Podocyte-specific expression of Cre recombinase promotes glomerular basement membrane thickening (original) (raw)
Abstract
Conditional gene targeting using Cre recombinase has offered a powerful tool to modify gene function precisely in defined cells/tissues and at specific times. However, in mammalian cells, Cre recombinase can be genotoxic. The importance of including Cre-expressing control mice to avoid misinterpretation and to maximize the validity of the experimental results has been increasingly recognized. While studying the role of podocytes in the pathogenesis of glomerular basement membrane (GBM) thickening, we used Cre recombinase driven by the podocyte-specific podocin promoter (NPHS2-Cre) to generate a conditional knockout. By conventional structural and functional measures (histology by periodic acid-Schiff staining, albuminuria, and plasma creatinine), we did not detect significant differences between NPHS2-Cre transgenic and wild-type control mice. However, surprisingly, the group that expressed Cre transgene alone developed signs of podocyte toxicity, including marked GBM thickening, loss of normal foot process morphology, and reduced Wilms tumor 1 expression. GBM thickening was characterized by altered expression of core structural protein laminin isoform α5β2γ1. RNA sequencing analysis of extracted glomeruli identified 230 genes that were significant and differentially expressed (applying a q < 0.05-fold change ≥ ±2 cutoff) in NPHS2-Cre mice compared with wild-type control mice. Many biological processes were reflected in the RNA sequencing data, including regulation of the extracellular matrix and pathways related to apoptosis and cell death. This study highlights the importance of including the appropriate controls for potential Cre-mediated toxicity in conditional gene-targeting experiments. Indeed, omitting the Cre transgene control can result in critical errors during interpretation of experimental data.
Keywords: Cre-mediated toxicity, Cre recombinase, glomerular basement membrane thickening, NPHS2-Cre, podocyte
INTRODUCTION
The ability to manipulate mice genetically via DNA editing has provided a potent tool for examining gene function. The Cre-loxP system is a well-established approach for studying the effects of spatially or temporally regulated genes in in vivo mouse models. In this system, modified from the P1 bacteriophage, a site-specific DNA recombinase enzyme called Cre efficiently catalyzes the recombination between two of its target recognition sites, termed loxP, a 34-bp consensus sequence made up of a directional 8-bp core space sequence and two 13-bp palindromic flanking sequences (84, 85). To achieve conditional control of gene deletion, an exon in the gene of interest is inserted between two loxP sites, and the modified gene is introduced via homologous recombination into its exact location in embryonic stem cells. Subsequently, the stem cells are injected into mouse blastocysts, yielding mice in which the gene is “floxed” but fully functional. Upon breeding the floxed mouse with a mouse transgenic for Cre recombinase, typically under the control of a tissue-specific promoter, the gene is inactivated by Cre recombinase because of excision of the exon. Conditional gene targeting using Cre recombinase has provided a valuable technique to achieve gene deletion precisely in defined cells/tissues and at specific times. This has permitted greater understanding of the roles of genes in vivo (38) and has avoided lethal and serious developmental defects that may result from their systemic mutation (71).
The highly specialized and terminally differentiated visceral glomerular epithelial cell or “podocyte,” a key component of the glomerular filtration barrier, is an early target of injury in both diabetic and nondiabetic glomerular diseases. Because of the role of podocytes in kidney development and function, podocyte-specific promoters have been identified to take advantage of the Cre-loxP system in podocyte-centric research (58, 93). As mutations in NPHS2, the gene encoding the glomerular protein podocin, were found to cause autosomal recessive steroid-resistant nephrotic syndrome (13), expression of Cre recombinase using the podocyte-specific promoter for podocin (NPHS2-Cre) has been previously described (57).
During development, the glomerular basement membrane (GBM) is built via a collective effort of the underlying glomerular endothelial cells and overlying podocytes. The immature GBM consists of collagen type IV-α1α2α1, synthesized by both podocytes and glomerular endothelial cells in early comma and S-shaped stages of glomerulogenesis (1). As the GBM matures, there is complex protein isoform switching. Indeed, the mature GBM contains isoforms of collagen type IV and laminin not found in most other basement membranes (56). Beginning at the capillary loop stage, podocytes alone appear to synthesize mature collagen type IV-α3α4α5, replacing the collagen type IV-α1α2α1 network (2). Both cell types may be responsible for the removal and replacement of immature laminin-α1β1γ1 (LAM-111) by mature laminin-α5β2γ1 (LAM-521) (83). After the mature GBM is fully assembled, the biosynthetic programs are downregulated (1).
Upon glomerular maturation, the podocyte is likely key in modifying the GBM and synthesizing and assembling basement membrane components, under both physiological and pathological conditions (51). While studying the role of podocyte biology in the pathogenesis of GBM thickening, we used NPHS2-Cre to generate a conditional knockout. Because we were aware of reports showing that Cre expression can cause toxicity in the absence of a floxed allele, irrespective of whether its expression is systemic, tissue specific, and/or ligand inducible (Table 1), we designed our experiments to include a control group that expressed the Cre transgene alone. We were surprised to find that GBM thickening was present in the transgenic mice that only expressed Cre, suggesting the presence of Cre-mediated toxicity.
Table 1.
Reports of Cre-mediated toxicity span many organ systems
| Target Cell/Tissue | Transgenic Mouse/Plant | Reported Toxic Effect(s) |
|---|---|---|
| Mouse models | ||
| Pancreatic β-cells and the hypothalamus | RIP-Cre (67) | β-cell dysfunction, glucose intolerance (42) |
| Myocardium | αMHC-Cre (5, 86) | Cardiac fibrosis and heart failure (11, 29, 35, 40, 43, 69) |
| p53−/− mouse (spontaneous lymphoma model) (24) | UBC-Cre-ERT2 (74) | Regression of established primary lymphoma (via apoptosis) (44) |
| Neuronal progenitors | Nestin-CreERT2 | Microencephaly, hydrocephaly (26, 70) |
| Skin epidermis | KRT5- and KRT14-Cre | Tetraploidy, apoptosis (17, 37) |
| Systemic, adult | CAGG-CreER | Gastric mucosal atrophy, metaplasia, and regenerative changes (34) |
| Lungs | Sftpc-Cre | Defective lung development with abnormal dilated cysts (38) |
| T lymphocytes | Lck-Cre | Increased T-cell apoptosis (CD4+ and CD8+ in thymus and spleen), acceleration of autoantibody production, lupus development (62) |
| Embryos | ERT2-Cre | Severe anemia, widespread apoptosis (60) |
| Systemic, adult and embryos | Rosa26-CreERT2 | Thymic atrophy; severe hematological toxicity, illegitimate chromosomal rearrangement in hematopoietic cells (32) |
| Systemic | PRKAR1A-Cre | Induction of PKA inhibitor causing global attenuation of the PKA signaling transduction pathway (27) |
| Plant models | ||
| Tomato, petunia, Nicotiana tabacum | CaMV 35S promoter-Cre | Growth retardation, distinct pattern of chlorosis in leaves (20) |
In the past, investigators have exploited the Cre-loxP system in mouse genetic manipulation studies without appreciating the potential for Cre-mediated toxicity. The assumption that Cre recombinase expression has minimal, if any, effects on the cell has been so pervasive that mice carrying only the Cre transgene have not always been used as controls (42). Lee et al. (42) systematically analyzed studies using a specific Cre recombinase mouse strain and observed that in greater than half the cases, the proper control for potential Cre-mediated toxicity, the use of the same mice lacking the loxP-flanked target gene, was not included. Omitting the Cre transgene control can result in critical misinterpretation of experimental data (26, 47, 60). For example, using tissue-specific Cre in pancreatic β-cells, at least 16 studies linking various genes to glucose intolerance and diabetes failed to recognize that the Cre transgene alone produced glucose intolerance (42). Indeed, Cre recombinase has been shown to induce apoptosis, growth inhibition, and chromosomal aberration in mammalian cells (38, 47, 66, 77, 81, 89).
In the present study, we sought formally to assess whether podocyte-restricted Cre recombinase expression modified glomerular biology in the absence of engineered loxP sites. We hypothesized that persistent NPHS2-Cre expression would mediate direct podocyte toxicity. To test our hypothesis, we followed an approach that combined functional, structural, molecular, and bioinformatics strategies. We wanted to emphasize that changes related to off-target effects of Cre recombinase may be missed when the relevant control cohort of mice is not included in conditional gene knockout studies.
MATERIALS AND METHODS
Primary antibodies.
Western blot and immunostaining experiments included use of the following primary antibodies: anti-actin (rabbit polyclonal, A2066, Sigma-Aldrich, St. Louis, MO), anti-laminin (rabbit polyclonal, ab11575, Abcam), anti-laminin γ1-subunit (rabbit monoclonal, ab233389, Abcam), and anti-Wilms tumor 1 (WT1; rabbit monoclonal, ab89901, Abcam).
NPHS2-Cre transgenic mice.
Protocols for animal use were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham (UAB), and all animals were monitored by the veterinary staff of the Animal Resources Program. All animal procedures were conducted in accordance with the Institutional Animal Care and Use Committee. Mice were housed in a specific pathogen-free, temperature-controlled environment on a 12:12-h (0600–1800 hours) light-dark cycle and had access to standard chow (Teklad 7904 irradiated natural ingredient NIH 31 open fixed formula diet) and water ad libitum. All mice were weaned at 21 days.
While studying the role of podocyte biology in the pathogenesis of GBM thickening, mice harboring the p2.5P-Cre transgene, with expression of Cre recombinase directed to podocytes within kidney glomeruli by the human podocin (NPHS2) promoter/enhancer region [29S6.CG-Tg(NPHS2-cre)295Lbh/BroJ] (catalog no. 008523, Jackson Laboratory, Bar Harbor, ME) (57) were used in generating a conditional knockout. For the purposes of genotyping, genomic DNA was isolated from tail biopsies after application of DNA digestion buffer (50 mM Tris-HCl, 100 mM EDTA, 1% SDS, and 100 mM NaCl) with proteinase K (Fisher Scientific, Suwanee, GA). Mice carrying the NPHS2-Cre transgene were identified via a PCR-based protocol using the primers (Integrated DNA Technologies, Coralville, IA) and probes (Life Technologies, Waltham, MA) shown in Table 2 [with sequences obtained from The Jackson Laboratory Genotyping Protocols Database (catalog no. 003724)], along with genomic prolactin as the internal positive control (Table 2). In the initial studies, mice harboring the NPHS2-Cre transgene alone were used as an experimental control.
Table 2.
Primer and probe sequences used for quantitative real-time PCR amplification
| Gene | Forward Sequence | Reverse Sequence | Probe |
|---|---|---|---|
| Cre | 5′-GCGGTCTGGCAGTAAAAACTATC-3′ | 5′-GTGAAACAGCATTGCTGTCACTT-3′ | 5′-AAACATGCTTCATCGTCGGTCCGG-3′ |
| Cygb | 5′-ACCCTCGCACAGCCTTCCT-3′ | 5′-CACAGCTGGATCTGGTTACAAACA-3′ | 5′-AACTGCCTGTCTTTTG-3′ |
| Itgb6 | 5′-TGGGCCGACAGAAGAACAGTT-3′ | 5′-CTCACGCCCTGGTCTCAATTTA-3′ | 5′-CATTGTTCAGATTGCTCC-3′ |
| Lama5 | 5′-GCAGGACGACGACGTCATCT-3′ | 5′-AAGTCTCGAAGTAACGGTGAGTAGGA-3′ | 5′-CCACAGAATACTCGCGAAT-3′ |
| Lamb2 | 5′-CAAGCACAATACTCGTGGACTCAA-3′ | 5′-TCCGACAGGCGTGAGTATGG-3′ | 5′-CAGTGTCAGGATTTCTATC-3′ |
| Lamc1 | 5′-AAGGCTGCCAACCCCATCT-3′ | 5′-AGACCACCGAGCTGAGGATCA-3′ | 5′-AAGATCCATGCGTGGCAT-3′ |
| Loxl2 | 5′-CCACAACAATGGCCAGTCTGA-3′ | 5′-AAGTGAAGACTTCCATGCTGTGGTA-3′ | 5′-TGCGTGGATTTGGCACG-3′ |
| Nov | 5′-CGGAAGCCACCGTAGGAGTT-3′ | 5′-CCACTCCGTCGTCTGCTCAA-3′ | 5′-ACTCCAGCATTAACTGC-3′ |
| Prl | 5′-TTATTGTTCAACATGGTGGATTAGC-3′ | 5′-CACTCGAGGACTGCACCAAA-3′ | 5′-TTCTGAACCTGATCCTCA-3′ |
| Wt1 | 5′-AGCTGTCCCACTTACAGATGCA-3′ | 5-TTGAAGTCACACTGGTATGGTTTCTC-3′ | 5′-CCGGAAGCACACTGG-3′ |
At specific time points and before death (baseline and 10, 16, and 24 wk), weight was recorded, and urine was collected from each mouse in metabolic cages to measure urinary albumin by ELISA according to the manufacturer’s protocol (Bethyl Laboratories, Montgomery, TX). Death was induced by following a two-step process (inhaled CO2 and exsanguination with bilateral thoracotomy). At death, blood was collected via cardiac puncture. Urinary and plasma creatinine levels were measured by liquid chromatography-tandem mass spectrometry at the UAB-University of California-San Diego O’Brien Center for Acute Kidney Injury Research bioanalytical core facility (87). Renal tissues were obtained (n = 6–8 per genotype), with wild-type mice as controls, achieved by breeding NPHS2-Cre heterozygous mice (NPHS2-Cre+/−) to yield mice that did not express the Cre transgene (NPHS2-Cre−/−). Histopathology was examined after periodic acid-Schiff staining. Only male mice were studied. To promote robust and reproducible results, accounting for the possibility of phenotypic variability in mice, more than one generation was studied across all groups (NPHS2-Cre+/−, NPHS2-Cre+/+, and wild-type control). For mice of the same genotype, there were no significant intergenerational differences in the parameters studied.
