Molecular Structure, Function, and Dynamics of Clathrin-Mediated Membrane Traffic (original) (raw)
Abstract
Clathrin is a molecular scaffold for vesicular uptake of cargo at the plasma membrane, where its assembly into cage-like lattices underlies the clathrin-coated pits of classical endocytosis. This review describes the structures of clathrin, major cargo adaptors, and other proteins that participate in forming a clathrin-coated pit, loading its contents, pinching off the membrane as a lattice-enclosed vesicle, and recycling the components. It integrates as much of the structural information as possible at the time of writing into a sketch of the principal steps in coated-pit and coated-vesicle formation.
The major route for endocytosis in most cells is mediated by clathrin. Numerous proteins participate in forming a clathrin-coated pit, loading cargo, pinching off the membrane as a vesicle, and recycling the components.
Reorganization of cellular membranes and transport of components from one lipid-bilayer-bounded compartment to another determine much of the internal structure of a cell. Clathrin, a principal molecular scaffold for processes of this kind, forms a lattice-like coat on and around membranes. Its best-studied role is in classical endocytosis—the subject of this collection. It also participates in certain phagocytic events (e.g., internalization of some bacteria). Much of the rest of the membrane-associated molecular apparatus may be different in the two cases. We refer to the clathrin-coated pits and -coated vesicles of classical endocytosis as the “canonical” clathrin pathway and to the clathrin-actin assemblies of phagocytic processes as a “noncanonical” pathway. Clathrin also operates along some intracellular traffic routes, such as transport between the _trans_-Golgi network (TGN) and endosomes. The physiology and the biochemistry of clathrin-scaffolded intracellular events are at present less precisely defined than those of transfers originating at the plasma membrane. This review describes the structure of clathrin-coated vesicles and introduces the major components that interact to organize clathrin-mediated endocytosis. Based on direct observations of processes in the canonical pathway at the plasma membrane, we outline events that occur during the genesis of clathrin-coated pits and vesicles. Many of these processes are discussed in much greater detail in other articles in this collection.
What we now call “clathrin-coated pits” were first observed by Roth and Porter in their studies of yolk-protein uptake by mosquito oocytes (Roth and Porter 1964). They found yolk-containing “bristle-coated” pits and vesicles at the cell surface and “naked” vesicles at more internal positions, sometimes fusing with large storage granules, and they outlined a pathway of invagination, pinching, uncoating, transport, and fusion. Pearse subsequently identified the bristle-coated structures with a particular vesicular fraction isolated from brain, characterized the coat protein, and named it clathrin, because of the cage-like character of its assembled state (Pearse 1976, 1975). The discovery by Anderson, Brown, and Goldstein that low-density lipoprotein (LDL) uptake proceeds through similar vesicles led to a general description of clathrin-mediated endocytosis and to proposals for many of the molecular mechanisms studied subsequently in specific detail (Anderson et al. 1977; Goldstein et al. 1979).
The coated structures isolated from brain vary in diameter from ∼700 to 1000 Å; the endocytic-coated vesicles observed in thin sections of insect or vertebrate epithelial cells and fibroblasts are generally slightly larger (1000–1500 Å). Heuser (1980), using rapid-freeze methods, examined the inner plasma-membrane surface of various types of cells adhering to a substrate and found large (1000–2000 Å in diameter), flat, hexagonal networks of clathrin, in addition to standard coated pits. Aggeler and Werb (1982) found similar extended arrays surrounding latex beads in the course of phagocytic uptake by macrophages. They detected such arrays where the plasma membrane of these cells contacted the substrate, but not elsewhere.
The apparent coexistence of closed or tightly curved structures with open, flat ones led some to suggest that the latter can transform into the former (Heuser 1980). The molecular organization of clathrin lattices shows that such a process would involve too drastic a deconstruction/ reconstruction to be a plausible mechanism (Kirchhausen 2009), and direct observations in living cells now show that canonical coated pits form de novo (Ehrlich et al. 2004; Perrais and Merrifield 2005; Saffarian et al. 2009; Cocucci et al. 2012; Aguet et al. 2013). Association of the larger arrays with distinct cellular processes (some of which may have endocytic function) resolves the confusion (Saffarian et al. 2009). Repeated suggestions in the literature that flat arrays can rearrange into curved lattices ignore the molecular properties of the clathrin trimer and the specificity of contacts between triskelions within a coat (Kirchhausen 2000).
CLATHRIN-REQUIRING PATHWAYS OF LIGAND UPTAKE
The Canonical Pathway
The uptake of transferrin (Tf) by the transferrin receptor (TfR) and of LDL by its receptor (LDLR) has come to define the canonical clathrin-dependent pathway. Early work showed that LDL and epidermal growth factor (Carpentier et al. 1982) or Tf and asialoorosomucoid (Neutra et al. 1985) bound to their respective receptors could occupy the same coated pit and hence that an individual coated structure could engulf multiple species of cargo. A large number of other receptors transit the same route. Sequences (“sorting signals”) in the receptor cytoplasmic tails determine inclusion, and various adaptors, especially the heterotetrameric adaptor complex AP2, bind specifically to these signal segments while also associating with clathrin (Ohno et al. 1995; see also Traub and Bonifacino 2013). Endocytic membrane reuptake after neurotransmitter release from synaptic vesicles appears to follow a closely related mechanism. Clathrin-coated vesicles from this recycling process that have been isolated from mammalian brain are the principal source of material for most biochemical and structural analyses. The presence of brain-specific isoforms of clathrin light chains (Jackson et al. 1987; Kirchhausen et al. 1987), auxilin (Umeda et al. 2000), dynamin (Urrutia et al. 1997), AP180 (Bushlin et al. 2008), and various other proteins of the presynaptic membrane-recycling pathway may reflect features of the clathrin cycle in synapses that are not shared with classical, receptor-mediated endocytosis as studied in epithelial cells and fibroblasts.
Noncanonical Pathways
Invasion of nonphagocytic cells by certain bacteria, such as Listeria, Yersinia, and Salmonella, requires clathrin (Veiga and Cossart 2005; Veiga et al. 2007; Bonazzi et al. 2012; see also Cossart and Helenius 2014). These pathogens enter by a so-called “zippering” mechanism, in which assembly of actin filaments pushes the host-cell membrane outward around the bacterium. The actin recruitment depends on formation of extended clathrin patches, which may resemble the arrays formed in macrophages during ingestion of latex beads or on the ventral surface of cells growing on a glass or plastic surface. Assembly of the actin pedestal organized by noninvasive enteropathogenic Escherichia coli (EPEC) also requires clathrin (Veiga et al. 2007). The direct association of clathrin recruitment with actin assembly is a characteristic of these clathrin-dependent processes that may distinguish them from the canonical endocytic pathway. Although present along with some of its interacting partners such as Abp1, Arp3, and cortactin (Boucrot et al. 2006; Boulant et al. 2011; Taylor et al. 2011), actin is not ordinarily necessary for uptake of transferrin receptor or normal coated-pit dynamics in various cells in culture (Boucrot et al. 2006; Cureton et al. 2010; Boulant et al. 2011). Actin becomes essential, however, if membrane tension or cargo size prevents vesicle closure (Boulant et al. 2011). In yeast cells, in which the clathrin lattice does not close off completely, actin is a constitutive participant in coat maturation (Engqvist-Goldstein and Drubin 2003), as it is for uptake of “coated plaques” in mammalian cells (see below) (Saffarian et al. 2009).
STRUCTURE AND BIOCHEMISTRY OF CLATHRIN-COATED VESICLES
The principal components of coated vesicles isolated from various sources are the heavy and light chains of clathrin and the four subunits of the heterotetrameric “adaptor complexes” (Kirchhausen 2000; Blondeau et al. 2004; Girard et al. 2005; Borner et al. 2006, 2012). The heterotetrameric adaptors link the clathrin coat and the membrane bilayer, and they are the principal cargo-recognition molecules. There are also several more-specialized adaptor proteins, which recognize particular receptors; the specialized adaptors often interact with both clathrin and with the heterotetrameric adaptors, increasing the repertoire of cargo that can be sorted (see Traub and Bonifacino 2013). Additional abundant proteins include components of the uncoating machinery (auxilin-1 and -2 and Hsc70), AP180/CALM, a molecule that recruits VAMP2,7 and 8, RME-6, a Rab5 GEF, and HIP1R (huntingtin-interacting-protein-1 related), an actin regulator that associates with clathrin light chains. Various “nonstructural” proteins that influence proper assembly and budding (e.g., Eps15, epsin, intersectin, and FCHo1/2) are generally excluded from the mature coated vesicle (Tebar et al. 1996; Blondeau et al. 2004; Henne et al. 2010).
Clathrin
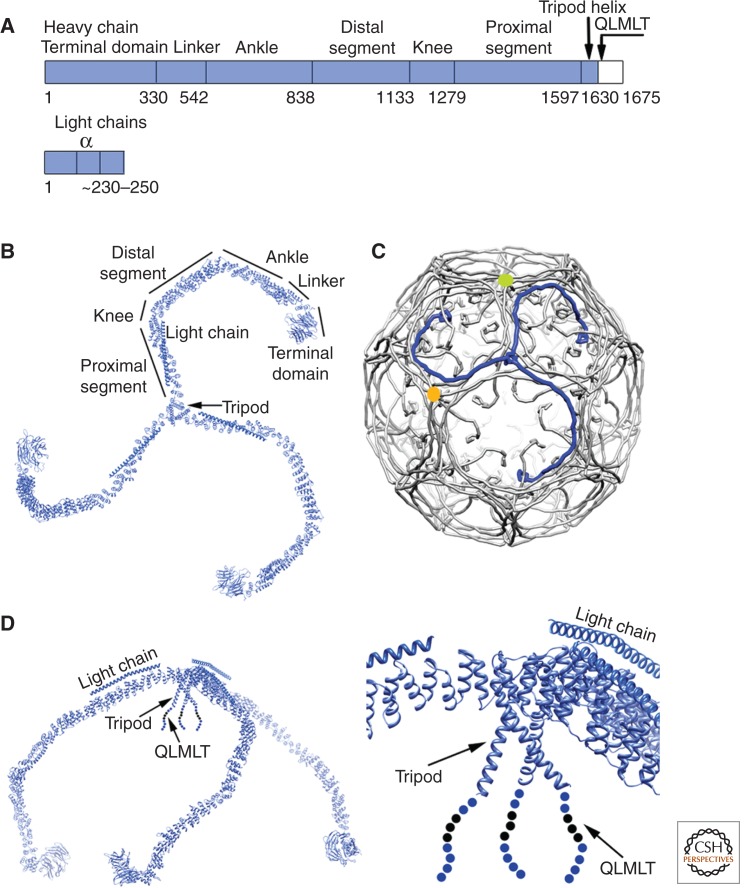
Clathrin is a trimer of three heavy chains, each with an associated light chain (Kirchhausen and Harrison 1981; Ungewickell and Branton 1981). The heavy chain has an amino-terminal, β-propeller domain with seven WD40 repeats, followed in the polypeptide chain by 42 α-helical zig-zags of roughly 30 amino acid residues each, a longer α-helix at the threefold contact, and a 45-residue carboxy-terminal segment with poorly defined structure (Fig. 1) (Fotin et al. 2004b). The carboxy-terminal segment contains a short region with an amino acid sequence that is preferentially recognized by Hsc70 and required for Hsc70-facilitated uncoating (Rapoport et al. 2008; Böcking et al. 2011). The 42 zig-zags that make up the bulk of the polypeptide chain create an extended, gently curved “leg” for the clathrin triskelion (from the Greek for “a three-legged structure”), with the β-propeller “terminal domain” at its tip. The light chains, of which there are two species in mammalian cells, each with alternative splice forms, associate with the threefold-proximal segment of the heavy-chain leg, through a long, central α-helix (Kirchhausen et al. 1987; Kirchhausen and Toyoda 1993; Kirchhausen 2000; Chen et al. 2002; Fotin et al. 2004b; Chen and Brodsky 2005; Wilbur et al. 2008). The amino- and carboxy-terminal light-chain regions are disordered; they may determine physiological partners, as shown by recruitment of HIP1R by the amino-terminal segment of both species (Chen and Brodsky 2005; Newpher et al. 2006).
Figure 1.
Clathrin. (A) Organization of the heavy- and light-chain polypeptide chains. The name or phase designating various segments appears above the corresponding region of the sequence. Human cells have two light chains (LCa and LCb), each with tissue-specific splice variants, yielding the range of lengths shown. There is no detectable preference of one or the other for association with a heavy chain; the central, α-helical segment, which mediates heavy-chain association, is almost completely conserved in all light-chain forms. A second heavy-chain gene in human cells encodes a paralog found in muscle and fat and involved in intracellular traffic, but not in endocytosis; it does not bind light chains. (B, left) Full α-carbon (Cα) representation of a clathrin triskelion, viewed along its threefold axis as if from the outside of a coat. The various segments of the heavy chain are labeled, and the central α-helical region of the light chain on each leg is also shown. (C) Packing of triskelions in a clathrin coat. The structure shown is that of a “D6 barrel,” one of the simplest and smallest coated-vesicle lattices. Each triskelion is drawn as a “worm” extending from the hook-like representation of the terminal domain on the inside of the shell to the vertex of the triskelion at the outside. (Blue) One triskelion, in an orientation similar to the one in B. (D, left) Side view of the triskelion, in the same representation and scale as in B. (Right) Blowup of the part of a triskelion close to the threefold axis, including the tripod and the disordered, carboxy-terminal segment with a site (QLMLT) for Hsc70 binding. (From Xing et al. 2010; adapted, with permission, from the authors.)
The Clathrin Lattice
Purified clathrin can reassemble (at pH < 6.5) into empty, coat-like structures called “cages” (Kirchhausen and Harrison 1981). The presence of AP2 or a fragment of one of its components enhances in vitro reassembly and allows it to occur at neutral pH, by forming small amorphous aggregates, around which clathrin assembles stably into “in vitro-assembled coats” (or, where clear from context, just “coats”) (Keen et al. 1991; Smith et al. 1998; Musacchio et al. 1999; Fotin et al. 2004b). The enhancement requires the β-chain hinge region (Shih et al. 1995), which protrudes from the AP2 adaptor core and hence projects from a small aggregate of AP2 protein and has a short motif that binds the amino-terminal domain of the clathrin heavy chain (see below). Various other proteins that bind and cluster clathrin can have the same effect.
When clathrin assembles, it forms a lattice in which the center of a triskelion is associated with each lattice point (Fig. 1B). The contour length of a leg is such that it reaches around nearly three edges, spiraling clockwise inward (if we follow it from hub to terminal domain) as it goes. If all the openings were hexagonal, the lattice would be an essentially flat array. The way the legs associate when forming coats or cages incorporates pentagonal openings as well as hexagonal ones. Exactly 12 pentagons are required for a closed shell, if all the other openings are hexagons. If some are heptagonal, then there must be a corresponding excess of pentagons. The number of hexagonal openings and the distribution of pentagonal openings among them define the size and shape of the overall structure.
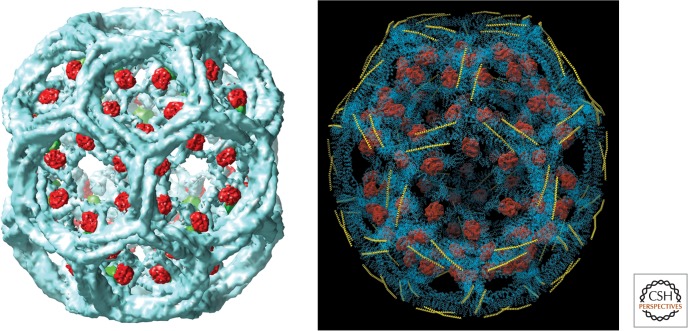
Assembly of coats under controlled conditions in vitro gives selective yield of a few, simple lattices, which can then be analyzed in molecular detail by single-particle cryoelectron microscopy (cryoEM) (Smith et al. 1998; Musacchio et al. 1999; Fotin et al. 2004b). Coats with one of these lattices, a barrel-like structure with D6 symmetry, are particularly regular, and the images have yielded a three-dimensional (3D) reconstruction with details extending to a resolution of ∼8 Å (Fig. 2A). The crystallographically derived structures and homology models (in the case of some of the repeats) can be positioned accurately within this reconstruction, and the interactions of various component triskelions can be examined. The pattern of interdigitating legs (Fig. 1B) is such that we can imagine the principal building block for each edge of the lattice to be a segment of “proximal leg” closely associated on its inward-facing surface with a segment of “distal” leg (Fig. 2B). (For nomenclature, see Fig. 1; “distal” and “proximal” refer to distance from the threefold center of the triskelion, so that “distal” is amino terminal to “proximal” in the polypeptide chain sequence.) The distal leg segment in an edge, which runs parallel to its associated proximal segment, belongs to a triskelion centered at a second-nearest-neighbor lattice point. The geometry of these interactions appears to be conserved at all edges, even when those edges are not related to each other by the D6 symmetry. The proximodistal contacts are also conserved in small-diameter coats. Thus, we believe that they make a principal contribution to coat assembly and stability. In contrast, the side-by-side contacts between antiparallel proximal segments and antiparallel distal segments along an edge appear quite tenuous, and the crossing angle between proximodistal pairs varies depending on the overall curvature of the lattice.
Figure 2.
A D6 clathrin coat. (A) Map from electron microscopy of a coat with bound auxilin (clathrin-binding and J-domain fragment) and Hsc70, showing the outer contour of clathrin density (blue), auxilin (red), and Hsc70 (green). (From Xing et al. 2010; with permission from the authors.) (B) Full Cα representation of the heavy chains in a coat (blue), with ribbon representation of light chain σ-helical region (yellow) and volume outline of auxilin clathrin-binding and J-domain fragment (red) (Fotin et al. 2004a,b).
Near the center of a clathrin trimer, each heavy chain projects inward as a long α-helix, followed by the less-ordered, carboxy-terminal segment. The three long helices (one from each heavy chain) splay apart from a set of close contacts at the outer radii, so that they form a tripod-like structure that holds the trimer together and links it noncovalently to more distal parts of other trimers that run beneath it. The “knee” between the proximal and distal legs curves gently enough to avoid the region immediately beneath the center of a neighboring triskelion, so the helical tripod can penetrate inward to contact the “ankles” of triskelions centered on second-nearest-neighbor lattice points, creating an extremely long-range pattern of interactions (Fig. 1B). Only the proximodistal contacts described in the preceding paragraph have extensive surface complementarity, however, and this “spot welding” of the extensively interwoven triskelions is probably one of the properties of the lattice that allows its rapid assembly and disassembly.
Clathrin triskelions can form sharply curved, small coats and more extended, flat arrays—a polymorphism that requires considerable flexibility. The pucker at the center of a triskelion appears to be relatively invariant, however, and bendability is instead a property of the α-zig-zag organization of most of the leg. Because only adjacent helices contact each other in this type of structure and because helices can “roll” against each other to some extent without substantially distorting the side-chain packing, iterated α-zig-zags produce readily bendable (compliant) rods. In assembled clathrin lattices, variation in curvature is concentrated in the knee regions and in the linker between the terminal domain and ankle, because the proximal and distal segments reinforce each other where they form strong contacts. Even within the D6 barrel structure, a range of different knee bends is present. The relatively narrow distribution of curvatures in spontaneously assembled cages in vitro indicates that the triskelion is domed at its vertex, with a preferred arching angle slightly more open than in a D6 barrel. Clathrin-coated “buds” formed on large unilamellar liposomes in vitro have an outer diameter of ∼900 Å, similar to the mean diameter of membrane-free cages. The wider range of diameters for cargo-incorporating coated pits in vivo shows that the legs can adapt to the nature of the surface against which the lattice is forming.
The terminal domain, protruding inward from the clathrin lattice, is free to contact adaptors and other membrane-interacting components (ter Haar et al. 1998, 2000; Drake et al. 2000; Drake and Traub 2001; Miele et al. 2004). It projects toward the axis of a vertex three lattice points removed from the one on which its triskelion is centered (Fig. 2A). Although the α-zig-zag linker connecting it to the main part of the clathrin leg is relatively stiff, the “fuzziness” of the terminal domain in the cryoEM reconstruction indicates some variability in its position with respect to the lattice as a whole, probably because of flexibility in the ankle regions that connect terminal domains to distal legs. The terminal domain interacts with heterotetrameric adaptors through a peptide motif in the long, flexible “hinge” of the β-chain having the consensus sequence LΦXΦD/E (“clathrin box”; Φ represents a hydrophobic residue) (Shih et al. 1995; ter Haar et al. 2000). Clathrin-box peptides bind in a groove between blades 1 and 2 of the terminal-domain β-propeller (ter Haar et al. 2000; Miele et al. 2004). Additional clathrin-binding motifs include PWXXW, which is present in about 25 residues carboxy terminal to a clathrin box in amphiphysin and Snx9 and binds to a nonoverlapping site on the “top” face of the β-propeller (Miele et al. 2004).
The clathrin light chains have no marked influence on coat assembly in vitro or coated vesicle formation in vivo although the 100 Å-long helical region might be expected to stiffen the proximal leg (Fig. 1). The amino-terminal, nonhelical region lies close to the midpoint of an edge; the carboxy-terminal, nonhelical region is close to a vertex.
Coated Vesicles
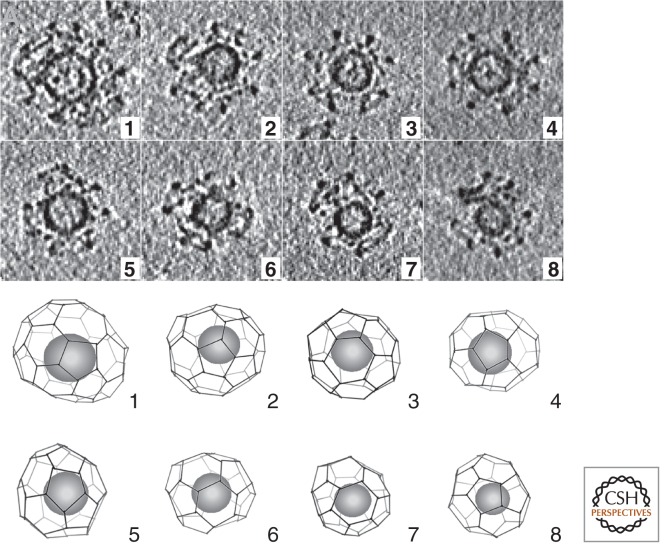
Coated vesicles isolated from cells or tissues are substantially more heterogeneous in size and shape than the coats assembled for cryoEM studies (Cheng et al. 2007; Heymann et al. 2013). Tomographic reconstructions of individual coated vesicles illustrate the variety of lattices present in a preparation from bovine brain (Fig. 3) (Cheng et al. 2007). Two of the coats in Figure 3 have a heptagonal opening in the lattice and hence 13 pentagonal ones. Most of these coated vesicles probably represent intermediates in presynaptic membrane uptake. Their clathrin shells average 700–800 Å in diameter, contain about 35–40 triskelions, and enclose a spherical vesicle, ∼400 Å in diameter, somewhat eccentrically placed. There are strong bridges of electron density between the coat and the vesicle on the side of closer approach—they are most likely caused by the bulky ∼300-kDa heterotetrameric AP2 adaptors, about 20–25 of which are present (on average) in each coat and which many proteomic studies have identified as the second most common CCV protein after clathrin itself. The asymmetry probably reflects the polarity of budding—adaptors arrive selectively during early phases of coat assembly as studied in cells in culture (see below). The gap between coat and vesicle can accommodate not only the various adaptors, but also the many other regulatory proteins that participate in cargo sorting, budding, and uncoating (see next section). Substantially larger coated vesicles can be seen in thin sections of non-neuronal cells taking up ligands, such as transferrin or LDL, that have receptors with large extracellular domains; most coated vesicles isolated from human placenta, for instance, range between 1000 and 1350 Å in diameter (Turkewitz and Harrison 1989).
Figure 3.
Coated vesicles, isolated from brain and examined by electron cryotomography. (Top) Gallery of central sections through tomograms of individual coated vesicles. (Bottom) Drawing of corresponding lattices, with position of vesicle shown (Cheng et al. 2007).
Nonclathrin Protein Components in the Coat
Nonclathrin proteins in a coated pit include clathrin adaptors, which bind both membrane and clathrin and link the coat with the lipid bilayer, and nonstructural proteins, which bind either clathrin, adaptors and/or the membrane. Nearly all the nonclathrin protein components have a membrane-proximal, folded domain, frequently with a membrane-interacting site or surface, and one or more segments of unstructured polypeptide chain. Some also have additional folded protein–protein interaction domains. The unstructured segments contain arrays of short, linear motifs that bind folded domains of other coated-vesicle proteins, generally with moderate affinity, allowing formation of dynamic networks. Clathrin coat assembly at the plasma membrane requires both recruitment of clathrin by lipid-associated adaptor proteins and cooperativity of lattice interactions. The nonstructural proteins, collectively termed endocytic accessory proteins, ensure efficient coated-pit assembly, cargo recruitment, and prompt coat disassembly after budding (see Traub and Bonifacino 2013; Merrifield and Kaksonen 2014).
Heterotetrameric Clathrin Adaptors
The heterotetrameric adaptors are the most abundant nonclathrin components of tissue- and cell-derived coated vesicles (Fig. 4) (for review, see Kirchhausen 1999; Robinson and Bonifacino 2001; Hirst et al. 2013). Vertebrate genomes encode five types, designated AP1 through AP5 (the last with a relatively divergent sequence and thus found only recently) (Hirst et al. 2013). AP2 is the principal type associated with traffic at the plasma membrane and thus with clathrin-mediated endocytosis; AP1 and AP3 appear to have roles in intracellular, clathrin-driven events (e.g., in traffic between the TGN and endosomes); AP4 and AP5 may not be coated-vesicle associated. The adaptors have two distinct, but homologous large chains (β1–5; α, γ, ∂, ε, and ς in AP1 through AP5, respectively); a medium chain (µ1–5); and a small chain (σ1–5).
Figure 4.
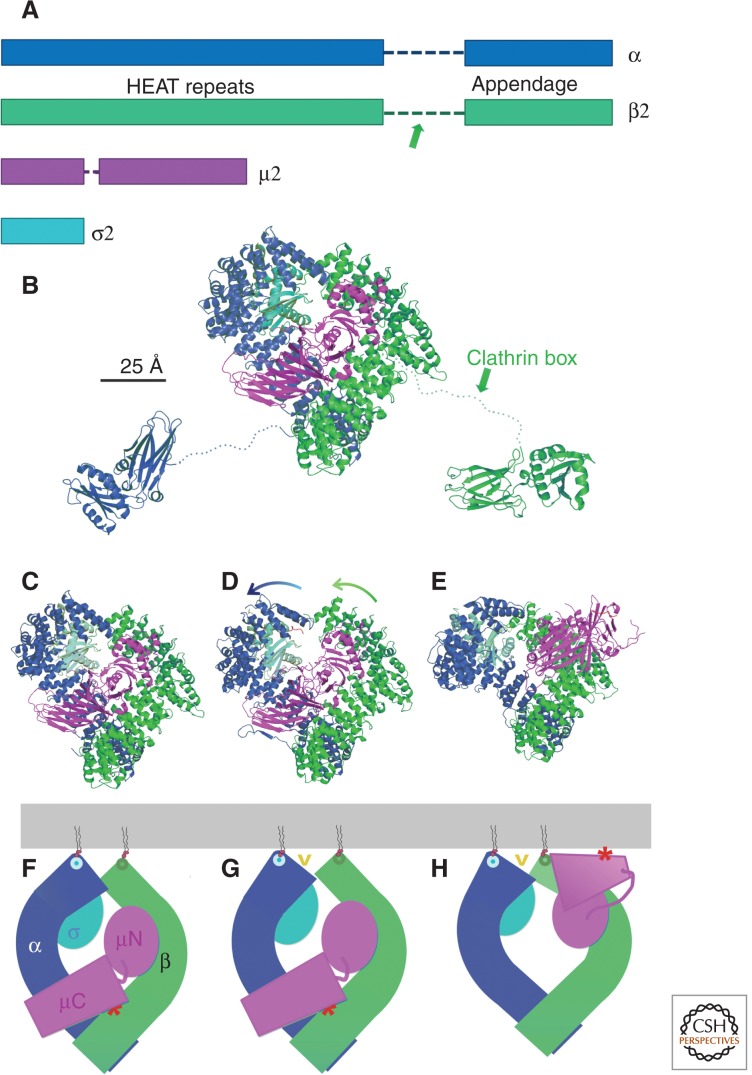
Heterotetrameric clathrin adaptor, AP2. (A) Domain organization of polypeptide chains of the four component proteins. The two heavy chains, α and β2, have amino-terminal HEAT-repeat domains connected by a flexible hinge (dashed line) to a carboxy-terminal appendage domain; their total length is about 940 amino acid residues. The arrow in the β2 diagram shows the approximate position of the clathrin-box motif that binds the clathrin terminal domain. The lengths of the bars are in approximate proportion to the number of residues in the corresponding domain. (B) Ribbon representation of the complete adaptor in its “locked” state. The flexible hinge regions of the heavy chains are shown as dotted lines. Colors as in A. (PDB: 2VGL, 1B9K, 1E42) (C–E) Ribbon representations of the AP2 “core” comprising the HEAT-repeat domains of the heavy chains and the complete µ and σ chains. The “locked” (PDB 2VGL) (Collins et al. 2002), “unlatched” (PDB:2JKR) (Kelly et al. 2008), and “open” (PDB:2XA7) (Jackson et al. 2010) conformations are in C, D, and E, respectively. Arrows in D show directions in which the heat-domain domains bend (bend concentrated mainly at the elbow) to achieve the open conformation, in which the μC domain also rotates substantially. (F–H) Diagrams corresponding to the molecular representations above them. (Gray bar) Membrane, with PtdIns(4,5)P2 (schematic). (Circles on heavy chains) Sites for PtdIns(4,5)P2 headgroup binding. Yellow “v” in G and H points to the position of the site for dileucine motif binding; (red asterisk on µ2-C domain) position of site for tyrosine-motif binding. Note that lipid-headgroup and cargo-recognition sites all line up near the membrane in this conformation.
Each large chain of AP2 and AP1 has a compactly folded amino-terminal region (roughly 60%–65% of the polypeptide chain), composed of a set of 14 “HEAT repeats,” α-helical zig-zags closely related to the clathrin zig-zag (Collins et al. 2002; Heldwein et al. 2004). The repeats form two gently curved arcs of seven zig-zags each, with a more sharply curved bend between the two halves, and the two chains are so disposed that they define a diamond-like frame that encloses the small (σ) chain and the amino-terminal part of the medium (µ) chain. These last two structures are homologous to each other. The entire assembly (referred to as the adaptor “bowl” because of the concavity on one side, that receives the carboxy-terminal part of the µ chain in one conformational state) comprises the initial about 600 residues of the two large chains, the amino-terminal domain of the µ-chain (µN), and the entire σ-chain. The bowl has an approximate twofold character, with the long diagonal of the diamond-shaped frame as the pseudo-twofold axis (Fig. 4D–I). An extended hinge segment of about 80–100 residues connects the stacked HEAT-repeats to an “appendage” domain at the carboxy-terminal end of each of the large chains (Fig. 4B,C) (Heuser and Keen 1988).
The heterotetramers of AP2 and AP1 have at least two distinct states—a closed or “locked” state and one or more open states (Rapoport et al. 1997; Collins et al. 2002; Jackson et al. 2010). In the closed state, two well-studied cargo-binding sites are buried (Fig. 4D,G); in at least one open state, both cargo-binding sites are exposed (Fig. 4F,I). AP2 also has also been trapped by crystal packing in an intermediate, “unlatched” state, in which only one of the cargo-binding sites is accessible (Fig. 4E,H). In the locked state, the diamond-shaped frame is closed at both ends of the long axis, although the contact between the two large chains is considerably more intimate at the carboxy-terminal end of the HEAT-repeats than at the amino-terminal end, and the unpaired, carboxy-terminal domain of the medium chain (µ2C)—a bilobed β-sandwich—rests in the bowl (Fig. 4D,G). In the open state, the amino-terminal halves of the large-chain HEAT-repeat regions bend away from each other, opening up one end of the diamond frame and displacing the µ2C domain outward (Fig. 4F,I). The µ2N domain and the σ2 chain also move away from each other, conserving their contacts with the amino-terminal parts of the β2 and α, respectively. The hinge between the two µ-chain domains reconfigures in the transition between locked and open conformations, allowing µ2C to rotate into a position aligned with the open end of the bowl. Packing of µ2C against σ2 and β2 probably makes this position a minimum energy conformation, but µ2C may sample the local environment tethered by the linker that connects it to μ2N, thereby increasing the likelihood that it will encounter and bind cargoes.
Association between AP2 and the plasma membrane requires phosphatidylinositol-4,5-bis-phosphate [PtdIns(4,5)P2]. Interaction of the phospholipid head group with basic patches on the amino-terminal ends of each of the large chains positions the heterotetramer with respect to the membrane (Fig. 4G). In the open state, μ2C also presents positively charged patches that probably interact with the negatively charged polar groups on the cytoplasmic side of the membrane bilayer, further stabilizing AP2 on the membrane. The two principal cargo-incorporation signals that AP2 recognizes are the so-called “tyrosine-based motif” YXXΦ (where Φ is a large, hydrophobic residue), found on the cytoplasmic tail of many endocytosed receptors including TfR, and the “acidic dileucine motif” [ED]xxxL[LI]. The site for the former motif is on the edge of µ2C (Fig. 4G–I) (Owen and Evans 1998). The pocket that receives the tyrosine, occluded in the closed states of AP2 and AP1, faces outward in the AP2 open state and aligns roughly with the PtdIns(4,5)P2 contact points. The site for the latter motif is mainly on μ2, just where it abuts the α-chain, and that position also aligns with the lipid-head-group contacts. Thus, the open state is compatible with all known membrane interactions of the adaptor. Transition to the open state is apparently favored not only by binding to PtdIns(4,5)P2 at multiple sites but also by binding to phosphoinositides phosphorylated at the 3′ position (Rapoport et al. 1997), by the interaction with clathrin (Matsui and Kirchhausen 1990; Rapoport et al. 1997), and by phosphorylation of Thr156, in the µ2 linker, by the clathrin-activated, α-subunit appendage domain-binding protein kinase AAK1 (Ricotta et al. 2002; Conner et al. 2003). The clathrin-box motif in the β-chain hinge is the principal site of interaction between heterotetrameric adaptors and clathrin (Fig. 4A) (Shih et al. 1995; Traub et al. 1999; Owen et al. 2000). The 80–100 residues of this flexible tether can easily span the gap of ∼100 Å between a clathrin lattice and the lipid bilayer.
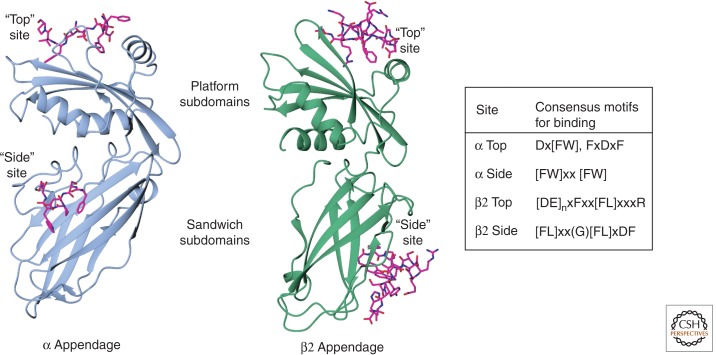
Heterotetrameric adaptors recruit a great variety of additional components, through contacts with their bilobed appendage domains (Fig. 5) (Owen et al. 1999, 2000; Traub et al. 1999; Praefcke et al. 2004; Ritter et al. 2004; Schmid et al. 2006; Schmid and McMahon 2007; Keyel et al. 2008). Each of the appendages has binding sites for sequence motifs found in the more than 20 known regulatory/accessory proteins (Praefcke et al. 2004; Schmid et al. 2006; Keyel et al. 2008). There is a binding site on each of the two subdomains of the α and β appendages; each of these sites recognizes a different motif (for examples, see Fig. 5).
Figure 5.
AP2 appendages. Ribbon representations of α (blue) and β2 (green) appendages with top site and side site cognate peptides (magenta stick representation). The motifs recognized are shown in the table to the right.
Other Adaptors
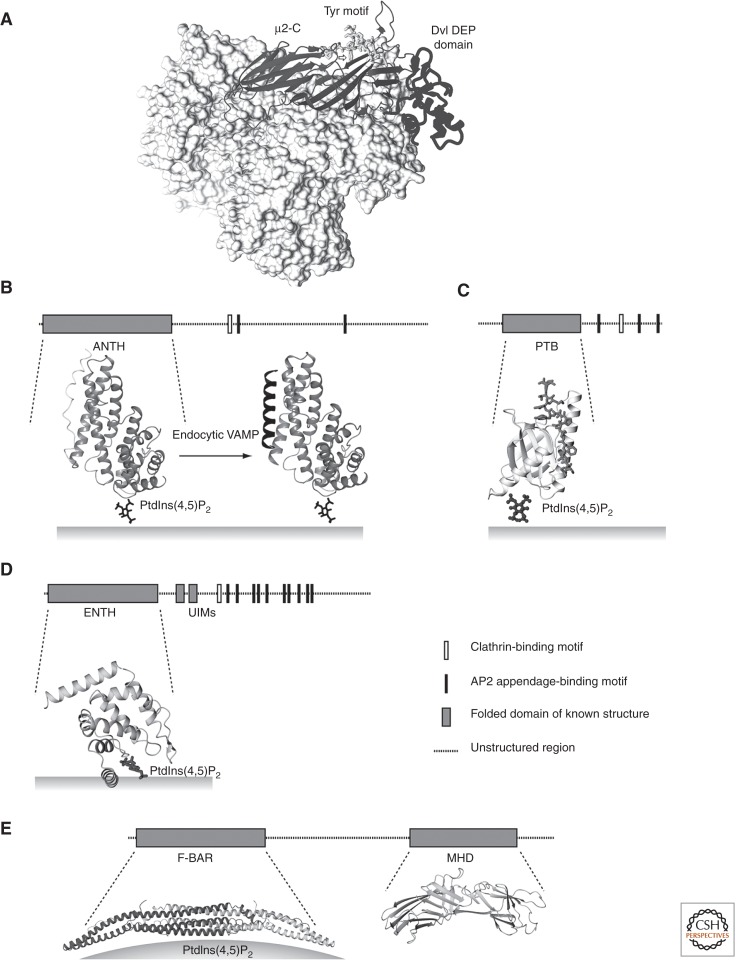
There are several other adaptors that interact directly or indirectly with clathrin and select specific types of cargo for incorporation into coated pits. These include the PTB-domain-containing proteins ARH, Dab2, and Fe65, which bind FxNPxY-motif-containing transmembrane proteins such as the LDL receptor, and members of the arrestin family, which associate with G-protein-coupled receptors (see Traub and Bonifacino 2013). A particularly abundant accessory protein in brain-coated vesicles is AP180, a 90-kDa species; its paralog in other tissues is known as CALM. Both have the ∼280 residue, AP180-amino-terminal-homology (ANTH) domain—a set of five α-helical, HEAT-repeat-like zig-zags of different length (Fig. 5) (Ford et al. 2001; Mao et al. 2001). The loops within the first and second zig-zags create a site for binding the head group of PtdIns(4,5)P2. The carboxy-terminal half of the molecules contains a clathrin-binding segment and motifs that bind the AP2 appendages. Many lower organisms have a single AP180/CALM ortholog, and deletion of these in Drosophila and Caenorhabditis elegans leads to an increase in the size of synaptic vesicles but to a decrease in their number and in transmitter release, causing unregulated movement (Nonet et al. 1999; Bao et al. 2005). Although overexpression of CALM inhibits clathrin-mediated endocytosis (Tebar et al. 1999), knockdown by RNAi in HeLa cells leads to formation of aberrant coated structures: very large “coated pits” with some attached tubular structures, rather than the ∼1000 Å budding vesicles seen in control cells (Meyerholz et al. 2005). Despite this aberration, the knockdown has little effect on endocytic rates of transferrin receptor under normal conditions (Huang et al. 2004; Meyerholz et al. 2005; T Maeda and T Kirchhausen, unpubl.). CALM/AP180 may select the synaptic-vesicle fusion proteins—the SNAREs VAMP2, VAMP3, and VAMP8—to help define the target membrane with which the uncoated vesicle will fuse and to drive those fusion events (Miller et al. 2011). Other proteins such as the epsins (Wendland 2002; Hawryluk et al. 2006), eps15 (Polo et al. 2002), dishevelled (Yu et al. 2010), β-arrestin (Lefkowitz and Whalen 2004), and Hrb (Chaineau et al. 2008; Pryor et al. 2008) may be classified as cargo adaptors, but they are present at only low levels in clathrin-coated vesicles.
Nonstructural Proteins
The nonstructural proteins (i.e., those largely excluded from the budded coated vesicle) appear at varying levels relative to clathrin during the lifetime of a coated pit (see Merrifield and Kaksonen 2014). For instance, levels of Eps15 and FCHo1/2 are maximal at early time points and drop off during late stages of coated-pit assembly; others such as dynamin, synaptojanin, and auxillin, which participate in vesicle pinching and uncoating, appear in substantial quantities only at subsequent stages.
BAR-Domain Proteins
Several proteins active in membrane traffic contain near their amino termini a BAR domain (named after Bin, amphiphysin, and Rvs—the initial set of proteins in which the conserved sequence signature of this structure was recognized) including the amphiphysins, endophilins, Snx9, and FCHo1/2. The BAR domain is an elongated bundle of three curved α-helices (Peter et al. 2004). Subgroups, with specific features of the BAR-domain modules, bear the designations N-BAR, F-BAR, and I-BAR (Frost et al. 2009; Mim and Unger 2012). Homodimerization generates a symmetrical, usually arc-like molecule (that of FCHo1 is shown in Fig. 5). Positively charged residues on the concave faces of N-BAR- and F-BAR-domain arcs interact with negatively charged lipid head groups in the cytosolic face of a suitably curved membrane. A number of BAR-domain-containing proteins tubulate liposomes when present at high concentrations and bind curved membranes preferentially. The former property is due to the BAR domain itself; the latter correlates with the presence of amphipathic helices at the ends or in the center of the BAR-domain arc (Farsad et al. 2001; Gallop et al. 2006; Bhatia et al. 2009; Mim and Unger 2012). Mammalian amphiphysins and endophilins are each a relatively extended set of proteins in which the amphipathic helix precedes the BAR domain (hence their assignment as N-BARs) (Gallop et al. 2006; Masuda et al. 2006). An unstructured linker has binding sites for AP2 appendages and clathrin; it connects the BAR domain to a carboxy-terminal SH3 domain, which binds the proline-rich, carboxy-terminal regions of two membrane-directed enzymes, the dynamin GTPase and the synaptojanin lipid phosphatase (David et al. 1996; Micheva et al. 1997; Verstreken et al. 2003). Snx9, found in most cell types, has the same domain structure and functionalities but arranged in the opposite order and with the addition of a PIP-binding PX domain just amino terminal to the BAR domain (Pylypenko et al. 2007; Wang et al. 2008; van Weering et al. 2012).
Eps15/Eps15R, Epsin 1/2/3, Intersectin, FCHo1/2
These four proteins, each of which has multiple isoforms, coassociate. There are multiple possibilities for cross-connections among them, and all have sequences that can bind AP2 appendages (Fig. 6). Eps15 and FCHo 1/2 localize to the rim of a growing coated pit (Tebar et al. 1996; Henne et al. 2010). In their absence, coat assembly aborts (Cocucci et al. 2012). Epsin, also present at the rim, may have a somewhat less restricted distribution. FCHo1/2 and the epsins contain an F-BAR domain and an ENTH domain, respectively, that bind preferentially to curved membranes. At high concentrations in vitro, they can drive tubulation of liposomes (Ford et al. 2002; Wu et al. 2010; Boucrot et al. 2012). The SH3 domain of intersectin may participate in dynamin recruitment. Epsin and Eps15 both bind ubiquitin through UIM domains and may thus help recruit ubiquitinated cargo molecules into coated pits (Polo et al. 2002).
Figure 6.
Adaptors and accessory proteins. A domain diagram of the full protein above the molecular structure shows the relationship of the domain illustrated to the complete polypeptide chain. (A) Disheveled (Dvl) DEP domain and tyrosine motif, bound to µ2-C (PDB 3ML6) (Yu et al. 2010), superposed onto the structure of the open AP2 core (PDB 2XA7) (Jackson et al. 2010). The tyrosine motif is represented as sticks; the DEP domain (dark gray) and µ2-C (lighter gray) as ribbons; the rest of the AP2 core as a surface. (B) CALM/AP180 ANTH domain, modeled bound with the PtdIns(4,5)P2 head group (PDB 1HG2) (Ford et al. 2001), and its complex with peptide from VAMP (PDB 3GYM) (Miller et al. 2011). (C) Autosomal recessive hypercholesterolemia (ARH) phosphotyrosine-binding (PTB) domain (ribbons) bound with peptide (sticks) from cytoplasmic tail of LDL receptor and modeled with the PtdIns(4,5)P2 head group (PDB 3SO6) (Dvir et al. 2012). (D) Epsin ENTH domain with the PtdIns(4,5)P2 head group (PDB 1H0A) (Ford et al. 2002). (E) FCHo F-BAR domain and μ-homology domain (MHD); structure of the former from FCHo2 (PDB 2V0O) (Henne et al. 2007) and of the latter from the yeast homolog Syp1 (PDB 3G9H) (Reider et al. 2009). The F-BAR domain binds preferentially to a curved membrane bilayer.
Synaptojanin
Synaptojanins (of which two isoforms are present in mammalian cells) are inositol phosphatases (McPherson et al. 1996). They have two separate phosphatase domains, one of which dephosphorylates PtdIns(4,5)P2 or PtdIns(3,4,5) P3 at the 5′ position of the inositol ring and one that dephosphrylates PI3P or PI4P. A proline-rich, carboxy-terminal tail binds the SH3 domain of the amphiphysins and the endophilins. One of two splice forms of synaptojanin 1 also has clathrin and AP2-binding motifs, distal to the proline-rich segment.
Dynamin
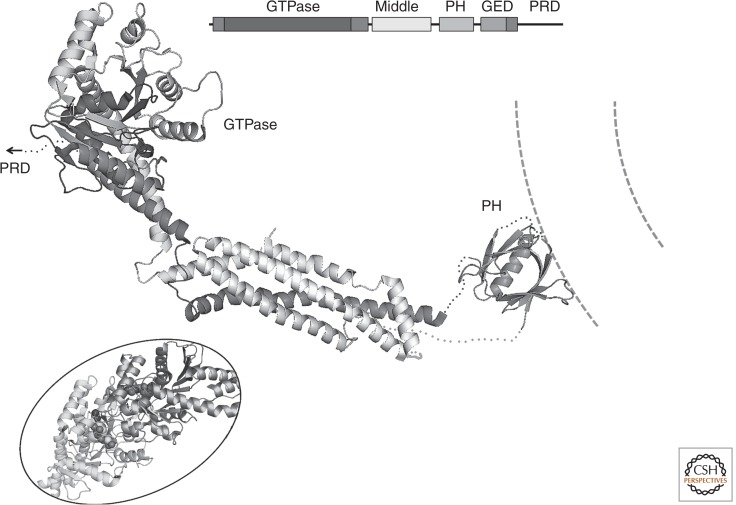
Dynamins are large, multidomain GTPases in which an extended, α-helical bundle, with a PH-domain at its tip, augments a p21-like catalytic domain (Chappie et al. 2009; Faelber et al. 2011; Ford et al. 2011; Chappie and Dyda 2013). In human cells, three isoforms—dynamins 1, 2, and 3—drive the pinching off of endocytic-coated vesicles at the plasma membrane, through a cycle of oligomerization and GTP hydrolysis. Dynamin does not appear to participate in budding of AP1-containing coated vesicles from internal membranes (Kural et al. 2012). Structurally related large GTPases participate in mitochondrial fission. Figure 7A shows the domain organization and structure of a mammalian dynamin 1.
Figure 7.
Dynamin. Domain diagram shows position in polypeptide chain of GTPase domain (somewhat augmented at both ends from the canonical small GTPase), “middle” domain, PH domain, GTPase effector domain (GED), and proline-rich domain (PRD). The large ribbon diagram shows a dynamin monomer, with PRD at the carboxyl terminus removed (PDB 3ZVR) (Ford et al. 2011). Note that the carboxy-terminal part of the GED region abuts the GTPase domain and influences its conformation. The PH domain interacts with the constricted membrane (dashed lines) at the neck of a budding coat. (Inset) GTP binding induces dimerization of the GTPase domains of two dynamins and switches the relative orientations of GTPase and GED. Two GMPPCP molecules bound at the dimer interface are shown as atomic models (spheres) (PDB 3ZYC) (Chappie et al. 2011). The orientation of one of the two GTPase domains (light gray) is the same as in the figure of the complete molecule; there distributed conformational changes, in addition to dimer formation, that accompany GTP binding.
In solution and in the cytosol, dynamin dimerizes readily, and the dimers can associate into tetramers at higher concentrations. From comparisons of various crystal structures and cryoEM reconstructions, two sets of dimer contacts appear to be critical (Chappie et al. 2011). An oblique contact between the stalk-like, α-helical bundles of two protomers yields an X-shaped dimer, with the GTPase domains at one end, projecting away from the mid-plane of the X, and with the PH domains flexibly tethered at the other end (Fig. 7B). GTPase–GTPase contacts, stabilized by GTP binding (Chappie et al. 2010), generate a very different twofold relationship, which coupled with the stem-dimer would produce a repeating polymer (Fig. 7C). GTP binds near the dimer interface, and dimerization of the GTPase domains contributes to nucleotide hydrolysis.
Dynamin assembles into helical structures along membrane tubules in vitro (Sweitzer and Hinshaw 1998), with contacts that include the stem–stem dimer contributing to the principal helical path and GTPase–GTPase dimer contacts bridging adjacent helical turns (Fig. 7D) (Zhang and Hinshaw 2001; Cheng et al. 2007; Chappie et al. 2011). The in vitro helical assemblies probably reflect the properties of the dynamin “collar” necessary for membrane fission, at the neck of a budding coated pit. The collar is linked to the constricted membrane bilayer through the dynamin PH domains. GTP hydrolysis is coupled to conformational changes in the connection between the GTPase domain and the α-helical stalk. The effects of these changes on the orientation of the stalk and on the organization of the collar presumably drive scission of the neck, but data are not yet available to distinguish clearly among various detailed models for this process.
Auxilin
Auxilin is a J-domain-containing protein, recruited to an endocytic-coated vesicle immediately after it has pinched off from the plasma membrane (Lee et al. 2006; Massol et al. 2006). The J-domain, in turn, recruits and activates the uncoating ATPase Hsc70, as described in the section on coated vesicle dynamics below. There are two auxilin isoforms in vertebrates: the brain-specific auxilin 1 and the ubiquitous auxilin 2 or GAK (Ungewickell et al. 1995; Umeda et al. 2000). The J-domain is at the carboxyl terminus of both, preceded by a clathrin-binding segment. At the amino terminus of auxilin 1 is a region with homology to the phosphoinositide phosphatase, PTEN (Guan et al. 2010). The homology extends to both a catalytic domain and a C2 domain (Fig. 5). The identity of residues in the catalytic domain critical for PTEN activity are different in auxilin, which is not a phosphatase, but the conserved shape of the pocket that contains these residues suggests that it could retain phosphoinositide-binding activity. In addition to the PTEN-like region, an amino-terminal protein kinase module (GAK) augments the polypeptide chain of auxilin 2. A fragment of auxilin that includes both the clathrin-binding and J-domains bind coats with a saturating stoichiometry of one auxilin per heavy chain and is sufficient to support Hsc70- and ATP-dependent uncoating in vitro (Holstein et al. 1996; Böcking et al. 2011).
Specific Lipids
Many of the coat-associated proteins have sites for interaction with polyphosphatidyl inositol polyphosphate (PIP) head groups. Specificity for the PIP that labels a particular membrane compartment helps determine intracellular localization. The AP2 α-chain recognizes PtdIns(4,5)P2, the principal species in the plasma membrane; the corresponding site on the AP1 β-chain binds PtdIns(4)P, which labels the TGN. Dynamin binds PtdIns(4,5)P2 through its PH domain; AP180, epsin, endophilin, and amphiphysin do so through their ANTH, ENTH, and BAR domains, respectively. Acute elimination of PtdIns(4,5)P2 from the plasma membrane, either by recruitment of a phosphatase (using a small-molecule dimerizer) or by treatment with butanol (which inhibits PtdIns(4,5)P2 production), results in nearly complete loss of clathrin-coated structures (Boucrot et al. 2006; Zoncu et al. 2007). Clathrin assembly at the plasma membrane thus requires both recruitment by lipid-associated adaptor proteins and cooperativity of lattice interactions. PtdIns(3,4)P2, generated by a type II PI3 kinase Cα, is required for coated-pit maturation and recruitment of Snx9 in the final stages of endocytic-coated vesicle formation (Posor et al. 2013).
STRUCTURAL DYNAMICS OF THE CANONICAL CLATHRIN PATHWAY
Coat Assembly
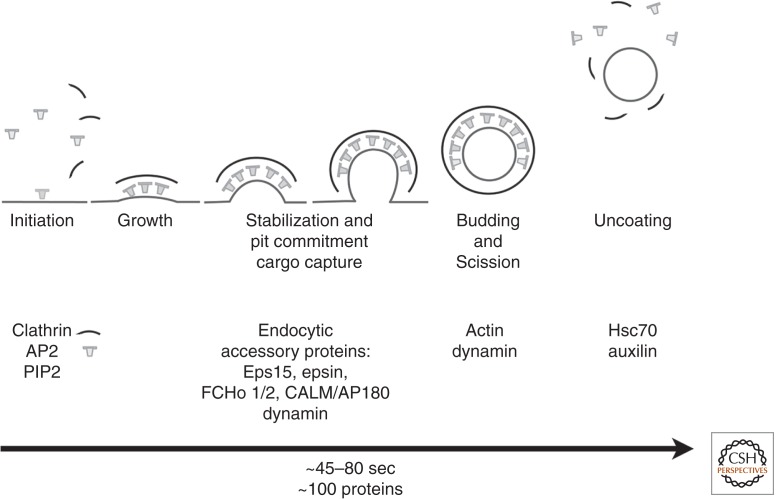
The kinetics of coated-pit assembly (restricting consideration to the diffraction-limited coats of “canonical” coated pits—we discuss larger coated “plaques” below) suggest that a nucleating event initiates the process, followed by steady accumulation of clathrin until a closed (or nearly closed) lattice has formed (Fig. 8). Initiation of coated-pit formation at the plasma membrane of cells in culture requires PtdIns(4,5)P2, which affords a transient initial docking point for AP2. Recent live-cell imaging studies with single-molecule detection suggest that assembly generally begins with two AP2s and one clathrin triskelion, or sometimes with four AP2s and two triskelions (Cocucci et al. 2012). The simplest model that can account for these data is that AP2s associate transiently with PtdIns (4,5)P2 on the inner leaflet of the plasma membrane and that capture of at least two by a clathrin triskelion increases their residency time and enhances the likelihood that further AP2 complexes and clathrin trimers will attach before the first ones dissociate. This nucleus can then recruit further untethered clathrin from the cytosolic pool and adaptors, including additional AP2s, from either the cytosol of the membrane. Any factor that stabilizes AP2 on the PtdIns(4,5)P2 membrane will enhance initiation, including nonclathrin components that link multiple AP2s. In cells reconstituted with AP2 complexes lacking the appendage domain and therefore defective in recruitment of endocytic accessory factors, a large fraction of clathrin-coated pits fail to invaginate and rapidly abort (Aguet et al. 2013). One obvious candidate for coated-vesicle stabilization is Eps15, which has about 15 AP2-appendage-binding sites. Eps15 is itself a dimer (Tebar et al. 1996), and it could cluster further through interaction with dimeric FCHo1/2. Early recruitment of FCHo1/2, Eps15, epsin, and intersectin to the rims of assembling coated pits is essential for their stability and further growth (Cocucci et al. 2012; Umasankar et al. 2012).
Figure 8.
Assembly and disassembly of a canonical, endocytic clathrin-coated pit. AP2 adaptor complexes, associated at the membrane with PtdIns(4,5)P2 (PIP2), recruit clathin triskelions to initiate lattice assembly. Stable growth and lattice closure require endocytic accessory proteins (Eps15, epsin, FCHo1/2, intersectin, CALM/AP180; some of which are also ancillary cargo adaptors). Dynamin, assisted by actin polymerization when the membrane is under tension, drives membrane scission and coated-vesicle release. Hsc70, recruited by the J-domain protein auxilin, mediates clathrin uncoating and release of a free vesicle, primed to fuse with a target membrane. Text beneath the diagram indicates the overall timescale and the stages at which various components appear to function. Short arcs, Clathrin triskelions; T shapes, AP2.
Clathrin accumulates at a constant rate throughout coat assembly (roughly one triskelion per second in BSC-1 cells at 37°C), so that the growth time of a coated pit is proportional to its final surface area. The average coat diameter of vesicles containing standard cargo molecules, such as Tf/TfR or EGF/EGFR, is ∼1000 Å, but larger vesicles form to accommodate larger cargo (such as a reovirus particle) (Ehrlich et al. 2004). That is, within certain limits, the curvature of the lattice appears to adapt to the contents. Resistance from within will decrease the probability of high curvature closing off a five-sided rather than a six-sided lattice opening. The rate of AP2 recruitment, relative to clathrin, diminishes substantially during subsequent phases of coat assembly (Saffarian and Kirchhausen 2008; Loerke et al. 2011). As the coated pit invaginates, the AP2 already incorporated moves away from the initial plane of the membrane (Saffarian and Kirchhausen 2008). AP2 is thereby enriched at one pole of the completed lattice (Fig. 3) (Cheng et al. 2007).
Membrane Invagination
When recruited to the surface of a large, unilamellar liposome by an anchored, unstructured peptide bearing a clathrin-box motif and a membrane attachment site, clathrin generates “coated buds” with nearly complete coats at 37°C (Dannhauser and Ungewickell 2012). Thus, contacts within the clathrin lattice are sufficient in this minimal system to drive membrane deformation, up to the stage of closure and scission of the neck connecting the bud with the parent liposome. The physical properties of lipid bilayers imply a substantial energy cost for invagination and budding of a vesicle. To divide a single, spherical phospholipid bilayer into two smaller vesicles requires ∼350 kT (∼250 kcal/mol), with the details depending on such factors as the intrinsic curvature imparted to the bilayer by its specific lipid composition (see Johannes et al. 2014). In a cell, rapid lateral diffusion of lipids means that some of the energy can be supplied by other processes, even at some distance—for example, processes that use or remove lipids for which the relevant curvature of the invagination is particularly unfavorable. But even 1.5–2 kcal/mol net favorable free energy of lattice interaction per clathrin heavy chain would be sufficient to recover 250 kcal/mol from a coat containing 60 triskelions—for example, the “soccer-ball” lattice, roughly 900 Å in outer diameter. This is the average diameter of an endocytic-coated pit in BSC-1 cells and of a cage formed by brain clathrin in vitro, when assembled (by lowering the pH) in the absence of any other protein component, and it therefore probably corresponds to the most stable curvature for a clathrin lattice. Molecular crowding from bulky adaptors and cytoplasmic domains of their associated cargo may also favor membrane invagination (Kirchhausen 2012; Stachowiak et al. 2012, 2013). The resistance of the membrane to invagination increases as the pit begins to constrict, and membrane tension appears to be an important factor contributing to the requirement in vivo for proteins other than the minimal set sufficient to release a coated vesicle assembled in vitro on a liposome.
The Role of Actin
Actin polymerization is not essential for canonical coated-pit dynamics in BSC-1 and HeLa cells under standard growth conditions in culture, nor is it required for normal rates of transferrin uptake (Fujimoto et al. 2000; Boucrot et al. 2006; Saffarian et al. 2009). But elevated membrane tension, created by a variety of mechanisms including cytoskeletal attachments and exposure of the cell to a hypo-osmolar medium, stalls coated-pit assembly and imposes a requirement for actin dynamics (Boulant et al. 2011). Uptake of elongated cargo such as a vesicular stomatitis virus (VSV) particle provides a particularly dramatic example (Cureton et al. 2010). A normally curved coated pit cannot close off around the virion, and growth stalls because of resistance from the effectively infinite tension generated by the particle shaft. Local actin polymerization finishes the engulfment process, perhaps by pushing the clathrin cap at one end of the invagination away from the mean plane of the surrounding membrane. Smaller, roughly spherical, defective VSV particles do not need actin to enter the cell, because their diameter is within the range of naturally occurring clathrin-coated vesicles.
One biochemical link between clathrin and actin is HIP1R (Bennett et al. 2001; Engqvist-Goldstein et al. 2001). A central coiled-coil in this protein binds a motif near the amino terminus of all clathrin light chains, a carboxy-terminal, vinculin-like domain binds F-actin, and an amino-terminal ANTH domain binds PtdIns(4,5)P2. The coiled-coil in the middle of HIP1R creates long, rod-like homodimers, as well as similar heterodimers with HIP1, with the ANTH domains at one end, the vinculin-like domains at the other, and the light-chain site between them (Niu et al. 2008). Thus, they have the properties expected of a membrane–clathrin–actin connector. Another, less-direct link between clathrin and actin is through dynamin, which can bind cortactin, an activator of Arp2/3 (Merrifield et al. 2005; Taylor et al. 2012).
Once uncoated, previously clathrin-coated vesicles can engage actin and move rapidly away from the site at which they formed (Merrifield et al. 2002). The molecular details of this presumably motor-driven, directed motion are not yet defined, although an obvious possibility is myosin VI (Buss et al. 2001; Hasson 2003). The organization and reorganization of cortical actin layers will clearly influence the fate of cargo molecules entrapped by coated pits.
Cargo Loading
Coated pits select cargo through interactions with the array of clathrin adaptors. The molecular mechanisms of these interactions are discussed extensively in Traub and Bonifacino (2013). For example, transferrin receptors bind AP2 directly, and LDLR binds ARH and Dab2, which, in turn, bind AP2. Live-cell imaging shows that these receptors diffuse on the cell surface until collision with an assembling clathrin lattice (Ehrlich et al. 2004; Cureton et al. 2012). The cargo-capturing pit is generally relatively “young” (e.g., <20 sec since initiation in BSC-1 cells at 37°C). Indeed, it would be substantially less likely that a diffusing transmembrane protein could enter a constricted, nearly mature pit than a gently domed one or a planar structure. Clathrin binding to AP2 is linked to its transition from a locked to an open conformation, and thus can couple cargo capture and lattice assembly (Rapoport et al. 1997; Jackson et al. 2010).
Scission
Clathrin assembly can provide the net free energy needed to generate a deeply invaginated bud in vitro (Dannhauser and Ungewickell 2012; for review, see Kirchhausen 2012), but membrane scission (fission) still presents a substantial barrier. Release of a coated vesicle thus requires that dynamin enable the neck of a coated pit to reorganize into a transition state that leads to pinching, although the mechanism remains unknown (for recent reviews, see Schmid and Frolov 2011; Chappie and Dyda 2013; Morlot and Roux 2013; Johannes et al. 2014). Acute inhibition of the dynamin GTPase activity with the compound dynasore causes accumulation of coated pits at two stages—pits with a fully constricted neck (“omega”-shaped cross sections) (Heuser 1980; Macia et al. 2006) as might be expected from the properties of dynamin collars and helices on lipid tubes in vitro, and pits just at the point at which a constriction is beginning to form (“U”-shaped cross sections). Thus, in addition to the well-known kinetic barrier between a narrow neck and a budded vesicle, there also appears to be a barrier between an unconstricted “dome” and the initial formation of a reentrant ring at its base.
Uncoating
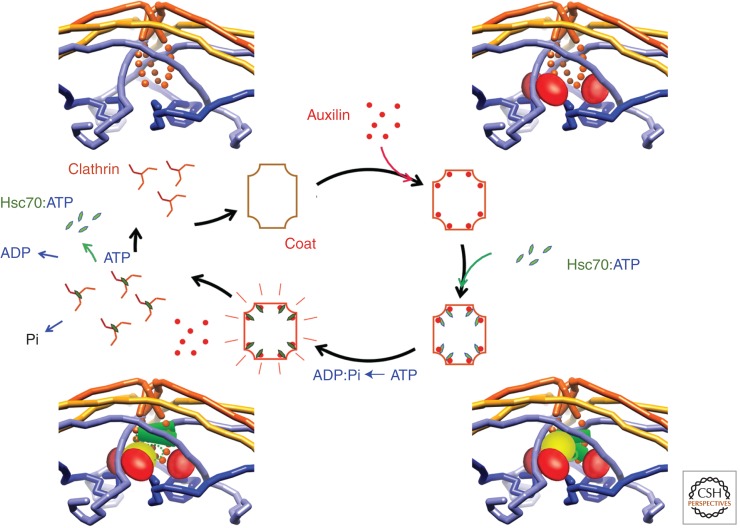
The positions of uncoated vesicles just interior to membranes with budding coated pits led Roth and Porter (1964) to conclude that uncoating followed promptly upon pinching off; live-cell fluorescence microscopy has confirmed this conjecture and shown that uncoating proceeds very rapidly following release of the coated vesicle (Ehrlich et al. 2004; Merrifield et al. 2005; Massol et al. 2006). The agent of clathrin uncoating is the cytosolic heat-shock cognate protein Hsc70 (Rothman and Schmid 1986). Like all members of the Hsp70 family, Hsc70 has an actin-like ATPase domain linked to a characteristic “molecular clamp” domain, which can capture hydrophobic peptides, exposed on an unfolded polypeptide chain or projecting from an assembly destined for dissociation (Jiang et al. 2005). When the enzyme is in the ATP-bound state, the clamp-domain/peptide association is relatively weak, ATP hydrolysis (to ADP and Pi) tightens the clamp, and nucleotide exchange (dissociation of ADP and binding of ATP) opens the clamp, releases the peptide, and completes the cycle. Hsc70 and its substrate are brought together by a J-domain-containing protein—auxilin in the case of a clathrin coat (Xing et al. 2010). One part of the J-domain protein associates with the substrate; the J-domain itself recruits Hsc70:ATP (Jiang et al. 2003). Thus, the initial weak encounter is strengthened by the J-domain protein as a bridge. The conformational change in Hsc70 that accompanies ATP hydrolysis releases the J-domain while closing the clamp on the substrate (Hartl and Hayer-Hartl 2002; Jiang et al. 2007).
Within the clathrin carboxy-terminal segment is a short, hydrophobic sequence, necessary for Hsc70- and ATP-dependent uncoating and very similar to an optimal Hsc70-interacting peptide (Rapoport et al. 2008). Auxilin associates with a clathrin lattice in such a way that an Hsc70 molecule, recruited by the J-domain, can, in turn, bind a nearby clathrin carboxy-terminal segment (Fig. 9) (Fotin et al. 2004a). Hsc70 binding is accompanied by a local distortion of the clathrin lattice, needed to make the interaction site fully accessible (Xing et al. 2010; Böcking et al. 2011). Thus, Hsc70, recruited by the auxilin J-domain to a location adjacent to a vertex, can capture a fluctuation in the lattice by binding its target segment and splitting ATP, thereby fastening the clamp and locking the transient distortion in place. Accumulation of local strain at multiple vertices can then lead to disassembly.
Figure 9.
Hsc70-mediated uncoating. Illustration of proposed mechanism. (Upper left) Vertex of a clathrin lattice, with clathrin polypeptide chains as continuous worms. (Orange) The hub of a triskelion centered on that vertex; with the carboxy-terminal Hsc-70-binding segment dotted; (yellow) the “knees” of triskelions centered on the neighboring vertices; (blue) the terminal domains and linkers of triskelions centered on second-nearest-neighbor vertices. See Figure 1 to place this vertex in the context of a full lattice. (Upper right) Binding of the carboxy-terminal region of auxilin, containing the clathrin-binding segment and the J-domain; the amino-terminal region, which contains the PTEN-homology domain, would project inward toward the vesicle membrane. (Lower right) Auxilin J-domain recruits Hsc70:ATP. (Lower left) Hsc70, in a conformational change driven by ATP hydrolysis, clamps tightly onto its recognition motif on one of the three clathrin carboxy-terminal segments. This event locks in a local distortion, necessary to expose the recognition motif enough to accommodate the Hsc70 clamp domain. Binding of Hsc70 at a critical number of vertices imposes sufficient distortion to destabilize the entire lattice. Rebinding of ATP to Hsc70 dissociates it from the free triskelions, which can then assemble into a new coat and complete the cycle. (From Xing et al. 2010 and Böcking et al. 2011; adapted, with permission, from the authors.)
Auxilin enters a coated vesicle just after membrane pinching (Lee et al. 2006; Massol et al. 2006; Taylor et al. 2011). Earlier arrival could lead to Hsc70-driven uncoating of an incomplete lattice and hence a futile assembly–disassembly cycle. Ongoing lipid modification provides a potential mechanism by which auxilin could detect that a vesicle has separated from the parent membrane. Only after pinching has generated membrane discontinuity will a lipid modified by a coat-associated enzyme remain in the vesicle, rather than diffuse rapidly out of the bud (Massol et al. 2006). The inactive, PTEN-like domain of auxilin, which is required for its recruitment to a budded coated vesicle, is likely to recognize some species of phosphoinositide head group. Moreover, the polyphosphoinositide phosphatase synaptojanin has many of the properties expected for the postulated modifying enzyme. In mammalian cells, a neuron-specific splice form of synaptojanin 1 appears in coated pits just at the time of pinching off, concomitantly with endophilin and dynamin (Gad et al. 2000; Perera et al. 2006); an alternative, ubiquitously expressed splice form, with carboxy-terminally located clathrin and AP2-binding motifs, in addition to the phosphatase domains and the endophilin-associating proline-rich region present in both forms, accumulates more steadily during coat assembly. Endophilin A and synaptojanin defects in C. elegans give rise to very similar neuronal phenotypes, including depletion of docked synaptic vesicles and apparent accumulation of clathrin-coated vesicles, consistent with an uncoating defect (Harris et al. 2000; Schuske et al. 2003). Depletion of PtdIns(4,5)P2 by synaptojanin will also weaken the affinity of AP2 for the vesicle membrane, promoting dissociation of the adaptor layer and reducing the likelihood of triskelion rebinding.
LARGER CLATHRIN LATTICES
Some cell types in culture (e.g., HeLa cells) elaborate abundant, relatively long-lived clathrin arrays at the interface with the substrate on which they are growing (Saffarian et al. 2009). When studied by deep-etch electron microscopy, the same cell types also show extended clathrin lattices, which can be of substantially greater diameter (up to 5000 Å) than the sharply invaginated coated pits seen in the same images. Although described as “puncta” in live-cell imaging studies and sometimes designated as “coated pits,” these long-lived objects are often larger than the ∼2500 Å diffraction limit, and they have very different dynamics from the more strictly “punctate,” canonical coated pits. These large noncanonical structures, now termed “coated plaques,” can engulf membrane, as shown by uptake of transferrin receptor. Actin assembly probably drives membrane engulfment, perhaps by a mechanism related to its role in clathrin-mediated yeast-cell membrane uptake. Clathrin dissociating from the edge of a coated plaque may also enhance local initiation of canonical coated-pit assembly (Merrifield et al. 2005; Saffarian et al. 2009; Taylor et al. 2011).
The EM images of extended arrays that correspond, at least in some cases, to coated plaques show some degree of short-range hexagonal order, but a high concentration of lattice defects (Heuser 1980). That is, the inherent curvature (pucker) of the clathrin triskelion limits the extent to which it can assemble into a lattice with only hexagonal facets (Fotin et al. 2004b). If the underlying support resists curvature, lattice defects will lead to a patchwork of hexagons with short-range order and long-range disorder. The same property accounts for the failure of clathrin to form tubular structures—for example, when engulfing VSV (Cureton et al. 2010): the threefold symmetry and approximately fixed pucker imparts curvature in two dimensions and prevents formation of a singly curved (tubular) lattice.
2014 AND BEYOND
The clathrin lattice and its heterotetrameric adaptors organize a large array of other molecular components—proteins and lipids. The interactions among the additional components and their association with clathrin and APs create a bewilderingly complex network of potential contacts. Not all these contacts can coexist, and we do not yet have the experimental data needed to define a hierarchy among them. Moreover, properties of tissue-specific isoforms of many of the associated proteins show that regulated expression modulates which components are present under specific physiological conditions or in particular differentiated cells. The interactions among these proteins are, in general, relatively weak, requiring coincident presence of several components to establish a meaningful residence time. The relatively high frequency of abortive events also shows that the contribution of this network of weak contacts to the stability of an assembling coat compensates for the inevitable stochasticity in arrival times of various proteins, so that absence of one or more critical participants often leads to dissolution of unstable, partial structures.
Expression from endogenous loci of proteins bearing genetically encoded fluorescent tags and application of powerful new imaging technologies are likely to provide ways to study patterns of recruitment of particular components, both in space and in time, and to link their biochemical and structural properties, described in this review, even more directly with their functional dynamics. Some of the literature cited in this work describes the biological phenomena that such experiments should seek to explain.
ACKNOWLEDGMENTS
T.K. is funded in part by NIH grant GM36548; D.O. is funded by a Wellcome Trust Principal Research Fellowship; and S.C.H. is a Howard Hughes Medical Insitute Investigator. We thank Scottie Robinson for discussion and Phil Evans and Lauren Jackson for help with figures. We also thank members of our laboratories and our colleagues for the opportunity to share good science and for many enlightening discussions. Finally, we apologize to colleagues whose work we have inadvertently failed to quote.
Footnotes
Editors: Sandra L. Schmid, Alexander Sorkin, and Marino Zerial
REFERENCES
*Reference is also in this collection.
- Aggeler J, Werb Z 1982. Initial events during phagocytosis by macrophages viewed from outside and inside the cell: Membrane–particle interactions and clathrin. J Cell Biol 94: 613–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguet F, Antonescu CN, Mettlen M, Schmid SL, Danuser G 2013. Advances in analysis of low signal-to-noise images link dynamin and AP2 to the functions of an endocytic checkpoint. Dev Cell 26: 279–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RG, Goldstein JL, Brown MS 1977. A mutation that impairs the ability of lipoprotein receptors to localise in coated pits on the cell surface of human fibroblasts. Nature 270: 695–699 [DOI] [PubMed] [Google Scholar]
- Bao H, Daniels RW, MacLeod GT, Charlton MP, Atwood HL, Zhang B 2005. AP180 maintains the distribution of synaptic and vesicle proteins in the nerve terminal and indirectly regulates the efficacy of Ca2+-triggered exocytosis. J Neurophysiol 94: 1888–1903 [DOI] [PubMed] [Google Scholar]
- Bennett EM, Chen CY, Engqvist-Goldstein AE, Drubin DG, Brodsky FM 2001. Clathrin hub expression dissociates the actin-binding protein Hip1R from coated pits and disrupts their alignment with the actin cytoskeleton. Traffic 2: 851–858 [DOI] [PubMed] [Google Scholar]
- Bhatia VK, Madsen KL, Bolinger P-Y, Kunding A, Hedegård P, Gether U, Stamou D 2009. Amphipathic motifs in BAR domains are essential for membrane curvature sensing. EMBO J 28: 3303–3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondeau F, Ritter B, Allaire PD, Wasiak S, Girard M, Hussain NK, Angers A, Legendre-Guillemin V, Roy L, Boismenu D, et al. 2004. Tandem MS analysis of brain clathrin-coated vesicles reveals their critical involvement in synaptic vesicle recycling. Proc Natl Acad Sci 101: 3833–3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böcking T, Aguet F, Harrison SC, Kirchhausen T 2011. Single-molecule analysis of a molecular disassemblase reveals the mechanism of Hsc70-driven clathrin uncoating. Nat Struct Mol Biol 18: 295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonazzi M, Kühbacher A, Toledo-Arana A, Mallet A, Vasudevan L, Pizarro-Cerdá J, Brodsky FM, Cossart P 2012. A common clathrin-mediated machinery co-ordinates cell–cell adhesion and bacterial internalization. Traffic 13: 1653–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner GHH, Harbour M, Hester S, Lilley KS, Robinson MS 2006. Comparative proteomics of clathrin-coated vesicles. J Cell Biol 175: 571–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner GHH, Antrobus R, Hirst J, Bhumbra GS, Kozik P, Jackson LP, Sahlender DA, Robinson MS 2012. Multivariate proteomic profiling identifies novel accessory proteins of coated vesicles. J Cell Biol 197: 141–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucrot E, Saffarian S, Massol R, Kirchhausen T, Ehrlich M 2006. Role of lipids and actin in the formation of clathrin-coated pits. Exp Cell Res 312: 4036–4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucrot E, Pick A, Camdere G, Liska N, Evergren E, McMahon HT, Kozlov MM 2012. Membrane fission is promoted by insertion of amphipathic helices and is restricted by crescent BAR domains. Cell 149: 124–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulant S, Kural C, Zeeh J-C, Ubelmann F, Kirchhausen T 2011. Actin dynamics counteract membrane tension during clathrin-mediated endocytosis. Nat Cell Biol 13: 1124–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushlin I, Petralia RS, Wu F, Harel A, Mughal MR, Mattson MP, Yao PJ 2008. Clathrin assembly protein AP180 and CALM differentially control axogenesis and dendrite outgrowth in embryonic hippocampal neurons. J Neurosci 28: 10257–10271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss F, Luzio JP, Kendrick-Jones J 2001. Myosin VI, a new force in clathrin mediated endocytosis. FEBS Lett 508: 295–299 [DOI] [PubMed] [Google Scholar]
- Carpentier JL, Gorden P, Anderson RG, Goldstein JL, Brown MS, Cohen S, Orci L 1982. Co-localization of 125I-epidermal growth factor and ferritin-low density lipoprotein in coated pits: A quantitative electron microscopic study in normal and mutant human fibroblasts. J Cell Biol 95: 73–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaineau M, Danglot L, Proux-Gillardeaux V, Galli T 2008. Role of HRB in clathrin-dependent endocytosis. J Biol Chem 283: 34365–34373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappie JS, Dyda F 2013. Building a fission machine—Structural insights into dynamin assembly and activation. J Cell Sci 126: 2773–2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappie JS, Acharya S, Liu Y-W, Leonard M, Pucadyil TJ, Schmid SL 2009. An intramolecular signaling element that modulates dynamin function in vitro and in vivo. Mol Biol Cell 20: 3561–3571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappie JS, Acharya S, Leonard M, Schmid SL, Dyda F 2010. G domain dimerization controls dynamin’s assembly-stimulated GTPase activity. Nature 465: 435–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappie JS, Mears JA, Fang S, Leonard M, Schmid SL, Milligan RA, Hinshaw JE, Dyda F 2011. A pseudoatomic model of the dynamin polymer identifies a hydrolysis-dependent powerstroke. Cell 147: 209–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-Y, Brodsky FM 2005. Huntingtin-interacting protein 1 (Hip1) and Hip1-related protein (Hip1R) bind the conserved sequence of clathrin light chains and thereby influence clathrin assembly in vitro and actin distribution in vivo. J Biol Chem 280: 6109–6117 [DOI] [PubMed] [Google Scholar]
- Chen C-Y, Reese ML, Hwang PK, Ota N, Agard D, Brodsky FM 2002. Clathrin light and heavy chain interface: α-Helix-binding superhelix loops via critical tryptophans. EMBO J 21: 6072–6082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Boll W, Kirchhausen T, Harrison SC, Walz T 2007. Cryoelectron tomography of clathrin-coated vesicles: Structural implications for coat assembly. J Mol Biol 365: 892–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocucci E, Aguet F, Boulant S, Kirchhausen T 2012. The first five seconds in the life of a clathrin-coated pit. Cell 150: 495–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins BM, McCoy AJ, Kent HM, Evans PR, Owen DJ 2002. Molecular architecture and functional model of the endocytic AP2 complex. Cell 109: 523–535 [DOI] [PubMed] [Google Scholar]
- Conner SD, Schröter T, Schmid SL 2003. AAK1-mediated micro2 phosphorylation is stimulated by assembled clathrin. Traffic 4: 885–890 [DOI] [PubMed] [Google Scholar]
- *.Cossart P, Helenius A 2014. Endocytosis of viruses and bacteria. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a016972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cureton DK, Massol RH, Whelan SPJ, Kirchhausen T 2010. The length of vesicular stomatitis virus particles dictates a need for actin assembly during clathrin-dependent endocytosis. PLoS Pathog 6: e1001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cureton DK, Harbison CE, Cocucci E, Parrish CR, Kirchhausen T 2012. Limited transferrin receptor clustering allows rapid diffusion of canine parvovirus into clathrin endocytic structures. J Virol 86: 5330–5340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke H, Baba T, Warnock DE, Schmid SL 1994. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol 127: 915–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannhauser PN, Ungewickell EJ 2012. Reconstitution of clathrin-coated bud and vesicle formation with minimal components. Nat Cell Biol 14: 634–639 [DOI] [PubMed] [Google Scholar]
- David C, McPherson PS, Mundigl O, de Camilli P 1996. A role of amphiphysin in synaptic vesicle endocytosis suggested by its binding to dynamin in nerve terminals. Proc Natl Acad Sci 93: 331–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake MT, Traub LM 2001. Interaction of two structurally distinct sequence types with the clathrin terminal domain β-propeller. J Biol Chem 276: 28700–28709 [DOI] [PubMed] [Google Scholar]
- Drake MT, Downs MA, Traub LM 2000. Epsin binds to clathrin by associating directly with the clathrin-terminal domain. Evidence for cooperative binding through two discrete sites. J Biol Chem 275: 6479–6489 [DOI] [PubMed] [Google Scholar]
- Dvir H, Shah M, Girardi E, Guo L, Farquhar MG, Zajonc DM 2012. Atomic structure of the autosomal recessive hypercholesterolemia phosphotyrosine-binding domain in complex with the LDL-receptor tail. Proc Natl Acad Sci 109: 6916–6921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M, Boll W, van Oijen A, Hariharan R, Chandran K, Nibert ML, Kirchhausen T 2004. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118: 591–605 [DOI] [PubMed] [Google Scholar]
- Engqvist-Goldstein AEY, Drubin DG 2003. Actin assembly and endocytosis: From yeast to mammals. Annu Rev Cell Dev Biol 19: 287–332 [DOI] [PubMed] [Google Scholar]
- Engqvist-Goldstein AE, Warren RA, Kessels MM, Keen JH, Heuser J, Drubin DG 2001. The actin-binding protein Hip1R associates with clathrin during early stages of endocytosis and promotes clathrin assembly in vitro. J Cell Biol 154: 1209–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faelber K, Posor Y, Gao S, Held M, Roske Y, Schulze D, Haucke V, Noé F, Daumke O 2011. Crystal structure of nucleotide-free dynamin. Nature 477: 556–560 [DOI] [PubMed] [Google Scholar]
- Farsad K, Ringstad N, Takei K, Floyd SR, Rose K, de Camilli P 2001. Generation of high curvature membranes mediated by direct endophilin bilayer interactions. J Cell Biol 155: 193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MG, Pearse BM, Higgins MK, Vallis Y, Owen DJ, Gibson A, Hopkins CR, Evans PR, McMahon HT 2001. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science 291: 1051–1055 [DOI] [PubMed] [Google Scholar]
- Ford MGJ, Mills IG, Peter BJ, Vallis Y, Praefcke GJK, Evans PR, McMahon HT 2002. Curvature of clathrin-coated pits driven by epsin. Nature 419: 361–366 [DOI] [PubMed] [Google Scholar]
- Ford MGJ, Jenni S, Nunnari J 2011. The crystal structure of dynamin. Nature 477: 561–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotin A, Cheng Y, Grigorieff N, Walz T, Harrison SC, Kirchhausen T 2004a. Structure of an auxilin-bound clathrin coat and its implications for the mechanism of uncoating. Nature 432: 649–653 [DOI] [PubMed] [Google Scholar]
- Fotin A, Cheng Y, Sliz P, Grigorieff N, Harrison SC, Kirchhausen T, Walz T 2004b. Molecular model for a complete clathrin lattice from electron cryomicroscopy. Nature 432: 573–579 [DOI] [PubMed] [Google Scholar]
- Frost A, Unger VM, De Camilli P 2009. The BAR domain superfamily: Membrane-molding macromolecules. Cell 137: 191–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto LM, Roth R, Heuser JE, Schmid SL 2000. Actin assembly plays a variable, but not obligatory role in receptor-mediated endocytosis in mammalian cells. Traffic 1: 161–171 [DOI] [PubMed] [Google Scholar]
- Gad H, Ringstad N, Löw P, Kjaerulff O, Gustafsson J, Wenk M, Di Paolo G, Nemoto Y, Crun J, Ellisman MH, et al. 2000. Fission and uncoating of synaptic clathrin-coated vesicles are perturbed by disruption of interactions with the SH3 domain of endophilin. Neuron 27: 301–312 [DOI] [PubMed] [Google Scholar]
- Gallop JL, Jao CC, Kent HM, Butler PJG, Evans PR, Langen R, McMahon HT 2006. Mechanism of endophilin N-BAR domain-mediated membrane curvature. EMBO J 25: 2898–2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard M, Allaire PD, McPherson PS, Blondeau F 2005. Non-stoichiometric relationship between clathrin heavy and light chains revealed by quantitative comparative proteomics of clathrin-coated vesicles from brain and liver. Mol Cell Proteomics 4: 1145–1154 [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Anderson RG, Brown MS 1979. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature 279: 679–685 [DOI] [PubMed] [Google Scholar]
- Guan R, Dai H, Han D, Harrison SC, Kirchhausen T 2010. Structure of the PTEN-like region of auxilin, a detector of clathrin-coated vesicle budding. Structure 18: 1191–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TW, Hartwieg E, Horvitz HR, Jorgensen EM 2000. Mutations in synaptojanin disrupt synaptic vesicle recycling. J Cell Biol 150: 589–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M 2002. Molecular chaperones in the cytosol: From nascent chain to folded protein. Science 295: 1852–1858 [DOI] [PubMed] [Google Scholar]
- Hasson T 2003. Myosin VI: Two distinct roles in endocytosis. J Cell Sci 116: 3453–3461 [DOI] [PubMed] [Google Scholar]
- Hawryluk MJ, Keyel PA, Mishra SK, Watkins SC, Heuser JE, Traub LM 2006. Epsin 1 is a polyubiquitin-selective clathrin-associated sorting protein. Traffic 7: 262–281 [DOI] [PubMed] [Google Scholar]
- Heldwein EE, Macia E, Wang J, Yin HL, Kirchhausen T, Harrison SC 2004. Crystal structure of the clathrin adaptor protein 1 core. Proc Natl Acad Sci 101: 14108–14113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne WM, Kent HM, Ford MGJ, Hegde BG, Daumke O, Butler PJG, Mittal R, Langen R, Evans PR, McMahon HT 2007. Structure and analysis of FCHo2 F-BAR domain: A dimerizing and membrane recruitment module that effects membrane curvature. Structure 15: 839–852 [DOI] [PubMed] [Google Scholar]
- Henne WM, Boucrot E, Meinecke M, Evergren E, Vallis Y, Mittal R, McMahon HT 2010. FCHo proteins are nucleators of clathrin-mediated endocytosis. Science 328: 1281–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J 1980. Three-dimensional visualization of coated vesicle formation in fibroblasts. J Cell Biol 84: 560–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser JE, Keen J 1988. Deep-etch visualization of proteins involved in clathrin assembly. J Cell Biol 107: 877–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann JB, Winkler DC, Yim Y-I, Eisenberg E, Greene LE, Steven AC 2013. Clathrin-coated vesicles from brain have small payloads: A cryoelectron tomographic study. J Struct Biol 184: 43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Irving C, Borner GHH 2013. Adaptor protein complexes AP4 and AP5: New players in endosomal trafficking and progressive spastic paraplegia. Traffic 14: 153–164 [DOI] [PubMed] [Google Scholar]
- Holstein SE, Ungewickell H, Ungewickell E 1996. Mechanism of clathrin basket dissociation: Separate functions of protein domains of the DnaJ homologue auxilin. J Cell Biol 135: 925–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Khvorova A, Marshall W, Sorkin A 2004. Analysis of clathrin-mediated endocytosis of epidermal growth factor receptor by RNA interference. J Biol Chem 279: 16657–16661 [DOI] [PubMed] [Google Scholar]
- Jackson AP, Seow HF, Holmes N, Drickamer K, Parham P 1987. Clathrin light chains contain brain-specific insertion sequences and a region of homology with intermediate filaments. Nature 326: 154–159 [DOI] [PubMed] [Google Scholar]
- Jackson LP, Kelly BT, McCoy AJ, Gaffry T, James LC, Collins BM, Honing S, Evans PR, Owen DJ 2010. A large-scale conformational change couples membrane recruitment to cargo binding in the AP2 clathrin adaptor complex. Cell 141: 1220–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Taylor AB, Prasad K, Ishikawa-Brush Y, Hart PJ, Lafer EM, Sousa R 2003. Structure–function analysis of the auxilin J-domain reveals an extended Hsc70 interaction interface. Biochemistry 42: 5748–5753 [DOI] [PubMed] [Google Scholar]
- Jiang J, Prasad K, Lafer EM, Sousa R 2005. Structural basis of interdomain communication in the Hsc70 chaperone. Mol Cell 20: 513–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Maes EG, Taylor AB, Wang L, Hinck AP, Lafer EM, Sousa R 2007. Structural basis of J cochaperone binding and regulation of Hsp70. Mol Cell 28: 422–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Johannes L, Wunder C, Bassereau P 2014. Bending “on the rocks”—A cocktail of biophysical modules to build endocytic pathways. Cold Spring Harb Perspect Biol 6: a016741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen JH, Beck KA, Kirchhausen T, Jarrett T 1991. Clathrin domains involved in recognition by assembly protein AP2. J Biol Chem 266: 7950–7956 [PubMed] [Google Scholar]
- Kelly BT, McCoy AJ, Späte K, Miller SE, Evans PR, Honing S, Owen DJ 2008. A structural explanation for the binding of endocytic dileucine motifs by the AP2 complex. Nature 456: 976–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyel PA, Thieman JR, Roth R, Erkan E, Everett ET, Watkins SC, Heuser JE, Traub LM 2008. The AP2 adaptor β2 appendage scaffolds alternate cargo endocytosis. Mol Biol Cell 19: 5309–5326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T 1999. Adaptors for clathrin-mediated traffic. Annu Rev Cell Dev Biol 15: 705–732 [DOI] [PubMed] [Google Scholar]
- Kirchhausen T 2000. Clathrin. Annu Rev Biochem 69: 699–727 [DOI] [PubMed] [Google Scholar]
- Kirchhausen T 2009. Imaging endocytic clathrin structures in living cells. Trends Cell Biol 19: 596–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T 2012. Bending membranes. Nat Cell Biol 14: 906–908 [DOI] [PubMed] [Google Scholar]
- Kirchhausen T, Harrison SC 1981. Protein organization in clathrin trimers. Cell 23: 755–761 [DOI] [PubMed] [Google Scholar]
- Kirchhausen T, Toyoda T 1993. Immunoelectron microscopic evidence for the extended conformation of light chains in clathrin trimers. J Biol Chem 268: 10268–10273 [PubMed] [Google Scholar]
- Kirchhausen T, Scarmato P, Harrison SC, Monroe JJ, Chow EP, Mattaliano RJ, Ramachandran KL, Smart JE, Ahn AH, Brosius J 1987. Clathrin light chains LCA and LCB are similar, polymorphic, and share repeated heptad motifs. Science 236: 320–324 [DOI] [PubMed] [Google Scholar]
- Kural C, Tacheva-Grigorova SK, Boulant S, Cocucci E, Baust T, Duarte D, Kirchhausen T 2012. Dynamics of intracellular clathrin/AP1- and clathrin/AP3-containing carriers. Cell Rep 10.1016/j.celrep.2012.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D-W, Wu X, Eisenberg E, Greene LE 2006. Recruitment dynamics of GAK and auxilin to clathrin-coated pits during endocytosis. J Cell Sci 119: 3502–3512 [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Whalen EJ 2004. β-Arrestins: Traffic cops of cell signaling. Curr Opin Cell Biol 16: 162–168 [DOI] [PubMed] [Google Scholar]
- Loerke D, Mettlen M, Schmid SL, Danuser G 2011. Measuring the hierarchy of molecular events during clathrin-mediated endocytosis. Traffic 12: 815–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T 2006. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell 10: 839–850 [DOI] [PubMed] [Google Scholar]
- Mao Y, Chen J, Maynard JA, Zhang B, Quiocho FA 2001. A novel all helix fold of the AP180 amino-terminal domain for phosphoinositide binding and clathrin assembly in synaptic vesicle endocytosis. Cell 104: 433–440 [DOI] [PubMed] [Google Scholar]
- Massol RH, Boll W, Griffin AM, Kirchhausen T 2006. A burst of auxilin recruitment determines the onset of clathrin-coated vesicle uncoating. Proc Natl Acad Sci 103: 10265–10270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda M, Takeda S, Sone M, Ohki T, Mori H, Kamioka Y, Mochizuki N 2006. Endophilin BAR domain drives membrane curvature by two newly identified structure-based mechanisms. EMBO J 25: 2889–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui W, Kirchhausen T 1990. Stabilization of clathrin coats by the core of the clathrin-associated protein complex AP2. Biochemistry 29: 10791–10798 [DOI] [PubMed] [Google Scholar]
- McPherson PS, Garcia EP, Slepnev VI, David C, Zhang X, Grabs D, Sossin WS, Bauerfeind R, Nemoto Y, de Camilli P 1996. A presynaptic inositol-5-phosphatase. Nature 379: 353–357 [DOI] [PubMed] [Google Scholar]
- *.Merrifield C, Kaksonen M 2014. Endocytic accessory factors and regulation of clathrin-mediated endocytosis. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a016733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield CJ, Feldman ME, Wan L, Almers W 2002. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat Cell Biol 4: 691–698 [DOI] [PubMed] [Google Scholar]
- Merrifield CJ, Perrais D, Zenisek D 2005. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell 121: 593–606 [DOI] [PubMed] [Google Scholar]
- Meyerholz A, Hinrichsen L, Groos S, Esk P-C, Brandes G, Ungewickell EJ 2005. Effect of clathrin assembly lymphoid myeloid leukemia protein depletion on clathrin coat formation. Traffic 6: 1225–1234 [DOI] [PubMed] [Google Scholar]
- Micheva KD, Ramjaun AR, Kay BK, McPherson PS 1997. SH3 domain-dependent interactions of endophilin with amphiphysin. FEBS Lett 414: 308–312 [DOI] [PubMed] [Google Scholar]
- Miele AE, Watson PJ, Evans PR, Traub LM, Owen DJ 2004. Two distinct interaction motifs in amphiphysin bind two independent sites on the clathrin terminal domain β-propeller. Nat Struct Mol Biol 11: 242–248 [DOI] [PubMed] [Google Scholar]
- Miller SE, Sahlender DA, Graham SC, Honing S, Robinson MS, Peden AA, Owen DJ 2011. The molecular basis for the endocytosis of small R-SNAREs by the clathrin adaptor CALM. Cell 147: 1118–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mim C, Unger VM 2012. Membrane curvature and its generation by BAR proteins. Trends Biochem Sci 37: 526–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlot S, Roux A 2013. Mechanics of dynamin-mediated membrane fission. Annu Rev Biophys 42: 629–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A, Smith CJ, Roseman AM, Harrison SC, Kirchhausen T, Pearse BM 1999. Functional organization of clathrin in coats: Combining electron cryomicroscopy and X-ray crystallography. Mol Cell 3: 761–770 [DOI] [PubMed] [Google Scholar]
- Neutra MR, Ciechanover A, Owen LS, Lodish HF 1985. Intracellular transport of transferrin- and asialoorosomucoid-colloidal gold conjugates to lysosomes after receptor-mediated endocytosis. J Histochem Cytochem 33: 1134–1144 [DOI] [PubMed] [Google Scholar]
- Newpher TM, Idrissi F-Z, Geli MI, Lemmon SK 2006. Novel function of clathrin light chain in promoting endocytic vesicle formation. Mol Biol Cell 17: 4343–4352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Q, Niu Q, Ybe JA, Ybe JA 2008. Crystal structure at 2.8 Å of huntingtin-interacting protein 1 (HIP1) coiled-coil domain reveals a charged surface suitable for HIP1 protein interactor (HIPPI). J Mol Biol 375: 1197–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet ML, Holgado AM, Brewer F, Serpe CJ, Norbeck BA, Holleran J, Wei L, Hartwieg E, Jorgensen EM, Alfonso A 1999. UNC-11, a Caenorhabditis elegans AP180 homologue, regulates the size and protein composition of synaptic vesicles. Mol Biol Cell 10: 2343–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS 1995. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science 269: 1872–1875 [DOI] [PubMed] [Google Scholar]
- Owen DJ, Evans PR 1998. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science 282: 1327–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DJ, Vallis Y, Noble ME, Hunter JB, Dafforn TR, Evans PR, McMahon HT 1999. A structural explanation for the binding of multiple ligands by the α-adaptin appendage domain. Cell 97: 805–815 [DOI] [PubMed] [Google Scholar]
- Owen DJ, Vallis Y, Pearse BM, McMahon HT, Evans PR 2000. The structure and function of the β2-adaptin appendage domain. EMBO J 19: 4216–4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse BM 1975. Coated vesicles from pig brain: Purification and biochemical characterization. J Mol Biol 97: 93–98 [DOI] [PubMed] [Google Scholar]
- Pearse BM 1976. Clathrin: A unique protein associated with intracellular transfer of membrane by coated vesicles. Proc Natl Acad Sci 73: 1255–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera RM, Zoncu R, Lucast L, De Camilli P, Toomre D 2006. Two synaptojanin 1 isoforms are recruited to clathrin-coated pits at different stages. Proc Natl Acad Sci 103: 19332–19337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrais D, Merrifield CJ 2005. Dynamics of endocytic vesicle creation. Dev Cell 9: 581–592 [DOI] [PubMed] [Google Scholar]
- Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJG, Evans PR, McMahon HT 2004. BAR domains as sensors of membrane curvature: The amphiphysin BAR structure. Science 303: 495–499 [DOI] [PubMed] [Google Scholar]
- Polo S, Sigismund S, Faretta M, Guidi M, Capua MR, Bossi G, Chen H, De Camilli P, Di Fiore PP 2002. A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature 416: 451–455 [DOI] [PubMed] [Google Scholar]
- Posor Y, Eichhorn-Gruenig M, Puchkov D, Schöneberg J, Ullrich A, Lampe A, Müller R, Zarbakhsh S, Gulluni F, Hirsch E, et al. 2013. Spatiotemporal control of endocytosis by phosphatidylinositol-3,4-bisphosphate. Nature 499: 233–237 [DOI] [PubMed] [Google Scholar]
- Praefcke GJK, Ford MGJ, Schmid EM, Olesen LE, Gallop JL, Peak-Chew S-Y, Vallis Y, Babu MM, Mills IG, McMahon HT 2004. Evolving nature of the AP2 α-appendage hub during clathrin-coated vesicle endocytosis. EMBO J 23: 4371–4383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor PR, Jackson L, Gray SR, Edeling MA, Thompson A, Sanderson CM, Evans PR, Owen DJ, Luzio JP 2008. Molecular basis for the sorting of the SNARE VAMP7 into endocytic clathrin-coated vesicles by the ArfGAP Hrb. Cell 134: 817–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylypenko O, Lundmark R, Rasmuson E, Carlsson SR, Rak A 2007. The PX-BAR membrane-remodeling unit of sorting nexin 9. EMBO J 26: 4788–4800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport I, Miyazaki M, Boll W, Duckworth B, Cantley LC, Shoelson S, Kirchhausen T 1997. Regulatory interactions in the recognition of endocytic sorting signals by AP2 complexes. EMBO J 16: 2240–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport I, Boll W, Yu A, Böcking T, Kirchhausen T 2008. A motif in the clathrin heavy chain required for the Hsc70/auxilin uncoating reaction. Mol Biol Cell 19: 405–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reider A, Barker SL, Mishra SK, Im YJ, Maldonado-Báez L, Hurley JH, Traub LM, Wendland B 2009. Syp1 is a conserved endocytic adaptor that contains domains involved in cargo selection and membrane tubulation. EMBO J 28: 3103–3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricotta D, Conner SD, Schmid SL, Figura von K, Honing S 2002. Phosphorylation of the AP2 μ subunit by AAK1 mediates high affinity binding to membrane protein sorting signals. J Cell Biol 156: 791–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter B, Denisov AY, Philie J, Deprez C, Tung EC, Gehring K, McPherson PS 2004. Two WXXF-based motifs in NECAPs define the specificity of accessory protein binding to AP1 and AP2. EMBO J 23: 3701–3710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS, Bonifacino JS 2001. Adaptor-related proteins. Curr Opin Cell Biol 13: 444–453 [DOI] [PubMed] [Google Scholar]
- Roth TF, Porter KR 1964. Yolk protein uptake in the oocyte of the mosquito Aedes aegypti L. J Cell Biol 20: 313–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE, Schmid SL 1986. Enzymatic recycling of clathrin from coated vesicles. Cell 46: 5–9 [DOI] [PubMed] [Google Scholar]
- Saffarian S, Kirchhausen T 2008. Differential evanescence nanometry: Live-cell fluorescence measurements with 10-nm axial resolution on the plasma membrane. Biophys J 94: 2333–2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffarian S, Cocucci E, Kirchhausen T 2009. Distinct dynamics of endocytic clathrin-coated pits and coated plaques. PLoS Biol 7: e1000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid SL, Frolov VA 2011. Dynamin: Functional design of a membrane fission catalyst. Annu Rev Cell Dev Biol 27: 79–105 [DOI] [PubMed] [Google Scholar]
- Schmid EM, McMahon HT 2007. Integrating molecular and network biology to decode endocytosis. Nature 448: 883–888 [DOI] [PubMed] [Google Scholar]
- Schmid EM, Ford MGJ, Burtey A, Praefcke GJK, Peak-Chew S-Y, Mills IG, Benmerah A, McMahon HT 2006. Role of the AP2 β-appendage hub in recruiting partners for clathrin-coated vesicle assembly. PLoS Biol 4: e262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuske KR, Richmond JE, Matthies DS, Davis WS, Runz S, Rube DA, van der Bliek AM, Jorgensen EM 2003. Endophilin is required for synaptic vesicle endocytosis by localizing synaptojanin. Neuron 40: 749–762 [DOI] [PubMed] [Google Scholar]
- Shih W, Gallusser A, Kirchhausen T 1995. A clathrin-binding site in the hinge of the β2 chain of mammalian AP2 complexes. J Biol Chem 270: 31083–31090 [DOI] [PubMed] [Google Scholar]
- Smith CJ, Grigorieff N, Pearse BM 1998. Clathrin coats at 21 A resolution: A cellular assembly designed to recycle multiple membrane receptors. EMBO J 17: 4943–4953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachowiak JC, Schmid EM, Ryan CJ, Ann HS, Sasaki DY, Sherman MB, Geissler PL, Fletcher DA, Hayden CC 2012. Membrane bending by protein–protein crowding. Nat Cell Biol 14: 944–949 [DOI] [PubMed] [Google Scholar]
- Stachowiak JC, Brodsky FM, Miller EA 2013. A cost-benefit analysis of the physical mechanisms of membrane curvature. Nat Cell Biol 15: 1019–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer SM, Hinshaw JE 1998. Dynamin undergoes a GTP-dependent conformational change causing vesiculation. Cell 93: 1021–1029 [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Perrais D, Merrifield CJ 2011. A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS Biol 9: e1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MJ, Lampe M, Merrifield CJ 2012. A feedback loop between dynamin and actin recruitment during clathrin-mediated endocytosis. PLoS Biol 10: e1001302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebar F, Sorkina T, Sorkin A, Ericsson M, Kirchhausen T 1996. Eps15 is a component of clathrin-coated pits and vesicles and is located at the rim of coated pits. J Biol Chem 271: 28727–28730 [DOI] [PubMed] [Google Scholar]
- Tebar F, Bohlander SK, Sorkin A 1999. Clathrin assembly lymphoid myeloid leukemia (CALM) protein: Localization in endocytic-coated pits, interactions with clathrin, and the impact of overexpression on clathrin-mediated traffic. Mol Biol Cell 10: 2687–2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Haar E, Musacchio A, Harrison SC, Kirchhausen T 1998. Atomic structure of clathrin: A β-propeller terminal domain joins an α zigzag linker. Cell 95: 563–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Haar E, Harrison SC, Kirchhausen T 2000. Peptide-in-groove interactions link target proteins to the β-propeller of clathrin. Proc Natl Acad Sci 97: 1096–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Traub LM, Bonifacino JS 2013. Cargo recognition in clathrin-mediated endocytosis. Cold Spring Harb Perspect Biol 5: a016790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub LM, Downs MA, Westrich JL, Fremont DH 1999. Crystal structure of the α appendage of AP2 reveals a recruitment platform for clathrin-coat assembly. Proc Natl Acad Sci 96: 8907–8912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkewitz AP, Harrison SC 1989. Concentration of transferrin receptor in human placental coated vesicles. J Cell Biol 108: 2127–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umasankar PK, Sanker S, Thieman JR, Chakraborty S, Wendland B, Tsang M, Traub LM 2012. Distinct and separable activities of the endocytic clathrin-coat components Fcho1/2 and AP2 in developmental patterning. Nat Cell Biol 14: 488–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda A, Meyerholz A, Ungewickell E 2000. Identification of the universal cofactor (auxilin 2) in clathrin coat dissociation. Eur J Cell Biol 79: 336–342 [DOI] [PubMed] [Google Scholar]
- Ungewickell E, Branton D 1981. Assembly units of clathrin coats. Nature 289: 420–422 [DOI] [PubMed] [Google Scholar]
- Ungewickell E, Ungewickell H, Holstein SE, Lindner R, Prasad K, Barouch W, Martin B, Greene LE, Eisenberg E 1995. Role of auxilin in uncoating clathrin-coated vesicles. Nature 378: 632–635 [DOI] [PubMed] [Google Scholar]
- Urrutia R, Henley JR, Cook T, McNiven MA 1997. The dynamins: Redundant or distinct functions for an expanding family of related GTPases? Proc Natl Acad Sci 94: 377–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Weering JRT, Sessions RB, Traer CJ, Kloer DP, Bhatia VK, Stamou D, Carlsson SR, Hurley JH, Cullen PJ 2012. Molecular basis for SNX-BAR-mediated assembly of distinct endosomal sorting tubules. EMBO J 31: 4466–4480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga E, Cossart P 2005. Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nat Cell Biol 7: 894–900 [DOI] [PubMed] [Google Scholar]
- Veiga E, Guttman JA, Bonazzi M, Boucrot E, Toledo-Arana A, Lin AE, Enninga J, Pizarro-Cerdá J, Finlay BB, Kirchhausen T, et al. 2007. Invasive and adherent bacterial pathogens co-opt host clathrin for infection. Cell Host Microbe 2: 340–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P, Koh T-W, Schulze KL, Zhai RG, Hiesinger PR, Zhou Y, Mehta SQ, Cao Y, Roos J, Bellen HJ 2003. Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating. Neuron 40: 733–748 [DOI] [PubMed] [Google Scholar]
- Wang Q, Kaan HYK, Hooda RN, Goh SL, Sondermann H 2008. Structure and plasticity of Endophilin and Sorting Nexin 9. Structure 16: 1574–1587 [DOI] [PubMed] [Google Scholar]
- Wendland B 2002. Epsins: Adaptors in endocytosis? Nat Rev Mol Cell Biol 3: 971–977 [DOI] [PubMed] [Google Scholar]
- Wilbur JD, Wilbur JD, Chen C-Y, Chen C-Y, Manalo V, Manalo V, Hwang PK, Hwang PK, Fletterick RJ, Fletterick RJ, et al. 2008. Actin binding by Hip1 (huntingtin-interacting protein 1) and Hip1R (Hip1-related protein) is regulated by clathrin light chain. J Biol Chem 283: 32870–32879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Huang B, Graham M, Raimondi A, Heuser JE, Zhuang X, De Camilli P 2010. Coupling between clathrin-dependent endocytic budding and F-BAR-dependent tubulation in a cell-free system. Nat Cell Biol 12: 902–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Böcking T, Wolf M, Grigorieff N, Kirchhausen T, Harrison SC 2010. Structure of clathrin coat with bound Hsc70 and auxilin: Mechanism of Hsc70-facilitated disassembly. EMBO J 29: 655–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A, Xing Y, Harrison SC, Kirchhausen T 2010. Structural analysis of the interaction between Dishevelled2 and clathrin AP2 adaptor, a critical step in noncanonical Wnt signaling. Structure 18: 1311–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Hinshaw JE 2001. Three-dimensional reconstruction of dynamin in the constricted state. Nat Cell Biol 3: 922–926 [DOI] [PubMed] [Google Scholar]
- Zoncu R, Perera RM, Sebastian R, Nakatsu F, Chen H, Balla T, Ayala G, Toomre D, De Camilli PV 2007. Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci 104: 3793–3798 [DOI] [PMC free article] [PubMed] [Google Scholar]