Biomolecular condensates: Organizers of cellular biochemistry (original) (raw)
. Author manuscript; available in PMC: 2020 Aug 18.
Published in final edited form as: Nat Rev Mol Cell Biol. 2017 Feb 22;18(5):285–298. doi: 10.1038/nrm.2017.7
Abstract
Biomolecular Condensates are micron-scale compartments in eukaryotic cells that lack surrounding membranes, but concentrate biomolecules including proteins and nucleic acids. They are involved in diverse processes, including RNA metabolism, ribosome biogenesis, the DNA damage response and signal transduction. Recent studies have shown that liquid-liquid phase separation driven by multivalent macromolecular interactions is an important organizing principle for Biomolecular Condensates. With this physical framework it is now possible to explain regulation of the assembly, composition, physical properties and biochemical and cellular functions of these important structures.
Keywords: Phase separation, nuclear bodies, membraneless-organelles, Biomolecular Condensate
eTOC
Apart from membrane-bound organelles eukaryotic cells feature various membraneless compartments, including the centrosome, the nucleolous and different granules. Many of these compartments form through liquid-liquid phase separation, and the principles, mechanisms and regulation of their assembly as well as their cellular functions are now starting to emerge.
Introduction
A fundamental problem in cell biology is how the densely packed cellular space is organized to allow control over complex biochemical reactions in space and time. One way to achieve spatiotemporal control is to regulate the localization of reaction components—concentrating components together can increase reaction kinetics, whereas segregating them apart can slow or inhibit reactions. These differences can alter flux through specific pathways and protect cells from damaging activities such as proteolysis, inappropriate covalent modifications and effects of low pH. Indeed, in vivo enzymatic reaction components are often packaged within distinct subcellular compartments.
Classical organelles, such as the endoplasmic reticulum or Golgi apparatus, are compartments defined by surrounding lipid bilayer membranes. These membranes are impermeable to most biological molecules. Thus, the interior and exterior of classical organelles are physically separated, and organelle compositions are regulated through specialized membrane transport machineries.
However, many cellular compartments are not bound by membranes (Fig. 1a). Examples include RNA–protein granules such as nucleoli, Cajal bodies [G], and PML nuclear bodies [G] in the nucleus1, as well as stress granules, and germ granules in the cytoplasm2,3. Clusters of signalling molecules at membranes, although two-dimensional by nature, can be viewed in a similar light. These micron-scale structures are all defined by their ability to concentrate proteins and nucleic acids at discrete cellular sites. As they lack a physical barrier separating their internal components from the surrounding medium, it remained elusive for many years how they concentrate molecules, maintain and regulate their structures, control their compositions and modulate internal biochemical activities.
Figure 1. Biomolecular Condensates in eukaryotic cells.
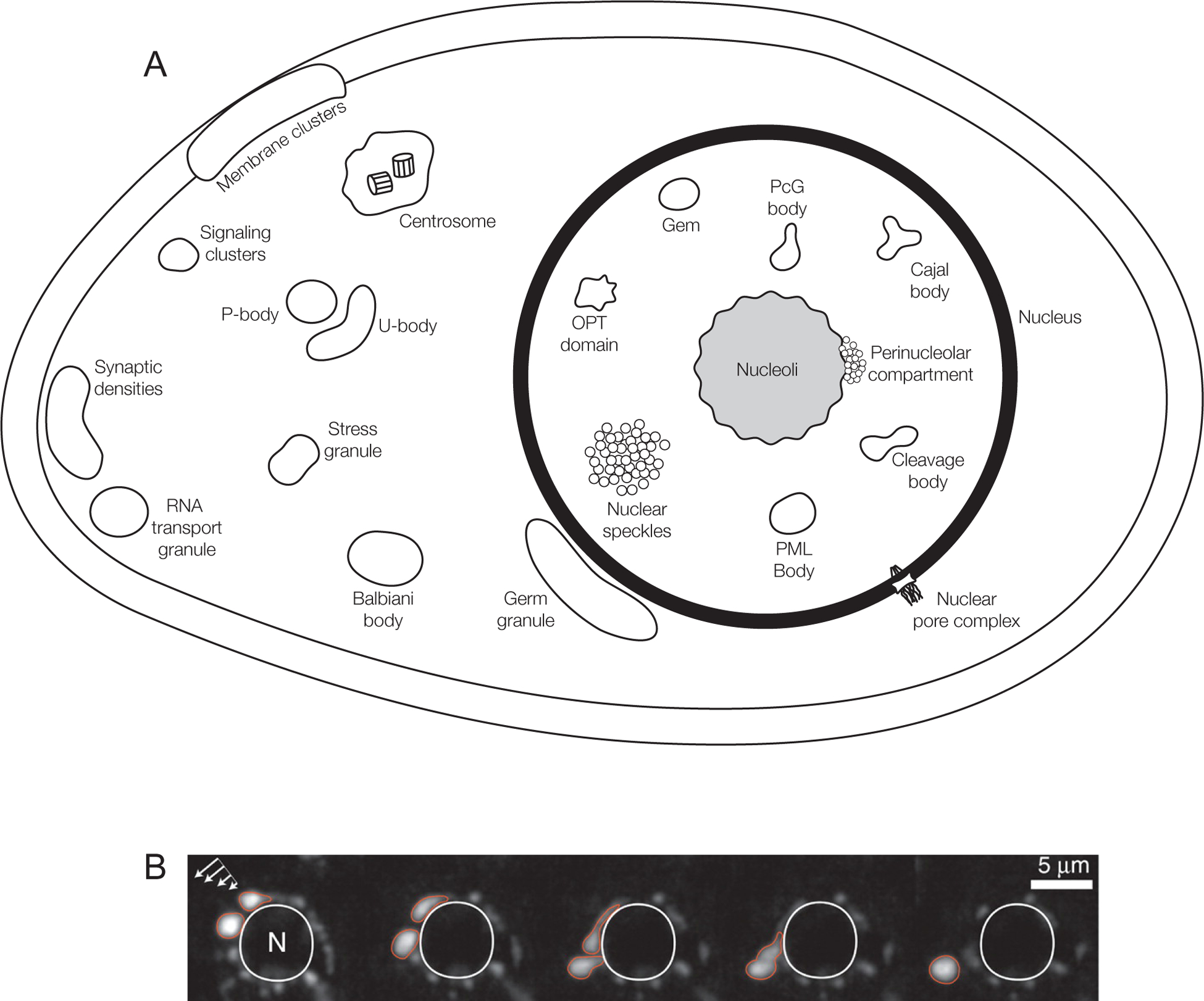
A) Schematic of the numerous Condensates in the nucleus, cytoplasm and membranes of eukaryotic cells. Some compartments only occur in specific cell types, but are shown here for completeness. For example, Balbiani bodies and germ granules are specific to germ cells (green hues), and RNA transport granules and synaptic densities are seen in neuronal cell types (pink hues). See Supplementary information S6 (Table) for more information on individual Condensates.
B) Caenorhabditis elegans germ granules, P granules, are perinuclear Condensates that behave like liquids. A montage of live time-lapse imaging of P granules under shear force (arrows, left top). P granules deform, drip, and fuse with one another around a nucleus (circular structure in the middle outlined in white) (Figure adapted with permissions from Brangwynne et al. 2009). See also Supplementary Information S1–S4 (Movies). Timepoints: 0s, 21s, 32s, 36s, 46s.
In this Review, we discuss cellular and biochemical observations that have led to a new physical model of membraneless compartments based on principles of polymer chemistry and soft matter physics. This model unites many of the observed behaviours of membraneless compartments, both membrane-associated molecular clusters and three-dimensional structures, under a common framework. We discuss how the model explains many aspects of the assembly and dissolution, composition, and function of membraneless compartments. We suggest mechanisms by which these features can be regulated in cells. Finally, we conclude with a series of major open questions in this exciting area of biology.
Phase separated liquid compartments
The first membraneless compartment was observed within the nucleus of neuronal cells in the 1830’s and was later termed the nucleolus4. Since then, many such compartments have been discovered in the nucleus, cytoplasm and on membranes of essentially all eukaryotic cells. Increasing resolution of microscopy, and description of their molecular components revealed similarities in their shape, dynamics and manner of assembly, despite differences in composition, location and function. Each type contains many molecular components. These can remain stably concentrated within the structures for hours to days, yet decades of photobleaching recovery experiments consistently showed that many of these organelles could exchange with the surrounding medium on timescales of seconds to minutes5–7. They also displayed unexpected behaviours such as two of the same type fusing upon contact8–14. (Supplementary information S1–S4 (Movies)). Until recently, it remained unclear how these properties could be explained in physical and molecular terms.
An important clue towards understanding the physical processes that drive the formation of membraneless organelles came from the discovery that P granules in Caenorhabditis elegans germ cells, are liquid-like compartments. P granules are perinuclear membraneless compartments composed of many proteins and RNAs (Fig. 1b). The relatively large size of P granules (2–4 μm diameter) compared to most other cellular bodies (200–1000 nm diameter) enabled quantitative analysis of their formation. P granules fuse with one another14 and subsequently relax back into a spherical shape (Fig. 1b, Supplementary information S1 (Movie)). Photobleaching experiments demonstrated that proteins are highly mobile within P granules, and also exchange rapidly with the surrounding cytoplasm14. Critically, under shear force, P granules can freely flow and deform around surfaces of other structures, as well as undergo fission14. Together, these observations suggested that P granules are liquids, which form through liquid-liquid demixing (phase separation) from the surrounding cytoplasm (see next section). The concept of phase separation suggested how P granules asymmetrically segregate during the first cell division of C.elegans embryos122. We note that such phase-separated structures are distinct in physical properties and functions from canonical macromolecular assemblies (such as ribosomes; for details see Supplementary information S5 (Box)). Since this study of P granules, other compartments such as nucleoli13, DNA damage repair sites15,16 and stress granules15 were also shown to exhibit liquid-like properties, highlighting the possibility that phase separation is a common mechanism by which membraneless compartments form17. As detailed below, principles of phase separation can indeed explain the formation of such structures with diverse material properties as well as complex organization, such as layers. The presence of a phase boundary explains how molecules can be concentrated in one place in a cell without a surrounding membrane, but still provide an environment suitable for cellular biochemistry, which depends on fast diffusion. Phase separation also provides a unifying principle that explains the formation of non-membrane bound compartments from diverse types of molecules.
Non-Membrane bound compartments are highly diverse in their physical properties, dimensionalities (membrane-associated or soluble), molecular compositions, subcellular locations and functions. Throughout the years they have been referred to by a variety of names, including cellular bodies, nuclear bodies, membraneless organelles, granules, speckles, aggregates, assemblages, membrane puncta, etc. Here we propose a new name_—Biomolecular Condensates—_which emphasizes the one feature common to all of the structures, namely their ability to concentrate molecules, independent of all other characteristics, and their composition by biomolecules. We apply this name to both membrane-associated structures and various non-membrane bound organelles and granules, as we believe that these are formed through similar mechanisms. The term also provides a link to concepts in condensed matter physics18, which as we will see below are important in understanding the formation of these structures.
Multivalency-driven phase separation
Molecules will be miscible in solution until they reach their solubility limit, the threshold concentration at which they phase separate. As detailed in Box 1, this behavior can be understood from classical thermodynamics. In the cell, the existence of separate phases enables the maintenance of chemical equilibrium between compartments of different chemical properties (e.g., concentration), through rapid movement of molecules between them.
Box 1. Thermodynamics of phase separation.
To understand phase separation we first consider free energy [G] of the solution (see Figure panel a) and the chemical potential (see Figure panel b), which is its first derivative (with respect to molecular composition). These properties are dictated by the energy possessed by each molecular species within its chemical bonds, its location, and its concentration in the system. For a simple system of non-interacting solute molecules in a solvent, free energy as a function of solute concentration is unimodal and the chemical potential is monotonic (see Figure, solid red curves). A given value of chemical potential corresponds to a unique solution composition. Under these conditions, the solute molecules are, on average, distributed homogeneously in order to maximize the entropy of the system. Fluctuations that produce transient inhomogeneities in concentration (and in chemical potential) are dissipated by diffusive flux, which equalizes the differences in chemical potential across the system and minimizes free energy (for further discussion, see18).
However, when solute molecules interact, the free energy curve becomes multimodal and the chemical potential curve becomes nonmonotocic (see Figure, dashed curves and arrow). Then, some values of chemical potential correspond to two different solute concentrations, and the free energy of the system can be minimized by separating the solute molecules into two compartments of different concentrations but equal chemical potentials (see Figure, solid teal curves)24,115.
In molecular terms, all macromolecules exhibit varying degrees of weak, non-specific interactions with each other and with solvent (water, in biology). These interactions tend to be very low in affinity, short-lived, lacking stereospecificity [G] and distributed throughout the surface of the molecule. Essentially, the solubility of macromolecules—the concentration at which they phase separate—is governed by the balance between the weak interactions between macromolecules versus those between the macromolecule and water. When interactions between macromolecules are weaker than those between macromolecules and water (so-called good solvent conditions), the macromolecules remain miscible in solution at all concentrations. However, when the macromolecule-macromolecule interactions are sufficiently stronger than macromolecule-water interactions (poor solvent conditions), the macromolecule has limited solubility and gains the propensity to phase separate116. In such systems, phase separation occurs at the concentration at which the favorable energetics of macromolecule–macromolecule interactions begin to overcome the entropic tendency of the solution to remain homogeneously mixed. At this solubility limit, the molecular mixture separates into two phases: a large-volume, low concentration dilute phase, and a small-volume, high concentration condensed phase. The phase separated state in such systems has the minimum free energy (equilibrium). The chemical potential in both phases is equal, eliminating net diffusive flux between the phases, while allowing individual molecules to move between them. Thus, the concentrated compartment is maintained persistently. At equilibrium, phase separated liquid systems allow a cell to maintain concentration differences without constant input of energy. On the other hand, gradients of soluble molecules in non-phase separating systems, as seen for instance in cell polarity systems117, require constant input of energy.

Biomolecular Condensates are often enriched with multivalent molecules—that is, molecules that harbour multiple elements that make intra- or inter-molecular interactions19–23. As detailed in Box 2, this is important because classical concepts in polymer science indicate that multivalent molecules naturally assemble into large oligomers or polymers when mixed, and that this assembly will inherently decrease the solubility of the molecules due to entropy [G] -driven effects24, promoting their phase separation. The coupled assembly and phase separation of multivalent macromolecules has emerged as an important organizing principle for Condensates. This idea can be applied broadly to understand the phase separation behavior of diverse multivalent molecules, including proteins composed of multiple modular interaction domains, proteins containing disordered regions that provide multiple weakly adhesive sequence elements, and also RNA and DNA molecules, which can harbour multiple regions that bind other nucleic acid molecules and proteins. Further, as we will see below, this mechanism naturally leads to biological means of regulating phase separation, as well as the composition, physical properties and biochemical functions of Condensates (Fig. 2).
Box 2. Multivalency promotes phase separation.
In addition to the very low affinity interactions dominating solubility (see Box 1), biological macromolecules also form complexes through relatively long-lived interactions that occur with high(er) affinity and high stereospecificity, for example the binding of modular signalling domains in proteins to their cognate ligands. When such interactions occur between multivalent molecules, they enable assembly into large oligomers or polymers, resulting in the formation of complexes with varying stoichiometries118. Increasing affinity between the interacting modules or the number of these modules (referred to as the molecule’s valency) promotes the formation of larger complexes24,119.
Importantly, interactions governing solubility and those governing formation of polymeric complexes are thermodynamically coupled, so that in poor solvent conditions (which appear to apply to many macromolecules in water) the solubility of a complex decreases as its size increases116,120. This phenomenon arises because the entropic cost of confining a complex into the condensed phase is lower than the cost of confining its components individually.
This phenomenon may also be viewed as increased avidity of the weak, solubility-determining interactions as the size of the assembly grows. Thus, oligomerization and phase separation are linked for non-covalently associating multivalent molecules. By increasing the average size of complexes, oligomerization can enhance the weak, non-specific interactions between molecules thereby decreasing solubility and promoting phase separation. Because phase separation concentrates molecules into a condensed phase, it further increases the degree of binding in that phase, thereby promoting formation of larger complexes.
We note that, to our knowledge, previous conceptions of the assembly of multidomain macromolecules have focused largely on the networks created by strong, specific interactions, without consideration of the extremely weak, non-specific interactions that govern solubility, and how they would be affected by the assembly process. We argue that considering the coupling between the strong and weak interactions, and thus the ability of multivalency to promote phase separation, is essential to understanding the behaviour of multivalent biological molecules121.
Finally, in some systems, such as disordered proteins, interactions may occupy intermediate regimes on the spectrum of strong, stereospecific contacts and weak, non-specific contacts. In such cases, the distinction between interactions that govern assembly and those that govern solubility is blurred. Such systems may be considered either through the lens of simple phase separation or multivalency-driven phase separation. Nevertheless, since disordered polymers become less soluble as they grow longer or become more adhesive, in either view, the presence of multiple points of contact between molecules provides an important driving force for phase separation.
Figure 2. Different modes of multivalent interactions in synthetic and natural systems undergoing liquid-liquid phase separation.
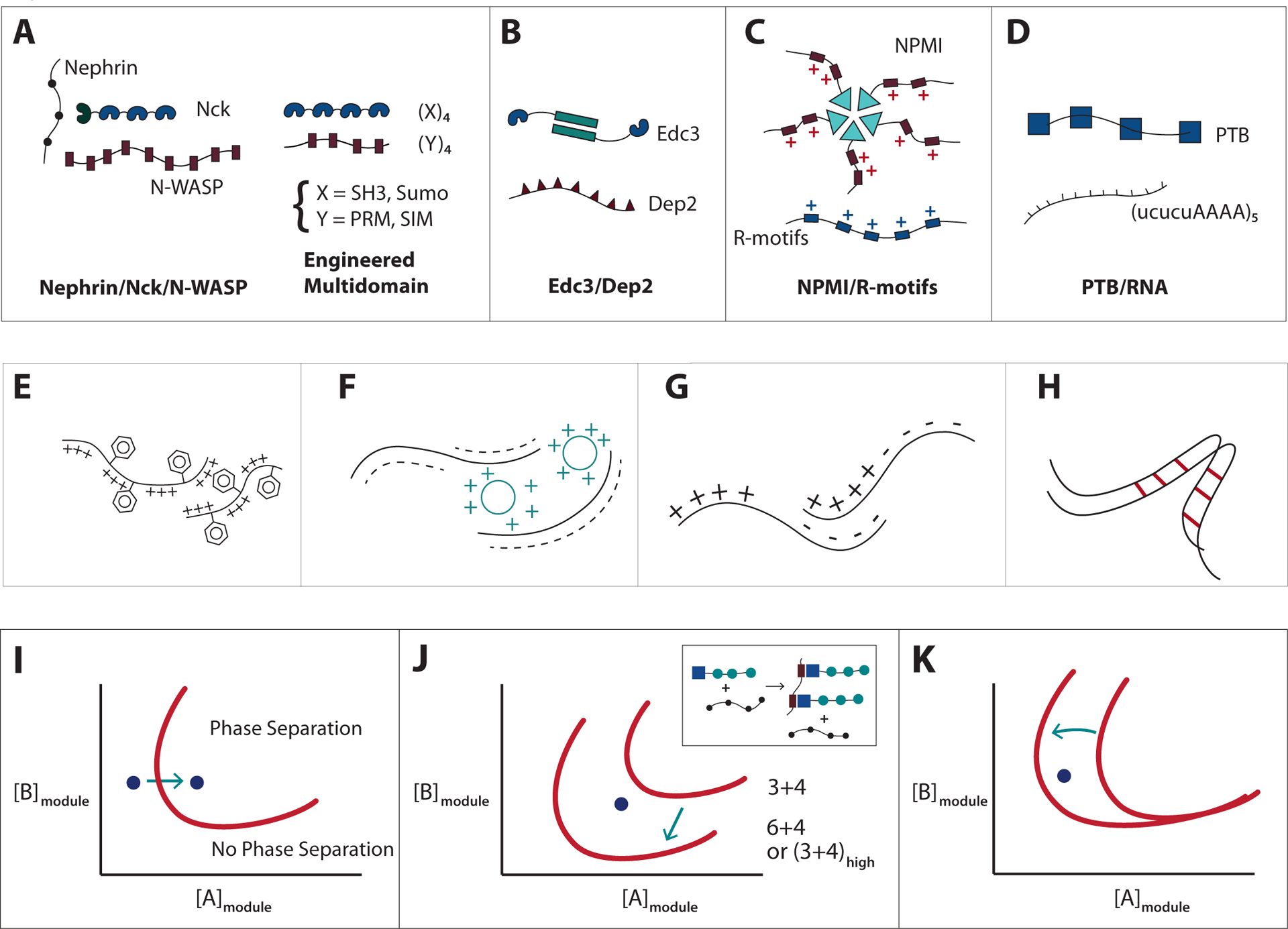
A) (Left) Nephrin contains three phospho-Tyr (pTyr) motifs (small blue circles), which interact with the SH2 domain (dark blue) on Nck. Nck also, contains three SH3 domains (blue), which bind to the numerous proline-rich motifs (PRM) (pink) in neural Wiskott-Aldrich syndrome protein (N-WASP). (Right) Engineered multivalent model systems, consisting of multiple SH3 or SUMO domains (blue), paired with multivalent ligands which contain multiple proline-rich or SUMO-interaction- motifs, PRM or SIM respectively (pink). See19 for details.
B) Edc3 dimerizes via its YJefN domain (green rectangles) and binds to the helical leucine-rich motifs (purple triangles) in Dcp2 via its LSm domain (blue). See27 for details.
C) Nucleophosmin (NPM1) assembles into pentamers via its oligomerizing domain (green triangles) and binds to proteins that contain positively charged Arg-rich linear motifs (R-motifs) (blue rectangles) via its negatively charged acidic, tracts (pink rectangles). NPM1 can also bind to potentially multivalent nucleic acids via its nucleotide binding domain (not shown). See23 for details.
D) RNA binding protein PTB interacts with UCUCU tracts in RNA (connected by AAAA linkers) via its RNA recognition motifs (blue squares). See19 for details.
E) Association of intrinsically disorder regions (IDRs) via cation-pi interactions between aromatic and basic residues, as in DDX422.
