Graves Disease: Practice Essentials, Pathophysiology, Epidemiology (original) (raw)
Practice Essentials
Graves disease is an autoimmune disorder characterized by hyperthyroidism due to circulating autoantibodies. Thyroid-stimulating immunoglobulins (TSIs) bind to and activate thyroid-stimulating hormone (TSH) receptors, causing the thyroid gland to grow and the thyroid follicles to increase synthesis of thyroid hormone. Graves disease, along with Hashimoto thyroiditis, is classified as an autoimmune thyroid disorder. Ultrasensitive (third-generation) TSH assays remain the best screening test for thyroid disorders. Treatment involves alleviation of symptoms and correction of the thyrotoxic state. Graves disease is named after Robert J. Graves, MD, [1] who described the condition in 1835.
In some patients, Graves disease represents a part of more extensive autoimmune processes leading to dysfunction of multiple organs (eg, polyglandular autoimmune syndromes). Graves disease is associated with pernicious anemia, vitiligo, diabetes mellitus type 1, autoimmune adrenal insufficiency, systemic sclerosis, myasthenia gravis, Sjögren syndrome, rheumatoid arthritis, and systemic lupus erythematosus. [2] Moreover, advances in cancer immunotherapy with immune checkpoint inhibitors (anti–CTLA-4, anti–PD-1, and anti–PD-L1 antibodies) have led to immune-related adverse effects, including problems affecting the thyroid glands (ie, thyroiditis [typically with a transient hyperthyroid phase followed by a permanent hypothyroid phase] and Graves disease). [3]
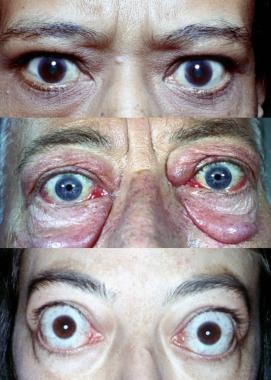
Graves ophthalmopathy (also known as Graves orbitopathy) is shown below.
Graves disease. Varying degrees of manifestations of Graves ophthalmopathy.
Signs and symptoms of Graves disease
Common physical findings in Graves disease, organized by anatomic region, are as follows:
- General - Increased basal metabolic rate, weight loss despite increase in or similar appetite
- Skin - Warm, most, fine skin; increased sweating; fine hair; vitiligo; alopecia; pretibial myxedema
- Head, eyes, ears, nose, and throat - Chemosis, conjunctival irritation, widening of the palpebral fissures, lid lag, lid retraction, proptosis, impairment of extraocular motion, visual loss in severe optic nerve involvement, periorbital edema
- Neck - Upon careful examination, the thyroid gland generally is diffusely enlarged and smooth; a well-delineated pyramidal lobe may be appreciated upon careful palpation; thyroid bruits and, rarely, thrills may be appreciated; thyroid nodules may be palpable.
- Chest - Gynecomastia, tachypnea; tachycardia; murmur; hyperdynamic precordium; S3, S4 heart sounds; ectopic beats; irregular heart rate and rhythm
- Abdomen - Hyperactive bowel sound
- Extremities - Edema, acropachy, onycholysis
- Neurologic - Hand tremor (fine and usually bilateral), hyperactive deep tendon reflexes
- Musculoskeletal - Kyphosis, lordosis, loss of height, proximal muscle weakness, hypokalemic periodic paralysis in persons of susceptible ethnic groups
- Psychiatric - Restlessness, anxiety, irritability, insomnia, depression
Workup in Graves disease
Ultrasensitive (third-generation) TSH assays remain the best screening test for thyroid disorders. With the exception of TSH-induced hyperthyroidism, subnormal or suppressed TSH levels are seen in most patients with thyrotoxicosis.
Liver function test results should be obtained to monitor for liver toxicity caused by thioamides (antithyroid medications).
A complete blood count (CBC) with differential should be obtained at baseline and with the development of fever or symptoms of infection. Graves disease may be associated with normocytic anemia, low-normal to slightly depressed total white blood cell (WBC) count with relative lymphocytosis and monocytosis, and low-normal to slightly depressed platelet count. Thioamides may rarely cause severe hematologic side effects, but routine screening for these rare events is not cost-effective.
Radioactive iodine scanning and measurements of iodine uptake are useful in differentiating the causes of hyperthyroidism. In Graves disease, the radioactive iodine uptake is increased, and the uptake is diffusely distributed over the entire gland. [4]
Management of Graves disease
Treatment involves alleviation of symptoms and correction of the thyrotoxic state. Adrenergic hyperfunction is treated with beta-adrenergic blockade. Correcting the high thyroid hormone levels can be achieved with antithyroid medications that block the synthesis of thyroid hormones or by treatment with radioactive iodine.
Radioactive iodine is, in fact, the most commonly used therapy for Graves disease. Indications for radioactive iodine over antithyroid agents include a large thyroid gland, multiple symptoms of thyrotoxicosis, high levels of thyroxine, and high titers of TSI.
Thyroidectomy is not the recommended first-line therapy for hyperthyroid Graves disease in the United States, although it may be appropriate in the presence of a thyroid nodule that is suggestive of carcinoma or in patients less than age 5 years. [5]

Pathophysiology
In Graves disease, B and T lymphocyte-mediated autoimmunity are known to be directed at 4 well-known thyroid antigens: thyroglobulin, thyroid peroxidase, sodium-iodide symporter and the TSH receptor. However, the TSH receptor itself is the primary autoantigen of Graves disease and is responsible for the manifestation of hyperthyroidism. In this disease, the antibody and cell-mediated thyroid antigen-specific immune responses are well defined. Direct proof of an autoimmune disorder that is mediated by autoantibodies is the development of hyperthyroidism in healthy subjects by transferring TSH-receptor antibodies in serum from patients with Graves disease and the passive transfer of TSH-receptor antibodies to the fetus in pregnant women.
The thyroid gland is under continuous stimulation by circulating autoantibodies against the TSH receptor, and pituitary TSH secretion is suppressed because of the increased production of thyroid hormones. The stimulating activity of TSH-receptor antibodies is found mostly in the immunoglobulin G1 subclass. These thyroid-stimulating antibodies cause release of thyroid hormone and thyroglobulin that is mediated by 3,'5'-cyclic adenosine monophosphate (cyclic AMP), and they also stimulate iodine uptake, protein synthesis, and thyroid gland growth.
The anti-sodium-iodide symporter, antithyroglobulin, and antithyroid peroxidase antibodies appear to have little role in the etiology of hyperthyroidism in Graves disease. However, they are markers of autoimmune disease against the thyroid. Intrathyroidal lymphocytic infiltration is the initial histologic abnormality in persons with autoimmune thyroid disease and can be correlated with the titer of thyroid antibodies. Besides being the source of autoantigens, the thyroid cells express molecules that mediate T cell adhesion and complement regulation (Fas and cytokines) that participate and interact with the immune system. In these patients, the proportion of CD4 lymphocytes is lower in the thyroid than in the peripheral blood. The increased Fas expression in intrathyroidal CD4 T lymphocytes may be the cause of CD4 lymphocyte reduction in these individuals.
Viral infection is an environmental factor linked to Graves disease. The prevalence of enteroviral proteins, the upregulation of human leukocyte antigen (HLA) class I expression, and co-localization with antiviral response proteins such as Stat1, VP1, and PKR all indicate an association between Graves disease and viral infection. [6] Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has also been linked to the development of subacute thyroiditis and Graves disease. [7, 8]
Several autoimmune thyroid disease susceptibility genes are considered to be linked to Graves disease, including CD40, CTLA-4, the thyroglobulin gene (TG), the TSH-receptor gene (TSHR), PTPN22, FOXP3, CD25, and VDR. The _RNASET2_-_FGFR1OP_-CCR6 region at 6q27 and an intergenic region at 4p14 have also been associated with the disorder. [9, 10, 11, 12, 13, 13] Similarly, in a genome-wide association study of more than 1500 patients with Graves disease and 1500 controls, six susceptibility loci were found to be related to the condition: the major histocompatibility complex (MHC), TSHR, CTLA-4, FCRL3, the RNASET2-FGFR1OP-CCR6 region at 6q27, and the intergenic region at 4p14. [14]
A genetic predisposition to thyroid autoimmunity may interact with environmental factors or events to precipitate the onset of Graves disease. Moreover, TSHR and MHC class II variants were found to be strongly associated with persistently TSH receptor autoantibody (TRAb)–positive Graves disease. [15] In terms of epigenetic regulation, non-coding RNA molecules such as miR-23a-3p and lncRNA MEG3 may participate in the pathophysiology of Graves disease by disrupting Th17/Treg cell balance. [16]
Gut microbiota have been shown to be associated with autoimmune thyroid diseases. [17] Decreases in Bifidobacterium and Lactobacillus may be linked to such disorders, but microbiome data in Graves disease are still limited. [17, 16]
Graves disease patients have a higher rate of peripheral blood mononuclear cell conversion into CD34+ fibrocytes compared with healthy controls. These cells may contribute to the pathophysiology of ophthalmopathy by accumulating in orbital tissues and producing inflammatory cytokines, including tumor necrosis factor alpha (TNF-alpha) and interleukin-6 (IL-6). [18]
The TSH receptor is detectable in orbital tissues, but whether it has a pathogenetic role in Graves ophthalmopathy is unclear. The insulin-like growth factor-1 (IGF1) receptor is overexpressed in orbital fibroblasts, B cells, and T cells of patients with Graves ophthalmopathy, and patients with this eye disorder may have circulating immunoglobulins that bind to IGF1 receptors. Activation of IGF1R will increase hyaluronan synthesis and cytokine release. Using monoclonal antibodies to block IGF1R can reduce the expression levels of TSHR and IGF1R on fibrocytes and antagonize TSH-induced expression of inflammation genes (IL6, IL8, and TNFA).
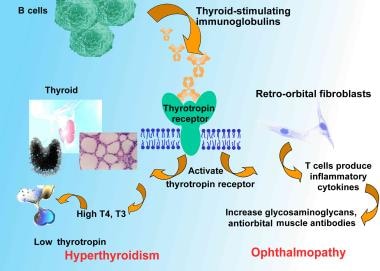
Pathophysiologic mechanisms are shown in the image below.
Pathophysiologic mechanisms of Graves disease relating thyroid-stimulating immunoglobulins to hyperthyroidism and ophthalmopathy. T4 is levothyroxine. T3 is triiodothyronine.

Epidemiology
Frequency
United States
Graves disease is the most common cause of hyperthyroidism in the United States. A study conducted in Olmstead County, Minnesota estimated the incidence to be approximately 30 cases per 100,000 persons per year. [19] The prevalence of maternal thyrotoxicosis is approximately 1 case per 500 persons, with maternal Graves disease being the most common etiology. Commonly, patients have a family history involving a wide spectrum of autoimmune thyroid diseases, such as Graves disease, Hashimoto thyroiditis, or postpartum thyroiditis, among others.
International
Among the causes of spontaneous thyrotoxicosis, Graves disease is the most common. Graves disease represents 60-90% of all causes of thyrotoxicosis in different regions of the world. In the Wickham Study in the United Kingdom, the incidence was reported to be 100-200 cases per 100,000 population per year. [20] The incidence in women in the UK has been reported to be 80 cases 100,000 per year. [21]
Mortality/Morbidity
If left untreated, Graves disease can cause severe thyrotoxicosis. A life-threatening thyrotoxic crisis (ie, thyroid storm) can occur. Long-standing severe thyrotoxicosis leads to severe weight loss with catabolism of bone and muscle. [22] Cardiac complications and psychocognitive complications can cause significant morbidity. Graves disease is also associated with ophthalmopathy, dermopathy, and acropachy.
Thyroid storm is an exaggerated state of thyrotoxicosis. [23] It occurs in patients who have unrecognized or inadequately treated thyrotoxicosis and a superimposed precipitating event such as thyroid surgery, nonthyroidal surgery, infection, or trauma. When thyroid storm was first described, the acute mortality rate was nearly 100%. In current practice, with aggressive therapy and early recognition of the syndrome, the mortality rate is approximately 20%. [24]
Long-term excess of thyroid hormone can lead to osteoporosis in men and women. The effect can be particularly devastating in women, in whom the disease may compound the bone loss secondary to chronic anovulation or menopause. Bone loss is accelerated in patients with hyperthyroidism. The increase in bone loss can be demonstrated by increased urinary pyridinoline cross-link excretion. Serum calcium and phosphate, plasma FGF-23 were significantly higher in the patients with Graves disease than in healthy control subjects, suggesting that FGF-23 is physiologically related to serum phosphate homeostasis in untreated Graves disease. [25]
Hyperthyroidism increases muscular energy expenditure and muscle protein breakdown. These abnormalities may explain the sarcopenia and myopathy observed in patients with hyperthyroid Graves disease.
