Function and regulation of Maskin, a TACC family protein, in microtubule growth during mitosis (original) (raw)
Abstract
The Xenopus protein Maskin has been previously identified and characterized in the context of its role in translational control during oocyte maturation. Maskin belongs to the TACC protein family. In other systems, members of this family have been shown to localize to centrosomes during mitosis and play a role in microtubule stabilization. Here we have examined the putative role of Maskin in spindle assembly and centrosome aster formation in the Xenopus egg extract system. Depletion and reconstitution experiments indicate that Maskin plays an essential role for microtubule assembly during M-phase. We show that Maskin interacts with XMAP215 and Eg2, the Xenopus Aurora A kinase in vitro and in the egg extract. We propose that Maskin and XMAP215 cooperate to oppose the destabilizing activity of XKCM1 therefore promoting microtubule growth from the centrosome and contributing to the determination of microtubule steady-state length. Further more, we show that Maskin localization and function is regulated by Eg2 phosphorylation.
Introduction
Microtubules are dynamic polymers that rearrange in different cell types and throughout the cell cycle to support cell organization and function. One of the most dramatic changes in microtubule organization occurs as the cell enters into mitosis. The relatively stable radial interphase microtubule network disassembles and reorganizes into a bipolar spindle-shaped apparatus that segregates the chromosomes between the two daughter cells. This process is finely coordinated in time and in space by the global and local regulation of different classes of proteins including microtubule-associated proteins (MAPs) and motors whose collective activity results in (1) a global increase of microtubule dynamics coupled to a local microtubule stabilization around the chromosomes, (2) an increase of microtubule nucleation activity at the centrosomes and around the chromosomes through a RanGTP-dependent pathway, and (3) the organization of the dynamic microtubules into a bipolar configuration around the chromosomes (Karsenti and Vernos, 2001; Wittmann et al., 2001; Gadde and Heald, 2004).
The increased dynamics of microtubules in mitosis is an essential prerequisite for spindle formation. It is driven by a change in the balance of the activities of stabilizing and destabilizing factors (Tournebize et al., 2000). Experiments performed in Xenopus egg extract have shown that it is essentially due to a severalfold increase in the frequency of transitions between the growing to the shrinking phase called catastrophe (Verde et al., 1992). The major catastrophe-promoting factor in M-phase egg extract is the kinesin-like protein XKCM1/MCAK, a member of the kinesin-13 family (Walczak et al., 1996). XKCM1 can catalytically depolymerize microtubules both from their plus and minus ends in vitro (Desai et al., 1999). XKCM1 like other members of this family is cytoplasmic and highly enriched at the centromeres, centrosomes, and spindle midzone during mitosis (for review see Moore and Wordeman, 2004).
In the egg extract, XKCM1 activity is antagonized by the microtubule plus-end–stabilizing factor XMAP215 that suppresses catastrophes (Gard and Kirschner, 1987; Tournebize et al., 2000; Kinoshita et al., 2002; Holmfeldt et al., 2004). XMAP215 is the founding member of large protein family of microtubule-associated proteins that accumulate at the microtubule organizing center during mitosis, promote microtubule assembly, and are essential for spindle assembly (Cullen and Ohkura, 2001; Popov et al., 2002; Gergely et al., 2003; Usui et al., 2003; Gard et al., 2004; Holmfeldt et al., 2004). Perturbations of either XKCM1 or XMAP215 activities have strong consequences on microtubule steady-state length and number in the M-phase egg extract and hinder spindle assembly (Walczak et al., 1996; Tournebize et al., 2000; Popov et al., 2001; Moore and Wordeman, 2004).
Recent studies have shown that chTOG/XMAP215 family members interact with members of the transforming acidic coiled-coil family (TACC family) in all species examined so far (Lee et al., 2001; Bellanger and Gonczy, 2003; Srayko et al., 2003 and for review see Gergely, 2002). These proteins are very diverse but share a conserved ∼200 aa COOH-terminal coiled-coil domain (TACC domain) that targets the protein to the centrosome and is involved in the interaction with chTOG/XMAP215 family members (for review see Gergely, 2002). Studies performed in several systems have highlighted the functional importance of this interaction (Lee et al., 2001; Giet et al., 2002; Bellanger and Gonczy, 2003; Gergely et al., 2003; Le Bot et al., 2003; Srayko et al., 2003; Usui et al., 2003). It has been proposed that the complex TACC/chTOG helps microtubules to elongate off the centrosome in M-phase by stabilizing their plus-ends (Bellanger and Gonczy, 2003; Giet et al., 2002; Le Bot et al., 2003). Interestingly some TACC proteins have been shown to interact with Aurora A (Giet et al., 2002; Conte et al., 2003; Pascreau et al., 2005), a kinase whose activity has been implicated in the increase in microtubule nucleation capacity of centrosomes in M-phase (Andrews et al., 2003).
Here we have examined the putative function of the Xenopus protein Maskin in spindle assembly using the egg extract system. Maskin is the only member of the TACC family in Xenopus. It was originally identified and characterized as a protein involved in translational control during oocyte maturation (Stebbins-Boaz et al., 1999; Groisman et al., 2001; Mendez and Richter, 2001) but its possible involvement in microtubule function has not been examined. We found that Maskin has an important role for microtubule assembly during M-phase, its localization and activity are regulated by the kinase Aurora A and it interacts with XMAP215. In addition, Maskin increases XMAP215 affinity for microtubules in vitro. Our data suggest that Maskin and XMAP215 cooperate to oppose the destabilizing activity of XKCM1 and this activity is essential for microtubule elongation from the centrosome.
Results
The Xenopus TACC family member Maskin associates with the centrosome and spindle microtubules in Xenopus cells and egg extract
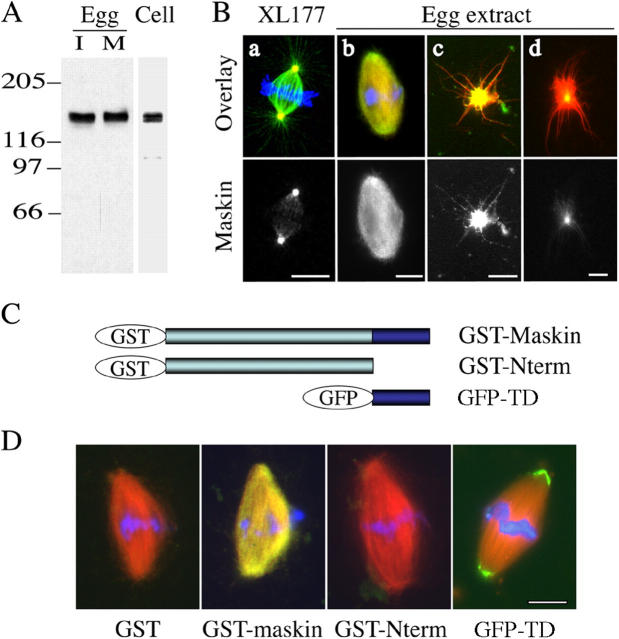
Maskin has been extensively characterized in the context of its role in translation regulation (Groisman et al., 2001; Stebbins-Boaz et al., 1999). It has been reported that it localizes to the mitotic spindle in Xenopus early embryos (Groisman et al., 2000) but no further data concerning its precise subcellular localization has been reported. To examine Maskin subcellular localization in tissue culture cells and egg extracts we raised polyclonal antibodies against the recombinant Maskin full-length protein expressed in bacteria. The affinity-purified antibody recognized one major band at ∼150 kD that sometimes appeared as a doublet, on Western blot of Xenopus XL177 cell lysates and cytostatic factor (CSF)–arrested egg extract (Fig. 1 A). Although Maskin predicted molecular weight is only 102 kD, this is in agreement with the previously reported migration of Maskin in SDS-PAGE (Stebbins-Boaz et al., 1999). We then examined Maskin localization by immunofluorescence microscopy on Xenopus XL177 cells and in egg extracts using the affinity-purified anti-Maskin antibodies (Fig. 1 B, a, and Fig. S1 available at http://www.jcb.org/cgi/content/full/jcb.200504037/DC1). In some interphase XL177 cells the anti-Maskin antibodies stained a single dot corresponding to the microtubule organizing center, presumably the centrosome. This staining increased significantly at the center of the two separating microtubule asters as the cells entered prophase and persisted throughout all mitotic phase until it became weaker again in telophase/cytokinesis. At metaphase (Fig. 1 B, a) the anti-Maskin antibody also decorated some spindle microtubules and in anaphase–telophase (Fig. S1) some microtubules extending from the centrosome to the separating chromosomes. Finally at cytokinesis (Fig. S1), they decorated the midbody (only in methanol-fixed cells). We did not observe any decoration of astral microtubules by the anti-Maskin antibodies at any stage during mitosis.
Figure 1.
