Mechanisms linking dietary fiber, gut microbiota and colon cancer prevention (original) (raw)
Minireviews Open Access
Copyright ©2014 Baishideng Publishing Group Co., Limited. All rights reserved.
World J Gastrointest Oncol. Feb 15, 2014; 6(2): 41-51
Published online Feb 15, 2014. doi: 10.4251/wjgo.v6.i2.41
Mechanisms linking dietary fiber, gut microbiota and colon cancer prevention
Huawei Zeng, United States Department of Agriculture, Agricultural Research Service, Grand Forks Human Nutrition Research Center, Grand Forks, ND 58203, United States
Darina L Lazarova, Michael Bordonaro, Department of Basic Sciences, The Commonwealth Medical College, Scranton, PA 18509, United States
ORCID number: $[AuthorORCIDs]
Author contributions: Zeng H conceived the topic, contributed to the writing and revising, and provided overall design and execution of the manuscript; Lazarova DL and Bordonaro M contributed to the writing and revising the manuscript.
Supported by The United States Department of Agriculture
Correspondence to: Huawei Zeng, PhD, United States Department of Agriculture, Agricultural Research Service, Grand Forks Human Nutrition Research Center, 2420 2nd Ave. North, Grand Forks, ND 58203, United States. huawei.zeng@ars.usda.gov
Telephone: +1-701-7958465 Fax: +1-701-7958220
Received: November 25, 2013
Revised: December 27, 2013
Accepted: January 15, 2014
Published online: February 15, 2014
Processing time: 82 Days and 7 Hours
Abstract
Many epidemiological and experimental studies have suggested that dietary fiber plays an important role in colon cancer prevention. These findings may relate to the ability of fiber to reduce the contact time of carcinogens within the intestinal lumen and to promote healthy gut microbiota, which modifies the host’s metabolism in various ways. Elucidation of the mechanisms by which dietary fiber-dependent changes in gut microbiota enhance bile acid deconjugation, produce short chain fatty acids, and modulate inflammatory bioactive substances can lead to a better understanding of the beneficial role of dietary fiber. This article reviews the current knowledge concerning the mechanisms via which dietary fiber protects against colon cancer.
Core tip: Dietary fiber modulates our health at nearly every level, and in every organ system, via complicated modes of action. This article reviews the mechanistic association of dietary fiber, gut microbiota and colon cancer prevention.
- Citation: Zeng H, Lazarova DL, Bordonaro M. Mechanisms linking dietary fiber, gut microbiota and colon cancer prevention. World J Gastrointest Oncol 2014; 6(2): 41-51
- URL: https://www.wjgnet.com/1948-5204/full/v6/i2/41.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v6.i2.41
INTRODUCTION
Colon cancer is one of the most common malignancies in the United States and accounts yearly for approximately 11% of all cancer deaths[1]. The incidence rates of colon cancer are higher in the Western world but are rapidly increasing in developing countries, and it is predicted that half of the Western population will develop at least one colorectal tumor by age of 70[1]. Although cancer treatments have made large strides in recent decades, prevention by diet and other healthy lifestyle factors and habits (e.g., physical exercise) offers a more desirable alternative. Genetic variation and environmental exposures (e.g., diet, physical activity), including diet, are the two main contributing factors influencing the occurrence of colon cancer[2]. Thus, colon cancer may be highly amenable to prevention through a dietary regimen, and dietary carbohydrates may play a critical role[3]. Carbohydrates can be separated into two basic groups based upon their digestibility in the gastrointestinal (GI) tract[4,5]. The first group is simple carbohydrates such as starch and simple sugars, which are easily hydrolyzed by enzymatic reactions and absorbed in the small intestine. The second group is composed of complex carbohydrates such as cellulose, lignin and pectin which are resistant to digestion in the small intestine and undergo bacterial fermentation in the colon. These complex carbohydrates, referred to as dietary fibers, are found in plants[4,5]. Many studies suggest that there is an association between high dietary fiber intake and a low incidence of colon cancer, and that dietary fiber has anticancer properties[6-8]. Furthermore, the US Food and Drug Administration has approved health claims supporting the role of dietary fiber in cancer prevention[9].
It is known that the human GI tract represents the most abundant reservoir of microbes with over 100 trillion bacteria grouped in about 1000 species[10,11]. The bacterial gut populations can be shifted to a healthier composition by fermentable dietary fiber that provides substrates for bacterial fermentation[10,11]. Dietary fiber decreases the risk for type 2 diabetes mellitus, obesity, cardiovascular disease, colon cancer, and improves immunity by modulating the gut microbiota landscape[6]. Dietary fiber modulates our health at nearly every level, and in every organ system, via complicated modes of action, many of which remain to be determined[10,11]. In the present review, we focus on the mechanistic association of dietary fiber, gut microbiota and colon cancer prevention.
