Molecular machines governing exocytosis of synaptic vesicles (original) (raw)
. Author manuscript; available in PMC: 2015 Jun 10.
Published in final edited form as: Nature. 2012 Oct 11;490(7419):201–207. doi: 10.1038/nature11320
Abstract
Calcium-dependent exocytosis of synaptic vesicles mediates the release of neurotransmitters. Important proteins in this process have been identified such as the SNAREs, synaptotagmins, complexins, Munc18 and Munc13. Structural and functional studies have yielded a wealth of information about the physiological role of these proteins. However, it has been surprisingly difficult to arrive at a unified picture of the molecular sequence of events from vesicle docking to calcium-triggered membrane fusion. Using mainly a biochemical and biophysical perspective, we briefly survey the molecular mechanisms in an attempt to functionally integrate the key proteins into the emerging picture of the neuronal fusion machine.
Exocytosis and recycling of synaptic vesicles define how much transmitter is released from nerve terminals during incoming action potentials (Fig. 1). Under resting conditions, synaptic vesicles are stored in the cytoplasm of the nerve terminal, with some of them attached to specialized sites at the presynaptic plasma membrane termed active zones. Active zones are composed of unique multidomain proteins that provide a scaffold for vesicle docking and participate in activating the release apparatus, referred to as priming. Priming probably involves several reactions, including some requiring metabolic energy. Docked and primed vesicles (termed readily releasable pool) are ready to go, and some do so spontaneously, with the transmitter released by a single vesicle giving rise to a miniature postsynaptic potential. When an action potential arrives, voltage-gated calcium channels open, with the resulting calcium influx stimulating the rate of exocytosis more than 100,000 fold in a highly cooperative manner (for review see ref. 1).
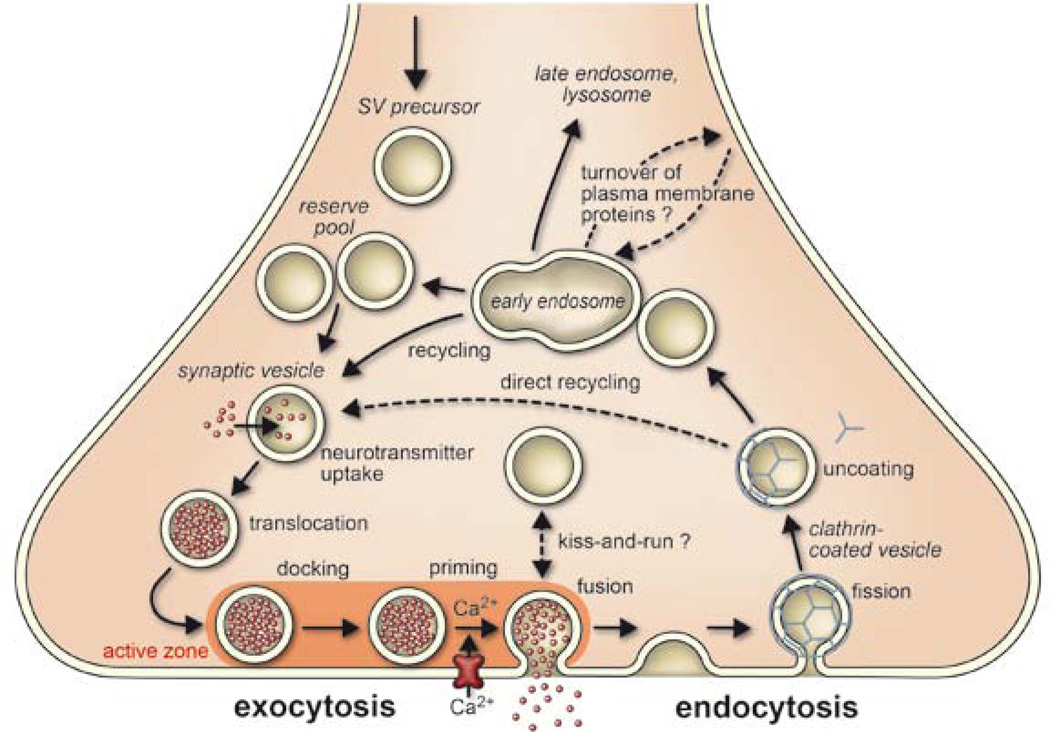
Figure 1.
Trafficking pathways in the nerve terminal. Synaptic vesicles are filled with neurotransmitter and stored in the cytoplasm. Active vesicles are translocated to release sites in the active zone where they dock. Priming involves all steps required to acquire release readiness of the exocytotic complex. Although usually assumed to occur after docking, priming and even triggering may precede docking during sustained activity, resulting in immediate fusion of an arriving vesicle. After exocytosis, the vesicle proteins probably remain clustered and are then retrieved by endocytosis. Despite some lingering controversies, consensus is emerging that retrieval is generally mediated by clathrin-mediated endocytosis. After clathrin uncoating, synaptic vesicles are regenerated within the nerve terminal, probably involving passage through an endosomal intermediate. Actively recycling vesicles are in slow exchange with the reserve pool. See text for more details
During the past two decades, the key proteins mediating neuronal exocytosis have been identified. Many of them belong to structurally conserved protein families including the SNAREs, Rab proteins, Sec1/ Munc18-like (SM) proteins, and a group of tethering proteins termed CATCHR (complex associated with tethering containing helical rods) proteins. Apparently, they form the core of an ancient intracellular fusion machine that diversified during evolution to adapt to the needs of specialized compartments. Neuronal exocytosis constitutes one of such adaptations, and specific regulatory proteins such as synaptotagmins and complexins evolved in the animal kingdom (for reviews see refs 2–8).
Despite such progress, there is still a gap in understanding between the functional properties of synaptic exocytosis and the molecular features of the key proteins. Modern electrophysiological9,10 and imaging approaches (11–13) provided a wealth of information about the number of docked and primed vesicles, the exchange rates of vesicles between different pools, their release probabilities, their kinetics of exocytosis, and the dependence of exocytosis on calcium. Thus, detailed job descriptions for the underlying molecular machines are available. However, whereas genetic perturbations were instrumental in defining the basic functions of the key proteins, it often proved difficult to assign them to a specific step in the exocytotic pathway. For instance, Munc18, synaptotagmin and even the SNAREs were shown to function in docking as well as in priming and triggering. Conversely, specific steps such as docking are controlled by multiple proteins (see refs 7 and 11 for a more detailed discussion). It also often proved difficult to reconcile the physiological effects of the perturbations with the physicochemical properties of the proteins. Thus, the molecular mechanisms responsible for the attachment of synaptic vesicles to the active zone, for the activation of the release machinery, and for calcium triggering of exocytosis on a millisecond timescale are only slowly emerging.
SNARE proteins, the engine of membrane fusion
The synaptic proteins synaptobrevin (also referred to as VAMP), syntaxin 1 and SNAP-25 belong to the SNARE protein family. Their defining feature is an extended coiled-coil stretch, which is referred to as a SNARE motif and falls into four subtypes, referred to as Qa, Qb, Qc and R-SNARE motif (for example ref. 14). In syntaxin, synaptobrevin and in most other SNAREs the SNARE motifs are connected by a short linker to a carboxy-terminal transmembrane region (TMR). SNAP-25 deviates from this general structure: here two SNARE motifs (Qb and Qc) are connected by a linker that is palmitoylated, whereas a TMR is lacking. Whereas synaptobrevin and SNAP-25 do not carry any other domains, syntaxin possesses an amino-terminal domain consisting of an antiparallel three-helix bundle, termed the Habc domain (15,16), connected to the SNARE motif by a flexible linker. Positioned N-terminally to the Habc domain is a short stretch that ends in the so-called N-peptide (see Fig. 2). SNAREs undergo a regulated assembly–disassembly cycle that is energized by the AAA1-ATPase NSF. Synaptobrevin is a synaptic vesicle protein, whereas syntaxin 1 and SNAP-25 are localized in the presynaptic plasma membrane. On contact, the SNAREs associate in trans at the N-terminal ends of the SNARE motifs. A tight bundle of four parallel a-helices is formed, each contributed by a different SNARE motif (17,18), which progresses towards the C-terminal membrane anchors (‘zippering’), thus pulling the membranes tightly together (19). Assembly is associated with a huge release of energy that is used to initiate membrane fusion (20,21). After fusion, the ternary SNARE complex resides in the plasma membrane in the low-energy cis configuration and is disassembled by NSF in conjunction with its SNAP cofactor. Next, synaptobrevin is endocytosed and recycled, thus being able to participate in another round of exocytosis (for reviews see refs 2–8).
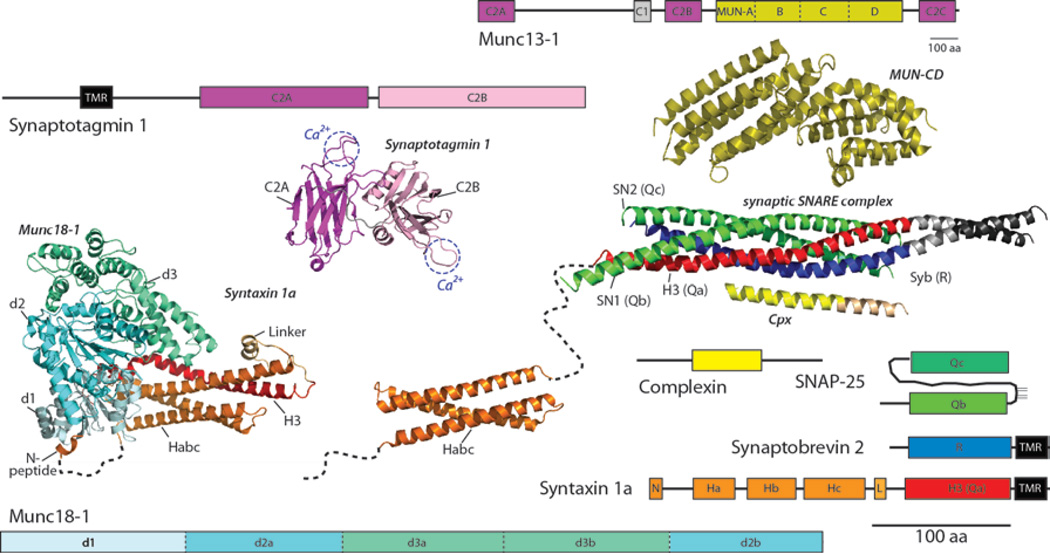
Figure 2.
Schematic depictions of domain structures and crystal structures of core proteins of the neuronal fusion machine. The dashed lines between the N-peptide (N) and Habc domain represent flexible regions in syntaxin. For synaptotagmin the two Ca2+ binding sites are indicated. Note that the domain structure of the large multi-domain protein Munc13 is shown five times smaller than those of the other domain structures. A high-resolution structure was obtained for the C-terminal half of the MUN domain. See text for details. The data for structures are from: Munc13-1 (C and D subdomains) (48),Synaptotagmin 1 (C2A and C2B domain) (117), Munc18-1 (blue-green, in complex with syntaxin (red)) (32),Habc domain (16), SNARE complex (18),complexin (63). aa, amino acid.
Despite the elegant simplicity and experimental support (22) of the zippering model, SNARE assembly proved to be an unexpectedly complex reaction, and there is still a lot to learn. In vitro, isolated SNARE motifs are unfolded but assemble into diverse homo-and hetero-oligomers that all are at least partially helical (reviewed in refs 4 and 8). For instance, SNAP-25 can bind sequentially two syntaxin molecules, thus blocking the binding site of synaptobrevin (23). Furthermore, syntaxin rapidly switches between an active open conformation and an inactive closed conformation in which the Habc domain folds against the N-terminal part of the SNARE motif (24,25). Such conformational dynamics and kinetic trapping of off-pathway intermediates explains why in vitro assembly of the ternary complex, although highly exergonic, lasts hours, far too slow to mediate fast exocytosis. On the other hand, if a complex of SNAP-25 and syntaxin with a free N-terminal binding site for synaptobrevin is stabilized, SNARE assembly is accelerated by orders of magnitude (26). The central problem is to delineate precisely the assembly pathway and to understand how the SNARE molecules are channeled along this pathway by regulatory proteins to execute fusion efficiently. Four proteins, each representing a small protein family, have emerged as such key regulators: Munc18 and Munc13 that prepare the SNARE engine for assembly, and synaptotagmin and complexin that govern calcium-dependent triggering.
Priming the SNARE engine
UNC-18 and UNC-13, the Caenorhabditis elegans orthologues of Munc18 and Munc13, respectively, were originally identified by S. Brenner in his classical screen uncovering genes involved in movement (27). Deletion of either Munc18 (ref. 28) or Munc13 (ref. 29) and their respective orthologues (30,31) completely inhibits neuronal exocytosis. Munc18 belongs to the conserved family of SM proteins. It possesses an arch-shaped architecture with a central cavity for high-affinity binding to syntaxin-1 (refs 32,33). By contrast, the large Munc13s belong to the CATCHR protein family (34). Munc13 also binds to syntaxin-1 but only with moderate affinity (35,36). Both proteins are involved in setting up the SNAREs for assembly and perhaps in guiding them through the initial part of the assembly pathway, but it is still not understood how exactly they operate, how many copies are required to carry out the reaction, and how the extraordinary phenotypes of the knockouts can be mechanistically explained.
Munc18
For many years, the molecular mechanism of Munc18 has been shrouded by a paradox because it locks syntaxin-1 in a closed conformation (24,33) (Fig. 2), in which syntaxin cannot enter SNARE complexes. Such inhibition is difficult to reconcile with the complete loss of exocytosis in deletion mutants, which suggests exactly the opposite, namely that SNARE zippering is absolutely dependent on Munc18. Indeed, Munc18 seems to be an oddity because other SM proteins, despite high structural similarity, bind instead tightly to the N-peptide of their cognate syntaxins, involving a binding site on the surface of the SM protein. This binding mode would enable these syntaxins to remain open, with SNARE assembly not being inhibited, whereas syntaxin-1 would need to be opened in the case of Munc18. To reconcile these discrepancies, it was proposed that binding of SM proteins to syntaxins, whether via the N-peptide or the Habc domain, merely serves to recruit the SM protein to the prospective fusion site. The SM proteins are then handed over to the SNARE motifs where they promote nucleation and/or zippering (for reviews see refs 3 and 37).
Recently, it has been recognized that SM proteins, including Munc18, generally bind to their respective syntaxins using both of the spatially distinct binding sites, but with different relative affinities (32,38,39). In fact, the two binding sites seem to act together in controlling SNARE complex formation (32). This sheds new light on the paradox, as full ‘opening’ of syntaxin may not be required for gating entry into SNARE complexes. In support of this view, a syntaxin mutant originally thought to be constitutively open (LE mutant) (24) is now known to bind Munc18 via both sites in an at least partially closed conformation, but without inhibiting formation of SNARE complexes (32,36). Indeed, when expressed as the only syntaxin 1 variant, the LE mutant results in enhanced spontaneous exocytosis, supporting that under resting conditions it is more reactive with respect to SNARE binding (40).
Thus it seems that binding of Munc18-1 to both the closed conformation and to the N-peptide of syntaxin 1a is an integral part of the pathway during which Munc18 guides syntaxin towards productive SNARE complex formation. Perhaps Munc18 first keeps syntaxin closed and inactive, thus preventing premature SNARE assembly, but allows for synchronization of a subsequent (calcium-dependent?) activation step (see for example ref. 3).
Despite such progress, it is still unclear why Munc18 is essential for efficient SNARE nucleation. Reconstitution experiments involving liposomes suggest that Munc18 participates in selecting the correct R-SNARE helix and thus guides nucleation of the ternary complex (for example refs 41 and 42). It is unclear whether it then remains associated with the ternary complex (43) as also suggested for other SM proteins (for example ref. 37) or dissociates upon zippering (44). In the latter case Munc18 might be interacting with a trans-SNARE complex only during its initiation, whereas progression of zippering would cause syntaxin to fully open, thus driving off Munc18.
Munc13
Munc13s are modular proteins sharing a conserved C-terminal region containing a phorbol-ester-binding C1 domain and two calcium-binding C2 domains that flank a larger, so-called MUN domain (Fig. 2, reviewed in ref. 45). Expression of the MUN domain alone partially rescues the total arrest of exocytosis in neurons lacking Munc13s (46), identifying it as a key functional element of the protein. MUN domains are shared with the proteins BAP3 and CAPS (Unc31) and with other proteins in most eukaryotes (47). The MUN domain is structurally strikingly similar to other CATCHR family members (48) that work in various trafficking steps. These proteins form elongated arrays of stacked a-helical bundles with flexible hinge regions, which tether transport vesicles to the site of fusion. It is conceivable that the conserved MUN domain serves as binding platforms that arrange the core fusion machinery, whereas the C1 and C2 domains mediate fine-tuning of its membrane recruitment, a feature ideally suited to Ca2+-regulated secretion.
CATCHR complexes are also thought to enable SNARE assembly, although their interplay with SNARE proteins seems to vary. For instance, Munc13 may participate directly by unlocking syntaxin from the grip of Munc18, because in C. elegans, the LE mutant of syntaxin (that is not inhibited by Munc18 binding) partially rescues neurotransmitter release in the absence of UNC-13 (refs 49 and 50). Furthermore, recent experiments have shown that the isolated MUN domain accelerates the transition of syntaxin-1 from the Munc18-1 complex to the SNARE complex (36). It should be kept in mind, however, that the LE mutant also partially rescues the block of exocytosis caused by deletion of RIM (also known as UNC-10) in C. elegans (51). RIM serves as central organizer of the active zone. It forms a tripartite complex with the N-terminal C2A domain of Munc13 and the small vesicular GTPases RAB3 and RAB27, thus orchestrating the attachment site of synaptic vesicles (reviewed in ref. 2).
Ca21-dependent triggering starts the SNARE engine
In contrast to the basic fusion reaction that is carried out by conserved proteins traced back to an ancient eukaryotic machine, the unique features of calcium-triggered exocytosis are primarily encoded in specialized proteins. Of these, synaptotagmins I, II and IX constitute the dominant calcium sensors whose deletion results in a complete loss of fast, calcium-triggered exocytosis (reviewed in refs 52–54). However, asynchronous (that is, slower) calcium-dependent release persists, showing that other calcium-binding proteins are involved, with candidates including other synaptotagmin isoforms or related proteins such as Doc2 (refs 55–57). Furthermore, complexins I and II are involved in triggering: deletion of complexins strongly reduces calcium-evoked exocytosis, whereas both stimulatory and inhibitory effects were observed on spontaneous release (for example see refs 58 and 59).
Synaptotagmins
The neuronal synaptotagmins are anchored to synaptic vesicles by a single TMR. Characteristic features of the synaptotagmins are two C2 domains, called C2A and C2B, that are connected to the membrane as well as to each other by flexible linkers. C2 domains are rigid, oval-shaped b-sandwiches that possess a cluster of calcium-binding loops, serving as partial coordination site for two (C2A) or three (C2B) calcium ions. In the presence of calcium, the C2 domains bind to membranes containing acidic phospholipids that complete the calcium coordination sites. In addition, the C2B domain contains a spatially separated basic patch that steers the domain to membranes enriched in phosphatidylinositol (4,5) bisphosphate (PI(4,5)P2). Membrane binding is primarily electrostatic and rapidly reversed by chelating calcium or increasing the ionic strength. Furthermore, the synaptotagmin C2 domains bind to syntaxin alone or syntaxin-containing SNARE complexes (for example see refs 60 and 61). Although binding occurs in the absence of calcium, it appears to be influenced by calcium (reviewed in refs 54 and 62).
Complexins
Complexins are small cytoplasmic proteins that bind via a central helix to a groove on the surface of the SNARE complex, which is formed by the helices of syntaxin and synaptobrevin (63,64) (Fig. 2). Because SNARE binding is required for their physiological action, complexins can only exert their function once SNAREs are at least partially assembled, placing them into the reaction sequence after zippering is initiated. Intriguingly, the central helix is not sufficient for complexin function. Rather, the N-terminal end pointing towards the membrane is needed for facilitation of fusion, whereas the regions flanking the central helix seem to have an inhibitory role. To accommodate the presumed dual stimulatory and inhibitory role of complexin, two alternative molecular mechanisms are discussed (3,9,65–67). First, binding to the surface of the SNARE complex may promote initiation and progression of zippering, for example, by stabilizing partially zippered SNARE complexes and sensitizing them to activation by synaptotagmin (‘super-priming’) (for example see ref. 58). Second, complexin acts as a clamp that blocks progression of SNARE-zippering, presumably by competing directly with synaptobrevin binding in the C-terminal part of the SNARE complex (for example see refs 59 and 68). The clamp is released upon calcium triggering, probably by synaptotagmin (see below) because complexins do not bind calcium.
Two models explain the action of calcium
Despite many years of research, it is still controversial as to how calcium influx brings about the extraordinary and highly cooperative acceleration of exocytosis. To some extent this is owing to the fact that the molecular status of a docked and primed vesicle, ready to respond to calcium by exocytosis in less than a millisecond, is not known with certainty.
Most authors seem to agree that SNAREs are already partially zippered in this state, with full zippering being prevented either by an energy barrier in the fusion pathway that the SNAREs alone cannot overcome (for example, electrostatic repulsion, transition towards a stalk intermediate, see below), and/or by an interfering protein, with prime candidates being complexins and/or synaptotagmins (Fig. 3, pathway I). During this state, Munc18, and perhaps also Munc13, may still be bound to the complex. It is debated whether such a complex is strained, that is, storing energy that is released during fusion, or whether it is relaxed, with the linkers connecting the zippered part of the complex to the membrane being flexible.
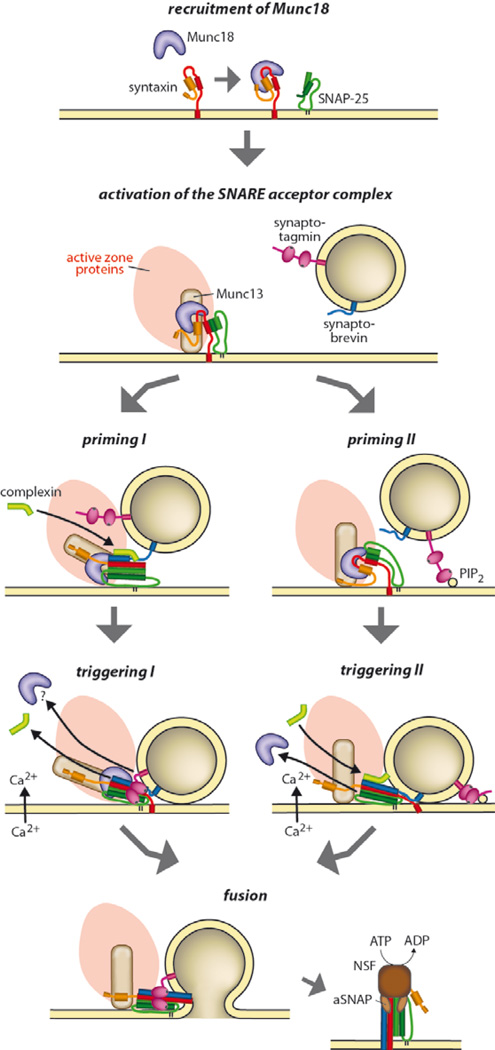
Figure 3.
Alternative models describing the steps between priming and fusion. Priming I involves arrest of a partially zippered SNARE complex, here shown with bound Munc18, Munc13 and synaptotagmin. Calcium influx triggers binding of synaptotagmin to the SNARE complex and to the plasma membrane (involving PI(4,5)P2, not shown here), associated with displacement of complexin and (possibly) Munc18 and/or Munc13. Priming II involves arrest after positioning of the vesicle with the aid of active zone components and (possibly) contact of synaptotagmin with PI(4,5)P2 in the plasma membrane, but no contact between the SNAREs. Ca2+-triggering pulls the vesicle closer via synaptotagmin-mediated cross-linking, resulting in SNARE assembly, associated with full opening of syntaxin and displacement of Munc18, and binding of complexin. See text for details.
Calcium binding to synaptotagmin would trigger fusion either by activating (disinhibiting) the SNAREs or by lowering the activation energy barrier in the fusion pathway through membrane interactions. Accordingly, synaptotagmin may act by (1) disengaging from the SNAREs, thus relieving the block (fusion clamp model) (69), (2) binding to the SNAREs, thus displacing the inhibitory complexin and/or promoting zippering (59), (3) binding to the membrane directly adjacent to the partially complexed SNAREs, thus destabilizing the bilayer at the fusion site (70–72), (4) increasing curvature stress by displacing lipids in the monolayer of the plasma membrane facing the vesicle (73,74), and (5) cross-linking the vesicle and the plasma membrane, thus accelerating fusion by charge compensation owing to the positive electrostatic potential of the C2 domains (75).
A wealth of evidence is invoked in support of a partially zippered and arrested SNARE complex; for instance, differential effects of SNARE mutations on fusion kinetics that affect nucleation and zippering, respectively (see for example refs 76–78). The model also allows for an integration of complexin into the fusion mechanism that needs at least partial SNARE assembly before it can bind and exert its action.
Furthermore, the model intuitively explains the fast fusion kinetics because only minor conformational rearrangements are required upon Ca2+-triggering, with all proteins already being correctly positioned for the final step.
On the other hand, there are problems with this model, which in our opinion have not been sufficiently appreciated. Experimentally, trans-complexes are difficult to capture. Similarly, despite hints, for example, from single-molecule experiments (4,79), an effect of synaptotagmin and/or complexin on the rate of SNARE assembly has remained elusive. Most importantly, the mechanisms proposed for arresting SNARE zippering somewhere in the middle are difficult to reconcile with the fact that SNARE assembly proceeds along a steep downhill energy gradient. For instance, a C-terminal fragment of synaptobrevin forms in vitro a stable complex with SNAP-25 and syntaxin, thus blocking, like a brake shoe, the C-terminal portion from assembling as envisioned in the partially zippered model. However, full-length synaptobrevin is able to rapidly displace this fragment (26) and, despite this additional energy barrier, to promote fusion in vitro even if only one of such partially inhibited SNARE complexes is involved (80). None of the proposed factors (including complexin) binds with an affinity even remotely comparable to that of the synaptobrevin fragment, questioning their ability to influence the strongly exergonic zippering reaction. Furthermore, we consider it unlikely that the control of the neuronal SNAREs is exerted by tinkering with its structurally highly conserved engine core, that is, the helical bundle whose major features (structure, stability, folding– unfolding hysteresis) are remarkably similar between SNAREs in regulated and non-regulated trafficking steps (8).
Similarly, a role of synaptotagmin in destabilizing membranes or inducing curvature stress is difficult to reconcile with Ca2+-dependent membrane binding being primarily electrostatic and reversible. Indeed, vesicle deformation in vitro requires saturation with C2 domain-containing synaptotagmin fragments (73,74), whereas only few phospholipid molecules are expected to be displaced by binding of single C2 domains, hardly sufficient to create even local tension. Generally, agents increasing positive spontaneous curvature of the proximal monolayers inhibit rather than enhance fusion(81), that is, exactly the opposite of what synaptotagmin is doing, requiring elaborate models of highly organized ‘bulges’ to explain promotion of fusion intermediates (82).
Recently, an alternative scenario for the docked and primed state has been envisaged that, despite being far from proven, we consider as an interesting alternative, as it overcomes several of the problems outlined above (70,83) (Fig. 3, pathway II, see also ref. 40). Supported by recent electron tomography data showing that docked vesicles appear to be a few nanometres away from the plasma membrane (11,12), it assumes that SNAREs do not connect in trans before the arrival of the calcium signal. Rather, the interaction of the vesicle with the active zone components (most notably Munc13 and RIM) would precisely position the vesicle on top of a patch of plasma membrane containing activated SNARE acceptor complexes, probably complexed with Munc18. In this state, vesicle-bound synaptotagmin may be already in contact with the plasma membrane, either by (calcium-independent) binding to the SNAREs or by loosely binding to PI(4,5)P2 patches colocalizing with syntaxin clusters. Calcium influx would trigger membrane binding and cross-linking of the vesicle and plasma membrane, thus nudging them a bit closer (75,83,84), sufficient to allow for rapid binding of synaptobrevin to the acceptor complex (78). Once nucleation is triggered, the SNAREs quickly progress through zippering and fusion.
This model places the entire control of the neuronal fusion machine upstream of SNARE nucleation, which has important consequences for our understanding of the partial reactions. Most importantly, it changes the view of SNARE function. Accordingly, SNAREs act as ‘single shot’ devices that, once nucleation is triggered, are unstoppable and flash through assembly to bring about fusion. ‘Misfiring’ of SNAREs (assembly without fusion) probably only occurs rarely, if at all, but is likely to increase in mutants affecting zippering (85). Also, it is possible that nucleation triggers the displacement of Munc18 and other factors (such as Munc13), thus allowing the SNAREs to carry out the work unhindered by bulky bound proteins. Such a simple and highly efficient mode of operation may explain why the SNARE engine was so successful in evolution. The proposed function for synaptotagmin is in line with the function of C2 domains in other proteins such as protein kinase C—they operate as electrostatic switches (62) mediating calcium-dependent rapid and reversible membrane binding.
The model also elegantly explains why solutions of high osmolarity (usually sucrose) trigger calcium-independent exocytosis of the readily releasable pool (86): the resulting water efflux creates negative pressure that draws docked vesicles closer to the plasma membrane, triggering SNARE firing. Furthermore, any destabilization of the overall architecture of the docking site, which increases Brownian fluctuations of the vesicle, would cause occasional spontaneous firing of SNAREs, which may explain changes in spontaneous release rates upon deletion or overexpression of some proteins (for a detailed discussion see for example refs 9 and 57).
Finally, the model provides for a fresh look at the molecular basis of the high cooperativity of calcium-triggered fusion. At non-saturating calcium concentrations, synaptotagmin binding may be less tight or transient, perhaps undergoing rapid and repetitive ‘on–off’ cycles, resulting in vesicle jittering. Accordingly, the probability for SNARE nucleation/firing would be reduced. Such a scenario may also explain the function of complexin, which is otherwise more difficult to integrate into this model. Complexin may increase the frequency of successful nucleation events by stabilizing correctly oriented syntaxin–synaptobrevin alignments. This hypothesis is in line with the ability of complexin to bind to SNARE complexes with fast, diffusion-limited kinetics (87).
Fusion—interplay between proteins and lipids
In the final step of exocytosis the vesicle membrane fuses with the plasma membrane. The merger of two bilayers involves non-bilayer intermediates at the contact site that ultimately develop into the opening of an aqueous channel, termed a fusion pore. During fusion the hydrophobic barrier separating the cytoplasm from both the vesicle content and the extracellular space must remain intact.
Key issues concerning the molecular rearrangements of proteins and membrane lipids along the fusion pathway are unresolved. Popular models requiring an oligomeric ring of SNARE complexes surrounding the prospective fusion pore as intermediate cannot be maintained in view of the fact that only one to two (or three) SNARE complexes are sufficient for fusion both in vitro and in vivo (80,88,89). Intriguingly, in vitro fusion can be mediated by trans assembly of artificially engineered molecules mimicking SNARE-zippering (even DNA) as long as they possess membrane anchors (for example see refs 91–94). Such a lack of structural specificity in catalysis is indeed a hallmark of membrane fusion, and it is likely that considerable structural variety is tolerated along the fusion pathway. This helps to explain why unrelated classes of fusion proteins evolved in parallel to the SNAREs, such as those fusing cells (95), viruses (96), mitochondria (97) or the endoplasmic reticulum (98). The stalk hypothesis, first developed 30 years ago (99), describes membrane fusion as an ordered sequence of steps initiated by an hourglass-shaped intermediate (the fusion stalk), followed by a hemifusion diaphragm and subsequent rupture, resulting in the formation of a fusion pore (Fig. 4). Indeed, stalk-like intermediates can be induced as a separate phase under mild conditions (100–102). However, the energy landscape as well as the intermediate molecular structures along the fusion pathway is unclear.
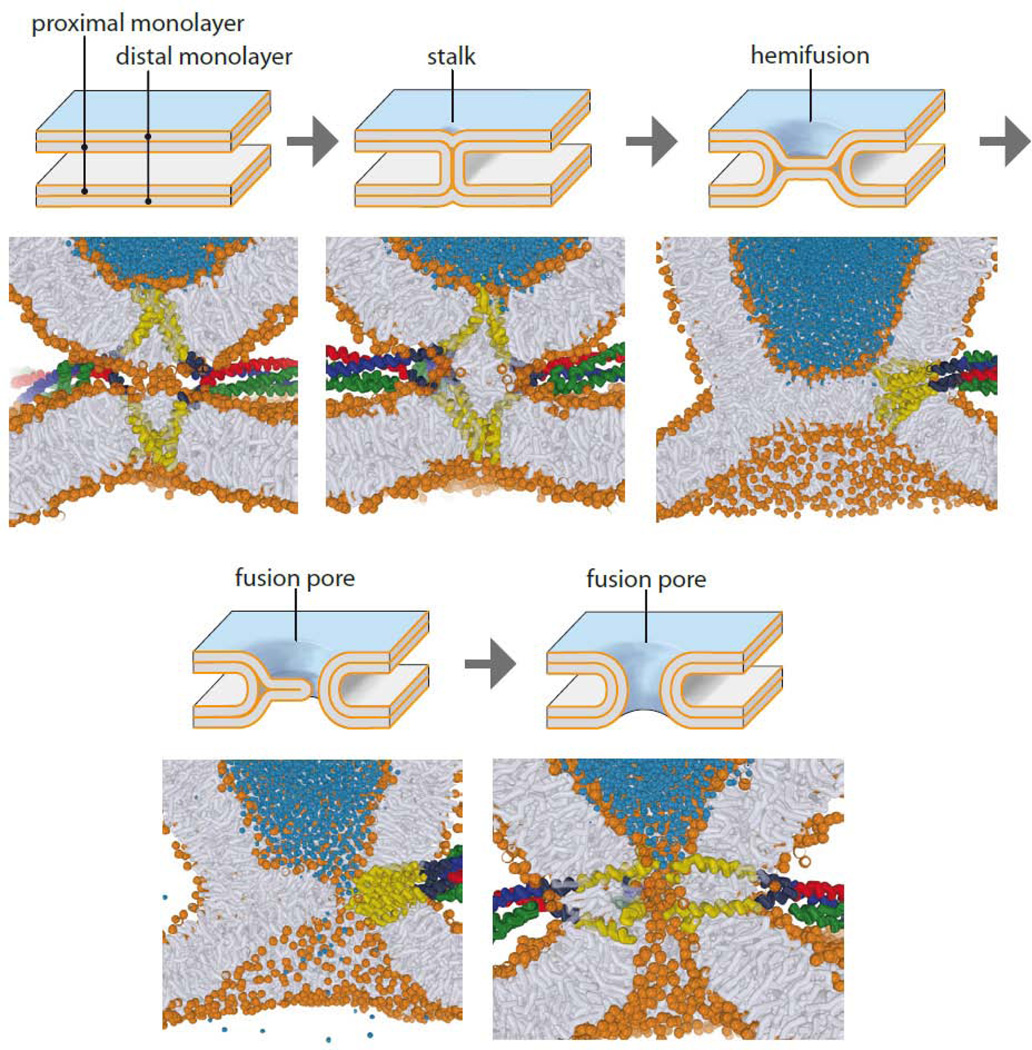
Figure 4.
Transition states during membrane fusion. Intermediates of the fusion pathway. The top drawings represent intermediate states of the membrane along the pathway as predicted by the elastic theory. Below, snapshots of intermediate states of a simulation of SNARE-mediated fusion are shown, which, although roughly corresponding to the elastic model, differ in detail and in their energy predictions (adapted from ref. 109, courtesy of J. Rissellada and H. Grubmüller).
Originally, the energy profile was modeled on the basis of the elastic properties of membranes, with the curvature stress of the intermediate model structures defining transition-state energies. However, these energies were unrealistically high, and molecular parameters were invoked to lower the energies (for review see ref. 103). More recently, coarse-grain or even atomistic simulations of fusion have provided detailed scenarios for intermediate structures (Fig. 4), with consequences for the energy landscape. For instance, it has been suggested that ‘splaying’ of phospholipid tails may form the first hydrophobic connection between the membranes (104) from where stalk formation proceed downhill an energy gradient. Furthermore, the enhanced fusogenicity of curved membranes can also be explained by the hydrophobic effect: owing to the increased spacing of the hydrophilic head groups the membrane surface is more hydrophobic. Lipid splaying requires the membranes to be at a critical distance of less than 1 nm (Fig. 4, see ref. 105 for a more detailed discussion).
These considerations have important consequences for the mechanism of SNARE-catalysed fusion. Certainly, zippering of the four-helix bundle brings the membranes in close proximity, but the question is how the SNAREs promote stalk formation and subsequent intermediate structures. If the main energy barrier is contributed by curvature stress (106), stiffness of the linkers connecting SNARE motifs and TMRs is essential for transmitting stress to the membranes. Indeed, mutagenesis of the linkers generally reduces fusion efficacy (see for example ref. 107), and at least syntaxin seems to have a stiff linker as a monomer (108). On the other hand, if close proximity, water removal, increase of local hydrophobicity and lipid splaying form the main energy barrier, bending stiffness of the SNARE linkers may not be as relevant, a view suggested by recent simulation studies (109). Instead, the pulling force exerted during zippering may drag the TMRs along with some phospholipids slightly out of the membrane, thus initiating phospholipid splaying once the critical distance has been reached.
What are the next steps? Transient hemifusion intermediates (experimentally defined, for example, by lipid mixing in the absence of content mixing) are observed upon SNARE-mediated fusion of liposomes, suggesting hemifusion as a metastable intermediate (for example see refs 110–114). However, it is experimentally difficult to differentiate between stalk and hemifusion intermediates. Hemifusion constitutes the lateral expansion of a stalk, leading to the formation of a hemifusion diaphragm (Fig. 4). It remains to be seen whether such diaphragms represent intermediates along the fusion pathway or whether they are dead-ends as previously suggested for viral fusion proteins (see ref. 115 for a review). In any case, the job of the SNAREs is not finished before the initial opening of the fusion pore, with interactions between the linkers as well as the TMRs probably being involved (116).
Conclusion
The molecular basis of synaptic exocytosis has fascinated scientists for decades. Since the initial discovery of quantal release in the 1950s by Katz and colleagues, and the elucidation of the synaptic vesicle-recycling pathway by Heuser and Ceccarelli in the 1970s, we have come a long way in deciphering the steps of the vesicle cycle at an increasingly detailed level. Although we have focused here on only a few key components, the vesicle cycle is governed by hundreds of proteins, and there are still new proteins being put on the map. We are only beginning to understand the rules by which individual protein–protein interactions work together in supramolecular machines to yield the synaptic vesicle cycle that reliably operates millions of times. These machines assemble on demand and disassemble when the task is completed. They are highly robust, tolerate varying stoichiometries, flexible compositions and other disturbances, and are controlled by an array of regulators such as protein kinases and phosphatases. Advances in technologies such as super-resolution microscopy, single-molecule measurements, fluorescent reporters and cryo-electron tomography are all contributing to closing the gap between our understanding of partial reactions in vitro and the fascinating efficiency of the vesicle cycle in intact synapses.
References
- 1.Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 2.Sudhof TC, Rizo J. Synaptic vesicle exocytosis. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science (New York, N.Y. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunger AT, Weninger K, Bowen M, Chu S. Single-molecule studies of the neuronal SNARE fusion machinery. Annu Rev Biochem. 2009;78:903–928. doi: 10.1146/annurev.biochem.77.070306.103621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wickner W, Schekman R. Membrane fusion. Nature structural & molecular biology. 2008;15:658–664. doi: 10.1038/nsmb.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rizo J, Rosenmund C. Synaptic vesicle fusion. Nature structural & molecular biology. 2008;15:665–674. doi: 10.1038/nsmb.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verhage M, Sorensen JB. Vesicle docking in regulated exocytosis. Traffic (Copenhagen, Denmark) 2008;9:1414–1424. doi: 10.1111/j.1600-0854.2008.00759.x. [DOI] [PubMed] [Google Scholar]
- 8.Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nature reviews. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 9.Sørensen J. Conflicting Views on the Membrane Fusion Machinery and the Fusion Pore. Annu Rev Cell Dev Biol. 2009;25:513–537. doi: 10.1146/annurev.cellbio.24.110707.175239. [DOI] [PubMed] [Google Scholar]
- 10.Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–872. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 11.Siksou L, Triller A, Marty S. Ultrastructural organization of presynaptic terminals. Current opinion in neurobiology. 2011;21:261–268. doi: 10.1016/j.conb.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Busnadiego R, et al. Insights into the molecular organization of the neuron by cryo-electron tomography. J Electron Microsc (Tokyo) 2011;60(Suppl 1):S137–148. doi: 10.1093/jmicro/dfr018. [DOI] [PubMed] [Google Scholar]
- 13.Sigrist SJ, Sabatini BL. Optical super-resolution microscopy in neurobiology. Current opinion in neurobiology. 2011 doi: 10.1016/j.conb.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Kloepper TH, Kienle CN, Fasshauer D. An elaborate classification of SNARE proteins sheds light on the conservation of the eukaryotic endomembrane system. Molecular biology of the cell. 2007;18:3463–3471. doi: 10.1091/mbc.E07-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez I, et al. Three-dimensional structure of an evolutionarily conserved N-terminal domain of syntaxin 1A. Cell. 1998;94:841–849. doi: 10.1016/s0092-8674(00)81742-0. [DOI] [PubMed] [Google Scholar]
- 16.Lerman JC, Robblee J, Fairman R, Hughson FM. Structural analysis of the neuronal SNARE protein syntaxin-1A. Biochemistry. 2000;39:8470–8479. doi: 10.1021/bi0003994. [DOI] [PubMed] [Google Scholar]
- 17.Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 18.Stein A, Weber G, Wahl MC, Jahn R. Helical extension of the neuronal SNARE complex into the membrane. Nature. 2009;460:525–528. doi: 10.1038/nature08156. This paper describes the X-ray structure of the synaptic SNARE complex with transmembrane regions.
- 19.Hanson PI, Heuser JE, Jahn R. Neurotransmitter release - four years of SNARE complexes. Curr. Opin. Neurobiol. 1997;7:310–315. doi: 10.1016/s0959-4388(97)80057-8. [DOI] [PubMed] [Google Scholar]
- 20.Li F, et al. Energetics and dynamics of SNAREpin folding across lipid bilayers. Nature structural & molecular biology. 2007;14:890–896. doi: 10.1038/nsmb1310. [DOI] [PubMed] [Google Scholar]
- 21.Wiederhold K, Fasshauer D. Is assembly of the SNARE complex enough to fuel membrane fusion? J. Biol. Chem. 2009;284:13143–13152. doi: 10.1074/jbc.M900703200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber T, et al. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 23.Fasshauer D, Margittai M. A Transient N-terminal Interaction of SNAP-25 and Syntaxin Nucleates SNARE Assembly. J. Biol. Chem. 2004;279:7613–7621. doi: 10.1074/jbc.M312064200. [DOI] [PubMed] [Google Scholar]
- 24.Dulubova I, et al. A conformational switch in syntaxin during exocytosis: role of munc18. Embo J. 1999;18:4372–4382. doi: 10.1093/emboj/18.16.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Margittai M, et al. Single-molecule fluorescence resonance energy transfer reveals a dynamic equilibrium between closed and open conformations of syntaxin 1. Proceedings of the National Academy of Sciences of the United States of America. 2003 doi: 10.1073/pnas.2331232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pobbati A, Stein A, Fasshauer D. N- to C-terminal SNARE complex assembly promotes rapid membrane fusion. Science (New York, N.Y. 2006;313:673–676. doi: 10.1126/science.1129486. [DOI] [PubMed] [Google Scholar]
- 27.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verhage M, et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science (New York, N.Y. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 29.Varoqueaux F, et al. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9037–9042. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richmond JE, Davis WS, Jorgensen EM. UNC-13 is required for synaptic vesicle fusion in C. elegans. Nat Neurosci. 1999;2:959–964. doi: 10.1038/14755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aravamudan B, Fergestad T, Davis WS, Rodesch CK, Broadie K. Drosophila UNC-13 is essential for synaptic transmission. Nat Neurosci. 1999;2:965–971. doi: 10.1038/14764. [DOI] [PubMed] [Google Scholar]
- 32.Burkhardt P, Hattendorf DA, Weis WI, Fasshauer D. Munc18a controls SNARE assembly through its interaction with the syntaxin N-peptide. Embo J. 2008;27:923–933. doi: 10.1038/emboj.2008.37. This paper reports that Munc18-1 interacts with two spatially separated binding sites of syntaxin-1a.
- 33.Misura KM, Scheller RH, Weis WI. Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature. 2000;404:355–362. doi: 10.1038/35006120. [DOI] [PubMed] [Google Scholar]
- 34.Yu IM, Hughson FM. Tethering factors as organizers of intracellular vesicular traffic. Annual review of cell and developmental biology. 2010;26:137–156. doi: 10.1146/annurev.cellbio.042308.113327. [DOI] [PubMed] [Google Scholar]
- 35.Betz A, Okamoto M, Benseler F, Brose N. Direct interaction of the rat unc-13 homologue Munc13-1 with the N terminus of syntaxin. The Journal of biological chemistry. 1997;272:2520–2526. doi: 10.1074/jbc.272.4.2520. [DOI] [PubMed] [Google Scholar]
- 36.Ma C, Li W, Xu Y, Rizo J. Munc13 mediates the transition from the closed syntaxin-Munc18 complex to the SNARE complex. Nature structural & molecular biology. 2011;18:542–549. doi: 10.1038/nsmb.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toonen RF, Verhage M. Munc18-1 in secretion: lonely Munc joins SNARE team and takes control. Trends Neurosci. 2007;30:564–572. doi: 10.1016/j.tins.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Furgason ML, et al. The N-terminal peptide of the syntaxin Tlg2p modulates binding of its closed conformation to Vps45p. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14303–14308. doi: 10.1073/pnas.0902976106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khvotchev M, et al. Dual modes of Munc18-1/SNARE interactions are coupled by functionally critical binding to syntaxin-1 N terminus. J Neurosci. 2007;27:12147–12155. doi: 10.1523/JNEUROSCI.3655-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerber SH, et al. Conformational switch of syntaxin-1 controls synaptic vesicle fusion. Science (New York, N.Y. 2008;321:1507–1510. doi: 10.1126/science.1163174. This paper and Ref. 49 describe the complex phenotype of the LE mutant of syntaxin on docking and fusion of synaptic vesicles.
- 41.Rathore SS, et al. Syntaxin N-terminal peptide motif is an initiation factor for the assembly of the SNARE-Sec1/Munc18 membrane fusion complex. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22399–22406. doi: 10.1073/pnas.1012997108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen J, Tareste DC, Paumet F, Rothman JE, Melia TJ. Selective Activation of Cognate SNAREpins by Sec1/Munc18 Proteins. Cell. 2007;128:183–195. doi: 10.1016/j.cell.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 43.Xu Y, Su L, Rizo J. Binding of Munc18-1 to Synaptobrevin and to the SNARE Four-Helix Bundle. Biochemistry. 2010 doi: 10.1021/bi9021878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zilly FE, Sorensen JB, Jahn R, Lang T. Munc18-bound syntaxin readily forms SNARE complexes with synaptobrevin in native plasma membranes. PLoS biology. 2006;4:e330. doi: 10.1371/journal.pbio.0040330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wojcik SM, Brose N. Regulation of membrane fusion in synaptic excitation-secretion coupling: speed and accuracy matter. Neuron. 2007;55:11–24. doi: 10.1016/j.neuron.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 46.Basu J, et al. A minimal domain responsible for Munc13 activity. Nature structural & molecular biology. 2005;12:1017–1018. doi: 10.1038/nsmb1001. [DOI] [PubMed] [Google Scholar]
- 47.Koch H, Hofmann K, Brose N. Definition of Munc13-homology-domains and characterization of a novel ubiquitously expressed Munc13 isoform. The Biochemical journal. 2000;349:247–253. doi: 10.1042/0264-6021:3490247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li W, et al. The crystal structure of a Munc13 C-terminal module exhibits a remarkable similarity to vesicle tethering factors. Structure. 2011;19:1443–1455. doi: 10.1016/j.str.2011.07.012. This crystal structure demonstrates that Munc13 is a member of the conserved CATCHR protein family involved in vesicle tethering.
- 49.Hammarlund M, Palfreyman MT, Watanabe S, Olsen S, Jorgensen EM. Open syntaxin docks synaptic vesicles. PLoS biology. 2007;5:e198. doi: 10.1371/journal.pbio.0050198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richmond JE, Weimer RM, Jorgensen EM. An open form of syntaxin bypasses the requirement for UNC-13 in vesicle priming. Nature. 2001;412:338–341. doi: 10.1038/35085583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koushika SP, et al. A post-docking role for active zone protein Rim. Nat Neurosci. 2001;4:997–1005. doi: 10.1038/nn732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kochubey O, Lou X, Schneggenburger R. Regulation of transmitter release by Ca(2+) and synaptotagmin: insights from a large CNS synapse. Trends in neurosciences. 2011;34:237–246. doi: 10.1016/j.tins.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 53.Pang ZP, Sudhof TC. Cell biology of Ca2+-triggered exocytosis. Current opinion in cell biology. 2010;22:496–505. doi: 10.1016/j.ceb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chapman ER. How does synaptotagmin trigger neurotransmitter release? Annu Rev Biochem. 2008;77:615–641. doi: 10.1146/annurev.biochem.77.062005.101135. [DOI] [PubMed] [Google Scholar]
- 55.Groffen AJ, et al. Doc2b Is a High-Affinity Ca2+ Sensor for Spontaneous Neurotransmitter Release. Science (New York, N.Y. 2010 doi: 10.1126/science.1183765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yao J, Gaffaney JD, Kwon SE, Chapman ER. Doc2 is a Ca2+ sensor required for asynchronous neurotransmitter release. Cell. 2011;147:666–677. doi: 10.1016/j.cell.2011.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walter AM, Groffen AJ, Sorensen JB, Verhage M. Multiple Ca2+ sensors in secretion: teammates, competitors or autocrats? Trends in neurosciences. 2011;34:487–497. doi: 10.1016/j.tins.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 58.Xue M, et al. Binding of the complexin N terminus to the SNARE complex potentiates synaptic-vesicle fusogenicity. Nature structural & molecular biology. 2010;17:568–575. doi: 10.1038/nsmb.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang X, Kaeser-Woo YJ, Pang ZP, Xu W, Sudhof TC. Complexin clamps asynchronous release by blocking a secondary Ca(2+) sensor via its accessory alpha helix. Neuron. 2010;68:907–920. doi: 10.1016/j.neuron.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lai AL, Huang H, Herrick DZ, Epp N, Cafiso DS. Synaptotagmin 1 and SNAREs form a complex that is structurally heterogeneous. Journal of molecular biology. 2011;405:696–706. doi: 10.1016/j.jmb.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vrljic M, et al. Molecular mechanism of the synaptotagmin-SNARE interaction in Ca2+-triggered vesicle fusion. Nature structural & molecular biology. 2010;17:325–331. doi: 10.1038/nsmb.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rizo J, Chen X, Arac D. Unraveling the mechanisms of synaptotagmin and SNARE function in neurotransmitter release. Trends in cell biology. 2006;16:339–350. doi: 10.1016/j.tcb.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 63.Chen X, et al. Three-dimensional structure of the complexin/SNARE complex. Neuron. 2002;33:397–409. doi: 10.1016/s0896-6273(02)00583-4. [DOI] [PubMed] [Google Scholar]
- 64.Bracher A, Kadlec J, Betz H, Weissenhorn W. X-ray structure of a neuronal complexin-SNARE complex from squid. The Journal of biological chemistry. 2002;277:26517–26523. doi: 10.1074/jbc.M203460200. [DOI] [PubMed] [Google Scholar]
- 65.Brose N. For better or for worse: complexins regulate SNARE function and vesicle fusion. Traffic (Copenhagen, Denmark) 2008;9:1403–1413. doi: 10.1111/j.1600-0854.2008.00758.x. [DOI] [PubMed] [Google Scholar]
- 66.Stein A, Jahn R. Complexins living up to their name--new light on their role in exocytosis. Neuron. 2009;64:295–297. doi: 10.1016/j.neuron.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 67.Neher E. Complexin: does it deserve its name? Neuron. 2010;68:803–806. doi: 10.1016/j.neuron.2010.11.038. [DOI] [PubMed] [Google Scholar]
- 68.Kummel D, et al. Complexin cross-links prefusion SNAREs into a zigzag array. Nature structural & molecular biology. 2011;18:927–933. doi: 10.1038/nsmb.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chicka MC, Hui E, Liu H, Chapman ER. Synaptotagmin arrests the SNARE complex before triggering fast, efficient membrane fusion in response to Ca2+ Nature structural & molecular biology. 2008;15:827–835. doi: 10.1038/nsmb.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stein A, Radhakrishnan A, Riedel D, Fasshauer D, Jahn R. Synaptotagmin activates membrane fusion through a Ca2+-dependent trans interaction with phospholipids. Nature structural & molecular biology. 2007;14:904–911. doi: 10.1038/nsmb1305. [DOI] [PubMed] [Google Scholar]
- 71.Xue M, Ma C, Craig TK, Rosenmund C, Rizo J. The Janus-faced nature of the C(2)B domain is fundamental for synaptotagmin-1 function. Nature structural & molecular biology. 2008;15:1160–1168. doi: 10.1038/nsmb.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee HK, et al. Dynamic Ca2+-dependent stimulation of vesicle fusion by membrane-anchored synaptotagmin 1. Science (New York, N.Y. 2010;328:760–763. doi: 10.1126/science.1187722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martens S, Kozlov MM, McMahon HT. How synaptotagmin promotes membrane fusion. Science (New York, N.Y. 2007;316:1205–1208. doi: 10.1126/science.1142614. [DOI] [PubMed] [Google Scholar]
- 74.Hui E, Johnson CP, Yao J, Dunning FM, Chapman ER. Synaptotagmin-mediated bending of the target membrane is a critical step in Ca(2+)-regulated fusion. Cell. 2009;138:709–721. doi: 10.1016/j.cell.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arac D, et al. Close membrane-membrane proximity induced by Ca(2+)-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nature structural & molecular biology. 2006;13:209–217. doi: 10.1038/nsmb1056. This study shows that synaptotagmin binds simultaneously to two membranes, bringing them into close proximity.
- 76.Sorensen JB, et al. Sequential N- to C-terminal SNARE complex assembly drives priming and fusion of secretory vesicles. Embo J. 2006;25:955–966. doi: 10.1038/sj.emboj.7601003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Walter AM, Wiederhold K, Bruns D, Fasshauer D, Sorensen JB. Synaptobrevin N-terminally bound to syntaxin-SNAP-25 defines the primed vesicle state in regulated exocytosis. J Cell Biol. 2010;188:401–413. doi: 10.1083/jcb.200907018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wiederhold K, et al. A coiled coil trigger site is essential for rapid binding of synaptobrevin to the SNARE acceptor complex. The Journal of biological chemistry. 2010;285:21549–21559. doi: 10.1074/jbc.M110.105148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Diao J, Ishitsuka Y, Bae WR. Single-molecule FRET study of SNARE-mediated membrane fusion. Bioscience reports. 2011;31:457–463. doi: 10.1042/BSR20110011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van den Bogaart G, et al. One SNARE complex is sufficient for membrane fusion. Nature structural & molecular biology. 2010 doi: 10.1038/nsmb.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chernomordik LV, Zimmerberg J. Bending membranes to the task: structural intermediates in bilayer fusion. Current opinion in structural biology. 1995;5:541–547. doi: 10.1016/0959-440x(95)80041-7. [DOI] [PubMed] [Google Scholar]
- 82.McMahon HT, Kozlov MM, Martens S. Membrane curvature in synaptic vesicle fusion and beyond. Cell. 2010;140:601–605. doi: 10.1016/j.cell.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 83.van den Bogaart G, et al. Synaptotagmin-1 may be a distance regulator acting upstream of SNARE nucleation. Nature structural & molecular biology. 2011;18:805–812. doi: 10.1038/nsmb.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuo W, Herrick DZ, Cafiso DS. Phosphatidylinositol 4,5-bisphosphate alters synaptotagmin 1 membrane docking and drives opposing bilayers closer together. Biochemistry. 2011;50:2633–2641. doi: 10.1021/bi200049c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schwartz ML, Merz AJ. Capture and release of partially zipped trans-SNARE complexes on intact organelles. The Journal of cell biology. 2009;185:535–549. doi: 10.1083/jcb.200811082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- 87.Pabst S, et al. Rapid and selective binding to the synaptic SNARE complex suggests a modulatory role of complexins in neuroexocytosis. J. Biol. Chem. 2002;277:7838–7848. doi: 10.1074/jbc.M109507200. [DOI] [PubMed] [Google Scholar]
- 88.Mohrmann R, de Wit H, Verhage M, Neher E, Sorensen JB. Fast vesicle fusion in living cells requires at least three SNARE complexes. Science (New York, N.Y. 2010;330:502–505. doi: 10.1126/science.1193134. Using a titration approach this study and Ref. 89 reveal that neurotransmitter release requires only few SNARE complexes.
- 89.Sinha R, Ahmed S, Jahn R, Klingauf J. Two synaptobrevin molecules are sufficient for vesicle fusion in central nervous system synapses. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14318–14323. doi: 10.1073/pnas.1101818108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shi L, et al. SNARE proteins: one to fuse and three to keep the nascent fusion pore open. Science (New York, N.Y. 2012;335:1355–1359. doi: 10.1126/science.1214984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chan YH, van Lengerich B, Boxer SG. Effects of linker sequences on vesicle fusion mediated by lipid-anchored DNA oligonucleotides. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:979–984. doi: 10.1073/pnas.0812356106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Simonsson L, Jonsson P, Stengel G, Hook F. Site-specific DNA-controlled fusion of single lipid vesicles to supported lipid bilayers. Chemphyschem. 2010;11:1011–1017. doi: 10.1002/cphc.200901010. [DOI] [PubMed] [Google Scholar]
- 93.Lygina AS, Meyenberg K, Jahn R, Diederichsen U. Transmembrane domain peptide/peptide nucleic acid hybrid as a model of a SNARE protein in vesicle fusion. Angewandte Chemie. 2011;50:8597–8601. doi: 10.1002/anie.201101951. [DOI] [PubMed] [Google Scholar]
- 94.Robson Marsden H, Elbers NA, Bomans PH, Sommerdijk NA, Kros A. A reduced SNARE model for membrane fusion. Angewandte Chemie. 2009;48:2330–2333. doi: 10.1002/anie.200804493. [DOI] [PubMed] [Google Scholar]
- 95.Avinoam O, Podbilewicz B. Eukaryotic cell-cell fusion families. Curr Top Membr. 2011;68:209–234. doi: 10.1016/B978-0-12-385891-7.00009-X. [DOI] [PubMed] [Google Scholar]
- 96.Harrison SC. Viral membrane fusion. Nature structural & molecular biology. 2008;15:690–698. doi: 10.1038/nsmb.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Westermann B. Mitochondrial fusion and fission in cell life and death. Nature reviews. Molecular cell biology. 2010;11:872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 98.Moss TJ, Daga A, McNew JA. Fusing a lasting relationship between ER tubules. Trends in cell biology. 2011;21:416–423. doi: 10.1016/j.tcb.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kozlov MM, Markin VS. [Possible mechanism of membrane fusion] Biofizika. 1983;28:242–247. [PubMed] [Google Scholar]
- 100.Yang L, Huang HW. Observation of a membrane fusion intermediate structure. Science (New York, N.Y. 2002;297:1877–1879. doi: 10.1126/science.1074354. [DOI] [PubMed] [Google Scholar]
- 101.Aeffner S, Reusch T, Weinhausen B, Salditt T. Structure, hydration barrier and curvature of membrane hemifusion stalks with varying lipid composition obtained by x-ray diffraction. PNAS. 2012 in press. [Google Scholar]
- 102.Qian S, Huang HW. A novel phase of compressed bilayers that models the prestalk transition state of membrane fusion. Biophysical journal. 2012;102:48–55. doi: 10.1016/j.bpj.2011.11.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chernomordik LV, Kozlov MM. Protein-lipid interplay in fusion and fission of biological membranes. Annual review of biochemistry. 2003;72:175–207. doi: 10.1146/annurev.biochem.72.121801.161504. [DOI] [PubMed] [Google Scholar]
- 104.Kinnunen PK. Fusion of lipid bilayers: a model involving mechanistic connection to HII phase forming lipids. Chem Phys Lipids. 1992;63:251–258. doi: 10.1016/0009-3084(92)90041-m. [DOI] [PubMed] [Google Scholar]
- 105.Risselada HJ, Grubmuller H. How SNARE molecules mediate membrane fusion: Recent insights from molecular simulations. Current opinion in structural biology. 2012;22:187–196. doi: 10.1016/j.sbi.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 106.Kozlov MM, McMahon HT, Chernomordik LV. Protein-driven membrane stresses in fusion and fission. Trends in biochemical sciences. 2010;35:699–706. doi: 10.1016/j.tibs.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kesavan J, Borisovska M, Bruns D. v-SNARE actions during Ca(2+)-triggered exocytosis. Cell. 2007;131:351–363. doi: 10.1016/j.cell.2007.09.025. This study systematically measures the effect of extending the juxtamembrane region of syntaptobrevin on neurotransmitter release.
- 108.Knecht V, Grubmuller H. Mechanical Coupling via the Membrane Fusion SNARE Protein Syntaxin 1A: A Molecular Dynamics Study. Biophysical journal. 2003;84:1527–1547. doi: 10.1016/S0006-3495(03)74965-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Risselada HJ, Kutzner C, Grubmuller H. Caught in the act: visualization of SNARE-mediated fusion events in molecular detail. Chembiochem : a European journal of chemical biology. 2011;12:1049–1055. doi: 10.1002/cbic.201100020. [DOI] [PubMed] [Google Scholar]
- 110.Xu Y, Zhang F, Su Z, McNew JA, Shin YK. Hemifusion in SNARE-mediated membrane fusion. Nature structural & molecular biology. 2005;12:417–422. doi: 10.1038/nsmb921. [DOI] [PubMed] [Google Scholar]
- 111.Wang T, Smith EA, Chapman ER, Weisshaar JC. Lipid mixing and content release in single-vesicle, SNARE-driven fusion assay with 1-5 ms resolution. Biophysical journal. 2009;96:4122–4131. doi: 10.1016/j.bpj.2009.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Giraudo CG, et al. SNAREs can promote complete fusion and hemifusion as alternative outcomes. J Cell Biol. 2005;170:249–260. doi: 10.1083/jcb.200501093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Reese C, Heise F, Mayer A. Trans-SNARE pairing can precede a hemifusion intermediate in intracellular membrane fusion. Nature. 2005;436:410–414. doi: 10.1038/nature03722. [DOI] [PubMed] [Google Scholar]
- 114.Chernomordik LV, Kozlov MM. Membrane hemifusion: crossing a chasm in two leaps. Cell. 2005;123:375–382. doi: 10.1016/j.cell.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 115.Chernomordik LV, Kozlov MM. Mechanics of membrane fusion. Nature structural & molecular biology. 2008;15:675–683. doi: 10.1038/nsmb.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Laage R, Rohde J, Brosig B, Langosch D. A conserved membrane-spanning amino acid motif drives homomeric and supports heteromeric assembly of presynaptic SNARE proteins. The Journal of biological chemistry. 2000;275:17481–17487. doi: 10.1074/jbc.M910092199. [DOI] [PubMed] [Google Scholar]
- 117.Fuson KL, Montes M, Robert JJ, Sutton RB. Structure of human synaptotagmin 1 C2AB in the absence of Ca2+ reveals a novel domain association. Biochemistry. 2007;46:13041–13048. doi: 10.1021/bi701651k. [DOI] [PMC free article] [PubMed] [Google Scholar]