Mitotic chromosomal instability and cancer: mouse modelling of the human disease (original) (raw)
. Author manuscript; available in PMC: 2017 Jul 25.
Published in final edited form as: Nat Rev Cancer. 2010 Feb;10(2):102–115. doi: 10.1038/nrc2781
Abstract
The stepwise progression from an early dysplastic lesion to full-blown metastatic malignancy is associated with increases in genomic instability. Mitotic chromosomal instability — the inability to faithfully segregate equal chromosome complements to two daughter cells during mitosis — is a widespread phenomenon in solid tumours that is thought to serve as the fuel for tumorigenic progression. How chromosome instability (CIN) arises in tumours and what consequences it has are still, however, hotly debated issues. Here we review the recent literature with an emphasis on models that recapitulate observations from human disease.
Since Boveri observed abnormal chromosome complements in tumour cells at the beginning of the twentieth century1,2, the role of chromosome instability (CIN) in tumour initiation and progression has been a central issue in cancer biology. Only recently, using sophisticated mouse modelling approaches, is it becoming clear that CIN is not simply a passenger phenotype but probably plays a causative part in a substantial proportion of malignancies. However, several questions and controversies still remain. Here we review these issues through the analysis of recent findings and their relevance to human disease. We focus on two crucial questions: first, how is aneuploidy generated? Second, what is the role of CIN in tumour initiation and/or progression?
Throughout this Review we will concentrate on the CIN that arises as a result of an abnormal mitosis. This CIN can occur because of alterations in mitotic timing, mitotic checkpoint control, or of microtubule or centrosome dynamics. Abnormalities in double-strand break repair or telomere maintenance can also eventually lead to CIN as a result of repeated chromosome breakage–fusion–bridge cycles. Because of space constraints, however, we will not cover the deregulation of these pathways, although we note that they may ultimately lead to mitotic abnormalities. Failure of the mitotic checkpoint machinery, which blocks the separation of sister chromatids before microtubule attachment (described below), has been an obvious candidate mechanism involved in the generation of CIN during mitosis. Disappointingly, the mitotic checkpoint is rarely found to be compromised in human tumours. It is, however, frequently hyperactivated in chromosomally unstable lesions and, importantly, this overactivation of the mitotic checkpoint is intricately linked to the inhibition of major tumour suppressive pathways and the acquisition of CIN.
As a point of nomenclature, distinctions have previously been made between whole chromosome instability (W-CIN) and CIN that includes translocations, interstitial deletions and amplifications (segmental chromosome instability or S-CIN)3. S-CIN is observed in several model systems into which mitotic defects have been introduced4–6. Some models of CIN only show W-CIN changes, yet human tumours often contain both abnormal chromosome complements and structural changes, and so we prefer to use the global definition of CIN in this Review. Where indicated, we will distinguish between the two forms as W-CIN and S-CIN. Other defects in genome integrity, such as microsatellite instability, defects in nucleotide excision repair or base excision repair, defects in telomere maintenance or the stability of larger repeats and abnormalities in the G2/M DNA damage checkpoint, also play a part in tumour initiation and progression and have been reviewed extensively elsewhere7–11.
Many of the studies that model mitotic CIN in mice are based on perturbations in the mitotic checkpoint pathway, which ensures the accurate segregation of chromosomes during mitosis. Because of its relevance to understanding genomic stability, a brief overview of the history of the identification of checkpoints is included in BOX 1. We reiterate, however, that although the identification of a DNA damage checkpoint and a mitotic checkpoint in yeast primed the cancer field to search for checkpoint mutations in human tumours, the requirement for the mitotic checkpoint in every cell division and the lethality observed in its absence make its loss in tumours an unlikely mechanism for the generation of CIN.
Box 1. A brief history of the identification of checkpoints.
In the late 1980s Ted Weinert and Leland Hartwell identified the existence of a DNA damage checkpoint in budding yeast (Saccharomyces cerevisiae)98. The Rad9 mutant strain they characterized was shown to be defective in the ability to arrest cell division as a result of irradiation-induced DNA damage. Importantly, Rad9 mutants were viable in the absence of DNA damage; only when irradiated with ionizing radiation did the lethality become evident. Moreover, ionizing radiation-induced lethality could be rescued by providing sufficient time for repair by growing the cells in the presence of the microtubule poison benzimidazole, which we now know leads to activation of the mitotic checkpoint. These studies provided experimental evidence for the existence of cell cycle checkpoints99. These signalling pathways are postulated to be non-essential in the absence of damage, and their main function is to arrest cell division until the damage is repaired or, in the case of mammalian cells, undergo programmed cell death when repair is not completed. Soon after these findings, two laboratories simultaneously identified an equivalent checkpoint responsible for arresting cell division in S. cerevisiae in response to mitotic spindle abnormalities induced by microtubule poisons. The mitotic arrest deficient (Mad)100 and budding uninhibited by benzimidazole (Bub)101 genes were identified in screens for sensitivity to spindle poisons and, as such, loss-of-function mutants were viable so long as cell division proceeded normally. These studies provided direct evidence for the existence of a spindle assembly checkpoint or mitotic checkpoint in budding yeast, the function of which is to arrest cell division at metaphase until all kinetochores are attached to microtubules from opposite spindle poles.
The later characterization of the mitotic checkpoint in mammalian cells102 revealed important differences relative to budding yeast. First, the mitotic checkpoint is essential in all normal or transformed mammalian cells examined49,53,103,104. This unexpected result may be due to karyotypic complexity and the transient requirement of the mitotic checkpoint during an unperturbed cell cycle to prevent an intolerable level of chromosome instability. In addition, non-kinetochore-bound human MAD2 and BUBR1 have checkpoint-independent functions that are required to prevent premature exit from mitosis, perhaps by blocking the degradation of key substrates early in the mitotic cycle23,28,105. Therefore, unlike the case in budding yeast, mitotic checkpoint genes are generally essential in each mammalian cell division and, in contrast to the DNA damage checkpoint, their complete loss is unlikely to account for the accumulation of genomic damage in human tumours.
An outline of the mitotic checkpoint
The molecular mechanisms responsible for the mitotic checkpoint have been thoroughly reviewed elsewhere12,13. Here, we present a broad outline (FIG. 1), emphasizing some of the recent controversies.
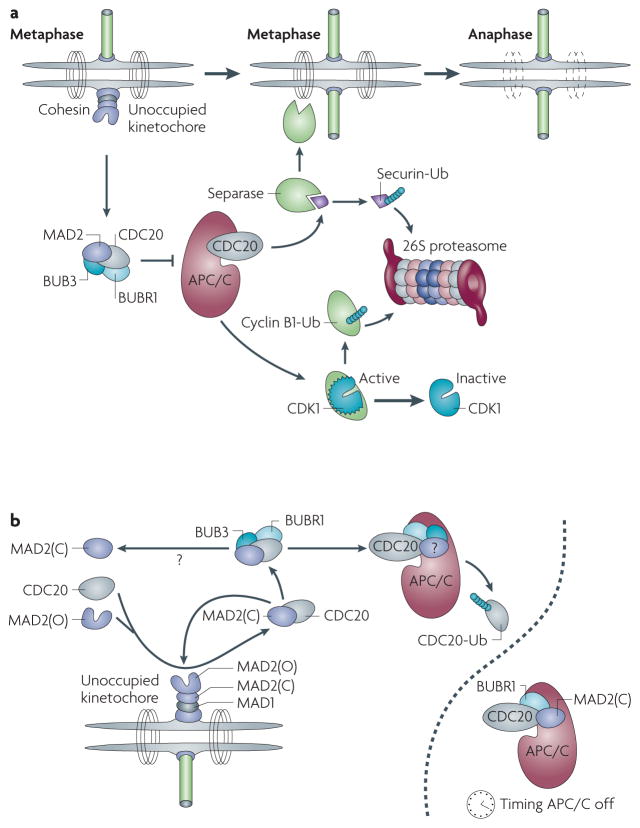
Figure 1. The mitotic checkpoint.
a | Outline of the mitotic checkpoint. An unattached kinetochore is shown on the left with the inner complex in purple, MAD1 in grey and MAD2 in its open and closed forms in purple. The mitotic checkpoint complex (MCC) is shown to inhibit the anaphase promoting complex/cyclosome (APC/C), which after attachment of the last kinetochore is activated and ubiquitylates securin and cyclin B1. More details of this pathway are described in the main text. b | The amplification of the unoccupied kinetochore signal is thought to depend on the conversion of MAD2 open complexes (MAD2(O)) to closed complexes (MAD2(C)) that bind to cell division cycle 20 (CDC20) and deliver it to the APC/C for ubiquitylation (Ub). The nature of the MCC is still debated, as indicated by question marks. A separate APC/C is shown to indicate its role in timing, independent of the kinetochore-derived signal. CDK1, cyclin-dependent kinase 1.
In its simplest form, the mitotic checkpoint is a mechanism by which eukaryotic cells arrest cell division at metaphase until all sister kinetochores are attached to microtubules from opposite spindle poles. In prometaphase, sister chromatids are topologically linked by the ring-like cohesin complexes14. In addition, the activity of cyclin-dependent kinase 1 (CDK1, also known as cell division cycle 2; CDC2), the main mitotic kinase, is high and maintains the mitotic state. As mammalian cells proceed from prometaphase to metaphase, a signalling complex that contains mitotic arrest deficient 1 (MAD1), MAD2, MPS1 (also known as TTK), BUB1, BUB3 and BUBR1 assembles at unoccupied kinetochores. This in turn leads to the generation of a diffusible signal that is dependent on MAD2 and BUBR1 (REFS 15–19), which prevents the E3 ubiquitin ligase complex anaphase promoting complex/cyclosome (APC/C)20 from degrading its mitotic targets cyclin B1 and securin (also known as pituitary tumour-transforming 1; PTTG1). In this state, exit from mitosis and the separation of sister chromatids are inhibited. As soon as the last kinetochore pair is attached to the microtubules at opposite spindle poles, the inhibitory diffusible signal is extinguished and the APC/C is fully activated through the release of inhibition of its cofactor, CDC20. This leads to the ubiquitylation of cyclin B1 and securin, the two crucial partners of CDK1 and the cysteine protease separase (also known as ESPL1), respectively. Degradation of cyclin B1 by the 26S proteasome leads to a rapid decline in CDK1 activity, allowing exit from mitosis. Securin is a small inhibitory chaperone of separase, the activity of which is essential for the dissolution of cohesin complexes at and near sister chromatid kinetochores. Degradation of securin by the 26S proteasome and release from inhibition by CDK1–cyclin B1 phosphorylation owing to cyclin B1 degradation leads conjunctly to activation of separase and cleavage of the SCC1 (also known as RAD21) component of cohesin; the net effect of this is the separation of sister chromatids. Both of these events, inhibition of CDK1 and activation of separase, are necessary for a correct metaphase-to-anaphase transition and faithful segregation of chromosomes.
Although it is clear that a diffusible inhibitory signal is generated at kinetochores and prevents APC/C acting on cyclin B1 and securin15, the nature of this event remains unclear. Musacchio and others have proposed a prion-like model based on two structural conformations adopted by MAD2 (REFS 21,22): open and closed. At this point the evidence for this model is biochemical but it accounts for the role of MAD2 at kinetochores, its interaction with CDC20 and the signal amplification required for the inhibition of the cell cycle by a single unoccupied kinetochore. Unoccupied kinetochores are known to recruit MAD1, which in turn binds with high affinity to MAD2 in its closed conformation. This MAD1–MAD2 complex is then thought to catalyse the conversion of open MAD2 monomers (the predominant form in the cytosol) to closed MAD2 forms that then bind to CDC20. This interaction serves a dual purpose: it inhibits the activity of APC/C (at least with regard to cyclin B1 and securin) and catalyses the further conversion of MAD2 open monomers to closed MAD2–CDC20 complexes, accounting for the required signal amplification.
Several observations substantially complicate this model. First, it is unclear what the role of BUBR1 is with regard to APC/C inhibition. BUBR1 is necessary for mitotic checkpoint function and is recruited to unoccupied kinetochores23. Its kinase domain is, however, dispensable for APC/C inhibition, and its amino (N)-terminal domain is sufficient to act as a pseudo-substrate inhibitor of the APC/C. Moreover, this APC/C inhibitory function is not dependent on the presence of BUBR1 at kinetochores. These findings have led to a modified MAD2 template model in which the heterodimeric CDC20–MAD2 closed conformer is required to deliver BUBR1 and perhaps BUB3 to the APC/C, where BUBR1 can then inhibit CDC20 function. The proposed mitotic checkpoint complex (MCC) composed of MAD2, CDC20, BUBR1 and BUB3 may be transient. MAD2 may depart, leaving behind a CDC20–BUBR1–BUB3 complex bound to the APC/C, although the duration of MAD2 persistence is still not completely resolved (for example, REFS 19,24).
Structural studies25 suggest that CDC20 is displaced from its active location on the APC/C when MCC components are present; an event that may in turn facilitate CDC20 ubiquitylation26, thereby maintaining a ‘checkpoint-on’ state. Here, ubiquitylation of CDC20 would continue so long as BUBR1–BUB3 (and perhaps MAD2) are still bound to the APC/C and would cease once the checkpoint is satisfied. Phosphorylation of MAD2 (REF. 27) and, recently, acetylation of BUBR1 (REF. 28) have been proposed to extinguish the binding to the APC/C and/or the stability of these components. This in turn would allow CDC20 to reoccupy its site on the APC/C, where it is protected from ubiquitylation. CDC20 would then assume its role in directing the APC/C to its principal downstream targets, securin and cyclin B1. Remarkably, cyclin A and NEK2 are ubiquitylated by the APC/C in the checkpoint-on state, adding further complexity to the inhibition of the APC/C. Substrate specificity and therefore substrate ordering seem to be key events in the different stages of mitosis but a molecular understanding of how substrate specificity arises remains limited.
In contrast to these findings, two independent reports have proposed that ubiquitylation of CDC20 by the E2 enzyme UBCH10 inactivates the checkpoint by blocking the association of CDC20 with MCC components29,30. The strongest evidence against this last model and in favour of one in which CDC20 ubiquitylation leads to its degradation during the checkpoint-on state comes from the finding that a form of CDC20 that lacks lysine and therefore cannot be ubiquitylated shows premature escape from mitotic arrest26.
Reconciling all of these findings and integrating them into the MAD2 template model will certainly require more complex biochemical models but, more importantly, these models will need to be tested in vivo to understand how a single unoccupied kinetochore can maintain a cell, at least for a certain period of time (as we discuss below), in mitosis.
Aneuploidy and CIN in tumours
The notion that CIN contributes to tumour initiation and/or progression is as old as our understanding of chromosomes. As mentioned, Boveri postulated more than 100 years ago that abnormalities in chromosome segregation could promote tumour formation1,2,26. Although some arguments can still be made for aneuploidy as simply a passenger event in tumours, three lines of observation argue otherwise.
First, in vitro transformation of cell lines through various genetic alterations that lead to CIN suggests aneuploidy has a direct causal role in tumorigenesis. Transformation of cells in culture has been a standard assay to determine the oncogenic or tumour suppressive nature of a gene for more than two decades31,32. Although the genetic events that must occur for a primary cell to become transformed may differ substantially from those that occur in human tumours, several now-established oncogenes and tumour suppressors were identified by transformation assays33–36. Among the mitotic checkpoint genes with roles that have been explored in in vitro transformation, securin overexpression in primary cells leads to marked aneuploidy and is sufficient for transformation37. Overexpression of aurora kinase A (AURKA), the function of which is required for centrosome maturation, bipolar spindle assembly and mitotic entry38, similarly leads to aneuploidy and transformation in human and rodent cells39,40 as a result of abnormal mitoses.
Second, perhaps the most robust causative data linking CIN to tumorigenesis comes from the study of mouse models of aneuploidy. Several laboratories have generated mouse models of aneuploidy based on mutations or transcriptional changes of mitotic checkpoint genes observed in tumours. An obvious caveat of all these individual studies is that these genes have non-mitotic functions that might explain their tumorigenic potential. In addition to their accepted mitotic functions, MAD2 (REF. 41) and RANBP2, a RAN GTPase binding protein that localizes to kinetochores during mitosis42, have been implicated in nuclear trafficking. Securin has also been linked to modification of p53 function43, and BUB1 and BUBR1 have been shown to play a part in the response to DNA damage44,45. Nevertheless, data from studies that have analysed various different genes involved in mitotic checkpoint control argue strongly for a contributory role of aneuploidy itself in tumour initiation and progression. TABLE 1 summarizes some of the mouse models of aneuploidy and their contributions to our understanding of CIN in human tumours. As we will describe in the next section, some of these models are more faithful to the mechanisms that are associated with CIN in human tumours than others but the message is the same: in general, CIN favours tumour formation.
Table 1.
Cancer models of CIN
| Gene | Cancer-associated mutation | Altered expression in tumours | Model | Tumour-associated phenotype in vivo |
|---|---|---|---|---|
| AURKA | Amplifications in different types of human cancer106–108 | Overexpressed in breast109, colorectal108,110, ovarian111, pancreatic112, gastric113, oesophageal, bladder107, cervical114, and head and neck cancer115 | Aurka+/− | Heterozygous mice develop lymphomas, hepatomas, lung adenocarcinomas and squamous cell carcinomas116 |
| Cre-CAT-Aurka; WAP-Cre | Overexpression induces mitotic abnormalities and mammary gland hyperplasia87 | |||
| MMTV-Aurka | Overexpression induces genetic instability preceeding mammary tumour formation40 | |||
| _Aurka_f/f | No tumour phenotype reported117 | |||
| AURKB | Overexpressed in astrocytomas118, seminomas119, ependymomas120, prostate cancer121 and non-small-cell lung carcinomas122; predictive factor for recurrence of hepatocellular carcinomas123 | No spontaneous models | Overexpression of a wild-type form or a non-degradable form in murine epithelial cells generates tumours in nude mice124 | |
| BUB1 | Mutated in colon, lung tumours125 and very low frequency of mutation in pancreatic cancer cells126; promoter hypermethylation in colon carcinoma127,128 | Reduced expression in AML128; overexpressed in breast cancer and cell lines129, in non-endometrioid endometrial carcinoma130, gastric cancer131, clear cell kidney carcinoma132, and thyroid carcinoma133; mutated in colon cancer cell lines and corresponding human samples134 | Bub1+/−, _Bub1_H/H, _Bub1_−/H | Heterozygous mice are more susceptible to DMBA-induced lung tumours; _Bub1_H/H mice develop spontaneous sarcomas and hepatocellular carcinomas; _Bub1_−/H mice have an increased incidence of lymphomas, lung adenomas and sarcomas54 |
| _Bub1_Δ2–3/Δ2–3 | 76% of mice (expressing hypomorphic BUB1 mutant that lacks exons 2 and 3) develop spontaneous lung and liver tumours135 | |||
| BUB1B (encodes BUBR1) | Promoter hypermethylation in colon carcinoma127 | Overexpressed in breast cancer and cell lines129, in gastric cancer131, clear cell kidney carcinoma132 and thyroid carcinoma133 | Bub1b+/− | No spontaneous tumours50; microadenomas and tubular adenomas of the colon, lung adenocarcinomas and liver neoplasms after AOM treatment51 |
| Bub1bH/H | No spontaneous tumours50; DMBA-treated mice are prone to lung tumours136 | |||
| BUB3 | Overexpressed in primary breast cancer129 and gastric carcinomas131 | _Bub3+/_− | Not determined53; no cancer predisposition137; no statistically significant increase in lung tumours after DMBA treatment52 | |
| _Bub3+/_−;_Trp53+/_− and _Bub3+/_−;_Rb1+/_− | No differences in the number or rate of tumours compared with single mutants137 | |||
| Bub3+/−;Rae1+/− | Increased incidence of lung tumours after DMBA treatment52 | |||
| CDC20 | Overexpressed in oral squamous cell carcinoma cell lines and in head and neck tumours138, pancreatic139, breast129, gastric140, ovarian cancer141, gliomas142 and in early-stage lung adenocarcinoma143 | Cdc20+/AAA (mutant cannot be inhibited by MAD2) | Spontaneous development of lymphomas and hepatomas at 24 months of age56 | |
| FZR1 (encodes CDH1) | Reduced expression in breast, colon and rectal tissue microarrays144; overexpressed in seminoma, neuroblasto ma, medulloblastoma, oesophageal adenoma, colon cancer, lung cancer, breast cancer and lymphoma145 | _Fzr1+/_− | 25% of Fzr1+/− mice develop epithelial neoplasias, such as adenocarcinoma and fibroadenoma of the mammary gland, lung, liver, kidney, testis and sebaceous gland tumours at long latencies5 | |
| CENPE | Low CENPE levels in benign tumours and increased levels in malignant pituitary neoplasias146 | _Cenpe+/_− | 10% of Cenpe+/− mice develop lymphomas and 10% develop lung adenomas with very long latencies147; decreases in the incidence of liver tumours and DMBA-induced tumours were reported for Cenpe+/− animals but neither was statistically significant | |
| _Cenpe+/_−;Cdkn2aARF_−/_− | Increased survival relative to single mutants148 | |||
| CCNB1 | Overexpressed in pulmonary adenocarcinoma149, non-small-cell lung cancer150,151, gastrointestinal stromal tumours152, oesophageal squamous cell carcinoma153,154, renal cell carcinoma155 and breast cancer156; correlates with poor survival in breast cancer157 | Ccnb1_−/_− | Die in utero158 | |
| NDC80 (encodes HEC1) | Overexpressed in lung cancer and correlates with poor prognosis159; overexpressed in lung, liver and brain tumours160 | CMV-TetO_Ndc80_ | 40% of mice develop tumours (lung and hepatocellular adenomas and sarcomas)71 | |
| MAD1L1 (encodes MAD1) | Mutated in cancer cells from lymphoid, pancreas, prostate, breast and lung tissues161,162 | Reduced expression in human gastric cancer, poorly differentiated tumours163,164 and hepatocellular carcinoma165; loss of MAD1 is implicated in tumour recurrence | _Mad1l1+/_− | 19% of mice develop spontaneous tumours at 18 months of age84 |
| _Mad1l1+/_−;_Mad2l1+/_−; _Trp53+/_− | Increased tumour frequency166 | |||
| MAD2L1 (encodes MAD2) | Rare mutations in bladder and breast cancer cells167,168 | Overexpressed in several tumour types68, such as malignant lymphoma169, liver cancer170, lung cancer171,172, soft tissue sarcoma173, hepatocellular carcinoma, gastric cancer174 and colorectal carcinoma175 | _Mad2l1+/_− | 27% develop lung tumours at 18 months of age104 |
| _Mad2l1+/−;Trp53+/_− | Increased tumour frequency166 | |||
| CMV-TetO_Mad2l1_ | MAD2 overexpression induces a wide range of neoplasias and accelerates tumorigenesis induced by MYC6. | |||
| TetO_Mad2l1_;TetO_Kras; Scgb1a1_-rtTA | MAD2 overexpression accelerates lung tumorigenesis induced by mutant KRAS200 | |||
| PTTG1 (encodes securin) | Overexpressed in pituitary37, pancreatic ductal carcinoma176, lung177,178, glioma179, hepatocellular carcinoma180,181, prostate182, ovarian183, colorectal184, thyroid185 cancers and multiple myeloma186; also a marker of metastatic tumours187,188 | _Pttg1_−/− | No tumours189 | |
| Pttg1_−/_−;_Rb1+/_− | Decrease in pituitary tumours relative to Rb1+/− (REF. 190) | |||
| Cga-Pttg1 | Hyperplasia and microadenomas of the pituitary191 | |||
| _Cga-Pttg1;Rb1+/_− | Increased frequency of anterior lobe tumours192 | |||
| PLK1 | Specific mutations in some cell lines alter protein stability193 | Upregulated in breast194, oesophageal194, lung195, colorectal cancer196 and anaplastic thyroid carcinoma197 | _Plk1+/_− | 27% develop lymphomas, lung adenocarcinomas, squamous cell carcinomas, and ovarian sarcomas198 |
| Plk1+/−;Trp53_−/_− | Higher frequency of tumours relative to single mutants198 | |||
| PLK4 | Loss of heterozygosity in hepatomas85 | Aberrant expression in colorectal cancer196 | _Plk4+/_− | Increased frequency of hepatocellular and lung carcinomas85 |
Third, a large amount of data collected from human tumours suggests that aneuploidy has a causative role in tumorigenesis by showing that CIN and chromosomal aberrations correlate with tumour grade and prognosis46,47. Further supporting this argument, genes involved in maintaining chromosome stability are frequently deregulated in human tumours48, as we will discuss in the next section. Finally, transcriptional expression profiles of aneuploid tumours have revealed a CIN signature that can be used to stratify lesions according to prognosis in an unbiased manner46. The fact that this CIN signature, which contains genes that are involved in a wide range of pathways, can predict clinical outcome even if genes that are regulated by the cell cycle are omitted provides further evidence that CIN plays a contributory part in the progression of these human tumours.
How is aneuploidy generated in human tumours?
Inevitably, most of the mechanistic studies that aim to answer this question have been carried out in mice and their results are summarized in TABLE 1. Many of these models have been generated based on the hypothesis that loss or downregulation of the mitotic checkpoint is responsible for CIN. Although this is largely the case in vitro and in model systems in vivo, as we discuss below, if these perturbations are to explain the mechanisms by which aneuploidy is generated in human cancer, there must be evidence for such changes in aneuploid human tumours. In other words, sufficiency for a cancer phenotype in mice or any other model cannot by itself be interpreted as an explanation for human disease without direct experimental evidence.
In mammalian cells a weakened mitotic checkpoint would be predicted to facilitate W-CIN as a result of premature exit from mitosis and premature separation of sister chromatids (FIG. 2). An overview of mouse models of aneuploidy reveals that this prediction is correct. Fibroblasts or lymphocytes derived from mice heterozygous for Mad2l1 (which encodes MAD2)49, Bub1b (which encodes BUBR1)50,51, Bub3 (REFS 52,53), Bub1 (REF. 54) and centromere protein E (Cenpe)55 or a _Cdc20_AAA mutant that cannot bind MAD2 (REF. 56) show varied levels of aneuploidy. In addition, several animal strains that have these genetic lesions develop tumours in various organs at late stages of life or are more prone to tumours in sensitized backgrounds. Nevertheless, several separate lines of evidence argue against the loss of mitotic checkpoint gene function as the main causative mechanism for aneuploidy in human tumours.
