GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model (original) (raw)
. Author manuscript; available in PMC: 2009 Feb 1.
Published in final edited form as: Cancer Cell. 2008 Feb;13(2):141–152. doi: 10.1016/j.ccr.2008.01.011
SUMMARY
How breast cancers are able to disseminate and metastasize is poorly understood. Using hyperplasia transplant system, we show that tumor dissemination and metastasis occur in discrete steps during tumor progression. Bioinformatic analysis revealed that loss of the transcription factor GATA-3 marked progression from adenoma to early carcinoma and onset of tumor dissemination. Restoration of GATA-3 in late carcinomas induced tumor differentiation suppressed tumor dissemination. Targeted deletion of GATA-3 in early tumors led to apoptosis of differentiated cells, indicating that its loss is not sufficient for malignant conversion. Rather, malignant progression occurred with an expanding GATA-3 negative tumor cell population. These data indicate that GATA-3 regulates tumor differentiation and suppresses tumor dissemination in breast cancer.
SIGNIFICANCE
During malignant progression, a primary tumor acquires the ability to form metastases. The differentiation status of a primary tumor strongly predicts its metastatic potential. The cell fate regulator GATA-3 is a strong and independent predictor of clinical outcome in human luminal breast cancer. Here we show that loss of GATA-3 marks the expansion of a stem cell-like population during the early stages of malignant progression in a mouse model of luminal breast cancer. Restoration of GATA-3 in undifferentiated mammary adenocarcinomas was sufficient to induce tumor differentiation and inhibit tumor dissemination. These data suggest that GATA-3 causally links the differentiation status of a tumor with its malignant potential during breast cancer progression and raise the possibility of its use in differentiation therapy.
INTRODUCTION
Mortality from breast cancer arises primarily from metastatic colonization of distant organs, but currently there is no curative treatment for metastatic disease. The term “malignant progression” describes the process by which a benign tumor acquires the ability to colonize distant organs to form metastases (Chambers et al., 2002; Gupta and Massague, 2006; Weigelt et al., 2005). Although malignant progression has been the subject of scrutiny for decades, it remains poorly understood owing to the complexity of the process and the difficulty of modeling it in the laboratory. Tumor cells must successfully accomplish a number of cellular processes to form metastases, including evasion of immune responses and programmed cell death, invasion of the host stroma, escape through vasculature and/or lymphatics, and survival and growth in distant sites (Chambers et al., 2002). Much of the work on metastasis is based on experimental systems, such as orthotopic xenografts or tail vein injections, that do not model all of the steps necessary for metastasis formation. As a result, many fundamental aspects of malignant progression remain poorly understood. For instance, at what time point during tumor progression does dissemination to distant sites begin? Of all the steps in metastasis formation, which is rate limiting?
One clue to a cellular basis of metastasis formation comes from pathologic analysis of epithelial cancers, which has established a strong correlation between the differentiation status of a primary tumor and its metastatic potential (Bloom and Richardson, 1957; Contesso et al., 1987; Liu et al., 2007). Well-differentiated breast tumors, such as fibroadenomas, tend to have a low capacity for metastasis formation, while poorly differentiated breast tumors, such as invasive ductal carcinomas, have a high capacity for metastasis. Although this correlation holds true for the large majority of solid tumors, there is currently little molecular understanding for why this is so. For instance, does the loss of differentiation causally lead to metastasis formation or are these events simply correlative?
To identify molecular markers of malignant progression, a series of 11 independent microarray profiling studies (1009 patients in total) compared the gene expression signatures of well-differentiated, low-metastatic breast tumors and poorly differentiated, high-metastatic breast tumors (Bertucci et al., 2000; Farmer et al., 2005; Gruvberger et al., 2001; Hoch et al., 1999; Mehra et al., 2005; Perou et al., 2000; Sorlie et al., 2003; Sotiriou et al., 2003; van 't Veer et al., 2002; Wang et al., 2005; West et al., 2001). The transcription factor GATA-3 emerged as a strong predictor of tumor grade, estrogen receptor status and prognosis in breast cancer. Meta-analysis of four microarray datasets found that low GATA-3 expression was a strong and independent predictor of metastasis formation and poor clinical outcome (Mehra et al., 2005). Further, logrank analysis human microarray data and patient survival identified GATA-3 as the second best predictor of breast cancer survival among 8024 genes (Jenssen et al., 2002). Immunohistochemical and tissue microarray studies confirmed these findings (Hoch et al., 1999; Jacquemier et al., 2005; Mehra et al., 2005). Taken together, these studies indicate that breast tumors with high GATA-3 expression tend to be well differentiated with low metastatic potential, while tumors with low GATA-3 expression tend to be poorly differentiated with high metastatic potential.
Recently, we and others have shown that GATA-3 regulates luminal cell differentiation in the mammary gland (Asselin-Labat et al., 2007; Kouros-Mehr et al., 2006). GATA-3 is necessary both for the differentiation of mammary stem cells into mature luminal cells and for the maintenance of the differentiated luminal epithelium in the adult mammary gland. In this report, we explore the role of GATA-3 in tumor progression using the mouse mammary tumor virus LTR-driven polyoma middle T antigen (MMTV-PyMT) transgenic mouse, which is an accurate and reliable model of human luminal breast cancer (Guy et al., 1992; Herschkowitz et al., 2007; Lin et al., 2003). Using a hyperplasia transplant system that enables the stereotyped dissection of the steps in metastasis formation, we show that GATA-3 is a critical regulator of tumor differentiation and that its loss underlies the onset of tumor dissemination during tumor progression.
RESULTS
A transplant model to study metastasis formation in breast cancer
The MMTV-PyMT mouse develops spontaneous mammary tumors that progress from hyperplasias to poorly differentiated adenocarcinomas (Guy et al., 1992; Lin et al., 2003). However, these mice develop hundreds of distinct tumor foci in their mammary glands, making it difficult to follow the metastatic potential of tumor foci over time. To examine the progression of single tumor foci over time, we generated bi-transgenic MMTV-PyMT; β-actin-enhanced green fluorescent protein (GFP) mice in the FVB/n strain and microdissected intact GFP-positive hyperplasias (0.7–1.0 mm) from 3-week-old females (Figure 1A). Intact hyperplasias were then transplanted into the gland-free mammary fat pads of wild type, syngeneic recipient mice and allowed to develop for 5, 8, 12, 15 or 18 weeks. The outgrowths exhibited a stereotyped tumor progression (Figure 1B–1D). By 5 weeks, the outgrowths progressed to well-differentiated adenomas that were encapsulated in basement membranes and expressed milk proteins, such as β-casein. By 8 weeks, there was a stereotyped progression to early carcinoma, characterized by the loss of basement membrane, epithelial architecture, and milk protein production. By 18 weeks, the outgrowths progressed to poorly differentiated adenocarcinomas. The median time to palpable tumor in the transplant model was 63 days (n = 24).
Figure 1. Dissemination and metastasis occurs at distinct progression steps in a breast cancer hyperplasia transplant model.
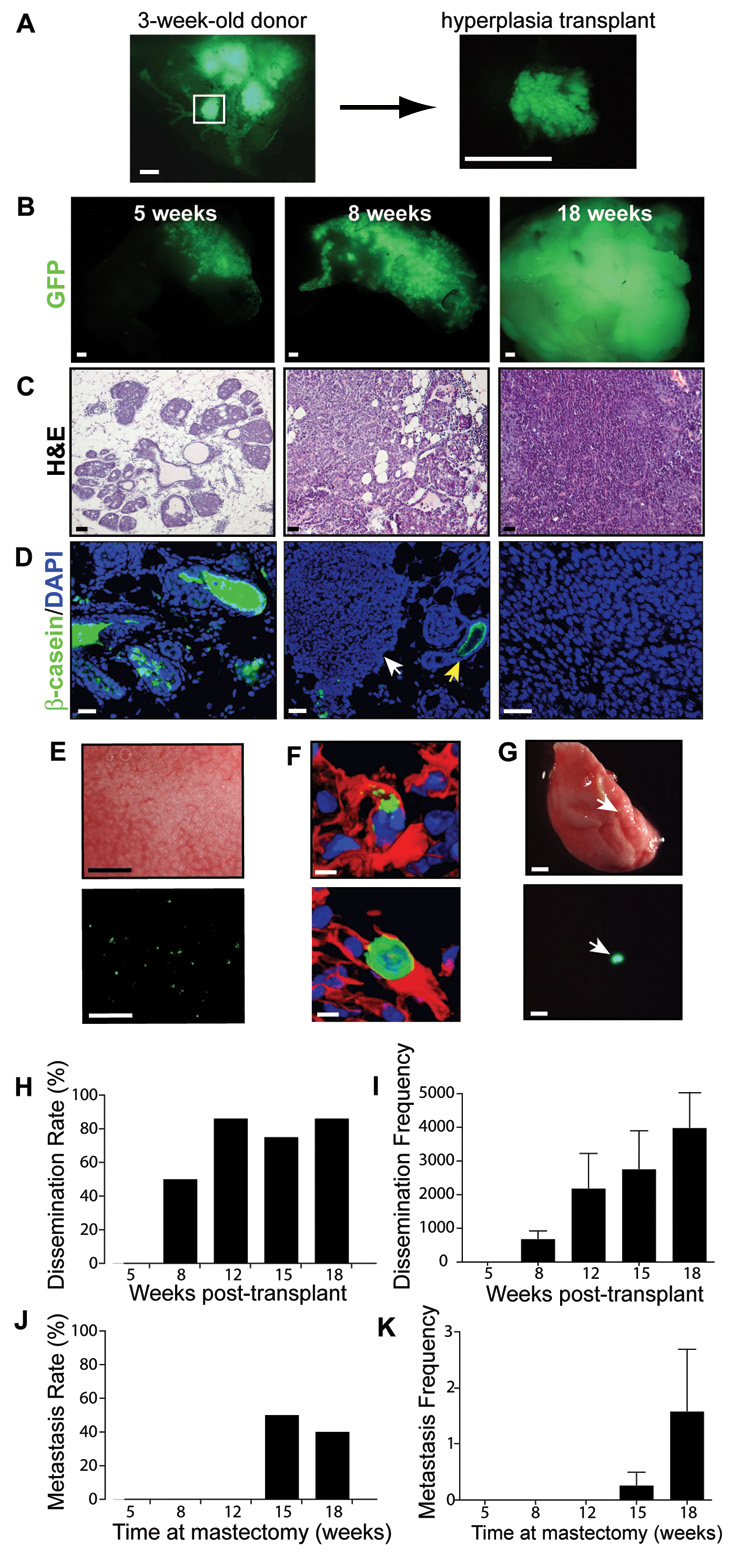
(A) Focal hyperplasia isolated from a 3-week-old MMTV-PyMT; β-actin GFP mouse.
