Primary cilia and signaling pathways in mammalian development, health and disease (original) (raw)
. Author manuscript; available in PMC: 2010 Jun 6.
Published in final edited form as: Nephron Physiol. 2009 Mar 10;111(3):p39–p53. doi: 10.1159/000208212
SUMMARY
Although first described 1898 and long considered a vestigial organelle of little functional importance, the primary cilium has become one of the hottest research topics in modern cell biology and physiology. Primary cilia are non-motile sensory organelles present in a single copy on the surface of most growth-arrested or differentiated mammalian cells, and defects in their assembly or function are tightly coupled to many developmental defects, diseases and disorders. In normal tissues the primary cilium coordinates a series of signal transduction pathways, including Hedgehog, Wnt, PDGFRα and integrin signaling. In the kidney the primary cilium may function as a mechano-, chemo- and osmosensing unit that probes the extracellular environment and transmits signals to the cell via e.g. polycystins, which depend on ciliary localization for appropriate function. Indeed, hypomorphic mutations in the mouse ift88 (previously called Tg737) gene, which encodes a ciliogenic intraflagellar transport (IFT) protein, result in malformation of primary cilia, and in the collecting ducts of kidney tubules this is accompanied by development of autosomal recessive polycystic kidney disease (PKD; (1)). While PKD was one of the first diseases to be linked to dysfunctional primary cilia, defects in this organelle have subsequently been associated with many other phenotypes, including cancer, obesity, diabetes as well as a number of developmental defects. Collectively, these disorders of the cilium are now referred to as the ciliopathies. In this review we provide a brief overview of the structure and function of primary cilia and some of their roles in coordinating signal transduction pathways in mammalian development, health and disease. This review was written in conjunction with the Takis Anagnostopoulos Symposium on Renal and Epithelial Physiology and Physiopathology at Faculté de Médecine Necker in Paris, June 26-27, 2008.
Keywords: Primary cilia, cellular GPS, signal transduction, development, tissue homeostasis, diseases, disorders, ciliopathies
STRUCTURE AND DIVERSITY OF CILIA
Cilia and flagella (the terms are equivalent) are antenna-like organelles that emanate from the surface of many growth-arrested or differentiated eukaryotic cells. They consist of a microtubule (MT)-based axoneme ensheathed by a bilayer lipid membrane that is continuous with the plasma membrane of the cell body, but which contains a distinct subset of receptors and other proteins involved in signaling. The axoneme grows out from the distal end of a modified centriole, the basal body, which provides a template for the formation of the nine-fold symmetry of the ciliary axoneme, and also serves to anchor the axoneme in the cell. Separating the ciliary and plasma membrane compartments is a region known as the ciliary necklace (2). The ciliary necklace is connected via fibers to the transition zone of the basal body, and these fibers are thought to be part of a ‘ciliary pore complex’ through which only select proteins are allowed to enter the ciliary compartment (3).
In general, cilia are classified as motile (9+2) or non-motile (9+0), where ‘9’ refers to the number of outer doublet MTs present in the ciliary axoneme and ‘2’ or ‘0’ refers to the number of central MTs present. Motility requires the presence of axoneme-associated dynein arms to generate power, and for most motile cilia additional accessory structures, e.g. radial spokes and central pair projections, are involved in regulating dynein-mediated motility (4). Some motile cilia, however, contain an extra central pair or lack the central pair entirely. For example, cilia with 9+4, 9+2, and 9+0 axonemes have been observed on the notochordal plate of rabbit embryos and these axonemes all contain dynein arms indicating that they are motile (5). Consistent with this, 9+0 monocilia on the embryonic mouse node as well as 9+0 cilia on Kuppfer’s vesicle in Medaka fish, were observed to perform rotational beating in a manner that generates a directional flow across the cell surface, which is required for establishment of the left-right axis (6-8).
In mammals, numerous motile 9+2 cilia are present on the surface epithelial cells lining the airways, brain ventricles, and oviducts. The main function of these 9+2 cilia is to promote the movement of fluids or substances, e.g. airway surface liquid, cerebrospinal fluid, or egg cells, across the epithelial surface; failure to do so may result in airway disease, hydrocephalus, or sterility (9). A single motile flagellum is present on the mammalian sperm cell, whereas the green alga Chlamydomonas, a commonly used model organism for studying ciliary assembly and function, contains two motile flagella that propel the cell towards or away from a light source (9, 10). In addition to their motile functions 9+2 cilia also have important sensory functions, which may in part play a role in regulating motility (4, 11). However, in terms of cilia-mediated signaling, it is the non-motile 9+0 cilia that have attracted the most attention in recent years.
Non-motile 9+0 cilia, also known as primary cilia, are present on most cells in the mammalian body (see http://www.bowserlab.org/primarycilia/ciliumpage2.htm for a comprehensive list of cells known to possess primary cilia). Like motile 9+2 cilia, the axoneme of 9+0 cilia consists of nine outer doublet MTs that are nucleated by the basal body, but the central MT pair and structures involved in motility (e.g. dynein arms, radial spokes) are lacking. The primary ciliary membrane is enriched for a number of receptors and ion channels, including platelet-derived growth factor receptor (PDGFR) α, somatostatin receptor 3, serotonin receptor 5, melanin-concentrating hormone receptor 1, polycystins 1 and 2, as well as components of the Hedgehog and Wnt signaling pathways (11). Therefore the primary cilium is considered to function mainly as a sensory organelle ((12); see also below). Some of the best examples of primary cilia that act as sensory organelles are the sensory cilia present in vertebrate olfactory organs and the outer segments of vertebrate photoreceptors. The latter are initially formed from primary cilia during development of the eye and remain connected to the inner segment in adult retina by a short ‘connecting cilium’ that is functionally and structurally equivalent to the transition zone of other types of cilia. The outer segment of photoreceptors turn over at a high rate and therefore large quantities of photo transduction proteins are continuously being transported from the inner to the outer segment, mainly via a process known as intraflagellar transport (IFT), which will be described in more detail below. Defects in IFT impair transport of photo transduction proteins from the inner to the outer segment and lead to degeneration of the outer segments, ultimately resulting in blindness (13). Likewise, the dysfunction of the cilium on olfactory neurons leads to anosmia and results in their degeneration (14).
In addition to differentiated cells of olfactory and visual organs, cells of many other organs and tissues in our body (e.g. kidney, liver, pancreas, brain, and oviduct) also display 9+0 primary cilia on their surface when the cells are in growth arrest. While these primary cilia are thought to serve as sensory ‘antennae’ that detect and transmit signals from the surrounding environment to the cell body in order to regulate embryonic development and tissue homeostasis in the adult (11, 12), there is a growing body of evidence suggesting that primary cilia also play a crucial role in cell cycle control. Since the primary cilium is subtended by the basal body, which is equivalent to one of the mitotic centrioles of the centrosome, a prerequisite for cell cycle entry is disassembly of the primary cilium, a tightly regulated and still not well understood process that appears to involve mitotic kinases such as Aurora and NIMA-related kinases (12, 15, 16). Consistent with a role for primary cilia in growth control, defective primary cilia were hypothesized to be associated with cancers resulting from abnormal mitogenic signaling or von Hippel-Lindau tumor suppressor signaling (17, 18).
ASSEMBLY OF THE PRIMARY CILIUM
Assembly of the primary cilium begins in G1 when Golgi-derived (primary) vesicles attach to the distal end of the older (mother) centriole of the centrosome. As ciliogenesis progresses, axonemal subunits are added directly onto the distal end of the mother centriole, and additional vesicles fuse with the primary vesicles eventually forming a membrane sheath around the nascent ciliary axoneme. In addition, the mother centriole acquires accessory structures and appendages that promote docking and attachment of the mother centriole to the apical plasma membrane of the cell (15, 19-21). Following docking of the mother centriole to the apical membrane, the axoneme continues to elongate within the membrane-enclosed compartment by addition of axonemal subunits to the distal end of the growing ciliary axoneme. Because the ciliary compartment lacks the capacity for de novo protein synthesis, axonemal assembly depends on transport of ciliary precursors from the base of the cilium to its distal tip. This transport is carried out by IFT, which is essential for the assembly and maintenance of almost all eukaryotic cilia and flagella (3).
IFT is a highly conserved process initially discovered in Chlamydomonas as a bi-directional movement of groups of large protein complexes (IFT particles) along the ciliary axoneme (22). Movement in the anterograde (base to tip) direction is mediated via kinesin-2 motors (Kif3a/Kif3b/KAP complex in vertebrates) whereas movement in the retrograde (tip to base) direction is mediated via cytoplasmic dynein 2 (3, 23-25). These motors attach to the IFT particles, which in turn are thought to be associated with axonemal cargo proteins entering and leaving the cilium (26). The IFT particles and motors as well as their cargo proteins accumulate near the site where transition fibers contact the ciliary membrane at the base of the cilium prior to entry into the ciliary compartment, and kinesin-2 then transports IFT particles, cargo proteins, and inactive cytoplasmic dynein 2 to the ciliary tip. At the tip, the IFT particles are remodeled, cargo is presumably unloaded, and kinesin-2 becomes inactive while cytoplasmic dynein 2 becomes active and transports the IFT particles and ciliary turn over products back to the cell body for recycling (3, 23, 24, 27). The mechanisms that regulate IFT at the ciliary base and tip are not well understood, although some key proteins involved, e.g. MAP kinases and IFT172, have been identified (24, 25).