Localization of endogenous and recombinant Maskin in Xenopus XL177 cells and egg extract. (A) Western blot of XL177 and of mitotic (M) and interphase (I) egg extracts probed with the anti-Maskin antibodies used in this work. (B) Immunofluorescence with anti-Maskin antibodies on spindles and asters assembled in cells and in egg extract. (a and b) Maskin localizes all along spindle microtubules and accumulates at spindle poles. (c) In asters nucleated by centrosomes in egg extract, Maskin accumulates strongly at the center and aligns along the microtubules as small dots. (d) In taxol-induced asters, a small amount of Maskin can be detected at the center. Microtubules are red, Maskin is green, and DNA in the spindle is blue. Bars, 10 μm. (C) Schematic representation of the recombinant proteins added to the egg extract. (D) Representative images of spindles assembled in extracts containing the recombinant proteins (200 μM, final concentration). Anti-GST antibodies were used to detect GST or the GST-tagged recombinant proteins by immunofluorescence. Images from the three spindles were taken with the same camera settings. The GFP-TD protein was visualized directly through GFP fluorescence. Microtubules are red, Maskin and GFP-TD are green, and DNA is blue. Bar, 10 μm.
We then examined the localization of Maskin in the egg extract (Fig. 1 B). Bipolar spindles were assembled around sperm nuclei in cycled egg extract containing rhodamine-labeled tubulin. Spindles were fixed, centrifuged onto coverslips, and processed for immunofluorescence with the affinity-purified anti-Maskin antibodies. Maskin localized to the whole spindle with some enrichment toward the spindle poles (Fig.1 B, b). Finally, we examined Maskin localization on microtubule asters formed by addition of purified centrosomes (Fig. 1 B, c) or taxol (Fig. 1 B, d) to CSF extracts. In both cases Maskin was found at the center of the asters. However, Maskin was strongly enriched at the center of centrosome asters and decorated microtubules in a dotty pattern (Fig. 1 B, c). Comparatively Maskin seemed to bind less efficiently to taxol asters (Fig. 1 B, d). We conclude that Maskin is strongly recruited to the centrosomes and also binds along dynamic microtubules during M-phase. These localization results fit very well with the localization reported for other TACC family members (Gergely et al., 2000a,b; Bellanger and Gonczy, 2003; Le Bot et al., 2003; Srayko et al., 2003).
The TACC domain targets to the spindle poles in egg extract
To determine which domains of Maskin are involved in its targeting to the spindle and centrosomes, we prepared various recombinant fragments tagged with either GST or GFP: the full-length, the TACC domain (TD) and the NH2-terminal region lacking the TACC domain (Fig. 1 C, GST-Nterm). These proteins were added at the same final concentration to cycled extract before spindle assembly. Spindles were fixed and centrifuged onto coverslips and processed for immunofluorescence with an anti-GST antibody to detect the GST fusion proteins or examined directly to visualize the GFP-TD localization (Fig. 1 D). GST-Maskin full-length localized along spindle microtubules and was enriched at the spindle poles like the endogenous protein. By contrast, the NH2-terminal domain of Maskin, GST-Nterm did not localize at all (Fig. 1 D). GFP-TD accumulated very strongly to the spindle poles (Fig. 1 D) and to the center of centrosome asters assembled in extract (unpublished data). GFP-TD localized strongly to one or two dots at the center of centrosome asters suggesting that it is recruited to the centrosome itself. Maskin is therefore targeted to the centrosome by its TACC domain and interacts with dynamic microtubules in M-phase egg extract. Although the NH2-terminal domain does not localize on its own it probably contributes to the localization of Maskin along spindle microtubules.
Maskin determines microtubule length and density in the spindle
As a first approach to examine the putative role of Maskin in spindle assembly in egg extract we tested whether our anti-Maskin antibodies would function for immunodepletion experiments. As shown in Fig. 2 A (left), three rounds of immunodepletion were required to deplete Maskin to more than 99% from CSF-arrested egg extract. Mock and Maskin-depleted extracts supplemented with sperm nuclei and rhodamine-labeled tubulin were sent into interphase by addition of calcium and cycled back into M-phase by addition or either mock and Maskin-depleted extract accordingly. After 45 min, samples were fixed and centrifuged onto coverslips. Both mock and Maskin-depleted extracts supported bipolar spindle assembly with similar efficiencies (70–80% in mock-depleted and 60–70% in Maskin-depleted extract; unpublished data). However, spindles assembled in Maskin-depleted extract were on average smaller than controls (Fig. 2 B). Their length was consistently reduced by 25–30% in all the independent experiments we performed **(**more than 10). The reduction of spindle width was more variable. We also found a variable but consistent reduction in the average tubulin fluorescence intensity in these spindles suggesting that they had a reduced microtubule density (Fig. 2 B, right). To check whether these effects were entirely due to Maskin depletion we tested whether we could rescue them by addition of recombinant Maskin to the depleted extract (Fig. 2). We found that indeed addition of GST-Maskin to the depleted extract rescued fully the spindle size to control values (Fig. 2). As the TACC domain is strongly targeted to the spindle poles we also tested whether it could rescue the depletion phenotype. We found that addition of GFP-TD to the Maskin-depleted extract was as efficient as addition of GST-Maskin for rescuing spindle size (Fig. 2). As reported above GFP-TD accumulated very strongly to the spindle poles whereas GST-Maskin localized along the spindle like the endogenous protein (Fig. 2 C).
Figure 2.
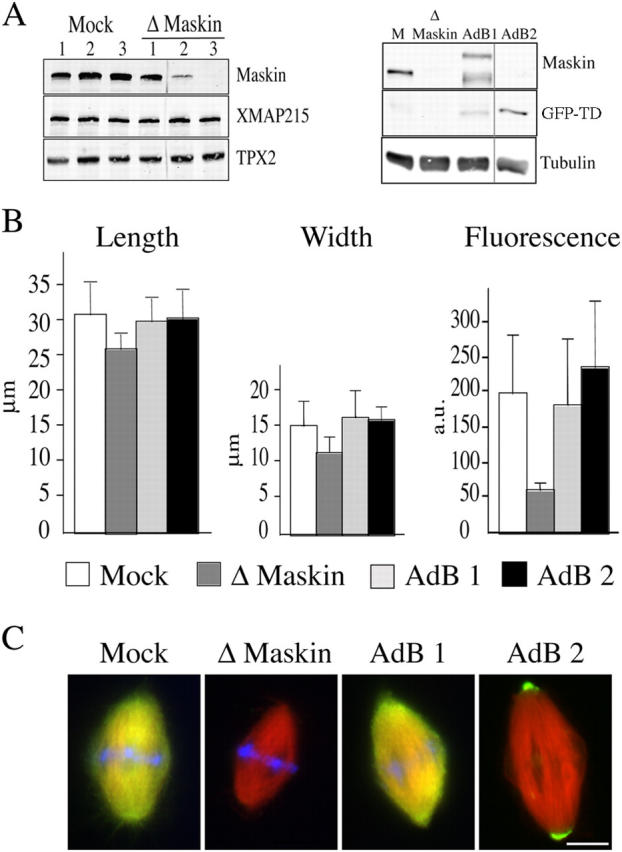
Spindle formed in Maskin-depleted extract are smaller. (A) Western blot of mock- and Maskin-depleted egg extract probed with the anti-Maskin antibodies (left). The three successive round of mock and Maskin depletion are shown. After the third round more than 99% of Maskin was depleted. The blot was reprobed with anti-XMAP215 and anti-TPX2 antibodies. There were no visible changes of XMAP215 concentration in the Maskin-depleted extract. The TPX2 band was used as loading control. (Right) Western blot of a typical rescue experiment. M, mock depleted extract; Δ Maskin, Maskin-depleted extract; AdB1, Maskin-depleted extract containing purified GST-Maskin; Adb2, Maskin-depleted extract containing GFP-TD protein. The blot was probed with the anti-Maskin antibody directed against the TACC domain and reprobed with an anti-tubulin antibody. The tubulin band was used as a loading control. (B) Graphs representing the average length (left), width (middle), and tubulin fluorescence intensity (right) of spindles assembled in the four types of egg extract. White bars, mock; dark gray bar, Maskin-depleted; light gray, AdB1; black bar, AdB2. (C) Immunofluorescence of spindles assembled in mock, Maskin-depleted, and AdB1 extracts with the anti-Maskin antibody (green) and GFP fluorescence (green) on spindles assembled in AdB2 conditions. Microtubules are red and DNA is blue. Bar, 10 μm.
To further confirm the role of Maskin in determining spindle size and density we examined spindles assembled in extracts containing GST-Maskin to increase the concentration of protein (Fig. S2 available at http://www.jcb.org/cgi/content/full/jcb.200504037/DC1). We found that the average size of spindles increased proportionally to the total Maskin concentration in the extract (Fig. S2).
Altogether these results suggested very strongly that Maskin has a role in microtubule assembly and/or stabilization in the M-phase egg extract.
