Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review (original) (raw)
Graphical abstract
Keywords: Smart drug delivery, Smart nanocarrier, Nanocarrier functionalization, Toxicity of nanocarrier, Cancer cell targeting, Drug release stimulus
Highlights
- •
Studied eight (8) promising nanocarriers for anti-cancer drug delivery. - •
Studied up-to-date strategies for cancer site targeting used in SDDSs. - •
Various stimulus techniques utilized for drug release at targeted sites are mentioned. - •
Studied toxicity of various nanocarriers used in SDDSs. - •
Challenges and research scope of nanocarriers in cancer therapy also highlighted.
Abstract
Nonspecific distribution and uncontrollable release of drugs in conventional drug delivery systems (CDDSs) have led to the development of smart nanocarrier-based drug delivery systems, which are also known as Smart Drug Delivery Systems (SDDSs). SDDSs can deliver drugs to the target sites with reduced dosage frequency and in a spatially controlled manner to mitigate the side effects experienced in CDDSs. Chemotherapy is widely used to treat cancer, which is the second leading cause of death worldwide. Site-specific drug delivery led to a keen interest in the SDDSs as an alternative to chemotherapy. Smart nanocarriers, nanoparticles used to carry drugs, are at the focus of SDDSs. A smart drug delivery system consists of smart nanocarriers, targeting mechanisms, and stimulus techniques. This review highlights the recent development of SDDSs for a number of smart nanocarriers, including liposomes, micelles, dendrimers, meso-porous silica nanoparticles, gold nanoparticles, super paramagnetic iron-oxide nanoparticles, carbon nanotubes, and quantum dots. The nanocarriers are described in terms of their structures, classification, synthesis and degree of smartness. Even though SDDSs feature a number of advantages over chemotherapy, there are major concerns about the toxicity of smart nanocarriers; therefore, a substantial study on the toxicity and biocompatibility of the nanocarriers has been reported. Finally, the challenges and future research scope in the field of SDDSs are also presented. It is expected that this review will be widely useful for those who have been seeking new research directions in this field and for those who are about to start their studies in smart nanocarrier-based drug delivery.
Nomenclature
ABC
accelerated blood clearance
BBB
blood brain barrier
BCM
bock copolymer micelle
CMC
critical micelle concentration
CNT
carbon nanotube
EPR
enhanced permeability and retention
IFP
interstitial fluid pressure
GNP
gold nanoparticle
GSH
glutathione sulfhydryl
LCST
lower critical stimulus temperature
MWCNT
multi-walled CNT
MDR
multidrug resistance
MPS
mononuclear phagocyte system
MSN
meso-porous silica nanoparticle
NP
nanoparticle
PEG
polyethylene glycol
PAMAM
poly (amidoamine)
QD
quantum dot
RES
reticuloendothelial system
SPR
surface plasma resonance
SPION
super paramagnetic iron oxide nanoparticle
SWCNT
single-walled CNT
SDDS
smart drug delivery system
VSSA
volume specific surface area
Introduction
Cancer is the second leading cause of death worldwide [1], [2]. Chemotherapy [3], [4] plays a vital role in treating undetectable cancer micro-focuses and free cancer cells. Chemotherapy uses chemicals to kill or block the growth of cancer cells [5]. As cancer cells grow faster than healthy ones, fast-growing cells are the main targets of chemotherapeutics; however, because there are healthy cells which are also fast-growing, the drugs used in chemotherapy also attack those fast-growing healthy cells. This unwanted attack results in the failure of conventional chemotherapy [6]. In addition, multi drug resistance (MDR) [7], [8], [9] is another major obstacle to successful chemotherapy. MDR enables the cancer cells to escape the effects of chemotherapeutics by developing resistance against the cytotoxic drugs during or shortly after the therapy. The limitations of conventional chemotherapy have led to the development of smart nanocarrier-based drug delivery systems, which are also known as Smart Drug Delivery System (SDDSs). SDDSs promise to apply drugs to specific and targeted sites [10]. Although, the magic bullet concept of Paul Ehrlich [11] is the cornerstone of the relationship between drug delivery and nanoparticles, the well-controlled release of drugs using a bead polymerization technique was reported first by Speiser et al. [12].
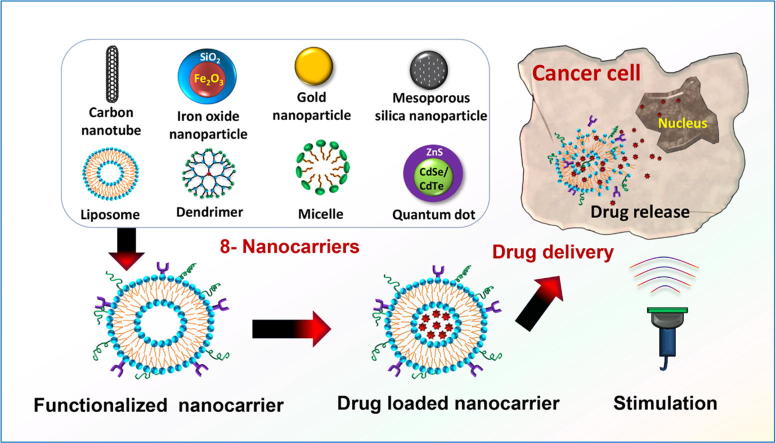
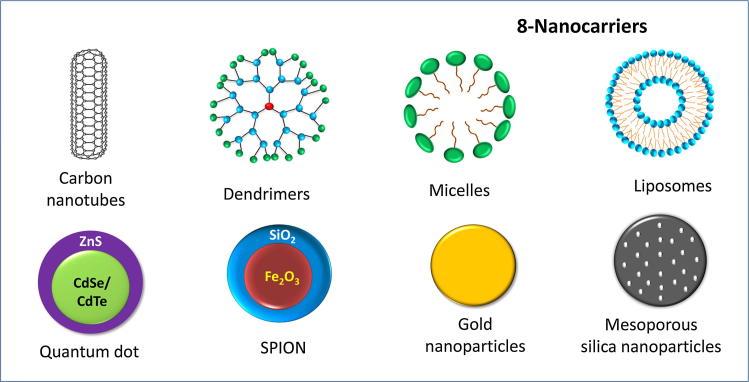
Nanocarriers are the base of SDDSs. Unfortunately, not all types of nanocarriers are reliable as drugs carriers in SDDSs. To qualify as an ideal nanocarrier in SDDSs, a nanocarrier should meet some basic criteria, discussed in detail in the subsequent sections. This review emphasizes the eight (8) most reported nanocarriers: (i) liposomes, (ii) micelles, (iii) dendrimers, (iv) meso-porous silica nanoparticles (MSNs), (v) gold nanoparticles (GNPs), (vi) super paramagnetic iron oxide nanoparticles (SPIONs), (vii) carbon nanotubes (CNTs), and (viii) quantum dots (QDs) in the context of their structures, classification, synthesis and degree of smartness. The schematic representation of these 8 nanocarriers is shown in Fig. 1.
Fig. 1.
Schematic representation of the 8 nanocarriers used in smart drug delivery systems.
Choosing the right strategies to identify cancer cells follows the selection of a suitable nanocarrier type. SDDS utilizes the physiochemical differences between cancer cells and healthy cells to identify cancer sites. To exactly identify the cancer cell site, there are two major approaches: passive targeting and active targeting. Passive targeting utilizes the Enhanced Permeability (EPR) [13] effect to specify the cancer site indirectly. Active targeting uses overexpressed cell surface receptors of cancer cells to target cancer cells directly like a guided missile [14]. Releasing drugs at the specific location at a precise concentration is the subsequent step. Drugs could be released from the nanocarriers by external or internal stimuli, depending on the type of nanocarriers and their smartness [15].
Though the prospect of SDDSs is quite promising, the toxicity of nanocarriers in human organs is a major concern; therefore, this review presents a table (Table 1) of eight (8) nanocarriers summarized in terms of their toxicity and biocompatibility. Furthermore, the existing challenges and future research scope in designing effective SDDSs are also highlighted in this review.
Table 1.
Different nanocarriers in terms of toxicity and bio-distribution.
| SDDS name | Toxicity | Bio-distribution of nanocarrier and renal excretion | Refs. | |
|---|---|---|---|---|
| Cytotoxicity | Immunogenicity | |||
| Liposome-based SDDS | •Cationic liposome affects the in vitro growth of different cell lines, such as L 1210, HepG2, A549, etc. | •Positively charged liposome has toxic effect on macrophages and U937 cells. | •Majority accumulates in the liver followed by spleen. | [214], [215], [216], [217], [218], [219], [220], [221], [222], [223], [224] |
| •In vivo study shows DNA damage due to the cationic surface charge. | •Rapid clearance with urine. | |||
| Micelle-based SDDS | •Kawaguchi investigated the toxicity of polymeric micelles, which show no pathological abnormalities. | •The Kawaguchi experiment finds that polymeric micelle-based drug carriers trigger transient immunogenicity in the MPS system. | •The in vivo toxicity screening of well characterized cationic polymeric micelles shows that particles could be found in major organs, such as lung, liver, kidney. | [59], [225], [226], [227], [228], [229], [230], [231] |
| •Many investigations show that polymeric micelles are less toxic. | •Polymeric micelles based on poly (ethylene oxide) and α-carbon substituted poly (ε-caprolactone) are found to be non-immunogenic to dendritic cells—the antigen presenting cell of the mammalian immune system. | •Peptide Amphiphile accumulates primarily in bladder then pass through the urine. | ||
| Dendrimer-based SDDS | •Dendrimers, such as PPI, PAMAM, and PLL, exert significant in vitro cytotoxicity due to their surface catatonic groups, but significantly lowered cytotoxicity is observed with the PEG-modified dendrimer. | •Dendrimers show no or little immunological response. Roberts et al. investigated the immunogenicity of the PAMAM dendrimer. | •They are present in the intracellular compartment of kidney, liver and lung. | [75], [88], [232], [233], [234], [235], [236], [237], [238], [239], [240], [241] |
| •Naha et al. study shows that PAMAM has adverse effects on mammalian cells. | ||||
| •Proper surface modification can reduce cytotoxicity. | ||||
| Meso-porous silica nanoparticle-based SDDS | •In vitro cytotoxicity is controversial. | •Functionalized mesoporous silica nanoparticles do not affect the viability of primary immune cells from the spleen in relevant concentrations. | •MSNs mainly distribute in the liver and spleen; minority can be found in the lungs, kidneys and heart. | [46], [100], [106], [242], [243], [244], [245], [246], [247], [248], [249] |
| •Pasqua et al. showed that MCM-41 and two of its functional analogs kill human neuroblastoma (SK–N–SH) cells. | •Potential adverse effects on the immune system are not clear and need further research. | •Silica nanoparticles have a toxic effect on the liver. | ||
| •Meso porous silica do not affect cell viability or the plasma membrane. | •PEGylated MSNs with smaller particle sizes possess longer blood circulation and lower gradated products in the urine. | |||
| •Silica nanoparticle cytotoxicity is size dependent; smaller particles have higher toxicity. | ||||
| Gold nanocarriers-based SDDS | •In vitro cytotoxicity screening of K562 leukemia cells shows that they do not exhibit an acute toxic effect based on the MTT assay—colorimetric assay for assessing cells’ metabolic activity. | •The immunological study of the RAW264.7 macrophage did not indicate any immunological toxicity. | •GSH coated GNP nanocarriers have lower accumulation in the kidneys and liver compared to bare GNPs. | [175], [250], [251], [252], [253], [254], [255], [256], [257], [258], [259], [260] |
| •Experiment on RAW264.7 also shows no considerable cytotoxicity based on the MTT assay. | •Villiers et al. also showed non-immunological toxicity. | •Mostly excreted with urine and no systemic toxicity. | ||
| •On the other hand Goodman in 2004 shows that cationic GNanocarriers shows toxicity. | •In vivo experiment showed size dependent toxicity; that is, nanoparticles with certain sizes show lethal toxicity while other sizes of nanoparticles show no considerable toxicity. | |||
| •Pan et al. in 2009 shows size dependent cytotoxicity. | ||||
| SPION-based SDDS | •SPIONs are toxic to brain cells with different coatings. | •The generation of ROS could trigger immunological toxicity. | •75% found in spleen | [130], [261], [262], [263], [264], [265] |
| •Compatible to kidney cells. | •Primarily found in the spleen and liver. | |||
| CNT-based SDDS | •Interaction of functionalized SWCNTs with CHO and 3T3 cells exhibited no toxicity. | •CNTs functionalized with peptides do not trigger anti-peptide antibodies. | •Well individualized MWCNTs with shorter lengths and higher degrees of oxidation escape the RES in organs (liver, spleen lungs) and clear through renal excretion. | [266], [267], [268], [269], [270], [271], [272] |
| Quantum dot-based SDDS | •QD-induced cytotoxicity is not observed in many in vivo and in vitro experiments. | •Immune response could be suppressed by CdSe/ZnS QDs. | •Salykin et al. report that QDs primarily deposit in the lung and atriums of heart. | [273], [274], [275], [276], [277], [278], [279], [280], [281] |
| •Not excreted with urine. |
Smart drug delivery system
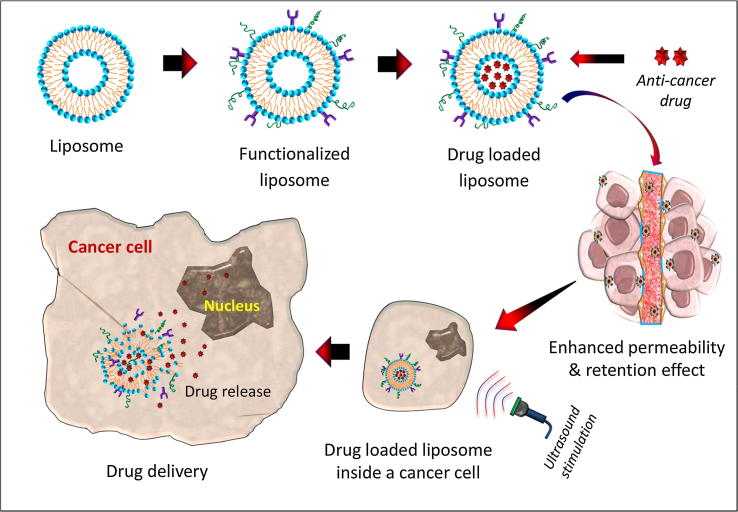
A smart drug delivery system, as illustrated in Fig. 2, using liposomes as nanocarriers, consists of (i) smart nanocarriers which carry anti-cancer drugs to the cancer site, (ii) targeting mechanisms to locate the cancerous site and (iii) stimulus techniques to release the payloads at the pre-located cancer cell site. Eight nanocarriers as well as their targeting mechanisms and stimulus techniques are discussed in detail in the subsequent sections.
Fig. 2.
Step-wise illustration of liposome-based smart drug delivery system for cancer therapy.
Smart nanocarriers
Particles with at least one dimension on the order of 1–100 nm are popularly known as nanoparticles. Currently, nanoparticles are defined in terms of volume specific surface area (VSSA). Typically, particles with VSSA equal to or greater than 60 m2/cm3 volume of the material are defined as nanoparticles [16]. When nanoparticles are used as transport modules for other substances, they are called nanocarriers. Conventional nanocarriers don’t have the ability to carry and release drugs at the right concentration at the targeted site under external or internal stimulation. Therefore, archetypical nanocarriers are not smart. They need to be modified or functionalized to make them smart. Smart nanocarriers should possess the following characteristics. First, smart nanocarriers should avoid the cleansing process of the body’s immune system. Second, they should be accumulated at the targeted site only. Third, smart nanocarrier should release the cargo at the targeted site at the right concentration under external or internal stimulation. In addition, finally, they should co-deliver chemotherapeutics and other substances, such as genetic materials, imaging agents, etc. [17], [18], [19].
