HDAC signaling in neuronal development and axon regeneration (original) (raw)
. Author manuscript; available in PMC: 2015 Aug 1.
Published in final edited form as: Curr Opin Neurobiol. 2014 Apr 12;0:118–126. doi: 10.1016/j.conb.2014.03.008
Summary
The development and repair of the nervous system requires the coordinated expression of a large number of specific genes. Epigenetic modifications of histones represent an essential principle by which neurons regulate transcriptional responses and adapt to environmental cues. The post-translational modification of histones by chromatin-modifying enzymes histone acetyltransferases (HATs) and histone deacetylases (HDACs) shapes chromatin to adjust transcriptional profiles during neuronal development. Recent observations also point to a critical role for histone acetylation and deacetylation in the response of neurons to injury. While HDACs are mostly known to attenuate transcription through their deacetylase activity and their interaction with co-repressors, these enzymes are also found in the cytoplasm where they display transcription-independent activities by regulating the function of diverse proteins. Here we discuss recent studies that go beyond the traditional use of HDAC inhibitors and have begun to dissect the roles of individual HDAC isoforms in neuronal development and repair after injury.
Introduction
Epigenetic changes refer to the modifications of chromatin, including histone and DNA that contribute to regulate the transcriptional response. One of the best-characterized epigenetic modifications is lysine acetylation of histones, which is mediated by two groups of enzymes, HATs and HDACs. The C-terminal tails of histones are normally positively charged to condense the DNA structure, thereby repressing gene expression. Whereas acetylation mediated by HATs opens the chromatin and allows transcription, HDACs have a general repressive effect on gene expression, restricting transcription factor access to regulatory regions [1,2]. HDACs can also have direct roles in transcription by deacetylating and regulating transcription factors or interacting with co-repressors [1,2]. In addition to these HDACs’ roles in regulating gene transcription, the acetylation and deacetylation of lysine residues is emerging as a mechanism analogous to phosphorylation to control the function, activity and stability of various proteins beyond histones. HDACs thus have the potential to be involved in multiple aspects of neuronal development and repair.
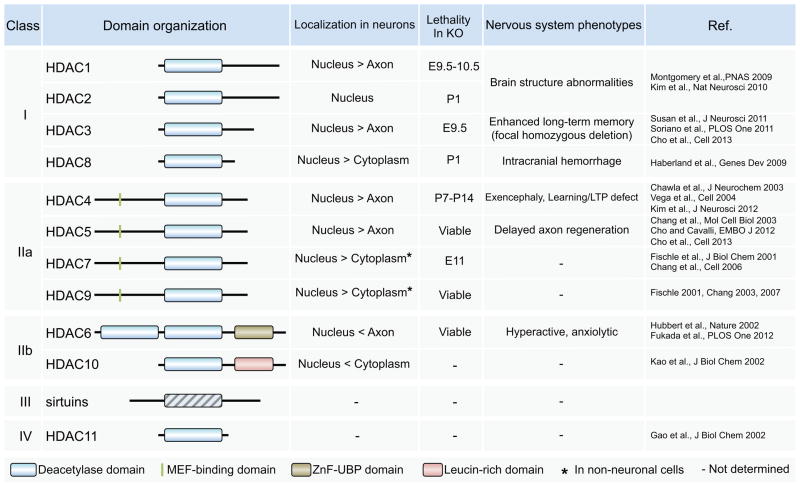
Mammalian HDACs are organized into a super-family of four classes based on domain organization (Figure 1) [1,3]. Class I members (HDAC1, -2, -3 and -8) are ubiquitously expressed with predominant nuclear localization. Class II and class IV members are more selectively expressed and can undergo stimulus-dependent shuttling between nucleus and cytoplasm. Class II members can be further subdivided into two classes. Class IIa members (HDAC4, -5, -7, and -9) contain, in addition to the HDAC domain, an extended N-terminal regulatory domain [4]. Class IIb is represented by HDAC6 and HDAC10, which possess distinct C-terminal domains. HDAC6 expression is mostly cytoplasmic and was the first identified tubulin deacetylase [5]. HDAC11, the only Class IV HDAC, has characteristics of both class I and class II HDACs, although little is known of its function. The sirtuins family of deacetylases represents class III but these are functionally unrelated to HDACs: their deacetylase activity depends on the co-factor NAD+, whereas other HDACs are known as zinc-dependent histone deacetylases. All HDACs classes display high structural conservation of their active sites. A catalytic tyrosine is conserved in all HDACs except for vertebrate class IIa enzymes where it is replaced by histidine [4], strongly reducing their catalytic activity. It has been suggested that vertebrate class IIa HDACs may have evolved to efficiently process restricted sets of specific substrates [4].
Figure 1. Comparison of classes I, II, III and IV HDAC protein structure and subcellular localization.
All HDACs contain a highly conserved catalytic domain known as zinc-dependent histone deacetylases. Class IIa enzymes are characterized by an N-terminal extension not found in other classes I. The sirtuins represents class III but are functionally unrelated to HDACs as their deacetylase activity depends on the co-factor NAD+. HDAC6 is the only HDAC that contains an identical duplication of two catalytic domains. It was traditionally thought that class I HDACs are located in the nucleus, whereas class II HDACs can shuttle between the nucleus and the cytoplasm. However, many studies have shown that specific signal transduction pathways can regulate the cellular localization of various HDACs, including class I HDACs. The localization of HDACs known from studies using neuronal cells, the lethality in total knockout mice and the neuronal phenotypes in loss-of-function mutant is indicated.
Although much has been learned through the use of HDAC inhibitors, recent studies are beginning to reveal the biological function of each of these individual enzymes. In this review we discuss recent findings on the role of HDACs in regulating gene expression during neuronal development and repair following injury, as well as studies that depart from this traditional focus and reveal new cytoplasmic functions for HDACs in neurons. The often divergent roles of specific HDAC family members are discussed. The role of HDACs in synaptic plasticity, brain function and neurodegeneration has been reviewed elsewhere [6–10] and will not be discussed here.
HDAC signaling in neuronal development
Neurogenesis in the developing brain
Neurogenesis is the process of generating new neurons from progenitor cells during development and throughout adulthood, which includes cell proliferation, migration, and differentiation. The differentiation of neuronal progenitor cells to neurons requires the transduction of signals to the genome to de-repress neuron-specific genes. It has been known for several years that HDAC inhibitors induce differentiation of both embryonic and adult cortical neuronal progenitor cells to neurons specifically [11–15]. The regional and cell-type specific expression of individual HDACs in the rat central nervous system (CNS) [16] suggests that each enzymes may play specific roles in neuronal development and emphasizes the need for more specific approaches to study the function of each HDAC isoform.
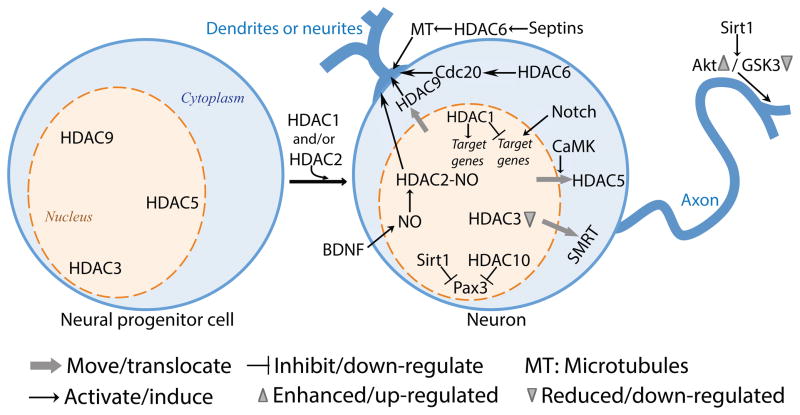
To study the role of HDAC1 in the progression of neural precursors to neurons during brain development, Montgomery et al. used a conditional deletion approach to avoid early lethality associated with global deletion. Using mice expressing Cre recombinase under the control of the human glial fibrillary acidic protein (GFAP) promoter, which is widely expressed in the central nervous system, including neural stem cells, conditional deletion of both HDAC1 and HDAC2 revealed a redundant role for these enzymes in the progression of neuronal precursors to neurons during brain development [17] (Figure 2). Neuronal progenitors lacking HDAC1 and HDAC2 are unable to differentiate into mature neurons and undergo cell death, resulting in defects in superficial cortical layering and death by postnatal day seven [17]. In zebrafish, HDAC1 represses Notch target genes expression during neurogenesis and favors the generation of motor neurons in response to hedgehog signaling [18], as well as to promote retinal neurogenesis [19] (Figure 2). Although HDACs are generally considered to repress gene expression, HDAC1 can also act as a positive regulator of gene transcription during development of the zebrafish CNS [20] (Figure 2). The HDAC1-regulated genes encode DNA-binding transcription factors that are implicated in promoting neuronal specification and CNS patterning, including the proneural bHLH proteins Ascl1, as well as Neurod and Neurod4 [20]. Chromatin immunoprecipitation experiments reveal that HDAC1 is specifically and stably associated with the promoter region of a subset of actively transcribed genes, including Ascl1, thereby promoting the expression of a neurogenic program in zebrafish embryos [20].
Figure 2. The roles of HDACs in neuron development.
The differentiation of neuronal progenitor cells to neurons requires the transduction of signals to the genome to de-repress neuron-specific genes. The nuclear export of HDAC3, HDAC5 and HDAC9 induces neurogenesis and differentiation by activating target genes. HDAC1 and HDAC2 are required for this process as well, in part by silencing progenitor transcripts and by promoting the expression of a neurogenic program. The development of axon and dendrites also depends upon HDACs function. In addition to a traditional role of HDACs in regulating gene expression, HDACs also influence dendrite and axon development through their action on the cytoskeleton (HDAC6) and signaling pathways (Sirt1).
HDAC3 is also a candidate in the control of gene expression during development and maintenance of the neural stem cell state. In rat cortical neurons HDAC3 maintains the nuclear localization of the transcriptional repressor Silencing Mediator of Retinoic acid and Thyroid hormone receptors (SMRT), which controls neuronal responsiveness of several transcription factors [21]. Knockdown of HDAC3 or inhibition of HDAC3 with valproate or apicidin triggers SMRT nuclear export [21] (Figure 2). Given that SMRT can regulate neurogenic pathways [21] future studies may reveal whether HDAC3 also controls neurogenesis.
These studies clearly point to a role for class I members HDACs in developmental neurogenesis through the control of gene expression. Other HDAC classes are likely involved in regulating this important developmental stage. Indeed, HDAC10 has been shown to interact with and maintain Pax3, a key transcription factor in stem cell maintenance and neurogenesis, in a deacetylated state [22] (Figure 2). Pax3 acetylation regulates its function by favoring Neurog2 transcription over Hes1 and thereby promoting neuron differentiation [23,24]. Interestingly, Sirt1 was also proposed to be a key component in regulating Pax3 acetylation levels [24].
Adult neurogenesis
In adult rodents, neurogenesis occurs mostly in the subventricular zone of the lateral ventricle and the subgranular zone of the hippocampal dentate gyrus. The distinct expression pattern of HDACs in the adult rat brain [16] suggests that HDAC isoforms expression may also be developmentally regulated. Hence, does adult neurogenesis employ the same battery of epigenetic mechanisms as developmental neurogenesis? Studies on the role of HDAC2 would argue that they do not. Indeed, the catalytic function of HDAC2 is required in adult brain, but not during embryonic neurogenesis [25] when HDAC1 can compensate for the lack of HDAC2 [17]. In mice lacking the catalytic activity of HDAC2 or in mice with conditional deletion of HDAC2, neurons derived from adult neurogenesis die at a specific maturation stage, whereas brain development and adult stem cell fate are normal [25]. This study suggests that HDAC2 is critically required to silence progenitor transcripts during neuronal differentiation of adult generated neurons [25]. In agreement with specific role for HDACs in adult neurogenesis, different subsets of neural stem cells and progenitors switch expression from HDAC1 to HDAC2 as they commit to a neurogenic lineage in the subventricular zone and the dentate gyrus [26], with HDAC1 enriched in neural stem cells and HDAC2 in neuroblasts. This study further demonstrates that HDAC inhibitors trichostatin (TSA) and valproate perturb postnatal neurogenesis in vivo and profoundly inhibit the production and expansion of neurospheres in vitro [26]. Together, these studies highlight the pleiotropic activities of HDACs in brain development.
