Chronic Kidney Disease in Children: Practice Essentials, Background, Etiology and Pathophysiology (original) (raw)
Overview
Practice Essentials
Chronic kidney disease (CKD) is characterized by an irreversible deterioration of renal function that gradually progresses to end-stage renal disease. Although children represent only a small proportion of all patients with CKD, they pose unique challenges because of the many extrarenal manifestations of CKD that complicate management.
Signs and symptoms
CKD is asymptomatic in its earliest stages (stage I and stage II), although urinalysis findings or blood pressure may be abnormal. As CKD progresses to more advanced stages, signs and symptoms greatly increase. Polydipsia and nocturia (secondary to a reduced capacity to concentrate the urine) may be some of the earliest symptoms that suggest CKD in an otherwise healthy-looking child who has tubulointerstitial kidney disease.
The signs and symptoms in advanced CKD may include the following:
- Anorexia, nausea, vomiting
- Bone disease (termed osteodystrophy)
- Cardiovascular disease
- Volume overload
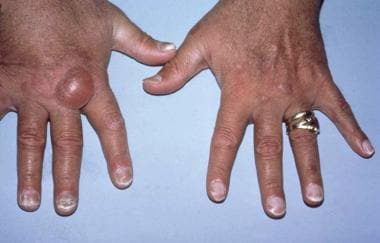
The image below illustrates several uremia-related cutaneous disorders.
Hands of a transfusion-dependent patient on long-term hemodialysis. Several uremia-related cutaneous disorders are visible. The pigmentary alteration results from retained urochromes and hemosiderin deposition. The large bullae are consistent with either porphyria cutanea tarda or the bullous disease of dialysis. All nails show the distal brown-red and proximal white coloring of half-and-half nails.
See Presentation for more detail.
Diagnosis
Initial testing in a child with suspected CKD must include an examination of the urine and estimation of the glomerular filtration rate. Anemia is an important clinical finding in CKD, and a complete blood cell count is an important investigation both in the initial evaluation and the subsequent follow-up in affected children.
Imaging studies such as ultrasonography and radionuclide studies help in confirming the diagnosis of CKD and may also provide clues to its etiology.
See Workup for more detail.
Management
Treatment of chronic kidney disease should include the following:
- Specific therapy based on diagnosis
- Evaluation and management of reversible causes of renal dysfunction
- Prevention and treatment of complications of decreased kidney function (eg, anemia, bone disease, cardiovascular manifestations, hypertension, growth failure)
- Evaluation and management of comorbid conditions
- Slowing the loss of kidney function
- Preparation for kidney failure therapy
- Replacement of kidney function with dialysis and transplantation if signs and symptoms of uremia are present
- Management of complications
See Treatment for more detail.

Background
Chronic kidney disease (CKD) and renal failure (RF) have been recognized as significant medical problems for most of the last 2 centuries and, until relatively recently, were uniformly fatal. Scientific and technologic improvements during the second half of the 20th century provided renal replacement therapy as a life-sustaining option for many individuals who otherwise may have died. The impact of these medical advancements has been remarkable.
Chronic kidney disease is characterized by an irreversible deterioration of renal function that gradually progresses to end-stage renal disease (ESRD). [1] Chronic kidney disease has emerged as a serious public health problem. Data from the United States Renal Data System (USRDS) show that incidence of kidney failure is rising among adults and is commonly associated with poor outcomes and high cost. [2] Moreover, in the past 2 decades, the incidence of chronic kidney disease in children has steadily increased, with poor and ethnic minority children disproportionately affected. [2, 3]
Children, adolescents, and young adults constitute less than 5% of the end-stage kidney disease (ESKD) population, and their 10-year survival ranges from 70% to 85%. [2, 4, 5] Although children represent only a small proportion of all patients with CKD, affected children pose unique challenges to the healthcare system and to their providers, who must address not only the primary renal disorder, but the many extrarenal manifestations of CKD that complicate management. [6, 7, 4, 1] Most importantly, the development of ESRD compromises the life expectancy of affected patients, with an age-specific mortality rate for children receiving dialysis that is 30 to 150 times higher than for healthy peers.
The major health consequences of chronic kidney disease include not only progression to kidney failure but also an increased risk of cardiovascular disease. Evidence-based clinical practice guidelines support early recognition and treatment of chronic kidney disease–related complications to improve growth and development and, ultimately, the quality of life in children with this chronic condition. Appropriate pediatric care may reduce the prevalence of this complex and expensive condition.
