International Union of Basic and Clinical Pharmacology. LXXVI. Current Progress in the Mammalian TRP Ion Channel Family (original) (raw)
Abstract
Transient receptor potential (TRP) channels are a large family of ion channel proteins, surpassed in number in mammals only by voltage-gated potassium channels. TRP channels are activated and regulated through strikingly diverse mechanisms, making them suitable candidates for cellular sensors. They respond to environmental stimuli such as temperature, pH, osmolarity, pheromones, taste, and plant compounds, and intracellular stimuli such as Ca2+ and phosphatidylinositol signal transduction pathways. However, it is still largely unknown how TRP channels are activated in vivo. Despite the uncertainties, emerging evidence using TRP channel knockout mice indicates that these channels have broad function in physiology. Here we review the recent progress on the physiology, pharmacology and pathophysiological function of mammalian TRP channels.
I. Introduction
Unlike most ion channels, TRP1 channel family members are identified by their sequence homology rather than by ligand function or ion selectivity. To date, ∼30 mammalian TRP channels have been identified and are grouped into six subfamilies on the basis of amino acid sequence homology: TRPC (“canonical”), TRPM (“melastatin”), TRPV (“vanilloid”), TRPA (“ankyrin”), TRPML (“mucolipin”), and TRPP (or PKD) (“polycystin”) (Clapham et al., 2005).
A. Common Features
TRP channels conduct cations and, when activated, depolarize cells. If, as a result, TRP channel-mediated intracellular Ca2+ induces increases above basal levels (∼100 nM), they initiate a plethora of cellular responses. They are commonly found in epithelial cells but can be found in all cell types. Most TRP channels are weakly voltage-sensitive and nonselective, with PCa/PNa < 10, with the exception of the monovalent-selective TRPM3α1, TRPM4, and TRPM5 (PCa/PNa < 0.05) and the Ca2+-selective TRPM3α2, TRPV5, and TRPV6 (PCa/PNa > 100). Based on extensive work on other members of the voltage-ligand-gated superfamily of ion channels (Yu et al., 2005) and TRP channel primary sequences, they are assumed to have six transmembrane (TM) spanning domains (S1–S6) with a pore domain between the fifth (S5) and sixth (S6) segments and both C and N termini located intracellularly. The cytoplasmic end of the S6 helices seem to form the lower gate, which opens and closes to regulate cation entry into the channel. The S1–S4 domain may gate the pore in response to ligand binding, but the paucity of positively charged arginines in S4 helices indicates weak voltage sensitivity of TRP channels. All elements outside the S5–S6 region provide means of either subunit association or act as linkers to elements that control gating. Other structural features of TRP channels include 1) a 25-amino acid TRP domain containing a TRP box (EWKFAR) just C-terminal to S6 in TRPC (also in TRPV and TRPM, but less conserved); 2) ankyrin repeats in the N-terminal cytoplasmic domain of TRPC, TRPV, and TRPA; and 3) proline-rich regions in the region just C-terminal to S6 in TRPC, TRPM, and in some TRPVs (Clapham, 2003; Ramsey et al., 2006).
Although some TRP channels clearly function as chemosensors for exogenous ligands, relatively few endogenous ligands are known for TRP channel activation. Therefore, one of the central unanswered questions in the field is how TRP channels are normally activated in vivo. Many TRP channels are potentiated by phospholipase C activation. Large classes of G protein-coupled receptors (Gq/11; linked to PLCβ) and tyrosine kinase receptors (linked to PLCγ) potentiate most TRP channels. However, the mechanism of this potentiation is not well understood (Trebak et al., 2007). Elements of the phosphatidylinositol signaling pathway are closely linked to the plasma membrane and also seem to regulate many TRP channels. In particular, PIP2, a common regulator of ion channels, potentiates most TRP channel activity (Voets and Nilius, 2007). In addition, intracellular Ca2+ increases the activity of some mammalian TRP channels and modulates practically all TRP channels. Regulation by phosphorylation, PIP2, and Ca2+ are common to ion channels and are not specific features of the TRP class of channels.
A common problem in the TRP field is the lack of specific pharmacological tools, leading to the dependence on highly nonspecific blockers, such as ruthenium red (which binds most Ca2+ binding sites in proteins), 2-APB, flufenamate, niflumic acid, and 1-(β-[3-(4-methoxyphenyl)propoxy]-4-methoxyphenethyl)-1_H_-imidazole (SKF96365). More useful tools include capsaicin, a fairly specific agonist of TRPV1, and 2-(1, 3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydro-7_H_-purin-7-yl)-_N_-(4-isopropylphenyl)acetamide (HC-030031), a relatively specific antagonist of TRPA1 (Caterina et al., 1997; McNamara et al., 2007). La3+ is useful in recognizing TRPC4 or TRPC5 because it potentiates these channels and blocks most other TRP and Ca2+-permeant channels (Strübing et al., 2001). TRP channel antagonists with higher selectivities and potencies are being developed in the pharmaceutical industry but most are currently unavailable for academic research. The dearth of useful pharmacological tools forces reliance on small interfering RNA and genetic strategies, but these methods do not replace the usefulness of blockers and antagonists. There is currently significant disagreement on the assembly, localization, and function of TRP channels. This confusion has been created by nonspecific antibodies, lack of precise pharmacological tools, and over-reliance on indirect Ca2+ measurements rather than direct measurement of currents.
Indeed, emerging evidence using knockout mice has revealed the very diverse functions of TRP channels (Moran et al., 2004; Desai and Clapham, 2005; Venkatachalam and Montell, 2007). Human genetics has further uncovered potential TRP channel functions. For example, 13 channelopathies have been proposed to stem from mutations in TRP genes: focal segmental glomerulosclerosis 2 (OMIM 603965), caused by TRPC6 mutations; Charcot-Marie-Tooth disease type 2C (OMIM 606071) and scapuloperoneal spinal muscular atrophy (OMIM 181405), caused by TRPV4 mutations; congenital stationary night blindness (OMIM 301500), caused by TRPM1 mutations; progressive familial heart block type 1 (OMIM 113990), caused by TRPM4 mutations; autosomal-recessive hypomagnesemia with secondary hypocalcemia (OMIM 602014), caused by TRPM6 mutations; amyotrophic lateral sclerosis-Parkinsonism/dementia complex (OMIM 105500), caused by TRPM2 or TRPM7 mutations; brachyolmia type 3 (OMIM 113500); mucolipidosis IV (OMIM 252650), caused by TRPML1 mutations; Kozlowski type of spondylometaphyseal dysplasia (OMIM 184252); metatropic dysplasia (OMIM 156530); congenital distal spinomuscular atrophy (OMIM 600175); autosomal dominant polycystic kidney disease (OMIM 173910), caused by TRPP1 or TRPP2 mutations; and familial episodic pain syndrome, caused by a TRPA1 mutation. The function of TRP channels in vivo is the current focus in the field. Here we summarize recent progress on the physiology, pharmacology, and pathophysiological function of mammalian TRP channels.
For detailed tables of TRP genes, accession numbers, splice variants, domains, biophysical properties, and pharmacology, see http://www.iuphar-db.org/DATABASE/FamilyIntroductionForward?familyId= 78 and http://clapham.tch.harvard.edu. There are many excellent comprehensive reviews on TRP channel domain structure, channelopathies, pharmacology, and neuronal TRPs (Venkatachalam and Montell, 2007; Talavera et al., 2008; Latorre et al., 2009; Nilius and Owsianik, 2010).
II. The Transient Receptor Potential (Canonical) Family
Seven mammalian TRPC proteins (TRPC1–7; Fig. 1) have been identified, but TRPC2 is a pseudogene in humans. These channels can be divided into three subgroups by sequence homology: C1/C4/C5, C3/C6/C7, and C2. All mammalian TRPC proteins seem to be potentiated by stimulation of G-protein-coupled receptors and receptor tyrosine kinases.
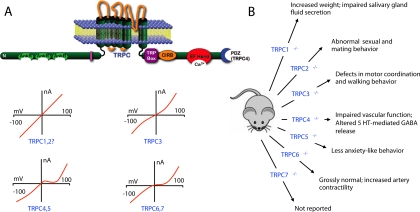
Fig. 1.
TRPC (canonical) family. A, molecular domains of TRPC channels and their current-voltage relationships. The TRP box is a conserved region in TRPC, TRPV, and TRPM families; its function is unclear, but it may bind PIP2. CIRB refers to a calmodulin/IP3R-binding (CIRB) domain. The EF hand is a helix-loop-helix structural domain found in a large family of calcium-binding proteins. PDZ (postsynaptic density 95/disc-large/zona occludens) is a common protein interaction motif that holds together signaling complexes. In this and the following figures, steady state current-voltage curves are shown. B, results of genetic deletion experiments. The TrpC7(−/−) phenotype has not been reported. TrpC2 is a pseudogene in humans.
A. Transient Receptor Potential C1/C4/C5 Subgroup
TRPC1 was the first member of the mammalian TRPCs purported to form an ion channel (Zitt et al., 1996). However, whether TRPC1 can form functional homomeric channels by itself remains debatable. Although homomeric TRPC1 was proposed to be a store-operated channel or stretch-activated channel (Zitt et al., 1996; Maroto et al., 2005), heterologous overexpression of TRPC1 has produced no measurable currents distinguishable from background leak (Lintschinger et al., 2000; Strübing et al., 2001). It is possible that TRPC1 homomeric channels are functional, but the activating stimulus has not yet been found. Alternatively, TRPC1 may function as a homomer in the endoplasmic reticulum and reach the plasma membrane only when coassembled with other TRP subunits. A more detailed examination of previously proposed homomeric TRPC1 channels is required before TRPC1 can be assumed to form a plasma membrane channel by itself.
TRPC1 forms heteromeric channels with C4 or C5, which have properties distinct from those of homomultimers (Lintschinger et al., 2000; Strübing et al., 2001). The existence of TRPC1 and C4 or C5 heteromeric channels is supported by the following evidence: 1) TRPC1 coexpressed with TRPC4 or TRPC5 form current-voltage relationships distinct from TRPC4 or TRPC5 expressed alone; 2) single-channel currents from TRPC1 coexpressed with TRPC4 or TRPC5 have clearly distinct conductances from TRPC4 or TRPC5 homomeric currents; 3) TRPC1 coimmunoprecipitates with TRPC4 or TRPC5 when purified from brain, and; 4) in hippocampal neurons, TRPC1/C4 or TRPC1/C5 heteromeric channels seem to localize to the cell bodies of neurons, whereas TRPC4 or TRPC5 can reside in both the cell body and the periphery (dendrites, axons) (Strübing et al., 2001, 2003). Therefore, given the wide expression of TRPC1 and its ability to coassemble with other TRPC subunits, TRPC1 might be a component of different heteromeric TRP complexes.
TRPC4 and TRPC5 are close homologs, sharing 64% identity. Both TRPC4 and TRPC5 contain a C-terminal PDZ-binding motif (VTTRL). PDZ domain scaffolding proteins, such as Na+/H+ exchanger regulator factor, as well as signaling molecules such as PLCβ1, have been reported to coimmunoprecipitate with TRPC4 and TRPC5 (Tang et al., 2000). TRPC4 and TRPC5 channels also share many functional characteristics, are both potentiated by GPCRs that couple to Gαq/11, and have similar current-voltage relationships (Okada et al., 1998; Schaefer et al., 2000). Although activation of these channels seems to require PLC enzymatic activity, their direct agonists are still unknown (Hofmann et al., 1999; Schaefer et al., 2000).
Homomeric TRPC4 or TRPC5 subunits underlie currents with a unique doubly rectifying current-voltage relationship with single-channel conductance of 28 and 38 pS, respectively. In addition, TRPC4 and TRPC5 channels are unique among TRP channels in that they are potentiated by micromolar concentrations of the trivalent cations La3+ or Gd3+ (Schaefer et al., 2000; Strübing et al., 2001). TRPC5 is dramatically potentiated by intracellular Ca2+, which does not seem to involve calmodulin (CaM). Intracellular Ca2+ also potentiates TRPC4 channel activity to a lesser degree (Blair et al., 2009). CaM itself was reported to accelerate TRPC5 agonist-activated current via a CaM-binding site located at the C terminus of TRPC5 but not the CaM/IP3R-binding (CIRB) domain (Ordaz et al., 2005). Other modulations include potentiation of TRPC5 channels by extracellular thioredoxin (Xu et al., 2008) and nitric oxide (Yoshida et al., 2006), inhibition of TRPC4 channels by PIP2 (Otsuguro et al., 2008), inhibition of outward TRPC5 current by intracellular Mg2+ (Obukhov and Nowycky, 2008), and desensitization of TRPC5 channels by PKC-mediated phosphorylation (Ordaz et al., 2005).
In heterologous expression systems, homomeric TRPC5 channels can be rapidly delivered to the plasma membrane after stimulation of growth factor receptors via Rac, phosphatidylinositol 3 kinase, and phosphatidylinositol 5 kinase (Bezzerides et al., 2004). In young hippocampal neurons, TRPC5 channel subunits seem to interact with growth cone-enriched protein stathmin 2, are packaged into vesicles, and then carried to newly formed growth cones, where TRPC5 expression modulates neurite extension and growth cone morphology (Greka et al., 2003). A recent study showed that the downstream signaling for TRPC5 in neurite growth and axon formation may involve CaM kinase kinase and CaM kinase I γ (Davare et al., 2009).
TRPC1, TRPC4, and TRPC5 are expressed in the brain, predominantly in the hippocampus, cortex, olfactory bulb, and amygdala as well as heart, lung, liver, spleen, and testis (Venkatachalam and Montell, 2007; Riccio et al., 2009). TRPC1 was reported to mediate store-operated current (Zitt et al., 1996), stretch-activated current (Maroto et al., 2005), or the metabotropic glutamate receptor 1 (mGluR1)-evoked slow EPSC (Kim et al., 2003). One group reported that TRPC1(−/−) mice have increased body size, and the store-operated current was not affected in vascular smooth muscle cells from the knockout mice (Dietrich et al., 2007), whereas another group reported that TRPC1(−/−) mice exhibit impaired salivary gland fluid secretion while the store-operated current was reduced (Liu et al., 2007). A recent study found that TRPC1(−/−) mice displayed normal mGluR1-mediated synaptic transmission, with no obvious impairment of slow EPSCs or store-operated current (Hartmann et al., 2008). These conclusions depend on one TRPC1(−/−) mouse, and further studies should be carried out to verify that the mice indeed lack TRPC1 protein. This issue is complicated by the lack of adequate TRPC1 antibodies.
TRPC4(−/−) mice are viable, fertile, and exhibit no gross abnormalities (Freichel et al., 2001). At the cellular level, TRPC4 plays an important role in Ca2+ signaling in endothelial cells, and TRPC4(−/−) mice have defects in acetylcholine-induced vasoregulation and lung microvascular permeability (Freichel et al., 2001; Tiruppathi et al., 2002). Another study using TRPC4(−/−) mice concluded that TRPC4 mediates the increase of 5-hydroxytryptamine 2 receptor-coupled GABA release in thalamic interneurons (Munsch et al., 2003). TRPC5(−/−) mice exhibit no obvious developmental or anatomical defects. However, upon behavioral testing, TRPC5(−/−) mice exhibit a reduced anxiety-like (innate fear) phenotype (Riccio et al., 2009). The cellular mechanism underlying this phenotype stems from reduced responses mediated by group 1 mGluR and cholecystokinin 2 receptors in neurons of the amygdala, a brain region that integrates sensory input with behaviors related to fear and other emotions (Riccio et al., 2009). It is noteworthy that mice lacking stathmin, a protein interacting with TRPC5, have a similar phenotype (Shumyatsky et al., 2005).
B. Transient Receptor Potential C3/C6/C7 Subgroup
TRPC3, TRPC6, and TRPC7 amino acid sequences are roughly 75% identical. When expressed in heterologous systems, these proteins are potentiated by Gq/11-coupled receptors or by direct application of diacylglycerol (DAG) analogs (Hofmann et al., 1999; Okada et al., 1999). TRPC3/C6/C7 channels generate nonselective, doubly rectifying cation currents with a single-channel conductance of 65, 35, and 25 pS, respectively. They have relatively low selectivity for Ca2+ over Na+ and are sensitive to intracellular Ca2+. TRPC3 may assemble with TRPC1 in some cells (Lintschinger et al., 2000; Strübing et al., 2003).
Several signaling molecules modulate TRPC3, TRPC6, and TRPC7 channel activities. DAG analogs potentiate TRPC3/C6/C7 channel activity but not via DAG's stimulation of PKC (Trebak et al., 2003). By phosphorylating Ser-712 in TRPC3, PKC itself negatively regulates TRPC3 function (Venkatachalam et al., 2003; Trebak et al., 2005). PKG directly phosphorylates TRPC3 and inhibits the channel activity (Kwan et al., 2004). The nonreceptor tyrosine kinases Src and Fyn positively regulate TRPC3 and TRPC6, respectively (Hisatsune et al., 2004; Vazquez et al., 2004). Intracellular Ca2+ stimulates TRPC6 but inhibits TRPC7 activity (Shi et al., 2004). TRPC3 reportedly interacts with syntaxin 3, which may be involved in channel trafficking or insertion (Singh et al., 2004). Translocation of TRPC3 may also be regulated by its interaction with the scaffolding protein Homer1 (Kim et al., 2006). TRPC3 was further found to associate via its N terminus with PLCγ1 to form a bimolecular PH domain, which binds PIP2 as well as sphingosine-1-phosphate (van Rossum et al., 2005). Thus, TRPC3/C6/C7 channels may serve as versatile downstream effectors for a wide range of hormone and neurotransmitter receptors.
TRPC3 is present in brain, with the highest expression in cerebellum, cortex, and hippocampus. In hippocampal neurons and pontine neurons, TRPC3 is reportedly activated through a pathway that is initiated by binding of brain-derived neurotrophic factor to TrkB, engagement of a PLCγ, and activation of the inositol trisphosphate receptor (Li et al., 1999; Amaral and Pozzo-Miller, 2007), whereas in striatal cholinergic neurons or cerebellar Purkinje neurons, mGluR1s activate TRPC3 (Berg et al., 2007; Hartmann et al., 2008). TRPC3 is also reportedly involved in brain-derived neurotrophic factor-induced axon guidance or neuronal survival in cerebellar granule cells (Li et al., 2005; Jia et al., 2007). Brain development in TRPC3(−/−) mice appears grossly normal. However, TRPC3(−/−) mice (but not TRPC1-TRPC4 double-knockout mice) lack mGluR1-mediated inward currents or slow synaptic potentials (Hartmann et al., 2008), suggesting that TRPC3 is responsible for the mGluR-evoked slow EPSCs in mouse cerebellar Purkinje cells. More importantly, a defect in walking behavior was found in TRPC3(−/−) mice, indicating a critical function for TRPC3 in motor coordination (Hartmann et al., 2008). Subsequently, “moonwalker” mice, which have motor and coordination defects with a characteristic backward walk, were shown to have a gain-of-function mutation (T635A) in TRPC3 (Becker et al., 2009). Gain of function resulting in constitutive activation of TRPC3 channels overloads cells with Ca2+. It is interesting that TRPC3 loss of function and gain of function have similar phenotypes (Trebak, 2010). Transgenic mice with cardiac-specific overexpression of TRPC3 display a cardiomyopathic phenotype with increased hypertrophy after pressure overload (Nakayama et al., 2006).
TRPC6 channels are abundant in smooth and cardiac muscle cells and thus are candidates for the receptor-activated nonselective cation channels long known to exist in these cells. TRPC6 is an essential part of the α1-adrenoreceptor-activated cation channel in rabbit portal vein myocytes (Inoue et al., 2001). As reported for numerous Ca2+ channels with constitutive activity, cardiac-specific overexpression of TRPC6 in transgenic mice results in cardiomyopathy (Kuwahara et al., 2006). Thus, it is important to find conditions in which TRPC6 is overexpressed or contains gain-of-function mutations. Indeed, gain-of-function mutations in TRPC6 are associated with the kidney disorder focal segmental glomerulosclerosis, characterized by proteinuria, nephrotic syndrome, and progressive loss of renal function in humans (Reiser et al., 2005; Winn et al., 2005). It would seem that this gain of function results in loss of normal podocyte function.
TRPC6(−/−) mice exhibit agonist-induced contractility of cerebral arteries, perhaps as a result of compensatory up-regulation of TRPC3 and TRPC7 (Dietrich et al., 2005). TRPC6 is reported to affect dendritic growth, synaptic formation, and neuronal survival (Li et al., 2005; Jia et al., 2007; Zhou et al., 2008); however, brain development appears normal in TRPC6(−/−) mice. Transgenic mice overexpressing TRPC6 in the forebrain show enhanced spatial learning and memory (Zhou et al., 2008).
TRPC7 is widely expressed. In brain, TRPC7 may couple to the activation of group 1 mGluR in cholinergic neurons of the striatum (Berg et al., 2007). In heart, angiotensin II may activate TRPC7 to produce Ca2+ overload, induce myocardial apoptosis, and contribute to heart failure (Satoh et al., 2007).
C. Transient Receptor Potential C2 Subgroup
TrpC2 is a pseudogene in humans, but its rodent ortholog encodes a functional TRPC2 channel important to pheromone sensing. In heterologous expression, TRPC2 forms homomeric channels permeant to cations and is potentiated by PLC-mediated signaling cascades (Vannier et al., 1999; Hofmann et al., 2000). In mouse, TRPC2 is predominantly expressed in the vomeronasal organ, a specialized region of the vertebrate brain involved in pheromone sensing (Liman et al., 1999; Vannier et al., 1999). In vomeronasal sensory neurons, DAG can directly activate TRPC2 (Lucas et al., 2003). Assumed homomeric TRPC2 current-voltage relationships are linear with a single channel conductance of 42 pS (Lucas et al., 2003).
The selective expression of TRPC2 in the vomeronasal organ hints at its potential role in pheromone signaling and sexual responses. Indeed, TRPC2(−/−) mice display radically altered response to pheromone cues and abnormal mating behavior (Leypold et al., 2002; Stowers et al., 2002). TRPC2(−/−) male mice fail to display male-male aggression, and they initiate sexual and courtship behaviors toward both male and female mice. TRPC2(−/−) female mice show a reduction in female-specific behavior but display unique characteristics of male sexual and courtship behaviors (Stowers et al., 2002; Kimchi et al., 2007). TRPC2 protein was detected in spermatogenic cells based on antibody staining (Wissenbach et al., 1998; Jungnickel et al., 2001), but TRPC2(−/−) mouse fertility is normal.