The class II HDAC5 has also been implicated in neuronal differentiation. In a screen for small molecules that induce a cardiogenic phenotype, Schneider et al. found a molecule, isoxazole, that triggers robust neuronal differentiation in adult neural stem cells via a mechanism involving calmodulin kinase-dependent nuclear export of HDAC5 and MEF2-dependent gene expression [27] (Figure 2). Given that nuclear export of class II HDACs can be trigggered in response to extracellular stimuli, other class II HDACs may be involved in activity-dependent adult neurogenesis.
In summary, both embryonic and adult neurogenesis engage HDAC family members to control gene expression and cell fate. The role of HDACs in directly controlling the activity of transcription factors is becoming apparent. Importantly, these studies emphasize that the clinical use of HDAC inhibitors to treat diseases ranging from schizophrenia to neurodegeneration could impair ongoing brain development and should be carefully considered.
Neurite formation, dendrite and axon growth
Breaking the symmetry of neurons to form axon and dendrites depends on cytoskeletal rearrangements [28]. Several HDAC family members deacetylate components of the microtubule and actin cytoskeleton, tubulin [5] and cortactin [29], respectively, and can impact neurite formation. Indeed, HDAC6 control microtubule dynamics to optimize neurite formation through septins, which provide a physical scaffold for HDAC6 to achieve efficient microtubule deacetylation [30]. Acute knockdown or knockout of septin7 in cerebrocortical neurons results in excessive stabilization of microtubules which inhibits their growth in vitro and impairs axon growth in vivo [30].
Compared to the mechanisms regulating axon development, relatively little is known about the mechanisms regulating the patterning of dendrites, which is essential for the establishment of neuronal connectivity. Several studies revealed that HDACs play an important role in dendrite development. Knock down of HDAC6 in cerebellar and hippocampal neurons decreases dendrite length and dendritic branching, suggesting that HDAC6 has a generalized dendritogenic function [31]. Interestingly, this HDAC6 function is independent of its deacetylase activity and rather requires a direct interaction between HDAC6 ZnF domain (Figure 1) and Csc20-APC, which stimulates Cdc20-APC activity via stabilization of Cdc20 polyubiquitination [31]. The observations that Aurora A kinase activates HDAC6 at the basal body to disassemble cliliary microtubules [32] and that Aurora A pathway regulates microtubule remodeling during neurite extension [33] suggest that HDAC6 could also regulate dendrite development via the Aurora kinase.
Using the Drosophila olfactory system, Tea et al. found a role for HDAC1 and HDAC2 in dendrite wiring. Rpd3, which is homologous to mammalian HDAC1 and HDAC2, but not, HDAC3, the only other class I HDAC in the fly, regulates dendrite targeting largely through the action of the transcription factor Pros [34]. In rat cortical neurons, HDAC2 regulates dendrite development in response to neurotrophic factors. Brain derived neurotrophic factor (BDNF) triggers nitric oxide synthesis and S-nitrosylation of HDAC2, resulting in dendritic growth and branching [35] (Figure 2). S-nitrosylation of HDAC2 does not affect its deacetylase activity, but induces its release from chromatin, which in turn increases acetylation of histones surrounding neurotrophin-dependent gene promoters [35]. Recent studies also indicate that the herbal derivative genipin increases S-nitrosylation of HDAC2 and has both neuroprotective and neurite outgrowth activities in retinal ganglion cells [36]. S-nitrosylation of HDAC2 occurs on cysteine residues (Cys!262 and Cys!274) that are conserved in HDAC1 and HDAC3, suggesting that nitrosylation might also control their function. HDAC9 activity has also been implicated in the regulation of dendrite development. In cultured mouse cortical neurons, increase of spontaneous firing activity causes HDAC9 translocation from nucleus to cytoplasm during postnatal development [37]. Introduction of a mutant HDAC9 that is trapped in the nucleus decreases the length of dendritic branches, whereas knockdown of HDAC9 promoted dendritic growth [37]. Although these studies point to HDAC9 functioning as a transcriptional repressor, whether HDAC9 also plays a role outside the nucleus to regulate dendrite growth remains to be determined.
Axon Regeneration: Axonal roles of HDACs
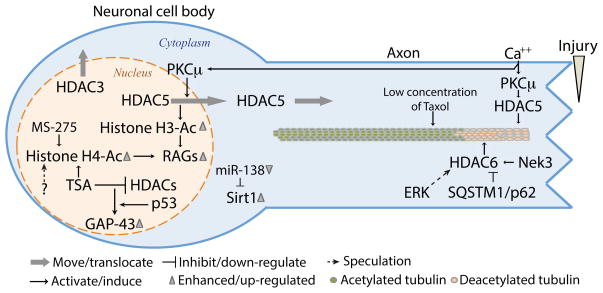
Injured axons need to transform their damaged axon tip into a new growth cone to initiate regeneration, a process that results in part from the reorganization and dynamic properties of the local microtubule cytoskeleton [38,39]. Studies in our laboratory revealed that HDAC5 controls microtubule dynamics in injured dorsal root ganglia (DRG) neurons [40]. Injury-induced calcium influx activates HDAC5 to decrease the level of acetylated tubulin, known to correlate with stable microtubules, in a gradient-like fashion from the site of injury. This pathway, which is not shared by axons in the CNS, locally decreases microtubule stability needed for axon regeneration [40] (Figure 3). It is worth noting that heightening microtubule stabilization with taxol treatment increases axon regeneration in the CNS [38,41,42], and induces axon formation in cultured neurons [43]. One explanation could be that in the injured CNS, stabilizing microtubules fortifies the microtubule array at the tip of the axon to generate more powerful driving force through the inhibitory environment, whereas in the PNS, local and controlled destabilization of the microtubule network optimizes growth cone dynamics.
Figure 3. The roles of HDACs in axon regeneration.
Axon injury triggers multiple events that engage several HDACs locally at the site of injury as well as distantly in the cell soma. In the injured axon, HDAC5 and HDAC6 regulate the necessary changes in the microtubule cytoskeleton to optimize growth cone dynamics and axon re-growth. Whereas HDAC5 activity is required for re-growth on permissive substrates, HDAC6 prevents growth on inhibitory substrates mimicking the environment of the injured CNS. In the cell soma, injury-induced nuclear export of HDAC5 and HDAC3 elicits changes in the epigenetic landscape that are required to activate a pro-regenerative gene expression program.