IMPACT OF DIETARY FIBER ON GUT MICROBIOTA
Dietary fiber constitutes a spectrum of non-digestible food ingredients including non-starch polysaccharides, oligosaccharides, lignin, and analogous polysaccharides with an associated health benefit[12,13]. Dietary fibers are not a static collection of undigestible plant materials that pass through the human GI tract without any function; instead, they bind potential nutrients, result in new metabolites, and modulate nutrient absorption/metabolism. Certain dietary fibers are fermentable, and in addition to their anaerobic degradation in the GI tract, there is also a concurrent anaerobic proteolytic fermentation[14]. Whereas the main fermentation products of fiber are thought to be beneficial (positive), the products of the proteolytic fermentation can be detrimental (negative), resulting in a ying-yang effect[14]. In healthy individuals, fermentation processes are primarily controlled by the amount and type of substrates accessible to bacteria in the colonic ecosystem[11]. The fate of fiber in the colon largely depends on the colonic microbiota and the physio-chemical characteristics of the fiber itself[15]. Fiber sources such as oat bran, pectin, and guar are highly fermented; whereas, cellulose and wheat bran may be poorly fermented[15,16]. On the other hand, the type of dietary fiber affects the microbial composition of the gut lumen. For example, inulin, a polymer of fructose monomers present in onions, garlic and asparagus[17], stimulates the growth of Bifidobacteria; whereas, it restricts the growth of potential pathogenic bacteria such as E. coli, Salmonella, and _Listeria_[17-19]. In experiments with a simulator of the human colon, dietary xylo-oligosaccharides decrease the major butyrate-producing bacteria Faecalibacterium prausnitzii, although total butyrate concentration is increased only in the distal vessel[20]. The same researchers reported that xylo-oligosaccharides also affect the levels of sulphate-reducing bacteria, Bacteroides fragilis, providing evidence that dietary carbohydrates modify the gut microbiota, and therefore, its ability to change the physiological properties of the colonic environment. In humans, diets high in nonstarch polysaccharides and/or resistant starch profoundly affect the types of fecal bacteria, including species related to Ruminococcus bromii, which can contribute to starch degradation and short chain fatty acid (SCFA) production[21].
There are over 50 bacterial phyla described to date but the human gut microbiota is dominated by two of them, the Bacteroidetes and the Firmicutes; whereas, the phyla Proteobacteria, Verrucomicrobia, Actinobacteria, Fusobacteria, and Cyanobacteria are present in minor proportions[22,23]. The taxonomic composition of the “ideal” microbiota, if such exists, remains to be identified. Presently, individuals are categorized into “enterotypes” or clusters based upon the abundance of key genera in the gut microbiota[24]. Recent studies showed that gut microbial communities are clustered into three types: Bacteroides (enterotype 1), Prevotella (enterotype 2) and Ruminococcus (enterotype 3), and these clusters seem unrelated to geographical origin, body mass index, age, or gender[25]. These findings suggest that there is not one ideal microbiota composition, but “a limited number of well balanced host-microbial symbiotic states”[25].
Much remains to be determined about what constitutes a healthy microbiota, but there are numerous diseases and conditions associated with a disturbed gut microbiota[26]. It has been generally accepted that the human gut contains approximately 500 to 1000 species[27], and the differential colonization suggests a relationship with disease susceptibility[28-30]. For example, the intestinal microbiota of children from Europe and rural Africa who are exposed to a modern Western diet and a rural diet respectively, exhibit significant differences in microbial composition. The major difference is that rural African children have microbiota enriched in Bacteroidetes and depleted in Firmicutes in comparison to European children[30].
Although amino acid fermenting bacteria and syntrophic species are present in the large intestine, the majority of colonic bacteria have predominantly saccharolytic metabolisms. Therefore, dietary fiber/carbohydrate availability is almost certainly the most important nutritional factor that determines the composition and metabolic activities of the gut microbiota, and many of the physiologic properties of the microbiota are attributed to the fermentation and production of SCFAs[31]. For example, lower dietary fiber intake and consistently lower SCFA production were observed in colon cancer risk subjects compared to healthy individuals, and these differences were accompanied by distinct profiles of the fecal microbiota communities of the two groups[32]. In the same study, Clostridium, Roseburia, and Eubacterium spp. were significantly less prevalent in the colon cancer risk group than the healthy individuals group; whereas, Enterococcus and Streptococcus spp. were more prevalent in the colon cancer risk group[32]. Consistent with these observations, the low pH conditions resulting from fiber fermentation increase biosynthetic requirements for nitrogen-containing precursors, and subsequently inhibit toxin accretion in the colon[33]. Taken together, individual properties such as body mass index, age, or gender may not explain the three observed gut bacterial enterotypes[25], but data-driven marker genes/microbial markers can be identified for certain diseases and conditions[30-32].
SCFA PRODUCTION
Dietary fiber consumption can have significant health benefits, particularly in laxation, mineral absorption, potential anticancer properties, lipid metabolism and anti-inflammatory effects[34]. Many of these health benefits can be attributed to the fermentation of dietary fiber into SCFAs in the colon. These SCFAs are generated by the colonic microbiota, and an equation outlining overall carbohydrate fermentation in the colon has been described[35]:
59 C6H12O6 + 38 H2O → 60 acetate + 22 propionate + 18 butyrate + 96 CO2 + 256 H+.
The significance of carbohydrate breakdown by intestinal bacteria is broad. For example, the increased input of carbohydrates allows for increased bacterial cell mass, which supports laxative effects and shorter colonic transit times. The decreased transit times decrease protein breakdown and the accumulation of putrefactive substances, such as ammonia, phenols, amines and hydrogen sulfide in the colon.
