quality of immune responses elicited by a particular vaccine. Indeed, generating the right class of immune response can be a matter of life and death, perhaps best illustrated by leprosy where the indolent tuberculoid form of the disease is characterized by a protective type 1 T cell (Th1 cell) response, whereas the lepromatous form induces an often lethal type 2 (Th2 cell) response.
The quality of CD4+ T cell immunity is essential for the quality of effector cells such as antibody-secreting plasma cells in preventive vaccination, and cytotoxic CD8+ T cells in therapeutic vaccination. CD4+ T cells also appear necessary for the efficient generation of memory CD8+ T cells (Janssen et al., 2003; Shedlock and Shen, 2003; Sun and Bevan, 2003). CD4+ T cells display a broad spectrum of phenotypes, which is likely due to the priming by antigen presenting cells (APCs), most often DCs (reviewed in (Bluestone et al., 2009)) (Figure 2). Thus, in response to intracellular microbes, such as viruses and certain bacteria, CD4+ T helper cells differentiate into Th1 cells, which secrete interferon-γ (IFN-γ) and possess a specific range of functions. In contrast, extracellular pathogens such as helminths induce the development of Th2 cells, whose cytokines [interleukin-4 (IL-4), IL-5, IL-10 and IL-13] direct immunoglobulin E- and eosinophil-mediated destruction of the pathogens (Mosmann et al., 1986).
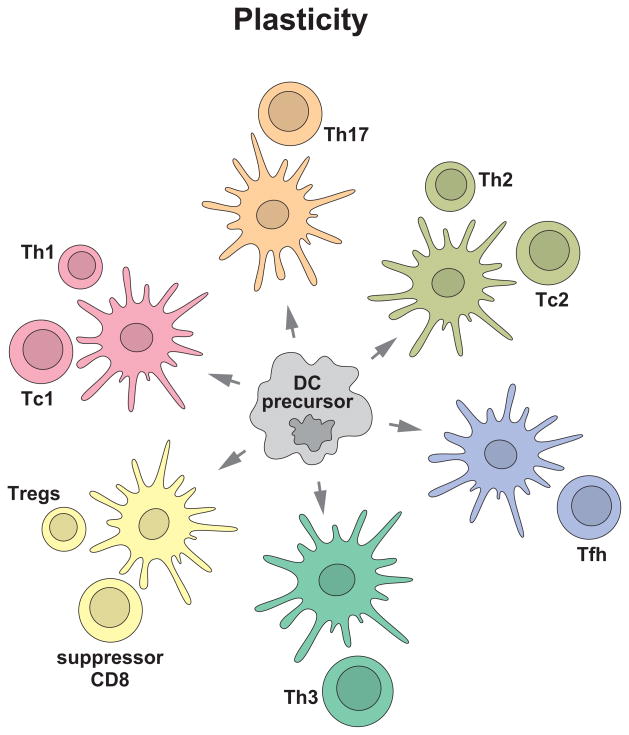
Figure 2. Distinct DC subsets generate distinct types of T cell immunity.
DC system has two cardinal features: 1) subsets; and 2) plasticity. This yields distinct types of immunity thereby allowing DCs to cope with protection against a variety of microbes and maintenance of tolerance to self. Understanding these two features is fundamental to develop vaccines that elicit the desired type of immune responses.
DCs regulate CD4+ T cell differentiation through a variety of molecules that belong to three major families: IL-12, TNF, and B7. The IL-12 family includes IL-12p70, which controls type 1 responses and the secretion of IFN-γ (Macatonia et al., 1995), IL-23, which contributes to the proliferation of inflammatory CD4+ T cells secreting IL-17 (Th17 cells) (Weaver et al., 2007), and IL-27, which appears to control IL-10 (Kastelein et al., 2007). Depending on the nature and time course of activation (maturation) by different agonists, DCs can express different molecules from the B7 family: CD80 (B7-1), CD86 (B7-2), ICOS-ligand, PD-L1 (B7-H1), PD-L2 (B7-DC), B7-H3, and B7-H4 (Chen, 2004; Greenwald et al., 2005). The B7 family includes members that can stimulate immune responses and others that can inhibit them (Chen, 2004). For instance, CD80 and CD86 bind to both CD28 and CTLA-4. whereas CD28 delivers signals for T cells to become functional effector cells, CTLA-4 delivers inhibitory signals that suppress their functions (Krummel and Allison, 1995). Furthermore, through its mode of action, one molecule might promote both the effector and the regulatory response, as exemplified by the ICOS ligand. Indeed, ICOS:ICOS ligand interaction helps the generation of regulatory T (Treg) cells (Ito et al., 2007) but also appears important in the stimulation of effector T cells and T-cell-dependent B-cell responses (Hutloff et al., 1999). A member of the TNF family, OX40L (which binds to OX40), shuts down the generation of IL-10-producing CD4+ type 1 regulatory T (Tr1) cells by DCs (Ito et al., 2006) but induces the differentiation of pro-inflammatory Th2 cells secreting TNF and IL-13. As we will discuss later, DCs and IL-12 are also essential regulators of another type of helper T cells so called T follicular helper (Tfh) cells, which in turn regulate humoral immunity (Schmitt et al., 2009).
A key cell population involved in the regulation of immune responses and homeostasis is the regulatory T (Treg) cell (Sakaguchi et al., 2010). Treg cells can be divided into two major subsets: thymus-derived naturally occurring Treg cells and periphery-induced Treg cells. Peripherally-induced Treg cells are thought to be derived from naive CD4+ T cells and include Tr1 cells, which mainly produce IL-10 (Roncarolo et al., 2001a), and Th3 cells, which mainly produce TGF-β (Fukaura et al., 1996). TGF-β1 synergizes with IL-21 to generate IgA-plasmablasts thereby playing a critical role in the development of mucosal immunity (Dullaers et al., 2009). The functional specialization of DC subsets in governing the differentiation of distinct types of Treg cells is currently a subject of active investigation. Peripheral Treg cells are generated by DCs that exist at the steady state, i.e., DCs that have not been activated by microbial stimuli or inflammatory mediators (Roncarolo et al., 2001b; Yamazaki et al., 2006). These DCs may not simply be unstimulated or immature. Activation of the Wnt and β-catenin signaling pathway in DCs has been shown to promote induced Treg cell production, at least in the mouse (Jiang et al., 2007). Similarly, in the thymus, production of thymic stroma lymphopoietin (TSLP) is essential for selection of naturally occurring CD4+CD25hi Treg cells (Watanabe et al., 2005).
Licensing Dendritic Cell Function: a word on DC “maturation”
DCs exist in distinct functional states including, resting and activated, also known as immature and mature. This is a key feature of DC biology and relates to the process of DC “maturation”, classically described as the morphological and functional alterations associated with the activation of DCs by microbial stimuli (e.g. via Toll-like receptor [TLR] agonists) (Trombetta and Mellman, 2005). Under steady state conditions, DCs in peripheral tissues are most often described as being “immature”, a phenotype characterized by the localization of MHC class II molecules to the late endosome-lysosomal compartment, a low surface expression of costimulatory molecules, low expression of chemokine receptors that trigger migration (e.g. CCR7), and an inability to release T cell-directed immunostimulatory cytokines (Trombetta and Mellman, 2005). Particularly adept at endocytosis, immature DCs are often associated with antigen uptake and sequestration, but not with antigen processing, the stable accumulation of peptide-MHC complexes, or their efficient presentation to T cells (Trombetta and Mellman, 2005). Maturation, as triggered by TLR agonists, upregulates surface MHC class II and costimulatory molecules on the DCs (Trombetta and Mellman, 2005) as well as promoting their migration to draining lymph nodes. It also enhances the ability of the DCs to interact with antigen-specific T cells, more efficient antigen processing and presentation, and cytokine release (Lanzavecchia and Sallusto, 2001). Thus, it is DC maturation, triggered by adjuvants, that links the innate and antigen specific arms of the immune response and thus allows the adaptive immunity to launch the response against a specific antigen (Steinman et al., 2003). Because agonist receptors, such as TLRs, are differentially expressed by different DC subsets and because different receptors may trigger qualitatively distinct forms of maturation (e.g. different patterns of cytokine release), understanding and accounting for DC maturation will be a key component of any attempt at rational vaccine design as it will determine the adjuvant used.
Maturation is a simple concept rendered complex by the likelihood that not all mature (or activated) DCs are equivalently immunogenic (Figure 3). For example, under steady state conditions particularly in lymphoid tissue one can find DC populations that display at least some of the features of mature DCs (e.g. elevated surface costimulatory molecules) despite the absence of overt inflammation or infection. The functional significance of these cells is unknown but it is not unreasonable to suspect that tolerogenic DCs may have to acquire the antigen presentation, migratory, and T cell interaction capacity of mature DCs in order to induce antigen-specific Treg cells or induce anergy or T cell apoptosis at high efficiency. As mentioned above, the priming of Treg cells either in the thymus or in the periphery may require activation by endogenous mediators such as TSLP or Wnt, respectively (Watanabe et al., 2005) (Manicassamy et al., 2010). Whether these mediators induce morphologically recognizable maturation in vivo is likely but not known. However, it is clear that resting or immature DCs can or must be “activated” in some way to induce T cell tolerance; hence, it is inaccurate to assume that the relevant steady state DCs are “immature” or resting.
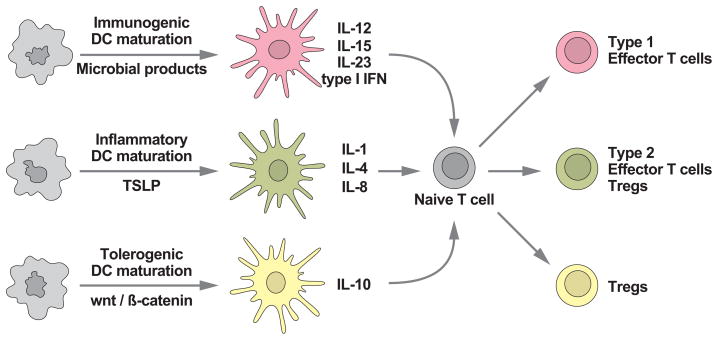
Figure 3. Many roads lead to DC maturation.
