HDL induces the expression of the M2 macrophage markers arginase 1 and Fizz-1 in a STAT6-dependent process - PubMed (original) (raw)
HDL induces the expression of the M2 macrophage markers arginase 1 and Fizz-1 in a STAT6-dependent process
Marie Sanson et al. PLoS One. 2013.
Abstract
Our lab has previously shown in a mouse model that normalization of a low HDL level achieves atherosclerotic plaque regression. This included the shift from a pro ("M1") to an anti-inflammatory ("M2") phenotypic state of plaque macrophages. Whether HDL can directly cause this phenotypic change and, if so, what the signaling mechanism is, were explored in the present studies. Murine primary macrophages treated with HDL showed increased gene expression for the M2 markers Arginase-1 (Arg-1) and Fizz-1, which are classically induced by IL-4. HDL was able to potentiate the IL-4-induced changes in Arg-1, and tended to do the same for Fizz-1, while suppressing the expression of inflammatory genes in response to IFNγ. The effects of either IL-4 or HDL were suppressed when macrophages were from STAT6(-/-) mice, but inhibitor studies suggested differential utilization of JAK isoforms by IL-4 and HDL to activate STAT6 by phosphorylation. Overall, our results describe a new function of HDL, namely its ability to directly enrich macrophages in markers of the M2, anti-inflammatory, state in a process requiring STAT6.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
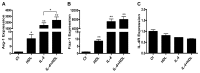
Figure 1. HDL promotes and enhances the expression of specific anti-inflammatory genes in primary macrophages.
BMDM were treated for 6h with HDL (50μg/mL), IL-4 (10ng/mL) or both. Gene expression of Arg-1 (A), Fizz-1 (B) and IL-4R (C) was assessed by real-time qPCR. Results are representative of four independent experiments. Asterisks indicate statistically significant differences, either compared to control, or between 2 conditions when linked by a bar (*p<0.05, **p<0.01).
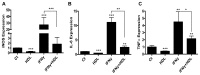
Figure 2. HDL inhibits the basal and induced expression of pro-inflammatory genes in primary macrophages.
BMDM were treated for 6h with HDL (50μg/mL), IFNγ (10ng/mL) or both. Gene expression for iNOS (A), IL-6 (B) or TNFα (C) was assessed by real-time qPCR. Results are representative of three independent experiments. Asterisks indicate statistically significant differences, either compared to control, or between 2 conditions when linked by a bar (*p<0.05, **p<0.01, ***p<0.001).
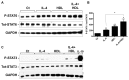
Figure 3. HDL induces STAT6 phosphorylation and enhances IL-4-induced STAT6 activation, but has no effect on STAT3 phosphorylation status.
BMDM were grown as previously described and stimulated for 15min with either HDL alone (50μg/mL), IL-4 alone (10ng/mL) or both. Phosphorylation state for STAT6 (A) and STAT3 (C) was assessed by western blot. As a positive control for STAT3 activation, cells were treated with IL-10 (10ng/mL) for 30 min. Signal quantification for phospho-STAT6 (B) is the result of three independent experiments. Asterisks indicate statistically significant difference, either compared to control, or between 2 conditions when linked by a bar (*p<0.05, **p<0.01).
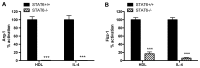
Figure 4. STAT6 inhibition affects macrophages alternative activation by HDL.
BMDM were isolated from STAT6+/+ (black bars) and STAT6-/- (hatched bars) mice and were stimulated for 6h with HDL (50μg/mL) or IL-4 (10ng/mL). The induction of Arg-1 or Fizz-1 mRNA in the STAT6+/+ cells by HDL was ~3X for each and by IL-4, ~1800 or 3300, respectively; these values were set to 100%. Results are representative of three independent experiments. Asterisks indicate statistically significant differences compared to corresponding STAT6+/+ conditions (***p<0.001).
Figure 5. Jak inhibition affects macrophages alternative activation by HDL.
BMDM were pre-treated for 2h with Jak pharmacological inhibitors (hatched bars. Ruxolitinib/Jak1/2 inhibitor: 1μM. TG101348/Jak2 inhibitor: 1μM: Kaempferol/Jak3 inhibitor: 40μM) or vehicle (black bars), and then treated for 6h with HDL (50μg/mL) or IL-4 (10ng/mL). Arg-1 (A, C, E) and Fizz-1 (B, D, F) expression are presented as the percentage of activation compared to non-inhibited conditions. The induction of Arg-1 and Fizz-1 by HDL was ~2X for each, and for IL-4 was >300 for Arg-1 and Fizz-1 and these values were set to 100%. Results are representative of three independent experiments. Asterisks indicate statistically significant differences compared to the vehicle-treated conditions (*p<0.05, **p<0.01, ***p<0.001).
Similar articles
- Pyropia yezoensis glycoprotein promotes the M1 to M2 macrophage phenotypic switch via the STAT3 and STAT6 transcription factors.
Choi JW, Kwon MJ, Kim IH, Kim YM, Lee MK, Nam TJ. Choi JW, et al. Int J Mol Med. 2016 Aug;38(2):666-74. doi: 10.3892/ijmm.2016.2656. Epub 2016 Jun 24. Int J Mol Med. 2016. PMID: 27353313 - Ginsenoside Rb1 enhances atherosclerotic plaque stability by skewing macrophages to the M2 phenotype.
