‘Men are but worms:’ neuronal cell death in C. elegans and vertebrates (original) (raw)
Introduction
Programmed cell death (PCD) is critical to the development and maintenance of many tissues. Deficiencies in PCD underlie some forms of oncogenesis, while excessive cell death may contribute to several pathological conditions, including stroke, autoimmune disorders, and certain neurodegenerative diseases. Cells undergoing PCD exhibit morphological and biochemical changes characteristic of apoptosis, including cytoplasmic shrinkage, plasma membrane blebbing, chromatin condensation, and DNA fragmentation.1 Phagocytes, both professional and nonprofessional, eventually engulf the dying cells, thereby preventing an inflammatory response.
Much of what we know today about the evolutionary conservation and genetic regulation of PCD arises from the pioneering work of Sydney Brenner, John Sulston, and Robert Horvitz, for which they were jointly awarded the 2002 Nobel Prize in Physiology or Medicine. From their studies of cell-lineage determination in the nematode Caenorhabditis elegans, Brenner, Sulston, and Horvitz found that of the 1090 somatic cells generated during development of the C. elegans hermaphrodite, only 959 remain in the adult. Moreover, 105 of the 131 cells that undergo PCD are neurons, a finding that Horvitz noted ‘particularly caught my attention’ in his Nobel address. In this manner, neuronal development in nematodes resembles that in their vertebrate counterparts, characterized by overproduction and then cell death, ostensibly (according to the neurotrophic hypothesis) to match the number of innervating neurons with the size of their target tissue. Perhaps it should come as no surprise then that some of the most striking similarities between the signal transduction pathways responsible for PCD in worms and vertebrates occur in neurons.
In this review, we shall discuss some of these similarities, as well as some important differences, between PCD in C. elegans and in vertebrate neurons, focusing on one of the most extensively studied models of neuronal PCD, nerve growth factor (NGF) deprivation in sympathetic neurons from superior cervical ganglia (SCG). Then, we will examine the potential contribution of neuronal cell death to the pathogenesis of both acute and chronic neurodegenerative diseases and discuss the role of PCD-targeted therapies in the treatment of these disorders.
The Genetics and Biochemistry of Programmed Cell Death
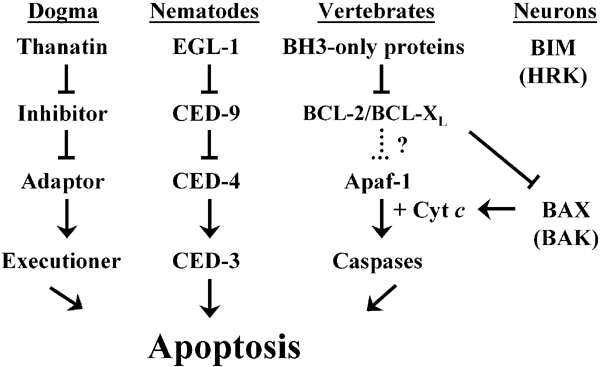
In a series of elegant genetic experiments that span three decades, Horvitz and his colleagues defined the ‘central dogma’ of PCD, depicted schematically in Figure 1. According to this model, in at least two types of neurons in C. elegans (i.e., the hermaphrodite-specific neurons (HSNs) in males and the neurosecretory motoneuron (NSM) sister cells), the ‘thanatin’ EGL-1 is induced in cells destined to die, interacts with the cell death inhibitor CED-9, thereby displacing the adaptor CED-4, which can then promote the activation of the executioner protease CED-3. Parallel studies in vertebrates conducted by numerous laboratories have identified the vertebrate counterparts of these nematode proteins as BH3-only BCL-2 proteins (e.g., BIM and HRK), antiapoptotic BCL-2 proteins (e.g., BCL-2 and BCL-XL), Apaf-1, and the caspase family of cysteine proteases, respectively.
Figure 1
The ‘central dogma’ of PCD. Based on the work of Horvitz and his colleagues in HSNs and NSM sister cells in nematodes, the thanatin EGL-1 is induced in cells that are destined to die, interacts with inhibitor CED-9, displaces the adaptor CED-4, which then activates executioner protease CED-3. This basic scheme is conserved in vertebrates in the form of BH3-only proteins (e.g., BIM and HRK), antiapoptotic BCL-2 proteins (e.g., BCL-2 and BCL-XL), Apaf-1, and the caspases. However, assuming that Apaf-1 is the one and only CED-4 ortholog in vertebrates*, two fundamental differences exist: First, caspase activation through this pathway requires cyt c release in vertebrates, but not in worms. And in sympathetic and CG neurons (and probably other neuronal subpopulations), this release requires BAX, whose ‘activation’ (see Figure 3) requires the BH3-only protein BIM, and probably others (e.g., HRK). Second, caspase activation in SCG (and probably other) neurons, requires BAX-dependent release of not only cyt c but also Smac/DIABLO, an IAP inhibitor. *(We equivocate here because the answers to certain questions may reveal even greater conservation than that described above: For example, is the mitochondrial localization of CED-9 reported by Chen et al.130 purely serendipitous or does it belie a BCL-2-like function in regulating mitochondrial permeability in the nematode? Furthermore, is Apaf-1 the one and only true vertebrate CED-4 ortholog as suggested by preliminary sequence analysis of the human genome131 or does another CED-4-like protein(s) exist whose activation of CED-3-like caspases truly can be inhibited at the level of the apoptosome by direct interaction with a CED-9-like antiapoptotic BCL-2 family member?)
Despite this remarkable evolutionary conservation, several fundamental differences appear to exist: First, caspase activation through this ‘intrinsic’ pathway during development requires cytochrome c (cyt c) in vertebrate, but not in nematode, neurons. (The structural basis for this difference seems apparent: CED–4 lacks the WD40 repeats required in Apaf-1 for interaction with cyt c, a potential caveat being that activation of the Drosophila Apaf-1-related killer (ARK), which also has WD40 repeats, may not require interaction with cyt c.2) Second, such cyt c release in multiple neuronal populations requires expression of at least one multidomain proapoptotic BCL-2 protein, usually BAX. And finally, caspase activation in sympathetic (and probably other) neurons in physiological settings requires not only BAX-dependent cyt c release but also inactivation of an inhibitor of apoptosis (IAP)-like activity by Smac/DIABLO, the phenomenon initially described as ‘competence-to-die.’3 Because this review, in highlighting the similarities between PCD in nematode and vertebrate neurons, will focus on the premitochondrial regulation of apoptosis, particularly by BCL-2 proteins, the interested reader is referred elsewhere for further discussion of the role of cyt c and IAPs in the postmitochondrial regulation of neuronal apoptosis.4,5,6,7,8,9
Premitochondrial Regulation of Intrinsic Pathway Apoptosis: The CED-9/BCL-2 Family
The CED-9/BCL-2 family of proteins consists of both antiapoptotic (e.g., BCL-2, BCL-XL, BCL-w, and MCL-1) and proapoptotic members; the latter are further divided into two subfamilies, ‘multidomain’ (e.g., BAX, BAK, and BOK) and ‘BH3-only’ (e.g., BAD, BID, and BIM) proteins. Four regions of homology known as BCL-2 homology (BH) domains 1−4 define membership in the BCL-2 family.10 Although certain exceptions exist, antiapoptotic BCL-2 proteins generally possess all four BH domains and a transmembrane domain (TMD), while multidomain proapoptotic members typically lack only the BH4 domain; and, true to their name, BH3-only proapoptotic proteins usually possess only a BH3 domain and (sometimes) a TMD. This conserved domain structure among the three subfamilies has functional significance because BH1–3 may mediate interactions between family members.
BCL-2 proteins can form certain homo- and heterodimers and multimers. Whether they do so in vivo or even in vitro in the absence of conformation-altering detergents that promote interactions (and may mimic a membrane environment) remains controversial. However, as a general rule, antiapoptotic BCL-2 proteins require BH1 and BH2 to prevent cell death and to heterodimerize with their proapoptotic counterparts.11,12,13,14 Conversely, proapoptotic proteins generally require BH3 to promote cell death and, accordingly, to heterodimerize with BH1 and BH2 of their antiapoptotic cousins.15,16,17,18 These in vitro findings from mutagenesis studies are consistent with the tertiary structure of BAK,19 BAD,20 and BIM21 heterodimers with BCL-XL showing that the BH3 domain of the proapoptotic members forms an amphipathic α-helix that associates through hydrophobic and electrostatic interactions with a ‘binding pocket’ formed by the BH1 and BH2 domains of BCL-XL. (One minor caveat to these conclusions may be that none of these structures includes the entire C-terminus or TMD (where present) of the respective proteins, and the tertiary structures of the proteins examined may differ in soluble versus membrane-bound forms.)
Consistent with studies such as these, Korsmeyer and co-workers22 proposed the ‘rheostat model’, according to which the ratio of proapoptotic to antiapoptotic BCL–2 family members within a cell determines whether that cell will die in response to a death signal. Although certain findings (e.g., the ability of both pro- and antiapoptotic family members to function independently to regulate cell death23,24,25) have cast doubt on certain elements of the original hypothesis, the basic premise of this model still holds true: the net balance of proapoptotic versus antiapoptotic BCL-2 proteins determines a cell's fate.
Nonetheless, the biochemical mechanisms by which BCL-2 family proteins regulate survival remain unclear. The three-dimensional structure of BCL-XL shows similarities to the pore-forming domains of bacterial toxins such as diphtheria toxin and the colicins.26 Accordingly, BCL-2, BCL-XL, BID, and BAX can form ion channels in synthetic lipid membranes.27,28,29,30 These channels are characterized by multiconductance states, pH sensitivity, voltage gating, and poor ion selectivity. Whether BCL-2 family members form pores in mitochondria in intact cells and what regulates this activity are unclear; however, these findings suggest that these proteins may either form or regulate the formation of channels in mitochondrial membranes. Alternatively, BCL-2 proteins may regulate the integrity of intracellular (especially mitochondrial) membranes, either alone or in concert with other proteins, such as VDAC.31,32,33 In any case, the molecular and biochemical basis for the regulation of apoptosis by BCL-2 proteins remains uncertain.