F) Patterned intermolecular electrostatic interactions between acidic and basic tracts, as in the interactions between the Nephrin intracellular domain (NICD) and positively charged partners, such as supercharged GFP (scGFP)43.
G) Patterned electrostatic interactions between acidic and basic tracts in a single molecular species, as in P granule protein Laf133.
H) Polypeptide backbone interactions between β-strands in the polypeptide, as in FUS and hnRNPA1/215,34,42,48.
I) Phase diagram as a function of the concentrations of modules present in polymerizing multivalent components that are essential for Condensate formation. Phase separation will be promoted by increasing cellular concentration of component A.
J) Regulation of Condensate formation by increase in critical concentration through increasing the valency of A and/or B or the affinity between A and B. Effective valency may be increased by the presence of a third interacting component as shown in the inset.
K) Regulation of Condensate formation by decrease in the intrinsic solubility of component A. As molecule A becomes less soluble, phase separation can occur at lower concentrations of A.
Phase separation of proteins with modular domains
There are now many examples of phase separation of natural proteins composed of modular interaction domains. The first example studied in detail was the actin-regulatory signalling pathway consisting of the multivalent proteins Nephrin, Nck and neural Wiskott-Aldrich Syndrome protein (N-WASP), which assembles into high order oligomers through the interactions between phosphotyrosines (pTyrs) in Nephrin and SH2 domains in Nck and between SH3 domains in Nck and proline-rich motifs (PRMs) in N-WASP (Fig.2a, left). This assembly produces both phase separated liquid droplets suspended in solution19 and phase separated clusters on lipid bilayers when Nephrin is attached to membranes in vitro25 or in cells (Soyeon Kim, Rosen lab, unpublished). An analogous system controlling actin organization in T cells, comprising the proteins Linker for Activation of T cells (LAT), Growth factor receptor-bound protein 2 (Grb2), Grb2-related adaptor downstream of Shc (Gads), Son of Sevenless (SOS) and Src homology 2 domain containing leukocyte protein of 76 kDa (Slp76) also forms membrane puncta in vitro and in cells in response to stimulation of the T cell receptor26. Apart from these signalling systems, also the P body components DCP2 and EDC3 (Fig. 2b), the nucleolar protein NPM1 (Fig. 2c) and the postsynaptic density proteins SynGAP and PSD95 were shown to phase separate through multivalent interactions of folded domains, in these cases with ligands harbouring disordered regions23,27,28.
Phase separation has also been explored in a variety of engineered proteins composed of repeated folded domains connected by flexible linkers. Use of such simplified model systems enables the influence of individual physical parameters to be isolated and understood in a more precise fashion than possible in more complex, naturally occurring proteins. Examples include polySH3 proteins binding to polyPRM ligands, proteins with multiple RNA-binding domains binding to repeated RNA oligonucleotides and proteins comprising multiple repeats of small ubiquitin-related modifier (SUMO) domains (polySUMO proteins) binding to polySUMO-Interaction-Motif (polySIM) ligands19,29 (Fig. 2a and 2d). Experiments with these molecules have identified valency (that is, the number of interaction modules) and affinity between the interacting modules as key parameters controlling phase separation. Higher valency and affinity both promote assembly into larger structures, enabling phase separation at lower concentrations (Fig. 2j), and decrease the dynamic rearrangements of molecules within phase separated droplets19,25.
We note that when molecules are highly soluble (that is, are characterized by a high solubility limit), assembly does not necessarily lead to phase separation. For example, engineered proteins consisting of tandem repeated WW domains [G] readily polymerize when mixed with multivalent PRM-containing partners. However, this assembly remains a single, macroscopically homogeneous phase30,31. These observations illustrate the idea that molecular assembly and phase separation of multivalent systems are distinct phenomena, even if often coupled.
Phase separation of proteins with intrinsically disordered regions
Proteins containing large intrinsically disordered regions (IDRs) represent a second, abundant class of macromolecules that can phase separate under physiologic conditions32. IDRs lack persistent three-dimensional structure but often contain repeated sequence elements that provide the basis for multivalent weakly adhesive intermolecular interactions.
IDR-containing proteins are enriched in many Condensates, particularly those that also concentrate RNA, such as P bodies, stress granules, germ granules and many nuclear structures. Many such proteins can phase separate on their own in vitro under a variety of solution conditions22,33–36. The IDRs of these proteins have low sequence complexity, and are enriched in a limited number of amino acid types—primarily glycine, serine, glutamine, asparagine, phenylalanine and tyrosine. Some also are enriched in charged residues—lysine, arginine, glutamate and aspartate. The lack of sequence diversity generates multiple Gly/Ser-Phe/Tyr-Gly/Ser sequences and/or poly-Gln and poly-Asn tracts in these molecules, as well as blocks of positive or negative charge20,22,37–40. These repetitive motifs are important for their targeting to RNA granules37–40 or the mitotic spindle41 and for phase separation in vitro and in cells22,34,35,41,42.
Several recent studies have pointed to a particularly important role of aromatic residues in the interactions that enable IDRs to phase separate (Fig. 2e). The IDR in DDX4, for example, contains numerous Phe-Gly repeats whose aromatic rings appear to promote phase separation by engaging in cation-pi interactions [G] with Arg residues intra- and intermolecularly22, and likely pi-stacking interactions [G] as well (not shown). Similarly, mutation of aromatic residues in BuGZ and in the Nephrin intracellular domain (NICD) decreases their ability to phase separate41,43. Sequences enriched in Gln, Asn or Ser residues also contribute to the driving force for phase separation through dipolar interactions [G] of their sidechains44,45. Finally, phase separation of IDR-containing proteins can also be promoted by interactions between blocks of oppositely charged residues—either between two different molecular types (Fig. 2f) or as alternating blocks in the same molecular type (Fig. 2g)15,16,22,33,43. In these systems the patterning of charged residues is important—for the same net molecular charge, when the charge is uniformly distributed phase separation is disfavoured, whereas when charged residues are clustered phase separation is promoted22,32,43,46. Notably, all of these interaction types—aromatic, polar and charge-charge—are short lived and provide little structural order to the peptide chain, consistent with the dynamic nature of phase separated liquids.
In addition to these amino acid sidechain interactions, interactions involving the polypeptide backbone also likely play an important role in phase separation of IDR-containing proteins. The IDRs from RNA-binding proteins FUS, Taf15, hnRNPA2, EWS, and CIRBP form solid-like hydrogels when concentrated in vitro21,40,47 (after initial liquid-liquid phase separation for some ot them, see below for a discussion of this temporal progression). Based on a combination of X-ray diffraction, electron microscopy, and chemical footprinting [G] data, these hydrogels contain long filaments that appear to be generated from interactions between stretches of b-strands, similar to those observed in amyloid fibres (Fig. 2h)21,40,47,48. This suggests that the interactions between b-strands that drive fibre and hydrogel formation when occurring thousands at a time, may provide the weak multivalent adhesions that drive liquid-liquid phase separation when occurring only a few at a time48. Relatedly, recent data demonstrated that short, evolutionarily conserved a-helical structures are important for phase separation of another RNA-binding protein, TDP4349–51. In any given IDR, the degree to which sidechain and backbone interactions contribute to phase separation will depend on the amino acid composition and overall sequence patterns of the protein. Predictive rules relating protein sequence to phase separation propensity are slowly emerging, but remain an important area of future research22,43,46,52.
IDRs can thus undergo a variety of types of homotypic and heterotypic interactions. While the individual interacting motifs are less well-defined in IDR-containing proteins than in multi-domain proteins, multivalency appears to play a central role in promoting phase separation of both types of molecules.
Regulation of assembly
The physical mechanisms promoting phase separation outlined above (for more details see Boxes 1 and 2), suggest means of controlling key features of Biomolecular Condensates, including their total volume, assembly and disassembly. Specifically, since molecules will phase separate until the values of the chemical potential [G] of both species are matched in the two phases, this control can be achieved by altering cellular concentration of Condensate components and/or their propensity to phase separate18.
Control of cellular concentration
Because Condensates form by phase separation, they appear sharply in cells when their essential components reach their solubility limit (Fig. 2i). For example, nucleoli in C. elegans embryos form only when the nucleolar component Fib1 (and perhaps other key molecules) is above a threshold concentration53. Under thermodynamic control, the total volume of the condensed phase (that is, the phase-separated entity) will then be determined by the extent to which the concentrations of its components exceed their solubility limits. This has, indeed, been observed quantitatively with engineered DDX4 and NICD as well as qualitatively with natural Condensates, such as PML bodies, nucleoli, P bodies, stress granules and centrosomes22,43, whose sizes scale with expression levels of key components. Similarly, the formation of Cajal bodies, PML bodies, histone locus bodies[G], nuclear speckles (artificial) and nucleoli (natural) can be induced by experimentally concentrating their key components at a particular cellular site54–58. Furthermore, expanding the volume of isolated nuclei by placing them in a hypotonic solution leads to a reversible dissolution of PML bodies and nucleoli59. Likewise, in C. elegans embryos decreasing the concentration of nucleolar components by increasing cell volume, leads to nucleolar size decrease53. Essentially, any mechanism that alters the local concentration of key components, including changes in protein expression, degradation, and localization, will influence the formation and total volume of the condensed phase.
Control of phase separation threshold
Condensate formation can also be controlled by modulating the phase separation threshold through changing the degree of molecular assembly (Fig. 2j) and/or intrinsic solubility (Fig. 2k) of key species. Post-translational modifications appear to be an important mechanism to achieve such control, as they can change both the valency and intrinsic solubility. In the Nephrin and LAT signalling assemblies (see above), for example, higher numbers of pTyr residues promote phase separation, enabling control of phase separation through modulating the activity of kinases and phosphatases19,25,26. Similarly, phase separation of the nuage [G] protein DDX4 is hindered by Arg methylation, which likely decreases the number of cation-pi interactions22. Moreover, the number and structure of PML nuclear bodies are influenced by the degree of SUMOylation of the PML protein, which can alter self-assembly through its SIM60. Relatedly, binding to interaction partners such as RNA can modulate protein solubility. For instance, the solubility of a P granule component, PGL3, decreases in vitro in the presence of RNA; in other words, PGL-3 phase separates at lower concentrations. Proteins that compete for RNA binding with PGL3, such as MEX-5, can then increase the solubility limit of PGL3 in the presence of RNA22,25,61. All these examples represent events that occur on rapid cellular timescales (in a matter of minutes). However, processes occurring on much slower timescales, for example alternative splicing or evolutionary processes could also alter phase separation propensity by modulating the interaction valency.
Regulation of composition
Individual Biomolecular Condensates have a specific composition, typically concentrating from ten to several hundred different proteins, and often also RNA molecules. Their composition is dynamically controlled; some components are constitutive, but many are recruited only transiently, for example during particular stages of the cell cycle or in response to stimuli60,62–65. How can we understand this complexity? Little work has been done so far to understand compositional control of Condensates in a general way. One recent attempt to develop a general framework to explain composition was based on dividing Condensate components into two qualitative classes29. The first are scaffolds, which are resident molecules essential for formation of the structure. Genetic studies have indicated that these are often only a small subset of Condensate components. For example, PML is the only protein known to be essential to form PML nuclear bodies66. Similarly, Spd5 is the key component necessary for formation of C. elegans centrosomes67, TIA1 for stress granules68, the NEAT1 non-coding RNA for paraspeckles [G], and mRNAs for P bodies2. The second class of components, termed clients, consists of molecules that are dispensable for Condensate assembly. These comprise the majority of components and often localize to Condensates in a regulated fashion through direct binding to scaffolds60,62,65.
