Dishevelled has a YAP nuclear export function in a tumor suppressor context-dependent manner - PubMed (original) (raw)
doi: 10.1038/s41467-018-04757-w.
Nam Hee Kim 2, Eunae Sandra Cho 1, Ji Hye Yang 1, Yong Hoon Cha 3, Hee Eun Kang 1, Jun Seop Yun 1, Sue Bean Cho 2, Seon-Hyeong Lee 4, Petra Paclikova 5, Tomasz W Radaszkiewicz 5, Vitezslav Bryja 5, Chi Gu Kang 1, Young Soo Yuk 1, So Young Cha 1, Soo-Youl Kim 4, Hyun Sil Kim 6, Jong In Yook 7
Affiliations
- PMID: 29895829
- PMCID: PMC5997650
- DOI: 10.1038/s41467-018-04757-w
Dishevelled has a YAP nuclear export function in a tumor suppressor context-dependent manner
Yoonmi Lee et al. Nat Commun. 2018.
Abstract
Phosphorylation-dependent YAP translocation is a well-known intracellular mechanism of the Hippo pathway; however, the molecular effectors governing YAP cytoplasmic translocation remains undefined. Recent findings indicate that oncogenic YAP paradoxically suppresses Wnt activity. Here, we show that Wnt scaffolding protein Dishevelled (DVL) is responsible for cytosolic translocation of phosphorylated YAP. Mutational inactivation of the nuclear export signal embedded in DVL leads to nuclear YAP retention, with an increase in TEAD transcriptional activity. DVL is also required for YAP subcellular localization induced by E-cadherin, α-catenin, or AMPK activation. Importantly, the nuclear-cytoplasmic trafficking is dependent on the p53-Lats2 or LKB1-AMPK tumor suppressor axes, which determine YAP phosphorylation status. In vivo and clinical data support that the loss of p53 or LKB1 relieves DVL-linked reciprocal inhibition between the Wnt and nuclear YAP activity. Our observations provide mechanistic insights into controlled proliferation coupled with epithelial polarity during development and human cancer.
Conflict of interest statement
The authors declare no competing interests.
Figures
Fig. 1
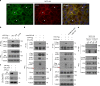
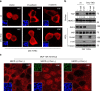
DVL interacts with phosphorylated YAP. a Confocal images of endogenous YAP (green) and DVL3 (red) in MCF-10A cells. Arrows indicate co-localized foci. Nuclear staining with TOPRO3 (blue) is shown in merged image. Scale bar, 10 μm. b DVL interacts with YAP in a phosphorylation-dependent manner. In all, 293 cells were transfected with His-tagged DVL3 and vector control (−) or flag-tagged YAP or mutants (5SA, S127A). Interactions between DVL and YAP were determined following immunoprecipitation (IP) with anti-flag antibody and immunoblotting with anti-HA. Whole-cell lysate (WCL) serves as input abundance for IP. c Lambda protein phosphatase (λ PPase) treatment to immunoprecipitated YAP abolishes DVL binding. The 293 cells were transfected with flag-tagged YAP and immunoprecipitated anti-flag beads were treated with λ PPase (+). The agarose beads were then subjected to binding to HA-tagged DVL. d Kinase-dead dominant negative Lats2 (Lats2-KR) or AMPK (AMPK-KD) or LKB1 (LKB1-KD) abolishes YAP and DVL interaction. Flag-tagged YAP and His-tagged DVL3 expression vectors were co-transfected with the dominant negative expression vectors as indicated in 293 cells. Interactions between DVL3 and YAP were determined as described above and phosphorylation status of YAP was determined by pS127-YAP antibody and mobility shift on a phos-tag gel. Black and red arrowheads correspond to the fully phosphorylated and active YAP on a phos-tag gel, respectively. e The MCF-10A cells were cultured under sparse and confluent states, and the whole-cell lysates (WCL) were subjected for immunoblot analysis and immunoprecipitation (IP) assay with anti-YAP antibody. Mouse IgG served as negative control. Unprocessed original scans of blots are shown in Supplementary Fig. 10
Fig. 2
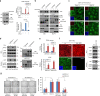
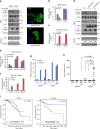