DCs exist in distinct functional states, resting and activated, or immature and mature. Depending on the signal DCs will undergo activation/maturation, the quality of which will determine the type of elicited adaptive immunity.
Virtually all DC subsets identified thus far, and discussed below, are capable of some form of activation, even if not all of them exhibit the dramatic cellular reorganizations observed for myeloid and monocyte-derived DCs. Plasmacytoid DCs, for example, do not dramatically relocalize their MHC class II molecules from lysosomes to the plasma membrane, but respond functionally (e.g. by interferon secretion) to a range of TLR agonists to facilitate immunity (Siegal et al., 1999). Because “mature” is usually associated with DCs that have undergone a morphological transition, we will use the more general term “activated” to describe the responses of DC subsets to adjuvants or endogenous activators when their phenotypic status (particularly in vivo) is unclear.
Human Dendritic Cell subsets
Although activation or maturation is a key factor determining DC function, the increasing number of distinct DC subsets being recognized indicates that the distribution of labor among DC subtypes is likely to be an equally important aspect of how DCs regulate T cell priming. We will concentrate on DC subsets that are associated with immunity, however even in peripheral tolerance induction, some subsets may be more effective than others (Siddiqui et al., 2010). Other subsets, notably DCs in B cell follicular regions, may be most adept at interacting with B cells, inducing humoral immunity to unprocessed soluble antigen trapped by these DCs (Wykes et al., 1998).
DC subsets in human blood
The evolution of knowledge of DC subsets has followed parallel tracks in mice and humans, and understanding them has become a major focus for many investigators over the past 15 years. Humans and mice display two major DC types: myeloid DCs (mDCs, also called conventional or classical DCs [cDCs]), and plasmacytoid DCs (pDCs). In mice, splenic mDCs were originally shown to comprise two major mDC subsets with marked differences in biological function: CD8α+CD11b− “lymphoid” DCs and CD8α−CD11b+ “myeloid” DCs. CD8α+ DCs are able to produce large amounts of IL-12 and polarize naive CD4+ T cells toward the Th1 cell phenotype, whereas CD8α− DCs preferentially induce Th2 cell responses (Maldonado-Lopez et al., 1999). Although the study of mouse DC subsets can make important contributions, it is crucial to do such studies with human cells because subtle but highly relevant differences exist between the human and mouse immune systems (Mestas and Hughes, 2004). Thus, to successfully generate human vaccines, we need to understand the diversity and biology of human DC subsets. DC subsets in the human blood can be distinguished by differential expression of three surface molecules: BDCA-1 (CD1c), BDCA-2 (CD303) and BDCA-3 (CD141) (Dzionek et al., 2000).
BDCA-2+ pDCs are considered the front line in anti-viral immunity owing to their capacity to rapidly produce high amounts of type I interferon in response to viruses (Siegal et al., 1999). They also express high amounts of IL-3Rα chain (CD123) and ILT-7 (Cao et al., 2006). pDCs are composed of at least two subsets with different functional properties (Matsui et al., 2009). They recognize viral components and self nucleic acids through TLR7 and TLR9, and possibly other as yet unidentified receptors. In their resting state, pDCs might play an important role in tolerance, including oral tolerance (Liu, 2005). The pDC presents three remarkable cell biological features to counteract viral infection: an extensive ER compartment that facilitates high capacity secretion of anti-viral factors, including type I interferons; an early endosomal compartment containing MHC class I molecules that appears to permit direct vesicular MHC class I loading for immediate activation of memory cytotoxic CD8+ T cells (Di Pucchio et al., 2008); and a late endosomal compartment containing MHC class II molecules, similar to that found in mDCs, which viral antigen presentation to CD4+ T cells. Thus, in both the MHC class I and class II pathways, pDCs may permit a rapid initial response to viral infections by utilizing pre-synthesized stores of MHC class I and II. In addition to their specialized role in the innate immune response to viruses (eg type I IFN release), pDCs are uniquely capable of rapidly expanding viral antigen specific CD8+ T effector cells (Di Pucchio et al., 2008). Thus, pDCs are poised to control the progress of a virus infection through nonspecific blockade of viral replication by type I IFN and the specific stimulation of adaptive antiviral responses via cytotoxic CD8+ T cells. pDCs are also critical for the generation of plasma cells and antibody responses (Jego et al., 2003). There, upon virus sensing, two pDC cytokines act sequentially: type I IFN is responsible for generation of non-Ig-secreting plasma blasts and IL-6 drives their differentiation into Ig-secreting plasma cells (Jego et al., 2003). pDCs can also amplify B cell response in a type I IFN-independent mechanism that is based on their stable expression of CD70 upon CpG activation (Shaw et al., 2010). CD70, a TNF family ligand, binds to its receptor CD27 expressed on memory B cells and promotes plasma cell differentiation and Ig secretion (Shaw et al., 2010). Finally, by virtue of their special capacity for secreting type I IFN, stimulating pDCs may provide an endogenous adjuvant that could promote the immunogenic maturation of other DC populations. Thus, strategies designed to prime pDCs may form the basis of a next generation of anti-viral vaccines.
In human blood there are two types of mDCs distinguished by reciprocal expression of BDCA-1 (CD1c) and BCDA-3 (CD141). Human CD141+ DCs represent the human counterpart of mouse CD8+ DCs. Indeed, they share with mouse CD8+ DCs the high capacity to capture exogenous antigens for presentation on HLA class I molecules (“cross-presentation”), typically reserved for the presentation of peptides from endogenous antigens. CD141+ DCs also share the expression of chemokine receptor XCR1 and of adhesion molecule, Necl2. Both human CD141+ DCs and mouse CD8+ DCs utilize XCR1 to migrate in response to the specific ligand XCL1, which is produced by NK cells and activated CD8+ T cells (Bachem et al., 2010; Crozat et al., 2010). Necl2 binds to class-I-restricted T-cell-associated molecule (CRTAM), a cell surface protein primarily expressed by NK cells, NK-T-cells, and activated CD8+ T cells. Thus, mouse CD8+ DCs and human CD141+ DCs appear well equipped for generation of CD8+ T cell immunity. In the mouse, gene ablation studies have also shown that the CD8+ subset plays a disproportionately important role in cross presentation (Shortman and Heath, 2010).
Similar to mouse CD8+ DCs, human CD141+ DCs express TLR3 and TLR8 and stimulation with their respective ligands, poly-I:C and poly-U, induces their maturation and cytokine secretion (Jongbloed et al., 2010; Poulin et al., 2010). The expression of other pattern recognition receptors (PRRs), such as NOD-like receptors and RIG-I-like receptors, on human CD141+ DCs is not yet clear. Although CD141+ DCs express a limited set of TLRs, CD1c+ DCs express a wide array of TLRs, including TLR4, 5, and 7. Blood CD1c+ DCs also display a capacity to cross-present antigens, and to secrete IL-12 (Jongbloed et al., 2010; Poulin et al., 2010). How these distinct blood mDC subsets contribute to shaping immunity remains to be established.
The identification of the human counterpart of mouse CD8+ DCs opens the possibility to translate into humans the knowledge generated in the mouse. One should however translate mouse data into clinical applications with a critical mind, as 65 million years of independent evolution have brought in many nuances that distinguish the human and the mouse immune systems (Mestas and Hughes, 2004). For example, other human DCs such as epidermal LCs (Klechevsky et al., 2008) can also cross-present antigens. Thus, it remains to be determined whether and how CD141+ blood mDCs are related to cutaneous mDCs subsets and how all those mDC subsets cooperate in shaping the adaptive immunity. Also, even if CD141+ DCs are far more adept at cross presentation than other DC subsets, “mass action” is a consideration because CD141+ DCs represent only a small fraction (~2%) of all DCs, at least in the blood.
DC subsets in human skin
In human skin, at least two different mDC subsets have been characterized: epidermal Langerhans cells (LCs) and dermal interstitial DCs (dermal DCs) (Valladeau and Saeland, 2005). Over the years, dermal DCs were further subdivided into at least two subsets CD1a+ DCs and CD14+ DCs (Valladeau and Saeland, 2005). The presence of two dermal DC subsets was also reported in mice that display a Langerin (CD207) subset in the dermis (Merad et al., 2008). Epidermal LCs and dermal CD14+ DCs express different sets of molecules. In particular, CD14+ DCs express a large number of surface C-type lectins including DC-SIGN, DEC-205, LOX-1, CLEC-6, Dectin-1 and DCIR. In contrast, LCs express the lectins Langerin and DCIR. Furthermore, whereas dermal CD14+ DCs express, at RNA level, a wide range of TLRs, including TLR-2, 4, 5, 6, 8, and 10 (Klechevsky et al., 2009; van der Aar et al., 2007); LCs exhibit a more restricted TLR expression including TLR-1,3, 6 and 10.
Studies suggest that human CD14+ DCs induce naïve T cells to differentiate into cells with properties of Tfh cells (Klechevsky et al., 2008). Thus, CD4+ T cells primed by CD14+ DCs are able to induce naïve B cells to produce larger amounts of IgM than those primed with LCs. Remarkably, only CD4+ T cells primed by CD14+ DCs induce naïve B cells to switch isotypes towards IgG and IgA. Furthermore, CD4+ T cells primed by CD14+ DCs secrete the chemokine CXCL13, a typical chemokine secreted by Tfh cells. Taken together, these data suggest that human dermal CD14+ DCs are specialized for the development of humoral responses (Klechevsky et al., 2008; Ueno et al., 2007). Along these lines, human monocyte-derived DCs activated with ligands of TLR 4, 5, and 7–8, heat-inactivated bacteria, or CD40 ligand, efficiently induce naïve CD4+ T cells to become IL-21-producers. IL-12 and to a minor extent IL-23 induced activated naïve CD4+ T cells to secrete IL-21 which in turn induce B cells to produce Ig (Schmitt et al., 2009).
