Real-world evidence: the devil is in the detail (original) (raw)
Abstract
Much has been written about real-world evidence (RWE), a concept that offers an understanding of the effects of healthcare interventions using routine clinical data. The reflection of diverse real-world practices is a double-edged sword that makes RWE attractive but also opens doors to several biases that need to be minimised both in the design and analytical phases of non-experimental studies. Additionally, it is critical to ensure that researchers who conduct these studies possess adequate methodological expertise and ability to accurately implement these methods. Critical design elements to be considered should include a clearly defined research question using a causal inference framework, choice of a fit-for-purpose data source, inclusion of new users of a treatment with comparators that are as similar as possible to that group, accurately classifying person-time and deciding censoring approaches. Having taken measures to minimise bias ‘by design’, the next step is to implement appropriate analytical techniques (for example propensity scores) to minimise the remnant potential biases. A clear protocol should be provided at the beginning of the study and a report of the results after, including caveats to consider. We also point the readers to readings on some novel analytical methods as well as newer areas of application of RWE. While there is no one-size-fits-all solution to evaluating RWE studies, we have focused our discussion on key methods and issues commonly encountered in comparative observational cohort studies with the hope that readers are better equipped to evaluate non-experimental studies that they encounter in the future.
Graphical abstract
Similar content being viewed by others
Introduction
Real-world evidence (RWE) remains one of the most enticing concepts in medicine, surrounded by much buzz. Recent developments, including the first-ever regulatory approval of label expansion of IBRANCE (palbociclib) for male breast cancer based on RWE, have brought in a new era in the applicability of RWE in healthcare [[1](/article/10.1007/s00125-020-05217-1#ref-CR1 "Wedam S, Fashoyin-Aje L, Bloomquist E et al (2020) FDA approval summary: palbociclib for male patients with metastatic breast cancer. Clin Cancer Res 26(6):1208–1212. https://doi.org/10.1158/1078-0432.CCR-19-2580
")]. RWE is defined as ‘clinical evidence about the usage and potential benefits or risks of a medical product derived from analysing real-world data (RWD)’ [[2](/article/10.1007/s00125-020-05217-1#ref-CR2 "U.S. FDA (2019) Webinar: framework for FDA’s Real-World Evidence Program – Mar 15, 2019. Available from https://www.fda.gov/drugs/webinar-framework-fdas-real-world-evidence-program-mar-15-2019
. Accessed 20 Dec 2019")\]. RWD are data relating to patient health status and/or the delivery of healthcare routinely collected from different sources and find application in many areas including therapeutic development to comparative effectiveness/safety, reimbursement, regulatory decision-making and clinical guideline development \[[2](/article/10.1007/s00125-020-05217-1#ref-CR2 "U.S. FDA (2019) Webinar: framework for FDA’s Real-World Evidence Program – Mar 15, 2019. Available from
https://www.fda.gov/drugs/webinar-framework-fdas-real-world-evidence-program-mar-15-2019
. Accessed 20 Dec 2019"), [3](/article/10.1007/s00125-020-05217-1#ref-CR3 "U.S. FDA (2019) Submitting documents using real-world data and real-world evidence to FDA for drugs and biologics guidance for industry - May 9, 2019. Available from
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/submitting-documents-using-real-world-data-and-real-world-evidence-fda-drugs-and-biologics-guidance
. Accessed 20 Dec 2019")\].The reflection of ‘diverse real-world practices’ enhances the appeal of RWD making it more relatable than data from RCTs. However, this very element that makes RWD attractive also makes it challenging to work with. Additionally, inaccurate application of methods and shortage of adequate methodological know-how potentially threaten the validity of RWD studies [[4](/article/10.1007/s00125-020-05217-1#ref-CR4 "Allie Nawrat, Pharma Technology Focus (2019) Real world data - how can it improve clinical trial outcomes. Available from https://www.pharmaceutical-technology.com/features/real-world-data-improving-outcomes/
. Accessed 20 Dec 2019")\]. In this paper we discuss commonly encountered issues and recommend key methodological considerations and potential solutions in the design, implementation and evaluation of real-world pharmacoepidemiological studies. This paper provides a general overview of a broad topic and because a detailed discussion on each subtopic is beyond the scope of this review, we have cited several references in relevant sections for interested readers to explore further.Defining the research question using a causal inference framework
It is a misconception that the entire purpose of RWD is to reduce the cost or complexity of trials (although this is feasible and done in some settings) or simply to get evidence ‘without randomisation’. RWE when done correctly is an important stand-alone source of evidence that complements RCTs and laboratory and other studies, which together inform decision-making. Understanding the research question is crucial to ensure that the right tools to generate robust RWE are employed.
Researchers should accurately describe the goals of real-world studies using a causal inference framework, like they would for an RCT, including any nuances (e.g. are we comparing initiation of treatment A vs treatment B or are we comparing patients switching from treatment A to treatment B vs patients staying on treatment A?) [[5](/article/10.1007/s00125-020-05217-1#ref-CR5 "Hernan MA (2018) The C-word: scientific euphemisms do not improve causal inference from observational data. Am J Public Health 108(5):616–619. https://doi.org/10.2105/AJPH.2018.304337
")]. While RWE is inherently relevant to clinical practice, it examines a different pattern of care (i.e. RWE and RCTs ask different questions). However, imagining an RWE study as a hypothetical trial forces a more stringent thought process about the intervention, comparators, timelines, outcomes and confounders [[6](/article/10.1007/s00125-020-05217-1#ref-CR6 "Hernan MA, Robins JM (2016) Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol 183(8):758–764. https://doi.org/10.1093/aje/kwv254
"), [7](/article/10.1007/s00125-020-05217-1#ref-CR7 "Hernan MA (2005) Invited commentary: hypothetical interventions to define causal effects--afterthought or prerequisite? Am J Epidemiol 162(7):618–620; discussion 621-612. https://doi.org/10.1093/aje/kwi255
")]. In estimating a causal effect, we ideally want to examine all potential outcomes in the same patients during the same time period, under contrasting treatments [[8](/article/10.1007/s00125-020-05217-1#ref-CR8 "Rubin DB (2005) Causal inference using potential outcomes: design, modeling, decisions. J Am Stat Assoc 100(469):322–331. https://doi.org/10.1198/016214504000001880
")]. However, this is impossible, as for each patient the outcome can be observed only under one treatment. We therefore compare the outcomes of two groups: treatment A vs an ‘exchangeable’ substitute treatment B [[9](/article/10.1007/s00125-020-05217-1#ref-CR9 "Nørgaard M, Ehrenstein V, Vandenbroucke JP (2017) Confounding in observational studies based on large health care databases: problems and potential solutions–a primer for the clinician. Clin Epidemiol 9:185–193. https://doi.org/10.2147/CLEP.S129879
"), [10](/article/10.1007/s00125-020-05217-1#ref-CR10 "Maldonado G, Greenland S (2002) Estimating causal effects. Int J Epidemiol 31(2):422–429. https://doi.org/10.1093/ije/31.2.422
")]. The validity of effect estimates depends on how valid the substitution is [[10](/article/10.1007/s00125-020-05217-1#ref-CR10 "Maldonado G, Greenland S (2002) Estimating causal effects. Int J Epidemiol 31(2):422–429. https://doi.org/10.1093/ije/31.2.422
")]. While there are no guarantees that an effect estimate can be causally interpreted, setting a causal goal for the study lays the foundation for robust design and analytical decisions [[5](#ref-CR5 "Hernan MA (2018) The C-word: scientific euphemisms do not improve causal inference from observational data. Am J Public Health 108(5):616–619. https://doi.org/10.2105/AJPH.2018.304337
"),[6](#ref-CR6 "Hernan MA, Robins JM (2016) Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol 183(8):758–764. https://doi.org/10.1093/aje/kwv254
"),[7](#ref-CR7 "Hernan MA (2005) Invited commentary: hypothetical interventions to define causal effects--afterthought or prerequisite? Am J Epidemiol 162(7):618–620; discussion 621-612. https://doi.org/10.1093/aje/kwi255
"),[8](/article/10.1007/s00125-020-05217-1#ref-CR8 "Rubin DB (2005) Causal inference using potential outcomes: design, modeling, decisions. J Am Stat Assoc 100(469):322–331. https://doi.org/10.1198/016214504000001880
")].
Data sources
Table 1 describes several data sources and provides examples of their application in diabetes research. RWD sources include administrative claims data [[11](#ref-CR11 "Nabhan C, Klink A, Prasad V (2019) Real-world evidence-what does it really mean? JAMA Oncol 5(6):781–783. https://doi.org/10.1001/jamaoncol.2019.0450
"),[12](#ref-CR12 "Sturmer T, Jonsson Funk M, Poole C, Brookhart MA (2011) Nonexperimental comparative effectiveness research using linked healthcare databases. Epidimiology 22(3):298–301. https://doi.org/10.1097/EDE.0b013e318212640c
"),[13](/article/10.1007/s00125-020-05217-1#ref-CR13 "Strom BL (2001) Data validity issues in using claims data. Pharmacoepidemiol Drug Saf 10(5):389–392. https://doi.org/10.1002/pds.610
")], electronic health records [[14](/article/10.1007/s00125-020-05217-1#ref-CR14 "Casey JA, Schwartz BS, Stewart WF, Adler NE (2016) Using electronic health records for population health research: a review of methods and applications. Annu Rev Public Health 37(1):61–81. https://doi.org/10.1146/annurev-publhealth-032315-021353
"), [15](/article/10.1007/s00125-020-05217-1#ref-CR15 "Farmer R, Mathur R, Bhaskaran K, Eastwood SV, Chaturvedi N, Smeeth L (2018) Promises and pitfalls of electronic health record analysis. Diabetologia 61(6):1241–1248. https://doi.org/10.1007/s00125-017-4518-6
")] and disease or treatment registries [[16](/article/10.1007/s00125-020-05217-1#ref-CR16 "Nelson EC, Dixon-Woods M, Batalden PB et al (2016) Patient focused registries can improve health, care, and science. BMJ 354:i3319. https://doi.org/10.1136/bmj.i3319
"), [17](/article/10.1007/s00125-020-05217-1#ref-CR17 "Diabetes Collaborative Registries (2019) The Diabetes Collaborative Registry. Transforming the future of diabetes care. Available from https://cvquality.acc.org/NCDR-Home/registries/outpatient-registries/the-diabetes-collaborative-registry
. Accessed 20 Dec 2019")\]. Additionally, patient-generated data from surveys, questionnaires, smartphone apps and social media are increasingly being considered for the purposes of pharmacovigilance, patient characterisation and disease understanding \[[11](/article/10.1007/s00125-020-05217-1#ref-CR11 "Nabhan C, Klink A, Prasad V (2019) Real-world evidence-what does it really mean? JAMA Oncol 5(6):781–783.
https://doi.org/10.1001/jamaoncol.2019.0450
"), [18](/article/10.1007/s00125-020-05217-1#ref-CR18 "McDonald L, Malcolm B, Ramagopalan S, Syrad H (2019) Real-world data and the patient perspective: the PROmise of social media? BMC Med 17(1):11. https://doi.org/10.1186/s12916-018-1247-8
"), [19](/article/10.1007/s00125-020-05217-1#ref-CR19 "Pierce CE, Bouri K, Pamer C et al (2017) Evaluation of facebook and twitter monitoring to detect safety signals for medical products: an analysis of recent FDA safety alerts. Drug Saf 40(4):317–331. https://doi.org/10.1007/s40264-016-0491-0
")]. However, these need careful evaluation as not all health apps are thoroughly validated and their pace of growth is fast outpacing the vetting process [[20](/article/10.1007/s00125-020-05217-1#ref-CR20 "Kuehn BM (2015) Is there an app to solve app overload? JAMA 313(14):1405–1407. https://doi.org/10.1001/jama.2015.2381
")]. Data linkages with appropriate safeguards offer opportunities to combine useful features from two or more data sources to conduct real-world studies [[21](/article/10.1007/s00125-020-05217-1#ref-CR21 "Rivera DR, Gokhale MN, Reynolds MW et al (2020) Linking electronic health data in pharmacoepidemiology: appropriateness and feasibility. Pharmacoepidemiol Drug Saf 29(1):18–29. https://doi.org/10.1002/pds.4918
")].