Isolation of mouse glomeruli using Dynabeads.
Glomeruli were extracted and purified from 24-wk-old NPHS2-Cre+/−, NPHS2-Cre+/+, and wild-type control mice using Dynabeads (n = 6 biological replicates/genotype, singly isolated). The protocol was a modification of previously described methods (88). Briefly, after intracardiac perfusion of Dulbecco’s PBS (Invitrogen, Waltham, MA) and 200 μl M450 tosylactivated Dynabeads (Invitrogen) diluted in 40 ml Dulbecco’s PBS, kidneys were harvested, decapsulated, placed on ice, minced, and transferred to a solution of collagenase type A (Sigma-Aldrich), deoxyribonuclease I (Worthington Biochemical, Lakewood, NJ), and HBSS (Invitrogen) for a 10-min incubation with gentle agitation in a 37°C water bath. After the digested tissue was filtered through a 100-μm Falcon cell strainer (Fisher Scientific), the resuspended tissue was serially applied to the magnetic DynaMag with intermittent washing to harvest the captured glomeruli. The specimen was examined under phase-contrast microscopy to ensure purity > 95%.
Isolation of ex vivo podocytes.
Ex vivo podocytes were isolated following a modification of previously described methods (59). Briefly, kidneys from 16-wk-old NPHS2-Cre+/−, NPHS2-Cre+/+, and wild-type control mice were extracted (n = 8–10 mice/genotype, pooled), decapsulated, placed on ice, minced, differentially filtered through 100-μm and 70-μm Falcon cell strainers (Fisher Scientific), and incubated with a digestion enzyme cocktail containing HBSS, collagenase type A, deoxyribonuclease I, and dispase II for 30 min with gentle agitation in a 37°C water bath. To achieve complete glomerular dissociation, chemical digestion was followed by physical agitation using the Miltenyi tissue dissociator (Miltenyi Biotec, Auburn, CA). Trypsin-EDTA (0.05%) was added for glomeruli that were particularly difficult to dissociate. A single cell suspension was obtained by passing the digested samples through a 20-μm filter (Miltenyi Biotec) to remove incompletely dissociated tissue. For the purification of podocytes, glomerular single cells were incubated for 30 min with anti-nephrin rabbit polyclonal antibody (2 μl/1 × 106 cells/50 μl, ab216692, Abcam) followed by a wash and incubation with anti-rabbit IgG microbeads (20 μl/107 total cells for 20 min, Miltenyi Biotec). Cells were positively selected using a MACS separator with LS columns (Miltenyi Biotec). Positively selected cells were then incubated with anti-podocalyxin goat polyclonal antibody (PA5-47502, ThermoFisher) for 15 min at 4°C followed by a wash and incubation with Alexa Fluor 647 donkey anti-goat IgG secondary antibody (Jackson ImmunoResearch, West Grove, PA), diluted 1:400, for 15 min at 4°C. Viability was confirmed using eBioscience 7-amino-actinomycin D viability staining solution (Invitrogen). Purity was confirmed with cell sorting by isolating fluorophore-labeled podocytes using the BD LSRII Analyzer at the UAB Comprehensive Flow Cytometry Core Facility.
Total RNA extraction, cDNA template generation, and quantitative real-time PCR.
The purified glomeruli and positively selected ex vivo podocyte subpopulation were harvested and processed for total RNA extraction, cDNA template generation, and quantitative real-time PCR analysis, as previously described (46). Briefly, samples were lysed and homogenized in TRIzol Reagent (Invitrogen). Total RNA was then isolated according to the manufacturer’s protocol after precipitation of RNA with isopropanol and glycogen from Mytilus edulis (Sigma-Aldrich), washing with 70% ethanol, and then air drying and resuspending the pellet in RNase-free water. To remove traces of genomic DNA from purified RNA samples, samples were treated with DNase (Invitrogen) according to the manufacturer’s protocol. cDNA templates were reverse transcribed using 1–2 μg total RNA/template, Superscript III (Invitrogen), and the Eppendorf Mastercycler Nexus Gradient Thermal Cycler. At the end of reverse transcription, Promega ribonuclease H (Fisher Scientific) was added, and templates were then stored at −20°C. Differential gene expression was quantified by real-time PCR performed in a 96-well format using the ABI Prism 7500 Sequence Detection System apparatus and software (Applied Biosystems). Sequences for primers and probes used for quantitative real-time PCR amplification are shown in Table 2. Relative gene expression levels were calculated after normalization to a set of standard TaqMan endogenous control genes, including eukaryotic 18S rRNA, GAPDH, c-abl oncogene 1, mitochondrially encoded ATP synthase 6, and β-actin (Life Technologies). Calculations were made via the ΔΔCT method (where CT is threshold cycle).
Immunostaining for WT1.
At 24 wk of age, mice were anesthetized with isoflurane and kidneys were preserved by in vivo perfusion fixation following a modification of previously described methods (92). Briefly, the left ventricle was perfused with PBS containing 3 ml of 2% lidocaine and 3,000 IU heparin sodium per 500 ml PBS followed by 2% paraformaldehyde-lysine-periodate fixation. Upon removal, the kidneys were postfixed on ice in the same fixative for 24–48 h and then stored at 4°C in PBS before being embedded in paraffin. For immunofluorescent staining for WT1, 4-μm tissue sections were deparaffinized in Histo-Clear (National Diagnostics, Atlanta, GA) and rehydrated in graded ethanol, as previously described (52). To enhance antigen retrieval, tissue sections were treated with Trilogy solution (Cell Marque, Rocklin, CA) and then incubated with 5% donkey serum (Jackson ImmunoResearch) in 0.1% Triton X-100 in PBS for 1 h. Sections were incubated overnight in a humidified chamber at 4°C with anti-WT1 rabbit monoclonal antibody diluted 1:200 (Abcam) in 1% donkey serum and 0.1% Triton X-100 in PBS. The following morning, after being washed, sections were incubated for 1 h at room temperature in a humidified chamber with Alexa Fluor 488-conjugated donkey anti-rabbit IgG secondary antibody diluted 1:500 (Jackson ImmunoResearch) in 1% donkey serum and 0.1% Triton X-100 in PBS. Nuclei were counterstained with Hoechst 33258 diluted 1:2,000 (Invitrogen) for 5 min. Sections were mounted in 0.2% _n_-propyl gallate in 9:1 glycerol-PBS. Image acquisition, processing, and analysis were applied uniformly across all treatment conditions in a blinded fashion. Images were captured using a Nikon Eclipse TE 2000 microscope, Perkin-Elmer ultraVIEW ERS rapid confocal imager, with Velocity software, at the UAB High Resolution Imaging Facility.
Transmission electron microscopy.
After whole kidney extraction and decapsulation, 1-mm cross sections were immersion fixed in Karnovsky fixative (paraformaldehyde-glutaraldehyde solution, Electron Microscopy Sciences, Hatfield, PA). Samples were rinsed in 0.1 M sodium cacodylate (pH 7.4) and then rinsed in 0.1 M sodium cacodylate containing 0.01 M 2-mercaptoethanol and 0.13 M sucrose (pH 7.4) (2-ME buffer), postfixed for 1 h in 1% osmium tetroxide in 2-ME buffer at 4°C, washed in 2-ME buffer, and then washed in 0.1 M sodium cacodylate. Samples were dehydrated in a graded series of ethanol and propylene oxide and embedded in epoxy resin (Embed 812 Resin, Electron Microscopy Sciences). Ultrathin (60–70 nm) sections were counterstained with uranyl acetate and lead citrate and observed using a Hitachi 7600 transmission electron microscope (Hitachi High-Technologies America, Schaumburg, IL) equipped with a Macrofire monochrome progressive scan charge-coupled device camera (Optronics, Goleta, CA) and AMTv image capture software (Advanced Microscopy Techniques, Danvers, MA).
Morphometric analysis.
A microscopist blinded to the mouse conditions collected all images of glomeruli and performed the morphometric analyses following a protocol that was a modification of previously published methods (50, 63). A systematic, randomized process was used for the selection of glomeruli and the points for measurement of GBM thickness. All visible peripheral capillary loops in each selected glomerulus were photographed at a primary magnification of approximately ×10,000. The exact magnifications and calibration of the digital measuring tool were calculated using electron micrographs of a grating replica grid containing 2,160 lines/mm (Electron Microscopy Sciences). Digital images were viewed with a standard grid overlay using Adobe Photoshop C3. In each capillary loop, GBM thickness was measured at 10 intercept points of the grid overlay and the epithelial side of the GBM, excluding the mesangial area of the capillary loop. For each mouse, 3–10 glomeruli were analyzed, and GBM thickness was measured at 185–351 points. All GBM thickness measurements were averaged to represent each mouse in statistical analyses.
Western blot analysis.
Western blot analysis, as previously described (19), was performed on protein extracts from Dynabead-isolated glomeruli. Briefly, extracted glomeruli from 24-wk-old wild-type, NPHS2-Cre+/−, and NPHS2-Cre+/+ mice (n = 5–6 mice/genotype, pooled) were resuspended in 2× SDS-PAGE sample buffer. Pierce broad-spectrum protease inhibitor and phosphatase inhibitor cocktails (Thermo Scientific) were added; samples were solubilized by vortexing. Insoluble material was removed by centrifugation at 15,000 g for 10 min. The protein concentration was measured by the Pierce BCA method (Thermo Scientific). An equal amount of protein was added to each lane followed by electrophoresis on 4–15% SDS-PAGE gels (Bio-Rad, Hercules, CA) and transfer to GVS nitrocellulose membranes (Fisher Scientific). After being blocked, membranes were incubated with antibodies to laminin (1:1,500) and the laminin γ1-subunit (1:1,500) followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:3,000, Bio-Rad). To ensure equal protein loading, the antibody to housekeeping protein actin (1:1,500) was used.
Reactions were developed with an enhanced chemiluminescent horseradish peroxidase substrate kit (Thermo Scientific), and blot chemiluminescent signals were captured using the ChemiDoc MP Imaging System (Bio-Rad), with densities of the positive bands measured with National Institutes of Health ImageJ software (National Institutes of Health, Bethesda, MD) (78). Three separate experiments were performed for each Western blot analysis, and the densitometry results were averaged, with normalization for differences in protein loading.
Next-generation sequencing on Illumina platforms.
After isolation of glomeruli using Dynabeads from NPHS2-Cre+/+ and wild-type control mice (n = 2 biological replicates/genotype, singly isolated), total RNA was extracted, and RNA samples were submitted to the Genomics Core Laboratory in the Heflin Center of Genomic Sciences at UAB for sample preparation and sequencing. mRNA sequencing was performed on the Illumina NextSeq500 as described by the manufacturer (Illumina, San Diego, CA). Briefly, samples were treated with DNase and assessed for quality of total RNA isolated from mouse glomeruli using the Agilent 2100 Bioanalyzer. RNA with an RNA integrity number of 7.0 or above was used for sequencing library preparation. We used the Agilent SureSelect Strand Specific mRNA library kit as per the manufacturer’s instructions (Agilent, Santa Clara, CA). Library construction began with two rounds of polyadenylate-positive selection using oligo-dT-containing magnetic beads. The resulting mRNA was randomly fragmented with cations and heat, which was followed by first-strand synthesis using random primers with inclusion of actinomycin D (final concentration: 2.4 ng/µl). Second-strand cDNA production was done with standard techniques, and the ends of the resulting cDNA were made blunt, A-tailed, and adaptors ligated for indexing to allow for multiplexing during sequencing. The cDNA libraries were quantitated using quantitative PCR in a Roche LightCycler 480 with the Kapa Biosystems kit for Illumina library quantitation (Kapa Biosystems, Woburn, MA) before cluster generation. Cluster generation was performed according to the manufacturer’s recommendations for onboard clustering (Illumina). We generated between 30 and 35 million paired-end 75-bp sequencing reads per sample to allow for better alignment of the sequences to the reference genome.
RNA sequencing bioinformatics analysis.
STAR (version 2.5.3a) was used to align the raw RNA sequencing Fastq reads to the reference genome from Gencode (23). After alignment, Cufflinks was used to count the number of reads mapping to each gene (91). Normalization and differential expression was then applied to the count files using Cuffdiff.
The data discussed in this publication have been deposited in the National Center for Biotechnology Information’s Gene Expression Omnibus (GEO) (25) and are accessible through GEO series Accession No. GSE117331 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE117331).
Functional enrichment analysis using the Protein Analysis Through Evolutionary Relationships system and Database for Annotation, Visualization, and Integrated Discovery.
The Protein Analysis Through Evolutionary Relationships (PANTHER) Classification System was used to identify significantly affected biological processes in the transcriptomic data sets (54). A list of detected genes was used as the data input, applying a q < 0.05 cutoff, along with a fold change ≥ ±2, such that only significant genes were considered for significant biological processes. Database for Annotation, Visualization, and Integrated Discovery (DAVID) 6.8 was also used to identify enriched biological themes (33).
Validation of RNA sequencing data using quantitative real-time PCR.
Quantitative real-time PCR was used to validate the RNA sequencing data for the following four specific matrix-related genes using cDNA templates generated from extracted glomeruli (n = 6 biological replicates/genotype, singly isolated): cytoglobin (Cygb), lysyl oxidase-like 2 (Loxl2), integrin-β6 (Itgb6), and nephroblastoma overexpressed gene (Nov). Sequences for primers and probes used for quantitative real-time PCR amplification are shown in Table 2.
Statistical analysis.
For comparison of mean values between two groups, an unpaired _t_-test was used. ANOVA was applied for comparison of data from more than two groups. A Tukey honest significant difference test was used to determine if the interaction among three or more variables was mutually statistically significant. All values are means ± SE except where otherwise indicated. Statistical significance was evaluated using GraphPad Prism version 7.01 for Windows (GraphPad Software, La Jolla, CA). The experimental findings were considered statistically significant if P < 0.5. All photomicrographs were made at similar intensity and background. During data analysis, the observer was blinded to the treatment categories. Genes identified from the RNA sequencing data were sorted by their significance. Significance was calculated as a corrected P cutoff or q value of 0.05 to select the regulated genes with the lowest false discovery rate.
RESULTS
Cre recombinase mRNA transcripts confirmed to be present in target tissue.
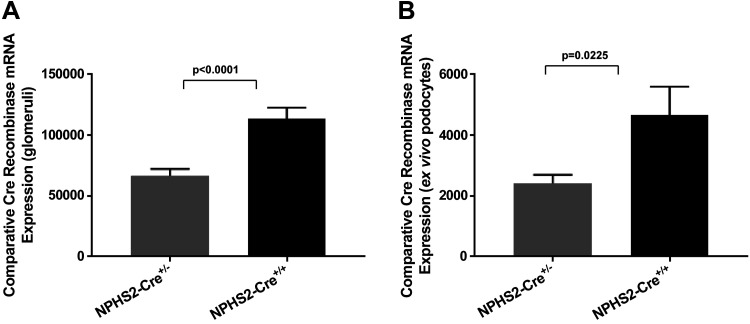
To confirm Cre expression in the target tissue of transgenic mice, glomeruli and ex vivo podocytes were harvested from NPHS2-Cre+/− and NPHS2-Cre+/+ mice (with Cre transgene heterozygosity and homozygosity established by genotyping of tail genomic DNA) and wild-type control mice. Cre recombinase gene expression was quantified by TaqMan real-time PCR. As expected, Cre recombinase expression was absent in both glomeruli and ex vivo podocytes of wild-type control mice. Normalized to an absence of Cre recombinase mRNA expression in glomeruli of wild-type control mice, expression of Cre transgene in glomeruli of NPHS2-Cre+/− and NPHS2-Cre+/+ mice was 66,702 ± 5,562 and 113,304 ± 9,407, respectively, and was statistically significantly different (P < 0.0001; Fig. 1_A_). Normalized to an absence of Cre recombinase mRNA expression in ex vivo podocytes harvested from wild-type control mice, expression of Cre transgene in ex vivo podocytes of NPHS2-Cre+/− and NPHS2-Cre+/+ mice was 2,418 ± 278.3 and 4,665 ± 931, respectively, and was statistically significantly different (P = 0.0225; Fig. 1_B_).
Fig. 1.
Comparative Cre recombinase mRNA expression in target tissue in NPHS2-Cre+/−, NPHS2-Cre+/+, and wild-type control mice. A: normalized to an absence of Cre recombinase mRNA expression in glomeruli of wild-type control mice, glomerular expression of the Cre transgene in NPHS2-Cre+/− and NPHS2-Cre+/+ mice was confirmed and was statistically significantly different (P < 0.0001) (n = 6 biological replicates/genotype, singly isolated). B: normalized to an absence of Cre recombinase mRNA expression in ex vivo podocytes isolated from wild-type control mice, podocyte expression of the Cre transgene in NPHS2-Cre+/− and NPHS2-Cre+/+ mice was confirmed and was statistically significantly different (P = 0.0225) (n = 8–10 mice/genotype, pooled).
Conventional functional and structural measures did not detect differences between NPHS2-Cre and wild-type control mice.
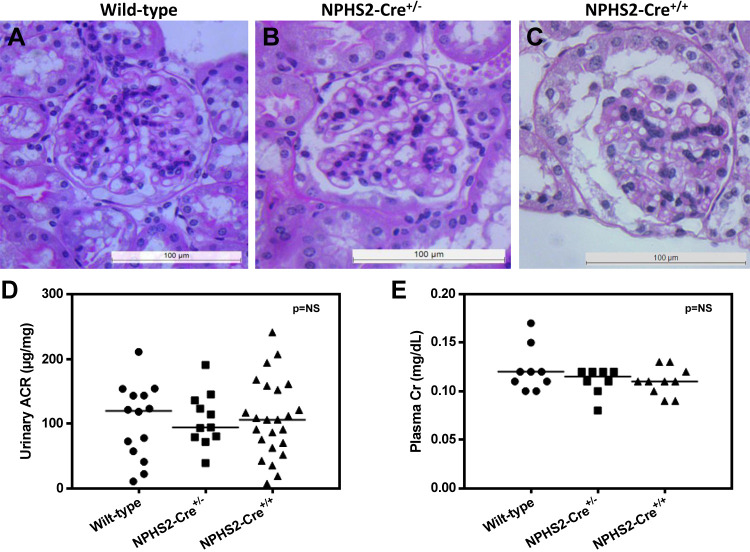
As the initial set of experiments were designed to include a Cre recombinase control group to discriminate clearly between phenotype(s) because of targeted mutation and the effects of Cre recombinase alone, NPHS2-Cre mice were compared with wild-type control mice using a set of standard measures of glomerular injury, including histopathology, urinary albumin excretion normalized to creatinine, and plasma creatinine. Histopathological changes by periodic acid-Schiff staining were minimal and nonspecific when we compared NPHS2-Cre+/−, NPHS2-Cre+/+, and wild-type control mice (Fig. 2, A–C). Biochemical parameters were assessed using two methods: the urinary albumin-to-creatinine ratio (ACR) and plasma creatinine. There were no statistically significant differences in urinary ACR (wild-type: 103.9 ± 15.46 μg/mg, NPHS2-Cre+/−: 106.4 ± 12.52 μg/mg, and NPHS2-Cre+/+: 108 ± 12.21 μg/mg; Fig. 2_D_). In addition, there were no statistically significant differences in plasma creatinine among the groups (wild-type: 0.122 ± 0.008 mg/dl, NPHS2-Cre+/−: 0.11 ± 0.005 mg/dl, and NPHS2-Cre+/+: 0.11 ± 0.005 mg/dl; Fig. 2_E_). Finally, there were no statistically significant differences in weights at 24 wk of age among the groups (wild-type: 30.49 ± 1.388 g, NPHS2-Cre+/−: 27.48 ± 0.553 g, and NPHS2-Cre+/+: 29.45 ± 0.694 g; data not shown).
Fig. 2.
Conventional functional and structural assessments of NPHS2-Cre+/−, NPHS2-Cre+/+, and wild-type control mice, aged to 24 wk. Shown is histopathology determined by periodic acid-Schiff (PAS) staining (n = 6 mice/group). A−C: representative micrographs for wild-type control (A), NPHS2-Cre+/− (B), and NPHS2-Cre+/+ (C). Histopathological changes by PAS staining were minimal and nonspecific between NPHS2-Cre transgenic and wild-type control mice. D: the urinary albumin-to-creatinine ratio (ACR) in NPHS2-Cre+/−, NPHS2-Cre+/+, and wild-type control mice (n > 10 mice/group). There were no significant differences in urinary albumin excretion normalized to creatinine among the groups. E: plasma creatinine in NPHS2-Cre+/−, NPHS2-Cre+/+, and wild-type control mice (n = 8–10 mice/group). There were no significant differences in plasma creatinine among the groups.
Reduced WT1 expression in NPHS2-Cre mice compared with wild-type control mice.
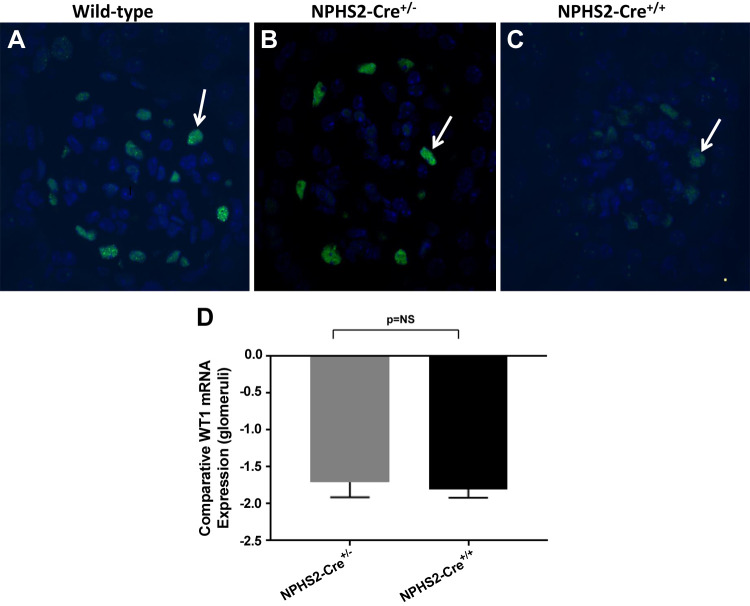
To determine whether expression of podocyte marker WT1 was affected by the expression of Cre transgene in NPHS2-Cre mice, immunofluorescent staining of paraffin-embedded tissue for WT1 was performed. By 24 wk of age, there was a notable decrease in the number of detectable WT1-positive cells in the glomeruli of NPHS2-Cre mice compared with wild-type control mice. Not only were there fewer glomerular cells that stained positive for WT1 in NPHS2-Cre mice but also the staining intensity was notably decreased in NPHS2-Cre+/+ mice compared with wild-type mice (Fig. 3, A–C). Comparative WT1 expression was then assessed by real-time PCR. When normalized to WT1 mRNA expression in the glomeruli of wild-type control mice, WT1 expression in the glomeruli of NPHS2-Cre+/− and NPHS2-Cre+/+ mice was downregulated 1.664 ± 0.246- and 1.77 ± 0.147-fold, respectively. There was no statistically significant difference in WT1 mRNA expression between NPHS2-Cre+/− and NPHS2-Cre+/+ glomeruli (Fig. 3_D_).
