Endocytosis and signalling: intertwining molecular networks (original) (raw)
. Author manuscript; available in PMC: 2010 Jul 1.
Published in final edited form as: Nat Rev Mol Cell Biol. 2009 Sep;10(9):609–622. doi: 10.1038/nrm2748
Abstract
Cell signalling and endocytic membrane trafficking have traditionally been viewed as distinct processes. Although our present understanding is incomplete and there are still great controversies, it is now recognized that these processes are intimately and bidirectionally linked in animal cells. Indeed, many recent examples illustrate how endocytosis regulates receptor signalling (including signalling from receptor tyrosine kinases and G protein-coupled receptors) and, conversely, how signalling regulates the endocytic pathway. The mechanistic and functional principles that underlie the relationship between signalling and endocytosis in cell biology are becoming increasingly evident across many systems.
Cells sense the environment and communicate with each other through the ligand-induced activation of signalling receptors at the cell surface. Signalling receptors, similar to other integral plasma membrane proteins, enter the endocytic pathway and are sorted into various endosomal compartments. Endocytosis regulates cell signalling most simply by controlling the number of receptors available for activation in the plasma membrane, and the activation of receptors or downstream effectors often stimulates receptor endocytosis. Studies of receptor tyrosine kinases (RTKs) and G protein-coupled receptors (GPCRs) have established many examples of this relationship, which functions as a homeostatic regulatory loop to prevent excessive ligand-induced activation of downstream effectors. This paradigm has also been expanded to other receptors, including those for transforming growth factor-β (TGFβ), cytokines, Wnt and Notch. Furthermore, a functionally important relationship between signalling and endocytosis has been convincingly established in worm, fly, zebrafish, frog and mouse models.
It is increasingly clear that endocytosis has many effects on signal transduction and, conversely, that receptor signalling regulates the endocytic machinery. This has blurred traditional lines that separate signalling and endocytosis at both the mechanistic and functional levels. Several cellular proteins that function in signalling and endocytosis have also been identified. This limits the degree to which signalling and endocytic machineries can be independently manipulated experimentally, which makes it challenging to precisely elucidate specific relationships between these machineries. There is now a vast collection of published primary literature on this topic, and numerous recent reviews have focused on its various aspects1,2. Instead, in this Review we discuss a limited subset of studies that illustrate the major features of the signalling–endocytosis nexus and represent the field more generally. We also attempt to organize individual examples in a way that emphasizes common mechanistic or functional themes. Although this approach may understate the full diversity and complexity of the signalling–endocytosis nexus, it allows us to describe the fundamental principles that apply across individual studies and systems. We suggest that the traditional duality of signalling and endocytosis, which is based on their classification as two independent processes, is beginning to give way to a view that these cellular processes are based on intertwining molecular networks.
Mechanisms of receptor endocytosis
The endocytosis of many signalling receptors is stimulated by ligand-induced activation. Activated signalling receptors use the same basic endocytic machinery as other endocytic cargo, as previously reviewed3–5 (BOX 1). Both the RTK epidermal growth factor receptor (EGFR) and various GPCRs, such as the β2-adrenergic receptor (β2AR), undergo rapid endocytosis through clathrin-coated pits. Indeed, there is now evidence that virtually every family of signalling receptor undergoes clathrin-dependent endocytosis (CDE), and such evidence has been predominantly obtained under physiological conditions. At the same time, there is also evidence showing that some receptors, including GPCRs, RTKs, TGFβ, Wnt and Notch receptors, undergo clathrin-independent endocytosis (CIE). However, the precise mechanisms of CIE and the extent of its contribution to receptor endocytosis in vivo remain unclear and are thus not discussed in detail in this Review.
Box 1. Pathways of receptor endocytosis.
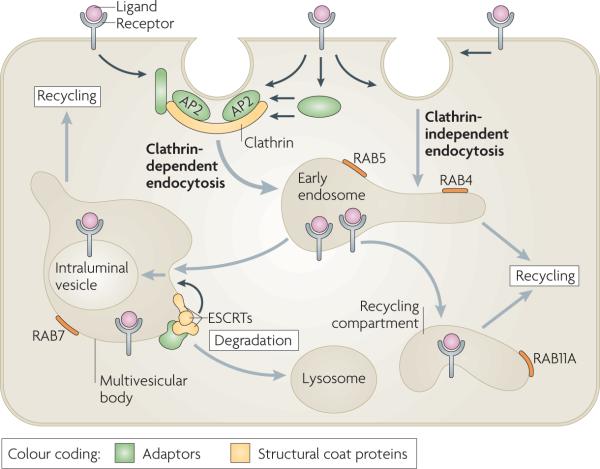
Endocytosis involves the capture of transmembrane proteins and their extracellular ligands into cytoplasmic vesicles that are pinched off from the plasma membrane (see the figure). The best-studied pathway of receptor internalization is mediated by clathrin-coated pits. These are small areas of the plasma membrane that are covered from the cytoplasmic surface with clathrin triskelions, which consist of three clathrin heavy chains and three clathrin light chains assembled into the polyhedral clathrin lattice. Receptors are recruited to clathrin-coated pits by directly interacting with the clathrin coat adaptor complex AP2 or by binding to other adaptor proteins, which in turn interacts with the clathrin heavy chain and/or AP2. Clathrin-coated pits invaginate inwards with the help of several accessory proteins and pinch off to form a clathrin-coated vesicle in a process that requires the GTPase dynamin. Several clathrin-independent pathways of endocytosis also exist, although the precise mechanisms and structural components involved in these pathways are not well understood.
Endocytic vesicles derived from both clathrin-dependent and clathrin-independent endocytosis fuse with early endosomes. Endosomal trafficking is controlled by several Rab proteins — small GTP-binding proteins of the Ras superfamily. Each GTP-bound Rab protein resides in a particular type of endosome and functions by recruiting specific effector proteins. Following their internalization into early RAB5-containing endosomes, receptors can rapidly recycle back to the plasma membrane by a RAB4-dependent mechanism, traffic to the recycling compartment that contains RAB11A or remain in endosomes, which mature into multivesicular bodies (MVBs) and late endosomes. MVBs are defined by the presence of intraluminal vesicles (ILVs) that are formed in a process of inward membrane invagination involving ESCRT (endosomal sorting complex required for transport) complexes. Early-to-late endosome maturation involves the acquisition of RAB7 and the removal of endosomal components that are capable of, and necessary for, recycling. In the MVBs, cargo destined for degradation is incorporated into ILVs. Fusion of late endosomes and MVBs with lysosomes carrying proteolytic enzymes results in cargo degradation.
Adaptor proteins in receptor endocytosis
Some RTKs and GPCRs, interferon (IFN), TGFβ and other receptors are thought to be recruited into clathrin-coated pits by the direct interaction of tyrosine- and di-leucine-based motifs in their cytoplasmic domains with the clathrin adaptor protein complex AP2 (REFS 4,6,7). Several other adaptor proteins, such as Epsin, epidermal growth factor receptor substrate 15 (EPS15) and Dishevelled are thought to link signalling receptors to a clathrin coat4,8,9. However, the molecular mechanisms regulating the CDE of many signalling receptors are not fully elucidated. The CDE of various GPCRs by β-arrestins (or non-visual arrestins) is among the most clearly established examples. β-arrestins bind to both activated GPCRs and to the clathrin coat components clathrin heavy chain and AP2, thus functioning as endocytic adaptors. This adaptor function is promoted by the ligand-induced activation of receptors and by GPCR kinase-induced phosphorylation of cytoplasmic serine and threonine residues in the receptor10,11. Activated phosphorylated GPCRs are thought to induce a conformational change in β-arrestins, which exposes their coat-binding helical domain and allows them to bind to receptors with high affinity12. β-arrestins also bind to phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2)13, which promotes the plasma membrane recruitment of β-arrestins and increases their endocytic activity. β-arrestins are regulated by phosphorylation and dephosphorlyation and they can undergo ubiquitylation that is catalysed by MDM2 (REF. 14), which also increases their membrane recruitment by an unknown mechanism.
Ubiquitylation in receptor endocytosis
Many signalling receptors are themselves modified by ubiquity lation (BOX 2). Monoubiquitylation of the GPCR Ste2 is known to promote endocytosis and vacuolar delivery in budding yeast15. Subsequent studies of various GPCRs and RTKs in animal cells suggested that ubiquitylation is not essential for their CDE. However, ubiquitin could serve as one of the clathrin-coated pit targeting signals for EGFR, high affinity nerve growth factor receptor (NTRK1; also known as TRKA) and Notch16–18. Typically, E3 ubiquitin ligases containing RING domains or HECT domains are recruited to activated receptors directly or through an intermediate adaptor. For example, growth factor receptor-bound protein 2 (GRB2) acts as an adaptor to recruit the E3 ubiquitin ligase Casitas B lineage lymphoma (CBl) to EGFRs and MET receptors19,20. ubiquitylated receptors are presumably recruited to clathrin-coated pits by interacting with ubiquitin-binding domains (UBDs) that are present in Epsin and EPS15 (REF. 21). ubiquitylation can also regulate the accessibility of receptor internalization motifs to AP2 (REFS 7,22).
Post-endocytic receptor trafficking
After internalization, signalling receptors are sorted to recycling and endosomal degradation pathways in the same way as other endocytic cargo (BOX 1). Although recycling has been considered a default cargo route from endosomes, it is now established that specific sequence motifs and specific interactions control the recycling of some signalling receptors23. An important feature of the endosomal sorting of many signalling receptors is the efficient targeting of these receptors to late endosomes and lysosomes for degradation. Ligand-induced ubiquitylation has a key role in the lysosomal targeting and downregulation of many signalling receptors. Ubiquitin-directed sorting into multivesicular bodies (MVB) is mediated by a set of multiprotein complexes that are associated with the endosomal membrane, which are collectively referred to as ESCRT 0 (endosomal sorting complex required for transport 0), ESCRT I, ESCRT II and ESCRT III (reviewed in REFS 24,25). One of the main components of ESCRT 0 is the HRS–STAM (hepatocyte growth factor-regulated tyrosine kinase substrate–signal transducing adapter molecule) complex, which is thought to interact directly with ubiquitylated receptors through the UBD of HRS and to facilitate the recruitment of ESCRT I to the MVB membrane. The ESCRT I–ESCRTIII complexes are required for the formation of intraluminal vesicles (ILVS) of MVBs (achieved by involution of the limiting membrane) and to recruit enzymes that deubiquitylate receptors before they are packaged into ILVs. ubiquitylation has thus emerged as a major post-translational modification that controls the trafficking of diverse signalling receptors after endocytosis.
Box 2. The ubiquitylation pathway.
Ubiquitylation is a post-translational modification that mediates the covalent conjugation of ubiquitin, a highly conserved protein of 76 amino acids, to substrate proteins126. The process of ubiquitylation involves the sequential action of E1 ubiquitin-activating enzymes, E2 ubiquitin-conjugating enzymes and E3 ubiquitin ligases that transfer ubiquitin to a lysine residue of the substrate.
Attachment of a single ubiquitin moiety to a single or multiple lysine residues on a substrate protein results in monoubiquitylation or multi-monoubiquitylation, respectively. Additional ubiquitin molecules can be attached to the lysine residues in ubiquitin itself, which leads to the formation of diubiquitin and polyubiquitin chains that are conjugated to a single lysine of the substrate. Although ubiquitin contains seven lysine residues, all capable of conjugating ubiquitin, lysine 48- and lysine 63-linked chains are the most abundant. Lysine 48-linked chains serve as a recognition signal for the proteasome and therefore target proteins for proteasomal degradation127. by contrast, lysine 63-linked ubiquitin chains typically do not target proteins to the proteasome but instead mediate interactions with the protein machinery that is involved in endocytic trafficking, the inflammatory response, protein translation and DNA repair. Similarly, monoubiquitylation does not target proteins to the proteasome but serves as a molecular recognition signal in membrane trafficking and possibly other cellular processes128. Several studies in mammalian cells and yeast indicate that the major internalization and endosomal sorting determinant is lysine 63-linked polyubiquitin rather than monoubiquitin, but there is evidence that both types of ubiquitylation can function in endocytosis.