Zhang X, Liu MH, Qiao L, Zhang XY, Liu XL, Dong M, Dai HY, Ni M, Luan XR, Guan J, Lu HX. Zhang X, et al. J Cell Mol Med. 2018 Jan;22(1):409-416. doi: 10.1111/jcmm.13329. Epub 2017 Sep 25. J Cell Mol Med. 2018. PMID: 28944992 Free PMC article. - CDK8/19 inhibitor enhances arginase-1 expression in macrophages via STAT6 and p38 MAPK activation.
Mizuno N, Shiga S, Tanaka Y, Kimura T, Yanagawa Y. Mizuno N, et al. Eur J Pharmacol. 2024 Sep 15;979:176852. doi: 10.1016/j.ejphar.2024.176852. Epub 2024 Jul 25. Eur J Pharmacol. 2024. PMID: 39067565 - High-density lipoprotein immunomodulates the functional activities of macrophage and cytokines produced during ex vivo macrophage-CD4+ T cell crosstalk at the recent-onset human type 1 diabetes.
Benghalem I, Meziane W, Hadjidj Z, Ysmail-Dahlouk L, Belamri A, Mouhadjer K, Aribi M. Benghalem I, et al. Cytokine. 2017 Aug;96:59-70. doi: 10.1016/j.cyto.2017.03.001. Epub 2017 Mar 16. Cytokine. 2017. PMID: 28324804 - STAT6 Upregulation Promotes M2 Macrophage Polarization to Suppress Atherosclerosis.
Gong M, Zhuo X, Ma A. Gong M, et al. Med Sci Monit Basic Res. 2017 Jun 15;23:240-249. doi: 10.12659/msmbr.904014. Med Sci Monit Basic Res. 2017. PMID: 28615615 Free PMC article.
Cited by
- C/EBP Homologous Protein (CHOP) Activates Macrophages and Promotes Liver Fibrosis in _Schistosoma japonicum_-Infected Mice.
Duan M, Yang Y, Peng S, Liu X, Zhong J, Guo Y, Lu M, Nie H, Ren B, Zhang X, Liu L. Duan M, et al. J Immunol Res. 2019 Dec 1;2019:5148575. doi: 10.1155/2019/5148575. eCollection 2019. J Immunol Res. 2019. PMID: 31886304 Free PMC article. - Establishment and Characterization of Free-Floating 3D Macrophage Programming Model in the Presence of Cancer Cell Spheroids.
Korotkaja K, Jansons J, Spunde K, Rudevica Z, Zajakina A. Korotkaja K, et al. Int J Mol Sci. 2023 Jun 28;24(13):10763. doi: 10.3390/ijms241310763. Int J Mol Sci. 2023. PMID: 37445941 Free PMC article. - High-density lipoproteins put out the fire.
Moore KJ, Fisher EA. Moore KJ, et al. Cell Metab. 2014 Feb 4;19(2):175-6. doi: 10.1016/j.cmet.2014.01.009. Cell Metab. 2014. PMID: 24506861 Free PMC article. - Redefining the transcriptional regulatory dynamics of classically and alternatively activated macrophages by deepCAGE transcriptomics.
Roy S, Schmeier S, Arner E, Alam T, Parihar SP, Ozturk M, Tamgue O, Kawaji H, de Hoon MJ, Itoh M, Lassmann T, Carninci P, Hayashizaki Y, Forrest AR, Bajic VB, Guler R; Fantom Consortium; Brombacher F, Suzuki H. Roy S, et al. Nucleic Acids Res. 2015 Aug 18;43(14):6969-82. doi: 10.1093/nar/gkv646. Epub 2015 Jun 27. Nucleic Acids Res. 2015. PMID: 26117544 Free PMC article. - ADAM17 Boosts Cholesterol Efflux and Downstream Effects of High-Density Lipoprotein on Inflammatory Pathways in Macrophages.
Kothari V, Tang J, He Y, Kramer F, Kanter JE, Bornfeldt KE. Kothari V, et al. Arterioscler Thromb Vasc Biol. 2021 Jun;41(6):1854-1873. doi: 10.1161/ATVBAHA.121.315145. Epub 2021 Apr 22. Arterioscler Thromb Vasc Biol. 2021. PMID: 33882688 Free PMC article.
References
- Moore KJ, Tabas I (2011) Macrophages in the pathogenesis of atherosclerosis. Cell 145: 341-355. doi:10.1016/j.cell.2011.04.005. PubMed: 21529710. - DOI - PMC - PubMed
- Reis ED, Li J, Fayad ZA, Rong JX, Hansoty D et al. (2001) Dramatic remodeling of advanced atherosclerotic plaques of the apolipoprotein E-deficient mouse in a novel transplantation model. J Vasc Surg 34: 541-547. doi:10.1067/mva.2001.115963. PubMed: 11533609. - DOI - PubMed
- Llodrá J, Angeli V, Liu J, Trogan E, Fisher EA et al. (2004) Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A 101: 11779-11784. doi:10.1073/pnas.0403259101. PubMed: 15280540. - DOI - PMC - PubMed
- Feig JE, Rong JX, Shamir R, Sanson M, Vengrenyuk Y et al. (2011) HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci U S A 108: 7166-7171. doi:10.1073/pnas.1016086108. PubMed: 21482781. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous