Cross Talk Between O-GlcNAcylation and Phosphorylation: Roles in Signaling, Transcription, and Chronic Disease (original) (raw)
. Author manuscript; available in PMC: 2012 Jun 7.
Abstract
O-GlcNAcylation is the addition of β-D-_N_-acetylglucosamine to serine or threonine residues of nuclear and cytoplasmic proteins. O-linked _N_-acetylglucosamine (O-GlcNAc) was not discovered until the early 1980s and still remains difficult to detect and quantify. Nonetheless, O-GlcNAc is highly abundant and cycles on proteins with a timescale similar to protein phosphorylation. O-GlcNAc occurs in organisms ranging from some bacteria to protozoans and metazoans, including plants and nematodes up the evolutionary tree to man. O-GlcNAcylation is mostly on nuclear proteins, but it occurs in all intracellular compartments, including mitochondria. Recent glycomic analyses have shown that O-GlcNAcylation has surprisingly extensive cross talk with phosphorylation, where it serves as a nutrient/stress sensor to modulate signaling, transcription, and cytoskeletal functions. Abnormal amounts of O-GlcNAcylation underlie the etiology of insulin resistance and glucose toxicity in diabetes, and this type of modification plays a direct role in neurodegenerative disease. Many oncogenic proteins and tumor suppressor proteins are also regulated by O-GlcNAcylation. Current data justify extensive efforts toward a better understanding of this invisible, yet abundant, modification. As tools for the study of O-GlcNAc become more facile and available, exponential growth in this area of research will eventually take place.
Keywords: O-GlcNAc, translation, diabetes, Alzheimer’s disease, cancer
INTRODUCTION
The modification of serine or threonine residues of nuclear and cytoplasmic proteins by the monosaccharide, β-D-_N_-acetylglucosamine was discovered in the early 1980s (1, 2) when bovine milk galactosyltransferase (GalT1) was used to probe for terminal _N_-acetylglucosamine moieties on glycoconjugates of living cells. Early studies found that O-GlcNAc is particularly enriched in chromatin and abundant on proteins on the nuclear envelope (2) but is present in most intracellular compartments. Over one thousand proteins are known to be O-GlcNAcylated, and this number continues to rapidly grow as the technology for detection of O-GlcNAc improves.
O-GlcNAcylation is an end point of the hexosamine biosynthetic pathway, which culminates in the production of UDP-GlcNAc (3–5), the high-energy donor substrate for the O-GlcNAc transferase (OGT) (Figure 1). Because the biosynthesis of UDP-GlcNAc is affected and regulated by nearly every metabolic pathway in the cell and because OGT-catalyzed O-GlcNAcylation is sensitive to insulin, to nutrients, and to cellular stress, it has been proposed that O-GlcNAcylation serves primarily to modulate cellular signaling and transcription regulatory pathways in response to nutrients and stress (6–9). Recent studies have shown that the modulation of these cellular pathways and functions by O-GlcNAcylation involves a very extensive cross talk with the pathways and mechanisms that are also regulated by protein phosphorylation signaling cascades (10–13). Thus, like phosphorylation, O-GlcNAcylation is directly involved in the regulation of many cellular processes (Figure 1). Also, like phosphorylation, O-GlcNAcylation is a rapidly cycling posttranslational modification, with OGT holoenzyme complexes regulating its addition and with O-GlcNAcase holoenzyme complexes regulating its removal. Sites of O-GlcNAcylation may often be directly at or located proximal to the same serine or threonine residues alternatively used by kinases, or the sugar may also occur at sites on a polypeptide distant from phosphoryation sites. This review highlights our current understanding of the critical role of O-GlcNAcylation in many cellular processes and its particular importance to chronic diseases of aging, such as diabetes, neurodegeneration, and cancer. Many reviews on O-GlcNAcylation have been published, which provide more details on earlier studies and on certain aspects of this broad and rapidly growing area of research (6–9, 13–23).
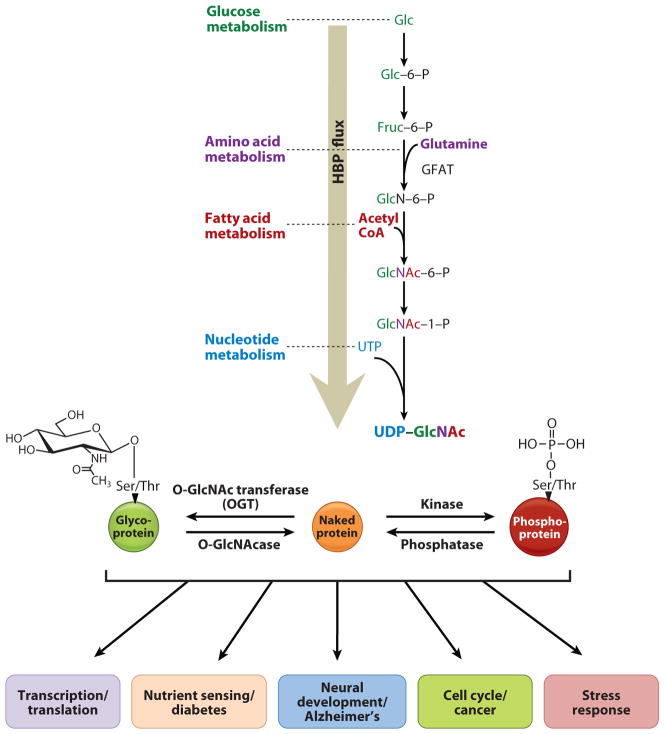
Figure 1.
The hexosamine biosynthetic pathway provides the sugar substrate for O-GlcNAcylation. When glucose enters into the cell, a small percentage is funneled directly into the hexosamine biosynthetic pathway, where it is converted into UDP-_N_-acetylglucosamine (UDP-GlcNAc). The enzyme O-GlcNAc transferase (OGT) catalyzes the addition of the amino sugar to nuclear and cytoplasmic proteins, whereas the enzyme O-GlcNAcase catalyzes the removal of the sugar. Modified proteins are involved in multiple cellular processes, such as transcription and translation, nutrient sensing, neuronal function, cell cycle, and stress. Acetyl-CoA, acetyl coenzyme A; Fru, fructose; GFAT, glucose:fructose 6-phosphate amidotransferase; Glc, glucose; NAc, _N_-acetyl; O-GlcNAc, O-linked _N_-acetylglucosamine.
DISTRIBUTION OF O-LINKED _N_-ACETYLGLUCOSAMINE CYCLING
Phylogeny
O-GlcNAcylation of nuclear and cytoplasmic proteins has been described in some bacteria, which modify their flagellins (24, 25). It is present in filamentous fungi (26), in Giardia (27), perhaps the oldest living eukaryote, and in many viruses that infect eukaryotic cells (see below). Thus far, O-GlcNAcylation has been documented in all metazoans, including Caenorhabditis elegans, insects, and plants (28). Although plants have two genes encoding different OGTs (29), animals usually have only a single gene encoding the catalytic polypeptide. In plants, O-GlcNAcylation is important for growth hormone signaling (30). Surprisingly, like tyrosine phosphorylation, O-GlcNAcylation has not yet been documented to occur in yeast. However, it remains possible that O-GlcNAcylation might occur in yeast via enzymes that have little or no homology to those presently known. It is also possible that yeast have a similar functional modification of serine and threonine residues on nuclear and cytoplasmic proteins but use a different sugar, such as mannose instead of _N_-acetylglucosamine.
Subcellular Localization
The highest density of O-GlcNAc occurs on nucleoporins (31, 32) and on some transcription factors (33), which have domains with clustered O-GlcNAc sites. Quantitatively, most O-GlcNAc occurs on chromatin proteins (2, 34). However, many cytosolic enzymes, including kinases (35–37), and glycolytic enzymes (38), most cytoskeleton regulatory proteins (39–42), and cytoskeleton proteins themselves (43–46) are also modified. In most cells, OGT is found mainly within the nucleus, and O-GlcNAcase is found mostly within the cytosol. However, both enzymes are found throughout the intracellular compartments, and little is known about the regulation of their intracellular trafficking. OGT does have a noncanonical nuclear localization sequence. The histone acetyltransferase (HAT) domain within the C-terminal half of O-GlcNAcase may play a role in its nuclear targeting (47–49). Within the nucleus and particularly at sites of transcription, the two O-GlcNAc cycling enzymes are often found within the same complex. Paradoxically, although OGT is mostly nuclear, it is excluded from the nucleolus, and O-GlcNAcase, which is mostly cytosolic, is highly enriched within the nucleolus (50). A splice variant of OGT is localized at the face of the inner mitochondrial membrane (51), but a mitochondrial form of O-GlcNAcase has not yet been described. However, the recent finding that increased O-GlcNAcylation of mitochondrial electron transport chain proteins is associated with diabetes (52) could be an important breakthrough in our understanding of the etiology of this disease (see below). While O-GlcNAcylation, catalyzed by OGT, is restricted to the cytosolic and nuclear compartments, a novel extracellular/ luminal glycosyltransferase, which catalyzes the addition of O-β-GlcNAc monosaccharide residues to extracellular domains of Notch receptor, has been reported recently (53). This so-called eOGT has no apparent homology to the nucleocytoplasmic OGT enzyme.
ENZYMES REGULATING O-LINKED _N_-ACETYLGLUCOSAMINE CYCLING
O-Linked _N_-Acetylglucosamine Transferase
Uridine diphospho-_N_-acetylglucosamine: peptide β-_N_-acetylglucosaminyltransferse (OGT) was first characterized in cytosolic preparations from rabbit reticulocytes, using synthetic peptide acceptor substrates (54), and was later purified over 30,000-fold from rat liver cytosol, using a combination of both conventional and UDP-Sepharose affinity chromatography (55). Purified liver OGT exhibited a 110-kDa α-subunit and a 78-kDa β-subunit and displayed an unusually high affinity for UDP-GlcNAc (_K_m = 545 nM). The ratio of the larger to the smaller subunit varies considerably between tissues, with the highest amount of the 78-kDa subunit in kidney and lower amounts of the smaller subunit in brain. Although OGT exhibits a high degree of sequence specificity with peptide substrates in vitro, there is no apparent absolute consensus sequence. Approximately one-half of the known O-GlcNAc sites contain a PVS (proline-valine-serine) type motif, but the other half have little in common except the presence of one or more serine or threonine moieties. Cloning of the rat (56), C. elegans (57), human (57), and plant (28, 30) OGT genes showed that it is highly conserved in all metazoans but has multiple splice variants. OGT maps to a locus near the centromere on the X-chromosome (58) (human Xq13.1), a region associated with Parkinson’s disease.
OGT is a bifunctional protein with a catalytic C-terminal domain, which apparently evolved from glycogen phosphorylase (59), and an N-terminal protein:protein interaction tetratricopeptide repeat (TPR) domain (60, 61), separated by a spacer region. Mammalian OGT is both tyrosine and serine phosphorylated (62) and contains up to 11.5 TPRs, which serve as protein:protein interaction docking sites for substrate targeting proteins. OGT appears to act by a random bi-bi kinetic mechanism with its multimerization, but not its catalytic activity, requiring the TPR repeats (63, 64). Surprisingly, OGT’s peptide substrate specificity is sensitive to the concentration of the donor substrate, UDP-GlcNAc (63). Upon insulin stimulation in insulin-responsive cells, OGT associates with the plasma membrane by binding to phosphoinositides (65) and is directly tyrosine phosphorylated by the insulin receptor, which activates the enzyme (62). OGT is also activated by the action of serine kinases, calcium calmodulin kinase IV (CAMKIV) (66), and by Src kinase, among others.
Although O-GlcNAc cycling is similar to phosphorylation in many respects, OGT’s action on its many substrates is very different than kinases. Serine or threonine phosphorylation is determined by the action of over 300 different genetically encoded kinases (67), each with its own peptide selectivity. In contrast, mammalian genomes contain only a single gene encoding the OGT catalytic subunit. OGT’s modification of its many substrates is regulated in a manner analogous to that for RNA polymerase II (68) or phosphatase targeting (69). The peptide sequence specificity of OGT is determined by its catalytic subunit and by UDP-GlcNAc concentrations, but targeting to specific proteins is regulated by myriad transient protein:protein interactions of the catalytic subunit to form holoenzyme complexes, each with unique protein specificity (20, 60, 63, 70–73). It is likely that OGT targeting proteins and the resulting holoenzyme complexes are different in various cell types and under different cellular conditions. Yeast two-hybrid analyses in brain tissue have identified some of these OGT targeting proteins. In many cases, OGT and protein phosphatases are found within the same complex (74), indicating that, in these cases, the same enzyme complex that adds O-GlcNAc concomitantly removes the phosphate moiety. Examples of OGT targeting proteins include Milton (OIP106) (75, 76), which is important for mitochondrial and receptor translocation in nerve axons; p38 MAP kinase, which plays a role in the dramatic increased O-GlcNAcylation of a subset of proteins during glucose starvation of nerve cells (70); the myosin phosphatase targeting subunit (MYPT1) (77), which also targets OGT to myosin (Figure 2); and PGC-1α, a key coactivator of transcription and the “master regulator of mitochondrial biogenesis,” which targets OGT to FOXO transcription factors in liver (78, 79), leading to inappropriate gluconeogenesis associated with diabetes. It is clear that these protein:protein interactions that target OGT to specific protein substrates might make the most specific and useful drug targets for the amelioration of conditions resulting from hyper-O-GlcNAcylation of specific proteins. Interestingly, of the several hundred O-GlcNAc sites mapped on intracellular proteins, only a few sites are in regions of the protein with an organized crystal structure, supporting the hypothesis that O-GlcNAcylation primarily occurs within regulatory domains of proteins.
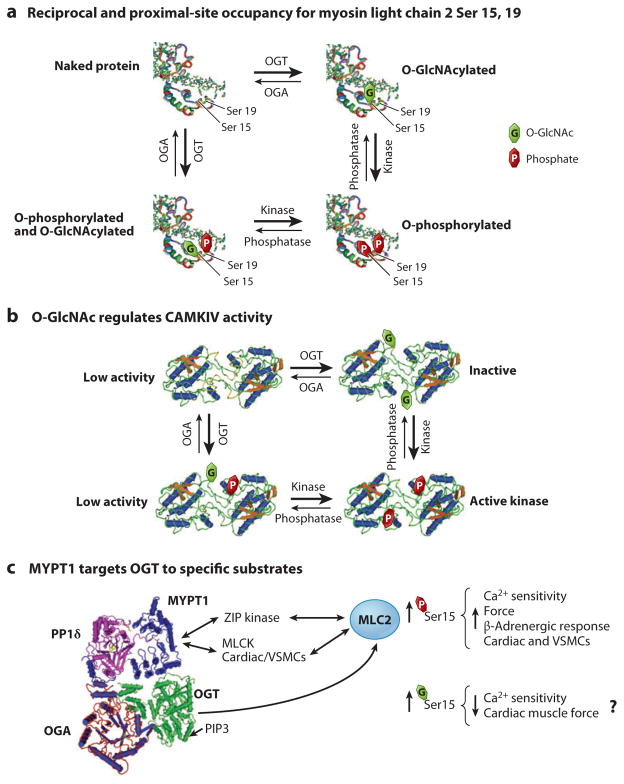
Figure 2.
O-GlcNAcylation and phosphorylation cross talk to regulate protein function. (a) O-linked _N_-acetylglucosamine (O-GlcNAc) and phosphate can modify the same amino acids reciprocally or amino acids proximal to each other. For example, serine 15 of myosin can be phosphorylated or O-GlcNAcylated. Additionally, serine 19, which is proximal to serine 15, can also be phosphorylated. Each of the modifications can potentially influence each other and alter the rate of addition and turnover of the other modification. (b) Kinases are targets for O-GlcNAcylation. The calcium calmodulin kinase IV (CaMKIV) structure is used to illustrate functions of corresponding CaMKIV-O-GlcNAcylated and -phosphorylated sites. In the inactive state, O-GlcNAc blocks a proximal activating phosphorylation. Upon activation, the O-GlcNAc residue is removed by O-GlcNAcase (OGA), and the phosphorylation site is accessible for kinase activation. (c) O-GlcNAc transferase (OGT) is targeted to substrates by interacting proteins. For this example, the MYPT1-PP1δhuman complex structure is used to illustrate a composite complex with structures of bacterial homologs of OGT and OGA. MYPT1 interacts with OGT and thus potentially targets the latter to myosin light chain for O-GlcNAcylation. MLCK, myosin light chain kinase; VSMC, vascular smooth muscle cell.
Several compounds that inhibit OGT in vitro, some with fairly good specificity, have been reported (80). Unfortunately, none of these compounds inhibits OGT very well when added to living cells. However, prospects for the development of inhibitors of OGT for either research or even pharmaceutical development appear bright because several talented groups are working toward this goal. The recent determination of the crystal structure of a bacterial homolog of OGT has significantly advanced our understanding of this enzyme (81, 82). A high-resolution structure of the human OGT was recently reported at an international meeting, but at the time of this writing, it remains unpublished. These structural studies largely support current models with respect to the mechanism of the enzyme and the roles of the TPR domains in substrate targeting.
O-GlcNAcase
O-GlcNAcase, a cytosolic, neutral β-_N_-acetylglucosamindase, was first identified in crude cellular extracts many years ago and was called hexosaminidase C to distinguish it from its lysosomal localized counterparts (83, 84). O-GlcNAcase was purified 22,000-fold from rat spleen cytosol (85) and subsequently from rat brain cytosol (48). The peptide sequence from the rat brain enzyme allowed for cloning of OGA, which was found to be identical to a previously identified gene, meningioma-expressed antigen 5 (MGEA5) (86, 87), which was identified owing to its association with meningioma and was originally thought to be a hyaluronidase. Like OGT, OGA is highly conserved and is expressed at the highest levels in pancreas, brain, and thymus, with lesser amounts in other tissues. O-GlcNAcase is also a bifunctional protein with both a catalytic domain and a HAT domain with homology to GCN5-type HATs, a type of HAT first described in yeast (49). It has been proposed that O-GlcNAcase indeed has HAT activity (88), but to date, this observation has not been replicated by several different groups. Nonetheless, it is likely that the HAT domain of O-GlcNAcase does play a role in O-GlcNAcase’s interactions with the transcription machinery. Yeast two-hybrid analyses have indicated that O-GlcNAcase is targeted to its many substrates in a manner similar to that described above for OGT targeting. During apoptosis, caspase 3, the “executioner caspase,” cleaves O-GlcNAcase into two nearly equal halves, but the different domains remain associated (89). This cleavage does not abrogate O-GlcNAcase activity, and surprisingly when each half is independently coexpressed in a cell, the two parts of the enzyme reassemble spontaneously. Over the past several years, some highly useful and highly specific inhibitors of O-GlcNAcase have been developed (90–93). Recently, a crystal structure of a homologous bacterial enzyme (which has been useful in elucidation of O-GlcNAcase’s mechanism) has been reported by two different groups (94–96).
DETECTION AND SITE MAPPING OF O-LINKED _N_-ACETYLGLUCOSAMINE
Even though O-GlcNAcylation is very abundant and widespread on most of the cell’s regulatory proteins, progress in understanding its roles in the cell has been slow. This is due largely to the lack of tools and methods to study the modification and the resulting paucity of people entering this area of research.
Why Did O-Linked _N_-Acetylglucosamine Remain Undetected?
If O-GlcNAcylation is so abundant and important, why did it remain undetected until the early 1980s? Importantly, why is O-GlcNAcylation still largely ignored by the signaling, transcription, and cell biological research communities, which study phosphorylation? First, O-GlcNAcylation is generally undetected by commonly used analytical protein methods, including gel electrophoresis and most forms of high-pressure liquid chromatography (97–100). For example, addition of the sugar does not generally affect migration of a polypeptide in gel electrophoresis or upon isoelectric focusing or even in high-resolution two-dimensional gels. Second, the sugar modification is rapidly hydrolyzed by cellular hexosaminidases upon cellular damage or during protein isolation if countermeasures are not employed. Third, O-GlcNAc is labile by conventional mass spectrometric methods (101, 102). It is lost at the source under conditions typically used in electrospray mass spectrometry (MS), and it is very labile in the gas phase upon collision-induced fragmentation. Most importantly, O-GlcNAc-peptide ion signals are strikingly suppressed in favor of ions derived from unmodified peptides when both are present within the same mixture, even when the unmodified peptides are in the minority. Finally, the identification, site mapping, and study of O-GlcNAcylation’s functions currently require many varied and fairly sophisticated methods and instrumentation not generally available to most laboratories.
Antibodies and Lectin Probes to O-Linked _N_-Acetylglucosamine
The lectin, wheat germ agglutinin (WGA) continues to be a useful tool to probe for O-GlcNAc (99). WGA’s binding to O-GlcNAcylated proteins is specifically competed with and released by excess _N_-acetylglucosamine (GlcNAc). Its shortcomings are that it has a relatively low affinity for terminal GlcNAc residues unless they are closely clustered together on the polypeptide. WGA also has much higher affinity for sialic acids, which are generally only on the outside of cells or within luminal compartments. Succinylation of WGA increases its specificity for GlcNAc but also reduces its affinity for the sugar. To date, several other lectins have been investigated as probes for O-GlcNAcylation, but none appear generally useful.
Enzymatic Probes for Detection and Enrichment
As indicated above, O-GlcNAc was first discovered by using bovine milk GalT1 to probe for terminal GlcNAc moieties on cells of the murine immune system (1). The specificity of galactosyltransferase for GlcNAc, using UDP-[3H]galactose, together with the alkali-induced β-elimination and product analysis, still makes this approach a powerful quantitative method to detect O-GlcNAc on proteins (97–99). Recently, using a mutant GalT1 with a larger active site, chemically reactive azido-or keto-sugars have been used to enzymatically tag O-GlcNAc residues (Figure 3) (103). These chemically reactive tags then allow for the attachment of biotin that allows for highly selective enrichment of O-GlcNAcylated peptides over unmodified peptides present in cellular extracts. As stated above, owing to severe ion suppression of the O-GlcNAc peptide ions by unmodified peptide ions, this high level of enrichment is key to successful MS analysis of O-GlcNAcylation (104).
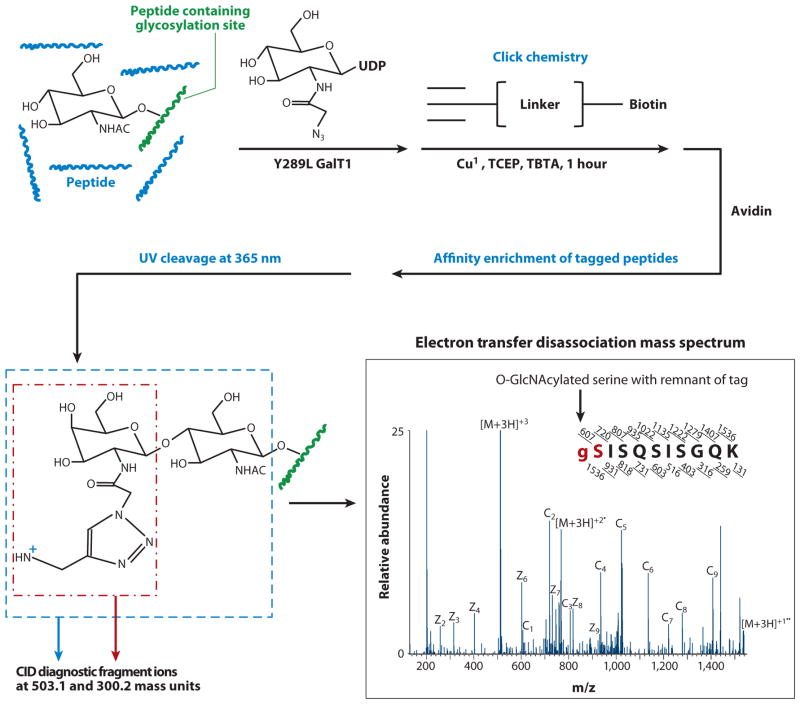
Figure 3.
Identification of O-linked _N_-acetylglucosamine (O-GlcNAc) sites is facilitated by photocleavable biotin tagging, combined with collision-assisted dissociation (CAD) and electron transfer dissociation (ETD) mass spectrometry. O-GlcNAc peptides are enriched by first labeling O-GlcNAc groups with _N_-azidoacetylgalactosamine (GalNAz) using a mutant galactosyltransferase (GalT1), followed by click chemistry addition of a photocleavable biotin tag. Tagged peptides are purified over an avidin column and subsequently are released from the beads by photochemical cleavage. CAD of tagged peptides generates diagnostic ions at mass/charge (m/z) 503.1 and 300.2. ETD enables peptide sequencing and O-GlcNAc site localization. An ETD spectrum of a sample peptide (SISQSISGQK) indicates that Ser1 is modified with a tagged GlcNAc-GalNAz moiety.
Glycomic/Mass Spectrometric Approaches to Quantify O-GlcNAcylation
Early studies, even with stoichiometrically modified synthetic O-GlcNAc peptides, showed that detection and site mapping of O-GlcNAc by electrospray MS, fast atom bombardment MS, matrix-assisted laser desorption ionization (MALDI)-induced dissociation MS were problematic and difficult (101, 105–108). Site mapping by collision-assisted dissociation (CAD) MS simply does not work for O-GlcNAc. The β-glycosidic linkage is very labile in the gas phase, and when the sugar is released, it carries much of the kinetic energy with it, resulting in poor peptide fragmentation. Given the sensitivity of O-GlcNAc to alkali-induced β-elimination, it is possible to map O-GlcNAc sites by determining the site of the β-elimination of the sugar by the loss of a water molecule [18 mass/charge (m/z)] within the sequence (109). However, because this approach does not involve specific enrichment of the O-GlcNAc peptides, it requires fairly large amounts of relatively pure O-GlcNAc peptides to work well. In addition, given that other moieties attached to serine or threonine could also be β-eliminated, producing false positives, any sites mapped by this method must be confirmed by other approaches, such as by site-directed mutagenesis. By combining β-elimination with Michael addition chemistry, using unlabeled or density-labeled dithiothreitol, the serine or threoine residues from which the O-GlcNAc is eliminated become covalently modified with a sulfhydryl group (110). The density-labeled dithiothreitol allows for comparative quantification of site occupancy, and importantly, the sulfhydryl groups allow for efficient thiol affinity enrichment of the tagged peptides. Another strength of this method is that the modified peptides fragment well in collision-induced fragmentation. The major weaknesses of this method are that it is still indirect and that the sites must be confirmed by another independent approach. A major breakthrough in the detection and site mapping of O-GlcNAc occurred first with the development of Fourier transform mass spectrometers capable of electron capture dissociation (ECD) (111) and subsequently with the development of ion-trap mass spectrometers, which could perform electron transfer dissociation mass spectrometry (ETD-MS) (112, 113). These instruments fragment peptides in a manner that does not result in the loss of labile posttranslational modifications, such as O-GlcNAc. In fact, ETD-MS studies of the phosphorylation of the cell adhesion protein paxillin, which also mapped some of its O-GlcNAcylation sites, may be the first times researchers who were not looking for O-GlcNAc actually detected it by MS (113). Unfortunately, the ECD-MS or ETD-MS methods do not solve the problem of ion suppression, discussed above. Therefore, enrichment of the O-GlcNAc peptides is still essential for high sensitivity analysis of mixtures. Recently, the combined chemical/enzymatic tagging/enrichment and subsequent mass spectrometric analysis of O-GlcNAcylation have been improved by the use of a UV light-cleavable alkyne-containing biotin tag, which allows for high-affinity enrichment of the O-GlcNAc peptides and their quantitative release from the streptavidin beads by exposure to UV light (Figure 3) (102, 114). Two critical features of this UV-cleavable tag are also of note: (a) Cleavage of the tag results in the generation of a positive charge on the tagged sugar, causing all tryptic O-GlcNAc peptides to have at least three positive charges, which is important to sensitive analysis by ETD-MS. (b) Upon standard collision-induced dissociation MS, the tag generates fragment ions that are diagnostic for the presence of O-GlcNAc on a peptide, which allows for rapid high-sensitivity screening of modified peptides by inexpensive ion-trap instruments. These new methods are dramatically increasing the number of identified O-GlcNAcylated proteins and sites.
CROSS TALK OR INTERPLAY WITH PHOSPHORYLATION IS EXTENSIVE
Recent applications of the new mass spectrometric methods for O-GlcNAc and more traditional metal ion affinity methods for the analysis of phosphorylation have shown that the dynamic cross talk between O-GlcNAcylation and phosphorylation is extensive (10, 11, 114). Inhibition of a single kinase, GSK3β, increases O-GlcNAcylation of many proteins (mostly cytoskeletal and heat shock proteins) and decreases O-GlcNAcylation of many other proteins (mostly transcription factors and RNA-binding proteins) (11). When phosphorylation site occupancy of 700 sites was determined after global O-GlcNAcylation was raised only about threefold in nonstimulated cells by incubating with an O-GlcNAcase inhibitor, virtually every actively cycling phosphorylation site (as determined by sensitivity to the phosphatase inhibitor okadaic acid) was either decreased or increased significantly by the altered O-GlcNAcylation (10). A twofold over-expression of OGT causes polyploidy in tissue culture cells and dramatically reduces proline-directed phosphorylation on many proteins (115). A concomitant glycomic and phosphoproteomic analysis of modification sites affected by this twofold overexpression of OGT identified hundreds of phosphorylation sites and O-GlcNAcylation sites. Many cytoskeletal proteins displayed reciprocal occupancy at the same serine or threonine residues, as did other classes of proteins. However, a majority of transcription factors displayed reciprocal occupancy of the two modifications at proximal sites on the polypeptide. Strikingly, this modest overexpression of OGT dramatically reduced phosphorylation by cyclin-dependent protein kinase 1 (CDK1) of its many important substrates involved in cell division (114). This reduction in CDK1-mediated phosphorylation was the result of several mechanisms, including altered expression of upstream regulatory kinases and altered phosphorylation of both upstream kinases and CDK1 itself. Overexpression of OGT also had similar effects on two other kinase cascades important to cell division, aurora kinase A and polo kinase. These findings underscore the importance of the extensive cross talk between these two most abundant nucleocytoplasmic protein modifications to the regulation of cellular function.
Interplay at Specific Serine or Threonine Residues on Proteins
To date, only about 1,500 O-GlcNAc sites have been reported from all organisms. However, this number will likely increase rapidly with the newer methods and instrumentation. Competition between O-GlcNAcylation and phosphoryation for occupancy of serine/ threonine sites occurs by several distinct mechanisms. Many proteins are reciprocally modified under different conditions at the same site by either O-GlcNAc or phosphate, such as at sites on the c-Myc oncogene protein (116), estrogen receptor β (117), some sites on RNA polymerase II (118, 119), endothelial nitric oxide synthase (120), and many others. Other proteins are competitively modified by either O-GlcNAc or phosphate at proximal sites but not at the same residue, such as vimentin (73), p53 (121), CAMKIV (35), and FOXO1 (78, 79). Presumably, this competitive occupancy results from either the large size of an O-GlcNAc residue (the Stokes radius is four to five times larger than a phosphate) or the negative charge of the phosphate moiety, or by induction of conformational changes in the protein by either modification (122–125). On other proteins, O-GlcNAcylation and phosphorylation occur at distant sites or even on completely different subpopulations of the molecules, such as on certain cytokeratins (43, 126). Yet on other proteins, both modifications exist simultaneously at different sites, such as on the insulin receptor substrate (IRS) proteins (127) and on cardiac myosin light chain (Figure 2) (45). Studies with synthetic O-GlcNAc peptides have suggested that addition of an O-GlcNAc moiety induces a β-turn confirmation and that addition of a phosphate tends to open up the peptide conformation (122–125). A recently developed Web site contains the most up-to-date list of published O-GlcNAc modification sites and an algorithm to predict if a site might be O-GlcNAcylated (http://cbsb.lombardi.georgetown.edu/hulab/OGAP.html).
O-GlcNAcylation of Phosphate Cycling Enzymes
In addition to their cross talk at the level of site occupancy, O-GlcNAcylation and phosphorylation dynamically modify the enzymes controlling each other’s cycling on polypeptides. Phosphatases are associated with the OGT (128), indicating that the same enzyme complex can both remove phosphate and add an O-GlcNAc residue on some proteins. Both OGT and O-GlcNAcase often occur in protein complexes containing both kinases and phosphatases (13, 73). An increasing number of kinases are not only known to be modified by O-GlcNAc, but also to be regulated by the sugar (36, 37, 129–131). CAMKIV, an important kinase in neurons and β-cells of the pancreas, which plays a key role in phosphorylation/activation of transcription factors, is O-GlcNAcylated at multiple residues at or near its activating phosphorylation site and within its ATP-binding pocket (Figure 2) (35). O-GlcNAcylation of CAMKIV keeps the enzyme in an inactive state. To be activated, CAMKIV must be first de-O-GlcNAcylated and then phosphorylated at a key regulatory site proximal to one of the major O-GlcNAc sites. O-GlcNAcylated CAMKIV has a reduced affinity for ATP. Mutation of the major O-GlcNAc site on CAMKIV to an alanine results in a constitutively active enzyme. Importantly, active CAMKIV phosphorylates OGT to activate it (66). Thus, in neurons, there is a cycle regulating both CAMKIV and OGT that sets up a two-step mechanism, perhaps to serve as a safety switch to prevent inappropriate activation of this important kinase. It is likely that similar mechanisms will be found for other kinases.
Phosphorylation of O-Linked _N_-Acetylglucosamine Cycling Enzymes
OGT is both tyrosine and serine phosphorylated. Phosphorylation by the insulin receptor, CAMKIV and other kinases, activates OGT and may play a role in OGT’s interaction with substrates or targeting subunits (62). O-GlcNAcase is also serine phosphorylated (132), but the consequence of this phosphorylation remains unknown (20).
Interplay with Other Posttranslational Modifications
It is likely that O-GlcNAc has interplay with other posttranslational modifications, but little work has been done in this area. It is already known that O-GlcNAcylation of the tumor suppressor p53 at serine149 prevents its ubiquitination (121), but this appears to be indirect because the sugar prevents phosphorylation at threonine155. Modest overexpression of OGT alters the acetylation and methylation patterns of histones, perhaps mediated by the OGT targeting protein and arginine methyltransferase, CARM1 (133). Of course, many proteins are both acetylated, and O-GlcNAcylated, but the relationship between these abundant modifications remains largely unknown (134). A major area in the future of biomedical research will concern elucidation of the roles of cross talk between posttranslational modifications in the regulation of cellular functions or dysfunctions.
CELLULAR FUNCTIONS OF O-LINKED _N_-ACETYLGLUCOSAMINE
Nutrient Modulation of Signaling
A generalization with respect to the roles of O-GlcNAcylation in cellular signaling has emerged during the past two decades. The primary function of O-GlcNAcylation appears to be the modulation of cellular processes in response to nutrients and to cellular stress. By analogy to an electrical circuit, if phosphorylation events represent “microswitches,” which turn on or turn off protein activity, O-GlcNAcylation could be thought of as a “rheostat” tuning the pathways and processes to accommodate nutrient status and cellular stress. Virtually all metabolic pathways influence the cellular concentrations of UDP-GlcNAc (Figure 1). OGT’s catalytic activity and peptide specificity are responsive to the concentrations of sugar nucleotide across a remarkable range, from nanomolars to up to 100 mM (63, 119)! Depending upon the cell type, as much as 2% to 5% of glucose is metabolized via the hexosamine biosynthetic pathway (135), culminating in the production of UDP-GlcNAc. It also appears that the association of OGT with its numerous targeting proteins, which serve to target it to specific substrates, is also regulated by nutrients (Figure 4) (71, 79). However, nothing is known about the mechanisms regulating these nutrient-sensitive protein:protein interactions.
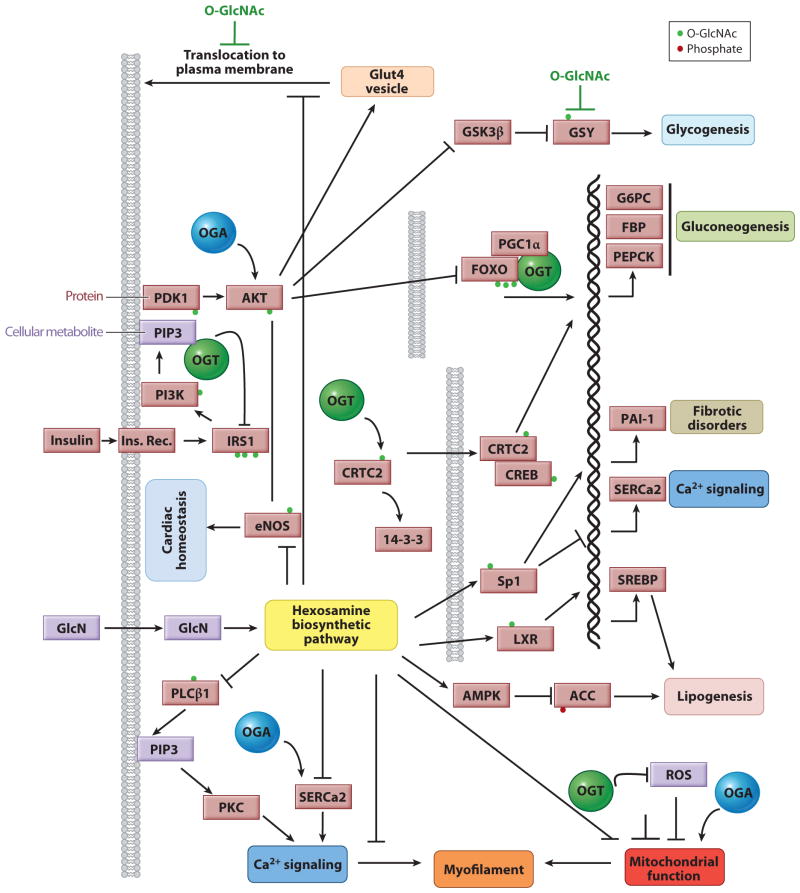
Figure 4.
O-linked _N_-acetylglucosamine (O-GlcNAc) is a nutrient sensor and underlies glucose toxicity. Chronic elevation in cellular nutrients (e.g., glucose) increases the glucose shunt into the hexosamine biosynthetic pathway, which elevates the availability of the metabolite substrate UDP-GlcNAc for O-GlcNAc transferase (OGT). Increased flux through this pathway is associated with altered O-GlcNAcylation of key proteins for cellular homeostasis and survival (IRS1, PI3K, Akt, and eNOS), resulting in alterations in multiple cellular pathways. Increased levels of O-GlcNAcylation, particularly on the effectors of the insulin/phosphoinositide signaling pathway, disrupt regulation of their activity by OGT, which results in key pathogenic contributions to glucose toxicity and insulin resistance, the two major hallmarks of diabetes mellitus. Note that proteins are shown as light red squares, and the cellular metabolites are light purple squares. OGT is shown as a green circle and O-GlcNAcase (OGA) as a blue circle. Arrows denote activation, whereas blunt ends denote inhibition.
O-Linked _N_-Acetylglucosamine and the Cellular Stress Response
O-GlcNAcylation of a large number of proteins rapidly increases when cells are exposed to almost any type of stress, including heat, high salt, heavy metals, UV light, hypoxia, and others (136, 137). Increased O-GlcNAcylation appears to contribute to the upregulation of chaperones and heat shock proteins both by increasing their expression and decreasing their turnover. If the extent of O-GlcNAcylation is artificially increased in cells, either by inhibition of O-GlcNAcase or by other means, the ability of cells to survive toxic stress strikingly increases. Importantly, many studies have shown that increased O-GlcNAcylation is protective against stress or trauma in vivo (138, 139). For example, in animal models, elevation of O-GlcNAcylation protects cardiac muscle from tissue damage after a heart attack (140–145). Protection of cardiac muscle from reperfusion injury appears to involve O-GlcNAcylation of mitochondrial proteins (142, 146).
Roles in Transcription
Early studies established that O-GlcNAcylation was highly abundant on chromatin proteins, and its concentration correlated with active sites of transcription on polytene chromosomes in Drosophila (34). Nearly every RNA polymerase II transcription factor is multiply O-GlcNAcylated, and WGA-Sepharose is commonly used to purify transcription factors (33, 147). The catalytic subunit of RNA polymerase is extensively O-GlcNAcylated within its C-terminal repeat domain (CTD) on a subset of the so-called IIa form (the form of the enzyme where the CTD is not phosphorylated) (118). Many components of the basal transcription machinery are also O-GlcNAcylated (119, 148, 149). Sometime ago, it was proposed that the formation of the preinitiation complex by RNA polymerase II at a promoter might require the presence of O-GlcNAc on the CTD and that the CTD O-GlcNAc residues would need to be removed prior to the phosphorylation of the CTD that allows transcriptional elongation to proceed (148). Recent data continue to support this model and further suggest that the poised polymerase II complexes (150), stalled at paused transcription sites, contain the O-GlcNAcylated forms of the enzyme. Recently, OGT was shown to be a polycomb gene; these genes are master regulators repressing the expression of subsets of genes during development (22, 151, 152). There are several studies indicating that the so-called housekeeping transcription factor, Sp1, which is very extensively O-GlcNAcylated, functions abnormally in diabetes, where it is likely hyper-O-GlcNAcylated (153–157). These studies suggest that Sp1’s promoter specificity may be directly affected by the site occupancy and/or the overall extent of its O-GlcNAcylation. The modification of histones by O-GlcNAc not only occurs both in their tails, where the well-studied modifications of the histone code reside, but also many of the O-GlcNAc sites on histones are located near the contact sites with DNA within the nucleosome. Histone O-GlcNAcylation cycles with the cell cycle and in response to heat stress (133). Thus, although much more work is required to elucidate O-GlcNAc’s roles in transcription, it is already clear that the regulation of transcription in response to nutrients or stress is a major function of this modification.
Roles in Translation
Early studies showed that the O-GlcNAcylated form of a 67-kDa protein blocked the phosphorylation of the α-subunit of the reticulocyte eukaryotic chain initiation factor 2 (eIF-2) by eIF-2 kinase, a heme-regulated protein synthesis inhibitor (158). Commercially available rabbit reticulocyte lysates, used for in vitro translation of polypeptides from mRNA, efficiently O-GlcNAcylate the newly synthesized polypeptides without addition of exogenous sugar nucleotide or OGT (159). In vitro transcription/translation combined with WGA-Sepharose affinity chromatography is a useful tool to first evaluate if low-abundance proteins, like transcription factors, are O-GlcNAcylated (99).
Cells contain ribonucleoprotein granules, called stress granules, and processing bodies, which coordinately regulate translation and mRNA turnover (160). O-GlcNAcylated proteins are major components of stress granules, but much less so for processing bodies. However, O-GlcNAc cycling appears to be required for both stress granule and processing body assembly. Stress-induced hyper-O-GlcNAcylation is important for the aggregation of untranslated messenger ribonucleoproteins into stress granules in mammalian cells (160).
O-GlcNAc is present on many proteins that form active polysome complexes, including at least 20 O-GlcNAcylated core ribosomal proteins (50). The ribosome protein S6, a component of the mammalian target of rapamycin signaling pathway, follows different dynamics of O-GlcNAcylation than its nutrient-induced phosphorylation. Both OGT and O-GlcNAcase strongly associate with ribosomes. O-GlcNAcase is enriched within the nucleolus, the site of ribosomal RNA production, but OGT is excluded from the nucleolus. Nuclear stress alters O-GlcNAcase nucleolar localization. Overexpression of OGT, but not of OGAse or a GFP control, causes a dramatic accumulation of 60S subunits and 80S monosomes (50). Current data strongly suggest that O-GlcNAc plays important roles in regulating translation and ribosome biogenesis, but much work remains to elucidate the functional details.
O-Linked _N_-Acetylglucosamine and the Cytoskeleton
O-GlcNAcylation is particularly abundant on “bridging” or regulatory proteins of the cytoskeleton, such as erythrocyte band 4.1 (161), talin (40), microtubule-associated proteins (39), and ankyrin (41), as well as on the major cytoskeleton proteins themselves, including actin, tubulin (44), myosin (38, 162), intermediate filament proteins, cytokeratins (43, 163), and neurofilaments. Upon mitotic arrest, the O-GlcNAcylation and phosphorylation of cytokeratins 8 and 18 concomitantly increase along with changes in keratin assembly. Plakoglobin, a linking protein that connects desmosomal and cadherins to the cytoskeleton, is O-GlcNAcylated at a site next to its destruction box, suggesting a role for the sugar in preventing degradation of this protein (164). Over-expression of OGT in keratinocytes stabilizes plakoglobin and increases cell-cell adhesion (165). A study using two-dimensional gels and MS identified 14 O-GlcNAcylated proteins in skeletal muscle, including signaling molecules, glycolytic enzymes, and contractile proteins (38). Loss of O-GlcNAcylation correlated with functional atrophy in the postural soleus muscle and reduced contraction force (166). Incubation of skeletal muscle skinned fibers in concentrations of _N_-acetylglucosamine, which inhibit O-GlcNAc-dependent processes, decreased calcium sensitivity and the affinity of muscle fibers. The authors proposed that O-GlcNAcylation regulates contractile protein interactions to modulate muscle contraction (167, 168). O-GlcNAcylation blocks calcium signaling pathways in C2C12 myoblasts by modification of phospholipase C β1, which negatively regulates its activity (169).
The inositol 1,4,5-trisphosphate (InsP3) receptor type I (InsP3R-I), which is the principle channel for intracellular calcium (Ca2+) release in many cell types, is O-GlcNAcylated. InsP3R-I channel activity is decreased by O-GlcNAcylation, suggesting that O-GlcNAcylation is an important regulator of the InsP3R-I (170). This study further suggests that O-GlcNAcylation of InsP3R-I might be a mechanism for neuronal dysfunction under conditions in which O-GlcNAc is high, such as diabetes or physiological stress.
O-GlcNAcylation even appears to be important in the cytoskeleton of some bacteria. Flagellins from Listeria monocytogenes are O-GlcNAcylated (24). The Listeria OGT, GmaR, is a bifunctional protein that plays a key role in suppressing the transcription of flagellin proteins, presumably independent of its enzymatic activity. However, GmaR also enzymatically O-GlcNAcylates the flagellins when they are synthesized (25).
Roles in Protein Trafficking and Degradation
In preparations of highly purified Drosophila proteasomes, 5 of 19 catalytic and 9 of 14 core subunits were found to be O-GlcNAcylated (171). O-GlcNAcylation of the Rpt2 ATPase, a component of the 19S cap of the proteasome, inhibits its ATPase activity and lowers the proteolytic activity of the proteasome on the transcription factor Sp1 and on a hydrophobic peptide substrate (172).
COPII proteins play an important role in endoplasmic reticulum to Golgi transport, which is blocked during mitosis (173). The COPII component, Sec24p, is extensively O-GlcNAcylated in interphase cells but loses the sugar modification and becomes phosphorylated when cells enter mitosis. The reciprocal cycling of O-GlcNAcylation and phosphorylation on Sec24p appears to not only regulate Golgi fragmentation during mitosis but also to block endoplasmic reticulum-Golgi transport during mitosis (173). Although O-GlcNAc is abundant on nuclear pore proteins, its role in nuclear transport remains unclear. The sugar may play a role in nuclear pore assembly/disassembly during mitosis. However, on certain proteins, O-GlcNAc has been suggested to be a nuclear targeting signal (174, 175).
O-Linked _N_-Acetylglucosamine and Development
Gene deletion of OGT in mice shows that O-GlcNAcylation is essential, even at the single-cell level in mammals (58). Cre-Lox tissue-targeted deletion of OGT results in death of the targeted tissue in mice (176). OGT deletion results in the loss of O-GlcNAcylation and causes T-cell apoptosis, neuronal tau hyperphosphorylation, and fibroblast growth arrest with altered expression of c-Fos, c-Jun, c-Myc, Sp1, and p27. These genetic studies (176) further establish that O-GlcNAcylation modulates protein phosphorylation and expression among essential and conserved cell signaling pathways. In C. elegans, deletion of OGT and O-GlcNAcase does not result in death of the organism but does result in a severe defect in metabolism, similar to diabetes (177, 178). O-GlcNAcylation also regulates cytoskeletal proteins and turnover during dauer formation in the worm (179).
Zebrafish have two OGT genes encoding at least six isoforms that are expressed both maternally and zygotically (180). Again, alterations of either OGT or O-GlcNAcase resulted in similar phenotypes, suggesting that cycling rates might be more important than absolute stoichiometry. Altering O-GlcNAcylation by over-expressing OGT or OGA, or by reducing OGT expression by using morpholinos, resulted in embryos with shortened body axes, reduced brains, and severe cellular disorganization after gastrulation. Overexpression of OGT and O-GlcNAcase delayed epiboly and caused a severe disorganization of the cytoskeleton. In zebrafish, O-GlcNAcylation controls the activity of proteins that regulate apoptosis and epiboly movements during development (180).
ROLES IN CHRONIC DISEASE
Given O-GlcNAc’s role as a nutrient sensor and its extensive cross talk with phosphorylation, it is not surprising that the protein modification plays fundamental roles in chronic diseases, particularly diabetes, neurodegeneration, cardiovascular disease, and cancer. In addition, many viruses that infect eukaryotic cells have key regulatory proteins that are also modified, presumably by the host O-GlcNAc cycling enzymes.
Diabetes and Glucose Toxicity
It has long been postulated that the hexosamine biosynthetic pathway played a role in the etiology of diabetes and glucose toxicity, with several hundred papers published on this topic (Figure 4) (21, 181–184). A seminal link between the conversion of glucose to glucosamine and glucose-induced insulin resistance was established by studies in 3T3 L1 adipocytes (4) and by work on glucose:fructose 6-phosphate amidotransferase (185–187), the rate limiting step in the biosynthesis of UDP-GlcNAc. Later studies indicated that increasing global O-GlcNAcylation by inhibiting O-GlcNAcase with a hexosaminidase inhibitor directly inhibited insulin-dependent glucose uptake (188). However, a recently developed specific inhibitor of O-GlcNAcase increased global O-GlcNAcylation but did not induce insulin resistance in 3T3 L1 adipocytes (189), and other studies showed that increased O-GlcNAcylation is not essential for glucose-induced insulin resistance (190), which is not surprising given the many possible mechanisms for inhibition of this complex regulatory pathway. Nonetheless, there are numerous genetic and other studies in insulin-sensitive tissues, such as those of muscle, liver, and fat, that strongly support a direct involvement of O-GlcNAcylation and the hexosamine biosynthetic pathway in insulin resistance. Importantly, the insulin receptor activates OGT, which is targeted to the plasma membrane by phosphoinositides, where OGT O-GlcNAcylates components of the insulin signaling cascade (Figure 4) (65). The IRS proteins are modified by O-GlcNAc at several sites (191), and increased O-GlcNAcylation of an IRS protein reduces its binding to phosphoinositide 3 kinase’s p85 regulatory subunit, which likely represents a major site of O-GlcNAc’s downregulation of insulin signaling (62).
Why hyperglycemia is so deleterious to cells and tissues has long been an enigma because glucose itself is not a toxic molecule. Evidence is emerging that the pleiotropic toxicity of hyperglycemia is due to a prolonged imbalance of the cross talk between O-GlcNAcylation and phosphorylation. Hyper-O-GlcNAcylation not only alters phosphorylation-mediated signaling cascades, but also alters the regulation of transcription. Several studies suggest that hyper-O-GlcNAcylation of transcription factors, such as Sp1, alters their activity, turnover, or protein:protein associations and causes abnormal gene expression in affected tissues (153–157). Individually, and in short periods, the effects of hyperglycemia-induced increases in O-GlcNAcylation are subtle, but when prolonged and cumulative, they lead to long-term detrimental alterations in cellular functions.
O-GlcNAcylation, Neuronal Function, and Neurodegeneration
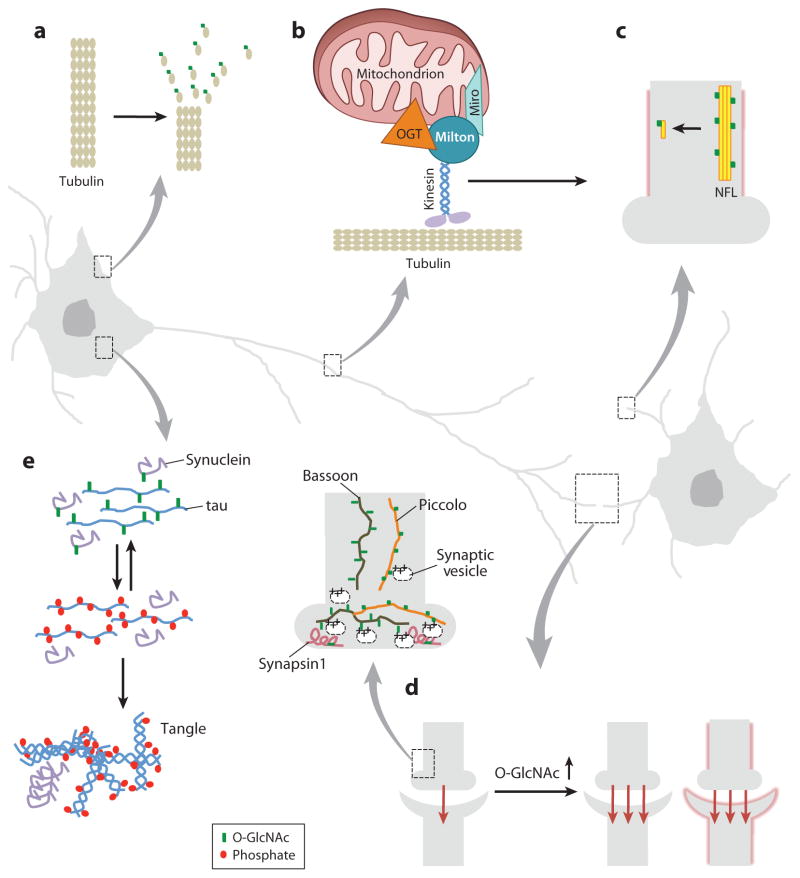
Except for the pancreas, O-GlcNAcylation is most abundant in the brain. During recent years, evidence has been mounting connecting defects in glucose metabolism in the brain with neurodegenerative diseases, such as Alzheimer’s disease (AD) and Parkinson’s disease (192). AD has even been referred to as “diabetes type 3” (193–195). Likewise, many studies indicate that O-GlcNAcylation plays an important role in both normal brain functions and the etiology of neurodegeneration (Figure 5). Some of the accumulated evidence supporting a direct role for O-GlcNAcylation in AD is listed below.
Figure 5.
O-linked _N_-acetylglucosamine (O-GlcNAc) signaling is crucial for proper neuronal function. (a) O-GlcNAcylation of tubulin is important in maintaining the soluble pool of the protein during cellular rearrangement. (b) O-GlcNAc transferase (OGT) is targeted to kinesin by the vesicle trafficking protein Milton, which regulates axonal transport. (c) Neuronal filament solubility is partially controlled by O-GlcNAcylation. (d ) Long-term potentiation is enhanced by increases in cellular O-GlcNAcylation. (e) At the synapse, many proteins are heavily modified by O-GlcNAc. The tau protein is O-GlcNAcylated in the normal physiological state, but in tau paired-helical tangles, associated with neurodegenerative disease, the tau is no longer O-GlcNAcylated but is heavily phosphorylated. Miro, Bassoon, and Piccolo are proteins.
- Virtually all proteins involved in AD are O-GlcNAcylated/phosphorylated (6, 18, 137).
- Glucose metabolism is impaired in AD neurons concomitant with reduced O-GlcNAc (196, 197).
- tau, the major component of neurofibrillary tangles associated with the disease, is extensively O-GlcNAcylated in normal brain and is hyperphosphorylated in AD brain (198, 199).
- Amyloid precursor proteins, whose degradation products form the neuronal plaques in the disease, are O-GlcNAcylated in their cytosolic domain (200), which may affect their processing to the toxic Aβ peptides found in AD plaques.
- Several studies in neuronal cells document interplay between O-GlcNAcylation and phosphorylation on tau (194, 199). For example, starved mice have hyperphosphorylated and hypo-O-GlcNAcylated tau, and a similar effect is observed on other neuronal proteins, which is reversed by feeding (201). Overexpression of OGT in neurons increases tau O-GlcNAcylation and concomitantly decreases tau phosphorylation at sites important to AD. In contrast, Cre-Lox brain-targeted deletion of OGT in mice led to tau hyperphosphorylation prior to neuron death (176).
- O-GlcNAcylation is indeed reduced in human AD brain tissue (199), and the human O-GlcNAcase gene (MGEA5) maps to chromosome 10q24.1, the late onset AD locus (202).
In addition to the known molecular players in AD, it is also clear that O-GlcNAcylation of many other proteins plays a role in brain function and disease. For example, myriad synaptosomal proteins are dynamically O-GlcNAcylated/phosphorylated, and profound synaptic loss occurs in AD (Figure 5) (108, 203, 204). O-GlcNAcylation was recently shown to play a role in neuronal plasticity (205, 206). Clathrin assembly proteins AP-180 and AP-3 are O-GlcNAcylated in normal brain, and modification of these proteins is decreased in AD brains (207, 208). The known neurodegeneration-associated protein, ataxin-10, interacts with OGT in brain to increase OGT’s activity on certain targets (209). Neurofilaments H, L, and M are extensively O-GlcNAcylated in normal brain (210, 211) and are hypo-O-GlcNAcylated in AD (201). O-GlcNAcylation is also reduced in amyotrophic lateral sclerosis (212), another type of neurodegeneration.
In addition to AD, data suggest that O-GlcNAcylation is also involved in Parkinson’s disease. For example, human OGT maps to X13.1, the Parkinson dystonia locus (48). A direct link between OGT and X-linked dystonia-parkinsonism has recently been established. Mutations in a transcription factor THAP1 were found to cause DYT6 dystonia (213). THAP1 interacts directly with OGT and also with an extensively O-GlcNAcylated transcription factor, HCF-1, recruiting HCF-1 to promoters regulating the cell cycle. These findings may explain OGT’s genetic link to X-linked dystonia-parkinsonism (213).
Pharmaceutical targeting, which decreased O-GlcNAcylation, to treat AD has indeed showed promise in animal models. Recent studies documented that a highly specific O-GlcNAcase inhibitor, which increases global O-GlcNAcylation in living mice and crosses the blood brain barrier, prevents the abnormal hyperphosphorylation of tau in a murine model of AD (92). Thus, although much more research needs to be done, it is clear that an imbalance in the extensive cross talk between O-GlcNAcylation and phosphorylation underlies many of the defects leading to neurodegenerative disease. However, unlike diabetes, in these cases, higher O-GlcNAcylation appears to prevent disease, and impaired glucose metabolism, with the resulting lower O-GlcNAcylation in brain, leads to hyperphosphorylation, which contributes to neuronal cell death.
Cardioprotection and Cardiovascular Disease
Like the paradox between O-GlcNAcylation’s apparently opposite roles in diabetes and neurodegeneration, in acute situations, increased O-GlcNAcylation is protective, preventing tissue damage in infarcted heart tissue (see above) (143, 145, 146). However, when O-GlcNAcylation is chronically elevated, as occurs in diabetes, the sugar modification contributes directly to cardiomyopathy. Hyperglycemic-induced increased O-GlcNAcylation results in prolonged calcium transient decays in cardiomyocytes and decreases the expression of the critical sarcoplasmic calcium ATPase, SERCA2a, which may be mediated by the hyper-O-GlcNAcylation of the Sp1 transcription factor (214). These effects of hyper-O-GlcNAcylation were reversed in cardiomyocytes and in perfused hearts by adenoviral overexpression of OGT to reduce O-GlcNAc levels (215). Recently, it has been shown that diabetes-associated mitochondrial dysfunction in cardiomyocytes is mediated by increased O-GlcNAcylation of mitochondrial proteins, including key proteins of the electron transport chain (52). It is also likely that excessive O-GlcNAcylation of contractile proteins within the heart muscle contributes to their malfunctions in diabetes (45). Thus, it is clear that hyper-O-GlcNAcylation, as occurs in diabetes, plays a fundamental role in the etiology of diabetic cardiomyopathy by several different mechanisms (216).
O-Linked _N_-Acetylglucosamine’s Roles in Cancer
Many nuclear oncogenes or tumor suppressors play a direct role in regulating transcription, and nearly all such proteins are modified by O-GlcNAc, such as c-Myc (116, 217, 218), retinoblastoma (Rb) (219), tumor suppressor HIC1 (220), β-catenin (221), estrogen receptors (117, 222, 223), and others. Given the extensive cross talk between O-GlcNAcylation and phosphorylation, and the known roles of phosphorylation in mechanisms underlying cancer, it is not surprising that O-GlcNAcylation is also involved in cancer etiology.
Some specific examples are illustrated below. The major O-GlcNAc site on the c-Myc oncoprotein is threonine58, which is also a site phosphorylated by the kinase, GSK3β, and is the major mutation site on the protein in human lymphomas (116). In nondividing cells, threonine58 of c-Myc is O-GlcNAcylated, but the same site is rapidly phosphorylated when the cells are stimulated to grow (224). The tumor suppressor Rb’s regulation of cell growth also appears to involve O-GlcNAcylation and phosphorylation cross talk. The E2F-1 transcription factor mediates the gene expression required for entry of cells into S phase of the cell cycle. The Rb protein is O-GlcNAcylated when it suppresses E2F-1 transcription, and this suppression is blocked when Rb is phosphoryated during mid- to late-G1 of the cell cycle (219). Interplay between O-GlcNAcylation and phosphorylation also regulates the abundance of the tumor suppressor p53. When p53 is O-GlcNAcylated at serine149, phosphorylation at serine155 is blocked, which is a site targeted by the COP9 signalsome, thus reducing ubiquitination and proteasome degradation of the protein (121). Reciprocal O-GlcNAcylation/phosphorylation likewise regulates the IκB (IKK)-NF-κB pathway, which plays an important role in cancer progression. O-GlcNAcylation of inhibitor of nuclear factor κ-B kinase subunit β at Ser733, an inactivating phosphorylation site, enhances its activity, especially in response to high glucose (225). Interplay between the two modifications also regulates the functions of estrogen receptors, important to the progression of breast cancer. For example, both the stability and transactivation activity of estrogen receptor β are differentially regulated by reciprocal O-GlcNAcylation/O-phosphorylation at Ser16 (117). Nuclear magnetic resonance and circular dichroism studies, together with molecular dynamics modeling, show that these alternative modifications induce different peptide conformations in the estrogen receptor (125).
O-GlcNAcylation not only regulates signaling and transcriptional processes relevant to cancer, but also affects the trafficking of cell adhesion molecules that are important to metastasis. For example, O-GlcNAcylation of β-catenin not only regulates its nuclear localization and transcriptional activity (221), but also blocks its association with E-cadherin, a cell adhesion molecule critical to epithelial cell adhesion. Loss of E-cadherin transport to the cell surface of epithelial cells is critically important to mechanisms underlying metastasis of cancer cells (226).
Several studies are beginning to document global alterations in O-GlcNAcylation in cancers. Breast cancer cells have up-regulated O-GlcNAcylation, and reduced O-GlcNAcylation inhibits tumor growth both in vitro and in vivo, as well as tumor invasiveness (227). These effects are mediated by O-GlcNAcylation of the oncogenic transcription factor FoxM1, which increases its expression and activity, suggesting that OGT might represent a therapeutic target for breast cancer. In contrast, O-GlcNAcase is upregulated in thyroid cancers, which would be expected to reduce global O-GlcNAcylation (228). O-GlcNAcylation is strikingly increased in chronic lymphocytic leukemic lymphocytes (229). Although all forms of chronic lymphocytic leukemia cells have high O-GlcNAcylation, the extent of the increased O-GlcNAcylation correlates directly with the aggressiveness of the disease. Patients with the highest levels of O-GlcNAcylation present with an indolent clinical behavior and a better prognosis, whereas those patients whose lymphocytes have lower levels of O-GlcNAcylation have a more aggressive form of the disease and a poor prognosis. Available data suggest that the highest levels of O-GlcNAcylation dampen signaling pathways controlling tumor cell growth. These and other findings certainly justify more research into the roles of O-GlcNAcylation in the etiology of cancer.
O-Linked _N_-Acetylglucosamine and Viruses
Analogous to O-GlcNAc’s localization in cells, unlike all other forms of protein glycosylation, which reside on the surface of viruses, O-GlcNAc is mostly localized on proteins surrounding the nucleic acid components of viruses. For example, a major tegument protein, the basic phosphoprotein of human cytomegalovirus, is O-GlcNAcylated at multiple sites (107, 230). O-GlcNAcylation of adenovirus fiber proteins appears to play a role in fiber assembly; after this, the sugar moieties appear buried within the trimer (231, 232). Proteins in rotavirus (233) and insect viruses, such as baculovirus (234), are also O-GlcNAcylated. Reciprocal phosphorylation and O-GlcNAcylation occur on the SV40 large T antigen, a key oncogenic protein that regulates viral gene expression (235). Host cell transcription factors play an important role in the expression of viral proteins. Excessive O-GlcNAcylation of the transcription factor Sp1 inhibits its activity at the human immunodeficiency virus-1 promoter (236). Although there are few studies of the O-GlcNAcylation of viral proteins, it is already clear that viruses represent powerful systems in which to elucidate the fundamental functions of O-GlcNAcylation.
CONCLUSION
Even though O-GlcNAcylation remained undiscovered until the early 1980s and is still essentially invisible by commonly used biochemical methods, it is now known from research in many laboratories that this ubiquitous protein modification plays an important role in regulating cellular functions in response to nutrients and stress. Despite the fact that O-GlcNAcylation functions on many proteins independently, especially in transcription, it is its extensive cross talk with phosphorylation that cements its fundamental roles in cell signaling. As a nutrient sensor, O-GlcNAcylation underlies fundamental mechanisms of chronic disease, especially in diabetes and neurodegenerative diseases. As the tools required for the study of O-GlcNAcylation become more facile and widely available, many more laboratories will explore its functions in their favorite system or on their favorite protein. As is evident in the review, there is now compelling evidence that justifies much further work on O-GlcNAcylation. We have only just begun to elucidate the functions of this important and essential posttranslational modification.
SUMMARY POINTS.
- O-GlcNAcylation is an essential cycling modification consisting of a single _N_-acetylglucosamine sugar attached to the serine or threonine residues of nuclear and cytoplasmic proteins.
- The enzymes that regulate O-linked _N_-acetylglucosamine cycling, O-GlcNAc transferase (OGT), and O-GlcNAcase are highly conserved from worms to man and are present in all multicellular organisms.
- O-GlcNAcylation has extensive cross talk with protein phosphorylation because of competitive site occupancy on polypeptides and as a result of each modification regulating the cycling enzymes of the other modification.
- Detection and site mapping of O-GlcNAc is difficult by standard methods, and in recent years, advanced mass spectrometric methods have been developed to quantify it with high sensitivity.
- O-GlcNAcylation and its cross talk with phosphorylation serve as a nutrient/stress sensor to regulate transcription, translation, signaling, and the cytoskeleton.
- O-GlcNAcylation and its cross talk with phosphorylation underlie the etiology of chronic diseases, such as diabetes, neurodegenerative diseases, and cancer.
- The paradox of O-GlcNAcylation is that, in acute conditions of stress, the sugar modification is protective allowing cell survival, but in the long-term, increased O-GlcNAcylation leads to abnormal signaling, transcription, and cell damage.
FUTURE ISSUES.
- Tools are needed to advance the field. The availability of site-specific, phosphosite antibodies led to an explosion in our understanding of the roles of protein phosphorylation. Such site-specific O-GlcNAc antibodies are critically needed to rapidly advance our understanding of O-GlcNAcylation.
- How are the O-GlcNAc cycling enzymes targeted to their substrates? How do enzymes that have only one or two genes in the genome specifically modify hundreds to thousands of different substrates? The regulation of OGT and O-GlcNAcase specificity remains a major challenge for the field.
- Why are so many proteins involved in transcription heavily O-GlcNAcylated? Nearly every transcription factor and RNA polymerase itself is extensively O-GlcNAcylated. Why?
- How are kinase signaling cascades integrated with O-GlcNAcylation? We know that the cross talk between O-GlcNAcylation and phosphorylation is extensive, but we know little about how the signaling pathways integrate these two modifications in response to nutrients or stress.
- Why is O-GlcNAc present in some bacteria, in ancient eukaryotes (e.g., Giardia), and in filamentous fungi but apparently absent in yeast? Do yeast use a different sugar to do the same function?
- What are the functions of O-GlcNAc on histones and on ribosomes? Does O-GlcNAcylation of these core cellular components play a role in linking metabolism to transcription and translation?
Acknowledgments
The authors thank former and current members of the laboratory and all of the people in this difficult field who have contributed to our knowledge about O-GlcNAcylation. Our original work was funded by National Institutes of Health grants, R01 CA42486 and R01 DK61671, awarded to G.W.H.
Glossary
O-GlcNAc
O-linked _N_-acetylglucosamine cycling
OGT
O-GlcNAc transferase
Cross talk
the action of one posttranslational modification influencing the addition or removal of another posttranslational modification
O-GlcNAc cycling
the regulated addition and removal of O-GlcNAc from polypeptides without degradation of the polypeptide. Catalyzed by O-GlcNAc transferase and O-GlcNAcase
CAMKIV
calcium calmodulin kinase IV
MGEA5
meningioma-expressed antigen 5
WGA
wheat germ agglutinin
Nutrient sensor
a modification or process that allows the cell to respond to the amounts of nutrients available
Glucose toxicity
prolonged exposure to high levels of glucose gradually causes many cellular processes to malfunction
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Gerald W. Hart, Email: gwhart@jhmi.edu.
Chad Slawson, Email: cslawso1@jhmi.edu.
Genaro Ramirez-Correa, Email: gramire2@jhmi.edu.
Olof Lagerlof, Email: olof.lagerlof@gmail.com.
LITERATURE CITED
- 1.Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem. 1984;259:3308–17. [PubMed] [Google Scholar]
- 2.Holt GD, Hart GW. The subcellular distribution of terminal N-acetylglucosamine moieties. Localization of a novel protein-saccharide linkage, O-linked GlcNAc. J Biol Chem. 1986;261:8049–57. [PubMed] [Google Scholar]
- 3.Dorfman A, Roseman S, Moses FE, Ludowieg J, Mayeda M. The biosynthesis of hyaluronic acid by group a streptococcus. III. Origin of the N-acetylglucosamine moiety. J Biol Chem. 1955;212:583–91. [PubMed] [Google Scholar]
- 4.Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem. 1991;266:4706–12. [PubMed] [Google Scholar]
- 5.Ghosh S, Blumenthal HJ, Davidson E, Roseman S. Glucosamine metabolism. V Enzymatic synthesis of glucosamine 6-phosphate. J Biol Chem. 1960;235:1265–73. [PubMed] [Google Scholar]
- 6.Wells L, Vosseller K, Hart GW. Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science. 2001;291:2376–78. doi: 10.1126/science.1058714. [DOI] [PubMed] [Google Scholar]
- 7.Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–22. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 8.Love DC, Hanover JA. The hexosamine signaling pathway: deciphering the “O-GlcNAc code”. Sci STKE. 2005:re13. doi: 10.1126/stke.3122005re13. [DOI] [PubMed] [Google Scholar]
- 9.Hanover JA. Glycan-dependent signaling: O-linked N-acetylglucosamine. FASEB J. 2001;15:1865–76. doi: 10.1096/fj.01-0094rev. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Gucek M, Hart GW. Cross-talk between GlcNAcylation and phosphorylation: site-specific phosphorylation dynamics in response to globally elevated O-GlcNAc. Proc Natl Acad Sci USA. 2008;105:13793–98. doi: 10.1073/pnas.0806216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Pandey A, Hart GW. Dynamic interplay between O-linked N-acetylglucosaminylation and glycogen synthase kinase-3-dependent phosphorylation. Mol Cell Proteomics. 2007;6:1365–79. doi: 10.1074/mcp.M600453-MCP200. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Udeshi ND, Slawson C, Compton PD, Sakabe K, et al. Extensive crosstalk between O-GlcNAcylation and phosphorylation regulates cytokinesis. Sci Signal. 2010;3:1–13. doi: 10.1126/scisignal.2000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeidan Q, Hart GW. The intersections between O-GlcNAcylation and phosphorylation: implications for multiple signaling pathways. J Cell Sci. 2010;123:13–22. doi: 10.1242/jcs.053678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hart GW, Haltiwanger RS, Holt GD, Kelly WG. Glycosylation in the nucleus and cytoplasm. Annu Rev Biochem. 1989;58:841–74. doi: 10.1146/annurev.bi.58.070189.004205. [DOI] [PubMed] [Google Scholar]
- 15.Hart GW. Dynamic O-linked glycosylation of nuclear and cytoskeletal proteins. Annu Rev Biochem. 1997;66:315–35. doi: 10.1146/annurev.biochem.66.1.315. [DOI] [PubMed] [Google Scholar]
- 16.Wells L, Hart GW. O-GlcNAc turns twenty: functional implications for post-translational modification of nuclear and cytosolic proteins with a sugar. FEBS Lett. 2003;546:154–58. doi: 10.1016/s0014-5793(03)00641-0. [DOI] [PubMed] [Google Scholar]
- 17.Slawson C, Housley MP, Hart GW. O-GlcNAc cycling: How a single sugar post-translational modification is changing the way we think about signaling networks. J Cell Biochem. 2006;97:71–83. doi: 10.1002/jcb.20676. [DOI] [PubMed] [Google Scholar]
- 18.Zachara NE, Hart GW. Cell signaling, the essential role of O-GlcNAc! Biochim Biophys Acta. 2006;1761:599–617. doi: 10.1016/j.bbalip.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Copeland RJ, Bullen JW, Hart GW. Cross-talk between GlcNAcylation and phosphorylation: roles in insulin resistance and glucose toxicity. Am J Physiol Endocrinol Metab. 2008;295:E17–28. doi: 10.1152/ajpendo.90281.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu P, Shimoji S, Hart GW. Site-specific interplay between O-GlcNAcylation and phosphorylation in cellular regulation. FEBS Lett. 2010;584:2526–38. doi: 10.1016/j.febslet.2010.04.044. [DOI] [PubMed] [Google Scholar]
- 21.Slawson C, Copeland RJ, Hart GW. O-GlcNAc signaling: a metabolic link between diabetes and cancer? Trends Biochem Sci. 2010;35:547–55. doi: 10.1016/j.tibs.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Love DC, Krause MW, Hanover JA. O-GlcNAc cycling: emerging roles in development and epigenetics. Semin Cell Dev Biol. 2010;21:646–54. doi: 10.1016/j.semcdb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanover JA, Krause MW, Love DC. The hexosamine signaling pathway: O-GlcNAc cycling in feast or famine. Biochim Biophys Acta. 2010;1800:80–95. doi: 10.1016/j.bbagen.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schirm M, Kalmokoff M, Aubry A, Thibault P, Sandoz M, Logan SM. Flagellin from Listeria monocytogenes is glycosylated with beta-O-linked N-acetylglucosamine. J Bacteriol. 2004;186:6721–27. doi: 10.1128/JB.186.20.6721-6727.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen A, Kamp HD, Grundling A, Higgins DE. A bifunctional O-GlcNAc transferase governs flagellar motility through anti-repression. Genes Dev. 2006;20:3283–95. doi: 10.1101/gad.1492606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woosley B, Xie M, Wells L, Orlando R, Garrison D, et al. Comprehensive glycan analysis of recombinant Aspergillus niger endo-polygalacturonase C. Anal Biochem. 2006;354:43–53. doi: 10.1016/j.ab.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Banerjee S, Robbins PW, Samuelson J. Molecular characterization of nucleocytosolic O-GlcNAc transferases of Giardia lamblia and Cryptosporidium parvum. Glycobiology. 2009;19:331–36. doi: 10.1093/glycob/cwn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olszewski NE, West CM, Sassi SO, Hartweck LM. O-GlcNAc protein modification in plants: evolution and function. Biochim Biophys Acta. 2010;1800:49–56. doi: 10.1016/j.bbagen.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartweck LM, Scott CL, Olszewski NE. Two O-linked N-acetylglucosamine transferase genes of Arabidopsis thaliana l. Heynh Have overlapping functions necessary for gamete and seed development. Genetics. 2002;161:1279–91. doi: 10.1093/genetics/161.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thornton TM, Swain SM, Olszewski NE. Gibberellin signal transduction presents ellipsis the spy who O-GlcNAc’d me. Trends Plant Sci. 1999;4:424–28. doi: 10.1016/s1360-1385(99)01485-5. [DOI] [PubMed] [Google Scholar]
- 31.Holt GD, Snow CM, Senior A, Haltiwanger RS, Gerace L, Hart GW. Nuclear pore complex glycoproteins contain cytoplasmically disposed O-linked N-acetylglucosamine. J Cell Biol. 1987;104:1157–64. doi: 10.1083/jcb.104.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanover JA, Cohen CK, Willingham MC, Park MK. O-linked N-acetylglucosamine is attached to proteins of the nuclear pore. Evidence for cytoplasmic and nucleoplasmic glycoproteins. J Biol Chem. 1987;262:9887–94. [PubMed] [Google Scholar]
- 33.Jackson SP, Tjian R. O-glycosylation of eukaryotic transcription factors: implications for mechanisms of transcriptional regulation. Cell. 1988;55:125–33. doi: 10.1016/0092-8674(88)90015-3. [DOI] [PubMed] [Google Scholar]
- 34.Kelly WG, Hart GW. Glycosylation of chromosomal proteins: localization of O-linked N-acetylglucosamine in Drosophila chromatin. Cell. 1989;57:243–51. doi: 10.1016/0092-8674(89)90962-8. [DOI] [PubMed] [Google Scholar]
- 35.Dias WB, Cheung WD, Wang Z, Hart GW. Regulation of calcium/calmodulin-dependent kinase IV by O-GlcNAc modification. J Biol Chem. 2009;284:21327–37. doi: 10.1074/jbc.M109.007310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gandy JC, Rountree AE, Bijur GN. Akt1 is dynamically modified with O-GlcNAc following treatments with pugnac and insulin-like growth factor-1. FEBS Lett. 2006;580:3051–58. doi: 10.1016/j.febslet.2006.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robles-Flores M, Melendez L, Garcia W, Mendoza-Hernandez G, Lam TT, et al. Posttranslational modifications on protein kinase c isozymes. Effects of epinephrine and phorbol esters. Biochim Biophys Acta. 2008;1783:695–712. doi: 10.1016/j.bbamcr.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 38.Cieniewski-Bernard C, Bastide B, Lefebvre T, Lemoine J, Mounier Y, Michalski JC. Identification of O-linked N-acetylglucosamine proteins in rat skeletal muscle using two-dimensional gel electrophoresis and mass spectrometry. Mol Cell Proteomics. 2004;3:577–85. doi: 10.1074/mcp.M400024-MCP200. [DOI] [PubMed] [Google Scholar]
- 39.Ding M, Vandre DD. High molecular weight microtubule-associated proteins contain O-linked-N-acetylglucosamine. J Biol Chem. 1996;271:12555–61. doi: 10.1074/jbc.271.21.12555. [DOI] [PubMed] [Google Scholar]
- 40.Hagmann J, Grob M, Burger MM. The cytoskeletal protein talin is o-glycosylated. J Biol Chem. 1992;267:14424–28. [PubMed] [Google Scholar]
- 41.Zhang X, Bennett V. Identification of O-linked N-acetylglucosamine modification of ankyring isoforms targeted to nodes of ranvier. J Biol Chem. 1996;271:31391–98. doi: 10.1074/jbc.271.49.31391. [DOI] [PubMed] [Google Scholar]
- 42.Arnold CS, Johnson GVW, Cole RN, Dong DLY, Lee M, Hart GW. The microtubule-associated protein tau is extensively modified with O-linked N-acetylglucosamine. J Biol Chem. 1996;271:28741–44. doi: 10.1074/jbc.271.46.28741. [DOI] [PubMed] [Google Scholar]
- 43.Chou CF, Smith AJ, Omary MB. Characterization and dynamics of O-linked glycosylation of human cytokeratin 8 and 18. J Biol Chem. 1992;267:3901–6. [PubMed] [Google Scholar]
- 44.Walgren JL, Vincent TS, Schey KL, Buse MG. High glucose and insulin promote O-GlcNAc modification of proteins, including alpha-tubulin. Am J Physiol Endocrinol Metab. 2003;284:E424–34. doi: 10.1152/ajpendo.00382.2002. [DOI] [PubMed] [Google Scholar]
- 45.Ramirez-Correa GA, Jin W, Wang Z, Zhong X, Gao WD, et al. O-linked GlcNAc modification of cardiac myofilament proteins: a novel regulator of myocardial contractile function. Circ Res. 2008;103:1354–58. doi: 10.1161/CIRCRESAHA.108.184978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cieniewski-Bernard C, Bastide B, Lefebvre T, Lemoine J, Mounier Y, Michalski JC. Identification of O-linked N-acetylglucosamine proteins in rat skeletal muscle using two-dimensional gel electrophoresis and mass spectrometry. Mol Cell Proteomics. 2004;3:577–85. doi: 10.1074/mcp.M400024-MCP200. [DOI] [PubMed] [Google Scholar]
- 47.Love DC, Kochran J, Cathey RL, Shin SH, Hanover JA. Mitochondrial and nucleocytoplasmic targeting of O-linked GlcNAc transferase. J Cell Sci. 2003;116:647–54. doi: 10.1242/jcs.00246. [DOI] [PubMed] [Google Scholar]
- 48.Gao Y, Wells L, Comer FI, Parker GJ, Hart GW. Dynamic O-glycosylation of nuclear and cytosolic proteins: cloning and characterization of a neutral, cytosolic β-N-acetylglucosaminidase from human brain. J Biol Chem. 2001;276:9838–45. doi: 10.1074/jbc.M010420200. [DOI] [PubMed] [Google Scholar]
- 49.Schultz J, Pils B. Prediction of structure and functional residues for O-GlcNAcase, a divergent homologue of acetyltransferases. FEBS Lett. 2002;529:179–82. doi: 10.1016/s0014-5793(02)03322-7. [DOI] [PubMed] [Google Scholar]
- 50.Zeidan Q, Wang Z, De Maio A, Hart GW. O-GlcNAc cycling enzymes associate with the translational machinery and modify core ribosomal proteins. Mol Biol Cell. 2010;21:1922–36. doi: 10.1091/mbc.E09-11-0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanover JA, Yu S, Lubas WB, Shin SH, Ragano-Caracciola M, et al. Mitochondrial and nucleocytoplasmic isoforms of O-linked GlcNAc transferase encoded by a single mammalian gene. Arch Biochem Biophys. 2003;409:287–97. doi: 10.1016/s0003-9861(02)00578-7. [DOI] [PubMed] [Google Scholar]
- 52.Hu Y, Suarez J, Fricovsky E, Wang H, Scott BT, et al. Increased enzymatic O-GlcNAcylation of mitochondrial proteins impairs mitochondrial function in cardiac myocytes exposed to high glucose. J Biol Chem. 2009;284:547–55. doi: 10.1074/jbc.M808518200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsuura A, Ito M, Sakaidani Y, Kondo T, Murakami K, et al. O-linked N-acetylglucosamine is present on the extracellular domain of notch receptors. J Biol Chem. 2008;283:35486–95. doi: 10.1074/jbc.M806202200. [DOI] [PubMed] [Google Scholar]
- 54.Haltiwanger RS, Holt GD, Hart GW. Enzymatic addition of O-GlcNAc to nuclear and cytoplasmic proteins. Identification of a uridine diphospho-N-acetylglucosamine:peptide beta-N-acetylglucosaminyltransferase. J Biol Chem. 1990;265:2563–68. [PubMed] [Google Scholar]
- 55.Haltiwanger RS, Blomberg MA, Hart GW. Glycosylation of nuclear and cytoplasmic proteins. Purification and characterization of a uridine diphospho-N-acetylglucosamine:polypeptide beta-N-acetylglucosaminyltransferase. J Biol Chem. 1992;267:9005–13. [PubMed] [Google Scholar]
- 56.Kreppel LK, Blomberg MA, Hart GW. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J Biol Chem. 1997;272:9308–15. doi: 10.1074/jbc.272.14.9308. [DOI] [PubMed] [Google Scholar]
- 57.Lubas WA, Frank DW, Krause M, Hanover JA. O-linked GlcNAc transferase is a conserved nucleocytoplasmic protein containing tetratricopeptide repeats. J Biol Chem. 1997;272:9316–24. doi: 10.1074/jbc.272.14.9316. [DOI] [PubMed] [Google Scholar]
- 58.Shafi R, Iyer SP, Ellies LG, O’Donnell N, Marek KW, et al. The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc Natl Acad Sci USA. 2000;97:5735–39. doi: 10.1073/pnas.100471497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wrabl JO, Grishin NV. Homology between O-linked GlcNAc transferases and proteins of the glycogen phosphorylase superfamily. J Mol Biol. 2001;314:365–74. doi: 10.1006/jmbi.2001.5151. [DOI] [PubMed] [Google Scholar]
- 60.Iyer SP, Hart GW. Roles of the tetratricopeptide repeat domain in O-GlcNAc transferase targeting and protein substrate specificity. J Biol Chem. 2003;278:24608–16. doi: 10.1074/jbc.M300036200. [DOI] [PubMed] [Google Scholar]
- 61.Blatch GL, Lassle M. The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. BioEssays. 1999;21:932–39. doi: 10.1002/(SICI)1521-1878(199911)21:11<932::AID-BIES5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 62.Whelan SA, Lane MD, Hart GW. Regulation of the O-linked beta-N-acetylglucosamine transferase by insulin signaling. J Biol Chem. 2008;283:21411–17. doi: 10.1074/jbc.M800677200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kreppel LK, Hart GW. Regulation of a cytosolic and nuclear O-GlcNAc transferase. Role of the tetratricopeptide repeats. J Biol Chem. 1999;274:32015–22. doi: 10.1074/jbc.274.45.32015. [DOI] [PubMed] [Google Scholar]
- 64.Lubas WA, Hanover JA. Functional expression of O-linked GlcNAc transferase. Domain structure and substrate specificity. J Biol Chem. 2000;275:10983–88. doi: 10.1074/jbc.275.15.10983. [DOI] [PubMed] [Google Scholar]
- 65.Yang X, Ongusaha PP, Miles PD, Havstad JC, Zhang F, et al. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008;451:964–69. doi: 10.1038/nature06668. [DOI] [PubMed] [Google Scholar]
- 66.Song M, Kim HS, Park JM, Kim SH, Kim IH, et al. O-GlcNAc transferase is activated by CaMKIV-dependent phosphorylation under potassium chloride-induced depolarization in NG-108-15 cells. Cell Signal. 2008;20:94–104. doi: 10.1016/j.cellsig.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 67.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–34. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 68.Schramm L, Hernandez N. Recruitment of RNA polymerase III to its target promoters. Genes Dev. 2002;16:2593–620. doi: 10.1101/gad.1018902. [DOI] [PubMed] [Google Scholar]
- 69.Cohen PTW. Protein phosphatase 1—targeted in many directions. J Cell Sci. 2002;115:241–56. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- 70.Cheung WD, Hart GW. AMP-activated protein kinase and p38 MAPK activate O-GlcNAcylation of neuronal proteins during glucose deprivation. J Biol Chem. 2008;283:13009–20. doi: 10.1074/jbc.M801222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheung WD, Sakabe K, Housley MP, Dias WB, Hart GW. O-linked beta-N-acetylglucosaminyltransferase substrate specificity is regulated by myosin phosphatase targeting and other interacting proteins. J Biol Chem. 2008;283:33935–41. doi: 10.1074/jbc.M806199200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Housley MP, Udeshi ND, Rodgers JT, Shabanowitz J, Puigserver P, et al. A PGC-1α-O-GlcNAc transferase complex regulates FoxO transcription factor activity in response to glucose. J Biol Chem. 2009;284:5148–57. doi: 10.1074/jbc.M808890200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Slawson C, Lakshmanan T, Knapp S, Hart GW. A mitotic GlcNAcylation/phosphorylation signaling complex alters the posttranslational state of the cytoskeletal protein vimentin. Mol Biol Cell. 2008;19:4130–40. doi: 10.1091/mbc.E07-11-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wells L, Kreppel LK, Comer FI, Wadzinski BE, Hart GW. O-GlcNAc transferase is in a functional complex with protein phosphatase 1 catalytic subunits. J Biol Chem. 2004;279:38466–70. doi: 10.1074/jbc.M406481200. [DOI] [PubMed] [Google Scholar]
- 75.Iyer SP, Akimoto Y, Hart GW. Identification and cloning of a novel family of coiled-coil domain proteins that interact with O-GlcNAc transferase. J Biol Chem. 2003;278:5399–409. doi: 10.1074/jbc.M209384200. [DOI] [PubMed] [Google Scholar]
- 76.Wang X, Schwarz TL. The mechanism of Ca2+-dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009;136:163–74. doi: 10.1016/j.cell.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheung WD, Sakabe K, Housley MP, Dias WB, Hart GW. O-GlcNAc transferase substrate specificity is regulated by MYPT1 and other interacting proteins. J Biol Chem. 2008;283:33935–41. doi: 10.1074/jbc.M806199200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Housley MP, Rodgers JT, Udeshi ND, Kelly TJ, Shabanowitz J, et al. O-GlcNAc regulates FoxO activation in response to glucose. J Biol Chem. 2008;283:16283–92. doi: 10.1074/jbc.M802240200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Housley MP, Udeshi ND, Rodgers JT, Shabanowitz J, Puigserver P, et al. A PGC-1alpha-O-GlcNAc transferase complex regulates FoxO transcription factor activity in response to glucose. J Biol Chem. 2009;284:5148–57. doi: 10.1074/jbc.M808890200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gross BJ, Kraybill BC, Walker S. Discovery of O-GlcNAc transferase inhibitors. J Am Chem Soc. 2005;127:14588–90. doi: 10.1021/ja0555217. [DOI] [PubMed] [Google Scholar]
- 81.Clarke AJ, Hurtado-Guerrero R, Pathak S, Schuttelkopf AW, Borodkin V, et al. Structural insights into mechanism and specificity of O-GlcNAc transferase. EMBO J. 2008;27:2780–88. doi: 10.1038/emboj.2008.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martinez-Fleites C, Macauley MS, He Y, Shen DL, Vocadlo DJ, Davies GJ. Structure of an O-GlcNAc transferase homolog provides insight into intracellular glycosylation. Nat Struct Mol Biol. 2008;15:764–65. doi: 10.1038/nsmb.1443. [DOI] [PubMed] [Google Scholar]
- 83.Braidman I, Carroll M, Dance N, Robinson D, Poenaru L, et al. Characterisation of human N-acetyl-beta-hexosaminidase C. FEBS Lett. 1974;41:181–84. doi: 10.1016/0014-5793(74)81206-8. [DOI] [PubMed] [Google Scholar]
- 84.Overdijk B, Van der Kroef WM, Van Steijn GJ, Lisman JJ. Isolation and further characterization of bovine brain hexosaminidase C. Biochim Biophys Acta. 1981;659:255–66. doi: 10.1016/0005-2744(81)90052-8. [DOI] [PubMed] [Google Scholar]
- 85.Dong DL, Hart GW. Purification and characterization of an O-GlcNAc selective N-acetyl-beta-d-glucosaminidase from rat spleen cytosol. J Biol Chem. 1994;269:19321–30. [PubMed] [Google Scholar]
- 86.Heckel D, Comtesse N, Brass N, Blin N, Zang KD, Meese E. Novel immunogenic antigen homologous to hyaluronidase in meningioma. Hum Mol Genet. 1998;7:1859–72. doi: 10.1093/hmg/7.12.1859. [DOI] [PubMed] [Google Scholar]
- 87.Comtesse N, Maldener E, Meese E. Identification of a nuclear variant of MGEA5, a cytoplasmic hyaluronidase and a beta-N-acetylglucosaminidase. Biochem Biophys Res Commun. 2001;283:634–40. doi: 10.1006/bbrc.2001.4815. [DOI] [PubMed] [Google Scholar]
- 88.Toleman C, Paterson AJ, Whisenhunt TR, Kudlow JE. Characterization of the histone acetyl-transferase (HAT) domain of a bifunctional protein with activable O-GlcNAcase and HAT activities. J Biol Chem. 2004;279:53665–73. doi: 10.1074/jbc.M410406200. [DOI] [PubMed] [Google Scholar]
- 89.Butkinaree C, Cheung WD, Park S, Park K, Barber M, Hart GW. Characterization of beta-N-acetylglucosaminidase cleavage by caspase-3 during apoptosis. J Biol Chem. 2008;283:23557–66. doi: 10.1074/jbc.M804116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dorfmueller HC, Borodkin VS, Schimpl M, Shepherd SM, Shpiro NA, van Aalten DM. GlcNAcstatin: a picomolar, selective O-GlcNAcase inhibitor that modulates intracellular O-GlcNAcylation levels. J Am Chem Soc. 2006;128:16484–85. doi: 10.1021/ja066743n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Whitworth GE, Macauley MS, Stubbs KA, Dennis RJ, Taylor EJ, et al. Analysis of PUGNAc and NAG-thiazoline as transition state analogues for human O-GlcNAcase: mechanistic and structural insights into inhibitor selectivity and transition state poise. J Am Chem Soc. 2007;129:635–44. doi: 10.1021/ja065697o. [DOI] [PubMed] [Google Scholar]
- 92.Yuzwa SA, Macauley MS, Heinonen JE, Shan X, Dennis RJ, et al. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol. 2008;4:483–90. doi: 10.1038/nchembio.96. [DOI] [PubMed] [Google Scholar]
- 93.Macauley MS, Vocadlo DJ. Increasing O-GlcNAc levels: an overview of small-molecule inhibitors of O-GlcNAcase. Biochim Biophys Acta. 2009;1800:107–21. doi: 10.1016/j.bbagen.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 94.Macauley MS, Whitworth GE, Debowski AW, Chin D, Vocadlo DJ. O-GlcNAcase uses substrate-assisted catalysis: kinetic analysis and development of highly selective mechanism-inspired inhibitors. J Biol Chem. 2005;280:25313–22. doi: 10.1074/jbc.M413819200. [DOI] [PubMed] [Google Scholar]
- 95.Dennis RJ, Taylor EJ, Macauley MS, Stubbs KA, Turkenburg JP, et al. Structure and mechanism of a bacterial beta-glucosaminidase having O-GlcNAcase activity. Nat Struct Mol Biol. 2006;13:365–71. doi: 10.1038/nsmb1079. [DOI] [PubMed] [Google Scholar]
- 96.Rao FV, Dorfmueller HC, Villa F, Allwood M, Eggleston IM, van Aalten DM. Structural insights into the mechanism and inhibition of eukaryotic O-GlcNAc hydrolysis. EMBO J. 2006;25:1569–78. doi: 10.1038/sj.emboj.7601026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roquemore EP, Chou TY, Hart GW. Detection of O-linked N-acetylglucosamine (O-GlcNAc) on cytoplasmic and nuclear proteins. Methods Enzymol. 1994;230:443–60. doi: 10.1016/0076-6879(94)30028-3. [DOI] [PubMed] [Google Scholar]
- 98.Zachara NE, Cole RN, Hart GW, Gao Y. Detection and analysis of proteins modified by O-linked N-acetylglucosamine. Curr Protocols Protein Sci. 2001;Chapter 12(Unit 12):8.1–8.25. doi: 10.1002/0471140864.ps1208s25. [DOI] [PubMed] [Google Scholar]
- 99.Zachara NE, Cheung WD, Hart GW. Nucleocytoplasmic glycosylation, O-GlcNAc: identification and site mapping. Methods Mol Biol. 2004;284:175–94. doi: 10.1385/1-59259-816-1:175. [DOI] [PubMed] [Google Scholar]
- 100.Whelan SA, Hart GW. Identification of O-GlcNAc sites on proteins. Methods Enzymol. 2006;415:113–33. doi: 10.1016/S0076-6879(06)15008-9. [DOI] [PubMed] [Google Scholar]
- 101.Greis KD, Hart GW. Analytical methods for the study of O-GlcNAc glycoproteins and glycopeptides. Methods Mol Biol. 1998;76:19–33. doi: 10.1385/0-89603-355-4:19. [DOI] [PubMed] [Google Scholar]
- 102.Wang Z, Udeshi ND, O’Malley M, Shabanowitz J, Hunt DF, Hart GW. Enrichment and site mapping of O-linked N-acetylglucosamine by a combination of chemical/enzymatic tagging, photochemical cleavage, and electron transfer dissociation mass spectrometry. Mol Cell Proteomics. 2010;9:153–60. doi: 10.1074/mcp.M900268-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Khidekel N, Arndt S, Lamarre-Vincent N, Lippert A, Poulin-Kerstien KG, et al. A chemoenzymatic approach toward the rapid and sensitive detection of O-GlcNAc posttranslational modifications. J Am Chem Soc. 2003;125:16162–63. doi: 10.1021/ja038545r. [DOI] [PubMed] [Google Scholar]
- 104.Whelan SA, Hart GW. Proteomic approaches to analyze the dynamic relationships between nucleocytoplasmic protein glycosylation and phosphorylation. Circ Res. 2003;93:1047–58. doi: 10.1161/01.RES.0000103190.20260.37. [DOI] [PubMed] [Google Scholar]
- 105.Reason AJ, Blench IP, Haltiwanger RS, Hart GW, Morris HR, et al. High-sensitivity FAB-MS strategies for O-GlcNAc characterization. Glycobiology. 1991;1:585–94. doi: 10.1093/glycob/1.6.585. [DOI] [PubMed] [Google Scholar]
- 106.Reason AJ, Morris HR, Panico M, Marais R, Treisman RH, et al. Localization of O-GlcNAc modification on the serum response transcription factor. J Biol Chem. 1992;267:16911–21. [PubMed] [Google Scholar]
- 107.Greis KD, Gibson W, Hart GW. Site-specific glycosylation of the human cytomegalovirus tegument basic phosphoprotein (UL32) at serine 921 and serine 952. J Virol. 1994;68:8339–49. doi: 10.1128/jvi.68.12.8339-8349.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cole RN, Hart GW. Glycosylation sites flank phosphorylation sites on synapsin I: O-linked N-acetylglucosamine residues are localized within domains mediating synapsin I interactions. J Neurochem. 1999;73:418–28. doi: 10.1046/j.1471-4159.1999.0730418.x. [DOI] [PubMed] [Google Scholar]
- 109.Greis KD, Hayes BK, Comer FI, Kirk M, Barnes S, et al. Selective detection and site-analysis of O-GlcNAc-modified glycopeptides by beta-elimination and tandem electrospray mass spectrometry. Anal Biochem. 1996;234:38–49. doi: 10.1006/abio.1996.0047. [DOI] [PubMed] [Google Scholar]
- 110.Wells L, Vosseller K, Cole RN, Cronshaw JM, Matunis MJ, Hart GW. Mapping sites of O-GlcNAc modification using affinity tags for serine and threonine post-translational modifications. Mol Cell Proteomics. 2002;1:791–804. doi: 10.1074/mcp.m200048-mcp200. [DOI] [PubMed] [Google Scholar]
- 111.Peter-Katalinic J. Methods in enzymology: O-glycosylation of proteins. Methods Enzymol. 2005;405:139–71. doi: 10.1016/S0076-6879(05)05007-X. [DOI] [PubMed] [Google Scholar]
- 112.Syka JE, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF. Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc Natl Acad Sci USA. 2004;101:9528–33. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schroeder MJ, Webb DJ, Shabanowitz J, Horwitz AF, Hunt DF. Methods for the detection of paxillin post-translational modifications and interacting proteins by mass spectrometry. J Proteome Res. 2005;4:1832–41. doi: 10.1021/pr0502020. [DOI] [PubMed] [Google Scholar]
- 114.Wang Z, Udeshi ND, Slawson C, Compton PD, Sakabe K, et al. Extensive crosstalk between O-GlcNAcylation and phosphorylation regulates cytokinesis. Sci Signal. 2010;3:ra2. doi: 10.1126/scisignal.2000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Slawson C, Zachara NE, Vosseller K, Cheung WD, Lane MD, Hart GW. Perturbations in O-linked beta-N-acetylglucosamine protein modification cause severe defects in mitotic progression and cytokinesis. J Biol Chem. 2005;280:32944–56. doi: 10.1074/jbc.M503396200. [DOI] [PubMed] [Google Scholar]
