Genome-Wide Analysis of Polymorphisms Associated with Cytokine Responses in Smallpox Vaccine Recipients (original) (raw)
. Author manuscript; available in PMC: 2014 Sep 22.
Published in final edited form as: Hum Genet. 2012 May 19;131(9):1403–1421. doi: 10.1007/s00439-012-1174-2
Abstract
The role that genetics plays in response to infection or disease is becoming increasingly clear as we learn more about immunogenetics and host-pathogen interactions. Here we report a genome-wide analysis of the effects of host genetic variation on cytokine responses to vaccinia virus stimulation in smallpox vaccine recipients. Our data show that vaccinia stimulation of immune individuals results in secretion of inflammatory and Th1 cytokines. We identified multiple SNPs significantly associated with variations in cytokine secretion. These SNPs are found in genes with known immune function, as well as in genes encoding for proteins involved in signal transduction, cytoskeleton, membrane channels and ion transport, as well as others with no previously identified connection to immune responses. The large number of significant SNP associations implies that cytokine secretion in response to vaccinia virus is a complex process controlled by multiple genes and gene families. Follow-up studies to replicate these findings and then pursue mechanistic studies will provide a greater understanding of how genetic variation influences vaccine responses.
Keywords: smallpox vaccine, vaccinia, GWAS, genome-wide association, SNP, immune response, cytokines
Introduction
Smallpox, a deadly disease caused by variola virus, plagued mankind until a decades-long effort by the World Health Organization led to its eradication in 1980 (Fenner 1988). Edward Jenner pioneered the use of cross-protective poxviruses as safe and effective vaccines (Jenner 1798). This work led to the eventual development of the vaccinia-based vaccines used successfully in the eradication effort. These live viral vaccines induced high levels of humoral and cell-mediated immunity in vaccinated subjects (Combadiere et al. 2004; Crotty et al. 2003; Frey et al. 2002; Hammarlund et al. 2003; Kennedy et al. 2009b), but also caused adverse events, some of which were life-threatening (Fulginiti 2003; Fulginiti et al. 2003), and resulted in the cessation of routine vaccination shortly after endemic smallpox was eradicated. A large percentage of the current U.S. population have conditions that contraindicate receipt of the vaccine (immunosuppression due to: cancer, organ transplants, HIV infection, heart conditions, and skin diseases such as eczema or psoriasis)(Fenner 1989). Altered cytokine responses to vaccinia inoculation in individuals with atopic dermatis are believed to be responsible for the higher incidence of eczema vaccinatum (Grigoryev et al. 2010; Howell et al. 2006; Scott et al. 2007). Furthermore, Th2 responses are correlated with impaired viral clearance,(Freyschmidt et al. 2007) while animal models where expression of key cytokines are intentionally over- or under-expressed clearly indicate that cytokine production in response to poxvirus infection or inoculation can greatly influence the course of the viral infection (Foong et al. 2009; Kohyama et al. 2007; Sharma et al. 1996; Tian et al. 2009; van Den Broek et al. 2000). Genetic variations can have a profound influence on disease susceptibility, progression, and resolution, as well as on vaccine-induced immune responses. Here we present data on genome-wide SNPs associated with variations in cytokine responses in a well-characterized cohort of over 1,000 healthy adult recipients of the smallpox vaccine. Identifying the genetic elements controlling cytokine secretion in response to viral infection or vaccination will assist in creation of next-generation vaccines that elicit immune responses with the optimal Th1/Th2 balance as well as other cytokines (Th17, inflammatory cytokines) necessary for robust immune protection.
Materials and Methods
Subject Recruitment
Study subjects were recruited from military personnel who were recent recipients of a single dose of the Dryvax® smallpox vaccine and from civilian healthcare workers who participated in the civilian smallpox immunization program at the Mayo Clinic in Rochester, MN. Military personnel were recruited from the Naval Health Research Center (NHRC) in San Diego, CA (Kennedy et al. 2009a). All participants were in good general health, had received one, and only one, dose of the smallpox vaccine within the last four years and had a documented vaccine “take”, indicating successful immunization. Approval from the Institutional Review Boards of both the Mayo Clinic and NHRC was obtained, as was written informed consent from each subject prior to all study procedures.
Isolation of peripheral blood mononuclear cells (PBMC)
Each subject underwent a single blood draw of approximately 100 mL, with blood collected in heparinized tubes and shipped overnight at room temperature. PBMCs from each subject were isolated within 24 hours of blood draw using Accuspin (Sigma, St. Louis, MO) tubes containing HISTOPAQUE®-1077 (Sigma) according to established procedures. Isolated PBMCs (1 × 107 cells/mL) were resuspended in culture medium supplemented with 10% dimethyl sulfoxide (Protide Pharmaceuticals, St. Paul, MN) and 20% fetal bovine serum (FBS; Hyclone, Logan, UT), frozen for 18hrs in controlled-rate freezing containers, and in liquid nitrogen for long-term storage (Ryan et al. 2009).
SNP typing and QC
The Gentra Puregene Blood kit (Gentra Systems Inc., Minneapolis, MN) was used to extract DNA from biospecimens prior to quantification by Picogreen (Molecular Probes, Carlsbad, CA). Genome-wide SNP typing was performed using the Infinium HumanHap550 or HumanHap650Y BeadChip arrays for the Caucasian and African-American subjects, respectively. After whole genome amplification, fragmentation and hybridization, samples were imaged on an Illumina BeadArray reader. The genotyping module of BeadStudio 2 was used to make the clustering and genotyping calls, which were then transferred to a SAS database for later analyses. Quality control checks included: genotyping reproducibility using paired samples, removal of monomorphic SNPs, call rate cutoffs of < 95% for both individual SNPs and subjects, and a check for Hardy-Weinberg Equilibrium (HWE). As subjects from two races were genotyped, tests for deviation from HWE were performed in a race-stratified fashion similar to the exact test of Schaid, et al (Schaid et al. 2006).
ELISA Assays
Frozen PBMC aliquots were recovered as previously described (Ovsyannikova et al. 2005; Ryan et al. 2009). Briefly, cells were thawed and resuspended in culture medium supplemented with 50IU/ml of IL-2 (Proleukin®, Chiron, Emeryville, CA) overnight. After washing, cells were resuspended at a concentration of 2 × 106 cells/mL for use in the cytokine secretion assays.
Vaccinia virus (NYCBOH) was grown and titered according to established procedures (Earl et al. 2001; Kennedy et al. 2009a). A single viral stock was prepared for all assays and was inactivated using psoralen and UV light in order to minimize viral modulation of immune responses (Ryan et al. 2009).
2 × 105 PBMCs were plated in each well of 96-well round bottom plates. Experimental conditions included: a single well containing PHA (5ug/ml) as a positive control, triplicate wells containing culture medium (unstimulated wells), and triplicate wells with vaccinia virus (stimulated wells). Cytokine-specific vaccinia stimulation was optimized based on multiplicity of infection (MOI) and length of time in culture as previously described (Ryan et al. 2009) and is as follows: IFNβ, IL-2, IL-18: MOI=5, 24 hours; IL-12p40, IL-12p70, TNFα, IL-1β: MOI 0.5, 24 hours; IFNα: MOI=0.05, 4 days; IL-4, IL-10: MOI=0.05, 7 days; IL-6: MOI=5, 8 days.
Cytokine levels in culture supernatant were detected using commercial ELISA-based kits for IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12p40, IL-12 p70, and TNF-α (all from BD Pharmingen), IFN-α (PBL Biomedical Laboratories, Piscataway, NJ), IFN-β (PBL Biomedical Laboratories) and IL-18 (MBL International, Woburn, MA). Optical density readings at 450 nm were converted to cytokine concentrations in pg/ml using the reference standards included in each assay.
Population stratification
As study participants were of more than one race, we selected 22,863 SNPs with >99% call rates that were spaced at approximately 100kb intervals and used these SNPs in the principal components (PC) implemented in the Eigenstrat software package to assess population structure (Price et al. 2006). Using the same genotype data, we employed a clustering procedure similar to what is implemented in the Structure software program (Pritchard et al. 2000) to assign Caucasian or African-American racial group membership to subjects who had not self-reported a conclusive racial declaration. After defining genetic racial groups, we extracted the first four Eigenstrat axes of variation (Price et al. 2006) within each group and used these as covariates in the race-specific analyses in order to account for residual population stratification.
Statistical analyses
These analytical methods are identical to those employed in our other genome-wide association study of smallpox vaccine response. Assessment of cytokines resulted in six recorded values: those from the three unstimulated and the three vaccinia stimulated wells. The median of the unstimulated wells was subtracted from the median value of the stimulated wells to create a summary measure of each cytokine secretion level for each individual.
Associations between the levels of each cytokine level and the SNP genotypes were assessed separately within the two racial groups using linear regression models. SNPs were modeled assuming an additive genotypic effect. Formal evaluations of significance utilized repeated measures analyses that used all of the multiple observations per subject while accounting for within-subject correlations using generalized estimating equations (GEEs). As there were measurements of cytokine levels in both the stimulated and unstimulated state for each individual, the primary test for genetic association between a SNP and the outcome of interest was a test for interaction between the ordinal SNP variable and a variable identifying whether the result arose from a stimulated or an unstimulated state. The tests of the significance of these interactions are similar to paired t-tests: both compare differences in the two stimulation states within an individual among genotype-defined groups of individuals. All analyses adjusted for gender; age at blood draw (quartiles); time from smallpox immunization to blood draw (quartiles); time from blood draw to assay (quartiles); shipping temperature of the sample (frozen or ambient); time of year when the sample was shipped (warm-weather months April-September vs. cold-weather months October-March); and the first four Eigenstrat axes of variation. An inverse cumulative normal (probit) transformation was used for the cytokine variables in order to correct for data skewness. We used q-q plots to compare the observed and expected distribution of p-values for a given outcome across all SNPs. Genomic control lambda values were calculated to assess, and correct for, any potential residual inflation of significance in the race-specific results. All statistical tests were two-sided, and were performed using the R software package (unless otherwise indicated) (Team 2008). Due to the existence of several significant SNPs for which few subjects had two copies of the minor allele, additional models were run to further assess the effect of the minor alleles. These sensitivity analyses involved grouping the homozygous minor subjects with the heterozygous subjects, resulting in a dominant rather than an ordinal model. The models were run with the same adjusting factors as the ordinal model.
