High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells (original) (raw)
- Letter
- Published: 23 June 2013
- Jennifer A Foden1,2,3,
- Cyd Khayter1,2,3,
- Morgan L Maeder1,2,3,5,
- Deepak Reyon1,2,3,4,
- J Keith Joung1,2,3,4,5 &
- …
- Jeffry D Sander1,2,3,4
Nature Biotechnology volume 31, pages 822–826 (2013)Cite this article
- 81k Accesses
- 2338 Citations
- 218 Altmetric
- Metrics details
Subjects
Abstract
Clustered, regularly interspaced, short palindromic repeat (CRISPR) RNA-guided nucleases (RGNs) have rapidly emerged as a facile and efficient platform for genome editing. Here, we use a human cell–based reporter assay to characterize off-target cleavage of CRISPR-associated (Cas)9-based RGNs. We find that single and double mismatches are tolerated to varying degrees depending on their position along the guide RNA (gRNA)-DNA interface. We also readily detected off-target alterations induced by four out of six RGNs targeted to endogenous loci in human cells by examination of partially mismatched sites. The off-target sites we identified harbored up to five mismatches and many were mutagenized with frequencies comparable to (or higher than) those observed at the intended on-target site. Our work demonstrates that RGNs can be highly active even with imperfectly matched RNA-DNA interfaces in human cells, a finding that might confound their use in research and therapeutic applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Additional access options:
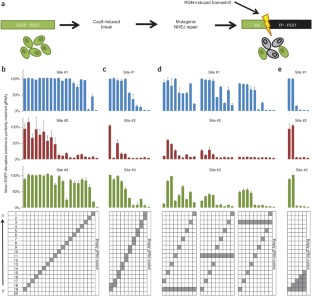
Figure 1: Activities of RGNs harboring variant mismatched sgRNAs in a human cell–based EGFP disruption assay.
Similar content being viewed by others
References
- Wiedenheft, B., Sternberg, S.H. & Doudna, J.A. RNA-guided genetic silencing systems in bacteria and archaea. Nature 482, 331–338 (2012).
Article CAS Google Scholar - Horvath, P. & Barrangou, R. CRISPR/Cas, the immune system of bacteria and archaea. Science 327, 167–170 (2010).
Article CAS Google Scholar - Terns, M.P. & Terns, R.M. CRISPR-based adaptive immune systems. Curr. Opin. Microbiol. 14, 321–327 (2011).
Article CAS Google Scholar - Wang, H. et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153, 910–918 (2013).
Article CAS Google Scholar - Shen, B. et al. Generation of gene-modified mice via Cas9/RNA-mediated gene targeting. Cell Res. 23, 720–723 (2013).
Article CAS Google Scholar - DiCarlo, J.E. et al. Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Res. 41, 4336–4343 (2013).
Article CAS Google Scholar - Jiang, W., Bikard, D., Cox, D., Zhang, F. & Marraffini, L.A. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat. Biotechnol. 31, 233–239 (2013).
Article CAS Google Scholar - Jinek, M. et al. RNA-programmed genome editing in human cells. Elife 2, e00471 (2013).
Article Google Scholar - Hwang, W.Y. et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 31, 227–229 (2013).
Article CAS Google Scholar - Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Article CAS Google Scholar - Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Article CAS Google Scholar - Cho, S.W., Kim, S., Kim, J.M. & Kim, J.S. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat. Biotechnol. 31, 230–232 (2013).
Article CAS Google Scholar - Gratz, S.J. et al. Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics doi:10.1534/genetics.113.152710 (24 May 2013).
- Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Article CAS Google Scholar - Reyon, D. et al. FLASH assembly of TALENs for high-throughput genome editing. Nat. Biotechnol. 30, 460–465 (2012).
Article CAS Google Scholar - Pattanayak, V., Ramirez, C.L., Joung, J.K. & Liu, D.R. Revealing off-target cleavage specificities of zinc-finger nucleases by in vitro selection. Nat. Methods 8, 765–770 (2011).
Article CAS Google Scholar - Perez, E.E. et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat. Biotechnol. 26, 808–816 (2008).
Article CAS Google Scholar - Gabriel, R. et al. An unbiased genome-wide analysis of zinc-finger nuclease specificity. Nat. Biotechnol. 29, 816–823 (2011).
Article CAS Google Scholar - Hockemeyer, D. et al. Genetic engineering of human pluripotent cells using TALE nucleases. Nat. Biotechnol. 29, 731–734 (2011).
Article CAS Google Scholar - Sugimoto, N. et al. Thermodynamic parameters to predict stability of RNA/DNA hybrid duplexes. Biochemistry 34, 11211–11216 (1995).
Article CAS Google Scholar
Acknowledgements
This work was supported by a US National Institutes of Health (NIH) Director's Pioneer Award DP1 GM105378, NIH R01 GM088040, NIH P50 HG005550, Defense Advanced Research Projects Agency (DARPA) W911NF-11-2-0056, and the Jim and Ann Orr Massachusetts General Hospital Research Scholar Award. We thank S.Q. Tsai for helpful discussions and encouragement.
Author information
Authors and Affiliations
- Molecular Pathology Unit, Massachusetts General Hospital, Charlestown, Massachusetts, USA
Yanfang Fu, Jennifer A Foden, Cyd Khayter, Morgan L Maeder, Deepak Reyon, J Keith Joung & Jeffry D Sander - Center for Cancer Research, Massachusetts General Hospital, Charlestown, Massachusetts, USA
Yanfang Fu, Jennifer A Foden, Cyd Khayter, Morgan L Maeder, Deepak Reyon, J Keith Joung & Jeffry D Sander - Center for Computational and Integrative Biology, Massachusetts General Hospital, Charlestown, Massachusetts, USA
Yanfang Fu, Jennifer A Foden, Cyd Khayter, Morgan L Maeder, Deepak Reyon, J Keith Joung & Jeffry D Sander - Department of Pathology, Harvard Medical School, Boston, Massachusetts, USA
Yanfang Fu, Deepak Reyon, J Keith Joung & Jeffry D Sander - Program in Biological and Biomedical Sciences, Harvard Medical School, Boston, Massachusetts, USA
Morgan L Maeder & J Keith Joung
Authors
- Yanfang Fu
You can also search for this author inPubMed Google Scholar - Jennifer A Foden
You can also search for this author inPubMed Google Scholar - Cyd Khayter
You can also search for this author inPubMed Google Scholar - Morgan L Maeder
You can also search for this author inPubMed Google Scholar - Deepak Reyon
You can also search for this author inPubMed Google Scholar - J Keith Joung
You can also search for this author inPubMed Google Scholar - Jeffry D Sander
You can also search for this author inPubMed Google Scholar
Contributions
Y.F., J.D.S. and J.K.J. designed experiments; Y.F., J.A.F., C.K., M.L.M., D.R. and J.D.S. performed experiments; Y.F., M.L.M., D.R., J.D.S. and J.K.J. wrote the manuscript.
Corresponding authors
Correspondence toJ Keith Joung or Jeffry D Sander.
Ethics declarations
Competing interests
J.K.J. has a financial interest in Transposagen Biopharmaceuticals. J.K.J.'s interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict-of-interest policies.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–14, Supplementary Note and Supplementary Methods (PDF 312 kb)
Supplementary Table 1
Sequences of oligonucleotides used to generate expression plasmids encoding sgRNAs/variant sgRNAs targeted to sites in the EGFP reporter gene and sgRNAs targeted to six endogenous human gene targets (XLSX 34 kb)
Supplementary Table 2
Sequences and characteristics of genomic on- and off-target sites for six RGENs targeted to endogenous human genes and primers and PCR conditions used to amplify these sites (XLSX 48 kb)
Rights and permissions
About this article
Cite this article
Fu, Y., Foden, J., Khayter, C. et al. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells.Nat Biotechnol 31, 822–826 (2013). https://doi.org/10.1038/nbt.2623
- Received: 01 April 2013
- Accepted: 03 June 2013
- Published: 23 June 2013
- Issue Date: September 2013
- DOI: https://doi.org/10.1038/nbt.2623