LCs induce more robust proliferation of naïve allogeneic CD4+ and CD8+ T cells when compared to CD14+ DCs (Klechevsky et al., 2008). LCs are also more efficient in cross-presenting peptides from protein antigens to CD8+ T cells and prime CD8+ T cells of high avidity when compared to CD14+ DCs. CD8+ T cells primed by LCs acquire more potent cytotoxicity than those primed by CD14+ DCs, and are able to efficiently kill target cells, including tumor cell lines that express peptide-HLA complex only at low amounts (Klechevsky et al., 2008). Dermal CD14+ DCs showed a very poor ability to induce their differentiation into potent CTL effectors. This is not due to the inability to generate peptide-MHC class I complexes, but rather the inability to induce the expression of the cytotoxic effector molecules (granzymes A and B and perforin) on the differentiating T cells. The limited ability of CD14+ DCs to cross-present proteins such as influenza matrix protein is not due to a lower ability to process proteins in general, as these cells are indeed more potent at processing MHC class II-restricted peptides from tetanus toxoid. The mechanistic basis for why some DCs are more efficient at cross presentation remains an important unknown. One possibility is that the increased concentrations of proteolytic enzymes found within the endocytic compartments of monocyte-derived DCs destroy internalized antigens before they have the chance to egress into the cytosol (McCurley and Mellman, 2010). In general, an attenuated capacity for proteolysis is a key feature in enhancing antigen processing and presentation by DCs, in both the MHC class I and class II pathways (Delamarre et al., 2005) (Trombetta and Mellman, 2005).
Although CD14+ DCs educate naïve CD4+ T cells to become Tfh-like cells, LCs polarize naïve CD4+ T cells into cells secreting Th2 cell-type cytokines such as IL-4, IL-5 and IL-13. This is consistent with mouse studies showing the preferential induction of Th2 cell responses upon delivery of an antigen to the LC-rich epidermis (Alvarez et al., 2005).
For many years, LCs have been viewed as a paradigm population in DC biology. Induction of potent CTL response by LCs is observed in mouse studies by subcutaneous injections of peptide-loaded epidermal LCs (Celluzzi and Falo, 1997). Mouse LCs can actually cross present antigens to CD8+ T cells in vivo (Stoitzner et al., 2006). In contrast, several mouse studies, for example models using herpes simplex virus (HSV), have questioned the contribution of LCs to the induction of antigen-specific responses in vivo. These studies attribute the HSV-specific immunity to CD8α+ DCs, rather than to LCs (Allan et al., 2003). Further ex vivo studies showed that dermal CD103+ DCs but not dermal CD11b+ nor LCs were able to present antigens to naïve TCR-transgenic CD8+ T cells ex vivo (Bedoui et al., 2009). In contrast, all DCs were able to present viral antigens to CD4+ T cells (Bedoui et al., 2009). These results suggest that although the three cutaneous DC populations acquired viral antigens only, CD103+ DCs were able to present viral antigens to CD8+ T cells. However, it remains to be determined whether these differences with regard to the function of LCs between mice and humans derive from the differences in their immune systems. One further unknown is the susceptibility of these DC subsets to virus infection, which may substantially modulate antigen presenting function.
Humoral vs. Cellular immunity regulated by two mDC subsets
Collectively, we hypothesize that two different components of adaptive immunity, i.e., humoral and cellular, are preferentially regulated by different mDC subsets, at least in the skin. Thus, although humoral immunity is preferentially regulated by CD14+ dermal DCs, cellular immunity is preferentially regulated by LCs (Figure 4). This idea is also supported by mouse studies showing that dermal DCs upon activation migrate into the outer paracortex just beneath the B cell follicles, whereas LCs migrate into the T cell rich inner paracortex (Kissenpfennig et al., 2005). Another human skin DC subset, dermal CD1a+ DCs are functionally intermediate between LCs and CD14+ DCs in our hands. Whether this DC subset shows a unique asset in the regulation of immune responses remains to be addressed. It will also be important to understand whether this paradigm applies to DCs localized to other peripheral and lymphoid tissues in humans.
Figure 4. Understanding human myeloid dendritic cell subsets for the rational design of DC-targeting vaccines.
Novel vaccines rely on rational immunological approaches and aim at activating both the cellular and the humoral arm. We envision that targeting antigens and activation of distinct mDC subsets, with different specializations, will result in the generation of a broad and long lived immune protection. Thus, the most efficient vaccines might be those that will target both LCs and dermal CD14+ DCs thereby allowing the maximal stimulation of cellular and humoral immune responses and the generation of long-term memory protection.
Plasticity of DCs and their precursors as key determinants of immunity
In addition to subsets with functional specialization, DCs and their precursors (monocytes) are endowed with functional plasticity (Figures 2 and 3). DC plasticity needs to be considered at three levels: 1) response to microbial signals; 2) sensing of tissue derived-factors; and 3) reciprocal interaction with other immune cells.
Upon microbial invasion, DC undergo an initial activation and maturation process that includes: i) direct signaling by microbial products; and ii) microenvironmental signals delivered by surrounding cells responding to the microbes (Reis e Sousa, 2006; Trombetta and Mellman, 2005). Pathogen-derived signals transform resting or immature DCs into activated or mature cells able to launch adaptive immunity. Microbial products can deliver signals via several molecules, PPRs, belonging to four major families: i) C-type lectins, ii) TLRs, iii) NOD like receptors and iv) RIG-I like receptors. These signals can differentially modulate DC function consequently yielding distinct immune responses (Manicassamy and Pulendran, 2009; Takeuchi and Akira, 2010). For example, some C-type lectins have signaling motifs in their cytoplasmic regions and deliver activation or suppression signals (Reis e Sousa, 2006). Similar to TLR expression, CLR expression differs between human and mouse (Flornes et al., 2004). These differences complicate the extrapolation of the knowledge obtained in mouse studies to humans. CLRs are also receptors for endogenous ligands. For example, Mincle and Clec9a (DNGR-1) recognize damaged cells, Mincle by detecting small nuclear ribonucleoprotein (Brown, 2008), which is released from damaged cells, and Clec9a by detecting as yet unidentified preformed ligand(s) exposed on necrotic cells (Sancho et al., 2009).
Similarly, different TLRs deliver different activation signals to DCs (Manicassamy and Pulendran, 2009). Thus, Escherichia coli lipopolysaccharide (LPS) stimulates DCs through TLR4, inducing a Th1 cell response by IL-12 secretion, whereas Porphyromonas gingivalis LPS activates DCs through TLR2, inducing DCs to secrete IL-10, and eventually resulting in Th2 cell development (Manicassamy and Pulendran, 2009).
Cytoplasmic sensors include RIG-I-like receptors and NOD-like receptors (Takeuchi and Akira, 2010). The former ones belong to the intracellular receptors for RNA viruses and include RIG-I, Melanoma differentiation associated gene 5 (MDA5) and LGP2 (Rehwinkel et al., 2010). NOD-like receptors (NLRs) are thought to recognize microbial components (reviewed in (Mariathasan and Monack, 2007)). NLRs, such as NALP1, NALP3, IPAF, and NAIP5, are components of a molecular complex called the inflammasome (Schroder and Tschopp, 2010). The inflammasome cleaves substrates, such as pro-IL-1β and pro-IL-18, to produce mature proteins. NOD1/2 are expressed in the cytosol of macrophages and DCs, NALP1 is absent in germinal center and interstitial DCs while is highly expressed in LCs within mucosal surfaces and skin (Schroder and Tschopp, 2010). A short DC-specific isoform of NALP3 has recently been identified (Schroder and Tschopp, 2010), yet its role in IL-1 production in DCs remains to be determined.
The concept of plasticity or flexibility of the DC system is further exemplified by monocytes and their response to environmental signals. Thus, different cytokines skew the in vitro differentiation of monocytes into DCs with different phenotypes and function. This might in fact reflect the inflammatory pathway of DC recruitment and generation in vivo (Dominguez and Ardavin, 2010; Geissmann et al., 2010). For example, when activated (for example by GM-CSF) monocytes encounter IL-4 they will yield IL-4-DCs (Romani et al., 1994). By contrast, after encounter with IFN-α, TNF or IL-15, activated monocytes will differentiate into IFN-DCs (Paquette et al., 1998), TNF-DCs or IL-15-DCs (Mohamadzadeh et al., 2001), respectively. This spectrum of DCs represents immunostimulatory DCs although their in vivo counterparts and precise identities are unknown. Furthermore, it has been argued that cytokine-driven DCs might not be as potent in the generation of adaptive immunity as are the DCs triggered directly via microbial signals through PRRs (Joffre et al., 2009).
Similarly, there is a whole repertoire of DCs that have been produced in vitro, that exhibit immunoregulatory or tolerogenic functions, for example DCs generated by culturing monocytes with IL-10 (Levings et al., 2005) or DCs generated in the presence of Vitamin A (Zapata-Gonzalez et al., 2007) or Vitamin D3 (Penna and Adorini, 2000), or DCs activated by E-cadherin-mediated signaling (Jiang et al., 2007). Should such diversity exist in vivo, these DC populations might well be important in the context of DCs role in maintaining peripheral tolerance. Tissue-localized mDCs are also polarized by other cells and their products including IFN-α from pDCs, IFN-γ from γδ T cells and NK cells, IL-4 and TNF from mast cells, IL-15 and TSLP from stromal cells, IL-10 from lymphocytes, and Wnt ligands from various cellular sources (reviewed in (Cheng et al., 2010; Ueno et al., 2010). In principle, these distinct DCs will induce distinct types of T cell immunity or tolerance.
