The future of cancer treatment: immunomodulation, CARs and combination immunotherapy (original) (raw)
. Author manuscript; available in PMC: 2017 Aug 10.
Published in final edited form as: Nat Rev Clin Oncol. 2016 Mar 15;13(5):273–290. doi: 10.1038/nrclinonc.2016.25
Abstract
In the past decade, advances in the use of monoclonal antibodies (mAbs) and adoptive cellular therapy to treat cancer by modulating the immune response have led to unprecedented responses in patients with advanced-stage tumours that would otherwise have been fatal. To date, three immune-checkpoint-blocking mAbs have been approved in the USA for the treatment of patients with several types of cancer, and more patients will benefit from immunomodulatory mAb therapy in the months and years ahead. Concurrently, the adoptive transfer of genetically modified lymphocytes to treat patients with haematological malignancies has yielded dramatic results, and we anticipate that this approach will rapidly become the standard of care for an increasing number of patients. In this Review, we highlight the latest advances in immunotherapy and discuss the role that it will have in the future of cancer treatment, including settings for which testing combination strategies and ‘armoured’ CAR T cells are recommended.
Immunotherapy is defined as the approach to treating cancer by generating or augmenting an immune response against it. This approach has been studied, mostly outside of mainstream cancer research, for over a century1. Nevertheless, cancer immunotherapy has only in the past decade been shown, in phase III clinical trials, to consistently improve the overall survival of patients with advanced-stage cancer2–5, bringing unprecedented interest to this field. Despite the breakthroughs of the past decade, the successes to date do not fully capture the promise of immunotherapy.
Antitumour immunotherapy has broad potential and could be used to treat many different types of advanced-stage cancer owing to the durable and robust responses it elicits across a diverse spectrum of malignancies. Two types of immunotherapy have emerged as particularly effective over the past decade: immune-cell-targeted monoclonal antibody (mAb) therapy and adoptive cellular therapy (ACT). In this Review, we present current clinical progress in both modalities, discuss how each of them might be particularly indicated for different types of cancer and we outline the potential therapeutic relevance of combination regimens.
Immune modulation with monoclonal antibodies
Immune modulation is based on the striking finding that stimulation of T-cell function with antibodies that block or activate regulatory receptors is sufficient to cause the regression of some tumours. Immunomodulatory mAbs target immune cells rather than cancer cells, and thus, are not necessarily specific to any cancer type. Indeed, the blockade of a single molecule, programmed cell-death protein 1 (PD-1), has resulted in antitumour activity and is now approved by the FDA to treat patients with mela-noma2,3 and non-small-cell lung cancer (NSCLC)6. PD-1 is one of the receptors involved in immune-checkpoint signalling; in particular, in lymphocyte maintenance of self-tolerance. Checkpoint blockade is a method by which T-cell function is stimulated with mAbs that block their inhibitory receptors, whereas T-cell co-stimulation is the method that aims at activating T-cell function with mAbs that target their stimulatory receptors. Some tumour types, however, are more likely than others to respond to checkpoint blockade, which raises the possibility that T-cell-stimulatory mAbs can be applied to a broad spectrum of cancer types if they are administered in the proper therapeutic context.
The generation of immunological memory is another unique feature of immune modulation as an effective cancer therapy7. A persistent memory response would have a role in both preventing disease recurrence and in guarding against the evolution of therapy-resistant malignant cancer clones. The precise implications of immunological memory formation remain undefined, but evidence for extremely durable remissions has been shown in some patients with unresectable or metastatic melanoma treated with immunotherapy8. Furthermore, complete and rapid tumour regression has been observed among a subset of these patients9,10, highlighting the fact that responses to immunotherapy are no less robust than those to cytotoxic chemotherapy and molecularly targeted therapy and can lead to tumour reduction and, in some cases, eradication.
The observation that mAbs targeting molecules on the T-cell surface are sufficient, in some patients, to mediate tumour regression is instructive. Therapeutic antitumour vaccination is based on the premise that an adaptive antitumour immune response can be elicited by presenting exogenous tumour antigens to the immune system. This strategy was at the forefront of cancer immunotherapy research in prior decades. Some vaccines were administered with so-called adjuvants, which, in the context of immunology, are agents designed to enhance the immune response to the antigen. One way to consider the current paradigm of cancer immunotherapy is a shift from administering an antigen to administering an adjuvant in the context of a pre-existing, but non therapeutic, vaccination event in situ, as will be described later.
The discovery that T-cell-stimulation alone (that is, without a co-administered vaccine to direct the immune response to a specific target) can have a therapeutic effect relies on a fundamental principle that surprised many in the field: it suggests that patients with cancer who derive benefit from T-cell-stimulatory therapy are immunologically primed, before treatment, to mount an anticancer immune response. Correspondingly, successful immunotherapy in these patients merely needs to unmask this latent potential.
Numerous research groups have invested substantial resources into identifying patients who are most likely to benefit from T-cell stimulatory therapy. Such knowledge would not only spare some patients from unnecessary treatment with associated toxicities, but it would also expand the use of immunotherapy to treat new types of cancer. Among the initial candidates for predictive biomarkers were C-reactive protein (CRP) and the absolute lymphocyte count11, because they correlated with improved outcomes. Subsequently, the measurement of circulating myeloid-derived suppressor cell (MDSC) levels before treatment emerged as a potential method to predict outcomes12. In 2015, elevated baseline levels of soluble CD25 were shown to correlate with poor survival outcomes13. In other studies14–16, extensive whole-exome sequencing was performed on samples from patients with melanoma and from patients with NSCLC treated with checkpoint blockade agents with the purpose of identifying genomic properties that might predict a response to these immunotherapies. Genetic features, such as mutation burden, were identified but no consensus has been reached regarding the identity of specific genetic alterations encoding so-called ‘neoepitopes’ that would make malignant cells recognizable to T cells and offer good predictive value for a response to checkpoint blockade16. The discovery of such alterations and their validation in prospective clinical trials would be of immense importance as they could enable immunotherapy to be given selectively to patients who would benefit from it, merging immunotherapy with precision medicine in a manner that could benefit innumerable patients with cancer.
Immune-checkpoint blockade
In this section, we discuss the well-established role of cytotoxic T-lymphocyte protein 4 (CTLA-4) and PD-1in T-cell activation. We also highlight other promising inhibitory T-cell receptors for which mAbs are being developed.
CTLA-4
T cells are primed to acquire effector function at the immunological synapse with an antigen-presenting cell (APC). The initiating event, so-called signal 1, is the recognition by the T-cell receptor (TCR) of a cognate antigen peptide presented in the context of an MHC molecule on the surface of an APC (FIG. 1). This interaction, however, is insufficient to activate T-cell function. In fact, the T cell will go on to become anergic or apoptotic if no second signal is received17. In order to adequately prime T cells, a second signal is required, typically in the form of the T-cell receptor CD28 binding with either CD80 or CD86 on the APC. Once this occurs, the T-cell inhibitory CTLA-4 receptor is shuttled to the cell surface where it binds CD80 or CD86 with greater affinity than CD28 (REFS 18,19). Thus, CTLA-4 translocation to the T-cell surface peaks after TCR stimulation, and activation of this receptor limits T-cell stimulation by TCR/CD28 co-ligation, both by preventing signalling downstream of the TCR and by outcompeting CD28 for its ligands. Phenotypic evidence of the ability of CTLA-4 to dampen T-cell activation is well demonstrated in _Ctla4_-knockout mice20,21. These animals developed fatal autoimmune disorders caused by a marked expansion of the T-cell population and infiltration into multiple tissue types20,21. CTLA-4 thus has a central role in suppressing T-cell function, thereby restricting T cell-mediated antitumour activity. Not surprisingly, CTLA-4 blockade has paved the way forward for modern cancer immunotherapy.
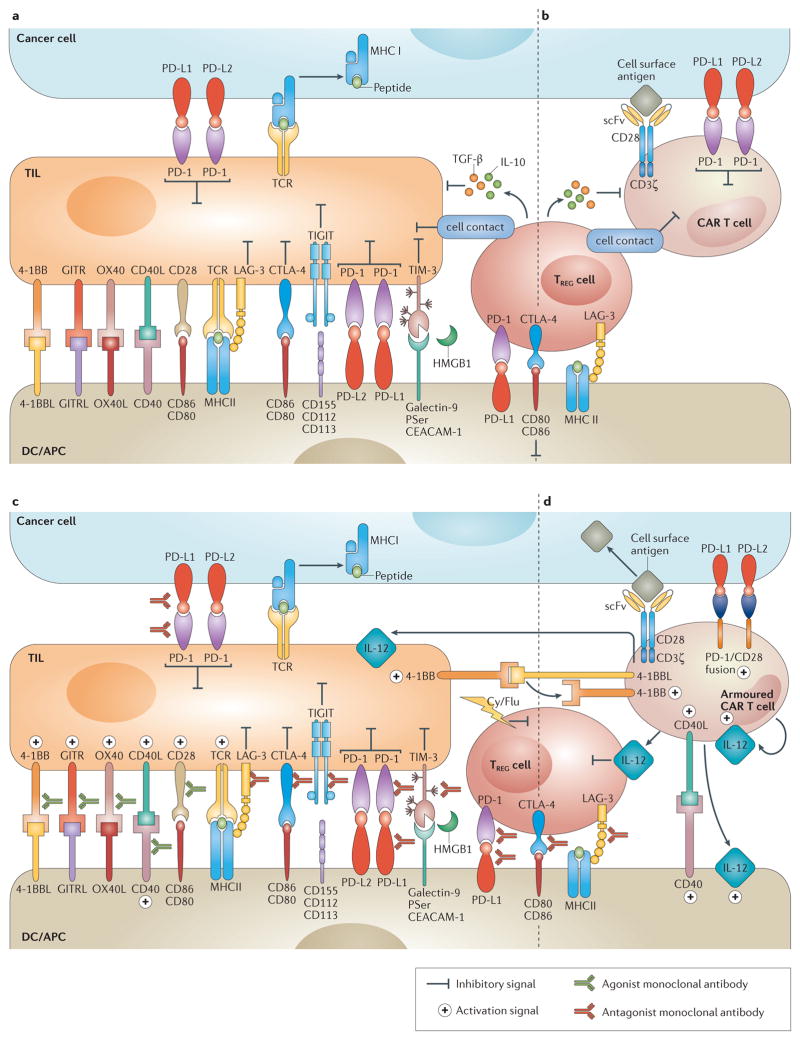
Figure 1. Immunomodulatory monoclonal antibodies and armoured chimeric antigen receptor (CAR) T cells overcome immune suppression.
a | Overview of the immune inhibitory molecules that compromise endogenous T–cell antitumour activity. T cells are susceptible to immune inhibitory factors associated within the microenvironment that prevent their full antitumour activity. Such factors include cell surface proteins (such as programmed cell death 1 ligands 1 and 2 (PD-L1 and PD-L2)) and cytokines (such as TGF-β and IL-10). Regulatory T (TREG) cells are representative of inhibitory cellular components of the tumour microenvironment (TME), which also include myeloid-derived suppressor cells, tumour-associated macrophages and other cell types non-depicted. b | Similarly to endogenous T cells, CAR T cells are susceptible to immune inhibitory factors present in the TME. c | Immunomodulatory monoclonal antibodies can be used to overcome local immunosuppression by either activating stimulatory receptors (such as TNFRSF9 (4-1BB) or OX40), or blocking suppressive receptors (for example, programmed cell-death 1 (PD-1) or cytotoxic T-lymphocyte antigen 4 (CTLA-4)). d | Armoured CAR T cells are engineered to express proteins that overcome immunosuppression associated with the TME (such as CD40L, IL-12 or TNFSF9 (4-1BBL)). Ag, antigen; APC, antigen-presenting cell; CD40L, CD40 ligand; Cy, cyclophosphamide; DC, dendritic cell; Flu, fludarabine; GITR, glucocorticoid-induced TNFR family related protein; GITRL, GITR ligand; HMGB1, high mobility group 1 protein; LAG-3, lymphocyte activation gene-3; PSer, phosphatidyl serine; scFv, single-chain variable fragment; TCR, T-cell receptor; TIGIT, T-cell immunoreceptor with immunoglobulin and ITIM domains; TIL, tumour -nfiltrating lymphocyte; TIM-3, T cell immunoglobulin and mucin domain 3.
Regulatory T (TREG) cells also use CTLA-4 to suppress antitumour immunity22,23. TREG cells constitutively express CTLA-4, typically at levels higher than those of conventional T cells24, and CTLA-4 is necessary for TREG cells to exert maximal immune-suppressive function23,25. Aside from disrupting CTLA-4 ligation on TREG cells, anti-CTLA-4 mAbs deplete intratumoural TREG cells, at least in mouse models26,27. In 2015 it was observed that CTLA-4 blockade reduces the interaction time of conventional T cells with TREG cells (REF. 22), thereby potentially freeing conventional T cells to be adequately primed by APCs.