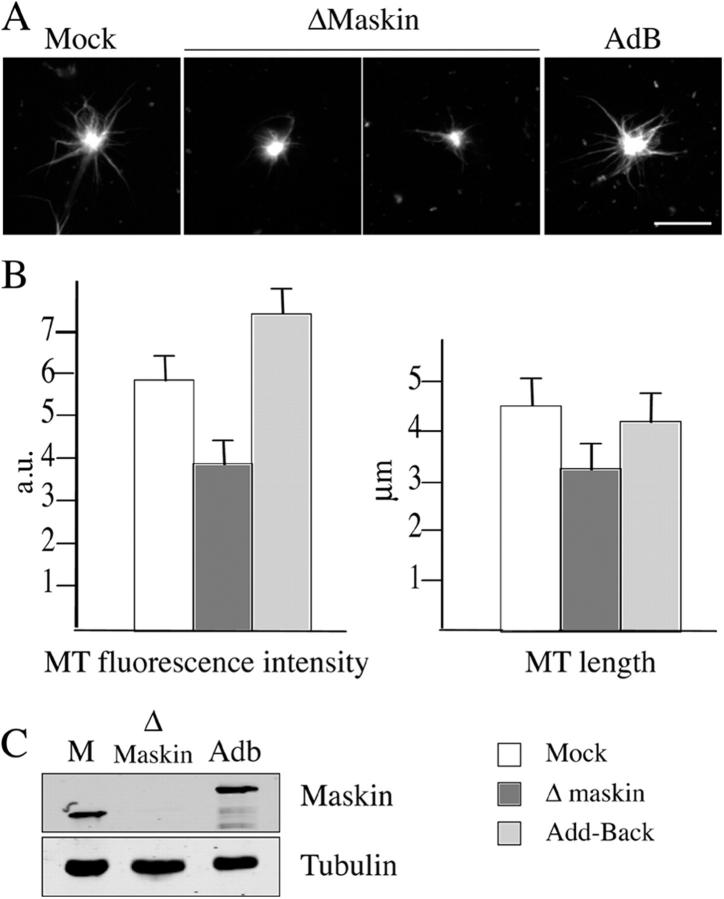
Maskin depletion impairs aster formation by centrosomes in extract
Spindles formed in egg extract around sperm nuclei consist of microtubules assembled through the RanGTP-dependent pathway around the chromatin and microtubules nucleated by the duplicated centrosomes (Karsenti and Vernos, 2001; Gruss and Vernos, 2004). Because the GFP-TD protein that targets to the centrosome/spindle poles was able to fully rescue the Maskin-depletion phenotypes it suggested that the main role of Maskin was performed at the centrosome. We therefore decided to examine the role of Maskin in microtubule aster formation by centrosomes in egg extract (Fig. 3). Purified centrosomes were added to mock or Maskin-depleted CSF-arrested egg extracts, sent to interphase, and cycled back into mitosis by addition of cyclin B Δ90. Samples were examined after 25 min of incubation. Microtubule asters formed both in mock or Maskin-depleted extracts (Fig. 3 A). However, asters formed in Maskin-depleted extracts were much weaker and smaller than asters assembled in the mock-depleted extract (Fig. 3 B). Addition of GST-Maskin to the Maskin-depleted extract at physiological concentrations (Fig. 3 C) rescued the size and microtubule fluorescence intensity of the asters (Fig. 3). Therefore, Maskin is essential for microtubule assembly from centrosomes in the M-phase extract.
Figure 3.
Maskin depletion impairs centrosome aster formation in egg extract. (A) Representative images of asters by purified centrosomes in mock-depleted extract (Mock), Maskin-depleted extract (ΔMaskin), and Maskin-depleted extract containing purified GST-Maskin protein (AdB). The extract was sent to interphase and cycled back into mitosis by addition of cyclin B Δ90. Bar, 10 μM. (B) Graphs showing the average microtubule fluorescence intensity (left) and microtubule length (right) in asters assembled under the three conditions as in A: mock (white bars), Δ Maskin (dark gray bars), Add-Back (light gray bars). (C) Western blot of the egg extracts used in A and B probed with anti-Maskin and anti-tubulin antibodies.
The reduction of centrosome aster size and density in Maskin-depleted extracts could be attributed to an effect on the microtubule nucleation capacity of the centrosomes. We therefore tested whether centrosome functionality was impaired in the absence of Maskin (Fig. S3 available at http://www.jcb.org/cgi/content/full/jcb.200504037/DC1). Sperm nuclei that are associated to an immature centriole were incubated in mock or Maskin-depleted extracts to reconstitute a functional centrosome for 30 min in the presence of nocodazole. They were then reisolated and placed in a solution of pure tubulin to test for their nucleation capacity in the absence of factors regulating microtubule dynamics. We found that centrosomes reconstituted in Maskin-depleted extract had similar microtubule nucleation capacity as those reconstituted in mock-depleted extract (Fig. S3). This shows that Maskin has no influence in microtubule nucleation by the centrosomes and therefore it suggests that its role is in microtubule stabilization and/or elongation from the centrosome.
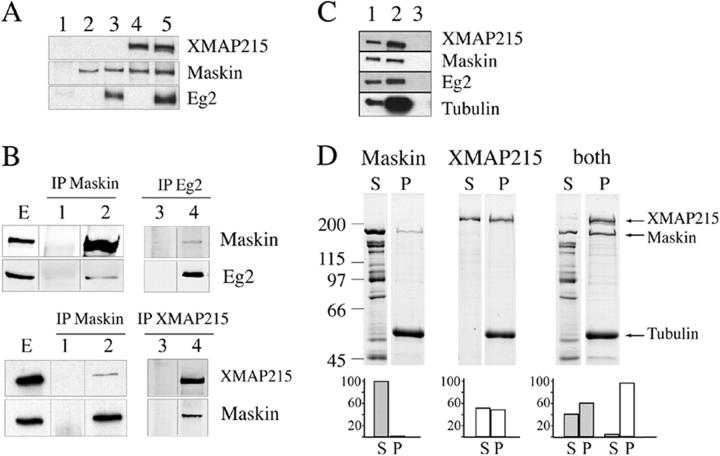
Maskin interacts with Aurora A/Eg2 and XMAP215 both in vitro and in the egg extract
Members of the TACC family have been reported to interact with two proteins that play important roles in microtubule dynamics and spindle assembly: chTOG/Msps (Lee et al., 2001; Bellanger and Gonczy, 2003; Srayko et al., 2003) and the kinase Aurora A (Giet et al., 2002). We first investigated whether Maskin interacts with the Xenopus chTOG/Msps family member XMAP215 and with the Xenopus Aurora A kinase Eg2 in vitro. GST-Maskin was incubated with purified his-XMAP215 or GFP-Eg2 or both proteins together. Proteins bound to Maskin were then pulled down using anti-GST–coupled beads and the presence of the different proteins tested by Western blot analysis. As shown in Fig. 4 A, Maskin associated efficiently with either Eg2 (lane 3) or XMAP215 (lane 4) in vitro. In addition, these interactions were not competitive as Maskin could also bind to both Eg2 and XMAP215 (lane 5).
Figure 4.
Maskin binds to microtubules and interacts with XMAP215 and Eg2 in vitro and in the egg extract. (A) Western blot of GST pull-downs in vitro with GST (lane 1) or GST-Maskin (lanes 2–5) in the presence of GFP-Eg2 (lane 3) or His-tagged XMAP215 (lane 4) or both (lane 5). Proteins recovered with the anti-GST antibody–coated beads were run on SDS-PAGE and blotted. The blot was probed with anti-XMAP215, anti-GST (to detect GST-Maskin), and anti-Eg2 antibodies to detect the corresponding proteins as indicated on the right. (B) Immunoprecipitations from CSF-arrested egg extract with different antibodies as indicated. (Lanes E) Total extract. (Lanes 1 and 3) Control immunoprecipitation with unspecific IgG. (Lanes 2 and 4) Immunoprecipitated proteins with specific antibodies as indicated. The blots were probed with anti-XMAP215, anti-Maskin, and anti-Eg2 antibodies to detect the presence of the corresponding proteins as indicated on the right. (C) Western blot analysis of a 0.5-μl total egg extract (lane 1), a microtubule pellet from 10 μl of egg extract incubated with taxol (lane 2), a pellet obtained from 10 μl of extract incubated with nocodazole (lane 3). The blot was probed with specific antibodies to detect the presence of the corresponding proteins as indicated on the right. Endogenous XMAP215, Maskin, and Eg2 copellet efficiently with microtubules in the extract. (D) Binding of Maskin or XMAP215 or both to microtubules in vitro. (D, top) Coomassie blue–stained gel from a representative microtubule pelleting experiment. GST-Maskin and XMAP215 at 1 μM final concentration were incubated with 3 μM taxol-stabilized microtubules either independently (left, Maskin; middle, XMAP215) or together (right, both). The supernatants (S) and pellets (P) obtained after centrifugation of the samples were run on SDS-PAGE and the gel stained with Coomassie blue. The positions of the XMAP215, Maskin, and tubulin bands are indicated on the right. Equivalent amount of the supernatants and pellets were run for direct comparison. (D, bottom) Quantification of the bands from the gel showing the proportion of proteins recovered in the supernatant and in the pellets (Maskin, gray bars; XMAP215, white bars) in each condition. The percentage of each protein in the pellet (P) and supernatant (S) was calculated over the total amount (S + P = 100%). This experiment was repeated five times and two gels were run for each experiment. Similar results were obtained in all independent experiments.
We then checked whether these interactions occurred also in the extract. The three proteins (Maskin, Eg2, and XMAP215) were immunoprecipitated independently from CSF-arrested egg extract using affinity-purified antibodies directed against each of the three proteins (Fig. 4 B). Anti-Maskin antibodies immunoprecipitated efficiently Maskin itself and very small amounts of XMAP215 and Eg2 (the amounts of these two proteins recovered in the immunoprecipitate were variable). Anti-XMAP215 antibodies coimmunoprecipitated Maskin efficiently. Finally the anti-Eg2 antibodies did coimmunoprecipitate a small amount of Maskin (this amount was also variable in different experiments). Therefore Maskin interacts efficiently with XMAP215 and Aurora A/Eg2 in vitro and more weakly in the egg extract. Pull-down experiments from egg extracts containing GFP-TD showed that Maskin interacts with XMAP215 through the TACC domain (unpublished data), as reported in other systems (Lee et al., 2001; Bellanger and Gonczy, 2003; Srayko et al., 2003).
Maskin interacts with microtubules in vitro and in the egg extract and increases XMAP215 affinity for microtubules in vitro
The role of Maskin in promoting microtubule elongation could be performed through its direct interaction with microtubules or by interacting with XMAP215 and/or other factors involved in microtubule stabilization. We therefore tested whether Maskin could interact directly with microtubules. Maskin copelleted efficiently with taxol-stabilized microtubules in the egg extract (Fig. 4 C).
To test whether Maskin could interact directly with microtubules, GST-Maskin was incubated with taxol-stabilized microtubules. Microtubules were pelleted through a glycerol cushion and the pellet run on SDS-PAGE and stained with Coomassie blue. A small fraction of Maskin copelleted with the microtubules showing that it can interact with them directly (Fig. 4 D). XMAP215 also bound to taxol microtubules under similar conditions with a slightly higher affinity than Maskin (Fig. 4 D). We then investigated the properties of the Maskin–XMAP215 complex. Both GST-Maskin and XMAP215 were incubated with taxol-stabilized microtubules. Microtubules were pelleted and analyzed. Interestingly, we found that when incubated together the affinity of the two proteins for the microtubules increased (Fig. 4 D). Quantification of the Coomassie-stained bands showed that 50% more of XMAP215 was recovered in the microtubule pellet in the presence of Maskin. Conversely, the amount of Maskin copelleting with microtubules was increased by a factor of six in the presence of XMAP215. These data suggested that Maskin's role in microtubule stabilization could be performed through the stimulation of XMAP215 binding to the microtubule lattice therefore increasing its ability to stabilize microtubules. Although Maskin on its own has a very low affinity for microtubules, in the presence of XMAP215 its affinity increases substantially and therefore another nonexclusive possibility is that Maskin could also stabilize microtubules directly.
Maskin phosphorylation by Eg2 is required for its localization to the spindle
Maskin is phosphorylated in CSF egg extract (unpublished data). Its sequence contains three sites that conform to the consensus sites for phosphorylation by Ipl1, a member of the Aurora kinase family, {KR}X{TS}{ILV} (Cheeseman et al., 2002) at positions Ser33, Ser620, and Ser626 (Fig. 5 A). These sites are conserved in its human ortholog TACC3 (positions Ser34, Ser552, and Ser558). To study the functional relevance of phosphorylation at these sites, we replaced Ser33, Ser620, and Ser626 to alanines by site-directed mutagenesis at each individual site, in paired combinations and on the three sites altogether. We then compared the phosphorylation activity of Eg2 on wild-type Maskin and on the different mutated proteins. As shown in Fig. 5 B, Eg2 phosphorylated efficiently wild-type Maskin in vitro. The three individual single mutants as well as the different double combinations only showed a mild decrease in overall phosphorylation (Fig. 5 B, see double mutant S620A, S626A). However, the triple alanine mutant (Maskin-3A) was not phosphorylated by Eg2 under the same conditions (Fig. 5 B). We conclude that Eg2 can phosphorylate Maskin on the three consensus sites in vitro_._ Furthermore Maskin cannot be phosphorylated by Eg2 on any other site.
Figure 5.
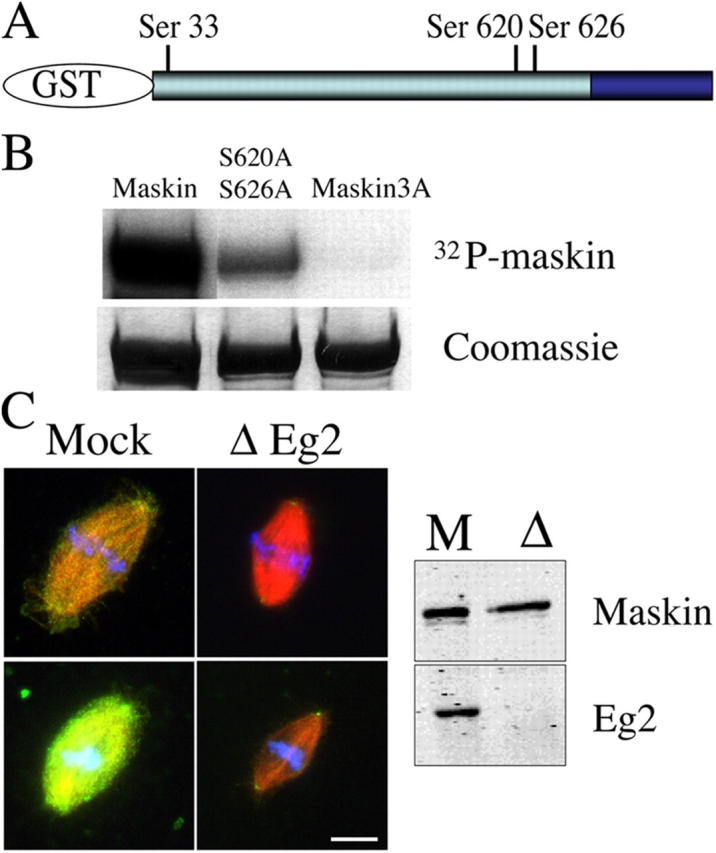
Eg2 phophorylates Maskin in vitro and is required for its localization in extract. (A) Schematic representation of GST-Maskin with the position of the three consensus sites for phosphorylation by Eg2. (B) Autoradiography (top) and Coomassie blue–stained gel (bottom) of GST-Maskin proteins incubated with purified Eg2. Three proteins are shown: GST-Maskin (Maskin), GST-Maskin mutated on Ser620 and Ser626 (S620A, S626A), and GST-Maskin mutated on the three sites Ser33, Ser620, and Ser626 (Maskin3A). (C) Effect of Eg2 depletion on the localization of Maskin in egg extract. Eg2 was depleted from the egg extract to more than 95% as shown by Western blot analysis (right). Spindles were assembled in cycled extract, fixed, and processed for immunofluorescence with the anti-Maskin antibody. Two pairs of spindles are shown taken with the same camera settings for comparison. Microtubules are red, Maskin is green, and DNA is blue. Bar, 10 μm.
To test for a possible role of Maskin phosphorylation by Eg2 we assembled spindles in Eg2-depleted egg extract and examined Maskin localization by immunofluorescence with the affinity-purified anti-Maskin antibodies (Fig. 5 C). We found that there was a dramatic reduction of Maskin in the spindles formed in Eg2-depleted extracts compared with the controls (Fig. 5 C). This indicated that the recruitment of Maskin to the spindle and spindle pole is dependent on Eg2 itself or on the phosphorylation of Maskin by Eg2.
