The role of lipoxygenases in pathophysiology; new insights and future perspectives (original) (raw)
Abstract
Lipoxygenases (LOXs) are dioxygenases that catalyze the formation of corresponding hydroperoxides from polyunsaturated fatty acids such as linoleic acid and arachidonic acid. LOX enzymes are expressed in immune, epithelial, and tumor cells that display a variety of physiological functions, including inflammation, skin disorder, and tumorigenesis. In the humans and mice, six LOX isoforms have been known. 15-LOX, a prototypical enzyme originally found in reticulocytes shares the similarity of amino acid sequence as well as the biochemical property to plant LOX enzymes. 15-LOX-2, which is expressed in epithelial cells and leukocytes, has different substrate specificity in the humans and mice, therefore, the role of them in mammals has not been established. 12-LOX is an isoform expressed in epithelial cells and myeloid cells including platelets. Many mutations in this isoform are found in epithelial cancers, suggesting a potential link between 12-LOX and tumorigenesis. 12R-LOX can be found in the epithelial cells of the skin. Defects in this gene result in ichthyosis, a cutaneous disorder characterized by pathophysiologically dried skin due to abnormal loss of water from its epithelial cell layer. Similarly, eLOX-3, which is also expressed in the skin epithelial cells acting downstream 12R-LOX, is another causative factor for ichthyosis. 5-LOX is a distinct isoform playing an important role in asthma and inflammation. This isoform causes the constriction of bronchioles in response to cysteinyl leukotrienes such as LTC4, thus leading to asthma. It also induces neutrophilic inflammation by its recruitment in response to LTB4. Importantly, 5-LOX activity is strictly regulated by 5-LOX activating protein (FLAP) though the distribution of 5-LOX in the nucleus. Currently, pharmacological drugs targeting FLAP are actively developing. This review summarized these functions of LOX enzymes under pathophysiological conditions in mammals.
Keywords: Lipoxygenase, Phenotype, Signal transduction, Resolvins
1. Introduction
Lipoxygenases (LOXs) catalyze the oxygenation of polyunsaturated fatty acids such as arachidonic acid and linoleic acid [1,2]. The oxygenated lipids initiate subsequent biological reactions, activate cellular signaling mechanisms through specific cell surface receptors, or are further metabolized into potent lipid mediators. LOX can be found not only in mammals, but also in plants. Historically, biochemical characterizations have been performed mainly on soybean LOX isoforms. While the overall structure of mammalian LOX enzymes seems to be similar, each isoform has unique properties, such as substrate specificity (Table 1, reviewed in [3]). In most cases, the structure depends on the shape of the substrate cavity and the coordination of histidine residues or alternatives to a non-heme iron atom at the catalytic center [4,5]. Importantly, LOX enzymes require a lag period for the activation of enzymes from an inactive ferrous form to an active ferric form by either molecular oxygen or lipid hydroperoxides. Enzymatic activity is also regulated by the N-terminal β-barrel region of polypeptides, where this region has a similar amino acid sequence to the C2-like domain; thus, Ca2+-mediated activation via interaction with the plasma membrane has been proposed. Earlier studies have shown that LOX enzymatic activity can be inhibited by phenolic antioxidants such as nordihydroguaiaretic acid and caffeic acid, suggesting a beneficial role of dietary polyphenol intake [6]. Alternatively, synthesized drugs for LOX are relatively limited thus far. The 5-LOX inhibitor zileuton has been accepted and used successfully for the control of asthma. Currently, inhibitors for 5-LOX activating protein are actively developed by many pharmaceutical companies [7]. These inhibitors essentially modulate the transportation of 5-LOX from the nucleus to the cytoplasm, leading to suppressive 5-hydroperoxyeicosatetraenoic acid (5-HPETE) production. This mode of action of 5-LOX inhibitor is unique, and there are no similar regulatory mechanisms and drugs for other LOX isoforms.
Table 1.
Properties of LOX enzymes.
| Proteins | 15-LOX | 15-LOX-2 | 12-LOX | 12R-LOX | eLOX-3 | 5-LOX | FLAP |
|---|---|---|---|---|---|---|---|
| Human | |||||||
| Gene | ALOX15 | ALOX15B | ALOX12 | ALOX12B | ALOXE3 | ALOX5 | ALOX5AP |
| Productsa | 15S-HPETE | 15S-HPETE | 12S-HPETE | 12R-HPETE | Epoxyalcohols | 5S-HPETE | NA |
| Expression | Leukocytes | Epithelium, leukocytes | Myeloids, skin, epithelium | Skin, epithelium | Skin, epithelium | Leukocytes | Leukocytes |
| Mouse | |||||||
| Gene | Alox15 | Alox15b | Alox12 | Alox12b | Aloxe3 | Alox5 | Alox5ap |
| Productsa | 12S-, 15S-HPETE | 8R-HPETE, epoxyalcohols | 15S-, 12S-HPETE | 12R-HPETE | Epoxyalcohols | 5S-HPETE | NA |
| Expression | Leukocytes | Skin, epithelium, leukocytes | Platelet, skin, epithelium | Skin, epithelium | Skin, epithelium | Leukocytes, epithelium | Leukocytes |
From a genetic point of view, the alignment of LOX isoform nucleotides encoded by arachidonate lipoxygenase (ALOX in humans and Alox in mice) genes has revealed that ALOX5 and other ALOX genes have separate origins. The other ALOX genes seem to have originated from fewer genes, as human ALOX genes are found in a cluster in chromosome 17p13.1 and murine Alox genes are found in chromosome 11 as active enzymes [8]. The expression levels of ALOX genes are partially controlled by cytokines, such as ALOX15, whose expression increases in response to Th2 cytokines. ALOX enzymatic activity is also regulated by tissue distribution and cell type. ALOX12B, ALOXE3, and ALOX15B are expressed mainly in the skin and other epithelial cells, whereas ALOX15, ALOX12, and ALOX5 are expressed in hematopoietic/immune cells. They are involved in atherosclerosis, neuronal disorder, immune modulation, skin diseases, and maintenance of the epithelium. The roles of human enzymes (Table 2) seem to be slightly different from what is expected from phenotypes of knockout mice (Table 3), which shows that these oxygenated lipids are uniquely and finely regulated in humans and mice.
Table 2.
Human diseases that potentially links to lipoxygenase genes.
| Genes | Atherosclerosis/heart disease | Immune response | Neurological disorder | Cancer | Skin disease | Others |
|---|---|---|---|---|---|---|
| ALOX15 | A near null mutant (T560M) in coronary artery disease [140] | Rectal cancer [100] | Bone mineral density↓[56,143–144] | |||
| Colon cancer [141] | ||||||
| Adenoma recurrence [142] | ||||||
| Breast cancer [39] | ||||||
| Prostate cancer [12] | ||||||
| ALOX15B | ↑ in carotid plaque with thrombosis [43] | Esophageal cancer [35] | ||||
| ↑ in carotid lesion [32] | Adrenocortical tumor [36] | |||||
| Variants in coronary artery disease [145] | Breast cancer [39] | |||||
| Epithelial tumors [37] | ||||||
| Prostate cancer cells [38] | ||||||
| Head and neck carcinoma [40] | ||||||
| ↑ in TAMs isolated from renal cell carcinoma [42] | ||||||
| ALOX12 | Variants in subclinical atherosclerosis [146] | Variants in Toxoplasmosis [147] | Bipolar disorder [49] | Methylation in AML[61] | Bone mineral density↓[54–57,150] | |
| Schizophrenia [50] | Esophageal squamous cell carcinoma [64] | Fat mass↑[53] | ||||
| Rectal cancer [100] | ||||||
| Adenoma recurrence [142] | ||||||
| Colorectal cancer [148] | ||||||
| E261R mutation | ||||||
| Breast cancer [48] | ||||||
| Colon cancer [47] | ||||||
| Colorectal cancer [65] | ||||||
| Colorectal adenoma [149] | ||||||
| ALOX12B | ARCI [81–82, 94,151] | |||||
| NCIE [84–86] | ||||||
| ALOXE3 | ARCI [82, 94,151] | |||||
| NCIE [84,86] | ||||||
| ALOX5 | Variants in atherosclerosis [152] | Variants in asthma [97,154,155] | Inverse correlation | |||
| ↑ in atherosclerosis [153] | Variants in AHR [98,99] | Rectal cancer [100] | ||||
| Variants in subclinical atherosclerosis [146] | Ovarian cancer [101] | |||||
| Colon cancer [47] | ||||||
| ALOX5AP | Variants in subclinical atherosclerosis [146] | Variants in asthma [126,156] | Rectal cancer [100] | |||
| Adenoma [100] |
Table 3.
Phenotypes of LOX-deficient mice.
| Genes | Atherosclerosis | Immune response | Neurological disorder | Others |
|---|---|---|---|---|
| Alox15 | ↓ in ApoE KO [19–21] | LTC4↑[26] | Peripheral diabetic neuropathy↓[163] | Osteoclast development↑[28] |
| ↓ in LDLR KO [22,23] | Arthritis↑[157] | Diabetic autonomic neuropathy→[163] | Insulin resistance↓[164] | |
| VSMC response↓[24] | Schistosoma mansoni infection→[158] | Angiogenesis↓[165] | ||
| Th1↓[159] | Myeloid differentiation↓[27] | |||
| IL-12 ↓, TNF-α → [160] | Erythrocyte development→[26] | |||
| Acute lung injury↓[161] | Angiogenesis↓[166] | |||
| Phagocytosis↑[162] | ER stress↓[167] | |||
| Hypertension↓[168] | ||||
| Inflammatory neovascularization↓[169] | ||||
| Nonalcoholic fatty liver disease↓[170] | ||||
| Diabetes associated pp38 and pErk↓[171] | ||||
| Obesity↓[29] | ||||
| Ischemic cardioprotection↓[25] | ||||
| Airway epithelial injury in asthma↓[172] | ||||
| Alox15b | Epidermal permeability barrier↓ [44] | |||
| Alox12 | Platelet sensitivity↑[67] | Carcinoma (B6/129)↓[68] | ||
| Papilloma (SENCAR)↓[68] | ||||
| Basal transepidermal water loss↑[69] | ||||
| Alox12b | Skin barrier↓[87,95] | |||
| Ichthyosiform↑[88] | ||||
| Aloxe3 | Skin barrier↓[95] | |||
| Alox5 | ↓ in LDLR KO [114] | PAF-induced lethal shock↓[102,103] | Anxiety-like behavior (C57BL/6)↑[117] | Inflammatory neovascularization↓[169] |
| OVA-induced asthma↓[108] | Synaptic dysfunction↓[119] | Endotoxin-induced Hypoxic pulmonary vasoconstriction↓[176] | ||
| Schistosoma mansoni infection↓[158] | Anxiety-like behavior (B6/129)↓[175] | _Apc_Δ_468_-induced intestinal polyposis↓[121–123]. | ||
| Early female mortality (MRL-lpr/lpr)↓[109] | ||||
| Toxoplasma gondii elimination↓[107] | ||||
| Tumor-infiltrating macrophages↑[121] | ||||
| OVA/alum-induced | ||||
| Th2↓[159] | ||||
| Peritonitis↓[105] | ||||
| LTB4↓[173] | ||||
| Acute pancreatits↓[104] | ||||
| Borrelia burgdorferi elimination↓[106] | ||||
| Histoplasma capsulatum elimination↓[174] | ||||
| Alox5ap | ↓ in COX-2 KO [115] | PAF-induced shock↓[127] | Improved Alzheimer's disease-like phenotype [133] | |
| Zymozan-induced peritonitis↓[127] | Anxiety-like behavior↑[134] | |||
| Collagen-induced arthritis↓[129] | ||||
| Cerebral inflammation↓[128] |
2. 15-Lipoxygenase (15-LOX)
15-LOX is a prototypical enzyme catalyzing oxygenation of polyunsaturated fatty acids. Among various mammalian species, rabbit reticulocyte LOX has been characterized from earlier studies and often used as standard for biochemical assays. When the potential link between atherosclerosis and its inhibition with antioxidants was explored, 15-LOX has been hypothesized to initiate and/or promote atherosclerosis through low density lipoprotein (LDL) oxidation. This is based on the “oxidative LDL theory” that oxidation of lipids induces atherosclerosis and its inhibition by antioxidants prevents atherogenesis. To initiate oxidation in vivo, there must be some initiators for lipid peroxidation. One potential candidate includes carbon-centered free radicals, which react with molecular oxygen to give rise to peroxyl radicals. Since these radicals trigger oxidation of lipids continuously, therefore, generation of such free radical-generating initiators must be tightly regulated in vivo. Lipid hydroperoxides generated from polyunsaturated fatty acids by LOX enzyme can induce this reaction since these oxidation products further decompose into other free radicals in the presence of metal ions. 15-LOX can be found in macrophages and other immune cells as well as epithelial cells. Human and mouse enzymes are known to be induced by Th2 cytokines such as IL-4 and IL-13 via STAT6-dependent manner [9,10].