Table 1 Examples of RWD sources and applications to diabetes research
Study design
Several classification schemes exist for real-world study designs [[22](/article/10.1007/s00125-020-05217-1#ref-CR22 "Pearce N (2012) Classification of epidemiological study designs. Int J Epidemiol 41(2):393–397. https://doi.org/10.1093/ije/dys049
")] but, broadly, cohort studies, case–control studies and self-controlled case series are the three basic types [23]. Cohort studies follow patients from the treatment to the outcome. Case–control studies select disease cases and controls from the source population and compare treatment histories in the two groups, thereby introducing several avenues for biases. Cohort design is a direct analogue of an experiment and has generally become the standard design for observational studies unless an alternate design is dictated by the research question. Self-controlled methods compare treatments and outcomes within the same individual rather than across groups of individuals by looking at different treatment times within the same person. They are a good fit in settings with acute recurrent or non-recurrent events and intermittent exposures and transient effects, assuming availability of precise timings [24]. Choice of a study design depends on several factors including the research question of interest, rarity of the exposure/outcome and avenues for biases. We direct interested readers to publications by Rothman et al [23] and Hallas and Pottegård [[25](/article/10.1007/s00125-020-05217-1#ref-CR25 "Hallas J, Pottegård A (2014) Use of self-controlled designs in pharmacoepidemiology. J Intern Med 275(6):581–589. https://doi.org/10.1111/joim.12186
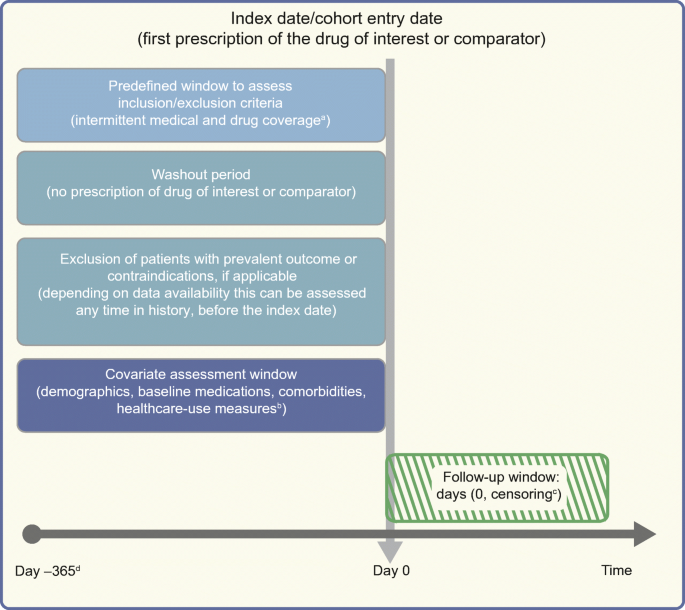
")] for further reading on this subject. As cohort studies are most intuitive when assessing incidence, natural history or comparative effectiveness/safety, we will focus much of our further discussion with this design in mind. Recently, clear design diagrams have been proposed to intuitively visualise studies conducted using healthcare databases/electronic medical records (Fig. 1) [[26](/article/10.1007/s00125-020-05217-1#ref-CR26 "Schneeweiss S, Rassen JA, Brown JS et al (2019) Graphical depiction of longitudinal study designs in health care databases. Ann Intern Med 170(6):398–406. https://doi.org/10.7326/M18-3079
")].
Fig. 1
Framework for a cohort study using an administrative claims or electronic medical record database, with methodology from Schneeweiss et al [[26](/article/10.1007/s00125-020-05217-1#ref-CR26 "Schneeweiss S, Rassen JA, Brown JS et al (2019) Graphical depiction of longitudinal study designs in health care databases. Ann Intern Med 170(6):398–406. https://doi.org/10.7326/M18-3079
")], and using templates from www.repeatinitiative.org/projects.html, which are licensed under a Creative Commons Attribution 3.0 Unported License. aTypically, a gap of up to 45 days in medical or pharmacy enrolment is allowed; bcovariates are measured in the 6 month period before entry into the cohort, and demographics are measured on day zero; cearliest of outcome of interest, switching or discontinuation of study drugs, death, disenrolment, or end of the study period; d365 days pre-index are shown for illustrative purposes; this could be any predefined time before the index date deemed appropriate, and tailored to the study question at hand. This figure is available as part of a downloadable slideset
Potential biases
The biggest criticism of real-world studies is their potential for systematic error (biases). These are broadly classified as confounding (due to lack of randomisation), selection bias (due to procedures used to select study population) and information bias (measurement error) [23].
Confounding
Confounding is the distortion of the treatment–outcome association when the groups being compared differ with respect to variables that influence the outcome. In comparing drug treatments, confounding by indication is a common issue that occurs because patients have an ‘indication’ for a particular drug [[27](/article/10.1007/s00125-020-05217-1#ref-CR27 "Blais L, Ernst P, Suissa S (1996) Confounding by indication and channeling over time: the risks of β2-agonists. Am J Epidemiol 144(12):1161–1169. https://doi.org/10.1093/oxfordjournals.aje.a008895
")]. As an example, comparing patients prescribed insulin vs oral glucose-lowering agents leads to confounding by indication as the two populations are imbalanced on the ‘indication’ (severe vs milder diabetes). When a treatment is known to be associated with an adverse event, confounding by contraindication is possible. In comparing thiazolidinediones with dipeptidyl peptidase-4 (DPP-4) inhibitors to assess the risk for heart failure, for example, patients with existing heart conditions are likely to be channelled away from thiazolidinediones. Restricting the study population to those without prevalent heart failure will therefore minimise intractable confounding by contraindication [[28](/article/10.1007/s00125-020-05217-1#ref-CR28 "Gokhale M, Buse JB, Jonsson Funk M et al (2017) No increased risk of cardiovascular events in older adults initiating dipeptidyl peptidase-4 inhibitors vs therapeutic alternatives. Diabetes Obes Metab 19(7):970–978. https://doi.org/10.1111/dom.12906
")].
In real-world studies confounding by frailty is possible. This is a particular problem in older adults, as frail, close-to-death patients are less likely to be treated with preventive treatments. Thus, when comparing users vs non-users of a particular drug to assess outcomes associated with frailty (e.g. mortality risk), the non-user group is likely to have higher mortality risk and make the drug look better than it really is [[29](/article/10.1007/s00125-020-05217-1#ref-CR29 "Glynn RJ, Knight EL, Levin R, Avorn J (2001) Paradoxical relations of drug treatment with mortality in older persons. Epidemiology 12(6):682–689. https://doi.org/10.1097/00001648-200111000-00017
"), [30](/article/10.1007/s00125-020-05217-1#ref-CR30 "Zhang H, McGrath L, Ellis A, Wyss R, Lund J, Stürmer T (2019) Restriction of pharmacoepidemiologic cohorts to initiators of unrelated preventive drug classes to reduce confounding by frailty in older adults. Am J Epidemiol 188(7):1371–1382. https://doi.org/10.1093/aje/kwz083
")].
Selection bias
This bias occurs when the selected population is not representative of the target population to which inference is to be drawn (due to selective survival rate, differential losses to follow-up, non-response, etc.) [31]. Selection bias is sometimes intertwined with confounding depending on the setting in which it occurs (e.g. epidemiologists sometimes use the term selection bias to mean ‘confounding by indication’, others use the term selection bias when confounders are unmeasured) [[32](/article/10.1007/s00125-020-05217-1#ref-CR32 "Hernán MA, Hernández-Díaz S, Robins JM (2004) A structural approach to selection bias. Epidimiology 15(5):615–625. https://doi.org/10.1097/01.ede.0000135174.63482.43
")].
Information bias
This arises due to inaccurate measurement or misclassification of treatments, outcome or confounders [23]. Its effect on results depends on whether misclassification is differential or non-differential across the treatments being compared. In a cohort study, non-differential exposure misclassification occurs when treatment status is equally misclassified among patients who develop or do not develop the outcome. As an illustration, if 10% of patients in both treatment A and treatment B groups received free drug samples (and therefore no prescription record in claims data), an equal proportion of patients in each group will be misclassified as ‘unexposed’. Non-differential outcome misclassification in a cohort study occurs when patients who develop the outcome are equally misclassified in treatment A and treatment B groups (e.g. 15% of healthy patients receiving treatment A or treatment B are misclassified as having lung cancer). Differential misclassification occurs when misclassification of treatment status is uneven between individuals that have or do not have the outcome, or when misclassification of the outcome is not the same between treatment A and treatment B. While non-differential misclassification of treatments and outcomes will generally bias estimates towards the null, differential misclassification can lead to spurious associations or can mask true effects. The effect of misclassification on results also depends on whether the results are reported as absolute or relative [23]. Absolute measures present difference in risk of the outcome in treatment A vs treatment B, while relative measures present the ratio of risk of outcome for treatment A vs treatment B. When misclassification is non-differential, studies reporting absolute measures should have outcome definitions with high sensitivity and specificity as low values for either can lead to bias. In studies reporting relative measures, near-perfect specificity, even at the cost of low sensitivity, is desired [[33](/article/10.1007/s00125-020-05217-1#ref-CR33 "Chubak J, Pocobelli G, Weiss NS (2012) Tradeoffs between accuracy measures for electronic health care data algorithms. J Clin Epidemiol 65(3):343–349. e342. https://doi.org/10.1016/j.jclinepi.2011.09.002
")].