The three major colonic SCFAs are acetate, propionate and butyrate, and the total concentration of SCFAs in colonic content may exceed 100 mmol/L [36]. The composition of diet and gut microbiota are the major factors in determining the molar proportion of SCFA species. In general, acetate makes up around 60%-75% of the total SFCA, and is generated by many of bacterial groups that inhabit the colon, with approximately one-third of the product coming from reductive acetogenesis[37]. The bacterial groups that form propionate and butyrate are specialized, and are of particular interest in terms of their health beneficial effects. The fact that a considerable number of bacterial species provide diverse molecular functions underscores the importance of a functional analysis to understand the composition of microbiota[25].
The data on the main propionate-producing bacteria in the human colon are still emerging, and several biochemical pathways for propionate formation are characterized[38,39]. The succinate route for propionate formation is generally employed by Bacteroides species, but the acrylate route from lactate is adopted by bacteria belonging to the clostridial cluster IX group. In addition, a third pathway is employed by the butyrate-producing bacterium R. inulinivorans with fucose as substrate[40].
Colonic bacteria that produce butyrate belong to the clostridial clusters I, III, IV, VI, XIVa, XV and XVI. Two particularly abundant groups that are estimated to consist 7%-24% of the total gut bacteria in healthy subjects are cluster IV bacteria related to Faecalibacterium prausnitzii, and cluster XIVa bacteria related to Eubacterium rectal and to _Roseburia spp_[41]. For example, reduced dietary intake of fiber by obese subject results in decreased concentrations of butyrate and butyrate-producing bacteria related to Eubacterium rectal and to _Roseburia spp_[42].
PHYSIOLOGICAL EFFECTS OF SCFA
Acetate (C2), propionate (C3) and butyrate (C4) are found in the human intestine at concentrations of approximately 13 mmol/L in the terminal ileum, approximately 130 mmol/L in caecum and approximately 80 mmol/L in the descending colon[36]. These SCFAs released in the intestinal lumen are readily absorbed and used as energy source by colonocytes (approximately 10% of basal energy requirements) and also by other tissues such as liver and muscle[43].
Acetate stimulates proliferation of normal crypt cell but reduces the frequency of spontaneous longitudinal muscle contractions in rat colonic smooth muscle[44]. Acetate enhances ileal motility, increases colonic blood flow, and plays a role in adipogenesis and host immune system through interacting with the G protein-coupled receptor (GPCR43, 41) in adipose tissue and immune cells[45,46]. In addition, it has been shown that acetate reduces lipopolysaccharide-stimulated tumor necrosis factor (TNF), interleukin (IL)-6 and nuclear factor (NF)-κB level while boosting peripheral blood antibody production in various different tissues[47].
Similar to acetate, propionate has been shown to exert a concentration-dependent effect on the frequency of spontaneous contractions in longitudinal muscle via enteric nerves in rat distal colon[44]. In both animal and human studies, it has been shown that propionate reduces food intake and increases satiety via augmentation of the satiety hormone leptin, and through activation of GPCR43, 41[48,49]. Also, propionate may be protective against carcinogenesis because it reduces human colon cancer cell growth and differentiation via hyperacetylation of histone proteins and stimulation of apoptosis[50,51]. In addition, propionate also inhibits the production of proinflammatory cytokines (e.g., TNF-α, NF-κB) in multiple tissues[52,53].
Although acetate, propionate, and butyrate are all metabolized to some extent by the epithelium to provide energy, butyrate plays the most critical role in maintaining colonic health and moderating cell growth and differentiation[54]. More than 70% of oxygen consumption in isolated colonocytes is due to butyrate oxidation, and the uptake and utilization of butyrate by the colonic epithelium have been demonstrated in a study on the SCFA levels in portal and arterial blood and in colonic contents[36]. Compared to acetate and propionate, butyrate exhibits strong anti-inflammatory properties, and this effect is likely mediated by inhibition of TNF-α production, NF-κB activation, and IL-8, -10, -12 expression in immune and colonic epithelial cells[55,56].
ANTI-INFLAMMATORY ACTION, SCFAS AND MICROBIOTA
Inflammation, a host defense mechanism, is an immediate response of the body to tissue injury caused by microbial infection and other noxious stimuli. However, inadequate resolution of inflammation and uncontrolled inflammatory reactions can evoke a state of chronic inflammation, which is a common etiologic factor for cancer[57].
Leukocyte recruitment and SCFAs
Leukocytes are recruited and migrate from the bloodstream to the inflamed tissue through a multistep process that involves expression and activation of several proteins such as adhesion molecules and chemokines[58], and SCFAs modify this leukocyte recruitment[59,60]. Several lines of evidence show that SCFAs induce directional migration of neutrophils, which is dependent upon the activation of GPR43, a G protein-coupled receptor[59,61]. The function of SCFAs as agonists of GPR43 may result in activation of protein kinase B (PKB) and mitogen activated protein kinases in neutrophils. Furthermore, the receptors GPR41 and GPR109A, both of which are related to GPR43, are activated by SCFAs[62]. These results support a role for the SCFAs in the movement of neutrophils[61].
