Hypoxia-induced methylation of a pontin chromatin remodeling factor (original) (raw)
Abstract
Pontin is a chromatin remodeling factor that possesses both ATPase and DNA helicase activities. Although Pontin is frequently overexpressed in human cancers of various types and implicated in oncogenic functions, the upstream signaling network leading to the regulation of Pontin that in turn affects transcription of downstream target genes has not been extensively studied. Here, we identify Pontin is methylated by G9a/GLP methyltransferases in hypoxic condition and potentiates HIF-1α-mediated activation by increasing the recruitment of p300 coactivator to a subset of HIF-1α target promoters. Intriguingly, Pontin methylation results in the increased invasive and migratory properties by activating downstream target gene, Ets1. In contrast, inhibition of Pontin methylation results in the suppression of tumorigenic and metastatic properties. Together, our data provide new approaches by targeting Pontin methylation and its downstream targets for the development of therapeutic agents for human cancers.
Keywords: epigenetics, transcriptional regulation, covalent nonhistone modification
Defining the molecular mechanisms that coordinate specific upstream signal to diverse transcriptional responses remains an important goal in biology. The main downstream effect of signaling cascades is the modulation of transcription factors and coregulators functioning in the nucleus in response to specific upstream signals (1–4). Oxygen deficiency affects not only physiological processes such as those involved in embryonic development, wound healing, and inflammation, but also in pathological conditions such as tumor progression, ischemic disease, and atherosclerosis (5, 6). Many hypoxic responses are mediated by hypoxia inducible factor 1 (HIF-1), a heterodimeric transcription factor that is comprised of an oxygen-regulated α subunit (HIF-1α or HIF-2α) and a constitutively expressed β subunit (HIF-1β) (7, 8). Under normoxic conditions, HIF-1α is unstable and subject to degradation mediated by the von Hippel–Lindau E3 ligase. However, under hypoxic conditions, HIF-1α is stabilized and it translocates into the nucleus and binds to HIF-1β. The HIF-1α/β heterodimer is then able to bind to the hypoxia response element (HRE) that contains ACGTG as a core sequence.
Although HIF-1α and HIF-2α share some common targets including VEGF and GLUT1 that are involved in regulating angiogenesis and glycolytic pathway, HIF-2α appears to have its unique targets during embryonic development by regulating factors such as Oct4 (9) and antioxidant enzymes, such as SOD2 (10). Both isoforms of HIFα interact with Sirt1 and the transcriptional activity of HIF-2α is enhanced upon deacetylation by Sirt1 whereas the transcriptional activity of HIF-1α is repressed by deacetylation (11, 12). Therefore, although the two isoforms of HIFα both execute hypoxic response, it appears that they have distinct functions through differential regulatory mechanisms.
Gene expression is not only influenced by presence of transcription factors, but also by chromatin structure regulated by chromatin modifiers. The transcription of most genes is regulated by the coordinate action of chromatin-remodeling complexes such as ATP-dependent SWI/SNF proteins as well as histone modifying enzymes. Numerous enzymatic activities are associated with coregulator functions and the activities are regulated by posttranslational modifications, including methylation, acetylation, phosphorylation, ubiquitylation, and small ubiquitin-like modifier (SUMO)-ylation (13–16). SUMOylation of Reptin has been shown to be crucial for transcriptional repression of a KAI1 metastasis suppressor gene (17, 18), whereas SUMOylation of Pontin has been shown to function as a transcriptional coactivator of androgen receptor-mediated transcription in prostate cancer (19). Recently, we reported that Reptin chromatin-remodeling factor negatively regulates a subset of hypoxia-responsive genes (20). Biochemical purification of Reptin-binding proteins identified G9a, and hypoxia-induced Reptin methylation turned out to participate in downregulating a subset of hypoxia target genes involved in metabolism and tumor development using a genome-wide analysis approach. In this manuscript, we provide evidence that Pontin chromatin-remodeling factor is methylated by G9a and GLP in hypoxic condition. We address a detailed molecular mechanism by which Pontin methylation mediates and elaborates the transcriptional regulation, thereby strongly activating a subset of hypoxia target genes differentially regulated by Pontin compared to those of Reptin.
Results
Pontin Is Methylated by G9a and GLP Methyltransferases.
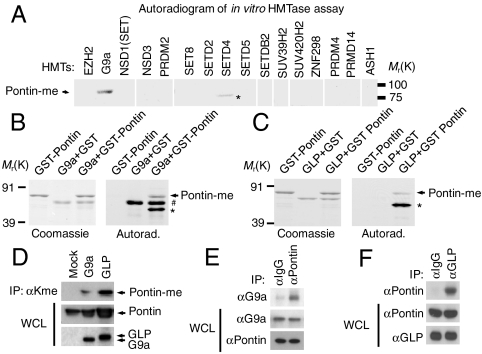
To screen for enzymes responsible for Pontin methylation, we performed in vitro methyltransferase assays and found that out of sixteen histone methyltransferases (HMTs), G9a was the only enzyme able to methylate Pontin (Fig. 1A). Because G9a often functions together with GLP (21, 22), we examined whether G9a and/or GLP are responsible for Pontin methylation. Further in vitro HMT assays using G9a and GLP revealed that Pontin is methylated by both enzymes (Fig. 1 B and C). To determine whether G9a/GLP can methylate Pontin in vivo, we examined Pontin methylation by performing immunoprecipitation using antimethyl lysine antibody by introducing either G9a or GLP. Pontin appears to have very low basal level of methylation, whereas its methylation status can be dramatically induced by G9a/GLP (Fig. 1D). This was supported by coimmunoprecipitation assays that showed strong Pontin interaction with both G9a and GLP at the endogenous level (Fig. 1 E and F).
Fig. 1.
G9a and GLP methylate Pontin. (A) Histone lysine methyltransferases were prepared as GST-fusion proteins and incubated with GST-Pontin in the presence of _S_-adenosyl-L-[_methyl_-3H] methionine (SAM). Reaction products were analyzed by autoradiogram. (B and C) In vitro methylation assay was performed using GST-G9a SET domain (B) or GST-GLP SET domain (C) with Coomassie brilliant blue staining (Left). Asterisks (*) indicate nonspecific bands, and the hash mark (#) indicates automethylation. (D) HEK293 cells were transfected with empty vector or with expression vector encoding G9a or GLP. Cell lysates were immunoprecipitated with antibody against antimethyl-lysine antibody followed by immunoblot analysis with anti-Pontin antibody to detect methylated Pontin. (E and F) Coimmunoprecipitation assay of endogenous Pontin with G9a (E) or GLP (F).
Pontin Methylation Is Induced by Hypoxia.
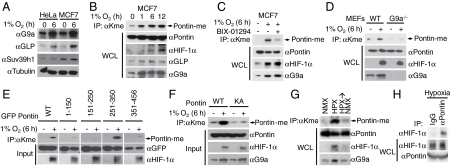
We then asked whether Pontin methylation can be induced by hypoxia as we have reported previously that Reptin methylation is induced by hypoxia as a result of increased G9a protein level and corresponding increase in its methyltransferase activity (20). Indeed, G9a protein levels were increased in both HeLa and MCF7 cells exposed to 6 h of hypoxia (1% O2) whereas there was no change in the levels of Suv39h1; another histone methyltransferase that targets histone H3K9 suggesting that there exist a specificity amongst several HMTs in response to hypoxia in upregulating their activity (Fig. 2A). We then examined whether Pontin methylation is induced in hypoxic condition, and found that Pontin methylation continued to increase with hypoxic exposure up to at least 12 h with corresponding accumulation of G9a and GLP in MCF7 breast cancer cells and HEK293 cells (Fig. 2B and Fig. S1). To further establish that the increase in Pontin methylation is directly caused by the increase in G9a and GLP enzymatic activity, we exposed cells to hypoxia in the presence and absence of G9a and GLP inhibitor, BIX-01294 (23, 24). BIX-01294 significantly reduced hypoxia-mediated Pontin methylation further demonstrating that the methyltransferase activity of G9a/GLP is required for Pontin methylation (Fig. 2C). Dependency of Pontin methylation on the expression of G9a was also demonstrated in G9a-deficient mouse embryonic fibroblasts (MEFs) (Fig. 2D). These data strongly suggest that G9a and GLP are required for hypoxia-mediated Pontin methylation. To identify specific methylation sites on Pontin, we performed in vivo methyltransferase assays on Pontin deletion mutants and found that a region containing the amino acid residues between 251 and 350, was methylated (Fig. 2E). Within this region, there were six lysine residues, and we mutated each one to alanine, however, these single mutants (K265A, K267A, K268A, K274A, K281A, and K285A) still retained some methylation (Fig. S2). Therefore we eventually had to mutate all 6 lysine residues to alanine to completely eliminate the residual methylation. We found that Pontin mutant that harbors lysine to alanine mutation (KA) did not show any increase in methylation whereas Pontin WT showed significant increase in methylation by hypoxia (Fig. 2F).