III. The Transient Receptor Potential (Vanilloid) Family
The TRPV (vanilloid) subfamily (Fig. 2) is named after vanilloid receptor 1 (Caterina et al., 1997). Six mammalian TRPV proteins (TRPV1–6) have been identified. They are commonly divided into two subgroups based on sequence homology, functional similarities, and Ca2+ selectivity: TRPV1–V4 and TRPV5/V6. The channel structure of the TRPV family contains intracellular N-terminal ankyrin repeats, prevalent protein interaction motifs that have been suggested to promote channel tetramerization (Erler et al., 2004) and regulate channel activity (Al-Ansary et al., 2010; Phelps et al., 2010). The pharmacology of the TRPV family has been detailed in a recent review (Vriens et al., 2009).
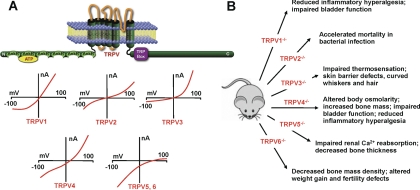
Fig. 2.
TRPV (vanilloid) family. A, molecular domains of TRPV channels and their current-voltage relationships. The ankyrin repeat is an ∼33-residue motif consisting of two α helices separated by loops. This region in TRPV1 binds ATP. B, results of genetic deletion experiments.
A. Transient Receptor Potential V1–V4 Subgroup
TRPV1–V4 subgroup members are weakly Ca2+-selective cation channels modulated by various intracellular signals, including Ca2+, CaM, and phosphoinositides (Zhu, 2005; Rohacs and Nilius, 2007). As for several members of the TRP superfamily and certain other ion channels (e.g., Hv1 and K2P), channels in this subgroup exhibit high temperature sensitivities (Q10 > 10), suggesting roles for TRPVs in thermal sensing by peripheral sensory neurons and other tissues. However, these channels are modulated by many different types of chemical and physical stimuli, indicating more complex roles in cellular sensing besides thermal sensing.
TRPV1 forms a voltage-gated outwardly rectifying weakly Ca2+-selective cation channel activated by noxious heat (>43°C) and low pH (Caterina et al., 1997; Tominaga et al., 1998; Jordt et al., 2000). As its name suggests, TRPV1 can also be activated by vanilloid compounds, such as capsaicin and capsinate found in hot (chili) and nonpungent (bell) peppers, respectively (Caterina et al., 1997; Iida et al., 2003), as well as by a myriad of endogenous compounds, such as anandamide (_N_-arachidonoylethanolamine) (Zygmunt et al., 1999), _N_-arachidonoyldopamine (Huang et al., 2002), _N_-oleoyldopamine (Chu et al., 2003), and arachidonic acid metabolites (12- and 15-hydroperoxyeicosatetraenoic acid, 5- and 15-hydroxyeicosatetraenoic acid) (Hwang et al., 2000). However, because of their lipophilicity, many of these second messengers may have broad effects on most ion channels. Many accumulate in plasma membranes but are also rapidly altered, making it difficult to test their activities under physiological conditions. A recent study found a peptide toxin, DkTx, from the Earth Tiger tarantula (Ornithoctonus huwena) that selectively and irreversibly activates TRPV1 (Bohlen et al., 2010). The toxin has a unique tandem repeat structure that binds to trap TRPV1 in the open state by interacting with residues in pore-forming region of the channel.
The activity of TRPV1 is modulated by a variety of intracellular molecules, including CaM, ATP, PIP2, and Ca2+-dependent phosphorylation and dephosphorylation. CaM interacts with both the C and N termini of the channel and cross-links them to desensitization (Numazaki et al., 2003; Rosenbaum et al., 2004; Lishko et al., 2007). Intracellular ATP competes with CaM for binding at overlapping sites in the TRPV1 ankyrin repeat domain, thereby opposing the actions of CaM and enhancing TRPV1 currents, and prevents desensitization (Lishko et al., 2007). Although the effect of PIP2 on TRPV1 modulation has been controversial, there is growing support that PIP2 sensitizes TRPV1. PIP2 binds the TRPV1 C terminus and competes with CaM for binding (Kwon et al., 2007). PIP2-mediated enhancement of TRPV1 current was also reported to require PIP2 binding to PIRT (phosphoinositide-interacting regulator of TRP), a putative auxiliary subunit of the channel (Kim et al., 2008a). TRPV1 activity is also regulated through the dynamic balance of Ca2+-dependent phosphorylation and dephosphorylation. Activation of the protein phosphatase calcineurin dephosphorylates the channel and enables channel desensitization (Docherty et al., 1996), whereas activation of protein kinase C (Premkumar and Ahern, 2000) and protein kinase A (De Petrocellis et al., 2001) seems to increase channel activity.
TRPV1 is highly expressed in myelinated (Aδ) and unmyelinated (C) nociceptive fibers of dorsal root, trigeminal, and nodose ganglion neurons (Helliwell et al., 1998; Caterina et al., 2000). Although there is a paucity of functional evidence for TRPV1 in the central nervous system, TRPV1 may be present in the brain (Steenland et al., 2006) and was proposed to play a role in synaptic plasticity, such as long-term depression (Gibson et al., 2008; Maione et al., 2009). TRPV1 is reportedly expressed in other tissues; like most of the TRP field, however, functional evidence lags behind error-prone antibody-determined localization data. Because TRPV1 is activated by heat and expressed in thermosensitive tissues, there is much interest in whether TRPV1 is important for thermosensation. Indeed, TRPV1(−/−) mice display reduced thermal hyperalgesia after inflammation and injury; however, whether TRPV1(−/−) mice have decreased responses to acute noxious heat is still debated (Caterina et al., 2000; Davis et al., 2000; Bölcskei et al., 2005). Many of the pro-inflammatory agents produced during injury reduce TRPV1 thresholds to noxious stimuli to as low as 30°C, so that normally nonpainful thermal stimuli are capable of activating TRPV1 (Sugiura et al., 2002). As such, TRPV1(−/−) mice show reduced thermal hyperalgesia in response to inflammatory mediators such as bradykinin or NGF (Caterina et al., 2000; Davis et al., 2000; Chuang et al., 2001). Drugs developed to antagonize TRPV1 by the pharmaceutical industry reduce sensitivity to heat stimuli in humans and initially raise body temperature (Gavva et al., 2008). This may be due to the tonic activation of visceral TRPV1 by nonthermal factors, which suppresses autonomic cold-defense effectors and body temperature; blockade of the activation by TRPV1 antagonists disinhibits thermoeffectors and causes hyperthermia (Romanovsky et al., 2009). In addition to important roles in thermosensation and thermoregulation, TRPV1 has been reported to be important for normal bladder function (Birder et al., 2002), gastrointestinal motility (Rong et al., 2004), behavioral responses to ethanol (Blednov and Harris, 2009; Ellingson et al., 2009), airway inflammation and disease (Geppetti et al., 2006), and detection of salt (Lyall et al., 2004).
TRPV2 is 50% identical to TRPV1 and forms a weakly Ca2+-selective cation channel. It is activated by temperatures >52°C when expressed in Xenopus laevis oocytes (Caterina et al., 1999; Kanzaki et al., 1999). There are, however, species-dependent differences in this activation, and human TRPV2 is apparently not activated by heat (Neeper et al., 2007). TRPV2 is reported to be present in a wide variety of tissues, including brain, pancreas, spleen, lung, stomach, intestine, bladder, prostate, and blood cells (Caterina et al., 1999; Kowase et al., 2002), with the usual caveat that antibody specificity has not been tested in knockout mice. It has been proposed that TRPV2 may serve as an endosomal calcium release channel that controls endosome fusion and/or exocytosis (Saito et al., 2007). Indeed, many studies suggest that activation of TRPV2 causes translocation of the channel to the plasma membrane (Kanzaki et al., 1999; Iwata et al., 2003; Nagasawa et al., 2007; Hisanaga et al., 2009), and TRPV2 inhibitor transilast (_N_-(3,4-dimethoxycinnamoyl) anthranilic acid) prevents this redistribution (Hisanaga et al., 2009). It is noteworthy that aberrant localization of TRPV2 is detected in rodent models of muscular dystrophy, and expression of dominant-negative TRPV2 reduced muscle damage (Iwata et al., 2009). TRPV2 has been shown to be expressed in macrophages and has a critical role in macrophage particle binding and phagocytosis. TRPV2(−/−) mice have been consistently shown to be more vulnerable when challenged with pathogens such as Listeria monocytogenes, mainly because of the greater organ bacterial load (Link et al., 2010).
TRPV3 also forms a voltage-sensitive weakly Ca2+-selective cation channel that is activated by warm temperatures (33–39°C) (Peier et al., 2002b; Smith et al., 2002; Xu et al., 2002) and a variety of botanical compounds including camphor, eugenol, thymol, and carvacrol (Moqrich et al., 2005; Xu et al., 2006). TRPV3 currents are unusual in two respects; they sensitize (grow larger) with repeated activation, and their temperature-dependent potentiation exhibits a marked hysteresis. In rodent skin keratinocytes, TRPV3 is proposed to sense warmth (Peier et al., 2002b; Chung et al., 2004); TRPV3(−/−) mice display altered behavioral responses to heat, including altered temperature preferences in thermotaxis assays (Moqrich et al., 2005). Unexpectedly, a recent study found that TRPV3 is required for epidermal growth factor receptor signaling in keratinocytes, and TRPV3(−/−) mice exhibit wavy hair coat and curly whiskers (Cheng et al., 2010a).
Many pro-inflammatory agents such as bradykinin, histamine, ATP, and prostaglandin E2 sensitize TRPV3 function (Xu et al., 2006; Huang et al., 2008; Mandadi et al., 2009). ATP interacts with the channel's N-terminal ankyrin repeats to regulate this sensitization (Phelps et al., 2010). Elevated TRPV3 activity can dramatically influence skin integrity; rodents with constitutively active TRPV3 channels have an increased susceptibility to dermatitis and skin lesions (Asakawa et al., 2006; Imura et al., 2007).
TRPV4 is activated by warm temperatures in the range of 27–34°C; consequently, at physiological temperatures, the channel should demonstrate significant constitutive activity (Liedtke et al., 2000; Güler et al., 2002; Watanabe et al., 2002). Activation of TRPV4 by heat may not be direct; in inside-out patches, TRPV4 cannot be activated by heat, yet it can still be activated by 4α-phorbol 12,13-didecanoate, a non–PKC-activating phorbol ester (Watanabe et al., 2002). TRPV4 is sensitive to osmotic and mechanical stimuli, such as cell swelling or fluid flow, and sensitivity of TRPV4 to these stimuli may depend on phospholipase A2 activation and the subsequent production of the arachidonic acid metabolite epoxyeicosatrienoic acid (EET) (Liedtke et al., 2000; Strotmann et al., 2000; Watanabe et al., 2003; Vriens et al., 2004, 2005; Fernandes et al., 2008). TRPV4 can also be activated by botanical and synthetic compounds such as 4α-phorbol-12,13-dihexanoate (Klausen et al., 2009), bisandrographolide (Smith et al., 2006), and (N_-((1_S)-1-{[4-((2_S_)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A) (Thorneloe et al., 2008). Like TRPV1, TRPV4 is modulated by CaM and ATP, C-terminal CaM binding potentiating the current (Strotmann et al., 2003) and Ca2+- dependent CaM binding to the N terminus desensitizing the current (Rosenbaum et al., 2004; Lishko et al., 2007). A variety of kinases also seem to modulate its activity (Gao et al., 2003; Chen et al., 2008a; Fan et al., 2009; Wegierski et al., 2009).
TRPV4 is widely distributed and was proposed to sense temperature in the hypothalamus, skin and primary sensory neurons (Liedtke et al., 2000; Güler et al., 2002; Peier et al., 2002b). However, three groups reported TRPV4(−/−) mice had normal behavioral responses to thermal stimulation in the hot plate and radiant paw heating assays, except under inflammatory conditions (Liedtke and Friedman, 2003; Suzuki et al., 2003; Todaka et al., 2004). A more recent study revealed that TRPV4(−/−) mice exhibited a strong preference for 34°C, whereas wild-type mice failed to discriminate between floor temperatures of 30°C and 34°C (Lee et al., 2005a). Such differences in findings may be due to strain differences.
The sensitivity of TRPV4 to osmotic stimuli may be important for cellular and systemic osmoregulation. TRPV4 was detected in putative osmoreceptive neurosensory cells around the ventricle (Liedtke et al., 2000), and TRPV4(−/−) mice display diminished drinking, elevated systemic osmotic pressure, and reduced synthesis of antidiuretic hormone in response to systemic hypertonicity induced by salt ingestion (Liedtke and Friedman, 2003). However, another study described an increase in antidiuretic hormone secretion in response to hypertonicity induced by water deprivation in TRPV4(−/−) mice (Mizuno et al., 2003). TRPV4 was also detected in cholangiocytes or the ciliated epithelial cells lining the bile duct, where it may play a key role in osmotic regulation of bile composition (Gradilone et al., 2007).
In support of a mechanosensing function for TRPV4, TRPV4(−/−) mice have a reduced behavioral response to persistent tail pressure as well as a reduced sensory neuronal discharge to pin prick on glabrous skin (Suzuki et al., 2003). TRPV4 may also contribute to the development of mechanical hyperalgesia after inflammation and injury (Alessandri-Haber et al., 2006). TRPV4 is expressed in urothelium and may play a role in urothelium-mediated transduction of intravesical mechanical pressure. In support of this hypothesis, TRPV4(−/−) mice display impaired bladder function (Birder et al., 2007; Gevaert et al., 2007). TRPV4 is expressed in inner and outer hair cells of the cochlea, but TRPV4(−/−) mice show no difference in the response to acoustic startle compared with wild-type mice (Liedtke and Friedman, 2003), indicating that the channel may not be the mechanotransduction channel in hair cells. In lung endothelial cells, TRPV4 may respond to unequal pressure across the alveolar septal barrier to regulate the permeability of these cells (Alvarez et al., 2006; Hamanaka et al., 2007). TRPV4(−/−) mice displayed significantly less lung edema in response to high peak inflation pressure ventilation compared with wild-type mice (Whitlock, 1995).
TRPV4 seems to regulate vascular tone (Earley et al., 2009; Zhang et al., 2009) and bone deposition and remodeling (Masuyama et al., 2008; Mizoguchi et al., 2008). It is noteworthy that mutations in TRPV4 have been identified in patients with three dominantly inherited skeletal phenotypes: autosomal-dominant brachyolmia, spondylometaphyseal dysplasia Kozlowski type, and metatropic dysplasia (Rock et al., 2008; Krakow et al., 2009). TRPV4 mutations have also been linked to patients with congenital distal spinomuscular atrophy, Charcot-Marie-Tooth disease type 2C, and scapuloperoneal spinal muscular atrophy (Auer-Grumbach et al., 2010; Deng et al., 2010; Landouré et al., 2010).
B. Transient Receptor Potential V5/V6 Subgroup
TRPV5 and TRPV6 are highly homologous proteins, sharing 74% identity (Clapham, 2003). Like other TRPV family members, they form Ca2+-permeable inwardly rectifying cation channels; unlike other TRPV family members, however, they are highly Ca2+ selective (PCa/PNa > 100) and are not heat-sensitive (Vennekens et al., 2000; Yue et al., 2001). Rather, they tend to be active at low Ca2+ concentrations and physiological membrane potentials. Both TRPV5 and TRPV6 inactivate to prevent Ca2+ overload, although with different kinetics (Suzuki et al., 2000; Hoenderop et al., 2001; Nilius et al., 2002). TRPV5 has a 100-fold higher affinity for the nonspecific blocker ruthenium red (IC50, 121 nM) than does TRPV6 (IC50, 9 μM) (Hoenderop et al., 2001; Voets et al., 2001).
The activity of TRPV5 and TRPV6 at the plasma membrane is regulated by a variety of second messengers, including Ca2+, CaM, Mg2+, ATP, PIP2, and protein kinases. Ca2+ acts as a negative feedback regulator of channel activity and contributes to channel inactivation (Vennekens et al., 2000; Hoenderop et al., 2001; Nilius et al., 2001; Yue et al., 2001). CaM interacts with TRPV5 and TRPV6 in a Ca2+-dependent manner (Lambers et al., 2004) and was proposed to mediate the slow component of Ca2+-dependent inactivation (Niemeyer et al., 2001). Intracellular Mg2+ causes a fast voltage-dependent block as well as a slower inhibition of TRPV5 and TRPV6 current (Nilius et al., 2000; Voets et al., 2003; Lee et al., 2005b). ATP binding sites have been identified within the ankyrin repeat domain, and the C terminus of TRPV6 and intracellular ATP stabilizes TRPV5 and TRPV6 currents (Hoenderop et al., 2001; Al-Ansary et al., 2010). PIP2 binding to the TRP box potentiates TRPV5 and TRPV6, and its depletion in the membrane via Ca2+-dependent activation of PLC contributes to channel inactivation (Rohács et al., 2005; Thyagarajan et al., 2008; Thyagarajan et al., 2009). A variety of other regulatory molecules may modulate its activity or membrane expression. Ca2+ binding proteins 80K-H/PRKCSH/hepatocystin and Calbindin-D28K tether to TRPV5 and prevent negative feedback of Ca2+ on the channels (Gkika et al., 2004; Lambers et al., 2006). B-box and SPRY-domain containing protein interacts with TRPV5 and decreases channel activity (van de Graaf et al., 2006), whereas RGS2 (Schoeber et al., 2006) and Nipsnap1 (Schoeber et al., 2008) bind to TRPV6 and inhibit the channel's activity.
TRPV5 is expressed in a number of tissues. In the kidney, TRPV5 is predominantly expressed in the distal convoluted and connecting tubule where it is important for transcellular transport and active reabsorption of Ca2+ in the kidney (Hoenderop et al., 1999). Indeed, ablation of the TRPV5 results in impaired Ca2+ resorption in the distal convoluted and connecting tubule; TRPV5(−/−) mice excrete approximately six times more Ca2+ in their urine and display compensatory increases in vitamin D levels and intestinal hyperabsorption of Ca2+. In addition, they display polyuria with significantly more acidic urine than that of wild-type mice (Hoenderop et al., 2003). TRPV5(−/−) mice also display bone abnormalities, including reduced trabecular and cortical bone thickness (Hoenderop et al., 2003) and increased osteoclast number and size (van der Eerden et al., 2005). Yet TRPV5(−/−) mice had low serum deoxypyridinoline levels, indicating decreased rate of bone breakdown and, unlike other mouse models with decreased osteoclast function, showed decreased bone thickness without osteopetrosis (van der Eerden et al., 2005).
TRPV6 is more widely distributed than TRPV5 (Peng et al., 2000; Hoenderop et al., 2001; Hirnet et al., 2003). In the intestine, TRPV6 localizes to the brush border membrane of enterocytes, where it is proposed to mediate transcellular Ca2+ entry (Peng et al., 1999; Zhuang et al., 2002). Indeed, TRPV6(−/−) mice that were fed a low Ca2+ diet exhibited decreased Ca2+ absorption and serum Ca2+ levels compared with wild-type mice; however, a disruption of closely adjacent EphB6 gene in the TRPV6(−/−) mice may complicate the interpretation of this phenotype (Bianco et al., 2007; Benn et al., 2008). In the kidney, TRPV6 is expressed in the convoluted tubules, connecting tubules, and cortical and medullary collecting ducts of the nephron, where it helps resorb Ca2+ (Nijenhuis et al., 2003). In the placental trophoblast, TRPV6 contributes to the transfer of Ca2+ from mother to fetus (Moreau et al., 2002; Suzuki et al., 2008) and may contribute to the reduced litter size of TRPV6(−/−) mice (Bianco et al., 2007).
IV. The Transient Receptor Potential (Melastatin) Family
The mammalian TRPM subfamily has eight members (Fig. 3) and is divided into three main groups based on similarities in amino acid sequence: TRPM1/M3, TRPM4/M5, and TRPM6/M7; TRPM2 and TRPM8 exhibit low sequence homology and therefore do not seem to warrant grouping. TRPM proteins have a TRP domain C-terminal to the transmembrane segments but lack ankyrin repeats in the N terminus. The N-terminal part of TRPM proteins is considerably longer than the corresponding regions in TRPC and TRPV members. The N terminus contains a large TRPM homology region (around 700 amino acids), which bears no homology to other known molecules. The biological significance of this region is still unknown. The C terminus can be divided into two regions, a coiled-coil domain and a second variable region. TRPM2, TRPM6, and TRPM7 are unique among known ion channels in that they encode enzymatically active protein domains in their C termini.
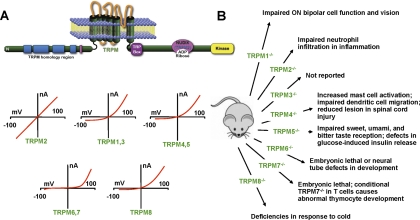
Fig. 3.
TRPM (melastatin) family. A, molecular domains of TRPM channels and their current-voltage relationships. NUDIX is a phosphohydrolase family homologous region in TRPM2 that binds ADP ribose. TRM6 and TRPM7 possess a C-terminal serine/threonine kinase that is similar in structure to protein kinase A. B, results of genetic deletion experiments.
A. Transient Receptor Potential M1/M3 Subgroup
TRPM1, the founding member of the TRPM subfamily, was discovered in a melanoma screen as a transcript that had decreased expression in highly metastatic, compared with less metastatic melanoma cells (Duncan et al., 1998). A recent analysis of mRNA showed that at least five human ion-channel–forming isoforms of TRPM1 could be detected in melanocytes, melanoma, brain, and retina (Oancea et al., 2009). In melanoma cells, TRPM1 is prevalent in highly dynamic intracellular vesicular structures. Total internal reflection fluorescent imaging of HEK cells expressing the GFP-TRPM1 splice variants suggests that the GFP-tagged isoforms did not reach the plasma membrane (Oancea et al., 2009). When expressed in SK-Mel22a melanoma cells, TRPM1 channels show a nonselective, outwardly rectifying current, which suggests that TRPM1 function as plasma membrane channels might depend on melanocyte-specific trafficking. Intriguingly, TRPM1 expression correlates with melanin content in neonatal human epidermal melanocytes, but how TRPM1 might regulate melanin is not known (Oancea et al., 2009).
Several independent studies have found that TRPM1 has a critical role in synaptic function in ON bipolar cells in the retina. Mutations in TRPM1 may contribute to congenital stationary night blindness (CSNB), a nonprogressive dark-adapted visual deficit (Morgans et al., 2009; Shen et al., 2009; Koike et al., 2010). TRPM1 is activated by the mGluR6 signaling cascade and thus is required for the depolarizing light response in ON bipolar cells. Consistent with the phenotype found in CSNB-affected humans, TRPM1(−/−) mice lack the b-wave normally recorded in electroretinograms. (Morgans et al., 2009; Shen et al., 2009; Koike et al., 2010). Additional support for TRPM1 in retinal function stems from human genetic studies by three independent groups, which showed that mutations in TRPM1 are associated with CSNB (Audo et al., 2009; Li et al., 2009; van Genderen et al., 2009).