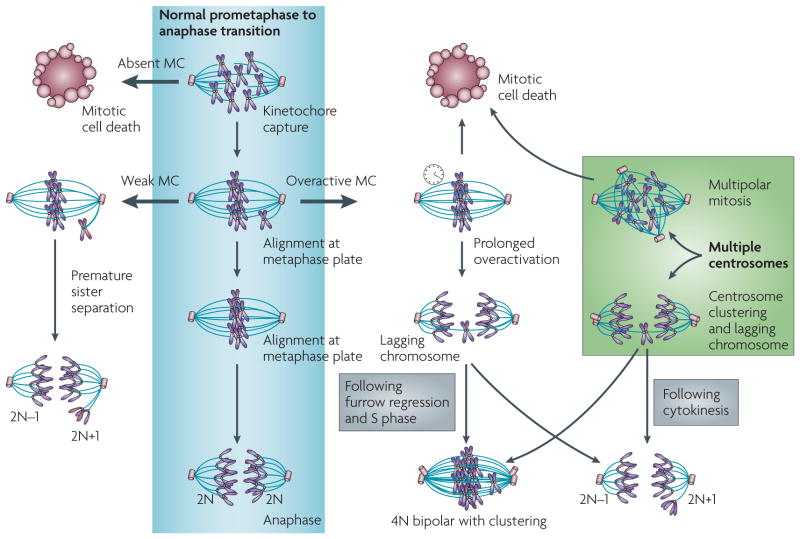
Figure 2. Multiple mechanisms leading to aneuploidy.
The normal mitotic checkpoint (MC) events from prometaphase to anaphase are shown in the centre. An absent checkpoint leads to mitotic cell death. A weak checkpoint (left) leads to premature sister chromatid separation and near-diploid aneuploidy. An overactive checkpoint (right) can lead either to mitotic cell death or lagging chromosomes and subsequent near-diploid aneuploidy or tetraploidy. Multiple centrosomes can have similar consequences to an overactive checkpoint. A multipolar mitosis leads to cell death unless centrosomes cluster, in which case the likelihood of lagging chromosomes is high.
Through extensive analyses of aneuploid human tumours, it is now increasingly clear that mutations in mitotic checkpoint genes are rare (TABLE 1 and reviewed in REF. 57). As we have mentioned, complete loss of the mitotic checkpoint is lethal at the cellular and organismal levels. Note that conditional inactivation of Bub1 in adult male mice58 impairs fertility without any decrease in viability but no other tissues were examined, and it is unclear what the penetrance of inactivation was in this case. It therefore remains possible that actively proliferating tissues were primarily affected in this model and that the conditional Bub1 mice survived because of incomplete penetrance. Downregulation of mitotic checkpoint genes, which is another putative mechanism for weakening the mitotic checkpoint, is also extremely rare. Importantly, the observations of decreased levels of mitotic checkpoint genes in cancer cell lines are often confounded by the lack of adequate controls, such as comparing the levels of MAD2 in various cancer cell lines with those of HeLa cells59–62. The expression of several genes that are required for mitosis and the mitotic checkpoint (MAD2L1, BUB3, polo-like kinases, CDC20, F-box protein 5 (FBXO5, also known as EMI1), NDC80 (also known as HEC1), PTTG1, cyclin B1 (CCNB1), CENPE and CENPA, among others63) is under control of the E2f family of transcription factors and therefore can vary depending on the level of inhibition of the Rb pathway, the number of cells in G2/M phase of the cell cycle in an asynchronous population and the number of quiescent cells. These aspects all vary markedly between cell lines. How one defines a normal level of expression is also a key point here. It is reasonable to assume that the only adequate normal value is that of normal adjacent tissue to the primary tumour, provided the adjacent tissue is proliferating (which is seldom the case). In the case of cancer cell lines, normal adjacent tissue cannot be procured. Further confounding this issue, the levels of a mitotic checkpoint protein in a non-primary cell line relative to HeLa cells say little about the mitotic checkpoint status of that cell line. Indeed, there are few well-documented examples of robust functional mitotic checkpoint defects in tumour cells that have reduced expression of checkpoint proteins.
Several heritable cancer predisposition syndromes result from loss-of-function mutations in genes essential for the DNA damage checkpoint and DNA repair pathways. Li–Fraumeni syndrome (TP53), hereditary non-polyposis colorectal cancer (MLH1 and MSH2), xeroderma pigmentosum (Xp family) and ataxia–telangiectasia (ataxia–telangiectasia mutated; ATM) are a few of the well-recognized ones. The existence of a range of these syndromes underscores two important points: first, that mutation of genes that control the DNA damage checkpoint and DNA repair pathways can be viable at a cellular, and often at an organismal level, and second, the accumulating DNA damage contributes to tumorigenesis. In the case of the mitotic checkpoint, only one genetic disorder has been associated with a mitotic checkpoint gene. Mosaic variegated aneuploidy (MVA) is an autosomal recessive disorder characterized by growth retardation, microcephaly and mosaic aneuploidies, predominantly monosomies and trisomies. Patients also show a high incidence of childhood tumours (Wilms’ tumour, rhabdomyosarcoma and leukaemia). The disease has been genetically mapped to BUBR1 (REF. 64), and CIN is thought to be the driving force for developmental defects and tumour formation. The severity of the phenotype in patients with MVA and the lack of other related syndromes reinforce the notion that the mitotic checkpoint is crucial for normal organism growth and not just for the prevention of genomic abnormalities that result from external stress.
Finally, several cancer cell lines with marked CIN have a robust mitotic checkpoint when treated with microtubule-stabilizing drugs65,66. The strongest evidence against loss or downregulation of the mitotic checkpoint as a cause of aneuploidy in tumours is evident by looking at the transcriptional profiles of aneuploid tumours. In most cases, genes essential for the mitotic checkpoint are upregulated, sometimes to very high levels (Oncomine48,67 and REF. 46). Although this may be the result of unrestricted proliferation in the absence of a functional Rb pathway, the consequence is not an absent or weakened checkpoint but, most likely, an overactive one66. Moreover, in retinoblastoma tissue samples, high levels of MAD2 are not confined to mitotic cells but are also found in interphase cells68, arguing that it is not only the high mitotic index that contributes to MAD2 overexpression after Rb pathway inhibition.
CIN has long been known to be a dominant phenotype in cancer cell lines69 and overactivation of the mitotic checkpoint in cancer cell lines fits this observation readily. It has also been proposed that aneuploidy might be an early event in cancer evolution, which induces a quasi-stable karyotypic state that is balanced by selection towards tumorigenesis70. Inhibition of the Rb pathway and the consequent overexpression of key mitotic checkpoint genes may efficiently initiate tumours because of this coupling of a loss of a tumour suppressor pathway to karyotypic instability.
Overexpression of MAD2 and HEC1 in inducible mouse models has already been shown to be sufficient for generating aneuploidy and initiating tumour formation6,71. In these models, hyperactivation of the mitotic checkpoint is predicted to lead to prolonged mitosis and the failure of one or more sister chromatids to separate on schedule. This would then increase the likelihood of merotelic attachments and lagging whole chromosomes or, in the extreme case, tetraploidy following mitotic slippage (also known as adaptation; FIG. 2). These events are readily seen in cells that overexpress MAD2L1 or NDC80. Although overexpression of these mitotic genes might have nonmitotic consequences or off-target effects, a recent elegant study72 has shown that prolonged activation of the mitotic checkpoint using spindle-stabilizing agents or the mitotic kinesin family member 11 (KIF11, also known as EG5) inhibitor monastrol also leads to lagging sister chromatids, merotelic attachments and aneuploidy after mitotic slippage. Given that mitotic slippage is a well-recognized response to prolonged mitotic arrest73, mitotic checkpoint overactivation (that is, prolonged inhibition of the APC/C and, consequently, abnormal stabilization of cyclin B1 and securin) could lead to an increased rate of aneuploidy by allowing lagging sister chromatids and merotelic attachments to accumulate. Eventually, mitotic slippage would occur, generating potentially tumorigenic aneuploid progeny. Interestingly, although the molecular events leading to mitotic slippage are still unclear, it is thought to result from the degradation of cyclin B1 in an APC/C-dependent manner despite the activation of the mitotic checkpoint74.
Therefore, overactivation of the mitotic checkpoint could be a widespread phenomenon in tumours with CIN. In vitro studies using nocodazole and monastrol, together with in vivo studies overexpressing mitotic checkpoint genes, favour the hypothesis that aneuploidy in tumours is largely a consequence of the upregulation of mitotic genes and subsequent mitotic checkpoint overactivation. It is of interest that, as mentioned above, several mitotic checkpoint genes are direct E2f targets, indicating that loss of a major tumour suppressor pathway (that is, Rb inhibition of E2f) leads not only to uncontrolled proliferation but is also directly associated with the generation of mitotic CIN75–79.
Centrosome amplification has also been tightly associated with aneuploidy as a result of aberrant mitoses, and recent studies have shed light on the mechanistic connection between the two. By looking at how cells that have more than two centrosomes and multipolar spindles survive, several cell lines have been found to preferentially cluster multiple centrosomes to two poles, thereby generating a functional bipolar spindle80,81. Inhibition of centrosome clustering by short-hairpin RNA (shRNA) targeting of KIFC1, which encodes a non-essential kinesin motor protein, led to lethality of multipolar cells81. Moreover, clustered bipolar spindles (in tetraploid cells that had more than two centrosomes) were shown to have an increased frequency of merotely, leading to lagging chromosomes and segregation errors82,83. Tetraploid cells that had two centrosomes were not observed to have an increased number of lagging chromatids compared with diploid cells, arguing that it is the initial microtubule attachment and subsequent clustering of multiple centrosomes that is conducive to the generation of aneuploidy. An interesting possibility then is that overexpression of AURKA, among other genes that regulate mitotic entry and centrosome homeostasis, results in mitotic abnormalities through centrosome amplifications. One can therefore propose that clustering of centrosomes in cells that have multipolar spindles leads to merotelic attachments and that these are conducive to aneuploidy; similar to what is observed during mitotic checkpoint overactivation.
Consequences of CIN
The notion that CIN serves as a tumorigenic driving force has been expanded by a series of observations from mouse models to include the idea that CIN might also be tumour suppressive in certain contexts. In this section we discuss the evidence from mouse models regarding the consequences of CIN at the cellular and organismal levels. These studies underscore the importance of generating mouse models that faithfully recapitulate the biology of human disease to draw physiologically meaningful conclusions.
As noted above, both mitotic checkpoint weakness and mitotic checkpoint overactivation can lead to CIN through different mechanisms. Haploinsufficiency for Cenpe55, Mad2l1 [REF. 49], Mad1l1 (REF. 84), Fzr1 (which encodes CDH1)5, Plk4 (REF. 85) and a hypomorphic allele of Bub1 (REF. 54) all lead to moderate levels of aneuploidy and an increase in the incidence of spontaneous late-onset tumours of lymphoid origin and tumours in some epithelial tissues (especially lung and liver) in mice. This is also the case for the Cdc20AAA mutant, the product of which fails to interact with MAD2; homozygous Cdc20AAA mice are embryonic lethal yet heterozygous adults are tumour prone56. Although spontaneous tumour onset does not necessarily result from CIN, as indicated by the lack of such a phenotype in Bub1b+/− (REFS 50,51), Bub3+/− (REFS 52,53) and heterozygous RNA export 1 (_Rae1+/_−)52 mice, CIN in these cases often increases sensitivity to carcinogen-induced tumours. Why spontaneous tumour onset differs between the different animal models of CIN is unclear but it does not seem to be related to the degree of CIN86. Other mitotic checkpoint-independent functions of these genes could account for these differences between the models but this has yet to be tested86.
Underscoring the previously mentioned observations from human tumours, spontaneous tumorigenesis is also a consequence of checkpoint overactivation, as seen when MAD2 (REF. 6) or HEC1 (REF. 71) is overexpressed, or in the presence of centrosome amplifications as seen when AURKA is overexpressed87. In the case of ubiquitous overexpression of MAD2 or HEC1, spontaneous tumour onset occurs earlier and in a wider range of tissues than that seen for partial loss-of-function mutations. AURKA was only overexpressed in transplanted mammary epithelial cells and so it is unclear whether ubiquitous overexpression of AURKA would show similar phenotypes to MAD2 and HEC1 overexpression in mice. As discussed above, both mitotic checkpoint overactivation and centrosome amplifications lead to lagging chromosomes and merotelic attachments, which, as discussed above, facilitate aneuploidy.
W-CIN and other collateral forms of DNA damage acquired during mitosis, such as chromosome breaks, deletions and amplifications (S-CIN), might together lead to more robust tumour penetrance. Interestingly, gene expression signatures derived from human tumours with CIN have shown that genes involved in DNA damage repair pathways are overexpressed in aneuploid tumours88. Overexpression of these genes seems to be necessary for resistance to chemotherapeutic agents that target microtubules, both for chromosomally unstable cell lines and in a subset of ovarian and breast tumours. These results suggest that the DNA damage repair pathway may be activated during the generation of CIN and is required for subsequent viability, although this hypothesis remains to be tested. MAD2 overexpression, which leads to transient mitotic arrest, has been shown to lead not only to W-CIN but also to double-strand breaks, interstitial deletions and amplifications6. The prevalence of mitotic checkpoint pathway hyperactivation through the overexpression of MAD2 or other components might therefore explain the common appearance of the DNA damage response in a wide spectrum of aneuploid tumours. Prolonged mitotic arrest through the chemical inhibition of microtubule function also leads to a high incidence of lagging chromatids, merotely and chromosome bridges, all of which could lead to DNA breaks as the cleavage furrow progresses during cytokinesis. Whether DNA damage also occurs in other models of aneuploidy has not been determined, but it might underlie some of the differences in tumour phenotypes observed between different models of CIN.
It is important to note that none of the genetic mechanisms used to generate aneuploidy in animal models results in as rapid an onset in tumorigenesis as seen with activating mutations of classic oncogenes, such as Ras family members89 and MYC90, or the loss of classic tumour suppressor genes, such as TP53 (REF. 91) and RB1 (REF. 92). This could be owing to the fact that the induced genomic instability is sufficient to induce transformation but is held in check by an uncharacterized surveillance mechanism that efficiently destroys transformed cells and persists for many months. Alternatively, low-level genomic instability may require multiple events to first establish the transformed state.
Three studies suggest that low-level aneuploidy such as that generated in the above-mentioned models has detrimental effects on the viability of primary cells. Thompson and Compton72 studied the effects of CIN generated by transient mitotic checkpoint overactivation using microtubule-stabilizing agents or monastrol on single-cell colonies of two sets of primary cell lines. Single-cell colonies were then analysed by chromosome-specific fluorescence in situ hybridization (FISH) to measure aneuploidy. Although mitotic checkpoint overactivation clearly increased the fraction of aneuploid cells in the first few passages, cells in later passages were remarkably euploid. It is still unclear whether the increase in the proportion of euploid cells is a result of a decrease in proliferation rate or increased cell death of aneuploid cells. Nevertheless, it is reasonable to conclude from these studies that aneuploidy is detrimental to the fitness of primary cells.
Williams et al.93 used a different strategy to generate isogenic lines of murine fibroblasts that carried trisomies for chromosomes 1, 13, 16 and 19 in the background of Robertsonian translocations. The decreased growth rates, immortalization rates and metabolic activity in most of the trisomic lines led the authors to conclude that low-level aneuploidy in primary cells has detrimental effects not only on organismal fitness but also on cellular fitness and viability. Although the Robertsonian translocations by themselves had no effect on immortalization times, it is possible that the combination of trisomies and Robertsonian translocations were both required for the observed properties of the cells.
Finally, although inducible MAD2 overexpression in mice leads to the appearance of tumours in a range of different organs, MAD2 overexpression in fibroblasts has a marked negative effect on cellular viability6. The overall conclusion from these studies is that in primary cells CIN is detrimental to viability and is therefore selected against. Whether a specific ‘aneuploidy sensor’ is responsible for this impaired fitness or whether it results from an alteration in global transcription remains to be determined. Evidently, this aneuploidy sensor is not 100% efficient, as some trisomies or monosomies are carried to term and, in the cases of Down’s, Turner’s and Klinefelter syndromes, tolerated with few global abnormalities. Nor is this sensor a ubiquitous property of primary cells, as mouse embryonic fibroblasts that lack any Rb family members (_Rb1_−/−, _Rbl1_−/− (which encodes p107) and _Rbl2_−/− (which encodes p130)) rapidly tend towards tetraploidy in the first 20 passages94, although the Rb pathway may be necessary for this sensor. Nevertheless, these findings may explain why mouse models of aneuploidy take so long to develop tumours. Most abnormal mitoses will not generate aneuploid cells that have a substantial growth advantage, and it is only after a particularly long period of time that transformed progeny arise.
A study in 2007 by the Cleveland lab55 suggested that aneuploidy could both promote and suppress tumorigenesis depending on the tissue and genetic contexts. Cenpe+/− mice developed CIN and spontaneous tumours in a similar pattern to other mitotic checkpoint genes. The evidence for the tumour suppressive role of aneuploidy was a 50% reduction in the incidence of liver tumours and a reduction in DMBA-induced tumours, neither of which were statistically significant and could therefore be due to chance alone. In addition, there was a statistically significant but only slight delay in tumour-free survival in _Cdkn2aARF-_null mice, which is a common tumour-prone background used to explore tumour suppressor effects. Nonetheless, other reports have since shown similar results. The incidence of small intestinal tumours was reduced twofold by Bub1b haploinsufficiency in the adenomatosis polyposis coli (Apc)Min/+ mouse model95. Remarkably, the incidence of colon tumours in this model was increased tenfold. The Malumbres group5 showed that, although Fzr1+/− animals have increased rates of aneuploidy and an increased incidence of spontaneous tumours compared with wild-type controls, treatment with the carcinogen DMBA results in fewer lung tumours.
If CIN has a tumour suppressive role as a result of excessive genomic damage and subsequent apoptosis or other forms of cell death, one would expect to see such events in the corresponding tissues. Except for the presence of apoptotic cells in the areas surrounding the small intestinal tumours of Bub1b+/−;ApcMin/+ mice, no such evidence exists. Perhaps more importantly, it is unclear whether it is CIN itself that contributes to the decreased tumour incidence. The ploidy status of tumours or earlier preneoplastic lesions needs to be examined carefully to draw such a conclusion. This type of analysis is confounded by the difficulty of growing tumour cells in vitro for metaphase chromosome counts, and the extrapolation of karyotypes from fibroblasts or lymphocytes from animals that develop intestinal (in the case of Bub1b+/−;ApcMin/+ mice) or lung and skin tumours (using DMBA) is not sufficient evidence for aneuploidy in tumour lesions. One way to obtain these data would be to use FISH or array-comparative genomic hybridization (CGH) to determine the extent of aneuploidy in the normal tissues and in the early lesions that arise in these models. However, CGH results are often confounded by the fact that only clonally expanded genomic lesions can be detected from a population of unstable cells and so in these cases only methods that offer single-cell resolution (such as FISH) would identify random CIN.
Finally, the possibility of non-cell-autonomous effects of adjacent tissues or infiltrating bone marrow-derived cells on tumour growth needs to be addressed. It is possible that tumour suppression is a result of non-cell-autonomous effects through which CIN in cells that compose the tumour microenvironment modulates the host response to the primary tumour. Conditional inactivation studies of selected cell types in mice and careful examination of ploidy changes early in the tumorigenic process need to be performed. Such non-cell-autonomous effects could also be responsible for the generation of tumours that result from organism-wide mitotic checkpoint partial loss-of-function or overactivation but, in general, changes in the tumour microenvironment that promote tumorigenesis on their own are much less common than those that lead to tumour suppression.
Ultimately, it is possible and even likely that excessive CIN inhibits cell viability and, as a result, tumour formation. Cell lethality is a consequence of complete loss of the mitotic checkpoint. Nevertheless, much like the case with ionizing radiation, it seems clear that moderate levels of genomic instability can be the evolutionary fuel that generates pro-tumorigenic changes. Most human tumours show clear evidence of CIN and, in these lesions, the tumour suppressive role that this level of genomic instability initially conferred is eventually overcome. The idea that moderate levels of CIN may have tumour suppressive effects remains an important observation in a few mouse models but a more careful analysis will be required to establish whether this is a general principle that is likely to apply to human disease.
Drugging the mitotic checkpoint pathway
Many chemotherapeutic agents result in activation of the mitotic checkpoint. Microtubule-stabilizing drugs (such as taxanes) and depolymerizing drugs (such as vinka alkaloids) are regularly used as mainstay therapy in several solid tumours, often having marked efficacy96. Nevertheless, the substantial side effects of these drugs, which result from myelosuppression and neurotoxicity, have spawned a search for newer, more specific drugs that might target cells that have an abnormal mitotic checkpoint. TABLE 2 summarizes some of the classic chemotherapeutic agents that target mitosis and some of the more recently developed anti-mitotic agents in clinical trials. Remarkably, a recent study65 has shown that the responses of cells to both classical anti-mitotic drugs and newer agents (such as EG5 inhibitors) show substantial variation, not only between different cell lines but also between cells of the same cell line. Irrespective of whether the cancer cell lines studied showed CIN or not, several responses were elicited following exposure to the drug, ranging from death during mitosis to mitotic slippage, death in the following interphase to a second round of mitosis. The authors propose a model in which DNA damage that is incurred during a prolonged mitotic arrest leads to caspase 9-mediated cell death, but that the timing of cell death depends on whether cells remain in mitosis or slip through it following degradation of cyclin B1. These two thresholds, activation of caspase 9 and degradation of cyclin B1, are thought to be responsible for the observed intra- and inter-line variations in drug response65. Given the pro-tumorigenic effects of mitotic checkpoint overactivation, it is possible that the use of microtubule drugs that overactivate the mitotic checkpoint might occasionally result in tumour progression after an initial response. In line with these findings, a recent study has shown that preventing mitotic slippage by downregulating CDC20 may increase the sensitivity of tumour cells to microtubule-targeting agents97, providing an alternative therapeutic window.
Table 2.
Drugs that target mitosis*
| Mechanism of action or target | Examples of drugs | Approved indications | Clinical trial stage | Company (clinical trials.gov identifier) |
|---|---|---|---|---|
| Microtubule stabilization | Docetaxel | Breast, prostate, non-small-cell lung cancer, gastric cancer, head and neck cancer | FDA approved | |
| Paclitaxel | Ovarian cancer, breast cancer, non-small-cell lung cancer and Kaposi’s sarcoma | FDA approved | ||
| Microtubule depolymerization | Vinblastine | Hodgkin’s and non-Hodgkin’s lymphoma, mycosis fungoides, testicular cancer and Kaposi’s sarcoma | FDA approved | |
| Vincristine | Leukaemias, Hodgkin’s and non-Hodgkin’s lymphoma, neuroblastoma, rhabdomyosarcoma, Wilms’ tumour and Kaposi’s sarcoma | FDA approved | ||
| Vinorelbine | Non-small-cell lung cancer | FDA approved | ||
| KIF11 kinesin inhibitors | Ispinesib | ND | Phase I/II in metastatic breast cancer, lymphoma and multiple other solid tumours | Cytokinetics (NCT00607841) —breast cancer |
| SB-743921 | ND | Phase I in solid tumours and Phase II in non-Hodgkin’s lymphoma | GlaxoSmithKline (NCT00136513) and Cytokinetics (NCT00343564) | |
| MK0731 | ND | Phase I in solid tumours | Merck (NCT00104364) | |
| ARRY-520 | ND | Phase I/II in advanced leukaemia and multiple myeloma | Array BioPharma (NCT00637052 and NCT00821249) | |
| AZD4877 | ND | Phase II in advanced bladder cancer | AstraZeneca (NCT00661609) | |
| Aurora kinase inhibitors | MLN8237 | ND | Phase II in AML, ALL, ovarian cancer and non-Hodgkin’s lymphoma | Millenium Pharmaceuticals (NCT00830518, NCT00739427, NCT00853307 and NCT00807495) |
| AT9283 | ND | Phase I and II in leukaemias | Astex Therapeutics (NCT00522990) | |
| AZD1152 | ND | Phase I and II in AML | AstraZeneca (NCT00952588) | |
| CENPE inhibitor | GSK923295 | ND | Phase I in refractory cancer | GlaxoSmithKline (NCT00504790) |
| PLK inhibitors | BI 2536 | ND | Phase I in non-Hodgkin’s lymphoma | Boehringer Ingelheim (NCT00243087) |
| ON 01910 | ND | Phase II in ovarian cancer and myelodysplastic syndrome | Onconova (NCT00856791 and NCT00906334) | |
| CDC2 inhibitor | P276-00 | ND | Phase II in malignant melanoma | Piramal Life Sciences (NCT00835419) |
The fact that cells with CIN have evolved to survive repeated rounds of mitotic arrests suggests that it might be preferable to inhibit the mitotic checkpoint or the centrosome abnormalities previously described. The MPS1 and AURKA kinases show some promise in this regard, and the cell lethality of mitotic checkpoint inhibition supports this approach although a means of specifically targeting tumour cells is not yet apparent. Targeted drug delivery or perhaps the hypersensitivity of tumour cells addicted to an overactive checkpoint might provide the therapeutic window required for drug efficacy.
In addition, centrosome clustering seems to be a survival mechanism used by cells that might otherwise carry out an abnormal and lethal multipolar mitosis81. Centrosome clustering therefore becomes an attractive target that is remarkably specific to cancer cells. Indeed, the normally non-essential kinesin motor protein KIFC1 is required for the viability of extra centrosome-containing cells81. It remains unclear, however, how efficient this targeting approach will be given the high rate of escape of cancer cells already observed in cell line analyses.
Future perspectives
The role of CIN in cancer remains filled with questions and some contradictions between the observations that arise from different laboratories and different model organisms. Although it now seems likely that CIN provides the evolutionary fuel to initiate and propagate the transformed state in several solid tumours, the oncogenic pathways that are activated or the tumour suppressor pathways compromised have yet to be elucidated. In addition, although the role of CIN in inhibiting tumour formation in humans is a possibility, the nature of this tumour suppressive role remains poorly defined. We stress that the observations made in model organisms must be viewed in relation to human cancer. The coming years will most likely see new answers to questions, such as when does mitotic CIN arise in human tumours and in which tumour types does it have an important role in growth, progression and/or metastasis? Can cells that have mitotic CIN be targeted efficiently from a therapeutic standpoint? On the 200th anniversary of the birth of Charles Darwin, it is striking that an analysis of evolution and natural selection — in this case in the form of CIN and cancer progression — is at the forefront of our battle against this devastating disease.
At a glance.
- Chromosomal instability (CIN), the inability to correctly segregate sister chromatids during mitosis, provides the evolutionary fuel to initiate and propagate the transformed state of multiple forms of cancer.
- The mitotic checkpoint is seldom lost or weakened in human tumours.
- Mitotic checkpoint overactivation is a more frequent observation in human tumours and is sufficient to generate CIN in vivo and in vitro. Mitotic checkpoint overactivation results in a prolonged mitosis, abnormal stabilization of cyclin B1 and securin, and an increased incidence of merotelic attachments and lagging chromosomes.
- Many of the key regulators of the mitotic checkpoint are downstream targets of the Rb tumour suppressor pathway and are therefore upregulated in most human tumours.
- The consequences of CIN are manifold and context-dependent. Although CIN can initiate tumour formation in many mouse models, under some conditions it decreases cellular fitness, providing a potential tumour suppressor effect. This effect is nevertheless often overcome, giving rise to the karyotypic complexity observed in advanced tumours.
- Mitotic checkpoint overactivation could prove effective as a novel therapeutic target as mitotic checkpoint loss is incompatible with cellular viability.
Acknowledgments
We apologize to those authors whose work is not cited because of space limitations. We would like to thank A. Unni and W. Forrester for helpful discussions of the manuscript. J.M.S. is supported by a Breast Cancer Research Program Predoctoral Traineeship Award from the Department of Defense (Congressionally Directed Medical Research Program). R.S. was supported by the Charles H. Revson Foundation. R.B. is supported by the National Institutes of Health.
Glossary
Chromosome instability
The inability to maintain a correct chromosome complement after cell division.
Aneuploidy
An abnormal chromosome number.
Mitotic checkpoint
A cell cycle checkpoint that arrests cell division at metaphase until all sister kinetochores are attached to microtubules from opposite spindle poles.
Breakage–fusion–bridge cycles
A process of amplification in which two centromeres of a dicentric chromosome are pulled to opposite poles during mitosis. If the chromosome breaks then the double-stranded breaks persist in the following S-phase and can contribute to translocations or form new dicentric chromosomes that continue the process of instability.
Whole chromosome instability
This describes CIN in terms of abnormal numbers of chromosomes.
Segmental chromosome instability
This describes CIN in terms of structural abnormalities, such as translocations, inversions, interstitial deletions and amplifications.
Kinetochore
The protein complex that assembles around centromeric chromosome regions and is the source of the signal that activates the mitotic checkpoint and the site of spindle fibre attachment.
Spindle pole
The site of origin of microtubule fibres in mitosis. In most cells this site is delineated by the presence of centrosomes that act as microtubule organizing centers.
Cohesin
A protein complex composed of structural maintenance of chromosomes 1A (SMC1A), SMC3, sister chromatid cohesion 1 (SSC1, also known as RAD21) and SSC3 (also known as stromal antigen 1), the function of which is to topologically link sister chromatids before metaphase.
Anaphase promoting complex/cyclosome
A large E3 ubiquitin ligase complex that degrades cyclin B1 and securin once the mitotic checkpoint is satisfied.
Spindle poison
A compound that affects microtubule function and therefore mitotic spindle formation by stabilizing (such as taxanes) or depolymerizing (such as vinka alkaloids or nocodazole) microtubules.
Transformation
A mechanistically defined process in which a primary cell acquires the ability to grow indefinitely in vitro (immortalization), form colonies in soft agar (anchorage-independent growth) and form tumour xenografts when implanted intradermally in nude mice.
Microcephaly
An abnormally small head circumference, which usually results from abnormal brain development.
Mosaic aneuploidy
A tissue in which groups of cells contain chromosome complements that differ from those of neighbouring cells.
Merotelic attachment
When a single kinetochore is attached to microtubules from two spindle poles rather than to one pole.
Mitotic slippage
The process by which a cell arrested in mitosis proceeds through anaphase despite an active mitotic checkpoint.
Monastrol
A small molecule inhibitor of the plus-end directed KIF11 kinesin motor, the function of which is required for chromosome segregation in mitosis.
Nocodazole
A chemical inhibitor of microtubule polymerization often used to activate the mitotic checkpoint and therefore arrest cells in the G2/M phase of the cell cycle.
Lagging chromosome
In anaphase, pairs of sister chromatids that remain at the metaphase plate, often as a consequence of merotelic attachment, can be the source of aneuploidy in the resulting daughter cells. This is distinct from a single chromatid that fails to segregate upon disjunction from its sister chromatid.
Chromosome bridge
In anaphase, a chromosome that bridges the two separating daughter nuclei as a result of abnormal attachments.
Robertsonian translocation
A non-reciprocal chromosomal translocation in which two distinct acrocentric chromosomes become fused and share a single centromere.
DMBA-induced tumour
An induced tumour model in mice in which the carcinogen DMBA (7,12-dimethylbenz(a) anthracene) is applied to the skin of 5–7 day old pups. This results in the appearance of skin and lung tumours once animals reach adulthood.
Array-comparative genomic hybridization
A genomic DNA hybridization technique that allows high-resolution analysis of copy number changes between two populations (such as normal versus tumour DNA).
Non-cell-autonomous effect
A phenotypic effect seen in a field of cells that are mediated by cells that are not part of that field, such as the clearance of tumour cells by bone marrow cells or cells of the tumour microenvironment.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
DATABASES
Entrez Gene: http://www.ncbi.nlm.nih.gov/gene
Apc | ATM | Cdkn2aARF | CENPA | Cenpe | FBXO5 | Fzr1 | KIFC1 | MLH1 | MSH2 | NDC80 | Plk4 | Rae1 | RB1 | Rbl1 | Rbl2
OMIM: http://www.ncbi.nlm.nih.gov/omim
ataxia–telangiectasia | Li–Fraumeni | mosaic variegated aneuploidy
UniProtKB: http://www.uniprot.org
AURKA | BUB1 | BUB3 | BUBR1 | CDC20 | CDK1 | cyclin A | cyclin B1 | KIF11 | MAD1 | MAD2 | MPS1 | NEK2 | p53 | RANBP2 | SCC1 | securin | separase | UBCH10
References
- 1.Boveri T. Über mehrpolige mitosen als mittel zur analyse des zellkerns. Verh Phys Med Ges Würzburg. 1902;35:67–90. [Google Scholar]
- 2.Boveri T. Zur Frage der Entstehung Maligner Tumoren. Gustav Fischer; Jena, Germany: 1914. [Google Scholar]
- 3.Ricke RM, van Ree JH, van Deursen JM. Whole chromosome instability and cancer: a complex relationship. Trends Genet. 2008;24:457–466. doi: 10.1016/j.tig.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Artandi SE, et al. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature. 2000;406:641–645. doi: 10.1038/35020592. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Higuera I, et al. Genomic stability and tumour suppression by the APC/C cofactor Cdh1. Nature Cell Biol. 2008;10:802–811. doi: 10.1038/ncb1742. [DOI] [PubMed] [Google Scholar]
- 6.Sotillo R, et al. Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell. 2007;11:9–23. doi: 10.1016/j.ccr.2006.10.019. The authors show that inducible MAD2L1 overexpression leads to widespread W-CIN and S-CIN in murine fibroblasts and tumour formation when expressed in mice under a ubiquitous promoter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleaver JE. Cancer in xeroderma pigmentosum and related disorders of DNA repair. Nature Rev Cancer. 2005;5:564–573. doi: 10.1038/nrc1652. [DOI] [PubMed] [Google Scholar]
- 8.Kunkel TA, Erie DA. DNA mismatch repair. Annu Rev Biochem. 2005;74:681–710. doi: 10.1146/annurev.biochem.74.082803.133243. [DOI] [PubMed] [Google Scholar]
- 9.Rustgi AK. The genetics of hereditary colon cancer. Genes Dev. 2007;21:2525–2538. doi: 10.1101/gad.1593107. [DOI] [PubMed] [Google Scholar]
- 10.Verdun RE, Karlseder J. Replication and protection of telomeres. Nature. 2007;447:924–931. doi: 10.1038/nature05976. [DOI] [PubMed] [Google Scholar]
- 11.Lobrich M, Jeggo PA. The impact of a negligent G2/M checkpoint on genomic instability and cancer induction. Nature Rev Cancer. 2007;7:861–869. doi: 10.1038/nrc2248. [DOI] [PubMed] [Google Scholar]
- 12.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nature Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 13.Nasmyth K. How do so few control so many? Cell. 2005;120:739–746. doi: 10.1016/j.cell.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Haering CH, Farcas AM, Arumugam P, Metson J, Nasmyth K. The cohesin ring concatenates sister DNA molecules. Nature. 2008;454:297–301. doi: 10.1038/nature07098. Cohesin complexes are shown conclusively to topologically link sister chromatids. [DOI] [PubMed] [Google Scholar]
- 15.Rieder CL, Cole RW, Khodjakov A, Sluder G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J Cell Biol. 1995;130:941–948. doi: 10.1083/jcb.130.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang G, Yu H, Kirschner MW. The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation. Genes Dev. 1998;12:1871–1883. doi: 10.1101/gad.12.12.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shannon KB, Canman JC, Salmon ED. Mad2 and BubR1 function in a single checkpoint pathway that responds to a loss of tension. Mol Biol Cell. 2002;13:3706–3719. doi: 10.1091/mbc.E02-03-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sudakin V, Chan GK, Yen TJ. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J Cell Biol. 2001;154:925–936. doi: 10.1083/jcb.200102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wassmann K, Benezra R. Mad2 transiently associates with an APC/p55Cdc complex during mitosis. Proc Natl Acad Sci USA. 1998;95:11193–11198. doi: 10.1073/pnas.95.19.11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sudakin V, et al. The cyclosome, a large complex containing cyclin-selective ubiquitin ligase activity, targets cyclins for destruction at the end of mitosis. Mol Biol Cell. 1995;6:185–197. doi: 10.1091/mbc.6.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Antoni A, et al. The Mad1/Mad2 complex as a template for Mad2 activation in the spindle assembly checkpoint. Curr Biol. 2005;15:214–225. doi: 10.1016/j.cub.2005.01.038. In vitro evidence for the MAD2 template model, which suggests an amplification mechanism by which kinetochore-bound MAD2 inhibits the APC/C. [DOI] [PubMed] [Google Scholar]
- 22.Varetti G, Musacchio A. The spindle assembly checkpoint. Curr Biol. 2008;18:R591–R595. doi: 10.1016/j.cub.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Malureanu LA, et al. BubR1 N terminus acts as a soluble inhibitor of cyclin B degradation by APC/C (Cdc20) in interphase. Dev Cell. 2009;16:118–131. doi: 10.1016/j.devcel.2008.11.004. The authors show that BUBR1 function is dispensable at the kinetochores and is only required in the MCC to inhibit the APC/C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braunstein I, Miniowitz S, Moshe Y, Hershko A. Inhibitory factors associated with anaphase-promoting complex/cyclosome in mitotic checkpoint. Proc Natl Acad Sci USA. 2007;104:4870–4875. doi: 10.1073/pnas.0700523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herzog F, et al. Structure of the anaphase-promoting complex/cyclosome interacting with a mitotic checkpoint complex. Science. 2009;323:1477–1481. doi: 10.1126/science.1163300. The authors provide structural evidence for the MCC and suggest a mechanism by which it inhibits the APC/C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nilsson J, Yekezare M, Minshull J, Pines J. The APC/C maintains the spindle assembly checkpoint by targeting Cdc20 for destruction. Nature Cell Biol. 2008;10:1411–1420. doi: 10.1038/ncb1799. CDC20 ubiquitylation by the APC/C during an active checkpoint is shown to be required to prevent satisfaction of the mitotic checkpoint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wassmann K, Liberal V, Benezra R. Mad2 phosphorylation regulates its association with Mad1 and the APC/C. EMBO J. 2003;22:797–806. doi: 10.1093/emboj/cdg071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi E, et al. BubR1 acetylation at prometaphase is required for modulating APC/C activity and timing of mitosis. EMBO J. 2009;28:2077–2089. doi: 10.1038/emboj.2009.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy SK, Rape M, Margansky WA, Kirschner MW. Ubiquitination by the anaphase-promoting complex drives spindle checkpoint inactivation. Nature. 2007;446:921–925. doi: 10.1038/nature05734. [DOI] [PubMed] [Google Scholar]
- 30.Stegmeier F, et al. Anaphase initiation is regulated by antagonistic ubiquitination and deubiquitination activities. Nature. 2007;446:876–881. doi: 10.1038/nature05694. [DOI] [PubMed] [Google Scholar]
- 31.Land H, Parada LF, Weinberg RA. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983;304:596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- 32.Perucho M, et al. Human-tumor-derived cell lines contain common and different transforming genes. Cell. 1981;27:467–476. doi: 10.1016/0092-8674(81)90388-3. [DOI] [PubMed] [Google Scholar]
- 33.Chang EH, Furth ME, Scolnick EM, Lowy DR. Tumorigenic transformation of mammalian cells induced by a normal human gene homologous to the oncogene of Harvey murine sarcoma virus. Nature. 1982;297:479–483. doi: 10.1038/297479a0. [DOI] [PubMed] [Google Scholar]
- 34.Goldfarb M, Shimizu K, Perucho M, Wigler M. Isolation and preliminary characterization of a human transforming gene from T24 bladder carcinoma cells. Nature. 1982;296:404–409. doi: 10.1038/296404a0. [DOI] [PubMed] [Google Scholar]
- 35.Land H, Parada LF, Weinberg RA. Cellular oncogenes and multistep carcinogenesis. Science. 1983;222:771–778. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- 36.Shih C, Padhy LC, Murray M, Weinberg RA. Transforming genes of carcinomas and neuroblastomas introduced into mouse fibroblasts. Nature. 1981;290:261–264. doi: 10.1038/290261a0. [DOI] [PubMed] [Google Scholar]
- 37.Pei L, Melmed S. Isolation and characterization of a pituitary tumor-transforming gene (PTTG) Mol Endocrinol. 1997;11:433–441. doi: 10.1210/mend.11.4.9911. [DOI] [PubMed] [Google Scholar]
- 38.Vader G, Lens SM. The Aurora kinase family in cell division and cancer. Biochim Biophys Acta. 2008;1786:60–72. doi: 10.1016/j.bbcan.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Katayama H, Brinkley WR, Sen S. The Aurora kinases: role in cell transformation and tumorigenesis. Cancer Metastasis Rev. 2003;22:451–464. doi: 10.1023/a:1023789416385. [DOI] [PubMed] [Google Scholar]
- 40.Zhang D, et al. Cre–loxP-controlled periodic Aurora-A overexpression induces mitotic abnormalities and hyperplasia in mammary glands of mouse models. Oncogene. 2004;23:8720–8730. doi: 10.1038/sj.onc.1208153. [DOI] [PubMed] [Google Scholar]
- 41.Lee SH, Sterling H, Burlingame A, McCormick F. Tpr directly binds to Mad1 and Mad2 and is important for the Mad1–Mad2-mediated mitotic spindle checkpoint. Genes Dev. 2008;22:2926–2931. doi: 10.1101/gad.1677208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dawlaty MM, et al. Resolution of sister centromeres requires RanBP2-mediated SUMOylation of topoisomerase IIα. Cell. 2008;133:103–115. doi: 10.1016/j.cell.2008.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernal JA, et al. Human securin interacts with p53 and modulates p53-mediated transcriptional activity and apoptosis. Nature Genet. 2002;32:306–311. doi: 10.1038/ng997. [DOI] [PubMed] [Google Scholar]
- 44.Fang Y, et al. BubR1 is involved in regulation of DNA damage responses. Oncogene. 2006;25:3598–3605. doi: 10.1038/sj.onc.1209392. [DOI] [PubMed] [Google Scholar]
- 45.Hein J, et al. Simian virus 40 large T antigen disrupts genome integrity and activates a DNA damage response via Bub1 binding. J Virol. 2009;83:117–127. doi: 10.1128/JVI.01515-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carter SL, Eklund AC, Kohane IS, Harris LN, Szallasi Z. A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nature Genet. 2006;38:1043–1048. doi: 10.1038/ng1861. Transcriptional analysis of human aneuploid tumours reveals a signature of CIN that has prognostic power independent of its relationship to cell cycle progression. [DOI] [PubMed] [Google Scholar]
- 47.Kronenwett U, et al. Improved grading of breast adenocarcinomas based on genomic instability. Cancer Res. 2004;64:904–909. doi: 10.1158/0008-5472.can-03-2451. [DOI] [PubMed] [Google Scholar]
- 48.Rhodes DR, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dobles M, Liberal V, Scott ML, Benezra R, Sorger PK. Chromosome missegregation and apoptosis in mice lacking the mitotic checkpoint protein Mad2. Cell. 2000;101:635–645. doi: 10.1016/s0092-8674(00)80875-2. [DOI] [PubMed] [Google Scholar]
- 50.Baker DJ, et al. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nature Genet. 2004;36:744–749. doi: 10.1038/ng1382. [DOI] [PubMed] [Google Scholar]
- 51.Dai W, et al. Slippage of mitotic arrest and enhanced tumor development in mice with BubR1 haploinsufficiency. Cancer Res. 2004;64:440–445. doi: 10.1158/0008-5472.can-03-3119. [DOI] [PubMed] [Google Scholar]
- 52.Babu JR, et al. Rae1 is an essential mitotic checkpoint regulator that cooperates with Bub3 to prevent chromosome missegregation. J Cell Biol. 2003;160:341–353. doi: 10.1083/jcb.200211048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalitsis P, Earle E, Fowler KJ, Choo KH. Bub3 gene disruption in mice reveals essential mitotic spindle checkpoint function during early embryogenesis. Genes Dev. 2000;14:2277–2282. doi: 10.1101/gad.827500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jeganathan K, Malureanu L, Baker DJ, Abraham SC, van Deursen JM. Bub1 mediates cell death in response to chromosome missegregation and acts to suppress spontaneous tumorigenesis. J Cell Biol. 2007;179:255–267. doi: 10.1083/jcb.200706015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weaver BA, Silk AD, Montagna C, Verdier-Pinard P, Cleveland DW. Aneuploidy acts both oncogenically and as a tumor suppressor. Cancer Cell. 2006;11:25–36. doi: 10.1016/j.ccr.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 56.Li M, Fang X, Wei Z, York JP, Zhang P. Loss of spindle assembly checkpoint-mediated inhibition of Cdc20 promotes tumorigenesis in mice. J Cell Biol. 2009;185:983–994. doi: 10.1083/jcb.200904020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pérez de Castro I, de Cárcer G, Malumbres M. A census of mitotic cancer genes: new insights into tumor cell biology and cancer therapy. Carcinogenesis. 2007;28:899–912. doi: 10.1093/carcin/bgm019. [DOI] [PubMed] [Google Scholar]
- 58.Perera D, et al. Bub1 maintains centromeric cohesion by activation of the spindle checkpoint. Dev Cell. 2007;13:566–579. doi: 10.1016/j.devcel.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 59.Jeong SJ, et al. Transcriptional abnormality of the hsMAD2 mitotic checkpoint gene is a potential link to hepatocellular carcinogenesis. Cancer Res. 2004;64:8666–8673. doi: 10.1158/0008-5472.CAN-03-3455. [DOI] [PubMed] [Google Scholar]
- 60.Sze KM, Ching YP, Jin DY, Ng IO. Association of MAD2 expression with mitotic checkpoint competence in hepatoma cells. J Biomed Sci. 2004;11:920–927. doi: 10.1007/BF02254377. [DOI] [PubMed] [Google Scholar]
- 61.Wang X, et al. Significance of MAD2 expression to mitotic checkpoint control in ovarian cancer cells. Cancer Res. 2002;62:1662–1668. [PubMed] [Google Scholar]
- 62.Wang X, et al. Correlation of defective mitotic checkpoint with aberrantly reduced expression of MAD2 protein in nasopharyngeal carcinoma cells. Carcinogenesis. 2000;21:2293–2297. doi: 10.1093/carcin/21.12.2293. [DOI] [PubMed] [Google Scholar]
- 63.Ren B, et al. E2F integrates cell cycle progression with DNA repair, replication, and G2/M checkpoints. Genes Dev. 2002;16:245–56. doi: 10.1101/gad.949802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hanks S, et al. Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nature Genet. 2004;36:1159. doi: 10.1038/ng1449. [DOI] [PubMed] [Google Scholar]
- 65.Gascoigne KE, Taylor SS. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell. 2008;14:111–122. doi: 10.1016/j.ccr.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 66.Tighe A, Johnson VL, Albertella M, Taylor SS. Aneuploid colon cancer cells have a robust spindle checkpoint. EMBO Rep. 2001;2:609–614. doi: 10.1093/embo-reports/kve127. References 65 and 66 provide evidence for a strong mitotic checkpoint in tumour cell lines and provide a mechanistic model for mitotic slippage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rhodes DR, et al. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9:166–180. doi: 10.1593/neo.07112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hernando E, et al. Rb inactivation promotes genomic instability by uncoupling cell cycle progression from mitotic control. Nature. 2004;430:797–802. doi: 10.1038/nature02820. [DOI] [PubMed] [Google Scholar]
- 69.Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997;386:623–627. doi: 10.1038/386623a0. [DOI] [PubMed] [Google Scholar]
- 70.Nicholson JM, Duesberg P. On the karyotypic origin and evolution of cancer cells. Cancer Genet Cytogenet. 2009;194:96–110. doi: 10.1016/j.cancergencyto.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 71.Diaz-Rodriguez E, Sotillo R, Schvartzman JM, Benezra R. Hec1 overexpression hyperactivates the mitotic checkpoint and induces tumor formation in vivo. Proc Natl Acad Sci USA. 2008;105:16719–16724. doi: 10.1073/pnas.0803504105. The authors show that overexpression of HEC1, an essential component of the kinetochore complex, leads to mitotic checkpoint overactivation, CIN and tumorigenesis in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thompson SL, Compton DA. Examining the link between chromosomal instability and aneuploidy in human cells. J Cell Biol. 2008;180:665–672. doi: 10.1083/jcb.200712029. Chemical overactivation of the mitotic checkpoint is shown to generate lagging chromosomes and aneuploidy, which are selected against in non-tumorigenic cell lines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rieder CL, Maiato H. Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev Cell. 2004;7:637–651. doi: 10.1016/j.devcel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 74.Brito DA, Rieder CL. Mitotic checkpoint slippage in humans occurs via cyclin B destruction in the presence of an active checkpoint. Curr Biol. 2006;16:1194–1200. doi: 10.1016/j.cub.2006.04.043. Mitotic slippage is shown to occur in an APC/C dependent manner in the presence of checkpoint activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iovino F, Lentini L, Amato A, Di Leonardo A. RB acute loss induces centrosome amplification and aneuploidy in murine primary fibroblasts. Mol Cancer. 2006;5:38. doi: 10.1186/1476-4598-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lentini L, Iovino F, Amato A, Di Leonardo A. Centrosome amplification induced by hydroxyurea leads to aneuploidy in pRB deficient human and mouse fibroblasts. Cancer Lett. 2006;238:153–160. doi: 10.1016/j.canlet.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 77.Lentini L, Pipitone L, Di Leonardo A. Functional inactivation of pRB results in aneuploid mammalian cells after release from a mitotic block. Neoplasia. 2002;4:380–387. doi: 10.1038/sj.neo.7900256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mayhew CN, et al. RB loss abrogates cell cycle control and genome integrity to promote liver tumorigenesis. Gastroenterology. 2007;133:976–984. doi: 10.1053/j.gastro.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 79.Zheng L, Flesken-Nikitin A, Chen PL, Lee WH. Deficiency of retinoblastoma gene in mouse embryonic stem cells leads to genetic instability. Cancer Res. 2002;62:2498–2502. [PubMed] [Google Scholar]
- 80.Quintyne NJ, Reing JE, Hoffelder DR, Gollin SM, Saunders WS. Spindle multipolarity is prevented by centrosomal clustering. Science. 2005;307:127–129. doi: 10.1126/science.1104905. [DOI] [PubMed] [Google Scholar]
- 81.Kwon M, et al. Mechanisms to suppress multipolar divisions in cancer cells with extra centrosomes. Genes Dev. 2008;22:2189–2203. doi: 10.1101/gad.1700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Silkworth WT, Nardi IK, Scholl LM, Cimini D. Multipolar spindle pole coalescence is a major source of kinetochore mis-attachment and chromosome missegregation in cancer cells. PLoS ONE. 2009;4:e6564. doi: 10.1371/journal.pone.0006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ganem NJ, Godinho SA, Pellman D. A mechanism linking extra centrosomes to chromosomal instability. Nature. 2009;460:278–282. doi: 10.1038/nature08136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Iwanaga Y, et al. Heterozygous deletion of mitotic arrest-deficient protein 1 (MAD1) increases the incidence of tumors in mice. Cancer Res. 2007;67:160–166. doi: 10.1158/0008-5472.CAN-06-3326. [DOI] [PubMed] [Google Scholar]
- 85.Ko MA, et al. Plk4 haploinsufficiency causes mitotic infidelity and carcinogenesis. Nature Genet. 2005;37:883–888. doi: 10.1038/ng1605. [DOI] [PubMed] [Google Scholar]
- 86.Holland AJ, Cleveland DW. Boveri revisited: chromosomal instability, aneuploidy and tumorigenesis. Nature Rev Mol Cell Biol. 2009;10:478–487. doi: 10.1038/nrm2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang X, et al. Overexpression of aurora kinase A in mouse mammary epithelium induces genetic instability preceding mammary tumor formation. Oncogene. 2006;25:7148–7158. doi: 10.1038/sj.onc.1209707. [DOI] [PubMed] [Google Scholar]
- 88.Swanton C, et al. Chromosomal instability determines taxane response. Proc Natl Acad Sci USA. 2009;106:8671–8676. doi: 10.1073/pnas.0811835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nature Rev Cancer. 2003;3:459–465. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 90.Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nature Rev Cancer. 2008;8:976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- 91.Donehower LA, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 92.Jacks T, et al. Effects of an Rb mutation in the mouse. Nature. 1992;359:295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- 93.Williams BR, et al. Aneuploidy affects proliferation and spontaneous immortalization in mammalian cells. Science. 2008;322:703–709. doi: 10.1126/science.1160058. Trisomy generated by Robertsonian translocations is shown to be detrimental to the viability of primary cell lines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gonzalo S, et al. Role of the RB1 family in stabilizing histone methylation at constitutive heterochromatin. Nature Cell Biol. 2005;7:420–428. doi: 10.1038/ncb1235. [DOI] [PubMed] [Google Scholar]
- 95.Rao CV, et al. Colonic tumorigenesis in BubR1+/− ApcMin/+ compound mutant mice is linked to premature separation of sister chromatids and enhanced genomic instability. Proc Natl Acad Sci USA. 2005;102:4365–4370. doi: 10.1073/pnas.0407822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nature Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 97.Huang HC, Shi J, Orth JD, Mitchison TJ. Evidence that mitotic exit is a better cancer therapeutic target than spindle assembly. Cancer Cell. 2009;16:347–358. doi: 10.1016/j.ccr.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weinert TA, Hartwell LH. The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cerevisiae. Science. 1988;241:317–322. doi: 10.1126/science.3291120. [DOI] [PubMed] [Google Scholar]
- 99.Weinert T, Hartwell L. Control of G2 delay by the rad9 gene of Saccharomyces cerevisiae. J Cell Sci. 1989;12(Suppl):145–148. doi: 10.1242/jcs.1989.supplement_12.12. [DOI] [PubMed] [Google Scholar]
- 100.Li R, Murray A. Feedback control of mitosis in budding yeast. Cell. 1991;66:519–531. doi: 10.1016/0092-8674(81)90015-5. [DOI] [PubMed] [Google Scholar]
- 101.Hoyt MA, Totis L, Roberts BT. S cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell. 1991;66:507–517. doi: 10.1016/0092-8674(81)90014-3. [DOI] [PubMed] [Google Scholar]
- 102.Li Y, Benezra R. Identification of a human mitotic checkpoint gene: hsMAD2. Science. 1996;274:246–248. doi: 10.1126/science.274.5285.246. References 101 and 102 were the first to identify mitotic checkpoint genes. [DOI] [PubMed] [Google Scholar]
- 103.Kops GJ, Foltz DR, Cleveland DW. Lethality to human cancer cells through massive chromosome loss by inhibition of the mitotic checkpoint. Proc Natl Acad Sci USA. 2004;101:8699–8704. doi: 10.1073/pnas.0401142101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Michel LS, et al. MAD2 haplo-insufficiency causes premature anaphase and chromosome instability in mammalian cells. Nature. 2001;409:355–359. doi: 10.1038/35053094. References 103 and 104 show that, unlike the case for the G2/M DNA damage checkpoint and the mitotic checkpoint in budding yeast, the mitotic checkpoint in vertebrates is essential for cell viability. [DOI] [PubMed] [Google Scholar]
- 105.Meraldi P, Draviam VM, Sorger PK. Timing and checkpoints in the regulation of mitotic progression. Dev Cell. 2004;7:45–60. doi: 10.1016/j.devcel.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 106.Zhou H, et al. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nature Genet. 1998;20:189–193. doi: 10.1038/2496. [DOI] [PubMed] [Google Scholar]
- 107.Sen S, et al. Amplification/overexpression of a mitotic kinase gene in human bladder cancer. J Natl Cancer Inst. 2002;94:1320–1329. doi: 10.1093/jnci/94.17.1320. [DOI] [PubMed] [Google Scholar]
- 108.Bischoff JR, et al. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 1998;17:3052–3065. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Miyoshi Y, Iwao K, Egawa C, Noguchi S. Association of centrosomal kinase STK15/BTAK mRNA expression with chromosomal instability in human breast cancers. Int J Cancer. 2001;92:370–373. doi: 10.1002/ijc.1200. [DOI] [PubMed] [Google Scholar]
- 110.Baba Y, et al. Aurora-A expression is independently associated with chromosomal instability in colorectal cancer. Neoplasia. 2009;11:418–4125. doi: 10.1593/neo.09154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gritsko TM, et al. Activation and overexpression of centrosome kinase BTAK/Aurora-A in human ovarian cancer. Clin Cancer Res. 2003;9:1420–1426. [PubMed] [Google Scholar]
- 112.Ablikim M, et al. Observation of Y(2175) in J/σπ psi →etaphif0 (980) Phys Rev Lett. 2008;100:102003. doi: 10.1103/PhysRevLett.100.102003. [DOI] [PubMed] [Google Scholar]
- 113.Sakakura C, et al. Tumour-amplified kinase BTAK is amplified and overexpressed in gastric cancers with possible involvement in aneuploid formation. Br J Cancer. 2001;84:824–831. doi: 10.1054/bjoc.2000.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Twu NF, et al. Expression of Aurora kinase A and B in normal and malignant cervical tissue: high Aurora A kinase expression in squamous cervical cancer. Eur J Obstet Gynecol Reprod Biol. 2009;142:57–63. doi: 10.1016/j.ejogrb.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 115.Reiter R, et al. Aurora kinase A messenger RNA overexpression is correlated with tumor progression and shortened survival in head and neck squamous cell carcinoma. Clin Cancer Res. 2006;12:5136–5141. doi: 10.1158/1078-0432.CCR-05-1650. [DOI] [PubMed] [Google Scholar]
- 116.Lu LY, et al. Aurora A is essential for early embryonic development and tumor suppression. J Biol Chem. 2008;283:31785–31790. doi: 10.1074/jbc.M805880200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cowley DO, et al. Aurora-A kinase is essential for bipolar spindle formation and early development. Mol Cell Biol. 2009;29:1059–1071. doi: 10.1128/MCB.01062-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Araki K, Nozaki K, Ueba T, Tatsuka M, Hashimoto N. High expression of Aurora-B/Aurora and Ipll-like midbody-associated protein (AIM-1) in astrocytomas. J Neurooncol. 2004;67:53–64. doi: 10.1023/b:neon.0000021784.33421.05. [DOI] [PubMed] [Google Scholar]
- 119.Chieffi P, et al. Aurora B expression in normal testis and seminomas. J Endocrinol. 2004;181:263–270. doi: 10.1677/joe.0.1810263. [DOI] [PubMed] [Google Scholar]
- 120.Gibson SE, Zeng WF, Weil RJ, Prayson RA. Aurora B kinase expression in ependymal neoplasms. Appl Immunohistochem Mol Morphol. 2008;16:274–278. doi: 10.1097/PAI.0b013e318126bff5. [DOI] [PubMed] [Google Scholar]
- 121.Chieffi P, et al. Aurora B expression directly correlates with prostate cancer malignancy and influence prostate cell proliferation. Prostate. 2006;66:326–333. doi: 10.1002/pros.20345. [DOI] [PubMed] [Google Scholar]
- 122.Smith SL, et al. Overexpression of aurora B kinase (AURKB) in primary non-small cell lung carcinoma is frequent, generally driven from one allele, and correlates with the level of genetic instability. Br J Cancer. 2005;93:719–729. doi: 10.1038/sj.bjc.6602779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tanaka S, et al. Aurora kinase B is a predictive factor for the aggressive recurrence of hepatocellular carcinoma after curative hepatectomy. Br J Surg. 2008;95:611–619. doi: 10.1002/bjs.6011. [DOI] [PubMed] [Google Scholar]
- 124.Nguyen HG, et al. Deregulated Aurora-B induced tetraploidy promotes tumorigenesis. FASEB J. 2009;23:2741–2748. doi: 10.1096/fj.09-130963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gemma A, et al. Somatic mutation of the hBUB1 mitotic checkpoint gene in primary lung cancer. Genes Chromosomes Cancer. 2000;29:213–218. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1027>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 126.Hempen PM, Kurpad H, Calhoun ES, Abraham S, Kern SE. A double missense variation of the BUB1 gene and a defective mitotic spindle checkpoint in the pancreatic cancer cell line Hs766T. Hum Mutat. 2003;21:445. doi: 10.1002/humu.9120. [DOI] [PubMed] [Google Scholar]
- 127.Shichiri M, Yoshinaga K, Hisatomi H, Sugihara K, Hirata Y. Genetic and epigenetic inactivation of mitotic checkpoint genes hBUB1 and hBUBR1 and their relationship to survival. Cancer Res. 2002;62:713–717. [PubMed] [Google Scholar]
- 128.Lin SF, et al. Expression of hBUB1 in acute myeloid leukemia. Leuk Lymphoma. 2002;43:385–391. doi: 10.1080/10428190290006206. [DOI] [PubMed] [Google Scholar]
- 129.Yuan B, et al. Increased expression of mitotic checkpoint genes in breast cancer cells with chromosomal instability. Clin Cancer Res. 2006;12:405–410. doi: 10.1158/1078-0432.CCR-05-0903. [DOI] [PubMed] [Google Scholar]
- 130.Moreno-Bueno G, et al. Differential gene expression profile in endometrioid and nonendometrioid endometrial carcinoma: STK15 is frequently overexpressed and amplified in nonendometrioid carcinomas. Cancer Res. 2003;63:5697–5702. [PubMed] [Google Scholar]
- 131.Grabsch H, et al. Overexpression of the mitotic checkpoint genes BUB1, BUBR1, and BUB3 in gastric cancer — association with tumour cell proliferation. J Pathol. 2003;200:16–22. doi: 10.1002/path.1324. [DOI] [PubMed] [Google Scholar]
- 132.Pinto M, et al. Overexpression of the mitotic checkpoint genes BUB1 and BUBR1 is associated with genomic complexity in clear cell kidney carcinomas. Cell Oncol. 2008;30:389–395. doi: 10.3233/CLO-2008-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wada N, et al. Overexpression of the mitotic spindle assembly checkpoint genes hBUB1, hBUBR1 and hMAD2 in thyroid carcinomas with aggressive nature. Anticancer Res. 2008;28:139–144. [PubMed] [Google Scholar]
- 134.Cahill DP, et al. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;392:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- 135.Schliekelman M, et al. Impaired Bub1 function in vivo compromises tension-dependent checkpoint function leading to aneuploidy and tumorigenesis. Cancer Res. 2009;69:45–54. doi: 10.1158/0008-5472.CAN-07-6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Baker DJ, et al. Early aging-associated phenotypes in Bub3/Rae1 haploinsufficient mice. J Cell Biol. 2006;172:529–540. doi: 10.1083/jcb.200507081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kalitsis P, et al. Increased chromosome instability but not cancer predisposition in haploinsufficient Bub3 mice. Genes Chromosomes Cancer. 2005;44:29–36. doi: 10.1002/gcc.20215. [DOI] [PubMed] [Google Scholar]
- 138.Mondal G, et al. Overexpression of Cdc20 leads to impairment of the spindle assembly checkpoint and aneuploidization in oral cancer. Carcinogenesis. 2007;28:81–92. doi: 10.1093/carcin/bgl100. [DOI] [PubMed] [Google Scholar]
- 139.Li D, et al. Overexpression of oncogenic STK15/BTAK/Aurora A kinase in human pancreatic cancer. Clin Cancer Res. 2003;9:991–997. [PubMed] [Google Scholar]
- 140.Kim JM, et al. Identification of gastric cancer-related genes using a cDNA microarray containing novel expressed sequence tags expressed in gastric cancer cells. Clin Cancer Res. 2005;11:473–482. [PubMed] [Google Scholar]
- 141.Ouellet V, et al. Tissue array analysis of expression microarray candidates identifies markers associated with tumor grade and outcome in serous epithelial ovarian cancer. Int J Cancer. 2006;119:599–607. doi: 10.1002/ijc.21902. [DOI] [PubMed] [Google Scholar]
- 142.Marucci G, et al. Gene expression profiling in glioblastoma and immunohistochemical evaluation of IGFBP-2 and CDC20. Virchows Arch. 2008;453:599–609. doi: 10.1007/s00428-008-0685-7. [DOI] [PubMed] [Google Scholar]
- 143.Singhal S, et al. Alterations in cell cycle genes in early stage lung adenocarcinoma identified by expression profiling. Cancer Biol Ther. 2003;2:291–298. doi: 10.4161/cbt.2.3.399. [DOI] [PubMed] [Google Scholar]
- 144.Fujita T, Liu W, Doihara H, Wan Y. Regulation of Skp2–p27 axis by the Cdh1/anaphase-promoting complex pathway in colorectal tumorigenesis. Am J Pathol. 2008;173:217–228. doi: 10.2353/ajpath.2008.070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lehman NL, et al. Oncogenic regulators and substrates of the anaphase promoting complex/cyclosome are frequently overexpressed in malignant tumors. Am J Pathol. 2007;170:1793–1805. doi: 10.2353/ajpath.2007.060767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wierinckx A, et al. A diagnostic marker set for invasion, proliferation, and aggressiveness of prolactin pituitary tumors. Endocr Relat Cancer. 2007;14:887–900. doi: 10.1677/ERC-07-0062. [DOI] [PubMed] [Google Scholar]
- 147.Weaver BA, Cleveland DW. Aneuploidy: instigator and inhibitor of tumorigenesis. Cancer Res. 2007;67:10103–10105. doi: 10.1158/0008-5472.CAN-07-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Weaver BA, Silk AD, Montagna C, Verdier-Pinard P, Cleveland DW. Aneuploidy acts both oncogenically and as a tumor suppressor. Cancer Cell. 2007;11:25–36. doi: 10.1016/j.ccr.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 149.Wikman H, et al. Identification of differentially expressed genes in pulmonary adenocarcinoma by using cDNA array. Oncogene. 2002;21:5804–5813. doi: 10.1038/sj.onc.1205726. [DOI] [PubMed] [Google Scholar]
- 150.Kettunen E, et al. Differentially expressed genes in nonsmall cell lung cancer: expression profiling of cancer-related genes in squamous cell lung cancer. Cancer Genet Cytogenet. 2004;149:98–106. doi: 10.1016/S0165-4608(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 151.Soria JC, et al. Overexpression of cyclin B1 in early-stage non-small cell lung cancer and its clinical implication. Cancer Res. 2000;60:4000–4004. [PubMed] [Google Scholar]
- 152.Koon N, et al. Molecular targets for tumour progression in gastrointestinal stromal tumours. Gut. 2004;53:235–240. doi: 10.1136/gut.2003.021238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Takeno S, et al. Prognostic value of cyclin B1 in patients with esophageal squamous cell carcinoma. Cancer. 2002;94:2874–2881. doi: 10.1002/cncr.10542. [DOI] [PubMed] [Google Scholar]
- 154.Yoshida T, Tanaka S, Mogi A, Shitara Y, Kuwano H. The clinical significance of Cyclin B1 and Wee1 expression in non-small-cell lung cancer. Ann Oncol. 2004;15:252–256. doi: 10.1093/annonc/mdh073. [DOI] [PubMed] [Google Scholar]
- 155.Ikuerowo SO, et al. Alteration of subcellular and cellular expression patterns of cyclin B1 in renal cell carcinoma is significantly related to clinical progression and survival of patients. Int J Cancer. 2006;119:867–874. doi: 10.1002/ijc.21869. [DOI] [PubMed] [Google Scholar]
- 156.Agarwal R, et al. Integrative analysis of cyclin protein levels identifies cyclin b1 as a classifier and predictor of outcomes in breast cancer. Clin Cancer Res. 2009;15:3654–3662. doi: 10.1158/1078-0432.CCR-08-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Aaltonen K, et al. High cyclin B1 expression is associated with poor survival in breast cancer. Br J Cancer. 2009;100:1055–1060. doi: 10.1038/sj.bjc.6604874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Brandeis M, et al. Cyclin B2-null mice develop normally and are fertile whereas cyclin B1-null mice die in utero. Proc Natl Acad Sci USA. 1998;95:4344–4349. doi: 10.1073/pnas.95.8.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hayama S, et al. Activation of CDCA1–KNTC2, members of centromere protein complex, involved in pulmonary carcinogenesis. Cancer Res. 2006;66:10339–10348. doi: 10.1158/0008-5472.CAN-06-2137. [DOI] [PubMed] [Google Scholar]
- 160.Perez de Castro I, de Carcer G, Malumbres M. A census of mitotic cancer genes: new insights into tumor cell biology and cancer therapy. Carcinogenesis. 2007;28:899–912. doi: 10.1093/carcin/bgm019. [DOI] [PubMed] [Google Scholar]
- 161.Nomoto S, et al. Search for in vivo somatic mutations in the mitotic checkpoint gene, hMAD1, in human lung cancers. Oncogene. 1999;18:7180–7183. doi: 10.1038/sj.onc.1203141. [DOI] [PubMed] [Google Scholar]
- 162.Tsukasaki K, et al. Mutations in the mitotic check point gene, MAD1L1, in human cancers. Oncogene. 2001;20:3301–3305. doi: 10.1038/sj.onc.1204421. [DOI] [PubMed] [Google Scholar]
- 163.Nishigaki R, et al. Proteomic identification of differentially-expressed genes in human gastric carcinomas. Proteomics. 2005;5:3205–3213. doi: 10.1002/pmic.200401307. [DOI] [PubMed] [Google Scholar]
- 164.Han S, et al. Clinical implication of altered expression of Mad1 protein in human breast carcinoma. Cancer. 2000;88:1623–1632. doi: 10.1002/(sici)1097-0142(20000401)88:7<1623::aid-cncr17>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 165.Nam CW, et al. Mitotic checkpoint gene MAD1 in hepatocellular carcinoma is associated with tumor recurrence after surgical resection. J Surg Oncol. 2008;97:567–571. doi: 10.1002/jso.20999. [DOI] [PubMed] [Google Scholar]
- 166.Chi YH, Ward JM, Cheng LI, Yasunaga J, Jeang KT. Spindle assembly checkpoint and p53 deficiencies cooperate for tumorigenesis in mice. Int J Cancer. 2009;124:1483–1489. doi: 10.1002/ijc.24094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hernando E, et al. Molecular analyses of the mitotic checkpoint components hsMAD2, hBUB1 and hBUB3 in human cancer. Int J Cancer. 2001;95:223–227. doi: 10.1002/1097-0215(20010720)95:4<223::aid-ijc1038>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 168.Percy MJ, et al. Expression and mutational analyses of the human MAD2L1 gene in breast cancer cells. Genes Chromosomes Cancer. 2000;29:356–362. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1044>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 169.Alizadeh AA, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 170.Chen X, et al. Gene expression patterns in human liver cancers. Mol Biol Cell. 2002;13:1929–1939. doi: 10.1091/mbc.02-02-0023.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Garber ME, et al. Diversity of gene expression in adenocarcinoma of the lung. Proc Natl Acad Sci USA. 2001;98:13784–13789. doi: 10.1073/pnas.241500798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Heighway J, et al. Expression profiling of primary non-small cell lung cancer for target identification. Oncogene. 2002;21:7749–7763. doi: 10.1038/sj.onc.1205979. [DOI] [PubMed] [Google Scholar]
- 173.Hisaoka M, Matsuyama A, Hashimoto H. Aberrant MAD2 expression in soft-tissue sarcoma. Pathol Int. 2008;58:329–333. doi: 10.1111/j.1440-1827.2008.02232.x. [DOI] [PubMed] [Google Scholar]
- 174.Wang L, et al. MAD2 as a key component of mitotic checkpoint: a probable prognostic factor for gastric cancer. Am J Clin Pathol. 2009;131:793–801. doi: 10.1309/AJCPBMHHD0HFCY8W. [DOI] [PubMed] [Google Scholar]
- 175.Rimkus C, et al. Expression of the mitotic checkpoint gene MAD2L2 has prognostic significance in colon cancer. Int J Cancer. 2007;120:207–211. doi: 10.1002/ijc.22155. [DOI] [PubMed] [Google Scholar]
- 176.Grutzmann R, et al. Gene expression profiling of microdissected pancreatic ductal carcinomas using high-density DNA microarrays. Neoplasia. 2004;6:611–622. doi: 10.1593/neo.04295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Saez C, et al. hpttg is over-expressed in pituitary adenomas and other primary epithelial neoplasias. Oncogene. 1999;18:5473–5476. doi: 10.1038/sj.onc.1202914. [DOI] [PubMed] [Google Scholar]
- 178.Honda S, et al. A role for the pituitary tumortransforming gene in the genesis and progression of non-small cell lung carcinomas. Anticancer Res. 2003;23:3775–3782. [PubMed] [Google Scholar]
- 179.Genkai N, Homma J, Sano M, Tanaka R, Yamanaka R. Increased expression of pituitary tumor-transforming gene (PTTG)-1 is correlated with poor prognosis in glioma patients. Oncol Rep. 2006;15:1569–1574. [PubMed] [Google Scholar]
- 180.Fujii T, et al. Overexpression of pituitary tumor transforming gene 1 in HCC is associated with angiogenesis and poor prognosis. Hepatology. 2006;43:1267–1275. doi: 10.1002/hep.21181. [DOI] [PubMed] [Google Scholar]
- 181.Su MC, Hsu HC, Liu YJ, Jeng YM. Overexpression of pituitary tumor-transforming gene-1 in hepatocellular carcinoma. Hepatogastroenterology. 2006;53:262–265. [PubMed] [Google Scholar]
- 182.Zhu X, et al. Significance of pituitary tumor transforming gene 1 (PTTG1) in prostate cancer. Anticancer Res. 2006;26:1253–1259. [PubMed] [Google Scholar]
- 183.Puri R, Tousson A, Chen L, Kakar SS. Molecular cloning of pituitary tumor transforming gene 1 from ovarian tumors and its expression in tumors. Cancer Lett. 2001;163:131–139. doi: 10.1016/s0304-3835(00)00688-1. [DOI] [PubMed] [Google Scholar]
- 184.Heaney AP, et al. Expression of pituitary-tumour transforming gene in colorectal tumours. Lancet. 2000;355:716–719. doi: 10.1016/S0140-6736(99)10238-1. [DOI] [PubMed] [Google Scholar]
- 185.Liang HS, et al. Comparative analysis of protein expression in differentiated thyroid tumours: a multicentre study. J Int Med Res. 2009;37:927–938. doi: 10.1177/147323000903700339. [DOI] [PubMed] [Google Scholar]
- 186.Chiriva-Internati M, et al. The pituitary tumor transforming gene 1 (PTTG-1): an immunological target for multiple myeloma. J Transl Med. 2008;6:15. doi: 10.1186/1479-5876-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nature Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 188.Yan S, et al. PTTG overexpression promotes lymph node metastasis in human esophageal squamous cell carcinoma. Cancer Res. 2009;69:3283–3290. doi: 10.1158/0008-5472.CAN-08-0367. [DOI] [PubMed] [Google Scholar]
- 189.Wang Z, Yu R, Melmed S. Mice lacking pituitary tumor transforming gene show testicular and splenic hypoplasia, thymic hyperplasia, thrombocytopenia, aberrant cell cycle progression, and premature centromere division. Mol Endocrinol. 2001;15:1870–1879. doi: 10.1210/mend.15.11.0729. [DOI] [PubMed] [Google Scholar]
- 190.Chesnokova V, Kovacs K, Castro AV, Zonis S, Melmed S. Pituitary hypoplasia in Pttg−/− mice is protective for Rb+/− pituitary tumorigenesis. Mol Endocrinol. 2005;19:2371–2379. doi: 10.1210/me.2005-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Abbud RA, et al. Early multipotential pituitary focal hyperplasia in the α-subunit of glycoprotein hormone-driven pituitary tumor-transforming gene transgenic mice. Mol Endocrinol. 2005;19:1383–1391. doi: 10.1210/me.2004-0403. [DOI] [PubMed] [Google Scholar]
- 192.Donangelo I, et al. Pituitary tumor transforming gene overexpression facilitates pituitary tumor development. Endocrinology. 2006;147:4781–4791. doi: 10.1210/en.2006-0544. [DOI] [PubMed] [Google Scholar]
- 193.Simizu S, Osada H. Mutations in the Plk gene lead to instability of Plk protein in human tumour cell lines. Nature Cell Biol. 2000;2:852–854. doi: 10.1038/35041102. [DOI] [PubMed] [Google Scholar]
- 194.Tokumitsu Y, et al. Prognostic significance of polo-like kinase expression in esophageal carcinoma. Int J Oncol. 1999;15:687–692. doi: 10.3892/ijo.15.4.687. [DOI] [PubMed] [Google Scholar]
- 195.Wolf G, et al. Prognostic significance of polo-like kinase (PLK) expression in non-small cell lung cancer. Oncogene. 1997;14:543–549. doi: 10.1038/sj.onc.1200862. [DOI] [PubMed] [Google Scholar]
- 196.Macmillan JC, Hudson JW, Bull S, Dennis JW, Swallow CJ. Comparative expression of the mitotic regulators SAK and PLK in colorectal cancer. Ann Surg Oncol. 2001;8:729–740. doi: 10.1007/s10434-001-0729-6. [DOI] [PubMed] [Google Scholar]
- 197.Nappi TC, et al. Identification of Polo-like kinase 1 as a potential therapeutic target in anaplastic thyroid carcinoma. Cancer Res. 2009;69:1916–1923. doi: 10.1158/0008-5472.CAN-08-1693. [DOI] [PubMed] [Google Scholar]
- 198.Lu LY, et al. Polo-like kinase 1 is essential for early embryonic development and tumor suppression. Mol Cell Biol. 2008;28:6870–6876. doi: 10.1128/MCB.00392-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jackson JR, Patrick DR, Dar MM, Huang PS. Targeted anti-mitotic therapies: can we improve on tubulin agents? Nature Rev Cancer. 2007;7:107–117. doi: 10.1038/nrc2049. [DOI] [PubMed] [Google Scholar]
- 200.Sotillo R, Schvartzman JM, Socci ND, Benezra R. Mad2-induced chromosome instability leads to lung tumor relapse after oncogene withdrawal. Nature. doi: 10.1038/nature08803. (in the press) [DOI] [PMC free article] [PubMed] [Google Scholar]