Fig. 3.
Expression of Wilms tumor 1 (WT1) in NPHS2-Cre+/−, NPHS2-Cre+/+, and wild-type control mice. By immunofluorescent staining of paraffin-embedded tissue (IF-P) for WT1 (original magnification ×60), the number of detectable WT1-positive cells was qualitatively decreased in glomeruli from NPHS2-Cre+/− and NPHS2-Cre+/+ mice at 24 wk of age compared with that seen in age-matched wild-type control mice. A−C: representative IF-P micrographs of WT1 staining in wild-type control (A), NPHS2-Cre+/− (B), and (C) NPHS2-Cre+/+ mice. Arrows indicate examples of WT1-positive cells. Both the number of detectable WT1-positive cells per glomerulus and the intensity of staining were decreased in NPHS2-Cre+/+ mice. D: comparative WT1 mRNA expression in glomeruli. When normalized to WT1 mRNA expression in glomeruli of wild-type control mice, glomerular expression of WT1 in NPHS2-Cre+/− and NPHS2-Cre+/+ was downregulated (−1.664 ± 0.246- and −1.77 ± 0.147-fold, respectively). However, there was no statistically significant difference in WT1 mRNA expression between NPHS2-Cre+/− and NPHS2-Cre+/+ glomeruli (n = 6 biological replicates/genotype, singly isolated). NS, not significant.
Marked GBM thickening present in NPHS2-Cre mice compared with wild-type control mice.
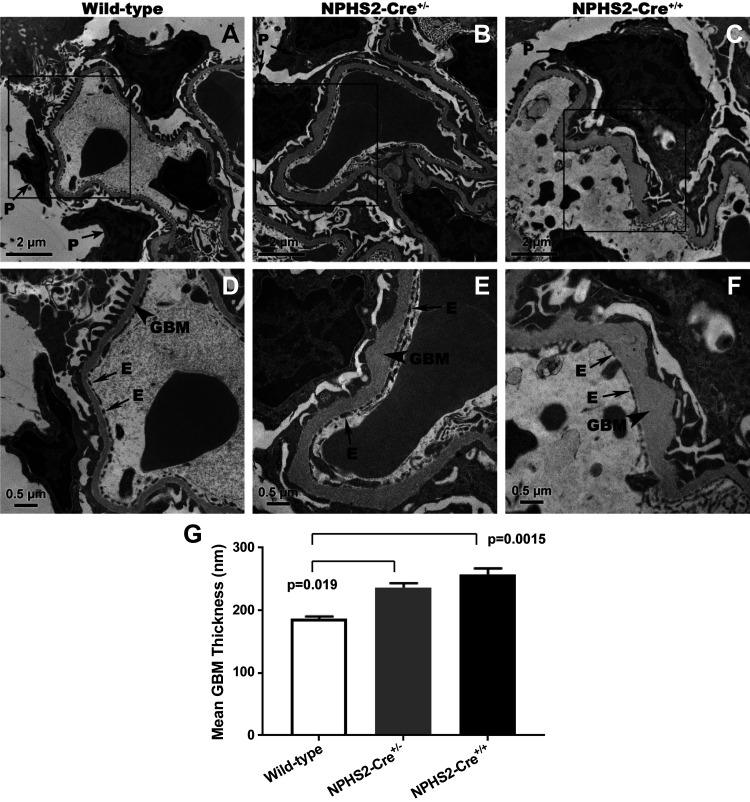
Morphometry of peripheral GBM was performed by examining glomeruli via transmission electron microscopy (Fig. 4). Compared with wild-type control mice (Fig. 4, A and D), strikingly abnormal GBMs were seen in transgenic mice aged to 24 wk (NPHS2-Cre+/−, Fig. 4, B and E; NPHS2-Cre+/+, Fig. 4, C and F), characterized by marked thickening with frequent large subepithelial GBM expansion, with discrete accumulation of basement membrane material of the same electron density as that seen in normal-appearing GBM. In addition, expanded regions of GBM coincided with loss of normal podocyte foot process morphology, including foot process broadening. At 24 wk, GBM thickness was significantly different among NPHS2-Cre+/− (236.9 ± 6.511 nm), NPHS2-Cre+/+ (256.8 ± 10.27 nm), and wild-type control mice (186.8 ± 3.576 nm, P = 0.0019). However, the difference in GBM thickness between NPHS2-Cre+/− and NPHS2-Cre+/+ mice was not statistically significant (Fig. 4_G_).
Fig. 4.
Morphometric analysis for glomerular basement membrane (GBM) thickness by transmission electron microscopy (TEM). By TEM, with boxes (top) corresponding to areas featured at higher magnification (bottom), there were strikingly abnormal GBMs seen in NPHS2-Cre+/− and NPHS2-Cre+/+ mice compared with wild-type control mice at 24 wk of age, characterized by marked thickening with frequent large subepithelial GBM expansion, with discrete accumulation of basement membrane material of the same electron density as that seen in the normal-appearing GBM. Expanded regions of GBM coincided with altered podocyte foot process morphology, including foot process broadening. A−F: representative electron micrographs of glomerular sections from wild-type control (A and D), NPHS2-Cre+/− (B and E), and NPHS2-Cre+/+ (C and F) mice. G: quantification of mean GBM thickness of NPHS2-Cre+/−, NPHS2-Cre+/+, and wild-type control mice shown in graphic form. There were statistically significant differences among mean GBM thickness as determined by one-way ANOVA [n = 3–5 mice/group, F(2,8) = 15.21, P = 0.0019]. There were statistically significant differences in mean GBM thickness between wild-type control and NPHS2-Cre+/− mice (Q = 4.9716, Tukey honest significant difference P = 0.019) and between wild-type control and NPHS2-Cre_+/+_ mice (Q = 7.7615, Tukey honest significant difference P = 0.0015) mice. E, endothelial cell; P, podocyte.
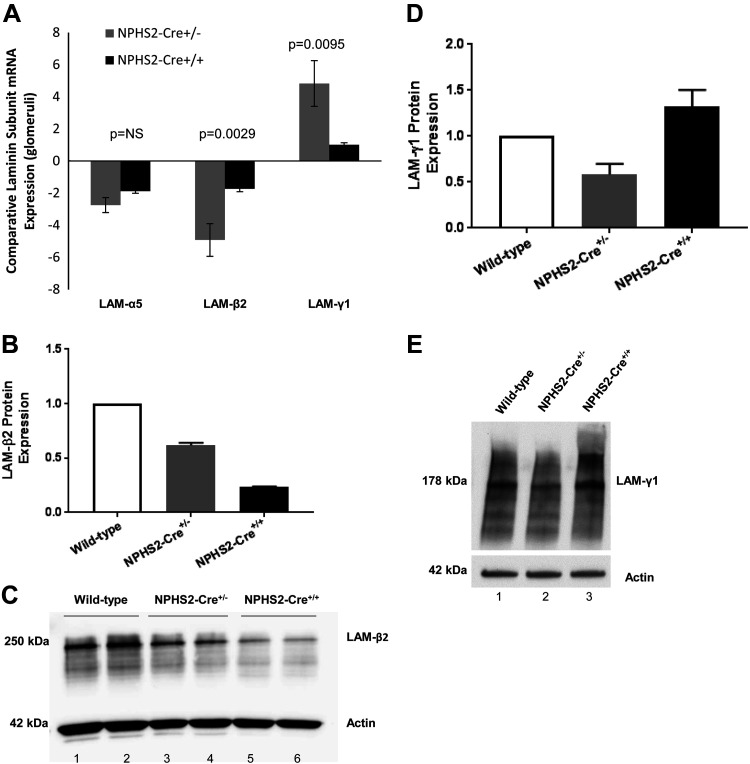
GBM thickening characterized by altered expression of core structural protein LAM-521.
To determine if changes in the core structural protein LAM-521 contributed to the GBM thickening seen in NPHS2-Cre transgenic mice, comparative mRNA expression by real-time PCR of the laminin chains that make up the mature isoform, namely, laminin-α5, laminin-β2, and laminin-γ1, was next assessed (n = 6 biological replicates/genotype, singly isolated). When normalized to expression levels in glomeruli of wild-type control mice, α5- and β2-subunits were downregulated (laminin-α5, NPHS2-Cre+/−: −2.744 ± 0.467-fold and NPHS2-Cre+/+: −1.88 ± 0.127-fold; and laminin-β2, NPHS2-Cre+/−: −4.918 ± 1.017-fold and NPHS2-Cre+/+: −1.733 ± 0.178-fold). In contrast, mRNA expression levels of the γ-subunit were upregulated (NPHS2-Cre+/−: 4.833 ± 1.424-fold and NPHS2-Cre+/+: 1.021 ± 0.124-fold; Fig. 5_A_).
Fig. 5.
Expression of mature isoform laminin-α5β2γ1 (LAM-521) in glomeruli of NPHS2-Cre+/−, NPHS2-Cre+/+, and wild-type control mice at 24 wk. A: comparative laminin chain mRNA expression in glomeruli (n = 6 biological replicates/genotype, singly isolated). When normalized to mRNA expression in glomeruli of wild-type control mice, glomerular mRNA expression of laminin-α5 and laminin-β2 was downregulated in NPHS2-Cre+/− and NPHS2-Cre+/+ mice, with laminin-β2 expression statistically significantly different between NPHS2-Cre+/− and NPHS2-Cre+/+ mice. mRNA expression of laminin-γ1 was upregulated in glomeruli from NPHS2-Cre+/− and NPHS2-Cre+/+ mice when normalized to glomerular mRNA expression in wild-type control mice, and laminin-γ1 expression level was significantly different between NPHS2-Cre+/− and NPHS2-Cre+/+ mice. B and C: Western blot analysis of laminin-β2 in glomeruli (n = 5–6 mice/genotype, pooled). When normalized to the protein expression in glomeruli of wild-type control mice, glomerular protein expression of laminin-β2 was decreased in NPHS2-Cre+/− and NPHS2-Cre+/+ (ratio: 0.618 ± 0.022 and 0.234 ± 0.004, respectively). The differences were statistically significant, as determined by one-way ANOVA (P < 0.0001). A representative Western blot for laminin-β2 is shown (wild-type control: lanes 1 and 2, NPHS2-Cre+/−: lanes 3 and 4, and NPHS2-Cre+/+: lanes 5 and 6). D and E: Western blot analysis of laminin-γ1 in glomeruli (n = 5–6 mice/genotype, pooled). When normalized to the protein expression in glomeruli of wild-type control mice, glomerular expression of laminin-γ1 was increased in NPHS2-Cre+/+ mice (ratio: 1.324 ± 0.180). The differences approached significant (P = 0.0534), as determined by one-way ANOVA. A representative Western blot for laminin-γ1 is shown (wild-type control: lane 1, NPHS2-Cre+/−: lane 2, and NPHS2-Cre+/+: lane 3). For Western blot analysis, values are averages of three separate experiments and are normalized for differences in protein loading, with actin used as the housekeeping protein.
Western blot analysis was next performed on protein isolated from glomeruli of NPHS2-Cre+/−, NPHS2-Cre+/+, and wild-type control mice (n = 5–6 mice/genotype, pooled) to assess the expression of laminin. When normalized to expression levels in glomeruli of wild-type mice, the band corresponding to laminin-β2 (based on the predicted molecular weight) was significantly decreased in NPHS2-Cre+/− and NPHS2-Cre+/+ mice, with ratios of 0.618 ± 0.022 and 0.234 ± 0.004, respectively (Fig. 5, B and C).
As the laminin γ1-subunit is common to most laminins, making up isoforms present during glomerulogenesis (LAM-111 and LAM-511) as well as upon maturation (LAM-521), and because the laminin γ1-unit was the only subunit that was upregulated in glomeruli of NPHS2-Cre transgenic mice by real-time PCR, Western blot analysis was performed to confirm upregulation of laminin-γ1. When normalized to expression levels in glomeruli of wild-type control mice, laminin-γ1 expression was increased in NPHS2-Cre+/+ mice, with a ratio of 1.324 ± 0.180 (Fig. 5, D and E).
Many biological processes reflected in RNA sequencing analysis of glomeruli isolated from NPHS2-Cre+/+ mice versus wild-type control mice.
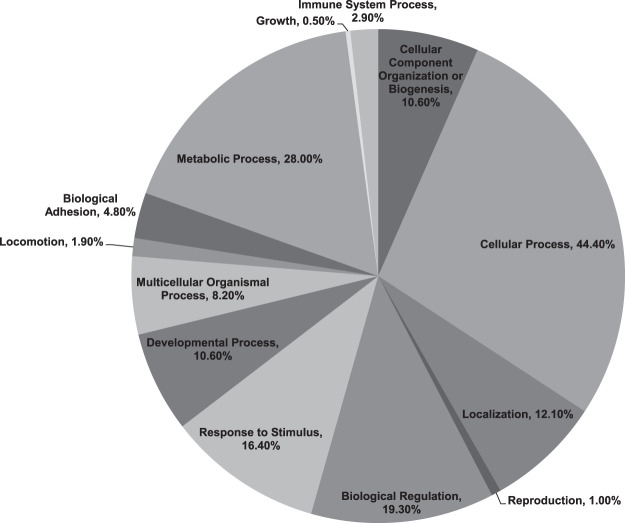
To investigate differential gene expression that might account for the differing glomerular phenotype of NPHS2-Cre+/+ mice compared with wild-type control mice, RNA sequencing analysis was performed on glomeruli extracted from both NPHS2-Cre+/+ and wild-type control mice. Two hundred thirty genes were identified that were considered to be significantly differentially expressed between the groups, meeting a false discovery rate value of <5% (q value < 0.05) and fold change ≥ ±2 cutoff (for details, see Supplemental Table S1; Supplemental Material is available at https://doi.org/10.17632/bky9jh6fn6.4). Of these significantly differentially regulated genes, 139 genes were upregulated and 91 genes were downregulated in glomeruli of NPHS2-Cre+/+ mice compared with wild-type control mice. To understand their biological significance better, Gene Ontology analysis was performed using both the PANTHER and DAVID classification systems (54). The biological processes predominantly reflected by the significant differentially regulated genes included cellular process (44.4%), metabolic process (28.0%), biological regulation (19.3%), response to stimulus (16.4%), localization (12.1%), developmental process (10.6%), and cellular component organization or biogenesis (10.6%), with some overlap across categories. Less-associated biological processes (<10%) included multicellular organismal process, biological adhesion, immune system process, locomotion, reproduction, and growth (Fig. 6). Some genes were not categorized by PANTHER and DAVID software and were classified as “uncharacterized” (Supplemental Table S1).
Fig. 6.
Biological processes reflected by the significant, differentially expressed glomerular genes. The Protein Analysis Through Evolutionary Relationships (PANTHER)-generated pie chart demonstrates the diverse repertoire of mouse glomerular biological processes implicated by the 230 significantly differentially expressed genes in NPHS2-Cre+/+ mice aged to 24 wk compared with age-matched wild-type control mice (n = 2 biological replicates/group, glomeruli singly isolated). Genes with a false discovery rate (FDR or q value) of <0.05 were considered to be significantly differentially expressed.
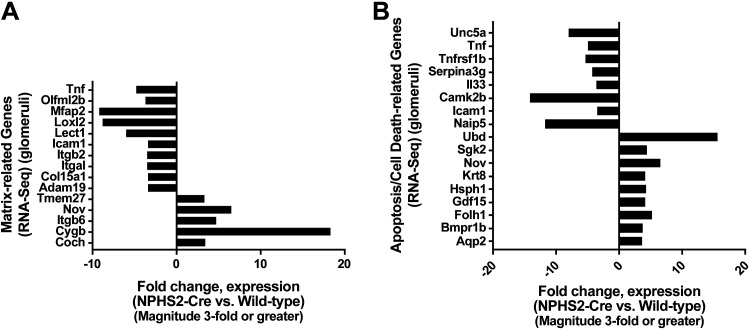
Significant differentially expressed matrix-related genes and apoptosis/cell death-related genes in NPHS2-Cre+/+ mice compared with wild-type control mice.
As increased GBM thickness was a prominent feature in NPHS2-Cre mouse glomeruli, extracellular matrix remodeling genes were considered particularly biologically important in the assessment of possible Cre-mediated toxicity. Of the 230 genes significantly differentially expressed in NPHS2-Cre+/+ versus wild-type control glomeruli, 15 matrix-related genes with a fold change of ≥ ±3 cutoff were identified (Fig. 7_A_). Interestingly, Cygb, a member of the globin superfamily noted to play an antifibrotic role in kidneys by inhibiting collagen synthesis via its radical scavenging function (55), was upregulated 18.232-fold in NPHS2-Cre+/+ mouse glomeruli. Furthermore, Loxl2, an amine oxidase that extracellularly promotes collagen type IV cross-linking and is essential for GBM stability and ultrafiltration function (7), was downregulated 8.581-fold in NPHS2-Cre+/+ mouse glomeruli. As the extracellular matrix communicates with cells through transmembrane cell surface receptors termed integrins, it is notable that Itgb6, more highly expressed during development than in the adult and increased in the kidney during periods of inflammation or repair (8), was upregulated 4.625-fold in NPHS2-Cre+/+ mouse glomeruli. Finally, Nov, a matrix-related protein detectable mainly in podocytes and collecting ducts in the adult kidney (10, 18) and shown to be profibrotic in an obstructive nephropathy mouse model (49), was upregulated 6.454-fold in NPHS2-Cre+/+ mouse glomeruli.
Fig. 7.
Significantly differentially expressed glomerular genes subclassified into matrix-related genes and apoptosis- or cell death-related genes. A: of the 230 significantly differentially expressed genes in NPHS2-Cre+/+ versus wild-type control glomeruli, 15 matrix-related genes with a fold change of ≥ ±3 cutoff (5 upregulated and 10 downregulated) were identified. B: of the 230 significantly differentially expressed genes in NPHS2-Cre+/+ versus wild-type control glomeruli, 17 genes related to apoptosis or cell death pathways with a fold change of ≥ ±3 cutoff (9 upregulated and 8 downregulated) were identified. Adam19, a disintegrin and metallopeptidase domain 19; Aqp2, aquaporin 2; Bmpr1b, bone morphogenetic protein receptor type 1B; Camk2b, Ca2+/calmodulin-dependent protein kinase IIβ; Coch, cochlin; Col15a1, collage type XV-α1; Cygb, cytoglobin; Folh1, folate hydrolase 1; Gdf15, growth differentiation factor 15; Hsph1, heat shock 105 kDa/110 kDa protein 1; Icam1, intercellular adhesion molecule-1; Il33, interleukin-33; Itgal, integrin-αL; Itgb2, integrin-β2; Itgb6, integrin-β6; Krt8, keratin 8; Lect1, leukocyte cell-derived chemotaxin 1; Loxl2, lysyl oxidase-like 2; Mfap2, microfibrillar-associated protein 2; Naip5, NLR family, apoptosis inhibitory protein 5; Nov, nephroblastoma overexpressed gene; Olfml2b, olfactomedin-like 2B; Serpina3g, serine (or cysteine) peptidase inhibitor clade A member 3G; Sgk2, serum/glucocorticoid-regulated kinase 2; Tmem27, transmembrane protein 27; Tnf, tumor necrosis factor; Tnfrsf1b, tumor necrosis factor receptor superfamily member 1b; Ubd, ubiquitin; Unc5a, Unc-5 netrin receptor A.
As the expression of the podocyte marker WT1 was decreased in NPHS2-Cre+/+ mouse glomeruli, suggesting podocyte injury and loss, genes associated with apoptosis and cell death were also considered biologically important in the assessment of possible Cre-mediated toxicity. Of the 230 genes significant and differentially expressed, 17 apoptosis- and cell death-related genes with a fold change of ≥ ±3 cutoff were identified (Fig. 7_B_). Genes that were downregulated in NPHS2-Cre+/+ mouse glomeruli included Unc-5 netrin receptor A (Unc5a; −7.693-fold), a DNA damage-inducible gene and downstream target of p53, and NLR family apoptosis inhibitory protein 5 (Naip5; −11.344-fold), a protein required for inflammasome activation and functioning upstream of caspase-1 activation (45). Inhibitors of the apoptotic process that were downregulated in NPHS2-Cre+/+ mouse glomeruli included TNF receptor superfamily member 1b (Tnfrsf1b; −4.935-fold), serine (or cysteine) peptidase inhibitor clade A member 3G (Serpina3g; −3.897-fold), and ICAM-1 (Icam1; −3.093-fold). Positive regulators of apoptosis that were upregulated in NPHS2-Cre+/+ mouse glomeruli included ubiquitin (Ubd; 15.611-fold), keratin 8 (Krt8; 4.148-fold), growth differentiation factor 15 (Gdf15; 4.153-fold), folate hydrolase 1 (Folh1; 5.160-fold), and bone morphogenetic protein receptor type 1B (Bmpr1b; 3.638-fold). Therefore, the changes in regulators of apoptosis and cell death pathways in NPHS2-Cre+/+ mouse glomeruli favored a shift toward a more proapoptotic/cell death phenotype.
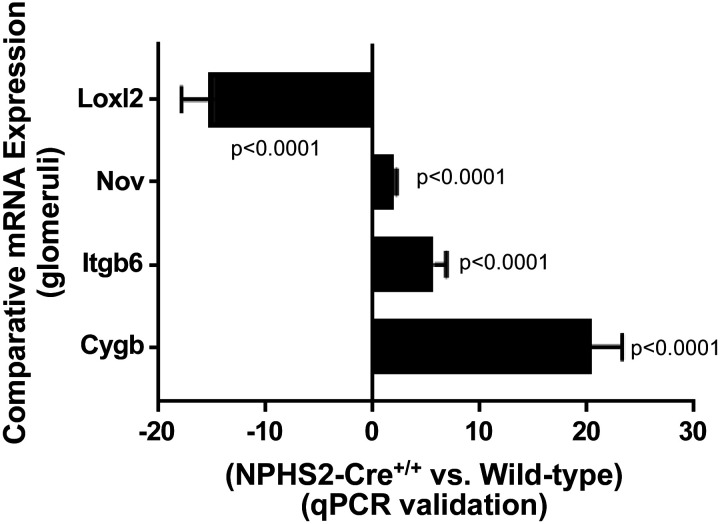
To validate RNA sequencing data, quantitative real-time PCR was performed using cDNA templates generated from extracted glomeruli for four specific matrix-related genes, with the following results (NPHS2-Cre+/+ compared with wild-type control): Cygb was upregulated 20.44 ± 2.845-fold, Loxl2 was downregulated 14.86 ± 3.06-fold, Itgb6 was upregulated 5.689 ± 1.148-fold, and Nov was upregulated 2.045 ± 0.167-fold (Fig. 8).
Fig. 8.
Validation of RNA sequencing results with quantitative PCR. Of the significantly differentially expressed glomerular genes that were subclassified into matrix-related genes and apoptosis- or cell death-related genes, four genes were further analyzed and validated with quantitative PCR: lysyl oxidase-like 2 (Loxl2), nephroblastoma overexpressed gene (Nov), integrin-β6 (Itgb6), and cytoglobin (Cygb). When normalized to expression levels in wild-type control glomeruli, mRNA expression levels in NPHS2-Cre+/+ glomeruli confirmed those observed in RNA sequencing data (Loxl2 was downregulated 14.86 ± 3.06-fold, P < 0.0001; Nov was upregulated 2.045 ± 0.167-fold, P < 0.0001; Itgb6 was upregulated 5.689 ± 1.148-fold, P < 0.0001; and Cygb was upregulated 20.44 ± 2.845-fold, P < 0.0001). Quantitative PCR experiments were performed on a new set of RNA samples (n = 6 biological replicates/group, glomeruli singly isolated).
DISCUSSION
For years, many investigators have exploited the Cre-loxP system in mouse genetic manipulation studies. Yet, mounting evidence challenges the widely held assumption that Cre recombinase, without loxP targets, is essentially inactive in vertebrate cells (42). This assumption may have persisted, in part, because of the absence of overt pathology in many Cre recombinase-expressing mice (42), overlooking the ability of mammals to tolerate considerable somatic cell death. This developmental plasticity is displayed by the Bcre32 transgenic mice, which have no described adult phenotypic abnormalities despite high levels of apoptosis in embryonic neural tissue (60). It is also possible that transgenic lines that express low levels of Cre have been inadvertently selected because embryos in the founder generation with high levels of Cre expression would either die or develop clear abnormalities and, therefore, be excluded (47). Increasingly, we are recognizing that the Cre-loxP system may pose several problems that can alter physiological parameters (30). Although it is certainly true that the extent of Cre effects will depend on the form of Cre, level of Cre expression, and cell type analyzed (22), an ever-increasing number of reports of Cre-mediated toxicity spanning many organ systems support the need to assess the independent effects of Cre-driven transgenes on experimental findings (Table 1).
Our present study provides an analysis of the glomerular phenotype of transgenic mice bearing the podocyte-specific Cre recombinase NPHS2-Cre. After confirming expression of NPHS2-Cre in target tissues of transgenic mice (purified glomeruli and ex vivo podocytes), we performed conventional measures of glomerular injury, including histopathology by light microscopy, urinary ACR, and plasma creatinine. These standard structural and functional assessments did not detect significant differences between NPHS2-Cre and wild-type control mice. However, when applying more sensitive methods for detection of glomerular injury, namely, assessment of expression of transcription factor and podocyte marker WT1 and ultrastructural assessment of glomerular morphology and GBM morphometry, we unexpectedly found decreased glomerular WT1 expression and marked GBM thickening in NPHS2-Cre transgenic mice compared with wild-type control mice. Moreover, GBM thickening was accompanied by loss of normal podocyte foot process morphology, including foot process broadening. Decreased WT1 expression and altered podocyte foot process morphology suggested Cre-mediated podocyte injury in NPHS2-Cre transgenic mice. As healthy podocytes are critical for maintaining normal GBM structure and function, marked GBM thickening in NPHS2-Cre transgenic mice provided further support for podocyte injury. Indeed, injured podocytes likely have a key role in upsetting the balance between synthetic and degradative pathways of the GBM, which actively remodels during times of injury and repair (51). GBM thickening is a structural lesion that occurs early in the natural history of diabetic nephropathy and precedes clinically evident albuminuria (64, 65). In fact, albuminuria and declining kidney function are considered insensitive biomarkers for detecting early diabetic nephropathy (61). Similarly, GBM structural abnormalities may precede the development of albuminuria and elevated plasma creatinine in the setting of nondiabetic injury, such as that because of Cre-mediated genotoxicity, representing a generalized response of the injured podocyte to stress (51, 72).
To determine if altered laminin expression accounted for the widened GBM seen in NPHS2-Cre transgenic mice, comparative mRNA expression studies of the laminin chains that make up the mature isoform LAM-521 were performed in glomeruli from Cre transgenic mice compared with wild-type control mice. Remarkably, in NPHS2-Cre transgenic mice, laminin α5- and β2-subunits were downregulated, whereas the laminin γ1-subunit was upregulated. Western blot analysis of protein isolated from glomeruli of NPHS2-Cre transgenic mice confirmed the downregulation of the laminin β2-subunit and upregulation of the laminin γ1-subunit. As the laminin γ1-subunit is common to most laminins (68), making up isoforms present in the GBM during glomerulogenesis (LAM-111 and LAM-511) as well as upon maturation (LAM-521) (83), laminin γ1-subunit upregulation in NPHS2-Cre transgenic mouse glomeruli, in the face of downregulation of the other components of mature LAM-521, may represent neosynthesis of laminin (41). We speculate that in NPHS2-Cre transgenic mice, podocytes, injured by Cre-induced cytotoxicity, may reverse or alter their normal laminin isoform pattern. Exactly how laminin isoform substitutions are regulated at either the gene or protein level remains incompletely understood (3).
As increased GBM thickness was a prominent feature in NPHS2-Cre mice, extracellular matrix remodeling genes were considered particularly biologically important in the assessment of possible Cre-mediated toxicity. Of the 230 genes significant and differentially regulated in RNA sequencing analysis of glomeruli isolated from NPHS2-Cre mice compared with wild-type control mice, 15 matrix-related genes with a fold change of ≥ ±3 cutoff were identified, providing evidence that GBM thickening in NPHS2-Cre mice may be a manifestation of Cre-mediated podocyte injury.
The degree of Cre-mediated toxicity may certainly depend on cumulative dose and exposure time. However, it is not entirely clear how Cre-mediated toxicity occurs. The promiscuity of Cre recombinase is a likely culprit, and recombination may occur at off-target cryptic or “pseudo” loxP sites, with Cre able to catalyze recombination at sites exhibiting up to 10 mismatches to the canonical loxP site (90). In fact, in bacterial assays, sequences from the human and mouse genome that are remarkably different from loxP can support Cre-mediated recombination at up to 100% of the efficiency of native loxP sites (90). When Semprini et al. (79) applied a bioinformatics tool to search the mouse genome for cryptic loxP sites, they found that these sites occur often and are homogenously distributed in the genome, with an overall frequency estimated to be 1.2 per megabase.
Cre recombinase has been associated with increased frequency of chromosomal rearrangements and signs of activation of DNA damage response pathways (34, 47, 77, 81). It is thought that Cre causes DNA damage at the cryptic loxP sites during cleavage, when Cre becomes attached to the DNA through a 3′-phosphate in a protein-DNA linkage reminiscent of that seen with DNA topoisomerases (79). In the presence of cryptic loxP sites, Cre recombinase tries to carry out recombination; however, the reaction is unsuccessful. If the reaction proceeds to the point where DNA nicks are generated, then the cell is left with a damaged DNA molecule covalently linked to a protein, a combination difficult to repair by the cell repair machinery (79). Janbandhu et al. (37) observed that Cre expression in cultured cells evokes a persistent DNA damage response. Indeed, unrelated to the targeting of any specific gene, Cre activity alone may induce cell death when Cre recombinase targets these cryptic loxP sites. In addition to cryptic loxP sites, Cre-mediated toxicity may also occur when endogenous genes are disrupted by a randomly integrated transgene, potentially generating off-target and unanticipated effects (Table 3).
Table 3.
Growing evidence challenges widely held assumptions regarding the Cre-loxP system
| Assumption | Challenged by Evidence |
|---|---|
| Assumption 1: Cre expression has minimal, if any, effects on the mammalian cell. | Cytotoxicity because of nonspecific endonuclease activity, absent from catalytically inactive Cre mutants (9, 37, 47, 66, 77, 81) |
| Growth arrest and/or reduced proliferation (22, 47, 66, 81) | |
| Increased apoptosis (60, 66) | |
| Chromosomal abnormalities (22, 47, 81) | |
| Downstream transcription can be blocked when Cre binds to loxP sites in the absence of recombination (36) | |
| Cre-mediated toxicity may become evident only after introducing a targeted mutation (e.g., Cre-mediated inactivation of an anti-apoptotic or DNA repair gene may lead to apoptosis because the mutant cells can no longer cope with Cre-mediated toxicity) (76) | |
| Assumption 2: Cre-loxP system has high fidelity. | Cre transgenes may alter gene expression at or near the site of transgene integration (76) |
| Pronuclear microinjection does not control for site of Cre recombinase integration into the genome (16) | |
| Unexpected expression profile of chosen promoter to drive recombination (53, 73) | |
| Assumption 3: Canonical recognition sites are not present in the mammalian genome. | Many pseudo-loxP sites have been identified in mammalian genome (90) |
| May catalyze illegitimate recombination (77) | |
| Assumption 4: There is higher recombination efficiency as compared with other strategies. | LoxP target genes can differ in their sensitivity to Cre-mediated recombination (76) |
| Inactivation of the gene may not coincide with the ablation of its product (i.e., RNA or protein) (76) | |
| Nonuniformity in Cre activity can lead to a mosaic pattern of recombination (75) and inefficient deletion of the gene by Cre (14, 28, 76, 80) | |
| Background strain-specific differences in efficiency of gene deletion (30) | |
| Diet and vivarium conditions may play a role in the Cre-associated phenotype (69) |
In our study, the expression of the podocyte marker WT1 was decreased in NPHS2-Cre transgenic mice compared with wild-type control mice, suggesting podocyte injury and loss. Additionally, of the 230 significantly differentially expressed genes in NPHS2-Cre mice compared with wild-type control mice, 17 apoptosis- and cell death-related genes with a fold change of ≥ ±3 cutoff were identified. Furthermore, the balance between anti- and proapoptotic/cell death regulators was shifted toward a more proapoptotic/cell death phenotype. These data support that persistent NPHS2-Cre expression may be targeting endogenous, noncanonical loxP sites, activating DNA damage response pathways and leading to cell death and reduced expression of the podocyte marker WT1. Although our study did not show a significant dose-dependent difference in GBM thickness between NPHS2-Cre+/− and NPHS2-Cre+/+ mice, others have reported that an optimal effective dose of Cre that allows true loxP site-specific recombination, without cryptic loxP-linked toxicity, can be titrated (9, 47). In our model, even NPHS2-Cre+/− mice achieved a Cre expression threshold that resulted in cytotoxicity and pathological remodeling of the GBM.
The use of the Cre-loxP system remains exceedingly important in our quest to understand normal physiology and disease. However, there should be increased awareness of the limitations of the system to include appropriate controls and to avoid compromising the validity of experimental results. Several assumptions have persisted, including 1) that the Cre-loxP system has high fidelity, 2) that canonical recognition sites are not present in the mammalian genome, and 3) that there is higher recombination efficiency when compared with other strategies (71). Yet, an ever-expanding body of literature now challenges these assumptions (Table 3) (4, 71). To avoid the complication of wrongly attributing a particular phenotype (that may be partly or wholly because of Cre-mediated toxicity) to the targeted gene mutation, strategies that may be considered during the design of experiments to minimize the potential for confounding effects of Cre recombinase are presented in Table 4.
Table 4.
Strategies to minimize the potential for confounding effects of Cre recombinase
| 1. Limit exposure time to Cre recombinase by using ligand-activated Cre transgenic lines (9, 15, 31, 32, 34). |
|---|
| 2. Self-deleting Cre expression vectors to combine gene ablation with Cre elimination (48, 66, 81). |
| 3. Screen for gene recombination rather than for the presence or absence of Cre (82). |
| 4. Include appropriate controls: Cre transgene alone, floxed control, and wild-type control (all on same strain background as the transgenic lines) (30). |
| 5. Examination of the Cre recombinase expression profile using appropriate reporter strain(s) (30). |
| 6. Use of viruses to target Cre expression to specific locations. In the brain, adeno-associated viruses are highly efficient for gene delivery (21), show minimal toxicity or immunogenic reaction (6, 12), and usually delete the relevant gene within 3–7 days postinjection (6, 30, 39). |
Under certain conditions, Cre can be a DNA-damaging agent and, therefore, toxic to mammalian cells (76). We conclude that persistent expression of Cre recombinase in podocytes may lead to Cre-mediated toxicity, cell-specific injury, and pathological remodeling of the GBM. At a time when the National Institutes of Health is increasingly highlighting the importance of rigor and reproducibility in scientific research, the potential for Cre-mediated toxicity underscores the importance of including Cre-expressing control mice to discriminate the effects of Cre clearly from those of the targeted mutation. When examined closely, it is possible that some seemingly healthy Cre transgenic mice may exhibit subtle Cre-dependent toxic phenotypes.
GRANTS
This work was supported by United States Department of Veterans Affairs, Biomedical Laboratory Research and Development Program, Career Development Award No. 2-5IK2BX001942 (to C. B. Marshall). The authors acknowledge support from the University of Alabama at Birmingham (UAB)-University of California-San Diego O’Brien Core Center for Acute Kidney Injury Research [National Institutes of Health (NIH) Grant P30-DK-079337], the UAB Comprehensive Flow Cytometry Core Facility (NIH Grants P30-AR-048311 and P30-AI-27667), and the UAB High Resolution Imaging Facility.
DISCLAIMERS
The contents do not represent the views of the United States Department of Veterans Affairs or the United States Government.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.B.M. conceived and designed research; R.S.B., C.C., M.R.C., and C.B.M. performed experiments; R.S.B., C.C., M.R.C., D.K.C., W.L.C., J.W.V., and C.B.M. analyzed data; R.S.B., D.K.C., W.L.C., J.W.V., and C.B.M. interpreted results of experiments; R.S.B., C.C., J.W.V., and C.B.M. prepared figures; C.B.M. drafted manuscript; R.S.B., M.R.C., D.K.C., W.L.C., J.W.V., and C.B.M. edited and revised manuscript; R.S.B., C.C., M.R.C., D.K.C., W.L.C., J.W.V., and C.B.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Drs. Paul W. Sanders and Anupam Agarwal for their helpful suggestions during the preparation of the manuscript; Dr. Sumant S. Chugh for sharing primer and probe sequences for genomic prolactin and Wilms tumor 1 and for helpful advice during design of real-time PCR studies; Dr. Camille Macé for technical assistance during optimization of glomerular isolation and Western blotting protocols; and Dr. James F. George for technical assistance during optimization of protocol for isolation and purification of ex vivo podocytes. They also apologize to those whose work was not included because of space limits.
REFERENCES
- 1.Abrahamson DR. Role of the podocyte (and glomerular endothelium) in building the GBM. Semin Nephrol 32: 342–349, 2012. doi: 10.1016/j.semnephrol.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrahamson DR, Hudson BG, Stroganova L, Borza DB, St John PL. Cellular origins of type IV collagen networks in developing glomeruli. J Am Soc Nephrol 20: 1471–1479, 2009. doi: 10.1681/ASN.2008101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abrahamson DR, Prettyman AC, Robert B, St John PL. Laminin-1 reexpression in Alport mouse glomerular basement membranes. Kidney Int 63: 826–834, 2003. doi: 10.1046/j.1523-1755.2003.00800.x. [DOI] [PubMed] [Google Scholar]
- 4.Adams DJ, van der Weyden L. Are we creating problems? Negative effects of Cre recombinase. Genesis 29: 115, 2001. doi: 10.1002/gene.1012. [DOI] [PubMed] [Google Scholar]
- 5.Agah R, Frenkel PA, French BA, Michael LH, Overbeek PA, Schneider MD. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest 100: 169–179, 1997. doi: 10.1172/JCI119509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed BY, Chakravarthy S, Eggers R, Hermens WT, Zhang JY, Niclou SP, Levelt C, Sablitzky F, Anderson PN, Lieberman AR, Verhaagen J. Efficient delivery of Cre-recombinase to neurons in vivo and stable transduction of neurons using adeno-associated and lentiviral vectors. BMC Neurosci 5: 4, 2004. doi: 10.1186/1471-2202-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Añazco C, López-Jiménez AJ, Rafi M, Vega-Montoto L, Zhang MZ, Hudson BG, Vanacore RM. Lysyl oxidase-like-2 cross-links collagen IV of glomerular basement membrane. J Biol Chem 291: 25999–26012, 2016. doi: 10.1074/jbc.M116.738856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arend LJ, Smart AM, Briggs JP. Mouse beta(6) integrin sequence, pattern of expression, and role in kidney development. J Am Soc Nephrol 11: 2297–2305, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Baba Y, Nakano M, Yamada Y, Saito I, Kanegae Y. Practical range of effective dose for Cre recombinase-expressing recombinant adenovirus without cell toxicity in mammalian cells. Microbiol Immunol 49: 559–570, 2005. doi: 10.1111/j.1348-0421.2005.tb03753.x. [DOI] [PubMed] [Google Scholar]
- 10.Bariety J, Mandet C, Hill GS, Bruneval P. Parietal podocytes in normal human glomeruli. J Am Soc Nephrol 17: 2770–2780, 2006. doi: 10.1681/ASN.2006040325. [DOI] [PubMed] [Google Scholar]
- 11.Bersell K, Choudhury S, Mollova M, Polizzotti BD, Ganapathy B, Walsh S, Wadugu B, Arab S, Kühn B. Moderate and high amounts of tamoxifen in αMHC-MerCreMer mice induce a DNA damage response, leading to heart failure and death. Dis Model Mech 6: 1459–1469, 2013. doi: 10.1242/dmm.010447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bessis N, GarciaCozar FJ, Boissier MC. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther 11, Suppl 1: S10–S17, 2004. doi: 10.1038/sj.gt.3302364. [DOI] [PubMed] [Google Scholar]
- 13.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- 14.Branda CS, Dymecki SM. Talking about a revolution: The impact of site-specific recombinases on genetic analyses in mice. Dev Cell 6: 7–28, 2004. doi: 10.1016/S1534-5807(03)00399-X. [DOI] [PubMed] [Google Scholar]
- 15.Bugeon L, Danou A, Carpentier D, Langridge P, Syed N, Dallman MJ. Inducible gene silencing in podocytes: a new tool for studying glomerular function. J Am Soc Nephrol 14: 786–791, 2003. doi: 10.1097/01.ASN.0000050222.86847.EA. [DOI] [PubMed] [Google Scholar]
- 16.Cartwright EJ. Transgenesis Techniques: Principles and Protocols. New York: Humana, 2009, p. xii. [Google Scholar]
- 17.Chan EL, Peace BE, Toney K, Kader SA, Pathrose P, Collins MH, Waltz SE. Homozygous K5Cre transgenic mice have wavy hair and accelerated malignant progression in a murine model of skin carcinogenesis. Mol Carcinog 46: 49–59, 2007. doi: 10.1002/mc.20192. [DOI] [PubMed] [Google Scholar]
- 18.Chevalier G, Yeger H, Martinerie C, Laurent M, Alami J, Schofield PN, Perbal B. novH: differential expression in developing kidney and Wilm’s tumors. Am J Pathol 152: 1563–1575, 1998. [PMC free article] [PubMed] [Google Scholar]
- 19.Chugh S, Yuan H, Topham PS, Haydar SA, Mittal V, Taylor GA, Kalluri R, Salant DJ. Aminopeptidase A: a nephritogenic target antigen of nephrotoxic serum. Kidney Int 59: 601–613, 2001. doi: 10.1046/j.1523-1755.2001.059002601.x. [DOI] [PubMed] [Google Scholar]
- 20.Coppoolse ER, de Vroomen MJ, Roelofs D, Smit J, van Gennip F, Hersmus BJ, Nijkamp HJ, van Haaren MJ. Cre recombinase expression can result in phenotypic aberrations in plants. Plant Mol Biol 51: 263–279, 2003. doi: 10.1023/A:1021174726070. [DOI] [PubMed] [Google Scholar]
- 21.Dayton RD, Wang DB, Klein RL. The advent of AAV9 expands applications for brain and spinal cord gene delivery. Expert Opin Biol Ther 12: 757–766, 2012. doi: 10.1517/14712598.2012.681463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Alboran IM, O’Hagan RC, Gärtner F, Malynn B, Davidson L, Rickert R, Rajewsky K, DePinho RA, Alt FW. Analysis of C-MYC function in normal cells via conditional gene-targeted mutation. Immunity 14: 45–55, 2001. doi: 10.1016/S1074-7613(01)00088-7. [DOI] [PubMed] [Google Scholar]
- 23.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21, 2013. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356: 215–221, 1992. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 25.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30: 207–210, 2002. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forni PE, Scuoppo C, Imayoshi I, Taulli R, Dastrù W, Sala V, Betz UA, Muzzi P, Martinuzzi D, Vercelli AE, Kageyama R, Ponzetto C. High levels of Cre expression in neuronal progenitors cause defects in brain development leading to microencephaly and hydrocephaly. J Neurosci 26: 9593–9602, 2006. doi: 10.1523/JNEUROSCI.2815-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gangoda L, Doerflinger M, Lee YY, Rahimi A, Etemadi N, Chau D, Milla L, O’Connor L, Puthalakath H. Cre transgene results in global attenuation of the cAMP/PKA pathway. Cell Death Dis 3: e365, 2012. doi: 10.1038/cddis.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gannon M, Herrera PL, Wright CV. Mosaic Cre-mediated recombination in pancreas using the pdx-1 enhancer/promoter. Genesis 26: 143–144, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 29.Hall ME, Smith G, Hall JE, Stec DE. Systolic dysfunction in cardiac-specific ligand-inducible MerCreMer transgenic mice. Am J Physiol Heart Circ Physiol 301: H253–H260, 2011. doi: 10.1152/ajpheart.00786.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harno E, Cottrell EC, White A. Metabolic pitfalls of CNS Cre-based technology. Cell Metab 18: 21–28, 2013. doi: 10.1016/j.cmet.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Heidmann D, Lehner CF. Reduction of Cre recombinase toxicity in proliferating Drosophila cells by estrogen-dependent activity regulation. Dev Genes Evol 211: 458–465, 2001. doi: 10.1007/s004270100167. [DOI] [PubMed] [Google Scholar]
- 32.Higashi AY, Ikawa T, Muramatsu M, Economides AN, Niwa A, Okuda T, Murphy AJ, Rojas J, Heike T, Nakahata T, Kawamoto H, Kita T, Yanagita M. Direct hematological toxicity and illegitimate chromosomal recombination caused by the systemic activation of CreERT2. J Immunol 182: 5633–5640, 2009. doi: 10.4049/jimmunol.0802413. [DOI] [PubMed] [Google Scholar]
- 33.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 34.Huh WJ, Mysorekar IU, Mills JC. Inducible activation of Cre recombinase in adult mice causes gastric epithelial atrophy, metaplasia, and regenerative changes in the absence of “floxed” alleles. Am J Physiol Gastrointest Liver Physiol 299: G368–G380, 2010. doi: 10.1152/ajpgi.00021.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hull TD, Bolisetty S, DeAlmeida AC, Litovsky SH, Prabhu SD, Agarwal A, George JF. Heme oxygenase-1 expression protects the heart from acute injury caused by inducible Cre recombinase. Lab Invest 93: 868–879, 2013. doi: 10.1038/labinvest.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iovino N, Denti MA, Bozzoni I, Cortese R. A loxP-containing pol II promoter for RNA interference is reversibly regulated by Cre recombinase. RNA Biol 2: 86–92, 2005. doi: 10.4161/rna.2.3.2045. [DOI] [PubMed] [Google Scholar]
- 37.Janbandhu VC, Moik D, Fässler R. Cre recombinase induces DNA damage and tetraploidy in the absence of loxP sites. Cell Cycle 13: 462–470, 2014. doi: 10.4161/cc.27271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeannotte L, Aubin J, Bourque S, Lemieux M, Montaron S, Provencher St-Pierre A. Unsuspected effects of a lung-specific Cre deleter mouse line. Genesis 49: 152–159, 2011. doi: 10.1002/dvg.20720. [DOI] [PubMed] [Google Scholar]
- 39.Kaspar BK, Vissel B, Bengoechea T, Crone S, Randolph-Moore L, Muller R, Brandon EP, Schaffer D, Verma IM, Lee KF, Heinemann SF, Gage FH. Adeno-associated virus effectively mediates conditional gene modification in the brain. Proc Natl Acad Sci USA 99: 2320–2325, 2002. doi: 10.1073/pnas.042678699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koitabashi N, Bedja D, Zaiman AL, Pinto YM, Zhang M, Gabrielson KL, Takimoto E, Kass DA. Avoidance of transient cardiomyopathy in cardiomyocyte-targeted tamoxifen-induced MerCreMer gene deletion models. Circ Res 105: 12–15, 2009. doi: 10.1161/CIRCRESAHA.109.198416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kootstra CJ, Bergijk EC, Veninga A, Prins FA, de Heer E, Abrahamson DR, Bruijn JA. Qualitative alterations in laminin expression in experimental lupus nephritis. Am J Pathol 147: 476–488, 1995. [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JY, Ristow M, Lin X, White MF, Magnuson MA, Hennighausen L. RIP-Cre revisited, evidence for impairments of pancreatic beta-cell function. J Biol Chem 281: 2649–2653, 2006. doi: 10.1074/jbc.M512373200. [DOI] [PubMed] [Google Scholar]
- 43.Lexow J, Poggioli T, Sarathchandra P, Santini MP, Rosenthal N. Cardiac fibrosis in mice expressing an inducible myocardial-specific Cre driver. Dis Model Mech 6: 1470–1476, 2013. doi: 10.1242/dmm.010470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y, Choi PS, Casey SC, Felsher DW. Activation of Cre recombinase alone can induce complete tumor regression. PLoS One 9: e107589, 2014. doi: 10.1371/journal.pone.0107589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lightfield KL, Persson J, Brubaker SW, Witte CE, von Moltke J, Dunipace EA, Henry T, Sun YH, Cado D, Dietrich WF, Monack DM, Tsolis RM, Vance RE. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol 9: 1171–1178, 2008. doi: 10.1038/ni.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu G, Clement LC, Kanwar YS, Avila-Casado C, Chugh SS. ZHX proteins regulate podocyte gene expression during the development of nephrotic syndrome. J Biol Chem 281: 39681–39692, 2006. doi: 10.1074/jbc.M606664200. [DOI] [PubMed] [Google Scholar]
- 47.Loonstra A, Vooijs M, Beverloo HB, Allak BA, van Drunen E, Kanaar R, Berns A, Jonkers J. Growth inhibition and DNA damage induced by Cre recombinase in mammalian cells. Proc Natl Acad Sci USA 98: 9209–9214, 2001. doi: 10.1073/pnas.161269798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mähönen AJ, Airenne KJ, Lind MM, Lesch HP, Ylä-Herttuala S. Optimized self-excising Cre-expression cassette for mammalian cells. Biochem Biophys Res Commun 320: 366–371, 2004. doi: 10.1016/j.bbrc.2004.05.175. [DOI] [PubMed] [Google Scholar]
- 49.Marchal PO, Kavvadas P, Abed A, Kazazian C, Authier F, Koseki H, Hiraoka S, Boffa JJ, Martinerie C, Chadjichristos CE. Reduced NOV/CCN3 Expression Limits Inflammation and Interstitial Renal Fibrosis after Obstructive Nephropathy in Mice. PLoS One 10: e0137876, 2015. doi: 10.1371/journal.pone.0137876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marquez B, Zouvani I, Karagrigoriou A, Anastasiades E, Pierides A, Kyriacou K. A simplified method for measuring the thickness of glomerular basement membranes. Ultrastruct Pathol 27: 409–416, 2003. doi: 10.1080/01913120390248728. [DOI] [PubMed] [Google Scholar]
- 51.Marshall CB. Rethinking glomerular basement membrane thickening in diabetic nephropathy: adaptive or pathogenic? Am J Physiol Renal Physiol 311: F831–F843, 2016. doi: 10.1152/ajprenal.00313.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marshall CB, Krofft RD, Pippin JW, Shankland SJ. CDK inhibitor p21 is prosurvival in adriamycin-induced podocyte injury, in vitro and in vivo. Am J Physiol Renal Physiol 298: F1140–F1151, 2010. doi: 10.1152/ajprenal.00216.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matthaei KI. Genetically manipulated mice: a powerful tool with unsuspected caveats. J Physiol 582: 481–488, 2007. doi: 10.1113/jphysiol.2007.134908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mi H, Huang X, Muruganujan A, Tang H, Mills C, Kang D, Thomas PD. PANTHER version 11: expanded annotation data from Gene Ontology and Reactome pathways, and data analysis tool enhancements. Nucleic Acids Res 45, D1: D183–D189, 2017. doi: 10.1093/nar/gkw1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mimura I, Nangaku M, Nishi H, Inagi R, Tanaka T, Fujita T. Cytoglobin, a novel globin, plays an antifibrotic role in the kidney. Am J Physiol Renal Physiol 299: F1120–F1133, 2010. doi: 10.1152/ajprenal.00145.2010. [DOI] [PubMed] [Google Scholar]
- 56.Miner JH. Developmental biology of glomerular basement membrane components. Curr Opin Nephrol Hypertens 7: 13–19, 1998. doi: 10.1097/00041552-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB. Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 35: 39–42, 2003. doi: 10.1002/gene.10164. [DOI] [PubMed] [Google Scholar]
- 58.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB. Two gene fragments that direct podocyte-specific expression in transgenic mice. J Am Soc Nephrol 13: 1561–1567, 2002. doi: 10.1097/01.ASN.0000015614.68893.0B. [DOI] [PubMed] [Google Scholar]
- 59.Murakami A, Oshiro H, Kanzaki S, Yamaguchi A, Yamanaka S, Furuya M, Miura S, Kanno H, Nagashima Y, Aoki I, Nagahama K. A novel method for isolating podocytes using magnetic activated cell sorting. Nephrol Dial Transplant 25: 3884–3890, 2010. doi: 10.1093/ndt/gfq323. [DOI] [PubMed] [Google Scholar]
- 60.Naiche LA, Papaioannou VE. Cre activity causes widespread apoptosis and lethal anemia during embryonic development. Genesis 45: 768–775, 2007. doi: 10.1002/dvg.20353. [DOI] [PubMed] [Google Scholar]
- 61.Najafian B, Mauer M. Morphologic features of declining renal function in type 1 diabetes. Semin Nephrol 32: 415–422, 2012. doi: 10.1016/j.semnephrol.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nelson RK, Gould KA. An Lck-cre transgene accelerates autoantibody production and lupus development in (NZB × NZW)F1 mice. Lupus 25: 137–154, 2016. doi: 10.1177/0961203315603139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Osawa G, Kimmelstiel P, Seiling V. Thickness of glomerular basement membranes. Am J Clin Pathol 45: 7–20, 1966. doi: 10.1093/ajcp/45.1.7. [DOI] [PubMed] [Google Scholar]
- 64.Osterby R, Gundersen HJ. Glomerular size and structure in diabetes mellitus. I. Early abnormalities. Diabetologia 11: 225–229, 1975. doi: 10.1007/BF00422326. [DOI] [PubMed] [Google Scholar]
- 65.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99: 342–348, 1997. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pfeifer A, Brandon EP, Kootstra N, Gage FH, Verma IM. Delivery of the Cre recombinase by a self-deleting lentiviral vector: efficient gene targeting in vivo. Proc Natl Acad Sci USA 98: 11450–11455, 2001. doi: 10.1073/pnas.201415498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, Shelton KD, Lindner J, Cherrington AD, Magnuson MA. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem 274: 305–315, 1999. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- 68.Pozzi A, Yurchenco PD, Iozzo RV. The nature and biology of basement membranes. Matrix Biol 57-58: 1–11, 2017. doi: 10.1016/j.matbio.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pugach EK, Richmond PA, Azofeifa JG, Dowell RD, Leinwand LA. Prolonged Cre expression driven by the α-myosin heavy chain promoter can be cardiotoxic. J Mol Cell Cardiol 86: 54–61, 2015. doi: 10.1016/j.yjmcc.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qiu L, Rivera-Pérez JA, Xu Z. A non-specific effect associated with conditional transgene expression based on Cre-loxP strategy in mice. PLoS One 6: e18778, 2011. doi: 10.1371/journal.pone.0018778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rajewsky K, Gu H, Kühn R, Betz UA, Müller W, Roes J, Schwenk F. Conditional gene targeting. J Clin Invest 98: 600–603, 1996. doi: 10.1172/JCI118828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Randles MJ, Collinson S, Starborg T, Mironov A, Krendel M, Königshausen E, Sellin L, Roberts IS, Kadler KE, Miner JH, Lennon R. Three-dimensional electron microscopy reveals the evolution of glomerular barrier injury. Sci Rep 6: 35068, 2016. doi: 10.1038/srep35068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rempe D, Vangeison G, Hamilton J, Li Y, Jepson M, Federoff HJ. Synapsin I Cre transgene expression in male mice produces germline recombination in progeny. Genesis 44: 44–49, 2006. doi: 10.1002/gene.20183. [DOI] [PubMed] [Google Scholar]
- 74.Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, Zediak VP, Velez M, Bhandoola A, Brown EJ. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell 1: 113–126, 2007. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ryding AD, Sharp MG, Mullins JJ. Conditional transgenic technologies. J Endocrinol 171: 1–14, 2001. doi: 10.1677/joe.0.1710001. [DOI] [PubMed] [Google Scholar]
- 76.Schmidt-Supprian M, Rajewsky K. Vagaries of conditional gene targeting. Nat Immunol 8: 665–668, 2007. doi: 10.1038/ni0707-665. [DOI] [PubMed] [Google Scholar]
- 77.Schmidt EE, Taylor DS, Prigge JR, Barnett S, Capecchi MR. Illegitimate Cre-dependent chromosome rearrangements in transgenic mouse spermatids. Proc Natl Acad Sci USA 97: 13702–13707, 2000. doi: 10.1073/pnas.240471297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Semprini S, Troup TJ, Kotelevtseva N, King K, Davis JR, Mullins LJ, Chapman KE, Dunbar DR, Mullins JJ. Cryptic loxP sites in mammalian genomes: genome-wide distribution and relevance for the efficiency of BAC/PAC recombineering techniques. Nucleic Acids Res 35: 1402–1410, 2007. doi: 10.1093/nar/gkl1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shao X, Somlo S, Igarashi P. Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. J Am Soc Nephrol 13: 1837–1846, 2002. doi: 10.1097/01.ASN.0000016444.90348.50. [DOI] [PubMed] [Google Scholar]
- 81.Silver DP, Livingston DM. Self-excising retroviral vectors encoding the Cre recombinase overcome Cre-mediated cellular toxicity. Mol Cell 8: 233–243, 2001. doi: 10.1016/S1097-2765(01)00295-7. [DOI] [PubMed] [Google Scholar]
- 82.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21: 70–71, 1999. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 83.St John PL, Abrahamson DR. Glomerular endothelial cells and podocytes jointly synthesize laminin-1 and -11 chains. Kidney Int 60: 1037–1046, 2001. doi: 10.1046/j.1523-1755.2001.0600031037.x. [DOI] [PubMed] [Google Scholar]
- 84.Sternberg N, Hamilton D. Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. J Mol Biol 150: 467–486, 1981. doi: 10.1016/0022-2836(81)90375-2. [DOI] [PubMed] [Google Scholar]
- 85.Sternberg N, Hamilton D, Hoess R. Bacteriophage P1 site-specific recombination. II. Recombination between loxP and the bacterial chromosome. J Mol Biol 150: 487–507, 1981. doi: 10.1016/0022-2836(81)90376-4. [DOI] [PubMed] [Google Scholar]
- 86.Subramaniam A, Jones WK, Gulick J, Wert S, Neumann J, Robbins J. Tissue-specific regulation of the alpha-myosin heavy chain gene promoter in transgenic mice. J Biol Chem 266: 24613–24620, 1991. [PubMed] [Google Scholar]
- 87.Takahashi N, Boysen G, Li F, Li Y, Swenberg JA. Tandem mass spectrometry measurements of creatinine in mouse plasma and urine for determining glomerular filtration rate. Kidney Int 71: 266–271, 2007. doi: 10.1038/sj.ki.5002033. [DOI] [PubMed] [Google Scholar]
- 88.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002. doi: 10.1016/S0002-9440(10)64239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thanos A, Morizane Y, Murakami Y, Giani A, Mantopoulos D, Kayama M, Roh MI, Michaud N, Pawlyk B, Sandberg M, Young LH, Miller JW, Vavvas DG. Evidence for baseline retinal pigment epithelium pathology in the Trp1-Cre mouse. Am J Pathol 180: 1917–1927, 2012. doi: 10.1016/j.ajpath.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thyagarajan B, Guimarães MJ, Groth AC, Calos MP. Mammalian genomes contain active recombinase recognition sites. Gene 244: 47–54, 2000. doi: 10.1016/S0378-1119(00)00008-1. [DOI] [PubMed] [Google Scholar]
- 91.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7: 562–578, 2012. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Verlander JW, Kim YH, Shin W, Pham TD, Hassell KA, Beierwaltes WH, Green ED, Everett L, Matthews SW, Wall SM. Dietary Cl(-) restriction upregulates pendrin expression within the apical plasma membrane of type B intercalated cells. Am J Physiol Renal Physiol 291: F833–F839, 2006. doi: 10.1152/ajprenal.00474.2005. [DOI] [PubMed] [Google Scholar]
- 93.Wong MA, Cui S, Quaggin SE. Identification and characterization of a glomerular-specific promoter from the human nephrin gene. Am J Physiol Renal Physiol 279: F1027–F1032, 2000. doi: 10.1152/ajprenal.2000.279.6.F1027. [DOI] [PubMed] [Google Scholar]