New insights into establishment and maintenance of DNA methylation imprints in mammals - PubMed (original) (raw)
Review
New insights into establishment and maintenance of DNA methylation imprints in mammals
Gavin Kelsey et al. Philos Trans R Soc Lond B Biol Sci. 2013.
Abstract
Fundamental to genomic imprinting in mammals is the acquisition of epigenetic marks that differ in male and female gametes at 'imprinting control regions' (ICRs). These marks mediate the allelic expression of imprinted genes in the offspring. Much has been learnt about the nature of imprint marks, the times during gametogenesis at which they are laid down and some of the factors responsible especially for DNA methylation. Recent work has revealed that transcription and histone modifications are critically involved in DNA methylation acquisition, and these findings allow us to propose rational models for methylation establishment. A completely novel perspective on gametic DNA methylation has emerged from epigenomic profiling. Far more differentially methylated loci have been identified in gametes than known imprinted genes, which leads us to revise the notion that methylation of ICRs is a specifically targeted process. Instead, it seems to obey default processes in germ cells, giving rise to distinct patterns of DNA methylation in sperm and oocytes. This new insight, together with the identification of proteins that preserve DNA methylation after fertilization, emphasizes the key role played by mechanisms that selectively retain differential methylation at imprinted loci during early development. Addressing these mechanisms will be essential to understanding the specificity and evolution of genomic imprinting.
Figures
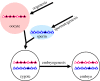
Figure 1.
Establishment and post-fertilization maintenance of imprinted DNA methylation. In the schematic presentation, the maternal and the paternal genomes are coloured red and blue, respectively. Triangles represent CpG islands, or CpG-rich sequences: black filling indicates DNA methylation, open triangles indicate absence of methylation. More than a thousand CpG islands become de novo methylated during oogenesis. Conversely, several hundred CpG-rich sequences acquire methylation during spermatogenesis, mostly at intergenic positions. Following fertilization and during early embryonic development, only at a few per cent of these sequence elements is the maternally or paternally derived DNA methylation selectively maintained. Although still poorly understood, it is through early embryonic maintenance mechanisms that the specificity of these germline differentially methylated regions (gDMRs) comes about. The gDMRs that are maintained during development correspond to ‘imprinting control regions’ (ICRs) of known or yet-to-be discovered imprinted domains.
Figure 2.
Gene-body methylation in oocytes revealed by whole genome bisulphite sequencing. The panel at the top represents the consensus annotation of transcripts at the imprinted Gnas locus. The locations of CpG islands are shown as open boxes, above the CpG density plot. Below, the methylation level determined from whole-genome bisulphite sequencing in wild-type oocytes, Dnmt3L−/− oocytes, sperm and blastocysts is plotted. Each dot on the displays represents the average methylation level of an individual CpG. The location of the gDMRs at Nespas/Gnasxl and exon 1A are marked by the pink columns. As expected, the gDMRs are unmethylated in sperm and have intermediate levels of methylation in blastocyst DNA. The DMR at Nesp55 (which acquires methylation on the paternal allele only after implantation) and the CpG island (CGI) at the canonical Gnas promoter are unmethylated in all samples. Note that gDMR methylation in oocytes is contiguous with the high level of gene body methylation; the whole domain is within a transcription unit in oocytes determined by activity of the Nesp55 promoter (boxed in the upper panel). All DNA methylation within the domain, not just at the gDMRs, depends upon the presence of DNMT3L in oocytes, as revealed by the very low level of methylation throughout the domain in the Dnmt3L−/− oocyte track. Reproduced, with very minor additional annotation, from Kobayashi et al. [34].
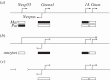
Figure 3.