The functions of ubiquitin are carried out by specific interactions with ubiquitin-binding domains (UBDs) that are found in many proteins. All of the helical UBDs interact with hydrophobic isoleucine 44 in ubiquitin, although there are several types of UBD that have different modes of recognition for monoubiquitin and polyubiquitin129. The isopeptidases responsible for the removal of ubiquitin from substrate proteins are called deubiquitylation enzymes130.
Endocytosis attenuates cell signalling
Ligand-induced endocytosis of signalling receptors is thought to be an important mechanism for negatively regulating signalling from the cell surface. Receptor endocytosis can attenuate the strength or duration of many plasma membrane-regulated signalling processes by physically reducing the concentration of cell surface receptors accessible to the ligand (FIG. 1a). In some cases, a reduction in the number of surface receptors does not attenuate the maximal signalling response that can be elicited by a ligand but instead shifts the dose response relationship so that a higher concentration of ligand is required to trigger a response of the same magnitude. This is of physiological importance in settings of limited ligand concentration. It is also of translational interest because it may contribute to time-dependent changes in the required doses of various drugs that are used in clinical practice.
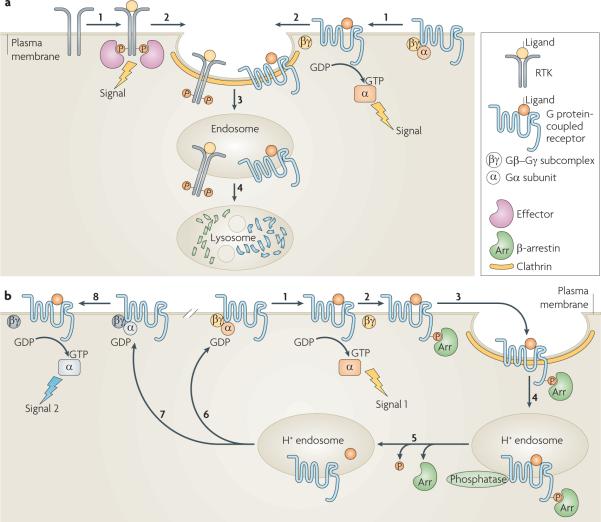
Figure 1. Endocytosis regulates signalling from the plasma membrane.
a | Schematic depicting signal attenuation. Ligand-induced activation of receptor tyrosine kinases (RTKs) or G protein-coupled receptors (GPCRs) promotes signalling from the plasma membrane by the receptor-mediated recruitment of signalling effectors to phosphorylated RTKs, or the activation of G proteins associated with GPCRs when the Gα subunit is bound to GTP (step 1). GPCR signalling can be mediated both by the GTP-bound Gα subunit (as depicted) and by the Gβ–Gγ subcomplex. Receptor recruitment into coated pits (step 2) and clathrin-dependent endocytosis (step 3) attenuate signalling by separating the receptors from plasma membrane-delimited substrates and/or mediators. Some receptors traffic to lysosomes after endocytosis, which results in their downregulation by proteolysis and further attenuates signalling (step 4). b | Schematic depicting signal desensitization, re-sensitization and pathway switching. The ability of some GPCRs to activate trimeric G proteins (signal 1; step 1) is attenuated before endocytosis by receptor phosphorylation and β-arrestin binding (step 2). Such desensitized receptor–β-arrestin complexes concentrate into clathrin-coated pits (step 3) and are endocytosed into acidic (H+) endosomes (step 4), which promotes various events that may include (depending on the receptor and cell type) ligand dissociation or destruction, dissociation of β-arrestin and phosphatase-catalysed dephosphorylation (step 5). Recycling (step 6) restores the receptors to the cell surface, re-sensitizing the cell for another round of signalling. In some cases GPCR recycling inserts receptors into a different G protein-containing environment (step 7), which produces a `switch' in signalling specificity following subsequent receptor activation (signal 2; step 8).
The endocytosis of signalling receptors can occur non-uniformly, such as in the regulation of the directional invasive migration of border cells during Drosophila melanogaster oogenesis26. RTK signalling is localized at the leading edge of border cells to convey guidance cues. Signalling by RTKs is delocalized in the absence of CBL, which is necessary for RTK endocytosis, and this results in severe cell migration defects. This suggests that RTK endocytosis is inefficient in the absence of CBL and therefore signalling is not attenuated at the leading edge. Thus, endocytosis ensures a localized response to guidance cues by spatially restricting signalling.
Receptor endocytosis can also attenuate cell signalling by separating the receptors from plasma membrane-delimited substrates or mediators (FIG. 1a). GPCR signalling through plasma membrane potassium channels, which is mediated by membrane-bound β- and γ-subunits of trimeric G proteins, requires that receptors and G proteins are present in the same membrane. GPCR-mediated signalling through phospholipase Cγ (PLCγ) requires its substrate PtdIns(4,5)P2, which is localized primarily in the plasma membrane27. Similarly, PLCγ1 and phosphoinositide 3-kinase (PI3K) signalling by EGFR is inhibited by receptor internalization owing to the lack of their lipid substrate, PtdIns(4,5)P2, in endosomes28. In the specialized case of Notch signalling (see BOX 3), in which the ligand is a transmembrane protein, the accessibility of the ligand to the receptor is also regulated by ligand endocytosis.
Box 3. Regulation of Notch signalling by endocytosis.
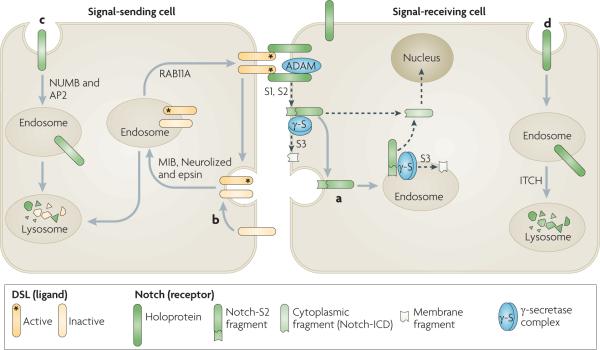
Dsl (Delta/Serrate/lag2 domain) proteins, which are Notch ligands, are transmembrane proteins that are present in the plasma membrane of a signal-sending cell. Dsl proteins interact with Notch located at the surface of a signal-receiving cell, which results in proteolytic S1 and S2 cleavages of the extracellular domain of Notch mediated by a disintegrin and metalloprotease (ADAM) enzyme and generates a Notch extracellular truncation fragment (Notch-S2) (see the figure, part a). Notch-S2 is further subject to intramembrane S3 cleavage by the γ-secretase complex, either in the plasma membrane or after endocytosis, to generate a membrane fragment and the cytoplasmic intracellular domain fragment (Notch-ICD), which translocates to the nucleus to activate the transcription of target genes. Evidence in Drosophila melanogaster and mammalian cells suggests that endocytosis is necessary for the S3 cleavage of recombinant and endogenous Notch16,76. Although γ-secretase is present in both the plasma membrane and endosomes131,132, it has optimal activity at the low pH levels of endosomes and lysosomes7,8,133.
The surface concentration of functional Dsl proteins is crucial for physiological levels of Notch signalling, and this is regulated by endocytosis (reviewed in REF. 134). The endocytosis and recycling of the Notch ligand Delta in signal-sending cells is also required for Delta activation, although the mechanism is not understood. Four E3 RING ubiquitin ligases, MIB1 and MIB2 and Neurolized 1 and Neurolized 2, as well as Epsin and RAB11A, are implicated in Dsl internalization and recycling (see the figure, part b)135–137.
The endocytic adaptors NUMB and AP2 promote the endocytosis of non-ligand bound Notch and its degradation in the lysosome of the signal-sending cell (see the figure, part c). In the D. melanogaster pupal retina, Notch is endocytosed and directed to late endosomes and lysosomes in the signal-receiving cell (see the figure, part d)138–140. In mammalian cells targeting of non-ligand bound Notch to lysosomes is promoted by the lysine 29-linked polyubiquitylation of Notch, which is mediated by the HECT domain-containing E3 ligase ITCH (also known as AIP4)141.
Sustained signalling in endosomes
Endosomes have numerous unique properties that together allow endosomal membranes to serve as important signalling platforms during signal transduction from various receptors. These properties include: a small volume that favours ligand–receptor association and the maintenance of receptor activity; a long residence time of active receptors with slow endosomal sorting; the ability to use microtubular transport to move for long distances and towards the nucleus; an enrichment in phosphatidylinositol 3-phosphate (PtdIns3P), which allows the assembly of complexes involving FYVE domain and PX domain-containing proteins29; the presence of specific resident proteins that can be used to assemble specific scaffold complexes; and an acidic pH, especially in late endosomes, which favours the activity of pro teolytic enzymes that participate in signalling. Numerous studies support the hypothesis that endosomal signalling can occur, and the types of endosomal signalling proposed can be divided into two groups: signalling that can take place in endosomes but can also occur at the plasma membrane (discussed in this section) and signalling that requires receptor endocytosis and/or occurs exclusively on endosomal membranes (discussed in the next section). It should be emphasized, however, that this division of endosomal signalling is not always straightforward. Endosomal signalling with an outcome that is qualitatively similar to that of plasma membrane signalling can be mediated by endosome-specific signalling complexes. By contrast, several examples of endosome-specific signalling are challenged by evidence indicating that similar events also occur in the plasma membrane.
Continuous RTK signalling in endosomes
In systems in which active receptors are rapidly internalized, the ability of a receptor to signal after endocytosis is important to ensure the sufficient duration and intensity of signalling — especially in vivo, where ligand is often limited. However, this capacity requires receptors to remain active in endosomes. Several RTKs, for example EGFR, remain ligand bound, phosphorylated and active in endosomes until late stages of endosomal trafficking30. The detection of all the components of the extracellular signal-regulated kinase (ERK)–mitogen-activated protein kinase (MAPK) activation cascade, including GRB2, SH2 domain-containing transforming protein (SHC), son of sevenless (SOS), Ras proteins, RAF, MAPK/ERK kinase 1 (MEK1) and MEK2, in endosomes, provides compelling evidence that EGFR can continue to signal following endocytosis (FIG. 2a). However, it has been challenging to unequivocally show that the endocytosis of EGFR and other RTKs is necessary for the full activation of ERK. Some experiments using dominant-negative mutants and small interfering RNAs (siRNAs) to target proteins involved in endocytosis suggest that this process is required for ERK activation by several RTKs31–34, but many similar experiments reach the opposite conclusion35–37. Although it is difficult to reconcile these data, it can be suggested that the contribution of signalling from RTKs to MAPKs in endosomes may depend on how fast internalized RTKs are sorted to degradative compartments in a particular cell type and on the specific experimental conditions.
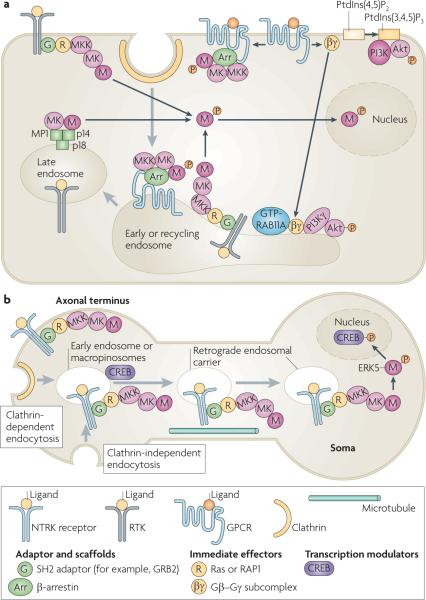
Figure 2. Signalling processes that begin at the cell surface and continue in endosomes.
a | Activation of mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K)–Akt signalling cascades by receptor tyrosine kinases (RTKs) and G protein-coupled receptors (GPCRs) occurs at the plasma membrane and in early, recycling and late endosomes. In all of these locations activated, phosphorylated MAPK dissociates from the MAPK kinase (MK), unless stably associated with a scaffold protein such as β-arrestin, and phosphorylates substrates in the cytoplasm and nucleus. When GPCRs are stimulated by lysophosphatidic acid, the activated G protein subcomplex Gβ–Gγ can activate PI3K and Akt at the plasma membrane and also translocate to the early and recycling endosomes to activate endosomal PI3K γ-isoform, which leads to the phosphorylation and activation of Akt in endosomes. b | Model of retrograde endosome signalling in neurons. Ligand-bound nerve growth factor receptors (NTRKs) are internalized by clathrin-dependent and clathrin-independent endocytosis, with associated components of the MAPK signalling cascade, into early endosomes or macropinosomes that have a multivesicular body-like morphology. NTRK signalling complexes are delivered to the soma in retrograde endosomal carriers, which are a population of early or late endosomes and macropinosomes, by dynein motor-mediated microtubular transport. The MAPK extracellular signal-regulated kinase 5 (ERK5) is then phosphorylated and activated in the soma. It phosphorylates the cAMP responsive element-binding protein (CREB), which regulates the transcription of anti-apoptotic genes. Grey arrows show trafficking pathways. Black arrows show protein translocations or complex assembly. GRB2, growth factor receptor-bound protein 2; MP1, MEK1 partner 1; PtdIns(3,4,5)P3, phosphatidylinositol-3,4,5-trisphosphate; PtdIns(4,5)P2, phosphatidylinositol-4,5-bisphosphate; SH2, Src homology 2.
The requirement of endocytosis for full ERK1 and ERK2 activation can be explained by the existence of a MAPK scaffold complex in late endosomes (FIG. 2a). This complex consists of MEK1 partner 1 (MP1) and p14 protein38. The MP1–p14 complex is anchored to the endosomal membrane by the adaptor p18 and binds MEK1, ERK1 and ERK2, thus facilitating the phosphorylation of ERK1 and ERK2 (preferentially ERK1) by MEK1 (REF. 39). Knockdown of p14 in cultured cells, or genetic knockout of p14 or p18 in mice, decreases the basal activity of MEK1, MEK2, ERK1 and ERK2 (REFS 38–40). The phenotype of mice in which p14 or p18 have been knocked out is somewhat different in that p14 seems to be necessary for prolonged ERK activity, whereas p18 is not necessary for EGF-mediated ERK1 and ERK2 activation, but instead is required for the maximal phosphorylation of MEK1 and MEK2 at early time points after EGF stimulation. In addition, it has been shown that MP1–p14 regulates p21-activated kinase 1 (PAK1)-dependent ERK activation during adhesion and cell spreading, but that this complex is not required for ERK activation by platelet-derived growth factor41. Thus, the role of the MP1–p14 complex in ERK regulation varies depending on the type of signalling system.
Continuous GPCR signalling in endosomes
It has also been proposed that the activation of MAPK cascades by GPCRs occurs from endosomes. Early evidence came from experiments using mutant dynamin to inhibit CDE, which showed that the endocytosis of the GPCR β2AR is required for full activation of ERK1 and ERK2 by the β2AR agonist isoproterenol42. Subsequently it was found that certain GPCRs remain associated with β-arrestins in endosomes43 and that β-arrestins can bind to various kinases in MAPK signalling cascades44. This led to the development of a `signalling endosome' hypothesis for the GPCR-mediated activation of ERK1, ERK2 and Jun N-terminal kinase. This concept is similar to that of RTK signalling described above, as β-arrestins function as membrane-associated scaffolds of MAPK modules and other protein kinase-dependent signalling processes on endosomes (FIG. 2a). It is also proposed that, by stably anchoring ERK to endosomes, β-arrestins can bias signalling towards predominantly cytoplasmic, rather than nuclear, ERK substrates45. In many cases it remains unclear whether signalling in which β-arrestin acts as a scaffold occurs from the endosomal membrane or the plasma membrane. Evidence that β-arrestin recruitment to the plasma membrane can also activate ERK1 and ERK2 (REF. 46) indicates that both situations probably occur. It should also be noted that, as for RTKs, different experiments show conflicting results regarding the requirement of endocytosis for GPCR signalling to MAPKs. It is likely that GPCRs can activate ERK both from the plasma membrane and endosomes, and that the contribution of endosomal signalling is proportional to the residence time of a GPCR–β-arrestin complex in an endosome.
Activation of the PI3K–Akt signalling axis is generally considered to occur at the cell surface, where stimulation of the GPCR by lysophosphatidic acid results in the activation of PI3K and Akt; this is mediated by the G protein Gβ1γ2 (REF. 47) (FIG. 2a). It is proposed that Gβ1γ2 can also translocate to endosomes, where it binds the small GTPase RAB11A and activates an endosomal pool of the PI3K γ-isoform and Akt, thus increasing the proliferative and anti-apoptotic effects of stimulation of the GPCR by lysophosphatidic acid47.
Retrograde endosome signalling in neurons
The distance between the site of receptor activation and effector function can be remarkably long in neuronal cells. Neurotrophins, such as nerve growth factor (NGF) and brain-derived nerve factor, are produced by post-synaptic cells. They activate pre-synaptic NTRKs in distal axonal termini to sustain survival signalling in various types of neuron. The signal must be transported from distal axons to the soma to promote the transcription of anti-apoptotic genes. Passive diffusion of signalling effectors is far too slow to account for such retrograde signalling. A prevailing model of retrograde signalling proposes a dynein-mediated microtubular transport of signalling endosomes containing activated NRTK signalling complexes to the soma (FIG. 2b). This model is based on several lines of experimental evidence. First, the retrograde transport of NGF-containing endosomes has been directly observed in living neurons48,49. Second, endocytic vesicles and endosomes isolated from rat pheochromocytoma PC12 cells and sciatic neurons contain phosphorylated NTRK1, PlCγ1, PI3K and proteins that are involved in the activation of Ras–ERK or RAP1– ERK signalling cascades50–53. Third, retrograde survival signalling that is induced by NGF and is applied to distal axons in compartmentalized neuronal cultures can be blocked by the expression of a dynamin mutant or dynamitin, which inhibit CDE and dynein-dependent transport, respectively54,55. Interestingly, endocytosis and retrograde transport of NGF were necessary for NGF-induced activation of ERK5 and phosphorylation of the cAmP responsive element-binding protein (CREB) in the neuronal soma. By contrast, activation of NTRK1 by NGF binding in the soma resulted in the activation of ERK1 and ERK2, which was not sufficient for the phosphorylation of CREB and the induction of survival signalling55.
In PC12 cells and sciatic and sympathetic neurons NTRK1 and NTRK2 are internalized by CDE, and signalling endosomes containing internalized NTRK have the early endosomal markers RAB5 and early endosome antigen 1 (EEA.1). This suggests that retrograde endocytic carriers are early endosomes51,55–57. An alternative model was proposed on the basis of the detection of the late endosomal marker RAB7 in retrograde carriers isolated from mouse motor neurons58. This study suggested that retrograde transport requires the replacement of RAB5 by RAB7 on the endosomal membrane and that retrograde carriers are a pool of late endosomes.
Another possible scenario of retrograde endosomal transport involves the internalization of activated NTRK1 by a clathrin-independent mechanism that requires plasma membrane ruffling and the expression of EH-domain containing 4 (EHD4; also known as pincher in rats)59,60. EHD4 mediates the formation of large macropinosomes of a heterogeneous shape that contain Ilvs, which is characteristic of mvBs, and are transported to the soma.
Although the existence of receptor-carrying retrograde endosomes is widely accepted, it is proposed that retrograde signalling, at least in some cases, can be mediated by the transport of downstream signalling effectors without transporting NGF and NTRK1 (REF. 61). Whether such transport involves endosomes is not established. Interestingly, it has been recently reported that CREB can be translated in distal axons and transported to the soma on signalling endosomes62.
Endosome-specific signalling
By providing a platform for the assembly of specific signalling complexes, endosomal membranes support signalling processes that cannot occur, or occur with low efficiency, at the plasma membrane. Several examples of endosome-specific signalling are described in this section.
TGFβ signalling in endosomes
The ability of endosomes to recruit proteins containing PtdIns3P-binding domains is used during TGFβ receptor signalling. Internalization of TGFβ receptors — heterotetramers consisting of a type I receptor dimer and a type II receptor dimer — allows the type I receptor to interact with the FYVE domain-containing adaptor SARA (SMAD anchor for receptor activation) in early endosomes63,64 (FIG. 3a). SARA is also associated with the receptor target SMAD2, and this allows the efficient phosphorylation of SMAD2 by the TGFβ receptor in endosomes. SMAD2 then dissociates from the complex and interacts with SMAD4, which results in the translocation of this SMAD2–SMAD4 complex to the nucleus, where it regulates gene transcription. Another FYVE-domain containing protein, endofin, interacts with TGFβ type I receptors and SMAD4, and therefore potentiates TGFβ signalling by facilitating the formation of a SMAD2–SMAD4 complex in endosomes65. However, it has been reported that SMAD signalling can be initiated from the plasma membrane and in some cases does not require endocytosis66,67.
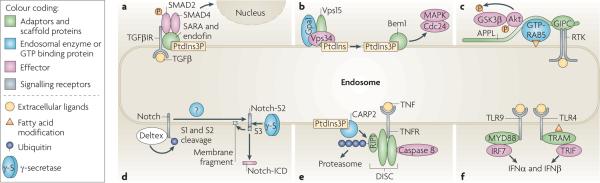
Figure 3. Signalling complexes specifically assembled in endosomes.
a | Endosomal recruitment of SMAD2 and SMAD4 by the FYVE domain adaptors SARA (SMAD anchor for receptor activation) and endofin, respectively, allows efficient phosphorylation of SMAD2 by internalized transforming growth factor-β receptor (TGFβR) and the formation of active SMAD2–SMAD4 complexes. b | Following the activation of the yeast protein Ste2, the GTP-binding α1 subunit (Gpa1) of the trimeric G protein complex binds to the endosomal protein Vps15. This activates the yeast phosphoinositide 3-kinase Vps34, which converts phosphatidylinositol (PtdIns) to phosphatidylinositol-3-phosphate (PtdIns3P). The FYVE domain-containing protein bud emergence protein 1 (Bem1) is subsequently recruited to PtdIns3P, where it potentiates the activation of Cdc42 and mitogen-activated protein kinase (MAPK). c | APPL1 (adaptor protein, phosphotyrosine interaction, PH domain and leucine zipper-containing 1) and APPL2 are targeted by GTP-bound RAB5 to early endosomes. They can also directly, or indirectly through GIPC (GAIP-interacting protein, C-terminus), interact with receptor tyrosine kinases (RTKs). Activated Akt and GSK3β (glycogen synthase kinase 3β) transiently associate with APPL, which leads to the phosphorylation of GSK3β in endosomes. d | Deltex promotes the ubiquitylation, endocytosis and accumulation of full-length Notch in late endosomes. The S1 and S2 Notch cleavages may be mediated by proteolytic enzymes in the late endosomes, and the Notch intracellular domain fragment (Notch-ICD) is released by γ-secretase present in late endosomes. e | Death-inducing signalling complex (DISC) is recruited to ligand-bound tumour necrosis factor receptor (TNFR). Receptor interacting protein 1 (RIP1), a component of DISC, is polyubiquitylated by the FYVE domain-containing E3 ligase CARP2 (caspases 8 and 10-associated RING finger protein) in endosomes and degraded by the proteasome, which allows the recruitment and activation of caspase 8. f | Activated Toll-like receptor 4 (TLR4) binds to the adaptor complex formed by myristoylated TRIF-related adaptor molecule (TRAM) and TIR-domain-containing adaptor protein inducing IFNβ (TRIF) only in endosomes, which allows the transcription of type I interferon (IFN) genes. Sustained interaction of ligand–TLR9 complexes with the adaptor protein myeloid differentiation primary response protein 88 (MYD88) and IFN regulatory factor 7 (IRF7) in endosomes is necessary for a potent type I IFN response.
GPCR signalling in endosomes
Specific targeting of the FYVE domain to early endosomes is also exploited by the yeast GPCR Ste2 to generate endosome-specific signalling68. Activation of Ste2 results in the activation of the trimeric G protein complex at the plasma membrane through the release of the Gβ–Gγ subcomplex from the GTP-binding α1 subunit (Gpa1); this mediates MAPK signalling from the plasma membrane. At the same time Gpa1 translocates to endosomes and, following GTP hydrolysis, can bind to the endosome-associated protein Vps15, which is structurally homologous to G protein β-subunits and is associated with the PI3K Vps34 (FIG. 3b). Gpa1 can subsequently be activated at the endosome by the cytoplasmic guanine nucleotide exchange factor Arr4, and GTP-bound Gpa1 is thought to activate Vps34 kinase activity, which converts PtdIns to PtdIns3P. This increases the concentration of PtdIns3P in endosomes, thereby promoting the recruitment of the FYVE domain-containing adaptor bud emergence protein 1 (Bem1) and other PtdIns3P-binding proteins that subsequently potentiate the activation of MAPKs and Cdc42 signalling cascades.
Two recent studies suggest that mammalian GPCRs may also signal from endosomes through trimeric G proteins, in a similar way to how GPCR signalling occurs from the plasma membrane. An immunosuppressive drug produces persistent endocytosis and Gi-mediated signalling of the sphingosine 1-phosphate GPCR69. Gs-mediated signalling of the thyroid-stimulating hormone GPCR is enhanced by receptor endocytosis, and a putative receptor-containing signalling endosome can be resolved by subcellular fractionation70.
RTK signalling in endosomes
Specific signalling complexes can be assembled through their recruitment to the early endosomal resident protein RAB5. It has been proposed that endosomes containing EGFR, RAB5 and the two homologous RAB5 effectors APPL1 (adaptor protein, phosphotyrosine interaction, PH domain and leucine zipper-containing 1) and APPL2 can serve as specialized signalling endosomes71. APPLs are anchored to the endosomal membrane by interacting with GTP-bound RAB5, as well as through their PH and BAR (Bin1/amphiphysin/Rvs167) domains, and they can also directly or indirectly interact with RTKs and other receptors72 (FIG. 3c). APPL-containing endosomes are a population of early endosomes that contain RAB5 but lack EEA.1; it was therefore proposed that APPLs and EEA.1 compete for binding to GTP-bound RAB5 (REF. 73). Acquisition of PtdIns3P through the recruitment and activity of human VPS34 (also known as PIK3C3), a homologue of the yeast Vps34 kinase that converts PtdIns into PtdIns3P, results in the accumulation of EEA.1 and the concomitant dissociation of APPLs in maturing early endosomes. Strikingly, downregulation of APPL1 by morpholinos in zebrafish leads to widespread apoptosis during development74. This effect is mediated by the inactivation of the Akt-GSK3β (glycogen synthase kinase 3β) signalling axis and can be rescued by the expression of functional APPL1. A small pool of Akt and GSK3β is found to transiently associate with endosomes. By contrast, the Akt-mTOR (mammalian target or rapamycin) signalling axis does not seem to be affected by APPL1 knockdown and does not involve endosomal signalling. In cultured mammalian Hela cells, however, APPL-containing endosomes seem to be necessary for ERK1 and ERK2 activation and Akt signalling to both GSK3β and mTOR73. In sympathetic neurons and PC12 cells, recruitment of APPL proteins, which interact with NTRK1 directly or indirectly through the adaptor protein GIPC (GAIP-interacting protein, C-terminus)72,75, to endosomal NTRK1 was also necessary for the activation of both ERK and Akt, and for NGF-induced neurite outgrowth75. Studies in zebrafish provide a strong case for a specific role for APPL in Akt anti-apoptotic signalling, but the mechanisms by which APPLs control ERK1 and ERK2 activation are not clear. It is possible that an increase in APPL concentration in endosomes could delay RTK trafficking and therefore result in increased ERK activity.
Notch signalling in endosomes
Several signalling systems take advantage of the presence and increased activity of transmembrane proteolytic enzymes in endosomes to carry out their function. For instance, it is proposed that the co-compartmentalization of Notch and γ-secretase in endosomes is necessary for the efficient S3 cleavage of the Notch intracellular domain and for physiological levels of Notch signalling16,76 (BOX 3).
Notch can also be activated in a ligand-independent fashion by the overexpression of the E3 ubiquitin ligase Deltex. Deltex promotes Notch ubiquitylation and endocytosis, leading to the accumulation of full-length Notch in MVBs that contain markers of late endosomes, which is required for Notch signalling during D. melanogaster embryonic development77 (FIG. 3d). It has been proposed that Notch ectodomain shedding is carried out in late endosomes by proteolytic enzymes in the lumen of these compartments. This is followed by the release of the Notch intracellular domain fragment by γ-secretase, which is thought to accumulate in the limiting membrane of late endosomes and lysosomes78. Interestingly, Deltex prevents the incorporation of Notch into the ILVs of MVBs by an unknown mechanism, thus making Notch accessible to γ-secretase and allowing the Notch intracellular domain fragment to be released into the cytoplasm.
Tumour necrosis factor signalling in endosomes
A combination of co-compartmentalization with a proteolytic enzyme in endosomes and endosomal FYVE–PtdIns3P interactions is used by the tumour necrosis factor receptor (TNFR) to promote apoptotic signalling (FIG. 3e). The components of the death-inducing signalling complex (DISC), FAS-associated death domain (FADD), TNFR-associated death-domain (TRADD) and receptor interacting protein 1 (RIP1), are recruited to the ligand-bound TNFR at the plasma membrane79. However, the cysteine protease caspase 8 is recruited to the DISC and activated by its autoproteolytic cleavage in endosomes, leading to apoptosis. The mechanisms that prevent caspase 8 activation at the plasma membrane are not known. Binding of caspase 8 to the DISC in endosomes seems to be accompanied by the removal of RIP1 from the complex. RIP1 is polyubiquitylated by the FYVE domain-containing E3 ligase CARP2 (caspases 8 and 10-associated RING finger protein; also known as RFLL) in endosomes and degraded by the proteosome80. It is further proposed that incorporation of the receptor–DISC–caspase 8 complex into ILVs of MVBs leads to the cleavage of pro-cathepsin D and the activation of neutral sphingomyelinase, which promotes additional apoptotic signalling involving caspase 9 (REF. 79). However, it is unclear how the DISC–caspase 8 complex, which would be located inside ILVs as a result of MVB involution, can gain access to pro-cathepsin D that is located in the lumen of MVBs.
Toll-like receptor signalling in endosomes
Several signalling systems use interactions that are based on the enrichment of PtdIns3P in endosomes for their function, whereas Toll-like receptor 4 (TLR4) takes advantage of the fact that another phospholipid, PtdIns(4,5)P2, is depleted in endosomes to switch from surface to endosome-specific signalling. The assembly of a ligand-binding complex of TLR4 is induced by the presence of lipopolysaccharide at the cell surface81. Subsequently, activated TLR4 interacts with an adaptor complex involving TIR-domain-containing adaptor protein (TIRAP) and myeloid differentiation primary response protein 88 (MYD88), resulting in the rapid induction of the activity of the MAPK p38 and of inhibitor of NF-κB kinase-β. The interaction of TIRAP with PtdIns(4,5)P2 facilitates the formation of the TIRAP–MYD88 complex at the plasma membrane. After its internalization, the TIRAP–MYD88 complex dissociates from TLR4, probably owing to a low concentration of PtdIns(4,5)P2 in endosomes. This allows the adaptor complex involving TRIF-related adaptor molecule (TRAM) and TIR-domain-containing adaptor protein inducing IFNβ (TRIF) to bind to the same motif in the internalized TLR4, resulting in the endosome-specific activation of IFN-regulatory factors (IRFs) and the induction of IFNb (FIG. 3f). Myristoylation of TRAM facilitates the interaction of the TRAM–TRIF adaptor complex with endosomal membranes. Interestingly, several other members of the TLR family, TLR3, TLR7 and TLR9, are found mostly in endosomal compartments, where they detect viral nucleic acids that have been released after viral degradation. Indeed, in plasmacytoid dendritic cells ligand-activated TLR9 directly interacts with MYD88 and the transcription factor IRF7, and these complexes are retained for a long time in endosomes and escape lysosomal degradation82. Such a sustained localization of TLR9 in endosomes is shown to be necessary for a potent type I IFN response.
Endosomal sorting regulates signalling
Receptor-mediated signalling can be terminated by the sorting of internalized ligand-activated receptors to ILVs of MVBs, effectively insulating receptors from cytoplasmic effectors (BOX 1; FIG. 1a) and promoting receptor proteolysis83,84. Receptor degradation can also occur in the absence of the ligand. D. melanogaster mutants with enhanced ligand-independent endocytosis and lysosomal targeting of Notch (BOX 3), for example, display loss-of-function phenotypes in their wings85.
Receptor sorting to ILVs, and the subsequent trafficking to lysosomes, generally correlates with the level of receptor ubiquitylation in endosomes. Inhibiting EGFR ubiquitylation by mutating the receptor ubiquitin-conjugation sites enhances EGFR signalling86. By contrast, increasing receptor ubiquitylation by inhibiting deubiquitylation accelerates the degradation of RTKs and down-regulates signalling. Mice in which the deubiquitylation enzyme UBPY (also known as USP8) has been conditionally knocked out have reduced expression levels of several RTKs, which results in decreased rates of cell proliferation and causes liver failure in adult mice87. However, RNA interference-mediated knockdown of UBPY causes the opposite effect (reduced EGFR degradation) in cultured mammalian cells, and knockdown of the distinct endosome-associated deubiquitylating enzyme AMSH (also known as STAMBP) enhanced degradation88. It was proposed that individual deubiquitylating enzymes have different effects on lysosomal targeting depending on their specificity for hydrolysing lysine 63 and lysine 48 linkages and their role in the regulation of ESCRT ubiquitylation88. RNA interference knockdown of the ESCRT 0 ubiquitin adaptor HRS in cultured mammalian cells, or HRS loss-of-function mutations in D. melanogaster, also result in enhanced signalling by RTKs89–91. Inhibition of HRS translation by microRNAs (specifically miR296) enhances the activity of vascular endothelial growth factor receptor and increases angiogenesis92. Inhibiting the function of ESCRT I by genetic knockout or by siRNA depletion of its component tumor suppressor protein 101 increases EGFR recycling and sustains signalling to ERK1 and ERK2, similarly to inhibiting ESCRT 0 by depleting HRS. By contrast, siRNA knockdown of VPS22 (an ESCRT II component) or VPS24 (an ESCRT III component) did not affect ERK activation. This suggests that EGFR signal termination occurs after endocytosis but upstream of ESCRT II89,93.
The trafficking of internalized receptors back to the plasma membrane, rather than to lysosomes, can sustain signalling. This is observed for various GPCRs such as the β2AR, the signalling of which through trimeric G proteins is inhibited by receptor phosphorylation in the plasma membrane before endocytosis (FIG. 1b). The subsequent endocytosis and RAB4-dependent recycling of these GPCRs `re-sensitizes' receptors, apparently by promoting their dephosphorylation by the endosome-associated phosphatase 2A94,95. Although much of this work was done in cultured cell models, it has been shown that RAB4-dependent recycling is required for sustaining normal physiological responsiveness of both β1ARs and β2ARs in intact cardiac muscle96,97. This principle has been reported for two other GPCRs, the calcitonin receptor-like receptor (CLR; also known as CALCRL) and neurokinin 1 receptor (NK1R; also known as TACR1), the activating ligands of which are degraded by the metalloendopeptidase endothelin-converting enzyme (ECE1) that is present in early endosomes. RNA interference-mediated depletion of cellular ECE1 inhibited ligand proteolysis in endosomes and reduced receptor recycling. This resulted in a prolonged reduction in subsequent receptor responsiveness, which suggests that ligand proteolysis promotes efficient receptor recycling and functional resensitization98,99. Furthermore, the notion that trafficking of internalized receptors back to the plasma membrane, rather than to lysosomes, can instead sustain signalling has been reported in studies of β2AR signalling in isolated cardiac myocytes. In this case ligand-induced activation elicits a transient increase in cellular contraction rate that is mediated by receptor coupling to Gs-type trimeric G proteins, followed by a decrease mediated by receptor coupling to distinct Gi-type trimeric G proteins. This switch of G protein–receptor coupling specificity requires β2AR endocytosis and recycling, which suggests that the recycling pathway inserts receptors into specific Gi-enriched surface domains100.
A process similar to GPCR re-sensitization has been observed for RTKs when they are activated by ligands that dissociate from them in endosomes. The EGFR agonist TGFα, for example, is released from the receptor in the acidic environment of endosomes. In this case, EGFR is not significantly downregulated, and each recycled receptor can be activated by TGFα many times101.
The fate of internalized signalling receptors may also be determined by the route of their internalization. EGFRs internalized through CDE are recycled back to the plasma membrane more efficiently, and degraded less efficiently, than receptors internalized by CIE. This difference in post-endocytic sorting explains the important role of the CDE of EGFR in ERK and Akt signalling34. Similar findings for a crucial role for the CDE of TGFβ receptors in SMAD signalling have also been reported102. Specifically, it was proposed that CDE increases the intensity of signalling because it redirects TGFβ receptors from constitutive lipid raft-dependent endocytosis, which usually targets receptors for ubiquitylation and degradation.
Signalling influences endocytosis
Although there is extensive published literature reporting that endocytosis affects receptor-mediated signalling, less is known about how signalling affects endocytosis. Nevertheless, it is clear that such regulation occurs, and several examples of signal-induced alterations in endocytic processes are described below.
Regulation of CDE by signalling
Early evidence for the regulation of CDE by signalling came from morphological studies of EGFR endocytosis in sympathetic neurons. ligand-induced activation of EGFR in these cells produced a rapid increase in the total number and surface density of clathrin-coated pits103. Subsequent studies show that EGFR or NTRK1 activation increases the pool of cellular clathrin that is associated with the plasma membrane of HeLa or PC12 cells, respectively, and this depends on Src kinase activity104,105. It was proposed that Src kinases function as downstream mediators of RTKs to increase the rate of de novo clathrin-coated pit formation, by phosphorylating the clathrin heavy chain in a region of its hub domain that controls the assembly of the triskelion104 (BOX 1). Depletion of AP2 reduces the number of clathrin-coated pits in HeLa cells, and activation of EGFR in these cells leads to de novo formation of c lathrin-coated pits that contain EGFR and the adaptor GRB2 (REF. 106). These clathrin-coated pits do not contain transferrin receptors, nutrient uptake receptors that undergo constitutive endocytosis through coated pits, which suggests that EGFR activation causes the formation of cargo-specific coated pits under these conditions. However, EGF has been shown to colocalize with other ligands in clathrin-coated pits, including transferrin, and different clathrin adaptors have been shown to be uniformly distributed in clathrin-coated pits107–109. Furthermore, live-cell imaging of EGFR endocytosis showed that, in HeLa cells expressing AP2 at physio logical levels, activated EGFRs are recruited into pre-existing clathrin-coated pits110. The degree to which EGFRs can normally mediate de novo coated pit formation or can associate with a subset of clathrin-coated pits that have a specialized composition remains unresolved.
GPCRs such as the β2ARs also undergo regulated endocytosis that is mediated by agonist concentration in pre-existing clathrin-coated pits111. β2ARs were found to concentrate uniformly in clathrin-coated pits in HEK293 cells that overexpress the specific adaptor β-arrestin but, in cells expressing β-arrestin at physiological levels, ligand-activated β2ARs selectively concentrated in a fraction of clathrin-coated pits. The presence of β2ARs regulated the period for which individual coated pits remained at the cell surface, as measured by the interval between clathrin coat deposition and membrane scission, by a mechanism involving receptor scaffolding to cortical actin112.
There is also evidence that signalling can alter the clathrin machinery more generally. Early evidence for this came from the study of CDE in the pre-synaptic nerve terminal. Depolarization of the pre-synaptic terminal produces rapid dephosphorylation of numerous endocytic proteins, including dynamin and amphiphysin, which is mediated by the activation of calcineurin and enhances the CDE of pre-synaptic vesicle membrane components113,114. Activation of the Ephrin B receptor by Ephrin B results in tyrosine phosphorylation of synaptojanin 1, a phosphoinositol 5-phosphatase that is involved in the uncoating of clathrin-coated vesicles after internalization115. Tyrosine phosphorylation decreases the activity of synaptojanin 1 and, therefore, affects the clathrin-mediated endocytosis of transferrin and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors.
Regulation of endosomal sorting by signalling
Signalling can also regulate later stages in the endocytic pathway. Indeed, EGFR activation was found to slow the process of endosome maturation116 and to increase the number of ILVs in MVBs117. The biochemistry of phospho inositides is proposed to have a role in endosome maturation at many stages, including during the initial formation of early endosomes through the recruitment of EEA1 and the release of APPL on the basis of their distinct lipid-interacting specificities73.
Signalling by downstream kinases affects endocytosis and endosomal trafficking at many points. Stress-induced p38 stimulates the formation of RAB5 and GDI (GDP dissociation inhibitor) complexes, which leads to the acceleration of endocytosis, presumably by delivering RAB5 to endocytic sites at the plasma membrane or to the sites of assembly of RAB5 micro-domains in early endosomes118. Activation of p38 also promotes the internalization of EGFR that is not bound to ligand119. ERK1 and ERK2 signalling seems to regulate endosomal maturation and cargo degradation, as knocking out MEK1 in fibroblasts abolishes endosome movement to the perinuclear area of the cell and delays receptor degradation40. It has been shown recently that the A-RAF isoform of the Raf kinase family is located in recycling endosomes and is required for the activity of the small GTPase ADP ribosylation factor 6 (ARF6)120. Knockdown of A-RAF, or overexpression of dominant-negative A-RAF mutants, reduces the recycling of the transferrin receptor, although this effect was not quantified. Because MEK inhibitors also affect transferrin recycling and ARF6 activity, A-RAF seems to mediate its effects through the activation of ERK1 and ERK2. Activation of protein kinase A (PKA) by β2AR regulates a rapid pathway of RAB4-dependent recycling, without affecting a slower recycling pathway, and this regulation is mediated by the phosphorylation of the receptor itself. Surprisingly, PKA-dependent signalling also regulates recycling of the transferrin receptor that is packaged in the same vesicles, which suggests that overall membrane flux is affected through a specific recycling pathway121.
Conclusions and future directions
Thousands of proteins are involved in intracellular signalling processes, and several hundreds comprise the machinery that controls endocytosis. Some of the key players in these two groups are discussed in this Review, and some examples of proteins with a role in both signalling and endocytosis are presented in TABLE 1. An adaptor protein can have dual functions by interacting with two alternative effectors. For instance, β-arrestins mediate GPCR endocytosis by binding to clathrin and AP2 while participating in signal transduction by scaffolding the components of the MAPK pathway. Similarly, GRB2 functions in endocytosis by interacting with the E3 ubiquitin ligase CBL, whereas its function in signalling to Ras proteins is mediated by its interaction with SOS. Enzymes carry out two seemingly independent functions by modifying two alternative substrates. Kinases with a large range of substrates, such as protein kinase C and Src kinases, are involved in various signalling cascades but they can also modulate the endocytosis of receptors. Although the precise mechanisms of function of many such proteins remain to be elucidated, it is becoming increasingly clear that these proteins represent nodes at which endocytic and signalling networks substantially overlap. Such integration of molecules and processes suggests that signalling and endocytosis may behave as, and should be analysed as, a single molecular network.
Table 1.
Examples of proteins with a dual function in signalling and endocytosis
| Protein | Role in endocytosis | Role in signalling |
|---|---|---|
| β-arrestins | Mediate internalization of GPCRs by linking receptors to clathrin and AP2 (REF. 11) | Desensitize GPCRs at the plasma membrane; scaffold MAPK signalling modules44,142 |
| GRB2 | Mediates internalization and ubiquitindependent degradation of RTKs20,143,19 | Mediates activation of Ras proteins by RTKs144 |
| MP1-p14-p18 complex | Involved in late endosome biogenesis and/or the transport of endosomes to the perinuclear area of the cell40,39 | Involved in ERK1 and ERK2 activation by scaffolding MEK1 and ERK1 and ERK2 (REFS 38–40) |
| APPL1 and APPL2 | Implicated in the endocytosis of NTRK1 (REFS 72,75) | Involved in the activation of Akt and ERK1 and ERK2 signalling pathways by RTKs72,74,75 |
| p38 MAPK | Stimulates ligand-independent endocytosis of EGFR and regulates RAB5 function (REFS 118,119) | Involved in the regulation of inflammatory and stress response in response to various stimuli by phosphorylating downstream targets145 |
| Epsin | Located in clathrin-coated pits and recruits ubiquitylated cargo17,146 | Involved in the activation of Cdc42 (REF. 147) |
| Intersectin | Involved in the regulation of clathrin-mediated endocytosis, and EGFR ubiquitylation and internalization148,149 | Functions as a guanine nucleotide exchange factor for CDC42 to regulate actin cytoskeleton dynamics150 |
| Dishevelled | Mediates internalization of Frizzled receptors by linking receptors to AP2 (REF. 9) | Mediates canonical and noncanonical Wnt signalling by interacting with various adaptors, kinases, small GTPase and other proteins151 |
Studies during the past decade have revealed that the functional interactions of cell signalling processes and endocytosis are important at all stages of morpho-genesis during animal development, as well as in the regulation of cell proliferation, metabolism, motility, survival, differentiation and the immune response in adult organisms. In the central nervous system, for example, regulation of signalling by endocytosis has been implicated in neurodegenerative diseases, drug abuse, nerve regeneration and neuronal plasticity (for example, long-term depression and potentiation)122–124. The endocytic machinery is often affected in cancer cells, and these aberrations may underlie the specific properties of tumours and the sensitivity of these tumours to therapeutics targeting signalling receptors125. many relationships between signalling and endocytosis have begun to be elucidated at the cellular level, but we have a limited understanding of the mechanisms of endocytosis and signalling crosstalk under in vivo conditions. In vivo analysis of the role of this crosstalk in human pathologies will certainly benefit from expanding the repertoire of mouse models with genetically altered endocytosis and signalling pathways. of particular importance are tissue-specific and inducible models that may help to avoid the embryonic lethality that is often associated with altering the components of the basic endocytic and signalling machineries. Needless to say, further elucidation of the specific mechanisms linking signalling and endocytosis is crucial for developing such strategies of in vivo analysis and manipulation.
Endosomal compartment
An intracellular acidic membrane compartment to which receptors are delivered after endocytosis, and from which receptors are sorted to different intracellular destinations.
Receptor tyrosine kinase
Transmembrane receptor that consists of extracellular ligand-binding domains, one helix membrane-spanning domain and a tyrosine kinase domain in the cytoplasmic part of the molecule.
G protein-coupled receptor
A transmembrane receptor containing seven membrane-spanning helical domains, which functions as a ligand-dependent guanine nucleotide exchange factor to activate trimeric G proteins to interact with other signalling proteins.
Clathrin-coated pit
The initial site of the recruitment of receptors destined for endocytosis and of the formation of a clathrin-coated vesicle.
Clathrin-dependent endocytosis
Endocytosis mediated by clathrin-coated pits and vesicles.
Clathrin-independent endocytosis
Endocytosis that does not require clathrin.
Clathrin heavy chain
The main structural protein of the clathrin coat (~170 kD) consisting of the hub, distal and proximal legs and the terminal domains.
E3 ubiquitin ligase
The final enzyme complex in the ubiquitin-conjugation pathway that transfers ubiquitin from previous components of the pathway to the substrate protein to form a covalently linked ubiquitin–substrate conjugate.
RING domain
A cysteine-rich tandem zinc-finger domain of 40–60 amino acids that is often found in E3 ubiquitin ligases.
HECT domain
A domain that contains ~350 amino acids and is highly conserved among a family of E3 enzymes. The name HECT for stands for homologous to E6-AP carboxyl terminus.
Multivesicular body
An endosomal intermediate containing small membrane vesicles that are formed by inward invagination and budding from the limiting membrane.
HRS–STAM
A ubiquitin-binding protein complex that functions as an adaptor for cargo sorting to the multivesicular body and lysosome pathway.
Intraluminal vesicle
A small vesicle located inside multivesicular bodies that are formed by ESCRT (endosomal sorting complex required for transport)-mediated invagination of the limiting membrane of these endosomes.
Trimeric G protein
A family of cytoplasmic signal mediators composed of an α-subunit containing a GTP-binding site and intrinsic GTPase activity, together with a hydrophobic and often acylated β- and γ-protein subcomplex. The β- and γ-protein subcomplex is activated by dissociation of α-GTP, which is initiated by GTP exchange on the α-subunit.
FYVE domain
Phosphatidylinositol 3-phosphate binding domain of ~60–65 amino acids that is named after four cysteine-rich proteins — Fab1, YOTB/ZK632.12, Vac1, and early endosome antigen 1 — that it has been found in.
PX domain
A lipid- and protein-interaction domain that consists of 100–130 amino acids and is defined by sequences that are found in two components of the phagocyte NADPH oxidase (PHOX) complex.
Lysophosphatidic acid
A phospholipid derivative that acts as a potent signalling molecule owing to the fact that it activates several G protein-coupled receptors. It is often formed by phospholipase D, which removes the choline group from lysophosphatidylcholine.
Soma
Cell body portion of a neuron that contains the nucleus.
Retrograde signalling
The process by which ligands released from a post-synaptic cell regulate events in a neuron synapsing onto that cell.
Pheochromocytoma
A rare catecholamine-secreting tumour derived from chromaffin cells, which are neuroendocrine cells found in the medulla of the adrenal gland.
Macropinosome
A large vesicle filled with extracellular fluid and formed through macropinocytosis.
PH domain
A phosphoinositol-binding protein domain that is characteristic of the RNase PH family of bacterial phosphate-dependent ribonucleases.
Leucine zipper
A leucine-rich domain in a protein that binds to other proteins with a similar domain.
Metalloendopeptidase
An enzyme which functions as an metalloproteinase endopeptidase.
Depolarization
A change in a cell's membrane potential, making it less negative, which in neurons and other excitatory cells may result in an action potential.
Acknowledgments
We thank past and present members of our laboratories, and many colleagues in other laboratories, who have contributed valuable data and ideas. A.S. is supported by National Institutes of Health (NIH) grants CA089151, CA112219 and DA014204, and M.v.Z. by NIH grants DA000439 and DA012864.
References
- 1.Polo S, Di Fiore PP. Endocytosis conducts the cell signaling orchestra. Cell. 2006;124:897–900. doi: 10.1016/j.cell.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 2.von Zastrow M, Sorkin A. signaling on the endocytic pathway. Curr. Opin. Cell Biol. 2007 doi: 10.1016/j.ceb.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 4.Sorkin A. Cargo recognition during clathrin-mediated endocytosis: a team effort. Curr. Opin. Cell Biol. 2004;16:392–399. doi: 10.1016/j.ceb.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 6.Yao D, Ehrlich M, Henis YI, Leof EB. Transforming growth factor-β receptors interact with AP2 by direct binding to β2 subunit. Mol. Biol. Cell. 2002;13:4001–4012. doi: 10.1091/mbc.02-07-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar KG, et al. Site-specific ubiquitination exposes a linear motif to promote interferon-α receptor endocytosis. J. Cell Biol. 2007;179:935–950. doi: 10.1083/jcb.200706034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brett TJ, Traub LM. Molecular structures of coat and coat-associated proteins: function follows form. Curr. Opin. Cell Biol. 2006;18:395–406. doi: 10.1016/j.ceb.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Yu A, et al. Association of dishevelled with the clathrin ap-2 adaptor is required for frizzled endocytosis and planar cell polarity signaling. Dev. Cell. 2007;12:129–141. doi: 10.1016/j.devcel.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurevich VV, et al. Arrestin interactions with G protein-coupled receptors. Direct binding studies of wild type and mutant arrestins with rhodopsin, β2-adrenergic, and m2 muscarinic cholinergic receptors. J. Biol. Chem. 1995;270:720–731. doi: 10.1074/jbc.270.2.720. [DOI] [PubMed] [Google Scholar]
- 11.Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu. Rev. Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 12.Edeling MA, et al. Molecular switches involving the AP-2 β2 appendage regulate endocytic cargo selection and clathrin coat assembly. Dev. Cell. 2006;10:329–342. doi: 10.1016/j.devcel.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Gaidarov I, Krupnick JG, Falck JR, Benovic JL, Keen JH. Arrestin function in G protein-coupled receptor endocytosis requires phosphoinositide binding. EMBO J. 1999;18:871–881. doi: 10.1093/emboj/18.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated β2-adrenergic receptor and β-arrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- 15.Hicke L, Riezman H. Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell. 1996;84:277–287. doi: 10.1016/s0092-8674(00)80982-4. [DOI] [PubMed] [Google Scholar]
- 16.Gupta-Rossi N, et al. Monoubiquitination and endocytosis direct γ-secretase cleavage of activated Notch receptor. J. Cell Biol. 2004;166:73–83. doi: 10.1083/jcb.200310098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kazazic M, et al. Epsin 1 is involved in recruitment of ubiquitinated EGF receptors into clathrin-coated pits. Traffic. 2009;10:235–245. doi: 10.1111/j.1600-0854.2008.00858.x. [DOI] [PubMed] [Google Scholar]
- 18.Arevalo JC, et al. Cell survival through Trk neurotrophin receptors is differentially regulated by ubiquitination. Neuron. 2006;50:549–559. doi: 10.1016/j.neuron.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 19.Peschard P, et al. Mutation of the c-Cbl TKB domain binding site on the Met receptor tyrosine kinase converts it into a transforming protein. Mol. Cell. 2001;8:995–1004. doi: 10.1016/s1097-2765(01)00378-1. [DOI] [PubMed] [Google Scholar]
- 20.Jiang X, Huang F, Marusyk A, Sorkin A. Grb2 regulates internalization of EGF receptors through clathrin-coated pits. Mol. Biol. Cell. 2003;14:858–870. doi: 10.1091/mbc.E02-08-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Traub LM, Lukacs GL. Decoding ubiquitin sorting signals for clathrin-dependent endocytosis by CLASPs. J. Cell Sci. 2007;120:543–553. doi: 10.1242/jcs.03385. [DOI] [PubMed] [Google Scholar]
- 22.Wolfe BL, Marchese A, Trejo J. Ubiquitination differentially regulates clathrin-dependent internalization of protease-activated receptor-1. J. Cell Biol. 2007;177:905–916. doi: 10.1083/jcb.200610154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu. Rev. Pharmacol. Toxicol. 2008;48:537–568. doi: 10.1146/annurev.pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- 24.Nickerson DP, Russell MR, Odorizzi G. A concentric circle model of multivesicular body cargo sorting. EMBO Rep. 2007;8:644–650. doi: 10.1038/sj.embor.7401004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–452. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- 26.Jekely G, Sung HH, Luque CM, Rorth P. Regulators of endocytosis maintain localized receptor tyrosine kinase signaling in guided migration. Dev. Cell. 2005;9:197–207. doi: 10.1016/j.devcel.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 27.McLaughlin S, Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 2005;438:605–611. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- 28.Haugh JM, Meyer T. Active EGF receptors have limited access to PtdIns(4, 5)P(2) in endosomes: implications for phospholipase C and PI 3-kinase signaling. J. Cell Sci. 2002;115:303–310. doi: 10.1242/jcs.115.2.303. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki T, Sasaki J, Sakai T, Takasuga S, Suzuki A. The physiology of phosphoinositides. Biol. Pharm. Bull. 2007;30:1599–1604. doi: 10.1248/bpb.30.1599. [DOI] [PubMed] [Google Scholar]
- 30.Sorkin A, Von Zastrow M. Signal transduction and endocytosis: close encounters of many kinds. Nature Rev. Mol. Cell Biol. 2002;3:600–614. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- 31.Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]; The requirement of clathrin-dependent endocytosis for the full activation of ERK1 and ERK2 by EGFR is shown for the first time.
- 32.Kermorgant S, Parker PJ. Receptor trafficking controls weak signal delivery: a strategy used by c-Met for STAT3 nuclear accumulation. J. Cell Biol. 2008;182:855–863. doi: 10.1083/jcb.200806076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J. Cell Biol. 2006;174:593–604. doi: 10.1083/jcb.200602080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sigismund S, et al. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev. Cell. 2008;15:209–219. doi: 10.1016/j.devcel.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 35.Galperin E, Sorkin A. Endosomal targeting of MEK2 requires RAF, MEK kinase activity and clathrin-dependent endocytosis. Traffic. 2008;9:1776–1790. doi: 10.1111/j.1600-0854.2008.00788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johannessen LE, Ringerike T, Molnes J, Madshus IH. Epidermal growth factor receptor efficiently activates mitogen-activated protein kinase in HeLa cells and hep2 cells conditionally defective in clathrin-dependent endocytosis. Exp. Cell Res. 2000;260:136–145. doi: 10.1006/excr.2000.5004. [DOI] [PubMed] [Google Scholar]
- 37.DeGraff JL, Gagnon AW, Benovic JL, Orsini MJ. Role of arrestins in endocytosis and signaling of α2-adrenergic receptor subtypes. J. Biol. Chem. 1999;274:11253–11259. doi: 10.1074/jbc.274.16.11253. [DOI] [PubMed] [Google Scholar]
- 38.Teis D, Wunderlich W, Huber LA. Localization of the MP1-MAPK scaffold complex to endosomes is mediated by p14 and required for signal transduction. Dev. Cell. 2002;3:803–814. doi: 10.1016/s1534-5807(02)00364-7. [DOI] [PubMed] [Google Scholar]
- 39.Nada S, et al. The novel lipid raft adaptor p18 controls endosome dynamics by anchoring the MEK-ERK pathway to late endosomes. EMBO J. 2009;28:477–489. doi: 10.1038/emboj.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teis D, et al. p14-MP1-MEK1 signaling regulates endosomal traffic and cellular proliferation during tissue homeostasis. J. Cell Biol. 2006;175:861–868. doi: 10.1083/jcb.200607025. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first demonstration of the assembly of a MAPK signalling complex in late endosomes and of the importance of this complex for the activation of ERK1 and ERK2 by EGFR.
- 41.Pullikuth A, McKinnon E, schaeffer HJ, Catling AD. The MEK1 scaffolding protein MP1 regulates cell spreading by integrating PAK1 and Rho signals. Mol. Cell. Biol. 2005;25:5119–5133. doi: 10.1128/MCB.25.12.5119-5133.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daaka Y, et al. Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J. Biol. Chem. 1998;273:685–688. doi: 10.1074/jbc.273.2.685. [DOI] [PubMed] [Google Scholar]; Early evidence supporting the hypothesis of endosomal signalling by GPCRs.
- 43.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Association of β-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J. Biol. Chem. 1999;274:32248–32257. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- 44.DeFea KA, et al. β-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J. Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides evidence that β-arrestins control the selectivity of GPCR signalling from the endocytic pathway by physically restricting the localization of a GPCR-activated MAPK.
- 45.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. β-arrestins and cell signaling. Annu. Rev. Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 46.Terrillon S, Bouvier M. Receptor activity-independent recruitment of βarrestin2 reveals specific signalling modes. EMBO J. 2004;23:3950–3961. doi: 10.1038/sj.emboj.7600387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garcia-Regalado A, et al. G protein-coupled receptor-promoted trafficking of Gβ1γ2 leads to AKT activation at endosomes via a mechanism mediated by Gβ1γ2-Rab11a interaction. Mol. Biol. Cell. 2008;19:4188–4200. doi: 10.1091/mbc.E07-10-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watson FL, et al. Rapid nuclear responses to target-derived neurotrophins require retrograde transport of ligand-receptor complex. J. Neurosci. 1999;19:7889–7900. doi: 10.1523/JNEUROSCI.19-18-07889.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cui B, et al. One at a time, live tracking of NGF axonal transport using quantum dots. Proc. Natl Acad. Sci. USA. 2007;104:13666–13671. doi: 10.1073/pnas.0706192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grimes ML, et al. Endocytosis of activated TrkA: evidence that nerve growth factor induces formation of signaling endosomes. J. Neuroscience. 1996;16:7950–7964. doi: 10.1523/JNEUROSCI.16-24-07950.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]; Biochemical evidence for the assembly of specific NTRK1 signalling complexes on endosomes that are involved in retrograde signalling in neurons.
- 51.Howe CL, Valletta JS, Rusnak AS, Mobley WC. NGF signaling from clathrin-coated vesicles: evidence that signaling endosomes serve as a platform for the Ras-MAPK pathway. Neuron. 2001;32:801–814. doi: 10.1016/s0896-6273(01)00526-8. [DOI] [PubMed] [Google Scholar]
- 52.Wu C, Lai CF, Mobley WC. Nerve growth factor activates persistent Rap1 signaling in endosomes. J. Neurosci. 2001;21:5406–5416. doi: 10.1523/JNEUROSCI.21-15-05406.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu C, Cui B, He L, Chen L, Mobley WC. The coming of age of axonal neurotrophin signaling endosomes. J. Proteomics. 2009;72:46–55. doi: 10.1016/j.jprot.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu C, et al. A functional dynein-microtubule network is required for NGF signaling through the Rap1/MAPK pathway. Traffic. 2007;8:1503–1520. doi: 10.1111/j.1600-0854.2007.00636.x. [DOI] [PubMed] [Google Scholar]
- 55.Watson FL, et al. Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nature Neurosci. 2001;4:981–988. doi: 10.1038/nn720. [DOI] [PubMed] [Google Scholar]; This study identifies ERK5 as being specifically involved in retrograde signalling by neurotrophins. It also shows the importance of clathrin-mediated endocytosis in the axonal termini and of retrograde endosomal transport for ERK5 activation in the neuronal soma.
- 56.Zheng J, et al. Clathrin-dependent endocytosis is required for TrkB-dependent Akt-mediated neuronal protection and dendritic growth. J. Biol. Chem. 2008;283:13280–13288. doi: 10.1074/jbc.M709930200. [DOI] [PubMed] [Google Scholar]
- 57.Delcroix JD, et al. NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron. 2003;39:69–84. doi: 10.1016/s0896-6273(03)00397-0. [DOI] [PubMed] [Google Scholar]
- 58.Deinhardt K, et al. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron. 2006;52:293–305. doi: 10.1016/j.neuron.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 59.Valdez G, et al. Pincher-mediated macroendocytosis underlies retrograde signaling by neurotrophin receptors. J. Neurosci. 2005;25:5236–5247. doi: 10.1523/JNEUROSCI.5104-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Valdez G, et al. Trk-signaling endosomes are generated by Rac-dependent macroendocytosis. Proc. Natl Acad. Sci. USA. 2007;104:12270–12275. doi: 10.1073/pnas.0702819104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mok SA, Campenot RB. A nerve growth factor-induced retrograde survival signal mediated by mechanisms downstream of TrkA. Neuropharmacology. 2007;52:270–278. doi: 10.1016/j.neuropharm.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 62.Cox LJ, Hengst U, Gurskaya NG, Lukyanov KA, Jaffrey SR. Intra-axonal translation and retrograde trafficking of CREB promotes neuronal survival. Nature Cell Biol. 2008;10:149–159. doi: 10.1038/ncb1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsukazaki T, Chiang TA, Davison AF, Attisano L, Wrana JL. SARA, a FYVE domain protein that recruits Smad2 to the TGFβ receptor. Cell. 1998;95:779–791. doi: 10.1016/s0092-8674(00)81701-8. [DOI] [PubMed] [Google Scholar]; The first demonstration of the role of a FYVE domain protein (SARA) and endosomes in TGFβ signalling.
- 64.Hayes S, Chawla A, Corvera S. TGF β receptor internalization into EEA1-enriched early endosomes: role in signaling to Smad2. J. Cell Biol. 2002;158:1239–1249. doi: 10.1083/jcb.200204088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen YG, Wang Z, Ma J, Zhang L, Lu Z. Endofin, a FYVE domain protein, interacts with Smad4 and facilitates TGF-β signaling. J. Biol. Chem. 2007;282:9688–9695. doi: 10.1074/jbc.M611704200. [DOI] [PubMed] [Google Scholar]
- 66.Hartung A, et al. Different routes of bone morphogenic protein (BMP) receptor endocytosis influence BMP signaling. Mol. Cell. Biol. 2006;26:7791–7805. doi: 10.1128/MCB.00022-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu Z, et al. Transforming growth factor β activates Smad2 in the absence of receptor endocytosis. J. Biol. Chem. 2002;277:29363–29368. doi: 10.1074/jbc.M203495200. [DOI] [PubMed] [Google Scholar]
- 68.Slessareva JE, Routt SM, Temple B, Bankaitis VA, Dohlman HG. Activation of the phosphatidylinositol 3-kinase Vps34 by a G protein α subunit at the endosome. Cell. 2006;126:191–203. doi: 10.1016/j.cell.2006.04.045. [DOI] [PubMed] [Google Scholar]; This study provides direct evidence linking trimeric G proteins to signalling from endosomes.
- 69.Mullershausen F, et al. Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nature Chem. Biol. 2009;5:428–434. doi: 10.1038/nchembio.173. [DOI] [PubMed] [Google Scholar]
- 70.Calebiro D, et al. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol. 2009 Aug 18; doi: 10.1371/journal.pbio.1000172. (doi:10.1371/journal.pbio.1000172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miaczynska M, et al. APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell. 2004;116:445–456. doi: 10.1016/s0092-8674(04)00117-5. [DOI] [PubMed] [Google Scholar]
- 72.Lin DC, et al. APPL1 associates with TrkA and GIPC1 and is required for nerve growth factor-mediated signal transduction. Mol. Cell. Biol. 2006;26:8928–8941. doi: 10.1128/MCB.00228-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zoncu R, et al. A phosphoinositide switch controls the maturation and signaling properties of APPL endosomes. Cell. 2009;136:1110–1121. doi: 10.1016/j.cell.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schenck A, et al. The endosomal protein Appl1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell. 2008;133:486–497. doi: 10.1016/j.cell.2008.02.044. [DOI] [PubMed] [Google Scholar]; The first in vivo evidence for the role of APPL proteins and endosomes in a specific Akt signalling pathway.
- 75.Varsano T, et al. GIPC is recruited by APPL to peripheral TrkA endosomes and regulates TrkA trafficking and signaling. Mol. Cell. Biol. 2006;26:8942–8952. doi: 10.1128/MCB.00305-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vaccari T, Lu H, Kanwar R, Fortini ME, Bilder D. Endosomal entry regulates Notch receptor activation in Drosophila melanogaster. J. Cell Biol. 2008;180:755–762. doi: 10.1083/jcb.200708127. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides evidence for the ubiquitylation-dependent internalization of activated Notch, and shows the importance of this internalization for the γ-secretase-mediated cleavage of Notch.
- 77.Wilkin M, et al. Drosophila HOPS and AP-3 complex genes are required for a Deltex-regulated activation of notch in the endosomal trafficking pathway. Dev. Cell. 2008;15:762–772. doi: 10.1016/j.devcel.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 78.Pasternak SH, et al. Presenilin-1, nicastrin, amyloid precursor protein, and γ-secretase activity are co-localized in the lysosomal membrane. J. Biol. Chem. 2003;278:26687–26694. doi: 10.1074/jbc.m304009200. [DOI] [PubMed] [Google Scholar]
- 79.Schneider-Brachert W, et al. Compartmentalization of TNF receptor 1 signaling: internalized TNF receptosomes as death signaling vesicles. Immunity. 2004;21:415–428. doi: 10.1016/j.immuni.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 80.Liao W, et al. CARP-2 is an endosome-associated ubiquitin ligase for RIP and regulates TNF-induced NF-κB activation. Curr. Biol. 2008;18:641–649. doi: 10.1016/j.cub.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kagan JC, et al. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-β. Nature Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]; The demonstration of the recruitment of specific signalling complexes to the activated Toll-like receptor, based on the change of the lipid composition of the membrane during endocytosis.
- 82.Honda K, et al. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005;434:1035–1040. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- 83.Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp. Cell Res. 2009;315:683–696. doi: 10.1016/j.yexcr.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 84.Marchese A, Paing MM, Temple BR, Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu. Rev. Pharmacol. Toxicol. 2008;48:601–629. doi: 10.1146/annurev.pharmtox.48.113006.094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weber U, Eroglu C, Mlodzik M. Phospholipid membrane composition affects EGF receptor and Notch signaling through effects on endocytosis during Drosophila development. Dev. Cell. 2003;5:559–570. doi: 10.1016/s1534-5807(03)00273-9. [DOI] [PubMed] [Google Scholar]
- 86.Huang F, Kirkpatrick D, Jiang X, Gygi S, Sorkin A. Differential regulation of EGF receptor internalization and degradation by multiubiquitination within the kinase domain. Mol. Cell. 2006;21:737–748. doi: 10.1016/j.molcel.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 87.Niendorf S, et al. Essential role of ubiquitin-specific protease 8 for receptor tyrosine kinase stability and endocytic trafficking in vivo. Mol. Cell. Biol. 2007;27:5029–5039. doi: 10.1128/MCB.01566-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Row PE, Prior IA, McCullough J, Clague MJ, Urbe S. The ubiquitin isopeptidase UBPY regulates endosomal ubiquitin dynamics and is essential for receptor down-regulation. J. Biol. Chem. 2006;281:12618–12624. doi: 10.1074/jbc.M512615200. [DOI] [PubMed] [Google Scholar]
- 89.Malerod L, Stuffers S, Brech A, Stenmark H. Vps22/EAP30 in ESCRT-II mediates endosomal sorting of growth factor and chemokine receptors destined for lysosomal degradation. Traffic. 2007;8:1617–1629. doi: 10.1111/j.1600-0854.2007.00630.x. [DOI] [PubMed] [Google Scholar]
- 90.Lloyd TE, et al. Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell. 2002;108:261–269. doi: 10.1016/s0092-8674(02)00611-6. [DOI] [PubMed] [Google Scholar]; This study shows in vivo that HRS and lysosomal sorting of RTKs have a role in the intensity of RTK signalling
- 91.Hammond DE, et al. Endosomal dynamics of Met determine signaling output. Mol. Biol. Cell. 2003;14:1346–1354. doi: 10.1091/mbc.E02-09-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wurdinger T, et al. miR-296 regulates growth factor receptor overexpression in angiogenic endothelial cells. Cancer Cell. 2008;14:382–393. doi: 10.1016/j.ccr.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bache KG, et al. The ESCRT-III subunit hVps24 is required for degradation but not silencing of the epidermal growth factor receptor. Mol. Biol. Cell. 2006;17:2513–2523. doi: 10.1091/mbc.E05-10-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pitcher JA, Payne ES, Csortos C, DePaoli-Roach AA, Lefkowitz RJ. The G-protein-coupled receptor phosphatase: a protein phosphatase type 2A with a distinct subcellular distribution and substrate specificity. Proc. Natl Acad. Sci. USA. 1995;92:8343–8347. doi: 10.1073/pnas.92.18.8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Seachrist JL, Anborgh PH, Ferguson SS. β2-adrenergic receptor internalization, endosomal sorting, and plasma membrane recycling are regulated by rab GTPases. J. Biol. Chem. 2000;275:27221–27228. doi: 10.1074/jbc.M003657200. [DOI] [PubMed] [Google Scholar]
- 96.Filipeanu CM, et al. Enhancement of the recycling and activation of β-adrenergic receptor by Rab4 GTPase in cardiac myocytes. J. Biol. Chem. 2006;281:11097–11103. doi: 10.1074/jbc.M511460200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Odley A, et al. Regulation of cardiac contractility by Rab4-modulated β2-adrenergic receptor recycling. Proc. Natl Acad. Sci. USA. 2004;101:7082–7087. doi: 10.1073/pnas.0308335101. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides evidence that RAB4-dependent recycling promotes GPCR signalling in vivo.
- 98.Padilla BE, et al. Endothelin-converting enzyme-1 regulates endosomal sorting of calcitonin receptor-like receptor and β-arrestins. J. Cell Biol. 2007;179:981–997. doi: 10.1083/jcb.200704053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cattaruzza F, Cottrell GS, Vaksman N, Bunnett NW. Endothelin-converting enzyme 1 promotes re-sensitization of neurokinin 1 receptor-dependent neurogenic inflammation. Br. J. Pharmacol. 2009;156:730–739. doi: 10.1111/j.1476-5381.2008.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang Y, Lauffer B, Von Zastrow M, Kobilka BK, Xiang Y. N-ethylmaleimide-sensitive factor regulates β2 adrenoceptor trafficking and signaling in cardiomyocytes. Mol. Pharmacol. 2007;72:429–439. doi: 10.1124/mol.107.037747. [DOI] [PubMed] [Google Scholar]
- 101.French AR, Tadaki DK, Niyogi SK, Lauffenburger DA. Intracellular trafficking of epidermal growth factor family ligands is directly influenced by the pH sensitivity of the receptor/ligand interaction. J. Biol. Chem. 1995;270:4334–4340. doi: 10.1074/jbc.270.9.4334. [DOI] [PubMed] [Google Scholar]
- 102.Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-β receptor signalling and turnover. Nature Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 103.Connolly JL, Green SA, Greene LA. Comparison of rapid changes in surface morphology and coated pit formation of PC12 cells in response to nerve growth factor, epidermal growth factor, and dibutyryl cyclic AMP. J. Cell Biol. 1984;98:457–465. doi: 10.1083/jcb.98.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wilde A, et al. EGF receptor signaling stimulates sRC kinase phosphorylation of clathrin, influencing clathrin redistribution and EGF uptake. Cell. 1999;96:1–20. doi: 10.1016/s0092-8674(00)80578-4. [DOI] [PubMed] [Google Scholar]
- 105.Beattie EC, Howe CL, Wilde A, Brodsky FM, Mobley WC. NGF signals through TrkA to increase clathrin at the plasma membrane and enhance clathrin-mediated membrane trafficking. J. Neurosci. 2000;20:7325–7333. doi: 10.1523/JNEUROSCI.20-19-07325.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Johannessen LE, Pedersen NM, Pedersen KW, Madshus IH, Stang E. Activation of the epidermal growth factor (EGF) receptor induces formation of EGF receptor- and grb2-containing clathrin-coated pits. Mol. Cell. Biol. 2006;26:389–401. doi: 10.1128/MCB.26.2.389-401.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hanover JA, Beguinot L, Willingham MC, Pastan IH. Transit of receptors for epidermal growth factor and transferrin through clathrin-coated pits. Analysis of the kinetics of receptor entry. J. Biol. Chem. 1985;260:15938–15945. [PubMed] [Google Scholar]
- 108.Carpentier J-L, et al. Co-localization of 125I-epidermal growth factor and ferritin-low density lipoprotein in coated pits: a quantitative electron microscopic study in normal and mutant human fibroblasts. J. Cell Biol. 1982;95:73–77. doi: 10.1083/jcb.95.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Keyel PA, et al. A single common portal for clathrin-mediated endocytosis of distinct cargo governed by cargo-selective adaptors. Mol. Biol. Cell. 2006;17:4300–4317. doi: 10.1091/mbc.E06-05-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rappoport JZ, Simon SM. Endocytic trafficking of activated EGFR is AP-2 dependent and occurs through preformed clathrin spots. J. Cell Sci. 2009;122:1301–1305. doi: 10.1242/jcs.040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Santini F, Gaidarov I, Keen JH. G protein-coupled receptor/arrestin3 modulation of the endocytic machinery. J. Cell Biol. 2002;156:665–676. doi: 10.1083/jcb.200110132. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides early evidence that activated GPCRs can change the properties of the clathrin-coated pits through which they are endocytosed.
- 112.Puthenveedu MA, von Zastrow M. Cargo regulates clathrin-coated pit dynamics. Cell. 2006;127:113–124. doi: 10.1016/j.cell.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 113.Bauerfeind R, Takei K, De Camilli P. Amphiphysin I is associated with coated endocytic intermediates and undergoes stimulation-dependent dephosphorylation in nerve terminals. J. Biol. Chem. 1997;272:30984–30992. doi: 10.1074/jbc.272.49.30984. [DOI] [PubMed] [Google Scholar]
- 114.Marks B, McMahon HT. Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Curr. Biol. 1998;8:740–749. doi: 10.1016/s0960-9822(98)70297-0. [DOI] [PubMed] [Google Scholar]
- 115.Irie F, Okuno M, Pasquale EB, Yamaguchi Y. EphrinB-EphB signalling regulates clathrin-mediated endocytosis through tyrosine phosphorylation of synaptojanin 1. Nature Cell Biol. 2005;7:501–509. doi: 10.1038/ncb1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 117.White IJ, Bailey LM, Aghakhani MR, Moss SE, Futter CE. EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J. 2006;25:1–12. doi: 10.1038/sj.emboj.7600759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cavalli V, et al. The stress-induced MAP kinase p38 regulates endocytic trafficking via the GDI:Rab5 complex. Mol. Cell. 2001;7:421–432. doi: 10.1016/s1097-2765(01)00189-7. [DOI] [PubMed] [Google Scholar]
- 119.Zwang Y, Yarden Y. p38 MAP kinase mediates stress-induced internalization of EGFR: implications for cancer chemotherapy. EMBO J. 2006;25:4195–4206. doi: 10.1038/sj.emboj.7601297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nekhoroshkova E, Albert S, Becker M, Rapp UR. A-RAF kinase functions in ARF6 regulated endocytic membrane traffic. PLoS ONE. 2009;4:e4647. doi: 10.1371/journal.pone.0004647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yudowski GA, Puthenveedu MA, Henry AG, von Zastrow M. Cargo-mediated regulation of a rapid Rab4-dependent recycling pathway. Mol. Biol. Cell. 2009;20:2774–2784. doi: 10.1091/mbc.E08-08-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zweifel LS, Kuruvilla R, Ginty DD. Functions and mechanisms of retrograde neurotrophin signalling. Nature Rev. Neurosci. 2005;6:615–625. doi: 10.1038/nrn1727. [DOI] [PubMed] [Google Scholar]
- 123.Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim JA, et al. Morphine-induced receptor endocytosis in a novel knockin mouse reduces tolerance and dependence. Curr. Biol. 2008;18:129–135. doi: 10.1016/j.cub.2007.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mosesson Y, Mills GB, Yarden Y. Derailed endocytosis: an emerging feature of cancer. Nature Rev. Cancer. 2008;8:835–850. doi: 10.1038/nrc2521. [DOI] [PubMed] [Google Scholar]
- 126.Hershko A, Ciechanover A. The ubiquitin system for protein degradation. Annu. Rev. Biochem. 1992;61:761–807. doi: 10.1146/annurev.bi.61.070192.003553. [DOI] [PubMed] [Google Scholar]
- 127.Pickart CM, Fushman D. Polyubiquitin chains: polymeric protein signals. Curr. Opin. Chem. Biol. 2004;8:610–616. doi: 10.1016/j.cbpa.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 128.Staub O, Rotin D. Role of ubiquitylation in cellular membrane transport. Physiol. Rev. 2006;86:669–707. doi: 10.1152/physrev.00020.2005. [DOI] [PubMed] [Google Scholar]
- 129.Hurley JH, Lee S, Prag G. Ubiquitin-binding domains. Biochem. J. 2006;399:361–372. doi: 10.1042/BJ20061138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Millard SM, Wood SA. Riding the DUBway: regulation of protein trafficking by deubiquitylating enzymes. J. Cell Biol. 2006;173:463–468. doi: 10.1083/jcb.200602082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Carey RM, Balcz BA, Lopez-Coviella I, Slack BE. Inhibition of dynamin-dependent endocytosis increases shedding of the amyloid precursor protein ectodomain and reduces generation of amyloid β protein. BMC Cell Biol. 2005;6:30. doi: 10.1186/1471-2121-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chyung JH, Raper DM, Selkoe DJ. γ-secretase exists on the plasma membrane as an intact complex that accepts substrates and effects intramembrane cleavage. J. Biol. Chem. 2005;280:4383–4392. doi: 10.1074/jbc.M409272200. [DOI] [PubMed] [Google Scholar]
- 133.Urra MS, et al. TrkA receptor activation by NGF induces shedding of the p75 neurotrophin receptor followed by endosomal γ-secretase-mediated release of the p75 intracellular domain. J. Biol. Chem. 2007;282:7606–7615. doi: 10.1074/jbc.M610458200. [DOI] [PubMed] [Google Scholar]
- 134.Nichols JT, Miyamoto A, Weinmaster G. Notch signaling — constantly on the move. Traffic. 2007;8:959–969. doi: 10.1111/j.1600-0854.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- 135.Pavlopoulos E, et al. Neuralized encodes a peripheral membrane protein involved in delta signaling and endocytosis. Dev. Cell. 2001;1:807–816. doi: 10.1016/s1534-5807(01)00093-4. [DOI] [PubMed] [Google Scholar]
- 136.Itoh M, et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev. Cell. 2003;4:67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- 137.Koo BK, et al. An obligatory role of mind bomb-1 in notch signaling of mammalian development. PLoS ONE. 2007;2:e1221. doi: 10.1371/journal.pone.0001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fehon RG, et al. Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF-homologous genes in Drosophila. Cell. 1990;61:523–534. doi: 10.1016/0092-8674(90)90534-l. [DOI] [PubMed] [Google Scholar]
- 139.Jekely G, Rorth P. Hrs mediates downregulation of multiple signalling receptors in Drosophila. EMBO Rep. 2003;4:1163–1168. doi: 10.1038/sj.embor.7400019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wilkin MB, et al. Regulation of notch endosomal sorting and signaling by Drosophila Nedd4 family proteins. Curr. Biol. 2004;14:2237–2244. doi: 10.1016/j.cub.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 141.Chastagner P, Israel A, Brou C. AIP4/Itch regulates Notch receptor degradation in the absence of ligand. PLoS ONE. 2008;3:e2735. doi: 10.1371/journal.pone.0002735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lefkowitz RJ, Pitcher J, Krueger K, Daaka Y. Mechanisms of β-adrenergic receptor desensitization and resensitization. Adv. Pharmacol. 1998;42:416–420. doi: 10.1016/s1054-3589(08)60777-2. [DOI] [PubMed] [Google Scholar]
- 143.Waterman H, et al. A mutant EGF-receptor defective in ubiquitylation and endocytosis unveils a role for Grb2 in negative signaling. EMBO J. 2002;21:303–313. doi: 10.1093/emboj/21.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rozakis-Adcock M, Fernley R, Wade J, Pawson T, Bowtell D. The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1. Nature. 1993;363:83–85. doi: 10.1038/363083a0. [DOI] [PubMed] [Google Scholar]
- 145.Loesch M, Chen G. The p38 MAPK stress pathway as a tumor suppressor or more? Front. Biosci. 2008;13:3581–3593. doi: 10.2741/2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shih SC, et al. Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nature Cell Biol. 2002;4:389–393. doi: 10.1038/ncb790. [DOI] [PubMed] [Google Scholar]
- 147.Aguilar RC, et al. Epsin N-terminal homology domains perform an essential function regulating Cdc42 through binding Cdc42 GTPase-activating proteins. Proc. Natl Acad. Sci. USA. 2006;103:4116–4121. doi: 10.1073/pnas.0510513103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Martin NP, et al. Intersectin regulates epidermal growth factor receptor endocytosis, ubiquitylation, and signaling. Mol. Pharmacol. 2006;70:1643–1653. doi: 10.1124/mol.106.028274. [DOI] [PubMed] [Google Scholar]
- 149.Simpson F, et al. SH3-domain-containing proteins function at distinct steps in clathrin-coated vesicle formation. Nature Cell Biol. 1999;1:119–124. doi: 10.1038/10091. [DOI] [PubMed] [Google Scholar]
- 150.Hussain NK, et al. Endocytic protein intersectin-l regulates actin assembly via Cdc42 and N-WASP. Nature Cell Biol. 2001;3:927–932. doi: 10.1038/ncb1001-927. [DOI] [PubMed] [Google Scholar]
- 151.Schulte G, Bryja V. The Frizzled family of unconventional G-protein-coupled receptors. Trends Pharmacol. Sci. 2007;28:518–525. doi: 10.1016/j.tips.2007.09.001. [DOI] [PubMed] [Google Scholar]