Fig. 2.
Hypoxia-induced Pontin methylation is mediated by G9a and GLP. (A) Protein expression levels of G9a, GLP, and Suv39h1 were examined by immunoblotting in HeLa and MCF7 cells exposed to normoxic and hypoxic conditions as indicated. (B and C) Pontin methylation was examined by immunoprecipitation of cell lysates from MCF7 cells exposed to hypoxia with anti-methyl-lysine antibody followed by immunoblotting with anti-Pontin, HIF-1α, G9a, and GLP antibodies for the indicated times (B) or in the presence and absence of an inhibitor of G9a and GLP, BIX-01294 (C). (D) Requirement of G9a for hypoxia-induced Pontin methylation was examined in WT and G9a-deficient MEFs exposed to normoxic and hypoxic condition. (E) HEK293 cells were cotransfected with plasmids encoding each GFP-tagged Pontin deletion constructs spanning the indicated amino acid residues exposed to normoxia or hypoxia for 6 h. Whole cell extracts were immunoprecipitated with antimethyl-lysine antibody followed by immunoblot assay using anti-GFP antibody to detect methylated Pontin. (F) Pontin methylation was examined as in (E) using constructs encoding Pontin WT or KA mutant exposed to normoxia or hypoxia for 6 h. (G) Dynamics of Pontin methylation was examined in 293HEK cells exposed to either normoxia, hypoxia, or hypoxia and normoxia and Pontin methylation was examined as in (E). (H) Endogenous interaction between Pontin and HIF-1α in MCF7 cells exposed to hypoxia using either anti-HIF-1α or normal IgG.
Next, to examine whether Pontin methylation is dynamic, we performed in vivo methylation assay in normoxic or hypoxic condition as well as a dynamic normoxia-hypoxia-normoxia condition. Immunoprecipitation assay with antimethyl lysine antibody showed that Pontin methylation was induced by hypoxia, and this methylation returned to its basal levels when the cells were returned to normoxic condition (Fig. 2G). The extent of Pontin methylation closely followed G9a protein levels as it was elevated following hypoxia and returned to basal levels once the cells were again exposed to normoxic condition. In an attempt to determine the functional link between Pontin methylation and hypoxic response, we examined the physical association between HIF-1α and Pontin at the endogenous expression level, and found that Pontin is able to readily interact with HIF-1α during hypoxic condition (Fig. 2H). Together, these data indicate that both Pontin methylation and the interaction of Pontin with HIF-1α are induced in hypoxic condition.
Identification of Pontin-Dependent Target Genes by Microarray Analysis.
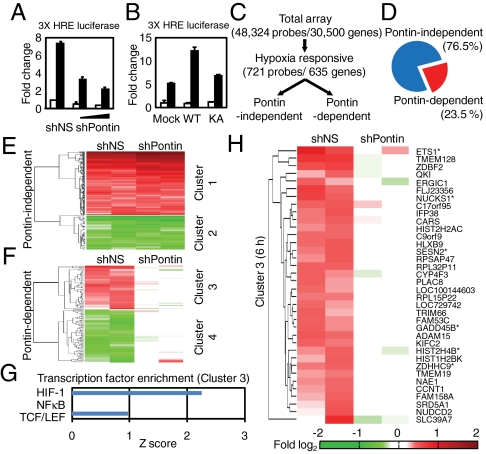
As Pontin is able to interact with HIF-1α, we hypothesized that Pontin might influence the transcriptional activity of HIF-1α. To this end, we attempted to determine the transcriptional role of Pontin methylation on the expression of hypoxia-responsive genes. We first performed a reporter assay using 3× HRE-luciferase and found that Pontin knockdown led to a reduction of hypoxia-mediated increase in 3× HRE-luciferase activity (Fig. 3A). Upon expressing Pontin WT, the activation of 3× HRE-luciferase activity was significantly increased whereas Pontin KA mutant failed to have any effect (Fig. 3B). We then performed a microarray analysis from RNAs isolated from MCF7 cells expressing either control shRNA (shNS) or Pontin shRNA (shPontin) in normoxic and hypoxic conditions to determine the effect of Pontin on the expression of hypoxic responsive genes across the whole genome (Fig. 3C). We used a Gaussian curve fitting to determine the applicable cut-offs in determining genes that showed significant change in response to hypoxia. Approximately 23.5% of the differentially expressed genes were affected by Pontin knockdown, indicating that these were in fact regulated both by hypoxia and Pontin (Fig. 3D). Hierarchical clustering was then performed and hypoxia-responsive genes could be categorized into several clusters (Fig. 3 E and F and Table S1). These clusters represented mainly into Pontin-independent group (clusters 1 and 2) and Pontin-dependent group (clusters 3 and 4) that lost sensitivity to hypoxic responsiveness as a result of Pontin knockdown.
Fig. 3.
Pontin-dependent target identification by microarray analysis. (A and B) Three × HRE-luciferase reporter assay with knockdown of Pontin (A) or overexpression with Pontin WT and KA mutant (B) under normoxia or hypoxia for 6 h. Values are expressed as mean ± SEM of three independent experiments. (C) Flow chart showing the strategy of cDNA microarray analysis and Pontin-dependent gene selection process. (D) Pontin-dependent genes represent about 23.5% of all differentially expressed genes by hypoxia. (E and F) Identification of Pontin-independent genes (E) and Pontin-dependent up-regulated (cluster 3, red) and down-regulated (cluster 4, green) genes (F) by hierarchical clustering and comparing fold change of hypoxia-induced genes from cells expressing shNS and shPontin. (G) Known target genes for HIF-1, NFκB and TCF/LEF were collected and the enrichment of these genes was analyzed for each cluster is shown as a Z score within each cluster. (H) Heatmap diagram of Pontin-dependent genes that are activated by hypoxia (6 h) showing gene names with known HIF-1α target genes marked with an asterisk. Also see Table S1 for Pontin-dependent target genes at 9 h of hypoxia.
Given that Pontin acts as an activator, we asked the question whether hypoxia and Pontin expression can synergistically act on hypoxia-responsive genes. To this end, we examined cluster 3 to determine which transcription factor targets are enriched. Upon searching for genes that are affected by transcription factors that have been previously reported to modulate transcription upon hypoxic stress such as HIF-1, NF-κB, and TCF/LEF (25), HIF-1 scored the highest in terms of the number of its target genes enriched in Pontin-dependent cluster 3 (from both 6 and 9 h of hypoxia) (Fig. 3G). In cluster 3, six out of 36 genes in 6 h dataset and four out of 34 genes in 9 h dataset were known HIF-1 target, and the promoters of 38 genes contained putative hypoxia response elements (Fig. 3H and Tables S1 and S2). These analyses strongly support the idea that Pontin-dependent clusters contained many HIF-1α targets (cluster 3). Intriguingly, genome-wide analysis of hypoxia-induced Pontin-dependent HIF-1α target genes revealed that Pontin-dependent target genes do not generally overlap with Reptin-dependent target genes upon hypoxia (20) (Fig. S3 and Table S2).
Pontin Methylation Is Required for Transcriptional Activation of a Subset of Hypoxia-Responsive Genes.
To validate Pontin-dependent target genes identified from our microarray analysis (Fig. 3H and Tables S1 and S2), we performed a quantitative RT-PCR analysis on both Pontin-dependent and -independent genes. Pontin knockdown led to an inhibition of hypoxia-mediated activation of Ets1, KDM4B, and IGFBP3 transcripts (Pontin-dependent) whereas no effect was observed for BNIP3L, HK2, and WSB1 transcripts (Pontin-independent) (Fig. 4 A and B). Further, introduction of shRNA-resistant Pontin WT (Pontin WT_R_) had an activating potential whereas shRNA-resistant Pontin KA mutant (Pontin KA_R_) appeared to have none. This activating function does not appear to be MCF7-specific event, as we have also observed regulation of Ets1 transcript levels in HeLa cells (Fig. S4). Together, these data provide evidence that Pontin methylation participates in hypoxia-driven activation of a subset of hypoxia target genes involved in oncogenesis and cell survival.
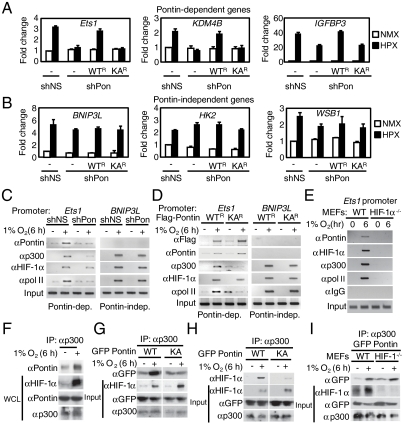
Fig. 4.
Pontin methylation potentiates HIF-1α transcriptional activity by enhancing interaction with p300. (A and B) Quantitative RT-PCR analysis of Pontin-dependent (A) and Pontin-independent (B) hypoxia target gene expressions identified (both 6 and 9 h). Results are expressed as relative mRNA levels compared to shNS under normoxic condition. Values are expressed as mean ± SEM of three independent experiments. (C and D) ChIP assays on the Ets1 and BNIP3L promoters in MCF7 cells with Pontin knockdown by shPontin (C) and reconstituted with exogenous shRNA-resistant Pontin WT_R_ and KA_R_ mutant (D) upon hypoxia. Promoter occupancy of proteins indicated was analyzed. (E) ChIP analysis performed in WT and HIF-1α-deficient (_HIF_-1_α_-/-) MEFs examining the recruitment of various proteins to Ets1 promoter as indicated. (F) Endogenous interaction between Pontin and p300 in MCF7 cells exposed to either normoxia or hypoxia using anti-p300 antibody. (G and H) MCF7 cells were transfected with either Pontin WT or KA mutant and immunoprecipitation assay was performed using anti-p300 antibody. (I) Pontin WT or KA mutant was immunoprecipitated with anti-p300 antibody in WT or _HIF_-1_α_-/- MEFs followed by immunoblotting using antibodies indicated.
To gain further insight into how Pontin modulates expression of a subset of HIF-1 target genes during hypoxic condition, we performed ChIP assays on Pontin-dependent and independent promoters in MCF7 cells expressing either control shRNA (shNS) or Pontin shRNA (shPontin). Hypoxia resulted in increased recruitment of Pontin on Ets1 promoter by binding to HIF-1α that is recruited to its response element whereas knockdown of Pontin resulted in reduced level of Pontin recruitment without affecting HIF-1α recruitment (Fig. 4C). The recruitment of p300 to Ets1 promoter appeared to be HIF-1α and Pontin-dependent as knockdown of Pontin caused a marked decrease in p300 recruitment. However, upon examining Pontin-independent target promoter; BNIP3L, Pontin recruitment was not observed nor the facilitation of p300 recruitment to the promoter (Fig. 4C). Similarly, exogenously introduced Pontin WT was able to enhance recruitment of p300 to Ets1 promoter whereas Pontin KA mutant failed to recruit p300 to the promoter, suggesting that Pontin methylation is crucial for the increased p300 localization with HIF-1α (Fig. 4D). Further, to determine whether the recruitment of Pontin and p300 to Ets1 promoter occurs in HIF-1α-dependent manner, we performed ChIP assays in HIF-1α-deficient MEFs. The recruitment of Pontin and p300 to Ets1 promoter was only observed in the presence of HIF-1α (WT MEFs) whereas no significant recruitment was observed in HIF-1α-deficient (HIF-1_α_-/-) MEFs (Fig. 4E).
To further investigate how methylated Pontin is involved in transcriptional activation, we tested the possibility that Pontin shows altered interaction with other coactivators that may affect HIF-1α transcriptional activity. As p300 conducts a critical role in HIF-1α-mediated transcriptional activation, we first examined whether methylated Pontin shows increased interaction with p300. Coimmunoprecipitation assays revealed that Pontin's ability to interact with p300 was enhanced in hypoxic condition at the endogenous level (Fig. 4F). This increase in p300 interaction was Pontin methylation-dependent as Pontin KA mutant did not show significant difference in response to hypoxia (Fig. 4G). Moreover, Pontin WT, but not Pontin KA mutant, was able to facilitate the interaction between HIF-1α and p300 (Fig. 4H). Pontin possesses its ability to interact with p300 directly without HIF-1α, as it shows increased binding with p300 in HIF-1α-deficient MEFs in a hypoxia-dependent manner (Fig. 4I). Collectively, these data indicate that Pontin methylation has a role in enhanced binding of p300 and thereby able to increase transcriptional activity of HIF-1α.
Pontin Methylation Enhances the Proliferative and Invasive Potential of Breast Cancer Cells.
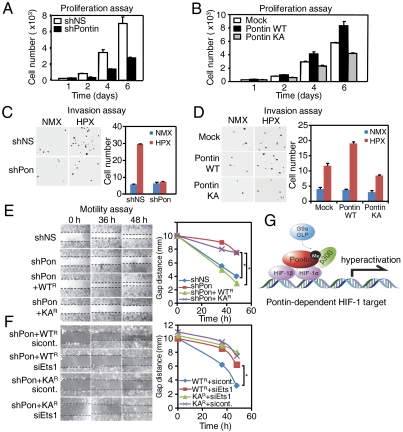
As many genes included in Pontin-dependent group contained genes that play a major role in proliferation, cell cycle, cell motility and invasion, we performed cell-based analyses to determine whether Pontin methylation plays an important role in regulating these processes. First, proliferation of MCF7 cells was inhibited upon Pontin knockdown suggesting that Pontin expression participates in upregulating proliferative potential during hypoxia (Fig. 5A). Consistently, ectopic expression of Pontin WT enhanced proliferation whereas expression of Pontin KA mutant lost its ability to increase proliferation (Fig. 5B). We also performed cell invasion assays in hypoxic condition to determine the invasive potential of MCF7 cells expressing shPontin or shNS. Invasion assays revealed that Pontin expression was not only important for proliferation of MCF7 cells, but also the invasive potential during hypoxia as knockdown of Pontin greatly impaired invasion of these cells through Matrigel (Fig. 5C). Similarly, invasion through Matrigel by MCF7 cells was markedly increased by introducing Pontin WT, whereas Pontin KA did not appear to confer increase in invasive potential (Fig. 5D). Together, these data confirmed that either Pontin knockdown or Pontin KA mutant overexpression significantly inhibited the invasive potential of MCF7 cells.
Fig. 5.
Pontin methylation increases tumorigenic properties via Ets1. (A and B) Proliferation was monitored over six days in hypoxic condition for MCF7 cells expressing shNS or shPontin (A) and expression of either Pontin WT or KA mutant (B). (C and D) Photomicrographs (40×) from Matrigel-coated transwell invasion assay of MCF7 cells expressing shNS and shPontin (C) and Pontin WT or Pontin KA (D) in normoxic and hypoxic conditions for 24 h. Bar graph shows mean number of cells per filter and p value is shown from student’s t_-test analysis. Error bars represent SEM; n = 5 (Right). (E and F) Photomicrographs from a scratch-mobility assay of MCF7 cells expressing either shNS or shPontin and reconstituted with shRNA-resistant Pontin WT_R or KA_R_ mutant (E) or Pontin WT_R_ or KA_R_ mutant either transfected with control siRNA or Ets1 siRNA (F) in the hypoxia for the indicated times (Left). Gap distance between the two migrating fronts is measured and shown as a graph (Right). Asterisk (*) is indicated for p < 0.001 by two-tailed students _t_-test. (G) Proposed model of transcriptional activation role for methylated Pontin during hypoxia. Pontin is methylated by G9a/GLP by hypoxia and enhances the recruitment of p300 to a subset of hypoxia target promoters, thereby potentiating the transcriptional activity of HIF-1α leading to increase in hypoxia-mediated cellular migration, proliferation, and invasion.
Ets1, which Is Regulated by Pontin Methylation, Is Responsible for Increased Cell Motility.
As part of examining other processes affecting metastatic potential of cancer cells, we examined the effect of hypoxia-mediated Pontin methylation on cell motility by performing scratch-mobility assay. Over 36–48 h, control shRNA transfected cells moved significantly into the scratch, whereas Pontin knockdown inhibited this movement (Fig. 5E). Adding back shRNA-resistant Pontin WT to these Pontin- knockdown cells resulted in rescuing Pontin knockdown phenotype, whereas introducing Pontin KA mutant failed to do so. These data strongly suggest that Pontin methylation is indeed important for conferring tumorigenic and metastatic potential of MCF7 cells. Because Ets1 was obtained from microarray analysis as a Pontin-dependent target gene that is responsible for cell motility and invasion (Fig. 3H), we decided to establish the contribution of Ets1 expression to cell motility. We then performed a scratch-mobility assay using MCF7 cells stably expressing Pontin WT_R_ or Pontin KA_R_ with either control siRNA or Ets1 siRNA. Pontin-dependent increase in cell motility was significantly decreased by Ets1 knockdown, demonstrating that Ets1 is required for Pontin-mediated increase in cell motility (Fig. 5F). Knockdown of Ets1 had little or no effect on cell motility of MCF7 cells stably expressing Pontin KA_R_. Collectively, our findings indicate that hypoxia-induced Pontin methylation is responsible for the regulation of a subset of HIF-1α target genes exemplified by Ets1 and thereby affects cell proliferation, tumor growth and invasive properties.
Discussion
In this study, we have identified Pontin as a nonhistone substrate methylated by hypoxia-induced G9a and GLP, leading to hyperactivation of a subset of hypoxia target genes by enhanced binding of p300 to HIF-1α and thereby increasing transcriptional activity of HIF-1α (Fig. 5G). Although Pontin and Reptin share high structural homology, they have distinct functions in regulating their specific target genes as a coactivator and as a corepressor, respectively (17–19). We found that this type of antagonistic regulation is also applied to the regulation of hypoxia target genes as well as the regulation of well-established Wnt target genes (20, 26). Hypoxia-induced G9a is responsible for methylation of both Pontin and Reptin, but the functional outcome appears to be in the opposite direction. G9a-mediated Reptin methylation exhibits high affinity for HIF-1α transcription factor as well as for HDAC1 corepressor, functioning as a negative regulator on a subset of hypoxia target genes (20). However, G9a/GLP-mediated Pontin methylation exhibits enhanced binding to p300 coactivator, and contributes to further activation of a subset of hypoxia target genes. It appears that Pontin is recruited to the target promoter at an early time point for transcriptional activation of HIF-1α target genes, compared to Reptin recruited on HIF-1α target promoters at a later time point for negative regulation. Because the ATPase activity of Pontin is important in exerting chromatin-remodeling function, we considered whether the ATPase activity is involved in regulating its methylation-dependent hypoxia targets. We found that there was no significant difference between Pontin WT and ATPase mutant in terms of its methylation, transcriptional activity and recruitment to Ets1 promoter (Fig. S5) suggesting that Pontin’s ATPase activity is not involved in regulating the hypoxia targets identified in this study.
Because methylation and demethylation processes are dynamic and thus the methylation status of nonhistone proteins as well as histones is likely to be determined by the net effect of these enzymes. Indeed, Pontin methylation is dynamic; the extent of Pontin methylation was elevated following hypoxia and returned to basal level once the cells are again exposed to normoxic condition. It can be speculated that certain demethylases may function to oppose Pontin methylation for a dynamic on-off switch for a subset of hypoxia-responsive genes. Further, hypoxia-induced Pontin methylation affects binding to HIF-1α and p300, and the association between methylated Pontin with HIF-1α or p300 appeared to be DNA-independent as treatment of ethidium bromide did not alter their binding (Fig. S6).
Together, our finding that G9a- and GLP-dependent Pontin methylation regulates a subset of hypoxia target genes represents a coordinate signaling pathway by which HIF-1α transcriptional activity can be modulated, thereby affecting cell migration, tumor growth, and cell survival pathway that are important for the progression of cancer. Ets1, which was selected as a Pontin-dependent target gene from microarray analysis, turned out to be important for exerting its effect on cell motility and growth regulation by Pontin methylation upon hypoxia. Our data shed light on the mechanism of Pontin’s potential regulatory role in tumor progression upon hypoxia and suggest a possibility of developing therapeutic agents that target Pontin methylation, providing an avenue for powerful anticancer therapeutic approaches in the future.
Methods
Antibodies.
The following commercially available antibodies were used: anti-Pontin, anti-GFP, anti-p300, anti-HDAC1, and anti-HIF-1α (Santa Cruz Biotechnology), anti-G9a, and anti-Suv39h1 (Upstate Biotechnology), antimethyl lysine and anti-GLP (Abcam), anti-FLAG (Sigma), and anti-RNA Polymerase II (Berkeley Antibody Company).
In Vitro Methyltransferase Assay.
All recombinant proteins (GST, GST-Pontin, GST-G9a SET, and GST-GLP SET) were purified from Escherichia coli. In vitro methyltransferase assays were performed with the reactions assembled in 5× lysine methyltransferase buffer including 3H-S-adenosylmethionine and purified proteins and incubated overnight at 37 °C as described in (20). Laemmli buffer was added to samples, and loaded on SDS-PAGE for autoradiography.
Quantitative Real-Time RT-PCR and ChIP Assays.
Quantitative RT-PCR and ChIP assays were conducted as previously described (20). Also see Table S3 for primer sequences used.
Statistical Analysis.
Statistical differences in test and control samples were determined by Student’s t test or ANOVA using the Statview package (Abacus Concepts, Inc.).
Supplementary Material
Supporting Information
Acknowledgments.
We thank G. Semenza for providing HIF-1α null MEFs, A. Tarakhovsky for G9a null MEFs, and Boehringer Ingleheim for G9a inhibitor. This work was supported by Creative Research Initiatives Program (Research Center for Chromatin Dynamics, 2009-0081563) (S.H.B.), the Converging Research Center Program (2010K001298) (D.H.), the Basic Science Research Program (3344-20100053) (J.S.L.), the National Junior Research Fellowship (NRF-2011-A01496-0001806) (H-J.R.S.), and Brain Korea 21 Fellowship (J.S.L., Y.K., and H.J.N.) from the National Research Foundation (NRF) Grant funded by the Ministry of Education, Science, and Technology (MEST) of Korea.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE27813).
References
- 1.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karin M, Lawrence T, Nizet V. Innate immunity gone awry: Linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823–835. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 3.Kastan MB, et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 4.Behrens J, et al. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 5.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 6.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 7.Wang GL, Semenza GL. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J Biol Chem. 1993;268:21513–21518. [PubMed] [Google Scholar]
- 8.Ema M, et al. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA. 1997;94:4273–4278. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol. 2008;9:285–296. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scortegagna M, et al. Multiple organ pathology, metabolic abnormalities, and impaired homeostasis of reactive oxygen species in Epas1-/- mice. Nat Genet. 2003;35:331–340. doi: 10.1038/ng1266. [DOI] [PubMed] [Google Scholar]
- 11.Dioum EM, et al. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324:1289–1293. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- 12.Lim JH, et al. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol Cell. 2010;38:864–878. doi: 10.1016/j.molcel.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 13.Wu SC, Zhang Y. Minireview: Role of protein methylation and demethylation in nuclear hormone signaling. Mol Endocrinol. 2009;23:1323–1334. doi: 10.1210/me.2009-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang XJ, Seto E. Lysine acetylation: Codified crosstalk with other posttranslational modifications. Mol Cell. 2008;31:449–461. doi: 10.1016/j.molcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han SJ, Lonard DM, O’Malley BW. Multi-modulation of nuclear receptor coactivators through posttranslational modifications. Trends Endocrinol Metab. 2009;20:8–15. doi: 10.1016/j.tem.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baek SH. When signaling kinases meet histones and histone modifiers in the nucleus. Mol Cell. 2011;42:274–284. doi: 10.1016/j.molcel.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Kim JH, et al. Transcriptional regulation of a metastasis suppressor gene by Tip60 and beta-catenin complexes. Nature. 2005;434:921–926. doi: 10.1038/nature03452. [DOI] [PubMed] [Google Scholar]
- 18.Kim JH, et al. Roles of sumoylation of a reptin chromatin-remodelling complex in cancer metastasis. Nat Cell Biol. 2006;8:631–639. doi: 10.1038/ncb1415. [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, et al. SUMOylation of pontin chromatin-remodeling complex reveals a signal integration code in prostate cancer cells. Proc Natl Acad Sci USA. 2007;104:20793–20798. doi: 10.1073/pnas.0710343105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JS, et al. Negative regulation of hypoxic responses via induced Reptin methylation. Mol Cell. 2010;39:71–85. doi: 10.1016/j.molcel.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins RE, et al. The ankyrin repeats of G9a and GLP histone methyltransferases are mono- and dimethyllysine binding modules. Nat Struct Mol Biol. 2008;15:245–250. doi: 10.1038/nsmb.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tachibana M, Matsumura Y, Fukuda M, Kimura H, Shinkai Y. G9a/GLP complexes independently mediate H3K9 and DNA methylation to silence transcription. EMBO J. 2008;27:2681–2690. doi: 10.1038/emboj.2008.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubicek S, et al. Reversal of H3K9me2 by a small-molecule inhibitor for the G9a histone methyltransferase. Mol Cell. 2007;25:473–481. doi: 10.1016/j.molcel.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 24.Chang Y, et al. Structural basis for G9a-like protein lysine methyltransferase inhibition by BIX-01294. Nat Struct Mol Biol. 2009;16:312–317. doi: 10.1038/nsmb.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cummins EP, Taylor CT. Hypoxia-responsive transcription factors. Pflugers Arch. 2005;450:363–371. doi: 10.1007/s00424-005-1413-7. [DOI] [PubMed] [Google Scholar]
- 26.McDonald SL, Silver A. The opposing roles of Wnt-5a in cancer. Br J Cancer. 2009;101:209–214. doi: 10.1038/sj.bjc.6605174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information