2.1. ALOX15
It is widely accepted that cyclooxygenase (COX) inhibitor non-steroidal anti-inflammatory drugs (NSAIDs) induce colon cancer in humans [11]. One suggested reason is that the balance between COX and LOX determines tumorigenesis critically. Under low COX activity, arachidonic acid released from cell membranes in response to external stimuli is preferentially metabolized by LOX enzymes. There is evidence that a 15-LOX metabolite 13S-HPODE (13S-hydroperoxyoctadecaenoic acid) generated from linoleic acid induces apoptosis in colon cancer cells; thus, defective expression of ALOX15 in colon cancers could promote tumorigenesis [11]. ALOX15 expression itself is controlled, at least in part, by the epigenetic process, as an alteration of methylation in the ALOX15 promoter has been observed in prostate cancer patients [12]. Furthermore, the expression of 15-LOX in epithelial cancer cells is tightly regulated by additional mechanisms. As mentioned, STAT6 is a critical regulator of ALOX15 expression regulated by its phosphorylation and acetylation, as well as histone modification [13,14]. Recent studies have also shown the ALOX15 expression can be modulated by the chromatin-dependent STAT6-independent mechanism [15,16]. Biochemically, the produced 13 S-HPETE interacts with PPAR-δ, followed by the induction of apoptosis prior to carcinogenic conditions [17]. The importance of 15-LOX-derived metabolites has also been defined by its aberrant failure in conditional transgenic mice, expressing it in the mouse prostate, inducing prostatic intraepithelial neoplasia once the apoptotic function is dysregulated [18].
2.2. Alox15
Atherosclerosis is an inflammatory disease characterized by an accumulation of lipid-loaded macrophages in blood vessels. Evidence has suggested a close link between Alox15 expression and atherosclerosis in the mouse, characterized mostly on the atherosclerosis-prone genetic background of mice lacking the ApoE and/or LDL receptor [19–23]. In both cases, the initiation and/or development of atherosclerosis depends on 15-LOX enzymatic activity. Recent studies have suggested that atherosclerosis is involved, at least in part, in sterile inflammation characterized by augmented IL-1β and the activation of caspases. The accumulation of lipids in blood vessels causes a failure of vascular function; therefore, disruption of Alox15 shows impaired migration of vascular smooth muscle cells [24]. In addition, ischemia-induced cardioprotection plays an important role in maintaining proper heart function. A previous study showed that Alox15 deficiency caused its impairment after reperfusion-mediated preconditioning by PKC activation in the heart [25]. These results suggest that this enzyme might also contribute to the pathogenesis of cardiovascular disease.
Lipid hydroperoxides, the primary reaction products of Alox15 from polyunsaturated fatty acids, readily induce oxidative stress through their decomposition to free radicals. Thus, _Alox15-_deficient mice might produce less oxidative stress under physiological conditions. However, _Alox15-_deficient mice have shown an increase in oxidative stress markers such as isoprostane 8-epi-prostaglandin F2a in a zymosan-induced peritonitis model [26]. These apparently paradoxical results might be explained by the concomitantly enhanced 5-LOX-, but not 15-LOX-, dependent mechanism, which facilitates the accumulation of neutrophils by 5-HPETE and leukotrienes, thereby leading to enhanced oxidative stress at the site of inflammation in _Alox15-_deficient mice.
Accumulating evidence suggests that Alox15 is critically involved in the regulation of cell differentiation. For example, the development of hematopoietic stem cells into the myeloid lineage is impaired in _Alox15-_deficient mice [27]. Alternatively, the formation of osteoclasts in _Alox15-_deficient mice and mice treated with enzymatic inhibitors has indicated its attenuation [28]. This impaired bone-desorbing osteoclastogenesis is negatively regulated by osteoblast formation, which derives from mesenchymal stem cells. Given that this bone-absorption is impaired in _Alox15-_deficient mice, bone-generating osteoblast formation is likely to be enhanced. In this case, adipocytes, which also stem from mesenchymal stem cells, would be impaired. Consistent with this speculation, _Alox15-_deficient mice have displayed impaired obesity [29], indicating a critical role of Alox15 in cell differentiation.
3. 15-Lipoxygenase, type B (15-LOX-2)
15-LOX-2 shows higher similarity to 15-LOX, initially discovered in the skin in humans [30]. Human 15-LOX-2 generates 12R-HPETE from arachidonic acid specifically, showing unusual specificity for the regioisomeric lipid mediators in contrast to most isozymes, which produce S-regioisomers. Furthermore, mouse ortholog of 15-LOX-2 generates 8S-HETE and 8S-, 15S-diHPETE from arachidonic acid, thus enzymologically murine Alox15b is considered to be similar to human ALOX12 [31]. Due to this, there are almost no overlapping diseases/phenotypes in the human and mouse in this isoform.
3.1. ALOX15B
Although a link between atherogenesis and _ALOX1_5 expression has long been hypothesized, it is known that the expression of ALOX15B in human carotid plaque macrophages is higher compared to ALOX15 [32,33]. An in vitro experiment of ALOX15B silencing reported an attenuated lipid accumulation in human macrophages, indicating that it is functional for lipid uptake into the cells [34]. Thus, it is suggested that ALOX15B plays an important role in the initiation and development of atherosclerosis in humans.
Several studies have shown the downregulation of ALOX15B in epithelial tumors, suggesting that ALOX15B has an antiproliferative role [35–40]. This reduction of 15-LOX-2 in tumor cells was restored by an inhibitor for COX enzyme, demonstrating that its expression is negatively regulated by prostaglandins, at least in part [35]. PPAR-γ, a nuclear receptor regulated by endogenous LOX products, was upregulated in some epithelial tumors, suggesting that the downregulation of ALOX15B is autonomously controlled by PPAR-γ in epithelial cancer cells [37]. In prostate epithelial cells, ALOX15 expression is positively regulated by transcription factor Sp1, whereas transcription factor Sp3, which is closely related to Sp1, negatively regulates its expression, suggesting that ALOX15B expression is critically regulated by multiple regulators [41]. Apart from epithelial cells, a separate study demonstrated increased ALOX15B expression in tumor-associated macrophages from renal cell carcinoma, suggesting that ALOX15B expression is distinctly regulated in epithelial cancer cells and macrophages [42]. Similar to tumor-associated macrophages, the upregulation of ALOX15B in carotid plaque macrophages has also been described [32,43].
3.2. Alox15b
There are a limited number of studies involving Alox15b. In chimeric mice transplanted with _Alox15b_-silenced bone marrow cells in mice lacking LDL receptor showed defective atherogenesis, suggesting that 15-LOX-2 is required in the murine atherosclerotic model [34]. As mentioned previously, the enzymatic action of murine 15-LOX-2 preferentially generates 12S-HPETE rather than 15S-HPETE from arachidonic acid, suggesting that mouse Alox15b and human ALOX12 have similar roles in vivo. The skin of _Alox15b-_deficient mice has been shown to display an ichthyosiform appearance, as in Alox12b- and _Aloxe3-_deficient mice [44]. This example clearly shows that the skin phenotype found in _Alox15b-_deficient mice seems to require specific oxidation products from polyunsaturated fatty acids. Furthermore, this result suggests that Alox15b could be a functional alternative for Alox12b and Aloxe3.
4. 12-Lipoxygenase (12-LOX)
12-LOX enzymes derived from ALOX12 in humans and its murine ortholog Alox12 (also known as platelet-typed 12S-LOX) produce 12S-HPETE from arachidonic acid. These enzymes are expressed in leukocytes in humans and in platelets, megakaryocytes, and skin in mice [45,46]. Genetic studies have suggested that ALOX12 gene polymorphisms are associated with cancers [47,48], neurological disorders [49,50], hypertension [51,52], fat mass [53], and bone mineral density [54–57]. The mechanism of regulation of 12-LOX expression has been studied in several models. The ALOX12 promoter region contains at least four binding sites for RUNX1, leading to its suppressive effect in human erythroleukemic cells [58]. Alox12 expression is also regulated by transcription factor p63 in the skin, which plays a key role in the development and terminal differentiation of the epidermis [59]. Similar to other isozymes, human 12-LOX has a catalytic domain in the C-terminal. Truncation of the N-terminal domain (which needs to be explored in terms of function) decreases its enzymatic activity at approximately 20% without altering substrate specificity [60].
4.1. ALOX12
Since the discovery of the high expression of LOX in platelets, the mechanism of its expression has been studied in detail. A previous study reported that ALOX12 expression was attenuated in platelets by the haplodeficiency of RUNX1, a hematopoietic transcription factor associated with familial thrombocytopenia, platelet dysfunction, and a predisposition to acute leukemia in patients with thrombocytopenia [58]. ALOX12 expression is also regulated epigenetically, as indicated by the increase in DNA methylation of ALOX12 genes in myelodysplastic syndrome and acute myeloid leukemia patients with megakaryocytic dysplasia [61,62]. It has been suggested that ALOX12 is associated with diminished bone mineral density as well [54–57]. Given that 12-LOX produces endogenous lipid ligands for nuclear receptors, such as PPAR-γ, which facilitate adipocyte differentiation from mesenchymal stem cells, the number of osteoblasts decreases, followed by impairment of bone mineral density [55].
An earlier study suggested that the 12-LOX-mediated pathway is associated with the risk of colorectal cancer [63]. The best-studied example includes mutation of E261R (835A>G), which causes an increase in 12-LOX activity, with a potential link to esophageal squamous cell carcinoma [64]. This mutation has also been associated with colorectal cancer [47,65] and breast cancer [48]. An in vitro and in vivo study has shown that 12-LOX plays a role in the proliferation and antiapoptosis of hepatocellular cells, suggesting that this carcinogenic function of ALOX12 requires endogenously generated lipid mediators [66].
4.2. Alox12
Consistent with the expression of ALOX12 in the platelets of humans, mice lacking Alox12 have shown increased platelet sensitivity and mortality due to thrombosis in response to the administration of adenosine diphosphate, whereas aggregation and secretion in response to most agonizts seemed normal [67]. Alox12 deficiency has led to a reduced incidence of carcinoma in a C57BL6/129 genetic background and of papilloma in a tumor-sensitive SENCAR genetic background, showing that Alox12 is involved in tumorigenesis in the skin in a context-dependent manner [68]. Most notably, Alox12 deficiency has caused basal transepidermal water loss in the skin with unaltered inflammatory responses in Alox12b- and _Aloxe3-_deficient mice [69]. This finding suggests that Alox12 has a critical role in the maintenance of the skin barrier in association with other isoforms, as explained in detail later.
5. 12-Lipoxygenase, 12R type (12R-LOX)
In humans, 12R-LOX has been detected in keratinocytes, tonsil squamous epithelial cells, bronchial epithelial cells, and psoriasis scales, as well as in B cells [8,70–74]. In mice, its expression has been induced at embryonic day (E) 15.5 in the epidermis, nasal epithelium, and surface of the tongue, suggesting that 12R-LOX is required for the proper development of neonates [71].
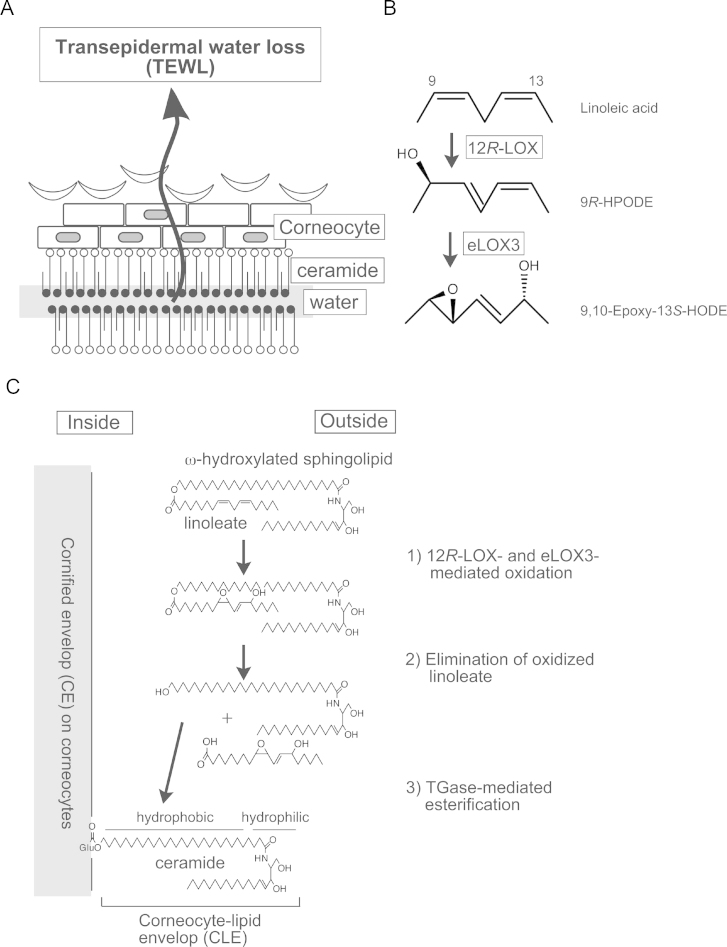
The physiological role of 12R-LOX is rather specific, in conjunction with its limited expression profile in epithelial cells. The best-characterized example is the skin. The important physiological role in the skin is to maintain appropriate moisture by preventing unnecessary water evaporation through epithelial cells, called transepithelial water loss (TEWL) (Fig. 1A). TEWL results in a loss of water from the skin, leading to excessively dry skin, as found in ichthyosis. In humans, this inherited disease is known as autosomal recessive congenital ichthyosis (ARCI) (MIM#s 190195, 242100, 242300). Biochemically, 12R-LOX reacts readily with linoleate rather than arachidonate to produce 9R-HPODE (Fig. 1B). This hydroperoxide is converted into its associated epoxide derivatives through the isomerase activity of eLOX-3 [75]. In the skin, the best substrate for these enzymes is linoleate, which is esterified with ω-hydroxylated sphingolipids, usually found outside corneocytes during the immature stage of skin development (Fig. 1C). As mentioned previously, this esterified linoleate is further converted into oxygenated linoleate, followed by elimination from sphingolipids by hydrolysis. The newly formed ceramide is ω-terminally hydroxylated; therefore, it is subsequently linked covalently to a carboxyl acid moiety of glutamine in cornified envelope (CE) proteins. The established lipid layer is called a corneocyte-lipid envelope (CLE), and it plays a crucial role in holding water in the hydrophobic group, in ceramides in the CLE. The completion of CLE formation requires transglutaminase-1, which catalyzes the cross-linking of the ceramides with the carboxylic acid in the side chain of glutamate in CE proteins, as many mutations of 12R-LOX enzymes cause ARCI in humans [76]. Mice lacking the transglutaminase-1 gene have consistently exhibited defective skin formation and high TEWL [77]. Both enzymes act critically prior to this transglutamination, as previous observations have suggested that a deficiency of 12R-LOX, as well as eLOX-3, causes failure of the skin barrier due to defective formation of enzymatic lipid oxidation products [78–80].
