Regulation of metabolic health by essential dietary amino acids (original) (raw)
. Author manuscript; available in PMC: 2020 Jan 1.
Published in final edited form as: Mech Ageing Dev. 2018 Jul 22;177:186–200. doi: 10.1016/j.mad.2018.07.004
Abstract
Although the beneficial effects of calorie restriction (CR) on health and aging were first observed a century ago, the specific macronutrients and molecular processes that mediate the effect of CR have been heavily debated. Recently, it has become clear that dietary protein plays a key role in regulating both metabolic health and longevity, and that both the quantity and quality - the specific amino acid composition - of dietary protein mediates metabolic health. Here, we discuss recent findings in model organisms ranging from yeast to mice and humans regarding the influence of dietary protein as well as specific amino acids on metabolic health, and the physiological and molecular mechanisms which may mediate these effects. We then discuss recent findings which suggest that the restriction of specific dietary amino acids may be a potent therapy to treat or prevent metabolic syndrome. Finally, we discuss the potential for dietary restriction of specific amino acids – or pharmaceuticals which harness these same mechanisms – to promote healthy aging.
Keywords: amino acids, diabetes, obesity, BCAAs, protein restriction
1. Calorie and protein restriction promote metabolic health and longevity
Over 100 years ago, studies performed by Osborne and colleagues began to hint that restricting calorie intake might promote longevity (Osborne et al., 1917). Seminal work undertaken in 1935 demonstrated that this was indeed the case, as calorie restriction (CR) with adequate nutrition significantly extended the lifespan of rats (McCay et al., 1935). Since that time, CR has been investigated and shown to promote healthy aging in a wide range of organisms including yeast (Saccharomyces cerevisiae) (Lin et al., 2002), nematode worms (Caenorhabditis elegans) (Hosono et al., 1989), fruit flies (Drosophila melanogaster) (Bross et al., 2005), and mice (Weindruch and Walford, 1982). Dietary restriction has also been shown to increase lifespan in other species, including spiders (Austad, 1989), rotifers (Gribble and Welch, 2013; Kirk, 2001) water striders (Kaitala, 1991), dogs (Lawler et al., 2008) and cows (Pinney et al., 1972).
A major outstanding question is whether or not the dramatic benefits of CR on health and longevity in other mammals will also apply to humans. Studies of CR on the longevity of non-human primates suggest that CR does promote healthy aging. A study undertaken at the Wisconsin National Primate Research Center has found that adult onset CR in non-human primates lowers the frequency of age-related deaths, delays the onset of age-associated diseases (Colman et al., 2009), and reduces all-cause and age-related mortality (Colman et al., 2014). In contrast, a second colony of non-human primates studied at the National Institute for Health showed no improvement in survival outcomes following CR; however, once again beneficial effects of CR on health were observed, including reduced onset of cancer and diabetes (Mattison et al., 2012). Differences in diet and baseline intake of calories likely explains the disparities between the two studies, and analysis of the combined data from both studies suggest that CR promotes both health span and lifespan relative to ad libitum fed non-human primates (Mattison et al., 2017).
In humans, there have been several clinical trials of CR, as well as post-hoc analysis of individuals who self-impose CR (Reveiwed in Most et al., 2017). In the Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE) randomized control trials, energy expenditure and fasting insulin were decreased, insulin sensitivity was increased, and a reduction in liver lipid deposition, oxidative stress, and other aging biomarkers was observed (Heilbronn et al., 2006; Il’yasova et al., 2018; Larson-Meyer et al., 2006). Non-obese humans from the Biosphere 2 experiments showed very similar responses as rodents to 20% CR, including decreases in insulin, cholesterol and triglycerides (Walford et al., 1992). Furthermore, in data from those who practice CR with optimal nutrition (CRON), CR reduces diseases of aging commonly associated with poor metabolic health, including type 2 diabetes, cardiovascular disease, cancer, stroke and vascular dementia (Most et al., 2017).
While it was originally hypothesized that CR increases longevity and improves metabolic health because of the reduction of calories, more recently it has been theorized that it is the concomitant reduction in protein that yields these beneficial effects (Fontana et al., 2008; Lee and Longo, 2016). Positive effects of a reduced protein diet on longevity were first reported in 1929 in brook trout (McCay et al., 1929), and it was later shown that male Sprague Dawley rats fed a diet in which 7.8% of calories were derived from protein lived almost 40% longer that rats fed a 20.4% protein diet (Ross, 1961). Drosophila fed a reduced yeast (protein) diet showed a similar lifespan increase to that of flies on CR, indicating that dietary protein may be a key mediator of the effects of a CR diet on longevity (Mair et al., 2005). Supporting this conclusion, a nutritional geometry study examining the effect of many different diets in Drosophila determined that a low protein:carbohydrate ratio promotes lifespan (Lee et al., 2008). Similar beneficial effects of reducing dietary protein on mice have also been observed, with Weindruch and colleagues finding that protein restriction (PR) increases the mean and maximal lifespan of female mice (Weindruch et al., 1986), and a very large study, utilizing a nutritional geometry approach to identify the optimal macronutrient ratio for mouse longevity, recently determined that male mice lived the longest on a low-protein, high-carbohydrate diet (Simpson et al., 2017; Solon-Biet et al., 2014).
While these results tilt strongly against traditional dietary advice for humans, a number of recent human studies have found that low protein intake is correlated with improved metabolic health and even increased longevity, and high protein intake is correlated with negative metabolic outcomes. A retrospective cohort analysis of humans between the ages of 50 and 65 found that a low protein diet (<10% calories from protein) is associated with reduction in insulin-like growth factor 1 (IGF-1), cancer and mortality, whereas high protein intake was associated with an increase in diabetes at all ages above 50 (Levine et al., 2014). A similar beneficial effect of a low protein diet was observed in a large prospective cohort study, with people in the highest quartile of protein consumption having twice the diabetes risk in the lowest quartile (Sluijs et al., 2010). Most recently, a randomized control trial found that even short-term protein restriction could significantly reduce fat mass and improved blood glucose levels in humans (Fontana et al., 2016).
2. How does PR promote metabolic health?
Evidence that in humans low protein diets can reduce fat mass, improve blood glucose, reduce IGF-1 levels, and decrease the incidence of cancer without CR is extremely encouraging. As such, several hypotheses exist about the potential mechanisms underlying the beneficial effects of PR on metabolic health (Overview of studies in Table 1). Studies in rodents have consistently found that PR results in an increase in food intake whilst improving metabolic parameters, therefore it has been postulated that many benefits of PR result from increased energy expenditure (Laeger et al., 2014; Morrison et al., 2007; White et al., 2000). Here, we discuss potential molecules and pathways that may be crucial in mediating the beneficial effects of PR and the mechanisms by which they interact with each other.
Table 1:
Recent studies examining the effects of protein restriction on metabolic health in rodents and humans. M=male, F=female, where not stated both sexes used. BAT = brown adipose tissue, BCAAs = branched chain amino acids, DIO = diet-induced obese, eIF2α = eukaryotic transcription factor 2α, FGF21 = fibroblast growth factor 21, IGF-1 = insulin-like growth factor 1, mTOR = mechanistic target of rapamycin, UCP1 = uncoupling protein 1, WAT = white adipose tissue.
| Species/Strain/Sex | Dietary Protein (%) | Metabolic effect | Length of intervention | Study |
|---|---|---|---|---|
| Mice C57BL/6 | 5% | Increased food intake | 14 months | (Solon-Biet et al., 2014) |
| Increased adiposity | ||||
| Improved glucose tolerance | ||||
| Reduced circulating insulin | ||||
| Reduced mTOR activation | ||||
| Rats Sprague-Dawley (M) | 9% | Increased food intake | 14 days | (Laeger et al., 2014) |
| Increased liver expression and circulating FGF21 | ||||
| Increased liver eIF2α expression | ||||
| Mice C57BL/6 (M) | 4% | Increased food intake | 14 days | (Laeger et al., 2014) |
| Increased energy expenditure | ||||
| Increased liver expression and circulating FGF21 | ||||
| Increased liver eIF2α expression | ||||
| Human | 5% | Increased circulating FGF21 | 28 days | (Laeger et al., 2014) |
| Sprague Dawley rats (M) | 10% | Increased food intake | 14 days | (Henagan et al., 2016) |
| Increased fat mass | ||||
| Increased energy expenditure | ||||
| Reduced hepatic lipogenic expression | ||||
| Increased hepatic autophagy | ||||
| Human (M) | 7–9% | Decreased body weight | 43 days | (Fontana et al., 2016) |
| Decreased fat mass | ||||
| Decreased fasting blood glucose | ||||
| Increased circulating FGF21 | ||||
| Decreased circulating BCAAs | ||||
| Mice C57BL/6J | 5–7% | Increased food intake | 12 weeks | (Fontana et al., 2016) |
| Reduced fat mass gain | ||||
| Weight loss | ||||
| Reduced lean mass | ||||
| Improved glucose and pyruvate tolerance | ||||
| Increased circulating FGF21 and adiponectin | ||||
| Mice C57BL/6 (M) | 4% | Increased food intake | 27 weeks | (Laeger et al., 2016) |
| Reduced body weight and fat mass | ||||
| Increased energy expenditure | ||||
| Increased circulating and hepatic FGF21 expression | ||||
| Reduced hepatic lipogenesis expression | ||||
| Increased UCP1 expression (BAT and WAT) | ||||
| Increased UCP1 in BAT | ||||
| Rats Obesity-prone Sprague Dawley (M) | 0–5% | Decreased body and lean mass | 3 weeks | (Pezeshki et al., 2016) |
| Increased energy expenditure | ||||
| Decreased plasma insulin, leptin and glucose | ||||
| Increased UCP-1, PGC1-α and FGF21 expression (BAT) | ||||
| Increased UCP-1 and FGF21 expression (muscle) | ||||
| Mice C57BL/6NCrl (M) | 5% | Increased energy intake | 16 weeks | (Maida et al., 2016) |
| Increased energy expenditure | ||||
| Reduced body, lean and fat mass | ||||
| Improved glucose metabolism | ||||
| Reduced circulating insulin, IGF-1 and leptin | ||||
| Increased circulating FGF21 | ||||
| Increased UCP1 expression (BAT and WAT) | ||||
| Human (M) | 9% | Improved insulin sensitivity | 7 days | (Maida et al., 2016) |
| Decreased circulating insulin and glucose | ||||
| Increased serum FGF21 | ||||
| Mice C57BL6/J | 5% | Reduced body mass | 14 months | (Solon-Biet et al., 2016) |
| Low circulating insulin | ||||
| Increased liver expression and circulating FGF21 | ||||
| Increased UCP1 expression (BAT) | ||||
| Mice C57BL/6 (M) | 5% | Increased food intake | 6 weeks | (Hill et al., 2017) |
| Decreased body, lean and fat mass | ||||
| Increased energy expenditure | ||||
| Increased circulating and hepatic Fgf21 expression | ||||
| Reduced hepatic lipogenesis expression | ||||
| Increased UCP1 expression (BAT) | ||||
| Mice C57BL/6J DIO (M) | 5% | Increased energy intake | 12 weeks | (Cummings et al., 2018) |
| Decreased body weight | ||||
| Decreased lean and fat mass | ||||
| Increased energy expenditure | ||||
| Improved glucose tolerance and insulin sensitivity | ||||
| Reduced hepatic lipogenesis expression | ||||
| Increased hepatic lipogenic expression | ||||
| Rats Wistar fatty (M) | 6% | Decreased fat and body weight | 24 weeks | (Kitada et al., 2018) |
| Reduced circulating glucose | ||||
| Improved insulin resistance | ||||
| Increased plasma FGF21 | ||||
| Increased UCP1 expression (BAT) |
FGF21
Recently, it has become clear that a key mediator of the effects of PR on metabolism, and in particular on insulin sensitivity and energy expenditure, is the hormone fibroblast growth factor 21 (FGF21) (Laeger et al., 2014). However, the effects of FGF21 on metabolism and metabolic health appear complex (Figure 1). In rats fed a low protein diet, hepatic mRNA expression of Fgf21 increases within 24 hours of PR initiation, and circulating levels of FGF21 increase 10-fold after 4 days (Laeger et al., 2014). Similar increases in levels of FGF21 are seen in mice (Fontana et al., 2016; Solon-Biet et al., 2016) and in humans fed PR diets (Fontana et al., 2016; Laeger et al., 2014). Importantly, Laeger and colleagues have shown that mice lacking Fgf21 do not exhibit alterations in food intake, energy expenditure, or weight gain when placed on a PR diet, demonstrating that FGF21 is a crucial mediator of the effects of PR (Laeger et al., 2014).
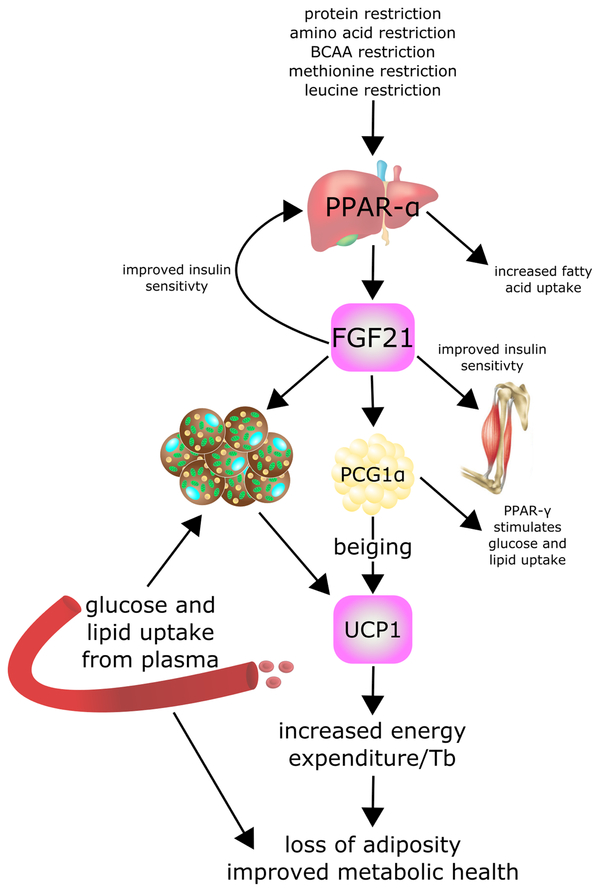
Figure 1:
FGF21 is a key mediator of the metabolic effects of protein and amino acid restriction. FGF21 = fibroblast growth factor 21, PCG1α = Peroxisome proliferator-activated receptor-γ coactivator-1α, PPAR-α = Peroxisome proliferator-activated receptor-α, UCP1 = uncoupling protein 1.