TRPM3 is most closely related to TRPM1. TRPM3 forms a constitutively active, Ca2+-permeable, nonselective cation channel with a reported near linear current-voltage relationship in heterologous expression systems (Grimm et al., 2003). TRPM3 is alternatively spliced; TRPM3α1 and TRPM3α2, which differ only in the presumed pore region, show significant differences in their channel properties (Oberwinkler et al., 2005): TRPM3α1 channels are poorly permeable to divalent cations, whereas TRPM3α2 channels conduct Ca2+ and Mg2+. In addition, extracellular Na+ inhibits TRPM3α2 but not TRPM3α1 channels. Both variants exhibit constitutively active, outwardly rectifying currents that are blocked by intracellular Mg2+, similar to TRPM6 and TRPM7 channels. Another short variant, TRPM31325 (shorter carboxyl terminus), mediates a spontaneous, nonselective cation current with PCa/PNa = 1.6 and detectable Mg2+ permeability. The single-channel conductance of TRPM31325 is ∼80 and 65 pS in the presence of extracellular Na+ and Ca2+, respectively. Their activities could be suppressed by 100 μM Gd3+ and La3+ and increased by hypotonicity or d-_erythro_-sphingosine, a metabolite of cellular sphingolipids (Grimm et al., 2003, 2005).
TRPM3 is most prominent in kidney, brain, and pituitary. However, the function of TRPM3 is poorly characterized, probably because of the existence of multiple variants with different properties. A TRPM3(−/−) mouse has not been reported to date. A recent report suggests that the steroid hormone pregnenolone sulfate can act as endogenous ligand for TRPM3 (Wagner et al., 2008). TRPM3 protein is expressed in pancreatic β cells, and pregnenolone could augment glucose-induced insulin secretion from pancreatic islets by activating TRPM3 (Wagner et al., 2008). High-resolution oligonucleotide arrays were used to suggest that TrpM3 is a candidate gene for the Kabuki syndrome, a congenital mental retardation syndrome (Kuniba et al., 2009).
B. Transient Receptor Potential M2
TRPM2 contains a C-terminal nudix hydrolase domain that is highly homologous to the ADP pyrophosphatase NUDT9. This domain binds ADP ribose (EC50, ∼100 μM) in a cleft in the NUDT9 domain of TRPM2 (Perraud et al., 2005) and hydrolyzes it (Perraud et al., 2001; Sano et al., 2001). ADP ribose arises from breakdown of β-NAD, CD38, or other enzymes acting on cyclic ADP ribose and hydrolysis of ADP polymers by poly-ADP ribose glycohydrolase. TRPM2 is also activated by oxidative or nitrosative stress (e.g., H2O2) (Hara et al., 2002), perhaps mediated by mitochondrial ADP-ribose (Perraud et al., 2005). High levels of intracellular Ca2+ have been proposed to activate TRPM2 (>10 μM) (Kraft and Harteneck, 2005; Du et al., 2009).
TRPM2 is a nonselective cation channel with a near-linear current-voltage relationship and has a single-channel conductance of ∼62 pS. TRPM2 current was insensitive to 100 μM La3+ but was inhibited by nonspecific channel blockers such as flufenamic acid or the antifungal agents clotrimazole or econazole (Hill et al., 2004). It is noteworthy that TRPM2 may function as a lysosomal Ca2+-release channel activated by intracellular ADP-ribose in addition to its role as a plasma membrane channel (Lange et al., 2009).
TRPM2 is highly expressed in cells of monocytic lineage. Because TRPM2 is regulated by signaling pathways responsive to oxidative stress and tumor necrosis factor-α, it has been assumed to be a sensor for intracellular oxidation (Hara et al., 2002; Kaneko et al., 2006). TRPM2 is proposed to function in monocyte chemotaxis, which is known to be regulated by ADP-ribose (Massullo et al., 2006). Functional TRPM2 has been reported in neurons, where it may be involved in H2O2-induced neuronal death (Kaneko et al., 2006; Olah et al., 2009), and in pancreatic β cells, where it may regulate insulin secretion (Togashi et al., 2006). Studies using TRPM2(−/−) mice suggest that the channel controls reactive oxygen species-induced chemokine production in monocytes and neutrophil infiltration in a mouse model of inflammation (Yamamoto et al., 2008). Human genetics studies indicate the potential involvement of TRPM2 in bipolar disorders (McQuillin et al., 2006). In addition, an inactivating proline-to-leucine substitution at position 1018 in TRPM2 is found in two related neurodegenerative disorders, amyotrophic lateral sclerosis and Parkinsonism/dementia complex, that have a high incidence on the Pacific Islands of Guam and Rota (Hermosura et al., 2008).
C. Transient Receptor Potential M4/M5 Subgroup
TRPM4 and TRPM5 are the only monovalent-selective ion channels of the TRP family (Launay et al., 2002; Hofmann et al., 2003; Liu and Liman, 2003; Prawitt et al., 2003). TRPM4 and TRPM5 have ∼40% sequence identity and exhibit similar channel properties. TRPM4 is expressed as two splice variants, TRPM4a (nonfunctional channel) and TRPM4b (functional channel) (Xu et al., 2001; Launay et al., 2002; Nilius et al., 2003). Both TRPM4b and TRPM5 channels have a single-channel conductance of ∼25 pS, their whole-cell currents are strongly outwardly rectified (Launay et al., 2002), and they are blocked by intracellular flufenamic acid and spermine (Ullrich et al., 2005). A short stretch of six acidic amino acids in the pore loop determines their monovalent selectivity (Nilius et al., 2003). Both are activated by relatively high Ca2+ levels in the cytosol (∼500 and 80 μM for TRPM4 and TRPM5, respectively) (Hofmann et al., 2003; Liu and Liman, 2003; Ullrich et al., 2005), and PIP2 reverses their Ca2+-dependent desensitization (Zhang et al., 2005; Nilius et al., 2006). Both TRPM4 and TRPM5 have been proposed to be preferentially sensitive to temperature in the range of 15 to 35°C (Talavera et al., 2005). TRPM4, but not TRPM5, is inhibited by intracellular ATP, whereas TRPM5 is inhibited by intracellular acidic pH (Nilius et al., 2004; Liu et al., 2005). TRPM5 activates and inactivates more rapidly than TRPM4. TRPM4 is also modulated by protein kinase C (PKC) phosphorylation, which enhances its sensitivity to intracellular Ca2+ (Nilius et al., 2005).
TRPM4 and TRPM5 probably underlie the often observed Ca2+-activated monovalent-selective cation current, and thus have attracted interest for their possible involvement in membrane potential oscillations (Launay et al., 2002; Prawitt et al., 2003). TRPM4 is ubiquitous, with highest expression in kidney and brain. Knockdown of TRPM4 decreases cerebral artery myogenic constrictions and thus may contribute to cerebral blood flow regulation (Reading and Brayden, 2007). Gain-of-function mutation of TRPM4 (E7K) causes impaired endocytosis and may be associated with human progressive familial heart block type 1 (Kruse et al., 2009). TRPM4(−/−) mice exhibit increased IgE-dependent mast cell activation and anaphylactic responses (Vennekens et al., 2007). Moreover, chemokine-dependent dendritic cell migration is considerably impaired in TRPM4(−/−) mice (Barbet et al., 2008). In a spinal cord injury model, TRPM4(−/−) mice were relatively protected compared with wild-type mice, and their neurological function improved more readily after injury (Gerzanich et al., 2009).
TRPM5 is expressed in taste receptor cells (Pérez et al., 2002), and sweet, umami, and bitter taste reception were reportedly abolished in TRPM5(−/−) mice, whereas sour or salty taste sensation was preserved (Zhang et al., 2003). The G protein PLCβ2-coupled receptors T1R and T2R may activate TRPM5 to produce these sensations (Zhang et al., 2003). Another group found markedly impaired but not complete absence of responses to bitter, sweet, and umami compounds (Damak et al., 2006). TRPM5 is expressed in pancreatic β-cells, where it may affect insulin release through PLC-dependent pathways (Gilon and Henquin, 2001). Indeed, recent studies from independent groups found defective glucose-induced insulin release in TRPM5(−/−) mice (Brixel et al., 2010; Colsoul et al., 2010). TRPM5 immunoreactivity was also seen in other chemosensory organs—the main olfactory epithelium and the vomeronasal organ, hinting at its potential functions in chemosensation (Kaske et al., 2007). Using TRPM5-GFP transgenic and TRPM5(−/−) mice, a recent study showed that TRPM5 is expressed in solitary enteroendocrine chemosensory cells in mouse duodenum and may be essential for the release of the endogenous opioids β-endorphin and Met-enkephalin and the release of uroguanylin from these cells (Kokrashvili et al., 2009b). It is noteworthy that some enteroendocrine cells express signaling elements involved in taste transduction (the gut's luminal glucose sensor), initiating the incretin response to elicit the release of glucagon-like peptide 1 (Kokrashvili et al., 2009a). Therefore, TRPM5's presence in these gut “taste cells” as well as in pancreatic β-cells will be interesting to explore in diabetes and obesity.
D. Transient Receptor Potential M6/M7 Subgroup
TRPM6 and TRPM7 are unique among ion channels because they possess both ion channel and protein kinase activities. TRPM6 and TRPM7 serine/threonine kinase domains are located at the extreme C terminus, and the catalytic core of the kinase domain is similar to that of other eukaryotic protein kinases and to enzymes with “ATP-grasp” domains. High-resolution structure of the M7 kinase alone demonstrates marked similarities to protein kinase A (Yamaguchi et al., 2001). The kinase domain does not seem to affect channel activity in any direct manner. Both proteins also share similar biophysical properties; these channels allow Mg2+ and Ca2+ into the cell (albeit at very low conductances) and allow primarily monovalent K+ out of the cell. They are strongly outwardly rectifying under physiological conditions. In the absence of divalent cations, their current-voltage relations are practically linear, indicating that divalent ions bind the pore to regulate conductance. At positive voltages, TRPM6 and TRPM7 have single-channel conductances of 84 and 105 pS, respectively. Both channels are inhibited by intracellular Mg2+ (0.3–1.0 mM) (Nadler et al., 2001; Voets et al., 2004b), but the inward current is strongly potentiated by extracellular acidic pH selectively (Jiang et al., 2005). However, homomeric TRPM6 and TRPM7 channels can be distinguished pharmacologically. For example, micromolar levels of 2-APB increase TRPM6 but inhibit TRPM7 channel activities, whereas millimolar concentrations of 2-APB potentiate TRPM7 channel activities (Li et al., 2006).
TRPM6 is primarily expressed in kidney and intestine, where it has been suggested to be responsible for epithelial Mg2+ reabsorption, based largely on the identification of TRPM6 mutants in a hereditary disease called hypomagnesemia with secondary hypocalcemia (Schlingmann et al., 2002; Walder et al., 2002). The symptom of the disease could be alleviated significantly by dietary supplements of high-dose Mg2+ (Schlingmann and Gudermann, 2005). TRPM6(−/−) mice were generated, but many of them died by embryonic day 12.5. These mice had neural tube defects with exencephaly and spina bifida occulta. Feeding dams a high-Mg2+ diet improved offspring survival. These results indicate a critical role for TRPM6 in neural tube closure in development (Walder et al., 2009).
TRPM7 is a large protein (1863 amino acids), identified in a yeast two-hybrid screen as a protein interacting with PLCβ1 (Runnels et al., 2001). In contrast to other GPCR-activated TRP channels, TRPM7 current increases slowly under whole-cell recording conditions and is inactivated by PIP2 hydrolysis by PLCβ or PLCγ (Runnels et al., 2002). TRPM7 autophosphorylates (Matsushita et al., 2005) and can phosphorylate proteins such as annexin 2 (Dorovkov and Ryazanov, 2004) and myosin IIA heavy chain (Clark et al., 2006), but its native substrates have not been identified.
Native activation of the TRPM7 channel is, as for most TRP channels, an unsolved mystery. Upon break-in during whole-cell recording, TRPM7 currents continually increase over time until they are quite large. This increase does not occur under perforated-patch conditions, in which intracellular perfusion is restricted to ions, suggesting that an intracellular inhibitor (in addition to Mg2+) normally limits current (L. J. Wu, B. Navarro, and D. E. Clapham, unpublished observations). TRPM7 expressed in a vascular smooth muscle cell line is subtly increased by shear stress apparently via insertion of additional TRPM7 into the plasma membrane (Oancea et al., 2006), but TRPM7 is not in any traditional sense a mechanosensitive channel. In addition to Mg2+ and Ca2+, TRPM7 is permeable to Zn2+, Co2+, and Mn2+, providing a potential ion channel mechanism for cellular entry of trace metal ions (Monteilh-Zoller et al., 2003).
TRPM7 is ubiquitously expressed but the expression level is low in most tissues. Suppression of TRPM7 expression reduced Ca2+-dependent anoxic death in neuronal culture, as well as in mice with stroke (Aarts et al., 2003; Sun et al., 2009). TRPM7 was proposed, based on knockout of TRPM7, in DT-40 chicken B-lymphocyte cell lines, to be important for Mg2+ homeostasis (Schmitz et al., 2003). Genetic deletion of TrpM7 is lethal before embryonic day 7.5, suggesting that TRPM7 is essential for embryonic development (Jin et al., 2008). Tissue-specific deletion of TRPM7 in the T-cell lineage results in a developmental block of thymocytes at the double-negative stage. Careful quantitation of intracellular Mg2+ changes in response to rapid changes in external Mg2+ levels did not alter global intracellular Mg2+. In addition, total Mg2+ levels in cells did not differ between T cells in wild-type and conditional TRPM7(−/−) mice (Jin et al., 2008). Thus, TRPM7 does not have an important function in Mg2+ homeostasis in T cells, although localized changes in intracellular Mg2+ may be relevant to its function. These findings also raise questions regarding the mechanism of hypomagnesemia (in hypomagnesemia with secondary hypocalcemia) that can be resolved by mutation of TRPM6 in animal models. A report that mutations in TRPM7 (threonine-to-isoleucine substitution at position 1482) in amyotrophic lateral sclerosis–Parkinsonism/dementia complex (Hermosura et al., 2005) has been challenged by a recent linkage study (Hara et al., 2010).
It is noteworthy that TRPM7 is localized to a distinct set of vesicles in some cells. TRPM7 resides in the membrane of synaptic vesicles of sympathetic neurons, forms molecular complexes with the synaptic vesicle proteins synapsin I and synaptotagmin I, and directly interacts with synaptic vesicular snapin. In sympathetic neurons, changes in TRPM7 levels and channel activity alter acetylcholine release. Thus, vesicular TRPM7 channel activity is critical to neurotransmitter release in sympathetic neurons (Krapivinsky et al., 2006). How would vesicle-localized TRPM7 mediate fusion? First, remember that ion channels being trafficked to the plasma membrane are assembled with the outer vestibule of the pore facing the inside of the vesicle. The cytoplasmic domains remain cytoplasmic before and after fusion. When TRPM7 is vesicular, its “outer” surface faces high potentiating (Li et al., 2007) pH. Vesicular membranes typically lack PIP2 in contrast to the PIP2-rich plasma membrane, and TRPM7 should be closed under this condition. When the vesicle approaches the membrane, its cytoplasmic domains are exposed to the high PIP2 levels of the plasma membrane, and the channel should open in its high divalent conductance state (low intravesicular pH). In this model, TRPM7 acts as a coincidence detector, opening only when vesicular pH is low, and PIP2 in the plasma membrane binds cytoplasmic domains of TRPM7. TRPM7 opening at this point would allow ion exchange between the vesicular and cytoplasmic spaces (Montell, 2006; Brauchi et al., 2008).
E. Transient Receptor Potential M8
Like TRPM1, TRPM8 was originally identified in a screen of cancer-related genes (Tsavaler et al., 2001). TRPM8 is permeant to Ca2+ (PCa/PNa ∼1–3) and has a single-channel conductance of ∼80 pS. Its sensory role was recognized when it was isolated by expression cloning of a menthol receptor from trigeminal neurons (McKemy et al., 2002) and by bioinformatics approaches using TRP channel sequence homology (Peier et al., 2002a). It can be activated by cold (8–28°C) and enhanced by cooling compounds such as menthol and icilin (McKemy et al., 2002; Peier et al., 2002a). Temperature modulates the voltage dependence of the channel, menthol and icilin mimicking this effect (Voets et al., 2004a). However, menthol and icilin may activate the TRPM8 through distinct mechanisms. For example, menthol activation is unaffected by intracellular pH and is inhibited by intracellular Ca2+, whereas icilin activation is inhibited by low pH and by the absence of intracellular Ca2+ (Andersson et al., 2004; Chuang et al., 2004). Mutational analyses indicate that residues in the S1 and S2 transmembrane segments are required for TRPM8 activation by menthol and icilin (Chuang et al., 2004; Bandell et al., 2006), whereas S4 and the S4–S5 linker of TRPM8 may mediate voltage sensing and some aspect of menthol binding (Voets et al., 2007).
TRPM8 is widely expressed, but its most clear-cut function is as a cold sensor in TrkA+ small-diameter primary sensory neurons. Indeed, three independent studies showed that TRPM8(−/−) mice have remarkable deficiencies to a range of cold responses (Bautista et al., 2007; Colburn et al., 2007; Dhaka et al., 2007). This suggests that TRPM8 is the predominant detector of cold temperatures in vivo, which has implication for somatosensation, nociception, and the development of analgesia. TRPM8 is also identified in other tissues; for example, the prostate epithelium (Tsavaler et al., 2001), where it may act as an androgen-responsive channel (Zhang and Barritt, 2004), and in arterial vascular smooth muscle, where it may regulate vascular tone (Johnson et al., 2009). High concentrations of menthol were used to argue that TRPM8 is also expressed in human sperm to regulate the acrosome reaction (De Blas et al., 2009), but TRPM8(−/−) fertility is normal (Bautista et al., 2007; Colburn et al., 2007; Dhaka et al., 2007).
V. The Transient Receptor Potential (Ankyrin) Family
TRPA1 (Fig. 4) is the only member of the mammalian family, but it seems to have arisen from larger families in insects that critically depend on chemosensation. The “A” in TRPA1 stands for ankyrin, because the protein contains at least 14 ankyrin repeats in its N terminus. These repeats are hypothesized to interact with cytoskeletal components (Howard and Bechstedt, 2004; Sotomayor et al., 2005) or to modulate ligand binding (Lishko et al., 2007). TRPA1 also contains an N-terminal Ca2+ binding EF hand domain. TRPA1 is selectively expressed in a subpopulation of neurons in the dorsal root, trigeminal, and nodose ganglia (Story et al., 2003; Diogenes et al., 2007; Brierley et al., 2009), as well as in hair and skin cells (Corey et al., 2004; Atoyan et al., 2009; Kwan et al., 2009). There it primarily acts as a chemosensor and, in some cases, may amplify Ca2+-entry through other channels (Jordt et al., 2004; Bautista et al., 2005; Zurborg et al., 2007). A recent study found that a gain-of-function mutation (N855S) in the S4 transmembrane segment of TRPA1 causes familial episodic pain syndrome, providing the first example of a human pain-associated TRP channelopathy (Kremeyer et al., 2010).
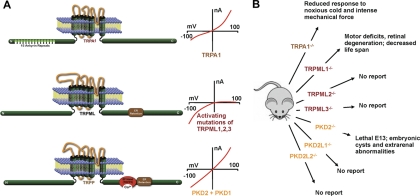
Fig. 4.
TRPA1 (ankyrin repeat), TRPML (mucolipin) and TRPP [polycystic kidney disease 2 (PKD2), also called polycystin 2 (PC2)] channels. A, “distal” TRP molecular domains and their current-voltage relationships. The ER retention signal is a small domain that presumably maintains the channel in the endoplasmic reticulum. Note that the current-voltage relationship for TRPA1 shows decay at positive potentials in most whole-cell recordings and is linear with electrophilic agonist. B, major phenotypes in “distal” TRP channel knockout mice. Note: the PKD1 refers to the 11-TM domain-containing protein of the polycystin 1 family. TRPP (PKD2, polycystin 2, PC2) refers to the 6-TM family of proteins.
TRPA1 is activated by a variety of chemicals, including cinnamaldehyde (in cinnamon) (Bandell et al., 2004), allicin, and diallyl disulfide (in garlic) (Bautista et al., 2005; Macpherson et al., 2005), isothiocyanates (in mustard oil, wasabi, and horseradish) (Bandell et al., 2004; Jordt et al., 2004), methyl salicylate (in winter green oil) (Bandell et al., 2004), acrolein (in smoke) (Bautista et al., 2006), and Δ9-tetrahydrocannabinol (in marijuana) (Jordt et al., 2004). In addition, the well known TRPM8 agonist menthol has a bimodal effect: it activates TRPA1 at low concentrations and inhibits it at high concentrations (Macpherson et al., 2006; Karashima et al., 2007). More recently, the endogenous compounds 4-hydroxynonenal and 15-deoxy-Δ12,14-prostaglandin J2, which can be released in response to tissue injury, inflammation, and oxidative stress were reported to be activators of TRPA1 (Macpherson et al., 2007b; Trevisani et al., 2007; Taylor-Clark et al., 2008). Many of the TRPA1 agonists are thiol-reactive electrophiles that activate TRPA1 through covalent interactions with cysteine residues in the channel N terminus, although other modifications are likely (Hinman et al., 2006; Macpherson et al., 2007a). Schmidt et al. (2009) recently reported that TRPA1 activation by mustard oil may be the result of increased protein kinase A/PLC-mediated trafficking to the membrane (Schmidt et al., 2009). TRPA1 activity is potentiated and subsequently inactivated by extracellular Ca2+. This modulation is indirect and attributed to Ca2+entry through TRPA1; the intracellular Ca2+-binding EF-hand motif is apparently not required (Wang et al., 2008).
In addition to chemical activation, it has been proposed that TRPA1 is directly activated by noxious cold (<17°C); however, the thermosensitivity of TRPA1 is debated. Numerous groups have reported that heterologously expressed TRPA1 is activated by noxious cold (Story et al., 2003; Bandell et al., 2004; Sawada et al., 2007; Karashima et al., 2009); however, other groups found no direct cold activation (Jordt et al., 2004; Nagata et al., 2005; Zurborg et al., 2007). Initial reports after the generation of two independent TRPA1(−/−) mice only contributed to the controversy. Whereas one study reported mild and sex-dependent alterations in the behavioral response to prolonged exposure to noxious cold in TRPA1(−/−) mice (Kwan et al., 2006), the second study found no sign of altered cold sensitivity in these mice (Bautista et al., 2006). A recent study identified a specific subset of cold-sensitive trigeminal ganglion neurons that is absent in TRPA1(−/−) mice and suggested that although TRPA1 is not required for sensing acute cold stimuli, it is required for behavioral responses to prolonged noxious cold (Karashima et al., 2009). The marked Ca2+ regulation of this channel under different conditions, strain differences, or the degree to which mice were back-crossed onto a common background, may underlie some of these discrepancies. It is noteworthy that TRPA1 orthologs from pit-bearing snakes are demonstrated to be the most heat-sensitive vertebrate ion channels and may play a role in detecting infrared radiation (Gracheva et al., 2010).
Detection of TRPA1 in hair cells in the ear (Corey et al., 2004; Nagata et al., 2005) led to the proposal that it forms the auditory mechanotransduction channel (Corey et al., 2004). However, heterologously expressed TRPA1 channels have not been shown to be mechanosensitive, and hair cells do not respond to mustard oil or other TRPA1 agonists (Corey, 2006). In addition, TRPA1(−/−) mice exhibit no overt vestibular defects, and their auditory responses are completely normal (Bautista et al., 2006; Kwan et al., 2006). In summary, there is no convincing evidence that TRPA1 itself is a mechanosensor in any cell type.
VI. The Transient Receptor Potential (Mucolipin) Family
The mucolipin TRP (TRPML; Fig. 4) proteins are primarily intracellular and are likely to be important for compartment trafficking and/or function (Bargal and Bach, 1997; Chen et al., 1998; Kim et al., 2009; Cheng et al., 2010b).
The founding member of the TRPML family, TRPML1, was first identified in linkage studies as the gene mutated in humans in Mucolipidosis type IV (MLIV), a progressive neurodegenerative disease of young children (Bargal et al., 2000; Bassi et al., 2000; Sun et al., 2000). MLIV is characterized clinically by severe motor deficits, mental retardation, retinal degeneration, iron-deficiency anemia, and elevated gastrin levels as a result of achlorhydria (Slaugenhaupt, 2002). At the cellular level, various materials [such as sphingolipids (mostly gangliosides), phospholipids, and acid mucopolysaccharides] accumulate in the lysosomes of patients with MLIV and appear as membrane-bound granular inclusions or lamellar concentric bodies. In contrast with other lysosomal storage diseases, the accumulation of heterogeneous storage material in MLIV lysosomes does not result from a block in catabolic pathways—lysosomal hydrolases are functional and correctly transported to the lysosomes; rather, it probably results from an ill-defined sorting, transport, or functional defect along the late endocytic pathway (Bargal and Bach, 1988; Chen et al., 1998).
Congruent with the ubiquitous lysosomal phenotype of MLIV patients, TRPML1 is expressed in cells of every tissue and localizes primarily to the lysosomal and late endosomal compartments (Manzoni et al., 2004; Kiselyov et al., 2005). TRPML1 contains two di-leucine motifs, one on its C terminus and one on its N terminus, that are likely to restrict its localization. In addition, TRPML1 has a large intraluminal loop between its first and second transmembrane domains that contains a putative serine-lipase site, a proline-rich domain, and a proteolytic cleavage site (Slaugenhaupt, 2002). This loop may interact with chaperone-mediated autophagy-related proteins, heat shock cognate protein of 70 kDa, and the 40-kDa heat shock protein (Venugopal et al., 2009). Currents recorded from late endosomes and lysosomes suggest that TRPML1 forms an inwardly rectifying, proton-impermeable, cation-selective channel with permeability to both Ca2+ and Fe2+. This permeability is potentiated by low luminal pH (Xu et al., 2007; Dong et al., 2008, 2009).
The inward rectification of TRPML1 indicates that when present in lysosomes, TRPML1 would primarily move cations out of the lysosomal lumen, depending on the translysosomal voltage and concentration gradients. This suggests that TRPML1 could function as a Ca2+ or Fe2+ release channel (Dong et al., 2008). Supporting this view, release of iron from late endosomes and lysosomes into the cytosol is essential for cellular iron metabolism and TRPML1(−/−) cells show altered iron homeostasis (Dong et al., 2008). Exocytosis from lysosomes are Ca2+-regulated, and one of the major sources of Ca2+ for this process is the lysosome itself (Peters and Mayer, 1998). Constitutively active TRPML1 mutants exhibit significant expression at the plasma membrane, whereas wild-type TRPML1 and non–gain-of-function mutants localize exclusively to the late endosomes and lysosomes (Dong et al., 2009). Consistent with a role for TRPML1 in Ca2+-dependent lysosomal exocytosis, surface staining of lysosomal-associated membrane protein type 1, a lysosomal marker, is dramatically increased in cells expressing constitutive active TRPML1 (Dong et al., 2009). In chaperone-mediated autophagy, proteins are directly transported through the lysosomal membrane, recognized by heat shock cognate protein of 70 kDa, and bound to the lysosomal membrane through interaction with LAMP-2A (Chiang et al., 1989; Cuervo and Dice, 1996). It is noteworthy that overexpression of the mammalian homolog of HSC70 in a fly model of MLIV rescued the motor deficits associated with TRPML1 deficiency (Venkatachalam et al., 2008). TRPML1(−/−) mice have been generated, and they largely recapitulated the phenotypes displayed in humans with MLIV, showing motor deficits, central nervous system inclusions, retinal degeneration, elevated plasma gastrin, and decreased life span (Venugopal et al., 2007).
Like TRPML1, TRPML2 is an inwardly rectifying Ca2+- and Fe2+-permeable cation-selective channel potentiated by low pH (Dong et al., 2008; Samie et al., 2009). TRPML2 is expressed in cells of all tissues, where it localizes primarily to intracellular compartments (Xu et al., 2007; Samie et al., 2009; Zeevi et al., 2009). Knockdown of endogenous TRPML2 expression in HEK293 cells leads to lysosomal storage and mitochondrial abnormalities (Zeevi et al., 2009). Functional studies suggest that TRPML2 may regulate the trafficking between recycling endosomes and the cell surface through an Arf6 clathrin-independent pathway (Karacsonyi et al., 2007). Generation of TRPML2(−/−) mice may help to elucidate the role of TRPML2 in vivo.
TRPML3 is an inwardly rectifying cation-selective channel that is regulated by extracellular/luminal pH (Grimm et al., 2007; Xu et al., 2007; Kim et al., 2008b). TRPML3 was discovered by positional cloning as the channel mutated in varitint-waddler mice, which are characterized by a variegated/dilute coat color owing to pigmentation defects, hearing loss, circling behavior caused by vestibular defects, hyperactivity, and embryonic lethality (Cable and Steel, 1998; Di Palma et al., 2002; Xu et al., 2007). The varitint-waddler phenotype is caused by the gain-of-function mutation (A419P) in the S6 of TRPML3 (Grimm et al., 2007; Kim et al., 2007; Xu et al., 2007). This mutation is a helix-breaking proline substitution that creates a constitutively active channel and eliminates regulation of the channel by extracytosolic cations (Grimm et al., 2007; Kim et al., 2007). Constitutive activation of the channel leads to increased Ca2+ influx and cell death (Xu et al., 2007); the loss of melanocytes in the cochlea and vestibulum probably underlies the deafness and the circling behavior of varitint-waddler mice (Cable and Steel, 1998; Xu et al., 2007). However, other than constitutive activation of the helix-break mutants, wild-type TRPML3 activation is not well understood. TRPML3 can be activated by preincubation in low-Na+ medium (Kim et al., 2008b). A recent report using a high-throughput chemical screen has identified a plethora of TRPML3 activators that will hopefully serve as useful tools (Grimm et al., 2010).
Mirroring its functional deficits, TRPML3 expression has been reported in the hair cells of the cochlea and the vestibulum, as well as in the melanocytes in skin hair follicles (Di Palma et al., 2002; van Aken et al., 2008). At the cellular level, TRPML3 can be detected in intracellular vesicular compartments and in the plasma membrane. Knockdown of TRPML3 expression or expression of a dominant-negative version of the channel stimulated endocytosis of transferrin and EGF/EGFR, whereas overexpression of TRPML3 inhibited these same processes (Kim et al., 2009). Knockdown of endogenous TRPML3 causes lysosomal storage and mitochondrial abnormalities (Zeevi et al., 2009).
VII. The Transient Receptor Potential (Polycystin)/Polycystic Kidney Disease 2 Family
TRPP refers to the polycystic kidney disease 2 (PKD2; Fig. 4) subset of the polycystins. Polycystins include putative 11-TM (PKD1, also called the PC1 family) and 6-TM subfamilies (PKD2, also called the PC2 family). The TRPP family nomenclature is confused by the previous inclusion of the 11-TM subfamily. Because there is little support for the 11TM group forming functional channels, we will only discuss the 6TM (PKD2, PC2) family. To avoid confusion, we use the PKD2 nomenclature but provide previous names associated with each.
Increasing evidence suggests that PKD1 subgroup members associate with PKD2 members to form heterocomplexes (Qian et al., 1997; Tsiokas et al., 1997) and that they share a notable number of physiological functions (Hanaoka et al., 2000; McGrath et al., 2003; LopezJimenez et al., 2006; Vogel et al., 2010). The PKD2 subgroup consists of three members, PKD2, PKD2L1, and PKD2L2, all of which have 6 TM-spanning domains and intracellular N and C termini. Based on their homology to other TRP family members, they are expected to assemble in a tetrameric structure to form Ca2+ permeable nonselective cation channels.
PKD2 (TRPP1, also called PC2, and TRPP2 in older nomenclature) was originally identified in linkage studies for autosomal dominant polycystic kidney disease (ADPKD) (Peters et al., 1993; Mochizuki et al., 1996). ADPKD is characterized by the progressive development of multiple fluid-filled cysts in the kidney, pancreas, and liver and an increased prevalence of cardiovascular abnormalities such as hypertension, mitral valve prolapse, and intracranial aneurysm (Gabow, 1993; Torra et al., 2000). Approximately 15% of clinical cases of ADPKD present with mutations in the PKD2 gene loci (Peters et al., 1993). The cystic phenotype and extrarenal abnormalities are largely recapitulated in PKD2(−/−) mice, and the mice die in utero between embryonic day 13.5 and parturition (Wu et al., 2000).
PKD2 is reported to form a Ca2+-permeable nonselective cation channel that can be activated by downstream of G protein-coupled receptor and/or receptor-tyrosine kinase at the cell surface (Ma et al., 2005; Bai et al., 2008b) and is regulated by phosphoinositides (Ma et al., 2005), Ca2+ (Vassilev et al., 2001; Koulen et al., 2002), and pH (Gonzalez-Perrett et al., 2002). Controversy surrounds PKD2 currents, because in most cell-based systems, PKD2 does not traffic to the plasma membrane and is retained in endoplasmic reticulum (Hanaoka et al., 2000; Vassilev et al., 2001; Koulen et al., 2002). There is good agreement, however, that PKD2 associates with PKD1 through its C-terminal coiled-coil domain (Bai et al., 2008a; Celić et al., 2008; Yu et al., 2009). The functional importance of the coiled-coil domain is underscored by the many naturally occurring ADPKD pathogenic truncations, including R742X, R807X, E837X, and R872X, in PKD2 (Sharif-Naeini et al., 2009), which eliminate the coiled-coil domain or the downstream open region and abolish the assembly of the PKD1-PKD2 complex.
PKD2 is widely expressed in both fetal and adult tissues. A pool of PKD2 has been proposed to localize to cilia through a motif in its N terminus (Geng et al., 2006). In the primary cilia of renal epithelial cells and vascular endothelial cells, PKD2, in conjunction with PKD1, may be required for transduction of extracellular shear stress induced by blood or urine flow into intracellular Ca2+ signals (Nauli et al., 2008; AbouAlaiwi et al., 2009). Thus, it was proposed that PKD2, perhaps in association with PKD1 and/or TRPV4, is a mechanosensitive channel. There is, however, very little direct support for this idea, and a recent study suggests that PKD2 is not itself a mechanosensitive channel but instead regulates mechanosensory channels (Sharif-Naeini et al., 2009), perhaps through the numerous reported interactions with the actin filament associated proteins. PKD2 is also expressed in nodal cilia, where it is required for the development of left-right asymmetry of the thoracic and visceral organs (McGrath et al., 2003).
PKD2 is present in the endoplasmic reticulum. The C terminus of PKD2 may bind PACS and PRKCSH/80K-H, which retain proteins in the endoplasmic reticulum (Köttgen et al., 2005) and protect it from homocysteine-induced endoplasmic reticulum protein–mediated degradation (Gao et al., 2010), respectively. In the endoplasmic reticulum, it has been proposed that PKD2 forms a Ca2+ release channel and modulates release of intracellular Ca2+ (Koulen et al., 2002; Geng et al., 2008). To reconcile disparate findings, an appealing model is one in which PKD1 on the plasma membrane interacts with PKD2 in the endoplasmic reticulum.
PKD2L1 (TRPP2, also called TRPP3 in older nomenclature) is reported to form an inwardly rectifying Ca2+-permeable nonselective cation channel with a large single channel conductance modulated by pH (Huang et al., 2006; Ishimaru et al., 2006; Shimizu et al., 2009). Concrete evidence that these currents are mediated by the PKD2L1 alone is lacking. The expression pattern of PKD2L1 is also debated. At both the mRNA and protein levels, PKD2L1 has been reported in multiple tissues, including the kidney, retina, liver, pancreas, heart, spleen, and brain (Nomura et al., 1998; Basora et al., 2002). More recently, the expression pattern of PKD2L1 seems to be restricted to testis, taste tissue, and in a specific subset of neurons surrounding the central canal of spinal cord (Huang et al., 2006; Ishimaru et al., 2006). In taste tissue, PKD2L1 colocalizes with PKD1L3 in a subpopulation of taste receptor cells, where they may function as sour (H+) receptors (LopezJimenez et al., 2006). In support of this idea, mice lacking PKD2L1-expressing cells, as a result of diphtheria toxin-mediated ablation, exhibit no gustatory nerve response to acidic stimuli in the chorda tympani nerves (Huang et al., 2006). However, in vivo behavior analyses revealed that PKD2L1(−/−) mice retain normal sensation to sour as well as sweet, bitter, and salty tastes (L. Guo and J. Zhou, unpublished observations). After PKD2L1 expression in a subset of pH-sensitive neurons surrounding the central canal of the spinal cord, it has also been proposed to serve as a chemosensor sensing the internal state of spinal fluid (Huang et al., 2006).
PKD2L2 (TRPP3, also called TRPP5 in older literature) is the least well understood member of the TRP family. Although PKD2L2 is expected to form a Ca2+-permeable nonselective cation channel (Guo et al., 2000), only limited support for this hypothesis exists. Overexpression of PKD2L2 in Madin-Darby canine kidney cells resulted in elevated levels of intracellular Ca2+ (Chen et al., 2008b), and outside-out patches from PKD2L2-transfected HEK293 cells revealed a channel with a single 25-pS conductance state that could not be measured in control cells (Sutton et al., 2006). Northern blot analysis indicates that PKD2L2 is expressed in mouse heart and testis, whereas reverse transcription-polymerase chain reaction analysis showed that in humans, PKD2L2 is expressed in brain, kidney, and testis (Guo et al., 2000). Immunohistochemical staining detects PKD2L2 in the plasma membrane of spermatocytes and round spermatids (Chen et al., 2008b), suggesting its potential role in spermatogenesis.
VIII. Summary
In this review, we have attempted to capture the current state of understanding of the function of the large class of mammalian TRP channels. The TRP literature has become so large that many works in the area could not be credited adequately. The TRP channels currently seem to have arisen in eukaryotes to fulfill cellular sensing in response to diverse environmental stimuli by reducing transmembrane (both intracellular and plasma membrane) voltages and often permeating divalent cations. The most common features are their weak voltage sensitivities, potentiation by phospholipase C-linked receptors, and modulation by positively charged intracellular divalent ions and the negatively charged molecules Ca2+/CaM and PIP2. One unusual features of this class of channels are their (often) dual functional relevance in both intracellular compartments and on the plasma membrane in response to extracellular stimuli, perhaps serving to deliver themselves to the plasma membrane by providing Ca2+ to intracellular synaptotagmins required for fusion. Another unusual feature is that several are permeant to Mg2+ and other divalent ions that are much too large (because of their large dehydration energies) to permeate other ion channels. The most significant development in the field is that knockout mice are now available for practically all TRP channels, which will enable more exacting determination of function and proper characterization of antibodies used in localization studies.
Acknowledgments.
This work is supported by the National Institutes of Health National Institute of Mental Health [Grant R01-MH090293–01] and a Harvard Medical School Lefler postdoctoral fellowship (to L.-J.W.).
1
Abbreviations:
2-APB
2-aminoethoxydiphenyl borate
ADPKD
autosomal dominant polycystic kidney disease
CaM
calmodulin
CIRB
CaM/IP3R-binding
CSNB
congenital stationary night blindness
DAG
diacylglycerol
EPSC
excitatory postsynaptic current
GSK1016790A
(N_-((1_S)-1-{[4-((2_S_)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide
HC-030031
2-(1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydro-7_H_-purin-7-yl)-_N_-(4-isopropylphenyl)acetamide
HEK
human embryonic kidney
IP3R
inositol trisphosphate receptor
mGluR1
metabotropic glutamate receptor 1
MLIV
mucolipidosis type IV
OMIM
Online Mendelian Inheritance in Man
PIP2
phosphatidylinositol 4,5-bisphosphate
PKD
polycystic kidney disease
PLC
phospholipase C
1-(β-[3-(4-methoxyphenyl)propoxy]-4-methoxyphenethyl)-1_H_-imidazole
TM
transmembrane
TRP
transient receptor potential
TRPA
transient receptor potential ankyrin
TRPC
transient receptor potential canonical
TRPM
transient receptor potential Melastatin
TRPML
transient receptor potential mucolipin
TRPP
transient receptor potential polycystin
TRPV
transient receptor potential vanilloid.
