MicroRNAs in neuronal function and dysfunction (original) (raw)
. Author manuscript; available in PMC: 2013 May 1.
Published in final edited form as: Trends Neurosci. 2012 Mar 19;35(5):325–334. doi: 10.1016/j.tins.2012.01.004
Abstract
MicroRNAs (miRNAs) are small noncoding RNA transcripts expressed throughout the brain that can regulate neuronal gene expression at the post-transcriptional level. Here, we provide an overview of the role for miRNAs in brain development and function, and review evidence suggesting that dysfunction in miRNA signaling contributes to neurodevelopment disorders such as Rett and fragile X syndromes, as well as complex behavioral disorders including schizophrenia, depression and drug addiction. A better understanding of how miRNAs influence the development of neuropsychiatric disorders may reveal fundamental insights into the causes of these devastating illnesses and offer novel targets for therapeutic development.
Keywords: microRNA, noncoding RNA, Rett syndrome, schizophrenia, depression, addiction
Introduction
Profiling genome-wide mammalian transcriptional output (known as the transcriptome) has provided important insights into the complexity of RNA biology. Notably, mammalian cells expend large amounts of energy producing vast quantities of small and large RNA transcripts that do not code for protein, termed noncoding RNAs (ncRNAs). Short (~22 nucleotides [nt]) ncRNAs, known as microRNAs (miRNAs), have been implicated in almost all aspects of cell biology. Here, we provide an overview of the role for miRNAs in brain development and function, and highlight recent findings implicating miRNAs in neurodevelopmental and neuropsychiatric disorders. We also discuss progress towards miRNA-based therapeutics to treat these conditions.
MicroRNAs in brain development and function
The enzymatic machinery and sequence of intracellular events involved in the biogenesis and maturation of miRNAs are highly conserved across animals and plants (Figure 1). In animals, miRNAs generally bind to the 3′ untranslated (3′UTR) region of target mRNA transcripts by incomplete complementation, particularly at the 5′ end of the miRNA, referred to as the seed region. Such pairing, which typically occurs via imperfect complementation in mammals, results in translational repression or degradation of target transcripts. This means that each miRNA can simultaneously regulate potentially hundreds or even thousands of mammalian mRNA transcripts, suggesting that miRNAs act as master regulators of gene expression.
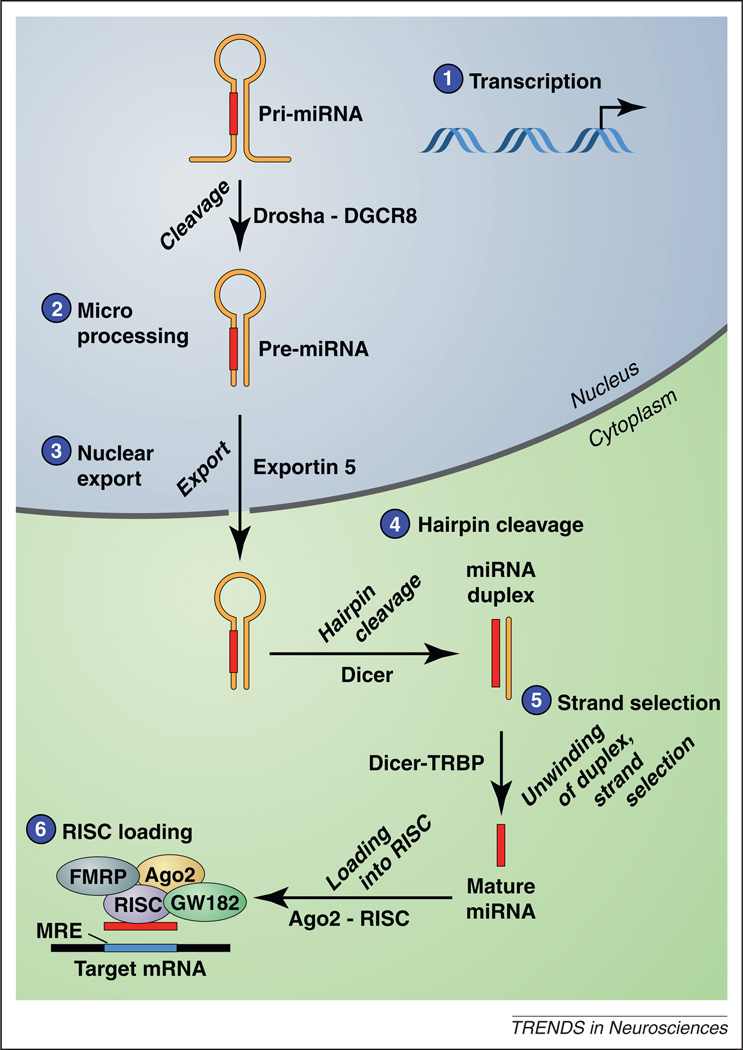
Figure 1.
MicroRNA biogenesis and function. (1) MicroRNA (miRNA) genes are transcribed by RNA polymerase II or III to generate primary miRNA (pri-miRNA) transcripts, which form a hairpin secondary structure 50–120 nt in length. (2) Pri-miRNAs are cleaved by a multiprotein complex that includes Drosha and DiGeorge syndrome critical region gene 8 (DCRC8), also known as Pasha. This cleavage event yields the pre-miRNA, which is exported from the nucleus to the cytoplasm by Exportin-5. (3) In the cytoplasm, Dicer cleaves the hairpin loop to generate a miRNA duplex, which is then unwound to yield a ~22 nt single-stranded mature miRNA from one arm of the pre-miRNA (4). (5) The mature miRNA is incorporated into the RNA-induced silencing complex (RISC) in a carefully orchestrated sequence of events regulated by Dicer, TAR RNA-binding protein (TRBP), the Argonaute proteins including Ago2, and other regulatory proteins such as GW182 [also known as trinucleotide repeat containing 6A (TNRC6A)] and fragile X mental retardation protein (FMRP) (6) Once incorporated into RISC, the miRNA guides this ribonucleoprotein complex to specific mRNA transcripts through complementary base-pairing interactions with regions in the 3′UTR of the target mRNA transcript, termed miRNA response elements (MREs).
Expression of many miRNAs is dynamically regulated during neurogenesis, neuronal maturation and brain development [1], and accumulating evidence supports a key role for miRNAs in these processes [2,3]. Genetic deletion of Dicer disrupts miRNA biogenesis (Figure 1) and is a widely used strategy to investigate the role for miRNAs in neurodevelopmental processes. Dicer-deficient zebrafish display marked defects in neural development [4], and injection of miR-430, an otherwise brain-abundant miRNA, rescues many of these defects [4]. In mice, deletion of Dicer results in deficits in brain development, failure to develop appropriate neuronal phenotype, neuronal atrophy, severe growth defects and early death [5–8]. Dicer deficiency often coincides with neuronal accumulation of proteins typically associated with neurodegenerative disorders [9]. For example, hyperphosphorylation of endogenous Tau [10], a microtubule-associated protein that is a major component of neurofibrillary tangles characteristic of Alzheimer’s disease (AD) and other dementias, is detected in mice with Dicer deletion throughout the forebrain [10].
Specific miRNAs have been implicated in neuronal differentiation and maintenance of neuronal phenotype [11–23]. For example, miR-133b is enriched in midbrain dopamine neurons of the mammalian brain [22], where it regulates the maturation and function of midbrain dopamine neurons through a negative feedback circuit involving the transcription factor Pituitary homeobox 3 (Pitx3) [23]. The brain-enriched miRNA, miR-124, promotes neuronal gene expression in differentiating neural progenitor cells [24]. Moreover, miR-124 expression is inhibited by repressor element 1-silencing transcription factor (REST) [25], a master repressor of neuronal gene expression [26,27]. Conversely, the function of REST is inhibited by miR-124 through an inhibitory action on small C-terminal domain phosphatase 1 (SCP1) [28], a major component of the REST complex. The REST–miR-124 signaling axis therefore plays a central role in controlling neuronal phenotype [12,29,30]. More recently, miR-132 was shown to be rapidly upregulated in the primary visual cortex of neonatal rodents after eye opening and was delayed by dark rearing [22]. Conversely, monocular deprivation reduced miR-132 expression in cortex contralateral to a light-deprived eye [23]. These findings show that miRNAs regulate the development and maintenance of healthy neurons and that miRNA dysfunction may contribute to neurodevelopmental abnormalities and neurodegenerative disorders.
MicroRNAs and neuroplasticity
Dicer deletion in Ca2+/calmodulin-dependent protein kinase II (CaMKII)-positive neurons in mice results in enhanced learning and memory [31], suggesting that miRNAs can maintain plasticity-related genes in translational repression until alleviated by neuronal activity. In fully differentiated neurons, miRNAs and much of the miRNA processing machinery is localized at dendritic spines and postsynaptic densities. This includes specialized cell granules known as processing bodies (P-bodies) that contain mRNA transcripts translationally arrested by miRNAs [32]. In Drosophila, neuronal activity triggers degradation of Armitage at the synapse, a key protein associated with the RNA-induced silencing complex (RISC) that regulates miRNA action [33]. This releases transcripts targeted for degradation in a RISC-dependent manner, such as CaMKII mRNA [33], and the induction of memory-dependent protein synthesis [33]. The mammalian homolog of Armitage, Moloney leukemia virus 10 (MOV10), is similarly localized at the synapse in rat hippocampal neurons and degraded by neuronal activity [34]. MOV10 degradation releases neuroplasticity-related genes from translational repression, including CaMKII, LIM domain kinase 1 (LimK1) and lysophospholipase 1 (Lypla1) mRNAs [34], by reducing their association with miRNAs including miR-138 [34].
Neuronal activity decreases the expression of most neuronal miRNAs, consistent with a regulatory framework in which miRNAs maintain plasticity-associated transcripts in a repressed state until alleviated by neuronal activity. Interestingly, miR-132 and miR-212 are two notable exceptions in which neuronal activity is necessary to maintain their expression, suggesting that increased expression of these two closely related miRNAs may drive synaptic plasticity [35]. Indeed, neuronal activity [36,37] and activity-dependent regulators of neuroplasticity such as the transcription factor cAMP response binding protein (CREB) [37] and brain-derived neurotrophic factor (BDNF) [37] increase miR-132 expression (Figure 2). Moreover, in cultured cortical and hippocampal neurons, miR-132 increases dendritic spine development [38–42] through repression of the GTPase-activating protein p250GAP [37], resulting in activation of the Ras-related C3 botulinum toxin substrate 1 (Rac1)-p21 protein activated kinase (PAK) signaling cascade [41] (Figure 2). Intriguingly, miR-132 can amplify CREB activity through a mechanism involving sensitization of adenylyl cyclases [43], and stimulation of neuronal morphogenesis by CREB is abolished by inhibition of miR-132 signaling [42]. CREB and miR-132 hence form a positive feedback loop that promotes dendritic spine formation (Figure 2). In addition, miR-132 also increases synaptic excitability, as measured by increased AMPA and NMDA glutamate receptor-mediated currents in postsynaptic cells, through an as yet unknown mechanism [38,44,45]. Consistent with the effects of miR-132 on cultured neurons described above, forebrain overexpression of miR-132 in mice increases dendritic spine density in the hippocampus [46]. However, miR-132 overexpression impairs novel object recognition memory [47]. This suggests that increased dendritic spine development resulting from miR-132 overexpression exerts an inhibitory influence on at least some aspects of memory, highlighting the importance of maintaining miR-132, and consequently dendritic complexity, within physiological boundaries.
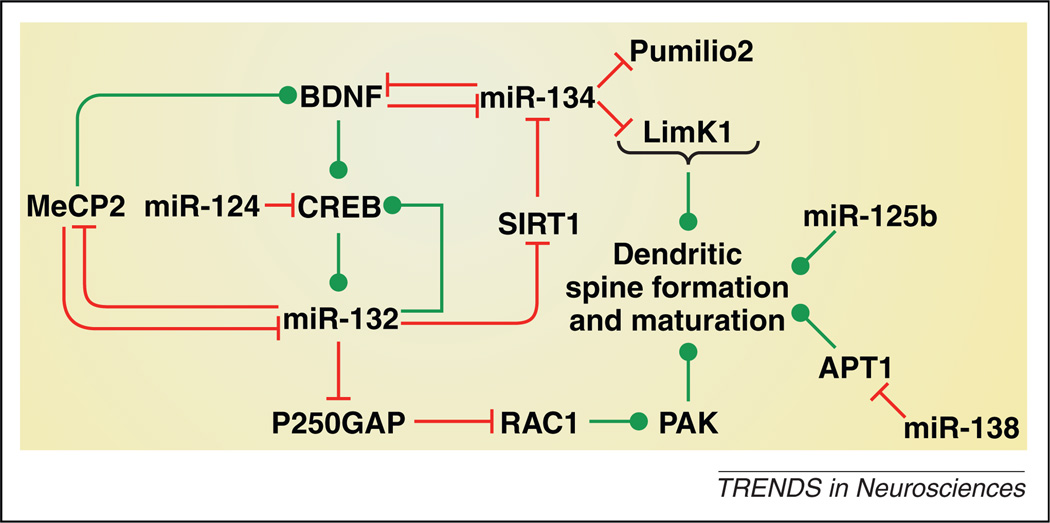
Figure 2.
MicroRNA regulation of dendritic spine formation and maturation. MicroRNAs (miRNAs) interact with transcription factors, deacetylases and kinases, growth factors and other components of intracellular machinery known to regulate dendritic spine formation and/or maturation, and thereby influence dendritic morphology. Some of the known interactions between miRNAs and regulators of dendritic morphology in vitro and in vivo are illustrated here. For the sake of clarity, not all interconnections between these miRNAs and target genes are shown; see text for details. Green lines indicate a stimulatory effect and red lines indicate an inhibitory effect. Abbreviations: APT1, acyl protein thioesterase; BDNF, brain-derived neurotrophic factor; CREB, cAMP response binding protein; LimK1, LIM domain kinase 1; MeCP2, methyl CpG binding protein 2; PAK, p21 protein activated kinase; RAC1, Ras-related C3 botulinum toxin substrate 1; Sirt1, sirtuin 1.
The deacetylase sirtuin 1 (SIRT1) is an established target for miR-132 [48]. SIRT1 was recently shown to enhance synaptic plasticity in mouse hippocampus through a mechanism involving repression of miR-134, resulting in increased expression of BDNF and CREB [46] (Figure 2). More recently it was shown that miR-34c has negative effects on hippocampus-dependent learning and memory by repressing SIRT1 expression [49]. Hence, it is an interesting possibility that miR-132, miR-134 and miR-34c may functionally interact to control synaptic plasticity. The transcription factor myocyte enhancing factor 2 (Mef2) increases miR-134 expression in response to neuronal activity [50]. Further, miR-134 is enriched in synaptodendritic regions of hippocampal neurons where it promotes dendritic outgrowth, but negatively regulates the size of dendritic spines, through inhibitory actions on Pumilio2 and LIM domain kinase 1 (LimK1), respectively [51,52] (Figure 2). Importantly, BDNF may trigger neuroplasticity by reversing the translational block of LimK1 by miR-134, which limits dendritic spine size [51]. Finally, other miRNAs shown to regulate dendritic spine formation include miR-138, which represses expression of acyl protein thioesterase 1 (APT1) to negatively regulate spine formation [53], miR-125b, which promotes spine formation [38], and miR-124, which inhibits CREB expression [54] (Figure 2). These findings demonstrate that miRNAs play a central role in neuronal morphogenesis, synaptic plasticity and also in learning and memory. It is therefore unsurprising that disruption of miRNA signaling has been implicated in a broad range of neurodevelopmental and psychiatric disorders.
MicroRNAs and neurodevelopment disorders
Fragile X syndrome
Fragile X syndrome (FXS) is an X chromosome-linked disorder that is the most common cause of inherited intellectual disability (mental retardation), and is also a cause of autism. FXS results from loss of function of fragile X mental retardation protein (FMRP), encoded by the FMR1 gene. This loss of function results from an expanded number of CGG triplet repeats in the 5′UTR of FMR1, which when expanded greater than 200 triplets results in hypermethylation of the FMR1 promoter and consequently diminished FMRP protein expression.
FMRP, together with the closely related fragile X-related proteins FXR1P and FXR2P, comprise a family of RNA-binding proteins that form messenger ribonucleoprotein (mRNP) complexes that associate with polyribosomes, and where FMRP can regulate mRNA transport and translation. FMRP interacts with core components of the RISC complex including Dicer and Argonaute [38,55,56]. FMRP also interacts with many plasticity-associated miRNAs, including miR-132, miR-125a and b, miR-128, miR-124, miR-219 and miR-9 [38,57]. This suggests that FMRP-miRNA interactions may control spatial and temporal translation of mRNAs involved in synaptic plasticity. Indeed, phosphorylated FMRP forms a complex with miR-125a and Argonaute-2 (Ago2) that inhibits the translation of postsynaptic density protein 95 (PSD-95) mRNA, a key postsynaptic component of glutamatergic synapses [58]. Moreover, FMRP dephosphorylation in response to metabotropic glutamate 5 (mGlu5) receptor activation releases PSD-95 mRNA from miR-125a-mediated repression and increases PSD-95 translation [58]. More recently, it was shown that FXRP1 was required for appropriate maturation of miR-9 and miR-124 [59]. These findings suggest that dynamic interactions between miRNAs, FMRP and FMRP-related proteins regulate microRNA biogenesis and function and thereby control neuroplasticity, with dysfunction in these interactions possibly contributing to FXS symptomatology [38].
Rett syndrome
Rett syndrome (RTT), an X-linked neurodevelopmental disorder, is a major cause of intellectual disability in females. Methyl CpG binding protein 2 (MeCP2) is a transcription factor that binds to methylated cytosine residues of CpG dinucleotides in DNA, recruiting histone deacetylases (HDACs) and other transcriptional repressors to silence target genes. Loss-of-function mutations or duplications of the MECP2 gene cause RTT. MeCP2 levels are closely correlated with those of BDNF in the brain, and restoring BDNF levels in the brains of MeCP2 mutant mice can ameliorate many of their RTT-like physiological and behavioral deficits [60]. The brain-enriched transcript of MeCP2 has a binding site for miR-132 in its 3′UTR, and is negatively regulated by this miRNA [61]. Furthermore, the DNA methyltransferase inhibitor 5-aza-2′-deoxycytidine (5-azadC), which decreases the methylation status of DNA and thereby reduces the inhibitory influence of MeCP2 on gene expression, increases miR-132 expression in cultured cells [62,63]. Hence, MeCP2 and miR-132 can each negatively regulate expression of the other, suggesting that homeostatic interactions between MeCP2 and miR-132 control their respective activities [61]. In addition to miR-132, MeCP2 also regulates expression of many other miRNAs (Table 1). Hence, disruption of MeCP2 regulation of miRNA signaling in the brain may contribute to RTT pathophysiology.
Table 1.
MicroRNA alterations in neurodevelopmental disorders
| Disorder | miRNAs | Proposed contribution to disorder | Refs |
|---|---|---|---|
| Fragile X syndrome | miR-124 | FMRP may regulate miR-124 levels in adult brain. | [57] |
| miR-9, miR-124, miR-125a,b, miR-128, miR-132, miR-219 | FMRP is bound to these miRNAs. Thinner dendritic spines are promoted by miR-125, and the opposite effect is induced by miR-132. The effects of both miRNAs are dependent upon FMRP. | [38] | |
| Rett syndrome | miR-132 | Negative feedback loop between MeCP2 and miR-132. Disruption of homeostatic interactions between these factors may contribute to RTT symptomatology. | [62,63] |
| let-7a, miR-7a*, miR-7i, miR-9*, miR-15a*, miR-24, miR-28, miR-29a*, miR-30d, miR-34b-3p, miR-34c, miR-101a, miR-106a, miR-124, miR-137, miR-206, miR-296-5p, miR-299*, miR-323-3p, miR-326, miR-328, miR-377, miR-455, miR-495, miR-543, miR-666-5p, miR-674, miR-744*, miR-874miR-137, miR-187, miR-197miR-132, miR-212miR-29b, miR-92, miR-329, miR-199b, miR-296, miR-221, miR-382miR-184 | MeCP2 represses the expression of these miRNAs | [104][105][61–63,104][106][107] | |
| miR-197, miR-199a, miR-221miR-122a, miR-130, miR-146a,b, miR-342, miR-409miR-7a*, miR-29c, miR-140 | MeCP2 increases the expression of these miRNAs. | [105][106][104] | |
| miR-132, miR-212miR-155, miR-802miR-302miR-130b | MeCP2 expression is regulated by these miRNAs. | [61–63,104,108][109][110][111] | |
| Autism spectrum disorder (ASD) | miR-132 | Downregulated in ASD postmortem brain. | [64] |
| miR-23a,b, miR-25, miR-29b, miR-30c, miR-93, miR-103, miR-106b, miR-107, miR-185, miR-186, miR-191, miR-194, miR-195, miR-205, miR-342, miR-346, miR-376a-AS, miR-451, miR-519c, miR-524 | Upregulated in lymphoblastoid cell lines derived from ASD patients. | [112] | |
| miR-16-2, miR-106b, miR-132, miR-133b, miR-136, miR-139, miR-148b, miR-153-1, miR-189, miR-190, miR-199b, miR-211, miR-219, miR-326, miR-367, miR-182-AS, miR-455, miR-495, miR518a, miR-520b | Downregulated in lymphoblastoid cell lines derived from ASD patients. | [112] |
Autism
Autism is prevalent in both FXS and RTT, suggesting that miRNAs contributing to these disorders may also contribute to autism (Table 1). Gene expression profiling from postmortem cortical tissues from individuals with autistic spectrum disorder (ASD) revealed that the expression of many miRNAs is dysregulated in their brains [64], including downregulated miR-132 expression. Considering that miR-132 may play a role in FXS and RTT, this raises the intriguing possibility that this miRNA contributes to autism symptomatology across neurodevelopmental disorders.
MicroRNAs and neuropsychiatric disorders
Depression
Depression is a chronic and potentially life-threatening disorder characterized by low mood and loss of interest in otherwise pleasurable activities (anhedonia). Depression is thought to be related, at least in part, to deficits in serotonin transmission [65], because many therapeutic agents that are clinically efficacious for depression elevate serotonin transmission in brain. Emerging evidence suggests that miRNAs may be involved in the manifestation of depression and the therapeutic actions of antidepressant drugs. For instance, genetic polymorphisms in the genes encoding miR-30e and miR-183 increase vulnerability to depression [66,67] (Table 2). In the case of miR-183, this increased vulnerability may be secondary to disrupted circadian rhythms [66]. More recently, miR-16 was shown to regulate negatively the expression of the serotonin transporter (SERT), the major target for the selective serotonin reuptake inhibitor (SSRI) class of antidepressant drugs [68]. In mice chronic treatment with the SSRI fluoxetine (Prozac®) increased levels of miR-16 in the raphe nuclei, from which serotonergic neurons arise, through a mechanism involving inhibition of Wnt signaling [68]. Moreover, increased miR-16 resulted in decreased SERT levels in the raphe, and consequently increased brain serotonin transmission, triggering anti-depressant-like effect on behavior [68].
Table 2.
MicroRNA alterations in neuropsychiatric disorders
| Disorder | miRNAs | Proposed contribution to disorder | Refs |
|---|---|---|---|
| Depression | miR-182miR-30e | May increase the risk of major depressive disorder. | [66][67] |
| miR-16 | Negatively regulates the expression of SERT, and hence may alter responsiveness to antidepressant treatments. | [68] | |
| miR-96 | Negatively regulates the expression of 5-HT1B receptor. | [101] | |
| miR-18a | Increased expression in the hypothalamic paraventricular nucleus of F344 rats with increased vulnerability to stress. | [113] | |
| Schizophrenia (Ref. [114] for a more comprehensive list). | miR-7, miR-106 let-7d, miR-7, miR-15a,b, miR-16, miR-20a, miR-26b, miR-27a, miR-29c, miR-31, miR-33, miR-101, miR-105, miR-107, miR-126*, miR-128a, miR-153, miR-181a,b,d, miR-184, miR-195, miR-199a, miR-219, miR-223, miR-302a*, b*, miR-338, miR-409-3p, miR-512-3p, miR-519b.miR-17-5p, miR-25, miR-92b, miR-105, miR-134, miR-148b, miR-150, miR-152, miR-154, miR-187, miR-193a, miR-199a*, b, miR-222, miR-328, miR-382, miR-409-3p, miR-423, miR-425-5p, miR-433, miR-452*, miR-487a, miR-495, miR-502, miR-512-3p, miR-519c, miR-532, miR-542-3p, miR-548b, miR-590, miR-592, miR-652, miR-767-5p.miR-7, miR-34a, miR-132, miR-132*, miR-154*, miR-212, miR-544. miR-22, miR-27b, miR-106b, miR-138, miR-148b, miR-151, miR-193b, miR-210, miR-301, miR-324-3p, miR-338, miR-339, miR-425, miR-545, miR-639. | These miRNAs are dysregulated in postmortem PFC from schizophrenia patients. | [71,115][70][102,116] |
| miR-181b | Upregulated in the superior temporal gyrus and dorsolateral PFC of schizophrenia patients. | [69–71] | |
| miR-219 | Expression is disrupted in the PFC of human schizophrenia patients.Exerts a tonic inhibitory influence on cortical NMDA receptor signaling. Expression in PFC of mice is decreased by psychotomimetics.Decreasing miR-219 signaling in brain attenuated schizophrenia-like behavioral deficits in mice. | [71][80] | |
| Drug addiction | let-7c,f, miR-22, miR-23b, miR-30b,d, miR-124a, miR-125b, miR-126, miR-128b, miR-129*, miR-132, miR-191, miR-212, miR-219, miR-328, miR-329, miR-338, miR-352, miR-376b, miR-383 | Altered expression in dorsal striatum after cocaine self-administration in rats. | [43,62] |
| miR-7a, miR-130a, miR-136, miR-137, miR-138, miR-148b, miR-154, miR-181a, miR-186, miR-301b, miR-324-5p, miR-337-3p, miR-369-3p, miR-376a,c, miR-380-3p, miR-384-5p miR-467a,b, miR-488, miR-500, miR-544, miR-665 | Increased expression in D2 receptor-expressing cells of striatum after cocaine treatment of mice. | [6] | |
| miR-29a,b, miR-34c, miR141, miR-154, miR-182, miR-183, miR-190b, miR-200a,b,c, miR-217, miR-298, miR-329, miR-380-3p, miR-381, miR-429, miR-467c,e, miR-496, miR-500, miR-504, miR-582, miR-680, miR-685, miR-743b, miR-874 | Altered expression in midbrain and PFC in mice receiving injections of nicotine, cocaine or amphetamine. | [103] | |
| let-7d, miR-124, miR-181a. | Cocaine alters the expression of these miRNAs in striatum and forebrain of mice, and in turn these miRNAs influence cocaine reward. | [117,118] | |
| miR-9 | May contribute to tolerance to the physiological and behavioral actions of alcohol. | [85] | |
| miR-23b | Negatively regulates the expression of the m opioid receptor. Expression is increased in cultured cells after chronic morphine exposure. | [119,120] | |
| let-7 | Negatively regulates m opioid receptor expression and may therefore regulate the development of opioid tolerance. | [121] | |
| miR-15b, miR-16, miR-21, miR-23a,b, miR-24, miR-26a,b, miR-30c, miR-99b, miR-103, miR-107, miR-132, miR-146a, miR-150, miR-155, miR-181b, miR-191, miR-221, miR-320a,c, miR-423-5p, miR-638, miR-1469, miR-1826, miR-1915 | Morphine alters the expression of these miRNAs in human macrophages. | [122] | |
| miR-190 | Decreased expression in cultures of hippocampal neurons of rats and mice after long-term treatment with the m opioid receptor agonist fentanyl but not after morphine treatment. | [123–125] | |
| miR-16, miR-21, miR-140* | Nicotine treatment increases the expression of these miRNAs in cultured cells. | [126,127] | |
| miR-133, miR-590 | Nicotine decreases the expression of these miRNAs in atrial tissues. | [128] |
Schizophrenia
Schizophrenia is a chronic psychiatric illness characterized by both positive and negative symptoms. Positive symptoms include hallucinations, delusions and disruption of logical thought patterns. Negative symptoms are phenomenologically similar to depression, and include affective flattening, avolition, alogia and/or anhedonia. In addition, schizophrenics also generally suffer from cognitive impairments.
There has been much interest in the possible involvement of miRNAs in schizophrenia (Table 2). A recent report demonstrated that the expression of miRNAs, particularly miR-181b, and components of the miRNA biogenic machinery, including DiGeorge syndrome critical region gene 8 (DGCR8), was dysregulated in the superior temporal gyrus and dorsolateral prefrontal cortex (PFC) of schizophrenic subjects [69–71]. Furthermore, polymorphisms in the genes for miR-206, miR-198, miR-30e and miR-137 were associated with schizophrenia vulnerability [67,72–76]. Disruption of miRNA signaling through altered biogenesis or genetic variation in miRNA genes may therefore contribute to schizophrenia.
In addition to the above clinical findings, preclinical studies also highlight the potential relevance of miRNAs to schizophrenia-related behavioral disturbances. Mice with genetic deletion of a chromosomal region that is syntenic to the human 22q11.2 locus [Df (16)A+/− mice], a region in humans in which microdeletions are associated with high risk of schizophrenia [77], display schizophrenia-like behavioral disturbances [78]. Dgcr8 haploinsufficient mice have reduced miRNA expression in the PFC [79], effects that are hypothesized to contribute to schizophrenia-like pathophysiological deficits including decreased morphological complexity of cortical neurons and reduced excitatory synaptic transmission in PFC. Expression of miR-219 is reduced in the PFC of mice treated with the psychotomimetic agent MK-801 [80]. This miRNA is similarly reduced in genetically modified mice with a hypomorphic mutation in Grin1 (the gene encoding the NR1 subunit of the NMDA receptor) that display schizophrenia-like behavioral deficits [80,81]. Importantly, the antipsychotic agents haloperidol and clozapine reversed the inhibitory effects of MK-801 on cortical miR-219 expression in mice [80]. Moreover, knockdown of miR-219 signaling through the brain, via infusions of a locked nucleic acid (LNA)-modified antisense oligonucleotide (discussed further below) attenuated the behavioral disturbances (e.g. hyperlocomotion and stereotyped behaviors) induced by MK-801 in mice [80]. It is known that miR-219 exerts an inhibitory influence on NMDA receptor-mediated transmission in cortical cells [45]. Thus, one possible explanation of these findings is that miR-219 exerts a tonic inhibitory influence on cortical NMDA receptor signaling, and when NMDA receptor signaling is disrupted by psychotomimetic agents such as MK-801 there is a compensatory decrease in miR-219 expression to counter the physiological and behavioral deficits associated with diminished NMDA receptor signaling. As such, boosting miR-219 signaling may be a novel therapeutic strategy to treat aspects of schizophrenia symptomatology. Intriguingly, expression of miR-219 appears to be disrupted in the PFC of human schizophrenia patients [71], consistent with a role for this miRNA in aspects of the disorder.
Drug addiction
Cocaine administration in rodents increases the expression of many miRNAs in addiction-relevant brain regions [6], and such cocaine-responsive miRNAs can influence sensitivity to the rewarding properties of the drug (Table 2). In particular, expression of miR-132 [36,43,62] and miR-212 [43,62] is increased in the dorsal striatum of rats with extended daily access to cocaine self-administration, an access condition that increases the expression of addiction-like cocaine-taking behaviors in rats [43,62]. Furthermore, striatal overexpression of miR-212 decreased, whereas its knockdown increased, the propensity to develop compulsive-like cocaine-taking behaviors in rats [43]. This suggests that miR-212 protects against the development of cocaine addiction, and it may coordinate the homeostatic responses of brain reward circuitries to counter the addictive properties of cocaine. This protective action of miR-212 was related to at least two effects of the miRNA in striatum. First, miR-212 sensitized the activity of adenylyl cyclase enzymes, and thereby amplified striatal CREB signaling that was engaged in response to cocaine consumption [43]. Importantly, an increase in the striatal CREB signaling pathway has been demonstrated to decrease the motivational properties of cocaine [82]. Second, striatal miR-212 negatively regulated the expression of MeCP2 and thereby reduced BDNF expression in this brain region [62]. Striatal BDNF signaling drives compulsive-like cocaine-taking behavior [62]. Hence, simultaneous amplification of CREB signaling and inhibition of MeCP2/BDNF signaling in the striatum contributes to the protective actions of miR-212 against the development of compulsive cocaine-taking. A role for miR-132, the other miRNA that is upregulated in the striatum of rats with extended cocaine access [43], in regulating drug-taking behavior has not yet been investigated.
Additional evidence supporting a role for miRNAs in regulating behavioral responses to cocaine is derived from studies of genetically-engineered mice in which the expression of Ago2 is disrupted specifically in dopamine D2 receptor-containing neurons in the striatum (i.e. Ago2-D2R conditional knockout mice). These mice exhibit dramatically decreased sensitivity to the rewarding properties of cocaine, as measured in a place-conditioning procedure, compared to wild-type mice [6]. Moreover, intravenous cocaine self-administration was far lower in the Ago2-D2R knockout mice compared to wild-type controls [6]. The enzymatic action of Ago2 plays an important role in the biogenesis of particular miRNAs ([6] and Refs therein), and Ago2 is an important component of the RISC complex necessary for the repressor action of miRNAs (Figure 1). Thus, these findings suggest that miRNAs, particularly in D2R-expressing neurons in the striatum, play a key role in regulating the addiction-related actions of cocaine.
Other drugs of abuse have also been shown to alter the expression of miRNAs. For example, nicotine and opiate administration in rodents cause changes in a variety of miRNAs (Table 2). Alcohol also induces changes in miRNA expression, both in vitro and in vivo. Concentrations of alcohol that are within the range of the blood alcohol level achieved in human social drinkers were found to alter miRNA expression in cultured mouse neurospheres [83], as well as increasing miR-212 expression in a human intestinal epithelial cell line [84] (Table 2). Ethanol administration in rats increased miR-9 expression in striatal neurons and the supraoptic nucleus (SON) [85], causing decreased expression of a subset of large Ca2+-activated K+ (BK) channel mRNA splice variants [85]. This resulted in translated BK channels with decreased sensitivity to alcohol [85], suggesting that miR-9 contributes to the development of tolerance to the physiological and behavioral actions of alcohol [85,86]. Taken together, these findings suggest that miRNAs are involved in cellular mechanisms that underlie the neuroplastic and behavioral changes that occur in response to drugs of abuse.
MicroRNAs as targets for the development of therapeutics
Abnormally increased or decreased expression of various miRNAs may contribute to the pathophysiology of many neurodevelopmental and psychiatric disorders (above). Hence, replacement or inhibition of downregulated or overactive miRNAs, respectively, may be clinically beneficial in the treatment these disorders (e.g. [87–90]). Much effort has been directed toward developing modified oligonucleotide mimetics to replace, or antisense oligonucleotides (AOs) to inhibit, targeted miRNAs. A major objective in these efforts is to develop oligonucleotides that achieve high in vivo efficacy without significant toxicity. Biostability and pharmacokinetics are therefore important considerations in their design and use. Locked nucleic acids (LNA; Figure 3a), a family of conformationally locked nucleotide analogs, are relatively resistant to nuclease activity and may prove to be a suitable platform for the development of miRNA-based therapeutics. For example, it is known that miR-122 regulates cholesterol metabolism and hepatitis C virus (HCV) replication [91]. An LNA-modified AO blocked miR-122 activity in the liver of African green monkeys without any evidence of toxicity [91], induced long-lasting decreases in plasma cholesterol levels [91], and decreased HCV levels in the blood of HCV-infected chimpanzees [92]. Moreover, an LNA-modified AO against miR-122 named miravirsen (SPC3649) has advanced to Phase II clinical trials in HCV-infected humans as a potential therapeutic for the disease (clinical trial identifier NCT01200420; http://www.clinicaltrials.gov). A major drawback associated with this strategy when considering neurological disorders is the relative difficulty in delivering AOs across the blood–brain barrier. Intriguingly, systemically injected exosomes, which are small transport vesicles secreted by many classes of mammalian cells, can be used to deliver small RNAs selectivity to the brain in mice [93] (Figure 3b). It will therefore be important to test whether this same approach can be used to deliver miRNA mimetics of AOs against targeted miRNAs selectively to the brain of mammalian species including humans.
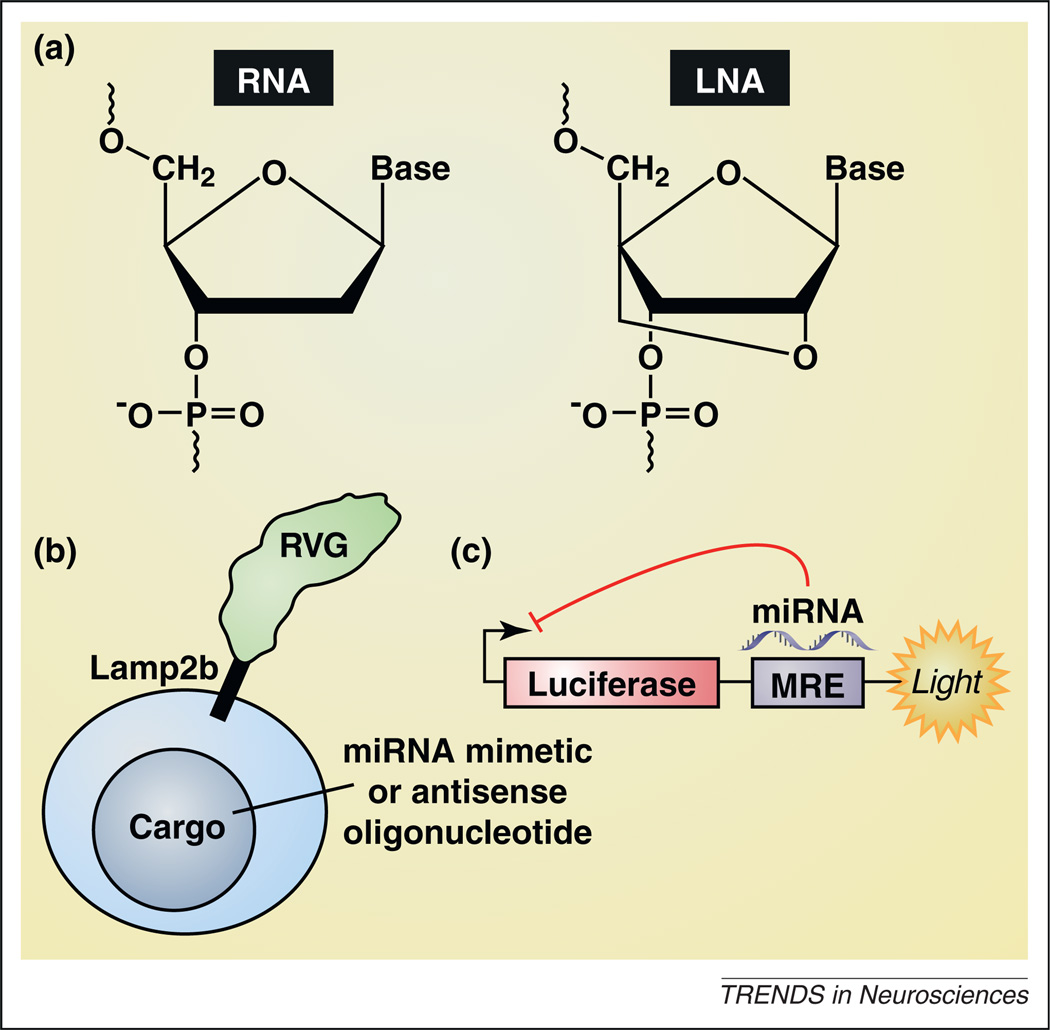
Figure 3.
Developing microRNA-based therapeutics. (a) The left panel shows an RNA monomer, and the right panel shows an LNA-modified oligonucleotide monomer in the locked 3′-endo conformation of the furanose ring. LNA-modified antisense oligonucleotides against targeted miRNAs are currently in Phase II clinical trials for hepatitis C virus (clinical trial identifier NCT01200420; http://www.clinicaltrials.gov). (b) Exosomes targeted to the brain, achieved by tethering the exosome-expressed protein Lamp2b (lysosome-associated membrane protein-2) with rabies virus glycoprotein (RVG), represent a potential mechanism to deliver miRNA mimetic or antisense oligonucleotide cargos selectively to the brain (e.g. [101]). (c) A screening strategy used successfully to identify novel small-molecule activators and inhibitors of discrete miRNAs [102,103]. The miRNA response element (MRE) sequence for a given miRNA is cloned downstream of the luciferase gene. Increases in the activity of a miRNA that binds to the MRE results in lower luciferase activity, reflected in diminished luminescence, whereas decreased activity results in increased luminescence.
In addition to mimetic and antisense oligonucleotides to modulate miRNA function, recent efforts have also been directed toward developing small-molecule drugs that can influence the biogenesis of miRNAs or directly influence their function [94]. The small-molecule anti-inflammatory drug enoxacin (Penetrex™) promotes biogenesis of endogenous miRNAs [95–97] (Figure 1). Conversely, compounds that disrupt miRNA biogenesis have been identified [98]. Most recently, a luciferase reporter-based system, in which MREs for discrete miRNAs are cloned upstream of the luciferase gene, has been used to detect small molecule drugs that selectively modulate miRNA action [99,100] (Figure 3c). It will be important to determine if similar screening approaches can be used to identify chemical probes, and potentially useful drugs, to modulate selectively the activity of miRNAs involved in neurodevelopmental and psychiatric disorders.
Concluding remarks
The findings reviewed here demonstrate that miRNAs play key roles in all aspects of neuronal development, function and plasticity. Moreover, dysfunction in miRNA signaling contributes to neurodevelopmental and psychiatric disorders. Nevertheless, much work remains to be done to understand the precise mechanisms through which miRNAs regulate temporal and spatial dynamics of neuronal function, and to translate this knowledge into novel therapeutics for the treatment of neurological disorders.
Acknowledgments
Supported by a grant from the National Institute on Drug Abuse (NIDA) to P.J.K. (DA025983). We thank the Department of Biomedical Graphics at The Scripps Research Institute for assistance with graphics. This is publication number 20973 from The Scripps Research Institute.
References
- 1.Kapsimali M, et al. MicroRNAs show a wide diversity of expression profiles in the developing and mature central nervous system. Genome Biol. 2007;8:R173. doi: 10.1186/gb-2007-8-8-r173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng LC, et al. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat. Neurosci. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu C, et al. Epigenetic regulation of miR-184 by MBD1 governs neural stem cell proliferation and differentiation. Cell Stem Cell. 2010;6:433–444. doi: 10.1016/j.stem.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giraldez AJ, et al. MicroRNAs regulate brain morphogenesis in zebrafish. Science. 2005;308:833–838. doi: 10.1126/science.1109020. [DOI] [PubMed] [Google Scholar]
- 5.Davis TH, et al. Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J. Neurosci. 2008;28:4322–4330. doi: 10.1523/JNEUROSCI.4815-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaefer A, et al. Argonaute 2 in dopamine 2 receptor-expressing neurons regulates cocaine addiction. J. Exp. Med. 2010;207:1843–1851. doi: 10.1084/jem.20100451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawase-Koga Y, et al. Different timings of Dicer deletion affect neurogenesis and gliogenesis in the developing mouse central nervous system. Dev. Dyn. 2009;238:2800–2812. doi: 10.1002/dvdy.22109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang T, et al. Wnt1-cre-mediated conditional loss of Dicer results in malformation of the midbrain and cerebellum and failure of neural crest and dopaminergic differentiation in mice. J. Mol. Cell Biol. 2010;2:152–163. doi: 10.1093/jmcb/mjq008. [DOI] [PubMed] [Google Scholar]
- 9.Bilen J, et al. A new role for microRNA pathways: modulation of degeneration induced by pathogenic human disease proteins. Cell Cycle. 2006;5:2835–2838. doi: 10.4161/cc.5.24.3579. [DOI] [PubMed] [Google Scholar]
- 10.Hebert SS, et al. Genetic ablation of Dicer in adult forebrain neurons results in abnormal tau hyperphosphorylation and neurodegeneration. Hum. Mol. Genet. 2010;19:3959–3969. doi: 10.1093/hmg/ddq311. [DOI] [PubMed] [Google Scholar]
- 11.Martino S, et al. MicroRNA implications across neurodevelopment and neuropathology. J. Biomed. Biotechnol. 2009;2009:654346. doi: 10.1155/2009/654346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vo NK, et al. MicroRNA pathways in neural development and plasticity. Curr. Opin. Neurobiol. 2010;20:457–465. doi: 10.1016/j.conb.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, et al. Effects of miR-541 on neurite outgrowth during neuronal differentiation. Cell Biochem. Funct. 2011;29:279–286. doi: 10.1002/cbf.1747. [DOI] [PubMed] [Google Scholar]
- 14.Abdelmohsen K, et al. miR-375 inhibits differentiation of neurites by lowering HuD levels. Mol. Cell. Biol. 2010;30:4197–4210. doi: 10.1128/MCB.00316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smrt RD, et al. MicroRNA miR-137 regulates neuronal maturation by targeting ubiquitin ligase mind bomb-1. Stem Cells. 2010;28:1060–1070. doi: 10.1002/stem.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kole AJ, et al. miR-29b is activated during neuronal maturation and targets BH3-only genes to restrict apoptosis. Genes Dev. 2011;25:125–130. doi: 10.1101/gad.1975411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaughwin P, et al. Stage-specific modulation of cortical neuronal development by Mmu-miR-134. Cereb. Cortex. 2011;21:1857–1869. doi: 10.1093/cercor/bhq262. [DOI] [PubMed] [Google Scholar]
- 18.Chen H, et al. miR-7 and miR-214 are specifically expressed during neuroblastoma differentiation, cortical development and embryonic stem cells differentiation, and control neurite outgrowth in vitro. Biochem. Biophys. Res. Commun. 2010;394:921–927. doi: 10.1016/j.bbrc.2010.03.076. [DOI] [PubMed] [Google Scholar]
- 19.Balzer E, et al. LIN28 alters cell fate succession and acts independently of the let-7 microRNA during neurogliogenesis in vitro. Development. 2010;137:891–900. doi: 10.1242/dev.042895. [DOI] [PubMed] [Google Scholar]
- 20.Coolen M, Bally-Cuif L. MicroRNAs in brain development and physiology. Curr. Opin. Neurobiol. 2009;19:461–470. doi: 10.1016/j.conb.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Sanuki R, et al. miR-124a is required for hippocampal axogenesis and retinal cone survival through Lhx2 suppression. Nat. Neurosci. 2011;14:1125–1134. doi: 10.1038/nn.2897. [DOI] [PubMed] [Google Scholar]
- 22.Mellios N, et al. miR-132, an experience-dependent microRNA, is essential for visual cortex plasticity. Nat. Neurosci. 2011;14:1240–1242. doi: 10.1038/nn.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tognini P, et al. Experience-dependent expression of miR-132 regulates ocular dominance plasticity. Nat. Neurosci. 2011;14:1237–1239. doi: 10.1038/nn.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smirnova L, et al. Regulation of miRNA expression during neural cell specification. Eur. J. Neurosci. 2005;21:1469–1477. doi: 10.1111/j.1460-9568.2005.03978.x. [DOI] [PubMed] [Google Scholar]
- 25.Conaco C, et al. Reciprocal actions of REST and a microRNA promote neuronal identity. Proc. Natl. Acad. Sci. U.S.A. 2006;103:2422–2427. doi: 10.1073/pnas.0511041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Battaglioli E, et al. REST repression of neuronal genes requires components of the hSWI.SNF complex. J. Biol. Chem. 2002;277:41038–41045. doi: 10.1074/jbc.M205691200. [DOI] [PubMed] [Google Scholar]
- 27.Andres ME, et al. CoREST: a functional corepressor required for regulation of neural-specific gene expression. Proc. Natl. Acad. Sci. U.S.A. 1999;96:9873–9878. doi: 10.1073/pnas.96.17.9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Visvanathan J, et al. The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev. 2007;21:744–749. doi: 10.1101/gad.1519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makeyev EV, et al. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol. Cell. 2007;27:435–448. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao X, et al. A functional study of miR-124 in the developing neural tube. Genes Dev. 2007;21:531–536. doi: 10.1101/gad.1519207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Konopka W, et al. MicroRNA loss enhances learning and memory in mice. J. Neurosci. 2010;30:14835–14842. doi: 10.1523/JNEUROSCI.3030-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cougot N, et al. Dendrites of mammalian neurons contain specialized P-body-like structures that respond to neuronal activation. J. Neurosci. 2008;28:13793–13804. doi: 10.1523/JNEUROSCI.4155-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ashraf SI, et al. Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila. Cell. 2006;124:191–205. doi: 10.1016/j.cell.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 34.Banerjee S, et al. A coordinated local translational control point at the synapse involving relief from silencing and MOV10 degradation. Neuron. 2009;64:871–884. doi: 10.1016/j.neuron.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 35.Krol J, et al. Characterizing light-regulated retinal microRNAs reveals rapid turnover as a common property of neuronal microRNAs. Cell. 2010;141:618–631. doi: 10.1016/j.cell.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 36.Nudelman AS, et al. Neuronal activity rapidly induces transcription of the CREB-regulated microRNA-132 in vivo. Hippocampus. 2010;20:492–498. doi: 10.1002/hipo.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vo N, et al. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc. Natl. Acad. Sci. U.S.A. 2005;102:16426–16431. doi: 10.1073/pnas.0508448102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edbauer D, et al. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron. 2010;65:373–384. doi: 10.1016/j.neuron.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magill ST, et al. microRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proc. Natl. Acad. Sci. U.SA. 2010;107:20382–20387. doi: 10.1073/pnas.1015691107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansen KF, et al. Transgenic miR132 alters neuronal spine density and impairs novel object recognition memory. PLoS ONE. 2010;5:e15497. doi: 10.1371/journal.pone.0015497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Impey S, et al. An activity-induced microRNA controls dendritic spine formation by regulating Rac1-PAK signaling. Mol. Cell. Neurosci. 2010;43:146–156. doi: 10.1016/j.mcn.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wayman GA, et al. An activity-regulated microRNA controls dendritic plasticity by down-regulating p250GAP. Proc. Natl. Acad. Sci. U.S.A. 2008;105:9093–9098. doi: 10.1073/pnas.0803072105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hollander JA, et al. Striatal microRNA controls cocaine intake through CREB signalling. Nature. 2010;466:197–202. doi: 10.1038/nature09202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lambert TJ, et al. MicroRNA132 modulates short-term synaptic plasticity but not basal release probability in hippocampal neurons. PLoS ONE. 2010;5:e15182. doi: 10.1371/journal.pone.0015182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng HY, et al. microRNA modulation of circadian-clock period and entrainment. Neuron. 2007;54:813–829. doi: 10.1016/j.neuron.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao J, et al. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Neill LA. Boosting the brain’s ability to block inflammation via microRNA-132. Immunity. 2009;31:854–855. doi: 10.1016/j.immuni.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Strum JC, et al. MicroRNA 132 regulates nutritional stress-induced chemokine production through repression of SirT1. Mol. Endocrinol. 2009;23:1876–1884. doi: 10.1210/me.2009-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zovoilis A, et al. microRNA-34c is a novel target to treat dementias. EMBO J. 2011;30:4299–4308. doi: 10.1038/emboj.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fiore R, et al. Mef2-mediated transcription of the miR379-410 cluster regulates activity-dependent dendritogenesis by fine-tuning Pumilio2 protein levels. EMBO J. 2009;28:697–710. doi: 10.1038/emboj.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schratt GM, et al. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- 52.Christensen M, et al. Recombinant adeno-associated virus-mediated microRNA delivery into the postnatal mouse brain reveals a role for miR-134 in dendritogenesis in vivo. Front. Neural Circuits. 2010;3:16. doi: 10.3389/neuro.04.016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siegel G, et al. A functional screen implicates microRNA-138-dependent regulation of the depalmitoylation enzyme APT1 in dendritic spine morphogenesis. Nat. Cell Biol. 2009;11:705–716. doi: 10.1038/ncb1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rajasethupathy P, et al. Characterization of small RNAs in Aplysia reveals a role for miR-124 in constraining synaptic plasticity through CREB. Neuron. 2009;63:803–817. doi: 10.1016/j.neuron.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheever A, Ceman S. Translation regulation of mRNAs by the fragile X family of proteins through the microRNA pathway. RNA Biol. 2009;6:175–178. doi: 10.4161/rna.6.2.8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jin P, et al. Biochemical and genetic interaction between the fragile X mental retardation protein and the microRNA pathway. Nat. Neurosci. 2004;7:113–117. doi: 10.1038/nn1174. [DOI] [PubMed] [Google Scholar]
- 57.Xu XL, et al. The steady-state level of the nervous-system-specific microRNA-124a is regulated by dFMR1 in Drosophila. J. Neurosci. 2008;28:11883–11889. doi: 10.1523/JNEUROSCI.4114-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muddashetty RS, et al. Reversible inhibition of PSD-95 mRNA translation by miR-125a, FMRP phosphorylation, and mGluR signaling. Mol. Cell. 2011;42:673–688. doi: 10.1016/j.molcel.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu XL, et al. FXR1P but not FMRP regulates the levels of mammalian brain-specific microRNA-9 and microRNA-124. J. Neurosci. 2011;31:13705–13709. doi: 10.1523/JNEUROSCI.2827-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kondo M, et al. Environmental enrichment ameliorates a motor coordination deficit in a mouse model of Rett syndrome – Mecp2 gene dosage effects and BDNF expression. Eur. J. Neurosci. 2008;27:3342–3350. doi: 10.1111/j.1460-9568.2008.06305.x. [DOI] [PubMed] [Google Scholar]
- 61.Klein ME, et al. Homeostatic regulation of MeCP2 expression by a CREB-induced microRNA. Nat. Neurosci. 2007;10:1513–1514. doi: 10.1038/nn2010. [DOI] [PubMed] [Google Scholar]
- 62.Im HI, et al. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat. Neurosci. 2010;13:1120–1127. doi: 10.1038/nn.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wada R, et al. miR-212 is downregulated and suppresses methyl-CpG-binding protein MeCP2 in human gastric cancer. Int. J. Cancer. 2010;127:1106–1114. doi: 10.1002/ijc.25126. [DOI] [PubMed] [Google Scholar]
- 64.Abu-Elneel K, et al. Heterogeneous dysregulation of microRNAs across the autism spectrum. Neurogenetics. 2008;9:153–161. doi: 10.1007/s10048-008-0133-5. [DOI] [PubMed] [Google Scholar]
- 65.Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat. Rev. Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 66.Saus E, et al. Genetic variants and abnormal processing of pre-miR-182, a circadian clock modulator, in major depression patients with late insomnia. Hum. Mol. Genet. 2010;19:4017–4025. doi: 10.1093/hmg/ddq316. [DOI] [PubMed] [Google Scholar]
- 67.Xu Y, et al. A polymorphism in the microRNA-30e precursor associated with major depressive disorder risk and P300 waveform. J. Affect. Disord. 2010;127:332–336. doi: 10.1016/j.jad.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 68.Baudry A, et al. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science. 2010;329:1537–1541. doi: 10.1126/science.1193692. [DOI] [PubMed] [Google Scholar]
- 69.Beveridge NJ, et al. Dysregulation of miRNA 181b in the temporal cortex in schizophrenia. Hum. Mol. Genet. 2008;17:1156–1168. doi: 10.1093/hmg/ddn005. [DOI] [PubMed] [Google Scholar]
- 70.Santarelli DM, et al. Upregulation of dicer and microRNA expression in the dorsolateral prefrontal cortex Brodmann area 46 in schizophrenia. Biol. Psychiatry. 2011;69:180–187. doi: 10.1016/j.biopsych.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 71.Beveridge NJ, et al. Schizophrenia is associated with an increase in cortical microRNA biogenesis. Mol. Psychiatry. 2010;15:1176–1189. doi: 10.1038/mp.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hansen T, et al. Brain expressed microRNAs implicated in schizophrenia etiology. PLoS ONE. 2007;2:e873. doi: 10.1371/journal.pone.0000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu Y, et al. MicroRNAs and target site screening reveals a pre-microRNA-30e variant associated with schizophrenia. Schizophr. Res. 2010;119:219–227. doi: 10.1016/j.schres.2010.02.1070. [DOI] [PubMed] [Google Scholar]
- 74.Sun G, et al. SNPs in human miRNA genes affect biogenesis and function. RNA. 2009;15:1640–1651. doi: 10.1261/rna.1560209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feng J, et al. Evidence for X-chromosomal schizophrenia associated with microRNA alterations. PLoS ONE. 2009;4:e6121. doi: 10.1371/journal.pone.0006121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ripke S, et al. Genome-wide association study identifies five new schizophrenia loci. Nat. Genet. 2011;43:969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karayiorgou M, et al. 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat. Rev. Neurosci. 2010;11:402–416. doi: 10.1038/nrn2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stark KL, et al. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat. Genet. 2008;40:751–760. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- 79.Schofield CM, et al. Monoallelic deletion of the microRNA biogenesis gene Dgcr8 produces deficits in the development of excitatory synaptic transmission in the prefrontal cortex. Neural Dev. 2011;6:11. doi: 10.1186/1749-8104-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kocerha J, et al. MicroRNA-219 modulates NMDA receptor-mediated neurobehavioral dysfunction. Proc. Natl. Acad. Sci. U.S.A. 2009;106:3507–3512. doi: 10.1073/pnas.0805854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mohn AR, et al. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- 82.Carlezon WA, Jr, et al. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- 83.Sathyan P, et al. Competing interactions between micro-RNAs determine neural progenitor survival and proliferation after ethanol exposure: evidence from an ex vivo model of the fetal cerebral cortical neuroepithelium. J. Neurosci. 2007;27:8546–8557. doi: 10.1523/JNEUROSCI.1269-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tang Y, et al. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol. Clin. Exp. Res. 2008;32:355–364. doi: 10.1111/j.1530-0277.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 85.Pietrzykowski AZ, et al. Posttranscriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron. 2008;59:274–287. doi: 10.1016/j.neuron.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Levitan IB. miXED messages in ion channel modulation. Neuron. 2008;59:188–189. doi: 10.1016/j.neuron.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 87.Liu XQ, et al. Targeted delivery of antisense inhibitor of miRNA for antiangiogenesis therapy using cRGD-functionalized nanoparticles. Mol. Pharm. 2011;8:250–259. doi: 10.1021/mp100315q. [DOI] [PubMed] [Google Scholar]
- 88.Wiggins JF, et al. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res. 2010;70:5923–5930. doi: 10.1158/0008-5472.CAN-10-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takeshita F, et al. Systemic delivery of synthetic microRNA-16 inhibits the growth of metastatic prostate tumors via downregulation of multiple cell-cycle genes. Mol. Ther. 2010;18:181–187. doi: 10.1038/mt.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kota J, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Elmen J, et al. LNA-mediated microRNA silencing in nonhuman primates. Nature. 2008;452:896–899. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
- 92.Lanford RE, et al. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alvarez-Erviti L, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 94.Li Y, et al. Emergence of chemical biology approaches to the RNAi/miRNA pathway. Chem. Biol. 2010;17:584–589. doi: 10.1016/j.chembiol.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Melo S, et al. Small molecule enoxacin is a cancer-specific growth inhibitor that acts by enhancing TAR RNA-binding protein 2-mediated microRNA processing. Proc. Natl. Acad. Sci. U.S.A. 2011;108:4394–4399. doi: 10.1073/pnas.1014720108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shan G, et al. A small molecule enhances RNA interference and promotes microRNA processing. Nat. Biotechnol. 2008;26:933–940. doi: 10.1038/nbt.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Q, et al. Enhancement of RNAi by a small molecule antibiotic enoxacin. Cell Res. 2008;18:1077–1079. doi: 10.1038/cr.2008.287. [DOI] [PubMed] [Google Scholar]
- 98.Watashi K, et al. Identification of small molecules that suppress microRNA function and reverse tumorigenesis. J. Biol. Chem. 2010;285:24707–24716. doi: 10.1074/jbc.M109.062976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gumireddy K, et al. Small-molecule inhibitors of microrna miR-21 function. Angew. Chem. Int. Ed. Engl. 2008;47:7482–7484. doi: 10.1002/anie.200801555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Young DD, et al. Small molecule modifiers of microRNA miR-122 function for the treatment of hepatitis C virus infection and hepatocellular carcinoma. J. Am. Chem. Soc. 2010;132:7976–7981. doi: 10.1021/ja910275u. [DOI] [PubMed] [Google Scholar]
- 101.Jensen KP, et al. A common polymorphism in serotonin receptor 1B mRNA moderates regulation by miR-96 and associates with aggressive human behaviors. Mol. Psychiatry. 2009;14:381–389. doi: 10.1038/mp.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moreau MP, et al. Altered microRNA expression profiles in postmortem brain samples from individuals with schizophrenia and bipolar disorder. Biol. Psychiatry. 2011;69:188–193. doi: 10.1016/j.biopsych.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lippi G, et al. Targeting of the Arpc3 actin nucleation factor by miR-29a/b regulates dendritic spine morphology. J. Cell Biol. 2011;194:889–904. doi: 10.1083/jcb.201103006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu H, et al. Genome-wide analysis reveals methyl-CpG-binding protein 2-dependent regulation of microRNAs in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. U.S.A. 2010;107:18161–18166. doi: 10.1073/pnas.1005595107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Szulwach KE, et al. Cross talk between microRNA and epigenetic regulation in adult neurogenesis. J. Cell Biol. 2010;189:127–141. doi: 10.1083/jcb.200908151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Urdinguio RG, et al. Disrupted microRNA expression caused by Mecp2 loss in a mouse model of Rett syndrome. Epigenetics. 2010;5:656–663. doi: 10.4161/epi.5.7.13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nomura T, et al. MeCP2-dependent repression of an imprinted miR-184 released by depolarization. Hum. Mol. Genet. 2008;17:1192–1199. doi: 10.1093/hmg/ddn011. [DOI] [PubMed] [Google Scholar]
- 108.Alvarez-Saavedra M, et al. miRNA-132 orchestrates chromatin remodeling and translational control of the circadian clock. Hum. Mol. Genet. 2011;20:731–751. doi: 10.1093/hmg/ddq519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kuhn DE, et al. Chromosome 21-derived microRNAs provide an etiological basis for aberrant protein expression in human Down syndrome brains. J. Biol. Chem. 2010;285:1529–1543. doi: 10.1074/jbc.M109.033407. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 110.Lin SL, et al. Regulation of somatic cell reprogramming through inducible mir-302 expression. Nucleic Acids Res. 2011;39:1054–1065. doi: 10.1093/nar/gkq850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Burmistrova OA, et al. MicroRNA in schizophrenia: genetic and expression analysis of miR-130b (22q11) Biochemistry (Mosc.) 2007;72:578–582. doi: 10.1134/s0006297907050161. [DOI] [PubMed] [Google Scholar]
- 112.Sarachana T, et al. Investigation of post-transcriptional gene regulatory networks associated with autism spectrum disorders by microRNA expression profiling of lymphoblastoid cell lines. Genome Med. 2010;2:23. doi: 10.1186/gm144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Uchida S, et al. Characterization of the vulnerability to repeated stress in Fischer 344 rats: possible involvement of microRNA-mediated down-regulation of the glucocorticoid receptor. Eur. J. Neurosci. 2008;27:2250–2261. doi: 10.1111/j.1460-9568.2008.06218.x. [DOI] [PubMed] [Google Scholar]
- 114.Beveridge NJ, Cairns MJ. MicroRNA dysregulation in schizophrenia. Neurobiol. Dis. 2011 doi: 10.1016/j.nbd.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 115.Perkins DO, et al. microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol. 2007;8:R27. doi: 10.1186/gb-2007-8-2-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim AH, et al. MicroRNA expression profiling in the prefrontal cortex of individuals affected with schizophrenia and bipolar disorders. Schizophr. Res. 2010;124:183–191. doi: 10.1016/j.schres.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chandrasekar V, Dreyer JL. Regulation of MiR-124, Let-7d, and MiR-181a in the accumbens affects the expression, extinction, and reinstatement of cocaine-induced conditioned place preference. Neuropsychopharmacology. 2011;36:1149–1164. doi: 10.1038/npp.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chandrasekar V, Dreyer JL. microRNAs miR-124, let-7d and miR-181a regulate cocaine-induced plasticity. Mol. Cell. Neurosci. 2009;42:350–362. doi: 10.1016/j.mcn.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 119.Wu Q, et al. Post-transcriptional regulation of mouse mu opioid receptor (MOR1) via its 3′ untranslated region: a role for microRNA23b. FASEB J. 2008;22:4085–4095. doi: 10.1096/fj.08-108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wu Q, et al. Long-term morphine treatment decreases the association of mu-opioid receptor (MOR1) mRNA with polysomes through miRNA23b. Mol. Pharmacol. 2009;75:744–750. doi: 10.1124/mol.108.053462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.He Y, et al. Regulation of opioid tolerance by let-7 family microRNA targeting the mu opioid receptor. J. Neurosci. 2010;30:10251–10258. doi: 10.1523/JNEUROSCI.2419-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dave RS, Khalili K. Morphine treatment of human monocyte-derived macrophages induces differential miRNA and protein expression: impact on inflammation and oxidative stress in the central nervous system. J. Cell. Biochem. 2010;110:834–845. doi: 10.1002/jcb.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zheng H, et al. Modulations of NeuroD activity contribute to the differential effects of morphine and fentanyl on dendritic spine stability. J. Neurosci. 2010;30:8102–8110. doi: 10.1523/JNEUROSCI.6069-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zheng H, et al. Yin Yang 1 phosphorylation contributes to the differential effects of mu-opioid receptor agonists on microRNA-190 expression. J. Biol. Chem. 2010;285:21994–22002. doi: 10.1074/jbc.M110.112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zheng H, et al. mu-Opioid receptor agonists differentially regulate the expression of miR-190 and NeuroD. Mol. Pharmacol. 2010;77:102–109. doi: 10.1124/mol.109.060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huang W, Li MD. Nicotine modulates expression of miR-140*, which targets the 3′-untranslated region of dynamin 1 gene (Dnm1) Int. J. Neuropsychopharmacol. 2009;12:537–546. doi: 10.1017/S1461145708009528. [DOI] [PubMed] [Google Scholar]
- 127.Shin VY, et al. NF-kappaB targets miR-16 and miR-21 in gastric cancer: involvement of prostaglandin E receptors. Carcinogenesis. 2011;32:240–245. doi: 10.1093/carcin/bgq240. [DOI] [PubMed] [Google Scholar]
- 128.Shan H, et al. Downregulation of miR-133 and miR-590 contributes to nicotine-induced atrial remodelling in canines. Cardiovasc. Res. 2009;83:465–472. doi: 10.1093/cvr/cvp130. [DOI] [PubMed] [Google Scholar]