FGF21 was identified as a novel, liver produced fibroblast growth factor almost two decades ago (Nishimura et al., 2000), and came to the notice of metabolism researchers when it was discovered to be a potent stimulator of insulin-independent glucose uptake by 3T3-L1 mouse cells and primary human adipocytes (Kharitonenkov et al., 2005). Administration of FGF21 improves the metabolic profile of several different mouse models of diabetes, including ob/ob, db/db (Kharitonenkov et al., 2005) and diet-induced obese (DIO) mice (Xu et al., 2009). In addition, metabolic parameters were improved in obese, diabetic patients after one month of treatment with the FGF21 analog LY2405319 (Gaich et al., 2013), suggesting this is a fairly robust intervention. One of the primary effects of FGF21 is to promote hepatic insulin sensitivity; administration of FGF21 suppresses hepatic glucose production in ob/ob and db/db mice (Berglund et al., 2009) as DIO mice (Xu et al., 2009). Similar effects are observed following the infusion of FGF21 into the brains of obese rats (Sarruf et al., 2010). While the mechanism underlying the effects of FGF21 on hepatic glucose metabolism is not completely clear, FGF21’s ability to promote hepatic insulin sensitivity requires suppression of the mechanistic Target Of Rapamycin Complex 1 (mTORC1) (Gong et al., 2016), a serine/threonine protein kinase which negatively regulates insulin sensitivity (Hsu et al., 2011; Rui et al., 2001; Yu et al., 2011). Several different FGF21 variants and agonists have now been tested as possible therapies for type 2 diabetes in mice and even in humans (Gaich et al., 2013; Sonoda et al., 2017).
In addition to improving hepatic insulin sensitivity directly, FGF21 has profound effects on energy balance and lipid metabolism. Energy expenditure is increased following administration of FGF21 to mice (Coskun et al., 2008; Xu et al., 2009), significantly reducing the body weight and adipose mass of DIO mice. It is believed that this effect is due to the normal physiological role of FGF21 in adaptive thermogenesis; FGF21 mRNA is strongly induced in brown (BAT) and white adipose tissue (WAT) in response to cold exposure, and promotes the browning or beiging of white adipose tissue (Fisher et al., 2012). Cold-activation of beiging in subcutaneous WAT is abolished in adipose-specific Fgf21 knockouts mice, highlighting a critical role for adipose-produced FGF21 in the acute thermogenic response (Huang et al., 2017); although it is worth noting that FGF21-independent mechanisms can compensate for the lack of FGF21 during the long-term adaptation to cold (Keipert et al., 2017). One of the primary fuels for cold-induced thermogenesis is fatty acids, and FGF21 is also a potent mediator of fatty acid oxidation and lipid metabolism, increasing lipolysis in white adipose tissue (WAT)(Inagaki et al., 2007) and substrate utilization in the liver (Badman et al., 2007). Fascinatingly, FGF21 is also induced by cold exposure in humans (Lee et al., 2014, 2013), and serum FGF21 levels correlate with BAT activity after acute cold exposure in males (Hanssen et al., 2015).
As mentioned above, knockout mouse studies have demonstrated that FGF21 is a key mediator of the metabolic effects of a PR diet. In mice, 14 days of PR is sufficient to increase energy expenditure, induce UCP1 expression in BAT and inguinal WAT, and induce morphological changes in WAT consistent with beiging; these changes are completely abrogated in Fgf21 −/− mice (Laeger et al., 2016). Similarly, the effects of PR on energy expenditure and food intake are blocked in mice lacking Ucp1 (Hill et al., 2017). The reduced metabolic effects of PR in Fgf21 −/− and Ucp1 −/− mice suggests that activation of the FGF21-UCP1 axis may play a role in the beneficial effects of PR.
GCN2
FGF21 is regulated in part through the serine/threonine kinase general control nonderepressible 2 (GCN2), which is activated by binding to uncharged transfer ribonucleic acids (tRNAs) (Wek et al., 1989, 1995). Activated GCN2 phosphorylates eukaryotic initiation factor 2-α (eIF2α), which blocks translation of most messenger RNAs (mRNAs) (Dever et al., 1992). However, eIF2α induces the translation of activating transcription factor 4 (ATF4), which is a key effector of the integrated stress response (Harding et al., 2000; Vattem and Wek, 2004), and is involved in several cell signaling pathways including inflammation (Zhong et al., 2012), autophagy (B’chir et al., 2013), and responses to mitochondrial stress (Quirós et al., 2017). Among its many effects, ATF4 initiates translation of Sestrin2, which can inhibit mTORC1 through blocking lysosomal localization (Ye et al., 2015). GCN2 is highly conserved across eukaryotes and activation leads to several changes that act in opposition to mTOR including blocking translation, inducing autophagy and arresting growth (Lehman et al., 2015).
Mice lacking Gcn2 initially have a delayed response to PR; however, after 14 days of PR feeding, changes in food intake, energy expenditure and body weight start to appear, likely in response to increased FGF21, the increase in which is similarly delayed (Laeger et al., 2016). Further work showed that although binding of ATF4 to the FGF21 promoter is blunted in Gcn2 knockout mice during the acute response to PR, chronic PR eventually leads to the binding of wild-type levels of ATF4 (Laeger et al., 2016). This suggests that whilst GCN2 is necessary for the acute response to PR, pathways upstream of ATF4 may act as auxiliary activators to induce FGF21 stimulation in the absence of GCN2 (Laeger et al., 2016).
mTOR
Conserved across all eukaryotes, the mechanistic Target Of Rapamycin (mTOR) is a phosphatidylinositol 3-kinase (PI3K)-like serine/threonine protein kinase which can be found in two complexes, mTOR complex 1 (mTORC1) and complex 2 (mTORC2), which phosphorylate a diverse set of substrates to regulate numerous cellular and physiological processes. To coordinate the catabolic or anabolic direction of metabolic processes at a given time, mTORC1 integrates nutritional information, including the availability of glucose, amino acids and oxygen, to control processes such as protein synthesis and autophagy. In contrast, mTORC2 acts to regulate metabolism in response to many hormonal cues, including insulin and IGF-1. Inhibition of mTORC1, either genetically or pharmacologically, extends lifespan in organisms ranging from yeast to mice, whereas inhibition of mTORC2 results in insulin resistance and shorter lifespan in male mice (Kennedy and Lamming, 2016; Lamming et al., 2014).
As amino acids are agonists of mTORC1, it is natural to conclude that reduced protein diets would reduce mTORC1 activity. Indeed, the weight of evidence of suggests that this is indeed the case. Drosophila on a low-protein, high-sugar diet have decreased TOR signaling (Sun et al., 2012), and Sprague-Dawley rats consuming a low protein, ketogenic diet have decreased mTORC1 signaling in the liver and hippocampus (McDaniel et al., 2011). Low protein intake is associated with reduced mTOR activity in the livers of mice (Solon-Biet et al., 2014), and mice fed a low protein diet have reduced mTOR activity in heart, skeletal muscle, and white adipose tissue (Lamming et al., 2015).
Amino acid activation of mTORC1 is a complex process that has only recently begun to be unraveled (Figure 2) (Goberdhan et al., 2016; Wolfson and Sabatini, 2017). In response to amino acids, mTORC1 is recruited to the lysosome by the Rag family of small GTPases (Sancak et al., 2010). The Rag GTPases are regulated in turn by the Ragulator complex, which has guanine nucleotide exchange factor (GEF) activity towards RagA and RagB (Bar-Peled et al., 2012). The Ragulator and v-ATPase interact with a low affinity amino acid transporter in the lysosome membrane (SLC38A9), which is required for mTORC1 activation by amino acids glutamine, arginine and leucine (Jung et al., 2015; Rebsamen et al., 2015; Wang et al., 2015). Recent work has found that arginine acts to stimulate the efflux of many of essential amino acids, including leucine, from the lysosome into the cytoplasm; sensing of leucine and other amino acids then occurs in the cytosol (Wyant et al., 2017). This stands in contrast to the previously “inside-out” model, in which the sensing of amino acids occurs inside the lysosome (Zoncu et al., 2011).
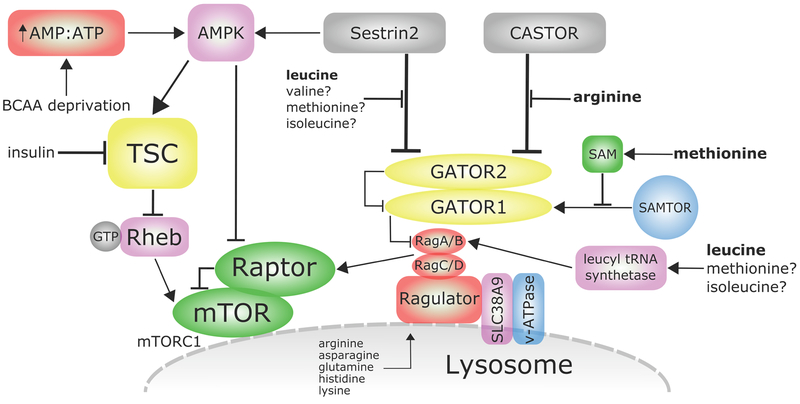
Figure 2:
Regulation of mTORC1 by amino acids. AMP = adenosine monophosphate, AMPK = AMP-activated protein kinase, ATP = adenosine triphosphate, mTORC1 = mechanistic target of rapamycin complex 1, Raptor = regulatory-associated protein of mTOR, Rheb = Ras homolog enriched in brain, SAMTOR = S-adenosyl methionine/target of rapamycin, TSC = tuberous sclerosis complex.
The Rag GTPases are also regulated by the GATOR complex, which is composed of two subcomplexes termed GATOR1 and GATOR2; GATOR1 is a GTPase-activating protein (GAP) for RagA and RagB, and acts to inhibit the lysosomal recruitment of mTORC1 in response to amino acids. In contrast, GATOR2 negatively regulates GATOR1 activity (Bar-Peled et al., 2013), and appears to be a major site of regulation by amino acids. Thus far, two inhibitors of GATOR2 have been described, Sestrin2 and CASTOR1. As shown in Figure 2, leucine and arginine disrupt the interaction between GATOR2 and Sestrin2 (leucine) and GATOR2 and CASTOR1 (arginine), thereby allowing lysosomal recruitment of mTORC1 (Chantranupong et al., 2016; Wolfson et al., 2016). In addition to inhibiting mTORC1 via this pathway, Sestrin2 also activates AMPK, which negatively regulates mTORC1 via the phosphorylation of the mTORC1 subunit RAPTOR and the Tuberous Sclerosis Complex (TSC) (Gwinn et al., 2008; Inoki et al., 2002). Recruitment of GATOR1 to the lysosome requires the KICSTOR complex, which is composed by KPTN, ITFG2, C12orf66, and SZT2 (Wolfson et al., 2017). It is not yet known if the KICSTOR complex plays a role in amino acid sensing upstream of mTORC1.
mTORC1 activity is also regulated by folliculin (FLCN), a tumor suppressor, associated with Birt-Hogg-Dubé (BHD) syndrome. FLCN and its binding partners folliculin-interacting proteins 1 and 2 (FNIP1/2) were recently discovered to positiviely regulate mTORC1 activity by acting as a GAP for RagC and RagD hetreodimers (Tsun et al., 2013). FLCN itself is recruited to the lysosome when amino acids are limiting, and also interacts with RagA (Petit et al., 2013). Suppression of FCLN reduces lysosomal leucine levels, which results in a decrease in mTORC1 activity. The reduction in lysosomal leucine may be due to FCLNs role in supression of Proton-Assisted Amino Acid Transporter 1 (PAT1), a lysosomal transporter of amino acids (Wu et al., 2016). In addition to amino acids, FLCN is also involved of the sensing of other environmental cues. At least in some cell types, FLCN is found in primary cilia, and links mTORC1 activity to flow stress through the regulation of liver kinase B1 (LKB1) and AMPK (Zhong et al., 2016). FLCN has recently been shown to regulate cytoplasmic retention of Transcription Factor Binding To IGHM Enhancer 3 (TFE3) by mTOR in response to amino acid signaling through RagC and RagD (Wada et al., 2016).
Activation of mTORC1 requires not only localization to the lysosome, but also interaction with GTP-bound Rheb, a small GTPase which is, like mTORC1, localized to the lysosome when amino acids are present (Fawal et al., 2015). However, in the absence of insulin/IGF-1 signaling, Rheb is kept in an inactive state by TSC, which acts as a GAP for Rheb; phosphorylation of TSC by Akt causes TSC to depart from the lysosome, permitting Rheb-GTP to activate mTORC1 (Menon et al., 2014). mTORC1 activation thus requires not only the availability of amino acids, but also a permissive hormonal state for anabolism, as signaled by insulin/IGF-1.
The relationship between mTORC1 and insulin sensitivity is complex; while genetic mouse models of altered liver mTORC1 activity generally have normal glucose tolerance (Lamming et al., 2012; Sengupta et al., 2010), mTORC1 may play a role in the regulation of fasting glucose levels (Caron et al., 2017). Further, it is widely accepted that hyperactive mTORC1 signaling in the liver contributes to insulin resistance via feedback inhibition of insulin receptor substrate (Saxton and Sabatini, 2017). Conversely, the activity of mTORC1 in skeletal muscle and adipose tissue may actually promote glucose tolerance – and in the context of adipose tissue, leanness as well (Bentzinger et al., 2008; Polak et al., 2008). The contribution of mTORC1 in different tissues to the effect of PR on glycemic control remains to be determined.
mTORC1 also plays an important role in regulating energy expenditure through the regulation of BAT as well being involved in the beiging of WAT. The loss of mTORC1 in adipocytes completely blocks BAT expansion in response to cold exposure and reduces mitochondrial biogenesis and oxidative metabolism (Labbé et al., 2016). Activation of mTORC1 by cold exposure was also associated with an increase in Akt (Labbé et al., 2016), which plays a critical role in the response to cold exposure by promoting glucose uptake (Albert et al., 2016).