Programmed Cell Death in the Nervous System: The Sympathetic Neuron Model
From approximately embryonic-day-16 (E16) to postnatal-day-7 (P7), sympathetic neurons require NGF for survival. 34 In vivo administration of NGF antiserum during this period induces extensive loss of SCG neurons.35 This cell death can be recapitulated in vitro: Neonatal sympathetic neurons, maintained in culture for 4–6 days in the presence of NGF, undergo an apoptotic cell death within 48 h of trophic factor withdrawal.36,37 This death, which requires de novo protein synthesis,38 expression of the proapoptotic BCL-2 family member Bax,39 and development of competence-to-die,3 is inhibited by caspase inhibitors40,41,42 and neuroprotective agents, such as KCl and cAMP.36,37,43,44
Transcriptional Regulation of BH3-only Proteins, the Long-Sought-after Thanatins
Nearly two decades ago, we reported that inhibitors of RNA and protein synthesis protect against trophic factor deprivation (TFD)-induced apoptosis in sympathetic neurons. 38 This observation suggests that, true to its name, ‘programmed’ cell death is a differentiation program like any other requiring selective new gene expression to direct a cell to its physiologically appropriate fate, in this case death. However, the identities of these genes, which we called ‘thanatins’45 and defined as genes induced during apoptosis whose principal, or sole, function is to mediate cell death, remained elusive. However, here again genetic studies of PCD in C. elegans proved illuminating: Conradt and Horvitz46 reported in 1998 that the BH3-only protein EGL-1, whose expression is induced in HSNs destined to die in male nematodes, physically interacts with CED-9, displacing the adaptor CED-4, which can then activate the caspase CED-3 (Figure 1). Subsequently, studies in sympathetic neurons identified a BH3-only protein – BIM – induced during NGF deprivation and required for TFD-induced apoptosis.47,48 However, Bim deletion,47unlike Bax deficiency,39 delays, but does not prevent, cyt c release, caspase activation, and cell death in this paradigm. These findings suggest the existence of at least one other functionally redundant, BH3-only protein in these neurons. Accordingly, in 1997, two groups reported the cloning of HRK/DP5, a BH3-only protein induced by NGF deprivation in SCG neurons49 with kinetics strikingly similar to those for BIM.50 Moreover, we recently have observed similar induction of another BH3-only protein, PUMA, during NGF deprivation in sympathetic neurons (C Besirli and EMJ, unpublished observations).
Cell death induced by overexpression of either Bim or Hrk/Dp5 (or any other BH3-only protein examined) requires BAX alone despite coexpression of BAK in cerebellar granule (CG) neurons,50,51,52 indicating that BH3-only proteins function genetically upstream of (or in parallel with) BAX-dependent cyt c release and its downstream sequelae in these neurons. Furthermore, although the physiological function of HRK and PUMA in neuronal cell death remains unknown, these findings suggest that recapitulation of the _Bax_−/− phenotype, in which cyt c release, caspase activation, and cell death are completely prevented long-term, will require inactivation of BIM, and almost certainly other BH3-only proteins (e.g., HRK and PUMA). However, two critical questions remain unanswered by these studies: What regulates the transactivation of BH3-only proteins and how (assuming Apaf-1 is the true vertebrate CED-4 ortholog), in the absence of an apoptosome complex resembling that in nematodes, do these BH3-only proteins function?
In C. elegans, in addition to the HSNs in males, the death of at least one other neuronal population, the NSM sister cells, requires transactivation of the BH3-only protein EGL-1. More recent studies in both cell types demonstrate that two zinc-finger transcription factors CES-1 and TRA-1 repress the transcription of egl-1.53,54,55 Of interest, CES-1 and TRA-1 have human orthologs, SLUG and GLI proteins, respectively. Although the potential role of SLUG and GLI proteins in regulating BH3-only protein expression in vertebrates is unknown, both have been implicated in the pathogenesis of certain human cancers.56 Nonetheless, recent findings shed some light on the transcriptional regulation of BH3-only proteins in vertebrate neurons, and (not surprisingly) the situation may be even more complex than that in worms.
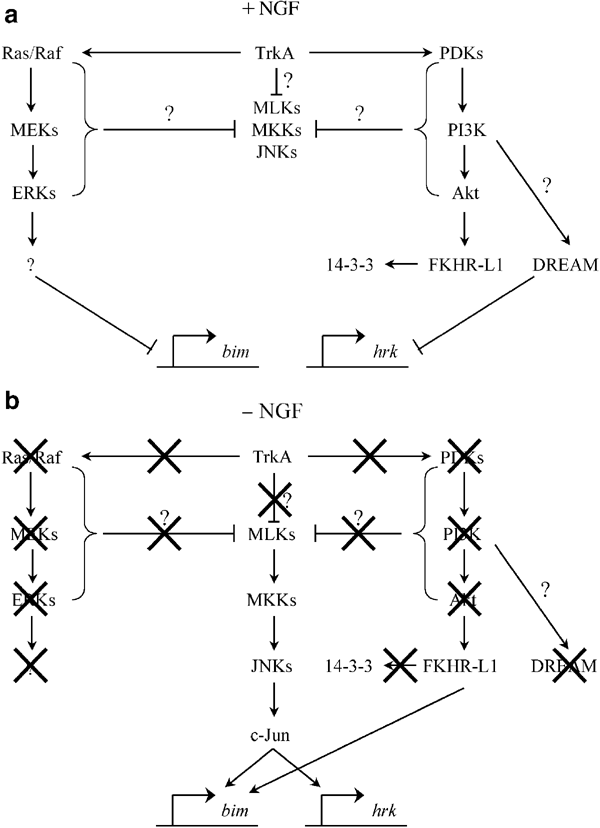
Multiple signaling pathways modulate the NGF-dependent survival of sympathetic neurons (Figure 2), including (but not necessarily limited to) those involving cAMP response element binding protein (CREB), NF-κB, phosphatidylinositol-3-kinase (PI3 K), and mitogen-activated protein kinases (MAPKs), including various MAPKs (e.g., p42/44 extracellular-regulated kinases (ERKs), c-Jun NH2-terminal kinases (JNKs), and p38 MAPKs), MAPK kinases (MAPKKs) (e.g., MKK3, 4, 6, and 7), and MAPKK kinase kinases (MAPKKKs) (e.g., mixed lineage kinases (MLKs)), as well as putative MAPKKK activators, such as small G-proteins (e.g., Cdc42/Rac). Some of these pathways, such as CREB,57 NF-κB,58,59 MEK/ERK,60,61 and PI3K/Akt,62,63,64 promote survival and/or differentiation in these neurons, whereas others, especially JNKs, promote cell death.65,66,67
Figure 2
Multiple signal transduction pathways coordinately regulate the expression of BH3-only proteins, such as BIM and HRK, in sympathetic neurons. (a) In NGF-maintained sympathetic neurons, BIM and HRK expression is suppressed by multiple mechanisms, including (but not necessarily limited to) those described below. First, NGF-dependent activation of TrkA causes activation of the Ras/MEK pathway, which (somehow) represses BIM expression. In parallel, PI3K-dependent activation of Akt causes phosphorylation of Forkhead transcription factors, such as FKHR-L1, which then can be sequestered in the cytosol by 14-3-3 proteins, thereby preventing BIM expression.132 Moreover, the transcriptional repressor DREAM, which can be activated by PI3 K signaling and/or elevated intracellular Ca2+, represses HRK expression. Finally, TrkA activation by NGF suppresses activation of the MLK/JNK pathway, although the precise molecular mechanisms are unknown (e.g., mutual negative regulation by the Ras/MEK and PI3K/Akt signaling modules may contribute). (b) In contrast, during NGF deprivation, ERK and PI3K activation decays, derepressing BIM and HRK expression. In parallel, as PI3K-dependent Akt activity decreases, Forkhead transcription factors, such as FKHR-L1, become dephosphorylated, dissociate from 14-3-3 proteins and translocate to the nucleus, where they transactivate BIM expression. And finally, NGF deprivation causes activation of the JNK pathway, culminating in AP-1-mediated BIM and HRK induction. Moreover, activation of the JNK pathway also causes phosphorylation of BIM, perhaps regulating the proapoptotic activity of BIM post-translationally by modulating proteasome-dependent degradation
Recent findings indicate that BCL-2 proteins such as BIM, HRK, and other BH3-only proteins are molecular targets for these kinase cascades that mediate at least some of their effects.47,48,50,68,69 For example, maximal transactivation of BIM requires activation of the JNK/c-Jun pathway,47,48,50,52 consistent with the identification of AP-1 sites in the Bim promoter.70 Also striking is the extent to which the transcripts for Bim and Hrk are coordinately regulated by the JNK/c-Jun pathway,50 and perhaps others, such as the PI3K/Akt module,71,72 consistent with a TFD-induced transcriptional ‘program’ culminating in BAX-dependent neuronal apoptosis. Accordingly, overexpression of known or hypothesized components of the JNK/c-Jun signaling module, including MLK1–3, MKK4, MKK7, and JNK1, requires BAX alone even when BAK is coexpressed.52 Similarly, in sympathetic neurons72 as in hematopoietic cells,69 in which TFD causes induction of both BIM and HRK, attenuation of signaling through the PI3K/Akt pathway during TFD causes dephosphorylation and nuclear translocation of FOXO transcription factors and FOXO-mediated Bim transcription. Consistent with this, ectopic expression of constitutively active FOXO transcription factors induces cell death that requires BIM and BAX in neurons72 (GVP and EMJ, unpublished observations). In addition to FOXO transcription factors, another potential target of PI3K signaling in this context may be DREAM, a Ca2+-dependent repressor that regulates hrk transcription.71 In this case, trophic factors such as NGF activate PI3K and increase intracellular Ca2+, both of which promote repression of hrk transcription by DREAM, which binds a downstream regulatory element (DRE) sequence in the 3′ untranslated region (UTR) of the hrk gene. In this manner, both the MLK/JNK and PI3K/Akt signaling modules may coordinately regulate the expression of the BH3-only proteins BIM and HRK during TFD-induced neuronal apoptosis. Finally, recent data suggest that activation of the Ras/MEK pathway, which may itself antagonize JNK signaling and promote CREB-mediated pro-survival signaling (see below), represses transcription of BIM. For example, inhibitors of MEK such as PD98059 and U0126 induce BIM expression in nonneuronal cell lines73,74,75,76 as well as in neurons (GVP and EMJ, unpublished observations). However, interpretation of these findings is complicated by crosstalk between the Ras/MEK, PI3K/Akt, and MLK/JNK modules, as well as recent reports on the post-translational regulation of BIM's proapoptotic activity by two of these pathways (i.e., ERK and JNK) via phosphorylation-dependent modulation of protein stability, probably involving the proteasome.52,73,77,78
However, conclusions based on these reports that activation of MLK/JNK signaling and/or suppression of PI3K/Akt and Ras/MEK signaling are the only pathways controlling BIM transcription likely under-represent the complexity of BIM transcriptional control. First, Liu and Greene79 report that Rb-dependent, E2F-mediated gene repression attenuates TFD-induced apoptosis in sympathetic neurons and that depression of certain E2F target genes, such as B- and C-myb, which are induced by NGF deprivation in SCG neurons,65 can induce cell death in these neurons. Since the BIM promoter contains potential binding sites for c-Myb,70 BIM may be one of the E2F target genes derepressed during TFD. Second, the transcriptional control of BH3-only proteins like BIM may vary in a cell type- and stimulus-specific manner. For example, NGF-deprived sympathetic neurons rely primarily on activation of the MLK/JNK pathway culminating in AP-1-dependent transactivation of BIM,47,48,50 with some modest contribution from loss of PI3K/Akt and Ras/MEK signaling.47 In contrast, in IL-3-deprived hematopoietic cells, suppression of the PI3K/Akt pathway culminating in FKHRL1-mediated BIM transcription predominates,68,69 with some contribution again from loss of Ras/MEK signaling.76 Even within a single cell type, control of BIM transcription may vary according to cellular context. For example, in the case of mass cultures of NGF-maintained sympathetic neurons, inhibition of the Ras/MEK or PI3K/Akt pathway by using U0126 or LY294002, respectively, causes some MLK/JNK-dependent induction of BIM; however, in the context of depolarization-induced survival of both SCG and CG neurons, which depends almost exclusively on PI3K/Akt signaling,80,81,82,83 LY294002 alone causes significant MLK/JNK-independent BIM induction (GVP and EMJ, unpublished observations) and BAX-dependent cell death,84 consistent with physiological crosstalk between the Ras/MEK, PI3K/Akt, and MLK/JNK pathways. Thus, coordinate transcriptional regulation of BH3-only proteins, such as that seen with BIM and HRK during neuronal apoptosis, will likely involve complex signaling networks that may be context dependent (i.e., cell- and stimulus-specific).
Finally, transcriptional targets other than BH3-only proteins may also contribute to survival responses mediated by some of the aforementioned signaling modules (e.g., Ras/MEK and PI3K/Akt). For example, the CREB family of transcription factors contributes to the trophic factor-mediated survival of both sympathetic and CG neurons (reviewed in Lonze and Ginty57) via a BAX-dependent pathway85 (GVP and EMJ, unpublished observations). A previous report suggests that this pro-survival activity involves CREB-mediated upregulation of bcl-2.86 However, as noted more recently,85 this is unlikely because both in vivo and in vitro, the trophic factor-dependent survival of both PNS and CNS neurons is only modestly affected by Bcl-2 deletion.87,88 Although we clearly lack a definitive compendium of CREB family-regulated genes, among the known or hypothesized targets, a more likely candidate is BCL-XL, which is critical for neuronal survival,89,90 and is a transcriptional target of the JAK-STAT signaling module,91 which includes at least one transcriptional target of CREB (i.e., STAT3).92 In addition, notwithstanding the added complexity of phosphorylation-independent regulatory events, the phosphorylation-dependent activation of CREB may involve multiple kinase cascades, including the Ras/MEK, PI3K/Akt, and p38 MAPK pathways mentioned above.57 Second, NGF-dependent activation of NF-κB contributes to the survival of sympathetic neurons.58,59 Such regulation may be both premitochondrial, involving transactivation of antiapoptotic BCL-2 proteins, such as BCL-XL and/or A1/BFL-1,93,94 and postmitochondrial, involving upregulation of IAPs.95,96 However, one important caveat exists regarding the importance of transcriptional regulation of antiapoptotic BCL-2 proteins during TFD in SCG and CG neurons: The expression of BCL-XL, BCL-2, and BCL-w remains essentially unchanged until very late in the cell death process (i.e., after the release of cyt c).51,87 Therefore, although NF-κB and CREB signaling clearly contributes to survival in these neurons, whether antiapoptotic BCL-2 proteins represent physiologically significant transcriptional targets of these pathways is unclear.
Enter the Interloper: Regulation of BAX during Neuronal Apoptosis
As mentioned above, one fundamental difference between developmental neuronal PCD in C. elegans and vertebrates is that the latter involves cyt c release for caspase activation, and at least in sympathetic and CG neurons (and probably many others), such release absolutely requires expression of multidomain proapoptotic BCL-2 protein BAX.3,97,98 Despite widespread expression of Bax in vivo, _Bax_-deficient mice exhibit relatively modest defects in non-neuronal lineages,99 likely secondary to functional redundancy with other multidomain proapoptotic BCL-2 family members.100,101 In contrast, their phenotype in neuronal lineages, whether central or peripheral, is dramatic.39,84,90,102 For example, despite coexpression of BAK, TFD-induced cell death in CG neurons depends solely on BAX.51 However, contrary to one report,103 neither the mRNA nor protein level of BAX increases during NGF deprivation-induced apoptosis in sympathetic neurons87 or K+-withdrawal-induced death in CG neurons.47,51,84,104 Therefore, BAX must be regulated post-translationally in these and other neuronal TFD paradigms,98,104,105,106,107 not via transactivation by p53, which only has a nominal role in TFD-induced neuronal apoptosis108,109,110,111 in contrast to its significant role in DNA damage-induced neuronal cell death.108,112,113,114,115 With regard to the contribution of p53 (or other family members) to TFD-induced neuronal PCD, we favor the hypothesis that reduced basal BAX expression underlies the modest delay in cell death observed in NGF-deprived SCG neurons deficient in p53 for two principal reasons: (1) p53 deletion is associated with reduced basal BAX expression in multiple neuronal populations including sympathetic neurons;116 and (2) reducing the gene dosage of Bax (e.g., in Bax+/− neurons), like p53 deficiency, delays TFD-induced apoptosis in SCG neurons.39,108
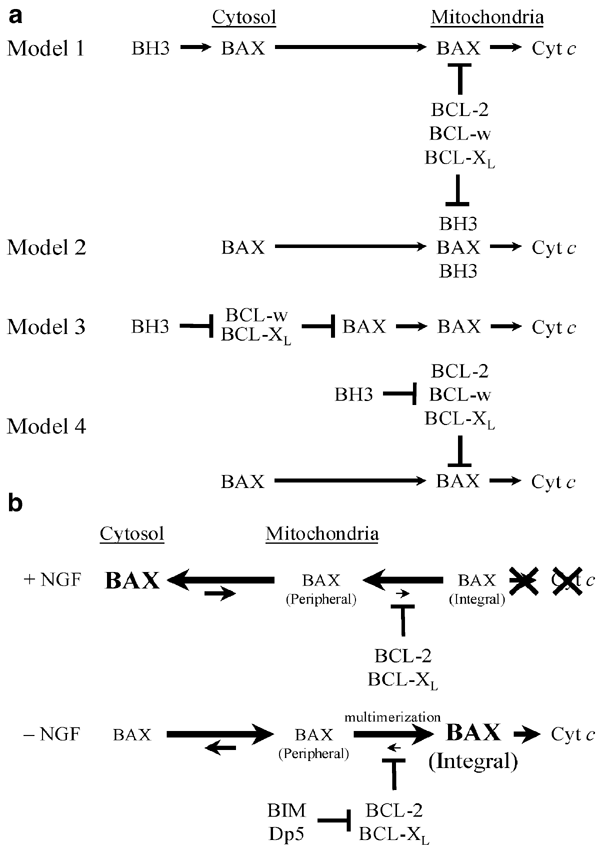
To our knowledge, TFD in sympathetic and CG neurons remains the only primary cell culture paradigms shown to be entirely dependent on the expression and translocation of BAX alone for cyt c release, caspase activation, and apoptosis,3,39,84,98,105,106 although this is probably true for other neuronal populations.39,107 Therefore, these models are excellent for studying the physiological mechanisms responsible for endogenous BAX translocation and BAX-dependent cyt c release. Moreover, macromolecular synthesis is required for BAX translocation, cyt c release, and apoptosis during TFD in SCG neurons;3,39,105,117 and, the BH3-only proteins BIM and HRK are induced in cells destined to die and require BAX to mediate cell death.47,48,50 Taken together, these findings clearly suggest that BH3-only proteins may trigger BAX translocation and its downstream sequelae. However, these results are consistent with several possibilities, which need not be mutually exclusive, for how this may occur (Figure 3a). (Please note that these arguments, which are similar to those previously presented by Putcha et al.,51 have only been strengthened by more recent findings, such as Liu et al.21)
Figure 3
BH3-only proteins indirectly trigger BAX translocation, cyt c release, caspase activation, and apoptosis during TFD-induced neuronal cell death. (a) Four proposed models depicting how BH3-only proteins, such as BIM and HRK, may promote BAX translocation and its downstream sequelae during TFD in sympathetic and CG neurons. Models 1 and 2 invoke direct interaction of BH3 donors with BAX, while Models 3 and 4 involve BH3-only proteins indirectly triggering BAX translocation via inactivating interactions with antiapoptotic BCL-2 proteins. We favor Model 4 for reasons discussed in the text. (b) A dynamic equilibrium model for BAX translocation. During TFD in sympathetic and CG neurons, BIM and HRK are induced and inactivate antiapoptotic BCL-2 proteins, thereby altering the equilibrium between cytosolic and mitochondrial pools of BAX, culminating in apparent BAX translocation, multimerization, and integration, followed by cyt c release, caspase activation, and apoptotic cell death. See text for more details. Because this hypothesis implicitly suggests that all BH3-only proteins function similarly (at least in neurons), one potential caveat is that structural and/or functional subfamilies may exist within the BH3-only subfamily of proapoptotic proteins32,133
First (Model 1), BH3-only proteins may interact directly with BAX in the cytosol to trigger its translocation to mitochondria, where it integrates and mediates the release of apoptogenic intermembrane-space proteins, such as cyt c. Alternatively (Model 2), BH3-only proteins may interact directly with BAX at mitochondria to form a multiprotein complex that mediates cyt c release. We do not favor these models for several reasons. First, the tertiary structure of soluble, cytosolic BAX is inconsistent with BAX interacting with BH3-only proteins in the cytosol, arguing against Model 1. Specifically, the BH1 and BH2 domains, which presumably would mediate interactions with a ‘BH3 donor,’ are not accessible for such interactions.118 (Whether the ‘BH3-binding pocket’ formed by BH1 and BH2 becomes accessible upon integration into the outer mitochondrial membrane by BAX or BAK is unclear.) Second, to date, only three known or putative BH3-only proteins may be cytosolic: BID, BAD, and MAP-1. Although the physiological role of MAP-1 is unknown, deletion of neither BID nor BAD altered the time course or extent of TFD-induced cyt c release or apoptosis in sympathetic or CG neurons,51 arguing against Model 1 for these proteins. Therefore, neither tBID nor BID nor BAD appears to be the cytosolic trigger for BAX translocation and its downstream sequelae in SCG and CG neurons. Third, with the potential exception of BID (for which only overexpression data exist), no BH3-only proteins interact directly with BAX at endogenous levels in primary cells or tissues undergoing cell death, nor have any been co-precipitated with BAX multimers, although the possibility of a ‘hit-and-run’ model for BAX activation remains.119 Finally, deletion of Bim (and presumably Hrk) attenuates BAX-dependent cyt c release.47 However, neither protein appears to be cytosolic,17,47 which argues against Model 1, nor can interact directly with BAX,17,120 which argues against Models 1 and 2.
Next, BH3-only proteins may indirectly promote BAX-dependent cyt c release by interacting with and inactivating antiapoptotic BCL-2 proteins, either in the cytosol (Model 3) or at mitochondria (Model 4), thereby freeing BAX to multimerize and integrate, forming structures that release cyt c. Both models are generally consistent with the genetics and biochemistry of PCD in C. elegans in which the BH3-only protein EGL-1 is induced in cells destined to die and interacts with and inactivates an antiapoptotic BCL-2 protein, leading to apoptosome formation, caspase activation, and apoptosis. (As discussed above, an important caveat is the requirement for cyt c release in vertebrates, but not in worms.) However, we favor Model 4 for several reasons. First, antiapoptotic BCL-2 proteins may not interact directly with BAX in the cytosol, except in the presence of conformation-altering nonionic detergents,121,122 which may mimic a membrane environment, arguing against Model 3, but for Model 4. Second, the tertiary structure of BAX is inconsistent with interactions with antiapoptotic BCL-2 proteins in the cytosol,118 which argues against Model 3, but not Model 4. Third, BIM and HRK, two BH3-only proteins that we propose are critical for TFD-induced apoptosis in neurons, do not interact directly with BAX and appear to localize to mitochondria. Moreover, the proapoptotic activity of these proteins generally correlates with their ability to interact with, and inactivate, their antiapoptotic cousins.16,17,120 Finally, this hypothesis may help explain a ‘threshold effect’ observed with both BIM and HRK. Specifically, neurons can tolerate a certain level of BIM and HRK induction without releasing cyt c or undergoing cell death.47,50 For example, during TFD in _Bcl-2_-overexpressing sympathetic and CG neurons, BIM levels increase with time (to a point greater than that seen in wild type littermates) and then decline precipitously as cells begin to die; in contrast, in _Bax_−/− neurons, BIM levels continue to increase (to levels exceeding even those seen in _Bcl-2_-overexpressing cells) and only begin to decline days later when significant decreases in global macromolecular synthesis begin to dismantle the synthetic machinery needed to sustain the induction. Accordingly, overexpression of Bcl-2 only attenuates TFD-induced cyt c release and apoptosis in SCG and CG neurons,105,123 whereas Bax deletion completely prevents both.3,39,84,98 The same is true for CGN death caused by ectopic overexpression of BH3-only proteins.50,51,52
Therefore, we propose that cellular BAX exists in equilibrium between two states (Figure 3b), soluble in the cytosol or peripherally associated with mitochondria, in NGF-maintained sympathetic neurons. With TFD, BH3-only proteins are induced and localize to intracellular membranes, including the outer mitochondrial membrane, where they interact with and inactivate antiapoptotic BCL-2 proteins, thereby allowing BAX peripherally associated with mitochondria or translocated from the cytosol to multimerize and integrate. This creates a thermodynamic ‘sink’ that shifts the BAX equilibrium strongly (and perhaps irreversibly) in favor of mitochondrial localization, resulting in further translocation and integration into the mitochondrial outer membrane. In this scenario, inhibitors of de novo protein synthesis should prevent BAX translocation (consistent with our earlier findings105) by preventing induction of BH3-only proteins.47 Therefore, we suggest that BH3-only proteins are triggers, albeit indirect, for BAX translocation and its downstream sequelae during TFD-induced neuronal apoptosis. In this manner, vertebrate neurons resemble their nematode counterparts insofar as caspase activation requires transactivation of BH3-only proteins, which in turn inactivate antiapoptotic BCL-2 proteins, the principal differences being the addition of an intervening step involving BAX-dependent cyt c release, which is required for apoptosome formation, and BAX-dependent release of Smac/DIABLO, which inactivates an IAP-like activity.7,124
The Next Step: Neuronal Cell Death and Neurodegeneration
Over 30 years have passed since the term ‘apoptosis’ was coined by Kerr, Wyllie, and Currie1 to describe cell death in response to physiological or pathological stimuli that has a particular morphology first described by Glucksman in 1951. However, only in the past decade or so has the study of apoptosis literally exploded, with over 50 000 articles published between 1990 and 2000. The reason for this tremendous interest is obvious: Dysregulated cell death may at the very least contribute to, and at most be responsible for, the pathogenesis of many of our most intractable disorders, ranging from heart disease and stroke to autoimmunity and cancer. Few, if any, areas in modern medicine, at least in theory, are not touched by apoptosis research. Similarly, the promise of apoptosis research is equally clear: By manipulating the cell-suicide program, we can prevent unwanted cell death (e.g., in stroke) or, conversely, induce death in unwanted cells (e.g., in cancer). However, even though the last decade witnessed amazing progress in the identification and, in some cases, our understanding of the regulation of the molecular machinery of cell death, many questions remain at both the theoretical and practical levels.
What is ‘apoptosis?’
Kerr et al. 1 initially defined apoptosis purely in morphological terms. Today, with our armamentarium of biochemical and molecular assays, a cynic might suggest that the word means everything and nothing. Since the field truly came in vogue in the early 1990s, it sometimes seems that every cell death is ‘apoptotic.’ Accordingly, one long-time cell death researcher recently lamented that everything in the Sigma catalog has been shown to cause apoptosis. Even to a purist, what was once the ‘gold standard’ assay for apoptosis – ultrastructural analysis – has been diminished by results-driven, not hypothesis-driven, experimentation. Although some may dismiss such concerns as semantics, we respectfully disagree. Describing a particular cell death as ‘apoptotic’ is helpful if, and only if, such classification provides information about the mechanisms contributing to that death and (presumably) how to manipulate it. Accordingly, an operational definition of apoptosis that we favor is as follows: cell death with the morphological hallmarks described by Kerr et al.1 that requires caspase activity for those morphological features. (Note that this definition allows for caspase-independent cell death, as well as ‘apoptotic’ morphology without caspase activation, but caspase-independent apoptosis would be an oxymoron.) However, any definition of apoptosis (including ours) will likely be criticized by some as either too narrow or too broad; therefore, future work in cell death may be best served by carefully describing and characterizing the death caused by a particular stimulus by using multiple morphological and biochemical criteria.
Does the cell death in a particular degenerative disorder, such as Alzheimer's disease or stroke, contribute to the clinical manifestations of the disease and, if so, is this death apoptotic?
Unfortunately, the best answer we can obtain for this question may be a correlative one. Such a correlation could be, but has rarely been, established by appropriate answers to the following questions. First, does cell death precede the onset of clinical signs and symptoms? Second, does the induction of comparable cell death in a spatial and temporal pattern similar to that seen in the disease recapitulate these signs and symptoms? And third, does preventing cell death – necrotic, apoptotic, or otherwise – ameliorate these signs and symptoms? The answer to this last question leads directly into the next question…
Regardless of whether excessive apoptosis per se is the proximate cause of the disease, does preventing this cell death alone without altering the primary pathogenic mechanisms provide sufficient therapeutic benefit to outweigh potential adverse effects? For example, how will antiapoptotic agents be administered to prevent the death of certain cells without pathologically disrupting homeostasis in other cell populations? Conversely, in situations (e.g., cancer) characterized by insufficient apoptosis, how will proapoptotic agents be delivered to ensure the death of intended, but not unintended, targets?
An early and reasonable premise of cell death research was that issues related to the pathogenesis of various degenerative conditions could be largely circumvented because execution of the cell must proceed through a ‘final, common pathway’ mediated by BCL-2 proteins and caspases. Accordingly, in a murine model of amyotrophic lateral sclerosis (ALS), Bcl-2 overexpression125 or caspase inhibition126 delays disease onset, prolongs the ‘symptomatic’ period, and delays mortality. However, neither of these interventions prevents dysfunction or death; and, the delay in disease progression, while real (∼10–15%), may represent a modest extension of lifespan, quality of life concerns notwithstanding. At a cellular level, these observations probably reflect several realities: First, neuronal cell death per se, vis-à-vis neuronal dysfunction, may not be the primary, let alone the sole, pathogenic mechanism responsible for the clinical signs and symptoms of the disease. Second, cell death that contributes to the pathophysiology of such degenerative conditions may not be purely apoptotic. Third, modulation of the BCL-2 and caspase checkpoints alone will probably only provide temporary protection from cell death, especially since cells may progress from apoptotic to nonapoptotic (e.g., autophagic, necrotic, etc.) modes of cell death, particularly if the pathogenic insult remains unabated. By no stretch of the imagination is caspase inhibition as a monotherapy likely to be the ‘magic bullet’ for chronic neurodegenerative disorders because neurons ‘saved’ by caspase inhibitors alone suffer (and ultimately will die from) a significant mitochondrial ‘hit’ that lies genetically downstream of the BAX/BCL-2 checkpoint. This ‘hit,’ which includes not only loss of mitochondrial cyt c but also may involve activation of the cyclosporin A-inhibitable permeability transition pore,127 defines a ‘window of opportunity’ during which trophic (and/or stimulus-abating) agents may be used in combination with antiapoptotic strategies to rescue cells from the brink of the abyss. And finally, degenerating cells, even when saved ‘in perpetuity’ (e.g., NGF-deprived _Bax_−/− neurons) are likely to be functionally compromised,128 a particularly vexing problem with neurons since a live, but dysfunctional (e.g., ‘disconnected’ or inexcitable), neuron may be no better functionally to the organism than a dead one.
Furthermore, few studies have examined the adverse effects of chronic antiapoptotic treatments, which could, if not selectively targeted to specific tissues, cause multiple significant problems (e.g., oncogenesis and autoimmunity) since naturally occurring PCD is a critical homeostatic mechanism in all organisms. In any case, findings such as those described above for ALS, while encouraging, suggest that antiapoptotic strategies alone are unlikely to provide dramatic functional benefits to patients in the context of chronic degenerative disorders in the absence of therapies designed to address the underlying pathogenic mechanisms. In contrast, antiapoptotic treatments may provide considerable benefit in certain situations (e.g., stroke and myocardial infarction) in which the insult is acute, transient, and spatially well defined. In such situations, preventing cell death for a critical window of time may allow cells to ‘ride out’ the insult and, probably in combination with other approaches, re-establish a normal milieu. In other words, even in acute degenerative conditions in which antiapoptotic therapies may have their greatest clinical utility, optimal treatment will probably require combination therapies with trophic (and/or stimulus-abating) agents. Therein lies the promise of therapies, such as the MLK inhibitor CEP-1347, which not only prevents TFD-induced apoptosis but also promotes neuronal growth and differentiation, at least in model systems.129
In summary, the study of apoptosis has progressed remarkably in the past decade, thanks in large part to the pioneering efforts of Brenner, Sulston, and Horvitz in nematodes, and promises to mature further still in the post-genomics era. The next few decades should demonstrate whether the reality of therapies designed to modulate cell death for therapeutic benefit will achieve the promise articulated by their most ardent advocates, including ourselves.
Abbreviations
BH:
BCL-2 homology (domain)
CG:
cerebellar granule (neuron)
CREB:
cAMP response element binding protein
Cyt c:
cytochrome c
DRE:
downstream regulatory element
ERK:
extracellular-regulated kinase
FOXO:
forkhead box
HSN:
hermaphrodite-specific neuron
JAK:
janus kinase
JNK:
c-Jun NH2-terminal kinase
MAPK:
mitogen-activated protein kinase
MAPKK:
mitogen-activated protein kinase kinase
MAPKKK:
mitogen-activated protein kinase kinase kinase
MLK:
mixed lineage kinase
NSM:
neurosecretory motoneuron
NGF:
nerve growth factor
PI3 K:
phosphatidylinositol-3-kinase
PCD:
programmed cell death
SCG:
superior cervical ganglion
STAT:
signal transducer and activator of transcription
TFD:
trophic factor deprivation
TMD:
transmembrane domain
UTR:
untranslated region
VDAC:
voltage-dependent anion channel
References
- Kerr JF, Wyllie AH and Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26: 239–257
Article CAS PubMed PubMed Central Google Scholar - Zimmermann KC, Ricci JE, Droin NM and Green DR (2002) The role of ARK in stress-induced apoptosis in Drosophila cells. J. Cell Biol. 156: 1077–1087
CAS PubMed PubMed Central Google Scholar - Deshmukh M and Johnson Jr EM (1998) Evidence of a novel event during neuronal death: development of competence-to-die in response to cytoplasmic cytochrome c. Neuron 21: 695–705
CAS PubMed Google Scholar - Wiese S, Digby MR, Gunnersen JM, Gotz R, Pei G, Holtmann B, Lowenthal J and Sendtner M (1999) The anti-apoptotic protein ITA is essential for NGF-mediated survival of embryonic chick neurons. Nat. Neurosci. 2: 978–983
CAS PubMed Google Scholar - Stefanis L, Park DS, Friedman WJ and Greene LA (1999) Caspase-dependent and -independent death of camptothecin-treated embryonic cortical neurons. J. Neurosci. 19: 6235–6247
CAS PubMed PubMed Central Google Scholar - Deshmukh M and Johnson Jr EM (2000) Caspase inhibition extends the commitment to neuronal death beyond cytochrome c release to the point of mitochondrial depolarization. J. Cell Biol. 150: 131–143
CAS PubMed PubMed Central Google Scholar - Deshmukh M, Du C, Wang X and Johnson Jr EM (2002) Exogenous Smac induces competence and permits caspase activation in sympathetic neurons. J. Neurosci. 22: 8018–8027
CAS PubMed PubMed Central Google Scholar - Chang LK, Putcha GV, Deshmukh M and Johnson EM (2002) Mitochondrial involvement in the point of no return in neuronal apoptosis. Biochimie 84: 223–231
CAS PubMed Google Scholar - Chang LK, Schmidt RE and Johnson Jr EM (2003) Alternating metabolic pathways in NGF-deprived sympathetic neurons affect caspase-independent death. J. Cell Biol. 162: 245–256
CAS PubMed PubMed Central Google Scholar - Adams JM and Cory S (2001) Life-or-death decisions by the Bcl-2 protein family. Trends Biochem. Sci. 26: 61–66
CAS PubMed Google Scholar - Borner C, Martinou I, Mattmann C, Irmler M, Schaerer E, Martinou JC and Tschopp J (1994) The protein Bcl-2 alpha does not require membrane attachment, but two conserved domains to suppress apoptosis. J. Cell Biol. 126: 1059–1068
CAS PubMed Google Scholar - Borner C, Olivier R, Martinou I, Mattmann C, Tschopp J and Martinou JC (1994) Dissection of functional domains in Bcl-2 alpha by site-directed mutagenesis. Biochem. Cell Biol. 72: 463–469
CAS PubMed Google Scholar - Sedlak TW, Oltvai ZN, Yang E, Wang K, Boise LH, Thompson CB and Korsmeyer SJ (1995) Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc. Natl. Acad. Sci. USA 92: 7834–7838
CAS PubMed PubMed Central Google Scholar - Yin XM, Oltvai ZN and Korsmeyer SJ (1994) BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature 369: 321–323
CAS PubMed Google Scholar - Boyd JM, Gallo GJ, Elangovan B, Houghton AB, Malstrom S, Avery BJ, Ebb RG, Subramanian T, Chittenden T and Lutz RJ (1995) Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene 11: 1921–1928
CAS PubMed Google Scholar - Wang K, Yin XM, Chao DT, Milliman CL and Korsmeyer SJ (1996) Bid: a novel BH3 domain-only death agonist. Genes Dev. 10: 2859–2869
CAS PubMed Google Scholar - Inohara N, Ding LY, Chen S and Nunez G (1997) Harakiri, a novel regulator of cell death, encodes a protein that activates apoptosis and interacts selectively with survival-promoting proteins Bcl-2 and Bcl-X-L. EMBO J. 16: 1686–1694
CAS PubMed PubMed Central Google Scholar - Kelekar A, Chang BS, Harlan JE, Fesik SW and Thompson CB (1997) Bad is a BH3 domain-containing protein that forms an inactivating dimer with Bcl-X-L. Mol. Cell. Biol. 17: 7040–7046
CAS PubMed PubMed Central Google Scholar - Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, Thompson CB and Fesik SW (1997) Structure of Bcl-X-L-Bak peptide complex: recognition between regulators of apoptosis. Science 275: 983–986
CAS PubMed Google Scholar - Petros AM, Nettesheim DG, Wang Y, Olejniczak ET, Meadows RP, Mack J, Swift K, Matayoshi ED, Zhang H, Thompson CB and Fesik SW (2000) Rationale for Bcl-X-L/Bad peptide complex formation from structure, mutagenesis, and biophysical studies. Protein Sci. 9: 2528–2534
CAS PubMed PubMed Central Google Scholar - Liu X, Dai S, Zhu Y, Marrack P and Kappler JW (2003) The structure of a Bcl-X-L/Bim fragment complex: implications for Bim function. Immunity 19: 341–352
CAS PubMed Google Scholar - Oltvai ZN and Korsmeyer SJ (1994) Checkpoints of dueling dimers foil death wishes. Cell 79: 189–192
CAS PubMed Google Scholar - Knudson CM and Korsmeyer SJ (1997) Bcl-2 and Bax function independently to regulate cell death. Nat. Genet. 16: 358–363
CAS PubMed Google Scholar - Otter I, Conus S, Ravn U, Rager M, Olivier R, Monney L, Fabbro D and Borner C (1998) The binding properties and biological activities of Bcl-2 and Bax in cells exposed to apoptotic stimuli. J. Biol. Chem. 273: 6110–6120
CAS PubMed Google Scholar - Middleton G, Cox SW, Korsmeyer S and Davies AM (2000) Differences in Bcl-2- and Bax-independent function in regulating apoptosis in sensory neuron populations. Eur. J. Neurosci. 12: 819–827
CAS PubMed Google Scholar - Muchmore SW, Sattler M, Liang H, Meadows RP, Harlan JE, Yoon HS, Nettesheim D, Chang BS, Thompson CB, Wong SL, Ng SL and Fesik SW (1996) X-ray and NMR structure of human Bcl-X-L, an inhibitor of programmed cell death. Nature 381: 335–341
CAS PubMed Google Scholar - Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod JJ, Mazzei G, Maundrell K, Gambale F, Sadoul R and Martinou JC (1997) Inhibition of Bax channel-forming activity by Bcl-2. Science 277: 370–372
CAS PubMed Google Scholar - Minn AJ, Velez P, Schendel SL, Liang H, Muchmore SW, Fesik SW, Fill M and Thompson CB (1997) Bcl-X-L forms an ion channel in synthetic lipid membranes. Nature 385: 353–357
CAS PubMed Google Scholar - Schendel SL, Xie Z, Montal MO, Matsuyama S, Montal M and Reed JC (1997) Channel formation by antiapoptotic protein Bcl-2. Proc. Natl. Acad. Sci. USA 94: 5113–5118
CAS PubMed PubMed Central Google Scholar - Schlesinger PH, Gross A, Yin XM, Yamamoto K, Saito M, Waksman G and Korsmeyer SJ (1997) Comparison of the ion channel characteristics of proapoptotic Bax and antiapoptotic Bcl-2. Proc. Natl. Acad. Sci. USA 94: 11357–11362
CAS PubMed PubMed Central Google Scholar - Cheng EH, Sheiko TV, Fisher JK, Craigen WJ and Korsmeyer SJ (2003) VDAC2 inhibits Bak activation and mitochondrial apoptosis. Science 301: 513–517
CAS PubMed Google Scholar - Sugiyama T, Shimizu S, Matsuoka Y, Yoneda Y and Tsujimoto Y (2002) Activation of mitochondrial voltage-dependent anion channel by a pro-apoptotic BH3-only protein Bim. Oncogene 21: 4944–4956
CAS PubMed Google Scholar - Basanez G, Sharpe JC, Galanis J, Brandt TB, Hardwick JM and Zimmerberg J (2002) Bax-type apoptotic proteins porate pure lipid bilayers through a mechanism sensitive to intrinsic monolayer curvature. J. Biol. Chem. 277: 49360–49365
CAS PubMed Google Scholar - Coughlin MD and Collins MB (1985) Nerve growth factor-independent development of embryonic mouse sympathetic neurons in dissociated cell culture. Dev. Biol. 110: 392–401
CAS PubMed Google Scholar - Levi-Montalcini R and Booker B (1960) Destruction of the sympathetic ganglia in mammals by an antiserum to the nerve growth-promoting factor. Proc. Natl. Acad. Sci. USA 46: 384–391
CAS PubMed PubMed Central Google Scholar - Deckwerth TL and Johnson Jr EM (1993) Temporal analysis of events associated with programmed cell death (apoptosis) of sympathetic neurons deprived of nerve growth factor. J. Cell Biol. 123: 1207–1222
CAS PubMed Google Scholar - Edwards SN, Buckmaster AE and Tolkovsky AM (1991) The death programme in cultured sympathetic neurones can be suppressed at the posttranslational level by nerve growth factor, cyclic AMP, and depolarization. J. Neurochem. 57: 2140–2143
CAS PubMed Google Scholar - Martin DP, Schmidt RE, DiStefano PS, Lowry OH, Carter JG and Johnson Jr EM (1988) Inhibitors of protein synthesis and RNA synthesis prevent neuronal death caused by nerve growth factor deprivation. J. Cell Biol. 106: 829–844
CAS PubMed Google Scholar - Deckwerth TL, Elliott JL, Knudson CM, Johnson EM, Snider WD and Korsmeyer SJ (1996) Bax is required for neuronal death after trophic factor deprivation and during development. Neuron 17: 401–411
CAS PubMed Google Scholar - Deshmukh M, Vasilakos J, Deckwerth TL, Lampe PA, Shivers BD and Johnson Jr EM (1996) Genetic and metabolic status of NGF-deprived sympathetic neurons saved by an inhibitor of ICE family proteases. J. Cell Biol. 135: 1341–1354
CAS PubMed Google Scholar - McCarthy MJ, Rubin LL and Philpott KL (1997) Involvement of caspases in sympathetic neuron apoptosis. J. Cell Sci. 110: 2165–2173
CAS PubMed Google Scholar - Troy CM, Stefanis L, Prochiantz A, Greene LA and Shelanski ML (1996) The contrasting roles of ICE family proteases and interleukin-1-β in apoptosis induced by trophic factor withdrawal and by copper/zinc superoxide dismutase down-regulation. Proc. Natl. Acad. Sci. USA 93: 5635–5640
CAS PubMed PubMed Central Google Scholar - Koike T, Martin DP and Johnson Jr EM (1989) Role of Ca2+ channels in the ability of membrane depolarization to prevent neuronal death induced by trophic-factor deprivation: evidence that levels of internal Ca2+ determine nerve growth factor dependence of sympathetic ganglion cells. Proc. Natl. Acad. Sci. USA 86: 6421–6425
CAS PubMed PubMed Central Google Scholar - Rydel RE and Greene LA (1988) cAMP analogs promote survival and neurite outgrowth in cultures of rat sympathetic and sensory neurons independently of nerve growth factor. Proc. Natl. Acad. Sci. USA 85: 1257–1261
CAS PubMed PubMed Central Google Scholar - Johnson Jr EM, Chang JY, Koike T and Martin DP (1989) Why do neurons die when deprived of trophic factor? Neurobiol. Aging 10: 549–552
PubMed Google Scholar - Conradt B and Horvitz HR (1998) The C. elegans protein EGL-1 is required for programmed cell death and interacts with the Bcl-2-like protein Ced-9. Cell 93: 519–529
CAS PubMed Google Scholar - Putcha GV, Moulder KL, Golden JP, Bouillet P, Adams JA, Strasser A and Johnson EM (2001) Induction of Bim, a proapoptotic BH3-only Bcl-2 family member, is critical for neuronal apoptosis. Neuron 29: 615–628
CAS PubMed Google Scholar - Whitfield J, Neame SJ, Paquet L, Bernard O and Ham J (2001) Dominant-negative c-Jun promotes neuronal survival by reducing Bim expression and inhibiting mitochondrial cytochrome c release. Neuron 29: 629–643
CAS PubMed Google Scholar - Imaizumi K, Tsuda M, Imai Y, Wanaka A, Takagi T and Tohyama M (1997) Molecular cloning of a novel polypeptide, Dp5, induced during programmed neuronal death. J. Biol. Chem. 272: 18842–18848
CAS PubMed Google Scholar - Harris CA and Johnson Jr EM (2001) BH3-only Bcl-2 family members are coordinately regulated by the Jnk pathway and require Bax to induce apoptosis in neurons. J. Biol. Chem. 276: 37754–37760
CAS PubMed Google Scholar - Putcha GV, Harris CA, Moulder KL, Easton RM, Thompson CB and Johnson Jr EM (2002) Intrinsic and extrinsic pathway signaling during neuronal apoptosis: Lessons from the analysis of mutant mice. J. Cell Biol. 157: 441–453
CAS PubMed PubMed Central Google Scholar - Putcha GV, Le S, Frank S, Besirli CG, Clark K, Chu B, Alix S, Youle RJ, LaMarche A, Maroney AC and Johnson Jr EM (2003) Jnk-mediated Bim phosphorylation potentiates Bax-dependent apoptosis. Neuron 38: 899–914
CAS PubMed Google Scholar - Conradt B and Horvitz HR (1999) The TRA-1A sex determination protein of C. elegans regulates sexually dimorphic cell deaths by repressing the Egl-1 cell death activator gene. Cell 98: 317–327
CAS PubMed Google Scholar - Metzstein MM and Horvitz HR (1999) The C. elegans cell death specification gene ces-1 encodes a snail family zinc finger protein. Mol. Cell 4: 309–319
CAS PubMed Google Scholar - Thellmann M, Hatzold J and Conradt B (2003) The Snail-like Ces-1 protein of C. elegans can block the expression of the BH3-only cell-death activator gene Egl-1 by antagonizing the function of bHLH proteins. Development 130: 4057–4071
CAS PubMed Google Scholar - Horvitz HR (2003) Worms, life, and death (Nobel Lecture). Chembiochem 4: 697–711
CAS PubMed Google Scholar - Lonze BE and Ginty DD (2002) Function and regulation of CREB family transcription factors in the nervous system. Neuron 35: 605–623
CAS PubMed Google Scholar - Maggirwar SB, Sarmiere PD, Dewhurst S and Freeman RS (1998) Nerve growth factor-dependent activation of NF-kappaB contributes to survival of sympathetic neurons. J. Neurosci. 18: 10356–10365
CAS PubMed PubMed Central Google Scholar - Sarmiere PD and Freeman RS (2001) Analysis of the NF-kappa B and PI 3-kinase/Akt survival pathways in nerve growth factor-dependent neurons. Mol. Cell. Neurosci. 18: 320–331
CAS PubMed Google Scholar - Creedon DJ, Johnson EM and Lawrence JC (1996) Mitogen-activated protein kinase-independent pathways mediate the effects of nerve growth factor and cAMP on neuronal survival. J. Biol. Chem. 271: 20713–20718
CAS PubMed Google Scholar - Virdee K and Tolkovsky AM (1996) Inhibition of p42 and p44 mitogen-activated protein kinase activity by PD98059 does not suppress nerve growth factor-induced survival of sympathetic neurones. J. Neurochem. 67: 1801–1805
CAS PubMed Google Scholar - Crowder RJ and Freeman RS (1998) Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and sufficient for the survival of nerve growth factor-dependent sympathetic neurons. J. Neurosci. 18: 2933–2943
CAS PubMed PubMed Central Google Scholar - Philpott KL, McCarthy MJ, Klippel A and Rubin LL (1997) Activated phosphatidylinositol 3-kinase and Akt kinase promote survival of superior cervical neurons. J. Cell Biol. 139: 809–815
CAS PubMed PubMed Central Google Scholar - Tsui-Pierchala BA, Putcha GV and Johnson Jr EM (2000) Phosphatidylinositol 3-kinase is required for the trophic, but not the survival-promoting, actions of NGF on sympathetic neurons. J. Neurosci. 20: 7228–7237
CAS PubMed PubMed Central Google Scholar - Estus S, Zaks WJ, Freeman RS, Gruda M, Bravo R and Johnson Jr EM (1994) Altered gene expression in neurons during programmed cell death: identification of c-jun as necessary for neuronal apoptosis. J. Cell Biol. 127: 1717–1727
CAS PubMed Google Scholar - Ham J, Babij C, Whitfield J, Pfarr CM, Lallemand D, Yaniv M and Rubin LL (1995) A c-Jun dominant negative mutant protects sympathetic neurons against programmed cell death. Neuron 14: 927–939
CAS PubMed Google Scholar - Eilers A, Whitfield J, Babij C, Rubin LL and Ham J (1998) Role of the Jun kinase pathway in the regulation of c-Jun expression and apoptosis in sympathetic neurons. J. Neurosci. 18: 1713–1724
CAS PubMed PubMed Central Google Scholar - Dijkers PF, Medema RH, Lammers JW, Koenderman L and Coffer PJ (2000) Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr. Biol. 10: 1201–1204
CAS PubMed Google Scholar - Dijkers PF, Birkenkamp KU, Lam EW, Thomas NS, Lammers JW, Koenderman L and Coffer PJ (2002) FKHR-L1 can act as a critical effector of cell death induced by cytokine withdrawal: protein kinase B-enhanced cell survival through maintenance of mitochondrial integrity. J. Cell Biol. 156: 531–542
CAS PubMed PubMed Central Google Scholar - Bouillet P, Zhang LC, Huang DC, Webb GC, Bottema CD, Shore P, Eyre HJ, Sutherland GR and Adams JM (2001) Gene structure alternative splicing, and chromosomal localization of pro-apoptotic Bcl-2 relative Bim. Mamm. Genome 12: 163–168
CAS PubMed Google Scholar - Sanz C, Mellstrom B, Link WA, Naranjo JR and Fernandez-Luna JL (2001) Interleukin 3-dependent activation of DREAM is involved in transcriptional silencing of the apoptotic Hrk gene in hematopoietic progenitor cells. EMBO J. 20: 2286–2292
CAS PubMed PubMed Central Google Scholar - Gilley J, Coffer PJ and Ham J (2003) FOXO transcription factors directly activate Bim gene expression and promote apoptosis in sympathetic neurons. J. Cell Biol. 162: 613–622
CAS PubMed PubMed Central Google Scholar - Ley R, Balmanno K, Hadfield K, Weston C and Cook SJ (2003) Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J. Biol. Chem. 278: 18811–18816
CAS PubMed Google Scholar - Reginato MJ, Mills KR, Paulus JK, Lynch DK, Sgroi DC, Debnath J, Muthuswamy SK and Brugge JS (2003) Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat. Cell Biol. 5: 733–740
CAS PubMed Google Scholar - Weston CR, Balmanno K, Chalmers C, Hadfield K, Molton SA, Ley R, Wagner EF and Cook SJ (2003) Activation of ERK1/2 by deltaRaf-1:ER* represses Bim expression independently of the JNK or PI3K pathways. Oncogene 22: 1281–1293
CAS PubMed Google Scholar - Shinjyo T, Kuribara R, Inukai T, Hosoi H, Kinoshita T, Miyajima A, Houghton PJ, Look AT, Ozawa K and Inaba T (2001) Downregulation of Bim, a proapoptotic relative of Bcl-2, is a pivotal step in cytokine-initiated survival signaling in murine hematopoietic progenitors. Mol. Cell. Biol. 21: 854–864
CAS PubMed PubMed Central Google Scholar - Lei K and Davis RJ (2003) Jnk phosphorylation of Bim-related members of the Bcl-2 family induces Bax-dependent apoptosis. Proc. Natl. Acad. Sci. USA 100: 2432–2437
CAS PubMed PubMed Central Google Scholar - Biswas SC and Greene LA (2002) Nerve growth factor (NGF) down-regulates the Bcl-2 homology 3 (BH3) domain-only protein Bim and suppresses its proapoptotic activity by phosphorylation. J. Biol. Chem. 277: 49511–49516
CAS PubMed Google Scholar - Liu DX and Greene LA (2001) Regulation of neuronal survival and death by E2F-dependent gene repression and derepression. Neuron 32: 425–438
CAS PubMed Google Scholar - Crowder RJ and Freeman RS (1999) The survival of sympathetic neurons promoted by potassium depolarization, but not by cyclic AMP, requires phosphatidylinositol 3- kinase and Akt. J. Neurochem. 73: 466–475
CAS PubMed Google Scholar - Vaillant AR, Mazzoni I, Tudan C, Boudreau M, Kaplan DR and Miller FD (1999) Depolarization and neurotrophins converge on the phosphatidylinositol 3- kinase-Akt pathway to synergistically regulate neuronal survival. J. Cell Biol. 146: 955–966
CAS PubMed PubMed Central Google Scholar - Miller TM, Tansey MG, Johnson Jr EM and Creedon DJ (1997) Inhibition of phosphatidylinositol 3-kinase activity blocks depolarization- and insulin-like growth factor I-mediated survival of cerebellar granule cells. J. Biol. Chem. 272: 9847–9853
CAS PubMed Google Scholar - Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR and Greenberg ME (1997) Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 275: 661–665
CAS PubMed Google Scholar - Miller TM, Moulder KL, Knudson CM, Creedon DJ, Deshmukh M, Korsmeyer SJ and Johnson Jr EM (1997) Bax deletion further orders the cell death pathway in cerebellar granule cells and suggests a caspase-independent pathway to cell death. J. Cell Biol. 139: 205–217
CAS PubMed PubMed Central Google Scholar - Lonze BE, Riccio A, Cohen S and Ginty DD (2002) Apoptosis, axonal growth defects, and degeneration of peripheral neurons in mice lacking CREB. Neuron 34: 371–385
CAS PubMed Google Scholar - Riccio A, Ahn S, Davenport CM, Blendy JA and Ginty DD (1999) Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science 286: 2358–2361
CAS PubMed Google Scholar - Greenlund LJ, Korsmeyer SJ and Johnson Jr EM (1995) Role of Bcl-2 in the survival and function of developing and mature sympathetic neurons. Neuron 15: 649–661
CAS PubMed Google Scholar - Michaelidis TM, Sendtner M, Cooper JD, Airaksinen MS, Holtmann B, Meyer M and Thoenen H (1996) Inactivation of Bcl-2 results in progressive degeneration of motoneurons, sympathetic and sensory neurons during early postnatal development. Neuron 17: 75–89
CAS PubMed Google Scholar - Motoyama N, Wang F, Roth KA, Sawa H, Nakayama K, Negishi I, Senju S, Zhang Q and Fujii S (1995) Massive cell death of immature hematopoietic cells and neurons in Bcl-X-deficient mice. Science 267: 1506–1510
CAS PubMed Google Scholar - Shindler KS, Latham CB and Roth KA (1997) Bax deficiency prevents the increased cell death of immature neurons in Bcl-X-deficient mice. J. Neurosci. 17: 3112–3119
CAS PubMed PubMed Central Google Scholar - Buettner R, Mora LB and Jove R (2002) Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin. Cancer Res. 8: 945–954
CAS PubMed Google Scholar - Mayr B and Montminy M (2001) Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell Biol. 2: 599–609
CAS PubMed Google Scholar - Chen C, Edelstein LC and Gelinas C (2000) The Rel/NF-kappaB family directly activates expression of the apoptosis inhibitor Bcl-X-L. Mol. Cell. Biol. 20: 2687–2695
PubMed PubMed Central Google Scholar - Zong WX, Edelstein LC, Chen C, Bash J and Gelinas C (1999) The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappaB that blocks TNFalpha-induced apoptosis. Genes Dev. 13: 382–387
CAS PubMed PubMed Central Google Scholar - Chu ZL, McKinsey TA, Liu L, Gentry JJ, Malim MH and Ballard DW (1997) Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc. Natl. Acad. Sci. USA 94: 10057–10062
CAS PubMed PubMed Central Google Scholar - Wang CY, Mayo MW, Korneluk RG, Goeddel DV and Baldwin Jr AS (1998) NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281: 1680–1683
CAS PubMed Google Scholar - Sun W, Gould TW, Vinsant S, Prevette D and Oppenheim RW (2003) Neuromuscular development after the prevention of naturally occurring neuronal death by Bax deletion. J. Neurosci. 23: 7298–7310
CAS PubMed PubMed Central Google Scholar - Putcha GV, Deshmukh M and Johnson Jr EM (2000) Inhibition of apoptotic signaling cascades causes loss of trophic factor dependence during neuronal maturation. J. Cell Biol. 149: 1011–1018
CAS PubMed PubMed Central Google Scholar - Knudson CM, Tung KS, Tourtellotte WG, Brown GA and Korsmeyer SJ (1995) Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science 270: 96–99
CAS PubMed Google Scholar - Lindsten T, Ross AJ, King A, Zong W, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR and Thompson CB (2000) The combined functions of proapoptotic BCL-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol. Cell 6: 1389–1399
CAS PubMed PubMed Central Google Scholar - Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB and Korsmeyer SJ (2001) Proapoptotic Bax and Bak: a requisite gateway to mitochondrial dysfunction and death. Science 292: 727–730
CAS PubMed PubMed Central Google Scholar - White FA, Kellerpeck CR, Knudson CM, Korsmeyer SJ and Snider WD (1998) Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. J. Neurosci. 18: 1428–1439
CAS PubMed PubMed Central Google Scholar - Aloyz RS, Bamji SX, Pozniak CD, Toma JG, Atwal J, Kaplan DR and Miller FD (1998) p53 is essential for developmental neuron death as regulated by the TrkA and p75 neurotrophin receptors. J. Cell Biol. 143: 1691–1703
CAS PubMed PubMed Central Google Scholar - Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B and Martinou J-C (1999) Bid-induced conformational change in Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol. 144: 891–901
CAS PubMed PubMed Central Google Scholar - Putcha GV, Deshmukh M and Johnson Jr EM (1999) Bax translocation is a critical event in neuronal apoptosis: regulation by neuroprotectants, Bcl-2, and caspases. J. Neurosci. 19: 7476–7485
CAS PubMed PubMed Central Google Scholar - McGinnis KM, Gnegy ME and Wang KK (1999) Endogenous Bax translocation in SH-SY5Y human neuroblastoma cells and cerebellar granule neurons undergoing apoptosis. J. Neurochem. 72: 1899–1906
CAS PubMed Google Scholar - Li L, Oppenheim RW and Milligan CE (2001) Characterization of the execution pathway of developing motoneurons deprived of trophic support. J. Neurobiol. 46: 249–264
CAS PubMed Google Scholar - Besirli CG, Deckwerth TL, Crowder RJ, Freeman RS and Johnson Jr EM (2003) Cytosine arabinoside rapidly activates Bax-dependent apoptosis and a delayed Bax-independent death pathway in sympathetic neurons. Cell Death Differ. 10: 1045–1058
CAS PubMed Google Scholar - Davies AM and Rosenthal A (1994) Neurons from mouse embryos with a null mutation in the tumour suppressor gene p53 undergo normal cell death in the absence of neurotrophins. Neurosci. Lett. 182: 112–114
CAS PubMed Google Scholar - Sadoul R, Quiquerez AL, Martinou I, Fernandez PA and Martinou JC (1996) p53 protein in sympathetic neurons: cytoplasmic localization and no apparent function in apoptosis. J. Neurosci. Res. 43: 594–601
CAS PubMed Google Scholar - Martinou I, Fernandez PA, Missotten M, White E, Allet B, Sadoul R and Martinou JC (1995) Viral proteins E1B19 K and p35 protect sympathetic neurons from cell death induced by NGF deprivation. J. Cell Biol. 128: 201–208
CAS PubMed Google Scholar - Chen RW, Saunders PA, Wei H, Li Z, Seth P and Chuang DM (1999) Involvement of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and p53 in neuronal apoptosis: evidence that GAPDH is upregulated by p53. J. Neurosci. 19: 9654–9662
CAS PubMed PubMed Central Google Scholar - Enokido Y, Araki T, Tanaka K, Aizawa S and Hatanaka H (1996) Involvement of p53 in DNA strand break-induced apoptosis in postmitotic CNS neurons. Eur. J. Neurosci. 8: 1812–1821
CAS PubMed Google Scholar - Enokido Y, Araki T, Aizawa S and Hatanaka H (1996) p53 involves cytosine arabinoside-induced apoptosis in cultured cerebellar granule neurons. Neurosci. Lett. 203: 1–4
CAS PubMed Google Scholar - Morris EJ, Keramaris E, Rideout HJ, Slack RS, Dyson NJ, Stefanis L and Park DS (2001) Cyclin-dependent kinases and p53 pathways are activated independently and mediate Bax activation in neurons after DNA damage. J. Neurosci. 21: 5017–5026
CAS PubMed PubMed Central Google Scholar - Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, Hoffman B and Reed JC (1994) Tumor suppressor p53 is a regulator of Bcl-2 and Bax gene expression in vitro and in vivo. Oncogene 9: 1799–1805
CAS PubMed Google Scholar - Neame SJ, Rubin LL and Philpott KL (1998) Blocking cytochrome c activity within intact neurons inhibits apoptosis. J. Cell Biol. 142: 1583–1593
CAS PubMed PubMed Central Google Scholar - Suzuki M, Youle RJ and Tjandra N (2000) Structure of Bax: coregulation of dimer formation and intracellular localization. Cell 103: 645–654
CAS PubMed Google Scholar - Sundararajan R and White E (2001) E1B 19K blocks Bax oligomerization and tumor necrosis factor alpha-mediated apoptosis. J. Virol. 75: 7506–7516
CAS PubMed PubMed Central Google Scholar - O’Connor L, Strasser A, O'Reilly LA, Hausmann G, Adams JM, Cory S and Huang DC (1998) BIM: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 17: 384–395
PubMed PubMed Central Google Scholar - Hsu YT and Youle RJ (1997) Nonionic detergents induce dimerization among members of the Bcl-2 family. J. Biol. Chem. 272: 13829–13834
CAS PubMed Google Scholar - Antonsson B, Montessuit S, Sanchez B and Martinou JC (2001) Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem. 276: 11615–11623
CAS PubMed Google Scholar - Tanabe H, Eguchi Y, Shimizu S, Martinou JC and Tsujimoto Y (1998) Death-signalling cascade in mouse cerebellar granule neurons. Eur. J. Neurosci. 10: 1403–1411
CAS PubMed Google Scholar - Yamaguchi H, Bhalla K and Wang HG (2003) Bax plays a pivotal role in thapsigargin-induced apoptosis of human colon cancer HCT116 cells by controlling Smac/Diablo and Omi/HtrA2 release from mitochondria. Cancer Res. 63: 1483–1489
CAS PubMed Google Scholar - Kostic V, Jackson-Lewis V, de Bilbao F, Dubois-Dauphin M and Przedborski S (1997) Bcl-2: prolonging life in a transgenic mouse model of familial amyotrophic lateral sclerosis. Science 277: 559–562
CAS PubMed Google Scholar - Li MW, Ona VO, Guegan C, Chen MH, Jackson-Lewis V, Andrews LJ, Olszewski AJ, Stieg PE, Lee JP, Przedborski S and Friedlander RM (2000) Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science 288: 335–339
CAS PubMed Google Scholar - Chang LK and Johnson Jr EM (2002) Cyclosporin A inhibits caspase-independent death of NGF-deprived sympathetic neurons: a potential role for mitochondrial permeability transition. J. Cell Biol. 157: 771–781
CAS PubMed PubMed Central Google Scholar - Werth JL, Deshmukh M, Cocabo J, Johnson EM and Rothman SM (2000) Reversible physiological alterations in sympathetic neurons deprived of NGF but protected from apoptosis by caspase inhibition or Bax deletion. Exp. Neurol. 161: 203–211
CAS PubMed Google Scholar - Harris CA, Deshmukh M, Tsui-Pierchala B, Maroney AC and Johnson Jr EM (2002) Inhibition of the c-Jun N-terminal kinase signaling pathway by the mixed lineage kinase inhibitor CEP-1347 (KT7515) preserves metabolism and growth of trophic factor-deprived neurons. J. Neurosci. 22: 103–113
CAS PubMed PubMed Central Google Scholar - Chen F, Hersh BM, Conradt B, Zhou Z, Riemer D, Gruenbaum Y and Horvitz HR (2000) Translocation of C. elegans Ced-4 to nuclear membranes during programmed cell death. Science 287: 1485–1489
CAS PubMed Google Scholar - Aravind L, Dixit VM and Koonin EV (2001) Apoptotic molecular machinery: vastly increased complexity in vertebrates revealed by genome comparisons. Science 291: 1279–1284
CAS PubMed Google Scholar - Tran H, Brunet A, Griffith EC and Greenberg ME (2003) The many forks in FOXO's road. Science STKE 2003: RE5
Google Scholar - Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S and Korsmeyer SJ (2002) Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2: 183–192
CAS PubMed Google Scholar
Acknowledgements
This work was supported by National Institutes of Health Grants R37AG-12947 and RO1NS38651 (EMJ). We thank D Ginty for helpful comments, PA Osborne for assistance with copy-editing, M Bloomgren for secretarial assistance, and members of the Johnson lab for their critical review of this manuscript. We apologize to our colleagues whose work was not discussed or cited due to length restrictions.
Author information
Author notes
- G V Putcha
Present address: Department of Pathology, Stanford University School of Medicine, 300 Pasteur Drive, L235, Stanford, CA, 94305-5324, USA
Authors and Affiliations
- Departments of Neurology and Molecular Biology & Pharmacology, Washington University School of Medicine, Saint Louis, MO, USA
G V Putcha & E M Johnson Jr
Authors
- G V Putcha
You can also search for this author inPubMed Google Scholar - E M Johnson Jr
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toE M Johnson Jr.
Additional information
Edited by G Melino
From Punch Magazine, 1882 (and H.R. Horvitz's Nobel lecture, 8 December 2002)
Rights and permissions
About this article
Cite this article
Putcha, G., Johnson, E. ‘Men are but worms:’ neuronal cell death in C. elegans and vertebrates.Cell Death Differ 11, 38–48 (2004). https://doi.org/10.1038/sj.cdd.4401352
- Received: 06 October 2003
- Accepted: 13 October 2003
- Published: 28 November 2003
- Issue Date: 01 January 2004
- DOI: https://doi.org/10.1038/sj.cdd.4401352