Definition of chronic renal disease
The definition and classification of chronic renal disease may help identify affected individuals, possibly resulting in the early institution of effective therapy. To achieve this goal, the Kidney Disease Outcomes Quality Initiative (KDOQI) working group of the National Kidney Foundation (NKF) defined chronic kidney disease as "evidence of structural or functional kidney abnormalities (abnormal urinalysis, imaging studies, or histology) that persist for at least 3 months, with or without a decreased glomerular filtration rate (GFR), as defined by a GFR of less than 60 mL/min per 1.73 m2." [5, 6, 7, 4, 1, 8]
Note, however, that the above definition is not applicable to children younger than 2 years, because they normally have a low GFR, even when corrected for body surface area. In these patients, calculated GFR based on serum creatinine can be compared with normative age-appropriate values to detect renal impairment.
See also Chronic Renal Failure, Renal Failure, Chronic and Dialysis Complications,Dermatologic Manifestations of Renal Disease, Renal Transplantation (Medical), and Perioperative Management of the Patient With Chronic Renal Failure.

Etiology and Pathophysiology
The chief causes of chronic kidney disease (CKD) in children include the following:
- Obstructive uropathy
- Hypoplastic or dysplastic kidneys
- Reflux nephropathy
- Focal segmental glomerulosclerosis as a variant of childhood nephritic syndrome
The distribution of causes varies with age. Whereas congenital anomalies of the kidney and urinary tract predominate in younger patients, glomerulonephritis is the leading cause in children older than 12 years of age.
Despite the diverse etiologies, once chronic kidney disease develops, the subsequent response of the failing kidney is similar. The kidney initially adapts to damage by increasing the filtration rate in the remaining normal nephrons, a process called adaptive hyperfiltration. As a result, patients with mild chronic kidney disease often have a normal or near-normal serum creatinine concentration. Additional homeostatic mechanisms (most frequently occurring within the renal tubules) permit the serum concentrations of sodium, potassium, calcium, and phosphorous and total body water to also remain within the reference range, particularly among those with mild to moderate stages of chronic kidney disease.
Adaptive hyperfiltration, although initially beneficial, appears to result in long-term damage to the glomeruli of the remaining nephrons, which is manifested by pathologic proteinuria and progressive kidney insufficiency. This irreversibility appears to be responsible for the development of end-stage kidney failure among persons in whom the original illness is either inactive or cured.
Although the underlying problem that initiated chronic kidney disease often cannot be treated primarily, extensive studies in experimental animals and preliminary studies in humans suggest that progression in chronic renal disease may be largely due to secondary factors that are unrelated to the activity of the initial disease. These include anemia, osteodystrophy, systemic and intraglomerular hypertension, glomerular hypertrophy, proteinuria, metabolic acidosis, hyperlipidemia, tubulointerstitial disease, systemic inflammation, and altered prostanoid metabolism. This common sequence of events in diverse types of chronic kidney disease is the basis for the common management plan for children with chronic kidney disease, irrespective of the etiology.
A prospective multicenter cohort study by Greenberg et al that assessed long-term kidney outcomes after pediatric cardiac surgery (between one and 18 months of age) reported that at follow-up after cardiopulmonary bypass, 17% of the 131 children in the study (22 children) had hypertension and 18% (21 children) had chronic kidney disease. [9]

Epidemiology
United States statistics
Based on data from the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) chronic renal insufficiency (CRI) database, 5651 patients aged 2-17 years were entered into this voluntary listing and had an estimated glomerular filtration rate (eGFR) of less than 75 mL/min per 1.73 m2. [10] In the past 2 decades, the incidence of the disease has steadily increased among all ethnic groups.
In a National Health and Nutrition Examination Survey (NHANES) on the prevalence of chronic kidney disease (CKD) in adolescents aged 12-18 years, the authors observed that the prevalence of persistent albuminuria was similar between 1988 and 2014 and ranged from 3.29% to 3.26%. However, the prevalence of both reduced and low eGFRs was higher in the most recent study period. [11] According to NHANES data from 2005 to 2020, 0.51% of US children aged 12-17 years had a low eGFR (< 60 mL/min/1.73 m2). [3]
International statistics
Globally, the prevalence of CKD stage II or lower in children is reported to be approximately 18.5-58.3 per million children. Disease prevalence is much lower than that in adults; in a study from India, children constituted 5.3% of all patients with chronic kidney disease seen in a referral hospital. [12] Data from the ItalKid study reported a mean incidence of 12.1 cases per year per million in the age-related population (age range, 8.8-13.9 y) and a prevalence of 74.7 per million in this population. [13] However, underreporting due to lack of recognition may suggest an even higher prevalence in children.
Sexual, racial, and age differences in incidence
In the United States, the incidence and rate of progression to end-stage renal disease (ESRD) are equal in both sexes, although obstructive uropathies are more common in males.
ESRD rates in Black individuals are 2.7 times higher than in White individuals, which may be due to genetic susceptibility; other factors may include socioeconomic problems and limited access to medical care. Such factors may result in the delivery of excessive numbers of low birth weight (LBW) babies, partially accounting for the observed increased incidence of ESRD, because chronic kidney disease is more common with increasing prematurity and survivorship.
Choi et al found that rates of ESRD among Black patients exceeded those among White patients at all levels of baseline eGFR. [14] Similarly, mortality rates among Black patients were equal to or higher than those among White patients at all levels of eGFR. Risk of ESRD among Black patients was highest at an eGFR of 45-59 mL/min/1.73 m2, as was the risk of mortality. [2, 3]
The frequency of chronic kidney disease increases with age and is much more common in adults than children. Among children, chronic kidney disease is more common in children older than 6 years than in those younger than 6 years. The percentages in the NAPRTCS cohort were 19% in children aged 0-1 years; 17% in those aged 6-12 years; 33% in children aged 2-5 years; and 31% in those older than 12 years. [10]

Genetics
Chronic kidney disease (CKD) is a heterogeneous disease, with possibly both genomic and environmental contributory factors. Various studies have shown a high CKD heritability (30-75%).
Genome-wide association studies (GWAS) and GWAS meta-analyses have identified several genetic loci, including variants in UMOD, SHROOM3, solute carriers, and E3 ubiquitin ligases. However, these genetic markers do not account for all the susceptibility to CKD, and other factors must contribute to the missing heritability.
A shorter telomere length has been associated with renal dysfunction and CKD progression, although most studies include small numbers of cases with variable findings. Copy number variants (CNVs) have been linked to congenital anomalies of the kidney and urinary tract, posterior urethral valves, nephronophthisis, and immunoglobulin A nephropathy. The A3243G mutation in the MT-TL1 gene has been associated with focal segmental glomerulosclerosis.
Only one GWAS has found associations between X chromosome and renal function (rs12845465 and rs5987107). No loci in the Y chromosome have reached genome-wide significance. Although additional biomarkers have been investigated in less common suspects such as telomeres, CNVs, mitochondrial DNA, and sex chromosomes, hidden heritability in CKD remains unexplained. [15]

Prognosis
Once chronic kidney disease (CKD) occurs, progression to end-stage renal disease (ESRD) appears certain. In a study by Warady et al, hypoalbuminemia, hypertension, dyslipidemia, male gender, anemia, nephrotic range proteinuria, dyslipidemia at baseline, hyperphosphatemia, and lower values for glomerular filtration rate (GFR) at baseline were predictors of rapid progression. [16] However, the rate of progression depends on the underlying diagnosis, on the successful implementation of secondary preventive measures, and on the individual patient.
About 70% of children with chronic kidney disease develop ESRD by age 20 years. Children with ESRD have a 10-year survival rate of about 80% and an age-specific mortality rate of about 30 times that seen in children without ESRD. The most common cause of death in these children is cardiovascular disease, followed by infection. Of the deaths due to cardiovascular causes, 25% were attributed to cardiac arrest (cause uncertain), 16% to stroke, 14% to myocardial ischemia, 12% to pulmonary edema, 11% to hyperkalemia, and 22% to other cardiovascular causes, including arrhythmia. Data from the Australia and New Zealand (ANZ) registry revealed that the risk of death was associated with the year in which renal replacement therapy was initiated, the age of patients at the start of that therapy, and the type of dialysis used. [17]
Once the estimated glomerular filtration rate (eGFR) declines to less than 30 mL/min per 1.73 m2 and the child has stage IV chronic kidney disease, the child and the family should be prepared for renal replacement therapy. The family should be provided with information related to preemptive kidney transplantation, peritoneal dialysis, and hemodialysis. When preemptive transplantation is not an option, the choice between the 2 forms of dialysis is generally dictated by technical, social, and compliance issues, as well as family preference. Peritoneal dialysis is much more common in infants and younger children.
Patients on long-term dialysis have a high incidence of morbidity and mortality. Preemptive renal transplantation should be the goal of management in these children.