This is confounding, as PR decreases the expression of mTOR and ribosomal protein S6 kinase 1 (S6K1), an important mTORC1 substrate (Ma et al., 2015; Xiao et al., 2011), but increases BAT activation in rodents (Elsukova et al., 2012; Selman et al., 2005), and increased energy expenditure through mitochondrial uncoupling is thought to have a positive effect on lifespan (Speakman et al., 2004). During protein restriction, energy expenditure is also increased via other mechanisms in response to FGF21, including the beiging of WAT. However, mTORC1 also play an important role in beiging; treatment with rapamycin or adipocyte-specific deletion of Raptor blocks the ability of β-adrenergic signaling to induce beiging in response to cold (Tran et al., 2016). The exact mechanism by which PR induces energy expenditure and the role of mTORC1 in this response thus remains to be determined.
Cross talk between metabolic pathways
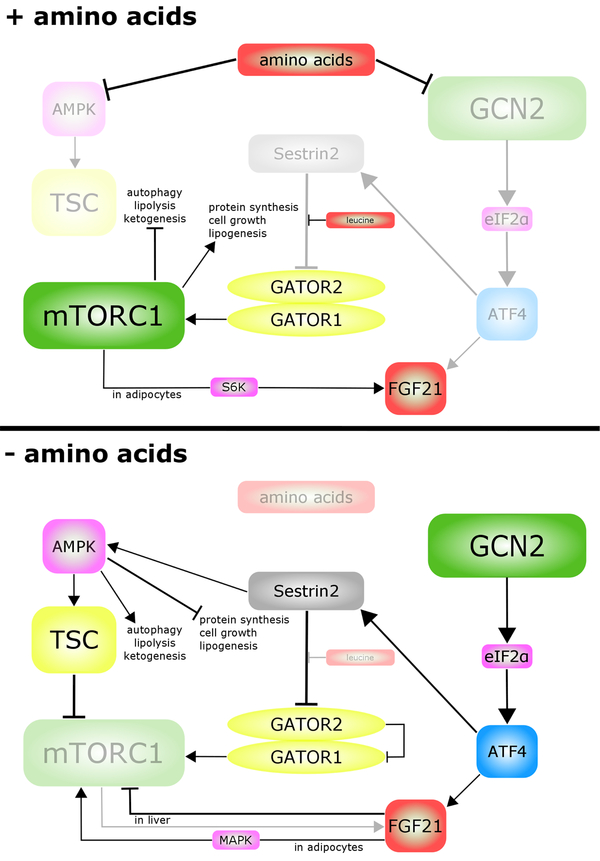
The interaction between these nutrient sensing and energy regulating pathways is complex (Figure 3). Amino acid availability regulates mTORC1 and GCN2 directly. Amino acid depletion activates GCN2, inhibiting eIF2α and thus stimulating the translation of ATF4. It was recently shown that ATF4 promotes the transcription of Sestrin2, thus inhibiting mTORC1 by preventing its lysosomal localization (Ye et al., 2015) and also by activating AMPK (Budanov and Karin, 2008). Cross-talk between FGF21 and mTORC1 has been well-documented, and each is believed to regulate the other, with some differences between cell types. In adipocytes, administration of FGF21 activates both mTORC1 and its downstream target S6K1 via mitogen-activated protein kinase (MAPK), with the presence of mTORC1 and S6K1 being essential for the ability of FGF21 to induce Ucp1 and stimulate glucose uptake (Minard et al., 2016). However, administration of FGF21 to mice promotes hepatic insulin sensitivity by suppressing mTORC1 activity in the liver; conversely, mice lacking Fgf21 have increased liver mTORC1 activity (Gong et al., 2016).
Figure 3:
Cross-talk between the mTORC1, GCN2 and FGF21 signaling pathways in the presence and absence of amino acids. AMPK = AMP-activated protein kinase, ATF4 = activating transcription factor 4, eIF2α = eukaryotic initiation factor 2, FGF21 = fibroblast-growth factor 21, GCN2 = general control nonderepressible 2, mTORC1 = mechanistic target of rapamycin complex 1, TSC = tuberous sclerosis complex.
In turn, mTORC1 plays an important role in the regulation of FGF21. Fgf21 transcription in hepatocytes is controlled physiologically by insulin and glucagon, which act to induce ATF4 by stimulating the activity of the PI3K/AKT/mTORC1 and cAMP/PKA signaling pathways, respectively (Alonge et al., 2017). This appears to be true in vivo as well, as mice lacking hepatic Tsc1, which have constitutively active mTORC1 signaling, have increased hepatic expression of Fgf21 and increased circulating levels of FGF21 (Cornu et al., 2014). Constitutive activation of mTORC1 signaling in skeletal muscle, via deletion of Tsc1 in skeletal muscle (Guridi et al., 2015) or by constitutively active 4E-BP1 (Tsai et al., 2015) leads to increased circulating levels of FGF21 as a direct result of increased muscle expression of Fgf21.
3. Amino acids act as metabolic health regulators
While both human and rodent studies demonstrate the metabolic benefits of a low protein diet, a long-standing question has been whether all types of dietary protein are equivalent. For instance, are plant-derived proteins healthier than animal proteins? Protein intake studies in humans have suggested that whilst high intake of animal proteins has a negative effect on metabolic health, this may not be the case for vegetable protein intake (Azemati et al., 2017; Sluijs et al., 2010). Indeed, clinical trials of vegan diets have found that they promote metabolic health, although the lower levels of proteins consumed by many vegans is a confounding factor (Barnard et al., 2009, 2006; Lee et al., 2016). While there are many differences between animal-derived protein and plant-based protein, one suggestion is that the beneficial metabolic effects of a vegan diet are driven by decreased levels of the sulfur containing amino acids, cysteine and methionine, which are particularly low in vegetable sources (Sosulski and Imafidon, 1990). More recently, work has highlighted the possibility that dietary branched-chain amino acids may be critical regulators of metabolic health. Below, we discuss emerging evidence that the specific amino acid composition of the diet is a critical regulator of metabolic health.
Methionine
For the past quarter of a century, reduced consumption of methionine has been theorized to be a key driver of the response to CR, as methionine restriction (MR) extends the lifespan of Fischer 344 rats (Orentreich et al., 1993). While the mechanism of this effect is unclear, MR increases blood glutathione levels, which could be indicative of improved resistance to oxidative stress (Richie et al., 1994). Restricting methionine by approximately 70% is sufficient to increase lifespan in mice, as well as mirroring some of the metabolic effects of CR, including decreased blood levels of insulin and IGF-1 (Miller et al., 2005). The naturally low levels of methionine in vegan foods has led to the suggestion that MR may be a more feasible lifespan-extending strategy than CR (McCarty et al., 2009). However, recent research suggests that at least in mice, a relatively large 70–80% reduction of dietary methionine levels is optimal for achieving the metabolic benefits of MR (Forney et al., 2017).
From a metabolic standpoint, MR has many beneficial effects in both rodents and humans; preventing weight gain and fat accretion, and improving control of blood glucose (Brown-Borg and Buffenstein, 2017; Cummings and Lamming, 2017; Miller et al., 2005; Plaisance et al., 2011). Many of these factors have been linked to FGF21, levels of which are upregulated by MR in both young and aged mice (Lees et al., 2014; Perrone et al., 2012). FGF21 is also thought to mediate the increased energy expenditure seen in response to MR, potentially by stimulating the beiging of white adipose tissue (WAT) to a more metabolically active adipose that resembles brown adipose tissue (BAT) (Douris et al., 2015).
Alterations in methionine intake appear to be strongly linked to the other sulfur-containing amino acid, cysteine. In addition to its role in protein translation, cysteine is a precursor for important cellular regulators such as glutathione (GSH), taurine, and hydrogen sulfide (H2S). Supplementation of cysteine is able to reverse the effects of MR on mouse and rat adiposity and energy intake; however cysteine supplementation alone does not appear to cause negative effects to metabolic health (Elshorbagy et al., 2011; Wanders et al., 2016). Further work indicated that cysteine reversed most of the genetic and metabolic changes induced by MR in the inguinal adipose tissue depots, and some of the changes in the liver, in addition to enhancing transcription of inflammation and carcinogenic genes. Moreover, metabolite levels returned to control fed levels in the liver, serum, muscle and fat depots of MR cysteine fed rats (Perrone et al., 2012). Additional work in the liver of rats indicated that cysteine reversed the decrease in mitochondrial reactive oxygen species (ROS), however cysteine supplementation with and without MR decreased mTORC1 activity, suggesting an alternative role for cysteine in mTOR signaling (Gomez et al., 2015). Addition of cysteine also reduced the increase in energy expenditure and serum FGF21 levels, and the reduction of fasting insulin and plasma glutathione in MR mice and reversed activation of eIF2α and protein kinase R–like endoplasmic reticulum (ER) kinase (PERK) (Wanders et al., 2016). This result supports evidence that glutathione depletion may mediate the beneficial phenotypes seen with MR via a GCN2-independent, PERK/eIF2α/ATF-dependent mechanism (Laeger et al., 2016; Wanders et al., 2016).
Recent work on sulfur amino acid restricted (SAAR) rats revealed that MR combined with cysteine deprivation for 12 weeks can halve hepatic protein synthesis rates relative to controls, despite a 40% increase in food intake. The restricted mice, though appearing healthy and maintaining normal habits, weighed half that of the controls, which indicated that an increase in energy expenditure, plus reduced protein synthesis were contributing to this phenotype. Furthermore, liver expression levels of eIF2α and phosphorylated eIF2α, which is activated by GCN2 and results in mTORC1 inhibition through activation of ATF4 (Figure 3), were increased in the SAAR mice, indicating that low levels of free hepatic methionine and cysteine repress the mTORC1 pathway (Nichenametla et al., 2018).
FGF21, which is strongly induced by MR (Wanders et al., 2016), is proposed to be one of the major effectors of MR on metabolic health. Indeed, in FGF21 −/− mice, MR induced increases in energy expenditure and thermogenic activation of WAT and BAT are lost, demonstrating the importance of FGF21 for the MR phenotype (Wanders et al., 2017). However, while investigating an alternative short-term methionine deprivation (MD) regimen, in which diet-induced obese (DIO) mice were fed an amino acid defined diet containing no methionine, we found that while MD increased energy expenditure in both male and female mice, plasma levels of FGF21 and WAT expression of Ucp1 were increased exclusively in male mice. This indicates that at least in females, the increased energy expenditure induced by short-term MD is independent of the FGF21-Ucp1 axis (Yu et al., 2018). The effects of MD may therefore be mediated via sex-specific mechanisms, or via an entirely FGF21-Ucp1 independent mechanism in mice of both sexes, as was recently shown to be the case for cold-induced thermogenesis (Keipert et al., 2017). Female DIO mice on a MD diet likewise showed equivalent improvements to male mice with respect to glucose tolerance and hepatic insulin sensitivity, supporting an FGF21-independent mechanism for these phenotypes (Yu et al., 2018). UCP1 has been shown to be required for increased energy expenditure on a MR diet, but not for the effects of MR on insulin sensitivity (Wanders et al., 2015).
Recently, a methionine derived metabolite, S-adenosyl-L-methionine (SAM) has been identified as a potent regulator of mTORC1 activity. A newly identified protein, which has been named SAMTOR, directly binds to SAM; SAMTOR interacts with GATOR1 to inhibit mTORC1 signaling, and in the presence of SAM this interaction is disrupted (Gu et al., 2017). In model organisms both diet and genetic changes to SAM have been investigated, and currently results appear conflicted. During the aging process, systemic SAM increases in Drosophila, and lifespan can be extended by increasing SAM catabolism (Obata and Miura, 2015). However, in mice, cognitive performance was improved with a SAM supplemented folate deficient diet (Montgomery et al., 2014) and in long-lived Snell dwarf mice levels of SAM were significantly higher than in normal mice (Vitvitsky et al., 2013). In yeast, overexpression of SAM synthetase increases lifespan, which may be a result of increased consumption of adenosine triphosphate (ATP) and methionine and activation of AMPK (Ogawa et al., 2016).
BCAAs
The branched-chain amino acids (BCAAs) have been of interest to metabolic researchers since at least the 1970’s, when it was first observed that leucine, isoleucine, and valine levels were elevated in the blood of obese humans (Felig et al., 1974). Since that time, it has become clear that the BCAAs are increased in obese adult humans relative to their lean counterparts (Newgard et al., 2009), and are also positively correlated with body mass index (BMI) in children and adolescents (McCormack et al., 2013). Circulating BCAAs are a positive predictor of diabetes in normoglycemic individuals (Wang et al., 2011). In both rodents and humans, research suggests that levels of circulating BCAAs are dependent on protein intake (Fontana et al., 2016; Noguchi et al., 2006; Solon-Biet et al., 2014), and are thus decreased in both rodents and humans on a PR diet.
In addition to this correlative data suggesting that BCAAs have a negative effect on metabolic health, supplementation of a Western diet with additional BCAAs further impairs insulin sensitivity in both rats and mice (Cummings et al., 2018; Newgard et al., 2009). Conversely, acute dietary deprivation of either leucine, isoleucine, or valine improves insulin sensitivity in mice (Xiao et al., 2014). Finally, we have recently shown that consumption of a diet with reduced levels of the BCAAs improves glucose tolerance in both lean and diet-induced obese mice (Cummings et al., 2018; Fontana et al., 2016), and a reduced BCAA diet also improves the insulin sensitivity of hyperphagic Zucker Fatty rats (White et al., 2016).
Although this evidence strongly indicates that BCAAs have an overall negative effect on metabolic health, several studies have identified positive effects of BCAA supplementation, particularly when started at old age in humans (Solerte et al., 2008a, 2008b) and rats (Pansarasa et al., 2008). BCAAs are widely sold as dietary supplements, particularly for athletes, and BCAA supplementation has been explored in both human and animal models as a treatment for sarcopenia (D’Antona et al., 2010; Pansarasa et al., 2008). Additionally, the catabolite of leucine, β-Hydroxy β-methylbutyrate, which decreases in rats with age (Shreeram et al., 2016), has been shown to stimulate genes associated with muscle repair in aged mice (Munroe et al., 2017). Similarly, the acute effects of leucine supplementation have been seen in exercised rats, where leucine fed animals have greater stimulation of skeletal muscle protein synthesis (Anthony et al., 1999). Leucine supplementation of aged humans for six months proved ineffective at increasing muscle mass, but also had no negative effects on glycemic control (Leenders et al., 2011). The differences in responses seen in BCAA studies may be due to differential requirements for BCAAs in catabolic (trauma) and anabolic (obesity) situations as well as the changing nutritional needs of mammals with age (Bifari and Nisoli, 2017). On balance, the evidence suggests that dietary BCAA consumption or supplementation is unhealthy in obese individuals eating an unhealthy diet.