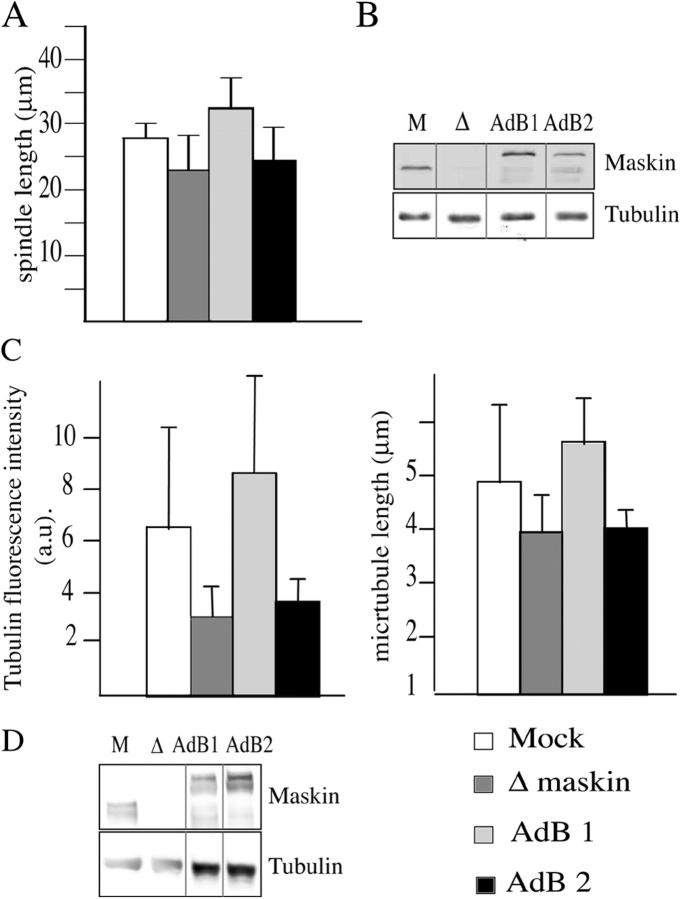
Maskin-3A does not rescue spindle size and centrosomal asters formation
To evaluate the functional role of Maskin phosphorylation by Eg2, we tested whether recombinant Maskin-3A could rescue the depletion phenotypes (Fig. 6). We first looked at spindle assembly. Spindles were assembled using mock and Maskin-depleted cycled extracts. GST-Maskin or GST-Maskin-3A were added to the depleted extract as the system was cycled back into M-phase. As shown in Fig. 6 A, GST-Maskin-3A was unable to rescue the size of spindles whereas GST-Maskin could. Consistently, GST-Maskin-3A could not rescue centrosome aster size in the depleted extract (Fig. 6 C). As a control we generated a Maskin triple E mutant (replacing Ser33, Ser620, and Ser626 to glutamic acids by site-directed mutagenesis) to mimic the phosphorylated state of the protein. We found that GST-Maskin-3E could rescue aster formation in Maskin-depleted extracts like the wild-type protein (Fig. S4 available at http://www.jcb.org/cgi/content/full/jcb.200504037/DC1). We conclude that Maskin phosphorylation by Eg2 is essential for its function in regulating microtubule assembly and/or stability.
Figure 6.
Maskin-3A does not rescue the spindle and aster size in Maskin-depleted extract. Spindles and centrosome asters were assembled in four different extracts. Mock (white bars), Maskin-depleted extract (dark gray bars), AdB1, Maskin-depleted extract–containing purified GST-Maskin (light gray bars), and AdB2, Maskin-depleted extract containing purified GST-Maskin3A (black bars). (A) Graph showing the average length of the spindles is shown for the four conditions. (B) Western blot of the four extracts used in A probed with the anti-Maskin and anti-tubulin antibodies. The tubulin band was used as a loading control. (C) Graphs showing the average tubulin fluorescence intensity (left) and length of microtubules (right) of asters assembled in the four conditions. (D) Western blot of the four types used in C probed with the anti-Maskin and anti-tubulin antibodies.
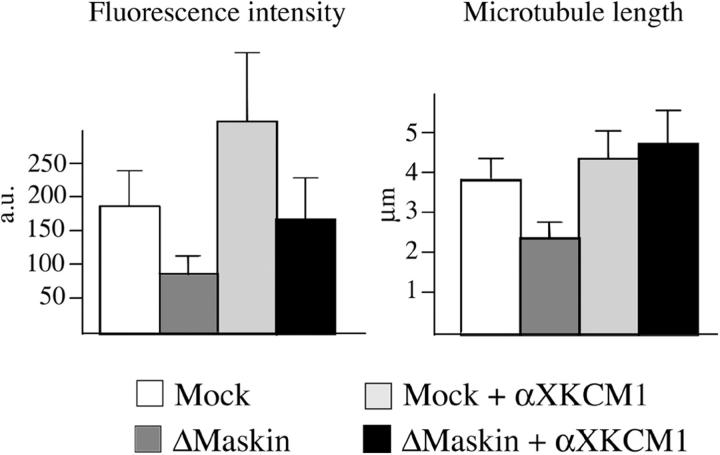
Inhibiting XKCM1 rescues microtubule aster formation in Maskin-depleted extracts
Microtubule dynamics in the M-phase egg extract has been shown to depend on the relative activities of two factors: the stabilizing factor XMAP215 and the catastrophe factor XKCM1. Here we found that Maskin interacts with XMAP215 and increases its affinity for microtubules. Therefore Maskin role in microtubule assembly/stabilization is at least in part achieved through XMAP215. We therefore tested whether Maskin depletion could be counteracted by inhibition of XKCM1. We performed a centrosome aster assembly assay in mock and Maskin-depleted extract as described above in the presence of inhibitory anti-XKCM1 antibodies (Fig. 7). We calibrated the concentration of anti-XKCM1 antibodies to get a partial inhibition of XKCM1 and a mild effect on microtubule length and density around centrosomes in control conditions. In Maskin-depleted extract the same degree of inhibition of XKCM1 also resulted in an increase of microtubule length and density therefore rescuing the Maskin depletion phenotype. Considering we have shown that in the presence of Maskin XMAP215 binds with higher affinity to microtubules, these results suggest that Maskin has a role in increasing the ability of XMAP215 to oppose XKCM1 depolymerizing activity.
Figure 7.
Inhibition of XKCM1 rescues aster size in Maskin-depleted extract. Microtubule asters were assembled as in Fig. 3 in mock-depleted extract (Mock, white bars), Maskin-depleted extract (ΔMaskin, dark gray bars), mock-depleted extract containing anti-XKCM1 antibodies (Mock + αXKCM1, light gray bar); Maskin-depleted extract containing anti-XKCM1 antibodies (ΔMaskin + αXKCM1, black bars). The average tubulin fluorescence intensity (left) and microtubule length (right) are shown.
Discussion
Maskin has been well characterized for its role in translational regulation during Xenopus oocyte maturation (Stebbins-Boaz et al., 1999; Groisman et al., 2001; Mendez and Richter, 2001). Here we show that like other TACC family members (Gergely et al., 2000a,b; Bellanger and Gonczy, 2003; Le Bot et al., 2003; Srayko et al., 2003) it is recruited by centrosomes and localizes to spindle poles and microtubules in Xenopus cells and M-phase egg extract. Our functional experiments in the egg extract system show that Maskin plays an essential role for microtubule growth and stability during M-phase. This role is dependent on its COOH-terminal TACC domain that targets to the centrosome and spindle pole and mediates its interaction with XMAP215. Our data suggest that Maskin together with XMAP215 at the centrosome and in the cytoplasm promotes microtubule assembly and counteracts the destabilizing activity of XKCM1. We also provide evidence for the regulation of Maskin localization and activity by the mitotic kinase Aurora A.
Role of Maskin in microtubule growth during mitosis
Like other members of the TACC family, Maskin is strongly enriched at the spindle poles and localizes to spindle microtubules during metaphase in Xenopus tissue culture cells and in the egg extract. In Maskin-depleted extracts centrosomes have a reduced efficiency for promoting microtubule aster formation although their intrinsic microtubule nucleation activity is intact. In addition, microtubules are shorter. Therefore our data indicate that the main role of Maskin is not in microtubule nucleation but in microtubule stabilization or growth. Interestingly, the effects we observe upon Maskin depletion are very similar to the phenotypes described in Drosophila embryos lacking a functional D-TACC: shorter spindles and the lack of astral microtubules (Gergely et al., 2000b).
Maskin does not localize very efficiently to spindle microtubules in cells and in egg extract but it does copellet efficiently with taxol-stabilized microtubules in the extract. As we found that it also pellets with pure microtubule in vitro although with low efficiency, one possibility is that Maskin stabilizes directly microtubules by binding to their lattice like some MAPs. Preliminary results indicate that Maskin has in fact only a minor stabilizing effect on microtubules in vitro (unpublished results). Because Maskin, like its orthologs, interacts with XMAP215, the main factor involved in microtubule stabilization in the M-phase egg extract, it is therefore possible that its stabilizing activity in the egg extract is directly linked to this interaction. Interestingly, partial depletion of XMAP215 from the egg extract induces phenotypes that are similar to the Maskin depletion. However we are certain that the Maskin depletion phenotypes are not due to a partial codepletion of XMAP215 as they were fully rescued by addition of recombinant Maskin. As a further support for the idea that Maskin is involved in microtubule assembly and/or stability, we have observed that increasing Maskin concentration in the extract results in the increase of spindle size.
Several data suggest that Maskin does not function efficiently in microtubule assembly/stabilization in the absence of XMAP215. Full depletion of XMAP215 results in the total absence of microtubule growth, a phenotype that is more dramatic than the Maskin depletion phenotype. Therefore Maskin activity is not sufficient for promoting microtubule growth in the absence of XMAP215. By contrast, XMAP215 still binds to microtubules on spindles and asters assembled in Maskin-depleted extract (unpublished results). However, it is less efficient for microtubule stabilization when Maskin is not present because spindles and asters are smaller. As we have shown that the complex both Maskin and XMAP215 have a higher affinity for microtubules when incubated together than each protein on its own in vitro this suggests that Maskin may increase microtubule stability through the modulation of XMAP215 activity.
We found that Maskin–XMAP215 interaction is more efficient in vitro than in the egg extract. It is possible that in the extract the interaction between the two proteins is regulated and/or other proteins may compete for the interaction. In this context, Maskin has been shown to be in a large complex with proteins involved in translation regulation like CPEB and elF-4E, and with RNA (Mendez and Richter, 2001). Because we can rescue the Maskin depletion phenotype with the TACC domain alone that does not contain the sequences involved in binding to these other components, it is likely that Maskin function in microtubule growth is independent from its role in translational regulation.
The rescue of the Maskin depletion phenotypes by the TACC domain alone suggests that the major site for Maskin activity is the centrosome/spindle pole where this domain can accumulate on its own. In addition, it is the domain of Maskin involved in the interaction with XMAP215. In other systems, it has been shown that TACC family members and XMAP215 family members depend on each other for their targeting to the centrosome/spindle poles (Lee et al., 2001; Srayko et al., 2003). Although we observed some reduction of XMAP215 localization to the centrosome in Maskin-depleted extract, this result is difficult to interpret because there is also a reduction of tubulin (unpublished data).
Our in vitro studies showing that the Maskin-XMAP215 complex binds to the microtubules with increased affinity suggest that if a similar mechanism occurs in the extract this would result in microtubule stabilization and protection from the depolymerizing activity of XKCM1. This could be particularly important at the centrosome where XKCM1 localizes. The Maskin–XMAP215 complex could protect the nascent microtubules promoting their elongation off the centrosome. In support of this model, we observed that a partial inhibition of XKCM1 in Maskin-depleted extract restored centrosome aster size.
A similar mechanism could function as well away from the centrosome and contribute to the determination of the steady-state length of microtubules in asters and spindles. Interestingly, while we were writing this work, O'Brien et al. (2005) published a paper describing a role for Maskin in the formation of RanGTP-dependent microtubule asters in the egg extract. It is therefore possible that Maskin has a general function in promoting microtubule stability through XMAP215 with a more prominent effect at the sites of microtubule nucleation like the centrosome or where the RanGTP-dependent microtubule nucleation occurs.
Regulation of Maskin by Aurora A/Eg2
We have shown that Maskin interacts with Eg2 and becomes phosphorylated by this kinase on three consensus sites outside the TACC domain in vitro. Recently it has been shown that phosphorylation of Maskin by Eg2 is involved in regulating its activity during oocyte maturation (Pascreau et al., 2005). Here we have shown that Eg2 also regulates Maskin localization and activity in M-phase egg extract. Indeed in the absence of Eg2 Maskin localizes very poorly to spindles and spindle poles. This suggests that in the full length protein, the TACC domain may not be competent for recruitment to the centrosome and microtubules and that phosphorylation by Eg2 may either induce a conformational change and/or release Maskin from other interactions that may prevent its recruitment to the spindle. In fact, the TACC domain that is not phosphorylated by Eg2, is very efficiently recruited to the centrosome and spindle poles.
Our data do not support the idea that phosphorylation could regulate directly the interaction between Maskin and XMAP215. This interaction is very efficient in vitro between the unphosphorylated recombinant proteins and we have not detected any significant increase in affinity in the presence of Eg2 under our experimental conditions. However, it is possible that the situation is different in the extract and phosphorylation of Maskin by Eg2 could promote the interaction of Maskin with XMAP215 for the same arguments exposed above for the centrosomal localization.
Interestingly, Aurora A is required for the increase of microtubule nucleation at the centrosomes during M-phase (Blagden and Glover, 2003). The recruitment of Maskin to the centrosome could be one of the mechanisms by which Aurora A functions in this process.
Materials and methods
Maskin constructs
The cDNA of Maskin in pET30a was obtained from Raul Mendez (CRG, Barcelona, Spain) (Groisman et al., 2000). The full-length coding region was excised by restriction digestion with BamHI and Xho1 and subcloned into pGEX-4T1 and pGEX-6P1 (Amersham Biosciences). Site directed mutagenesis (Stratagene) was performed on the pGEX-6P1 construct to introduce alanines at positions Ser33, Ser620, and Ser626 either independently, in the three possible paired combinations, or all three together. The GST-NH2-terminal Maskin construct was prepared by introducing a stop codon corresponding to aa 717 in pGEX-6P1. The GFP-TACC construct was made by amplifying the region TACC domain (aa 723–aa 931) introducing EcoRI and BamHI restriction site at 5′ and 3′, respectively. The PCR product was subcloned into a modified pHAT-2 vector contains the EGFP sequence after the Histidine tag (Wittmann et al., 2000). All constructs were fully sequenced.
Expression and purification of recombinant proteins
The GST fusion proteins were expressed in Escherichia coli and purified by glutathione affinity chromatography using standard protocols. His-tagged Maskin full-length and GFP-TD proteins were purified as described in Wittmann et al. (2000). Purified proteins were dialyzed against CSF-XB (10 mM K-Hepes, pH 7.7, 50 mM sucrose, 100 mM KCl, 2 mM MgCl2, and 5 mM EGTA), frozen in aliquots in liquid nitrogen, and stored at −80°C. Protein concentration was estimated using Coomassie-stained SDS gels using BSA as a standard. For cleavage of GST, GST-Maskin was incubated with PreSission Protease (Amersham Biosciences) overnight at 4°C.
The full-length XMAP215 cDNA was cloned and expressed using the Bac-to-bac baculovirus system (Invitrogen). SF9 cells were used to express the His-tagged full length protein that was purified using affinity chromatography following the manufacturer's instructions.
Antibodies and Western blots
Polyclonal anti-Maskin antibodies were produced in rabbit by injection of bacterially expressed recombinant His-Maskin full-length protein. The serum was affinity-purified over a column of His-Maskin full-length or GST-Maskin full-length or GFP-TACC with similar results. Monoclonal anti-tubulin antibody was from Sigma-Aldrich (DM1A). The 1C1 monoclonal anti-Eg2 antibody was a gift from C. Prigent (CNRS, Rennes, France). A polyclonal anti-Eg2 antibody was raised in rabbit by injection of bacterially expressed EGFP-Eg2 full-length protein and affinity purified on bacterially expressed His-Eg2. Polyclonal anti-GST antibody was generated in-house and the affinity-purified polyclonal anti-XKCM1 antibody was generated as described previously (Walczak et al., 1996).
Blots were developed using the ECL chemiluminescence detection system (GE Healthcare) or using Alexa Fluor 680 (Invitrogen)–labeled antibodies with the Odyssey Infrared imaging system (Li-Cor). Extracts from Xenopus XL177 (Miller and Daniel, 1977) were prepared as described previously (Le Bot et al., 1998).
Immunoprecipitation
For immunoprecipitation experiments, protein A–conjugated Dynabeads 280 (Dynal) were coated with the appropriate antibodies (5 μg/20 μl of beads), in 300 μl PBS-T (PBS, 0.1% Triton X-100). Beads were washed twice with PBS-T and twice with CSF-XB (10 mM K-Hepes, pH 7.7, 50 mM sucrose, 100 mM KCl, 2 mM MgCl2, 0.1 mM CaCl2, and 5 mM EGTA). Antibody-coated beads were incubated in 40 μl of extract on ice for 1 h, retrieved on a magnet for 10 min, and washed twice with CSF-XB and twice with PBS-T. Proteins were eluted from the beads by incubation in sample buffer for 10 min at room temperature. The samples were then subjected to SDS-PAGE and analyzed by Western blotting.
Egg extracts, spindle assembly, and asters formation
Cytostatic factor arrested extracts (CSF extracts) were prepared as previously described (Murray, 1991). For cycled spindle assembly, 0.2 mg/ml rhodamine-labeled tubulin (Hyman et al., 1991) and demembranated sperm nuclei (∼500 nuclei/μl) were added to CSF extract on ice. The extract was then released into interphase by the addition of 0.4 mM Ca2+ and incubated for 90 min at 20°C. This extract was then cycled back into mitosis by addition of one volume of CSF-arrested extract. After 45 min, spindles were fixed in 1 ml BRB80 (80 mM K-Pipes, pH 6.8, 1 mM EGTA, 1 mM MgCl2) containing 30% glycerol, 0.25% glutaraldehyde, and 0.1% Triton X-100 and centrifuged onto coverslips (12,000 rpm, 12 min, 16°C; JS-13.1 rotor; Beckman Coulter) through a 40% glycerol cushion in BRB80, as described previously (Wittmann et al., 2000).
Microtubule asters were assembled in egg extract by addition of centrosomes purified from KE37 lymphoid cells as described previously (Moudjou and Bornens, 1998) or 1 μM taxol (paclitaxel; Invitrogen) in the presence of 0.2 mg/ml rhodamine-labeled tubulin. The reactions were incubated for 20 min at 20°C, fixed, and centrifuged onto coverslip as described previously (Wittmann et al., 1998). Alternatively, purified centrosomes were added to CSF extract, the extract was sent into interphase by addition of 0.4 mM Ca2+, 0.2 mg/ml cycloheximide (Sigma-Aldrich), and 0.4 mg/ml rhodamine-labeled tubulin for 45 min at 20°C. The extract was then cycled back into mitosis by addition of 38 μg/ml of cyclin B Δ90 (Glotzer et al., 1991). After 25 min asters were fixed and centrifuged onto coverslips as described above.
For Maskin domains localization studies, recombinant GST-Maskin, GST-NH2-terminal and GFP-TD were added at 200 nM as the extract was cycled back into mitosis.
Immunodepletion and add-back experiments
Immunodepletions were performed as previously described (Antonio et al., 2000) except that three successive rounds of depletion were required to deplete Maskin efficiently from the egg extract. In brief, three volumes of protein A–conjugated Dynabeads (Dynal) coupled to antibodies were used to deplete one volume of extract. The depletion efficiency was assayed by Western blot.
For rescue experiments GST-Maskin, GST-Maskin3A, or GFP-TD were added to restore endogenous concentration (as estimated by Western blot analysis) as the reaction was cycled back into mitosis. For aster experiments recombinant proteins were added as the extract was sent into interphase. For inhibition of XKCM1, 10 μg/ml of affinity purified anti-XKCM1 antibodies (a gift from E. Karsenti, EMBL, Heidelberg, Germany) were added to 20 μl of extract. Eg2 depletion was performed as described above using the polyclonal anti-Eg2 antibodies and two successive rounds of 45 min.
In vitro kinase assay
In vitro kinase assays with recombinant Eg2 were performed as previously described (Bayliss et al., 2003). Full-length recombinant GST-Maskin or the different mutated GST-Maskin proteins at 10 μM were incubated with full-length recombinant GFP-Aurora-A/Eg2 in kinase buffer (20 mM Hepes, pH 7.5, 200 mM KCl, 5 mM MgCl2, 0.5 mM EGTA, 1 mM DTT, 0.05% Triton X-100, 50 μM ATP) containing γ32P-ATP (GE Healthcare), for 10 min at 30°C. After separation by SDS-PAGE, phosphorylated proteins were detected by autoradiography.
In vitro pull-down and microtubule binding assay
For the pull-down assay, GST protein or GST-Maskin (10 μg protein/20 μl beads) were incubated with anti-GST antibody coupled beads (Dynal) as described above. After three washes with kinase assay buffer (50 mM Hepes, pH 7.5, 0.1 mM EGTA, 0.015% brij35), GST-Eg2 (0.5 μM), and ATP mix (0.15 mM ATP, 30 mM MgCl2 in kinase assay buffer) were added and the mixture incubated for 25 min at 25°C. BSA (10 mg/ml) and XMAP215 (10 μM) were then added and the mixture further incubated for 30 min at 25°C. The beads were retrieved and washed three times with wash buffer (0.5% Triton X-100, 0.25 mM Na3VO3, 10 mM NaF, in TBS). Proteins associated to the beads were eluted by incubation in sample buffer and analyzed by SDS-PAGE.
Protein binding to taxol-stabilized microtubules in vitro was assayed as described in Gard and Kirschner (1987) with the following modification. 3 μM taxol-stabilized microtubules were incubated with 0.25 μM, 0.5 μM, and 1 μM GST, GST-Maskin or XMAP215 separately or 1 μM of both GST-Maskin and XMAP215 for 30 min at 25°C. Microtubules were then pelleted through a 30% (vol/vol) glycerol cushion (in BRB80/5 μM taxol) at 100,000 g in TLA-100 rotor (Beckman Coulter) for 20 min at 23°C. Quantification of Coomassie blue–stained bands in the gel was performed by scanning the gel and measuring the intensity of all the bands with Adobe Photoshop. In the case of microtubule pellets of Maskin and XMAP215 the values were normalized with the value of the corresponding tubulin bands.
For the microtubule pelleting experiment in egg extract, 20 μM of taxol or 40 μM Nocodazole were added to CSF-extract. After 20 min at 20°C the extracts were diluted three times in BRB80, 10% glycerol, 1 mM GTP, and 5 μl of taxol (in the taxol sample) and centrifuged for 12 min at 20°C, 12,000 rpm in a TLS-55 rotor, through a 40% glycerol cushion in BRB80. The cushion was washed once with water and the pellets analyzed by Western blotting.
Immunofluorescence and quantifications
Immunofluorescence on Xenopus XL177 was performed as previously described (Wittmann et al., 1998) with the affinity-purified anti-Maskin antibodies (1.4 μg/ml). Cells were fixed in methanol at −20°C or in 0.25% glutaraldehyde. All pictures were taken on a Leica TCS Sp2 confocal microscopy at 63× magnification making average projection of layers and processed identically with Adobe Photoshop.
Spindles and aster assembled in egg extract was processed for immunofluorescence as described (Wittmann et al., 1998) with the affinity-purified anti-Maskin antibodies to detect endogenous Maskin or anti-GST antibodies for detection of exogenous GST-tagged Maskin. GFP-TD was visualized directly. Pictures were taken with a 12-bit greyscale cooled CCD camera (F-View, Soft Imaging System) mounted on an Axioskop-2 (Carl Zeiss MicroImaging Inc.) at 63× magnification (NA 1.4) and the image were processed with the analysis software (soft image system) and Adobe Photoshop.
For quantifications, nonsaturated images of randomly selected spindles and asters were taken using the same camera settings. Measurements of spindle length and width were performed using Analysis (Soft Imaging System). The total microtubule fluorescence associated with each spindle was then measured using Image J. Determination of aster average length and average of tubulin content was performed using a custom made macro running on Matlab software (Math Works) as described in Gruss et al. (2002).
Online supplemental material
Fig. S1 shows Maskin localization in XL177 cells. Fig. S2 shows the increase of spindle size by addition of Maskin to the extract. Fig. S3 shows that the microtubule nucleation capacity of centrosomes is unaffected by Maskin depletion. Fig. S4 shows that Maskin-3E rescues centrosomal aster formation. Online supplemental materials are available at http://www.jcb.org/cgi/content/full/jcb.200504037/DC1.
Acknowledgments
We thank R. Mendez for the gift of the pET30-Maskin construct, E. Karsenti for the gift of anti-XKCM1 antibodies, and C. Prigent for the gift of 1C1 monoclonal anti-Eg2 antibody.
T. Sardon was supported by a postdoctoral fellowship from the Spanish Ministerio de Educación y Ciencia (EX2004-1011).
I. Peset, L.A. Bejarano, and I. Vernos' present address is Center for Genomic Regulation (CRG), Passeig Marítim, 37-49, 08003 Barcelona, Spain.
Abbreviations used in this paper: CSF, cytostatic factor; MAP, microtubule-associated protein; TACC, transforming acidic coiled-coil; TD, TACC domain.
References
- Andrews, P.D., E. Knatko, W.J. Moore, and J.R. Swedlow. 2003. Mitotic mechanics: the auroras come into view. Curr. Opin. Cell Biol. 15:672–683. [DOI] [PubMed] [Google Scholar]
- Antonio, C., I. Ferby, H. Wilhelm, M. Jones, E. Karsenti, A.R. Nebreda, and I. Vernos. 2000. Xkid, a chromokinesin required for chromosome alignment on the metaphase plate. Cell. 102:425–435. [DOI] [PubMed] [Google Scholar]
- Bayliss, R., T. Sardon, I. Vernos, and E. Conti. 2003. Structural basis of Aurora-A activation by TPX2 at the mitotic spindle. Mol. Cell. 12:851–862. [DOI] [PubMed] [Google Scholar]
- Bellanger, J.M., and P. Gonczy. 2003. TAC-1 and ZYG-9 form a complex that promotes microtubule assembly in C. elegans embryos. Curr. Biol. 13:1488–1498. [DOI] [PubMed] [Google Scholar]
- Blagden, S.P., and D.M. Glover. 2003. Polar expeditions–provisioning the centrosome for mitosis. Nat. Cell Biol. 5:505–511. [DOI] [PubMed] [Google Scholar]
- Cheeseman, I.M., S. Anderson, M. Jwa, E.M. Green, J. Kang, J.R. Yates III, C.S. Chan, D.G. Drubin, and G. Barnes. 2002. Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell. 111:163–172. [DOI] [PubMed] [Google Scholar]
- Conte, N., B. Delaval, C. Ginestier, A. Ferrand, D. Isnardon, C. Larroque, C. Prigent, B. Seraphin, J. Jacquemier, and D. Birnbaum. 2003. TACC1-chTOG-Aurora A protein complex in breast cancer. Oncogene. 22:8102–8116. [DOI] [PubMed] [Google Scholar]
- Cullen, C.F., and H. Ohkura. 2001. Msps protein is localized to acentrosomal poles to ensure bipolarity of Drosophila meiotic spindles. Nat. Cell Biol. 3:637–642. [DOI] [PubMed] [Google Scholar]
- Desai, A., S. Verma, T.J. Mitchison, and C.E. Walczak. 1999. Kin I kinesins are microtubule-destabilizing enzymes. Cell. 96:69–78. [DOI] [PubMed] [Google Scholar]
- Gadde, S., and R. Heald. 2004. Mechanisms and molecules of the mitotic spindle. Curr. Biol. 14:R797–R805. [DOI] [PubMed] [Google Scholar]
- Gard, D., and M. Kirschner. 1987. A microtubule-associated protein from Xenopus eggs that specifically promotes assembly at the plus end. J. Cell Biol. 105:2203–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard, D.L., B.E. Becker, and S.J. Romney. 2004. MAPping the eukaryotic tree of life: structure, function, and evolution of the MAP215/Dis1 family of microtubule-associated proteins. Int. Rev. Cytol. 239:179–272. [DOI] [PubMed] [Google Scholar]
- Gergely, F. 2002. Centrosomal TACCtics. Bioessays. 24:915–925. [DOI] [PubMed] [Google Scholar]
- Gergely, F., C. Karlsson, I. Still, J. Cowell, J. Kilmartin, and J.W. Raff. 2000. a. The TACC domain identifies a family of centrosomal proteins that can interact with microtubules. Proc. Natl. Acad. Sci. USA. 97:14352–14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergely, F., D. Kidd, K. Jeffers, J.G. Wakefield, and J.W. Raff. 2000. b. D-TACC: a novel centrosomal protein required for normal spindle function in the early Drosophila embryo. EMBO J. 19:241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergely, F., V.M. Draviam, and J.W. Raff. 2003. The ch-TOG/XMAP215 protein is essential for spindle pole organization in human somatic cells. Genes Dev. 17:336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giet, R., D. McLean, S. Descamps, M.J. Lee, J.W. Raff, C. Prigent, and D.M. Glover. 2002. Drosophila Aurora A kinase is required to localize D-TACC to centrosomes and to regulate astral microtubules. J. Cell Biol. 156:437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer, M., A.W. Murray, and M.W. Kirschner. 1991. Cyclin is degraded by the ubiquitin pathway. Nature. 349:132–138. [DOI] [PubMed] [Google Scholar]
- Groisman, I., Y.S. Huang, R. Mendez, Q. Cao, W. Theurkauf, and J.D. Richter. 2000. CPEB, Maskin, and cyclin B1 mRNA at the mitotic apparatus: implications for local translational control of cell division. Cell. 103:435–447. [DOI] [PubMed] [Google Scholar]
- Groisman, I., Y.S. Huang, R. Mendez, Q. Cao, and J.D. Richter. 2001. Translational control of embryonic cell division by CPEB and Maskin. Cold Spring Harb. Symp. Quant. Biol. 66:345–351. [DOI] [PubMed] [Google Scholar]
- Gruss, O.J., and I. Vernos. 2004. The mechanism of spindle assembly: functions of Ran and its target TPX2. J. Cell Biol. 166:949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss, O.J., M. Wittmann, H. Yokoyama, R. Pepperkok, T. Kufer, H. Sillje, E. Karsenti, I.W. Mattaj, and I. Vernos. 2002. Chromosome-induced microtubule assembly mediated by TPX2 is required for spindle formation in HeLa cells. Nat. Cell Biol. 4:871–879. [DOI] [PubMed] [Google Scholar]
- Holmfeldt, P., S. Stenmark, and M. Gullberg. 2004. Differential functional interplay of TOGp/XMAP215 and the KinI kinesin MCAK during interphase and mitosis. EMBO J. 23:627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman, A., D. Drechsel, D. Kellogg, S. Salser, K. Sawin, P. Steffen, L. Wordeman, and T. Mitchison. 1991. Preparation of modified tubulins. Methods Enzymol. 196:478–485. [DOI] [PubMed] [Google Scholar]
- Karsenti, E., and I. Vernos. 2001. The mitotic spindle: a self-made machine. Science. 294:543–547. [DOI] [PubMed] [Google Scholar]
- Kinoshita, K., B. Habermann, and A.A. Hyman. 2002. XMAP215: a key component of the dynamic microtubule cytoskeleton. Trends Cell Biol. 12:267–273. [DOI] [PubMed] [Google Scholar]
- Le Bot, N., C. Antony, J. White, E. Karsenti, and I. Vernos. 1998. Role of xklp3, a subunit of the Xenopus kinesin II heterotrimeric complex, in membrane transport between the endoplasmic reticulum and the Golgi apparatus. J. Cell Biol. 143:1559–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bot, N., M.C. Tsai, R.K. Andrews, and J. Ahringer. 2003. TAC-1, a regulator of microtubule length in the C. elegans embryo. Curr. Biol. 13:1499–1505. [DOI] [PubMed] [Google Scholar]
- Lee, M.J., F. Gergely, K. Jeffers, S.Y. Peak-Chew, and J.W. Raff. 2001. Msps/XMAP215 interacts with the centrosomal protein D-TACC to regulate microtubule behaviour. Nat. Cell Biol. 3:643–649. [DOI] [PubMed] [Google Scholar]
- Mendez, R., and J.D. Richter. 2001. Translational control by CPEB: a means to the end. Nat. Rev. Mol. Cell Biol. 2:521–529. [DOI] [PubMed] [Google Scholar]
- Miller, L., and J.C. Daniel. 1977. Comparison of in vivo and in vitro ribosomal RNA synthesis in nucleolar mutants of Xenopus laevis. In Vitro. 13:557–563. [DOI] [PubMed] [Google Scholar]
- Moore, A., and L. Wordeman. 2004. The mechanism, function and regulation of depolymerizing kinesins during mitosis. Trends Cell Biol. 14:537–546. [DOI] [PubMed] [Google Scholar]
- Moudjou, M., and M. Bornens. 1998. Method of centrosome isolation from culture animal cells. Cell Biology: A Laboratory Handbook. Vol. 2. J.E. Celis, editor. Academic Press, London. 111–119.
- Murray, A.W. 1991. Cell cycle extracts. Methods Cell Biol. 36:581–605. [PubMed] [Google Scholar]
- O'Brien, L.L., A.J. Albee, L. Liu, W. Tao, P. Dobrzyn, S.B. Lizarraga, and C. Wiese. 2005. The Xenopus TACC homologue, Maskin, functions in mitotic spindle assembly. Mol Biol Cell. 16:2836–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascreau, G., J.G. Delcros, J.Y. Cremet, C. Prigent, and Y. Arlot-Bonnemains. 2005. Phosphorylation of Maskin by aurora-A participates to the control of sequential protein synthesis during Xenopus laevis oocyte maturation. J. Biol. Chem. 280:13415–13423. [DOI] [PubMed] [Google Scholar]
- Popov, A.V., A. Pozniakovsky, I. Arnal, C. Antony, A.J. Ashford, K. Kinoshita, R. Tournebize, A.A. Hyman, and E. Karsenti. 2001. XMAP215 regulates microtubule dynamics through two distinct domains. EMBO J. 20:397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov, A.V., F. Severin, and E. Karsenti. 2002. XMAP215 is required for the microtubule-nucleating activity of centrosomes. Curr. Biol. 12:1326–1330. [DOI] [PubMed] [Google Scholar]
- Srayko, M., S. Quintin, A. Schwager, and A.A. Hyman. 2003. Caenorhabditis elegans TAC-1 and ZYG-9 form a complex that is essential for long astral and spindle microtubules. Curr. Biol. 13:1506–1511. [DOI] [PubMed] [Google Scholar]
- Stebbins-Boaz, B., Q. Cao, C.H. de Moor, R. Mendez, and J.D. Richter. 1999. Maskin is a CPEB-associated factor that transiently interacts with elF-4E. Mol. Cell. 4:1017–1027. [DOI] [PubMed] [Google Scholar]
- Tournebize, R., A. Popov, K. Kinoshita, A.J. Ashford, S. Rybina, A. Pozniakovsky, T.U. Mayer, C.E. Walczak, E. Karsenti, and A.A. Hyman. 2000. Control of microtubule dynamics by the antagonistic activities of XMAP215 and XKCM1 in Xenopus egg extracts. Nat. Cell Biol. 2:13–19. [DOI] [PubMed] [Google Scholar]
- Usui, T., H. Maekawa, G. Pereira, and E. Schiebel. 2003. The XMAP215 homologue Stu2 at yeast spindle pole bodies regulates microtubule dynamics and anchorage. EMBO J. 22:4779–4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde, F., M. Dogterom, E. Stelzer, E. Karsenti, and S. Leibler. 1992. Control of microtubule dynamics and length by cyclin A- and cyclin B-dependent kinases in Xenopus egg extracts. J. Cell Biol. 118:1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak, C., T.J. Mitchison, and A.B. Desai. 1996. XKCM1: a Xenopus kinesin-related protein that regulates microtubule dynamics during mitotic spindle assembly. Cell. 84:37–47. [DOI] [PubMed] [Google Scholar]
- Wittmann, T., M. Wilm, E. Karsenti, and I. Vernos. 2000. TPX2, A novel xenopus MAP involved in spindle pole organization. J. Cell Biol. 149:1405–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann, T., H. Boleti, C. Antony, E. Karsenti, and I. Vernos. 1998. Localization of the kinesin-like protein Xklp2 to spindle poles requires a leucine zipper, a microtubule-associated protein, and dynein. J. Cell Biol. 143:673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann, T., A. Hyman, and A. Desai. 2001. The spindle: a dynamic assembly of microtubules and motors. Nat. Cell Biol. 3:E28–E34. [DOI] [PubMed] [Google Scholar]