The IFT particles are composed of ca. 16 different polypeptides, which in Chlamydomonas can be separated biochemically into two distinct complexes termed complex A and B (28). Almost all of the genes encoding IFT particle polypeptides have been cloned and sequenced, and bioinformatic analyses of IFT polypeptide sequences have revealed that many of them contain motifs and domains known to be involved in transient protein-protein interactions, similar to components of coat protein I (COPI) and clathrin-coated vesicles (29, 30). Functional studies of individual IFT particle proteins in diverse ciliated organisms have confirmed a requirement for these proteins in ciliary assembly, and have further indicated that components of IFT complex B are associated with anterograde IFT while components of IFT complex A primarily function during retrograde IFT (24, 27, 29). For example, inactivation of the complex B polypeptide IFT88/Polaris, which is encoded by the ift88 (previously called Tg737) gene, impairs primary cilia formation in the mouse, presumably because ciliary building blocks fail to enter the ciliary compartment via anterograde IFT (1). In contrast, inactivation of the complex A polypeptide IFT139/THM1 in the mouse leads to the formation of stunted, bulbous cilia, presumably due to defective retrograde IFT resulting in accumulation of IFT particles within the cilium (31).
Because of their essential role in building the primary cilium, IFT proteins are required for appropriate functioning of a variety of cilia-mediated signaling pathways such as Hedgehog (32) and PDGFRα (33) signaling. However, there is growing evidence that IFT also plays a more direct role in signaling, both in Chlamydomonas as well as in vertebrates, although the exact mechanisms involved are still somewhat obscure (34, 35).
INTRODUCTION TO PRIMARY CILIA AND CILIOPATHIES
The finding that the gene whose function was disrupted in the Oak Ridge Polycystic Kidney mouse (ORPK mouse, ift88orpk, or ift88Tg737NRpw) encodes an ortholog of Chlamydomonas IFT88 was the eye opener for many cell and developmental biologists that primary cilia function as sensory devices that probe the extracellular environment and send on information to the cell body in order to control developmental processes and tissue homeostasis. Mutations in ift88 in mice were known to cause a series of developmental defects (36), including cystic kidney disease, but the function of the gene was mysterious until its involvement in IFT and cilia assembly was demonstrated. Thus in 2000, Pazour and colleagues first showed that Chlamydomonas IFT88 is an ortholog of the previously characterized mouse Tg737 gene and demonstrated a role for IFT88 in flagellar assembly (1). Consistent with these findings it was shown that primary cilia in the renal tubules (1) and cilia of the node (37) of tg737/ift88 mutant mice were abnormally short or missing, which suggested that PKD and defects in the establishment of left-right asymmetry during embryonic development could be ciliary diseases. Another key finding that further substantiated the link between cilia and PKD was the observation that GFP-tagged versions of the C. elegans orthologs of mammalian polycystin 1 (PKD1) and polycystin 2 (PKD2) localize to ciliated endings of male sensory neurons (38). This prompted other investigators to reexamine the sub cellular localization of mammalian PKD1 and PKD2, leading to the demonstration that both of these proteins indeed localize to primary cilia in cultured human and mouse kidney cells (39, 40). While Chlamydomonas, C. elegans and mice were crucial in establishing the first link between primary cilia and PKD, additional model systems have subsequently proven to be of great benefit for investigation of cilia assembly, ciliopathies and ciliary signaling. These other important model organisms include Tetrahymena, Drosophila, Trypanosomes and zebrafish, which are easy to manipulate and analyze in the laboratorium. Zebrafish has become an excellent genetic tool in the study of ciliary genes involved in vertebrate embryogenesis and human disease, since its transparent embryo develops outside the mother’s body and it has many of the same organ systems affected in human ciliopathies. Collectively, studies on several model organisms as well as cultures and tissues of mouse and human origin have not only provided important insight into the molecular mechanisms of PKD, but have also revealed a link between primary cilia and a growing list of other diseases and syndromes, including Nephronophthisis and Bardet Biedl, Orofacial-digital type 1, Meckel Gruber and Von Hippel-Lindau syndromes (Figure 1). Doubtless, many more ciliopathies will be added to this list, since limited information is available on primary cilia in many organs and tissues during development and in the adult.
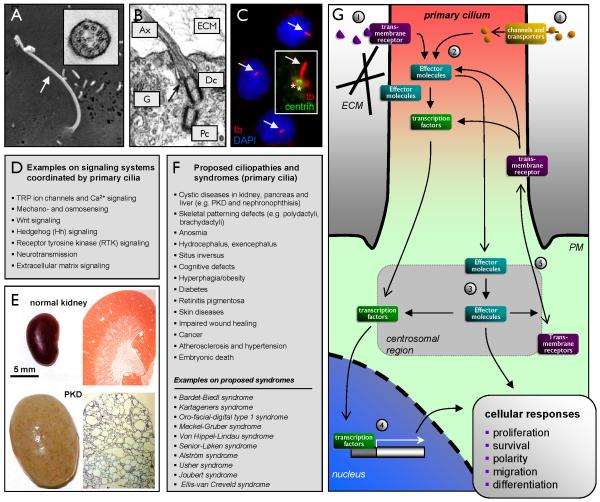
Figure 1. Overview of primary cilia in signaling pathways and ciliopathies.
(A) Scanning electron microscopy of a primary cilium (arrow) emanating from the surface of a human embryonic stem cell (hESC). The insert shows a cross section of the hESC cilium with a 9+0 microtubules ultra structure analyzed by transmission electron microscopy (TEM). Reprinted from (140) with permission from JCB. (B) TEM analysis of the structural relationship between the primary ciliary axoneme (Ax), the distal (Dc) and proximal (Pc) centrioles and the Golgi apparatus (G) in a chicken chondrocyte. ECM: Extracellular Matrix. Reprinted from (97) with permission from Elsevier. (C) Immunofluorescence microscopy analysis of primary cilia (anti-acetylated α-tubulin (tb), red, arrow) in cultures of growth arrested NIH3T3 cells. The nucleus with DAPI (blue). Inset: an NIH3T3 primary cilium co-stained with anti-centrin-2 (green) that marks to two centrioles (asterisks) (D) List of various signal transduction systems being coordinated by the primary cilium. (E) Gross morphology (left panels) and histological sections (right panels) of a normal (top panels) and an ift88tm1Bky cystic kidney mutant (bottom panels) at 3 weeks of age. Tissue sections (right panels) were stained with hematoxylin and eosin. (F) List of proposed ciliopathy phenotypes and human syndromes caused by defects in assembly or function of primary cilia in mammals. (G) Schematic overview of signal transduction systems being coordinated by the primary cilium and the centrosome in regulation of cell proliferation, survival, polarity, migration and differentiation. Activation of transmembrane receptors (e.g. by interaction with extracellular matrix (ECM), by binding to soluble ligands such as PDGF-AA (see also Figure 2A) and morphogens, or due to mechanostimulation (see also Figure 2C) 1) leads to activation of effector molecules in the cilium 2) or at the centrosome 3) followed by activation of specific transcription factors for de novo gene expression 4). Effector molecules may also activate downstream components in signal transduction independent of the nucleus in regulation of cellular processes. As part of this there is a continuous turnover of transmembrane receptors in the cilium, partly regulated by effector molecules that controls the trafficking of signaling components into and out of cilium (see also Figure 3B) 5), such as the concerted movement of Ptc out of and Smo into the cilium in response to Hh stimulation. PM: Plasma membrane.
Kidney diseases
Great attention has been given to the nephronal disorders arising from ciliary dysfunction, such as Polycystic kidney disease (PKD), due to which a connection between ciliary malfunctions and aberrant tissue homeostasis was first hypothesized (1, 40, 41). Both the autosomal dominant (ADPKD) and recessive (ARPKD) variants of PKD are characterized by formation of large fluid filled cysts and greatly enlarged kidneys that are associated with increased proliferation late in the disease process (42). ADPKD is mainly due to mutations in the PKD1 and −2 genes, whereas ARPKD is caused by mutations in the gene encoding Fibrocystin (42). Another complex of recessive cystic kidney diseases is nephronophthisis (NPHP), resulting from defects in the genes encoding Nephrocystin (Nphp) 1-9, and being the main genetic cause of end-stage renal failure within the first three decades of life. As opposed to PKD, tubular cyst formation in NPHP does not result in enlarged kidneys, but phenotypic characteristics include degradation of tubular basement membranes, tubular collapse and interstitial fibrosis (43-45).The cystic kidney phenotype in NPHP is frequently combined with other defects such as cerebellar hypoplasia and ataxia in Joubert Syndrome, or retinitis pigmentosa in Senior-Løken Syndrome (43-45). Interestingly, NPHP-2 resulting from mutations in the INVS gene encoding Inversin has an earlier onset than the other NPHP-types, and combines the phenotypic characteristics of NPHP, such as renal interstitial and glomerular fibrosis and tubular cysts, with features of ADPKD, including enlargement of the kidneys due to cysts outside of the medullary region (46). Moreover, microarrays and immunoblotting analysis of kidneys from mice containing a homozygous carboxy-terminal deletion of invs showed increased proliferation and cell cycle progression as compared to wt mice (47).
Many proteins whose functions are disturbed in cystic diseases have been localized to the cilium or the ciliary basal body, where they might contribute to regulating kidney development and function. Included herein are the polycystins (39, 40), the nephrocystins (46, 48-57), Fibrocystin (58), and proteins regulating Wnt signaling and planar cell polarity (59-62), which in different ways coordinate a series of signal transduction pathways in the kidney. It was proposed that PKD1 and PKD2 form a protein complex in the primary cilium to function as a mechanosensor to elicit a calcium signal in response to fluid movement through the renal tubules (63) (Figure 2), where loss of cilia or mutations in the polycystins lead to cyst formation. In line with this, earlier studies showed that bending of primary cilia in cultures of renal epithelial duct cells by fluid shear or mechanical stimulation causes intracellular Ca2+ to increase (64-66). This suggests that ciliary mechanotransduction is important for normal function of the kidney epithelium and that loss of the cilium leads to PKD.
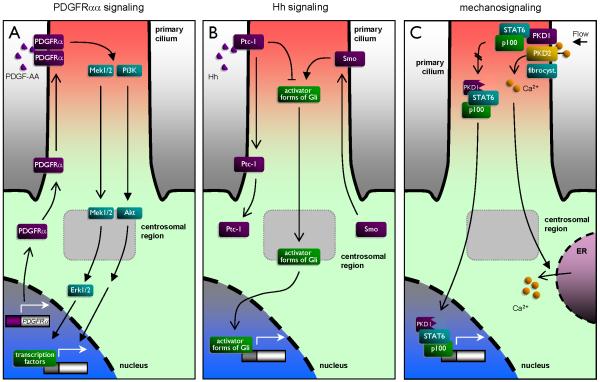
Figure 2. Simplistic models on different signaling systems being coordinated by the primary cilium.
A: Ciliary PDGFRaa signaling. Upon growth arrest expression of PDGFRa is up-regulated and the receptor is targeted to the primary cilium. Stimulation with PDGF-AA leads to PDGFRa homodimerization in the cilium followed by activation of PI3K/Akt and Mek1/2-Erk1/2 pathways leading to cell migration and transcriptional control of cell cycle entry. B: Ciliary Hedgehog (Hh) signaling. Binding of Hh ligands to Ptc-1 in the primary cilium results in translocation of Ptc-1 out of the cilium and targeting of Smo into the cilium. This favors the formation of active forms of Gli transcription factors in the cilium followed by translocation of the active Gli forms to the nucleus for activation of the Hh response. C: Bending of the primary cilium by fluid flow leads to activation of the PKD1/2 complex in the cilium and influx of Ca2+ into the cilium followed by activation af Ca2+ channels in endoplasmic reticulum (ER). Fibrocystin may assist PKD2 function. In the absence of ciliary bending PKD1 is processed and translocated to the nucleus in a protein complex with p100 and STAT6 to activate transcriptional activity in cystic diseases. Please see text for references.
More recent studies in mice have revealed that the rate of cyst formation and cystic disease severity is dependant on when cilia or polycystin function is disrupted (67, 68). Using conditional alleles of ift88, kif3a (component of the heterotrimeric IFT kinesin-2 motor complex), or PKD1, and inducible Cre deletor mouse lines it was shown that disruption in early postnatal life (P1 to P12) results in rapid cyst formation within three weeks of loss of the gene. In contrast, if cilia or polycystin-1 function is disrupted after P12, cyst formation requires 6 months to a year to form. Another surprising finding from these studies was that there was no marked increase in proliferation rates between the mutant and control kidneys, even in the cystic animals where function was disrupted in perinatal periods. Together these data indicate that there is a critical time point at which cilia dysfunction causes a rapid or slow progressing cystogenic phenotype and raised concern about the simple pathogenic model whereby loss of cilia-mediated mechanosensation is the cause of cyst formation. In further studies, it was revealed that this switch point might be associated with completion of renal differentiation, which occurs at about two weeks of age in mice, and changes in the proliferative environment and a large change in the gene expression profile that occurs at around P12. The importance of having a proliferative environment for cyst formation in the cilia mutants was analyzed by inducing cilia loss in adult mice followed by renal injury through obstruction or ischemic reperfusion. This injury reinitiates proliferation in the adult kidney as part of the repair process and was found to result in rapid cyst formation similar to that seen in the perinatally induced cilia mutants. Cysts were not present in the contra-lateral non-injured cilia mutant kidney. A possible mechanism connecting ciliary dysfunction, cell proliferation, and cyst formation was further defined by studies of Fischer et al (2006) (69). They showed that mitotic spindles in the perinatal kidney normally align along the axis of the nephron such that cells divisions increase nephron length. In contrast, in cystic disease mouse and rat models the orientation of cells divisions occur randomly with many resulting in expansion of the diameter of the nephron. Deletion of the ciliary protocadherin and planar cell polarity (PCP) protein Fat4 was recently shown to elicit a similar disruption of spindle orientation during renal tubular elongation in mice (61). Thus, these data suggest that cilia or the basal body are needed for normal orientation of the spindle. Whether this is in response to fluid flow and the polycystin generated calcium signal, and how this is coupled to PCP has yet to be fully addressed.
SIGNALING PATHWAYS COORDINATED BY THE PRIMARY CILIUM
In normal tissues the primary cilium coordinates a series of signal transduction pathways, including Hedgehog, Wnt, and PDGFRα pathways as well as functioning as a photo-, mechano- and osmosensing unit that probes and relays information from the extracellular environment into the cell. In many cases, proper signaling is tightly coupled to the correct translocation of receptors and down-stream effector molecules involved in signal transduction to the cilium. We still know very little about the mechanisms that control trafficking of signal components into and out of the cilium, although IFT is likely to be involved in several aspects of receptor trafficking. Further, a single primary cilium may contain many different signal transduction systems in order to carry out diverse signaling processes in development and homeostasis of tissues. Therefore it is likely that the composition of signal systems closely reflects the functionality of the cell type in different tissues, i.e., that some ciliary signal systems are tissue specific. This may be particularly relevant when comparing cilia situated deep inside various tissues and organs versus cilia that protrude from the apical surface into the lumen of e.g. tubular structures as seen on epithelial and endothelial cells. Also, during development, the composition of ciliary signal systems may change as part of the dynamic process that controls cell differentiation, allocating different signal systems to different cell types to determine cell fate and function.
In the following we will present a brief overview of some of the signal transduction pathways that have been shown to be coordinated by the primary cilium to control cellular processes during development and in tissue homeostasis.
Signaling in primary cilia on epithelial and endothelial cells
As outlined in the above, PKD1 and PKD2 may form a mechanosensory complex that coordinate a flow-sensing response in kidney primary cilia. However, ciliary polycystins may well have other functions in relaying this response to control development and homeostasis of the kidney (42). The channel activity and positioning of PKD2 in the cilium is regulated by Fibrocystin, which is indirectly linked to the N-terminus of PKD2 through Kif3a/b of the Kinesin 2 complex (70-73). In addition to their role in Ca2+-signaling, the polycystins contribute to maintaining homeostasis through p53 and JNK (74) and also negatively regulate the JAK/STAT (75, 76) and the mTOR pathways (77). Alterations in mechanostimulation induces cleavage of PKD1, releasing tuberin and mTOR from their flow-dependent inhibition by PKD1 (77), and allowing the transcription factor STAT6 and co-factor P100 to translocate from the cilium to the nucleus (76). By itself, the PKD1 C-terminal fragment may influence gene transcription and perhaps modulate Wnt signaling (76, 78, 79), all processes that promote dedifferentiation and proliferation (reviewed in(80).
The Nephrocystins are also assumed to exert their function through the primary cilium in renal development and maintenance, although the exact mechanisms remain elusive (43-45). More than half of the Nphps (1, 4, 5, 6 and -8) have been localized specifically to the ciliary transition zone at the base of the cilium, suggesting a role in ciliary assembly and/or transport of specific proteins into the ciliary compartments (48, 50, 52-54, 56, 57). As such, Nphp-1 and -4 have also been proposed a role in axonemal or IFT modeling (81), whereas Nphp-9/Nek8 is necessary for expression and ciliary positioning of Polycystin-1 and -2 (82, 83). Nphp-3 may also impact on ciliary length in mammalian cells (81, 84), and was recently demonstrated to interact directly and genetically with Nphp-2/Inversin in the establishment of bilateral asymmetry and promotion of tissue polarity (84, 85). The latest hypothesis proposes the existence of one or more nephrocystin complexes at the ciliary transition zone, in line with the BBSome, which may interact with or be part of a ciliary pore complex (43, 45, 56, 86). In addition, the activation of the transcription factor ATF4/CREB-2 by Nphp-6/CEP290 (55) indicates that the actions of the nephrocystins are complex and may affect ciliary functions on several levels. Furthermore, studies of Nphp-2 and -3 (84, 85) as well as recent findings with ift88, kif3a, and bbs mutants (62, 87) suggest an important role for the primary cilium in regulating Wnt signaling, which will be described in further detail below.
Primary cilia on cholangiocytes that extend from the epithelium into the bile duct lumen also possess a series of receptors and signaling molecules that control tissue homeostasis. These include PKD1, PKD2, Fibrocystin, TRPV4 and G protein-coupled purinergic receptor, P2Y12, that ultimately coordinate mechano-, osmo-, and chemo-sensory functions, which when defective cause e.g. cystic and fibrotic liver diseases (88). Primary cilia on endothelial cells (EC) in blood vessels and in endocardium were proposed to function as shear stress sensors. In cultured human umbilical vein EC the cilia were found to disassemble in response to laminar shear stress (89). PKD1 strongly localized to EC cilia in embryonic mouse aorta. In cultures of embryonic ECs fluid shear stress cleaves PKD1 and ultimately leads to changes in Ca2+ signaling and NO synthesis (90) as well as expression of shear responsive genes such as Krüppel-like factor-2 (91). Consequently, dysfunctional cilia in the cardiovascular system may increase the risk for artherosclerosis and hypertension (90, 92).
PDGFRα signaling in cycle control and migration
Signaling via Platelet-Derived Growth Factors (PDGF) and their receptors (PDGFRs) plays an essential role in cell survival, growth control and cell migration during gastrulation, fetal development and in maintenance of tissues in the adult, with defects causing a range of diseases, including cancer, vascular disorders and fibrosis (93). Recently, it was shown that PDGFRα-signaling is coordinated by the primary cilium in mouse embryonic fibroblasts (MEFs), i.e., expression of PDGFRα is up-regulated during growth arrest and targeted to the cilium where PDGF-AA-dependent activation of the receptor and its initial down-stream effectors such as Mek1/2 and Akt occurs (33, 94) (Figure 2). In wild type cells these signaling events stimulate cell cycle entrance, which is blocked in ift88orpk MEFs that lack the primary cilium (33). Consequently, PDGFRα signaling through the fibroblast primary cilium may be important in tissue homeostasis while perturbations in this pathway could lead to oncogenesis.
The fibroblast primary cilium may function as a cellular GPS that coordinates directional migration and PDGFRα-mediated chemotaxis. Using micropipettes to generate a PDGF-AA gradient wild type growth-arrested MEFs respond immediately to PDGF-AA injection, and migrate uniformly towards the pipette, while ift88orpk MEFs do not respond to PDGF-AA and move around randomly. In in vitro wound healing assays primary cilia in wild type MEFs orient parallel to one another, perpendicular to the wound and incubation with PDGF-AA increases the migration speed and the directional movement of the cells. In contrast, in ift88orpk cells the migration speed is unaffected by PDGF-AA incubation and cells have decreased directionality (94). PDGFRα-mediated migration is associated with activation of the ubiquitous plasma membrane Na+/H+ exchanger, NHE1, and inhibition of NHE1 reduces PDGF-AA-mediated cell migration speed and directionality of wt MEFs, whereas this inhibition is markedly reduced in ift88orpk MEFs (95). These results support the conclusion that the primary cilium represents an alternative mechanism of sensing chemotactic gradients and is part of the positioning machinery that coordinates directed migration in wound healing and developmental processes.
Primary cilia may also directly interact with extracellular matrix (ECM) proteins as the cell moves, transmitting mechanical information from the outside milieu to the cell. In vascular smooth muscle cells (VSMCs) primary cilia that contain PKD1, PKD2 and integrins are critical for cell-ECM interaction and mechanosensing that allow the cilia to project into the ECM and potentially control wound healing (96). Also, in chondrocytes primary cilia make direct physical contact with ECM components via specific ECM receptors, suggesting that mechanical stimuli may be transmitted through the cilium to control tissue development and to construct a mechanically robust skeletal system (97, 98). Although speculative at this point, the directional migration of fibroblasts may also be similarly regulated through interactions between the cilium and ECM, and potentially in concert with chemoattractants in embryonic patterning and adult tissue reorganization.
Wnt signaling
As indicated above, the primary cilium has been proposed a role in Wnt signaling that regulates cell proliferation, cell fate determination and migration. Recently, Wnt signaling was described as a network of interacting rather than individual pathways (99). Traditionally Wnt signals are divided into at least three distinct pathways, all initiated by binding of a ligand of the Wnt family to a 7 transmembrane Frizzled (Fz) receptor. Canonical Wnt signaling involves Dishevelled (Dvl) mediated stabilization of β-catenin, which serves as a transcriptional co-activator and in turn induces cell cycle progression, proliferation, differentiation and growth in addition to migration and regulation of embryonic development (100). Non-canonical, or β-catenin independent Wnt signaling pathways act through aPCK, CamK and JNK to control cellular polarity, migration and PCP, necessary for convergence extension during e.g. gastrulation and neurulation (reviewed by (101)).
The primary cilium and basal body have been assigned a role in regulating both the canonical and the noncanonical Wnt signaling pathways due to the ciliary/basal body localization of the PCP proteins Inversin (49, 85) Vangl-2 (102) and Fat4 (61)in addition to members of the degradation complex, GSK-3β (59) and APC (62). Vangl-2 appears to interact with Prickle-1 and Inversin (85) both of which can suppress Wnt/β-catenin signaling through Dvl degradation (85, 103), the latter in concert with another ciliary protein Nephrocystin-3, as mentioned above (84). PCP-like phenotypes have been observed in the inner ear of mice where ift88 has been disrupted (104), and in Bbs4 deficient mice and zebrafish (102). Further, loss of primary cilia in the ift88Tg737Rpw (previously called ORPK or Tg737orpk) mouse causes a series of abnormalities in the pancreas, including extensive cyst formation in ducts associated with defects in cell cycle control (105, 106). In the dilated ducts and cysts the cytosolic localization of β-Catenin is increased and there is an increased expression of Tcf/Lef (105), which activates transcription of Wnt target genes (107-109). These observations support the conclusion that primary cilia are associated with regulation of Wnt signaling in the pancreas.
More recently, two different approaches where used to disrupt cilia/basal body function and demonstrated the importance of these structures for regulation of canonical Wnt signaling (62, 87). RNAi-induced basal body disruption impaired gastrulation in zebrafish due to obstructed convergence extension (CE) movements, and was accompanied by moderately increased canonical signaling. The latter was also the response to shRNA knock-down of either BBS4 or BBS6/MKKS in HEK293 cells, which impaired noncanonical WNT5a’s ability to suppress the canonical Wnt3a activity. An identical effect was seen in cells where cilia were ablated by knock-down of KIF3A with shRNA (87). A corresponding hyper-response to Wnt3a was observed in kif3a or ift88Tg737Rpw mouse embryonic fibroblasts (MEF) using a BATgal canonical Wnt reporter as well as in MEFs and _Ofd1_-deficient murine embryonic stem cells, albeit not in the absence of Wnt3a (62). In vivo there was a general increase in canonical Wnt signaling activity in kif3a mutants, although spatially the activity was normal. The phenocopying of these effects by specific inhibition of the proteasomal subunit RPN10, which associates with BBS4 (87), suggests that proteasomal targeting of β-catenin is a process that requires the basal body. Notably, whereas basal body disruption impaired non-canonical signaling and CE (87), the noncanonical Wnt5a was still able to induce cytoskeletal rearrangements indicative of PCP equally well in heterozygous and _kif3a_−/− MEFs (62), suggesting that repression of the canonical pathway and activation of non-canonical Wnt signal are two independent processes involving the cilium or basal body. Further insights into the connection between cilia/basal body and Wnt signaling have been revealed by Bergmann and colleagues who demonstrated that the ciliary protein Nphp3 binds to Inversin and can inhibit Inversin mediated canonical Wnt signaling (84).
Hedgehog signaling
Another critical signal transduction pathway that is coordinated by the primary cilium is the hedgehog (Hh) pathway. In additional to its general roles in tissue homeostasis, this pathway is crucial in tissue differentiation during embryonic development, and dysfunction of the Hh pathway is responsible for e.g. basal cell carcinoma, the most common form of cancer in humans.
The hedgehog ligand comes in three different varieties (sonic (Shh), indian (Ihh) and desert (Dhh)) that are spatially and temporally regulated and whose concentration gradient ultimately helps determine the eventual cell fate and proliferation rate (for review see (110)). The ligands work through two transmembrane proteins, Patched (Ptc) and Smoothened (Smo) that in turn regulate the activity of three transcription factors of the Gli family (Gli1,2,3) (111). Ptc is the receptor for Hh ligands, and in the absence of Hh ligands it negatively regulates Hh signaling by suppressing the activity of Smo. Upon binding of Hh ligand to Ptc, the inhibition of Smo is relieved, preventing processing of Gli3 to a repressor and activating the Gli-2 transcription factor, which in turn induces the hedgehog pathway through their nuclear transcriptional targets (112).
A series of observations have now shown that primary cilia are critical regulators of the Hh pathway where the regulated concerted movement of Ptc out of and Smo into the cilium may create a switch by which cells can turn Hh signaling on and off during development and in control of tissue homeostasis (reviewed in (111, 113)). In this scenario, binding of ligands to Ptc in the cilium activate the Hh pathway by removal of Ptc from the cilium (114) in a process that is associated with ciliary enrichment of Smo (115) (Figure 2). In vitro activation of Smo in cells exposed to Shh is shown to be blocked in MEFs lacking IFT172 or the dynein retrograde motor, Dync2h1 (116). The translocation of Smo into the primary cilium upon Shh stimulation can also be blocked by knocking down Kif3A or β-arrestins, which are thought to be adapter proteins for the Smo protein (117).
Mutations in IFT proteins required for ciliary assembly results in dysfunctional Hh signaling and severe developmental disorders in mammals (reviewed in (111)). Removal of Kif3A causes aberrant hedgehog signaling which has been shown to have an impact on skeletogenesis (118, 119), neural tube formation (120), and cerebellar development (121, 122). Mutations in the IFT139 homologue (Thm1) specifically results in abnormal Gli3 activator/repressor ratios which in turn results in defects in the neural tube formation (31). Similarly, mutations in a basal body protein (Ftm1) also resulted in abnormal ratios of Gli3 activator/repressor which lead to defects in left-right symmetry, neural tube formation and limb development (123). The inhibition of Gli3 cleavage for subsequent activation of the Hh pathway was shown to require other ciliary proteins also: the retrograde IFT dynein motor subunit, Dnchc2 (124), the IFT172 protein (125) and the ciliary Arl13b (a small GTPase of the Arf/Arl family) (126). Recently, a siRNA screen identified different mediators of the Hh pathway, among them genes controlling ciliogeneis: Nek1 (NIMA-like kinase) and Prka (a kinase participating in miRNA processing and thought to localize at the base of the cilium) (127). Further, deletion of ift88 in ovary using Cre-Lox recombination in mice resulted in a severe delay in mammary gland development and defects in ovarian function (128), and in IFT57-deficient mice, there were defects in ventral neural tube formation (exencephaly) due to aberrant Shh signaling (129). These findings highlight the importance of IFT proteins in the hedgehog pathway.
One example of the in vivo changes in the levels of Hh molecules in the cilium comes from studies on the development of the pancreas, which is controlled by the graded response to Hh signaling (130-133). Interestingly, Smo and Gli2 are absent from pancreatic primary cilia at human embryonic stage week 7.5, i.e. before formation of the endocrine system, but highly concentrated in cilia in 14 and 18 week old fetuses (134). This increase in ciliary localization of Smo and Gli2 is accompanied by loss of Gli3 ductal epithelium, suggesting that a graded Hh signaling response coordinated by the primary cilium regulates the development of the human pancreas. Therefore, disruption of pancreatic development in mice with defects in primary ciliary (105, 106, 135) may be due to loss of both coordinated Hh and Wnt signaling during genesis of the pancreas. The canonical Wnt and Ihh pathways may also help to coordinate osteoblast and chondrocyte differentiation during bone development ((136, 137); for review, see (138, 139)). One known disorder resulting from developmental skeletal problems is chrondoectodermal dysplasia in Ellis-van Creveld syndrome, which arises from mutations in Evc, which localizes to the base of the chondrocyte primary cilium (140).
STEM CELLS AND PRIMARY CILIA
Stem cells hold great promises for their potential therapeutical abilities. Starting off in the pluripotent states, they have the potential to give rise to all three germinal layers and can differentiate to form specific cell types dependent on the environment and specific factors present. Thus far, it is thought that they could be used for therapies directed against Alzheimer’s disease, Parkinson’s disease, diabetes, and a host of other conditions (141). Also, a credible paradigm has stem cells as important targets against cancer since cancer stem cells are the true progenitors of cancers (142).
Recently, human embryonic stem cells were shown to possess primary cilia with the classic 9+0 architecture. Furthermore, it was shown that the Hh pathway was functional through the primary cilium as evidenced by the movement of Smo into and Ptc out of the primary cilium upon treatment with an agonist (143). This implies that primary cilia could play pivotal roles in the earliest stages of development in terms of cell differentiation and proliferation. In addition, PDGFRα, which helps maintain hESCs in an undifferentiated state (144), and components of the Wnt signaling cascade, which maintains the self-renewal in both mESC and hESC (145), have been shown to localize to the primary cilia and/or in the centrosomal region at the base of the cilium of hESCs (146). Further, it was reported that the traditional stem cell markers (Oct4, Sox2, and Nanog) localize to the primary cilium in a subpopulation of cultured hESC (146). While their exact function in the cilium is not known, one hypothesis is that processing of these transcription factors could occur in the primary cilium analogous to that suggested for the Gli proteins. These data further hint at the primary cilium’s critical role in coordinating pathways determining cell differentiation and proliferation.
In later stages of development, it has now been reported that the primary cilia are critical for the development of neural stem cells needed for proper development of the hippocampal region. In mice lacking the Kif3A or the Smo proteins, there is a failure in the maturation of radial astrocytes which would normally develop into the dentate gyrus and be responsible for maintenance of adult neurogenesis (147). Similarly, removal of either of these proteins from the cerebellar granule cell precursors results in the improper development of the cerebellum (121). A similar effect is seen when the IFT88 protein is knocked down (122). Cilia are also important for proper neurogenesis in the hippocampal region as shown in mutants lacking the stumpy protein (Gli3 processing was altered). Knocking down the Smo or Shh proteins was also shown to lead to a failure in development of the neocortex (148). Postnatally, this leads to a lack of a specific subtype of neural stem cells (149).
CONCLUSIONS
Recent research in primary cilia and their function in coordinating cellular signal transduction pathways, developmental processes and tissue homeostasis have moved this organelle to a central position in human pathophysiology. While PKD was one of the first diseases to be linked to dysfunctional primary cilia, defects in these organelles have subsequently been associated with many other phenotypes, including cystic pancreatic and liver diseases, retinitis pigmentosa, anosmia, defective neurogenesis, polydactyly, and other developmental defects now referred to as the ciliopathies, and there are also indications that the primary cilium is important in behavioral and mental disorders and oncogenesis. Recent work has further shown that stem cells possess primary cilia with signal transduction components that control maintenance of stem cell pluripotentiality and regulate early differentiation and proliferation. These findings imply that primary cilia play pivotal roles in the earliest stages of embryonic development, which could be important in regenerative medicine. Future studies on the mechanisms in ciliary assembly, translocation of signal components in and out of cilium and coordination of ciliary signaling transduction pathways in developmental processes and tissue homeostasis will add further and important insight into the intrinsic biology of primary cilia in human health and disease.
ACKNOWLEDGEMENTS
This work was supported by The Lundbeck Foundation #R9-A969, Novo Nordisk Foundation, The Danish Natural Science Research Council #272-07-0530 and #272-07-0411 (STC and LBP), Novo Nordisk Scholarship (IRV), The Lundbeck Foundation #150/05 (AA) and NIH RO1 DK065655 and HD050327 (BKY). We apologize to those whose work is not described in this review owing to restricted space.
REFERENCES
- 1.Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilula NB, Satir P. The ciliary necklace. A ciliary membrane specialization. J Cell Biol. 1972;53:494–509. doi: 10.1083/jcb.53.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 4.Salathe M. Regulation of mammalian ciliary beating. Annu Rev Physiol. 2007;69:401–422. doi: 10.1146/annurev.physiol.69.040705.141253. [DOI] [PubMed] [Google Scholar]
- 5.Feistel K, Blum M. Three types of cilia including a novel 9+4 axoneme on the notochordal plate of the rabbit embryo. Dev Dyn. 2006;235:3348–3358. doi: 10.1002/dvdy.20986. [DOI] [PubMed] [Google Scholar]
- 6.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 7.Okada Y, Takeda S, Tanaka Y, Belmonte JC, Hirokawa N. Mechanism of nodal flow: a conserved symmetry breaking event in left-right axis determination. Cell. 2005;121:633–644. doi: 10.1016/j.cell.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Hirokawa N, Tanaka Y, Okada Y, Takeda S. Nodal flow and the generation of left-right asymmetry. Cell. 2006;125:33–45. doi: 10.1016/j.cell.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Ibanez-Tallon I, Heintz N, Omran H. To beat or not to beat: roles of cilia in development and disease. Hum Mol Genet. 2003;12:R27–35. doi: 10.1093/hmg/ddg061. [DOI] [PubMed] [Google Scholar]
- 10.Silflow CD, Lefebvre PA. Assembly and motility of eukaryotic cilia and flagella. Lessons from Chlamydomonas reinhardtii. Plant Physiol. 2001;127:1500–1507. [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen ST, Pedersen LB, Schneider L, Satir P. Sensory cilia and integration of signal transduction in human health and disease. Traffic. 2007;8:97–109. doi: 10.1111/j.1600-0854.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 12.Pazour GJ, Witman GB. The vertebrate primary cilium is a sensory organelle. Curr Opin Cell Biol. 2003;15:105–110. doi: 10.1016/s0955-0674(02)00012-1. [DOI] [PubMed] [Google Scholar]
- 13.Insinna C, Besharse JC. Intraflagellar transport and the sensory outer segment of vertebrate photoreceptors. Dev Dyn. 2008;237:1982–1992. doi: 10.1002/dvdy.21554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulaga HM, Leitch CC, Eichers ER, Badano JL, Lesemann A, Hoskins BE, Lupski JR, Beales PL, Reed RR, Katsanis N. Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nat Genet. 2004;36:994–998. doi: 10.1038/ng1418. [DOI] [PubMed] [Google Scholar]
- 15.Pan J, Snell W. The primary cilium: keeper of the key to cell division. Cell. 2007;129:1255–1257. doi: 10.1016/j.cell.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Quarmby LM, Parker JD. Cilia and the cell cycle? J Cell Biol. 2005;169:707–710. doi: 10.1083/jcb.200503053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mans DA, Voest EE, Giles RH. All along the watchtower: Is the cilium a tumor suppressor organelle? Biochim Biophys Acta. 2008 doi: 10.1016/j.bbcan.2008.02.002. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Christensen ST, Pedersen SF, Satir P, Veland IR, Schneider L. The primary cilium coordinates signaling pathways in cell cycle control and migration during development and tissue repair. Curr Top Dev Biol. 2008 doi: 10.1016/S0070-2153(08)00810-7. In Press. [DOI] [PubMed] [Google Scholar]
- 19.Dawe HR, Farr H, Gull K. Centriole/basal body morphogenesis and migration during ciliogenesis in animal cells. J Cell Sci. 2007;120:7–15. doi: 10.1242/jcs.03305. [DOI] [PubMed] [Google Scholar]
- 20.Sorokin S. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Biol. 1962;15:363–377. doi: 10.1083/jcb.15.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedersen LB, Veland IR, Schrøder JM, Christensen ST. Assembly of primary cilia. Dev Dyn. 2008;237:1993–2006. doi: 10.1002/dvdy.21521. [DOI] [PubMed] [Google Scholar]
- 22.Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci USA. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scholey JM. Intraflagellar transport. Annu Rev Cell Dev Biol. 2003;19:423–443. doi: 10.1146/annurev.cellbio.19.111401.091318. [DOI] [PubMed] [Google Scholar]
- 24.Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT): role in ciliary assembly, resorption and signalling. Curr Top Dev Biol. 2008 doi: 10.1016/S0070-2153(08)00802-8. In press. [DOI] [PubMed] [Google Scholar]
- 25.Scholey JM. Intraflagellar transport motors in cilia: moving along the cell’s antenna. J Cell Biol. 2008;180:23–29. doi: 10.1083/jcb.200709133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin H, Diener DR, Geimer S, Cole DG, Rosenbaum JL. Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J Cell Biol. 2004;164:255–266. doi: 10.1083/jcb.200308132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blacque OE, Cevik S, Kaplan OI. Intraflagellar transport: from molecular characterisation to mechanism. Front Biosci. 2008;13:2633–2652. doi: 10.2741/2871. [DOI] [PubMed] [Google Scholar]
- 28.Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cole DG. The intraflagellar transport machinery of Chlamydomonas reinhardtii. Traffic. 2003;4:1–8. doi: 10.1034/j.1600-0854.2003.t01-1-00103.x. [DOI] [PubMed] [Google Scholar]
- 30.Jekely G, Arendt D. Evolution of intraflagellar transport from coated vesicles and autogenous origin of the eukaryotic cilium. Bioessays. 2006;28:191–198. doi: 10.1002/bies.20369. [DOI] [PubMed] [Google Scholar]
- 31.Tran PV, Haycraft CJ, Besschetnova TY, Turbe-Doan A, Stottmann RW, Herron BJ, Chesebro AL, Qiu H, Scherz PJ, Shah JV, Yoder BK, Beier DR. THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nat Genet. 2008;40:403–410. doi: 10.1038/ng.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 33.Schneider L, Clement CA, Teilmann SC, Pazour GP, Hoffmann EK, Satir P, Christensen ST. PDGFRαα signaling is regulated through the primary cilium in fibroblasts. 2005;15:1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Wang Q, Pan J, Snell WJ. Intraflagellar transport particles participate directly in cilium-generated signaling in Chlamydomonas. Cell. 2006;125:549–62. doi: 10.1016/j.cell.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 35.Scholey JM, Anderson KV. Intraflagellar transport and cilium-based signaling. Cell. 2006;125:439–442. doi: 10.1016/j.cell.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 36.Lehman JM, Michaud EJ, Schoeb TR, Aydin-Son Y, Miller M, Yoder BK. The Oak Ridge Polycystic Kidney mouse: Modeling ciliopathies of mice and men. Dev Dyn. 2008;237:1960–1971. doi: 10.1002/dvdy.21515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murcia NS, Richards WG, Yoder BK, Mucenski ML, Dunlap JR, Woychik RP. The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development. 2000;127:2347–2355. doi: 10.1242/dev.127.11.2347. [DOI] [PubMed] [Google Scholar]
- 38.Barr MM, Sternberg PW. A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature. 1999;401:386–389. doi: 10.1038/43913. [DOI] [PubMed] [Google Scholar]
- 39.Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol. 2002;12:R378–R380. doi: 10.1016/s0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- 40.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002;13:2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- 41.Taulman PD, Haycraft CJ, Balkovetz DF, Yoder BK. Polaris, a protein involved in left-right axis patterning, localizes to basal bodies and cilia. Mol Biol Cell. 2001;12:589–599. doi: 10.1091/mbc.12.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolb RJ, Nauli SM. Ciliary dysfunction in polycystic kidney disease: an emerging model with polarizing potential. Front Biosci. 2008;13:4451–4466. doi: 10.2741/3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hildebrandt F, Zhou W. Nephronophthisis-associated ciliopathies. J Am Soc Nephrol. 2007;18:1855–1871. doi: 10.1681/ASN.2006121344. [DOI] [PubMed] [Google Scholar]
- 44.Adams M, Smith UM, Logan CV, Johnson CA. Recent advances in the molecular pathology, cell biology and genetics of ciliopathies. J Med Genet. 2008;45:257–267. doi: 10.1136/jmg.2007.054999. [DOI] [PubMed] [Google Scholar]
- 45.Salomon R, Saunier S, Niaudet P. Nephronophthisis. Pediatr Nephrol. 2008 doi: 10.1007/s00467-008-0840-z. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Otto EA, Schermer B, Obara T, O’Toole JF, Hiller KS, Mueller AM, Ruf RG, Hoefele J, Beekmann F, Landau D, Foreman JW, Goodship JA, Strachan T, Kispert A, Wolf MT, et al. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet. 2003;34:413–420. doi: 10.1038/ng1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sugiyama N, Yokoyama T. Sustained cell proliferation of renal epithelial cells in mice with inv mutation. Genes Cells. 2006;11:1213–1224. doi: 10.1111/j.1365-2443.2006.01011.x. [DOI] [PubMed] [Google Scholar]
- 48.Hong DH, Pawlyk B, Sokolov M, Strissel KJ, Yang J, Tulloch B, Wright AF, Arshavsky VY, Li T. RPGR isoforms in photoreceptor connecting cilia and the transitional zone of motile cilia. Invest Ophthalmol Vis Sci. 2003;44:2413–2421. doi: 10.1167/iovs.02-1206. [DOI] [PubMed] [Google Scholar]
- 49.Morgan D, Eley L, Sayer J, Strachan T, Yates LM, Craighead AS, Goodship JA. Expression analyses and interaction with the anaphase promoting complex protein Apc2 suggest a role for inversin in primary cilia and involvement in the cell cycle. Hum Mol Genet. 2002;11:3345–3350. doi: 10.1093/hmg/11.26.3345. [DOI] [PubMed] [Google Scholar]
- 50.Otto EA, Loeys B, Khanna H, Hellemans J, Sudbrak R, Fan S, Muerb U, O’Toole JF, Helou J, Attanasio M, Utsch B, Sayer JA, Lillo C, Jimeno D, Coucke P, et al. Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior-Loken syndrome and interacts with RPGR and calmodulin. Nat Genet. 2005;37:282–288. doi: 10.1038/ng1520. [DOI] [PubMed] [Google Scholar]
- 51.Mollet G, Silbermann F, Delous M, Salomon R, Antignac C, Saunier S. Characterization of the nephrocystin/nephrocystin-4 complex and subcellular localization of nephrocystin-4 to primary cilia and centrosomes. Hum Mol Genet. 2005;14:645–656. doi: 10.1093/hmg/ddi061. [DOI] [PubMed] [Google Scholar]
- 52.Winkelbauer ME, Schafer JC, Haycraft CJ, Swoboda P, Yoder BK. The C. elegans homologs of nephrocystin-1 and nephrocystin-4 are cilia transition zone proteins involved in chemosensory perception. J Cell Sci. 2005;118:5575–5587. doi: 10.1242/jcs.02665. [DOI] [PubMed] [Google Scholar]
- 53.Chang B, Khanna H, Hawes N, Jimeno D, He S, Lillo C, Parapuram SK, Cheng H, Scott A, Hurd RE, Sayer JA, Otto EA, Attanasio M, O’Toole JF, Jin G, et al. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum Mol Genet. 2006;15:1847–1857. doi: 10.1093/hmg/ddl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fliegauf M, Horvath J, von Schnakenburg C, Olbrich H, Muller D, Thumfart J, Schermer B, Pazour GJ, Neumann HP, Zentgraf H, Benzing T, Omran H. Nephrocystin specifically localizes to the transition zone of renal and respiratory cilia and photoreceptor connecting cilia. J Am Soc Nephrol. 2006;17:2424–2433. doi: 10.1681/ASN.2005121351. [DOI] [PubMed] [Google Scholar]
- 55.Sayer JA, Otto EA, O’Toole JF, Nurnberg G, Kennedy MA, Becker C, Hennies HC, Helou J, Attanasio M, Fausett BV, Utsch B, Khanna H, Liu Y, Drummond I, Kawakami I, et al. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet. 2006;38:674–681. doi: 10.1038/ng1786. [DOI] [PubMed] [Google Scholar]
- 56.von Schnakenburg C, Fliegauf M, Omran H. Nephrocystin and ciliary defects not only in the kidney? Pediatr Nephrol. 2007;22:765–769. doi: 10.1007/s00467-007-0434-1. [DOI] [PubMed] [Google Scholar]
- 57.Delous M, Baala L, Salomon R, Laclef C, Vierkotten J, Tory K, Golzio C, Lacoste T, Besse L, Ozilou C, Moutkine I, Hellman NE, Anselme I, Silbermann F, Vesque C, et al. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet. 2007;39:875–881. doi: 10.1038/ng2039. [DOI] [PubMed] [Google Scholar]
- 58.Ward CJ, Yuan D, Masyuk TV, Wang X, Punyashthiti R, Whelan S, Bacallao R, Torra R, LaRusso NF, Torres VE, Harris PC. Cellular and subcellular localization of the ARPKD protein; fibrocystin is expressed on primary cilia. Hum Mol Genet. 2003;12:2703–2710. doi: 10.1093/hmg/ddg274. [DOI] [PubMed] [Google Scholar]
- 59.Wilson NF, Lefebvre PA. Regulation of flagellar assembly by glycogen synthase kinase 3 in Chlamydomonas reinhardtii. Eukaryot Cell. 2004;3:1307–1319. doi: 10.1128/EC.3.5.1307-1319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qian CN, Knol J, Igarashi P, Lin F, Zylstra U, Teh BT, Williams BO. Cystic renal neoplasia following conditional inactivation of apc in mouse renal tubular epithelium. J Biol Chem. 2005;280:3938–3945. doi: 10.1074/jbc.M410697200. [DOI] [PubMed] [Google Scholar]
- 61.Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, Quaggin SE, Harrison R, Mount R, McNeill H. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet. 2008;40:1010–1015. doi: 10.1038/ng.179. [DOI] [PubMed] [Google Scholar]
- 62.Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, Chuang PT, Reiter JF. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol. 2008;10:70–76. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- 63.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 64.Schwartz EA, Leonard ML, Bizios R, Bowser SS. Analysis and modeling of the primary cilium bending response to fluid shear. Am J Physiol. 1997;272:F132–F138. doi: 10.1152/ajprenal.1997.272.1.F132. [DOI] [PubMed] [Google Scholar]
- 65.Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- 66.Praetorius HA, Spring KR. Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol. 2003;191:69–76. doi: 10.1007/s00232-002-1042-4. [DOI] [PubMed] [Google Scholar]
- 67.Davenport JR, Watts AJ, Roper VC, Croyle MJ, van Groen T, Wyss JM, Nagy TR, Kesterson RA, Yoder BK. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol. 2007;17:1586–1594. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Piontek K, Menezes LF, Garcia-Gonzalez MA, Huso DL, Germino GG. A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat Med. 2007;13:1490–1495. doi: 10.1038/nm1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M. Defective planar cell polarity in polycystic kidney disease. Nat Genet. 2006;38:21–23. doi: 10.1038/ng1701. [DOI] [PubMed] [Google Scholar]
- 70.Wu Y, Dai XQ, Li Q, Chen CX, Mai W, Hussain Z, Long W, Montalbetti N, Li G, Glynne R, Wang S, Cantiello HF, Wu G, Chen XZ. Kinesin-2 mediates physical and functional interactions between polycystin-2 and fibrocystin. Hum Mol Genet. 2006;15:3280–3292. doi: 10.1093/hmg/ddl404. [DOI] [PubMed] [Google Scholar]
- 71.Wang S, Zhang J, Nauli SM, Li X, Starremans PG, Luo Y, Roberts KA, Zhou J. Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol Cell Biol. 2007;27:3241–3252. doi: 10.1128/MCB.00072-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim I, Li C, Liang D, Chen XZ, Coffy RJ, Ma J, Zhao P, Wu G. Polycystin-2 expression is regulated by a PC2-binding domain of intracellular portion of fibrocystin. J Biol Chem. 2008 doi: 10.1074/jbc.M805452200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim I, Fu Y, Hui K, Moeckel G, Mai W, Li C, Liang D, Zhao P, Ma J, Chen XZ, George AL, Jr., Coffey RJ, Feng ZP, Wu G. Fibrocystin/polyductin modulates renal tubular formation by regulating polycystin-2 expression and function. J Am Soc Nephrol. 2008;19:455–468. doi: 10.1681/ASN.2007070770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nishio S, Hatano M, Nagata M, Horie S, Koike T, Tokuhisa T, Mochizuki T. Pkd1 regulates immortalized proliferation of renal tubular epithelial cells through p53 induction and JNK activation. J Clin Invest. 2005;115:910–918. doi: 10.1172/JCI22850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bhunia AK, Piontek K, Boletta A, Liu L, Qian F, Xu PN, Germino FJ, Germino GG. PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell. 2002;109:157–168. doi: 10.1016/s0092-8674(02)00716-x. [DOI] [PubMed] [Google Scholar]
- 76.Low SH, Vasanth S, Larson CH, Mukherjee S, Sharma N, Kinter MT, Kane ME, Obara T, Weimbs T. Polycystin-1, STAT6, and P100 function in a pathway that transduces ciliary mechanosensation and is activated in polycystic kidney disease. Dev Cell. 2006;10:57–69. doi: 10.1016/j.devcel.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 77.Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A. 2006;103:5466–5471. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim E, Arnould T, Sellin LK, Benzing T, Fan MJ, Gruning W, Sokol SY, Drummond I, Walz G. The polycystic kidney disease 1 gene product modulates Wnt signaling. J Biol Chem. 1999;274:4947–4953. doi: 10.1074/jbc.274.8.4947. [DOI] [PubMed] [Google Scholar]
- 79.Chauvet V, Tian X, Husson H, Grimm DH, Wang T, Hiesberger T, Igarashi P, Bennett AM, Ibraghimov-Beskrovnaya O, Somlo S, Caplan MJ. Mechanical stimuli induce cleavage and nuclear translocation of the polycystin-1 C terminus. J Clin Invest. 2004;114:1433–1443. doi: 10.1172/JCI21753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weimbs T. Polycystic kidney disease and renal injury repair: common pathways, fluid flow, and the function of polycystin-1. Am J Physiol Renal Physiol. 2007;293:F1423–F1432. doi: 10.1152/ajprenal.00275.2007. [DOI] [PubMed] [Google Scholar]
- 81.Jauregui AR, Nguyen KC, Hall DH, Barr MM. The Caenorhabditis elegans nephrocystins act as global modifiers of cilium structure. J Cell Biol. 2008;180:973–988. doi: 10.1083/jcb.200707090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sohara E, Luo Y, Zhang J, Manning DK, Beier DR, Zhou J. Nek8 regulates the expression and localization of polycystin-1 and polycystin-2. J Am Soc Nephrol. 2008;19:469–476. doi: 10.1681/ASN.2006090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Otto EA, Trapp ML, Schultheiss UT, Helou J, Quarmby LM, Hildebrandt F. NEK8 mutations affect ciliary and centrosomal localization and may cause nephronophthisis. J Am Soc Nephrol. 2008;19:587–592. doi: 10.1681/ASN.2007040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bergmann C, Fliegauf M, Bruchle NO, Frank V, Olbrich H, Kirschner J, Schermer B, Schmedding I, Kispert A, Kranzlin B, Nurnberg G, Becker C, Grimm T, Girschick G, Lynch SA, et al. Loss of nephrocystin-3 function can cause embryonic lethality, Meckel-Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am J Hum Genet. 2008;82:959–970. doi: 10.1016/j.ajhg.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 87.Gerdes JM, Liu Y, Zaghloul NA, Leitch CC, Lawson SS, Kato M, Beachy PA, Beales PL, DeMartino GN, Fisher S, Badano JL, Katsanis N. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet. 2007;39:1350–1360. doi: 10.1038/ng.2007.12. [DOI] [PubMed] [Google Scholar]
- 88.Masyuk AI, Masyuk TV, LaRusso NF. Cholangiocyte primary cilia in liver health and disease. Dev Dyn. 2008;237:2007–2012. doi: 10.1002/dvdy.21530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iomini C, Tejada K, Mo W, Vaananen H, Piperno G. Primary cilia of human endothelial cells disassemble under laminar shear stress. J Cell Biol. 2004;164:811–817. doi: 10.1083/jcb.200312133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation. 2008;117:1161–1171. doi: 10.1161/CIRCULATIONAHA.107.710111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hierck BP, Van der Heiden K, Alkemade FE, Van de Pas S, Van Thienen JV, Groenendijk BC, Bax WH, Van der Laarse A, Deruiter MC, Horrevoets AJ, Poelmann RE. Primary cilia sensitize endothelial cells for fluid shear stress. Dev Dyn. 2008;237:725–735. doi: 10.1002/dvdy.21472. [DOI] [PubMed] [Google Scholar]
- 92.Van der Heiden K, Hierck BP, Krams R, de Crom R, Cheng C, Baiker M, Pourquie MJ, Alkemade FE, DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE. Endothelial primary cilia in areas of disturbed flow are at the base of atherosclerosis. Atherosclerosis. 2008;196:542–550. doi: 10.1016/j.atherosclerosis.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 93.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schneider L, Cammer M, Lehman J, Nielsen SK, Guerra CF, Veland IR, Stock C, Hoffmann EK, Yoder BK, Schwab A, Satir P, Christensen ST. Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. doi: 10.1159/000276562. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schneider L, Stock C, Dieterich P, Jensen BH, Pedersen LB, Satir P, Schwab A, Christensen ST, Pedersen SF. The Na+/H+ exchanger NHE1 plays a central role in directional migration stimulated via PDGFRα in the primary cilium. doi: 10.1083/jcb.200806019. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lu CJ, Du H, Wu J, Jansen DA, Jordan KL, Xu N, Sieck GC, Qian Q. Non-random distribution and sensory functions of primary cilia in vascular smooth muscle cells. Kidney Blood Press Res. 2008;31:171–184. doi: 10.1159/000132462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jensen CG, Poole CA, McGlashan SR, Marko M, Issa ZI, Vujcich KV, Bowser SS. Ultrastructural, tomographic and confocal imaging of the chondrocyte primary cilium in situ. Cell Biol Int. 2004;28:101–110. doi: 10.1016/j.cellbi.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 98.McGlashan SR, Jensen CG, Poole CA. Localization of extracellular matrix receptors on the chondrocyte primary cilium. J Histochem Cytochem. 2006;54:1005–1014. doi: 10.1369/jhc.5A6866.2006. [DOI] [PubMed] [Google Scholar]
- 99.Kestler HA, Kuhl M. From individual Wnt pathways towards a Wnt signalling network. Philos Trans R Soc Lond B Biol Sci. 2008;363:1333–1347. doi: 10.1098/rstb.2007.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vlad A, Rohrs S, Klein-Hitpass L, Muller O. The first five years of the Wnt targetome. Cell Signal. 2008;20:795–802. doi: 10.1016/j.cellsig.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 101.Kikuchi A, Yamamoto H. Tumor formation due to abnormalities in the beta-catenin-independent pathway of Wnt signaling. Cancer Sci. 2008;99:202–208. doi: 10.1111/j.1349-7006.2007.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, Tan PL, Phillips HM, Leroux MR, Henderson DJ, Murdoch JN, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 103.Chan DW, Chan CY, Yam JW, Ching YP, Ng IO. Prickle-1 negatively regulates Wnt/beta-catenin pathway by promoting Dishevelled ubiquitination/degradation in liver cancer. Gastroenterology. 2006;131:1218–1227. doi: 10.1053/j.gastro.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 104.Jones C, Roper VC, Foucher I, Qian D, Banizs B, Petit C, Yoder BK, Chen P. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat Genet. 2008;40:69–77. doi: 10.1038/ng.2007.54. [DOI] [PubMed] [Google Scholar]
- 105.Cano DA, Murcia NS, Pazour GJ, Hebrok M. Orpk mouse model of polycystic kidney disease reveals essential role of primary cilia in pancreatic tissue organization. Development. 2004;131:3457–3467. doi: 10.1242/dev.01189. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Q, Davenport JR, Croyle MJ, Haycraft CJ, Yoder BK. Disruption of IFT results in both exocrine and endocrine abnormalities in the pancreas of Tg737(orpk) mutant mice. Lab Invest. 2005;85:45–64. doi: 10.1038/labinvest.3700207. [DOI] [PubMed] [Google Scholar]
- 107.Willert K, Nusse R. Beta-catenin: a key mediator of Wnt signaling. Curr Opin Genet Dev. 1998;8:95–102. doi: 10.1016/s0959-437x(98)80068-3. [DOI] [PubMed] [Google Scholar]
- 108.Roose J, Clevers H. TCF transcription factors: molecular switches in carcinogenesis. Biochim Biophys Acta. 1999;1424:M23–37. doi: 10.1016/s0304-419x(99)00026-8. [DOI] [PubMed] [Google Scholar]
- 109.Nusse R. WNT targets. Repression and activation. Trends Genet. 1999;15:1–3. doi: 10.1016/s0168-9525(98)01634-5. [DOI] [PubMed] [Google Scholar]
- 110.Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22:2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- 111.Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu Rev Cell Dev Biol. 2007;23:345–373. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu A, Wang B, Niswander LA. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development. 2005;132:3103–3111. doi: 10.1242/dev.01894. [DOI] [PubMed] [Google Scholar]
- 113.Christensen ST, Ott CM. Cell signaling. A ciliary signaling switch. Science. 2007;317:330–331. doi: 10.1126/science.1146180. [DOI] [PubMed] [Google Scholar]
- 114.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 115.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 116.Ocbina PJ, Anderson KV. Intraflagellar transport, cilia, and mammalian Hedgehog signaling: analysis in mouse embryonic fibroblasts. Dev Dyn. 2008;237:2030–2038. doi: 10.1002/dvdy.21551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kovacs JJ, Whalen EJ, Liu R, Xiao K, Kim J, Chen M, Wang J, Chen W, Lefkowitz RJ. Beta-arrestin-mediated localization of smoothened to the primary cilium. Science. 2008;320:1777–1781. doi: 10.1126/science.1157983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Koyama E, Young B, Nagayama M, Shibukawa Y, Enomoto-Iwamoto M, Iwamoto M, Maeda Y, Lanske B, Song B, Serra R, Pacifici M. Conditional Kif3a ablation causes abnormal hedgehog signaling topography, growth plate dysfunction, and excessive bone and cartilage formation during mouse skeletogenesis. Development. 2007;134:2159–2169. doi: 10.1242/dev.001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Haycraft CJ, Zhang Q, Song B, Jackson WS, Detloff PJ, Serra R, Yoder BK. Intraflagellar transport is essential for endochondral bone formation. Development. 2007;134:307–316. doi: 10.1242/dev.02732. [DOI] [PubMed] [Google Scholar]
- 120.Cho A, Ko HW, Eggenschwiler JT. FKBP8 cell-autonomously controls neural tube patterning through a Gli2- and Kif3a-dependent mechanism. Dev Biol. 2008;321:27–39. doi: 10.1016/j.ydbio.2008.05.558. [DOI] [PubMed] [Google Scholar]
- 121.Spassky N, Han YG, Aguilar A, Strehl L, Besse L, Laclef C, Ros MR, Garcia-Verdugo JM, Alvarez-Buylla A. Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool. Dev Biol. 2008;317:246–259. doi: 10.1016/j.ydbio.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chizhikov VV, Davenport J, Zhang Q, Shih EK, Cabello OA, Fuchs JL, Yoder BK, Millen KJ. Cilia proteins control cerebellar morphogenesis by promoting expansion of the granule progenitor pool. J Neurosci. 2007;27:9780–9789. doi: 10.1523/JNEUROSCI.5586-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vierkotten J, Dildrop R, Peters T, Wang B, Ruther U. Ftm is a novel basal body protein of cilia involved in Shh signalling. Development. 2007;134:2569–2577. doi: 10.1242/dev.003715. [DOI] [PubMed] [Google Scholar]
- 124.May SR, Ashique AM, Karlen M, Wang B, Shen Y, Zarbalis K, Reiter J, Ericson J, Peterson AS. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev Biol. 2005;287:378–389. doi: 10.1016/j.ydbio.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 125.Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci U S A. 2005;102:11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell. 2007;12:767–778. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 127.Evangelista M, Lim T, Lee J, Parker L, Ashique A, Peterson A, Ye W, Davis D, de Sauvage F. Kinome siRNA screen identifies regulators of ciliogenesis and hedgehog signal transduction. Sci Signal. 2008;1:ra7. doi: 10.1126/scisignal.1162925. [DOI] [PubMed] [Google Scholar]
- 128.Johnson ET, Nicola T, Roarty K, Yoder BK, Haycraft CJ, Serra R. Role for primary cilia in the regulation of mouse ovarian function. Dev. Dyn. 2008;237:2053–2060. doi: 10.1002/dvdy.21612. [DOI] [PubMed] [Google Scholar]
- 129.Houde C, Dickinson RJ, Houtzager VM, Cullum R, Montpetit R, Metzler M, Simpson EM, Roy S, Hayden MR, Hoodless PA, Nicholson DW. Hippi is essential for node cilia assembly and Sonic hedgehog signaling. Dev Biol. 2006;300:523–533. doi: 10.1016/j.ydbio.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kawahira H, Scheel DW, Smith SB, German MS, Hebrok M. Hedgehog signaling regulates expansion of pancreatic epithelial cells. Dev Biol. 2005;280:111–121. doi: 10.1016/j.ydbio.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 131.Lau J, Kawahira H, Hebrok M. Hedgehog signaling in pancreas development and disease. Cell Mol Life Sci. 2006;63:642–652. doi: 10.1007/s00018-005-5357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cano DA, Hebrok M, Zenker M. Pancreatic development and disease. Gastroenterology. 2007;132:745–762. doi: 10.1053/j.gastro.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 133.van den Brink GR. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol Rev. 2007;87:1343–1375. doi: 10.1152/physrev.00054.2006. [DOI] [PubMed] [Google Scholar]
- 134.Nielsen SK, Mollgard K, Clement CA, Veland IR, Awan A, Yoder BK, Novak I, Christensen ST. Characterization of primary cilia and Hedgehog signaling during development of the human pancreas and in human pancreatic duct cancer cell lines. Dev Dyn. 2008;237:2039–2052. doi: 10.1002/dvdy.21610. [DOI] [PubMed] [Google Scholar]
- 135.Cano DA, Sekine S, Hebrok M. Primary cilia deletion in pancreatic epithelial cells results in cyst formation and pancreatitis. Gastroenterology. 2006;131:1856–1869. doi: 10.1053/j.gastro.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 136.Allan EH, Ho PW, Umezawa A, Hata J, Makishima F, Gillespie MT, Martin TJ. Differentiation potential of a mouse bone marrow stromal cell line. J Cell Biochem. 2003;90:158–169. doi: 10.1002/jcb.10614. [DOI] [PubMed] [Google Scholar]
- 137.Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development. 2005;132:49–60. doi: 10.1242/dev.01564. [DOI] [PubMed] [Google Scholar]
- 138.Day TF, Yang Y. Wnt and hedgehog signaling pathways in bone development. J Bone Joint Surg Am. 2008;90(Suppl 1):19–24. doi: 10.2106/JBJS.G.01174. [DOI] [PubMed] [Google Scholar]
- 139.Serra R. Role of intraflagellar transport and primary cilia in skeletal development. Anat Rec (Hoboken) 2008;291:1049–1061. doi: 10.1002/ar.20634. [DOI] [PubMed] [Google Scholar]
- 140.Ruiz-Perez VL, Blair HJ, Rodriguez-Andres ME, Blanco MJ, Wilson A, Liu YN, Miles C, Peters H, Goodship JA. Evc is a positive mediator of Ihh-regulated bone growth that localises at the base of chondrocyte cilia. Development. 2007;134:2903–2912. doi: 10.1242/dev.007542. [DOI] [PubMed] [Google Scholar]
- 141.Nishikawa S, Goldstein RA, Nierras CR. The promise of human induced pluripotent stem cells for research and therapy. Nat Rev Mol Cell Biol. 2008;9:725–729. doi: 10.1038/nrm2466. [DOI] [PubMed] [Google Scholar]
- 142.Chumsri S, Burger AM. Cancer stem cell targeted agents: therapeutic approaches and consequences. Curr Opin Mol Ther. 2008;10:323–333. [PubMed] [Google Scholar]
- 143.Kiprilov EN, Awan A, Desprat R, Velho M, Clement CA, Byskov AG, Andersen CY, Satir P, Bouhassira EE, Christensen ST, Hirsch RE. Human embryonic stem cells in culture possess primary cilia with hedgehog signaling machinery. J Cell Biol. 2008;180:897–904. doi: 10.1083/jcb.200706028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pebay A, Wong RC, Pitson SM, Wolvetang EJ, Peh GS, Filipczyk A, Koh KL, Tellis I, Nguyen LT, Pera MF. Essential roles of sphingosine-1-phosphate and platelet-derived growth factor in the maintenance of human embryonic stem cells. Stem Cells. 2005;23:1541–1548. doi: 10.1634/stemcells.2004-0338. [DOI] [PubMed] [Google Scholar]
- 145.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 146.Awan A, Olivieri RO, Jensen PL, Christensen ST, Andersen CY. Characterization of Human Embryonic Stem Cells (hESCs) grown under feeder-free conditions. Methods Mol Biol. 2008 doi: 10.1007/978-1-60761-369-5_11. In press. [DOI] [PubMed] [Google Scholar]
- 147.Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, Alvarez-Buylla A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- 148.Komada M, Saitsu H, Kinboshi M, Miura T, Shiota K, Ishibashi M. Hedgehog signaling is involved in development of the neocortex. Development. 2008;135:2717–2727. doi: 10.1242/dev.015891. [DOI] [PubMed] [Google Scholar]
- 149.Breunig JJ, Sarkisian MR, Arellano JI, Morozov YM, Ayoub AE, Sojitra S, Wang B, Flavell RA, Rakic P, Town T. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc Natl Acad Sci U S A. 2008;105:13127–13132. doi: 10.1073/pnas.0804558105. [DOI] [PMC free article] [PubMed] [Google Scholar]