Depending on the types and applications of nanocarriers, there are some steps to transform conventional nanocarriers into smart ones. First, nanocarriers face many biological barriers, including cleansing by the reticuloendothelial system (RES) on the way to the targeted site. The RES takes the nanocarrier out of circulation shortly and accumulates those anti-cancer drug-carrying nanocarriers in the liver, spleen or bone marrow. PEGylation is a unique solution to avoid this cleansing process. PEGylation helps nanocarriers escape the RES. Davies and Abuchowsky reported the PEGylation for the first time [20]. Unfortunately, PEGylation reduces the drug uptake significantly by the cells [21], [22]. This twist is known as the PEGylation dilemma [23], [24]. Second, nanocarriers can be functionalized to identify the cancer cells precisely out of healthy ones. The physiochemical differences between cancer cells and healthy ones are the identification marks to separate the two types of cells. The surface of cancer cells overexpresses some proteins. The overexpressed proteins are the key targets of the smart nanocarrier. Nanocarriers are modified with ligands matching the overexpressed proteins. The ligands of smart nanocarriers identify the cells with the receptor proteins. Third, conveying the drug to the target site is not the termination of the process. Releasing the drug from the smart carrier under stimulation is the next big challenge. To make nanocarriers responsive to the stimulus system, various chemical groups can be grafted on the surface of the nanocarriers. Fourth, modifications are also done for the co-delivery of anti-cancer drugs together with other substances, including genetic materials [25], imaging agents or even additional anti-cancer drugs. Liposomes, micelles, dendrimers, GNPs, quantum dots and MSNs show promise for co-delivery [26], [27], [28], [29], [30]. Eight promising nanocarriers are discussed in detail below in terms of their structure, classification, synthesis and smartness.
Liposome and its smartness
Liposomes [31], illustrated in Fig. 3, are naturally occurring phospholipid-based amphipathic nanocarriers. Phospholipids, a major component of the cell membrane, consist of a fatty acid-based hydrophobic tail and a phosphate-based hydrophilic head. In 1973, Gregory Gregordians showed that when phospholipids are introduced in an aqueous medium, they self-assemble into a bi-layer vesicle with the non-polar ends forming a bilayer and the polar ends facing the water. The core formed by the bilayer can entrap water or water-soluble drugs [32]. On the basis of the number of bilayers and the size of the liposome, there are two types: multi-lamellar vesicles and uni-lamellar vesicles. Uni-lamellar vesicles can be further divided into two groups, namely, large uni-lamellar vesicles (LUV) and small uni-lamellar vesicles (SUV) [33], [34].
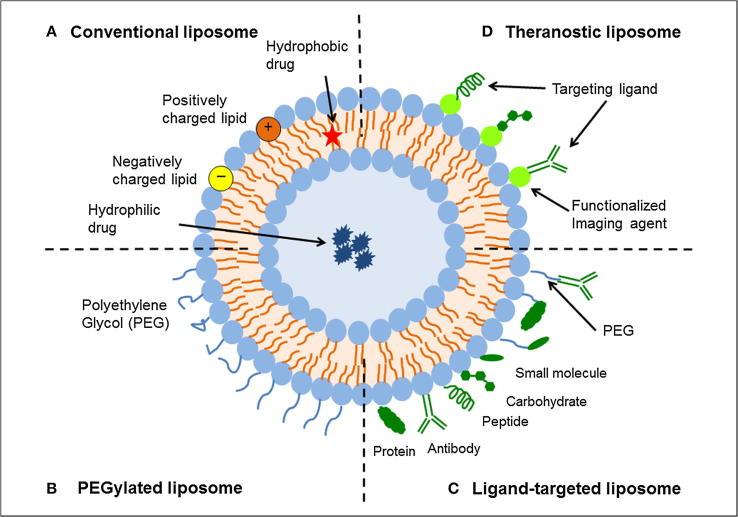
Fig. 3.
Schematic representation of the different types of liposomal drug delivery systems. (A) Conventional liposome, (B) liposome with PEGylation, (C) ligand-targeted liposome, and (D) theranostic liposome. Reprinted with permission [43], under CC BY 4.0 license.
There are several methods to prepare liposomes [35], [36], namely, the thin film hydration method or Bangham method [37], reverse phase evaporation [38], solvent injection technique [39], and detergent dialysis [40]. Conventional methods have many setbacks. To remove those limitations, some novel technologies have been devised, such as supercritical fluid technology, the supercritical anti-solvent method [41], and supercritical reverse phase evaporation [42].
Conventional liposomes have many problems including instability, insufficient drug loading, faster drug release and shorter circulation times in the blood; therefore, they are not smart. Functionalization of conventional liposomes, as shown in Fig. 3 [44], makes them smart. Like other nanocarriers, liposomes also need to overcome the challenge presented by the RES. PEGylation helps liposomes escape the RES. Therefore, PEGylated liposomes have longer blood circulation time [45]. Smart nanocarriers can determine the difference between healthy cells and cancerous ones. Monoclonal antibodies, antibody fragments, proteins, peptides, vitamins, carbohydrates and glycoproteins are usually grafted on the liposome to actively target the cancer site [46], [47], [48], [49]. Smart liposomes are responsive to various external and internal stimulation, including pH change, enzyme transformation, redox reaction, light, ultrasound and microwaves [50], [51], [52]. A liposome functionalized with a radio-ligand is known as a radiolabeled liposome. Radiolabeled liposomes [53] can be used to determine the bio-distribution of liposomes in the body and to diagnose the tumor along with carrying out therapy. Liposomes that can carry both therapeutics and imaging agents [54] are known as theranostic liposomes [55], [56]. In addition to delivering imaging agents together with chemotherapeutics, liposomes are promising in the co-delivery of two chemotherapeutic drugs, gene agents [57] with chemotherapeutics as well as chemotherapeutics with anti-cancer metals [58].
Micelles and their smartness
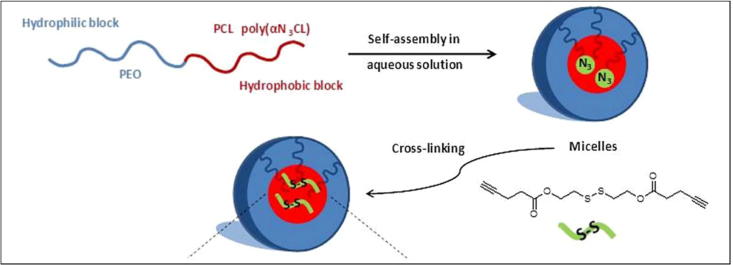
Amphiphilic molecules, having both hydrophilic and hydrophobic portions, show unique characteristics of self-assembly when exposed to a solvent. If the solvent is hydrophilic and its concentration exceeds the critical micelle concentration (CMC), the polar parts of the co-polymer are attracted toward the solvent, while hydrophobic parts orient away from the solvent. In this way, the hydrophobic portions form a core, while hydrophilic portions form a corona. This type of arrangement is called a direct or regular polymeric micelle [59], [60], depicted in Fig. 4. On the other hand, amphiphilic molecules exposed to a hydrophobic solvent produce a reverse structure known as a reverse micelle. That is, the hydrophilic portions make the core and the hydrophobic portions make the corona in a reverse micelle [61], [62], [63]. PG-PCL, PEEP-PCL [64], PEG-PCL [65] and PEG-DSPE are examples of some micelles [66]. The preparation of micelles depends on the solubility of the co-polymer used [67]. For a relatively water-soluble co-polymer, two methods are used, namely, the direct dissolution method and the film casting method. In contrast, dialysis or an oil in water procedure is used if the co-polymer is not readily water-soluble [68], [69].
Fig. 4.
Schematic diagram of cross-linked micelle formation in aqueous solution. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission [70].
Micelles may face immature drug release by crossing the CMC. In addition, interaction with blood and absorption of unimers to plasma protein may disrupt the equilibrium between micelle and blood. The solution to this problem is a smart micelle. To overcome the problems mentioned, micelles are usually cross-linked; that is, linking two polymer chains by disulfide formation [70]. There are two types of cross-linking schemes: core cross-linked polymer micelles and the shell cross-linked polymer micelles. To actively target cancer cells, different types of ligands are used to decorate the micelle surface, namely, folic acid, peptides, carbohydrates, antibodies, aptamers, etc. [66]. To release the anti-cancer drug at the right concentration, the core or the corona of the micelle can be functionalized. The stimuli used in micelle based SDDSs are pH gradients, temperature changes, ultrasound [71], enzymes, and oxidation [66]. Using a multifunctional micelle, the co-delivery strategy is very important for the synergetic effects in cancer treatment. Seo et al. reported a temperature-responsive micelle-based co-delivery system which can carry genes along with anti-cancer drugs [72]. In cancer diagnosis and monitoring, single photon emission computed tomography (SPECT), magnetic resonance imaging (MRI), computed tomography (CT), positron emission tomography (PET), and ultrasonography play vital roles. The surface of micelle can be decorated with the imaging agent [73]. Combined delivery of doxorubicin and the imaging of tumors via ultrasound has been reported by Kennedy and coworkers [74].
Dendrimers and their smartness
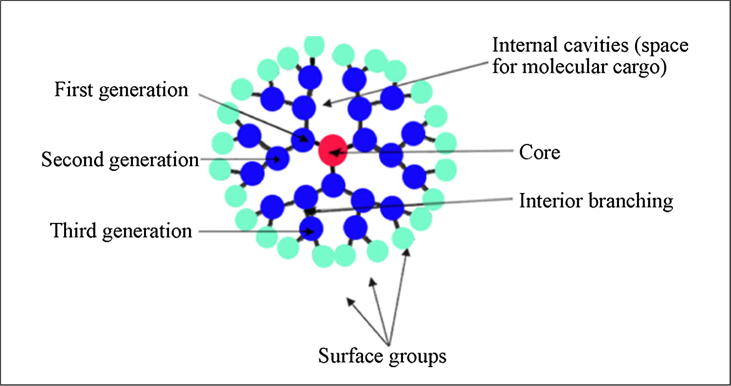
Polymers with many branches are known as dendrimers, which can be graphically presented as a suction ball. As shown in Fig. 5, a dendrimer has three distinguishable parts: a core, branching dendrons and surface-active groups [75]. The active groups on the dendrimer surface determine the physiochemical properties of the dendrimer. Based on the surface groups, it may be either hydrophobic or hydrophilic. Due to its nanoscale size, monodisperse nature [76], water solubility, bio-compatibility, and highly branched structure, it is of high interest. Because of the nanoscale size, it can be used as a drug carrier [77]. The branched structure makes the dendrimer versatile. Moreover, all of its active groups on the surface face outward, which results in a higher drug encapsulation rate. Various types of dendrimer, such as poly (propylene-imine) (PPI or POPAM), PAMAM, POPAM, POMAM [78], polylysine dendrimer, dendritic hydrocarbon, carbon/oxygen-based dendrimer, porphyrin-based dendrimer, ionic dendrimer, silicon-based dendrimer, phosphorus-based [79] dendrimer, and Newkome dendrimer [80] have been reported. The commonly reported methods to produce dendrimers include the divergent method [81] and the convergent method [82]. Dendrimers were introduced for the first time by Fritz Vogtle et al. in 1978 [83]. The dendritic structures that have been thoroughly investigated and received widespread focus are Tomalia’s poly (amidoamine) (PAMAM) [84], [85] and Newcome’s ‘arboreal system’ [86], [87].
Fig. 5.
General structure of dendrimer. Reprinted with permission [88].
Conventional dendrimers face rapid clearance by the immune system and lower uptake by cancer cells. Modification of the dendrimer is the solution to these limitations. Chemical modification, copolymerization with a linear polymer, and hybridization with other nanocarriers are options to overcome these limitations as reported so far [89]. To actively target the cancer site, the surface of dendritic structures can be modified by peptides, proteins, carbohydrates, aptamers, antibodies, etc. The dendrimer surface can also be modified for various stimuli responsive systems, such as light, heat, pH change, protein, and enzyme transformation [90], [91]. Among other dendrimers, the cationic nature of PAMAM makes it highly useful for the delivery of genetic materials. Delivery efficiency depends on the generation of PAMAM. Haensler and Szoka were the first to report PAMAM-based nucleic acid delivery in 1993 [92], [75]. The dendritic contrast agent for tumor imaging is very promising [93].
Meso-porous silica nanoparticles (MSNs) and their smartness
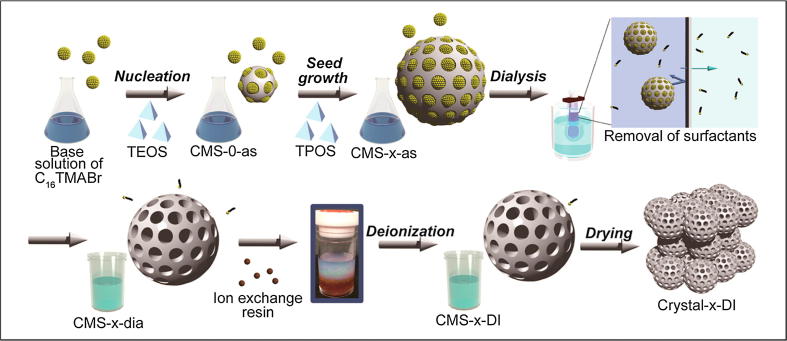
Meso-porous materials are materials containing pores with diameters between 2 and 50 nm, as defined by the IUPAC [94]. MSNs [95] have the honeycomb-like porous structure of silica (SiO2), as shown in Fig. 6. The term MSN was coined forty years ago to describe zeolite-silica gel mixtures with well-defined and uniform porosity [96]. MSNs are widely studied because of their tunable particle size (50 nm through 300 nm), uniform and tunable pore size (2–6 nm) [97], high surface area, high pore volume and biocompatibility [98], [99], [100]. Tunable particle size is an essential criterion to be a smart nanocarrier, and tunable pore size allows drugs of different molecular shapes to be loaded. The high surface areas of the internal surface (pores) and external surface are suitable for grafting different functional groups on MSNs. Apart from bio-compatibility, adhesion of this carrier to cancer cells by the EPR effect makes them an ideal choice [101]. There are mainly two types of MSNs, namely, (1) ordered meso-porous silica NPs (MCM-41, MCM-48, and SBA-15), and (2) hollow or rattle-type meso-porous silica NPs [102]. Among those MSNs, MCM-41, synthesized by a Mobil Corporation scientist, is the most investigated MSN for biomedical applications. The controlled drug delivery capability of MCM-41 was known in 2001 [96]. The ways to fabricate MSNs are the soft template method and hard template method.
Fig. 6.
Schematic for the synthesis of monodisperse colloidal MSNs and the fabrication of colloidal crystals. Reprinted with permission [103], © American Chemical Society (2014).
Conventional MSNs have limited blood circulation half-lives due to the hemolysis of human red blood cells (HRBCs), non-specific binding to human serum protein (HSA) and the phagocytosis of human THP-1 mono-cytic leukemia cell line-derived macrophages (THP-1 macrophages). PEGylation helps offset those causes [104]. The pore openings of smart MSNs can be controlled by grafting co-polymers on their surfaces. Grafted co-polymers work as gatekeepers. Polymer-grafted MSNs show zero premature release of loaded drugs [105]. For active targeting, the surface of meso-porous silica nanoparticles (MSNs) can be modified using folate, mannose, transferrin and peptides. Stealth behavior can be achieved by PEGylation [106]. MSN can release the loaded drugs in response to diverse stimuli, including pH change, redox reaction, enzyme transformation, temperature change, light, magnetic field, etc. [107], [108]. Positively charged MSN could be used for gene delivery with higher transfection efficiency [109]. Hsiao et al. designed and constructed a MSN-based theranostic drug delivery system which can be used for cancer imaging along with drug delivery [110].
Gold nanocarriers and their smartness
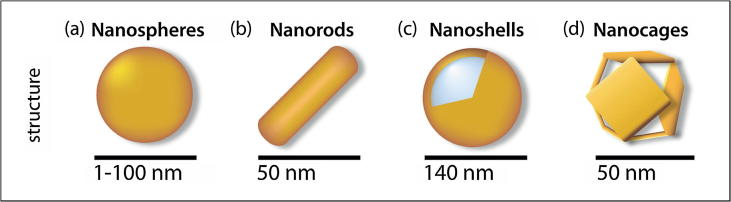
Metallic nanocarriers are a matter of significant interest because of their unique characteristics, such as customizable size, large surface to volume ratio, easy synthesis, noble optical properties, thermal ablation of cancer cell and easy surface functionalization [111]. Studies show that the intercellular uptake of nanocarriers depends on the size and shape of colloidal nanocarriers [112]. GNPs [113] are metallic nanocarriers available in custom shapes and sizes, as shown in Fig. 7. GNPs have great prospects as metallic candidates for delivering payloads. Payloads could be drug molecules or large biomolecules, such as proteins, DNA and RNA. GNPs are also interesting due to the surface plasmon resonance (SPR) phenomenon [114], [115], which enable them to convert light to heat and scatter the produced heat to kill the cancer cells. GNPs are mainly synthesized via a number of routes, including (1) chemical [116], (2) physical [117], and (3) biological methods [118], [119]. The grafting of the surfaces of GNPs with proper ligands could significantly overcome the blood brain barrier (BBB) [120].
Fig. 7.
Schematic diagram of GNPs with different sizes and shapes. Reprinted with permission from [121].
Smart nanocarriers should be chemically stable in biological media, biocompatible, efficient in targeting and responsive to external or internal stimuli. GNPs without modification are unstable in blood and face higher uptake by the RES. To overcome these limitations, gold nanocarriers need to be PEGylated. Under physiological conditions, PEGylated GNPs show enhanced solubility and stability [122]. For targeted drug delivery, the surface of GNPs can be modified by various ligands. For example, transferrin (TF) can be grafted onto the surface of GNPs, as many tumors overexpress the TF receptor on their surface [123]. The GNP surface could also be modified by folic acid, as folic acid receptors are also overexpressed on various tumor cells [124], [125]. The drug can be unloaded from GNPs either by (1) external stimuli (laser, ultrasound and X-ray, light [126]) or by (2) internal stimuli (pH, redox condition, matrix metalloproteinase) [127]. Various studies show the promise for gene transfection by GNPs to silence the gene responsible for the cancer [128]. GNPs modified with fluorescently labeled heparin could be used to diagnose the cancer site [129].
Super paramagnetic iron oxide nanoparticles (SPIONs) and their smartness
Freeman et al. introduced the concept of using of magnetic materials along with magnetic fields in medicine in 1960 [109]. The magnetic materials include the widely studied SPIONs. Small synthetic maghemite and magnetite (Fe3O4) particles with cores ranging between 10 and 100 nm in diameter are two SPIONs. Mixed iron oxides with transition metals, such as copper, cobalt, and nickel also belong to the category of SPIONs. When magnetic particles are reduced to 10–20 nm, they show a unique phenomenon called super para-magnetism. On the application of a magnetic field, the magnetic nanoparticles are magnetized up to their saturation, but show no residual magnetism upon removal of the magnetic field [130], [131]. The fabrication of SPIONs includes three methods, including a physical method, wet chemical method and microbial method [132]. There are various methods to synthesis SPIONs, namely, co-precipitation, thermal decomposition, hydrothermal, micro-emulsion, sono-chemical, microwave-assisted synthesis methods [133]. Among those, chemical synthesis is the most predominant one.
The smartness of post-fabricated SPIONs depends on the functionalization (as shown in Fig. 8). Functionalization reduces the aggregation of SPIONs, protects their surfaces from oxidation, provides a surface to conjugate drugs and targeting ligands, increases the blood circulation by avoiding the RES, and reduces nonspecific targets [130]. Stimuli-responsive polymer-coated SPIONs are under intensive investigation for targeted drug delivery. Responsive polymers undergo physical and chemical transitions such as phase, solubility and hydrophobicity conformation. A recent study has shown that polymer-modified SPIONs have dual responsiveness to pH gradients and temperature changes [135]. This carrier can be controlled by an external magnetic field. Because of the presence of phosphate group, nucleic acids are negatively charged; therefore, SPIONs can be modified with cationic lipids and polymers to carry genetic materials [136]. SPIONs are members of the family of nanocarriers that have theranostic properties. As a magnetic nanocarrier, it can be detected by an external magnetic field [137], [138].
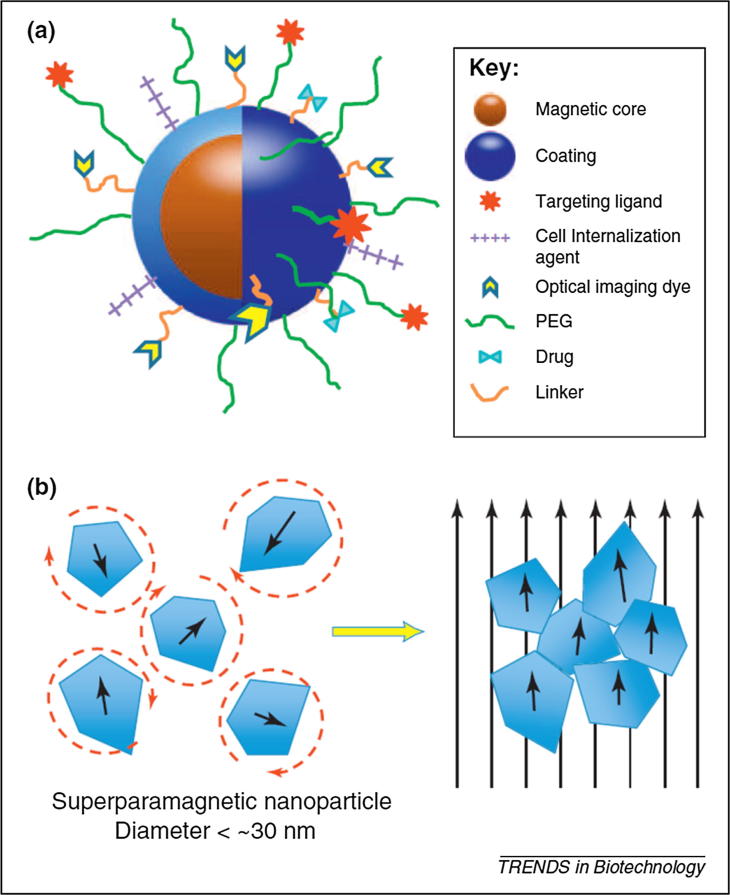
Fig. 8.
(a) Schematic representation of the ‘core–shell’ structure of magnetic nanocarriers and multi-functional surface decoration, (b) illustration of super paramagnetic MNP response to applied magnetic fields. Reproduced with permission [134], under CC BY 3.0 license.
Carbon nanotubes (CNTs) and their smartness
CNTs are a type of fullerene, a class of carbon allotropes in the shape of hollow spheres, ellipsoid, tubes and many other forms [139], [140]. When a graphene sheet is rolled up into a seamless cylindrical tube, the shape is known as a CNT. There are two types of CNTs: single walled (SWCNT) and multi-walled (MWCNT) [141], [142]. The strong optical absorption in the near-infrared region by the CNT makes this particle a strong candidate for photo thermal ablation; furthermore, nanoparticles with sizes ranging from 50 to 100 nm are easy to be engulfed. MWCNTs can pass through the barrier of various cellular compartments, and PEGylated SWCNTs are able to localize in a specific cellular compartment. CNTs can be synthesized via heating carbon black and graphite in a controlled flame environment. However, this process cannot control the shape, size, mechanical strength, quality and purity of the synthesized CNTs. To address the limitations of the controlled flame environment, electric arc discharges [142], the chemical vapor deposition method [143] and the laser ablation method have been reported. Due to the better defined walls of SWCNTs and relatively more structural defects of MWCNTs, SWCNTs are more efficient than MWCNTs in drug delivery [5], [144].
CNTs should be functionalized [146] either chemically or physically, as illustrated in Fig. 9, to make them smart. PEGylation is a very important step to increase solubility, avoid the RES and to lower the toxicity [147]. Poly (N-isopropyl acrylamide) (PNIPAM) is a temperature-sensitive polymer. Due to their low critical stimulus temperature (LCST), PNIPAM could be used to modify CNTs for temperature stimulus. The disulfide cross-linker, based on methacrylate cysteine, is used for enzyme responsive drug release. For pH responsiveness, an ionizable polymer with a pKa value between 3 and 10 is an ideal candidate. Weak acids and bases show a change in the ionization state upon pH variation [148]. Recent studies exhibit that functionalized CNTs can overcome the BBB [149], [150]. CNTs have shown promise in carrying plasmid DNA, small-interfering ribonucleic acid (siRNA), antisense oligonucleotides, and aptamers [151]. In addition to gene delivery, it can also be used for the thermal ablation of a cancer site [152]. Functionalized CNTs can be used as diagnostic tools for the early detection of cancer [153].
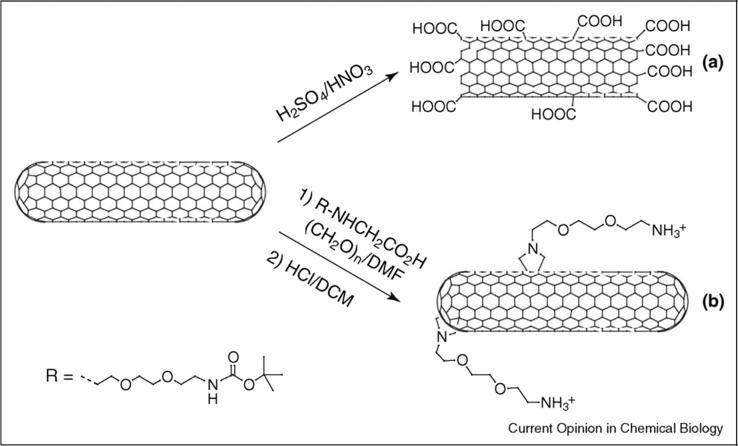
Fig. 9.
Organic functionalization of carbon nanotubes. Pristine single- or multi-walled carbon nanotubes can be (a) treated with acids to purify them and generate carboxylic groups at the terminal parts, or (b) reacted with amino acid derivatives and aldehydes to add solubilizing moieties around the external surface. Reprinted with permission [145].
Quantum dots (QDs) and their smartness
Quantum dots [154], fluorescent semiconducting nanocarriers, are often made of hundreds to thousands of atoms of group II and group VI molecule and have unique photophysical properties [155]. This nanocarrier could be used to visualize the tumor while the drug is being released at the targeted site. Most commercially available QDs consist of three parts: a core, a shell, and a capping material. The core consists of a semiconductor material, e.g., CdSe. Another semiconductor, such as ZnS, is used to build up shell surround the semiconductor core. A cap encapsulates the double layer QDs with different materials [156]. QD-based SDDSs have attracted significant interest for several reasons. First, QDs possess an extremely small core size of 2–10 nm in diameter. This feature makes it useful as a tracer in other drug delivery systems. Second, versatile surface chemistry allows different approaches for the surface modification of QDs. Third, their photophysical properties provide QDs extra mileage for real-time monitoring of drug-carrying and drug release [157]. To synthesize QDs, either a top-down approach or a bottom-up method can be employed. Molecular beam epitaxy (MBE) [158], ion implantation, e-beam lithography and X-ray lithography [159] belong to top-down processing; on the other hand, colloidal QDs are prepared by self-assembly in solution following chemical reduction, which is a bottom-up approach [160].
Functionalization of archetypical QDs also bears a significant importance similar to other smart nanocarriers. As reported for other nanocarriers, QDs also experience non-specific uptake by the RES. PEGylation is an excellent solution for QDs as well. Properly PEGylated QDs are able to accumulate in tumor sites by an enhanced permeability and retention (EPR) effect without a targeting ligand. To actively target a tumor site, various ligands, such as peptides, folate, and large proteins (monoclonal antibodies) can be grafted on the QD surface [162]. Recently, Iannazzo et al. showed the bright prospects of graphene QD-based targeted drug delivery. They covalently linked QDs to the tumor targeting module biotin to find the biotin receptor overexpressed on tumor cells. This system can successfully release a drug under pH stimulus, as shown in Fig. 10 [163]. QDs are specially known for cancer imaging due to their inherent florescence. A folic acid complex has been used to diagnose ovarian cancer [164]. To combat MDR, co-delivery of chemotherapeutics and siRNA was developed [165]. Bio-conjugated and polymer-encapsulated QD probes for cancer imaging and targeting were studied by Gao et al. [166], [167].
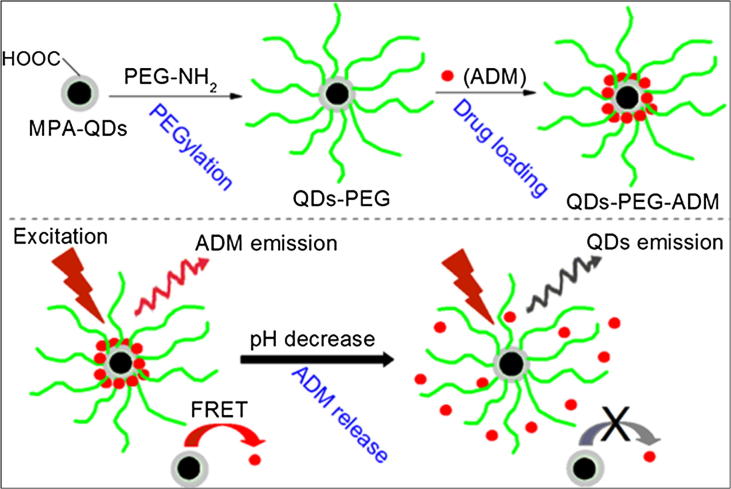
Fig. 10.
Schematic diagram of the preparation of QD-PEG-ADM and the drug release mechanism of quantum dots (QDs). Reprinted with permission [161].
Cancer cell targeting mechanism
If the anti-cancer drug-carrying smart nanocarrier survives the cleansing process of our body’s immune system, the smart nanocarrier then finds the cancerous area of the body. A smart drug delivery system utilizes two types of targeting: passive targeting and active targeting [168], [169]. Passive targeting employs the EPR effect [170] to locate cancer sites. Active targeting utilizes the ligand-receptor technique to locate the ultimate target – the individual cancer cell.
Passive targeting
Accumulation rate of drug-loaded nanocarriers into a tumor is much higher than in normal tissue due to the leaky endothelium of the tumor vasculature. This phenomenon is known as the enhanced permeability effect. The lymphatic system is the drainage system of the body. A deficiency of the lymphatic system leads to the retention of the nanoparticles in the tumor. This retention is known as the enhanced retention effect. Both the phenomena are collectively known as the EPR effect [171]. Using this EPR effect, the concentration of anti-cancer drugs in the tumor could be increased many times compared to the healthy tissue of the body. Interstitial fluid pressure (IFP) is another barrier in the way of successful accumulation of drug-loaded nanocarriers in the solid tumor [172], [173]; however, efficient modifications of nanocarriers can overcome many biological barriers, including IFP and the RES [174].
Active targeting
Active targeting means guiding the drug-carrying nanocarriers to the cancer cells such as guided missiles [175]. Cancer cells and normal cells can be separated in terms of cell surface receptor and antigen expression. Cell surface receptors are embedded proteins in the cell membrane responsible for trans-membrane communication. Cancer cells show the amplification or overexpression of various cell surface receptors otherwise known as cell markers, such as folic acid and cell surface antigen. Drug-loaded nanocarriers are conjugated with targeting ligands. These ligands identify their matching target overexpressed on the cancer cell surface. Folate, transferrin, antibodies, peptides and aptamers are some investigated ligands.
Stimulus for drug release
Exogenous and endogenous are the two types of stimuli. An extra-corporal signal to release drugs from nanocarriers, such as a magnetic field, ultrasound waves, an electric field, a temperature change is known as exogenous stimulus. A signal produced from inside the body to release anti-cancer drugs is known as an endogenous stimulus. pH change, enzyme transformation, temperature and redox reactions are the examples of endogenous stimuli [176].
Endogenous stimulus
Endogenous stimulus is also known as intrinsic stimulus. In the case of endogenous stimulus, the triggering signal comes from the internal pH level, enzyme activity and redox activity of the body. Different types of endogenous stimuli are discussed below in detail [177].
The pH-responsive stimulus
According to the Warburg effect, the tumor cells predominantly produce energy due to enhanced glycolysis followed by lactic acid fermentation in the cytosol [178]. This extra acid production leads to lower pH in cancer cells. The pH-responsive drug delivery system is interesting because the pH level varies from organ to organ, even from tissue to tissue. The extracellular pH in tumors has an acidic environment compare to more slightly basic intracellular pH [179]. Therefore, pH has been established as an effective physiological property for smart drug delivery to tumor sites by many studies. This acidic extracellular pH results from poor blood flow, hypoxia and lactic acid in tumors [180]. The extracellular pH range is approximately 6–7 [181]. In addition to this pH gradient across the cell, there is a pH change across cell compartments. The lysosomal pH level is approximately 5, whereas the cytosol has a pH level of 7.2 [182]. The pH-sensitive nanocarriers usually store and stabilize anti-cancer drugs at physiological pH, but rapidly release the drug at a pH trigger point, which ensures that intracellular drug concentration reaches a peak. The target can be reached by different approaches, including the introduction of ionizable chemical groups, such as amines, phosphoric acid and carboxyl groups, among others. These groups undergo pH-dependent physical and chemical changes which result in drug release.
Redox sensitive stimulus
Glutathione sulfhydryl (GSH) is a highly effective antioxidant. It consists of three amino acids. GSH is found at higher concentrations in all mammalian tissue [183]. GSH controls the reductive microenvironment. The concentration of GSH in a tumor site is at least 4 times higher than in normal cells. The intra-cellular concentration of GSH is 1000 times higher than in the blood stream [70], [184]. GSH, a functional group with the structure R-S-S-, can reduce the disulfide bonds of nanocarriers. Reduction of disulfide bonds leads to the release of an encapsulated drug [185]; for example, the disulfide bond of cross-linked micelles could be reduced by the cell-site GHS. The reduction of disulfide bonds leads to the precise cargo unload from nano-vehicles [186].
Enzyme stimulus
Nanocarriers whose surfaces are modified to make the nanocarriers responsive to the bio-catalytic action of enzymes are known as enzyme-stimulus nanocarriers. Enzymes are catalysts for biochemical reactions produced by living organisms. Enzymes play a vital role in cell function regulation; therefore, they are very important targets for drug delivery. Enzyme-triggered strategies utilize the overexpressed enzyme of the extracellular environment of tumor sites. This strategy is not applicable for intracellular drug release because the intracellular enzyme concentrations of cancer cells and healthy cells are almost same [187]. Proteases, an enzyme that breaks down protein and peptides, is an ideal candidate for releasing drugs from liposomes [188], [189].
Exogenous stimulus
In extrinsic stimulus systems, contrast agents are used to visualize the accumulation of nanocarriers in cancer sites. The accumulated drug-loaded nanocarriers are stimulated by an external factor, such as a magnetic field, ultrasound waves, light and electric fields [190] to release drugs at the right concentration.
Magnetic field responsive stimulus
In magnetically induced systems, an extracorporeal magnetic field is used to accumulate drug-loaded nanocarriers in tumor sites after the injection of nanocarriers. Core-shell structured nanoparticles coated with silica, polymer or magnetoliposome (maghemite nanocrystals encapsulated in liposomes) are some ideal candidates for magnetic stimulus [191], [192]. Magnetically guided nanocarriers can also carry genetic materials. Magnetic nanocarriers produce heat in the surrounding medium when they are placed under an oscillating magnetic field. This heat brings changes in the structures of nanocarriers [193], [194], [195].
Thermo-responsive stimulus
In this method, drug-loaded nanocarriers release their payloads in response to temperature change. At a predetermined temperature, the nanocarriers change their conformation, solubility or hydrophilic and hydrophobic balance. There are some nanocarriers which release their cargo whenever they go through a temperature change. Thermo-sensitive nanocarriers show the lower critical solution temperature (LCST) phenomena [196], [197]. Polymer aqueous solutions show one phase below LCST and phase separation above the temperature. Micelles with thermo-responsiveness are being widely studied [198], [199]. Thermo-sensitive hydrogels and poly (N-isopropyl acrylamide) (PNIPAAm) show temperature responsive sol-gel transitions [200].
Light-triggered stimulus
The recent development of light-triggered drug delivery is a new avenue for on-demand drug delivery. The light may be in the ultraviolet, visible or near-infrared ranges. The stimulus is achieved by making the nanocarriers sensitive to light [201], [202], [203]. CNTs and GNPs are good candidates for light-triggered stimulus, especially for the near-infrared (NIR) range. Metallic nanocarriers absorb light and convert the absorbed light to heat in order to kill cancer cells [204].
Ultrasound-responsive stimulus
Ultrasound is under intense investigation to release drugs from nanocarriers because of its non-invasiveness characteristics, deep penetration into the body and non-ionizing irradiation [205]. By using ultrasound, both thermal and mechanical effects can be induced in the nanocarriers to release the loaded-drug. The release of drugs from temperature-sensitive liposomes was investigated by Dromi el al. in 2007 using high intensity focused ultrasound waves [206], [207], [208].
Electric field-responsive stimulus
This stimulus system uses an electric field to release payloads. The thermo-responsive, light-triggered and ultrasound-responsive stimulus systems discussed already require large or specialized equipment to release drugs. On the contrary, electric fields are easy to generate and control [209]. Conducting polymers such as polypyrole (PPy) are in consideration for electric-responsive stimulus. Conducting polymers are used to modify nanocarriers, and the success of conducting polymers depends on the choice of dopant and the molecular weight of the drug. Biotin is a dopant that has been studied experimentally [210]. MWCNTs can be used as a conductive additive to increase electrical conductivity [211]; in addition, polyelectrolyte hydrogels are also in consideration [212], [213].
Toxicity study of eight nanocarriers
Currently, the toxicity of nanocarriers in the human body is the most important issue for investigation. To give the current status of toxicity research on nanocarriers loaded with anti-cancer drugs to the relevant researchers, a study is presented in Table 1.
Factors affecting the toxicity of nanocarriers
Table 1 shows that all the engineered nanocarriers exhibit some degree of toxicity. The toxicity of the nanocarriers depends on their size, shape (tube, films, rods, etc.) [282] surface charge and the presence or absence of a shell. In addition, the route of administration of drugs [283] and the dose of drugs also determine the toxicity of nanocarriers [284]. Size is the most important parameter in toxicity assessments of nanocarriers. The toxicity and the size of nanocarriers are inversely related; that is, the smaller the size of the nanocarriers, the higher the toxicity and vice versa [250], [285]. Shape also has a very important role in toxicity. For example, spherical gold nanocarriers are almost safe for the human body, while rod-shaped ones are very toxic [286], [287]. The surface charge of nanocarriers is another challenge to SDDS design, as the surface charge largely determines the interaction between the body and nanocarriers. Nanocarriers with positive charges, or cationic nanocarriers, show greater toxicity compared to ones with negative surface charges [214], [288]. A shell around the nanocarriers plays a vital role in reducing the toxicity of nanocarriers. Research shows that intravenous (IV) administration brings more medical complications than oral administration.
Challenges and the future research scope
Every opportunity comes with some challenges. SDDSs are no exception. The barriers in the of way of successful SDDSs are the toxicity of nanocarriers in the human body, cost-effectiveness of the system, the diversities and heterogeneities of cancer, and lack of specific regulatory guidelines [289], [290], [291].
To kill the cancer cell, nanocarriers carry and release the anti-cancer drugs at the targeted sites. The concern is with the ultimate fate of the drug-carrying nanocarriers. Depending on the chemical composition, size, shape, specific surface area, surface charge as well as the presence and absence of a shell around the nanocarrier, conventional nanocarriers accumulate in different vital organs such as the lungs, spleen, kidneys, liver and heart. A comprehensive study of the bio-distribution of nanocarriers is summarized in Table 1 above. Table 1 shows that the majority of nanocarriers are not discharged from body; instead, they accumulate in the vital organs mentioned above [292]. This deposition leads to toxicity, which is a gigantic barrier in the way of the success of SDDSs. Many in vitro and in vivo studies of toxicities in the cases of animals have been performed; unfortunately, toxicity studies in the human body are very limited. The research scope for toxicity studies is still wide open [293], [294].
Cancer varies in diversity and heterogeneity; that is, the types of cancers are still undetermined. Moreover, the physical nature of cancer may vary from person to person. Therefore, personalization of anti-cancer treatment is also a major challenge. DNA/RNA-based anti-cancer treatments have a bright future to make medication personalized and safer. Thus, the development of nanocarriers as carriers of DNA/RNA to remove cancer cells could be a promising research area [295], [296], [297].
In the way of finding of cancer cells, conventional nanocarriers face many biological challenges, such as the RES, accelerated blood clearance (ABC), etc. To address those hurdles, conventional nanocarriers are modified using various processes, including PEGylation, grafting ligands on the surface of nanocarriers; in addition, the nanocarriers need to be functionalized in order to release the drugs at target sites under stimulation. These modifications lead to increased manufacturing steps, which in turn lead to an increased cost of the final product. The cost-benefit balance should be positive for any launched product to be sustainable in the market [298], [299], [300].
Securing approval from regulatory authorities is the ultimate challenge in the way of the commercialization of SDDSs. The FDA and European medicines authority (EMA) have very strong roles in the approval process. Twenty-three years after the first smart nanocarrier-based anti-cancer drug, Doxil, has been reported in 1995 the number of FDA-approved nanocarrier-based anti-cancer drugs is very limited, though there are many products in the pipeline. For regulatory approval, the manufactures are supposed to prove the safety of the products for the human body both in the short term and long term. Therefore, it is very time consuming and laborious to launch a product following all the necessary steps. The lack of specific guidelines sometimes complicates the approval process. Therefore, an accord among researchers, industry and regulatory authorities is necessary to overcome these barriers [301], [302].
Conclusions and future perspectives
Nanocarriers, a wonder of modern science, play vital roles in biomedical applications, especially in anti-cancer drug delivery. To conquer the limitations associated with conventional chemotherapy, smart nanocarrier-based drug delivery systems, also known as SDDSs. have been introduced. However, there are still many challenges ahead for SDDSs to be effectively applied as a promising alternative to chemotherapy for cancer treatment; therefore, the technology behind SDDSs is under continuous research. The toxicity of the nanocarriers is a major barrier in the way of a successful SDDS. Studies have been conducted to either optimize the toxicity of existing nanocarriers or to develop some other new nanocarriers with lower toxicity. This review considers the gravity of toxicity and makes a bio-distribution assessment of the eight most common nanocarriers used in SDDSs. Our study on toxicity along with bio-distribution shows that almost every nanocarrier, from liposomes to QDs, show some degree of toxicity. The toxicity suggests that more extensive research is needed for SDDSs. The associated challenges and future research scope in SDDSs, which may favor the enduring perspectives and development of nanocarrier-based SDDSs for cancer treatment, have also been discussed.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics requirements
This article does not contain any studies with human or animal subjects.
Acknowledgements
The authors would like to express their gratitude to the researchers who have been referenced to successfully present this review. The authors acknowledge that, without their outstanding and continuous efforts to shed light in smart drug delivery, it would be impossible to write this review. This research work is based on the personal contribution of the authors without any grant from any specific funding agency.
Biographies

Sarwar Hossen received his B.Sc. and M.Sc. degree in Applied physics, Electronics and Communication Engineering from the Islamic University, Kushtia, Bangladesh in 2007 and 2008, respectively. He has been teaching physics since 2011 in the National University, Bangladesh as a member of BCS (General Education), a prestigious civil service in Bangladesh. He is enthusiastic about nanoparticles and the application of nanoparticles for biomedical applications, especially in smart drug delivery.

M. Khalid Hossain has received his Master of Science (M.Sc) degree in Applied Physics, Electronics and Communication Engineering from the Islamic University, Kushtia, Bangladesh in 2009. During his M.Sc program, he mainly focused on the preparation of amorphous Fe73.5Cu1Nb3Si13.5B9 magnetic ribbon by a rapid quenching method; then, the nanostructure and ultra-soft magnetic properties were developed by heat treatment. He has been a research scientist of the Institute of Electronics, Bangladesh Atomic Energy Commission (BAEC), Dhaka, Bangladesh since 2012. His research interests include energy materials, micro/nano fabrication, thin films, photovoltaic devices and advanced functional materials. He has published 17SCI(E) articles as author and co-author in various reputable peer reviewed journals.

M. Khairul Basher received his B.Sc. and M.Sc. degree in Applied Physics, Electronics & Communication Engineering from the University of Chittagong, Chittagong, Bangladesh in 2007 and 2008, respectively. He also completed a M. Phil degree in Material Science from the Bangladesh University of Engineering and Technology, Dhaka, Bangladesh. After completing M.Sc., he joined the Bangladesh Atomic Energy Commission as a scientist in 2012. He is now working as a research scientist in the Institute of Electronics, Bangladesh Atomic Energy Commission. His research interest mainly focuses on nanostructured materials and energy materials.

M. Nasrul Haque Mia received his B.Sc. and M.Sc. degree in Applied Physics, Electronics & Communication Engineering from the Islamic University, Kushtia, Bangladesh in 2003 and 2004, respectively. After completing his M.Sc., he joined the Bangladesh Atomic Energy Commission as a scientist in 2009. He is now working as a research scientist and divisional head of the VLSI technology laboratory in the Bangladesh Atomic Energy Commission. His research interest mainly focuses on nanostructured thin films for device applications.

M. Tayebur Rahman received his B.Sc. (Eng.) and M.Sc. (Eng.) degrees in Materials Science and Engineering from the University of Rajshahi, Bangladesh in 2014 and 2015, respectively. During his B.Sc. program, he explored the area of nanotechnology in medicine sectors, and, during his M.Sc. program, he fabricated ceramic nanoparticles (Fe2O3, TiO2 and NiFe2O4) dispersed in HDPE and UPR polymer matrix as novel nanocomposites to evaluate the mechanical, thermal, optical, and electrical properties. Currently, he is working as a research fellow in the Bangladesh Atomic Energy Commission (BAEC) and Bangladesh Council of Scientific and Industrial Research (BCSIR). His research interest mainly focuses on nanomaterials for bio-medical applications, nanocomposites, thin films and photovoltaic devices.

M. Jalal Uddin received his Master of Science (MS) degree in Nanomolecular Science from Jacobs University Bremen, Germany in 2013. During his MS program, he mainly focused on the preparation of metal-insulator-semiconductor (MIS) structures on flexible and cost-effective PET substrates and developed the modeling of a self-assembled monolayer (SAM) to quantify its electrical and physical properties. He has been a senior faculty member of the department of Electrical and Electronic Engineering, Islamic University, Kushtia, Bangladesh since 2004. Currently, he is researching as a PhD fellow at the Nano & Bio-IT Convergence Lab, KwangWoon University, Seoul, Republic of Korea. His research interests include photovoltaic devices, nano/micro sensors and smart-biochips for environmental and bio-medical applications.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society Cancer facts and figures 2017. Genes Dev. 2017;21:2525–2538. [Google Scholar]
- 3.Chabner B.A., Roberts T.G. Timeline: chemotherapy and the war on cancer. Nat Rev Cancer. 2005;5:65–72. doi: 10.1038/nrc1529. [DOI] [PubMed] [Google Scholar]
- 4.DeVita V.T., Chu E. A history of cancer chemotherapy. Cancer Res. 2008;68:8643–8653. doi: 10.1158/0008-5472.CAN-07-6611. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W., Zhang Z., Zhang Y. The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Res Lett. 2011;6:555. doi: 10.1186/1556-276X-6-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmad S.S., Reinius M.A., Hatcher H.M., Ajithkumar T.V. Anticancer chemotherapy in teenagers and young adults: managing long term side effects. BMJ. 2016;354:i4567. doi: 10.1136/bmj.i4567. [DOI] [PubMed] [Google Scholar]
- 7.Gillet J., Gottesman M.M. Multi-drug resistance in cancer. Humana Press; Totowa, NJ: 2010. [Google Scholar]
- 8.Alfarouk K.O., Stock C.-M., Taylor S., Walsh M., Muddathir A.K., Verduzco D. Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cell Int. 2015;15:71. doi: 10.1186/s12935-015-0221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nooter K., Stoter G. Molecular mechanisms of multidrug resistance in cancer chemotherapy. Pathol Res Pract. 1996;192:768–780. doi: 10.1016/S0344-0338(96)80099-9. [DOI] [PubMed] [Google Scholar]
- 10.Gupta P.K. Drug targeting in cancer chemotherapy: a clinical perspective. J Pharm Sci. 1990;79:949–962. doi: 10.1002/jps.2600791102. [DOI] [PubMed] [Google Scholar]
- 11.Kreuter J. Nanoparticles-a historical perspective. Int J Pharm. 2007;331:1–10. doi: 10.1016/j.ijpharm.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Khanna S.C., Jecklin T., Speiser P. Bead polymerization technique for sustained-release dosage form. J Pharm Sci. 1970;59:614–618. doi: 10.1002/jps.2600590508. [DOI] [PubMed] [Google Scholar]
- 13.Matsumura Y., Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 14.Bae Y.H., Park K. Targeted drug delivery to tumors: myths, reality and possibility. J Control Release. 2011;153:198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding C., Tong L., Feng J., Fu J. Recent advances in stimuli-responsive release function drug delivery systems for tumor treatment. Molecules. 2016;21:1715. doi: 10.3390/molecules21121715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreyling W.G., Semmler-Behnke M., Chaudhry Q. A complementary definition of nanomaterial. Nano Today. 2010;5:165–168. [Google Scholar]
- 17.Peer D., Karp J.M., Hong S., Farokhzad O.C., Margalit R., Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 18.Lee B.K., Yun Y.H., Park K. Smart nanoparticles for drug delivery: boundaries and opportunities. Chem Eng Sci. 2015;125:158–164. doi: 10.1016/j.ces.2014.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu D., Yang F., Xiong F., Gu N. The smart drug delivery system and its clinical potential. Theranostics. 2016;6:1306–1323. doi: 10.7150/thno.14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abuchowski A., McCoy J.R., Palczuk N.C., van Es T., Davis F.F. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem. 1977;252:3582–3586. [PubMed] [Google Scholar]
- 21.Moghimi S.M., Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog Lipid Res. 2003;42:463–478. doi: 10.1016/s0163-7827(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 22.Moghimi S.M., Hunter A.C., Murray J.C. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 23.Knop K., Hoogenboom R., Fischer D., Schubert U. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chemie Int Ed. 2010;49:6288–6308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 24.Verhoef J.J.F., Anchordoquy T.J. Questioning the use of PEGylation for drug delivery. Drug Deliv Transl Res. 2013;3:499–503. doi: 10.1007/s13346-013-0176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu H., Li Z., Si J. Nanocarriers in gene therapy: a review. J Biomed Nanotechnol. 2014;10:3483–3507. doi: 10.1166/jbn.2014.2044. [DOI] [PubMed] [Google Scholar]
- 26.Qi S.-S., Sun J.-H., Yu H.-H., Yu S.-Q. Co-delivery nanoparticles of anti-cancer drugs for improving chemotherapy efficacy. Drug Deliv. 2017;24:1909–1926. doi: 10.1080/10717544.2017.1410256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang L., Gao Z., Huang W., Jin M., Wang Q. Nanocarrier-mediated co-delivery of chemotherapeutic drugs and gene agents for cancer treatment. Acta Pharm Sin B. 2015;5:169–175. doi: 10.1016/j.apsb.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janib S.M., Moses A.S., MacKay J.A. Imaging and drug delivery using theranostic nanoparticles. Adv Drug Deliv Rev. 2010;62:1052–1063. doi: 10.1016/j.addr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srinivasan M., Rajabi M., Mousa S. Multifunctional nanomaterials and their applications in drug delivery and cancer therapy. Nanomaterials. 2015;5:1690–1703. doi: 10.3390/nano5041690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parvanian S., Mostafavi S.M., Aghashiri M. Multifunctional nanoparticle developments in cancer diagnosis and treatment. Sens Bio-Sensing Res. 2017;13:81–87. [Google Scholar]
- 31.Bangham A.D., Standish M.M., Weissmann G. The action of steroids and streptolysin S on the permeability of phospholipid structures to cations. J Mol Biol. 1965;13:253–259. doi: 10.1016/s0022-2836(65)80094-8. [DOI] [PubMed] [Google Scholar]
- 32.Gregoriadis G. Drug entrapment in liposomes. FEBS Lett. 1973;36:292–296. doi: 10.1016/0014-5793(73)80394-1. [DOI] [PubMed] [Google Scholar]
- 33.Akbarzadeh A., Rezaei-Sadabady R., Davaran S., Joo S.W., Zarghami N., Hanifehpour Y. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8:102. doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma A. Liposomes in drug delivery: progress and limitations. Int J Pharm. 1997;154:123–140. [Google Scholar]
- 35.Huang Z., Li X., Zhang T., Song Y., She Z., Li J. Progress involving new techniques for liposome preparation. Asian J Pharm Sci. 2014;9:176–182. [Google Scholar]
- 36.Carugo D., Bottaro E., Owen J., Stride E., Nastruzzi C. Liposome production by microfluidics: potential and limiting factors. Sci Rep. 2016;6:25876. doi: 10.1038/srep25876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bangham A.D. Properties and uses of lipid vesicles: an overview. Ann N Y Acad Sci. 1978;308:2–7. doi: 10.1111/j.1749-6632.1978.tb22010.x. [DOI] [PubMed] [Google Scholar]
- 38.Szoka F., Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci U S A. 1978;75:4194–4198. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deamer D.W. Preparation and properties of ether-injection liposomes. Ann N Y Acad Sci. 1978;308:250–258. doi: 10.1111/j.1749-6632.1978.tb22027.x. [DOI] [PubMed] [Google Scholar]
- 40.Zumbuehl O., Weder H.G. Liposomes of controllable size in the range of 40 to 180 nm by defined dialysis of lipid/detergent mixed micelles. BBA. 1981;640:252–262. doi: 10.1016/0005-2736(81)90550-2. [DOI] [PubMed] [Google Scholar]
- 41.Lesoin L., Crampon C., Boutin O., Badens E. Preparation of liposomes using the supercritical anti-solvent (SAS) process and comparison with a conventional method. J Supercrit Fluids. 2011;57:162–174. [Google Scholar]
- 42.Otake K., Shimomura T., Goto T., Imura T., Furuya T., Yoda S. Preparation of liposomes using an improved supercritical reverse phase evaporation method. Langmuir. 2006;22:2543–2550. doi: 10.1021/la051654u. [DOI] [PubMed] [Google Scholar]
- 43.Sercombe L., Veerati T., Moheimani F., Wu S.Y., Sood A.K., Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286. doi: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bozzuto G., Molinari A. Liposomes as nanomedical devices. Int J Nanomed. 2015;10:975. doi: 10.2147/IJN.S68861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allen T.M., Cullis P.R. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 46.Noble G.T., Stefanick J.F., Ashley J.D., Kiziltepe T., Bilgicer B. Ligand-targeted liposome design: challenges and fundamental considerations. Trends Biotechnol. 2014;32:32–45. doi: 10.1016/j.tibtech.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 47.Sapra P., Allen T.M. Ligand-targeted liposomal anticancer drugs. Prog Lipid Res. 2003;42:439–462. doi: 10.1016/s0163-7827(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 48.Sawant R.R., Torchilin V.P. Challenges in development of targeted liposomal therapeutics. AAPS J. 2012;14:303–315. doi: 10.1208/s12248-012-9330-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruoslahti E. Peptides as targeting elements and tissue penetration devices for nanoparticles. Adv Mater. 2012;24:3747–3756. doi: 10.1002/adma.201200454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee Y., Thompson D.H. Stimuli-responsive liposomes for drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9:e1450. doi: 10.1002/wnan.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang S.L., MacDonald R.C. Acoustically active liposomes for drug encapsulation and ultrasound-triggered release. Biochim Biophys Acta – Biomembr. 2004;1665:134–141. doi: 10.1016/j.bbamem.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 52.Jin Y., Liang X., An Y., Dai Z. Microwave-triggered smart drug release from liposomes co-encapsulating doxorubicin and salt for local combined hyperthermia and chemotherapy of cancer. Bioconjug Chem. 2016;27:2931–2942. doi: 10.1021/acs.bioconjchem.6b00603. [DOI] [PubMed] [Google Scholar]
- 53.Ogihara-Umeda I., Sasaki T., Kojima S., Nishigori H. Optimal radiolabeled liposomes for tumor imaging. J Nucl Med. 1996;37:326–332. [PubMed] [Google Scholar]
- 54.Petersen A.L., Hansen A.E., Gabizon A., Andresen T.L. Liposome imaging agents in personalized medicine. Adv Drug Deliv Rev. 2012;64:1417–1435. doi: 10.1016/j.addr.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 55.Li S., Goins B., Zhang L., Bao A. Novel multifunctional theranostic liposome drug delivery system: construction, characterization, and multimodality MR, near-infrared fluorescent, and nuclear imaging. Bioconjug Chem. 2012;23:1322–1332. doi: 10.1021/bc300175d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muthu M.S., Feng S.-S. Theranostic liposomes for cancer diagnosis and treatment: current development and pre-clinical success. Expert Opin Drug Deliv. 2013;10:151–155. doi: 10.1517/17425247.2013.729576. [DOI] [PubMed] [Google Scholar]
- 57.Samson A.A.S., Park S., Kim S.-Y., Min D.-H., Jeon N.L., Song J.M. Liposomal co-delivery-based quantitative evaluation of chemosensitivity enhancement in breast cancer stem cells by knockdown of GRP78/CLU. J Liposome Res. 2018:1–9. doi: 10.1080/08982104.2017.1420081. [DOI] [PubMed] [Google Scholar]
- 58.Zununi Vahed S., Salehi R., Davaran S., Sharifi S. Liposome-based drug co-delivery systems in cancer cells. Mater Sci Eng C. 2017;71:1327–1341. doi: 10.1016/j.msec.2016.11.073. [DOI] [PubMed] [Google Scholar]
- 59.Shin D.H., Tam Y.T., Kwon G.S. Polymeric micelle nanocarriers in cancer research. Front Chem Sci Eng. 2016;10:348–359. [Google Scholar]
- 60.Cagel M., Tesan F.C., Bernabeu E., Salgueiro M.J., Zubillaga M.B., Moretton M.A. Polymeric mixed micelles as nanomedicines: achievements and perspectives. Eur J Pharm Biopharm. 2017;113:211–228. doi: 10.1016/j.ejpb.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 61.Trivedi R., Kompella U.B. Nanomicellar formulations for sustained drug delivery: strategies and underlying principles. Nanomedicine. 2010;5:485–505. doi: 10.2217/nnm.10.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kataoka K., Harada A., Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev. 2001;47:113–131. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 63.Chen Y., Liu Y., Yao Y., Zhang S., Gu Z. Reverse micelle-based water-soluble nanoparticles for simultaneous bioimaging and drug delivery. Org Biomol Chem. 2017;15:3232–3238. doi: 10.1039/c7ob00169j. [DOI] [PubMed] [Google Scholar]
- 64.Tang L.-Y., Wang Y.-C., Li Y., Du J.-Z., Wang J. Shell-detachable micelles based on disulfide-linked block copolymer as potential carrier for intracellular drug delivery. Bioconjug Chem. 2009;20:1095–1099. doi: 10.1021/bc900144m. [DOI] [PubMed] [Google Scholar]
- 65.Deng H., Liu J., Zhao X., Zhang Y., Liu J., Xu S. PEG-b-PCL copolymer micelles with the ability of pH-controlled negative-to-positive charge reversal for intracellular delivery of doxorubicin. Biomacromolecules. 2014;15:4281–4292. doi: 10.1021/bm501290t. [DOI] [PubMed] [Google Scholar]
- 66.Sutton D., Nasongkla N., Blanco E., Gao J. Functionalized micellar systems for cancer targeted drug delivery. Pharm Res. 2007;24:1029–1046. doi: 10.1007/s11095-006-9223-y. [DOI] [PubMed] [Google Scholar]
- 67.Letchford K., Burt H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: micelles, nanospheres, nanocapsules and polymersomes. Eur J Pharm Biopharm. 2007;65:259–269. doi: 10.1016/j.ejpb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 68.Liu J., Xiao Y., Allen C. Polymer–drug compatibility: a guide to the development of delivery systems for the anticancer agent, ellipticine. J Pharm Sci. 2004;93:132–143. doi: 10.1002/jps.10533. [DOI] [PubMed] [Google Scholar]
- 69.Kohori F., Yokoyama M., Sakai K., Okano T. Process design for efficient and controlled drug incorporation into polymeric micelle carrier systems. J Control Release. 2002;78:155–163. doi: 10.1016/s0168-3659(01)00492-8. [DOI] [PubMed] [Google Scholar]
- 70.Cajot S., Schol D., Danhier F., Préat V., Gillet De Pauw M.-C., Jérôme C. In vitro investigations of smart drug delivery systems based on redox-sensitive cross-linked micelles. Macromol Biosci. 2013;13:1661–1670. doi: 10.1002/mabi.201300250. [DOI] [PubMed] [Google Scholar]
- 71.Husseini G.a., Runyan C.M., Pitt W.G. Investigating the mechanism of acoustically activated uptake of drugs from Pluronic micelles. BMC Cancer. 2002;2:20. doi: 10.1186/1471-2407-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seo S.-J., Lee S.-Y., Choi S.-J., Kim H.-W. Tumor-targeting co-delivery of drug and gene from temperature-triggered micelles. Macromol Biosci. 2015;15:1198–1204. doi: 10.1002/mabi.201500137. [DOI] [PubMed] [Google Scholar]
- 73.Blanco E., Kessinger C.W., Sumer B.D., Gao J. Multifunctional micellar nanomedicine for cancer therapy. Exp Biol Med. 2009;234:123–131. doi: 10.3181/0808-MR-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rapoport N., Gao Z., Kennedy A. Multifunctional nanoparticles for combining ultrasonic tumor imaging and targeted chemotherapy. JNCI J Natl Cancer Inst. 2007;99:1095–1106. doi: 10.1093/jnci/djm043. [DOI] [PubMed] [Google Scholar]
- 75.Palmerston Mendes L., Pan J., Torchilin V. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules. 2017;22:1401. doi: 10.3390/molecules22091401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jackson C.L., Chanzy H.D., Booy F.P., Drake B.J., Tomalia D.A., Bauer B.J. Visualization of dendrimer molecules by transmission electron microscopy (TEM): staining methods and cryo-TEM of vitrified solutions. Macromolecules. 1998;31:6259–6265. [Google Scholar]
- 77.Nanjwade B.K., Bechra H.M., Derkar G.K., Manvi F.V., Nanjwade V.K. Dendrimers: emerging polymers for drug-delivery systems. Eur J Pharm Sci. 2009 doi: 10.1016/j.ejps.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 78.Majoros I.J., Williams C.R., Tomalia D.A., Baker J.R. New dendrimers: synthesis and characterization of POPAM-PAMAM hybrid dendrimers. Macromolecules. 2008;41:8372–8379. doi: 10.1021/ma801843a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Caminade A.-M. Phosphorus dendrimers for nanomedicine. Chem Commun. 2017;53:9830–9838. doi: 10.1039/c7cc04949h. [DOI] [PubMed] [Google Scholar]
- 80.Richardt G., Werner N., Fritz V. Types of dendrimers and their syntheses. Dendrimer chem. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2009. pp. 81–167. [Google Scholar]
- 81.Tomalia D.A., Baker H., Dewald J., Hall M., Kallos G., Martin S. A new class of polymers: starburst-dendritic macromolecules. Polym J. 1985;17:117–132. [Google Scholar]
- 82.Hawker C.J., Frechet J.M.J. Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. J Am Chem Soc. 1990;112:7638–7647. [Google Scholar]
- 83.Buhleier E., Wehner W., Vögtle F. “Cascade”- and “nonskid-chain-like” syntheses of molecular cavity topologies. Synthesis (Stuttg) 1978;1978:155–158. [Google Scholar]
- 84.Bosman A.W., Janssen H.M., Meijer E.W. About dendrimers: structure, physical properties, and applications. Chem Rev. 1999;99:1665–1688. doi: 10.1021/cr970069y. [DOI] [PubMed] [Google Scholar]
- 85.Esfand R., Tomalia D.A. Poly(amidoamine) (PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discov Today. 2001;6:427–436. doi: 10.1016/s1359-6446(01)01757-3. [DOI] [PubMed] [Google Scholar]
- 86.Newkome G.R., Yao Z., Baker G.R., Gupta V.K. Micelles. Part 1. Cascade molecules: a new approach to micelles. A [27]-arborol. J Org Chem. 1985;50:2003–2004. [Google Scholar]
- 87.Abbasi E., Aval S., Akbarzadeh A., Milani M., Nasrabadi H., Joo S. Dendrimers: synthesis, applications, and properties. Nanoscale Res Lett. 2014;9:247. doi: 10.1186/1556-276X-9-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jain K., Kesharwani P., Gupta U., Jain N.K. Dendrimer toxicity: let’s meet the challenge. Int J Pharm. 2010;394:122–142. doi: 10.1016/j.ijpharm.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 89.Bugno J., Hsu H., Hong S. Tweaking dendrimers and dendritic nanoparticles for controlled nano-bio interactions: potential nanocarriers for improved cancer targeting. J Drug Target. 2015;23:642–650. doi: 10.3109/1061186X.2015.1052077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang H., Huang Q., Chang H., Xiao J., Cheng Y. Stimuli-responsive dendrimers in drug delivery. Biomater Sci. 2016;4:375–390. doi: 10.1039/c5bm00532a. [DOI] [PubMed] [Google Scholar]
- 91.Ramireddy R., Raghupathi K.R., Torres D.A., Thayumanavan S. Stimuli sensitive amphiphilic dendrimers. New J Chem. 2012;36:340. doi: 10.1039/C2NJ20879B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pandita D., Poonia N., Kumar S., Lather V., Madaan K. Dendrimers in drug delivery and targeting: drug-dendrimer interactions and toxicity issues. J Pharm Bioallied Sci. 2014;6:139. doi: 10.4103/0975-7406.130965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ye M., Qian Y., Tang J., Hu H., Sui M., Shen Y. Targeted biodegradable dendritic MRI contrast agent for enhanced tumor imaging. J Control Release. 2013;169:239–245. doi: 10.1016/j.jconrel.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 94.Brühwiler D. Postsynthetic functionalization of mesoporous silica. Nanoscale. 2010;2:887–892. doi: 10.1039/c0nr00039f. [DOI] [PubMed] [Google Scholar]
- 95.Watermann A. Mesoporous silica nanoparticles as drug delivery vehicles in cancer. Nanomaterials. 2017;7:189. doi: 10.3390/nano7070189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roggers R., Kanvinde S., Boonsith S., Oupický D. The practicality of mesoporous silica nanoparticles as drug delivery devices and progress toward this goal. AAPS PharmSciTech. 2014;15:1163–1171. doi: 10.1208/s12249-014-0142-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nandiyanto A.B.D., Kim S.G., Iskandar F., Okuyama K. Synthesis of spherical mesoporous silica nanoparticles with nanometer-size controllable pores and outer diameters. Microporous Mesoporous Mater. 2009;120:447–453. [Google Scholar]
- 98.Slowing I., Viveroescoto J., Wu C., Lin V. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv Drug Deliv Rev. 2008;60:1278–1288. doi: 10.1016/j.addr.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 99.Asefa T., Tao Z. Biocompatibility of mesoporous silica nanoparticles. Chem Res Toxicol. 2012;25:2265–2284. doi: 10.1021/tx300166u. [DOI] [PubMed] [Google Scholar]
- 100.Lin Y.-S., Haynes C.L. Synthesis and characterization of biocompatible and size-tunable multifunctional porous silica nanoparticles. Chem Mater. 2009;21:3979–3986. [Google Scholar]
- 101.Popat A., Liu J., Lu G.Q. (Max), Qiao S.Z. A pH-responsive drug delivery system based on chitosan coated mesoporous silica nanoparticles. J Mater Chem. 2012;22:11173. [Google Scholar]
- 102.Tang F., Li L., Chen D. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv Mater. 2012;24:1504–1534. doi: 10.1002/adma.201104763. [DOI] [PubMed] [Google Scholar]
- 103.Yamamoto E., Kitahara M., Tsumura T., Kuroda K. Preparation of size-controlled monodisperse colloidal mesoporous silica nanoparticles and fabrication of colloidal crystals. Chem Mater. 2014;26:2927–2933. [Google Scholar]
- 104.He Q., Zhang J., Shi J., Zhu Z., Zhang L., Bu W. The effect of PEGylation of mesoporous silica nanoparticles on nonspecific binding of serum proteins and cellular responses. Biomaterials. 2010;31:1085–1092. doi: 10.1016/j.biomaterials.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 105.Paris J.L., Cabañas M.V., Manzano M., Vallet-Regí M. Polymer-grafted mesoporous silica nanoparticles as ultrasound-responsive drug carriers. ACS Nano. 2015;9:11023–11033. doi: 10.1021/acsnano.5b04378. [DOI] [PubMed] [Google Scholar]
- 106.Yanes R.E., Tamanoi F. Development of mesoporous silica nanomaterials as a vehicle for anticancer drug delivery. Ther Deliv. 2012;3:389–404. doi: 10.4155/tde.12.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nadrah P., Planinšek O., Gaberšček M. Stimulus-responsive mesoporous silica particles. J Mater Sci. 2014;49:481–495. [Google Scholar]
- 108.Song Y., Li Y., Xu Q., Liu Z. Mesoporous silica nanoparticles for stimuli-responsive controlled drug delivery: advances, challenges, and outlook. Int J Nanomed. 2016;12:87–110. doi: 10.2147/IJN.S117495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hergt R., Andrä W. Magnetism in medicine. J Appl Phys. 2007;404:550–570. [Google Scholar]
- 110.Hsiao S.-M., Peng B.-Y., Tseng Y.S., Liu H.-T., Chen C.-H., Lin H.-M. Preparation and characterization of multifunctional mesoporous silica nanoparticles for dual magnetic resonance and fluorescence imaging in targeted cancer therapy. Microporous Mesoporous Mater. 2017;250:210–220. [Google Scholar]
- 111.Conde J., Doria G., Baptista P. Noble metal nanoparticles applications in cancer. J Drug Deliv. 2012;2012:751075. doi: 10.1155/2012/751075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chithrani B.D., Ghazani A.A., Chan W.C.W. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 113.Applications I. Unique roles of gold nanoparticles in drug delivery, targeting and imaging applications. Molecules. 2017;22:1445. doi: 10.3390/molecules22091445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Noguez C. Surface plasmons on metal nanoparticles: the influence of shape and physical environment. J Phys Chem C. 2007;111:3806–3819. [Google Scholar]
- 115.El-Sayed I.H., Huang X., El-Sayed M.A. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer. Nano Lett. 2005;5:829–834. doi: 10.1021/nl050074e. [DOI] [PubMed] [Google Scholar]
- 116.Kimling J., Maier M., Okenve B., Kotaidis V., Ballot H., Plech A. Turkevich method for gold nanoparticle synthesis revisited. J Phys Chem B. 2006;110:15700–15707. doi: 10.1021/jp061667w. [DOI] [PubMed] [Google Scholar]
- 117.Mafune F., Kohno J.Y., Taked Y., Kondow T. Full physical preparation of size-selected gold nanoparticles in solution: laser ablation and Laser induced size control. J Phys Chem B. 2002;106:7575–7577. [Google Scholar]
- 118.Song J.Y., Jang H.K., Kim B.S. Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochem. 2009;44:1133–1138. [Google Scholar]
- 119.Khan A., Rashid R., Murtaza G., Zahra A. Gold nanoparticles: synthesis and applications in drug delivery. Trop J Pharm Res. 2014;13:1169. [Google Scholar]
- 120.Clark A.J., Davis M.E. Increased brain uptake of targeted nanoparticles by adding an acid-cleavable linkage between transferrin and the nanoparticle core. Proc Natl Acad Sci U S A. 2015;112:12486–12491. doi: 10.1073/pnas.1517048112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dreaden E.C., Austin L.A., Mackey M.A., El-Sayed M.A. Size matters: gold nanoparticles in targeted cancer drug delivery. Ther Deliv. 2012;3:457–478. doi: 10.4155/tde.12.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Qian W., Murakami M., Ichikawa Y., Che Y. Highly efficient and controllable PEGylation of gold nanoparticles prepared by femtosecond laser ablation in water. J Phys Chem C. 2011;115:23293–23298. [Google Scholar]
- 123.Yang P.-H., Sun X., Chiu J.-F., Sun H., He Q.-Y. Transferrin-mediated gold nanoparticle cellular uptake. Bioconjug Chem. 2005;16:494–496. doi: 10.1021/bc049775d. [DOI] [PubMed] [Google Scholar]
- 124.Han G., Ghosh P., Rotello V.M. Functionalized gold nanoparticles for drug delivery. Nanomedicine. 2007;2:113–123. doi: 10.2217/17435889.2.1.113. [DOI] [PubMed] [Google Scholar]
- 125.Dixit V., Van den Bossche J., Sherman D.M., Thompson D.H., Andres R.P. Synthesis and grafting of thioctic acid-PEG-folate conjugates onto Au nanoparticles for selective targeting of folate receptor-positive tumor cells. Bioconjug Chem. 2006;17:603–609. doi: 10.1021/bc050335b. [DOI] [PubMed] [Google Scholar]
- 126.Yao C., Zhang L., Wang J., He Y., Xin J., Wang S. Gold nanoparticle mediated phototherapy for cancer. J Nanomater. 2016;2016:1–29. [Google Scholar]
- 127.Tian L., Lu L., Qiao Y., Ravi S., Salatan F., Melancon M. Stimuli-responsive gold nanoparticles for cancer diagnosis and therapy. J Funct Biomater. 2016;7:19. doi: 10.3390/jfb7030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mendes R., Fernandes A., Baptista P. Gold nanoparticle approach to the selective delivery of gene silencing in cancer—the case for combined delivery? Genes (Basel) 2017;8:94. doi: 10.3390/genes8030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tiwari P., Vig K., Dennis V., Singh S. Functionalized gold nanoparticles and their biomedical applications. Nanomaterials. 2011;1:31–63. doi: 10.3390/nano1010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wahajuddin Arora S. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int J Nanomed. 2012;7:3445–3471. doi: 10.2147/IJN.S30320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kodama R. Magnetic nanoparticles. J Magn Magn Mater. 1999;200:359–372. [Google Scholar]
- 132.Cano M., Núñez-Lozano R., Dumont Y., Larpent C., de la Cueva-Méndez G. Synthesis and characterization of multifunctional superparamagnetic iron oxide nanoparticles (SPION)/C 60 nanocomposites assembled by fullerene–amine click chemistry. RSC Adv. 2016;6:70374–70382. [Google Scholar]
- 133.Kandasamy G., Maity D. Recent advances in superparamagnetic iron oxide nanoparticles (SPIONs) for in vitro and in vivo cancer nanotheranostics. Int J Pharm. 2015;496:191–218. doi: 10.1016/j.ijpharm.2015.10.058. [DOI] [PubMed] [Google Scholar]
- 134.Cole A.J., Yang V.C., David A.E. Cancer theranostics: the rise of targeted magnetic nanoparticles. Trends Biotechnol. 2011;29:323–332. doi: 10.1016/j.tibtech.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Patra S., Roy E., Karfa P., Kumar S., Madhuri R., Sharma P.K. Dual-responsive polymer coated superparamagnetic nanoparticle for targeted drug delivery and hyperthermia treatment. ACS Appl Mater Interfaces. 2015;7:9235–9246. doi: 10.1021/acsami.5b01786. [DOI] [PubMed] [Google Scholar]
- 136.Mok H., Zhang M. Superparamagnetic iron oxide nanoparticle-based delivery systems for biotherapeutics. Expert Opin Drug Deliv. 2013;10:73–87. doi: 10.1517/17425247.2013.747507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Laurent S., Saei A.A., Behzadi S., Panahifar A., Mahmoudi M. Superparamagnetic iron oxide nanoparticles for delivery of therapeutic agents: opportunities and challenges. Expert Opin Drug Deliv. 2014;11:1449–1470. doi: 10.1517/17425247.2014.924501. [DOI] [PubMed] [Google Scholar]
- 138.Santhosh P.B., Ulrih N.P. Multifunctional superparamagnetic iron oxide nanoparticles: promising tools in cancer theranostics. Cancer Lett. 2013;336:8–17. doi: 10.1016/j.canlet.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 139.Jeffreys A.J., Wilson V., Thein S.L. Individual-specific “fingerprints” of human DNA. Nature. 1985;316:76–79. doi: 10.1038/316076a0. [DOI] [PubMed] [Google Scholar]
- 140.Krätschmer W., Lamb L.D., Fostiropoulos K., Huffman D.R. Solid C60: a new form of carbon. Nature. 1990;347:354–358. [Google Scholar]
- 141.Liu Z., Robinson J.T., Tabakman S.M., Yang K., Dai H. Carbon materials for drug delivery & cancer therapy. Mater Today. 2011;14:316–323. [Google Scholar]
- 142.Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–58. [Google Scholar]
- 143.Cantoro M., Hofmann S., Pisana S., Scardaci V., Parvez A., Ducati C. Catalytic chemical vapor deposition of single-wall carbon nanotubes at low temperatures. Nano Lett. 2006;6:1107–1112. doi: 10.1021/nl060068y. [DOI] [PubMed] [Google Scholar]
- 144.Eatemadi A., Daraee H., Karimkhanloo H., Kouhi M., Zarghami N. Carbon nanotubes: properties, synthesis, purification, and medical applications. Nanoscale Res Lett. 2014:1–13. doi: 10.1186/1556-276X-9-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bianco A., Kostarelos K., Prato M. Applications of carbon nanotubes in drug delivery. Curr Opin Chem Biol. 2005;9:674–679. doi: 10.1016/j.cbpa.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 146.Li Z., de Barros A.L.B., Soares D.C.F., Moss S.N., Alisaraie L. Functionalized single-walled carbon nanotubes: cellular uptake, biodistribution and applications in drug delivery. Int J Pharm. 2017;524:41–54. doi: 10.1016/j.ijpharm.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 147.Lay C.L., Liu J., Liu Y. Functionalized carbon nanotubes for anticancer drug delivery. Expert Rev Med Devices. 2011;8:561–566. doi: 10.1586/erd.11.34. [DOI] [PubMed] [Google Scholar]
- 148.Schmaljohann D. Thermo- and pH-responsive polymers in drug delivery. Adv Drug Deliv Rev. 2006;58:1655–1670. doi: 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 149.Wang J.T., Al-Jamal K.T. Functionalized carbon nanotubes: revolution in brain delivery. Nanomedicine. 2015;10:2639–2642. doi: 10.2217/nnm.15.114. [DOI] [PubMed] [Google Scholar]
- 150.Kafa H., Wang J.T.-W., Rubio N., Venner K., Anderson G., Pach E. The interaction of carbon nanotubes with an in vitro blood-brain barrier model and mouse brain in vivo. Biomaterials. 2015;53:437–452. doi: 10.1016/j.biomaterials.2015.02.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Son K.H., Hong J.H., Lee J.W. Carbon nanotubes as cancer therapeutic carriers and mediators. Int J Nanomed. 2016;11:5163–5185. doi: 10.2147/IJN.S112660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Seifalian A. A new era of cancer treatment: carbon nanotubes as drug delivery tools. Int J Nanomed. 2011;6:2963. doi: 10.2147/IJN.S16923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen Z., Zhang A., Wang X., Zhu J., Fan Y., Yu H. The advances of carbon nanotubes in cancer diagnostics and therapeutics. J Nanomater. 2017;2017:1–13. [Google Scholar]
- 154.Matea C., Mocan T., Tabaran F., Pop T., Mosteanu O., Puia C. Quantum dots in imaging, drug delivery and sensor applications. Int J Nanomed. 2017;12:5421–5431. doi: 10.2147/IJN.S138624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zrazhevskiy P., Sena M., Gao X. Designing multifunctional quantum dots for bioimaging, detection, and drug delivery. Chem Soc Rev. 2010;39:4326–4354. doi: 10.1039/b915139g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ghasemi Y., Peymani P., Afifi S. Quantum dot: magic nanoparticle for imaging, detection and targeting. Acta Biomed. 2009;80:156–165. [PubMed] [Google Scholar]
- 157.Qi L., Gao X. Emerging application of quantum dots for drug delivery and therapy. Expert Opin Drug Deliv. 2008;5:263–267. doi: 10.1517/17425247.5.3.263. [DOI] [PubMed] [Google Scholar]
- 158.Nakata Y., Mukai K., Sugawara M., Ohtsubo K., Ishikawa H., Yokoyama N. Molecular beam epitaxial growth of InAs self-assembled quantum dots with light-emission at 1.3μm. J Cryst Growth. 2000;208:93–99. [Google Scholar]
- 159.Bertino M.F., Gadipalli R.R., Martin L.A., Rich L.E., Yamilov A., Heckman B.R. Quantum dots by ultraviolet and X-ray lithography. Nanotechnology. 2007;18:315603. [Google Scholar]
- 160.Valizadeh A., Mikaeili H., Samiei M., Farkhani S., Zarghami N., Kouhi M. Quantum dots: synthesis, bioapplications, and toxicity. Nanoscale Res Lett. 2012;7:480. doi: 10.1186/1556-276X-7-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gui R., Wan A., Zhang Y., Li H., Zhao T. Ratiometric and time-resolved fluorimetry from quantum dots featuring drug carriers for real-time monitoring of drug release in situ. Anal Chem. 2014;86:5211–5214. doi: 10.1021/ac501293e. [DOI] [PubMed] [Google Scholar]
- 162.Zhang H., Yee D., Wang C. Quantum dots for cancer diagnosis and therapy: biological and clinical perspectives. Nanomedicine (Lond) 2008;3:83–91. doi: 10.2217/17435889.3.1.83. [DOI] [PubMed] [Google Scholar]
- 163.Iannazzo D., Pistone A., Salamò M., Galvagno S., Romeo R., Giofré S.V. Graphene quantum dots for cancer targeted drug delivery. Int J Pharm. 2017;518:185–192. doi: 10.1016/j.ijpharm.2016.12.060. [DOI] [PubMed] [Google Scholar]
- 164.Zhao M.-X., Zhu B.-J. The research and applications of quantum dots as nano-carriers for targeted drug delivery and cancer therapy. Nanoscale Res Lett. 2016;11:207. doi: 10.1186/s11671-016-1394-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kamal M.A., Jabir N.R.N., Tabrez Ashraf, Shakil Damanhouri. Nanotechnology-based approaches in anticancer research. Int J Nanomed. 2012;7:4391. doi: 10.2147/IJN.S33838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Senapati S., Mahanta A.K., Kumar S., Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther. 2018;3:7. doi: 10.1038/s41392-017-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gao X., Cui Y., Levenson R.M., Chung L.W.K., Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22:969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 168.Danhier F., Feron O., Préat V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148:135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 169.Mohanty C., Das M., Kanwar J.R., Sahoo S.K. Receptor mediated tumor targeting: an emerging approach for cancer therapy. Curr Drug Deliv. 2011;8:45–58. doi: 10.2174/156720111793663606. [DOI] [PubMed] [Google Scholar]
- 170.Matsumura Y., Maeda H.A. A new concept for macromolecular therapeutics in cancer-chemotherapy – mechanism of tumoritropic acumulation of proteins and the antitumor agent Smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 171.Nakamura Y., Mochida A., Choyke P.L., Kobayashi H. Nanodrug delivery: is the enhanced permeability and retention effect sufficient for curing cancer? Bioconjug Chem. 2016;27:2225–2238. doi: 10.1021/acs.bioconjchem.6b00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jain R.K. Transport of molecules across tumor vasculature. Cancer Metastasis Rev. 1987;6:559–593. doi: 10.1007/BF00047468. [DOI] [PubMed] [Google Scholar]
- 173.Heldin C.-H., Rubin K., Pietras K., Östman A. High interstitial fluid pressure—an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 174.Blanco E., Shen H., Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Villiers C.L., Freitas H., Couderc R., Villiers M.-B., Marche P.N. Analysis of the toxicity of gold nano particles on the immune system: effect on dendritic cell functions. J Nanoparticle Res. 2010;12:55–60. doi: 10.1007/s11051-009-9692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mura S., Nicolas J., Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 177.Liu M., Du H., Zhang W., Zhai G. Internal stimuli-responsive nanocarriers for drug delivery: design strategies and applications. Mater Sci Eng C. 2017;71:1267–1280. doi: 10.1016/j.msec.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 178.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gerweck L.E., Seetharaman K. Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res. 1996;56:1194–1198. [PubMed] [Google Scholar]
- 180.Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 181.Engin K., Leeper D.B., Cater J.R., Thistlethwaite A.J., Tupchong L., McFarlane J.D. Extracellular pH distribution in human tumours. Int J Hyperthermia. 1995;11:211–216. doi: 10.3109/02656739509022457. [DOI] [PubMed] [Google Scholar]
- 182.Nilsson C., Kågedal K., Johansson U., Ollinger K. Analysis of cytosolic and lysosomal pH in apoptotic cells by flow cytometry. Methods Cell Sci. 2003;25:185–194. doi: 10.1007/s11022-004-8228-3. [DOI] [PubMed] [Google Scholar]
- 183.Gamcsik M.P., Kasibhatla M.S., Teeter S.D., Colvin O.M. Glutathione levels in human tumors. Biomarkers. 2012;17:671–691. doi: 10.3109/1354750X.2012.715672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Meng F., Hennink W.E., Zhong Z. Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials. 2009;30:2180–2198. doi: 10.1016/j.biomaterials.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 185.Wen H. Redox sensitive nanoparticles with disulfide bond linked sheddable shell for intracellular drug delivery. Med Chem (Los Angeles) 2014;4:748–755. [Google Scholar]
- 186.Cheng R., Feng F., Meng F., Deng C., Feijen J., Zhong Z. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J Control Release. 2011;152:2–12. doi: 10.1016/j.jconrel.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 187.Andresen T.L., Thompson D.H., Kaasgaard T. Enzyme-triggered nanomedicine: drug release strategies in cancer therapy. Mol Membr Biol. 2010;27:353–363. doi: 10.3109/09687688.2010.515950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Andresen T.L., Jensen S.S., Jørgensen K. Advanced strategies in liposomal cancer therapy: problems and prospects of active and tumor specific drug release. Prog Lipid Res. 2005;44:68–97. doi: 10.1016/j.plipres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 189.Meers P. Enzyme-activated targeting of liposomes. Adv Drug Deliv Rev. 2001;53:265–272. doi: 10.1016/s0169-409x(01)00205-8. [DOI] [PubMed] [Google Scholar]
- 190.Yao J., Feng J., Chen J. External-stimuli responsive systems for cancer theranostic. Asian J Pharm Sci. 2016;11:585–595. [Google Scholar]
- 191.Hua M.-Y., Liu H.-L., Yang H.-W., Chen P.-Y., Tsai R.-Y., Huang C.-Y. The effectiveness of a magnetic nanoparticle-based delivery system for BCNU in the treatment of gliomas. Biomaterials. 2011;32:516–527. doi: 10.1016/j.biomaterials.2010.09.065. [DOI] [PubMed] [Google Scholar]
- 192.Plassat V., Wilhelm C., Marsaud V., Ménager C., Gazeau F., Renoir J.-M. Anti-estrogen-loaded superparamagnetic liposomes for intracellular magnetic targeting and treatment of breast cancer tumors. Adv Funct Mater. 2011;21:83–92. [Google Scholar]
- 193.Bringas E., Köysüren Ö., Quach D.V., Mahmoudi M., Aznar E., Roehling J.D. Triggered release in lipid bilayer-capped mesoporous silica nanoparticles containing SPION using an alternating magnetic field. Chem Commun. 2012;48:5647. doi: 10.1039/c2cc31563g. [DOI] [PubMed] [Google Scholar]
- 194.Hu S.-H., Chen S.-Y., Liu D.-M., Hsiao C.-S. Core/single-crystal-shell nanospheres for controlled drug release via a magnetically triggered rupturing mechanism. Adv Mater. 2008;20:2690–2695. doi: 10.1002/adma.200800193. [DOI] [PubMed] [Google Scholar]
- 195.Hu S.-H., Chen S.-Y., Gao X. Multifunctional nanocapsules for simultaneous encapsulation of hydrophilic and hydrophobic compounds and on-demand release. ACS Nano. 2012;6:2558–2565. doi: 10.1021/nn205023w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shao P., Wang B., Wang Y., Li J., Zhang Y. The application of thermosensitive nanocarriers in controlled drug delivery. J Nanomater. 2011;2011:1–12. [Google Scholar]
- 197.Kost J., Langer R. Responsive polymeric delivery systems. Adv Drug Deliv Rev. 2012;64:327–341. doi: 10.1016/s0169-409x(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 198.Yokoyama M., Okano T. Targetable drug carriers: present status and a future perspective. Adv Drug Deliv Rev. 1996;21:77–80. [Google Scholar]
- 199.Topp M.D.C., Dijkstra P.J., Talsma H., Feijen J. Thermosensitive micelle-forming block copolymers of poly(ethylene glycol) and poly(N-isopropylacrylamide) Macromolecules. 1997;30:8518–8520. [Google Scholar]
- 200.Klouda L., Mikos A.G. Thermoresponsive hydrogels in biomedical applications. Eur J Pharm Biopharm. 2008;68:34–45. doi: 10.1016/j.ejpb.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lu J., Choi E., Tamanoi F., Zink J.I. Light-activated nanoimpeller-controlled drug release in cancer cells. Small. 2008;4:421–426. doi: 10.1002/smll.200700903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yuan J., Duan, Yang, Luo, Xi M. Detection of serum human epididymis secretory protein 4 in patients with ovarian cancer using a label-free biosensor based on localized surface plasmon resonance. Int J Nanomed. 2012;7:2921. doi: 10.2147/IJN.S32641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yan H., Teh C., Sreejith S., Zhu L., Kwok A., Fang W. Functional mesoporous silica nanoparticles for photothermal-controlled drug delivery in vivo. Angew Chemie Int Ed. 2012;51:8373–8377. doi: 10.1002/anie.201203993. [DOI] [PubMed] [Google Scholar]
- 204.Yang G., Liu J., Wu Y., Feng L., Liu Z. Near-infrared-light responsive nanoscale drug delivery systems for cancer treatment. Coord Chem Rev. 2016;320–321:100–117. [Google Scholar]
- 205.Rapoport N.Y., Kennedy A.M., Shea J.E., Scaife C.L., Nam K.-H. Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. J Control Release. 2009;138:268–276. doi: 10.1016/j.jconrel.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Dromi S., Frenkel V., Luk A., Traughber B., Angstadt M., Bur M. Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin Cancer Res. 2007;13:2722–2727. doi: 10.1158/1078-0432.CCR-06-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Schroeder A., Kost J., Barenholz Y. Ultrasound, liposomes, and drug delivery: principles for using ultrasound to control the release of drugs from liposomes. Chem Phys Lipids. 2009;162:1–16. doi: 10.1016/j.chemphyslip.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 208.Geers B., Dewitte H., De Smedt S.C., Lentacker I. Crucial factors and emerging concepts in ultrasound-triggered drug delivery. J Control Release. 2012;164:248–255. doi: 10.1016/j.jconrel.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 209.Ge J., Neofytou E., Cahill T.J., Beygui R.E., Zare R.N. Drug release from electric-field-responsive nanoparticles. ACS Nano. 2012;6:227–233. doi: 10.1021/nn203430m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.George P.M., LaVan D.A., Burdick J.A., Chen C.-Y., Liang E., Langer R. Electrically controlled drug delivery from biotin-doped conductive polypyrrole. Adv Mater. 2006;18:577–581. [Google Scholar]
- 211.Im J.S., Bai B.C., Lee Y.-S. The effect of carbon nanotubes on drug delivery in an electro-sensitive transdermal drug delivery system. Biomaterials. 2010;31:1414–1419. doi: 10.1016/j.biomaterials.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 212.Murdan S. Electro-responsive drug delivery from hydrogels. J Control Release. 2003;92:1–17. doi: 10.1016/s0168-3659(03)00303-1. [DOI] [PubMed] [Google Scholar]
- 213.Abidian M.R., Kim D.-H., Martin D.C. Conducting-polymer nanotubes for controlled drug release. Adv Mater. 2006;18:405–409. doi: 10.1002/adma.200501726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Panzner E.A., Jansons V.K. Control of in vitro cytotoxicity of positively charged liposomes. J Cancer Res Clin Oncol. 1979;95:29–37. doi: 10.1007/BF00411106. [DOI] [PubMed] [Google Scholar]
- 215.Parnham M.J., Wetzig H. Toxicity screening of liposomes. Chem Phys Lipids. 1993;64:263–274. doi: 10.1016/0009-3084(93)90070-j. [DOI] [PubMed] [Google Scholar]
- 216.Knudsen K.B., Northeved H., Kumar E.K.P., Permin A., Gjetting T., Andresen T.L. In vivo toxicity of cationic micelles and liposomes. Nanomed Nanotechnol Biol Med. 2015;11:467–477. doi: 10.1016/j.nano.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 217.Filion M.C., Phillips N.C. Toxicity and immunomodulatory activity of liposomal vectors formulated with cationic lipids toward immune effector cells. BBA. 1997;1329:345–356. doi: 10.1016/s0005-2736(97)00126-0. [DOI] [PubMed] [Google Scholar]
- 218.Szebeni J., Moghimi S.M. Liposome triggering of innate immune responses: a perspective on benefits and adverse reactions. J Liposome Res. 2009;19:85–90. doi: 10.1080/08982100902792855. [DOI] [PubMed] [Google Scholar]
- 219.Haber E., Afergan E., Epstein H., Gutman D., Koroukhov N., Ben-David M. Route of administration-dependent anti-inflammatory effect of liposomal alendronate. J Control Release. 2010;148:226–233. doi: 10.1016/j.jconrel.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 220.Goldsmith M., Mizrahy S., Peer D. Grand challenges in modulating the immune response with RNAi nanomedicines. Nanomedicine. 2011;6:1771–1785. doi: 10.2217/nnm.11.162. [DOI] [PubMed] [Google Scholar]
- 221.Dokka S., Toledo D., Shi X., Castranova V., Rojanasakul Y. Oxygen radical-mediated pulmonary toxicity induced by some cationic liposomes. Pharm Res. 2000;17:521–525. doi: 10.1023/a:1007504613351. [DOI] [PubMed] [Google Scholar]
- 222.Mozafari M.R., Reed C.J., Rostron C. Cytotoxicity evaluation of anionic nanoliposomes and nanolipoplexes prepared by the heating method without employing volatile solvents and detergents. Pharmazie. 2007;62:205–209. [PubMed] [Google Scholar]
- 223.Landesman-Milo D., Peer D. Altering the immune response with lipid-based nanoparticles. J Control Release. 2012;161:600–608. doi: 10.1016/j.jconrel.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 224.Roursgaard M., Knudsen K.B., Northeved H., Persson M., Christensen T., Kumar P.E.K. In vitro toxicity of cationic micelles and liposomes in cultured human hepatocyte (HepG2) and lung epithelial (A549) cell lines. Toxicol In Vitro. 2016;36:164–171. doi: 10.1016/j.tiv.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 225.Kawaguchi T., Honda T., Nishihara M., Yamamoto T., Yokoyama M. Histological study on side effects and tumor targeting of a block copolymer micelle on rats. J Control Release. 2009;136:240–246. doi: 10.1016/j.jconrel.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 226.Liu F., Huang H., Gong Y., Li J., Zhang X., Cao Y. Evaluation of in vitro toxicity of polymeric micelles to human endothelial cells under different conditions. Chem Biol Interact. 2017;263:46–54. doi: 10.1016/j.cbi.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 227.Kumar R., Kulkarni A., Nagesha D.K., Sridhar S. In vitro evaluation of theranostic polymeric micelles for imaging and drug delivery in cancer. Theranostics. 2012;2:714–722. doi: 10.7150/thno.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Gupta R., Shea J., Scaife C., Shurlygina A., Rapoport N. Polymeric micelles and nanoemulsions as drug carriers: therapeutic efficacy, toxicity, and drug resistance. J Control Release. 2015;212:70–77. doi: 10.1016/j.jconrel.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 229.Li X., Yang Z., Yang K., Zhou Y., Chen X., Zhang Y. Self-assembled polymeric micellar nanoparticles as nanocarriers for poorly soluble anticancer drug ethaselen. Nanoscale Res Lett. 2009;4:1502–1511. doi: 10.1007/s11671-009-9427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Knudsen K.B., Northeved H., Ek P.K., Permin A., Andresen T.L., Larsen S. Differential toxicological response to positively and negatively charged nanoparticles in the rat brain. Nanotoxicology. 2013;8:1–33. doi: 10.3109/17435390.2013.829589. [DOI] [PubMed] [Google Scholar]
- 231.Chung E.J., Mlinar L.B., Sugimoto M.J., Nord K., Roman B.B., Tirrell M. In vivo biodistribution and clearance of peptide amphiphile micelles. Nanomed Nanotechnol Biol Med. 2015;11:479–487. doi: 10.1016/j.nano.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 232.Malik N., Wiwattanapatapee R., Klopsch R., Lorenz K., Frey H., Weener J. Dendrimers. J Control Release. 2000;65:133–148. doi: 10.1016/s0168-3659(99)00246-1. [DOI] [PubMed] [Google Scholar]
- 233.Leroueil P.R., Berry S.A., Duthie K., Han G., Rotello V.M., McNerny D.Q. Wide varieties of cationic nanoparticles induce defects in supported lipid bilayers. Nano Lett. 2008;8:420–424. doi: 10.1021/nl0722929. [DOI] [PubMed] [Google Scholar]
- 234.Asthana A., Chauhan A.S., Diwan P.V., Jain N.K. Poly(amidoamine) (PAMAM) dendritic nanostructures for controlled sitespecific delivery of acidic anti-inflammatory active ingredient. AAPS PharmSciTech. 2005;6:E536–E542. doi: 10.1208/pt060367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Agrawal P., Gupta U., Jain N.K. Glycoconjugated peptide dendrimers-based nanoparticulate system for the delivery of chloroquine phosphate. Biomaterials. 2007;28:3349–3359. doi: 10.1016/j.biomaterials.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 236.Ziemba B., Janaszewska A., Ciepluch K., Krotewicz M., Fogel W.A., Appelhans D. In vivo toxicity of poly(propyleneimine) dendrimers. J Biomed Mater Res Part A. 2011;99A:261–268. doi: 10.1002/jbm.a.33196. [DOI] [PubMed] [Google Scholar]
- 237.Sadekar S., Ghandehari H. Transepithelial transport and toxicity of PAMAM dendrimers: implications for oral drug delivery. Adv Drug Deliv Rev. 2012;64:571–588. doi: 10.1016/j.addr.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Pereira V.H., Salgado A.J., Oliveira J.M., Cerqueira S.R., Frias A.M., Fraga J.S. In vivo biodistribution of carboxymethylchitosan/poly(amidoamine) dendrimer nanoparticles in rats. J Bioact Compat Polym. 2011;26:619–627. [Google Scholar]
- 239.Albertazzi L., Gherardini L., Brondi M., Sulis Sato S., Bifone A., Pizzorusso T. In vivo distribution and toxicity of PAMAM dendrimers in the central nervous system depend on their surface chemistry. Mol Pharm. 2013;10:249–260. doi: 10.1021/mp300391v. [DOI] [PubMed] [Google Scholar]
- 240.Naha P., Mukherjee S., Byrne H. Toxicology of engineered nanoparticles: focus on poly(amidoamine) dendrimers. Int J Environ Res Public Health. 2018;15:338. doi: 10.3390/ijerph15020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Padilla De Jesús O.L., Ihre H.R., Gagne L., Fréchet J.M.J., Szoka F.C. Polyester dendritic systems for drug delivery applications: in vitro and in vivo evaluation. Bioconjug Chem. 2002;13:453–461. doi: 10.1021/bc010103m. [DOI] [PubMed] [Google Scholar]
- 242.Di Pasqua A.J., Sharma K.K., Shi Y.-L., Toms B.B., Ouellette W., Dabrowiak J.C. Cytotoxicity of mesoporous silica nanomaterials. J Inorg Biochem. 2008;102:1416–1423. doi: 10.1016/j.jinorgbio.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 243.Napierska D., Thomassen L.C.J., Rabolli V., Lison D., Gonzalez L., Kirsch-Volders M. Size-dependent cytotoxicity of monodisperse silica nanoparticles in human endothelial cells. Small. 2009;5:846–853. doi: 10.1002/smll.200800461. [DOI] [PubMed] [Google Scholar]
- 244.Ye Y., Liu J., Chen M., Sun L., Lan M. In vitro toxicity of silica nanoparticles in myocardial cells. Environ Toxicol Pharmacol. 2010;29:131–137. doi: 10.1016/j.etap.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 245.Heidegger S., Gößl D., Schmidt A., Niedermayer S., Argyo C., Endres S. Immune response to functionalized mesoporous silica nanoparticles for targeted drug delivery. Nanoscale. 2016;8:938–948. doi: 10.1039/c5nr06122a. [DOI] [PubMed] [Google Scholar]
- 246.Bibi S., Lattmann E., Mohammed A.R., Perrie Y. Trigger release liposome systems: local and remote controlled delivery? J Microencapsul. 2012;29:262–276. doi: 10.3109/02652048.2011.646330. [DOI] [PubMed] [Google Scholar]
- 247.So S.J., Jang I.S., Han C.S. Effect of micro/nano silica particle feeding for mice. J Nanosci Nanotechnol. 2008;8:5367–5371. doi: 10.1166/jnn.2008.1347. [DOI] [PubMed] [Google Scholar]
- 248.He Q., Shi J. Mesoporous silica nanoparticle based nano drug delivery systems: synthesis, controlled drug release and delivery, pharmacokinetics and biocompatibility. J Mater Chem. 2011;21:5845. [Google Scholar]
- 249.Ivanov S., Zhuravsky S., Yukina G., Tomson V., Korolev D., Galagudza M. In vivo toxicity of intravenously administered silica and silicon nanoparticles. Materials (Basel) 2012;5:1873–1889. [Google Scholar]
- 250.Pan Y., Neuss S., Leifert A., Fischler M., Wen F., Simon U. Size-dependent cytotoxicity of gold nanoparticles. Small. 2007;3:1941–1949. doi: 10.1002/smll.200700378. [DOI] [PubMed] [Google Scholar]
- 251.Connor E.E., Mwamuka J., Gole A., Murphy C.J., Wyatt M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325–327. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 252.Shukla R., Bansal V., Chaudhary M., Basu A., Bhonde R.R., Sastry M. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: a microscopic overview. Langmuir. 2005;21:10644–10654. doi: 10.1021/la0513712. [DOI] [PubMed] [Google Scholar]
- 253.Goodman C.M., McCusker C.D., Yilmaz T., Rotello V.M. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug Chem. 2004;15:897–900. doi: 10.1021/bc049951i. [DOI] [PubMed] [Google Scholar]
- 254.Alkilany A.M., Murphy C.J. Toxicity and cellular uptake of gold nanoparticles: what we have learned so far? J Nanoparticle Res. 2010;12:2313–2333. doi: 10.1007/s11051-010-9911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Chen Y.-S., Hung Y.-C., Liau I., Huang G.S. Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Res Lett. 2009;4:858–864. doi: 10.1007/s11671-009-9334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.De Jong W.H., Hagens W.I., Krystek P., Burger M.C., Sips A.J.A.M., Geertsma R.E. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29:1912–1919. doi: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 257.Zhang G., Yang Z., Lu W., Zhang R., Huang Q., Tian M. Influence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted mice. Biomaterials. 2009;30:1928–1936. doi: 10.1016/j.biomaterials.2008.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Balasubramanian S.K., Jittiwat J., Manikandan J., Ong C.-N., Yu L.E., Ong W.-Y. Biodistribution of gold nanoparticles and gene expression changes in the liver and spleen after intravenous administration in rats. Biomaterials. 2010;31:2034–2042. doi: 10.1016/j.biomaterials.2009.11.079. [DOI] [PubMed] [Google Scholar]
- 259.Jia Y.-P., Ma B.-Y., Wei X.-W., Qian Z.-Y. The in vitro and in vivo toxicity of gold nanoparticles. Chin Chem Lett. 2017;28:691–702. [Google Scholar]
- 260.Fratoddi I., Venditti I., Cametti C., Russo M.V. How toxic are gold nanoparticles? The state-of-the-art. Nano Res. 2015;8:1771–1799. [Google Scholar]
- 261.Singh N., Jenkins G.J.S., Asadi R., Doak S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION) Nano Rev. 2010;1:5358. doi: 10.3402/nano.v1i0.5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Edge D., Shortt C.M., Gobbo O.L., Teughels S., Prina-Mello A., Volkov Y. Pharmacokinetics and bio-distribution of novel super paramagnetic iron oxide nanoparticles (SPIONs) in the anaesthetized pig. Clin Exp Pharmacol Physiol. 2016;43:319–326. doi: 10.1111/1440-1681.12533. [DOI] [PubMed] [Google Scholar]
- 263.Jarockyte G., Daugelaite E., Stasys M., Statkute U., Poderys V., Tseng T.-C. Accumulation and Toxicity of superparamagnetic iron oxide nanoparticles in cells and experimental animals. Int J Mol Sci. 2016;17:1193. doi: 10.3390/ijms17081193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Patil R.M., Thorat N.D., Shete P.B., Bedge P.A., Gavde S., Joshi M.G. Comprehensive cytotoxicity studies of superparamagnetic iron oxide nanoparticles. Biochem Biophys Reports. 2018;13:63–72. doi: 10.1016/j.bbrep.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wei Y., Zhao M., Yang F., Mao Y., Xie H., Zhou Q. Iron overload by superparamagnetic iron oxide nanoparticles is a high risk factor in cirrhosis by a systems toxicology assessment. Sci Rep. 2016;6:29110. doi: 10.1038/srep29110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Shi Kam N.W., Jessop T.C., Wender P.A., Dai H. Nanotube molecular transporters: internalization of carbon nanotube−protein conjugates into mammalian cells. J Am Chem Soc. 2004;126:6850–6851. doi: 10.1021/ja0486059. [DOI] [PubMed] [Google Scholar]
- 267.Sayes C.M., Liang F., Hudson J.L., Mendez J., Guo W., Beach J.M. Functionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitro. Toxicol Lett. 2006;161:135–142. doi: 10.1016/j.toxlet.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 268.Gaillard C., Duval M., Dumortier H., Bianco A. Carbon nanotube-coupled cell adhesion peptides are non-immunogenic: a promising step toward new biomedical devices. J Pept Sci. 2011;17:139–142. doi: 10.1002/psc.1290. [DOI] [PubMed] [Google Scholar]
- 269.Jain S., Thakare V.S., Das M., Godugu C., Jain A.K., Mathur R. Toxicity of multiwalled carbon nanotubes with end defects critically depends on their functionalization density. Chem Res Toxicol. 2011;24:2028–2039. doi: 10.1021/tx2003728. [DOI] [PubMed] [Google Scholar]
- 270.Allegri M., Perivoliotis D.K., Bianchi M.G., Chiu M., Pagliaro A., Koklioti M.A. Toxicity determinants of multi-walled carbon nanotubes: the relationship between functionalization and agglomeration. Toxicol Rep. 2016;3:230–243. doi: 10.1016/j.toxrep.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Hatami M. Toxicity assessment of multi-walled carbon nanotubes on Cucurbita pepo L. under well-watered and water-stressed conditions. Ecotoxicol Environ Saf. 2017;142:274–283. doi: 10.1016/j.ecoenv.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 272.Fujita K., Fukuda M., Endoh S., Maru J., Kato H., Nakamura A. Size effects of single-walled carbon nanotubes on in vivo and in vitro pulmonary toxicity. Inhal Toxicol. 2015;27:207–223. doi: 10.3109/08958378.2015.1026620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Hardman R. A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ Health Perspect. 2006;114:165–172. doi: 10.1289/ehp.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Pelley J.L., Daar A.S., Saner M.A. State of academic knowledge on toxicity and biological fate of quantum dots. Toxicol Sci. 2009;112:276–296. doi: 10.1093/toxsci/kfp188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Bottrill M., Green M. Some aspects of quantum dot toxicity. Chem Commun. 2011;47:7039. doi: 10.1039/c1cc10692a. [DOI] [PubMed] [Google Scholar]
- 276.Wang X., Tian J., Yong K.-T., Zhu X., Lin M.C.-M., Jiang W. Immunotoxicity assessment of CdSe/ZnS quantum dots in macrophages, lymphocytes and BALB/c mice. J Nanobiotechnol. 2016;14:10. doi: 10.1186/s12951-016-0162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Wu T., Tang M. Toxicity of quantum dots on respiratory system. Inhal Toxicol. 2014;26:128–139. doi: 10.3109/08958378.2013.871762. [DOI] [PubMed] [Google Scholar]
- 278.Salykina YF, Zherdeva VV, Dezhurov SV, Wakstein MS, Shirmanova MV, Zagaynova EV, et al. Biodistribution and clearance of quantum dots in small animals. In: Tuchin VV, Genina EA, editors. 2010. p. 799908.
- 279.Choi H.S., Liu W., Misra P., Tanaka E., Zimmer J.P., Itty Ipe B. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Libralato G., Galdiero E., Falanga A., Carotenuto R., de Alteriis E., Guida M. Toxicity effects of functionalized quantum dots, gold and polystyrene nanoparticles on target aquatic biological models: a review. Molecules. 2017;22:1439. doi: 10.3390/molecules22091439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Manshian B.B., Abdelmonem A.M., Kantner K., Pelaz B., Klapper M., Nardi Tironi C. Evaluation of quantum dot cytotoxicity: interpretation of nanoparticle concentrations versus intracellular nanoparticle numbers. Nanotoxicology. 2016;10:1318–1328. doi: 10.1080/17435390.2016.1210691. [DOI] [PubMed] [Google Scholar]
- 282.Zhao Y., Wang Y., Ran F., Cui Y., Liu C., Zhao Q. A comparison between sphere and rod nanoparticles regarding their in vivo biological behavior and pharmacokinetics. Sci Rep. 2017;7:4131. doi: 10.1038/s41598-017-03834-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Bednarski M., Dudek M., Knutelska J., Nowiński L., Sapa J., Zygmunt M. The influence of the route of administration of gold nanoparticles on their tissue distribution and basic biochemical parameters: in vivo studies. Pharmacol Rep. 2015;67:405–409. doi: 10.1016/j.pharep.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 284.Naqvi Naqvi, Samim M., Abdin M.Z., Ahmad F.J., prashant C.K. Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int J Nanomed. 2010;5:983. doi: 10.2147/IJN.S13244. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 285.Bahadar H., Maqbool F., Niaz K., Abdollahi M. Toxicity of nanoparticles and an overview of current experimental models. Iran Biomed J. 2016;20:1–11. doi: 10.7508/ibj.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Zhang B., Sai Lung P., Zhao S., Chu Z., Chrzanowski W., Li Q. Shape dependent cytotoxicity of PLGA-PEG nanoparticles on human cells. Sci Rep. 2017;7:1–8. doi: 10.1038/s41598-017-07588-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Wang S., Lu W., Tovmachenko O., Rai U.S., Yu H., Ray P.C. Challenge in understanding size and shape dependent toxicity of gold nanomaterials in human skin keratinocytes. Chem Phys Lett. 2008;463:145–149. doi: 10.1016/j.cplett.2008.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Kermanizadeh A., Jacobsen N.R., Roursgaard M., Loft S., Møller P. Hepatic toxicity assessment of cationic liposome exposure in healthy and chronic alcohol fed mice. Heliyon. 2017;3:e00458. doi: 10.1016/j.heliyon.2017.e00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5:161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 290.Sanhai W.R., Sakamoto J.H., Canady R., Ferrari M. Seven challenges for nanomedicine. Nat Nanotechnol. 2008;3:242–244. doi: 10.1038/nnano.2008.114. [DOI] [PubMed] [Google Scholar]
- 291.Shi J., Kantoff P.W., Wooster R., Farokhzad O.C. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Tsoi K.M., MacParland S.A., Ma X.-Z., Spetzler V.N., Echeverri J., Ouyang B. Mechanism of hard-nanomaterial clearance by the liver. Nat Mater. 2016;15:1212–1221. doi: 10.1038/nmat4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Oberdörster G. Safety assessment for nanotechnology and nanomedicine: concepts of nanotoxicology. J Intern Med. 2010;267:89–105. doi: 10.1111/j.1365-2796.2009.02187.x. [DOI] [PubMed] [Google Scholar]
- 294.Yang Y., Qin Z., Zeng W., Yang T., Cao Y., Mei C. Toxicity assessment of nanoparticles in various systems and organs. Nanotechnol Rev. 2017;6:279–289. [Google Scholar]
- 295.Huang W., Chen L., Kang L., Jin M., Sun P., Xin X. Nanomedicine-based combination anticancer therapy between nucleic acids and small-molecular drugs. Adv Drug Deliv Rev. 2017;115:82–97. doi: 10.1016/j.addr.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 296.Das S.K., Menezes M.E., Bhatia S., Wang X.-Y., Emdad L., Sarkar D. Gene therapies for cancer: strategies, challenges and successes. J Cell Physiol. 2015;230:259–271. doi: 10.1002/jcp.24791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Naldini L. Gene therapy returns to centre stage. Nature. 2015;526:351–360. doi: 10.1038/nature15818. [DOI] [PubMed] [Google Scholar]
- 298.Bosetti R. Cost–effectiveness of nanomedicine: the path to a future successful and dominant market? Nanomedicine. 2015;10:1851–1853. doi: 10.2217/nnm.15.74. [DOI] [PubMed] [Google Scholar]
- 299.Hare J.I., Lammers T., Ashford M.B., Puri S., Storm G., Barry S.T. Challenges and strategies in anti-cancer nanomedicine development: an industry perspective. Adv Drug Deliv Rev. 2017;108:25–38. doi: 10.1016/j.addr.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 300.Ventola C.L. Progress in nanomedicine: approved and investigational nanodrugs. P T. 2017;42:742–755. [PMC free article] [PubMed] [Google Scholar]
- 301.Bobo D., Robinson K.J., Islam J., Thurecht K.J., Corrie S.R. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33:2373–2387. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- 302.Pillai G. Nanomedicines for cancer therapy: an update of FDA approved and those under various stages of development. SOJ Pharm Pharm Sci. 2014;1:1–13. [Google Scholar]