Such plasticity is associated with distinct signaling pathways as shown by a recent study (Arima et al., 2010). There, TSLP via activation of NF-kB leads DCs to express OX40L allowing the induction of Th2 cell differentiation, whereas the activation of signal transducer and activator of transcription 6 (STAT6) triggered DCs to secrete chemokines necessary for the recruitment of Th2 cells. In addition, TSLP signaling limited the activation of STAT4 and interferon regulatory factor 8 (IRF-8), which are essential factors for the production of the Th1 cell-polarizing cytokine IL-12. This Th1 cell-inducing pathway was instead activated by TLRs and CD40 ligand. Thus, the functional plasticity of DCs relies on elaborate signal codes that are generated by different stimuli. As alluded to above, the DC activation or maturation process is more sophisticated than just licensing DCs for T cell stimulation, it enables DCs to sense their environment and to assume an activated phenotype that carefully instructs the qualitative nature of the T cell responses induced.
DCs also have a reciprocal interaction with innate immune cells. The interaction of DCs with NK, NKT, and γδ T cells can occur in the periphery and the secondary lymphoid organs (reviewed in (Munz et al., 2005)). A recent mouse study suggested that the activation of NK cells is totally dependent on the interaction with DCs at the secondary lymphoid organs (Lucas et al., 2007). Activated NK cells enhance their cytotoxicity and capacity to secrete IFN-γ, which render DCs to induce type 1 responses (Munz et al., 2005). Mature DCs also activate NKT and γδ T cells, inducing the secretion of IFN-γ and IL-4 from NKT cells (Hermans et al., 2003) and IFN-γ and TNF-α from γδ T cells (Leslie et al., 2002). In particular, activated NKT cells acquire the capacity to kill tumor cells (Smyth et al., 2002). In return, CD40L expressed on NKT cells induces the strong activation of DCs (Munz et al., 2005).
Thus, subsets and plasticity allow DCs to cope with the challenges of their environment. These two features also dictate the quality of the response to vaccine adjuvants and can be harnessed for improved vaccination.
Targeting of Dendritic Cell Subsets to Improve Vaccines
Translating the accumulating knowledge on DC subsets and their unique functional attributes into the design of novel vaccines is becoming an exciting topic in human immunology. Active immunization has long been a successful strategy for the prevention of infectious diseases. The question now is how to capitalize on our new understanding of DCs to improve vaccines to the point where they can now also be used more effectively as therapeutic strategies.
Antigens can be delivered directly to DCs in vivo using various types of fusion proteins including cytokines (for example GM-CSF), chemokines and toxins, or more specifically antibodies against specific DC surface receptor(s). Studies in mice demonstrate that the specific targeting of antigen to DCs in vivo results in considerable potentiation of antigen-specific CD4+ and CD8+ T cell immunity. The induction of immunity is observed only when a DC maturation signal was provided (Bonifaz et al., 2002; Hawiger et al., 2001); otherwise, tolerance ensued (Hawiger et al., 2001). Furthermore, in vivo targeting of murine DC subsets revealed intrinsic differences in antigen processing and presentation of different populations (Dudziak et al., 2007). As discussed earlier, the CD8+CD205+ population was found to be more adept at cross presentation of exogenous antigen on MHC class I than the CD8−33D1+ DC population, which was somewhat more efficient at MHC class II presentation. Would targeting antigens to the CD205+ DCs be more efficient at generating CD8+ T cell responses? How do the mouse studies relate to the human immune system?
Targeting LCs for antigen delivery may be an optimal strategy for the induction of potent antigen-specific CTL responses. LC-specific molecule, such as Langerin, can be used as a target DC receptor (Idoyaga et al., 2008). Dermal CD14+ DCs might represent the appropriate target for the induction of potent humoral responses (Figure 4). Selection of an appropriate adjuvant is also a critical parameter for the induction of the immunity of the desired type. For example, although TLR-ligands are widely considered to promote protective immunity against infectious agents, selecting the appropriate ligand will be critical. For instance, TLR2 ligation, which promotes the induction of Treg cells rather than Th1 or Th17 cells (Manicassamy et al., 2009), does not appear to be a preferred option for cancer vaccines. Thus, the challenge is to match the molecular target on DCs with the desired immune outcome, mimicking in many ways the natural role of these DC receptors to fine tune responses appropriate to the infection. Another strategy to target DCs is the usage of probiotic lactic acid bacteria to target mucosal DCs in the gut upon oral administration. Genetic manipulation of such bacteria could allow coupling of antigen expression and adjuvant effect of microbial products (Mohamadzadeh et al., 2008).
DCs originating from a specific tissue have the capacity to instruct T cells to home back to that tissue (Mora et al., 2003), while different DC subsets might provide even more detailed instructions. Furthermore, DCs activated by different adjuvants could induce T cells with entirely different migration properties. Addressing this aspect is critical for the design of vaccines, where optimal sites for T cell migration may vary in different disease states. For example, whereas vaccines against melanoma are expected to induce T cells that migrate into tumor sites including skin, vaccines against influenza virus are desired to induce T cells to migrate into airway mucosal surfaces. Therefore, multiple parameters need to be considered for the development of DC targeting vaccines. These include: 1) biological function of target DC subsets (induction of humoral vs cellular immunity), 2) the tissue distribution and receptors expressed by the target population to ensure antigen delivery, and 3) and the activation receptors expressed by a given DC subset so as to guide the choice of adjuvant. Thus, a more complete understanding of the human DC subset biology will be necessary for the next generation of efficient DC-based vaccines. Other essential components of a successful vaccine are the selection of antigen and its formulation. These issues will be discussed below in the context of therapeutic cancer vaccines.
Therapeutic vaccines in cancer
The prospect of DC targeted vaccines for the treatment of infectious disease seems promising. Recently, active immunization against an infectious agent has been shown to hold great promise in cancer, namely the prevention of HPV-positive cervical cancer by vaccinating with a recombinant viral capsid protein (Harper et al., 2006). Unfortunately, the vast majority of human cancers do not have an obvious etiologic agent, so vaccine approaches in oncology would have to be therapeutic. In cancer, however, this task comes with a number of special challenges. First and foremost, most cancer antigens are non-mutated self-proteins and thus the repertoire is depleted of high avidity clones through negative selection (Finn, 2003). As a result, tolerance must be overcome, and overcome in the context of patients whose tumors often induce a tolerogenic milieu.
Numerous approaches for the therapeutic vaccination of humans with cancer have been developed including: autologous and allogeneic tumor cells (which are often modified to express various cytokines), peptides, proteins and DNA vaccines (reviewed in (Dougan and Dranoff, 2009)) (Figure 5). The observed results have been variable, yet in many cases, a tumor-specific immune response could be measured. The clinical efficacy of therapeutic vaccination in cancer has been questioned (Rosenberg et al., 2004) because of the limited rate of objective tumor regressions observed in clinical trials. At least two issues need to be considered: 1) the quality of immune responses that these early cancer vaccines were capable of eliciting; and 2) definitions of clinical endpoints allowing assessment of efficacy.
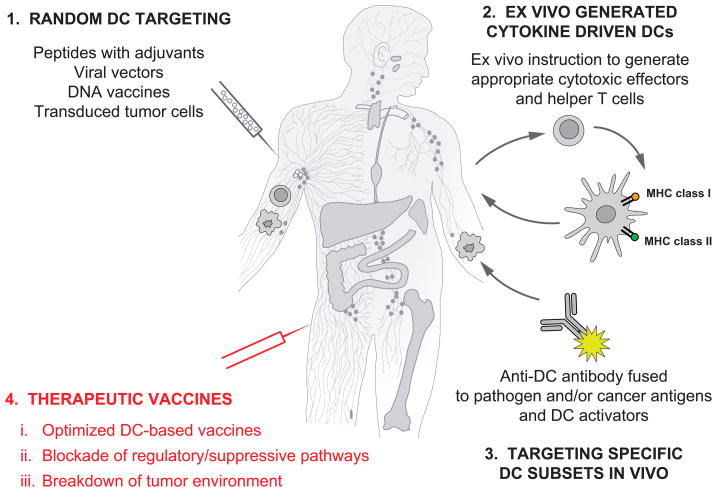
Figure 5. Approaches to DC-based therapeutic vaccination in cancer and chronic infections.
- Vaccines based on antigen with or without adjuvant that target DCs randomly. That might result in vaccine antigens being taken up by a “wrong” type of DCs in the periphery which might lead to “unwanted” type of immune response. Vaccine antigens could also flow to draining lymph nodes where they can be captured by resident DCs; 2) Vaccines based on ex-vivo generated antigen-loaded cytokine-driven DCs that are injected back into patients; and 3) specific in vivo DC targeting with anti-DC antibodies linked (by fusion or conjugation) fused antigens and with DC activators. 4) Next generation clinical trials will test optimized DC vaccines combined with patient-adjusted approaches to block Tregs and to breakdown the suppressive tumor environment. These therapies will be tested in pre-selected patients thereby leading to personalized therapy.
Concerning the first point, the vast majority of early attempts at cancer vaccines were performed in the absence of any firm understanding of DCs or their role in immunization. The targeting of untargeted peptides, often in weak or ineffective adjuvants, was (and still is, even in some large ongoing clinical trials) a commonplace. It should be clear that such approaches should have had, a priori, a low likelihood of even generating a robust immune response much less one that is therapeutically protective.
Concerning the second point, defining clinical endpoints, the use of conventional RECIST (“response evaluation criteria in solid tumors”) measures to judge efficacy has been challenged by recent clinical trials testing anti-CTLA4 (ipilumimab) in patients with stage IV melanoma. There, in a randomized phase III clinical trial a two-fold improved overall survival in patients who received anti-CTLA4 was observed, but without early indications of tumor shrinkage (Hodi et al., 2010). In another indication an active immunotherapy product, sipuleucel-T (APC8015), based on the PBMCs activated with a fusion protein of prostate cancer antigen such as prostatic acid phosphatase PAP with GM-CSF, resulted in approximately 4 month-prolonged median survival in phase III trials in patients with prostate cancer (Higano et al., 2009). In both studies, the analysis of survival curves shows the separation only after 4–6 months suggesting a certain delay in the treatment effect, just as one would expect if efficacy could only occur after the induction or re-direction of anti-tumor immunity. Many questions remain concerning the therapeutic mechanisms underlying the results obtained in these trials. For example, there is no data in the sipuleucel-T trial that antigen-specific immunity was even achieved in responding patients. Yet, these modest but very real successes will help define the basic principles of active immunotherapy which set this treatment modality apart from chemotherapy, radiotherapy, targeted therapies and even adoptive T cell transfer.
Unlike what happens when conventional cytotoxic therapies are used, the time in which it takes to build tumor immunity tumors might progress before they actually regress; and tumors might appear clinically enlarged due to inflammation associated with active immune responses and lymphocyte infiltration. Thus, the clinical oncologist’s and drug developer’s expectation of instantaneous tumor “melting” may have to be managed, as may also be the case even for many ultimately effective non-immune-based targeted therapies. Although it may be tempting to conclude that overall survival may be the only true parameter of clinical efficacy, such a situation would greatly impede progress and patient access to new therapies since survival-based trials can be exceedingly long and costly. The need for modernized objective, quantifiable response criteria cannot be overemphasized. In this context, a number of studies demonstrated in small groups of patients with cancer that a success or failure of therapeutic vaccination is correlated with the expansion of antigen-specific effector T cells (Paczesny et al., 2004; Welters et al., 2010). Patients who fail are those in whom antigen-specific CD4+CD25+Foxp3+ regulatory T cells outnumber the antigen-specific effector T cells (Welters et al., 2010). Thus, antigen-specific immune responses should remain among the key parameters of efficacy. A better understanding of how effective vaccines for example Influenza vaccine or yellow fever vaccine stimulate protective immune responses (Gaucher et al., 2008; Querec et al., 2009), might contribute to a better understanding of immune parameters of vaccine efficacy in cancer and chronic infections. Indeed, engineering vaccines to precisely target pathogens and cancer cells requires establishing the laws of immunity (Yewdell, 2010).
Cell-based vaccines
Ex vivo-generated DCs have been used as therapeutic vaccines in patients with metastatic cancer for over a decade and early studies have been reviewed elsewhere (Palucka et al., 2007). Importantly, a number of clinical studies have shown that DCs can expand T cells specific for non-mutated self proteins that are over-expressed in cancer. The analysis of immunological and clinical responses yields three patient groups: 1) one with no response; 2) one with immunological response but no clinical responses; and 3) the third one with both immunological and clinical responses. This third group is currently the smallest one but these patients are essential and they need to be studied in-depth as they will eventually permit us to understand the immune mechanisms that need to be established to control tumor growth and eliminate established tumors.
From the analysis of vaccinated patients, four parameters emerge as critical to understanding if a vaccine-induced immune response can be protective: 1) the quality of elicited CTLs; 2) the quality of induced CD4+ helper T cells; 3) the elimination and/or non-activation of Tregs; and 4) the breakdown of the immunosuppressive tumor microenvironment. Indeed, CD8+ T cells play important roles in clearance of tumor cells and infected cells and are the actual drug elicited by vaccines. The immune responses elicited by the first generation DC vaccines might not be of the quality required to allow the rejection of bulky tumors. For example, the induced CD8+ T cells might not migrate into the tumor lesions (Appay et al., 2008; Harlin et al., 2009). Furthermore, low avidity CD8+ T cells might not be able to recognize peptide-MHC class I complexes on tumor cells and/or to kill them (Appay et al., 2008). Finally, the tumor micro-environment might inhibit effector CD8+ T cell functions, for example, through myeloid-derived suppressor cells and Treg cells (for review (Gabrilovich and Nagaraj, 2009)). In this context, the quality of CD4+ T cells also represents a parameter essential for the outcome of immune response. CD4+ T cells can contribute to anti-tumor immunity (Pardoll and Topalian, 1998) through different mechanisms including i) provision of help in the expansion of tumor antigen-specific CTLs (Antony et al., 2005); ii) activation of macrophages at tumor sites (Corthay et al., 2005); iii) active killing of tumor cells (Quezada et al.); and iv) the induction of long-term memory CD8+ T cells (Sun and Bevan, 2003). However, CD4+ T cells can also be detrimental, be it in the form of Treg cells that might dampen elicited CD8+ T cell responses (Roncarolo et al., 2001a), or pro-tumor type 2 cytokine secreting CD4+ T cells that counteract anti-tumor immunity by promoting tumor development (Aspord et al., 2007) and/or by polarizing tumor associated macrophages (DeNardo et al., 2009).
The recent progress in immunomonitoring of specific immune responses in the blood (Palucka et al., 2006) and at the tumor site should help us address these questions. Modern approaches including polychromatic flow cytometry rather than the analysis of a single cytokine (e.g., IFN-γ ELISPOT) and/or frequency of tetramer positive cells will contribute to a better assessment of the quality of the immune responses elicited in the patients (Seder et al., 2008). Indeed, several studies, mostly performed in the context of HIV vaccines, have led to the conclusion that a mere measurement of the frequency of IFN-γ secreting CD8+ T cells is insufficient to evaluate the quality of vaccine-elicited immunity (Appay et al., 2008).
Antibody-based vaccines
The experimental success of using DC-specific antibodies to target antigens to individual DC subsets in conjunction with appropriately chosen adjuvants has appealing potential for the design of anti-cancer vaccines. Combined with a powerful adjuvant, vaccinating with one or multiple tumor-derived antigens coupled to DC-specific antibodies may amplify existing responses or break tolerance enabling the generation of protective responses. Because such responses would have to be MHC class I-restricted, the approach might be more efficient if directed at DC populations adapted for cross presentation, together with adjuvants that will activate their particular TLRs. Studies to date demonstrate the targeting of tumor antigens to DCs and LCs (Flacher et al., 2009) and the generation of therapeutic anti-tumor immunity (Sancho et al., 2008) in animal models. The BDCA3+ subpopulation of myeloid DCs, as the likely human homolog of the CD8α+ DC subpopulation, may be of special interest with respect to their potential for priming CD8+ T cell responses.
Furthermore, targeting both tumor and control antigens to human DCs ex vivo can lead to efficient antigen presentation and generation of CD4+ T cell (Birkholz et al., 2010) and CD8+ T cell (Bozzacco et al., 2007; Klechevsky et al., 2010) responses. Importantly, certain lectins, including Dectin-1, LOX-1 and DC-SIGN, as well as other DC surface molecules (e.g. CD40), also provide activation signals (Brown, 2006; Delneste et al., 2002; Figdor et al., 2002; Geijtenbeek et al., 2004). They can thus be exploited for both antigen delivery and activation pathway in a single targeted vaccine. The therapeutic success of these vaccines will build on the recent knowledge and progresses in our understanding of the biology of human DC subsets, cutaneous mDCs in particular.
A major challenge of this approach will be not only achieving T cell responses, but T cell responses that are sufficiently robust and long lasting so as to be clinically active. In the case of cancer, however, it will be possible to treat patients repeatedly and with more aggressive adjuvant combinations than is traditionally the case when developing prophylactic vaccines for infectious agents. In addition, it will almost certainly be beneficial to combine any such vaccination approaches with other agents, both immune and non-immune, as discussed below.
Other antigen delivery systems are also under active investigation, particularly viral vector-based. However, less is known regarding how such vectors enable antigen and adjuvant delivery to DCs.
The problem of antigen selection
Another major challenge remains the selection of antigen. The problem is relatively straight forward for prophylactic vaccines, assuming one understands which epitopes are neutralizing, expressed during human infection, and immunogenic. In the case of cancer antigens, the choice is less clear.
Candidate tumor antigens include: i) unique (mutated) antigens; and ii) shared self-antigens (Parmiani et al., 2007). The choice between these types of antigens for vaccination could be viewed as choice between inducing immunity (mutated antigens, antigens not expressed during negative selection in the thymus) or breaking tolerance and inducing autoimmunity (overexpressed antigen, differentiation antigens). The debate about which type of antigen will be most effective is still open, and will likely remain open until optimized delivery vehicles and adjuvants are developed for use in humans. The presumed advantages of mutated antigens are based on their potential to be recognized as non-self by the immune system and their potential resistance to negative selection in case the mutated protein is essential for cell survival, such as the B-Raf V600E epitope in melanoma (Parmiani et al., 2007). Furthermore, mutated antigens may select T cell receptors of higher affinity than shared antigens (due to the absence of thymic negative selection) and minimize the prevalence of antigen-specific Tregs (unless the tumor has already induced self-tolerance in the periphery) (Parmiani et al., 2007).
An example of a very potent anti-tumor and autoreactive response against self antigen is provided by studies on paraneoplastic diseases and onconeuronal antigens (Darnell, 1994). Onconeural antigens which are normally expressed in immune privileged sites, such as neurons, can also be expressed in some cases of breast and ovarian cancer. In these patients a strong antigen-specific CD8+ T cell response is generated (Albert et al., 1998), which provides effective tumor control but also autoreactive neurologic disease, paraneoplastic cerebellar degeneration. It is also the case that in melanoma patients, the existence of robust T cell responses to tumor-associated antigens (even shared antigens) is common (Nagorsen et al., 2003). Thus, immunity has occurred, it is just not protective, either due to T cell anergy or Treg cell prevalence. The example proves, however, that it is possible to generate T cell responses, even endogenously, to tumor antigens. Indeed recent results have demonstrated that antigen-specific T cells accumulate within tumor beds in melanoma patients (Rosenberg and Dudley, 2009). A vaccine would try to amplify or re-direct these responses to therapeutic efficacy.
Various groups have attempted to rank the potential of the numerous cancer-associated antigens that have been described to date (Cheever et al., 2009). In the absence of objective data in humans, it is difficult to make such assessments, so another approach has been to score either the expression of genes giving rise to tumor antigens or the physical presence of individual peptide-MHC class I complexes expressed at the surface of tumor cells. To obtain optimal coverage, even for a tumor in an individual patient, it may be necessary to immunize with several antigens simultaneously, although a single strong response may be sufficient. It is also possible that the best antigens will not be abundant as peptides at the tumor cell surface, and therefore not detectable by biochemical approaches. Absent approaches that enable the DC targeting of complex mixtures of tumor antigens, it seems most reasonable to begin this effort by using those antigens that can be objectively identified in the hope that improved delivery approaches and adjuvants will yield positive, protective immune responses. Focused pre-clinical and clinical studies should be employed to test this hypothesis.