Fig. 1.
A. Schematic representation of transepidermal water loss (TEWL) from the skin. B. Reaction of 12R-LOX and eLOX-3. C. Development of corneocyte-lipid envelop (CLE) over cornified envelop (CE) on corneocyetes.
5.1. ALOX12B
Genetic failure of the above process leads to ARCI, a heterogeneous skin disease characterized by rough and scaly skin, with a prevalence of one in 200,000 newborns throughout the world [81–86]. The affected skin generally improves during either childhood or puberty, and these patients have a normal life span. Among various ARCI diseases, ALOX12B is mainly, but not solely, involved in nonbullous congenital ichthyosiform erythroderma. Mutations, mostly found as missense, termination, and frameshift in ALOX12B, are widely found in its entire molecule, involving both in a C-terminal catalytic domain and an N-terminal β-barrel structure [82]. Some mutants have lost enzymatic activity, as confirmed by biochemical assays.
5.2. Alox12b
Disruption of the Alox12b gene in the murine model provides an effective means of studying human ichthyosis. _Alox12b-_deficient mice suffer postnatal death characterized by a severely impaired barrier function of the skin [87]. This defective epidermal barrier appears around E17.5 in wild-type (WT) controls, prior to which the expression of Alox12b reaches its maximal level beginning at E15.5 and continues after birth. Thus, there is a strong correlation between Alox12b expression and the formation of functional epidermis.
A study using skin transplantation from _Alox12b_-deficient neonates into nude mice revealed ichthyosiform formation, typically characterized by a thickening of the epidermis and severe hyperkeratosis, with a phenotype similar to that of patients with ALOX12B mutations in the grafted mice [88]. Essentially, the skin grafted from the neonates became thicker than that from the WT controls, with a hyperplastic histology displaying epidermal acanthosis and severe hyperkeratosis. Further investigation of this hyperkeratosis by electron microscopy revealed that the stratum corneum of skin grafted from _Alox12b-_deficient mice was abnormally overlaid, indicative of aberrant proliferation. In addition, both the size and number of keratohyaline granules increased, indicating hypergranulosis in mutant skin grafts. Functional assays that measured TEWL identified a marked increase in the skin from the neonates, as well as a marginal but significant increase in mature skin grafted in these mice, demonstrating that Alox12b plays a critical role in the maintenance of barrier function in the skin (Fig. 1A). Among genetically manipulated mice with defects in barrier function in the skin, such as KLF4- and _Claudin-_deficient mice [89,90], _Alox12b-_deficient mice displayed an extremely defective phenotype in epidermal barrier function, but not in tight junctions.
6. Epidermal lipoxygenase 3 (eLOX-3)
As mentioned previously, the conversion of linoleoyl ceramide in CLE into CE plays a crucial role in the proper maintenance of epidermal barrier formation. The eLOX-3 enzyme plays a major role in the second step, which involves the conversion of 9R-HPODE esterified with ω-hydroxyacyl-sphingosine into its related epoxylated derivative (Fig. 1B) [91–93]. Biochemical reactions catalyzed by eLOX-3 are essential, and their failure leads to ARCI.
6.1. ALOXE3
Genetically, many mutations in ALOXE3, together with ALOX12B, have been found in ichthyosis [81–84,86,94]. The outcome of disease in ALOXE3 variants seems to be similar to that found in ALOX12B variants, showing clearly that this sequential oxidation by ALOXE3 and ALOX12B are equally important. As a substrate, it is known that eLOX-3 favors oxygenated lipids like 12R-HPETE rather than unoxidized compound such as arachidonic acid (Fig. 1B). Other than ARCI, other diseases associated with ALOXE3 variants have not been reported.
6.2. Aloxe3
A previous study showed that _Aloxe3-_deficient mice also exhibit a similarly severe ichthyosis phenotype with the loss of covalently bound ceramides and impaired CLE development [86]. As expected from its hepoxilin synthase activity of eLOX-3, in vivo results also showed a marked reduction of its metabolites in the skin. Similar to _Alox12b-_deficient mice, _Aloxe3-_deficient mice displayed a postnatal lethal phenotype [95].
7. 5-Lipoxygenase (5-LOX)
5-LOX plays an important role in the control of asthma. Under asthmatic conditions, activated immune cells first produce arachidonic acid by an enzymatic action of phospholipase A2 from the plasma membrane, followed by 5-HPETE production through the 5-LOX enzyme. The produced 5-HPETE then converts into leukotrienes that have a potent biological effect on the constriction of bronchioles through cysteinyl leukotriene receptor 1, which is expressed exclusively onto bronchiolar smooth muscle cells, but not epithelial cells [96]. Conversely, cysteinyl leukotriene receptor 2 is expressed strongly in pulmonary interstitial macrophages and weakly in smooth muscle cells.
7.1. ALOX5
The expression of the ALOX5 gene is transcriptionally regulated at a basal level and regulated by external stimuli such as Ca2+. Numerous agents have been developed as antiasthmatics. A potent LOX-5 inhibitor zileuton introduced in the United States blocks a considerable amount of cysteinyl leukotriene production for a short period of time. Although the prevalence is low, a genetic E254K (760G>A) mutation in ALOX5 has been reported in bronchiolar asthma patients [97]. This mutation causes an alteration in the electronic charge of the C-terminal catalytic domain from negative to positive, implicating defective changes in enzymatic activity and protein interaction. Mutations in the Sp1 binding site in the ALOX5 promoter has been associated with airway hyperresponsiveness, but not with asthma [98,99]. Some evidence suggests that low 5-LOX expression in tumors found in humans might lead to greater 15-LOX expression followed by cancer formation through impaired apoptotic activity [47,100,101].
7.2. Alox5
7.2.1. Inflammation
As mentioned previously, immune cells express leukotriene receptor 1 and 2 as well as cysteinyl leukotriene receptor 2; as such, the role of Alox5 could account for the migration of these immune cells. As expected, neutrophilic inflammation is one of the apparent phenotypes in _Alox5-_deficient mice. These mice have been shown to be resistant to anaphylaxis induced by platelet-activating factor (PAF), showing that Alox5 seems to be closely involved in this process [102,103]. Similarly, _Alox5-_deficient mice exhibit a suppressed response to chemically induced local inflammation [104,105]. These mice are also susceptible to _Borrelia burgdorferi_-induced arthritis [106]. In this study, the authors showed that enzymatic activity of 5-LOX is not required for the initiation of infection, but it is required for earlier joint swelling and retarded arthritis recovery, suggesting a potential increase in the accumulation of neutrophils. Similarly, in a Toxoplasma gondii infection model, _Alox5-_deficient mice exhibited suppressed leukotriene A4 production and increased interleukin-12 and interferon-γ production, followed by an increase in mortality rate due to marked encephalitis [107]. Such altered cytokine production seems to be explained, at least in part, by impaired neutrophilic inflammation.
In an ovalbumin-induced asthma model, _Alox5-_deficient mice exhibited a suppressed methacholine-induced response to airway hyperresponsiveness with impaired eosinophilic inflammation in the lung [108]. Thus, the production of lipid products from 5-LOX plays an important role under physiological conditions, and its level is tightly regulated by the balance between LOX and other enzymes, such as COX, as shown in an earlier study. Another study reported an increased mortality rate in _Alox5-_deficient male mice with an autoimmune-prone MRL-lpr/lpr genetic background, raising the possibility that an enhanced renal autoimmune inflammation could be involved in this process [109].
Recently, the roles of oxidation products derived from eicosapentaenoic acid (20:5) and docosahexaenoic acid (22:6) have been studied extensively (Fig. 2). As seen in their chemical structures, 20:5 and 22:6 have four and five bisallylic carbon atoms, respectively, in their molecules, providing a variety of oxidation products through free radical-mediated mechanisms. For example, 20:5 is a primary substrate for conventional LOX enzymes such as 15-LOX, 12-LOX, and 5-LOX. In addition, 18-hydroperoxy-5,8,11,14,16-eicosapentaenoic acid (18-HPETE), a free radical-mediated oxidation product of 20:5, can be further metabolized by 5-LOX to produce a novel class of oxidation product collectively called resolvins (Fig. 2) [110,111]. Emerging evidence has shown that resolvins assist in terminating inflammation through specific GPCR ChemR23 at nM concentrations in vitro [112]. The formation of resolvins seems to be critically regulated by local O2 concentration, as well as the expression and activity of multiple LOX enzymes, both of which influence the final yield of resolvins from its initial substrate 20:5. Due to its anti-inflammatory function, whether these oxidized lipids might modulate the functions of microRNAs is under investigation [113].
Fig. 2.
Formation of resolvins from eicosapentaenoic acid (20:5).
7.2.2. Atherosclerosis
Apart from reactions in asthma and neutrophilic inflammation, there is some research showing that 5-LOX plays a key role in the initiation and/or development of atherosclerosis. Impaired expression of functional 5-LOX in LDL receptor-deficient mice has revealed suppressed atherogenesis, suggesting that 5-LOX plays a causative role in this disease [114]. Consistently, another atherosclerotic model induced by COX-2 disruption attenuated disease formation in _Alox5-_deficient mice [115]. Given that LOX-15 is involved in atherosclerosis, these studies provide examples that atherosclerosis can be induced by lipid peroxidation products formed from any isoforms. This finding is entirely consistent with observations indicating that antioxidants generally exhibit protective effects in experimental models.
7.2.3. Neuronal disorder
Alox5 is known to be highly expressed in neuronal tissue, particularly in Alzheimer’s disease; thus, its role in neuronal disorders has been actively characterized [116]. A recent study reported that aged female _Alox5-_deficient mice exhibited protective effects against anxiety-like behavior on a C57BL/6 genetic background, raising the possibility that Alox5 could modulate neuronal function [117]. A subsequent study using a transgenic mouse model of Alzheimer’s disease demonstrated the efficacy of a 5-LOX inhibitor zileuton and hypothesized that Alox5 could facilitate the initiation or progression of this disease [118]. Using such a disease model, Alox5 deficiency consistently improved disease phenotypes [119].
7.2.4. Tumor
Colorectal cancer is often caused by mutation of the tumor suppressor Adenomatous polyposis coli (APC) gene. Among many mouse models generated by Apc mutations, Apc_Δ_468 mice specifically bear a truncated Apc gene that develops severe polyposis by four months [120]. Interestingly, immunohistochemistry showed an increase in Alox5 expression in Apc_Δ_468 mice, suggesting that LOX-5 might contribute to tumorigenesis in colorectal cancer [121–123]. Mast cells play an important role in the development of colorectal cancer in this animal model, as they induce epithelial proliferation [122]. Consistently, the number of mast cells increased in APC_Δ_468 mice compared to WT controls. In this model, a deficiency in Alox5 led to impairment, implying that LOX5 acts as an important role in colorectal tumorigenesis [122].
8. 5-Lipoxygenase activating protein (FLAP)
FLAP is a small protein that activates 5-LOX through protein interaction. The formed complex of 5-LOX and FLAP in the nucleus efficiently generates 5-HEPE from arachidonic acid, which is subsequently converted into various leukotrienes. FLAP protein stays on the nuclear membrane and acts as a transporter for 5-LOX. FLAP expression is limited in myeloid cells.
8.1. ALOX5AP
Drugs targeting FLAP protein have been actively developed in humans. DG-031 (veliflapon, BAY x 1005), first licensed by DeCode Genetics and then developed by Bayer, is one example. This compound also reduces the incidence of ischemic myocardial infarction by reducing LTB4 production [124]. A recently developed AM-103/GSK2190914 was designed based on the three-dimensional structure of FLAP protein [125]. In asthma, two intronic single-nucleotide polymorphisms have been associated with ALOX5AP, suggesting that these mutations can be used for diagnostic markers [126].