The effect of dietary BCAAs on longevity and robustness remains unclear. Low levels of circulating BCAAs are seen in the long lived dwarf Ames mice (Wijeyesekera et al., 2012), and blood levels of the BCAAs inversely correlate with the longevity of C57BL/6J mice (Solon-Biet et al., 2014). However, supplementation with BCAAs increases the chronological lifespan of yeast (Alvers et al., 2009) and C. elegans (Mansfeld et al., 2015), while BCAA supplementation begun at 9 months of age increases the average, but not maximum lifespan of male mice (D’Antona et al., 2010). The effect of specifically restricting dietary BCAAs on mammalian health and longevity remains to be determined.
The physiological and molecular mechanisms by which the BCAAs regulate metabolic health are complex. The BCAAs, particularly leucine, are well described as potent agonists of mTORC1, and leucine restriction reduces mTORC1 activity in vivo (Lees et al., 2017). However, while leucine deprivation promotes metabolic health in wild-type mice through improved glucose homeostasis and reduced fat mass (Lees et al., 2017), Gcn2 −/− mice deprived of leucine for one week exhibit liver steatosis and increased triglycerides, and the beneficial effects of leucine deprivation were all but abolished, suggesting that these effects are primarily mediated by GCN2 (Guo and Cavener, 2007). Xiao and colleagues have shown that short-term leucine deprivation improves hepatic insulin sensitivity via sequential activation of GCN2 and inhibition of mTORC1 signaling (Xiao et al., 2011).
At the physiological level, a direct comparison between 80% restriction of both leucine and methionine in ten month old C57BL/6J male mice indicated that both decreased body fat and body mass, however MR had a greater effect on body mass (Lees et al., 2017). Although both interventions increased food intake, WAT lipid cycling, whole body glucose metabolism, and hepatic insulin sensitivity, MR had a greater positive impact on glucose and lipid homeostasis (Lees et al., 2017). These results suggest that BCAAs such as leucine and other amino acids influence metabolic health through both distinct and overlapping mechanisms. In addition, recent work has found that a subset of neurons in mediobasal hypothalamus can rapidly respond to physiological changes in extracellular leucine concentration (Heeley et al., 2018). Leucine was shown to activate both POMC and NPY/AGRP human and mice hypothalamic neurons. Interestingly, this mechanisms was not modulated by either KATP channels or mTOR but by activation and inhibition of Ca2+ channels (Heeley et al., 2018). This may suggest a completely unique method by which leucine can exert its effect compared to other BCAAs, which may explain some of the idiosyncratic results seen with deprivation and supplementation with this amino acid.
Though amino acids themselves can act as signaling molecules, their catabolites can also induce metabolic changes, for example the catabolite, 3-hydroxy-isobutyrate (3-HIB), of the BCAA valine has also recently been implicated in insulin resistance (Jang et al., 2016). It has been found that 3-HIB increases the uptake of fatty acids into the muscle through the transcriptional activator peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC-1α) promoting accumulation of lipids in muscle and insulin resistance in mice. Interestingly, in the diabetic db/db mouse model and in diabetic humans 3-HIB is increased in the muscle (Jang et al., 2016). This study is important in recognizing that the flux of BCAA catabolites, rather than the BCAAs themselves, may be key regulators of the metabolic response of PR and amino acid restriction.
To explore the role of BCAA catabolism in metabolic health, mice were created that lack the branched chain amino acid transaminase 2 (Bcat2 −/−) gene. Bcat2 −/− This resulted in decreased body weight and adiposity despite increased food consumption, improved glucose and insulin tolerance and increased energy expenditure even though circulating BCAAs were increased (She et al., 2007). Conversely, transgenic expression of Bcat2 is hepatocytes, where it is not normally expressed, reduced hepatic levels of BCAAs attenuated mTOR signaling in high fat fed mice, and impaired blood glucose tolerance, suggesting that BCAAs regulate blood glucose during diet-induced obesity (Ananieva et al., 2017).
In addition to “canonical” leucine activation by mTORC1 via Sestrin2 (Figure 2), it was recently shown that leucyl-tRNA synthetase, which can sense intracellular levels of leucine, can activate mTOR in both yeast (Bonfils et al., 2012) and humans (Han et al., 2012). Aminoacyl-tRNA synthetases are the enzymes that catalyze the reaction between an amino acid and its cognate tRNA, and are able to discriminate between specific amino acids. The leucyl-tRNA synthetase in particular may act to regulate mTORC1 signaling; the leucyl-tRNA synthetase may function as a GAP for RagD (Han et al., 2012), and can regulate the ability of RagA and RagB to recruit mTORC1 to the lysosome through the leucylation of specific lysine residues on RagA and RagB (He et al., 2018).
Tryptophan
In addition to methionine and the BCAAs, the amino acid tryptophan has been the most thoroughly studied in terms of metabolic health. Most investigations have concluded that tryptophan restriction is beneficial for longevity. Disruption of tryptophan pathways in S. cerevisiae, C. elegans and Drosophila increases longevity through destabilization of a tryptophan transporter, which inhibits uptake (He et al., 2014). In mammals, tryptophan restriction also promotes longevity; in rats it has been shown to delay tumor onset and increase mean and maximal lifespan (De Marte and Enesco, 1986; Ooka et al., 1988; Segall and Timiras, 1976). Conversely, activation of tryptophan catabolism has been implicated in the progression of Alzheimer’s (Anderson and Ojala, 2010; Gulaj et al., 2010) and Parkinson’s disease (Ogawa et al., 1992; Widner et al., 2002). However, other studies in mammalian animal models have suggested that tryptophan may be beneficial. In growth-retarded rats, tryptophan supplementation was able to rescue the phenotype and return rats to normal size within 6–22 months and increased lifespan (Segall, 1977); however, this may be due to correction of early growth retardation.
Taken together, these results suggest that even single amino acid supplementation or deprivation can strongly alter metabolic phenotypes, and that these processes are mediated through a myriad of downstream pathways. In addition to activation by specific amino acids, the catabolites of amino acid degradation, or the absence of amino acids can also regulate metabolic processes. Furthermore, amino acids can act as metabolic substrates and be degraded into the tricarboxylic acid (TCA) cycle during periods of low carbohydrate availability. There are several potential targets including methionine, tryptophan and BCAAs that may have clinical relevance for the prevention of metabolic diseases (Table 2).
Table 2:
Recent studies of the effect of amino acid restricted diets on the metabolic health of humans and rodents. M=male, F=female, where not stated both sexes used, FGF21 = fibroblast growth factor 21, UCP1 = uncoupling protein 1, eIF2α = eukaryotic transcription factor 2α, BCAAs = branched chain amino acids, BAT = brown adipose tissue, WAT = white adipose tissue, DIO = diet induced obesity, S6K1 = S6 protein kinase 1, Met = methionine, Val = valine, Ile = isoleucine.
| Species/Strain/Sex | Restricted amino acid | Level of intake | Metabolic effect | Length of intervention | Study |
|---|---|---|---|---|---|
| Mice CB6F1 (F) | Met | 23–35% | Decreased circulating IGF-I, insulin and glucose | Longevity study | (Miller et al., 2005) |
| Increased resistance to liver stress | |||||
| Mice C57BL/6J (M) | Leu | 0% | Increased oxygen consumption | 7 days | (Cheng et al., 2010) |
| Mice C57BL/6J (M) | Leu | 0% | Improved glucose tolerance | 7 days | (Xiao et al., 2011) |
| Improved hepatic insulin sensitivity | |||||
| Decreases mTOR/S6K1 signaling | |||||
| Activates GCN2 | |||||
| Mice C57BL/6J (M) | Leu/Val/Ile | 0% | Improved insulin sensitivity | 1 day | (Xiao et al., 2014) |
| Mice C57BL/6J (M) | Val | 0% | Improved glucose and insulin sensitivity | 7 days | (Xiao et al., 2014) |
| Decreased hepatic mTOR activation | |||||
| Increased hepatic GCN2 activation | |||||
| Increased hepatic AMPK activation | |||||
| Mice C57BL/6J (M) | Ile | 0% | Improved glucose and insulin sensitivity | 7 days | (Xiao et al., 2014) |
| Decreased hepatic mTOR activation | |||||
| Increased hepatic AMPK activation | |||||
| Mice C57BL/6J (M) | Met | 20% | Improved insulin sensitivity | 8 weeks | (Stone et al., 2014) |
| Suppresses hepatic glucose production | |||||
| Increased hepatic expression of FGF21 | |||||
| Increased circulating FGF21 | |||||
| Mice C57BL/6J (M) | Met | 20% | Increased food intake | 8 weeks | (Lees et al., 2014) |
| Reduced body weight | |||||
| Increased physical activity | |||||
| Improved hepatic insulin sensitivity | |||||
| Remodeling of WAT metabolism | |||||
| Decreased hepatic lipogenic gene expression | |||||
| Increased circulating and hepatic expression of FGF21 | |||||
| Rats Zucker-fatty (M) | BCAAs | 55% | Improved skeletal muscle glucose disposal | 15 weeks | (White et al., 2016) |
| Improves skeletal muscle insulin sensitivity | |||||
| Mice C57BL/6J | Leu | 33% | No body mass change | 13 weeks | (Fontana et al., 2016) |
| Increased adiposity | |||||
| Improved glucose tolerance | |||||
| Mice C57BL/6J | BCAAs | 33% | Increased food intake | 13 weeks | (Fontana et al., 2016) |
| Improved glucose and pyruvate tolerance | |||||
| Decreased fasting blood glucose and insulin secretion | |||||
| Mice C57BL/6J (M) | Met | 15% | Increased in energy intake | 10 weeks | (Wanders et al., 2017) |
| Increased energy expenditure | |||||
| Lower accumulation of body weight | |||||
| Lower accumulation of fat mass | |||||
| Reduction in adipocyte size | |||||
| Mice C57BL/6J (M) | Met | 20% | Increased food intake | 8 weeks | (Lees et al., 2017) |
| Decreased body and fat mass | |||||
| Improved whole body glucose metabolism | |||||
| Decreased fasting blood glucose and insulin | |||||
| Elevated lipid cycling in WAT | |||||
| Reduced hepatic lipogenic gene expression | |||||
| Elevated fasting serum FGF21 | |||||
| Mice C57BL/6J (M) | Leu | 20% | Increased food intake | 8 weeks | (Lees et al., 2017) |
| Decreased body and fat mass | |||||
| Improved whole body glucose metabolism | |||||
| Decreased fasting insulin | |||||
| Elevated lipid cycling in WAT | |||||
| Mice C57BL/6J | Met | 0% | Increased food intake | 5 weeks | (Yu et al., 2018) |
| Weight and adiposity loss | |||||
| Improved insulin sensitivity | |||||
| Improved pyruvate tolerance | |||||
| Mice C57BL/6J (DIO) | Met | 0% | Increased food intake (females) | 5 weeks | (Yu et al., 2018) |
| Increased energy expenditure | |||||
| Restores body weight | |||||
| Reduced adiposity | |||||
| Normalized glucose tolerance | |||||
| Improved insulin sensitivity | |||||
| Induces FGF21 (males) | |||||
| Mice C57BL/6J (DIO) | BCAAs | 33% | Rapid weight loss | 14 weeks | (Cummings et al., 2018) |
| Loss of fat and lean mass | |||||
| Loss of dermis WAT | |||||
| Improved glucose and insulin sensitivity | |||||
| Increases energy expenditure | |||||
| Decreased liver droplet size | |||||
| Transiently increases FGF21 | |||||
| Decreased hepatic lipogenic gene expression |
4. Amino acid restriction as an intervention in obesity and diabetes
In humans, plasma amino acid concentrations are biomarkers for several diseases (Roth and Druml, 2011). A comparison of obese versus lean humans indicated that serum levels of BCAAs, aromatic amino acids, glutamate and alanine were increased (Newgard et al., 2009) and further work indicated that increased plasma levels of BCAAs are correlated with development of insulin resistance and diabetes in humans (Newgard, 2012). Furthermore, BCAAs can predict the development of diabetes 12 years later in normoglycemic individuals (Wang et al., 2011). Weight loss diets have been shown to consistently reduce levels of plasma amino acids in humans including BCAAs (Zheng et al., 2016). It has also been suggested that visceral white adipose tissue may be involved in the accumulation of BCAAs in obese humans and mice (Lackey et al., 2013)
While it has been known for almost a decade that supplementing BCAAs to rats fed a high fat diet results in insulin resistance, possibly due to hyperactivation of mTORC1 in skeletal muscle (Newgard et al., 2009), it has only recently become apparent that normal dietary levels of the BCAAs play a key role in maintaining an obese, insulin resistant state. We recently demonstrated that specifically reducing dietary levels of the BCAAs by 67% in diet-induced obese mice caused a rapid reduction in weight due to fat mass loss (DIO) mice, even as the mice continued to eat an otherwise high fat, high sugar Western diet (Cummings et al., 2018). Glucose tolerance and insulin sensitivity were also rapidly restored. This phenomena is thought to be mediated at least in part by increased energy expenditure, which may be initiated by transient activation of FGF21 in response to reduced levels of the BCAAs (Cummings et al., 2018). This provides evidence that by altering the precise macronutrient composition of diets can improve health parameters, without calorie restriction.
In both humans and mice, MR promotes leanness and improves metabolic health, however, long term adherence in humans to such diets is poor. We recently established that a short term methionine deprivation (MD) regimen that rapidly improves the metabolic health of DIO mice, rapidly reducing adiposity and improving glycemic control. This did not occur through reduced calorie intake, but through increased energy expenditure, which mirrors responses we previously saw to BCAA restriction in obese mice. Intriguingly, this effect appears to be metabolically distinct from the effects of MR, as the effects of MR are dependent upon FGF21; while male mice on a MD diet showed strong induction of FGF21 and evidence of WAT beiging (induction of Ucp1), female mice on a MD diet had similar improvements in glucose homeostasis and adiposity without engaging the FGF21-UCP1 axis (Yu et al., 2018).
5. Conclusions
As obesity and diabetes become increasingly common, the necessity for new interventions that improve metabolic health is becoming increasingly essential (Centers for Disease Control and Prevention, 2014; Fothergill et al., 2016). In particular, as diets based on reduced calorie intake have poor long-term adherence, recent findings suggesting that diets based on altered macronutrient content in humans and mice have greater long-term compliance may point to strategies based on these diets as a step in the right direction. Recent work demonstrates that dietary protein, and indeed specific dietary amino acids, are powerful mediators of metabolic health. In particular, dietary restriction of the amino acids including leucine, isoleucine, methionine, tryptophan, and valine promote metabolic in rodents; further studies will need to take place to determine the effect of restriction these dietary amino acids on human health.
Though it is clear that reducing amino acid intake has benefits on metabolic health parameters such as weight, adiposity, and insulin sensitivity, there is still no consensus on how this benefit is conferred. To understand these mechanisms we need a deeper molecular understanding of the downstream effects of amino acid deprivation and supplementation, which may allow the development of novel therapies for obesity and diabetes. While many of the effects of a PR diet and amino acid restriction may be mediated by GCN2 and mTOR, there is little consensus on how this is achieved. In Gcn2 −/− mice, strong activation of mTORC1 is seen in the liver after injection with arginase, suggesting GCN2 is a powerful mTOR inhibitor (Nikonorova et al., 2018). However, other studies have suggested that PR mediated inactivation of mTOR is regulated by eIF2, but not ATF4, which may indicate that different amino acids modulate mTOR through distinct cell signaling pathways (Averous et al., 2016; Nikonorova et al., 2018). New potential avenues for research include investigating the sexually dimorphic molecular effects of methionine restriction (Yu et al., 2018), and identifying the molecular basis for the different effects of methionine and leucine restriction (Lees et al., 2017). Finally, the ability of aminoacyl-tRNA synthetases to aminoacylate lysines of diverse substrates suggest that this may be a novel mechanism by which the abundance of individual amino acids may regulate diverse metabolic processes (He et al., 2018).
There are still many questions to answer with regards to the regulation of metabolic health via amino acid deprivation. Development of clinical therapies involving these mechanisms will require a greater understanding of the sexual dimorphism between responses to amino acid restriction, as different mechanisms may be induced in different sexes. In addition, though there have been many studies analyzing the effects of amino acid changes on the liver, muscle, BAT and WAT, it is important to understand how these interventions affect other tissues, for example how they may affect signaling in areas of the brain, such as the hypothalamus, which is involved in hunger signaling and energy homeostasis. Furthermore, though restriction of BCAAs generally appears beneficial, studies in aged mammals suggest that lifelong restriction may not be optimal, and that as animals age and nutritional requirements change, diets may need to be tailored for maintenance of bone and muscle. This may shed some light on potential uses of increasing beiging of WAT to increase energy expenditure and weight loss. Developing our knowledge of the mechanisms behind these effects is imperative for developing novel therapeutic approaches to diabetes and obesity.
Acknowledgements
This manuscript was supported in part by grants from the NIH (AG050135, AG051974, and AG056771 to D.W.L.), a New Investigator Program Award (D.W.L.) from the Wisconsin Partnership Program, and startup funds from the UW-Madison School of Medicine and Public Health and the UW-Madison Department of Medicine (D.W.L.). This research was conducted while D.W.L. was an AFAR Research Grant recipient from the American Federation for Aging Research. This work was supported using facilities and resources from the William S. Middleton Memorial Veterans Hospital. This work does not represent the views of the Department of Veterans Affairs or the United States Government.
References
- Albert V, Svensson K, Shimobayashi M, Colombi M, Munoz S, Jimenez V, Handschin C, Bosch F, Hall MN, 2016. mTORC2 sustains thermogenesis via Akt-induced glucose uptake and glycolysis in brown adipose tissue. EMBO Mol. Med 8, 232–246. 10.15252/emmm.201505610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonge KM, Meares GP, Hillgartner FB, 2017. Glucagon and Insulin Cooperatively Stimulate Fibroblast Growth Factor 21 Gene Transcription by Increasing the Expression of Activating Transcription Factor 4. J. Biol. Chem 292, 5239–5252. 10.1074/jbc.M116.762922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvers AL, Fishwick LK, Wood MS, Hu D, Chung HS, Dunn WA Jr, Aris JP, 2009. Autophagy and amino acid homeostasis are required for chronological longevity in Saccharomyces cerevisiae. Aging Cell 8, 353–369. 10.1111/j.1474-9726.2009.00469.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananieva EA, Van Horn CG, Jones MR, Hutson SM, 2017. Liver BCATm transgenic mouse model reveals the important role of the liver in maintaining BCAA homeostasis. J. Nutr. Biochem 40, 132–140. 10.1016/J.JNUTBIO.2016.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G, Ojala J, 2010. Alzheimer’s and seizures: interleukin-18, indoleamine 2,3-dioxygenase and quinolinic Acid. Int. J. Tryptophan Res 3, 169–73. 10.4137/IJTR.S4603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony JC, Anthony TG, Layman DK, 1999. Leucine Supplementation Enhances Skeletal Muscle Recovery in Rats Following Exercise. J. Nutr 129, 1102–1106. 10.1093/jn/129.6.1102 [DOI] [PubMed] [Google Scholar]
- Austad SN, 1989. Life extension by dietary restriction in the bowl and doily spider, Frontinella pyramitela. Exp. Gerontol 24, 83–92. 10.1016/0531-5565(89)90037-5 [DOI] [PubMed] [Google Scholar]
- Averous J, Lambert-Langlais S, Mesclon F, Carraro V, Parry L, Jousse C, Bruhat A, Maurin A-C, Pierre P, Proud CG, Fafournoux P, 2016. GCN2 contributes to mTORC1 inhibition by leucine deprivation through an ATF4 independent mechanism. Sci. Rep 6, 27698 10.1038/srep27698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azemati B, Rajaram S, Jaceldo-Siegl K, Sabate J, Shavlik D, Fraser GE, Haddad EH, 2017. Animal-Protein Intake Is Associated with Insulin Resistance in Adventist Health Study 2 (AHS-2) Calibration Substudy Participants: A Cross-Sectional Analysis. Curr. Dev. Nutr 1, e000299 10.3945/cdn.116.000299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- B’chir W, Maurin A-C, Carraro V, Averous J, Jousse C, Muranishi Y, Parry L, Stepien G, Fafournoux P, Bruhat A, 2013. The eIF2α/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res 41, 7683–7699. 10.1093/nar/gkt563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E, 2007. Hepatic Fibroblast Growth Factor 21 Is Regulated by PPARα and Is a Key Mediator of Hepatic Lipid Metabolism in Ketotic States. Cell Metab. 5, 426–437. 10.1016/J.CMET.2007.05.002 [DOI] [PubMed] [Google Scholar]
- Bar-Peled L, Chantranupong L, Cherniack AD, Chen WW, Ottina KA, Grabiner BC, Spear ED, Carter SL, Meyerson M, Sabatini DM, 2013. A Tumor Suppressor Complex with GAP Activity for the Rag GTPases That Signal Amino Acid Sufficiency to mTORC1. Science (80-.) 340, 1100–1106. 10.1126/science.1232044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM, 2012. Ragulator Is a GEF for the Rag GTPases that Signal Amino Acid Levels to mTORC1. Cell 150, 1196–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard ND, Cohen J, Jenkins DJ, Turner-McGrievy G, Gloede L, Green A, Ferdowsian H, 2009. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am. J. Clin. Nutr 89, 1588S–1596S. 10.3945/ajcn.2009.26736H [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard ND, Cohen J, Jenkins DJA, Turner-McGrievy G, Gloede L, Jaster B, Seidl K, Green AA, Talpers S, 2006. A Low-Fat Vegan Diet Improves Glycemic Control and Cardiovascular Risk Factors in a Randomized Clinical Trial in Individuals With Type 2 Diabetes. Diabetes Care 29, 1777–1783. 10.2337/dc06-0606 [DOI] [PubMed] [Google Scholar]
- Bentzinger CF, Romanino K, Cloëtta D, Lin S, Mascarenhas JB, Oliveri F, Xia J, Casanova E, Costa CF, Brink M, Zorzato F, Hall MN, Rüegg MA, 2008. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab 8, 411–24. 10.1016/j.cmet.2008.10.002 [DOI] [PubMed] [Google Scholar]
- Berglund ED, Li CY, Bina HA, Lynes SE, Michael MD, Shanafelt AB, Kharitonenkov A, Wasserman DH, 2009. Fibroblast Growth Factor 21 Controls Glycemia via Regulation of Hepatic Glucose Flux and Insulin Sensitivity. Endocrinology 150, 4084–4093. 10.1210/en.2009-0221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bifari F, Nisoli E, 2017. Branched-chain amino acids differently modulate catabolic and anabolic states in mammals: a pharmacological point of view. Br. J. Pharmacol 174, 1366–1377. 10.1111/bph.13624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfils G, Jaquenoud M, Bontron S, Ostrowicz C, Ungermann C, De Virgilio C, 2012. Leucyl-tRNA Synthetase Controls TORC1 via the EGO Complex. Mol. Cell 46, 105–110. [DOI] [PubMed] [Google Scholar]
- Bross TG, Rogina B, Helfand SL, 2005. Behavioral, physical, and demographic changes in Drosophila populations through dietary restriction. Aging Cell 4, 309–17. 10.1111/j.1474-9726.2005.00181.x [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Buffenstein R, 2017. Cutting back on the essentials: Can manipulating intake of specific amino acids modulate health and lifespan? Ageing Res. Rev 39, 87–95. 10.1016/j.arr.2016.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budanov AV, Karin M, 2008. p53 Target Genes Sestrin1 and Sestrin2 Connect Genotoxic Stress and mTOR Signaling. Cell 134, 451–460. 10.1016/j.cell.2008.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron A, Mouchiroud M, Gautier N, Labbé SM, Villot R, Turcotte L, Secco B, Lamoureux G, Shum M, Gélinas Y, Marette A, Richard D, Sabatini DM, Laplante M, 2017. Loss of hepatic DEPTOR alters the metabolic transition to fasting. Mol. Metab 6, 447–458. 10.1016/j.molmet.2017.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2014. National Diabetes Statistics Report: Estimates of Diabetes and its Burden in the United States. US Dep. Heal. Hum. Serv. 2014 10.1177/1527154408322560 [DOI] [Google Scholar]
- Chantranupong L, Scaria SM, Saxton RA, Gygi MP, Shen K, Wyant GA, Wang T, Harper JW, Gygi SP, Sabatini DM, 2016. The CASTOR Proteins Are Arginine Sensors for the mTORC1 Pathway. Cell 165, 153–164. 10.1016/j.cell.2016.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Meng Q, Wang C, Li H, Huang Z, Chen S, Xiao F, Guo F, 2010. Leucine Deprivation Decreases Fat Mass by Stimulation of Lipolysis in White Adipose Tissue and Upregulation of Uncoupling Protein 1 (UCP1) in Brown Adipose Tissue. Diabetes 59, 17–25. 10.2337/db09-0929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R, 2009. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325, 201–4. 10.1126/science.1173635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM, 2014. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat. Commun 5, 3557 10.1038/ncomms4557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornu M, Oppliger W, Albert V, Robitaille AM, Trapani F, Quagliata L, Fuhrer T, Sauer U, Terracciano L, Hall MN, 2014. Hepatic mTORC1 controls locomotor activity, body temperature, and lipid metabolism through FGF21. Proc. Natl. Acad. Sci. U. S. A 111, 11592–9. 10.1073/pnas.1412047111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A, 2008. Fibroblast Growth Factor 21 Corrects Obesity in Mice. Endocrinology 149, 6018–6027. 10.1210/en.2008-0816 [DOI] [PubMed] [Google Scholar]
- Cummings NE, Lamming DW, 2017. Regulation of metabolic health and aging by nutrient-sensitive signaling pathways. Mol. Cell. Endocrinol 455, 13–22. 10.1016/J.MCE.2016.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings NE, Williams EM, Kasza I, Konon EN, Schaid MD, Schmidt BA, Poudel C, Sherman DS, Yu D, Arriola Apelo SI, Cottrell SE, Geiger G, Barnes ME, Wisinski JA, Fenske RJ, Matkowskyj KA, Kimple ME, Alexander CM, Merrins MJ, Lamming DW, 2018. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J. Physiol 596, 623–645. 10.1113/JP275075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Antona G, Ragni M, Cardile A, Tedesco L, Dossena M, Bruttini F, Caliaro F, Corsetti G, Bottinelli R, Carruba MO, Valerio A, Nisoli E, 2010. Branched-Chain Amino Acid Supplementation Promotes Survival and Supports Cardiac and Skeletal Muscle Mitochondrial Biogenesis in Middle-Aged Mice. Cell Metab 12, 362–372. 10.1016/j.cmet.2010.08.016 [DOI] [PubMed] [Google Scholar]
- De Marte ML, Enesco HE, 1986. Influence of low tryptophan diet on survival and organ growth in mice. Mech. Ageing Dev 36, 161–71. [DOI] [PubMed] [Google Scholar]
- Dever TE, Feng L, Wek RC, Cigan AM, Donahue TF, Hinnebusch AG, 1992. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell 68, 585–96. [DOI] [PubMed] [Google Scholar]
- Douris N, Stevanovic DM, Fisher FM, Cisu TI, Chee MJ, Nguyen NL, Zarebidaki E, Adams AC, Kharitonenkov A, Flier JS, Bartness TJ, Maratos-Flier E, 2015. Central Fibroblast Growth Factor 21 Browns White Fat via Sympathetic Action in Male Mice. Endocrinology 156, 2470–2481. 10.1210/en.2014-2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshorbagy AK, Valdivia-Garcia M, Mattocks DAL, Plummer JD, Smith AD, Drevon CA, Refsum H, Perrone CE, 2011. Cysteine supplementation reverses methionine restriction effects on rat adiposity: significance of stearoyl-coenzyme A desaturase. J. Lipid Res 52, 104–12. 10.1194/jlr.M010215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsukova EI, Medvedev LN, Mizonova OV, Taidonov SV, 2012. Effect of calorie restricted diet on brown adipose tissue in mice. Bull. Exp. Biol. Med 152, 286–8. [DOI] [PubMed] [Google Scholar]
- Fawal M-A, Brandt M, Djouder N, 2015. MCRS1 Binds and Couples Rheb to Amino Acid-Dependent mTORC1 Activation. Dev. Cell 33, 67–81. 10.1016/j.devcel.2015.02.010 [DOI] [PubMed] [Google Scholar]
- Felig P, Wahren J, Hendler R, Brundin T, 1974. Splanchnic glucose and amino acid metabolism in obesity. J. Clin. Invest 53, 582–590. 10.1172/JCI107593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, Spiegelman BM, 2012. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 26, 271–81. 10.1101/gad.177857.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Cummings NE, Arriola Apelo SI, Neuman JC, Kasza I, Schmidt BA, Cava E, Spelta F, Tosti V, Syed FA, Baar EL, Veronese N, Cottrell SE, Fenske RJ, Bertozzi B, Brar HK, Pietka T, Bullock AD, Figenshau RS, Andriole GL, Merrins MJ, Alexander CM, Kimple ME, Lamming DW, 2016. Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health. Cell Rep 16, 520–30. 10.1016/j.celrep.2016.05.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO, 2008. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell 7, 681–7. 10.1111/J.1474-9726.2008.00417.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forney LA, Wanders D, Stone KP, Pierse A, Gettys TW, 2017. Concentration-dependent linkage of dietary methionine restriction to the components of its metabolic phenotype. Obesity 25, 730–738. 10.1002/oby.21806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, Chen KY, Skarulis MC, Walter M, Walter PJ, Hall KD, 2016. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity 24, 1612–1619. 10.1002/oby.21538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, Kharitonenkov A, Bumol T, Schilske HK, Moller DE, 2013. The Effects of LY2405319, an FGF21 Analog, in Obese Human Subjects with Type 2 Diabetes. Cell Metab 18, 333–340. [DOI] [PubMed] [Google Scholar]
- Goberdhan DCI, Wilson C, Harris AL, 2016. Amino Acid Sensing by mTORC1: Intracellular Transporters Mark the Spot. Cell Metab 23, 580–589. 10.1016/j.cmet.2016.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez A, Gomez J, Torres ML, Naudi A, Mota-Martorell N, Pamplona R, Barja G, 2015. Cysteine dietary supplementation reverses the decrease in mitochondrial ROS production at complex I induced by methionine restriction. J. Bioenerg. Biomembr 47, 199–208. 10.1007/s10863-015-9608-x [DOI] [PubMed] [Google Scholar]
- Gong Q, Hu Z, Zhang F, Cui A, Chen X, Jiang H, Gao J, Chen X, Han Y, Liang Q, Ye D, Shi L, Chin YE, Wang Y, Xiao H, Guo F, Liu Y, Zang M, Xu A, Li Y, 2016. Fibroblast growth factor 21 improves hepatic insulin sensitivity by inhibiting mammalian target of rapamycin complex 1 in mice. Hepatology 64, 425–38. 10.1002/hep.28523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble KE, Welch DB, 2013. Life-span extension by caloric restriction is determined by type and level of food reduction and by reproductive mode in Brachionus manjavacas (Rotifera). J Gerontol A Biol Sci Med Sci 68, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Orozco JM, Saxton RA, Condon KJ, Liu GY, Krawczyk PA, Scaria SM, Harper JW, Gygi SP, Sabatini DM, 2017. SAMTOR is anS-adenosylmethionine sensor for the mTORC1 pathway. Science 358, 813–818. 10.1126/science.aao3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulaj E, Pawlak K, Bien B, Pawlak D, 2010. Kynurenine and its metabolites in Alzheimer’s disease patients. Adv. Med. Sci 55, 204–11. 10.2478/v10039-010-0023-6 [DOI] [PubMed] [Google Scholar]
- Guo F, Cavener DR, 2007. The GCN2 eIF2alpha kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab 5, 103–14. 10.1016/j.cmet.2007.01.001 [DOI] [PubMed] [Google Scholar]
- Guridi M, Tintignac LA, Lin S, Kupr B, Castets P, Rüegg MA, 2015. Activation of mTORC1 in skeletal muscle regulates whole-body metabolism through FGF21. Sci. Signal 8, ra113 10.1126/scisignal.aab3715 [DOI] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ, 2008. AMPK Phosphorylation of Raptor Mediates a Metabolic Checkpoint. Mol. Cell 30, 214–226. 10.1016/j.molcel.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S, 2012. Leucyl-tRNA Synthetase Is an Intracellular Leucine Sensor for the mTORC1-Signaling Pathway. Cell 149, 410–424. 10.1016/J.CELL.2012.02.044 [DOI] [PubMed] [Google Scholar]
- Hanssen MJW, Broeders E, Samms RJ, Vosselman MJ, van der Lans AAJJ, Cheng CC, Adams AC, van Marken Lichtenbelt WD, Schrauwen P, 2015. Serum FGF21 levels are associated with brown adipose tissue activity in humans. Sci. Rep 5, 10275 10.1038/srep10275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D, 2000. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6, 1099–108. [DOI] [PubMed] [Google Scholar]
- He C, Tsuchiyama SK, Nguyen QT, Plyusnina EN, Terrill SR, Sahibzada S, Patel B, Faulkner AR, Shaposhnikov MV, Tian R, Tsuchiya M, Kaeberlein M, Moskalev AA, Kennedy BK, Polymenis M, 2014. Enhanced Longevity by Ibuprofen, Conserved in Multiple Species, Occurs in Yeast through Inhibition of Tryptophan Import. PLoS Genet 10, e1004860 10.1371/journal.pgen.1004860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X-D, Gong W, Zhang J-N, Nie J, Yao C-F, Guo F-S, Lin Y, Wu X-H, Li F, Li J, Sun W-C, Wang E-D, An Y-P, Tang H-R, Yan G-Q, Yang P-Y, Wei Y, Mao Y-Z, Lin P-C, Zhao J-Y, Xu Y, Xu W, Zhao S-M, 2018. Sensing and Transmitting Intracellular Amino Acid Signals through Reversible Lysine Aminoacylations. Cell Metab 27, 151–166. e6 10.1016/j.cmet.2017.10.015 [DOI] [PubMed] [Google Scholar]
- Heeley N, Kirwan P, Darwish T, Arnaud M, Evans ML, Merkle FT, Reimann F, Gribble FM, Blouet C, 2018. Rapid sensing of l-leucine by human and murine hypothalamic neurons: Neurochemical and mechanistic insights. Mol. Metab 10.1016/J.MOLMET.2018.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E, 2006. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA 295, 1539–48. 10.1001/jama.295.13.1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henagan TM, Laeger T, Navard AM, Albarado D, Noland RC, Stadler K, Elks CM, Burk D, Morrison CD, 2016. Hepatic autophagy contributes to the metabolic response to dietary protein restriction. Metabolism 65, 805–815. 10.1016/J.METABOL.2016.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CM, Laeger T, Albarado DC, McDougal DH, Berthoud HR, Münzberg H, Morrison CD, 2017. Low protein-induced increases in FGF21 drive UCP1-dependent metabolic but not thermoregulatory endpoints. Sci. Rep 7, 8209 10.1038/s41598-017-07498-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosono R, Nishimoto S, Kuno S, 1989. Alterations of life span in the nematode Caenorhabditis elegans under monoxenic culture conditions. Exp. Gerontol 24, 251–264. 10.1016/0531-5565(89)90016-8 [DOI] [PubMed] [Google Scholar]
- Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D, Peterson TR, Choi Y, Gray NS, Yaffe MB, Marto JA, Sabatini DM, 2011. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science 332, 1317–22. 10.1126/science.1199498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Zhong L, Lee JTH, Zhang J, Wu D, Geng L, Wang Y, Wong C-M, Xu A, 2017. The FGF21-CCL11 Axis Mediates Beiging of White Adipose Tissues by Coupling Sympathetic Nervous System to Type 2 Immunity. Cell Metab 26, 493–508. e4 10.1016/j.cmet.2017.08.003 [DOI] [PubMed] [Google Scholar]
- Il’yasova D, Fontana L, Bhapkar M, Pieper CF, Spasojevic I, Redman LM, Das SK, Huffman KM, Kraus WE, CALERIE Study Investigators, 2018. Effects of 2 years of caloric restriction on oxidative status assessed by urinary F2-isoprostanes: The CALERIE 2 randomized clinical trial. Aging Cell e12719 10.1111/acel.12719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA, 2007. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab 5, 415–25. 10.1016/j.cmet.2007.05.003 [DOI] [PubMed] [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan K-L, 2002. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol 4, 648–657. 10.1038/ncb839 [DOI] [PubMed] [Google Scholar]
- Jang C, Oh SF, Wada S, Rowe GC, Liu L, Chan MC, Rhee J, Hoshino A, Kim B, Ibrahim A, Baca LG, Kim E, Ghosh CC, Parikh SM, Jiang A, Chu Q, Forman DE, Lecker SH, Krishnaiah S, Rabinowitz JD, Weljie AM, Baur JA, Kasper DL, Arany Z, 2016. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat. Med 22, 421–6. 10.1038/nm.4057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Genau HM, Behrends C, 2015. Amino Acid-Dependent mTORC1 Regulation by the Lysosomal Membrane Protein SLC38A9. Mol. Cell. Biol 35, 2479–2494. 10.1128/MCB.00125-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaitala A, 1991. Phenotypic Plasticity in Reproductive Behaviour of Waterstriders: Trade-Offs Between Reproduction and Longevity During Food Stress. Funct. Ecol 5, 12 10.2307/2389551 [DOI] [Google Scholar]
- Keipert S, Kutschke M, Ost M, Schwarzmayr T, van Schothorst EM, Lamp D, Brachthäuser L, Hamp I, Mazibuko SE, Hartwig S, Lehr S, Graf E, Plettenburg O, Neff F, Tschöp MH, Jastroch M, 2017. Long-Term Cold Adaptation Does Not Require FGF21 or UCP1. Cell Metab 26, 437–446. e5 10.1016/j.cmet.2017.07.016 [DOI] [PubMed] [Google Scholar]
- Kennedy BK, Lamming DW, 2016. The Mechanistic Target of Rapamycin: The Grand ConducTOR of Metabolism and Aging. Cell Metab 23, 990–1003. 10.1016/j.cmet.2016.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li D-S, Mehrbod F, Jaskunas SR, Shanafelt AB, 2005. FGF-21 as a novel metabolic regulator. J. Clin. Invest 115, 1627–35. 10.1172/JCI23606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk KL, 2001. Dietary Restriction and Aging: Comparative Tests of Evolutionary Hypotheses. Journals Gerontol. Ser. A Biol. Sci. Med. Sci 56, B123–B129. 10.1093/gerona/56.3.B123 [DOI] [PubMed] [Google Scholar]
- Kitada M, Ogura Y, Suzuki T, Monno I, Kanasaki K, Watanabe A, Koya D, 2018. A low-protein diet exerts a beneficial effect on diabetic status and prevents diabetic nephropathy in Wistar fatty rats, an animal model of type 2 diabetes and obesity. Nutr. Metab. (Lond) 15, 20 10.1186/s12986-018-0255-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé SM, Mouchiroud M, Caron A, Secco B, Freinkman E, Lamoureux G, Gélinas Y, Lecomte R, Bossé Y, Chimin P, Festuccia WT, Richard D, Laplante M, 2016. mTORC1 is Required for Brown Adipose Tissue Recruitment and Metabolic Adaptation to Cold. Sci. Rep 6, 37223 10.1038/srep37223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackey DE, Lynch CJ, Olson KC, Mostaedi R, Ali M, Smith WH, Karpe F, Humphreys S, Bedinger DH, Dunn TN, Thomas AP, Oort PJ, Kieffer DA, Amin R, Bettaieb A, Haj FG, Permana P, Anthony TG, Adams SH, 2013. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am. J. Physiol. - Endocrinol. Metab 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeger T, Albarado DC, Burke SJ, Trosclair L, Hedgepeth JW, Berthoud H-R, Gettys TW, Collier JJ, Münzberg H, Morrison CD, 2016. Metabolic Responses to Dietary Protein Restriction Require an Increase in FGF21 that Is Delayed by the Absence of GCN2. Cell Rep 16, 707–16. 10.1016/j.celrep.2016.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, Münzberg H, Hutson SM, Gettys TW, Schwartz MW, Morrison CD, 2014. FGF21 is an endocrine signal of protein restriction. J. Clin. Invest 124, 3913–3922. 10.1172/JCI74915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming DW, Cummings NE, Rastelli AL, Gao F, Cava E, Bertozzi B, Spelta F, Pili R, Fontana L, 2015. Restriction of dietary protein decreases mTORC1 in tumors and somatic tissues of a tumor-bearing mouse xenograft model. Oncotarget 6, 31233–40. 10.18632/oncotarget.5180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming DW, Mihaylova MM, Katajisto P, Baar EL, Yilmaz OH, Hutchins A, Gultekin Y, Gaither R, Sabatini DM, 2014. Depletion of Rictor, an essential protein component of mTORC2, decreases male lifespan. Aging Cell 13, 911–917. 10.1111/acel.12256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, Guertin DA, Sabatini DM, Baur JA, 2012. Rapamycin-Induced Insulin Resistance Is Mediated by mTORC2 Loss and Uncoupled from Longevity. Science (80-.) 335, 1638–1643. 10.1126/science.1215135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, Alfonso A, Ravussin E, Team PC, 2006. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care 29, 1337–44. 10.2337/dc05-2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler DF, Larson BT, Ballam JM, Smith GK, Biery DN, Evans RH, Greeley EH, Segre M, Stowe HD, Kealy RD, 2008. Diet restriction and ageing in the dog: major observations over two decades. Br. J. Nutr 99, 793–805. 10.1017/S0007114507871686 [DOI] [PubMed] [Google Scholar]
- Lee C, Longo V, 2016. Dietary restriction with and without caloric restriction for healthy aging. F1000Research 5 10.12688/f1000research.7136.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JWO, Taylor PW, Soran N, Raubenheimer D, 2008. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc. Natl. Acad. Sci. U. S. A 105, 2498–503. 10.1073/pnas.0710787105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Brychta RJ, Linderman J, Smith S, Chen KY, Celi FS, 2013. Mild cold exposure modulates fibroblast growth factor 21 (FGF21) diurnal rhythm in humans: relationship between FGF21 levels, lipolysis, and cold-induced thermogenesis. J. Clin. Endocrinol. Metab 98, E98–102. 10.1210/jc.2012-3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, Perron RM, Werner CD, Phan GQ, Kammula US, Kebebew E, Pacak K, Chen KY, Celi FS, 2014. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab 19, 302–9. 10.1016/j.cmet.2013.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y-M, Kim S-A, Lee I-K, Kim J-G, Park K-G, Jeong J-Y, Jeon J-H, Shin J-Y, Lee D-H, 2016. Effect of a Brown Rice Based Vegan Diet and Conventional Diabetic Diet on Glycemic Control of Patients with Type 2 Diabetes: A 12-Week Randomized Clinical Trial. PLoS One 11, e0155918 10.1371/journal.pone.0155918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Hartgens F, Wodzig WKWH, Saris WHM, van Loon LJC, 2011. Prolonged leucine supplementation does not augment muscle mass or affect glycemic control in elderly type 2 diabetic men. J. Nutr 141, 1070–6. 10.3945/jn.111.138495 [DOI] [PubMed] [Google Scholar]
- Lees EK, Banks R, Cook C, Hill S, Morrice N, Grant L, Mody N, Delibegovic M, 2017. Direct comparison of methionine restriction with leucine restriction on the metabolic health of C57BL/6J mice. Sci. Rep 7, 9977 10.1038/s41598-017-10381-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees EK, Król E, Grant L, Shearer K, Wyse C, Moncur E, Bykowska AS, Mody N, Gettys TW, Delibegovic M, 2014. Methionine restriction restores a younger metabolic phenotype in adult mice with alterations in fibroblast growth factor 21. Aging Cell 13, 817–827. 10.1111/acel.12238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman SL, Cerniglia GJ, Johannes GJ, Ye J, Ryeom S, Koumenis C, 2015. Translational Upregulation of an Individual p21Cip1 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress. PLOS Genet 11, e1005212 10.1371/journal.pgen.1005212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng C-W, Madia F, Fontana L, Mirisola MG, Guevara-Aguirre J, Wan J, Passarino G, Kennedy BK, Wei M, Cohen P, Crimmins EM, Longo VD, 2014. Low Protein Intake Is Associated with a Major Reduction in IGF-1, Cancer, and Overall Mortality in the 65 and Younger but Not Older Population. Cell Metab 19, 407–417. 10.1016/j.cmet.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S-J, Kaeberlein M, Andalis AA, Sturtz LA, Defossez P-A, Culotta VC, Fink GR, Guarente L, 2002. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature 418, 344–348. 10.1038/nature00829 [DOI] [PubMed] [Google Scholar]
- Ma L, Dong W, Wang R, Li Y, Xu B, Zhang J, Zhao Z, Wang Y, 2015. Effect of caloric restriction on the SIRT1/mTOR signaling pathways in senile mice. Brain Res. Bull 116, 67–72. 10.1016/j.brainresbull.2015.06.004 [DOI] [PubMed] [Google Scholar]
- Maida A, Zota A, Sjøberg KA, Schumacher J, Sijmonsma TP, Pfenninger A, Christensen MM, Gantert T, Fuhrmeister J, Rothermel U, Schmoll D, Heikenwälder M, Iovanna JL, Stemmer K, Kiens B, Herzig S, Rose AJ, 2016. A liver stress-endocrine nexus promotes metabolic integrity during dietary protein dilution. J. Clin. Invest 126, 3263–3278. 10.1172/JCI85946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Piper MDW, Partridge L, 2005. Calories Do Not Explain Extension of Life Span by Dietary Restriction in Drosophila. PLOS Biol 3, e223 10.1371/JOURNAL.PBIO.0030223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfeld J, Urban N, Priebe S, Groth M, Frahm C, Hartmann N, Gebauer J, Ravichandran M, Dommaschk A, Schmeisser S, Kuhlow D, Monajembashi S, Bremer-Streck S, Hemmerich P, Kiehntopf M, Zamboni N, Englert C, Guthke R, Kaleta C, Platzer M, Sühnel J, Witte OW, Zarse K, Ristow M, 2015. Branched-chain amino acid catabolism is a conserved regulator of physiological ageing. Nat. Commun 6, 10043 10.1038/ncomms10043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Colman RJ, Beasley TM, Allison DB, Kemnitz JW, Roth GS, Ingram DK, Weindruch R, de Cabo R, Anderson RM, 2017. Caloric restriction improves health and survival of rhesus monkeys. Nat. Commun 8, 14063 10.1038/ncomms14063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R, 2012. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 489, 318–21. 10.1038/nature11432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty MF, Barroso-Aranda J, Contreras F, 2009. The low-methionine content of vegan diets may make methionine restriction feasible as a life extension strategy. Med. Hypotheses 72, 125–128. 10.1016/j.mehy.2008.07.044 [DOI] [PubMed] [Google Scholar]
- McCay CM, Crowell MF, Maynard LA, 1935. The effect of retarded growth upon the length of life span and upon the ultimate body size. Nutrition 5, 155–71; discussion 172. [PubMed] [Google Scholar]
- McCay CM, Dilley WE, Crowell MF, 1929. Growth rates of brook trout reared upon purified rations,upon dry skim milk diets,and upon feed combinations of cereal grains. J. Nutr 1, 233–246. [Google Scholar]
- McCormack SE, Shaham O, McCarthy MA, Deik AA, Wang TJ, Gerszten RE, Clish CB, Mootha VK, Grinspoon SK, Fleischman A, 2013. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr. Obes 8, 52–61. 10.1111/j.2047-6310.2012.00087.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel SS, Rensing NR, Thio LL, Yamada KA, Wong M, 2011. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia 52, e7–e11. 10.1111/j.1528-1167.2011.02981.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon S, Dibble CC, Talbott G, Hoxhaj G, Valvezan AJ, Takahashi H, Cantley LC, Manning BD, 2014. Spatial Control of the TSC Complex Integrates Insulin and Nutrient Regulation of mTORC1 at the Lysosome. Cell 156, 771–785. 10.1016/j.cell.2013.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M, 2005. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell 4, 119–125. 10.1111/j.1474-9726.2005.00152.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minard AY, Tan S-X, Yang P, Fazakerley DJ, Domanova W, Parker BL, Humphrey SJ, Jothi R, Stöckli J, James DE, 2016. mTORC1 Is a Major Regulatory Node in the FGF21 Signaling Network in Adipocytes. Cell Rep 17, 29–36. 10.1016/j.celrep.2016.08.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SE, Sepehry AA, Wangsgaard JD, Koenig JE, 2014. The Effect of S-Adenosylmethionine on Cognitive Performance in Mice: An Animal Model Meta-Analysis. PLoS One 9, e107756 10.1371/journal.pone.0107756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CD, Xi X, White CL, Ye J, Martin RJ, 2007. Amino acids inhibit Agrp gene expression via an mTOR-dependent mechanism. Am. J. Physiol. Metab 293, E165–E171. 10.1152/ajpendo.00675.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most J, Tosti V, Redman LM, Fontana L, 2017. Calorie restriction in humans: An update. Ageing Res. Rev 39, 36–45. 10.1016/j.arr.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munroe M, Pincu Y, Merritt J, Cobert A, Brander R, Jensen T, Rhodes J, Boppart MD, 2017. Impact of β-hydroxy β-methylbutyrate (HMB) on age-related functional deficits in mice. Exp. Gerontol 87, 57–66. 10.1016/J.EXGER.2016.11.010 [DOI] [PubMed] [Google Scholar]
- Newgard CB, 2012. Interplay between Lipids and Branched-Chain Amino Acids in Development of Insulin Resistance. Cell Metab 15, 606–614. 10.1016/j.cmet.2012.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP, 2009. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 9, 311–26. 10.1016/j.cmet.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichenametla SN, Mattocks DAL, Malloy VL, Pinto JT, 2018. Sulfur amino acid restriction-induced changes in redox-sensitive proteins are associated with slow protein synthesis rates. Ann. N. Y. Acad. Sci 10.1111/nyas.13556 [DOI] [PubMed] [Google Scholar]
- Nikonorova IA, Mirek ET, Signore CC, Goudie MP, Wek RC, Anthony TG, 2018. Time-resolved analysis of amino acid stress identifies eIF2 phosphorylation as necessary to inhibit mTORC1 activity in liver. J. Biol. Chem jbc.RA117.001625. 10.1074/jbc.RA117.001625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Nakatake Y, Konishi M, Itoh N, 2000. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim. Biophys. Acta 1492, 203–6. [DOI] [PubMed] [Google Scholar]
- Noguchi Y, Zhang Q-W, Sugimoto T, Furuhata Y, Sakai R, Mori M, Takahashi M, Kimura T, 2006. Network analysis of plasma and tissue amino acids and the generation of an amino index for potential diagnostic use. Am. J. Clin. Nutr 83, 513S–519S. 10.1093/ajcn/83.2.513S [DOI] [PubMed] [Google Scholar]
- Obata F, Miura M, 2015. Enhancing S-adenosyl-methionine catabolism extends Drosophila lifespan. Nat. Commun 6, 8332 10.1038/ncomms9332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Matson WR, Beal MF, Myers RH, Bird ED, Milbury P, Saso S, 1992. Kynurenine pathway abnormalities in Parkinson’s disease. Neurology 42, 1702–6. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Tsubakiyama R, Kanai M, Koyama T, Fujii T, Iefuji H, Soga T, Kume K, Miyakawa T, Hirata D, Mizunuma M, 2016. Stimulating S-adenosyl-l-methionine synthesis extends lifespan via activation of AMPK. Proc. Natl. Acad. Sci. U. S. A 113, 11913–11918. 10.1073/pnas.1604047113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooka H, Segall PE, Timiras PS, 1988. Histology and survival in age-delayed low-tryptophan-fed rats. Mech. Ageing Dev 43, 79–98. [DOI] [PubMed] [Google Scholar]
- Orentreich N, Matias JR, DeFelice A, Zimmerman JA, 1993. Low methionine ingestion by rats extends life span. J. Nutr 123, 269–274. [DOI] [PubMed] [Google Scholar]
- Osborne TB, Mendel LB, Ferry EL, 1917. The effect of retardation of growth upon the breeding period and duration of life of rats. Science 45, 294–5. 10.1126/science.45.1160.294 [DOI] [PubMed] [Google Scholar]
- Pansarasa O, Flati V, Corsetti G, Brocca L, Pasini E, D’Antona G, 2008. Oral Amino Acid Supplementation Counteracts Age-Induced Sarcopenia in Elderly Rats. Am. J. Cardiol 101, S35–S41. 10.1016/J.AMJCARD.2008.02.079 [DOI] [PubMed] [Google Scholar]
- Perrone CE, Mattocks DAL, Plummer JD, Chittur SV, Mohney R, Vignola K, Orentreich DS, Orentreich N, 2012. Genomic and Metabolic Responses to Methionine-Restricted and Methionine-Restricted, Cysteine-Supplemented Diets in Fischer 344 Rat Inguinal Adipose Tissue, Liver and Quadriceps Muscle. J. Nutrigenet. Nutrigenomics 5, 132–157. 10.1159/000339347 [DOI] [PubMed] [Google Scholar]
- Petit CS, Roczniak-Ferguson A, Ferguson SM, 2013. Recruitment of folliculin to lysosomes supports the amino acid–dependent activation of Rag GTPases. J. Cell Biol 202, 1107–1122. 10.1083/jcb.201307084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezeshki A, Zapata RC, Singh A, Yee NJ, Chelikani PK, 2016. Low protein diets produce divergent effects on energy balance. Sci. Rep 6, 25145 10.1038/srep25145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney DO, Stephens DF, Pope LS, 1972. Lifetime effects of winter supplemental feed level and age at first parturition on range beef cows. J. Anim. Sci 34, 1067–74. [DOI] [PubMed] [Google Scholar]
- Plaisance EP, Greenway FL, Boudreau A, Hill KL, Johnson WD, Krajcik RA, Perrone CE, Orentreich N, Cefalu WT, Gettys TW, 2011. Dietary Methionine Restriction Increases Fat Oxidation in Obese Adults with Metabolic Syndrome. J. Clin. Endocrinol. Metab 96, E836–E840. 10.1210/jc.2010-2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak P, Cybulski N, Feige JN, Auwerx J, Rüegg MA, Hall MN, 2008. Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab 8, 399–410. 10.1016/j.cmet.2008.09.003 [DOI] [PubMed] [Google Scholar]
- Quirós PM, Prado MA, Zamboni N, D’Amico D, Williams RW, Finley D, Gygi SP, Auwerx J, 2017. Multi-omics analysis identifies ATF4 as a key regulator of the mitochondrial stress response in mammals. J. Cell Biol 216, 2027–2045. 10.1083/jcb.201702058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebsamen M, Pochini L, Stasyk T, de Araújo MEG, Galluccio M, Kandasamy RK, Snijder B, Fauster A, Rudashevskaya EL, Bruckner M, Scorzoni S, Filipek PA, Huber KVM, Bigenzahn JW, Heinz LX, Kraft C, Bennett KL, Indiveri C, Huber LA, Superti-Furga G, 2015. SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature 519, 477–81. 10.1038/nature14107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richie JJ, Leutzinger Y, Parthasarathy S, Malloy V, Orentreich N, Zimmerman J, 1994. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J 8, 1302–1307. [DOI] [PubMed] [Google Scholar]
- Ross MH, 1961. Length of life and nutrition in the rat. J. Nutr 75, 197–210. [DOI] [PubMed] [Google Scholar]
- Roth E, Druml W, 2011. Plasma amino acid imbalance: dangerous in chronic diseases? Curr. Opin. Clin. Nutr. Metab. Care 14, 67–74. 10.1097/MCO.0b013e328341368c [DOI] [PubMed] [Google Scholar]
- Rui L, Fisher TL, Thomas J, White MF, 2001. Regulation of Insulin/Insulin-like Growth Factor-1 Signaling by Proteasome-mediated Degradation of Insulin Receptor Substrate-2. J. Biol. Chem 276, 40362–40367. 10.1074/jbc.M105332200 [DOI] [PubMed] [Google Scholar]
- Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM, 2010. Ragulator-Rag Complex Targets mTORC1 to the Lysosomal Surface and Is Necessary for Its Activation by Amino Acids. Cell 141, 290–303. 10.1016/j.cell.2010.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarruf DA, Thaler JP, Morton GJ, German J, Fischer JD, Ogimoto K, Schwartz MW, 2010. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes 59, 1817–24. 10.2337/db09-1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton RA, Sabatini DM, 2017. mTOR Signaling in Growth, Metabolism, and Disease. Cell 168, 960–976. 10.1016/j.cell.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall P, 1977. Long-term tryptophan restriction and aging in the rat. Aktuelle Gerontol 7, 535–8. [PubMed] [Google Scholar]
- Segall PE, Timiras PS, 1976. Patho-physiologic findings after chronic tryptophan deficiency in rats: a model for delayed growth and aging. Mech. Ageing Dev 5, 109–24. [DOI] [PubMed] [Google Scholar]
- Selman C, Phillips T, Staib JL, Duncan JS, Leeuwenburgh C, Speakman JR, 2005. Energy expenditure of calorically restricted rats is higher than predicted from their altered body composition. Mech. Ageing Dev 126, 783–793. 10.1016/j.mad.2005.02.004 [DOI] [PubMed] [Google Scholar]
- Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM, 2010. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature 468, 1100–4. 10.1038/nature09584 [DOI] [PubMed] [Google Scholar]
- She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, Hutson SM, 2007. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab 6, 181–94. 10.1016/j.cmet.2007.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreeram S, Ramesh S, Puthan JK, Balakrishnan G, Subramanian R, Reddy MT, Pereira SL, 2016. Age associated decline in the conversion of leucine to β-Hydroxy-β-Methylbutyrate in rats. Exp. Gerontol 80, 6–11. 10.1016/j.exger.2016.03.021 [DOI] [PubMed] [Google Scholar]
- Simpson SJ, Le Couteur DG, James DE, George J, Gunton JE, Solon-Biet SM, Raubenheimer D, 2017. The Geometric Framework for Nutrition as a tool in precision medicine. Nutr. Heal. aging 4, 217–226. 10.3233/NHA-170027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluijs I, Beulens JWJ, van der A DL, Spijkerman AMW, Grobbee DE, van der Schouw YT, 2010. Dietary intake of total, animal, and vegetable protein and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-NL study. Diabetes Care 33, 43–8. 10.2337/dc09-1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solerte SB, Fioravanti M, Locatelli E, Bonacasa R, Zamboni M, Basso C, Mazzoleni A, Mansi V, Geroutis N, Gazzaruso C, 2008a. Improvement of Blood Glucose Control and Insulin Sensitivity During a Long-Term (60 Weeks) Randomized Study with Amino Acid Dietary Supplements in Elderly Subjects with Type 2 Diabetes Mellitus. Am. J. Cardiol 101, S82–S88. 10.1016/J.AMJCARD.2008.03.006 [DOI] [PubMed] [Google Scholar]
- Solerte SB, Gazzaruso C, Bonacasa R, Rondanelli M, Zamboni M, Basso C, Locatelli E, Schifino N, Giustina A, Fioravanti M, 2008b. Nutritional Supplements with Oral Amino Acid Mixtures Increases Whole-Body Lean Mass and Insulin Sensitivity in Elderly Subjects with Sarcopenia. Am. J. Cardiol 101, S69–S77. 10.1016/j.amjcard.2008.03.004 [DOI] [PubMed] [Google Scholar]
- Solon-Biet SM, Cogger VC, Pulpitel T, Heblinski M, Wahl D, McMahon AC, Warren A, Durrant-Whyte J, Walters KA, Krycer JR, Ponton F, Gokarn R, Wali JA, Ruohonen K, Conigrave AD, James DE, Raubenheimer D, Morrison CD, Le Couteur DG, Simpson SJ, 2016. Defining the Nutritional and Metabolic Context of FGF21 Using the Geometric Framework. Cell Metab 24, 555–565. 10.1016/j.cmet.2016.09.001 [DOI] [PubMed] [Google Scholar]
- Solon-Biet SM, McMahon AC, Ballard JWO, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, Gokarn R, Khalil M, Turner N, Cooney GJ, Sinclair DA, Raubenheimer D, Le Couteur DG, Simpson SJ, 2014. The Ratio of Macronutrients, Not Caloric Intake, Dictates Cardiometabolic Health, Aging, and Longevity in Ad Libitum-Fed Mice. Cell Metab 19, 418–430. 10.1016/j.cmet.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda J, Chen MZ, Baruch A, 2017. FGF21-receptor agonists: an emerging therapeutic class for obesity-related diseases. Horm. Mol. Biol. Clin. Investig 30 10.1515/hmbci-2017-0002 [DOI] [PubMed] [Google Scholar]
- Sosulski FW, Imafidon GI, 1990. Amino acid composition and nitrogen-to-protein conversion factors for animal and plant foods. J. Agric. Food Chem 38, 1351–1356. 10.1021/jf00096a011 [DOI] [Google Scholar]
- Stone KP, Wanders D, Orgeron M, Cortez CC, Gettys TW, 2014. Mechanisms of Increased In Vivo Insulin Sensitivity by Dietary Methionine Restriction in Mice. Diabetes 63, 3721–3733. 10.2337/db14-0464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Komatsu T, Lim J, Laslo M, Yolitz J, Wang C, Poirier L, Alberico T, Zou S, 2012. Nutrient-dependent requirement for SOD1 in lifespan extension by protein restriction in Drosophila melanogaster. Aging Cell 11, 783–793. 10.1111/j.1474-9726.2012.00842.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran CM, Mukherjee S, Ye L, Frederick DW, Kissig M, Davis JG, Lamming DW, Seale P, Baur JA, 2016. Rapamycin Blocks Induction of the Thermogenic Program in White Adipose Tissue. Diabetes 65, 927–41. 10.2337/db15-0502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S, Sitzmann JM, Dastidar SG, Rodriguez AA, Vu SL, McDonald CE, Academia EC, O’Leary MN, Ashe TD, La Spada AR, Kennedy BK, 2015. Muscle-specific 4E-BP1 signaling activation improves metabolic parameters during aging and obesity. J. Clin. Invest 125, 2952–64. 10.1172/JCI77361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsun Z-Y, Bar-Peled L, Chantranupong L, Zoncu R, Wang T, Kim C, Spooner E, Sabatini DM, 2013. The Folliculin Tumor Suppressor Is a GAP for the RagC/D GTPases That Signal Amino Acid Levels to mTORC1. Mol. Cell 52, 495–505. 10.1016/j.molcel.2013.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vattem KM, Wek RC, 2004. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl. Acad. Sci. U. S. A 101, 11269–74. 10.1073/pnas.0400541101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitvitsky V, Martinov M, Ataullakhanov F, Miller RA, Banerjee R, 2013. Sulfur-based redox alterations in long-lived Snell dwarf mice. Mech. Ageing Dev 134, 321–30. 10.1016/j.mad.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada S, Neinast M, Jang C, Ibrahim YH, Lee G, Babu A, Li J, Hoshino A, Rowe GC, Rhee J, Martina JA, Puertollano R, Blenis J, Morley M, Baur JA, Seale P, Arany Z, 2016. The tumor suppressor FLCN mediates an alternate mTOR pathway to regulate browning of adipose tissue. Genes Dev 30, 2551–2564. 10.1101/gad.287953.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walford RL, Harrist SB, Guniont MW, 1992. The calorically restricted low-fat nutrient-dense diet in Biosphere 2 significantly lowers blood glucose, total leukocyte count, cholesterol, and blood pressure in humans. Med. Sci 89, 11533–11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders D, Burk DH, Cortez CC, Van NT, Stone KP, Baker M, Mendoza T, Mynatt RL, Gettys TW, 2015. UCP1 is an essential mediator of the effects of methionine restriction on energy balance but not insulin sensitivity. FASEB J 29, 2603–2615. 10.1096/fj.14-270348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders D, Forney LA, Stone KP, Burk DH, Pierse A, Gettys TW, 2017. FGF21 Mediates the Thermogenic and Insulin-Sensitizing Effects of Dietary Methionine Restriction but Not Its Effects on Hepatic Lipid Metabolism. Diabetes 66, 858–867. 10.2337/db16-1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders D, Stone KP, Forney LA, Cortez CC, Dille KN, Simon J, Xu M, Hotard EC, Nikonorova IA, Pettit AP, Anthony TG, Gettys TW, 2016. Role of GCN2-Independent Signaling Through a Noncanonical PERK/NRF2 Pathway in the Physiological Responses to Dietary Methionine Restriction. Diabetes 65, 1499–510. 10.2337/db15-1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Tsun ZY, Wolfson RL, Shen K, Wyant GA, Plovanich ME, Yuan ED, Jones TD, Chantranupong L, Comb W, Wang T, Bar-Peled L, Zoncu R, Straub C, Kim C, Park J, Sabatini BL, Sabatini DM, 2015. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science (80-.) 347, 188–194. 10.1126/science.1257132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O’Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE, 2011. Metabolite profiles and the risk of developing diabetes. Nat. Med 17, 448–453. 10.1038/nm.2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Walford RL, 1982. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science 215, 1415–8. 10.1126/SCIENCE.7063854 [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL, Fligiel S, Guthrie D, 1986. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J. Nutr 116, 641–654. [DOI] [PubMed] [Google Scholar]
- Wek RC, Jackson BM, Hinnebusch AG, 1989. Juxtaposition of domains homologous to protein kinases and histidyl-tRNA synthetases in GCN2 protein suggests a mechanism for coupling GCN4 expression to amino acid availability. Proc. Natl. Acad. Sci. U. S. A 86, 4579–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek SA, Zhu S, Wek RC, 1995. The histidyl-tRNA synthetase-related sequence in the eIF-2 alpha protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Mol. Cell. Biol 15, 4497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White BD, Porter MH, Martin RJ, 2000. Effects of age on the feeding response to moderately low dietary protein in rats. Physiol. Behav 68, 673–681. 10.1016/S0031-9384(99)00229-2 [DOI] [PubMed] [Google Scholar]
- White PJ, Lapworth AL, An J, Wang L, McGarrah RW, Stevens RD, Ilkayeva O, George T, Muehlbauer MJ, Bain JR, Trimmer JK, Brosnan MJ, Rolph TP, Newgard CB, 2016. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol. Metab 5, 538–551. 10.1016/j.molmet.2016.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widner B, Leblhuber F, Fuchs D, 2002. Increased neopterin production and tryptophan degradation in advanced Parkinson’s disease. J. Neural Transm 109, 181–189. 10.1007/s007020200014 [DOI] [PubMed] [Google Scholar]
- Wijeyesekera A, Selman C, Barton RH, Holmes E, Nicholson JK, Withers DJ, 2012. Metabotyping of Long-Lived Mice using 1H NMR Spectroscopy. J. Proteome Res 11, 2224–2235. 10.1021/pr2010154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson RL, Chantranupong L, Saxton RA, Shen K, Scaria SM, Cantor JR, Sabatini DM, 2016. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science (80-.) 351, 43–48. 10.1126/science.aab2674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson RL, Chantranupong L, Wyant GA, Gu X, Orozco JM, Shen K, Condon KJ, Petri S, Kedir J, Scaria SM, Abu-Remaileh M, Frankel WN, Sabatini DM, 2017. KICSTOR recruits GATOR1 to the lysosome and is necessary for nutrients to regulate mTORC1. Nature 543, 438–442. 10.1038/nature21423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson RL, Sabatini DM, 2017. The Dawn of the Age of Amino Acid Sensors for the mTORC1 Pathway. Cell Metab 26, 301–309. 10.1016/j.cmet.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zhao L, Chen Z, Ji X, Qiao X, Jin Y, Liu W, 2016. FLCN Maintains the Leucine Level in Lysosome to Stimulate mTORC1. PLoS One 11, e0157100 10.1371/journal.pone.0157100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyant GA, Abu-Remaileh M, Wolfson RL, Chen WW, Freinkman E, Danai LV, Vander Heiden MG, Sabatini DM, 2017. mTORC1 Activator SLC38A9 Is Required to Efflux Essential Amino Acids from Lysosomes and Use Protein as a Nutrient. Cell 171, 642–654. e12 10.1016/j.cell.2017.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F, Huang Z, Li H, Yu J, Wang C, Chen S, Meng Q, Cheng Y, Gao X, Li J, Liu Y, Guo F, 2011. Leucine deprivation increases hepatic insulin sensitivity via GCN2/mTOR/S6K1 and AMPK pathways. Diabetes 60, 746–56. 10.2337/db10-1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F, Yu J, Guo Y, Deng J, Li K, Du Y, Chen S, Zhu J, Sheng H, Guo F, 2014. Effects of individual branched-chain amino acids deprivation on insulin sensitivity and glucose metabolism in mice. Metabolism 63, 841–850. 10.1016/J.METABOL.2014.03.006 [DOI] [PubMed] [Google Scholar]
- Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li Y-S, Lindberg RA, Chen J-L, Jung DY, Zhang Z, Ko H-J, Kim JK, Véniant MM, 2009. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58, 250–9. 10.2337/db08-0392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Palm W, Peng M, King B, Lindsten T, Li MO, Koumenis C, Thompson CB, 2015. GCN2 sustains mTORC1 suppression upon amino acid deprivation by inducing Sestrin2. Genes Dev 29, 2331–6. 10.1101/gad.269324.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Yang SE, Miller BR, Wisinski JA, Sherman DS, Brinkman JA, Tomasiewicz JL, Cummings NE, Kimple ME, Cryns VL, Lamming DW, 2018. Short-term methionine deprivation improves metabolic health via sexually dimorphic, mTORC1-independent mechanisms. FASEB J fj.201701211R. 10.1096/fj.201701211R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Yoon S-O, Poulogiannis G, Yang Q, Ma XM, Villén J, Kubica N, Hoffman GR, Cantley LC, Gygi SP, Blenis J, 2011. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science 332, 1322–6. 10.1126/science.1199484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Ceglarek U, Huang T, Li L, Rood J, Ryan DH, Bray GA, Sacks FM, Schwarzfuchs D, Thiery J, Shai I, Qi L, 2016. Weight-loss diets and 2-y changes in circulating amino acids in 2 randomized intervention trials. Am. J. Clin. Nutr 103, 505–511. 10.3945/ajcn.115.117689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong M, Zhao X, Li J, Yuan W, Yan G, Tong M, Guo S, Zhu Y, Jiang Y, Liu Y, Jiang Y, 2016. Tumor Suppressor Folliculin Regulates mTORC1 through Primary Cilia. J. Biol. Chem 291, 11689–11697. 10.1074/jbc.M116.719997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Li J, Chen Y, Wang JJ, Ratan R, Zhang SX, 2012. Activation of Endoplasmic Reticulum Stress by Hyperglycemia Is Essential for Muller Cell-Derived Inflammatory Cytokine Production in Diabetes. Diabetes 61, 492–504. 10.2337/db11-0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM, 2011. mTORC1 Senses Lysosomal Amino Acids Through an Inside-Out Mechanism That Requires the Vacuolar H+-ATPase. Science (80-.) 334, 678–683. 10.1126/science.1207056 [DOI] [PMC free article] [PubMed] [Google Scholar]