The role of transcription in promoting methylation of gDMRs in the Gnas locus. Schematic of the mouse Gnas locus. (a) The alternative transcription start sites, associated with the promoters for Nesp55, Gnasxl, exon 1A and Gnas, are indicated by the arrows; on the antisense strand, the promoter for the non-coding antisense transcript Nespas is indicated. Below the line, the DMRs and the CpG island at the Gnas promoter are indicated as boxes, with methylation on the maternal (Mat) or paternal (Pat) alleles shown by the filled boxes. (b) In growing oocytes, prior to or during de novo methylation, the DMR-associated promoters at Nespas, Gnasxl and 1A are not expressed, while the Gnas promoter is. In addition, the Nesp promoter is expressed, placing the Nespas/Gnasxl and 1A DMRs and Gnas promoter CpG island within an active transcription unit. Repression of the Nespas, Gnasxl and 1A promoters may be necessary for de novo methylation, whereas activity of the Gnas promoter may protect it from methylation. (c) Deletion of the Nesp55 promoter region or insertion of a transcription termination cassette downstream of the Nesp55 exon to ablate transcription through the DMRs results in failure to establish DNA methylation at the DMRs in oocytes [30,78,79]. Not drawn to scale: the distance between the Nespas and Gnas promoters is approximately 45 kb.
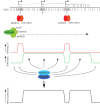
Figure 4.
A model for DNA methylation in oocytes incorporating transcription and histone modification state. At the top, an idealized transcription unit active in oocytes, defined by an upstream, oocyte-specific transcription start site (A), and two intragenic CpG island promoter regions, one (B) that is inactive in oocytes, and a second (C) that is expressed in oocytes. Directly below, individual CpG dinucleotides are indicated, with dense clusters of CpGs at CpG islands. CpG islands A and C are predicted to be bound by CxxC-containing proteins KDM2A and CFP1 that have H3K36 demethylase and H3K4 methyltransferase activities, respectively (the latter via recruitment of the SET1 complex). Transcription through the locus brings the H3K36 methyltransferase SETD2 and the H3K4 demethylases KDM5B and KDM1B, as these activities are associated with the elongating RNA polII complex. (Note that these predictions are based on the properties of these proteins established in somatic cells, only KDM1B has thus far been shown to be involved in gDMR methylation in oocytes. Other members of the respective protein families may perform these roles in oocytes.) The combination of these activities generates distinct states of H3K4 and H3K36 methylation within transcribed regions, active and inactive CpG island promoters and intergenic regions. This profile of H3K4 and H3K36 methylation is read by the DNMT3A/DNMT3L de novo DNA methyltransferase complex to establish methylation at gene bodies and silent, intragenic CpG islands and generate the DNA methylation profile indicated at the bottom. By this mechanism, CpG island B could become a gDMR; differential acquisition of DNA methylation of intragenic CpG islands B and C may be determined by promoter activity or other factors that promote binding of proteins such as KDM2A and CFP1 at CpG island C that are hostile to DNA methylation.
Similar articles
- The specification of imprints in mammals.
Hanna CW, Kelsey G. Hanna CW, et al. Heredity (Edinb). 2014 Aug;113(2):176-83. doi: 10.1038/hdy.2014.54. Epub 2014 Jun 18. Heredity (Edinb). 2014. PMID: 24939713 Free PMC article. Review. - DNA methylation dynamics during the mammalian life cycle.
Hackett JA, Surani MA. Hackett JA, et al. Philos Trans R Soc Lond B Biol Sci. 2013 Jan 5;368(1609):20110328. doi: 10.1098/rstb.2011.0328. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 23166392 Free PMC article. Review. - Germline-derived DNA methylation and early embryo epigenetic reprogramming: The selected survival of imprints.
Monk D. Monk D. Int J Biochem Cell Biol. 2015 Oct;67:128-38. doi: 10.1016/j.biocel.2015.04.014. Epub 2015 May 9. Int J Biochem Cell Biol. 2015. PMID: 25966912 Review. - Histone methylation is mechanistically linked to DNA methylation at imprinting control regions in mammals.
Henckel A, Nakabayashi K, Sanz LA, Feil R, Hata K, Arnaud P. Henckel A, et al. Hum Mol Genet. 2009 Sep 15;18(18):3375-83. doi: 10.1093/hmg/ddp277. Epub 2009 Jun 10. Hum Mol Genet. 2009. PMID: 19515852 - The parental non-equivalence of imprinting control regions during mammalian development and evolution.
Schulz R, Proudhon C, Bestor TH, Woodfine K, Lin CS, Lin SP, Prissette M, Oakey RJ, Bourc'his D. Schulz R, et al. PLoS Genet. 2010 Nov 18;6(11):e1001214. doi: 10.1371/journal.pgen.1001214. PLoS Genet. 2010. PMID: 21124941 Free PMC article.
Cited by
- Heterochromatin delays CRISPR-Cas9 mutagenesis but does not influence the outcome of mutagenic DNA repair.
Kallimasioti-Pazi EM, Thelakkad Chathoth K, Taylor GC, Meynert A, Ballinger T, Kelder MJE, Lalevée S, Sanli I, Feil R, Wood AJ. Kallimasioti-Pazi EM, et al. PLoS Biol. 2018 Dec 12;16(12):e2005595. doi: 10.1371/journal.pbio.2005595. eCollection 2018 Dec. PLoS Biol. 2018. PMID: 30540740 Free PMC article. - Is ZFP57 binding to H19/IGF2:IG-DMR affected in Silver-Russell syndrome?
Sparago A, Cerrato F, Riccio A. Sparago A, et al. Clin Epigenetics. 2018 Feb 21;10:23. doi: 10.1186/s13148-018-0454-7. eCollection 2018. Clin Epigenetics. 2018. PMID: 29484033 Free PMC article. - Adaptive variability in the duration of critical windows of plasticity: Implications for the programming of obesity.
Wells JC. Wells JC. Evol Med Public Health. 2014 Aug 5;2014(1):109-21. doi: 10.1093/emph/eou019. Evol Med Public Health. 2014. PMID: 25095791 Free PMC article. Review. - Imprinting control regions (ICRs) are marked by mono-allelic bivalent chromatin when transcriptionally inactive.
Maupetit-Méhouas S, Montibus B, Nury D, Tayama C, Wassef M, Kota SK, Fogli A, Cerqueira Campos F, Hata K, Feil R, Margueron R, Nakabayashi K, Court F, Arnaud P. Maupetit-Méhouas S, et al. Nucleic Acids Res. 2016 Jan 29;44(2):621-35. doi: 10.1093/nar/gkv960. Epub 2015 Sep 22. Nucleic Acids Res. 2016. PMID: 26400168 Free PMC article. - Methylation of the C19MC microRNA locus in the placenta: association with maternal and chilhood body size.
Prats-Puig A, Xargay-Torrent S, Carreras-Badosa G, Mas-Parés B, Bassols J, Petry CJ, Girardot M, D E Zegher F, Ibáñez L, Dunger DB, Feil R, López-Bermejo A. Prats-Puig A, et al. Int J Obes (Lond). 2020 Jan;44(1):13-22. doi: 10.1038/s41366-019-0450-9. Epub 2019 Sep 25. Int J Obes (Lond). 2020. PMID: 31554916
References
- Ferguson-Smith AC. 2011. Genomic imprinting: the emergence of an epigenetic paradigm. Nat. Rev. Genet. 12, 565–57510.1038/nrg3032 (doi:10.1038/nrg3032) - DOI - DOI - PubMed
- McGrath J, Solter D. 1984. Completion of mouse embryogenesis requires both the maternal and paternal genomes. Cell 37, 179–18310.1016/0092-8674(84)90313-1 (doi:10.1016/0092-8674(84)90313-1) - DOI - DOI - PubMed
- Surani MA, Barton SC, Norris ML. 1984. Development of reconstituted mouse eggs suggests imprinting of the genome during gametogenesis. Nature 308, 548–55010.1038/308548a0 (doi:10.1038/308548a0) - DOI - DOI - PubMed
- Cattanach BM, Kirk M. 1985. Differential activity of maternal and paternally derived chromosome regions in mice. Nature 315, 496–49810.1038/315496a0 (doi:10.1038/315496a0) - DOI - DOI - PubMed
- Barlow DP, Stoger R, Herrmann BG, Saito K, Schweifer N. 1991. The mouse insulin-like growth factor type-2 receptor is imprinted and closely linked to the Tme locus. Nature 349, 84–8710.1038/349084a0 (doi:10.1038/349084a0) - DOI - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BBS/E/B/0000M233/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G0800013/MRC_/Medical Research Council/United Kingdom
- MR/K011332/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources