Endocytic regulation of TGF-β signaling (original) (raw)
Introduction
Transforming growth factor-β (TGF-β) is a member of the structurally related growth factor family, which includes TGF-β, activin, Nodal, bone morphogenetic proteins (BMPs), myostatin and others. These factors play key roles in regulating a wide range of biological responses during development and tissue homeostasis in adults, and deregulation of their signal transduction has been associated with many human diseases, including cancer and tissue fibrosis 1, 2, 3, 4, 5, 6, 7, 8, 9, 10. TGF-β elicits its signaling by binding to its cell surface Ser/Thr kinase receptors, leading to the formation of the receptor heterocomplexes, in which the constitutively active type II receptor (TβRII) phosphorylates the type I receptor (TβRI). The phosphorylated TβRI adopts a conformation that facilitates the docking of receptor-regulated Smad proteins (R-Smad) and activates R-Smad through phosphorylation. Then R-Smad forms a complex with Co-Smad Smad4, and together they are accumulated in the nucleus and regulate the expression of target genes 11, 12, 13, 14, 15, 16, 17.
Ligand binding to its receptor at the cell surface not only initiates signaling events but also triggers internalization of both ligand and receptors. Receptor-mediated endocytosis has both positive and negative functions in signal transduction 18, 19, 20, 21, 22, 23. It can sequester the receptors in intracellular compartments, target ligand-receptor complexes to lysosomes for degradation or increase the access of receptors to their intracellular substrates. Receptors internalized in endosomes can also be recycled back to the cell surface for re-use 24.
Two major endocytic pathways mediate internalization of cell surface receptors: clathrin-mediated endocytosis and non-clathrin-mediated endocytosis, the latter including lipid-raft- or caveolae-mediated endocytosis 19, 25, 26. Clathrin-mediated endocytosis is utilized by many cell surface receptors such as G protein-coupled receptors, tyrosine kinase receptors (epidermal growth factor receptor, platelet-derived growth factor receptor and insulin receptor) and other non-kinase, single transmembrane receptors (low-density lipoprotein receptor, transferring receptor) 27, and is the best characterized pathway. The internalizing receptors are first concentrated on the clathrin-coated pits, which are assembled on the cytoplasmic face of the plasma membrane by the recruitment of the adaptor complex AP2 (consisting of α, β2, μ2 and σ2 subunits), triskelial clathrin (comprising three heavy chains and 3 light chains) and other accessory proteins such as Eps15, epsin, disabled-2, synaptotagmin and amphiphysin 28, 29, 30. Then the pits undergo invagination and pinch off from the plasma membrane in a dynamin GTPase-dependent way 31. After uncoating of the clathrin coat, the vesicle is finally fused with and the receptors are transported to early endosomes.
Besides clathrin-coated pits, cholesterol- and sphingolipids-enriched lipid rafts can also be found in the plasma membrane 32, 33. These specialized detergent-insoluble, low-density membrane microdomains are called caveolae when the structural protein caveolin is associated. Lipid rafts can serve as signaling centers for nitric oxide, calcium, G protein-coupled receptors and protein tyrosine kinases, or as virus entrance 32, 34. They also mediate the internalization of various proteins; cholera toxin, glycosylphosphatidylinositol (GPI)-anchored proteins, endothelin receptor and growth hormone receptor are some of the examples 34, 35, 36. The internalized cargos are thought to be transported to not-well-characterized caveosomes and eventually to other organelles such as endosomes or lysosomes. Therefore, lipid rafts/caveolae regulate multiple cell signaling events.
Like most of the cell surface proteins, TGF-β receptors undergo endocytosis 37. In this article, TGF-β receptor endocytosis, and the roles of endocytosis in TGF-β signaling, receptor degradation and signal inactivation will be reviewed. The role of endocytosis in establishment of the BMP morphogen gradient has been extensively reviewed 18, 38, 39, 40.
Internalization of TGF-β receptors
Receptor internalization
An early study that followed the turnover of 125I-labelled TGF-β in BALB/c 3T3 mouse fibroblasts suggested that TGF-β is rapidly internalized via its receptors and degraded in lysosomes 41. Using a similar approach, receptor-mediated TGF-β internalization has also been investigated in mink lung epithelial Mv1Lu cells and human embryonic kidney HEK293 cells, and both TβRI and TβRII were indicated to be required for optimal internalization of TGF-β 42. However, by following ligand endocytosis, it is difficult to elucidate the contribution of individual receptors in this process and to investigate the role of endocytosis in TGF-β signaling, as there may be several TGF-β-binding proteins on the plasma membrane besides the signaling type I and type II receptors 16, 43. Therefore, it is important to directly investigate the internalization process of TGF-β receptors.
Endocytosis of TGF-β receptors and its role in TGF-β signaling have been studied extensively by multiple approaches. Owing to the lack of good antibodies for TGF-β receptors, it is difficult to specifically trace the trafficking of endogenous TGF-β receptors. To overcome this problem and to examine the role of the receptor kinase activity in modulating receptor endocytosis, Leof and colleagues have cleverly engineered artificial receptor constructs by fusing the extracellular domains of the granulocyte/macrophage colony-stimulating factor (GM-CSF) α and β receptors to the transmembrane and cytoplasmic domains of TGF-β type I and type II receptors and followed internalization of the GM-CSF ligand 44. Using this system, they showed that both TGF-β type I and type II receptors are rapidly endocytosed 45, and that the kinase activity of TβRII, but not TβRI, is required for optimal internalization of the heteromeric receptor complexes 46. Consistent with this, deletion of the kinase domain of TβRI had no effect on downregulation of the heteromeric receptor complexes 47.
Like most of the other cell surface receptors, TGF-β receptors are mainly internalized via clathrin-dependent endocytosis (Figure 1). Several lines of evidence support this notion. First, using GM-CSF receptor-TGF-β receptor chimeric constructs, Leof and colleagues found that TGF-β receptor endocytosis is mediated by clathrin-dependent pits as it is specifically dampened by potassium depletion, hypertonic medium and cytosol acidification in mouse fibroblast AKR-2B cells 45, 48. Secondly, by following biotinylated cell surface TβRI, it has been documented that rapid internalization of TβRI can be blocked by the dynamin K44A mutant or by potassium depletion 49. Thirdly, both TβRI and TβRII can associate with the β2 subunit of the clathrin-associated adaptor complex AP2 48. The direct interaction of TGF-β receptors with β2 adaptin is mediated by the intracellular domain of the receptors and the β2 adaptin N-terminal trunk domain. This interaction is different from that of most other plasma membrane receptors that interact with the μ2 subunit of AP2 30.
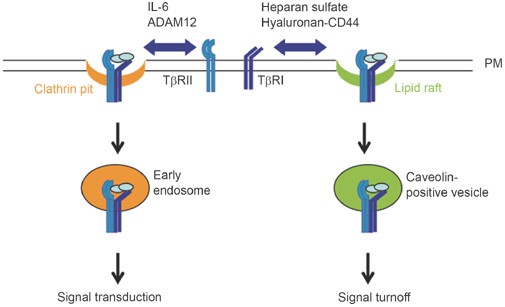
Figure 1
TGF-β receptors are distributed in both lipid rafts/caveolae and nonraft membrane microdomains. Internalization of TGF-β receptors via clathrin-coated pits can enhance TGF-β signaling, whereas lipid rafts-mediated endocytosis of TGF-β receptors facilitates receptor degradation and thus the turnoff of signaling. The subcompartmental distribution of TGF-β receptors on the plasma membrane can be regulated: interleukin-6 and ADAM12 were shown to shift the receptors to the nonraft fractions, while heparan sulfate and hyaluronan-CD44 were shown to promote the lipid raft/caveolae localization of TGF-β receptors.
However, TGF-β has also been reported to be endocytosed independently of the clathrin-mediated pathway as the internalization was blocked by phenylarsine oxide, a nonspecific inhibitor, but not affected by reagents which were thought to interfere with clathrin-mediated endocytosis, such as the primary amine monodansylcadaverine and the GTPase-deficient dynamin K44A mutant 42. In addition, both TGF-β and BMP receptors have been shown to interact with caveolin 50, 51, 52 and can also be endocytosed in a lipid rafts/caveolae-dependent manner 51, 52, 53 (Figure 1), but the detailed mechanism remains to be elucidated.
Receptor endocytosis can take place constitutively or be activated by ligand, and the ligand-dependent endocytosis usually involves receptor phosphorylation or ubiquitination 30, 54. Both type I and type II TGF-β receptors seem to constantly undergo internalization 49, 52, 55. Once internalized into the cytoplasmic compartments, TGF-β receptor molecules seem to be recycled back to the cell surface in the absence of ligand 56. The recycling of the internalized TGF-β receptors is impaired by dominant-negative Rab11 57, suggesting that the recycling takes place in the perinuclear recycling endosomes 58 (Figure 2). As the activated receptors are more likely to be subjected to degradation, it is surprising to note that ligand treatment has no effect on the rates of internalization and recycling of TGF-β receptors 57.
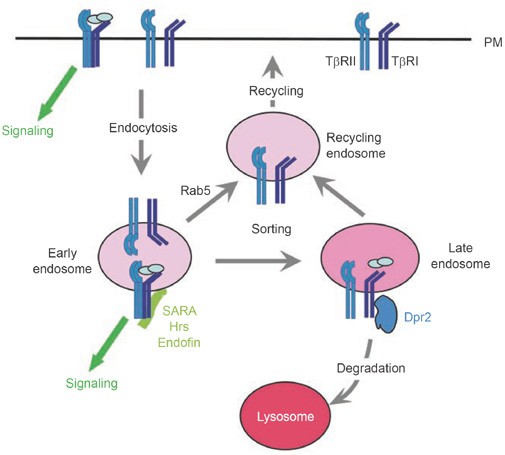
Figure 2
Subcellular localization of receptors regulates TGF-β signaling. TGF-β receptors are endocytosed constitutively in the absence of ligand. After entering the early endosomes, they are sorted to recycling endosomes and recycled back to the plasma membrane. Upon ligand binding, the receptors can initiate signaling by activating the Smad pathway. The ligand-bound receptors can continue and even maximize their signaling activity in early endosomes, where the signaling-promoting factors such as SARA, Hrs and endofin reside. Some of the ligand-bound receptors are recycled back to the plasma membrane for re-use, or sorted to late endosomes, where they interact with Dapper2 and are targeted to lysosomes for degradation. The GDP-bound form of Rab5, Rab5 S34N, which interferes with endosome fusion, has been shown to enhance ligand-independent Smad signaling.
Internalization signals of TGF-β receptors
Internalization of most cell surface receptors is mediated by short specific sequences in their cytoplasmic domains 29, 30. Two types of such internalization signals with distinct consensus sequences mediate clathrin-dependent endocytosis: one with tyrosine-containing sequences such as NPXY and YXXΦ (X is any amino acid and Φ is a hydrophobic amino acid) and the other known as di-leucine-based motifs [DE]XXXL[LI]. These sequences can directly bind to the endocytic machinery: all of them are recognized by the μ2 subunit of AP2; NPXY can also interact with clathrin and the phosphotyrosine-binding domain of the adaptor protein disabled-2; and di-leucine-based signal can also associate with the β2 subunit of AP2 29, 30. Other variable internalization signals have also been identified, such as acidic clusters 59 and ubiquitination 54, 60, and shown to be essential for the internalization of the respective receptors. The conjugated ubiquitin can be recognized by ubiquitin-binding motifs in the accessory proteins, such as eps15 and epsin 30, 54. Therefore, these internalization signals play an important role in cargo enrichment on the clathrin-coated pits as well as in vesicle formation.
Such internalization signals have also been identified in TGF-β receptors. By following the endocytosis of truncation, deletion and substitution mutants of TβRII from the cell surface, Ehrlich et al. 55 have found that a di-leucine-like signal in the cytoplasmic region proximal to the transmembrane domain, Ile218Ile219Leu220, is necessary for TβRII endocytosis. In accordance with the recognition of the di-leucine signal by the clathrin endocytic machinery, TβRII is constitutively internalized in a clathrin-dependent manner 55. Furthermore, both TβRI and TβRII can interact with the β2 subunit of AP2 via their intracellular domains 48. However, it is currently unclear whether the di-leucine-like signal of TβRII is directly involved in this interaction.
The internalization signal of TβRI is distinct from the classical consensus sequences. There are two putative tyrosine-based YXXΦ motifs in the intracellular domain of TβRI: Y182DMT and Y249QTV. However, mutation of either tyrosine residue had no effect on TβRI endocytosis 56. Instead, deletion and substitution mutation studies have identified a motif (R482LTALRIKKTL492) close to the C-terminal tail that is important for TβRI downregulation 47. This motif was referred as the NANDOR (no activating non-down-regulating) Box. Although this motif is highly conserved among all the type I receptors of the TGF-β family, it does not belong to any of the two types of well-characterized internalization signals. Ala replacement of any single amino acid in this box had no effect on ALK4 internalization, indicating that cooperation between the amino acid residues or certain conformation of the NANDOR Box is important for mediating receptor endocytosis/downregulation 61, but mutation of two to four amino acids blocked receptor downregulation 47. Interestingly, this motif seems to be essential for TβRI activation as its deletion abolished TβRI phosphorylation by TβRII and the ability of TβRI to mediate TGF-β signaling 47. As the kinase activity of TβRI may not be necessary for receptor internalization 46, the function of this motif in coupling receptor activation and internalization/downregulation needs further investigation. Supporting the importance of the COOH-terminus of type I receptors in mediating receptor endocytosis, a study with the type I activin receptor ALK4 showed that a conserved Trp, six amino acids upstream of the NANDOR Box (W477 in ALK4), is essential for ALK4 internalization, and substitution of this Trp with Ala blocked ligand-induced endocytosis of ALK4 61.
Regulation of receptor internalization
Few studies have touched on how internalization of TGF-β receptors is regulated. The FK506-interacting protein FKBP12 can directly associate with TβRI through a hydrophobic interaction with the Leu-Leu motif in the GS domain of the receptor 62, and has been proposed to inhibit the basal activity of TGF-β receptors resulting from randomly encountered receptor interactions 63. FKBP12 may also function as a negative regulator of TβRI internalization as rapamycin, which disrupts the TβRI-FKBP12 interaction, can enhance the internalization of wild-type TβRI, but not the mutant deficient in FKBP12 binding 64. Internalization of endoglin and TβRII has been shown to be controlled by thrombin in human endothelial cells. Thrombin and a peptide agonist of its receptor PAR1 promote endocytosis of endoglin and TβRII, but not TβRI; and the endocytosis, which negatively regulates TGF-β signaling, is dependent on protein kinase C-ζ 65. Whether other mechanisms are involved in regulation of TGF-β receptor endocytosis is unclear. For instance, ubiquitination has been shown to be important in regulating endocytosis of receptor tyrosine kinases 66, but the possibility of its involvement in TGF-β receptor internalization remains to be investigated.
Cell type-specific receptor internalization
Internalization of TGF-β receptors has been suggested to be cell-specifically regulated. Although the heteromeric receptor complexes of type I and type II receptors are efficiently internalized and downregulated in both Mv1Lu epithelial cells and AKR-2B fibroblasts, the homomeric type I or type II receptors are endocytosed differently 45, 67. In AKR-2B cells, the homomeric receptors exhibit a similar internalization rate as the heteromeric receptor complexes, whereas in Mv1Lu cells, the homomeric receptors are internalized less efficiently in comparison to the heteromeric receptor complexes 67. In both cell lines, the homomeric receptor complexes seem to be recycled back to the cell surface as they do not undergo downregulation. However, the underlying mechanism is presently unknown.
Contrary to what is observed in fibroblasts 46, the kinase activity of TβRII is not essential for downregulation of heteromeric TGF-β receptor complexes in epithelial cells 56. In addition to this, a signaling-incompetent mutation of TβRI – T200V – abolishes endocytosis/receptor downregulation in fibroblasts, whereas it has no such effect in epithelial cells 56.
Promoting effect of endocytosis on TGF-β signaling
Promotion of TGF-β signaling by endocytosis
Receptor internalization can play either a positive or a negative role in signal transduction. By reducing the number of cell surface receptors and inducing receptor degradation, endocytosis regulates signaling negatively, whereas endocytosis can also increase the proximity of the internalized receptors to their substrates or other signaling intermediaries in the cytoplasma 18, 20, 21, 22, 37. Several studies have investigated the role of endocytosis in TGF-β signaling and yielded different conclusions.
By using the GM-CSFR-TGF-β receptor fusion system in combination with endogenous TGF-β receptors, Penheiter et al. 68 found that TGF-β receptors can form a complex with SARA (Smad Anchor for Receptor Activation) and Smad, and TβRI phosphorylation can take place even when clathrin-coated vesicle formation is blocked by low-temperature treatment, potassium depletion or the dominant-negative K44A dynamin mutant, while the receptor-mediated phosphorylation and thus activation of Smad2/3 require receptor internalization, suggesting that receptor endocytosis plays an essential role in TGF-β signaling. However, it is unclear how clathrin-mediated endocytosis initiates Smad2/3 phosphorylation in the receptor-SARA-Smad complex, which has been pre-assembled presumably at the plasma membrane. Although clathrin-dependent endocytosis has also been suggested to be essential for TGF-β/Smad signaling in human kidney mesangial cells, a different mechanism was proposed 69. Runyan et al. 69 found that inhibition of clathrin-mediated endocytosis only slightly affects TGF-β-induced Smad2 phosphorylation and Smad2-Smad4 association, but decreases the nuclear accumulation of Smad2 and therefore attenuates Smad-mediated transcriptional responses. It was proposed that endocytosis is possibly required to release Smad2 from the SARA complex as inhibition of clathrin-dependent endocytosis by potassium depletion prolonged the SARA-Smad2 complex formation. The positive role of clathrin-mediated endocytosis in TGF-β signaling is supported by immunofluorescence and cell fractionation studies 52. TGF-β receptors can be internalized via both clathrin-coated pits and cholesterol-rich lipid rafts/caveolae as they are found in both EEA1-positive early endosomes and caveolin-1-positive compartments. Disruption of clathrin-coated pits or clathrin-mediated endocytosis by potassium depletion, dominant-negative mutants of Eps15 or dynamin attenuates TGF-β-induced Smad2 phosphorylation, whereas disruption of lipid rafts by the cholesterol-depleting agent Nystatin can shift TGF-β receptors to nonraft compartments and enhances TGF-β/Smad signaling. These data suggest that clathrin-mediated endocytosis promotes, whereas caveolae-mediated internalization turns off, TGF-β/Smad signaling (see below). Similar results showing the localization of TGF-β receptors in EEA1-positive vesicles and the stimulatory role of clathrin-dependent endocytosis in TGF-β/Smad signaling were also reported by Hayes et al. 70.
Although the above-described studies showed an important role of clathrin-mediated endocytosis in TGF-β signaling, several lines of evidence indicate that TGF-β signaling can take place on the plasma membrane. First, it was reported that blocking clathrin-dependent internalization of TGF-β receptors by potassium depletion or mutant dynamin (K44A) had no effect on the phosphorylation or nuclear accumulation of Smad2 and Smad-mediated transcriptional response 49. Consistent with this, Smad2 can associate with TGF-β receptors under conditions of potassium depletion. It is still unclear why different results were obtained from similar experimental systems. Secondly, a dispensable role of clathrin-mediated endocytosis in BMP-induced Smad1/5 activation has been suggested 51. Both type I and type II BMP receptors undergo internalization via the clathrin-coated pits, but only type II BMP receptor can be endocytosed via caveolae. BMP-induced phosphorylation of Smad1/5 was not influenced by dynamin K44A or by chlorpromazine, a disruptor of clathrin-coated pits, although prolonged treatment of chlorpromazine decreased the transcriptional responses of BMP. Interestingly, BMP-induced alkaline phosphatase production seems to need intact lipid rafts as decrease of cholesterol level specifically blocked alkaline phosphatase production 51. Thirdly, activin-induced Smad2 phosphorylation is not dependent on receptor endocytosis, as ALK4 W477A, which is unable to undergo ligand-dependent internalization, can still mediate activin-stimulated Smad2 phosphorylation and transcriptional responses even under the condition of potassium depletion 61.
TGF-β signaling in endosomes
Endosomes are important signaling centers for TGF-β signal transduction. Several positive regulators of TGF-β signaling are localized in endosomes. For instance, three FYVE (Fab1, YOTB/ZK632.12, Vac1, and EEA1) domain-containing proteins, SARA, endofin and Hrs/Hgs ((hepatocyte growth factor-regulated tyrosine kinase substrate), have been suggested to promote TGF-β, activin and decapentaplegic (Dpp) signaling 71, 72, 73, 74, 75. The FYVE domain is a zinc-binding motif known to bind phosphatidylinositol 3-phosphate, a lipid enriched in early endosomes, and can specifically recruit the FYVE-domain-containing proteins to early endosomes 76. The FYVE domain is essential for the stimulatory effect of these proteins on TGF-β signaling 71, 73, 75, 77, indicating that their localization in early endosomes is important to fulfill their functions. It was shown that SARA functions as a scaffold protein by specifically interacting with Smad2/3 and TGF-β receptors to facilitate Smad2/3 phosphorylation by the TGF-β receptor 71. Similarly, Hrs can interact with Smad2 and cooperate with SARA to promote activin-mediated activation of Smad2 72. Endofin can also facilitate TGF-β signaling. However, unlike SARA or Hrs, endofin binds to Smad4 and, in cooperation with SARA, may assist the heterocomplex formation between receptor-phosphorylated Smad2/3 and Smad4 75 (Figure 3). Endofin has also been suggested to promote BMP signaling by helping recruit Smad1 to BMP receptors 78. In this regard, endofin acts very similarly as SARA to function as a Smad anchor for receptor activation.
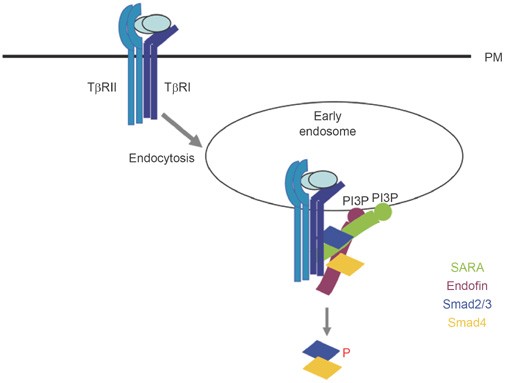
Figure 3
Early endosomes function as a signaling organelle to promote TGF-β signaling. Early endosomes are enriched with phosphatidylinositol-3 phosphate, which recruits to the membrane the FYVE domain-containing proteins, such as SARA, Hrs and endofin. SARA and Hrs have been shown to function as scaffold proteins to bring Smad2/3 to TGF-β/activin receptors and facilitate receptor-mediated Smad phosphorylation. Endofin associates with Smad4 as well as TGF-β receptors to promote Smad activation in collaboration with SARA.
Different from its positive role in TGF-β signaling, SARA and Hrs have also been shown to negatively regulate Dpp signaling in Drosophila. SARA can bring the catalytic subunit of protein phosphatase 1 to and dephosphorylate the Dpp receptor Thickveins (Tkv) 79, whereas loss of Hrs leads to enhanced Dpp signaling and an increased level of Tkv 80. Interestingly, endofin has been suggested to recruit protein phosphatase 1 to dephosphorylate BMP type I receptors and therefore attenuate BMP signaling 78. It is currently unknown how the opposite functions of endofin in BMP signaling are controlled.
Another function of SARA in Drosophila developing wing epithelial cells is to target molecules of Dpp signaling, such as its receptor Tkv, to endosomes associated with the spindle machinery to ensure appropriate distribution of the signaling molecules in the two daughter cells after mitosis 74. By doing so, SARA ensures the maintenance of Dpp signaling levels across mitosis.
The small GTPase Rab5 is a crucial regulator of early endosome dynamics 58. Like all other GTPases, it cycles between the inactive GDP-binding form and the active GTP-binding form. It was shown that Rab5 S34N, a GDP-bound dominant-negative Rab5 mutant, can stimulate the expression of a Smad3-responsive reporter in a ligand-independent manner, whereas the GTP-bound Rab5 Q79L mutant attenuates the transcriptional activity induced by activin 77. As Rab5 S34N inhibits clathrin-mediated recycling and early endosome fusion 81, it was suggested that the stimulatory effect of Rab5 S34N on Smad-dependent signaling could be a consequence of decreased degradative or recycling trafficking of TGF-β/activin receptors, which results in their accumulation in early endosomes, where other TGF-β/activin signaling-promoting factors SARA, endofin and Hrs reside 77 (Figure 2). However, this hypothesis remains to be tested as RN-tre, a specific Rab5 GTPase-activating protein that facilitates GTP hydrolysis, does not increase the expression of the Smad-responsive reporter 77. Nevertheless, these data strongly indicate that early endosomes are a signaling organelle for the TGF-β/Smad pathway.
Endocytosis and turnoff of TGF-β signaling
Receptor degradation via the endocytic pathway
TGF-β receptor activity is tightly regulated by a set of their interacting proteins 7, 43, 82. Endocytosis, which has long been regarded as a means to turn off signaling by mediating receptor degradation, plays an important role in controlling the amount of receptors on the plasma membrane. Several studies have examined the half-life of TGF-β receptors and yielded different results. Using the 125I-TGF-β1-binding assay, Centrella et al. 83 found that the half-life of cell surface TβRI and TβRII is about 2 h in primary osteoblasts. However, the half-life of the newly synthesized mature (endoglycosidase H-resistant) form of TβRII in MvlLu lung epithelial cells, as analyzed by 35S-Met metabolic labeling, varied from about 1 h 84 to 2.5 h 85. TGF-β1 treatment reduced the half-life of TβRII to 45 min 84 or 1.7 h 85. Interestingly, the half-life of the newly synthesized mature TβRI seems to be longer than that of TβRII and is also reduced by ligand treatment 85. When the constitutively active TβRI (caTβRI) was overexpressed in HEK293T cells, the half-life of the cell surface caTβRI is about 4 h 86. Nonetheless, these studies together suggest that TGF-β receptors are constantly turned over and that their downregulation is enhanced by ligand treatment.
Although the formation of TβRI/TβRII complexes is required for TGF-β signal transduction, downregulation of either TβRI or TβRII was observed when they were expressed separately 45, 55, 86. Together with the observation that these receptors may have different turnover dynamics, it is likely that either receptor alone can undergo downregulation. However, cooperation between receptors or receptor complex formation is needed for effective receptor downregulation. Using receptor-deficient cell lines in combination with an ectopic expression system, it was shown that downregulation of TβRII and betaglycan (a TGF-β type III receptor) needs TβRI 87. Additional evidence that effective receptor downregulation may require the functional interaction between type I and type II receptors was provided by the GM-CSF-TGF-β receptor chimeric constructs: both homomeric and heteromeric TGF-β receptors undergo fast internalization, but only the heteromeric receptors are downregulated 45, 67.
The turnover of cell surface TGF-β receptors is subject to their regulation by other molecules. For instance, H-8, an inhibitor of cAMP-dependent protein kinase and cGMP-dependent protein kinase, and staurosporine, a potent protein kinase C inhibitor, have been shown to enhance TGF-β binding to its receptors, whereas phosphatase inhibitors okadaic acid, vanadate and fluoride decrease the binding 83, indicating that the turnover of TGF-β receptors is modulated by other signaling events. The oncogene H-ras may also modulate the downregulation of cell surface TGF-β receptors 88.
Turnoff of TGF-β signaling in lipid rafts/caveolae
Lipid rafts/caveolae are generally regarded as signaling centers for nitric oxide, calcium, G protein-coupled receptors and protein tyrosine kinases 32, 34, 89. However, several lines of evidence suggest that these membrane microdomains negatively regulate TGF-β signaling. Caveolin-1, a critical component of caveolae, has been shown to attenuate TGF-β signaling 50. It physically associates with TβRI and interferes with TGF-β-induced Smad2 phosphorylation and subsequent downstream events.
The inhibitory Smad Smad7 inhibits TGF-β signaling via multiple mechanisms, acting as a decoy substrate to form a stable complex with receptors to prevent recruitment of R-Smads 90, 91, engaging protein phosphatase 1 to dephosphorylate receptors 92, and functioning in the nucleus to recruit HDAC to repress transcription 93, or disrupt the functional Smad-DNA complex formation 94. Another way in which Smad7 can inhibit TGF-β signaling is by recruiting E3 ubiquitin ligases Smurf1 and Smurf2 and inducing ubiquitination and degradation of type I receptors 95, 96. The interaction of Smad7 and Smurf proteins with TGF-β receptors targets the complexes to lipid rafts/caveolae, and caveolin-dependent internalization of TGF-β receptors could promote receptor turnover and signaling termination 52. Consistent with this, cholesterol has been suggested to inhibit Smad2 activation, promote TGF-β receptor degradation, and therefore inhibit TGF-β signaling; and this effect may result from the shifted localization of TGF-β receptors from nonraft to lipid raft microdomains in the plasma membrane 53.
As lipid rafts-mediated internalization of TGF-β receptors promotes receptor degradation, modulation of the receptor distribution in lipid rafts vs nonraft microdomains is a mechanism to regulate TGF-β signaling. Interleukin (IL)-6 enhances TGF-β/Smad signaling in kidney tubular epithelial cells, and this enhancement was shown to be because of IL-6-induced partitioning of TGF-β receptors to nonraft microdomains and thus decreased degradation of TGF-β receptors 97 (Figure 1). In support of this, potassium depletion attenuates the stimulatory effect of IL-6 on TGF-β/Smad signaling, whereas Nystatin increases it. ADAM12 (a disintegrin and metalloproteinase 12) is a glycoprotein with zinc protease and integrin-binding activities in its extracellular domain and has been implicated in the control of membrane fusion, cytokine and growth factor shedding, and cell migration 98. A recent study reported that it can interact with the extracellular domain of TβRII and enhance TGF-β signaling 99. Interestingly, neither its protease activity nor the intracellular domain is required for this stimulatory effect. Rather, it induces the accumulation of TβRII in EEA1-positive early endosomes and stabilizes the TβRII protein probably by preventing the association between TβRII and Smad7 and the internalization of TβRII into caveolin1-positive vesicles.
Two polysaccharides have been shown to negatively influence TGF-β/Smad signaling by shifting the receptors to lipid rafts/caveolae fractions. Heparan sulfate, a polysaccharide that is a component of proteoglycans, could stimulate lipid rafts-mediated endocytosis and rapid degradation of TGF-β receptors 100. Consistent with this, more receptors were found in nonraft fractions in heparan sulfate-deficient cells than in wild-type cells. Similarly, hyaluronan functions through its receptor CD44 to partition TGF-β receptors to caveolin-1-positive caveolae fractions and enhance the interaction of caveolin-1 with Smad7, leading to attenuation of TGF-β signaling 101. These observations suggest another layer of regulation of TGF-β signaling by other pathways; however, the mechanistic details remain to be established, such as how IL-6 and ADAM12 divert the trafficking of TGF-β receptors from caveolin-dependent endocytosis to clathrin-dependent endocytosis, and how the polysaccharides shift the receptors to lipid raft fractions.
Lipid rafts/caveolae may not only serve as a place to promote Smad7-mediated degradation of TGF-β receptors, but are also involved in some of the specific TGF-β signal pathways. For instance, a recent study showed that ALK1, a TGF-β type I receptor that signals via Smad1/5 in endothelial cells 102, is localized in caveolae, and caveolin-1 associates with ALK1 and promotes TGF-β/ALK1 signaling 103. Caveolin-1 can also enhance TGF-β-induced expression of type I procollagen via the PI3K/Akt pathway although it inhibits Smad3 activation 104. In addition, association of TGF-β receptors with endothelial nitric oxide synthase (eNOS) in lipid rafts/caveolae fractions is important for the activation of eNOS as TGF-β-induced dissociation of TGF-β receptors from eNOS resulted in a decrease of eNOS phosphorylation and its enzymatic activity 105. Lipid rafts may also be important for ligand-induced heterocomplex formation of TGF-β receptors 106.
It should be noted that in contrast to the model that clathrin-mediated endocytosis of TGF-β receptors promotes TGF-β signaling whereas caveolae/lipid rafts-mediated internalization is required for receptor degradation 52, data from the studies with GM-CSF receptor-TGF-β receptor chimeric constructs as well as endogenous TGF-β receptors suggested that TGF-β receptor degradation may be mediated by clathrin-dependent endocytosis, but does not require the cholesterol-rich caveolae/lipid rafts as receptor turnover was blocked by potassium depletion, but not by the cholesterol-depleting agent Nystatin 57.
The mechanisms regulating the stability of TGF-β receptors and Smad proteins are reviewed in detail by Lönn et al. [107](/articles/cr2008315#ref-CR107 "Lönn P, Morén A, Raja E, Dahl M, Moustakas A . Regulating the stability of TGFβ receptors and Smads. Cell Res 25 November 2008; doi: 10.1038/cr.2008.308
").Dapper2
Dapper was initially identified as an interacting protein of Dishevelled (Dvl) and functions as a general antagonist to block both the canonical β-catenin-mediated and noncanonical JNK-mediated Wnt signaling in Xenopus 108. Its fish ortholog Dapper2, which shares several conserved domains with the Xenopus Dapper (Dapper1), can attenuate TGF-β/activin/Nodal signaling 86. Overexpression of Dapper2 in zebrafish embryos led to phenotypes mimicking the ones in the loss-of-function mutants of the Nodal co-receptor oep, pinhead and eye fusion; and knockdown of its expression by morpholino-antisense oligonucleotides resulted in expanded mesoderm formation, suggesting that Dapper2 modulates mesoderm formation by acting as a negative regulator of activin/Nodal signaling. Consistent with its function in zebrafish embryos, fish Dapper2 could also attenuate TGF-β and activin signaling in mammalian cells, as shown by Smad phosphorylation and reporter assays 86. Biochemical analysis further revealed that Dapper2 can interact with TβRI and the activin/Nodal type I receptor ALK4 and exhibit a high binding affinity to the activated receptors, and overexpression of Dapper2 led to lysosomal degradation of the cell surface TβRI. As Dapper2 protein was found in late endosomes, it was proposed that Dapper2 modulates the levels of cell surface ALK4 or TβRI by targeting the endocytosed activated receptors for lysosomal degradation (Figure 2). The mouse Dapper2 has been shown to attenuate TGF-β/activin signaling 109. Thus, although Dapper2 has been suggested to regulate Wnt signaling in embryos 110, the inhibitory function of Dapper2 in TGF-β/activin/Nodal signaling is evolutionally conserved from fish to mouse. In agreement with its function in TGF-β/activin/Nodal signaling, Dapper2 expression depends on Nodal signals in fish embryos 86.
β-Arrestin 2
β-Arrestins bind to G protein-coupled receptors and function to attenuate their signaling activities, to act as scaffold proteins to connect to other signaling pathways such as the mitogen-activated protein kinase (MAPK) cascades and to facilitate clathrin-mediated internalization of cell surface receptors 111. β-Arrestin 2 can bind to the type III TGF-β receptor betaglycan, and this binding is triggered by TβRII-mediated phosphorylation on the intracellular domain of betaglycan 112. More importantly, the association of betaglycan with β-arrestin 2 leads to the internalization of both TβRII and betaglycan and thus downregulation of TGF-β signaling. However, it is unclear whether the downregulation of TGF-β signaling is due to the intracellular sequestration or increased turnover of receptors.
ARIP2
Internalization of the type II activin receptor (ActRII) was shown to be promoted by activin receptor-interacting protein 2 (ARIP2) 113. Overexpression of ARIP2 attenuated the activin-induced reporter expression, indicating that internalization of ActRII negatively regulates activin signaling. ARIP2 associates with ActRII via its NH2-terminal PDZ domain and with Ral-binding protein 1 (RalBP1) via its COOH-terminal region. RalBP1 has been shown, as an effector of the GTPase Ral, to regulate endocytosis of epidermal growth factor and insulin receptors 114. Interestingly, unlike ARIP2, which contains only one PDZ domain, ARIP1, which has five PDZ domains and two WW domains, was reported to promote activin signaling by acting as a scaffold protein to bring ActRIIA and Smad3 proteins together 115.
Concluding remarks
Although endocytosis of TGF-β receptors has been extensively investigated, many important questions still remain unsolved. The current prevailing view is that lipid rafts/caveolae negatively modulate TGF-β signaling by promoting TGF-β receptor turnover, probably via Smad7-mediated recruitment of Smurf, which catalyzes receptor ubiquitination 52. But it is unclear how Smad7 can target the activated TGF-β receptors to lipid rafts/caveolae, or, alternatively, how the TGF-β receptors-Smad7 interaction preferentially occurs in lipid rafts/caveolae. It seems that Smad7-Smurf-mediated degradation of TGF-β receptors depends on both proteosomal and lysosomal activities 96. Then, how does ubiquitination promote lysosomal turnover of TGF-β receptors? Does ubiquitination play any role in TGF-β receptor endocytosis? Degradation of TGF-β receptors occurs not only via caveolae-mediated endocytosis, but also via clathrin-dependent internalization to early endosomes and then to late endosomes. It is totally unknown how the endosomal sorting of TGF-β receptors to different destinations (plasma membrane for re-use or late endosomes/lysosome for degradation) is regulated. Lipid rafts/caveolae are signaling centers for membrane-attached tyrosine kinases, while they play a negative role in TGF-β signaling by promoting receptor degradation. In addition to the canonical Smad pathway, TGF-β can also activate other signaling molecules such as small GTPases (Ras, Rac, Rho and CDC42), MAPKs, PI3K/Akt, PAK and others in a cell type-dependent manner 8, 116. Whether the activation of any of these molecules is dependent on compartment-specific localization of TGF-β receptors is another important subject of future investigation.
References
- Blobe GC, Schiemann WP, Lodish HF . Role of transforming growth factor beta in human disease. N Engl J Med 2000; 342:1350–1358.
Article CAS PubMed Google Scholar - Massague J, Chen YG . Controlling TGF-beta signaling. Genes Dev 2000; 14:627–644.
CAS PubMed Google Scholar - Massague J, Blain SW, Lo RS . TGFbeta signaling in growth control, cancer, and heritable disorders. Cell 2000; 103:295–309.
Article CAS PubMed Google Scholar - Miyazono K, Suzuki H, Imamura T . Regulation of TGF-beta signaling and its roles in progression of tumors. Cancer Sci 2003; 94:230–234.
Article CAS PubMed Google Scholar - Moustakas A, Souchelnytskyi S, Heldin CH . Smad regulation in TGF-beta signal transduction. J Cell Sci 2001; 114:4359–4369.
CAS PubMed Google Scholar - Whitman M . Smads and early developmental signaling by the TGFbeta superfamily. Genes Dev 1998; 12:2445–2462.
Article CAS PubMed Google Scholar - Chen YG, Meng AM . Negative regulation of TGF-beta signaling in development. Cell Res 2004; 14:441–449.
Article CAS PubMed Google Scholar - Derynck R, Zhang YE . Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003; 425:577–584.
Article CAS PubMed Google Scholar - ten Dijke P, Arthur HM . Extracellular control of TGFbeta signalling in vascular development and disease. Nat Rev Mol Cell Biol 2007; 8:857–869.
Article CAS PubMed Google Scholar - Derynck R, Miyazono K . The TGF-Beta Family. Cold Spring Harbor: Cold Spring Harbor Laboratory Press, 2008.
- Shi Y, Massague J . Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003; 113:685–700.
Article CAS PubMed Google Scholar - Wrana JL . Regulation of Smad activity. Cell 2000; 100:189–192.
Article CAS PubMed Google Scholar - ten Dijke P, Hill CS . New insights into TGF-beta-Smad signalling. Trends Biochem Sci 2004; 29:265–273.
Article CAS PubMed Google Scholar - Feng XH, Derynck R . Specificity and versatility in TGF-beta signaling through Smads. Annu Rev Cell Dev Biol 2005; 21:659–693.
Article CAS PubMed Google Scholar - Heldin CH, Miyazono K, ten Dijke P . TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 1997; 390:465–471.
Article CAS PubMed Google Scholar - Massague J . TGF-beta signal transduction. Annu Rev Biochem 1998; 67:753–791.
Article CAS PubMed Google Scholar - Schmierer B, Hill CS . TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol 2007; 8:970–982.
Article CAS PubMed Google Scholar - Gonzalez-Gaitan M . Signal dispersal and transduction through the endocytic pathway. Nat Rev Mol Cell Biol 2003; 4:213–224.
Article CAS PubMed Google Scholar - Conner SD, Schmid SL . Regulated portals of entry into the cell. Nature 2003; 422:37–44.
Article CAS PubMed Google Scholar - von Zastrow M, Sorkin A . Signaling on the endocytic pathway. Curr Opin Cell Biol 2007; 19:436–445.
Article CAS PubMed PubMed Central Google Scholar - Ceresa BP, Schmid SL . Regulation of signal transduction by endocytosis. Curr Opin Cell Biol 2000; 12:204–210.
Article CAS PubMed Google Scholar - Sorkin A, Von Zastrow M . Signal transduction and endocytosis: close encounters of many kinds. Nat Rev Mol Cell Biol 2002; 3:600–614.
Article CAS PubMed Google Scholar - Piddini E, Vincent JP . Modulation of developmental signals by endocytosis: different means and many ends. Curr Opin Cell Biol 2003; 15:474–481.
Article CAS PubMed Google Scholar - Maxfield FR, McGraw TE . Endocytic recycling. Nat Rev Mol Cell Biol 2004; 5:121–132.
Article CAS PubMed Google Scholar - Mukherjee S, Ghosh RN, Maxfield FR . Endocytosis. Physiol Rev 1997; 77:759–803.
Article CAS PubMed Google Scholar - Brown DA, London E . Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol 1998; 14:111–136.
Article CAS PubMed Google Scholar - Schmid SL . Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem 1997; 66:511–548.
Article CAS PubMed Google Scholar - Takei K, Haucke V . Clathrin-mediated endocytosis: membrane factors pull the trigger. Trends Cell Biol 2001; 11:385–391.
Article CAS PubMed Google Scholar - Bonifacino JS, Lippincott-Schwartz J . Coat proteins: shaping membrane transport. Nat Rev Mol Cell Biol 2003; 4:409–414.
Article CAS PubMed Google Scholar - Bonifacino JS, Traub LM . Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem 2003; 72:395–447.
Article CAS PubMed Google Scholar - Hinshaw JE . Dynamin and its role in membrane fission. Annu Rev Cell Dev Biol 2000; 16:483–519.
Article CAS PubMed PubMed Central Google Scholar - Anderson RG, Jacobson K . A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science 2002; 296:1821–1825.
Article CAS PubMed Google Scholar - Munro S . Lipid rafts: elusive or illusive? Cell 2003; 115:377–388.
Article CAS PubMed Google Scholar - van Deurs B, Roepstorff K, Hommelgaard AM, Sandvig K . Caveolae: anchored, multifunctional platforms in the lipid ocean. Trends Cell Biol 2003; 13:92–100.
Article CAS PubMed Google Scholar - Nabi IR, Le PU . Caveolae/raft-dependent endocytosis. J Cell Biol 2003; 161:673–677.
Article CAS PubMed PubMed Central Google Scholar - Nichols BJ, Lippincott-Schwartz J . Endocytosis without clathrin coats. Trends Cell Biol 2001; 11:406–412.
Article CAS PubMed Google Scholar - Le Roy C, Wrana JL . Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol 2005; 6:112–126.
Article CAS PubMed Google Scholar - Vincent JP, Dubois L . Morphogen transport along epithelia, an integrated trafficking problem. Dev Cell 2002; 3:615–623.
Article CAS PubMed Google Scholar - Kicheva A, Gonzalez-Gaitan M . The decapentaplegic morphogen gradient: a precise definition. Curr Opin Cell Biol 2008; 20:137–143.
Article CAS PubMed Google Scholar - O'Connor MB, Umulis D, Othmer HG, Blair SS . Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing. Development 2006; 133:183–193.
Article CAS PubMed Google Scholar - Massague J, Kelly B . Internalization of transforming growth factor-beta and its receptor in BALB/c 3T3 fibroblasts. J Cell Physiol 1986; 128:216–222.
Article CAS PubMed Google Scholar - Zwaagstra JC, El-Alfy M, O'Connor-McCourt MD . Transforming growth factor (TGF)-beta 1 internalization: modulation by ligand interaction with TGF-beta receptors types I and II and a mechanism that is distinct from clathrin-mediated endocytosis. J Biol Chem 2001; 276:27237–27245.
Article CAS PubMed Google Scholar - Derynck R, Feng XH . TGF-beta receptor signaling. Biochim Biophys Acta 1997; 1333:F105–F150.
CAS PubMed Google Scholar - Anders RA, Leof EB . Chimeric granulocyte/macrophage colony-stimulating factor/transforming growth factor-beta (TGF-beta) receptors define a model system for investigating the role of homomeric and heteromeric receptors in TGF-beta signaling. J Biol Chem 1996; 271:21758–21766.
Article CAS PubMed Google Scholar - Anders RA, Arline SL, Dore JJ, Leof EB . Distinct endocytic responses of heteromeric and homomeric transforming growth factor beta receptors. Mol Biol Cell 1997; 8:2133–2143.
Article CAS PubMed PubMed Central Google Scholar - Anders RA, Dore JJ Jr, Arline SL, Garamszegi N, Leof EB . Differential requirement for type I and type II transforming growth factor beta receptor kinase activity in ligand-mediated receptor endocytosis. J Biol Chem 1998; 273:23118–23125.
Article CAS PubMed Google Scholar - Garamszegi N, Dore JJ Jr, Penheiter SG, Edens M, Yao D, Leof EB . Transforming growth factor beta receptor signaling and endocytosis are linked through a COOH terminal activation motif in the type I receptor. Mol Biol Cell 2001; 12:2881–2893.
Article CAS PubMed PubMed Central Google Scholar - Yao D, Ehrlich M, Henis YI, Leof EB . Transforming growth factor-beta receptors interact with AP2 by direct binding to beta2 subunit. Mol Biol Cell 2002; 13:4001–4012.
Article CAS PubMed PubMed Central Google Scholar - Lu Z, Murray JT, Luo W, et al. Transforming growth factor beta activates Smad2 in the absence of receptor endocytosis. J Biol Chem 2002; 277:29363–29368.
Article CAS PubMed Google Scholar - Razani B, Zhang XL, Bitzer M, von Gersdorff G, Bottinger EP, Lisanti MP . Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor. J Biol Chem 2001; 276:6727–6738.
Article CAS PubMed Google Scholar - Hartung A, Bitton-Worms K, Rechtman MM, et al. Different routes of bone morphogenic protein (BMP) receptor endocytosis influence BMP signaling. Mol Cell Biol 2006; 26:7791–7805.
Article CAS PubMed PubMed Central Google Scholar - Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL . Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol 2003; 5:410–421.
Article CAS PubMed Google Scholar - Chen CL, Liu IH, Fliesler SJ, Han X, Huang SS, Huang JS . Cholesterol suppresses cellular TGF-beta responsiveness: implications in atherogenesis. J Cell Sci 2007; 120:3509–3521.
Article CAS PubMed Google Scholar - Hicke L, Dunn R . Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol 2003; 19:141–172.
Article CAS PubMed Google Scholar - Ehrlich M, Shmuely A, Henis YI . A single internalization signal from the di-leucine family is critical for constitutive endocytosis of the type II TGF-beta receptor. J Cell Sci 2001; 114:1777–1786.
CAS PubMed Google Scholar - Dore JJ Jr, Yao D, Edens M, Garamszegi N, Sholl EL, Leof EB . Mechanisms of transforming growth factor-beta receptor endocytosis and intracellular sorting differ between fibroblasts and epithelial cells. Mol Biol Cell 2001; 12:675–684.
Article CAS PubMed PubMed Central Google Scholar - Mitchell H, Choudhury A, Pagano RE, Leof EB . Ligand-dependent and -independent transforming growth factor-beta receptor recycling regulated by clathrin-mediated endocytosis and Rab11. Mol Biol Cell 2004; 15:4166–4178.
Article CAS PubMed PubMed Central Google Scholar - Zerial M, McBride H . Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2001; 2:107–117.
Article CAS PubMed Google Scholar - Voorhees P, Deignan E, van Donselaar E, et al. An acidic sequence within the cytoplasmic domain of furin functions as a determinant of trans-Golgi network localization and internalization from the cell surface. Embo J 1995; 14:4961–4975.
Article CAS PubMed PubMed Central Google Scholar - Govers R, ten Broeke T, van Kerkhof P, Schwartz AL, Strous GJ . Identification of a novel ubiquitin conjugation motif, required for ligand-induced internalization of the growth hormone receptor. Embo J 1999; 18:28–36.
Article CAS PubMed PubMed Central Google Scholar - Zhou Y, Scolavino S, Funderburk SF, Ficociello LF, Zhang X, Klibanski A . Receptor internalization-independent activation of Smad2 in activin signaling. Mol Endocrinol 2004; 18:1818–1826.
Article CAS PubMed Google Scholar - Huse M, Chen YG, Massague J, Kuriyan J . Crystal structure of the cytoplasmic domain of the type I TGF beta receptor in complex with FKBP12. Cell 1999; 96:425–436.
Article CAS PubMed Google Scholar - Chen YG, Liu F, Massague J . Mechanism of TGFbeta receptor inhibition by FKBP12. Embo J 1997; 16:3866–3876.
Article CAS PubMed PubMed Central Google Scholar - Yao D, Dore JJ Jr, Leof EB . FKBP12 is a negative regulator of transforming growth factor-beta receptor internalization. J Biol Chem 2000; 275:13149–13154.
Article CAS PubMed Google Scholar - Tang H, Low B, Rutherford SA, Hao Q . Thrombin induces endocytosis of endoglin and type-II TGF-beta receptor and down-regulation of TGF-beta signaling in endothelial cells. Blood 2005; 105:1977–1985.
Article CAS PubMed Google Scholar - Marmor MD, Yarden Y . Role of protein ubiquitylation in regulating endocytosis of receptor tyrosine kinases. Oncogene 2004; 23:2057–2070.
Article CAS PubMed Google Scholar - Dore JJ Jr, Edens M, Garamszegi N, Leof EB . Heteromeric and homomeric transforming growth factor-beta receptors show distinct signaling and endocytic responses in epithelial cells. J Biol Chem 1998; 273:31770–31777.
Article CAS PubMed Google Scholar - Penheiter SG, Mitchell H, Garamszegi N, Edens M, Dore Jr JJ, Leof EB . Internalization-dependent and -independent requirements for transforming growth factor beta receptor signaling via the Smad pathway. Mol Cell Biol 2002; 22:4750–4759.
Article CAS PubMed PubMed Central Google Scholar - Runyan CE, Schnaper HW, Poncelet AC . The role of internalization in transforming growth factor beta1-induced Smad2 association with Smad anchor for receptor activation (SARA) and Smad2-dependent signaling in human mesangial cells. J Biol Chem 2005; 280:8300–8308.
Article CAS PubMed Google Scholar - Hayes S, Chawla A, Corvera S . TGF beta receptor internalization into EEA1-enriched early endosomes: role in signaling to Smad2. J Cell Biol 2002; 158:1239–1249.
Article CAS PubMed PubMed Central Google Scholar - Tsukazaki T, Chiang TA, Davison AF, Attisano L, Wrana JL . SARA, a FYVE domain protein that recruits Smad2 to the TGFbeta receptor. Cell 1998; 95:779–791.
Article CAS PubMed Google Scholar - Miura S, Takeshita T, Asao H, et al. Hgs (Hrs), a FYVE domain protein, is involved in Smad signaling through cooperation with SARA. Mol Cell Biol 2000; 20:9346–9355.
Article CAS PubMed PubMed Central Google Scholar - Itoh F, Divecha N, Brocks L, et al. The FYVE domain in Smad anchor for receptor activation (SARA) is sufficient for localization of SARA in early endosomes and regulates TGF-beta/Smad signalling. Genes Cells 2002; 7:321–331.
Article CAS PubMed Google Scholar - Bokel C, Schwabedissen A, Entchev E, Renaud O, Gonzalez-Gaitan M . Sara endosomes and the maintenance of Dpp signaling levels across mitosis. Science 2006; 314:1135–1139.
Article PubMed Google Scholar - Chen YG, Wang Z, Ma J, Zhang L, Lu Z . Endofin, a FYVE domain protein, interacts with Smad4 and facilitates transforming growth factor-beta signaling. J Biol Chem 2007; 282:9688–9695.
Article CAS PubMed Google Scholar - Gillooly DJ, Simonsen A, Stenmark H . Cellular functions of phosphatidylinositol 3-phosphate and FYVE domain proteins. Biochem J 2001; 355:249–258.
Article CAS PubMed PubMed Central Google Scholar - Panopoulou E, Gillooly DJ, Wrana JL, et al. Early endosomal regulation of Smad-dependent signaling in endothelial cells. J Biol Chem 2002; 277:18046–18052.
Article CAS PubMed Google Scholar - Shi W, Chang C, Nie S, Xie S, Wan M, Cao X . Endofin acts as a Smad anchor for receptor activation in BMP signaling. J Cell Sci 2007; 120:1216–1224.
Article CAS PubMed Google Scholar - Bennett D, Alphey L . PP1 binds Sara and negatively regulates Dpp signaling in Drosophila melanogaster. Nat Genet 2002; 31:419–423.
Article CAS PubMed Google Scholar - Jekely G, Rorth P . Hrs mediates downregulation of multiple signalling receptors in Drosophila. EMBO Rep 2003; 4:1163–1168.
Article CAS PubMed PubMed Central Google Scholar - Stenmark H, Parton RG, Steele-Mortimer O, Lutcke A, Gruenberg J, Zerial M . Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. Embo J 1994; 13:1287–1296.
Article CAS PubMed PubMed Central Google Scholar - Miyazono K . Positive and negative regulation of TGF-beta signaling. J Cell Sci 2000; 113 (Pt 7):1101–1109.
CAS PubMed Google Scholar - Centrella M, Ji C, Casinghino S, McCarthy TL . Rapid flux in transforming growth factor-beta receptors on bone cells. J Biol Chem 1996; 271:18616–18622.
Article CAS PubMed Google Scholar - Koli KM, Arteaga CL . Processing of the transforming growth factor beta type I and II receptors. Biosynthesis and ligand-induced regulation. J Biol Chem 1997; 272:6423–6427.
Article CAS PubMed Google Scholar - Wells RG, Yankelev H, Lin HY, Lodish HF . Biosynthesis of the type I and type II TGF-beta receptors. Implications for complex formation. J Biol Chem 1997; 272:11444–11451.
Article CAS PubMed Google Scholar - Zhang L, Zhou H, Su Y, et al. Zebrafish Dpr2 inhibits mesoderm induction by promoting degradation of nodal receptors. Science 2004; 306:114–117.
Article CAS PubMed Google Scholar - Zwaagstra JC, Kassam Z, O'Connor-Mccourt MD . Down-regulation of transforming growth factor-beta receptors: cooperativity between the types I, II, and III receptors and modulation at the cell surface. Exp Cell Res 1999; 252:352–362.
Article CAS PubMed Google Scholar - Zhao J, Buick RN . Regulation of transforming growth factor beta receptors in H-ras oncogene-transformed rat intestinal epithelial cells. Cancer Res 1995; 55:6181–6188.
CAS PubMed Google Scholar - Simons K, Toomre D . Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 2000; 1:31–39.
Article CAS PubMed Google Scholar - Nakao A, Afrakhte M, Moren A, et al. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature 1997; 389:631–635.
Article CAS PubMed Google Scholar - Hayashi H, Abdollah S, Qiu Y, et al. The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell 1997; 89:1165–1173.
Article CAS PubMed Google Scholar - Shi W, Sun C, He B, et al. GADD34-PP1c recruited by Smad7 dephosphorylates TGFbeta type I receptor. J Cell Biol 2004; 164:291–300.
Article CAS PubMed PubMed Central Google Scholar - Bai S, Cao X . A nuclear antagonistic mechanism of inhibitory Smads in transforming growth factor-beta signaling. J Biol Chem 2002; 277:4176–4182.
Article CAS PubMed Google Scholar - Zhang S, Fei T, Zhang L, et al. Smad7 antagonizes transforming growth factor beta signaling in the nucleus by interfering with functional Smad-DNA complex formation. Mol Cell Biol 2007; 27:4488–4499.
Article CAS PubMed PubMed Central Google Scholar - Ebisawa T, Fukuchi M, Murakami G, et al. Smurf1 interacts with transforming growth factor-beta type I receptor through Smad7 and induces receptor degradation. J Biol Chem 2001; 276:12477–12480.
Article CAS PubMed Google Scholar - Kavsak P, Rasmussen RK, Causing CG, et al. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation. Mol Cell 2000; 6:1365–1375.
Article CAS PubMed Google Scholar - Zhang XL, Topley N, Ito T, Phillips A . Interleukin-6 regulation of transforming growth factor (TGF)-beta receptor compartmentalization and turnover enhances TGF-beta1 signaling. J Biol Chem 2005; 280:12239–12245.
Article CAS PubMed Google Scholar - Seals DF, Courtneidge SA . The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev 2003; 17:7–30.
Article CAS PubMed Google Scholar - Atfi A, Dumont E, Colland F, et al. The disintegrin and metalloproteinase ADAM12 contributes to TGF-beta signaling through interaction with the type II receptor. J Cell Biol 2007; 178:201–208.
Article CAS PubMed PubMed Central Google Scholar - Chen CL, Huang SS, Huang JS . Cellular heparan sulfate negatively modulates transforming growth factor-beta1 (TGF-beta1) responsiveness in epithelial cells. J Biol Chem 2006; 281:11506–11514.
Article CAS PubMed Google Scholar - Ito T, Williams JD, Fraser DJ, Phillips AO . Hyaluronan regulates transforming growth factor-beta1 receptor compartmentalization. J Biol Chem 2004; 279:25326–25332.
Article CAS PubMed Google Scholar - Lebrin F, Deckers M, Bertolino P, Ten Dijke P . TGF-beta receptor function in the endothelium. Cardiovasc Res 2005; 65:599–608.
Article CAS PubMed Google Scholar - Santibanez JF, Blanco FJ, Garrido-Martin EM, Sanz-Rodriguez F, del Pozo MA, Bernabeu C . Caveolin-1 interacts and cooperates with the transforming growth factor-beta type I receptor ALK1 in endothelial caveolae. Cardiovasc Res 2008; 77:791–799.
Article CAS PubMed Google Scholar - Kim S, Lee Y, Seo JE, Cho KH, Chung JH . Caveolin-1 increases basal and TGF-beta1-induced expression of type I procollagen through PI-3 kinase/Akt/mTOR pathway in human dermal fibroblasts. Cell Signal 2008; 20:1313–1319.
Article CAS PubMed Google Scholar - Schwartz EA, Reaven E, Topper JN, Tsao PS . Transforming growth factor-beta receptors localize to caveolae and regulate endothelial nitric oxide synthase in normal human endothelial cells. Biochem J 2005; 390:199–206.
Article CAS PubMed PubMed Central Google Scholar - Ma XY, Wang Q, Jiang YX, Xiao ZY, Fang XH, Chen YG . Lateral diffusion of TGF-beta type I receptor studied by single-molecule imaging. Biochem Biophys Res Commun 2007; 356:67–71.
Article CAS PubMed Google Scholar - Lönn P, Morén A, Raja E, Dahl M, Moustakas A . Regulating the stability of TGFβ receptors and Smads. Cell Res 25 November 2008; doi:10.1038/cr.2008.308
- Cheyette BN, Waxman JS, Miller JR, et al. Dapper, a Dishevelled-associated antagonist of beta-catenin and JNK signaling, is required for notochord formation. Dev Cell 2002; 2:449–461.
Article CAS PubMed Google Scholar - Su Y, Zhang L, Gao X, et al. The evolutionally conserved activity of Dapper2 in antagonizing TGF-beta signaling. Faseb J 2007; 21:682–690.
Article CAS PubMed Google Scholar - Waxman JS, Hocking AM, Stoick CL, Moon RT . Zebrafish Dapper1 and Dapper2 play distinct roles in Wnt-mediated developmental processes. Development 2004; 131:5909–5921.
Article CAS PubMed Google Scholar - Lefkowitz RJ, Shenoy SK . Transduction of receptor signals by beta-arrestins. Science 2005; 308:512–517.
Article CAS PubMed Google Scholar - Chen W, Kirkbride KC, How T, et al. Beta-arrestin 2 mediates endocytosis of type III TGF-beta receptor and down-regulation of its signaling. Science 2003; 301:1394–1397.
Article CAS PubMed Google Scholar - Matsuzaki T, Hanai S, Kishi H, et al. Regulation of endocytosis of activin type II receptors by a novel PDZ protein through Ral/Ral-binding protein 1-dependent pathway. J Biol Chem 2002; 277:19008–19018.
Article CAS PubMed Google Scholar - Nakashima S, Morinaka K, Koyama S, et al. Small G protein Ral and its downstream molecules regulate endocytosis of EGF and insulin receptors. Embo J 1999; 18:3629–3642.
Article CAS PubMed PubMed Central Google Scholar - Shoji H, Tsuchida K, Kishi H, et al. Identification and characterization of a PDZ protein that interacts with activin type II receptors. J Biol Chem 2000; 275:5485–5492.
Article CAS PubMed Google Scholar - Moustakas A, Heldin CH . Non-Smad TGF-beta signals. J Cell Sci 2005; 118:3573–3584.
Article CAS PubMed Google Scholar
Acknowledgements
The work in Ye-Guang Chen's laboratory is supported by grants from the National Natural Science Foundation of China (30430360, 30671033) and the Ministry of Sciences and Technology of China 973 Program (2004CB720002, 2006CB943401, 2006CB910102) and 863 Program (2006AA02Z172).
Author information
Authors and Affiliations
- Department of Biological Sciences and Biotechnology, State Key Laboratory of Biomembrane and Membrane Biotechnology, Tsinghua University, 100084, Beijing, China
Ye-Guang Chen
Authors
- Ye-Guang Chen
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toYe-Guang Chen.
Rights and permissions
About this article
Cite this article
Chen, YG. Endocytic regulation of TGF-β signaling.Cell Res 19, 58–70 (2009). https://doi.org/10.1038/cr.2008.315
- Published: 02 December 2008
- Issue Date: January 2009
- DOI: https://doi.org/10.1038/cr.2008.315