- 116.Chou TY, Hart GW, Dang CV. c-Myc is glycosylated at threonine 58, a known phosphorylation site and a mutational hot spot in lymphomas. J Biol Chem. 1995;270:18961–65. doi: 10.1074/jbc.270.32.18961. [DOI] [PubMed] [Google Scholar]
- 117.Cheng X, Hart GW. Alternative O-glycosylation/O-phosphorylation of serine-16 in murine estrogen receptor beta: Post-translational regulation of turnover and transactivation activity. J Biol Chem. 2001;276:10570–75. doi: 10.1074/jbc.M010411200. [DOI] [PubMed] [Google Scholar]
- 118.Kelly WG, Dahmus ME, Hart GW. RNA polymerase II is a glycoprotein. Modification of the COOH-terminal domain by O-GlcNAc. J Biol Chem. 1993;268:10416–24. [PubMed] [Google Scholar]
- 119.Comer FI, Hart GW. Reciprocity between O-GlcNAc and O-phosphate on the carboxyl terminal domain of RNA polymerase II. Biochemistry. 2001;40:7845–52. doi: 10.1021/bi0027480. [DOI] [PubMed] [Google Scholar]
- 120.Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Investig. 2001;108:1341–48. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang WH, Kim JE, Nam HW, Ju JW, Kim HS, et al. Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat Cell Biol. 2006;8:1074–83. doi: 10.1038/ncb1470. [DOI] [PubMed] [Google Scholar]
- 122.Wu WG, Pasternack L, Huang DH, Koeller KM, Lin CC, et al. Structural study on O-glycopeptides: Glycosylation-induced conformational changes of O-GlcNAc, O-LacNAc, O-sialyl-LacNAc, and O-sialyl-Lewis-X peptides of the mucin domain of MAdCAM-1. J Am Chem Soc. 1999;121:2409–17. [Google Scholar]
- 123.Liang FC, Chen RP, Lin CC, Huang KT, Chan SI. Tuning the conformation properties of a peptide by glycosylation and phosphorylation. Biochem Biophys Res Commun. 2006;342:482–88. doi: 10.1016/j.bbrc.2006.01.168. [DOI] [PubMed] [Google Scholar]
- 124.Hart GW, Sakabe K. Fine-tuning ER-beta structure with PTMs. Chem Biol. 2006;13:923–24. doi: 10.1016/j.chembiol.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 125.Chen YX, Du JT, Zhou LX, Liu XH, Zhao YF, et al. Alternative O-GlcNAcylation/O-phosphorylation of Ser16 induce different conformational disturbances to the N terminus of murine estrogen receptor beta. Chem Biol. 2006;13:937–44. doi: 10.1016/j.chembiol.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 126.Omary MB, Ku NO, Liao J, Price D. Keratin modifications and solubility properties in epithelial cells and in vitro. Subcell Biochem. 1998;31:105–40. [PubMed] [Google Scholar]
- 127.Ball LE, Berkaw MN, Buse MG. Identification of the major site of O-linked beta-N-acetylglucosamine modification in the C terminus of insulin receptor substrate-1. Mol Cell Proteomics. 2006;5:313–23. doi: 10.1074/mcp.M500314-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wells L, Kreppel LK, Comer FI, Wadzinski BE, Hart GW. O-GlcNAc transferase is in a functional complex with protein phosphatase 1 catalytic subunits. J Biol Chem. 2004;279:38466–70. doi: 10.1074/jbc.M406481200. [DOI] [PubMed] [Google Scholar]
- 129.Matthews JA, Acevedo-Duncan M, Potter RL. Selective decrease of membrane-associated PKC-alpha and PKC-epsilon in response to elevated intracellular O-GlcNAc levels in transformed human glial cells. Biochim Biophys Acta. 2005;1743:305–15. doi: 10.1016/j.bbamcr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 130.Luo B, Soesanto Y, McClain DA. Protein modification by O-linked GlcNAc reduces angiogenesis by inhibiting Akt activity in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:651–57. doi: 10.1161/ATVBAHA.107.159533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kang ES, Han D, Park J, Kwak TK, Oh MA, et al. O-GlcNAc modulation at Akt1 Ser473 correlates with apoptosis of murine pancreatic beta cells. Exp Cell Res. 2008;314:2238–48. doi: 10.1016/j.yexcr.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 132.Beausoleil SA, Jedrychowski M, Schwartz D, Elias JE, Villen J, et al. Large-scale characterization of HeLa cell nuclear phosphoproteins. Proc Natl Acad Sci USA. 2004;101:12130–35. doi: 10.1073/pnas.0404720101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sakabe K, Hart GW. O-GlcNAc transferase regulates mitotic chromatin dynamics. J Biol Chem. 2010;285:34460–68. doi: 10.1074/jbc.M110.158170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–40. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 135.Bouche C, Serdy S, Kahn CR, Goldfine AB. The cellular fate of glucose and its relevance in type 2 diabetes. Endocr Rev. 2004;25:807–30. doi: 10.1210/er.2003-0026. [DOI] [PubMed] [Google Scholar]
- 136.Zachara NE, O’Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem. 2004;279:30133–42. doi: 10.1074/jbc.M403773200. [DOI] [PubMed] [Google Scholar]
- 137.Zachara NE, Hart GW. O-GlcNAc a sensor of cellular state: the role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochim Biophys Acta. 2004;1673:13–28. doi: 10.1016/j.bbagen.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 138.Xing D, Feng W, Not LG, Miller AP, Zhang Y, et al. Increased protein O-GlcNAc modification inhibits inflammatory and neointimal responses to acute endoluminal arterial injury. Am J Physiol Heart Circ Physiol. 2008;295:H335–42. doi: 10.1152/ajpheart.01259.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang S, Zou LY, Bounelis P, Chaudry I, Chatham JC, Marchase RB. Glucosamine administration during resuscitation improves organ function after trauma hemorrhage. Shock. 2006;25:600–7. doi: 10.1097/01.shk.0000209563.07693.db. [DOI] [PubMed] [Google Scholar]
- 140.Liu J, Pang Y, Chang T, Bounelis P, Chatham JC, Marchase RB. Increased hexosamine biosynthesis and protein O-GlcNAc levels associated with myocardial protection against calcium paradox and ischemia. J Mol Cell Cardiol. 2006;40:303–12. doi: 10.1016/j.yjmcc.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 141.Jones SP, Zachara NE, Ngoh GA, Hill BG, Teshima Y, et al. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation. 2008;117:1172–82. doi: 10.1161/CIRCULATIONAHA.107.730515. [DOI] [PubMed] [Google Scholar]
- 142.Ngoh GA, Watson LJ, Facundo HT, Dillmann W, Jones SP. Non-canonical glycosyltransferase modulates post-hypoxic cardiac myocyte death and mitochondrial permeability transition. J Mol Cell Cardiol. 2008;45:313–25. doi: 10.1016/j.yjmcc.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chatham JC, Marchase RB. The role of protein O-linked beta-N-acetylglucosamine in mediating cardiac stress responses. Biochim Biophys Acta. 2010;1800:57–66. doi: 10.1016/j.bbagen.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fülöp N, Zhang Z, Marchase RB, Chatham JC. Glucosamine cardioprotection in perfused rat hearts associated with increased O-linked N-acetylglucosamine protein modification and altered p38 activation. Am J Physiol Heart Circ Physiol. 2007;292:H2227–36. doi: 10.1152/ajpheart.01091.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fülöp N, Marchase RB, Chatham JC. Role of protein O-linked N-acetyl-glucosamine in mediating cell function and survival in the cardiovascular system. Cardiovasc Res. 2007;73:288–97. doi: 10.1016/j.cardiores.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jones SP. A bittersweet modification: O-GlcNAc and cardiac dysfunction. Circ Res. 2005;96:925–36. doi: 10.1161/01.RES.0000168039.61228.67. [DOI] [PubMed] [Google Scholar]
- 147.Jackson SP, Tjian R. Purification and analysis of RNA polymerase II transcription factors by using wheat germ agglutinin affinity chromatography. Proc Natl Acad Sci USA. 1989;86:1781–85. doi: 10.1073/pnas.86.6.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Comer FI, Hart GW. O-GlcNAc and the control of gene expression. Biochim Biophys Acta. 1999;1473:161–71. doi: 10.1016/s0304-4165(99)00176-2. [DOI] [PubMed] [Google Scholar]
- 149.Hart GW, Kreppel LK, Comer FI, Arnold CS, Snow DM, et al. O-GlcNAcylation of key nuclear and cytoskeletal proteins: reciprocity with O-phosphorylation and putative roles in protein multimerization. Glycobiology. 1996;6:711–16. doi: 10.1093/glycob/6.7.711. [DOI] [PubMed] [Google Scholar]
- 150.Price DH. Poised polymerases: On your mark…Get set…Go! Mol Cell. 2008;30:7–10. doi: 10.1016/j.molcel.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 151.Sinclair DA, Syrzycka M, Macauley MS, Rastgardani T, Komljenovic I, et al. Drosophila O-GlcNAc transferase (OGT) is encoded by the polycomb group (PcG) gene, super sex combs (sxc) Proc Natl Acad Sci USA. 2009;106:13427–32. doi: 10.1073/pnas.0904638106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gambetta MC, Oktaba K, Muller J. Essential role of the glycosyltransferase Sxc/Ogt in polycomb repression. Science. 2009;325:93–96. doi: 10.1126/science.1169727. [DOI] [PubMed] [Google Scholar]
- 153.McClain DA, Paterson AJ, Roos MD, Wei X, Kudlow JE. Glucose and glucosamine regulate growth factor gene expression in vascular smooth muscle cells. Proc Natl Acad Sci USA. 1992;89:8150–54. doi: 10.1073/pnas.89.17.8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Goldberg HJ, Scholey J, Fantus IG. Glucosamine activates the plasminogen activator inhibitor 1 gene promoter through Sp1 DNA binding sites in glomerular mesangial cells. Diabetes. 2000;49:863–71. doi: 10.2337/diabetes.49.5.863. [DOI] [PubMed] [Google Scholar]
- 155.Kudlow JE. Post-translational modification by O-GlcNAc: another way to change protein function. J Cell Biochem. 2006;98:1062–75. doi: 10.1002/jcb.20926. [DOI] [PubMed] [Google Scholar]
- 156.Solomon SS, Majumdar G, Martinez-Hernandez A, Raghow R. A critical role of Sp1 transcription factor in regulating gene expression in response to insulin and other hormones. Life Sci. 2008;83:305–12. doi: 10.1016/j.lfs.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 157.Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, et al. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci USA. 2000;97:12222–26. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ray MK, Datta B, Chakraborty A, Chattopadhyay A, Meza-Keuthen S, Gupta NK. The eukaryotic initiation factor 2-associated 67-kDa polypeptide (p67) plays a critical role in regulation of protein synthesis initiation in animal cells. Proc Natl Acad Sci USA. 1992;89:539–43. doi: 10.1073/pnas.89.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Starr CM, Hanover JA. Glycosylation of nuclear pore protein p62. Reticulocyte lysate catalyzes O-linked N-acetylglucosamine addition in vitro. J Biol Chem. 1990;265:6868–73. [PubMed] [Google Scholar]
- 160.Ohn T, Kedersha N, Hickman T, Tisdale S, Anderson P. A functional RNAi screen links O-GlcNAc modification of ribosomal proteins to stress granule and processing body assembly. Nat Cell Biol. 2008;10:1224–31. doi: 10.1038/ncb1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Holt GD, Haltiwanger RS, Torres CR, Hart GW. Erythrocytes contain cytoplasmic glycoproteins. O-linked GlcNAc on band 4.1. J Biol Chem. 1987;262:14847–50. [PubMed] [Google Scholar]
- 162.Ramirez-Correa GA, Jin W, Wang Z, Zhong X, Gao WD, et al. O-linked GlcNAc modification of cardiac myofilament proteins. A novel regulator of myocardial contractile function. Circ Res. 2008;103:1354–58. doi: 10.1161/CIRCRESAHA.108.184978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Srikanth B, Vaidya MM, Kalraiya RD. O-GlcNAcylation determines the solubility, filament organization, and stability of keratins 8 and 18. J Biol Chem. 2010;285:34062–71. doi: 10.1074/jbc.M109.098996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hatsell SJ, Medina L, Merola J, Haltiwanger R, Cowin P. Plakoglobin is O-glycosylated close to the N-terminal destruction box. J Biol Chem. 2003;278:37745–52. doi: 10.1074/jbc.M301346200. [DOI] [PubMed] [Google Scholar]
- 165.Hu P, Berkowitz P, Madden VJ, Rubenstein DS. Stabilization of plakoglobin and enhanced keratinocyte cell-cell adhesion by intracellular O-glycosylation. J Biol Chem. 2006;281:12786–91. doi: 10.1074/jbc.M511702200. [DOI] [PubMed] [Google Scholar]
- 166.Cieniewski-Bernard C, Mounier Y, Michalski JC, Bastide B. O-GlcNAc level variations are associated with the development of skeletal muscle atrophy. J Appl Physiol. 2006;100:1499–505. doi: 10.1152/japplphysiol.00865.2005. [DOI] [PubMed] [Google Scholar]
- 167.Hedou J, Cieniewski-Bernard C, Leroy Y, Michalski JC, Mounier Y, Bastide B. O-linked N-acetylglucosaminylation is involved in the Ca2+ activation properties of rat skeletal muscle. J Biol Chem. 2007;282:10360–69. doi: 10.1074/jbc.M606787200. [DOI] [PubMed] [Google Scholar]
- 168.Cieniewski-Bernard C, Montel V, Stevens L, Bastide B. O-GlcNAcylation, an original modulator of contractile activity in striated muscle. J Muscle Res Cell Motil. 2009;30:281–87. doi: 10.1007/s10974-010-9201-1. [DOI] [PubMed] [Google Scholar]
- 169.Kim YH, Song M, Oh YS, Heo K, Choi JW, et al. Inhibition of phospholipase C-beta1-mediated signaling by O-GlcNAc modification. J Cell Physiol. 2006;207:689–96. doi: 10.1002/jcp.20609. [DOI] [PubMed] [Google Scholar]
- 170.Rengifo J, Gibson CJ, Winkler E, Collin T, Ehrlich BE. Regulation of the inositol 1,4,5-trisphosphate receptor type I by O-GlcNAc glycosylation. J Neurosci. 2007;27:13813–21. doi: 10.1523/JNEUROSCI.2069-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sumegi M, Hunyadi-Gulyas E, Medzihradszky KF, Udvardy A. 26S proteasome subunits are O-linked N-acetylglucosamine-modified in Drosophila melanogaster. Biochem Biophys Res Commun. 2003;312:1284–89. doi: 10.1016/j.bbrc.2003.11.074. [DOI] [PubMed] [Google Scholar]
- 172.Zhang F, Su K, Yang X, Bowe DB, Paterson AJ, Kudlow JE. O-GlcNAc modification is an endogenous inhibitor of the proteasome. Cell. 2003;115:715–25. doi: 10.1016/s0092-8674(03)00974-7. [DOI] [PubMed] [Google Scholar]
- 173.Dudognon P, Maeder-Garavaglia C, Carpentier JL, Paccaud JP. Regulation of a COPII component by cytosolic O-glycosylation during mitosis. FEBS Lett. 2004;561:44–50. doi: 10.1016/S0014-5793(04)00109-7. [DOI] [PubMed] [Google Scholar]
- 174.Sterne-Marr R, Blevitt JM, Gerace L. O-linked glycoproteins of the nuclear pore complex interact with a cytosolic factor required for nuclear protein import. J Cell Biol. 1992;116:271–80. doi: 10.1083/jcb.116.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Duverger E, Carpentier V, Roche A-C, Monsigny M. Sugar-dependent nuclear import of glyco-conjugates from the cytosol. Exp Cell Res. 1993;207:197–201. doi: 10.1006/excr.1993.1181. [DOI] [PubMed] [Google Scholar]
- 176.O’Donnell N, Zachara NE, Hart GW, Marth JD. Ogt-dependent X-chromosome-linked protein glycosylation is a requisite modification in somatic cell function and embryo viability. Mol Cell Biol. 2004;24:1680–90. doi: 10.1128/MCB.24.4.1680-1690.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Forsythe ME, Love DC, Lazarus BD, Kim EJ, Prinz WA, et al. Caenorhabditis elegans ortholog of a diabetes susceptibility locus: Oga-1 (O-GlcNAcase) knockout impacts O-GlcNAc cycling, metabolism, and dauer. Proc Natl Acad Sci USA. 2006;103:11952–57. doi: 10.1073/pnas.0601931103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hanover JA, Forsythe ME, Hennessey PT, Brodigan TM, Love DC, et al. A Caenorhabditis elegans model of insulin resistance: altered macronutrient storage and dauer formation in an OGT-1 knockout. Proc Natl Acad Sci USA. 2005;102:11266–71. doi: 10.1073/pnas.0408771102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lee J, Kim KY, Paik YK. Regulation of dauer formation by O-GlcNAcylation in Caenorhabditis elegans. J Biol Chem. 2010;285:2930–39. doi: 10.1074/jbc.M109.022665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Webster DM, Teo CF, Sun Y, Wloga D, Gay S, et al. O-GlcNAc modifications regulate cell survival and epiboly during zebrafish development. BMC Dev Biol. 2009;9:28. doi: 10.1186/1471-213X-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.McClain DA, Crook ED. Hexosamines and insulin resistance. Diabetes. 1996;45:1003–9. doi: 10.2337/diab.45.8.1003. [DOI] [PubMed] [Google Scholar]
- 182.McClain DA. Hexosamines as mediators of nutrient sensing and regulation in diabetes. J Diabetes Complicat. 2002;16:72–80. doi: 10.1016/s1056-8727(01)00188-x. [DOI] [PubMed] [Google Scholar]
- 183.Buse MG. Hexosamines, insulin resistance, and the complications of diabetes: current status. Am J Physiol Endocrinol Metab. 2006;290:E1–8. doi: 10.1152/ajpendo.00329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wells L, Vosseller K, Hart GW. A role for N-acetylglucosamine as a nutrient sensor and mediator of insulin resistance. Cell Mol Life Sci. 2003;60:222–28. doi: 10.1007/s000180300017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Daniels MC, Ciaraldi TP, Nikoulina S, Henry RR, McClain DA. Glutamine:fructose-6-phosphate amidotransferase activity in cultured human skeletal muscle cells: relationship to glucose disposal rate in control and non-insulin-dependent diabetes mellitus subjects and regulation by glucose and insulin. J Clin Investig. 1996;97:1235–41. doi: 10.1172/JCI118538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hebert LF, Jr, Daniels MC, Zhou JX, Crook ED, Turner RL, et al. Overexpression of glutamine:fructose-6-phosphate amidotransferase in transgenic mice leads to insulin resistance. J Clin Investig. 1996;98:930–36. doi: 10.1172/JCI118876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cooksey RC, Hebert LF, Jr, Zhu JH, Wofford P, Garvey WT, McClain DA. Mechanism of hexosamine-induced insulin resistance in transgenic mice overexpressing glutamine:fructose-6-phosphate amidotransferase: decreased glucose transporter GLUT4 translocation and reversal by treatment with thiazolidinedione. Endocrinology. 1999;140:1151–57. doi: 10.1210/endo.140.3.6563. [DOI] [PubMed] [Google Scholar]
- 188.Vosseller K, Wells L, Lane MD, Hart GW. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc Natl Acad Sci USA. 2002;99:5313–18. doi: 10.1073/pnas.072072399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Macauley MS, Bubb AK, Martinez-Fleites C, Davies GJ, Vocadlo DJ. Elevation of global O-GlcNAc levels in 3T3-L1 adipocytes by selective inhibition of O-GlcNAcase does not induce insulin resistance. J Biol Chem. 2008;283:34687–95. doi: 10.1074/jbc.M804525200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Robinson KA, Ball LE, Buse MG. Reduction of O-GlcNAc protein modification does not prevent insulin resistance in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab. 2006;292:E884–90. doi: 10.1152/ajpendo.00569.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Klein AL, Berkaw MN, Buse MG, Ball LE. O-linked N-acetylglucosamine modification of insulin receptor substrate-1 occurs in close proximity to multiple SH2 domain binding motifs. Mol Cell Proteomics. 2009;8:2733–45. doi: 10.1074/mcp.M900207-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Dias WB, Hart GW. O-GlcNAc modification in diabetes and Alzheimer’s disease. Mol Biosyst. 2007;3:766–72. doi: 10.1039/b704905f. [DOI] [PubMed] [Google Scholar]
- 193.Kroner Z. The relationship between Alzheimer’s disease and diabetes: type 3 diabetes? Altern Med Rev. 2009;14:373–79. [PubMed] [Google Scholar]
- 194.Lefebvre T, Dehennaut V, Guinez C, Olivier S, Drougat L, et al. Dysregulation of the nutrient/stress sensor O-GlcNAcylation is involved in the etiology of cardiovascular disorders, type-2 diabetes and Alzheimer’s disease. Biochim Biophys Acta. 2010;1800:67–79. doi: 10.1016/j.bbagen.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 195.Pilcher H. Alzheimer’s disease could be “Type 3 diabetes. Lancet Neurol. 2006;5:388–89. doi: 10.1016/s1474-4422(06)70434-3. [DOI] [PubMed] [Google Scholar]
- 196.Alexander GE, Chen K, Pietrini P, Rapoport SI, Reiman EM. Longitudinal PET evaluation of cerebral metabolic decline in dementia: a potential outcome measure in Alzheimer’s disease treatment studies. Am J Psychiatry. 2002;159:738–45. doi: 10.1176/appi.ajp.159.5.738. [DOI] [PubMed] [Google Scholar]
- 197.Liu F, Shi J, Tanimukai H, Gu J, Grundke-Iqbal I, et al. Reduced O-GlcNAcylation links lower brain glucose metabolism and tau pathology in Alzheimer’s disease. Brain. 2009;132:1820–32. doi: 10.1093/brain/awp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Arnold CS, Johnson GV, Cole RN, Dong DL, Lee M, Hart GW. The microtubule-associated protein tau is extensively modified with O-linked N-acetylglucosamine. J Biol Chem. 1996;271:28741–44. doi: 10.1074/jbc.271.46.28741. [DOI] [PubMed] [Google Scholar]
- 199.Liu F, Iqbal K, Grundke-Iqbal I, Hart GW, Gong CX. O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer’s disease. Proc Natl Acad Sci USA. 2004;101:10804–9. doi: 10.1073/pnas.0400348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Griffith LS, Mathes M, Schmitz B. Beta-amyloid precursor protein is modified with O-linked N-acetylglucosamine. J Neurosci Res. 1995;41:270–78. doi: 10.1002/jnr.490410214. [DOI] [PubMed] [Google Scholar]
- 201.Deng Y, Li B, Liu Y, Iqbal K, Grundke-Iqbal I, Gong CX. Dysregulation of insulin signaling, glucose transporters, O-GlcNAcylation, and phosphorylation of tau and neurofilaments in the brain: implication for Alzheimer’s disease. Am J Pathol. 2009;175:2089–98. doi: 10.2353/ajpath.2009.090157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bertram L, Blacker D, Mullin K, Keeney D, Jones J, et al. Evidence for genetic linkage of Alzheimer’s disease to chromosome 10q. Science. 2000;290:2302–3. doi: 10.1126/science.290.5500.2302. [DOI] [PubMed] [Google Scholar]
- 203.Cole RN, Hart GW. Cytosolic O-glycosylation is abundant in nerve terminals. J Neurochem. 2001;79:1080–89. doi: 10.1046/j.1471-4159.2001.00655.x. [DOI] [PubMed] [Google Scholar]
- 204.Vosseller K, Trinidad JC, Chalkley RJ, Specht CG, Thalhammer A, et al. O-linked N-acetylglucosamine proteomics of postsynaptic density preparations using lectin weak affinity chromatography and mass spectrometry. Mol Cell Proteomics. 2006;5:923–34. doi: 10.1074/mcp.T500040-MCP200. [DOI] [PubMed] [Google Scholar]
- 205.Tallent MK, Varghis N, Skorobogatko Y, Hernandez-Cuebas L, Whelan K, et al. In vivo modulation of O-GlcNAc levels regulates hippocampal synaptic plasticity through interplay with phosphorylation. J Biol Chem. 2009;284:174–81. doi: 10.1074/jbc.M807431200. [DOI] [PubMed] [Google Scholar]
- 206.Skorobogatko YV, Deuso J, Adolf-Bergfoyle J, Nowak MG, Gong Y, et al. Human Alzheimer’s disease synaptic O-GlcNAc site mapping and iTRAQ expression proteomics with ion trap mass spectrometry. Amino Acids. 2011;40:765–79. doi: 10.1007/s00726-010-0645-9. [DOI] [PubMed] [Google Scholar]
- 207.Yao PJ, Coleman PD. Reduced O-glycosylated clathrin assembly protein AP180: implication for synaptic vesicle recycling dysfunction in Alzheimer’s disease. Neurosci Lett. 1998;252:33–36. doi: 10.1016/s0304-3940(98)00547-3. [DOI] [PubMed] [Google Scholar]
- 208.Yao PJ, Coleman PD. Reduction of O-linked N-acetylglucosamine-modified assembly protein-3 in Alzheimer’s disease. J Neurosci. 1998;18:2399–411. doi: 10.1523/JNEUROSCI.18-07-02399.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Marz P, Stetefeld J, Bendfeldt K, Nitsch C, Reinstein J, et al. Ataxin-10 interacts with O-linked beta-N-acetylglucosamine transferase in the brain. J Biol Chem. 2006;281:20263–70. doi: 10.1074/jbc.M601563200. [DOI] [PubMed] [Google Scholar]
- 210.Dong DL, Xu ZS, Chevrier MR, Cotter RJ, Cleveland DW, Hart GW. Glycosylation of mammalian neurofilaments. Localization of multiple O-linked N-acetylglucosamine moieties on neurofilament polypeptides L and M. J Biol Chem. 1993;268:16679–87. [PubMed] [Google Scholar]
- 211.Dong DL, Xu ZS, Hart GW, Cleveland DW. Cytoplasmic O-GlcNAc modification of the head domain and the KSP repeat motif of the neurofilament protein neurofilament-H. J Biol Chem. 1996;271:20845–52. doi: 10.1074/jbc.271.34.20845. [DOI] [PubMed] [Google Scholar]
- 212.Ludemann N, Clement A, Hans VH, Leschik J, Behl C, Brandt R. O-glycosylation of the tail domain of neurofilament protein M in human neurons and in spinal cord tissue of a rat model of amyotrophic lateral sclerosis (ALS) J Biol Chem. 2005;280:31648–58. doi: 10.1074/jbc.M504395200. [DOI] [PubMed] [Google Scholar]
- 213.Mazars R, Gonzalez-de-Peredo A, Cayrol C, Lavigne AC, Vogel JL, et al. The THAP-zinc finger protein THAP1 associates with coactivator HCF-1 and O-GlcNAc transferase: a link between DYT6 and DYT3 dystonias. J Biol Chem. 2010;285:13364–71. doi: 10.1074/jbc.M109.072579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, et al. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J Biol Chem. 2003;278:44230–37. doi: 10.1074/jbc.M303810200. [DOI] [PubMed] [Google Scholar]
- 215.Hu Y, Belke D, Suarez J, Swanson E, Clark R, et al. Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ Res. 2005;96:1006–13. doi: 10.1161/01.RES.0000165478.06813.58. [DOI] [PubMed] [Google Scholar]
- 216.Fülöp N, Mason MM, Dutta K, Wang P, Davidoff AJ, et al. Impact of type 2 diabetes and aging on cardiomyocyte function and O-linked N-acetylglucosamine levels in the heart. Am J Physiol Cell Physiol. 2007;292:C1370–78. doi: 10.1152/ajpcell.00422.2006. [DOI] [PubMed] [Google Scholar]
- 217.Chou TY, Dang CV, Hart GW. Glycosylation of the c-Myc transactivation domain. Proc Natl Acad Sci USA. 1995;92:4417–21. doi: 10.1073/pnas.92.10.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kamemura K, Hart GW. Dynamic interplay between O-glycosylation and O-phosphorylation of nucleocytoplasmic proteins: a new paradigm for metabolic control of signal transduction and transcription. Prog Nucleic Acid Res Mol Biol. 2003;73:107–36. doi: 10.1016/s0079-6603(03)01004-3. [DOI] [PubMed] [Google Scholar]
- 219.Wells L, Slawson C, Hart GW. The E2F-1 associated retinoblastoma-susceptibility gene product is modified by O-GlcNAc. Amino Acids. 2011;40:877–83. doi: 10.1007/s00726-010-0709-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lefebvre T, Pinte S, Guérardel C, Deltour S, Martin-Soudant N, et al. The tumor suppressor HIC1 (hypermethylated in cancer 1) is O-GlcNAc glycosylated. Eur J Biochem. 2004;271:3843–54. doi: 10.1111/j.1432-1033.2004.04316.x. [DOI] [PubMed] [Google Scholar]
- 221.Sayat R, Leber B, Grubac V, Wiltshire L, Persad S. O-GlcNAc-glycosylation of beta-catenin regulates its nuclear localization and transcriptional activity. Exp Cell Res. 2008;314:2774–87. doi: 10.1016/j.yexcr.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 222.Jiang MS, Hart GW. A subpopulation of estrogen receptors are modified by O-linked N-acetylglucosamine. J Biol Chem. 1997;272:2421–28. doi: 10.1074/jbc.272.4.2421. [DOI] [PubMed] [Google Scholar]
- 223.Cheng X, Cole RN, Zaia J, Hart GW. Alternative O-glycosylation/O-phosphorylation of the murine estrogen receptor beta. Biochemistry. 2000;39:11609–20. doi: 10.1021/bi000755i. [DOI] [PubMed] [Google Scholar]
- 224.Kamemura K, Hayes BK, Comer FI, Hart GW. Dynamic interplay between O-glycosylation and O-phosphorylation of nucleocytoplasmic proteins: Alternative glycosylation/phosphorylation of THR-58, a known mutational hot spot of c-Myc in lymphomas, is regulated by mitogens. J Biol Chem. 2002;277:19229–35. doi: 10.1074/jbc.M201729200. [DOI] [PubMed] [Google Scholar]
- 225.Kawauchi K, Araki K, Tobiume K, Tanaka N. Loss of p53 enhances catalytic activity of IKKbeta through O-linked beta-N-acetyl glucosamine modification. Proc Natl Acad Sci USA. 2009;106:3431–36. doi: 10.1073/pnas.0813210106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zhu W, Leber B, Andrews DW. Cytoplasmic O-glycosylation prevents cell surface transport of E-cadherin during apoptosis. EMBO J. 2001;20:5999–6007. doi: 10.1093/emboj/20.21.5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Caldwell SA, Jackson SR, Shahriari KS, Lynch TP, Sethi G, et al. Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene. 2010;29:2831–42. doi: 10.1038/onc.2010.41. [DOI] [PubMed] [Google Scholar]
- 228.Krzeslak A, Pomorski L, Lipinska A. Elevation of nucleocytoplasmic beta-N-acetylglucosaminidase (O-GlcNAcase) activity in thyroid cancers. Int J Mol Med. 2010;25:643–48. doi: 10.3892/ijmm_00000387. [DOI] [PubMed] [Google Scholar]
- 229.Shi Y, Tomic J, Wen F, Shaha S, Bahlo A, et al. Aberrant O-GlcNAcylation characterizes chronic lymphocytic leukemia. Leukemia. 2010;24:1588–98. doi: 10.1038/leu.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Benko DM, Haltiwanger RS, Hart GW, Gibson W. Virion basic phosphoprotein from human cytomegalovirus contains O-linked N-acetylglucosamine. Proc Natl Acad Sci USA. 1988;85:2573–77. doi: 10.1073/pnas.85.8.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Caillet-Boudin ML, Strecker G, Michalski JC. O-linked GlcNAc in serotype-2 adenovirus fibre. Eur J Biochem. 1989;184:205–11. doi: 10.1111/j.1432-1033.1989.tb15008.x. [DOI] [PubMed] [Google Scholar]
- 232.Mullis KG, Haltiwanger RS, Hart GW, Marchase RB, Engler JA. Relative accessibility of N-acetylglucosamine in trimers of the adenovirus types 2 and 5 fiber proteins. J Virol. 1990;64:5317–23. doi: 10.1128/jvi.64.11.5317-5323.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Gonzalez SA, Burrone OR. Rotavirus ns26 is modified by addition of single O-linked residues of N-acetylglucosamine. Virology. 1991;182:8–16. doi: 10.1016/0042-6822(91)90642-o. [DOI] [PubMed] [Google Scholar]
- 234.Whitford M, Faulkner P. A structural polypeptide of the baculovirus Autographa californica nuclear polyhedrosis virus contains O-linked N-acetylglucosamine. J Virol. 1992;66:3324–29. doi: 10.1128/jvi.66.6.3324-3329.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Medina L, Grove K, Haltiwanger RS. SV40 large T antigen is modified with O-linked N-acetylglucosamine but not with other forms of glycosylation. Glycobiology. 1998;8:383–91. doi: 10.1093/glycob/8.4.383. [DOI] [PubMed] [Google Scholar]
- 236.Jochmann R, Thurau M, Jung S, Hofmann C, Naschberger E, et al. O-linked N-acetylglucosaminylation of Sp1 inhibits the human immunodeficiency virus type 1 promoter. J Virol. 2009;83:3704–18. doi: 10.1128/JVI.01384-08. [DOI] [PMC free article] [PubMed] [Google Scholar]