(B–D) Representative (B) GFP whole mount, (C) H&E staining and (D) β–casein (green)/DAPI(blue) immunofluorescence of 5-, 8-, and 18-week tumor outgrowths. White arrowhead denotes early carcinoma adjacent to adenoma (yellow arrow) in an 8-week outgrowth.
(E) Brightfield (upper) and GFP (lower) whole mount of the lungs of an 18-week tumor-bearing mouse.
(F) Immunofluorescence of GFP (green), DAPI counterstain (blue) and tomato lectin (vasculature, red) of disseminated tumor cells in the lung of a tumor-bearing mouse (18-week outgrowth).
(G) Brightfield (upper) and GFP (lower) whole mount of a lung with metastasis (arrowhead). Shown is the lung of a mouse sacrificed 8 weeks after removal of an 18-week tumor outgrowth.
(H,I) Tumor dissemination rate (% mice with dissemination) (H), and frequency (total projected number of disseminated cells)(I) in the lungs of tumor-bearing mice at 5- (n=5), 8- (n=8), 12- (n=7), 15- (n=4), and 18-weeks (n= 7) post-transplant.
(J,K) Lung metastasis rate (% mice with lung metastases) (J) and frequency (total number of lung metastases) (K) of tumor outgrowths. Mice with tumor outgrowths of 5 (n=5), 8 (n=5), 12 (n=5), 15 (n=6), and 18 weeks (n=5) underwent mastectomy and were sacrificed 8 weeks later.
Data are represented as mean ± s.e.m. (I,K). Scale bars correspond to 1 mm (A, B, E, G), 25 µm (C) and 5 µm (D, F).
Tumor dissemination and metastasis are distinct events during malignant progression
To characterize malignant progression in the transplant model, we determined the onset of tumor cell dissemination during neoplastic progression. We detected single GFP+ tumor cells in the lung, liver, spleen, brain, and kidneys of host mice beginning at 8 weeks post-transplantation, suggesting that tumor dissemination began precisely during the transition from adenoma to early carcinoma (Figure 1E, Figure S1 for negative control). Tomato-lectin staining revealed that the disseminated cells in the lungs were entrapped in the lung vasculature (n = 20, Figure 1F), suggesting that the cells had reached the lungs by a hematogenous route. The number of disseminated cells in the lungs of tumor-bearing mice increased steadily from 8 to 18 weeks post-transplant (Figure 1H and 1I). By 18 weeks post-transplant, several thousand disseminated cells were present in the lungs of tumor-bearing mice.
We then performed a pulse-chase experiment to track the metastatic capability of these disseminated cells. Tumor transplants were allowed to grow for a varying pulse period (5, 8, 12, 15, and 18 weeks) and were then removed by mastectomy. Mice were sacrificed after a constant chase period (8 weeks post-mastectomy) to allow the single cells to grow into metastases, and their organs were analyzed for metastasis. Interestingly, metastases were detected after the 8-week chase period from the 15- and 18-week outgrowths and not from the 5-, 8-, or 12-week outgrowths (Figure 1G, 1J and 1K). Thus, although tumor dissemination began at 8 weeks post-transplant, the capacity to form metastases was acquired later, at 15 weeks post-transplant. We did not detect any single disseminated tumor cells in any tumor-bearing mouse after the 8-week chase period (n = 208 sections from 26 mice). Moreover, we only detected an average of 1–2 metastases per mouse in the lungs of 18-week tumor mice after the 8-week chase period, suggesting that the overwhelming majority of disseminated cells had been destroyed (Figure 1K). This suggests that the rate-limiting step in metastasis formation is the extravasation and growth of disseminated cells that arrive in distant sites. A subset of mice in which the tumors recurred locally after mastectomy died within the 8-week chase period due to heavy metastatic burden in lungs (n = 4). Metastases were not detected in other organs, suggesting that there is a tissue-specific tropism for metastasis formation in this model. Taken together, these data indicate that tumor dissemination and metastasis occur at distinct times during tumor progression in the MMTV-PyMT model.
Loss of GATA-3 marks loss of differentiation and tumor progression in mammary adenocarcinomas
We used a bioinformatics strategy to identify molecular markers of malignant progression in this model. The RNA expression profiles of the adenomas (5-week outgrowths) and late carcinomas (18-week outgrowths) were compared by microarray analysis. Analysis of the data revealed that the most differentially expressed genes between adenomas and carcinomas were luminal differentiation genes. Of the six most differentially expressed genes between adenomas and carcinomas, five were associated with luminal differentiation (Table 1). Milk protein genes (whey acidic protein, α-lactalbumin and several caseins) and estrogen receptors were markedly down-regulated in carcinomas as compared to adenomas (Figure 2A). As in human breast cancer, tumor progression in this model strongly coincided with a loss of luminal cell differentiation. Importantly, the luminal cell fate regulator GATA-3 was down-regulated in carcinomas and was the only member of the GATA family to be differentially expressed. Immunofluorescence staining showed that nearly all tumor cells in 5-week adenomas were positive for GATA-3 and that 8-week early carcinomas displayed partial loss of GATA-3, but the tumor cells in 18-week late carcinomas were GATA-3 negative (Figure 2B). Interestingly, the small fraction of 18-week tumor outgrowths that failed to disseminate were GATA-3 positive (Figure 1H and Figure 2D). Disseminated tumor cells (n = 10) and metastases (n = 6) in the lungs of tumor-bearing mice were consistently GATA-3 negative (Figure 2E and 2F). Thus, the loss of GATA-3 marked the loss of tumor differentiation and the onset of tumor dissemination, suggesting that GATA-3 loss is a bona fide marker of malignant progression in this model.
Table 1.
Most differentially expressed genes during tumor progression
| Name | Symbol | n1* | n2 | n3 | Avg |
|---|---|---|---|---|---|
| Casein alpha‡ | Csna | 4.3 | 3.6 | 4.0 | 3.9 |
| Fatty acid bp4 | Fabp4 | 3.9 | 2.8 | 3.8 | 3.5 |
| Casein gamma‡ | Csng | 2.8 | 4.0 | 3.6 | 3.5 |
| Carbonic anhydrase 6‡ | Car6 | 2.3 | 2.3 | 3.1 | 2.6 |
| Whey acidic protein‡ | Wap | 2.8 | 2.0 | 2.6 | 2.4 |
| Carbonic anhydrase 3‡ | Car3 | 2.6 | 2.0 | 2.2 | 2.3 |
| Lipoprotein lipase | Lpl | 2.4 | 2.0 | 2.0 | 2.1 |
| Zinc finger prot 503 | Zfp503 | 2.1 | 2.2 | 1.5 | 1.9 |
| Riken 3021401L19 | 2.4 | 1.9 | 1.4 | 1.9 | |
| Adipsin | And | 2.3 | 1.2 | 2.2 | 1.9 |
| Thyroid SPOT14 hom | Thrsp | 2.1 | 1.5 | 1.9 | 1.8 |
| Lactalbumin alpha‡ | Lalba | 1.0 | 2.3 | 1.9 | 1.7 |
| Sterol carrier prot 2 | Scp2 | 2.2 | 1.7 | 1.3 | 1.7 |
| Acyl-CoA synth L4 | Acsl4 | 1.9 | 1.6 | 1.6 | 1.7 |
| CDC-like kinase 1 | Clk1 | 1.6 | 1.8 | 1.7 | 1.7 |
| Catenin delta 1‡ | Ctnnd1 | 2.3 | 1.1 | 1.6 | 1.7 |
| Tumor diff exp 1 | Tde1 | 1.6 | 1.5 | 1.8 | 1.6 |
Figure 2. Loss of GATA-3 marks malignant progression in breast cancer.
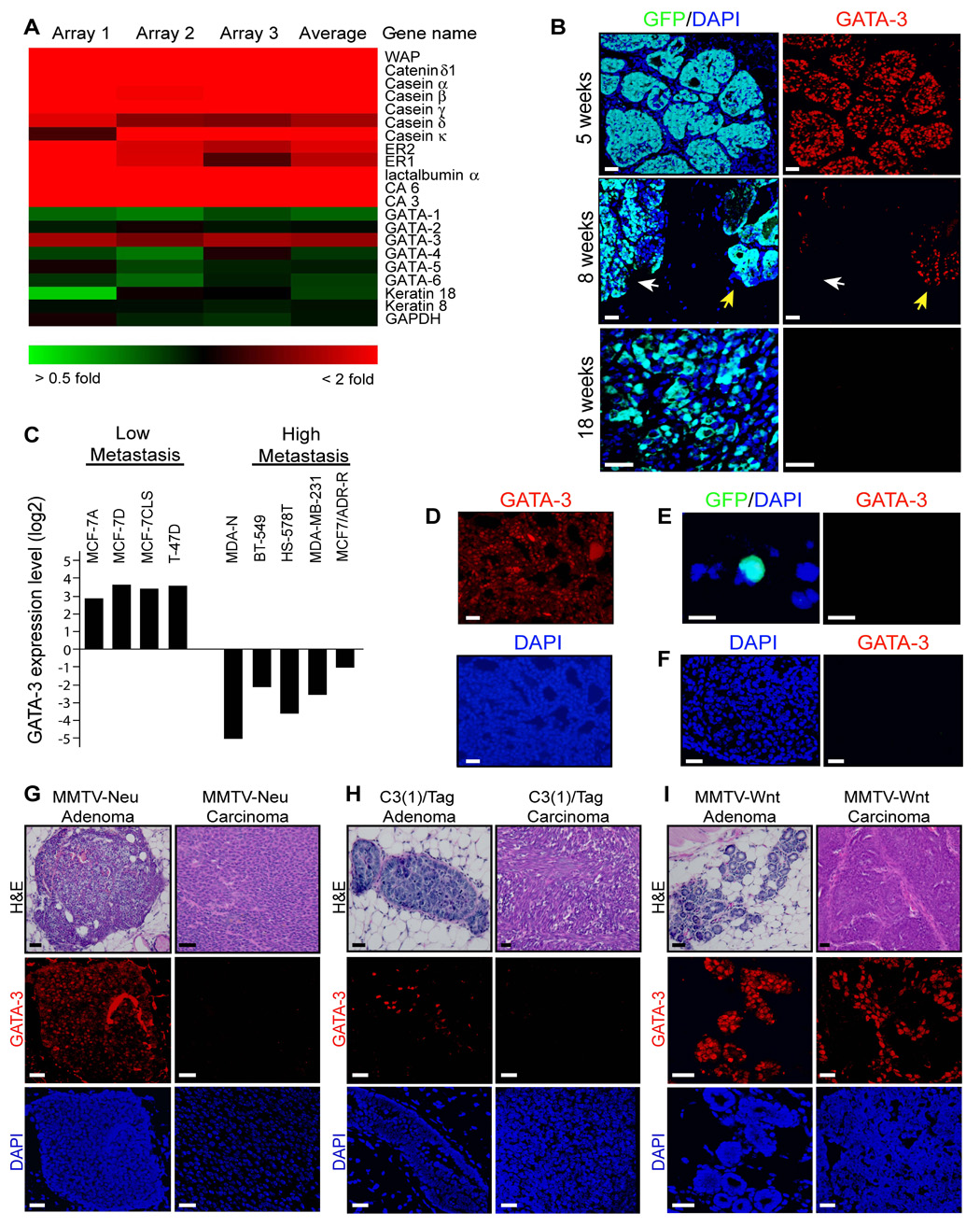
(A) Clustered image map of microarray data comparing Cy5-labelled (red) 5-week tumor outgrowth and Cy3-labelled (green) 18-week outgrowth (n = 3 microarrays). Color intensity represents M = log2 (Cy5 fluorescence / Cy3 fluorescence). ER, estrogen receptor; CA, carbonic anhydrase; WAP, whey acidic protein.
(B) Immunofluorescence staining for GFP (green) and GATA-3 (red) and DAPI counterstain (blue) in 5-, 8-, and 18-week tumor outgrowths (n = 3 per condition). White arrow denotes early carcinoma in 8-week tumor outgrowth adjacent to an adenoma (yellow arrow).
(C) Relative GATA-3 expression levels in nine breast cancer cell lines from a microarray dataset (Ross et al., 2000). Data are represented as log2 ratios of GATA-3 expression relative to control genes from a reference RNA in the pool of 60 cell lines.
(D) Immunofluorescence staining for GATA-3 (red) and DAPI (blue) counterstain in a representative 18-week tumor outgrowth in which tumor dissemination to lungs was undetectable by histologic analysis.
(E) Immunofluorescence staining of a representative disseminated cell in the lung of an 8-week tumor-bearing mouse (same color scheme as B).
(F) Immunofluorescence staining for GATA-3 (red) and DAPI (blue) in a representative lung metastasis from a MMTV-PyMT mouse.
(G–I) H&E staining and immunofluorescence for GATA-3 and DAPI in the MMTV-Wnt, MMTV-Neu, and C3(1)/Tag mouse models of breast cancer. Representative adenomas and carcinomas are shown from 9-month-old MMTV-Neu mice in the FVB/n strain (n = 12 mammary glands from 3 mice), 6-month-old C3(1)/Tag mice in the FVB/n strain (n =11 mammary glands from 3 mice), and 4.5-month-old MMTV-Wnt1 mice in the FVB/n strain (n = 8 mammary glands from 3 mice) that were analyzed for GATA-3 expression.
Scale bars correspond to 25 µm (B, D, F–I) and 10 µm (E).
We further analyzed GATA-3 expression in a series of human breast cancer cell lines and mouse models of breast cancer (Figure 2C). Using a gene expression database of the NCI-60 cell lines (Ross et al., 2000), we observed that the human breast cancer cell lines MCF-7 and T-47D, which have features of differentiated mammary cells and have low metastatic potential, displayed high GATA-3 expression. In contrast, the poorly differentiated, highly metastatic cell lines MDA-N, BT-549, HS-578T, and MDA-MD-231 all demonstrated significant (>60-fold) down-regulation of GATA-3 expression relative to the differentiated cell lines. Loss of GATA-3 also coincided with tumor progression in three additional mouse models of breast cancer, MMTV-Neu, MMTV-Wnt1, and C3(1)/Tag, but the rate and extent of GATA-3 loss varied between the models (Figure 2G–2I). In the MMTV-Neu model, which is a luminal mammary adenocarcinoma model similar to the MMTV-PyMT model, early adenomas expressed GATA-3, while carcinomas lost GATA-3 expression (Figure 2G). In C3(1)/Tag mice, adenomas displayed partial GATA-3 loss while carcinomas were GATA-3 negative. The SV40T-antigen in this model inhibits cellular differentiation directly (Goetz et al., 2001), in agreement with our observation (Figure 2H). In MMTV-Wnt1 mice, which develop multi-lineage tumors, adenomas were GATA-3 positive and the loss of GATA-3 was first detected in carcinomas. However, approximately one-third of the tumor cells in carcinomas were GATA-3 positive (Figure 2I). Since MMTV-Wnt1 neoplasias contain clonal populations of mature luminal and myoepithelial progenitor cells, the Wnt1-driven progenitor cells likely continue to produce differentiated progeny even late in tumor progression (Li et al., 2003).
GATA-3 is sufficient to induce tumor differentiation and repress dissemination in breast cancer
Given the essential role of GATA-3 in maintaining mammary luminal cell differentiation (Kouros-Mehr et al., 2006), we assessed its ability to regulate tumor differentiation and metastasis in mammary adenocarcinomas using a retroviral delivery strategy. Primary cultures of non-fluorescent MMTV-PyMT carcinomas were infected with empty vector retrovirus or retrovirus containing mouse GATA-3 cloned into an IRES-GFP cassette and transplanted into the cleared mammary fat pads of wild-type mice, by published methods (Welm et al., 2005). After 6 weeks of growth, the tumor cells infected with empty vector formed poorly differentiated adenocarcinomas with no ductal architecture (Figure 3A). In contrast, the GATA-3-infected tumors expressed milk proteins (β-casein) and basement membrane components (perlecan) and formed clusters of ducts indicating that the tumor cells were functionally differentiated (Figure 3A). Microarray analysis of GATA-3-infected and control-infected tumor outgrowths confirmed that GATA-3 induced the expression of a broad range of luminal differentiation markers, including milk proteins such as caseins, α-lactalbumin and whey acidic protein (WAP) (Figure 3B). FOXA1, a transcription factor and downstream effector of GATA-3 (Kouros-Mehr et al., 2006), was also up-regulated in GATA-3-infected tumor outgrowths relative to controls (M = 0.6, 1.5-fold change; data not shown).
Figure 3. GATA-3 causally regulates tumor differentiation and dissemination in breast cancer.
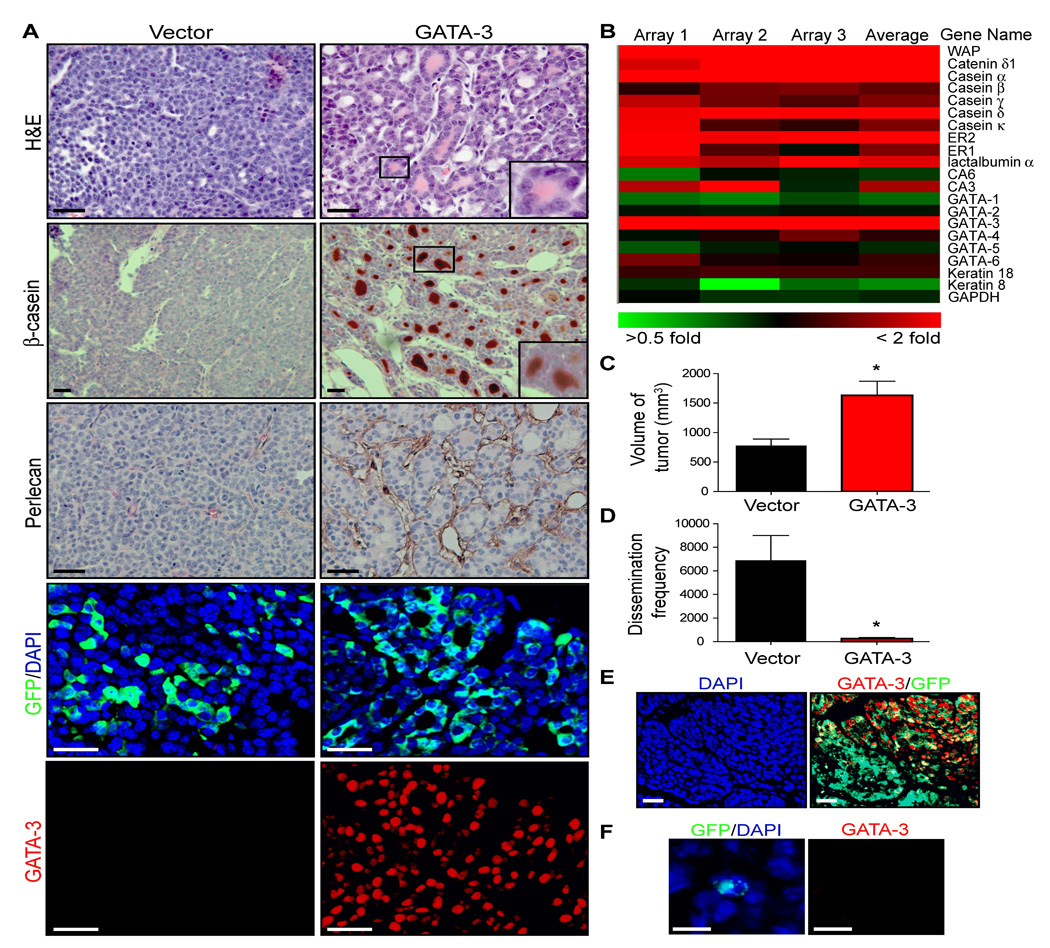
(A) H&E staining and immunocytochemistry for β-casein, perlecan (heparan sulfate proteoglycan), GFP, GATA-3, and nuclei (DAPI) in vector control and GATA-3 retrovirally transduced tumor outgrowths. Primary cultures of adenocarcinomas from 14-week-old MMTV-PyMT mice (non-fluorescent) were transduced with retrovirus, transplanted into the mammary fat pads of wild-type mice, and grown for 6 weeks.
(B) Clustered image map of microarray data comparing Cy5-labelled (red) GATA-3 retrovirally-infected tumor outgrowths and Cy3-labelled (green) control-infected tumor outgrowths (n = 3 microarrays). Color intensity represents M = log2 (Cy5 fluorescence /Cy3 fluorescence). ER, estrogen receptor; CA, carbonic anhydrase.
(C, D) Volume of tumor outgrowths and frequency of tumor dissemination in lungs of vector control- (n = 5) and GATA-3- (n = 5) infected tumor outgrowths.
(E,F) Immunofluorescence for GATA-3 (red), GFP (green) and DAPI (blue) in (E) GATA-3-infected tumors and (F) in a disseminated tumor cell in lung arising from a GATA-3-infected tumor.
Error bars indicate s.e.m.; *, P < 0.01 (Student’s t-test). Scale bars correspond to 25 µm (A, E), 10 µm (F).
We then determined whether GATA-3 restoration affected the metastatic capability of the resulting tumors. GATA-3-infected tumors had abundant cystic structures filled with secretory material and were two-fold larger in tumor size relative to controls (n = 5 per group; p<0.01, Figure 3C). Despite being larger than controls, the GATA-3-infected tumors exhibited a 27-fold reduction in tumor dissemination in the lungs of tumor-bearing mice (p<0.01, Figure 3D). This difference was not due to a difference in GFP expression, as the GATA-3 and control tumor outgrowths expressed similar levels of GFP (data not shown). Interestingly, the few disseminated cells found in the lungs of GATA-3 transduced tumor-bearing mice were GATA-3 negative (n = 10) (Figure 3F). Moreover, immunostaining of the GATA-3-infected primary tumors revealed regions that were GATA-3 negative (Figure 3E), suggesting that there is a negative selection pressure on GATA-3 during breast cancer progression. These data indicate that GATA-3 is sufficient to differentiate mammary ductal adenocarcinomas and that the absence of GATA-3 is necessary for tumor dissemination in breast cancer.
The premature loss of GATA-3 in early tumors is not tolerated
We considered the silencing of the GATA-3 locus in differentiated tumor cells as a potential mechanism for the loss of GATA-3 during malignant progression. We therefore characterized the status of the GATA-3 locus in MMTV-PyMT late carcinomas. Sequencing of the GATA-3 promoter and full-length gene in 18-week carcinomas failed to identify somatic mutations (data not shown). However, bisulfite sequencing of the same regions identified a number of methylated cytosine residues in the GATA-3 promoter and first exon (Figure S2). The highest concentration of methylated cytosines occurred in the first exon of GATA-3, consistent with an earlier study of human breast tumors (Yan et al., 2000).
To test the silencing hypothesis further, we assessed the consequences of GATA-3 loss in differentiated neoplastic cells through inducible, targeted deletion of the GATA-3 locus in early MMTV-PyMT adenomas. The floxed GATA-3 construct (GATA-3_fl_) was designed such that Cre-mediated recombination leads to the induction of GFP expression (Pai et al., 2003). We targeted expression to differentiated luminal mammary epithelial cells with the doxycycline-inducible WAP-rtTA-Cre (Kouros-Mehr et al., 2006; Utomo et al., 1999). First, we tested our deletion strategy by administering a 7-day course of doxycycline to triple transgenic MMTV-PyMT; WAP-rtTA-Cre; GATA-3_fl/+_ mice with mixed early and late carcinomas and analyzing GFP expression to assess recombination (Figure S3). As expected, GATA-3 positive (differentiated) areas of the tumor were also GFP positive, indicating recombination of the floxed GATA-3 locus. Undifferentiated areas of the tumor lacked GATA-3 and remained GFP negative, indicating that recombination only occurred in well-differentiated tumor cells.
We then assessed the acute and long-term consequences of GATA-3 deletion in mice with early tumors. After a two-week course of doxycycline, the tumors of 10-week-old control MMTV-PyMT; WAP-rtTA-Cre; GATA-3_fl/+_ mice appeared well differentiated with cystic elements and high GATA-3 and milk protein (β-casein) expression (Figure 4A). In contrast, MMTV-PyMT; WAP-rtTA-Cre; GATA-3_fl/fl_ mice displayed premature loss of differentiated tumor cells, characterized by loss of milk protein expression and widespread tumor cell detachment from basement membrane. These cells underwent apoptotic cell death in the ductal lumen as indicated by cleaved caspase-3 immunostaining. The resulting tumors contained elongated ducts that were devoid of cells or secretory material. After an additional 6 weeks of doxycycline (8 weeks total), control mice displayed a heavy tumor burden with progression to invasive carcinoma and concomitant loss of GATA-3 (Figure 4B and 4C). In contrast, MMTV-PyMT; WAP-rtTA-Cre; GATA-3_fl/fl_ littermates had significantly smaller tumors, presumably due to widespread cell loss. Interestingly, the resulting tumors contained a population of cells that were GATA-3 positive, GFP negative and cytokeratin 18 positive, suggesting these luminal cells had avoided DNA recombination and remained GATA-3 positive. Taken together, these data suggest that the premature loss of GATA-3 in well-differentiated adenomas is not sufficient to promote malignant progression. Thus, the mechanism by which GATA-3 is lost during tumor progression may not involve the silencing of the GATA-3 locus in differentiated cells.
Figure 4. Deletion of GATA-3 is not tolerated in differentiated tumors.
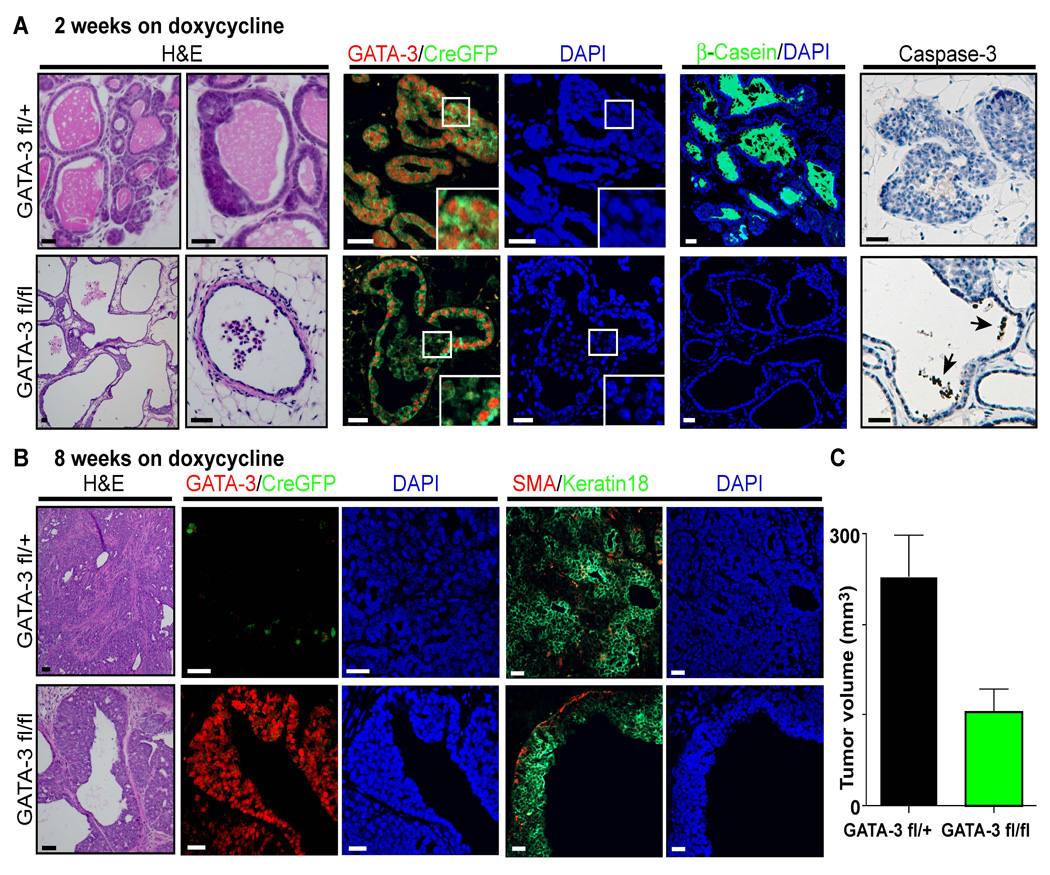
(A,B) H&E staining and immunofluorescence for GATA-3, GFP as a marker of Cre-mediated deletion (CreGFP), β-casein as a differentiation marker, Keratin 18 as a luminal epithelial marker, a-smooth muscle actin (SMA) as a marker of myoepithelial cells, cleaved caspase-3 as a marker of apoptosis, and DAPI counterstain in tumors of 10-week-old MMTV-PyMT; WAP-rtTA-Cre; GATA-3_fl/+_ and MMTV-PyMT; WAP-rtTA-Cre; GATA-3_fl/fl_ mice treated with doxycycline for (A) 2 weeks (n = 8 tumors) or (B) 8 weeks (n = 16 tumors). Black arrows indicate caspase-3 positive cells in the ductal lumen. Inserts are higher magnification views. Scale bars correspond to 25 µm.
(C) Mean tumor volume of tumors at 8 weeks of doxycycline for MMTV-PyMT; WAP-rtTA-Cre; GATA-3_fl/+_ (GATA-3_fl/+) and MMTV-PyMT; WAP-rtTA-Cre; GATA-3_fl/fl (GATA-3_fl/fl_). Error bars indicate s.e.m. (n = 16 tumors); *, P< 0.01 (Student’s t-test).
Tumor progression involves the expansion of a GATA-3 negative cell population with cell surface markers of mammary stem-like cells
An alternative hypothesis to account for the loss of GATA-3 during tumor progression is the expansion of a tumor population that is GATA-3 negative. A recent study found that mammary epithelial stem cells are GATA-3 negative (Asselin-Labat et al., 2007). This population can be isolated through sorting of mammary epithelial cells for the cell-surface markers CD24, CD29 (β1 integrin) and CD61 (β3 integrin). The CD24+CD29loCD61+ subpopulation of mammary epithelial cells is enriched for luminal progenitor cells, while the CD24+CD29+CD61+ subpopulation, characterized by serial passage and functional assays, is enriched for mammary stem cells (Asselin-Labat et al., 2007; Shackleton et al., 2006; Stingl et al., 2006). Accordingly, we characterized primary epithelial cells from early and late MMTV-PyMT tumors and normal mammary glands using these markers. We found that the populations of CD24+CD29+ and CD29+CD61+ cells progressively increased in carcinomas (93% and 76%, respectively), compared to early tumors/adenomas (55% and 33%, respectively) and normal mammary epithelial cells (45% and 16%, respectively) (p< 0.001, Figure 5A and 5B). Further immunohistochemical staining showed that the CD29+CD61+ tumor cells in early and late tumors were GATA-3 negative (Figure 6). Thus, early tumors and carcinomas contained a progressively enriched subpopulation of CD24+CD29+CD61+ cells. Similar results were found in MMTV-PyMT tumors from the C57BL/6 (Figure 5A) and FVB/n strains (Figure S4).
Figure 5. A stem cell-like population is enriched in late carcinomas of in MMTV-PyMT tumors.
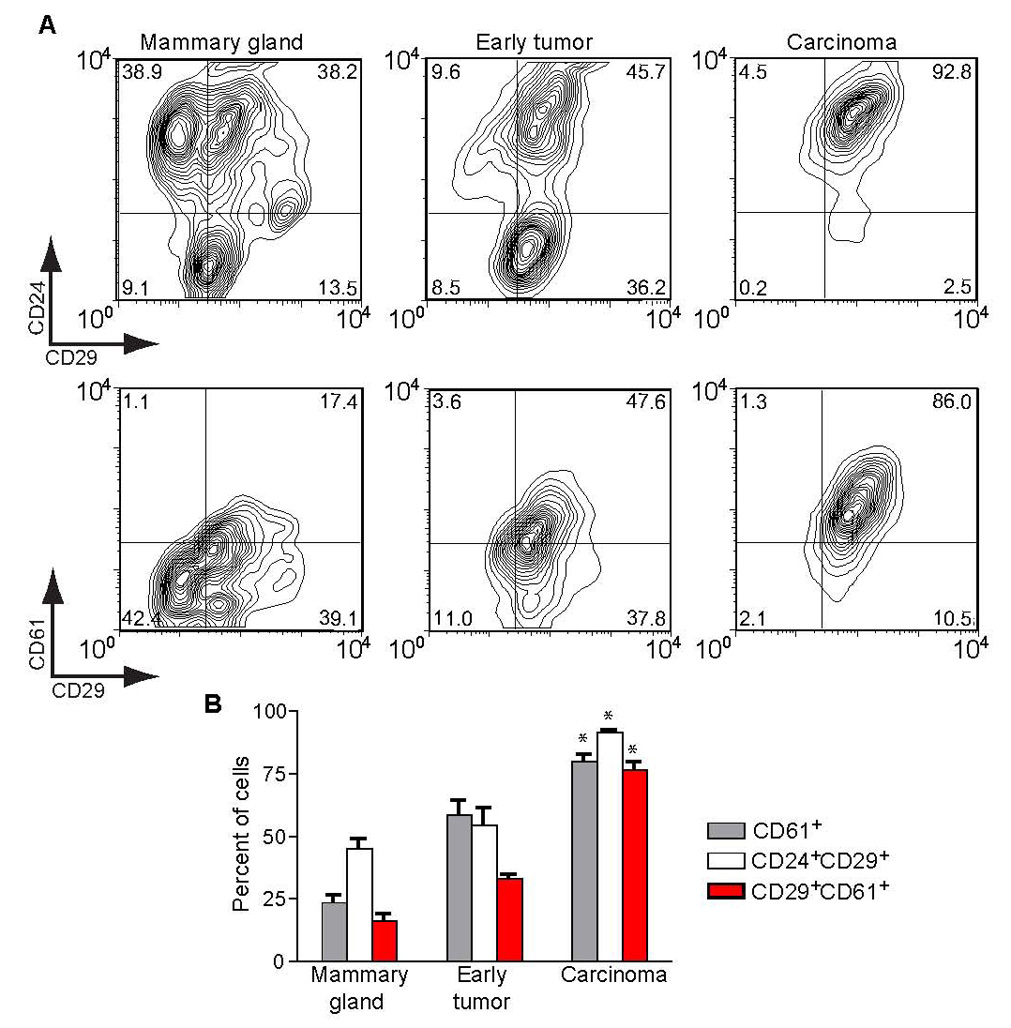
(A) CD24/CD29 and CD61/CD29 contour plots of C57BL/6 primary cells isolated from wild-type mammary glands, 11-week early, nonpalpable MMTV-PyMT tumor and a 5-month MMTV-PyMT tumor measuring 13×20×8mm (C57BL/6). Values in each quadrant indicate the percentage of cells in each condition (n = 10,000 events).
(B) Percentage of CD61+, CD24+CD29+ double positive, and CD24+CD61+ double positive cells in samples described in (A). A total of 3–4 samples were analyzed per condition. Error bars indicate s.e.m.; *, P < 0.001 (Analysis of Variance).
Figure 6. Immunohistochemical staining of CD29, CD61 and GATA-3 in MMTV-PyMT tumors.
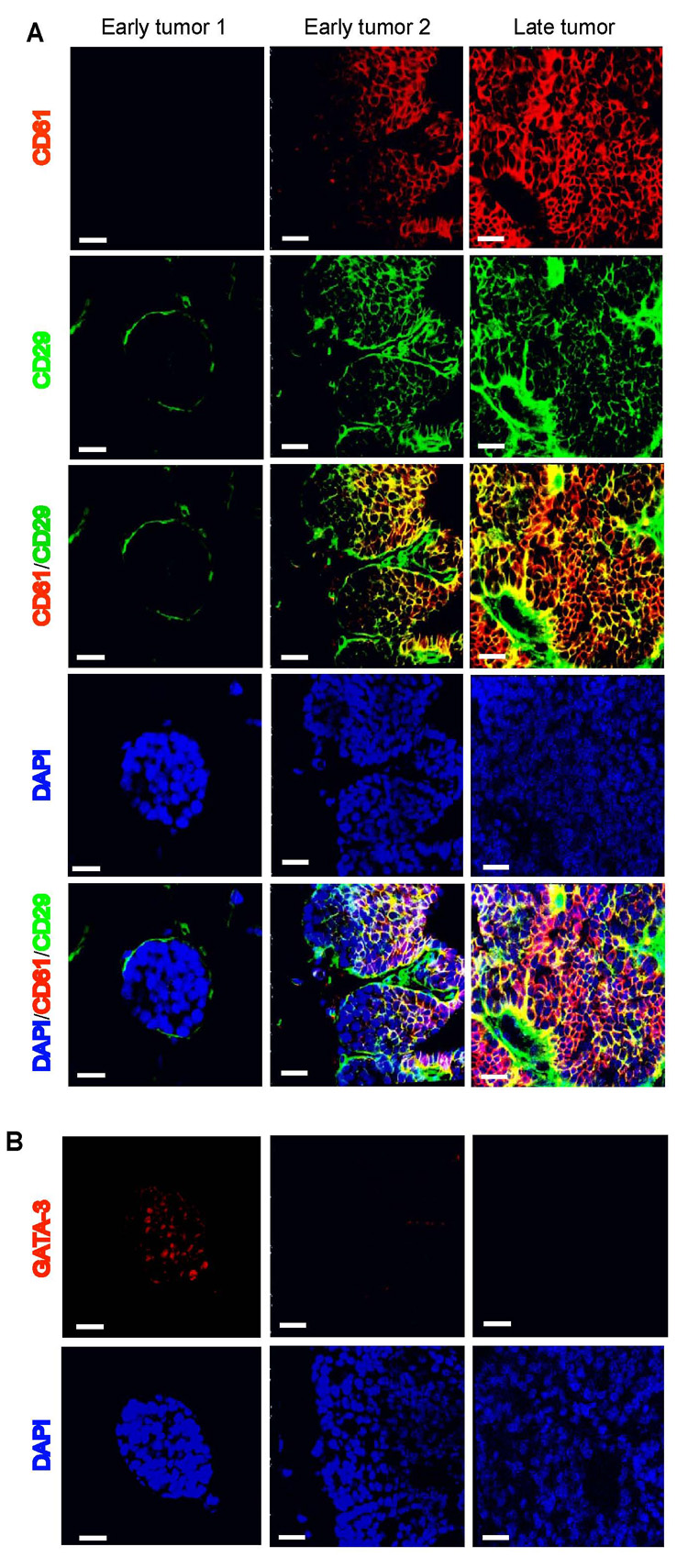
(A) Immunoflourescence for CD29 (green), CD61 (red), and DAPI (blue) on frozen sections of 7-week-old (early) and 14-week-old (late) MMTV-PyMT tumors. Early1 and Early2 refers to two distinct areas within the same tumor. (B) Immuflourescence for GATA-3 (red) and DAPI (blue) on serial sections of the tumors in (A). Adjacent, acetone-fixed frozen sections were thawed, processed with microwave antigen retrieval, and labelled with GATA-3 as described (Kouros-Mehr, 2006). Scale bar, 25 µm.
We isolated the populations enriched in putative stem-like cells (CD24+CD29+CD61+) and differentiated cells (CD24+CD29−CD61−) from early tumors and normal mammary glands by FACS and assessed cellular motility in a transwell migration assay (Figure 7A). Interestingly, after 72 hours of incubation, the CD24+CD29+CD61+ cell population from normal mammary glands and early tumors displayed a significantly enhanced migratory potential compared to CD24+CD29−CD61− cells. Compared to the CD24+CD29−CD61− population, the CD24+CD29+CD61+ cell fractions from normal mammary glands and early tumors exhibited greatly enhanced migration potential (Figure 7B).
Figure 7. Stem cell-like populations from MMTV-PyMT tumors show increased motility and ability to differentiate into luminal and myoepithelial cells.
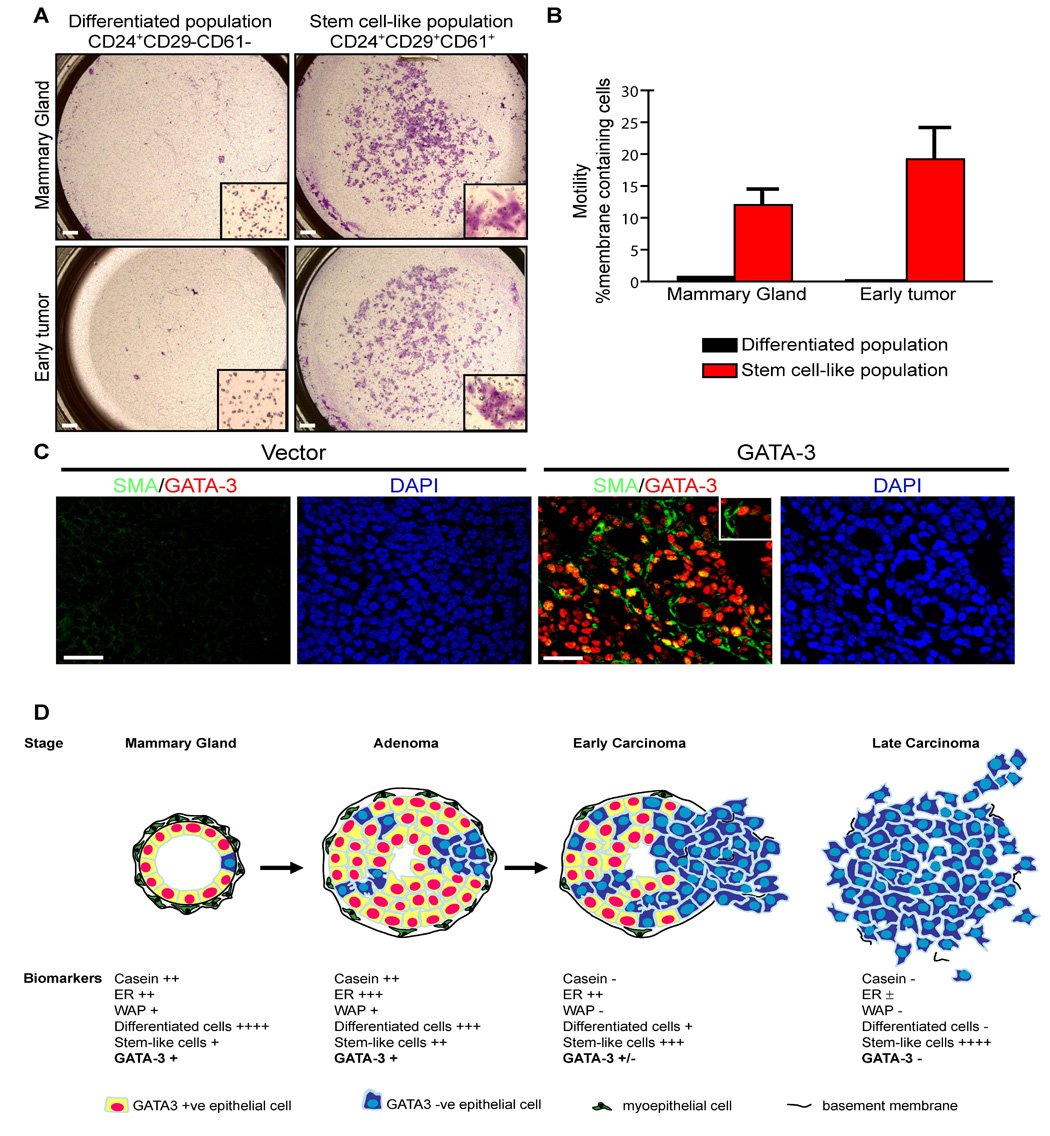
(A, B) Analysis of motility among distinct cell populations purified from wild-type mammary glands and early MMTV-PyMT tumors. (A) Transwell migration assays performed on CD24+CD29+CD61+ (stem cell-like) and CD24+CD29−CD61− (differentiated cell) subpopulations purified from normal mammary glands (C57BL/6, n = 2) and adenomatous MMTV-PyMT tumors (C57BL/6, n = 2). Cells were isolated with a FACSAria cell sorter and 1.5 × 105 cells were used per condition. After 72 hours, (A) the membranes containing migratory cells were stained and (B) the fraction of the membranes containing stained cells were determined using Adobe Photoshop. Insets are higher magnifications of cells on the membranes. Scale bar, 0.5 mm. Error bars indicate s.e.m.
(C) GATA-3 transduced tumor outgrowths have both luminal and myoepithelial cells. Primary cultures of adenocarcinomas from 14-week-old MMTV-PyMT mice (non-fluorescent) were transduced with retroviruses encoding vector alone (Vector) or GATA-3 (GATA-3), transplanted into the mammary fat pads of wild-type mice, grown for 6 weeks, and then stained for GATA-3 (red) and α-smooth-muscle-actin (SMA, green) by immunofluorescence, and nuclear staining with DAPI. Scale bar, 25 µm.
(D) Model showing how GATA-3 expression and differentiated luminal epithelial markers may be lost through expansion of a GATA-3 negative stem cell-like cell population during MMTV-PyMT tumor progression. ER, estrogen receptor; WAP, whey acidic protein.
If the tumor cells of late carcinomas have features of stem-like cells, we predicted that they should be bipotential, capable of giving rise to both luminal and myoepithelial cells. Further examination of the late carcinomas retrovirally infected with GATA-3 (Figure 3A) revealed that these carcinoma cells had given rise to multiple cell lineages (Figure 7C). The reverted tumors contained GATA-3 positive luminal cells as well as GATA-3 negative, smooth muscle-actin positive myoepithelial cells. Taken together, these results indicate that MMTV-PyMT early tumors and carcinoma cells contain a progressively enriched subpopulation of highly motile cells that shares some properties with mammary stem cells, which were defined as GATA-3 negative (Asselin-Labat et al., 2007). Our data suggest that expansion of this tumor subpopulation underlies the emergence of the GATA-3 negative state during tumor progression.
DISCUSSION
GATA-3 causally links tumor differentiation and tumor dissemination
In the present study, we developed a modified model of the MMTV-PyMT mouse that facilitates the study of breast cancer progression, including the events that lead to tumor dissemination and metastasis. We found that malignant conversion begins with the loss of tumor differentiation and the onset of tumor dissemination that coincides with the transition from a GATA-3 positive adenoma state to a GATA-3 negative early carcinoma state, as shown in the model in Figure 7D. Disseminated tumor cells in distant sites were GATA-3 negative, suggesting that the GATA-3 negative state is required for tumor dissemination to commence. Restoring GATA-3 was sufficient to induce tumor differentiation and suppress tumor dissemination. These data suggest that GATA-3 normally controls the differentiation of luminal tumor cells and that selection against GATA-3 expression is a critical event in the early steps of malignant progression. The importance of tumor differentiation (tumor grade) in predicting malignant potential of human breast tumors has been appreciated for decades, and our data suggest that GATA-3 is a molecular link between these processes.
In agreement with our observations, GATA-3 is a strong and independent predictor of clinical outcome in breast cancer, with its low expression indicating a poor prognosis (Bertucci et al., 2000; Farmer et al., 2005; Gruvberger et al., 2001; Hoch et al., 1999; Mehra et al., 2005; Perou et al., 2000; Sorlie et al., 2003; Sotiriou et al., 2003; van 't Veer et al., 2002; Wang et al., 2005; West et al., 2001). Low GATA-3 expression is strongly associated with higher histologic grade, positive lymph nodes, larger tumor size, estrogen receptor (ER) and progesterone receptor (PR) loss, and HER2 overexpression. Indeed, the prognostic utility of GATA-3 exceeds that of conventional variables such as ER status (Jenssen et al., 2002). These studies correlatively link low GATA-3 expression with poor tumor differentiation and metastatic formation in human breast tumors. Our study suggests that GATA-3, in fact, plays a causal role in tumor differentiation and metastasis formation in breast cancer.
Metastatic capability of disseminated tumor cells in breast cancer
Our transplant model allowed us to follow the progression of a single tumor focus growing orthotopically in an immune competent mouse. We have shown that tumor dissemination and metastasis formation occur in discrete steps during tumor progression. The process of metastasis formation is highly inefficient, as <1/4000 disseminated cells can successfully give rise to a metastasis. Since most disseminated cells appear to be trapped in the vasculature, the rate-limiting step in metastasis formation is likely to involve extravasation, survival and proliferation of disseminated cells in distant sites. In keeping with these data, microarray analysis of human breast cancers indicate that GATA-3 expression levels in metastases are consistently lower than their corresponding primary tumors (Weigelt et al., 2003). Thus, although progression to a GATA-3 negative state underlies the onset of tumor dissemination, further events are likely to be necessary for successful formation of metastases from disseminated cells.
In the MMTV-PyMT transgenic mouse model, these events likely do not arise from random mutagenesis of key target genes because the model has a very low rate of somatic mutagenesis (Jakubczak et al., 1996) and genomic instability (Hodgson et al., 2005). One possibility is that the development of a pre-metastatic niche in distant sites is necessary for the successful formation of a metastasis (Kaplan et al., 2005). Alternatively, it is possible that only a rare cell within the primary tumor, such as a tumor stem cell, is capable of successfully completing all of the various steps of metastasis (Pardal et al., 2003). A similar kinetic analysis of metastasis in other mouse models of breast cancer, such as the MMTV-Wnt1 and MMTV-Neu mice, may further shed light on the nature of metastasis formation in breast cancer.
Expansion of GATA-3 negative tumor cells during breast cancer progression
We found that the targeted loss of GATA-3 in early tumors is not sufficient to induce malignant progression. This suggests that the silencing of the GATA-3 locus in differentiated tumor cells is not the primary molecular mechanism that leads to the GATA-3 negative state in breast cancer. An alternative mechanism for the development of GATA-3 negative tumor cells is the expansion of tumor population that has not activated the GATA-3 locus. We found that adenomas and carcinoma cells were progressively enriched for cell-surface markers of mammary stem-like cells, which were shown previously to be GATA-3 negative (Asselin-Labat et al., 2007). Early adenomatous tumors contained an increased population of these GATA-3 negative stem-like cells, suggesting that a small population of these cells reside in early tumors and that selective pressures promote their expansion growth over time. In late carcinomas in the MMTV-PyMT model, such cells comprised nearly the entire tumor mass and are capable of differentiating into both luminal and myoepithelial cells. These putative tumor stem-like cells exhibited significantly enhanced motility compared to the differentiated cell population, suggesting that tumor invasion and dissemination may be carried out by this expanding tumor cell subpopulation. Interestingly, the restoration of GATA-3 in late carcinomas led to the differentiation of both luminal and myoepithelial lineages, suggesting that carcinoma cells are indeed bipotential. A major question that arises is what elements in the tumor microenvironment foster the selection and dominance of this GATA-3 negative cell population. Further work will be necessary both to characterize this cell population and to delineate its role during malignant progression and the mechanisms that underlie their expansion during tumor progression.
Differentiation therapy in breast cancer
GATA-3 is critical for cell survival, terminal cell differentiation, or cell-type specific function in many nontransformed tissues such as CD4 T cells, NK cells, skin and developing mammary gland (Ho and Pai, 2007), and our data indicate that it functions as a differentiation agent in carcinomas as well. Differentiation therapy describes the enforced differentiation of primary tumors with therapeutic compounds. In acute promyelocytic leukemia, a t(15;17) translocation leads to deficiencies in retinoic acid receptor signaling, a block in myeloid differentiation and neoplastic transformation (Weis et al., 1994). High dose retinoic acid therapy and chemotherapy are sufficient to restore tumor differentiation and achieve remission in patients (Tallman et al., 1997).
Our work suggests that compounds that activate GATA-3 may be sufficient to differentiate mammary ductal adenocarcinomas. Alternatively, compounds that prevent the loss of GATA-3 may prevent breast cancer progression in patients. Though it is technically feasible to screen drug libraries to identify such compounds, a more rational approach to drug discovery is to identify upstream regulators of GATA-3 signaling. In development, the specification of GATA expression is mediated by secreted factors, notably the Wnt genes (Davidson et al., 2002). In the developing mammary gland, specific Wnt members are expressed at sites of luminal specification (Kouros-Mehr and Werb, 2006). A molecular understanding of GATA-3-mediated luminal cell specification could reveal further insights into a differentiation therapy of breast cancer.
METHODS
Mice
MMTV-PyMT (FVB/n), MMTV-Neu (FVB/n), MMTV-Wnt (FVB/n) and β-actin-GFP (FVB/n) mice were from Jackson Labs. MMTV-PyMT (C57BL/6) mice were provided by Dr. Kasper Almholt. WAP-rtTA-Cre (C57BL/6) and C3(1)/Tag mice (FVB/n) were obtained from the NCI mouse models consortium. GATA-3_fl/+_ mice (on the C57BL/6 background) were generated as described (Pai et al., 2003). Although tumor progression is significantly delayed in MMTV-PyMT mice crossed onto the C57BL/6 strain relative to the FVB/n strain, although the tumors are indistinguishable when compared at relative stages.
Tumor transplantation
All animal experiments were performed with protocols approved by the UCSF IACUC. MMTV-PyMT (Guy et al., 1992) (FVB/n) mice were bred with β-actin-GFP reporter mice (FVB/n) to allow visualization of the mammary epithelium. Focal hyperplasias (0.7–1 mm) were microdissected from 3-week-old female MMTV-PyMT; β-actin-GFP mice using a fluorescence dissecting microscope. The intact hyperplasias were transplanted into the cleared mammary fat pads of 3-week-old female FVB/n recipients. Tissues were fixed by cardiac perfusion fixation of mice followed by overnight fixation in 4 % paraformaldehyde (PFA) at 4° C. Biotinylated tomato lectin (100 µl, 1 mg/ml, Vector Laboratories) was injected into the tail vein prior to cardiac fixation for visualization of vasculature.
Analysis of tumor dissemination and metastasis
The PFA-fixed lungs of tumor-bearing mice were OCT embedded and frozen sections prepared such that all five lobes were analyzed for tumor dissemination and metastasis. 25-µm lung sections separated by 200-µm intervals were analyzed for GFP expression using a Leica DMR microscope with a broad-pass filter that allowed the distinction between auto-fluorescence (orange/red color) and GFP (green). Only GFP positive cells with nuclei (DAPI) were included in the analysis. The total number of disseminated cells per lung was calculated by averaging the number of GFP+ cells in eight 25-µm sections (~5% of total lung volume) and extrapolating to the full thickness of the lung. Metastases were detected by whole lung analysis with a fluorescence-dissecting microscope and then by the same histologic analysis.
Microarray analysis
RNA was extracted with Trizol Reagent (Tel-Test), reverse transcribed in the presence of aminoallyl-dUTP, and coupled to CyScribe dyes (Amersham), as described (Barczak et al., 2003). Cy5 and Cy3 labeled samples were hybridized onto 70-mer oligonucleotide spotted microarrays with 38,784 features (MEEBO set, UCSF Functional Genomics Core). Lowess print-tip normalization of microarray data and analysis was performed using the Acuity software package.
Immunofluorescence
Immunofluorescence was performed on PFA-fixed, paraffin-embedded tumor sections with antibodies and protocols as previously described (Kouros-Mehr et al., 2006). Additionally, immunostaining with anti-cleaved-caspase-3 (Cell Signaling) was performed at a dilution of 1:250. CD29 (ABD Serotec, clone HM beta 1-1) and CD61 (BD, clone 2C9.G2) immunostaining were performed on acetone-fixed frozen tumor sections at dilutions of 1:50 and 1:100, respectively, and on adjacent acetone-fixed frozen sections with anti-GATA-3 (Santa Cruz Biotechnology, clone HG3-31) at 1:20 after sodium citrate antigen retrieval.
Conditional deletion of GATA-3
MMTV-PyMT (C57BL/6) mice were bred with GATA-3_fl/+_ mice and the resulting offspring were bred with WAP-rtTA-Cre; GATA-3_fl/+_ mice. Genotyping and doxycycline administration was performed as previously described (Kouros-Mehr et al., 2006).
Retroviral transduction of tumors
Full length GATA-3 (Zhang et al., 1999) was cloned into the pMIG vector for retroviral production (Van Parijs et al., 1999). Primary cultures of adenocarcinomas from nontransplanted 14-week-old MMTV-PyMT mice (FVB/n) were infected with retrovirus and cultured for an additional 3 days (Welm et al., 2005). GFP positive-cells were isolated using a FACSAria Cell sorter and 500,000 cells were transplanted into the cleared mammary fat pads of 3-week-old female FVB recipients. Mice were harvested 6 weeks later. Tumor size was determined by measuring with calipers and calculated as length × width × height/2.
Methylation analysis
Genomic DNA was isolated from an 18-week tumor outgrowth and bisulfite treatment was carried out, as described (Frommer et al., 1992). The promoter and first exon of GATA-3 were amplified using primers (F–GGAATCAGTGTGCAGTGTGG, R-TTAGGAGCTTTGGCTTGCTC and F–TGCAGTTTCCTTGTGCTGAG, R-CACCTGCAAGGGAGAGAAAG) and then sequenced for analysis.
Mammary cell preparation, FACS analysis and cell sorting
Primary tumors from 3- to 6-month-old MMTV-PyMT mice (FVB/n and C57BL/6 strains) and primary mammary glands from 3- to 10-month-old wild type FVB/n and C57BL/6 mice were harvested and epithelial cell suspensions were prepared as previously described (Welm et al., 2005), except that organoids were trypsinized and the resulting suspensions were filtered through a 70 µM filter. Single cells were labeled with CD24-PE (BD Biosciences), CD61-biotin (BD), and CD29-FITC (ABD Serotec) at 1:100 dilution, and streptavidin-APC (BD Biosciences) at 1:100 was used for secondary detection. FACS analysis and cell sorting were performed using a FACSCalibur or FACSAria and the FlowJo software package.
Cell culture and migration studies
Cell invasion assays were performed using the cell invasion assay kit (Chemicon International). Primary tumors were harvested and cell populations were sorted by FACS as described above. 1.5 × 105 cells in 300 µl of serum-free media were placed over the inner gel chamber in a 24-well tissue culture plate, and 500 µl of serum-containing media was placed in the outer chamber. After 72 hours, the membranes containing migratory cells were developed and the fraction of the membranes containing stained cells was determined using Adobe Photoshop.
Supplementary Material
01
ACKNOWLEDGEMENTS
We thank Elena Atamaniuc for providing mouse mammary tumor samples and the Sandler/UCSF Genomics Core Facility for assistance with microarray profiling and data analysis. This work was supported by grants to Z.W. (CA057621, CA105379 and ES012801) from the National Cancer Institute and National Institute of Environmental Health Sciences, by a UCSF Comprehensive Cancer Center Intramural Award from the Alexander and Margaret Stewart Trust (Z.W.), by the UCSF Medical Scientist Training Program (MSTP) (H.K.-M. and S.K.B.), a California Breast Cancer Research Program (CBCRP) dissertation award (H.K.-M.), the Paul and Daisy Soros Fellowship for New Americans (H.K.-M.), a CBCRP postdoctoral fellowship (A.J.E.) and an American Cancer Society fellowship (L.E.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession numbers Microarray data were submitted to the GEO Omnibus Repository with accession numbers GSE5223 and GSE5221.
REFERENCES
- Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- Barczak A, Rodriguez MW, Hanspers K, Koth LL, Tai YC, Bolstad BM, Speed TP, Erle DJ. Spotted long oligonucleotide arrays for human gene expression analysis. Genome Res. 2003;13:1775–1785. doi: 10.1101/gr.1048803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertucci F, Houlgatte R, Benziane A, Granjeaud S, Adelaide J, Tagett R, Loriod B, Jacquemier J, Viens P, Jordan B, et al. Gene expression profiling of primary breast carcinomas using arrays of candidate genes. Hum Mol Genet. 2000;9:2981–2991. doi: 10.1093/hmg/9.20.2981. [DOI] [PubMed] [Google Scholar]
- Bloom HJ, Richardson WW. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957;11:359–377. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- Contesso G, Mouriesse H, Friedman S, Genin J, Sarrazin D, Rouesse J. The importance of histologic grade in long-term prognosis of breast cancer: a study of 1,010 patients, uniformly treated at the Institut Gustave-Roussy. J Clin Oncol. 1987;5:1378–1386. doi: 10.1200/JCO.1987.5.9.1378. [DOI] [PubMed] [Google Scholar]
- Davidson EH, Rast JP, Oliveri P, Ransick A, Calestani C, Yuh CH, Minokawa T, Amore G, Hinman V, Arenas-Mena C, et al. A genomic regulatory network for development. Science. 2002;295:1669–1678. doi: 10.1126/science.1069883. [DOI] [PubMed] [Google Scholar]
- Farmer P, Bonnefoi H, Becette V, Tubiana-Hulin M, Fumoleau P, Larsimont D, Macgrogan G, Bergh J, Cameron D, Goldstein D, et al. Identification of molecular apocrine breast tumours by microarray analysis. Oncogene. 2005;24:4660–4671. doi: 10.1038/sj.onc.1208561. [DOI] [PubMed] [Google Scholar]
- Frommer M, McDonald LE, Millar DS, Collis CM, Watt F, Grigg GW, Molloy PL, Paul CL. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc Natl Acad Sci U S A. 1992;89:1827–1831. doi: 10.1073/pnas.89.5.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz F, Tzeng YJ, Guhl E, Merker J, Graessmann M, Graessmann A. The SV40 small t-antigen prevents mammary gland differentiation and induces breast cancer formation in transgenic mice; truncated large T-antigen molecules harboring the intact p53 and pRb binding region do not have this effect. Oncogene. 2001;20:2325–2332. doi: 10.1038/sj.onc.1204355. [DOI] [PubMed] [Google Scholar]
- Gruvberger S, Ringner M, Chen Y, Panavally S, Saal LH, Borg A, Ferno M, Peterson C, Meltzer PS. Estrogen receptor status in breast cancer is associated with remarkably distinct gene expression patterns. Cancer Res. 2001;61:5979–5984. [PubMed] [Google Scholar]
- Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschkowitz JI, Simin K, Weigman VJ, Mikaelian I, Usary J, Hu Z, Rasmussen KE, Jones LP, Assefnia S, Chandrasekharan S, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8:R76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho IC, Pai SY. GATA-3 - not just for Th2 cells anymore. Cell Mol Immunol. 2007;4:15–29. [PubMed] [Google Scholar]
- Hoch RV, Thompson DA, Baker RJ, Weigel RJ. GATA-3 is expressed in association with estrogen receptor in breast cancer. Int J Cancer. 1999;84:122–128. doi: 10.1002/(sici)1097-0215(19990420)84:2<122::aid-ijc5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Hodgson JG, Malek T, Bornstein S, Hariono S, Ginzinger DG, Muller WJ, Gray JW. Copy number aberrations in mouse breast tumors reveal loci and genes important in tumorigenic receptor tyrosine kinase signaling. Cancer Res. 2005;65:9695–9704. doi: 10.1158/0008-5472.CAN-05-0755. [DOI] [PubMed] [Google Scholar]
- Jacquemier J, Ginestier C, Rougemont J, Bardou VJ, Charafe-Jauffret E, Geneix J, Adelaide J, Koki A, Houvenaeghel G, Hassoun J, et al. Protein expression profiling identifies subclasses of breast cancer and predicts prognosis. Cancer Res. 2005;65:767–779. [PubMed] [Google Scholar]
- Jakubczak JL, Merlino G, French JE, Muller WJ, Paul B, Adhya S, Garges S. Analysis of genetic instability during mammary tumor progression using a novel selection-based assay for in vivo mutations in a bacteriophage lambda transgene target. Proc Natl Acad Sci U S A. 1996;93:9073–9078. doi: 10.1073/pnas.93.17.9073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenssen TK, Kuo WP, Stokke T, Hovig E. Associations between gene expressions in breast cancer and patient survival. Hum Genet. 2002;111:411–420. doi: 10.1007/s00439-002-0804-5. [DOI] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell. 2006;127:1041–1055. doi: 10.1016/j.cell.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouros-Mehr H, Werb Z. Candidate regulators of mammary branching morphogenesis identified by genome-wide transcript analysis. Dev Dyn. 2006;235:3404–3412. doi: 10.1002/dvdy.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Welm B, Podsypanina K, Huang S, Chamorro M, Zhang X, Rowlands T, Egeblad M, Cowin P, Werb Z, et al. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci U S A. 2003;100:15853–15858. doi: 10.1073/pnas.2136825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ, Pollard JW. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163:2113–2126. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Wang X, Chen GY, Dalerba P, Gurney A, Hoey T, Sherlock G, Lewicki J, Shedden K, Clarke MF. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med. 2007;356:217–226. doi: 10.1056/NEJMoa063994. [DOI] [PubMed] [Google Scholar]
- Mehra R, Varambally S, Ding L, Shen R, Sabel MS, Ghosh D, Chinnaiyan AM, Kleer CG. Identification of GATA3 as a breast cancer prognostic marker by global gene expression meta-analysis. Cancer Res. 2005;65:11259–11264. doi: 10.1158/0008-5472.CAN-05-2495. [DOI] [PubMed] [Google Scholar]
- Pai SY, Truitt ML, Ting CN, Leiden JM, Glimcher LH, Ho IC. Critical roles for transcription factor GATA-3 in thymocyte development. Immunity. 2003;19:863–875. doi: 10.1016/s1074-7613(03)00328-5. [DOI] [PubMed] [Google Scholar]
- Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Ross DT, Scherf U, Eisen MB, Perou CM, Rees C, Spellman P, Iyer V, Jeffrey SS, Van de Rijn M, Waltham M, et al. Systematic variation in gene expression patterns in human cancer cell lines. Nat Genet. 2000;24:227–235. doi: 10.1038/73432. [DOI] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A, Martiat P, Fox SB, Harris AL, Liu ET. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A. 2003;100:10393–10398. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Ogden A, Shepherd L, Willman C, Bloomfield CD, Rowe JM, et al. All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med. 1997;337:1021–1028. doi: 10.1056/NEJM199710093371501. [DOI] [PubMed] [Google Scholar]
- Utomo AR, Nikitin AY, Lee WH. Temporal, spatial, and cell type-specific control of Cre-mediated DNA recombination in transgenic mice. Nat Biotechnol. 1999;17:1091–1096. doi: 10.1038/15073. [DOI] [PubMed] [Google Scholar]
- van 't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- Van Parijs L, Refaeli Y, Lord JD, Nelson BH, Abbas AK, Baltimore D. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity. 1999;11:281–288. doi: 10.1016/s1074-7613(00)80103-x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME, Yu J, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- Weigelt B, Glas AM, Wessels LF, Witteveen AT, Peterse JL, van't Veer LJ. Gene expression profiles of primary breast tumors maintained in distant metastases. Proc Natl Acad Sci U S A. 2003;100:15901–15905. doi: 10.1073/pnas.2634067100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigelt B, Peterse JL, van 't Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- Weis K, Rambaud S, Lavau C, Jansen J, Carvalho T, Carmo-Fonseca M, Lamond A, Dejean A. Retinoic acid regulates aberrant nuclear localization of PML-RAR alpha in acute promyelocytic leukemia cells. Cell. 1994;76:345–356. doi: 10.1016/0092-8674(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Welm AL, Kim S, Welm BE, Bishop JM. MET and MYC cooperate in mammary tumorigenesis. Proc Natl Acad Sci U S A. 2005;102:4324–4329. doi: 10.1073/pnas.0500470102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West M, Blanchette C, Dressman H, Huang E, Ishida S, Spang R, Zuzan H, Olson JA, Jr, Marks JR, Nevins JR. Predicting the clinical status of human breast cancer by using gene expression profiles. Proc Natl Acad Sci U S A. 2001;98:11462–11467. doi: 10.1073/pnas.201162998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan PS, Perry MR, Laux DE, Asare AL, Caldwell CW, Huang TH. CpG island arrays: an application toward deciphering epigenetic signatures of breast cancer. Clin Cancer Res. 2000;6:1432–1438. [PubMed] [Google Scholar]
- Zhang DH, Yang L, Cohn L, Parkyn L, Homer R, Ray P, Ray A. Inhibition of allergic inflammation in a murine model of asthma by expression of a dominant-negative mutant of GATA-3. Immunity. 1999;11:473–482. doi: 10.1016/s1074-7613(00)80122-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01