Another common criticism of RWD concerns ‘missing data’. The commonly used strategy of excluding records with missing data can severely bias results. Multiple imputation methods for mitigating the effect of missing data have been shown to decrease bias and improve precision in a variety of disease areas including diabetes [[34](/article/10.1007/s00125-020-05217-1#ref-CR34 "Read SH, Lewis SC, Halbesma N, Wild SH (2017) Measuring the association between body mass index and all-cause mortality in the presence of missing data: analyses from the Scottish National Diabetes Register. Am J Epidemiol 185(8):641–649. https://doi.org/10.1093/aje/kww162
"), [35](/article/10.1007/s00125-020-05217-1#ref-CR35 "Harel O, Mitchell EM, Perkins NJ et al (2018) Multiple imputation for incomplete data in epidemiologic studies. Am J Epidemiol 187(3):576–584. https://doi.org/10.1093/aje/kwx349
")]. Methods for addressing missing data should be based on a careful consideration of reasons for missingness and availability of validation datasets needed for imputation methods [[36](/article/10.1007/s00125-020-05217-1#ref-CR36 "Hughes RA, Heron J, Sterne JAC, Tilling K (2019) Accounting for missing data in statistical analyses: multiple imputation is not always the answer. Int J Epidemiol 48(4):1294–1304. https://doi.org/10.1093/ije/dyz032
")].
Time-related biases
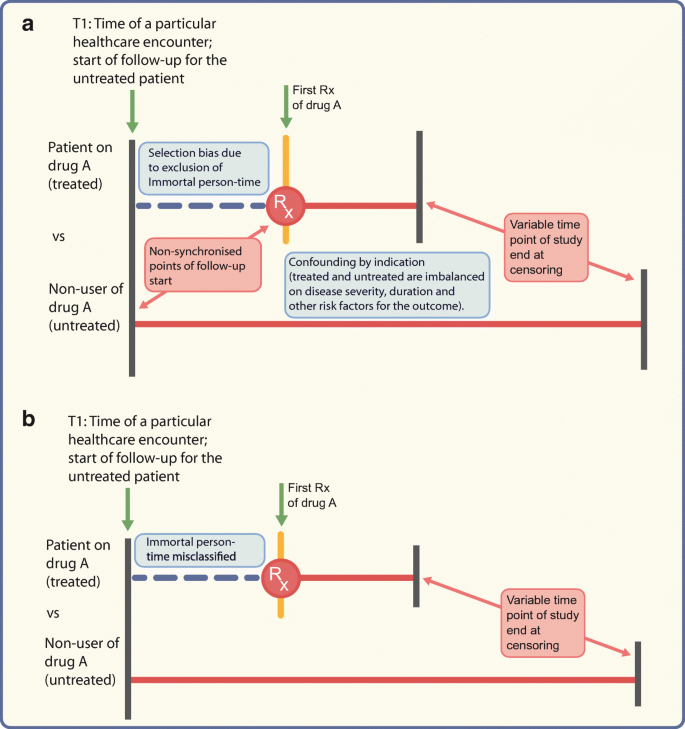
These are biases that misclassify person-time attributed to the treatment. Immortal time bias is one such bias arising from misclassification of the time before the treatment during which a patient, by design, could not have experienced the outcome and the patients have to be event-free until treatment starts [[37](/article/10.1007/s00125-020-05217-1#ref-CR37 "Suissa S (2007) Immortal time bias in pharmacoepidemiology. Am J Epidemiol 167(4):492–499. https://doi.org/10.1093/aje/kwm324
")]. Misclassifying this time or excluding it from the analysis altogether leads to immortal time bias [[37](/article/10.1007/s00125-020-05217-1#ref-CR37 "Suissa S (2007) Immortal time bias in pharmacoepidemiology. Am J Epidemiol 167(4):492–499. https://doi.org/10.1093/aje/kwm324
"), [38](/article/10.1007/s00125-020-05217-1#ref-CR38 "Lévesque LE, Hanley JA, Kezouh A, Suissa S (2010) Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ 340:b5087. https://doi.org/10.1136/bmj.b5087
")]. This is exacerbated in studies comparing treatment users vs non-users (Fig. 2) but can occur when comparing active treatments without careful consideration of person-time. Consider an example comparing a sulfonylurea vs metformin, where metformin users consisted of patients with or without prior sulfonylurea use. For the metformin patients with prior sulfonylurea use, their time on sulfonylureas before metformin was misclassified as ‘metformin-exposed’ which led to immortal time bias and spuriously suggested the protective effect of metformin on mortality risk, since they had to survive to switch to metformin [[39](/article/10.1007/s00125-020-05217-1#ref-CR39 "Bowker SL, Majumdar SR, Veugelers P, Johnson JA (2006) Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 29(2):254–258. https://doi.org/10.2337/diacare.29.02.06.dc05-1558
"), [40](/article/10.1007/s00125-020-05217-1#ref-CR40 "Suissa S, Azoulay L (2012) Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care 35(12):2665–2673. https://doi.org/10.2337/dc12-0788
")].
Fig. 2
Depiction of problems encountered when comparing treated vs untreated (not using active comparator) patients; drug A could, as an example, be a DPP-4 inhibitor. (a) Different times of follow-up (starting at the initiation date for the treated patients or time of healthcare encounter T1 for the untreated patients) will lead to selection bias if immortal person-time is excluded from the analysis. Confounding by indication may arise from the imbalance between the two groups on ‘indication’. (b) Even if the follow-up for both groups starts from time T1, the time between T1 and drug initiation would be misclassified as ‘time on drug A’ when in reality the patient was not on drug A before the first prescription. Red horizontal lines represent study timeline. Rx, prescription. This figure is available as part of a downloadable slideset
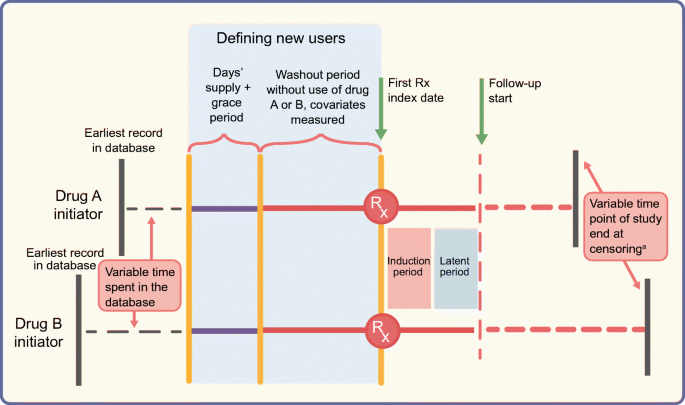
In studies assessing cancer outcomes, events occurring shortly after initiation may not be meaningfully attributed to the exposed period, particularly since carcinogenic exposures typically have long induction periods [[41](/article/10.1007/s00125-020-05217-1#ref-CR41 "Pottegard A, Friis S, Sturmer T, Hallas J, Bahmanyar S (2018) Considerations for pharmacoepidemiological studies of drug-cancer associations. Basic Clin Pharmacol Toxicol 122(5):451–459. https://doi.org/10.1111/bcpt.12946
")]. Not counting person-time and events during a predefined time-lag after drug initiation accounts for both these periods (Fig. 3). Similarly, it is unlikely that patients stop being ‘at risk’ on the day after drug discontinuation and a period of latency should also be considered to provide an opportunity to capture the outcome that was potentially present subclinically before treatment discontinuation [[41](/article/10.1007/s00125-020-05217-1#ref-CR41 "Pottegard A, Friis S, Sturmer T, Hallas J, Bahmanyar S (2018) Considerations for pharmacoepidemiological studies of drug-cancer associations. Basic Clin Pharmacol Toxicol 122(5):451–459. https://doi.org/10.1111/bcpt.12946
")]. As an example, a recent study exploring the incidence of breast cancer with insulin glargine vs intermediate-acting insulin used induction and lag periods to account for cancer latency [[42](/article/10.1007/s00125-020-05217-1#ref-CR42 "Stürmer T, Marquis MA, Zhou H et al (2013) Cancer incidence among those initiating insulin therapy with glargine versus human NPH insulin. Diabetes Care 36(11):3517–3525. https://doi.org/10.2337/dc13-0263
")].
Fig. 3
Schematic diagram of the active comparator new-user design comparing two glucose-lowering drugs. The top and bottom horizontal lines represent study timelines for initiators of drug A (e.g. DPP-4 inhibitor) and a therapeutically equivalent drug B (e.g. pioglitazone), respectively. Both groups of patients spend a variable amount of time in the database before ‘new use’ is defined. To define the new-use period for both groups, we need a predefined period equal to ‘expected days’ supply plus the grace period’ without a prescription being filled for treatment A or treatment B (indicated by solid purple lines) prior to the start of the washout period (solid orange lines). The washout period should also be free of any prescriptions for A or B and covariates are measured during this time. The index date indicates the date of the first prescription (Rx) and is followed by induction and latent periods during which person-time and outcomes are not counted. Solid red lines represent this time after the first prescription. Follow-up, indicated by the dashed red lines, starts at the end of the latent period and ends at censoring. Note that patients’ timelines for start of follow-up are intuitively synchronised by the active comparator new-user design, even though the patients can have variable start points of enrolment in the database or variable time points for end of follow-up/study end. aIf censoring is due to drug discontinuation, a predefined lag period should be considered before stopping to count outcomes and person-time. This figure is available as part of a downloadable slideset
Prevalent-user biases
Prevalent users are patients already on treatment before follow-up starts and therefore more tolerant of the drug. Methodological problems due to inclusion of prevalent users are illustrated by inconsistent results from studies examining cancer incidence with insulin glargine, depending on the study design used [[43](/article/10.1007/s00125-020-05217-1#ref-CR43 "Habel LA, Danforth KN, Quesenberry CP et al (2013) Cohort study of insulin glargine and risk of breast, prostate, and colorectal cancer among patients with diabetes. Diabetes Care 36(12):3953–3960. https://doi.org/10.2337/dc13-0140
"), [44](/article/10.1007/s00125-020-05217-1#ref-CR44 "Bradley MC, Chillarige Y, Lee H et al (2020) Similar breast cancer risk in women older than 65 years initiating glargine, detemir, and NPH Insulins. Diabetes Care 43(4):785–792. https://doi.org/10.2337/dc19-0614
")]. However, the most striking and frequently cited example illustrating these issues is a series of studies highlighting the discrepancies in the estimated effects of hormone therapy on cardiovascular outcomes between new users and prevalent users in the same data [[45](#ref-CR45 "Agency for Healthcare Research and Quality (2012) The incident user design in comparative effectiveness research. Available from https://effectivehealthcare.ahrq.gov/products/incident-user-design/research
. Accessed 13 Dec 2019"),[46](#ref-CR46 "Johnson ES, Bartman BA, Briesacher BA et al (2013) The incident user design in comparative effectiveness research. Pharmacoepidemiol Drug Saf 22(1):1–6.
https://doi.org/10.1002/pds.3334
"),[47](#ref-CR47 "Petitti DB, Freedman DA (2005) Invited commentary: how far can epidemiologists get with statistical adjustment? Am J Epidemiol 162(5):415–418. https://doi.org/10.1093/aje/kwi224
"),[48](/article/10.1007/s00125-020-05217-1#ref-CR48 "Ray WA (2003) Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 158(9):915–920. https://doi.org/10.1093/aje/kwg231
")]. The Nurses’ Health cohort study reported a decreased risk of major CHD in prevalent users of oestrogen and progestin compared with non-users, in contrast to results from the RCT which showed an increased risk in the oestrogen + progestin arm relative to placebo [[49](/article/10.1007/s00125-020-05217-1#ref-CR49 "Grodstein F, Stampfer MJ, Manson JE et al (1996) Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med 335(7):453–461. https://doi.org/10.1056/NEJM199608153350701
"), [50](/article/10.1007/s00125-020-05217-1#ref-CR50 "Manson JE, Hsia J, Johnson KC et al (2003) Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 349(6):523–534. https://doi.org/10.1056/NEJMoa030808
")]. A re-analysis of the Nurses’ Health study cohort comparing new users of hormone therapy vs non-initiators demonstrated results in line with the RCT, highlighting the issues due to inclusion of prevalent users [[51](/article/10.1007/s00125-020-05217-1#ref-CR51 "Hernán MA, Alonso A, Logan R et al (2008) Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidimiology 19(6):766–779. https://doi.org/10.1097/EDE.0b013e3181875e61
")]. As the prevalent users have ‘survived’ treatment, any patients who experienced early events (susceptible patients) will be automatically excluded in prevalent-user studies, introducing substantial bias if the hazard for the outcome varies depending on time spent on treatment [[48](/article/10.1007/s00125-020-05217-1#ref-CR48 "Ray WA (2003) Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 158(9):915–920. https://doi.org/10.1093/aje/kwg231
"), [52](/article/10.1007/s00125-020-05217-1#ref-CR52 "Yola M, Lucien A (1994) Evidence of the depletion of susceptibles effect in non-experimental pharmacoepidemiologic research. J Clin Epidemiol 47(7):731–737. https://doi.org/10.1016/0895-4356(94)90170-8
")]. In studies with a mix of prevalent and incident users, the differential proportion of prevalent users across two groups being compared leads to selection bias and also obscures early events if the prevalent users contribute more person-time. Moreover, since confounders are affected by prior treatment, they are mediators in a causal pathway between the treatment and outcome, and any analytical adjustment would worsen the bias [[48](/article/10.1007/s00125-020-05217-1#ref-CR48 "Ray WA (2003) Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 158(9):915–920. https://doi.org/10.1093/aje/kwg231
")].
Methods for minimising bias by study design and analysis
Active comparator new-user design
To avoid prevalent-user biases, new-user design has been recommended as almost a default strategy, except in settings where inclusion of prevalent users is preferable (e.g. describing burden of illness) [[53](/article/10.1007/s00125-020-05217-1#ref-CR53 "Cox E, Martin BC, Van Staa T, Garbe E, Siebert U, Johnson ML (2009) Good research practices for comparative effectiveness research: approaches to mitigate bias and confounding in the design of nonrandomized studies of treatment effects using secondary data sources: the International Society for Pharmacoeconomics and Outcomes Research Good Research Practices for Retrospective Database Analysis Task Force Report—Part II. Value Health 12(8):1053–1061. https://doi.org/10.1111/j.1524-4733.2009.00601.x
")]. This design includes initiators of a drug, after a washout period without that drug (treatment-naivety not necessary) and provides an intuitive timeline to start follow-up [[48](/article/10.1007/s00125-020-05217-1#ref-CR48 "Ray WA (2003) Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 158(9):915–920. https://doi.org/10.1093/aje/kwg231
")]. Because new-user designs restrict the population to drug initiators, concerns have been expressed about reduced generalisability and precision at the cost of high internal validity. Modifications of the new-user design, such as the prevalent new-user designs, have recently been proposed to address this (e.g. while comparing new-to-market vs older drugs) [[54](/article/10.1007/s00125-020-05217-1#ref-CR54 "Suissa S, Moodie EE, DellʼAniello S (2017) Prevalent new-user cohort designs for comparative drug effect studies by time-conditional propensity scores. Pharmacoepidemiol Drug Saf 26(4):459–468. https://doi.org/10.1002/pds.4107
")]. Such designs propose including patients switching from older to newer drugs to increase precision. However, precision gain needs to be carefully weighed against the mixing of research questions (initiation vs switching) and the potential for biases introduced by comparing switchers with patients who remain on treatment [55].
The merits of a new-user design are further amplified by comparing drugs in clinical equipoise [[56](/article/10.1007/s00125-020-05217-1#ref-CR56 "Kramer MS, Lane DA, Hutchinson TA (1987) Analgesic use, blood dyscrasias, and case-control pharmacoepidemiology: a critique of the International Agranulocytosis and Aplastic Anemia Study. J Chronic Dis 40(12):1073–1081. https://doi.org/10.1016/0021-9681(87)90073-7
")]. Comparing treated and untreated patients opens the door to a host of biases (Fig. 2), which can be overcome by the active comparator new-user design comparing new users of therapeutically equivalent drugs (active comparators; Fig. 3). This design makes the two cohorts ‘exchangeable’ with respect to baseline disease severity and outcome risk and the follow-up can start from an intuitive, synchronised time point [[57](/article/10.1007/s00125-020-05217-1#ref-CR57 "Lund JL, Richardson DB, Stürmer T (2015) The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep 2(4):221–228. https://doi.org/10.1007/s40471-015-0053-5
"), [58](/article/10.1007/s00125-020-05217-1#ref-CR58 "Yoshida K, Solomon DH, Kim SC (2015) Active-comparator design and new-user design in observational studies. Nat Rev Rheumatol 11(7):437–441. https://doi.org/10.1038/nrrheum.2015.30
")]. The demonstrated balance of measured characteristics may also increase the probability of balance of unmeasured covariates, although this cannot be empirically demonstrated [[28](/article/10.1007/s00125-020-05217-1#ref-CR28 "Gokhale M, Buse JB, Jonsson Funk M et al (2017) No increased risk of cardiovascular events in older adults initiating dipeptidyl peptidase-4 inhibitors vs therapeutic alternatives. Diabetes Obes Metab 19(7):970–978. https://doi.org/10.1111/dom.12906
"), [59](/article/10.1007/s00125-020-05217-1#ref-CR59 "Gokhale M, Buse JB, Gray CL, Pate V, Marquis MA, Stürmer T (2014) Dipeptidyl-peptidase-4 inhibitors and pancreatic cancer: a cohort study. Diabetes Obes Metab 16(12):1247–1256. https://doi.org/10.1111/dom.12379
")]. Often there may be situations in diabetes research where an active comparator is not available (e.g. an RWE study emulating a placebo-controlled trial). In such cases, synchronising cohorts based on any healthcare encounters that make the two cohorts as substitutable as possible is still preferred over comparing with non-users [[57](/article/10.1007/s00125-020-05217-1#ref-CR57 "Lund JL, Richardson DB, Stürmer T (2015) The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep 2(4):221–228. https://doi.org/10.1007/s40471-015-0053-5
")]. In a recent example illustrating this principle, the risk of cardiovascular outcomes with the sodium–glucose cotransporter-2 (SGLT2) inhibitor canagliflozin was assessed relative to non-SGLT2-inhibitors rather than to non-users of canagliflozin (which could have led to inclusion of diabetes patients not on pharmacological therapy and therefore caused imbalance of patient characteristics) [[60](/article/10.1007/s00125-020-05217-1#ref-CR60 "Ryan PB, Buse JB, Schuemie MJ et al (2018) Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non-SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: a real-world meta-analysis of 4 observational databases (OBSERVE-4D). Diabetes Obes Metab 20(11):2585–2597. https://doi.org/10.1111/dom.13424
")].
The active comparator new-user design is analogous to a head-to-head RCT comparing two drugs in equipoise. It allows following patients by ignoring treatment changes over time, analogous to the ‘intent-to-treat’ analyses in RCTs. This may introduce treatment misclassification bias towards the null and should be avoided, particularly in studies assessing harm, to avoid masking actual treatment-associated harm. Another option is the ‘as-treated’ approach where follow-up is censored at treatment discontinuation, switching or augmentation. A caveat with the ‘as-treated’ approach is potential selection bias introduced because of informative censoring (i.e. patients censored because they made treatment changes are not representative of patients who remain on treatment). This needs to be addressed in the analysis using inverse probability of censoring weights [[32](/article/10.1007/s00125-020-05217-1#ref-CR32 "Hernán MA, Hernández-Díaz S, Robins JM (2004) A structural approach to selection bias. Epidimiology 15(5):615–625. https://doi.org/10.1097/01.ede.0000135174.63482.43
")].
Recent applications of these designs in diabetes research include new-user studies on SGLT2 inhibitors demonstrating no increased risk of amputations relative to non-SGLT2 inhibitors, but increased risk relative to the most appropriate DPP-4 inhibitor comparator using restrictive study criteria and robust analytic techniques [[60](/article/10.1007/s00125-020-05217-1#ref-CR60 "Ryan PB, Buse JB, Schuemie MJ et al (2018) Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non-SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: a real-world meta-analysis of 4 observational databases (OBSERVE-4D). Diabetes Obes Metab 20(11):2585–2597. https://doi.org/10.1111/dom.13424
"), [61](/article/10.1007/s00125-020-05217-1#ref-CR61 "Yang JY, Wang T, Pate V et al (2019) Sodium-glucose co-transporter-2 inhibitor use and risk of lower-extremity amputation: evolving questions, evolving answers. Diabetes Obes Metab 21(5):1223–1236. https://doi.org/10.1111/dom.13647
")].
Analysis
An example that naturally fits with the active comparator new-user study is the use of propensity scores, a powerful tool for controlling measured confounding [[62](#ref-CR62 "Stürmer T, Wyss R, Glynn RJ, Brookhart MA (2014) Propensity scores for confounder adjustment when assessing the effects of medical interventions using nonexperimental study designs. J Intern Med 275(6):570–580. https://doi.org/10.1111/joim.12197
"),[63](#ref-CR63 "Brookhart MA, Wyss R, Layton JB, Stürmer T (2013) Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes 6(5):604–611. https://doi.org/10.1161/CIRCOUTCOMES.113.000359
"),[64](#ref-CR64 "Glynn RJ, Schneeweiss S, Stürmer T (2006) Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol 98(3):253–259. https://doi.org/10.1111/j.1742-7843.2006.pto_293.x
"),[65](#ref-CR65 "Stürmer T, Joshi M, Glynn RJ, Avorn J, Rothman KJ, Schneeweiss S (2006) A review of the application of propensity score methods yielded increasing use, advantages in specific settings, but not substantially different estimates compared with conventional multivariable methods. J Clin Epidemiol 59(5):437–447. https://doi.org/10.1016/j.jclinepi.2005.07.004
"),[66](/article/10.1007/s00125-020-05217-1#ref-CR66 "Winkelmayer WC, Kurth T (2004) Propensity scores: help or hype? Nephrol Dial Transplant 19(7):1671–1673. https://doi.org/10.1093/ndt/gfh104
")]. A propensity score is a summary score estimating the probability of treatment A vs treatment B based on patients’ baseline characteristics. Once estimated, propensity scores can be implemented by matching, weighting and stratification on the score [[62](/article/10.1007/s00125-020-05217-1#ref-CR62 "Stürmer T, Wyss R, Glynn RJ, Brookhart MA (2014) Propensity scores for confounder adjustment when assessing the effects of medical interventions using nonexperimental study designs. J Intern Med 275(6):570–580. https://doi.org/10.1111/joim.12197
"), [63](/article/10.1007/s00125-020-05217-1#ref-CR63 "Brookhart MA, Wyss R, Layton JB, Stürmer T (2013) Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes 6(5):604–611. https://doi.org/10.1161/CIRCOUTCOMES.113.000359
"), [67](/article/10.1007/s00125-020-05217-1#ref-CR67 "Stürmer T, Schneeweiss S, Brookhart MA, Rothman KJ, Avorn J, Glynn RJ (2005) Analytic strategies to adjust confounding using exposure propensity scores and disease risk scores: nonsteroidal antiinflammatory drugs and short-term mortality in the elderly. Am J Epidemiol 161(9):891–898. https://doi.org/10.1093/aje/kwi106
"), [68](/article/10.1007/s00125-020-05217-1#ref-CR68 "Desai RJ, Rothman KJ, Bateman BT, Hernandez-Diaz S, Huybrechts KF (2017) A Propensity score based fine stratification approach for confounding adjustment when exposure is infrequent. Epidimiology 28(2):249–257. https://doi.org/10.1097/EDE.0000000000000595
")], all of which allow empirical demonstration of covariate balance before and after implementation. Propensity scores can also be included in an outcome model, although this takes away the ability to empirically ‘see’ the adjustment and has other disadvantages so is therefore discouraged [[67](/article/10.1007/s00125-020-05217-1#ref-CR67 "Stürmer T, Schneeweiss S, Brookhart MA, Rothman KJ, Avorn J, Glynn RJ (2005) Analytic strategies to adjust confounding using exposure propensity scores and disease risk scores: nonsteroidal antiinflammatory drugs and short-term mortality in the elderly. Am J Epidemiol 161(9):891–898. https://doi.org/10.1093/aje/kwi106
")]. The choice of method used to implement propensity scores (matching, stratification, different types of weighting) depends on the target population to which inference needs to be drawn and the extent of unmeasured residual confounding [[62](/article/10.1007/s00125-020-05217-1#ref-CR62 "Stürmer T, Wyss R, Glynn RJ, Brookhart MA (2014) Propensity scores for confounder adjustment when assessing the effects of medical interventions using nonexperimental study designs. J Intern Med 275(6):570–580. https://doi.org/10.1111/joim.12197
"), [69](#ref-CR69 "Sato T, Matsuyama Y (2003) Marginal structural models as a tool for standardization. Epidimiology 14(6):680–686. https://doi.org/10.1097/01.EDE.0000081989.82616.7d
"),[70](#ref-CR70 "Yoshida K, Hernández-Díaz S, Solomon DH et al (2017) Matching weights to simultaneously compare three treatment groups: comparison to three-way matching. Epidimiology 28(3):387–395. https://doi.org/10.1097/EDE.0000000000000627
"),71,[72](#ref-CR72 "Crump RK, Hotz VJ, Imbens GW, Mitnik OA (2009) Dealing with limited overlap in estimation of average treatment effects. Biometrika 96(1):187–199. https://doi.org/10.1093/biomet/asn055
"),73,[74](/article/10.1007/s00125-020-05217-1#ref-CR74 "Glynn RJ, Lunt M, Rothman KJ, Poole C, Schneeweiss S, Stürmer T (2019) Comparison of alternative approaches to trim subjects in the tails of the propensity score distribution. Pharmacoepidemiol Drug Saf 28(10):1290–1298. https://doi.org/10.1002/pds.4846
")].
Other methods such as disease risk scores (summary scores based on baseline outcome risk) can have advantages in specific settings [[75](/article/10.1007/s00125-020-05217-1#ref-CR75 "Arbogast PG, Ray WA (2009) Use of disease risk scores in pharmacoepidemiologic studies. Stat Methods Med Res 18(1):67–80. https://doi.org/10.1177/0962280208092347
"), [76](/article/10.1007/s00125-020-05217-1#ref-CR76 "Glynn RJ, Gagne JJ, Schneeweiss S (2012) Role of disease risk scores in comparative effectiveness research with emerging therapies. Pharmacoepidemiol Drug Saf 21(Suppl 2):138–147. https://doi.org/10.1002/pds.3231
")]. When substantial unmeasured confounding is expected, instrumental variable methods might be used to obtain unbiased effect estimates [[77](/article/10.1007/s00125-020-05217-1#ref-CR77 "Brookhart MA, Rassen JA, Schneeweiss S (2010) Instrumental variable methods in comparative safety and effectiveness research. Pharmacoepidemiol Drug Saf 19(6):537–554. https://doi.org/10.1002/pds.1908
"), [78](/article/10.1007/s00125-020-05217-1#ref-CR78 "Ertefaie A, Small DS, Flory JH, Hennessy S (2017) A tutorial on the use of instrumental variables in pharmacoepidemiology. Pharmacoepidemiol Drug Saf 26(4):357–367. https://doi.org/10.1002/pds.4158
")]. Instrumental variables are variables that affect treatment choice but not the outcome of interest other than through treatment. Examples include physicians’ preference and any rapid change in treatments (e.g. due to market access, guideline changes, warnings about serious side effects). All of these methods, however, are based on a number of assumptions that should be evaluated when conducting and interpreting real-world studies. Further, more than one method can be considered as supplementary sensitivity analyses after clearly specifying the reasons a priori. Given the dynamic treatment patterns in routine clinical practice (discontinuation, re-initiation, switching treatment, etc.), analyses often need to account for time-varying treatments and confounders depending on the research question of interest.
Recently, the utility of machine learning in causal inference has been explored [[79](/article/10.1007/s00125-020-05217-1#ref-CR79 "Blakely T, Lynch J, Simons K, Bentley R, Rose S (2019) Reflection on modern methods: when worlds collide—prediction, machine learning and causal inference. Int J Epidemiol. https://doi.org/10.1093/ije/dyz132
"), [80](/article/10.1007/s00125-020-05217-1#ref-CR80 "Wyss R, Schneeweiss S, van der Laan M, Lendle SD, Ju C, Franklin JM (2018) Using super learner prediction modeling to improve high-dimensional propensity score estimation. Epidimiology 29(1):96–106. https://doi.org/10.1097/EDE.0000000000000762
")]. Machine learning algorithms have been shown to perform well in estimating propensity scores in certain settings by reducing model mis-specification [[81](/article/10.1007/s00125-020-05217-1#ref-CR81 "Westreich D, Lessler J, Funk MJ (2010) Propensity score estimation: neural networks, support vector machines, decision trees (CART), and meta-classifiers as alternatives to logistic regression. J Clin Epidemiol 63(8):826–833. https://doi.org/10.1016/j.jclinepi.2009.11.020
"), [82](/article/10.1007/s00125-020-05217-1#ref-CR82 "Bi Q, Goodman KE, Kaminsky J, Lessler J (2019) What is machine learning? A primer for the epidemiologist. Am J Epidemiol 188(12):2222–2239. https://doi.org/10.1093/aje/kwz189
")] but can amplify bias in certain settings (e.g. if use of instrumental variables in propensity score estimation is encouraged) [[83](/article/10.1007/s00125-020-05217-1#ref-CR83 "Myers JA, Rassen JA, Gagne JJ et al (2011) Effects of adjusting for instrumental variables on bias and precision of effect estimates. Am J Epidemiol 174(11):1213–1222. https://doi.org/10.1093/aje/kwr364
")]. A concern with these methods is the lack of easy interpretability and the risk of being data-driven rather than being informed by substantive knowledge and therefore need careful consideration before being used.
Newer avenues for applicability of RWE
RWD are increasingly being used to predict outcomes from clinical trials, which supports efficient resource management, faster drug approval times and making medicines available sooner for patients. A recent example is a study comparing linagliptin vs glimepiride using RWD from Medicare and commercial data [[84](/article/10.1007/s00125-020-05217-1#ref-CR84 "Patorno E, Schneeweiss S, Gopalakrishnan C, Martin D, Franklin JM (2019) Using real-world data to predict findings of an ongoing phase IV cardiovascular outcome trial–cardiovascular safety of linagliptin vs. glimepiride. Diabetes Care 42(12):2204–2210. https://doi.org/10.2337/dc19-0069
")]. While this study demonstrated linagliptin’s ‘non-inferiority’ in line with the findings of the CAROLINA trial, the magnitude was smaller than that observed in the trial. This was likely due to differences in the nature of treatments being compared rather than a lack of robust methodology. Despite the difference in magnitude of results, this supports the value that RWD brings. Another application is the use of an RWD-based comparator for single-arm trials when using a randomised control arm is not feasible, as was done with BLINCYTO (blinatumomab) indicated for leukaemia treatment [[2](/article/10.1007/s00125-020-05217-1#ref-CR2 "U.S. FDA (2019) Webinar: framework for FDA’s Real-World Evidence Program – Mar 15, 2019. Available from https://www.fda.gov/drugs/webinar-framework-fdas-real-world-evidence-program-mar-15-2019
. Accessed 20 Dec 2019")\]. We use blinatumomab as a powerful example of use of RWD in regulatory decision-making. It is not inconceivable that RWD may find applications in the future in areas where randomised trials may be deemed unethical, such as treatment of heart failure without background SGLT2 inhibitor therapy, or for the prevention of rare events where sample sizes could become prohibitive. The main challenge to address here is the differences between trial participants vs patients in routine practice, including the potential for differential recording of characteristics, warranting deeper design and analytical considerations depending on the nature and extent of differences. Efforts are also ongoing to map the potential effects of RCT data to real-world populations, although we are not aware of examples of this in diabetes research. Pragmatic trials (that measure effectiveness of the treatment/intervention in routine clinical practice) including RWD are also increasingly being explored in a number of disease areas \[[85](/article/10.1007/s00125-020-05217-1#ref-CR85 "Dal-Ré R, Janiaud P, Ioannidis JPA (2018) Real-world evidence: how pragmatic are randomized controlled trials labeled as pragmatic? BMC Med 16(1):49.
https://doi.org/10.1186/s12916-018-1038-2
"), [86](/article/10.1007/s00125-020-05217-1#ref-CR86 "Zuidgeest MGP, Goetz I, Groenwold RHH, Irving E, van Thiel G, Grobbee DE (2017) Series: Pragmatic trials and real world evidence: Paper 1. Introduction. J Clin Epidemiol 88:7–13. https://doi.org/10.1016/j.jclinepi.2016.12.023
")]. Finally, we may be at the verge of a paradigm shift with respect to classification of validity and hierarchy of study designs. The approach of prioritising internal validity (getting unbiased estimates) at the cost of external validity (generalisability or transportability of results), and our current thinking of internal validity as a prerequisite for external validity can negatively affect the value that RWE brings. Westreich et al recently proposed a joint measure of the validity of effect estimates (target validity) and defined target bias as any deviation of the estimated treatment effect from the true treatment effect in a target population rather than the current distinction between internal and external validity [[87](/article/10.1007/s00125-020-05217-1#ref-CR87 "Westreich D, Edwards JK, Lesko CR, Cole SR, Stuart EA (2018) Target Validity and the hierarchy of study designs. Am J Epidemiol 188(2):438–443. https://doi.org/10.1093/aje/kwy228
")].
Conclusion
The value of RWE lies in going beyond the constraints of RCTs to understand the effects in real-world populations. However, the hopes of ‘quick wins’ with RWE need to be balanced with a knowledge of robust methodology. We have focused our discussion on key concepts, methods and recommendations in the hope that readers are better informed of the utility and limitations of a particular RWE study that they encounter. The following key points should be looked for when evaluating an RWE study: a clearly articulated research question; a fit-for-purpose data source; a state-of-the-art design including appropriate comparators; covariate balance; analysis methods including sensitivity analyses; and the likelihood of being able to reasonably replicate the study in another similar setting. Several guidelines have come into existence to assist investigators with proper conduct, interpretation and reporting of real-world studies [88,89,[90](/article/10.1007/s00125-020-05217-1#ref-CR90 "Berger ML, Sox H, Willke RJ et al (2017) Good practices for real-world data studies of treatment and/or comparative effectiveness: recommendations from the joint ISPOR-ISPE Special Task Force on real-world evidence in health care decision making. Pharmacoepidemiol Drug Saf 26(9):1033–1039. https://doi.org/10.1002/pds.4297
")]. As the field continues to grow, it is important for scientific journals and regulatory agencies to use peer reviewers with adequate methodological know-how to ensure dissemination of high-quality RWE and maximise its utility in decision-making.
Abbreviations
DPP-4:
Dipeptidyl peptidase-4
RWD:
Real-world data
RWE:
Real-world evidence
SGLT2:
Sodium–glucose cotransporter-2
References
- Wedam S, Fashoyin-Aje L, Bloomquist E et al (2020) FDA approval summary: palbociclib for male patients with metastatic breast cancer. Clin Cancer Res 26(6):1208–1212. https://doi.org/10.1158/1078-0432.CCR-19-2580
Article PubMed Google Scholar - U.S. FDA (2019) Webinar: framework for FDA’s Real-World Evidence Program – Mar 15, 2019. Available from https://www.fda.gov/drugs/webinar-framework-fdas-real-world-evidence-program-mar-15-2019. Accessed 20 Dec 2019
- U.S. FDA (2019) Submitting documents using real-world data and real-world evidence to FDA for drugs and biologics guidance for industry - May 9, 2019. Available from https://www.fda.gov/regulatory-information/search-fda-guidance-documents/submitting-documents-using-real-world-data-and-real-world-evidence-fda-drugs-and-biologics-guidance. Accessed 20 Dec 2019
- Allie Nawrat, Pharma Technology Focus (2019) Real world data - how can it improve clinical trial outcomes. Available from https://www.pharmaceutical-technology.com/features/real-world-data-improving-outcomes/. Accessed 20 Dec 2019
- Hernan MA (2018) The C-word: scientific euphemisms do not improve causal inference from observational data. Am J Public Health 108(5):616–619. https://doi.org/10.2105/AJPH.2018.304337
Article PubMed PubMed Central Google Scholar - Hernan MA, Robins JM (2016) Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol 183(8):758–764. https://doi.org/10.1093/aje/kwv254
Article PubMed PubMed Central Google Scholar - Hernan MA (2005) Invited commentary: hypothetical interventions to define causal effects--afterthought or prerequisite? Am J Epidemiol 162(7):618–620; discussion 621-612. https://doi.org/10.1093/aje/kwi255
Article PubMed Google Scholar - Rubin DB (2005) Causal inference using potential outcomes: design, modeling, decisions. J Am Stat Assoc 100(469):322–331. https://doi.org/10.1198/016214504000001880
Article CAS Google Scholar - Nørgaard M, Ehrenstein V, Vandenbroucke JP (2017) Confounding in observational studies based on large health care databases: problems and potential solutions–a primer for the clinician. Clin Epidemiol 9:185–193. https://doi.org/10.2147/CLEP.S129879
Article PubMed PubMed Central Google Scholar - Maldonado G, Greenland S (2002) Estimating causal effects. Int J Epidemiol 31(2):422–429. https://doi.org/10.1093/ije/31.2.422
Article PubMed Google Scholar - Nabhan C, Klink A, Prasad V (2019) Real-world evidence-what does it really mean? JAMA Oncol 5(6):781–783. https://doi.org/10.1001/jamaoncol.2019.0450
Article PubMed Google Scholar - Sturmer T, Jonsson Funk M, Poole C, Brookhart MA (2011) Nonexperimental comparative effectiveness research using linked healthcare databases. Epidimiology 22(3):298–301. https://doi.org/10.1097/EDE.0b013e318212640c
Article Google Scholar - Strom BL (2001) Data validity issues in using claims data. Pharmacoepidemiol Drug Saf 10(5):389–392. https://doi.org/10.1002/pds.610
Article CAS PubMed Google Scholar - Casey JA, Schwartz BS, Stewart WF, Adler NE (2016) Using electronic health records for population health research: a review of methods and applications. Annu Rev Public Health 37(1):61–81. https://doi.org/10.1146/annurev-publhealth-032315-021353
Article PubMed Google Scholar - Farmer R, Mathur R, Bhaskaran K, Eastwood SV, Chaturvedi N, Smeeth L (2018) Promises and pitfalls of electronic health record analysis. Diabetologia 61(6):1241–1248. https://doi.org/10.1007/s00125-017-4518-6
Article PubMed Google Scholar - Nelson EC, Dixon-Woods M, Batalden PB et al (2016) Patient focused registries can improve health, care, and science. BMJ 354:i3319. https://doi.org/10.1136/bmj.i3319
Article PubMed PubMed Central Google Scholar - Diabetes Collaborative Registries (2019) The Diabetes Collaborative Registry. Transforming the future of diabetes care. Available from https://cvquality.acc.org/NCDR-Home/registries/outpatient-registries/the-diabetes-collaborative-registry. Accessed 20 Dec 2019
- McDonald L, Malcolm B, Ramagopalan S, Syrad H (2019) Real-world data and the patient perspective: the PROmise of social media? BMC Med 17(1):11. https://doi.org/10.1186/s12916-018-1247-8
Article PubMed PubMed Central Google Scholar - Pierce CE, Bouri K, Pamer C et al (2017) Evaluation of facebook and twitter monitoring to detect safety signals for medical products: an analysis of recent FDA safety alerts. Drug Saf 40(4):317–331. https://doi.org/10.1007/s40264-016-0491-0
Article PubMed PubMed Central Google Scholar - Kuehn BM (2015) Is there an app to solve app overload? JAMA 313(14):1405–1407. https://doi.org/10.1001/jama.2015.2381
Article PubMed Google Scholar - Rivera DR, Gokhale MN, Reynolds MW et al (2020) Linking electronic health data in pharmacoepidemiology: appropriateness and feasibility. Pharmacoepidemiol Drug Saf 29(1):18–29. https://doi.org/10.1002/pds.4918
Article PubMed Google Scholar - Pearce N (2012) Classification of epidemiological study designs. Int J Epidemiol 41(2):393–397. https://doi.org/10.1093/ije/dys049
Article PubMed Google Scholar - Rothman KJ, Greenland GS, Lash TL (2008) Modern epidemiology, 3rd edn. Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadephlia
Google Scholar - Petersen I, Douglas I, Whitaker H (2016) Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ 354:i4515
Article PubMed Google Scholar - Hallas J, Pottegård A (2014) Use of self-controlled designs in pharmacoepidemiology. J Intern Med 275(6):581–589. https://doi.org/10.1111/joim.12186
Article CAS PubMed Google Scholar - Schneeweiss S, Rassen JA, Brown JS et al (2019) Graphical depiction of longitudinal study designs in health care databases. Ann Intern Med 170(6):398–406. https://doi.org/10.7326/M18-3079
Article PubMed Google Scholar - Blais L, Ernst P, Suissa S (1996) Confounding by indication and channeling over time: the risks of β2-agonists. Am J Epidemiol 144(12):1161–1169. https://doi.org/10.1093/oxfordjournals.aje.a008895
Article CAS PubMed Google Scholar - Gokhale M, Buse JB, Jonsson Funk M et al (2017) No increased risk of cardiovascular events in older adults initiating dipeptidyl peptidase-4 inhibitors vs therapeutic alternatives. Diabetes Obes Metab 19(7):970–978. https://doi.org/10.1111/dom.12906
Article CAS PubMed PubMed Central Google Scholar - Glynn RJ, Knight EL, Levin R, Avorn J (2001) Paradoxical relations of drug treatment with mortality in older persons. Epidemiology 12(6):682–689. https://doi.org/10.1097/00001648-200111000-00017
Article CAS PubMed Google Scholar - Zhang H, McGrath L, Ellis A, Wyss R, Lund J, Stürmer T (2019) Restriction of pharmacoepidemiologic cohorts to initiators of unrelated preventive drug classes to reduce confounding by frailty in older adults. Am J Epidemiol 188(7):1371–1382. https://doi.org/10.1093/aje/kwz083
Article PubMed PubMed Central Google Scholar - Coggon D, Barker D, Rose G (2009) Epidemiology for the uninitiated, 5th edn. Wiley, New York
Google Scholar - Hernán MA, Hernández-Díaz S, Robins JM (2004) A structural approach to selection bias. Epidimiology 15(5):615–625. https://doi.org/10.1097/01.ede.0000135174.63482.43
Article Google Scholar - Chubak J, Pocobelli G, Weiss NS (2012) Tradeoffs between accuracy measures for electronic health care data algorithms. J Clin Epidemiol 65(3):343–349. e342. https://doi.org/10.1016/j.jclinepi.2011.09.002
Article PubMed Google Scholar - Read SH, Lewis SC, Halbesma N, Wild SH (2017) Measuring the association between body mass index and all-cause mortality in the presence of missing data: analyses from the Scottish National Diabetes Register. Am J Epidemiol 185(8):641–649. https://doi.org/10.1093/aje/kww162
Article PubMed Google Scholar - Harel O, Mitchell EM, Perkins NJ et al (2018) Multiple imputation for incomplete data in epidemiologic studies. Am J Epidemiol 187(3):576–584. https://doi.org/10.1093/aje/kwx349
Article PubMed Google Scholar - Hughes RA, Heron J, Sterne JAC, Tilling K (2019) Accounting for missing data in statistical analyses: multiple imputation is not always the answer. Int J Epidemiol 48(4):1294–1304. https://doi.org/10.1093/ije/dyz032
Article PubMed PubMed Central Google Scholar - Suissa S (2007) Immortal time bias in pharmacoepidemiology. Am J Epidemiol 167(4):492–499. https://doi.org/10.1093/aje/kwm324
Article PubMed Google Scholar - Lévesque LE, Hanley JA, Kezouh A, Suissa S (2010) Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ 340:b5087. https://doi.org/10.1136/bmj.b5087
Article PubMed Google Scholar - Bowker SL, Majumdar SR, Veugelers P, Johnson JA (2006) Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 29(2):254–258. https://doi.org/10.2337/diacare.29.02.06.dc05-1558
Article PubMed Google Scholar - Suissa S, Azoulay L (2012) Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care 35(12):2665–2673. https://doi.org/10.2337/dc12-0788
Article CAS PubMed PubMed Central Google Scholar - Pottegard A, Friis S, Sturmer T, Hallas J, Bahmanyar S (2018) Considerations for pharmacoepidemiological studies of drug-cancer associations. Basic Clin Pharmacol Toxicol 122(5):451–459. https://doi.org/10.1111/bcpt.12946
Article CAS PubMed PubMed Central Google Scholar - Stürmer T, Marquis MA, Zhou H et al (2013) Cancer incidence among those initiating insulin therapy with glargine versus human NPH insulin. Diabetes Care 36(11):3517–3525. https://doi.org/10.2337/dc13-0263
Article CAS PubMed PubMed Central Google Scholar - Habel LA, Danforth KN, Quesenberry CP et al (2013) Cohort study of insulin glargine and risk of breast, prostate, and colorectal cancer among patients with diabetes. Diabetes Care 36(12):3953–3960. https://doi.org/10.2337/dc13-0140
Article CAS PubMed PubMed Central Google Scholar - Bradley MC, Chillarige Y, Lee H et al (2020) Similar breast cancer risk in women older than 65 years initiating glargine, detemir, and NPH Insulins. Diabetes Care 43(4):785–792. https://doi.org/10.2337/dc19-0614
Article PubMed Google Scholar - Agency for Healthcare Research and Quality (2012) The incident user design in comparative effectiveness research. Available from https://effectivehealthcare.ahrq.gov/products/incident-user-design/research. Accessed 13 Dec 2019
- Johnson ES, Bartman BA, Briesacher BA et al (2013) The incident user design in comparative effectiveness research. Pharmacoepidemiol Drug Saf 22(1):1–6. https://doi.org/10.1002/pds.3334
Article PubMed Google Scholar - Petitti DB, Freedman DA (2005) Invited commentary: how far can epidemiologists get with statistical adjustment? Am J Epidemiol 162(5):415–418. https://doi.org/10.1093/aje/kwi224
Article PubMed Google Scholar - Ray WA (2003) Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 158(9):915–920. https://doi.org/10.1093/aje/kwg231
Article PubMed Google Scholar - Grodstein F, Stampfer MJ, Manson JE et al (1996) Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med 335(7):453–461. https://doi.org/10.1056/NEJM199608153350701
Article CAS PubMed Google Scholar - Manson JE, Hsia J, Johnson KC et al (2003) Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 349(6):523–534. https://doi.org/10.1056/NEJMoa030808
Article CAS PubMed Google Scholar - Hernán MA, Alonso A, Logan R et al (2008) Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidimiology 19(6):766–779. https://doi.org/10.1097/EDE.0b013e3181875e61
Article Google Scholar - Yola M, Lucien A (1994) Evidence of the depletion of susceptibles effect in non-experimental pharmacoepidemiologic research. J Clin Epidemiol 47(7):731–737. https://doi.org/10.1016/0895-4356(94)90170-8
Article PubMed Google Scholar - Cox E, Martin BC, Van Staa T, Garbe E, Siebert U, Johnson ML (2009) Good research practices for comparative effectiveness research: approaches to mitigate bias and confounding in the design of nonrandomized studies of treatment effects using secondary data sources: the International Society for Pharmacoeconomics and Outcomes Research Good Research Practices for Retrospective Database Analysis Task Force Report—Part II. Value Health 12(8):1053–1061. https://doi.org/10.1111/j.1524-4733.2009.00601.x
Article PubMed Google Scholar - Suissa S, Moodie EE, DellʼAniello S (2017) Prevalent new-user cohort designs for comparative drug effect studies by time-conditional propensity scores. Pharmacoepidemiol Drug Saf 26(4):459–468. https://doi.org/10.1002/pds.4107
Article CAS PubMed Google Scholar - Garry E, Buse JB, Gokhale M, Lund JL, Pate V, Sturmer T (2018) Implementation of the prevalent new user study design in the US Medicare population: benefit versus harm. Pharmacoepidemiol Drug Saf 27:167–167
Google Scholar - Kramer MS, Lane DA, Hutchinson TA (1987) Analgesic use, blood dyscrasias, and case-control pharmacoepidemiology: a critique of the International Agranulocytosis and Aplastic Anemia Study. J Chronic Dis 40(12):1073–1081. https://doi.org/10.1016/0021-9681(87)90073-7
Article CAS PubMed Google Scholar - Lund JL, Richardson DB, Stürmer T (2015) The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep 2(4):221–228. https://doi.org/10.1007/s40471-015-0053-5
Article PubMed PubMed Central Google Scholar - Yoshida K, Solomon DH, Kim SC (2015) Active-comparator design and new-user design in observational studies. Nat Rev Rheumatol 11(7):437–441. https://doi.org/10.1038/nrrheum.2015.30
Article CAS PubMed PubMed Central Google Scholar - Gokhale M, Buse JB, Gray CL, Pate V, Marquis MA, Stürmer T (2014) Dipeptidyl-peptidase-4 inhibitors and pancreatic cancer: a cohort study. Diabetes Obes Metab 16(12):1247–1256. https://doi.org/10.1111/dom.12379
Article CAS PubMed PubMed Central Google Scholar - Ryan PB, Buse JB, Schuemie MJ et al (2018) Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non-SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: a real-world meta-analysis of 4 observational databases (OBSERVE-4D). Diabetes Obes Metab 20(11):2585–2597. https://doi.org/10.1111/dom.13424
Article CAS PubMed PubMed Central Google Scholar - Yang JY, Wang T, Pate V et al (2019) Sodium-glucose co-transporter-2 inhibitor use and risk of lower-extremity amputation: evolving questions, evolving answers. Diabetes Obes Metab 21(5):1223–1236. https://doi.org/10.1111/dom.13647
Article CAS PubMed PubMed Central Google Scholar - Stürmer T, Wyss R, Glynn RJ, Brookhart MA (2014) Propensity scores for confounder adjustment when assessing the effects of medical interventions using nonexperimental study designs. J Intern Med 275(6):570–580. https://doi.org/10.1111/joim.12197
Article PubMed PubMed Central Google Scholar - Brookhart MA, Wyss R, Layton JB, Stürmer T (2013) Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes 6(5):604–611. https://doi.org/10.1161/CIRCOUTCOMES.113.000359
Article PubMed PubMed Central Google Scholar - Glynn RJ, Schneeweiss S, Stürmer T (2006) Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol 98(3):253–259. https://doi.org/10.1111/j.1742-7843.2006.pto_293.x
Article CAS PubMed PubMed Central Google Scholar - Stürmer T, Joshi M, Glynn RJ, Avorn J, Rothman KJ, Schneeweiss S (2006) A review of the application of propensity score methods yielded increasing use, advantages in specific settings, but not substantially different estimates compared with conventional multivariable methods. J Clin Epidemiol 59(5):437–447. https://doi.org/10.1016/j.jclinepi.2005.07.004
Article PubMed Google Scholar - Winkelmayer WC, Kurth T (2004) Propensity scores: help or hype? Nephrol Dial Transplant 19(7):1671–1673. https://doi.org/10.1093/ndt/gfh104
Article PubMed Google Scholar - Stürmer T, Schneeweiss S, Brookhart MA, Rothman KJ, Avorn J, Glynn RJ (2005) Analytic strategies to adjust confounding using exposure propensity scores and disease risk scores: nonsteroidal antiinflammatory drugs and short-term mortality in the elderly. Am J Epidemiol 161(9):891–898. https://doi.org/10.1093/aje/kwi106
Article PubMed Google Scholar - Desai RJ, Rothman KJ, Bateman BT, Hernandez-Diaz S, Huybrechts KF (2017) A Propensity score based fine stratification approach for confounding adjustment when exposure is infrequent. Epidimiology 28(2):249–257. https://doi.org/10.1097/EDE.0000000000000595
Article Google Scholar - Sato T, Matsuyama Y (2003) Marginal structural models as a tool for standardization. Epidimiology 14(6):680–686. https://doi.org/10.1097/01.EDE.0000081989.82616.7d
Article Google Scholar - Yoshida K, Hernández-Díaz S, Solomon DH et al (2017) Matching weights to simultaneously compare three treatment groups: comparison to three-way matching. Epidimiology 28(3):387–395. https://doi.org/10.1097/EDE.0000000000000627
Article Google Scholar - Li F, Thomas LE, Li F (2018) Addressing extreme propensity scores via the overlap weights. Am J Epidemiol 188(1):250–257
Google Scholar - Crump RK, Hotz VJ, Imbens GW, Mitnik OA (2009) Dealing with limited overlap in estimation of average treatment effects. Biometrika 96(1):187–199. https://doi.org/10.1093/biomet/asn055
Article Google Scholar - Yoshida K, Solomon DH, Haneuse S et al (2018) Multinomial extension of propensity score trimming methods: a simulation study. Am J Epidemiol 188(3):609–616
Article PubMed Central Google Scholar - Glynn RJ, Lunt M, Rothman KJ, Poole C, Schneeweiss S, Stürmer T (2019) Comparison of alternative approaches to trim subjects in the tails of the propensity score distribution. Pharmacoepidemiol Drug Saf 28(10):1290–1298. https://doi.org/10.1002/pds.4846
Article PubMed Google Scholar - Arbogast PG, Ray WA (2009) Use of disease risk scores in pharmacoepidemiologic studies. Stat Methods Med Res 18(1):67–80. https://doi.org/10.1177/0962280208092347
Article PubMed Google Scholar - Glynn RJ, Gagne JJ, Schneeweiss S (2012) Role of disease risk scores in comparative effectiveness research with emerging therapies. Pharmacoepidemiol Drug Saf 21(Suppl 2):138–147. https://doi.org/10.1002/pds.3231
Article PubMed PubMed Central Google Scholar - Brookhart MA, Rassen JA, Schneeweiss S (2010) Instrumental variable methods in comparative safety and effectiveness research. Pharmacoepidemiol Drug Saf 19(6):537–554. https://doi.org/10.1002/pds.1908
Article PubMed PubMed Central Google Scholar - Ertefaie A, Small DS, Flory JH, Hennessy S (2017) A tutorial on the use of instrumental variables in pharmacoepidemiology. Pharmacoepidemiol Drug Saf 26(4):357–367. https://doi.org/10.1002/pds.4158
Article PubMed Google Scholar - Blakely T, Lynch J, Simons K, Bentley R, Rose S (2019) Reflection on modern methods: when worlds collide—prediction, machine learning and causal inference. Int J Epidemiol. https://doi.org/10.1093/ije/dyz132
- Wyss R, Schneeweiss S, van der Laan M, Lendle SD, Ju C, Franklin JM (2018) Using super learner prediction modeling to improve high-dimensional propensity score estimation. Epidimiology 29(1):96–106. https://doi.org/10.1097/EDE.0000000000000762
Article Google Scholar - Westreich D, Lessler J, Funk MJ (2010) Propensity score estimation: neural networks, support vector machines, decision trees (CART), and meta-classifiers as alternatives to logistic regression. J Clin Epidemiol 63(8):826–833. https://doi.org/10.1016/j.jclinepi.2009.11.020
Article PubMed PubMed Central Google Scholar - Bi Q, Goodman KE, Kaminsky J, Lessler J (2019) What is machine learning? A primer for the epidemiologist. Am J Epidemiol 188(12):2222–2239. https://doi.org/10.1093/aje/kwz189
Article PubMed Google Scholar - Myers JA, Rassen JA, Gagne JJ et al (2011) Effects of adjusting for instrumental variables on bias and precision of effect estimates. Am J Epidemiol 174(11):1213–1222. https://doi.org/10.1093/aje/kwr364
Article PubMed PubMed Central Google Scholar - Patorno E, Schneeweiss S, Gopalakrishnan C, Martin D, Franklin JM (2019) Using real-world data to predict findings of an ongoing phase IV cardiovascular outcome trial–cardiovascular safety of linagliptin vs. glimepiride. Diabetes Care 42(12):2204–2210. https://doi.org/10.2337/dc19-0069
Article CAS PubMed PubMed Central Google Scholar - Dal-Ré R, Janiaud P, Ioannidis JPA (2018) Real-world evidence: how pragmatic are randomized controlled trials labeled as pragmatic? BMC Med 16(1):49. https://doi.org/10.1186/s12916-018-1038-2
Article PubMed PubMed Central Google Scholar - Zuidgeest MGP, Goetz I, Groenwold RHH, Irving E, van Thiel G, Grobbee DE (2017) Series: Pragmatic trials and real world evidence: Paper 1. Introduction. J Clin Epidemiol 88:7–13. https://doi.org/10.1016/j.jclinepi.2016.12.023
Article PubMed Google Scholar - Westreich D, Edwards JK, Lesko CR, Cole SR, Stuart EA (2018) Target Validity and the hierarchy of study designs. Am J Epidemiol 188(2):438–443. https://doi.org/10.1093/aje/kwy228
Article PubMed Central Google Scholar - Langan SM, Schmidt SA, Wing K et al (2018) The reporting of studies conducted using observational routinely collected health data statement for pharmacoepidemiology (RECORD-PE). BMJ 363:k3532
Article PubMed PubMed Central Google Scholar - Public Policy Committee ISoP (2016) Guidelines for good pharmacoepidemiology practice (GPP). Pharmacoepidemiol Drug Saf 25(1):2–10
Article Google Scholar - Berger ML, Sox H, Willke RJ et al (2017) Good practices for real-world data studies of treatment and/or comparative effectiveness: recommendations from the joint ISPOR-ISPE Special Task Force on real-world evidence in health care decision making. Pharmacoepidemiol Drug Saf 26(9):1033–1039. https://doi.org/10.1002/pds.4297
Article PubMed PubMed Central Google Scholar - Pawaskar M, Bonafede M, Johnson B, Fowler R, Lenhart G, Hoogwerf B (2013) Medication utilization patterns among type 2 diabetes patients initiating Exenatide BID or insulin glargine: a retrospective database study. BMC Endocr Disord 13(1):20. https://doi.org/10.1186/1472-6823-13-20
Article PubMed PubMed Central Google Scholar - Bron M, Guerin A, Latremouille-Viau D et al (2014) Distribution and drivers of costs in type 2 diabetes mellitus treated with oral hypoglycemic agents: a retrospective claims data analysis. J Med Econ 17(9):646–657. https://doi.org/10.3111/13696998.2014.925905
Article PubMed Google Scholar - Jacobs E, Hoyer A, Brinks R, Kuss O, Rathmann W (2017) Burden of mortality attributable to diagnosed diabetes: a nationwide analysis based on claims data from 65 million people in Germany. Diabetes Care 40(12):1703–1709. https://doi.org/10.2337/dc17-0954
Article PubMed Google Scholar - Reed M, Huang J, Brand R et al (2013) Implementation of an outpatient electronic health record and emergency department visits, hospitalizations, and office visits among patients with diabetes. JAMA 310(10):1060–1065. https://doi.org/10.1001/jama.2013.276733
Article CAS PubMed PubMed Central Google Scholar - Reed M, Huang J, Graetz I et al (2012) Outpatient electronic health records and the clinical care and outcomes of patients with diabetes mellitus. Ann Intern Med 157(7):482–489. https://doi.org/10.7326/0003-4819-157-7-201210020-00004
Article PubMed PubMed Central Google Scholar - WellDoc (2017) WellDoc receives FDA 510(k) clearance to offer a non-prescription version of BlueStar Digital Therapeutic for Type 2 Diabetes. Available from https://www.welldoc.com/news/welldoc-receives-fda-510k-clearance-to-offer-a-non-prescription-version-of-bluestar-digital-therapeutic-for-type-2-diabetes/. Accessed 20 Dec 2019
- Dennis S, Taggart J, Yu H, Jalaludin B, Harris MF, Liaw ST (2019) Linking observational data from general practice, hospital admissions and diabetes clinic databases: can it be used to predict hospital admission? BMC Health Serv Res 19(1):526. https://doi.org/10.1186/s12913-019-4337-1
Article PubMed PubMed Central Google Scholar - Williams R, van Staa TP, Gallagher AM, Hammad T, Leufkens HGM, de Vries F (2018) Cancer recording in patients with and without type 2 diabetes in the Clinical Practice Research Datalink primary care data and linked hospital admission data: a cohort study. BMJ Open 8(5):e020827. https://doi.org/10.1136/bmjopen-2017-020827
Article PubMed PubMed Central Google Scholar - Gordon JP, Evans M, Puelles J, McEwan PC (2015) Factors predictive of weight gain and implications for modeling in type 2 diabetes patients initiating metformin and sulfonylurea combination therapy. Diabetes Ther 6(4):495–507. https://doi.org/10.1007/s13300-015-0134-y
Article CAS PubMed PubMed Central Google Scholar
Authors’ relationships and activities
MG was a full-time employee of GlaxoSmithKline at the time of drafting this manuscript. She is now a full-time employee of and owns stocks in Merck and has no potential conflicts of interest relevant to this article. JBB’s contracted consulting fees and travel support for contracted activities are paid to the University of North Carolina by Adocia, AstraZeneca, Dance Biopharm, Dexcom, Eli Lilly, Fractyl, GI Dynamics, Intarcia Therapeutics, Lexicon, MannKind, Metavention, NovaTarg, Novo Nordisk, Orexigen, PhaseBio, Sanofi, Senseonics, vTv Therapeutics and Zafgen. JBB reports grant support from AstraZeneca, Eli Lilly, Intarcia Therapeutics, Johnson & Johnson, Lexicon, Medtronic, Novo Nordisk, Sanofi, Theracos, Tolerion and vTv Therapeutics and is a consultant to Cirius Therapeutics Inc., CSL Behring, Mellitus Health, Neurimmune AG, Pendulum Therapeutics and Stability Health. JBB holds stock/options in Mellitus Health, Pendulum Therapeutics, PhaseBio and Stability Health and is supported by grants from the National Institutes of Health (UL1TR002489, U01DK098246, UC4DK108612, U54DK118612, P30DK124723), PCORI and ADA. TS receives investigator-initiated research funding and support from the National Institute on Aging as a principal investigator (R01 AG056479) and from the National Institutes of Health as co-investigator (R01 CA174453, R01 HL118255 and R21-HD080214). He also receives salary support as co-Director of the Biostatistics, Epidemiology, and Research Design (BERD), North Carolina Translational and Clinical Sciences Institute (UL1TR002489) and from the Center for Pharmacoepidemiology (current members are GlaxoSmithKline, UCB BioSciences, Merck and Takeda). TS also receives research support from pharmaceutical companies (Amgen, AstraZeneca and Novo Nordisk), and from a generous contribution from N. A. Dreyer to the Department of Epidemiology, University of North Carolina at Chapel Hill. TS does not accept personal compensation of any kind from any pharmaceutical company. He owns stock in Novartis, Roche, BASF, AstraZeneca and Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
- Pharmacoepidemiology, Center for Observational & Real-World Evidence, Merck, 770 Sumneytown Pike, West Point, PA, 19486, USA
Mugdha Gokhale - Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, NC, USA
Mugdha Gokhale & Til Stürmer - Department of Medicine, University of North Carolina, Chapel Hill, NC, USA
John B. Buse
Authors
- Mugdha Gokhale
You can also search for this author inPubMed Google Scholar - Til Stürmer
You can also search for this author inPubMed Google Scholar - John B. Buse
You can also search for this author inPubMed Google Scholar
Contributions
All authors made substantial contributions to the conception of this manuscript, drafting the article and revising it critically for important intellectual content. All authors approved the version to be published.
Corresponding author
Correspondence toMugdha Gokhale.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Gokhale, M., Stürmer, T. & Buse, J.B. Real-world evidence: the devil is in the detail.Diabetologia 63, 1694–1705 (2020). https://doi.org/10.1007/s00125-020-05217-1
- Received: 07 January 2020
- Accepted: 01 May 2020
- Published: 15 July 2020
- Issue Date: September 2020
- DOI: https://doi.org/10.1007/s00125-020-05217-1