Cardiac hypertrophy has been reported in thyrotoxicosis of different etiologies. Rhythm disturbances such as extrasystolic arrhythmia, atrial fibrillation, and flutter are common. Cardiomyopathy and congestive heart failure can occur. [26]
Psychiatric manifestations such as mood and anxiety disorders are common. [27] Subjective cognitive dysfunction is often reported by Graves disease patients and may be due to affective and somatic manifestations of thyrotoxicosis, which remit after treatment of Graves thyrotoxicosis. [28]
A study by Folkestad et al reported Graves disease and toxic nodular goiter to be a risk factors for dementia. The investigators found that every 6 months of reduced TSH was linked to a 16% rise in dementia risk in hyperthyroid individuals. [29]
Nonpitting edema is the most prevalent form of dermopathy (about 40%) and are primarily in the pretibial area. The nearly all (>95%) patients with dermopathy had ophthalmopathy. [30] Advanced forms of dermopathy are elephantiasis or thyroid acropachy. Severe acropachy can be disabling and can lead to total loss of hand function.
Progression of ophthalmopathy can lead to compromised vision and blindness. Visual loss due to corneal lesions or optic nerve compression can be seen in severe Graves ophthalmopathy.
In a study of 1128 patients with Graves ophthalmopathy, Kim et al found the prevalence of ocular hypertension (OHT) to be 6.8% and the prevalence of open-angle glaucoma (OAG) to be 1.6%. The prevalences were higher in patients over age 40 years, being 9.5% and 3.4%, respectively. The investigators also reported the prevalence of OHT in Graves ophthalmopathy to be associated with male sex, duration of the ophthalmopathy, a clinical activity score of 3 or above, extraocular muscle involvement, and lid retraction. Male sex and duration of the ophthalmopathy were associated with the prevalence of OAG in Graves ophthalmopathy. [31]
Maternal Graves disease can lead to neonatal hyperthyroidism by transplacental transfer of thyroid-stimulating antibodies. Approximately 1-5% of children of mothers with Graves disease (usually with high TSI titer) are affected. Usually, the TSI titer falls during pregnancy.
Elderly individuals may develop apathetic hyperthyroidism, and the only presenting features may be unexplained weight loss or cardiac symptoms such as atrial fibrillation and congestive heart failure.
Boelaert et al investigated the prevalence of and relative risks for coexisting autoimmune diseases in patients with Graves disease (2791 patients) or Hashimoto thyroiditis (495 patients). The authors found coexisting disorders in 9.7% of patients with Graves disease and in 14.3% of those with Hashimoto thyroiditis, with rheumatoid arthritis being the most common of these (prevalence = 3.15% and 4.24% in Graves disease and Hashimoto thyroiditis, respectively). Relative risks of greater than 10 were found for pernicious anemia, systemic lupus erythematosus, Addison disease, celiac disease, and vitiligo. The authors also reported a tendency for parents of patients with Graves disease or Hashimoto thyroiditis to have a history of hyperthyroidism or hypothyroidism, respectively. [32]
Race
In whites, autoimmune thyroid diseases are, based on linkage analysis, linked with the following loci: AITD1, CTLA4, GD1, GD2, GD3, HT1, and HT2. Different loci have been reported to be linked with autoimmune thyroid diseases in persons of other races.
Susceptibility is influenced by genes in the human leukocyte antigen (HLA) region on chromosome 6 and in CTLA4 on band 2q33. Association with specific HLA haplotypes has been observed and is found to vary with ethnicity.
Sex
As with most autoimmune diseases, susceptibility is increased in females. Hyperthyroidism due to Graves disease has a female-to-male ratio of 7-8:1.
The female-to-male ratio for pretibial myxedema is 3.5:1. Only 7% of patients with localized myxedema have thyroid acropachy.
Unlike the other manifestations of Graves disease, the female-to-male ratio for thyroid acropachy is 1:1.
Age
Typically, Graves disease is a disease of young women, but it may occur in persons of any age.
The typical age range is 20-40 years.
Most affected women are aged 30-60 years.
Although pediatric cases are rare, Beliard et al reported on Graves disease in a 12-month-old infant. [33]

Prognosis
The natural history of Graves disease is that most patients become hypothyroid and require replacement. Similarly, the ophthalmopathy generally becomes quiescent. On occasion, hyperthyroidism returns because of persisting thyroid tissue after ablation and high antibody titers of anti-TSI. Further therapy may be necessary in the form of surgery or radioactive iodine ablation.
A study by Tun et al indicated that in patients with Graves disease receiving thionamide therapy, high TSH receptor–stimulating antibody (TRab) levels at diagnosis of the disease and/or high TRab levels at treatment cessation are risk factors for relapse, particularly within the first two years. The study included 266 patients. [34]
A retrospective study by Rabon et al indicated that in children with Graves disease, antithyroid drugs usually do not induce remission, although most children who do achieve remission through these agents do not relapse. Of 268 children who were started on an antithyroid drug, 57 (21%) experienced remission, with 16 of them (28%) relapsing. [35]
A study by Song et al indicated that in patients with long-standing Graves disease, following a 2-year course of antithyroid drugs, the risk of developing diabetes mellitus is 1.18 times higher than that of controls. More specifically, the investigators reported that in patients who, following a 2-year course of antithyroid drugs, continued on such medications for at least 1 more year, with no radioactive iodine ablation, the risk for diabetes was 1.17 times higher. For those who at some point after the initial 24-month drug course underwent radioactive iodine ablation, the risk was 1.88 times higher. It was also found that the diabetes risk rose as the duration of antithyroid drug treatment increased. [36]

Patient Education
Awareness of the symptoms related to hyperthyroidism and hypothyroidism is important, especially in the titration of antithyroid agents and in replacement therapy for hypothyroidism.
Patients also should be aware of the potential adverse effects of these medicines. They should watch for fever, sore throat, and throat ulcers.
Patients also must be instructed to avoid cold medicines that contain alpha-adrenergic agonists such as ephedrine or pseudoephedrine.

- Ellis H. Robert Graves: 1796-1852. Br J Hosp Med (Lond). 2006 Jun. 67(6):313. [QxMD MEDLINE Link].
- Cruz AA, Akaishi PM, Vargas MA, de Paula SA. Association between thyroid autoimmune dysfunction and non-thyroid autoimmune diseases. Ophthal Plast Reconstr Surg. 2007 Mar-Apr. 23(2):104-8. [QxMD MEDLINE Link].
- Yeung SJ, Qdaisat A, Chaftari P, et al. Diagnosis and management of immune-related adverse effects of immune checkpoint therapy in the emergency department. J Am Coll Emerg Physicians Open. 2020 Dec. 1 (6):1637-59. [QxMD MEDLINE Link]. [Full Text].
- Al-Muqbel KM, Tashtoush RM. Patterns of thyroid radioiodine uptake: Jordanian experience. J Nucl Med Technol. 2010 Mar. 38(1):32-6. [QxMD MEDLINE Link].
- Quintanilla-Dieck L, Khalatbari HK, Dinauer CA, Rastatter JC, Chelius DC Jr, Katowitz WR, et al. Management of Pediatric Graves Disease: A Review. JAMA Otolaryngol Head Neck Surg. 2021 Dec 1. 147 (12):1110-8. [QxMD MEDLINE Link].
- Weider T, Richardson SJ, Morgan NG, Paulsen TH, Dahl-Jørgensen K, Hammerstad SS. HLA Class I Upregulation and Antiviral Immune Responses in Graves Disease. J Clin Endocrinol Metab. 2021 Mar 25. 106 (4):e1763-74. [QxMD MEDLINE Link]. [Full Text].
- Duntas LH, Jonklaas J. COVID-19 and Thyroid Diseases: A Bidirectional Impact. J Endocr Soc. 2021 Aug 1. 5 (8):bvab076. [QxMD MEDLINE Link]. [Full Text].
- Murugan AK, Alzahrani AS. SARS-CoV-2 plays a pivotal role in inducing hyperthyroidism of Graves' disease. Endocrine. 2021 Aug. 73 (2):243-54. [QxMD MEDLINE Link]. [Full Text].
- Chu X, Pan CM, Zhao SX, et al. A genome-wide association study identifies two new risk loci for Graves' disease. Nat Genet. 2011 Aug 14. 43(9):897-901. [QxMD MEDLINE Link].
- Jacobson EM, Tomer Y. The CD40, CTLA-4, thyroglobulin, TSH receptor, and PTPN22 gene quintet and its contribution to thyroid autoimmunity: back to the future. J Autoimmun. 2007 Mar-May. 28(2-3):85-98. [QxMD MEDLINE Link].
- Iwama S, Ikezaki A, Kikuoka N, et al. Association of HLA-DR, -DQ genotype and CTLA-4 gene polymorphism with Graves' disease in Japanese children. Horm Res. 2005. 63(2):55-60. [QxMD MEDLINE Link].
- Tan G, Wang X, Zheng G, et al. Meta-analysis reveals significant association between FOXP3 polymorphisms and susceptibility to Graves' disease. J Int Med Res. 2021 Apr. 49 (4):3000605211004199. [QxMD MEDLINE Link]. [Full Text].
- Zhou F, Liang Z, Wang X, et al. The VDR gene confers a genetic predisposition to Graves' disease and Graves' ophthalmopathy in the Southwest Chinese Han population. Gene. 2021 Aug 15. 793:145750. [QxMD MEDLINE Link]. [Full Text].
- Chu X, Pan CM, Zhao SX, et al. A genome-wide association study identifies two new risk loci for Graves' disease. Nat Genet. 2011 Aug 14. 43(9):897-901. [QxMD MEDLINE Link].
- Bell L, Hunter AL, Kyriacou A, Mukherjee A, Syed AA. Clinical diagnosis of Graves' or non-Graves' hyperthyroidism compared to TSH receptor antibody test. Endocr Connect. 2018 Mar 12. 2017:7354673. [QxMD MEDLINE Link]. [Full Text].
- Zhou F, Wang X, Wang L, et al. Genetics, Epigenetics, Cellular Immunology, and Gut Microbiota: Emerging Links With Graves' Disease. Front Cell Dev Biol. 2021. 9:794912. [QxMD MEDLINE Link]. [Full Text].
- Gong B, Wang C, Meng F, et al. Association Between Gut Microbiota and Autoimmune Thyroid Disease: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne). 2021. 12:774362. [QxMD MEDLINE Link]. [Full Text].
- Douglas RS, Afifiyan NF, Hwang CJ, et al. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2010 Jan. 95(1):430-8. [QxMD MEDLINE Link]. [Full Text].
- Furszyfer J, Kurland LT, McConahey WM, Elveback LR. Graves' disease in Olmsted County, Minnesota, 1935 through 1967. Mayo Clin Proc. 1970 Sep. 45(9):636-44. [QxMD MEDLINE Link].
- Tunbridge WM, Evered DC, Hall R, et al. The spectrum of thyroid disease in a community: the Whickham survey. Clin Endocrinol (Oxf). 1977 Dec. 7(6):481-93. [QxMD MEDLINE Link].
- Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf). 1995 Jul. 43(1):55-68. [QxMD MEDLINE Link].
- Riis AL, Jørgensen JO, Gjedde S, et al. Whole body and forearm substrate metabolism in hyperthyroidism: evidence of increased basal muscle protein breakdown. Am J Physiol Endocrinol Metab. 2005 Jun. 288(6):E1067-73. [QxMD MEDLINE Link].
- Nayak B, Burman K. Thyrotoxicosis and thyroid storm. Endocrinol Metab Clin North Am. 2006 Dec. 35(4):663-86, vii. [QxMD MEDLINE Link].
- Burch HB, Wartofsky L. Life-threatening thyrotoxicosis. Thyroid storm. Endocrinol Metab Clin North Am. 1993 Jun. 22(2):263-77. [QxMD MEDLINE Link].
- Park SE, Cho MA, Kim SH, Rhee Y, Kang ES, Ahn CW. The adaptation and relationship of FGF-23 to changes in mineral metabolism in Graves' disease. Clin Endocrinol (Oxf). 2007 Jun. 66(6):854-8. [QxMD MEDLINE Link].
- Uchida T, Takeno K, Goto M, et al. Superior thyroid artery mean peak systolic velocity for the diagnosis of thyrotoxicosis in Japanese patients. Endocr J. 2010 Mar 6. [QxMD MEDLINE Link]. [Full Text].
- Bunevicius R, Prange AJ Jr. Psychiatric manifestations of Graves' hyperthyroidism: pathophysiology and treatment options. CNS Drugs. 2006. 20(11):897-909. [QxMD MEDLINE Link].
- Vogel A, Elberling TV, Hørding M, Dock J, Rasmussen AK, Feldt-Rasmussen U. Affective symptoms and cognitive functions in the acute phase of Graves' thyrotoxicosis. Psychoneuroendocrinology. 2007 Jan. 32(1):36-43. [QxMD MEDLINE Link].
- Folkestad L, Brandt F, Lillevang-Johansen M, Brix TH, Hegedus L. Graves' Disease and Toxic Nodular Goiter, Aggravated by Duration of Hyperthyroidism, Are Associated with Alzheimer's and Vascular Dementia: A Registry-Based Long-Term Follow-Up of Two Large Cohorts. Thyroid. 2020 Mar 3. [QxMD MEDLINE Link].
- Schwartz KM, Fatourechi V, Ahmed DD, Pond GR. Dermopathy of Graves' disease (pretibial myxedema): long-term outcome. J Clin Endocrinol Metab. 2002 Feb. 87(2):438-46. [QxMD MEDLINE Link].
- Kim JW, Ko J, Woo YJ, Bae HW, Yoon JS. Prevalence of Ocular Hypertension and Glaucoma as well as Associated Factors in Graves' Orbitopathy. J Glaucoma. 2018 Mar 19. [QxMD MEDLINE Link].
- Boelaert K, Newby PR, Simmonds MJ, et al. Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med. 2010 Feb. 123(2):183.e1-9. [QxMD MEDLINE Link].
- Alex-Ann Beliard K, Shyamkumar S, Brar PC, Rapaport R. Graves disease in infancy: a patient presentation and literature review. Endocrinol Diabetes Metab Case Rep. 2021 Jun 1. 2021:[QxMD MEDLINE Link]. [Full Text].
- Tun NN, Beckett G, Zammitt NN, Strachan MW, Seckl JR, Gibb FW. Thyrotropin Receptor Antibody Levels at Diagnosis and After Thionamide Course Predict Graves' Disease Relapse. Thyroid. 2016 Jul 6. [QxMD MEDLINE Link].
- Rabon S, Burton AM, White PC. Graves' Disease in Children: Long Term Outcomes of Medical Therapy. Clin Endocrinol (Oxf). 2016 May 12. [QxMD MEDLINE Link].
- Song E, Koo MJ, Noh E, et al. Risk of Diabetes in Patients with Long-Standing Graves' Disease: A Longitudinal Study. Endocrinol Metab (Seoul). 2021 Dec. 36 (6):1277-86. [QxMD MEDLINE Link]. [Full Text].
- Chen JL, Chiu HW, Tseng YJ, Chu WC. Hyperthyroidism is characterized by both increased sympathetic and decreased vagal modulation of heart rate: evidence from spectral analysis of heart rate variability. Clin Endocrinol (Oxf). 2006 Jun. 64(6):611-6. [QxMD MEDLINE Link].
- Kung AW. Clinical review: Thyrotoxic periodic paralysis: a diagnostic challenge. J Clin Endocrinol Metab. 2006 Jul. 91(7):2490-5. [QxMD MEDLINE Link].
- Ryan DP, da Silva MR, Soong TW, et al. Mutations in potassium channel Kir2.6 cause susceptibility to thyrotoxic hypokalemic periodic paralysis. Cell. 2010 Jan 8. 140(1):88-98. [QxMD MEDLINE Link]. [Full Text].
- Tanda ML, Piantanida E, Liparulo L, Veronesi G, Lai A, Sassi L, et al. Prevalence and Natural History of Graves' Orbitopathy in a Large Series of Patients with Newly Diagnosed Graves' Hyperthyroidism Seen at a Single Center. J Clin Endocrinol Metab. 2013 Feb 13. [QxMD MEDLINE Link].
- Chung JO, Cho DH, Chung DJ, et al. Ultrasonographic features of papillary thyroid carcinoma in patients with Graves' disease. Korean J Intern Med. 2010 Mar. 25(1):71-6. [QxMD MEDLINE Link]. [Full Text].
- Pellegriti G, Mannarino C, Russo M, Terranova R, Marturano I, Vigneri R. Increased Mortality in Patients with Differentiated Thyroid Cancer Associated With Graves' Disease. J Clin Endocrinol Metab. 2013 Jan 24. [QxMD MEDLINE Link].
- Brandt F, Thvilum M, Almind D, Christensen K, Green A, Hegedus L, et al. Graves´ disease and toxic nodular goiter are both associated with increased mortality but differ with respect to the cause of death. A Danish population-based register study. Thyroid. 2012 Dec 20. [QxMD MEDLINE Link].
- Zaletel K, Krhin B, Gaberscek S, Pirnat E, Hojker S. The influence of the exon 1 polymorphism of the cytotoxic T lymphocyte antigen 4 gene on thyroid antibody production in patients with newly diagnosed Graves' disease. Thyroid. 2002 May. 12(5):373-6. [QxMD MEDLINE Link].
- Zaletel K, Krhin B, Gaberscek S, Hojker S. Thyroid autoantibody production is influenced by exon 1 and promoter CTLA-4 polymorphisms in patients with Hashimoto's thyroiditis. Int J Immunogenet. 2006 Apr. 33(2):87-91. [QxMD MEDLINE Link].
- Wang PW, Chen IY, Liu RT, Hsieh CJ, Hsi E, Juo SH. Cytotoxic T lymphocyte-associated molecule-4 gene polymorphism and hyperthyroid Graves' disease relapse after antithyroid drug withdrawal: a follow-up study. J Clin Endocrinol Metab. 2007 Jul. 92(7):2513-8. [QxMD MEDLINE Link].
- Ban Y, Tozaki T, Taniyama M, Tomita M, Ban Y. Association of a C/T single-nucleotide polymorphism in the 5' untranslated region of the CD40 gene with Graves' disease in Japanese. Thyroid. 2006 May. 16(5):443-6. [QxMD MEDLINE Link].
- Heward JM, Brand OJ, Barrett JC, Carr-Smith JD, Franklyn JA, Gough SC. Association of PTPN22 haplotypes with Graves' disease. J Clin Endocrinol Metab. 2007 Feb. 92(2):685-90. [QxMD MEDLINE Link].
- Minich WB, Dehina N, Welsink T, Schwiebert C, Morgenthaler NG, Köhrle J. Autoantibodies to the IGF1 Receptor in Graves' Orbitopathy. J Clin Endocrinol Metab. 2013 Feb. 98(2):752-60. [QxMD MEDLINE Link].
- Benvenga S, Guarneri F, Vaccaro M, et al. Homologies between proteins of Borrelia burgdorferi and thyroid autoantigens. Thyroid. 2004. 14:964-6. [QxMD MEDLINE Link].
- Gangi E, Kapatral V, El-Azami El-Idrissi M, et al. Characterization of a recombinant Yersinia enterocolitica lipoprotein; implications for its role in autoimmune response against thyrotropin receptor. Autoimmunity. 2004 Sep-Nov. 37(6-7):515-20. [QxMD MEDLINE Link].
- De Bellis A, Sansone D, Coronella C, et al. Serum antibodies to collagen XIII: a further good marker of active Graves' ophthalmopathy. Clin Endocrinol (Oxf). 2005 Jan. 62(1):24-9. [QxMD MEDLINE Link].
- Cappelli C, Pirola I, De Martino E, Agosti B, Delbarba A, Castellano M. The role of imaging in Graves' disease: A cost-effectiveness analysis. Eur J Radiol. 2007 Apr 23. [QxMD MEDLINE Link].
- Markovic V, Eterovic D. Thyroid echogenicity predicts outcome of radioiodine therapy in patients with graves' disease. J Clin Endocrinol Metab. 2007 Sep. 92(9):3547-52. [QxMD MEDLINE Link].
- Yasuda K, Miyoshi Y, Tachibana M, et al. Relationship between dose of antithyroid drugs and adverse events in pediatric patients with Graves' disease. Clin Pediatr Endocrinol. 2017 Jan. 26 (1):1-7. [QxMD MEDLINE Link]. [Full Text].
- Kubota S, Ohye H, Yano G, Nishihara E, Kudo T, Ito M. Two-day thionamide withdrawal prior to radioiodine uptake sufficiently increases uptake and does not exacerbate hyperthyroidism compared to 7-day withdrawal in Graves' disease. Endocr J. 2006 Oct. 53(5):603-7. [QxMD MEDLINE Link].
- Bonnema SJ, Bennedbaek FN, Veje A, et al. Propylthiouracil before 131I therapy of hyperthyroid diseases: effect on cure rate evaluated by a randomized clinical trial. J Clin Endocrinol Metab. 2004. 89:4439-44. [QxMD MEDLINE Link].
- Read CH Jr, Tansey MJ, Menda Y. A 36-year retrospective analysis of the efficacy and safety of radioactive iodine in treating young Graves' patients. J Clin Endocrinol Metab. 2004 Sep. 89(9):4229-33. [QxMD MEDLINE Link].
- Ceccarelli C, Canale D, Battisti P, Caglieresi C, Moschini C, Fiore E. Testicular function after 131I therapy for hyperthyroidism. Clin Endocrinol (Oxf). 2006 Oct. 65(4):446-52. [QxMD MEDLINE Link].
- Lutterman SL, Zwaveling-Soonawala N, Verberne HJ, Verburg FA, van Trotsenburg ASP, Mooij CF. The Efficacy and Short- and Long-Term Side Effects of Radioactive Iodine Treatment in Pediatric Graves' Disease: A Systematic Review. Eur Thyroid J. 2021 Jul. 10 (5):353-63. [QxMD MEDLINE Link]. [Full Text].
- Rivkees SA, Dinauer C. An optimal treatment for pediatric Graves' disease is radioiodine. J Clin Endocrinol Metab. 2007 Mar. 92(3):797-800. [QxMD MEDLINE Link].
- Chen YK, Lin CL, Chang YJ, Cheng FT, Peng CL, Sung FC. Cancer risk in patients with Graves' disease: A nationwide cohort study. Thyroid. 2013 Feb 19. [QxMD MEDLINE Link].
- Kim BW. Does Radioactive Iodine Therapy for Hyperthyroidism Cause Cancer?. J Clin Endocrinol Metab. 2022 Jan 18. 107 (2):e448-57. [QxMD MEDLINE Link]. [Full Text].
- Stein JD, Childers D, Gupta S, Talwar N, Nan B, Lee BJ, et al. Risk factors for developing thyroid-associated ophthalmopathy among individuals with Graves disease. JAMA Ophthalmol. 2015 Mar. 133 (3):290-6. [QxMD MEDLINE Link].
- Lanzolla G, Sabini E, Leo M, et al. Statins for Graves' orbitopathy (STAGO): a phase 2, open-label, adaptive, single centre, randomised clinical trial. Lancet Diabetes Endocrinol. 2021 Nov. 9 (11):733-42. [QxMD MEDLINE Link].
- Lanzolla G, Sabini E, Leo M, et al. Statins for Graves' orbitopathy (STAGO): a phase 2, open-label, adaptive, single centre, randomised clinical trial. Lancet Diabetes Endocrinol. 2021 Nov. 9 (11):733-42. [QxMD MEDLINE Link].
- Watanabe N, Noh JY, Kozaki A, Iwaku K, Sekiya K, Kosuga Y, et al. Radioiodine-Associated Exacerbation of Graves' Orbitopathy in the Japanese Population: Randomized Prospective Study. J Clin Endocrinol Metab. 2015 Jul. 100 (7):2700-8. [QxMD MEDLINE Link].
- Shiber S, Stiebel-Kalish H, Shimon I, Grossman A, Robenshtok E. Glucocorticoid regimens for prevention of Graves' ophthalmopathy progression following radioiodine treatment: systematic review and meta-analysis. Thyroid. 2014 Oct. 24 (10):1515-23. [QxMD MEDLINE Link].
- Bartalena L, Marcocci C, Bogazzi F, et al. Relation between therapy for hyperthyroidism and the course of Graves' ophthalmopathy. N Engl J Med. 1998 Jan 8. 338(2):73-8. [QxMD MEDLINE Link].
- Bartalena L, Marcocci C, Bogazzi F, Panicucci M, Lepri A, Pinchera A. Use of corticosteroids to prevent progression of Graves' ophthalmopathy after radioiodine therapy for hyperthyroidism. N Engl J Med. 1989 Nov 16. 321(20):1349-52. [QxMD MEDLINE Link].
- Bartalena L, Tanda ML, Piantanida E, Lai A, Pinchera A. Relationship between management of hyperthyroidism and course of the ophthalmopathy. J Endocrinol Invest. 2004 Mar. 27(3):288-94. [QxMD MEDLINE Link].
- Quah Qin Xian N, Alnahrawy A, Akshikar R, Lee V. Real-World Efficacy and Safety of Mycophenolate Mofetil in Active Moderate-to-Sight-Threatening Thyroid Eye Disease. Clin Ophthalmol. 2021. 15:1921-32. [QxMD MEDLINE Link]. [Full Text].
- Bartalena L, Kahaly GJ, Baldeschi L, et al. The 2021 European Group on Graves' orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves' orbitopathy. Eur J Endocrinol. 2021 Aug 27. 185 (4):G43-G67. [QxMD MEDLINE Link]. [Full Text].
- Rajendram R, Bunce C, Lee RW, Morley AM. Orbital radiotherapy for adult thyroid eye disease. Cochrane Database Syst Rev. 2012 Jul 11. 7:CD007114. [QxMD MEDLINE Link].
- Wakelkamp IM, Tan H, Saeed P, et al. Orbital irradiation for Graves' ophthalmopathy: Is it safe? A long-term follow-up study. Ophthalmology. 2004 Aug. 111(8):1557-62. [QxMD MEDLINE Link].
- Seals KF, Lee EW, Cagnon CH, Al-Hakim RA, Kee ST. Radiation-Induced Cataractogenesis: A Critical Literature Review for the Interventional Radiologist. Cardiovasc Intervent Radiol. 2015 Sep 24. [QxMD MEDLINE Link].
- Kahaly GJ, Douglas RS, Holt RJ, Sile S, Smith TJ. Teprotumumab for patients with active thyroid eye disease: a pooled data analysis, subgroup analyses, and off-treatment follow-up results from two randomised, double-masked, placebo-controlled, multicentre trials. Lancet Diabetes Endocrinol. 2021 Jun. 9 (6):360-72. [QxMD MEDLINE Link].
- Salvi M, Vannucchi G, Campi I, Currò N, Dazzi D, Simonetta S. Treatment of Graves' disease and associated ophthalmopathy with the anti-CD20 monoclonal antibody rituximab: an open study. Eur J Endocrinol. 2007 Jan. 156(1):33-40. [QxMD MEDLINE Link].
- Stan MN, Garrity JA, Carranza Leon BG, Prabin T, Bradley EA, Bahn RS. Randomized controlled trial of rituximab in patients with Graves' orbitopathy. J Clin Endocrinol Metab. 2015 Feb. 100 (2):432-41. [QxMD MEDLINE Link].
- Salvi M, Vannucchi G, Currò N, Campi I, Covelli D, Dazzi D, et al. Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves' orbitopathy: a randomized controlled study. J Clin Endocrinol Metab. 2015 Feb. 100 (2):422-31. [QxMD MEDLINE Link].
- Ebner R, Devoto MH, Weil D, et al. Treatment of thyroid associated ophthalmopathy with periocular injections of triamcinolone. Br J Ophthalmol. 2004 Nov. 88(11):1380-6. [QxMD MEDLINE Link]. [Full Text].
- Finamor FE, Martins JR, Nakanami D, Paiva ER, Manso PG, Furlanetto RP. Pentoxifylline (PTX)--an alternative treatment in Graves' ophthalmopathy (inactive phase): assessment by a disease specific quality of life questionnaire and by exophthalmometry in a prospective randomized trial. Eur J Ophthalmol. 2004 Jul-Aug. 14(4):277-83. [QxMD MEDLINE Link].
- Durrani OM, Reuser TQ, Murray PI. Infliximab: a novel treatment for sight-threatening thyroid associated ophthalmopathy. Orbit. 2005 Jun. 24(2):117-9. [QxMD MEDLINE Link].
- Grodski S, Stalberg P, Robinson BG, Delbridge LW. Surgery versus Radioiodine Therapy as Definitive Management for Graves' Disease: The Role of Patient Preference. Thyroid. 2007 Feb. 17(2):157-60. [QxMD MEDLINE Link].
- Liu X, Wong CKH, Chan WWL, et al. Outcomes of Graves' Disease Patients Following Antithyroid Drugs, Radioactive Iodine, or Thyroidectomy as the First-line Treatment. Ann Surg. 2021 Jun 1. 273 (6):1197-206. [QxMD MEDLINE Link]. [Full Text].
- Genovese BM, Noureldine SI, Gleeson EM, Tufano RP, Kandil E. What is the best definitive treatment for graves' disease? A systematic review of the existing literature. Ann Surg Oncol. 2013 Feb. 20(2):660-7. [QxMD MEDLINE Link].
- Pradeep PV, Agarwal A, Baxi M, Agarwal G, Gupta SK, Mishra SK. Safety and efficacy of surgical management of hyperthyroidism: 15-year experience from a tertiary care center in a developing country. World J Surg. 2007 Feb. 31(2):306-12; discussion 313. [QxMD MEDLINE Link].
- Panzer C, Beazley R, Braverman L. Rapid preoperative preparation for severe hyperthyroid Graves' disease. J Clin Endocrinol Metab. 2004 May. 89(5):2142-4. [QxMD MEDLINE Link].
- Piantanida E. Preoperative management in patients with Graves' disease. Gland Surg. 2017 Oct. 6 (5):476-81. [QxMD MEDLINE Link]. [Full Text].
- Erbil Y, Ozluk Y, Giris M, Salmaslioglu A, Issever H, Barbaros U. Effect of lugol solution on thyroid gland blood flow and microvessel density in the patients with Graves' disease. J Clin Endocrinol Metab. 2007 Jun. 92(6):2182-9. [QxMD MEDLINE Link].
- Randle RW, Bates MF, Long KL, Pitt SC, Schneider DF, Sippel RS. Impact of potassium iodide on thyroidectomy for Graves' disease: Implications for safety and operative difficulty. Surgery. 2018 Jan. 163 (1):68-72. [QxMD MEDLINE Link]. [Full Text].
- Calissendorff J, Falhammar H. Lugol's solution and other iodide preparations: perspectives and research directions in Graves' disease. Endocrine. 2017 Dec. 58 (3):467-73. [QxMD MEDLINE Link]. [Full Text].
- Apaydin T, Gogas Yavuz D. Preoperative plasmapheresis in patients with Graves' disease intolerant to antithyroid drugs. Ther Apher Dial. 2021 Dec. 25 (6):877-83. [QxMD MEDLINE Link].
- Zhang Y, Dong Z, Li J, Yang J, Yang W, Wang C. Comparison of endoscopic and conventional open thyroidectomy for Graves' disease: A meta-analysis. Int J Surg. 2017 Feb 22. 40:52-9. [QxMD MEDLINE Link].
- Liao SL, Huang SW. Correlation of retrobulbar volume change with resected orbital fat volume and proptosis reduction after fatty decompression for Graves ophthalmopathy. Am J Ophthalmol. 2011 Mar. 151(3):465-9.e1. [QxMD MEDLINE Link].
- Alsuhaibani AH, Carter KD, Policeni B, Nerad JA. Effect of orbital bony decompression for Graves' orbitopathy on the volume of extraocular muscles. Br J Ophthalmol. 2011 Sep. 95(9):1255-8. [QxMD MEDLINE Link].
- Hiraiwa T, Ito M, Imagawa A, et al. High diagnostic value of a radioiodine uptake test with and without iodine restriction in Graves' disease and silent thyroiditis. Thyroid. 2004 Jul. 14(7):531-5. [QxMD MEDLINE Link].
- Anagnostis P, Adamidou F, Polyzos SA, Katergari S, Karathanasi E, Zouli C, et al. Predictors of long-term remission in patients with Graves' disease: a single center experience. Endocrine. 2013 Feb 11. [QxMD MEDLINE Link].
- Sato H, Sasaki N, Minamitani K, Minagawa M, Kazukawa I, Sugihara S, et al. Higher dose of methimazole causes frequent adverse effects in the management of Graves' disease in children and adolescents. J Pediatr Endocrinol Metab. 2012. 25(9-10):863-7. [QxMD MEDLINE Link].
- Lanzolla G, Marinò M, Marcocci C. Selenium in the Treatment of Graves' Hyperthyroidism and Eye Disease. Front Endocrinol (Lausanne). 2020. 11:608428. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid. 2016 Oct. 26 (10):1343-1421. [QxMD MEDLINE Link]. [Full Text].
- Rivkees SA, Stephenson K, Dinauer C. Adverse events associated with methimazole therapy of Graves' disease in children. Int J Pediatr Endocrinol. 2010. 2010:176970. [QxMD MEDLINE Link]. [Full Text].
- Mohlin E, Filipsson Nyström H, Eliasson M. Long-term prognosis after medical treatment of Graves' disease in a northern Swedish population 2000-2010. Eur J Endocrinol. 2014 Mar. 170 (3):419-27. [QxMD MEDLINE Link].
- Ye X, Liu J, Wang Y, Bin L, Wang J. Increased serum VEGF and b-FGF in Graves' ophthalmopathy. Graefes Arch Clin Exp Ophthalmol. 2014 Oct. 252 (10):1639-44. [QxMD MEDLINE Link].
- Macchia PE, Bagattini M, Lupoli G, et al. High-dose intravenous corticosteroid therapy for Graves' ophthalmopathy. J Endocrinol Invest. 2001. 24:152-8. [QxMD MEDLINE Link].
- Gibson A, Czyz CN. Graves Disease, Orbital Decompression. 2018 Jan. [QxMD MEDLINE Link]. [Full Text].
- Sisti E, Coco B, Menconi F, Leo M, Rocchi R, Latrofa F, et al. Intravenous glucocorticoid therapy for Graves' ophthalmopathy and acute liver damage: an epidemiological study. Eur J Endocrinol. 2015 Mar. 172 (3):269-76. [QxMD MEDLINE Link].
- Dickinson AJ, Vaidya B, Miller M, Coulthard A, Perros P, Baister E. Double-blind, placebo-controlled trial of octreotide long-acting repeatable (LAR) in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2004 Dec. 89(12):5910-5. [QxMD MEDLINE Link].
- Wemeau JL, Caron P, Beckers A, et al. Octreotide (long-acting release formulation) treatment in patients with graves' orbitopathy: clinical results of a four-month, randomized, placebo-controlled, double-blind study. J Clin Endocrinol Metab. 2005. 90:841-8. [QxMD MEDLINE Link].
- Stan MN, Garrity JA, Bradley EA, Woog JJ, Bahn MM, Brennan MD. Randomized, double-blind, placebo-controlled trial of long-acting release octreotide for treatment of Graves' ophthalmopathy. J Clin Endocrinol Metab. 2006 Dec. 91(12):4817-24. [QxMD MEDLINE Link].
- Yang YT, Chen JF, Tung SC, et al. Long-term outcome and prognostic factors of single-dose Radioiodine Therapy in patients with Graves' disease. J Formos Med Assoc. 2020 Feb 10. [QxMD MEDLINE Link]. [Full Text].
- Liu X, Shi B, Li H. Valuable predictive features of relapse of Graves' disease after antithyroid drug treatment. Ann Endocrinol (Paris). 2015 Oct 26. [QxMD MEDLINE Link].
- Villagelin D, Romaldini JH, Santos RB, Milkos AB, Ward LS. Outcomes in Relapsed Graves' Disease Patients Following Radioiodine or Prolonged Low Dose of Methimazole Treatment. Thyroid. 2015 Oct 20. [QxMD MEDLINE Link].
- Salvi M, Campi I. Medical Treatment of Graves' Orbitopathy. Horm Metab Res. 2015 Sep. 47 (10):779-88. [QxMD MEDLINE Link].
- Prasek K, Płazińska MT, Krolicki L. Diagnosis and treatment of Graves' disease with particular emphasis on appropriate techniques in nuclear medicine. General state of knowledge. Nucl Med Rev Cent East Eur. 2015. 18 (2):110-6. [QxMD MEDLINE Link].
- Jankauskiene J, Jarusaitiene D. The Influence of Juvenile Graves' Ophthalmopathy on Graves' Disease Course. J Ophthalmol. 2017. 2017:4853905. [QxMD MEDLINE Link]. [Full Text].
Author
Sai-Ching Jim Yeung, MD, PhD, FACP Professor of Medicine, Department of Emergency Medicine, Department of Endocrine Neoplasia and Hormonal Disorders, The University of Texas MD Anderson Cancer Center
Sai-Ching Jim Yeung, MD, PhD, FACP is a member of the following medical societies: American Association for Cancer Research, American College of Physicians, American Medical Association, American Thyroid Association, Endocrine Society
Disclosure: Serve(d) as a director, officer, partner, employee, advisor, consultant or trustee for: Celgene, Inc.
Received research grant from: DepoMed and Bristol-Myer-Squibb.
Coauthor(s)
Alice Cua Chiu, MD Associate Affiliate, Department of Internal Medicine, Division of Endocrinology, Bayshore Medical Center
Alice Cua Chiu, MD is a member of the following medical societies: American Medical Association, Endocrine Society
Disclosure: Nothing to disclose.
Specialty Editor Board
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Received salary from Medscape for employment. for: Medscape.
Chief Editor
Romesh Khardori, MD, PhD, FACP (Retired) Professor, Division of Endocrinology, Diabetes and Metabolism, Department of Internal Medicine, Eastern Virginia Medical School
Romesh Khardori, MD, PhD, FACP is a member of the following medical societies: American Association of Clinical Endocrinology, American College of Physicians, American Diabetes Association, Endocrine Society
Disclosure: Nothing to disclose.
Additional Contributors
Steven R Gambert, MD Professor of Medicine, Johns Hopkins University School of Medicine; Director of Geriatric Medicine, University of Maryland Medical Center and R Adams Cowley Shock Trauma Center
Steven R Gambert, MD is a member of the following medical societies: Alpha Omega Alpha, American Association for Physician Leadership, American College of Physicians, American Geriatrics Society, Endocrine Society, Gerontological Society of America, Association of Professors of Medicine
Disclosure: Nothing to disclose.