8.2. Alox5ap
_Alox5ap-_deficient mice exhibited unique phenotypes similarly observed in _Alox5-_deficient mice, such as an impaired response to PAF-induced anaphylaxis and zymosan-induced peritonitis [127]. In a collagen-induced arthritis model, _Alox5ap-_deficient mice displayed impaired arthritis, whereas the accumulation of antibody against collagen remained unchanged, suggesting that 5-LOX positively regulated inflammation without affecting the immune response. In a cerebral artery occlusion model, disruption of the Alox5ap gene caused impaired median infarct size and a better functional score, demonstrating that FLAP protein positively regulates cerebral inflammation [128]. The expression of Alox5ap is independent of the expression of 5-LOX. Alox5ap induces 12S-HETE production in the 12-LOX-induced signaling pathway, suggesting that 5-HPETE or downstream leukotriene metabolites might be involved in this process [129].
In atherosclerosis, FLAP inhibitor MK-886 and BAY x 1005 effectively attenuated disease formation in mice lacking ApoE and LDL receptors [130,131]. Similarly, another atherosclerotic model developed by transgenic mice expressing a dominant negative form of TGFβ receptor II in _ApoE-_deficient mice was suppressed by MK-886 [132]. These examples strongly suggested that FLAP protein is required for atherogenesis in these mouse models. Consistently, _Alox5ap-_deficient mice exhibited attenuated disease formation in an experimental model generated by _Cox-2-_deficient mice [115].
_Alox5ap-_deficient mice displayed an improved Alzheimer’s disease-like phenotype [133] and an apparent increase in anxiety-like behavior in aged mice [134]. These results supported the phenotype of _Alox5-_deficient mice, showing that both 5-LOX and FLAP mutually and collaboratively play critical roles in the leukotriene pathway in neurological disorders.
9. Clinical Trials
There are many studies reporting synthesis and characterization of lipoxygenase inhibitors (reviewed in [135,136]). Generally, substances sharing similarity to either lipids or phenolic antioxidants have lower inhibitory activity for LOX enzyme. Furthermore, due to the presence of multiple isoforms, the development of selective inhibitor seems to be challenging. Therefore, there is a limited number of successful drugs that can be used for therapeutic purposes. One clinically applicable example includes zileuton that acts as anti-asthmatic agent by inhibiting 5-LOX. Apart from direct regulation of enzymes, there are some studies targeting FLAP for modulating 5-LOX under pathophysiological conditions in clinical trials.
9.1. GSK2190915
This is a FLAP inhibitor that inhibits the production of LTB4 and other cysteinyl leukotrienes. The results of Phase I study showed that there was no clear difference in adverse events between placebo and drug-treated subjects in Western Europe (EURDACT2007-00484872) and Japan (NCT00955383) [137]. Plasma concentration of GSK2190915 reaches maximal at two hours after oral administration. Consistently, LTB4 production in drug-treated subjects was significantly impaired (EC50 in plasma is approximately 85 nM). Its beneficial effect is proven in adults and adolescents with persistent asthma (NCT01147744) [138]. Comparison between GSK2190915 and established asthma treatment such as montelukast and the inhaled corticosteroid fluticasone propionate revealed that GSK2190915 30 mg once daily has similar effect compared to montelukast 10 mg once daily as assessed by forced expiratory volume in 1 s (FEV1), a widely used measure for asthma evaluation. A subsequent study reported that GSK2190915 50 mg daily showed clear attenuation in early asthmatic response induced by inhaled allergens in a placebo-controlled double-blind randomized study in UK (NCT00812773) [139].
10. Conclusions
The physiological roles of LOX enzymes have been studied extensively, due to the close link between them and various diseases. Apparently, the best characterized example includes the relation between 5-LOX and asthma, because cysteinyl leukotriene receptor 1, a GPCR activated by leukotrienes produced by 5-LOX, is widely expressed on bronchiolar smooth muscle cells. Genetically, both ALOX12B and ALOXE3 play a critical role in the development of ichthyosis through TEWL. In humans, some mutations in ALOX12 are found in tumor cells, suggesting this isoform might have anti-tumor effect. An increased expression of LOX enzymes in response to Th2 cytokines has been well established; how their expression is controlled and its consequences need to be investigated in the future.
Acknowledgment
We thank Professor Emeritus Etsuo Niki of the University of Tokyo for suggesting the importance of the study of resolvins mentioned in this article.
References
- 1.Brash A.R. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J. Biol. Chem. 1999;274:23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- 2.Radmark O., Samuelsson B. 5-Lipoxygenase: mechanisms of regulation. J. Lipid Res. 2009;50(Suppl):S40–S45. doi: 10.1194/jlr.R800062-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schneider C., Pratt D.A., Porter N.A., Brash A.R. Control of oxygenation in lipoxygenase and cyclooxygenase catalysis. Chem. Biol. 2007;14:473–488. doi: 10.1016/j.chembiol.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu S., Mueser T.C., Marnett L.J., Funk M.O., Jr. Crystal structure of 12-lipoxygenase catalytic-domain-inhibitor complex identifies a substrate-binding channel for catalysis. Structure. 2012;20:1490–1497. doi: 10.1016/j.str.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbert N.C., Bartlett S.G., Waight M.T., Neau D.B., Boeglin W.E., Brash A.R., Newcomer M.E. The structure of human 5-lipoxygenase. Science. 2011;331:217–219. doi: 10.1126/science.1197203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werz O. Inhibition of 5-lipoxygenase product synthesis by natural compounds of plant origin. Planta medica. 2007;73:1331–1357. doi: 10.1055/s-2007-990242. [DOI] [PubMed] [Google Scholar]
- 7.Pettersen D., Davidsson O., Whatling C. Recent advances for FLAP inhibitors. Bioorg. Med. Chem. Lett. 2015;25:2607–2612. doi: 10.1016/j.bmcl.2015.04.090. [DOI] [PubMed] [Google Scholar]
- 8.Krieg P., Marks F., Furstenberger G. A gene cluster encoding human epidermis-type lipoxygenases at chromosome 17p13.1: cloning, physical mapping, and expression. Genomics. 2001;73:323–330. doi: 10.1006/geno.2001.6519. [DOI] [PubMed] [Google Scholar]
- 9.Nassar G.M., Morrow J.D., Roberts L.J., 2nd, Lakkis F.G., Badr K.F. Induction of 15-lipoxygenase by interleukin-13 in human blood monocytes. J. Biol. Chem. 1994;269:27631–27634. [PubMed] [Google Scholar]
- 10.Conrad D.J., Lu M. Regulation of human 12/15-lipoxygenase by Stat6-dependent transcription. Am. J. Respir. Cell Mol. Biol. 2000;22:226–234. doi: 10.1165/ajrcmb.22.2.3786. [DOI] [PubMed] [Google Scholar]
- 11.Shureiqi I., Chen D., Lee J.J., Yang P., Newman R.A., Brenner D.E., Lotan R., Fischer S.M., Lippman S.M. 15-LOX-1: a novel molecular target of nonsteroidal anti-inflammatory drug-induced apoptosis in colorectal cancer cells. J. Natl. Cancer Inst. 2000;92:1136–1142. doi: 10.1093/jnci/92.14.1136. [DOI] [PubMed] [Google Scholar]
- 12.Kelavkar U.P., Harya N.S., Hutzley J., Bacich D.J., Monzon F.A., Chandran U., Dhir R., O’Keefe D.S. DNA methylation paradigm shift: 15-lipoxygenase-1 upregulation in prostatic intraepithelial neoplasia and prostate cancer by atypical promoter hypermethylation. Prostaglandins Other Lipid Mediat. 2007;82:185–197. doi: 10.1016/j.prostaglandins.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Shankaranarayanan P., Chaitidis P., Kuhn H., Nigam S. Acetylation by histone acetyltransferase CREB-binding protein/p300 of STAT6 is required for transcriptional activation of the 15-lipoxygenase-1 gene. J. Biol. Chem. 2001;276:42753–42760. doi: 10.1074/jbc.M102626200. [DOI] [PubMed] [Google Scholar]
- 14.Liu C., Xu D., Han H., Fan Y., Schain F., Xu Z., Claesson H.E., Bjorkholm M., Sjoberg J. Transcriptional regulation of 15-lipoxygenase expression by histone h3 lysine 4 methylation/demethylation. PLoS One. 2012;7:e52703. doi: 10.1371/journal.pone.0052703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zuo X., Morris J.S., Shureiqi I. Chromatin modification requirements for 15-lipoxygenase-1 transcriptional reactivation in colon cancer cells. J. Biol. Chem. 2008;283:31341–31347. doi: 10.1074/jbc.M803729200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zuo X., Morris J.S., Broaddus R., Shureiqi I. 15-LOX-1 transcription suppression through the NuRD complex in colon cancer cells. Oncogene. 2009;28:1496–1505. doi: 10.1038/onc.2008.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shureiqi I., Jiang W., Zuo X., Wu Y., Stimmel J.B., Leesnitzer L.M., Morris J.S., Fan H.Z., Fischer S.M., Lippman S.M. The 15-lipoxygenase-1 product 13-S-hydroxyoctadecadienoic acid down-regulates PPAR-delta to induce apoptosis in colorectal cancer cells. Proc. Natl. Acad. Sci. USA. 2003;100:9968–9973. doi: 10.1073/pnas.1631086100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelavkar U.P., Parwani A.V., Shappell S.B., Martin W.D. Conditional expression of human 15-lipoxygenase-1 in mouse prostate induces prostatic intraepithelial neoplasia: the FLiMP mouse model. Neoplasia. 2006;8:510–522. doi: 10.1593/neo.06202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cyrus T., Witztum J.L., Rader D.J., Tangirala R., Fazio S., Linton M.F., Funk C.D. Disruption of the 12/15-lipoxygenase gene diminishes atherosclerosis in apo E-deficient mice. J. Clin. Investig. 1999;103:1597–1604. doi: 10.1172/JCI5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao L., Pratico D., Rader D.J., Funk C.D. 12/15-Lipoxygenase gene disruption and vitamin E administration diminish atherosclerosis and oxidative stress in apolipoprotein E deficient mice through a final common pathway. Prostaglandins Other Lipid Mediat. 2005;78:185–193. doi: 10.1016/j.prostaglandins.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Huo Y., Zhao L., Hyman M.C., Shashkin P., Harry B.L., Burcin T., Forlow S.B., Stark M.A., Smith D.F., Clarke S., Srinivasan S., Hedrick C.C., Pratico D., Witztum J.L., Nadler J.L., Funk C.D., Ley K. Critical role of macrophage 12/15-lipoxygenase for atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;110:2024–2031. doi: 10.1161/01.CIR.0000143628.37680.F6. [DOI] [PubMed] [Google Scholar]
- 22.Rong S., Cao Q., Liu M., Seo J., Jia L., Boudyguina E., Gebre A.K., Colvin P.L., Smith T.L., Murphy R.C., Mishra N., Parks J.S. Macrophage 12/15 lipoxygenase expression increases plasma and hepatic lipid levels and exacerbates atherosclerosis. J. Lipid Res. 2012;53:686–695. doi: 10.1194/jlr.M022723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.George J., Afek A., Shaish A., Levkovitz H., Bloom N., Cyrus T., Zhao L., Funk C.D., Sigal E., Harats D. 12/15-Lipoxygenase gene disruption attenuates atherogenesis in LDL receptor-deficient mice. Circulation. 2001;104:1646–1650. doi: 10.1161/hc3901.095772. [DOI] [PubMed] [Google Scholar]
- 24.Reddy M.A., Kim Y.S., Lanting L., Natarajan R. Reduced growth factor responses in vascular smooth muscle cells derived from 12/15-lipoxygenase-deficient mice. Hypertension. 2003;41:1294–1300. doi: 10.1161/01.HYP.0000069011.18333.08. [DOI] [PubMed] [Google Scholar]
- 25.Gabel S.A., London R.E., Funk C.D., Steenbergen C., Murphy E. Leukocyte-type 12-lipoxygenase-deficient mice show impaired ischemic preconditioning-induced cardioprotection. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H1963–H1969. doi: 10.1152/ajpheart.2001.280.5.H1963. [DOI] [PubMed] [Google Scholar]
- 26.Sun D., Funk C.D. Disruption of 12/15-lipoxygenase expression in peritoneal macrophages. Enhanced utilization of the 5-lipoxygenase pathway and diminished oxidation of low density lipoprotein. J. Biol. Chem. 1996;271:24055–24062. [PubMed] [Google Scholar]
- 27.Kinder M., Thompson J.E., Wei C., Shelat S.G., Blair I.A., Carroll M., Pure E. Interferon regulatory factor-8-driven myeloid differentiation is regulated by 12/15-lipoxygenase-mediated redox signaling. Exp. Hematol. 2010;38(1036–1046):e1031–e1034. doi: 10.1016/j.exphem.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kronke G., Uderhardt S., Katzenbeisser J., Schett G. The 12/15-lipoxygenase pathway promotes osteoclast development and differentiation. Autoimmunity. 2009;42:383–385. doi: 10.1080/08916930902832488. [DOI] [PubMed] [Google Scholar]
- 29.Nunemaker C.S., Chen M., Pei H., Kimble S.D., Keller S.R., Carter J.D., Yang Z., Smith K.M., Wu R., Bevard M.H., Garmey J.C., Nadler J.L. 12-Lipoxygenase-knockout mice are resistant to inflammatory effects of obesity induced by Western diet. Am. J. Physiol. Endocrinol. Metab. 2008;295:E1065–E1075. doi: 10.1152/ajpendo.90371.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brash A.R., Boeglin W.E., Chang M.S. Discovery of a second 15S-lipoxygenase in humans. Proc. Natl. Acad. Sci. USA. 1997;94:6148–6152. doi: 10.1073/pnas.94.12.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jisaka M., Kim R.B., Boeglin W.E., Brash A.R. Identification of amino acid determinants of the positional specificity of mouse 8S-lipoxygenase and human 15S-lipoxygenase-2. J. Biol. Chem. 2000;275:1287–1293. doi: 10.1074/jbc.275.2.1287. [DOI] [PubMed] [Google Scholar]
- 32.Gertow K., Nobili E., Folkersen L., Newman J.W., Pedersen T.L., Ekstrand J., Swedenborg J., Kuhn H., Wheelock C.E., Hansson G.K., Hedin U., Haeggstrom J.Z., Gabrielsen A. 12- and 15-lipoxygenases in human carotid atherosclerotic lesions: associations with cerebrovascular symptoms. Atherosclerosis. 2011;215:411–416. doi: 10.1016/j.atherosclerosis.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 33.Hulten L.M., Olson F.J., Aberg H., Carlsson J., Karlstrom L., Boren J., Fagerberg B., Wiklund O. 15-Lipoxygenase-2 is expressed in macrophages in human carotid plaques and regulated by hypoxia-inducible factor-1alpha. Eur. J. Clin. Investig. 2010;40:11–17. doi: 10.1111/j.1365-2362.2009.02223.x. [DOI] [PubMed] [Google Scholar]
- 34.Magnusson L.U., Lundqvist A., Karlsson M.N., Skalen K., Levin M., Wiklund O., Boren J., Hulten L.M. Arachidonate 15-lipoxygenase type B knockdown leads to reduced lipid accumulation and inflammation in atherosclerosis. PLoS One. 2012;7:e43142. doi: 10.1371/journal.pone.0043142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu X.C., Shappell S.B., Liang Z., Song S., Menter D., Subbarayan V., Iyengar S., Tang D.G., Lippman S.M. Reduced 15S-lipoxygenase-2 expression in esophageal cancer specimens and cells and upregulation in vitro by the cyclooxygenase-2 inhibitor, NS398. Neoplasia. 2003;5:121–127. doi: 10.1016/s1476-5586(03)80003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soon P.S., Libe R., Benn D.E., Gill A., Shaw J., Sywak M.S., Groussin L., Bertagna X., Gicquel C., Bertherat J., McDonald K.L., Sidhu S.B., Robinson B.G. Loss of heterozygosity of 17p13, with possible involvement of ACADVL and ALOX15B, in the pathogenesis of adrenocortical tumors. Ann. Surg. 2008;247:157–164. doi: 10.1097/SLA.0b013e318153ff55. [DOI] [PubMed] [Google Scholar]
- 37.Subbarayan V., Xu X.C., Kim J., Yang P., Hoque A., Sabichi A.L., Llansa N., Mendoza G., Logothetis C.J., Newman R.A., Lippman S.M., Menter D.G. Inverse relationship between 15-lipoxygenase-2 and PPAR-gamma gene expression in normal epithelia compared with tumor epithelia. Neoplasia. 2005;7:280–293. doi: 10.1593/neo.04457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng Y., Bai X., Yang Q., Wu H., Wang D. Downregulation of 15-lipoxygenase 2 by glucocorticoid receptor in prostate cancer cells. Int. J. Oncol. 2010;36:1541–1549. doi: 10.3892/ijo_00000641. [DOI] [PubMed] [Google Scholar]
- 39.Jiang W.G., Watkins G., Douglas-Jones A., Mansel R.E. Reduction of isoforms of 15-lipoxygenase (15-LOX)-1 and 15-LOX-2 in human breast cancer. Prostaglandins Leukot. Essent. Fatty Acids. 2006;74:235–245. doi: 10.1016/j.plefa.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Wang D., Chen S., Feng Y., Yang Q., Campbell B.H., Tang X., Campbell W.B. Reduced expression of 15-lipoxygenase 2 in human head and neck carcinomas. Tumour Biol. 2006;27:261–273. doi: 10.1159/000094761. [DOI] [PubMed] [Google Scholar]
- 41.Tang S., Bhatia B., Zhou J., Maldonado C.J., Chandra D., Kim E., Fischer S.M., Butler A.P., Friedman S.L., Tang D.G. Evidence that Sp1 positively and Sp3 negatively regulate and androgen does not directly regulate functional tumor suppressor 15-lipoxygenase 2 (15-LOX2) gene expression in normal human prostate epithelial cells. Oncogene. 2004;23:6942–6953. doi: 10.1038/sj.onc.1207913. [DOI] [PubMed] [Google Scholar]
- 42.Daurkin I., Eruslanov E., Stoffs T., Perrin G.Q., Algood C., Gilbert S.M., Rosser C.J., Su L.M., Vieweg J., Kusmartsev S. Tumor-associated macrophages mediate immunosuppression in the renal cancer microenvironment by activating the 15-lipoxygenase-2 pathway. Cancer Res. 2011;71:6400–6409. doi: 10.1158/0008-5472.CAN-11-1261. [DOI] [PubMed] [Google Scholar]
- 43.Vijil C., Hermansson C., Jeppsson A., Bergstrom G., Hulten L.M. Arachidonate 15-lipoxygenase enzyme products increase platelet aggregation and thrombin generation. PLoS One. 2014;9:e88546. doi: 10.1371/journal.pone.0088546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moran J.L., Qiu H., Turbe-Doan A., Yun Y., Boeglin W.E., Brash A.R., Beier D.R. A mouse mutation in the 12R-lipoxygenase, Alox12b, disrupts formation of the epidermal permeability barrier. J. Investig. Dermatol. 2007;127:1893–1897. doi: 10.1038/sj.jid.5700825. [DOI] [PubMed] [Google Scholar]
- 45.Funk C.D. The molecular biology of mammalian lipoxygenases and the quest for eicosanoid functions using lipoxygenase-deficient mice. Biochim. Biophys. Acta. 1996;1304:65–84. doi: 10.1016/s0005-2760(96)00107-5. [DOI] [PubMed] [Google Scholar]
- 46.Funk C.D., Chen X.S., Johnson E.N., Zhao L. Lipoxygenase genes and their targeted disruption. Prostaglandins Other Lipid Mediat. 2002;68–69:303–312. doi: 10.1016/s0090-6980(02)00036-9. [DOI] [PubMed] [Google Scholar]
- 47.Goodman J.E., Bowman E.D., Chanock S.J., Alberg A.J., Harris C.C. Arachidonate lipoxygenase (ALOX) and cyclooxygenase (COX) polymorphisms and colon cancer risk. Carcinogenesis. 2004;25:2467–2472. doi: 10.1093/carcin/bgh260. [DOI] [PubMed] [Google Scholar]
- 48.Prasad V.V., Kolli P., Moganti D. Association of a functional polymorphism (Gln261Arg) in 12-lipoxygenase with breast cancer. Exp. Ther. Med. 2011;2:317–323. doi: 10.3892/etm.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fridman C., Ojopi E.P., Gregorio S.P., Ikenaga E.H., Moreno D.H., Demetrio F.N., Guimaraes P.E., Vallada H.P., Gattaz W.F., Dias Neto E. Association of a new polymorphism in ALOX12 gene with bipolar disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2003;253:40–43. doi: 10.1007/s00406-003-0404-y. [DOI] [PubMed] [Google Scholar]
- 50.Kim T., Kim H.J., Park J.K., Kim J.W., Chung J.H. Association between polymorphisms of arachidonate 12-lipoxygenase (ALOX12) and schizophrenia in a Korean population. Behav. Brain Funct. 2010;6:44. doi: 10.1186/1744-9081-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quintana L.F., Guzman B., Collado S., Claria J., Poch E. A coding polymorphism in the 12-lipoxygenase gene is associated to essential hypertension and urinary 12(S)-HETE. Kidney Int. 2006;69:526–530. doi: 10.1038/sj.ki.5000147. [DOI] [PubMed] [Google Scholar]
- 52.Gonzalez-Nunez D., Claria J., Rivera F., Poch E. Increased levels of 12(S)-HETE in patients with essential hypertension. Hypertension. 2001;37:334–338. doi: 10.1161/01.hyp.37.2.334. [DOI] [PubMed] [Google Scholar]
- 53.Xiao W.J., He J.W., Zhang H., Hu W.W., Gu J.M., Yue H., Gao G., Yu J.B., Wang C., Ke Y.H., Fu W.Z., Zhang Z.L. ALOX12 polymorphisms are associated with fat mass but not peak bone mineral density in Chinese nuclear families. Int. J. Obes. 2011;35:378–386. doi: 10.1038/ijo.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mullin B.H., Spector T.D., Curtis C.C., Ong G.N., Hart D.J., Hakim A.J., Worthy T., Wilson S.G. Polymorphisms in ALOX12, but not ALOX15, are significantly associated with BMD in postmenopausal women. Calcif. Tissue Int. 2007;81:10–17. doi: 10.1007/s00223-007-9023-3. [DOI] [PubMed] [Google Scholar]
- 55.Harslof T., Husted L.B., Nyegaard M., Carstens M., Stenkjaer L., Brixen K., Eiken P., Jensen J.E., Borglum A.D., Mosekilde L., Rejnmark L., Langdahl B.L. Polymorphisms in the ALOX12 gene and osteoporosis. Osteoporos. Int. 2011;22:2249–2259. doi: 10.1007/s00198-010-1472-2. [DOI] [PubMed] [Google Scholar]
- 56.Xiao W.J., Ke Y.H., He J.W., Zhang H., Yu J.B., Hu W.W., Gu J.M., Gao G., Yue H., Wang C., Hu Y.Q., Li M., Liu Y.J., Fu W.Z., Zhang Z.L. Polymorphisms in the human ALOX12 and ALOX15 genes are associated with peak bone mineral density in Chinese nuclear families. Osteoporos. Int. 2012;23:1889–1897. doi: 10.1007/s00198-011-1835-3. [DOI] [PubMed] [Google Scholar]
- 57.Ichikawa S., Koller D.L., Johnson M.L., Lai D., Xuei X., Edenberg H.J., Klein R.F., Orwoll E.S., Hui S.L., Foroud T.M., Peacock M., Econs M.J. Human ALOX12, but not ALOX15, is associated with BMD in white men and women. J. Bone Miner. Res. 2006;21:556–564. doi: 10.1359/jbmr.051212. [DOI] [PubMed] [Google Scholar]
- 58.Kaur G., Jalagadugula G., Mao G., Rao A.K. RUNX1/core binding factor A2 regulates platelet 12-lipoxygenase gene (ALOX12): studies in human RUNX1 haplodeficiency. Blood. 2010;115:3128–3135. doi: 10.1182/blood-2009-04-214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim S., Choi I.F., Quante J.R., Zhang L., Roop D.R., Koster M.I. p63 directly induces expression of Alox12, a regulator of epidermal barrier formation. Exp. Dermatol. 2009;18:1016–1021. doi: 10.1111/j.1600-0625.2009.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aleem A.M., Jankun J., Dignam J.D., Walther M., Kuhn H., Svergun D.I., Skrzypczak-Jankun E. Human platelet 12-lipoxygenase, new findings about its activity, membrane binding and low-resolution structure. J. Mol. Biol. 2008;376:193–209. doi: 10.1016/j.jmb.2007.11.086. [DOI] [PubMed] [Google Scholar]
- 61.Ohgami R.S., Ma L., Ren L., Weinberg O.K., Seetharam M., Gotlib J.R., Arber D.A. DNA methylation analysis of ALOX12 and GSTM1 in acute myeloid leukaemia identifies prognostically significant groups. Br. J. Haematol. 2012;159:182–190. doi: 10.1111/bjh.12029. [DOI] [PubMed] [Google Scholar]
- 62.Jiang Y., Dunbar A., Gondek L.P., Mohan S., Rataul M., O’Keefe C., Sekeres M., Saunthararajah Y., Maciejewski J.P. Aberrant DNA methylation is a dominant mechanism in MDS progression to AML. Blood. 2009;113:1315–1325. doi: 10.1182/blood-2008-06-163246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kamitani H., Geller M., Eling T. The possible involvement of 15-lipoxygenase/leukocyte type 12-lipoxygenase in colorectal carcinogenesis. Adv. Exp. Med. Biol. 1999;469:593–598. doi: 10.1007/978-1-4615-4793-8_86. [DOI] [PubMed] [Google Scholar]
- 64.Guo Y., Zhang X., Tan W., Miao X., Sun T., Zhao D., Lin D. Platelet 12-lipoxygenase Arg261Gln polymorphism: functional characterization and association with risk of esophageal squamous cell carcinoma in combination with COX-2 polymorphisms. Pharmacogenet. Genom. 2007;17:197–205. doi: 10.1097/FPC.0b013e328010bda1. [DOI] [PubMed] [Google Scholar]
- 65.Tan W., Wu J., Zhang X., Guo Y., Liu J., Sun T., Zhang B., Zhao D., Yang M., Yu D., Lin D. Associations of functional polymorphisms in cyclooxygenase-2 and platelet 12-lipoxygenase with risk of occurrence and advanced disease status of colorectal cancer. Carcinogenesis. 2007;28:1197–1201. doi: 10.1093/carcin/bgl242. [DOI] [PubMed] [Google Scholar]
- 66.Xu X.M., Yuan G.J., Deng J.J., Guo H.T., Xiang M., Yang F., Ge W., Chen S.Y. Inhibition of 12-lipoxygenase reduces proliferation and induces apoptosis of hepatocellular carcinoma cells in vitro and in vivo. Hepatobiliary Pancreat. Dis. Int. 2012;11:193–202. doi: 10.1016/s1499-3872(12)60147-7. [DOI] [PubMed] [Google Scholar]
- 67.Johnson E.N., Brass L.F., Funk C.D. Increased platelet sensitivity to ADP in mice lacking platelet-type 12-lipoxygenase. Proc. Natl. Acad. Sci. USA. 1998;95:3100–3105. doi: 10.1073/pnas.95.6.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Virmani J., Johnson E.N., Klein-Szanto A.J., Funk C.D. Role of’‘platelet-type’ 12-lipoxygenase in skin carcinogenesis. Cancer Lett. 2001;162:161–165. doi: 10.1016/s0304-3835(00)00634-0. [DOI] [PubMed] [Google Scholar]
- 69.Johnson E.N., Nanney L.B., Virmani J., Lawson J.A., Funk C.D. Basal transepidermal water loss is increased in platelet-type 12-lipoxygenase deficient mice. J. Investig. Dermatol. 1999;112:861–865. doi: 10.1046/j.1523-1747.1999.00595.x. [DOI] [PubMed] [Google Scholar]
- 70.Boeglin W.E., Kim R.B., Brash A.R. A 12R-lipoxygenase in human skin: mechanistic evidence, molecular cloning, and expression. Proc. Natl. Acad. Sci. USA. 1998;95:6744–6749. doi: 10.1073/pnas.95.12.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun D., McDonnell M., Chen X.S., Lakkis M.M., Li H., Isaacs S.N., Elsea S.H., Patel P.I., Funk C.D. Human 12(R)-lipoxygenase and the mouse ortholog. Molecular cloning, expression, and gene chromosomal assignment. J. Biol. Chem. 1998;273:33540–33547. doi: 10.1074/jbc.273.50.33540. [DOI] [PubMed] [Google Scholar]
- 72.Schneider C., Keeney D.S., Boeglin W.E., Brash A.R. Detection and cellular localization of 12R-lipoxygenase in human tonsils. Arch. Biochem. Biophys. 2001;386:268–274. doi: 10.1006/abbi.2000.2217. [DOI] [PubMed] [Google Scholar]
- 73.Garcia-Verdugo I., BenMohamed F., Tattermusch S., Leduc D., Charpigny G., Chignard M., Ollero M., Touqui L. A role for 12R-lipoxygenase in MUC5AC expression by respiratory epithelial cells. Eur. Respir. J. 2012;40:714–723. doi: 10.1183/09031936.00023111. [DOI] [PubMed] [Google Scholar]
- 74.Krieg P., Heidt M., Siebert M., Kinzig A., Marks F., Furstenberger G. Epidermis-type lipoxygenases. Adv. Exp. Med. Biol. 2002;507:165–170. doi: 10.1007/978-1-4615-0193-0_26. [DOI] [PubMed] [Google Scholar]
- 75.Zheng Y., Yin H., Boeglin W.E., Elias P.M., Crumrine D., Beier D.R., Brash A.R. Lipoxygenases mediate the effect of essential fatty acid in skin barrier formation: a proposed role in releasing omega-hydroxyceramide for construction of the corneocyte lipid envelope. J. Biol. Chem. 2011;286:24046–24056. doi: 10.1074/jbc.M111.251496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Herman M.L., Farasat S., Steinbach P.J., Wei M.H., Toure O., Fleckman P., Blake P., Bale S.J., Toro J.R. Transglutaminase-1 gene mutations in autosomal recessive congenital ichthyosis: summary of mutations (including 23 novel) and modeling of TGase-1. Hum. Mutat. 2009;30:537–547. doi: 10.1002/humu.20952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matsuki M., Yamashita F., Ishida-Yamamoto A., Yamada K., Kinoshita C., Fushiki S., Ueda E., Morishima Y., Tabata K., Yasuno H., Hashida M., Iizuka H., Ikawa M., Okabe M., Kondoh G., Kinoshita T., Takeda J., Yamanishi K. Defective stratum corneum and early neonatal death in mice lacking the gene for transglutaminase 1 (keratinocyte transglutaminase) Proc. Natl. Acad. Sci. USA. 1998;95:1044–1049. doi: 10.1073/pnas.95.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brash A.R., Niraula N.P., Boeglin W.E., Mashhadi Z. An ancient relative of cyclooxygenase in cyanobacteria is a linoleate 10S-dioxygenase that works in tandem with a catalase-related protein with specific 10S-hydroperoxide lyase activity. J. Biol. Chem. 2014;289:13101–13111. doi: 10.1074/jbc.M114.555904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Munoz-Garcia A., Thomas C.P., Keeney D.S., Zheng Y., Brash A.R. The importance of the lipoxygenase-hepoxilin pathway in the mammalian epidermal barrier. Biochim. Biophys. Acta. 2014;1841:401–408. doi: 10.1016/j.bbalip.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krieg P., Furstenberger G. The role of lipoxygenases in epidermis. Biochim. Biophys. Acta. 2014;1841:390–400. doi: 10.1016/j.bbalip.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 81.Lesueur F., Bouadjar B., Lefevre C., Jobard F., Audebert S., Lakhdar H., Martin L., Tadini G., Karaduman A., Emre S., Saker S., Lathrop M., Fischer J. Novel mutations in ALOX12B in patients with autosomal recessive congenital ichthyosis and evidence for genetic heterogeneity on chromosome 17p13. J. Investig. Dermatol. 2007;127:829–834. doi: 10.1038/sj.jid.5700640. [DOI] [PubMed] [Google Scholar]
- 82.Eckl K.M., de Juanes S., Kurtenbach J., Natebus M., Lugassy J., Oji V., Traupe H., Preil M.L., Martinez F., Smolle J., Harel A., Krieg P., Sprecher E., Hennies H.C. Molecular analysis of 250 patients with autosomal recessive congenital ichthyosis: evidence for mutation hotspots in ALOXE3 and allelic heterogeneity in ALOX12B. J. Investig. Dermatol. 2009;129:1421–1428. doi: 10.1038/jid.2008.409. [DOI] [PubMed] [Google Scholar]
- 83.Li H., Lorie E.P., Fischer J., Vahlquist A., Torma H. The expression of epidermal lipoxygenases and transglutaminase-1 is perturbed by NIPAL4 mutations: indications of a common metabolic pathway essential for skin barrier homeostasis. J. Investig. Dermatol. 2012;132:2368–2375. doi: 10.1038/jid.2012.160. [DOI] [PubMed] [Google Scholar]
- 84.Jobard F., Lefevre C., Karaduman A., Blanchet-Bardon C., Emre S., Weissenbach J., Ozguc M., Lathrop M., Prud’homme J.F., Fischer J. Lipoxygenase-3 (ALOXE3) and 12(R)-lipoxygenase (ALOX12B) are mutated in non-bullous congenital ichthyosiform erythroderma (NCIE) linked to chromosome 17p13.1. Hum. Mol. Genet. 2002;11:107–113. doi: 10.1093/hmg/11.1.107. [DOI] [PubMed] [Google Scholar]
- 85.Harting M., Brunetti-Pierri N., Chan C.S., Kirby J., Dishop M.K., Richard G., Scaglia F., Yan A.C., Levy M.L. Self-healing collodion membrane and mild nonbullous congenital ichthyosiform erythroderma due to 2 novel mutations in the ALOX12B gene. Arch. Dermatol. 2008;144:351–356. doi: 10.1001/archderm.144.3.351. [DOI] [PubMed] [Google Scholar]
- 86.Yu Z., Schneider C., Boeglin W.E., Brash A.R. Mutations associated with a congenital form of ichthyosis (NCIE) inactivate the epidermal lipoxygenases 12R LOX and eLOX3. Biochim. Biophys. Acta. 2005;1686:238–247. doi: 10.1016/j.bbalip.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 87.Epp N., Furstenberger G., Muller K., de Juanes S., Leitges M., Hausser I., Thieme F., Liebisch G., Schmitz G., Krieg P. 12R-lipoxygenase deficiency disrupts epidermal barrier function. J. Cell Biol. 2007;177:173–182. doi: 10.1083/jcb.200612116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Juanes S., Epp N., Latzko S., Neumann M., Furstenberger G., Hausser I., Stark H.J., Krieg P. Development of an ichthyosiform phenotype in Alox12b-deficient mouse skin transplants. J. Invest. Dermatol. 2009;129:1429–1436. doi: 10.1038/jid.2008.410. [DOI] [PubMed] [Google Scholar]
- 89.Segre J.A., Bauer C., Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat. Genet. 1999;22:356–360. doi: 10.1038/11926. [DOI] [PubMed] [Google Scholar]
- 90.Furuse M., Hata M., Furuse K., Yoshida Y., Haratake A., Sugitani Y., Noda T., Kubo A., Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J. Cell Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zheng Y., Brash A.R. On the role of molecular oxygen in lipoxygenase activation: comparison and contrast of epidermal lipoxygenase-3 with soybean lipoxygenase-1. J. Biol. Chem. 2010;285:39876–39887. doi: 10.1074/jbc.M110.180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zheng Y., Brash A.R. Dioxygenase activity of epidermal lipoxygenase-3 unveiled: typical and atypical features of its catalytic activity with natural and synthetic polyunsaturated fatty acids. J. Biol. Chem. 2010;285:39866–39875. doi: 10.1074/jbc.M110.155374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu Z., Schneider C., Boeglin W.E., Marnett L.J., Brash A.R. The lipoxygenase gene ALOXE3 implicated in skin differentiation encodes a hydroperoxide isomerase. Proc. Natl. Acad. Sci. USA. 2003;100:9162–9167. doi: 10.1073/pnas.1633612100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vahlquist A., Bygum A., Ganemo A., Virtanen M., Hellstrom-Pigg M., Strauss G., Brandrup F., Fischer J. Genotypic and clinical spectrum of self-improving collodion ichthyosis: ALOX12B, ALOXE3, and TGM1 mutations in Scandinavian patients. J. Invest. Dermatol. 2010;130:438–443. doi: 10.1038/jid.2009.346. [DOI] [PubMed] [Google Scholar]
- 95.Krieg P., Rosenberger S., de Juanes S., Latzko S., Hou J., Dick A., Kloz U., van der Hoeven F., Hausser I., Esposito I., Rauh M., Schneider H. Aloxe3 knockout mice reveal a function of epidermal lipoxygenase-3 as hepoxilin synthase and its pivotal role in barrier formation. J. Invest. Dermatol. 2013;133:172–180. doi: 10.1038/jid.2012.250. [DOI] [PubMed] [Google Scholar]
- 96.Duroudier N.P., Tulah A.S., Sayers I. Leukotriene pathway genetics and pharmacogenetics in allergy. Allergy. 2009;64:823–839. doi: 10.1111/j.1398-9995.2009.02015.x. [DOI] [PubMed] [Google Scholar]
- 97.Bai C., Matsui E., Ohnishi H., Kimata K., Kasahara K., Kaneko H., Kato Z., Fukao T., Kondo N. A novel polymorphism, E254K, in the 5-lipoxygenase gene associated with bronchial asthma. Int. J. Mol. Med. 2008;21:139–144. [PubMed] [Google Scholar]
- 98.Kawagishi Y., Mita H., Taniguchi M., Maruyama M., Oosaki R., Higashi N., Kashii T., Kobayashi M., Akiyama K. Leukotriene C4 synthase promoter polymorphism in Japanese patients with aspirin-induced asthma. J. Allergy Clin. Immunol. 2002;109:936–942. doi: 10.1067/mai.2002.124466. [DOI] [PubMed] [Google Scholar]
- 99.Kim S.H., Bae J.S., Suh C.H., Nahm D.H., Holloway J.W., Park H.S. Polymorphism of tandem repeat in promoter of 5-lipoxygenase in ASA-intolerant asthma: a positive association with airway hyperresponsiveness. Allergy. 2005;60:760–765. doi: 10.1111/j.1398-9995.2005.00780.x. [DOI] [PubMed] [Google Scholar]
- 100.Kleinstein S.E., Heath L., Makar K.W., Poole E.M., Seufert B.L., Slattery M.L., Xiao L., Duggan D.J., Hsu L., Curtin K., Koepl L., Muehling J., Taverna D., Caan B.J., Carlson C.S., Potter J.D., Ulrich C.M. Genetic variation in the lipoxygenase pathway and risk of colorectal neoplasia. Genes Chromosom. Cancer. 2013;52:437–449. doi: 10.1002/gcc.22042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.White K.L., Schildkraut J.M., Palmieri R.T., Iversen E.S., Jr., Berchuck A., Vierkant R.A., Rider D.N., Charbonneau B., Cicek M.S., Sutphen R., Birrer M.J., Pharoah P.P., Song H., Tyrer J., Gayther S.A., Ramus S.J., Wentzensen N., Yang H.P., Garcia-Closas M., Phelan C.M., Cunningham J.M., Fridley B.L., Sellers T.A., Goode E.L. Ovarian cancer risk associated with inherited inflammation-related variants. Cancer Res. 2012;72:1064–1069. doi: 10.1158/0008-5472.CAN-11-3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen X.S., Sheller J.R., Johnson E.N., Funk C.D. Role of leukotrienes revealed by targeted disruption of the 5-lipoxygenase gene. Nature. 1994;372:179–182. doi: 10.1038/372179a0. [DOI] [PubMed] [Google Scholar]
- 103.Goulet J.L., Snouwaert J.N., Latour A.M., Coffman T.M., Koller B.H. Altered inflammatory responses in leukotriene-deficient mice. Proc. Natl. Acad. Sci. USA. 1994;91:12852–12856. doi: 10.1073/pnas.91.26.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cuzzocrea S., Rossi A., Serraino I., Di Paola R., Dugo L., Genovese T., Britti D., Sciarra G., De Sarro A., Caputi A.P., Sautebin L. 5-lipoxygenase knockout mice exhibit a resistance to acute pancreatitis induced by cerulein. Immunology. 2003;110:120–130. doi: 10.1046/j.1365-2567.2003.01715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Segal B.H., Kuhns D.B., Ding L., Gallin J.I., Holland S.M. Thioglycollate peritonitis in mice lacking C5, 5-lipoxygenase, or p47(phox): complement, leukotrienes, and reactive oxidants in acute inflammation. J. Leukoc. Biol. 2002;71:410–416. [PubMed] [Google Scholar]
- 106.Blaho V.A., Zhang Y., Hughes-Hanks J.M., Brown C.R. 5-Lipoxygenase-deficient mice infected with Borrelia burgdorferi develop persistent arthritis. J. Immunol. 2011;186:3076–3084. doi: 10.4049/jimmunol.1003473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aliberti J., Serhan C., Sher A. Parasite-induced lipoxin A4 is an endogenous regulator of IL-12 production and immunopathology in Toxoplasma gondii infection. J. Exp. Med. 2002;196:1253–1262. doi: 10.1084/jem.20021183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Irvin C.G., Tu Y.P., Sheller J.R., Funk C.D. 5-Lipoxygenase products are necessary for ovalbumin-induced airway responsiveness in mice. Am. J. Physiol. 1997;272:L1053–L1058. doi: 10.1152/ajplung.1997.272.6.L1053. [DOI] [PubMed] [Google Scholar]
- 109.Goulet J.L., Griffiths R.C., Ruiz P., Spurney R.F., Pisetsky D.S., Koller B.H., Coffman T.M. Deficiency of 5-lipoxygenase abolishes sex-related survival differences in MRL-lpr/lpr mice. J. Immunol. 1999;163:359–366. [PubMed] [Google Scholar]
- 110.Arita M., Yoshida M., Hong S., Tjonahen E., Glickman J.N., Petasis N.A., Blumberg R.S., Serhan C.N. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc. Natl. Acad. Sci. USA. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tjonahen E., Oh S.F., Siegelman J., Elangovan S., Percarpio K.B., Hong S., Arita M., Serhan C.N. Resolvin E2: identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem. Biol. 2006;13:1193–1202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 112.Arita M., Bianchini F., Aliberti J., Sher A., Chiang N., Hong S., Yang R., Petasis N.A., Serhan C.N. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Recchiuti A., Krishnamoorthy S., Fredman G., Chiang N., Serhan C.N. MicroRNAs in resolution of acute inflammation: identification of novel resolvin D1-miRNA circuits. FASEB J. 2011;25:544–560. doi: 10.1096/fj.10-169599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mehrabian M., Allayee H., Wong J., Shi W., Wang X.P., Shaposhnik Z., Funk C.D., Lusis A.J. Identification of 5-lipoxygenase as a major gene contributing to atherosclerosis susceptibility in mice. Circ. Res. 2002;91:120–126. doi: 10.1161/01.res.0000028008.99774.7f. [DOI] [PubMed] [Google Scholar]
- 115.Yu Z., Crichton I., Tang S.Y., Hui Y., Ricciotti E., Levin M.D., Lawson J.A., Pure E., FitzGerald G.A. Disruption of the 5-lipoxygenase pathway attenuates atherogenesis consequent to COX-2 deletion in mice. Proc. Natl. Acad. Sci. USA. 2012;109:6727–6732. doi: 10.1073/pnas.1115313109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Joshi Y.B., Pratico D. The 5-lipoxygenase pathway: oxidative and inflammatory contributions to the Alzheimer's disease phenotype. Front. Cell. Neurosci. 2014;8:436. doi: 10.3389/fncel.2014.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Joshi Y.B., Pratico D. Knockout of 5-lipoxygenase results in age-dependent anxiety-like behavior in female mice. PLoS One. 2011;6:e29448. doi: 10.1371/journal.pone.0029448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chu J., Li J.G., Pratico D. Zileuton improves memory deficits, amyloid and tau pathology in a mouse model of Alzheimer's disease with plaques and tangles. PLoS One. 2013;8:e70991. doi: 10.1371/journal.pone.0070991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Giannopoulos P.F., Chu J., Joshi Y.B., Sperow M., Li J.G., Kirby L.G., Pratico D. Gene knockout of 5-lipoxygenase rescues synaptic dysfunction and improves memory in the triple-transgenic model of Alzheimer's disease. Mol. Psychiatry. 2014;19:511–518. doi: 10.1038/mp.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 120.Gounari F., Chang R., Cowan J., Guo Z., Dose M., Gounaris E., Khazaie K. Loss of adenomatous polyposis coli gene function disrupts thymic development. Nat. Immunol. 2005;6:800–809. doi: 10.1038/ni1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cheon E.C., Strouch M.J., Krantz S.B., Heiferman M.J., Bentrem D.J. Genetic deletion of 5-lipoxygenase increases tumor-infiltrating macrophages in Apc(Delta468) mice. J. Gastrointest. Surg. 2012;16:389–393. doi: 10.1007/s11605-011-1761-x. [DOI] [PubMed] [Google Scholar]
- 122.Cheon E.C., Khazaie K., Khan M.W., Strouch M.J., Krantz S.B., Phillips J., Blatner N.R., Hix L.M., Zhang M., Dennis K.L., Salabat M.R., Heiferman M., Grippo P.J., Munshi H.G., Gounaris E., Bentrem D.J. Mast cell 5-lipoxygenase activity promotes intestinal polyposis in APCDelta468 mice. Cancer Res. 2011;71:1627–1636. doi: 10.1158/0008-5472.CAN-10-1923. [DOI] [PubMed] [Google Scholar]
- 123.Wimberly A.L., Forsyth C.B., Khan M.W., Pemberton A., Khazaie K., Keshavarzian A. Ethanol-induced mast cell-mediated inflammation leads to increased susceptibility of intestinal tumorigenesis in the APC Delta468 min mouse model of colon cancer. Alcohol Clin. Exp. Res. 2013;37(Suppl 1):E199–E208. doi: 10.1111/j.1530-0277.2012.01894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Funk C.D. Leukotriene modifiers as potential therapeutics for cardiovascular disease. Nat. Rev. Drug Discov. 2005;4:664–672. doi: 10.1038/nrd1796. [DOI] [PubMed] [Google Scholar]
- 125.Sampson A.P. FLAP inhibitors for the treatment of inflammatory diseases. Curr. Opin. Investig. Drugs. 2009;10:1163–1172. [PubMed] [Google Scholar]
- 126.Holloway J.W., Barton S.J., Holgate S.T., Rose-Zerilli M.J., Sayers I. The role of LTA4H and ALOX5AP polymorphism in asthma and allergy susceptibility. Allergy. 2008;63:1046–1053. doi: 10.1111/j.1398-9995.2008.01667.x. [DOI] [PubMed] [Google Scholar]
- 127.Byrum R.S., Goulet J.L., Griffiths R.J., Koller B.H. Role of the 5-lipoxygenase-activating protein (FLAP) in murine acute inflammatory responses. J. Exp. Med. 1997;185:1065–1075. doi: 10.1084/jem.185.6.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Strom J.O., Strid T., Hammarstrom S. Disruption of the alox5ap gene ameliorates focal ischemic stroke: possible consequence of impaired leukotriene biosynthesis. BMC Neurosci. 2012;13:146. doi: 10.1186/1471-2202-13-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Griffiths R.J., Smith M.A., Roach M.L., Stock J.L., Stam E.J., Milici A.J., Scampoli D.N., Eskra J.D., Byrum R.S., Koller B.H., McNeish J.D. Collagen-induced arthritis is reduced in 5-lipoxygenase-activating protein-deficient mice. J. Exp. Med. 1997;185:1123–1129. doi: 10.1084/jem.185.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jawien J., Gajda M., Rudling M., Mateuszuk L., Olszanecki R., Guzik T.J., Cichocki T., Chlopicki S., Korbut R. Inhibition of five lipoxygenase activating protein (FLAP) by MK-886 decreases atherosclerosis in apoE/LDLR-double knockout mice. Eur. J. Clin. Investig. 2006;36:141–146. doi: 10.1111/j.1365-2362.2006.01606.x. [DOI] [PubMed] [Google Scholar]
- 131.Jawien J., Gajda M., Olszanecki R., Korbut R. BAY x 1005 attenuates atherosclerosis in apoE/LDLR-double knockout mice. J. Physiol. Pharmacol. 2007;58:583–588. [PubMed] [Google Scholar]
- 132.Back M., Sultan A., Ovchinnikova O., Hansson G.K. 5-Lipoxygenase-activating protein: a potential link between innate and adaptive immunity in atherosclerosis and adipose tissue inflammation. Circ. Res. 2007;100:946–949. doi: 10.1161/01.RES.0000264498.60702.0d. [DOI] [PubMed] [Google Scholar]
- 133.Giannopoulos P.F., Chu J., Joshi Y.B., Sperow M., Li J.G., Kirby L.G., Pratico D. 5-lipoxygenase activating protein reduction ameliorates cognitive deficit, synaptic dysfunction, and neuropathology in a mouse model of Alzheimer's disease. Biol. Psychiatry. 2013;74:348–356. doi: 10.1016/j.biopsych.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 134.Joshi Y.B., Chu J., Pratico D. Knockout of 5-lipoxygenase prevents dexamethasone-induced tau pathology in 3xTg mice. Aging Cell. 2013;12:706–711. doi: 10.1111/acel.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Steinhilber D., Hofmann B. Recent advances in the search for novel 5-lipoxygenase inhibitors. Basic Clin. Pharmacol. Toxicol. 2014;114:70–77. doi: 10.1111/bcpt.12114. [DOI] [PubMed] [Google Scholar]
- 136.van Leyen K. Lipoxygenase: an emerging target for stroke therapy. CNS Neurol. Disord. Drug Targets. 2013;12:191–199. doi: 10.2174/18715273112119990053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bain G., King C.D., Brittain J., Hartung J.P., Dearmond I., Stearns B., Truong Y.P., Hutchinson J.H., Evans J.F., Holme K. Vol. 52. 2012. Pharmacodynamics, pharmacokinetics, and safety of AM211: a novel and potent antagonist of the prostaglandin D2 receptor type 2; pp. 1482–1493. (J. Clin. Pharmacol.). [DOI] [PubMed] [Google Scholar]
- 138.Follows R.M., Snowise N.G., Ho S.Y., Ambery C.L., Smart K., McQuade B.A. Efficacy, safety and tolerability of GSK2190915, a 5-lipoxygenase activating protein inhibitor, in adults and adolescents with persistent asthma: a randomised dose-ranging study. Respir. Res. 2013;14:54. doi: 10.1186/1465-9921-14-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Singh D., Boyce M., Norris V., Kent S.E., Bentley J.H. Inhibition of the early asthmatic response to inhaled allergen by the 5-lipoxygenase activating protein inhibitor GSK2190915: a dose-response study. Int. J. Gen. Med. 2013;6:897–903. doi: 10.2147/IJGM.S51364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Assimes T.L., Knowles J.W., Priest J.R., Basu A., Borchert A., Volcik K.A., Grove M.L., Tabor H.K., Southwick A., Tabibiazar R., Sidney S., Boerwinkle E., Go A.S., Iribarren C., Hlatky M.A., Fortmann S.P., Myers R.M., Kuhn H., Risch N., Quertermous T. A near null variant of 12/15-LOX encoded by a novel SNP in ALOX15 and the risk of coronary artery disease. Atherosclerosis. 2008;198:136–144. doi: 10.1016/j.atherosclerosis.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Habermann N., Ulrich C.M., Lundgreen A., Makar K.W., Poole E.M., Caan B., Kulmacz R., Whitton J., Galbraith R., Potter J.D., Slattery M.L. PTGS1, PTGS2, ALOX5, ALOX12, ALOX15, and FLAP SNPs: interaction with fatty acids in colon cancer and rectal cancer. Genes Nutr. 2013;8:115–126. doi: 10.1007/s12263-012-0302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kraus S., Hummler S., Toriola A.T., Poole E.M., Scherer D., Kotzmann J., Makar K.W., Kazanov D., Galazan L., Naumov I., Coghill A.E., Duggan D., Gigic B., Arber N., Ulrich C.M. Impact of genetic polymorphisms on adenoma recurrence and toxicity in a COX2 inhibitor (celecoxib) trial: results from a pilot study. Pharmacogenet. Genom. 2013;23:428–437. doi: 10.1097/FPC.0b013e3283631784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Urano T., Shiraki M., Fujita M., Hosoi T., Orimo H., Ouchi Y., Inoue S. Association of a single nucleotide polymorphism in the lipoxygenase ALOX15 5′-flanking region (-5229G/A) with bone mineral density. J. Bone Miner. Metab. 2005;23:226–230. doi: 10.1007/s00774-004-0588-x. [DOI] [PubMed] [Google Scholar]
- 144.Cheung C.L., Chan V., Kung A.W. A differential association of ALOX15 polymorphisms with bone mineral density in pre- and post-menopausal women. Hum. Hered. 2008;65:1–8. doi: 10.1159/000106057. [DOI] [PubMed] [Google Scholar]
- 145.Wuest S.J., Horn T., Marti-Jaun J., Kuhn H., Hersberger M. Association of polymorphisms in the ALOX15B gene with coronary artery disease. Clin. Biochem. 2014;47:349–355. doi: 10.1016/j.clinbiochem.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 146.Burdon K.P., Rudock M.E., Lehtinen A.B., Langefeld C.D., Bowden D.W., Register T.C., Liu Y., Freedman B.I., Carr J.J., Hedrick C.C., Rich S.S. Human lipoxygenase pathway gene variation and association with markers of subclinical atherosclerosis in the diabetes heart study. Mediat. Inflamm. 2010;2010:170153. doi: 10.1155/2010/170153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Witola W.H., Liu S.R., Montpetit A., Welti R., Hypolite M., Roth M., Zhou Y., Mui E., Cesbron-Delauw M.F., Fournie G.J., Cavailles P., Bisanz C., Boyer K., Withers S., Noble A.G., Swisher C.N., Heydemann P.T., Rabiah P., Muench S.P., McLeod R. ALOX12 in human toxoplasmosis. Infect. Immun. 2014;82:2670–2679. doi: 10.1128/IAI.01505-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Resler A.J., Makar K.W., Heath L., Whitton J., Potter J.D., Poole E.M., Habermann N., Scherer D., Duggan D., Wang H., Lindor N.M., Passarelli M.N., Baron J.A., Newcomb P.A., Le Marchand L., Ulrich C.M. Genetic variation in prostaglandin synthesis and related pathways, NSAID use and colorectal cancer risk in the Colon Cancer Family Registry. Carcinogenesis. 2014;35:2121–2126. doi: 10.1093/carcin/bgu119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gong Z., Hebert J.R., Bostick R.M., Deng Z., Hurley T.G., Dixon D.A., Nitcheva D., Xie D. Common polymorphisms in 5-lipoxygenase and 12-lipoxygenase genes and the risk of incident, sporadic colorectal adenoma. Cancer. 2007;109:849–857. doi: 10.1002/cncr.22469. [DOI] [PubMed] [Google Scholar]
- 150.Xiong D.H., Shen H., Zhao L.J., Xiao P., Yang T.L., Guo Y., Wang W., Guo Y.F., Liu Y.J., Recker R.R., Deng H.W. Robust and comprehensive analysis of 20 osteoporosis candidate genes by very high-density single-nucleotide polymorphism screen among 405 white nuclear families identified significant association and gene-gene interaction. J. Bone Miner. Res. 2006;21:1678–1695. doi: 10.1359/JBMR.060808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Israeli S., Goldberg I., Fuchs-Telem D., Bergman R., Indelman M., Bitterman-Deutsch O., Harel A., Mashiach Y., Sarig O., Sprecher E. Non-syndromic autosomal recessive congenital ichthyosis in the Israeli population. Clin. Exp. Dermatol. 2013;38:911–916. doi: 10.1111/ced.12148. [DOI] [PubMed] [Google Scholar]
- 152.Dwyer J.H., Allayee H., Dwyer K.M., Fan J., Wu H., Mar R., Lusis A.J., Mehrabian M. Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N. Engl. J. Med. 2004;350:29–37. doi: 10.1056/NEJMoa025079. [DOI] [PubMed] [Google Scholar]
- 153.Spanbroek R., Grabner R., Lotzer K., Hildner M., Urbach A., Ruhling K., Moos M.P., Kaiser B., Cohnert T.U., Wahlers T., Zieske A., Plenz G., Robenek H., Salbach P., Kuhn H., Radmark O., Samuelsson B., Habenicht A.J. Expanding expression of the 5-lipoxygenase pathway within the arterial wall during human atherogenesis. Proc. Natl. Acad. Sci. USA. 2003;100:1238–1243. doi: 10.1073/pnas.242716099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kalayci O., Birben E., Sackesen C., Keskin O., Tahan F., Wechsler M.E., Civelek E., Soyer O.U., Adalioglu G., Tuncer A., Israel E., Lilly C. ALOX5 promoter genotype, asthma severity and LTC production by eosinophils. Allergy. 2006;61:97–103. doi: 10.1111/j.1398-9995.2006.00979.x. [DOI] [PubMed] [Google Scholar]
- 155.Choi J.H., Park H.S., Oh H.B., Lee J.H., Suh Y.J., Park C.S., Shin H.D. Leukotriene-related gene polymorphisms in ASA-intolerant asthma: an association with a haplotype of 5-lipoxygenase. Hum. Genet. 2004;114:337–344. doi: 10.1007/s00439-004-1082-1. [DOI] [PubMed] [Google Scholar]
- 156.Via M., De Giacomo A., Corvol H., Eng C., Seibold M.A., Gillett C., Galanter J., Sen S., Tcheurekdjian H., Chapela R., Rodriguez-Santana J.R., Rodriguez-Cintron W., Thyne S., Avila P.C., Choudhry S., Gonzalez Burchard E. The role of LTA4H and ALOX5AP genes in the risk for asthma in Latinos. Clin. Exp. Allergy. 2010;40:582–589. doi: 10.1111/j.1365-2222.2009.03438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kronke G., Katzenbeisser J., Uderhardt S., Zaiss M.M., Scholtysek C., Schabbauer G., Zarbock A., Koenders M.I., Axmann R., Zwerina J., Baenckler H.W., van den Berg W., Voll R.E., Kuhn H., Joosten L.A., Schett G. 12/15-lipoxygenase counteracts inflammation and tissue damage in arthritis. J. Immunol. 2009;183:3383–3389. doi: 10.4049/jimmunol.0900327. [DOI] [PubMed] [Google Scholar]
- 158.Secor W.E., Powell M.R., Morgan J., Wynn T.A., Funk C.D. Mice deficient for 5-lipoxygenase, but not leukocyte-type 12-lipoxygenase, display altered immune responses during infection with Schistosoma mansoni. Prostaglandins Other Lipid Mediat. 1998;56:291–304. doi: 10.1016/s0090-6980(98)00059-8. [DOI] [PubMed] [Google Scholar]
- 159.Jeon S.G., Moon H.G., Kim Y.S., Choi J.P., Shin T.S., Hong S.W., Tae Y.M., Kim S.H., Zhu Z., Gho Y.S., Kim Y.K. 15-lipoxygenase metabolites play an important role in the development of a T-helper type 1 allergic inflammation induced by double-stranded RNA. Clin. Exp. Allergy. 2009;39:908–917. doi: 10.1111/j.1365-2222.2009.03211.x. [DOI] [PubMed] [Google Scholar]
- 160.Zhao L., Cuff C.A., Moss E., Wille U., Cyrus T., Klein E.A., Pratico D., Rader D.J., Hunter C.A., Pure E., Funk C.D. Selective interleukin-12 synthesis defect in 12/15-lipoxygenase-deficient macrophages associated with reduced atherosclerosis in a mouse model of familial hypercholesterolemia. J. Biol. Chem. 2002;277:35350–35356. doi: 10.1074/jbc.M205738200. [DOI] [PubMed] [Google Scholar]
- 161.Zarbock A., Distasi M.R., Smith E., Sanders J.M., Kronke G., Harry B.L., von Vietinghoff S., Buscher K., Nadler J.L., Ley K. Improved survival and reduced vascular permeability by eliminating or blocking 12/15-lipoxygenase in mouse models of acute lung injury (ALI) J. Immunol. 2009;183:4715–4722. doi: 10.4049/jimmunol.0802592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Uderhardt S., Herrmann M., Oskolkova O.V., Aschermann S., Bicker W., Ipseiz N., Sarter K., Frey B., Rothe T., Voll R., Nimmerjahn F., Bochkov V.N., Schett G., Kronke G. 12/15-lipoxygenase orchestrates the clearance of apoptotic cells and maintains immunologic tolerance. Immunity. 2012;36:834–846. doi: 10.1016/j.immuni.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 163.Obrosova I.G., Stavniichuk R., Drel V.R., Shevalye H., Vareniuk I., Nadler J.L., Schmidt R.E. Different roles of 12/15-lipoxygenase in diabetic large and small fiber peripheral and autonomic neuropathies. Am. J. Pathol. 2010;177:1436–1447. doi: 10.2353/ajpath.2010.100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sears D.D., Miles P.D., Chapman J., Ofrecio J.M., Almazan F., Thapar D., Miller Y.I. 12/15-lipoxygenase is required for the early onset of high fat diet-induced adipose tissue inflammation and insulin resistance in mice. PLoS One. 2009;4:e7250. doi: 10.1371/journal.pone.0007250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kundumani-Sridharan V., Niu J., Wang D., Van Quyen D., Zhang Q., Singh N.K., Subramani J., Karri S., Rao G.N. 15(S)-hydroxyeicosatetraenoic acid-induced angiogenesis requires Src-mediated Egr-1-dependent rapid induction of FGF-2 expression. Blood. 2010;115:2105–2116. doi: 10.1182/blood-2009-09-241802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Singh N.K., Kundumani-Sridharan V., Rao G.N. 12/15-Lipoxygenase gene knockout severely impairs ischemia-induced angiogenesis due to lack of Rac1 farnesylation. Blood. 2011;118:5701–5712. doi: 10.1182/blood-2011-04-347468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cole B.K., Kuhn N.S., Green-Mitchell S.M., Leone K.A., Raab R.M., Nadler J.L., Chakrabarti S.K. 12/15-Lipoxygenase signaling in the endoplasmic reticulum stress response. Am. J. Physiol. Endocrinol. Metab. 2012;302:E654–E665. doi: 10.1152/ajpendo.00373.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kriska T., Cepura C., Magier D., Siangjong L., Gauthier K.M., Campbell W.B. Mice lacking macrophage 12/15-lipoxygenase are resistant to experimental hypertension. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H2428–H2438. doi: 10.1152/ajpheart.01120.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Leedom A.J., Sullivan A.B., Dong B., Lau D., Gronert K. Endogenous LXA4 circuits are determinants of pathological angiogenesis in response to chronic injury. Am. J. Pathol. 2010;176:74–84. doi: 10.2353/ajpath.2010.090678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Martinez-Clemente M., Ferre N., Titos E., Horrillo R., Gonzalez-Periz A., Moran-Salvador E., Lopez-Vicario C., Miquel R., Arroyo V., Funk C.D., Claria J. Disruption of the 12/15-lipoxygenase gene (Alox15) protects hyperlipidemic mice from nonalcoholic fatty liver disease. Hepatology. 2010;52:1980–1991. doi: 10.1002/hep.23928. [DOI] [PubMed] [Google Scholar]
- 171.Stavniichuk R., Shevalye H., Hirooka H., Nadler J.L., Obrosova I.G. Interplay of sorbitol pathway of glucose metabolism, 12/15-lipoxygenase, and mitogen-activated protein kinases in the pathogenesis of diabetic peripheral neuropathy. Biochem. Pharmacol. 2012;83:932–940. doi: 10.1016/j.bcp.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mabalirajan U., Rehman R., Ahmad T., Kumar S., Leishangthem G.D., Singh S., Dinda A.K., Biswal S., Agrawal A., Ghosh B. 12/15-lipoxygenase expressed in non-epithelial cells causes airway epithelial injury in asthma. Sci. Rep. 2013;3:1540. doi: 10.1038/srep01540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fabre J.E., Goulet J.L., Riche E., Nguyen M., Coggins K., Offenbacher S., Koller B.H. Transcellular biosynthesis contributes to the production of leukotrienes during inflammatory responses in vivo. J. Clin. Investig. 2002;109:1373–1380. doi: 10.1172/JCI14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Secatto A., Rodrigues L.C., Serezani C.H., Ramos S.G., Dias-Baruffi M., Faccioli L.H., Medeiros A.I. 5-Lipoxygenase deficiency impairs innate and adaptive immune responses during fungal infection. PLoS One. 2012;7:e31701. doi: 10.1371/journal.pone.0031701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Uz T., Dimitrijevic N., Tueting P., Manev H. 5-lipoxygenase (5LOX)-deficient mice express reduced anxiety-like behavior. Restor. Neurol. Neurosci. 2002;20:15–20. [PubMed] [Google Scholar]
- 176.Ichinose F., Zapol W.M., Sapirstein A., Ullrich R., Tager A.M., Coggins K., Jones R., Bloch K.D. Attenuation of hypoxic pulmonary vasoconstriction by endotoxemia requires 5-lipoxygenase in mice. Circ. Res. 2001;88:832–838. doi: 10.1161/hh0801.089177. [DOI] [PubMed] [Google Scholar]