References
- Aarts et al., 2003.Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. (2003) A key role for TRPM7 channels in anoxic neuronal death. Cell 115:863–877 [DOI] [PubMed] [Google Scholar]
- AbouAlaiwi et al., 2009.AbouAlaiwi WA, Takahashi M, Mell BR, Jones TJ, Ratnam S, Kolb RJ, Nauli SM. (2009) Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circ Res 104:860–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ansary et al., 2010.Al-Ansary D, Bogeski I, Disteldorf BM, Becherer U, Niemeyer BA. (2010) ATP modulates Ca2+ uptake by TRPV6 and is counteracted by isoform-specific phosphorylation. FASEB J 24:425–435 [DOI] [PubMed] [Google Scholar]
- Alessandri-Haber et al., 2006.Alessandri-Haber N, Dina OA, Joseph EK, Reichling D, Levine JD. (2006) A transient receptor potential vanilloid 4-dependent mechanism of hyperalgesia is engaged by concerted action of inflammatory mediators. J Neurosci 26:3864–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez et al., 2006.Alvarez DF, King JA, Weber D, Addison E, Liedtke W, Townsley MI. (2006) Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury. Circ Res 99:988–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral and Pozzo-Miller, 2007.Amaral MD, Pozzo-Miller L. (2007) TRPC3 channels are necessary for brain-derived neurotrophic factor to activate a nonselective cationic current and to induce dendritic spine formation. J Neurosci 27:5179–5189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson et al., 2004.Andersson DA, Chase HW, Bevan S. (2004) TRPM8 activation by menthol, icilin, and cold is differentially modulated by intracellular pH. J Neurosci 24:5364–5369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa et al., 2006.Asakawa M, Yoshioka T, Matsutani T, Hikita I, Suzuki M, Oshima I, Tsukahara K, Arimura A, Horikawa T, Hirasawa T, et al. (2006) Association of a mutation in TRPV3 with defective hair growth in rodents. J Invest Dermatol 126:2664–2672 [DOI] [PubMed] [Google Scholar]
- Atoyan et al., 2009.Atoyan R, Shander D, Botchkareva NV. (2009) Non-neuronal expression of transient receptor potential type A1 (TRPA1) in human skin. J Invest Dermatol 129:2312–2315 [DOI] [PubMed] [Google Scholar]
- Audo et al., 2009.Audo I, Kohl S, Leroy BP, Munier FL, Guillonneau X, Mohand-Saïd S, Bujakowska K, Nandrot EF, Lorenz B, Preising M, et al. (2009) TRPM1 is mutated in patients with autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet 85:720–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer-Grumbach et al., 2010.Auer-Grumbach M, Olschewski A, Papić L, Kremer H, McEntagart ME, Uhrig S, Fischer C, Fröhlich E, Bálint Z, Tang B, et al. (2010) Alterations in the ankyrin domain of TRPV4 cause congenital distal SMA, scapuloperoneal SMA and HMSN2C. Nat Genet 42:160–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai et al., 2008a.Bai CX, Giamarchi A, Rodat-Despoix L, Padilla F, Downs T, Tsiokas L, Delmas P. (2008a) Formation of a new receptor-operated channel by heteromeric assembly of TRPP2 and TRPC1 subunits. EMBO Rep 9:472–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai et al., 2008b.Bai CX, Kim S, Li WP, Streets AJ, Ong AC, Tsiokas L. (2008b) Activation of TRPP2 through mDia1-dependent voltage gating. EMBO J 27:1345–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell et al., 2006.Bandell M, Dubin AE, Petrus MJ, Orth A, Mathur J, Hwang SW, Patapoutian A. (2006) High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat Neurosci 9:493–500 [DOI] [PubMed] [Google Scholar]
- Bandell et al., 2004.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. (2004) Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41:849–857 [DOI] [PubMed] [Google Scholar]
- Barbet et al., 2008.Barbet G, Demion M, Moura IC, Serafini N, Léger T, Vrtovsnik F, Monteiro RC, Guinamard R, Kinet JP, Launay P. (2008) The calcium-activated nonselective cation channel TRPM4 is essential for the migration but not the maturation of dendritic cells. Nat Immunol 9:1148–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargal et al., 2000.Bargal R, Avidan N, Ben-Asher E, Olender Z, Zeigler M, Frumkin A, Raas-Rothschild A, Glusman G, Lancet D, Bach G. (2000) Identification of the gene causing mucolipidosis type IV. Nat Genet 26:118–123 [DOI] [PubMed] [Google Scholar]
- Bargal and Bach, 1988.Bargal R, Bach G. (1988) Phospholipids accumulation in mucolipidosis IV cultured fibroblasts. J Inherit Metab Dis 11:144–150 [DOI] [PubMed] [Google Scholar]
- Bargal and Bach, 1997.Bargal R, Bach G. (1997) Mucolipidosis type IV: abnormal transport of lipids to lysosomes. J Inherit Metab Dis 20:625–632 [DOI] [PubMed] [Google Scholar]
- Basora et al., 2002.Basora N, Nomura H, Berger UV, Stayner C, Guo L, Shen X, Zhou J. (2002) Tissue and cellular localization of a novel polycystic kidney disease-like gene product, polycystin-L. J Am Soc Nephrol 13:293–301 [DOI] [PubMed] [Google Scholar]
- Bassi et al., 2000.Bassi MT, Manzoni M, Monti E, Pizzo MT, Ballabio A, Borsani G. (2000) Cloning of the gene encoding a novel integral membrane protein, mucolipidin-and identification of the two major founder mutations causing mucolipidosis type IV. Am J Hum Genet 67:1110–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista et al., 2006.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. (2006) TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124:1269–1282 [DOI] [PubMed] [Google Scholar]
- Bautista et al., 2005.Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Högestätt ED, Julius D, Jordt SE, Zygmunt PM. (2005) Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA 102:12248–12252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista et al., 2007.Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. (2007) The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448:204–208 [DOI] [PubMed] [Google Scholar]
- Becker et al., 2009.Becker EB, Oliver PL, Glitsch MD, Banks GT, Achilli F, Hardy A, Nolan PM, Fisher EM, Davies KE. (2009) A point mutation in TRPC3 causes abnormal Purkinje cell development and cerebellar ataxia in moonwalker mice. Proc Natl Acad Sci USA 106:6706–6711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benn et al., 2008.Benn BS, Ajibade D, Porta A, Dhawan P, Hediger M, Peng JB, Jiang Y, Oh GT, Jeung EB, Lieben L, et al. (2008) Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-D9k. Endocrinology 149:3196–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg et al., 2007.Berg AP, Sen N, Bayliss DA. (2007) TrpC3/C7 and Slo2.1 are molecular targets for metabotropic glutamate receptor signaling in rat striatal cholinergic interneurons. J Neurosci 27:8845–8856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzerides et al., 2004.Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. (2004) Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol 6:709–720 [DOI] [PubMed] [Google Scholar]
- Bianco et al., 2007.Bianco SD, Peng JB, Takanaga H, Suzuki Y, Crescenzi A, Kos CH, Zhuang L, Freeman MR, Gouveia CH, Wu J, et al. (2007) Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. J Bone Miner Res 22:274–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder et al., 2007.Birder L, Kullmann FA, Lee H, Barrick S, de Groat W, Kanai A, Caterina M. (2007) Activation of urothelial transient receptor potential vanilloid 4 by 4alpha-phorbol 12,13-didecanoate contributes to altered bladder reflexes in the rat. J Pharmacol Exp Ther 323:227–235 [DOI] [PubMed] [Google Scholar]
- Birder et al., 2002.Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, et al. (2002) Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci 5:856–860 [DOI] [PubMed] [Google Scholar]
- Blair et al., 2009.Blair NT, Kaczmarek JS, Clapham DE. (2009) Intracellular calcium strongly potentiates agonist-activated TRPC5 channels. J Gen Physiol 133:525–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov and Harris, 2009.Blednov YA, Harris RA. (2009) Deletion of vanilloid receptor (TRPV1) in mice alters behavioral effects of ethanol. Neuropharmacology 56:814–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen et al., 2010.Bohlen CJ, Priel A, Zhou S, King D, Siemens J, Julius D. (2010) A bivalent tarantula toxin activates the capsaicin receptor, TRPV1, by targeting the outer pore domain. Cell 141:834–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölcskei et al., 2005.Bölcskei K, Helyes Z, Szabó A, Sándor K, Elekes K, Németh J, Almási R, Pintér E, Petho G, Szolcsányi J. (2005) Investigation of the role of TRPV1 receptors in acute and chronic nociceptive processes using gene-deficient mice. Pain 117:368–376 [DOI] [PubMed] [Google Scholar]
- Brauchi et al., 2008.Brauchi S, Krapivinsky G, Krapivinsky L, Clapham DE. (2008) TRPM7 facilitates cholinergic vesicle fusion with the plasma membrane. Proc Natl Acad Sci USA 105:8304–8308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley et al., 2009.Brierley SM, Hughes PA, Page AJ, Kwan KY, Martin CM, O'Donnell TA, Cooper NJ, Harrington AM, Adam B, Liebregts T, et al. (2009) The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology 137:2084–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brixel et al., 2010.Brixel LR, Monteilh-Zoller MK, Ingenbrandt CS, Fleig A, Penner R, Enklaar T, Zabel BU, Prawitt D. (2010) TRPM5 regulates glucose-stimulated insulin secretion. Pflugers Arch 460:69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cable and Steel, 1998.Cable J, Steel KP. (1998) Combined cochleo-saccular and neuroepithelial abnormalities in the Varitint-waddler-J (VaJ) mouse. Hear Res 123:125–136 [DOI] [PubMed] [Google Scholar]
- Caterina et al., 2000.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288:306–313 [DOI] [PubMed] [Google Scholar]
- Caterina et al., 1999.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. (1999) A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398:436–441 [DOI] [PubMed] [Google Scholar]
- Caterina et al., 1997.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824 [DOI] [PubMed] [Google Scholar]
- Celić et al., 2008.Celić A, Petri ET, Demeler B, Ehrlich BE, Boggon TJ. (2008) Domain mapping of the polycystin-2 C-terminal tail using de novo molecular modeling and biophysical analysis. J Biol Chem 283:28305–28312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al., 1998.Chen CS, Bach G, Pagano RE. (1998) Abnormal transport along the lysosomal pathway in mucolipidosis, type IV disease. Proc Natl Acad Sci USA 95:6373–6378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al., 2008a.Chen L, Liu C, Liu L. (2008a) Changes in osmolality modulate voltage-gated calcium channels in trigeminal ganglion neurons. Brain Res 1208:56–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al., 2008b.Chen Y, Zhang Z, Lv XY, Wang YD, Hu ZG, Sun H, Tan RZ, Liu YH, Bian GH, Xiao Y, et al. (2008b) Expression of Pkd2l2 in testis is implicated in spermatogenesis. Biol Pharm Bull 31:1496–1500 [DOI] [PubMed] [Google Scholar]
- Cheng et al., 2010a.Cheng X, Jin J, Hu L, Shen D, Dong XP, Samie MA, Knoff J, Eisinger B, Liu ML, Huang SM, et al. (2010a) TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell 141:331–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng et al., 2010b.Cheng X, Shen D, Samie M, Xu H. (2010b) Mucolipins: Intracellular TRPML1–3 channels. FEBS Lett 584:2013–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang et al., 1989.Chiang HL, Terlecky SR, Plant CP, Dice JF. (1989) A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science 246:382–385 [DOI] [PubMed] [Google Scholar]
- Chu et al., 2003.Chu CJ, Huang SM, De Petrocellis L, Bisogno T, Ewing SA, Miller JD, Zipkin RE, Daddario N, Appendino G, Di Marzo V, et al. (2003) N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J Biol Chem 278:13633–13639 [DOI] [PubMed] [Google Scholar]
- Chuang et al., 2004.Chuang HH, Neuhausser WM, Julius D. (2004) The super-cooling agent icilin reveals a mechanism of coincidence detection by a temperature-sensitive TRP channel. Neuron 43:859–869 [DOI] [PubMed] [Google Scholar]
- Chuang et al., 2001.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. (2001) Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 411:957–962 [DOI] [PubMed] [Google Scholar]
- Chung et al., 2004.Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. (2004) TRPV3 and TRPV4 mediate warmth-evoked currents in primary mouse keratinocytes. J Biol Chem 279:21569–21575 [DOI] [PubMed] [Google Scholar]
- Clapham, 2003.Clapham DE. (2003) TRP channels as cellular sensors. Nature 426:517–524 [DOI] [PubMed] [Google Scholar]
- Clapham et al., 2005.Clapham DE, Julius D, Montell C, Schultz G. (2005) International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev 57:427–450 [DOI] [PubMed] [Google Scholar]
- Clark et al., 2006.Clark K, Langeslag M, van Leeuwen B, Ran L, Ryazanov AG, Figdor CG, Moolenaar WH, Jalink K, van Leeuwen FN. (2006) TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J 25:290–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colburn et al., 2007.Colburn RW, Lubin ML, Stone DJ, Jr, Wang Y, Lawrence D, D'Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N. (2007) Attenuated cold sensitivity in TRPM8 null mice. Neuron 54:379–386 [DOI] [PubMed] [Google Scholar]
- Colsoul et al., 2010.Colsoul B, Schraenen A, Lemaire K, Quintens R, Van Lommel L, Segal A, Owsianik G, Talavera K, Voets T, Margolskee RF, et al. (2010) Loss of high-frequency glucose-induced Ca2+ oscillations in pancreatic islets correlates with impaired glucose tolerance in Trpm5−/− mice. Proc Natl Acad Sci USA 107:5208–5213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey, 2006.Corey DP. (2006) What is the hair cell transduction channel? J Physiol 576:23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey et al., 2004.Corey DP, García-Añoveros J, Holt JR, Kwan KY, Lin SY, Vollrath MA, Amalfitano A, Cheung EL, Derfler BH, Duggan A, et al. (2004) TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 432:723–730 [DOI] [PubMed] [Google Scholar]
- Cuervo and Dice, 1996.Cuervo AM, Dice JF. (1996) A receptor for the selective uptake and degradation of proteins by lysosomes. Science 273:501–503 [DOI] [PubMed] [Google Scholar]
- Damak et al., 2006.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Pérez CA, Shigemura N, Yoshida R, Mosinger B, Jr, Glendinning JI, Ninomiya Y, et al. (2006) Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses 31:253–264 [DOI] [PubMed] [Google Scholar]
- Davare et al., 2009.Davare MA, Fortin DA, Saneyoshi T, Nygaard S, Kaech S, Banker G, Soderling TR, Wayman GA. (2009) Transient receptor potential canonical 5 channels activate Ca2+/calmodulin kinase Igamma to promote axon formation in hippocampal neurons. J Neurosci 29:9794–9808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis et al., 2000.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, et al. (2000) Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 405:183–187 [DOI] [PubMed] [Google Scholar]
- De Blas et al., 2009.De Blas GA, Darszon A, Ocampo AY, Serrano CJ, Castellano LE, Hernández-González EO, Chirinos M, Larrea F, Beltrán C, Treviño CL. (2009) TRPM8, a versatile channel in human sperm. PLoS One 4:e6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis et al., 2001.De Petrocellis L, Harrison S, Bisogno T, Tognetto M, Brandi I, Smith GD, Creminon C, Davis JB, Geppetti P, Di Marzo V. (2001) The vanilloid receptor (VR1)-mediated effects of anandamide are potently enhanced by the cAMP-dependent protein kinase. J Neurochem 77:1660–1663 [DOI] [PubMed] [Google Scholar]
- Deng et al., 2010.Deng HX, Klein CJ, Yan J, Shi Y, Wu Y, Fecto F, Yau HJ, Yang Y, Zhai H, Siddique N, et al. (2010) Scapuloperoneal spinal muscular atrophy and CMT2C are allelic disorders caused by alterations in TRPV4. Nat Genet 42:165–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai and Clapham, 2005.Desai BN, Clapham DE. (2005) TRP channels and mice deficient in TRP channels. Pflugers Arch 451:11–18 [DOI] [PubMed] [Google Scholar]
- Dhaka et al., 2007.Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. (2007) TRPM8 is required for cold sensation in mice. Neuron 54:371–378 [DOI] [PubMed] [Google Scholar]
- Di Palma et al., 2002.Di Palma F, Belyantseva IA, Kim HJ, Vogt TF, Kachar B, Noben-Trauth K. (2002) Mutations in Mcoln3 associated with deafness and pigmentation defects in varitint-waddler (Va) mice. Proc Natl Acad Sci USA 99:14994–14999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich et al., 2007.Dietrich A, Kalwa H, Storch U, Mederos y, Schnitzler M, Salanova B, Pinkenburg O, Dubrovska G, Essin K, Gollasch M, Birnbaumer L, et al. (2007) Pressure-induced and store-operated cation influx in vascular smooth muscle cells is independent of TRPC1. Pflugers Arch 455:465–477 [DOI] [PubMed] [Google Scholar]
- Dietrich et al., 2005.Dietrich A, Mederos Y, Schnitzler M, Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E, Salanova B, Kalwa H, et al. (2005) Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol Cell Biol 25:6980–6989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogenes et al., 2007.Diogenes A, Akopian AN, Hargreaves KM. (2007) NGF up-regulates TRPA1: implications for orofacial pain. J Dent Res 86:550–555 [DOI] [PubMed] [Google Scholar]
- Docherty et al., 1996.Docherty RJ, Yeats JC, Bevan S, Boddeke HW. (1996) Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflugers Arch 431:828–837 [DOI] [PubMed] [Google Scholar]
- Dong et al., 2008.Dong XP, Cheng X, Mills E, Delling M, Wang F, Kurz T, Xu H. (2008) The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature 455:992–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong et al., 2009.Dong XP, Wang X, Shen D, Chen S, Liu M, Wang Y, Mills E, Cheng X, Delling M, Xu H. (2009) Activating mutations of the TRPML1 channel revealed by proline-scanning mutagenesis. J Biol Chem 284:32040–32052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorovkov and Ryazanov, 2004.Dorovkov MV, Ryazanov AG. (2004) Phosphorylation of annexin I by TRPM7 channel-kinase. J Biol Chem 279:50643–50646 [DOI] [PubMed] [Google Scholar]
- Du et al., 2009.Du J, Xie J, Yue L. (2009) Intracellular calcium activates TRPM2 and its alternative spliced isoforms. Proc Natl Acad Sci USA 106:7239–7244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan et al., 1998.Duncan LM, Deeds J, Hunter J, Shao J, Holmgren LM, Woolf EA, Tepper RI, Shyjan AW. (1998) Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res 58:1515–1520 [PubMed] [Google Scholar]
- Earley et al., 2009.Earley S, Pauyo T, Drapp R, Tavares MJ, Liedtke W, Brayden JE. (2009) TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am J Physiol Heart Circ Physiol 297:H1096–H1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingson et al., 2009.Ellingson JM, Silbaugh BC, Brasser SM. (2009) Reduced oral ethanol avoidance in mice lacking transient receptor potential channel vanilloid receptor 1. Behav Genet 39:62–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erler et al., 2004.Erler I, Hirnet D, Wissenbach U, Flockerzi V, Niemeyer BA. (2004) Ca2+-selective transient receptor potential V channel architecture and function require a specific ankyrin repeat. J Biol Chem 279:34456–34463 [DOI] [PubMed] [Google Scholar]
- Fan et al., 2009.Fan HC, Zhang X, McNaughton PA. (2009) Activation of the TRPV4 ion channel is enhanced by phosphorylation. J Biol Chem 284:27884–27891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes et al., 2008.Fernandes J, Lorenzo IM, Andrade YN, Garcia-Elias A, Serra SA, Fernández-Fernández JM, Valverde MA. (2008) IP3 sensitizes TRPV4 channel to the mechano- and osmotransducing messenger 5′-6′-epoxyeicosatrienoic acid. J Gen Physiol 131:i2. [DOI] [PubMed] [Google Scholar]
- Freichel et al., 2001.Freichel M, Suh SH, Pfeifer A, Schweig U, Trost C, Weissgerber P, Biel M, Philipp S, Freise D, Droogmans G, et al. (2001) Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/− mice. Nat Cell Biol 3:121–127 [DOI] [PubMed] [Google Scholar]
- Gabow, 1993.Gabow PA. (1993) Autosomal dominant polycystic kidney disease. N Engl J Med 329:332–342 [DOI] [PubMed] [Google Scholar]
- Gao et al., 2010.Gao H, Wang Y, Wegierski T, Skouloudaki K, Pütz M, Fu X, Engel C, Boehlke C, Peng H, Kuehn EW, et al. (2010) PRKCSH/80K-H, the protein mutated in polycystic liver disease, protects polycystin-2/TRPP2 against HERP-mediated degradation. Hum Mol Genet 19:16–24 [DOI] [PubMed] [Google Scholar]
- Gao et al., 2003.Gao X, Wu L, O'Neil RG. (2003) Temperature-modulated diversity of TRPV4 channel gating: activation by physical stresses and phorbol ester derivatives through protein kinase C-dependent and -independent pathways. J Biol Chem 278:27129–27137 [DOI] [PubMed] [Google Scholar]
- Gavva et al., 2008.Gavva NR, Treanor JJ, Garami A, Fang L, Surapaneni S, Akrami A, Alvarez F, Bak A, Darling M, Gore A, et al. (2008) Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain 136:202–210 [DOI] [PubMed] [Google Scholar]
- Geng et al., 2008.Geng L, Boehmerle W, Maeda Y, Okuhara DY, Tian X, Yu Z, Choe CU, Anyatonwu GI, Ehrlich BE, Somlo S. (2008) Syntaxin 5 regulates the endoplasmic reticulum channel-release properties of polycystin-2. Proc Natl Acad Sci USA 105:15920–15925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng et al., 2006.Geng L, Okuhara D, Yu Z, Tian X, Cai Y, Shibazaki S, Somlo S. (2006) Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J Cell Sci 119:1383–1395 [DOI] [PubMed] [Google Scholar]
- Geppetti et al., 2006.Geppetti P, Materazzi S, Nicoletti P. (2006) The transient receptor potential vanilloid 1: role in airway inflammation and disease. Eur J Pharmacol 533:207–214 [DOI] [PubMed] [Google Scholar]
- Gerzanich et al., 2009.Gerzanich V, Woo SK, Vennekens R, Tsymbalyuk O, Ivanova S, Ivanov A, Geng Z, Chen Z, Nilius B, Flockerzi V, et al. (2009) De novo expression of Trpm4 initiates secondary hemorrhage in spinal cord injury. Nat Med 15:185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevaert et al., 2007.Gevaert T, Vriens J, Segal A, Everaerts W, Roskams T, Talavera K, Owsianik G, Liedtke W, Daelemans D, Dewachter I, et al. (2007) Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest 117:3453–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson et al., 2008.Gibson HE, Edwards JG, Page RS, Van Hook MJ, Kauer JA. (2008) TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron 57:746–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilon and Henquin, 2001.Gilon P, Henquin JC. (2001) Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocr Rev 22:565–604 [DOI] [PubMed] [Google Scholar]
- Gkika et al., 2004.Gkika D, Mahieu F, Nilius B, Hoenderop JG, Bindels RJ. (2004) 80K-H as a new Ca2+ sensor regulating the activity of the epithelial Ca2+ channel transient receptor potential cation channel V5 (TRPV5). J Biol Chem 279:26351–26357 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Perrett et al., 2002.Gonzalez-Perrett S, Batelli M, Kim K, Essafi M, Timpanaro G, Moltabetti N, Reisin IL, Arnaout MA, Cantiello HF. (2002) Voltage dependence and pH regulation of human polycystin-2-mediated cation channel activity. J Biol Chem 277:24959–24966 [DOI] [PubMed] [Google Scholar]
- Gracheva et al., 2010.Gracheva EO, Ingolia NT, Kelly YM, Cordero-Morales JF, Hollopeter G, Chesler AT, Sánchez EE, Perez JC, Weissman JS, Julius D. (2010) Molecular basis of infrared detection by snakes. Nature 464:1006–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradilone et al., 2007.Gradilone SA, Masyuk AI, Splinter PL, Banales JM, Huang BQ, Tietz PS, Masyuk TV, Larusso NF. (2007) Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proc Natl Acad Sci USA 104:19138–19143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greka et al., 2003.Greka A, Navarro B, Oancea E, Duggan A, Clapham DE. (2003) TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat Neurosci 6:837–845 [DOI] [PubMed] [Google Scholar]
- Grimm et al., 2007.Grimm C, Cuajungco MP, van Aken AF, Schnee M, Jörs S, Kros CJ, Ricci AJ, Heller S. (2007) A helix-breaking mutation in TRPML3 leads to constitutive activity underlying deafness in the varitint-waddler mouse. Proc Natl Acad Sci USA 104:19583–19588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm et al., 2010.Grimm C, Jörs S, Saldanha SA, Obukhov AG, Pan B, Oshima K, Cuajungco MP, Chase P, Hodder P, Heller S. (2010) Small molecule activators of TRPML3. Chem Biol 17:135–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm et al., 2003.Grimm C, Kraft R, Sauerbruch S, Schultz G, Harteneck C. (2003) Molecular and functional characterization of the melastatin-related cation channel TRPM3. J Biol Chem 278:21493–21501 [DOI] [PubMed] [Google Scholar]
- Grimm et al., 2005.Grimm C, Kraft R, Schultz G, Harteneck C. (2005) Activation of the melastatin-related cation channel TRPM3 by d-erythro-sphingosine [corrected]. Mol Pharmacol 67:798–805 [DOI] [PubMed] [Google Scholar]
- Güler et al., 2002.Güler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. (2002) Heat-evoked activation of the ion channel, TRPV4. J Neurosci 22:6408–6414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo et al., 2000.Guo L, Schreiber TH, Weremowicz S, Morton CC, Lee C, Zhou J. (2000) Identification and characterization of a novel polycystin family member, polycystin-L2, in mouse and human: sequence, expression, alternative splicing, and chromosomal localization. Genomics 64:241–251 [DOI] [PubMed] [Google Scholar]
- Hamanaka et al., 2007.Hamanaka K, Jian MY, Weber DS, Alvarez DF, Townsley MI, Al-Mehdi AB, King JA, Liedtke W, Parker JC. (2007) TRPV4 initiates the acute calcium-dependent permeability increase during ventilator-induced lung injury in isolated mouse lungs. Am J Physiol Lung Cell Mol Physiol 293:L923–L932 [DOI] [PubMed] [Google Scholar]
- Hanaoka et al., 2000.Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. (2000) Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature 408:990–994 [DOI] [PubMed] [Google Scholar]
- Hara et al., 2010.Hara K, Kokubo Y, Ishiura H, Fukuda Y, Miyashita A, Kuwano R, Sasaki R, Goto J, Nishizawa M, Kuzuhara S, et al. (2010) TRPM7 is not associated with amyotrophic lateral sclerosis-parkinsonism dementia complex in the Kii peninsula of Japan. Am J Med Genet B Neuropsychiatr Genet 153B:310–313 [DOI] [PubMed] [Google Scholar]
- Hara et al., 2002.Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, et al. (2002) LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell 9:163–173 [DOI] [PubMed] [Google Scholar]
- Hartmann et al., 2008.Hartmann J, Dragicevic E, Adelsberger H, Henning HA, Sumser M, Abramowitz J, Blum R, Dietrich A, Freichel M, Flockerzi V, et al. (2008) TRPC3 channels are required for synaptic transmission and motor coordination. Neuron 59:392–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell et al., 1998.Helliwell RJ, McLatchie LM, Clarke M, Winter J, Bevan S, McIntyre P. (1998) Capsaicin sensitivity is associated with the expression of the vanilloid (capsaicin) receptor (VR1) mRNA in adult rat sensory ganglia. Neurosci Lett 250:177–180 [DOI] [PubMed] [Google Scholar]
- Hermosura et al., 2008.Hermosura MC, Cui AM, Go RC, Davenport B, Shetler CM, Heizer JW, Schmitz C, Mocz G, Garruto RM, Perraud AL. (2008) Altered functional properties of a TRPM2 variant in Guamanian ALS and PD. Proc Natl Acad Sci USA 105:18029–18034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermosura et al., 2005.Hermosura MC, Nayakanti H, Dorovkov MV, Calderon FR, Ryazanov AG, Haymer DS, Garruto RM. (2005) A TRPM7 variant shows altered sensitivity to magnesium that may contribute to the pathogenesis of two Guamanian neurodegenerative disorders. Proc Natl Acad Sci USA 102:11510–11515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill et al., 2004.Hill K, Benham CD, McNulty S, Randall AD. (2004) Flufenamic acid is a pH-dependent antagonist of TRPM2 channels. Neuropharmacology 47:450–460 [DOI] [PubMed] [Google Scholar]
- Hinman et al., 2006.Hinman A, Chuang HH, Bautista DM, Julius D. (2006) TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA 103:19564–19568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirnet et al., 2003.Hirnet D, Olausson J, Fecher-Trost C, Bödding M, Nastainczyk W, Wissenbach U, Flockerzi V, Freichel M. (2003) The TRPV6 gene, cDNA and protein. Cell Calcium 33:509–518 [DOI] [PubMed] [Google Scholar]
- Hisanaga et al., 2009.Hisanaga E, Nagasawa M, Ueki K, Kulkarni RN, Mori M, Kojima I. (2009) Regulation of calcium-permeable TRPV2 channel by insulin in pancreatic beta-cells. Diabetes 58:174–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisatsune et al., 2004.Hisatsune C, Kuroda Y, Nakamura K, Inoue T, Nakamura T, Michikawa T, Mizutani A, Mikoshiba K. (2004) Regulation of TRPC6 channel activity by tyrosine phosphorylation. J Biol Chem 279:18887–18894 [DOI] [PubMed] [Google Scholar]
- Hoenderop et al., 1999.Hoenderop JG, van der Kemp AW, Hartog A, van de Graaf SF, van Os CH, Willems PH, Bindels RJ. (1999) Molecular identification of the apical Ca2+ channel in 1, 25-dihydroxyvitamin D3-responsive epithelia. J Biol Chem 274:8375–8378 [DOI] [PubMed] [Google Scholar]
- Hoenderop et al., 2003.Hoenderop JG, van Leeuwen JP, van der Eerden BC, Kersten FF, van der Kemp AW, Mérillat AM, Waarsing JH, Rossier BC, Vallon V, Hummler E, et al. (2003) Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J Clin Invest 112:1906–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenderop et al., 2001.Hoenderop JG, Vennekens R, Müller D, Prenen J, Droogmans G, Bindels RJ, Nilius B. (2001) Function and expression of the epithelial Ca(2+) channel family: comparison of mammalian ECaC1 and 2. J Physiol 537:747–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann et al., 2003.Hofmann T, Chubanov V, Gudermann T, Montell C. (2003) TRPM5 is a voltage-modulated and Ca(2+)-activated monovalent selective cation channel. Curr Biol 13:1153–1158 [DOI] [PubMed] [Google Scholar]
- Hofmann et al., 1999.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. (1999) Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397:259–263 [DOI] [PubMed] [Google Scholar]
- Hofmann et al., 2000.Hofmann T, Schaefer M, Schultz G, Gudermann T. (2000) Cloning, expression and subcellular localization of two novel splice variants of mouse transient receptor potential channel 2. Biochem J 351:115–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard and Bechstedt, 2004.Howard J, Bechstedt S. (2004) Hypothesis: a helix of ankyrin repeats of the NOMPC-TRP ion channel is the gating spring of mechanoreceptors. Curr Biol 14:R224–R226 [DOI] [PubMed] [Google Scholar]
- Huang et al., 2006.Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Tränkner D, Ryba NJ, Zuker CS. (2006) The cells and logic for mammalian sour taste detection. Nature 442:934–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang et al., 2002.Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros TJ, Krey JF, Chu CJ, et al. (2002) An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci USA 99:8400–8405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang et al., 2008.Huang SM, Lee H, Chung MK, Park U, Yu YY, Bradshaw HB, Coulombe PA, Walker JM, Caterina MJ. (2008) Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2. J Neurosci 28:13727–13737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang et al., 2000.Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, et al. (2000) Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci USA 97:6155–6160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida et al., 2003.Iida T, Moriyama T, Kobata K, Morita A, Murayama N, Hashizume S, Fushiki T, Yazawa S, Watanabe T, Tominaga M. (2003) TRPV1 activation and induction of nociceptive response by a non-pungent capsaicin-like compound, capsiate. Neuropharmacology 44:958–967 [DOI] [PubMed] [Google Scholar]
- Imura et al., 2007.Imura K, Yoshioka T, Hikita I, Tsukahara K, Hirasawa T, Higashino K, Gahara Y, Arimura A, Sakata T. (2007) Influence of TRPV3 mutation on hair growth cycle in mice. Biochem Biophys Res Commun 363:479–483 [DOI] [PubMed] [Google Scholar]
- Inoue et al., 2001.Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. (2001) The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha(1)-adrenoceptor-activated Ca2+-permeable cation channel. Circ Res 88:325–332 [DOI] [PubMed] [Google Scholar]
- Ishimaru et al., 2006.Ishimaru Y, Inada H, Kubota M, Zhuang H, Tominaga M, Matsunami H. (2006) Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc Natl Acad Sci USA 103:12569–12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata et al., 2003.Iwata Y, Katanosaka Y, Arai Y, Komamura K, Miyatake K, Shigekawa M. (2003) A novel mechanism of myocyte degeneration involving the Ca2+-permeable growth factor-regulated channel. J Cell Biol 161:957–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata et al., 2009.Iwata Y, Katanosaka Y, Arai Y, Shigekawa M, Wakabayashi S. (2009) Dominant-negative inhibition of Ca2+ influx via TRPV2 ameliorates muscular dystrophy in animal models. Hum Mol Genet 18:824–834 [DOI] [PubMed] [Google Scholar]
- Jia et al., 2007.Jia Y, Zhou J, Tai Y, Wang Y. (2007) TRPC channels promote cerebellar granule neuron survival. Nat Neurosci 10:559–567 [DOI] [PubMed] [Google Scholar]
- Jiang et al., 2005.Jiang J, Li M, Yue L. (2005) Potentiation of TRPM7 inward currents by protons. J Gen Physiol 126:137–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin et al., 2008.Jin J, Desai BN, Navarro B, Donovan A, Andrews NC, Clapham DE. (2008) Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science 322:756–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson et al., 2009.Johnson CD, Melanaphy D, Purse A, Stokesberry SA, Dickson P, Zholos AV. (2009) Transient receptor potential melastatin 8 channel involvement in the regulation of vascular tone. Am J Physiol Heart Circ Physiol 296:H1868–H1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt et al., 2004.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Högestätt ED, Meng ID, Julius D. (2004) Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427:260–265 [DOI] [PubMed] [Google Scholar]
- Jordt et al., 2000.Jordt SE, Tominaga M, Julius D. (2000) Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci USA 97:8134–8139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungnickel et al., 2001.Jungnickel MK, Marrero H, Birnbaumer L, Lémos JR, Florman HM. (2001) Trp2 regulates entry of Ca2+ into mouse sperm triggered by egg ZP3. Nat Cell Biol 3:499–502 [DOI] [PubMed] [Google Scholar]
- Kaneko et al., 2006.Kaneko S, Kawakami S, Hara Y, Wakamori M, Itoh E, Minami T, Takada Y, Kume T, Katsuki H, Mori Y, et al. (2006) A critical role of TRPM2 in neuronal cell death by hydrogen peroxide. J Pharmacol Sci 101:66–76 [DOI] [PubMed] [Google Scholar]
- Kanzaki et al., 1999.Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I. (1999) Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol 1:165–170 [DOI] [PubMed] [Google Scholar]
- Karacsonyi et al., 2007.Karacsonyi C, Miguel AS, Puertollano R. (2007) Mucolipin-2 localizes to the Arf6-associated pathway and regulates recycling of GPI-APs. Traffic 8:1404–1414 [DOI] [PubMed] [Google Scholar]
- Karashima et al., 2007.Karashima Y, Damann N, Prenen J, Talavera K, Segal A, Voets T, Nilius B. (2007) Bimodal action of menthol on the transient receptor potential channel TRPA1. J Neurosci 27:9874–9884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karashima et al., 2009.Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, Nilius B, Voets T. (2009) TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci USA 106:1273–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaske et al., 2007.Kaske S, Krasteva G, König P, Kummer W, Hofmann T, Gudermann T, Chubanov V. (2007) TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci 8:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al., 2008a.Kim AY, Tang Z, Liu Q, Patel KN, Maag D, Geng Y, Dong X. (2008a) Pirt, a phosphoinositide-binding protein, functions as a regulatory subunit of TRPV1. Cell 133:475–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al., 2007.Kim HJ, Li Q, Tjon-Kon-Sang S, So I, Kiselyov K, Muallem S. (2007) Gain-of-function mutation in TRPML3 causes the mouse Varitint-Waddler phenotype. J Biol Chem 282:36138–36142 [DOI] [PubMed] [Google Scholar]
- Kim et al., 2008b.Kim HJ, Li Q, Tjon-Kon-Sang S, So I, Kiselyov K, Soyombo AA, Muallem S. (2008b) A novel mode of TRPML3 regulation by extracytosolic pH absent in the varitint-waddler phenotype. EMBO J 27:1197–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al., 2009.Kim HJ, Soyombo AA, Tjon-Kon-Sang S, So I, Muallem S. (2009) The Ca(2+) channel TRPML3 regulates membrane trafficking and autophagy. Traffic 10:1157–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al., 2006.Kim JY, Zeng W, Kiselyov K, Yuan JP, Dehoff MH, Mikoshiba K, Worley PF, Muallem S. (2006) Homer 1 mediates store- and inositol 1,4,5-trisphosphate receptor-dependent translocation and retrieval of TRPC3 to the plasma membrane. J Biol Chem 281:32540–32549 [DOI] [PubMed] [Google Scholar]
- Kim et al., 2003.Kim SJ, Kim YS, Yuan JP, Petralia RS, Worley PF, Linden DJ. (2003) Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature 426:285–291 [DOI] [PubMed] [Google Scholar]
- Kimchi et al., 2007.Kimchi T, Xu J, Dulac C. (2007) A functional circuit underlying male sexual behaviour in the female mouse brain. Nature 448:1009–1014 [DOI] [PubMed] [Google Scholar]
- Kiselyov et al., 2005.Kiselyov K, Chen J, Rbaibi Y, Oberdick D, Tjon-Kon-Sang S, Shcheynikov N, Muallem S, Soyombo A. (2005) TRP-ML1 is a lysosomal monovalent cation channel that undergoes proteolytic cleavage. J Biol Chem 280:43218–43223 [DOI] [PubMed] [Google Scholar]
- Klausen et al., 2009.Klausen TK, Pagani A, Minassi A, Ech-Chahad A, Prenen J, Owsianik G, Hoffmann EK, Pedersen SF, Appendino G, Nilius B. (2009) Modulation of the transient receptor potential vanilloid channel TRPV4 by 4alpha-phorbol esters: a structure-activity study. J Med Chem 52:2933–2939 [DOI] [PubMed] [Google Scholar]
- Koike et al., 2010.Koike C, Obara T, Uriu Y, Numata T, Sanuki R, Miyata K, Koyasu T, Ueno S, Funabiki K, Tani A, et al. (2010) TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc Natl Acad Sci USA 107:332–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokrashvili et al., 2009a.Kokrashvili Z, Mosinger B, Margolskee RF. (2009a) Taste signaling elements expressed in gut enteroendocrine cells regulate nutrient-responsive secretion of gut hormones. Am J Clin Nutr 90:822S–825S [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokrashvili et al., 2009b.Kokrashvili Z, Rodriguez D, Yevshayeva V, Zhou H, Margolskee RF, Mosinger B. (2009b) Release of endogenous opioids from duodenal enteroendocrine cells requires Trpm5. Gastroenterology 137:598–606, 606.e1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köttgen et al., 2005.Köttgen M, Benzing T, Simmen T, Tauber R, Buchholz B, Feliciangeli S, Huber TB, Schermer B, Kramer-Zucker A, Höpker K, et al. (2005) Trafficking of TRPP2 by PACS proteins represents a novel mechanism of ion channel regulation. EMBO J 24:705–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulen et al., 2002.Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S. (2002) Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol 4:191–197 [DOI] [PubMed] [Google Scholar]
- Kowase et al., 2002.Kowase T, Nakazato Y, Yoko-O H, Morikawa A, Kojima I. (2002) Immunohistochemical localization of growth factor-regulated channel (GRC) in human tissues. Endocr J 49:349–355 [DOI] [PubMed] [Google Scholar]
- Kraft and Harteneck, 2005.Kraft R, Harteneck C. (2005) The mammalian melastatin-related transient receptor potential cation channels: an overview. Pflugers Arch 451:204–211 [DOI] [PubMed] [Google Scholar]
- Krakow et al., 2009.Krakow D, Vriens J, Camacho N, Luong P, Deixler H, Funari TL, Bacino CA, Irons MB, Holm IA, Sadler L, et al. (2009) Mutations in the gene encoding the calcium-permeable ion channel TRPV4 produce spondylometaphyseal dysplasia, Kozlowski type and metatropic dysplasia. Am J Hum Genet 84:307–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapivinsky et al., 2006.Krapivinsky G, Mochida S, Krapivinsky L, Cibulsky SM, Clapham DE. (2006) The TRPM7 ion channel functions in cholinergic synaptic vesicles and affects transmitter release. Neuron 52:485–496 [DOI] [PubMed] [Google Scholar]
- Kremeyer et al., 2010.Kremeyer B, Lopera F, Cox JJ, Momin A, Rugiero F, Marsh S, Woods CG, Jones NG, Paterson KJ, Fricker FR, et al. (2010) A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron 66:671–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse et al., 2009.Kruse M, Schulze-Bahr E, Corfield V, Beckmann A, Stallmeyer B, Kurtbay G, Ohmert I, Schulze-Bahr E, Brink P, Pongs O. (2009) Impaired endocytosis of the ion channel TRPM4 is associated with human progressive familial heart block type I. J Clin Invest 119:2737–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuniba et al., 2009.Kuniba H, Yoshiura K, Kondoh T, Ohashi H, Kurosawa K, Tonoki H, Nagai T, Okamoto N, Kato M, Fukushima Y, et al. (2009) Molecular karyotyping in 17 patients and mutation screening in 41 patients with Kabuki syndrome. J Hum Genet 54:304–309 [DOI] [PubMed] [Google Scholar]
- Kuwahara et al., 2006.Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA, Olson EN. (2006) TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest 116:3114–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan et al., 2004.Kwan HY, Huang Y, Yao X. (2004) Regulation of canonical transient receptor potential isoform 3 (TRPC3) channel by protein kinase G. Proc Natl Acad Sci USA 101:2625–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan et al., 2006.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. (2006) TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50:277–289 [DOI] [PubMed] [Google Scholar]
- Kwan et al., 2009.Kwan KY, Glazer JM, Corey DP, Rice FL, Stucky CL. (2009) TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J Neurosci 29:4808–4819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon et al., 2007.Kwon Y, Hofmann T, Montell C. (2007) Integration of phosphoinositide- and calmodulin-mediated regulation of TRPC6. Mol Cell 25:491–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers et al., 2006.Lambers TT, Mahieu F, Oancea E, Hoofd L, de Lange F, Mensenkamp AR, Voets T, Nilius B, Clapham DE, Hoenderop JG, et al. (2006) Calbindin-D28K dynamically controls TRPV5-mediated Ca2+ transport. EMBO J 25:2978–2988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers et al., 2004.Lambers TT, Weidema AF, Nilius B, Hoenderop JG, Bindels RJ. (2004) Regulation of the mouse epithelial Ca2(+) channel TRPV6 by the Ca(2+)-sensor calmodulin. J Biol Chem 279:28855–28861 [DOI] [PubMed] [Google Scholar]
- Landouré et al., 2010.Landouré G, Zdebik AA, Martinez TL, Burnett BG, Stanescu HC, Inada H, Shi Y, Taye AA, Kong L, Munns CH, et al. (2010) Mutations in TRPV4 cause Charcot-Marie-Tooth disease type 2C. Nat Genet 42:170–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange et al., 2009.Lange I, Yamamoto S, Partida-Sanchez S, Mori Y, Fleig A, Penner R. (2009) TRPM2 functions as a lysosomal Ca2+-release channel in beta cells. Sci Signal 2:ra23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre et al., 2009.Latorre R, Zaelzer C, Brauchi S. (2009) Structure-functional intimacies of transient receptor potential channels. Q Rev Biophys 42:201–246 [DOI] [PubMed] [Google Scholar]
- Launay et al., 2002.Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. (2002) TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 109:397–407 [DOI] [PubMed] [Google Scholar]
- Lee et al., 2005a.Lee H, Iida T, Mizuno A, Suzuki M, Caterina MJ. (2005a) Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J Neurosci 25:1304–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al., 2005b.Lee J, Cha SK, Sun TJ, Huang CL. (2005b) PIP2 activates TRPV5 and releases its inhibition by intracellular Mg2+. J Gen Physiol 126:439–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leypold et al., 2002.Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. (2002) Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci USA 99:6376–6381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al., 1999.Li HS, Xu XZ, Montell C. (1999) Activation of a TRPC3-dependent cation current through the neurotrophin BDNF. Neuron 24:261–273 [DOI] [PubMed] [Google Scholar]
- Li et al., 2007.Li M, Du J, Jiang J, Ratzan W, Su LT, Runnels LW, Yue L. (2007) Molecular determinants of Mg2+ and Ca2+ permeability and pH sensitivity in TRPM6 and TRPM7. J Biol Chem 282:25817–25830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al., 2006.Li M, Jiang J, Yue L. (2006) Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol 127:525–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al., 2005.Li Y, Jia YC, Cui K, Li N, Zheng ZY, Wang YZ, Yuan XB. (2005) Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature 434:894–898 [DOI] [PubMed] [Google Scholar]
- Li et al., 2009.Li Z, Sergouniotis PI, Michaelides M, Mackay DS, Wright GA, Devery S, Moore AT, Holder GE, Robson AG, Webster AR. (2009) Recessive mutations of the gene TRPM1 abrogate ON bipolar cell function and cause complete congenital stationary night blindness in humans. Am J Hum Genet 85:711–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke et al., 2000.Liedtke W, Choe Y, Martí-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. (2000) Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103:525–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke and Friedman, 2003.Liedtke W, Friedman JM. (2003) Abnormal osmotic regulation in trpv4−/− mice. Proc Natl Acad Sci USA 100:13698–13703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman et al., 1999.Liman ER, Corey DP, Dulac C. (1999) TRP2: a candidate transduction channel for mammalian pheromone sensory signaling. Proc Natl Acad Sci USA 96:5791–5796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link et al., 2010.Link TM, Park U, Vonakis BM, Raben DM, Soloski MJ, Caterina MJ. (2010) TRPV2 has a pivotal role in macrophage particle binding and phagocytosis. Nat Immunol 11:232–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintschinger et al., 2000.Lintschinger B, Balzer-Geldsetzer M, Baskaran T, Graier WF, Romanin C, Zhu MX, Groschner K. (2000) Coassembly of Trp1 and Trp3 proteins generates diacylglycerol- and Ca2+-sensitive cation channels. J Biol Chem 275:27799–27805 [DOI] [PubMed] [Google Scholar]
- Lishko et al., 2007.Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R. (2007) The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron 54:905–918 [DOI] [PubMed] [Google Scholar]
- Liu and Liman, 2003.Liu D, Liman ER. (2003) Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci USA 100:15160–15165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al., 2005.Liu D, Zhang Z, Liman ER. (2005) Extracellular acid block and acid-enhanced inactivation of the Ca2+-activated cation channel TRPM5 involve residues in the S3–S4 and S5–S6 extracellular domains. J Biol Chem 280:20691–20699 [DOI] [PubMed] [Google Scholar]
- Liu et al., 2007.Liu X, Cheng KT, Bandyopadhyay BC, Pani B, Dietrich A, Paria BC, Swaim WD, Beech D, Yildrim E, Singh BB, et al. (2007) Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(−/−) mice. Proc Natl Acad Sci USA 104:17542–17547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LopezJimenez et al., 2006.LopezJimenez ND, Cavenagh MM, Sainz E, Cruz-Ithier MA, Battey JF, Sullivan SL. (2006) Two members of the TRPP family of ion channels, Pkd1l3 and Pkd2l1, are co-expressed in a subset of taste receptor cells. J Neurochem 98:68–77 [DOI] [PubMed] [Google Scholar]
- Lucas et al., 2003.Lucas P, Ukhanov K, Leinders-Zufall T, Zufall F. (2003) A diacylglycerol-gated cation channel in vomeronasal neuron dendrites is impaired in TRPC2 mutant mice: mechanism of pheromone transduction. Neuron 40:551–561 [DOI] [PubMed] [Google Scholar]
- Lyall et al., 2004.Lyall V, Heck GL, Vinnikova AK, Ghosh S, Phan TH, Alam RI, Russell OF, Malik SA, Bigbee JW, DeSimone JA. (2004) The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. J Physiol 558:147–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma et al., 2005.Ma R, Li WP, Rundle D, Kong J, Akbarali HI, Tsiokas L. (2005) PKD2 functions as an epidermal growth factor-activated plasma membrane channel. Mol Cell Biol 25:8285–8298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson et al., 2007a.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. (2007a) Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445:541–545 [DOI] [PubMed] [Google Scholar]
- Macpherson et al., 2005.Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, Patapoutian A. (2005) The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol 15:929–934 [DOI] [PubMed] [Google Scholar]
- Macpherson et al., 2006.Macpherson LJ, Hwang SW, Miyamoto T, Dubin AE, Patapoutian A, Story GM. (2006) More than cool: promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci 32:335–343 [DOI] [PubMed] [Google Scholar]
- Macpherson et al., 2007b.Macpherson LJ, Xiao B, Kwan KY, Petrus MJ, Dubin AE, Hwang S, Cravatt B, Corey DP, Patapoutian A. (2007b) An ion channel essential for sensing chemical damage. J Neurosci 27:11412–11415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maione et al., 2009.Maione S, Cristino L, Migliozzi AL, Georgiou AL, Starowicz K, Salt TE, Di Marzo V. (2009) TRPV1 channels control synaptic plasticity in the developing superior colliculus. J Physiol 587:2521–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandadi et al., 2009.Mandadi S, Sokabe T, Shibasaki K, Katanosaka K, Mizuno A, Moqrich A, Patapoutian A, Fukumi-Tominaga T, Mizumura K, Tominaga M. (2009) TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflugers Arch 458:1093–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni et al., 2004.Manzoni M, Monti E, Bresciani R, Bozzato A, Barlati S, Bassi MT, Borsani G. (2004) Overexpression of wild-type and mutant mucolipin proteins in mammalian cells: effects on the late endocytic compartment organization. FEBS Lett 567:219–224 [DOI] [PubMed] [Google Scholar]
- Maroto et al., 2005.Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. (2005) TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol 7:179–185 [DOI] [PubMed] [Google Scholar]
- Massullo et al., 2006.Massullo P, Sumoza-Toledo A, Bhagat H, Partida-Sánchez S. (2006) TRPM channels, calcium and redox sensors during innate immune responses. Semin Cell Dev Biol 17:654–666 [DOI] [PubMed] [Google Scholar]
- Masuyama et al., 2008.Masuyama R, Vriens J, Voets T, Karashima Y, Owsianik G, Vennekens R, Lieben L, Torrekens S, Moermans K, Vanden Bosch A, et al. (2008) TRPV4-mediated calcium influx regulates terminal differentiation of osteoclasts. Cell Metab 8:257–265 [DOI] [PubMed] [Google Scholar]
- Matsushita et al., 2005.Matsushita M, Kozak JA, Shimizu Y, McLachlin DT, Yamaguchi H, Wei FY, Tomizawa K, Matsui H, Chait BT, Cahalan MD, et al. (2005) Channel function is dissociated from the intrinsic kinase activity and autophosphorylation of TRPM7/ChaK1. J Biol Chem 280:20793–20803 [DOI] [PubMed] [Google Scholar]
- McGrath et al., 2003.McGrath J, Somlo S, Makova S, Tian X, Brueckner M. (2003) Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell 114:61–73 [DOI] [PubMed] [Google Scholar]
- McKemy et al., 2002.McKemy DD, Neuhausser WM, Julius D. (2002) Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416:52–58 [DOI] [PubMed] [Google Scholar]
- McNamara et al., 2007.McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, et al. (2007) TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA 104:13525–13530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillin et al., 2006.McQuillin A, Bass NJ, Kalsi G, Lawrence J, Puri V, Choudhury K, Detera-Wadleigh SD, Curtis D, Gurling HM. (2006) Fine mapping of a susceptibility locus for bipolar and genetically related unipolar affective disorders, to a region containing the C21ORF29 and TRPM2 genes on chromosome 21q22.3. Mol Psychiatry 11:134–142 [DOI] [PubMed] [Google Scholar]
- Mizoguchi et al., 2008.Mizoguchi F, Mizuno A, Hayata T, Nakashima K, Heller S, Ushida T, Sokabe M, Miyasaka N, Suzuki M, Ezura Y, et al. (2008) Transient receptor potential vanilloid 4 deficiency suppresses unloading-induced bone loss. J Cell Physiol 216:47–53 [DOI] [PubMed] [Google Scholar]
- Mizuno et al., 2003.Mizuno A, Matsumoto N, Imai M, Suzuki M. (2003) Impaired osmotic sensation in mice lacking TRPV4. Am J Physiol Cell Physiol 285:C96–C101 [DOI] [PubMed] [Google Scholar]
- Mochizuki et al., 1996.Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, et al. (1996) PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272:1339–1342 [DOI] [PubMed] [Google Scholar]
- Monteilh-Zoller et al., 2003.Monteilh-Zoller MK, Hermosura MC, Nadler MJ, Scharenberg AM, Penner R, Fleig A. (2003) TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol 121:49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell, 2006.Montell C. (2006) An exciting release on TRPM7. Neuron 52:395–397 [DOI] [PubMed] [Google Scholar]
- Moqrich et al., 2005.Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, Patapoutian A. (2005) Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 307:1468–1472 [DOI] [PubMed] [Google Scholar]
- Moran et al., 2004.Moran MM, Xu H, Clapham DE. (2004) TRP ion channels in the nervous system. Curr Opin Neurobiol 14:362–369 [DOI] [PubMed] [Google Scholar]
- Moreau et al., 2002.Moreau R, Daoud G, Bernatchez R, Simoneau L, Masse A, Lafond J. (2002) Calcium uptake and calcium transporter expression by trophoblast cells from human term placenta. Biochim Biophys Acta 1564:325–332 [DOI] [PubMed] [Google Scholar]
- Morgans et al., 2009.Morgans CW, Zhang J, Jeffrey BG, Nelson SM, Burke NS, Duvoisin RM, Brown RL. (2009) TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc Natl Acad Sci USA 106:19174–19178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsch et al., 2003.Munsch T, Freichel M, Flockerzi V, Pape HC. (2003) Contribution of transient receptor potential channels to the control of GABA release from dendrites. Proc Natl Acad Sci USA 100:16065–16070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler et al., 2001.Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, et al. (2001) LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature 411:590–595 [DOI] [PubMed] [Google Scholar]
- Nagasawa et al., 2007.Nagasawa M, Nakagawa Y, Tanaka S, Kojima I. (2007) Chemotactic peptide fMetLeuPhe induces translocation of the TRPV2 channel in macrophages. J Cell Physiol 210:692–702 [DOI] [PubMed] [Google Scholar]
- Nagata et al., 2005.Nagata K, Duggan A, Kumar G, García-Añoveros J. (2005) Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci 25:4052–4061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama et al., 2006.Nakayama H, Wilkin BJ, Bodi I, Molkentin JD. (2006) Calcineurin-dependent cardiomyopathy is activated by TRPC in the adult mouse heart. Faseb J 20:1660–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauli et al., 2008.Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J. (2008) Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation 117:1161–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper et al., 2007.Neeper MP, Liu Y, Hutchinson TL, Wang Y, Flores CM, Qin N. (2007) Activation properties of heterologously expressed mammalian TRPV2: evidence for species dependence. J Biol Chem 282:15894–15902 [DOI] [PubMed] [Google Scholar]
- Niemeyer et al., 2001.Niemeyer BA, Bergs C, Wissenbach U, Flockerzi V, Trost C. (2001) Competitive regulation of CaT-like-mediated Ca2+ entry by protein kinase C and calmodulin. Proc Natl Acad Sci USA 98:3600–3605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijenhuis et al., 2003.Nijenhuis T, Hoenderop JG, van der Kemp AW, Bindels RJ. (2003) Localization and regulation of the epithelial Ca2+ channel TRPV6 in the kidney. J Am Soc Nephrol 14:2731–2740 [DOI] [PubMed] [Google Scholar]
- Nilius et al., 2006.Nilius B, Mahieu F, Prenen J, Janssens A, Owsianik G, Vennekens R, Voets T. (2006) The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. EMBO J 25:467–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius and Owsianik, 2010.Nilius B, Owsianik G. (2010) Transient receptor potential channelopathies. Pflugers Arch 460:437–450 [DOI] [PubMed] [Google Scholar]
- Nilius et al., 2003.Nilius B, Prenen J, Droogmans G, Voets T, Vennekens R, Freichel M, Wissenbach U, Flockerzi V. (2003) Voltage dependence of the Ca2+-activated cation channel TRPM4. J Biol Chem 278:30813–30820 [DOI] [PubMed] [Google Scholar]
- Nilius et al., 2002.Nilius B, Prenen J, Hoenderop JG, Vennekens R, Hoefs S, Weidema AF, Droogmans G, Bindels RJ. (2002) Fast and slow inactivation kinetics of the Ca2+ channels ECaC1 and ECaC2 (TRPV5 and TRPV6). Role of the intracellular loop located between transmembrane segments 2 and 3. J Biol Chem 277:30852–30858 [DOI] [PubMed] [Google Scholar]
- Nilius et al., 2005.Nilius B, Prenen J, Tang J, Wang C, Owsianik G, Janssens A, Voets T, Zhu MX. (2005) Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. J Biol Chem 280:6423–6433 [DOI] [PubMed] [Google Scholar]
- Nilius et al., 2001.Nilius B, Prenen J, Vennekens R, Hoenderop JG, Bindels RJ, Droogmans G. (2001) Modulation of the epithelial calcium channel, ECaC, by intracellular Ca2+. Cell Calcium 29:417–428 [DOI] [PubMed] [Google Scholar]
- Nilius et al., 2004.Nilius B, Prenen J, Voets T, Droogmans G. (2004) Intracellular nucleotides and polyamines inhibit the Ca2+-activated cation channel TRPM4b. Pflugers Arch 448:70–75 [DOI] [PubMed] [Google Scholar]
- Nilius et al., 2000.Nilius B, Vennekens R, Prenen J, Hoenderop JG, Bindels RJ, Droogmans G. (2000) Whole-cell and single channel monovalent cation currents through the novel rabbit epithelial Ca2+ channel ECaC. J Physiol 527 (Pt 2):239–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura et al., 1998.Nomura H, Turco AE, Pei Y, Kalaydjieva L, Schiavello T, Weremowicz S, Ji W, Morton CC, Meisler M, Reeders ST, et al. (1998) Identification of PKDL, a novel polycystic kidney disease 2-like gene whose murine homologue is deleted in mice with kidney and retinal defects. J Biol Chem 273:25967–25973 [DOI] [PubMed] [Google Scholar]
- Numazaki et al., 2003.Numazaki M, Tominaga T, Takeuchi K, Murayama N, Toyooka H, Tominaga M. (2003) Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc Natl Acad Sci USA 100:8002–8006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oancea et al., 2009.Oancea E, Vriens J, Brauchi S, Jun J, Splawski I, Clapham DE. (2009) TRPM1 forms ion channels associated with melanin content in melanocytes. Sci Signal 2:ra21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oancea et al., 2006.Oancea E, Wolfe JT, Clapham DE. (2006) Functional TRPM7 channels accumulate at the plasma membrane in response to fluid flow. Circ Res 98:245–253 [DOI] [PubMed] [Google Scholar]
- Oberwinkler et al., 2005.Oberwinkler J, Lis A, Giehl KM, Flockerzi V, Philipp SE. (2005) Alternative splicing switches the divalent cation selectivity of TRPM3 channels. J Biol Chem 280:22540–22548 [DOI] [PubMed] [Google Scholar]
- Obukhov and Nowycky, 2008.Obukhov AG, Nowycky MC. (2008) TRPC5 channels undergo changes in gating properties during the activation-deactivation cycle. J Cell Physiol 216:162–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada et al., 1999.Okada T, Inoue R, Yamazaki K, Maeda A, Kurosaki T, Yamakuni T, Tanaka I, Shimizu S, Ikenaka K, Imoto K, et al. (1999) Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. Ca2+-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. J Biol Chem 274:27359–27370 [DOI] [PubMed] [Google Scholar]
- Okada et al., 1998.Okada T, Shimizu S, Wakamori M, Maeda A, Kurosaki T, Takada N, Imoto K, Mori Y. (1998) Molecular cloning and functional characterization of a novel receptor-activated TRP Ca2+ channel from mouse brain. J Biol Chem 273:10279–10287 [DOI] [PubMed] [Google Scholar]
- Olah et al., 2009.Olah ME, Jackson MF, Li H, Perez Y, Sun HS, Kiyonaka S, Mori Y, Tymianski M, MacDonald JF. (2009) Ca2+-dependent induction of TRPM2 currents in hippocampal neurons. J Physiol 587:965–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordaz et al., 2005.Ordaz B, Tang J, Xiao R, Salgado A, Sampieri A, Zhu MX, Vaca L. (2005) Calmodulin and calcium interplay in the modulation of TRPC5 channel activity. Identification of a novel C-terminal domain for calcium/calmodulin-mediated facilitation. J Biol Chem 280:30788–30796 [DOI] [PubMed] [Google Scholar]
- Otsuguro et al., 2008.Otsuguro K, Tang J, Tang Y, Xiao R, Freichel M, Tsvilovskyy V, Ito S, Flockerzi V, Zhu MX, Zholos AV. (2008) Isoform-specific inhibition of TRPC4 channel by phosphatidylinositol 4,5-bisphosphate. J Biol Chem 283:10026–10036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peier et al., 2002a.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, et al. (2002a) A TRP channel that senses cold stimuli and menthol. Cell 108:705–715 [DOI] [PubMed] [Google Scholar]
- Peier et al., 2002b.Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, et al. (2002b) A heat-sensitive TRP channel expressed in keratinocytes. Science 296:2046–2049 [DOI] [PubMed] [Google Scholar]
- Peng et al., 1999.Peng JB, Chen XZ, Berger UV, Vassilev PM, Tsukaguchi H, Brown EM, Hediger MA. (1999) Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. J Biol Chem 274:22739–22746 [DOI] [PubMed] [Google Scholar]
- Peng et al., 2000.Peng JB, Chen XZ, Berger UV, Weremowicz S, Morton CC, Vassilev PM, Brown EM, Hediger MA. (2000) Human calcium transport protein CaT1. Biochem Biophys Res Commun 278:326–332 [DOI] [PubMed] [Google Scholar]
- Pérez et al., 2002.Pérez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF. (2002) A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci 5:1169–1176 [DOI] [PubMed] [Google Scholar]
- Perraud et al., 2001.Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R, et al. (2001) ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 411:595–599 [DOI] [PubMed] [Google Scholar]
- Perraud et al., 2005.Perraud AL, Takanishi CL, Shen B, Kang S, Smith MK, Schmitz C, Knowles HM, Ferraris D, Li W, Zhang J, et al. (2005) Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J Biol Chem 280:6138–6148 [DOI] [PubMed] [Google Scholar]
- Peters and Mayer, 1998.Peters C, Mayer A. (1998) Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature 396:575–580 [DOI] [PubMed] [Google Scholar]
- Peters et al., 1993.Peters DJ, Spruit L, Saris JJ, Ravine D, Sandkuijl LA, Fossdal R, Boersma J, van Eijk R, Nørby S, Constantinou-Deltas CD. (1993) Chromosome 4 localization of a second gene for autosomal dominant polycystic kidney disease. Nat Genet 5:359–362 [DOI] [PubMed] [Google Scholar]
- Phelps et al., 2010.Phelps CB, Wang RR, Choo SS, Gaudet R. (2010) Differential regulation of TRPV1, TRPV3, and TRPV4 sensitivity through a conserved binding site on the ankyrin repeat domain. J Biol Chem 285:731–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prawitt et al., 2003.Prawitt D, Monteilh-Zoller MK, Brixel L, Spangenberg C, Zabel B, Fleig A, Penner R. (2003) TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i. Proc Natl Acad Sci USA 100:15166–15171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar and Ahern, 2000.Premkumar LS, Ahern GP. (2000) Induction of vanilloid receptor channel activity by protein kinase C. Nature 408:985–990 [DOI] [PubMed] [Google Scholar]
- Qian et al., 1997.Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, Germino GG. (1997) PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet 16:179–183 [DOI] [PubMed] [Google Scholar]
- Ramsey et al., 2006.Ramsey IS, Delling M, Clapham DE. (2006) An introduction to TRP channels. Annu Rev Physiol 68:619–647 [DOI] [PubMed] [Google Scholar]
- Reading and Brayden, 2007.Reading SA, Brayden JE. (2007) Central role of TRPM4 channels in cerebral blood flow regulation. Stroke 38:2322–2328 [DOI] [PubMed] [Google Scholar]
- Reiser et al., 2005.Reiser J, Polu KR, Möller CC, Kenlan P, Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C, et al. (2005) TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet 37:739–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio et al., 2009.Riccio A, Li Y, Moon J, Kim KS, Smith KS, Rudolph U, Gapon S, Yao GL, Tsvetkov E, Rodig SJ, et al. (2009) Essential role for TRPC5 in amygdala function and fear-related behavior. Cell 137:761–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock et al., 2008.Rock MJ, Prenen J, Funari VA, Funari TL, Merriman B, Nelson SF, Lachman RS, Wilcox WR, Reyno S, Quadrelli R, et al. (2008) Gain-of-function mutations in TRPV4 cause autosomal dominant brachyolmia. Nat Genet 40:999–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohács et al., 2005.Rohács T, Lopes CM, Michailidis I, Logothetis DE. (2005) PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci 8:626–634 [DOI] [PubMed] [Google Scholar]
- Rohacs and Nilius, 2007.Rohacs T, Nilius B. (2007) Regulation of transient receptor potential (TRP) channels by phosphoinositides. Pflugers Arch 455:157–168 [DOI] [PubMed] [Google Scholar]
- Romanovsky et al., 2009.Romanovsky AA, Almeida MC, Garami A, Steiner AA, Norman MH, Morrison SF, Nakamura K, Burmeister JJ, Nucci TB. (2009) The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol Rev 61:228–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong et al., 2004.Rong W, Hillsley K, Davis JB, Hicks G, Winchester WJ, Grundy D. (2004) Jejunal afferent nerve sensitivity in wild-type and TRPV1 knockout mice. J Physiol 560:867–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum et al., 2004.Rosenbaum T, Gordon-Shaag A, Munari M, Gordon SE. (2004) Ca2+/calmodulin modulates TRPV1 activation by capsaicin. J Gen Physiol 123:53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnels et al., 2001.Runnels LW, Yue L, Clapham DE. (2001) TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 291:1043–1047 [DOI] [PubMed] [Google Scholar]
- Runnels et al., 2002.Runnels LW, Yue L, Clapham DE. (2002) The TRPM7 channel is inactivated by PIP(2) hydrolysis. Nat Cell Biol 4:329–336 [DOI] [PubMed] [Google Scholar]
- Saito et al., 2007.Saito M, Hanson PI, Schlesinger P. (2007) Luminal chloride-dependent activation of endosome calcium channels: patch clamp study of enlarged endosomes. J Biol Chem 282:27327–27333 [DOI] [PubMed] [Google Scholar]
- Samie et al., 2009.Samie MA, Grimm C, Evans JA, Curcio-Morelli C, Heller S, Slaugenhaupt SA, Cuajungco MP. (2009) The tissue-specific expression of TRPML2 (MCOLN-2) gene is influenced by the presence of TRPML1. Pflugers Arch 459:79–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano et al., 2001.Sano Y, Inamura K, Miyake A, Mochizuki S, Yokoi H, Matsushime H, Furuichi K. (2001) Immunocyte Ca2+ influx system mediated by LTRPC2. Science 293:1327–1330 [DOI] [PubMed] [Google Scholar]
- Satoh et al., 2007.Satoh S, Tanaka H, Ueda Y, Oyama J, Sugano M, Sumimoto H, Mori Y, Makino N. (2007) Transient receptor potential (TRP) protein 7 acts as a G protein-activated Ca2+ channel mediating angiotensin II-induced myocardial apoptosis. Mol Cell Biochem 294:205–215 [DOI] [PubMed] [Google Scholar]
- Sawada et al., 2007.Sawada Y, Hosokawa H, Hori A, Matsumura K, Kobayashi S. (2007) Cold sensitivity of recombinant TRPA1 channels. Brain Res 1160:39–46 [DOI] [PubMed] [Google Scholar]
- Schaefer et al., 2000.Schaefer M, Plant TD, Obukhov AG, Hofmann T, Gudermann T, Schultz G. (2000) Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J Biol Chem 275:17517–17526 [DOI] [PubMed] [Google Scholar]
- Schlingmann and Gudermann, 2005.Schlingmann KP, Gudermann T. (2005) A critical role of TRPM channel-kinase for human magnesium transport. J Physiol 566:301–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlingmann et al., 2002.Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D, et al. (2002) Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet 31:166–170 [DOI] [PubMed] [Google Scholar]
- Schmidt et al., 2009.Schmidt M, Dubin AE, Petrus MJ, Earley TJ, Patapoutian A. (2009) Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron 64:498–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz et al., 2003.Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM. (2003) Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell 114:191–200 [DOI] [PubMed] [Google Scholar]
- Schoeber et al., 2008.Schoeber JP, Topala CN, Lee KP, Lambers TT, Ricard G, van der Kemp AW, Huynen MA, Hoenderop JG, Bindels RJ. (2008) Identification of Nipsnap1 as a novel auxiliary protein inhibiting TRPV6 activity. Pflugers Arch 457:91–101 [DOI] [PubMed] [Google Scholar]
- Schoeber et al., 2006.Schoeber JP, Topala CN, Wang X, Diepens RJ, Lambers TT, Hoenderop JG, Bindels RJ. (2006) RGS2 inhibits the epithelial Ca2+ channel TRPV6. J Biol Chem 281:29669–29674 [DOI] [PubMed] [Google Scholar]
- Sharif-Naeini et al., 2009.Sharif-Naeini R, Folgering JH, Bichet D, Duprat F, Lauritzen I, Arhatte M, Jodar M, Dedman A, Chatelain FC, Schulte U, et al. (2009) Polycystin-1 and -2 dosage regulates pressure sensing. Cell 139:587–596 [DOI] [PubMed] [Google Scholar]
- Shen et al., 2009.Shen Y, Heimel JA, Kamermans M, Peachey NS, Gregg RG, Nawy S. (2009) A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. J Neurosci 29:6088–6093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi et al., 2004.Shi J, Mori E, Mori Y, Mori M, Li J, Ito Y, Inoue R. (2004) Multiple regulation by calcium of murine homologues of transient receptor potential proteins TRPC6 and TRPC7 expressed in HEK293 cells. J Physiol 561:415–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu et al., 2009.Shimizu T, Janssens A, Voets T, Nilius B. (2009) Regulation of the murine TRPP3 channel by voltage, pH, and changes in cell volume. Pflugers Arch 457:795–807 [DOI] [PubMed] [Google Scholar]
- Shumyatsky et al., 2005.Shumyatsky GP, Malleret G, Shin RM, Takizawa S, Tully K, Tsvetkov E, Zakharenko SS, Joseph J, Vronskaya S, Yin D, et al. (2005) stathmin, a gene enriched in the amygdala, controls both learned and innate fear. Cell 123:697–709 [DOI] [PubMed] [Google Scholar]
- Singh et al., 2004.Singh BB, Lockwich TP, Bandyopadhyay BC, Liu X, Bollimuntha S, Brazer SC, Combs C, Das S, Leenders AG, Sheng ZH, et al. (2004) VAMP2-dependent exocytosis regulates plasma membrane insertion of TRPC3 channels and contributes to agonist-stimulated Ca2+ influx. Mol Cell 15:635–646 [DOI] [PubMed] [Google Scholar]
- Slaugenhaupt, 2002.Slaugenhaupt SA. (2002) The molecular basis of mucolipidosis type IV. Curr Mol Med 2:445–450 [DOI] [PubMed] [Google Scholar]
- Smith et al., 2002.Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, et al. (2002) TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 418:186–190 [DOI] [PubMed] [Google Scholar]
- Smith et al., 2006.Smith PL, Maloney KN, Pothen RG, Clardy J, Clapham DE. (2006) Bisandrographolide from Andrographis paniculata activates TRPV4 channels. J Biol Chem 281:29897–29904 [DOI] [PubMed] [Google Scholar]
- Sotomayor et al., 2005.Sotomayor M, Corey DP, Schulten K. (2005) In search of the hair-cell gating spring elastic properties of ankyrin and cadherin repeats. Structure 13:669–682 [DOI] [PubMed] [Google Scholar]
- Steenland et al., 2006.Steenland HW, Ko SW, Wu LJ, Zhuo M. (2006) Hot receptors in the brain. Mol Pain 2:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story et al., 2003.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, et al. (2003) ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112:819–829 [DOI] [PubMed] [Google Scholar]
- Stowers et al., 2002.Stowers L, Holy TE, Meister M, Dulac C, Koentges G. (2002) Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science 295:1493–1500 [DOI] [PubMed] [Google Scholar]
- Strotmann et al., 2000.Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. (2000) OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol 2:695–702 [DOI] [PubMed] [Google Scholar]
- Strotmann et al., 2003.Strotmann R, Schultz G, Plant TD. (2003) Ca2+-dependent potentiation of the nonselective cation channel TRPV4 is mediated by a C-terminal calmodulin binding site. J Biol Chem 278:26541–26549 [DOI] [PubMed] [Google Scholar]
- Strübing et al., 2001.Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE. (2001) TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 29:645–655 [DOI] [PubMed] [Google Scholar]
- Strübing et al., 2003.Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE. (2003) Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem 278:39014–39019 [DOI] [PubMed] [Google Scholar]
- Sugiura et al., 2002.Sugiura T, Tominaga M, Katsuya H, Mizumura K. (2002) Bradykinin lowers the threshold temperature for heat activation of vanilloid receptor 1. J Neurophysiol 88:544–548 [DOI] [PubMed] [Google Scholar]
- Sun et al., 2009.Sun HS, Jackson MF, Martin LJ, Jansen K, Teves L, Cui H, Kiyonaka S, Mori Y, Jones M, Forder JP, et al. (2009) Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat Neurosci 12:1300–1307 [DOI] [PubMed] [Google Scholar]
- Sun et al., 2000.Sun M, Goldin E, Stahl S, Falardeau JL, Kennedy JC, Acierno JS, Jr., Bove C, Kaneski CR, Nagle J, Bromley MC, Colman M, Schiffmann R, Slaugenhaupt SA. (2000) Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum Mol Genet 9:2471–2478 [DOI] [PubMed] [Google Scholar]
- Sutton et al., 2006.Sutton KA, Jungnickel MK, Ward CJ, Harris PC, Florman HM. (2006) Functional characterization of PKDREJ, a male germ cell-restricted polycystin. J Cell Physiol 209:493–500 [DOI] [PubMed] [Google Scholar]
- Suzuki et al., 2000.Suzuki M, Ishibashi K, Ooki G, Tsuruoka S, Imai M. (2000) Electrophysiologic characteristics of the Ca-permeable channels, ECaC and CaT, in the kidney. Biochem Biophys Res Commun 274:344–349 [DOI] [PubMed] [Google Scholar]
- Suzuki et al., 2003.Suzuki M, Mizuno A, Kodaira K, Imai M. (2003) Impaired pressure sensation in mice lacking TRPV4. J Biol Chem 278:22664–22668 [DOI] [PubMed] [Google Scholar]
- Suzuki et al., 2008.Suzuki Y, Kovacs CS, Takanaga H, Peng JB, Landowski CP, Hediger MA. (2008) Calcium channel TRPV6 is involved in murine maternal-fetal calcium transport. J Bone Miner Res 23:1249–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera et al., 2008.Talavera K, Nilius B, Voets T. (2008) Neuronal TRP channels: thermometers, pathfinders and life-savers. Trends Neurosci 31:287–295 [DOI] [PubMed] [Google Scholar]
- Talavera et al., 2005.Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, Margolskee RF, Nilius B. (2005) Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 438:1022–1025 [DOI] [PubMed] [Google Scholar]
- Tang et al., 2000.Tang Y, Tang J, Chen Z, Trost C, Flockerzi V, Li M, Ramesh V, Zhu MX. (2000) Association of mammalian trp4 and phospholipase C isozymes with a PDZ domain-containing protein, NHERF. J Biol Chem 275:37559–37564 [DOI] [PubMed] [Google Scholar]
- Taylor-Clark et al., 2008.Taylor-Clark TE, Undem BJ, Macglashan DW, Jr., Ghatta S, Carr MJ, McAlexander MA. (2008) Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1). Mol Pharmacol 73:274–281 [DOI] [PubMed] [Google Scholar]
- Thorneloe et al., 2008.Thorneloe KS, Sulpizio AC, Lin Z, Figueroa DJ, Clouse AK, McCafferty GP, Chendrimada TP, Lashinger ES, Gordon E, Evans L, et al. (2008) N_-((1_S)-1-{[4-((2_S_)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropa noyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: Part I. J Pharmacol Exp Ther 326:432–442 [DOI] [PubMed] [Google Scholar]
- Thyagarajan et al., 2009.Thyagarajan B, Benn BS, Christakos S, Rohacs T. (2009) Phospholipase C-mediated regulation of transient receptor potential vanilloid 6 channels: implications in active intestinal Ca2+ transport. Mol Pharmacol 75:608–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyagarajan et al., 2008.Thyagarajan B, Lukacs V, Rohacs T. (2008) Hydrolysis of phosphatidylinositol 4,5-bisphosphate mediates calcium-induced inactivation of TRPV6 channels. J Biol Chem 283:14980–14987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiruppathi et al., 2002.Tiruppathi C, Freichel M, Vogel SM, Paria BC, Mehta D, Flockerzi V, Malik AB. (2002) Impairment of store-operated Ca2+ entry in TRPC4(−/−) mice interferes with increase in lung microvascular permeability. Circ Res 91:70–76 [DOI] [PubMed] [Google Scholar]
- Todaka et al., 2004.Todaka H, Taniguchi J, Satoh J, Mizuno A, Suzuki M. (2004) Warm temperature-sensitive transient receptor potential vanilloid 4 (TRPV4) plays an essential role in thermal hyperalgesia. J Biol Chem 279:35133–35138 [DOI] [PubMed] [Google Scholar]
- Togashi et al., 2006.Togashi K, Hara Y, Tominaga T, Higashi T, Konishi Y, Mori Y, Tominaga M. (2006) TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J 25:1804–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga et al., 1998.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. (1998) The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21:531–543 [DOI] [PubMed] [Google Scholar]
- Torra et al., 2000.Torra R, Badenas C, Pérez-Oller L, Luis J, Millán S, Nicolau C, Oppenheimer F, Milà M, Darnell A. (2000) Increased prevalence of polycystic kidney disease type 2 among elderly polycystic patients. Am J Kidney Dis 36:728–734 [DOI] [PubMed] [Google Scholar]
- Trebak, 2010.Trebak M. (2010) The puzzling role of TRPC3 channels in motor coordination. Pflugers Arch 459:369–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebak et al., 2005.Trebak M, Hempel N, Wedel BJ, Smyth JT, Bird GS, Putney JW., Jr (2005) Negative regulation of TRPC3 channels by protein kinase C-mediated phosphorylation of serine 712. Mol Pharmacol 67:558–563 [DOI] [PubMed] [Google Scholar]
- Trebak et al., 2007.Trebak M, Lemonnier L, Smyth JT, Vazquez G, Putney JW., Jr (2007) Phospholipase C-coupled receptors and activation of TRPC channels. Handb Exp Pharmacol (179):593–614 [DOI] [PubMed] [Google Scholar]
- Trebak et al., 2003.Trebak M, St J Bird G, McKay RR, Birnbaumer L, Putney JW., Jr (2003) Signaling mechanism for receptor-activated canonical transient receptor potential 3 (TRPC3) channels. J Biol Chem 278:16244–16252 [DOI] [PubMed] [Google Scholar]
- Trevisani et al., 2007.Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andrè E, Patacchini R, Cottrell GS, et al. (2007) 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA 104:13519–13524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsavaler et al., 2001.Tsavaler L, Shapero MH, Morkowski S, Laus R. (2001) Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res 61:3760–3769 [PubMed] [Google Scholar]
- Tsiokas et al., 1997.Tsiokas L, Kim E, Arnould T, Sukhatme VP, Walz G. (1997) Homo- and heterodimeric interactions between the gene products of PKD1 and PKD2. Proc Natl Acad Sci USA 94:6965–6970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich et al., 2005.Ullrich ND, Voets T, Prenen J, Vennekens R, Talavera K, Droogmans G, Nilius B. (2005) Comparison of functional properties of the Ca2+-activated cation channels TRPM4 and TRPM5 from mice. Cell Calcium 37:267–278 [DOI] [PubMed] [Google Scholar]
- van Aken et al., 2008.van Aken AF, Atiba-Davies M, Marcotti W, Goodyear RJ, Bryant JE, Richardson GP, Noben-Trauth K, Kros CJ. (2008) TRPML3 mutations cause impaired mechano-electrical transduction and depolarization by an inward-rectifier cation current in auditory hair cells of varitint-waddler mice. J Physiol 586:5403–5418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Graaf et al., 2006.van de Graaf SF, van der Kemp AW, van den Berg D, van Oorschot M, Hoenderop JG, Bindels RJ. (2006) Identification of BSPRY as a novel auxiliary protein inhibiting TRPV5 activity. J Am Soc Nephrol 17:26–30 [DOI] [PubMed] [Google Scholar]
- van der Eerden et al., 2005.van der Eerden BC, Hoenderop JG, de Vries TJ, Schoenmaker T, Buurman CJ, Uitterlinden AG, Pols HA, Bindels RJ, van Leeuwen JP. (2005) The epithelial Ca2+ channel TRPV5 is essential for proper osteoclastic bone resorption. Proc Natl Acad Sci USA 102:17507–17512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Genderen et al., 2009.van Genderen MM, Bijveld MM, Claassen YB, Florijn RJ, Pearring JN, Meire FM, McCall MA, Riemslag FC, Gregg RG, Bergen AA, et al. (2009) Mutations in TRPM1 are a common cause of complete congenital stationary night blindness. Am J Hum Genet 85:730–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rossum et al., 2005.van Rossum DB, Patterson RL, Sharma S, Barrow RK, Kornberg M, Gill DL, Snyder SH. (2005) Phospholipase Cgamma1 controls surface expression of TRPC3 through an intermolecular PH domain. Nature 434:99–104 [DOI] [PubMed] [Google Scholar]
- Vannier et al., 1999.Vannier B, Peyton M, Boulay G, Brown D, Qin N, Jiang M, Zhu X, Birnbaumer L. (1999) Mouse trp2, the homologue of the human trpc2 pseudogene, encodes mTrp2, a store depletion-activated capacitative Ca2+ entry channel. Proc Natl Acad Sci USA 96:2060–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev et al., 2001.Vassilev PM, Guo L, Chen XZ, Segal Y, Peng JB, Basora N, Babakhanlou H, Cruger G, Kanazirska M, Ye Cp, et al. (2001) Polycystin-2 is a novel cation channel implicated in defective intracellular Ca2+ homeostasis in polycystic kidney disease. Biochem Biophys Res Commun 282:341–350 [DOI] [PubMed] [Google Scholar]
- Vazquez et al., 2004.Vazquez G, Wedel BJ, Kawasaki BT, Bird GS, Putney JW., Jr. (2004) Obligatory role of Src kinase in the signaling mechanism for TRPC3 cation channels. J Biol Chem 279:40521–40528 [DOI] [PubMed] [Google Scholar]
- Venkatachalam et al., 2008.Venkatachalam K, Long AA, Elsaesser R, Nikolaeva D, Broadie K, Montell C. (2008) Motor deficit in a Drosophila model of mucolipidosis type IV due to defective clearance of apoptotic cells. Cell 135:838–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam and Montell, 2007.Venkatachalam K, Montell C. (2007) TRP channels. Annu Rev Biochem 76:387–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam et al., 2003.Venkatachalam K, Zheng F, Gill DL. (2003) Regulation of canonical transient receptor potential (TRPC) channel function by diacylglycerol and protein kinase C. J Biol Chem 278:29031–29040 [DOI] [PubMed] [Google Scholar]
- Vennekens et al., 2000.Vennekens R, Hoenderop JG, Prenen J, Stuiver M, Willems PH, Droogmans G, Nilius B, Bindels RJ. (2000) Permeation and gating properties of the novel epithelial Ca(2+) channel. J Biol Chem 275:3963–3969 [DOI] [PubMed] [Google Scholar]
- Vennekens et al., 2007.Vennekens R, Olausson J, Meissner M, Bloch W, Mathar I, Philipp SE, Schmitz F, Weissgerber P, Nilius B, Flockerzi V, et al. (2007) Increased IgE-dependent mast cell activation and anaphylactic responses in mice lacking the calcium-activated nonselective cation channel TRPM4. Nat Immunol 8:312–320 [DOI] [PubMed] [Google Scholar]
- Venugopal et al., 2007.Venugopal B, Browning MF, Curcio-Morelli C, Varro A, Michaud N, Nanthakumar N, Walkley SU, Pickel J, Slaugenhaupt SA. (2007) Neurologic, gastric, and opthalmologic pathologies in a murine model of mucolipidosis type IV. Am J Hum Genet 81:1070–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal et al., 2009.Venugopal B, Mesires NT, Kennedy JC, Curcio-Morelli C, Laplante JM, Dice JF, Slaugenhaupt SA. (2009) Chaperone-mediated autophagy is defective in mucolipidosis type IV. J Cell Physiol 219:344–353 [DOI] [PubMed] [Google Scholar]
- Voets et al., 2004a.Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. (2004a) The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 430:748–754 [DOI] [PubMed] [Google Scholar]
- Voets et al., 2003.Voets T, Janssens A, Prenen J, Droogmans G, Nilius B. (2003) Mg2+-dependent gating and strong inward rectification of the cation channel TRPV6. J Gen Physiol 121:245–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets and Nilius, 2007.Voets T, Nilius B. (2007) Modulation of TRPs by PIPs. J Physiol 582:939–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets et al., 2004b.Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, Bindels RJ, Hoenderop JG. (2004b) TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem 279:19–25 [DOI] [PubMed] [Google Scholar]
- Voets et al., 2007.Voets T, Owsianik G, Janssens A, Talavera K, Nilius B. (2007) TRPM8 voltage sensor mutants reveal a mechanism for integrating thermal and chemical stimuli. Nat Chem Biol 3:174–182 [DOI] [PubMed] [Google Scholar]
- Voets et al., 2001.Voets T, Prenen J, Fleig A, Vennekens R, Watanabe H, Hoenderop JG, Bindels RJ, Droogmans G, Penner R, Nilius B. (2001) CaT1 and the calcium release-activated calcium channel manifest distinct pore properties. J Biol Chem 276:47767–47770 [DOI] [PubMed] [Google Scholar]
- Vogel et al., 2010.Vogel P, Read R, Hansen GM, Freay LC, Zambrowicz BP, Sands AT. (2010) Situs inversus and related ciliopathies in Dpcd−/−, Pkd1l1−/− and Nme7−/− mice. Vet Pathol 47:120–131 [DOI] [PubMed] [Google Scholar]
- Vriens et al., 2009.Vriens J, Appendino G, Nilius B. (2009) Pharmacology of vanilloid transient receptor potential cation channels. Mol Pharmacol 75:1262–1279 [DOI] [PubMed] [Google Scholar]
- Vriens et al., 2005.Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, Morisseau C, Hammock BD, Fleming I, Busse R, et al. (2005) Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res 97:908–915 [DOI] [PubMed] [Google Scholar]
- Vriens et al., 2004.Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. (2004) Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci USA 101:396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner et al., 2008.Wagner TF, Loch S, Lambert S, Straub I, Mannebach S, Mathar I, Düfer M, Lis A, Flockerzi V, Philipp SE, et al. (2008) Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat Cell Biol 10:1421–1430 [DOI] [PubMed] [Google Scholar]
- Walder et al., 2002.Walder RY, Landau D, Meyer P, Shalev H, Tsolia M, Borochowitz Z, Boettger MB, Beck GE, Englehardt RK, Carmi R, et al. (2002) Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet 31:171–174 [DOI] [PubMed] [Google Scholar]
- Walder et al., 2009.Walder RY, Yang B, Stokes JB, Kirby PA, Cao X, Shi P, Searby CC, Husted RF, Sheffield VC. (2009) Mice defective in Trpm6 show embryonic mortality and neural tube defects. Hum Mol Genet 18:4367–4375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al., 2008.Wang YY, Chang RB, Waters HN, McKemy DD, Liman ER. (2008) The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J Biol Chem 283:32691–32703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe et al., 2003.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. (2003) Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424:434–438 [DOI] [PubMed] [Google Scholar]
- Watanabe et al., 2002.Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. (2002) Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem 277:47044–47051 [DOI] [PubMed] [Google Scholar]
- Wegierski et al., 2009.Wegierski T, Lewandrowski U, Müller B, Sickmann A, Walz G. (2009) Tyrosine phosphorylation modulates the activity of TRPV4 in response to defined stimuli. J Biol Chem 284:2923–2933 [DOI] [PubMed] [Google Scholar]
- Whitlock, 1995.Whitlock MC. (1995) Two-locus drift with sex chromosomes: the partitioning and conversion of variance in subdivided populations. Theor Popul Biol 48:44–64 [DOI] [PubMed] [Google Scholar]
- Winn et al., 2005.Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, et al. (2005) A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308:1801–1804 [DOI] [PubMed] [Google Scholar]
- Wissenbach et al., 1998.Wissenbach U, Schroth G, Philipp S, Flockerzi V. (1998) Structure and mRNA expression of a bovine trp homologue related to mammalian trp2 transcripts. FEBS Lett 429:61–66 [DOI] [PubMed] [Google Scholar]
- Wu et al., 2000.Wu G, Markowitz GS, Li L, D'Agati VD, Factor SM, Geng L, Tibara S, Tuchman J, Cai Y, Park JH, van Adelsberg J, Hou H, Jr., Kucherlapati R, Edelmann W, Somlo S. (2000) Cardiac defects and renal failure in mice with targeted mutations in Pkd2. Nat Genet 24:75–78 [DOI] [PubMed] [Google Scholar]
- Xu et al., 2006.Xu H, Delling M, Jun JC, Clapham DE. (2006) Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci 9:628–635 [DOI] [PubMed] [Google Scholar]
- Xu et al., 2007.Xu H, Delling M, Li L, Dong X, Clapham DE. (2007) Activating mutation in a mucolipin transient receptor potential channel leads to melanocyte loss in varitint-waddler mice. Proc Natl Acad Sci USA 104:18321–18326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al., 2002.Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, et al. (2002) TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 418:181–186 [DOI] [PubMed] [Google Scholar]
- Xu et al., 2008.Xu SZ, Sukumar P, Zeng F, Li J, Jairaman A, English A, Naylor J, Ciurtin C, Majeed Y, Milligan CJ, et al. (2008) TRPC channel activation by extracellular thioredoxin. Nature 451:69–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al., 2001.Xu XZ, Moebius F, Gill DL, Montell C. (2001) Regulation of melastatin, a TRP-related protein, through interaction with a cytoplasmic isoform. Proc Natl Acad Sci USA 98:10692–10697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi et al., 2001.Yamaguchi H, Matsushita M, Nairn AC, Kuriyan J. (2001) Crystal structure of the atypical protein kinase domain of a TRP channel with phosphotransferase activity. Mol Cell 7:1047–1057 [DOI] [PubMed] [Google Scholar]
- Yamamoto et al., 2008.Yamamoto S, Shimizu S, Kiyonaka S, Takahashi N, Wajima T, Hara Y, Negoro T, Hiroi T, Kiuchi Y, Okada T, et al. (2008) TRPM2-mediated Ca2+influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med 14:738–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida et al., 2006.Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y, Tominaga M, Shimizu S, Sato Y, Mori Y. (2006) Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol 2:596–607 [DOI] [PubMed] [Google Scholar]
- Yu et al., 2005.Yu FH, Yarov-Yarovoy V, Gutman GA, Catterall WA. (2005) Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacol Rev 57:387–395 [DOI] [PubMed] [Google Scholar]
- Yu et al., 2009.Yu Y, Ulbrich MH, Li MH, Buraei Z, Chen XZ, Ong AC, Tong L, Isacoff EY, Yang J. (2009) Structural and molecular basis of the assembly of the TRPP2/PKD1 complex. Proc Natl Acad Sci USA 106:11558–11563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue et al., 2001.Yue L, Peng JB, Hediger MA, Clapham DE. (2001) CaT1 manifests the pore properties of the calcium-release-activated calcium channel. Nature 410:705–709 [DOI] [PubMed] [Google Scholar]
- Zeevi et al., 2009.Zeevi DA, Frumkin A, Offen-Glasner V, Kogot-Levin A, Bach G. (2009) A potentially dynamic lysosomal role for the endogenous TRPML proteins. J Pathol 219:153–162 [DOI] [PubMed] [Google Scholar]
- Zhang et al., 2009.Zhang DX, Mendoza SA, Bubolz AH, Mizuno A, Ge ZD, Li R, Warltier DC, Suzuki M, Gutterman DD. (2009) Transient receptor potential vanilloid type 4-deficient mice exhibit impaired endothelium-dependent relaxation induced by acetylcholine in vitro and in vivo. Hypertension 53:532–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang and Barritt, 2004.Zhang L, Barritt GJ. (2004) Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res 64:8365–8373 [DOI] [PubMed] [Google Scholar]
- Zhang et al., 2003.Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. (2003) Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112:293–301 [DOI] [PubMed] [Google Scholar]
- Zhang et al., 2005.Zhang Z, Okawa H, Wang Y, Liman ER. (2005) Phosphatidylinositol 4,5-bisphosphate rescues TRPM4 channels from desensitization. J Biol Chem 280:39185–39192 [DOI] [PubMed] [Google Scholar]
- Zhou, 2009.Zhou J. (2009) Polycystins and primary cilia: primers for cell cycle progression. Annu Rev Physiol 71:83–113 [DOI] [PubMed] [Google Scholar]
- Zhou et al., 2008.Zhou J, Du W, Zhou K, Tai Y, Yao H, Jia Y, Ding Y, Wang Y. (2008) Critical role of TRPC6 channels in the formation of excitatory synapses. Nat Neurosci 11:741–743 [DOI] [PubMed] [Google Scholar]
- Zhu, 2005.Zhu MX. (2005) Multiple roles of calmodulin and other Ca(2+)-binding proteins in the functional regulation of TRP channels. Pflugers Arch 451:105–115 [DOI] [PubMed] [Google Scholar]
- Zhuang et al., 2002.Zhuang L, Peng JB, Tou L, Takanaga H, Adam RM, Hediger MA, Freeman MR. (2002) Calcium-selective ion channel, CaT1, is apically localized in gastrointestinal tract epithelia and is aberrantly expressed in human malignancies. Lab Invest 82:1755–1764 [DOI] [PubMed] [Google Scholar]
- Zitt et al., 1996.Zitt C, Zobel A, Obukhov AG, Harteneck C, Kalkbrenner F, Lückhoff A, Schultz G. (1996) Cloning and functional expression of a human Ca2+-permeable cation channel activated by calcium store depletion. Neuron 16:1189–1196 [DOI] [PubMed] [Google Scholar]
- Zurborg et al., 2007.Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. (2007) Direct activation of the ion channel TRPA1 by Ca2+. Nat Neurosci 10:277–279 [DOI] [PubMed] [Google Scholar]
- Zygmunt et al., 1999.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Di Marzo V, Julius D, Högestätt ED. (1999) Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400:452–457 [DOI] [PubMed] [Google Scholar]