Once it emerged that CTLA-4 constrains T-cell activity, the use of agents that block this receptor became an attractive candidate ‘adjuvant’ for therapeutic cancer vaccination28. This approach was used in preclinical studies to enhance the potency of the immune response generated by the administration of DNA vaccines29 and of vaccines consisting of irradiated tumour cells transduced to express the granulocyte-macrophage colony-stimulating factor (GM-CSF)30. These results were followed by a phase III trial in which patients were randomly assigned to receive ipilimumab (anti-CTLA-4 human mAb) plus gp100 peptide vaccine, or each agent alone4. The overall survival of the patients in the ipilimumab group was improved compared with patients that received the peptide vaccine only, and addition of the peptide vaccine to ipilimumab did not confer any additional advantages. This result was a breakthrough for several reasons. Firstly, no treatment before ipilimumab had demonstrated any significant improvement of overall survival for patients with advanced-stage melanoma in the setting of a phase III clinical trial. Secondly, these results revealed for the first time that immune-checkpoint blockade can be sufficient to improve survival in patients with advanced-stage cancer. Finally, the survival plot in this study shows a plateau at approximately 2 years, after which the patients that survived (20%) went on to experience durable benefit for the remainder of the study4. This pattern was atypical of melanoma and, in fact, of other malignancies treated with conventional cancer therapy.
These clinical findings suggest that, once immune-checkpoint blockade successfully engages a patient’s immune system to control tumour growth, the immune response can be sustained even after the course of treatment has ended. This observation is consistent with the fact that such immunotherapeutic agents do not target the tumour itself, but rather they modify the patient’s immune system to control tumour progression, even after the exogenous agent has been withdrawn.
The introduction of ipilimumab in the clinic brought a new and different set of drug adverse effects now known as immune-related adverse events (irAE)31. These are defined as the mechanism-based toxicities that result from a ‘disinhibited’ immune response. Given the unique aetiology of these adverse events, limited overlap exists between irAEs and the toxicities associated with most other forms of cancer therapy in terms of type or severity of symptoms31. In principle, the immune stimulation caused by immune-checkpoint blockade can affect any organ system; however, some organ systems are particularly susceptible to the adverse effects of immune modulation by anti-CTLA-4 treatment (TABLE 1). Fortunately, even clinically moderate to severe irAEs can generally be controlled with medical management without altering the antitumour effects of the therapy. Whereas most irAEs are typically reversible, endocrinopathies such as hypophysitis and thyroiditis frequently require chronic hormone replacement. Discontinuation of ipilimumab should be considered if corticosteroids cannot be tapered below 10 mg daily, which is the case for a minority of patients. Clinicians, however, should keep in mind the unique response kinetics of ipilimumab and other checkpoint-blocking agents. Some patients who ultimately derive benefit from ipilimumab will often experience an initial phase of tumour growth on commencing therapy32, a phenomenon that has not been observed to the same extent with cytotoxic chemotherapy or molecularly targeted anticancer therapy, which are directed against the tumour and not the patient’s immune system. An appropriate use of checkpoint blocking agents (particularly anti-CTLA-4 monotherapy), thus, requires careful clinical judgment to avoid discontinuing the therapy too early in the treatment course. Immune-related response criteria (irRC) have been developed to provide guidance for avoiding premature discontinuation of therapy under specific circumstances33. For example, whereas the development of a small new lesion during therapy would be designated as progression of disease by standard RECIST criteria34 and imply treatment failure; however, such a designation by irRC criteria would also take into account the overall disease burden33.
Table 1.
Adverse events associated with immune-checkpoint blockade
| Immune-mediated adverse event | Manifestations | Management |
|---|---|---|
| Enterocolitis | Diarrhoea, abdominal pain, mucus or blood in stool | Antidiarrhoeals followed by systemic corticosteroids if persistent; infliximab if refractory |
| Pneumonitis | Dyspnoea, cough | Systemic corticosteroids |
| Hepatitis | ALT/AST, bilirubin elevation | Systemic corticosteroids; mycophenolate mofetil if refractory |
| Dermatitis | Pruritic/macular/papular rash, Stevens–Johnson syndrome (rare), toxic epidermal necrolysis (rare) | Topical betamethasone or oral antihistamines; systemic corticosteroids if refractory |
| Neuropathy | Sensory/motor neuropathy, Guillain–Barre syndrome (rare), myasthenia gravis (rare) | Systemic corticosteroids |
| Endocrinopathy | Hypothyroidism, hyperthyroidism, hypopituitarism, adrenal insufficiency, hypogonadism, Cushing’s syndrome (rare) | Systemic corticosteroids, appropriate hormone replacement (potentially long-term) |
| Other irAEs | Arthritis, nephritis, meningitis, pericardidits, uveitis, iritis, anaemia, neutropenia | Organ-system specific |
Ipilimumab was approved by the FDA for the treatment of metastatic melanoma in 2011. This agent’s antitumour properties, however, are not limited to melanoma35 nor even to cancers that are historically thought to be immune responsive, such as renal-cell carcinoma (RCC)36. Nevertheless, in our opinion, the future role of CTLA-4 blockade in cancer therapy will be primarily in the context of combination regimens. CTLA-4 blockade might enable local antitumour therapy to trigger a systemic response; for example, there is anecdotal evidence of widespread tumour regression after localized radiation therapy in the setting of systemic ipilimumab treatment37. Furthermore, preclinical evidence exists showing that intratumoural viral therapy38 and cryo-ablation39 can trigger a systemic response in the setting of CTLA-4 blockade.
PD-1 axis
PD-1 is a second inhibitory receptor expressed on T cells. The PD-1 ligands, PD-L1 and PD-L2, are expressed on the surface of APCs and malignant cells, particularly in response to local inflammatory cytokines, such as IFNγ. Similarly to CTLA-4, PD-1 ligation inhibits signalling downstream of the TCR40,41 (FIG. 1). PD-L1 can also ligate to CD80 expressed on T cells as a second mechanism of T-cell suppression42,43. Autoimmune processes developed in pd1 knockout mice include arthritis, nephritis, and myocarditis44,45. PD-1 ligands present within tumours can function as potent mediators of T-cell suppression and intratumoural PD-L1 expression is associated with a poor prognosis in some tumour types, including lung, ovarian or colon cancer, among others46.
PD-1 and PD-L1 blockade are currently among the most promising endeavours in clinical oncology. Two anti-PD-1 mAbs, pembrolizumab and nivolumab, were approved by the FDA in 2014 after the publication of robust data showing that up to 40% of patients with advanced-stage melanoma, including those who previously had no response to ipilimumab, experienced objective responses when treated with these agents, compared with approximately 12% for ipilimumab monotherapy3. In 2015, the combination of ipilimumab and nivolumab was approved by the FDA for the treatment of patients with advanced-stage melanoma based on phase III data showing improved response rates and progression-free survival rates compared with either agent alone47. Nivolumab was also approved in 2015 for the treatment of squamous-cell lung cancer that is refractory to platinum-based therapy based on the results of a phase III study showing a 3.2-month improvement in overall survival, and a 17% improvement in 2-year survival for patients with advanced-stage squamous-cell NSCLC receiving nivolumab compared with those receiving docetaxel for disease48. The indication for the use of nivolumab was then expanded to patients with other types of advanced-stage NSCLC49, as was pembrolizumab for patients with PD-L1-positive NSCLC50. Thus, antibodies that target the PD-1 axis have been approved for the treatment of patients with melanoma3,51 and NSCLC48, and ongoing efforts are seeking to expand the indication for the treatment of RCC51,52, bladder cancer53, ovarian cancer52, Hodgkin lymphoma54, and a growing list of other malignancies.
Intratumoural PD-L1 can suppress T-cell activity through interactions with both PD-1 and CD80 on T cells42,43, and for this reason some investigators have predicted that anti-PD-1 and anti-PD-L1 therapies might have distinct antitumour effects and adverse-effect profiles55. PD-1 blockade would leave the CD80–PD-L1 interaction intact, whereas PD-L1 blockade would leave the PD-L2–PD-1 interaction intact. In spite of these mechanistic differences, distinct clinical features of each approach have not yet become apparent.
Some similarity is shared between the inflammatory toxicities associated with the blockade of the PD-1 axis and CTLA-4; high-grade toxicities, however, are much less common with PD-1 pathway blockade47, with the exception of pneumonitis, which is a particular concern for patients receiving anti-PD-1 or anti-PD-L1 mAbs6,51. Earlier in the development of anti-PD-1 therapy, 3% of the treated patients developed pulmonary toxicity, which was fatal for approximately one third of them6,51. The vigilance for pulmonary toxicities has increased as a result of these observations, and their management has improved47 (TABLE 1). The development of life-threatening pneumonitis can be avoided in the vast majority of patients by early intervention with corticosteroids and withholding anti-PD-1 treatment when appropriate; clinical experiences reflect an evolved approach to pneumonitis management, with no deaths from this toxicity reported in two phase III trials of anti-PD-1 therapy in 2015 (REFS 47,48).
Anti-CTLA-4/anti-PD-1 combinations
We anticipate that the greatest antitumour effect of blocking CTLA-4 as well as the PD-1 axis will come in the form of combination therapy. Dual anti-CTLA-4 and anti-PD-1 therapy has already shown significant promise9,10,47. For example, this approach has demonstrated an unprecedented 58% response rate and an 11.5% complete response rate in patients with advanced-stage melanoma in a global phase III trial47. Combinations with other forms of immune modulation and agents historically thought not to function through immune modulation are being actively investigated for cancer therapy.
Lymphocyte-activation gene 3 (LAG-3)
LAG-3 is expressed on activated conventional T cells, TREG cells, B cells and plasmacytoid dendritic cells56. Upon binding MHC class II molecules on APCs, LAG-3 transmits an inhibitory signal in conventional T cells57, whereas this signalling event enhances the suppressive function of TREG cells (REFS 58,59) (FIG. 1). Co-expression of LAG-3 and PD-1 is a marker of exhausted T cells (dysfunctional T cells classically associated with chronic infection) and, therefore, the blockade of both receptors confers additive therapeutic activity in preclinical models of chronic infection and cancer60–62. Interestingly, a soluble form of LAG-3 has been detected in the serum of patients with breast cancer and its presence correlates with a more-favourable prognosis63. A soluble LAG-3-Ig fusion protein, designed to promote dendritic-cell (DC) maturation through MHC II binding, has been tested in patients with RCC in a phase I clinical trial, resulting in disease stabilization and enhanced CD8+ T-cell activation64. Finally, a blocking mAb targeting LAG-3 is currently being tested in the clinic (NCT01968109)65.
T-cell membrane protein 3 (TIM-3)
TIM-3 (also known as hepatitis A virus cellular receptor 2; HAVCR2) is another exhaustion-associated inhibitory receptor that serves to blunt T-cell-effector function66 and induce apoptosis of T cells66. To date, four natural ligands of TIM-3 have been identified: galectin-9 (REF. 67), HMGB1 (REF. 68), phosphatidyl serine69 and CEACAM-1 (REF. 70) (FIG. 1). TIM-3 blockade has demonstrated antitumour activity in mouse models of colon adenocarcinoma, melanoma, and sarcoma, particularly when combined with PD-L1 blockade71,72. Furthermore, anti-TIM-3 treatment has been shown to enhance the proliferation and cytokine production of CD8+ T cells derived from patients with melanoma73.
T-cell immunoreceptor with Ig and ITIM domains (TIGIT)
TIGIT is expressed on CD4+ T cells, in which it marks TREG cells; on CD8+ T cells, in which it is a marker of exhausted cytotoxic cells; and on other immune cells74,75. TIGIT blockade in animal models mediates antitumour activity in combination with anti-TIM-3 or anti-PD-L1 mAbs. By contrast, a clear autoimmune phenotype has not been described for _Tigit_-deficient mice74,76, which suggests that TIGIT blockade might have a potential role in future immunotherapy regimens without adding significant toxicity.
T-cell co-stimulation
In part as a response to the potent antitumour activity observed when inhibitory T-cell receptors are blocked, substantial interest has been generated towards the activation of co-stimulatory T-cell receptors to control cancer. Upon engagement of the TCR by peptides presented on MHC by APCs, co-stimulatory receptors on T-cells receive a crucial second signal from cell-surface proteins on APCs that enable T-cell activation77 (FIG. 1). The encouraging results of several preclinical studies prompted clinical trials to investigate several agonist mAbs targeting co-stimulatory molecules. These co-stimulatory receptors are members of the tumour necrosis factor receptor (TNFR) family, a group of non-enzymatic cell-surface proteins that mediate proliferation, activation and differentiation responses in T cells.
T-cell antigen 4-1BB homologue (4-1BB)
Therapeutic mAbs targeting the co-stimulatory molecule 4-1BB (also known as CD137 or TNFR superfamily member 9) are among the most advanced co-stimulatory agonists currently in clinical development. 4-1BB is expressed on T cells, natural killer (NK) cells and monocytes78. Stimulation of T cells by 4-1BBL, its cognate ligand expressed on dendritic cells, results in proliferation and upregulation of the antiapoptotic proteins Bcl-2-like protein 1 (Bcl-xL)79, Bcl-2-related protein A1 (Bfl-1)79,80 and CASP8 and FADD-like apoptosis regulator (c-FLIP)80, which protect T cells from activation-induced cell death79–82. Data from preclinical studies have shown the antitumour activity of anti-4-1BB mAb therapy alone and in combination with CTLA-4 blockade83, CD40 activation84, cellular vaccines83,85 or radiation therapy86. Urelumab, a fully human anti-4-1BB agonistic mAb, has demonstrated antitumour activity in patients with melanoma87. A subsequent clinical study, however, was suspended owing to the development of severe hepatotoxicity88. Further clinical testing of urelumab at reduced doses in combination with several regimens is ongoing89. Given the role of 4-1BB in augmenting NK-cell activity, therapies that combine anti-4-1BB mAbs with mAbs targeting tumour cells (such as rituximab or cetuximab) are being developed to increase the ability of tumour-targeting mAbs to mediate antibody-dependent cell-mediated cytotoxicity via NK cells90,91.
Glucocorticoid-induced TNFR-related protein (GITR)
GITR (also known as TNFR superfamily member 18) is another clinically relevant co-stimulatory receptor92. GITR is upregulated when conventional T cells are activated, and is constitutively expressed by TREG cells (REF. 92). GITR activation augments effector T-cell proliferation, cytokine production and resistance to TREG-cell-mediated suppression93–95. Treatment with anti-GITR agonist mAbs has been shown to mediate tumour rejection and the generation of immunological memory in syngeneic mouse models of fibrosarcoma96, colorectal carcinoma97 and melanoma98. GITR ligation is also able to disrupt TREG-cell lineage stability and impart T-effector function99. Treatments using anti-GITR approaches are currently being evaluated in early phase clinical trials across a broad spectrum of malignancies100,101.
CD40
CD40 is a member of the TNFR family, but it is predominantly expressed on dendritic cells, macrophages, monocytes and B cells, and also in malignant melanoma, lymphoma, leukaemia, and carcinoma cells102,103. CD4+ T cells express CD40L, the ligand for CD40, which enables APCs to activate T cells. Upon CD40 ligation, dendritic cells upregulate MHC class II and secrete proinflammatory cytokines, such as IL-12 (REF. 103) (FIG. 1). On B cells, CD40 is important for immunoglobulin class switching104. Therapeutic mAbs that target CD40 on tumour cells have been developed105; however, CD40 agonist mAbs that target nonmalignant immune cells to elicit an antitumour response are of potentially even broader applicability. The macrophage-mediated robust antitumour activity that agonist CD40 mAbs show when combined with chemotherapy for the treatment of patients with pancreatic cancer provides a striking example of their efficacy106.
OX40
OX40 (also known as tumour necrosis factor ligand superfamily member 4) is present on the surface of T cells, NK cells and neutrophils, whereas its ligand, OX40L, is expressed on a number of different immune cells, including APCs107. OX40 engagement on T cells promotes proliferation, survival, and the secretion of cytokines associated with both type 1 and type 2 T helper cell responses108,109. OX40 ligation also blunts the suppressive effects and promotes activation-induced cell death of TREG cells (REFS 110,111). In preclinical models, the ligation of OX40 exerts antitumour activity mediated by CD4+ and CD8+ T cells, and confers immunological memory manifested as resistance to tumour rechallenge112. Results of a phase I trial113 demonstrated that an agonist mAb targeting OX40 given as monotherapy has antitumour activity in patients with melanoma or RCC. A number of follow-up clinical trials using OX40 agonists in combination regimens are currently underway114–117.
Checkpoint blockade plus co-stimulation
The concept of augmenting T-cell activity with co-stimulatory mAbs and concurrently liberating activated T cells to lyse malignant cells by blocking PD-1 or PD-L1 is an appealing antitumour approach. Several ongoing clinical trials involving patients across a broad range of solid organ and haematological malignancies are investigating this possibility; with most of them employing agents that target the PD-1 axis as the means of checkpoint blockade118. In the near future, we will have more data to evaluate the toxicity–benefit ratio of such immunotherapeutic combinations; we anticipate that this knowledge will ultimately lead to the design of new therapies for a wide range of advanced-stage cancers.
CAR-T-cell therapy
The goal of adoptive T-cell therapy is to generate a robust immune-mediated antitumour response through the ex vivo manipulation of T cells. This aim can be accomplished through the selection and expansion of tumour-infiltrating lymphocytes (TILs), or through gene transfer of a synthetic TCR (sTCR) or a chimeric antigen receptor (CAR) into T cells. We will focus on CAR T-cell therapy, which differs from TIL or sTCR-based therapies in that it uses a single-chain variable fragment (scFv) derived from the variable heavy and variable light chains of an antibody to target an extracellular antigen independent of the peptide–HLA complex119,120. In its simplest form, a CAR is encoded by a single gene consisting of an scFv, a transmembrane region, and the CD3ζ chain (the signalling domain of the TCR complex)119. This molecule, a so-called first-generation CAR, provides only activation signal 1 to T cells, and has been shown to lead to T-cell anergy upon repeated antigen stimulation120,121. Second-generation CARs contain an additional co-stimulatory domain that provides activation signal 2 upon the scFv engaging the target antigen121. The most frequently used co-stimulatory molecules, to date, have been the signalling domains of CD28 (REFS 121,122) or 4-1BB123,124, although others125,126 have also been studied. Almost all clinical trials performed to date, and all the trials discussed herein, have used second-generation CARs. In third-generation CARs, two co-stimulatory domains are added to the above design, although direct comparisons with second-generation CARs have not yet been conducted in the clinical setting. Finally, ‘armoured’ CAR T cells have been evaluated in preclinical experiments; the first clinical trials using armoured CAR T cells are now enrolling patients (NCT02498912)127. An armoured CAR vector includes a second gene, encoding a protein that either provides the resulting T cell with a survival or cytotoxicity advantage, or modulates the tumour microenvironment. Examples of such proteins include the proinflammatory cytokine IL-12 (REF. 128), or the immunostimulatory molecules 4-1BBL129 or CD40L130.
CD19-targeted CAR-T-cell therapy
Initial clinical trials using CAR T cells have all focused on targeting CD19, which is an ideal antigen because it is ubiquitously expressed on a broad range of differentiated B cells (from pro-B cells to memory B cells), but it is not expressed on haematopoietic stem cells or any other essential cell types, limiting potential ‘on target-off tumour’ toxicity. For this reason, CD19-targeted CAR T cells have been used to treat diseases from B-cell acute lymphoblastic leukaemia (B-ALL) to more-differentiated non-Hodgkin lymphomas. We and others have found that the expected normal B-cell aplasia is well tolerated and can be managed with monthly administration of intravenous immunoglobulin122,124,131.
B-ALL
To date, the single greatest success of ACT has been achieved with CD19-targeted CAR T cells for the treatment of relapsed and/or refractory paediatric and adult B-ALL122,124,131. The efficacy of CD19-targeted CAR T-cell therapy can be put in perspective when considering the results of a large US–UK cooperative pre-CAR-T-cell therapy era group study that treated adults with ALL after first relapse (n = 609): the median survival was 24 weeks, and the 5-year overall survival was 7%132. Complete response rates from investigations conducted at several institutions testing CAR T-cell therapy have been reported in the range of ~70–90% in heavily pretreated patients122,124,131. Similar response rates have been reported in studies across institutions; however, understanding the key similarities and differences in CAR design, gene transfer technology, and the effects of different trial designs on patient outcomes are key for the field to advance.
Different scFv, co-stimulatory domains, and gene transfer methods have been employed by researchers from several institutions involved in several trials with CAR-T-cell therapy133 (TABLE 2). Most of these trials included patients with relapsed or refractory B-ALL who received salvage chemotherapy, and regardless of the response, were administered CD19-targeted second-generation CAR T cells, most often after lymphodepleting preconditioning chemotherapy. Each study used one of two different anti-CD19 scFvs, different gene-transfer methods and infused either bulk or selected CAR-T-cell populations.
Table 2.
CD19-targeted CAR T-cell design by institution120
| Study | Anti-CD19 scFv clone | Spacer regions | Signalling domains | Genetic modification method | T-cell population |
|---|---|---|---|---|---|
| Baylor165 | FMC63 | Human IgG1 (CH2 and CH3 domains) | CD28 and CD3ζ or CD3ζ alone | Retroviral transduction | Bulk |
| Fred Hutchinson139 | FMC63 | Modified human IgG4 | 4-1BB co-stimulatory CD3ζ activation | Lentiviral transduction | 1:1 CD4+: CD8+ Tcm |
| MD Anderson140 | FMC63 | Modified human IgG4 | CD28 co-stimulatory CD3ζ activation | Sleeping beauty transposon electroporation | Bulk |
| MSKCC122,134,135,147,164 | SJ25C1 | No hinge | CD28 co-stimulatory CD3ζ activation | Retroviral transduction | Bulk |
| NCI131,138,162 | FMC63 | No hinge | CD28 co-stimulatory CD3ζ activation | Retroviral transduction | Bulk |
| UPenn/CHOP124,141,142,152,153,163 | FMC63 | CD8a hinge | 4-1BB co-stimulatory CD3ζ activation | Lentiviral transduction | Bulk |
Several large clinical trials have used CD19-targeted CAR-modified T cells to treat B-ALL at various research centres in the USA, including the Memorial Sloan Kettering Cancer Center (MSKCC)122,134,135, the University of Pennsylvania (UPenn)124,136,137, the US National Cancer Institute (NCI)131,138, the Fred Hutchinson Cancer Center139 and the MD Anderson Cancer Center140 (TABLE 3). Differences in design between these trials included patient populations, conditioning therapies, tumour burden, and CAR-T-cell dose, among other variables. Additionally, the end points defined for each study and the methods for reporting clinical efficacy and safety varied between different institutions122,124,131,134–140.
Table 3.
Clinical outcomes of CD19-targeted CAR-T-cell therapy for patients with B-ALL
| Study/CAR design | Conditioning/CAR-T-cell dose | Patient population | Clinical responses | CAR-T-cell persistence |
|---|---|---|---|---|
| Fred Hutchinson139FMC63-BBζ | Cy 60 mg/kg + Flu 25 mg/m2 daily × 3–5 (46%) or other Cy-based conditioning (54%) 0.2–20 × 106 CAR T cells/kg | n = 24 adults with R/R disease Age range: 22–71 years | 91% CR 87% MRD (by PCR) 29% sCRS | Persistence in responding patients >1 month Improved with Flu conditioning |
| MD Anderson140FMC63-28ζ | No conditioning 1–500 × 106 CAR T cells/m2 | n = 10 Post-alloHSCT uniquely, no active disease | 5 month DFS: 30% No GVHD or sCRS | Detectable up to 3 months |
| MSKCC122,134,135SJ25-28ζ | Cy 1.5–3 g/m2 × 1 dose 1–3 × 106 CAR T cells/kg (fractionated over 2 days) | Adults with R/R disease Median age: 45 years (22–74 years) n = 38 evaluable for safety (n = 28 evaluable for response) | 87% CR 81% MRD-negative (by deep sequencing) Median time to CR: 23 days 6-month DFS: 50% 6-month OS: 59% 14% CD19-negative relapses 23% sCRS | Peak day: 7–14 Persistence 1–3 months |
| NCI131,138FMC63-28ζ | Cy 900 mg/m2 × 1 dose + Flu 25 mg/m2 × 3 doses 1–3 × 106 CAR T cells/kg | Paediatric/young adults with R/R disease Median age: 13 years (1–30 years) n = 21 (n = 20 with B-ALL) Uniquely reported intention-to-treat-analysis | 67% CR 10-month OS: 52% Two CD19-negative relapses 29% sCRS | Detectable up to day 68 (by qPCR) |
| UPenn/CHOP124,141,142FMC63-BBζ | Variable conditioning:>50% received Flu 30 mg/m2 daily ×4 doses + Cy 500 mg/m2 daily × 2 doses 0.76–20.6 × 106 CAR T cells/kg | Paediatric patients with R/R disease Median age: 11 years n = 48 evaluable | 94% CR 82% MRD-negative (by flow cytometry) 6-month DFS: 76% 6-month OS: 78% 67% CD19-negative relapses 29% sCRS | Range detectable:1 month–2 years (by qPCR) |
In the trials conducted at MSKCC122,134,135, UPenn124,141,142, NCI131,138, and Fred Hutchinson139, investigators reported similar remarkably high overall response rates, complete remission rates, minimal residual disease (MRD) negativity (when reported) and comparable toxicity. This is in contrast with the design of the trial carried out at MD Anderson140, in which, uniquely, lymphodepleting conditioning chemotherapy was omitted and an electroporation method of gene transfer that involved co-culture on artificial antigen presenting cells (aAPCs) was employed. Conditioning chemotherapy is likely to deplete immune-suppressive regulatory cells, a process that might be essential to the effectiveness of CAR-T-cell therapy. Similarly, an electroporation process that includes ex vivo culture on aAPCs can potentially exhaust T cells before they are infused.
CAR T-cell persistence is likely an important factor in determining the efficacy of the antitumoural response, although the optimal time of survival of CAR T cells required to eradicate disease in patients is not known, and likely highly variable between tumour types and individual patients. Most clinical trials conducted to date have not routinely detected, as might have been expected, the occurrence of lifelong memory against the target antigen; this is evidenced by only transient B-cell aplasias observed in the majority of patients treated122,131,134,135,138. Investigators conducting the UPenn study uniquely reported persistence of B-cell aplasia of >26 months in the patient with the longest ongoing response (range 1–26 months)124,136. Data from few patients with follow-up durations of over 1 year have been reported to date; however, it is estimated that B-cell aplasia at 6 months was 73% in this trial124,136. This persistence could be caused by several factors that were unique to the UPenn study: the young paediatric population treated (median age 11), the use of fludarabine-based conditioning chemotherapy in the majority of patients (see further discussion below), or the use of 4-1BB as opposed to the CD28 co-stimulatory domain. Paediatric B-ALL is very different compared with B-ALL in adult patients: as adults have a far greater rate of relapse and associated mortality in response to standard of care cancer therapies than children with B-ALL143. Moreover, differences between paediatric and adult patients’ thymic function144 and T-cell-subset populations and immunosenescence145 could potentially explain why CAR T cells might have superior persistence in paediatric patients. In addition, 4-1BB is considered a ‘late’ co-stimulatory signal146 and thus might have a role in increased persistence. Whether any of these factors, alone or in combination with each other and/or conditioning chemotherapy, contribute to increased persistence must be validated by further preclinical and clinical studies.
Despite the differences between the clinical trials discussed earlier122,124,131,134–140, the efficacy and safety outcomes were remarkably similar. Therefore, drawing generalizable conclusions about optimal trial and vector design from the comparison of these studies is difficult. Important lessons on the influence of the conditioning regimen on CAR T-cell persistence and efficacy, however, can be garnered from the results of the trial conducted at Fred Hutchinson, in which changes to the lymphodepleting conditioning regimen were made as the trial progressed139. Preclinical studies had shown that lymphodepleting conditioning chemotherapy is necessary before CAR-T-cell infusion to obtain maximal antitumour efficacy128, an observation also noted in the first cohort of patients with CLL treated with this therapy147,148. Inferior response rates were noted in trials that did not include conditioning chemotherapy, such as those carried out at MD Anderson140 and Baylor149 (TABLE 3).
In the clinical trial carried out at Fred Hutchinson, the addition of fludarabine to the use of cyclophosphamide-based conditioning regimens was investigated in patients with B-ALL (as well non-Hodgkin lymphoma (NHL)). These trials offered a unique opportunity to compare the effects of changes in conditioning regimens in patient cohorts that had otherwise been treated identically139. In this trial, 13 patients with B-ALL received the same dose of CAR T cells, but eight were given fludarabine and the other five were given conditioning regimens lacking fludarabine139. In patients that received fludarabine, CAR-T-cell numbers were found to peak earlier and expand to numbers >100-fold greater than those of patients treated with conditioning regimens lacking fludarabine. At day 28 post-CAR-T-cell infusion, this difference was even more apparent because, in patients who received conditioning without fludarabine, CAR T cells became minimally detectable139. This increased cell expansion and persistence correlated with enhanced clinical responses and toxicity, indicating its functional relevance. Similarly, in a trial that included 19 patients with NHL, nine of them received fludarabine conditioning. In these patients, an identical trend of increased peak CAR-T-cell expansion, and persistence at 28 days was observed. The overall response rate of patients with NHL that received a fludarabine-containing regimen was 83%, compared with 50% of patients treated with conditioning regimens lacking fludarabine. Interestingly, no CD19+ relapses and only one CD19− relapse was observed139.
Another open-ended question is whether CART-cell therapy should serve as a ‘bridge’ to an allogeneic haematopietic stem cell transplantion (alloHSCT), or whether alloHSCT could be avoided. The standard of care for adults with relapsed B-ALL who develop a second complete response is alloHSCT150,151. For patients who would not be eligible for an alloHSCT owing to their disease burden, CAR T-cell therapy helps to reach a second or third complete response, making them newly eligible for transplant. A group of MSKCC investigators have reported on the largest series of adult patients with relapsed B-ALL treated to date (n = 38), with a strategy that encouraged patients to undergo an alloHSCT whenever possible135. However, two-thirds of patients achieving a complete response to CAR-T-cell therapy did not proceed to alloHSCT because of the lack of an available donor, a personal preference, or, most commonly, because of medical co-morbidities that excluded alloHSCT. The overall survival of the patients with >6 months of follow-up who achieved a complete response and proceeded to alloHSCT was 70%135. This is similar to the overall survival of patients who were ineligible or declined alloHSCT (62%; _P_= 0.5). Additionally, a subset of patients who were followed expectantly without alloHSCT have had long-term disease-free survival of >12 months post-CAR-T-cell therapy135. This study is the largest of its kind performed to address this question, but these values were certainly underpowered and the patients in the study were not randomly allocated, thus precluding any firm conclusions on post-CAR-T-cell alloHSCT. These results135, however, suggest that CART-cell therapy might replace alloHSCT for patients with B-ALL in the future.
CLL
The initial trials investigating CAR T-cell therapy and, therefore, the first responses to this therapy were reported in patients with chronic lymphocytic leukaemia (CLL)147,152. The responses in patients treated with CAR T cells for CLL have been more modest than those observed in patients with B-ALL; although exceptional responses have been reported initially among the first two out of three patients achieving a complete response152,153. In an updated analysis by investigators from UPenn, five (inclusive of the initial two) out of 23 evaluable patients achieved a complete response141. In a study carried out at MSKCC, in which nine patients were treated, the first three patients did not receive conditioning therapy, and the following six patients received cyclophosphamide or bendamustine conditioning147,148. None of the patients who did not receive conditioning therapy had a response, and only one of the six patients who received it achieved a complete response147,148.
There are several potential causes for this relative paucity of responses seen in CLL when compared with B-ALL. One reason is that CAR T cells generated from patients with CLL might have inherent effector T-cell dysfunction154,155. Furthermore, it remains to be confirmed whether CAR T cells migrate towards, and penetrate into lymph nodes as efficiently as they do bone marrow. Additionally, immune suppression via T-cell checkpoint inhibitory receptors156, cell types associated with immunosuppression, such as TREG cells (REF.157) and MDSCs158 or supportive cell types, such as CLL-nurse cells159,160, and inhibitory cytokine production161 might influence CAR-T-cell efficacy in patients with CLL to varying degrees. CLL cells might exist in a tumour microenvironment that is suppressive to CAR-T-cell function (FIG. 2). Additional modifications in the design of CAR-T-cell therapies are needed to further optimize their efficacy for the treatment of CLL.
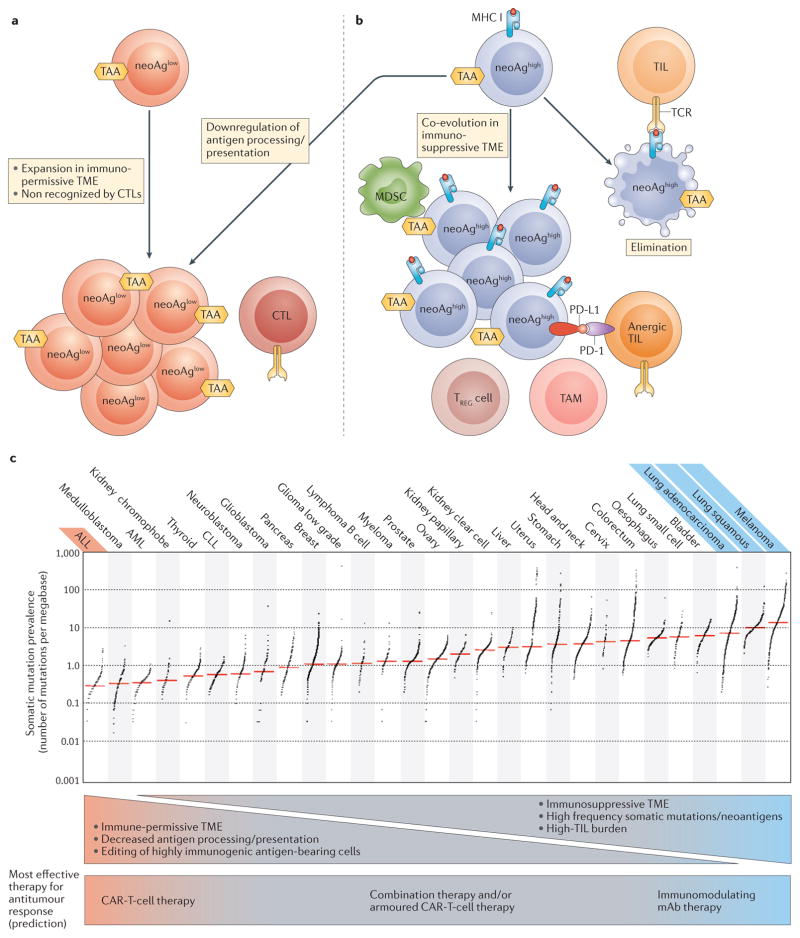
Figure 2. Neoantigen presentation in the tumour microenvironment.
a | Tumour cells can avoid elimination by immunoediting because of low neoantigen presentation (neoAglow). This occurs as a result of a low level of somatic mutations, or because tumour cells might downregulate antigen processing or presentation. NeoAglow tumours might escape detection by endogenous CTLs early in tumorigenesis, and therefore would expand without the pressure to co-evolve within an immunosuppressive microenvironment. This is the ideal setting for CAR T cells to direct a robust antitumour response. b | Tumours that present a high neoantigen burden (neoAghigh) can be eliminated during the immunoediting process (far right). Alternatively, neoAghigh tumour cells might co-evolve within an immunosuppressive tumour microenvironment (TME) that mediates avoidance of T-cell surveillance. This setting is the ideal situation for immune-modulating therapies with monoclonal antibodies to direct a robust antitumour response. c | Tumour types can be ranked by their relative presence of somatic mutations209, and this classification can be used to indirectly estimate neoantigen burden. Tumours can be stratified on the basis of somatic mutation prevalence; tumour types with the most robust clinical responses to CAR T-cell therapy (red) or immune modulating mAbs (blue) are stratified by somatic mutation prevalence. ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia; CAR, chimeric antigen receptor; CLL, chronic lymphocytic leukaemia; CTL, cytotoxic T cell; mAb, monoclonal antibody; MDSC, myeloid-derived suppressor cell; neoAg, neoantigen; PD-1, programmed cell death 1; PD-L1, programmed cell death 1 ligand 1; TAA, tumour-associated antigen; TAM, tumour-associated macrophage; TCR, T-cell receptor; TIL, tumour infiltrating lymphocyte; TREG cell, regulatory T cell. Image reproduced from Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013),
NHL
Several groups have reported their initial experience treating patients with relapsed and/or refractory NHL (mostly of aggressive histology subtypes) using the same CARs as those used to treat B-cell leukaemias139,162–165. Investigators from the NCI carried out a trial that included nine patients with aggressive NHL; either diffuse-large B-cell lymphoma (DLBCL) or primary mediastinal B-cell lymphoma (PMBCL)162. Using cyclophosphamide-conditioning chemotherapy before administration of CAR T cells, four out of seven evaluable patients with aggressive NHL achieved a complete response, which included three patients who had a persistent complete response 9–22 months after treatment. The T-cell counts peaked in the peripheral blood 7–17 days after infusion and persisted for a period between 2 weeks and 2 months162.
Similar to the NCI trial, investigators from UPenn also reported on their CAR-T-cell trials for aggressive NHL with a cohort of 13 patients with DLBCL that had been heavily pretreated163. Different non-myeloablative preconditioning chemotherapy regimens were used in this trial. Five patients achieved a complete response, in all cases ongoing at the time of reporting between 6 months to >1 year163.
At the same time as UPenn investigators reported their results from treating patients with aggressive NHL with CAR-T-cell therapy, researchers at MSKCC reported on 10 evaluable patients with relapsed, aggressive-histology NHL who were chemosensitive to salvage therapy, but with tumour presence, as confirmed by post-treatment PET scans164. Unlike at the NCI or UPenn, the protocol investigated administering CAR T cells in the setting of high-dose chemotherapy (BEAM regimen; carmustine, etoposide, cytarabine, melphalan) followed by autologous stem-cell rescue (ASCR). CAR T cells were administered on days +2 and +3 post-ASCR. Six out of 10 evaluable patients achieved a complete response, with four of them remaining free from disease progression after 13–21 months of follow-up164.
As described earlier, investigators at the Fred Hutchinson conducted a trial that included 19 patients with NHL of different grades139. These patients’ T cells were separated into CD4+ and CD8+, and independently transduced in parallel with a 4-1BB containing CAR. CD8+ cells were further selected to have a potentially favourable central memory immunophenotype (Tcm). The final infusion product was formulated to consist of 1:1 CD4+:CD8+ Tcm cells. The conditioning regimen included included various combinations involving cyclophosphamide and/or fludarabine. The overall response rate in all patients with NHL who received treatment was 63%. Similarly as observed in the trial for B-ALL139, these researchers oberved that T-cell persistence and the complete response rate were significantly improved in the group of patients that received a conditioning treatment containing fludarabine, as previously described.
Finally, investigators at Baylor reported on a trial that included five patients with NHL165. The design of this trial was unique because it involved infusing two CAR-T-cell products simultaneously: one containing a CD28-bearing CAR and the other consisting of an equal dose of T cells containing a first-generation CAR with a CD3ζ signalling domain but without an additional co-stimulatory domain. Furthermore, in contrast with other groups, it did not include lymphodepleting preconditioning chemotherapy. The efficacy of this design was limited, and no sustained remissions were observed in the patients who received treatment. The investigators determined that second-generation CAR T cells persisted longer (up to 9 months as detected in peripheral blood using quantitative PCR) than first-generation CAR T cells165.
Toxicity
The adoption of CAR-T-cell therapy, has been followed by the emergence of a novel set of adverse effects including cytokine-release-syndrome (CRS), macrophage activation syndrome (MAS; or haemophagocytic lymphohistiocytosis, HLH), and neurological toxicities. CRS is a constellation of symptoms derived from the cytokines released by activated T cells and/or activated macrophages122,138,166. A concomitant MAS is evidenced by the presence of elevated levels of ferritin and, in some cases, hypofibrinogenaemia166 in addition to elevated levels of cytokines, such as IL-6 and IL-10. MAS can occur owing to a positive feedback loop that affects signalling pathways activated by the cytokines released by activated CAR T cells138,166. These potential cytokine mediated toxicities range from mild, with isolated fever, to severe (sCRS), with symptoms that include hypotension and respiratory distress requiring the intervention of an intensive care unit122,138. CRP is a parameter that can be measured in routine clinical laboratory tests and serves as a surrogate marker of CRS in patients with B-ALL. A monitored rise in CRP levels in serum is indicative of a high likelihood of impending sCRS122. sCRS and MAS are managed with the administration of antibodies targeting the IL-6 pathway, and with lymphodepleting doses of corticosteroids122,138. Neurological toxicity seems to be distinct from CRS and can occur together with, or independent from, sCRS. The incidence of both toxicities (neurological and sCRS) seems to correlate with disease burden, tumour histology, CAR-T-cell dose, and conditioning chemotherapy. The timing of CRS-mediated toxicity correlates with T-cell expansion in blood because the onset occurs around the time of peak T-cell expansion and usually resolves as T cells contract122,138.
Major differences between CD19-targeted CART-cell trials exist, including differences in how sCRS is defined. In reports from the four major clinical trials for B-ALL mentioned above131,135,137,139, however, authors report similar rates of grade 3/4 CRS or sCRS, ranging from 23–29%. Treatment-related mortality from CRS and neurotoxicity is low. In adults treated for B-ALL, treatment-related mortality is reported as 8% (three out of 38 patients), 4% (one out of 24 patients), and 25% (three out of 12 patients) by MSKCC135, Fred Hutchinson139, and UPenn167 investigators, respectively. Treatment of paediatric B-ALL and of other indications (such as CLL and NHL) in adults have even lower mortality rates, with UPenn investigators reporting no mortalities out of 85 non-adult B-ALL patients treated for all other indications167 and NCI investigators reporting no mortalities in 21 paediatric or young-adult patients treated for B-ALL131. Given the heavy pretreatment history and poor prognosis of the patients included in these trials, the toxicity data need to be considered in the proper context when weighing this type of intervention in a risk–benefit discussion with patients. The modulation of CAR-T-cell infusion doses (for example, administering lower doses to patients with larger tumour burdens), and the selection of patients with lower tumour burdens might be promising approaches to minimize the occurrence of CRS and related toxicities in the future.
Additional targets in haematologic cancer
CD19 is an excellent target for CAR-T-cell therapy because it is expressed across a broad range of B-cell differentiation stages, and is ubiquitously expressed in many patients with a range of B-cell malignancies. Additional potential CAR targets for patients with haematological malignancies, however, have been identified and their use has been validated, with substantial preclinical data available; some of these potential targets have already been applied in the clinic. A selected list of these targets include, for the treatment of B-cell malignancies: CD22 (REF. 168), ROR1 (inactive tyrosine-protein kinase transmembrane receptor ROR1)169,170, CD30 (REF. 171) and Ig kappa (κ) light chain149; for the treatment of multiple myeloma: B-cell maturation antigen (BCMA)172, SLAMF7 (CS1)173, CD38 (REFS 174,175) and CD138 (REF. 176); and for the treatment of AML: CD33 (REF. 177) and CD123 (REFS 178–180). The discussion of these and other targets is beyond the scope of this Review, but they have been discussed in detail elsewhere181.
CAR T cells for solid tumours
The demonstration of clinical efficacy in trials using CAR-T-cell therapy are, at present, limited to haematological malignancies, but this modality is beginning to be explored clinically in the treatment of solid tumours. Solid tumours present three unique challenges not seen in B-ALL. Firstly, when compared with B-ALL, their microenvironment can be considerably more immunosuppressive (FIG. 2). Secondly, antigen selection is, in general, more difficult because the antigen heterogeneity across the same malignancy is generally higher in solid tumours182,183. Thirdly, ‘on-target, off-tumour’ toxicity is more problematic because potential target antigens in solid tumours are more likely to be expressed in other essential organs. New targets for solid tumours that are beginning to enter clinical studies include mesothelin for the treatment of mesothelioma184–186, pancreatic142,186 and ovarian cancer186; disialoganglioside GD2 (REFS 187,188) and EGFRvIII189 for CNS malignancies; and mucin-16 (REFS 190,191) for the treatment of ovarian cancer. The results of the initial clinical trials for these and other targets are awaited. A more detailed discussion of CAR-T-cell therapy for solid tumours can be found elsewhere181.
Potential escape mechanisms
Different physiological mechanisms can prevent durable CAR-T-cell-mediated antitumour responses. These mechanisms include target tumour-antigen escape, lack of CAR-T-cell persistence, and lack of CAR T-cell function. Antigen escape occurs in the setting of an initial response to CAR T cells, in which the target extracellular tumour-associated antigen is down regulated or when a minor tumour subclone that lacks antigen expression outgrows the other clones192. In either situation, malignant cells become undetectable to CAR T cells. Antigen escape was reported as a major cause of relapse in the UPenn trial for paediatric B-ALL, in which 10 out of 15 relapses involved this mechanism and resulted in the expansion of CD19-negative malignant cell populations137. Antigen escape could be addressed by targeting multiple antigens. Lack of CAR T-cell persistence has been previously discussed in this Review. The ideal length of CAR T-cell persistence is unknown; however, it seems that some minimum degree of persistence (weeks to months) is required for optimal CAR-T-cell efficacy and complete tumour eradication. Conditioning chemotherapy, T-cell immunophenotype, the patients’ age and health status, the CAR-T-cell host and CAR vector design are among the factors that appear to influence persistence. Finally, lack of function can be further divided into two main causes. The first is an inability of CAR T cells to access the site of disease owing to a lack of homing signals and/or the presence of exclusion signals in the site of disease. The second is the suppression of CAR-T cell-mediated cytotoxicity at the site of disease by signals from the microenvironment. The lack of persistence or function can be addressed by administering CAR T cells in combination with immunomodulatory antibodies or by administering armoured CAR T cells.
Armoured CAR T cells
In an immunosuppressive tumour microenvironment, CAR T cells are likely to suffer the same loss of cytotoxic functionality as endogenous T cells (FIG. 1A). This phenomenon has been demonstrated by in vivo experiments, in which the injection of CAR T cells into mice bearing large, established tumours led to the upregulation at the protein level of the T-cell inhibitory enzymes diacylglycerol kinase and SHP-1, the cell surface expression of the inhibitory receptors PD-1, LAG-3, and TIM-3, and the inability to clear the tumour193. One strategy to overcome the effects of an immunosuppressive microenvironment is through the further modification of CAR T cells to additionally express immune-modulatory proteins, including ligands and cytokines (FIG. 1B). Examples of three classes of armoured CAR T cells that are currently in preclinical development are described below.
The first example is the inclusion of a second chimeric gene in the CAR vector, in which the PD-1 extracellular receptor domain is fused to the CD28 intracellular signalling domain. This design was tested in the setting of a synthetic TCR (sTCR), but could equally apply to CAR T cells194,195. T cells that included both the PD-1/CD28 fusion gene and the sTCR outperformed T cells that included the sTCR alone in in vitro studies of cytokine secretion and proliferation194,195. In murine xenograft models, and in a syngeneic model of human melanoma, the PD-1/CD28 chimera expressing targeted T cells demonstrated increased clearance rechallenge194,195.
Another strategy involves CAR T cells that are genetically modified to constitutively express stimulatory ligands. T cells co-expressing CD40L130 or 4-1BBL129 with a second-generation CAR have been shown to increase survival of mice with difficult-to-treat systemic lymphoma xenograft. CD40L mediates its effect on CD40+ tumours through T cells directly, by enhancing their immunogenicity, and via stimulation of dendritic cells130. 4-1BBL expression has been shown to bind T-cell co-stimulatory receptors and stimulate, not only the transduced cells themselves, but also through _trans_-co-stimulation of adjacent T cells129.
A third example is the inclusion of a gene construct that leads to the secretion of a pro-inflammatory cytokine. IL-2 (REF. 196), IL-15 (REF. 197) and IL-12 (REF. 128) have all been studied in this context. The first trial using armoured CAR T cells secreting IL-12 has recently opened and is testing a CAR targeting mucin-16 in patients with ovarian cancer (NCT02498912)191,127. Systemic administration of IL-12 was shown to be toxic in early phase clinical trials198, but local administration delivered to the site of the tumour can be achieved through secretion by CAR-targeted T cells. In this capacity, T-cells act as ‘micropharmacies’ and, owing to the low IL-12 levels achieved through localized secretion, systemic toxicities might be avoided. IL-12 secretion benefits CAR T cells through pleotropic effects. In preclinical studies, IL-12 secretion has been shown to obviate the need for preconditioning chemotherapy128, enhance CAR T-cell persistence199, provide resistance to TREG cell (REF. 128) and MDSC200,202 inhibition and result in enhanced antitumour efficacy128,190,199–202.
Given that ovarian cancer, similarly to many other solid tumours, has a high TIL burden, which is indicative of a strongly immunosuppressive microenvironment, we are investigating the efficacy of mucin-16-targeted IL-12-secreting CAR T cells in a phase I trial for patients with relapsed ovarian cancer191.
mAbs, CAR T cells, or combined therapy?
Durable responses are seen using immune checkpoint blockade for the treatment of patients with metastatic melanoma or NSCLC, and CAR T-cell therapy has produced dramatic responses in B-ALL. An appreciation of why each therapy has been so effective for these malignancies, and less so in others (FIG. 2), might lead to more rational design in clinical trials to investigate immunotherapy for other tumour types.
It is understood that, as tumour cells evolve, they are eliminated by immune surveillance, particularly by T cells that respond to tumour neoantigen-derived peptides presented by MHCs203–208. Tumour types such as melanoma and NSCLC, which harbour a high frequency of somatic mutations209, leading to increased presentation of neoantigens, are more likely to escape immune surveillance through co-evolution in an immunosuppressive microenvironment. This same immunosuppressive microenvironment, which thwarts endogenous TILs, might also prevent CAR T cells from generating a robust antitumour response through the same suppressive mechanisms193,210 (FIG. 1).
Thus, we hypothesize that, for tumours with high neoantigen-presenting capacity in an immunosuppressive microenvironment, immune-modulating mAbs, such as those that confer checkpoint blockade, will likely be necessary for the generation of immune-mediated antitumour responses. Additionally, as evidenced by the high rates of durable responses observed in patients with melanoma1–3,9,47 and NSCLC48,211 treated with PD-1 targeting mAbs alone, and especially in patients with melanoma treated with PD-1/CTLA-4 dual targeting10,47, immune-modulating mAbs are frequently sufficient to induce such a response in this setting. Evidence for this hypothesis is supported by data from studies in which a subset of lung adenocarcinomas with higher levels of somatic mutations had increased levels of inflammation- related gene expression and immune-checkpoint effector molecules, including PD-L1 (REF. 212). Furthermore, even within a given malignancy, the prevalence of neo-antigens can be predictive of the response to checkpoint blockade with PD-1-targeted therapy in patients with NSCLC14, and with CTLA-4-targeted therapy in those with melanoma15,16.
We further hypothesize that tumours with low neoantigen-presenting capacity, such as those that have a reduced number of potentially immunogenic somatic mutations (for example, B-ALL209) or, otherwise, do not present neoantigens through downregulated antigen processing, presentation or HLA expression might be overlooked by endogenous T cells. These tumour types might not have had the pressure to co-evolve in an immunosuppressive microenvironment. In this situation, antigen presentation and TIL burden will likely be low, and immune-modulating mAbs alone would be less likely to generate a robust antitumour response. CAR T cells, however, are not inhibited by these barriers, and, as demonstrated with CD19-targeted CAR-T-cell therapy for B-ALL, can induce rapid complete responses in up to 90% of patients in this tumour type, which has a low somatic mutation rate209.
For tumours between both extremes of the neoantigen spectrum, immunotherapies involving either CAR T cells or immune-modulating mAbs alone have not shown the dramatic results seen with melanoma and lung cancer on the one hand, and B-ALL on the other. The limited available preclinical data supports the use of combination cellular and mAb therapy in syngeneic models of sarcoma and breast cancer, in which the combination of PD-1 blockade with murine CAR T cells showed a significantly enhanced antitumour effect compared with either intervention alone210. We predict that, in patients with tumour types of an intermediate neoantigen-presentation capacity, the maximal immune-mediated antitumour responses might be best achieved in patients in which both the microenvironment can be modified and HLA-independent targeted effectors added, such as with combination immune modulating mAbs plus CAR-T-cell therapy or, potentially, armoured CAR-T-cell therapy. However, a risk of toxicities being exacerbated does exist when these approaches are used. We eagerly await the results of the first trial investigating the combination of CTLA-4 blockade with CAR T cells (NCT00586391)213, and the first trial using an armoured CAR vector (NCT02498912)127.
This neoantigen burden-based response hypothesis is not predicted to apply to other types of ACT. Unlike CAR-T-cell therapy, bulk TIL-based therapy requires tumour antigen peptides to be presented on MHCs. Thus, TIL therapy would more likely be successful in a tumour type with high neoantigen burden, as has been demonstrated with the successful use of TIL therapy in treatment of melanoma214–216. This requirement would be overcome, however, if T cells targeting a specific neoantigen epitope in a particular patient could be identified and their numbers expanded ex vivo217. In such patients, the efficacy of neoantigen-directed TILs or sTCR-based therapies would remain subject to downregulation of HLA, defects in antigen processing and/or presentation machinery, or an immunosuppressive microenvironment, all of which can be found more frequently in tumour types with a high neoantigen burden (FIG. 2). Overall, this might partially explain the uniquely robust success among ACT, at least to date, of CAR-T-cell monotherapy in B-ALL.
Conclusions
The remarkable clinical results observed in trials investigating immunotherapy since 2010 have generated a large amount of interest in this therapeutic modality. Clinical trials using checkpoint blockade inhibitors to treat patients with metastatic mela-noma1–3,9,47 and NSCLC48,211, and trials using CAR T cells to treat relapsed or refractory B-ALL122,124,131 have demonstrated that treating cancer ‘indirectly’ by acting on the immune system can yield durable disease control in patients with malignancies previously thought to be uniformly fatal (BOX 1). We have focused on mAbs and CAR T-cell therapy, but a number of other modalities of immunotherapy also offer great hope. These include immunomodulatory small molecules218, oncolytic viruses38, vaccines219, and tumour-targeting mAbs220, as well as attempts to overcome T-cell exclusion221, and to exploit the immunomodulatory potential of chemotherapy and radiation therapy. The efficacy of these methods can be complementary to that of the technologies discussed here. Conceptualizing which tumour types are most likely to respond to different immunotherapies by categorizing those tumours according to their neoantigen presentation ability and their microenvironment will help investigators choose the appropriate combinations of immunotherapy for each particular cancer; and with the ongoing advances in precision medicine, to facilitate personalized therapeutic selection for each patient.
Box 1. Anticipated challenges.
Immunomodulatory antibodies
- Identify optimal combinations of immunomodulatory monoclonal antibodies with each other or with other forms of cancer therapy for individual cancer types. Such combinations are key to expanding the role of immunotherapy
- Minimize impact of ‘on-target, off-tumour’ adverse effects. Immunomodulatory antibodies are not inherently cancer-specific, and therefore inflammation in benign tissues can occur. With new combinations emerging, investigators must remain wary of new or more-severe adverse effects
- Define ideal treatment duration. Unlike many cancer treatments, the activity of immunotherapy can persist after the drugs have been cleared
CAR T cells
- The challenge for relapsed/refractory B-ALL is to routinely translate deep remissions into cures. Fine-tuning conditioning chemotherapy regimens and targeting multiple antigens simultaneously to avoid antigen escape might address this issue
- The challenge for CLL and solid tumours is to achieve response rates similar to those for B-ALL. Obstacles are microenvironment-mediated immunosuppression, lack of persistence, lack of appropriate homing and/or access to the site of disease and, in some cases, lack of tumour-specific ubiquitously expressed target antigens. Modulation of the microenvironment with armoured CAR T cells or with co-administration of immunomodulatory antibodies might minimize these hurdles
- To mitigate cytokine-release syndrome and related toxicities: modifying cell-dose and frequency of administration, including administering lower doses of CAR T cells initially to patients with bulky disease followed by larger subsequent doses and treating patients in earlier stages of disease. Additionally, with the improvement of biomarkers of CRS, early intervention for this adverse event might be possible
Combinations
- Identify the safest/most efficacious combinations of immunomodulatory antibodies and/or adoptive cellular therapeutics. Rational preclinical evidence-based approaches need to be taken, but optimization in patients will be time-consuming
- In addition to selecting the appropriate combinations of agents, unique challenges to be addressed in phase I trials include selecting initial doses, determining sequencing and timing of agents, estimating maximum tolerated dose, identifying agents with limited single agent activity/toxicity, but with considerable potential for synergy
B-ALL, B-cell acute lymphoblastic leukaemia; CAR, chimeric antigen receptor; CLL, chronic lymphocytic leukaemia; CRS, cytokine-release syndrome.
Key points.
- Cancer immunotherapies have the potential to generate robust antitumour responses; this can be achieved through several methods, such as modulatory antibodies or adoptive cellular therapy
- Since 2010, clinical trials using different immunotherapeutic approaches to treat patients with several tumour types have yielded unprecedented results
- In contrast with therapies that act on the tumour itself, immunotherapy-dependent antitumour responses can be sustained after the treatment has finished
- The optimal efficacy of immunotherapy will likely be achieved with designs that include combinations of different immunotherapeutic approaches, or immunotherapy combined with other cancer treatments
Acknowledgments
The authors would like to thank their funders. D.N.K. receives support through the American Association for Cancer Research Amgen fellowship in Clinical/Translational Cancer Research and the American Philosophical Society Daland Fellowship in Clinical Investigation. E.L.S. receives support from the Conquer Cancer Foundation of ASCO, Lymphoma Research Foundation, MSKCC Technology Development Fund, and the Multiple Myeloma Research Foundation. R.B.J. receives support from the Annual Terry Fox Run for Cancer Research (New York, NY) organized by the Canada Club of New York, Carson Family Charitable Trust, Emerald Foundation, the Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center (Innovations in the structures, functions and targets of monoclonal antibody-based drugs for cancer), Kate’s Team, National Institutes of Health Grants (R01CA138738-05, PO1CA059350, PO1CA190174-01), and the William Lawrence and Blanche Hughes Foundation. J.D.W. receives funding support from Bristol-Myers Squibb, Emerald Foundation, Genentech, the Ludwig Center for Cancer Immunotherapy, Medimmune, Merck Pharmaceuticals, Polynoma Pharmaceuticals and Swim Across America.
Footnotes
Author contributions
All authors researched data for article, contributed to discussion of the content, wrote the manuscript and reviewed/edited the article before submission.
Competing interests statement
D.N.K. and E.L.S. and declare no competing interests. R.J.B. is a co-founder, stockholder, and consultant for Juno Therapeutics Inc. J.D.W. is a consultant for Bristol Myers Squibb, Genentech, Medimmune, Merck Pharmaceuticals and Polynoma Pharmaceuticals.
References
- 1.Coley WB. The treatment of inoperable sarcoma by bacterial toxins (the mixed toxins of the Streptococcus erysipelas and the Bacillus prodigiosus) Proc R Soc Med. 1910;3:1–48. [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2014;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 3.Robert C, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 4.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kantoff PW, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 6.Rizvi NA, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pedicord VA, Montalvo W, Leiner IM, Allison JP. Single dose of anti-CTLA-4 enhances CD8+ T-cell memory formation, function, and maintenance. Proc Natl Acad Sci USA. 2011;108:266–271. doi: 10.1073/pnas.1016791108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schadendorf D, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33:1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman PB, D’Angelo SP, Wolchok JD. Rapid eradication of a bulky melanoma mass with one dose of immunotherapy. N Engl J Med. 2015;372:2073–2074. doi: 10.1056/NEJMc1501894. [DOI] [PubMed] [Google Scholar]
- 10.Postow MA, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilgenhof S, et al. Single-center experience with ipilimumab in an expanded access program for patients with pretreated advanced melanoma. J Immunother. 2013;36:215–222. doi: 10.1097/CJI.0b013e31828eed39. [DOI] [PubMed] [Google Scholar]
- 12.Kitano S, et al. Computational algorithm-driven evaluation of monocytic myeloid-derived suppressor cell frequency for prediction of clinical outcomes. Cancer Immunol Res. 2014;2:812–821. doi: 10.1158/2326-6066.CIR-14-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hannani D, et al. Anticancer immunotherapy by CTLA-4 blockade: obligatory contribution of IL-2 receptors and negative prognostic impact of soluble CD25. Cell Res. 2015;25:208–224. doi: 10.1038/cr.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rizvi NA, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snyder A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Allen EM, et al. Genomic correlates of response to CTLA4 blockade in metastatic melanoma. Science. 2015;350:207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonifaz L, et al. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walunas TL, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 19.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tivol EA, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 21.Waterhouse P, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 22.Matheu MP, et al. Imaging regulatory T cell dynamics and CTLA4-mediated suppression of T cell priming. Nat Commun. 2015;6:6219. doi: 10.1038/ncomms7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wing K, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi T, et al. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Read S, Malmström V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simpson TR, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selby MJ, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1:32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- 28.Phan GQ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gregor PD, et al. CTLA-4 blockade in combination with xenogeneic DNA vaccines enhances T-cell responses, tumor immunity and autoimmunity to self antigens in animal and cellular model systems. Vaccine. 2004;22:1700–1708. doi: 10.1016/j.vaccine.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 30.Quezada SA. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 32.Wolchok JD, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 33.Wolchok JD, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 34.Eisenhauer EA, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 35.Yang JC, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30:825–830. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Royal RE, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Postow MA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zamarin D, et al. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med. 2014;6:226ra32–226ra32. doi: 10.1126/scitranslmed.3008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waitz R, Fassò M, Allison JP. CTLA-4 blockade synergizes with cryoablation to mediate tumor rejection. Oncoimmunology. 2014;1:544–546. doi: 10.4161/onci.19442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chemnitz JM, et al. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 41.Parry RV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park JJ, et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood. 2010;116:1291–1298. doi: 10.1182/blood-2010-01-265975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paterson AM, et al. The programmed death-1 ligand 1:B7-1 pathway restrains diabetogenic effector T cells in vivo. J Immunol. 2011;187:1097–1105. doi: 10.4049/jimmunol.1003496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishimura H, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 45.Nishimura H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 46.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 47.Larkin J, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brahmer J, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–134. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.U.S. Food and Drug Administation. FDA expands approved use of Opdivo in advanced lung cancer. [online] 2014 http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm466413.htm.
- 50.U.S. Food and Drug Administation. FDA approves Keytruda for advanced non-small cell lung cancer. [online] 2015 http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm465444.htm.
- 51.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brahmer JR, et al. Nivolumab (anti-PD-1, BMS-936558, ONO-4538) in patients (pts) with advanced non-small-cell lung cancer (NSCLC): survival and clinical activity by subgroup analysis [abstract] J Clin Oncol. 2014;32(Suppl):8112. [Google Scholar]
- 53.Powles T, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 54.Ansell SM, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2014;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huard B, et al. Cellular expression and tissue distribution of the human LAG-3-encoded protein, an MHC class II ligand. Immunogenetics. 1994;39:213–217. doi: 10.1007/BF00241263. [DOI] [PubMed] [Google Scholar]
- 57.Huard B, et al. CD4/major histocompatibility complex class II interaction analyzed with CD4- and lymphocyte activation gene-3 (LAG-3)–Ig fusion proteins. Eur J Immunol. 1995;25:2718–2721. doi: 10.1002/eji.1830250949. [DOI] [PubMed] [Google Scholar]
- 58.Huang CT, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 59.Okamura T, et al. CD4+CD25−LAG3+ regulatory T cells controlled by the transcription factor Egr-2. Proc Natl Acad Sci USA. 2009;106:13974–13979. doi: 10.1073/pnas.0906872106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blackburn SD, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Butler NS, et al. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2012;13:188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woo SR, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Triebel F, Hacene K, Pichon MF. A soluble lymphocyte activation gene-3 (sLAG-3) protein as a prognostic factor in human breast cancer expressing estrogen or progesterone receptors. Cancer Lett. 2006;235:147–153. doi: 10.1016/j.canlet.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 64.Brignone C, et al. A phase I pharmacokinetic and biological correlative study of IMP321, a novel MHC class II agonist, in patients with advanced renal cell carcinoma. Clin Cancer Res. 2009;15:6225–6231. doi: 10.1158/1078-0432.CCR-09-0068. [DOI] [PubMed] [Google Scholar]
- 65.US National Library of Science. ClinicalTrials.gov [online] 2016 https://www.clinicaltrials.gov/ct2/show/NCT01968109.
- 66.Jin HT, et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci USA. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu C, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 68.Chiba S, et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol. 2012;13:832–842. doi: 10.1038/ni.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakayama M, et al. Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood. 2009;113:3821–3830. doi: 10.1182/blood-2008-10-185884. [DOI] [PubMed] [Google Scholar]
- 70.Huang YH, et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2015;517:386–390. doi: 10.1038/nature13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ngiow SF, et al. Anti-TIM3 antibody promotes T cell IFN-γ-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71:3540–3551. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- 72.Sakuishi K, et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fourcade J, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnston RJ, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8+ T cell effector function. Cancer Cell. 2014;26:923–937. doi: 10.1016/j.ccell.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 75.Lozano E, Dominguez-Villar M, Kuchroo V, Hafler DA. The TIGIT/CD226 axis regulates human T cell function. J Immunol. 2012;188:3869–3875. doi: 10.4049/jimmunol.1103627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kurtulus S, et al. Mechanisms of TIGIT-driven immune suppression in cancer. J Immunother Cancer. 2014;2:O13. [Google Scholar]
- 77.Khalil DN, et al. The new era of cancer immunotherapy: manipulating T-cell activity to overcome malignancy. Adv Cancer Res. 2015;128:1–68. doi: 10.1016/bs.acr.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 78.Bartkowiak T, Curran MA. 4-1BB agonists: multi-potent potentiators of tumor immunity. Front Oncol. 2015;5:117. doi: 10.3389/fonc.2015.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee HW, et al. 4-1BB promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xL and Bfl-1. J Immunol. 2002;169:4882–4888. doi: 10.4049/jimmunol.169.9.4882. [DOI] [PubMed] [Google Scholar]
- 80.Stärck L, Scholz C, Dörken B, Daniel PT. Costimulation by CD137/4-1BB inhibits T cell apoptosis and induces Bcl-xL and c-FLIPshort via phosphatidylinositol 3-kinase and AKT/protein kinase B. Eur J Immunol. 2005;35:1257–1266. doi: 10.1002/eji.200425686. [DOI] [PubMed] [Google Scholar]
- 81.Shuford WW, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vinay DS, Kwon BS. 4-1BB (CD137), an inducible costimulatory receptor, as a specific target for cancer therapy. BMB Rep. 2014;47:122–129. doi: 10.5483/BMBRep.2014.47.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Curran MA, et al. Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PLoS ONE. 2011;6:e19499. doi: 10.1371/journal.pone.0019499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Uno T, et al. Eradication of established tumors in mice by a combination antibody-based therapy. Nat Med. 2006;12:693–698. doi: 10.1038/nm1405. [DOI] [PubMed] [Google Scholar]
- 85.Tirapu I, et al. Improving efficacy of interleukin-12-transfected dendritic cells injected into murine colon cancer with anti-CD137 monoclonal antibodies and alloantigens. Int J Cancer. 2004;110:51–60. doi: 10.1002/ijc.20093. [DOI] [PubMed] [Google Scholar]
- 86.Shi W, Siemann DW. Augmented antitumor effects of radiation therapy by 4-1BB antibody (BMS-469492) treatment. Anticancer Res. 2006;26:3445–3453. [PubMed] [Google Scholar]
- 87.Molckovsky A, Siu LL. First-in-class, first-in-human phase I results of targeted agents: highlights of the 2008 American Society of Clinical Oncology meeting. J Hematol Oncol. 2008;1:20. doi: 10.1186/1756-8722-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Garber K. Beyond ipilimumab: new approaches target the immunological synapse. J Natl Cancer Inst. 2011;103:1079–1082. doi: 10.1093/jnci/djr281. [DOI] [PubMed] [Google Scholar]
- 89.US National Library of Science. ClinicalTrials.gov [online] 2016 https://clinicaltrials.gov/ct2/show/NCT02253992.
- 90.James AM, Cohen AD, Campbell KS. Combination immune therapies to enhance anti-tumor responses by NK cells. Front Immunol. 2013;4:481. doi: 10.3389/fimmu.2013.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kohrt HE, et al. Targeting CD137 enhances the efficacy of cetuximab. J Clin Invest. 2014;124:2668–2682. doi: 10.1172/JCI73014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 92.Schaer DA, Cohen AD, Wolchok JD. Anti-GITR antibodies — potential clinical applications for tumor immunotherapy. Curr Opin Investig Drugs. 2010;11:1378–1386. [PubMed] [Google Scholar]
- 93.Kanamaru F, et al. Costimulation via glucocorticoid-induced TNF receptor in both conventional and CD25+ regulatory CD4+ T cells. J Immunol. 2004;172:7306–7314. doi: 10.4049/jimmunol.172.12.7306. [DOI] [PubMed] [Google Scholar]
- 94.Ronchetti S, et al. Glucocorticoid-induced TNFR-related protein lowers the threshold of CD28 costimulation in CD8+ T cells. J Immunol. 2007;179:5916–5926. doi: 10.4049/jimmunol.179.9.5916. [DOI] [PubMed] [Google Scholar]
- 95.Valzasina B, et al. Triggering of OX40 (CD134) on CD4+CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105:2845–2851. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 96.Mitsui J, et al. Two distinct mechanisms of augmented antitumor activity by modulation of immunostimulatory/inhibitory signals. Clin Cancer Res. 2010;16:2781–2791. doi: 10.1158/1078-0432.CCR-09-3243. [DOI] [PubMed] [Google Scholar]
- 97.Bulliard Y, et al. Activating Fc γ receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J Exp Med. 2013;210:1685–1693. doi: 10.1084/jem.20130573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cohen AD, et al. Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. PLoS ONE. 2010;5:e10436. doi: 10.1371/journal.pone.0010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schaer DA, et al. GITR pathway activation abrogates tumor immune suppression through loss of regulatory T cell lineage stability. Cancer Immunol Res. 2013;1:320–331. doi: 10.1158/2326-6066.CIR-13-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT02583165.
- 101.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT02628574.
- 102.Eliopoulos AG, Young LS. The role of the CD40 pathway in the pathogenesis and treatment of cancer. Curr Opin Pharmacol. 2004;4:360–367. doi: 10.1016/j.coph.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 103.Van Kooten C, Banchereau J. CD40–CD40 ligand. J Leukoc Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 104.Kawabe T, et al. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 105.Burington B, et al. CD40 pathway activation status predicts response to CD40 therapy in diffuse large B cell lymphoma. Sci Transl Med. 2011;3:74ra22. doi: 10.1126/scitranslmed.3001620. [DOI] [PubMed] [Google Scholar]
- 106.Beatty GL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baumann R, et al. Functional expression of CD134 by neutrophils. Eur J Immunol. 2004;34:2268–2275. doi: 10.1002/eji.200424863. [DOI] [PubMed] [Google Scholar]
- 108.Rogers PR, et al. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 2001;15:445–455. doi: 10.1016/s1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 109.Arestides RSS, et al. Costimulatory molecule OX40L is critical for both Th1 and Th2 responses in allergic inflammation. Eur J Immunol. 2002;32:2874–2880. doi: 10.1002/1521-4141(2002010)32:10<2874::AID-IMMU2874>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 110.Griseri T, Asquith M, Thompson C, Powrie F. OX40 is required for regulatory T cell-mediated control of colitis. J Exp Med. 2010;207:699–709. doi: 10.1084/jem.20091618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hirschhorn-Cymerman D, et al. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J Exp Med. 2009;206:1103–1116. doi: 10.1084/jem.20082205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pan PY, et al. OX40 ligation enhances primary and memory cytotoxic T lymphocyte responses in an immunotherapy for hepatic colon metastases. Mol Ther. 2002;6:528–536. doi: 10.1006/mthe.2002.0699. [DOI] [PubMed] [Google Scholar]
- 113.Curti BD, et al. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res. 2013;73:7189–7198. doi: 10.1158/0008-5472.CAN-12-4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT01862900.
- 115.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT01303705.
- 116.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT02205333.
- 117.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT02221960.
- 118.Naidoo J, Page DB, Wolchok JD. Immune modulation for cancer therapy. Br J Cancer. 2014;111:2214–2219. doi: 10.1038/bjc.2014.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci USA. 1989;86:10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brentjens RJ, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 121.Brentjens RJ, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res. 2007;13:5426–5435. doi: 10.1158/1078-0432.CCR-07-0674. [DOI] [PubMed] [Google Scholar]
- 122.Davila ML, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Imai C, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 124.Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hombach AA, et al. OX40 costimulation by a chimeric antigen receptor abrogates CD28 and IL-2 induced IL-10 secretion by redirected CD4+ T cells. Oncoimmunology. 2012;1:458–466. doi: 10.4161/onci.19855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guedan S, et al. ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood. 2014;124:1070–1080. doi: 10.1182/blood-2013-10-535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.US National Library of Science. ClinicalTrials.gov [online] 2015 http://www.clinicaltrials.gov/ct2/show/NCT02498912.
- 128.Pegram HJ, et al. Tumor-targeted T cells modified tosecrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119:4133–4141. doi: 10.1182/blood-2011-12-400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhao Z, et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell. 2015;28:415–428. doi: 10.1016/j.ccell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Curran KJ, et al. Enhancing antitumor efficacy of chimeric antigen receptor T cells through constitutive CD40L expression. Mol Ther. 2015;23:769–778. doi: 10.1038/mt.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee DW, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2014;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fielding AK, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109:944–950. doi: 10.1182/blood-2006-05-018192. [DOI] [PubMed] [Google Scholar]
- 133.Pegram HJ, Smith EL, Rafiq S, Brentjens RJ. CAR therapy for hematological cancers: can success seen in the treatment of B-cell acute lymphoblastic leukemia be applied to other hematological malignancies? Immunotherapy. 2015;7:545–561. doi: 10.2217/imt.15.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Park JH, et al. CD19-Targeted 19-28z CAR modified autologous T cells induce high rates of complete remission and durable responses in adult patients with relapsed, refractory B-cell ALL. Blood. 2014;124:382. [Google Scholar]
- 135.Park JH, et al. Efficacy and safety of CD19-targeted 19-28z CAR modified T cells in adult patients with relapsed or refractory B-ALL. J Clin Oncol. 2015;33:7010. [Google Scholar]
- 136.Grupp SA, et al. T cells engineered with a chimeric antigen receptor (CAR) targeting CD19 (CTL019) have long term persistence and induce durable remissions in children with relapsed, refractory ALL. Blood. 2014;124:380. [Google Scholar]
- 137.Grupp SA. Immunotherapy for childhood leukemia. Presented at the 2015 ASCO Annual Meeting; 2015. [Google Scholar]
- 138.Lee DW, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Turtle C, et al. Immunotherapy with CD19-specific chimeric antigen receptor (CAR)-modified T cells of defined subset composition. J Clin Oncol. 2015;33:3006. [Google Scholar]
- 140.Kebriaei P, et al. Adoptive therapy using sleeping beauty gene transfer system and artificial antigen presenting cells to manufacture T cells expressing CD19-specific chimeric antigen receptor. Blood. 2014;124:311. [Google Scholar]
- 141.Porter DL, et al. Randomized, phase II dose optimization study of chimeric antigen receptor modified T cells directed against CD19 (CTL019) in patients with relapsed, refractory CLL. Blood. 2014;124:1982. [Google Scholar]
- 142.Beatty GL, et al. Safety and antitumor activity of chimeric antigen receptor modified T cells in patients with chemotherapy refractory metastatic pancreatic cancer [abstract] J Clin Oncol. 2015;33(Suppl):3007. [Google Scholar]
- 143.Howlader N, et al. SEER Cancer Statistics Review, 1975–2012. National Cancer Institute [online] 2015 http://seer.cancer.gov/crs/1975_2012/
- 144.Aspinall R, Andrew D. Thymic involution in aging. J Clin Immunol. 2000;20:250–256. doi: 10.1023/a:1006611518223. [DOI] [PubMed] [Google Scholar]
- 145.Goronzy JJ, Li G, Yu M, Weyand CM. Signaling pathways in aged T cells — a reflection of T cell differentiation, cell senescence and host environment. Semin Immunol. 2012;24:365–372. doi: 10.1016/j.smim.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 147.Brentjens RJ, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Park JH, et al. Impact of the conditioning chemotherapy on outcomes in adoptive T cell therapy: results from a phase I clinical trial of autologous CD19-targeted T cells for patients with relapsed CLL. Blood. 2012;120:1797. [Google Scholar]
- 149.Ramos C, et al. Clinical responses in patients infused with T lymphocytes redirected to target κ-light immunoglobulin chain. Blood. 2013;122:506. [Google Scholar]
- 150.Hahn T, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute lymphoblastic leukemia in adults: an evidence-based review. Biol Blood Marrow Transplant. 2006;12:1–30. doi: 10.1016/j.bbmt.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 151.Eapen M, et al. Outcomes after HLA-matched sibling transplantation or chemotherapy in children with B-precursor acute lymphoblastic leukemia in a second remission: a collaborative study of the Children’s Oncology Group and the Center for International Blood and Marrow Transplant Research. Blood. 2006;107:4961–4967. doi: 10.1182/blood-2005-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Porter DL, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Porter DL, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Christopoulos P, et al. Definition and characterization of the systemic T-cell dysregulation in untreated indolent B-cell lymphoma and very early CLL. Blood. 2011;117:3836–3846. doi: 10.1182/blood-2010-07-299321. [DOI] [PubMed] [Google Scholar]
- 155.Riches JC, et al. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2013;121:1612–1621. doi: 10.1182/blood-2012-09-457531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.McClanahan F, et al. Mechanisms of PD-L1/PD-1 mediated CD8 T-cell dysfunction in the context of aging-related immune defects in the E -TCL1 CLL mouse model. Blood. 2015;126:212–221. doi: 10.1182/blood-2015-02-626754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.D’Arena G, et al. Regulatory T-cell number is increased in chronic lymphocytic leukemia patients and correlates with progressive disease. Leuk Res. 2011;35:363–368. doi: 10.1016/j.leukres.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 158.Jitschin R, et al. CLL-cells induce IDOhi CD14+HLA-DRlo myeloid-derived suppressor cells that inhibit T-cell responses and promote TRegs. Blood. 2014;124:750–760. doi: 10.1182/blood-2013-12-546416. [DOI] [PubMed] [Google Scholar]
- 159.Boissard F, et al. Nurse like cells: chronic lymphocytic leukemia associated macrophages. Leuk Lymphoma. 2015;56:1570–1572. doi: 10.3109/10428194.2014.991731. [DOI] [PubMed] [Google Scholar]
- 160.Burger JA, et al. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96:2655–2663. [PubMed] [Google Scholar]
- 161.Saulep-Easton D, et al. The BAFF receptor TACI controls IL-10 production by regulatory B cells and CLL B cells. Leukemia. 2015;30:163–172. doi: 10.1038/leu.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kochenderfer JN, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2014;33:540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Schuster SJ, et al. Phase IIa trial of chimeric antigen receptor modified T cells directed against CD19 (CTL019) in patients with relapsed or refractory CD19+ lymphomas. J Clin Oncol. 2015;33:8516. [Google Scholar]
- 164.Sauter CS, et al. Phase I trial of 19-28z chimeric antigen receptor modified T cells (19-28z CAR-T) post-high dose therapy and autologous stem cell transplant (HDT-ASCT) for relapsed and refractory (rel/ref) aggressive B-cell non-Hodgkin lymphoma (B-NHL) J Clin Oncol. 2015;33:8515. [Google Scholar]
- 165.Savoldo B, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20:119–122. doi: 10.1097/PPO.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Frey NV, et al. Refractory cytokine release syndrome in recipients of chimeric antigen receptor (CAR) T cells. Blood. 2014;124:2296. [Google Scholar]
- 168.Haso W, et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood. 2013;121:1165–1174. doi: 10.1182/blood-2012-06-438002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Berger C, et al. Safety of targeting ROR1 in primates with chimeric antigen receptor-modified T cells. Cancer Immunol Res. 2015;3:206–216. doi: 10.1158/2326-6066.CIR-14-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hudecek M, et al. The B-cell tumor-associated antigen ROR1 can be targeted with T cells modified to express a ROR1-specific chimeric antigen receptor. Blood. 2010;116:4532–4541. doi: 10.1182/blood-2010-05-283309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ying Z-T, et al. First-in-patient proof of safety and efficacy of a 4th generation chimeric antigen receptor-modified T cells for the treatment of relapsed or refractory CD30 positive lymphomas [poster]. Presented at the 13th International Conference on Malignant Lymphoma; 2015. [Google Scholar]
- 172.Carpenter RO, et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. 2013;19:2048–2060. doi: 10.1158/1078-0432.CCR-12-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chu J, et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia. 2014;28:917–927. doi: 10.1038/leu.2013.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mihara K, et al. T-cell immunotherapy with a chimeric receptor against CD38 is effective in eliminating myeloma cells. Leukemia. 2012;26:365–367. doi: 10.1038/leu.2011.205. [DOI] [PubMed] [Google Scholar]
- 175.Drent E, et al. CD38 chimeric antigen receptor engineered T cells as therapeutic tools for multiple myeloma. Blood. 2014;124:4759. [Google Scholar]
- 176.Guo B, et al. CD138-directed adoptive immunotherapy of chimeric antigen receptor (CAR)-modified T cells for multiple myeloma. J Cell Immunother. 2015 http://dx.doi.org/10.1016/j.jocit.2014.11.001.
- 177.Kenderian SS, et al. CD33 specific chimeric antigen receptor T cells exhibit potent preclinical activity against human acute myeloid leukemia. Leukemia. 2015;29:1637–1647. doi: 10.1038/leu.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gill S, et al. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood. 2014;123:2343–2354. doi: 10.1182/blood-2013-09-529537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tettamanti S, et al. Targeting of acute myeloid leukaemia by cytokine-induced killer cells redirected with a novel CD123-specific chimeric antigen receptor. Br J Haematol. 2013;161:389–401. doi: 10.1111/bjh.12282. [DOI] [PubMed] [Google Scholar]
- 180.Mardiros A, et al. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood. 2013;122:3138–3148. doi: 10.1182/blood-2012-12-474056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jackson HJ, Rafiq S, Brentjens RJ. Driving CAR T cells forward. Nat Rev Clin Oncol. 2016 doi: 10.1038/nrclinonc.2016.36. http://dx.doi.org/10.1038/nrclinonc.2016.36. [DOI] [PMC free article] [PubMed]
- 182.Marusyk A, Polyak K. Tumor heterogeneity: causes and consequences. Biochim Biophys Acta. 2010;1805:105–117. doi: 10.1016/j.bbcan.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Fidler IJ, Hart IR. Biological diversity in metastatic neoplasms: origins and implications. Science. 1982;217:998–1003. doi: 10.1126/science.7112116. [DOI] [PubMed] [Google Scholar]
- 184.Adusumilli PS, et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med. 2014;6:261ra151. doi: 10.1126/scitranslmed.3010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Beatty GL, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res. 2014;2:112–120. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tanyi J, et al. Safety and feasibility of chimeric antigen receptor modified T cells directed against mesothelin (CART-meso) in patients with mesothelin expressing cancers [abstract] Cancer Res. 2015;75(Suppl):CT105. [Google Scholar]
- 187.Louis CU, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Singh N, et al. Nature of tumor control by permanently and transiently modified GD2 chimeric antigen receptor T cells in xenograft models of neuroblastoma. Cancer Immunol Res. 2014;2:1059–1070. doi: 10.1158/2326-6066.CIR-14-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Johnson LA, et al. Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci Transl Med. 2015;7:275ra22. doi: 10.1126/scitranslmed.aaa4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Koneru M, et al. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology. 2015;4:e994446. doi: 10.4161/2162402X.2014.994446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Koneru M, et al. A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16ecto directed chimeric antigen receptors for recurrent ovarian cancer. J Transl Med. 2015;13:102. doi: 10.1186/s12967-015-0460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Grupp SA, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Moon EK, et al. Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin Cancer Res. 2014;20:4262–4273. doi: 10.1158/1078-0432.CCR-13-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ankri C, et al. Human T cells engineered to express a programmed death 1/28 costimulatory retargeting molecule display enhanced antitumor activity. J Immunol. 2013;191:4121–4129. doi: 10.4049/jimmunol.1203085. [DOI] [PubMed] [Google Scholar]
- 195.Kobold S, et al. Impact of a new fusion receptor on PD-1-mediated immunosuppression in adoptive T cell therapy. J Natl Cancer Inst. 2015;107:djv146. doi: 10.1093/jnci/djv146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Heemskerk B, et al. Adoptive cell therapy for patients with melanoma, using tumor-infiltrating lymphocytes genetically engineered to secrete interleukin-2. Hum Gene Ther. 2008;19:496–510. doi: 10.1089/hum.2007.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hoyos V, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24:1160–1170. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Leonard JP, et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-γ production. Blood. 1997;90:2541–2548. [PubMed] [Google Scholar]
- 199.Chinnasamy D, et al. Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clin Cancer Res. 2012;18:1672–1683. doi: 10.1158/1078-0432.CCR-11-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kerkar SP, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest. 2011;121:4746–4757. doi: 10.1172/JCI58814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kerkar SP, et al. Collapse of the tumor stroma is triggered by IL-12 induction of Fas. Mol Ther. 2013;21:1369–1377. doi: 10.1038/mt.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Pegram HJ, et al. IL-12-secreting CD19-targeted cord blood-derived T cells for the immunotherapy of B-cell acute lymphoblastic leukemia. Leukemia. 2015;29:415–422. doi: 10.1038/leu.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Dunn GP, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 204.Koebel CM, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 205.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 206.Shankaran V, et al. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 207.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 208.Matsushita H, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482:400–404. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Alexandrov LB, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.John LB, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res. 2013;19:5636–5646. doi: 10.1158/1078-0432.CCR-13-0458. [DOI] [PubMed] [Google Scholar]
- 211.Garon EB, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 212.Skoulidis F, et al. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov. 2015;5:860–877. doi: 10.1158/2159-8290.CD-14-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.US National Library of Science. ClinicalTrials.gov [online] 2015 http://www.clinicaltrials.gov/ct2/show/NCT00586391.
- 214.Robbins PF, et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med. 2013;19:747–752. doi: 10.1038/nm.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Rosenberg SA, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Dudley ME, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tran E, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Stafford JH, et al. Colony stimulating factor 1 receptor inhibition delays recurrence of glioblastoma after radiation by altering myeloid cell recruitment and polarization. Neuro Oncol. 2015 doi: 10.1093/neuonc/nov272. http://dx.doi.org/10.1093/neuonc/nov272. [DOI] [PMC free article] [PubMed]
- 219.Lipson EJ, et al. Safety and immunologic correlates of melanoma GVAX, a GM-CSF secreting allogeneic melanoma cell vaccine administered in the adjuvant setting. J Transl Med. 2015;13:214. doi: 10.1186/s12967-015-0572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.DiLillo DJ, Ravetch JV. Differential Fc-receptor engagement drives an anti-tumor vaccinal effect. Cell. 2015;161:1035–1045. doi: 10.1016/j.cell.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kraman M, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-α. Science. 2010;330:827–830. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]