DVL suppresses YAP nuclear abundance and TEAD transcriptional activity. a YAP was co-transfected with HA-DVL3 into MCF-7 cells, and protein abundance (left), TEAD reporter activity (right upper), and CTGF transcript abundance (right lower) were determined. Data of reporter assay and RT-PCR are normalized to negative control empty vector (−) and presented as mean ± SD. b The 293 and MCF-7 cells were transfected with DVL3, and protein abundance of YAP in nuclear and cytoplasmic fraction was determined by immunoblot analysis. YAP phosphorylation status in cytoplasmic and nuclear fraction was determined by pS127-YAP antibody and mobility shift on a phos-tag gel. Red arrowhead indicates active YAP on a phos-tag gel. Tubulin and HDAC1 served as loading controls of cytosolic fraction and nuclear lysates, respectively. Relative nuclear YAP abundance compared to control was measured by ImageJ. c, d Inducible knockdown of DVL3 increases nuclear YAP abundance. The MCF-10A cells expressing tetracycline-inducible shRNA against DVL3 were generated with lentiviral system, and endogenous YAP localization and abundance without or with doxycycline (Dox) were determined by immunofluorescence (c) and immunoblot analysis from whole-cell lysate (WCL) and nuclear fraction (d). The cells were serum-starved for 16 h before harvest. Tubulin and HDAC1 served as loading controls of cytosolic fraction and nuclear lysates, respectively. e DVL3 was knockdowned with doxycycline (Dox), and the TEAD reporter activity (upper panel) and CTGF transcript abundance (lower panel) were determined by reporter assay and qRT-PCR, respectively. f The wt and DVL-TKO cells were cultured in confluent condition and subcellular localization of endogenous YAP and DVL3 were determined by confocal microscopy (left panels) and immunoblot analysis (right panels). The cells were serum-starved for 16 h before examination. Inset, DAPI nuclear stain; Scale bar, 10 μm. g Knockdown of DVL3 increases anchorage-independent growth of MCF-7 cells. The MCF-7 cells expressing inducible shRNA for DVL3 were seeded onto a soft agar without (Dox-) or with (Dox+) doxycycline in combination with shControl or shYAP for 3 weeks. The colonies were stained with crystal violet and quantified. Data presented as mean ± SD, n = 5
Fig. 3
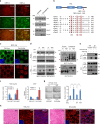
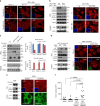
NES (nuclear export sequence) in DVL is responsible for YAP trafficking. a The confluent MCF-7 cells were treated with Leptomycin B (LMB, 5 ng ml−1) for 4 h, and endogenous YAP and DVL localization and abundance were determined by confocal microscopy (left panels) and immunoblotting (right panels). Scale bar, 10 μm. b Schematic representation of the conserved NES and point mutant (NES-mutant-ASA) in DVL of human (h), mouse (m), and xenopus (x). c The MCF-7 cells were transfected with wt or NES-ASA mutant DVL3 and DVL-YAP localization was determined by confocal microscopy. The cells were serum-starved for 16 h before harvest. Scale bar, 5 μm. d MCF-7 and 293 cells expressing inducible HA-tagged wt or NES-mutant (ASA) of DVL3 were treated with doxycycline, and YAP and DVL abundance were determined (left panels). YAP phosphorylation status in cytoplasmic and nuclear fraction was determined by pS127-YAP antibody and mobility shift on a phos-tag gel (right panels). Red arrowhead indicates active YAP. e The 293 cells were transfected with flag-tagged YAP with NES-mutant or wt DVL3. The nuclear protein form NES-mutant transfectant was subjected for immunoprecipitation, whole-cell lysate of wt DVL serving as control. Fifty micro grams of nuclear protein and 10 μg of whole-cell lysates were used for immunoprecipitation assay to adjust YAP abundance. f TEAD reporter activity and CTGF transcript abundance were analyzed from MCF-7 cells expressing inducible wt or NES-mutant of DVL3 (mean ± SD, n = 3). g The MCF-7 cells were transfected with YAP in combination with wt or NES-ASA mutant of DVL3, then seeded onto a soft agar for 3 weeks. The colonies were stained with crystal violet and quantified (mean ± SD, n = 5). h The 293 cells stably expressing tet-inducible wt or NES-mutant of DVL were injected into athymic nude mice subcutaneously. When the tumor volume reached around 500 mm3 (n = 1), the mice were treated with doxycycline (50 mg/kg) intraperitoneally prior sacrifice to 24 h. The tissues were examined by H/E staining (scale bar, 50 μm) and YAP localization was determined from frozen sections (scale bar, 10 μm). Unprocessed original scans of blots are shown in Supplementary Fig. 10
Fig. 4
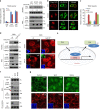
DVL has nuclear export function on phosphorylated YAP. a Relative fold repression of reporter activities and nuclear YAP abundance by DVL on wt or phospho-resistant mutants of YAP (S127A, 5SA) were measured with TEAD reporter assay (left panel) and immunoblot analysis (right panels), respectively. Relative nuclear YAP abundance compared to control was measured by ImageJ. b The MCF-7 cells were transfected with HA-tagged DVL3 in combination with flag-tagged wt or phospho-resistant mutants YAP (S127A, 5SA), and nuclear localizations of YAP and DVL were examined by confocal immunofluorescence microscopy. To minimize the overexpression issue, 10 ng of YAP expression vectors was used. Scale bar, 10 μm. c YAP and DVL3 were co-transfected in combination with vector control or dominant negative Lats2 (Lats2-KR), and relative TEAD reporter activity was measured. Data presented as mean ± SD, n = 3. d DVL3 was transfected in wt or Lats1/2 double-knockout (Lats1/2−/−) 293A cells, and nuclear YAP abundance by DVL3 was determined by immunoblot analysis (left panels) and immunofluorescence study (right panels). The cells were serum-starved for 16 h before harvest. e Schematic diagram of nuclear export of phosphorylated YAP by DVL. f Wnt ligands activate YAP resulting in decreased interaction with DVL. The 293A cells transfected with HA-tagged DVL3 were serum-starved and then stimulated by Wnt1 and Wnt3a ligands for 4 h. The whole-cell lysates (WCL) were subjected for immunoblot analysis and immunoprecipitation (IP) assay with anti-HA antibody. g Soluble Wnt1 and Wnt3a induce YAP nuclear localization. The confluent 293A cells were serum-starved and then stimulated by Wnt ligands for 4 h. Endogenous YAP and DVL localization were determined by confocal immunofluorescence microscopy. Scale bar, 10 μm. Unprocessed original scans of blots are shown in Supplementary Fig. 10
Fig. 5
DVL is required for cytoplasmic trafficking of YAP induced by E-cadherin or α-catenin. a The wt and DVL-TKO 293 T-REx cells were transfected with flag-tagged YAP (50 ng) in combination with vector control (1 μg) or E-cadherin (1 μg) or α-catenin (1 μg), and YAP localization was determined by confocal microscopy. Inset, DAPI nuclear stain; Scale bar, 5 μm. b The wt and DVL-TKO 293 cells were transfected with vector control (−) or E-cadherin (E-cad) or α-catenin (α-cat), and endogenous YAP abundance in nuclear fraction and whole-cell lysates (WCL) was determined by immunoblot analysis. Relative nuclear YAP abundance compared to control was measured by ImageJ. c The MCF-10A cells were cultured under confluent contact inhibition and intracellular YAP localization was monitored by confocal microscopy. The cells were incubated with a mouse IgG (HECD-) or a neutralizing monoclonal antibody (HECD, 10 μg/ml) that disrupts homophilic binding of E-cadherin for 16 h. Immunofluorescence images showing YAP staining of MCF-10A cells having tetracycline-inducible DVL3 in the absence (Dox-) or presence (Dox+) of doxycycline. Upper inset, HECD antibody detected by fluorescent-conjugated secondary antibody; lower Inset, DAPI nuclear stain; Scale bar, 5 μm. Unprocessed original scans of blots are shown in Supplementary Fig. 10
Fig. 6
Loss of p53/Lats2 tumor suppressor allows co-activation of YAP and canonical Wnt activity by DVL. a The 293 cells were transfected with control (dsRed) or shRNA for p53 (dsRed-shp53) in combination with HA-tagged DVL3 expression vector. Whole-cell lysates (WCL) were immunoblotted with indicated antibodies, and nuclear fractions were used for nuclear YAP abundance (left panels). Endogenous YAP localization was determined by confocal microscopy (right panels). Inset, DAPI nuclear stain; Scale bar, 10 μm. b, c The TEAD (b) or TCF/LEF (c) reporter constructs were co-transfected with YAP and DVL3 in control transfected cell (dsRed) or shRNA for p53 transfected cells, and the relative reporter activity was measured from triplicate experiments (mean ± SD). d The 293 cells were transfected with control (−) or mutants p53 (p53-R175H, p53-R273H) in combination with HA-tagged DVL3 expression vector. Whole-cell lysates (WCL) were immunoblotted, and nuclear fractions were used for nuclear YAP abundance. e, f The TEAD (e) and TCF/LEF (f) reporter construct was co-transfected with DVL in empty vector transfected cell (−) or p53 mutants transfected cells, and the relative reporter activity was measured from triplicate experiments (mean ± SD). g The TEAD or TCF/LEF reporter constructs were co-transfected with YAP and DVL as indicated in control transfected cell (dsRed) or shRNA for p53 transfected cells. The relative reporter activity was measured from triplicate experiments. Data presented as mean ± SD. h The 293 cells (1 × 106) were transiently transfected with YAP and DVL3 as indicated in combination with dsRed control or shRNA for p53, and the cells were inoculated into the flank of athymic nude mice (n = 10). Tumor volume was measured 5 weeks post-injection. Statistical significance was determined by Mann–Whitney test. i Kaplan–Meier survival graphs for breast cancer patients with wt or mutant p53 status on the basis of CTGF and Axin2 transcript abundances at an optimal threshold indicated by percentile numbers. Samples with high abundance of CTGF and Axin2 are represented with red lines. See Supplementary Fig. S8c for scatter plot of CTGF and Axin2 transcript abundance. A log-rank test was used to calculate statistical significances
Fig. 7
Role of LKB1/AMPK tumor suppressor axis on DVL’s function on YAP. a The MCF-10A cells expressing tetracycline-inducible shRNA against DVL3 were cultured under sparse culture condition and treated with 2-deoxyglucose (2DG, 3 mM) and metformin (Met, 5 mM) for 16 h period in absence (−) or presence (+) of doxycycline (Dox). Endogenous YAP localization was determined by confocal microscopy. Inset, DAPI nuclear stain; Scale bar, 10 μm. b The wt and DVL-TKO 293 T-REx cells were treated with 2DG and Met, and nuclear YAP abundance was then determined by immunoblot analysis (left panels) and confocal microscopy (right panels). Inset, DAPI nuclear stain; Scale bar, 5 μm. c Kinase-dead GFP-fused AMPK or flag-tagged LKB1 mutant was co-transfected with control (−) or HA-tagged DVL3 (+) in 293 cells. Whole-cell lysates (WCL) were immunoblotted with indicated antibodies, and nuclear fractions were used for nuclear YAP abundance (left panels). The TEAD (right upper) or TCF/LEF (right lower) reporter was co-transfected with DVL in empty vector transfected cell (vector) or kinase-dead AMPK (AMPK-KD) or LKB mutant (LKB-KD), and the relative fold repression of reporter activity was measured from triplicate experiments (mean ± SD). d The wt and AMPKα1/α2 double-knockout (DKO) MEFs were stably transfected with inducible DVL3 and nuclear YAP abundance was examined by immunoblot analysis (left panels) and immunofluorescence (right panels) in the absence (−) or presence (+) of doxycycline for a 48 h period. Upper inset, DAPI nuclear stain; Lower inset, HA (DVL3); Scale bar, 5 μm. e The A549 cells were stably transfected with inducible DVL3, and nuclear YAP abundance and DVL3 localization under sparse or confluent culture condition were examined by immunoblot analysis (left panels) and immunofluorescence (right panels) in presence of doxycycline. Inset, DAPI nuclear stain; Scale bar, 5 μm. f The 293 cells (1 × 106) were transiently transfected with YAP and DVL3 in combination with vector control or LKB-KD mutant, and the cells were inoculated into the flank of athymic nude mice (n = 10). The empty vector or LKB-KD transfected cells served as control. Tumor volume was measured 5 weeks end-point (Mann–Whitney test)
Similar articles
- Dishevelling Wnt and Hippo.
Kim NH, Lee Y, Yook JI. Kim NH, et al. BMB Rep. 2018 Sep;51(9):425-426. doi: 10.5483/BMBRep.2018.51.9.179. BMB Rep. 2018. PMID: 30078391 Free PMC article. - Restriction of intestinal stem cell expansion and the regenerative response by YAP.
Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS, Fuchs CS, Magness ST, Smits R, Ogino S, Kuo CJ, Camargo FD. Barry ER, et al. Nature. 2013 Jan 3;493(7430):106-10. doi: 10.1038/nature11693. Epub 2012 Nov 25. Nature. 2013. PMID: 23178811 Free PMC article. - Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway.
Mo JS, Meng Z, Kim YC, Park HW, Hansen CG, Kim S, Lim DS, Guan KL. Mo JS, et al. Nat Cell Biol. 2015 Apr;17(4):500-10. doi: 10.1038/ncb3111. Epub 2015 Mar 9. Nat Cell Biol. 2015. PMID: 25751140 Free PMC article. - Dishevelled: The hub of Wnt signaling.
Gao C, Chen YG. Gao C, et al. Cell Signal. 2010 May;22(5):717-27. doi: 10.1016/j.cellsig.2009.11.021. Epub 2009 Dec 13. Cell Signal. 2010. PMID: 20006983 Review. - Angiomotin'g YAP into the nucleus for cell proliferation and cancer development.
Hong W. Hong W. Sci Signal. 2013 Sep 3;6(291):pe27. doi: 10.1126/scisignal.2004573. Sci Signal. 2013. PMID: 24003252 Review.
Cited by
- PSME4 Degrades Acetylated YAP1 in the Nucleus of Mesenchymal Stem Cells.
Kim YS, Kim M, Cho DI, Lim SY, Jun JH, Kim MR, Kang BG, Eom GH, Kang G, Yoon S, Ahn Y. Kim YS, et al. Pharmaceutics. 2022 Aug 9;14(8):1659. doi: 10.3390/pharmaceutics14081659. Pharmaceutics. 2022. PMID: 36015285 Free PMC article. - MAML1/2 promote YAP/TAZ nuclear localization and tumorigenesis.
Kim J, Kwon H, Shin YK, Song G, Lee T, Kim Y, Jeong W, Lee U, Zhang X, Nam G, Jeung HC, Kim W, Jho EH. Kim J, et al. Proc Natl Acad Sci U S A. 2020 Jun 16;117(24):13529-13540. doi: 10.1073/pnas.1917969117. Epub 2020 Jun 1. Proc Natl Acad Sci U S A. 2020. PMID: 32482852 Free PMC article. - Atypical function of a centrosomal module in WNT signalling drives contextual cancer cell motility.
Luo Y, Barrios-Rodiles M, Gupta GD, Zhang YY, Ogunjimi AA, Bashkurov M, Tkach JM, Underhill AQ, Zhang L, Bourmoum M, Wrana JL, Pelletier L. Luo Y, et al. Nat Commun. 2019 May 29;10(1):2356. doi: 10.1038/s41467-019-10241-w. Nat Commun. 2019. PMID: 31142743 Free PMC article. - Intracellular Signals Activated by Canonical Wnt Ligands Independent of GSK3 Inhibition and β-Catenin Stabilization.
García de Herreros A, Duñach M. García de Herreros A, et al. Cells. 2019 Sep 25;8(10):1148. doi: 10.3390/cells8101148. Cells. 2019. PMID: 31557964 Free PMC article. Review. - Hippo-Yap/Taz signaling: Complex network interactions and impact in epithelial cell behavior.
van Soldt BJ, Cardoso WV. van Soldt BJ, et al. Wiley Interdiscip Rev Dev Biol. 2020 May;9(3):e371. doi: 10.1002/wdev.371. Epub 2019 Dec 11. Wiley Interdiscip Rev Dev Biol. 2020. PMID: 31828974 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- NFR-2017R1A2B3002241, NRF-2016R1E1A1A01942724, NRF-2017R1C1B1012464, NRF-2014R1A6A3A04055110/National Research Foundation of Korea (NRF)/International
- HI17C2586/Korea Health Industry Development Institute (KHIDI)/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous