Macrophage-mediated inflammation in metabolic disease (original) (raw)
. Author manuscript; available in PMC: 2012 Jun 26.
Published in final edited form as: Nat Rev Immunol. 2011 Oct 10;11(11):738–749. doi: 10.1038/nri3071
Abstract
Metabolism and immunity are two fundamental systems of metazoans. The presence of immune cells, such as macrophages, in metabolic tissues, suggests dynamic, on-going crosstalk between these two regulatory systems. Here, we discuss how changes in recruitment and activation of macrophages contribute to metabolic homeostasis. In particular, we focus our discussion on the pathogenic and protective functions of classically (M1) and alternatively (M2) activated macrophages, respectively, in experimental models of obesity and metabolic disease.
Introduction
During the last 25 years, the incidence of obesity has increased dramatically throughout the world. In 2008, it was estimated that there were 1.45 billion overweight adults in the world, of which ~500 million were obese1. The number of overweight individuals now exceeds the number of malnourished individuals by ~525 million, a statistic that, in part, reflects the global acceptance of energy-dense foods that are rich in fats but are often lacking in vitamins and other micronutrients2. Consequently, the rise in worldwide obesity has resulted in an explosion of obesity-related health problems, including insulin resistance, type 2 diabetes, coronary artery disease, fatty liver disease, and some cancers and degenerative diseases3-5. Since behavioural and dietary approaches have been ineffective in combating obesity6, a greater emphasis is being placed on understanding the molecular links between obesity and chronic metabolic diseases. In this context, chronic, low-grade inflammation, primarily mediated by innate and adaptive immune cells, has emerged as a key pathogenic link between obesity and its metabolic sequelae7-12.
Almost two decades ago, Spiegelman and colleagues identified the first links between inflammation, obesity, and insulin resistance13, 14. In these initial reports, adipose tissue of obese animals was shown to express tumour necrosis factor (TNF), an inflammatory cytokine that promoted insulin resistance via serine phosphorylation of IRS113, 14. While these initial descriptions laid the groundwork for how inflammation in adipose tissue mediates insulin resistance, the true nature of this inflammatory process was not explored for another decade. In 2003, two reports by Ferrante and Chen demonstrated that obesity leads to infiltration of adipose tissue by macrophages, immune cells that are primarily responsible for the inflammatory response in this metabolic tissue 15, 16 (Figure 1). Subsequently, there has been an avalanche of reports demonstrating that the activation state of macrophages, along with infiltration of other innate and adaptive immune cells, contributes to the inflammatory milieu in adipose tissue that mediates insulin resistance. In this review, we provide a general framework for understanding the dynamic crosstalk between macrophages, metabolic tissues and macronutrient metabolism, and how this crosstalk is altered in the disease state of obesity.
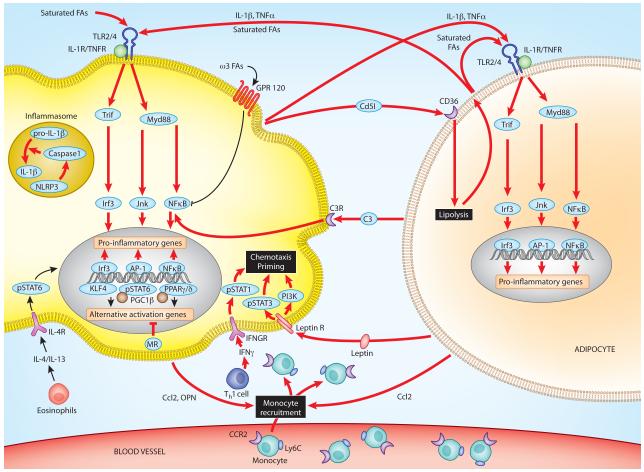
Figure 1. Classically activated (M1) macrophages contribute to adipose tissue inflammation and insulin resistance.
Obesity results in de novo recruitment of macrophages into adipose tissue, which promote adipose tissue inflammation and insulin resistance. In part, dietary saturated fatty acids activate Toll-like receptor 2 (TLR2) and TLR4 in adipose tissue macrophages, resulting in activation of interferon regulatory factor 3 (IRF3), activator protein 1 (AP1) and nuclear factor-κB (NF-κB) inflammatory signaling cascades. These pathways induce the production of pro-inflammatory cytokines such as tumour necrosis factor (TNF). Production of IL-1β results from activation of the NLRP3 inflammasome, potentially by ceramides, the synthesis of which is increased in obesity. These pro-inflammatory cytokines inhibit insulin action in adipocytes by activation of IKKb and JNK signaling pathways. Once initiated, these inflammatory cascades are perpetuated by the crosstalk between the inflamed adipocytes, classically activated (M1) adipose tissue macrophages and T and B cells via elaboration of various chemokines and chemotactic factors. Some of the identified chemotactic factors include CC-chemokine ligand 2 (CCL2) and osteopontin (OPN), the expression of which is induced in adipocytes and macrophages during obesity. Importantly, CCL2 leads to recruitment of Ly6C+CCR2+ inflammatory monocytes, which differentiate into classically activated adipose tissue macrophages to enhance adipose tissue inflammation. In addition, adipose tissue macrophages release CD5-like antigen (CD5L), which promotes lipolysis in adipocytes after being taken up by adipocytes via CD36-mediated endocytosis. In a feed-forward loop, the released fatty acids induce the expression of chemokines, leading to recruitment of Ly6C+CCR2+ inflammatory monocytes and macrophages into adipose tissue. Reciprocally, saturated fatty acids, and inflammatory cytokines (TNF and IL-1β) from adipocytes sustain activation of inflammatory cascades in classically activated adipose tissue macrophages. Mineralocorticoid (MR) signaling also contributes to classical activation of adipose tissue macrophages by inhibiting the IL-4- and peroxisome proliferator activated receptor-γ (PPARγ)-driven program of alternative activation, whereas decreases in adipose tissue eosinophils and circulating levels of adipokines (adiponectin) impair alternative activation and release the break on the inflammatory activation of adipose tissue macrophages, respectively.
Obesity-induced insulin resistance
Resistance of metabolic tissues, such as adipose tissue, liver and muscle, to the anabolic actions of insulin is termed insulin resistance, a characteristic feature of obesity-induced metabolic dysfunction17. Insulin resistance in muscle and adipose tissue manifests as impaired glucose disposal and enhanced triglyceride lipolysis, respectively, resulting in hyperinsulinemia, hyperglycemia, and hyperlipidemia18. In contrast, the liver exhibits partial insulin resistance, as evidenced by impaired suppression of glucose production but not lipogenesis19. Collectively, this peripheral insulin resistance in metabolic tissues causes β-cells in the pancreas to secrete more insulin, a process that is termed compensatory hyperinsulinemia. However, with worsening of insulin resistance, β-cell exhaustion often ensues, resulting in sustained hyperglycemia and type 2 diabetes17, 18.
Because insulin resistance plays a pivotal role in the pathogenesis of type 2 diabetes, considerable effort has been made to elucidate the causal factors responsible for obesity-induced insulin resistance. In general, a number of cell extrinsic and intrinsic mechanisms have been identified that exhibit a cause-and-effect relationship between weight gain and peripheral insulin resistance20. The category of cell intrinsic mechanisms includes mitochondrial dysfunction, oxidative and endoplasmic reticulum (ER) stress, and ectopic lipid deposition, whereas alterations in circulating adipokines and fatty acids, and metabolic tissue inflammation are the dominant cell extrinsic pathways that modulate peripheral insulin action. Since detailed discussion about these topics is beyond the scope of this review, readers are referred to other excellent reviews on these related topics7, 8, 17, 20. We focus our discussion on the relationship between macrophage-mediated inflammation and insulin resistance.
Inflammation and insulin resistance
Among the immune cells that infiltrate obese adipose tissue, macrophages are functionally and numerically dominant12, 21. In lean mice, approximately 10-15% of cells express the macrophage marker F4/80, whereas 45-60% of cells in adipose tissues of obese animals are F4/80+, indicating that obesity significantly alters the ratio of macrophages to adipocytes15. In addition to their numbers, adipose tissue macrophages in lean and obese animals exhibit distinct cellular localization and inflammatory potential22. Adipose tissue macrophages in lean animals have an alternatively activated (M2) phenotype, are less inflammatory than classically activated macrophages and are uniformly dispersed throughout the adipose tissue, whereas adipose tissue macrophages of obese mice have a pro-inflammatory, classical (M1) phenotype and are primarily found in “crown-like” structures around dying adipocytes22, 23. As discussed in detail below, alternatively activated macrophages found in lean adipose tissue (Arginase-1+, CD206+, CD301+) play a critical role in maintaining insulin sensitivity of adipocytes via secretion of IL-1021, 23, 24, a regulatory cytokine that potentiates insulin signaling in adipocytes (Figure 2). In contrast, classically activated macrophages in obese adipose tissue (Nos2+, TNFα+) secrete pro-inflammatory cytokines8, 12, 25(Figure 1), which induce insulin resistance via IKKβ and JNK by serine phosphorylation of IRS proteins (Box 1). Although segregating macrophages into these two states is experimentally very useful, it also has its limitations, as macrophage phenotypes in vivo exhibit plasticity along the entire spectrum of activation states26, 27
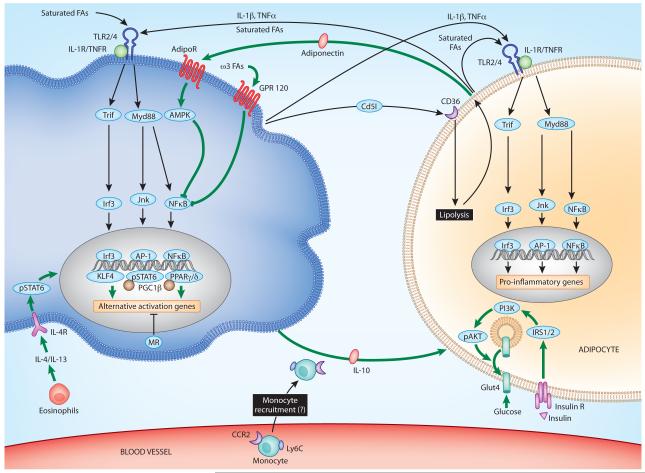
Figure 2. Alternatively activated (M2) macrophages protect against obesity and insulin resistance.
In lean animals, adipose tissue macrophages display an alternatively activated phenotype with reduced inflammatory potential and increased production of the insulin sensitizing cytokine interleukin-10 (IL-10). Eosinophils secrete IL-4 to induce alternative macrophage activation. Activation of signal transducer and activator of transcription 6 (STAT6) by IL-4 induces the transcriptional cascade involving the fatty acid sensors peroxisome proliferator-activated receptor δ (PPARδ), PPARγ and Kruppel-like factor 4 (KLF4), which synergize with STAT6 to sustain the alternative activation of ATMs. Adiponectin released by adipocytes also synergizes with IL-4 signaling to enhance alternative macrophage activation and reduce macrophage-mediated inflammation. Concurrently, unsaturated free fatty acids, such as omega-3 fatty acids, signal via G-protein coupled receptor 120 (GPR120) to dampen nuclear factor-κB (NF-κB) activation in adipose tissue macrophages. Disruption of mineralocorticoid signaling introduces an alternative bias in macrophage activation, whereas agonists of mineralocorticoid receptor (MR) potentiate classical activation.
Box 1. Stress kinases mediate insulin resistance.
While the molecular mediators for each of the cell intrinsic and extrinsic pathways are distinct, many of them converge on a series of stress-activated kinases, including JUN N-terminal kinase (JNK) and inhibitor of nuclear factor-κB (NF-κB) kinase-β (IKKβ), that target intermediates in the insulin signaling pathway (Figure 1)93. For instance, oxidative and ER stress, inflammatory cytokines and saturated fatty acids activate JNK and IKKβ signaling pathways in liver, skeletal muscle and fat, resulting in inhibitory serine phosphorylation of insulin receptor substrate-1 (IRS1) and IRS234, 94-96. In addition, adipokines and cytokines induce the expression of SOCS (suppressor of cytokine signaling) proteins, which interfere with tyrosine phosphorylation of IRS1 and IRS2 or target them for proteasomal degradation20. Thus, the net effect JNK and IKKβ activation in parenchymal cells is the inhibition of insulin signaling, as evidenced by decreased tyrosine phosphorylation of IRS1 and IRS2. In a feed-forward loop, transcriptional activation of NF-κB and activator protein 1 (AP1) by IKKβ and JNK exacerbates insulin resistance by inducing expression of inflammatory genes, which then act in a paracrine or autocrine manner to inhibit insulin action93.
Classically activated macrophages
Temporal analysis of obese adipose tissue has revealed that recruitment of classically activated macrophages coincides with the appearance of necrotic adipocytes and the onset of insulin resistance28. During obesity, adipose tissue mass increases by hyperplasia and hypertrophy, the latter being associated with activation of stress signaling pathways in adipocytes that can result in cell death. For instance, hypertrophic adipocytes are under constant stress, as evidenced by increase in ER stress, hypoxic responses, release of free fatty acids and increased production of ROS7. Interestingly, immunohistochemical studies demonstrate that the inflammatory macrophages expressing the dendritic cell marker CD11c circumscribe necrotic adipocytes23, 29, thereby forming “crown-like” structures15. Moreover, these classically activated CD11c+ macrophages phagocytize released lipids and develop into lipid engorged, multinucleated giant cells28, which are reminiscent of inflammatory macrophages found in atherosclerotic plaques30. However, as recent studies suggest potential dissociation between adipocyte death and macrophage recruitment into obese adipose tissue31, additional work will be required to determine whether ingestion of necrotic debris is sufficient to serve as separate stimulus for triggering macrophage-mediated inflammation and insulin resistance in adipose tissue.
Four distinct lines of evidence suggest that classically activated, inflammatory macrophages contribute to the pathogenesis of obesity-induced insulin resistance (Figure 1). First, mice lacking CC-chemokine receptor 2 (CCR2), which is required for the trafficking of inflammatory monocytes and macrophages into tissues, are protected from obesity-induced inflammation and insulin resistance32. Second, selective depletion of the CD11c+ classically activated macrophages in CD11c-DTR mice reduces adipose tissue inflammation and improves insulin action without having a significant impact on diet-induced obesity33. Third, genetic ablation of the pro-inflammatory signalling molecule IKKβ in myeloid cells or reconstitution of mice with JNK-deficient bone marrow reduces myeloid cell-mediated inflammation in adipose tissue, resulting in preservation of insulin sensitivity34, 35. Lastly, loss of GPR120, a G-protein coupled receptor that mediates the anti-inflammatory actions of omega-3 (unsaturated) fatty acids, worsens adipose tissue inflammation and insulin resistance36 (Figure 1).
While these distinct genetic manipulations provide strong support for the causative role of macrophage-mediated inflammation in insulin resistance, they raise two important questions: what are the triggers for inflammatory activation of macrophages in obesity and what signaling pathways control trafficking of these cells into adipose tissue?
Triggers of macrophage activation in obesity
Since dysregulation of fatty acid homeostasis contributes to obesity-induced insulin resistance, fatty acids have been postulated to be a potential trigger of mediating classical activation of macrophages in obesity (Figure 1). In support of this, saturated, but not unsaturated, fatty acids promote inflammatory activation of macrophages. This is, partly, mediated by ligation of the pattern-recognition receptor TLR437. For instance, acute infusion of lipids into wild type but not TLR4-deficient mice is sufficient to promote adipose tissue inflammation and systemic insulin resistance38. This was further supported by bone marrow chimera studies demonstrating that TLR4 expression by hematopoietic cells is necessary for high fat diet-induced insulin resistance in adipose tissue and liver39. Based on these and other studies, TLR4 seemed like an attractive candidate for linking dietary fatty acids with adipose tissue inflammation and insulin resistance40, 41.
However, recent reports suggest that the link between dietary fatty acids and insulin resistance might not be a direct consequence of activation of canonical TLR4 signaling pathways42. In support of this, mice lacking MyD88, the primary mediator of TLR and IL-1R signaling, have a more severe metabolic disease in response to a high fat diet than wild-type control mice43, suggesting a protective role for MyD88 signaling in obesity-induced metabolic disease. Similarly, TLR4 deficiency on the C57BL/10 genetic background exacerbated diet-induced obesity, steatosis, and insulin resistance44. Thus, additional investigations will be necessary to pinpoint the factors and signaling pathways involved in triggering metabolic inflammation in obesity, and clarifying the involvement of TLR4-MyD88 pathway in adipose tissue inflammation and insulin resistance.
Chemotactic factors
A key event in initiation of adipose tissue inflammation is the recruitment of inflammatory Ly6ChiCCR2+ monocytes, which differentiate into classically activated M1 macrophages45 (Figure 1). While several chemotactic factors have been implicated in this process, CCR2 and its ligands, such as CCL2, seem to play a dominant role in the recruitment of inflammatory monocytes and macrophages into adipose tissue32, 46. Absence of CCR2 reduces obesity-induced macrophage infiltration and adipose tissue inflammation, resulting in improvement in insulin action and hepatic steatosis32. Although transgenic expression of CCL2 in adipose tissue is sufficient to enhance macrophage recruitment and potentiate obesity-induced insulin resistance46, 47, it does not seem to be required for initiation of macrophage-mediated inflammation in adipose tissue48, 49, potentially suggesting functional redundancy amongst CCR2 ligands.
In addition to CCL2 and CCR2, several other factors have been shown to modulate macrophage chemotaxis and adipose tissue inflammation. For instance, osteopontin, which is a secreted matrix glycoprotein that acts as a pro-inflammatory cytokine, promotes adipose tissue inflammation, insulin resistance and hepatic steatosis50-52 (Figure 1). Although osteopontin is not a prototypical chemotactic factor, macrophages lacking osteopontin display impaired chemotaxis to CCL2, potentially providing an explanation for the reduced numbers of adipose tissue macrophages in obese osteopontin-deficient mice52.
Fatty acids released by adipocytes during lipolysis were also shown to promote recruitment of macrophage into adipose tissue. In a weight loss model, the acute trafficking of macrophages into adipose tissue correlates with lipolysis of stored triglycerides53. Moreover, treatment with β3-adrenergic agonists, which increases lipolysis of stored triglycerides in adipocytes, or genetic disruption of Atgl, an adipocyte lipase required for breaking down triglycerides in response to β3-adrenergic signaling, results in increased or decreased recruitment of adipose tissue macrophages, respectively. Similarly, mice lacking CD5-like antigen (CD5L), a factor released by macrophages that promotes lipolysis in adipocytes, have reduced macrophage infiltration of their adipose depots after high fat diet challenge54 (Figure 1). In this case, at least part of the chemotactic activity of CD5L is mediated by induction of chemokine gene expression (CCL2, CCL5, CCL7 and CCL8) in adipocytes.
Inflammasome
The inflammasome — a cytosolic multiprotein complex consisting of a NLRP (NOD-, LRR- and pyrin domain-containing) family member, the inflammasome adaptor molecule ASC, and the effector subunit caspase 1 — can be activated in myeloid and other cells by a variety of “danger” signals, including pathogen-derived molecular patterns, noxious foreign substances and endogenous molecules55. Although the composition of the inflammasome depends on the nature of the “danger” signal, its activation results in processing of pro-caspase 1 into activated caspase 1, which then cleaves the pro-IL-1β and pro-IL-18 into the secreted cytokines IL-1β and IL-18, respectively. Interestingly, it was recently reported that obesity leads to activation of the inflammasome in adipose tissue, resulting in IL-1β mediated inflammation and insulin resistance56-58 (Figure 1). While there are some discrepancies between the reported results, such as the relative contribution of adipocytes and macrophages to obesity-induced inflammasome activation, all groups reported improvement in insulin action and glucose disposal in mice lacking NLRP3 or caspase 1. Moreover, Vandanmagsar et al. suggested that ceramides, the synthesis of which is increased in obesity, might be potential triggers for activating the NLRP3 inflammasome57. Alternatively, increased ROS production, which is known to occur in obesity resulting from mitochondrial dysfunction, might also activate the NLRP3 inflammasome59. However, the mechanisms linking ROS to activation of NLRP3 inflammasomes remain controversial60, 61. Nonetheless, since targeting of the inflammasome is clinically efficacious in restoring insulin action in obese mice and humans with type 2 diabetes62, it will be important to identify the pathways that trigger inflammasome activation in obesity.
T cell activation in obese adipose tissue
While the initial defence against pathogens is innate, the continual persistence of antigenic stimulus triggers the activation and deployment of the adaptive immune system. Interestingly, the immune response in adipose tissue during obesity follows a parallel course. For instance, the initial increase in nutrient intake is accompanied by increased nutrient storage in adipocytes. However, as each adipocyte reaches its maximal capacity for storage, a small proportion undergo necrotic cell death, resulting in recruitment of macrophages to clear the necrotic debris and remodel the enlarging adipose tissue21, 28. In this regard, the infiltrating CD11c+ classically activated macrophages would facilitate the clearance of the dying cells and the remodeling of the extracellular matrix28, factors that ultimately favor the differentiation of new adipocytes to store excess nutrients. However, with persistence of increased nutrient intake, more adipocytes undergo cell death, thereby providing the antigenic stimulus for macrophages to activate the adaptive immune system (Figure 3). Indeed, as discussed below, antigen presentation via MHC class I and II molecules by CD11c+ adipose tissue macrophages may participate in activation and maintenance of adaptive immune responses in adipose tissue of obese mice, which collectively amplify adipose tissue inflammation and insulin resistance. In support of this, deletion of CD40L or blockade of CD40, a costimulatory molecule expressed by macrophages that is involved in antigen presentation, prevented infiltration of adipose tissue by pathogenic T cells and macrophages, and normalized metabolic abnormalities associated with obesity63.
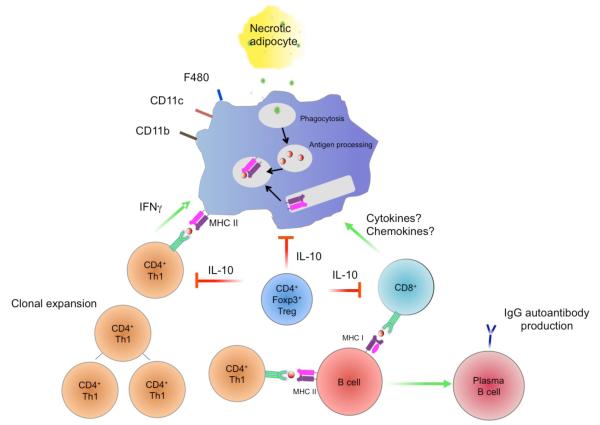
Figure 3.
Crosstalk between innate and adaptive immune cells in adipose tissue. CD4+ FOXP3+ Treg cells and alternatively activated macrophages, enriched in the visceral adipose tissue of lean mice, secrete IL-10 to enhance insulin action and glucose disposal in adipocytes. With over nutrition, engorged adipocytes undergo necrotic cell death, resulting in recruitment of classically activated macrophages to clear debris. In this context, adipose tissue macrophages expressing the prototypical molecules (MHC class II, CD1d, co-stimulatory molecules) and markers (CD11c) of antigen-presenting cells are potentially capable of presenting to T and B cells to promote adaptive immune responses. This is postulated to promote clonal expansion of CD4+ Th1 cells and increase infiltration by CD8+ T cells. In a feed forward loop, IFNγ production by CD4+ Th1 cells, and secretion of inflammatory cytokines and chemotactic factors by CD8+ T cells results in increased recruitment and classical activation of macrophages. Concomitant with this, numbers of immunosuppressive Treg cells decrease in adipose tissue with obesity, further contributing to the adipose tissue inflammation and insulin resistance. B cells, which infiltrate obese adipose tissue, can present antigens on MHC class I and II molecules to naïve T cells. IgG2c antibodies produced by mature B cells further amplifies adipose tissue inflammation and insulin resistance.
Two recent papers have provided evidence for activation of pathogenic adaptive immune responses in diet-induced obesity64, 65. Winer et al. reported that absolute numbers of CD4+ T cells increased with obesity in mice, and that this was largely due to accumulation of interferon-γ (IFNγ) producing Th1 cells65. Moreover, the increased production of IFNγ by these cells contributed to classical activation of adipose tissue macrophages, resulting in increased inflammation in adipose and progression of insulin resistance (Figure 3). In agreement with this, depletion of pathogenic Th1 cells by anti-CD3 immunotherapy resulted in a sustained improvement in insulin sensitivity65. Similarly, reconstitution of _Rag1_-/- mice with CD4+ but not CD8+ cells reduced obesity-induced insulin resistance65. Lastly, since a shift from regulatory T (Treg) cells to pathogenic Th1 cells was also observed in humans with body mass index (BMI) > 30, anti-CD3 therapy might be potentially efficacious in the treatment of insulin resistance and type 2 diabetes in obese humans. Thus, while macrophages are necessary for initiation of diet-induced inflammation in adipose tissue, the maintenance of inflammation during chronic obesity seems to be also dependent on adaptive immunity.
In contrast, Nishimura et al. focused on changes in CD8+ T cells, the numbers of which in adipose tissue increased in dietary and genetic models of obesity64. Like CD11c+ adipose tissue macrophages, CD8+ T cells localized to “crown-like structures” in obese adipose tissue, suggesting potential crosstalk between CD8+ T cells and macrophages (Figure 3). Depletion of CD8+ T cells by anti-CD8 or genetic CD8a deficiency protected mice from obesity-induced inflammation and insulin resistance, whereas adoptive transfer of CD8+ T cells worsened the local inflammatory response in adipose tissue, insulin sensitivity, and glucose disposal. Moreover, since the infiltration by CD8+ T cells preceded the recruitment of macrophages to adipose tissue, the authors suggest that the release of chemotactic factors by CD8+ T cells contributes to macrophage-mediated inflammation in adipose tissue. Taken together, these studies suggest that, in obesity, adipose tissue is an active site for both MHC class I and II-mediated antigen presentation by macrophages to CD8+ and CD4+ T cells, respectively, which contribute to adipose tissue inflammation and peripheral insulin resistance (Figure 3).
In the preceding sections, we have discussed how overnutrition results in recruitment of monocytes into the enlarging adipose tissue, where they differentiate into classically activated macrophages. The elaboration of pro-inflammatory molecules by infiltrating macrophages, such as TNFα and IL-1β, contributes to adipose tissue inflammation and insulin resistance. This inflammatory milieu is further amplified by the crosstalk between pathogenic CD4+ and CD8+ T cells, and CD11c+ classically activated macrophages in obese adipose tissue. However, as in many instances of inflammation, counter-regulatory mechanisms are also in place to prevent excessive inflammation. In adipose tissue, these include Tregs (Box 2), Th2 cells and alternatively activated macrophages, which are discussed in detail below.
Alternatively activated macrophages
The inherent plasticity of macrophage, in part, stems from their ability to enact distinct activation programs. Unlike classically activated macrophages, which exhibit high inflammatory potential, the Th2 cytokines IL-4 and IL-13 promote the maturation of the less inflammatory alternatively activated macrophages with distinct secretory and functional capacity66. Alternatively activated macrophages in tissues or at sites of Th2- type inflammation can be identified by their unique enzymatic (encoded by Arg1), secretory (encoded by Chi3l3/Chi3l4, and Retnla) and phagocytic (encoded by Mrc1, Clec7a and Clec10A) activities66. Indeed, these characteristic features of classically and alternatively activated macrophages have permitted detailed analysis of their functions in metabolic inflammation and insulin resistance. While classically activated macrophages promote metabolic inflammation and insulin resistance8, resident alternatively activated macrophages attenuate inflammation and confer protection against the deleterious effects of diet-induced obesity21. As discussed below, this has been discerned by identification of transcriptional regulators that sustain alternative macrophage activation in tissues.
PPARs, alternative activation and metabolic disease
Peroxisome proliferator activated receptors (PPARs) are ligand-dependent transcription factors that function as the body’s sensors of fatty acids to regulate glucose and lipid metabolism67. Among the three PPAR subtypes (α, δ, and γ), PPARδ and PPARγ are abundantly expressed in murine and human monocytes and macrophages68. Several laboratories have reported that activation of PPARγ and PPARδ attenuates expression of pro-inflammatory genes in macrophages, thus implicating PPARs in the negative regulation of classical macrophage activation68, 69. However, the importance of PPARγ in alternative macrophage activation was not investigated until recently.
The induction of PPARγ by IL-4 in monocytes and macrophages suggested potential involvement in alternative macrophage activation70. In 2007 Odegaard and colleagues were the first to demonstrate that PPARγ transcriptional signaling is required for orchestrating the metabolic programs of alternative activation, including β-oxidation of fatty acids and mitochondrial biogenesis24. In addition, PPARγ was found to directly regulate the expression of arginase 1, a hallmark of alternatively activated macrophages (Figure 2). Congruent with this, myeloid cell-specific disruption of PPARγ impaired alternative activation in vitro and in vivo, and reduced the numbers of arginase 1+ macrophages in adipose tissue of lean mice24. As a consequence, when challenged with a high fat diet, mice lacking PPARγ in their myeloid cells were predisposed to developing obesity and insulin resistance in part due to mitochondrial dysfunction and altered glucose metabolism in adipose tissue24, 71. These results thus established that alternatively activated macrophages, which are predominantly found in adipose tissue of lean mice, perform a protective role in obesity-induced metabolic disease.
IL-4 also induces expression of PPARδ in macrophages72, which synergizes with STAT6 to coordinate the function of alternatively activated macrophages72, 73. However, unlike PPARγ, PPARδ is not required for the oxidative metabolism that fuels their alternative activation24. However, it is required for amplifying and coordinating many other facets of alternative activation, such as inducing the expression of signature genes (Arg1, Mgl1, Mgl2, Mrc1, Retlna, Chi3l3 and Pdcd1lg2), transducing the mitogenic action of IL-4, and suppressing the expression of pro-inflammatory genes72, 73 (Figure 2). Consistent with its role in the maturation of alternatively activated macrophages, disruption of PPARδ in myeloid cells or generation of PPARδ-deficient bone marrow chimeras reduces alternative activation of adipose tissue macrophages and liver macrophages, thereby predisposing mice to the metabolic sequelae of obesity, including insulin resistance and hepatic steatosis. These findings thus provide independent verification for the non-redundant roles of PPARγ and PPARδ in alternative macrophage activation. However, generation of chimeras with PPARγ or PPARδ null bone marrow failed to protect C57BL/6J mice from diet-induced insulin resistance74, potentially reflecting strain differences or the relative resistance of resident tissue macrophages to transplantation-induced replacement75. Lastly, while the instructive cues for alternative activation are provided by the Th2-type cytokines IL-4 and IL-13, PPARγ and PPARδ primarily function to amplify and sustain this program of macrophage activation.
KLF4, alternative activation and insulin resistance
Similar to PPARγ and PPARδ, Kruppel-like factor 4 (KLF4) was found to cooperate with IL-4–STAT6 signaling to promote alternative macrophage activation and ameliorate obesity-induced insulin resistance. Liao et al. found that expression of KLF4 was induced in murine and human macrophages by IL-4, and overexpression of KLF4 synergized with IL-4 to promote alternative macrophage activation, whereas its deficiency was associated with impairment in alternative polarization of macrophages76 (Figure 2). In fact, KLF4-deficient macrophages exhibited a profound classical bias that contributed to their enhanced bactericidal activity. Moreover, in context of metabolic disease, the phenotype of mice lacking KLF4 in myeloid cells mirrored the metabolic phenotype of PPARγ-deficient myeloid cells: both genotypes were prone to diet-induced obesity and insulin resistance that was associated with impairment in alternative activation of adipose tissue macrophages.
Mineralocorticoid receptor and alternative activation
In contrast to PPARs and KLF4, mineralocorticoid signaling potentiates classical and suppresses alternative activation of macrophages77 (Figure 2). For instance, aldosterone, a mineralocorticoid receptor agonist, induces pro-inflammatory gene expression in macrophages and potentiates the lipopolysaccharide driven program of classical macrophage activation77, 78. The converse was observed in macrophages lacking mineralocorticoid receptor or after stimulation of wild-type macrophages with RU26752, an antagonist of mineralocorticoid receptor. So, inhibition of mineralocorticoid signaling enhanced expression of alternative activation markers, while suppressing classical macrophage activation. Interestingly, transcriptional profiling revealed that the alternative bias in mineralocorticoid receptor-deficient macrophages was, in part, mediated by activation of PPARγ signaling, indicating that mineralocorticoid receptor and PPARγ regulate diametrically opposing facets of macrophage activation. Since mineralocorticoid receptor antagonists are already in clinical use for the treatment of congestive heart failure and hypertension79, it would be important to explore the clinical utility of these drugs in obesity-induced inflammation and insulin resistance.
The source of IL-4 in white adipose tissue
Although adipocytes have been implicated in secreting IL-4 and IL-13, a recent study demonstrates that eosinophils are the primary source of IL-4 in adipose tissue. Using the 4get reporter mice, Wu and colleagues found that 90% of the cells competent for IL-4 secretion in adipose tissue of lean mice were eosinophils, and their presence in adipose tissue was inversely correlated with body mass80 (Figure 2). Importantly, in mice lacking eosinophils, alternative activation of adipose tissue macrophages was severely compromised, indicating that eosinophils are the primary source of IL-4 in adipose tissue80. Accordingly, the presence of eosinophils in adipose tissue displayed a tight correlation with protection from obesity and insulin resistance. For instance, eosinophil-deficient mice gained more weight on a high fat diet and were worse off in their metabolic profiles, whereas mice with tissue eosinophilia, such as IL-5-transgenic mice, had lower levels of adiposity and improved glucose tolerance.
Taken together, these findings have established that alternatively activated macrophages and eosinophils protect against the metabolic sequelae of obesity. However, the precise mechanisms by which these cells communicate with metabolic tissues to improve glucose homeostasis remain unknown. Two plausible mechanisms include: the anti-inflammatory effects of alternatively activated macrophages on adipose tissue inflammation, and the secretion of insulin sensitizing factors by alternatively activated macrophages that improve parenchymal cell glucose homeostasis. The precise nature of the insulin sensitizing factors and the stimuli that trigger alternative macrophage activation in metabolic tissues are two important areas for future investigations.
Infection, immunity and insulin resistance
As discussed above, evidence gathered over the last decade demonstrates a causal relationship between macrophage-mediated inflammation and insulin resistance. Since this crosstalk between the immune and metabolic systems is conserved from flies to humans7, it suggests that, under some circumstances, inflammation-mediated insulin resistance might be beneficial. One such scenario is acute bacterial infection, when insulin resistance provides a mechanism for diverting nutrients away from non-essential functions, such as storage, and towards fueling immunity.
Insulin resistance: an adaptive mechanism (bacterial infections)
In mammals, cells of the innate (neutrophils, macrophages and dendritic cells) and adaptive (T and B cells) immune system utilize glucose upon activation81-84. In the case of T and B cells, glycolysis fuels their clonal proliferation85-87, whereas for macrophages, aerobic glycolysis provides the building blocks for their secretory and respiratory burst83. For instance, the production of reactive oxygen species (ROS) by the NADPH oxidase complex in macrophages requires continual generation of NADPH88, which is only made by the pentose phosphate pathway, a branch pathway of glycolysis89. Interestingly, this same pathway generates riboses and aromatic amino acid precursors, the building blocks necessary for synthesis of DNA, RNA and proteins, which sustain cellular activation and proliferation89, 90. Thus, the bioenergetic demands of the activated immune system are fuelled primarily by glucose. In this circumstance, peripheral insulin resistance functions as an adaptive mechanism that allows the organism to mobilize fuel from sites of storage to combat infection (Figure 4). For instance, in liver and adipose tissue, insulin resistance drives gluconeogenesis and lipolysis to provide glucose and fatty acids to support immune activation and peripheral metabolism, respectively17, 18. Similarly, insulin resistance in skeletal muscle, the primary site of glucose disposal, reduces storage of glucose as glycogen, thereby providing the activated immune system with unfettered access to nutrients (Figure 4). Therefore, infection-induced insulin resistance is an adaptive strategy that supports the bioenergetic demands of the activated immune system to ensure survival of the organism.
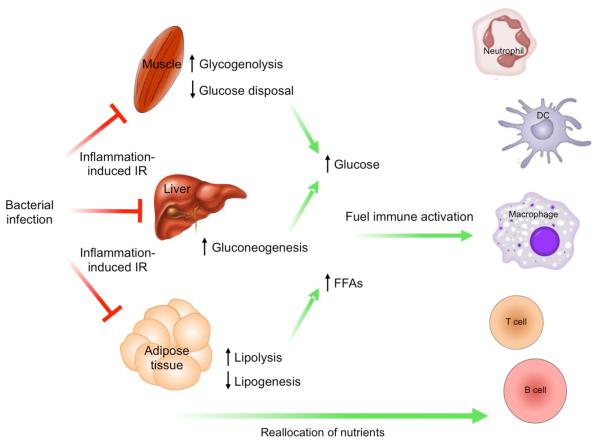
Figure 4. Infection-induced insulin resistance is adaptive.
Bacterial infection of the host activates innate immune cells, resulting in the release of pro-inflammatory cytokines that mediate insulin resistance in metabolic tissues. Insulin resistance in the liver increases gluconeogenesis, whereas in muscle it decreases glucose disposal and increases breakdown of stored glycogen. This has the net effect of increasing circulating levels of glucose, a nutrient that is preferentially used by innate and adaptive immune cells to fuel their activation. In parallel, insulin resistance in adipose tissue decreases lipogenesis and increases lipolysis. The free fatty acids (FFAs) released by adipocytes are used to support the metabolic demands of immune and non-immune cells.
An important corollary of these observations is that, in response to infection or inflammation, organisms ranging from flies to humans are “hard-wired” to develop insulin resistance. This suggests that this adaptive strategy in the setting of infection has the potential of being maladaptive if inflammation is prolonged and chronic. This is indeed the case in obesity, where excess nutrient intake leads to chronic macrophage-mediated inflammation and insulin resistance7-10, 12. As discussed above, cellular and molecular pathways employed in sensing and eradication of bacterial pathogens also contribute to obesity-induced inflammation and insulin resistance.
Th2-type immunity enhances insulin action (helminth infection)
In contrast to bacterial infections, parasitic infections by extracellular helminths are chronic and to a large extent, do not acutely affect survival91. However, this chronic parasitism poses a metabolic challenge for the host, as the helminths continually parasitize host nutrients for their own growth. This raises the important question whether immunity against helminths is somehow coupled to regulation of peripheral metabolism.
In humans and experimental models, helminths are potent inducers of Th2-type immune responses, consisting of infiltration of tissues by eosinophils, alternatively activated macrophages and Th2 cells, and increased production of Th2-type cytokines (IL-4, IL-13, and IL-5)91. Interestingly, these aspects of the Th2-type immune response have been demonstrated to enhance insulin action and promote nutrient storage (Figure 5). First, Th2-type cytokine IL-4 confers protection against diet-induced obesity and insulin resistance in mice92. Second, mice lacking the Th2 cell-associated signalling molecule STAT6 (signal transducer and activator of transcription 6) are more prone to diet-induced insulin resistance and completely refractory to the glucose lowering effects of IL-492. Third, polarization of the immune response towards the Th2 axis in a model of allergic inflammation improves glucose tolerance and insulin resistance92. Fourth, infection with the migratory helminth Nippostrongylus brasiliensis conferred long-term protection from obesity-induced glucose intolerance and insulin resistance80. Lastly, as elaborated above, alternatively activated macrophage and adipose tissue eosinophils are necessary for maintaining glucose homeostasis during diet-induced obesity24, 72, 73, 76, 80. Collectively, these findings indicate that the Th2-type immune response invoked by helminths sequesters nutrients for long-term storage, a strategy that is likely advantageous to the host as it prevents the growth of parasites.
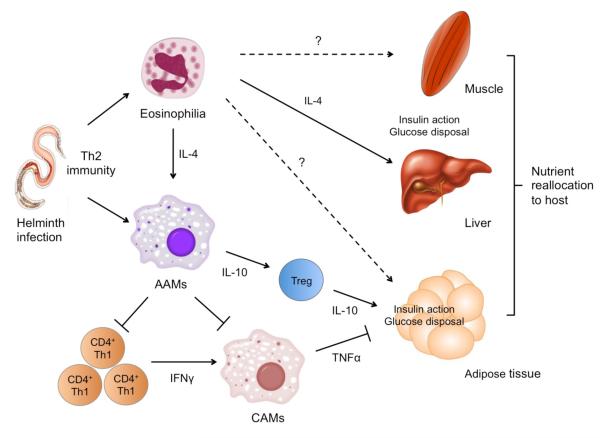
Figure 5.
Th2-type immunity enhances insulin action. Parasitic helminths induce the prototypical Th2-type immune responses characterized by tissue eosinophilia, alternative macrophage activation, and increased production of Th2-type cytokines (IL-4 and IL-13), as well as IL-10. Each aspect of this anti-helminth immunity enhances insulin action in liver, adipose tissue, and potentially, muscle, the three primary organs involved in metabolic homeostasis. Lean adipose tissue contains abundant numbers of eosinophils (which are the primary producers of IL-4 and IL-13) that sustain alternative activation of adipose tissue macrophages. In a paracrine manner, alternatively activated macrophages protect against insulin resistance by directly suppressing the clonal expansion of Th1 cells and dampening the inflammation mediated by classically activated macrophages. While local production of IL-4 and IL-10 enhances insulin-stimulated glucose disposal in fat, IL-4 stimulates the anabolic actions of insulin in liver. The net effect of Th2-type immunity is to enhance nutrient storage by potentiating the anabolic actions of insulin in tissues.
Conclusion
In all metazoans, immunity and metabolism are two fundamental systems. The former provides an effective defence against invading pathogens and permits colonization by commensals, whereas the latter allows the organism to adapt to changes in nutrient availability. Interestingly, studies over the last decade suggest that macrophages, the sentinels of innate immunity, mediate a dynamic crosstalk between these two essential systems.
The functions of macrophages in regulation of metabolism have principally been investigated in white adipose tissue, the primary site for storage of excess nutrients. Although macrophages take residence in white adipose tissue of both lean and obese mice, their activation phenotypes and functional roles vary with the metabolic status of the animal. In lean mice, alternatively activated macrophages predominate and enhance the anabolic actions of insulin. In striking contrast, obese adipose tissue is largely infiltrated with classically activated macrophages, which promote insulin resistance via secretion of pro-inflammatory cytokines. While adipose tissue macrophages clearly play an important role in pathogenesis of metabolic disease, the crosstalk between macrophages and metabolic tissues likely evolved to modulate insulin action and nutrient availability during times of infection. Lastly, since innate and adaptive immune cells normally reside in adipose tissue, the enlarging adipose tissue is not only a site for storage of fats or secretion of adipokines, but also a tertiary lymphoid organ for macrophage-mediated antigen presentation and lymphocyte activation.
Box 2. Additional roles of adaptive immune cells in metabolic disease.
Tregs
Treg cells, which are involved in tolerance to self-antigens, are highly enriched in visceral adipose tissue of lean mice compared which other lymphoid tissues, such as spleen or lymph nodes, and have a distinct expression profile, including higher expression of IL-1097. Interestingly, high fat feeding induced a gradual loss of CD4+FOXP3+ Treg cells, which correlated with a decrease in an index of insulin sensitivity. Furthermore, depletion of CD4+FOXP3+ Treg cells led to worsening of obesity-induced insulin resistance. Conversely, in situ expansion of Treg cells led to partial improvement in glucose homeostasis. Although these loss- and gain-of-function studies implicate Treg cell function in the proper maintenance of adipose tissue function (Figure 3), additional investigations is necessary to address the primary functions of Treg cells in adipose tissue and the mechanisms underlying their gradual disappearance from fat during obesity.
B cells
B cells, which mediate humoral immunity and participate in antigen presentation to T cells, have also been implicated in obesity-induced metabolic pathologies. Winer et al. found that adipose tissue of obese mice was rich in B2 cells, leading to increased production of IgG2c in visceral adipose tissue and serum98. Absence of mature B cells or their depletion by anti-CD20 immunotherapy conferred significant protection from obesity-induced insulin resistance98. This was, in part, due to decrease in the numbers of pathogenic T cells and inflammatory macrophages in visceral adipose tissues of B cell-deficient mice, potentially reflecting the contribution of antigen presentation by B cells to CD4+ and CD8+ T cells (Figure 3). Remarkably, adoptive transfer of serum IgG, but not IgM, from obese mice was sufficient to induce insulin resistance in absence of mature B cells, suggesting contribution of pathogenic antibodies to the development of obesity-induced metabolic disease. In support of this, analysis of sera from insulin resistant and insulin sensitive subjects demonstrated that a number of antibodies targeting intracellular antigens were specifically enriched in insulin resistant subjects. Together, these findings suggest that although the initial immune response in adipose tissue to weight gain is innate, chronic overnutrition results in activation of adaptive immunity in adipose tissue that not only modifies the innate response, but also independently contributes to progression of obesity-induced insulin resistance (Figure 3).
Acknowledgments
We thank members of the Chawla laboratory and A. Loh for valuable comments on the manuscript, and Katie Vicari and Randy Levinson for assistance with illustrations. The author’s work was supported by grants from: NIH (DK076760, HL076746), Larry L. Hillblom Foundation Network Grant and an NIH Director’s Pioneer Award (DP1OD006415) to A.C. Support was provided by Stanford Graduate Fellowship to K.D.N, and A-STAR Fellowship to Y.P.G. Due to space limitations, we regret that we are unable to cite all relevant publications on this topic from our colleagues.
Glossary
Insulin resistance
A physiological or pathophysiological state in which insulin becomes less effective at lowering serum glucose due to decreased responsiveness of insulin target tissues, such as adipose tissue, skeletal muscle and liver.
Adipokines
Hormones and/or cytokines secreted by adipose tissue, such as leptin and adiponectin.
Alternatively activated macrophages (M2)
A macrophage stimulated by Th2 cytokines IL-4 or IL-13 that expresses arginase 1, mannose receptor (CD206) and CD301. Chronic states of helminth infections are associated with alternatively activated macrophages.
Classical phenotype (M1)
A pro-inflammatory, anti-microbial program of macrophage activation induced by interferon-γ and Toll-like receptor ligands.
CD11c-DTR mice
Transgenic mice that express the diphtheria toxin receptor from the CD11c/Itgax promoter. Injection of diphtheria toxin allows for specific ablation of CD11c+ cells.
CD4+ T cells
A T cell subset that expresses the glycoprotein CD4, which assists the TCR in recognition of antigens presented on MHC II molecules by antigen presenting cells.
Th1 cells
Th1 (helper type 1 cells) secrete IFNg and TNFa to promote cell-mediated immunity via supporting classical macrophage activation and proliferation of cytotoxic CD8+ T cells.
CD8+ T cells
CD8+ T cells express the co-receptor CD8, which together with the TCR recognizes antigens bound to MHC class I molecules. Activated CD8+ T cells induce death of virally-infected or damaged cells.
Th2 cells
Th2 (helper type 2 cells) secrete the Th2 cytokines IL4, IL5, and IL13 to stimulate humoral immunity (B cells) and alternative macrophage activation.
Mineralocorticoid
A steroid hormone that regulates concentration of minerals, such as sodium and potassium, in extracellular fluids. It binds and activates the mineralcorticoid receptor to mediate its transcriptional effects.
4get reporter mice
A knock-in mouse model that expresses eGFP from the IL-4 locus, allowing for detection of cells competent for IL-4 production.
pentose phosphate pathway
This pathway utilizes glucose to generate NADPH and pentoses (also known as riboses). The first oxidative phase converts glucose-6-phosphate to ribulose-5-phosphate and generates NADPH. The second non-oxidative phase synthesizes other 5-carbon sugars from ribulose-5-phosphate.
B2 cells
Conventional bone marrow-derived B cells that make up the bulk of splenic B cells. They express high levels of B220+ and membrane bound IgD.
Footnotes
Competing interests statement The authors declare that they have no competing financial interests.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Finucane MM, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–67. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berrington de Gonzalez A, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–9. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. Jama. 2007;298:2028–37. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 5.Zheng W, et al. Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med. 2011;364:719–29. doi: 10.1056/NEJMoa1010679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leibel RL. Molecular physiology of weight regulation in mice and humans. Int J Obes (Lond) 2008;32(Suppl 7):S98–108. doi: 10.1038/ijo.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 8.Olefsky J, Glass C. Macrophages, Inflammation, and Insulin Resistance. Annu Rev Physiol. 2010;72:1–28. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 9.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odegaard JI, Chawla A. Mechanisms of macrophage activation in obesity-induced insulin resistance. Nat Clin Pract Endocrinol Metab. 2008;4:619–26. doi: 10.1038/ncpendmet0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–7. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrante AW., Jr. Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262:408–14. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 13.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 14.Hotamisligil GS, et al. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–8. doi: 10.1126/science.271.5249.665. This was the first evidence for serine phophorylation of IRS proteins underlies the inhibition of insulin signaling by inflammatory cytokines.
- 15.Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. These two papers provide the initial evidence for involvement of macrophages in obesity-induced adipose tissue inflammation and insulin resistance.
- 17.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–81. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–6. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–6. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 2007;21:1443–55. doi: 10.1101/gad.1550907. An assessment of various cellular pathways and molecular mechanisms that contribute to obesity-associated insulin resistance.
- 21.Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annu Rev Pathol. 2011;6:275–97. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–46. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84. doi: 10.1172/JCI29881. This paper demonstrates that adipose tissues of lean and obese mice are preferentially populated by alternatively and classically activated macrophages, respectively.
- 24.Odegaard JI, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–20. doi: 10.1038/nature05894. This was the first demonstration that PPARgamma regulates alternative activation of adipose tissue macrophages, which ameliorates obesity-induced insulin resistance.
- 25.Lumeng CN, Deyoung SM, Saltiel AR. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am J Physiol Endocrinol Metab. 2007;292:E166–74. doi: 10.1152/ajpendo.00284.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol. 2004;76:509–13. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet--induced obesity in mice. Diabetes. 2010;59:1171–81. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strissel KJ, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–8. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 29.Cinti S, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–55. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Cho HJ, et al. Induction of dendritic cell-like phenotype in macrophages during foam cell formation. Physiol Genomics. 2007;29:149–60. doi: 10.1152/physiolgenomics.00051.2006. [DOI] [PubMed] [Google Scholar]
- 31.Feng D, et al. High-fat diet-induced adipocyte cell death occurs through a cyclophilin d intrinsic signaling pathway independent of adipose tissue inflammation. Diabetes. 2011;60:2134–43. doi: 10.2337/db10-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weisberg SP, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–24. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patsouris D, et al. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–9. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arkan MC, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–8. doi: 10.1038/nm1185. This paper shows that deletion of IKKbeta in myeloid cells confers protection from obesity-induced insulin resistance, thereby implicating classically activated macrophages in pathogenesis of metabolic disease.
- 35.Solinas G, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–97. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Oh DY, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–98. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konner AC, Bruning JC. Toll-like receptors: linking inflammation to metabolism. Trends Endocrinol Metab. 2011;22:16–23. doi: 10.1016/j.tem.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Shi H, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–25. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saberi M, et al. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 2009;10:419–29. doi: 10.1016/j.cmet.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poggi M, et al. C3H/HeJ mice carrying a toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia. 2007;50:1267–76. doi: 10.1007/s00125-007-0654-8. [DOI] [PubMed] [Google Scholar]
- 41.Tsukumo DM, et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–98. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 42.Erridge C, Samani NJ. Saturated fatty acids do not directly stimulate Toll-like receptor signaling. Arterioscler Thromb Vasc Biol. 2009;29:1944–9. doi: 10.1161/ATVBAHA.109.194050. [DOI] [PubMed] [Google Scholar]
- 43.Hosoi T, Yokoyama S, Matsuo S, Akira S, Ozawa K. Myeloid differentiation factor 88 (MyD88)-deficiency increases risk of diabetes in mice. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vijay-Kumar M, et al. Loss of function mutation in toll-like receptor-4 does not offer protection against obesity and insulin resistance induced by a diet high in trans fat in mice. J Inflamm (Lond) 2011;8:2. doi: 10.1186/1476-9255-8-2. These papers describe the paradoxical worsening of metabolic disease in mice lacking TLR4 or MyD88 signaling.
- 45.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 46.Kanda H, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamei N, et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006;281:26602–14. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 48.Inouye KE, et al. Absence of CC Chemokine Ligand 2 Does Not Limit Obesity-Associated Infiltration of Macrophages into Adipose Tissue. Diabetes. 2007 doi: 10.2337/db07-0425. [DOI] [PubMed] [Google Scholar]
- 49.Kirk EA, Sagawa ZK, McDonald TO, O’Brien KD, Heinecke JW. Macrophage chemoattractant protein-1 deficiency fails to restrain macrophage infiltration into adipose tissue. Diabetes. 2008;57:1254–61. doi: 10.2337/db07-1061. [DOI] [PubMed] [Google Scholar]
- 50.Chapman J, et al. Osteopontin is required for the early onset of high fat diet-induced insulin resistance in mice. PLoS One. 2010;5:e13959. doi: 10.1371/journal.pone.0013959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kiefer FW, et al. Osteopontin deficiency protects against obesity-induced hepatic steatosis and attenuates glucose production in mice. Diabetologia. 2011;54:2132–42. doi: 10.1007/s00125-011-2170-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nomiyama T, et al. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J Clin Invest. 2007;117:2877–88. doi: 10.1172/JCI31986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kosteli A, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120:3466–79. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kurokawa J, et al. Apoptosis inhibitor of macrophage (AIM) is required for obesity-associated recruitment of inflammatory macrophages into adipose tissue. Proc Natl Acad Sci U S A. 2011;108:12072–7. doi: 10.1073/pnas.1101841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19:615–22. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 56.Stienstra R, et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12:593–605. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vandanmagsar B, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–88. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wen H, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–15. doi: 10.1038/ni.2022. These three papers describe the links between inflammasome activation, obesity-induced inflammation and insulin resistance.
- 59.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–37. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–40. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 61.Masters SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Nardo D, Latz E. NLRP3 inflammasomes link inflammation and metabolic disease. Trends Immunol. 2011;32:373–9. doi: 10.1016/j.it.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poggi M, et al. CD40L Deficiency Ameliorates Adipose Tissue Inflammation and Metabolic Manifestations of Obesity in Mice. Arterioscler Thromb Vasc Biol. 2011 doi: 10.1161/ATVBAHA.111.231357. [DOI] [PubMed] [Google Scholar]
- 64.Nishimura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–20. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 65.Winer S, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–9. doi: 10.1038/nm.2001. These two papers show the involvement of adaptive immune responses in modulation of obesity-induced metabolic disease.
- 66.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 67.Evans RM, Barish GD, Wang Y-X. PPARs and the complex journey to obesity. Nat Med. 2004;10:355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- 68.Chawla A. Control of macrophage activation and function by PPARs. Circ Res. 2010;106:1559–69. doi: 10.1161/CIRCRESAHA.110.216523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Glass CK, Saijo K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat Rev Immunol. 2010;10:365–76. doi: 10.1038/nri2748. [DOI] [PubMed] [Google Scholar]
- 70.Huang JT, et al. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400:378–82. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 71.Hevener AL, et al. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest. 2007;117:1658–69. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kang K, et al. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–95. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Odegaard JI, et al. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marathe C, et al. Preserved glucose tolerance in high-fat-fed C57BL/6 mice transplanted with PPARgamma-/-, PPARdelta-/-, PPARgammadelta-/-, or LXRalphabeta-/- bone marrow. J Lipid Res. 2009;50:214–24. doi: 10.1194/jlr.M800189-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kennedy DW, Abkowitz JL. Kinetics of central nervous system microglial and macrophage engraftment: analysis using a transgenic bone marrow transplantation model. Blood. 1997;90:986–93. [PubMed] [Google Scholar]
- 76.Liao X, et al. Kruppel-like factor 4 regulates macrophage polarization. J Clin Invest. 2011;121:2736–49. doi: 10.1172/JCI45444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Usher MG, et al. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest. 2010;120:3350–64. doi: 10.1172/JCI41080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gilbert KC, Brown NJ. Aldosterone and inflammation. Curr Opin Endocrinol Diabetes Obes. 2010;17:199–204. doi: 10.1097/med.0b013e3283391989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McManus F, McInnes GT, Connell JM. Drug Insight: eplerenone, a mineralocorticoid-receptor antagonist. Nat Clin Pract Endocrinol Metab. 2008;4:44–52. doi: 10.1038/ncpendmet0676. [DOI] [PubMed] [Google Scholar]
- 80.Wu D, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–7. doi: 10.1126/science.1201475. This paper demonstrates the protective effects of eosinophils and Th2 parasitic inflammation in metabolic disease.
- 81.Cramer T, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–57. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–52. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 83.Newsholme P, Curi R, Gordon S, Newsholme EA. Metabolism of glucose, glutamine, long-chain fatty acids and ketone bodies by murine macrophages. Biochem J. 1986;239:121–5. doi: 10.1042/bj2390121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Krawczyk CM, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–9. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Doughty CA, et al. Antigen receptor-mediated changes in glucose metabolism in B lymphocytes: role of phosphatidylinositol 3-kinase signaling in the glycolytic control of growth. Blood. 2006;107:4458–65. doi: 10.1182/blood-2005-12-4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Frauwirth KA, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–77. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 87.Hume DA, Radik JL, Ferber E, Weidemann MJ. Aerobic glycolysis and lymphocyte transformation. Biochem J. 1978;174:703–9. doi: 10.1042/bj1740703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.El-Benna J, Dang PM, Gougerot-Pocidalo MA, Elbim C. Phagocyte NADPH oxidase: a multicomponent enzyme essential for host defenses. Arch Immunol Ther Exp (Warsz) 2005;53:199–206. [PubMed] [Google Scholar]
- 89.Wamelink MM, Struys EA, Jakobs C. The biochemistry, metabolism and inherited defects of the pentose phosphate pathway: a review. J Inherit Metab Dis. 2008;31:703–17. doi: 10.1007/s10545-008-1015-6. [DOI] [PubMed] [Google Scholar]
- 90.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 91.Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733–44. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 92.Ricardo-Gonzalez RR, et al. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc Natl Acad Sci U S A. 2010;107:22617–22. doi: 10.1073/pnas.1009152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–9. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cai D, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–90. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hirosumi J, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–6. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 96.Yuan M, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–7. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 97.Feuerer M, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–9. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Winer DA, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–7. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]