A dynamin mutant defines a super-constricted pre-fission state (original) (raw)
. Author manuscript; available in PMC: 2015 Aug 7.
SUMMARY
Dynamin is a 100 kDa GTPase that organizes into helical assemblies at the base of nascent clathrin-coated vesicles. Formation of these oligomers stimulates the intrinsic GTPase activity of dynamin, which is necessary for efficient membrane fission during endocytosis. Recent evidence suggests that the transition-state of dynamin's GTP hydrolysis reaction serves as a key determinant of productive fission. Here we present the structure of a transition-state-defective dynamin mutant, K44A, trapped in a pre-fission state, at 12.5 Å resolution. This structure constricts to 3.7 nm, reaching the theoretical limit required for spontaneous membrane fission. Computational docking indicates that the ground state conformation of the dynamin polymer is sufficient to achieve this super-constricted pre-fission state and reveals how a 2-start helical symmetry promotes the most efficient packing of dynamin tetramers around the membrane neck. These data suggest a new model for the assembly and regulation of the minimal dynamin fission machine.
INTRODUCTION
Dynamin is a mechanochemical GTPase that assembles around the necks of invaginated clathrin-coated pits to catalyze membrane fission during the final stages of clathrin-mediated endocytosis (CME) (Ferguson and De Camilli, 2012; Schmid and Frolov, 2011; van der Bliek and Payne, 2010). Dynamin consists of a catalytic G domain connected to a membrane-binding pleckstrin homology (PH) domain through a helical stalk (middle domain and GTPase effector domain, GED) (Figure 1A and B) (Faelber et al., 2011; Ford et al., 2011). At the C-teminus a proline- and arginine-rich domain (PRD) is present through which dynamin partners interact. In this arrangement, the GED's C-terminus (CGED) associates with the N- and C-termini of the G domain (NGTPase and CGTPase, respectively) to form the bundle signaling element (BSE) (Chappie et al., 2009) (Figure 1A and 1B, cyan). Structural analysis of a minimal G domain-GED fusion protein (GG) revealed that G domain dimerization optimally positions dynamin's catalytic machinery, leading to enhanced catalytic turnover (Chappie et al., 2010). In addition, the BSE undergoes a dramatic hydrolysis-dependent conformational change that may function as a dynamin powerstroke (Chappie et al., 2011). G domain dimerization is an intermolecular interaction and only occurs between tetramers in adjacent rungs of the helical assembly (Chappie et al., 2011; Faelber et al., 2011; Ford et al., 2011). Thus, the architecture of the dynamin polymer ensures that assembly and stimulated turnover are tightly coupled.
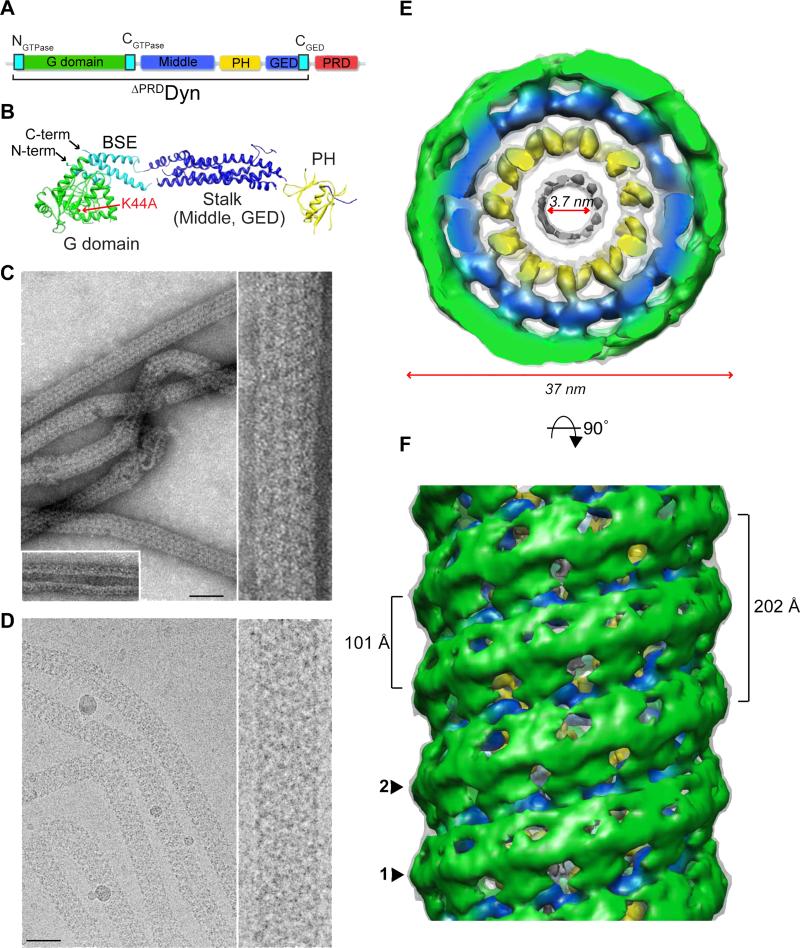
Figure 1. Three-dimensional reconstruction of super-constricted K44ADynGTP lipid tubes.
(A) Domain structure of dynamin, illustrating the conserved domains; G domain (green), middle domain (blue), pleckstrin homology domain (PH, yellow), GTPase effector domain (GED, blue) and the proline- and arginine-rich domain (PRD, red). The G domain N- and C-termini (NGTPase, CGTPase) and GED C-terminus (CGED) assemble into the bundle-signaling element (BSE) (cyan). (B) Crystal structure of nucleotide-free human dynamin 1 (Faelber et al., 2011) (PDB: 3SNH) showing the organization of the protein with an alternative PH domain assignment (Chappie and Dyda, 2013). The position of the K44A single mutation in the G domain (red arrow), the assembled BSE (cyan) and the N- and C-termini are labeled. (C, D) Negative-stain (C) and cryo (D) electron microscopy images of well-ordered K44ADynGTP tubes at low (left) and high (right) magnifications. Inset in C shows K44ADyn tubes in the absence of GTP. Scale bars, 50 nm. (E) Top view of the K44ADynGTP 3D density map. The map is subdivided into three radial densities colored green, blue and yellow. The outer diameter is 37 nm, while the inner lumen is 3.7 nm. (F) Side view of the K44ADynGTP 3D map shows how K44ADynGTP assembles as a 2-start helix labeled 1 and 2 with a helical pitch of 202 Å and an axial distance between neighboring subunits of 101 Å.
It remains unclear how the GTP hydrolysis cycle of dynamin translates to structural changes to promote membrane fission. Dynamin generates high membrane curvature and imposes localized strain on the inner monolayer of the membrane when assembled (Bashkirov et al., 2008; Roux et al., 2010; Schmid and Frolov, 2011). This asymmetric distribution of membrane stress has been predicted to promote a hemi-fission intermediate if the inner luminal diameter of the neck approaches the bilayer thickness (~4 nm) (Kozlovsky and Kozlov, 2003). Therefore, in the absence of physical constraints, an inner lumen of 4 nm could lead to spontaneous membrane fission (Bashkirov et al., 2008; Kozlovsky and Kozlov, 2003). Previous studies have visualized intermediates along the fission pathway. Wild-type dynamin (WTDyn) in the absence of nucleotide and truncated dynamin in the presence of GMPPCP (ΔPRDDynGMPPCP) form protein-lipid tubes with inner luminal diameters of 20 nm and 7 nm respectively (Chappie et al., 2011; Sweitzer and Hinshaw, 1998). The structure of the final pre-fission state where the inner lumen reaches 4 nm and the conformational changes necessary to achieve it remain a mystery.
Recent findings suggest that structural rearrangements in the dynamin polymer associated with the transition-state may serve as a key determinant of productive fission (Schmid and Frolov, 2011; Chappie and Dyda, 2013). To examine these structural changes and understand how they contribute to membrane fission, we solved the structure of a transition-state-defective human dynamin 1 mutant (K44ADyn). This structure displays a 2-start helical symmetry and is tightly constricted with an inner luminal diameter of 3.7 nm, reaching the theoretical limit for spontaneous fission. Computational docking reveals that a ground state conformation of the hydrolysis reaction is sufficient to achieve this final ‘super-constricted’ state and shows how the 2-start helical arrangement generates the most efficient packing of dynamin tetramers around the membrane neck. Together these data support a model for dynamin-catalyzed membrane fission that requires a transition-state-dependent conformational change to proceed successfully.
RESULTS
K44A dynamin tubes constrict to 3.7 nm in the presence of GTP
K44ADyn elicits a strong dominant negative effect in vivo that blocks CME and impairs the assembly-stimulated GTPase activity of dynamin in vitro (Damke et al., 1994). K44ADyn retains the ability to tubulate liposomes (Figure 1C and 1D) making it an ideal candidate for examining nucleotide-dependent conformations of the assembled polymer. Like WTDyn, K44ADyn generates protein-lipid tubes with an inner luminal diameter of ~20 nm in the absence of nucleotide (Figure 1C, inset). Addition of GTP to K44ADyn tubes (K44ADynGTP) induces a constriction far beyond what has previously been reported for other dynamin variants (Figure 1C and 1D). Sedimentation assays indicate that K44ADyn super-constricted tubes are stable in the presence of GTP whereas WTDyn disassociates from the lipid rapidly (Figure S1A) (Warnock et al., 1996). WTDyn also forms super-constricted tubes in the presence of GTP though they are short-lived and less stable (Figures S1B). Tilt series of dynamin tubes (K44ADyn , ΔPRDDynGTP, ΔPRD DynGMPPCP, WTDynGTP) revealed them all to be right-handed (Figure S1C; Chappie et al., 2011; Chen et al., 2004; Zhang and Hinshaw, 2001), suggesting this property is inherent to the assembled dynamin oligomer and does not change as a consequence of super-constriction.
The prolonged stability of K44ADynGTP tubes allowed us to solve its structure by cryo-EM (Figure 1D). The 3D map was calculated using the iterative helical real-space method (IHRSR) (Egelman, 2007) to yield a 12.5 Å reconstruction (Figures 1E and 1F) with 11.8 subunits per turn (Table S1). The map was generated from tube segments with outer diameters of 35-36 nm and with an even projection distribution (Figures S1D and S1E). As with previous reconstructions (Chappie et al., 2011; Chen et al., 2004; Zhang and Hinshaw, 2001), the K44ADynGTP polymer contains three radial densities (Figures 1E and 2): an inner density embedded in the outer leaflet of the lipid bilayer (yellow); a middle density that stabilizes the helical packing (blue); and an outer density that provides connectivity between the rungs of the helix (green). This new map, however, presents two unique features. First, K44ADyn assembles into a 2-start helix in the presence of GTP, whereas all previous reconstructions display a 1-start helical symmetry (Figure 1F, labeled 1 and 2). The difference between the 1-start and 2-start helix is evident from the Fourier transforms of the helical tubes (Figure S2A, dotted lines). The 2-start helix effectively doubles the helical pitch to 202 Å (Figures 1F and Table S1) without altering the lateral packing distance between subunits along the helical path (~100 Å) (Figure 2, section 4 and Table S1) or the distance between neighboring rungs (~100 Å) (Figure 2, section 3 and Table S1). Second, the K44ADyn polymer has an outer diameter of 37 nm and an inner luminal diameter of 3.7 nm (Figure 1E and Table S1). WTDynGTP tubes also display a 2-start helical symmetry and constrict to ~4-6 nm, albeit transiently (Figure S2B).
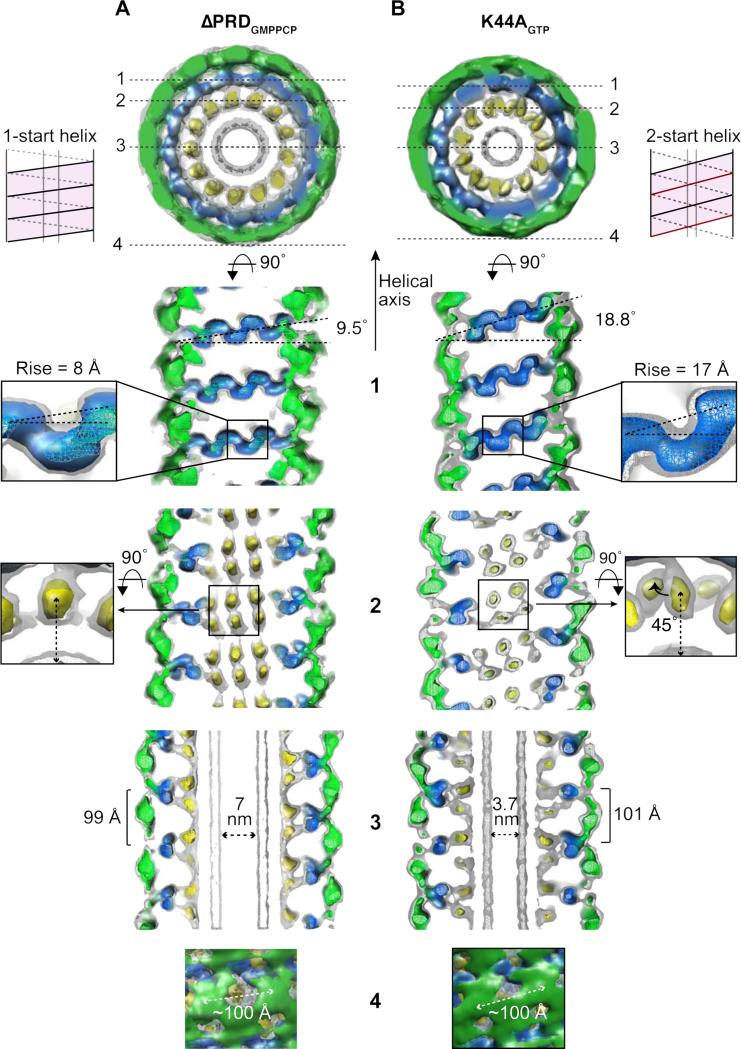
Figure 2. Structural changes that promote super-constriction.
Sections (1-4) through the ΔPRDDynGMPPCP (A) and K44ADynGTP (B) 3D maps, as marked in the top view, are shown in greater detail below. Schematic diagrams (pink) illustrate the respective 1-start (ΔPRDGMPPCP) and 2-start (K44AGTP) helical symmetries. (1) The rise per subunit of the helix differs from 8 Å (A) to 17 Å (B) (insets). The angle perpendicular to the tube axis increases from 9.5° (A) to 18.8° (B). (2) In the inner radial region of K44ADynGTP, one density of the PH domain pair is tilted 45° out of the membrane (insets). (3) The inner luminal diameter is significantly smaller in the K44ADyn tubes, 3.7 nm, compared to ΔPRDDynGMPPCP tubes, 7 nm. The axial distance between neighboring subunits is slightly larger in K44ADynGTP tubes, 101 Å compared to 99 Å. (4) The lateral packing (~100 Å) between the subunits remains constant.
Structural changes that promote super-constriction
To identify architectural changes leading to super-constriction, we compared our K44ADynGTP 3D map to ‘constricted’ ΔPRDDyn tubes stabilized by GMPPCP (Chappie et al., 2011). ΔPRDDynGMPPCP tubes form a 1-start helix with an inner luminal diameter of 7 nm, an outer diameter of 40 nm, with 13.2 subunits per turn and a pitch of 99 Å (Table S1). Juxtaposition of K44ADynGTP and ΔPRDDynGMPPCP shows that formation of a 2-start helix in K44ADynGTP is driven by an increase in the subunit rise from ~8 Å to ~17 Å (Figure 2, section 1, insets and Table S1). This reduces the number of subunits per turn without significantly altering the distance between subunits (Table S1). Instead, the entire stalk density of K44ADynGTP within each rung tilts ~19° relative to the helical axis (Figures 2B, section 1, blue and S2C, Leg), which causes a displacement of the inner leg densities in the same direction (Figure 2B, section 2, yellow). In addition, one leg density within a pair rotates 45° out of the plane of the membrane (Figure 2B, section 2, inset).
K44ADynGTP super-constricted tubes are trapped in a ground state conformation
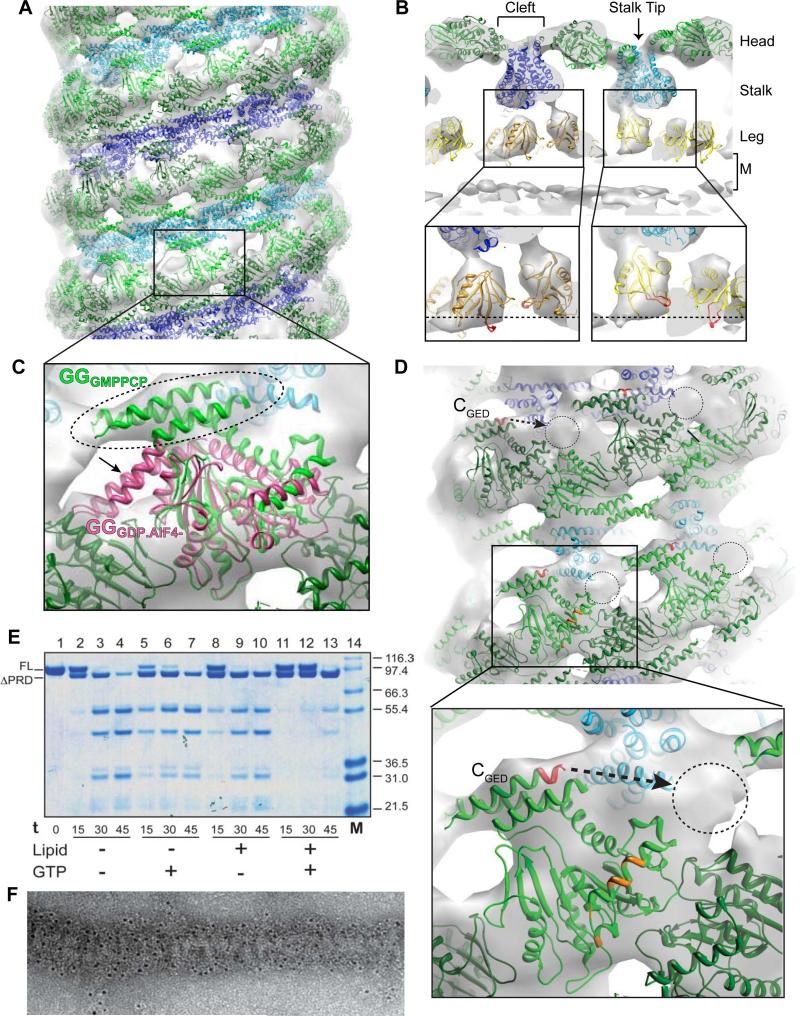
To describe the molecular changes that occur within individual dynamin subunits of the super-constricted K44ADynGTP map, we performed computational docking using the YUP software package (Tan et al., 2008) to generate a pseudo-atomic model of the K44ADynGTP polymer (Figure 3). The cryo-EM density could not accommodate the available intact dynamin crystal structures (Faelber et al., 2011; Ford et al., 2011) (data not shown) and thus individual dynamin domain structures were positioned using a combination of manual and flexible fitting procedures (Mears et al., 2007; Tan et al., 2008). As with previous reports (Chappie et al., 2011; Faelber et al., 2011; Ford et al., 2011; Mears et al., 2007) the G domain and BSE fit into the outer head density (green), the middle domain and GED fit into the stalk density (blue), and the PH domain occupies the leg density (yellow) (Figures 3A and 3B). Half of the PH domains are tilted such that variable loop 1 (Figure 3C, inset, colored red), which normally penetrates the outer leaflet of the bilayer (Burger et al., 2000; Ramachandran and Schmid, 2008) is partially pulled out, weakening dynamin's association with the membrane (Figure 3B, insets, dashed lines estimate lipid bilayer boundary). The PH domains disrupt the outer leaflet of the bilayer, leading to a less defined density for the outer leaflet.
Figure 3. Pseudo-atomic model of the K44ADynGTP polymer.
(A) Side view of K44ADynGTP 3D map showing docked GG dimers (PDB: 3ZYC, green), stalks (truncated from PDB: 3SNH, blue and cyan), and the PH domains (PDB: 1DYN, yellow). (B) Cross-section ‘T-view’ of the 3D map with the three radial densities (Head, Stalk and Leg) marked and the membrane labeled M. The cleft between the G domains and the stalk tip are also noted. PH domains are positioned with variable loop 1 (colored red) either inserted into the outer leaflet or tilted away from the membrane by 45° (insets). Dashed lines shows presumed position of the outer boundary of the lipid bilayer (insets). (C) The GGGMPPCP (PDB: 3ZYC; green) crystal structure fits well into the 3D map with the BSE in the upward configuration (dashed oval) while the GGGDP/AlF- BSE (magenta; PDB: 2X2E) extends out of the density (dashed arrow). (D) Side views of the K44ADynGTP docking reveals an empty density (dashed circles) that may be occupied by the PRD. The relative position of GED's C-terminus (red) and proximity (dashed arrow) is shown in the inset. The putative PRD site (dashed circle) is also close to a conserved, surface-accessible region in the G domain (inset, orange). (E) Limited proteolysis of K44ADyn (FL indicates full-length) by trypsin over time (0-45 min) in the absence (lanes 2-7) and presence (lanes 8-13) of lipid, and with (lanes 5-7; 11-13) or without GTP (lanes 2-4; 8-10). The assembled state of K44ADynGTP protects the protein from trypsin digest with the exception of the PRD (ΔPRD; lanes 11-13). (F) Immunogold labeling of K44ADynGTP lipid tubes using a PRD specific antibody specifically labeled the outer surface of K44ADynGTP lipid tubes.
Multiple crystallographic models are available for dynamin in different nucleotide-bound states (Chappie et al., 2010; Chappie et al., 2011; Faelber et al., 2011; Ford et al., 2011). These differ in the position of the BSE relative to the G domain core, with the helix bundle up in the nucleotide-loaded, pre-hydrolysis ground state and down in both the transition- and nucleotide-free states, assumed to represent a post-hydrolysis state (Chappie and Dyda, 2013). Only the GMPPCP-bound conformation of the GG structure fits into our K44ADynGTP map (Figure 3C, green). The BSE of GGGPD.AlF4- protrudes from the density (Figure 3C, magenta), suggesting that the individual subunits within super-constricted K44ADynGTP tubes adopt a ground state-like conformation. This is consistent with the K44 side chain being one of three critical catalytic components responsible for stabilizing dynamin's transition-state (Chappie et al., 2011) (Figure S3A and S3B) and with our findings that mutating it in GG abolishes dimerization and catalytic activity (Figures S3C and S3D).
Putative location of the PRD in theK44ADynGTP 3D map
Docking revealed a strong unoccupied density on the outer surface of the K44ADynGTP tubes that cannot be accounted for with any of existing dynamin crystal structures (Figures 3D). By way of exclusion and considering its proximity to the CGED (Figure 3D, dashed arrow), we interpret this density to be a portion of the flexible PRD. The following results support such placement of the PRD: i) limited proteolysis indicates the PRD is exposed and cleavable in super-constricted K44ADynGTP tubes (Figure 3E, compare lanes 8-10 to 11-13) and ii) an antibody against the PRD specifically labels the outer surface of constricted tubes (3±0.08 gold particles/μm2 tube versus 0.66±0.048 gold particles/μm2 background, average±SEM, n=97, p<0.001) (Figure 3F). Little proteolysis is observed during the 30-min incubation with GTP, confirming that K44ADyn is protected in the super-constricted state and does not disassociate and rebind to the lipid (Figure 3E, compare lanes 6 and 12). The putative position of the PRD is close to a conserved surface-accessible region of the G domain (Figure 3D, inset, orange) found only in dynamin and not in other dynamin-related proteins that lack a PRD.
The location and accessibility of the PRD may allow partners to modulate function even in the final stages of CME. To examine this, we superimposed the endophilin cryo-EM map (EMD-5367, Mim et al., 2012) with our K44A super-constricted map and measured the distance between the presumed positions of the PRD and the endophilin SH3 domain (Figure S4A). These segments are separated by ~8 nm. Biochemical mapping indicates that there are ~28 amino acids (residues 750 and 778 in human dynamin 1) between the end of the GED and the endophilin's binding motif in the PRD (Anggono et al., 2007). This region is predicted to be unstructured (Figure S4B) and as such could span over 10.4 nm. Thus it is conceivable that the two regions could physically interact within the constraints of the super-constricted state.
A 2-start helical symmetry generates a preferred packing of dynamin tetramers
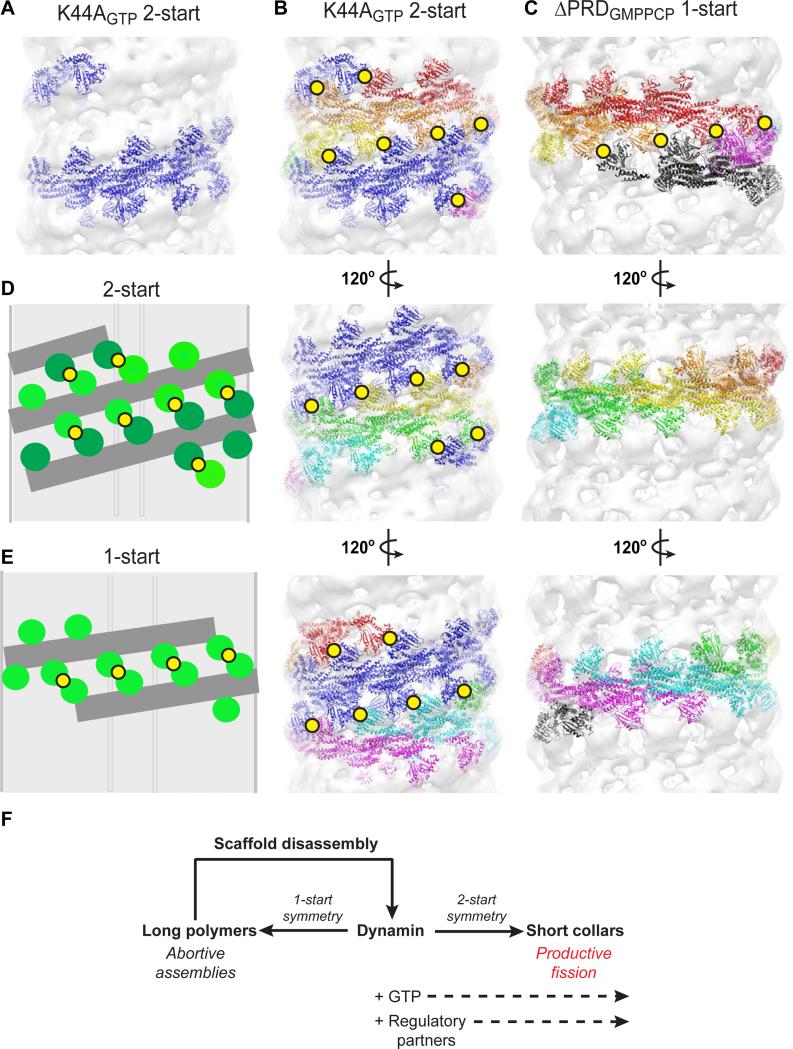
To determine how the 2-start symmetry of the super-constricted state impacts G domain dimerization, we colored the individual docked structures in the K44ADynGTP map according to the presumed boundaries of the underlying tetramers (the building block of dynamin) and examined their arrangement within a single helical turn (Figure 4). While only six tetramers are required for one turn of the K44ADynGTP helix, the increased pitch prevents G domain dimerization from occurring within a single strand (Figure 4A) as occurs in the 1-start ΔPRDDynGMPPCP polymer (Figure 4C). Instead, the 2-start symmetry dictates that the G domain dimers form between the two helical strands of the K44ADynGTP assembly and generates a total of 19 such parings that are distributed equally around the neck (Figure 4B and 4D). A single turn of the 1-start ΔPRDDynGMPPCP helix forms only 4 G domain dimers that are asymmetrically localized on one side of the tube (Figure 4C and 4E). Each dimer buries a surface area of 2,561Å2 (Chappie et al., 2010), representing a significant free energy gain and additional physical association that could stabilize the assembly further in the longitudinal direction.
Figure 4. A 2-start helical symmetry generates efficient packing of dynamin tetramers.
(A) Illustration of a single strand of the K44ADynGTP 2-start helix showing that G domain dimerization does not occur in a single strand wrapping around the membrane. (B, C) Side-by-side comparison of a single turn of the K44ADynGTP 2-start polymer (B) and a single turn of the δPRDDynGMPPCP 1-start polymer (C). Three rotations of each helix are shown (120 degrees each). In the K44ADynGTP polymer, one strand is colored blue and the other is colored rainbow. Yellow circles denote G domain dimers formed in each assembly. (D, E) Cartoon illustration of the subunits organization in a 2-start (D) and 1-start (E) helix. (F) Assembly of short dynamin collars with a 2-start helical symmetry facilitate membrane fission. Long dynamin polymers assembled in the absence of GTP are abortive and unable to efficiently mediate fission.
Structural superposition shows only minor differences between the docked K44ADyn (green) and ΔPRDDyn (blue) tetramers (Figure S4C), indicating that the overall domain organization and intramolecular interactions are preserved. The changes in packing instead appear to arise from different intermolecular interactions between tetramers, which cause them to slide and twist as rigid bodies to accommodate the helical expansion and radial compression associated with the super-constricted state (Figure S4D, dashed circles).
DISCUSSION
Dynamin family GTPases mediate numerous membrane fission and fusion events in the cell (Heymann and Hinshaw, 2009). In vitro studies have shown that dynamin alone can cause membrane fission in the presence of GTP (Bashkirov et al., 2008; Morlot et al., 2012; Pucadyil and Schmid, 2008) and have suggested that a transition-state of dynamin's GTP hydrolysis reaction may be the key determinant of fission (Shnyrova et al., 2013). Here we have structurally characterized a transition-state-defective dynamin mutant (K44ADyn) that consequently becomes trapped in a pre-fission complex. K44ADynGTP helical tubes adopt a super-constricted conformation with an inner luminal diameter of only 3.7 nm. Similar results were observed with WTDynGTP, albeit transiently. Super-constriction is achieved by dynamin adopting a 2-start helical organization, which has not been observed in any previous reconstructions of dynamin polymers.
A 2-start helical assembly offers several advantages for efficient membrane fission. First, the rise between subunits in the 2-start helix is doubled, allowing for constriction to 3.7 nm without major changes in the basic building block of the polymer. Second, the 2-start symmetry allows G domain dimers to form between the adjacent helical strands concomitant with polymer assembly. This maximizes the number of G domain dimers that can be formed within a single helical turn and ensures even distribution of these dimers around the membrane neck, effectively priming the system for coordinated stimulated GTP hydrolysis. The dynamin-related protein DNM1, which catalyzes mitochondrial fission in yeast, also adopts a 2-start helical arrangement on membranes (Mears et al., 2011). How conserved this architecture is among other dynamin family members remains to be seen.
Computational docking shows that dynamin tetramers in the super-constricted state are stabilized in a ground state conformation. This implies that constriction to the fission limit occurs without input from stimulated GTP hydrolysis and that subsequent fission requires progression through the transition-state. Docking also identified an empty density on the surface of the K44ADynGTP polymer that we predict represents the PRD. Localization of the PRD in this region allows for dynamin partners to bind throughout the entire endocytic process. These partners may facilitate dynamin assembly and the efficiency of a minimal fission complex. For example, endophilin has been shown to regulate dynamin assembly during synaptic vesicle recycling and may prime dynamin to assemble into a 2-start helix at the necks of coated-pits by increasing the pitch of the dynamin helix from 10-20 nm (Sundborger et al., 2011).
Our pseudo-atomic model also shows that one of the PH domains in the asymmetric unit of the K44ADynGTP map is tilted 45° perpendicular to the membrane axis, consistent with the concept that the PH domain undergoes a conformational change during fission. PH domain insertion into the plasma membrane is crucial for endocytosis as mutants lacking this ability are dominant negative (Ramachandran et al., 2009). The PH domain can also actively destabilize the lipid bilayer, possibly promoting the fission reaction (Bethoney et al., 2009; Ramachandran and Schmid, 2008; Shnyrova et al., 2013). We suggest that the PH domain rearrangement we observe in the super-constricted state represents either the initiation of the PH domain ‘tilting’ proposed to evoke fission (Shnyrova et al., 2013) or the observed ‘retracted’ state of the PH domain proposed to keep dynamin on the membrane surface during the GTP hydrolysis cycle (Mehrotra et al, 2014).
Recent studies estimate that the minimal fission machine consists of two turns of a 1-start dynamin helix (Liu et al., 2013; Shnyrova et al., 2013). Based on our findings, we interpret the optimal minimal machinery to be a single turn of a 2-start dynamin helix. We anticipate that the constant presence of GTP in the cell will bias dynamin toward the formation of short 2-start helices (Figure 4F). Supporting this we show that in vitro both WTDynGTP and K44AGTP DynGTP assemble only into 2-start helices. The 1-start and 2-start helical symmetries are discrete structural states and likely do not interconvert without complete disassembly of one scaffold and reassembly with the alternative symmetry. Therefore, the long 1-start helices observed in vitro (Chappie et al., 2011, Chen et al., 2004, Zhang and Hinshaw, 2001) might not represent ‘true’ fission intermediates. This distinction provides an explanation for in vitro observations that long dynamin assemblies inhibit fission and must undergo cycles of GTP hydrolysis to reach a critical minimal length to be productive (Bashkirov et al., 2008; Pucadyil and Schmid, 2008). These changes could be interpreted to represent a symmetry switch to the more efficient 2-start packing (Figure 4F).
The K44ADynGTP structure presented here provides new insights into the mechanisms of dynamin-catalyzed membrane fission during CME (Graphical Abstract). In the cell, assembly of dynamin in the presence of GTP promotes the formation of a 2-start helix at the necks of coated pits that can reach the 4 nm fission barrier. The 2-start helical symmetry also provides the optimal minimal configuration for G domain dimerization. G domain dimerization induces stimulated GTP hydrolysis and progression through the transition-state, which serves as the key determinant of productive fission and leads to conformational changes that promote the physical scission event.
Though our findings shed light on the final pre-fission state of the dynamin polymer, the precise transition-state-dependent conformational changes within this assembly that trigger fission still remains to be elucidated. Previous structural studies revealed a dynamin powerstroke consisting of a hydrolysis-dependent BSE conformational change (Chappie et al., 2011). How the powerstroke propagates through the rest of the dynamin polymer and whether it is sufficient for the GTP induced supercoiling and fragmentation observed in vitro remains to be determined (Danino et al., 2004; Roux et al., 2006). Moreover, biochemical studies suggest fission may be triggered by more subtle GTP-induced membrane remodeling events such as scaffold loosening and/or disassembly (Bashkirov et al., 2008; Pucadyil and Schmid, 2008), curvature-dependent changes in membrane elastic energy at the edge of the dynamin polymer (Morlot et al., 2012), or PH domain tilting within short meta-stable dynamin scaffolds (Shnyrova et al., 2013). These mechanisms are likely not mutually exclusive and dynamin may use a combination of both mechanical force and membrane remodeling to drive fission. Future structural and biophysical studies will assist in unraveling the global conformational changes that are needed to move beyond the final super-constricted pre-fission state.
EXPERIMENTAL PROCEDURES
Cryo-electron microscopy imaging
A 3.5 μl sample of K44ADynGTP lipid tubes was placed on a plasma-cleaned (Fishione Inc.) Quantifoil holey carbon EM grid (SPI Supplies), blotted with filter paper, and flash-frozen in liquid ethane using a Leica EM GP (Leica Microsystems). The vitrified samples were imaged at liquid nitrogen temperature on a Polara FEG electron microscope (FEI) operating at 200 kV and recorded at 49,000X magnification. Images were recorded on Kodak SO163 film under low-dose conditions with defocus values ranging from −0.5 to 2 μm. Images were digitized using a NIKON supercool 9000 scanner at 80 ppm, 2.55 Å/pixel.
3D reconstruction of K44ADynGTP lipid tubes
Images were individually CTF corrected using the bsoft image-processing package (bshow and bctf, Cs=2.26) (Heymann and Belnap, 2007). Well-ordered and straight K44ADynGTP tubes were manually selected and processed with Iterative Helical Real Space Reconstruction (IHRSR) methodology (Egelman, 2007) (see supplemental info). The final map merged to a rotation angle of 30.59° and rise of 17.19 Å. The resolution of the final map was determined to be 12.5 Å by Fourier shell correlation (FSC=0.5) and was calculated, using the new ‘gold standard’ (Henderson, 2013) from 3D maps generated from half of the data randomized with two different reference models (a cylinder and a modified ΔPRDDyn map) and two different starting parameters (rise and rotation).