Results
SNP Typing Results
Subjects were genotyped using the Illumina Infinium HumanHap550 (Caucasians) or the HumanHap 650Y (African-American) BeadChip arrays. The HumanHap550 is a subset of SNPs from the HumanHap650Y; the following summary is for all SNPs, with the understanding that some SNPs were only run for the African-American subjects. Overall genotype concordance was high (97.9% including missing genotypes, 99.9% after excluding missing genotypes). SNPs with call rates below 95% were excluded (7.3%), as were 71 subjects with call rates less than 95%. Additional exclusions involved removing SNPs with minor allele frequencies below 1% and those that appeared to be out of HWE (p<10−8). Of these 1,000 subjects, 580 formed the Caucasian racial cluster and 217 formed the African-American cluster. During analysis, SNPs with fewer than 10 observed minor alleles for a given outcome and racial cohort (Caucasian or African-American) were excluded from that outcome/race specific analysis.
Immune Outcomes
Cytokine responses for each subject were quantitated by ELISA assays and are outlined in Table 1. PBMCs from vaccine recipients exhibited a predominantly Th1 type response to viral stimulation characterized by robust secretion of IL-2, IL-12p40, TNFα, and IFNγ as well as high levels of proinflammatory cytokines IFNα, IL-1β, and IL-6 (Umlauf et al. 2011). In contrast, we saw low levels IL-10 and negligible amounts of IL-4, IL-12p70, IL-18, and IFNβ. Although we did find statistically significant associations for IL-4, IL-12p70, IL-18, and IFNβ the small differences in cytokine secretion are unlikely to be biologically meaningful, therefore results from these cytokines are not reported.
Table 1.
Cytokine Response to Vaccinia Stimulation in Smallpox Vaccinees.
| Caucasian Subjects | African-American Subjects | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cytokine | N | Mean | Std Dev | Median | IQR | N | Mean | Std Dev | Median | IQR |
| IL-1β | 450 | 113.6 | 209.0 | 55.6 | 28.1–148.3 | 185 | 100.7 | 145.1 | 46.9 | 24.9–102.2 |
| IL-2 | 420 | 28.3 | 38.0 | 18.7 | 5.0–39.6 | 178 | 20.1 | 33.4 | 15.0 | 1.2–33.6 |
| IL-4 | 490 | 2.1 | 15.6 | 0.6 | −1.5–3.5 | 197 | 1.9 | 21.5 | 0.5 | −3.0–3.0 |
| IL-6 | 407 | 1253.8 | 1173.1 | 996.0 | 434.7–1914.7 | 177 | 1431.8 | 1143.2 | 1256.5 | 595.6–2173.0 |
| IL-10 | 492 | 11.7 | 36.3 | 2.8 | −0.3–11.2 | 198 | 9.1 | 27.0 | 2.0 | −1.3–13.0 |
| IL-12p40 | 435 | 87.9 | 109.4 | 62.9 | 27.4–115.2 | 182 | 93.8 | 120.1 | 65.6 | 30.1–146.6 |
| IL-12p70 | 434 | 3.9 | 8.4 | 2.9 | 0.6–5.5 | 182 | 2.0 | 19.1 | 2.7 | 0.0–5.8 |
| IL-18 | 420 | 1.1 | 20.2 | 0.7 | −1.9–2.8 | 178 | 2.4 | 22.0 | 1.3 | −0.8–3.9 |
| IFNα | 512 | 92.5 | 94.3 | 66.0 | 20.8–141.7 | 199 | 76.7 | 86.7 | 49.5 | 12.0–120.8 |
| IFN β | 429 | 2.9 | 15.1 | 1.7 | −2.9–7.3 | 178 | 1.6 | 14.0 | 1.3 | −4.8–7.1 |
| TNFα | 450 | 221.0 | 234.1 | 168.6 | 91.3–336.7 | 185 | 198.3 | 202.3 | 162.9 | 78.8–314.4 |
Genome Wide Analysis Results
Our results indicated that recall responses in smallpox vaccine recipients are primarily Th1, and that viral stimulation also induces a strong proinflammatory response in PBMCs. We found a number of significant genetic associations with variations in Th1-type cytokine production. After correction for the small degree of inflation of significance present in the p-value distributions, a number of SNPs for the phenotypes of interest were identified as meeting initial thresholds of significance. The QQ-plots and Manhattan plots indicating associations with IL-2 secretion for both our Caucasian and African-American cohorts are illustrated in Figure 1. SNP associations with Th1 cytokine secretion (IL-2, TNFα, IL-12p40) that reached a high level of significance (p<5×10−7) are listed in Tables 2–3 for Caucasians and African Americans respectively. Tables 5- 7 outline the significant associations found with the pro- inflammatory cytokines IL-1β, IFNα, and IL-6 respectively. Table 8 documents the genetic associations for IL-10, the only Th2 cytokine that was consistently secreted upon viral stimulation of PBMCs from vaccinated subjects. For each of the tables we have listed the function of the protein product (where known) underneath the gene name.
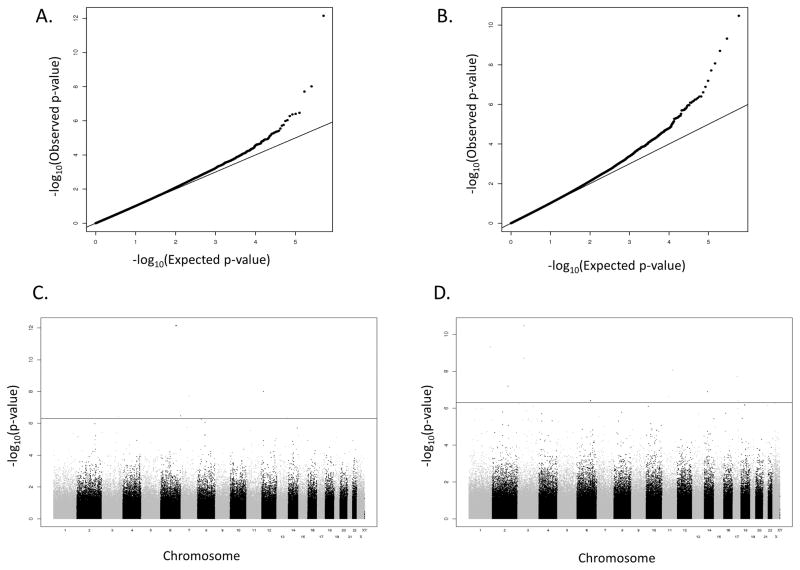
Figure 1. Quantile-Quantile and Manhattan plots of the expected (x-axis) and observed (y-axis) -log10 p-values for genotype associations with IL-2 secretion.
a) the Caucasian cohort and b) the African-American cohort. The x-axis displays the –log10 of the p-value for each SNP association and the y-axis displays the chromosomes in alternating black and gray. p-values were adjusted for gender, age quartile at enrollment, time from immunization to blood draw, season and temperature sample was sent, time from blood draw to assay in quartiles, and the first 4 eigenvectors from the principal component analysis. c and d) Manhattan plot summary of GWAS results for IL-2 secretion for the Caucasian cohort (panel c) and the African-American cohort (panel d).
Table 2.
SNPs showing significant association with secreted IL-2.
| SNP IDa | Chromosomeb | Genec | Locationd | Distance from Gene | MAFe | Genotype | Nf | Median (IQR)g | p-valueh |
|---|---|---|---|---|---|---|---|---|---|
| SNP associations in the Caucasian cohort | |||||||||
| rs9389316 | 6 | GAPDHL19 | 5′upstream | 59695 | 17 | CC | 391 | 19.7 (5.7,40.2) | 7.12E-13 |
| GAPDH-like pseudogene | CA | 17 | 4.3 (−1.7,12.1) | ||||||
| AA | 0 | (,) | |||||||
| rs2268118 | 12 | GRIN2B | intron | 0 | 45 | CC | 363 | 20.7 (6.2,40.8) | 9.70E-09 |
| ionotropic glutamate receptor | CA | 37 | 14 (2.6,29.6) | ||||||
| AA | 4 | 0.2 (−3.2,1.1) | |||||||
| rs1403155* | 7 | AUTS2 | 5′upstream | 1013906 | 13 | GG | 395 | 19.1 (5.5,39.6) | 1.95E-08 |
| Autism susceptibility gene 2 | GA | 11 | 3.1 (−0.3,14.3) | ||||||
| AA | 1 | −1.4 (−1.4, −1.4) | |||||||
| rs1473500 | 6 | FRMD1 | 5′upstream | 31177 | 26 | GG | 382 | 18.9 (5.8,39.3) | 3.44E-07 |
| Protein interacting with angiotension II receptor | GA | 26 | 7.8 (1.6,36.2) | ||||||
| AA | 0 | (,) | |||||||
| rs10513432* | 3 | P2RY1 | 5′upstream | 17371 | 31 | AA | 378 | 19.8 (5.8,40.5) | 3.94E-07 |
| G-protein coupled purinergic receptor | AG | 29 | 9.2 (2.5,15.1) | ||||||
| GG | 1 | −17.5 (−17.5, −17.5) | |||||||
| rs1372791 | 13 | LOC341604 | 5′upstream | 292856 | 11 | GG | 397 | 19.3 (5.2,39.7) | 4.26E-07 |
| GA | 11 | 6.3 (1.8,14.4) | |||||||
| AA | 0 | (,) | |||||||
| SNP associations in the African-American cohort | |||||||||
| rs13088281 | 3 | RFT1 | intron | 0 | 15 | CC | 161 | 13.2 (0.6,30.4) | 3.36E-11 |
| Oligosaccharide translocase | CA | 15 | 41.8 (25.2,63.8) | ||||||
| AA | 0 | (,) | |||||||
| rs908327* | 1 | TOMM20 | 3′downstream | 180060 | 16 | AA | 160 | 17.9 (1.6,35.9) | 4.74E-10 |
| Mitochondrial membrane translocase | AC | 14 | −5.2 (−10.9,9.8) | ||||||
| CC | 1 | −119.8 (−119.8, −119.8) | |||||||
| rs17331151 | 3 | ITIH3 | 3′downstream | 1509 | 13 | GG | 163 | 14.1 (0.7,30.5) | 1.98E-09 |
| ITIH4 | 3′downstream | 2472 | GA | 13 | 49.5 (30.8,60.9) | ||||
| Serine protease inhibitors | AA | 0 | (,) | ||||||
| rs11223581* | 11 | CNTN5 | intron | 0 | 12 | AA | 163 | 14.2 (0.7,32.6) | 8.51E-09 |
| Neuronal cell adhesion molecule | AG | 10 | 26.4 (21.4,51.5) | ||||||
| GG | 1 | 59.5 (59.5,59.5) | |||||||
| rs16948200* | 17 | NGFR | 5′upstream | 6355 | 30 | GG | 144 | 19.2 (2.7,36.5) | 1.93E-08 |
| Nerve growth factor receptor | GA | 26 | 1.5 (−3.8,15.4) | ||||||
| AA | 2 | −4.8 (−7.2, −2.4) | |||||||
| rs10432496 | 2 | LOC100129594 | 5′upstream | 29486 | 13 | GG | 163 | 17.1 (1.6,34.3) | 6.41E-08 |
| GA | 13 | 0.8 (−8.2,2.3) | |||||||
| AA | 0 | (,) | |||||||
| rs11845208 | 14 | ATP5GP2 | 5′upstream | 377106 | 18 | AA | 157 | 18.8 (2.8,35.8) | 1.30E-07 |
| ATP synthase | AG | 18 | −3.2 (−11.5,2.1) | ||||||
| GG | 0 | (,) | |||||||
| rs4963243 | 11 | DAGLA | intron | 0 | 10 | GG | 166 | 15.7 (1.4,35.2) | 2.45E-07 |
| Diacylglycerol lipase | GA | 10 | 2.8 (−2.2,14.4) | ||||||
| AA | 0 | (,) | |||||||
| rs1392089 | 6 | LOC728727 | intron | 0 | 10 | GG | 165 | 17 (1.6,35.4) | 3.97E-07 |
| GA | 10 | −7.5 (−25.1, −1.1) | |||||||
| AA | 0 | (,) | |||||||
| rs7224438 | 17 | BCAS3 | intron | 0 | 127 | AA | 71 | 23.7 (6.9,45.7) | 4.03E-07 |
| Breast cancer amplified sequence 3 | AG | 79 | 9.6 (0.5,29.1) | ||||||
| GG | 24 | 1.2 (−7.1,21.3) | |||||||
| rs3796352 | 3 | TMEM110 | intron | 0 | 13 | GG | 163 | 14.2 (0.9,30.9) | 4.75E-07 |
| Transmembrane protein 110 | GA | 13 | 41.8 (19.5,60.7) | ||||||
| AA | 0 | (,) |
Table 3.