Patient Education
Children with chronic kidney disease and their families should receive education about the importance of compliance with secondary preventative measures, natural disease progression, prescribed medications (highlighting their potential benefits and adverse effects), diet, and types of long-term renal replacement modalities.
Information related to preemptive kidney transplantation, peritoneal dialysis, and hemodialysis should be provided to the family once the child's estimated glomerular filtration rate (eGFR) declines to less than 30 mL/min per 1.73 m2 and the child has stage IV chronic kidney disease.
For patient education information, see Chronic Kidney Disease and Kidney Transplant.

- [Guideline] Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021 Oct. 100 (4S):S1-S276. [QxMD MEDLINE Link].
- Saran R, Li Y, Robinson B, et al. US Renal Data System 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2015 Jul. 66 (1 Suppl 1):Svii, S1-305. [QxMD MEDLINE Link].
- United States Renal Data System. Chronic Kidney Disease: Chapter 5. Kidney and urologic disease among children and adolescents. 2023 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2023. [Full Text].
- [Guideline] Ikizler TA, Burrowes JD, Byham-Gray LD, et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am J Kidney Dis. 2020 Sep. 76 (3 Suppl 1):S1-S107. [QxMD MEDLINE Link].
- [Guideline] Kopple JD. National Kidney Foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis. 2001 Jan. 37(1 Suppl 2):S66-70. [QxMD MEDLINE Link].
- [Guideline] National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002 Feb. 39(2 Suppl 1):S1-266. [QxMD MEDLINE Link].
- [Guideline] KDOQI. KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 update. Executive summary. Am J Kidney Dis. 2009 Mar. 53(3 Suppl 2):S11-104. [QxMD MEDLINE Link].
- Beck LH Jr, Ayoub I, Caster D, et al. KDOQI US Commentary on the 2021 KDIGO Clinical Practice Guideline for the Management of Glomerular Diseases. Am J Kidney Dis. 2023 Aug. 82 (2):121-75. [QxMD MEDLINE Link].
- Greenberg JH, Zappitelli M, Devarajan P, Thiessen-Philbrook HR, Krawczeski C, Li S, et al. Kidney Outcomes 5 Years After Pediatric Cardiac Surgery: The TRIBE-AKI Study. JAMA Pediatr. 2016 Nov 1. 170 (11):1071-1078. [QxMD MEDLINE Link].
- Seikaly MG, Ho PL, Emmett L, et al. Chronic renal insufficiency in children: the 2001 Annual Report of the NAPRTCS. Pediatr Nephrol. 2003 Aug. 18(8):796-804. [QxMD MEDLINE Link].
- Saydah SH, Xie H, Imperatore G, Burrows NR, Pavkov ME. Trends in albuminuria and GFR among adolescents in the United States, 1988-2014. Am J Kidney Dis. 2018 Nov. 72 (5):644-52. [QxMD MEDLINE Link].
- Gulati S, Mittal S, Sharma RK, Gupta A. Etiology and outcome of chronic renal failure in Indian children. Pediatr Nephrol. 1999 Sep. 13(7):594-6. [QxMD MEDLINE Link].
- Ardissino G, Dacco V, Testa S, et al. Epidemiology of chronic renal failure in children: data from the ItalKid project. Pediatrics. 2003 Apr. 111(4 Pt 1):e382-7. [QxMD MEDLINE Link].
- Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Hernandez GT, O''Hare AM. White/black racial differences in risk of end-stage renal disease and death. Am J Med. 2009 Jul. 122(7):672-8. [QxMD MEDLINE Link]. [Full Text].
- Cañadas-Garre M, Anderson K, Cappa R, et al. Genetic susceptibility to chronic kidney disease - some more pieces for the heritability puzzle. Front Genet. 2019. 10:453. [QxMD MEDLINE Link].
- Warady BA, Abraham AG, Schwartz GJ, et al. Predictors of rapid progression of glomerular and nonglomerular kidney disease in children and adolescents: the Chronic Kidney Disease in Children (CKiD) cohort. Am J Kidney Dis. 2015 Jun. 65 (6):878-88. [QxMD MEDLINE Link].
- Craven AM, Hawley CM, McDonald SP, et al. Predictors of renal recovery in Australian and New Zealand end-stage renal failure patients treated with peritoneal dialysis. Perit Dial Int. 2007 Mar-Apr. 27(2):184-91. [QxMD MEDLINE Link].
- Hsu CW, Yamamoto KT, Henry RK, De Roos AJ, Flynn JT. Prenatal risk factors for childhood CKD. J Am Soc Nephrol. 2014 Sep. 25(9):2105-11. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Hogg RJ, Furth S, Lemley KV, et al. National Kidney Foundation's Kidney Disease Outcomes Quality Initiative clinical practice guidelines for chronic kidney disease in children and adolescents: evaluation, classification, and stratification. Pediatrics. 2003 Jun. 111(6 Pt 1):1416-21. [QxMD MEDLINE Link].
- Eknoyan G. The importance of early treatment of the anaemia of chronic kidney disease. Nephrol Dial Transplant. 2001. 16 Suppl 5:45-9. [QxMD MEDLINE Link].
- Greenberg JH, Kakajiwala A, Parikh CR, Furth S. Emerging biomarkers of chronic kidney disease in children. Pediatr Nephrol. 2018 Jun. 33 (6):925-33. [QxMD MEDLINE Link].
- Mian AN, Schwartz GJ. Measurement and estimation of glomerular filtration rate in children. Adv Chronic Kidney Dis. 2017 Nov. 24 (6):348-56. [QxMD MEDLINE Link].
- Pierce CB, Muñoz A, Ng DK, Warady BA, Furth SL, Schwartz GJ. Age- and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney Int. 2021 Apr. 99 (4):948-56. [QxMD MEDLINE Link]. [Full Text].
- Sanchez CP. Secondary hyperparathyroidism in children with chronic renal failure: pathogenesis and treatment. Paediatr Drugs. 2003. 5(11):763-76. [QxMD MEDLINE Link].
- [Guideline] Noordzij M, Korevaar JC, Boeschoten EW, Dekker FW, Bos WJ, Krediet RT. The Kidney Disease Outcomes Quality Initiative (K/DOQI) Guideline for Bone Metabolism and Disease in CKD: association with mortality in dialysis patients. Am J Kidney Dis. 2005 Nov. 46(5):925-32. [QxMD MEDLINE Link].
- Seeherunvong W, Abitbol CL, Chandar J, Zilleruelo G, Freundlich M. Vitamin D insufficiency and deficiency in children with early chronic kidney disease. J Pediatr. 2009 Jun. 154(6):906-11.e1. [QxMD MEDLINE Link].
- Salusky IB. A new era in phosphate binder therapy: what are the options?. Kidney Int Suppl. 2006 Dec. (105):S10-5. [QxMD MEDLINE Link].
- Saland JM, Ginsberg H, Fisher EA. Dyslipidemia in pediatric renal disease: epidemiology, pathophysiology, and management. Curr Opin Pediatr. 2002 Apr. 14(2):197-204. [QxMD MEDLINE Link].
- Soergel M, Schaefer F. Effect of hypertension on the progression of chronic renal failure in children. Am J Hypertens. 2002 Feb. 15(2 Pt 2):53S-56S. [QxMD MEDLINE Link].
- Swinford RD, Portman RJ. Measurement and treatment of elevated blood pressure in the pediatric patient with chronic kidney disease. Adv Chronic Kidney Dis. 2004 Apr. 11(2):143-61. [QxMD MEDLINE Link].
- Kari JA, El Desoky SM, Farag YM, Singh AK. Predictors of renal replacement therapy and mortality in children with chronic kidney disease. Saudi Med J. 2015 Jan. 36 (1):32-9. [QxMD MEDLINE Link].
- Haffner D, Schaefer F, Nissel R, et al. Effect of growth hormone treatment on the adult height of children with chronic renal failure. German Study Group for Growth Hormone Treatment in Chronic Renal Failure. N Engl J Med. 2000 Sep 28. 343(13):923-30. [QxMD MEDLINE Link].
- Hodson EM, Willis NS, Craig JC. Growth hormone for children with chronic kidney disease. Cochrane Database Syst Rev. 2012 Feb 15. CD003264. [QxMD MEDLINE Link].
- Drube J, Wan M, Bonthuis M, et al. Clinical practice recommendations for growth hormone treatment in children with chronic kidney disease. Nat Rev Nephrol. 2019 Sep. 15 (9):577-89. [QxMD MEDLINE Link].
- Hui WF, Betoko A, Savant JD, et al. Assessment of dietary intake of children with chronic kidney disease. Pediatr Nephrol. 2017 Mar. 32 (3):485-94. [QxMD MEDLINE Link].
- Mak RH. Chronic kidney disease in children: state of the art. Pediatr Nephrol. 2007 Oct. 22(10):1687-8. [QxMD MEDLINE Link].
- Fogo AB. Mechanisms of progression of chronic kidney disease. Pediatr Nephrol. 2007 Dec. 22 (12):2011-22. [QxMD MEDLINE Link].
- Muscheites J, Wigger M, Drueckler E, Fischer DC, Kundt G, Haffner D. Cinacalcet for secondary hyperparathyroidism in children with end-stage renal disease. Pediatr Nephrol. 2008 Oct. 23(10):1823-9. [QxMD MEDLINE Link].
- Major clinical predictors to be used for the perioperative management of a patient with chronic renal failure. CHF = congestive heart failure.
- Intermediate clinical predictors to be used for the perioperative management of a patient with chronic renal failure. CHF = congestive heart failure; METs = metabolic equivalents of task; MI = myocardial infarction.
- Minor clinical predictors to be used for the perioperative management of a patient with chronic renal failure. ECG = electrocardiogram; METs = metabolic equivalents of task.
- Hands of a transfusion-dependent patient on long-term hemodialysis. Several uremia-related cutaneous disorders are visible. The pigmentary alteration results from retained urochromes and hemosiderin deposition. The large bullae are consistent with either porphyria cutanea tarda or the bullous disease of dialysis. All nails show the distal brown-red and proximal white coloring of half-and-half nails.


Author
Sanjeev Gulati, MD, MBBS, DNB(Peds), DM, DNB(Neph), FIPN(Australia), FICN, FRCPC(Canada) Additional Professor, Department of Nephrology, Sanjay Gandhi Post Graduate Institute of Medical Sciences; Senior Consultant in Pediatric Nephrology and Additional Director, Department of Nephrology and Transplant Medicine, Fortis Institute of Renal Sciences Transplantation, India
Sanjeev Gulati, MD, MBBS, DNB(Peds), DM, DNB(Neph), FIPN(Australia), FICN, FRCPC(Canada) is a member of the following medical societies: American Society of Pediatric Nephrology, International Society of Nephrology, Royal College of Physicians and Surgeons of Canada, Indian Academy of Pediatrics
Disclosure: Nothing to disclose.
Specialty Editor Board
Mary L Windle, PharmD Adjunct Associate Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Nothing to disclose.
Frederick J Kaskel, MD, PhD Director of the Division and Training Program in Pediatric Nephrology, Vice Chair, Department of Pediatrics, Montefiore Medical Center and Albert Einstein School of Medicine
Frederick J Kaskel, MD, PhD is a member of the following medical societies: American Association for the Advancement of Science, Eastern Society for Pediatric Research, Renal Physicians Association, American Academy of Pediatrics, American Pediatric Society, American Physiological Society, American Society of Nephrology, American Society of Pediatric Nephrology, American Society of Transplantation, Federation of American Societies for Experimental Biology, International Society of Nephrology, National Kidney Foundation, New York Academy of Sciences, Sigma Xi, The Scientific Research Honor Society, Society for Pediatric Research
Disclosure: Nothing to disclose.
Chief Editor
Craig B Langman, MD Tenured Professor of Pediatrics, The First Isaac A Abt, MD, Professor of Kidney Diseases (Emeritus), Northwestern University, The Feinberg School of Medicine; Staff Nephrologist, The Ann and Robert H Lurie Children’s Hospital of Chicago
Craig B Langman, MD is a member of the following medical societies: American Academy of Pediatrics, American Federation for Clinical Research, American Society for Bone and Mineral Research, American Society of Nephrology, American Society of Pediatric Nephrology, Association of Clinical Scientists, Cochrane Collaboration, International Bone and Mineral Society, International Conferences on Calcium Regulating Hormones, International Pediatric Nephrology Association, International Society of Nephrology, International Society of Renal Nutrition and Metabolism, Midwest Society for Pediatric Research, Pediatric Academic Societies, ROCK (Research on Calculus Kinetics) Society, Society for Pediatric Research
Disclosure: Received income in an amount equal to or greater than $250 from: Alexion Pharmaceuticals; Horizon Pharmaceuticals); Dicerna Pharmaceuticals
Incyte Pharmaceuticals; Eli Lilly for: Federation bio; Dicerna pharmaceuticals.
Additional Contributors
Laurence Finberg, MD Clinical Professor, Department of Pediatrics, University of California, San Francisco, School of Medicine and Stanford University School of Medicine
Laurence Finberg, MD is a member of the following medical societies: American Medical Association
Disclosure: Nothing to disclose.