Whereas HDAC5 is involved in injury-induced tubulin deacetylation and axon regeneration in DRG neurons, HDAC6 does not play a major role in this process [40]. In contrast, when DRG neurons are cultured in the presence of myelin-associated glycoprotein to mimic the inhibitory environment of the CNS, inhibiting HDAC6 enhances tubulin acetylation levels and promotes axon regeneration [44] (Figure 3). This is intriguing because inhibition of HDAC6 in hippocampal neurons increases tubulin acetylation, but does not affect axon growth properties [43]. Similarly, mice lacking HDAC6 display hyperacetylated microtubules but are otherwise viable with no apparent developmental defects [45]. Interestingly, HDAC6, but no other class II HDACs, is robustly up-regulated in cultured cortical neurons undergoing oxidative stress or neurite growth inhibition [44]. These studies suggest that HDAC6 may play a specific role following axon injury or in other pathological conditions in the CNS, but not during normal neuronal development or in the peripheral nervous system (PNS).
The apparent opposite roles of HDAC5 and HDAC6 may also result from different substrate specificity beyond tubulin. Indeed, unlike HDAC5, HDAC6 has two deacetylase domains (Figure 1), which are required for its interaction with the F-actin-binding protein cortactin [29]. Cortactin deacetylation increases its affinity with F-actin and enhances actin-dependent cell motility [29]. The role of cortactin in growth cone morphology and spreading in hippocampal neurons [46] suggests that HDAC6 regulation of axon regeneration may involve actin-based mechanisms. HDAC6 also possesses a ZnF-UBP C-terminal domain that mediates its interaction with the Cdc20-APC complex to regulate dendrite development [31] and possibly axon growth [47].
The upstream signals regulating HDAC5 and HDAC6 activities are also likely to differ. Whereas HDAC5 activity in injured DRG neurons depends on calcium activated PKCμ [40], whether and how HDAC6 activity towards tubulin or other substrates is regulated to control growth on inhibitory substrates remains elusive. The extracellular signal-regulated kinase (ERK) may represent a candidate regulator of HDAC6. ERK-mediated phosphorylation of HDAC6 promotes migration of embryonic fibroblasts via deacetylation of α-tubulin [48] and axon injury was shown to activate ERK [49]. Another candidate regulator of HDAC6 involves the scaffolding protein sequestosome 1/p62, which was shown to negatively regulate HDAC6 deacetylase activity [50]. In the absence of p62, HDAC6 is hyper-activated and deacetylation of α-tubulin and cortactin occurs [50]. Finally, the NIMA-related kinases Nek3 was also shown to control HDAC6-dependent deacetylation of microtubules in neurons [51]. Future studies will elucidate the signaling pathways controlling HDAC6 activity, and define the protein ensembles regulated by HDAC5 and HDAC6 in axons. These findings may shed light on the apparent opposite functions these enzymes play in axon regeneration.
Axon Regeneration: Nuclear roles of HDACs
Studies based on HDAC inhibitors have suggested a role for histone acetylation, or the lack thereof, in the poor regenerative ability of CNS neurons. HDAC inhibition was shown to improve locomotion in vivo after spinal cord injury [52], improve regeneration in injured retinal ganglion cells [53], partially overcome neurite outgrowth of cerebellar granule neurons cultured on inhibitory substrates [54] and increase regeneration of ascending sensory axons following spinal cord injury [55]. These studies suggest that if provided with the appropriate epigenetic landscape, CNS neurons can express the genes required to stimulate axon regeneration. In contrast, peripheral neurons possess the intrinsic ability to increase histone acetylation in response to injury. Our laboratory revealed a calcium-initiated signaling mechanism in DRG neurons that elicits HDAC5 nuclear export via PKCμ activation, thereby increasing the level of acetylated histone H3 and accelerating axon regeneration via expression of pro-regenerative genes [56] (Figure 3). In addition to histone H3 acetylation, Finelli et al. showed that injury to the peripherally projecting DRG axons induces histone H4 acetylation, which also correlates with expression of regeneration-associated genes [55] (Figure 3). Whether acetylation of histone H4 in response to injury also depends on the calcium-PKC-HDAC5 pathway remains to be determined.
CNS neurons appear unable to respond to incoming injury signals and elicit the appropriate epigenetic response. Indeed, key elements of the pathway leading to HDAC5 nuclear export in peripheral neurons fail to be activated in retinal ganglion cells (RGCs) [56], suggesting that failure to export HDAC5 in these cells limits their regenerative ability. Similarly, injury to the sensory axon ascending into the spinal cord fails to increase histone H4 acetylation in DRG neurons [55]. Furthermore, whereas HDAC3 is exported to the cytoplasm in injured DRG [56], HDAC3 accumulates in the nucleus of injured RGCs [57], which may contribute to decrease histone acetylation and repress gene transcription. Interestingly, in an HDAC5 knockout mouse, HDAC3 localizes mostly to the nucleus of injured DRG neurons, rather than to the cytoplasm in the wild type [56]. It appears that in response to injury, HDAC5 knockout DRG neurons behave similarly to RGCs, with respect to HDCA3 localization. Could HDAC5 be required for injury-induced HDAC3 nuclear export? The known interaction between HDAC5 and HDAC3 [58] suggests that injury-induced HDAC5 nuclear export may be coupled to HDAC3 localization.
Which genes are associated with increased histone acetylation to control axon regeneration? Using chromatin immunoprecipitation approaches, inhibition of HDACs with TSA in cerebellar neurons was found to induce expression of the well-known axonal growth associated marker GAP-43, as well as the HATs, CBP, p300 and P/CAF [54]. With a similar approach, Finelli et al. found that in DRG neurons, injury-induced histone H4 acetylation is associated with promoter regions of regeneration-associated genes [55], including Smad1, Atf3, Sprr1a. Interestingly, inhibition of HDAC1/3 with MS-275 treatment did not increase the acetylated levels of histone H4 at the promoters of Smad1 or Atf3 [55]. By creating a mutant HDAC5 that is trapped in the nucleus (HDAC5nuc), our laboratory compared the effects of injury in wild type and HDAC5nuc-expressing DRG neurons and identified a number of genes that are activated by injury in manner that depends on HDAC5 nuclear export, including several transcription factors implicated in the regenerative response, such as jun, fos, KLFs, Gadd45a [56]. A large proportion of the HDAC5-dependent genes have prominent roles in the regulation of transcription [56], suggesting that HDAC5 controls an early response phase to injury. These studies emphasize that multiple regulatory mechanisms control the epigenetic landscape of injured neurons. The activity of multiple HDACs likely converges to regulate a favorable, pro-regenerative epigenetic landscape around regeneration-associated gene promoters. Furthermore, HDACs can deacetylate transcription factors in addition to histones and inhibit transcription via interaction with co-repressors, providing an additional level of transcriptional control in injured neurons. Future studies are needed to explore how the nuclear localization and activity of multiple HDACs is regulated in response to injury, which may in part underlies the epigenetic regenerative ability of central and peripheral neurons.
Sirtuins in neuronal development and regeneration
The sirtuins family of deacetylases also influences neuronal development and repair. During neurogenesis, the Notch pathway represses neuron-specific genes [59] and hence impairs the transition of neuronal progenitors to neurons. The oncogene BCL6 allows epigenetic silencing of Hes5 and hence neuronal differentiation despite active Notch signaling by recruiting Sirt1 and excluding the co-activator Mastermind-like 1 at Hes promoter region [60].
During neurite formation, Sirt2 controls the levels of tubulin acetylation. Sirt2 overexpression reduces the levels of acetylated, stable, microtubules and strongly impairs neurite formation in cultured hippocampal neurons [61]. In contrast to Sirt2, up-regulation of Sirt1 promotes the formation and elongation of axons, whereas inhibition of Sirt1 retards axonal development in hippocampal neurons [62]. Sirt1’s effect in axon development relies on its deacetylase activity towards Akt and thereby activating the Akt/GSK3 pathway [62].
Sirt1 also plays a role in injured axons to stimulate axon regeneration. Sirt1 is the target of miR-138 in DRG neurons and down-regulation of miR-138 after axon injury correlates with an increase in Sirt1 levels, which is required for axon regeneration [63]. The precise mechanism by which Sirt1 regulates axon regeneration awaits further clarifications, but given the role of Sirt1 in deacetylating cortactin [64], Sirt1 may increase actin-dependent motility [29].
Conclusion
The complexity of HDACs’ functions during development and in mature neurons is rapidly coming to light. It is clear that HDAC family members have very specific functions and that studies using HDAC inhibitors need careful interpretation. It is also clear that different roles of HDACs are isoform-specific, rather than class-specific. Furthermore, a given HDAC can also play different roles depending on the developmental stage. The ability of HDACs to influences other histone modifications will also have to be considered, since HDACs regulate other histone post-translational modifications, i.e. by interacting with DNA methyltransferases [65]. Given that epigenetic regulation affects globally, yet specifically, an ensemble of genes, targeting specific HDAC pathways represent an ideal strategy to promote neural repair. Therefore, identifying the HDAC-dependent epigenetic signaling mechanisms involved in neuronal development and repair will be critical to our understanding and approaches to the treatment of injuries in the nervous system. The emerging functions of HDACs as regulatory enzymes in the cytoplasm will also need to be carefully investigated and may lead to new strategies to improve axon growth and repair following damage.
Highlights.
- HDACs function in the differentiation of neuronal progenitor cells to neurons during development as well as in the adult brain.
- HDAC family members have very specific functions: different roles of HDACs are isoform-specific rather than class-specific.
- In injured neurons, HDACs function locally at the site of injury to control cytoskeleton dynamics and in the nucleus to activate a pro-regenerative program.
- HDAC family members emerge as regulatory enzymes in the cytoplasm that can also function independently of their deacetylase activity.
Acknowledgments
We thank Drs. Andrew Yoo and Vitaly Klyachko for helpful discussions and for critical reading of the manuscript. We thank members of the Cavalli lab for helpful comments. This work was supported in part by grants from NIH (DE022000 and NS082446), and from the University of Missouri Spinal Cord Injuries Research Program (to VC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J Mol Biol. 2004;338:17–31. doi: 10.1016/j.jmb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang XJ, Seto E. The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat Rev Mol Cell Biol. 2008;9:206–218. doi: 10.1038/nrm2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lahm A, Paolini C, Pallaoro M, Nardi MC, Jones P, Neddermann P, Sambucini S, Bottomley MJ, Lo Surdo P, Carfi A, et al. Unraveling the hidden catalytic activity of vertebrate class IIa histone deacetylases. Proc Natl Acad Sci U S A. 2007;104:17335–17340. doi: 10.1073/pnas.0706487104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 6.Riccio A. Dynamic epigenetic regulation in neurons: enzymes, stimuli and signaling pathways. Nat Neurosci. 2010;13:1330–1337. doi: 10.1038/nn.2671. [DOI] [PubMed] [Google Scholar]
- 7.Ronan JL, Wu W, Crabtree GR. From neural development to cognition: unexpected roles for chromatin. Nat Rev Genet. 2013;14:347–359. doi: 10.1038/nrg3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dulac C. Brain function and chromatin plasticity. Nature. 2010;465:728–735. doi: 10.1038/nature09231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pirooznia SK, Elefant F. Targeting specific HATs for neurodegenerative disease treatment: translating basic biology to therapeutic possibilities. Front Cell Neurosci. 2013;7:30. doi: 10.3389/fncel.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mielcarek M, Landles C, Weiss A, Bradaia A, Seredenina T, Inuabasi L, Osborne GF, Wadel K, Touller C, Butler R, et al. HDAC4 Reduction: A Novel Therapeutic Strategy to Target Cytoplasmic Huntingtin and Ameliorate Neurodegeneration. PLoS Biol. 2013;11:e1001717. doi: 10.1371/journal.pbio.1001717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsieh J, Nakashima K, Kuwabara T, Mejia E, Gage FH. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc Natl Acad Sci U S A. 2004;101:16659–16664. doi: 10.1073/pnas.0407643101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaked M, Weissmuller K, Svoboda H, Hortschansky P, Nishino N, Wolfl S, Tucker KL. Histone deacetylases control neurogenesis in embryonic brain by inhibition of BMP2/4 signaling. PLoS One. 2008;3:e2668. doi: 10.1371/journal.pone.0002668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balasubramaniyan V, Boddeke E, Bakels R, Kust B, Kooistra S, Veneman A, Copray S. Effects of histone deacetylation inhibition on neuronal differentiation of embryonic mouse neural stem cells. Neuroscience. 2006;143:939–951. doi: 10.1016/j.neuroscience.2006.08.082. [DOI] [PubMed] [Google Scholar]
- 14.Kim HJ, Leeds P, Chuang DM. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J Neurochem. 2009;110:1226–1240. doi: 10.1111/j.1471-4159.2009.06212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siebzehnrubl FA, Buslei R, Eyupoglu IY, Seufert S, Hahnen E, Blumcke I. Histone deacetylase inhibitors increase neuronal differentiation in adult forebrain precursor cells. Exp Brain Res. 2007;176:672–678. doi: 10.1007/s00221-006-0831-x. [DOI] [PubMed] [Google Scholar]
- 16*.Broide RS, Redwine JM, Aftahi N, Young W, Bloom FE, Winrow CJ. Distribution of histone deacetylases 1–11 in the rat brain. J Mol Neurosci. 2007;31:47–58. doi: 10.1007/BF02686117. This study reveals a comprehensive atlas of all eleven HDAC isoforms in the rat brain. Using high-resolution in situ hybridization and imaging technology, the authors present a quantitative analysis and cell type localization of HDAC isoforms expression. [DOI] [PubMed] [Google Scholar]
- 17.Montgomery RL, Hsieh J, Barbosa AC, Richardson JA, Olson EN. Histone deacetylases 1 and 2 control the progression of neural precursors to neurons during brain development. Proc Natl Acad Sci U S A. 2009;106:7876–7881. doi: 10.1073/pnas.0902750106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunliffe VT. Histone deacetylase 1 is required to repress Notch target gene expression during zebrafish neurogenesis and to maintain the production of motoneurones in response to hedgehog signalling. Development. 2004;131:2983–2995. doi: 10.1242/dev.01166. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi M, Tonou-Fujimori N, Komori A, Maeda R, Nojima Y, Li H, Okamoto H, Masai I. Histone deacetylase 1 regulates retinal neurogenesis in zebrafish by suppressing Wnt and Notch signaling pathways. Development. 2005;132:3027–3043. doi: 10.1242/dev.01881. [DOI] [PubMed] [Google Scholar]
- 20**.Harrison MR, Georgiou AS, Spaink HP, Cunliffe VT. The epigenetic regulator Histone Deacetylase 1 promotes transcription of a core neurogenic programme in zebrafish embryos. BMC Genomics. 2011;12:24. doi: 10.1186/1471-2164-12-24. This study revelas that HDAC1 acts as a positive regulator of gene transcription during development of the zebrafish CNS, in addition to its more well-established function in transcriptional repression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soriano FX, Hardingham GE. In cortical neurons HDAC3 activity suppresses RD4-dependent SMRT export. PLoS One. 2011;6:e21056. doi: 10.1371/journal.pone.0021056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai IL, Lin TP, Yao YL, Lin CY, Hsieh MJ, Yang WM. Histone deacetylase 10 relieves repression on the melanogenic program by maintaining the deacetylation status of repressors. J Biol Chem. 2010;285:7187–7196. doi: 10.1074/jbc.M109.061861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichi S, Nakazaki H, Boshnjaku V, Singh RM, Mania-Farnell B, Xi G, McLone DG, Tomita T, Mayanil CS. Fetal neural tube stem cells from Pax3 mutant mice proliferate, differentiate, and form synaptic connections when stimulated with folic acid. Stem Cells Dev. 2012;21 :321–330. doi: 10.1089/scd.2011.0100. [DOI] [PubMed] [Google Scholar]
- 24.Ichi S, Boshnjaku V, Shen YW, Mania-Farnell B, Ahlgren S, Sapru S, Mansukhani N, McLone DG, Tomita T, Mayanil CS. Role of Pax3 acetylation in the regulation of Hes1 and Neurog2. Mol Biol Cell. 2011;22:503–512. doi: 10.1091/mbc.E10-06-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jawerka M, Colak D, Dimou L, Spiller C, Lagger S, Montgomery RL, Olson EN, Wurst W, Gottlicher M, Gotz M. The specific role of histone deacetylase 2 in adult neurogenesis. Neuron Glia Biol. 2010;6:93–107. doi: 10.1017/S1740925X10000049. [DOI] [PubMed] [Google Scholar]
- 26**.Foti SB, Chou A, Moll AD, Roskams AJ. HDAC inhibitors dysregulate neural stem cell activity in the postnatal mouse brain. Int J Dev Neurosci. 2013;31:434–447. doi: 10.1016/j.ijdevneu.2013.03.008. This study reveals that neural stem cells and progenitors switch expression from HDAC1 to HDAC2 as they commit to a neurogenic lineage. This study also demonstrates that HDAC inhibition perturbs postnatal neurogenesis in vivo and profoundly inhibit the production and expansion of neurospheres in vitro. [DOI] [PubMed] [Google Scholar]
- 27*.Schneider JW, Gao Z, Li S, Farooqi M, Tang TS, Bezprozvanny I, Frantz DE, Hsieh J. Small-molecule activation of neuronal cell fate. Nat Chem Biol. 2008;4:408–410. doi: 10.1038/nchembio.95. This study shows that the small molecules isoxazole induces robust neuronal differentiation in adult neural stem cells via calcium-ion influx, CaMK activation and nuclear export of HDAC5.. The authors suggest that adult neural stem cells may be able to respond to neurotransmission signals despite their immaturity. [DOI] [PubMed] [Google Scholar]
- 28.Tahirovic S, Bradke F. Neuronal polarity. Cold Spring Harb Perspect Biol. 2009;1:a001644. doi: 10.1101/cshperspect.a001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Yuan Z, Zhang Y, Yong S, Salas-Burgos A, Koomen J, Olashaw N, Parsons JT, Yang XJ, Dent SR, et al. HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol Cell. 2007;27:197–213. doi: 10.1016/j.molcel.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Ageta-Ishihara N, Miyata T, Ohshima C, Watanabe M, Sato Y, Hamamura Y, Higashiyama T, Mazitschek R, Bito H, Kinoshita M. Septins promote dendrite and axon development by negatively regulating microtubule stability via HDAC6-mediated deacetylation. Nat Commun. 2013;4:2532. doi: 10.1038/ncomms3532. This study demonstrates that septins provide a physical scaffold to facilitate the interaction between HDAC6 and a-tubulin and to achieve an efficient microtubule dea cetylation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31**.Kim AH, Puram SV, Bilimoria PM, Ikeuchi Y, Keough S, Wong M, Rowitch D, Bonni A. A centrosomal Cdc20-APC pathway controls dendrite morphogenesis in postmitotic neurons. Cell. 2009;136:322–336. doi: 10.1016/j.cell.2008.11.050. This study reveals a role for HDCA6 in stimulating the activity of centrosomal Cdc20-APC, and thus driving the differentiation of dendrites. This HDAC function is independent of its enzymatic activity and involves HDCA6 C-terminal domain ZnF domain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mori D, Yamada M, Mimori-Kiyosue Y, Shirai Y, Suzuki A, Ohno S, Saya H, Wynshaw-Boris A, Hirotsune S. An essential role of the aPKC-Aurora A-NDEL1 pathway in neurite elongation by modulation of microtubule dynamics. Nat Cell Biol. 2009;11:1057–1068. doi: 10.1038/ncb1919. [DOI] [PubMed] [Google Scholar]
- 34.Tea JS, Chihara T, Luo L. Histone deacetylase Rpd3 regulates olfactory projection neuron dendrite targeting via the transcription factor Prospero. J Neurosci. 2010;30:9939–9946. doi: 10.1523/JNEUROSCI.1643-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Nott A, Watson PM, Robinson JD, Crepaldi L, Riccio A. S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature. 2008;455:411–415. doi: 10.1038/nature07238. This study reveals that by stimulating nitric oxide production and S-nitrosylation of HDAC2, neurotrophic factors promote chromatin remodelling and the activation of genes that are associated with neuronal development. [DOI] [PubMed] [Google Scholar]
- 36.Koriyama Y, Takagi Y, Chiba K, Yamazaki M, Sugitani K, Arai K, Suzuki H, Kato S. Requirement of retinoic acid receptor beta for genipin derivative-induced optic nerve regeneration in adult rat retina. PLoS One. 2013;8:e71252. doi: 10.1371/journal.pone.0071252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugo N, Oshiro H, Takemura M, Kobayashi T, Kohno Y, Uesaka N, Song WJ, Yamamoto N. Nucleocytoplasmic translocation of HDAC9 regulates gene expression and dendritic growth in developing cortical neurons. Eur J Neurosci. 2010;31:1521–1532. doi: 10.1111/j.1460-9568.2010.07218.x. [DOI] [PubMed] [Google Scholar]
- 38.Erturk A, Hellal F, Enes J, Bradke F. Disorganized microtubules underlie the formation of retraction bulbs and the failure of axonal regeneration. J Neurosci. 2007;27:9169–9180. doi: 10.1523/JNEUROSCI.0612-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bradke F, Fawcett JW, Spira ME. Assembly of a new growth cone after axotomy: the precursor to axon regeneration. Nat Rev Neurosci. 2012;13:183–193. doi: 10.1038/nrn3176. [DOI] [PubMed] [Google Scholar]
- 40**.Cho Y, Cavalli V. HDAC5 is a novel injury-regulated tubulin deacetylase controlling axon regeneration. Embo J. 2012;31:3063–3078. doi: 10.1038/emboj.2012.160. This study identifies HDAC5 as an injury-regulated tubulin deacetylase, which functions downstream of a calcium-PKC signaling cascade to control growth cone dynamics and axon regeneration. This study thus reveals a signaling mechanism for the spatial control of microtubule modification, and hence dynamics, which are required to promote axon regeneration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sengottuvel V, Leibinger M, Pfreimer M, Andreadaki A, Fischer D. Taxol facilitates axon regeneration in the mature CNS. J Neurosci. 2011;31:2688–2699. doi: 10.1523/JNEUROSCI.4885-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42*.Hellal F, Hurtado A, Ruschel J, Flynn KC, Laskowski CJ, Umlauf M, Kapitein LC, Strikis D, Lemmon V, Bixby J, et al. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science. 2011;331:928–931. doi: 10.1126/science.1201148. This study describes that application of low concentrations of taxol promotes functional improvement after spinal cord injury, by reducing scar formation and also enhancing intrinsic axon growth capacity via microtubule stabilization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Witte H, Neukirchen D, Bradke F. Microtubule stabilization specifies initial neuronal polarization. J Cell Biol. 2008;180:619–632. doi: 10.1083/jcb.200707042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44**.Rivieccio MA, Brochier C, Willis DE, Walker BA, D’Annibale MA, McLaughlin K, Siddiq A, Kozikowski AP, Jaffrey SR, Twiss JL, et al. HDAC6 is a target for protection and regeneration following injury in the nervous system. Proc Natl Acad Sci U S A. 2009;106:19599–19604. doi: 10.1073/pnas.0907935106. This study reveals that specific inhibition of HDAC6 increases neuronal viability under oxidative stress and enhances axon outgrowth on inhibitory substrate. This study suggests that selective inhibition of HDAC6 is a potential target for axon regeneration after CNS injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Kwon S, Yamaguchi T, Cubizolles F, Rousseaux S, Kneissel M, Cao C, Li N, Cheng HL, Chua K, et al. Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol Cell Biol. 2008;28:1688–1701. doi: 10.1128/MCB.01154-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kurklinsky S, Chen J, McNiven MA. Growth cone morphology and spreading are regulated by a dynamin-cortactin complex at point contacts in hippocampal neurons. J Neurochem. 2011;117:48–60. doi: 10.1111/j.1471-4159.2011.07169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Konishi Y, Stegmuller J, Matsuda T, Bonni S, Bonni A. Cdh1-APC controls axonal growth and patterning in the mammalian brain. Science. 2004;303:1026–1030. doi: 10.1126/science.1093712. [DOI] [PubMed] [Google Scholar]
- 48*.Williams KA, Zhang M, Xiang S, Hu C, Wu JY, Zhang S, Ryan M, Cox AD, Der CJ, Fang B, et al. Extracellular Signal-regulated Kinase (ERK) Phosphorylates Histone Deacetylase 6 (HDAC6) at Serine 1035 to Stimulate Cell Migration. J Biol Chem. 2013;288:33156–33170. doi: 10.1074/jbc.M113.472506. This study reveals that EGF-activated ERK phosphorylates HDAC6 and thereby enhance its tubulin deacetylatse activity. Non-phosphorylable mutant HDAC6 shows reduced tubulin deacetylase activity but no change towards histone deacetylation in the nucleus, suggesting that HDAC6 has distinct regulatory mechanisms to different substrates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perlson E, Hanz S, Ben-Yaakov K, Segal-Ruder Y, Seger R, Fainzilber M. Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron. 2005;45:715–726. doi: 10.1016/j.neuron.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 50.Yan J, Seibenhener ML, Calderilla-Barbosa L, Diaz-Meco MT, Moscat J, Jiang J, Wooten MW, Wooten MC. SQSTM1/p62 Interacts with HDAC6 and Regulates Deacetylase Activity. PLoS One. 2013;8:e76016. doi: 10.1371/journal.pone.0076016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang J, Baloh RH, Milbrandt J. The NIMA-family kinase Nek3 regulates microtubule acetylation in neurons. J Cell Sci. 2009;122:2274–2282. doi: 10.1242/jcs.048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lv L, Han X, Sun Y, Wang X, Dong Q. Valproic acid improves locomotion in vivo after SCI and axonal growth of neurons in vitro. Exp Neurol. 2012;233:783–790. doi: 10.1016/j.expneurol.2011.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Biermann J, Grieshaber P, Goebel U, Martin G, Thanos S, Di Giovanni S, Lagreze WA. Valproic acid-mediated neuroprotection and regeneration in injured retinal ganglion cells. Invest Ophthalmol Vis Sci. 2010;51:526–534. doi: 10.1167/iovs.09-3903. [DOI] [PubMed] [Google Scholar]
- 54*.Gaub P, Tedeschi A, Puttagunta R, Nguyen T, Schmandke A, Di Giovanni S. HDAC inhibition promotes neuronal outgrowth and counteracts growth cone collapse through CBP/p300 and P/CAF-dependent p53 acetylation. Cell Death Differ. 2010;17:1392–1408. doi: 10.1038/cdd.2009.216. This study reveals that HDAC inhibitors increases regenerative capacity of cerebellar neurons cultured on inhibitory substrates by activating a pro-neuronal outgrowth transcriptional program through CBP/p300 and P/CAF-mediated histone H3 and p53 acetylation. [DOI] [PubMed] [Google Scholar]
- 55**.Finelli MJ, Wong JK, Zou H. Epigenetic regulation of sensory axon regeneration after spinal cord injury. J Neurosci. 2013;33:19664–19676. doi: 10.1523/JNEUROSCI.0589-13.2013. This study establishes a correlation between reduced axon growth potential and histone H4 hypoacetylation. Peripheral, but not central sensory axon injury increases histone H4 acetylation, which regulates the expression of regeneration-associated genes in DRG neurons. The authors also show that HDAC inhibitor (MS-275) treatment enhances axon growth in a mouse model of spinal cord injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56**.Cho Y, Sloutsky R, Naegle KM, Cavalli V. Injury-Induced HDAC5 Nuclear Export Is Essential for Axon Regeneration. Cell. 2013;155:894–908. doi: 10.1016/j.cell.2013.10.004. This study demonstrates that an injury-induced calcium back propagating wave triggers epigenetic changes through the nuclear export of the histone deacetylase HDAC5 to promote regeneration-promoting gene transcription. This study also highlights some key differences between peripheral and central neurons that may contribute to the differences in their regenerative capacities. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pelzel HR, Schlamp CL, Nickells RW. Histone H4 deacetylation plays a critical role in early gene silencing during neuronal apoptosis. BMC Neurosci. 2010;11:62. doi: 10.1186/1471-2202-11-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58*.Fischle W, Dequiedt F, Hendzel MJ, Guenther MG, Lazar MA, Voelter W, Verdin E. Enzymatic activity associated with class II HDACs is dependent on a multiprotein complex containing HDAC3 and SMRT/N-CoR. Mol Cell. 2002;9:45–57. doi: 10.1016/s1097-2765(01)00429-4. The authors show that the class II HDAC4 and HDAC5 do not possess high intrisinc enzymatic activity as isloated polypeptides. However, HDAC activity is provided in the context of a complex with HDAC3 and corepressors N-CoR and SMRT. [DOI] [PubMed] [Google Scholar]
- 59.Pierfelice T, Alberi L, Gaiano N. Notch in the vertebrate nervous system: an old dog with new tricks. Neuron. 2011;69:840–855. doi: 10.1016/j.neuron.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 60.Tiberi L, van den Ameele J, Dimidschstein J, Piccirilli J, Gall D, Herpoel A, Bilheu A, Bonnefont J, Iacovino M, Kyba M, et al. BCL6 controls neurogenesis through Sirt1-dependent epigenetic repression of selective Notch targets. Nat Neurosci. 2012;15:1627–1635. doi: 10.1038/nn.3264. [DOI] [PubMed] [Google Scholar]
- 61.Pandithage R, Lilischkis R, Harting K, Wolf A, Jedamzik B, Luscher-Firzlaff J, Vervoorts J, Lasonder E, Kremmer E, Knoll B, et al. The regulation of SIRT2 function by cyclin-dependent kinases affects cell motility. J Cell Biol. 2008;180:915–929. doi: 10.1083/jcb.200707126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62*.Li XH, Chen C, Tu Y, Sun HT, Zhao ML, Cheng SX, Qu Y, Zhang S. Sirt1 Promotes Axonogenesis by Deacetylation of Akt and Inactivation of GSK3. Mol Neurobiol. 2013 doi: 10.1007/s12035-013-8437-3. This study shows that Sirt1 is enriched in the axons and growth cones of hippocampal neurons and promotes axon outgrowth through Akt-GSK3 pathway. Sirt1 associates with and deacetylates Akt, leading to inactivation of GSK3. [DOI] [PubMed] [Google Scholar]
- 63.Liu CM, Wang RY, Saijilafu, Jiao ZX, Zhang BY, Zhou FQ. MicroRNA-138 and SIRT1 form a mutual negative feedback loop to regulate mammalian axon regeneration. Genes Dev. 2013;27:1473–1483. doi: 10.1101/gad.209619.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y, Zhang M, Dong H, Yong S, Li X, Olashaw N, Kruk PA, Cheng JQ, Bai W, Chen J, et al. Deacetylation of cortactin by SIRT1 promotes cell migration. Oncogene. 2009;28:445–460. doi: 10.1038/onc.2008.388. [DOI] [PubMed] [Google Scholar]
- 65.Bai S, Ghoshal K, Datta J, Majumder S, Yoon SO, Jacob ST. DNA methyltransferase 3b regulates nerve growth factor-induced differentiation of PC12 cells by recruiting histone deacetylase 2. Mol Cell Biol. 2005;25:751–766. doi: 10.1128/MCB.25.2.751-766.2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]