SNPs showing significant association with secreted TNFα
| SNP IDa | Chromosomeb | Genec | Locationd | Distance from Gene | MAFe | Genotype | Nf | Median (IQR)g | p-valueh |
|---|---|---|---|---|---|---|---|---|---|
| SNP associations in the Caucasian cohort | |||||||||
| rs738968* | 22 | LOC441996 | 3′downstream | 95977 | 74 | AA | 364 | 181.1 (93.6,344.8) | 8.43E-08 |
| AC | 70 | 160.4 (84.1,310.9) | |||||||
| CC | 2 | 108.5 (103.6,113.3) | |||||||
| rs8141914* | 22 | LOC441996 | 3′downstream | 105844 | 74 | GG | 363 | 179.6 (93.2,345) | 9.78E-08 |
| GA | 70 | 160.4 (84.1,310.9) | |||||||
| AA | 2 | 108.5 (103.6,113.3) | |||||||
| rs16994335* | 22 | LOC441996 | 3′downstream | 52775 | 77 | GG | 361 | 182.6 (93.9,344.5) | 1.30E-07 |
| GA | 73 | 158.7 (82.6,319.2) | |||||||
| AA | 2 | 108.5 (103.6,113.3) | |||||||
| rs11889798* | 2 | C2orf83 | intron | 0 | 64 | GG | 375 | 188 (97.3,346.6) | 3.87E-07 |
| LOC729968 | 3′downstream | 295 | 64 | GA | 58 | 123.3 (58.8,274.6) | |||
| AA | 3 | 101.6 (83.1,111.4) | |||||||
| rs13006863* | 2 | SLC4A5 | intron | 0 | 257 | GG | 209 | 212.3 (106.9,374.9) | 4.83E-07 |
| GA | 189 | 147.8 (89,289.1) | |||||||
| AA | 34 | 148.9 (100.9,292.5) | |||||||
| SNP associations in the African-American cohort | |||||||||
| rs4251424 | 12 | IRAK4 | intron | 0 | 11 | GG | 170 | 175.8 (94.3,323.4) | 1.66E-08 |
| PUS7L | 5′upstream | 1230 | GA | 11 | 89.7 (39.2,114.1) | ||||
| IL-1R associated kinase 4 | AA | 0 | (,) | ||||||
| rs13414205 | 2 | CAMKMT | intron | 0 | 37 | GG | 149 | 153 (72.2,298.1) | 7.06E-08 |
| Calmodulin lysine N-methyltransferase | GA | 29 | 224.7 (161.5,343.9) | ||||||
| AA | 4 | 616.8 (443.4,702.6) | |||||||
| rs758386 | 3 | SLC6A20 | synonymous | 0 | 10 | GG | 170 | 171.6 (82,322.6) | 2.41E-07 |
| Sodium bicarbonate cotransporter protein | GA | 10 | 125.5 (48.4,213.5) | ||||||
| AA | 0 | (,) |
Table 5.
SNPs showing significant association with secreted IL-1β.
| SNP IDa | Chromosomeb | Genec | Locationd | Distance from Gene | MAFe | Genotype | Nf | Median (IQR)g | p-valueh |
|---|---|---|---|---|---|---|---|---|---|
| SNP associations in the Caucasian cohort | |||||||||
| rs10998624* | 10 | SUPV3L1 | 5′upstream | 192 | 12 | GG | 425 | 58.2 (29.7,156.6) | 1.06E-09 |
| VPS26A | 3′downstream | 7184 | GA | 10 | 32.9 (14.5,57.9) | ||||
| AA | 1 | 19 (19,19) | |||||||
| rs16850864 | 4 | MTHFD2L | intron | 0 | 10 | GG | 426 | 58 (29.7,151.2) | 7.30E-09 |
| GA | 10 | 23.6 (7,70.6) | |||||||
| AA | 0 | (,) | |||||||
| rs16850885 | 4 | EPGN | 5′upstream | 4064 | 10 | GG | 426 | 58 (29.7,151.2) | 7.30E-09 |
| MTHFD2L | 3′downstream | 1324 | GA | 10 | 23.6 (7,70.6) | ||||
| AA | 0 | (,) | |||||||
| rs16850918 | 4 | EPGN | 3′downstream | 5454 | 10 | AA | 426 | 58 (29.7,151.2) | 7.30E-09 |
| AG | 10 | 23.6 (7,70.6) | |||||||
| GG | 0 | (,) | |||||||
| rs572987 | 4 | MTHFD2L | intron | 0 | 10 | AA | 426 | 58 (29.7,151.2) | 7.30E-09 |
| AG | 10 | 23.6 (7,70.6) | |||||||
| GG | 0 | (,) | |||||||
| rs9582259 | 13 | SLC15A1 | intron | 0 | 44 | AA | 391 | 60.7 (31.3,153.6) | 2.99E-08 |
| AC | 44 | 30.2 (16,79) | |||||||
| CC | 0 | (,) | |||||||
| rs9835973 | 3 | RAB6B | intron | 0 | 152 | GG | 301 | 52.6 (25.5,125.5) | 7.45E-08 |
| GA | 118 | 70.5 (35.3,172) | |||||||
| AA | 17 | 75.5 (46.1,141.8) | |||||||
| rs902464 | 4 | DCHS2 | 5′upstream | 3673 | 10 | GG | 426 | 58.4 (29.9,151.2) | 3.04E-07 |
| GA | 10 | 22.8 (16.9,48.9) | |||||||
| AA | 0 | (,) | |||||||
| rs9883650 | 3 | MDS1 | intron | 0 | 71 | AA | 368 | 61.1 (31.8,158.7) | 3.91E-07 |
| AC | 61 | 34.5 (17.1,89.4) | |||||||
| CC | 5 | 32.4 (21.7,48.9) | |||||||
| rs16853574 | 3 | MDS1 | intron | 0 | 62 | AA | 376 | 60.8 (31.5,158.7) | 4.76E-07 |
| AG | 52 | 33.2 (17.3,89.9) | |||||||
| GG | 5 | 32.4 (21.7,48.9) | |||||||
| SNP associations in the African-American cohort | |||||||||
| rs12247397* | 10 | LOC389936 | 5′upstream | 85153 | 21.0 | AA | 160 | 53.9 (25.6,109.6) | 3.33E-09 |
| AG | 19 | 37.6 (24.7,75.7) | |||||||
| GG | 1 | 44.7 (44.7,44.7) | |||||||
| rs17000918* | 22 | FLJ44385 | 5′upstream | 134939 | 15.0 | CC | 167 | 55.8 (28.5,117.8) | 3.93E-09 |
| CA | 11 | 29.7 (21.9,35.2) | |||||||
| AA | 2 | 45 (30.3,59.7) | |||||||
| rs11564024* | 7 | LOC392008 | intron | 0 | 31.0 | AA | 154 | 62.4 (30.8,125.8) | 2.82E-08 |
| AC | 25 | 23.7 (8.7,44.1) | |||||||
| CC | 3 | 22.2 (14.8,62.2) | |||||||
| rs17168526* | 7 | COL28A1 | synonymous | 0 | 21.0 | AA | 162 | 51.4 (28.8,118) | 6.40E-08 |
| AG | 17 | 62.3 (14.8,90.1) | |||||||
| GG | 2 | 10.6 (5.3,15.9) | |||||||
| rs12542677* | 8 | XKR4 | intron | 0 | 11.0 | GG | 172 | 52.7 (25.6,114.7) | 2.32E-07 |
| GA | 9 | 36.4 (24.7,74.2) | |||||||
| AA | 1 | 43 (43,43) | |||||||
| rs4827947* | X | LOC100128151 | 3′downstream | 186536 | 21.0 | GG | 162 | 61.5 (28.4,124.5) | 3.18E-07 |
| GA | 19 | 33.3 (19.7,39.9) | |||||||
| AA | 1 | 22.2 (22.2,22.2) |
Table 7.
SNPs showing significant association with secreted IL-6
| SNP IDa | Chromosomeb | Genec | Locationd | Distance from Gene | MAFe | Genotype | Nf | Median (IQR)g | p-valueh |
|---|---|---|---|---|---|---|---|---|---|
| SNP associations in the Caucasian cohort | |||||||||
| None | |||||||||
| SNP associations in the African-American cohort | |||||||||
| rs8096445 | 18 | MEX3C | intron | 0 | 15 | GG | 157 | 1175.9 (595.6,2063.9) | 9.23E-09 |
| GA | 15 | 2308.4 (1429,3259.6) | |||||||
| AA | 0 | (,) | |||||||
| rs17444059 | 5 | PDE4D | intron | 0 | 12 | AA | 163 | 1337.4 (677.6,2276.4) | 2.03E-08 |
| AG | 12 | 507.8 (320.3,918.3) | |||||||
| GG | 0 | (,) | |||||||
| rs6728021* | 2 | LTBP1 | 5′upstream | 50888 | 20 | AA | 157 | 1129.4 (595.5,2032.7) | 4.48E-08 |
| AG | 16 | 2052 (1514,2798.5) | |||||||
| GG | 2 | 3014.5 (2879.6,3149.4) | |||||||
| rs1516489 | 3 | LOC100129725 | 3′downstream | 148218 | 10 | AA | 165 | 1337.4 (680.8,2274.6) | 7.72E-08 |
| AC | 10 | 274.3 (77.7,871.1) | |||||||
| CC | 0 | (,) | |||||||
| rs17290760* | 9 | NDUFB6 | intron | 0 | 15 | AA | 161 | 1321.6 (674.3,2274.6) | 1.17E-07 |
| TOPORS | 5′upstream | 3779 | AG | 13 | 933.7 (424,1552.7) | ||||
| GG | 1 | 329.6 (329.6,329.6) | |||||||
| rs17299841* | 6 | C6orf190 | 3′downstream | 7542 | 11 | AA | 164 | 1336.2 (679.2,2251) | 1.59E-07 |
| AC | 9 | 570 (366.5,891.2) | |||||||
| CC | 1 | 329.6 (329.6,329.6) | |||||||
| rs2501276* | 1 | CDC42 | 5′upstream | 5496 | 14 | GG | 162 | 1234.1 (595.5,2056.1) | 1.85E-07 |
| GA | 12 | 2314 (1190.5,2983.4) | |||||||
| AA | 1 | 2991.9 (2991.9,2991.9) | |||||||
| rs2255327 | 8 | BLK | intron | 0 | 11 | GG | 162 | 1202.5 (595.5,2068.6) | 2.90E-07 |
| GA | 11 | 2390.3 (1463.8,3051.1) | |||||||
| AA | 0 | (,) | |||||||
| rs2973662* | 5 | ODZ2 | intron | 0 | 16 | GG | 159 | 1139 (593.6,2048.3) | 4.51E-07 |
| GA | 14 | 2314.6 (1374.1,3053.6) | |||||||
| AA | 1 | 3594.4 (3594.4,3594.4) |
Table 8.
SNPs showing significant association with secreted IL-10
| SNP IDa | Chromosomeb | Genec | Locationd | Distance from Gene | MAFe | Genotype | Nf | Median (IQR)g | p-valueh |
|---|---|---|---|---|---|---|---|---|---|
| SNP associations in the Caucasian cohort | |||||||||
| rs16970881* | 16 | DNAH3 | intron | 0 | 39 | GG | 441 | 2.9 (−0.3,11.4) | 1.27E-07 |
| GA | 35 | 0.6 (−0.9,4) | |||||||
| AA | 2 | −0.7 (−1, −0.5) | |||||||
| SNP associations in the African-American cohort | |||||||||
| rs13111850* | 4 | SPOCK3 | 5′upstream | 108508 | 12.0 | AA | 177 | 2 (−1.1,12.9) | 3.17E-11 |
| AG | 8 | 0 (−2.2,5.1) | |||||||
| GG | 2 | −2.5 (−2.7, −2.2) | |||||||
| rs13231718* | 7 | DYNC1I1 | intron | 0 | 10.0 | AA | 186 | 1.7 (−1.5,12.2) | 5.38E-09 |
| AC | 8 | 4 (2.1,20) | |||||||
| CC | 1 | 10.6 (10.6,10.6) | |||||||
| rs17231212 | 7 | LOC100129730 | 5′upstream | 27454 | 11.0 | AA | 184 | 2.3 (−0.8,13.4) | 1.81E-08 |
| AG | 11 | −2.2 (−5, −0.1) | |||||||
| GG | 0 | (,) | |||||||
| rs10055544* | 5 | LOC100131236 | 5′upstream | 347030 | 11.0 | GG | 181 | 2 (−0.9,12.3) | 1.36E-07 |
| GA | 9 | −2.8 (−4.5, −0.8) | |||||||
| AA | 1 | −8.1 (−8.1, −8.1) | |||||||
| rs6679454 | 1 | DAB1 | intron | 0 | 108 | AA | 100 | 3.8 (0.5,21.9) | 2.89E-07 |
| AG | 80 | 0.5 (−3.9,3.2) | |||||||
| GG | 14 | −1.9 (−4.7,11.1) |
Discussion
Both animal models and large scale vaccination studies have demonstrated that poxviruses elicit a strong Th1 response. Our results indicate that this Th1 response is maintained in the vaccinia virus-specific memory T cell population as well. We have previously reported on genome-wide associations with markers of cellular immunity, namely IFNγ ELISPOT and quantitation of secreted IFNγ: in this report we extend our findings to additional measures including Th1, Th2, and inflammatory cytokines. Interestingly, we found considerable variations in cytokine responses to viral stimulation of PBMCs from vaccinated individuals (Table 1).
The relative absence of IFNβ or IL-18 secretion by PBMCs in response to vaccinia infection may be due to the immunomodulatory proteins encoded by poxviruses such as those described below. We inactivated the virus stock used in our experiments with psoralen and UV irradiation to crosslink viral DNA, resulting in >6 log reduction in infectivity. It is likely that this treatment inhibited viral production of immunomodulatory proteins (and we have previously reported on the detection of IFNg using live vs inactivated vaccinia virus)(Ryan et al. 2009), however we cannot rule out the possibility that NYCBOH-encoded proteins (A52R, A53R, B13R, B16R, B19R, C12L) affected secretion patterns of the cytokines assayed. A53R encodes CrmC, a soluble TNFR protein capable of sequestering TNFα and inhibiting TNF signaling. The NYCBOH genome also contains two additional genes for soluble TNFR proteins (C22L, B28R), although they contain mutations and are likely nonfunctional (Goebel et al. 1990). Some strains of vaccinia (Lister, USSR, Evans) also contain the K3R gene which encodes CrmE, yet another soluble TNFR.(Alcami et al. 1999; Reading et al. 2002) The B19R glycoprotein is a soluble receptor for IFN α/β, inhibiting IFNα/β from binding to cellular receptors and dampening antiviral responses (Colamonici et al. 1995; Symons et al. 1995). The E3L protein binds to dsRNA and inhibits PKR signaling, (Chang et al. 1992), IRF-dependent type I IFN synthesis, and TNFα production (Myskiw et al. 2009; Smith et al. 2001). Vaccinia viruses, including the NYCBOH strain, also encode C12L which is an IL-18 binding protein that inhibit IL-18 receptor activation, and may have hampered antibody-based detection of IL-18 in culture supernatant (Reading and Smith 2003; Smith et al. 2000; Symons et al. 2002). NYCBOH also encodes both B16R (an IL-1β inhibitor) and C10L (an IL-1R antagonist) which both block IL-1β signaling. We found high levels of TNFα, IFNα, IFNγ, IL-1β in spite of the virally encoded immunomodulatory proteins affecting each of these cytokines, providing support for our hypothesis that viral proteins are not interfering with cytokine secretion.
Although the median concentration of secreted IL-18 was low (0.7pg/ml in Caucasians and 1.3pg/ml in African Americans) we have previously reported a number SNPs in both IL18 and IL18R that are significantly associated with vaccinia neutralizing antibody titer after smallpox immunization (Haralambieva et al. 2011). Our analyses here have focused on the entire cohort, and it is quite possible that subsets of our cohort (for example: those making larger quantities of IL-18) behave differently from the group as a whole, and thus more focused studies may provide novel insights into the genetic control of smallpox vaccine responses.
One of the strengths of genome-wide association studies is the ability to identify novel genes/processes involved in control of diseases or biologic responses. Our results indicate that a large number of SNPs were associated with variations in cytokine response to vaccinia virus in subjects who had received the smallpox vaccine. One such SNP is rs16948200 in the nerve growth factor receptor gene NGFR. African-American individuals homozygous for the major allele (G) secrete over 12-fold more IL-2 than heterozygotes, while those homozygous for the minor allele (A) secrete essentially no IL-2. NGFR shares a similar structure to other members of the TNFR superfamily including the involvement of the downstream NF-kB and apoptotic pathways (Lotz et al. 1996). Nerve growth factor is produced by a variety of lymphoid cells and can influence proliferation, survival, differentiation and effector function immune cells (Garaci et al. 1999; Lambiase et al. 1997; Otten et al. 1989; Torcia et al. 1996). Our results indicate a possible connection between NGFR and IL-2, which may account for the proliferative and survival effects of NGF on immune cells. The intronic SNP rs4251424, in the interleukin-1 receptor-associated kinase 4 (IRAK4) gene, was associated with differential secretion of TNFα. In fact, African-Americans homozygous for the major allele (G) secreted nearly twice as much TNFα as heterozygous individuals. IRAK4 initiates a cascade of phosphorylation and signaling events in response to viral stimulation through TLRs (TLR7,8, and 9), resulting in cytokine production (Yang et al. 2005). IRAK4 has also been implicated in T cell activation and function (Suzuki et al. 2006). Thus, a SNP influencing IRAK4 expression or function may lead to distinct differences in T cell production of TNFα.
Rs11242417, an intronic SNP in the glial cell line-derived neurotrophic factor receptor alpha (GFRA3) gene, is associated with variations in IL-12p40 secretion in our Caucasian cohort. The GFRA3 gene encodes for a cellular receptor involved in neuronal development (Nishino et al. 1999) and although this same SNP is associated with schizophrenia (Souza et al. 2010) its’ role in immune function is not known. In ourAfrican-America cohort, we identified rs859267 in ADORA2B as being associated with variations in IL-12p40 in an allele dose-dependent manner. Possession of the minor allele (C) leads to a 30% reduction in IL-12p40 for heterozygotes and a 70% reduction in IL-12p40 secretion in homozygotes (Table 4). ADORA2B encodes for the adenosine A2B G protein coupled receptor. Blockade of ADORA2B signaling enhances macrophage phagocytosis, cytokine production, and chemokine synthesis (Belikoff et al. 2011). Adenosine also inhibits IL-12 and TNFα release by dendritic cells through activation of the adenosine A2 receptor (Ben Addi et al. 2008; Panther et al. 2003), providing supporting evidence for our results showing that genetic polymorphisms in ADORA2B may affect IL-12 production by PBMCs.
Table 4.
SNPs showing significant association with secreted IL-12p40.
| SNP IDa | Chromosomeb | Genec | Locationd | Distance from Gene | MAFe | Genotype | Nf | Median (IQR)g | p-valueh |
|---|---|---|---|---|---|---|---|---|---|
| SNP associations in the Caucasian cohort | |||||||||
| rs7771911 | 6 | LOC100131805 | 3′downstream | 333903 | 11 | CC | 400 | 65.6 (27.9,116.7) | 1.89E-12 |
| CA | 11 | 39.9 (19.4,44.1) | |||||||
| AA | 0 | (,) | |||||||
| rs17142462 | 7 | KCND2 | 5′upstream | 307090 | 10 | GG | 402 | 64 (27.6,116.1) | 3.32E-09 |
| GA | 10 | 36.7 (5.6,69.2) | |||||||
| AA | 0 | (,) | |||||||
| rs7105056 | 11 | BCO2 | intron | 0 | 14 | GG | 408 | 65 (28.7,116) | 9.71E-09 |
| GA | 14 | 28.2 (10.2,35.9) | |||||||
| AA | 0 | (,) | |||||||
| rs7658486* | 4 | ARHGAP10 | intron | 0 | 43 | GG | 375 | 66.4 (29.3,121.5) | 1.47E-08 |
| GA | 39 | 39.1 (19.3,67.4) | |||||||
| AA | 2 | 56 (41.6,70.4) | |||||||
| rs11242417 | 5 | GFRA3 | intron | 0 | 142 | AA | 289 | 71.5 (31.5,133) | 5.56E-08 |
| AC | 118 | 47.2 (18.8,88.6) | |||||||
| CC | 12 | 41 (20.8,66.9) | |||||||
| rs1584468 | 5 | PRR16 | intron | 0 | 19 | GG | 403 | 66.4 (29,117.5) | 8.64E-08 |
| GA | 19 | 26.3 (11,43.5) | |||||||
| AA | 0 | (,) | |||||||
| rs11034653 | 11 | LOC100132631 | 3′downstream | 507609 | 10 | GG | 412 | 62.7 (27.2,114.5) | 1.72E-07 |
| GA | 10 | 105.4 (89.6,142.3) | |||||||
| AA | 0 | (,) | |||||||
| rs6484985 | 11 | LOC100132631 | 3′downstream | 527429 | 10 | AA | 412 | 62.7 (27.2,114.5) | 1.72E-07 |
| AG | 10 | 105.4 (89.6,142.3) | |||||||
| GG | 0 | (,) | |||||||
| rs3736638* | 7 | COL1A2 | intron | 0 | 10 | CC | 411 | 63.2 (27.2,115.6) | 2.81E-07 |
| CA | 8 | 74.2 (58.8,102.2) | |||||||
| AA | 1 | 121.4 (121.4,121.4) | |||||||
| SNP associations in the African-American cohort | |||||||||
| rs859267* | 17 | ADORA2B | 5′upstream | 22210 | 46 | AA | 135 | 78 (39.8,156.2) | 2.52E-07 |
| AC | 40 | 53.7 (21.7,71.6) | |||||||
| CC | 3 | 21.7 (5.5,67.7) | |||||||
| rs6943090* | 7 | FKBP6 | intron | 0 | 44 | GG | 137 | 74.9 (39.8,155.5) | 3.17E-07 |
| TRIM50 | 5′upstream | 4563 | GA | 40 | 43.2 (18.8,113.2) | ||||
| AA | 2 | -9.8 (-10.2,-9.3) |
IFNα production was also significantly associated with a number of SNPs in both of the racial groups we studied. In Caucasian subjects, possession of a single minor allele (A) at rs542631 in the LTBP1 gene led to a 260% increase in IFNα secretion upon vaccinia stimulation. LTBP1 is involved in the trafficking and activation of latent TGFB complexes (Keski-Oja et al. 2004). Our data also show that another SNP near the same gene, rs6728024, is associated with variations in IL-6 secretion in African-American subjects, resulting in an almost three-fold, minor allele dose-dependent increase of IL-6. These associations are not surprising given the myriad effects TGF-β can have on cytokine production by immune cells, including the induction of proinflammatory cytokines (TNFα, IL-1, IL-6) by monocytes (Bogdan and Nathan 1993). We also found a number of SNPs significantly associated with IFNα secretion in our African-American subjects, however, many of these SNPs (rs9493873 near SGK1, rs3095748 in DAPK1, rs2043599 near NLRP8 and NLRP13, rs10517025 in ATP8A1, and rs17221323 in DIP2C) failed to reach genome-wide significance during our sensitivity analyses, indicating that these associations were likely driven by extremely high or low IFNα secretion measured in only a few subjects homozygous for the minor allele. Thus, these associations should be interpreted with caution.
Of particular interest with IL-6 is our finding that individuals heterozygous (GA) at rs2255327 in BLK, the B lymphoid tyrosine kinase, had levels of IL-6 two-fold higher than those homozygous for the major allele (GG). In addition to B cells, BLK is also expressed in granulocytes, monocytes, and macrophages (Okutani et al. 2006). BLK plays a critical role in B cell receptor signaling and subsequent development. Related Src family kinases activate cytokine production (IL-1, IL-6, TNFα) in macrophages in response to pathogen recognition by TLRs (Lowell 2004). SNPs in BLK are associated with autoimmune and inflammatory disorders such as: systemic lupus erythematosum (Harley et al. 2008; Ito et al. 2009; Yang et al. 2009), Sjogren’s syndrome (Nordmark et al. 2011), systemic sclerosis (Gourh et al. 2010), and antiphospholipid antibody syndrome (Yin et al. 2009). Our results indicate that genetic variation in BLK may affect downstream IL-6 production.
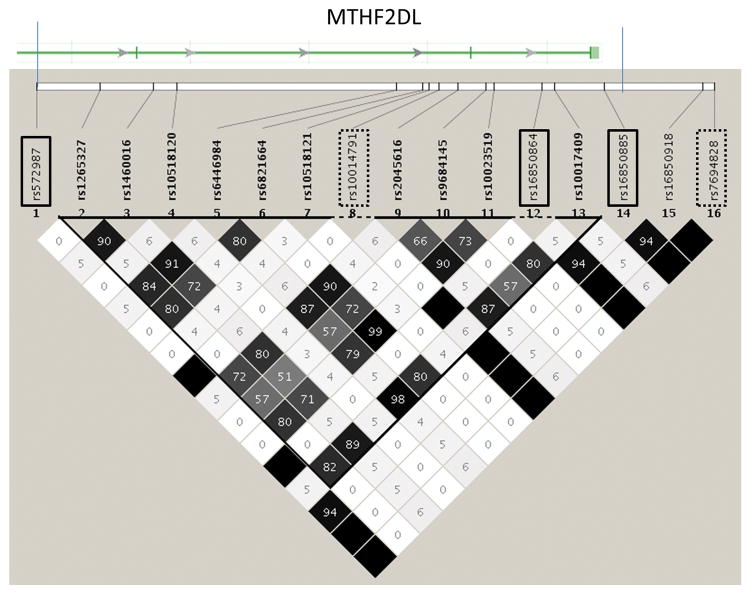
We found a number of SNPs located near (rs16850885) or within (rs16850864, rs572987) the MTHFD2L gene associated with variations in IL-1β secretion in our Caucasian subjects. MTHFD2L codes for a methylenetetrahydrofolate dehydrogenase 2-like protein involved in carbon metabolism and conversion of folate to formate in the mitochondria (Bolusani et al. 2011). As shown in Figure 2, these SNPs are all in tight LD with one another. Another nearby SNP, rs7694828, is in tight LD with each of these three identified SNPs. Rs7694828 is located in a binding site of the transcriptional regulator CTCF (Kim et al. 2007). CTCF functions as an insulator, preventing the influence of cis-acting enhancers on gene activation (Bell et al. 1999). Yet another SNP (rs10014791) is also in high LD (r2=1) with the three identified MTHFD2L SNPs and resides in a binding site for v-MAF. Thus, the identified SNPs may not directly affect the associated immune outcome, but rather tag nearby SNPs whose functional effects lead to variations in the downstream immune outcome. Most of the SNPs reported here are non-coding SNPs. This is due, in large part, to the composition of the HumanHap 550/650 chips: the vast majority of the SNPs on these platforms are in non-coding regions of the genome. Non-coding SNPs in splice sites, promoters, and regulatory regions can have as profound an influence on gene expression/function as coding SNPs, furthermore the SNPs we have identified may not be the causal SNPs, but rather tag coding/regulatory SNPs that actually impact gene/protein expression or function. We view this initial GWAS as a screening tool to identify genetic regions of interest for the future replication and fine-mapping efforts that we believe will be critical to elucidating the mechanisms behind the reported genetic associations.
Figure 2. Genetic region containing a portion of the MTHFD2L gene and 3 SNPs significantly associated with variations in total IL-1β secretion in the Caucasian subjects.
r2 relationships between each pair of SNPs is indicated by the shading and number within each diamond on the LD plot. LD blocks (Gabriel definition) are represented by the bold triangle. SNPs showing significant associations in this study are highlighted in solid boxes, additional SNPs of interest are highlighted in hatched boxes. The vertical bar on top of the LD plot shows the introns/exons of the MTHFD2L gene aligned with the SNPs from the LD plot.
Our study utilized individuals with documented evidence of vaccinia immunization, simultaneously analyzed multiple cytokines involved in both innate and adaptive responses to poxviruses, and allowed us to assess associations within genetically defined racial subgroups. However, our study was limited in that: 1) we had a relatively small number of African-American subjects, 2) we examined a large, but not comprehensive, set of cytokines, and 3) we do not yet have an external validation data set. With these limitations in mind, we have nevertheless identified a number of novel genes containing SNPs associated with vaccinia-specific cytokine responses to smallpox vaccination. These SNPs are found in genes with known immune function, as well as in genes with no previously identified connection to immune responses, and in intergenic regions of the genome. We examined each significant (p< 5 × 10−7) race-specific association in the other racial group in a preliminary manner, using a cut-off of p < 0.01 in the second racial group. While we found a number of SNPs associated with similar trends in immune outcome, we did not find any SNP associations that reached this significance threshold. This initial replication assessment was likely hampered by racial effects and low sample sizes. Thus, important next steps are to expand the selection of cytokines examined (such as IL-17), fine-mapping to identify causal variants (both coding and regulatory), replication of the identified associations and fine-mapped variants in an independent cohort. Our findings may pinpoint novel means of immune regulation, and these newly identified SNPs may be excellent candidates for functional studies aimed at discovering the mechanisms behind such regulation.
Table 6.
SNPs showing significant association with secreted IFNα.
| SNP IDa | Chromosomeb | Genec | Locationd | Distance from Gene | MAFe | Genotype | Nf | Median (IQR)g | p-valueh |
|---|---|---|---|---|---|---|---|---|---|
| SNP associations in the Caucasian cohort | |||||||||
| rs13181561 | 5 | ECSM2 | 5′upstream | 8585 | 248 | AA | 272 | 88.4 (35.8,174) | 2.71E-14 |
| TMEM173 | 3′downstream | 4644 | AG | 192 | 51.5 (12.4,112.1) | ||||
| GG | 28 | 5.6 (1.3,17.2) | |||||||
| rs6573333 | 14 | MNAT1 | intron | 0 | 14 | CC | 482 | 67.6 (22.5,142.8) | 3.09E-10 |
| CA | 14 | 37.9 (8.5,56) | |||||||
| AA | 0 | (,) | |||||||
| rs6576443 | 15 | ATP10A | 3′downstream | 29201 | 341 | GG | 212 | 77.7 (39.1,149.9) | 6.20E-09 |
| GA | 229 | 57.7 (16.8,140.2) | |||||||
| AA | 56 | 31.9 (5.4,84.9) | |||||||
| rs6573332 | 14 | MNAT1 | intron | 0 | 16 | GG | 482 | 67.6 (22.5,142.8) | 1.26E-08 |
| GA | 16 | 38.4 (11.2,57.9) | |||||||
| AA | 0 | (,) | |||||||
| rs7150492 | 14 | MNAT1 | intron | 0 | 16 | GG | 481 | 67.7 (22.5,143) | 1.33E-08 |
| GA | 16 | 38.4 (11.2,57.9) | |||||||
| AA | 0 | (,) | |||||||
| rs2925296 | 15 | ATP10A | 3′downstream | 36278 | 356 | GG | 203 | 75.7 (39.3,149.1) | 1.72E-08 |
| GA | 234 | 59.8 (16.5,138.7) | |||||||
| AA | 61 | 34.1 (7.6,101.5) | |||||||
| rs10195263* | 2 | LOC344332 | 5′upstream | 307939 | 20 | AA | 479 | 67.5 (22.8,142.6) | 3.14E-08 |
| AG | 18 | 23.7 (2.9,65.8) | |||||||
| GG | 1 | 5.9 (5.9,5.9) | |||||||
| rs542631 | 2 | LTBP1 | intron | 0 | 10 | CC | 487 | 64.5 (20.6,139.2) | 1.11E-07 |
| CA | 10 | 168.5 (96.4,200.8) | |||||||
| AA | 0 | (,) | |||||||
| rs261532 | 5 | UBE2D2 | intron | 0 | 304 | CC | 245 | 83.7 (32.1,167) | 2.50E-07 |
| CA | 202 | 55.7 (18.3,114.9) | |||||||
| AA | 51 | 31.8 (3.6,84.3) | |||||||
| rs8012779* | 14 | MNAT1 | intron | 0 | 22 | GG | 477 | 69.6 (22.1,143) | 4.03E-07 |
| TRMT5 | 3′downstream | 6543 | GA | 20 | 49 (27.7,63.1) | ||||
| AA | 1 | 7.1 (7.1,7.1) | |||||||
| SNP associations in the African-American cohort | |||||||||
| rs4078978* | 2 | WDR92 | intron | 0 | 12.0 | GG | 186 | 56 (16.7,122.3) | 2.24E-18 |
| GA | 8 | 1.6 (−0.2,7.7) | |||||||
| AA | 2 | −2.5 (−4.3, −0.7) | |||||||
| rs381365 | X | LOC100128265 | 3′downstream | 47772 | 12.0 | CC | 184 | 56 (17.4,122.8) | 2.30E-12 |
| CA | 12 | 3.4 (2.3,21.9) | |||||||
| AA | 0 | (,) | |||||||
| rs2048161 | 4 | ZNF827 | intron | 0 | 15.0 | GG | 181 | 61.9 (18.4,124.1) | 5.78E-12 |
| GA | 15 | 8.8 (−0.7,17.3) | |||||||
| AA | 0 | (,) | |||||||
| rs17252936* | X | MAMLD1 | intron | 0 | 13.0 | AA | 184 | 50.6 (14.3,122.3) | 5.51E-10 |
| AG | 11 | 30.6 (9.5,63.1) | |||||||
| GG | 1 | 0 (0,0) | |||||||
| rs11171846 | 12 | TIMELESS | intron | 0 | 10.0 | GG | 186 | 56 (15.5,122.3) | 6.14E-10 |
| GA | 10 | 14.2 (3.2,22) | |||||||
| AA | 0 | (,) | |||||||
| rs12044963 | 1 | KCND3 | intron | 0 | 12.0 | CC | 184 | 53 (15.7,122.8) | 9.43E-10 |
| CA | 12 | 12.9 (6.8,34.3) | |||||||
| AA | 0 | (,) | |||||||
| rs6778194* | 3 | LOC152118 | 5′upstream | 17628 | 12.0 | AA | 185 | 50.6 (15.4,122.2) | 2.43E-09 |
| AC | 8 | 23.1 (6.8,70) | |||||||
| CC | 2 | 9.7 (−0.2,19.6) | |||||||
| rs2272205* | 2 | COL4A4 | intron | 0 | 20.0 | AA | 178 | 59.6 (14.9,122.3) | 2.84E-09 |
| AG | 16 | 23.9 (10.5,49.8) | |||||||
| GG | 2 | 10 (5,15) | |||||||
| rs9408928 | 9 | RAB14 | intron | 0 | 13.0 | AA | 183 | 53.6 (15.6,123.2) | 4.07E-09 |
| AG | 13 | 4.1 (2.7,24.4) | |||||||
| GG | 0 | (,) | |||||||
| rs1540283 | X | PHEX | intron | 0 | 15.0 | AA | 181 | 60.6 (17.7,124.1) | 9.40E-09 |
| AG | 15 | 11 (−0.4,30.5) | |||||||
| GG | 0 | (,) | |||||||
| rs2269466 | X | PHEX | intron | 0 | 15.0 | AA | 181 | 60.6 (17.7,124.1) | 9.40E-09 |
| AG | 15 | 11 (−0.4,30.5) | |||||||
| GG | 0 | (,) | |||||||
| rs17007761 | 2 | LOC728241 | 3′downstream | 263187 | 17.0 | CC | 179 | 45.5 (11.1,111.1) | 9.86E-09 |
| CA | 17 | 114.8 (65.8,180.1) | |||||||
| AA | 0 | (,) | |||||||
| rs4713226* | 6 | OR2H1 | 3′downstream | 2315 | 14.0 | GG | 184 | 51.6 (15.2,122.3) | 2.45E-08 |
| GA | 10 | 17.1 (9,51.1) | |||||||
| AA | 2 | 7 (0.4,13.5) | |||||||
| rs9408926 | 9 | CEP110 | synonymous | 0 | 12.0 | GG | 184 | 53 (15.2,122.8) | 3.96E-08 |
| GA | 12 | 11.6 (2.2,29.6) | |||||||
| AA | 0 | (,) | |||||||
| rs210359 | 14 | BMP4 | 3′downstream | 248982 | 10.0 | CC | 186 | 51.6 (14.5,122.3) | 7.61E-08 |
| CA | 10 | 27.6 (4.8,48.5) | |||||||
| AA | 0 | (,) | |||||||
| rs13067593* | 3 | LPP | intron | 0 | 35.0 | AA | 162 | 63.3 (23.9,125.6) | 7.88E-08 |
| AG | 29 | 18.4 (1.1,58.5) | |||||||
| GG | 3 | 4.1 (−3,12.3) | |||||||
| rs5925760 | X | PTCHD1 | intron | 0 | 10.0 | GG | 184 | 51.6 (13.5,122.8) | 1.22E-07 |
| GA | 10 | 28.3 (14.4,46.4) | |||||||
| AA | 0 | (,) | |||||||
| rs7060947 | X | ODZ1 | intron | 0 | 24.0 | AA | 169 | 63 (19.5,125.6) | 1.31E-07 |
| AG | 24 | 15.9 (2.2,38.2) | |||||||
| GG | 0 | (,) | |||||||
| rs17221323* | 10 | DIP2C | intron | 0 | 14.0 | AA | 183 | 52.5 (15.6,123.2) | 1.39E-07 |
| AG | 12 | 16.9 (2.9,50.8) | |||||||
| GG | 1 | 0.9 (0.9,0.9) | |||||||
| rs8127571 | 21 | LOC100129027 | 3′downstream | 84485 | 10.0 | GG | 186 | 53 (14.9,122.3) | 1.86E-07 |
| GA | 10 | 20.3 (4.4,37.7) | |||||||
| AA | 0 | (,) | |||||||
| rs4839431* | 1 | CYMP | rna_exon | 0 | 32.0 | GG | 163 | 53.6 (16.1,124.9) | 2.11E-07 |
| GA | 28 | 29.2 (3.2,62.1) | |||||||
| AA | 2 | −0.5 (−1.3,0.3) | |||||||
| rs9807334 | 18 | ELAC1 | 3′downstream | 9671 | 10.0 | GG | 186 | 51.6 (14.9,122.3) | 2.16E-07 |
| GA | 10 | 14.7 (2.3,25.3) | |||||||
| AA | 0 | (,) | |||||||
| rs10517025* | 4 | ATP8A1 | intron | 0 | 22.0 | GG | 176 | 47.6 (10.9,120.6) | 2.17E-07 |
| GA | 18 | 74.6 (52.6,127) | |||||||
| AA | 2 | 156.7 (100.9,212.5) | |||||||
| rs17714988 | 13 | hCG_1820717 | 5′upstream | 4677 | 10.0 | AA | 186 | 56 (14.9,122.3) | 3.29E-07 |
| AG | 10 | 15.7 (3.2,34.5) | |||||||
| GG | 0 | (,) | |||||||
| rs9493873* | 6 | SGK1 | 5′upstream | 80509 | 28.0 | AA | 168 | 62.6 (20.4,127.5) | 4.18E-07 |
| AG | 24 | 14.6 (2.5,48.5) | |||||||
| GG | 2 | 4.8 (4.2,5.4) | |||||||
| rs3095748* | 9 | DAPK1 | intron | 0 | 13.0 | AA | 184 | 50.6 (15.2,122.3) | 4.64E-07 |
| AG | 11 | 20.1 (2.1,71.3) | |||||||
| GG | 1 | 0.4 (0.4,0.4) | |||||||
| rs2043599* | 19 | NLRP13 | 5′upstream | 7347 | 26.0 | AA | 171 | 58.5 (18.1,125.6) | 4.77E-07 |
| NLRP8 | 5′upstream | 8149 | AG | 24 | 18.9 (3.2,74.6) | ||||
| GG | 1 | 0.8 (0.8,0.8) | |||||||
| rs10517038 | 4 | ATP8A1 | intron | 0 | 18.0 | GG | 178 | 48.3 (11.1,119.3) | 4.89E-07 |
| GA | 18 | 74.6 (50.3,136.6) | |||||||
| AA | 0 | (,) | |||||||
| rs3811769 | 4 | ATP8A1 | intron | 0 | 18.0 | AA | 178 | 48.3 (11.1,119.3) | 4.89E-07 |
| AG | 18 | 74.6 (50.3,136.6) | |||||||
| GG | 0 | (,) |
Acknowledgments
We extend our thanks to Drs. Meg Ryan and Kevin L. Russell, the Naval Health Research Center team, and the Mayo Vaccine Research Group nurses and study coordinators for their efforts in subject recruitment. We thank Julie M. Cunningham and the Mayo Advanced Genomic Technology Center for genotyping efforts, as well as Megan O’Byrne and David Watson for assistance with the statistical analysis. Funding support was provided by the National Institute of Allergies and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, Contract No. HHSN266200400065C. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute Of Allergy And Infectious Diseases or the National Institutes of Health.
Footnotes
Ethical Standards
All experiments described here comply with the current, applicable U.S. laws.
Conflict of Interests
The authors do not have any conflicts of interest to report.
References
- Alcami A, Khanna A, Paul NL, Smith GL. Vaccinia virus strains Lister, USSR and Evans express soluble and cell-surface tumour necrosis factor receptors. J Gen Virol. 1999;80 (Pt 4):949–59. doi: 10.1099/0022-1317-80-4-949. [DOI] [PubMed] [Google Scholar]
- Belikoff BG, Hatfield S, Georgiev P, Ohta A, Lukashev D, Buras JA, Remick DG, Sitkovsky M. A2B adenosine receptor blockade enhances macrophage-mediated bacterial phagocytosis and improves polymicrobial sepsis survival in mice. J Immunol. 2011;186:2444–53. doi: 10.4049/jimmunol.1001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AC, West AG, Felsenfeld G. The protein CTCF is required for the enhancer blocking activity of vertebrate insulators. Cell. 1999;98:387–96. doi: 10.1016/s0092-8674(00)81967-4. [DOI] [PubMed] [Google Scholar]
- Ben Addi A, Lefort A, Hua X, Libert F, Communi D, Ledent C, Macours P, Tilley SL, Boeynaems JM, Robaye B. Modulation of murine dendritic cell function by adenine nucleotides and adenosine: involvement of the A(2B) receptor. Eur J Immunol. 2008;38:1610–20. doi: 10.1002/eji.200737781. [DOI] [PubMed] [Google Scholar]
- Bogdan C, Nathan C. Modulation of macrophage function by transforming growth factor beta, interleukin-4, and interleukin-10. Ann N Y Acad Sci. 1993;685:713–39. doi: 10.1111/j.1749-6632.1993.tb35934.x. [DOI] [PubMed] [Google Scholar]
- Bolusani S, Young BA, Cole NA, Tibbetts AS, Momb J, Bryant JD, Solmonson A, Appling DR. Mammalian MTHFD2L encodes a mitochondrial methylenetetrahydrofolate dehydrogenase isozyme expressed in adult tissues. J Biol Chem. 2011;286:5166–74. doi: 10.1074/jbc.M110.196840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HW, Watson JC, Jacobs BL. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc Natl Acad Sci U S A. 1992;89:4825–9. doi: 10.1073/pnas.89.11.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colamonici OR, Domanski P, Sweitzer SM, Larner A, Buller RM. Vaccinia virus B18R gene encodes a type I interferon-binding protein that blocks interferon alpha transmembrane signaling. J Biol Chem. 1995;270:15974–8. doi: 10.1074/jbc.270.27.15974. [DOI] [PubMed] [Google Scholar]
- Combadiere B, Boissonnas A, Carcelain G, Lefranc E, Samri A, Bricaire F, Debre P, Autran B. Distinct time effects of vaccination on long-term proliferative and IFN-gamma-producing T cell memory to smallpox in humans. J Exp Med. 2004;199:1585–93. doi: 10.1084/jem.20032083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–73. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- Earl PL, Moss B, Wyatt LS, Carroll MW. Generation of recombinant vaccinia viruses. Curr Protoc Protein Sci. 2001;Chapter 5(Unit 5):13. doi: 10.1002/0471140864.ps0513s13. [DOI] [PubMed] [Google Scholar]
- Fenner F. Smallpox and its eradication. World Health Organization; Geneva: 1988. [Google Scholar]
- Fenner F. Risks and benefits of vaccinia vaccine use in the worldwide smallpox eradication campaign. Res Virol. 1989;140:465–6. doi: 10.1016/s0923-2516(89)80126-8. discussion 487–91. [DOI] [PubMed] [Google Scholar]
- Foong YY, Jans DA, Rolph MS, Gahan ME, Mahalingam S. Interleukin-15 mediates potent antiviral responses via an interferon-dependent mechanism. Virology. 2009;393:228–37. doi: 10.1016/j.virol.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Frey SE, Newman FK, Cruz J, Shelton WB, Tennant JM, Polach T, Rothman AL, Kennedy JS, Wolff M, Belshe RB, Ennis FA. Dose-related effects of smallpox vaccine. N Engl J Med. 2002;346:1275–80. doi: 10.1056/NEJMoa013431. [DOI] [PubMed] [Google Scholar]
- Freyschmidt EJ, Mathias CB, MacArthur DH, Laouar A, Narasimhaswamy M, Weih F, Oettgen HC. Skin inflammation in RelB(−/−) mice leads to defective immunity and impaired clearance of vaccinia virus. J Allergy Clin Immunol. 2007;119:671–9. doi: 10.1016/j.jaci.2006.12.645. [DOI] [PubMed] [Google Scholar]
- Fulginiti VA. Risks of smallpox vaccination. Jama. 2003;290:1452. doi: 10.1001/jama.290.11.1452-a. author reply 1452. [DOI] [PubMed] [Google Scholar]
- Fulginiti VA, Papier A, Lane JM, Neff JM, Henderson DA. Smallpox vaccination: a review, part II. Adverse events. Clin Infect Dis. 2003;37:251–71. doi: 10.1086/375825. [DOI] [PubMed] [Google Scholar]
- Garaci E, Caroleo MC, Aloe L, Aquaro S, Piacentini M, Costa N, Amendola A, Micera A, Calio R, Perno CF, Levi-Montalcini R. Nerve growth factor is an autocrine factor essential for the survival of macrophages infected with HIV. Proc Natl Acad Sci U S A. 1999;96:14013–8. doi: 10.1073/pnas.96.24.14013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel SJ, Johnson GP, Perkus ME, Davis SW, Winslow JP, Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990;179:247–66. 517–63. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- Gourh P, Agarwal SK, Martin E, Divecha D, Rueda B, Bunting H, Assassi S, Paz G, Shete S, McNearney T, Draeger H, Reveille JD, Radstake TR, Simeon CP, Rodriguez L, Vicente E, Gonzalez-Gay MA, Mayes MD, Tan FK, Martin J, Arnett FC. Association of the C8orf13-BLK region with systemic sclerosis in North-American and European populations. J Autoimmun. 2010;34:155–62. doi: 10.1016/j.jaut.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryev DN, Howell MD, Watkins TN, Chen YC, Cheadle C, Boguniewicz M, Barnes KC, Leung DY. Vaccinia virus-specific molecular signature in atopic dermatitis skin. J Allergy Clin Immunol. 2010;125:153–159. e28. doi: 10.1016/j.jaci.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–7. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- Haralambieva IH, Ovsyannikova IG, Dhiman N, Kennedy RB, O’Byrne M, Pankratz VS, Jacobson RM, Poland GA. Common SNPs/Haplotypes in IL18R1 and IL18 Genes Are Associated With Variations in Humoral Immunity to Smallpox Vaccination in Caucasians and African Americans. J Infect Dis. 2011;204:433–41. doi: 10.1093/infdis/jir268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley JB, Alarcon-Riquelme ME, Criswell LA, Jacob CO, Kimberly RP, Moser KL, Tsao BP, Vyse TJ, Langefeld CD, Nath SK, Guthridge JM, Cobb BL, Mirel DB, Marion MC, Williams AH, Divers J, Wang W, Frank SG, Namjou B, Gabriel SB, Lee AT, Gregersen PK, Behrens TW, Taylor KE, Fernando M, Zidovetzki R, Gaffney PM, Edberg JC, Rioux JD, Ojwang JO, James JA, Merrill JT, Gilkeson GS, Seldin MF, Yin H, Baechler EC, Li QZ, Wakeland EK, Bruner GR, Kaufman KM, Kelly JA. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet. 2008;40:204–10. doi: 10.1038/ng.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell MD, Gallo RL, Boguniewicz M, Jones JF, Wong C, Streib JE, Leung DY. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006;24:341–8. doi: 10.1016/j.immuni.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Ito I, Kawasaki A, Ito S, Hayashi T, Goto D, Matsumoto I, Tsutsumi A, Hom G, Graham RR, Takasaki Y, Hashimoto H, Ohashi J, Behrens TW, Sumida T, Tsuchiya N. Replication of the association between the C8orf13-BLK region and systemic lupus erythematosus in a Japanese population. Arthritis Rheum. 2009;60:553–8. doi: 10.1002/art.24246. [DOI] [PubMed] [Google Scholar]
- Jenner E. An inquiry into the causes and effects of the variolae vaccinae, a disease discovered in some of the western counties of England, particularly Gloucestershire, and known by the name of the cow pox. Law; London: 1798. [Google Scholar]
- Kennedy R, Pankratz VS, Swanson E, Watson D, Golding H, Poland GA. Statistical approach to estimate vaccinia- specific neutralizing antibody titers using a high throughput assay. Clin Vaccine Immunol. 2009a doi: 10.1128/CVI.00109-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy RB, Ovsyannikova IG, Jacobson RM, Poland GA. The immunology of smallpox vaccines. Curr Opin Immunol. 2009b;21:314–20. doi: 10.1016/j.coi.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keski-Oja J, Koli K, von Melchner H. TGF-beta activation by traction? Trends Cell Biol. 2004;14:657–9. doi: 10.1016/j.tcb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Kim TH, Abdullaev ZK, Smith AD, Ching KA, Loukinov DI, Green RD, Zhang MQ, Lobanenkov VV, Ren B. Analysis of the vertebrate insulator protein CTCF-binding sites in the human genome. Cell. 2007;128:1231–45. doi: 10.1016/j.cell.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohyama S, Ohno S, Isoda A, Moriya O, Belladonna ML, Hayashi H, Iwakura Y, Yoshimoto T, Akatsuka T, Matsui M. IL-23 enhances host defense against vaccinia virus infection via a mechanism partly involving IL-17. J Immunol. 2007;179:3917–25. doi: 10.4049/jimmunol.179.6.3917. [DOI] [PubMed] [Google Scholar]
- Lambiase A, Bracci-Laudiero L, Bonini S, Bonini S, Starace G, D’Elios MM, De Carli M, Aloe L. Human CD4+ T cell clones produce and release nerve growth factor and express high-affinity nerve growth factor receptors. J Allergy Clin Immunol. 1997;100:408–14. doi: 10.1016/s0091-6749(97)70256-2. [DOI] [PubMed] [Google Scholar]
- Lotz M, Setareh M, von Kempis J, Schwarz H. The nerve growth factor/tumor necrosis factor receptor family. J Leukoc Biol. 1996;60:1–7. doi: 10.1002/jlb.60.1.1. [DOI] [PubMed] [Google Scholar]
- Lowell CA. Src-family kinases: rheostats of immune cell signaling. Mol Immunol. 2004;41:631–43. doi: 10.1016/j.molimm.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Myskiw C, Arsenio J, van Bruggen R, Deschambault Y, Cao J. Vaccinia virus E3 suppresses expression of diverse cytokines through inhibition of the PKR, NF-kappaB, and IRF3 pathways. J Virol. 2009;83:6757–68. doi: 10.1128/JVI.02570-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino J, Mochida K, Ohfuji Y, Shimazaki T, Meno C, Ohishi S, Matsuda Y, Fujii H, Saijoh Y, Hamada H. GFR alpha3, a component of the artemin receptor, is required for migration and survival of the superior cervical ganglion. Neuron. 1999;23:725–36. doi: 10.1016/s0896-6273(01)80031-3. [DOI] [PubMed] [Google Scholar]
- Nordmark G, Kristjansdottir G, Theander E, Appel S, Eriksson P, Vasaitis L, Kvarnstrom M, Delaleu N, Lundmark P, Lundmark A, Sjowall C, Brun JG, Jonsson MV, Harboe E, Goransson LG, Johnsen SJ, Soderkvist P, Eloranta ML, Alm G, Baecklund E, Wahren-Herlenius M, Omdal R, Ronnblom L, Jonsson R, Syvanen AC. Association of EBF1, FAM167A(C8orf13)-BLK and TNFSF4 gene variants with primary Sjogren’s syndrome. Genes Immun. 2011;12:100–9. doi: 10.1038/gene.2010.44. [DOI] [PubMed] [Google Scholar]
- Okutani D, Lodyga M, Han B, Liu M. Src protein tyrosine kinase family and acute inflammatory responses. Am J Physiol Lung Cell Mol Physiol. 2006;291:L129–41. doi: 10.1152/ajplung.00261.2005. [DOI] [PubMed] [Google Scholar]
- Otten U, Ehrhard P, Peck R. Nerve growth factor induces growth and differentiation of human B lymphocytes. Proc Natl Acad Sci U S A. 1989;86:10059–63. doi: 10.1073/pnas.86.24.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovsyannikova IG, Jacobson RM, Ryan JE, Vierkant RA, Pankratz VS, Jacobsen SJ, Poland GA. HLA class II alleles and measles virus-specific cytokine immune response following two doses of measles vaccine. Immunogenetics. 2005;56:798–807. doi: 10.1007/s00251-004-0756-0. [DOI] [PubMed] [Google Scholar]
- Panther E, Corinti S, Idzko M, Herouy Y, Napp M, la Sala A, Girolomoni G, Norgauer J. Adenosine affects expression of membrane molecules, cytokine and chemokine release, and the T-cell stimulatory capacity of human dendritic cells. Blood. 2003;101:3985–90. doi: 10.1182/blood-2002-07-2113. [DOI] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–9. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–59. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading PC, Khanna A, Smith GL. Vaccinia virus CrmE encodes a soluble and cell surface tumor necrosis factor receptor that contributes to virus virulence. Virology. 2002;292:285–98. doi: 10.1006/viro.2001.1236. [DOI] [PubMed] [Google Scholar]
- Reading PC, Smith GL. Vaccinia virus interleukin-18-binding protein promotes virulence by reducing gamma interferon production and natural killer and T-cell activity. J Virol. 2003;77:9960–8. doi: 10.1128/JVI.77.18.9960-9968.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JE, Dhiman N, Ovsyannikova IG, Vierkant RA, Pankratz VS, Poland GA. Response surface methodology to determine optimal cytokine responses in human peripheral blood mononuclear cells after smallpox vaccination. J Immunol Methods. 2009;341:97–105. doi: 10.1016/j.jim.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaid DJ, Batzler AJ, Jenkins GD, Hildebrandt MA. Exact tests of Hardy-Weinberg equilibrium and homogeneity of disequilibrium across strata. Am J Hum Genet. 2006;79:1071–80. doi: 10.1086/510257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JE, ElKhal A, Freyschmidt EJ, MacArthur DH, McDonald D, Howell MD, Leung DY, Laouar A, Manjunath N, Bianchi T, Boes M, Oettgen HC, Geha RS. Impaired immune response to vaccinia virus inoculated at the site of cutaneous allergic inflammation. J Allergy Clin Immunol. 2007;120:1382–8. doi: 10.1016/j.jaci.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Sharma DP, Ramsay AJ, Maguire DJ, Rolph MS, Ramshaw IA. Interleukin-4 mediates down regulation of antiviral cytokine expression and cytotoxic T-lymphocyte responses and exacerbates vaccinia virus infection in vivo. J Virol. 1996;70:7103–7. doi: 10.1128/jvi.70.10.7103-7107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EJ, Marie I, Prakash A, Garcia-Sastre A, Levy DE. IRF3 and IRF7 phosphorylation in virus-infected cells does not require double-stranded RNA-dependent protein kinase R or Ikappa B kinase but is blocked by Vaccinia virus E3L protein. J Biol Chem. 2001;276:8951–7. doi: 10.1074/jbc.M008717200. [DOI] [PubMed] [Google Scholar]
- Smith VP, Bryant NA, Alcami A. Ectromelia, vaccinia and cowpox viruses encode secreted interleukin-18-binding proteins. J Gen Virol. 2000;81:1223–30. doi: 10.1099/0022-1317-81-5-1223. [DOI] [PubMed] [Google Scholar]
- Souza RP, Romano-Silva MA, Lieberman JA, Meltzer HY, MacNeil LT, Culotti JG, Kennedy JL, Wong AH. Genetic association of the GDNF alpha-receptor genes with schizophrenia and clozapine response. J Psychiatr Res. 2010;44:700–6. doi: 10.1016/j.jpsychires.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Suzuki S, Millar DG, Unno M, Hara H, Calzascia T, Yamasaki S, Yokosuka T, Chen NJ, Elford AR, Suzuki J, Takeuchi A, Mirtsos C, Bouchard D, Ohashi PS, Yeh WC, Saito T. A critical role for the innate immune signaling molecule IRAK-4 in T cell activation. Science. 2006;311:1927–32. doi: 10.1126/science.1124256. [DOI] [PubMed] [Google Scholar]
- Symons JA, Adams E, Tscharke DC, Reading PC, Waldmann H, Smith GL. The vaccinia virus C12L protein inhibits mouse IL-18 and promotes virus virulence in the murine intranasal model. J Gen Virol. 2002;83:2833–44. doi: 10.1099/0022-1317-83-11-2833. [DOI] [PubMed] [Google Scholar]
- Symons JA, Alcami A, Smith GL. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell. 1995;81:551–60. doi: 10.1016/0092-8674(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Team RDC. R: A language and environment for statistical computing. 2008. [Google Scholar]
- Tian T, Liu L, Freyschmidt EJ, Murphy GF, Kupper TS, Fuhlbrigge RC. Overexpression of IL-1alpha in skin differentially modulates the immune response to scarification with vaccinia virus. J Invest Dermatol. 2009;129:70–8. doi: 10.1038/jid.2008.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torcia M, Bracci-Laudiero L, Lucibello M, Nencioni L, Labardi D, Rubartelli A, Cozzolino F, Aloe L, Garaci E. Nerve growth factor is an autocrine survival factor for memory B lymphocytes. Cell. 1996;85:345–56. doi: 10.1016/s0092-8674(00)81113-7. [DOI] [PubMed] [Google Scholar]
- Umlauf BJ, Ovsyannikova IG, Haralambieva IH, Kennedy RB, Vierkant RA, Pankratz VS, Jacobson RM, Poland GA. Correlations between vaccinia-specific immune responses within a cohort of armed forces members. Viral Immunol. 2011;24:415–20. doi: 10.1089/vim.2011.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Den Broek M, Bachmann MF, Kohler G, Barner M, Escher R, Zinkernagel R, Kopf M. IL-4 and IL-10 antagonize IL-12-mediated protection against acute vaccinia virus infection with a limited role of IFN-gamma and nitric oxide synthetase 2. J Immunol. 2000;164:371–8. doi: 10.4049/jimmunol.164.1.371. [DOI] [PubMed] [Google Scholar]
- Yang K, Puel A, Zhang S, Eidenschenk C, Ku CL, Casrouge A, Picard C, von Bernuth H, Senechal B, Plancoulaine S, Al-Hajjar S, Al-Ghonaium A, Marodi L, Davidson D, Speert D, Roifman C, Garty BZ, Ozinsky A, Barrat FJ, Coffman RL, Miller RL, Li X, Lebon P, Rodriguez-Gallego C, Chapel H, Geissmann F, Jouanguy E, Casanova JL. Human TLR-7-, -8-, and -9-mediated induction of IFN-alpha/beta and -lambda Is IRAK-4 dependent and redundant for protective immunity to viruses. Immunity. 2005;23:465–78. doi: 10.1016/j.immuni.2005.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Ng P, Zhao M, Hirankarn N, Lau CS, Mok CC, Chan TM, Wong RW, Lee KW, Mok MY, Wong SN, Avihingsanon Y, Lee TL, Ho MH, Lee PP, Wong WH, Lau YL. Population differences in SLE susceptibility genes: STAT4 and BLK, but not PXK, are associated with systemic lupus erythematosus in Hong Kong Chinese. Genes Immun. 2009;10:219–26. doi: 10.1038/gene.2009.1. [DOI] [PubMed] [Google Scholar]
- Yin H, Borghi MO, Delgado-Vega AM, Tincani A, Meroni PL, Alarcon-Riquelme ME. Association of STAT4 and BLK, but not BANK1 or IRF5, with primary antiphospholipid syndrome. Arthritis Rheum. 2009;60:2468–71. doi: 10.1002/art.24701. [DOI] [PubMed] [Google Scholar]