SCFAs also modulate the expression and secretion of cell adhesion molecules and chemokines that play a central role in leukocyte recruitment[52,60]. Cell adhesion molecules such as selectins, integrins, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1 are critical for adhesion and transendothelial migration of leukocytes[63]. Recent studies have shown that SCFAs reduce the adherence of monocytes and lymphocytes to human umbilical vein endothelial cells, and this is associated with an attenuation of NF-κB and PPARγ activities and adhesion molecule expression (ICAM-1 and VCAM-1)[52,63]. In addition, butyrate reduces the constitutive and IFN-γ-induced expression of LFA-3 and ICAM-1; the LPS-stimulated production of CXCL-2, 3, and macrophage chemoattractant protein-1, IL-8 by neutrophils and macrophages[64,65]. Therefore, by modulating the amount or type of adhesion molecules and chemokines, SCFAs may alter the recruitment of leukocytes, and in part, reduce the chronic GI tract inflammatory response.
Proinflammatory mediators, SCFAs
A wide variety of cytokines and other proinflammatory mediators contribute to both extrinsic and intrinsic pathways of inflammation-associated carcinogenesis, and macrophages are the major source of inflammatory mediators[57]. Once activated, macrophages produce significant amounts of mediators such as TNF-α, Il-1β, IFN-γ and IL-6, chemokines, and nitric oxide (NO)[57,66]. SCFAs, mainly butyrate, reduce the LPS- and cytokine-stimulated production of pro-inflammatory mediators such as TNF-α, IL-6, IFN-γ and NO while increase the release of the anti-inflammatory cytokine IL-10[66,67]. The histone deacetylases (HDACs) and histone acetyltransferases control the degree of protein acetylation and gene expression, and the ability of butyrate to inhibit HDAC activity is the main mechanism via which the acid affects the expression of proinflammatory mediators[66-68]. In addition to increasing net histone acetylation and therefore, influencing gene expression, butyrate also augments the acetylation of nonhistone proteins such as NF-κB, MyoD, and p53[66].
Gastrointestinal barriers and microbiota
Gut microbiota contribute to the maintenance of an intact GI barrier, and the disruption of this barrier can cause an inflammatory process[10]. The primary or innate barrier is an interaction between the microbiota and the gut epithelial cell layer. This interaction is an active process, in which certain inflammatory mediators are produced. For example, the ligands of toll like receptors (TLRs) such as LPS and flagellin are microbially derived, and they activate respectively, TLR-4 and -5 to modulate distinct aspects of host metabolism and immune response[69]. The secondary physical barrier is formed by epithelial cell secretion of mucus, and this intestinal mucus layer is a critical physical barrier protecting the intestinal epithelium from the intestinal microbiota, including invasive microbes[70]. The mucus layer is composed by mucin proteins produced by Goblet cells[10], whereas, in the small intestine, the Paneth cells directly sense enteric bacteria through TLR activation, and release various antimicrobial peptides[71]. Therefore, mucus not only forms a physical barrier and provides a nutrition source for the microbiota, but it also contains protective mediators such as secreted antimicrobial peptides and Ig A[70,72]. Thus, the mucosal immune system and the homeostasis of gut microbiota are interdependent, and a balance between them maintains a stable intestinal environment.
EFFECT OF SCFAS ON CELL CYCLE, MIGRATION AND APOPTOSIS
Although SCFAs stimulate normal colonocyte proliferation at low concentrations (e.g., 0.05 mmol/L-0.1 mmol/L butyrate), SCFAs also inhibit the growth of most human colon cancer cells by cell cycle arrest and apoptosis through a complex molecular regulation[73,74]. Several in vitro studies have demonstrated that butyrate inhibits HDACs, and allow histone hyperacetylation that leads to transcription of many genes including p21/Cip1, and cyclin D3[75]. The induction of the cyclin-dependent kinase inhibitory protein p21/Cip1 accounts for cell arrest in the G1 phase of the cell cycle[75]. In addition, we and others have also observed that at 0.5 or higher mmol/L concentration, butyrate inhibits the migration and invasion rate of cancer cells by increasing the expression of anti-metastasis genes (e.g., metalloproteinases) and inhibiting the activation of pro-metastatic genes (e.g., matrix metalloproteinases)[76,77].
There is also overwhelming evidence that dietary fiber counteracts the earliest stages of colonic carcinogenesis. For example, carbohydrates may protect colonocytes against the genotoxicity of a typical Western diet, which is characterized by increased levels of protein and fat intake. Thus, resistant starch decreases by 70% the DNA damage manifested by single-strand breaks in colonocytes of rats fed a Western diet[78]; significantly, when such DNA damage is not repaired, it may initiate colonic carcinogenesis. This interpretation is supported by experimental data that resistant starch protects rodents against tumors induced by the carcinogen azoxymethane[79,80]. The protective effect of resistant starch against such DNA alterations could be attributed to the increased production of SCFAs, and the decreased phenol and ammonia levels[78]. Among the SCFAs, butyrate has been demonstrated to have a significant physiological effect on neoplastic colonic cells[81]; however, acetate has also been implicated in protection against genotoxic agents[20] Interestingly, different carbohydrates affect differentially the extent of DNA damage; for example, dietary xylo-oligosaccharides but not inulin may alter the genotoxicity of the colonic environment. Utilizing a human colonic simulator inoculated with human feces and a soy protein isolate, the researchers have reported that xylo-oligosaccharides reduce genotoxicity of the liquid phase in the proximal vessel, but increase genotoxicity in the distal vessel[20].
It is evident that the DNA-protective effects of the carbohydrates are mediated by (1) their ability to sustain the existence of specific colonic microbiota; and (2) by the fermentation products resulting from the presence of the colonic bacterial species. In rats, a resistant starch-enriched diet increases the numbers of bifidobacteria and lactobacilli species; whereas, it decreases coliforms and results in higher levels of SCFAs[82]. However, the levels of the short-chain fatty acids are dependent not only upon the type and amount of dietary carbohydrates, but also by the present colonic bacterial species. Such two-way interactions explain the observations that rats fed resistant starch diet supplemented with the probiotic Bifidobacterium lactis exhibit a stronger apoptotic response to a genotoxic carcinogen in the colon than those fed the same diet without the probiotic supplement[82].
Evidence for a protective role of butyrate against colon cancer comes mostly from studies in carcinogen-induced rodent models of this malignancy. Thus, the effects of diets containing guar gum and oat bran (both highly fermentable, but associated with low butyrate levels in the distal colon) have been compared to these of a diet with wheat bran (resulting in high butyrate concentrations) in a rat dimethylhydrazine model of colon cancer[83]. The researchers reported the highest protection against colonic tumors in the group of rats fed the wheat bran diet. Similarly, rats fed diet with resistant starch exhibited a lesser burden of colonic adenocarcinomas after exposure to azoxymethane, and this protective effect seemed to be related to the production of butyrate in the colon[79]. It has been observed that in rats with tumors induced by azoxymethane and deoxycholic acid, dietary sodium gluconate increases butyrate levels and decreases the numbers of tumors in the colon[84]. Also, oral administration of the butyrate-producing bacteria Butyrivibrio fibrisolvens augmented butyrate levels, and reduced the formation of aberrant crypt foci, an early colonic lesion, in the colon and rectum of mice treated with dimethylhydrazine[85].
However, not all reports support a chemopreventive effect for butyrate[15]. Some epidemiological studies have also shown no relationship between fiber intake and colon cancer incidence, and no effect of SCFAs (e.g., butyrate) on colonic tumorigenesis[86,87]. These observations were initially counter-intuitive given the reported anticancer-effects of dietary fiber/SCFAs. However, molecular analyses on the effect of SCFAs in colonic tumorigenesis may partly explain these seemingly controversial observations.
First, the constitutive activation of the canonical WNT signaling pathway is a common characteristic of colon cancer, and the beta-catenin- Tcf (BCT) transcriptional complexes are the downstream mediators of this pathway[88,89]. It has been proposed that WNT/beta-catenin activity exists as a gradient, within which absence of WNT signal results in terminal differentiation and apoptosis, relatively low levels of signaling lead to controlled self-renewal, moderate levels of signaling promote uncontrolled cell proliferation, and relatively high levels of WNT signaling lead to apoptosis[90]. Therefore, hyper-activation of WNT/beta-catenin signaling in butyrate-treated colon cancer cells is a required event to achieve high levels of apoptosis in these cells[91].
Second, studies on human colon cancer cell lines with different WNT/beta-catenin signaling mutations have identified two classes of cell lines: those which respond to butyrate treatment with (1) a high fold; and (2) a low fold induction of WNT/beta-catenin activity and apoptosis[91]. Thus, discrepancies in the literature as to the protective nature of fiber intake against colon cancer[5,15,92] may be due to the fact that only a subset of colonic lesions responds to butyrate with hyper-activation of WNT/beta-catenin signaling and enhanced apoptosis. Further, colonic lesions may become resistant to the effects of butyrate through exposure to suboptimal levels of this agent; for example, butyrate-resistant cells produced in vitro exhibit suppressed WNT/catenin hyperactivation and inhibited induction of apoptosis upon exposure to butyrate and other HDAC inhibitors[93]. This butyrate-resistant cell line may reflect the in vivo existence of human tumors that are resistant or partially resistant to the effects of butyrate, and suggests that a high dietary fiber intake is required for an effective protective action against colon cancer. Differences in the responsiveness of colonic neoplastic cells to the effects of butyrate on WNT/catenin signaling may be mediated through the differential expression and activity of transcriptional coactivators that influence WNT/catenin activity, particularly CBP and p300[94,95]. For example, a butyrate-resistant cell line has been shown to be defective in p300 expression, which likely mediates effects of butyrate on WNT/catenin signaling and cell physiology[95].
Third, the composition of gut microbiota and diet (e.g., fat) are factors that affect the SCFA productions and their action[15,96,97], and the effect of SCFAs on colon neoplastic cells might be modifiable by other dietary compounds and metabolites; thus, adding a particular type of oil (e.g., fish oil vs corn oil) results in a variable reduction of colon tumors in rat azoxymethane model of carcinogenesis[98]. Finally, the effect of fiber and butyrate on colon carcinogenesis is likely dependent upon the timing of fiber and butyrate administration with respect to the stage of cancer development[15]. Several studies have shown that a high fiber intake specifically affects early tumor development in the colon; however, progression to advanced adenomas is unlikely to be influenced by fiber intake[7,86]. These data clearly support a multifaceted role of SCFA production/action, and more in vivo studies are warranted to further dissect the role of fiber intake in modulating colon cell cycle and apoptosis pathways.
FUNCTIONAL ROLE OF FIBER SOURCE PER SE
Although gut microbiota and fiber fermentation to SCFAs play a critical role in cancer prevention, the fiber source per se may have independent effects on colonic health. First, dietary fiber increases viscosity and fecal bulking (diluting potential carcinogens), and it therefore shortens the time for proteolytic fermentation (and production of harmful substances) and also decreases the contact between potential carcinogens and mucosal cells[4,99]. In addition, dietary fiber could bind/excrete potential luminal carcinogens (e.g., secondary bile acids) and lower fecal pH in the colon[4,100,101]. Second, dietary fiber is not only a substrate for fermentation, but it is also a source of vitamins, minerals and slowly digestible energy; for example, bran fractions are rich in minerals, vitamin B6, thiamine, folate and vitamin E[102]. Third, dietary fiber is associated with phytochemicals such as phenolics, carotenoids, lignans, beta-glucan and inulin[102,103]. For example, arabinoxylan, a constituent of hemicelluloses, is an important source of phenolic compounds that may be released in the colon during fermentation of complexed fibers[4,102]. These bioactive substances may protect the GI tract from oxidative damage, although this possibility is controversial due to the anaerobic environment in the colon and the fact that the fiber-associated phytochemicals (e.g., carotenoids) do not seem to be absorbed through the GI tract into the rest of the body, even though the colon is the primary site for fiber fermentation and the release of these chemicals[104]. However, since the concentrations of bioactive substances derived from dietary fiber sources can be much higher in the colonic lumen than in plasma and other tissue, these phytochemicals may delay the onset of colon cancer.
CONCLUSIONS AND PERSPECTIVES
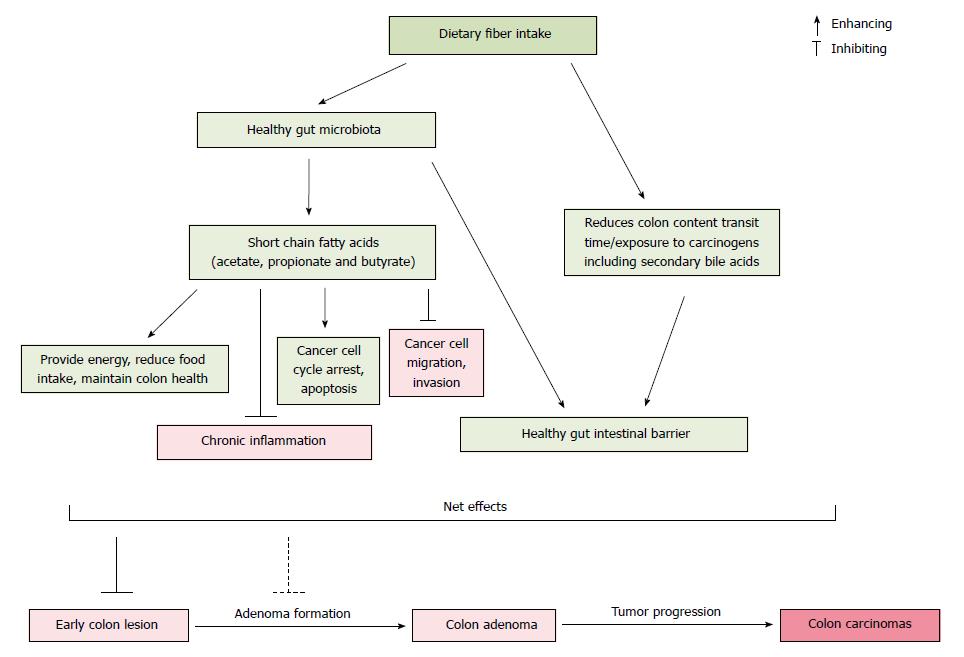
A large amount of research has reported an inverse relationship between dietary fiber intake and colon cancer risk. The protective effect of fiber against colon cancer derives from a multi-layered system of mechanistic checks and balances, which may explain why not all studies report this beneficial effect. Although the anticancer mechanisms of dietary fiber are not fully understood, several modes of action have been proposed (Figure 1). First, dietary fiber resists digestion in the small intestine, and enters the colon where it is fermented to produce SCFAs that may enhance the healthy composition of gut microbiota. Second, SCFAs have anticancer properties which include the promotion of cancer cell cycle arrest, apoptosis, and the inhibition of chronic inflammatory process and cancer cell migration/invasion in the colon. Importantly, these molecular activities are effective only within a certain physiological concentration range of the SCFAs. Third, dietary fiber increases fecal bulking and viscosity, reduces the time for proteolytic fermentation that results in harmful substances, and shortens the contact between potential carcinogens and mucosal cells. In addition, dietary fiber can bind/excrete potential luminal carcinogens (e.g., secondary bile acids), lower fecal pH in the colon, and thus provide a healthy intestinal environment.
Figure 1 The proposed interaction of primary pathways related to dietary fiber consumption, gut microbiota and colon cancer risk.
Not all fibers have the same properties; therefore, the characteristics and components of dietary fibers (e.g., arabinoxylan, β-glucan) may determine their modes of action against colon cancer cells. Future studies on the type of fiber and fiber components may provide a better understanding of how and why dietary fiber decreases the risk of colon cancer. Furthermore, evidence from many lines of research demonstrates that fiber consumption modifies the composition of gut microbiota, and a well balanced colonic microbiota influences the host at nearly every level including immunity and neoplastic development. Metagenomics is one of the newest approaches to determine gut microbiota composition, but it is still difficult to characterize the interactions between hosts and their microbiota. The combination of several “meta” analyses such as metagenomics, metabolomics, metatranscriptomics, and the shift of focus from a “who is there” to a “why are they there” will advance our understanding of the relationship between dietary fiber consumption, microbiota composition, and human health. Future studies are required to unravel the microbiota changes that correlate with the beneficial effects of fiber, although it is likely that such changes in the gut bacteria may be dose-, time-, and strain-dependent. These efforts may lead to identification of microbiota signatures that are causal or correlative biomarkers for fiber consumption and colon cancer prevention.
If butyrate is indeed the key mediator for the protective effect of fiber against colon cancer, then the effects of diet and microbiota on the butyrate levels in the colon, and our ability to manipulate these levels via dietary supplements, will be important for designing effective colon cancer preventive strategies. The levels of fecal butyrate among individuals differ widely (3.5-32.6 mmol/kg), and these inter-individual differences have been explained in part by body-mass index and dietary intake of protein, fiber, and fat[105]; however, there are additional factors that remain to be determined.
ACKNOWLEDGMENTS
The United States Department of Agriculture, Agricultural Research Service, Northern Plains Area, is an equal opportunity/affirmative action employer and all agency services are available without discrimination. Mention of a trademark or proprietary product does not constitute a guarantee or warranty of the product by the United States Department of Agriculture and does not imply its approval to the exclusion of other products that may also be suitable.
Footnotes
P- Reviewers: Arasaradnam RP, Gurkan A, M'Koma AE S- Editor: Wen LL L- Editor: A E- Editor: Wang CH
References
| 2. | Chambers WM, Warren BF, Jewell DP, Mortensen NJ. Cancer surveillance in ulcerative colitis. Br J Surg. 2005;92:928-936. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 3. | Kushi LH, Byers T, Doyle C, Bandera EV, McCullough M, McTiernan A, Gansler T, Andrews KS, Thun MJ. American Cancer Society Guidelines on Nutrition and Physical Activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2006;56:254-281; quiz 313-314. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 7. | Peters U, Sinha R, Chatterjee N, Subar AF, Ziegler RG, Kulldorff M, Bresalier R, Weissfeld JL, Flood A, Schatzkin A. Dietary fibre and colorectal adenoma in a colorectal cancer early detection programme. Lancet. 2003;361:1491-1495. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 8. | Nomura AM, Hankin JH, Henderson BE, Wilkens LR, Murphy SP, Pike MC, Le Marchand L, Stram DO, Monroe KR, Kolonel LN. Dietary fiber and colorectal cancer risk: the multiethnic cohort study. Cancer Causes Control. 2007;18:753-764. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 15. | Lupton JR. Microbial degradation products influence colon cancer risk: the butyrate controversy. J Nutr. 2004;134:479-482. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 16. | McBurney MI, Thompson LU. Fermentative characteristics of cereal brans and vegetable fibers. Nutr Cancer. 1990;13:271-280. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 17. | Bosscher D, Breynaert A, Pieters L, Hermans N. Food-based strategies to modulate the composition of the intestinal microbiota and their associated health effects. J Physiol Pharmacol. 2009;60 Suppl 6:5-11. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 18. | Gibson GR, Beatty ER, Wang X, Cummings JH. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. 1995;108:975-982. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 19. | Chong ES. A potential role of probiotics in colorectal cancer prevention: review of possible mechanisms of action. World J Microbiol Biotechnol. 2014;30:351-374. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 23. | Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635-1638. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 27. | Dethlefsen L, Eckburg PB, Bik EM, Relman DA. Assembly of the human intestinal microbiota. Trends Ecol Evol. 2006;21:517-523. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 28. | Kalliomäki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol. 2001;107:129-134. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 29. | Ouwehand AC, Isolauri E, He F, Hashimoto H, Benno Y, Salminen S. Differences in Bifidobacterium flora composition in allergic and healthy infants. J Allergy Clin Immunol. 2001;108:144-145. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 31. | Cummings JH, Macfarlane GT. The control and consequences of bacterial fermentation in the human colon. J Appl Bacteriol. 1991;70:443-459. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 33. | Smith EA, Macfarlane GT. Enumeration of human colonic bacteria producing phenolic and indolic compounds: effects of pH, carbohydrate availability and retention time on dissimilatory aromatic amino acid metabolism. J Appl Bacteriol. 1996;81:288-302. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 37. | Miller TL, Wolin MJ. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl Environ Microbiol. 1996;62:1589-1592. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 43. | McNeil NI. The contribution of the large intestine to energy supplies in man. Am J Clin Nutr. 1984;39:338-342. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 47. | Tedelind S, Westberg F, Kjerrulf M, Vidal A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J Gastroenterol. 2007;13:2826-2832. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 49. | Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA. 2008;105:16767-16772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1035] [Cited by in F6Publishing: 1084] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
|---|
| 50. | Hinnebusch BF, Meng S, Wu JT, Archer SY, Hodin RA. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J Nutr. 2002;132:1012-1017. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 51. | Jan G, Belzacq AS, Haouzi D, Rouault A, Métivier D, Kroemer G, Brenner C. Propionibacteria induce apoptosis of colorectal carcinoma cells via short-chain fatty acids acting on mitochondria. Cell Death Differ. 2002;9:179-188. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 52. | Zapolska-Downar D, Naruszewicz M. Propionate reduces the cytokine-induced VCAM-1 and ICAM-1 expression by inhibiting nuclear factor-kappa B (NF-kappaB) activation. J Physiol Pharmacol. 2009;60:123-131. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 56. | Lührs H, Gerke T, Müller JG, Melcher R, Schauber J, Boxberge F, Scheppach W, Menzel T. Butyrate inhibits NF-kappaB activation in lamina propria macrophages of patients with ulcerative colitis. Scand J Gastroenterol. 2002;37:458-466. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 58. | Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182-1190. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 62. | Tazoe H, Otomo Y, Karaki S, Kato I, Fukami Y, Terasaki M, Kuwahara A. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed Res. 2009;30:149-156. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 63. | Böhmig GA, Krieger PM, Säemann MD, Wenhardt C, Pohanka E, Zlabinger GJ. n-butyrate downregulates the stimulatory function of peripheral blood-derived antigen-presenting cells: a potential mechanism for modulating T-cell responses by short-chain fatty acids. Immunology. 1997;92:234-243. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 65. | Cox MA, Jackson J, Stanton M, Rojas-Triana A, Bober L, Laverty M, Yang X, Zhu F, Liu J, Wang S. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World J Gastroenterol. 2009;15:5549-5557. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 68. | Waldecker M, Kautenburger T, Daumann H, Busch C, Schrenk D. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J Nutr Biochem. 2008;19:587-593. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 74. | Heerdt BG, Houston MA, Augenlicht LH. Potentiation by specific short-chain fatty acids of differentiation and apoptosis in human colonic carcinoma cell lines. Cancer Res. 1994;54:3288-3293. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 76. | Zeng H, Briske-Anderson M. Prolonged butyrate treatment inhibits the migration and invasion potential of HT1080 tumor cells. J Nutr. 2005;135:291-295. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 77. | Emenaker NJ, Calaf GM, Cox D, Basson MD, Qureshi N. Short-chain fatty acids inhibit invasive human colon cancer by modulating uPA, TIMP-1, TIMP-2, mutant p53, Bcl-2, Bax, p21 and PCNA protein expression in an in vitro cell culture model. J Nutr. 2001;131:3041S-3046S. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 79. | Le Leu RK, Brown IL, Hu Y, Esterman A, Young GP. Suppression of azoxymethane-induced colon cancer development in rats by dietary resistant starch. Cancer Biol Ther. 2007;6:1621-1626. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 82. | Le Leu RK, Brown IL, Hu Y, Bird AR, Jackson M, Esterman A, Young GP. A synbiotic combination of resistant starch and Bifidobacterium lactis facilitates apoptotic deletion of carcinogen-damaged cells in rat colon. J Nutr. 2005;135:996-1001. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 84. | Kameue C, Tsukahara T, Yamada K, Koyama H, Iwasaki Y, Nakayama K, Ushida K. Dietary sodium gluconate protects rats from large bowel cancer by stimulating butyrate production. J Nutr. 2004;134:940-944. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 85. | Ohkawara S, Furuya H, Nagashima K, Asanuma N, Hino T. Oral administration of butyrivibrio fibrisolvens, a butyrate-producing bacterium, decreases the formation of aberrant crypt foci in the colon and rectum of mice. J Nutr. 2005;135:2878-2883. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 90. | Bordonaro M, Lazarova DL, Sartorelli AC. Hyperinduction of Wnt activity: a new paradigm for the treatment of colorectal cancer. Oncol Res. 2008;17:1-9. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 99. | Macfarlane GT, Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int. 2012;95:50-60. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 100. | Kern F, Birkner HJ, Ostrower VS. Binding of bile acids by dietary fiber. Am J Clin Nutr. 1978;31:S175-S179. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 101. | Courtney ED, Melville DM, Leicester RJ. Review article: chemoprevention of colorectal cancer. Aliment Pharmacol Ther. 2004;19:1-24. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 103. | Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. 2003;78:517S-520S. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 104. | Halliwell B, Zhao K, Whiteman M. The gastrointestinal tract: a major site of antioxidant action. Free Radic Res. 2000;33:819-830. [PubMed] [DOI] [Cited in This Article: ] |
|---|