Computational docking
All-atom structures were refined using the YUP.SCX method of the YUP software package (Tan et al., 2008). Initial fitting was performed using GGGMPPCP monomers (PDB: 3ZYC; Chappie et al., 2011), dynamin middle/GED stalk monomers excised from (PDB: 3SNH; Faelber et al., 2011), and PH domain monomers (PDB: 1DYN; Ferguson et al., 1994). Orientations of the middle/GED and PH monomers were largely unchanged compared to previous docked maps (Chappie et al., 2011; Faelber et al., 2011; Ford et al., 2011) and the GGGMPPCP placement refined to a single orientation that best matched the K44ADynGTP cryo-EM structure.
Limited Proteolysis
K44ADyn in HCB100 at 0.25 mg/ml was incubated with 1:500 w/w trypsin (Sigma Aldrich) at room temperature in the presence and absence of synthetic DOPS extruded liposomes, and in the presence and absence of 1 mM GTP. Small aliquots were taken at intervals of 15 min after addition of trypsin to examine the amount of proteolysis that occurred over the course of 45 min. Samples were run on an SDS-PAGE and visualized using Colloidal Blue (Invitrogen).
Immunogold Labeling
Immunogold labeling of K44ADynGTP lipid tubes was performed as previously described (Mears et al., 2007). Briefly, K44ADyn tubes were prepared as described above and incubated with 1 mM GTP for 30 minutes. The sample was applied to carbon-coated 400 mesh Cu/Rh grids for 1 min and subsequently blocked with HCB100 containing 1% BSA for 45 min. The grids were then incubated on a drop of Hudy-1 antibody (Upstate Biotech Inc.) for 1 hour and washed 6 x 5 min with HCB100. The grids were incubated with 6 nm colloidal gold rabbit anti-mouse secondary antibody for 1 hour followed by 6 × 5 min washes with HCB100. The grids were stained with 1% uranyl acetate for 30 sec. Images were taken on a CM120 transmission electron microscope (FEI Company) using low-dose conditions at 100 kV with a LaB6 filament. Images were recorded using a Gatan 1k × 1k CCD camera. Labeling density was quantified as number of gold particles in a 500-ìm2 area (gold particles/ìm2) and compared between dynamin lipid tubes, background and samples labeled with secondary antibody alone.
Supplementary Material
01
02
HIGHLIGHTS.
- Cryo-EM structure of a super-constricted dynamin polymer that reaches the fission limit
- Dynamin super-constriction occurs without GTP hydrolysis
- 2-start symmetry yields preferred packing of dynamin subunits on a membrane surface
- PRD localizes to the exterior of the super-constricted dynamin tube and is accessible
ACKOWLEDGEMENTS
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Intramural Research Program. Joshua S. Chappie is a Nancy and Peter Meinig Family Investigator in the Life Sciences. We thank Drs. Jeanne Morin-Leisk, Paula Flicker and Toshi Kawate for insightful discussions and Dr. Fred Dyda for his continued support and generosity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
A.C.S., J.A.H., P.R., and J.S.C. purified proteins and carried out biochemical assays. A.C.S. and J.E.H. collected EM data. S.F. and J.E.H. performed all image processing and computational docking, assisted by J.S.C. and A.C.S. A.C.S., J.S.C., and J.E.H. prepared the manuscript.
ACCESSION CODES The K44ADynGTP tube density map has been deposited in the EM Data Bank with accession code EMD-XXXX. Coordinates for the complete docked model consisting of GGGMPPCP, the human dynamin 1 middle/GED stalk, and human dynamin 1 PH domain have been deposited in the Protein Data Bank with accession code XXXX.
REFERENCES
- Anggono V, Robinson PJ. Syndapin I and endophilin I bind overlapping proline-rich regions of dynamin I: role in synaptic vesicle endocytosis. J Neurochem. 2007;102:931–943. doi: 10.1111/j.1471-4159.2007.04574.x. [DOI] [PubMed] [Google Scholar]
- Bashkirov PV, Akimov SA, Evseev AI, Schmid SL, Zimmerberg J, Frolov VA. GTPase cycle of dynamin is coupled to membrane squeeze and release, leading to spontaneous fission. Cell. 2008;135:1276–1286. doi: 10.1016/j.cell.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethoney KA, King MC, Hinshaw JE, Ostap EM, Lemmon MA. A possible effector role for the pleckstrin homology (PH) domain of dynamin. Proc Natl Acad Sci U S A. 2009;106:13359–13364. doi: 10.1073/pnas.0906945106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger KN, Demel RA, Schmid SL, de Kruijff B. Dynamin is membrane-active: lipid insertion is induced by phosphoinositides and phosphatidic acid. Biochemistry. 2000;39:12485–12493. doi: 10.1021/bi000971r. [DOI] [PubMed] [Google Scholar]
- Chappie JS, Acharya S, Leonard M, Schmid SL, Dyda F. G domain dimerization controls dynamin's assembly-stimulated GTPase activity. Nature. 2010;465:435–440. doi: 10.1038/nature09032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappie JS, Acharya S, Liu YW, Leonard M, Pucadyil TJ, Schmid SL. An intramolecular signaling element that modulates dynamin function in vitro and in vivo. Mol Biol Cell. 2009;20:3561–3571. doi: 10.1091/mbc.E09-04-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappie JS, Dyda F. Building a fission machine--structural insights into dynamin assembly and activation. J Cell Sci. 2013;126:2773–2784. doi: 10.1242/jcs.108845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappie JS, Mears JA, Fang S, Leonard M, Schmid SL, Milligan RA, Hinshaw JE, Dyda F. A pseudoatomic model of the dynamin polymer identifies a hydrolysis-dependent powerstroke. Cell. 2011;147:209–222. doi: 10.1016/j.cell.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Zhang P, Egelman EH, Hinshaw JE. The stalk region of dynamin drives the constriction of dynamin tubes. Nat Struct Mol Biol. 2004;11:574–575. doi: 10.1038/nsmb762. [DOI] [PubMed] [Google Scholar]
- Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danino D, Moon KH, Hinshaw JE. Rapid constriction of lipid bilayers by the mechanochemical enzyme dynamin. J Struct Biol. 2004;147:259–267. doi: 10.1016/j.jsb.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Egelman EH. The iterative helical real space reconstruction method: Surmounting the problems posed by real polymers. J Struct Biol. 2007;157:83–94. doi: 10.1016/j.jsb.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Faelber K, Posor Y, Gao S, Held M, Roske Y, Schulze D, Noé F, Daumke O. Crystal structure of nucleotide-free dynamin. Nature. 2011;477:556–560. doi: 10.1038/nature10369. [DOI] [PubMed] [Google Scholar]
- Ferguson KM, Lemmon MA, Schlessinger J, Sigler PB. Crystal structure at 2.2 A resolution of the pleckstrin homology domain from human dynamin. Cell. 1994;79:199–209. doi: 10.1016/0092-8674(94)90190-2. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, De Camilli P. Dynamin, a membrane-remodelling GTPase. Nat Rev Mol Cell Biol. 2012;13:75–88. doi: 10.1038/nrm3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MG, Jenni S, Nunnari J. The crystal structure of dynamin. Nature. 2011;477:561–566. doi: 10.1038/nature10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R. Avoiding the pitfalls of single particle cryo-electron microscopy: Einstein from noise. Proc Natl Acad Sci U S A. 2013;110:18037–18041. doi: 10.1073/pnas.1314449110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann JB, Belnap DM. Bsoft: image processing and molecular modeling for electron microscopy. J Struc Biol. 2007;157:3–18. doi: 10.1016/j.jsb.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Heymann JA, Hinshaw JE. Dynamins at a glance. J Cell Sci. 2009;122:3427–3431. doi: 10.1242/jcs.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlovsky Y, Kozlov MM. Membrane fission: model for intermediate structures. Biophys J. 2003;85:85–96. doi: 10.1016/S0006-3495(03)74457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YW, Mattila JP, Schmid SL. Dynamin-catalyzed membrane fission requires coordinated GTP hydrolysis. PLoS One. 2013;8:e55691. doi: 10.1371/journal.pone.0055691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears JA, Lackner LL, Fang S, Ingerman E, Nunnari J, Hinshaw JE. Conformational changes in Dnm1 support a contractile mechanism for mitochondrial fission. Nat Struct Mol Biol. 2011;18:20–26. doi: 10.1038/nsmb.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears JA, Ray P, Hinshaw JE. A corkscrew model for dynamin constriction. Structure. 2007;15:1190–1202. doi: 10.1016/j.str.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrotra N, Nichols J, Ramachandran R. Alternate pleckstrin homology domain orientations regulate dynamin-catalyzed membrane fission. Mol Biol Cell. 2014;25:879–890. doi: 10.1091/mbc.E13-09-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mim C, Cui H, Gawronsk-Salerno JA, Frost A, Voth GA, Unger VM. Structural basis of membrane bending by the N-BAR protein endophilin. Cell. 2012;149:137–145. doi: 10.1016/j.cell.2012.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlot S, Galli V, Klein M, Chiaruttini N, Manzi J, Humbert F, Dinis L, Lenz M, Cappello G, Roux A. Membrane shape at the edge of the dynamin helix sets location and duration of the fission reaction. Cell. 2012;151:619–629. doi: 10.1016/j.cell.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucadyil TJ, Schmid SL. Real-time visualization of dynamin catalyzed membrane fission and vesicle release. Cell. 2008;135:1263–1275. doi: 10.1016/j.cell.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Schmid SL. Real-time detection reveals that effectors couple dynamin's GTP-dependent conformational changes to the membrane. EMBO J. 2008;27:27–37. doi: 10.1038/sj.emboj.7601961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Pucadyil TJ, Liu YW, Acharya S, Leonard M, Lukiyanchuk V, Schmid SL. Membrane insertion of the pleckstrin homology domain variable loop 1 is critical for dynamin-catalyzed vesicle scission. Mol Biol Cell. 2009;20:4630–4639. doi: 10.1091/mbc.E09-08-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux A, Koster G, Lenz M, Sorre B, Manneville JB, Nassoy P, Bassereau P. Membrane curvature controls dynamin polymerization. Proc Natl Acad Sci U S A. 2010;107:4141–4146. doi: 10.1073/pnas.0913734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux A, Uyhazi K, Frost A, De Camilli P. GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature. 2006;441:528–531. doi: 10.1038/nature04718. [DOI] [PubMed] [Google Scholar]
- Schmid SL, Frolov VA. Dynamin: Functional Design of a Membrane Fission Catalyst. Annu Rev Cell Dev Biol. 2011;27:79–105. doi: 10.1146/annurev-cellbio-100109-104016. [DOI] [PubMed] [Google Scholar]
- Shnyrova AV, Bashkirov PV, Akimov SA, Pucadyil TJ, Zimmerberg J, Schmid SL, Frolov V. Geometric catalysis of membrane fission driven by flexible dynamin rings. Science. 2013;339:1433–1436. doi: 10.1126/science.1233920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundborger A, Soderblom C, Vorontsova O, Evergren E, Hinshaw JE, Shupliakov O. An endophilin-dynamin complex promotes budding of clathrin-coated vesicles during synaptic vesicle recycling. J Cell Sci. 2011;124:133–143. doi: 10.1242/jcs.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer SM, Hinshaw JE. Dynamin undergoes a GTP-dependent conformational change causing vesiculation. Cell. 1998;93:1021–1029. doi: 10.1016/s0092-8674(00)81207-6. [DOI] [PubMed] [Google Scholar]
- Tan RK, Devkota B, Harvey SC. YUP.SCX: coaxing atomic models into medium resolution electron density maps. J Struct Biol. 2008;163:163–174. doi: 10.1016/j.jsb.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Bliek AM, Payne GS. Dynamin subunit interactions revealed. Dev Cell. 2010;18:687–688. doi: 10.1016/j.devcel.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnock DE, Hinshaw JE, Schmid SL. Dynamin self-assembly stimulates its GTPase activity. J Biol Chem. 1996;271:22310–22314. doi: 10.1074/jbc.271.37.22310. [DOI] [PubMed] [Google Scholar]
- Zhang P, Hinshaw JE. Three-dimensional reconstruction of dynamin in the constricted state. Nat Cell Biol. 2001;3:922–926. doi: 10.1038/ncb1001-922. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01
02