Thus far, most focus has been placed on protein antigens whose peptides can be presented on the cell surface in complexes with classical MHC molecules (Townsend et al., 1985). However, tumors also express altered lipids and sugars that are presented by CD1 molecules (Hava et al., 2005). These can also be harnessed for improved vaccination, for example NKT cells that are thought to recognize lipid antigens can generate protective response with IFN-γ secretion (Fujii et al., 2002). Accordingly, injection of cancer patients with DCs loaded with NKT cell ligand, alpha-galactosyl-ceramide leads to sustained expansion of antigen-specific T cells (Chang et al., 2005).
A potentially interesting approach in the selection of antigen targets is suggested by the possibility that tumors are maintained by specialized subpopulations of “cancer stem cells” (Lobo et al., 2007). Although the definition and identity of these cells remains highly controversial, tumor cells that routinely survive conventional chemotherapy or targeted therapies are the ones that are responsible for tumor relapse and death. If these cells have special properties, or even if not, combining vaccine approaches with non-lymphoablative front line therapies may provide an optimal setting for generating protective immune responses.
Antigen formulation
An additional important problem is the form of antigen that should be delivered in the context of a vaccine, either preventative or therapeutic. Although peptides have often been used for immunization, as free entities peptides have poor pharmacokinetic properties and are rapidly cleared. Coupling them to carriers helps somewhat, but chemical or genetic coupling to DC targeted antibodies would appear the most efficient approach to get them to their required destinations. The use of peptides, of course, may pre-suppose the identification of relevant T cell epitopes, so conceivably the use of proteins may be preferable, or protein-peptide mixtures contained within an antibody-targeted carrier device (e.g. nanoparticle), which would enable the use of multiple potential antigens. In this context, recent studies indicate improved immunogenicity when viral antigens from HPV (Kenter et al., 2009) or HIV (Pialoux et al., 2001) are delivered in the form of long peptides together with adjuvants.
A further consideration is antigen stability. DCs exhibit a remarkably attenuated capacity for protein degradation, which serves to extend the longevity of internalized antigens enabling a constant supply of endogenously produced peptides for loading on to both MHC class I and class II molecules (Delamarre et al., 2005, Science). The simple rule, then, is that antigens (even endogenous ones) that are long lived are generally better than antigens that are more rapidly degraded (Delamarre et al., 2006, JEM). The extracellular and intracellular fates of antigens therefore will matter, and attention needs to be paid towards providing administered antigens in a form that maximizes half life.
Are DCs enough?
In view of the remarkable diversity of suppressive pathways present in patients with metastatic cancer, any durable clinical response elicited by vaccination is already an achievement. However, to improve the outcomes, DC vaccines need to be combined, in particular for patients at advanced stages, with other therapies that offset the suppressive tumor environment (Dougan and Dranoff, 2009; Melief, 2008). Such combination regimens will involve several intervention strategies that target different pathways. In particular, blocking antibodies or soluble receptors can be exploited for the blockade of suppressive cytokines in the tumor microenvironment for example IL-10 (Moore et al., 2001), IL-13 (Terabe et al., 2000), or TGF-β (Li et al., 2005). Tumor cells can often express surface molecules that inherently suppress T cell activity, notably PD-L1, which comes up especially in tumors that express oncogenic mutations in the PI3 kinase pathway. Antibodies to PD-1 on activated T cells, or to PD-L1, might thus reverse T cell exhaustion or anergy (Pilon-Thomas et al., 2010), and in early clinical studies, treatment with anti-PD1 exerts some beneficial clinical effect (Brahmer et al., 2010). It is a common observation in melanoma (and other cancers) that patients exhibit pre-existing T cell responses without a vaccine ever having been purposefully administered. These T cells are not protective, or at least not sufficiently protective despite the fact that they can often be recovered from tumor beds (Rosenberg and Dudley, 2009), suggesting that re-activation strategies may be useful on their own.
These examples emphasize that DCs may not be enough, and in some cases, may not even be strictly necessary, at least from the treating physician’s point of view. An endogenous vaccine may be created by necrotic or apoptotic death of tumor cells following chemotherapy or targeted therapy, where tumor antigens released in conjunction with “danger signals” from the dying cells are internalized by infiltrating DCs and then presented to T cells. A further therapeutic vaccine may help amplify these responses, or perhaps re-tool them to be more immunoprotective than immunoregulatory, but a more effective approach in such instances might be to target the T cells themselves. This is the goal of anti-CTLA4-based therapies (Peggs et al., 2009). Conceivably, antibodies to PD1-PDL1 might also achieve this goal, and in a fashion with less autoimmune toxicity since only those T cells encountering their cognate antigen in the context of PD1-PDL1 interactions would be stimulated.
Just as different tumors are currently treated with different combinations of cytostatic drugs and targeted therapies, we foresee the development of clinical protocols combining DC vaccines with individualized adjunct therapies, most likely those involving non-lymphoablative cytotoxic or targeted therapies (Figure 5). In melanoma, the recent demonstration of dramatic but transient responses in patients expressing the V600E oncogenic mutation with a specific B-Raf inhibitor (Boni et al., 2010) creates a remarkable opportunity to implement just such combination therapies. For such complex therapies to be designed rationally, however, careful attention will have to be paid towards profiling the immunological status of individual patients and their tumors before, during, and after therapy. Patient selection and immunological markers attesting to the effects of a given therapeutic attempt will be key to understanding why an approach does or does not work.
We have a dream
Studies performed in the last decade have highlighted the commonalities and uniqueness of the various DC subsets. This new knowledge represents a fertile ground to work on to design better strategies for intervening in numerous clinical situations. The capacity of LCs and CD14+ DCs to preferentially prime cellular immunity and humoral immunity respectively has significant implications, most particularly in the context of novel human vaccines. Thus, targeting LCs will be important for the design of vaccines that aim at eliciting strong cellular immunity. Such vaccines might be particularly useful at preventing, and perhaps even treating, chronic diseases including viral (HIV, Hepatitis C Virus), bacterial (mycobacteria) and parasitic (malaria) diseases, as well as cancer. The most efficient vaccines might actually be those that will target both CD14+ DCs and LCs, thereby allowing the maximal stimulation of both humoral and cellular immune responses. In this regard it is intriguing to consider that one of the most effective vaccines, smallpox vaccine, acts through a combination of strong cellular and humoral immunity and requires scarification of the skin, a procedure that injures both epidermis and dermis and that is likely to mobilize and activate LCs as well as dermal DCs. Likewise, one of the most potent vaccines ever generated against Yellow Fever (YF17D) activate multiple DC subsets (Querec et al., 2006) and leads to integrated immune response that include both humoral and cellular immunity (Gaucher et al., 2008).
We foresee that the improved vaccines that target DCs will permit us to treat and prevent many chronic diseases, and likewise, manipulation of DCs will also permit to dampen overly enhanced immune responses as occurs in allergy and autoimmunity possibly by turning on regulatory mechanisms.
Box 1. Key areas for future research to facilitate design of novel vaccines.
KEY AREAS OF INVESTIGATION TO DEVELOP NOVEL PROTECTIVE AND THERAPEUTIC VACCINES
Protective antigens and their formulations
How DCs acquire and process antigens
Adjuvants
Molecular mechanisms by which DCs translate membrane and cytoplasmic signals to adaptive immunity
Which DC subsets can be linked with which subset of regulatory/suppressor cells
Acknowledgments
Dedicated to patients and volunteers who participated in our studies and clinical trials at BIIR and Genentech. We thank former and current members of BIIR, Genentech, and Yale Medical School for their contributions, in particular: Hideki Ueno, Joseph Fay, Sangkon Oh, Virginia Pascual, Lee Roberts, Gerard Zurawski, Lelia Delamarre, and Sergio Trombetta. Supported by the NIH (P01 CA084514, U19 AIO57234, R01 CA089440 and CA078846), the Dana Foundation, the Susan Komen Foundation, the Baylor Health Care System; the Baylor Health Care System Foundation, the ANRS and the INSERM. KP holds the Michael A. Ramsay Chair for Cancer Immunology Research. JB holds the Caruth Chair for Transplant Immunology Research. We also thank Allison Bruce (Genentech) for excellent assistance with artwork.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- Albert ML, Darnell JC, Bender A, Francisco LM, Bhardwaj N, Darnell RB. Tumor-specific killer cells in paraneoplastic cerebellar degeneration. Nat Med. 1998;4:1321–1324. doi: 10.1038/3315. [DOI] [PubMed] [Google Scholar]
- Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, Carbone FR. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- Alvarez D, Harder G, Fattouh R, Sun J, Goncharova S, Stampfli MR, Coyle AJ, Bramson JL, Jordana M. Cutaneous antigen priming via gene gun leads to skin-selective Th2 immune-inflammatory responses. J Immunol. 2005;174:1664–1674. doi: 10.4049/jimmunol.174.3.1664. [DOI] [PubMed] [Google Scholar]
- Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW, et al. CD8+ T cell immunity against a tumor/self-antigen Is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appay V, Douek DC, Price DA. CD8+ T cell efficacy in vaccination and disease. Nat Med. 2008;14:623–628. doi: 10.1038/nm.f.1774. [DOI] [PubMed] [Google Scholar]
- Arima K, Watanabe N, Hanabuchi S, Chang M, Sun SC, Liu YJ. Distinct signal codes generate dendritic cell functional plasticity. Science signaling. 2010;3:ra4. doi: 10.1126/scisignal.2000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspord C, Pedroza-Gonzalez A, Gallegos M, Tindle S, Burton EC, Su D, Marches F, Banchereau J, Palucka AK. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med. 2007;204:1037–1047. doi: 10.1084/jem.20061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachem A, Guttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, Salama A, Movassaghi K, Opitz C, Mages HW, et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1273–1281. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, Allan RS, Wojtasiak M, Shortman K, Carbone FR, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- Birkholz K, Schwenkert M, Kellner C, Gross S, Fey G, Schuler-Thurner B, Schuler G, Schaft N, Dorrie J. Targeting of DEC-205 on human dendritic cells results in efficient MHC class II-restricted antigen presentation. Blood. 2010 doi: 10.1182/blood-2010-02-268425. [DOI] [PubMed] [Google Scholar]
- Bluestone JA, Mackay CR, O’Shea JJ, Stockinger B. The functional plasticity of T cell subsets. Nat Rev Immunol. 2009;9:811–816. doi: 10.1038/nri2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, Ferrone CR, Flaherty KT, Lawrence DP, Fisher DE, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70:5213–5219. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzacco L, Trumpfheller C, Siegal FP, Mehandru S, Markowitz M, Carrington M, Nussenzweig MC, Piperno AG, Steinman RM. DEC-205 receptor on dendritic cells mediates presentation of HIV gag protein to CD8+ T cells in a spectrum of human MHC I haplotypes. Proc Natl Acad Sci U S A. 2007;104:1289–1294. doi: 10.1073/pnas.0610383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- Brown GD. Sensing necrosis with Mincle. Nat Immunol. 2008;9:1099–1100. doi: 10.1038/ni1008-1099. [DOI] [PubMed] [Google Scholar]
- Cao W, Rosen DB, Ito T, Bover L, Bao M, Watanabe G, Yao Z, Zhang L, Lanier LL, Liu YJ. Plasmacytoid dendritic cell-specific receptor ILT7-Fc epsilonRI gamma inhibits Toll-like receptor-induced interferon production. J Exp Med. 2006;203:1399–1405. doi: 10.1084/jem.20052454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celluzzi CM, Falo LD., Jr Epidermal dendritic cells induce potent antigen-specific CTL-mediated immunity. J Invest Dermatol. 1997;108:716–720. doi: 10.1111/1523-1747.ep12292095. [DOI] [PubMed] [Google Scholar]
- Chang DH, Osman K, Connolly J, Kukreja A, Krasovsky J, Pack M, Hutchinson A, Geller M, Liu N, Annable R, et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201:1503–1517. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- Cheng P, Zhou J, Gabrilovich D. Regulation of dendritic cell differentiation and function by Notch and Wnt pathways. Immunol Rev. 2010;234:105–119. doi: 10.1111/j.0105-2896.2009.00871.x. [DOI] [PubMed] [Google Scholar]
- Corthay A, Skovseth DK, Lundin KU, Rosjo E, Omholt H, Hofgaard PO, Haraldsen G, Bogen B. Primary antitumor immune response mediated by CD4+ T cells. Immunity. 2005;22:371–383. doi: 10.1016/j.immuni.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Crozat K, Guiton R, Contreras V, Feuillet V, Dutertre CA, Ventre E, Vu Manh TP, Baranek T, Storset AK, Marvel J, et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207:1283–1292. doi: 10.1084/jem.20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell RB. Paraneoplastic syndromes. In: Feldmann E, editor. Current diagnosis in neurobiology. 1994. pp. 137–141. [Google Scholar]
- Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307:1630–1634. doi: 10.1126/science.1108003. [DOI] [PubMed] [Google Scholar]
- Delneste Y, Magistrelli G, Gauchat J, Haeuw J, Aubry J, Nakamura K, Kawakami-Honda N, Goetsch L, Sawamura T, Bonnefoy J, Jeannin P. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–362. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pucchio T, Chatterjee B, Smed-Sorensen A, Clayton S, Palazzo A, Montes M, Xue Y, Mellman I, Banchereau J, Connolly JE. Direct proteasome-independent cross-presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class I. Nat Immunol. 2008;9:551–557. doi: 10.1038/ni.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez PM, Ardavin C. Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol Rev. 2010;234:90–104. doi: 10.1111/j.0105-2896.2009.00876.x. [DOI] [PubMed] [Google Scholar]
- Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- Dullaers M, Li D, Xue Y, Ni L, Gayet I, Morita R, Ueno H, Palucka KA, Banchereau J, Oh S. A T cell-dependent mechanism for the induction of human mucosal homing immunoglobulin A-secreting plasmablasts. Immunity. 2009;30:120–129. doi: 10.1016/j.immuni.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- Figdor CG, van Kooyk Y, Adema GJ. C-type lectin receptors on dendritic cells and Langerhans cells. Nat Rev Immunol. 2002;2:77–84. doi: 10.1038/nri723. [DOI] [PubMed] [Google Scholar]
- Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- Flacher V, Sparber F, Tripp CH, Romani N, Stoitzner P. Targeting of epidermal Langerhans cells with antigenic proteins: attempts to harness their properties for immunotherapy. Cancer Immunol Immunother. 2009;58:1137–1147. doi: 10.1007/s00262-008-0563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flornes LM, Bryceson YT, Spurkland A, Lorentzen JC, Dissen E, Fossum S. Identification of lectin-like receptors expressed by antigen presenting cells and neutrophils and their mapping to a novel gene complex. Immunogenetics. 2004;56:506–517. doi: 10.1007/s00251-004-0714-x. [DOI] [PubMed] [Google Scholar]
- Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat Immunol. 2002;3:867–874. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- Fukaura H, Kent SC, Pietrusewicz MJ, Khoury SJ, Weiner HL, Hafler DA. Induction of circulating myelin basic protein and proteolipid protein-specific transforming grwoth factor-b1-secreting Th3 cells by oral administration of myelin in multiple sclerosis patients. J Clin Invest. 1996;98:70–77. doi: 10.1172/JCI118779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaucher D, Therrien R, Kettaf N, Angermann BR, Boucher G, Filali-Mouhim A, Moser JM, Mehta RS, Drake DR, 3rd, Castro E, et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med. 2008;205:3119–3131. doi: 10.1084/jem.20082292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek TB, van Vliet SJ, Engering A, t Hart BA, van Kooyk Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu Rev Immunol. 2004;22:33–54. doi: 10.1146/annurev.immunol.22.012703.104558. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol. 2010;10:453–460. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, McKee M, Gajewski TF. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, Jenkins D, Schuind A, Costa Clemens SA, Dubin G. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367:1247–1255. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- Hava DL, Brigl M, van den Elzen P, Zajonc DM, Wilson IA, Brenner MB. CD1 assembly and the formation of CD1-antigen complexes. Curr Opin Immunol. 2005;17:88–94. doi: 10.1016/j.coi.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Hawiger D, Inaba K, Dorsett Y, Guo K, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–780. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, Schmidt R, Harris AL, Old L, Cerundolo V. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171:5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, Provost N, Frohlich MW. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010 doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- Idoyaga J, Cheong C, Suda K, Suda N, Kim JY, Lee H, Park CG, Steinman RM. Cutting edge: langerin/CD207 receptor on dendritic cells mediates efficient antigen presentation on MHC I and II products in vivo. J Immunol. 2008;180:3647–3650. doi: 10.4049/jimmunol.180.6.3647. [DOI] [PubMed] [Google Scholar]
- Ito T, Wang YH, Duramad O, Hanabuchi S, Perng OA, Gilliet M, Qin FX, Liu YJ. OX40 ligand shuts down IL-10-producing regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:13138–13143. doi: 10.1073/pnas.0603107103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, Qin XF, Liu YJ, Gilliet M. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- Jiang A, Bloom O, Ono S, Cui W, Unternaehrer J, Jiang S, Whitney JA, Connolly J, Banchereau J, Mellman I. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity. 2007;27:610–624. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffre O, Nolte MA, Sporri R, Reis e Sousa C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev. 2009;227:234–247. doi: 10.1111/j.1600-065X.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, Chen CJ, Dunbar PR, Wadley RB, Jeet V, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med. 2010;207:1247–1260. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastelein RA, Hunter CA, Cua DJ. Discovery and Biology of IL-23 and IL-27: Related but Functionally Distinct Regulators of Inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM, Offringa R, Drijfhout JW, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Klechevsky E, Flamar AL, Cao Y, Blanck JP, Liu M, O’Bar A, Agouna-Deciat O, Klucar P, Thompson-Snipes L, Zurawski S, et al. Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR. Blood. 2010 doi: 10.1182/blood-2010-01-264960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klechevsky E, Liu M, Morita R, Banchereau R, Thompson-Snipes L, Palucka AK, Ueno H, Banchereau J. Understanding human myeloid dendritic cell subsets for the rational design of novel vaccines. Hum Immunol. 2009;70:281–288. doi: 10.1016/j.humimm.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, Chaussabel D, Zurawski G, Palucka AK, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–266. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- Leslie DS, Vincent MS, Spada FM, Das H, Sugita M, Morita CT, Brenner MB. CD1-mediated γ/δ T cell maturation of dendritic cells. J Exp Med. 2002;196:1575–1584. doi: 10.1084/jem.20021515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levings MK, Gregori S, Tresoldi E, Cazzaniga S, Bonini C, Roncarolo MG. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood. 2005;105:1162–1169. doi: 10.1182/blood-2004-03-1211. [DOI] [PubMed] [Google Scholar]
- Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming Growth Factor-beta Regulation of Immune Responses. Annu Rev Immunol. 2005 doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–699. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic Cells Prime Natural Killer Cells by trans-Presenting Interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh C-S, Culpepper JA, Wysocka M, Trinchieri G, Murphy KM, O’Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- Maldonado-Lopez R, De Smedt T, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M. CD8α+ and CD8α− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manicassamy S, Pulendran B. Modulation of adaptive immunity with Toll-like receptors. Semin Immunol. 2009;21:185–193. doi: 10.1016/j.smim.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manicassamy S, Ravindran R, Deng J, Oluoch H, Denning TL, Kasturi SP, Rosenthal KM, Evavold BD, Pulendran B. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat Med. 2009;15:401–409. doi: 10.1038/nm.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang YC, Pulendran B. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- Matsui T, Connolly JE, Michnevitz M, Chaussabel D, Yu CI, Glaser C, Tindle S, Pypaert M, Freitas H, Piqueras B, et al. CD2 distinguishes two subsets of human plasmacytoid dendritic cells with distinct phenotype and functions. J Immunol. 2009;182:6815–6823. doi: 10.4049/jimmunol.0802008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurley N, Mellman I. Monocyte-derived dendritic cells exhibit increased levels of lysosomal proteolysis as compared to other human dendritic cell populations. PLoS ONE. 2010;5:e11949. doi: 10.1371/journal.pone.0011949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372–383. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- Mestas J, Hughes CCW. Of mice and men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Mohamadzadeh M, Berard F, Essert G, Chalouni C, Pulendran B, Davoust J, Bridges G, Palucka AK, Banchereau J. Interleukin 15 skews monocyte differentiation into dendritic cells with features of Langerhans cells. J Exp Med. 2001;194:1013–1020. doi: 10.1084/jem.194.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamadzadeh M, Duong T, Hoover T, Klaenhammer TR. Targeting mucosal dendritic cells with microbial antigens from probiotic lactic acid bacteria. Expert Rev Vaccines. 2008;7:163–174. doi: 10.1586/14760584.7.2.163. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- Munz C, Steinman RM, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med. 2005;202:203–207. doi: 10.1084/jem.20050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagorsen D, Scheibenbogen C, Marincola FM, Letsch A, Keilholz U. Natural T cell immunity against cancer. Clin Cancer Res. 2003;9:4296–4303. [PubMed] [Google Scholar]
- Paczesny S, Banchereau J, Wittkowski KM, Saracino G, Fay J, Palucka AK. Expansion of melanoma-specific cytolytic CD8+ T cell precursors in patients with metastatic melanoma vaccinated with CD34+ progenitor-derived dendritic cells. J Exp Med. 2004;199:1503–1511. doi: 10.1084/jem.20032118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucka AK, Ueno H, Connolly J, Kerneis-Norvell F, Blanck JP, Johnston DA, Fay J, Banchereau J. Dendritic cells loaded with killed allogeneic melanoma cells can induce objective clinical responses and MART-1 specific CD8+ T-cell immunity. J Immunother. 2006;29:545–557. doi: 10.1097/01.cji.0000211309.90621.8b. [DOI] [PubMed] [Google Scholar]
- Palucka AK, Ueno H, Fay JW, Banchereau J. Taming cancer by inducing immunity via dendritic cells. Immunol Rev. 2007;220:129–150. doi: 10.1111/j.1600-065X.2007.00575.x. [DOI] [PubMed] [Google Scholar]
- Paquette RL, Hsu NC, Kiertscher SM, Park AN, Tran L, Roth MD, Glaspy JA. Interferon-alpha and granulocyte-macrophage colony-stimulating factor differentiate peripheral blood monocytes into potent antigen-presenting cells. J Leukoc Biol. 1998;64:358–367. doi: 10.1002/jlb.64.3.358. [DOI] [PubMed] [Google Scholar]
- Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol. 1998;10:588–594. doi: 10.1016/s0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- Parmiani G, De Filippo A, Novellino L, Castelli C. Unique human tumor antigens: immunobiology and use in clinical trials. J Immunol. 2007;178:1975–1979. doi: 10.4049/jimmunol.178.4.1975. [DOI] [PubMed] [Google Scholar]
- Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- Pialoux G, Gahery-Segard H, Sermet S, Poncelet H, Fournier S, Gerard L, Tartar A, Gras-Masse H, Levy JP, Guillet JG. Lipopeptides induce cell-mediated anti-HIV immune responses in seronegative volunteers. AIDS. 2001;15:1239–1249. doi: 10.1097/00002030-200107060-00005. [DOI] [PubMed] [Google Scholar]
- Pilon-Thomas S, Mackay A, Vohra N, Mule JJ. Blockade of Programmed Death Ligand 1 Enhances the Therapeutic Efficacy of Combination Immunotherapy against Melanoma. J Immunol. 2010 doi: 10.4049/jimmunol.0904114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin LF, Salio M, Griessinger E, Anjos-Afonso F, Craciun L, Chen JL, Keller AM, Joffre O, Zelenay S, Nye E, et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207:1261–1271. doi: 10.1084/jem.20092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R, Akira S, Ahmed R, Pulendran B. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med. 2006;203:413–424. doi: 10.1084/jem.20051720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, Pirani A, Gernert K, Deng J, Marzolf B, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10:116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, et al. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010 doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehwinkel J, Tan CP, Goubau D, Schulz O, Pichlmair A, Bier K, Robb N, Vreede F, Barclay W, Fodor E, Reis e Sousa C. RIG-I detects viral genomic RNA during negative-strand RNA virus infection. Cell. 2010;140:397–408. doi: 10.1016/j.cell.2010.01.020. [DOI] [PubMed] [Google Scholar]
- Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- Romani N, Gruner S, Brang D, Kampgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch PO, Steinman RM, Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001a;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- Roncarolo MG, Levings MK, Traversari C. Differentiation of T regulatory cells by immature dendritic cells. J Exp Med. 2001b;193:F5–9. doi: 10.1084/jem.193.2.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- Sancho D, Joffre OP, Keller AM, Rogers NC, Martinez D, Hernanz-Falcon P, Rosewell I, Reis e Sousa C. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho D, Mourao-Sa D, Joffre OP, Schulz O, Rogers NC, Pennington DJ, Carlyle JR, Reis e Sousa C. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest. 2008;118:2098–2110. doi: 10.1172/JCI34584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt N, Morita R, Bourdery L, Bentebibel SE, Zurawski SM, Banchereau J, Ueno H. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 2009;31:158–169. doi: 10.1016/j.immuni.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- Shaw J, Wang YH, Ito T, Arima K, Liu YJ. Plasmacytoid dendritic cells regulate B-cell growth and differentiation via CD70. Blood. 2010;115:3051–3057. doi: 10.1182/blood-2009-08-239145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- Siddiqui KR, Laffont S, Powrie F. E-cadherin marks a subset of inflammatory dendritic cells that promote T cell-mediated colitis. Immunity. 2010;32:557–567. doi: 10.1016/j.immuni.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Crowe NY, Hayakawa Y, Takeda K, Yagita H, Godfrey DI. NKT cells --- conductors of tumor immunity? Curr Opin Immunol. 2002;14:165–171. doi: 10.1016/s0952-7915(02)00316-3. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- Stoitzner P, Tripp CH, Eberhart A, Price KM, Jung JY, Bursch L, Ronchese F, Romani N. Langerhans cells cross-present antigen derived from skin. Proc Natl Acad Sci U S A. 2006;103:7783–7788. doi: 10.1073/pnas.0509307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, Carbone DP, Paul WE, Berzofsky JA. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1:515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- Townsend AR, Gotch FM, Davey J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell. 1985;42:457–467. doi: 10.1016/0092-8674(85)90103-5. [DOI] [PubMed] [Google Scholar]
- Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- Ueno H, Klechevsky E, Morita R, Aspord C, Cao T, Matsui T, Di Pucchio T, Connolly J, Fay JW, Pascual V, et al. Dendritic cell subsets in health and disease. Immunol Rev. 2007;219:118–142. doi: 10.1111/j.1600-065X.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- Ueno H, Schmitt N, Klechevsky E, Pedroza-Gonzalez A, Matsui T, Zurawski G, Oh S, Fay J, Pascual V, Banchereau J, Palucka K. Harnessing human dendritic cell subsets for medicine. Immunol Rev. 2010;234:199–212. doi: 10.1111/j.0105-2896.2009.00884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladeau J, Saeland S. Cutaneous dendritic cells. Semin Immunol. 2005;17:273–283. doi: 10.1016/j.smim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- van der Aar AM, Sylva-Steenland RM, Bos JD, Kapsenberg ML, de Jong EC, Teunissen MB. Loss of TLR2, TLR4, and TLR5 on Langerhans cells abolishes bacterial recognition. J Immunol. 2007;178:1986–1990. doi: 10.4049/jimmunol.178.4.1986. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Wang YH, Lee HK, Ito T, Cao W, Liu YJ. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–1185. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 Family Cytokines and the Expanding Diversity of Effector T Cell Lineages. Annu Rev Immunol. 2007 doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- Welters MJ, Kenter GG, de Vos van Steenwijk PJ, Lowik MJ, Berends-van der Meer DM, Essahsah F, Stynenbosch LF, Vloon AP, Ramwadhdoebe TH, Piersma SJ, et al. Success or failure of vaccination for HPV16-positive vulvar lesions correlates with kinetics and phenotype of induced T-cell responses. Proc Natl Acad Sci U S A. 2010;107:11895–11899. doi: 10.1073/pnas.1006500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes M, Pombo A, Jenkins C, MacPherson GG. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J Immunol. 1998;161:1313–1319. [PubMed] [Google Scholar]
- Yamazaki S, Inaba K, Tarbell KV, Steinman RM. Dendritic cells expand antigen-specific Foxp3CD25CD4 regulatory T cells including suppressors of alloreactivity. Immunol Rev. 2006;212:314–329. doi: 10.1111/j.0105-2896.2006.00422.x. [DOI] [PubMed] [Google Scholar]
- Yewdell JW. Designing CD8+ T cell vaccines: it’s not rocket science (yet) Curr Opin Immunol. 2010;22:402–410. doi: 10.1016/j.coi.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata-Gonzalez F, Rueda F, Petriz J, Domingo P, Villarroya F, de Madariaga A, Domingo JC. 9-cis-Retinoic acid (9cRA), a retinoid X receptor (RXR) ligand, exerts immunosuppressive effects on dendritic cells by RXR-dependent activation: inhibition of peroxisome proliferator-activated receptor gamma blocks some of the 9cRA activities, and precludes them to mature phenotype development. J Immunol. 2007;178:6130–6139. doi: 10.4049/jimmunol.178.10.6130. [DOI] [PubMed] [Google Scholar]