The study used simple model systems composed of multivalent scaffolds and their cognate low valency clients to elucidate principles of compositional control29. Both in vitro and in cells, phase separated droplets formed by polySUMO–polySIM scaffolds differentially recruited low valency clients (e.g. GFP-SUMO or GFP-SIM) depending on the relative stoichiometries of the scaffold components (Fig.3a and 3b). Changes in composition could be induced rapidly by altering the SUMO to SIM ratio in the scaffolds. Particularly, around stoichiometric equality even very small changes in relative concentrations of polySUMO and polySIM could drive large changes in client recruitment. In addition, clients with higher valency (for example containing more than one SUMO domain) were recruited more strongly (Fig. 3c). Analogous behaviors were also observed in mammalian PML nuclear bodies and yeast P bodies. In both cases, perturbing scaffold stoichiometries (by mutating SUMOylation sites or modulating cellular mRNA levels, respectively), resulted in changes in client recruitment. Thus, despite the complexity of Condensates, their compositions may be explained, at least in part, by simple principles.
Figure 3. A Model for Compositional Control of Biomolecular Condensates.
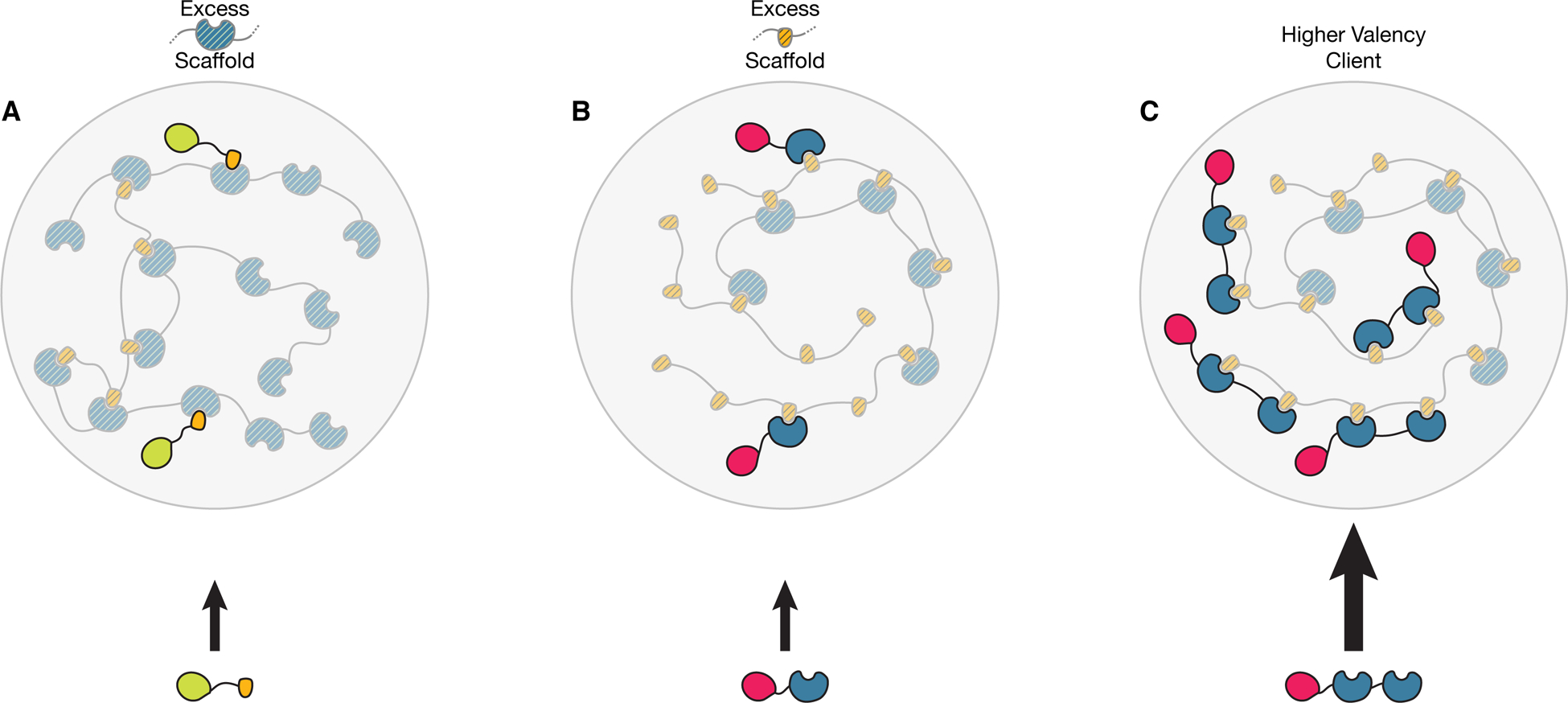
Multivalent molecules comprising the scaffold of the Condensate contain complementary modules (blue and yellow, for example small ubiquitin-related modifier (SUMO) domains and SUMO-interaction motifs, respectively)) which allow the assembly of the scaffold to form the phase separated structure (large circles). Client molecules in this example harbour interaction modules complementary to the scaffold components but at lower valency, and are recruited to the structure through binding to free cognate sites in the scaffold (owing to stoichiometric excess of one of the modules).
A) Stoichiometric excess of the scaffold component containing blue modules yields free blue scaffold sites. Clients containing yellow modules can be recruited to the body by binding to the blue scaffold sites that are unoccupied by scaffold–scaffold interactions.
B) Stoichiometric excess of the scaffold component containing yellow modules yields free yellow scaffold sites. Clients containing blue modules can be recruited to the body by binding to the yellow scaffold sites that are unoccupied by scaffold–scaffold interactions.
C) Higher valency of the blue client promotes stronger recruitment of this client when the yellow scaffold module is in stoichiometric excess (but not when the blue scaffold is in excess (not shown)). Figure modified with permissions from Banani, S. F. et al. Cell (2016).
In addition to specific binding interactions, general electrostatic properties also can influence client recruitment to Condensates. This was shown recently in reconstituted LAT signalling clusters, which selectively exclude negatively charged proteins and concentrate positively charged proteins, probably because the scaffold components of this Condensate are highly negatively charged26. The relative importance of specific binding and electrostatic interactions is likely to vary between systems.
IDR-based phase separating systems also show selective recruitment of clients. In some cases, this can be understood analogously to domain based systems. For example, decreasing the number of Gly/Ser-Phe/Tyr-Gly/Ser motifs in hnRNPA2 and FUS decreases the efficiency of their recruitment into IDR-based droplets in vitro48 and into stress granules in cells40, respectively by reducing the valency of these clients. In other systems, however, the molecular mechanisms governing selectivity of client recruitment are not yet understood42,22.
Control of physical properties
Many Biomolecular Condensates possess liquid-like properties. However, some appear to behave more like solids69 or have solid-like elements51. Moreover, physical properties and organization of phase separated droplets can change over time. As these properties likely influence Condensate functions (see below), they are probably regulated in vivo.
Maturation of IDR-Based Phases
Phase separated droplets of FUS and hnRNPA1 rapidly exchange molecules with the soluble phase (as assessed by fluorescence recovery after photobleaching), contain largely disordered protein (shown for FUS by nuclear magnetic resonance), and behave macroscopically as liquids15,34–36,42,48. However, many droplets formed by IDR-containing proteins that are initially fluid become more viscoelastic over the course of several hours (e.g., FUS, Pub1, LSm4, eIF4GII, Tia1, hnRNPA1, Whi3, and Fib1), eventually behaving as solids and ceasing to exchange molecules with the surroundings. This process is referred to as maturation, or hardening15,34,36,42,48,70. The material properties of these hardened states observed in vitro are still unclear, but could be gels, glasses or two phase solids. Maturation also likely occurs in vivo, as some Condensates behave as solids (e.g. Balbiani bodies [G]71 and yeast stress granules69) or contain solid-like substructures (see below). Balbiani bodies are particularly interesting in this regard. They are large membrane-less structures that are present in immature oocytes and are thought to protect organelles during the many decades of oocyte dormancy. In Xenopus oocytes they are formed from a prion-domain containing protein called XVelo, which when expressed from baculovirus forms solid-like structures. Other proteins containing prion-like domain, such as FUS, form liquids in vitro15. Indeed, expression of the prion-like domain of FUS forms liquids, while that of Xvelo forms solids71. Therefore, it seems that the material properties are encoded in part by the prion-like domains. However, whether Xvelo forms a solid-like Balbiani body de novo, or matures through a more liquid-like state is not known. Note that maturation is not observed with phase separated liquids formed by proteins composed of modular domains; in these systems dynamic behaviours are constant, and determined by the affinity and kinetics of the modular domain–ligand interactions23.
Several potential mechanisms could account for maturation (Figure 4). Unfolded proteins have a propensity to form amyloid fibres through b-strand interactions72. This behavior should be enhanced for IDRs within phase separated droplets because of the high protein concentrations42,43 and the tendency of polymers to adopt extended conformations in the condensed phase, which predispose the polypeptide chain to make β-strand-like contacts24,73,74. Thus, phase separation could promote increased rates of nucleation and/or growth of amyloid fibres, which could further crosslink through lateral contacts. Indeed, droplet maturation occurs in vitro concomitant with macroscopic formation of filamentous structures15,34–36,42,48, and fully matured droplets contain amyloid-like filaments observable by electron microscopy21,42,71. Balbiani bodies are rich in b-sheet as assessed by Thioflavin T staining. Chemical footprinting studies suggest that fibre formation in Condensates also occurs in cells48. As an alternative to fibre formation, some systems may be kinetically trapped, or “vitrified” in an amorphous, cross-linked state if their b-strand (or sidechain) interactions form quickly and dissociate slowly, preventing progression to regularized amyloid74 (Fig. 4). Finally, increased entanglement of polymer chains (whereby the chains wrap around each other and cannot cross) could also change Condensate properties in a manner akin to maturation75. These latter mechanisms may account for observations that yeast stress granules behave as solids but do not appear to contain fibers69. Although detailed experimental studies are still lacking, the slowed molecular dynamics and increasing hardness of droplets as maturation proceeds in these scenarios would likely result from increases in i) fibre length, numbers, crosslinking density and strength, ii) the density and strength of b-strand or sidechain interactions, or iii) the degree of entanglement.
Figure 4. Changing material properties of Biomolecular Condensates.
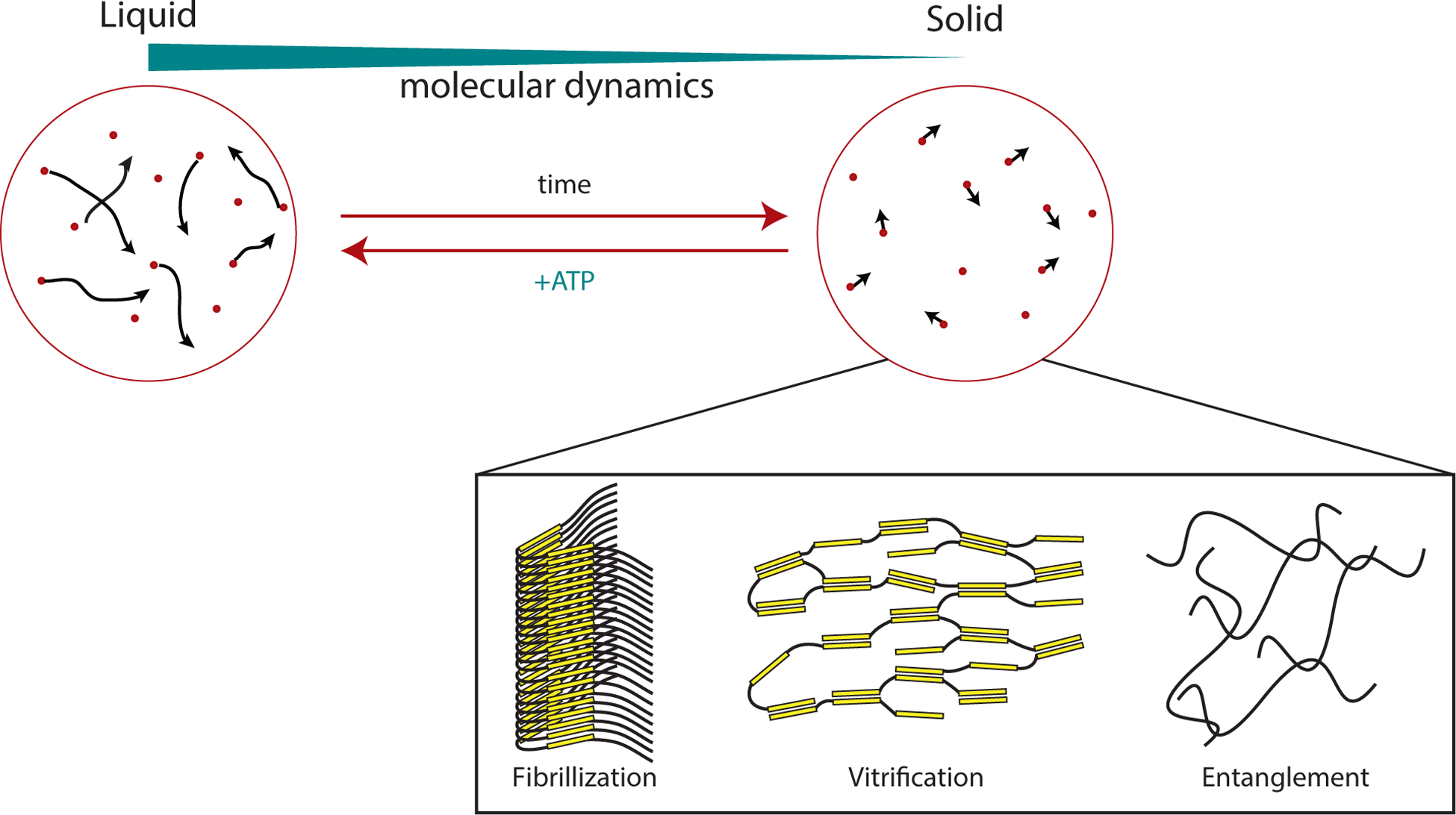
Condensates composed of intrinsically disordered regions (IDRs) have the propensity to mature, changing their properties from liquid-like to solid-like. Initially, the components in the condensed phase exhibit only transient interactions and lack appreciable order. Thus the molecules freely rearrange (and exchange with the surrounding solution) and the molecular dynamics can be described as that of a liquid. Over time, the liquid becomes more solid-like. Several potential mechanisms for this ‘hardening’ and the concomitant decrease in molecular dynamics have been proposed, as described in the text. Briefly, these could include nucleation and elongation of amyloid-like fibres, kinetic trapping into amorphous glasses (‘vitrification’) or entanglement of the disordered polypeptides. ATP-dependent machineries such as chaperones and disaggregases are expected to act against these processes (other mechanisms that do not depend on ATP may act similarly).
Regulation of physical properties by energy consuming processes
Cells likely have mechanisms to limit the tendency of IDRs to mature in order to tune the dynamics and liquid-like properties of Condensates into functionally appropriate regimes (see below). One likely mechanism involves the use of energy-dependent processes or machines to control the degree of fibre and/or crosslink formation within Condensates, limiting these structures when Condensates need to be dynamic and enabling their formation and/or growth when Condensates need to be static (Fig. 4). This may explain why chaperones, and ATP-dependent disaggregases as well as molecular motors are present in many RNA granules51,69. Indeed, depletion of ATP increases the viscosity of stress granules and nucleoli13,51. Moreover, several ATPases can regulate the dynamics of stress granules, increasing or decreasing their persistence49,51.
Recently, it has been suggested that imbalances between the thermodynamic drive of IDRs to form fibres and the opposing disaggregase machineries could lead to disease. In fact, a large body of data links dysregulation of RNA-containing Condensates with neurodegenerative diseases. We direct readers to numerous recent reviews focused on this important topic20,76–81.
In addition to controlling maturation, it is likely that energy utilizing systems modulate many additional features of Condensates. For example, the transcription of rRNA influences the nucleation and spatial distribution of condensing nucleoli in C. elegans embryos58,82. Further, the actin cytoskeleton, dynamics of which are controlled by ATP hydrolysis in actin filaments, motors and nucleation factors, affects the size distribution of nucleoli and histone locus bodies83. The actin cytoskeleton can also affect localization of Condensates. For example, phase separated LAT clusters are moved radially at the T-cell–antigen presenting cell interface by dynamic movements of the actin cytoskeleton84,85. Because energy-consumption influences virtually all biological processes, these initial observations are likely exemplary of a more general phenomenon in the regulation of Condensates. The study of energy consuming, non-equilibrium materials—“active matter”—is an area of great current interest in physics and materials science86–89. The application of physical theories should provide insight into the influence of cellular energy on the equilibrium processes of phase separation13,90.
Multi-phase Biomolecular Condensates
The studies we have discussed so far involve a single condensed phase and a more dilute surrounding phase. However, some Biomolecular Condensates are composed of distinct subcompartments, that is they contain secondary condensed phases within the primary condensed phase51,70,91,92. A recent study examined this in detail for nucleoli, demonstrating that the subcompartments have distinct viscosities, surface tensions and compositions70. The encapsulation of one subcompartment by another is enabled by the distinct surface tensions of the phases, which arises from distinct multivalent interactions based on IDRs or folded domains of the components. How the composition of such multiphase Condensates is regulated and how the assembly of such structures is initiated in cells remain open questions.
The different subcompartments may have different propensities for maturation based on their compositions. The (inner) dense fibrillar subcompartment of the nucleolus, for instance, is more prone to maturation than the (outer) granular component and may exhibit a spectrum of viscoelastic behaviours in cells, which are likely subject to regulation13,70. As indicated by their names, electron microscopy images of these subcompartments show distinct textures93. Similarly, Cajal bodies sometimes exhibit coiled structures that can be visualized by electron microscopy22,42,94 and other times appear more isotropic. These coiled elements may represent fibrous structures embedded within a larger liquid phase. Recent biochemical and high resolution imaging studies have revealed that other Condensates also contain substructures that behave as solids13,51,91,92. Though the function of these subcompartments remains to be determined, it seems likely that cells regulate the relative amounts of solid versus liquid material to yield a functional effect, such as regulating reaction kinetics or stabilizing the structure against mechanical forces (see below).
Implications for function
We have so far discussed the physicochemical and molecular mechanisms that drive formation of Biomolecular Condensates and how their assembly, composition, and material properties can be regulated. These characteristics of Condensates present unique opportunities, distinct from those provided by macromolecular complexes (Supplementary information S5 (Box)), for controlling the biochemical environment of the cell95. In this section, we describe how the properties of Condensates can translate to their biological functions.
Effects of Biomolecular Condensates on reaction kinetics
Condensates substantially increase the local concentration of resident chemical species. In the simplest case, the increase in concentration should accelerate reactions inside the structure (Fig. 5a) (note, however, that the overall reaction rate will increase only if both enzyme and substrate of a reaction are concentrated in a condensed phase, but not if either is concentrated alone). This has been observed for some cellular systems. For example, the rate of histone mRNA-processing is significantly reduced when key components of this process fail to concentrate within the histone locus body96. Similar effects have been shown for zebrafish Cajal body components97 consistent with a computational model of small nuclear ribonucleoprotein [G] assembly in these structures98. Acceleration of reactions by phase separation has also been observed biochemically. For example, the total solution activity of the Hammerhead ribozyme [G] can increase up to ~70-fold when it is concentrated along with its substrate RNA strand into phase separated droplets in vitro99. Actin polymerization rates can also be substantially accelerated by concentrating the Arp2/3 complex and N-WASP into Nephrin–Nck–N-WASP-based droplets or clusters on model membranes19,25.
Figure 5. Functional consequences of forming Biomolecular Condensates.
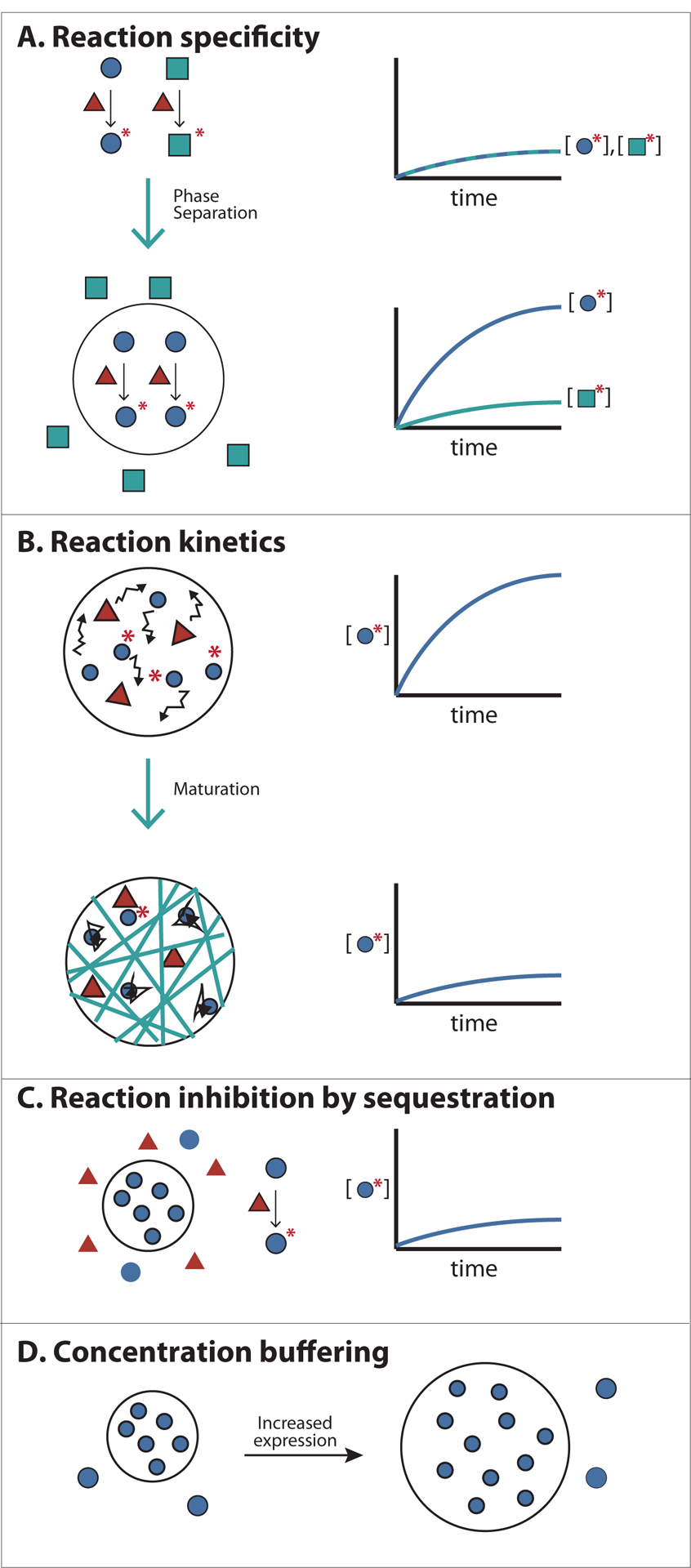
A) Concentrating reactants inside Condensates can increase reaction kinetics and specificity. An enzyme with two alternative substrates is shown. Colocalizing the enzyme with one of its substrates within the condensed phase (black circle) accelerates rates of reaction with that substrate. Additionally, excluding the substrate of an alternative pathway can direct a specific reaction to occur inside Condensates.
B) Changes in the physical properties of cellular bodies can affect the kinetics of reactions. For example, increased viscosity of cellular bodies by fibre formation (or other mechanisms of maturation, see text), may slow diffusion of molecules, decreasing reaction kinetics.
C) Sequestering molecules inside Condensates can prevent reactions involving partners present in the bulk phase. This could control substrate flux through various pathways.
D) The concentration of essential Condensates components in the bulk phase is clamped at the phase separation threshold (defined by the solubility limit of the molecule). Thus the concentration of these components in the bulk phase can be maintained despite fluctuations in expression or degradation.
However, concentration into a condensed phase does not always produce overall reaction rate acceleration. As an example, essential factors for small nuclear RNA modification, called guide RNAs, are normally concentrated inside Cajal bodies. However, disrupting Cajal body formation and thereby dispersing guide RNAs in the nucleoplasm does not seem to affect the efficiency of small nuclear RNA modification22,100. Similarly, the activity of enzymes in the purine biosynthetic pathway was not significantly enhanced when they were concentrated with their substrate into phase separated droplets in vitro101. This lack of enhancement was due to the lower specific activities of the enzymes towards their substrates within the droplets as compared to their activity in solution. In unpublished work, we have observed that the highly concentrated scaffolds and enzymes within phase separated droplets frequently interfere with each other, with scaffold components inhibiting enzyme activities and enzymes dispersing droplets by covalently modifying scaffolds. In cells, mechanisms likely exist to prevent or take advantage of such interference.
Many physical features of Condensates could affect reactions that occur within them. Molecular crowding—the decrease in accessible volume owing to high macromolecule concentration—can affect allosteric regulation and binding affinity, altering enzymatic activities102. Furthermore, Condensates are porous structure (see below) and this will also have complex effects on the movement of molecules within them. A solution containing high concentrations of a small molecule (e.g. glycerol) will slow the movement of all molecules within it, and thus decrease reaction rates. But a solution containing a concentrated polymer matrix will behave differently. In this case, the free volume between the condensed scaffold components will behave as pores, through which small proteins will move as though the polymer were absent; only large molecules, not fitting into these pores or molecules that bind the polymer will move slowly103. The impact of such effects on Condensates—particularly those containing RNA—is likely to be significant, since they are composed of combinations of large RNA molecules, proteins of various sizes, and small organic compounds. In addition, the various viscoelastic properties of Condensates, controlled for example by the degree of IDR maturation, interaction kinetics of multidomain scaffolds, RNA composition104,36 or active (energy consuming) processes, will likely influence the dynamics of molecules within them (Fig. 5b) and at the phase boundary. Changes in viscoelasticity could also affect Condensate composition. Understanding these behaviours will greatly benefit from additional experimental and theoretical work describing chemistry within complex, heterogeneous media.
Regulating specificity of biochemical reactions
Phase separated compartments could concentrate a protein with a subset of its potential interaction partners while excluding others, imparting specificity to biochemical processes. For example, a Condensate could concentrate an enzyme with a particular subset of its possible substrates, conferring specificity to a potentially promiscuous reaction (Fig. 5a). Relatedly, a Condensate could concentrate (and thus accelerate the chemistry of) molecules that act in one particular biological pathway, while excluding components of alternative pathways, controlling biochemical flux. In this way, Condensates could act analogously to classical scaffolding molecules in signalling pathways, which bind multiple, selected pathway components simultaneously to provide spatial proximity and structural organization, thus enhancing flux and selectivity105. Consistent with this idea, clustering the metabolic branch point enzyme, carB, with one downstream enzyme, pyrB, but not another, argI, was shown to direct the metabolic flux of carbamoyl phosphate to favour pyrimidine and disfavour arginine in Escherichia coli106. Many metabolic enzymes localize to Condensate-like puncta in response to nutrient starvation, suggesting that such effects may be generally important in metabolic control107,108. In a related example from mammalian cells, reconstituted T-cell receptor signalling clusters concentrate kinases but exclude phosphatases, stabilizing the phosphorylation-dependent clusters26.
Sequestration of molecules
Condensates could also in principle sequester molecules, thus effectively inhibiting their activity outside the structure (Fig. 5c), as has been suggested for sequestration of the transcription factor DAXX in PML bodies109. Stress and RNA transport granules have been ascribed similar storage functions2. An important consideration for such models is that for sequestration to be effective, most copies of the desired species must be captured in the Condensate. Since one type of Condensate typically constitutes only 1–2 % of the cellular volume (unpublished observations from Rosen and Hyman labs), strong inhibition (high depletion of the molecule from the surrounding nucleo- or cytoplasm) would require very high partition coefficients [G] for the sequestered component. Further quantitative analyses of Condensates will be necessary to test these sequestration models.
Buffering cellular concentration of molecules
Once a phase separated structure has formed, the volume of the condensed phase will grow as more scaffold components are added to the system, but the scaffold component concentration in the surrounding solution will remain clamped at the solubility threshold value (Fig. 5d). This phenomenon could be used to buffer against biological fluctuations (for example in gene expression), making certain pathways more robust to noise or repressing pathways that require noise for their proper function110.
Controlling function through dynamic regulation of phase separation
One important advantage of phase separated structures is that all of these potential functions can be switched on and off extremely rapidly by controlling the formation and dissolution of a condensed phase. At the solubility limit of a molecule, even minute changes in a physical parameter (such as concentration or temperature) can sharply induce phase transitions. For instance, changes of 1 °C can cause condensation or dissolution of BUgZ, DDX4, hnRNPA1 or FUS droplets22,34,41. Modest changes in salt concentration can have similar effects22,42. In addition, as described above, Condensate composition can also be regulated in switch-like fashion with small changes in relative stoichiometries of scaffold components.
In conclusion, the biochemical environment within Condensates may be fundamentally different than that in the surrounding cytoplasm or nucleoplasm, and it may endow the cells with unique ways of regulating cellular reactions.
Conclusions and perspectives
Research in the last several years has made significant strides toward understanding the molecular mechanisms that underlie the formation, regulation, and function of Biomolecular Condensates. It appears that many of these structures form through liquid-liquid phase separation, driven by interactions of multivalent molecules. This mechanism naturally leads to routes to control the assembly and disassembly, composition, and physical properties of Condensates. These routes in turn have implications for the biochemistry that occurs within them and thus their cellular functions.
The phase boundary allows molecules to be concentrated within Condensates while continuously exchanging with the surroundings, without the complications of transport through a membranous barrier. Therefore, composition of Condensates can be regulated in a more flexible manner than that of classical organelles—without specialized molecules and signals to import and export. For instance, a more general mechanism, such as charge, can be used to target certain molecules to specific phases26 even in the absence of high affinity binding interactions. Further, components within Condensates can freely diffuse, providing ideal conditions to regulate the rates of biochemical reactions while spatially constraining them.
Given that cells can form compartments by phase separation, why would cells need intracellular membranes at all? Membrane-bound compartments can provide long-term stability that may be difficult to maintain with Condensates, since the local environment of a Condensate is constantly changing owing to fluctuations in gene expression, molecule turnover, etc. For instance, homeostatic reactions that are on-going require long-term separation from the bulk cytoplasm. In addition to long-term storage, cytotoxic reactions need to be kept structurally separate in order to protect the integrity of the surrounding cytoplasm or nucleoplasm. Finally, very small molecules, such as ions, will be difficult to retain inside Condensates. For instance, a pH gradient could not be stably maintained without a membrane. Thus, these two ways of organizing a cell–membranes or phase separation–are complementary and allow maximal possibilities in organizing cellular contents.
Many important questions remain in the study of Condensates. Most importantly, we do not understand in most cases what biochemical or cellular functions uniquely emerge from organizing molecules into such structures. In many cases we can infer function from the collection of Condensate components, but we do not understand how the activities of those components change by virtue of being in the structure rather than being more uniformly distributed in the cell. Where examined, the phenotypes resulting from disruption of Condensates are relatively subtle and the structures do not appear to be essential for the viability of cells or organisms111–114. Yet, they are conserved over evolution, suggesting they do play important functional roles, perhaps in response to particular stimuli or stresses.
We also do not understand the relationship between the microscopic properties of the component molecules and the macroscopic properties of Condensates. Further, it is not known how the latter relate to biochemical and cellular functions, or if cells regulate these properties to functional effect.
Although at low resolution many Condensates appear to be homogeneous, as described above, electron microscopy and super-resolution light microscopy have both indicated that many contain internal organization at multiple scales51,91–93. Does this organization occur in other Condensates, and, in general, how does it arise? Is it dynamically controlled? Is it functionally important?
What are all of the factors that control the composition of a given Condensate? We have discussed the importance of direct binding interactions and electrostatic effects, but are there other considerations, perhaps related to active processes? What do we need to know about a Condensate (or even a simplified phase separated droplet) to quantitatively predict how other molecules will partition into it? How is composition finely tuned so that distinct Condensates can coexist in a cell with shared components but functional differences? Is there a sequence- or structure-based code for recruitment of IDRs into phase separated droplets?
Does the idea that Condensates are generated through phase separation and multivalent assemblies have implications for disease, and could this enable novel clinical opportunities? Existing data suggests that Condensates may lie across a continuum of material and compositional states. Moreover, aberrations in this natural spectrum, some of which may involve misregulation of fibre formation, are implicated in neurodegeneration. How do these aberrations affect cell physiology? This is probably only one of many instances where a mechanistic understanding of Condensates could have medical implications.
Finally, what other cellular structures might be organized by phase separation? In principle, any system composed of interactions between multivalent entities should have the propensity to phase separate under appropriate solvent conditions. Chromatin biology is an intriguing area of cell biology that is enriched in multivalent interactions. Chromatin can be considered as long arrays of nucleosomes modified with specific marks on their component histones. Those marks are read by specific modular domains that also often appear in multivalent arrays in chromatin binding proteins. It thus seems reasonable that modified nucleosomes and histone tail readers may phase separate and that this process could affect aspects of chromatin organization and function.
Addressing these questions will likely require new technologies and new conceptual approaches, drawing on disciplines ranging from genetics to biochemistry to physics. Their answers promise to explain how nanometer-scale molecules can give rise to micron-scale cellular organization and the function of this organization in biology.
Supplementary Material
Supplementary Box S5
Supplementary Information contents
Supplementary movie 3
Supplementary movie 1
Supplementary movie 4
Supplementary table S6
Supplementary movie 2
Key points.
- In addition to canonical membrane-bound organelles, eukaryotic cells contain numerous membraneless compartments, or Biomolecular Condensates, that concentrate specific collections of proteins and nucleic acids.
- Biomolecular Condensates behave as phase separated liquids, and are enriched in multivalent molecules.
- Theoretical concepts from polymer and physical chemistry regarding the behaviour of multivalent molecules provide a mechanistic framework that can explain a wide range of cellular behaviors exhibited by Biomolecular Condensates, including plausible mechanisms by which their assembly, composition, and biochemical and cellular functions can be regulated.
Acknowledgments
We thank Robert Duronio and Christoph Weber for discussion and critical comments on the review. Research on multivalency driven phase separation is supported in the Hyman lab by the Max Planck Society, and in the Rosen lab by the Howard Hughes Medical Institute, the Welch Foundation (I-1544) and a Sara and Frank McKnight Graduate Fellowship (to SFB).
Author biographies
Salman Banani received his B.S. in Chemical Biology from the University of California, Berkeley. He is currently a candidate for the M.D. and Ph.D. degrees at the University of Texas Southwestern Medical Center. He completed his thesis work in the laboratory of Michael Rosen, where he focused on the mechanisms of compositional regulation of phase separated assemblies.
Hyun Lee received her B.S. in Molecular Biology from the University of Wisconsin, Madison, and her Ph.D. in Genetics and Molecular Biology from the University of North Carolina, Chapel Hill. She is a post-doctoral fellow at the Max Planck Institute for Molecular Cell Biology and Genetics in Dresden. She studies the role of self-organizing proteins and RNA in neurodegeneration and ageing.
Tony Hyman received his Ph.D. from Cambridge University, and was a post-doctoral fellow at the University of California San Francisco. He is a director of the Max Planck Institute of Molecular Cell Biology and Genetics in Dresden. His lab focuses on the function and formation of non-membrane bound organelles, Biomolecular Condensates.
Michael Rosen received his Ph.D. from Harvard University, and was a post-doctoral fellow at the Samuel Lunenfeld Research Institute and University of Toronto. He is Chair of the Department of Biophysics at UT Southwestern Medical Center and an Investigator of the Howard Hughes Medical Institute. His lab studies the physical mechanisms and functional consequences of macromolecular phase separation.
Footnotes
The authors declare no competing interests
References
- 1.Mao YS, Zhang B & Spector DL Biogenesis and function of nuclear bodies. Trends in Genetics 27, 295–306 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Decker CJ & Parker R P-bodies and stress granules: possible roles in the control of translation and mRNA degradation. Cold Spring Harb Perspect Biol 4, a012286 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu H Higher-order assemblies in a new paradigm of signal transduction. Cell 153, 287–292 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pederson T The nucleolus. Cold Spring Harb Perspect Biol 3, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dundr M et al. In vivo kinetics of Cajal body components. J Cell Biol 164, 831–842 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phair RD & Misteli T High mobility of proteins in the mammalian cell nucleus. Nature 404, 604–609 (2000). [DOI] [PubMed] [Google Scholar]
- 7.Weidtkamp-Peters S et al. Dynamics of component exchange at PML nuclear bodies. 121, 2731–2743 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Platani M, Goldberg I, Swedlow JR & Lamond AI In vivo analysis of Cajal body movement, separation, and joining in live human cells. J Cell Biol 151, 1561–1574 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw PJ & Jordan EG The nucleolus. Annu Rev Cell Dev Biol 11, 93–121 (1995). [DOI] [PubMed] [Google Scholar]
- 10.Fu L et al. Nuclear aggresomes form by fusion of PML-associated aggregates. Mol Biol Cell 16, 4905–4917 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y-CM, Kappel C, Beaudouin J, Eils R & Spector DL Live cell dynamics of promyelocytic leukemia nuclear bodies upon entry into and exit from mitosis. Mol Biol Cell 19, 3147–3162 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dellaire G, Ching RW, Dehghani H, Ren Y & Bazett-Jones DP The number of PML nuclear bodies increases in early S phase by a fission mechanism. J Cell Sci 119, 1026–1033 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Brangwynne CP, Mitchison TJ & Hyman AA Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proceedings of the National Academy of Sciences 108, 4334–4339 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brangwynne CP et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324, 1729–1732 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Patel A et al. A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell 162, 1066–1077 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Altmeyer M et al. Liquid demixing of intrinsically disordered proteins is seeded by poly(ADP-ribose). Nat Commun 6, 8088 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyman AA & Brangwynne CP Beyond stereospecificity: liquids and mesoscale organization of cytoplasm. Developmental Cell 21, 14–16 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Hyman AA, Weber CA & Jülicher F Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol 30, 39–58 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Li P et al. Phase transitions in the assembly of multivalent signalling proteins. Nature 483, 336–340 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King OD, Gitler AD & Shorter J The tip of the iceberg: RNA-binding proteins with prion-like domains in neurodegenerative disease. Brain Res. 1462, 61–80 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han TW et al. Cell-free formation of RNA granules: bound RNAs identify features and components of cellular assemblies. Cell 149, 768–779 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Nott TJ et al. Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol Cell 57, 936–947 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitrea DM et al. Nucleophosmin integrates within the nucleolus via multi-modal interactions with proteins displaying R-rich linear motifs and rRNA. Elife 5, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flory PJ Principles of Polymer Chemistry. (Cornell University Press, 1953). [Google Scholar]
- 25.Banjade S & Rosen MK Phase transitions of multivalent proteins can promote clustering of membrane receptors. Elife 3, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su X et al. Phase separation of signaling molecules promotes T cell receptor signal transduction. Science (2016). doi: 10.1126/science.aad9964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fromm SA et al. In vitro reconstitution of a cellular phase-transition process that involves the mRNA decapping machinery. Angew. Chem. Int. Ed. Engl 53, 7354–7359 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng M et al. Phase Transition in Postsynaptic Densities Underlies Formation of Synaptic Complexes and Synaptic Plasticity. Cell 166, 1163–1175.e12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banani SF et al. Compositional Control of Phase-Separated Cellular Bodies. Cell (2016). doi: 10.1016/j.cell.2016.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foo CTSWP, Lee JS, Mulyasasmita W, Parisi-Amon A & Heilshorn SC Two-component protein-engineered physical hydrogels for cell encapsulation. 106, 22067–22072 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mulyasasmita W, Lee JS & Heilshorn SC Molecular-Level Engineering of Protein Physical Hydrogels for Predictive Sol–Gel Phase Behavior. Biomacromolecules 12, 3406–3411 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brangwynne CP, Tompa P & Pappu RV Polymer physics of intracellular phase transitions. Nat Phys 11, 899–904 (2015). [Google Scholar]
- 33.Elbaum-Garfinkle S et al. The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proceedings of the National Academy of Sciences (2015). doi: 10.1073/pnas.1504822112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molliex A et al. Phase Separation by Low Complexity Domains Promotes Stress Granule Assembly and Drives Pathological Fibrillization. Cell 163, 123–133 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burke KA, Janke AM, Rhine CL & Fawzi NL Residue-by-Residue View of In Vitro FUS Granules that Bind the C-Terminal Domain of RNA Polymerase II. Mol Cell 60, 231–241 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H et al. RNA Controls PolyQ Protein Phase Transitions. Mol Cell 60, 220–230 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilks N et al. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol Biol Cell 15, 5383–5398 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Decker CJ, Teixeira D & Parker R Edc3p and a glutamine/asparagine-rich domain of Lsm4p function in processing body assembly in Saccharomyces cerevisiae. J Cell Biol 179, 437–449 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reijns MAM, Alexander RD, Spiller MP & Beggs JD A role for Q/N-rich aggregation-prone regions in P-body localization. J Cell Sci 121, 2463–2472 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kato M et al. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 149, 753–767 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang H et al. Phase Transition of Spindle-Associated Protein Regulate Spindle Apparatus Assembly. Cell 163, 108–122 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin Y, Protter DSW, Rosen MK & Parker R Formation and Maturation of Phase-Separated Liquid Droplets by RNA-Binding Proteins. Mol Cell (2015). doi: 10.1016/j.molcel.2015.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pak CW et al. Sequence Determinants of Intracellular Phase Separation by Complex Coacervation of a Disordered Protein. Mol Cell 63, 72–85 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crick SL, Jayaraman M, Frieden C, Wetzel R & Pappu RV Fluorescence correlation spectroscopy shows that monomeric polyglutamine molecules form collapsed structures in aqueous solutions. Proc Natl Acad Sci USA 103, 16764–16769 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crick SL, Ruff KM, Garai K, Frieden C & Pappu RV Unmasking the roles of N- and C-terminal flanking sequences from exon 1 of huntingtin as modulators of polyglutamine aggregation. Proceedings of the National Academy of Sciences 110, 20075–20080 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Das RK & Pappu RV Conformations of intrinsically disordered proteins are influenced by linear sequence distributions of oppositely charged residues. Proceedings of the National Academy of Sciences 110, 13392–13397 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwon I et al. Phosphorylation-regulated binding of RNA polymerase II to fibrous polymers of low-complexity domains. Cell 155, 1049–1060 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiang S et al. The LC Domain of hnRNPA2 Adopts Similar Conformations in Hydrogel Polymers, Liquid-like Droplets, and Nuclei. Cell 163, 829–839 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buchan JR, Kolaitis R-M, Taylor JP & Parker R Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function. Cell 153, 1461–1474 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.ALS Mutations Disrupt Phase Separation Mediated by α-Helical Structure in the TDP43 Low-Complexity C-Terminal Domain. 24, 1537–1549 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jain S et al. ATPase-Modulated Stress Granules Contain a Diverse Proteome and Substructure. Cell 164, 487–498 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quiroz FG & Chilkoti A Sequence heuristics to encode phase behaviour in intrinsically disordered protein polymers. Nat Mater 14, 1164–1171 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weber SC & Brangwynne CP Inverse size scaling of the nucleolus by a concentration-dependent phase transition. Curr Biol 25, 641–646 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shevtsov SP & Dundr M Nucleation of nuclear bodies by RNA. Nat Cell Biol 13, 167–U134 (2011). [DOI] [PubMed] [Google Scholar]
- 55.Kaiser TE, Intine RV & Dundr M De novo formation of a subnuclear body. Science 322, 1713–1717 (2008). [DOI] [PubMed] [Google Scholar]
- 56.Mao YS, Sunwoo H, Zhang B & Spector DL Direct visualization of the cotranscriptional assembly of a nuclear body by noncoding RNAs. Nat Cell Biol 13, 95–101 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chung I, Leonhardt H & Rippe K De novo assembly of a PML nuclear subcompartment occurs through multiple pathways and induces telomere elongation. 124, 3603–3618 (2011). [DOI] [PubMed] [Google Scholar]
- 58.Berry J, Weber SC, Vaidya N, Haataja M & Brangwynne CP RNA transcription modulates phase transition-driven nuclear body assembly. Proceedings of the National Academy of Sciences 112, E5237–45 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hancock R A role for macromolecular crowding effects in the assembly and function of compartments in the nucleus. J Struct Biol 146, 281–290 (2004). [DOI] [PubMed] [Google Scholar]
- 60.Dellaire G, Eskiw C, Dehghani H, Ching R & Bazett-Jones D Mitotic accumulations of PML protein contribute to the re-establishment of PML nuclear bodies in G1. J Cell Sci 119, 1034–1042 (2006). [DOI] [PubMed] [Google Scholar]
- 61.Polar Positioning of Phase-Separated Liquid Compartments in Cells Regulated by an mRNA Competition Mechanism. 166, 1572–1584.e16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grousl T et al. Robust heat shock induces eIF2alpha-phosphorylation-independent assembly of stress granules containing eIF3 and 40S ribosomal subunits in budding yeast, Saccharomyces cerevisiae. J Cell Sci 122, 2078–2088 (2009). [DOI] [PubMed] [Google Scholar]
- 63.Buchan JR & Parker R Eukaryotic stress granules: the ins and outs of translation. Mol Cell 36, 932–941 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoyle NP, Castelli LM, Campbell SG, Holmes LEA & Ashe MP Stress-dependent relocalization of translationally primed mRNPs to cytoplasmic granules that are kinetically and spatially distinct from P-bodies. J Cell Biol 179, 65–74 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Louria-Hayon I et al. The promyelocytic leukemia protein protects p53 from Mdm2-mediated inhibition and degradation. J Biol Chem 278, 33134–33141 (2003). [DOI] [PubMed] [Google Scholar]
- 66.Ishov AM et al. PML is critical for ND10 formation and recruits the PML-interacting protein daxx to this nuclear structure when modified by SUMO-1. J Cell Biol 147, 221–234 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Centrosome maturation and mitotic spindle assembly in C. elegans require SPD-5, a protein with multiple coiled-coil domains 3, 673–684 (2002). [DOI] [PubMed] [Google Scholar]
- 68.RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. 147, 1431–1442 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kroschwald S et al. Promiscuous interactions and protein disaggregases determine the material state of stress-inducible RNP granules. Elife 4, e06807 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feric M et al. Coexisting Liquid Phases Underlie Nucleolar Subcompartments. Cell (2016). doi: 10.1016/j.cell.2016.04.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boke E et al. Amyloid-like Self-Assembly of a Cellular Compartment. Cell 166, 637–650 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fändrich M, Fletcher MA & Dobson CM Amyloid fibrils from muscle myoglobin. Nature 410, 165–166 (2001). [DOI] [PubMed] [Google Scholar]
- 73.Vitalis A, Wang X & Pappu RV Quantitative characterization of intrinsic disorder in polyglutamine: insights from analysis based on polymer theories. Biophysj 93, 1923–1937 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Halfmann R A glass menagerie of low complexity sequences. Curr Opin Struc Biol 38, 9–16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Watanabe H Viscoelasticity and dynamics of entangled polymers. Progress in Polymer Science (1999). [Google Scholar]
- 76.Ramaswami M, Taylor JP & Parker R Altered ribostasis: RNA-protein granules in degenerative disorders. Cell 154, 727–736 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li YR, King OD, Shorter J & Gitler AD Stress granules as crucibles of ALS pathogenesis. J Cell Biol 201, 361–372 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weber SC & Brangwynne CP Getting RNA and protein in phase. Cell 149, 1188–1191 (2012). [DOI] [PubMed] [Google Scholar]
- 79.Wolozin B Physiological protein aggregation run amuck: stress granules and the genesis of neurodegenerative disease. Discov Med 17, 47–52 (2014). [PMC free article] [PubMed] [Google Scholar]
- 80.Aguzzi A & Altmeyer M Phase Separation: Linking Cellular Compartmentalization to Disease. Trends Cell Biol 26, 547–558 (2016). [DOI] [PubMed] [Google Scholar]
- 81.Alberti S & Hyman AA Are aberrant phase transitions a driver of cellular aging? Bioessays 38, 959–968 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oakes CC, La Salle S, Smiraglia DJ, Robaire B & Trasler JM A unique configuration of genome-wide DNA methylation patterns in the testis. Proc Natl Acad Sci USA 104, 228–233 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feric M & Brangwynne CP A nuclear F-actin scaffold stabilizes ribonucleoprotein droplets against gravity in large cells. Nat Cell Biol 15, 1253–1259 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaizuka Y, Douglass AD, Varma R, Dustin ML & Vale RD Mechanisms for segregating T cell receptor and adhesion molecules during immunological synapse formation in Jurkat T cells. Proceedings of the National Academy of Sciences 104, 20296–20301 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yi J, Wu XS, Crites T & Hammer JA Actin retrograde flow and actomyosin II arc contraction drive receptor cluster dynamics at the immunological synapse in Jurkat T cells. Mol Biol Cell 23, 834–852 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee CF, Brangwynne CP, Gharakhani J, Hyman AA & Jülicher F Spatial organization of the cell cytoplasm by position-dependent phase separation. Phys Rev Lett 111, 088101 (2013). [DOI] [PubMed] [Google Scholar]
- 87.Zwicker D, Hyman AA & Jülicher F Suppression of Ostwald ripening in active emulsions. Phys. Rev. E 92, 012317 (2015). [DOI] [PubMed] [Google Scholar]
- 88.Hagan MF & Baskaran A Emergent self-organization in active materials. Curr Opin Cell Biol 38, 74–80 (2016). [DOI] [PubMed] [Google Scholar]
- 89.Popkin G The physics of life. Nature 529, 16–18 (2016). [DOI] [PubMed] [Google Scholar]
- 90.Sanchez T, Chen DTN, DeCamp SJ, Heymann M & Dogic Z Spontaneous motion in hierarchically assembled active matter. Nature 491, 431–434 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang JT et al. Regulation of RNA granule dynamics by phosphorylation of serine-rich, intrinsically disordered proteins in C. elegans. Elife 3, e04591 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lang M et al. Three-dimensional organization of promyelocytic leukemia nuclear bodies. 123, 392–400 (2010). [DOI] [PubMed] [Google Scholar]
- 93.Boisvert F-M, van Koningsbruggen S, Navascués J & Lamond AI The multifunctional nucleolus. Nat Rev Mol Cell Biol 8, 574–585 (2007). [DOI] [PubMed] [Google Scholar]
- 94.Fine structural organization of the interphase nucleus in some mammalian cells. 27, 266–288 (1969). [DOI] [PubMed] [Google Scholar]
- 95.Hyman AA & Simons K Cell biology. Beyond oil and water--phase transitions in cells. Science 337, 1047–1049 (2012). [DOI] [PubMed] [Google Scholar]
- 96.Concentrating pre-mRNA processing factors in the histone locus body facilitates efficient histone mRNA biogenesis. 213, 557–570 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Coilin-dependent snRNP assembly is essential for zebrafish embryogenesis. 17, 403–409 (2010). [DOI] [PubMed] [Google Scholar]
- 98.In vivo kinetics of U4/U6·U5 tri-snRNP formation in Cajal bodies. 22, 513–523 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Strulson CA, Molden RC, Keating CD & Bevilacqua PC RNA catalysis through compartmentalization. Nat Chem 4, 941–946 (2012). [DOI] [PubMed] [Google Scholar]
- 100.Small Cajal body-specific RNAs of Drosophila function in the absence of Cajal bodies. 20, 5250–5259 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Davis BW et al. Colocalization and Sequential Enzyme Activity in Aqueous Biphasic Systems: Experiments and Modeling. Biophys J 109, 2182–2194 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kuznetsova IM, Zaslavsky BY, Breydo L, Turoverov KK & Uversky VN Beyond the excluded volume effects: mechanistic complexity of the crowded milieu. Molecules 20, 1377–1409 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cai L-H, Panyukov S & Rubinstein M Mobility of Nonsticky Nanoparticles in Polymer Liquids. Macromolecules 44, 7853–7863 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Elbaum-Garfinkle S & Brangwynne CP Liquids, Fibers, and Gels: The Many Phases of Neurodegeneration. Developmental Cell 35, 531–532 (2015). [DOI] [PubMed] [Google Scholar]
- 105.Good MC, Zalatan JG & Lim WA Scaffold proteins: hubs for controlling the flow of cellular information. Science 332, 680–686 (2011).21551057 [Google Scholar]
- 106.Castellana M et al. Enzyme clustering accelerates processing of intermediates through metabolic channeling. Nat Biotechnol 32, 1011–1018 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.O’Connell JD, Zhao A, Ellington AD & Marcotte EM Dynamic reorganization of metabolic enzymes into intracellular bodies. Annu Rev Cell Dev Biol 28, 89–111 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Noree C, Monfort E, Shiau AK & Wilhelm JE Common regulatory control of CTP synthase enzyme activity and filament formation. Mol Biol Cell 25, 2282–2290 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li H et al. Sequestration and inhibition of Daxx-mediated transcriptional repression by PML. Mol Cell Biol 20, 1784–1796 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eldar A & Elowitz MB Functional roles for noise in genetic circuits. Nature 467, 167–173 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kshirsagar M & Parker R Identification of Edc3p as an enhancer of mRNA decapping in Saccharomyces cerevisiae. Genetics (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dunckley T & Parker R The DCP2 protein is required for mRNA decapping in Saccharomyces cerevisiae and contains a functional MutT motif. EMBO J 18, 5411–5422 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bernardi R & Pandolfi PP Role of PML and the PML-nuclear body in the control of programmed cell death. Oncogene 22, 9048–9057 (2003). [DOI] [PubMed] [Google Scholar]
- 114.Nakagawa S, Naganuma T, Shioi G & Hirose T Paraspeckles are subpopulation-specific nuclear bodies that are not essential in mice. J Cell Biol 193, 31–39 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dill KA & Bromberg S Molecular Driving Forces. (Garland Pub, 2011). [Google Scholar]
- 116.Flory PJ Thermodynamics of High Polymer Solutions. J Chem Phys 10, 51 (1942). [Google Scholar]
- 117.Griffin EE, Odde DJ & Seydoux G Regulation of the MEX-5 Gradient by a Spatially Segregated Kinase/Phosphatase Cycle. Cell 146, 955–968 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stockmayer W Molecular distribution in condensation polymers. Journal of polymer science 9, 69–71 (1952). [Google Scholar]
- 119.Cohen R & Benedek G Equilibrium and kinetic theory of polymerization and the sol-gel transition. The Journal of Physical Chemistry 86, 3696–3714 (1982). [Google Scholar]
- 120.Huggins ML Solutions of long chain compounds. (The Journal of chemical physics, 1941). [Google Scholar]
- 121.Semenov A & Rubinstein M Thermoreversible gelation in solutions of associative polymers. 1. Statics. Macromolecules 31, 1373–1385 (1998). [Google Scholar]
- 122.Saha S et al. Polar positioning of phase-separated liquid compartments in cells regulated by an mRNA competition mechanism. Cell 166, 1572–1584 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Box S5
Supplementary Information contents
Supplementary movie 3
Supplementary movie 1
Supplementary movie 4
Supplementary table S6
Supplementary movie 2